Patents
Literature
235 results about "Healthy individuals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
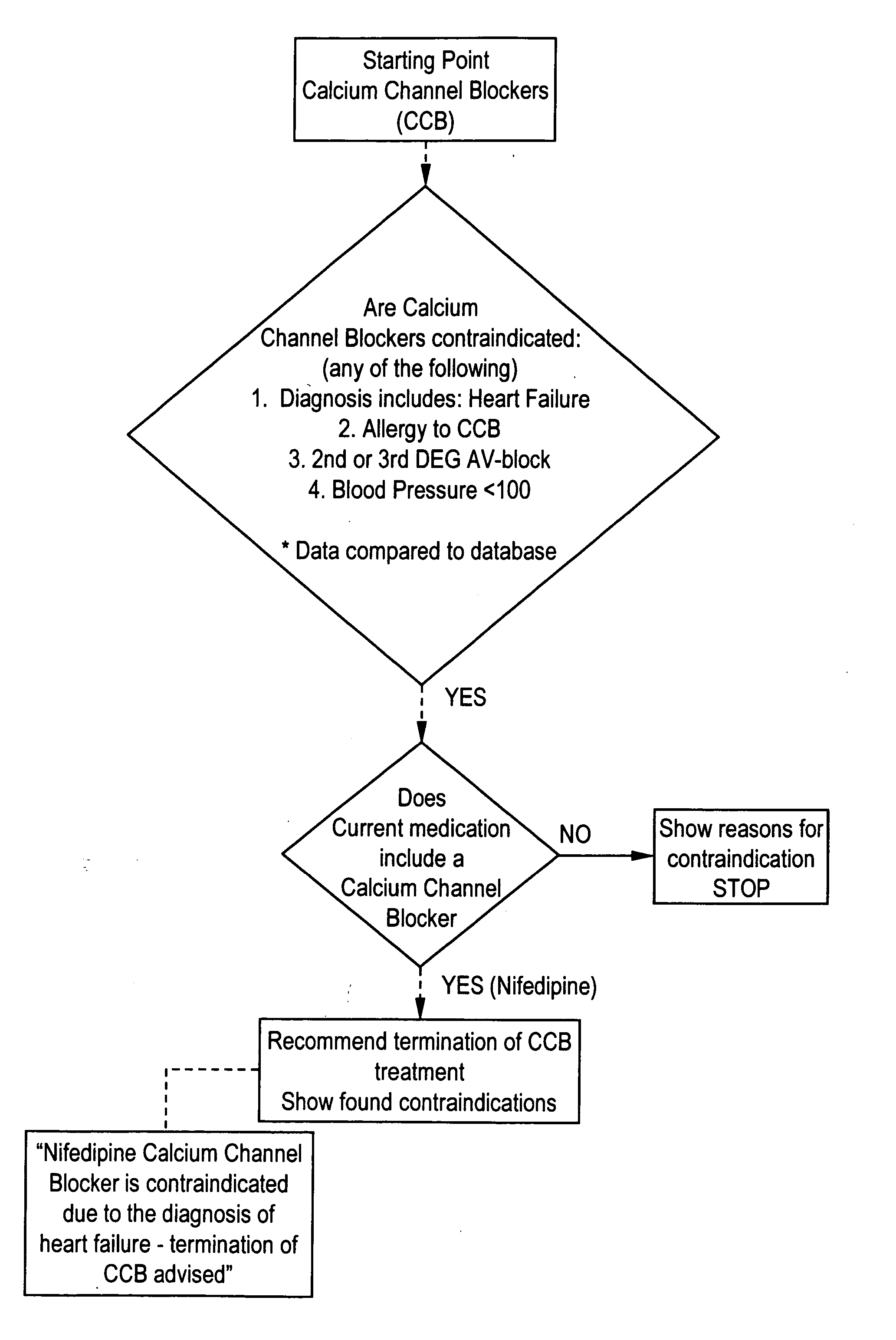
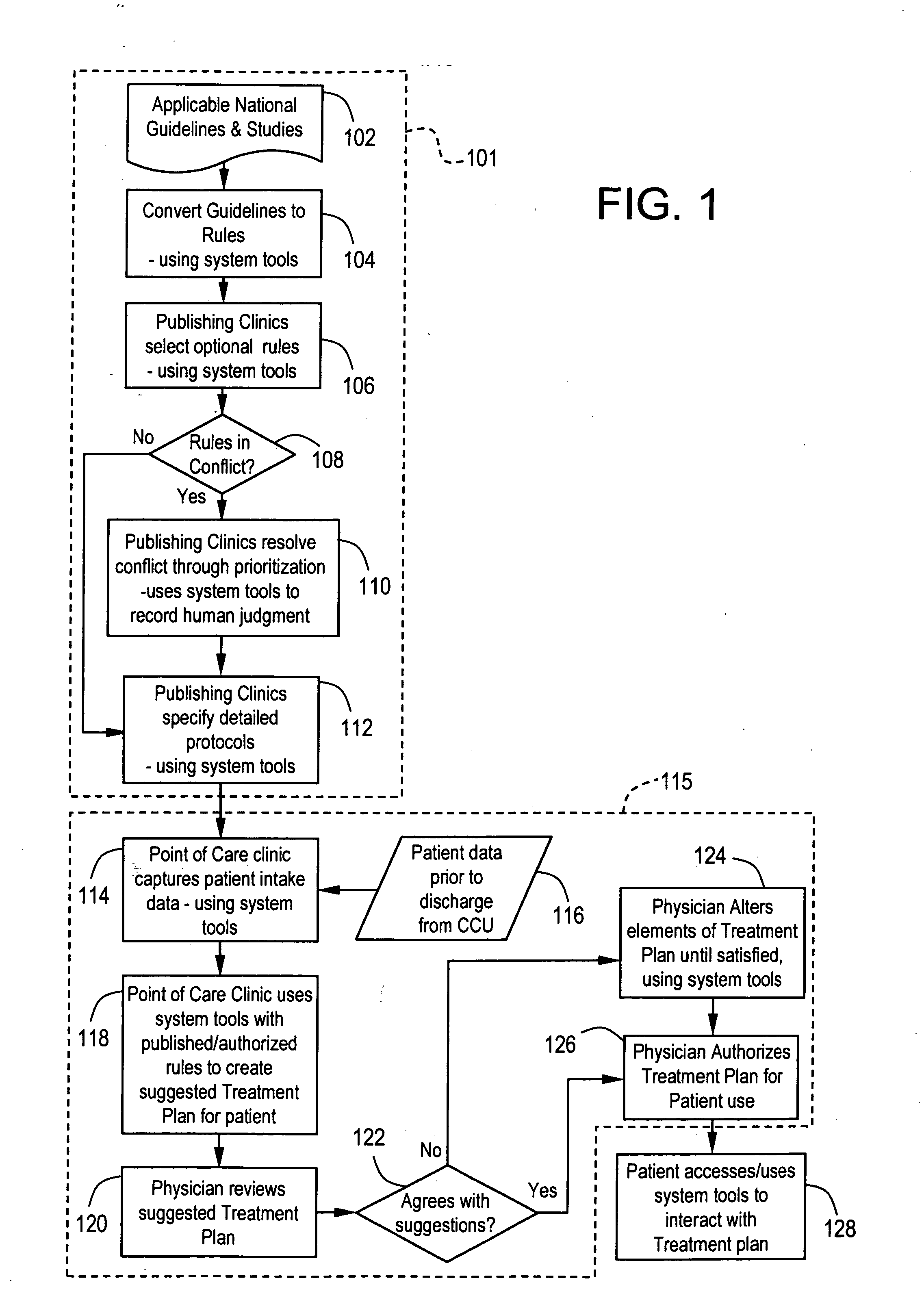
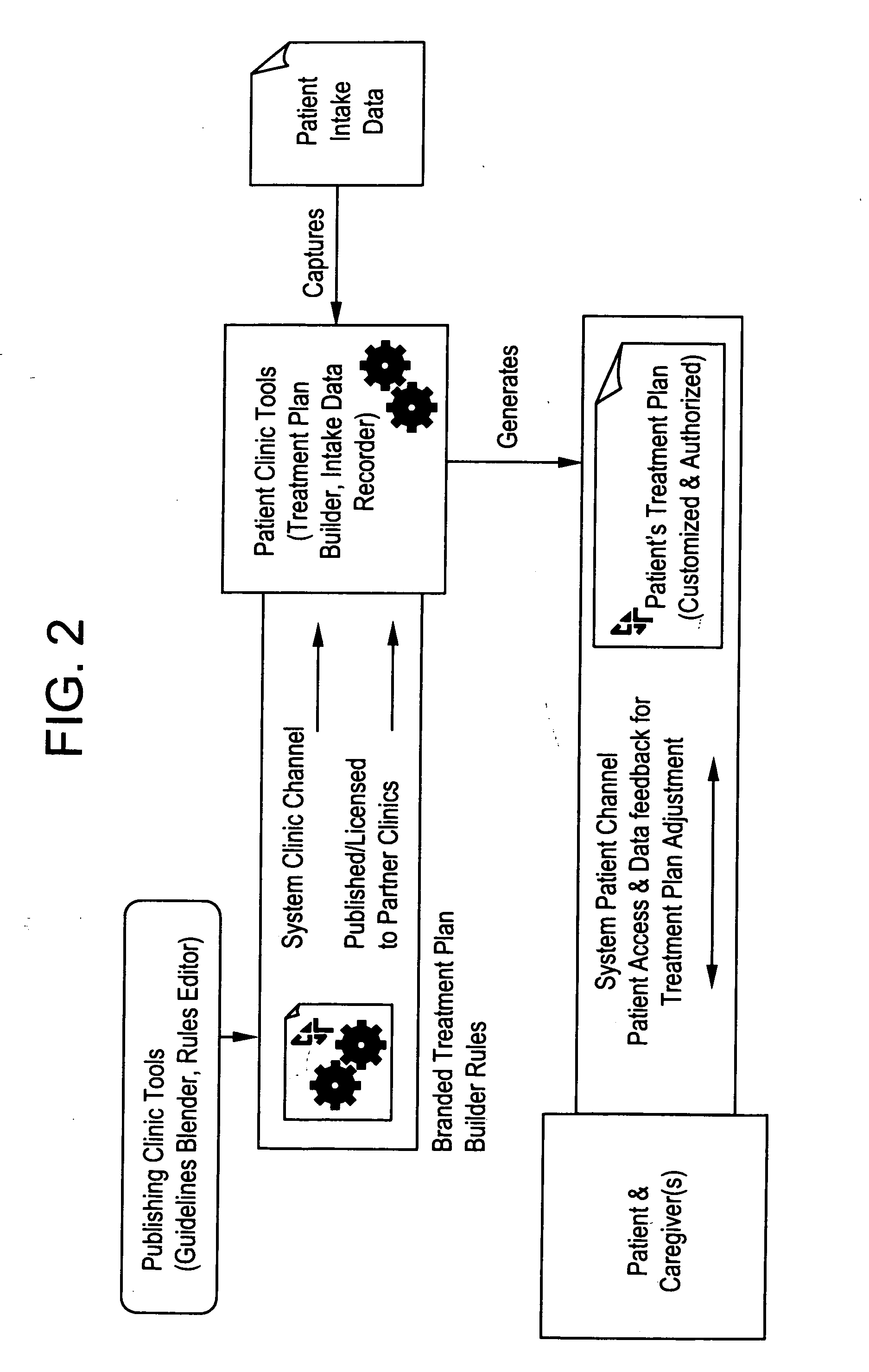
Methods and apparatus for automated interactive medical management
InactiveUS20050043965A1Data processing applicationsDrug and medicationsTherapy planningHealthy individuals
The present invention provides computerized tools for disigning and implementing one or more treatment plans for automated interactive management of one or more individuals having one or more diagnosed medical conditions or for health maintenance of one or more apparently healthy individuals.
Owner:PHEMI
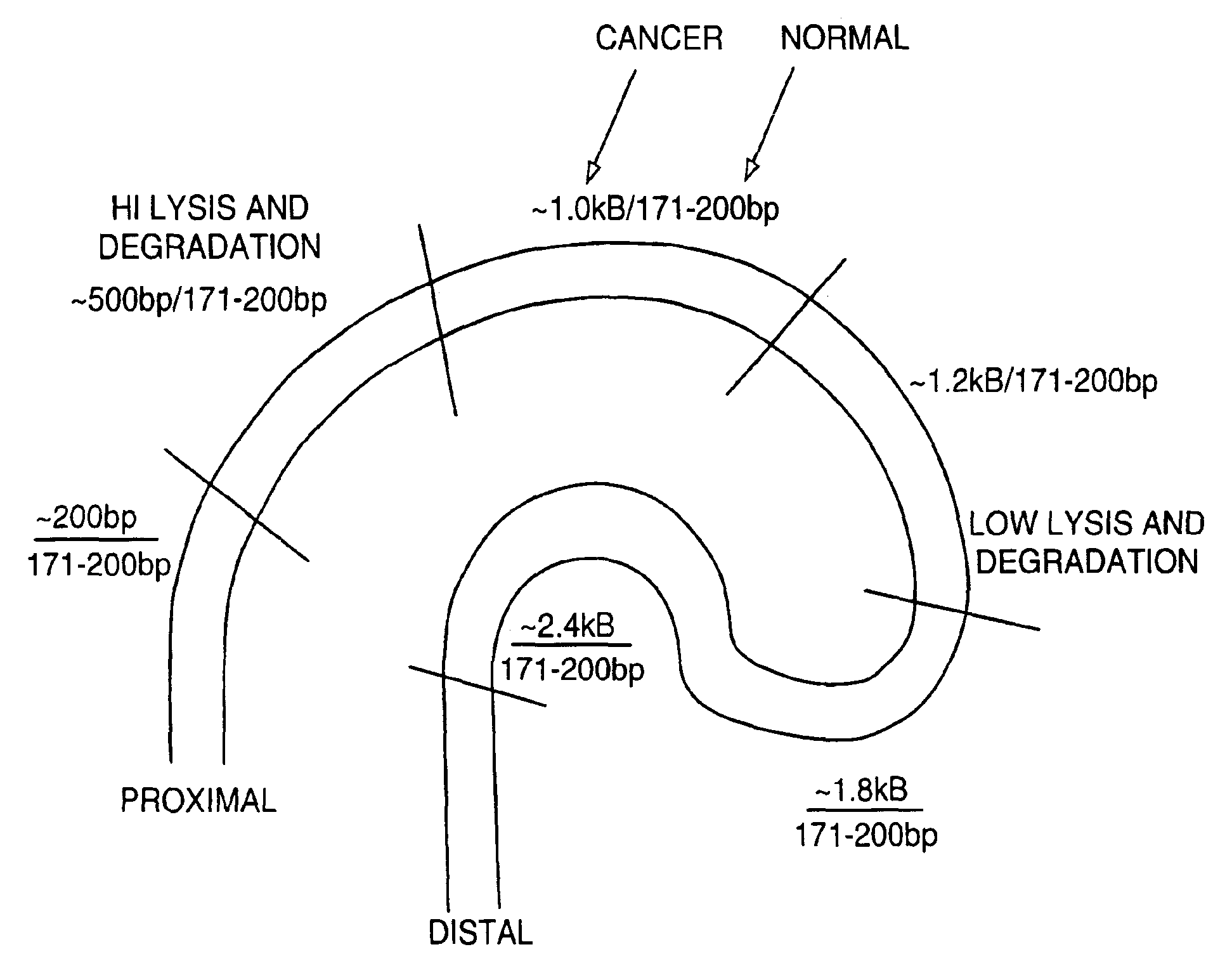
Methods for detecting nucleic acids indicative of cancer
InactiveUS6964846B1High molecular weightMicrobiological testing/measurementRecombinant DNA-technologyCellular DebrisBody fluid sample
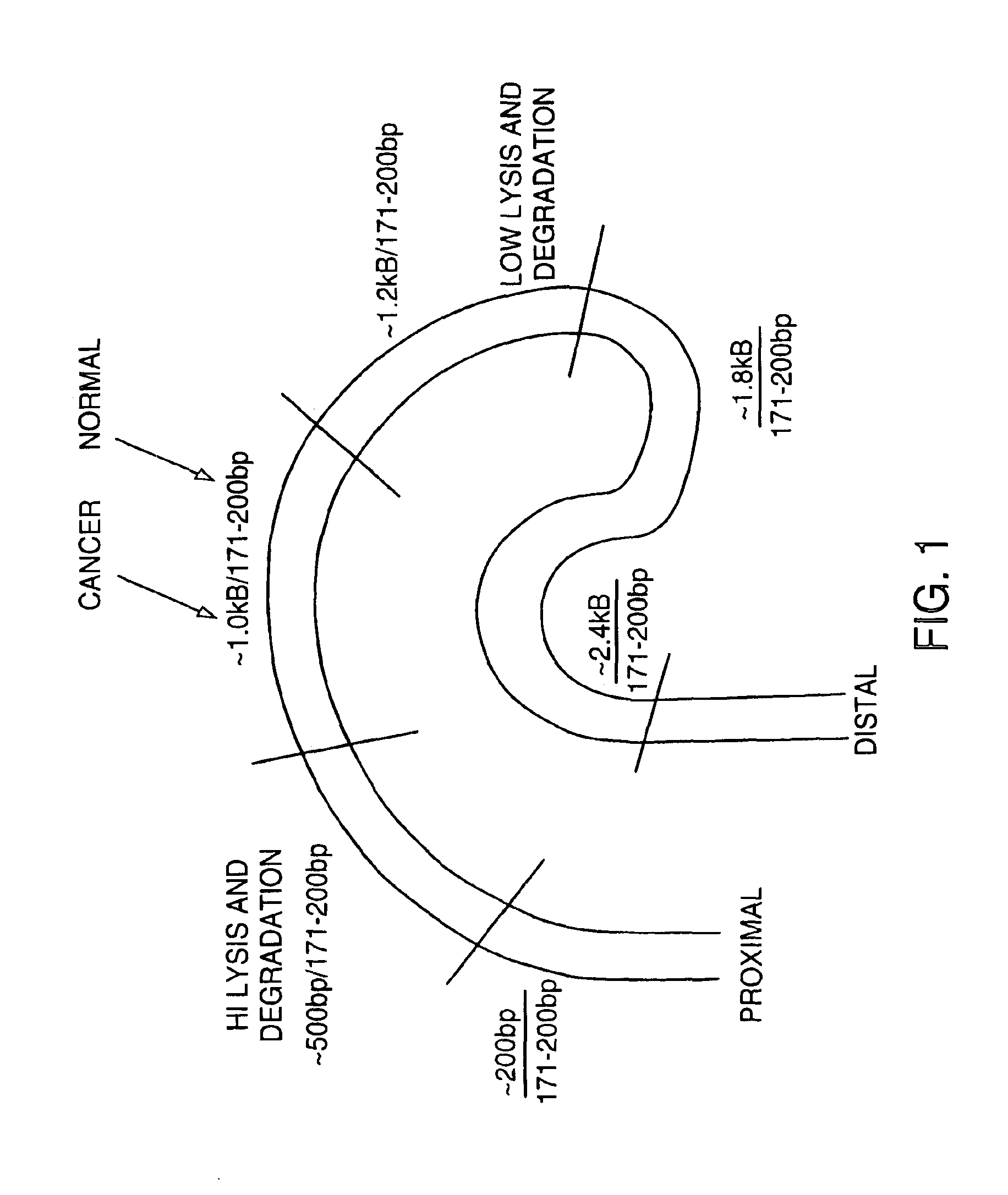
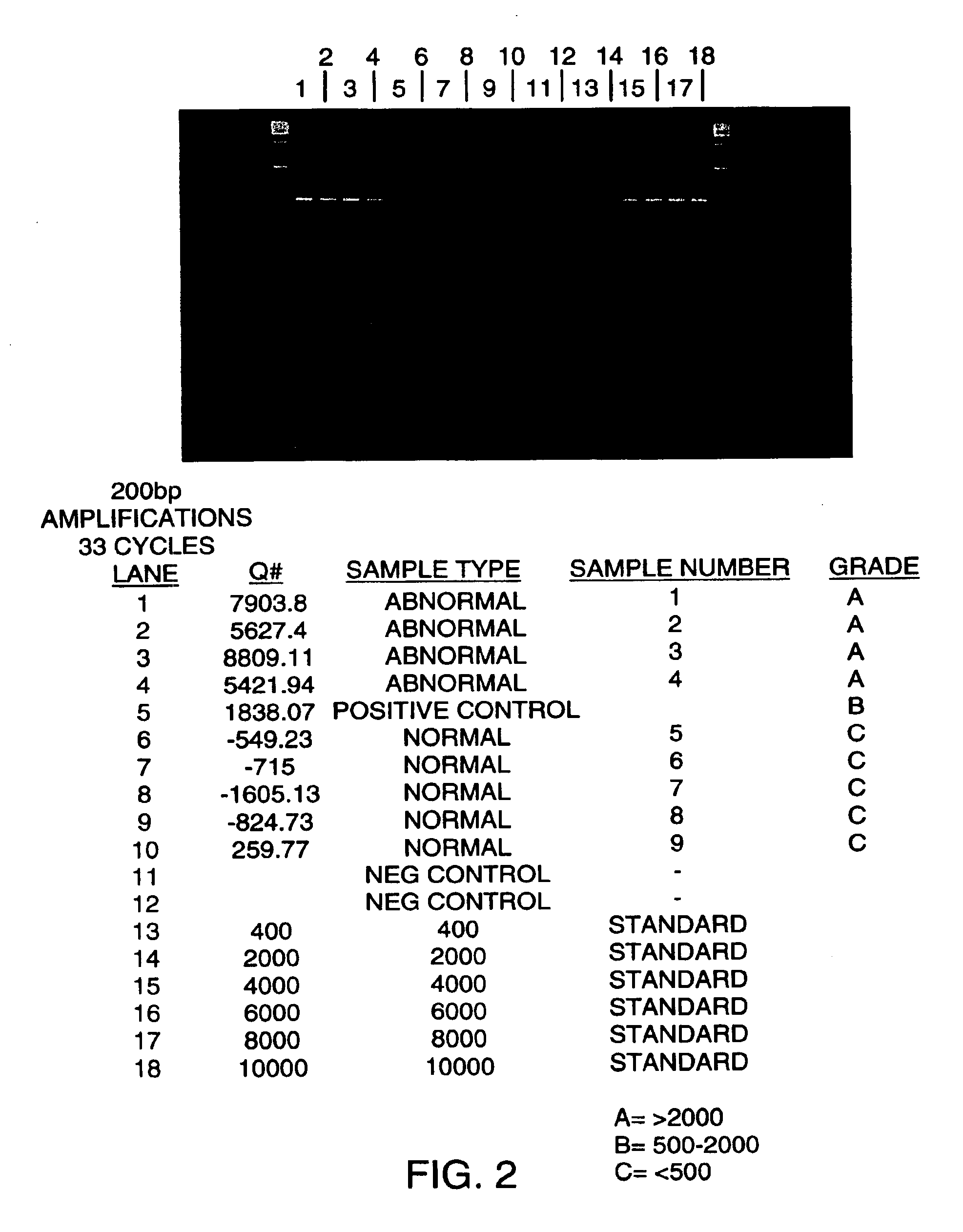
The invention provides methods for screening tissue or body fluid samples for nucleic acid indicia of cancer or precancer.Method are provided for screening a patient for cancer or precancer by detecting the presence of nucleic acid fragments that are longer than nucleic acid fragments expected to be present in a sample obtained from a healthy individual. In one embodiment, a positive screen for cancer or precancer is identified when a patient tissue or body fluid sample comprising exfoliated cells or cellular debris contains an amount of nucleic acid of a length greater than about 200 base pairs that exceeds a predetermined amount.
Owner:EXACT SCI CORP
Biomarkers useful for diagnosing prostate cancer, and methods thereof
InactiveUS20090127454A1Improve diagnostic capabilitiesImpact to detectParticle separator tubesComponent separationMetaboliteProstate cancer
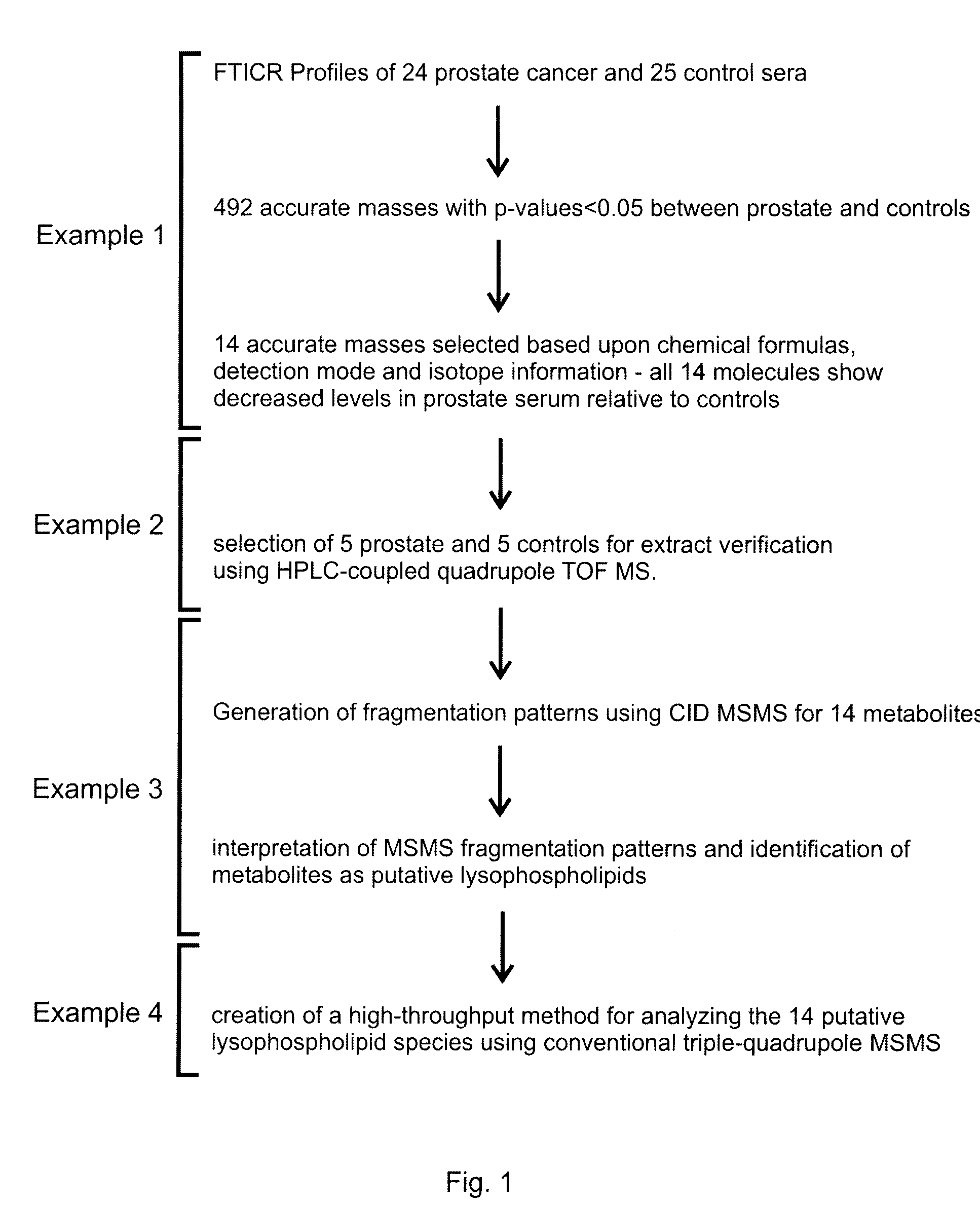
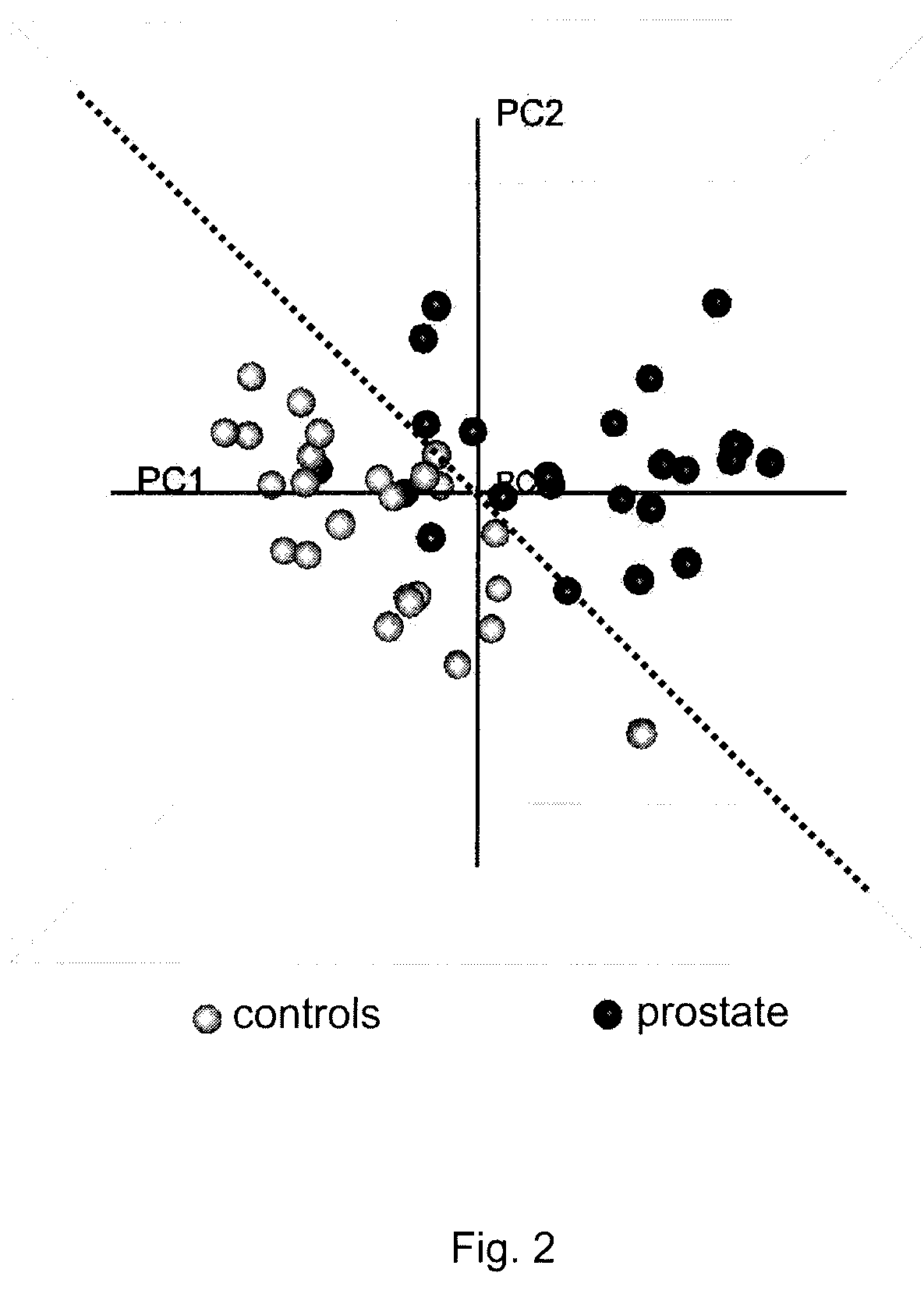
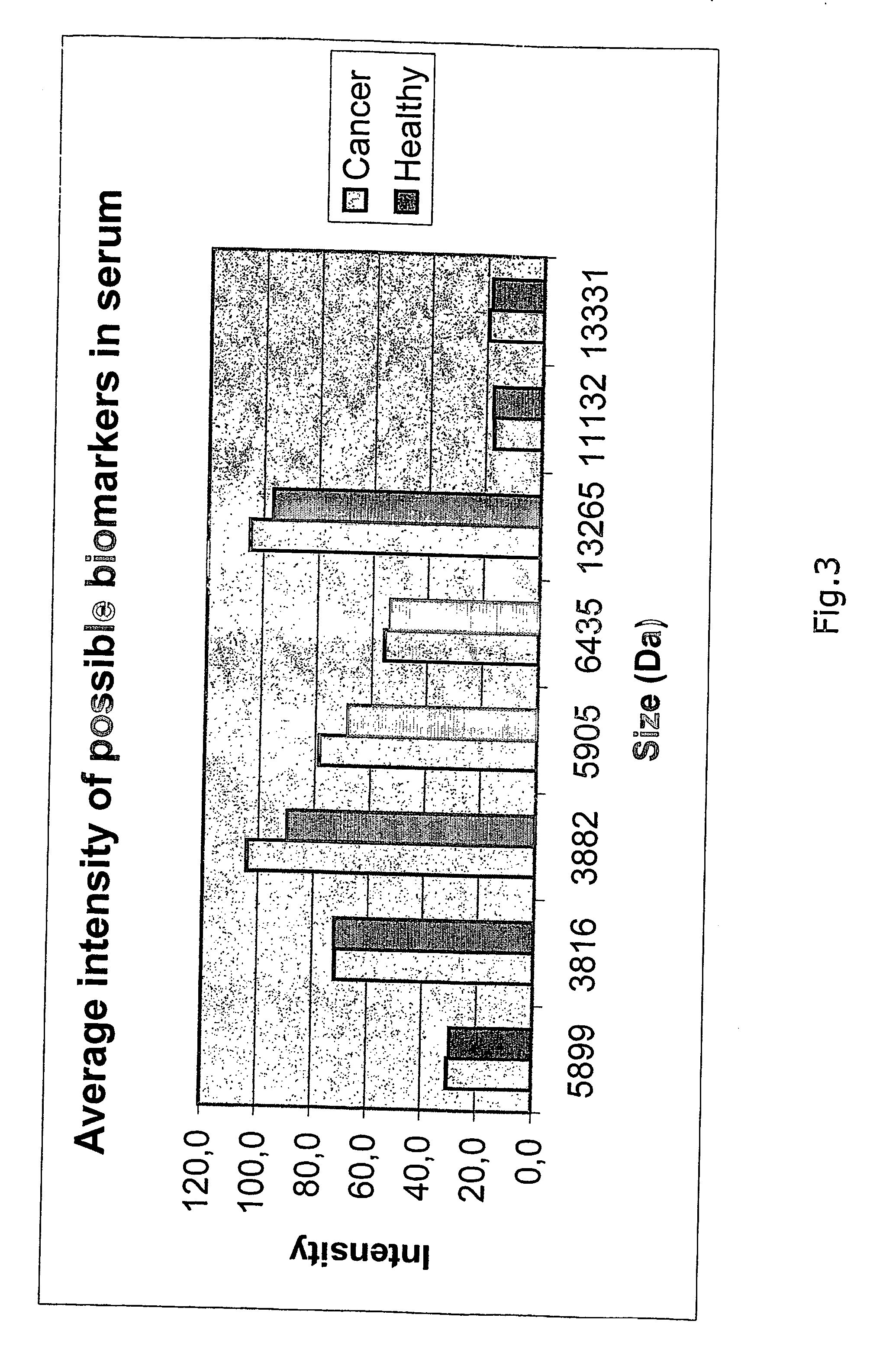
The present invention describes a method for predicting a health-state indicative of the presence of prostate cancer. The method measures the intensities of specific small biochemicals, called metabolites, in a blood sample from a patient with an undetermined health-state, and compares these intensities to the intensities observed in a population of healthy individuals and / or to the intensities previously observed in a population of confirmed prostate cancer-positive individuals. The method enables a practitioner to determine the probability that a screened patient is positive for prostate cancer.
Owner:MED LIFE DISCOVERIES LP
Diagnosis of disease state using MRNA profiles in peripheral leukocytes
InactiveUS6190857B1High sensitivitySugar derivativesMicrobiological testing/measurementWhite blood cellProstate cancer
Disclosed are diagnostic techniques for the detection of human disease states that affect gene expression in peripheral leukocytes. The invention relates particularly to probes and methods for evaluating the presence of RNA species that are differentially expressed in the peripheral blood of individuals with such a disease state compared to normal healthy individuals. The invention further relates to methods for detection of protein species that are differentially expressed in the peripheral blood of individuals with such a disease state compared to normal healthy individuals. Genetic probes, antibody probes and methods useful in monitoring the progression and diagnosis of two specific disease states, prostatic cancer and breast cancer, are described.
Owner:LAB OF AMERICA HLDG
Adam12 as a biomarker for bladder cancer
InactiveUS20090029372A1Improve the level ofLower Level RequirementsMicrobiological testing/measurementMaterial analysisTissue ArraysAffymetrix genechip
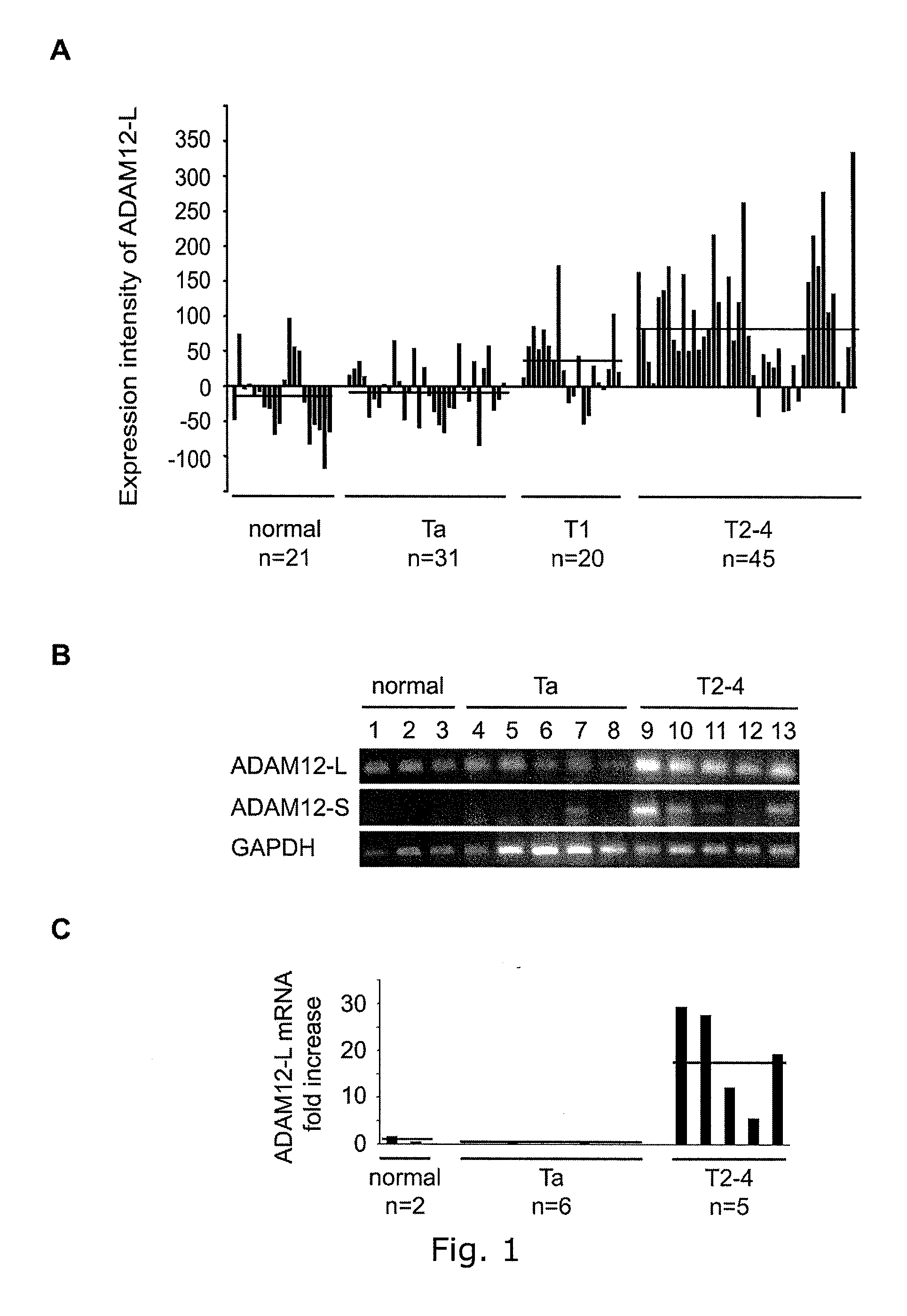
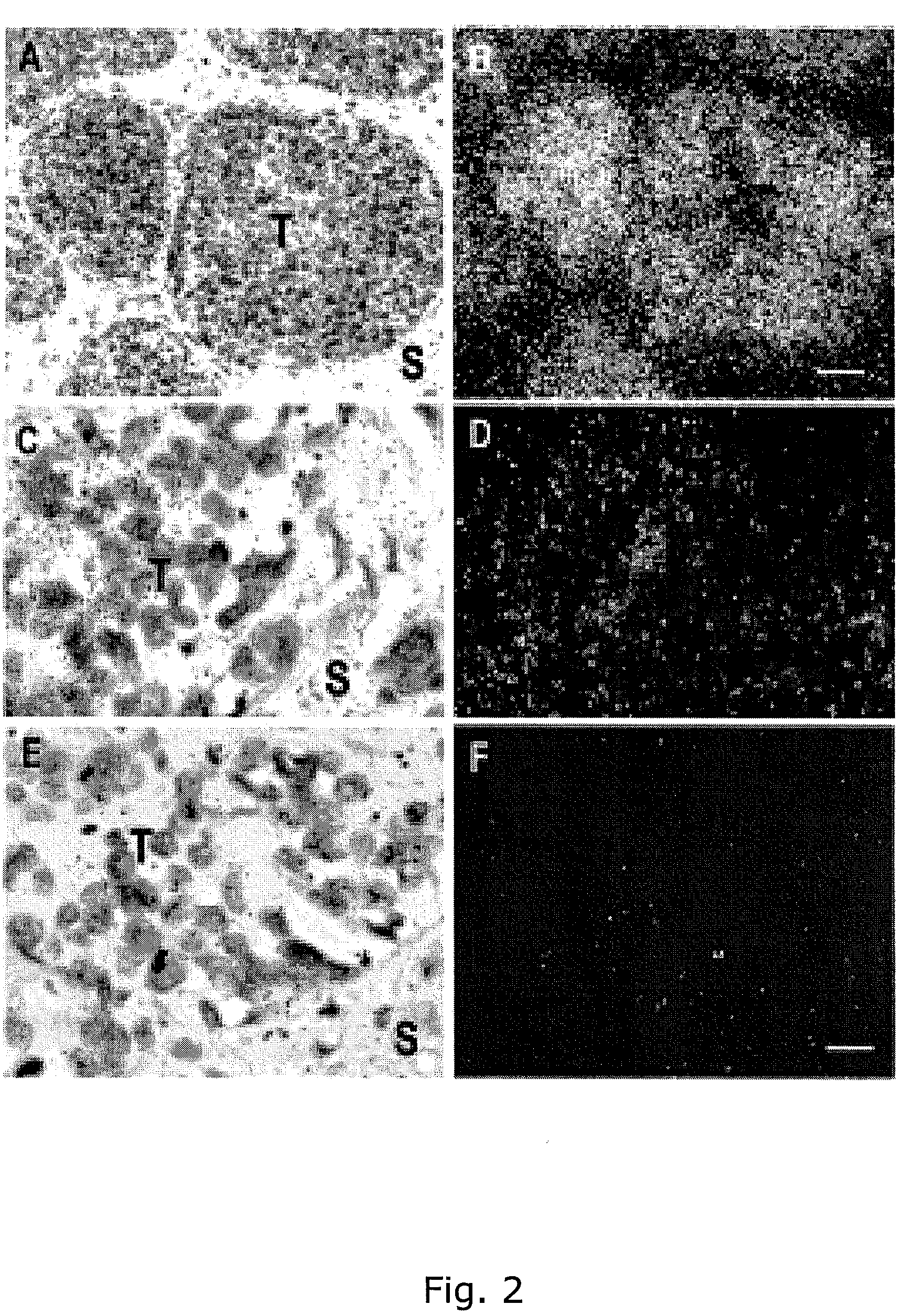
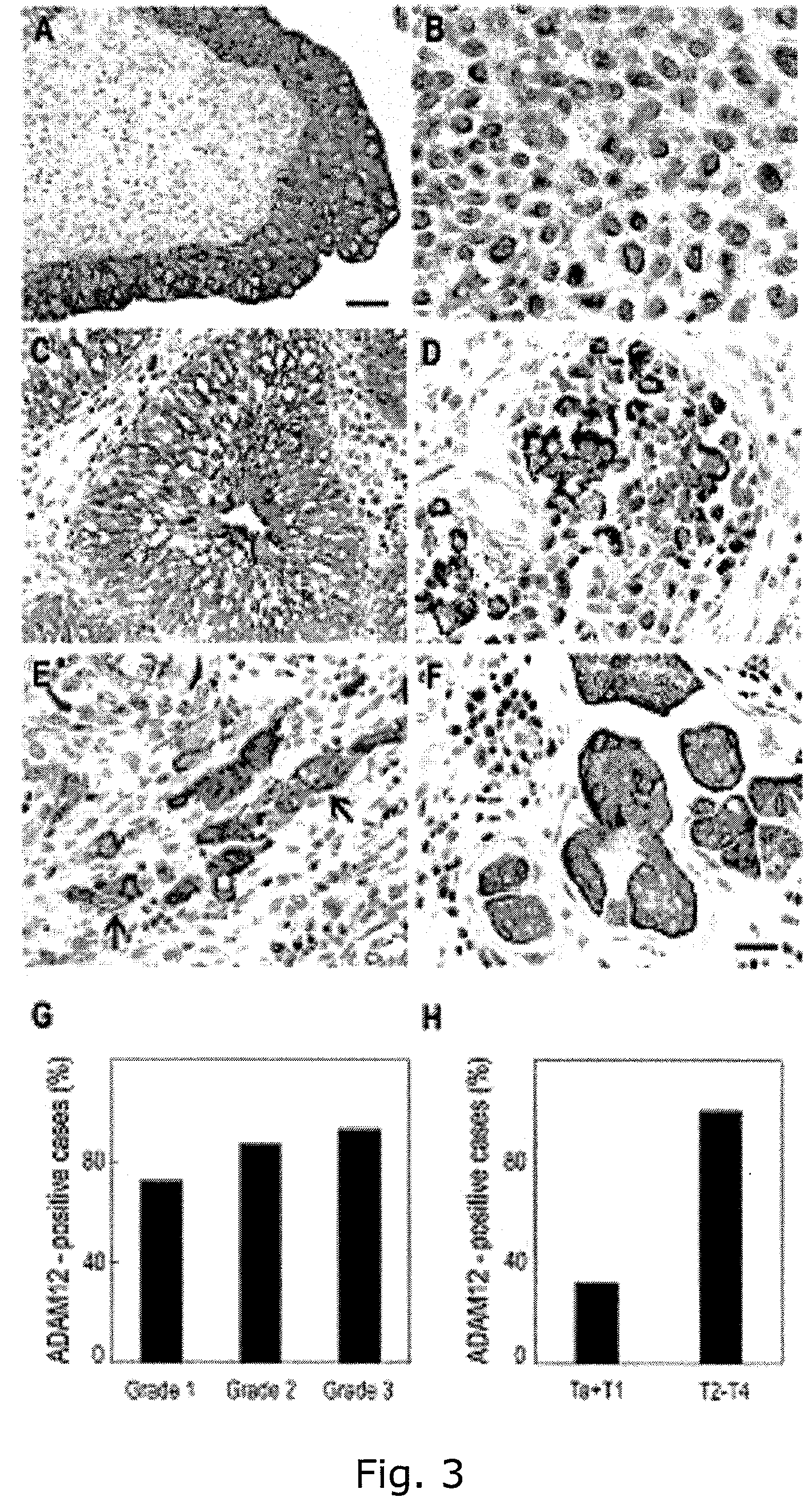
The present inventors have shown that the gene and protein expression profiles of ADAM8, ADAM10 and ADAM12 in different grades and stages of bladder cancer.ADAM12 gene expression was evaluated in tumors from 96 patients with bladder cancer using a customized Affymetrix GeneChip. Gene expression in bladder cancer was validated using reverse transcription-polymerase chain reaction (RT-PCR), quantitative PCR, and in situ hybridization. Protein expression was evaluated by immunohistochemical staining on tissue arrays of bladder cancers.The presence and relative amount of ADAM12 in the urine of cancer patients were determined by Western blotting and densitometric measurements, respectively.Particularly ADAM12 mRNA expression was significantly upregulated in bladder cancer, as determined by microarray analysis, and the level of ADAM12 mRNA correlated with disease stage. ADAM12 protein expression correlated with tumor stage and grade. ADAM12 was present in higher levels in the urine from bladder cancer patients than in urine from healthy individuals. Significantly, following removal of tumor by surgery, in most bladder cancer cases examined the level of ADAM12 in the urine decreased and, upon recurrence of tumor, increased.
Owner:PHYSICIANS CHOICE LAB SERVICES +1
Low level light therapy for enhancement of neurologic function
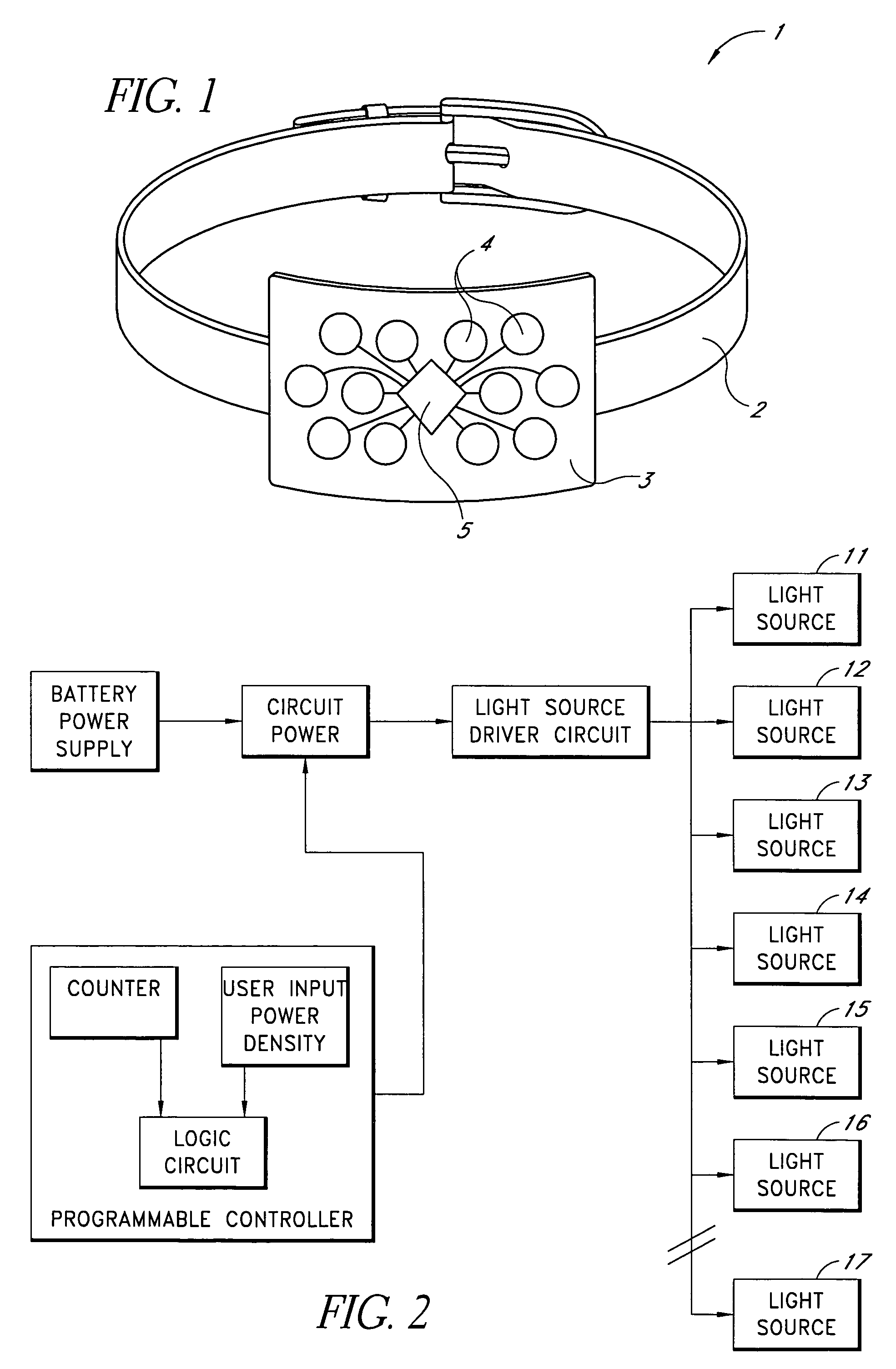
ActiveUS7534255B1Improve neurological functionIncreases ATP productionDiagnosticsSurgeryLight energyRisk stroke
Therapeutic methods for enhancing neurologic function such as may be desired in individuals having motor and / or cognitive impairment, including that resulting from Alzheimer's disease, dementia, head trauma, mental disease such as depression, stroke and neurodegeneration, as well as in healthy individuals are described, the methods including delivering a cognitive enhancing effective amount of light energy having a wavelength in the visible to near-infrared wavelength range to a target area of the brain. The neurologic function enhancing effective amount of light energy, in accordance with a preferred embodiment, is a predetermined power density (mW / cm2) at the level of the brain tissue being treated, and is delivered by determining a surface power density of the light energy that is sufficient to deliver the predetermined power density of light energy to the target brain tissue. In one embodiment, progenitor cells are treated using light energy and implanted into the central nervous system of a patient.
Owner:PHOTOTHERA IP HLDG
Antisense composition and method for treating muscle atrophy
A method and compound for treating skeletal muscle mass deficiency in a human subject are disclosed. The composition is an oligomer of morpholino subunits and phosphorus-containing intersubunit linkages joining a morpholino nitrogen of one subunit to a 5′ exocyclic carbon of an adjacent subunit, contains between 10-40 nucleotide bases, has a base sequence effective to hybridize to an expression-sensitive region of processed or preprocessed human myostatin RNA transcript, identified, in its processed form, by SEQ ID NO:6, and is capable of uptake by target muscle cells in the subject. In practicing the method, the compound is administered in an amount and at a dosage schedule to produce an overall reduction in the level of serum myostatin measured in the patient, and preferably to bring the myostatin level within the a range determined for normal, healthy individuals.
Owner:AVI BIOPHARMA
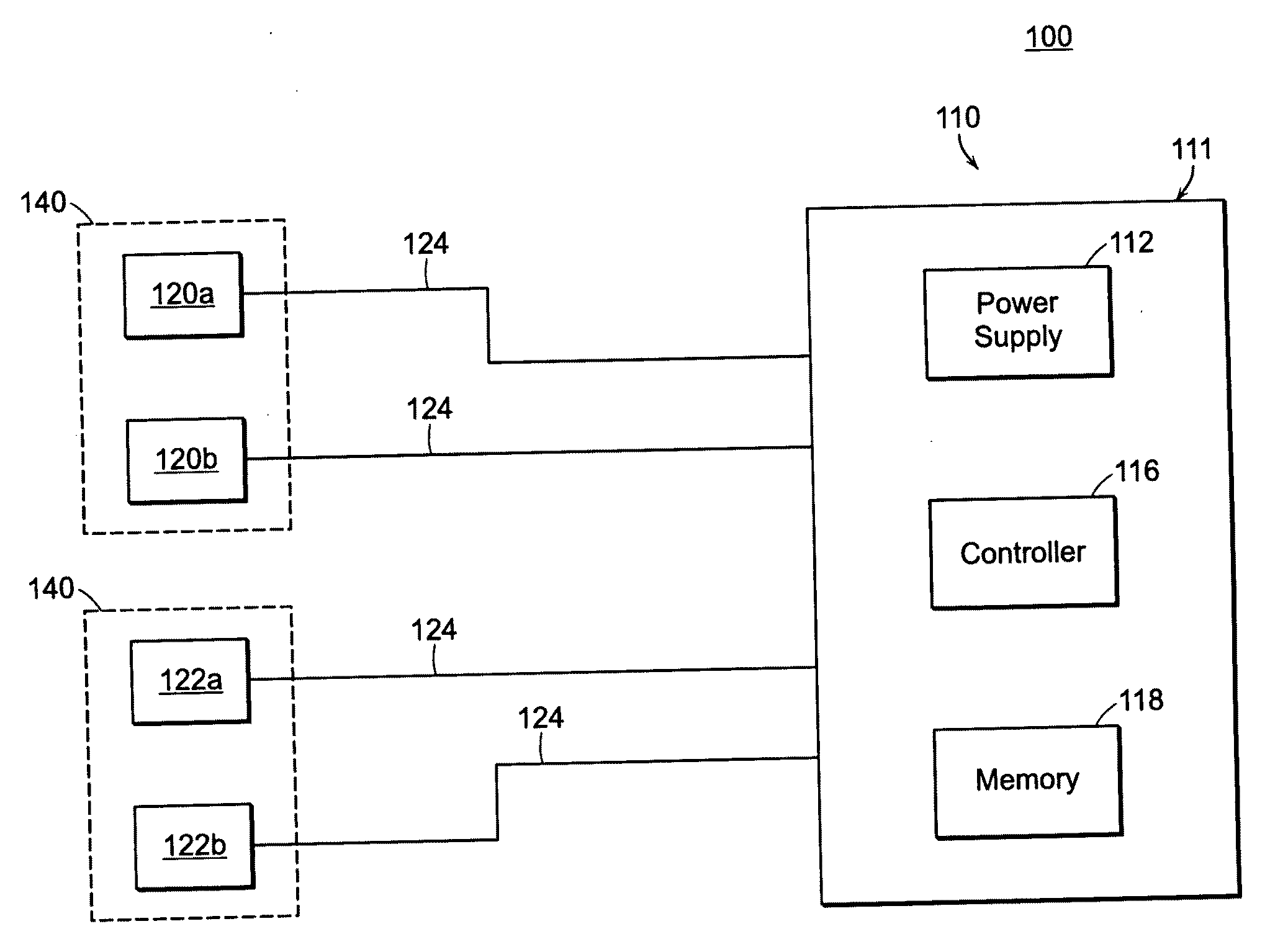
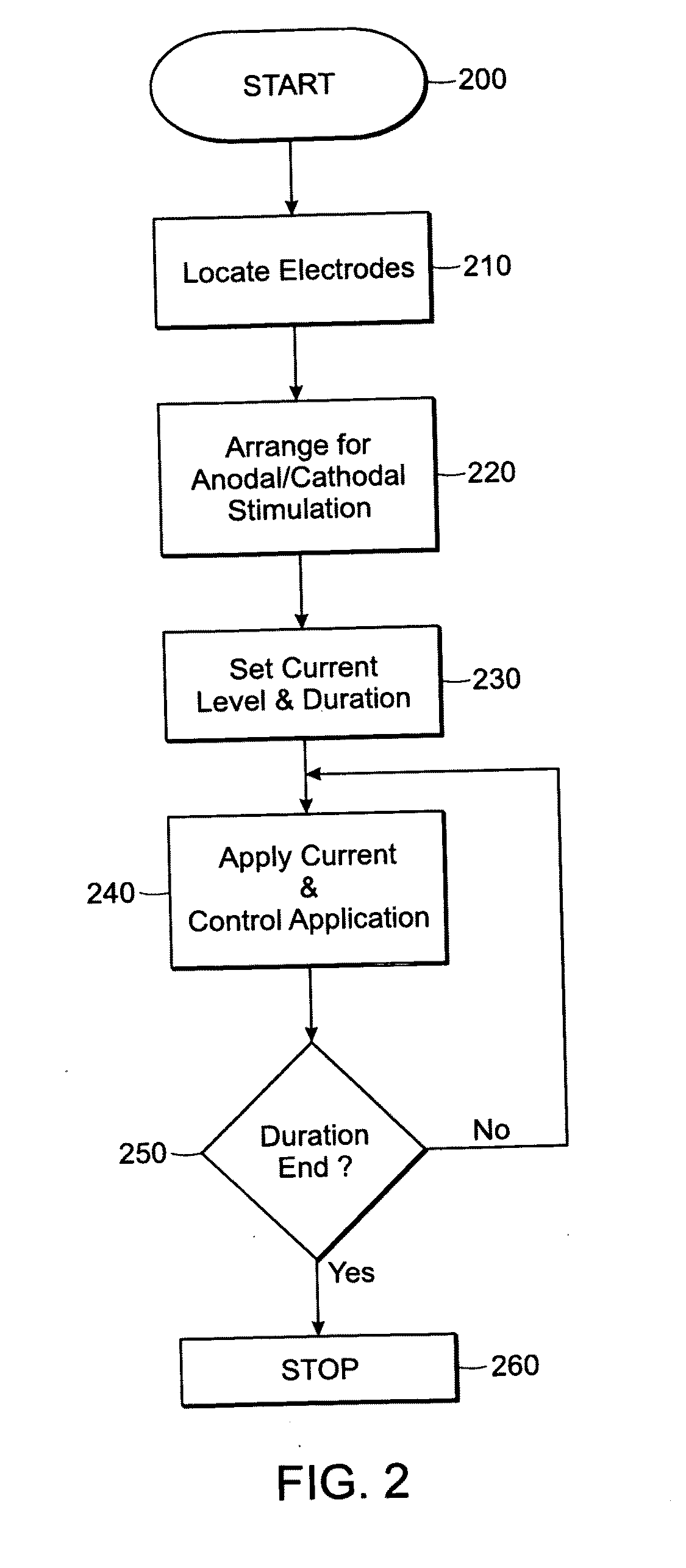
Methods and devices for increasing learning and effects of training in healthy individuals and patients after brain lesions using DC stimulation and apparatuses and systems related thereto
InactiveUS20100268287A1Easy to learnImprove abilitiesInternal electrodesExternal electrodesPower flowMedicine
Featured is a method and device for increasing learning and effects of training. Such methods include locating a pair of electrodes on a head of a person in relation to a specific area of the brain, applying a desired DC current to the electrodes at a level sufficient to stimulate the brain tissue; and controlling the DC current application so the current is applied to the specific brain area at least one of before, during or after such a learning or training event. In this way, application of the DC current to the brain area improves a subject's ability to acquire one of motor skills or knowledge of the learning or training event or the ability to retain the motor skills or knowledge of the learning or training event. The person to which the electrodes are attached can be healthy or a patient after brain lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for the treatment of fabry disease using pharmacological chaperones
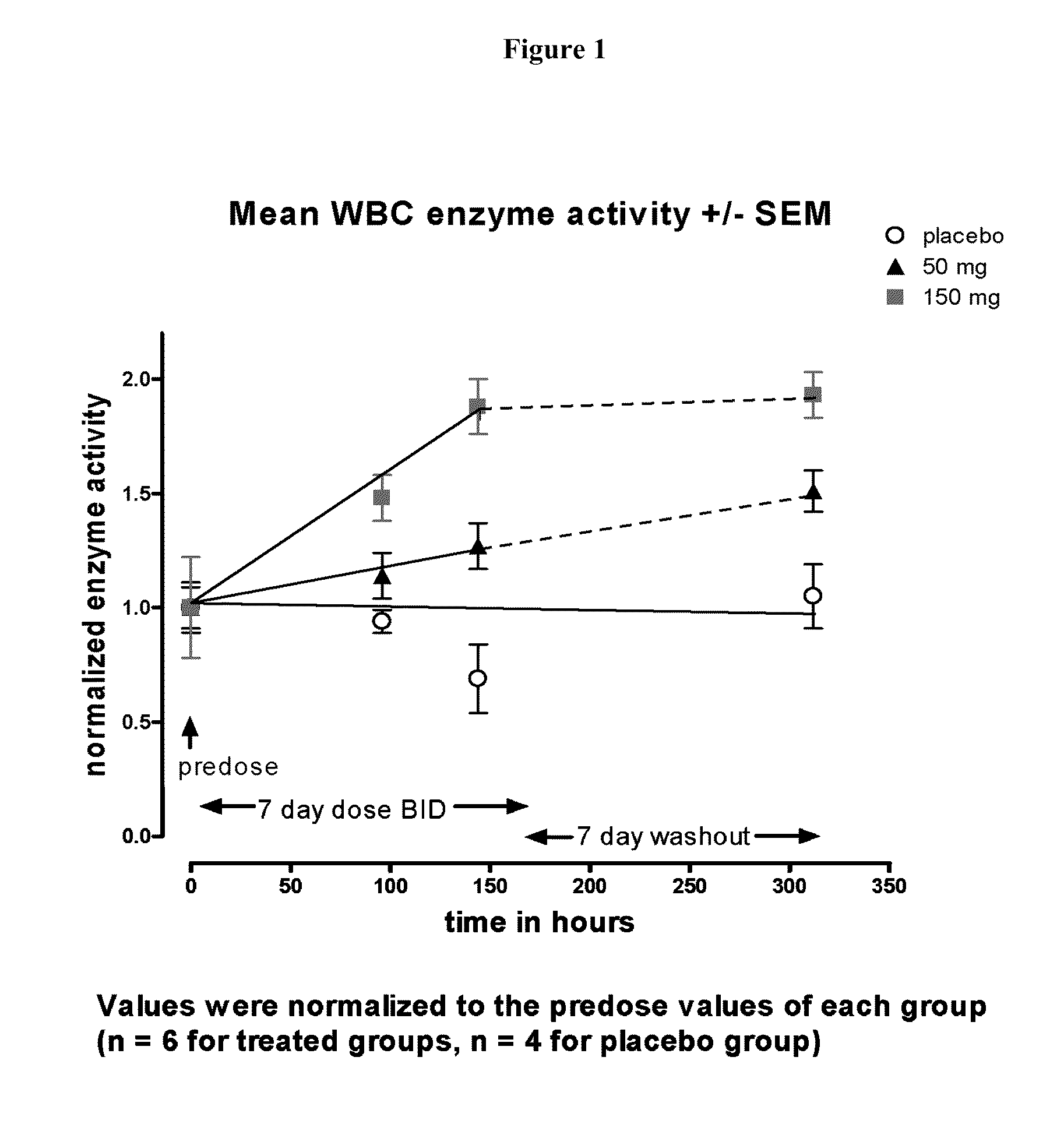
InactiveUS20100113517A1High activityHigh protein activityBiocidePeptide/protein ingredientsPharmacometricsCytoplasmic staining
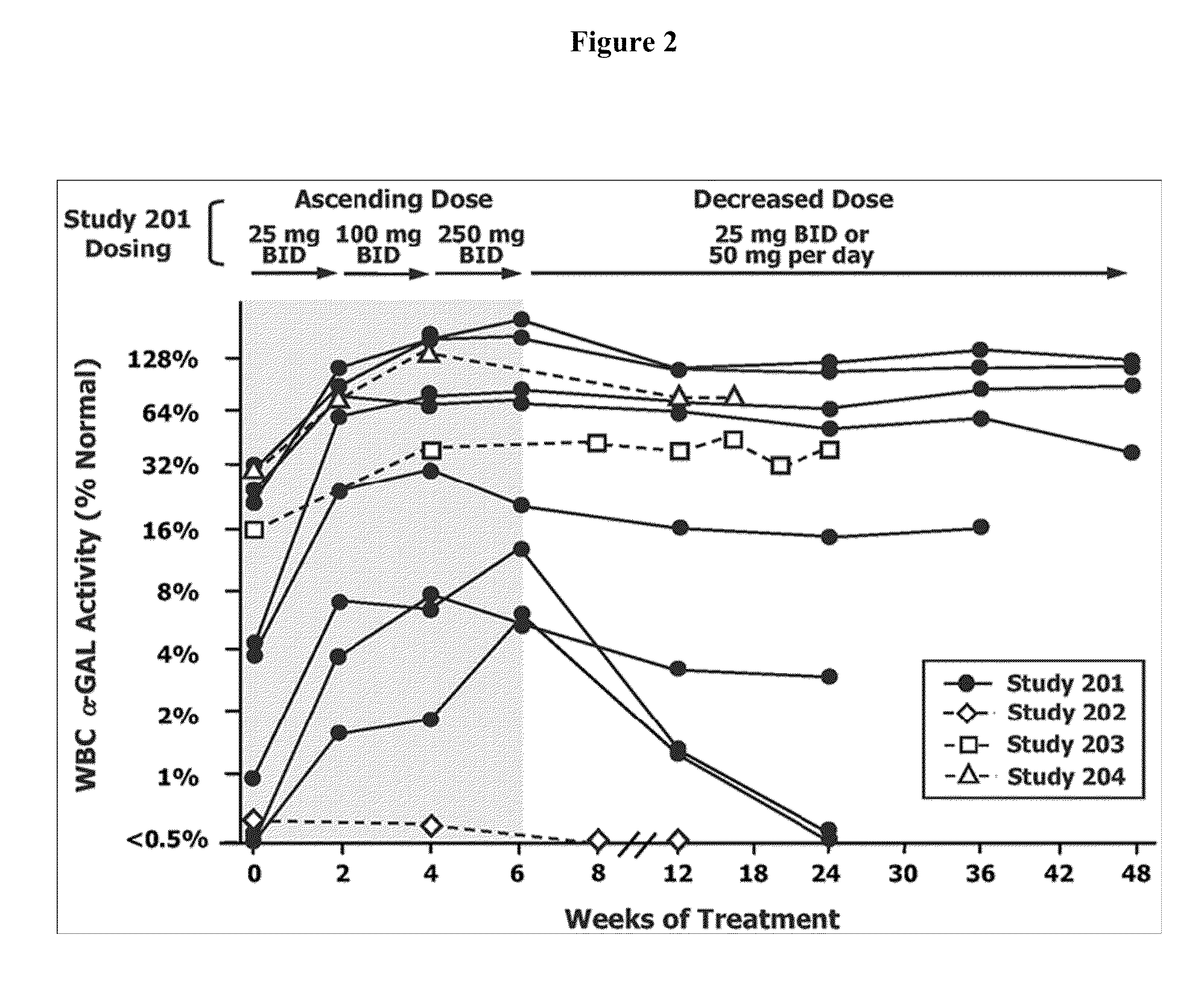
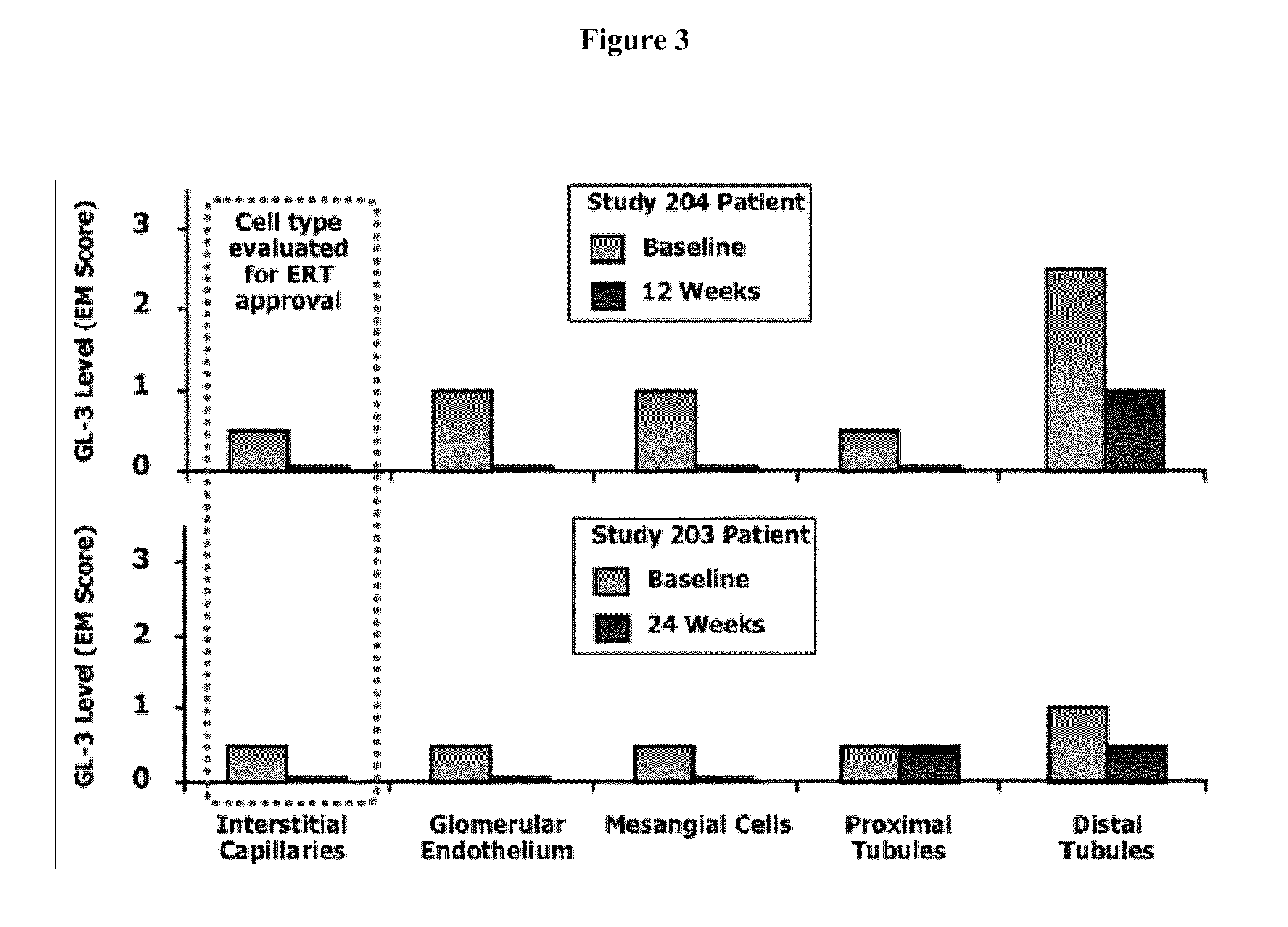
The present invention provides a method treating a patient with Fabry disease by determining whether there is an improvement of a surrogate marker that is associated with Fabry disease following administration of a specific pharmacological chaperone of α-galactosidase A. The method includes administering an effective amount of 1-deoxygalactonojirimycn to the individual, wherein the 1-deoxygalactonojirimycin binds to alpha-galactosidase A in an amount effective to increase activity of the alpha-galactosidase A. The present invention also provides a method for monitoring and increasing a therapeutic response of a patient with Fabry disease following administration of a specific pharmacological chaperone of α-galactosidase A by evaluating the effect on the cytoplasmic staining pattern of a cell from the patient, wherein detection of a staining pattern in the cell that is similar to the staining pattern in a cell from a healthy individual indicates that the individual with Fabry disease is a responder.
Owner:AMICUS THERAPEUTICS INC
Compositions and Methods for Improving Mitochondrial Function and Treating Neurodegenerative Diseases and Cognitive Disorders
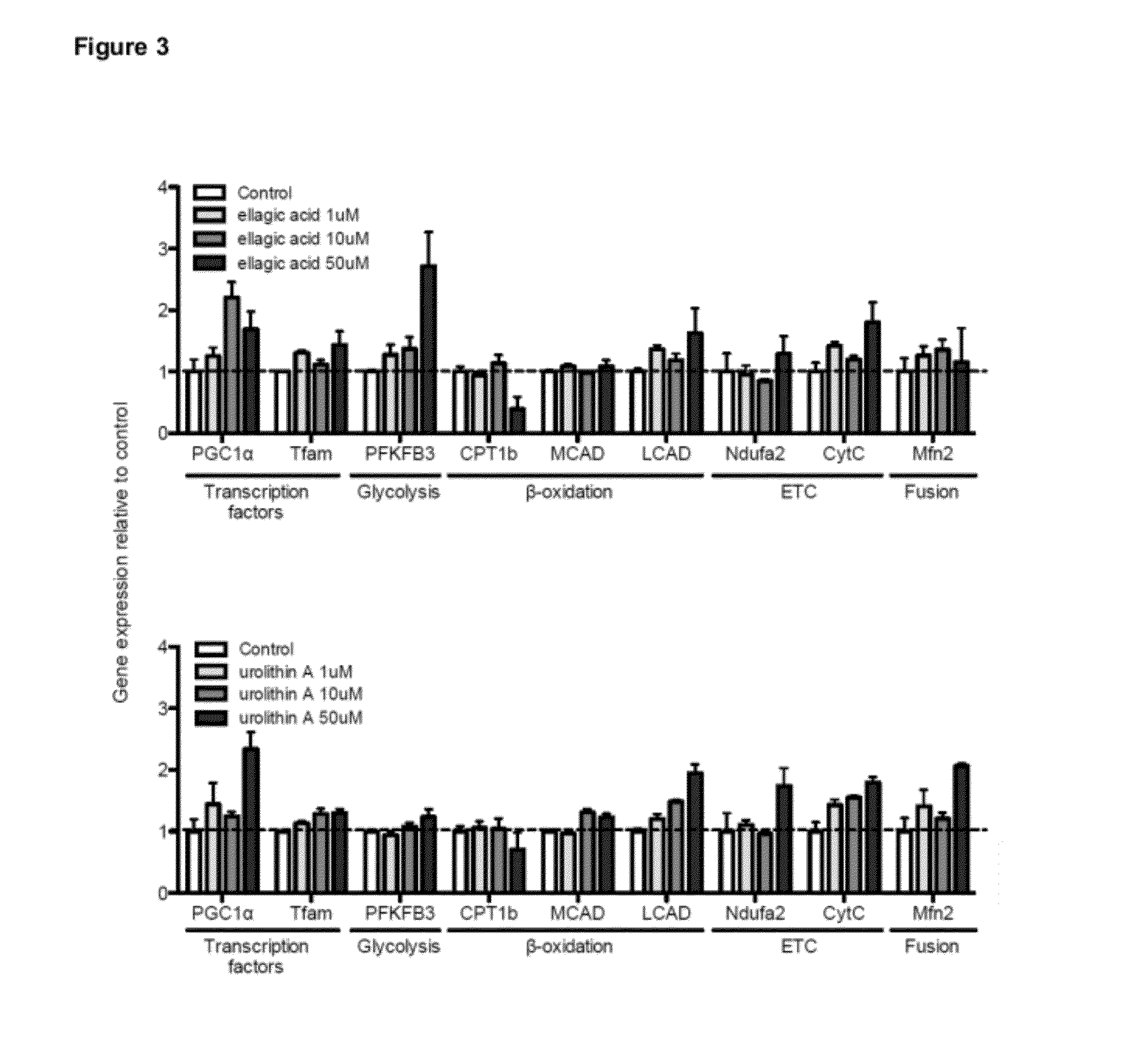
ActiveUS20120164243A1Increase and maintain metabolic rateImprove and maintain mental performanceBiocideNervous disorderPhysiologyUrolithin
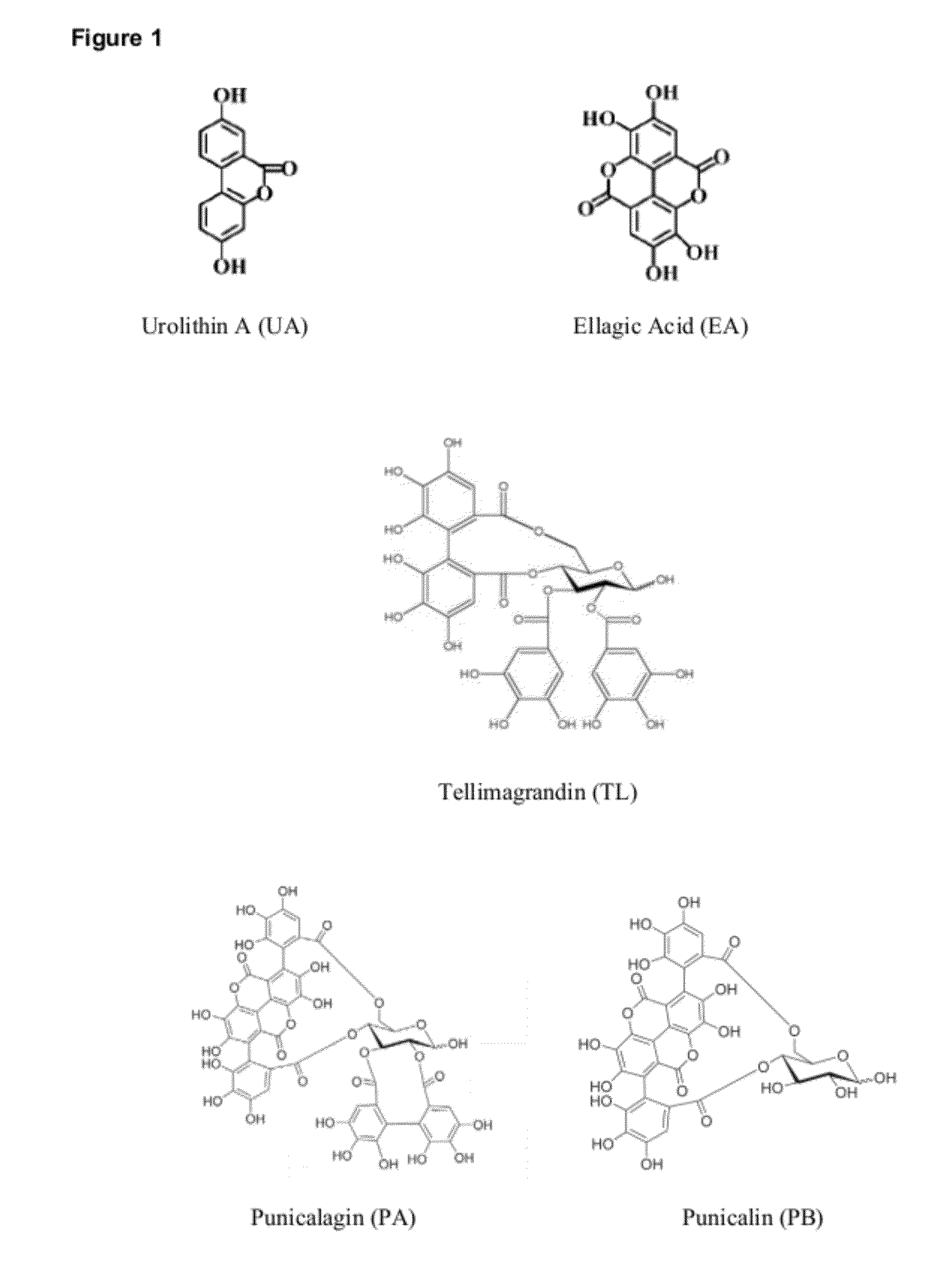
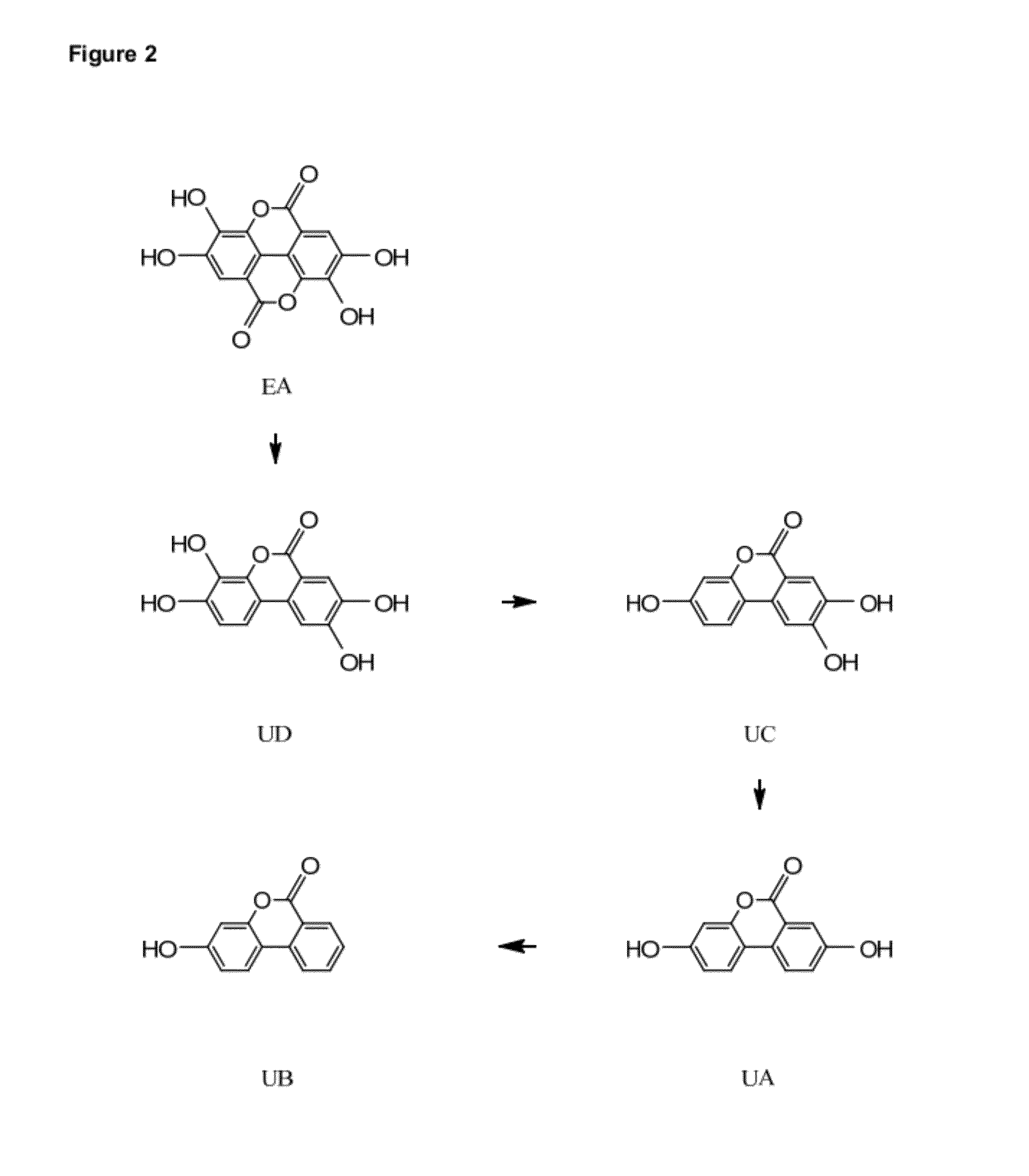
Provided are compositions comprising compounds or precursors to compounds which may be used for a variety of therapeutic applications including, for example, treating and / or preventing a disease or disorder related to reduced or inadequate mitochondrial activity, including aging or stress, diabetes, obesity, and neurodegenerative diseases. The compounds relate generally to urolithins and precursors thereof, including but not limited to ellagitannins and urolithin A. In certain embodiments the compositions are presented in or as food products or nutritional supplements. These same compounds and compositions can also be used advantageously in generally healthy individuals to increase or maintain metabolic rate, decrease percent body fat, increase or maintain muscle mass, manage body weight, improve or maintain mental performance (including memory), improve or maintain muscle performance, improve or maintain mood, and manage stress.
Owner:AMAZENTIS
Use Of Genes As Molecular Markers In Diagnosis Of Schizophrenia And Diagnostic Kit For The Same
InactiveUS20080274455A1Sugar derivativesMicrobiological testing/measurementQuantitative Real Time PCRScreening method
Drug-naive and drug-free schizophrenic PBL were screened to identify additional markers that are differentially expressed compared to healthy individuals using microarray and quantitative real-time PCR (QRT-PCR) techniques. Genes for dopamine D2 receptor (DRD2) and inwardly rectifying potassium channel (Kir2.3) were found to be overexpressed in microarray analysis. Increased mRNA levels were confirmed by QRT-PCR using SybrGreen method and dual labeled TaqMan probes.The invention relates to a method for diagnosing schizophrenia in a subject comprising assessing the level or the expression level of at least one of the following genes or proteins: Kir2.3 or DRD2 or a gene encoding Kir2.3 or DRD2. The invention further relates to agents and uses thereof, said agents specifically binding to said proteins or nucleic acids encoding them, diagnostic kits and screening methods.Use of both molecular markers allow prediction of schizophrenia and help to follow efficiency of drugs in therapy in order to provide a more tailored medication for schizophrenic patients.
Owner:THE BIOLOGICAL RES CENT OF THE HUNGARIAN ACAD OF SCI
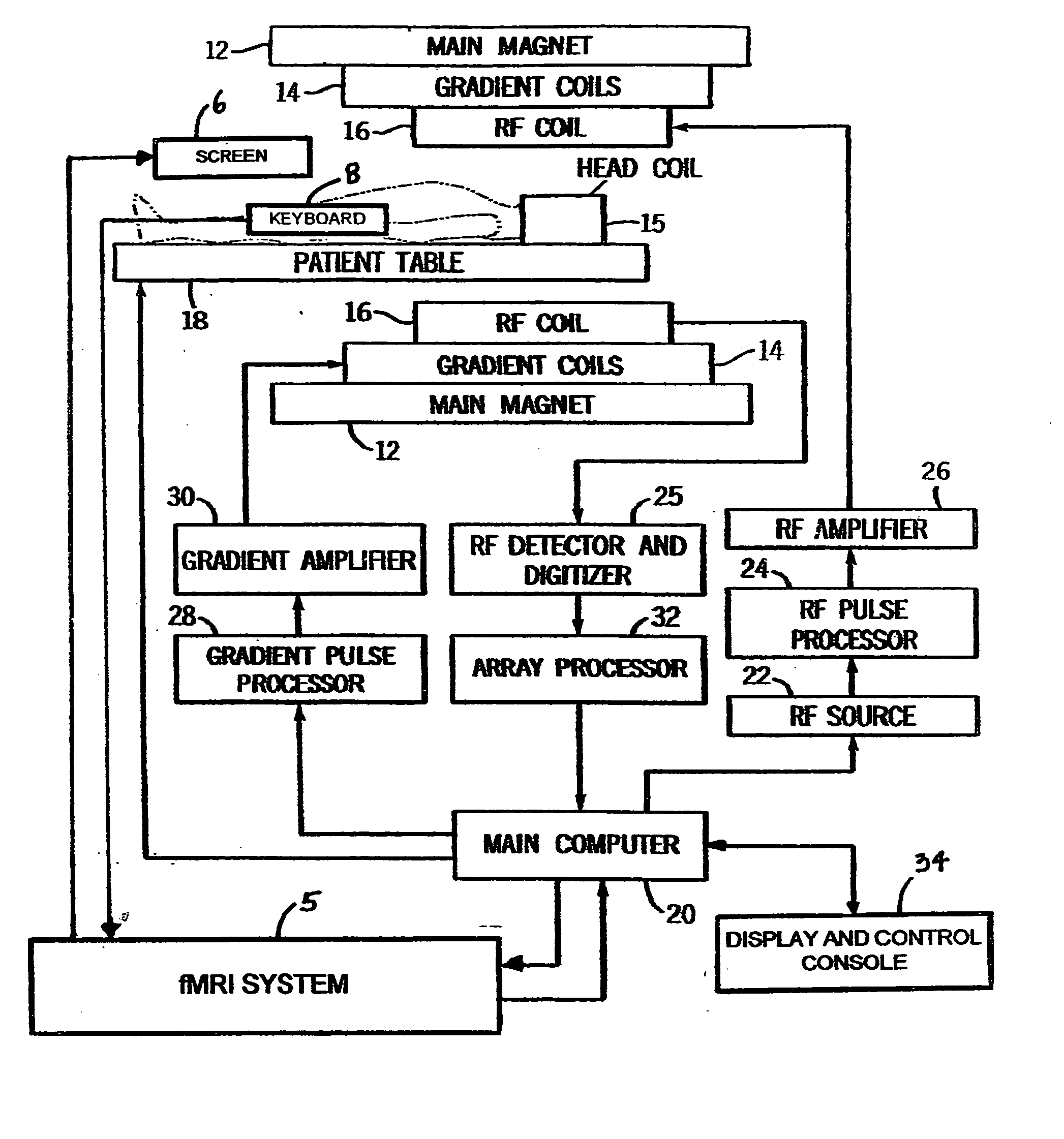
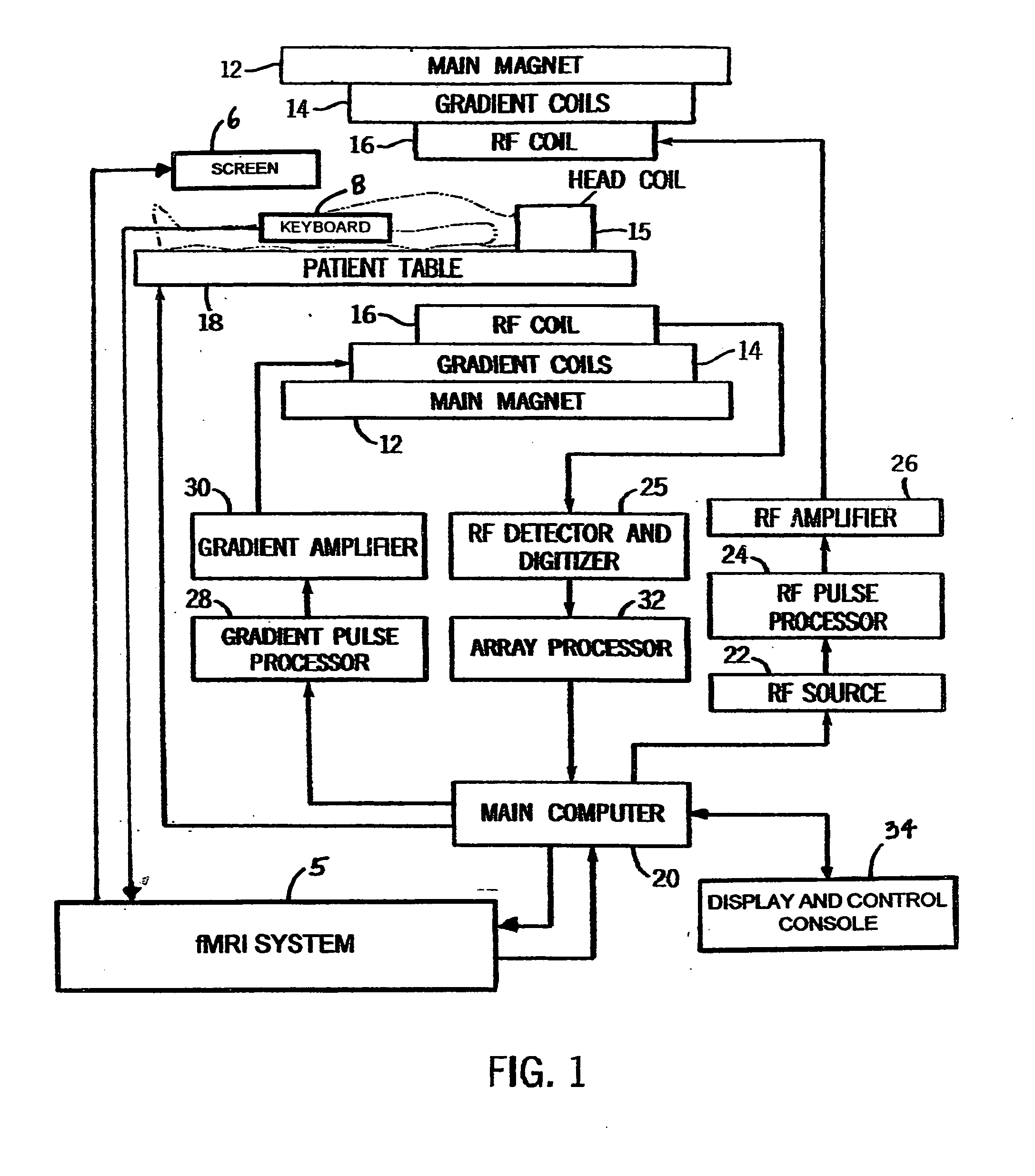
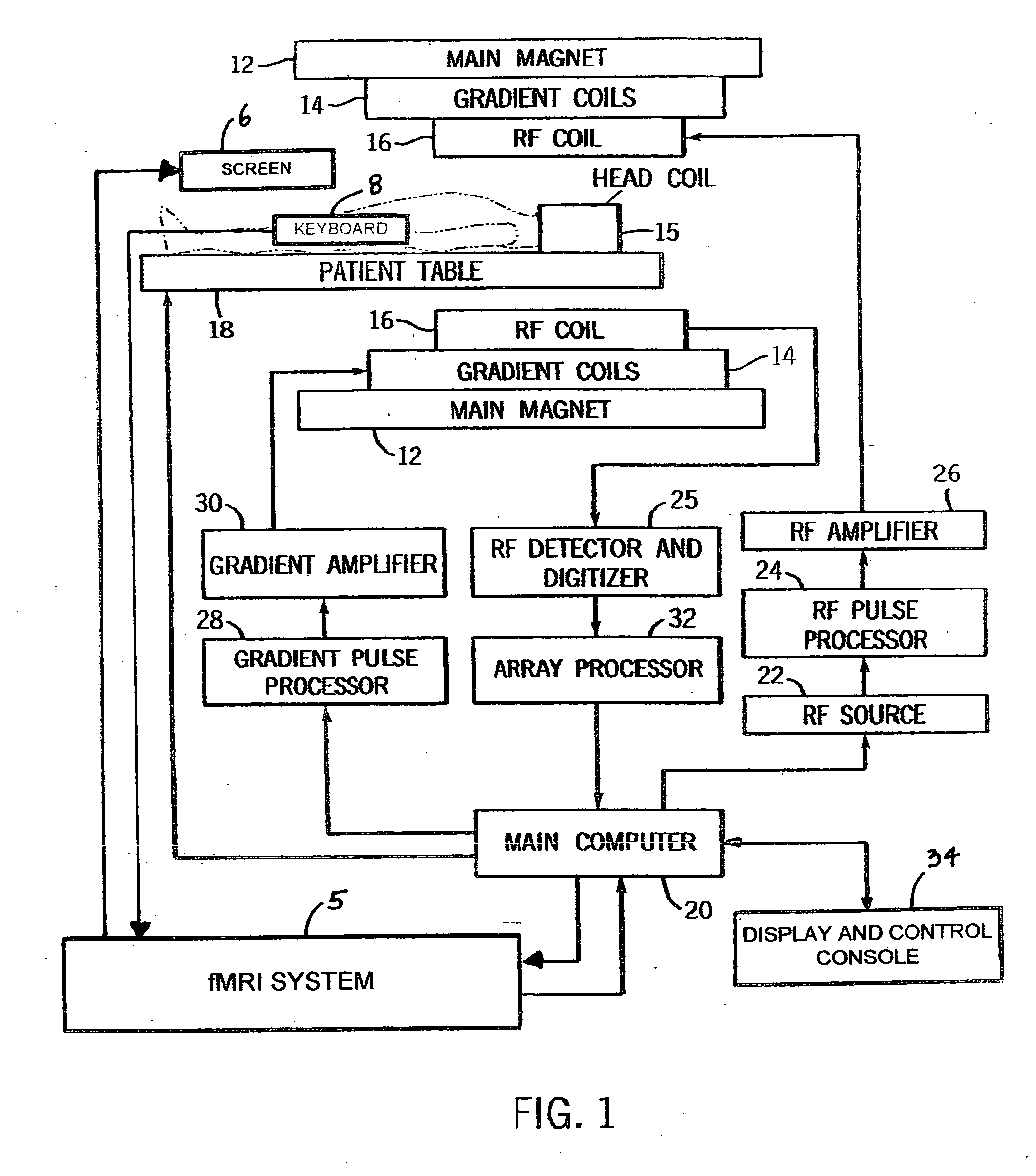
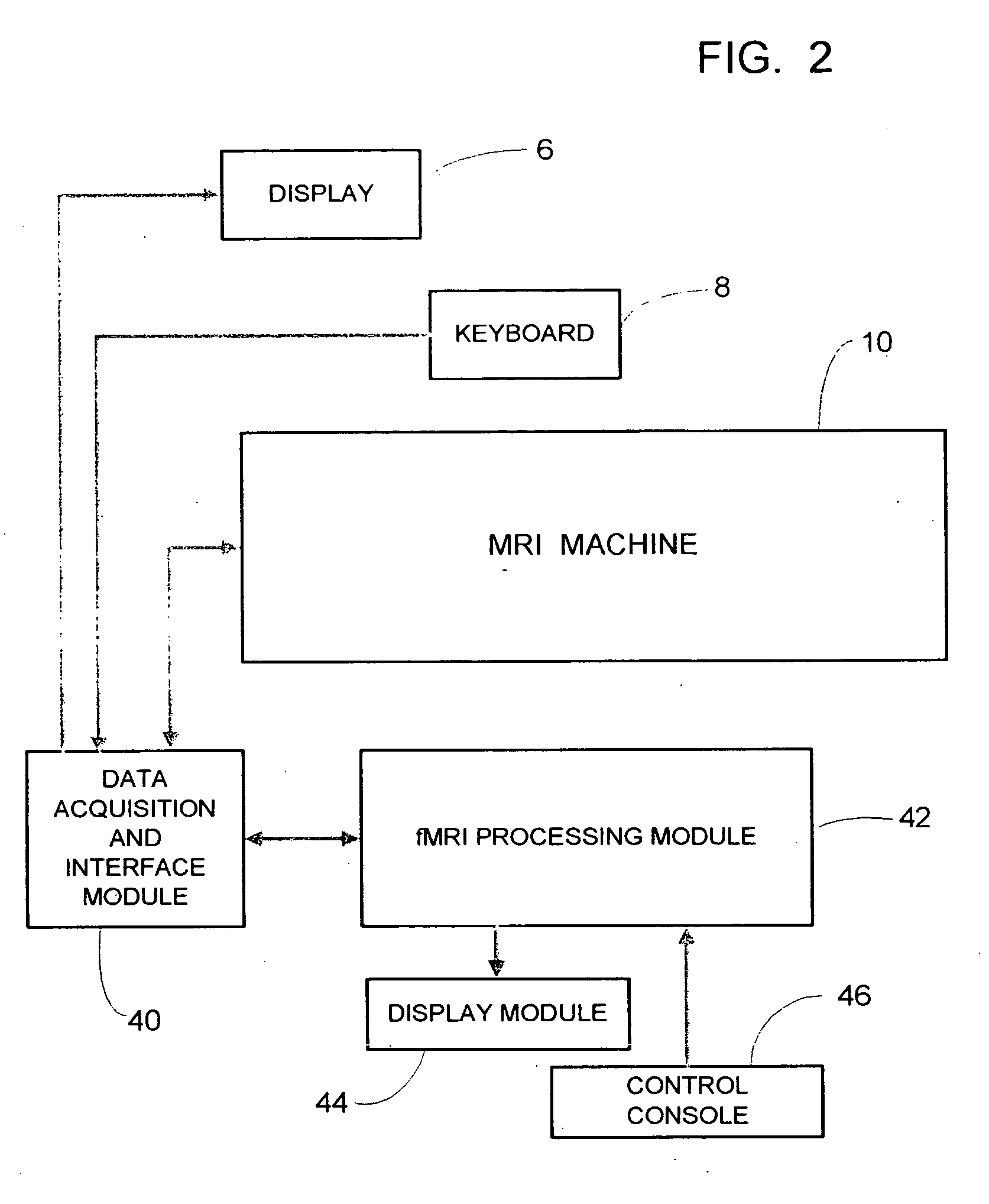
fMRI system for use in assessing the efficacy of therapies in treating CNS disorders
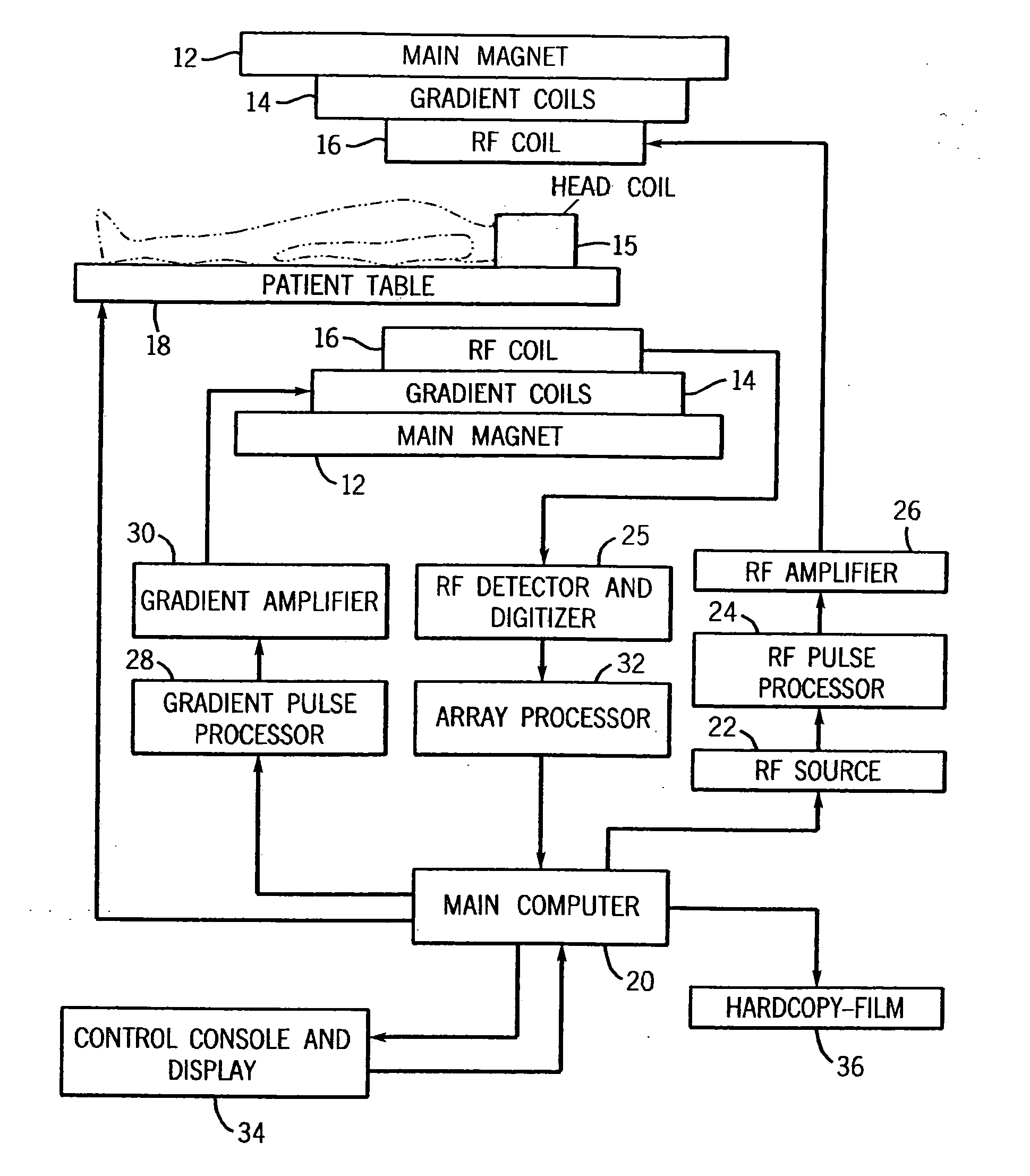
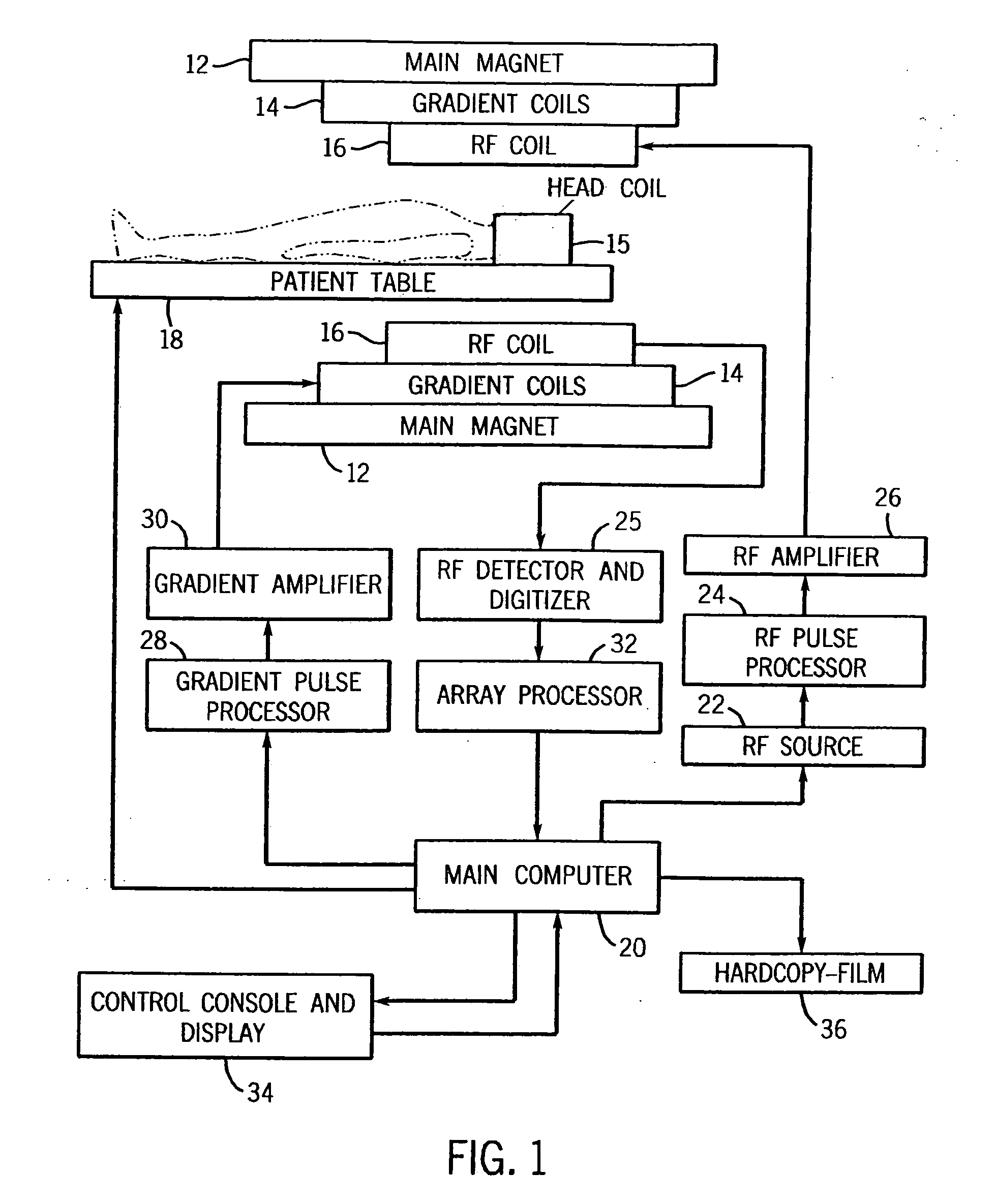
InactiveUS20050107682A1High level of sensitivityStrong specificityDiagnostic recording/measuringSensorsDiseaseMedicine
A system for assessing the medical effectiveness of therapies such as pharmaceutical medications for use in treating central nervous system disorders. Functional magnetic resonance imaging techniques are utilized in measuring neural activity induced by specific activation tasks in specific regions of the brain that are known to be affected by a central nervous system disorder. The levels of neural activity induced in patients subject to the disorder when on and off of the therapy are compared and then compared with normative data generated with respect to healthy individuals under comparable conditions. These comparisons quantify and assess the efficacy of the therapy.
Owner:RAO STEPHEN M +1
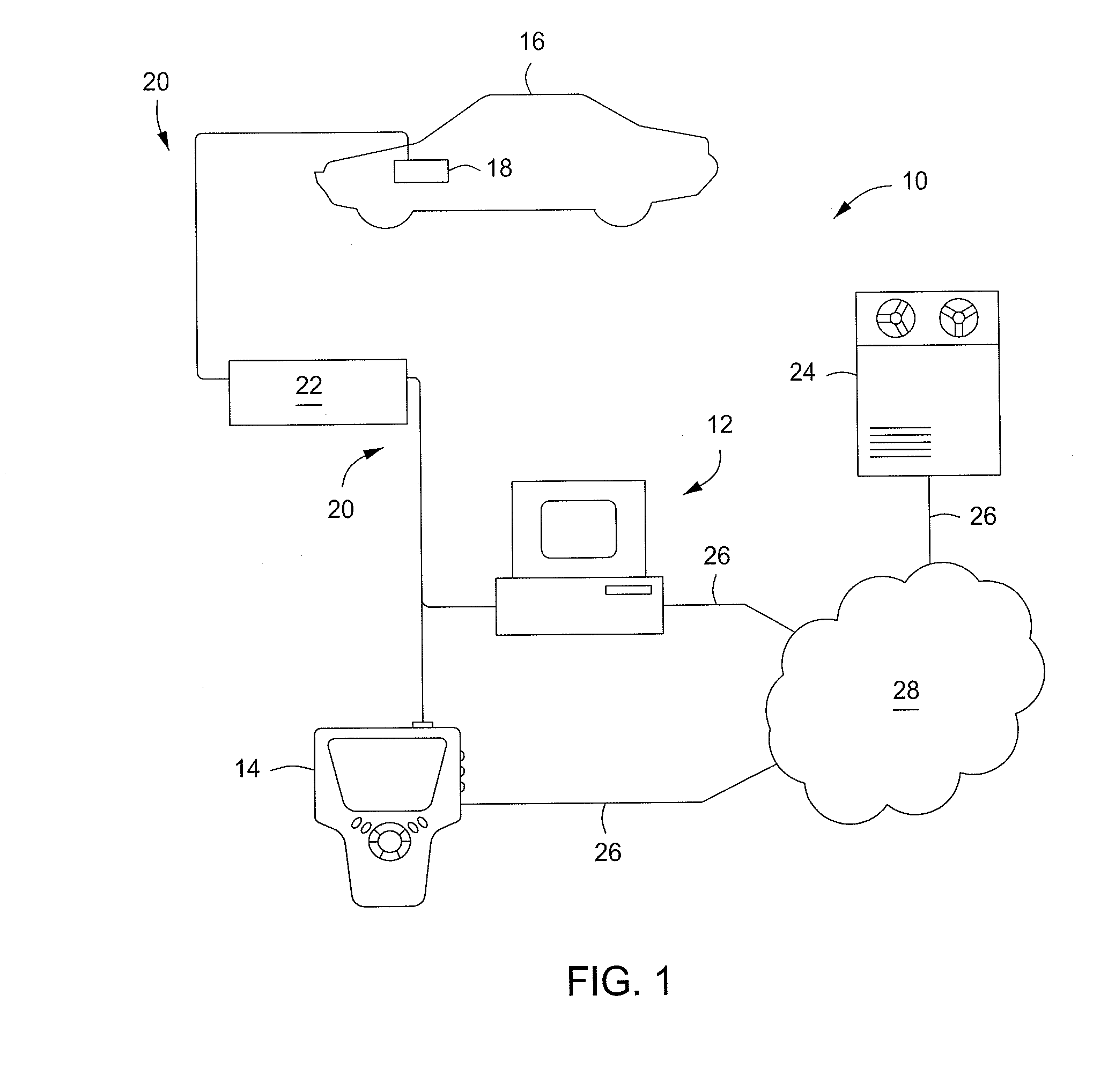
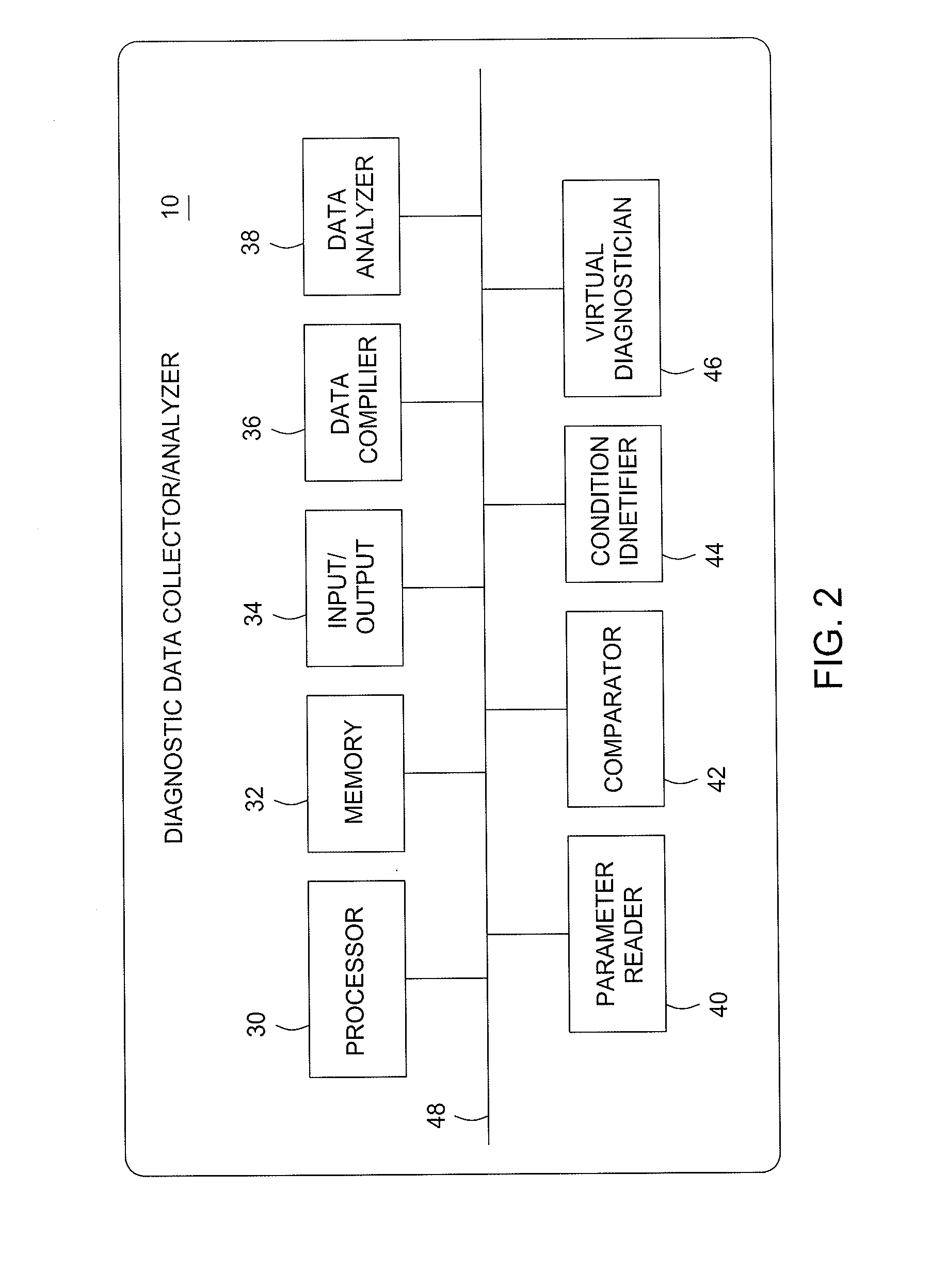
Diagnostics Data Collection and Analysis Method and Apparatus
InactiveUS20100324376A1Data processing applicationsRegistering/indicating working of vehiclesStatistical analysisHealthy individuals
A medical diagnostic data collector / analyzer compiles historical vehicle diagnostic data, including measured vital signs from a number of different people from a variety of population types, and performs statistical analyses on various vital sign combinations to establish ranges corresponding to healthy individuals and various disease conditions.
Owner:SPX CORP
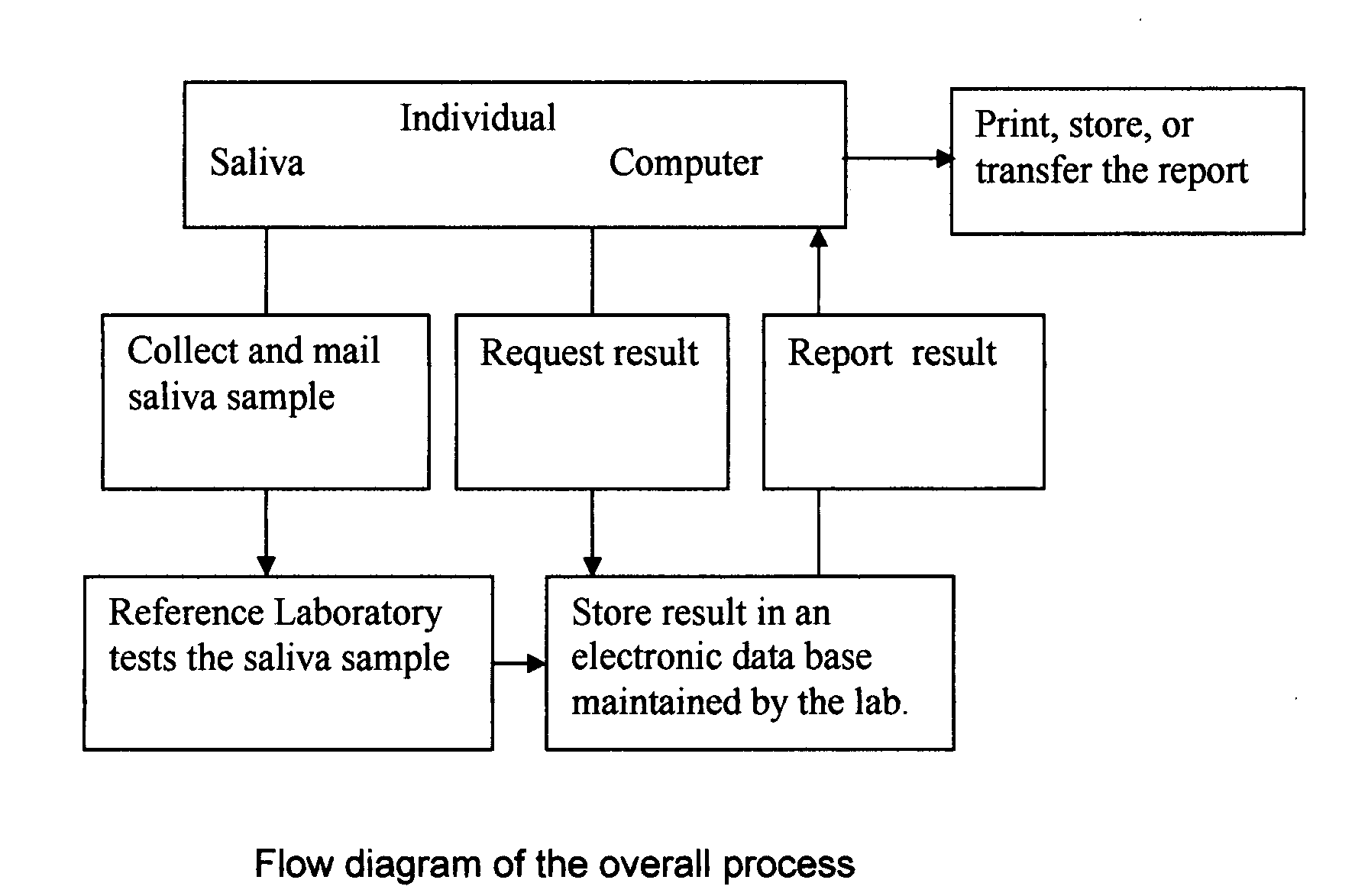
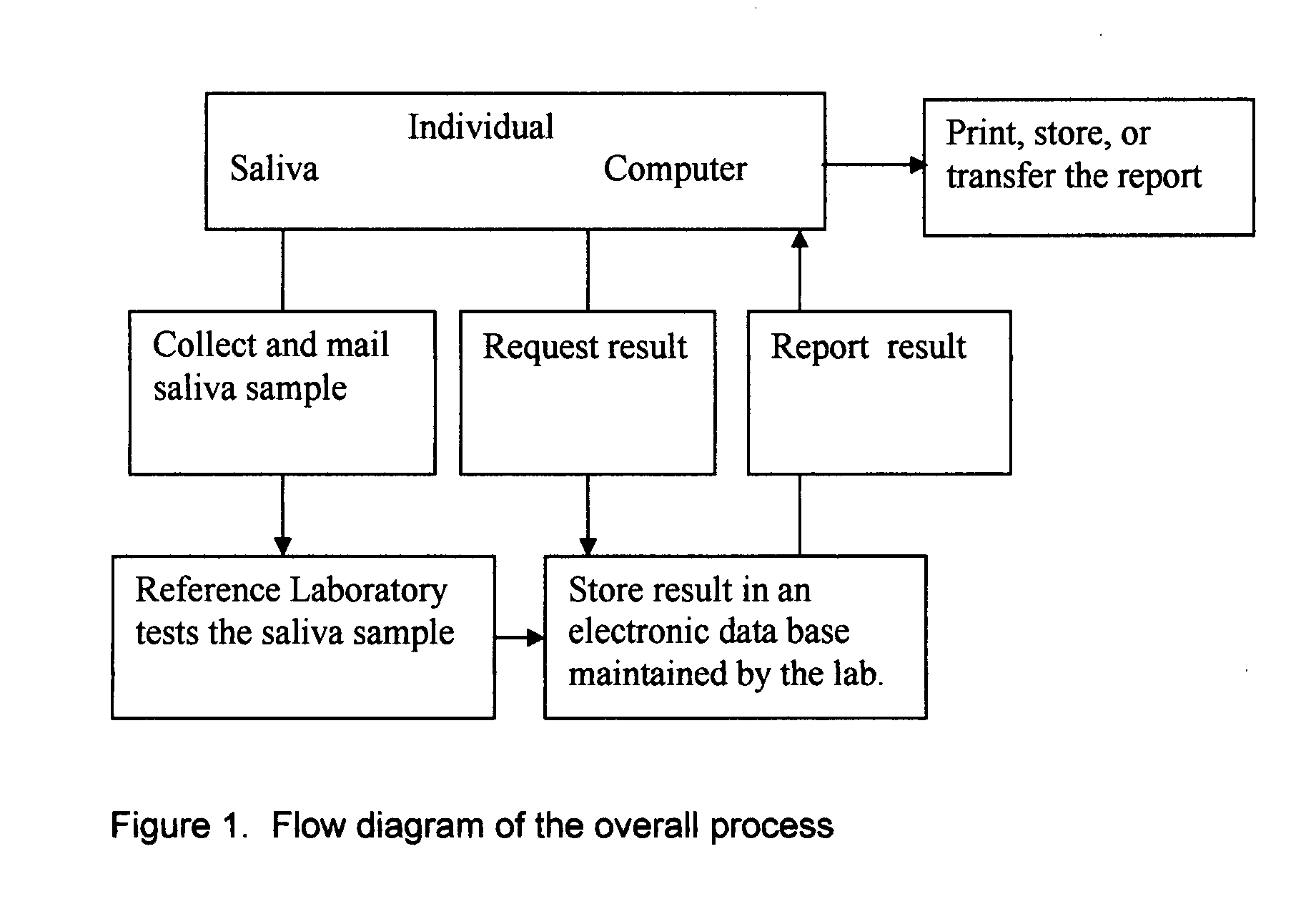
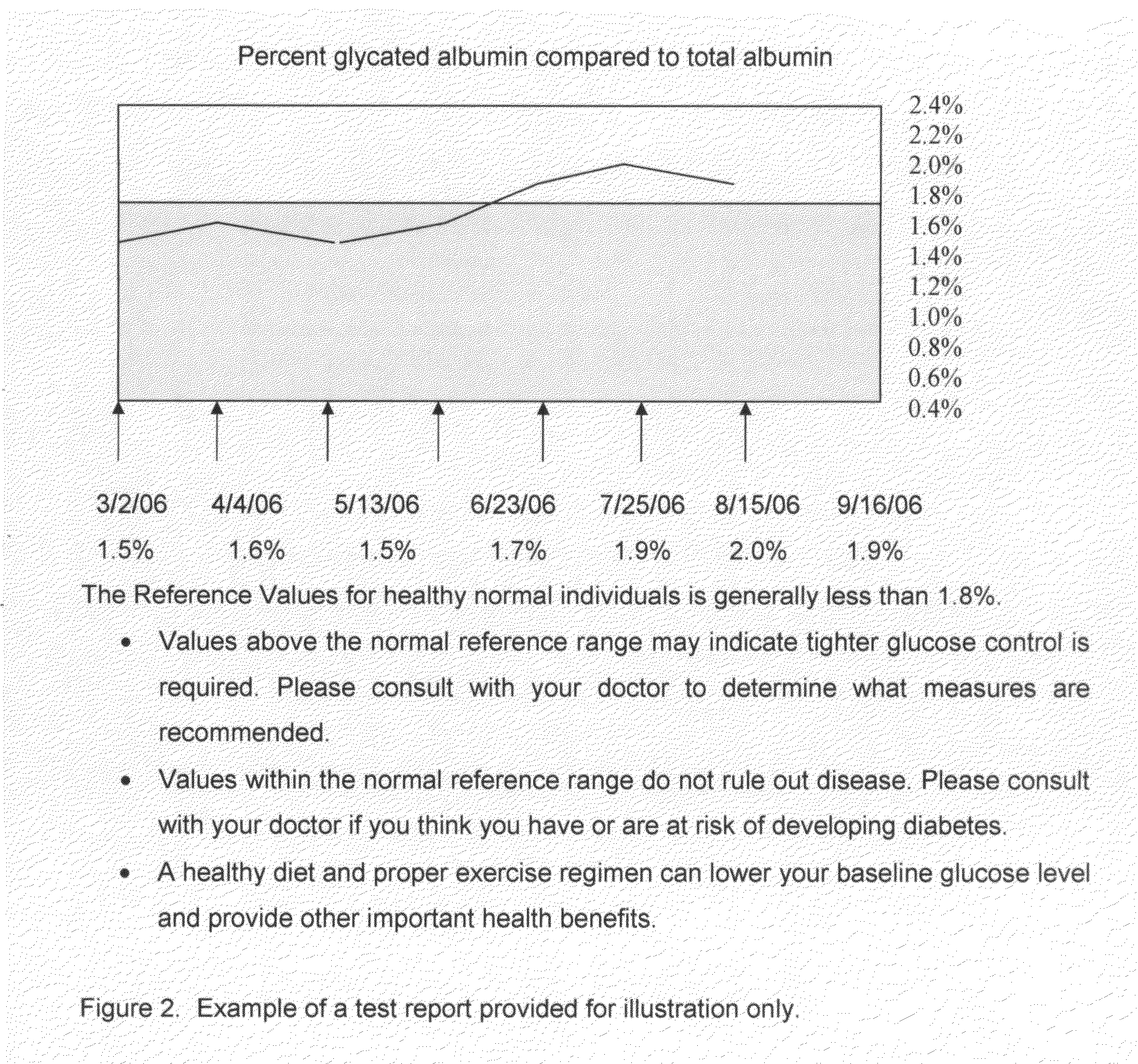
Home test for glycated albumin in saliva
InactiveUS20080227210A1Withdrawing sample devicesVaccination/ovulation diagnosticsSaliva sampleSaliva collection
A home test for measuring glycated albumin levels in saliva. The saliva sample is collected at home using a standardized saliva collection kit and mailed to a testing laboratory that performs the test and reports the result directly back to the customer via the internet. The home test can be used to monitor glucose control in diabetics and in healthy individuals. It may also be used as a diagnostic aid in identifying individuals with diabetes, or who are at risk of developing diabetes.
Owner:SMITH HENRY
Antisense composition and method for treating muscle atrophy
A method and compound for treating skeletal muscle mass deficiency in a human subject are disclosed. The composition is an oligomer of morpholino subunits and phosphorus-containing intersubunit linkages joining a morpholino nitrogen of one subunit to a 5′ exocyclic carbon of an adjacent subunit, contains between 10-40 nucleotide bases, has a base sequence effective to hybridize to an expression-sensitive region of processed or preprocessed human myostatin RNA transcript, identified, in its processed form, by SEQ ID NO:6, and is capable of uptake by target muscle cells in the subject. In practicing the method, the compound is administered in an amount and at a dosage schedule to produce an overall reduction in the level of serum myostatin measured in the patient, and preferably to bring the myostatin level within the a range determined for normal, healthy individuals.
Owner:AVI BIOPHARMA
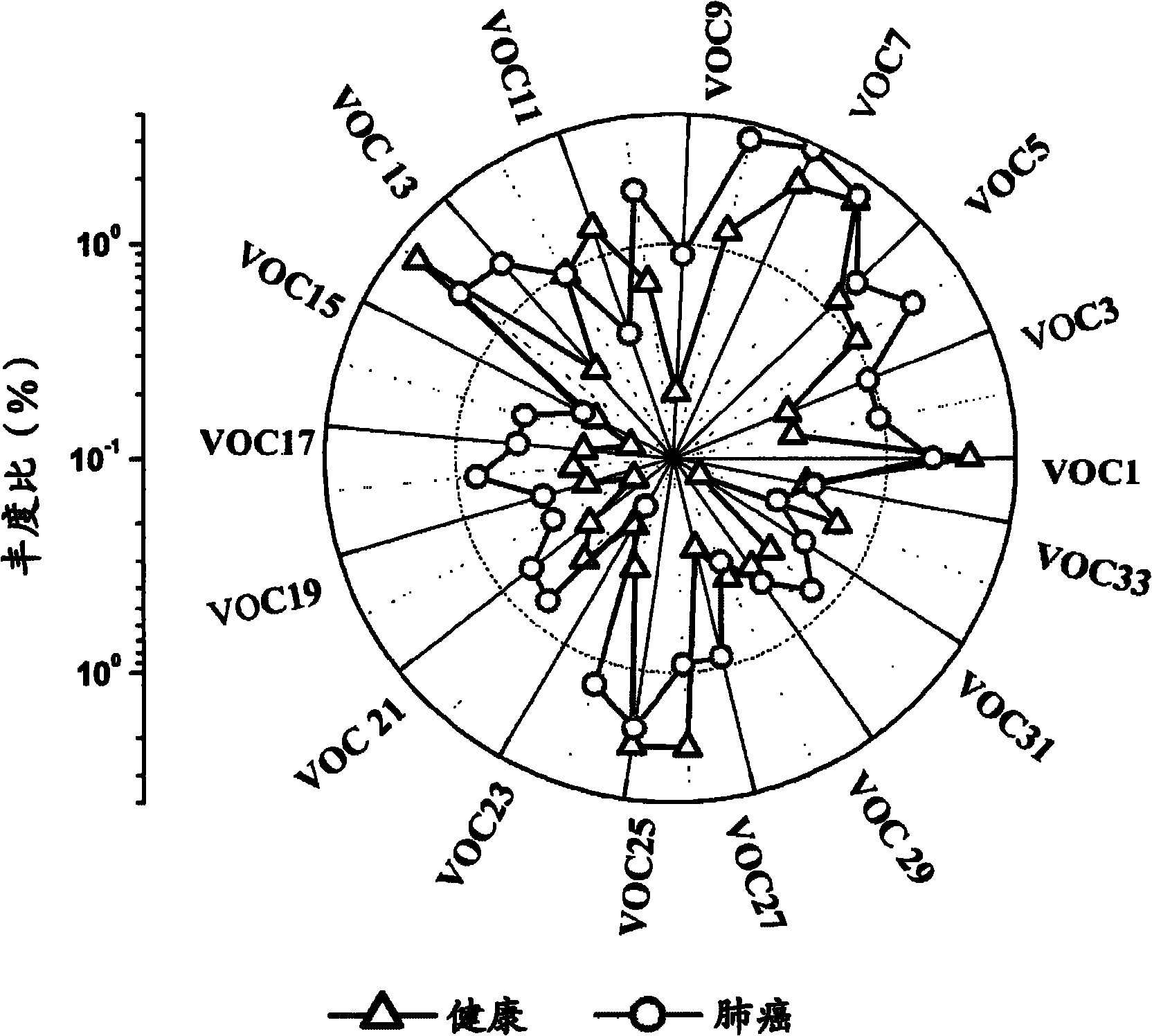
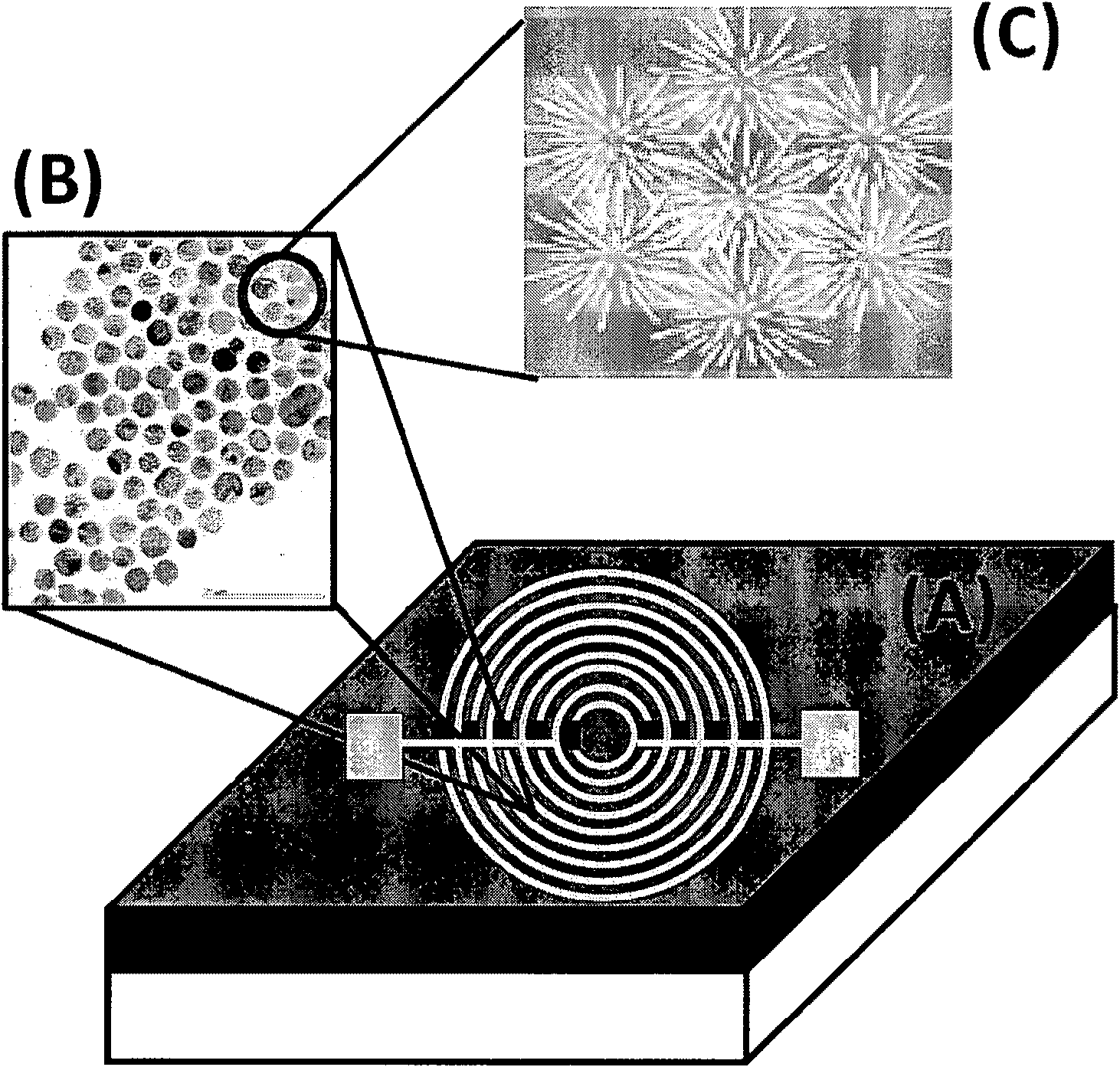
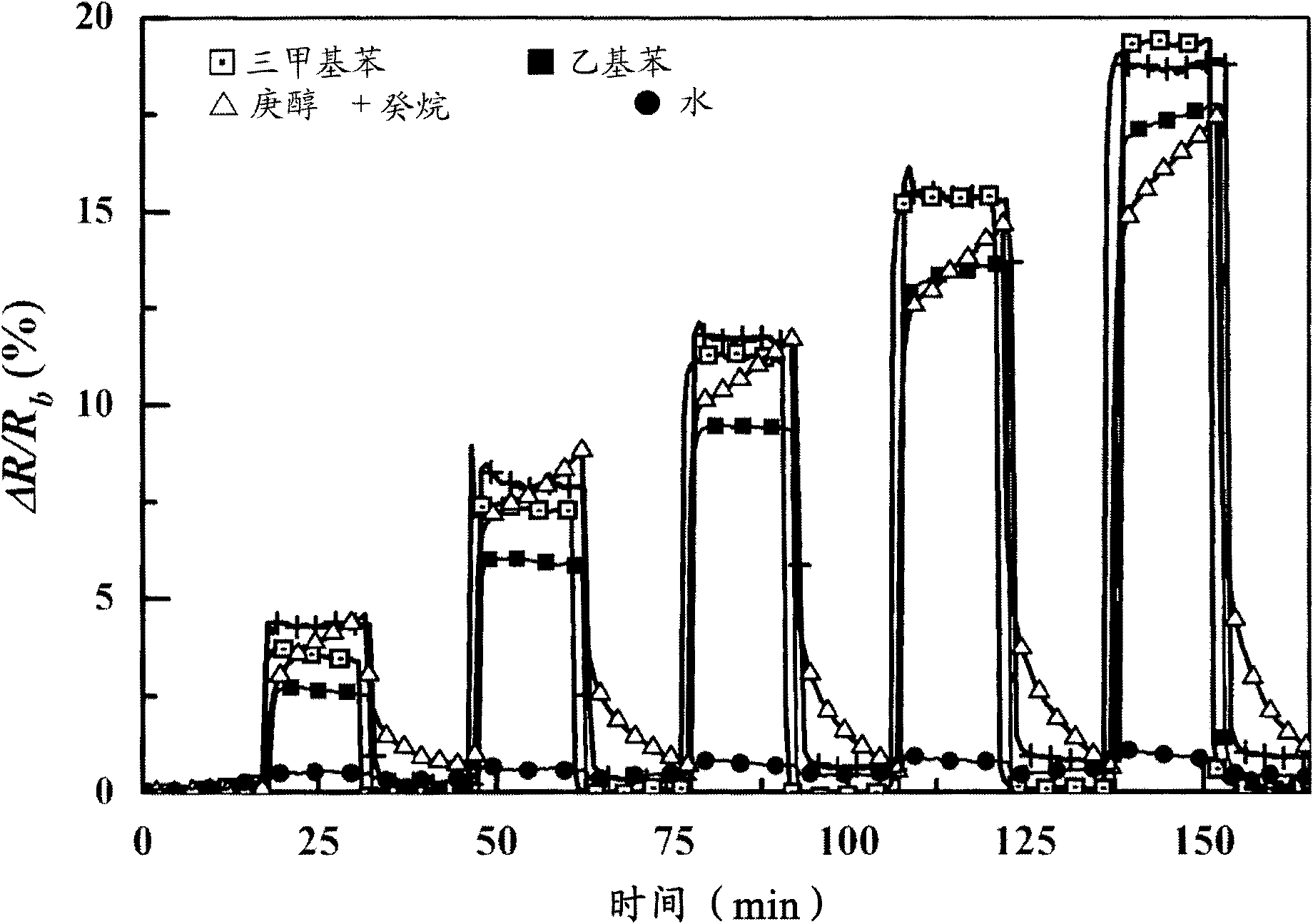
Volatile organic compounds as diagnostic markers in the breath for lung cancer
Owner:TECHNION RES & DEV FOUND LTD
System for detecting symptoms, determining staging and gauging drug efficacy in cases of Alzheimer's disease
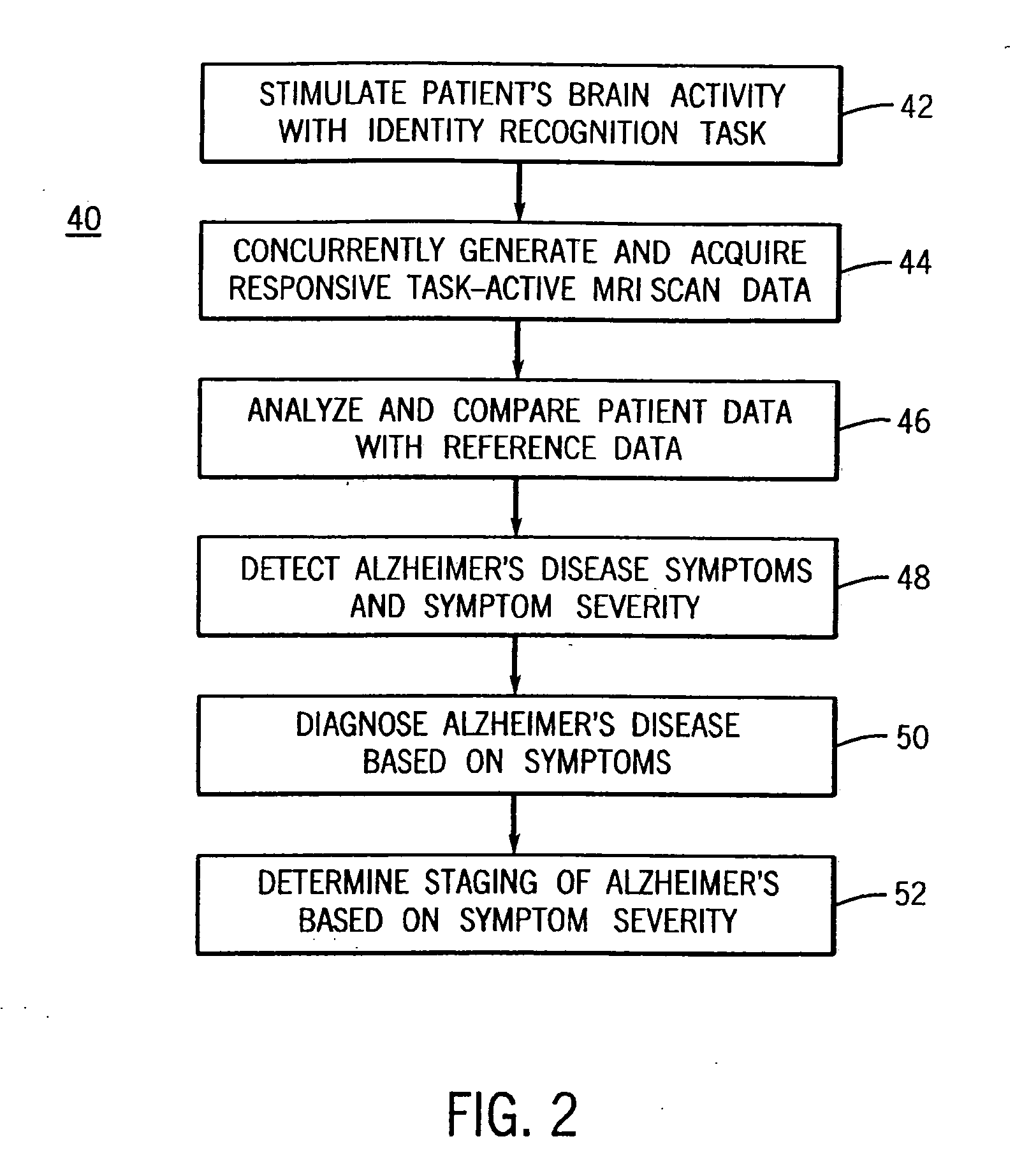
InactiveUS20050197560A1Accurate diagnosisEfficient and consistent and reliable mannerMagnetic measurementsDiagnostic recording/measuringIdentity recognitionPatients symptoms
A system for using functional magnetic resonance imaging (fMRI) for detecting symptoms indicative of Alzheimer's disease, diagnosing Alzheimer's disease and gauging the efficacy of medications used in treating Alzheimer's disease. The system includes steps involving activating a selected region of the brain which may be affected by Alzheimer's disease using an identity recognition type task, concurrently acquiring task-active MRI data responsive to the task, comparing the patient's task-active MRI data to reference data derived from a database of task-active data from healthy individuals and detecting whether the patient has symptoms related to Alzheimer's disease. The severity of the patient's symptoms and the staging of the disease may also be determined. Also, a medication may be administered to the patient and the efficacy of the medication may be gauged based on the severity of the patient's symptoms.
Owner:RAO STEPHEN M +1
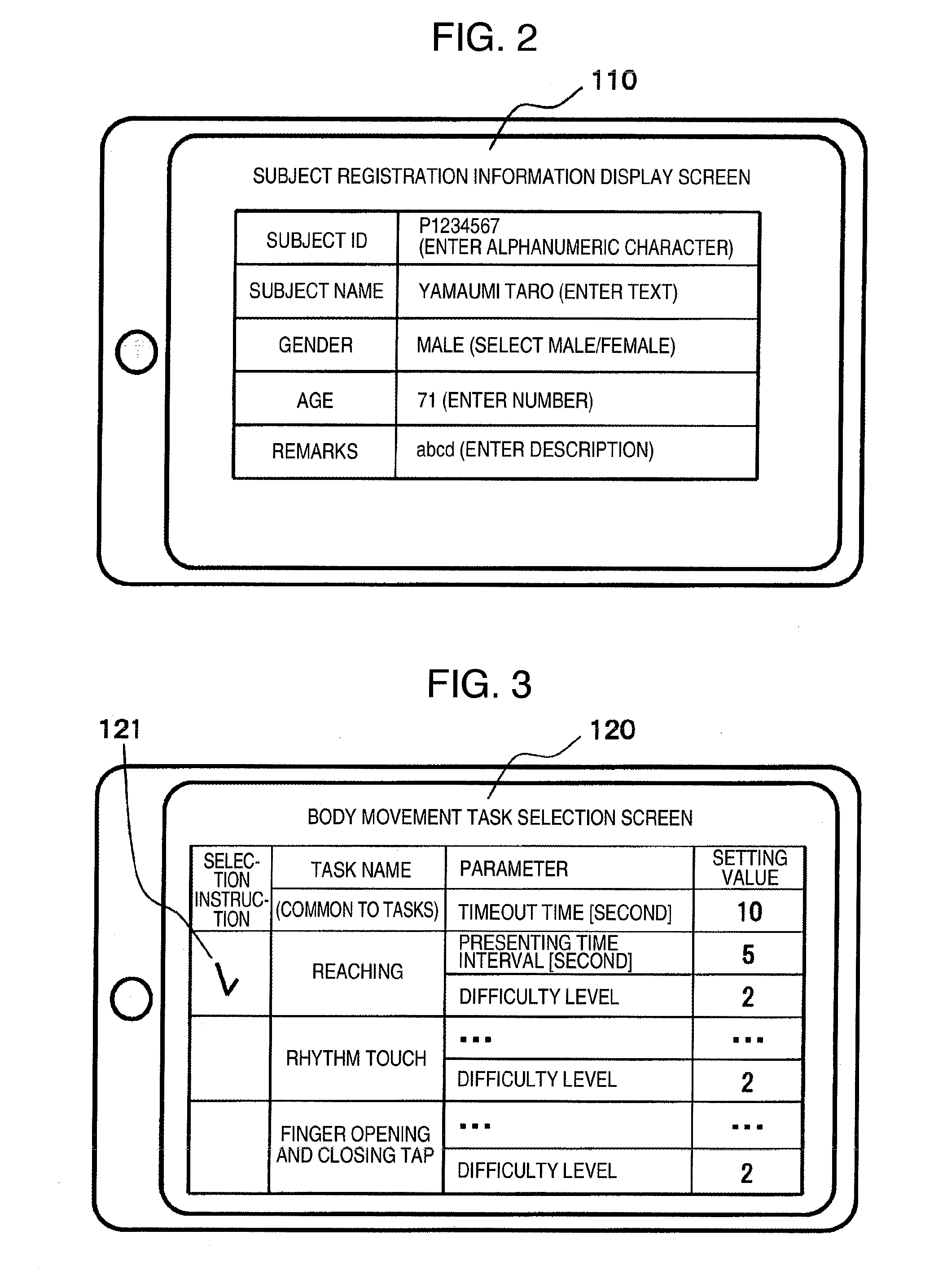
Brain dysfunction assessment method, brain dysfunction assessment device, and program thereof
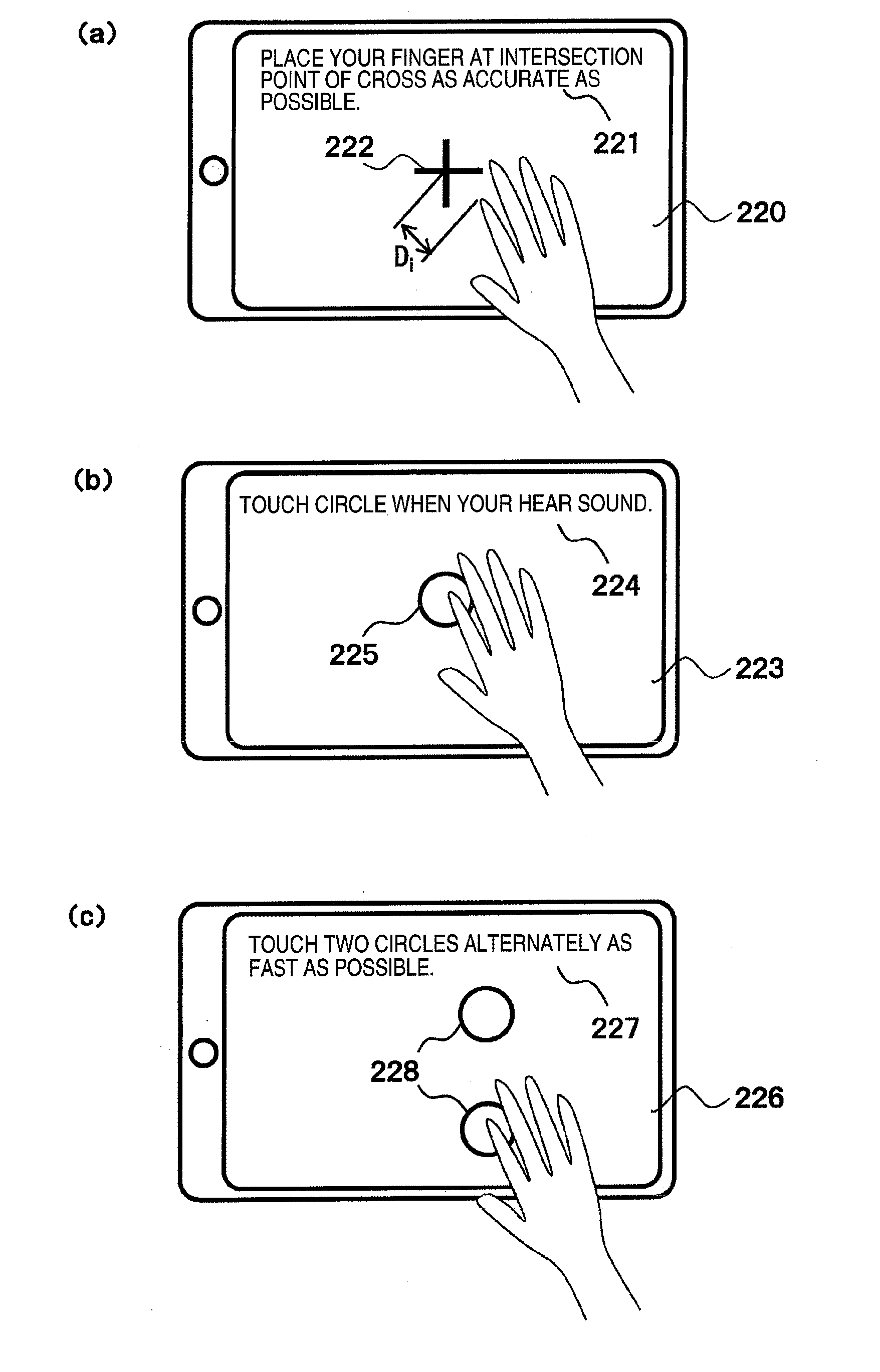
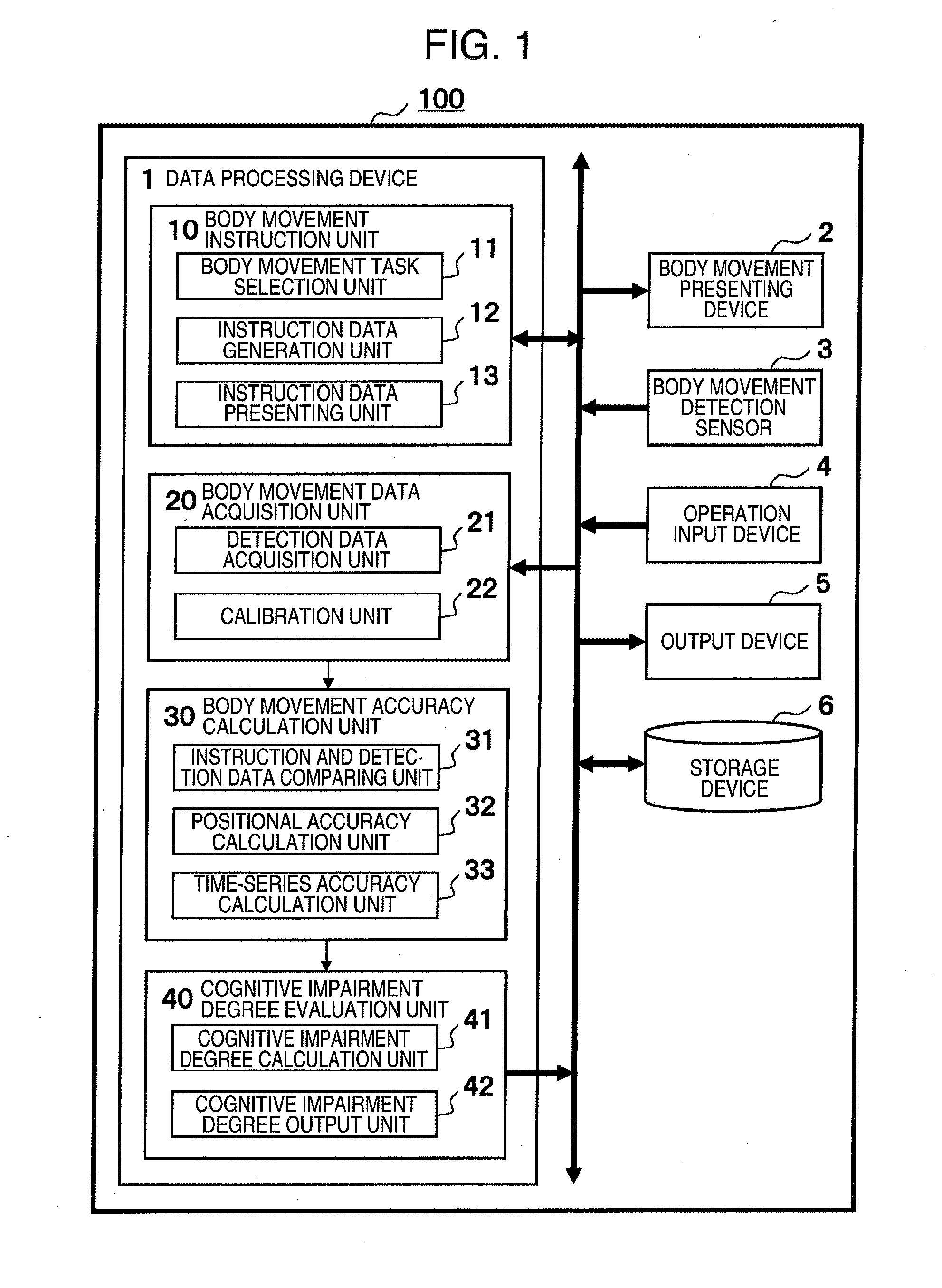
ActiveUS20160100788A1Easy assessment processPerson identificationAudiometeringPhysical medicine and rehabilitationBody movement
A brain dysfunction assessment device (100) comprises: a body movement indicator (10) which presents a body movement to be performed to a subject with a body movement presentation device (2); a body movement data acquisition unit (20) which acquires the subject's body movement data with a body movement detection sensor (3); a body movement accuracy calculation unit (30) which calculates a positional accuracy and a time sequence accuracy of the subject's body movement from the acquired body movement data; and a cognitive dysfunction assessment unit (40) which assesses the level of cognitive dysfunction of the subject by comparing a value which indicates the accuracy of the subject's body movement that is obtained from the calculated positional accuracy and time sequence accuracy with statistical data which indicates the accuracy of body movement of healthy individuals.
Owner:MAXELL HLDG LTD
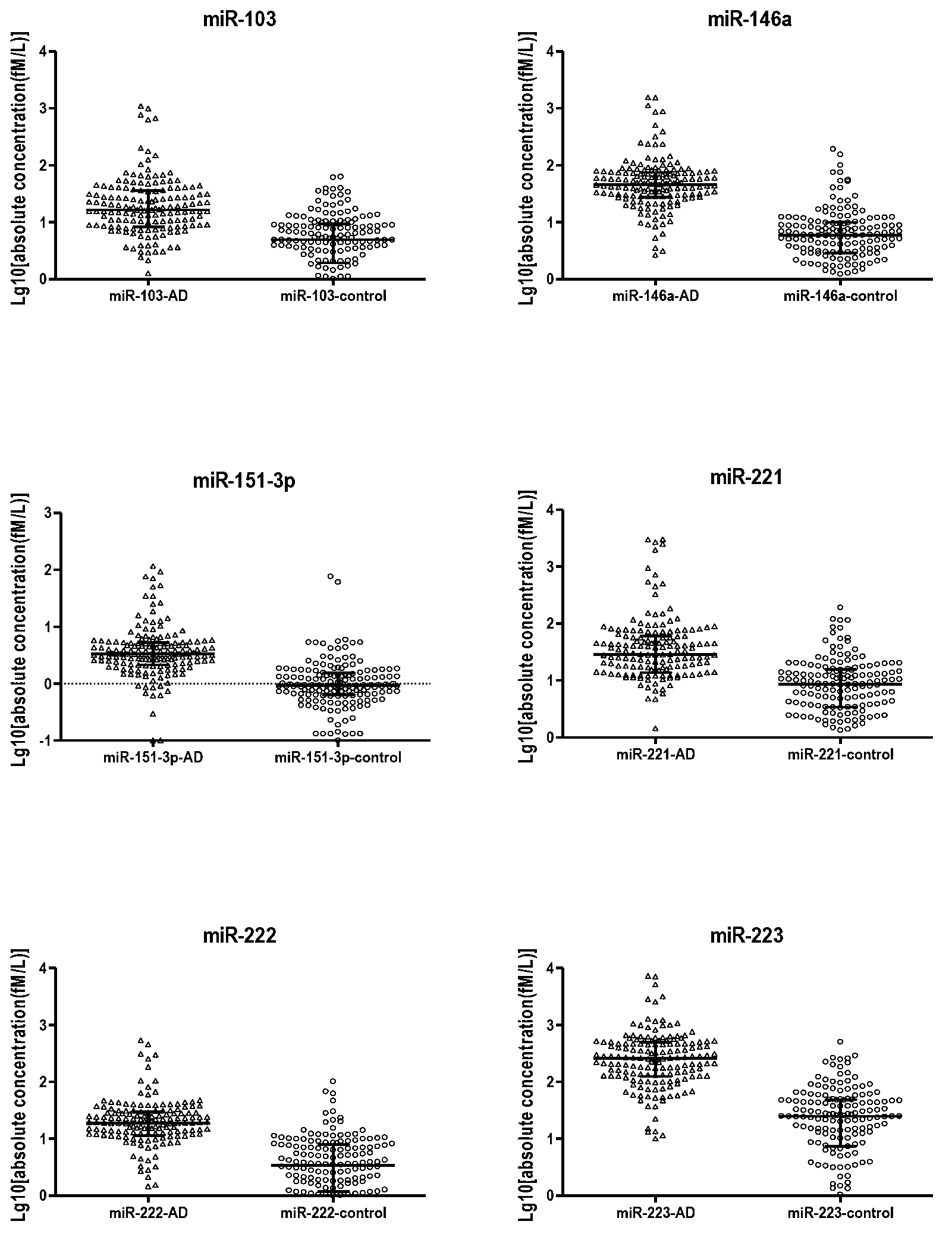
Early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and reverse transcription primer and application thereof
InactiveCN103173448AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationTumor BiomarkersAdenocarcinoma
The invention belongs to the field of biological and medicine examination, relates to a tumor biomarker, and in particular relates to an early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and a reverse transcription primer and an application thereof. The early-stage lung adenocarcinoma miRNA specific expression profile comprises more than one of up-regulated expression miR-103, miR-146a, miR-151, miR-221, miR-222 and miR-223. The invention provides an early-stage lung adenocarcinoma diagnosis model, a reverse transcription primer of specific expression miRNA and a kit or biochip for early-stage lung adenocarcinoma diagnosis based on the specific expression profile. The technical scheme provided by the invention can realize accurate diagnosis of early-stage lung adenocarcinoma by detecting a small amount of miRNA, and provides possibility to the production of accurate, efficient and economical detection products. The established lung adenocarcinoma prediction model can distinguish lung adenocarcinoma from a healthy individual, and has great practical significance in clinic.
Owner:SHANGHAI CHEST HOSPITAL
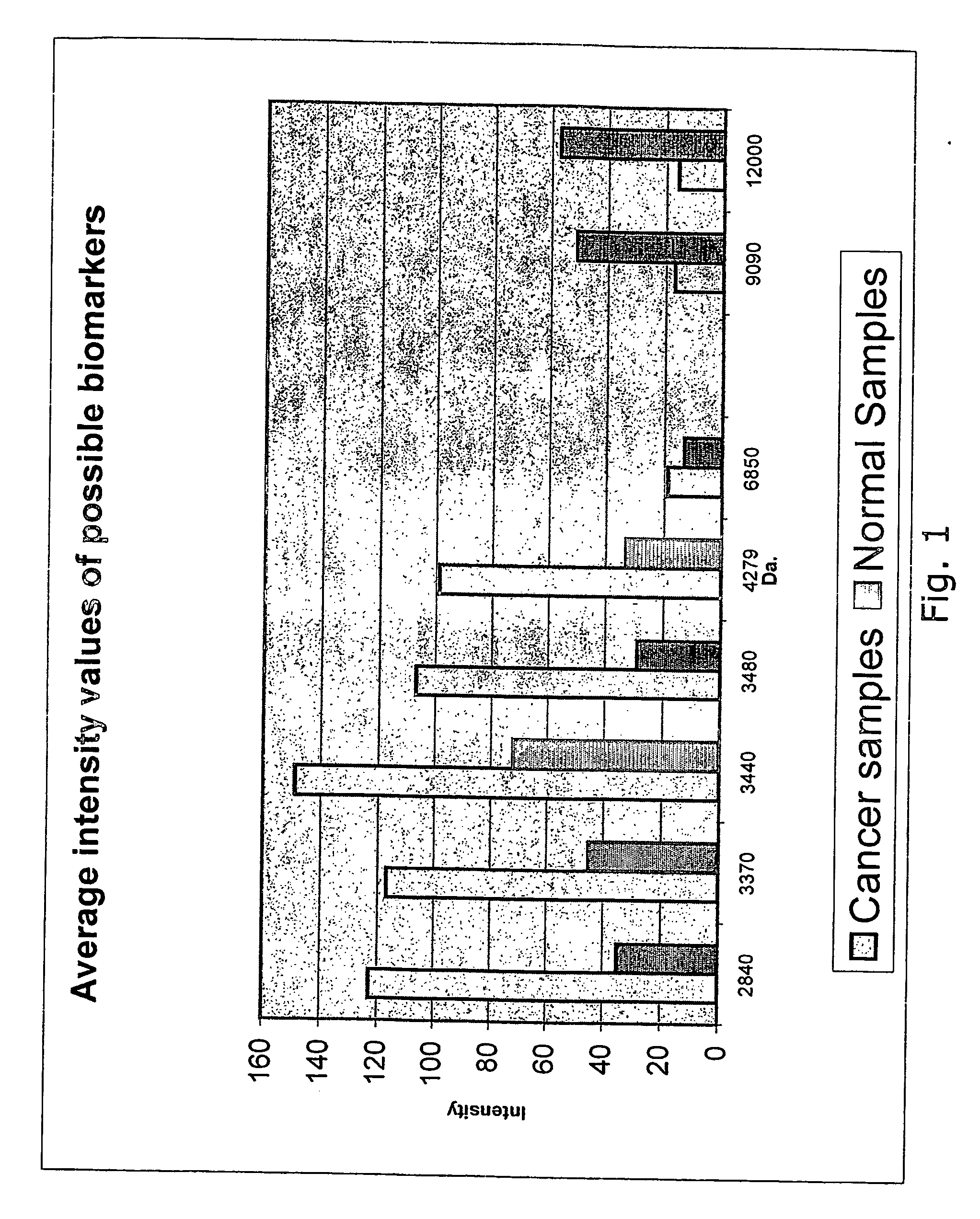
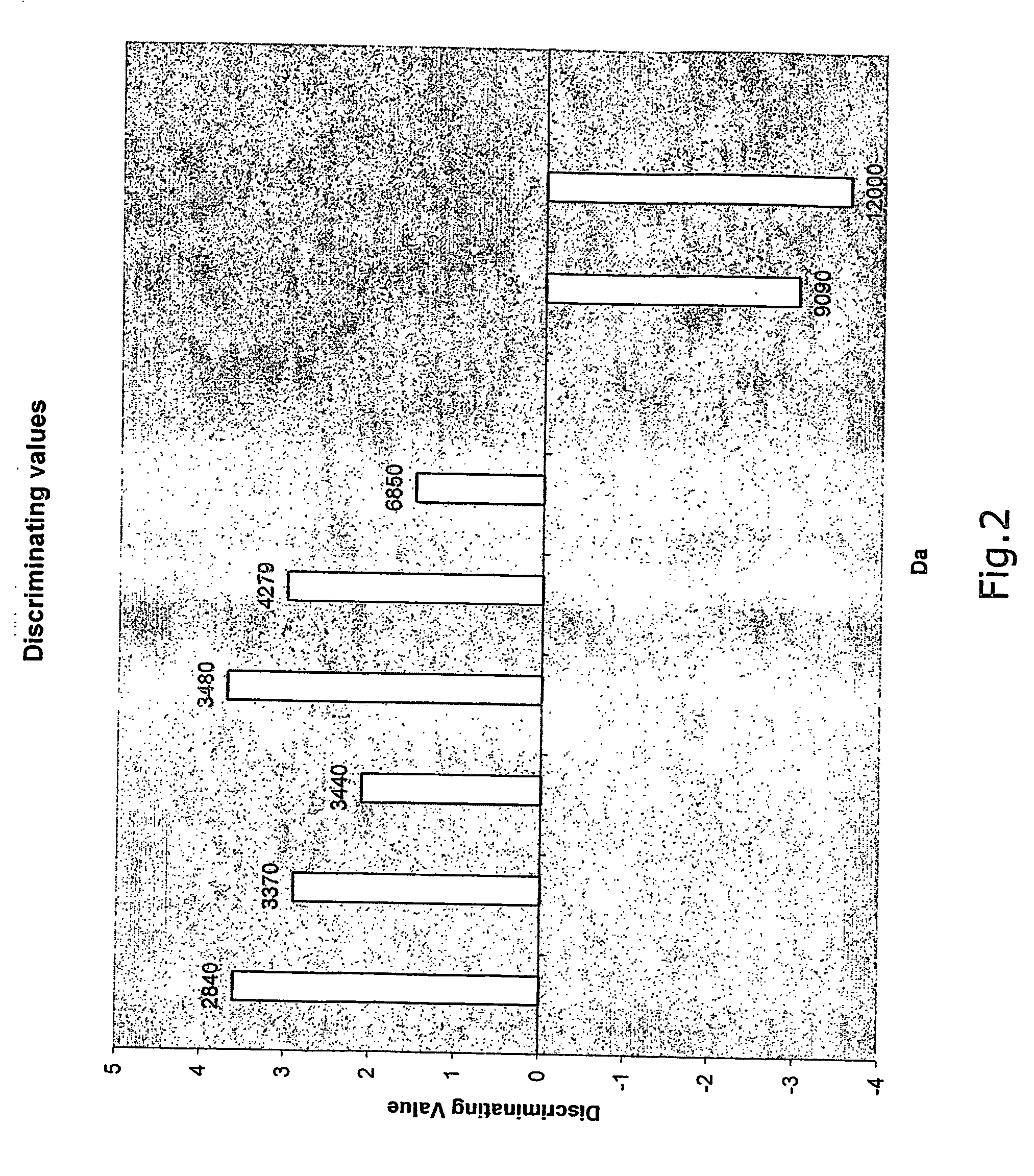
Method for detection of colorectal cancer in human samples
InactiveUS20070117164A1Reduce signalingReduce intensityBiological material analysisSerum samplesMass Spectrometry-Mass Spectrometry
The present invention relates to a method of diagnosing colorectal cancer in human samples using several novel protein markers. The markers have been identified by assaying a number of tissue and serum samples from healthy individuals and persons diagnosed with colorectal cancer by means of protein chip technology using mass spectrometry. Differential expression pattern of these markers are indicative of a person having colorectal cancer patient. The diagnosis is based on comparing at least one intensity value, obtained using the method, to a reference value.
Owner:RASKOV HANS HENRIK +1
Methods and systems for evaluating health risk factors by measurement of DNA damage and DNA repair
InactiveUS20070269824A1Microbiological testing/measurementBiological material analysisHealth riskIncreased risk
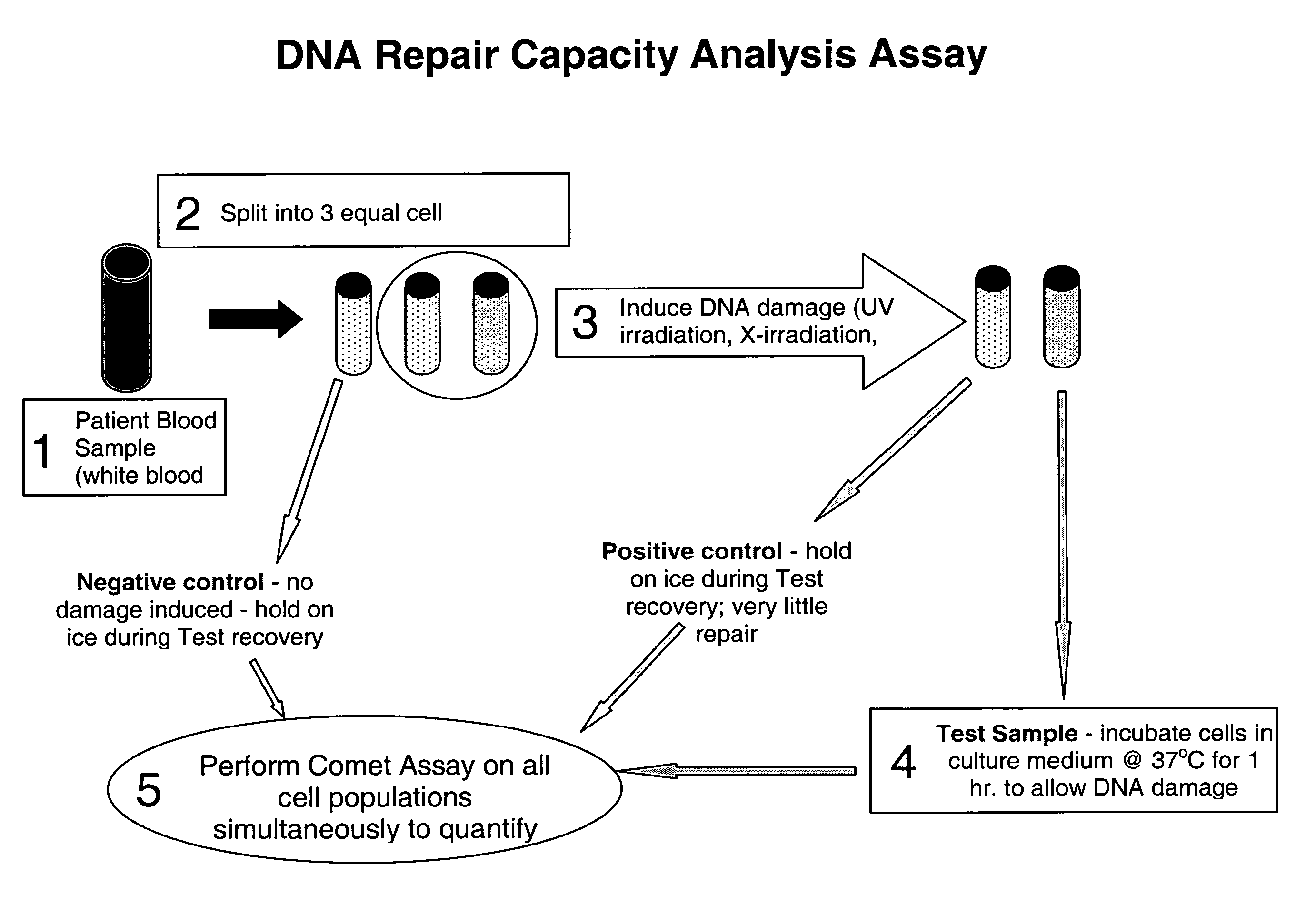
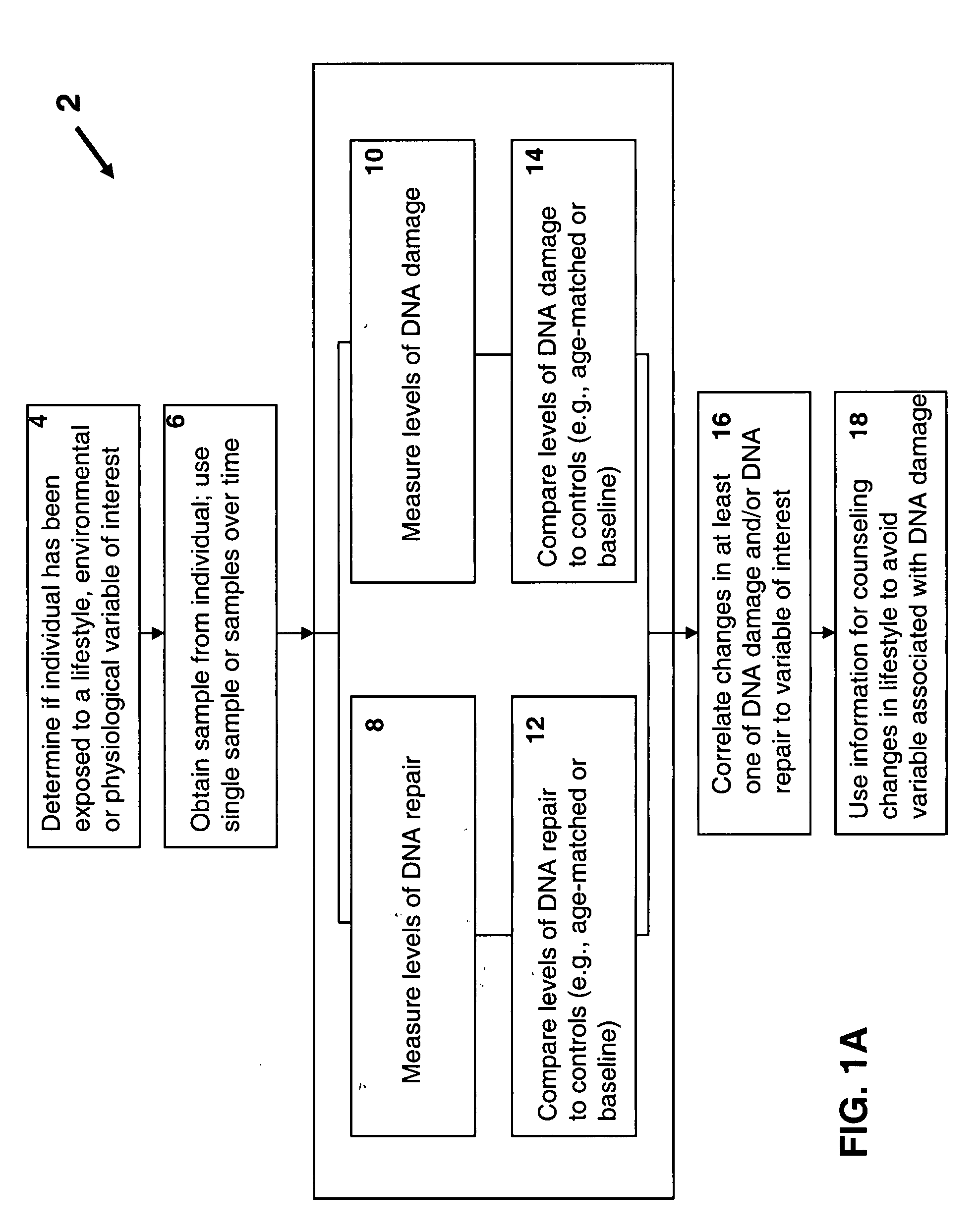
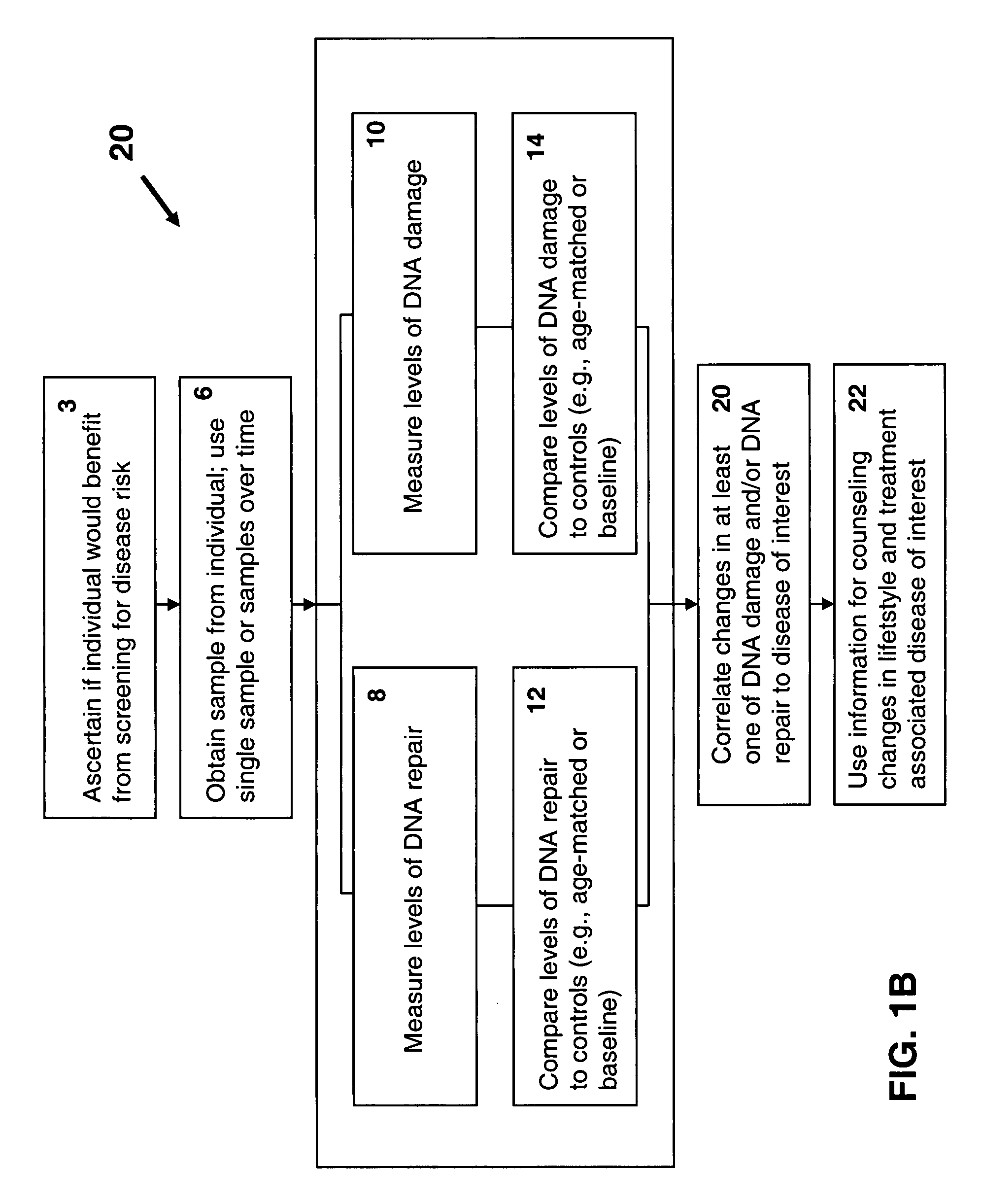
Disclosed are methods for measuring the effects of environmental, physiological, or lifestyle variables on DNA damage and DNA repair activity as well as the use of measurements of DNA damage and DNA repair activity to predict increased risk for disease. Embodiments of the methods involve the use of a combination of assays to measure DNA damage and DNA repair activity in an individual and comparing these measurements to suitable controls using the selected assays for normal healthy individuals of varying ages. In other embodiments, the methods may comprise a comparison of DNA damage levels to DNA repair levels to obtain an apparent net measurement of DNA damage accumulation.
Owner:ALBRECHT JEFFREY +3
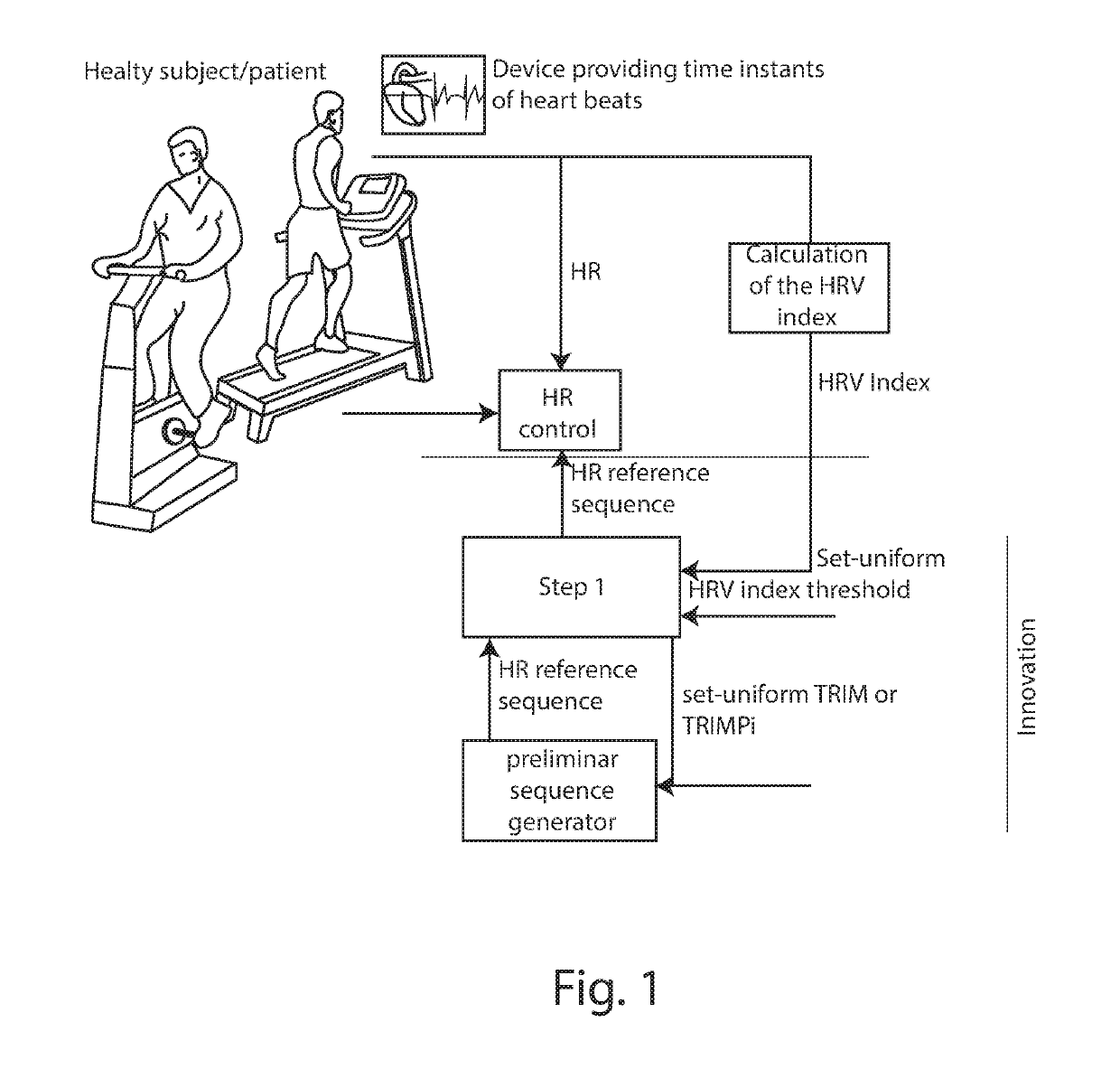
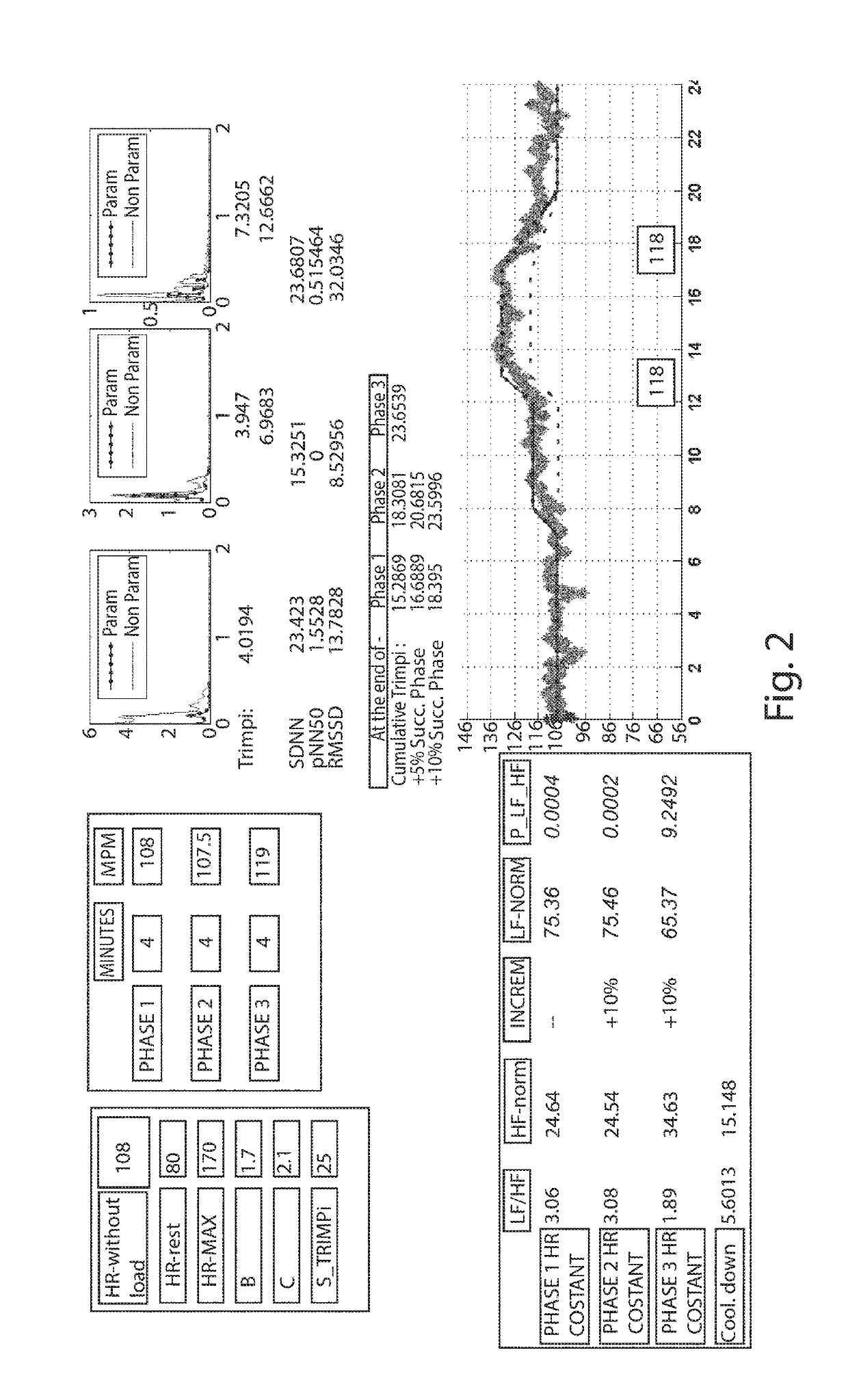
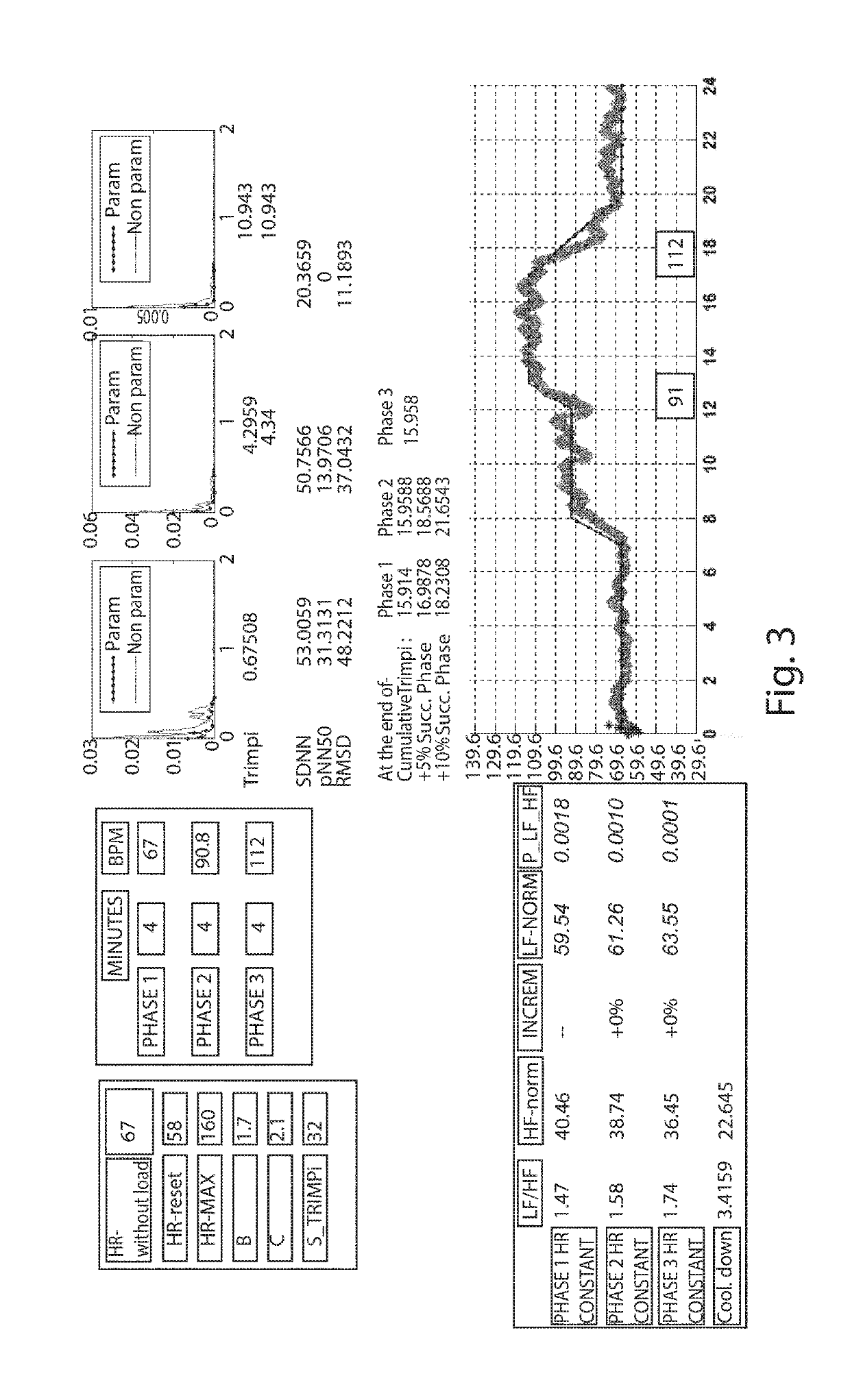
Individually tailored exercise training and rehabilitation technique: medical personal trainer
The present invention concerns a new individually tailored exercise training and rehabilitation technique, referred to as “MPT: Medical Personal Trainer”. More precisely, the invention concerns an innovative method to be used in the field of physical activity and exercise training / rehabilitation programs for healthy individuals and for patients with chronic diseases. It merges together, in an interactive way, advanced notions from medicine, engineering, mathematics, artificial intelligence, in order to provide new clinical perspectives for automatic personalized health care as well as novel insights on individually-tailored management and advanced treatment, in a user-familiar setting, of healthy subjects (including elderly) and patients with chronic diseases.
Owner:SAN RAFFAELE ROMA SRL
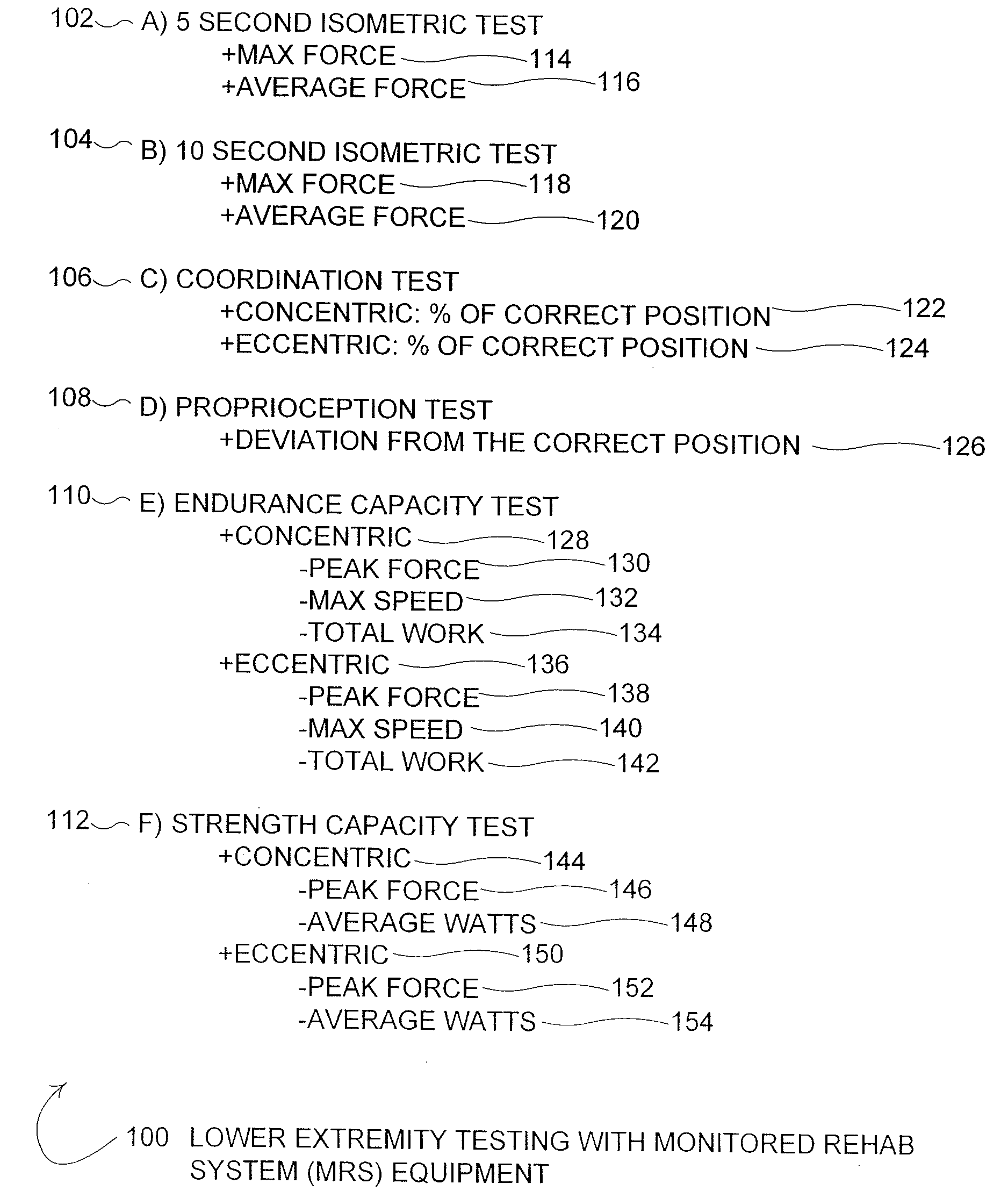
Method for measuring physical fitness and creating athletic training regimens for particular sports
InactiveUS20090062627A1ClubsDiagnostic recording/measuringRegimenPhysical medicine and rehabilitation
Disclosed is a method for creating a fitness training program based on performing neuromuscular and muscular performance tests on an individual, analyzing the results of the neuromuscular and muscular performance tests, and creating the fitness training program based on the analysis of the neuromuscular and muscular performance test results. The equipment used to perform the neuromuscular and muscular performance tests may be equipment designed for rehabilitation physical therapy used for recovery from injuries and surgical procedures that permits general neuromuscular and muscular performance testing and testing of the difference in muscle performance between a right side and a left side of the body of the individual. The individual is tested to create a fitness training program to increase neuromuscular and muscular performance, not for purposes of injury and / or surgery rehabilitation. The individual tested may be a healthy individual. The method is particularly well suited for an athlete that desires to increase performance in a particular sport. The test equipment should objectively, quantitatively, and accurately measure the general neuromuscular and muscular performance and the difference in muscle performance between the right side and left side of the body. Neuromuscular and muscular performance tests may include a variety of exercise movements with each movement tested for isometric, coordination, proprioceptive, endurance capacity, and strength capacity response. The fitness training regimen is created for the individual based on the analysis of the neuromuscular and muscular performance test results.
Owner:YOUNGER J KEVIN
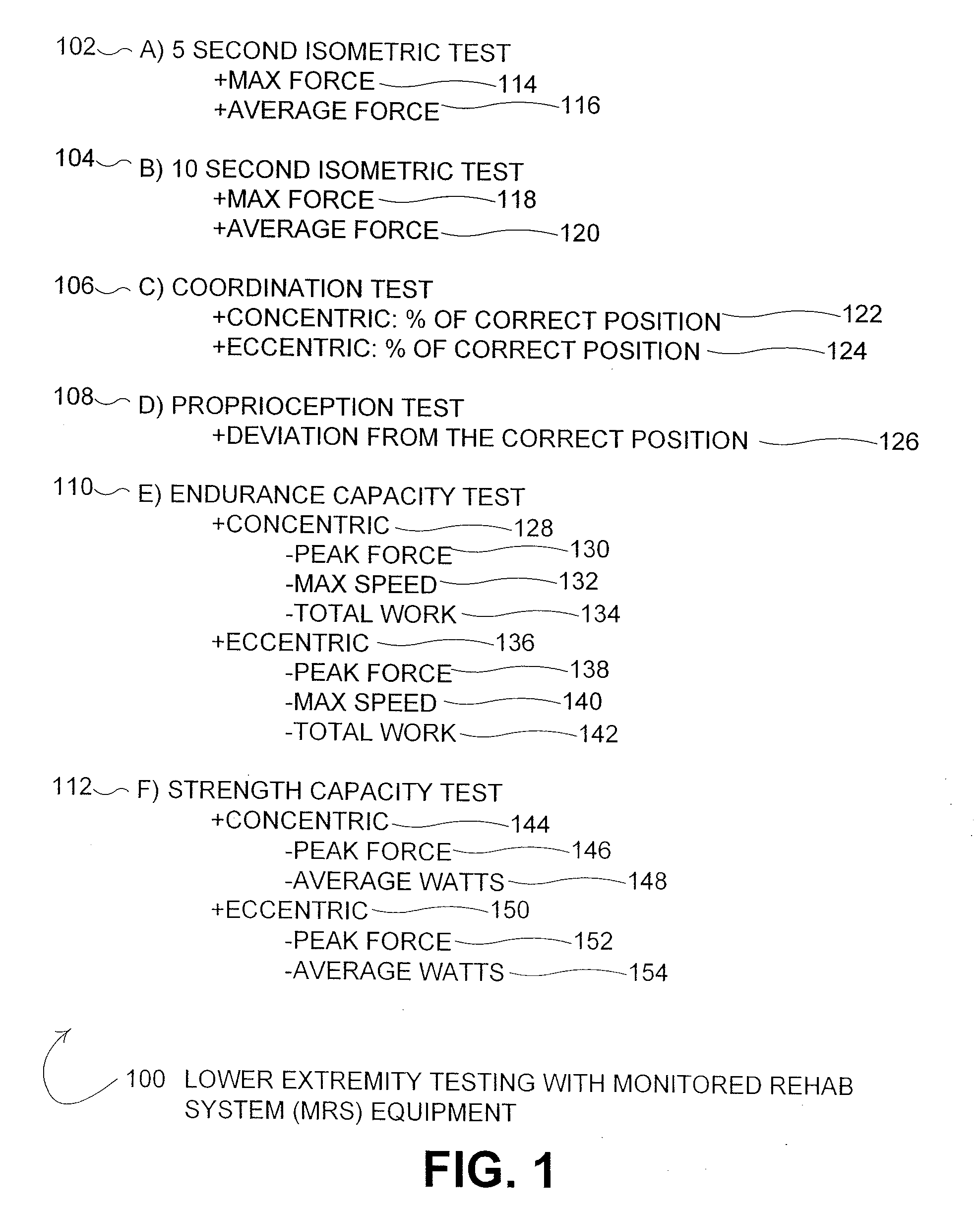
Immunodiversity Assessment Method and Its Use
Disclosed is a method for distinguishing the immunorepertoires of normal, healthy individuals from those of individuals who have symptomatic and / or non-symptomatic disease.
Owner:HAN JIAN
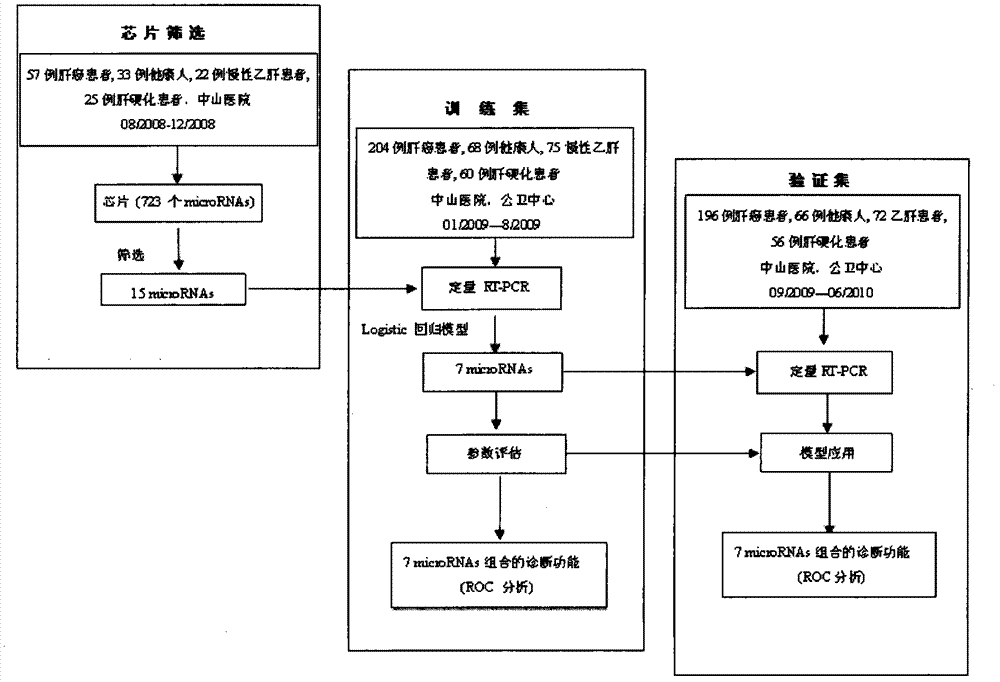
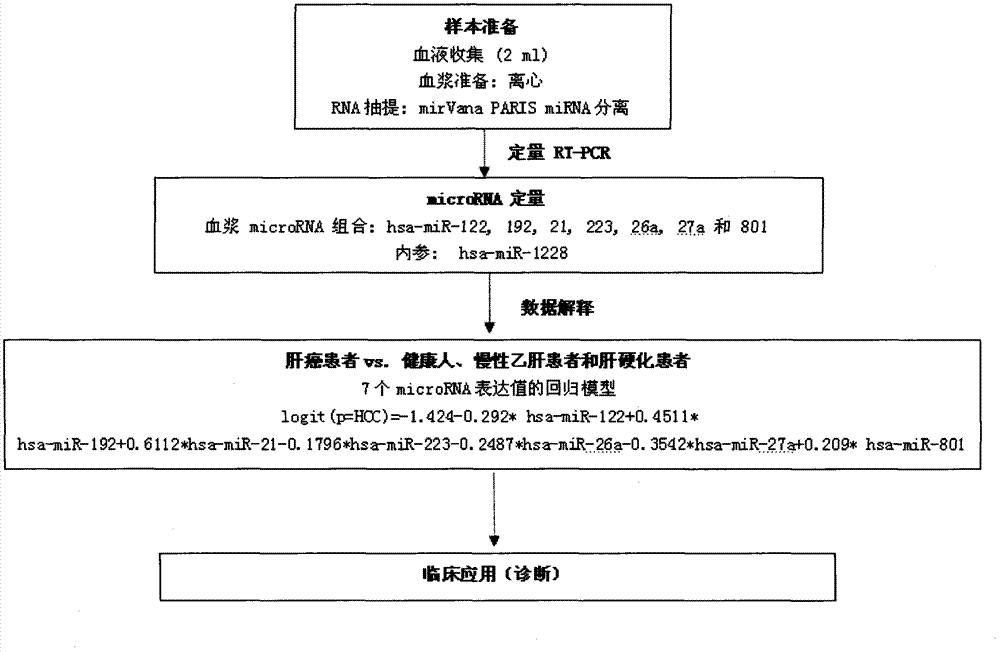
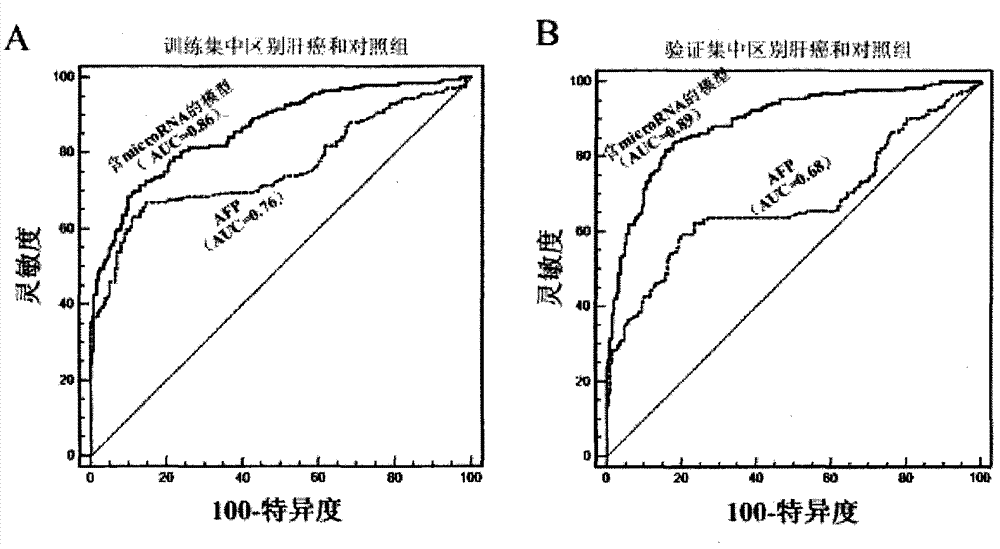
Liver cancer diagnostic marker composed of blood plasma microRNA (micro ribonucleic acid) and new method for diagnosing liver cancer
ActiveCN102776185AMicrobiological testing/measurementDNA/RNA fragmentationChronic hepatitisEarly Hepatocellular Carcinoma
The invention relates to a liver cancer diagnostic marker composed of blood plasma microRNA (micro ribonucleic acid), a kit containing the marker and a new method for diagnosing liver cancer (in particular early liver cancer). The liver cancer diagnostic marker is composed of a plurality of nucleic acid molecules, wherein each nucleic acid molecule is used for coding at least one microRNA sequence; and preferably, the liver cancer diagnostic marker is composed of nucleic acid molecules for coding hsa-miR-122, hsa-miR-192, hsa-miR-21, hsa-miR-223, hsa-miR-26a, hsa-miR-27a, hsa-miR-801 and hsa-miR-1228. The kit can be applied to diagnosing hepatocellular carcinoma, in particular early hepatocellular carcinoma, or to differentiating the blood plasma of at least one hepatocellular carcinoma sufferer from the blood plasma of at least one healthy individual, and the blood plasma of at least one chronic hepatitis b sufferer or the blood plasma of at least one cirrhosis sufferer.
Owner:JUSBIO SCI SHANGHAI CO LTD
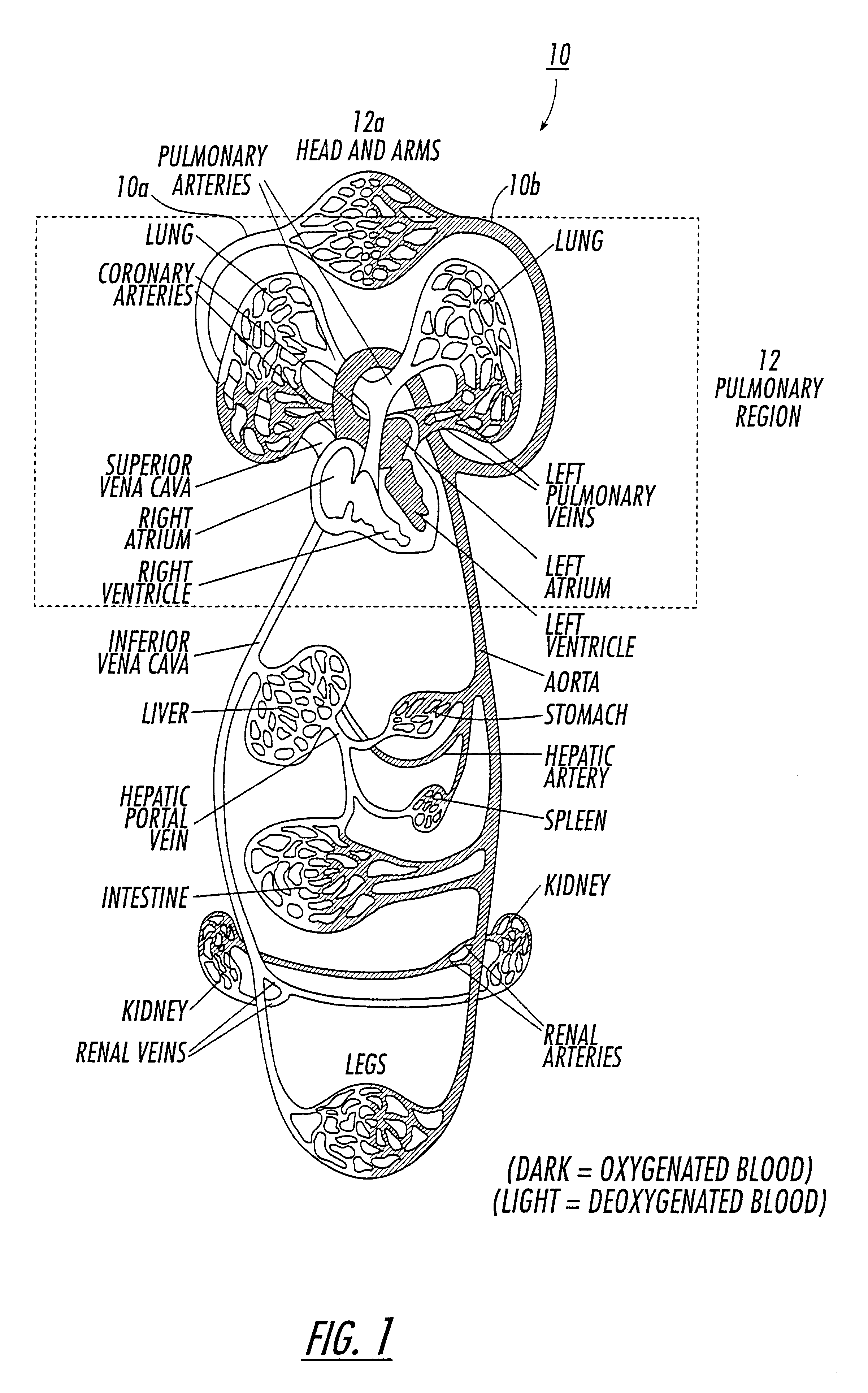
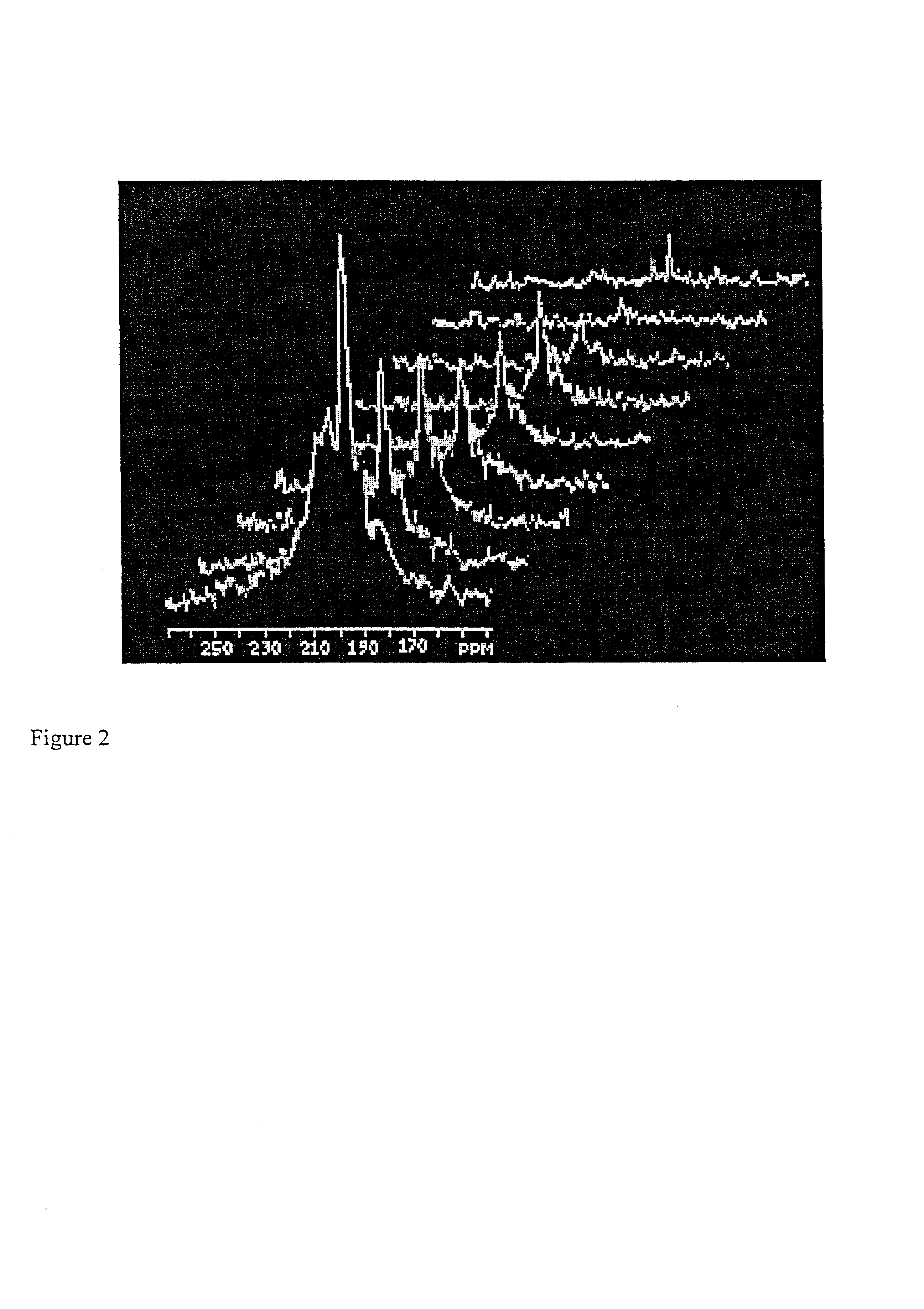
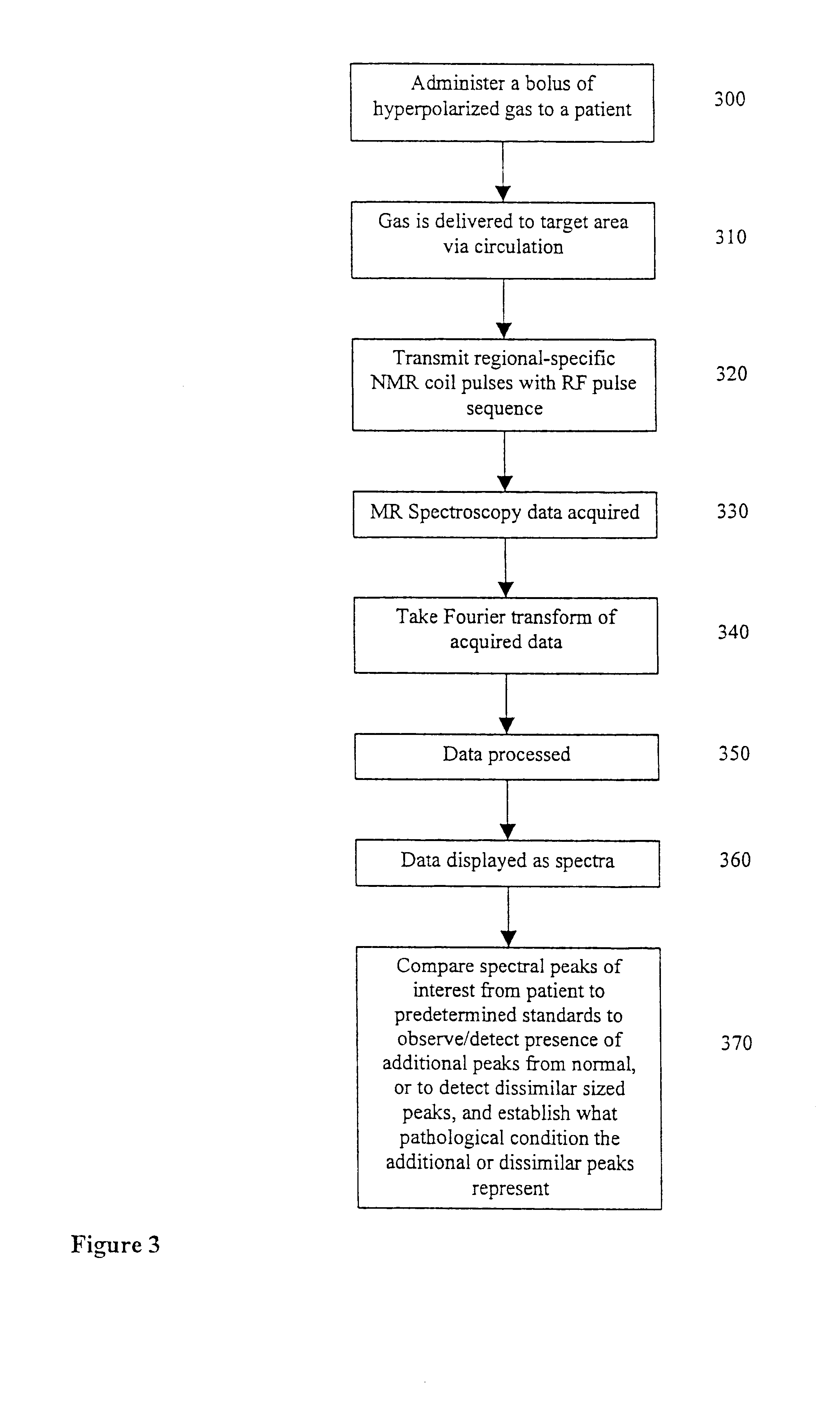
Diagnostic procedures using 129Xe spectroscopy characteristic chemical shift to detect pathology in vivo
InactiveUS6696040B2Reduce impactLarger flip anglesMeasurements using NMR spectroscopySensorsMedicineSpectroscopy
An in vivo non-invasive method for detecting and / or diagnosing a pathological condition using hyperpolarized <129>Xe spectroscopy is disclosed. Generally stated, the method includes determining the magnitude of spectral peaks which represent particular chemical shifts and comparing the observed magnitudes to those of healthy individuals. Preferably, the method includes subtracting substantial backgrounds and accounting for secondary conditions such as the polarization of hyperpolarized gas administered. Additionally, a quantitative analysis of hyperpolarized <129>Xe spectra advantageously allows a physician to establish the extent of disease progression. Advantageously, this method can be used regardless of the method of hyperpolarized <129>Xe administration.
Owner:POLAREAN
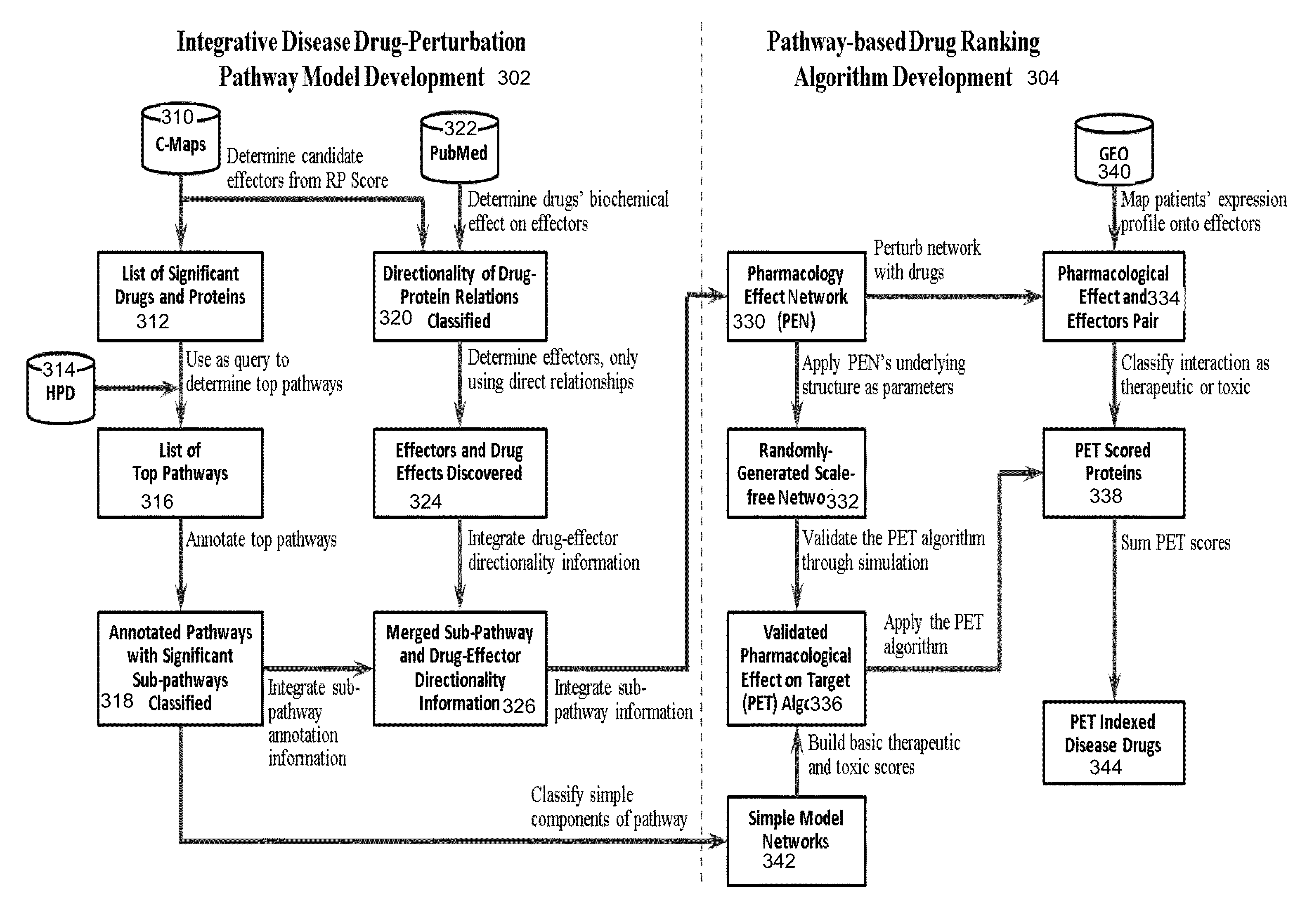
Integrative pathway modeling for drug efficacy prediction
InactiveUS20130144887A1Quantitative and accurate pathway/network modelingEvaluate drug efficacyChemical property predictionDigital data processing detailsDiseaseMedicine
An integrative pathway modeling approach and ranking / evaluating algorithms based on disease-specific pathway models can predict drug efficacy for patients based on their gene expression profiles. A disease-specific pathway model is first constructed with proteins and drugs important to the disease by using computational connectivity maps (C-Maps). Through the pathway model-based ranking algorithm, ideal drugs or optimized drug combination can be discovered for a patient to modulate the gene expression profile of this patient close to those in healthy individuals at pathway-level.
Owner:MEDEOLINX
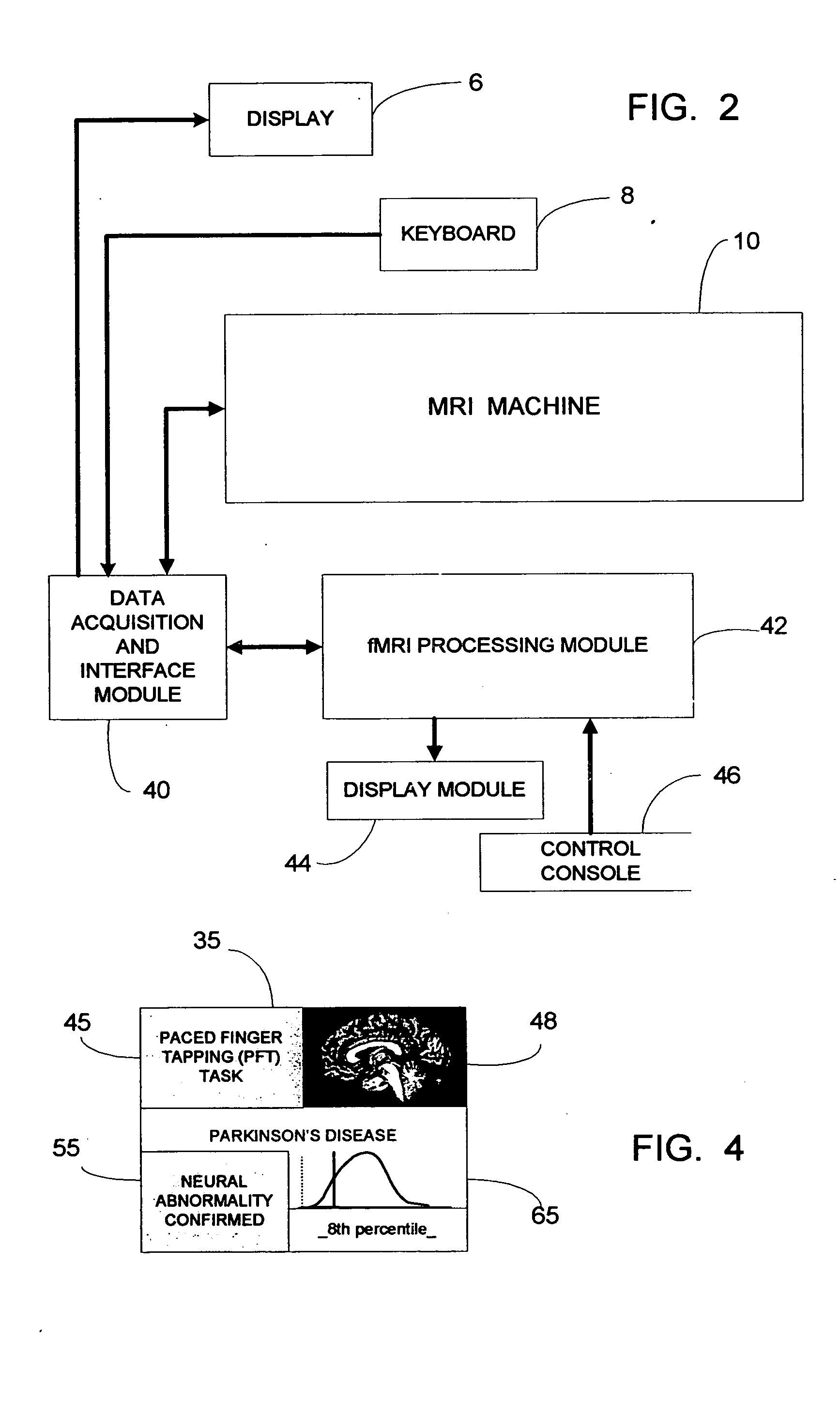
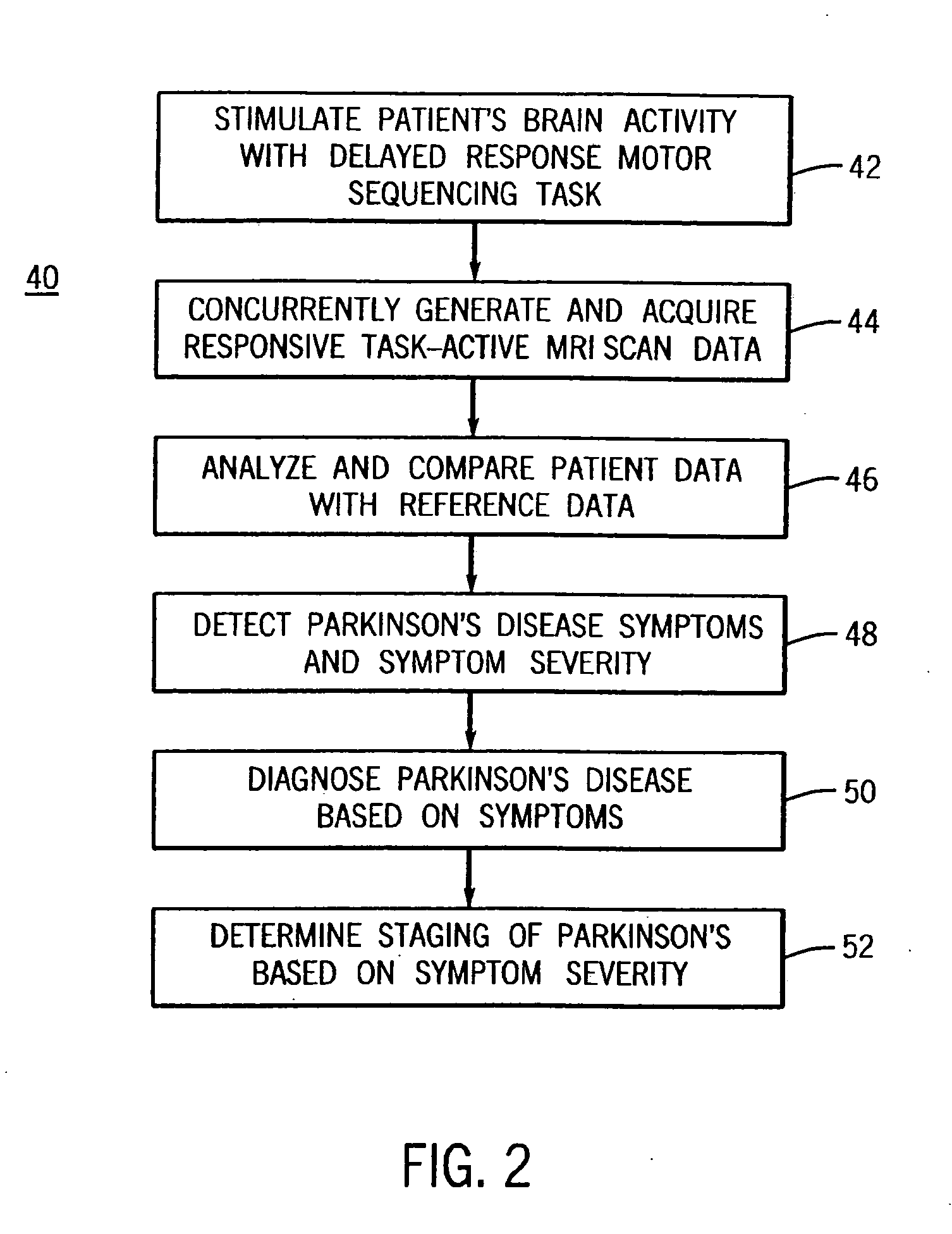
System for detecting symptoms, determining staging and gauging drug efficacy in cases of Parkinson's disease
InactiveUS20050197561A1Efficient and consistent and reliable mannerDiagnostic recording/measuringMeasurements using NMR imaging systemsDelayed responseMedicine
A system for using functional magnetic resonance imaging (fMRI) for detecting symptoms indicative of Parkinson's disease, diagnosing Parkinson's disease and gauging the efficacy of medications used in treating Parkinson's disease. The system includes process steps involving activating a selected region of the brain which may be affected by Parkinson's disease using a delayed response motor sequence type task, concurrently acquiring task-active MRI data responsive to the task, comparing the patient's task-active MRI data to reference data derived from a database of task-active data from healthy individuals and detecting whether the patient has symptoms related to Parkinson's disease. The severity of the patient's symptoms and the staging of the disease may also be determined. Also, a medication may be administered to the patient and the efficacy of the medication may gauged based on the severity of the patient's symptoms on and off of medication.
Owner:ELSINGER CATHERINE L +1
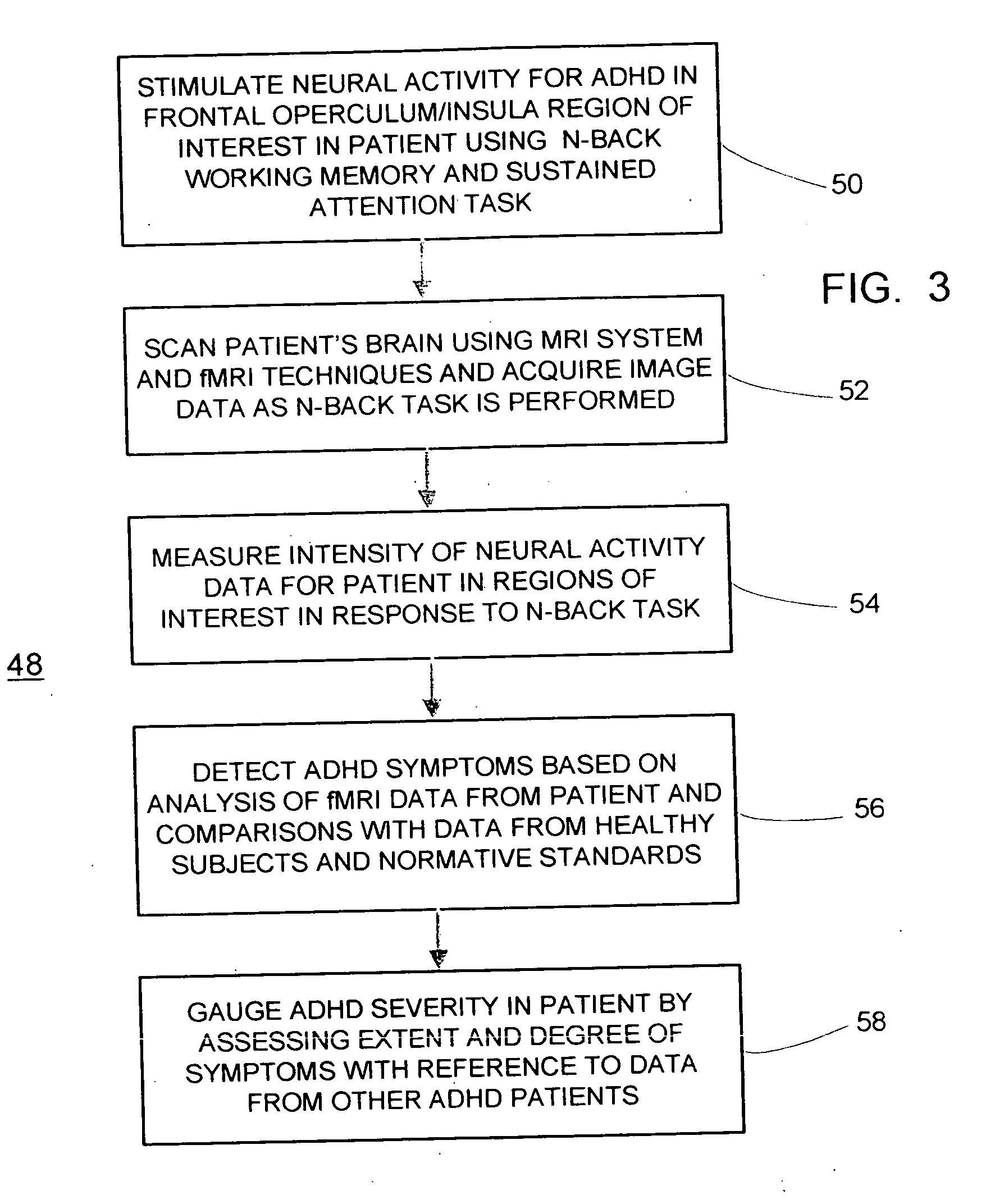
fMRI system for detecting symptoms associated with Attention Deficit Hyperactivity Disorder
InactiveUS20050267357A1Efficient and consistent and reliable mannerAccurate diagnosisComputer-assisted medical data acquisitionDiagnostic recording/measuringSustaining attentionPatients symptoms
A system based on the use of fMRI techniques for use in detecting neurological abnormalities indicative of Attention Deficit Hyperactivity Disorder (ADHD), in determining the severity of ADHD and in gauging the efficacy of medications used in treating ADHD. The system includes the steps of activating a selected region of the brain which is known to be affected by ADHD using a working memory and sustained attention task such as an N-Back task and concurrently acquiring fMRI image data responsive to the task. The patient's task-active fMRI data is then compared to reference fMRI data derived from a database of task-active fMRI data acquired from healthy individuals and determining whether the patient has symptoms related to ADHD. The extent of the patient's ADHD related symptoms and the severity of the disorder may also be assessed. Additionally, patients who are affected by ADHD may be administered medications intended to address their symptoms and based on comparing the severity of the patient's symptoms on and off therapy, the efficiency of the medication may be gauged and a measure may be provided of how well individual patients respond to a given medication.
Owner:RAO STEPHEN M +1
Methods of treating degenerative bone conditions
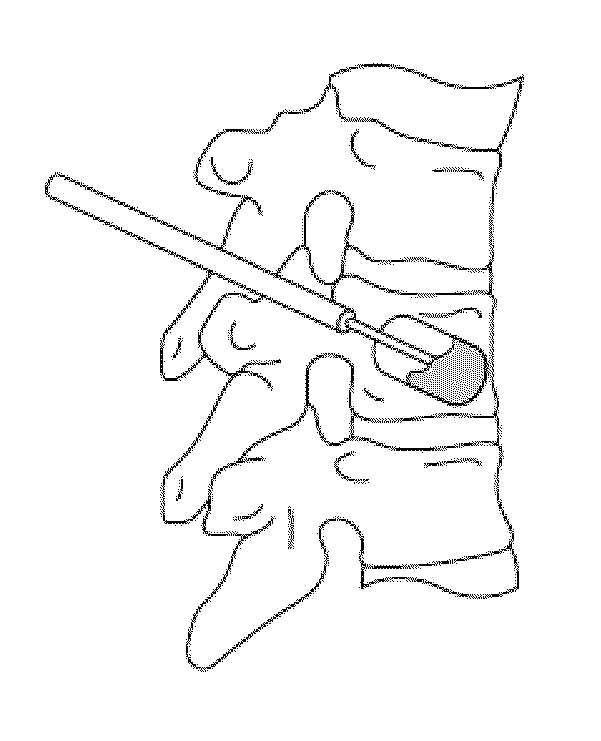
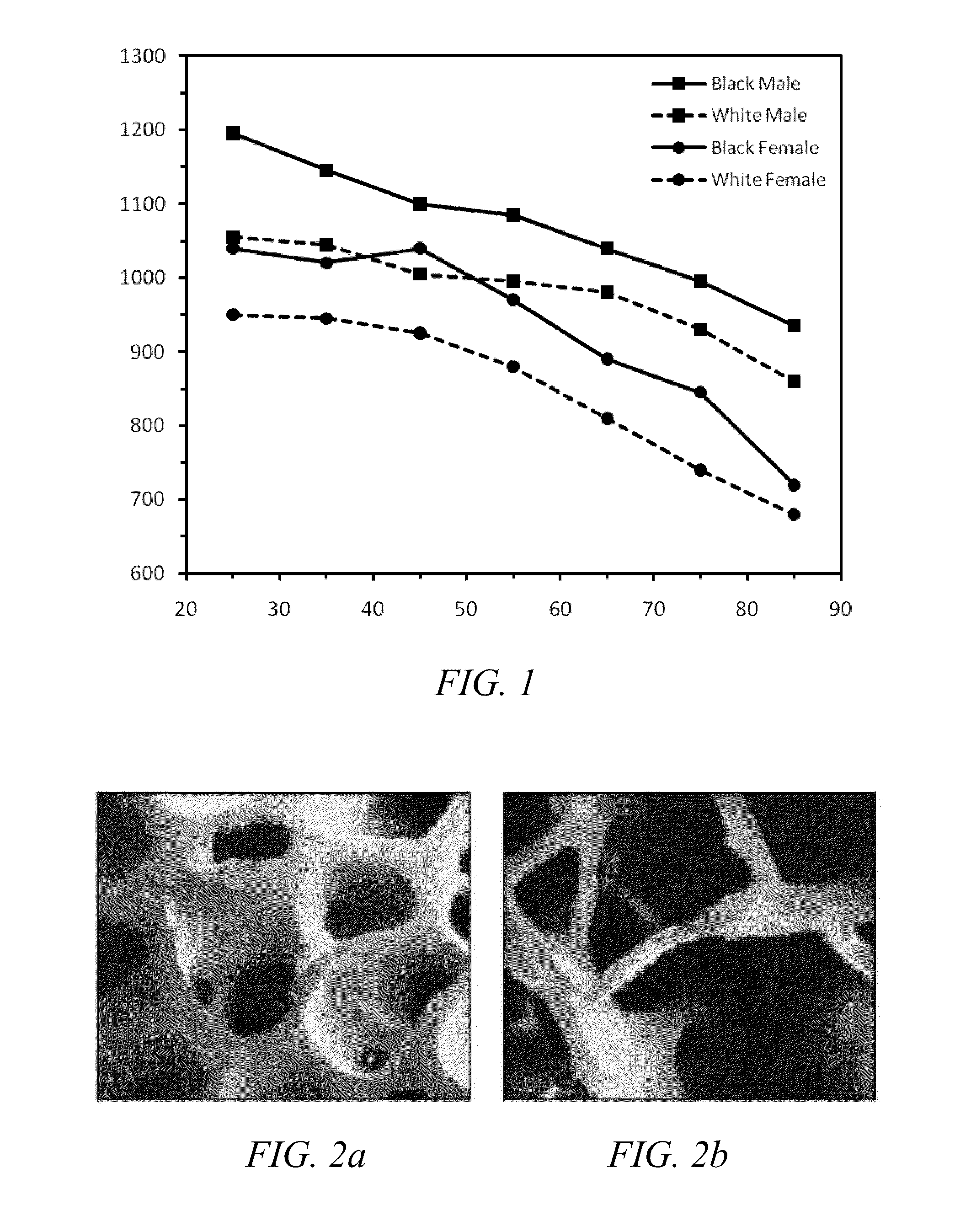
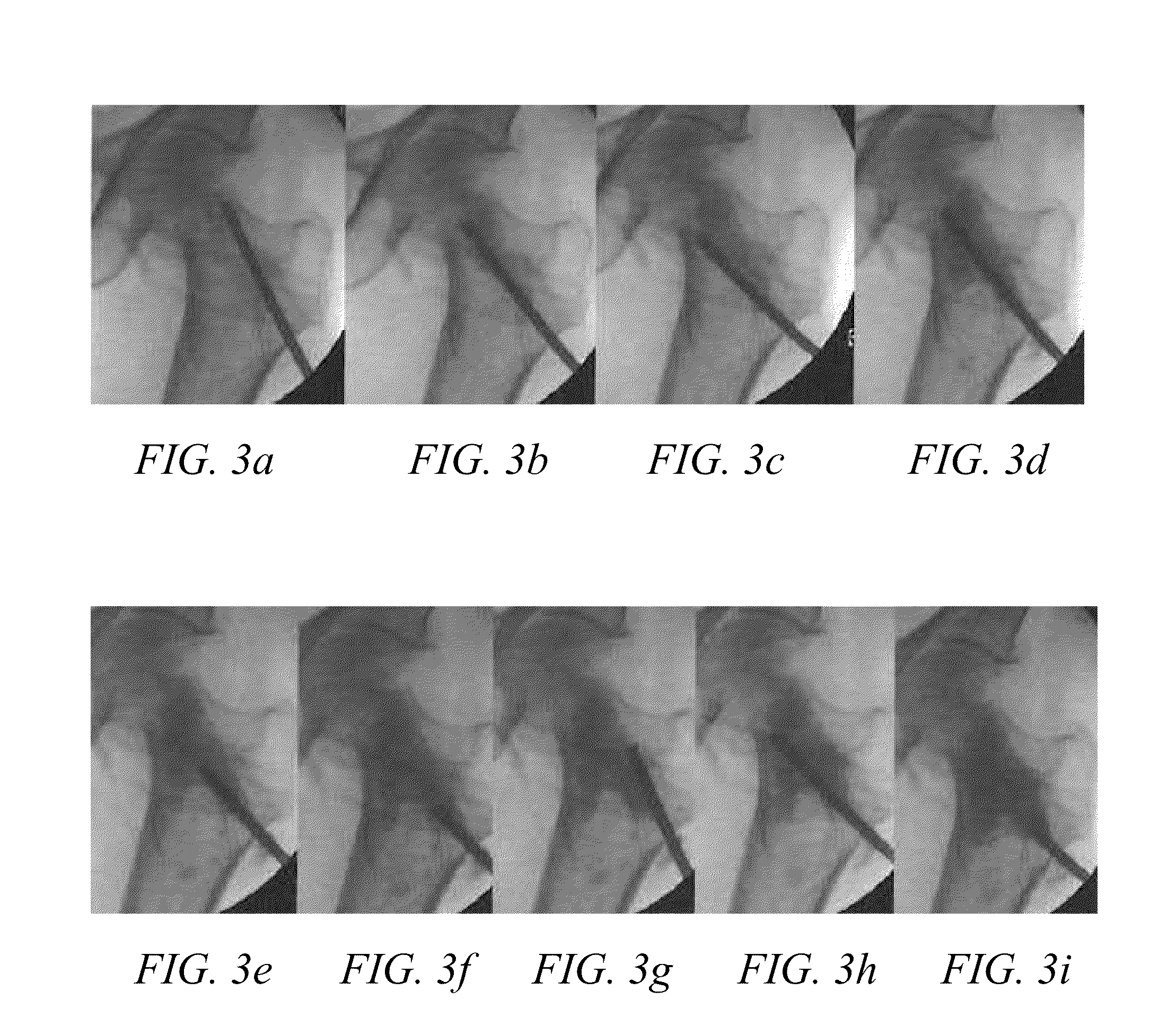
ActiveUS20120004594A1Improve bone qualityIncrease bone densitySurgical adhesivesPharmaceutical delivery mechanismNatural boneOsteoporotic bone
Methods and articles of manufacture for treating patients with degenerative bone are provided. The methods are useful to improve bone quality in a localized area of a degenerative bone (e.g., osteopenic or osteoporotic bone), such as by improving BMD to be substantially similar to BMD in an average, healthy individual at the age of peak BMD. The methods can comprise forming a void in a localized area of the degenerative bone and filling the void with a bone regenerative material that causes generation of new, healthy, natural bone material. The articles of manufacture can comprise kits formed of various materials useful in the inventive methods for improving bone quality.
Owner:AGNOVOS HEALTHCARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com