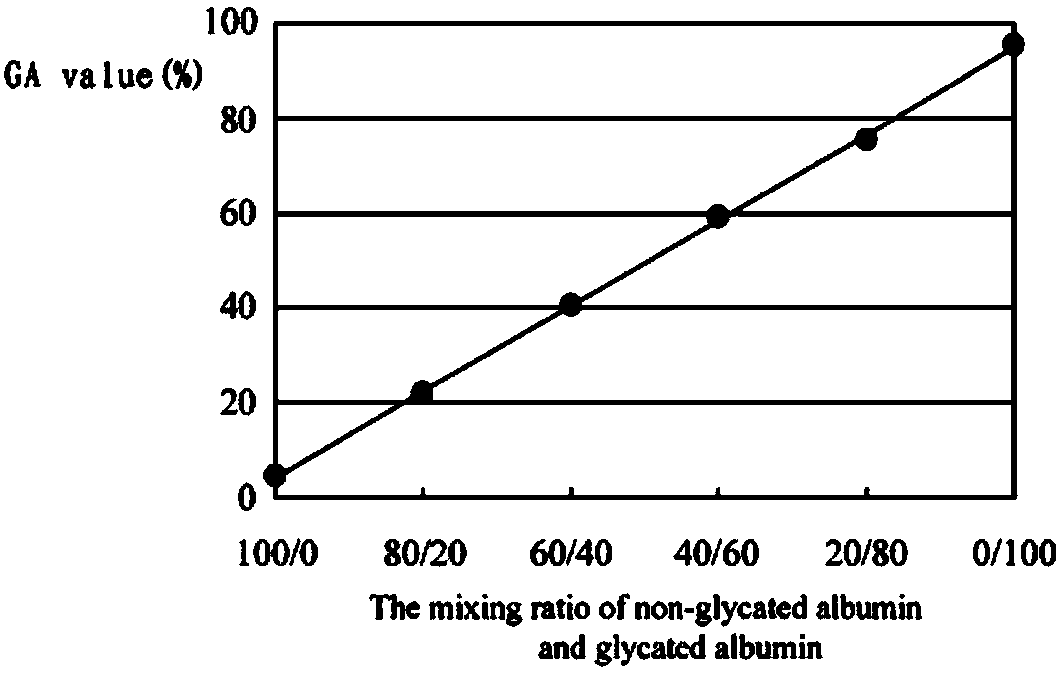
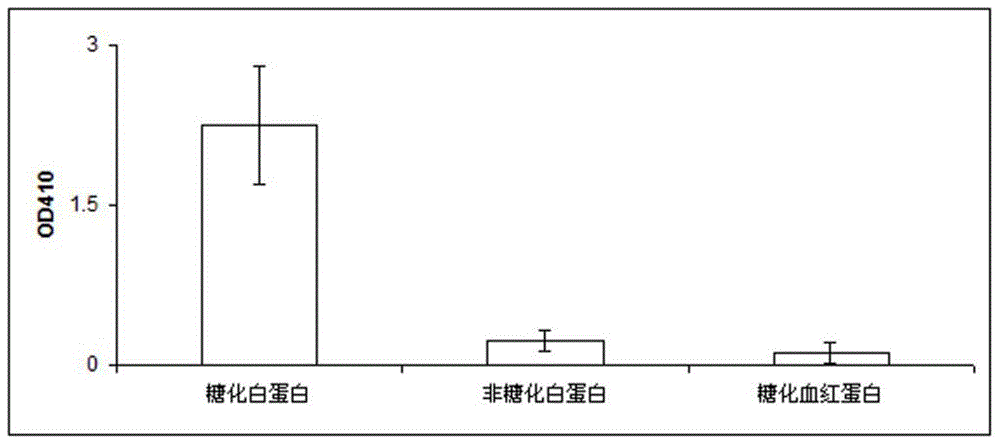
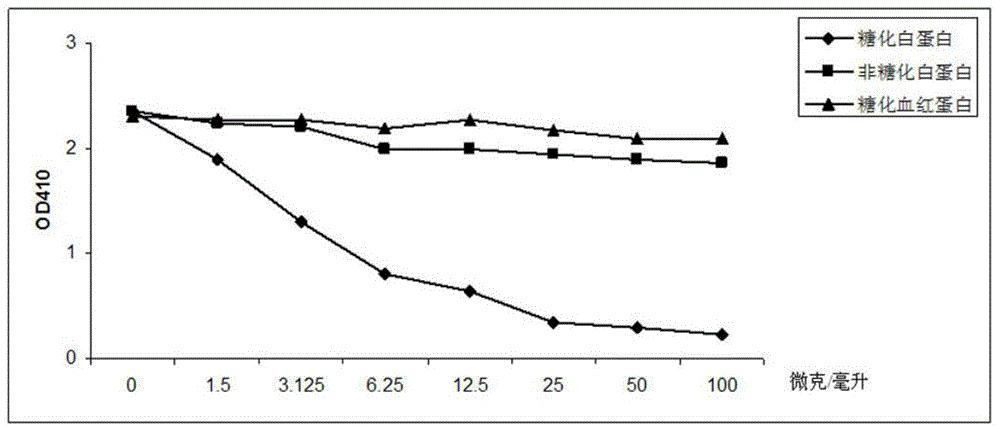
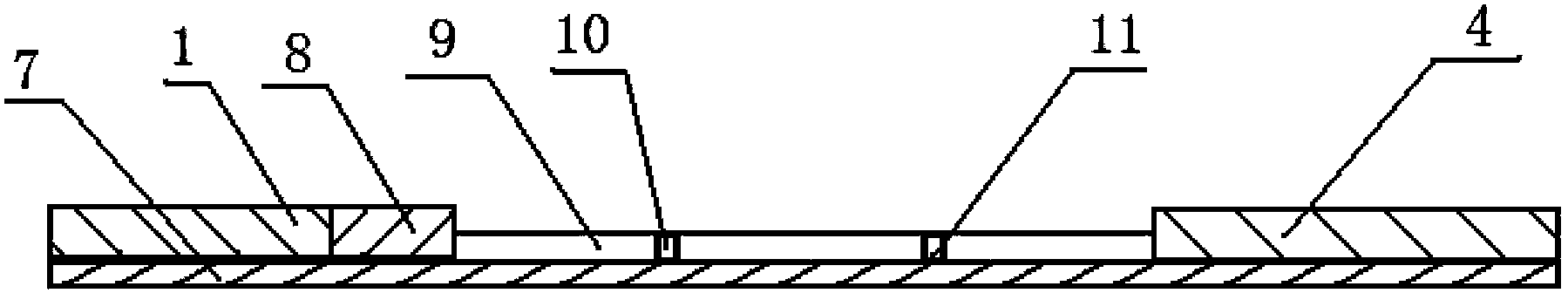
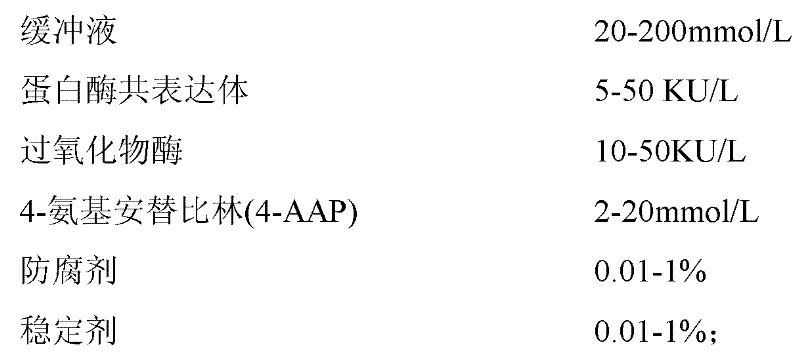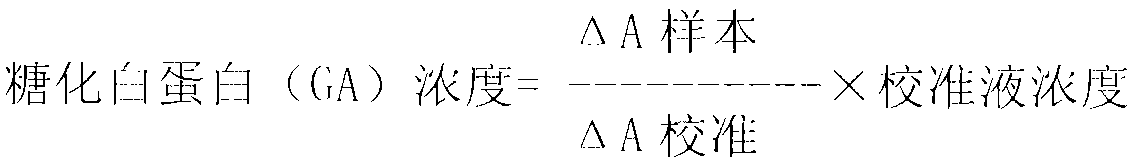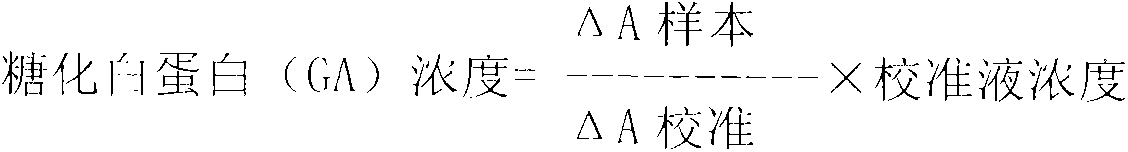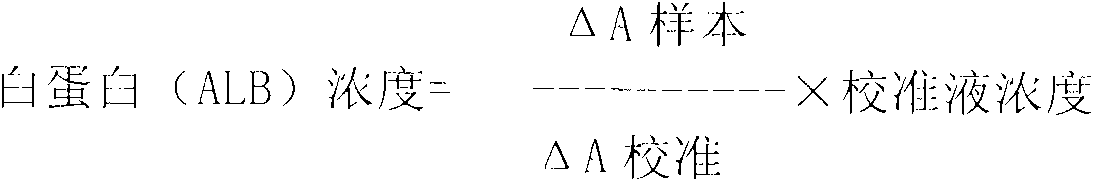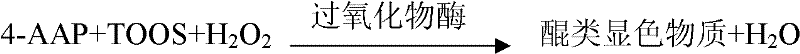Patents
Literature
65 results about "Glycated albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycated albumin is albumin that is bound to sugar. Since about 80 percent of circulating proteins found in blood are albumin, it is the most common type of protein found in blood. Albumin is naturally replaced in the body every 20-25 days.
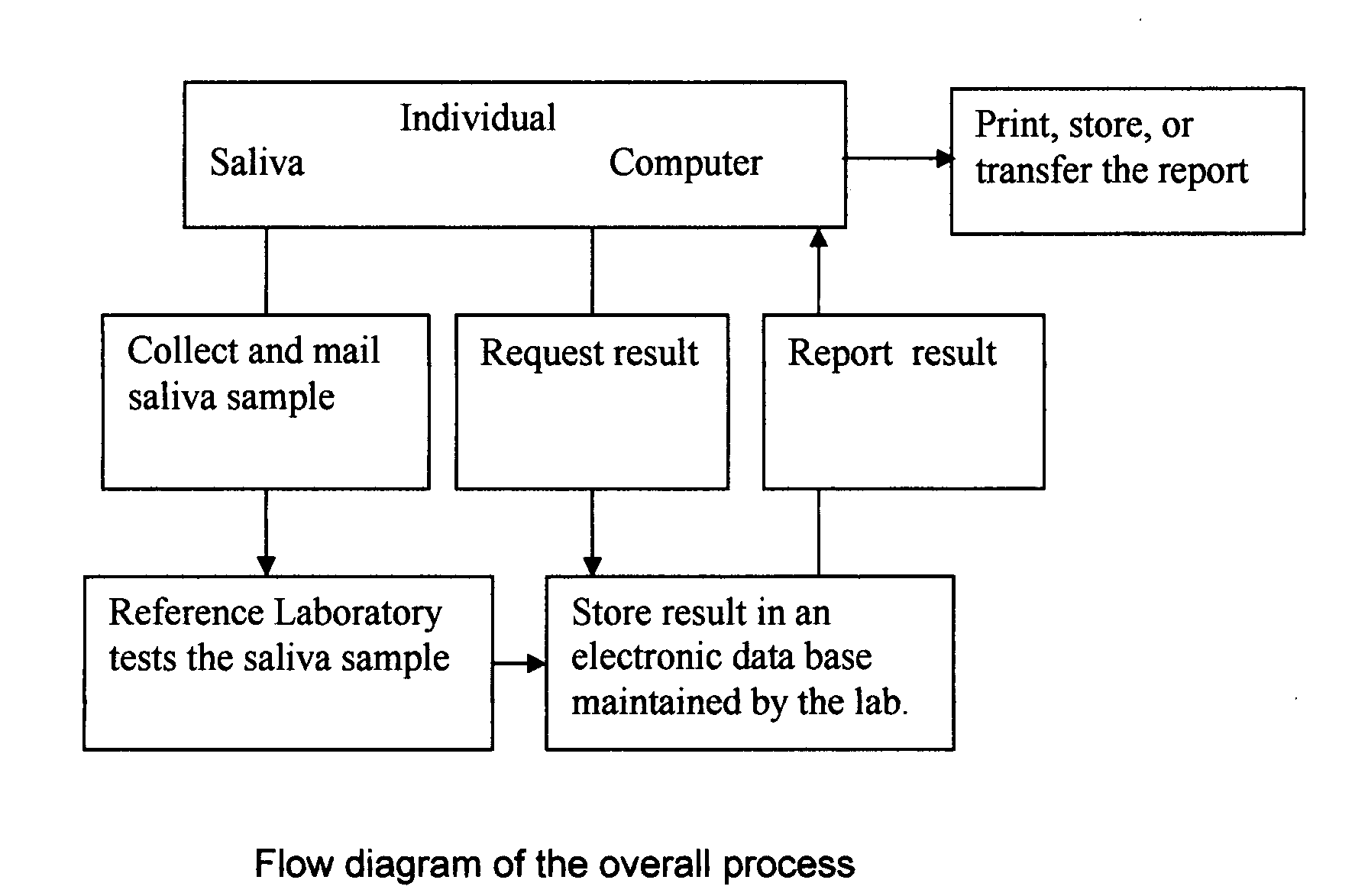
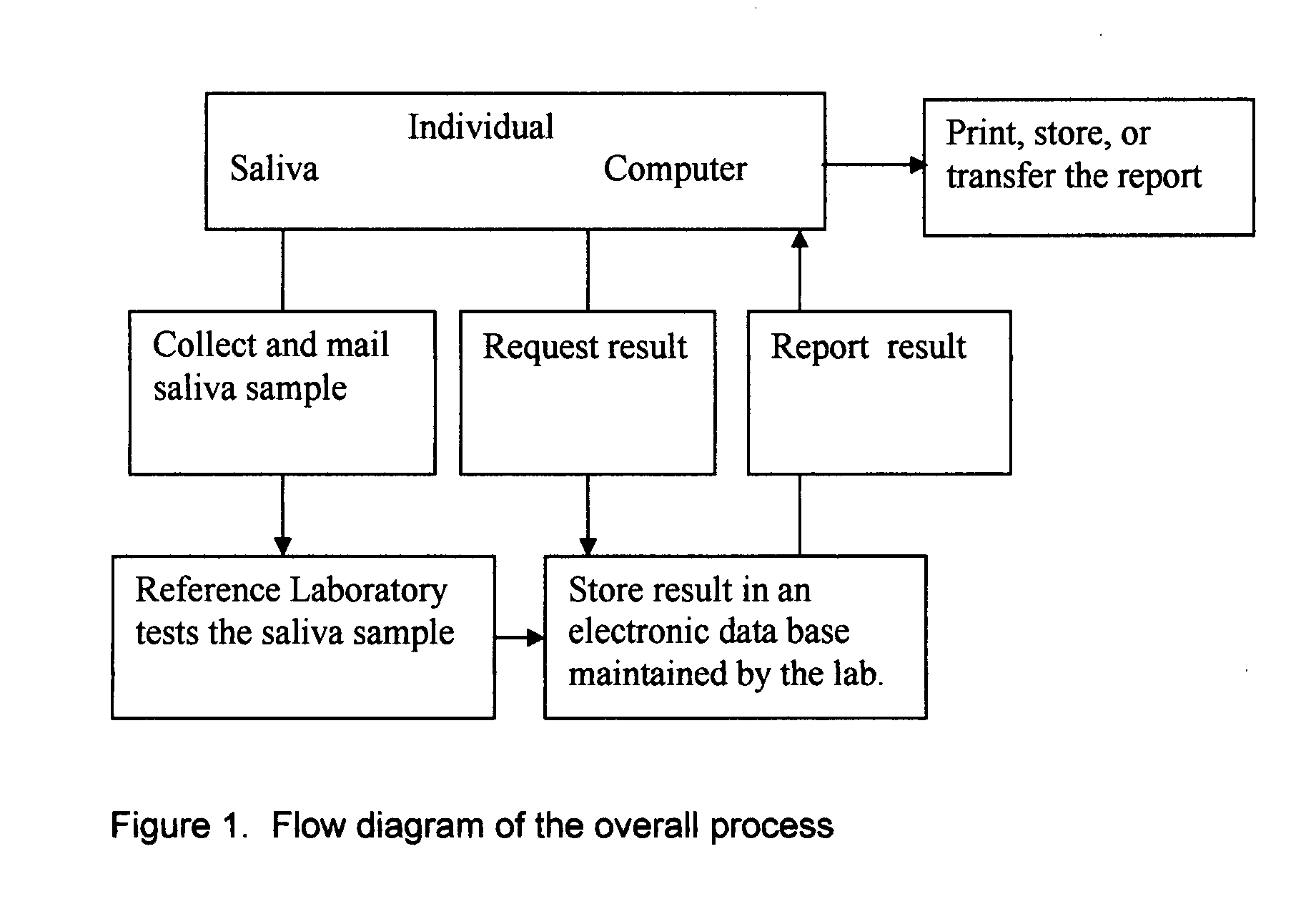
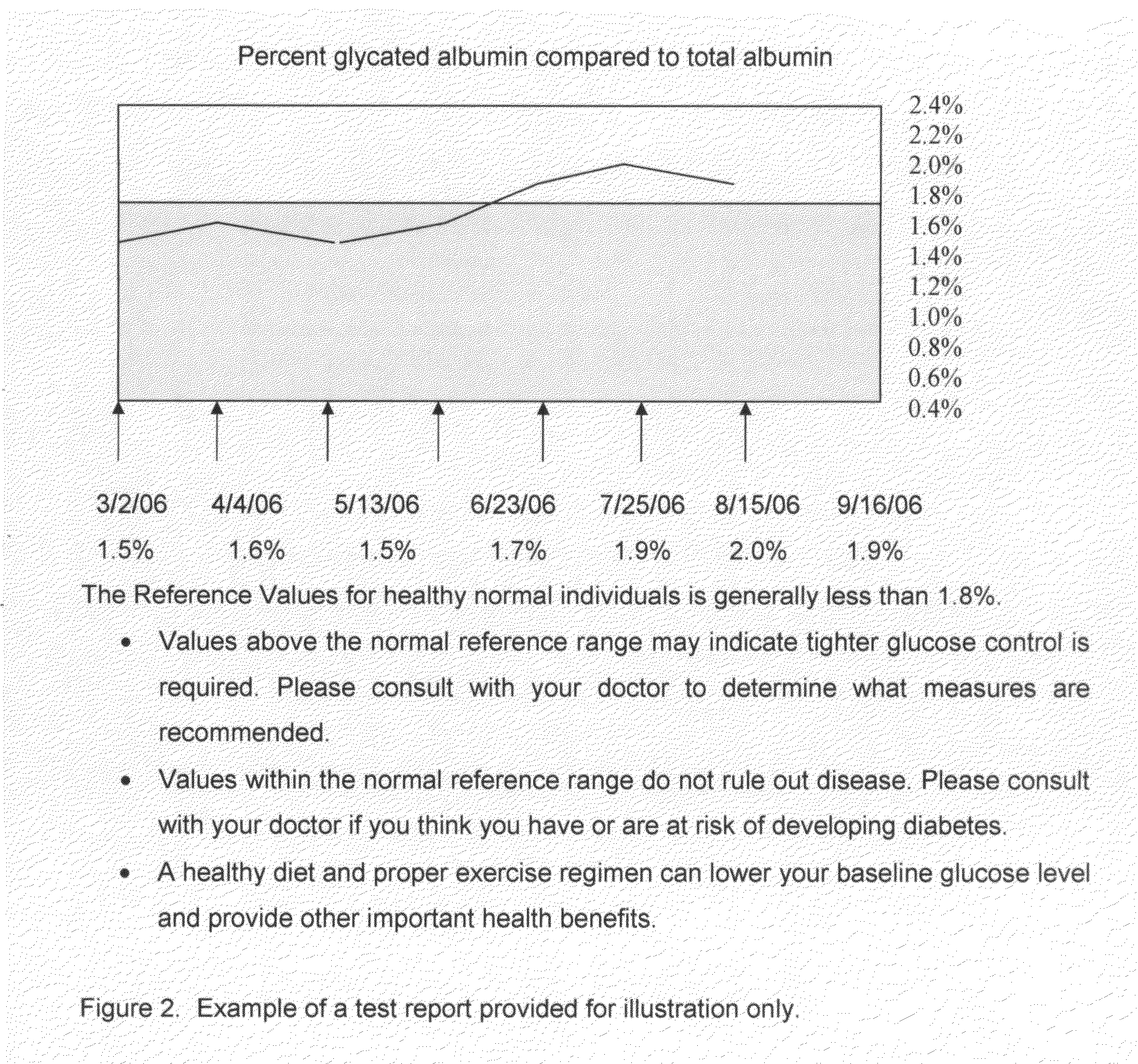
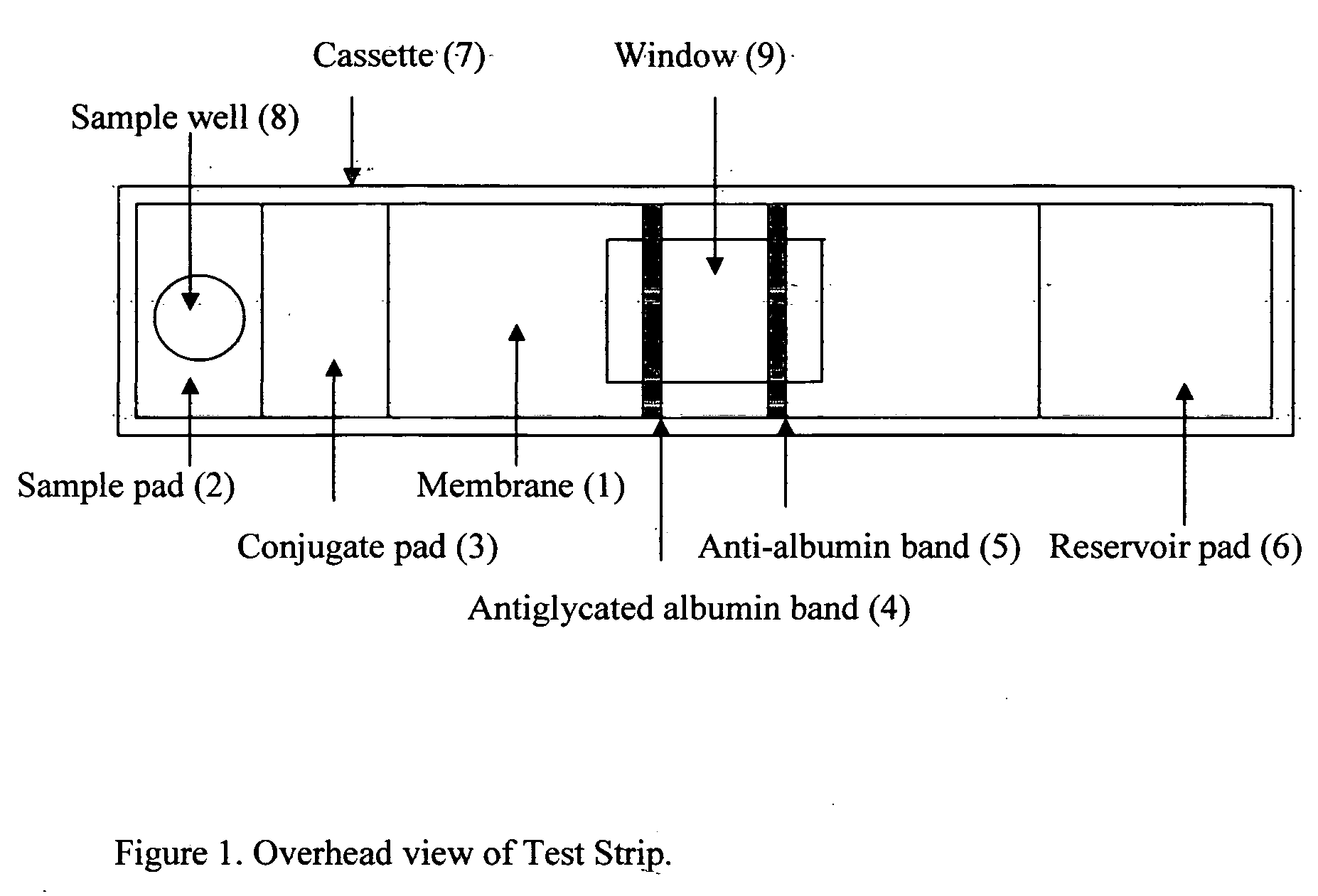
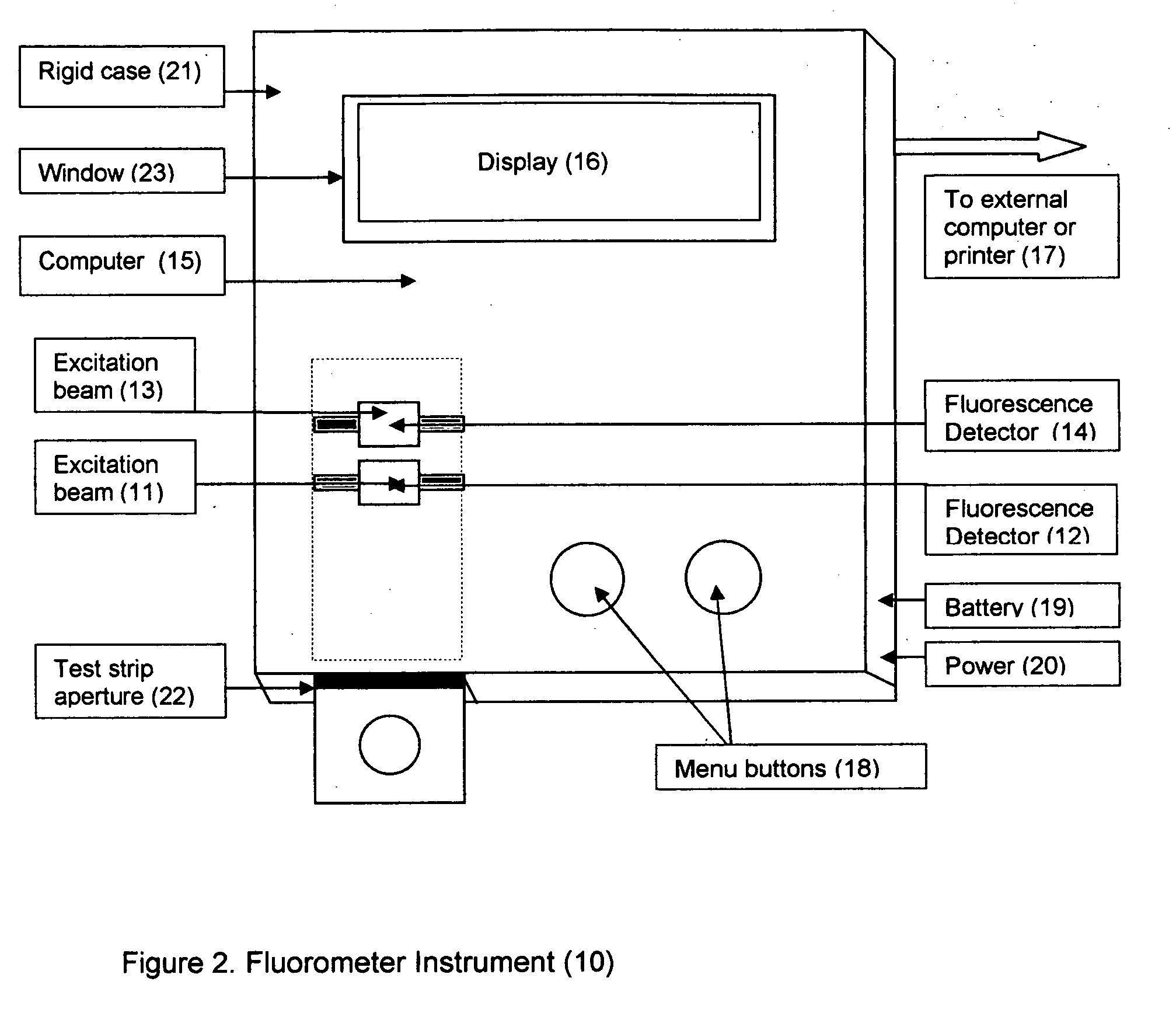
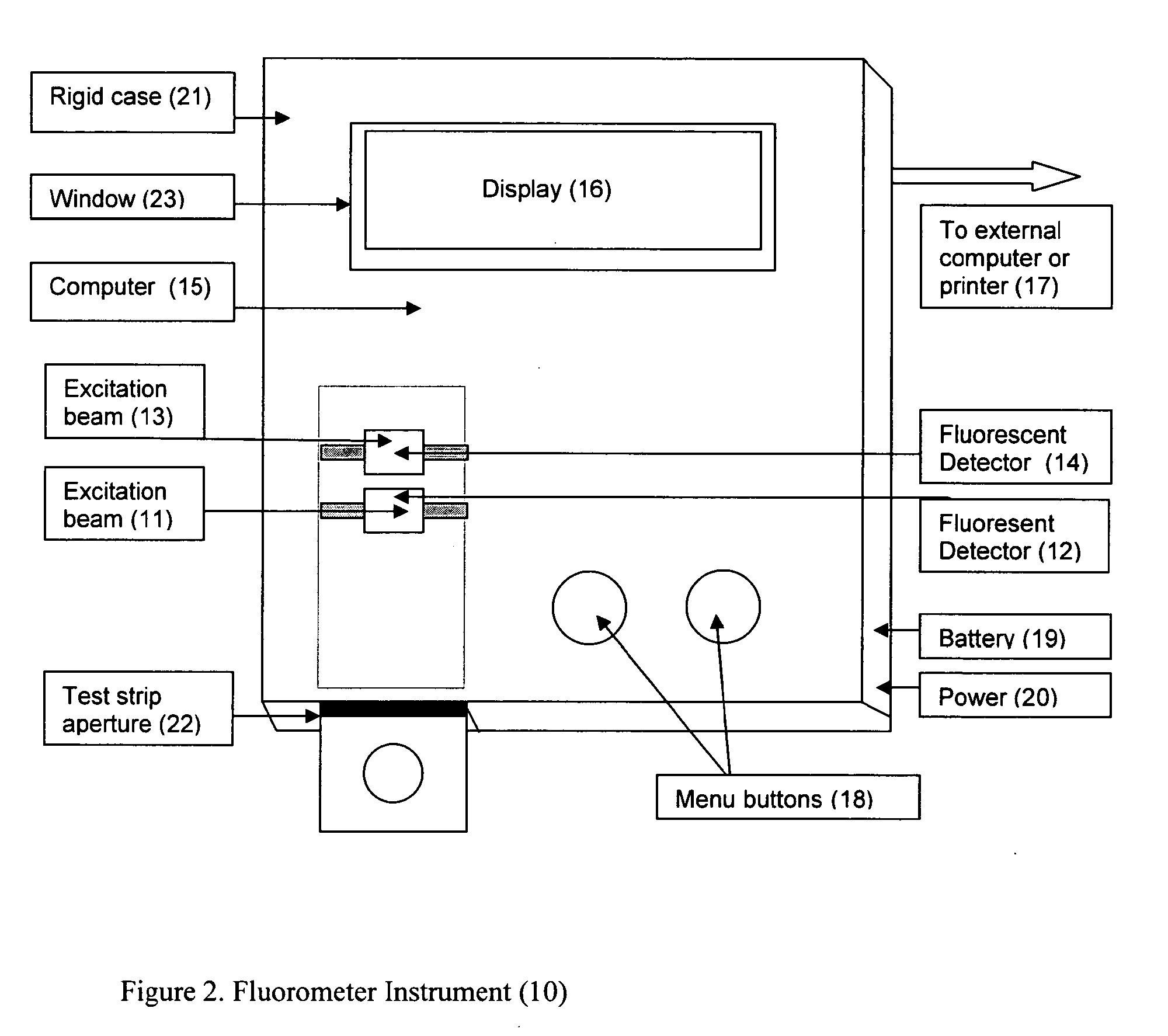
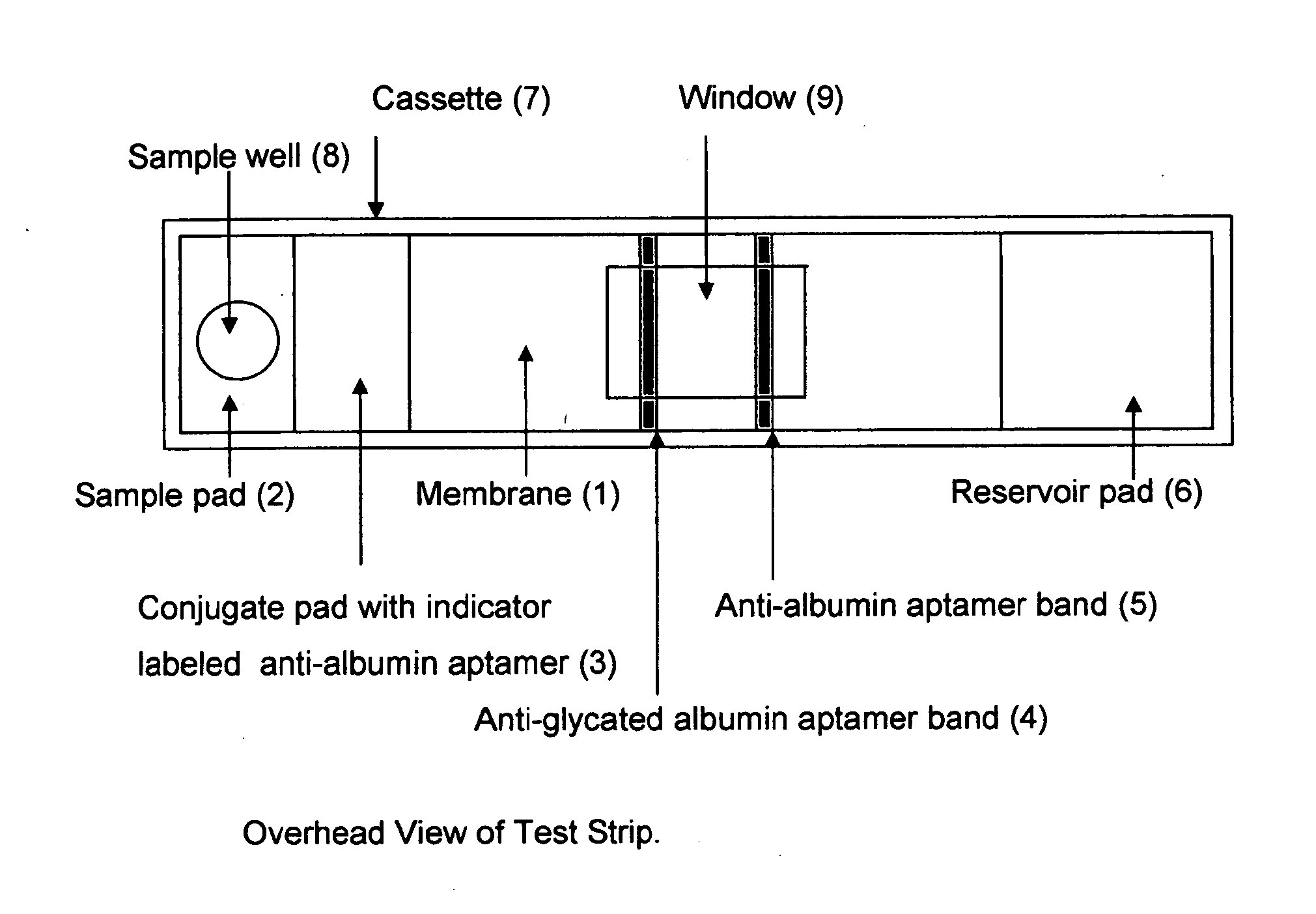
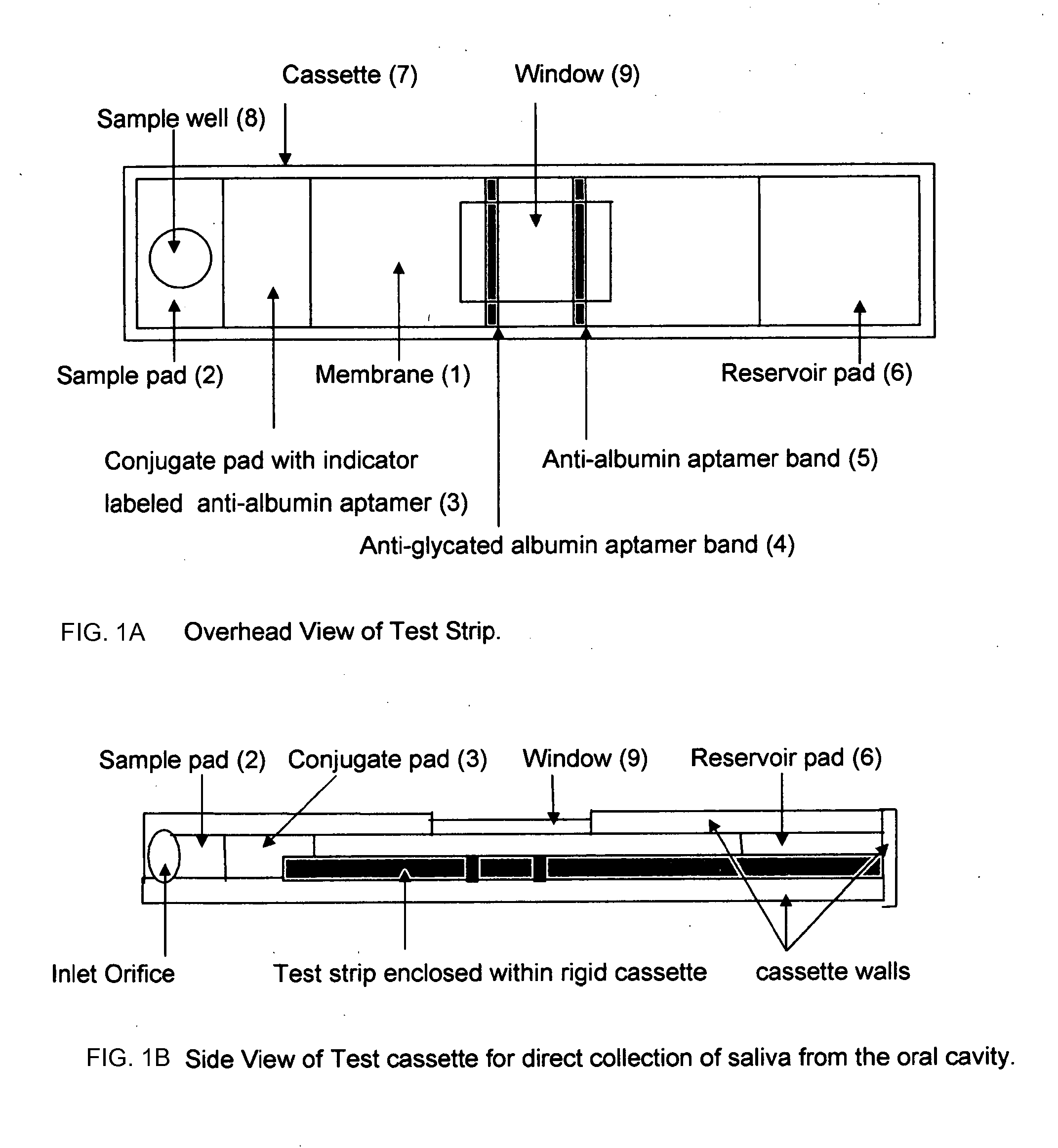
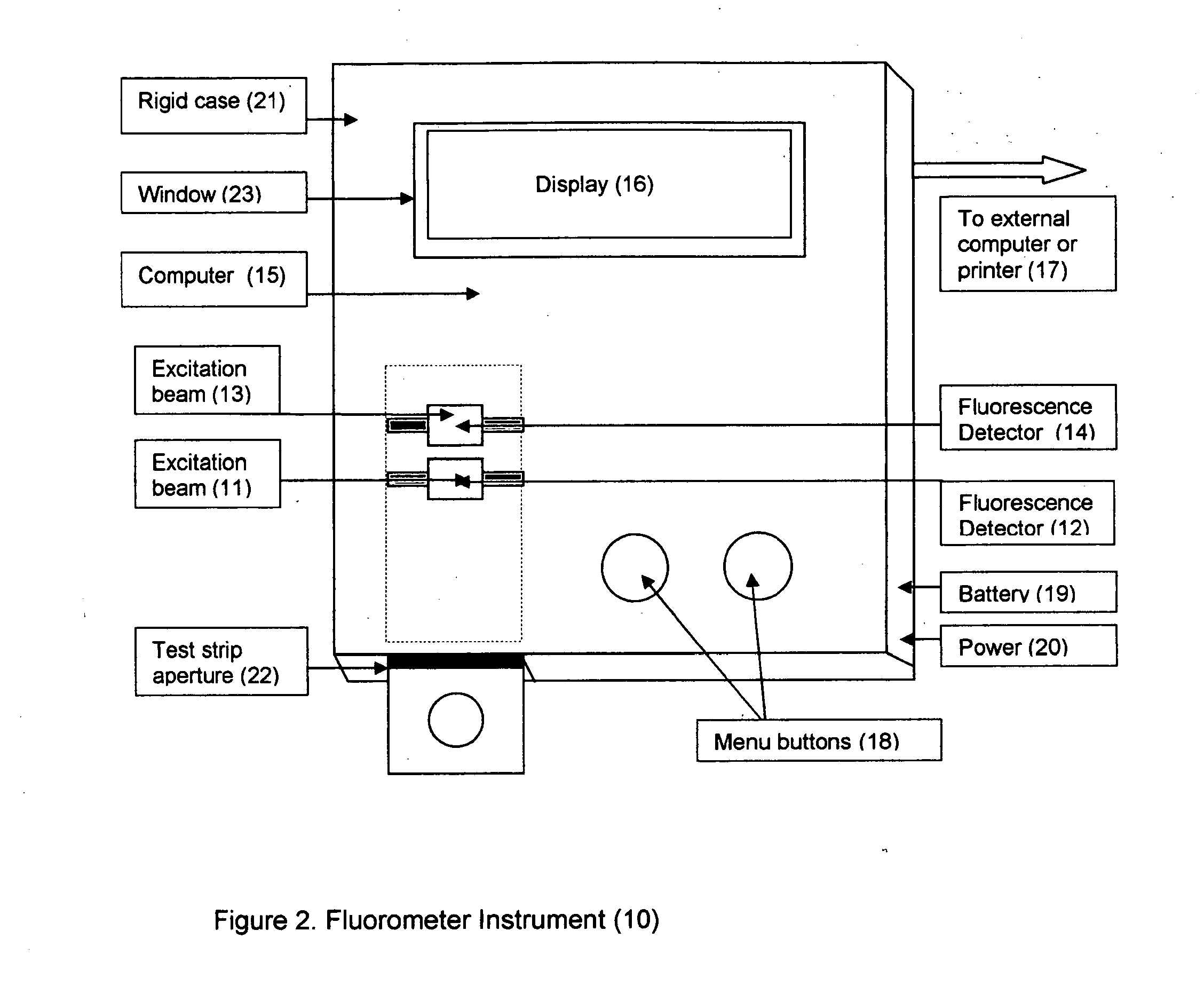
Home test for glycated albumin in saliva
InactiveUS20080227210A1Withdrawing sample devicesVaccination/ovulation diagnosticsSaliva sampleSaliva collection
A home test for measuring glycated albumin levels in saliva. The saliva sample is collected at home using a standardized saliva collection kit and mailed to a testing laboratory that performs the test and reports the result directly back to the customer via the internet. The home test can be used to monitor glucose control in diabetics and in healthy individuals. It may also be used as a diagnostic aid in identifying individuals with diabetes, or who are at risk of developing diabetes.
Owner:SMITH HENRY
Composition for assaying glycoprotein
InactiveUS20050101771A1Reduced responseLose their reactivityMicrobiological testing/measurementPeptide preparation methodsProteinase activityBiology
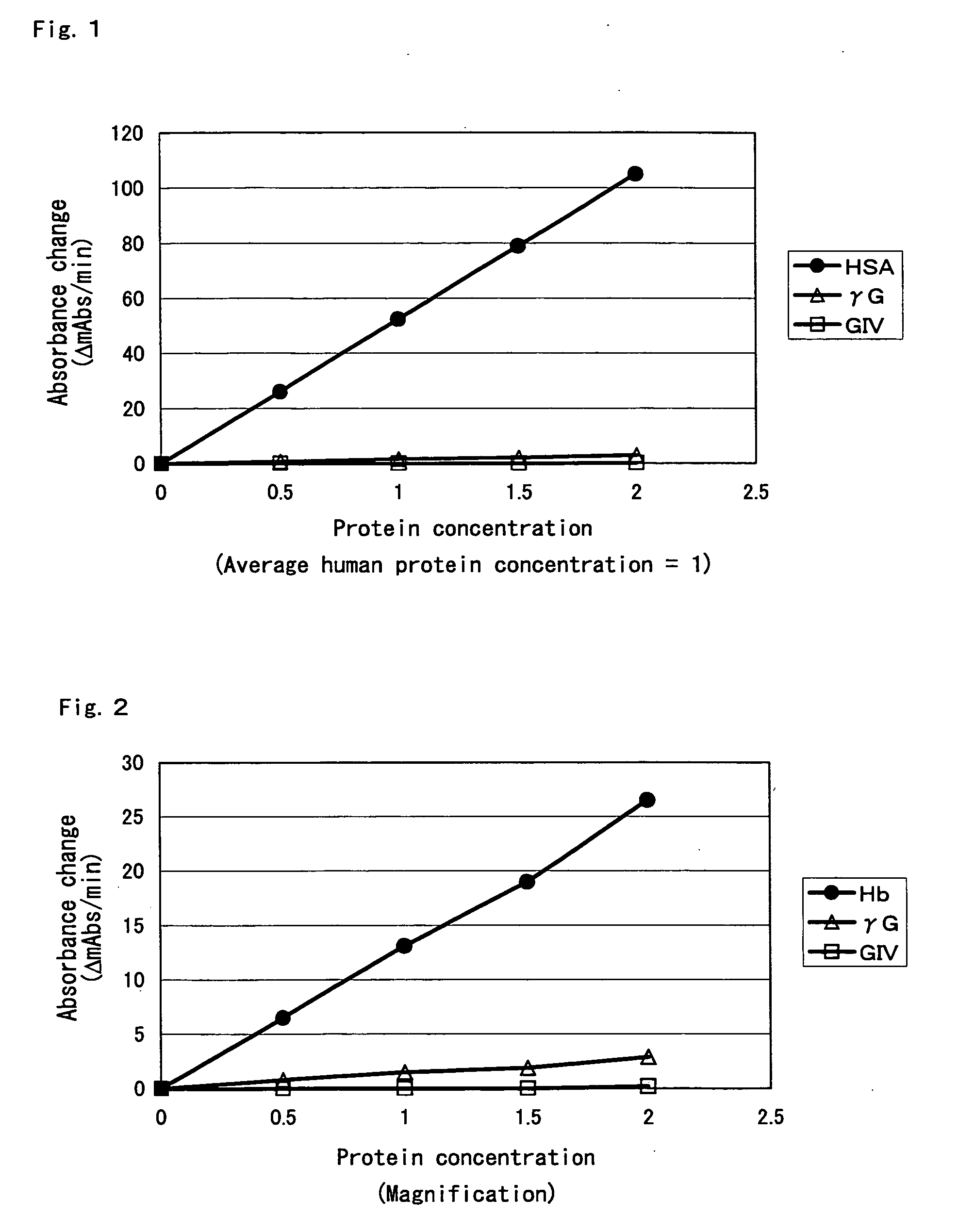
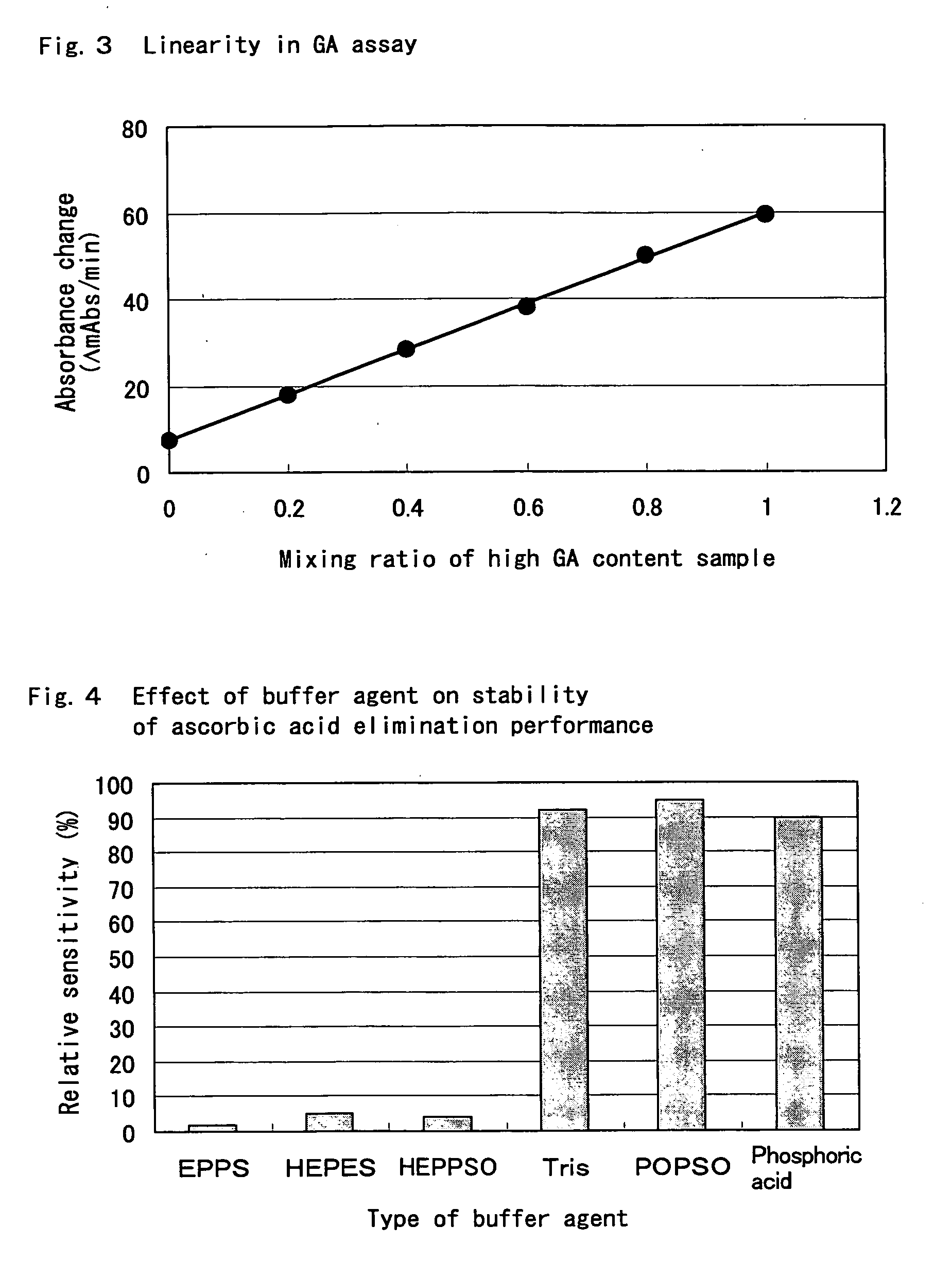
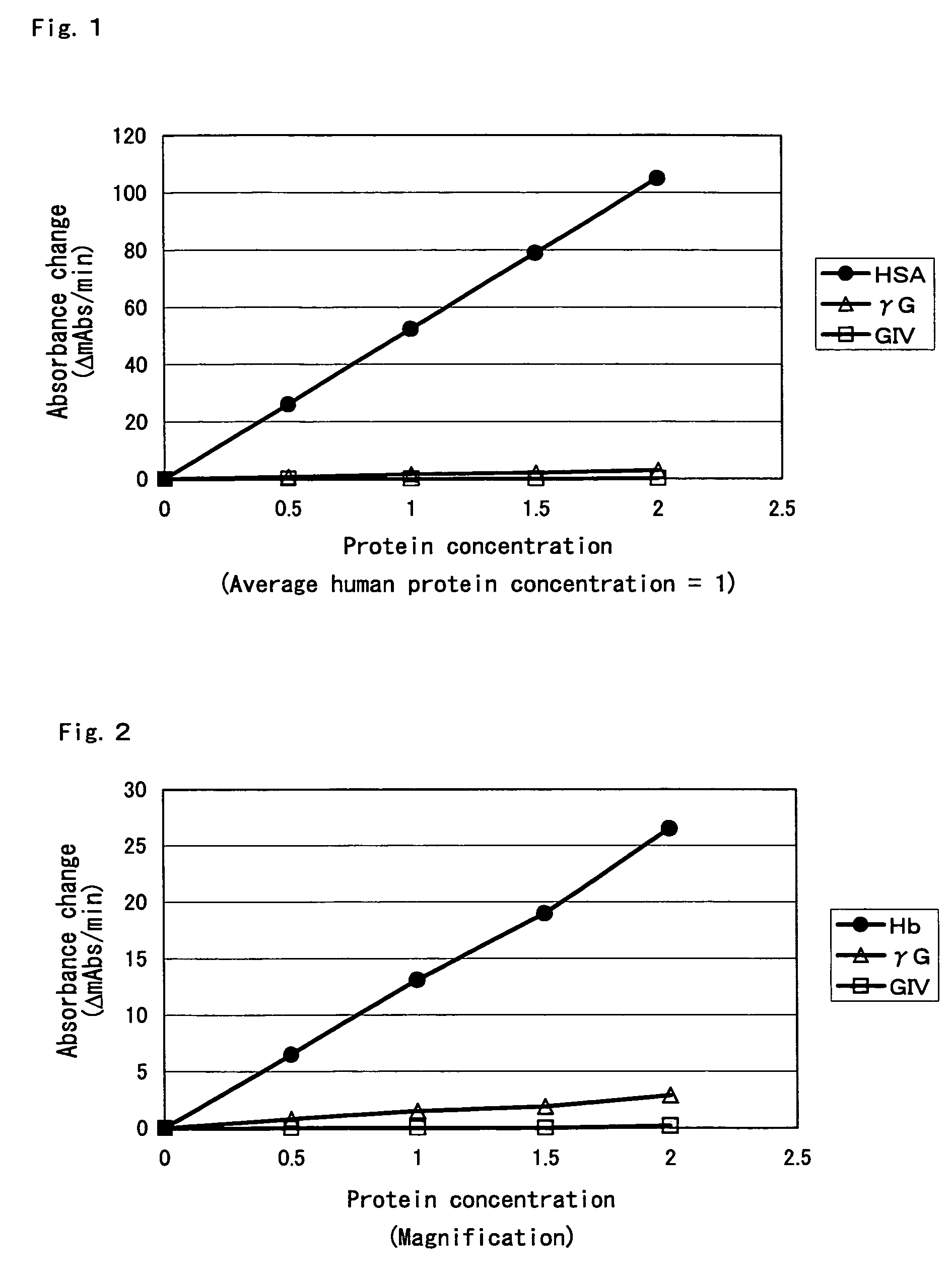
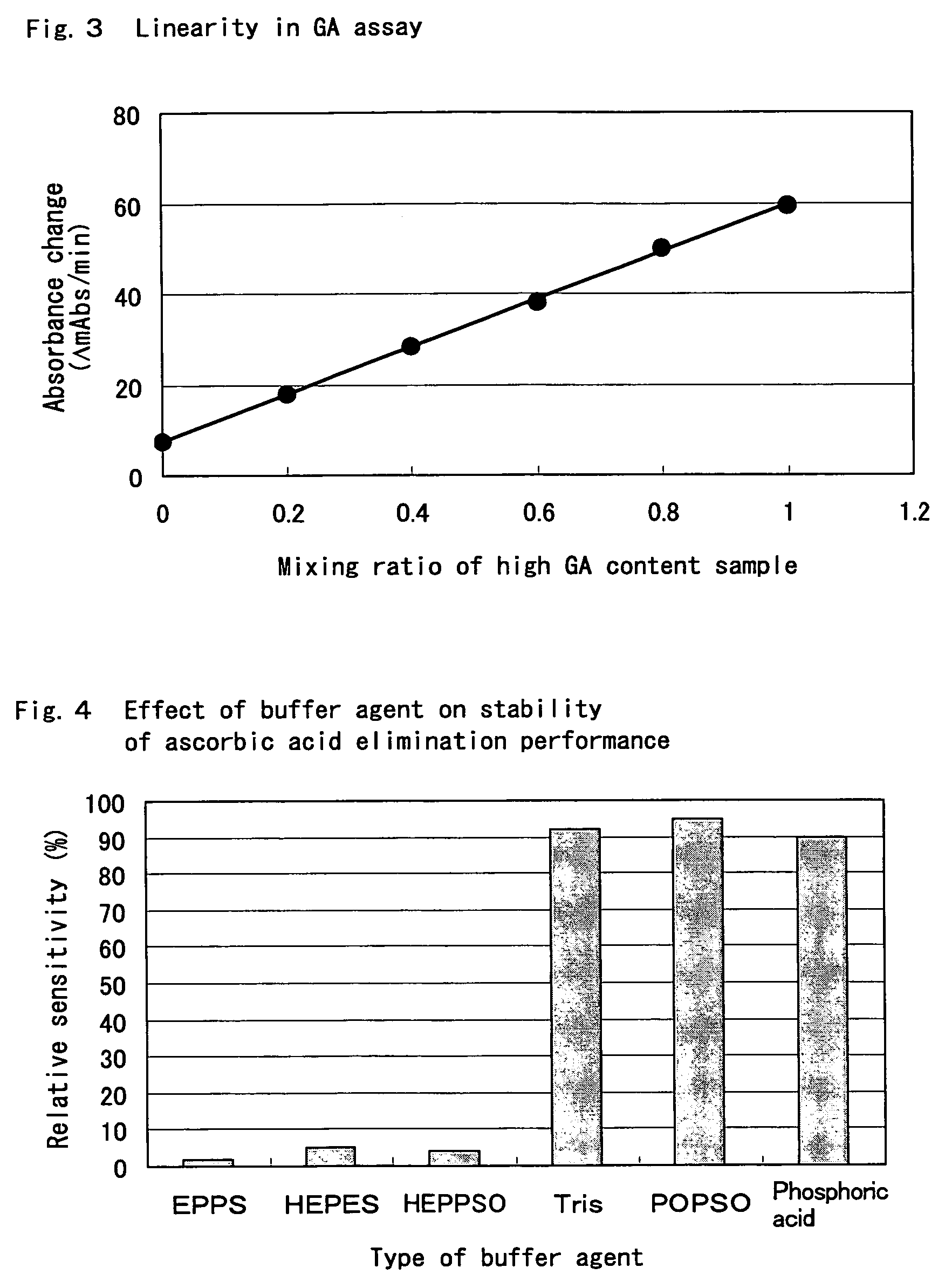
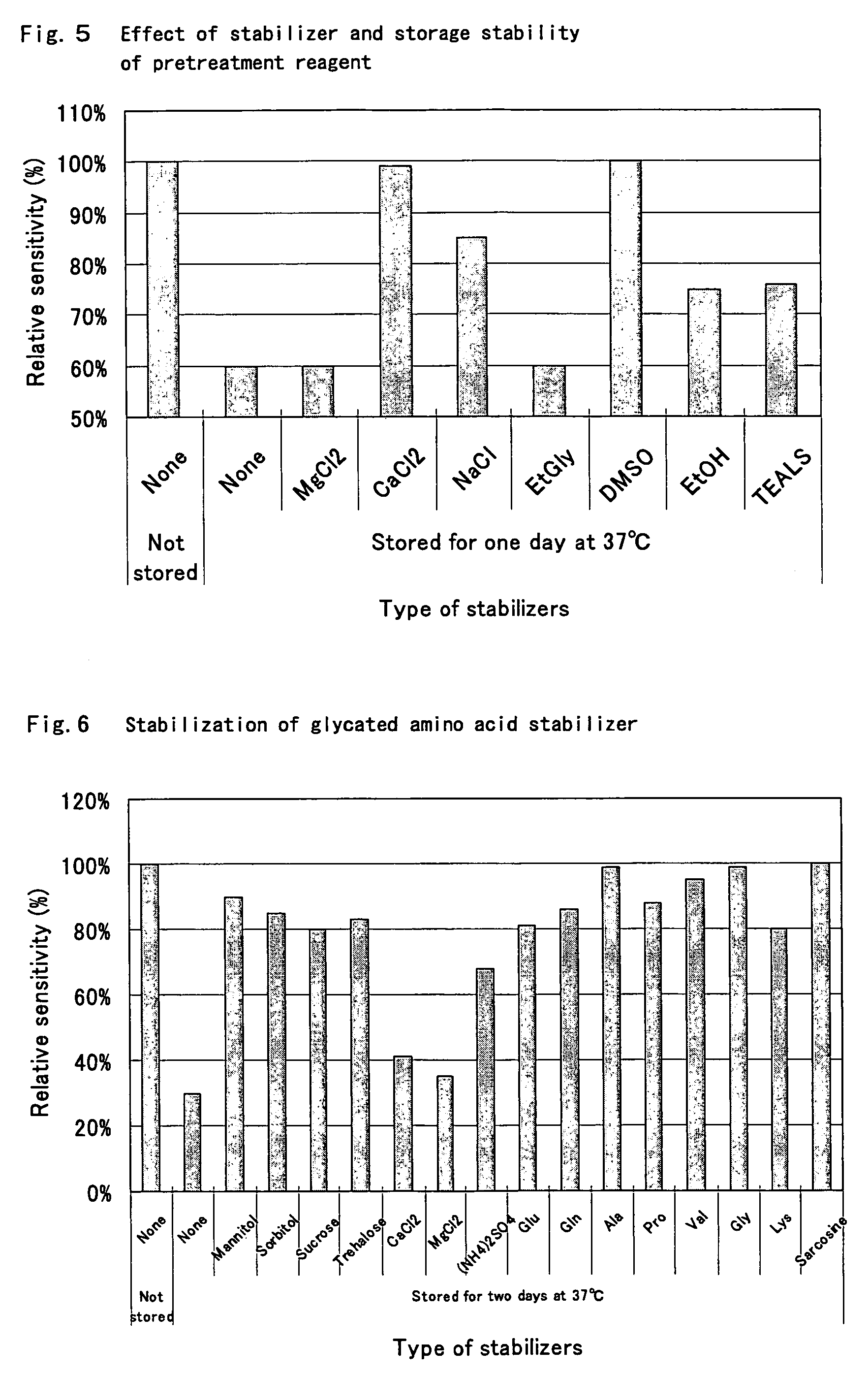
Compositions for accurately assaying a glycated protein by: 1) avoiding effects of globulin and ascorbic acid components, 2) siabilizing proteases and at least enzymes acting on glycated amino acids; 3) accurately assaying albumin; and 4) assaying glycated albumin while avoiding the effects of glycated hemoglobin, and an assay method are provided. Thus, the contents of a glycated protein and glycated albumin can be more accurately determined.
Owner:ASAHI KASEI PHARMA
Rapid test for glycated albumin in saliva
InactiveUS20060270060A1Accurate assessmentBioreactor/fermenter combinationsBiological substance pretreatmentsMeasuring instrumentPlasma Albumin
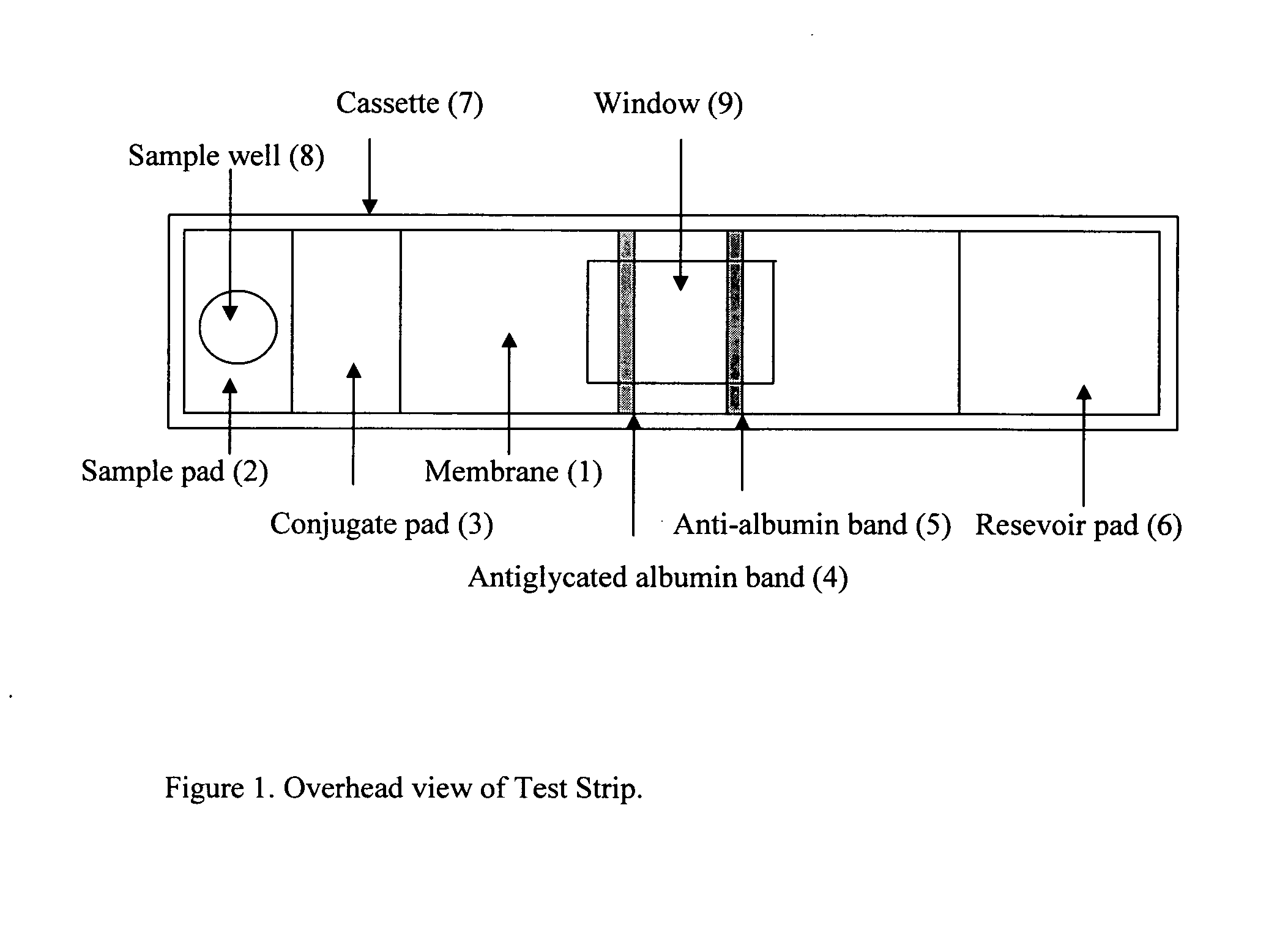
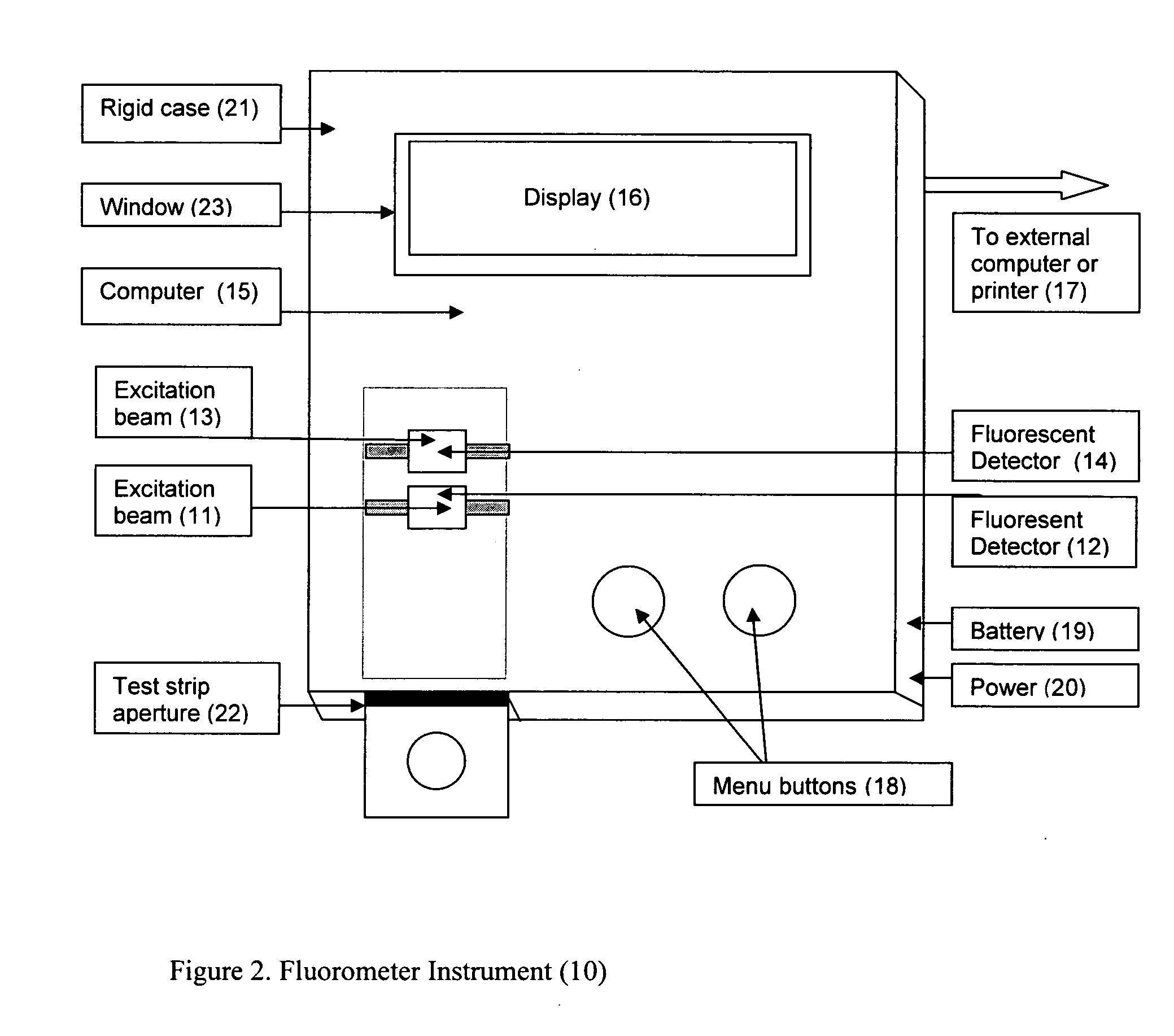
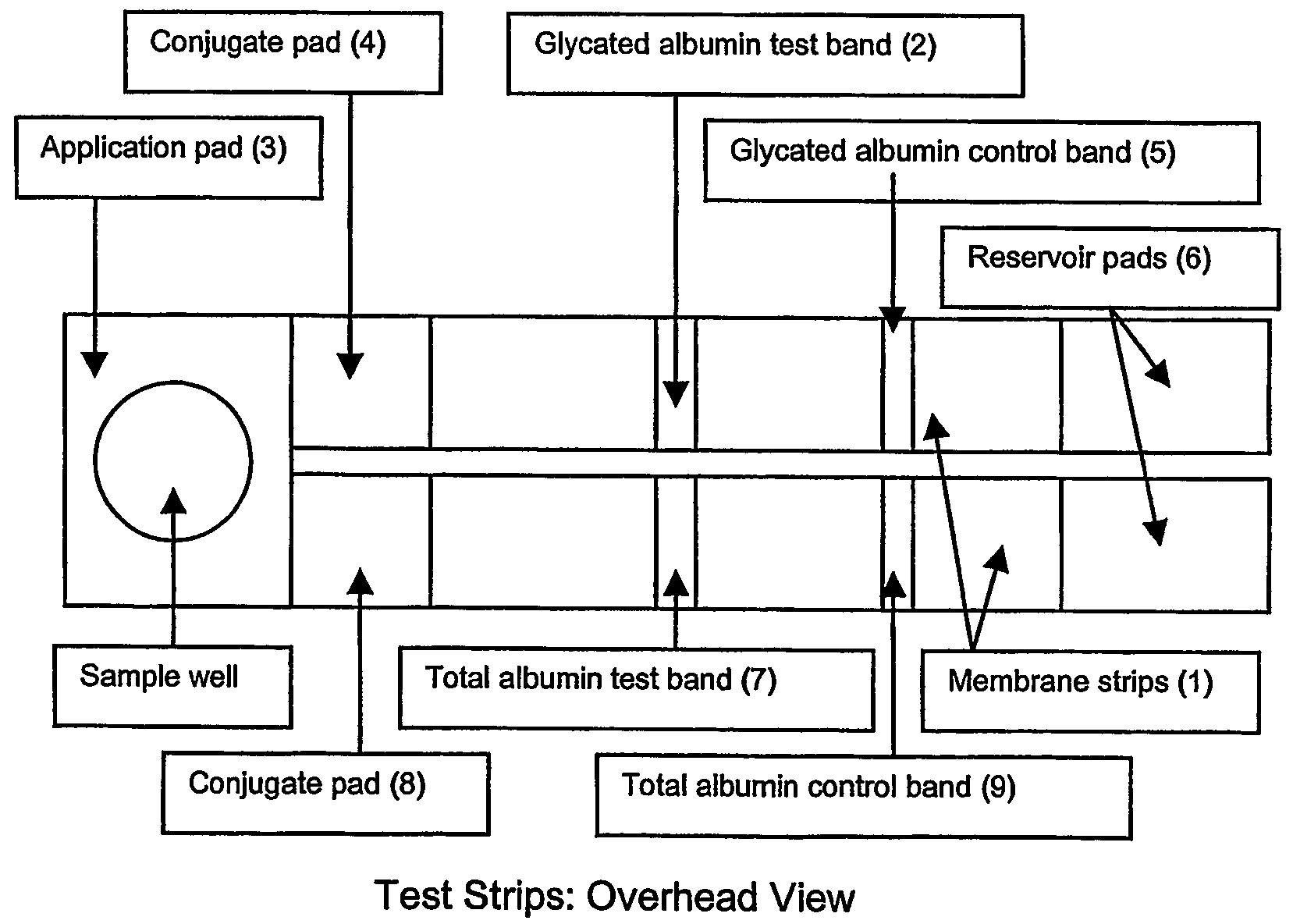
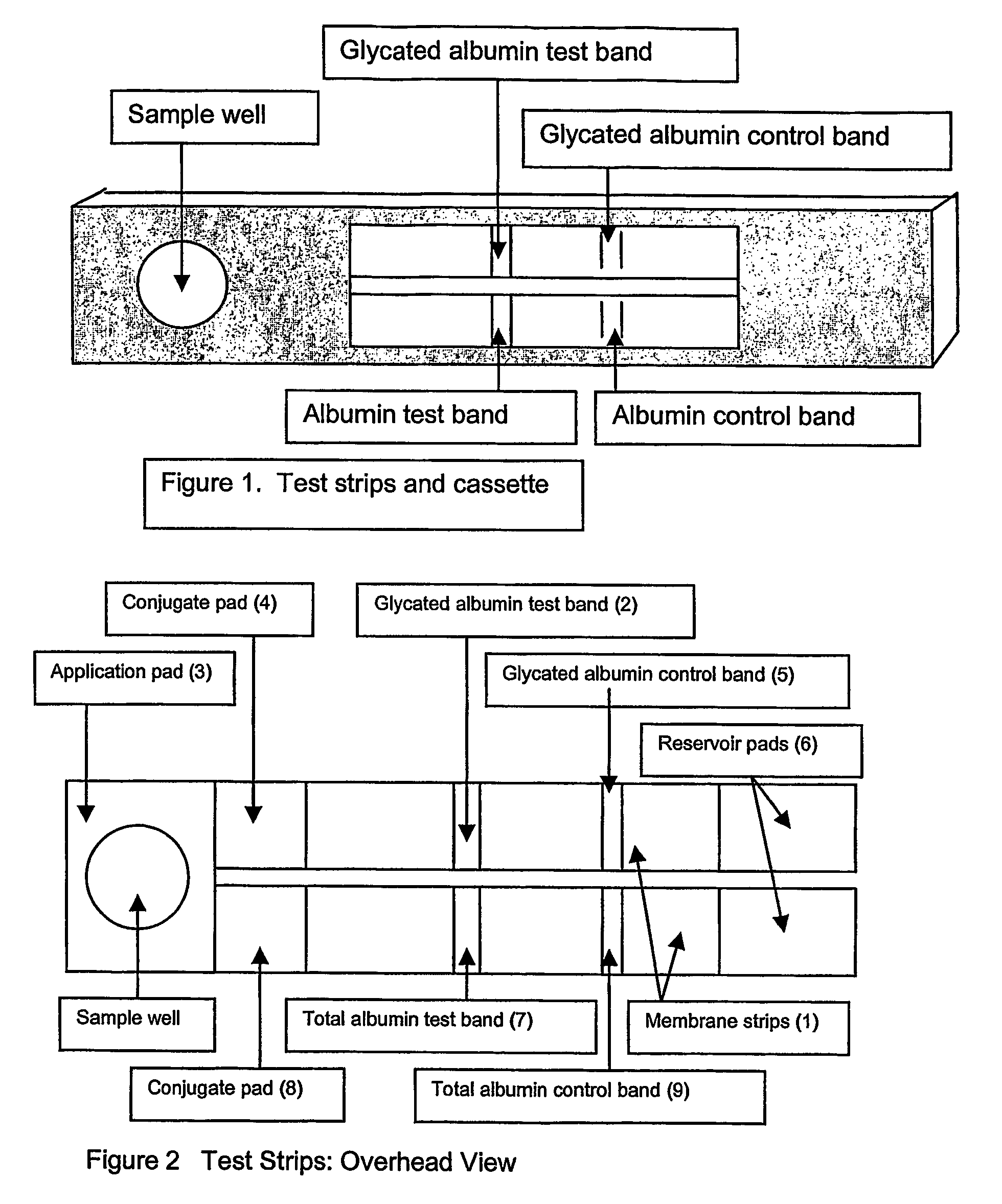
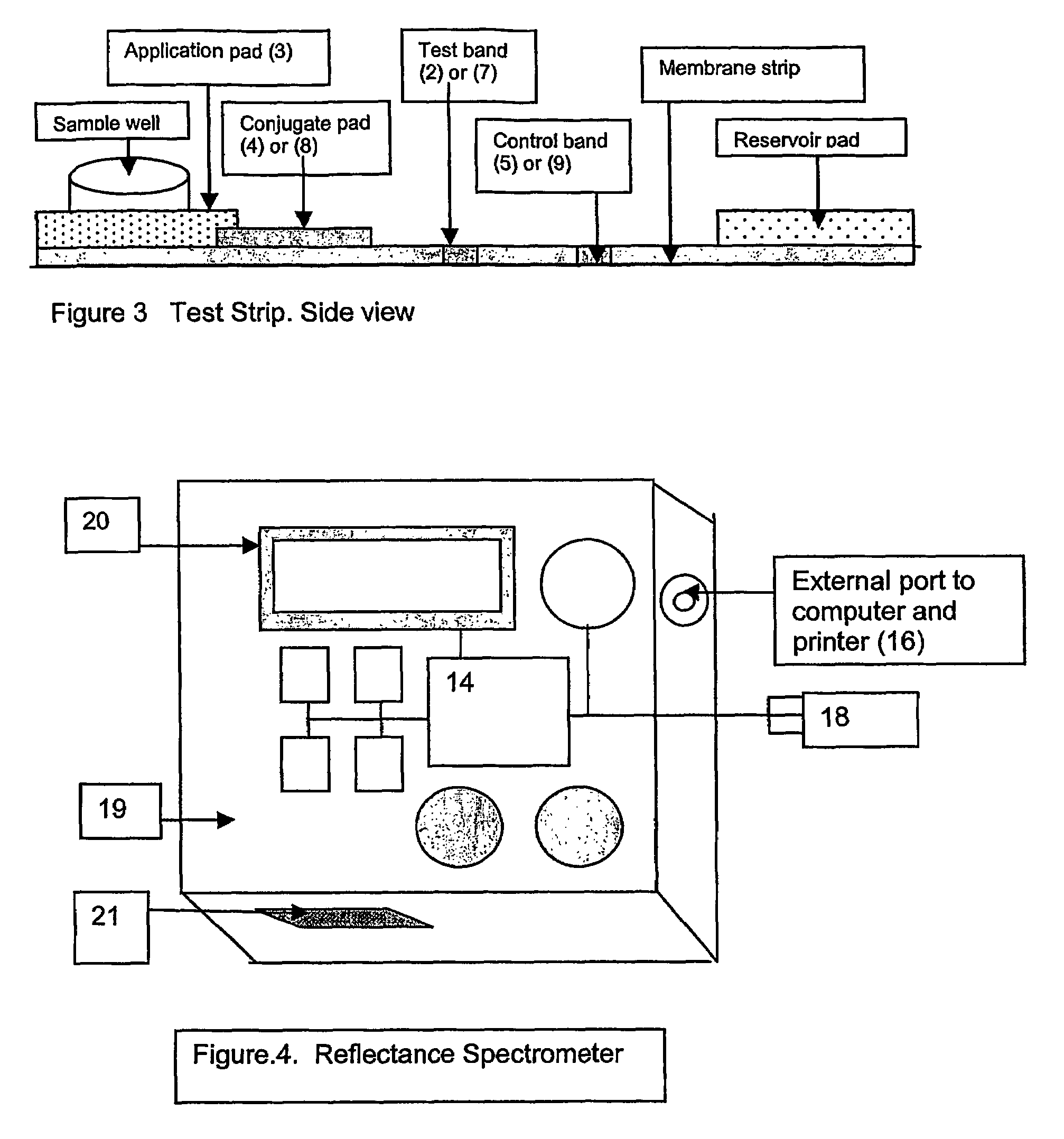
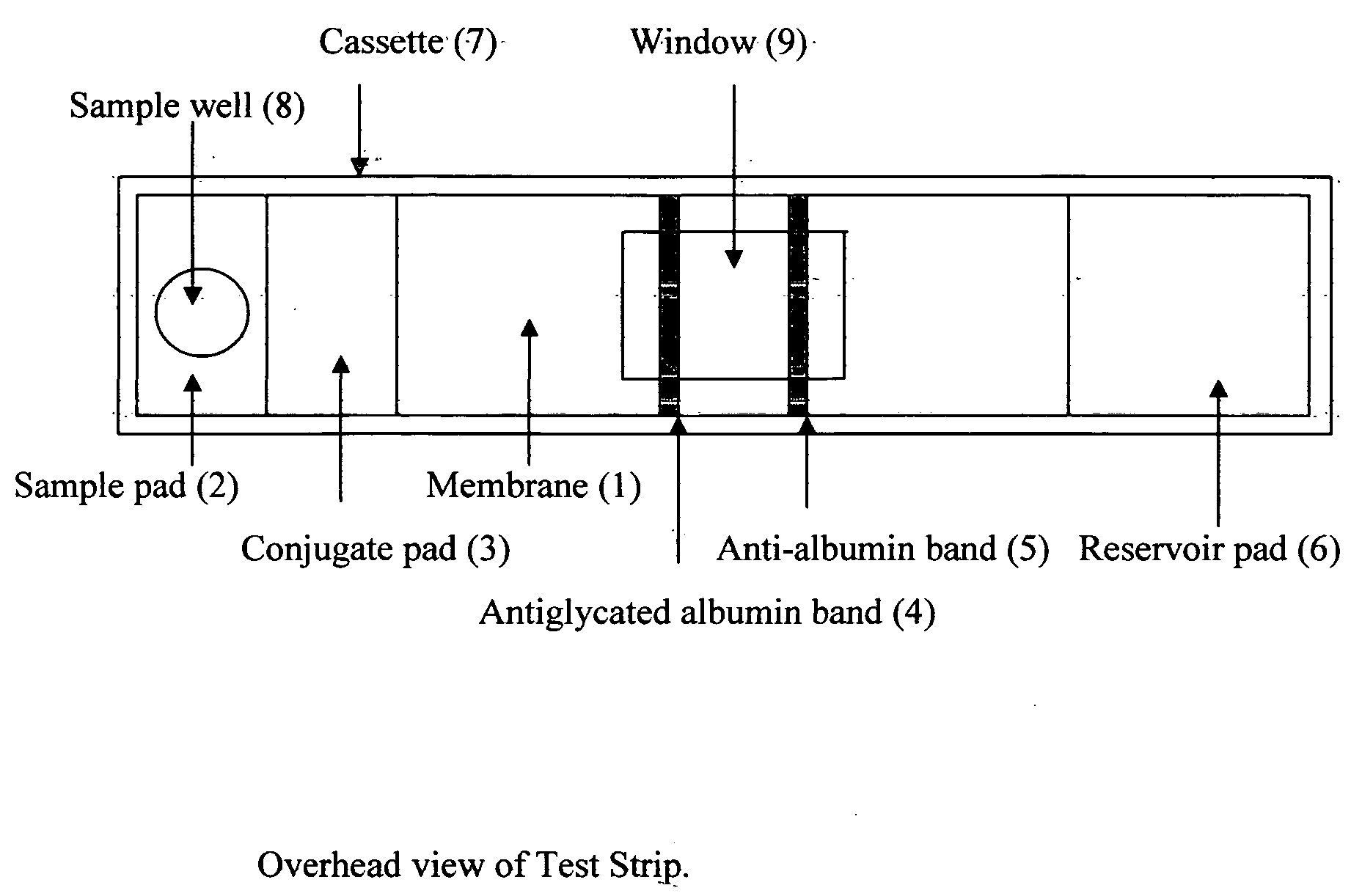
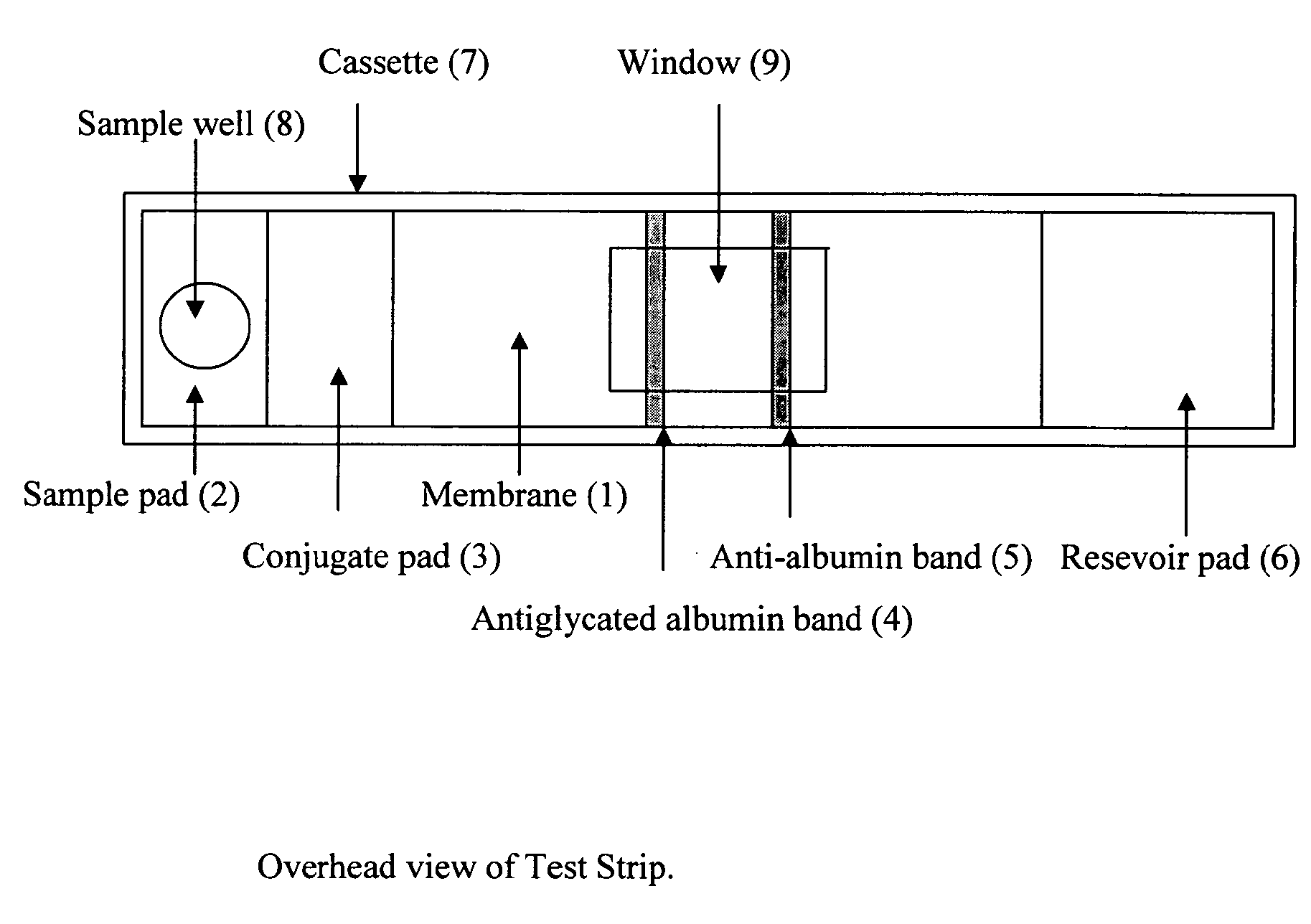
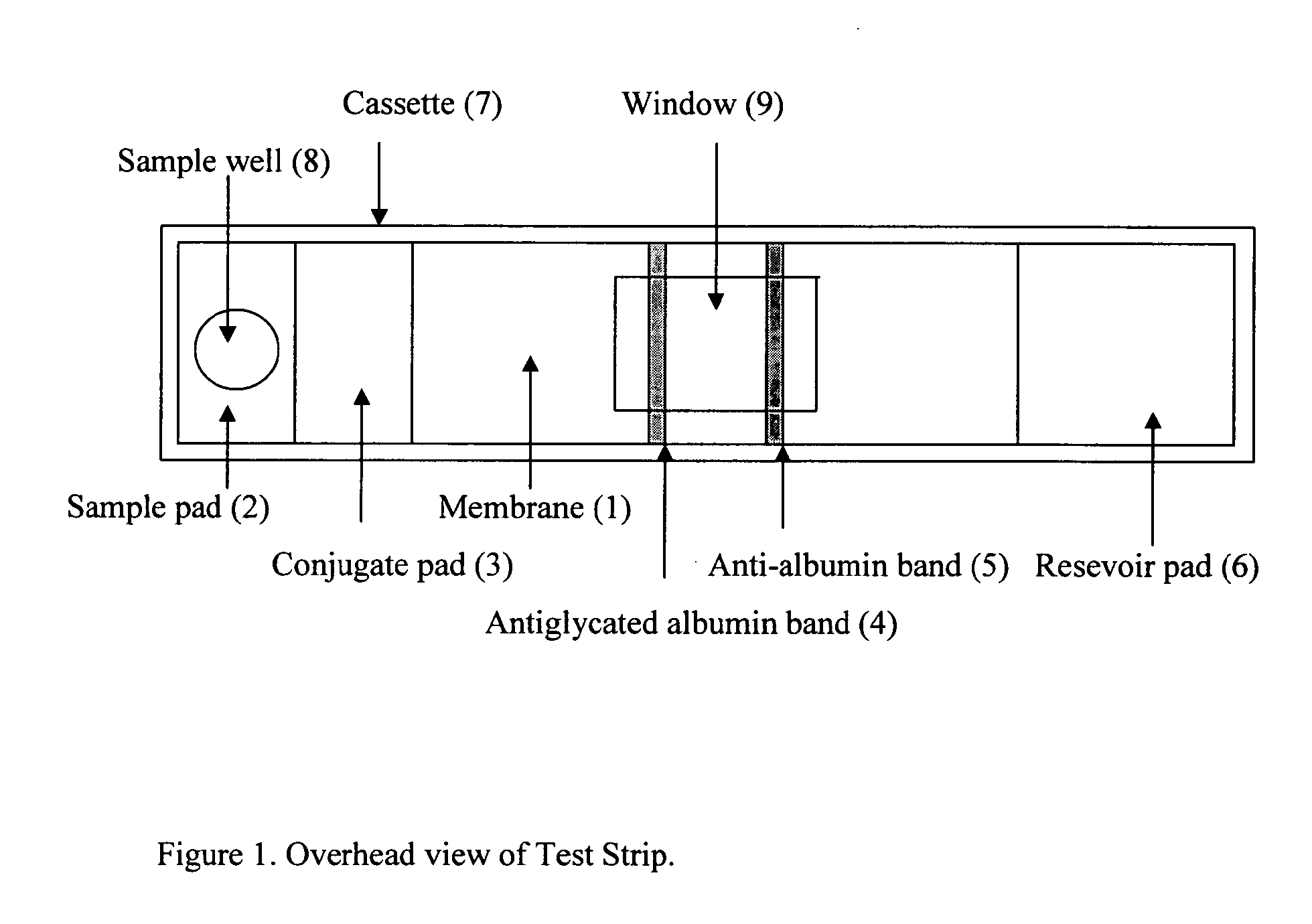
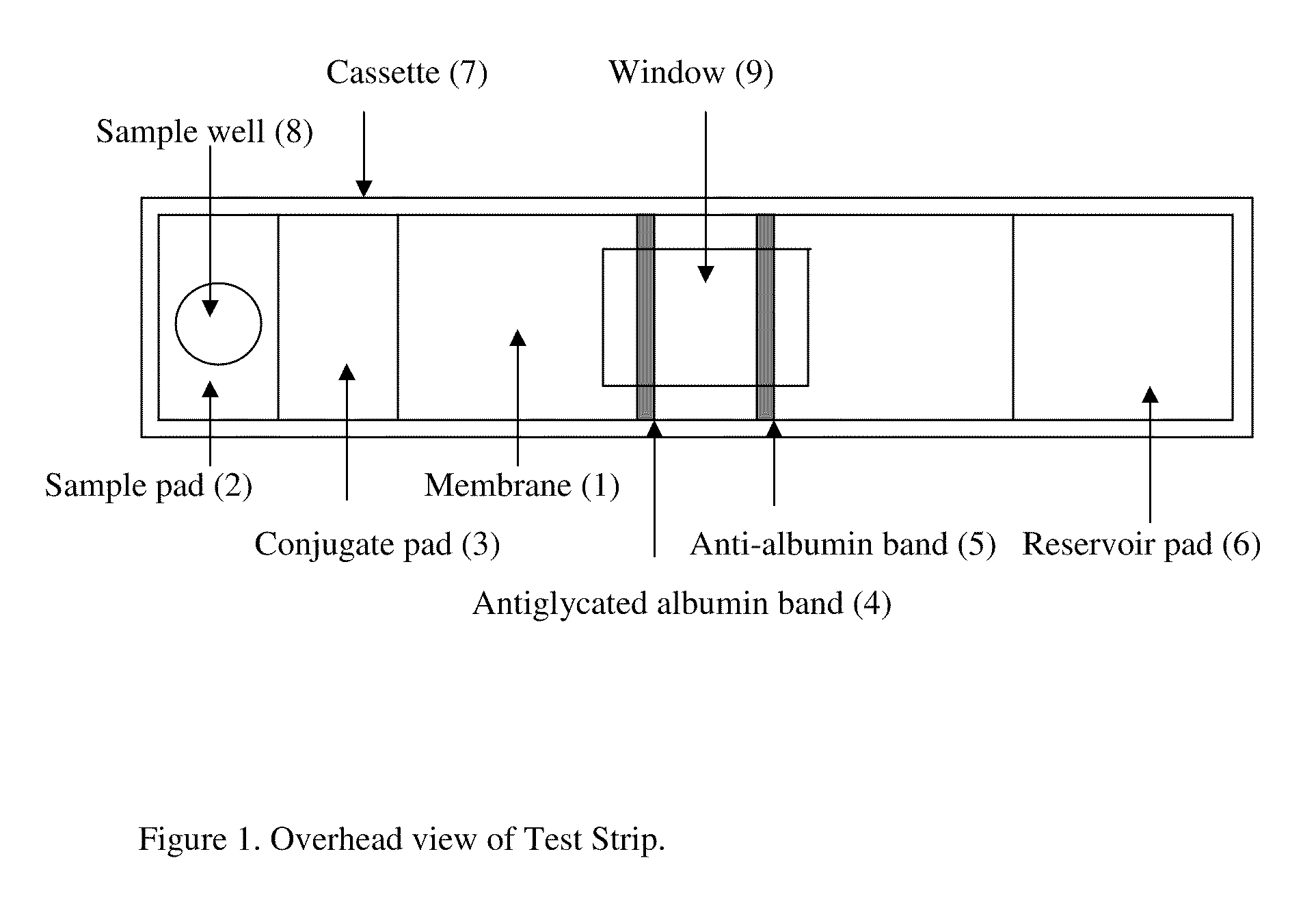
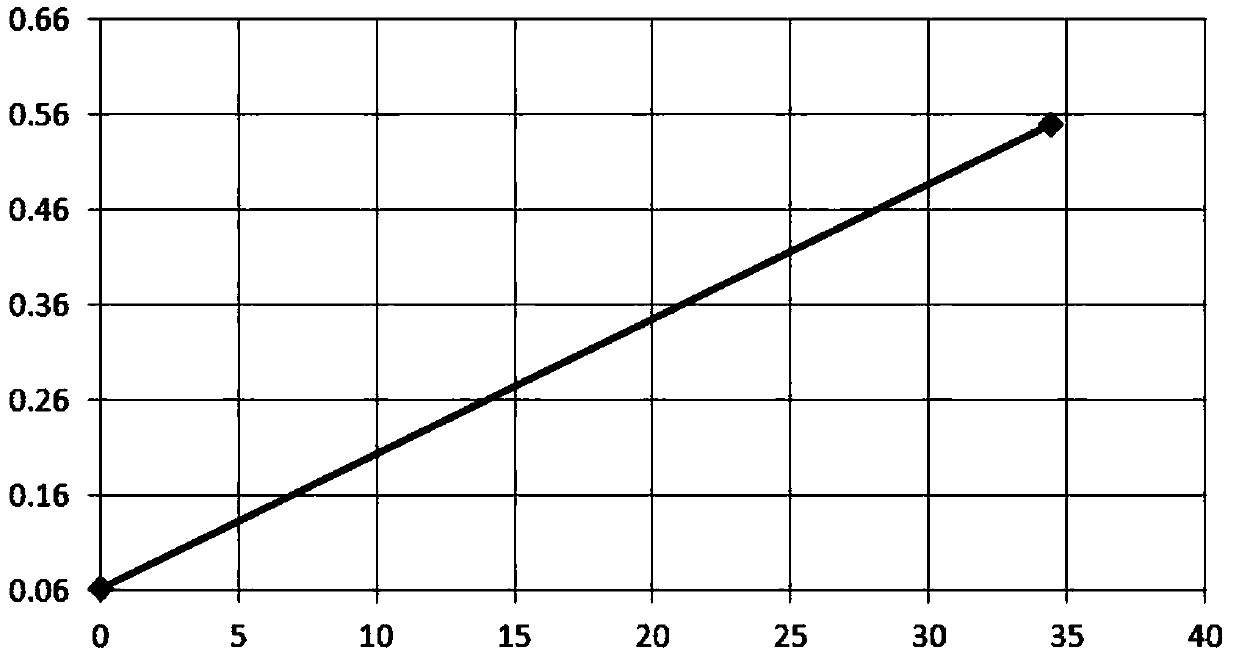
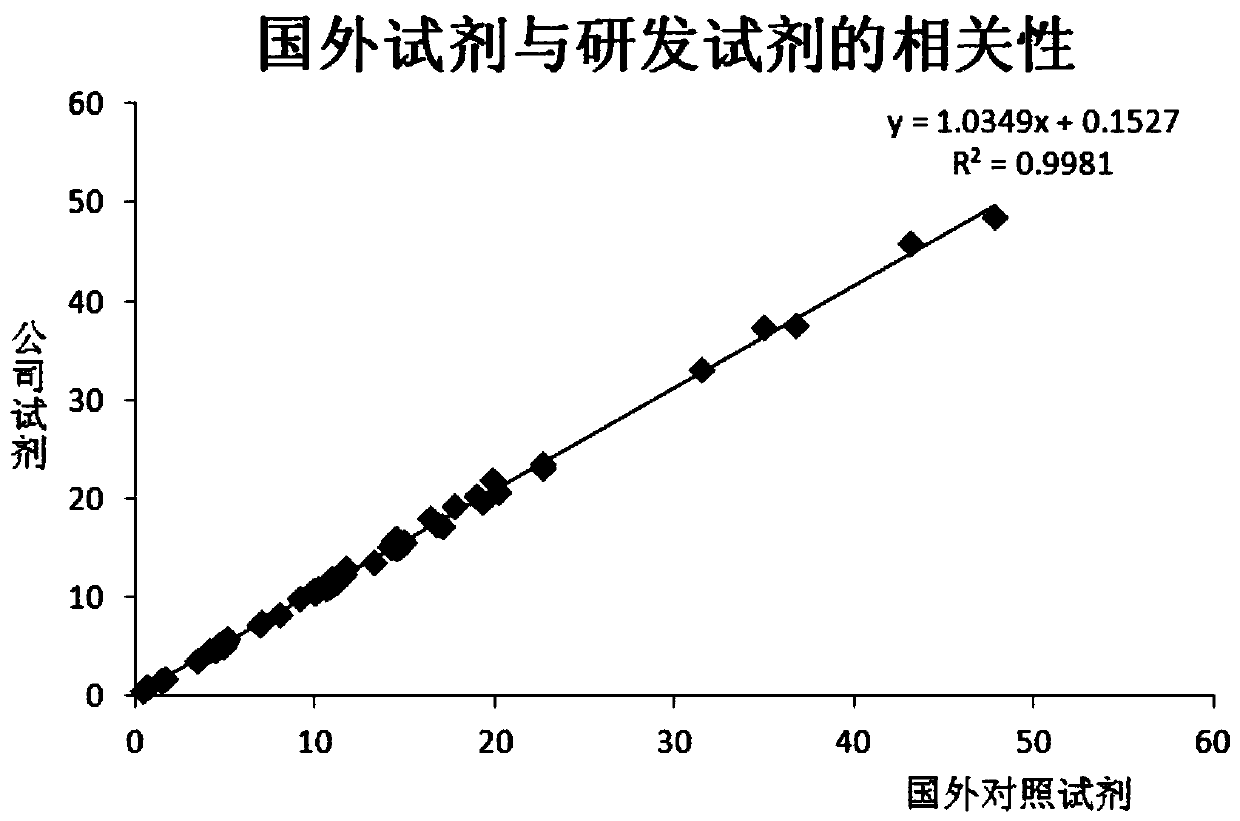
This invention describes a rapid immunochromatographic assay for measuring the ratio of glycated albumin to total albumin in saliva. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:SMITH HENRY JOHN
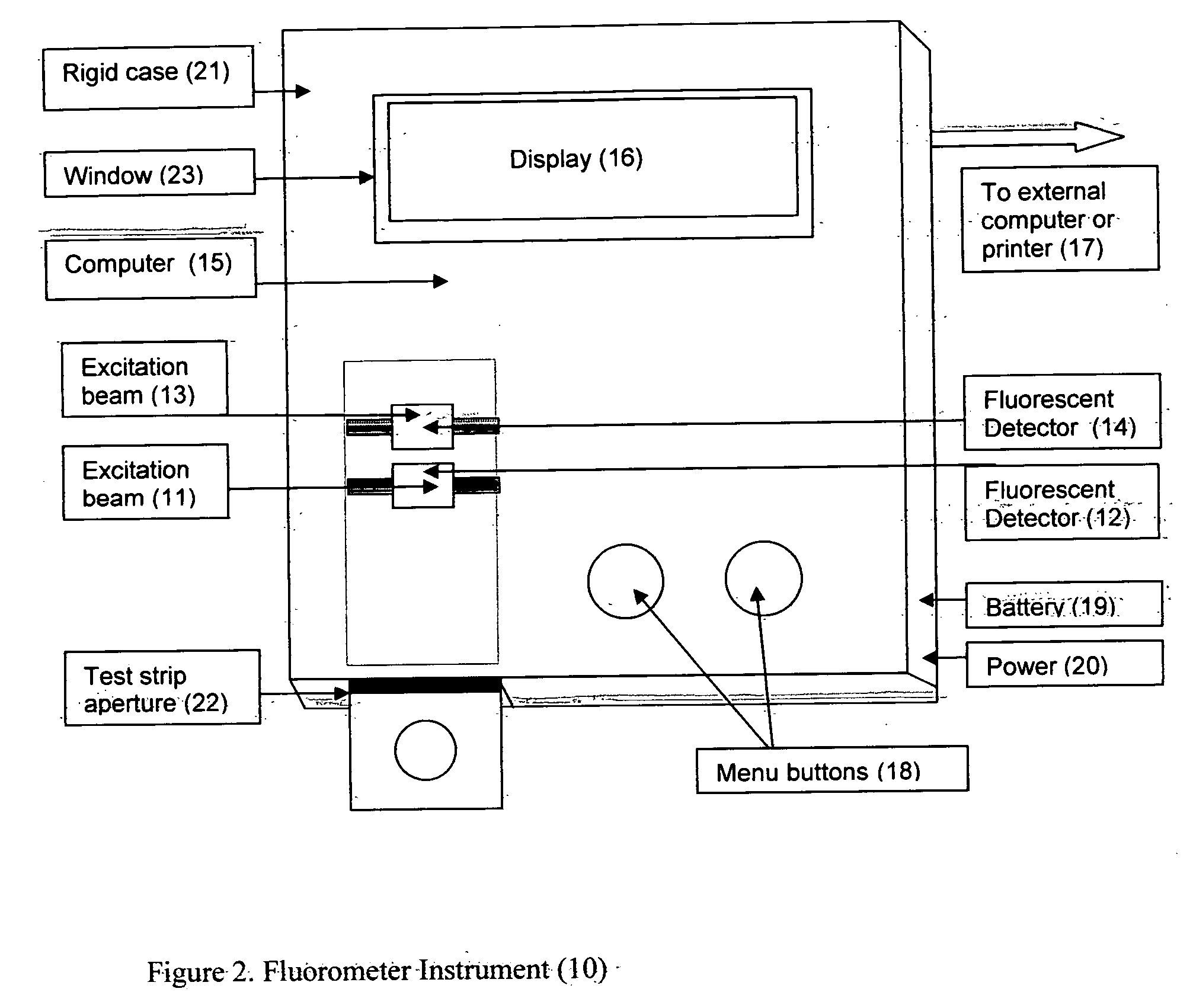
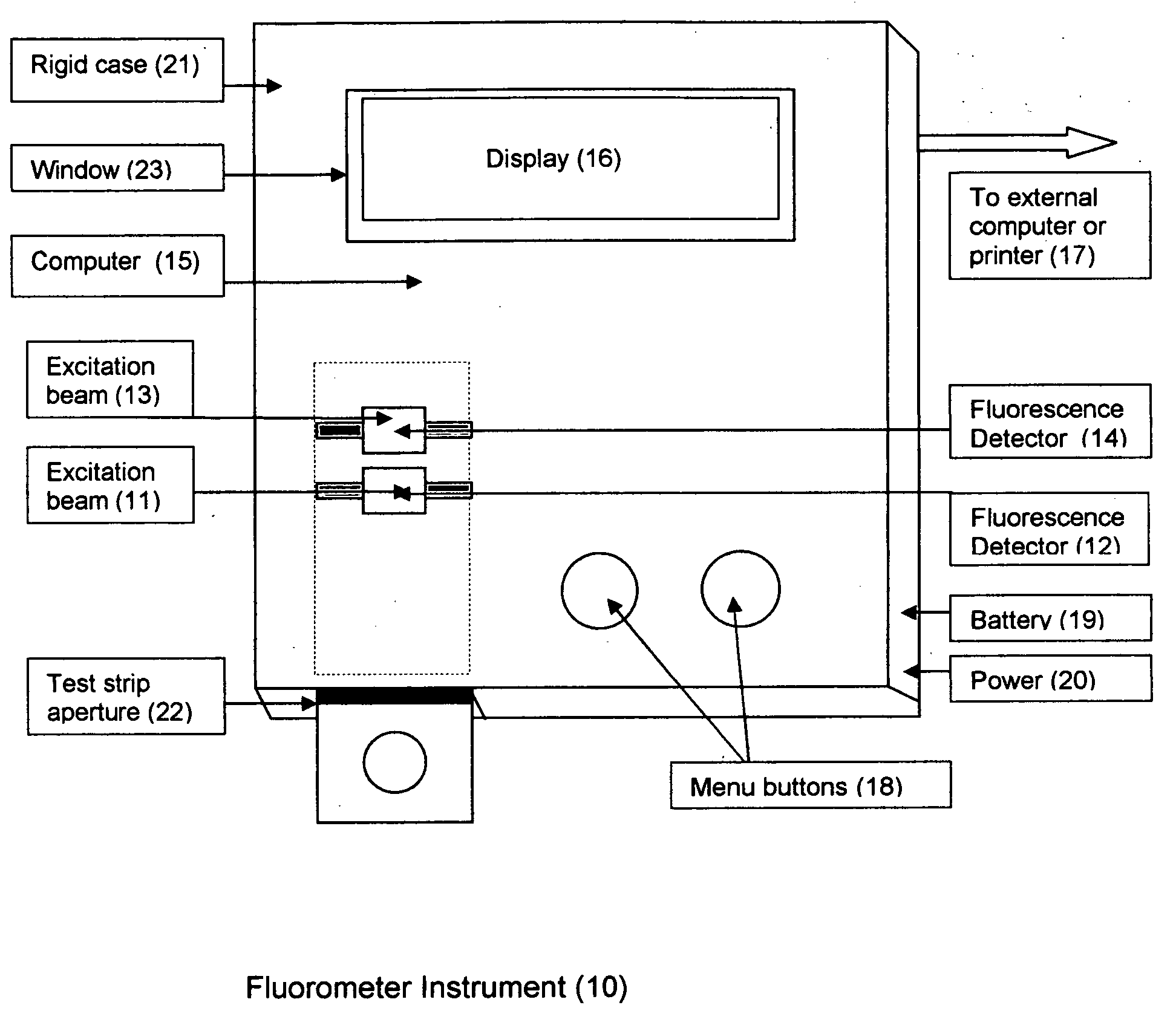
Rapid test for glycated albumin
ActiveUS7659107B2Bioreactor/fermenter combinationsBiological substance pretreatmentsALBUMIN TESTSingle sample
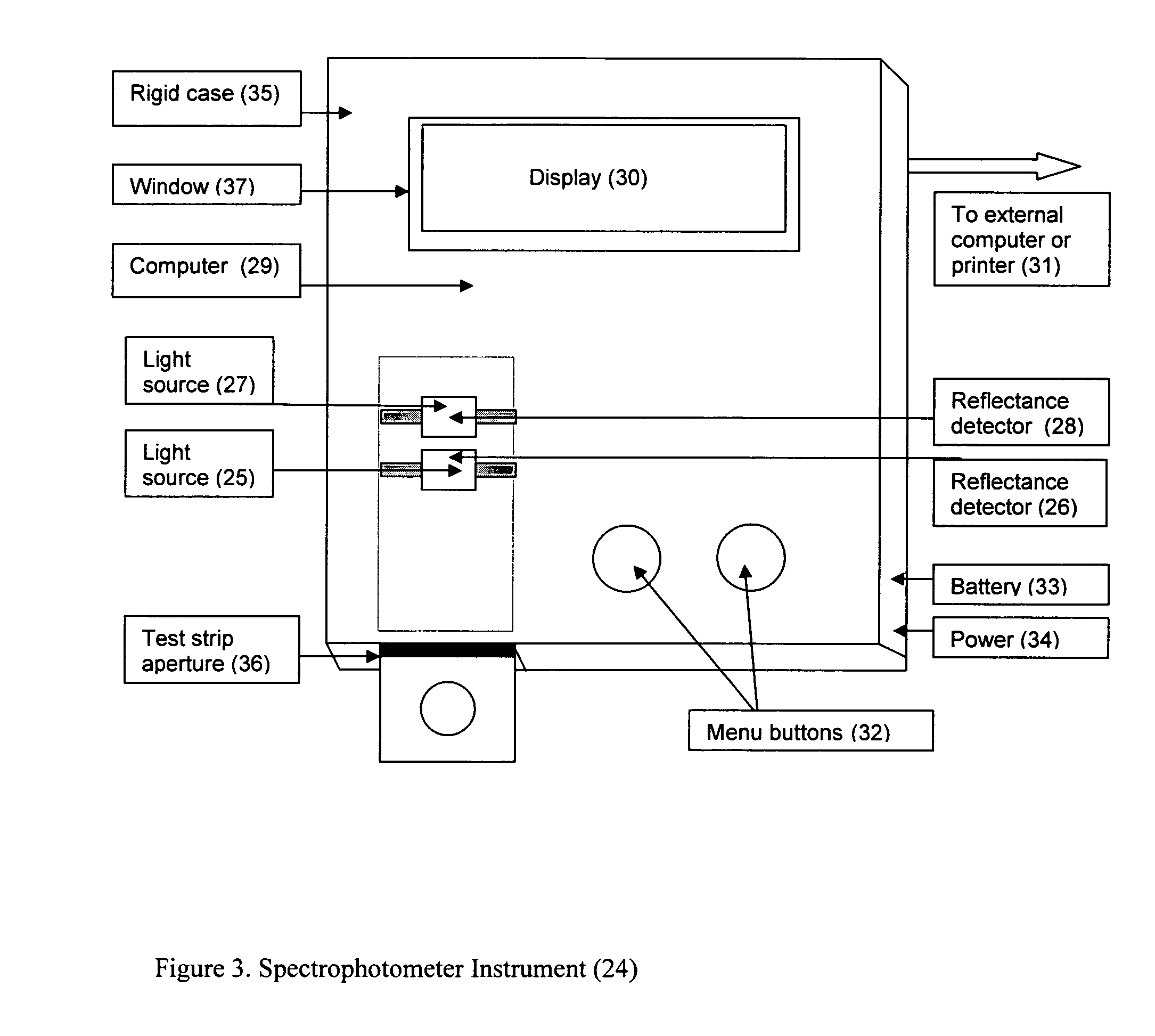
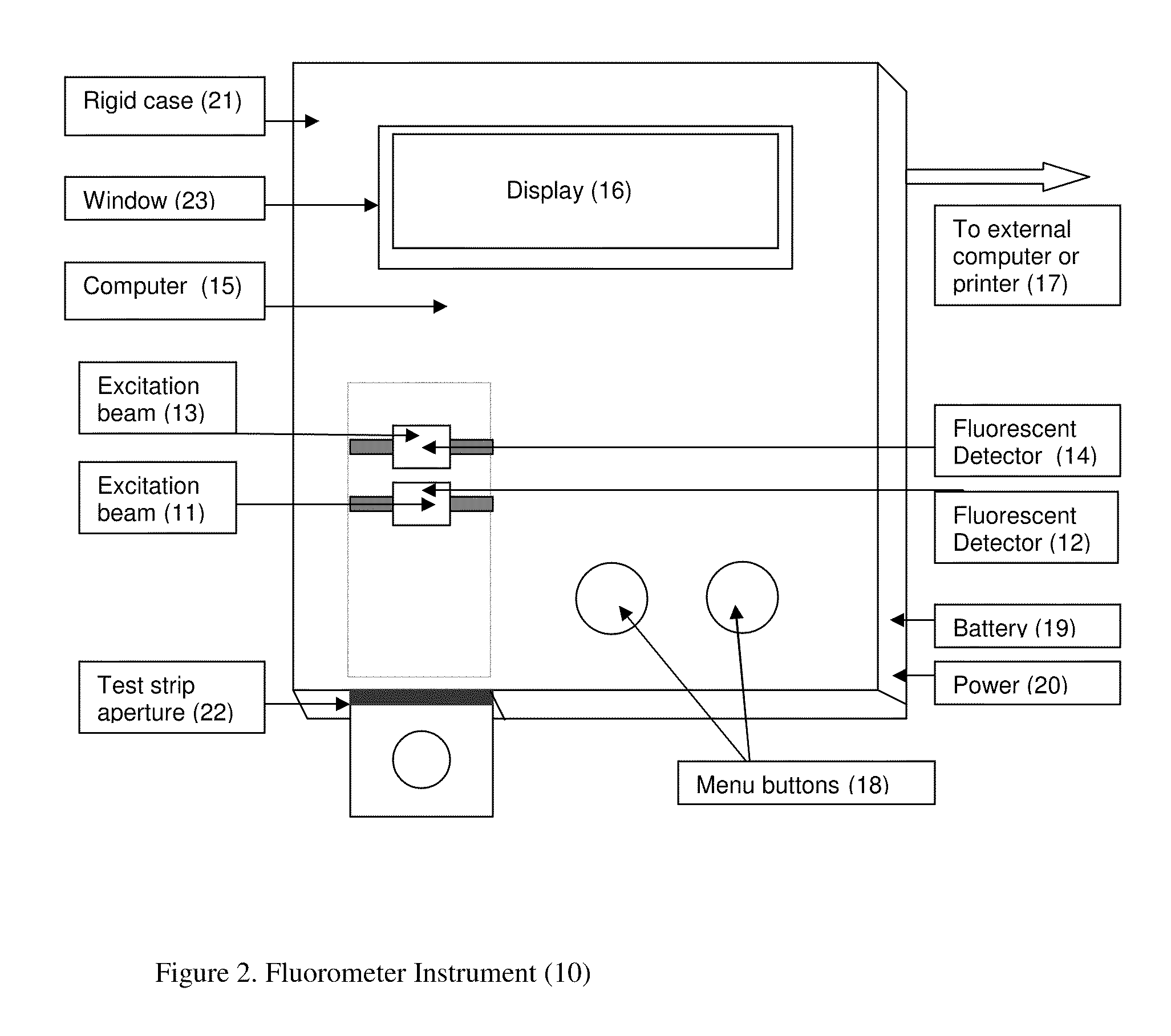
A rapid immunochromatographic assay system is provided for measuring the amount of glycated albumin in a blood sample relative to the total level of albumin in the sample. The assay system is comprised of a disposable cassette that contains the test strips and testing reagents, and a measurement device that automatically reads, calculates and displays the test results over a period of time. The test cassette contains two test strips that are used to measure glycated albumin and total albumin respectively. The strips are contiguous beneath the single sample application well so that the same sample is tested simultaneously by both test strips. Part of the sample will migrate thru the glycated albumin test strip where it will react with the glycated albumin test reagents to yield a glycated albumin result, while part of the sample will migrate thru the total albumin test strip where it will react with the total albumin test reagents to yield a total albumin result. The test cassette is placed within a measuring device such as a reflectance spectrometer or fluorometer, that reads, calculates and expresses the result as the percentage of glycated albumin relative to total albumin in the sample. The results of successive testing that are performed over a period of time are stored in the instrument's memory and displayed in a numerical or graphical format so that the individual's glycated albumin levels can be monitored over time.
Owner:MEDYTOX SOLUTIONS
Composition for assaying glycoprotein
InactiveUS7250269B2InhibitionMicrobiological testing/measurementDepsipeptidesProteinase activityGlycoprotein i
Compositions for accurately assaying a glycated protein by: 1) avoiding effects of globulin and ascorbic acid components, 2) siabilizing proteases and at least enzymes acting on glycated amino acids; 3) accurately assaying albumin; and 4) assaying glycated albumin while avoiding the effects of glycated hemoglobin, and an assay method are provided. Thus, the contents of a glycated protein and glycated albumin can be more accurately determined.
Owner:ASAHI KASEI PHARMA
Rapid test for glycated albumin in blood
InactiveUS20070015291A1Accurate assessmentBiological material analysisMeasuring instrumentBlood plasma
This invention describes a rapid assay for measuring the ratio of glycated albumin to total albumin in blood. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. The ratio of glycated albumin to total albumin in blood will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads; calculates and displays the final result: The results of tests performed over a period of time are stored in the instrument's memory presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:EPINEX DIAGNOSTICS
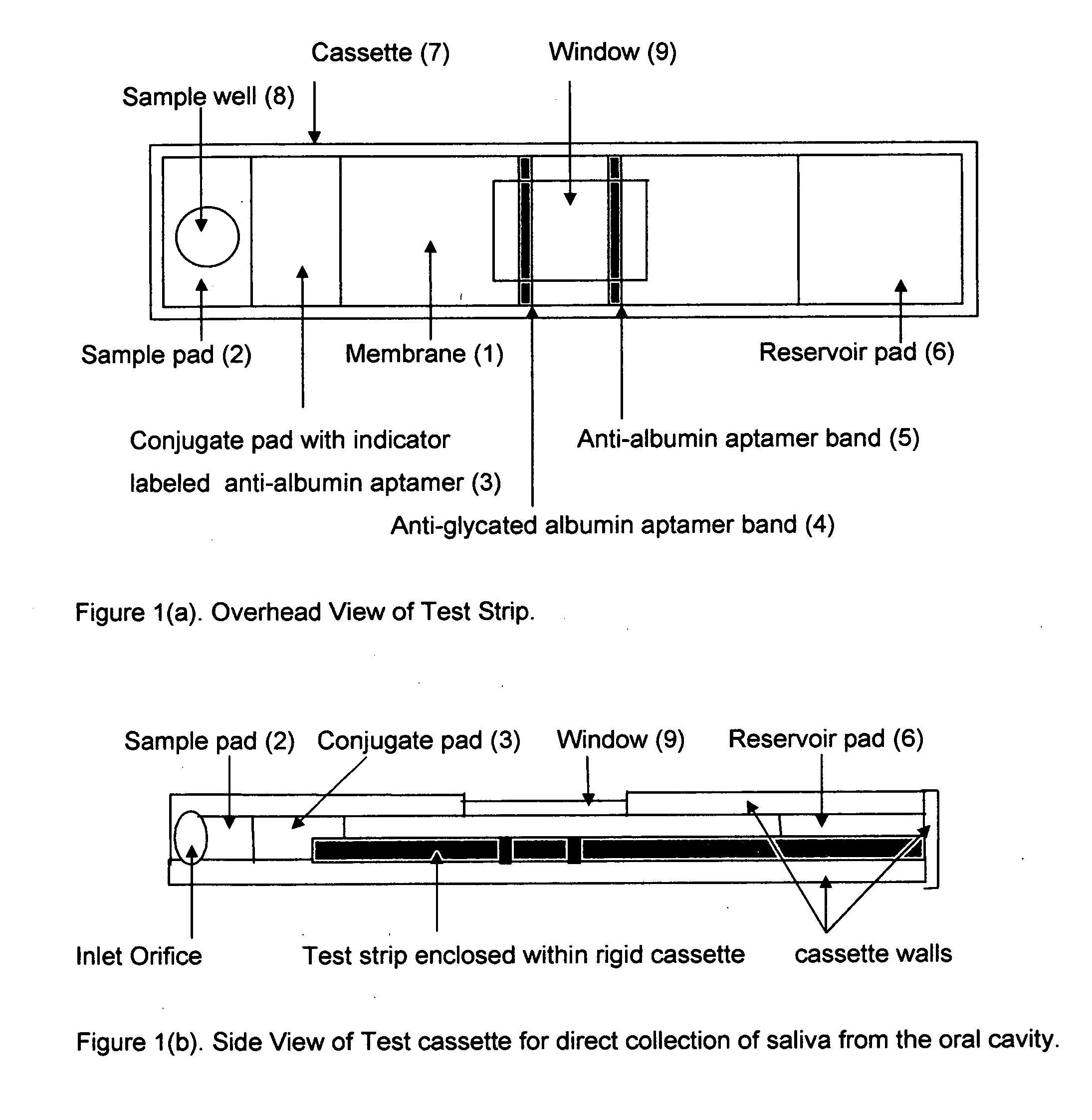
Aptamer based point-of-care test for glycated albumin
InactiveUS20090042237A1Accurate assessmentBioreactor/fermenter combinationsRadiation pyrometryPoint of careMeasuring instrument
This invention describes a point-of-care or home use device for measuring the ratio of glycated albumin to total albumin in saliva and other body fluids. Diabetics and prediabetics may have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:EPINEX DIAGNOSTICS
Rapid test for glycated albumin in saliva
InactiveUS20100167306A1Accurate assessmentMicrobiological testing/measurementBiological material analysisMeasuring instrumentPlasma Albumin
This invention describes a rapid immunochromatographic assay for measuring the ratio of glycated albumin to total albumin in saliva. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. Saliva albumin is derived from plasma albumin and therefore contains glycated and non-glycated albumin fractions that can be measured. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:SMITH HENRY JOHN
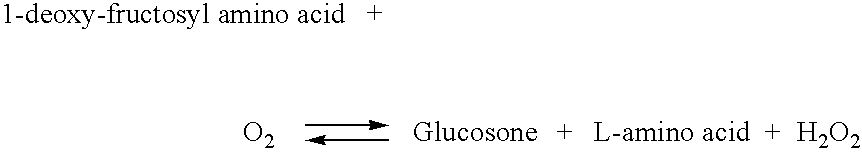
Fructose amino acid oxidase, preparation method and glycatedalbumin detection kit comprising oxidase
ActiveCN103695380AReduce performanceGood dilution linearityMicrobiological testing/measurementBiological material analysisD-amino acid oxidaseGlutamic acid
The invention discloses fructose amino acid oxidase which has an amino acid sequence shown as SEQID.No.1 (sequence identifier number 1) or has above 80% homology with the amino acid sequence. One or more amino acid residues in corresponding positions of amino acid selected from (a) to (f) are substituted. The obtained fructose amino acid oxidase has higher thermostability: (a) 59-site glutamic acid, (b) 98-site glutamic acid, (c) 225-site glycine, (d) 277-site lysine, (e) 283-site glutamic acid and (f) 355-site aspartic acid. The invention further discloses a preparation method of the oxidase and a kit comprising the oxidase and used for determining glycatedalbumin. The kit has higher thermostability and can accurately determine the glycatedalbumin.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Human serum glycated albumin array kit
ActiveCN102565420AUnchanged vitalityStrong specificityBiological testingMethylanilineFructose lysine
The invention provides a human serum glycated albumin assay kit which comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises the following components: 20-200mmol / L of buffer solution, 5-50KU / L of protease co-expression vector, 10-50KU / L of peroxisome, 2-20mmol / L of 4-amino antipyrine, 0.01-1% of preservative and 0.01-1% of stabilizer, and the protease co-expression vector is obtained by cloning a protease gene and an ascorbic acid oxidase gene onto a same vector for performing co-expression; and the reagent 2 comprises the following components: 20-200mmol / L of buffer solution, 5-50U / mL of fructose lysine enzyme, 1-10mmol / L of N, N-bis(4-sulfobutyl ether)-3-methylaniline, 0.01-1% of preservative and 0.01-1% of stabilizer. The kit can remove the interferences of globulin and ascorbic acid in human serum and has good stability and low cost.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Extended risk assessment panel for individualized treatment of cardiovascular disease, and methods related thereto
ActiveUS20140088072A1More treatmentDetailed informationBiocideOrganic active ingredientsSterolIndividualized treatment
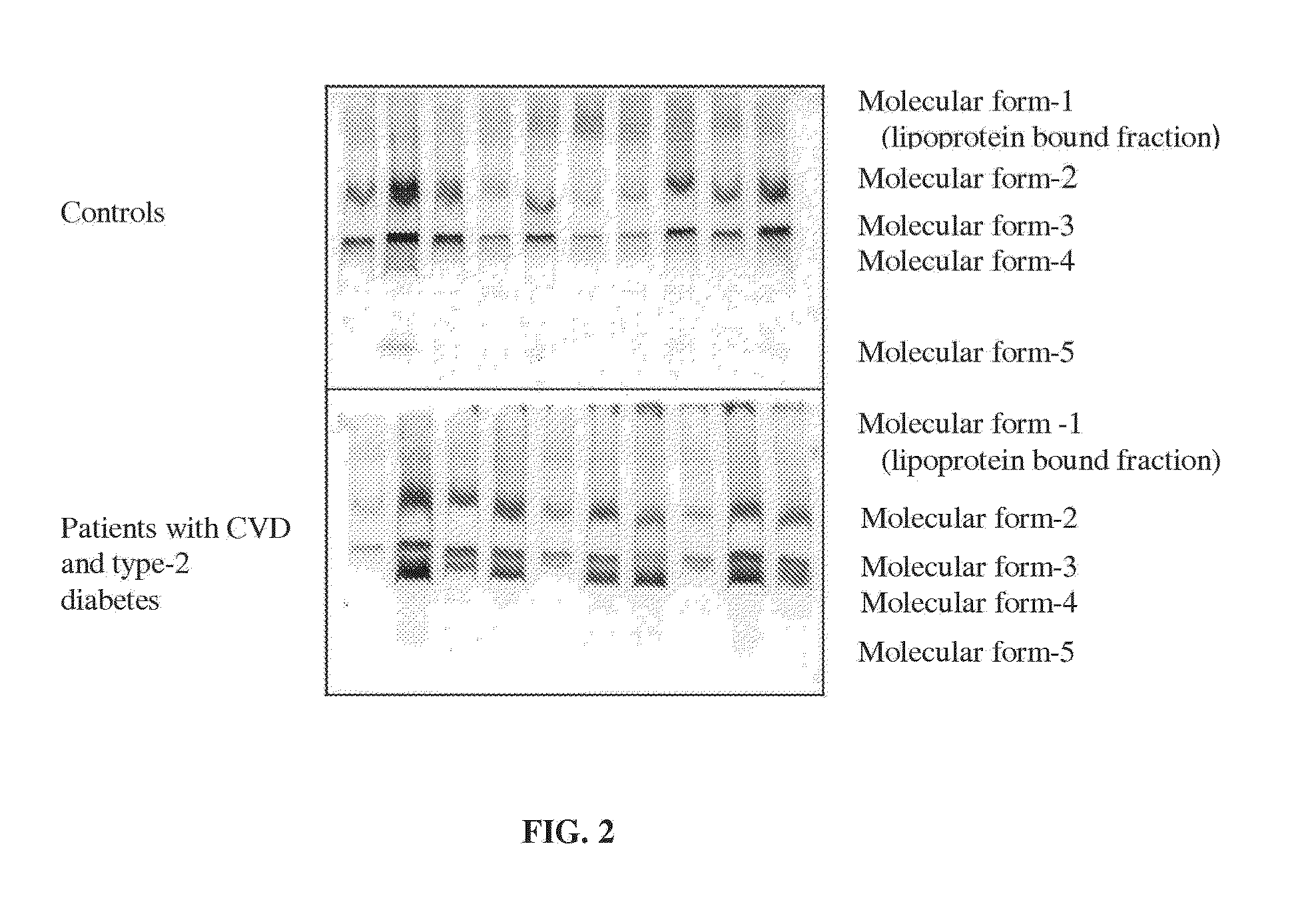
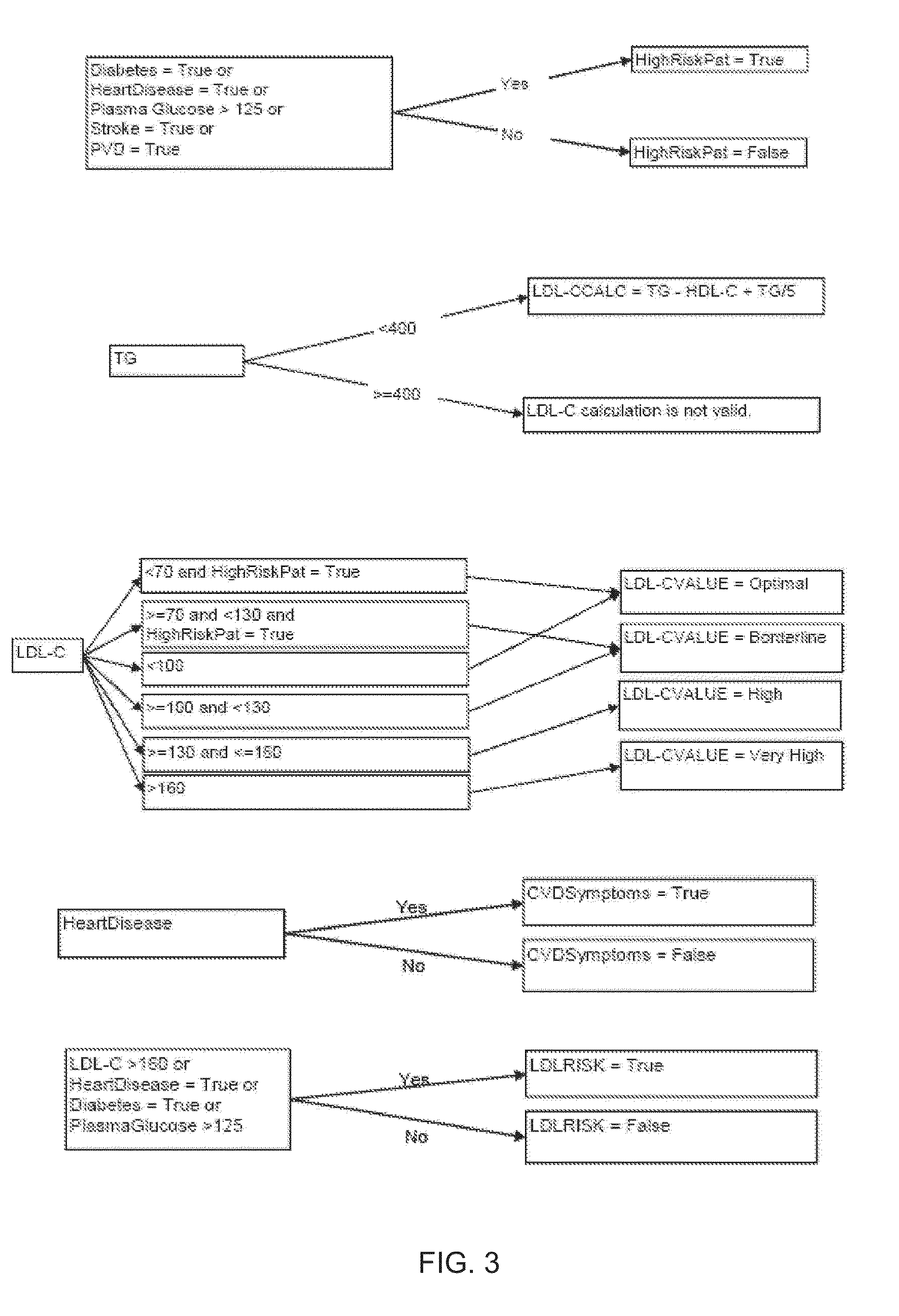
Disclosed is a personalized diagnostic and treatment solution for cardiovascular disease. The invention comprises an extended CVD risk assessment panel, combining tests for traditional and new important risk markers, and methods for devising a personalized treatment plan for a patient via the use of a CVD diagnosis and treatment protocol algorithm. The new important risk markers include HDL subpopulation profile by two-dimensional gel-electrophoresis, plasma sterols, direct measurement of sdLDL-C, determination of CRP molecular forms, glycated albumin as a percentile of total albumin, and other specialized testing pertaining to apolipoprotein E and Factor V Leiden genotyping, NT-proBNP and adiponectin. This solution provides a more complete risk assessment of an individual than merely measuring traditional CVD risk markers, and enables the healthcare practitioner to optimize therapy for patients with or without established CVD. This solution presents the advantages of greater accuracy, savings in time and cost over existing testing and treatment methods.
Owner:BOSTON HEART DIAGNOSTICS
Preparation method of stable glycated albumin calibrating material and quality control material
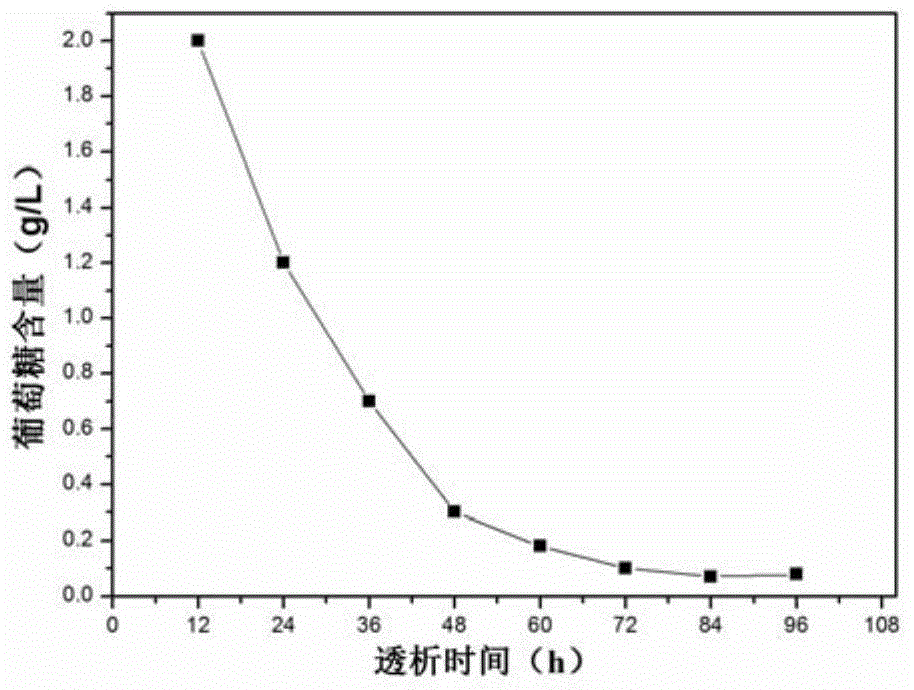
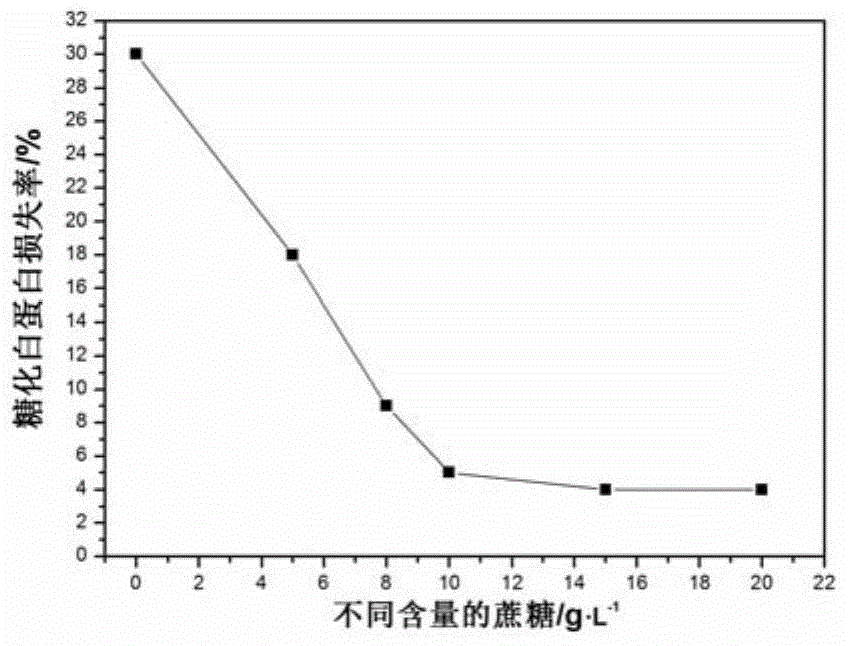
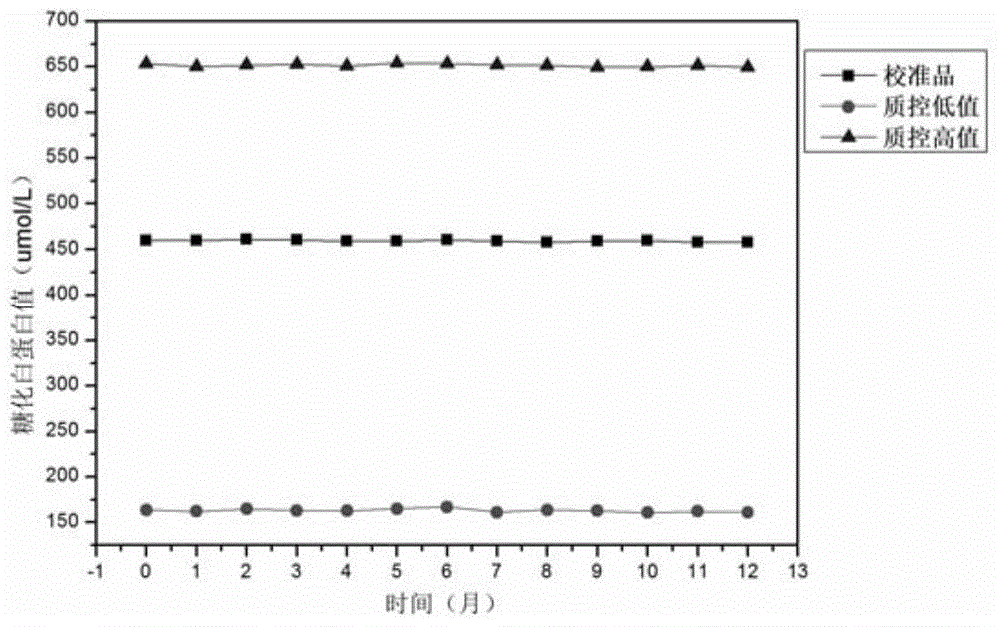
PendingCN105092336AAvoid the defect of fluctuating concentrationAvoid being HIVPeptide/protein ingredientsMetabolism disorderWater bathsFreeze-drying
The invention relates to a preparation method of a stable glycated albumin calibrating material and a quality control material. The preparation method comprises the following steps of selecting serum, and removing settling and disturbance matters; adding glucose and preservative into the treated serum, arranging in a constant-temperature water bath with temperature of 35-38 DEG C, saccharifying, and respectively obtaining the glycated albumin calibrating material and the quality control material according to the saccharifying time; respectively packaging the prepared glycated albumin calibrating material and the quality control material, arranging in dialysates with temperature of 2-6 DEG C, dialyzing, and removing excessive glucose, until the content of the remained glucose is less than 0.1g / L; finally, adding the preservative and 10-50g / L of freeze-drying protecting agent into the dialyzed glycated albumin calibrating material and quality control material, freezing and drying to obtain a freeze-drying powder, dissolving again, and detecting the fixed value by a glycated albumin detection kit. The preparation method has the advantages that the sources of raw materials are sufficient, easy to obtain, the preparation technology is simple, and the stable glycated albumin calibrating material and the quality control material can be prepared by the serum of a healthy person.
Owner:NINGBO RUI BIO TECH
Rapid test for glycated albumin in blood
InactiveUS20090246801A1Accurate assessmentBiological material analysisBiological testingMeasuring instrumentBlood plasma
This invention describes a rapid assay for measuring the ratio of glycated albumin to total albumin in blood. Patients with diabetes have elevated levels of glucose in their blood that can react with plasma albumin to form glycated albumin. The amount of glycated albumin formed is directly correlated with the level of plasma glucose that the albumin has been exposed to over a period of time. The ratio of glycated albumin to total albumin in blood will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period.The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:MEDYTOX SOLUTIONS
Method for assaying glycated albumin
ActiveUS7871789B2Efficient digestionAssay the glycated albumin more efficientlyHydrolasesMicrobiological testing/measurementCarbon numberAlbuminous degeneration
An albumin denaturing agent for digesting an albumin by a protease efficiently is provided. The albumin denaturing agent contains quaternary ammonium having a hydrocarbon group with a carbon number of 12 or more, or a salt of the quaternary ammonium. The albumin in a sample is digested by the protease in the presence of the albumin denaturing agent, a glycated part of the thus obtained albumin digestion product and a FAOD effect a reaction, and a redox reaction between the glycated part and the FAOD is measured, thereby determining a ratio (GA (%)) of the glycated albumin of the glycated albumin with respect to the albumin.
Owner:ARKRAY INC
Stable kit for detecting glycation albumin
ActiveCN104198472AThe detection method is simpleFast detection methodMaterial analysis by observing effect on chemical indicatorPeroxidaseSurface-active agents
The invention provides a stable kit for detecting glycation albumin. The stable kit consists of a reagent R1, a reagent R2 and a reagent R3, wherein the reagent R1 comprises the following components: a buffering solution, glycation amino acid oxidase, albumin protease, peroxidase, catalase, 4-aminoantipyrene, a stabilizing agent and a preservative; the reagent R2 comprises the following components: a buffering solution, albumin protease, peroxidase, chromogen, a stabilizing agent and a preservative; the reagent R3 comprises the following components: a buffering solution, bromocresol green, a surface active agent and a preservative. The stable kit provided by the invention has the advantages that the influence on the detection result caused by endogenous glycation amino acid is removed by adding glycation amino acid oxidase, so that the result is accurate; the stable kit adopts an enzyme method to detect the content of the glycation albumin, and is a simple, fast, sensitive and accurate detection method.
Owner:上海睿康生物科技有限公司
Aptamer Based Point-of-Care Test for Glycated Albumin
InactiveUS20140170766A1Accurate assessmentColor/spectral properties measurementsBiological testingPoint of careMeasuring instrument
Disclosed herein is a point-of-care or home use device for measuring the ratio of glycated albumin to total albumin in saliva and other body fluids. The ratio of glycated albumin to total albumin in saliva will provide an indication of the average amount of protein glycation that occurred over the preceding 2-3 week period. The test is performed using a disposable strip or cassette that contains the testing reagents and the results are measured in a measuring instrument that automatically reads, calculates and displays the final result. The results of tests performed over a period of time are stored in the instrument's memory and presented in a numerical or graphical format so that the individual's glycated albumin level can be monitored over time.
Owner:MEDYTOX SOLUTIONS
Glycated albumin enzymatic detection kit and detection method thereof
The invention relates to a glycated albumin (GA) enzymatic detection kit, which comprises a GA reagent 1 and a GA reagent 2. The GA reagent 1 comprises tris(hydroxymethyl)aminomethane, aminoantipyrine (4-AAP), protease K, calcium acetate (CaAc2), potassium ferrocyanide trihydrate (K4Fe(CN)6.3H2O), copper acetate (CuAc2) and Cholic acid. The GA reagent 2 includes tris(hydroxymethyl)aminomethane, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline sodium salt (TOOS), fructosaminase, Triton X-100 and peroxidase. The invention aims to provide the glycated albumin enzymatic detection kit and its detection method with the characteristics of strong specificity, high sensitivity, short time, simple operation mode, and accurate and reliable detection result.
Owner:BEIJING HOMA BIOLOGICAL ENG
Method for detecting serum glycated albumin and a special candidate reference substance thereof
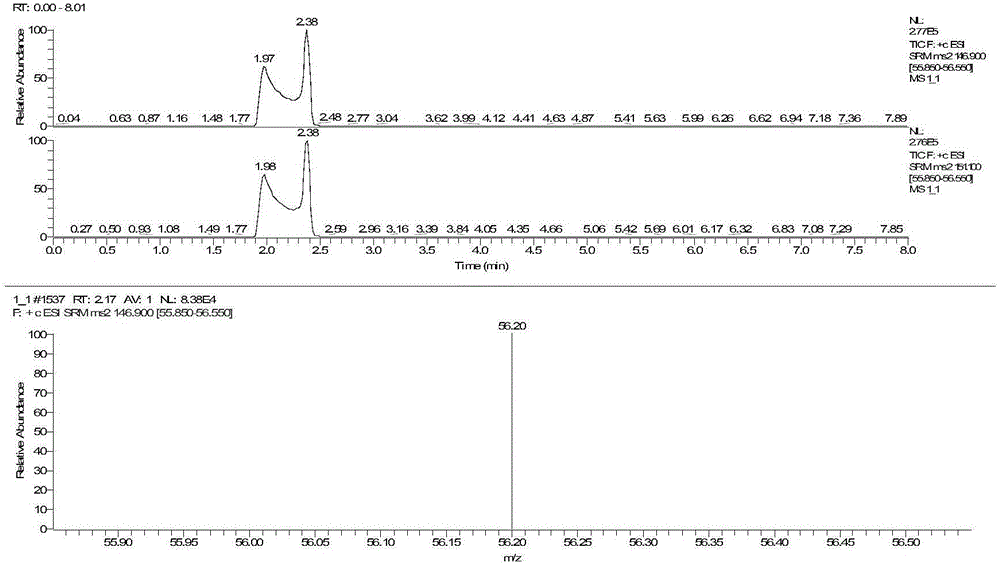
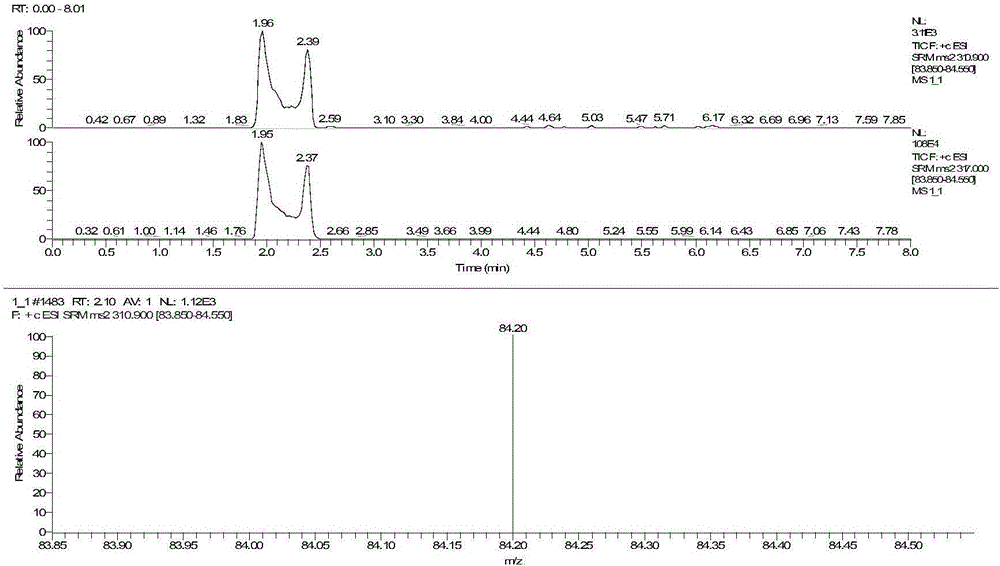
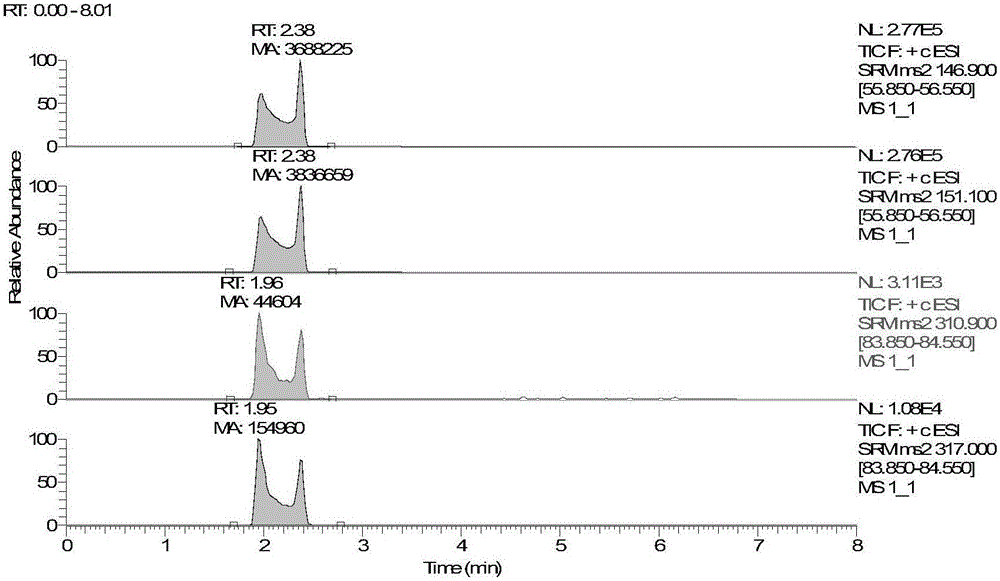
The invention discloses a method for detecting serum glycated albumin and a special candidate reference substance thereof. The present invention provides a method using an isotope dilution liquid chromatography tandem mass spectrometry method for the determination of glycated albumin content in a sample to be detected. The method comprises the following steps: 1) preparing a standard solution, and separating and purifying the albumin in a sample to be detected; 2) preparing a working standard solution and a working sample to be detected; 3) respectively detecting the working standard solution and the working sample to be detected by using a liquid chromatography tandem mass spectrometer to obtain the glycated albumin content in the sample to be detected. The experiment of the invention has proved that based on a JSCC recommendation method, a candidate reference method for accurate determination of glycated albumin is established by using the liquid chromatography tandem triple quadrupole. On this basis, a GA candidate reference substance for freezing and mixing human serum matrix is developed, and the invention is expected to be further used for the chamber evaluation and validation of the GA project.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
Kit for measuring glycolated serum albumin by enzymatic chemiluminescence method
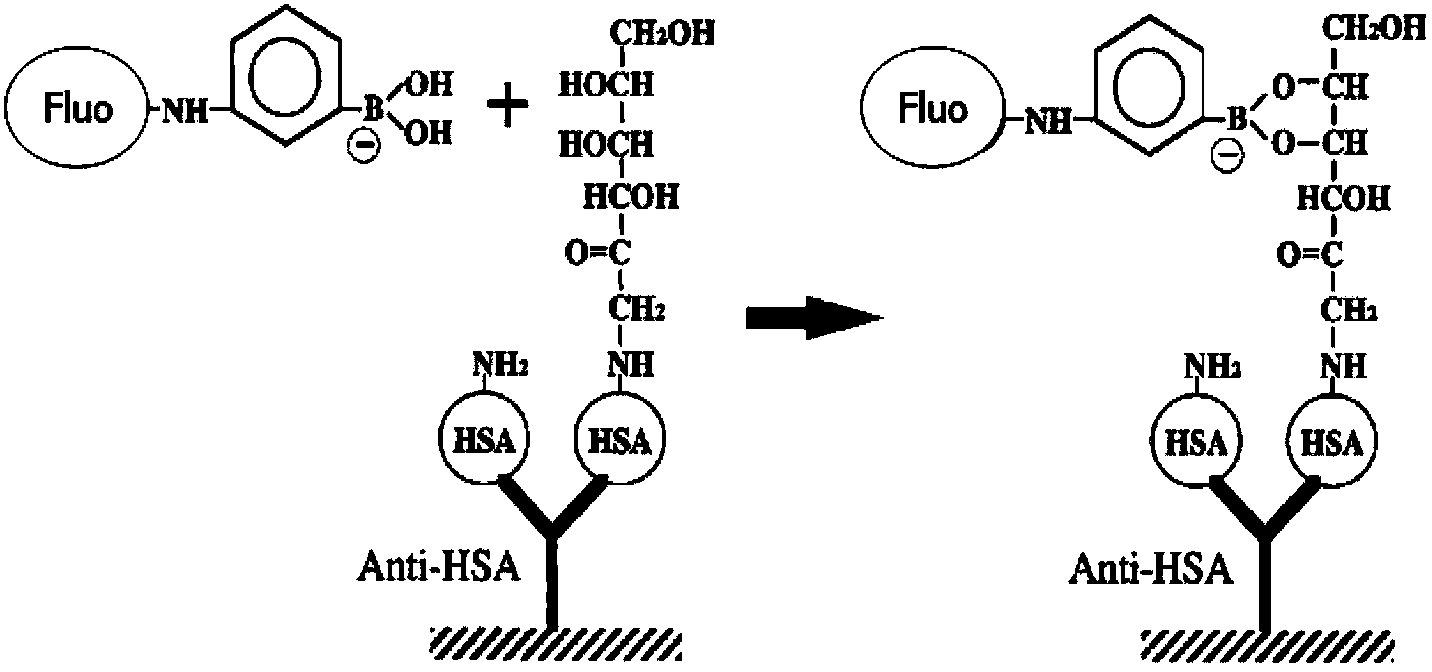
ActiveCN107870170AImprove transgender abilityStrong specificityChemiluminescene/bioluminescenceConcentration ratioBoronic acid
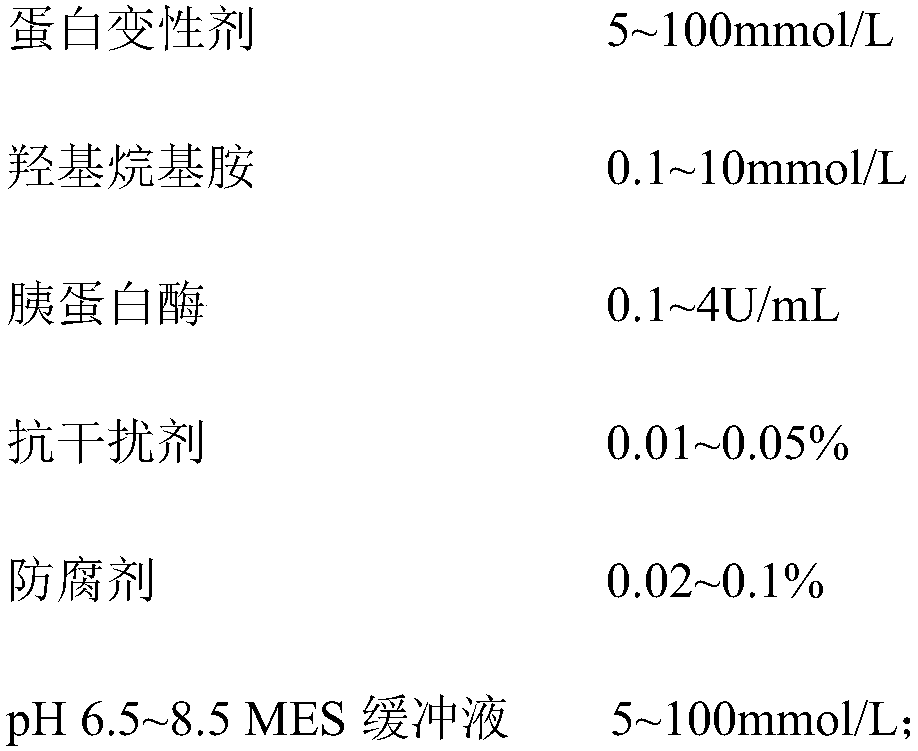
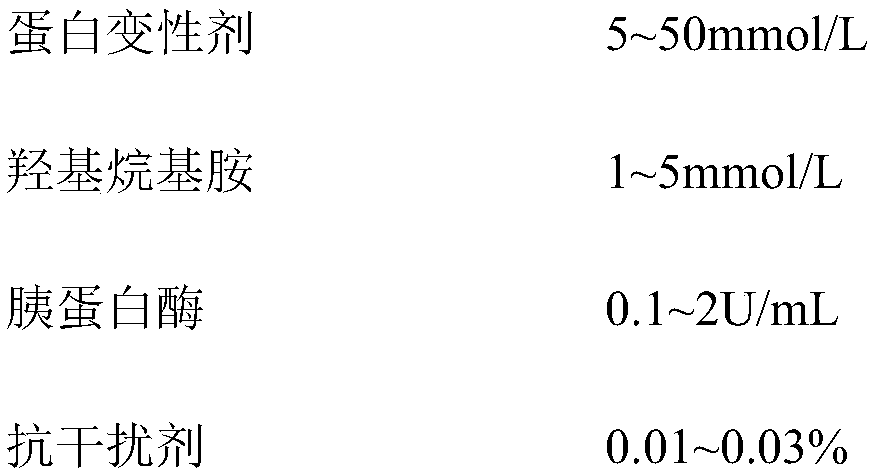
The invention belongs to the technical field of medical examination and particularly relates to a kit for measuring a concentration ratio of glycolated serum albumin and albumin. The kit comprises a reagent R1, a reagent R2, a reagent R3 and a reagent R4, wherein the reagent I contains a protein denaturant, hydroxyl-alkyl amine, trypsin, an anti-interference agent, a preservative and a buffer solution; the reagent II contains fructose amino acid oxidase, trehalose and a buffer solution; the reagent III contains amino acid oxidase, trehalose and a buffer solution; and the reagent IIII is a luminescent substrate 1,2-dioxetane boronic acid. The kit provided by the invention is similar with an existing enzymatic method detection kit in detection principle, but is lower in cost, high in interference resistance, accurate in detection, stable in property and convenient for extensive use.
Owner:GUANGZHOU JINDE BIOTECH
Method for preparing fructose lysine enzyme and application of preparing fructose lysine enzyme
ActiveCN102559643AHigh specific vitalityEasy extractionHydrolasesMicrobiological testing/measurementFructoseFractional Precipitation
The invention provides a method for preparing fructose lysine enzyme and a glycated albumin detection kit, wherein the glycated albumin detection kit is prepared by utilizing the fructose lysine enzyme that is prepared by adopting the method. The method comprises the operation steps as follows: strains are screened so as to obtain 11 to 82 aspergillus strains of the high-activity fructose lysine enzyme; the 11 to 82 aspergillus strains are cultivated, and mycelia are collected; the mycelia are suspended in buffer solution, and then are processed through cell disruption and centrifugation, and supernatant fluid is collected; the supernatant fluid is processed through fractional precipitation by adopting ammonium sulfate solution, and deposits are collected so as to obtain crude extracts; the crude extracts are processed through hydrophobic chromatography, and eluant is collected; in addition, the collected eluant is processed through affinity chromatography, and then eluant is collected, that is, fructose lysine enzyme solution is obtained. The method has the advantages as follows: the preparation steps are simple, and the obtained fructose lysine enzyme achieves high purity, high activity and high reaction specificity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
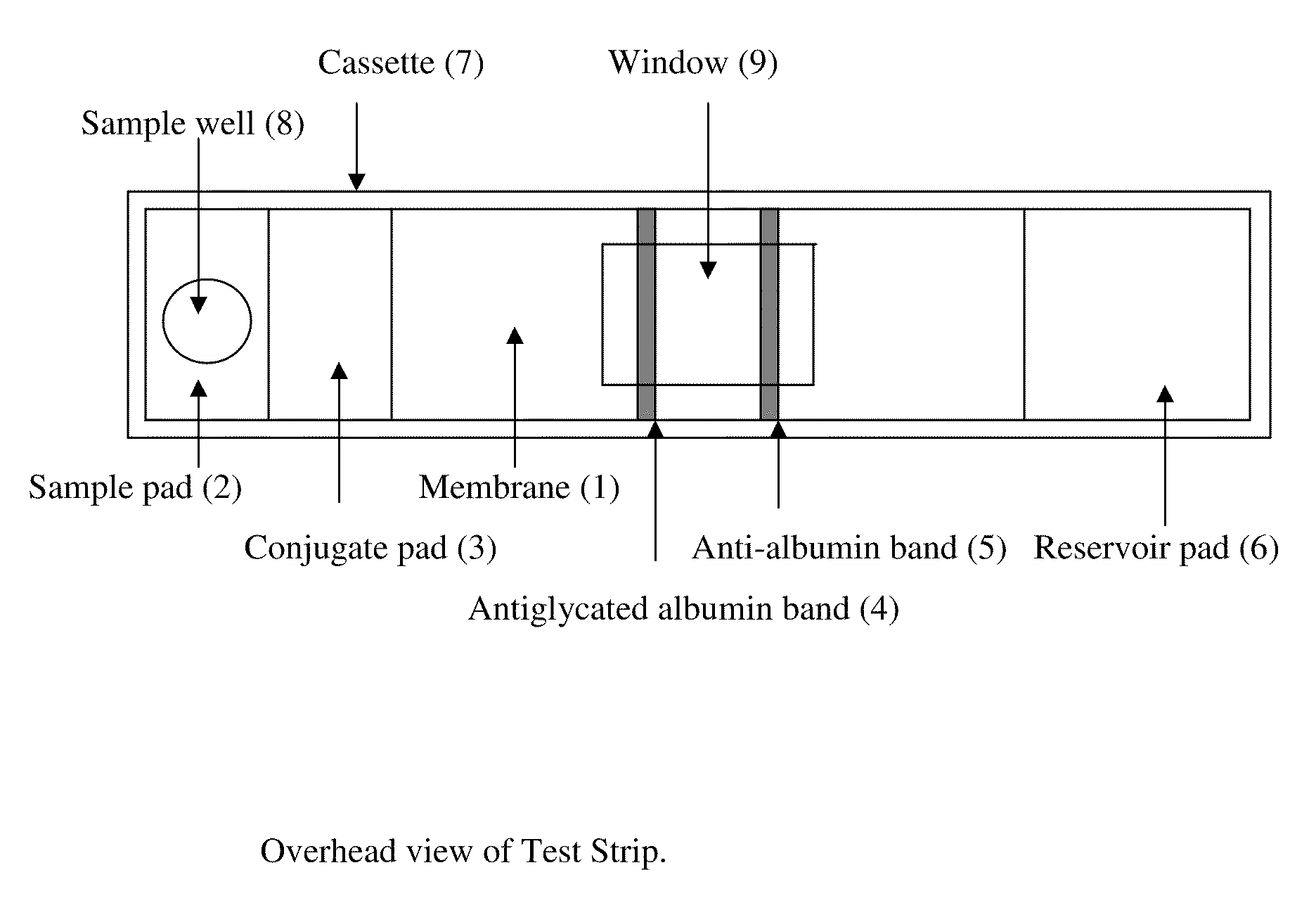
Glycated albumin detection immunochromatography test trip and preparation method thereof
InactiveCN104345150AEasy to prepareHigh speedDisease diagnosisBiological testingPhenylboronic acidTrace Amounts
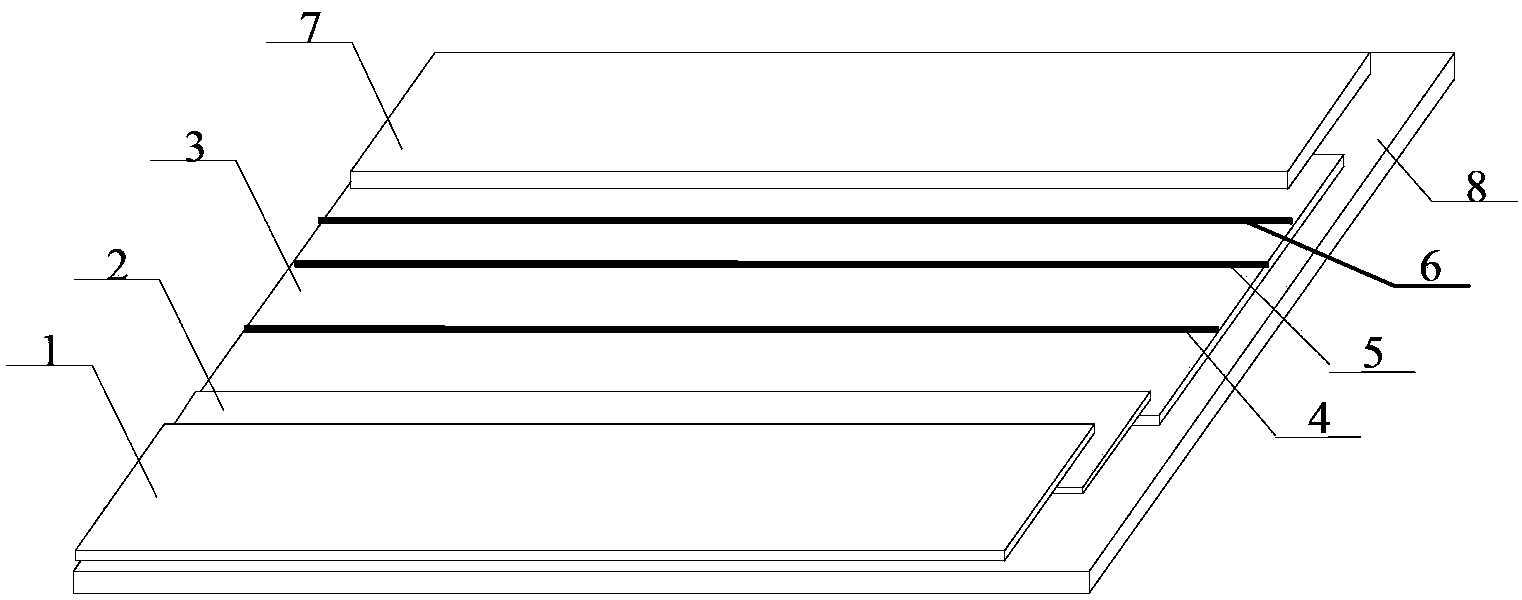
The invention provides a glycated albumin detection immunochromatography test trip and a preparation method thereof. The test trip comprises a liner, an analysis membrane disposed in the middle part of the liner, a water absorption pad arranged at one end of the analysis membrane upper part, a conjugate pad arranged at the other end of the analysis membrane upper part, and a sample pad disposed at one end of the conjugate pad upper part. The analysis membrane is provided with detection lines and a quality control line. The detection lines include a glycated albumin detection line and a hemoglobin detection line. The preparation method comprises: preparation of a phenylboronic acid marker, preparation of the sample pad, preparation of the conjugate pad, preparation of the detection line and quality control line on the analysis membrane, preparation of the water absorption pad and preparation of the glycated albumin detection immunochromatography test trip. The glycated albumin detection immunochromatography test trip provided by the invention can realize quantitative detection of the glycated albumin content in 3-5min only with a trace amount of a whole blood sample, greatly improves the screening speed, and has the advantages of high sensitivity, good specificity and simple structure.
Owner:SHENZHEN AIRUI BIO TECH
Anti-human glycated albumin monoclonal antibody and use thereof
ActiveCN103554256AIncreased sensitivityImprove stabilityImmunoglobulins against animals/humansBiological testingHeavy chainNucleotide
The invention discloses an anti-human glycated albumin monoclonal antibody comprising a heavy chain variable region and a light chain variable region, wherein an amino acid sequence of the heavy chain variable region is shown in SEQ ID NO:2, and an amino acid sequence of the light chain variable region is shown in SEQ ID NO:4. The anti-human glycated albumin monoclonal antibody is an IgG1 subtype. The invention also discloses a gene used for encoding the anti-human glycated albumin monoclonal antibody, wherein a nucleotide sequence encoding the heavy chain variable region is shown in SEQ ID NO:1, and a nucleotide sequence encoding the light chain variable region is shown in SEQ ID NO:3. The anti-human glycated albumin monoclonal antibody can be used for measuring human glycated albumin.
Owner:宁波医杰生物科技有限公司
Preparation method of glycated albumin calibrator
The invention discloses a preparation method of a glycated albumin calibrator. The preparation method includes following steps: (1) preparing a phosphate buffer liquid being 7.0-8.0 in pH, which comprises certain amounts of a glycosylation accelerator, sodium chloride, EDTA, sodium azide and glucose; (2) adding recombination human serum albumin and performing a glycosylation reaction for a certain time to prepare a solution; (3) transferring the solution to a tangential flow filtering system to remove glycosylated polypeptide, glycosylated amino acids and glucose; (4) diluting the filtered solution to a required concentration of the calibrator; and (5) adding an excipient, and packaging the calibrator separately and freeze-drying the calibrator. According to the method, the prepared glycated albumin calibrator is free of the glycosylated polypeptide, the glycosylated amino acids and the glucose. The method is suitable for mass production of the glycated albumin calibrator.
Owner:浙江夸烨生物科技有限公司
Test card for detecting human glycated albumin
The invention discloses a binary-channel test card for detecting a human glycated albumin. The binary-channel test card is characterized in that a sample pad, a bonding pad, a cellulose nitrate membrane and a water absorption pad are orderly adhered to a PVC backing; special monoclonal antibodies and aptamers of a human glycated albumin and an albumin are used as detection reagents and are used for detecting content of the human glycated albumin and the total albumins. The binary-channel test card can detect a ratio of the glycated albumin to the total albumins in a human biological sample such as a blood sample and a saliva sample, utilizes saliva replacing blood to carry out non-invasive detection, and prevents inconvenience and danger of blood sampling. The binary-channel test card is convenient for fast, accurate and simple control and monitoring of a blood sugar level.
Owner:TIANJIN HUASHENGYUAN TECH
New application of bifidobacterium tetravaccine composition
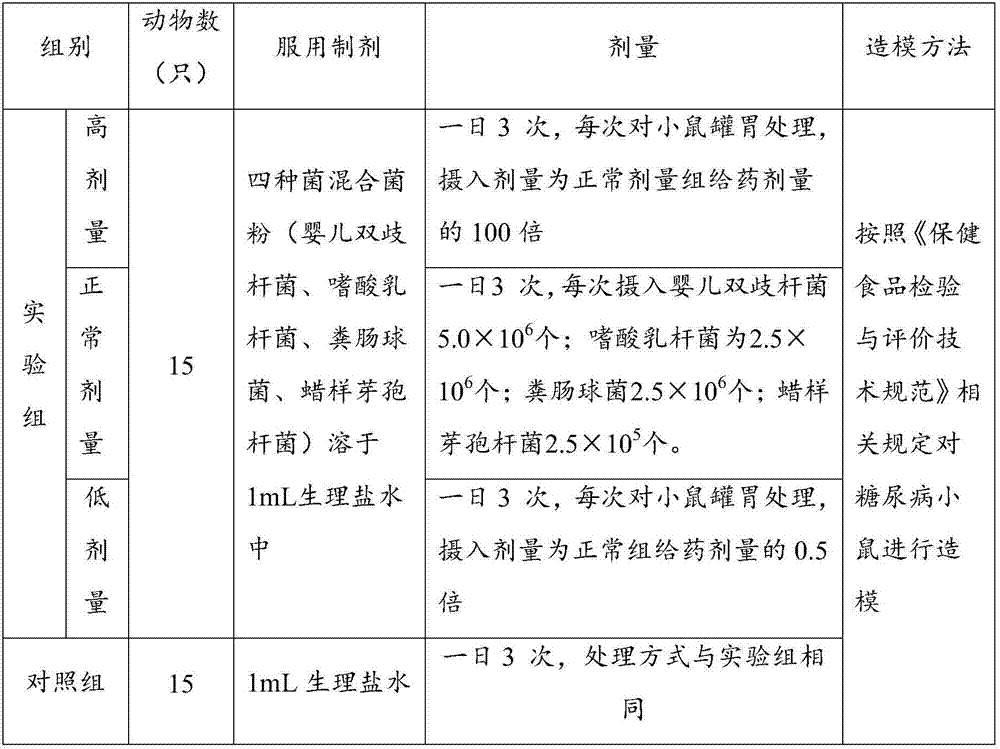
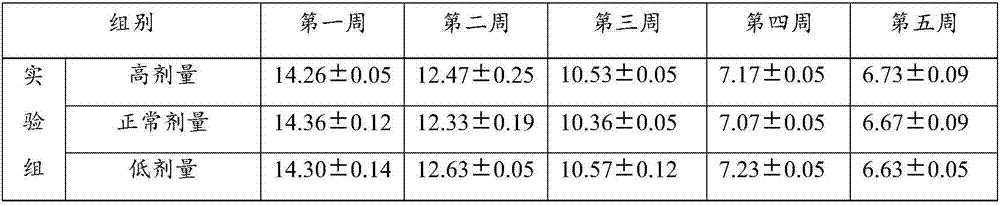
ActiveCN107050065ANo side effectsIngredients are harmlessMetabolism disorderUnknown materialsEndocrinology departmentSide effect
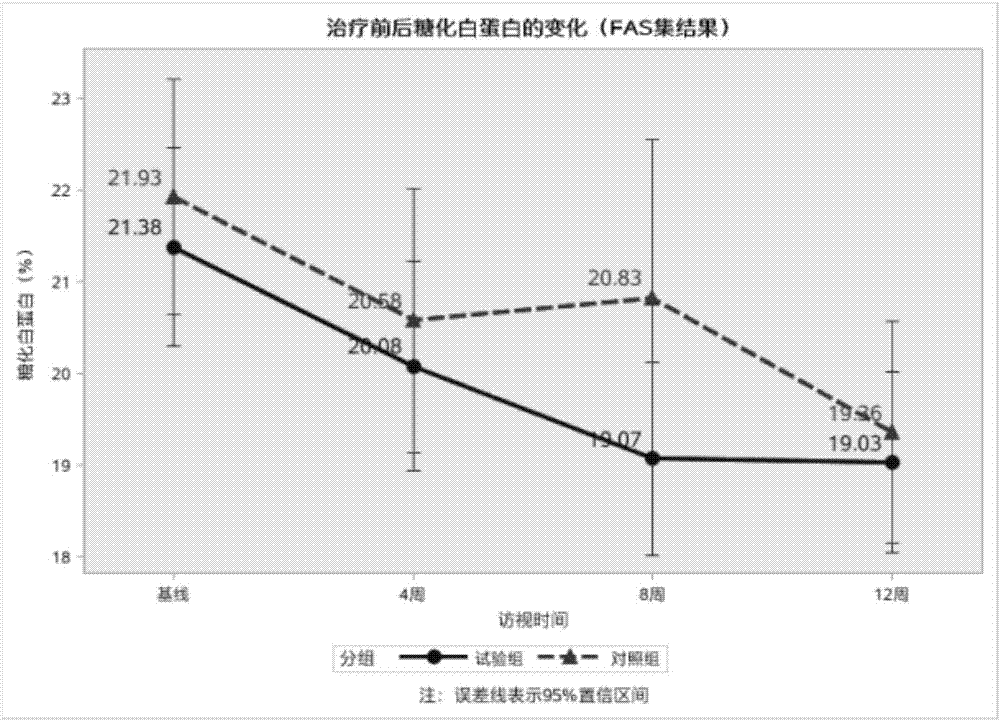
The invention discloses new application of a bifidobacterium tetravaccine composition, and belongs to the technical field of medicines. The invention discloses application of the bifidobacterium tetravaccine composition in preparing medicines or health-care foods or foods for assisting blood glucose reducing, application of the bifidobacterium tetravaccine composition in preparing medicines or health-care foods or foods for assisting glycated albumin reducing and assisting glycated hemoglobin reducing, and application of the bifidobacterium tetravaccine composition in preparing medicines or health-care foods or foods for blood glucose reducing for type II diabetes patients or hyperinsulinemia patients. The new application of the bifidobacterium tetravaccine composition has the advantages that the application range of the bifidobacterium tetravaccine composition is widened, and the clinical application range of the bifidobacterium tetravaccine composition is widened from the gastroenterology department to new diabetes endocrinology department; the composition is a probiotics microecological preparation, the components are harmless, and the side effect to a human body is avoided.
Owner:HANGZHOU GRAND BIOLOGIC PHARMA INC
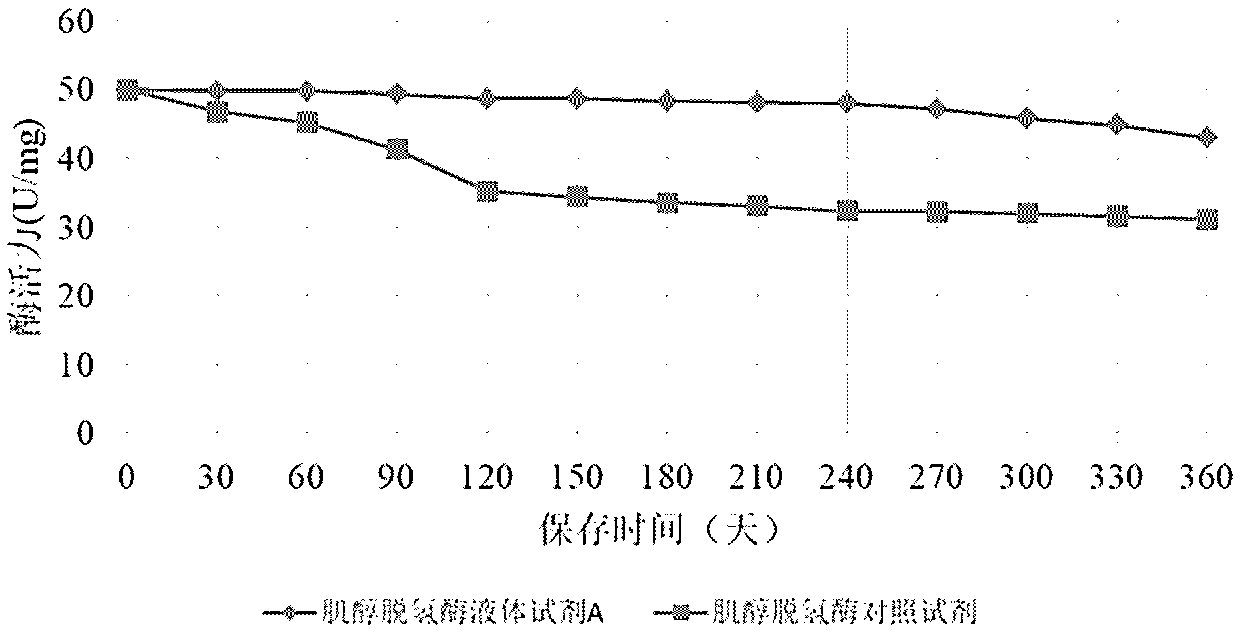
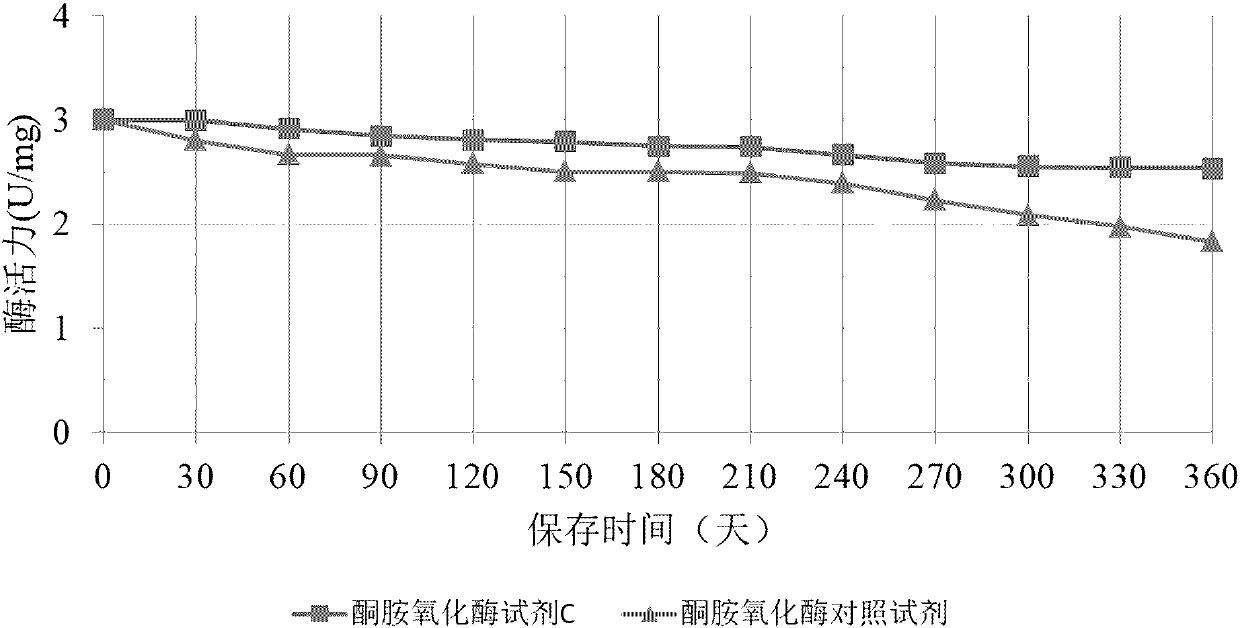
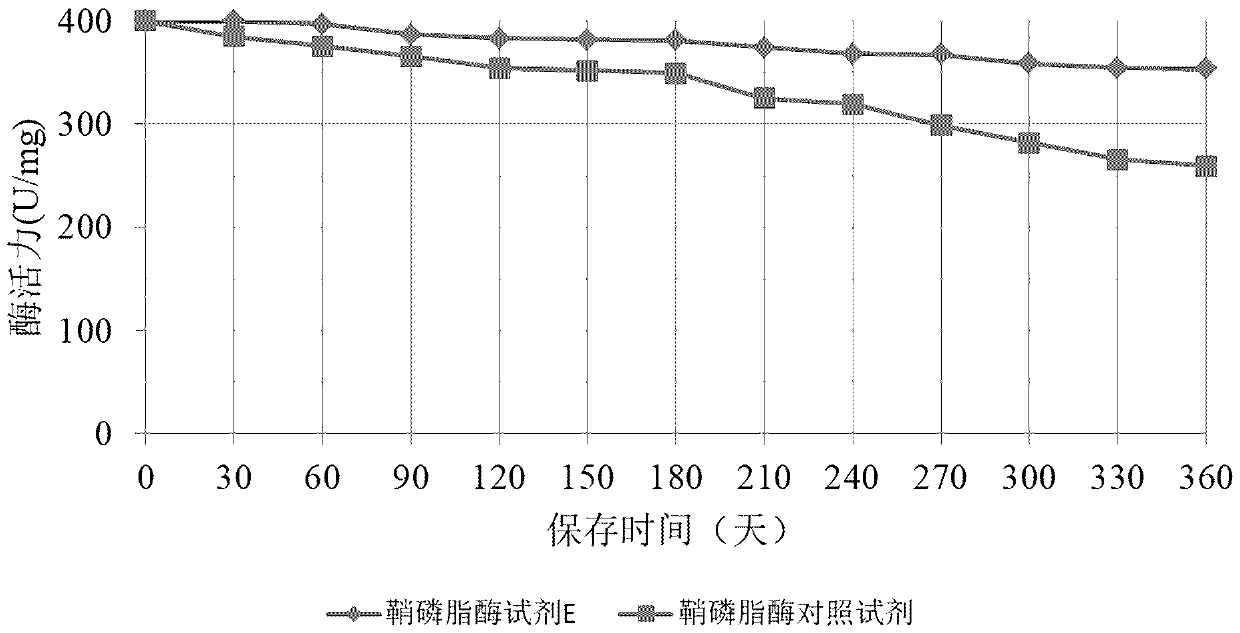
Method for long-term stabilization of inositol dehydrogenase, ketoamine oxidase and sphingomyelinase in liquid
The present invention provides a method for long-term stabilization of inositol dehydrogenase, ketoamine oxidase and sphingomyelinase in liquid. The method is used to prepare an enzymatic biochemicalkit for determining inositol, glycated albumin and small and dense low density lipoprotein cholesterol for a purpose of enabling the enzymatic biochemical kit to have a shelf life of 12 months or more. The method is characterized in that a composition of 4-formylbenzeneboronic acid and benzyldimethylphenol polyoxyethylene ether is added to a reagent solution for preserving enzymes, and concentrations of the 4-formylbenzeneboronic acid and benzyldimethylphenol polyoxyethylene ether are preferably 0.008-0.012 w / V% and 0.1-0.2 w / V%, respectively.
Owner:深圳市安帝宝科技有限公司
Method for quickly purifying glycated albumin coarse solution
ActiveCN104729907AAvoid decompositionShorten the timePreparing sample for investigationCentrifugationFilter system
The invention discloses a method for quickly purifying a glycated albumin coarse solution. The method comprises the following steps: (1) performing low-temperature centrifugation on the glycated albumin coarse solution, and taking a supernatant solution; (2) transferring the supernatant solution to a tangent flow filtering system for concentration treatment to obtain a coarse concentrated glycated albumin coarse solution; (3) performing dilution treatment in the tangent flow filtering system to remove glycosylated peptides, glycated amino acid and glucose from the coarse concentrated glycated albumin coarse solution; and (4) diluting the purified glycated albumin coarse solution to the required concentration by a buffering solution. The glycated albumin coarse solution is purified by the method, so that small-molecular impurities such as glycosylated peptides can be quickly removed; the method has the characteristics of simplicity, convenience and quickness in operation and the like and is suitable for large-scale purification of the glycated albumin coarse solution.
Owner:浙江夸烨生物科技有限公司
Kit for determining glycated albumin
ActiveCN109212238AConsistent accuracyConsistent linear rangeBiological testingEnzyme activatorAssay sensitivity
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method for improving thermal stability of liquid fructosaminase
ActiveCN104611319AImprove thermal stabilityImprove stabilityEnzyme stabilisationSodium azideHydroxymethyl
Owner:浙江夸烨生物科技有限公司
Diabetes detection analyzer
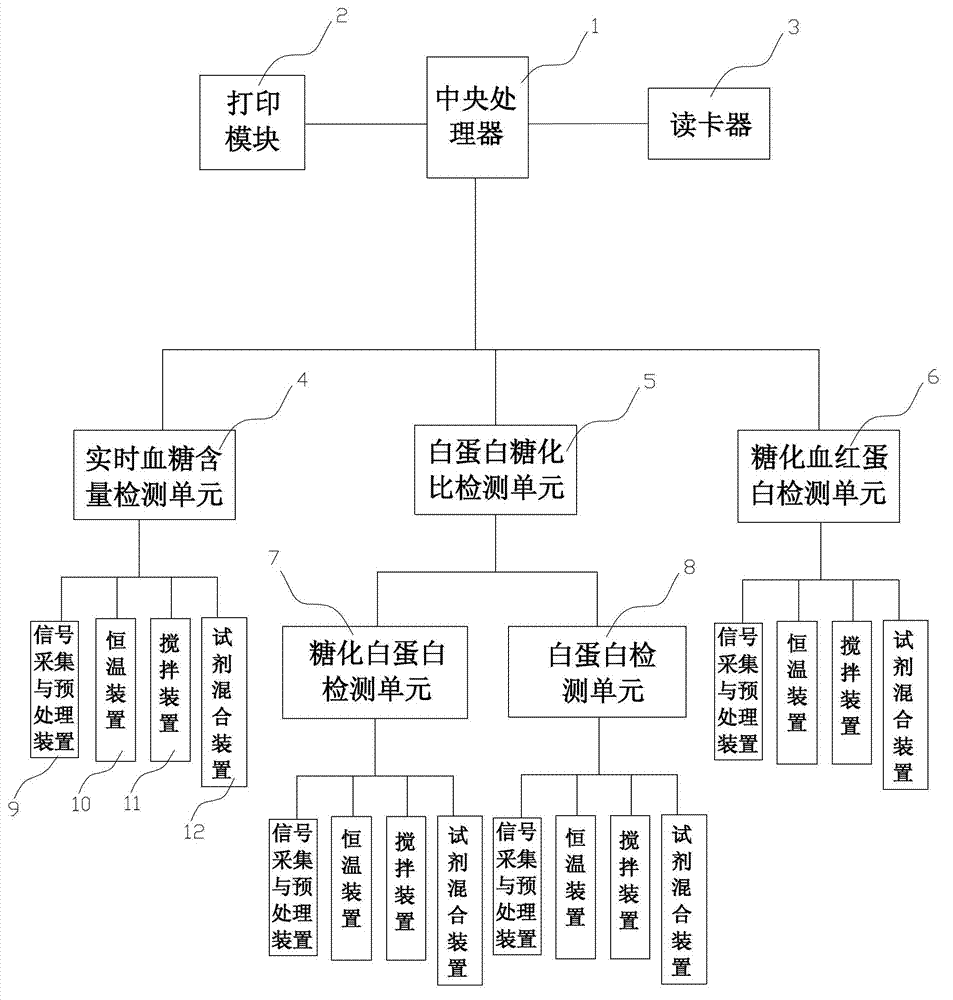
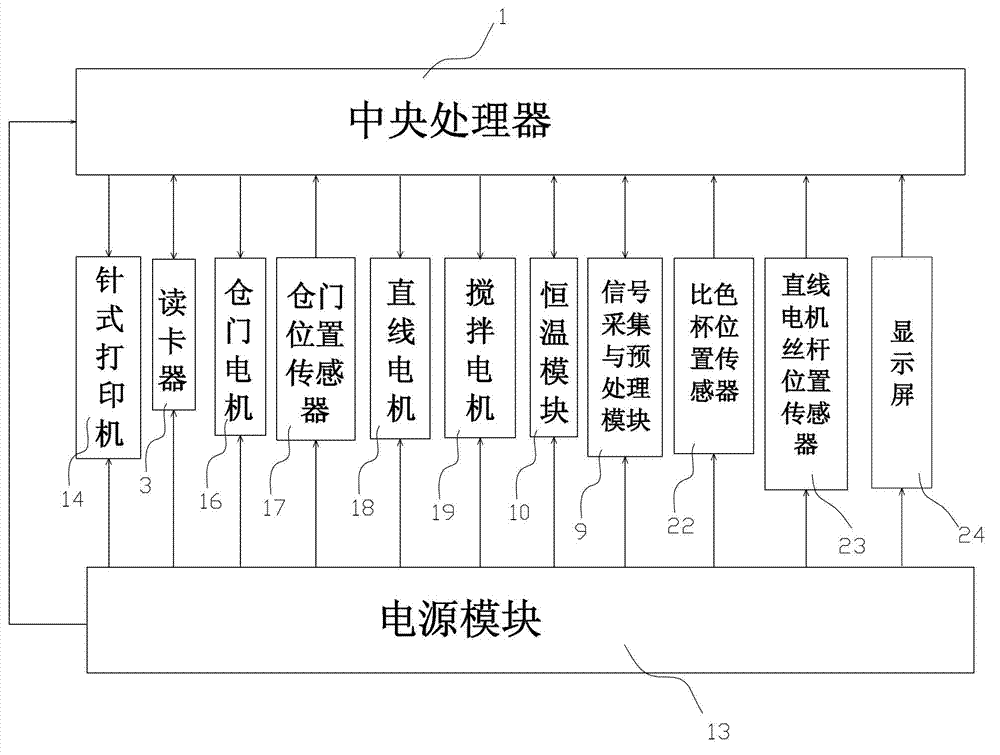
The invention relates to a diabetes detection analyzer, which comprises a detection analyzer body; a central processor, a power module and a real-time blood sugar content detection unit are arranged in the detection analyzer body; the real-time blood sugar content detection unit comprises a signal acquisition and preprocessing device based on a reagent absorbance analysis principle, a constant temperature device and a stirring device; a reagent cavity used for mounting a reagent to be tested is arranged on the constant temperature device; an albumin glycation ratio detection unit used for detecting the average blood sugar content of a patient in 2-3 weeks is also arranged in the detection analyzer body; and the albumin saccharification ratio detection unit comprises a glycated albumin detection unit and an albumin detection unit which have the same structure as the real-time blood sugar content detection unit. The diabetes detection analyzer is low in price and simple and convenient to use, and can be used for detecting the real-time blood sugar content and the average blood sugar content of 2-3 weeks in a blood sample of the patient at the same time.
Owner:宁波美康盛德生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com