Preparation method of stable glycated albumin calibrating material and quality control material
A technology of glycosylated albumin and calibrator, which is applied in the field of medical testing, can solve problems such as pollution and glycosylated albumin concentration fluctuations, and achieve the effect of rich raw material sources, good long-term stability, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] First of all, the serum of healthy people was used, and the HIV, HCV and HTLV were all negative. Then centrifuge at 18000rpm*30min to remove the precipitate, and then dialyze with the dialysate to remove some small molecule interferences; wherein, the composition of the dialysate is: tris buffer (pH7.5): 50mM, sodium chloride: 9g / L, solvent: distilled water.
[0032] Secondly, add 5g / L glucose and 0.5g / L sodium azide to the dialyzed serum, place it in a constant temperature water bath at 37°C for 1 day to obtain a quality control product with a low quality control value, and 2 days to obtain a calibration product , 4 days to get quality control products with high quality control values.
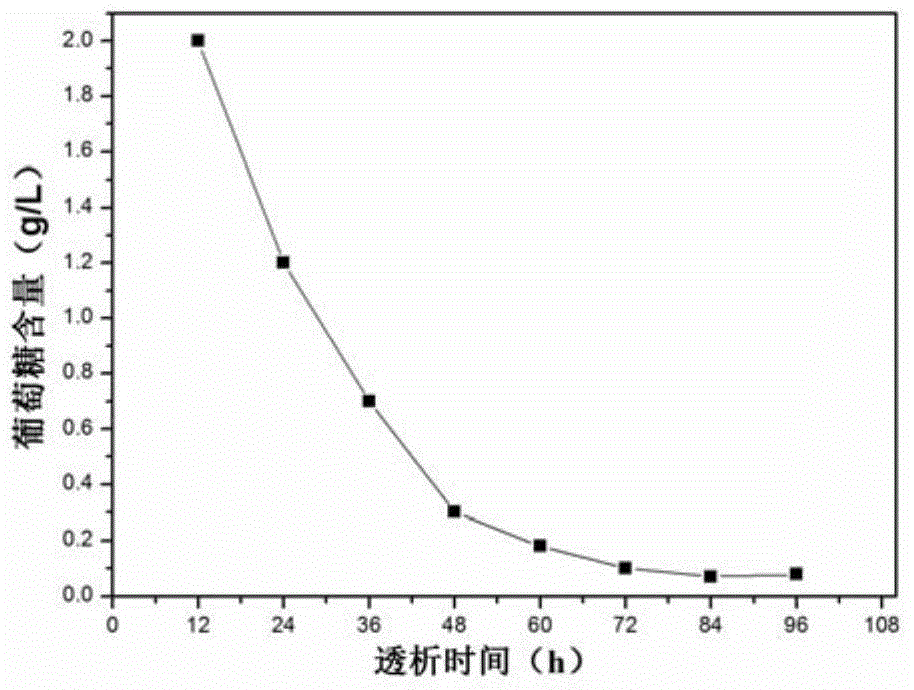
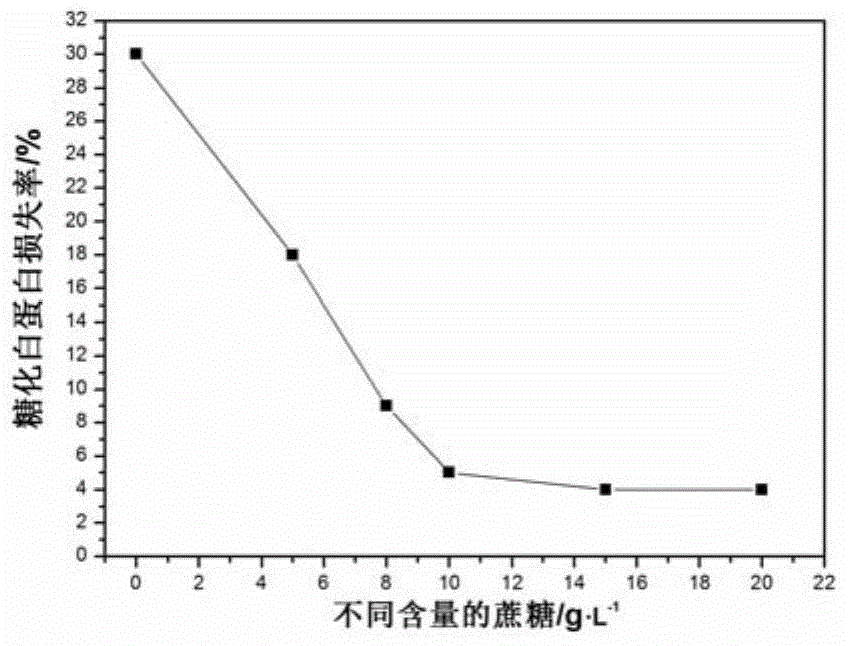
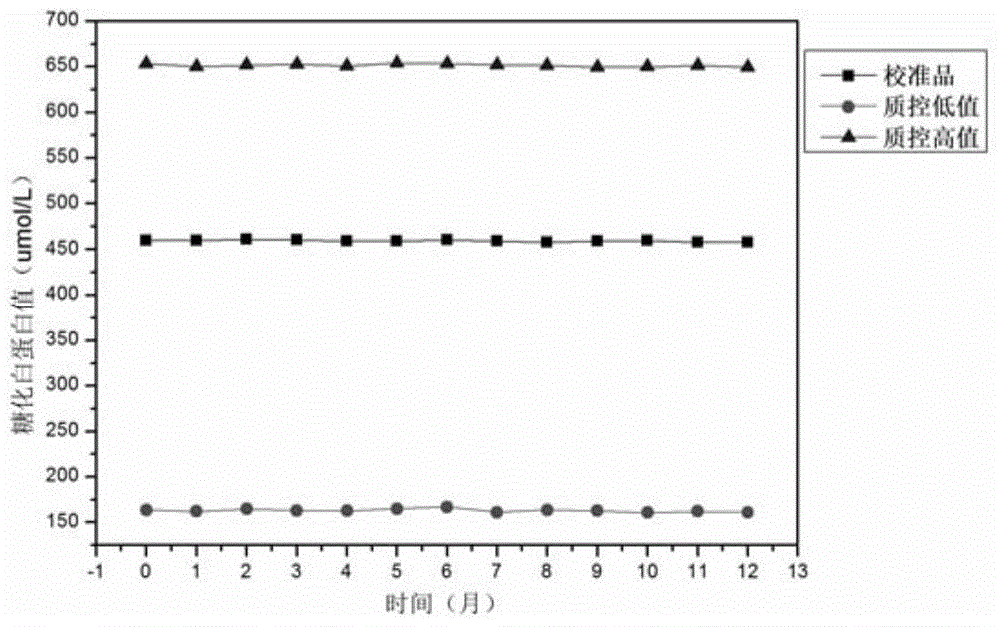
[0033] Again, put the prepared calibration products and quality control products into dialysis bags respectively, place them in the dialysate at 4°C, change the dialysate every 12 hours, dialyze to remove excess glucose, and prevent further saccharification until glucose is used The ...
Embodiment 2
[0036] First of all, the serum of healthy people was used, and the HIV, HCV and HTLV were all negative. Then centrifuge at 18000rpm*30min to remove the precipitate, and then use the dialysate to dialyze to remove some small molecule interferers, wherein the composition of the dialysate is: tris buffer (pH7.5): 50mM, sodium chloride: 9g / L, solvent: distilled water.
[0037] Secondly, add 5g / L glucose and 0.5g / L sodium azide to the dialyzed serum, place it in a constant temperature water bath at 37°C for 1 day to obtain a quality control product with a low quality control value, and 2 days to obtain a calibration product , 4 days to get quality control products with high quality control values.
[0038] Again, put the prepared calibration products and quality control products into dialysis bags respectively, place them in the dialysate at 4°C, change the dialysate every 12 hours, dialyze to remove excess glucose, and prevent further saccharification until used The remaining gl...
Embodiment 3
[0041]First of all, the serum of healthy people was used, and the HIV, HCV and HTLV were all negative. Then centrifuge at 18000rpm*30min to remove the precipitate, and then use the dialysate to dialyze to remove some small molecule interferers, wherein the composition of the dialysate is: tris buffer (pH7.5): 50mM, sodium chloride: 9g / L, solvent: distilled water.
[0042] Secondly, add 5g / L glucose and 0.5g / L sodium azide to the dialyzed serum, place it in a constant temperature water bath at 37°C for 1 day to obtain a quality control product with a low quality control value, and 2 days to obtain a calibration product , 4 days to get quality control products with high quality control values.
[0043] Again, put the prepared calibration products and quality control products into dialysis bags respectively, place them in the dialysate at 4°C, change the dialysate every 12 hours, dialyze to remove excess glucose, and prevent further saccharification until glucose is used The re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com