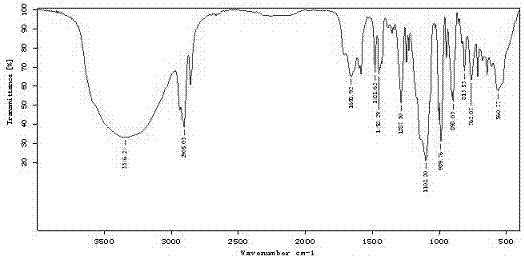
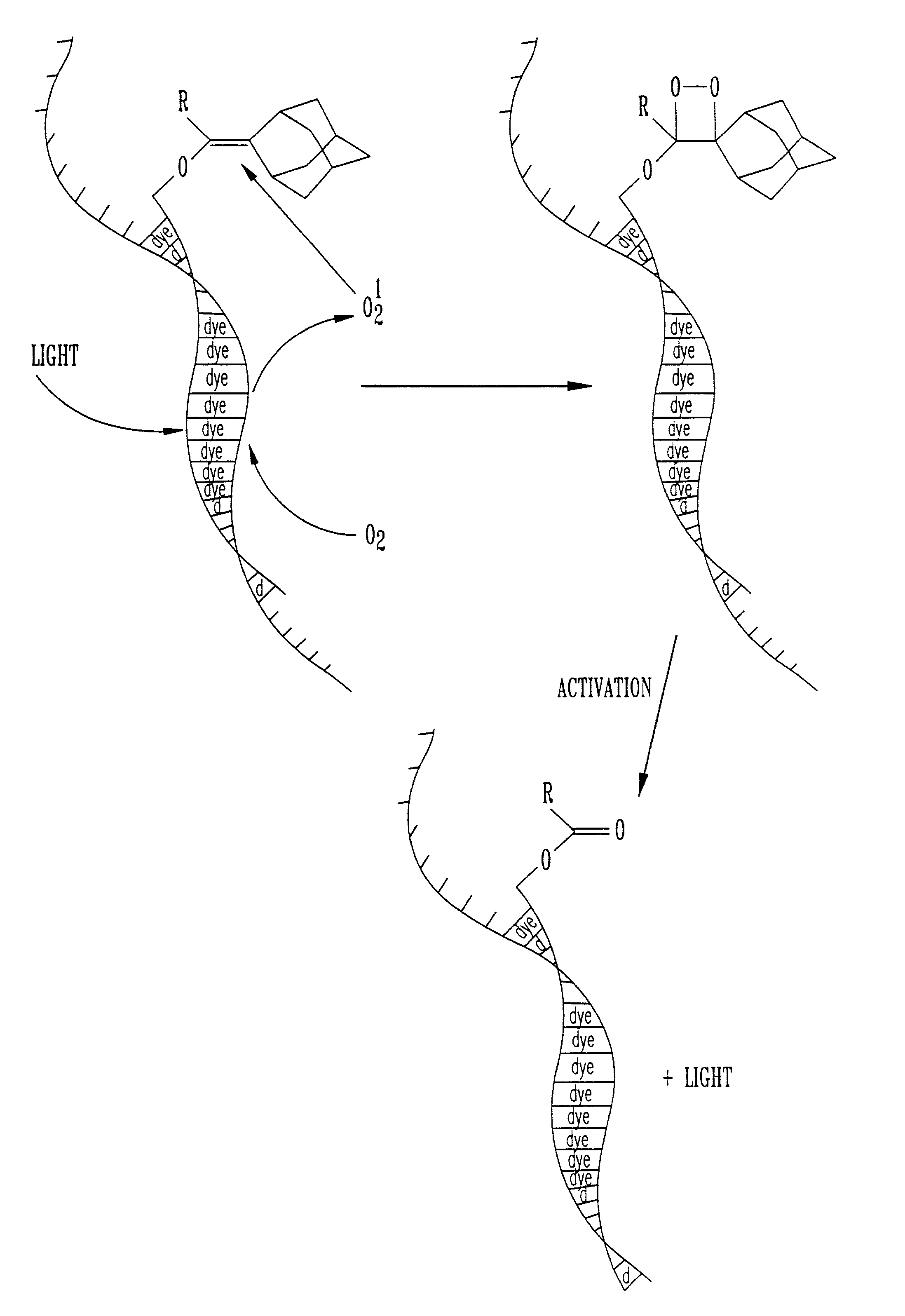
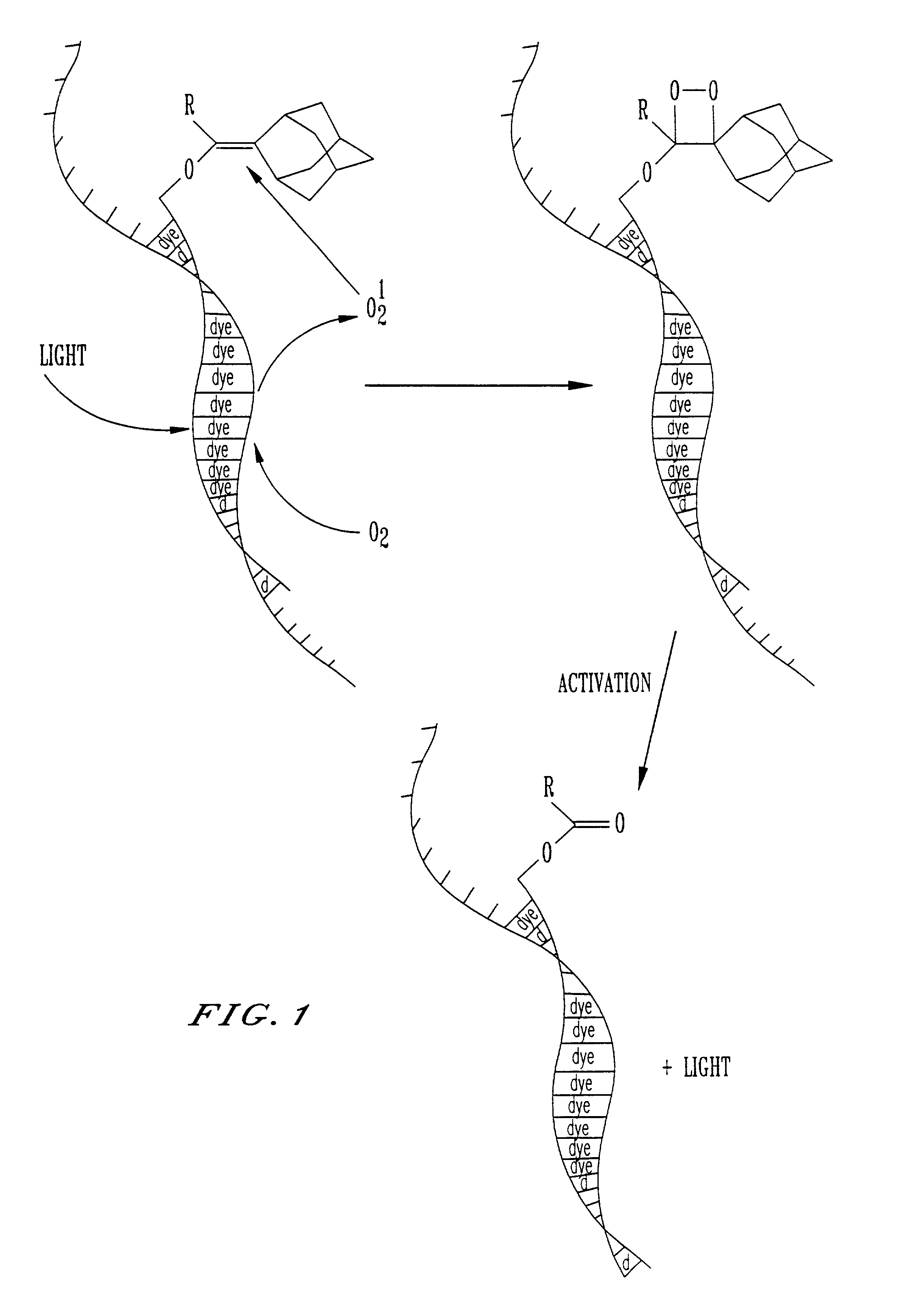
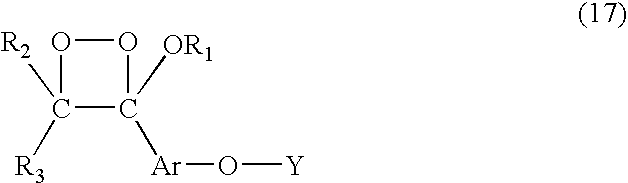Patents
Literature
49 results about "Dioxetane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
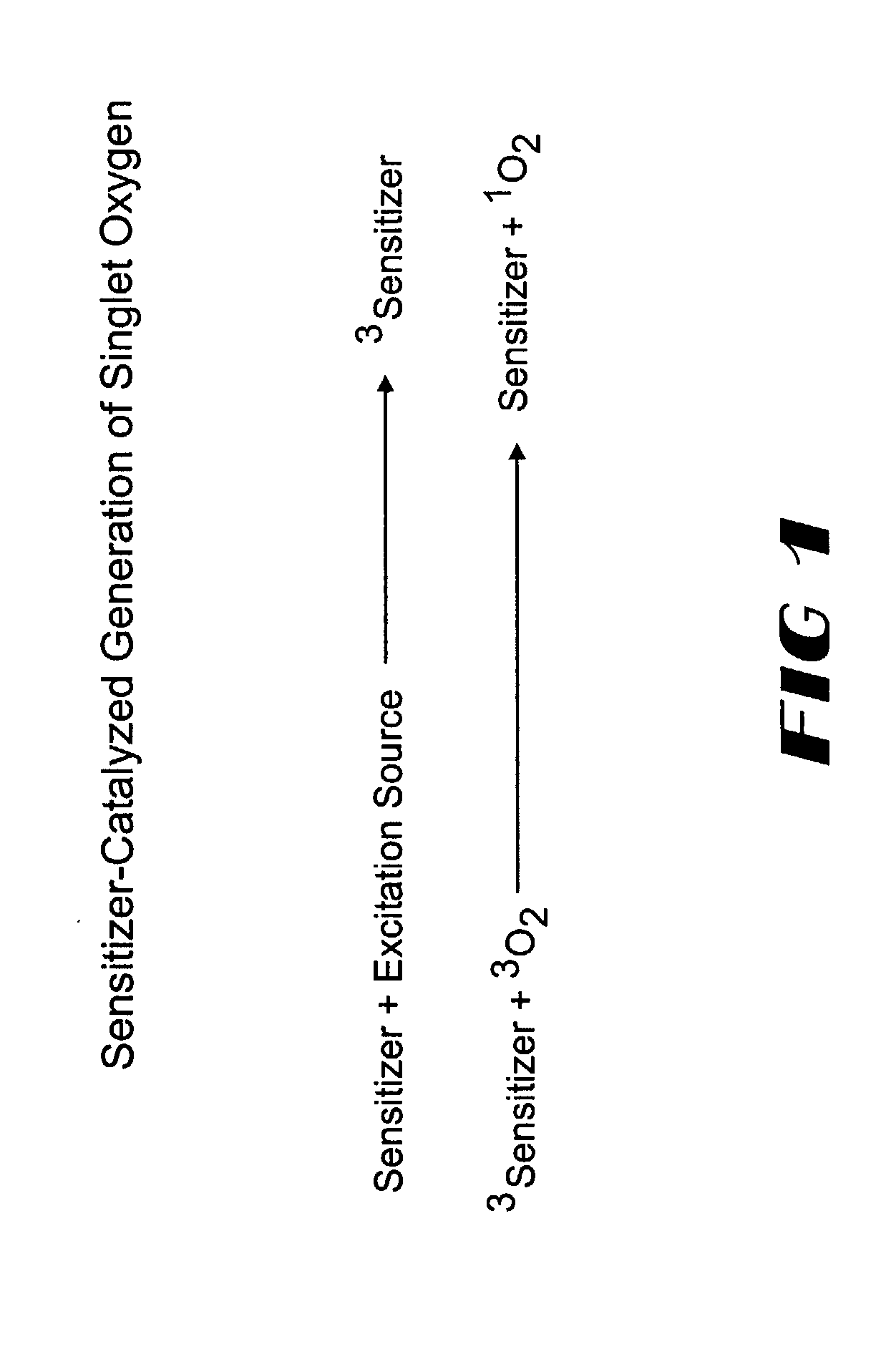
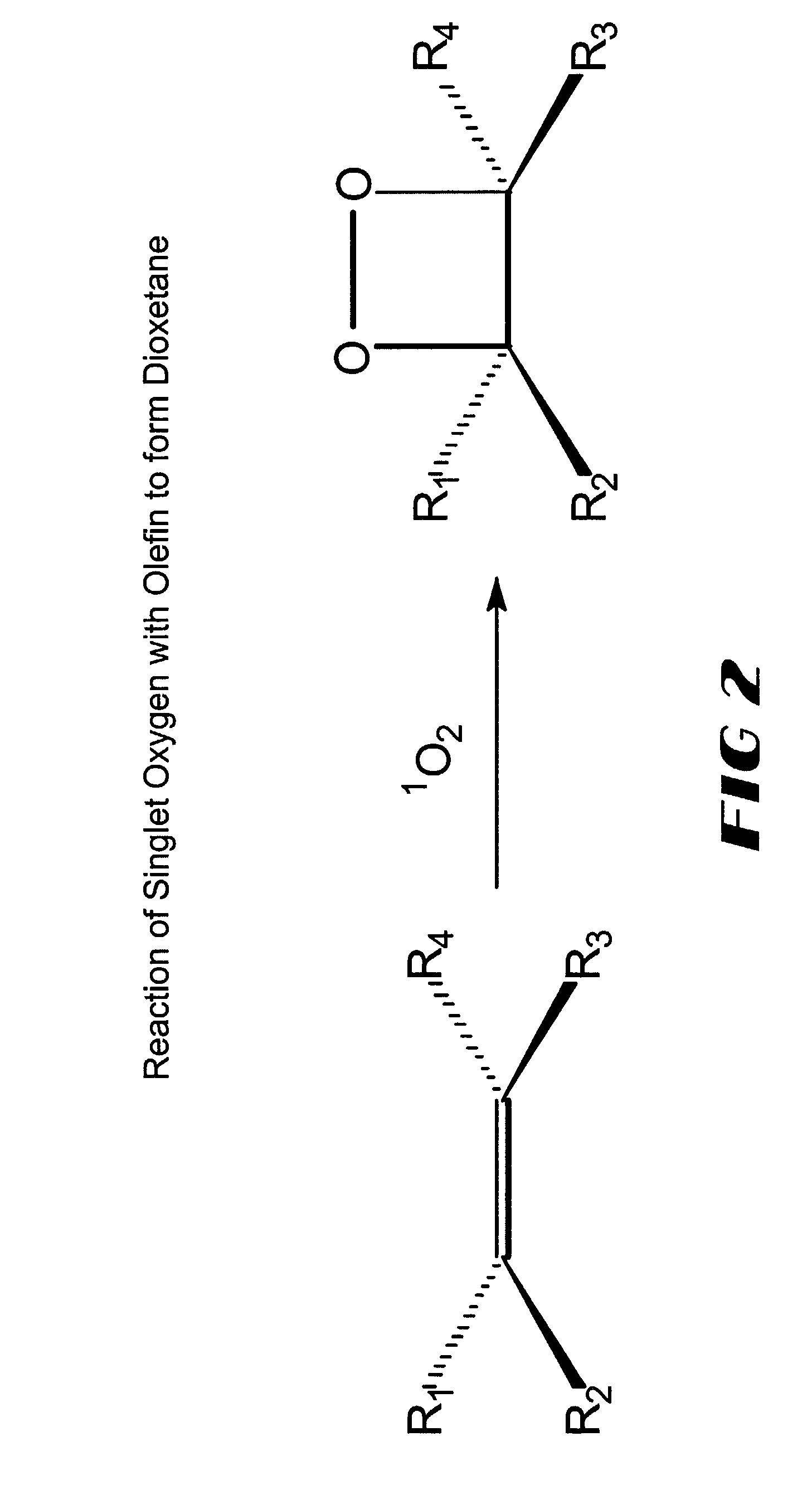
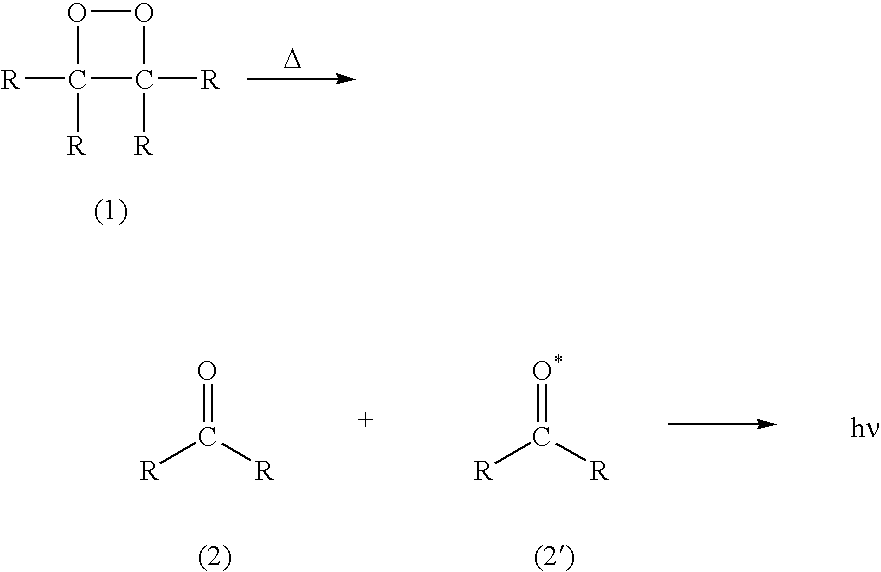
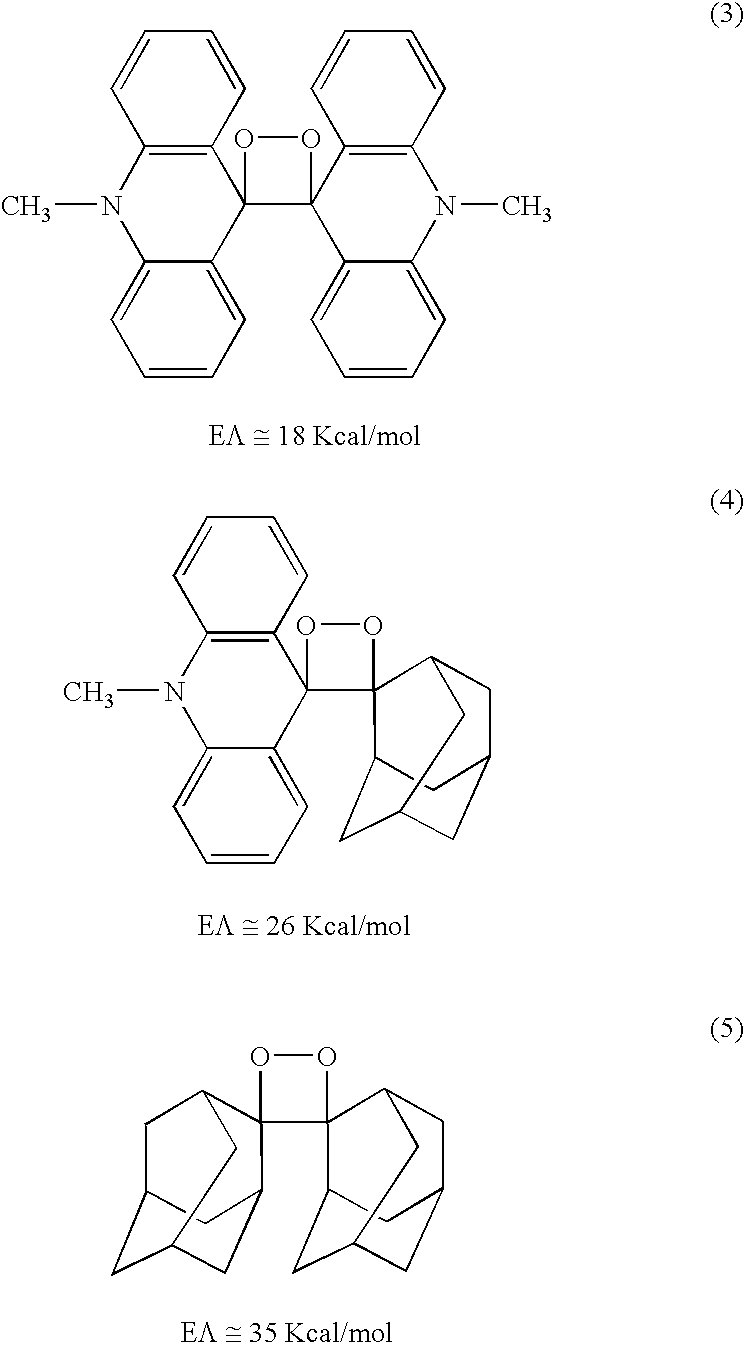
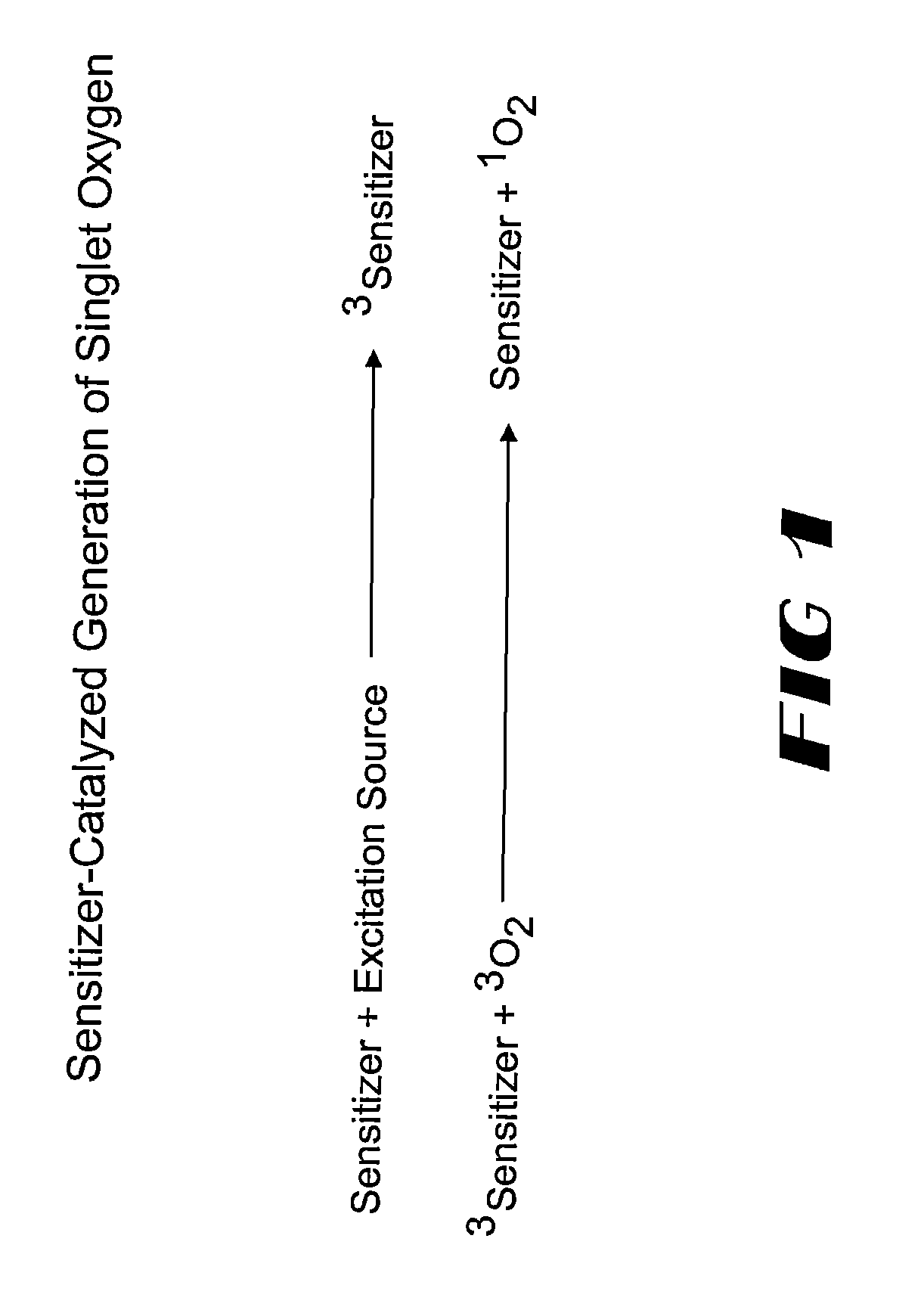
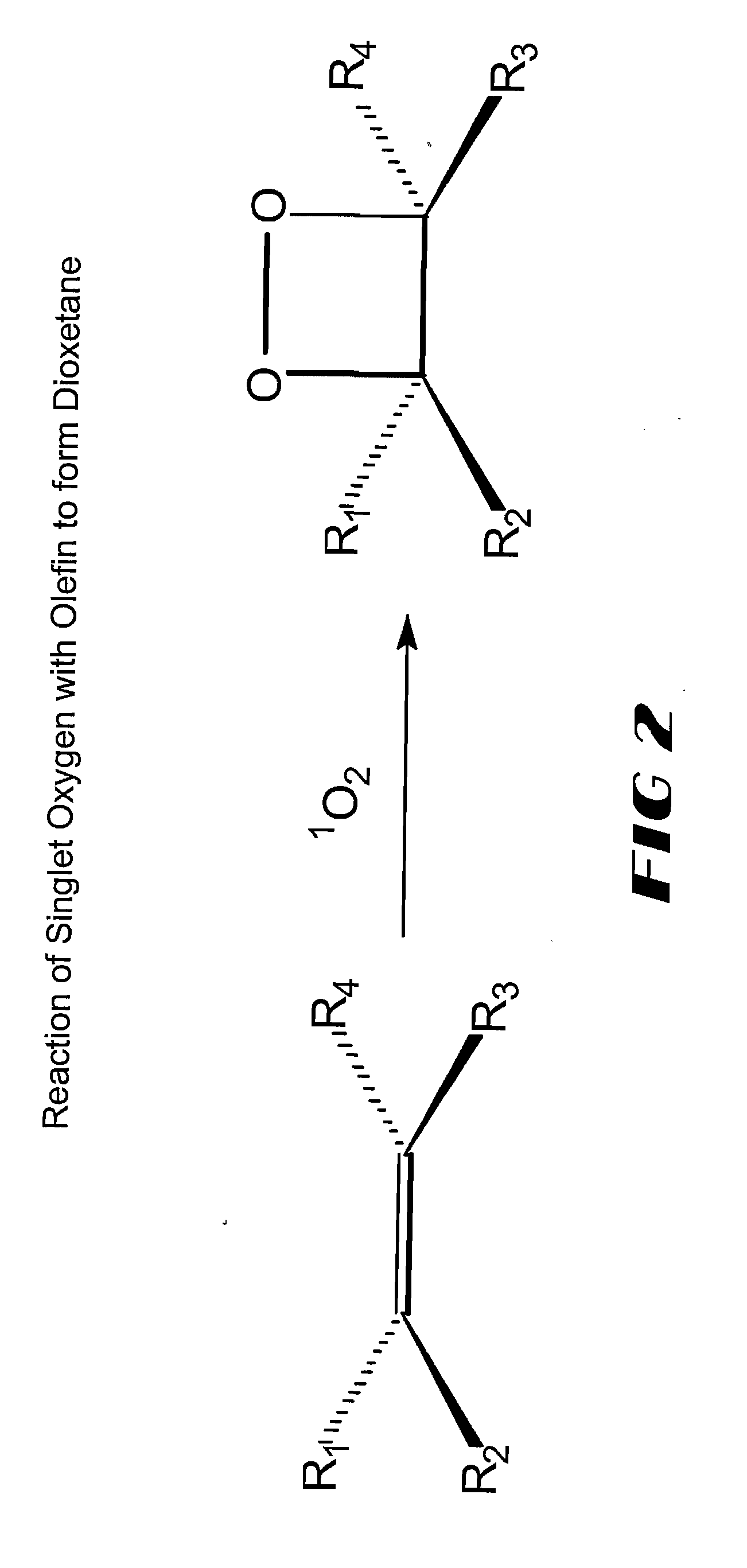
A dioxetane or dioxacyclobutane is an organic compound with formula C₂O₂H₄, whose backbone is a four-membered ring of two oxygen atoms and two carbon atoms.
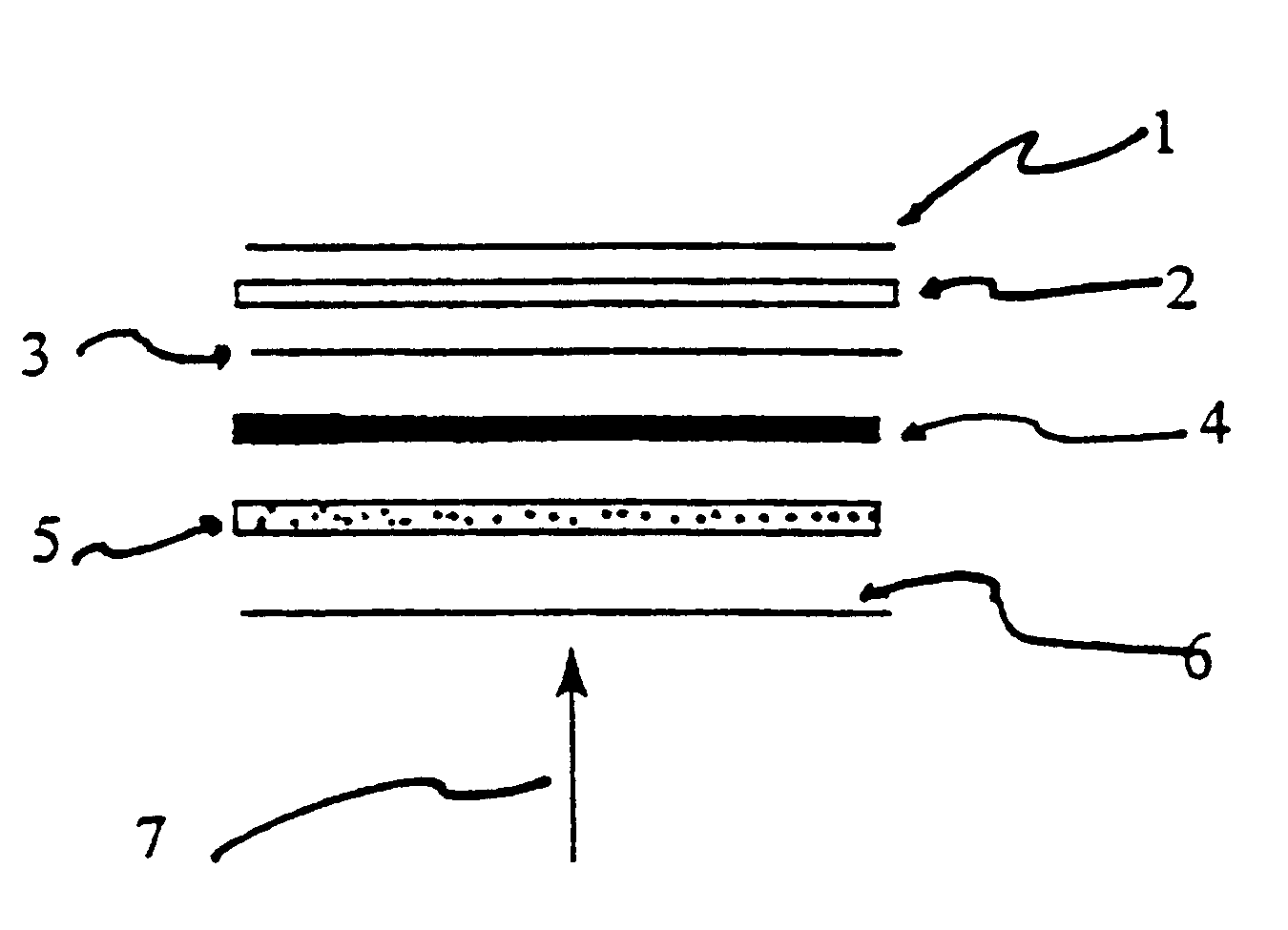
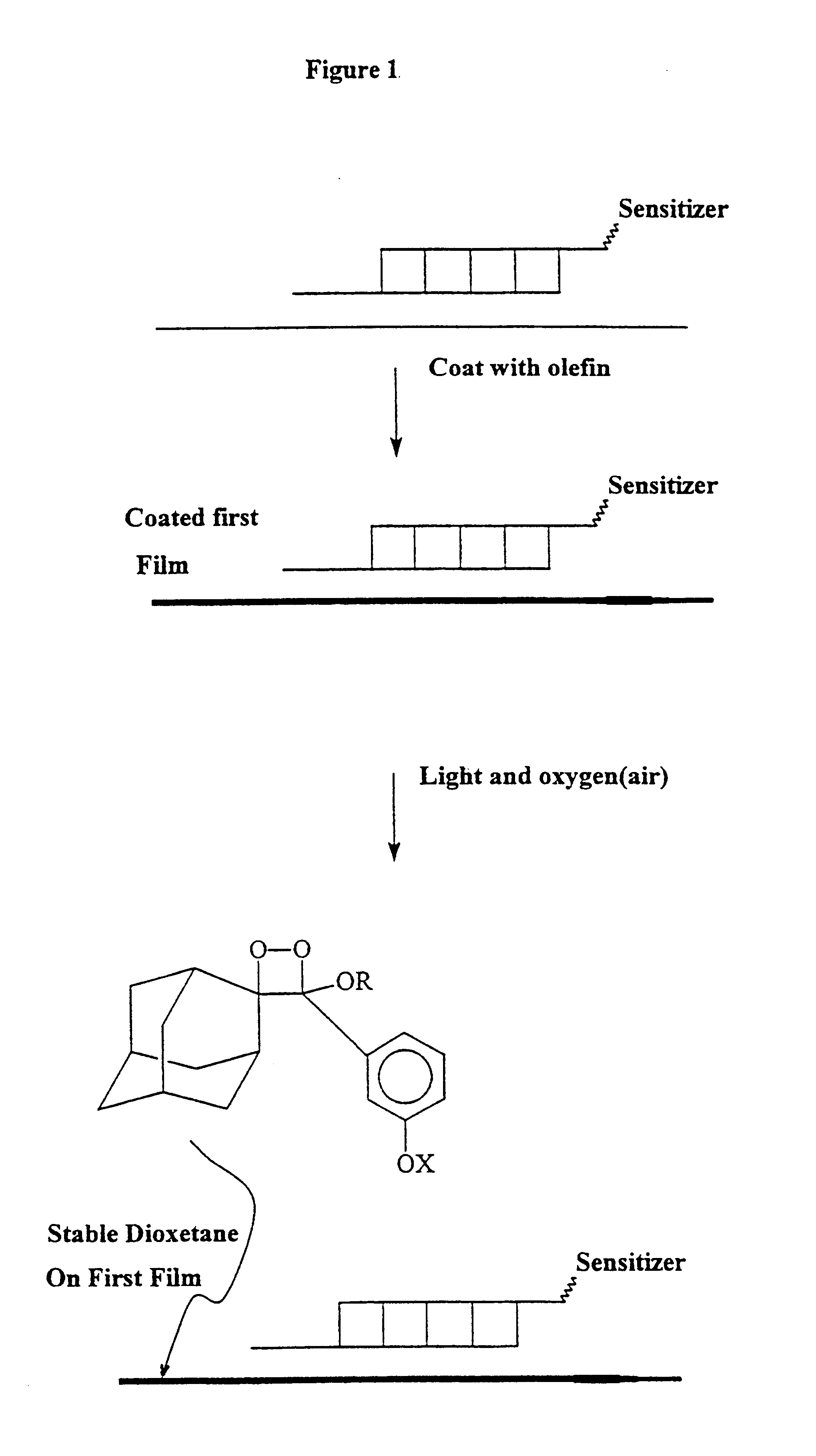
Activating film for chemiluminescent assays and methods for use
InactiveUS6613578B1Chemiluminescene/bioluminescenceLuminescent dosimetersChemical treatmentSolid-state chemistry
The present invention relates to chemiluminescent assays which incorporate a second film or membrane which includes a solid chemical component for activation of a stable dioxetane. Decomposition of the stable dioxetane can be accomplished using a combination of heat and chemical treatment.
Owner:EMP BIOTECH
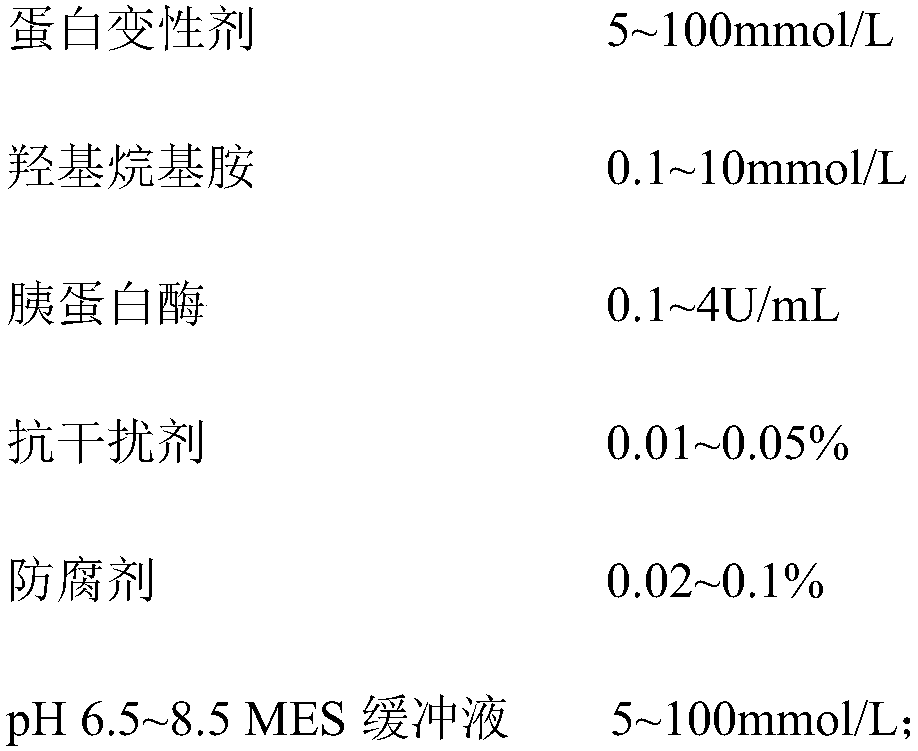
Kit for measuring glycolated serum albumin by enzymatic chemiluminescence method
ActiveCN107870170AImprove transgender abilityStrong specificityChemiluminescene/bioluminescenceConcentration ratioBoronic acid
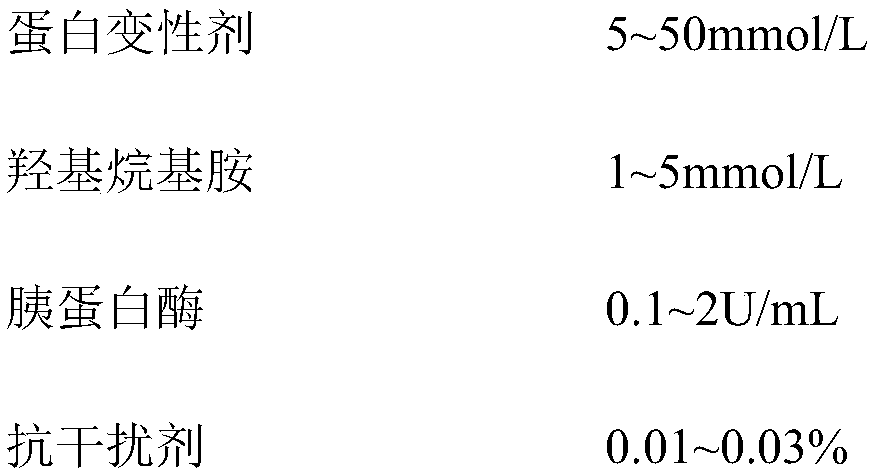
The invention belongs to the technical field of medical examination and particularly relates to a kit for measuring a concentration ratio of glycolated serum albumin and albumin. The kit comprises a reagent R1, a reagent R2, a reagent R3 and a reagent R4, wherein the reagent I contains a protein denaturant, hydroxyl-alkyl amine, trypsin, an anti-interference agent, a preservative and a buffer solution; the reagent II contains fructose amino acid oxidase, trehalose and a buffer solution; the reagent III contains amino acid oxidase, trehalose and a buffer solution; and the reagent IIII is a luminescent substrate 1,2-dioxetane boronic acid. The kit provided by the invention is similar with an existing enzymatic method detection kit in detection principle, but is lower in cost, high in interference resistance, accurate in detection, stable in property and convenient for extensive use.
Owner:GUANGZHOU JINDE BIOTECH
Chemiluminescent influenza diagnostic kit
InactiveUS7081352B2Sugar derivativesMicrobiological testing/measurementAbsorbent materialN-Acetylneuraminic acid
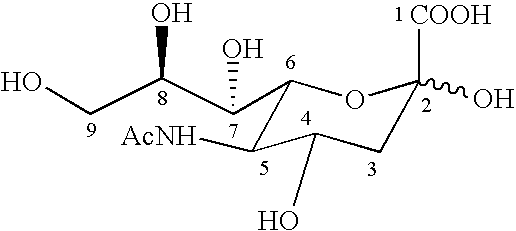
A chemiluminescent system for detecting the presence of influenza virus in a biological fluid sample is provided. An influenza diagnostic kit is provided which includes (1) a sampling device for obtaining the biological fluid from a subject, (2) a chemiluminescent substrate material which, in the presence of influenza virus in the biological sample, will generate a chemiluminescent product that will produce detectable light, and (3) a means for detecting any generated light. A liquid sample containing the biological fluid, and preferably a diluent, are contacted with the an absorbent material containing the chemiluminescent substrate material. The substrate responds to neuraminidase activity intrinsic to influenza A and influenza B virus particles, such that when the substrate is in contact with influenza virus, the substrate is cleaved to yield a chemiluminescent product that then decomposes to produce light which can then be detected. The chemiluminescent substrate materials include enzymatically triggerable 1,2-dioxetane derivatives of 4-alkoxy-N-acetylneuraminic acid and 4,7-dialkoxy-N-acetyineuraminic acid. The system is sufficiently simple that it can reliably be used by a layperson in a nonmedical setting. The biological fluid generally originates from the oral cavity, the pharyngeal cavity, or the nasopharyngeal cavity.
Owner:ZYMETX
Polymeric ammonium and or phosphonium salts having added pi-electrons and higher molecular weight as enhancers for chemiluminescent systems
InactiveUS7300766B2Reduce yieldImprovement of chemiluminescenceMicrobiological testing/measurementChemiluminescene/bioluminescencePhosphoniumPhosphonium salt
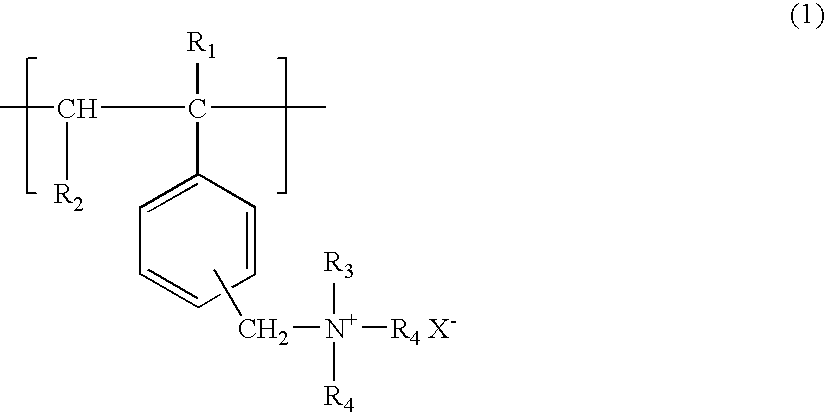
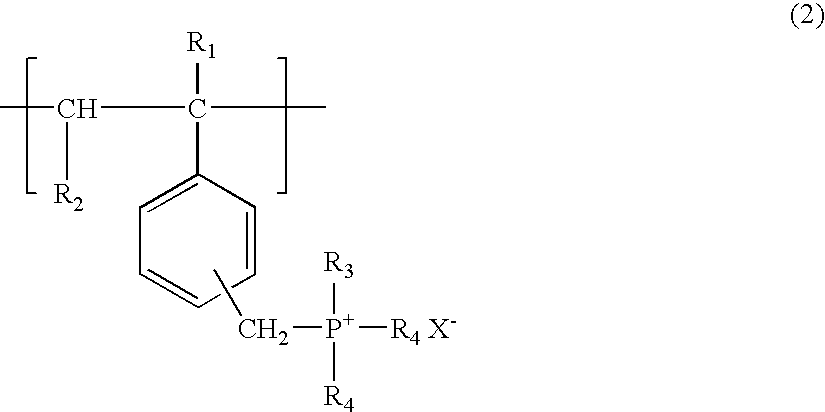
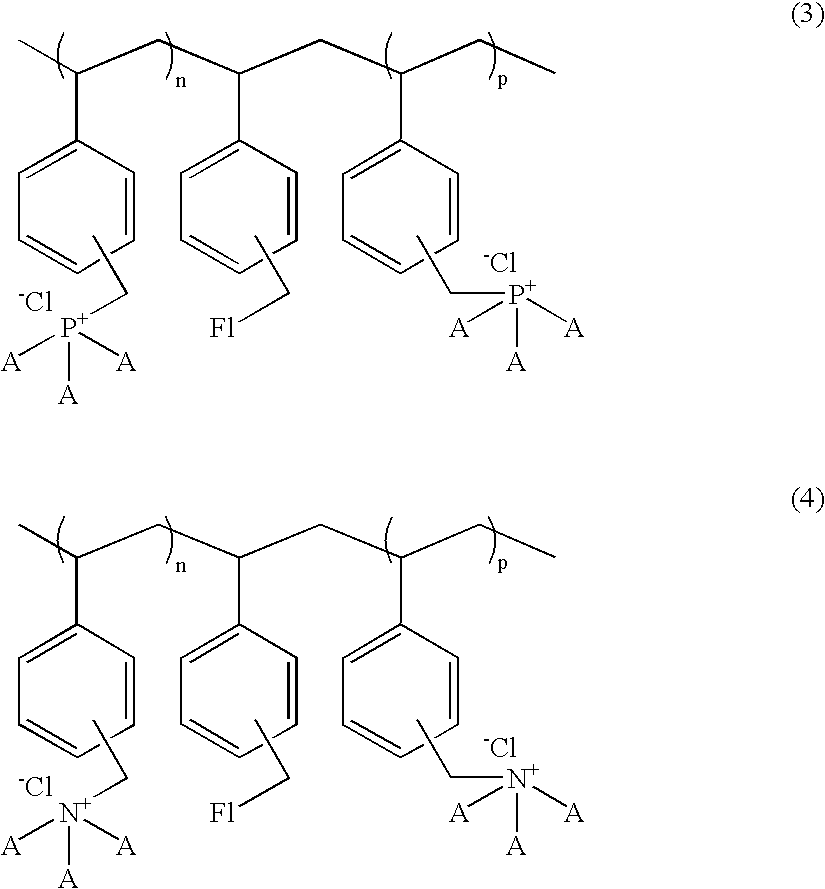
A compound for enhancing the light output of a chemiluminescent system and, in particular, a stabilized, triggerable 1,2-dioxetane, is obtained by reacting a polyvinylbenzyl halide and a bilinker with either a trisubstituted amine or trisubstituted phosphine. A fluorescent molecule may be attached to polymer along with the trisubstituted amine or trisubstituted phosphine. Also, the benzyl halide may be reacted with a π-electron donor, such as a vinyl naphthalene or vinylanthracene to form a co-polymer which is polymerizable with the trisubstituted amine and / or trisubstituted phosphine. By controlling the reaction parameters it is possible to obtain either a water-soluble or partial water soluble or insoluble compound crosslink polymer.
Owner:GIRI BRIJ P
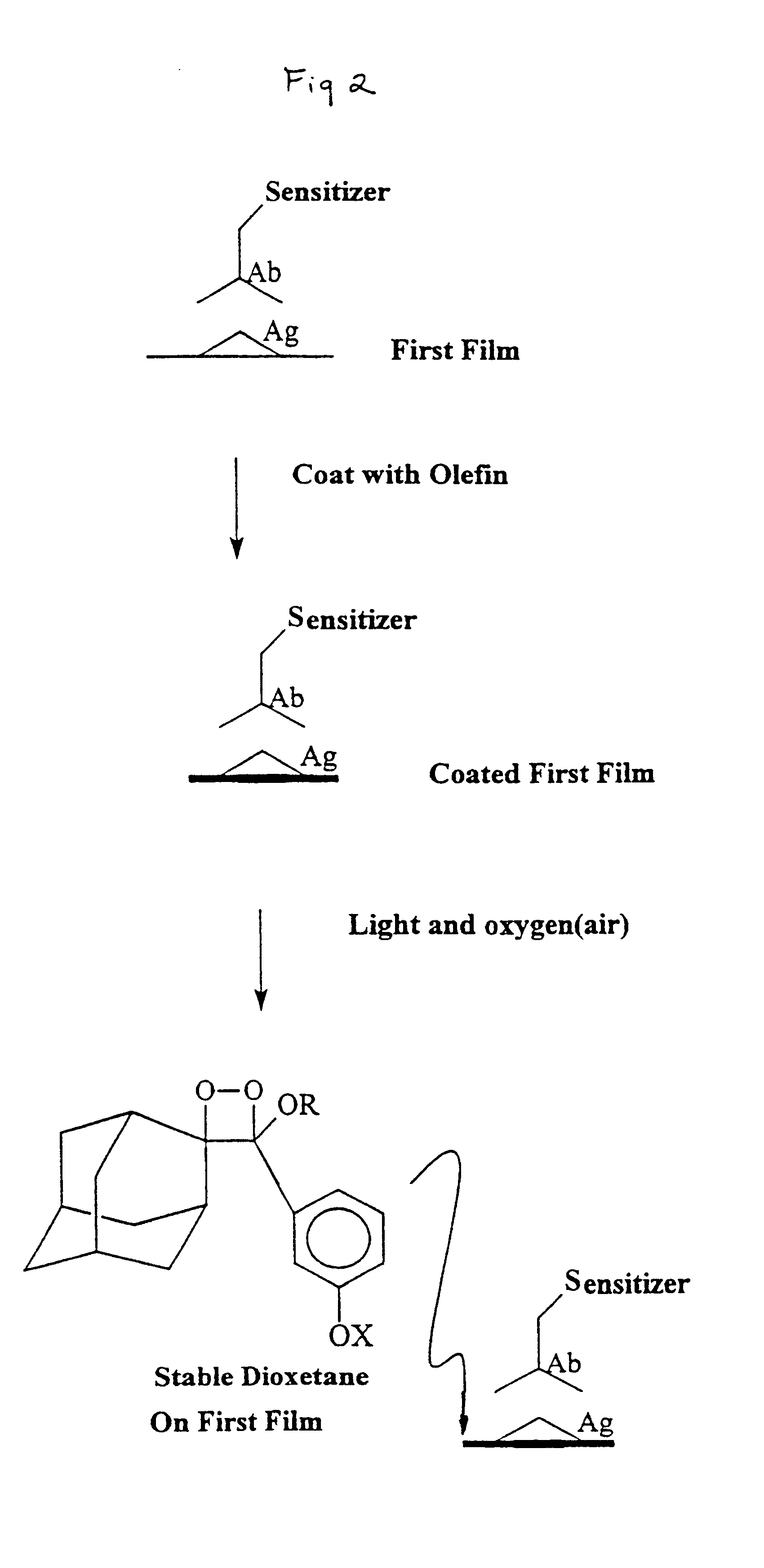
Sensitizer-labeled analyte detection
InactiveUS20040014043A1Microbiological testing/measurementChemiluminescene/bioluminescenceAnalyteChemical compound
The invention provides methods for detecting an analyte in a sample including the steps of: (a) exciting a sensitizer label on an analyte; (b) permitting energy from the excited sensitizer label to be transferred to and excite an acceptor molecule, whereby the sensitizer label returns to an unexcited state; (c) reacting the excited acceptor molecule with a chemiluminescent precursor to form a chemiluminescent compound which emits light in response to an activation source; (d) exposing the chemiluminescent compound to the activating source to produce a detectable signal; (e) detecting the signal; and (f) correlating the signal with the presence or absence of the analyte. The chemiluminescent precursor is desirably an olefin capable of being converted to a 1,2-dioxetane. Target amplification techniques, such as PCR, may be used to directly label a target analyte with a sensitizer.
Owner:EMP BIOTECH
Synthetic method of 1,2-dioxetane compound
ActiveCN102875600AHigh purityImprove luminosityGroup 5/15 element organic compoundsLuminescent compositionsPhosphoric Acid EstersPhosphate
The invention provides a synthetic method of 1,2-dioxetane compound. The synthetic method includes the steps: A) preparation of a carboxylic compound II; B) preparation of an ester compound III; C) preparation of an enol methyl ether compound IV; D) preparation of a phenol compound V; E) preparation of a phosphate ester compound; and F) preparation of a target compound VII. The synthetic method of 1,2-dioxetane compound is short in synthesis route, few in step, mild in reaction condition, simple and convenient to operate and high in yield, and the synthesized 1,2-dioxetane compound is high in purity and fine in luminescent performance and can be used as substrate in chemiluminescence reaction to be applied to the technical field of chemiluminescence immunoassay.
Owner:广东派特埃尔生物科技有限公司
Dioxetane labeled probes and detection assays employing the same
InactiveUS7053208B2Bioreactor/fermenter combinationsBiological substance pretreatmentsSinglet oxygenSteroid Compound
Probes labeled with 1,2-dioxetane precursors can be employed in a variety of assays. The probes may be nucleic acid, peptide nucleic acid, proteins including enzyme, antibody or antigen, steroid, carbohydrate, drug or non-drug hapten. The probe is provided with a 1,2-dioxetane precursor bound thereto, generally either covalently, or a strong ligand bond. The dioxetane precursor moiety is converted to a bound 1,2-dioxetane by exposure to singlet oxygen. These dioxetane (labels) either spontaneously decompose, or are induced to decompose by an appropriate trigger to release light. The trigger may be a change in pH temperature, or an agent which removes a protective group. Assay formats in which these 1,2-dioxetane labeled probes and referents may be used to include hybridization assays, immuno assays, gel-based assays and Capillary Zone Electrophoresis.
Owner:APPL BIOSYSTEMS INC
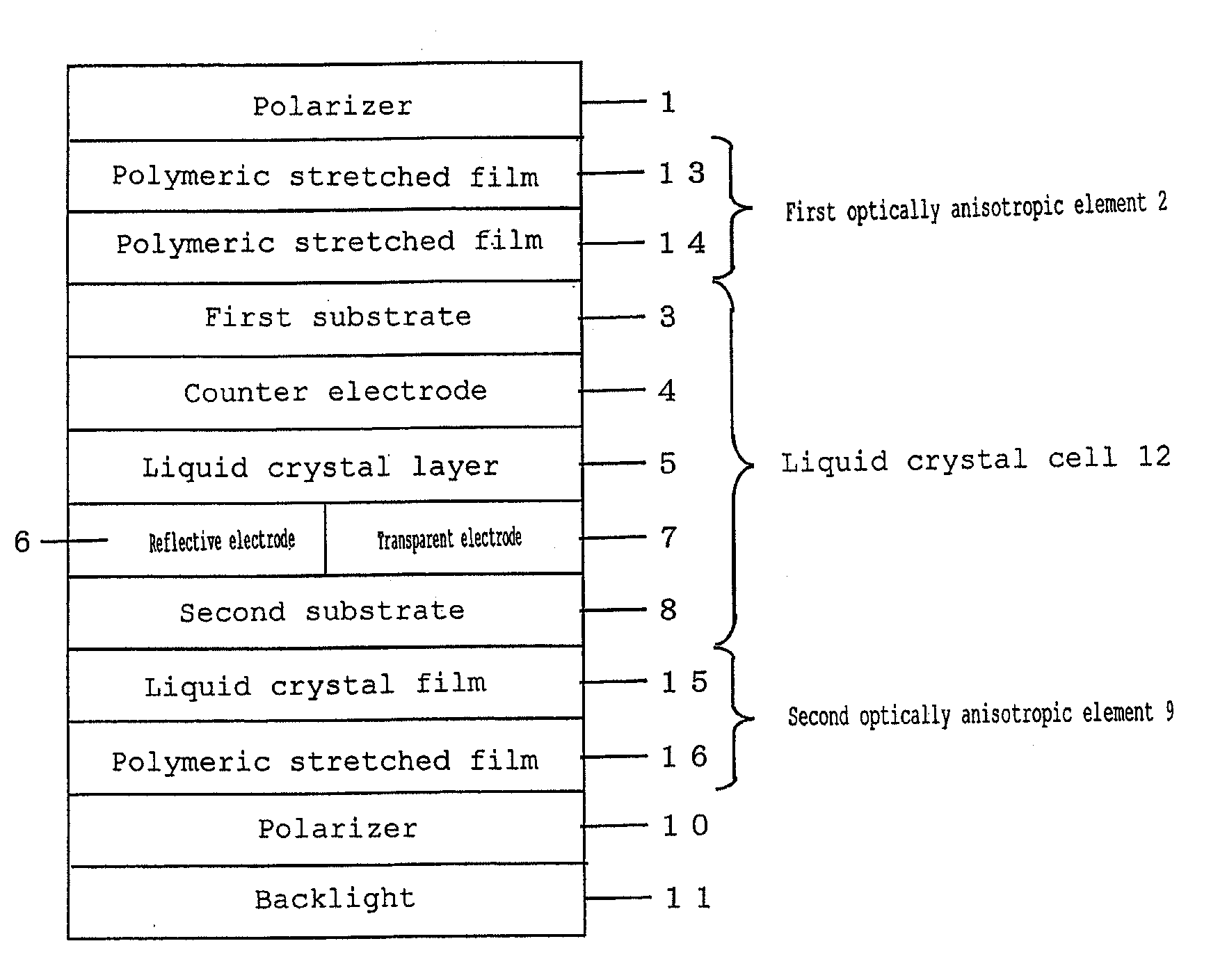
Dioxetane compound, cationically polymerizable composition, optical film, and liquid crystal display device
ActiveUS20090068379A1Easy to aggregateImprove retentionLiquid crystal compositionsOrganic chemistryLiquid crystallineCrystallography
A novel dioxetane compound is provided having a cationically polymerizable oxetane group, which compound is excellent in compatibility with a liquid crystalline compound and a non-liquid crystalline compound. An optical film is also provided with excellent liquid crystal orientation retention properties and mechanical strength, produced by aligning a composition of the dioxetane compound and a cationically polymerizable compound in a liquid crystal orientation and fixing the liquid crystal orientation by polymerization. Further, a liquid crystal display device is provided with the optical film.
Owner:NIPPON OIL CORP
Chemiluminescent 1,2-dioxetanes
InactiveUS7422908B2Easy to handleEasy to triggerDiffusing elementsChemiluminescene/bioluminescenceChemical reactionReactive site
A method of generating light through chemiluminescence involves providing a stable 1,2-dioxetane of the formula:Wherein (a) R1 and R2 are each, individually, a chemical reactive site or when fused together form a chemical reactive site, and R3 and R4 are each, individually, a chemical reactive site or when fused together form a chemical reactive or (b) R1 has at least two hetero atoms with chemical reactive site and R3 and R4 are inactive site and R2 is a chemical reactive site.
Owner:GIRI BRIJ P
Dioxetane compound, cationically polymerizable composition, optical film, and liquid crystal display device
InactiveUS7998543B2Easy to synthesizeImprove compatibilityLiquid crystal compositionsOrganic chemistryCrystallographyLiquid crystalline
A novel dioxetane compound is provided having a cationically polymerizable oxetane group, which compound is excellent in compatibility with a liquid crystalline compound and a non-liquid crystalline compound. An optical film is also provided with excellent liquid crystal orientation retention properties and mechanical strength, produced by aligning a composition of the dioxetane compound and a cationically polymerizable compound in a liquid crystal orientation and fixing the liquid crystal orientation by polymerization. Further, a liquid crystal display device is provided with the optical film.
Owner:NIPPON OIL CORP
Ultra-sensitive chemiluminescent substrates for enzymes and their conjugates
New chemiluminescent compounds, stable in aqueous buffers, for use in biological assaying include acridanebased compounds and (1,2)-dioxetanes. Among the new acridanebased compounds are water-soluble acridanes, enhancer coupled acridanes, bis and trisacridanes as well as acridane-(1,2)-dioxetanes. Among the new (1,2)-dioxetanes are electron deficient group-containing dioxetanes and tethered bis-1,2-dioxetanes. The (1,2)-dioxetanes are useful as substrates for various enzymes. The acridanes can be admixed with an oxidizing agent. An aqueous buffer and, optionally, a stabilizer to form a substrate or reagent formulation useful for assaying, inter alia, HRP.
Owner:MICHIGAN DIAGNOSTICS
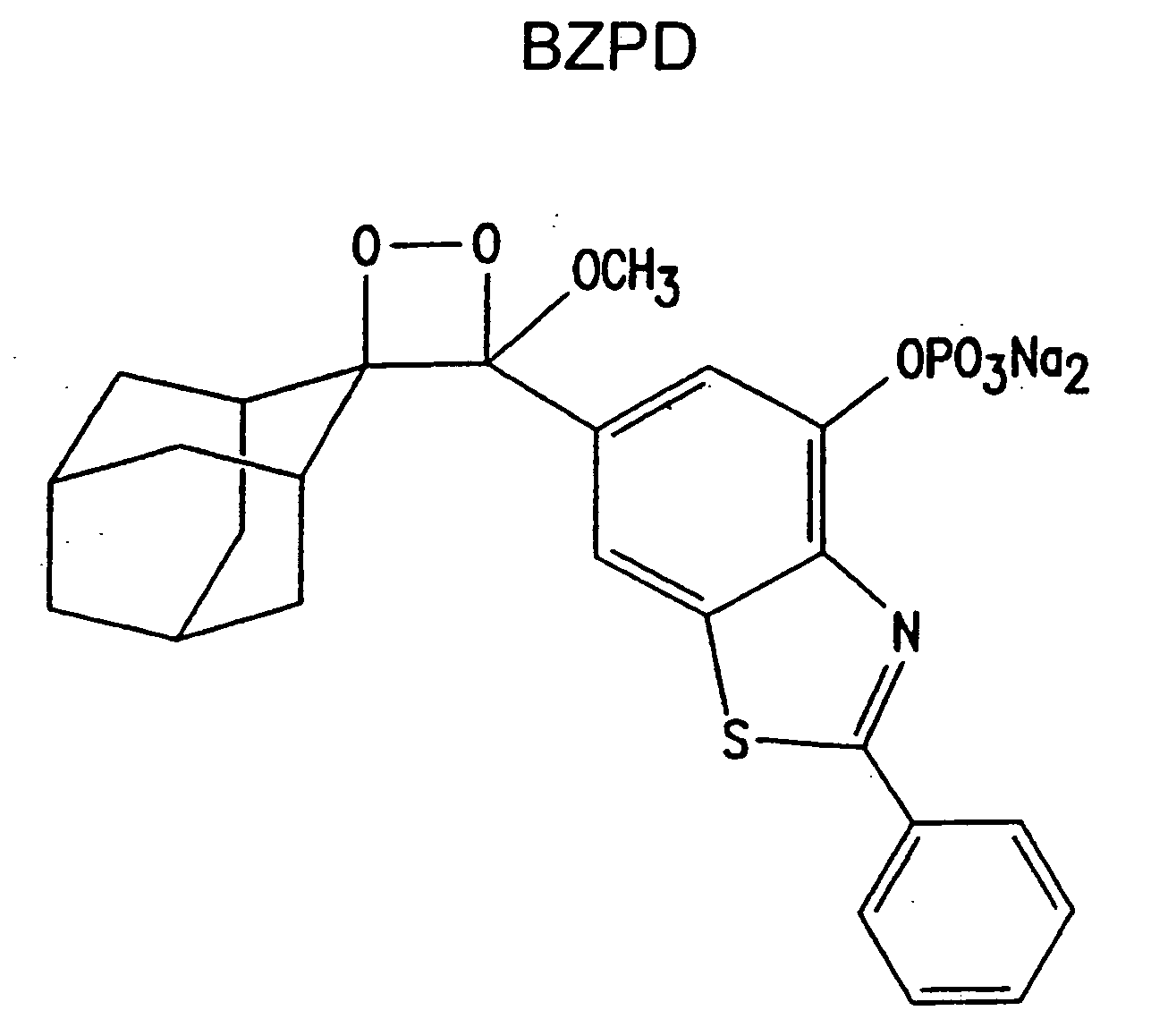
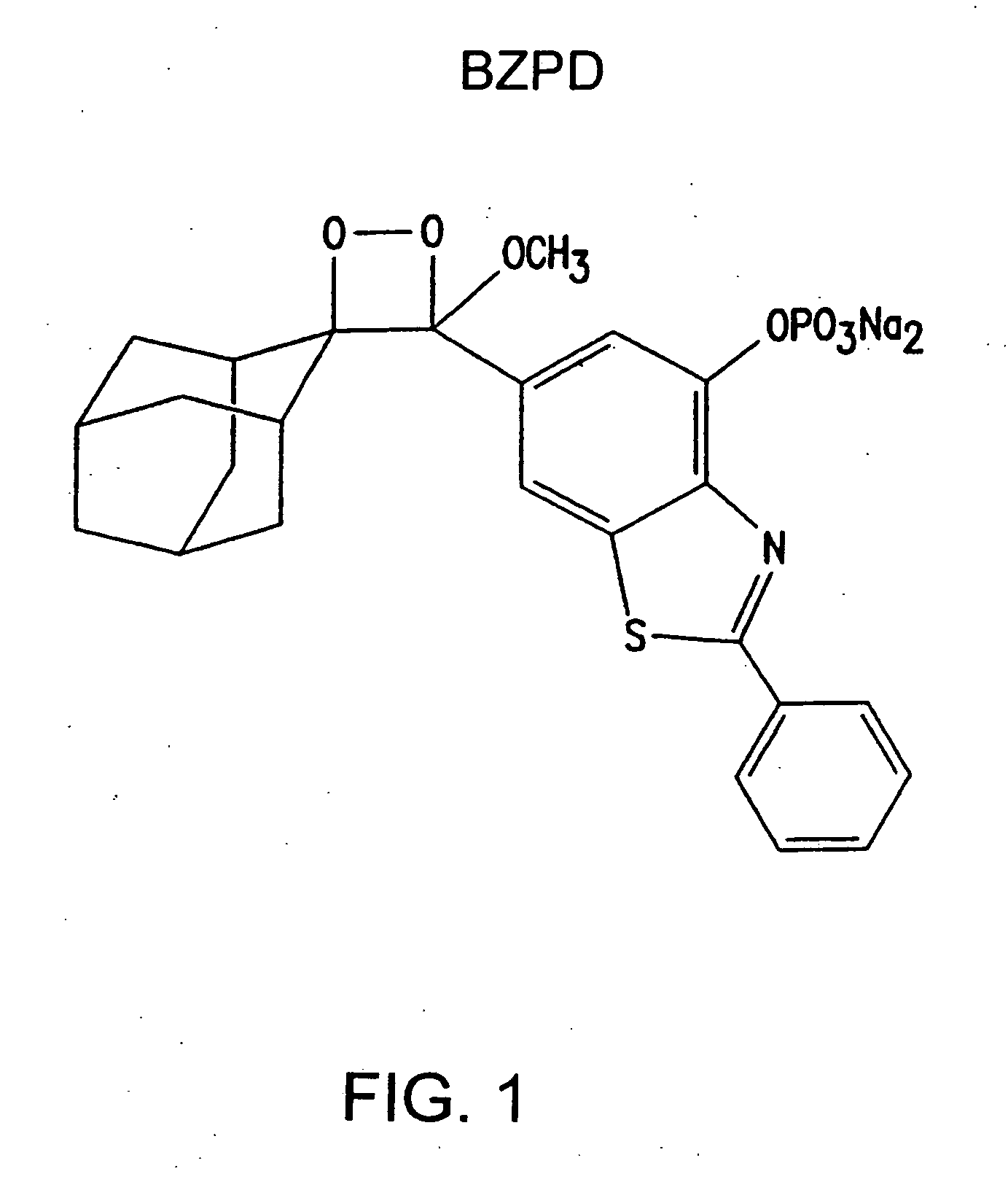
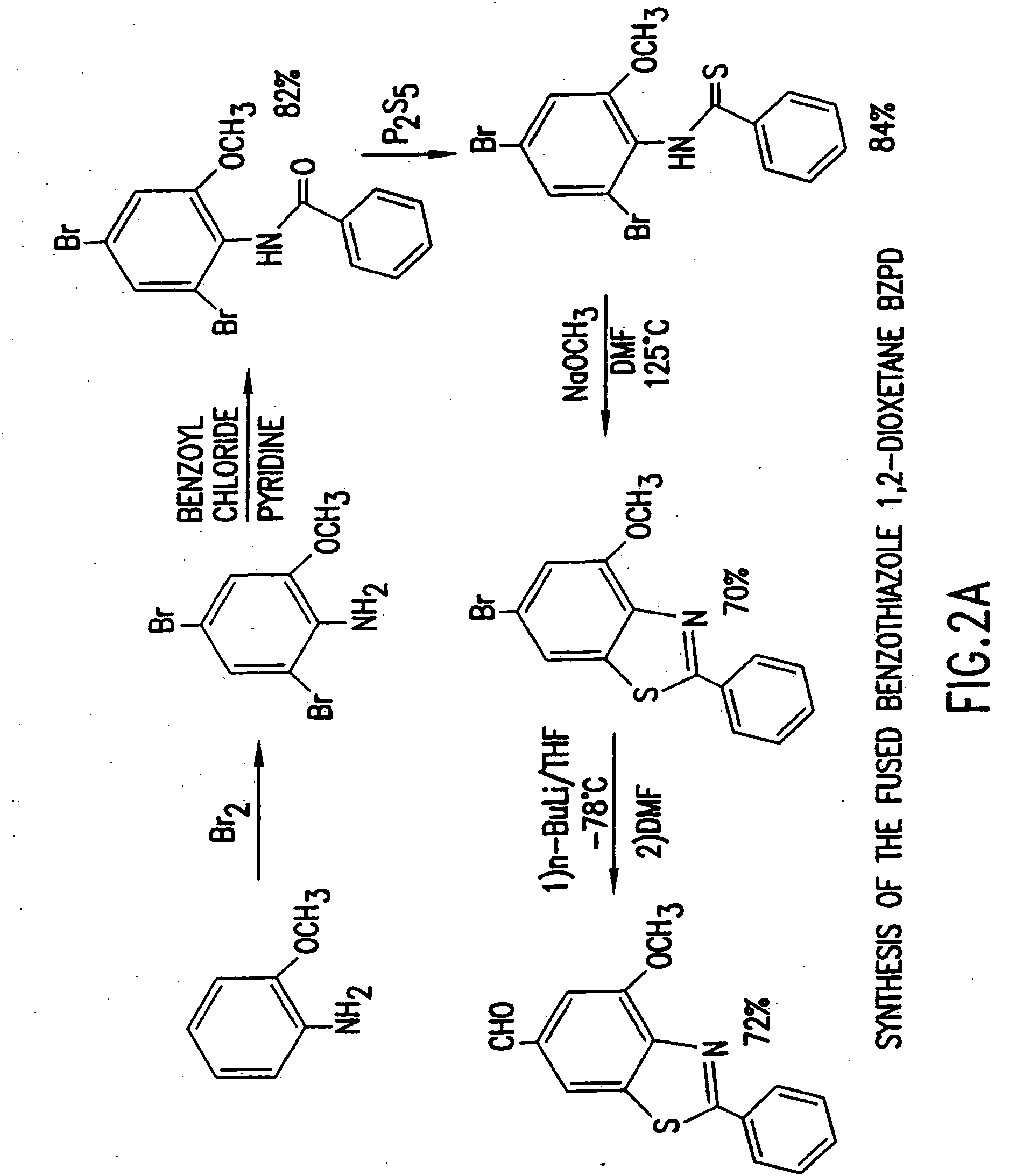
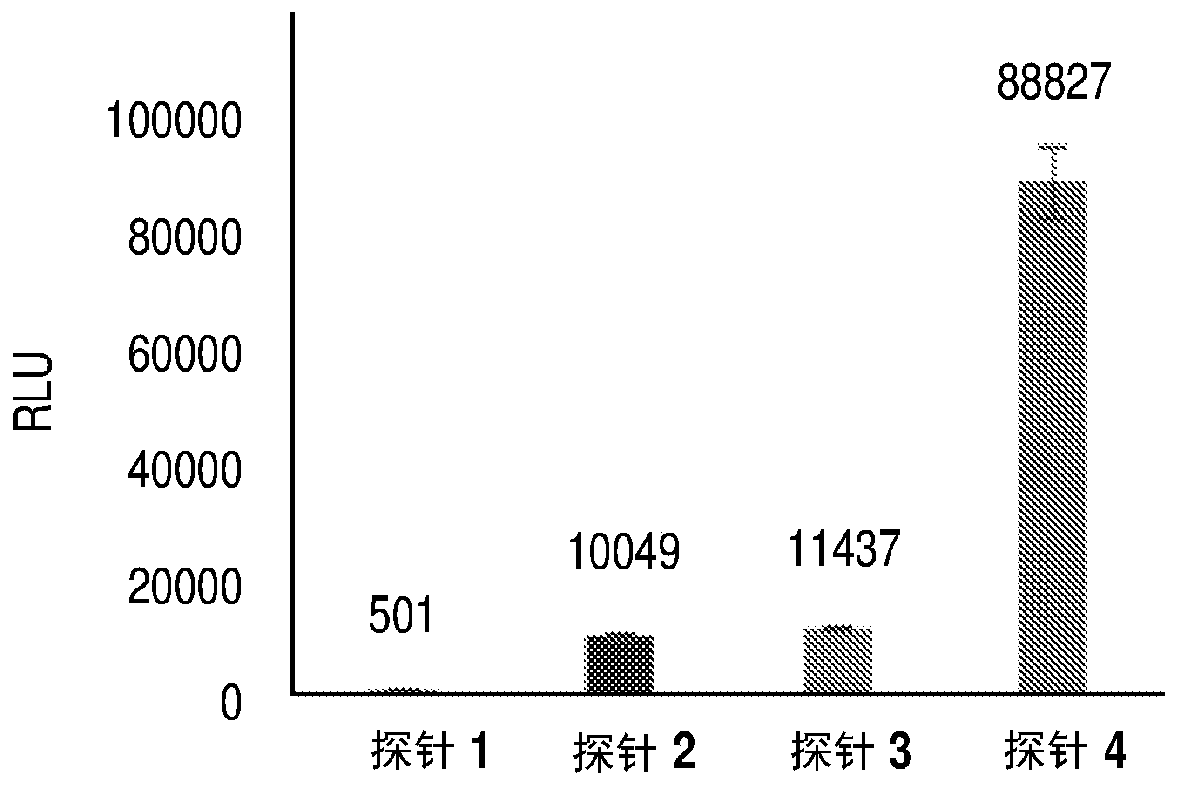
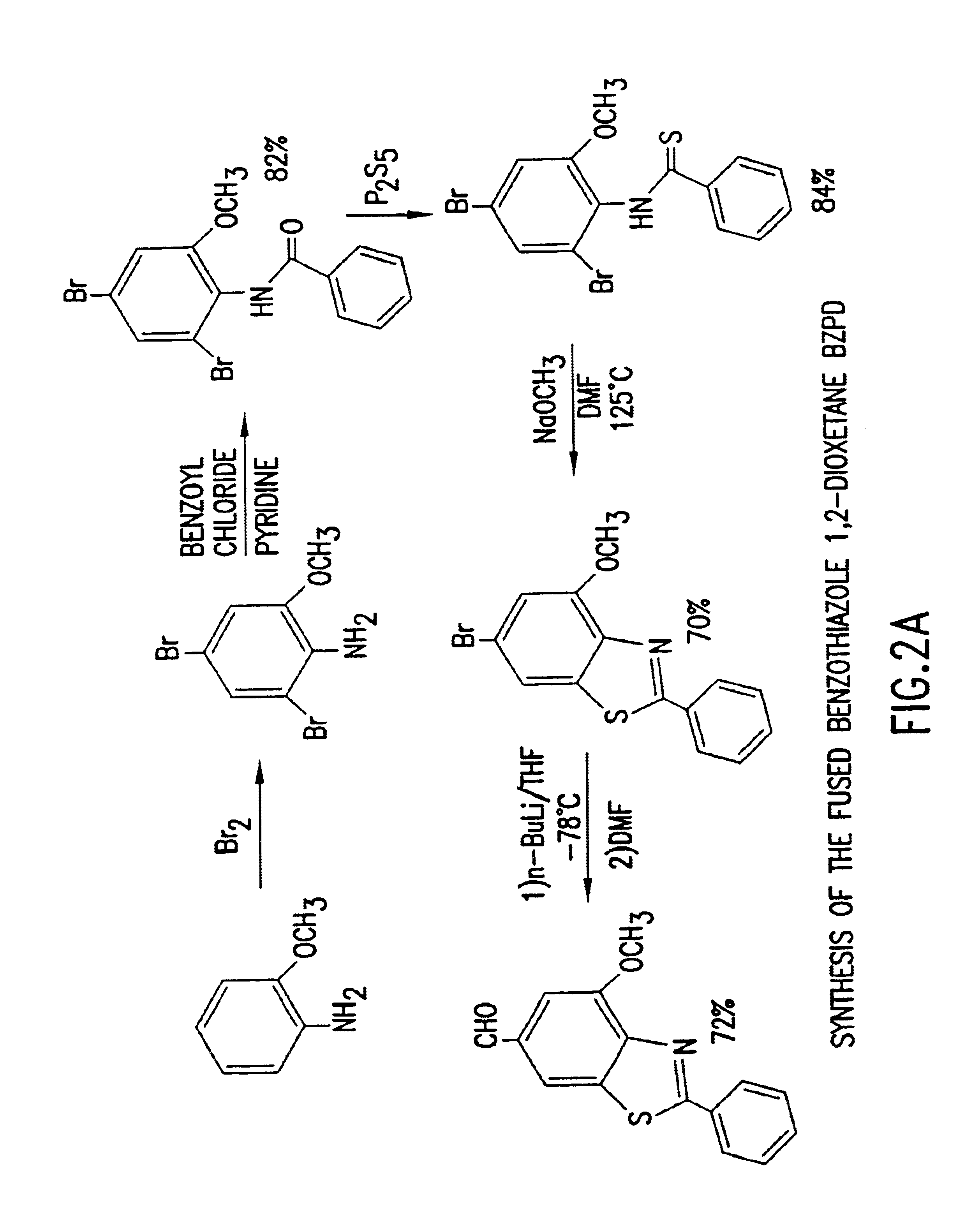
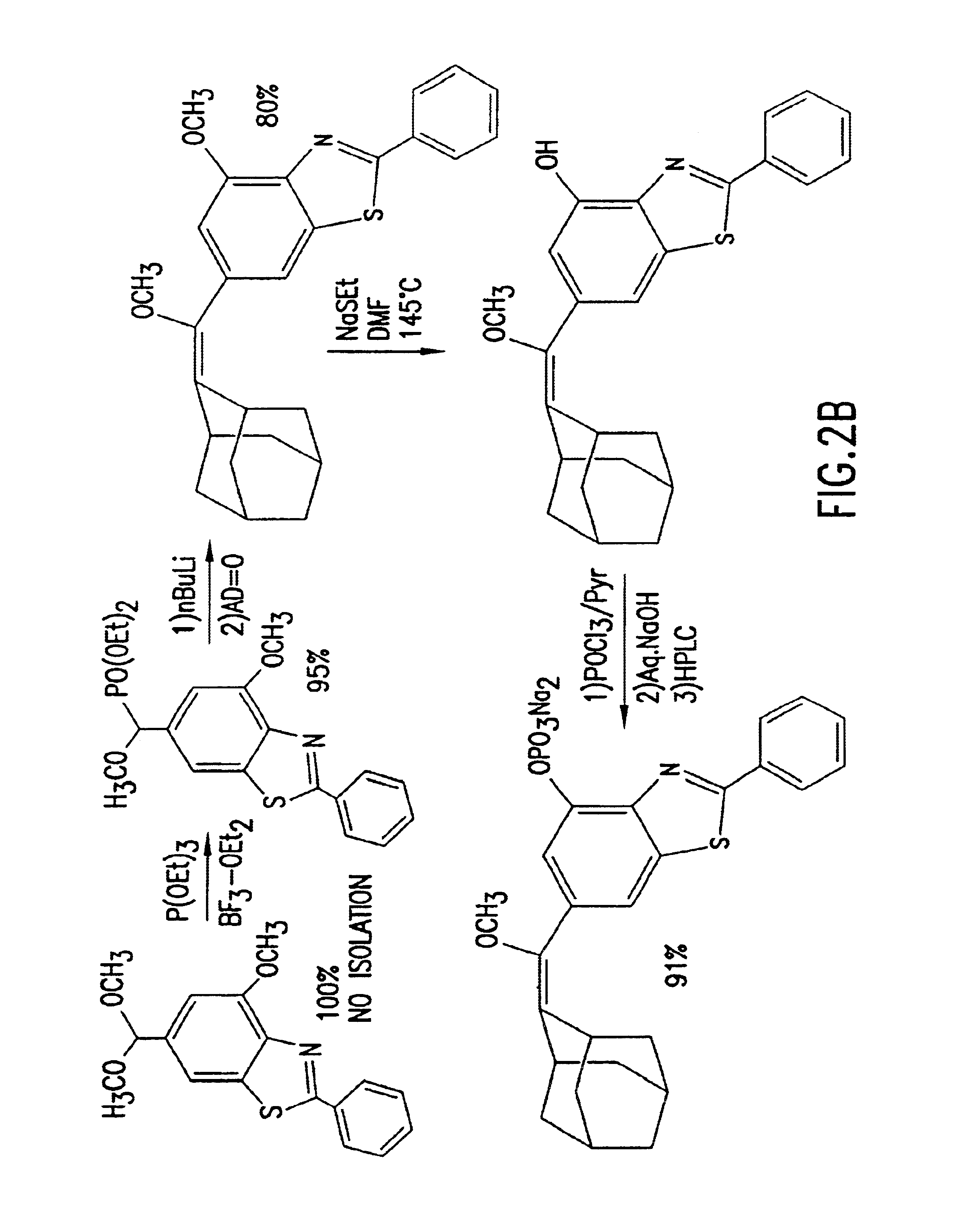
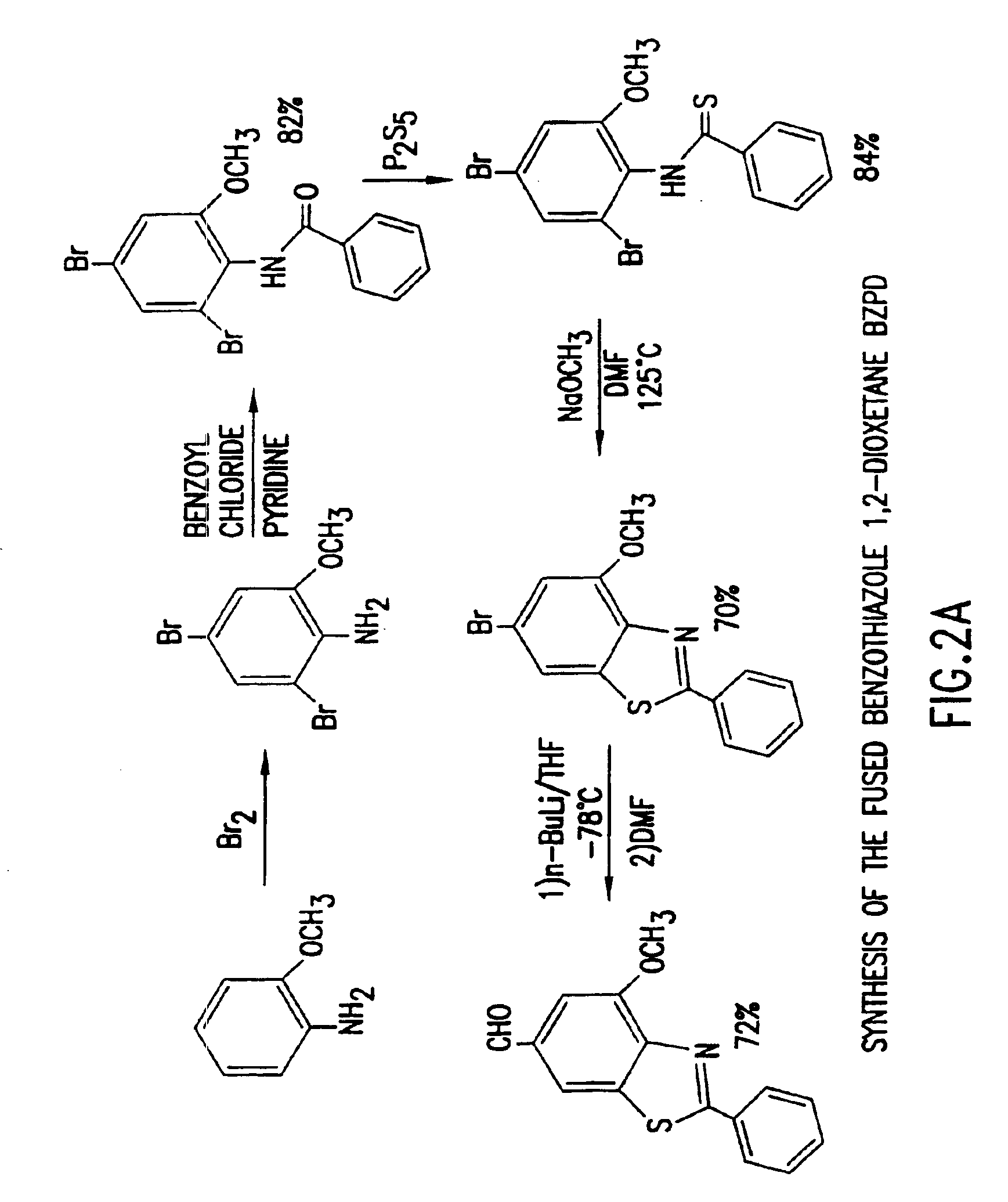
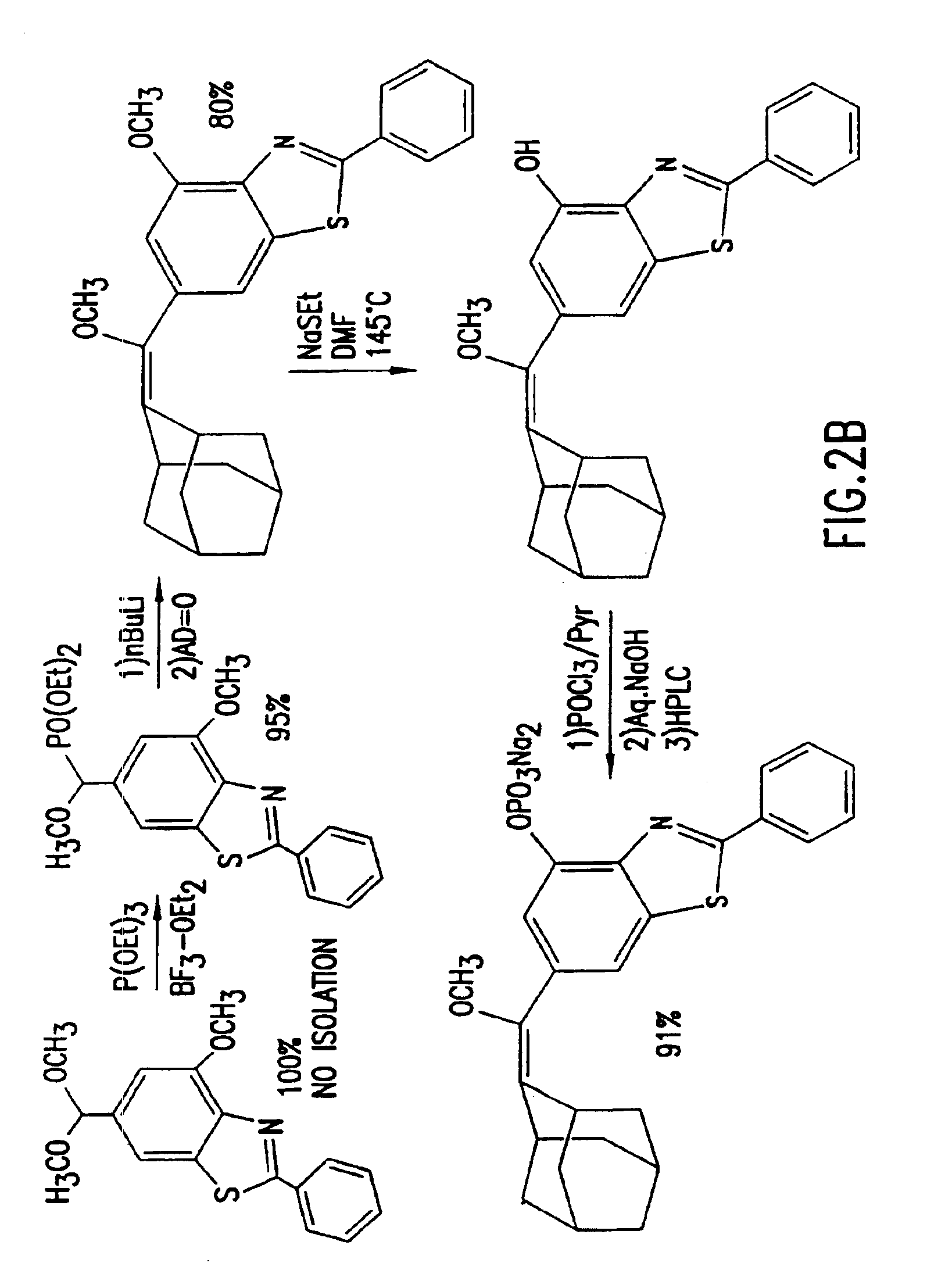
Heteroaryl substituted benzothiazole dioxetanes
Chemiluminescent heteroaryl substituted benzothiazole 1,2-dioxetane compounds capable of producing light energy when decomposed are provided. These chemiluminescent compounds are represented by the general formula: The heteroaryl substituent Y can be, for example, a pyridyl group or a benzothiazolyl group. The heteroaryl substituted benzothiazole compounds are substantially stable at room temperature. Kits including the heteroaryl substituted dioxetane compounds as well as methods for using these compounds for detecting the presence of one or more analytes in a sample are also provided.
Owner:EDWARDS BROOKS +2
Long wavelength emitting chemiluminescent probes
InactiveCN112469705AMaterial analysis by optical meansGroup 3/13 element organic compoundsAtomic physicsChemistry
The present invention relates to dioxetane compounds of Formula la or lb, a compound of Formula II and their applications.
Owner:尼米斯技术公司 +1
Synthetic method of 1, 2-dioxetane compound
InactiveCN103073589AReduce riskThe process is easy to realizeGroup 5/15 element organic compoundsOrganic solventUltrasound irradiation
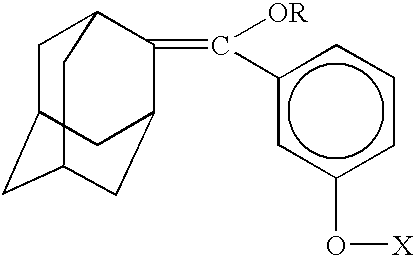
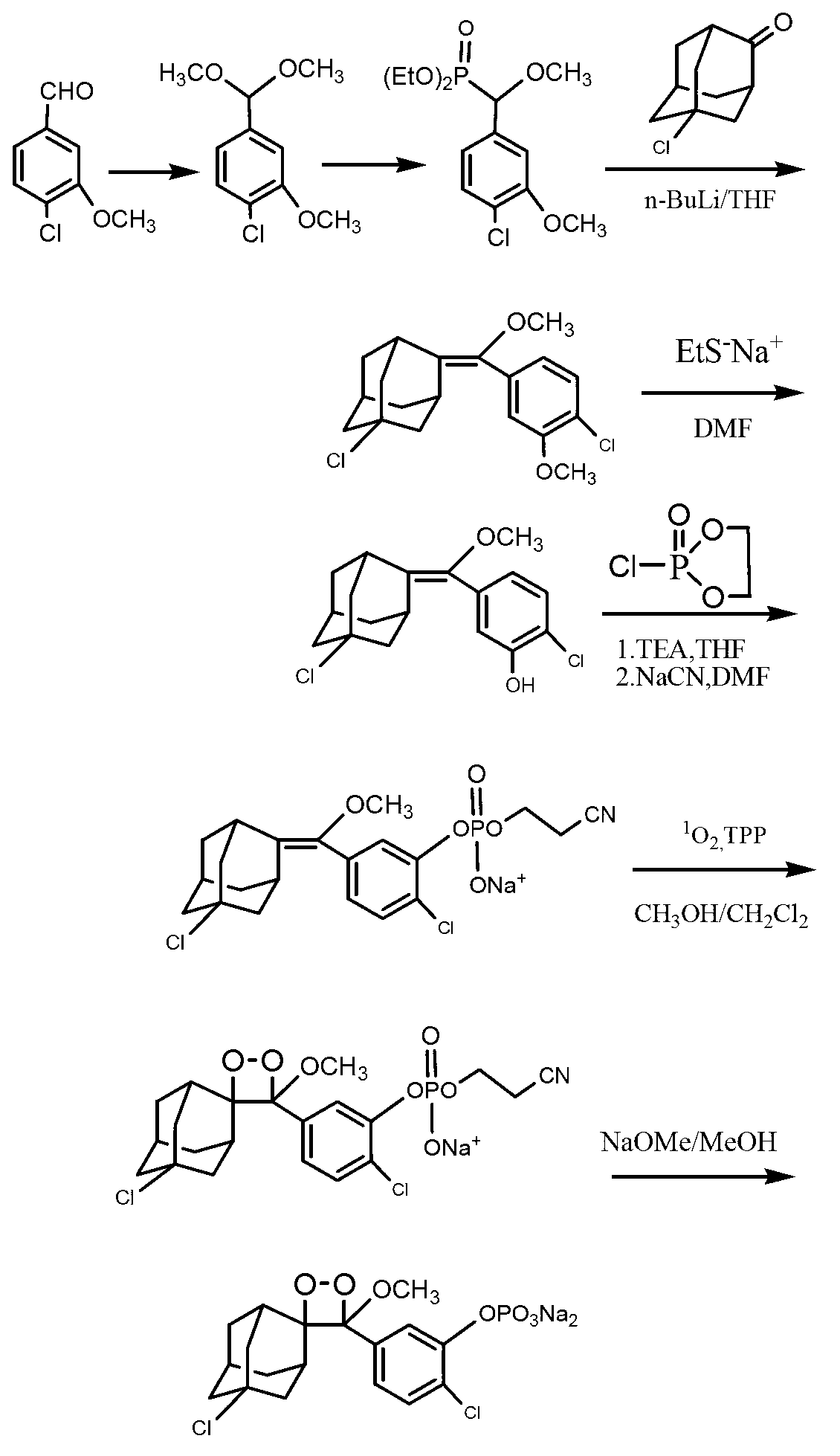
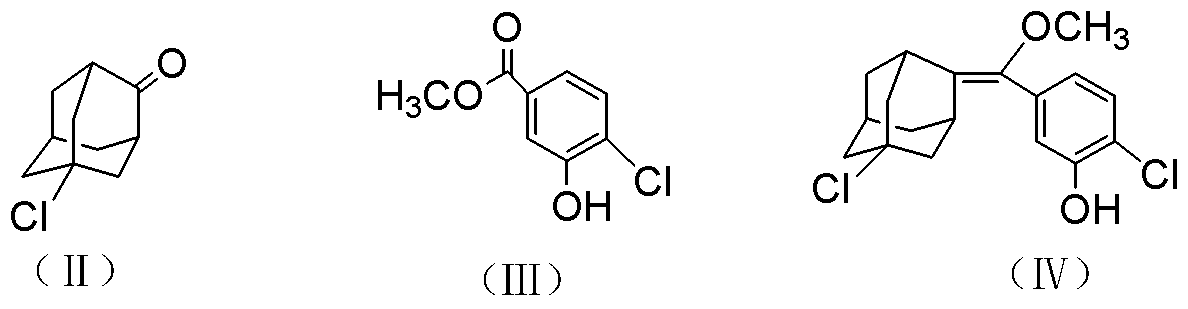
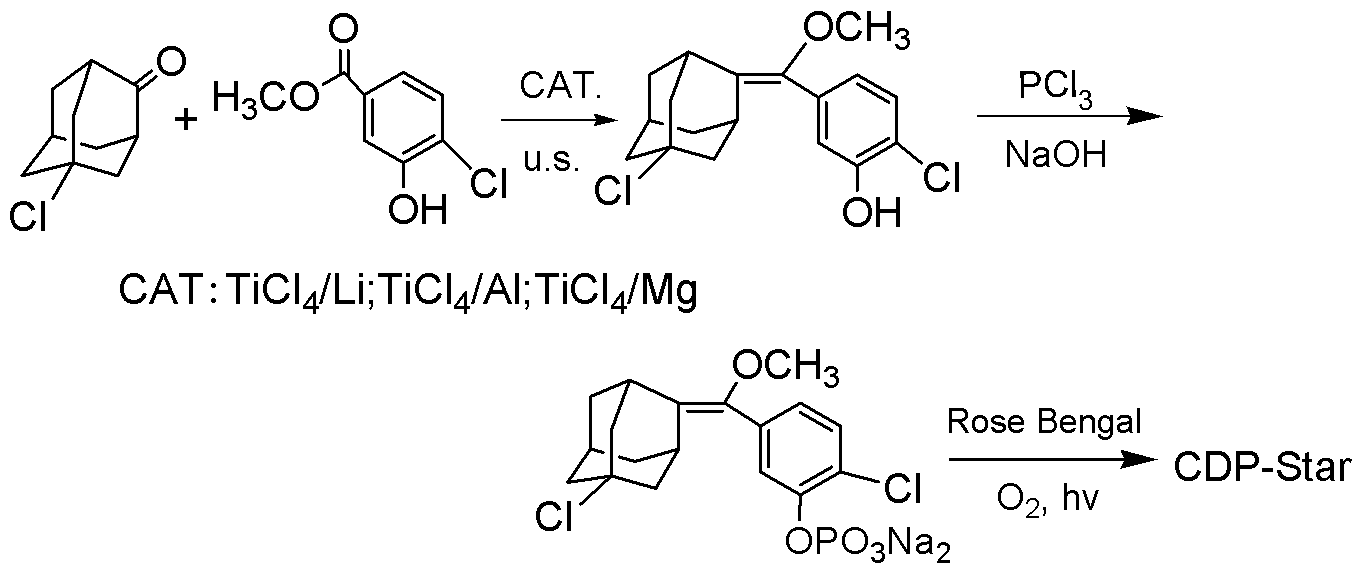
The invention discloses a synthetic method of a 1, 2-dioxetane compound. The method takes 5-chloro-2-adamantanone and 4-chloro-3-methyl hydroxybenzoate as the raw materials, and includes the steps of reacting to prepare substituted phenyl methoxymethene adamantine in an organic solvent under ultrasonic and the actions of a catalyst and triethylamine; and subjecting substituted phenyl methoxymethene adamantine to a photosensitized oxidation reaction to prepare the 1, 2-dioxetane compound. The new catalytic systems used in the method include general goods all in mass production, therefore the risk is low, and technical magnification and industrialization are easy to achieve; compared with the traditional organic synthesis method, the synthetic method adopts ultrasound irradiation, so that reaction conditions can be improved, the reaction temperature can be reduced, the reaction can be accelerated, and the reaction yield can be improved.
Owner:HANGZHOU NORMAL UNIVERSITY
Chemiluminescent probe for detecting fibroblast activating protein as well as synthesis method and application of chemiluminescent probe
ActiveCN113024523ARealize detectionOrganic chemistryChemiluminescene/bioluminescenceBlood plasmaPhotochemistry
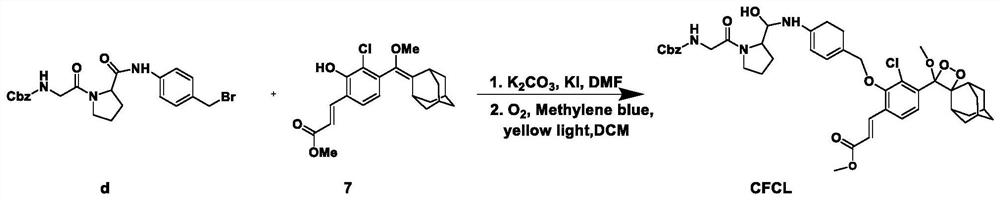
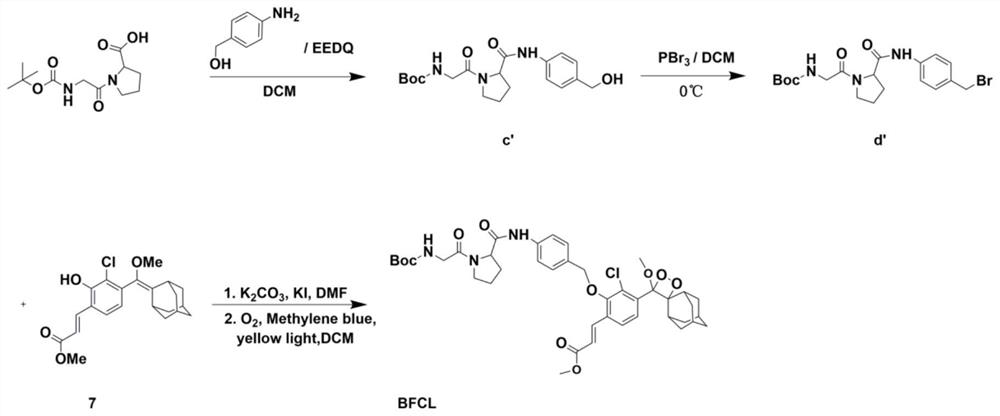
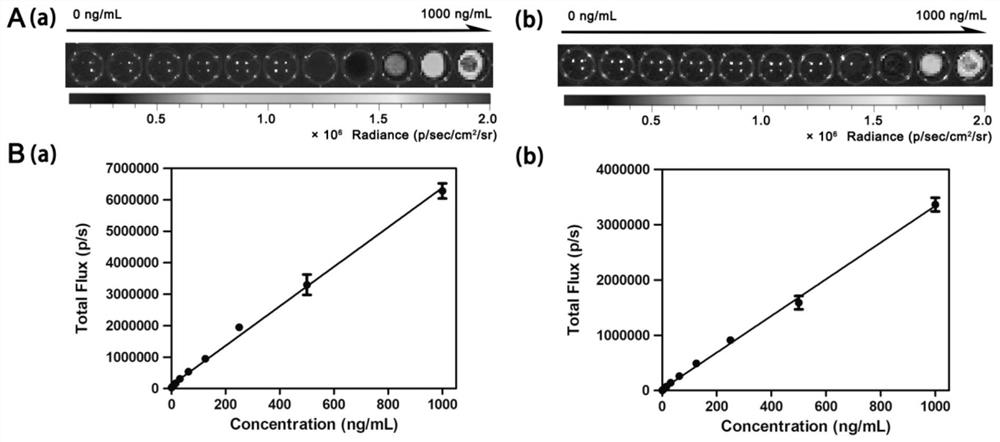
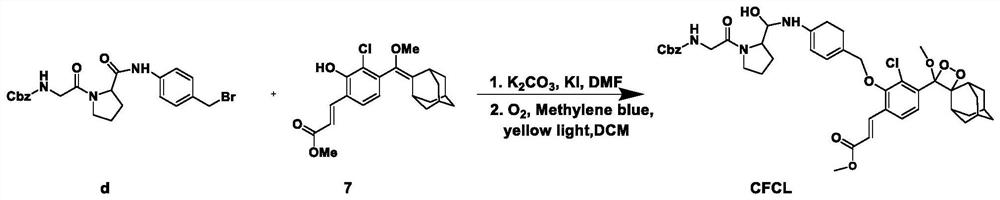
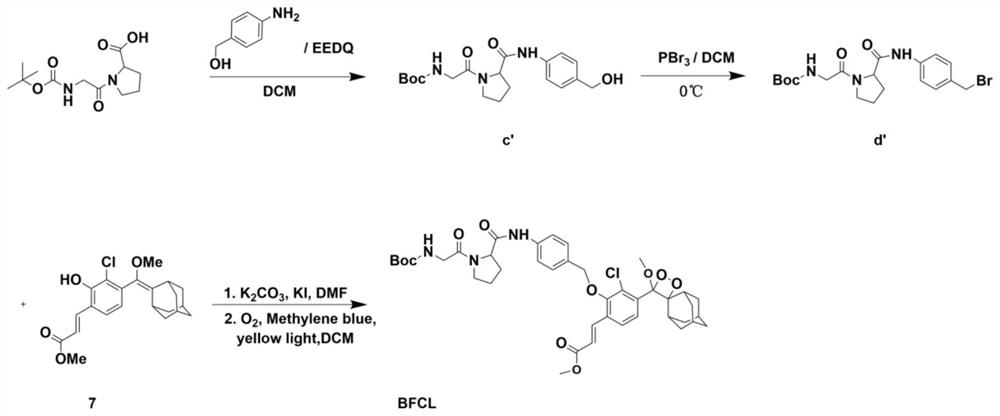
The invention belongs to the technical field of small-molecule chemiluminescence probes, and particularly relates to a chemiluminescence probe for detecting fibroblast activation protein (FAP[alpha]) as well as a synthesis method and application of the chemiluminescence probe. The chemiluminescent probe disclosed by the invention is an adamantane-dioxetane chemiluminescent probe and comprises two types, namely CFCL and BFCL. An adamantane-dioxetane derivative and an FAP[alpha] sensitive group are subjected to a high performance liquid chromatography monitoring reaction in an anhydrous inert solvent in the presence of an acid-binding agent and a catalyst, and the adamantane-dioxetane chemiluminescent probe is prepared. The chemiluminescent probe can be used for in-vivo and in-vitro detection of FAP[alpha], including determination of FAP[alpha] in cells, tissues or plasma, and imaging of FAP[alpha] in animal bodies. The chemiluminescent probe can also be used for evaluating and screening FAP[alpha] inhibitors. According to the present invention, the adamantane-dioxetane is improved to obtain the chemiluminescence probe, such that the FAP[alpha] detection is achieved, the important technical breakthrough is provided, and the application range of the chemiluminescence probe is further expanded.
Owner:FUDAN UNIV +1
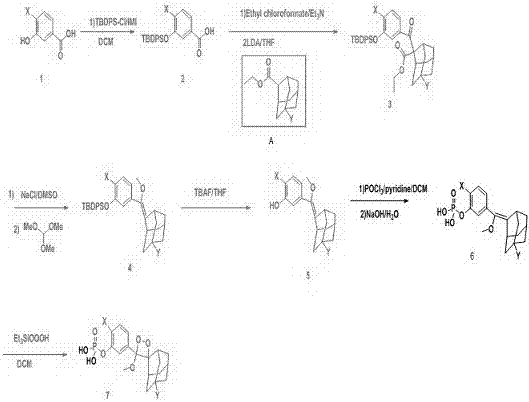
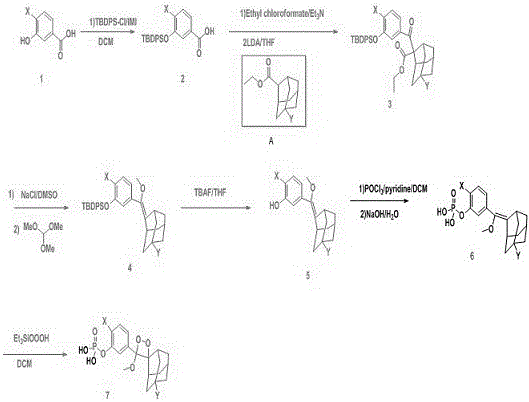
Preparation method of 1, 2-dioxetane derivative
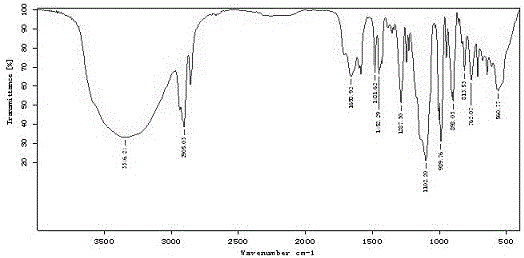
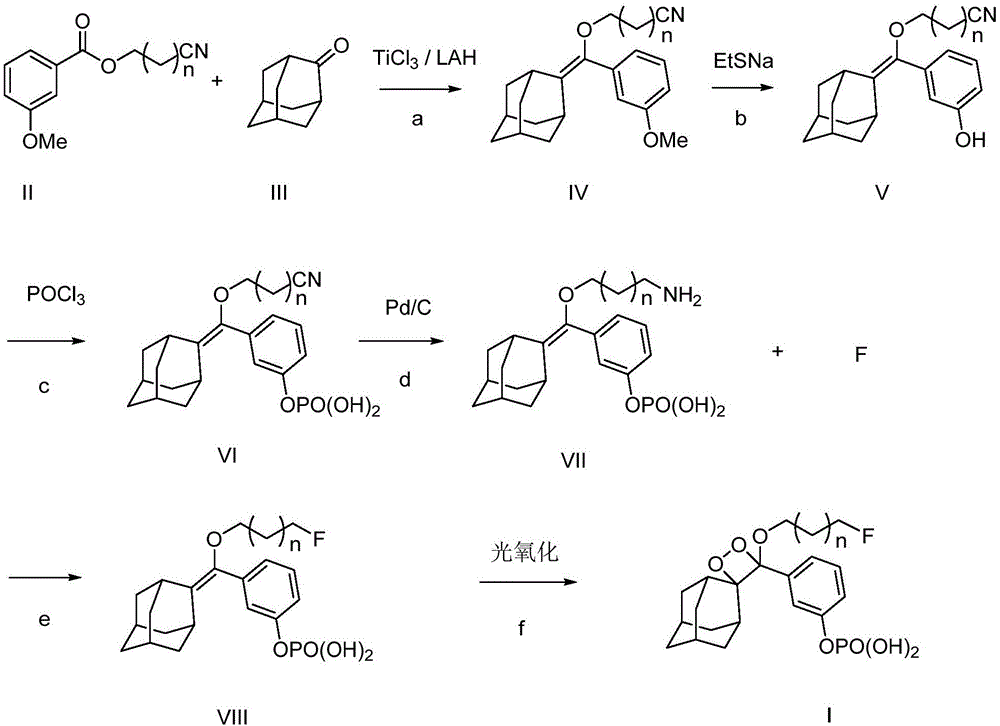
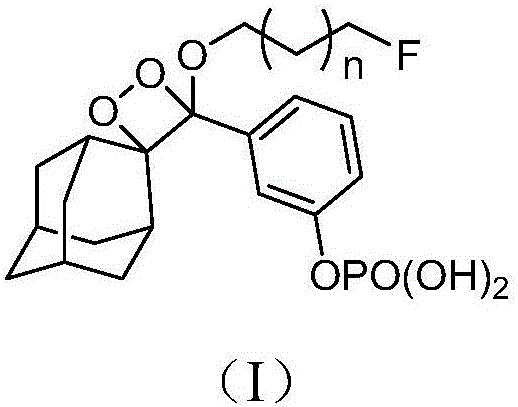
ActiveCN105085569AAvoid uneven responseAvoid safety hazardsGroup 5/15 element organic compoundsWittig reactionPhosphorylation
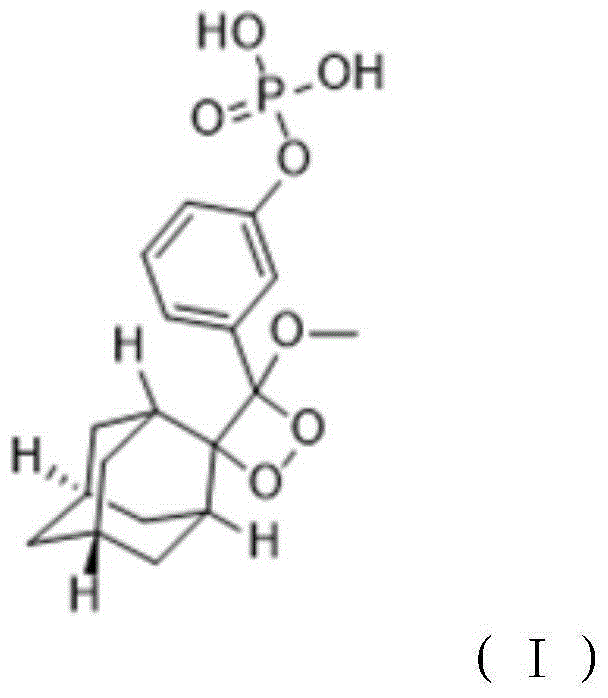
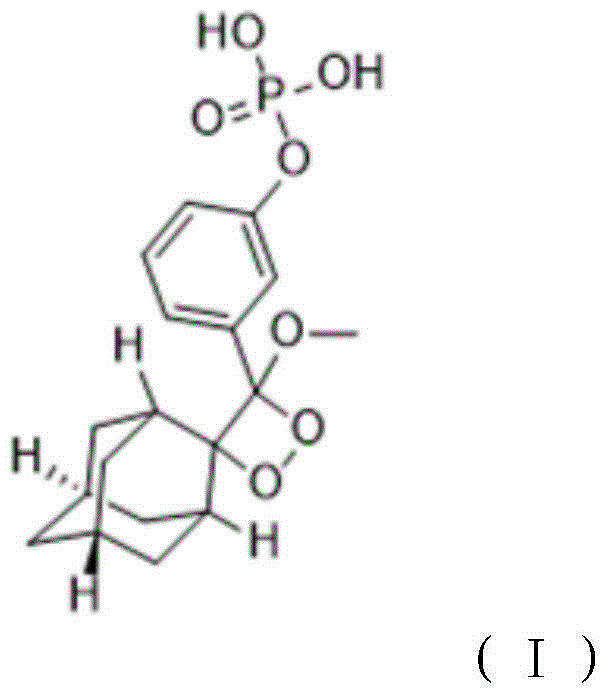
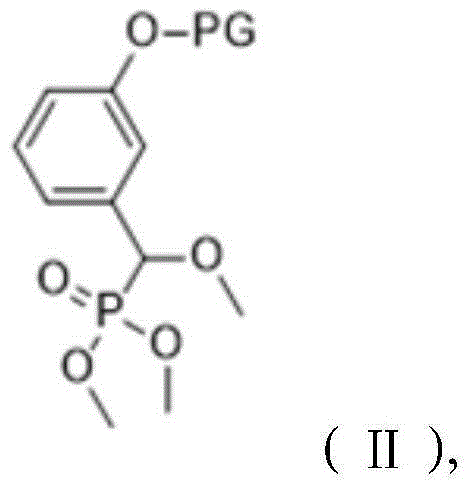
The invention relates to a preparation method of a 1, 2-dioxetane derivative. The 1, 2-dioxetane derivative is a compound shown in a formula I. The preparation method comprises the following steps of taking a compound shown in a formula II and adamantanone; performing Wittig reaction to synthesize a compound shown in a formula III; performing phosphorylation to obtain a compound shown in a formula IV; and performing oxidation reaction to obtain the 1, 2-dioxetane derivative. The preparation method of the 1, 2-dioxetane derivative has the advantages that safety is high; production cost is low; and industrial production of the 1, 2-dioxetane derivative is facilitated.
Owner:SUZHOU YACOO SCI CO LTD
Chemiluminiscence substrate with high luminescence intensity, long wavelength and good stability and preparation method and application of chemiluminiscence substrate
PendingCN110804009AHigh strengthImprove stabilityChemiluminescene/bioluminescenceGroup 3/13 element organic compoundsLiving bodyLuminescence
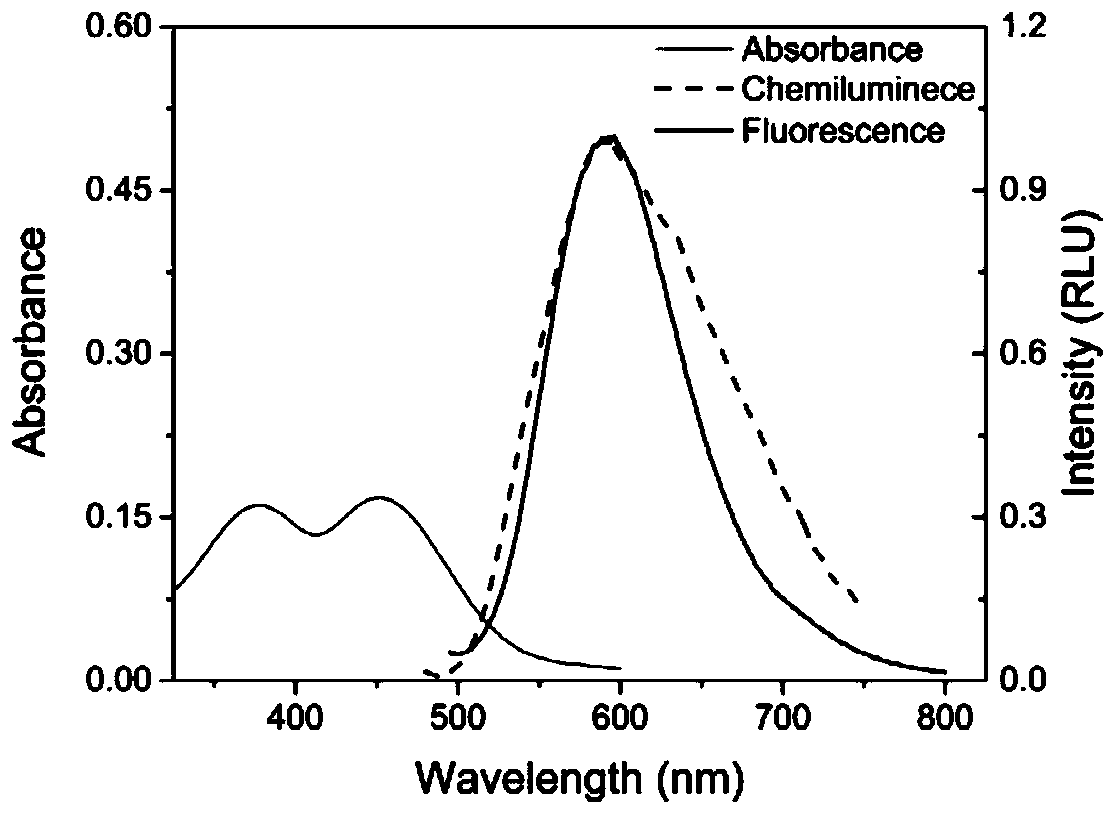
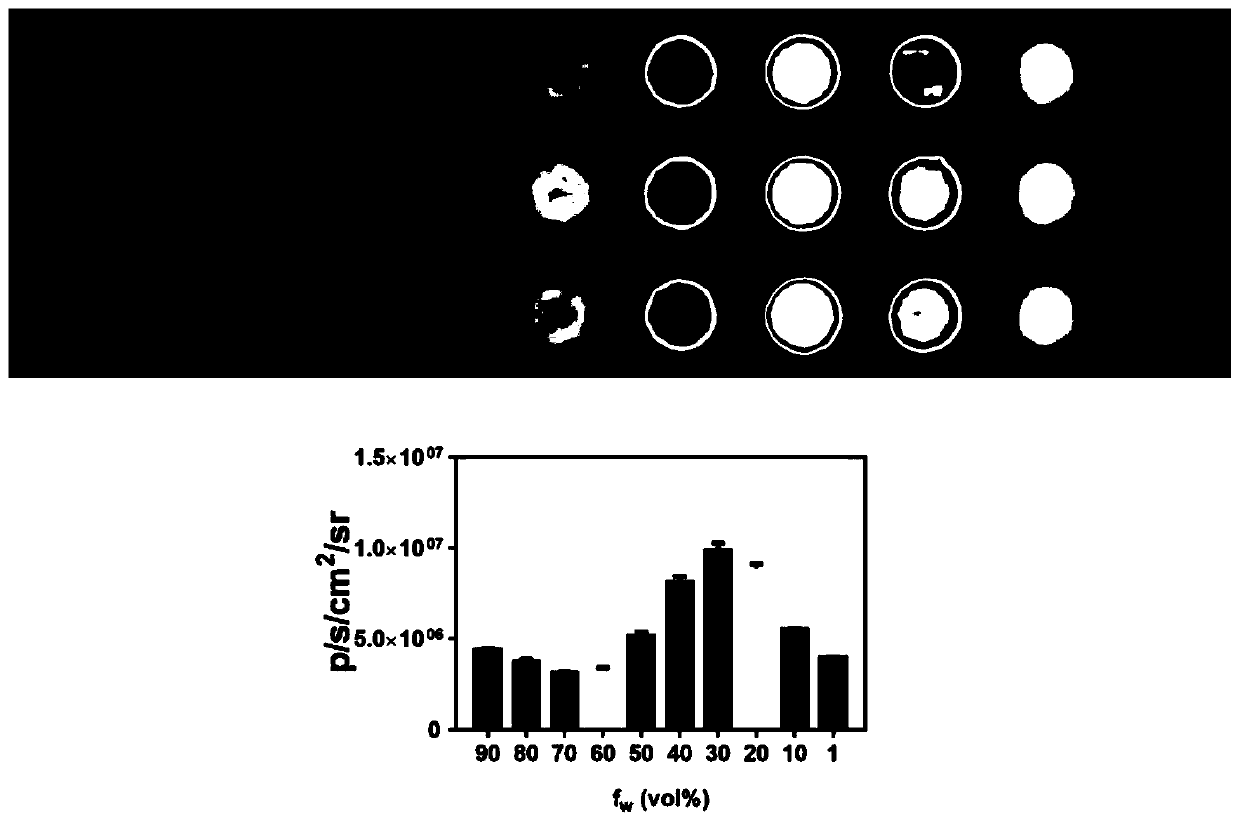
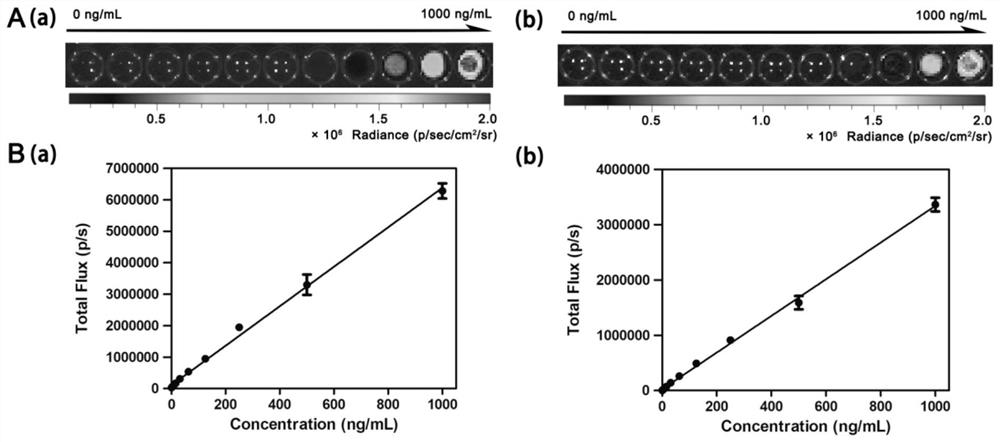
The invention provides a chemiluminiscence substrate with high luminescence intensity, a long wavelength and good stability and a preparation method and application of the chemiluminiscence substrate.The chemiluminiscence substrate is of a structure of a formula I shown in the description. In addition, the invention provides an autofluorescence wavelength and intensity of a chemiluminiscence probe in a physiological environment and application of the chemiluminiscence probe in living imaging. Testing results shown that the chemiluminiscence probe provided by the invention has autofluorescenceof a long wavelength (600nm), is capable of effectively penetrating skin tissue, and has high autofluorescence intensity (greater than 10<7>p / s / cm<2> / sr), excellent thermal stability (without 1,2-dioxy-heterocyclobutane structure) and diverse detection groups (applicable to different detection models).
Owner:EAST CHINA UNIV OF SCI & TECH
Chemiluminescent probes for imaging/detection of proteases
ActiveCN110944985APolypeptide with localisation/targeting motifDipeptide ingredientsBiochemistryProteases
The present invention provides turn-ON dioxetane-based chemiluminescence probes based on the Schapp's adamantylidene-dioxetane probe, which are capable of detecting or imaging, more specifically, determining the presence, or measuring the level, of proteases, as well as compositions and uses thereof.
Owner:特拉维夫大学拉玛特有限公司
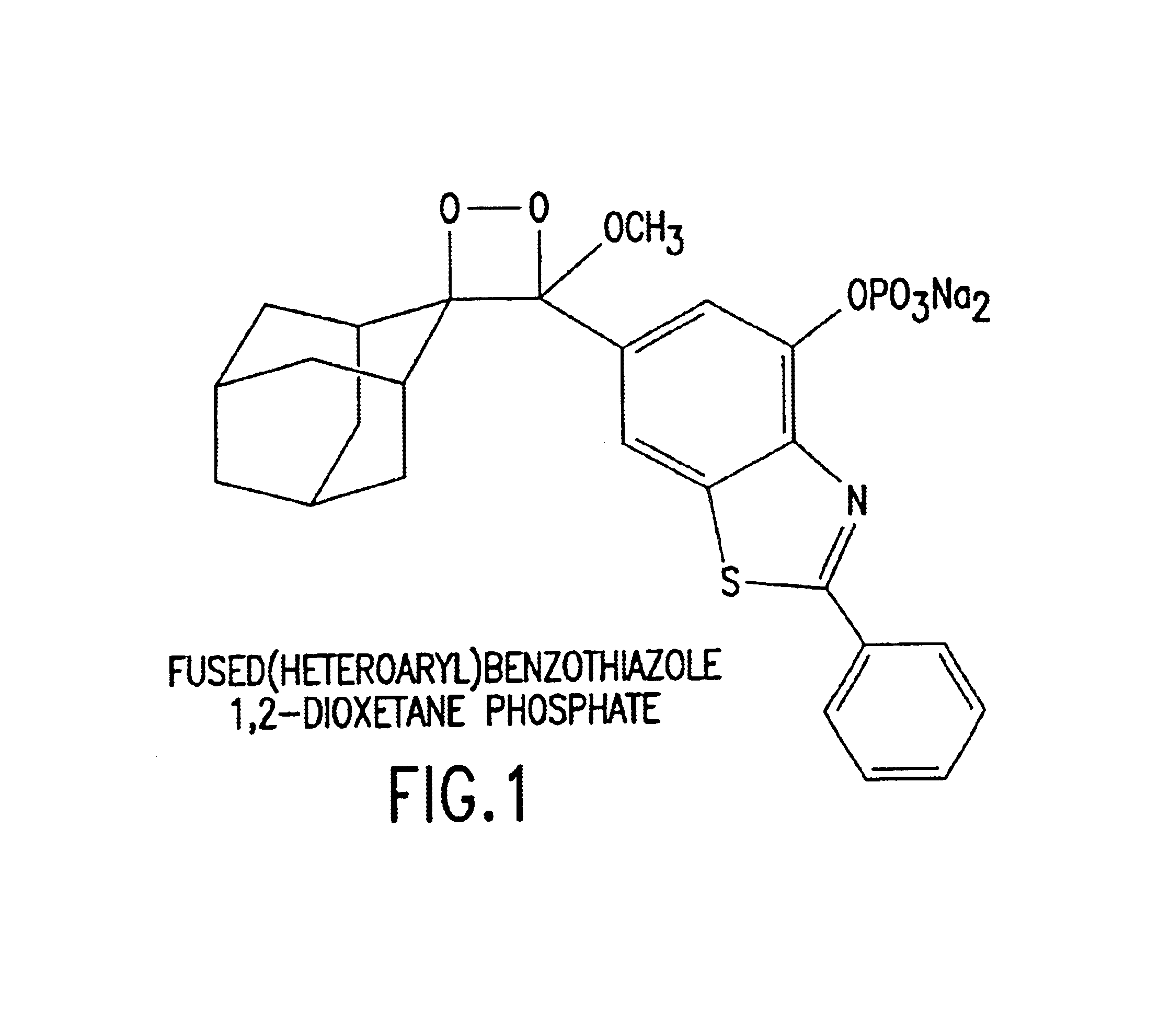
Benzothiazole dioxetanes
InactiveUS6852548B2Material analysis by observing effect on chemical indicatorGroup 5/15 element organic compoundsLight energyRoom temperature
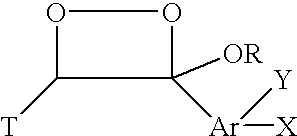
Chemiluminescent 1,2-dioxetane compounds capable of producing light energy when decomposed, substantially stable at room temperature, represented by the formulas I or II: wherein T is:
Owner:APPL BIOSYSTEMS INC
Near-infrared chemiluminescent probes for in-vivo imaging
ActiveUS20200093942A1Efficient emissionsLess scatteringSugar derivativesGroup 3/13 element organic compoundsPhotochemistryAnalytical chemistry
The present invention provides turn-ON dioxetane-based chemiluminescence probes based on the Schapp's adamantylidene-dioxetane probe, which emit light in the near-infrared (NIR) region and are therefore useful for in vivo imaging, as well as compositions and uses thereof.
Owner:RAMOT AT TEL AVIV UNIV LTD
Sensitizer-labeled analyte detection
InactiveUS20080113380A1Microbiological testing/measurementChemiluminescene/bioluminescenceEnergy transferAnalyte
Owner:EMP BIOTECH
Chemiluminescent probe for detecting fibroblast activation protein, its synthesis method and application
ActiveCN113024523BRealize detectionOrganic chemistryChemiluminescene/bioluminescenceIntracellularFibroblast
The invention belongs to the technical field of small molecule chemiluminescent probes, in particular to a chemiluminescent probe for detecting fibroblast activation protein (FAPα), a synthesis method and application thereof. The chemiluminescence probe of the present invention is a kind of adamantane-dioxetane chemiluminescence probe, including two kinds of CFCL and BFCL; In the presence of anhydrous inert solvents, it is prepared by reacting with a sensitive group of FAPα through high-performance liquid chromatography; the chemiluminescent probe can be used for the detection of FAPα in vivo and in vitro, including the determination of FAPα in cells, tissues or plasma. FAPα, for imaging of FAPα in animals. The chemiluminescent probe can also be used to evaluate and screen FAPα inhibitors. The invention obtains a chemiluminescent probe by improving the adamantane-dioxetane to realize the detection of FAPα, which is an important technological breakthrough and further expands its application range.
Owner:FUDAN UNIV +1
Stress and luminescence enhanced force-induced luminescence composite film and preparation method
The invention discloses a force-induced luminescent composite film with enhanced stress and luminescence and a preparation method thereof. The preparation method is: adding N,N'-dimethylformamide to a conjugated microporous polymer TPE-CMP or a conjugated microporous polymer In the microporous polymer TPA-CMP, use a ball mill, ball mill, dilute with tetrahydrofuran, and ultrasonically obtain a dispersion; take linear polymethyl acrylate, add the dispersion, heat and stir; put it into a mold, add double adamantane substituted 1 , 2-dioxetane, azobisisobutyronitrile and methyl acrylate, stirred evenly, protected by an inert gas, reacted, and vacuum-dried at room temperature to obtain a force-induced luminescent composite film with enhanced stress and luminescence. The stress- and luminescent-enhanced force-induced luminescent composite thin film of the present invention has high mechanical strength, and the fluorescent conjugated microporous polymer acts as an energy transfer acceptor, effectively improving the luminous intensity during the fracture process of the composite thin film and changing the Glow color.
Owner:TIANJIN UNIV
Heteroaryl substituted benzothiazole dioxetanes
InactiveUS7112413B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementArylLight energy
Chemiluminescent heteroaryl substituted benzothiazole 1,2-dioxetane compounds capable of producing light energy when decomposed are provided. These chemiluminescent compounds are represented by the general formula: The heteroaryl substituent Y can be, for example, a pyridyl group or a benzothiazolyl group. The heteroaryl substituted benzothiazole compounds are substantially stable at room temperature. Kits including the heteroaryl substituted dioxetane compounds as well as methods for using these compounds for detecting the presence of one or more analytes in a sample are also provided.
Owner:APPL BIOSYSTEMS INC
Synthetic method of 1,2-dioxetane compound
ActiveCN102875600BHigh purityImprove luminosityGroup 5/15 element organic compoundsLuminescent compositionsPhosphoric Acid EstersPhosphate
The invention provides a synthetic method of 1,2-dioxetane compound. The synthetic method includes the steps: A) preparation of a carboxylic compound II; B) preparation of an ester compound III; C) preparation of an enol methyl ether compound IV; D) preparation of a phenol compound V; E) preparation of a phosphate ester compound; and F) preparation of a target compound VII. The synthetic method of 1,2-dioxetane compound is short in synthesis route, few in step, mild in reaction condition, simple and convenient to operate and high in yield, and the synthesized 1,2-dioxetane compound is high in purity and fine in luminescent performance and can be used as substrate in chemiluminescence reaction to be applied to the technical field of chemiluminescence immunoassay.
Owner:广东派特埃尔生物科技有限公司
1,2-dioxetane derivative and preparation method thereof
ActiveCN106588990ASimple preparation processGroup 5/15 element organic compoundsLuminescent compositionsLuminous intensityFluorescence
The invention provides a 1,2-dioxetane derivative and a preparation method thereof, which solve the problems of weak luminous intensity of a chemiluminescence substrate prepared by AMPPD in the prior art. A structural general formula of the 1,2-dioxetane derivative is shown as a formula (I): in the formula, n is an integer between 0 and 5; and F is a fluorescent group. The 1,2-dioxetane derivative contains the fluorescent group, is directly coupled with a chemiluminescence reinforcing agent, luminescence enhancement effect is provided, a plurality of chemiluminescence reinforcing agents are not required, and the preparation process is simple.
Owner:SICHUAN ORIENTER BIOLOGICAL TECH
Dioxetane compounds and their use for the detection of microorganisms
ActiveCN112204020ASugar derivativesGroup 5/15 element organic compoundsMicroorganismChemical compound
The present invention relates to dioxetane compounds, their use in the detection of presence or absence, quantification and identification of microorganisms including bacteria, bacterial fragments (e.g., LPS, endotoxin), viruses, fungi as well as other pathogens by means of chemiluminescence and to corresponding methods.
Owner:尼米斯技术公司 +1
Single-component near-infrared afterglow imaging nanoprobe and preparation method thereof
PendingCN114591730AImprove stabilityGood tumor enrichment abilityEnergy efficient lightingLuminescent compositionsPolyethylene glycolPorphyrin
The invention provides a single-component near-infrared afterglow imaging nanoprobe and a preparation method thereof, and the nanoprobe is formed by taking polyphenylene ethylene (PPV) as a main chain and modifying polyethylene glycol (PEG) and 5, 10, 15, 20-tetra (4-hydroxyphenyl) porphyrin with different proportions on a side chain. The probe can be self-assembled in water to form nanoparticles, and the particle size is 10-100 nanometers. Under white light irradiation, PPV and 5, 10, 15, 20-tetra (4-hydroxyphenyl) porphyrin generate singlet oxygen, the singlet oxygen can oxidize a PPV main chain to form a dioxetane intermediate, the intermediate is then broken to release chemical energy and excite PPV to emit light, then energy is transmitted to 5, 10, 15, 20-tetra (4-hydroxyphenyl) porphyrin through fluorescence resonance energy transfer, and finally a near-infrared afterglow signal is emitted. And afterglow imaging is realized.
Owner:NANJING UNIV OF POSTS & TELECOMM
A kind of preparation method of 1,2-dioxane derivatives
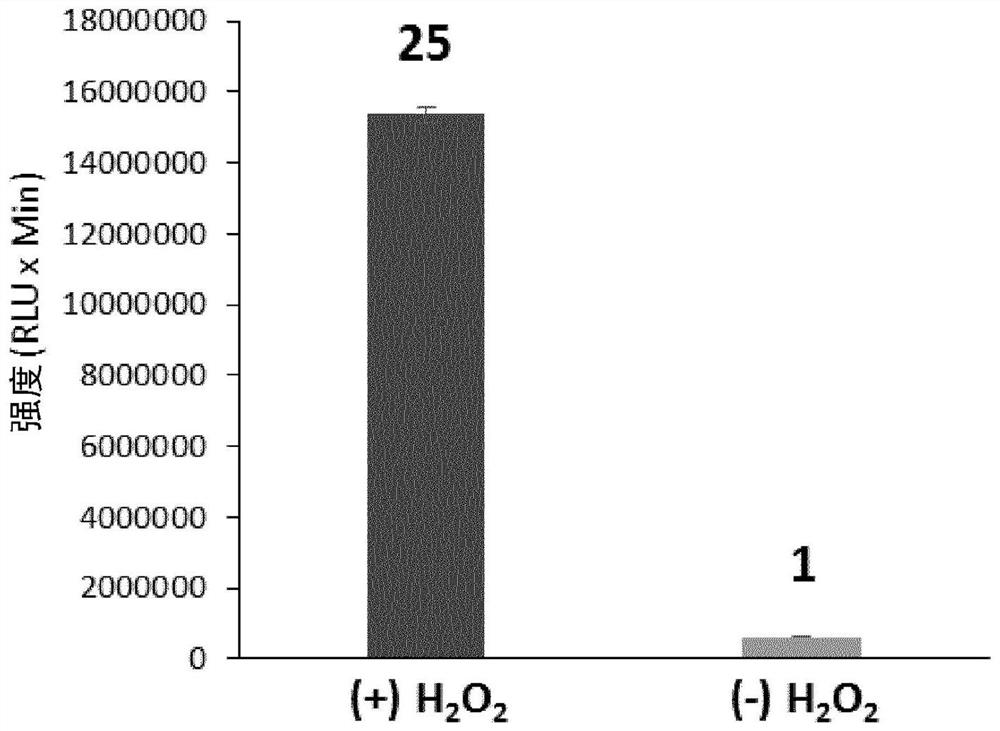
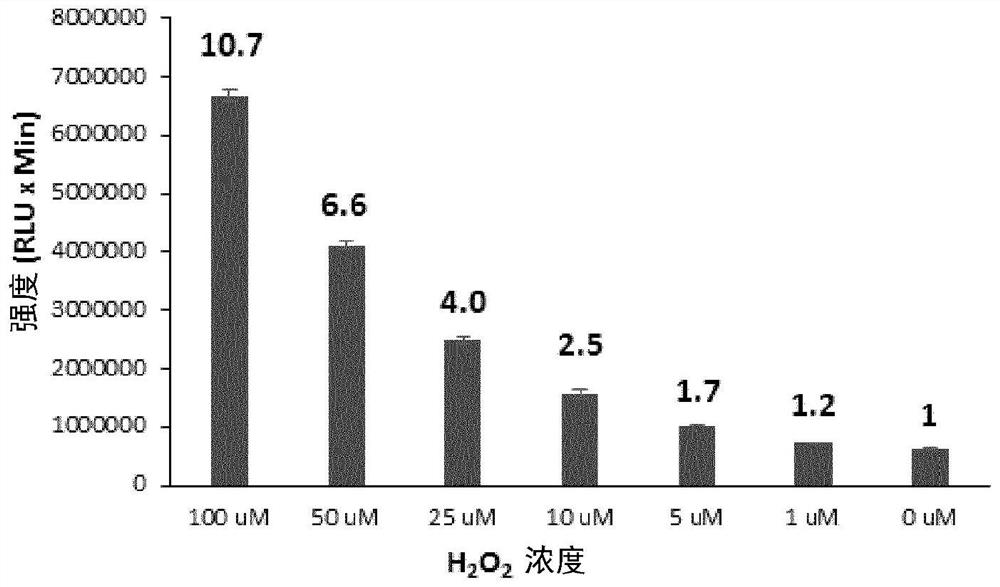
ActiveCN105085569BAvoid uneven responseAvoid safety hazardsGroup 5/15 element organic compoundsWittig reactionPhosphorylation
The invention relates to a preparation method of a 1, 2-dioxetane derivative. The 1, 2-dioxetane derivative is a compound shown in a formula I. The preparation method comprises the following steps of taking a compound shown in a formula II and adamantanone; performing Wittig reaction to synthesize a compound shown in a formula III; performing phosphorylation to obtain a compound shown in a formula IV; and performing oxidation reaction to obtain the 1, 2-dioxetane derivative. The preparation method of the 1, 2-dioxetane derivative has the advantages that safety is high; production cost is low; and industrial production of the 1, 2-dioxetane derivative is facilitated.
Owner:SUZHOU YACOO SCI CO LTD
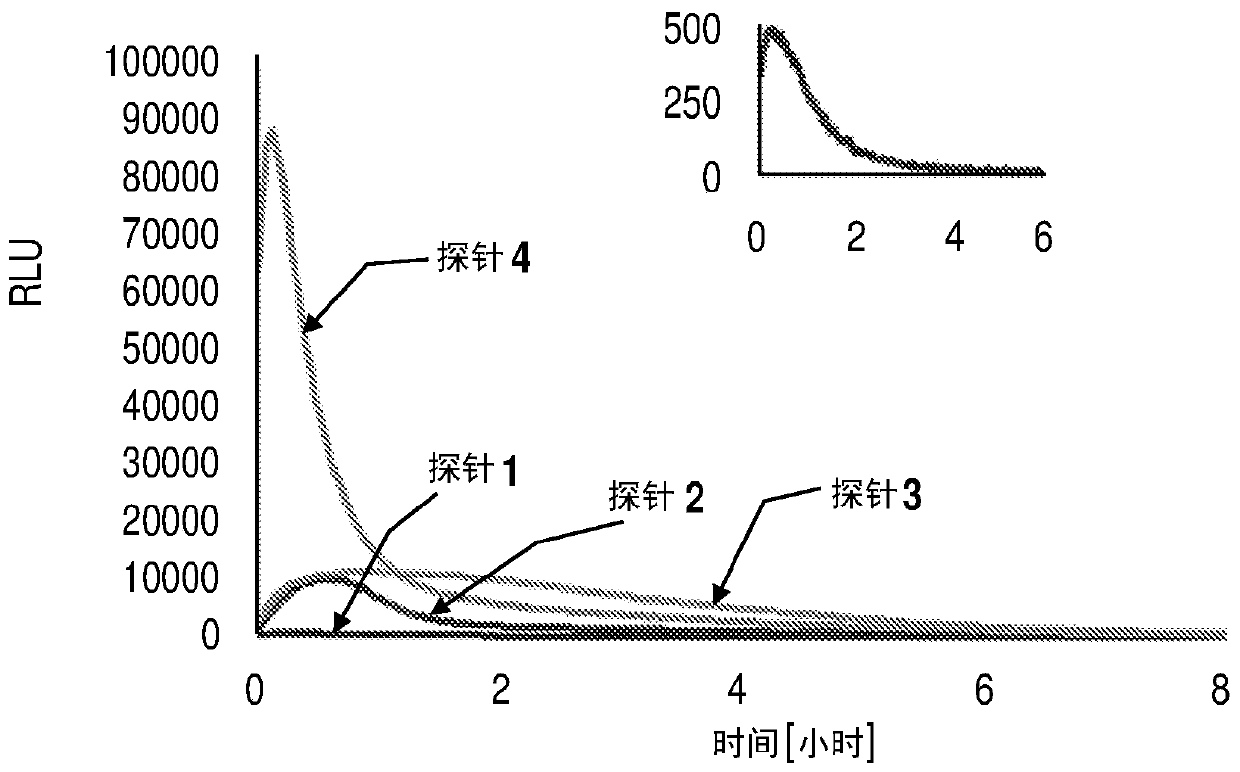
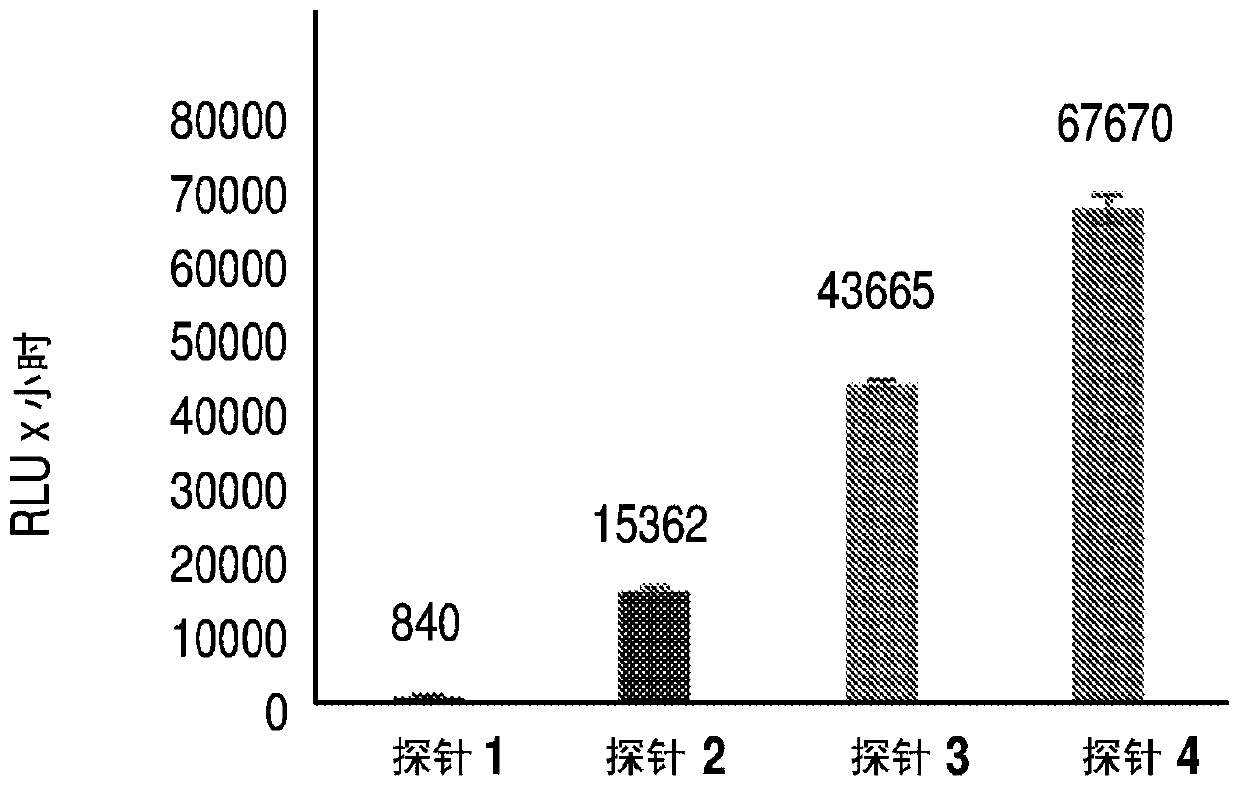
Chemiluminescent probes for diagnostics and in vivo imaging
ActiveUS10660974B2Enhanced chemiluminescenceGroup 4/14 element organic compoundsMethine/polymethine dyesFluorophoreBiochemistry
Dioxetane-based chemiluminescence probes, more specifically fluorophore-tethered dioxetane-based chemiluminescence probes and π* acceptor group-containing dioxetane based chemiluminescence probes can be included in compositions. The chemiluminescence probes are useful for both diagnostics and in vivo imaging.
Owner:RAMOT AT TEL AVIV UNIV LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com