Chemiluminiscence substrate with high luminescence intensity, long wavelength and good stability and preparation method and application of chemiluminiscence substrate
A chemiluminescence, high-intensity technology, used in chemiluminescence/bioluminescence, chemical instruments and methods, and analysis by chemically reacting materials, etc., can solve the problem of structural instability, susceptibility to environmental interference, and low probe luminescence intensity. and other problems, to achieve the effect of enhancing chemiluminescence intensity, excellent stability and high intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Taking quinoline nitrile chemiluminescent substrate as an example, the specific synthetic route is as follows:
[0038]
[0039] 1. Synthesis of 4-bromo-3-(dimethoxymethyl)phenol
[0040]
[0041] Add 4-bromo-3-hydroxyl-phenol (1g, 4.55mmol) and 20mL methanol into a 50mL dry single-necked bottle, stir to dissolve; then add p-toluenesulfonic acid (171.3mg, 0.91mmol), and reflux for 6h; After completion, the reaction solution was poured into 200ml of dilute sodium carbonate solution (2g), and extracted with ethyl acetate (200mL×2), the extracts were combined, dried over anhydrous sodium sulfate, spin-dried, purified by column chromatography, pale pink liquid 0.7 g, yield 57%.
[0042] 1 H NMR (400MHz, CDCl 3 -d 1 , ppm): δ=7.40-7.38(d, J=8.4Hz, 1H, Ph-H), δ=7.12-7.11(d, J=3.2Hz, 1H, Ph-H), δ=6.73-6.70( d-d, J 1 =8.8Hz,J 2 =3.2Hz,1H,Ph-H),δ=5.84(s,1H,Ph-OH),δ=5.50(s,1H,-CH-O-),δ=3.41(s,6H,-O- CH 3 ). 13 C NMR (100MHz, CDCl 3 -d 1,ppm): 155.51, 133.74, 117...
Embodiment 2
[0069] Other chemiluminescent hydroxyl substrates, the specific synthetic route is as follows:
[0070] 1. Synthesis of Self-luminescent Indole Substrates
[0071]
[0072] Add sulfonate indole salt (322mg, 1.08mmol), quinoline nitrile (300mg, 0.90mmol) and 50ml acetonitrile into a 100mL round bottom flask, stir and mix well; then add sodium acetate (74mg, 0.90mmol); heat to reflux 10h, point the plate to monitor the reaction progress. After the reaction was completed, a red crude product was obtained by rotary evaporation, which was purified by a flash column to obtain 270 mg of a light red solid product with a yield of 53%.
[0073] 1 H NMR (400MHz, CDCl 3 -d 1 , ppm): δ=7.82-7.79(m, 1H, Ph-H), δ=7.59-7.57(d, J=8Hz, 1H, Ph-H), δ=7.53-7.49(m, 3H, Ph-H) H), δ=7.48-7.44(d, J=16Hz, 1H, Alkene-H), δ=7.12(s, 1H, Ph-H), δ=7.02-6.98(d, J=16Hz, 1H, Alkene -H),δ=6.92-6.89(d-d,J 1 =8.4Hz,J 2 =2.8Hz,1H, Ph-H),δ=6.81-6.80(d,J=2.8Hz,1H,Ph-H),δ=4.42-4.36(q,J=6.8Hz,2H,-N-CH 2 -C...
Embodiment 3
[0082] Absorption, Fluorescence and Chemiluminescence Spectra of F-QM-OH in Aggregated State
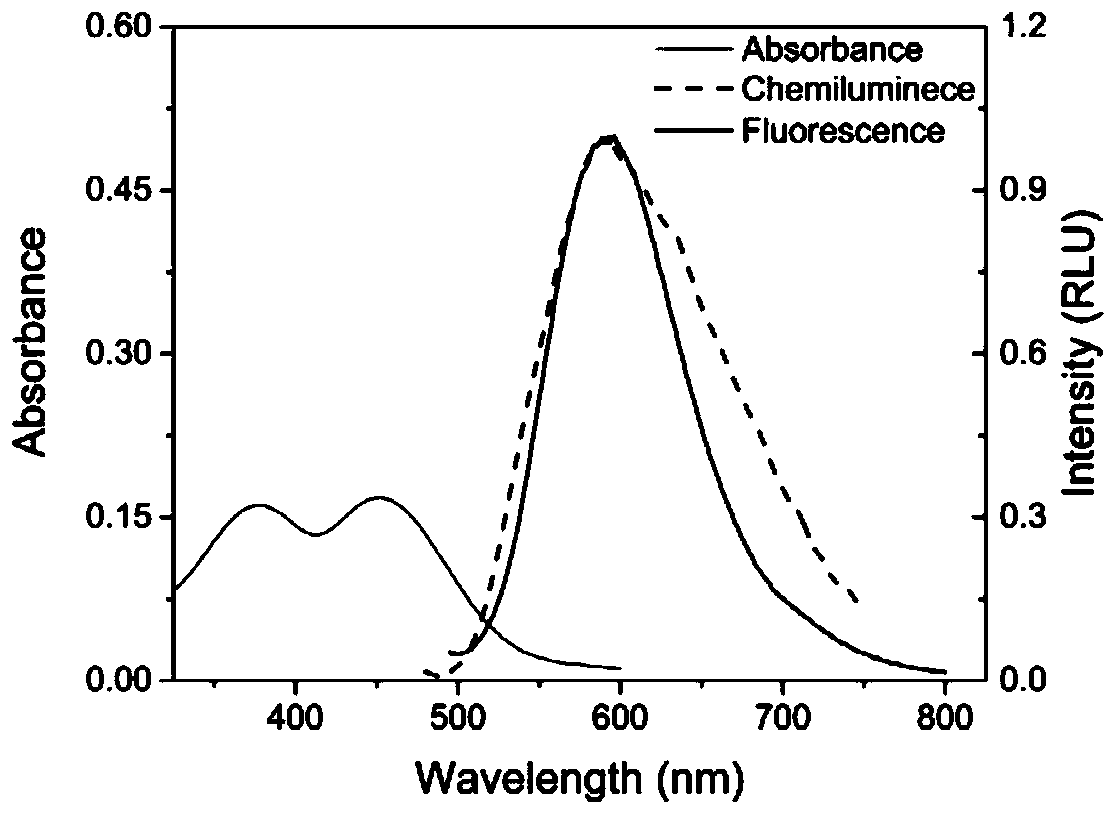
[0083] Get the F-QM-OH prepared in Example 1 and dissolve it in analytically pure dimethyl sulfoxide to make 1.0 × 10 -2 stock solution of M. Then prepare water (H 2 O) 99% DMSO / H 2 O mixed solvent 2mL. Take 20 μL of the above stock solution and add it to the prepared DMSO / HO 2 O mixed solvent, mixed evenly and transferred to an optical quartz cuvette (10 × 10mm) to test its fluorescence spectrum. Such as figure 1 As shown, with 480nm as the excitation wavelength, the maximum emission peak of the F-QM-OH substrate is located at about 600nm in the near-infrared region, and the Stokes shift is 120nm; the chemiluminescence spectrum of F-QM-OH is basically consistent with the fluorescence spectrum .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com