1,2-dioxetane derivative and preparation method thereof
A technology of dioxetane and its derivatives, which is applied in the field of 1,2-dioxetane derivatives and its preparation, can solve problems such as weak luminous intensity, and achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
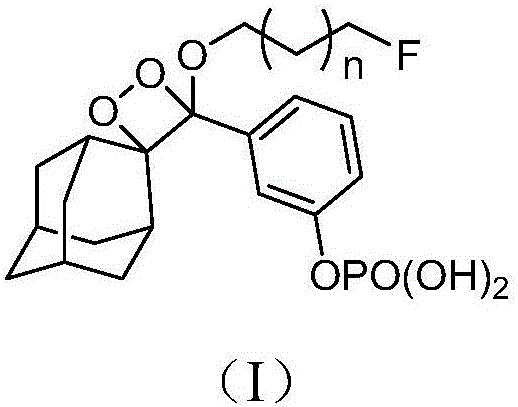
[0056] A kind of 1,2-dioxetane derivative, general structural formula is as shown in formula (I):
[0057]
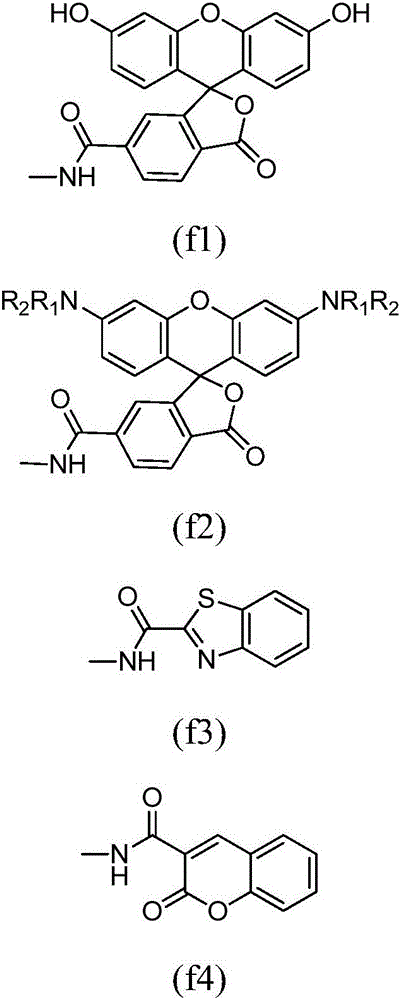
[0058] Wherein, n is an integer between 0 and 5; F is a fluorescent group, and the structural formula of the fluorescent group F is the following formula (f1):
[0059]
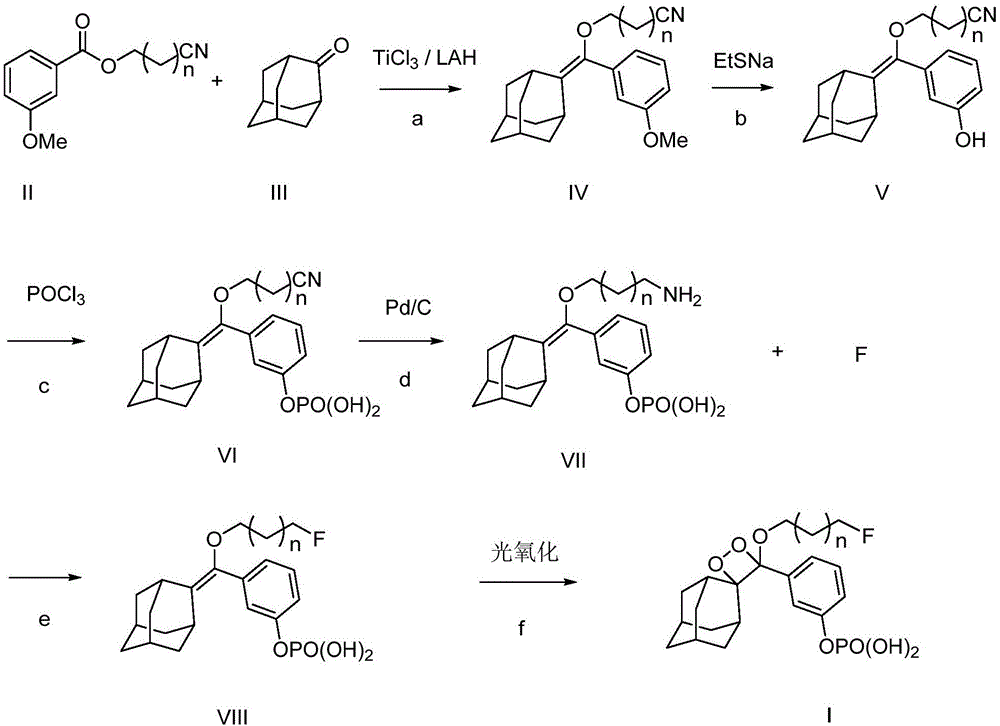
[0060] refer to figure 1 , the preparation method of 1,2-dioxetane derivative is as follows:
[0061] a, compound (II) and compound (III) generate compound (IV) under the action of titanium trichloride and lithium aluminum hydride; wherein,
[0062] Compound (II) structural formula is as follows:
[0063]
[0064] Compound (III) structural formula is as follows:
[0065]
[0066] Compound (IV) structural formula is as follows:
[0067]
[0068] The preparation process of compound (IV) is as follows:
[0069] Add 200ml tetrahydrofuran to a dry three-necked flask, stir in an ice-water bath for 30 minutes, add titanium trichloride (24.5g, 0.16mol), add lithium aluminum hydride (3.0g, 0.0...
Embodiment 2
[0089] A kind of 1,2-dioxetane derivative, general structural formula is as shown in formula (I):
[0090]
[0091] Wherein, n is an integer between 0 and 5; F is a fluorescent group, and the structural formula of the fluorescent group F is the following formula (f2):
[0092]
[0093] refer to figure 1 , the preparation method of 1,2-dioxetane derivative is as follows: comprise the following steps successively:
[0094] a, compound (II) and compound (III) generate compound (IV) under the action of titanium trichloride and lithium aluminum hydride; wherein,
[0095] Compound (II) structural formula is as follows:
[0096]
[0097] Compound (III) structural formula is as follows:
[0098]
[0099] Compound (IV) structural formula is as follows:
[0100]
[0101] The preparation process of compound (IV) is as follows:
[0102] Add 160ml of tetrahydrofuran to a dry three-necked flask, stir in an ice-water bath for 23 minutes, add 22g of titanium trichloride, a...
Embodiment 3
[0120] Embodiment 3: A 1,2-dioxetane derivative, the general structural formula is shown in formula (I):
[0121]
[0122] Wherein, n is an integer between 0 and 5; F is a fluorescent group, and the structural formula of the fluorescent group F is the following formula (f3):
[0123]
[0124] refer to figure 1 , the preparation method of 1,2-dioxetane derivative is as follows:
[0125] a, compound (II) and compound (III) generate compound (IV) under the action of titanium trichloride and lithium aluminum hydride; wherein,
[0126] Compound (II) structural formula is as follows:
[0127]
[0128] Compound (III) structural formula is as follows:
[0129]
[0130] Compound (IV) structural formula is as follows:
[0131]
[0132] The preparation process of compound (IV) is as follows:
[0133] Add 230ml of tetrahydrofuran into a dry three-necked flask, stir in an ice-water bath for 40 minutes, add 27g of titanium trichloride, add 4g of lithium aluminum hydride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com