Patents
Literature
1229 results about "Medical testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
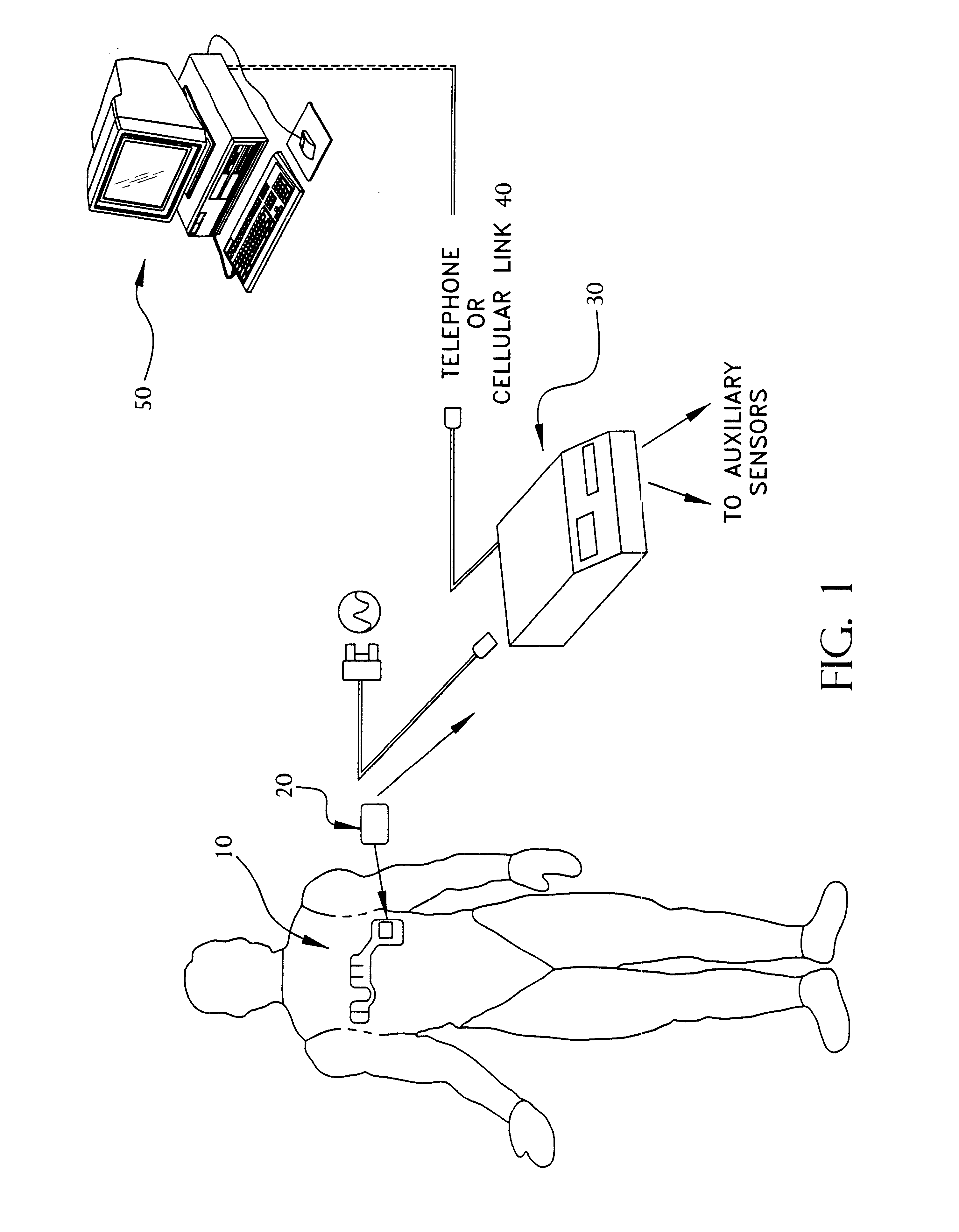
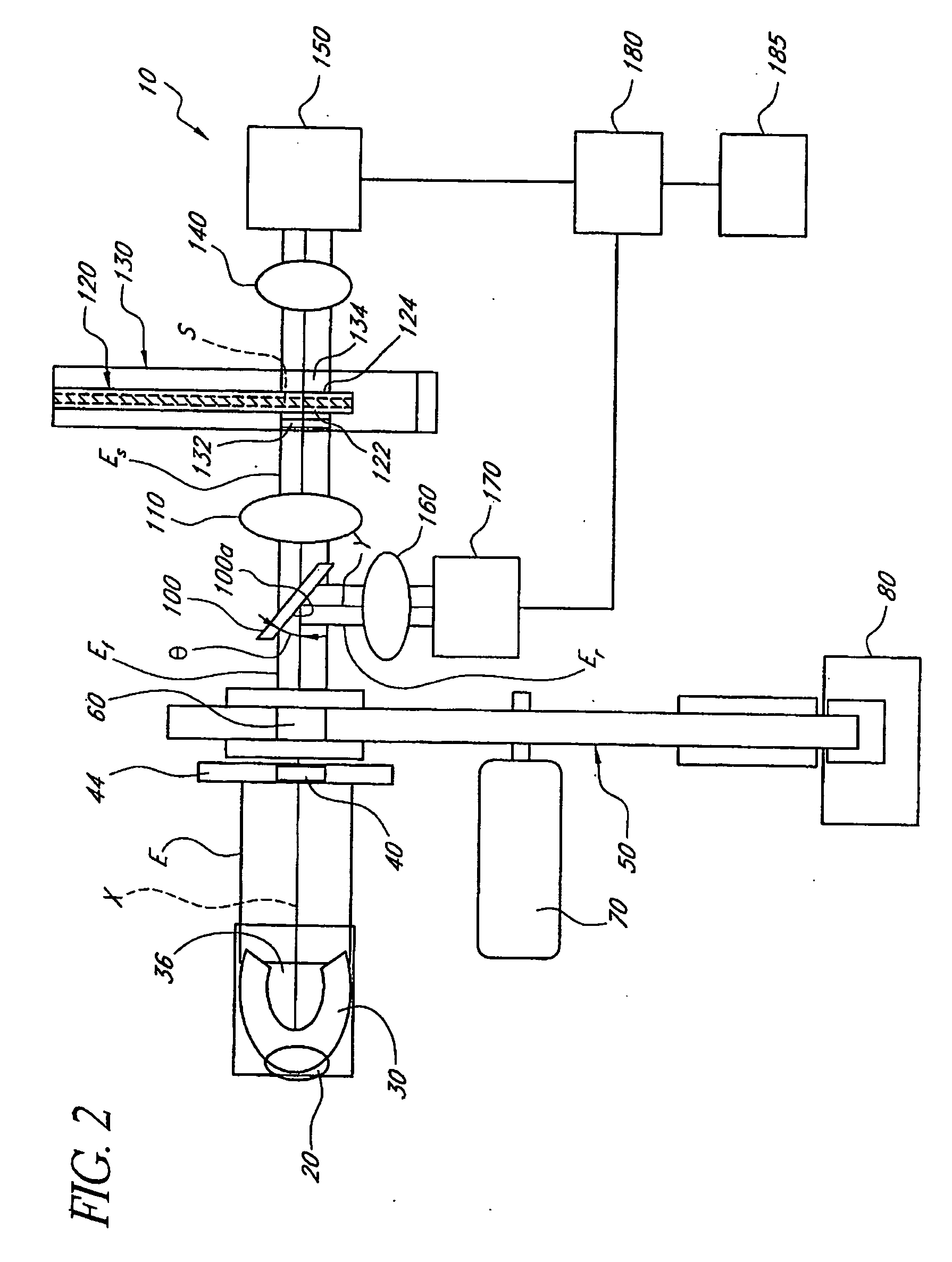
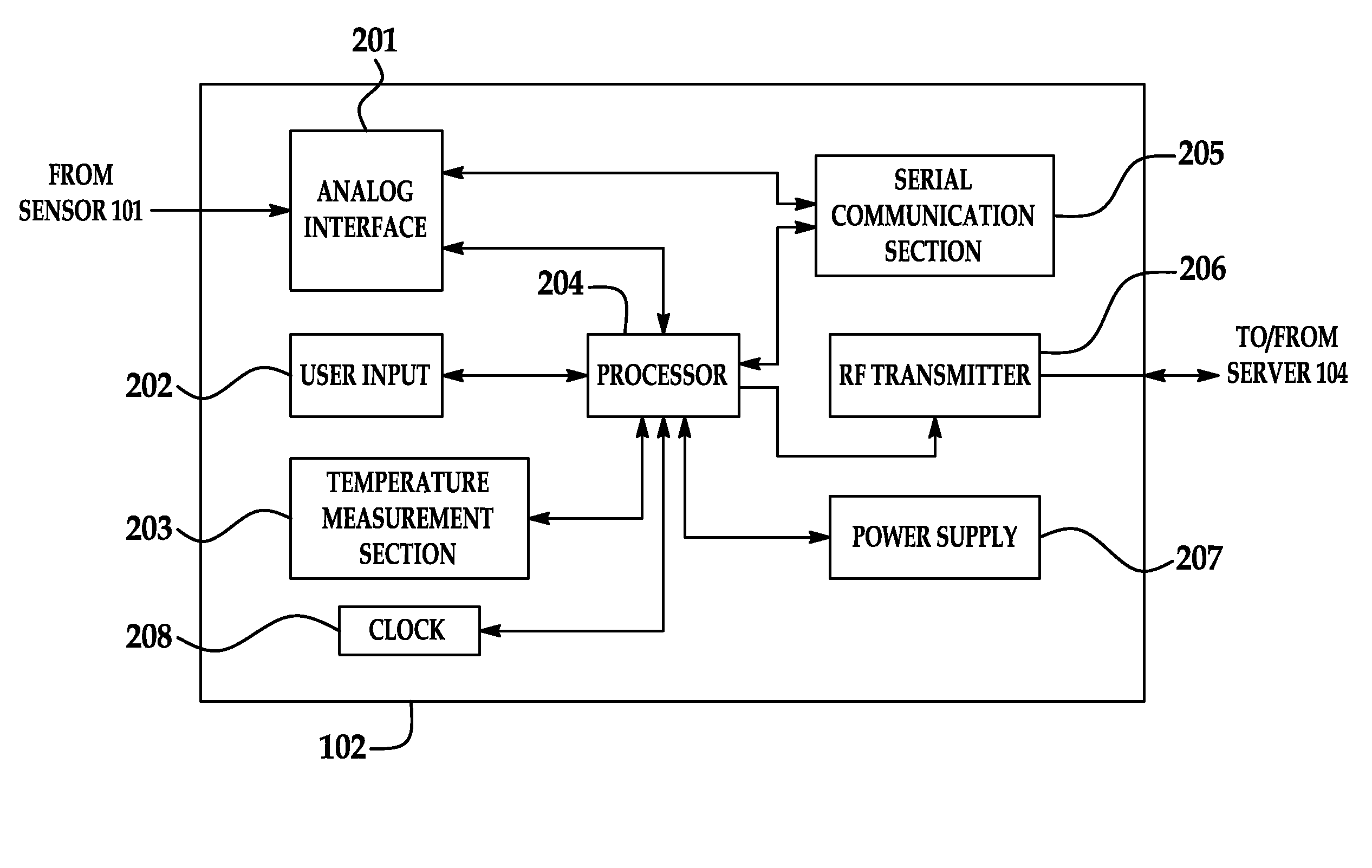
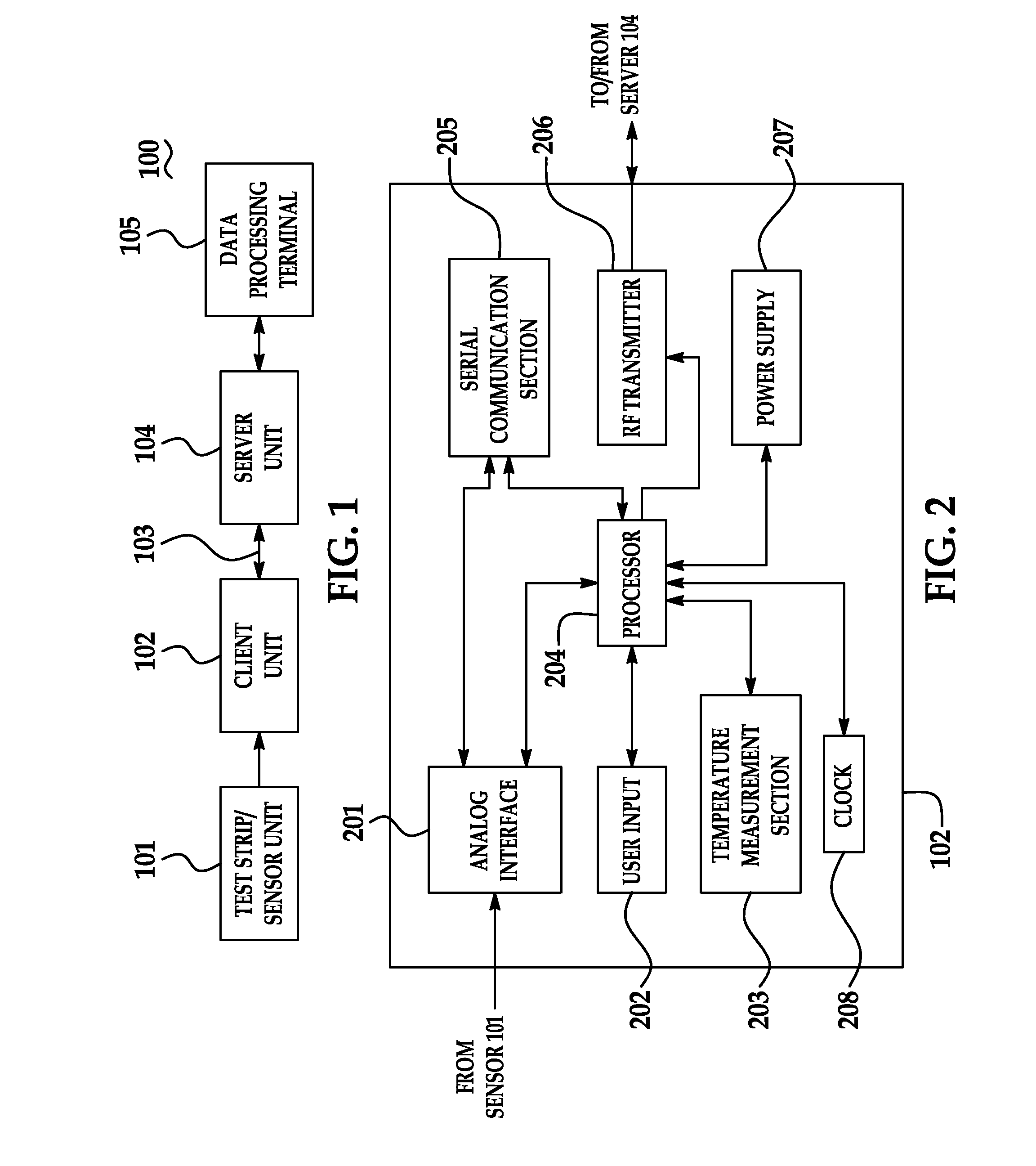
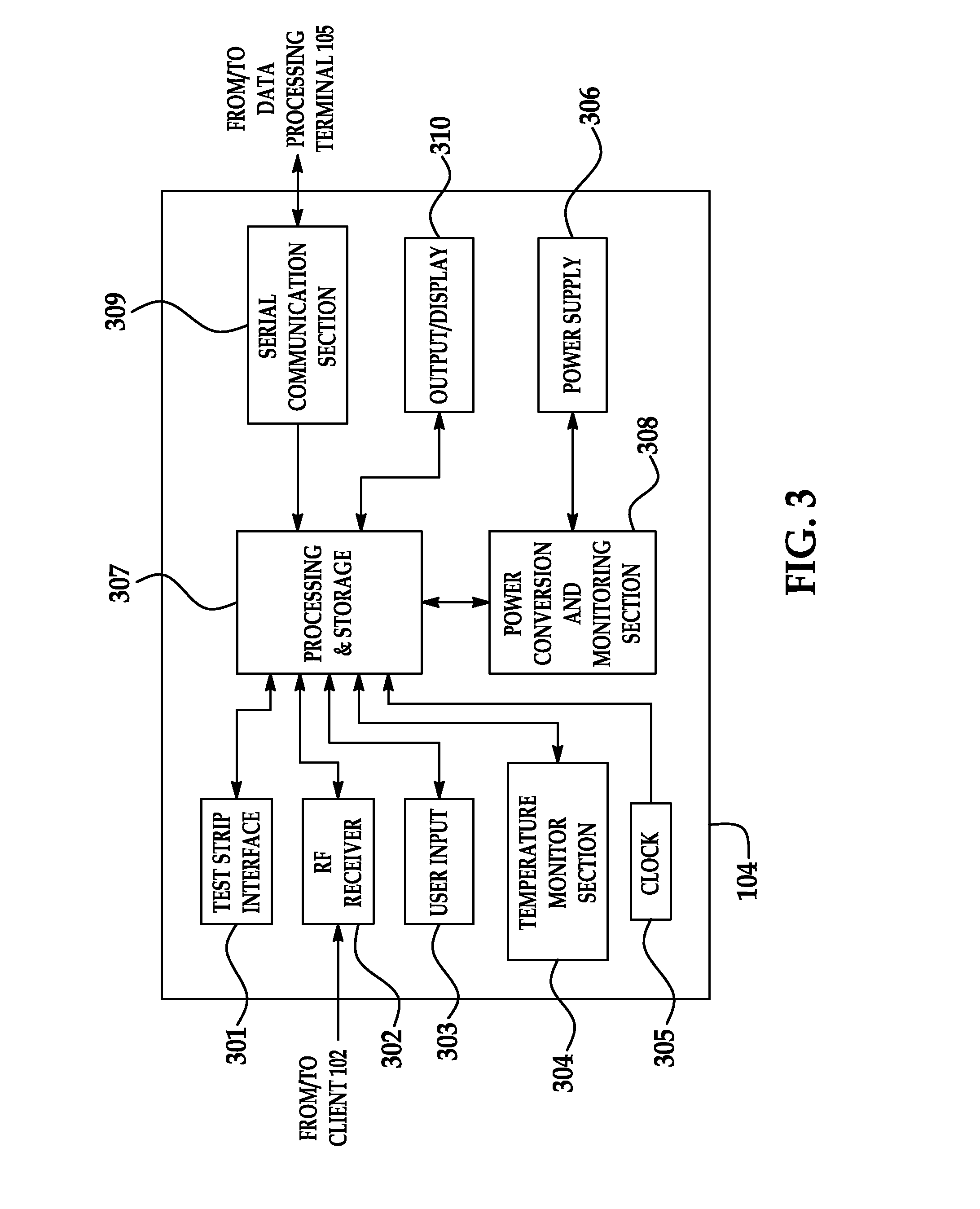
Portable remote patient telemonitoring system
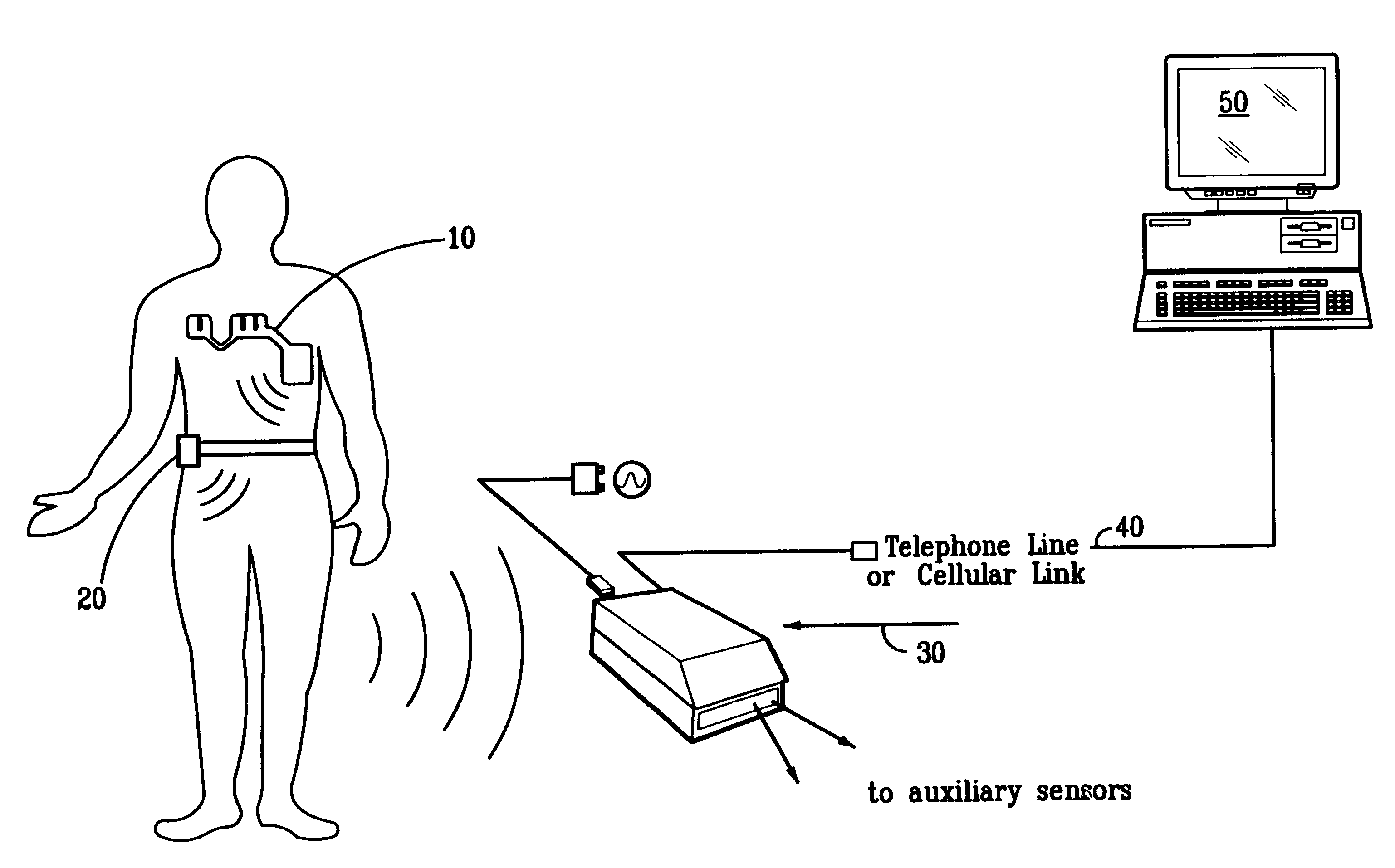
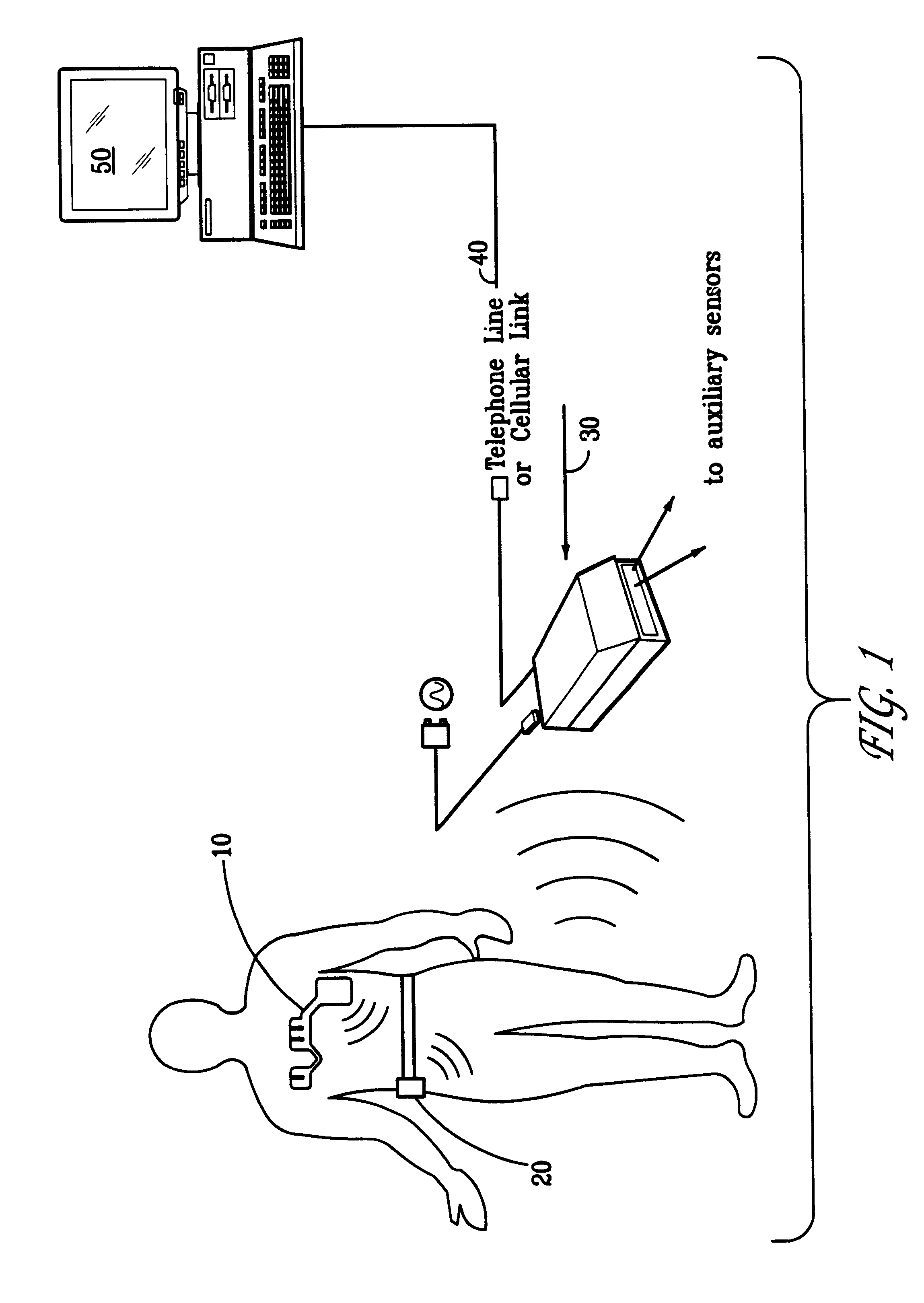
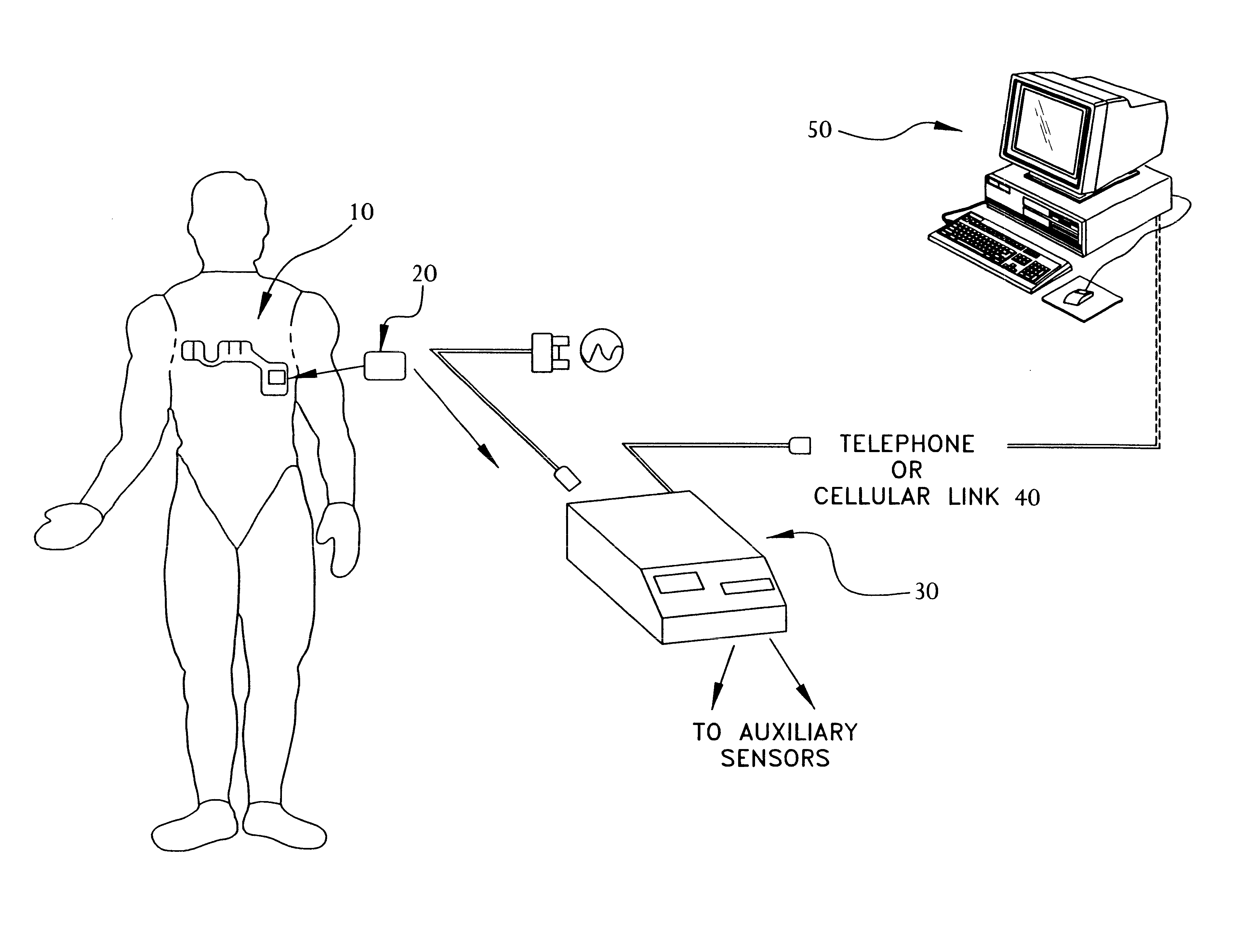
A system and method for monitoring vital signs and capturing data from a patient remotely using radiotelemetry techniques. The system is characterized by a cordless, disposable sensor band with sensors form measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and transmission circuitry for the detection and transmission of vital signs data of the patient. A small signal transfer unit that can either be worn by the patient, e.g., on his or her belt, or positioned nearby receives data from the sensor band, which it then forwards by e.g., radio transmission to a base station that can be located up to 60 meters away. The base station receives data transmissions from the signal transfer unit and is designed to connect to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Patient safety is enhanced by the ability of the base station to compare clinical data, e.g., ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. Such events are indicated to the physician and could be indicated to the patient by reverse transmission to the signal transfer unit. A remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. The system of the invention has useful application to the collection of patient clinical data during drug trials and medical testing for regulatory approvals as well as management of patients with chronic diseases.
Owner:CLEARPATH PARTNERS
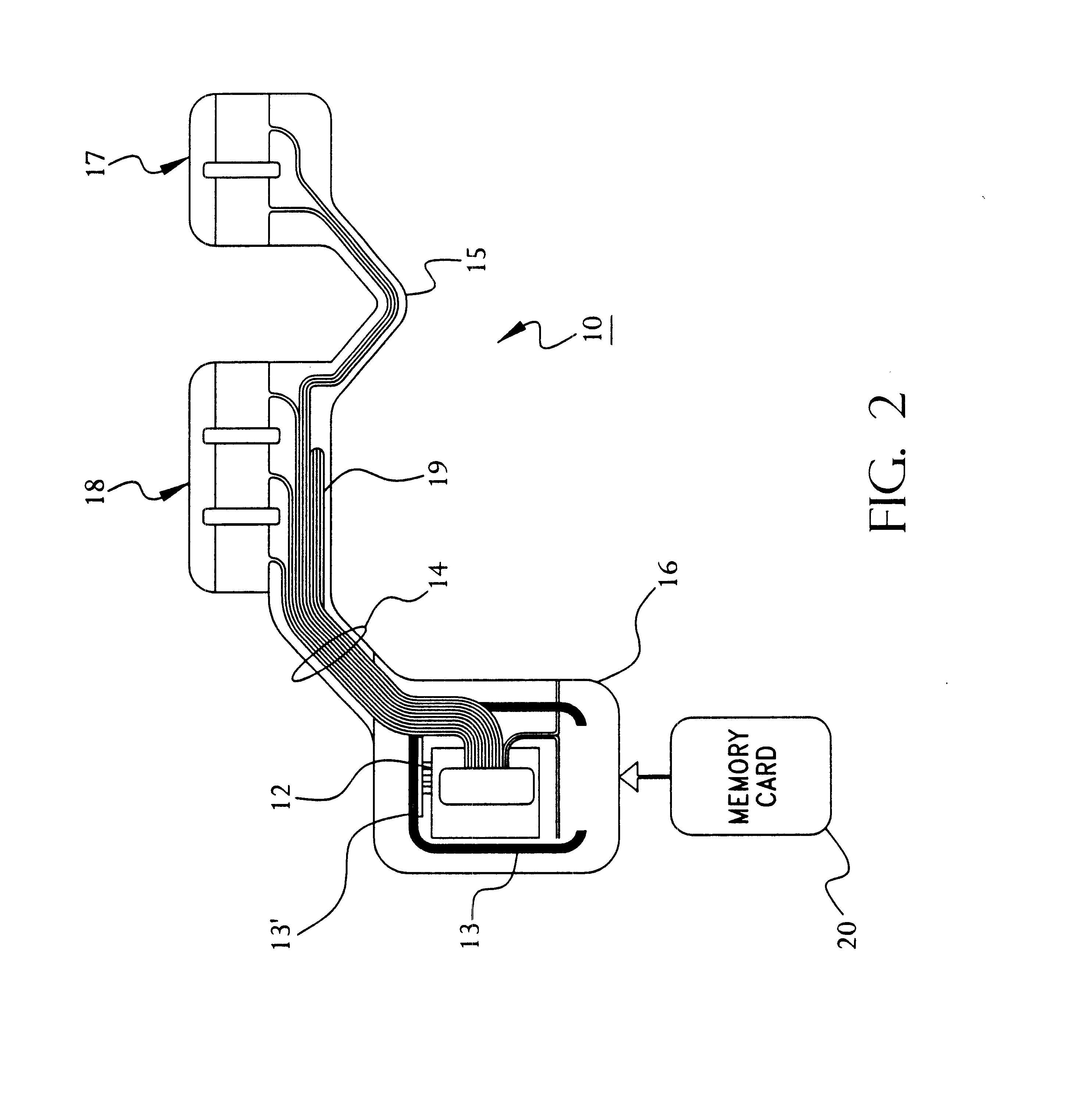
Portable remote patient telemonitoring system using a memory card or smart card
InactiveUS6454708B1Low costIncrease the number ofSurgeryRespiratory organ evaluationSmart cardFull waveform
A system and method for monitoring health parameters and capturing data from a subject. The system is characterized by a cordless, disposable sensor band with sensors for measuring full waveform ECG, full waveform respiration, skin temperature, and motion, and a connector which accepts a memory card or a smart card for storage of the measured data. After a predetermined period of time, such as when the sensor band is removed, the memory card or smart card is removed and inserted into a monitoring device which reads the stored health parameter data of the subject. The monitoring device includes a base station that includes a memory / smart card reader and is connected to conventional phone lines for transferring the collected data to a remote monitoring station. The base station may also capture additional clinical data, such as blood pressure data, and to perform data checks. Subject safety is enhanced by the ability of the base station to compare clinical data, e.g. ECG, against given profiles and to mark events when appropriate or when the base station is programmed to do so. The remote monitoring station allows the presentation and review of data (including events) forwarded by the sensor band. ECG analysis software and a user-friendly graphical user interface are provided to remotely analyze the transmitted data and to permit system maintenance and upkeep. In alternative embodiments, a smart card includes the sensor band's electronics and / or signal transmission circuitry in conjunction with a portable data logger so that the electronics may be reused from one disposable sensor band to the next without limiting the patient's range of movement. The system of the invention has useful application to the collection of subject clinical data during drug trials and medical testing for regulatory approvals as well as management of subjects with chronic diseases.
Owner:CLEARPATH PARTNERS
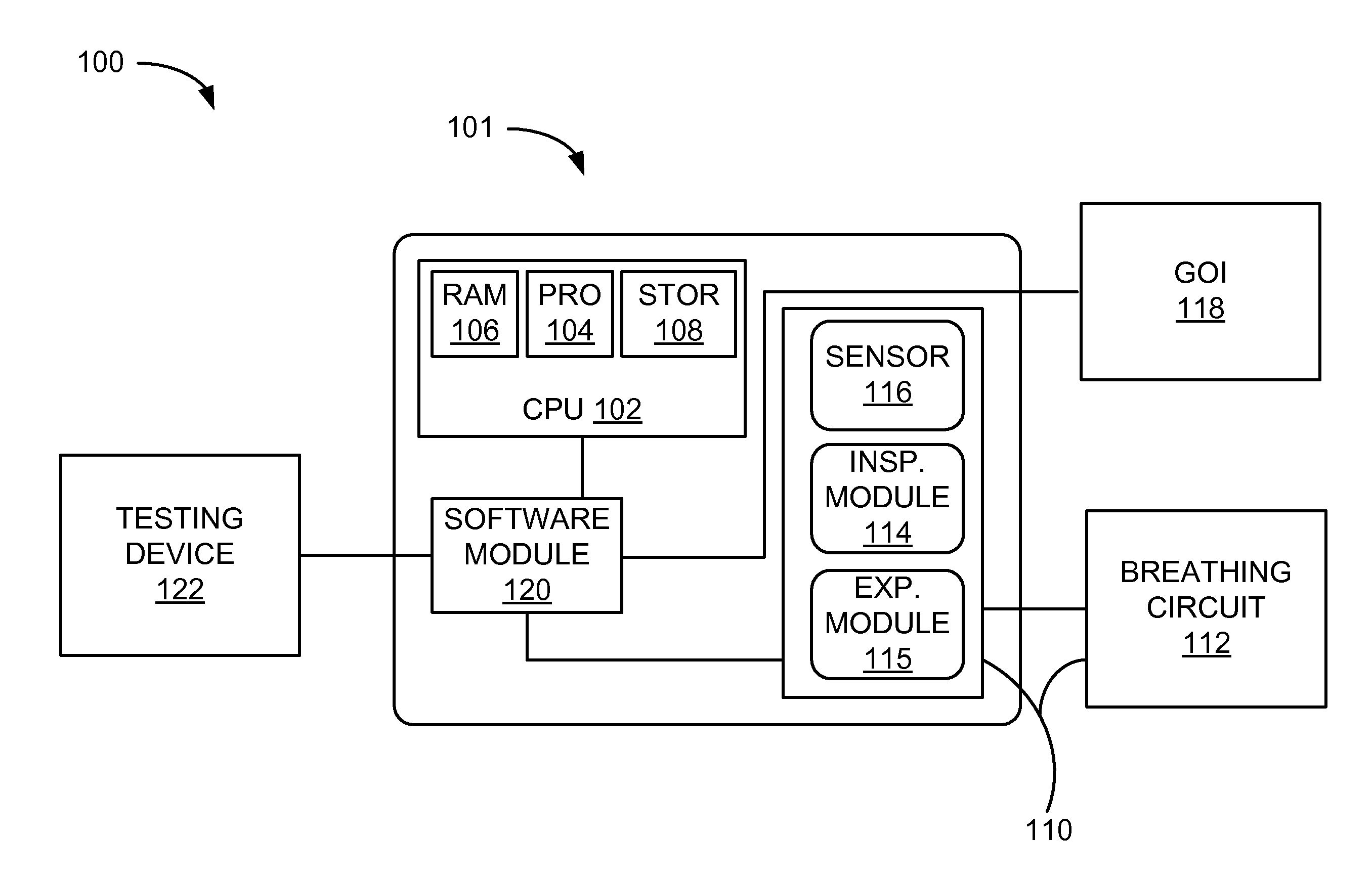
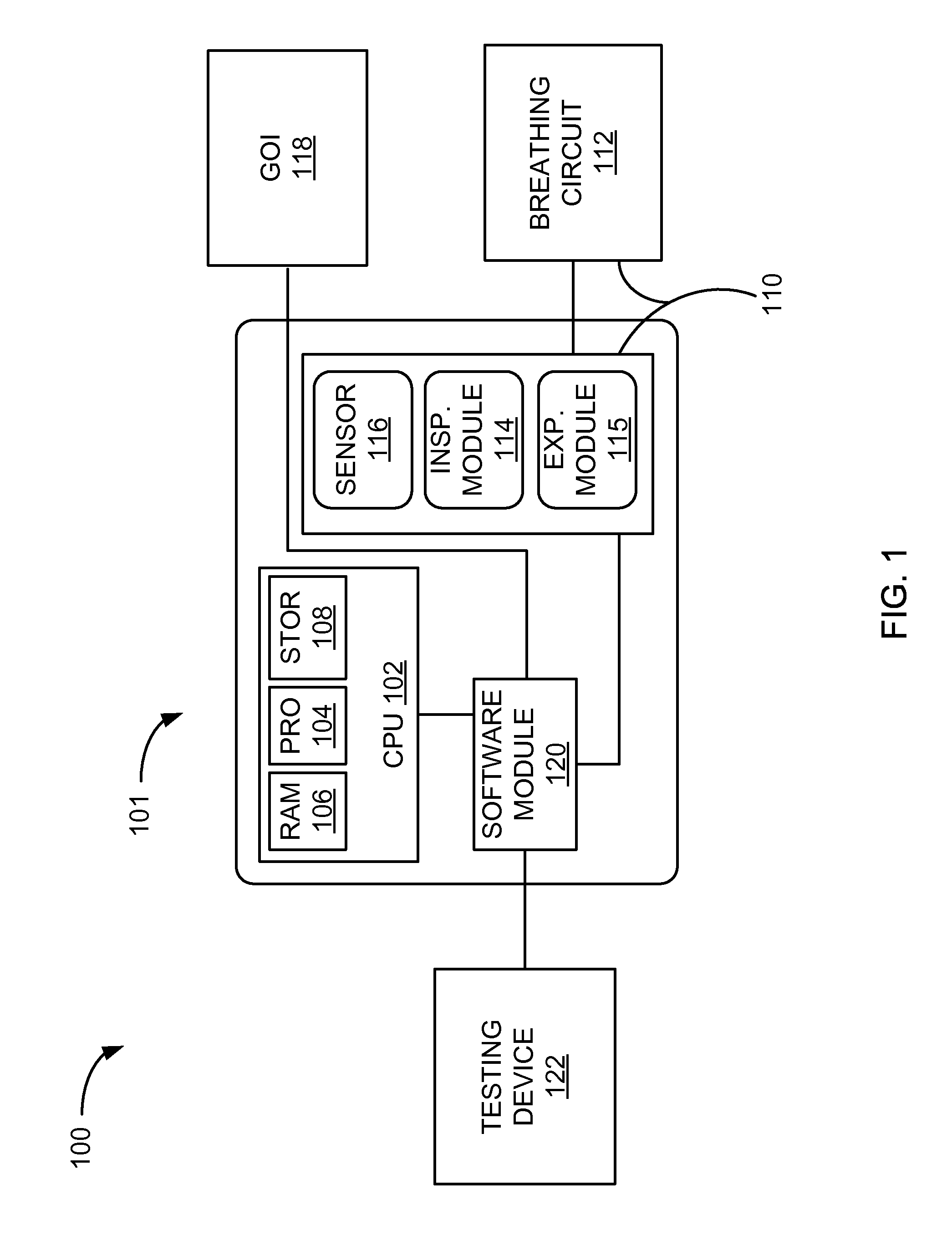
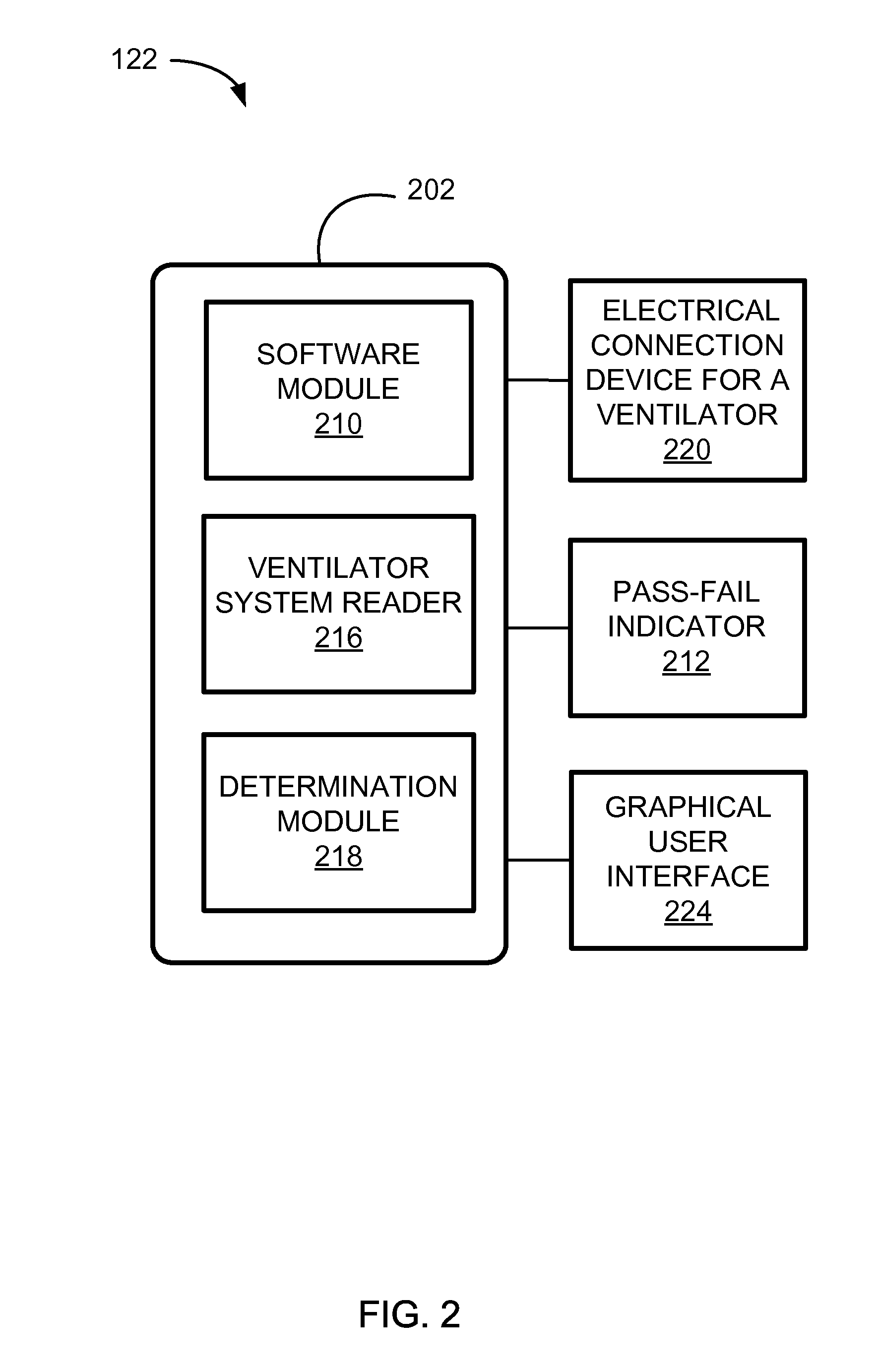
Systems And Methods For Medical Device Testing
ActiveUS20120197580A1Guaranteed functionVehicle testingRespiratory device testingMedicineMedical testing
This disclosure describes systems and methods for testing a medical device. The disclosure describes a novel approach determining if the ventilator system is functioning properly without having to connect the medical device to a patient.
Owner:TYCO HEALTHCARE GRP LP
System and method for the automated presentation of system data to, and interaction with, a computer maintained database
InactiveUS20030061072A1Improve efficiencyEasy to take care ofComputer-assisted medical data acquisitionDiagnostic recording/measuringDiseaseDiscussion group
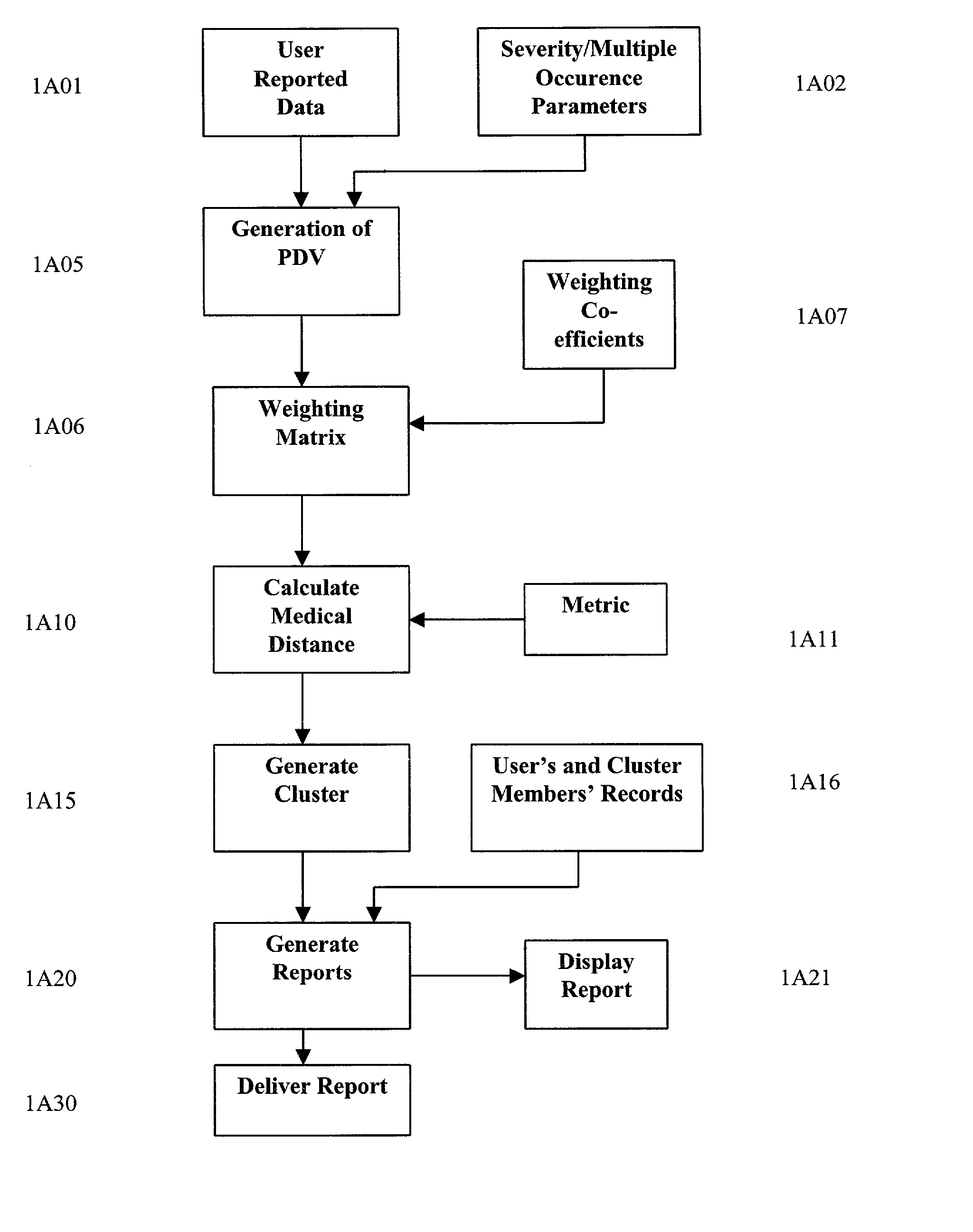
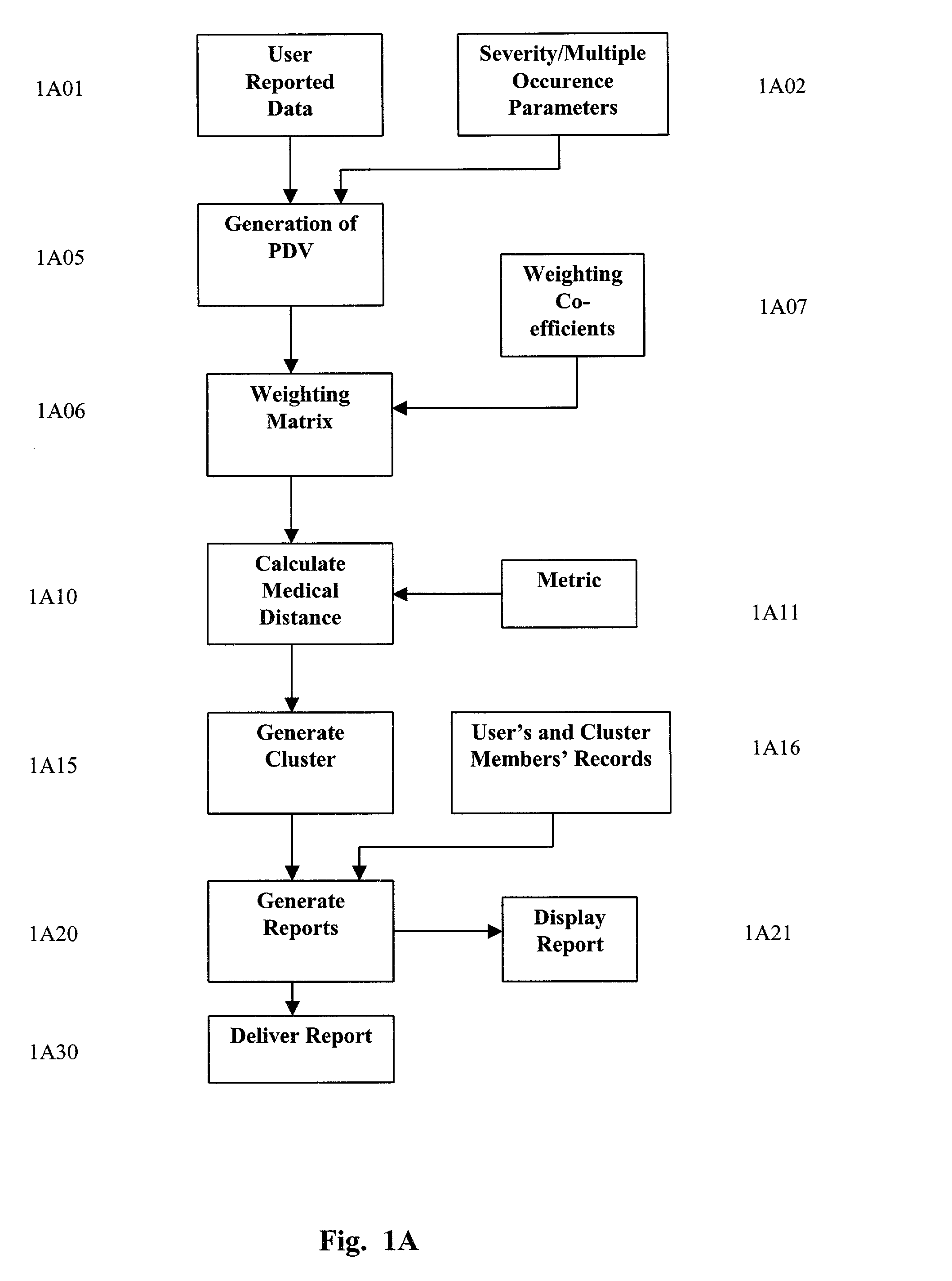
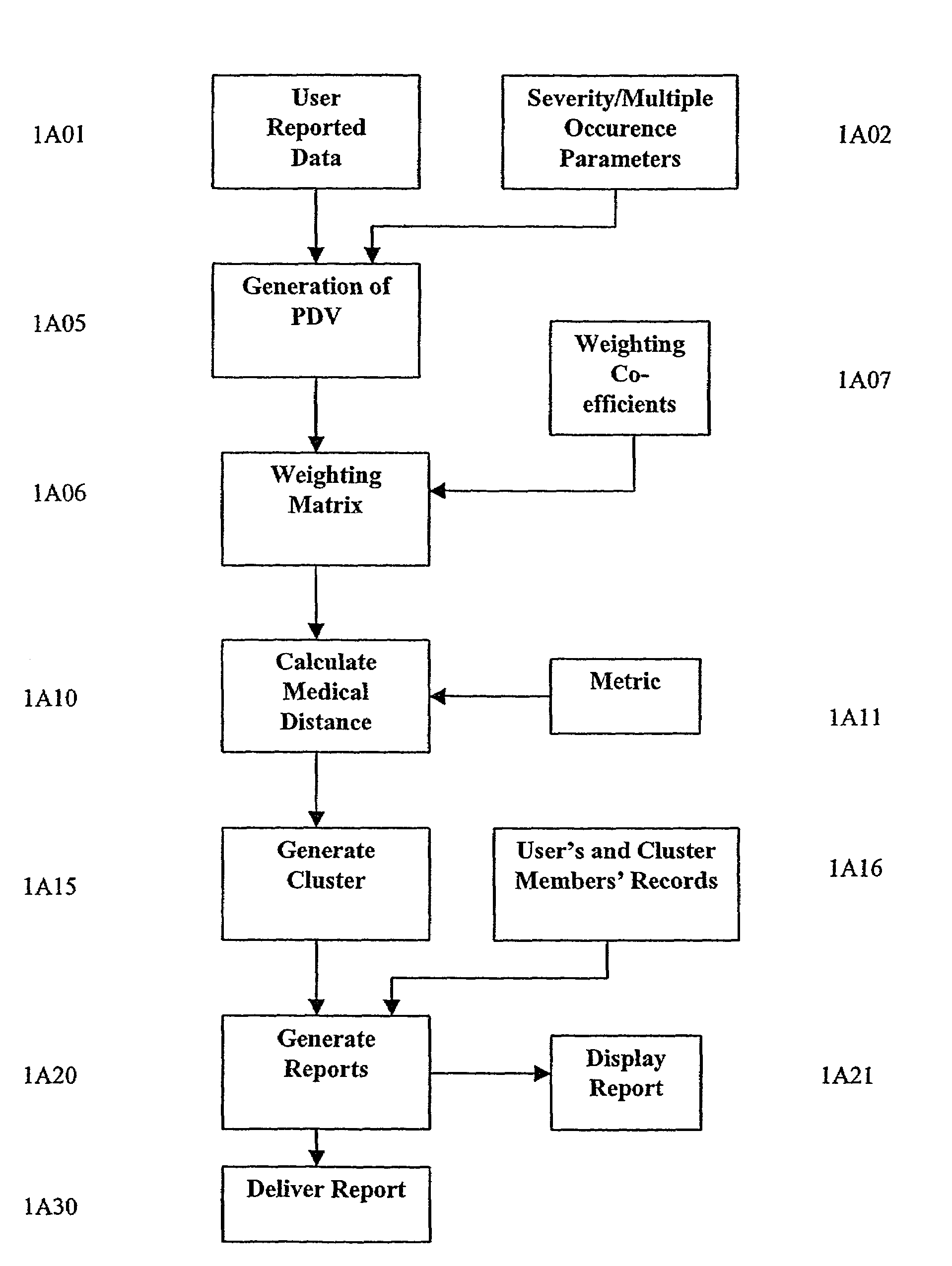
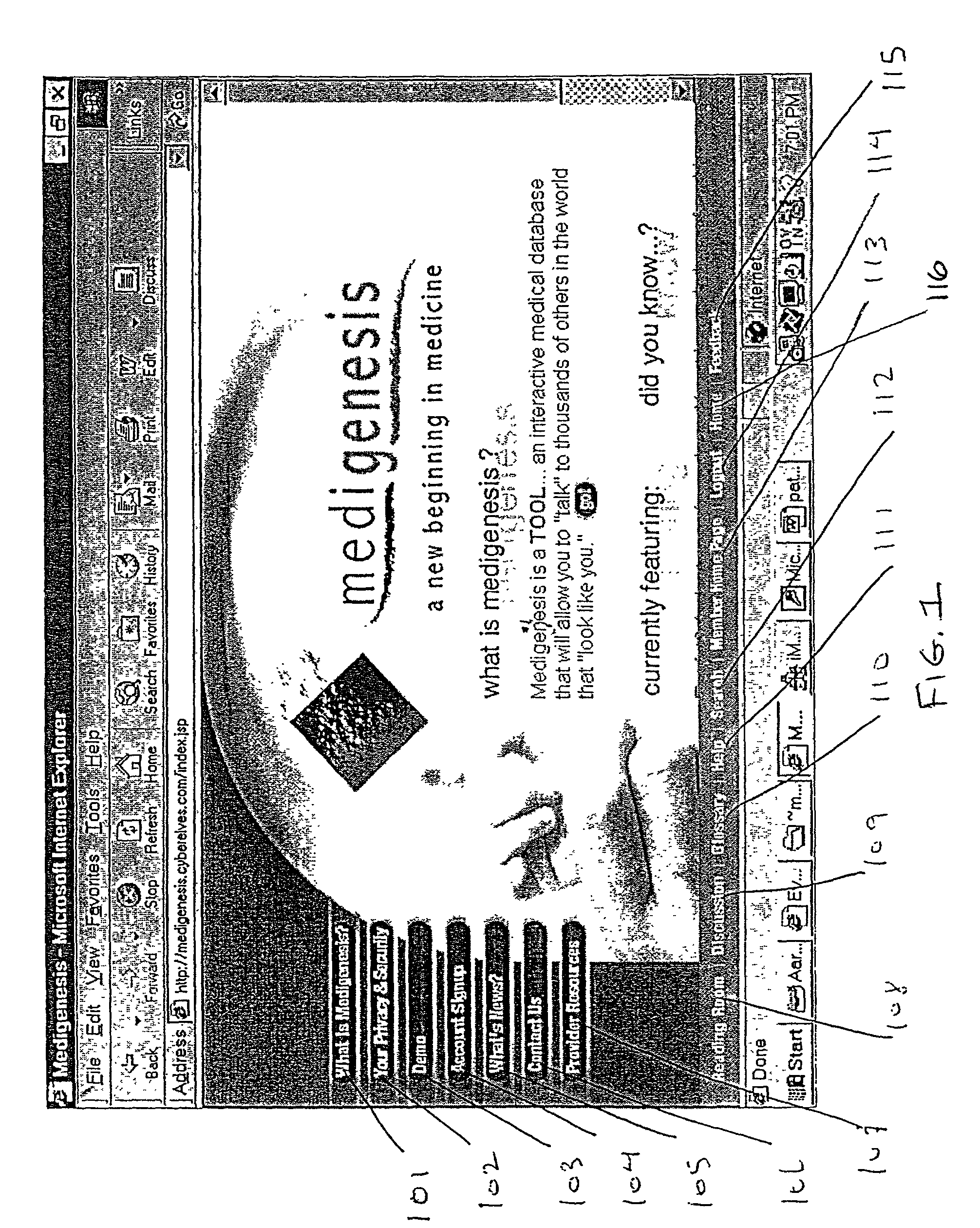
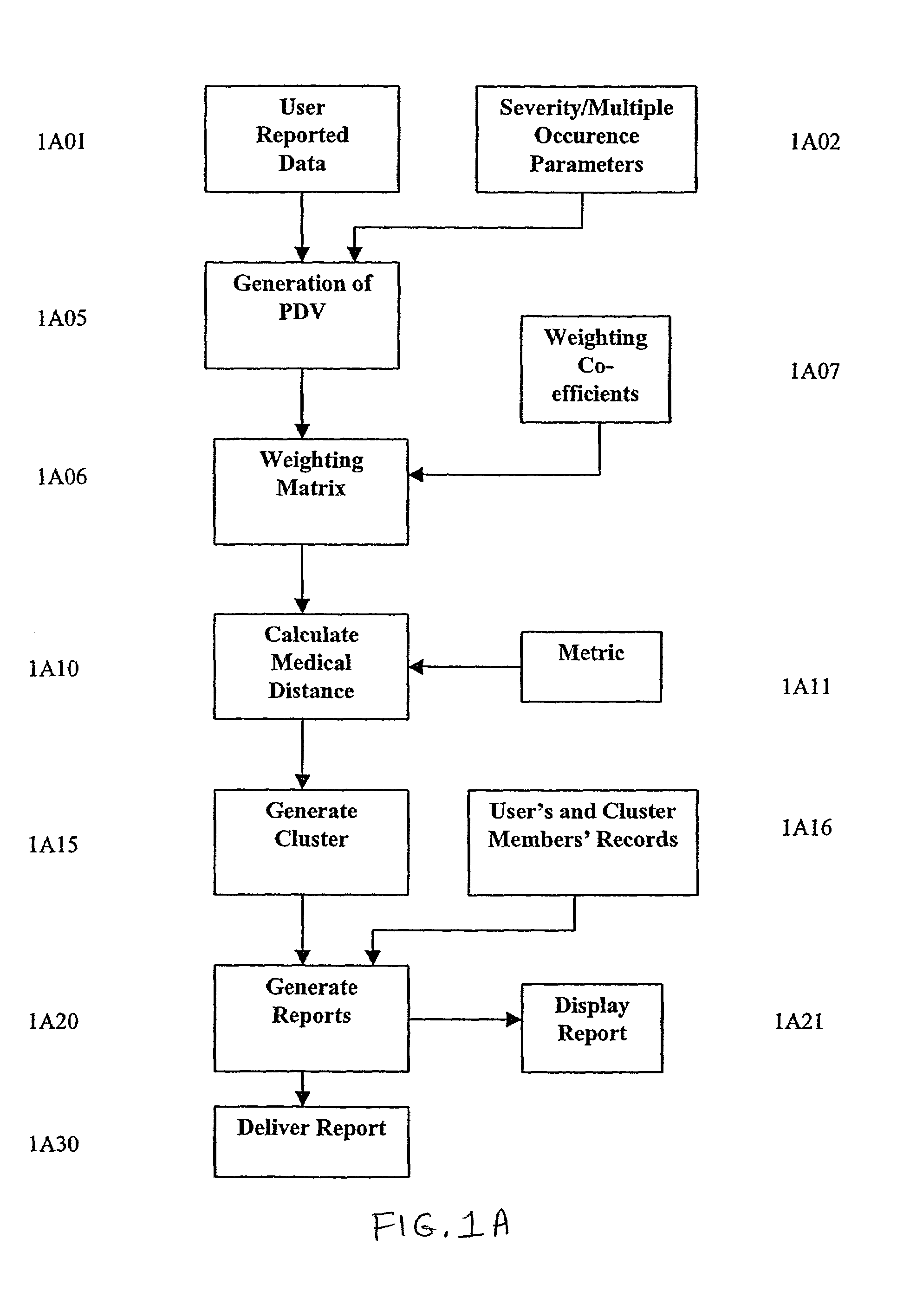
A system and method are provided for extracting a set of data from a system user descriptive of the complete health snapshot of the user's to interact with a database of numerous other users so as to generate a cluster of similar user's exhibiting a similar (within some system defined distance metric) health snapshot. The system guides the user to present his or her data via a complex questionnaire based upon a novel descriptive taxonomy, based upon the principles of "cyberhealth" as opposed to the standard medical "disease oriented" singular cause and effect model. The system generates the cluster of similar users, analyzes the cluster to obtain a ranked list of possible remedies or therapies to assist the user in dealing with health problems. The system further creates a computer networked virtual community of users with common health problems / interests, facilitates online chat, discussion groups, and the trading of health information. Additionally, the system provides listings of and links to health care providers and medical testing laboratories who are able to assist users of the system.
Owner:MEDIGENESIS
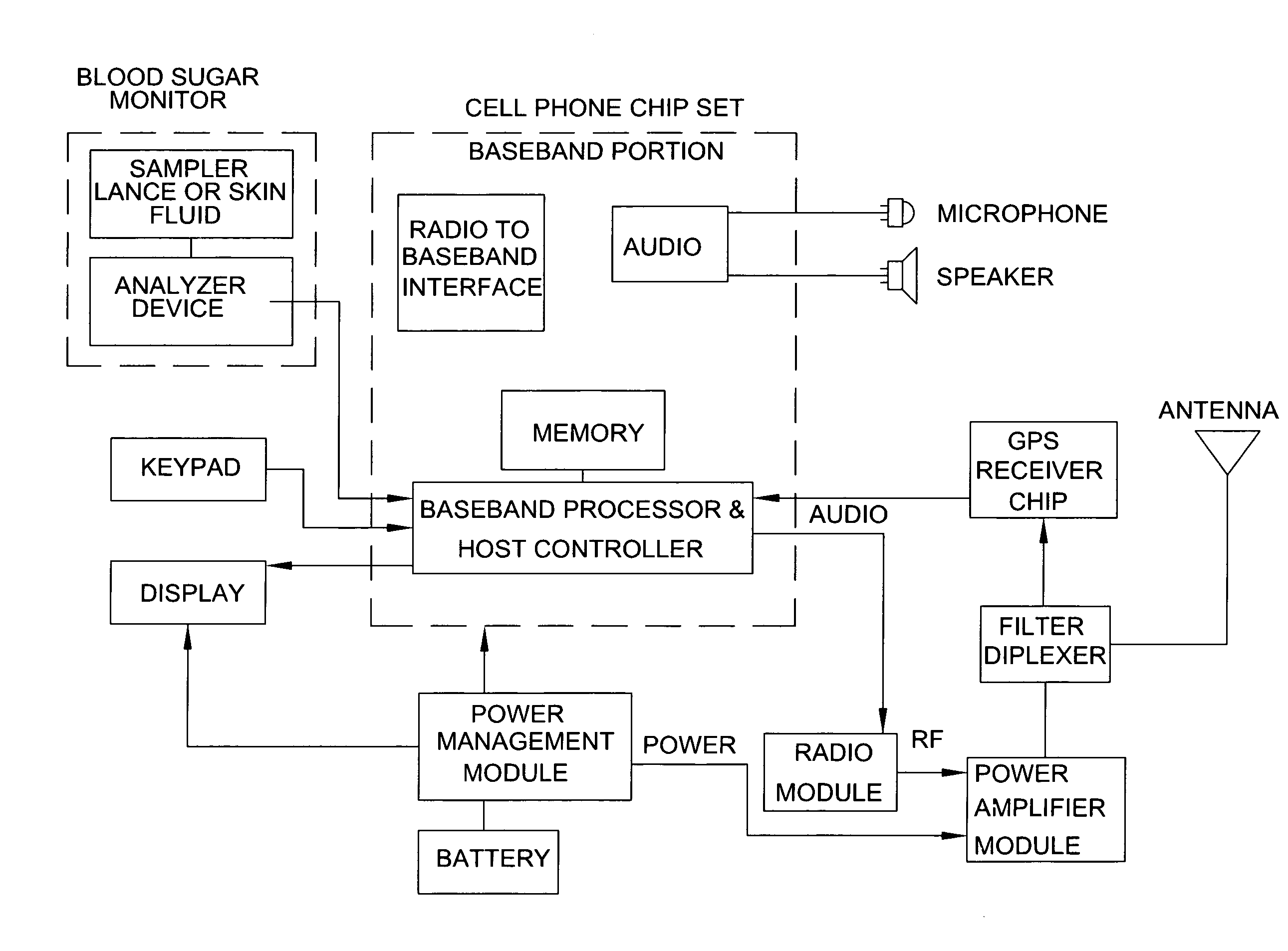
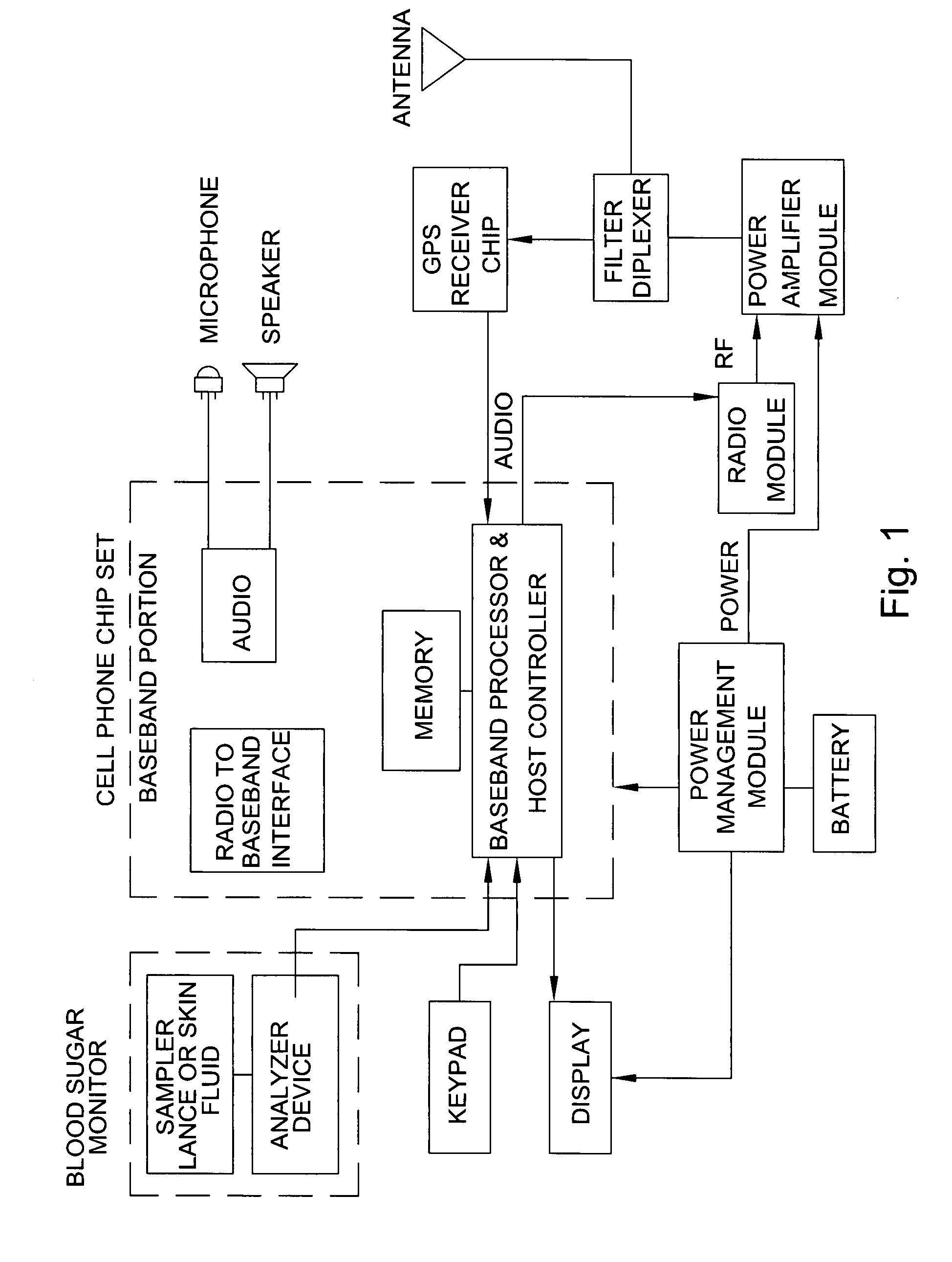
Combined cell phone and medical monitoring apparatus
InactiveUS20080070599A1The result is accurateEasily identifiableEvaluation of blood vesselsSensorsComputer hardwareMedical equipment
The present invention discloses an apparatus that combines a cellular phone or other wireless device with one or more medical monitoring devices, wherein the two devices share a single housing, power source, display, memory chip and data processor. The apparatus is capable of functioning as a separate medical apparatus and a normal cellular phone and addresses the need for combining multi-function portable medical testing and condition indicator devices into a device that is already carried by the majority of the population. The preferred embodiment of the invention combines a portable device of blood glucose monitoring with a cellular phone and is capable of audibly alerting the user of abnormal test results and storing multiple test results for monitoring and managing chronic conditions. In addition the apparatus is capable of storing and transmitting medical information to healthcare providers or emergency responders if highly abnormal results are obtained.
Owner:APODACA JENNIFER +1
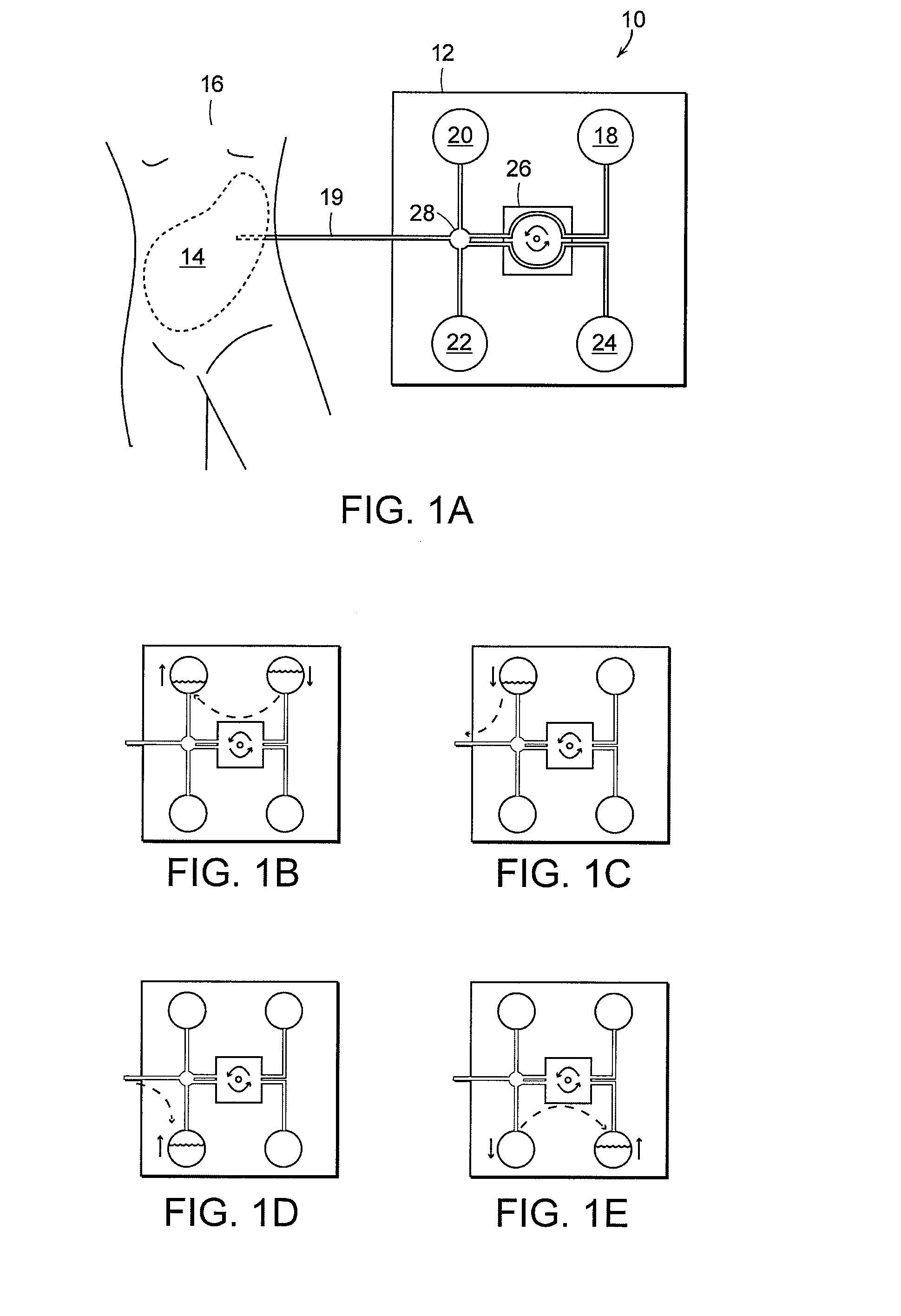
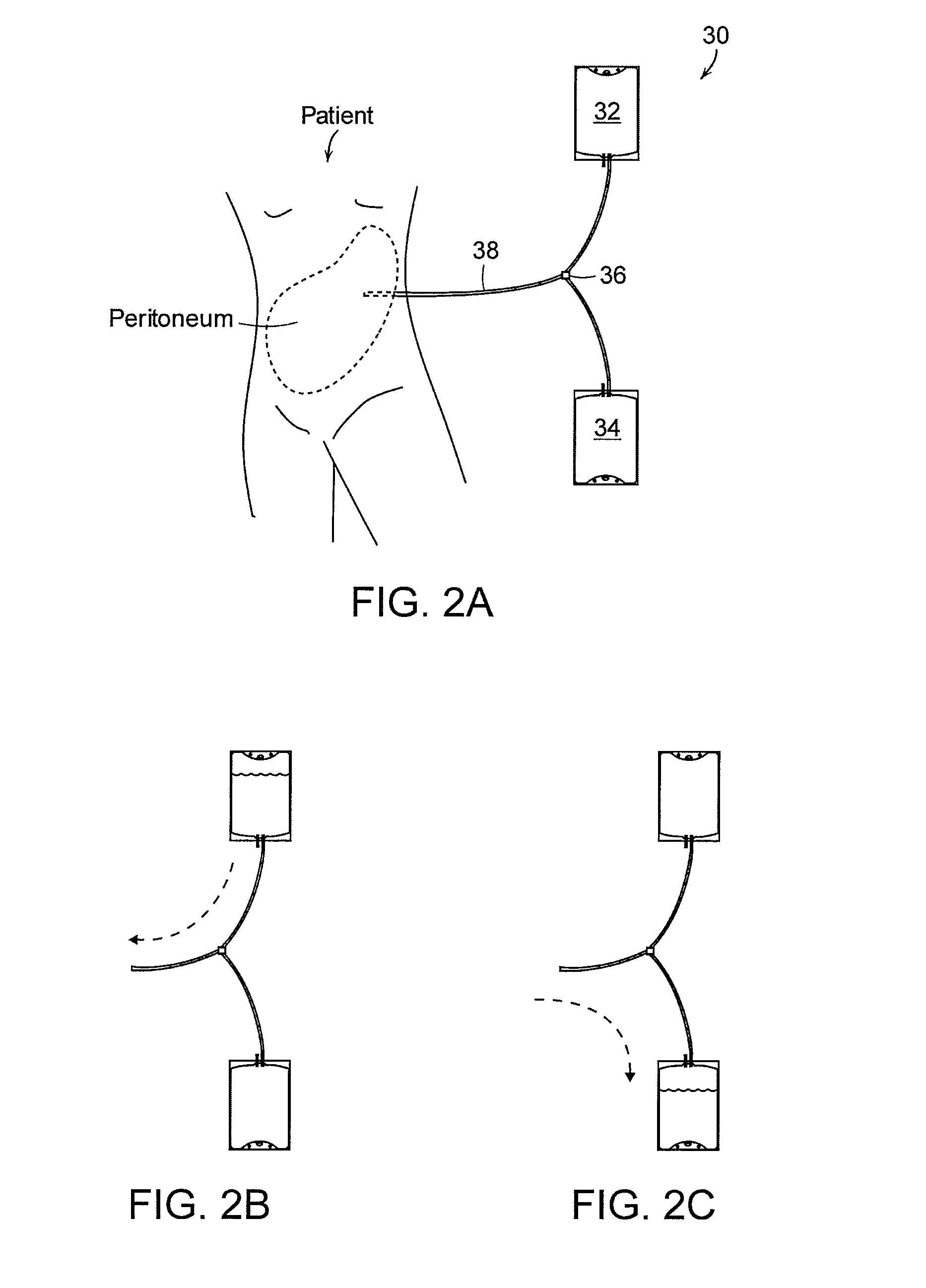
Apparatus and methods for early stage peritonitis detection and for in vivo testing of bodily fluid
InactiveUS20080183126A1Easy to cleanIncreased turbulenceDiagnostics using lightMedical devicesFiberIn vivo
The invention provides, inter alia, automated medical methods and apparatus that test PD effluent in a flow path (e.g., with an APD system or CAPD setup) to detect, for example, the onset of peritonitis, based on optical characteristics of the effluent resolved at cellular scales of distance. For example, according to one aspect of the invention, an APD machine includes, in an effluent flow path, apparatus for early stage peritonitis detection comprising an illumination source and a detector. The source is arranged to illuminate peritoneal effluent in a chamber that forms part of the flow path, and the detector is arranged to detect illuminant scattered by the effluent. The detector detects that reflected or scattered illuminant at a cellular scale of resolution, e.g., on a scale such that separate cellular-sized biological (or other) components in the effluent can be distinguished from one another based on scattering events detected by the detector. Other aspects of the invention provide automated medical testing methods and apparatus that detect the onset of peritonitis and other bodily conditions by testing fluids in the body in vivo, e.g., the patient's peritoneum. Such apparatus and methods utilize a first fiber optic bundle to carry illuminant from a source of the type described above into a bodily organ or cavity, and a second fiber optic bundle to carry illuminant scattered by fluid in that organ or cavity to a detector as described above.
Owner:FRESENIUS MEDICAL CARE HLDG INC
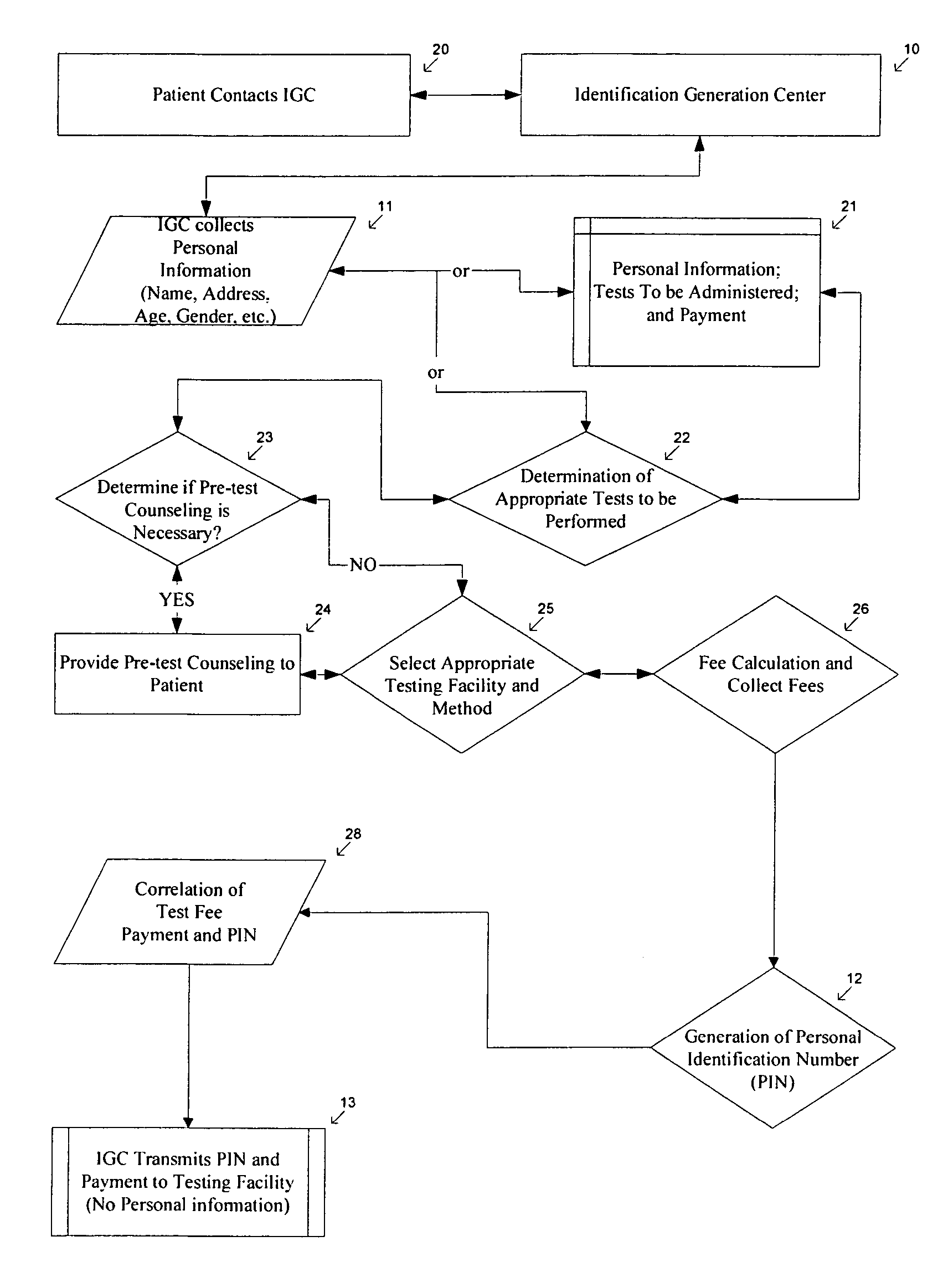
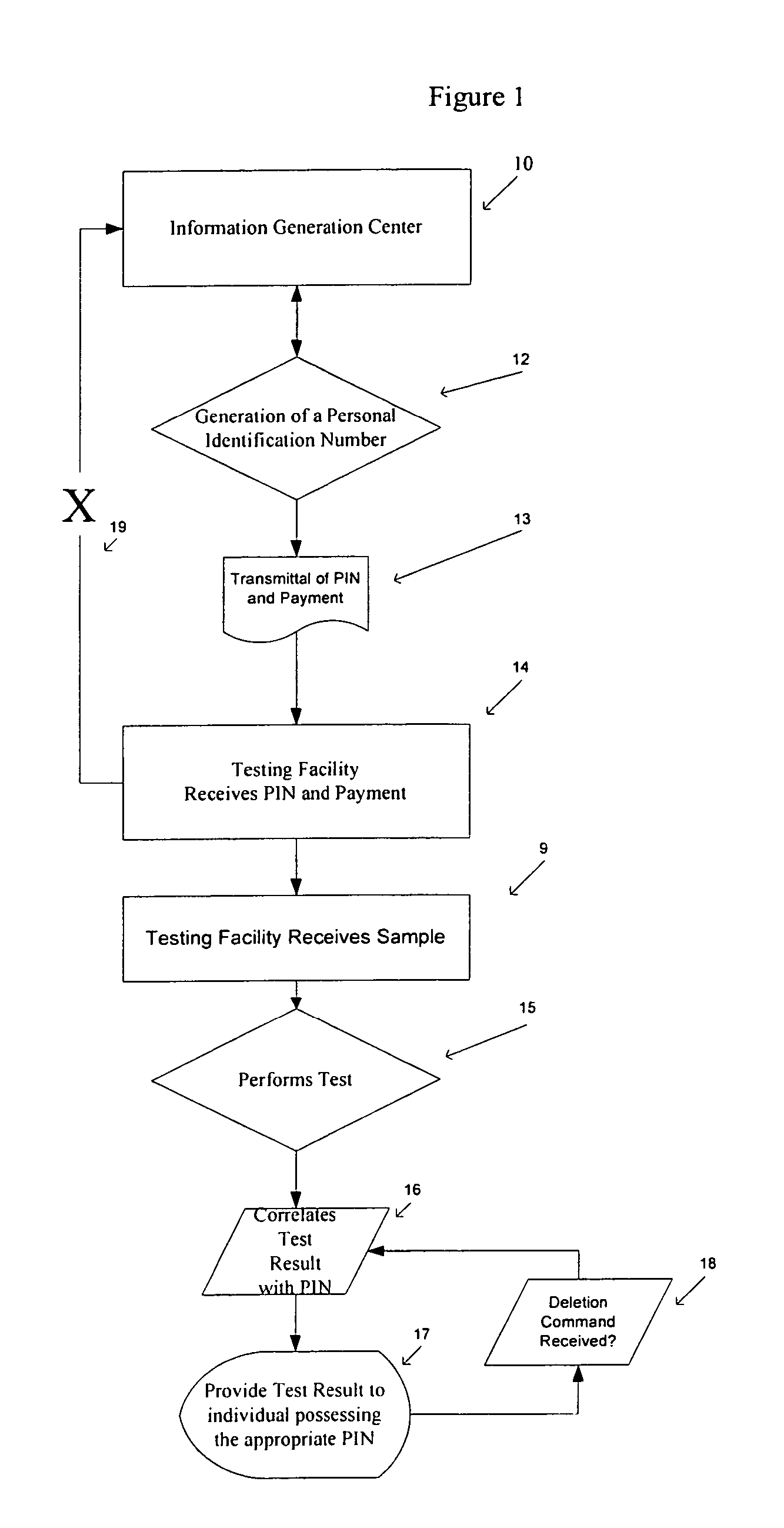
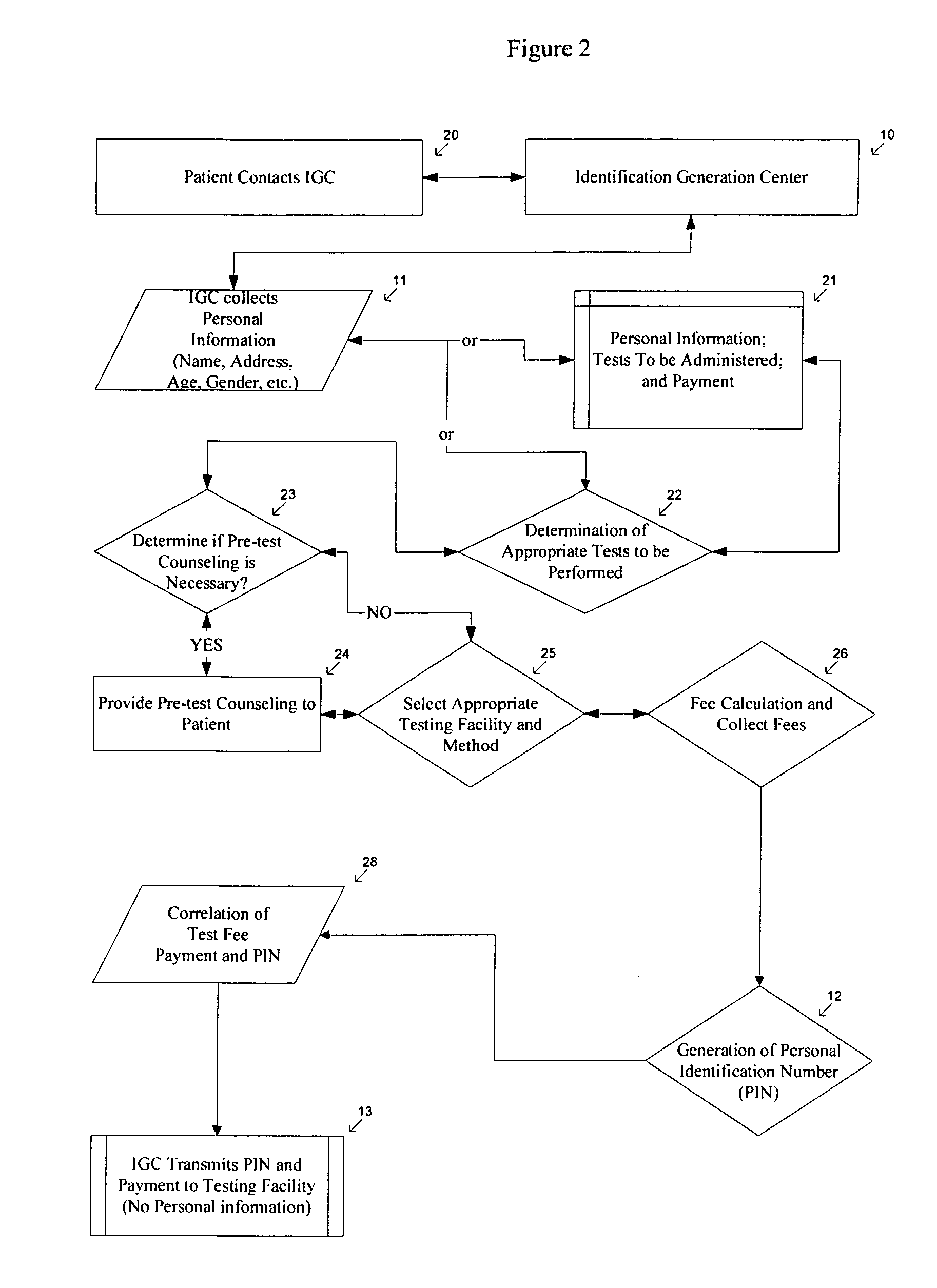
Method of anonymous medical testing and providing the patient with the test results
InactiveUS20050075543A1Maintaining subject 's anonymityMaintaining anonymityComputer-assisted medical data acquisitionDiagnostic recording/measuringPersonal identification numberTest sample
A system for delivering confidential test results to anonymous subjects is described. The system includes procuring a unique personal identifier or personal identification number (PIN) from a first source, which communicates that PIN to a second source that is capable of taking and / or testing a suitable test specimen from the patient being tested. The second source receives a biological sample identified by the PIN from the patient or a patient representative and performs a test on the biological sample to detect genetic and / or medical information and provides a test result. The test result is then provided to the patient through an internet site, phone system or in person, and accessed by the patient through the unique PIN.
Owner:CALABRESE CHARLES A
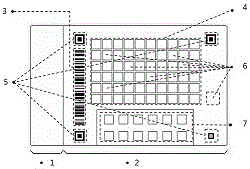
Test paper detection card intelligent detection system and test paper detection card intelligent analysis method
ActiveCN106546581AEliminate distracting factorsHigh sensitivityImage enhancementImage analysisColored whiteBarcode
The invention provides a test paper detection card intelligent detection system and a test paper detection card intelligent analysis method. The test paper detection card intelligent detection system comprises a test card detection card and an intelligent terminal; the test paper detection card includes a test paper detection area; four corner positions of the test paper detection area are each provided with a positioning mark; a standard color area formed by a plurality of standard color blocks with different colors is arranged at one side of the test paper detection area; a reaction area formed by arraying a plurality of reaction blocks is arranged at the other side of the test paper detection area; the test paper detection area also is distributed with a validation area formed by a plurality of white shadow validation color blocks; a bar code area is arranged in the test paper detection area. The test paper detection card intelligent detection system and the test paper detection card intelligent analysis method have the advantages that environmental interference factors during image shooting are effectively eliminated, and the sensitivity and accuracy of detection results are improved; and in addition, a detection and analysis algorithm is simple, so the detection time can be shortened, and the system and the method can play an important role in urine routine, blood routine and other medical detection.
Owner:长沙云知检信息科技有限公司
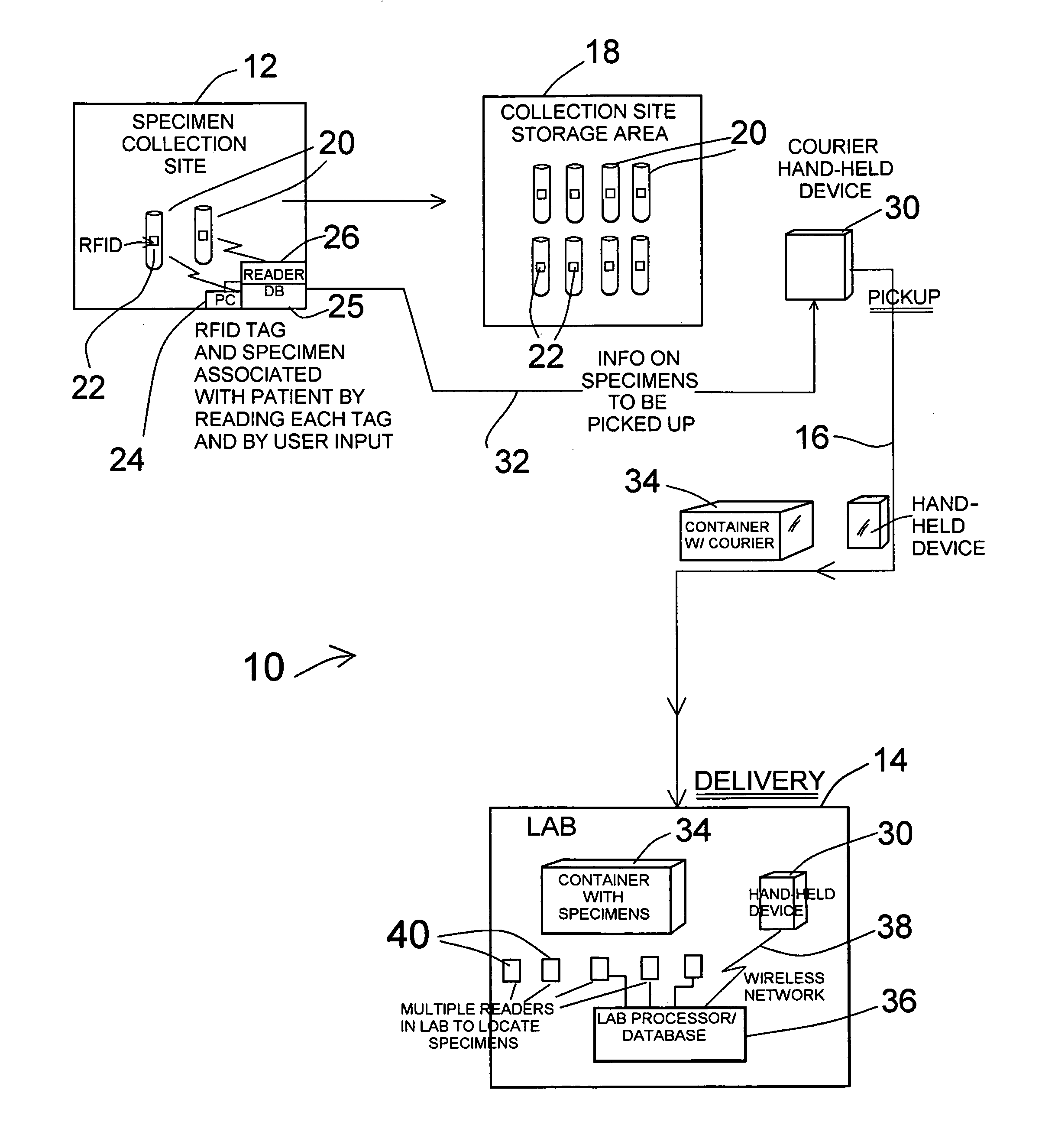
RFID tracking of patient specimen samples
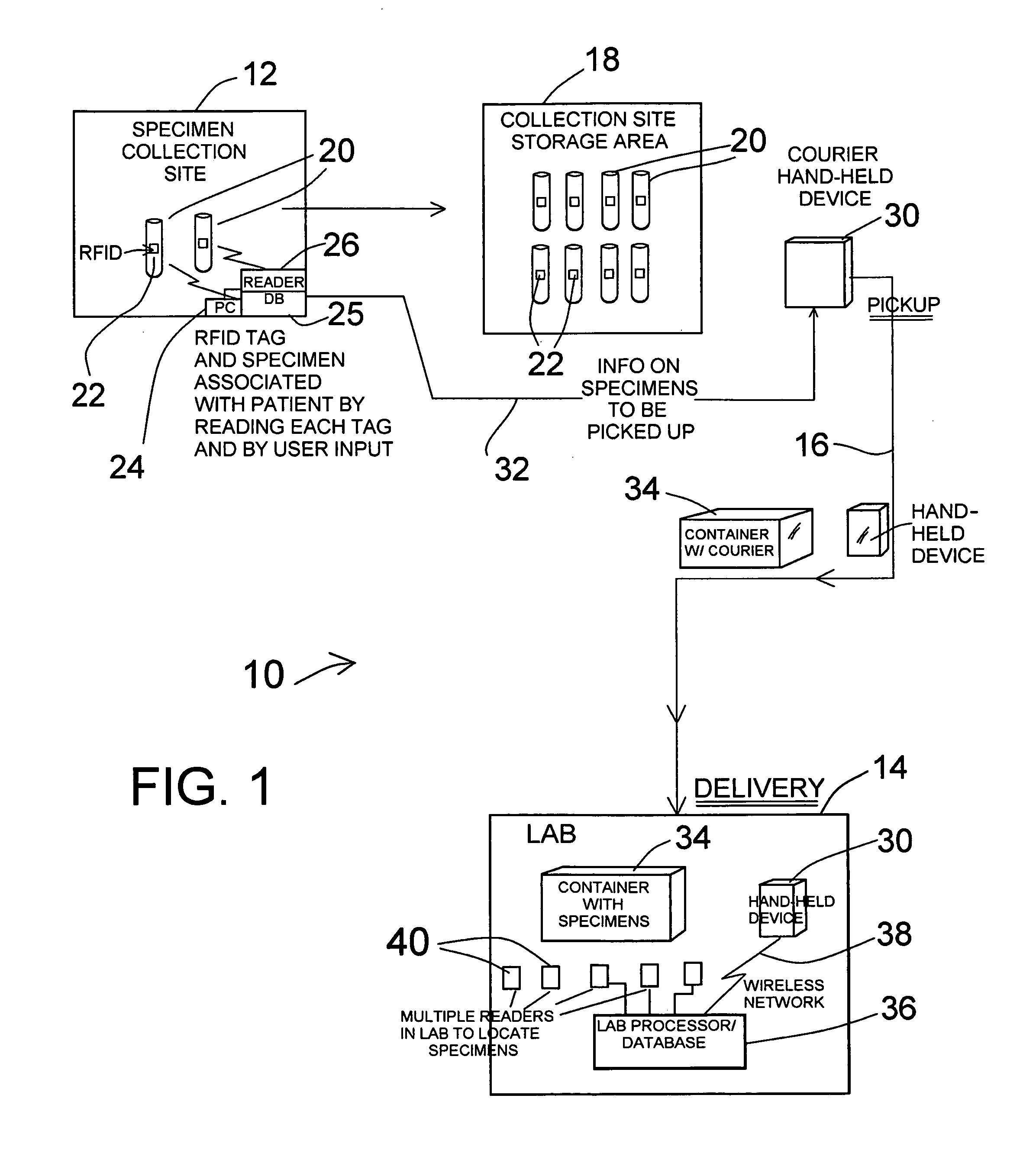
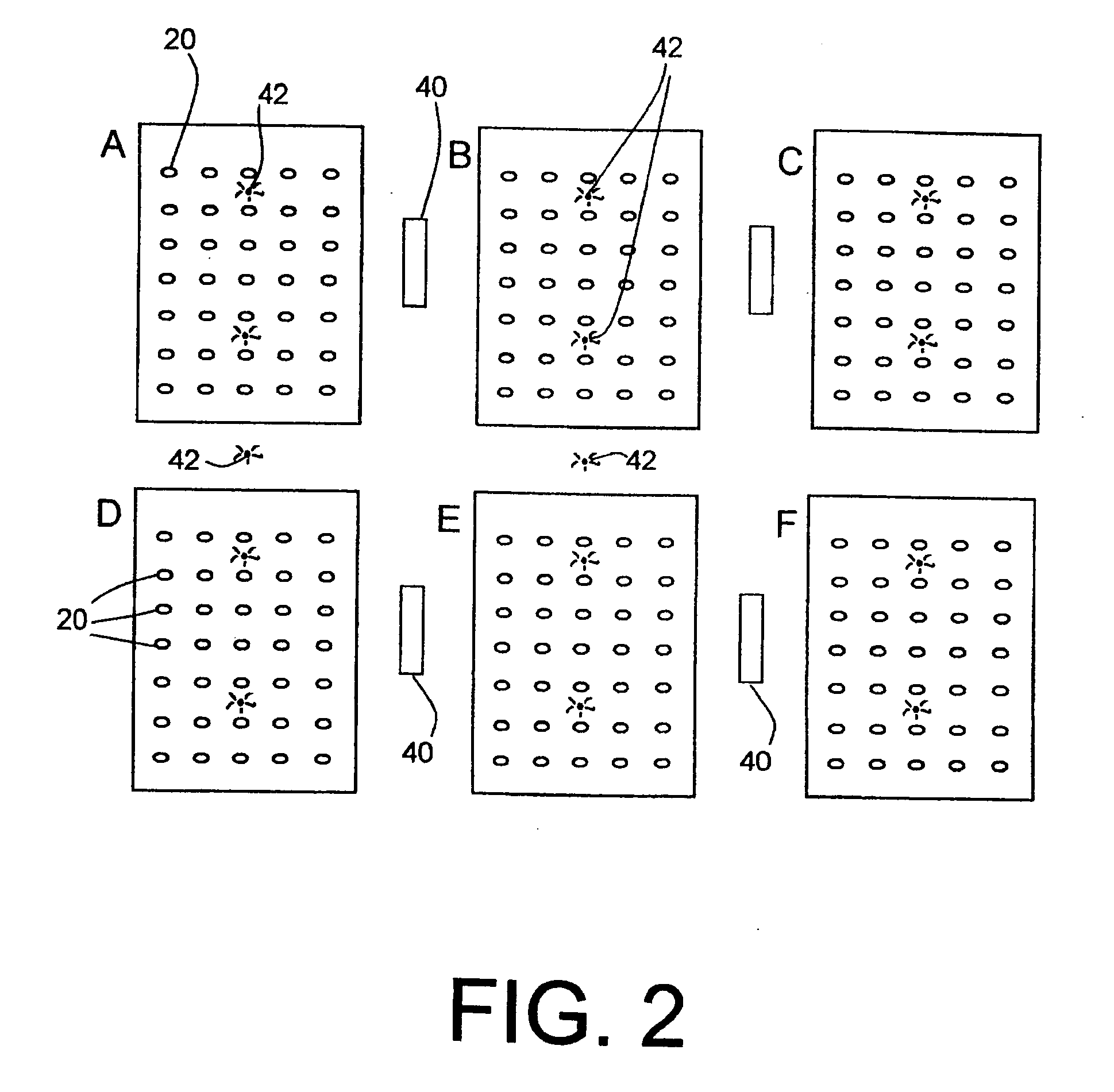
InactiveUS20120025985A1Precise positioningInterference minimizationComputer-assisted medical data acquisitionLogisticsHand heldHand held devices
Miniature RFID tags are used in a system for identifying, locating, tracking and inventorying patient specimens pursuant to medical testing. The RFID tags are attached to specimen vessels, and at a point of collection for patient specimens each RFID tag of a vessel is associated with patient and test data, in a collection site database. When a series of vessels are to go to a laboratory, a hand-held device receives all data on the specimens via download from the collection site PC / database. A courier picks up a container with the specimen vessels and delivers it to the laboratory, along with the hand-held device. At the lab a reader reads all specimen tags, and the data stored in the hand-held device is downloaded to a lab processor / database to verify all specimens are present. Location of specimens can be done by reading or powering up different zones, and the hand-held device can have a power node for selectively powering one or several specimen tags for identification or location of specific specimens.
Owner:BOLANDER JARIE G +2
Dermal lance with nerve stimulus
In some arrangements, a dermal lance apparatus is adapted to pierce a person's skin to obtain bodily fluids for medical testing. The lance can stimulate nerves in the person's skin to reduce or eliminate pain during the lancing procedure. After the nerves are stimulated, the lance can form the opening at a lance point. The lance can use mechanical motion to stimulate the person's skin. Alternatively, the lance can affect the temperature of the person's skin for effective stimulation.
Owner:OPTISCAN BIOMEDICAL
System and method for the automated presentation of system data to, and interaction with, a computer maintained database
InactiveUS7676384B2Computer-assisted medical data acquisitionDiagnostic recording/measuringDiseaseDiscussion group
A system and method are provided for extracting a set of data from a system user descriptive of the complete health snapshot of the user's to interact with a database of numerous other users so as to generate a cluster of similar user's exhibiting a similar (within some system defined distance metric) health snapshot. The system guides the user to present his or her data via a complex questionnaire based upon a novel descriptive taxonomy, based upon the principles of “cyberhealth” as opposed to the standard medical “disease oriented” singular cause and effect model. The system generates the cluster of similar users, analyzes the cluster to obtain a ranked list of possible remedies or therapies to assist the user in dealing with health problems. The system further creates a computer networked virtual community of users with common health problems / interests, facilitates online chat, discussion groups, and the trading of health information. Additionally, the system provides listings of and links to health care providers and medical testing laboratories who are able to assist users of the system.
Owner:MEDIGENESIS
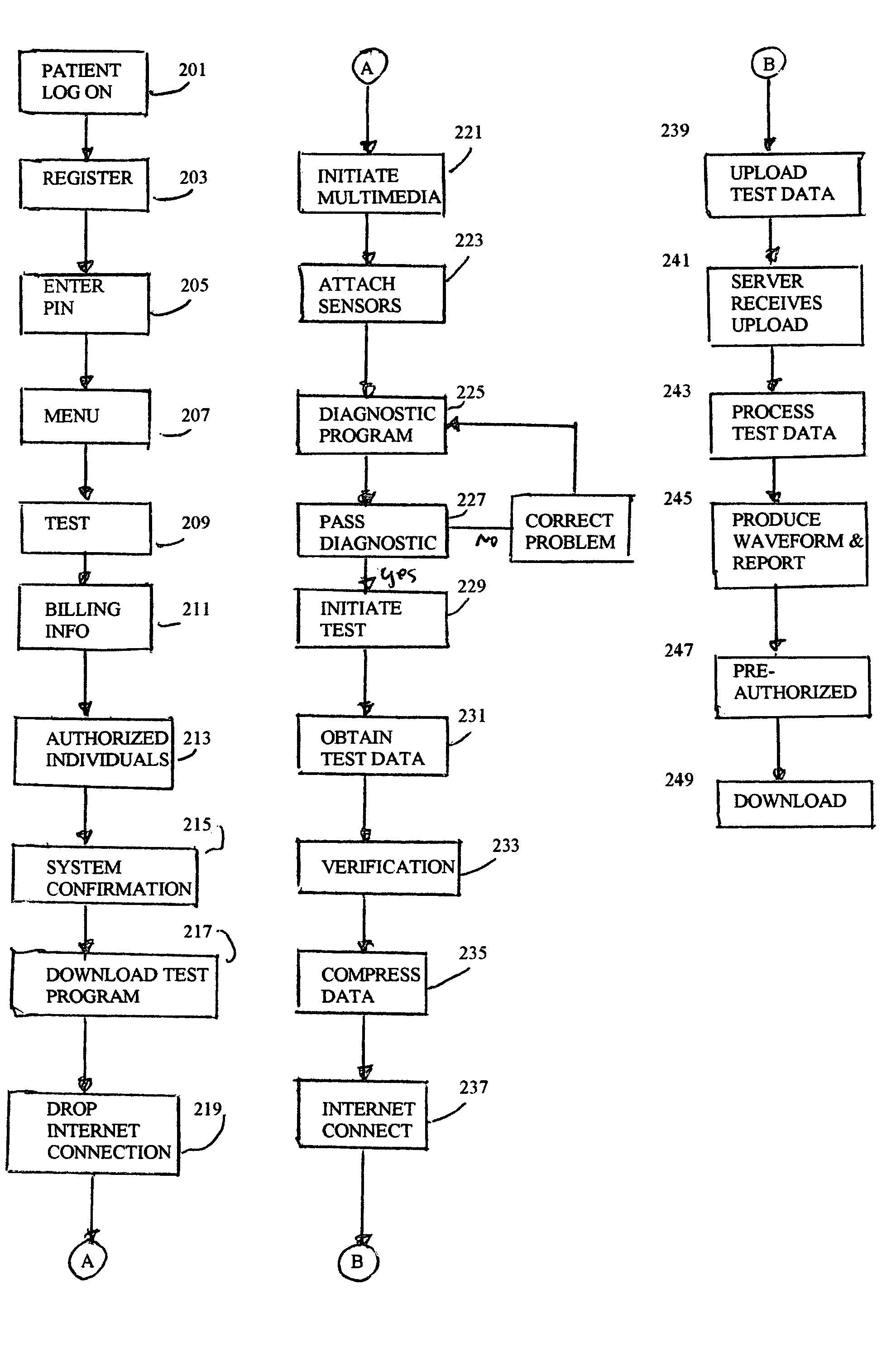
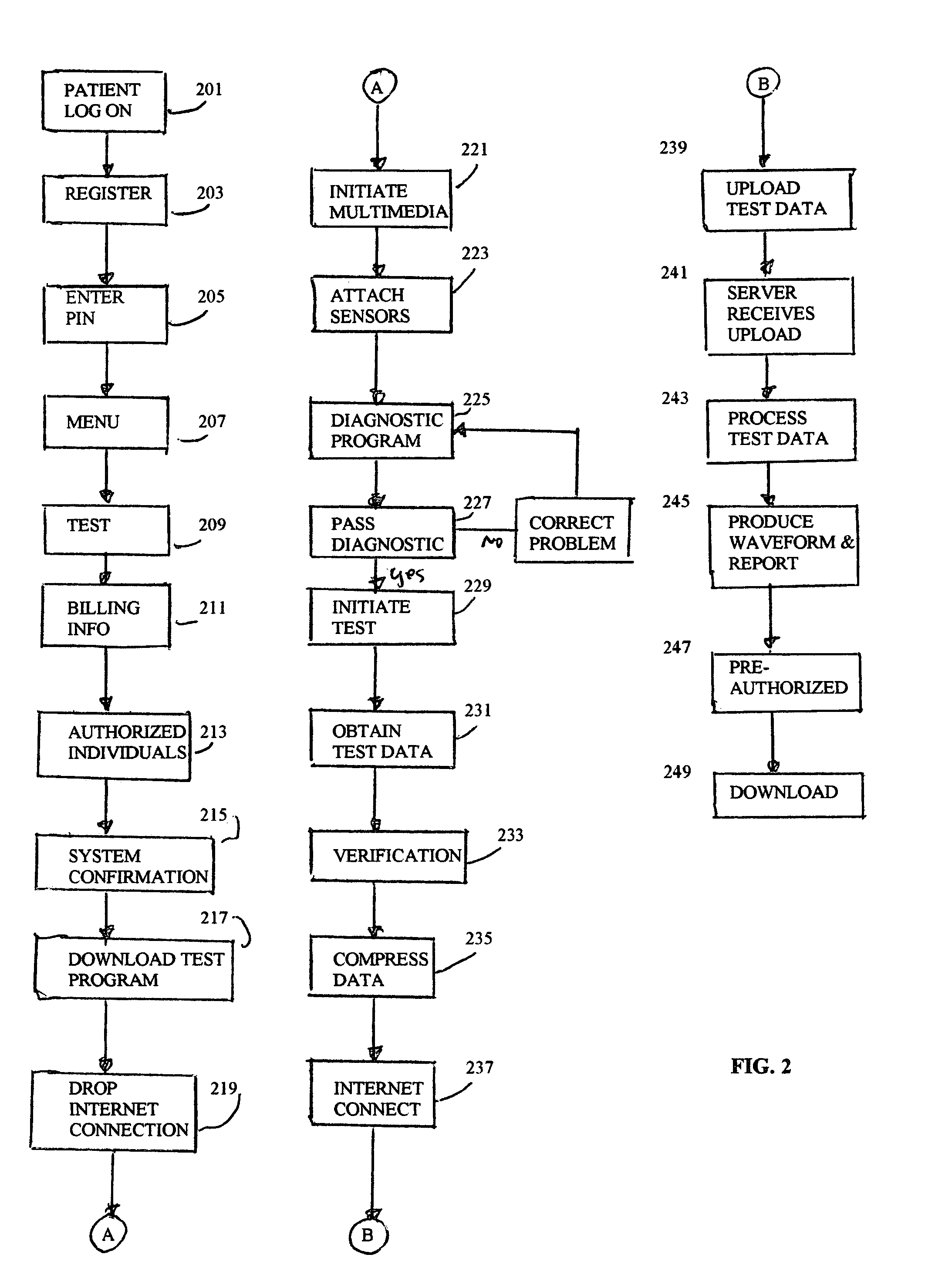
Internet device operation for medical testing
InactiveUS6963907B1Wide rangeDecision supportUltrasonic/sonic/infrasonic diagnosticsCatheterTest measurementThe Internet
A method of operating an Internet device, includes downloading via the Internet a medical testing program from a server. At least one sensor is coupled to the Internet device. The sensor is attached or otherwise coupled to a patient. The Internet device executes the test program to obtain test measurement data from the at least one sensor. The test measurement data is uploaded to the server via the Internet. The Internet device receives processed test data from the server as a download from the server via the Internet. The Internet device displays the processed data.
Owner:CARDIOBEAT COM
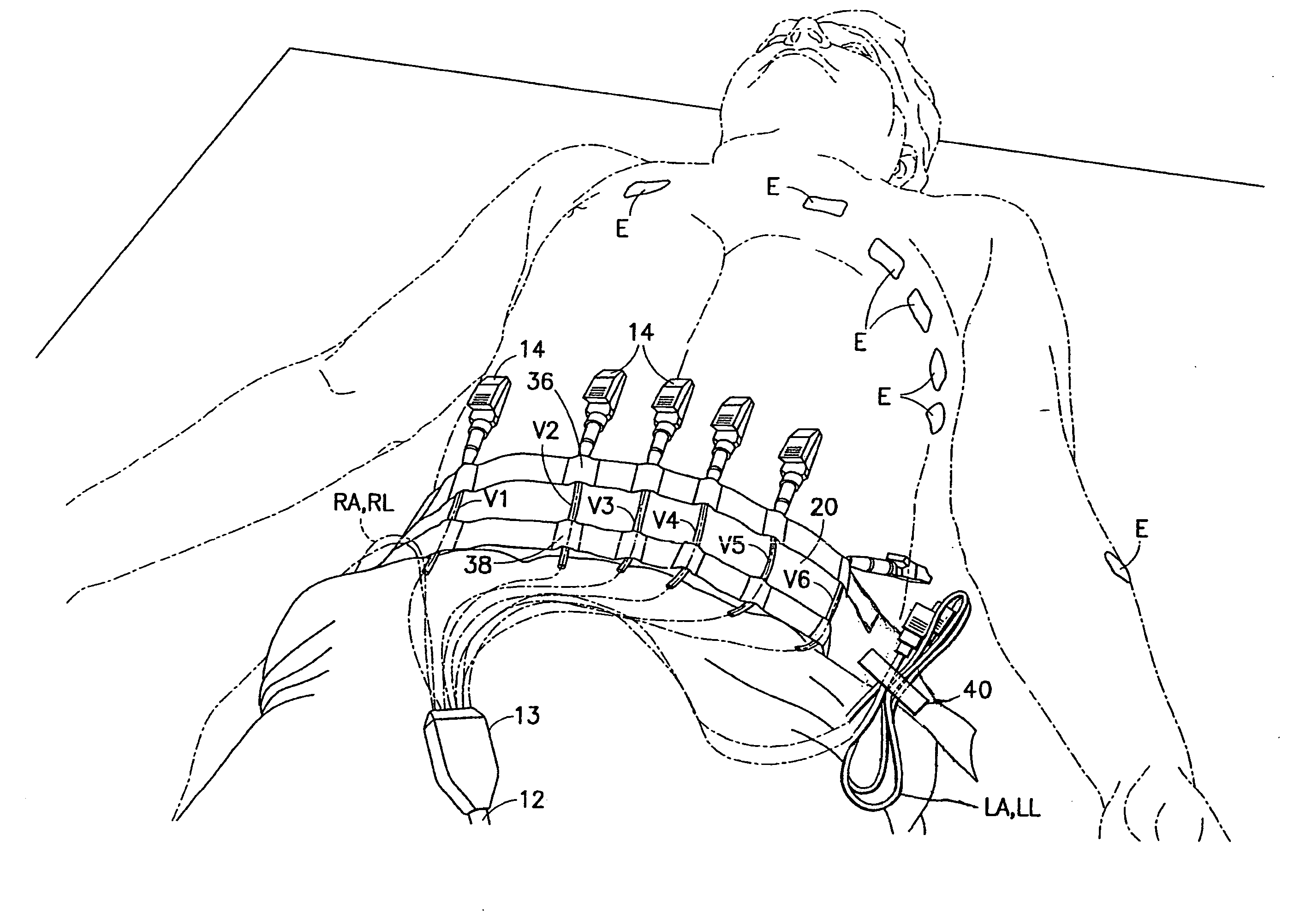
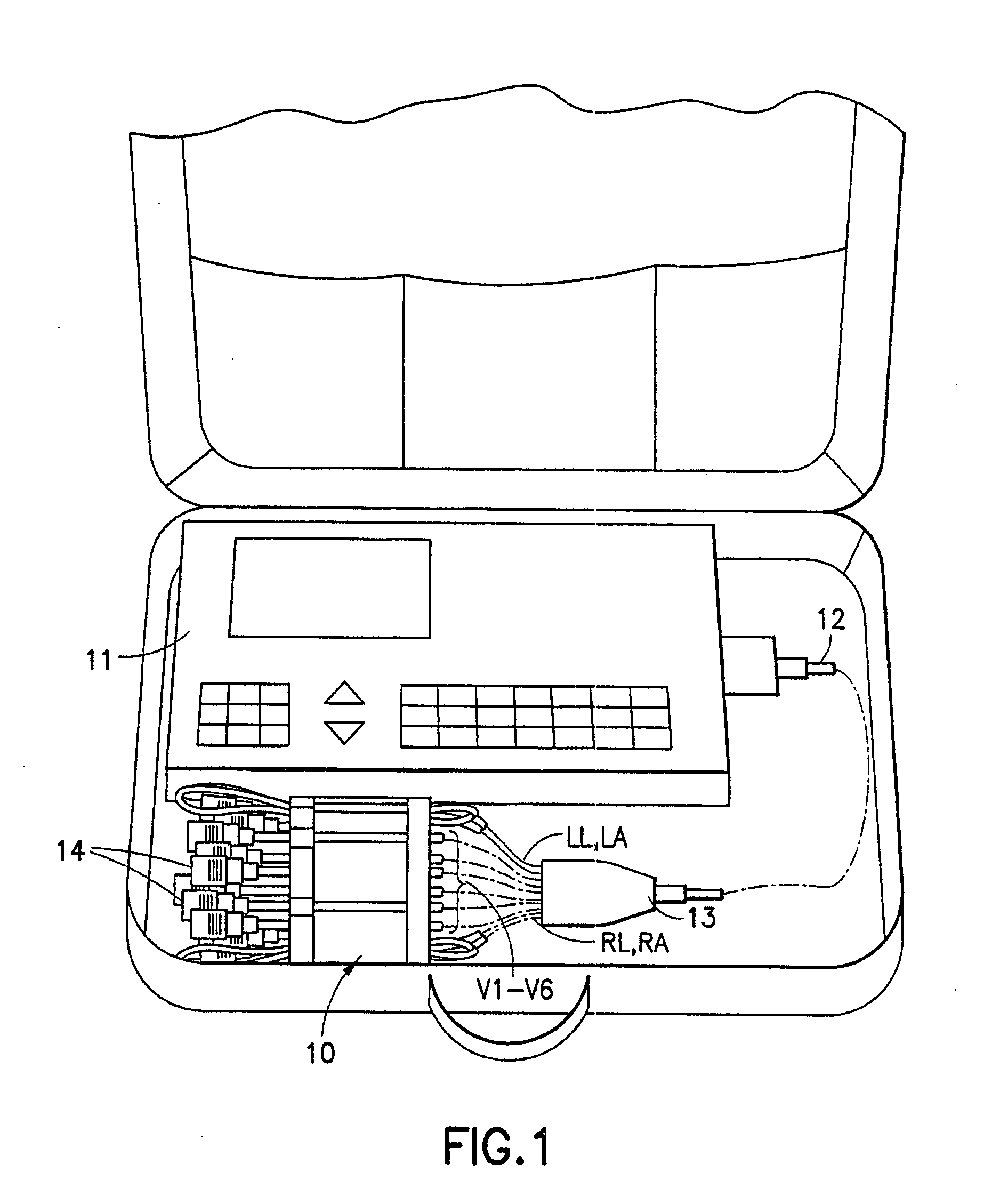
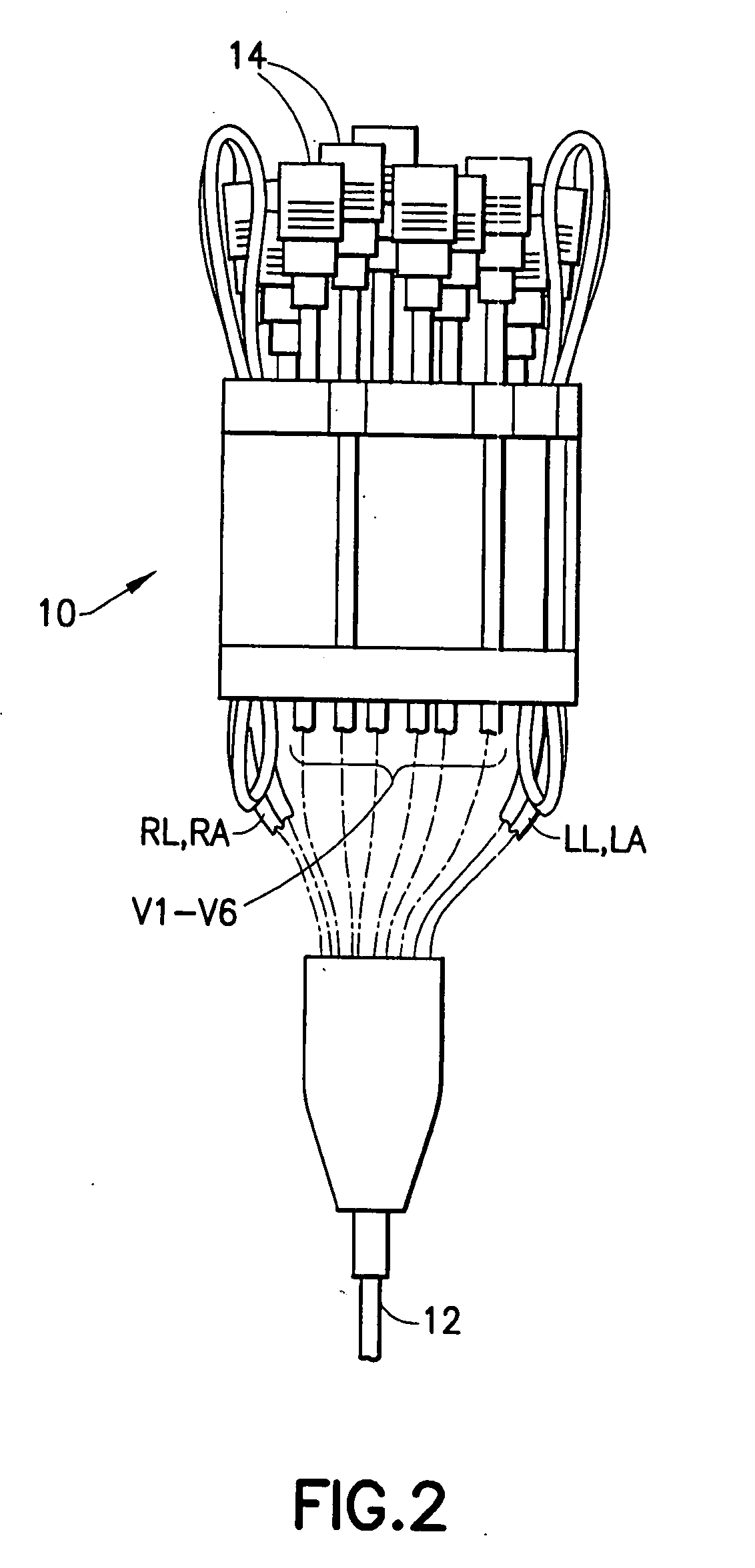
Device for arranging wire leads from medical testing apparatus
InactiveUS20070282350A1Easy to reachAvoid separationElectrocardiographySurgeryEngineeringMedical testing
A portable lead alignment device is provided for aligning, organizing and storing in a pre-assembled fashion the leads extending from an EKG apparatus. The device includes an elongate flexible support with at least one linear array of chest lead alignment tunnels disposed thereon. Each tunnel snugly accommodates a chest lead so that the lead can be slid longitudinally relative to the support. However, the tunnels prevent the leads from being separated from the device. The lead alignment device may further include a plurality of ground lead organizers for releasably retaining the ground leads in a neat looped arrangement on the support.
Owner:HERNEST LYNNE J
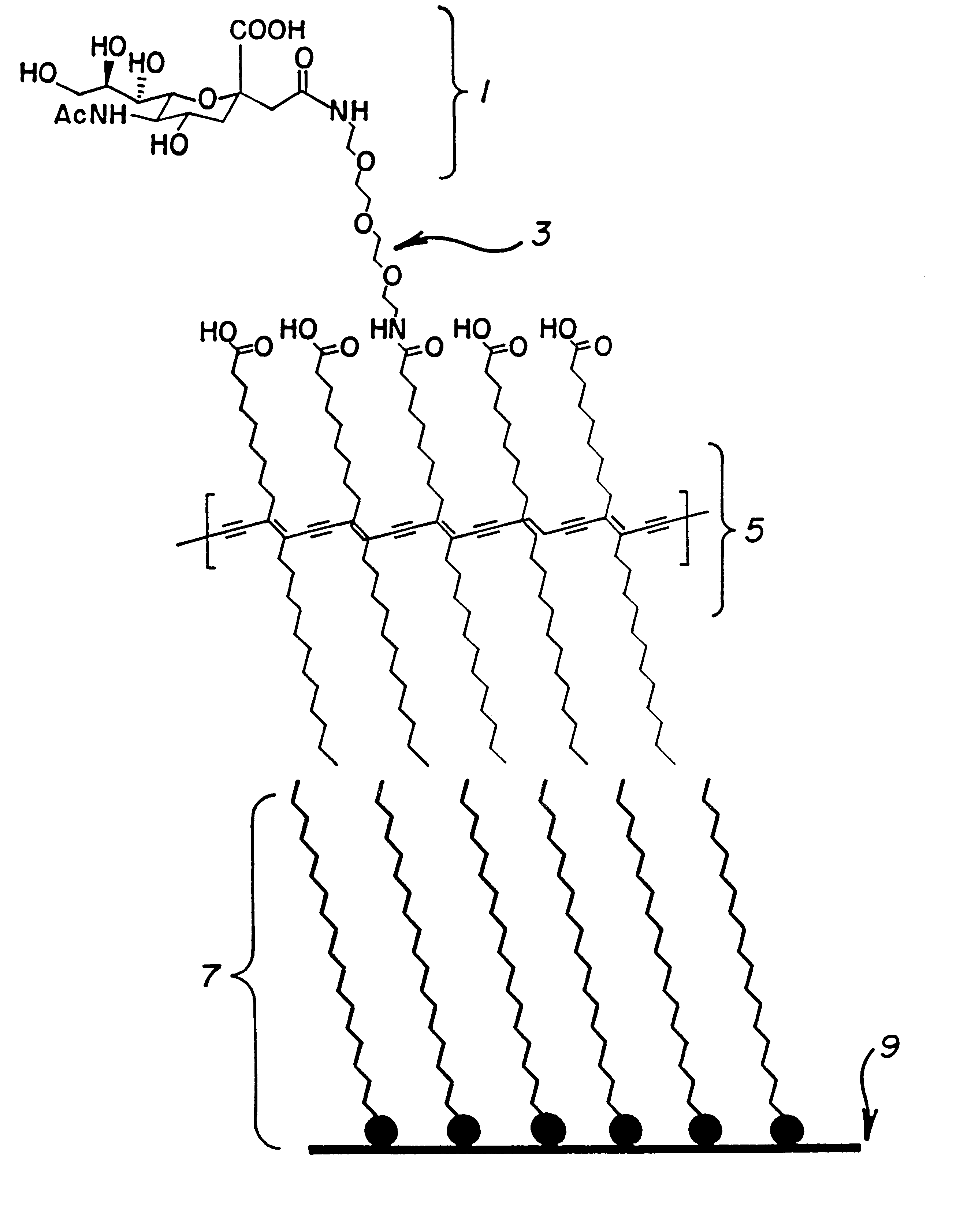
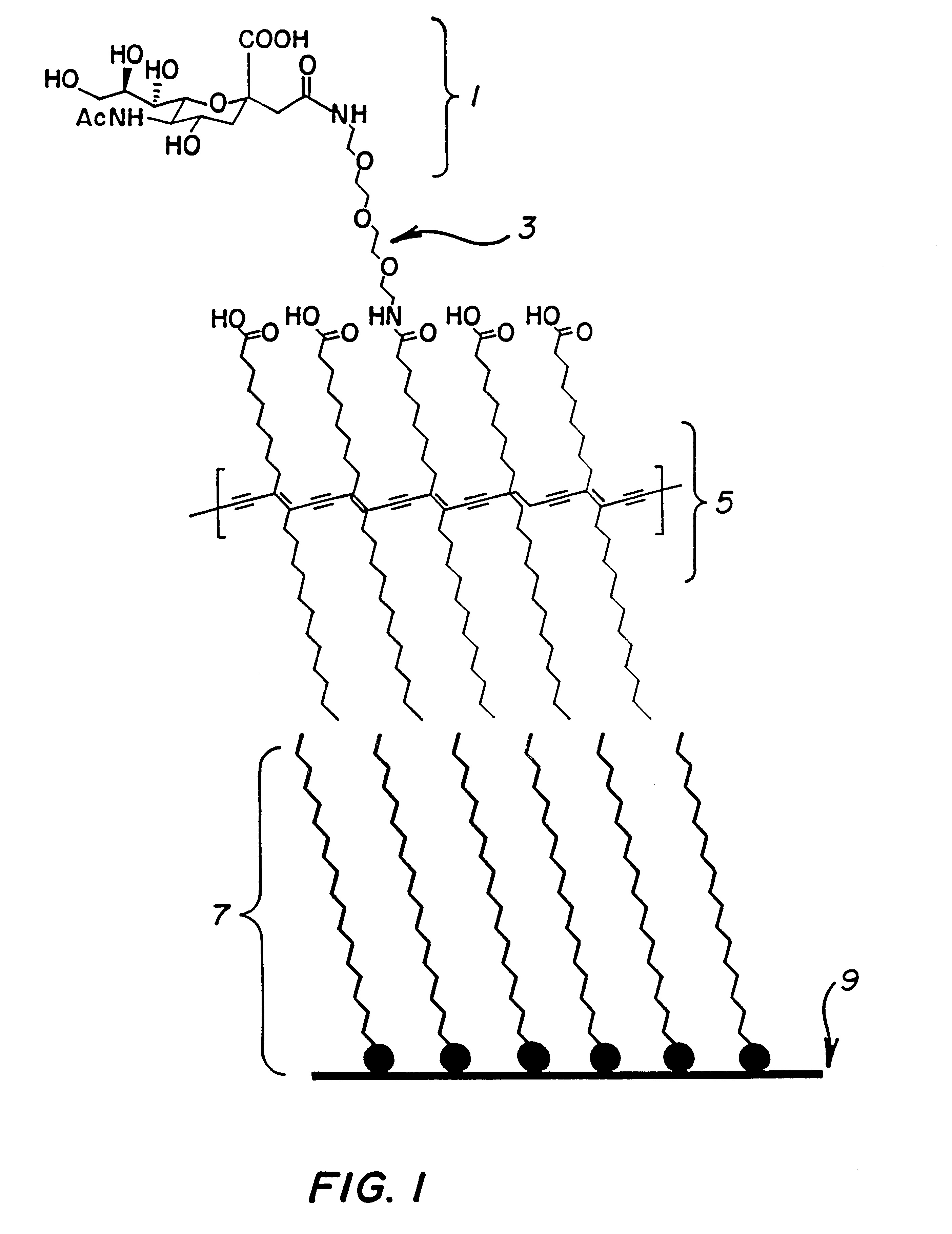
Polymeric assay film for direct colorimetric detection
InactiveUS6395561B1Limited applicabilityMaintain its infectivityMaterial nanotechnologyMicrobiological testing/measurementAnalyteDrug development
A lipid bilayer with affinity to an analyte, which directly signals binding by a changes in the light absorption spectra. This novel assay means and method has special applications in the drug development and medical testing fields. Using a spectrometer, the system is easily automated, and a multiple well embodiment allows inexpensive screening and sequential testing. This invention also has applications in industry for feedstock and effluent monitoring.
Owner:RGT UNIV OF CALIFORNIA
Quantitative determination RBP4 kit by chemiluminescence magnetic enzymoimmune method
ActiveCN101452001AExtended storage timeStable LuminescenceChemiluminescene/bioluminescenceBiological testingImmunocompetenceMagnetic bead
The invention relates to a medical testing kit for performing quantitative detection on human serum RBP4 using chemiluminescence magnetic-enzyme immunotherapy. The kit is composed of four reagent parts: specificity mouse anti-human RBP4 custodite immunomagnetic beads, enzyme labelling specificity mouse anti-human RBP4 antibody II, chemiluminescence substrate, corresponding titer and quality control liquid. The using method of the kit comprises: using bead particulates as solid phase carrier, combining specificity mouse anti-human RBP4 antibody I on the surface, forming RBP4 specificity immunocompetence beads, capturing antigen RBP4 to be detected in the enzyme labelling specificity mouse anti-human RBP4 antibody II, forming double antibody sandwich composite on the surface of the beads, wherein enzyme marked on the composite reacts with corresponding irradiance substrate in the reaction system to form stable luminous signals, thereby reaching quantitative detection and analysis on RBP4 through strength of the detection light signals. The invention has the advantages of high sensitivity, high specificity, simple and fast operation.
Owner:WUHAN EASYDIAGNOSIS BIOMEDICINE
Early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and reverse transcription primer and application thereof
InactiveCN103173448AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationTumor BiomarkersAdenocarcinoma
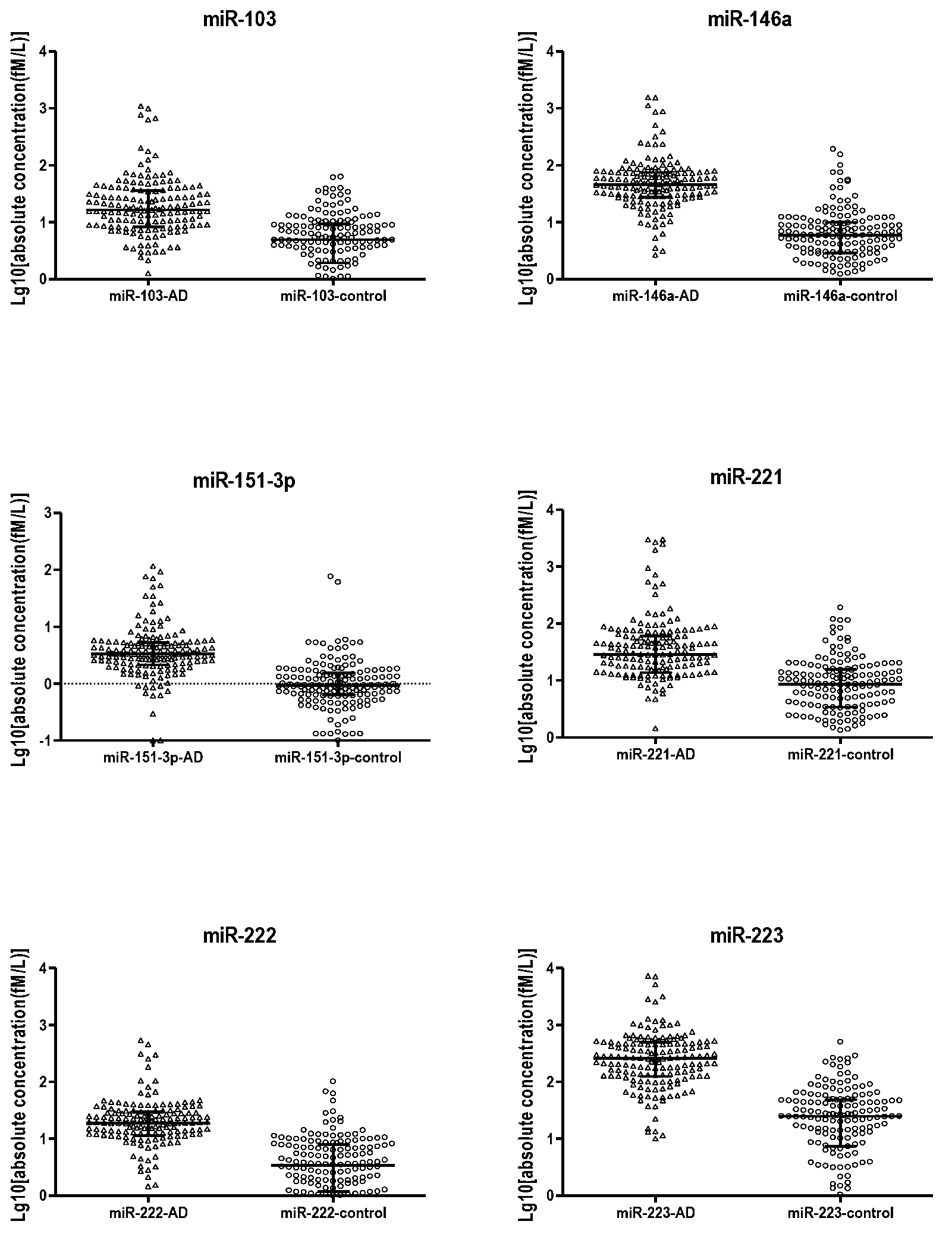
The invention belongs to the field of biological and medicine examination, relates to a tumor biomarker, and in particular relates to an early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and a reverse transcription primer and an application thereof. The early-stage lung adenocarcinoma miRNA specific expression profile comprises more than one of up-regulated expression miR-103, miR-146a, miR-151, miR-221, miR-222 and miR-223. The invention provides an early-stage lung adenocarcinoma diagnosis model, a reverse transcription primer of specific expression miRNA and a kit or biochip for early-stage lung adenocarcinoma diagnosis based on the specific expression profile. The technical scheme provided by the invention can realize accurate diagnosis of early-stage lung adenocarcinoma by detecting a small amount of miRNA, and provides possibility to the production of accurate, efficient and economical detection products. The established lung adenocarcinoma prediction model can distinguish lung adenocarcinoma from a healthy individual, and has great practical significance in clinic.
Owner:SHANGHAI CHEST HOSPITAL
Low Energy Communication of Medical Monitoring Information
InactiveUS20140118104A1Electric testing/monitoringDiagnostic recording/measuringAnalyteWireless communication protocol
Methods, systems, and devices for low energy communication of medical testing information are provided. Low energy communication of medical testing information may include detecting an analyte sample, determining an analyte concentration associated with the detected analyte sample, and transmitting an indication of the analyte concentration to an external device using a low energy wireless communication protocol. Transmitting an indication of the analyte concentration to an external device using a low energy wireless communication protocol may include generating an audio indication of the analyte concentration, packetizing the audio indication, and transmitting the packetized audio indication.
Owner:ABBOTT DIABETES CARE INC
Community based managed health kiosk system for soliciting medical testing and health study participants
InactiveUS20090240115A1Drug and medicationsComputer-assisted medical data acquisitionCommunity-based managementCommunity based
The present invention is directed to community based managed health kiosk systems for the solicitation of patients for medical testing and health studies, and more particularly to facilitating a kiosk user's access to current and upcoming medical tests and trials that would be appropriate for the given user based upon kiosk health assessments.
Owner:COMPIZED SCREENING
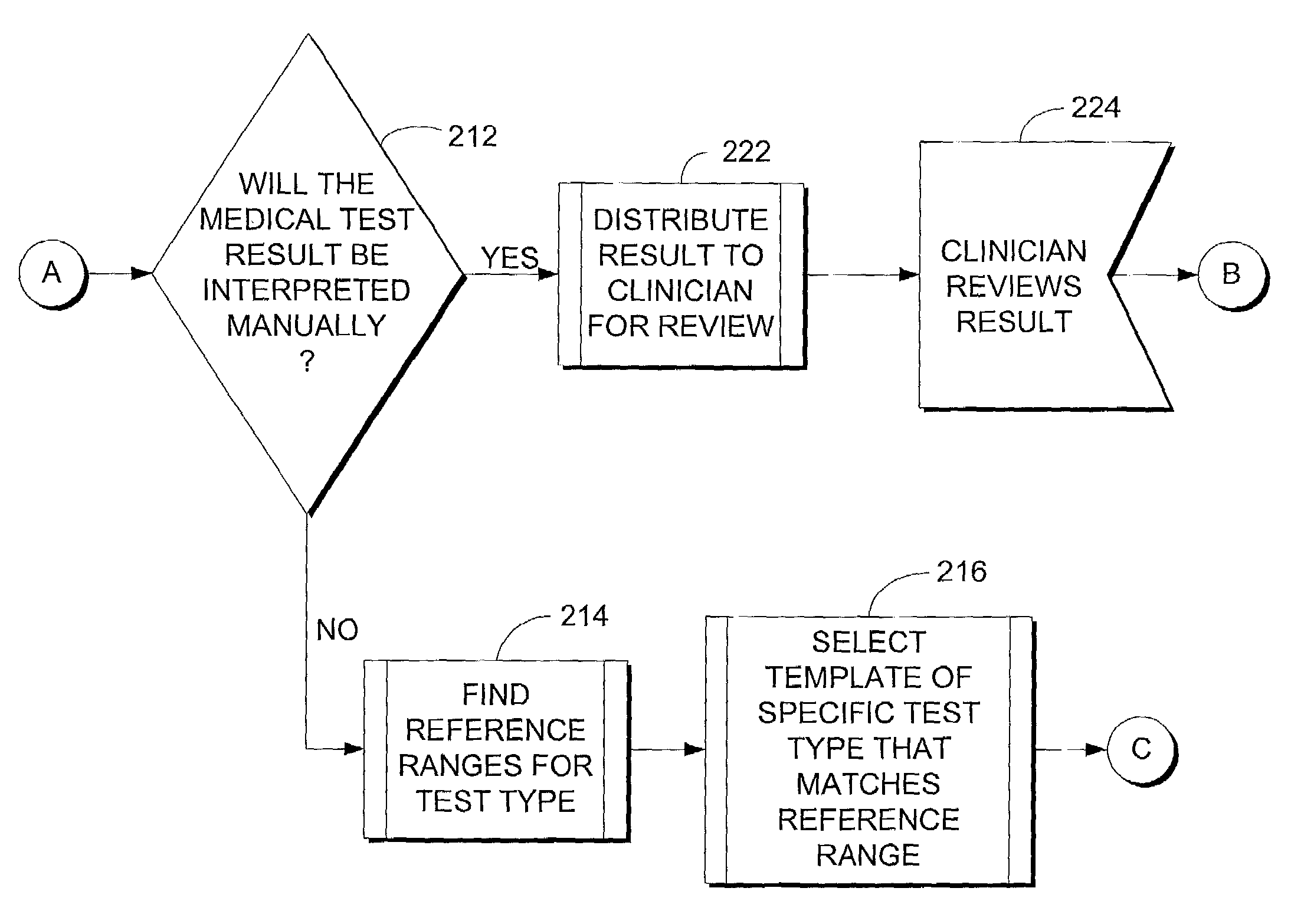
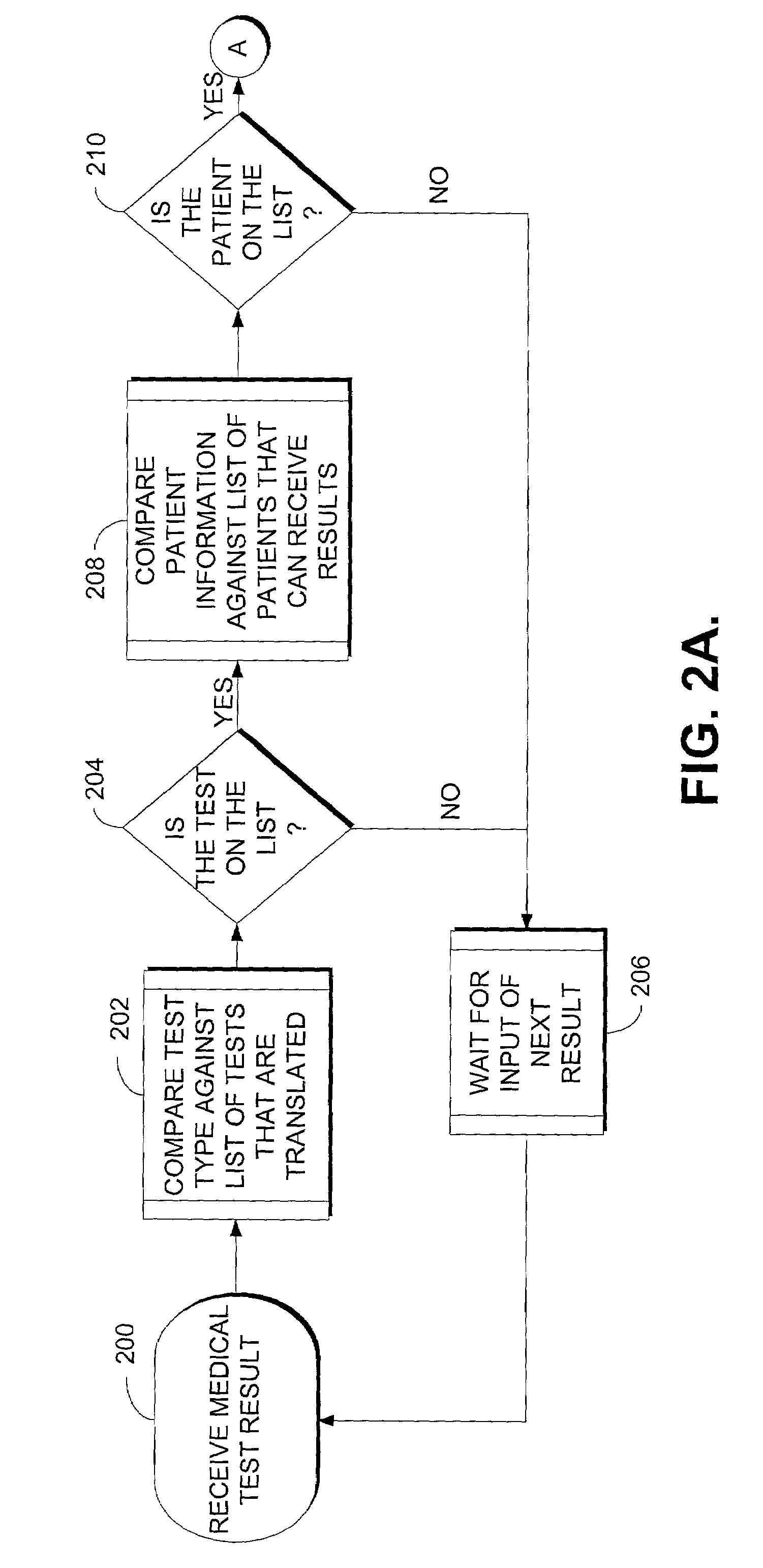
Computer system for translating medical test results into plain language
A method for translating medical test results into plain language is provided. The method includes receiving a medical test result for a type of medical test and, after making a threshold determination whether the medical test result will initially be automatically interpreted by the computer system independent of clinician input, identifying a template or set of templates associated with the type of medical test. The method also includes selecting the template matching the medical test result and outputting a plain language explanation based on the selected template.
Owner:CERNER INNOVATION
Breath ketone detector
InactiveUS8871521B2Analysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorKetoacidosisPowder mixture
Ketoacidosis is an extreme and uncontrolled form of ketosis, which is a normal response to prolonged fasting. Embodiments of this invention test the ketone level of a patient by measuring the ketone bodies in breath condensation. Some embodiments include a device for medical testing comprising a hollow container, comprising powder mixture of sodium nitroferricyanide, ammonium sulfate and silica and a liquid including an ammonium hydroxide solution.
Owner:AKERS BIOSCI
Blood ammonia content determination method and blood ammonia diagnosis kit
InactiveCN1769481AStrong specificityLess susceptible to interferenceMicrobiological testing/measurementLactate dehydrogenaseMedical testing
The invention relates to a method for determining the content of blood ammonia, and also the reagent kit for blood ammonia diagnosis. The reagent kit comprises cushioning solution, adenosine triphosphate, phosphoenolpyruvate phosphatase, deacidized type coenzyme, ammonia kinase, pyruvate kinase, lactate dehydrogenase, and stabilizer. By mixing sample and reagent of a predetermiend volumetric ratio, generating coupling reaction between them, subjecting the final reactant to biochemiscal analyser, the main wavelength absorbancy variance ratio (speed) can be detected, and the blood ammonia content can thus be measured. The method of the invention can be used to obtain the needed measurement result purely through biochemical analytic instruments, and advantages of the method include higher sensibility, better accuracy, less susceptibility to contamination of internal or external materials, and easy application.
Owner:王尔中
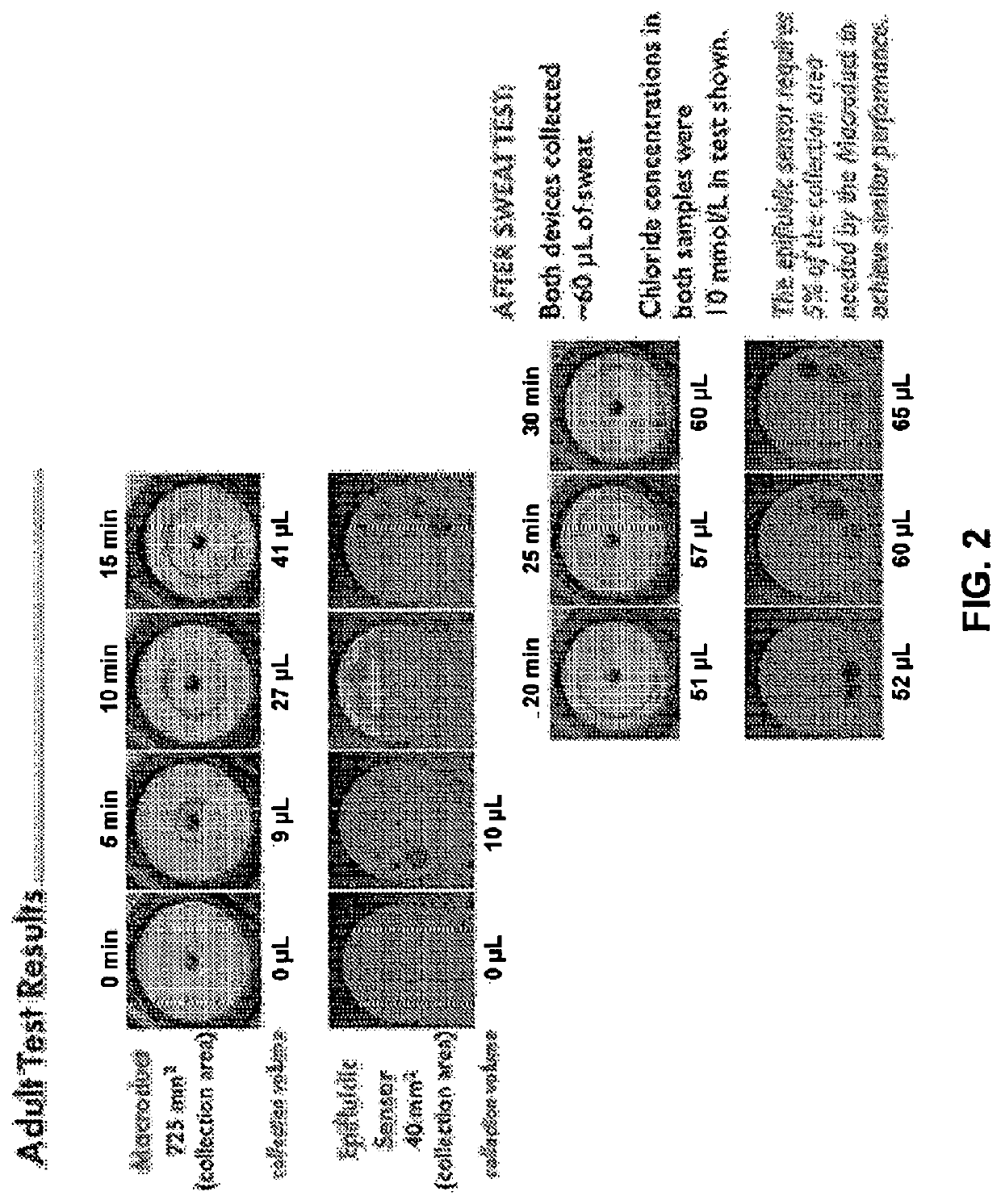
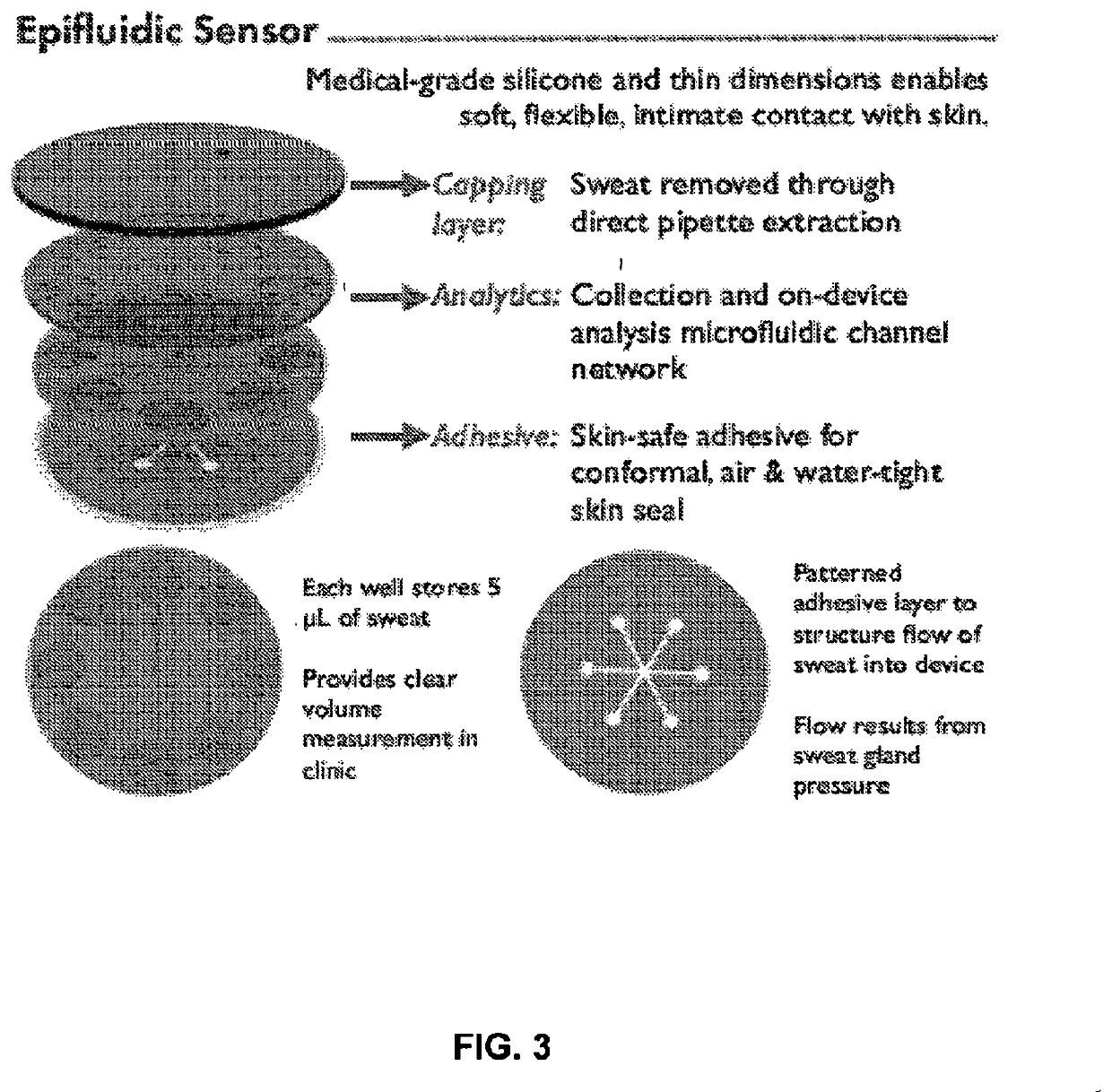
Thin, soft, skin-mounted microfluidic networks for detection and analysis of targets of interest in sweat
Provided herein are flexible, microfluidic epidermal systems and methods useful in the analysis of biofluids for biomarkers corresponding to a variety of conditions and methods of use. The provided systems configured to create conformal contact with the skin to allow for medical testing or screening, either in situ or later external laboratory testing. The described devices and methods may be used for cystic fibrosis screening, glucose monitoring, drug and / or alcohol testing, creatinine monitoring, urea monitoring, pH measurement and dialysis treatment efficacy testing.
Owner:NORTHWESTERN UNIV
Multinomial liquid quality control material and preparation method thereof
ActiveCN101762710AAnalytical composition is stableSo as not to damageBiological testingBlood plasmaMedical testing
The invention relates to the field of the medical test quality control, in particular to a multinomial liquid quality control material and a preparation method thereof. The preparation method comprises the following steps: collecting waste blood of a blood bank; carrying out clinical examination on a waste blood sample or mammal blood; separating serum or blood plasma; analyzing the content of each quality control components in the serum or the blood plasma; adjusting each analysis component; adopting a methylene blue photosensitive inactivation method to inactivate various potential pathogenic microorganisms; and adding a composite protective agent to prepare the quality control material. As the invention adds the composite protective agent consisting of glycol and glycerol into the quality control material, each analysis component in the quality control material is stable, can be stable for one month at room temperature, can be stable for 2 to 4 months at a temperature of 4 DEG C, and can be stable for 12 months at a temperature of minus 20 DEG C. Moreover, the quality control material of the invention adopts the methylene blue photosensitive inactivation method to inactivate various potential pathogenic microorganisms, thereby having no potential pathogenic microorganism harmfulness.
Owner:明德松
Diatom inspection method in medical jurisprudence
ActiveCN101776623ADoes not affect detectabilityDoes not affect species identificatioMaterial analysis using wave/particle radiationPreparing sample for investigationFiltrationScanning electron microscope
The invention relates to the field of medical jurisprudence inspection, in particular to a diatom inspection method in medical jurisprudence. The method at least comprises the following steps: (1) microwave digestion: adding concentrated nitric acid and hydrogen peroxide solution into the inspected sample to carry out microwave digestion; (2) vacuum filtration: carrying out vacuum filtration on the digestion solution, adding ultrapure water, carrying out vacuum filtration continually until the surface of the filter membrane is approximately neutral, adding absolute ethyl alcohol, and carrying out vacuum filtration to remove moisture from the filter membrane; (3) a scanning electron microscope automatically takes pictures and stores the pictures; and (4) qualitative and quantitative analysis on diatom: carrying out inspection, classification and statistic treatment on the diatom in the on-spot pictures in an artificial identification way or computer automated identification way. The invention has the advantages of high detection sensitivity, high accuracy of qualitative and quantitative analysis, simpleness, high efficiency and environmental protection, can effectively avoid pollution, greatly improves the working environment for diatom inspection technicians in medical jurisprudence, and reduces the labor intensity, thereby having wide application prospects in drowning diagnosis practice in medical jurisprudence.
Owner:GUANGZHOU CRIMINAL SCI & TECH RES INST
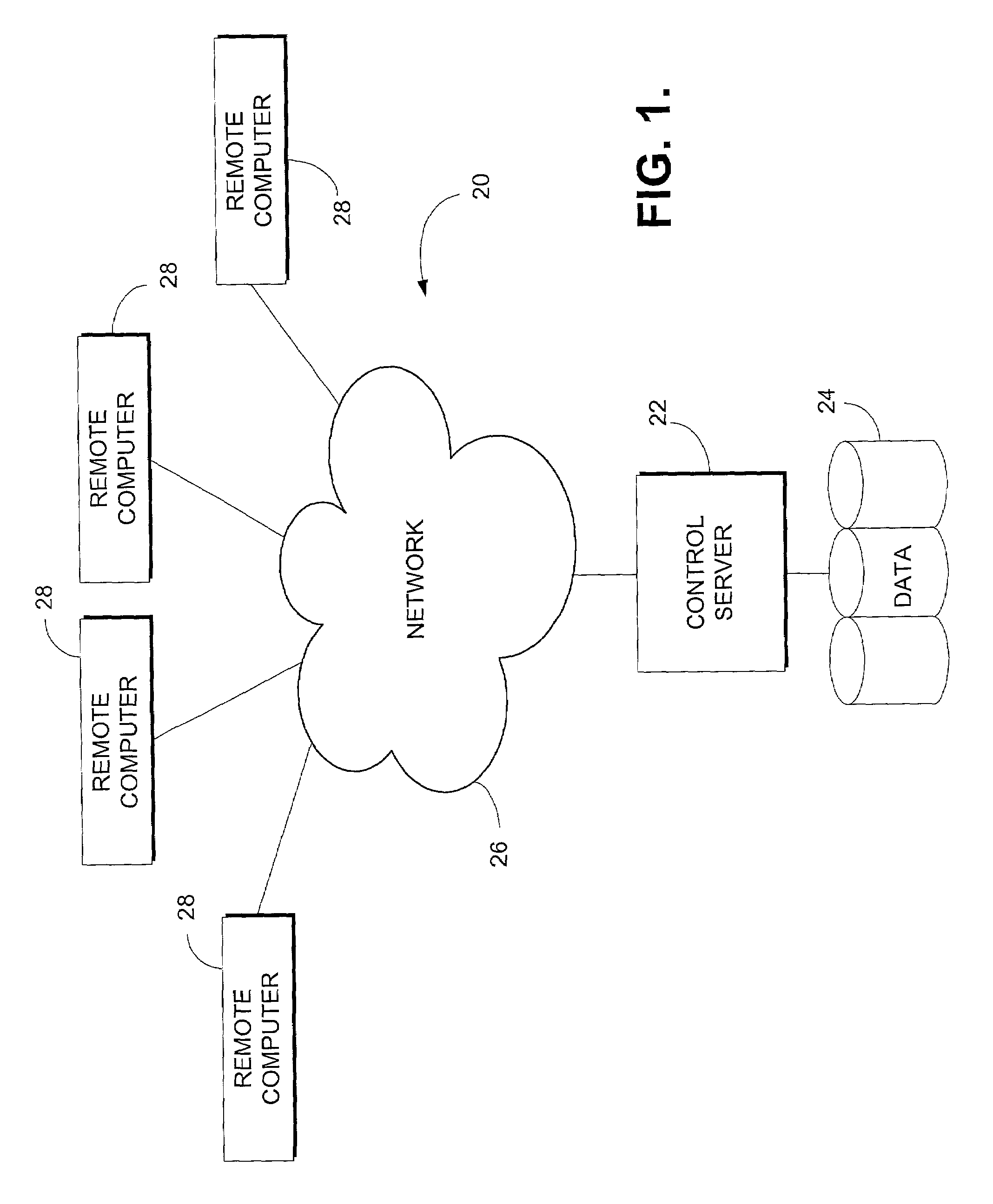
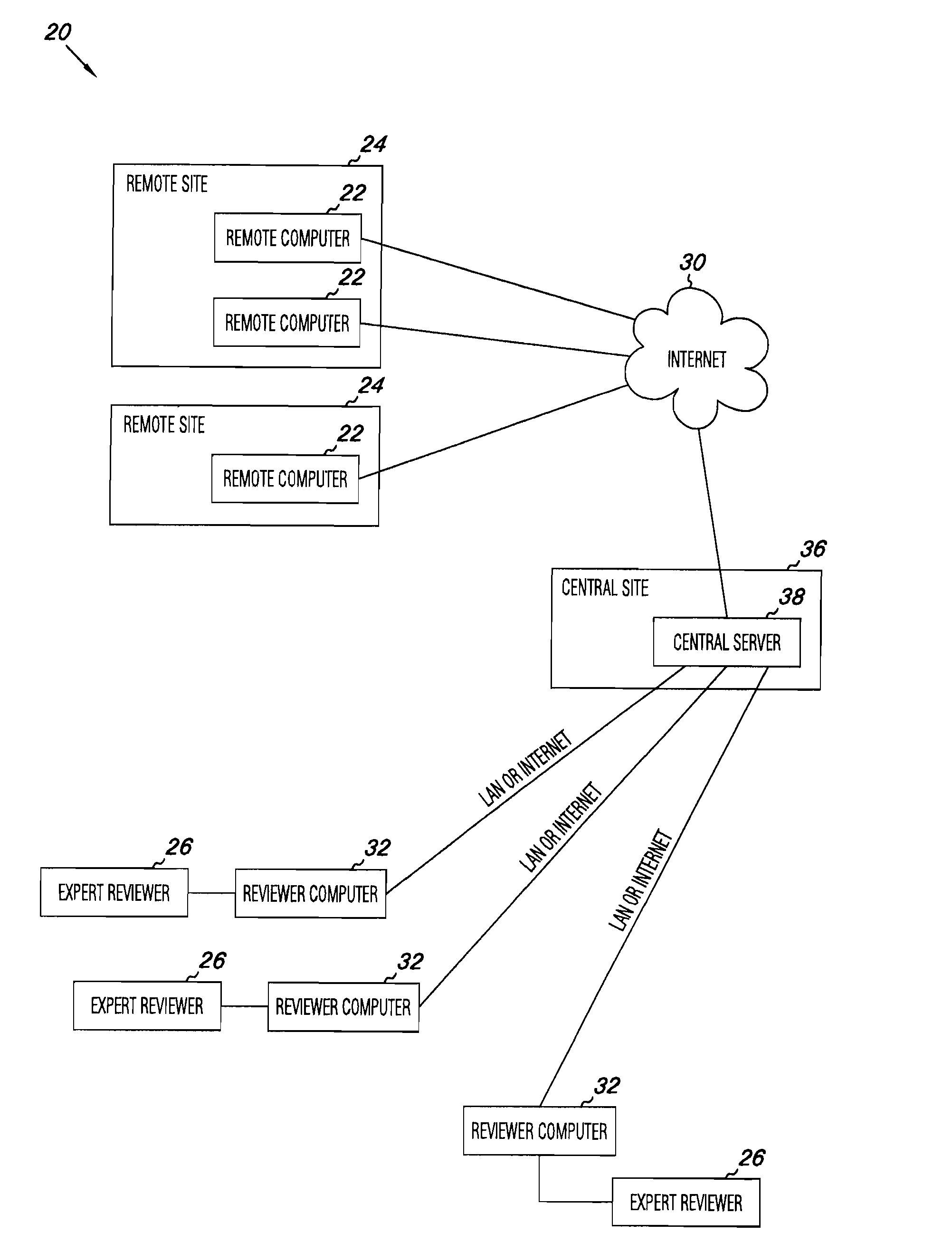
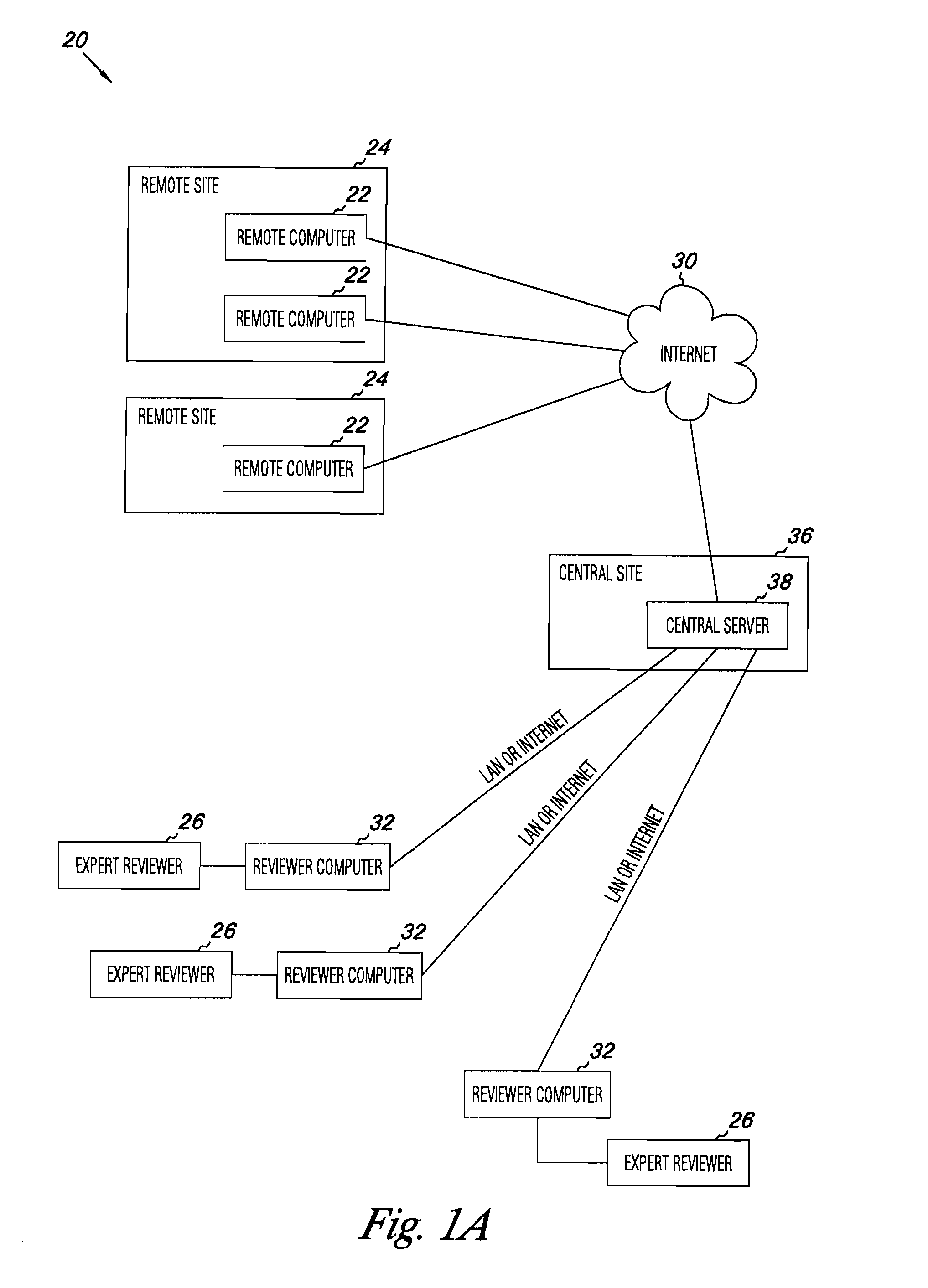
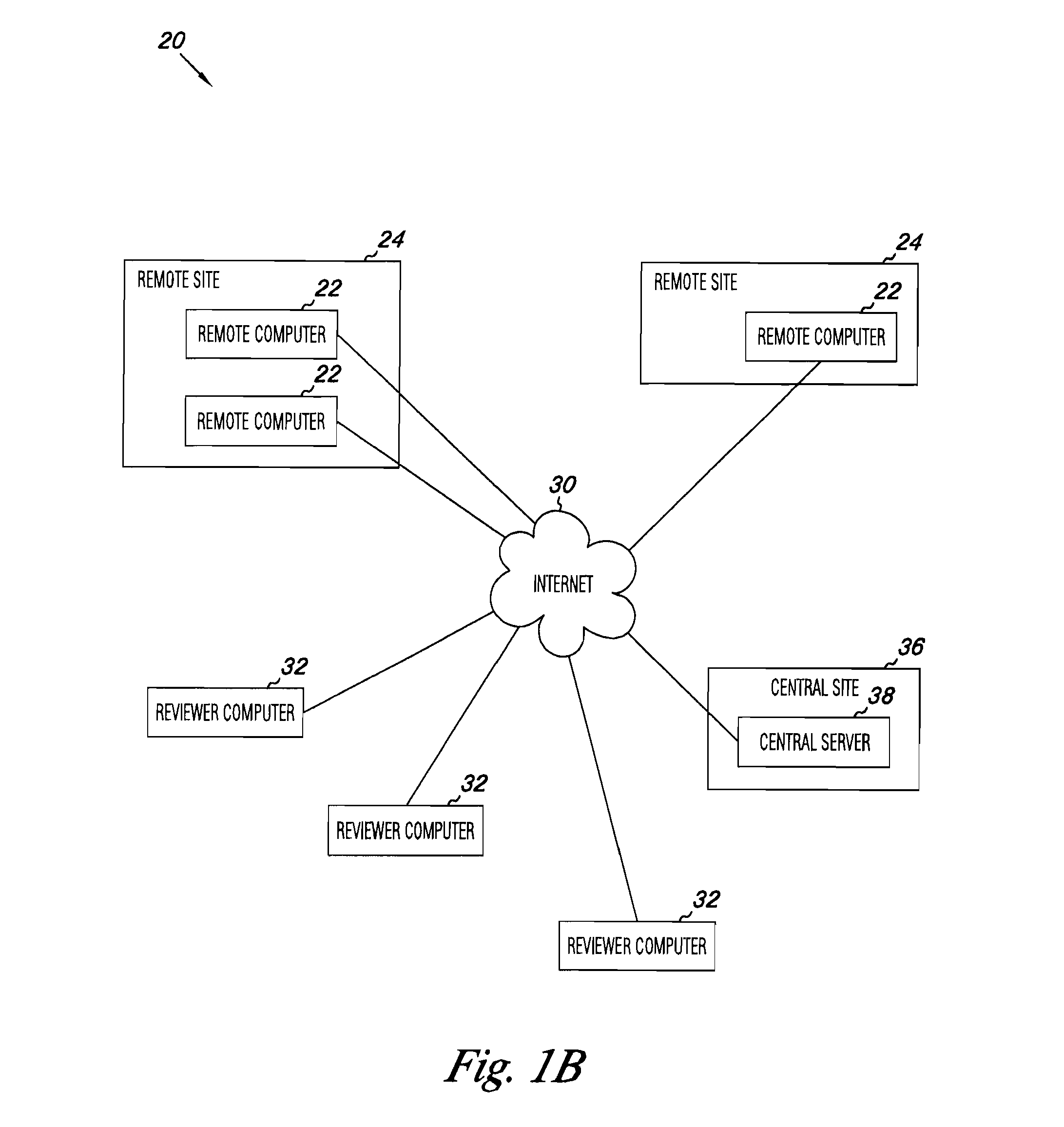
Apparatus and methods for medical testing
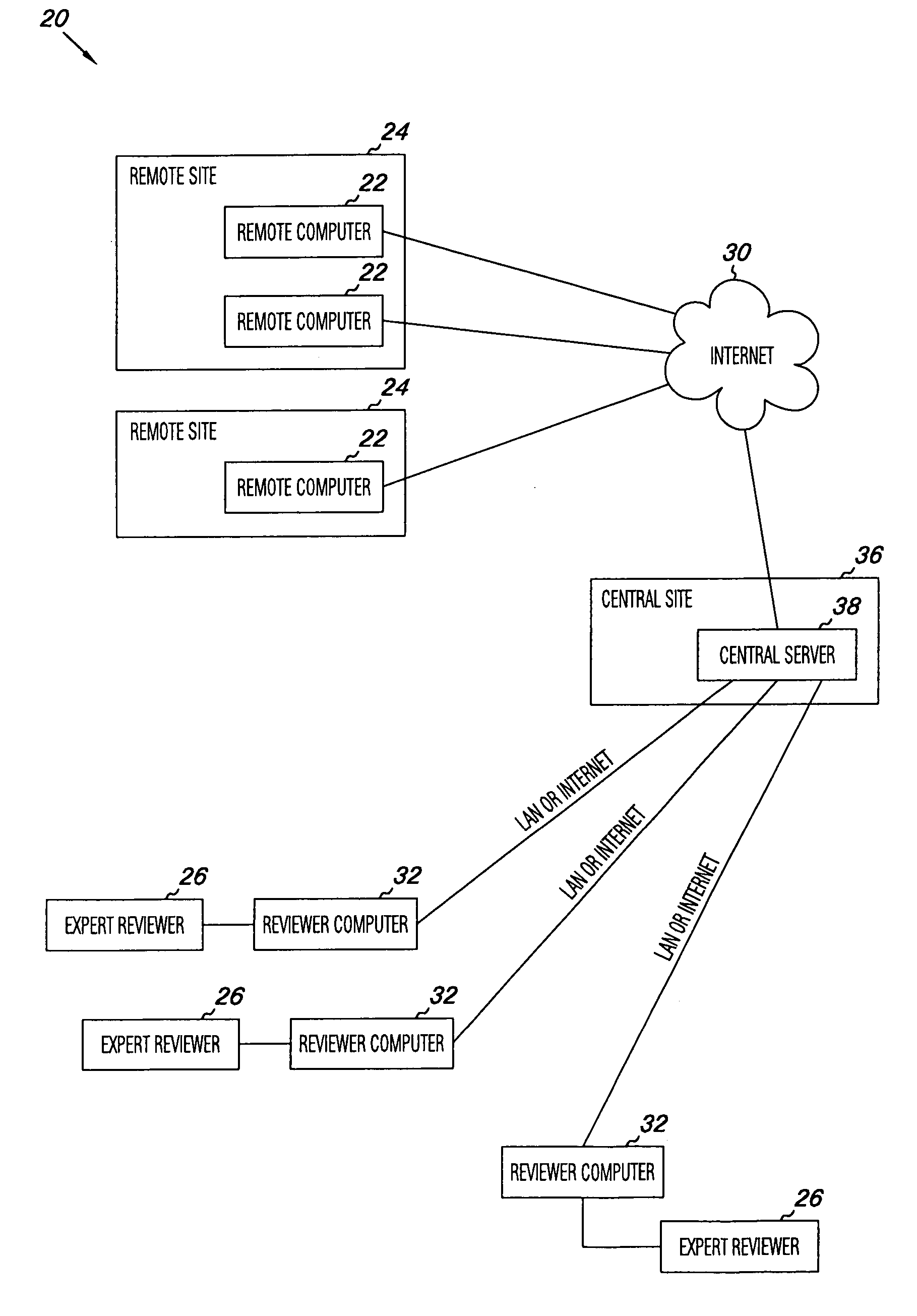
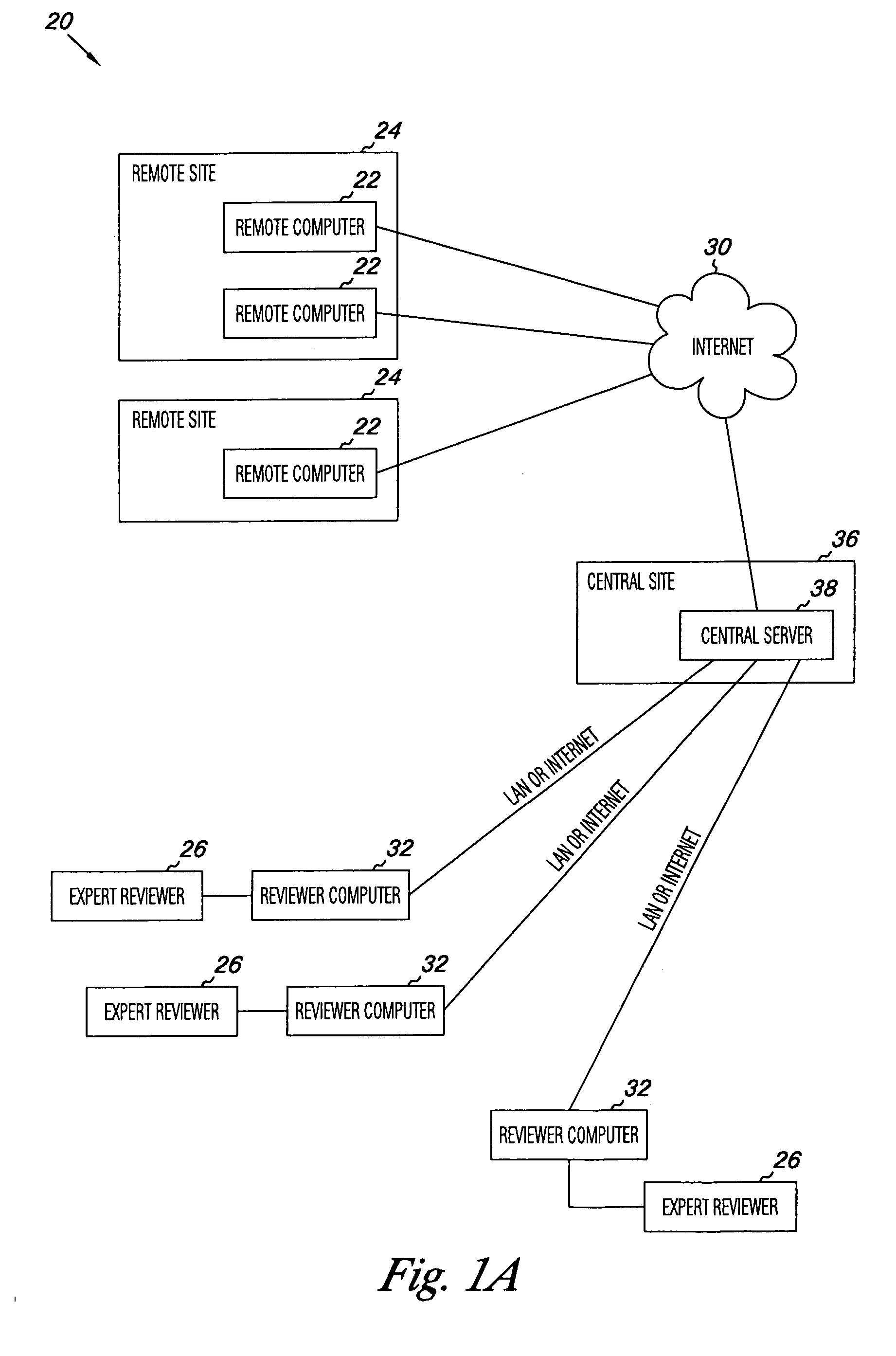
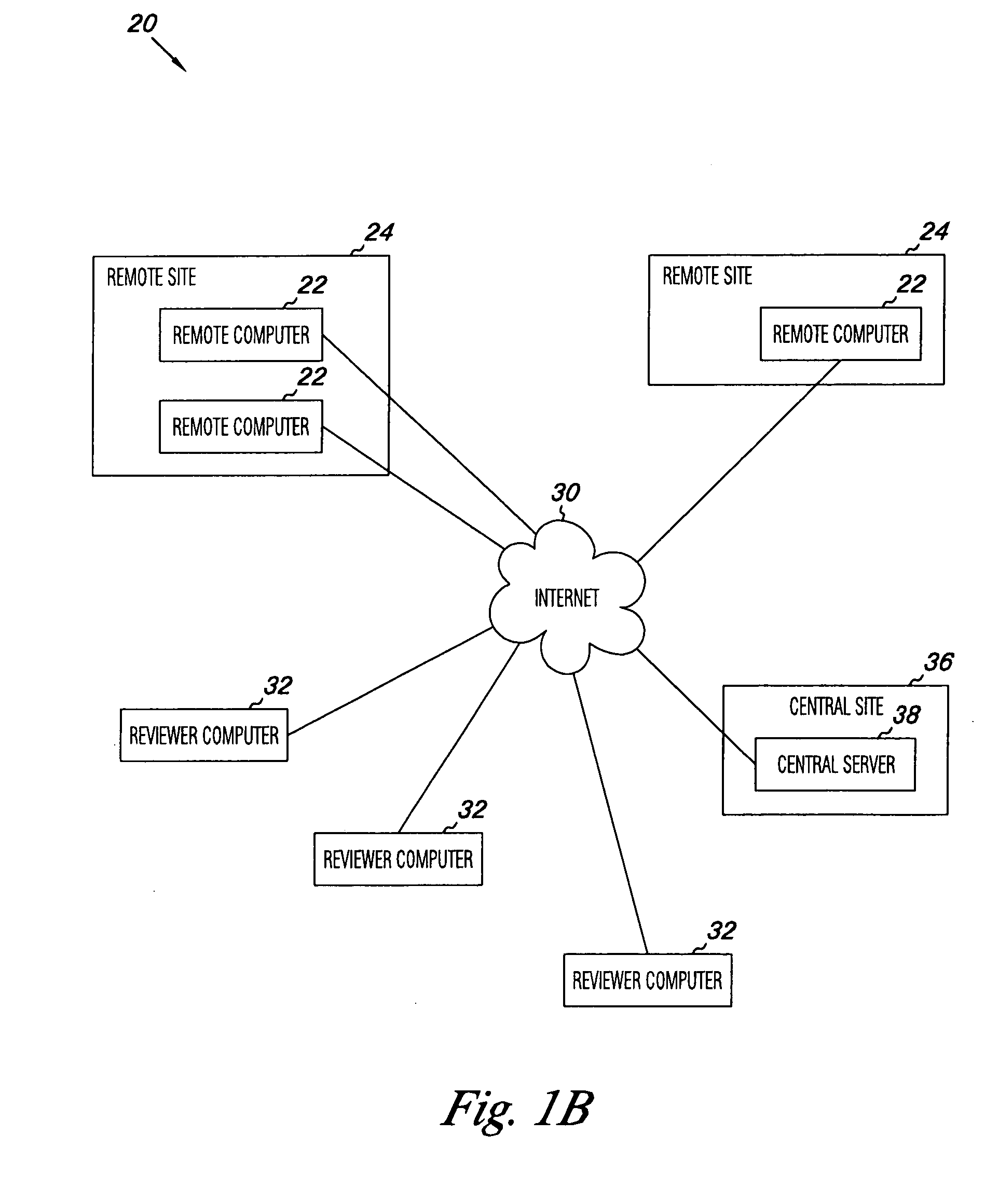
Apparatus and methods for practicing telemedicine in the form of software systems acting over a network and kits containing laboratory supplies and equipment to organize the laboratory operations and interpret the results of molecular diagnostic testing are disclosed. At least two computers in communication over the Internet or other network are used, a remote computer located at a remote site and a central server located at a central site. The remote site may be geographically distant from the central site. A specimen is procured from a patient proximate to the remote site. Laboratory operations are conducted on the specimen at the remote site. The laboratory data resulting from the laboratory operations is interpreted by an expert reviewer who may be located at the central site, and a report is then transmitted back to the remote site.
Owner:MCGLENNEN RONALD C +5
Biochip for detecting drug resistant genes of helicobacter pylori clarithromycin and preparation method and application thereof
InactiveCN101665824ADetermining drug resistanceSimple methodNucleotide librariesMicrobiological testing/measurementResistant genesClarithromycin
The invention belongs to the fields of genetic engineering and medical inspection, and relates to a biochip for detecting drug resistant genes of drug resistant genes and a preparation method and an application thereof. The biochip is prepared by the following steps: carrying out aldehyde processing for a chip base; connecting a 5' end of a probe with a molecule arm of poly (dT)10; then carrying out amino modification for the 5' end of the probe; aiming at the mutants at the following positions of helicobacter pylori 23S rRNA genes by the probe: A2115G, A2142G, A2142C, A2143G, A2143C, T2182C and G2224A; and fixing the probe on the chip base. The biochip has high sensitivity, good specificity and low detection cost, can distinguish the difference of monobasic bases, and is suitable for being popularized and used in establishment units.
Owner:周玉贵
Apparatus and methods for medical testing
InactiveUS20110178814A1Accurately conductData processing applicationsDigital data processing detailsSoftware systemThe Internet
Apparatus and methods for practicing telemedicine in the form of software systems acting over a network and kits containing laboratory supplies and equipment to organize the laboratory operations and interpret the results of molecular diagnostic testing are disclosed. At least two computers in communication over the Internet or other network are used, a remote computer located at a remote site and a central server located at a central site. The remote site may be geographically distant from the central site. A specimen is procured from a patient proximate to the remote site. Laboratory operations are conducted on the specimen at the remote site. The laboratory data resulting from the laboratory operations is interpreted by an expert reviewer who may be located at the central site, and a report is then transmitted back to the remote site.
Owner:MCGLENNEN RONALD C +5
Method for detecting blood ammonia content and blood ammonia diagnostic reagent kit
InactiveCN1749756AStrong specificityImprove test accuracyColor/spectral properties measurementsBiological testingAdenosinePeroxidase
The present invention belongs to the field of medical detection technology. The reagent kit for blood ammonia diagnosis includes buffering solution, adenosine triphophate, glutamic acid, pyruvic acid, alcohol, oxidized coenzyme, glutamine synthetase, pyruvate oxidase, hydrogen peroxidase, aldehyde dehydrogenase and stabilizer. Through mixing the sample and reagent in certain volume ratio to produce enzyme coupling reaction, and detecting in biochemical analyzer the main wavelength absorbency change speed, the blood ammonia content is measured. The present invention can obtain the measurement result in biochemical analyzer in high sensitivity, high precision and no contamination of various foreign and internal matters.
Owner:王尔中
Hybridoma cell line and anti-human erythrocyte surface H antigen monoclonal antibodies generated thereof
InactiveCN101037671AImmunoglobulins against cell receptors/antigens/surface-determinantsFused cellsAntigenGene engineering
The invention discloses a hybridization tumor cell stem 2E8Z CGMCC NO.1942 and monoclonal antibody of human red blood cell surface H antigen thereof. The monoclonal antibody 2E8 of human red blood cell surface H antigen genereated by hybridization tumor cell stem has advantages of high effect, strong differentia, extensive distribution of antigen. The flexible peptide is easy to be bended, so mixed albumen of the invnetion is feld correctly and without influence each activity combination area of the mixed albumen. Experiment evidence, the mixed albumen can specially combine with human red blood cell surface H antigen; moreover, the mixed albumen with high expression amount, easy to be purified, short production cycle, large production scale and low cost. Base on these advantages, the invention plans to use gene engineering method to produce double function molecule and establish good base of treating based on red blood cell and testing platform, having more actual sense and wider application foreground in medical testing field.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
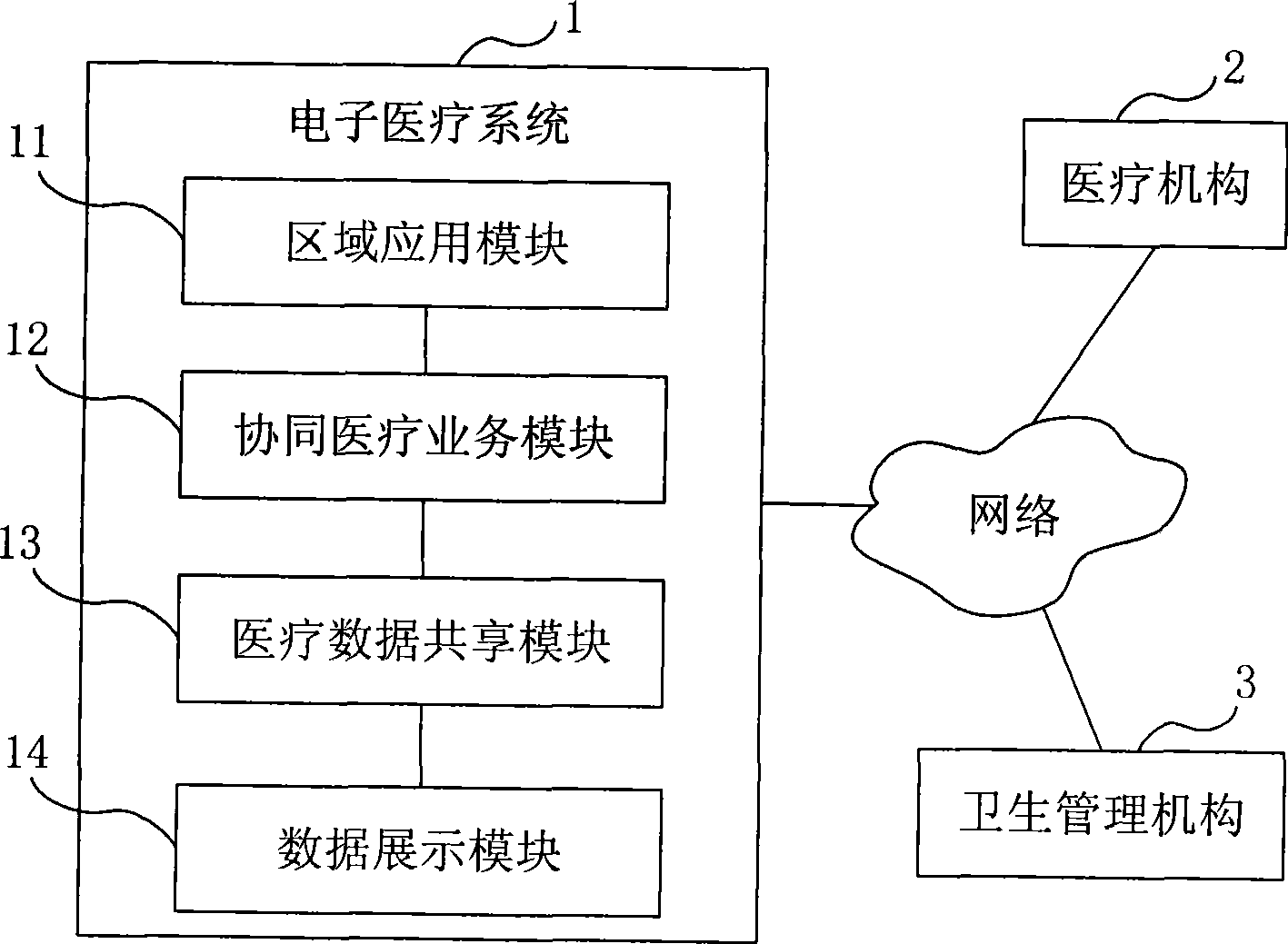
Electronic medical system
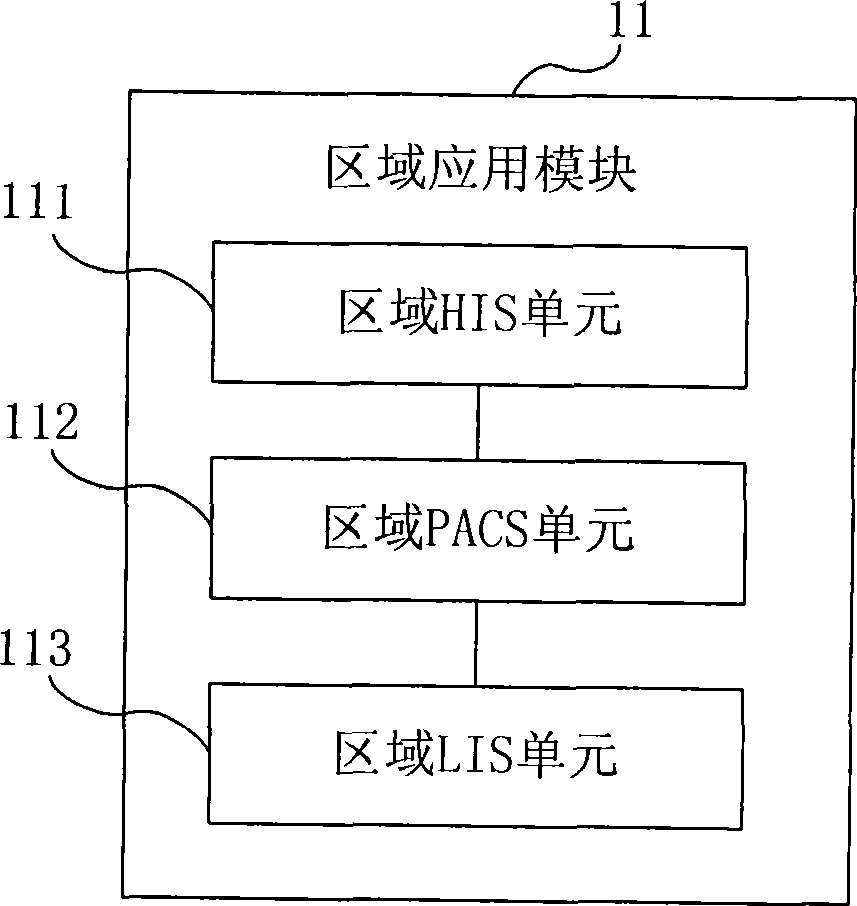
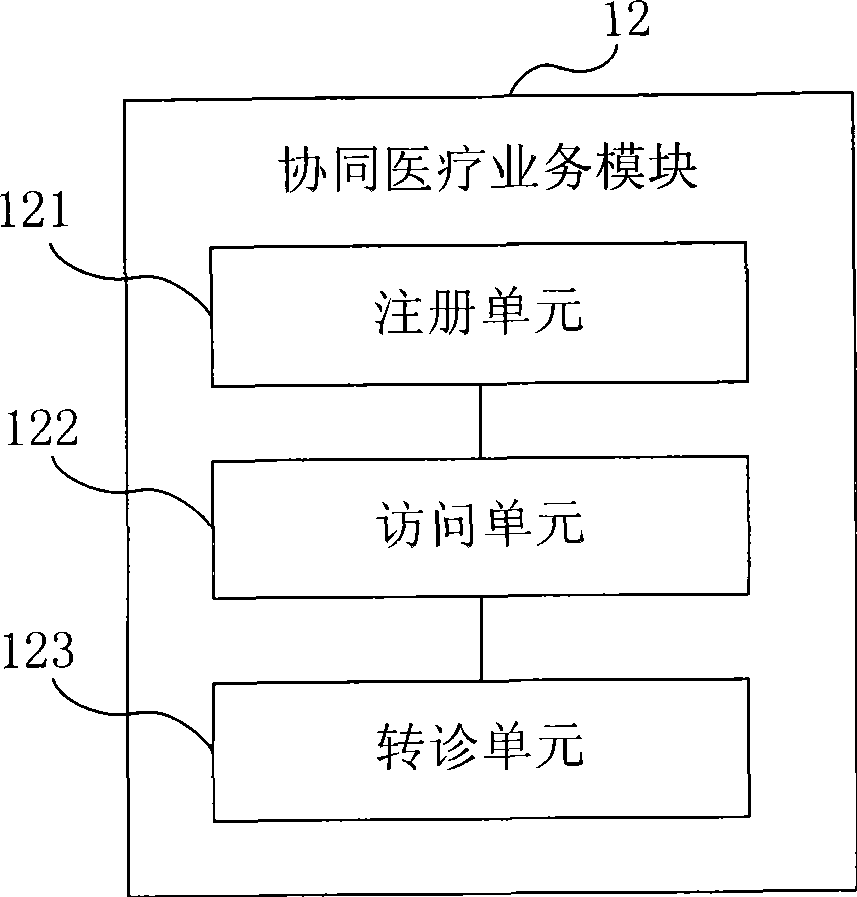
ActiveCN101414324ARealization of Collaborative Medical ServicesSpecial data processing applicationsData sharingMedical testing
The embodiment of the invention provides an electronic medical system. The electronic medical system is connected with a medical mechanism network in a set region and comprises a region application module used for collecting and storing the medical mechanism information in the set region as well as the medical image information and the medical checking information of the medical mechanism in the set region; a cooperative medical business module used for cooperating with different medical mechanisms in the set region to carry out cooperative medical business according to the information stored by the region application module; a medical data sharing module used for storing and managing the data in the cooperative medical business. The embodiment of the invention realizes the cooperative medical business between different medical mechanisms in the region by carrying out cooperative medical business by one special cooperative medical business module based on sharing the information like the medical mechanism information, the medical image information and the medical checking information in one region.
Owner:CHINA UNITED NETWORK COMM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com