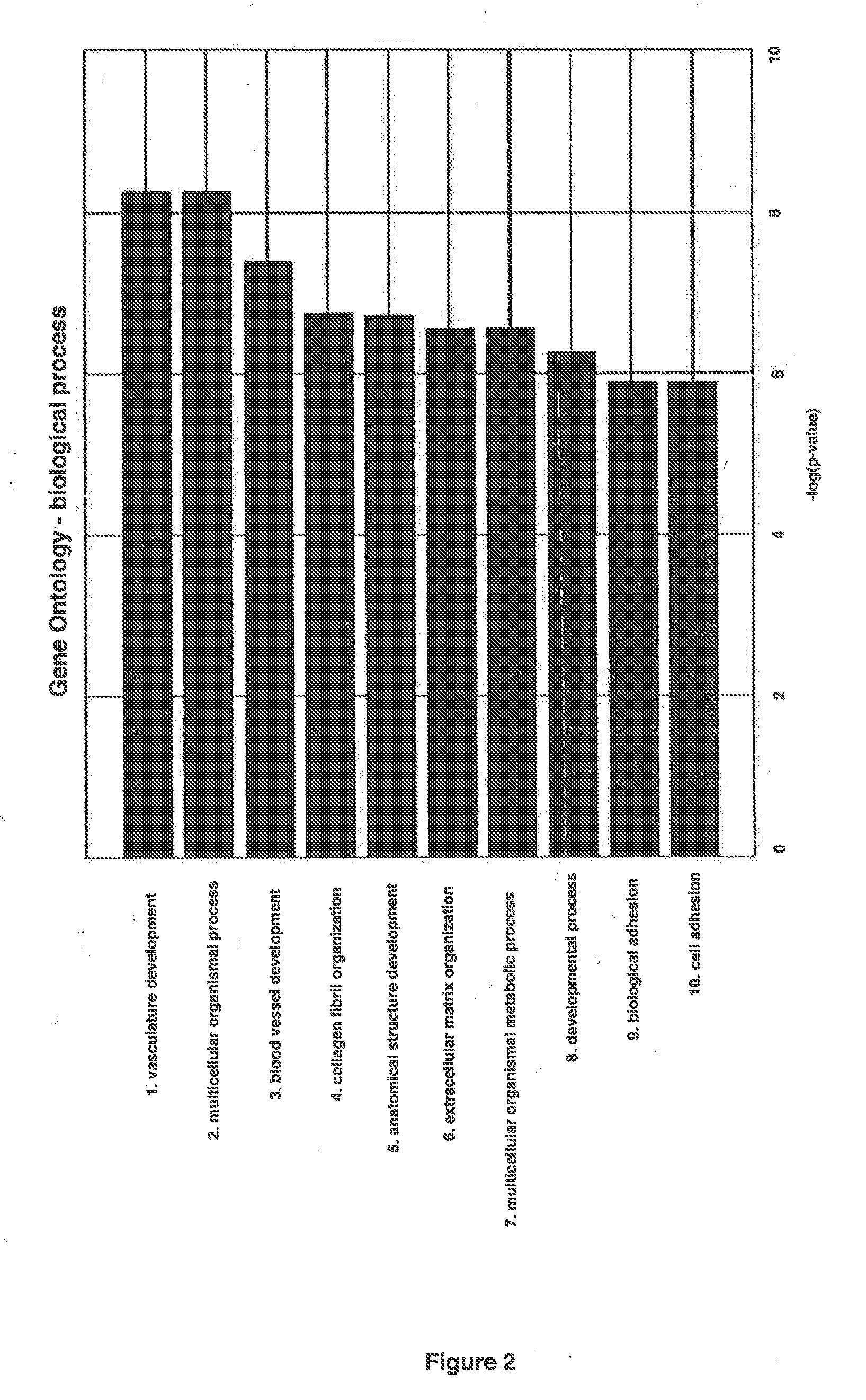
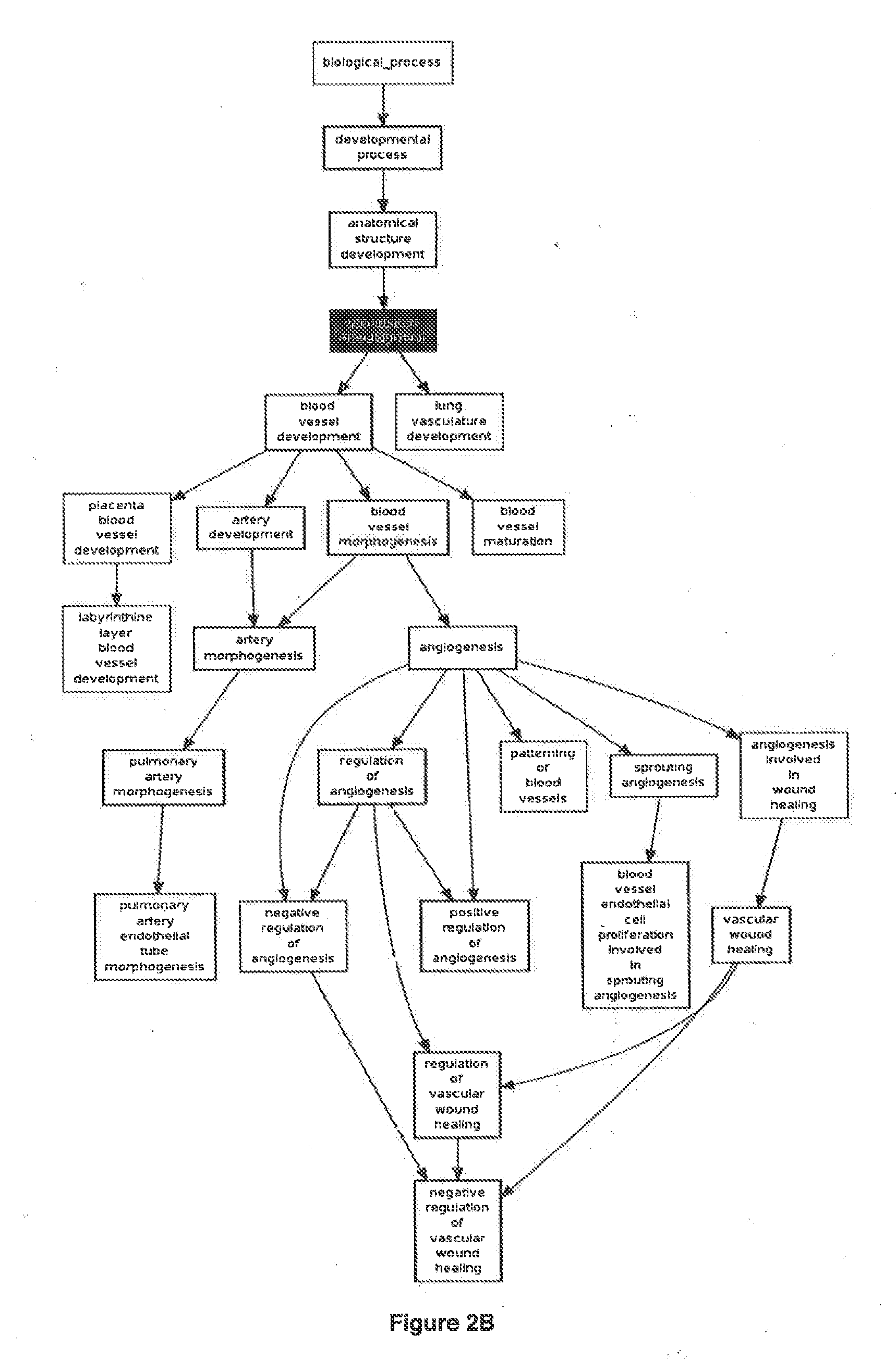
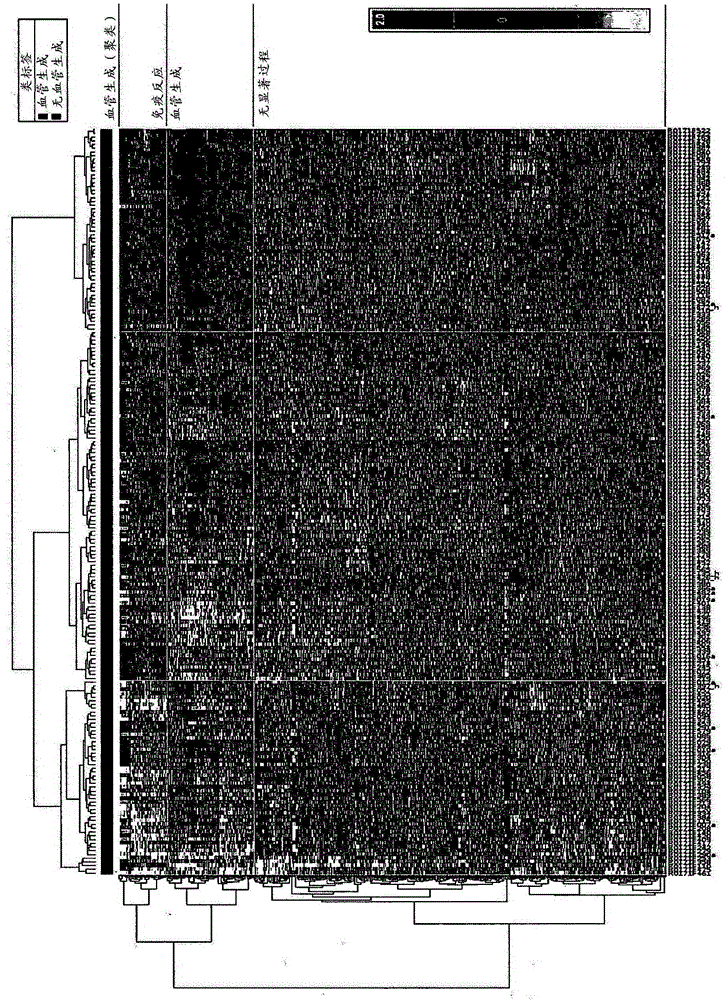
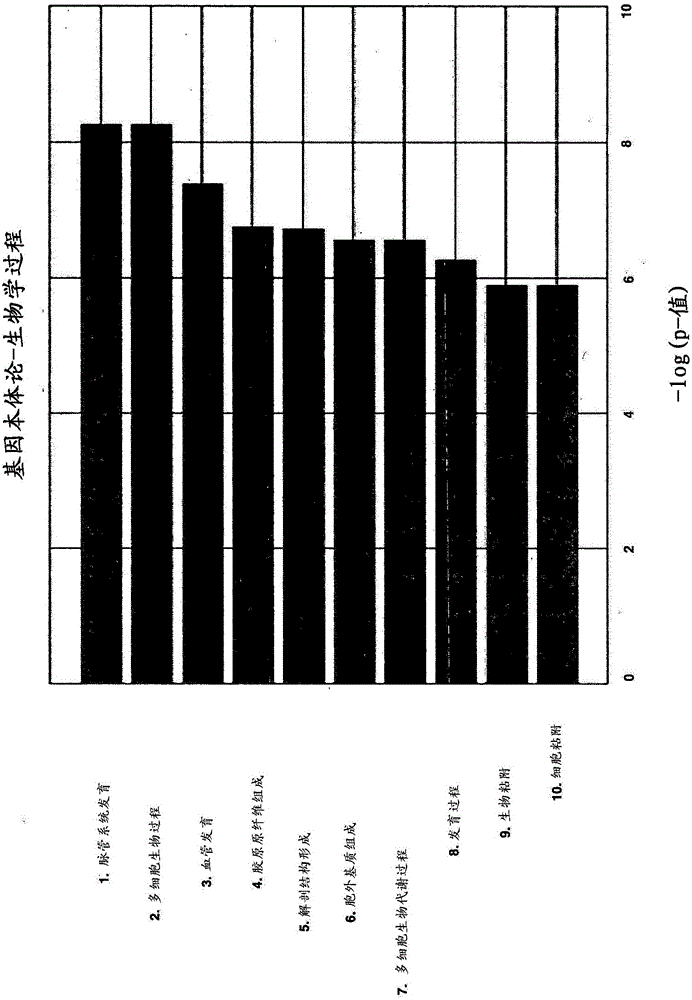
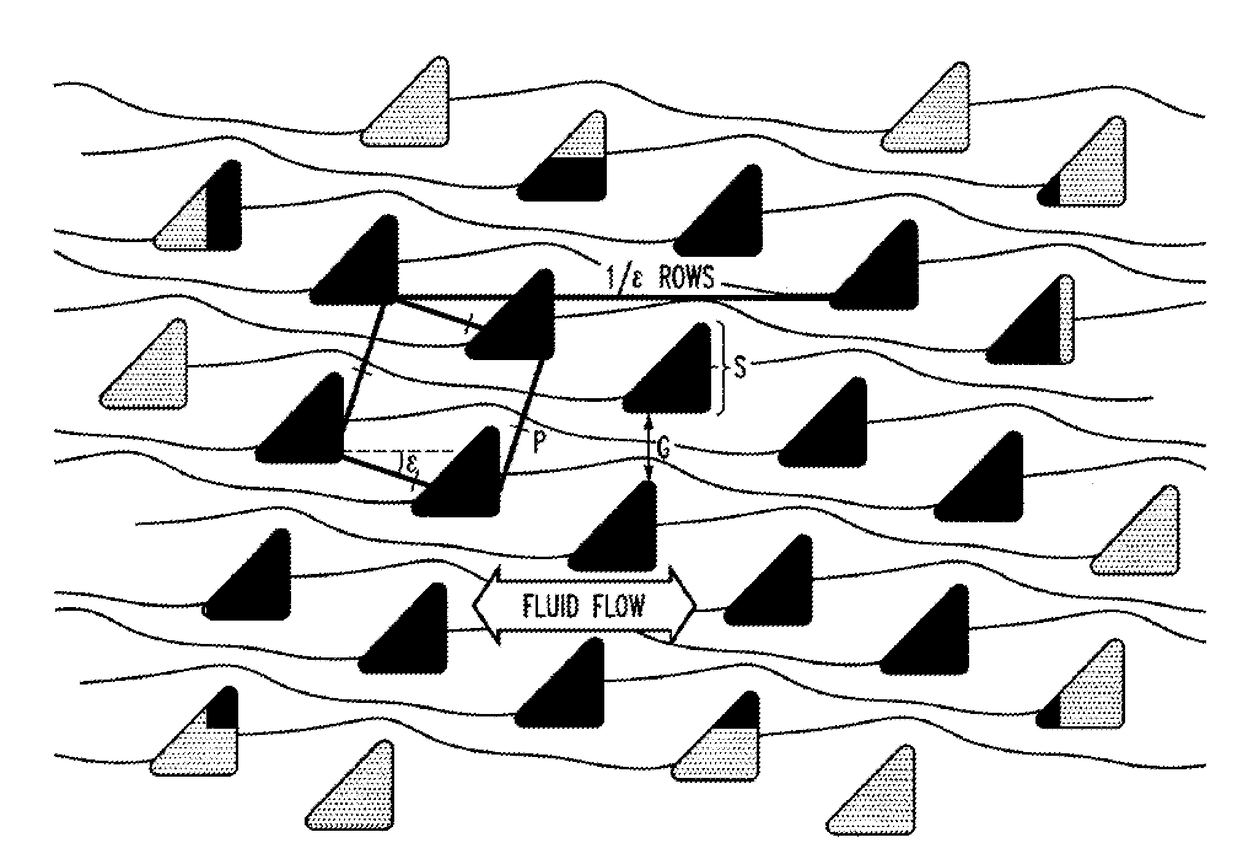
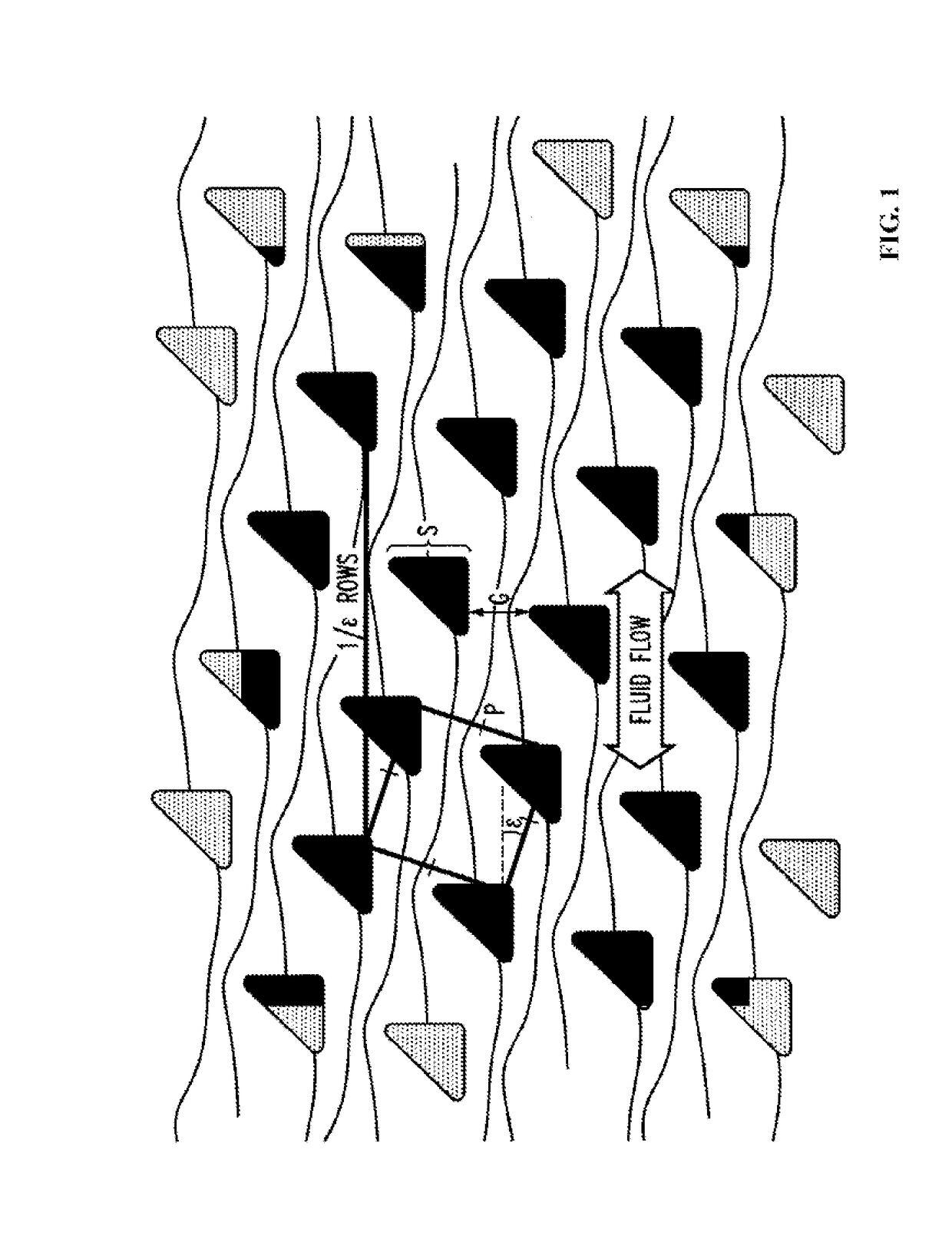
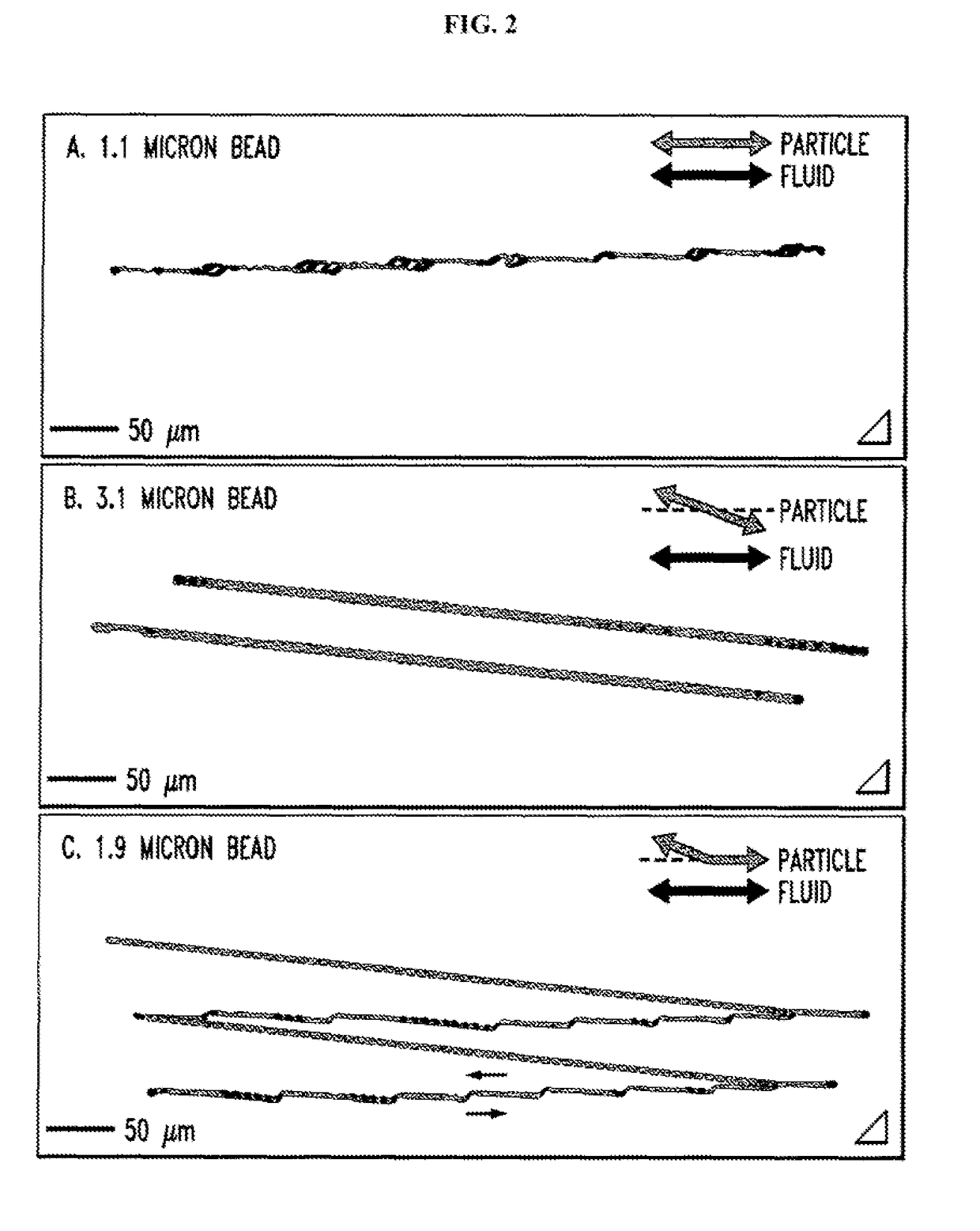
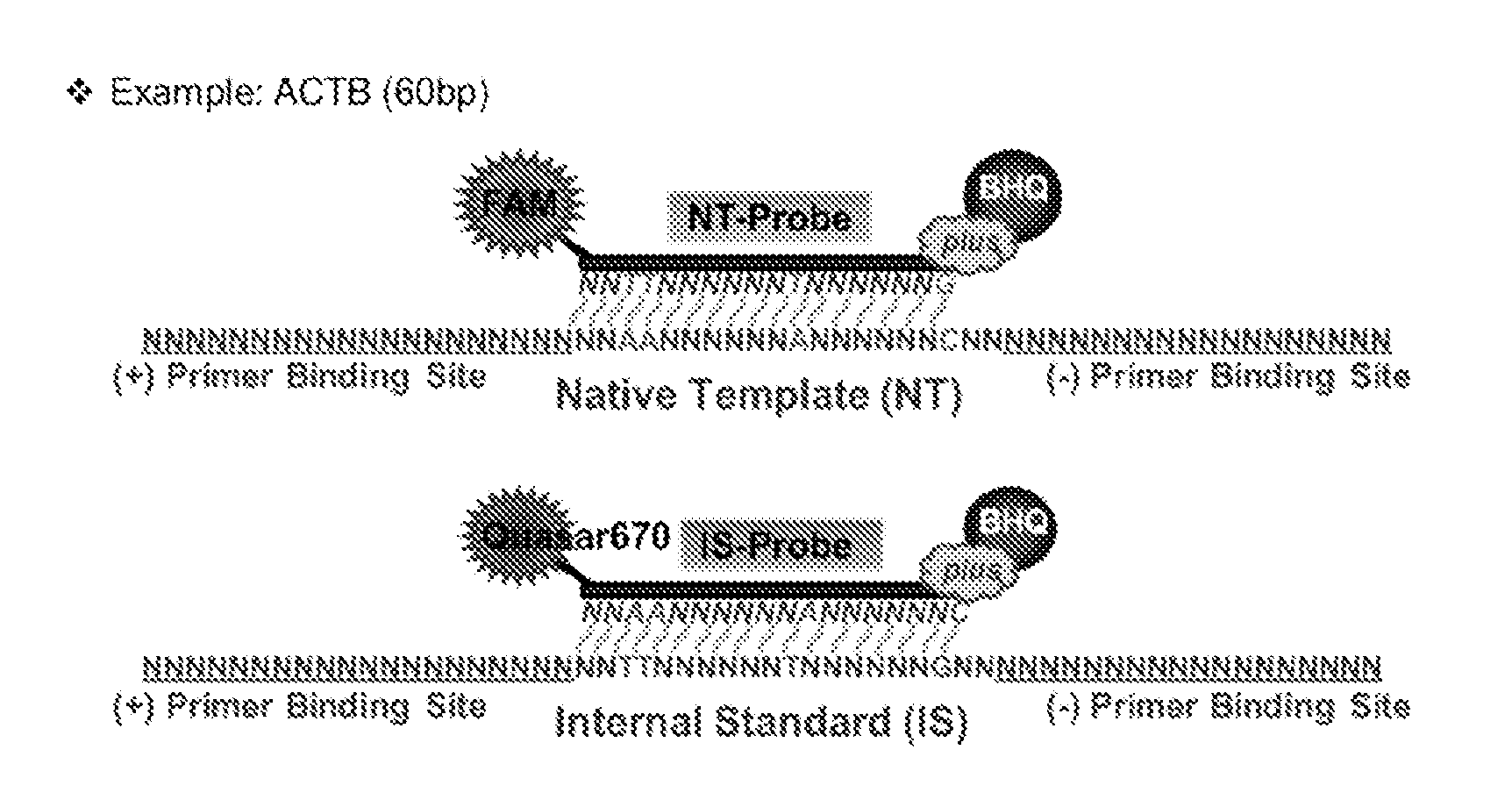
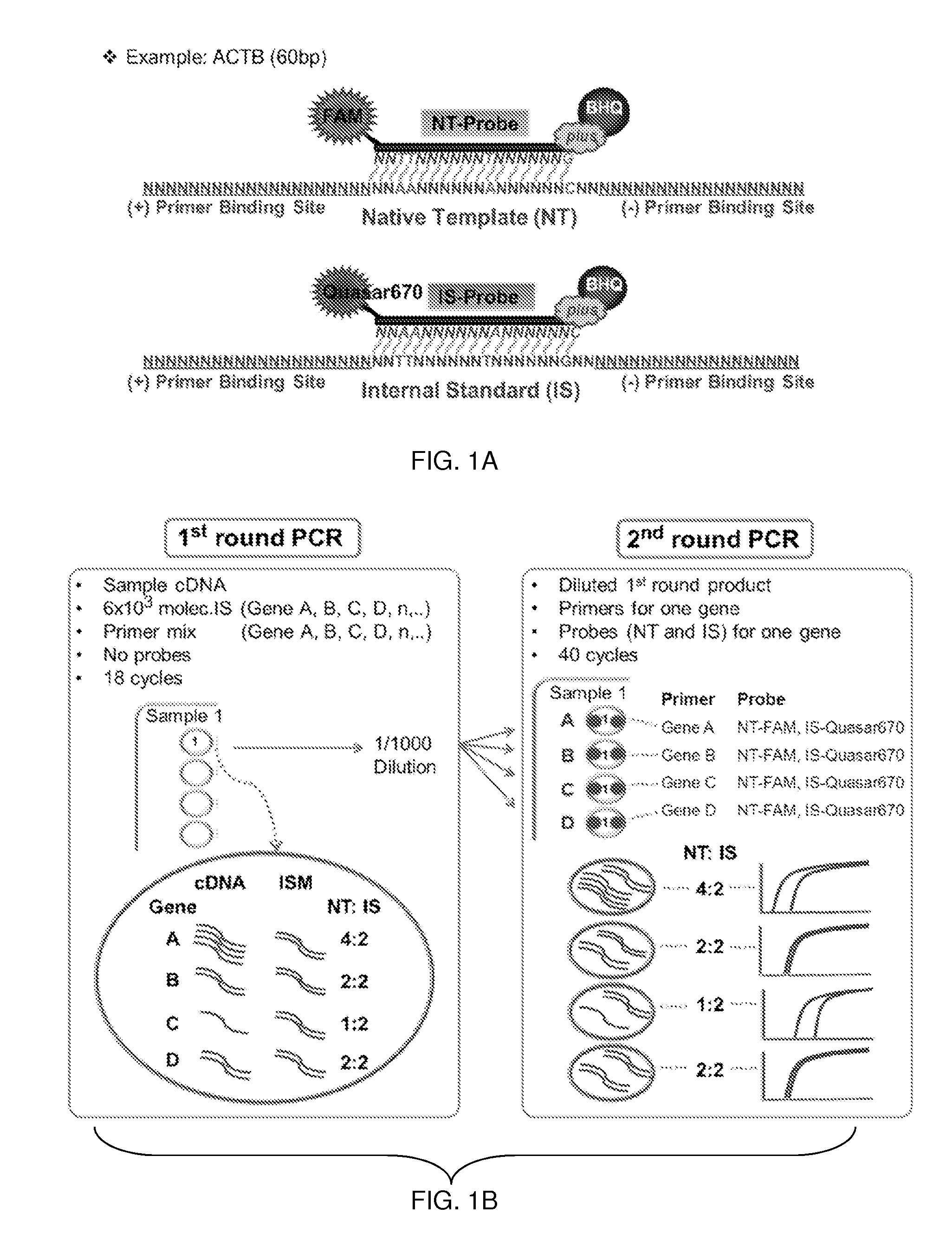
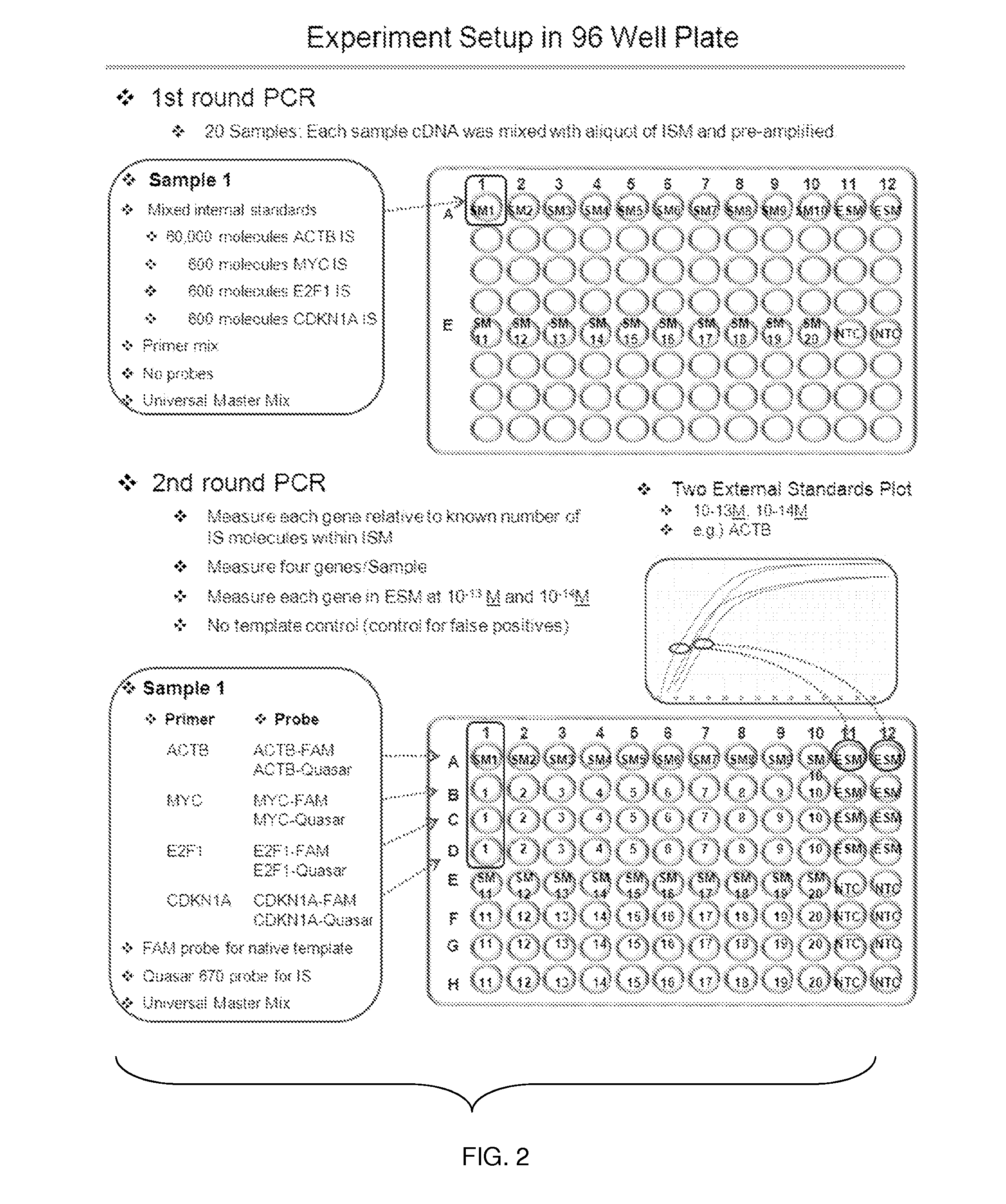
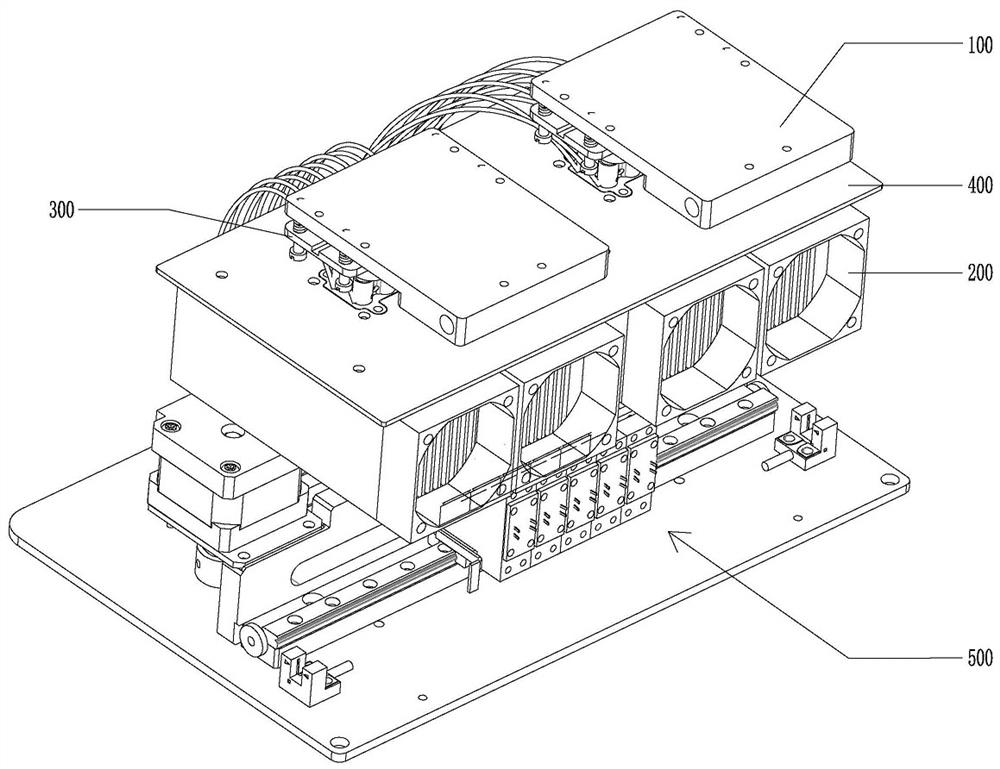
Patents
Literature
33 results about "Molecular Diagnostic Testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
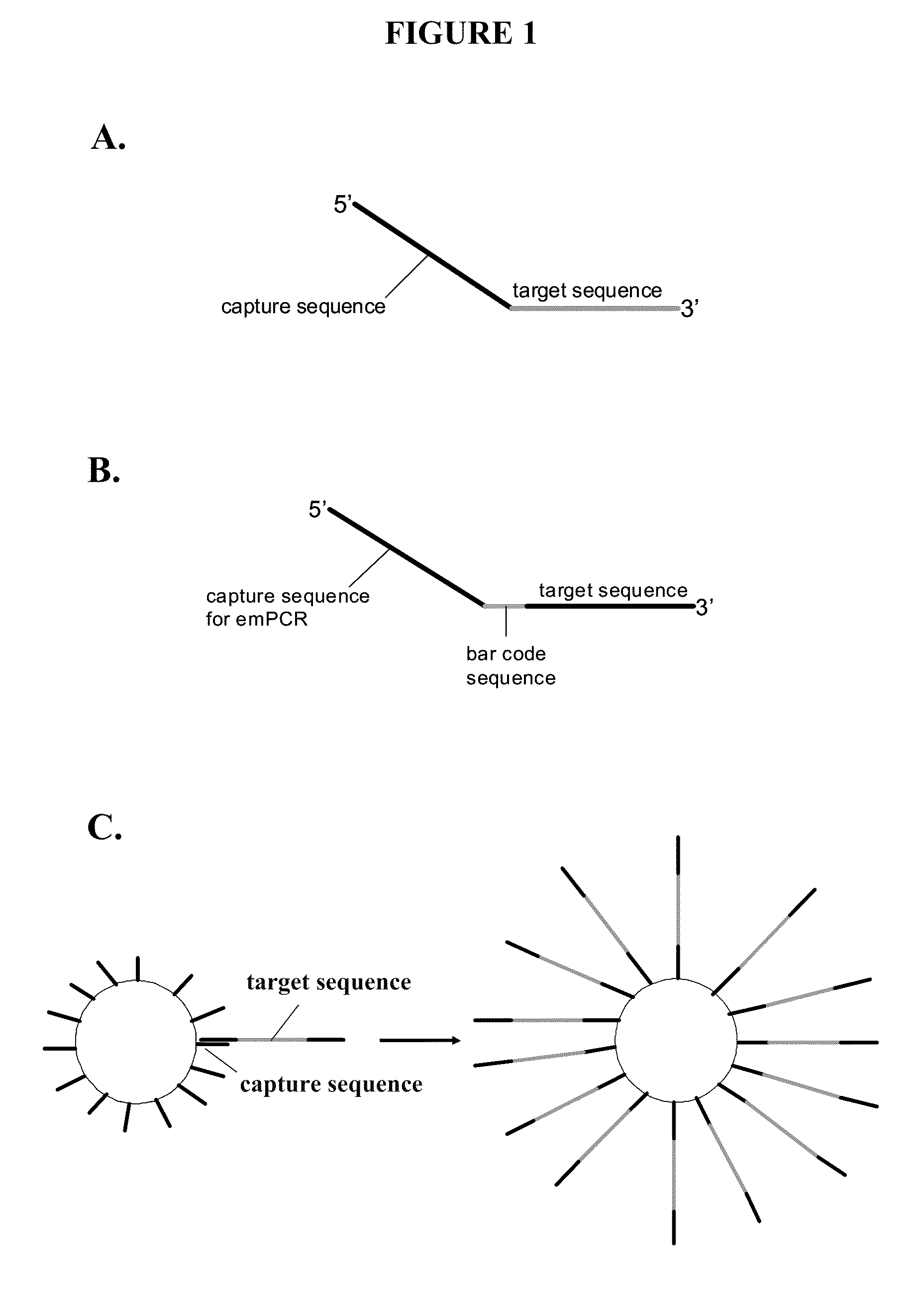
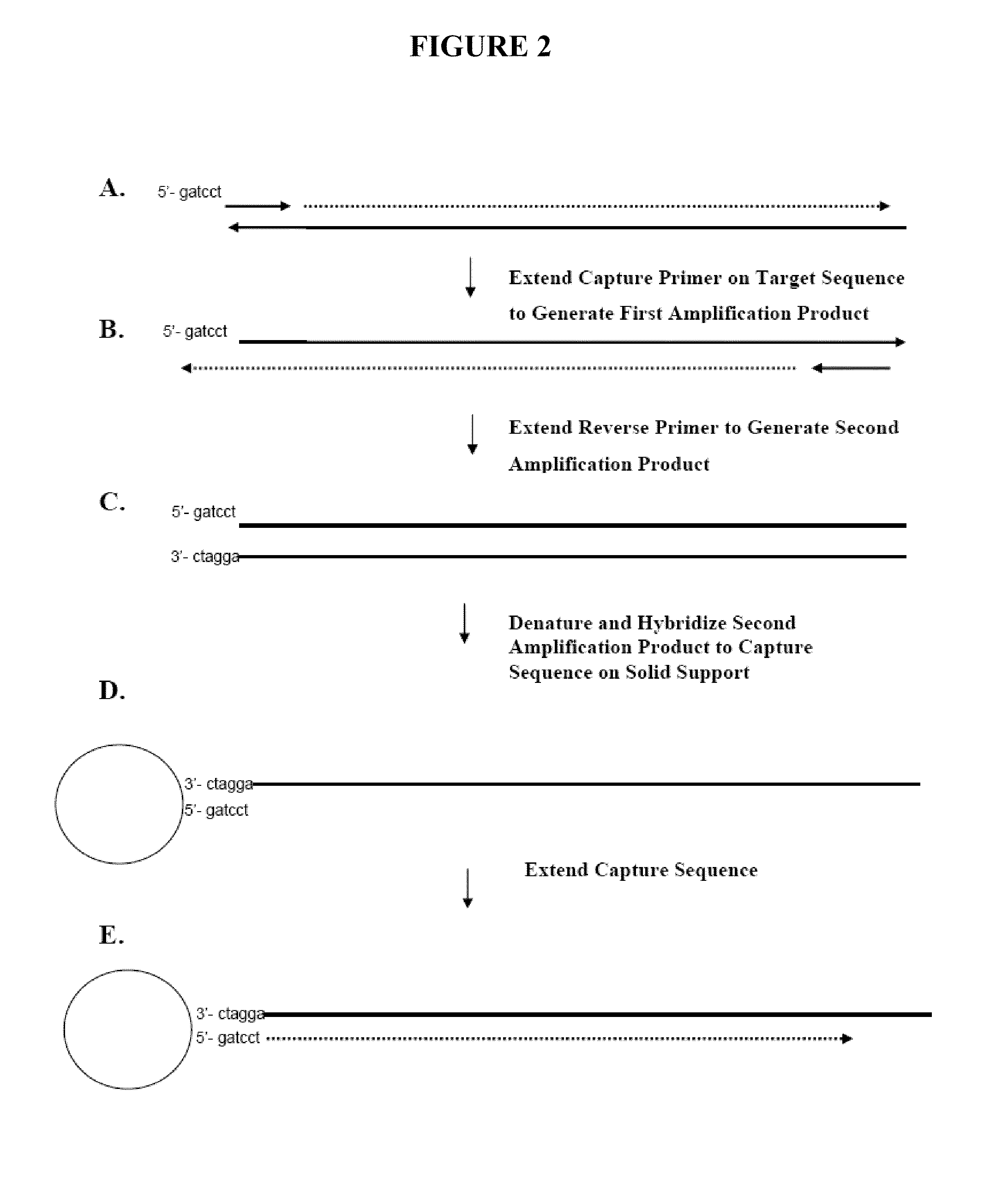
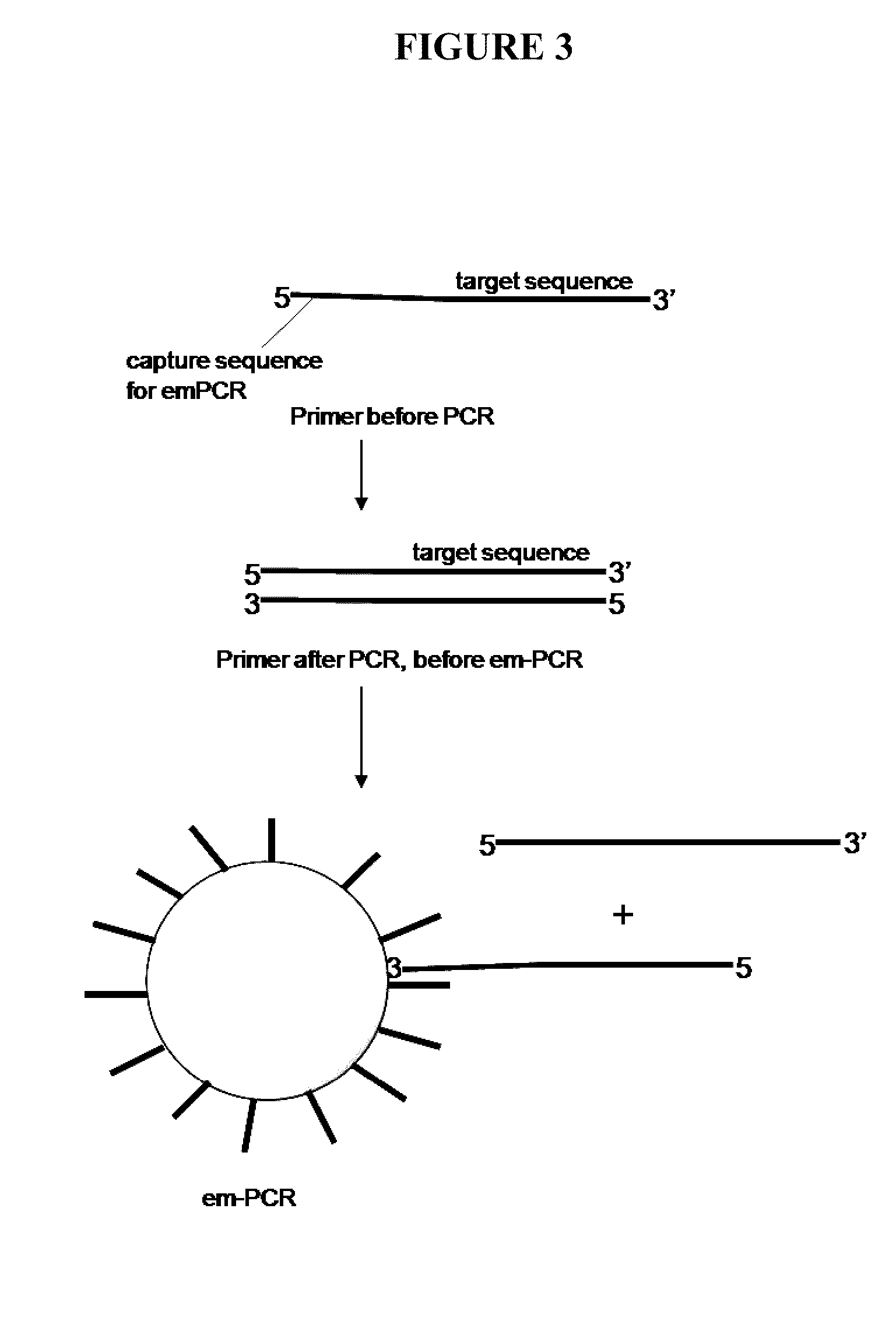
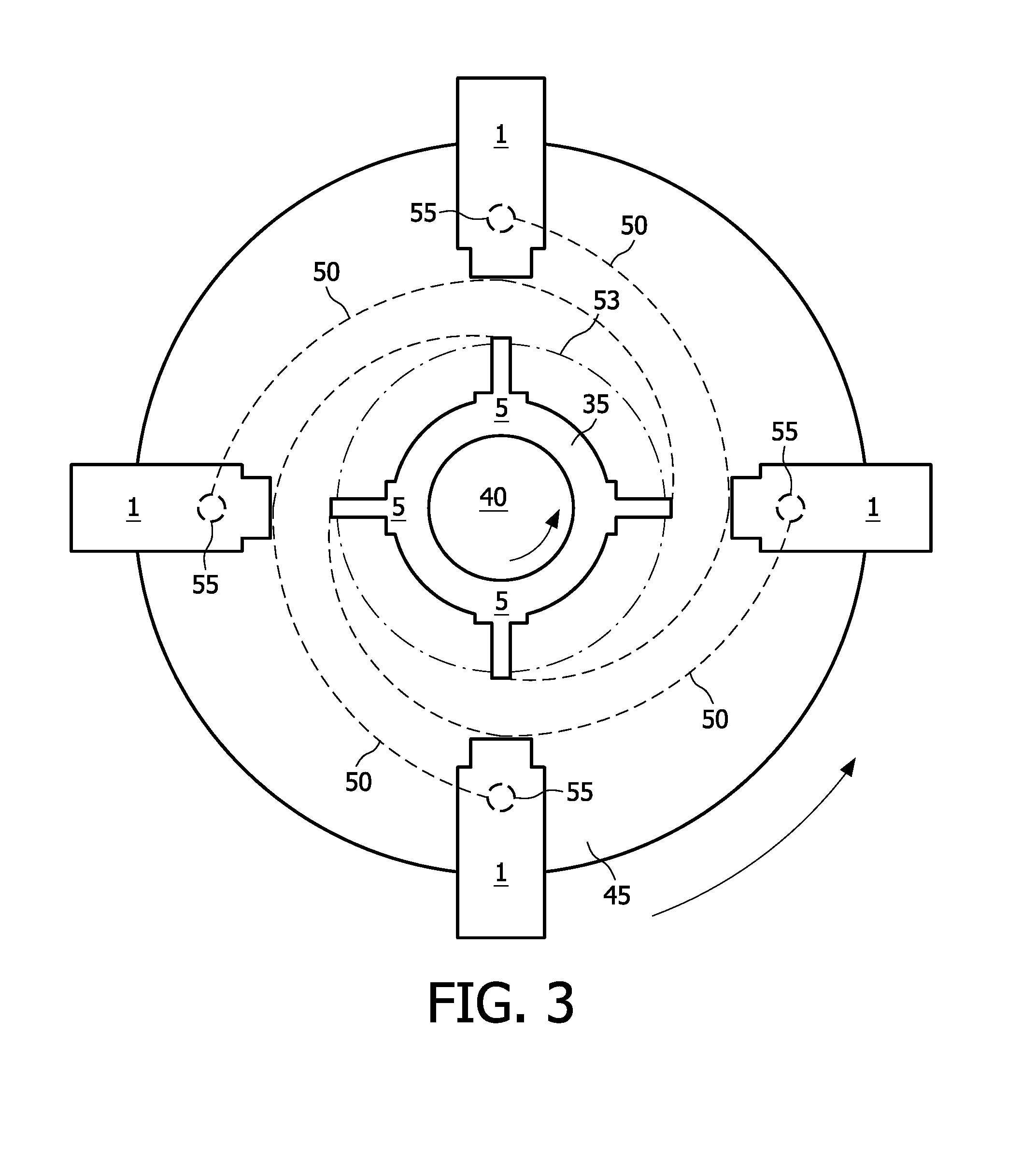
Capture primers and capture sequence linked solid supports for molecular diagnostic tests
InactiveUS9416409B2Microbiological testing/measurementSequence analysisMolecular diagnosticsMolecular Diagnostic Testing
The present invention provides systems, methods, and compositions for performing molecular tests. In particular, the present invention provides methods, compositions and systems for generating target sequence-linked solid supports (e.g., beads) using a solid support linked to a plurality of capture sequences and capture primers composed of a 3′ target-specific portion and a 5′ capture sequence portion. In certain embodiments, the target sequence linked solid support is used in sequencing methods (e.g., pyrosequencing, zero-mode waveguide type sequencing, nanopore sequencing, etc.) to determine the sequence of the target sequence (e.g., in order to detect the identity of a target nucleic acid in sample).
Owner:IBIS BIOSCI
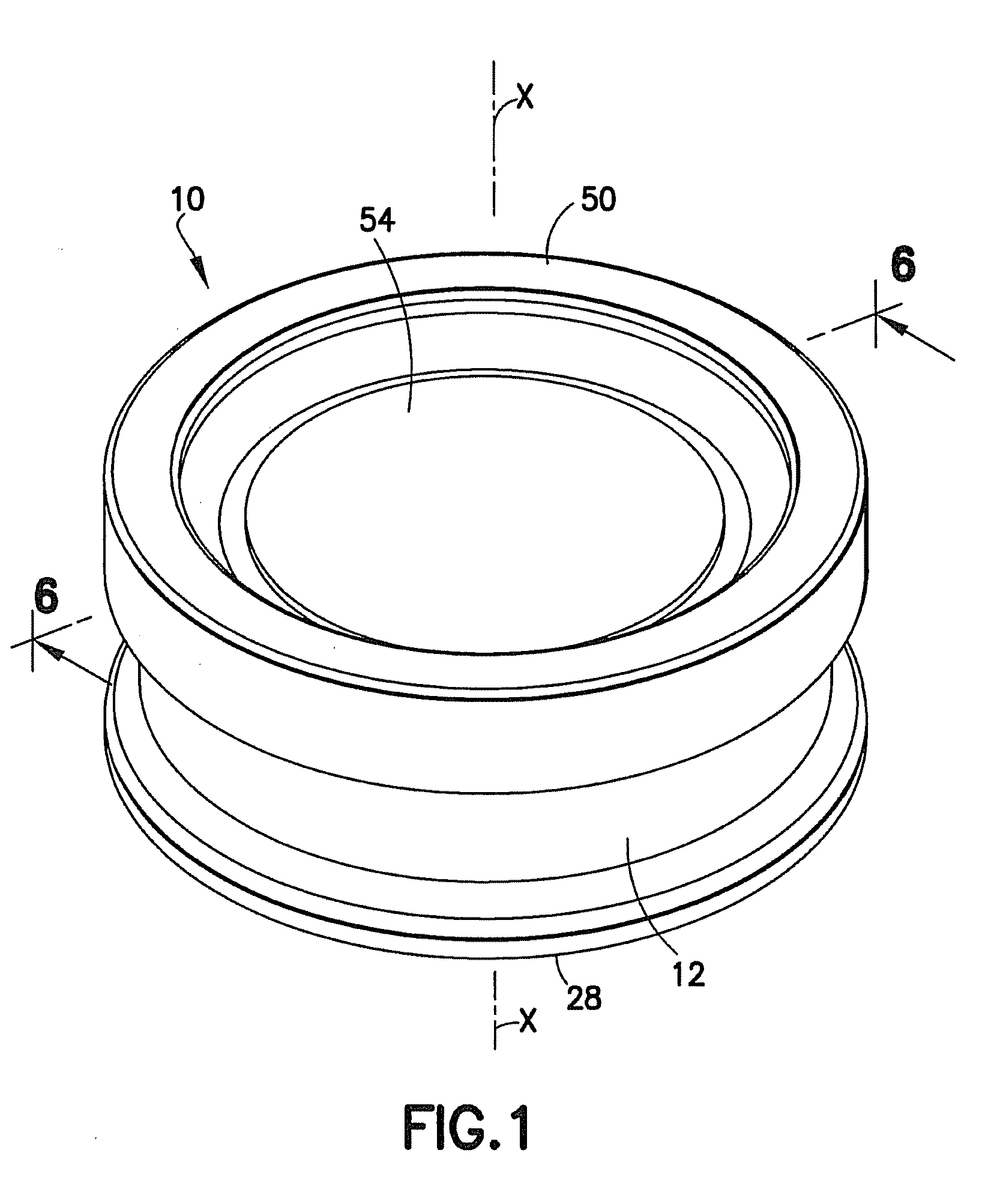
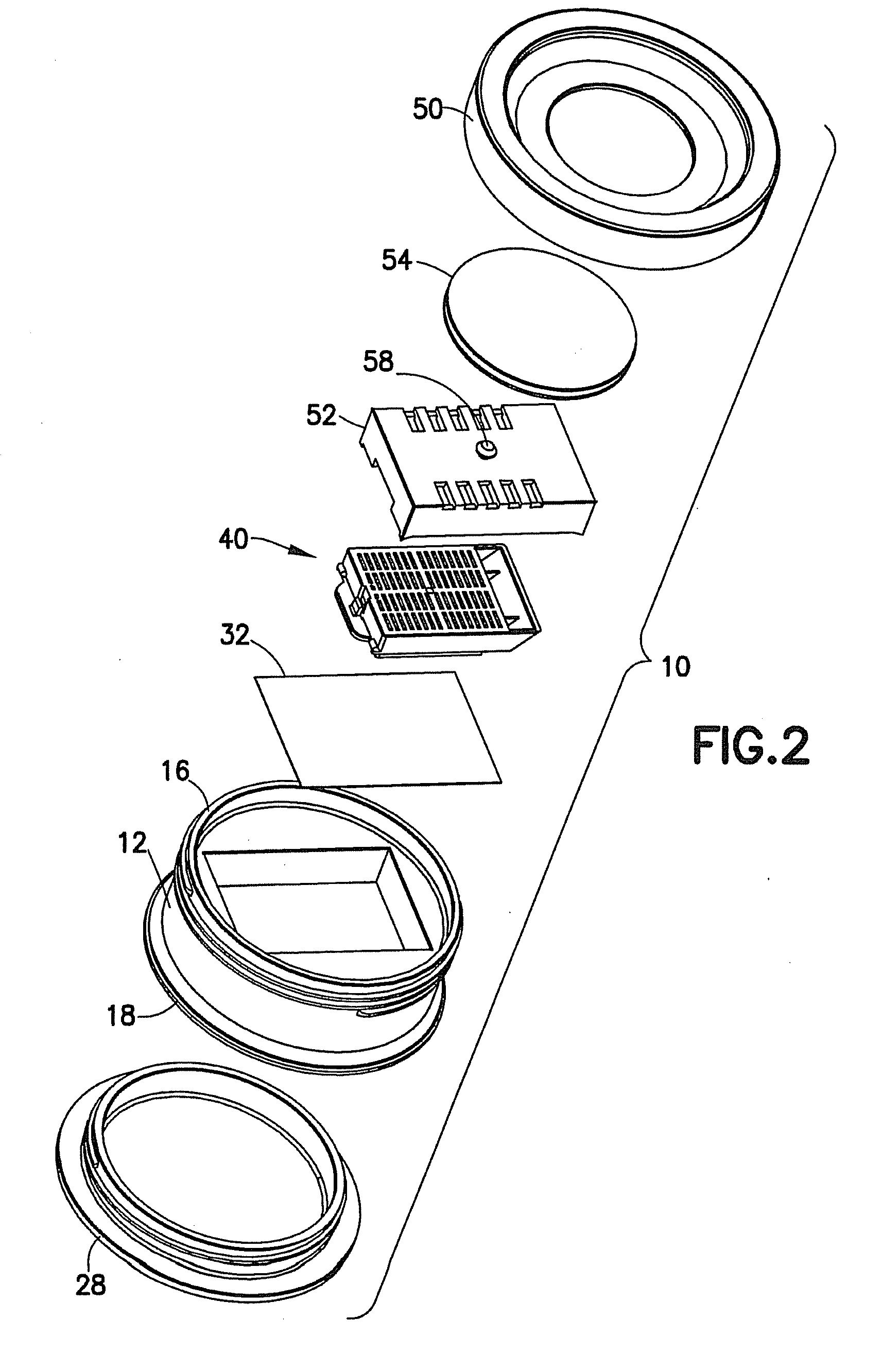
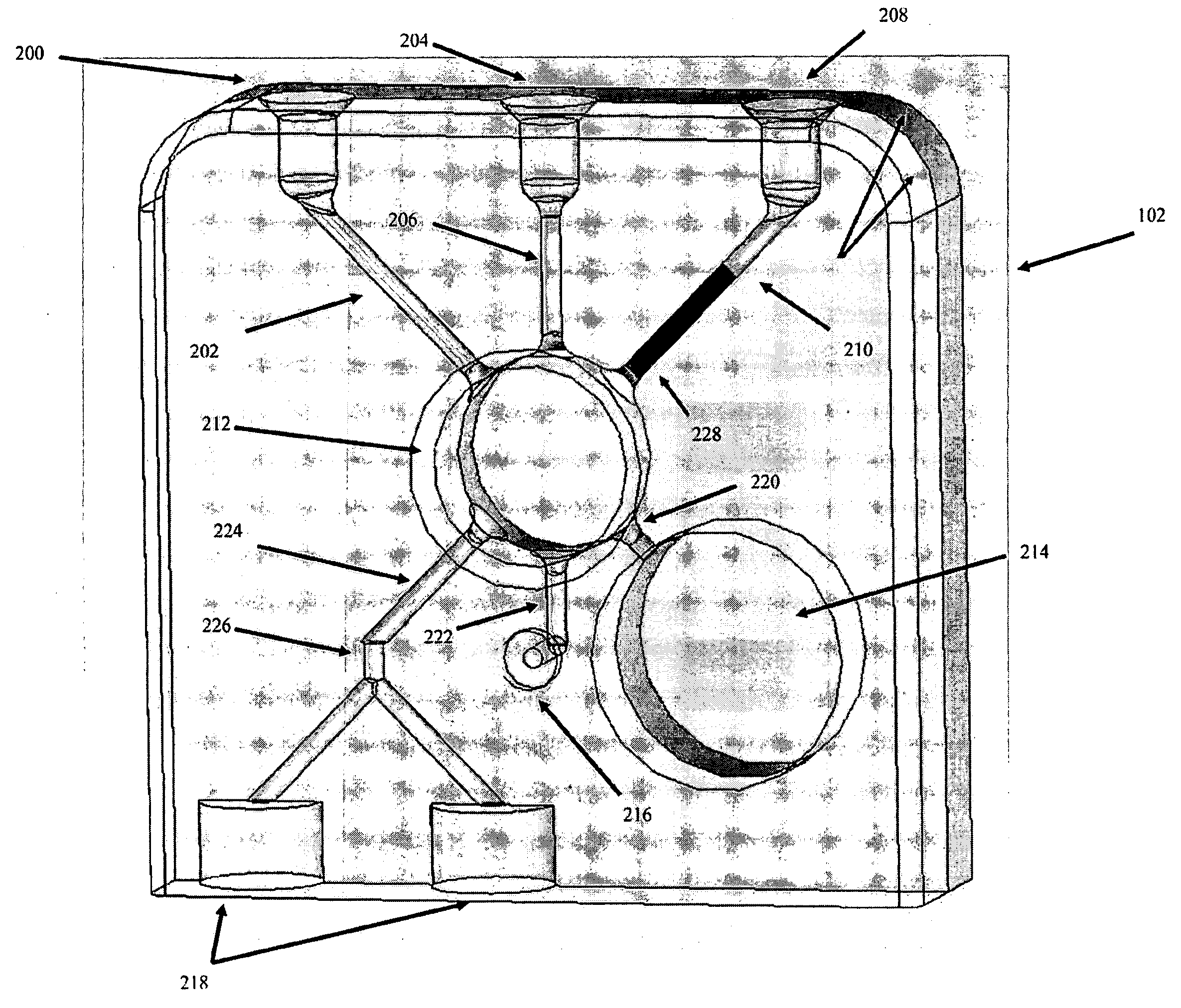
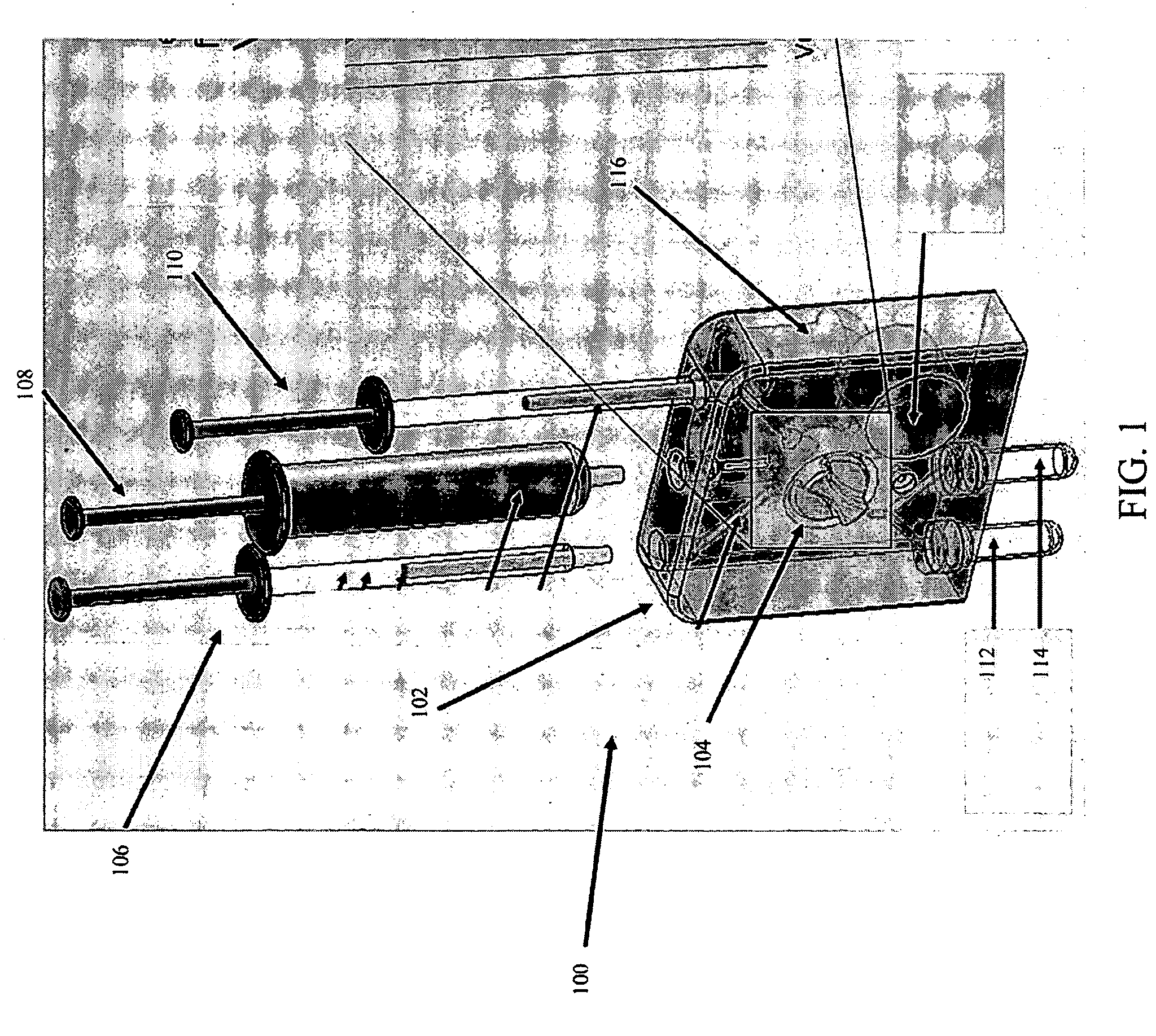
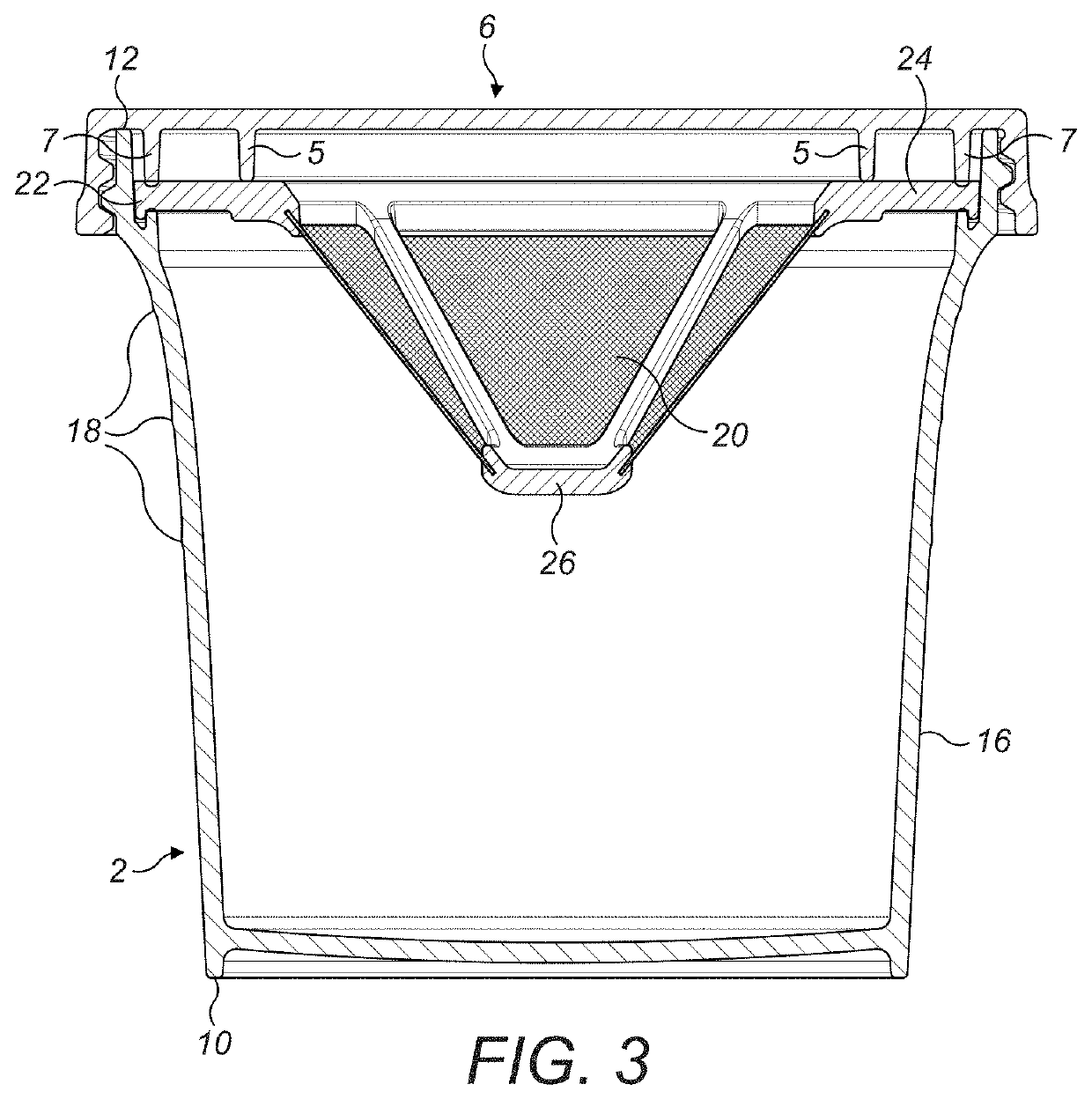
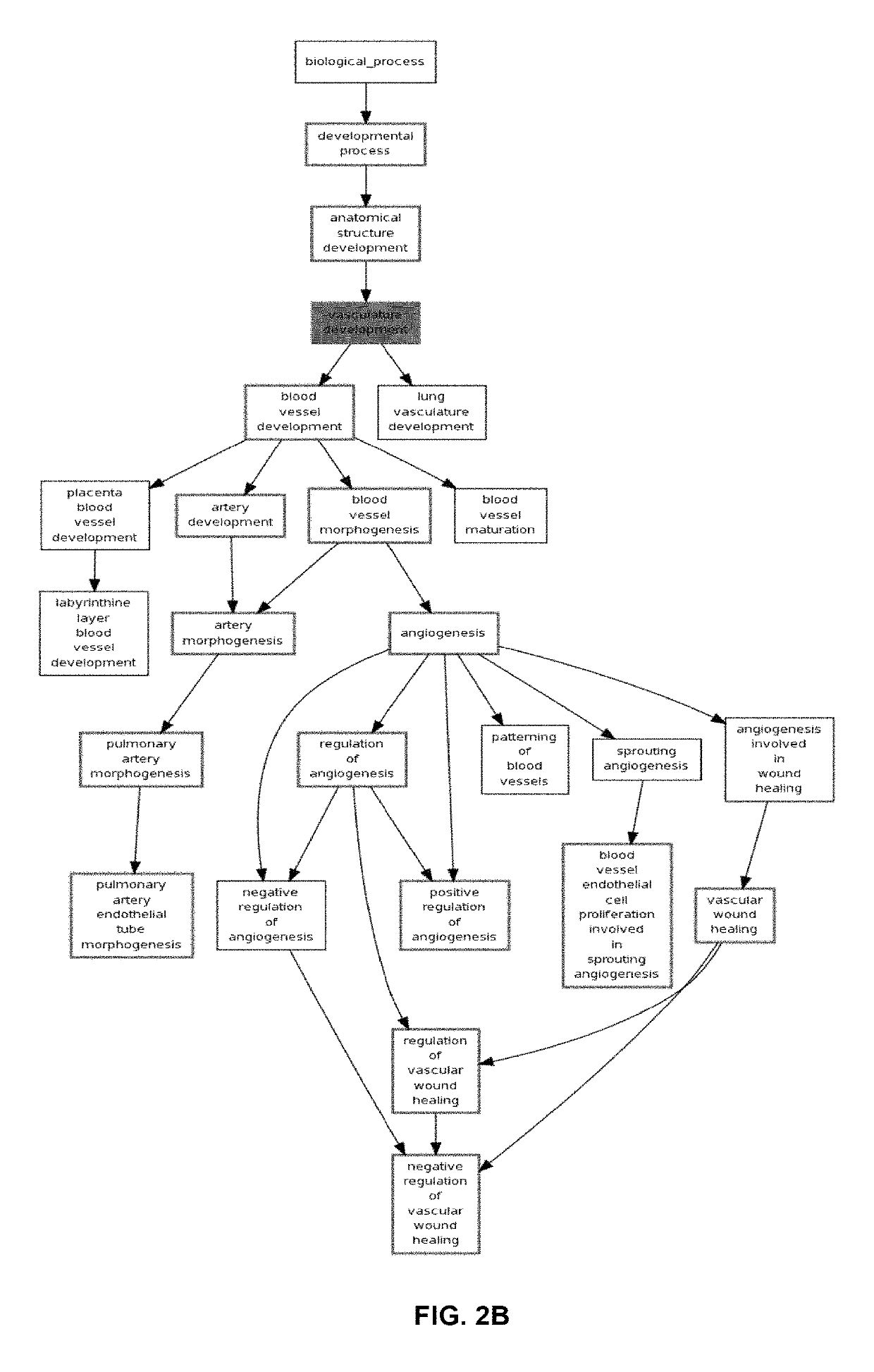
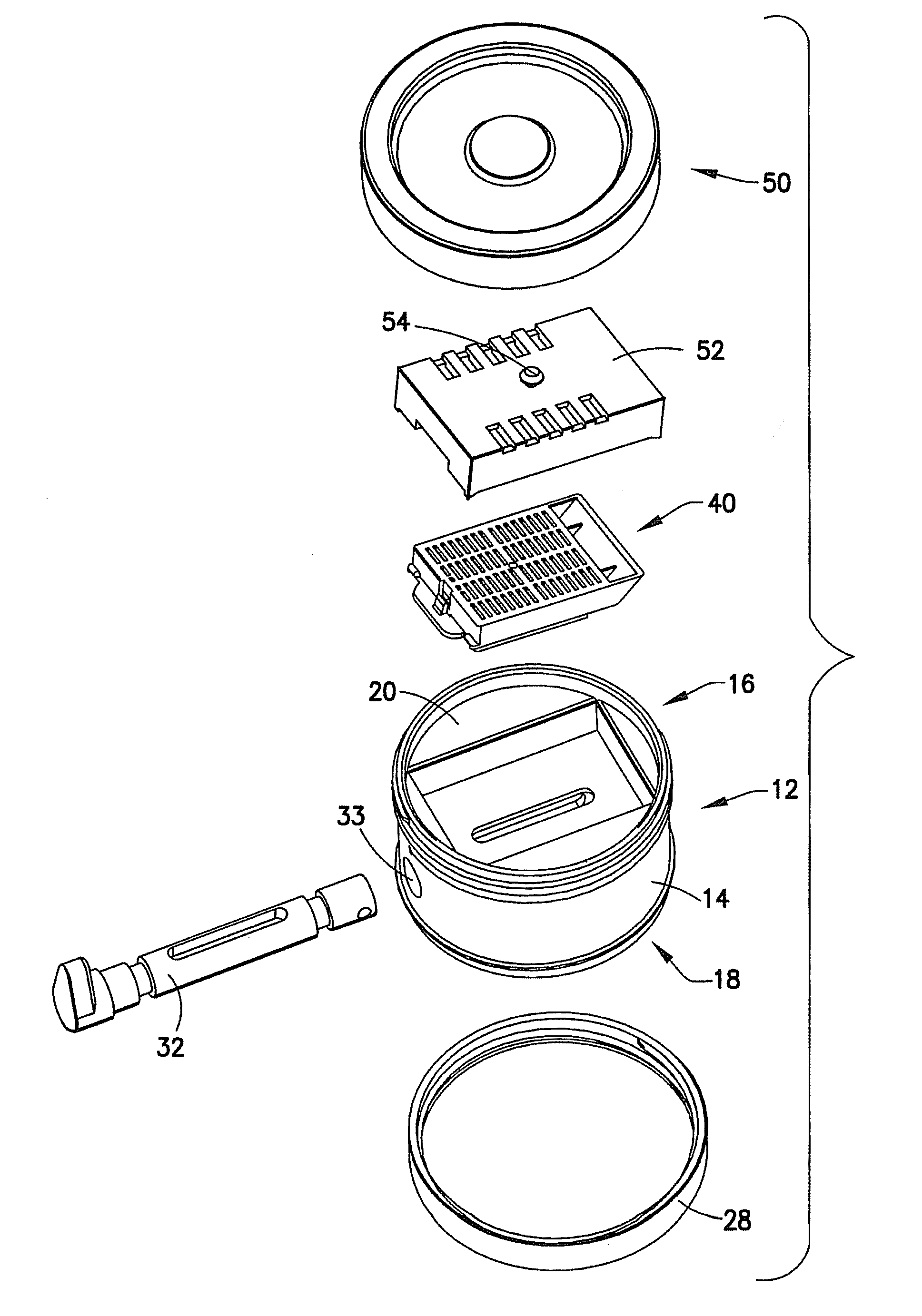
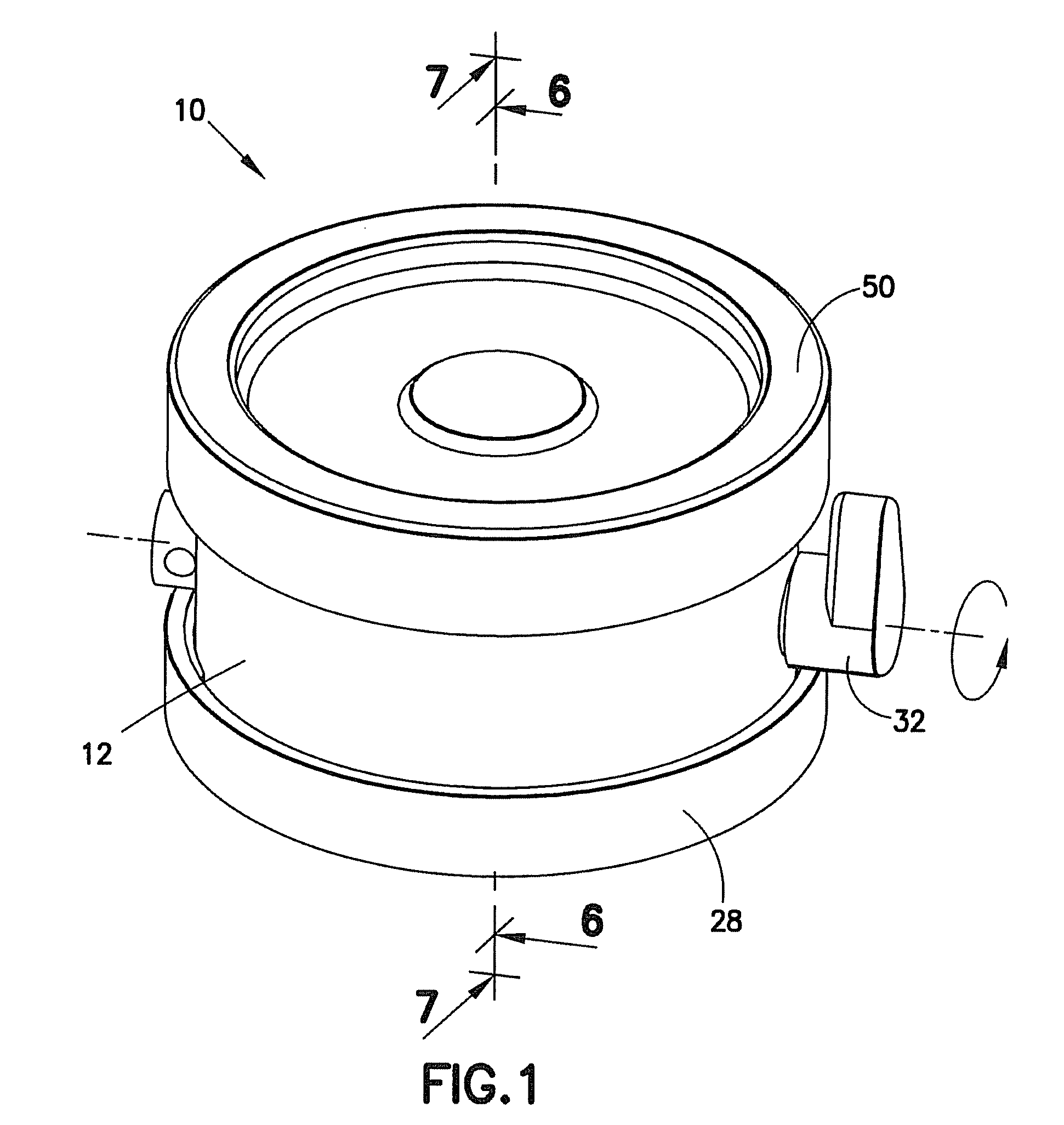
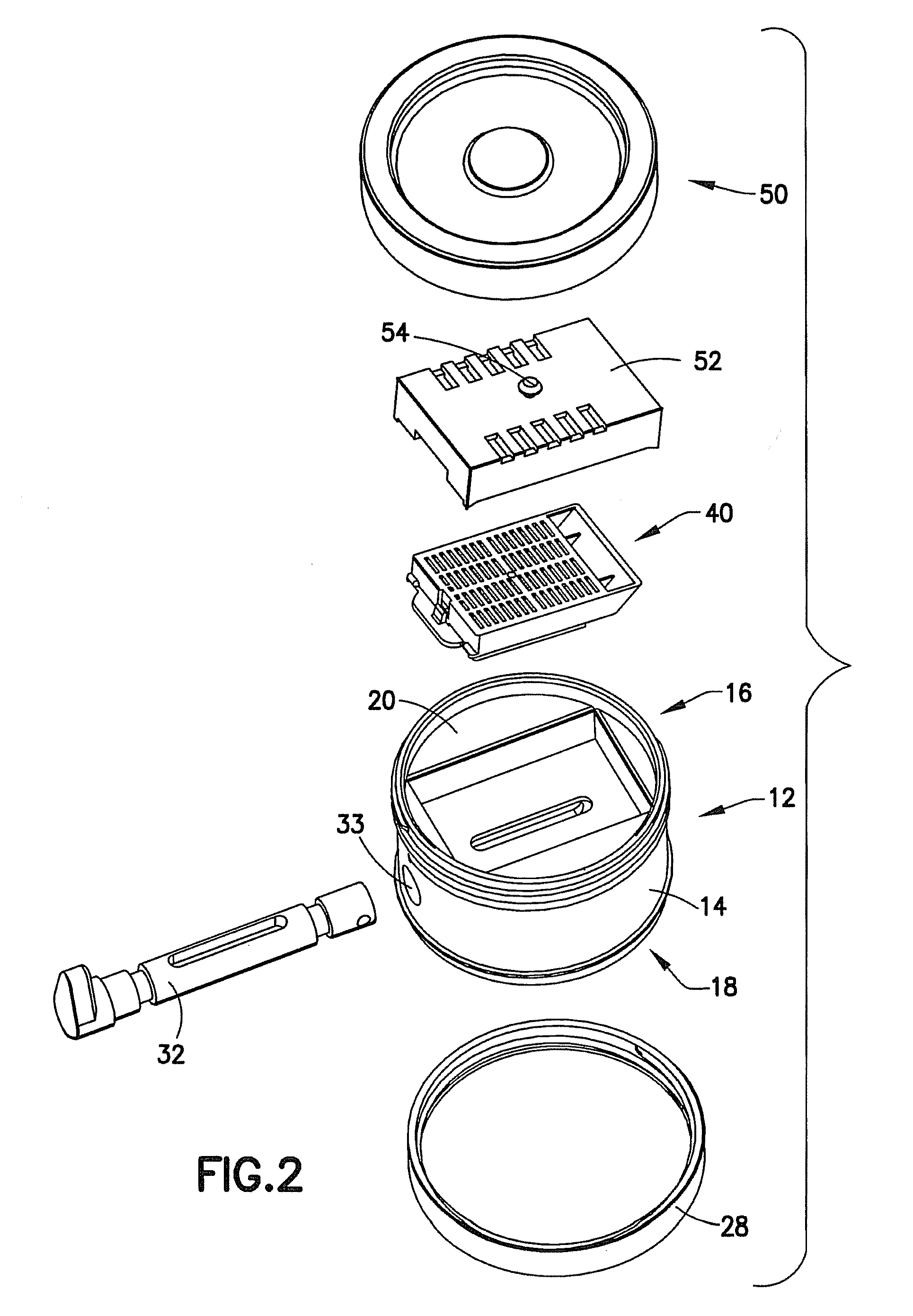
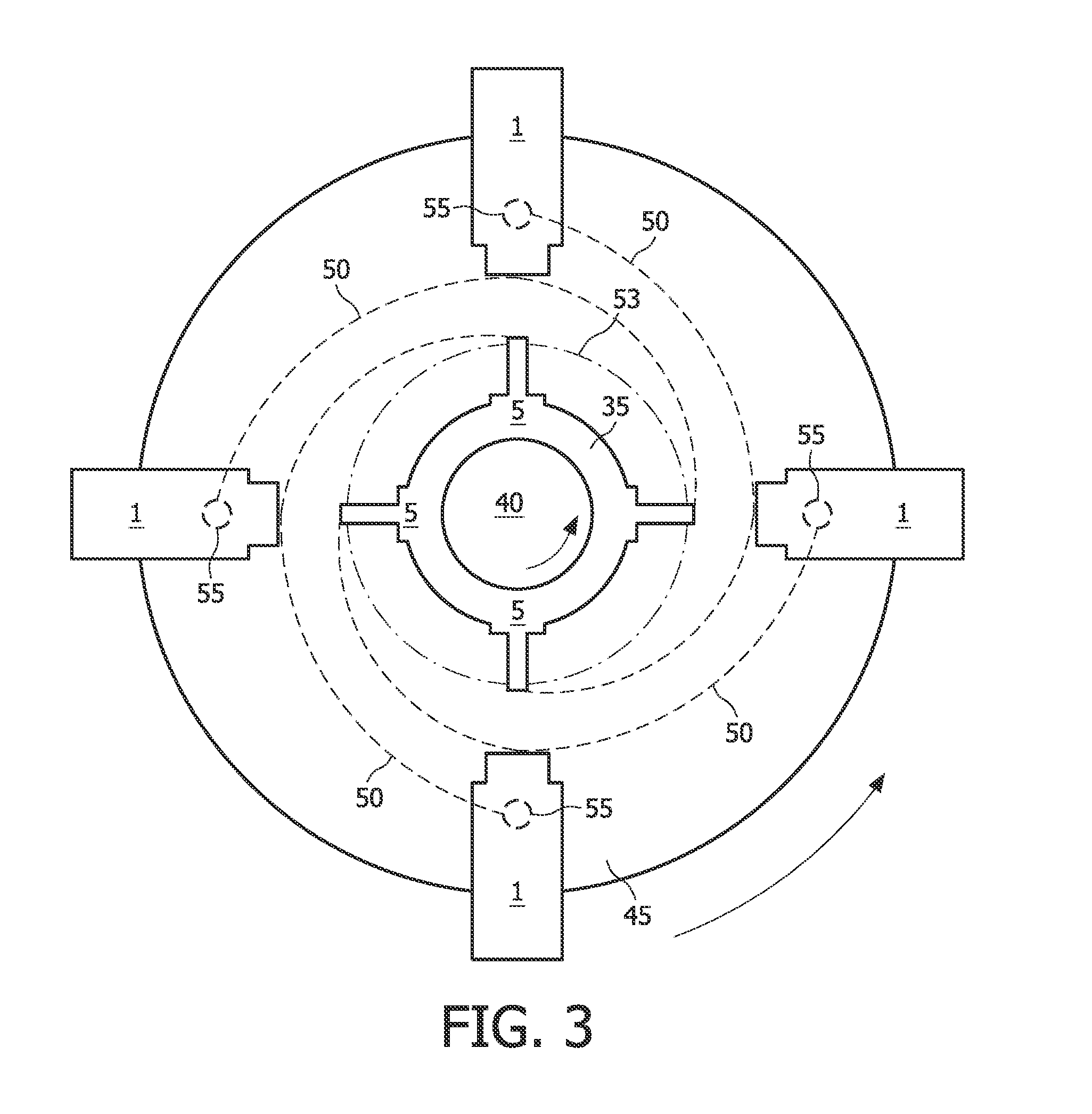
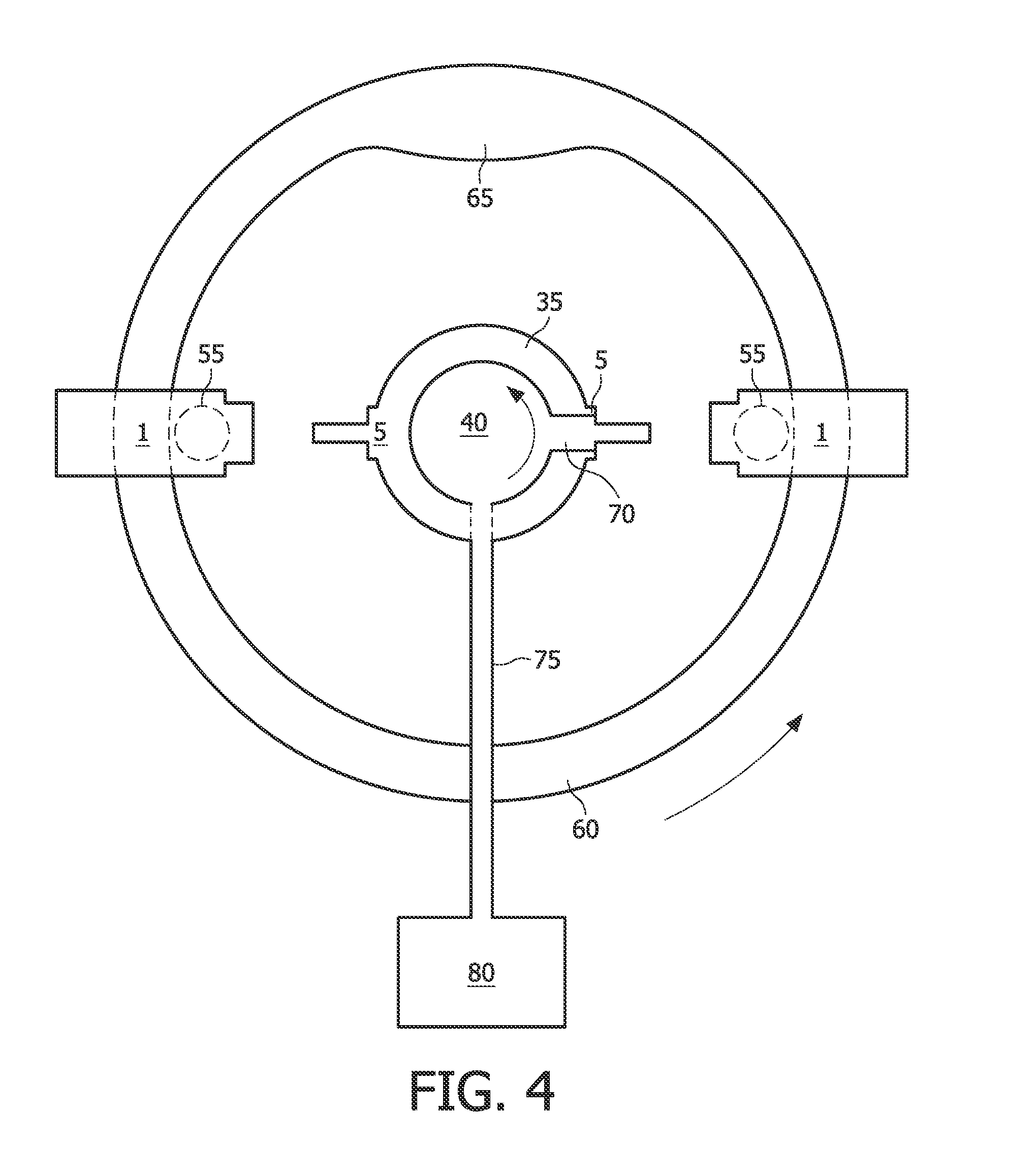
Tissue Container for Molecular and Histology Diagnostics Incorporating a Breakable Membrane
ActiveUS20090104692A1Prevent movementBioreactor/fermenter combinationsBiological substance pretreatmentsHistological diagnosisMolecular Diagnostic Testing
A container for storing a biological sample for molecular diagnostic testing and / or histological testing is provided. The container includes a first chamber for receiving a sample holder therein, a second chamber, and a closure for enclosing the container. A breakable membrane, such as a piercable foil, extends within the container and separates the two chambers. When the breakable membrane is broken, fluid can pass between the first and second chambers. The membrane may be broken through an activator on the closure, such as a depressible member or a rotatable carrier, causing the sample holder to break through the membrane.
Owner:BECTON DICKINSON & CO
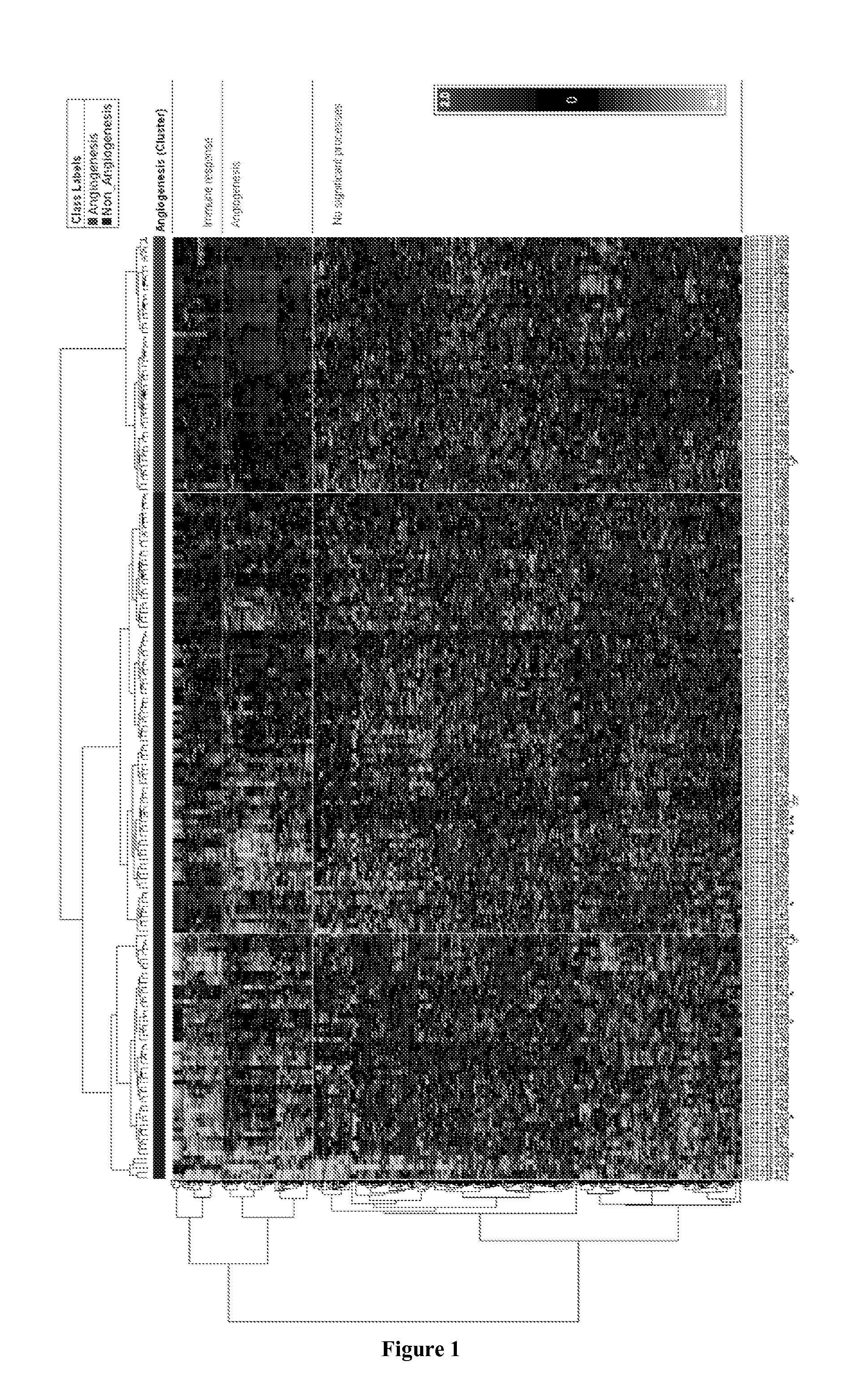
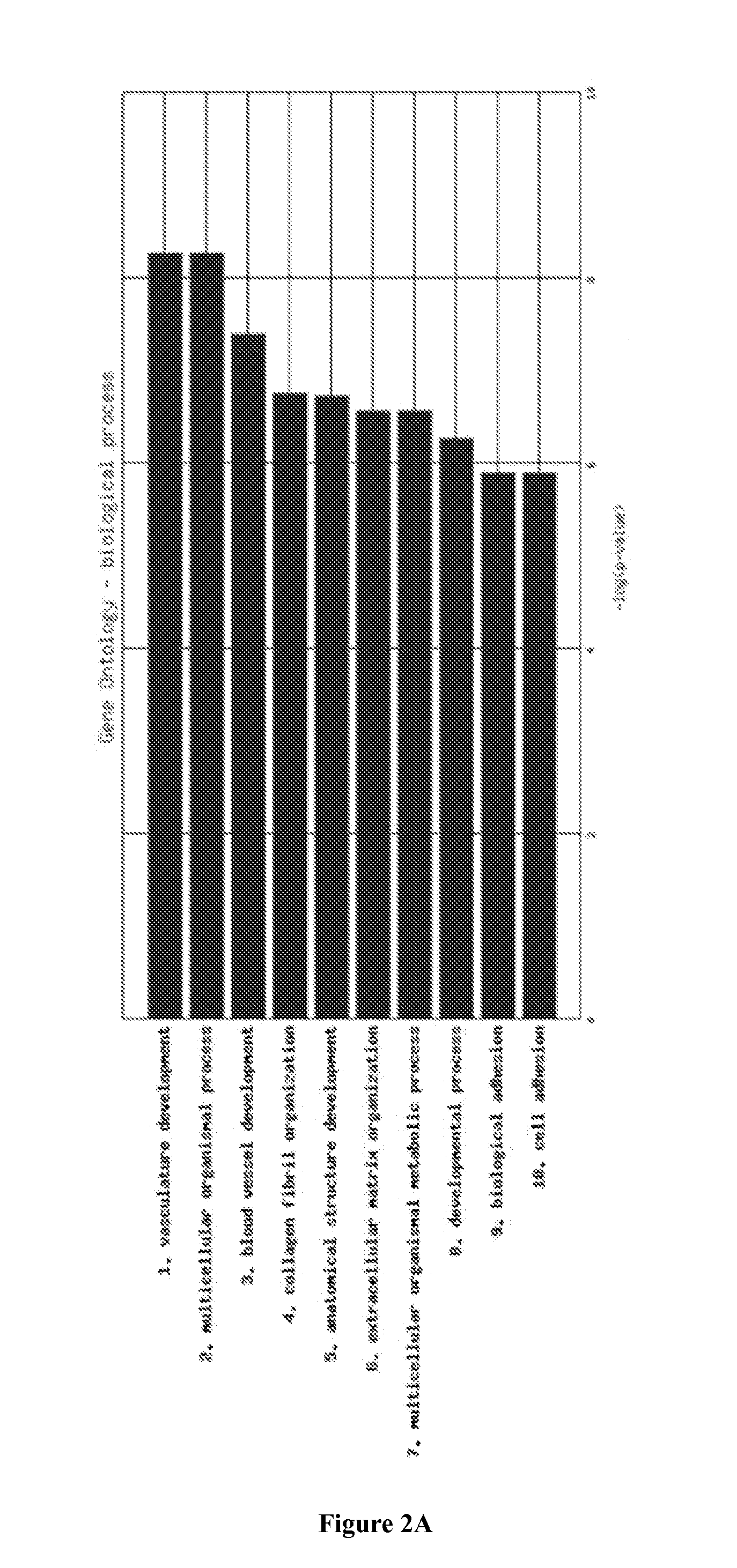
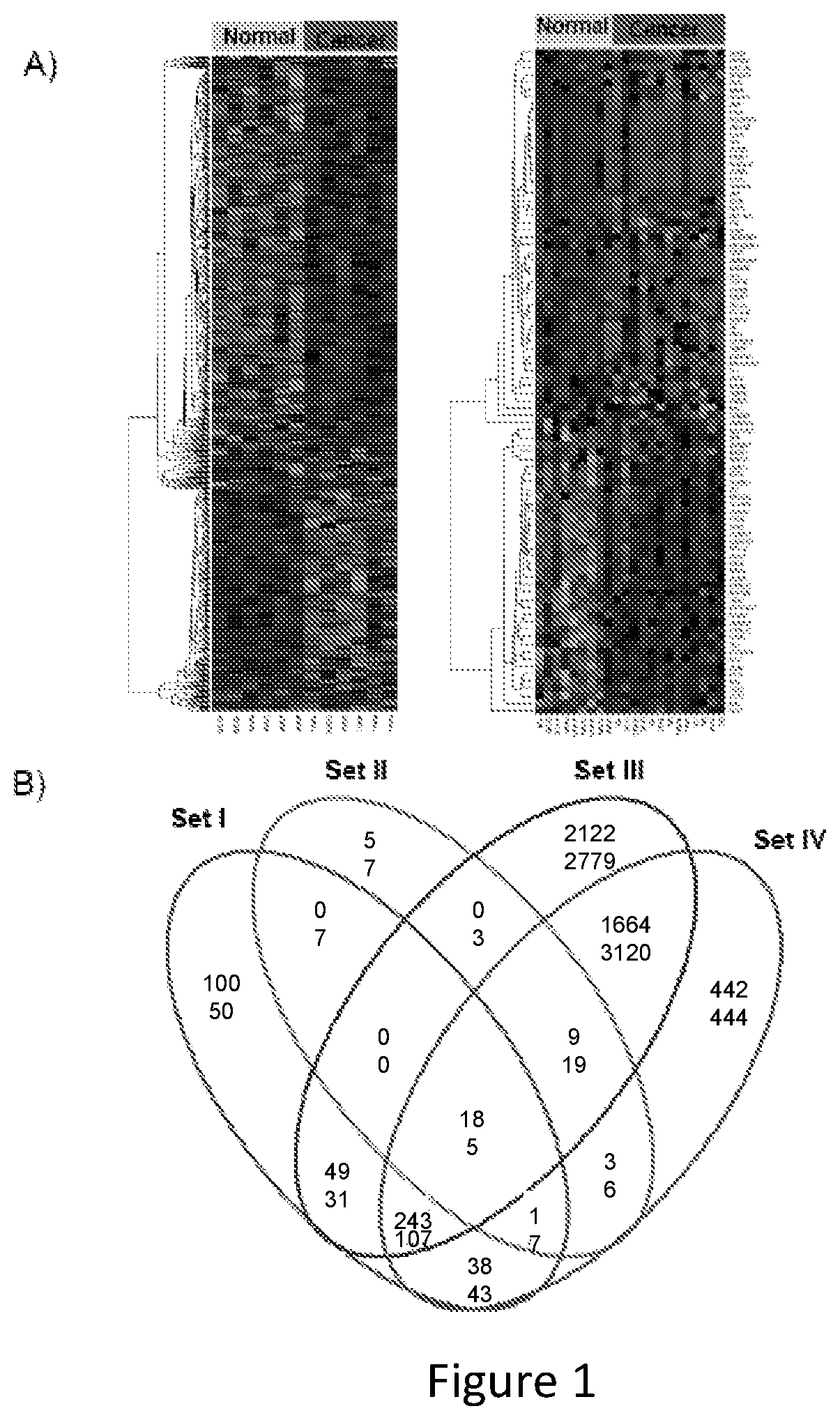
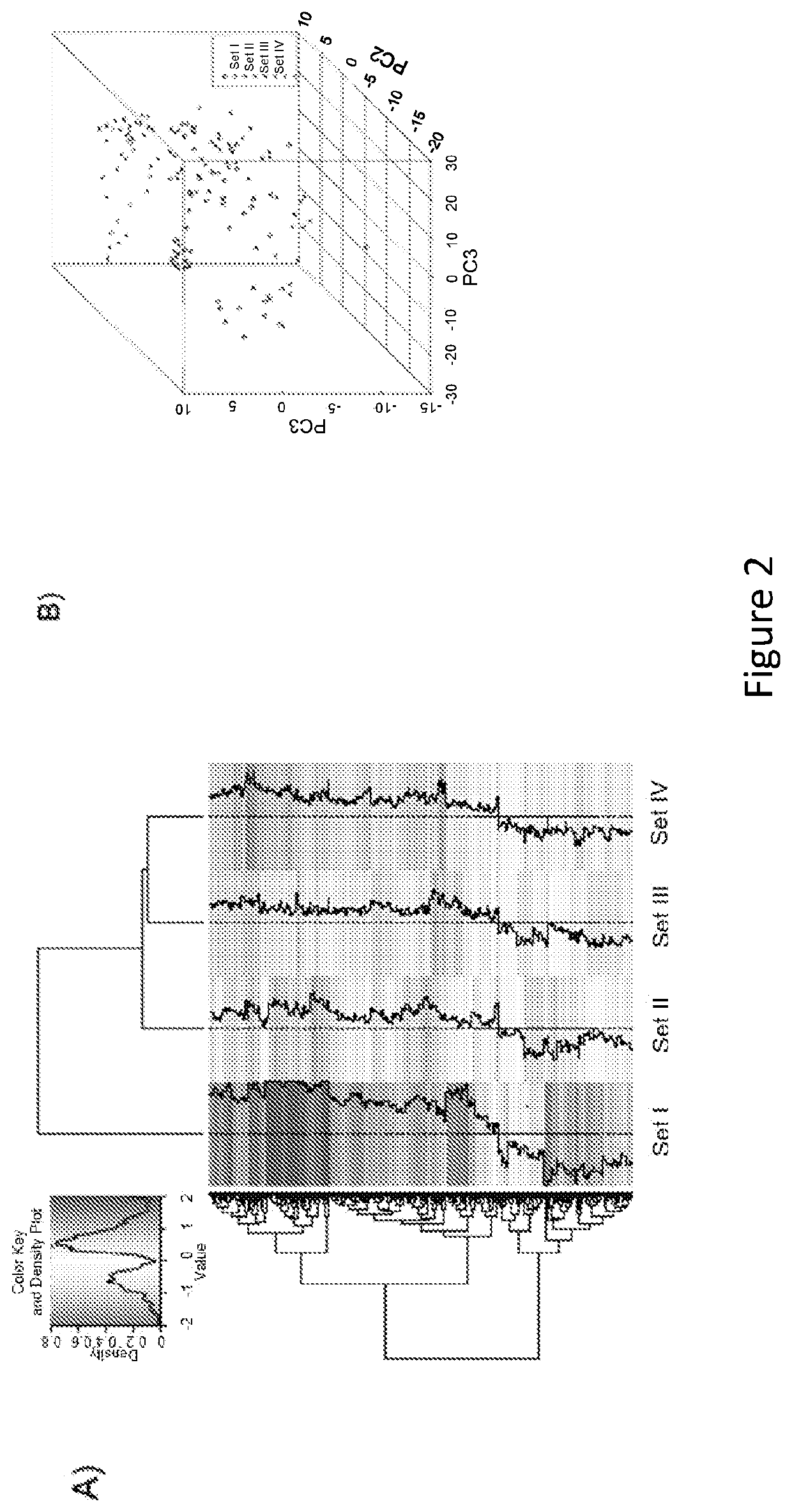
Molecular Diagnostic Test for Cancer
InactiveUS20140342924A1Microbiological testing/measurementLibrary screeningCancer typeAntiangiogenic therapy
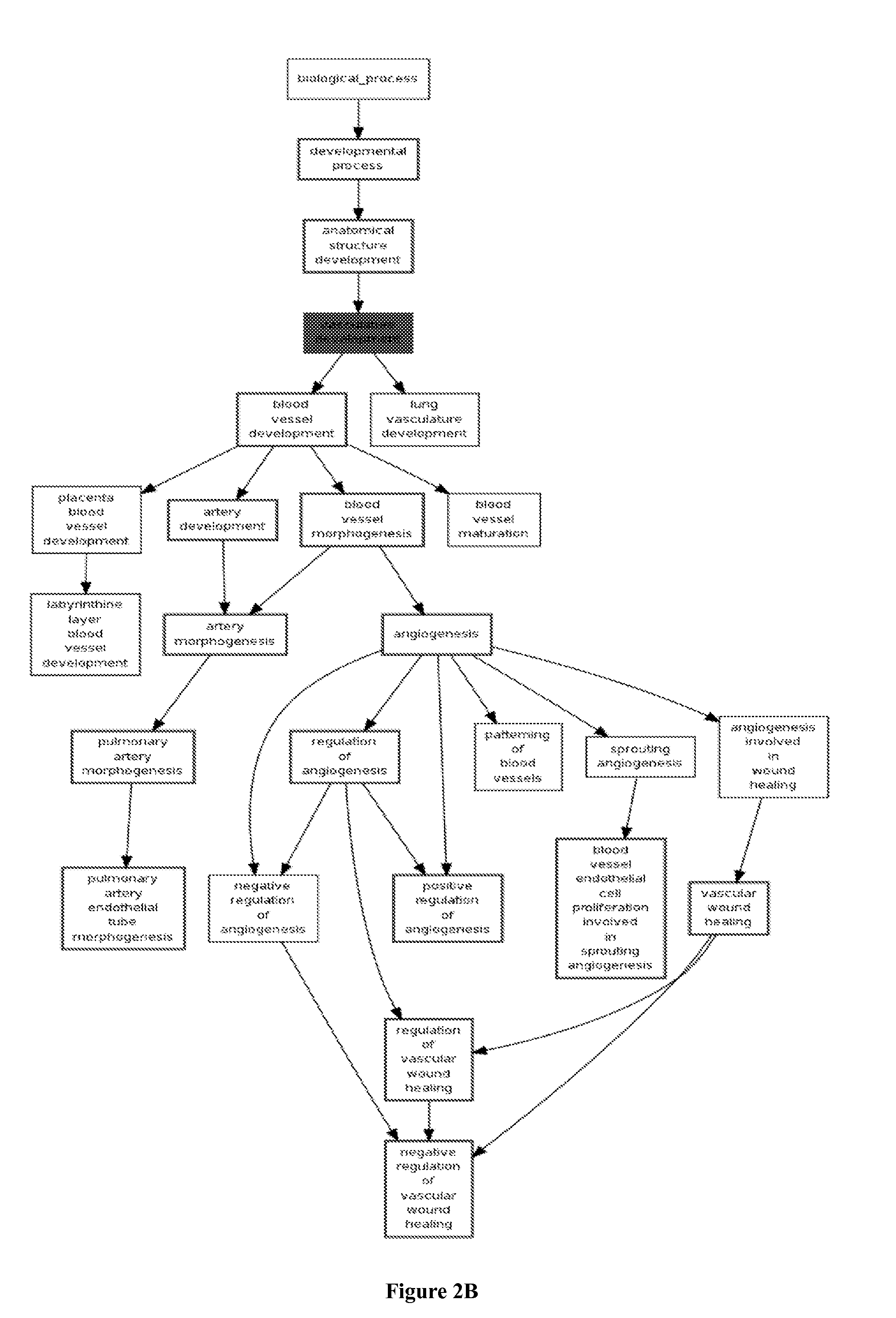
Methods and compositions are provided for the identification of a molecular diagnostic test for cancer. The test identifies cancer subtypes that are responsive to anti-angiogenesis therapeutics and enables classification of a patient within this subtype. The present invention can be used to determine whether patients with cancer are clinically responsive or non-responsive to a therapeutic regimen prior to administration of any anti-angiogenic agent. This test may be used in different cancer types and with different drugs that directly or indirectly affect angiogenesis or angiogenesis signalling. In addition, the present invention may be used as a prognostic indicator for certain cancer types. In particular, the present invention is directed to the use of certain combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen.
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
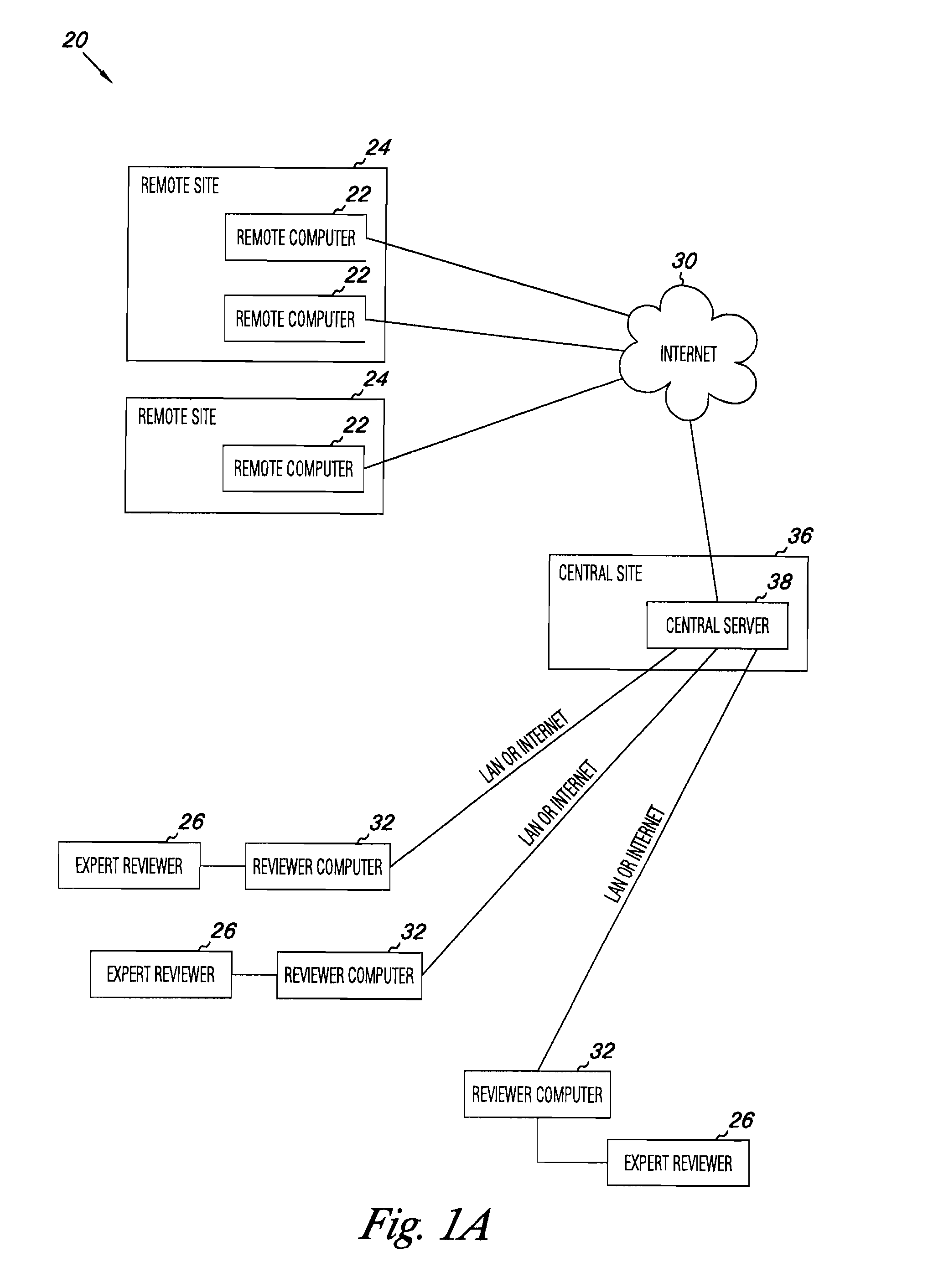
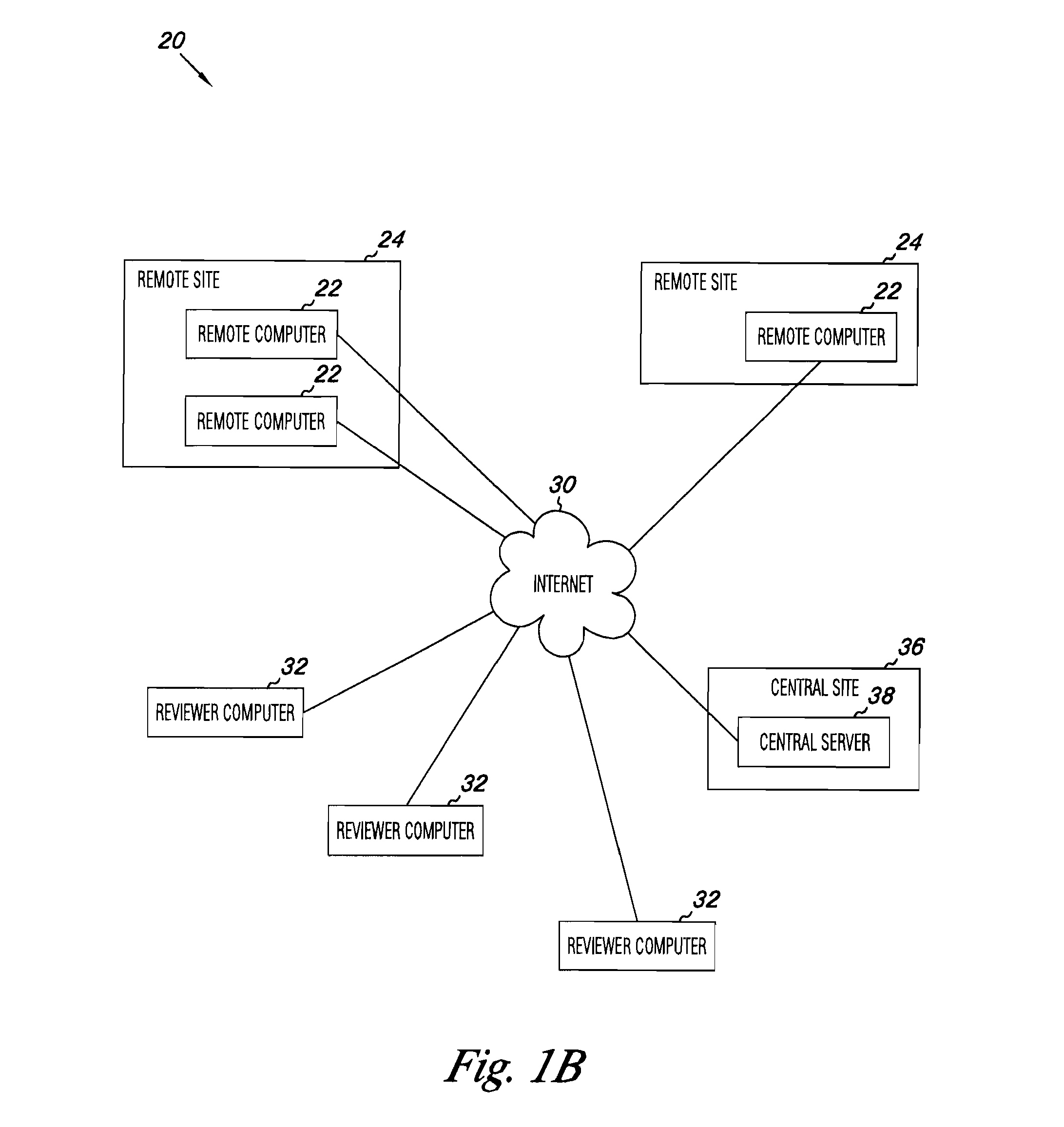
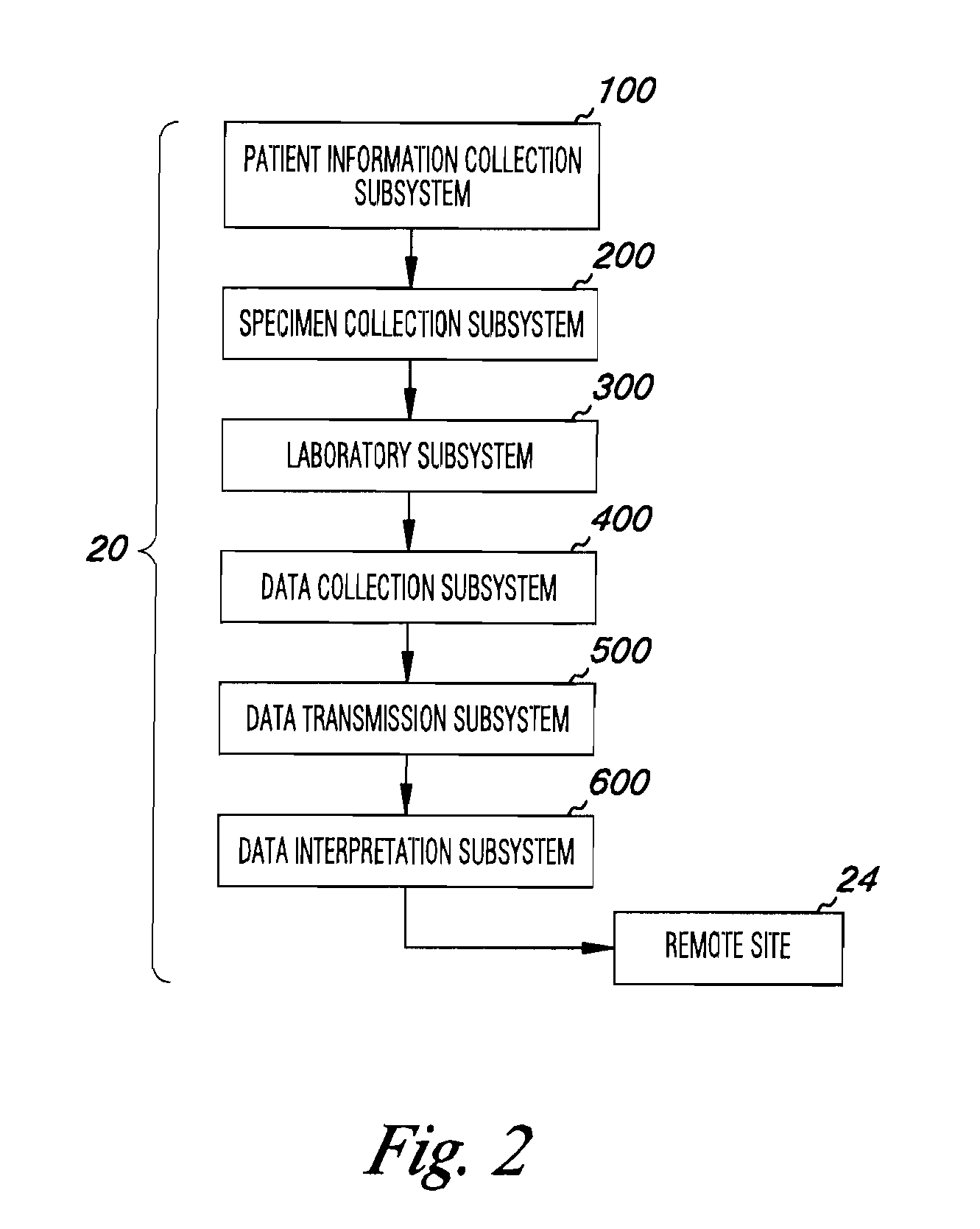
Apparatus and methods for medical testing
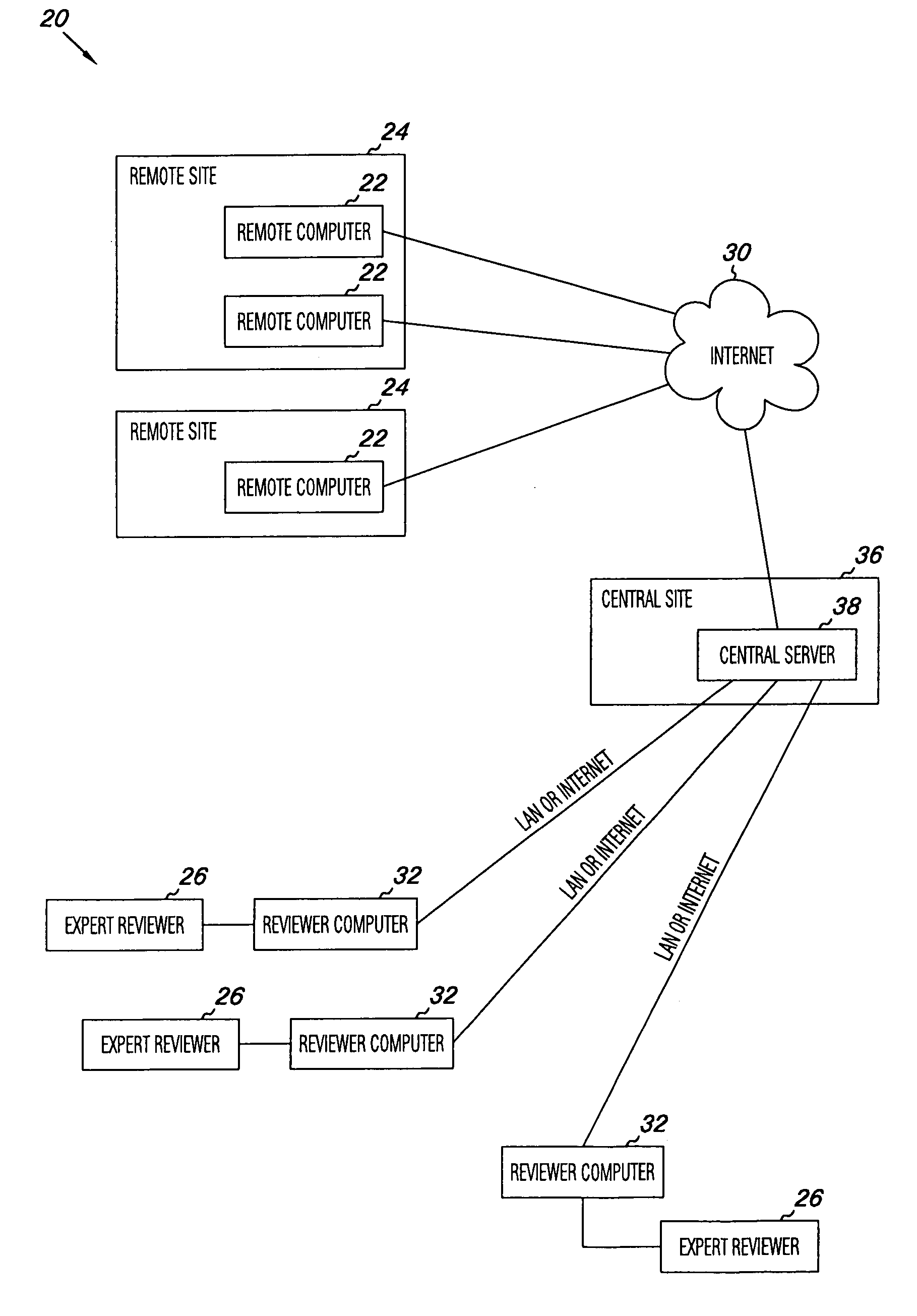
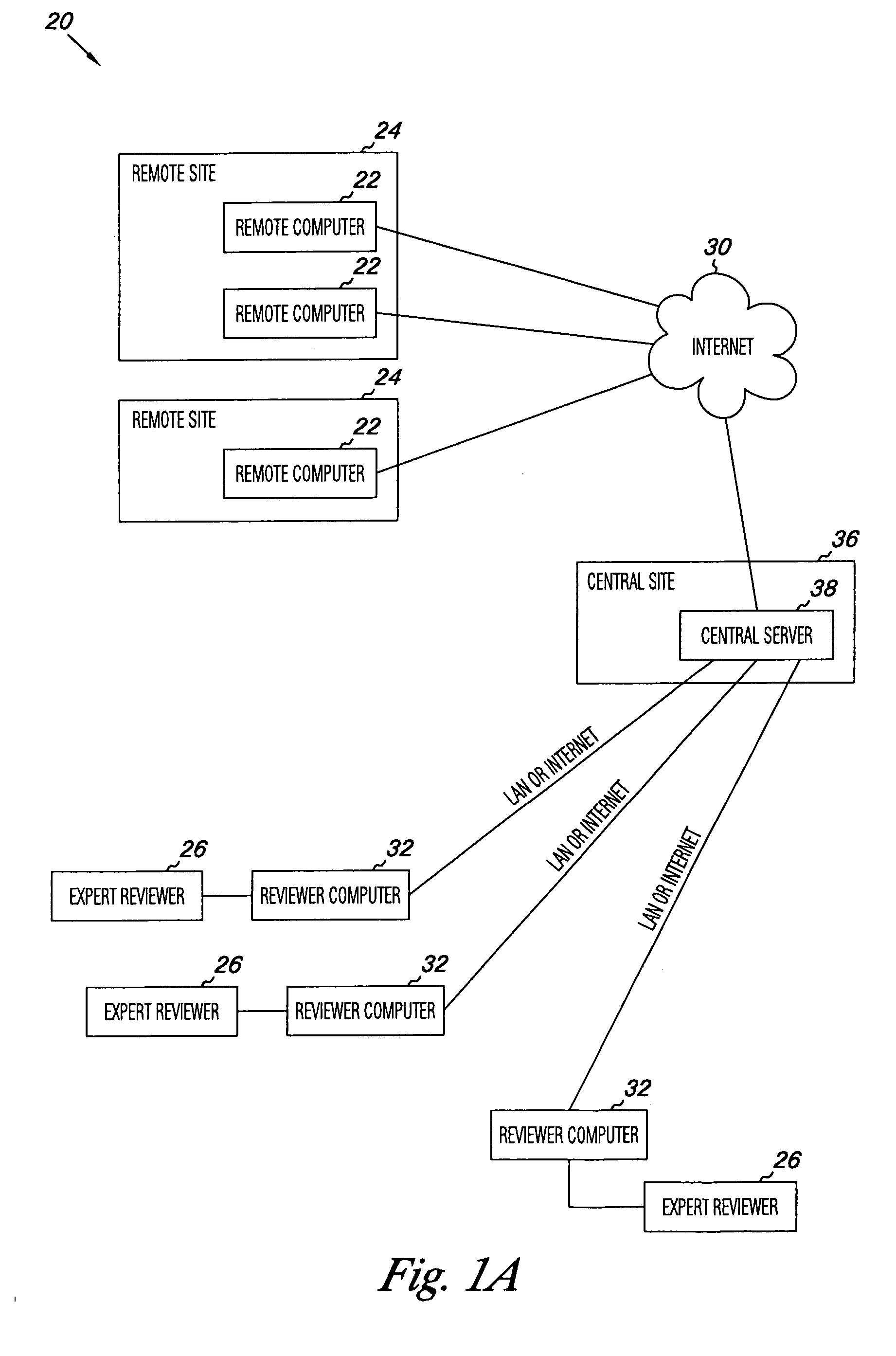
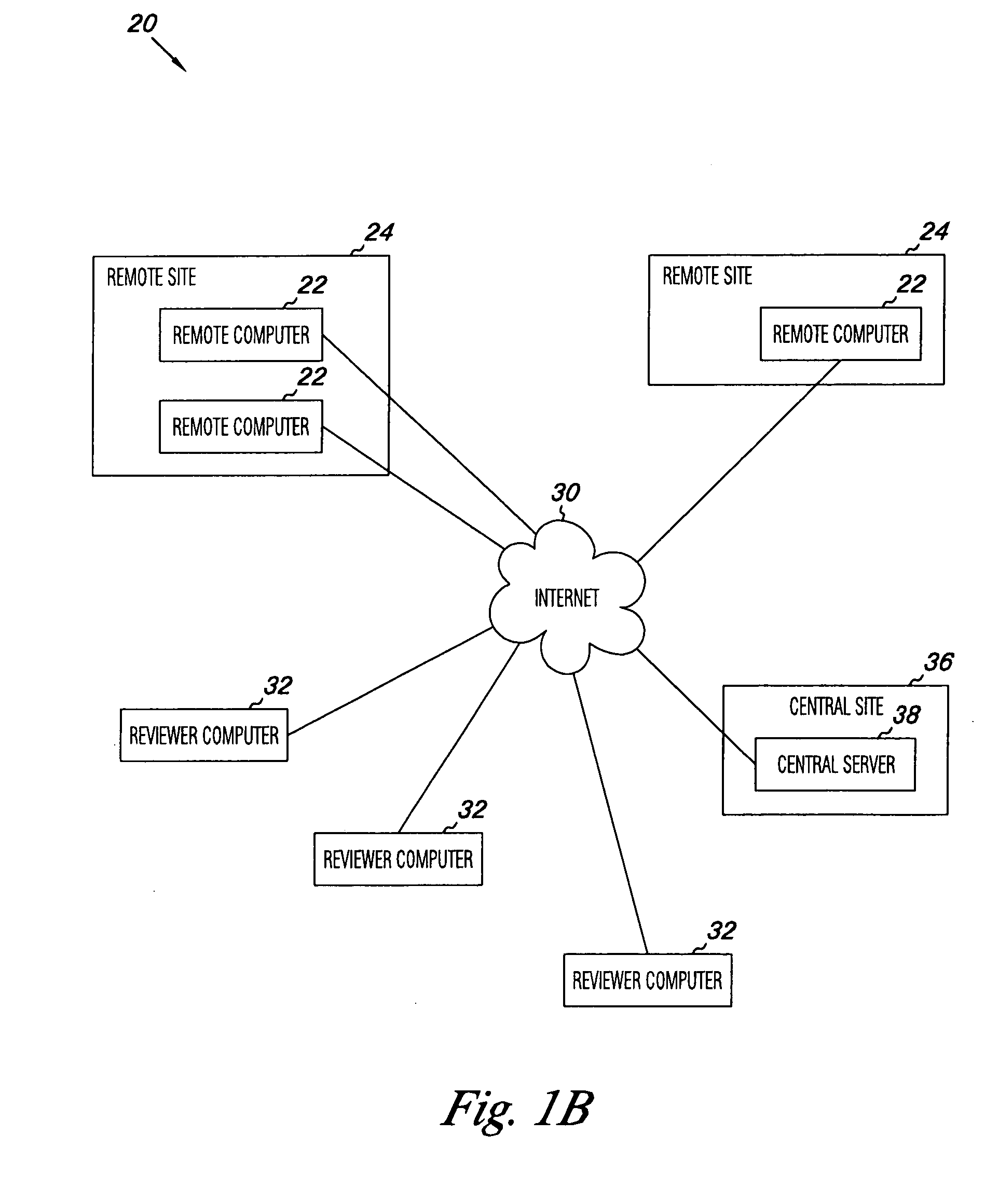
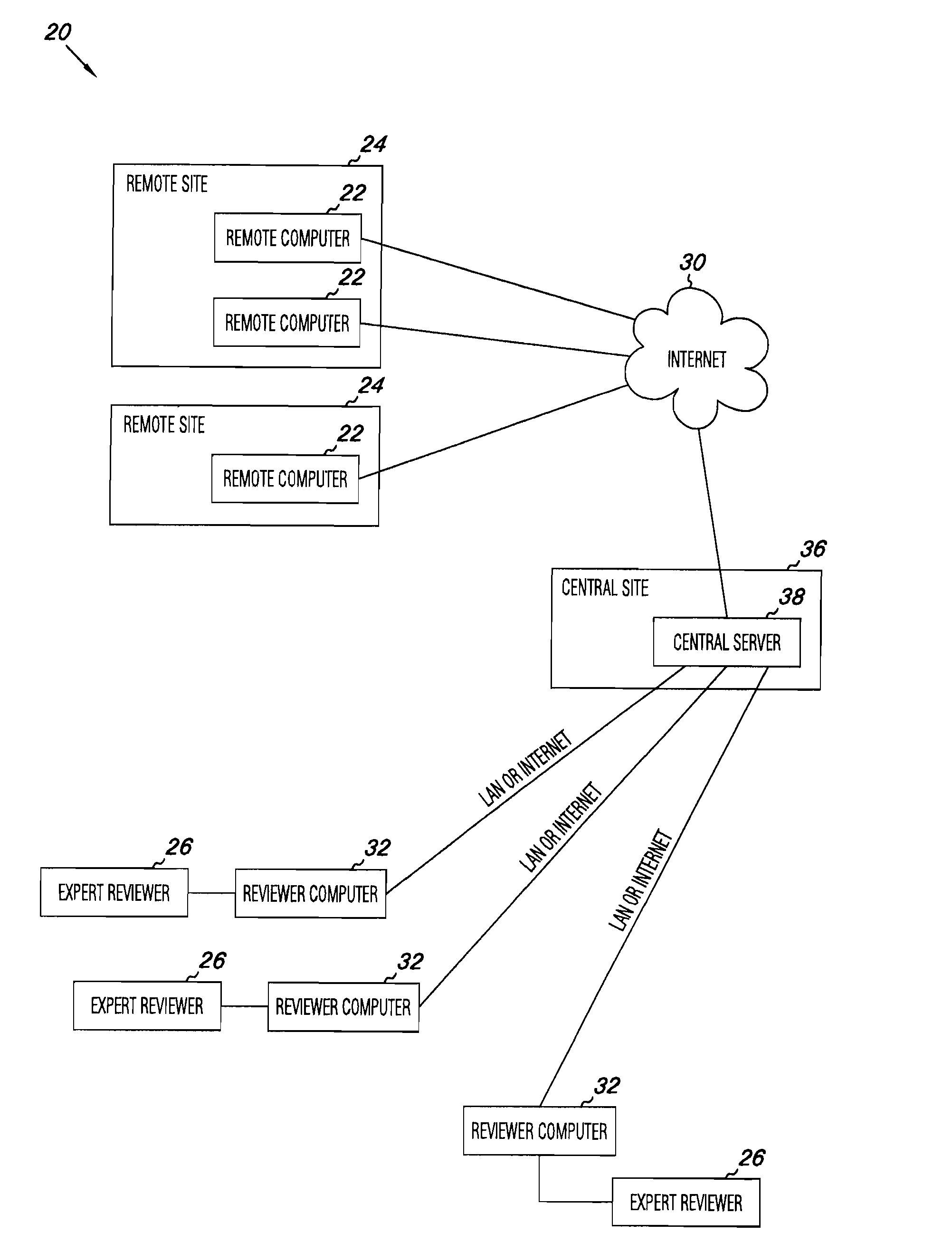
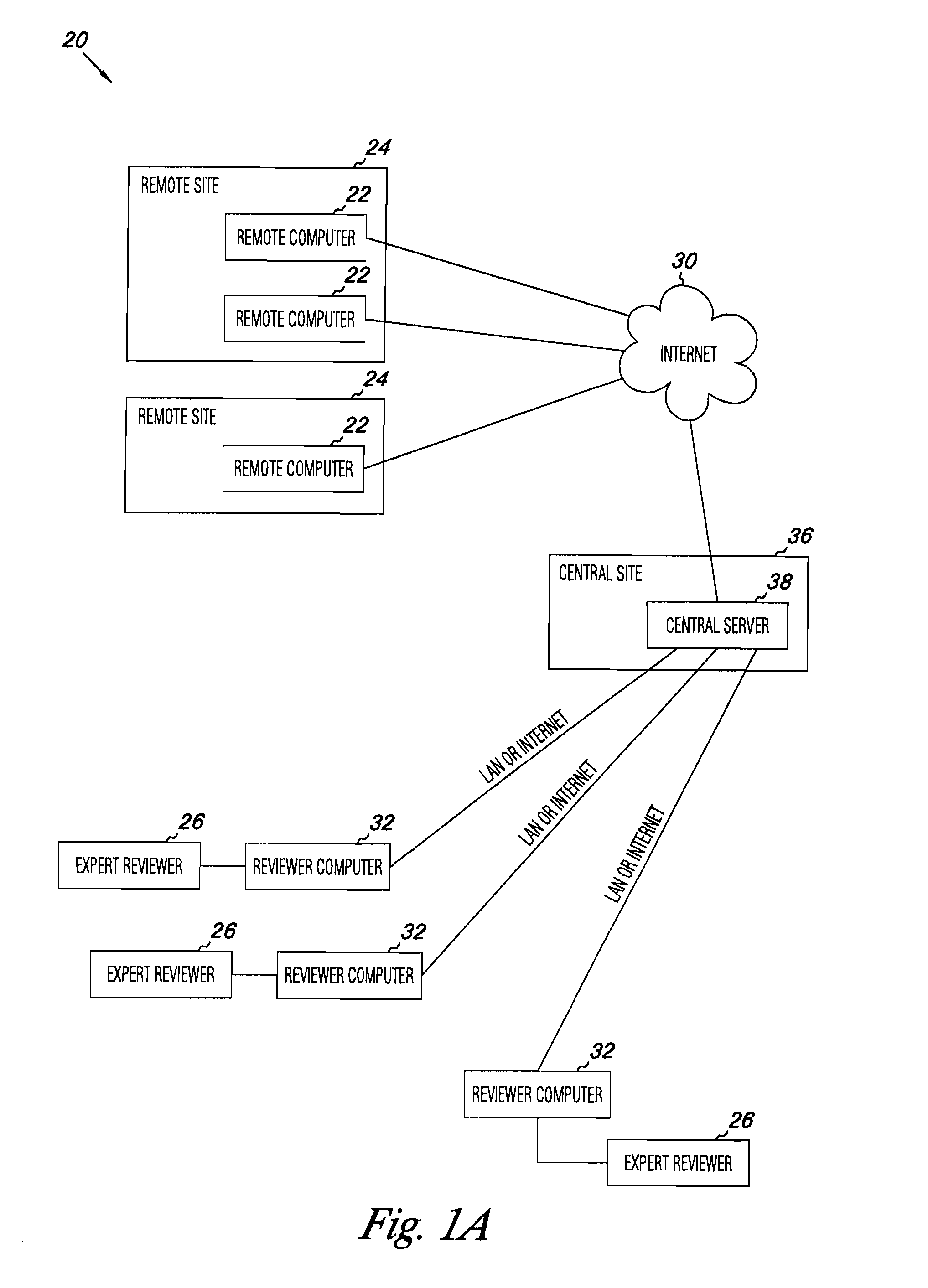
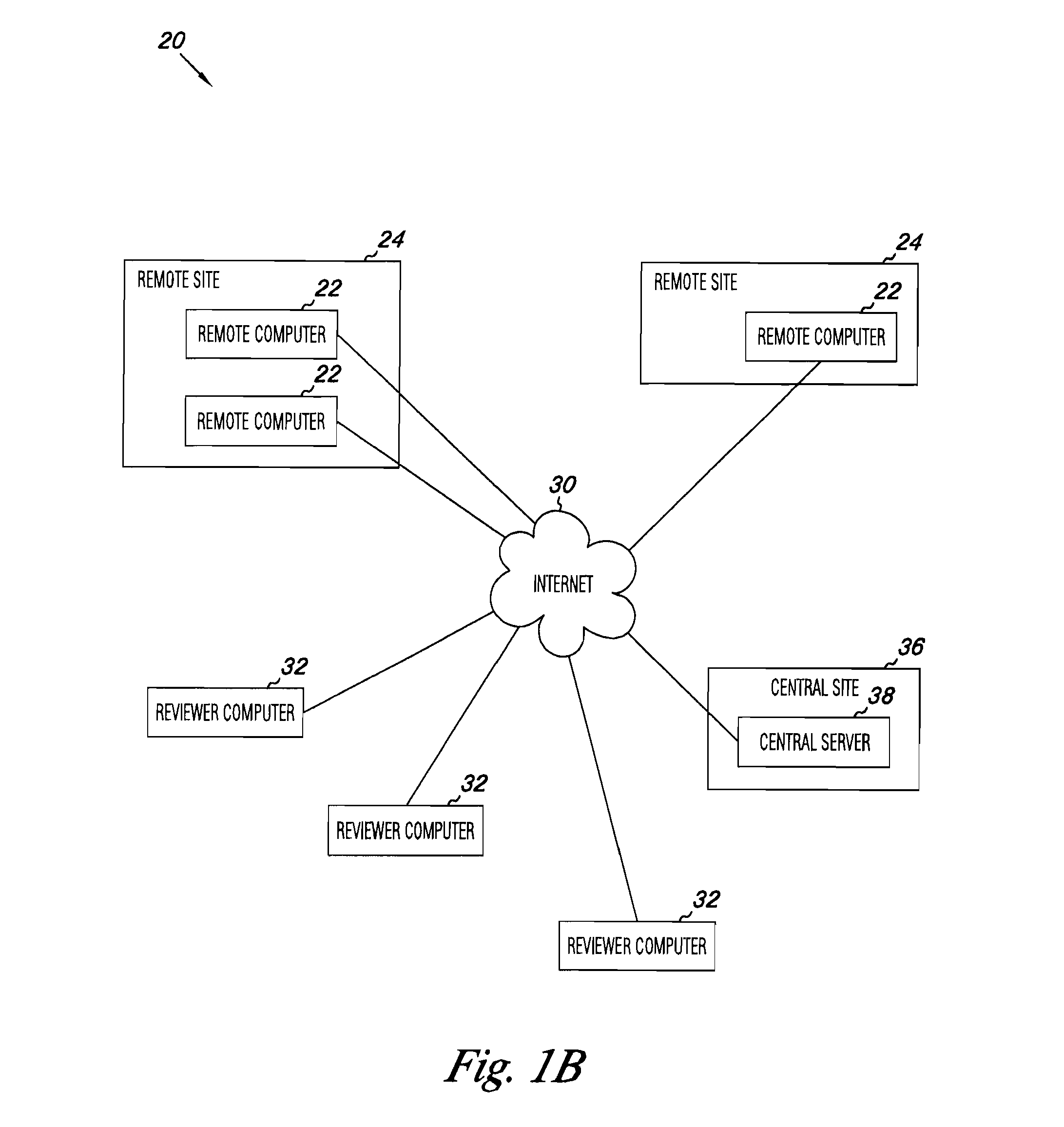
Apparatus and methods for practicing telemedicine in the form of software systems acting over a network and kits containing laboratory supplies and equipment to organize the laboratory operations and interpret the results of molecular diagnostic testing are disclosed. At least two computers in communication over the Internet or other network are used, a remote computer located at a remote site and a central server located at a central site. The remote site may be geographically distant from the central site. A specimen is procured from a patient proximate to the remote site. Laboratory operations are conducted on the specimen at the remote site. The laboratory data resulting from the laboratory operations is interpreted by an expert reviewer who may be located at the central site, and a report is then transmitted back to the remote site.
Owner:MCGLENNEN RONALD C +5
Apparatus and methods for medical testing
InactiveUS20110178814A1Accurately conductData processing applicationsDigital data processing detailsSoftware systemThe Internet
Apparatus and methods for practicing telemedicine in the form of software systems acting over a network and kits containing laboratory supplies and equipment to organize the laboratory operations and interpret the results of molecular diagnostic testing are disclosed. At least two computers in communication over the Internet or other network are used, a remote computer located at a remote site and a central server located at a central site. The remote site may be geographically distant from the central site. A specimen is procured from a patient proximate to the remote site. Laboratory operations are conducted on the specimen at the remote site. The laboratory data resulting from the laboratory operations is interpreted by an expert reviewer who may be located at the central site, and a report is then transmitted back to the remote site.
Owner:MCGLENNEN RONALD C +5
Molecular diagnostic test for cancer
ActiveUS20160002732A1Microbiological testing/measurementLibrary screeningCancer typeAngiogenesis growth factor
Methods and compositions are provided for the identification of a molecular diagnostic test for cancer. The test identifies cancer subtypes that have an up-regulation or a down-regulation in biomarker expression related to angiogenesis and vascular development. The present invention can be used to determine whether patients with cancer are clinically responsive or non-responsive to a therapeutic regimen prior to administration of any anti-angiogenic agent. This test may be used in different cancer types and with different drugs that directly or indirectly affect angiogenesis or angiogenesis signalling. In addition, the present invention may be used as a prognostic indicator for certain cancer types. In particular, the present invention is directed to the use of certain combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen.
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
Molecular diagnostic test for cancer
InactiveCN105102631AMicrobiological testing/measurementBiostatisticsCancer typeAngiogenesis growth factor
Methods and compositions are provided for the identification of a molecular diagnostic test for cancer. The test identifies cancer subtypes that have an up-regulation or a down- regulation in biomarker expression related to angiogenesis and vascular development. The present invention can be used to determine whether patients with cancer are clinically responsive or non-responsive to a therapeutic regimen prior to administration of any anti- angiogenic agent. This test may be used in different cancer types and with different drugs that directly or indirectly affect angiogenesis or angiogenesis signalling. In addition, the present invention may be used as a prognostic indicator for certain cancer types. In particular, the present invention is directed to the use of certain combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen.
Owner:ALMAC DIAGNOSTICS LIMITED
Molecular diagnostic test for cancer
ActiveUS20140051591A1Inorganic active ingredientsHealth-index calculationCancer typeChemotherapeutic drugs
Methods and compositions are provided for the identification of a molecular diagnostic test for cancer. The test defines a novel DNA damage repair deficient molecular subtype and enables classification of a patient within this subtype. The present invention can be used to determine whether patients with cancer are clinically responsive or non-responsive to a therapeutic regimen prior to administration of any chemotherapy. This test may be used in different cancer types and with different drugs that directly or indirectly affect DNA damage or repair, such as many of the standard cytotoxic chemotherapeutic drugs currently in use. In particular, the present invention is directed to the use of certain combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen.
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
Microfluidic Processing of Leukocytes for Molecular Diagnostic Testing
InactiveUS20170333900A1Improve cell qualitySimple working processLaboratory glasswaresIndividual particle analysisDisease areaWhite blood cell
Owner:UNIV OF MARYLAND BALTIMORE +2
Disposable Sample Processing Unit
InactiveUS20090036665A1Analysis using chemical indicatorsHeating or cooling apparatusPoint of careRNA extraction
A low-cost, non-instrumented, easy-to-use disposable platform for extraction, stabilization, and preservation of viral RNA in specimens at the point of collection is described. The system may use chemical heating. The platform performs the following steps: specimen lysis, RNA extraction, and RNA stabilization in a modular approach. This modular approach confers versatility to the product for application to multiple targets such as avian flu, and HIV, specimens such as blood, nasal swabs, and downstream applications such as PCR or transcription-mediated amplification. The technology described is a point-of-care specimen-processing platform generically applicable to both emerging point-of-care and central-facility molecular diagnostic tests, as well as to surveillance applications.
Owner:PROGRAM FOR APPROPRIATE TECHNOLOGY IN HEALTH
Sputum collection and washing apparatus and a method of using it
ActiveUS10898169B1Improve reliabilityImproving laboratory supportSurgical needlesPreparing sample for investigationStainingEngineering
A medical apparatus (FDA Classifications: “Sputum Specimen [In-Home] Self-Collection Device” and “In Vitro Medical Diagnostic Device”) and method for self-collection, transport, decontamination and storage preservation of expectorated sputum specimens, to optimize specimen quality and reliability of clinical laboratory stain interpretation, culture and molecular diagnostic tests, thereby improving laboratory support for pathogen-specific treatment (antibiotic stewardship).
Owner:PS SYST
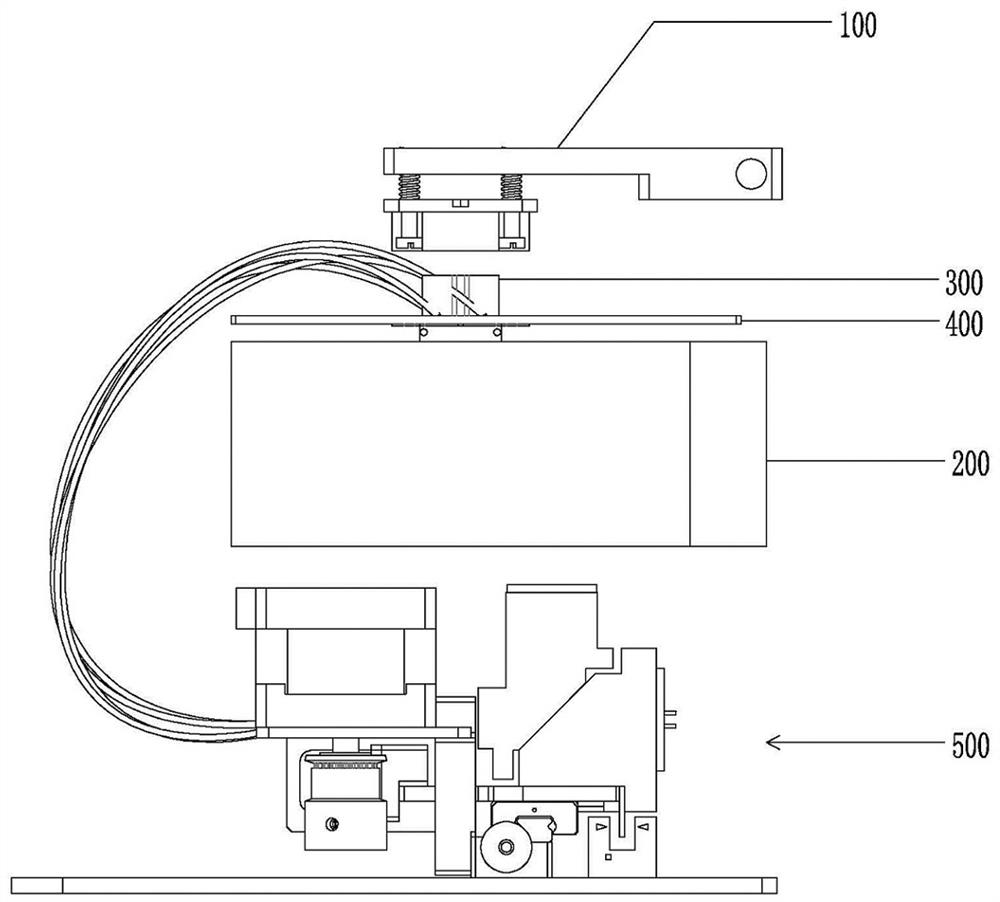
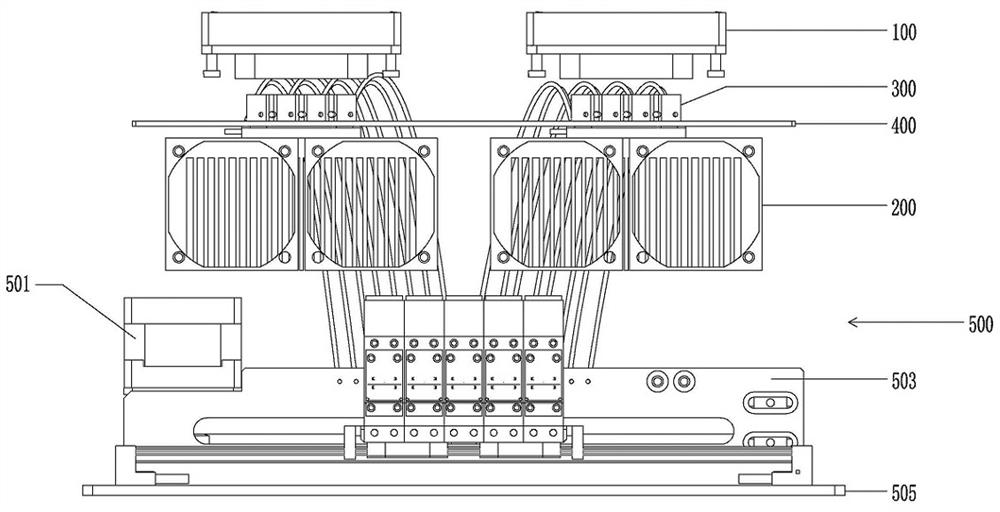
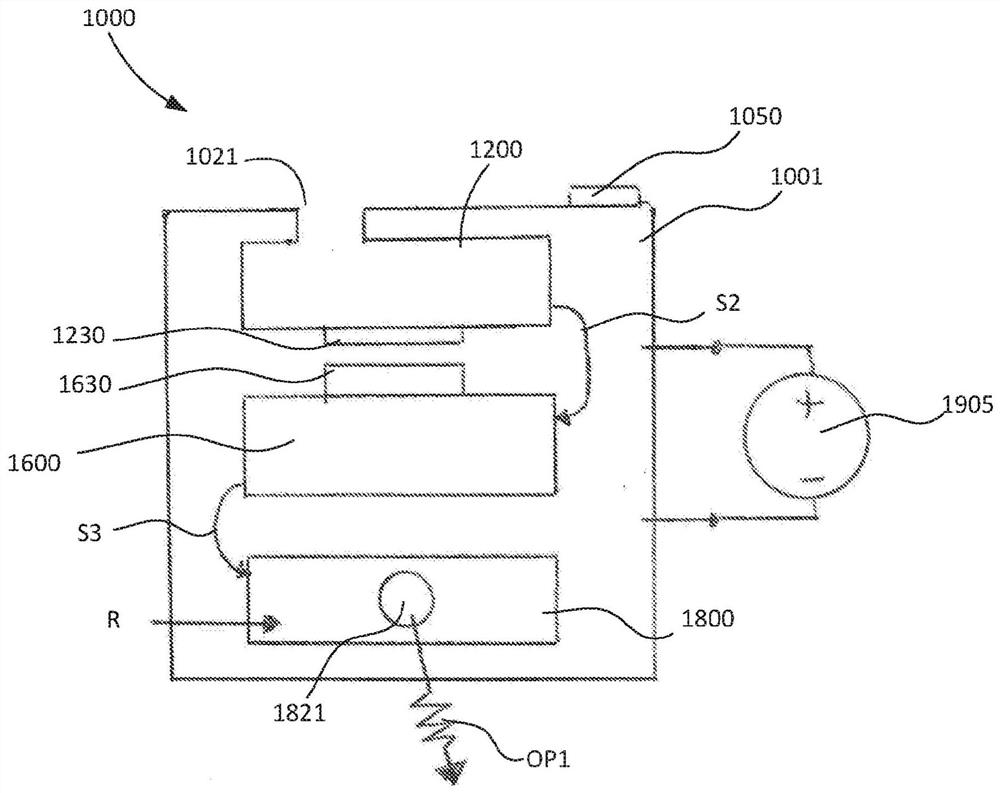
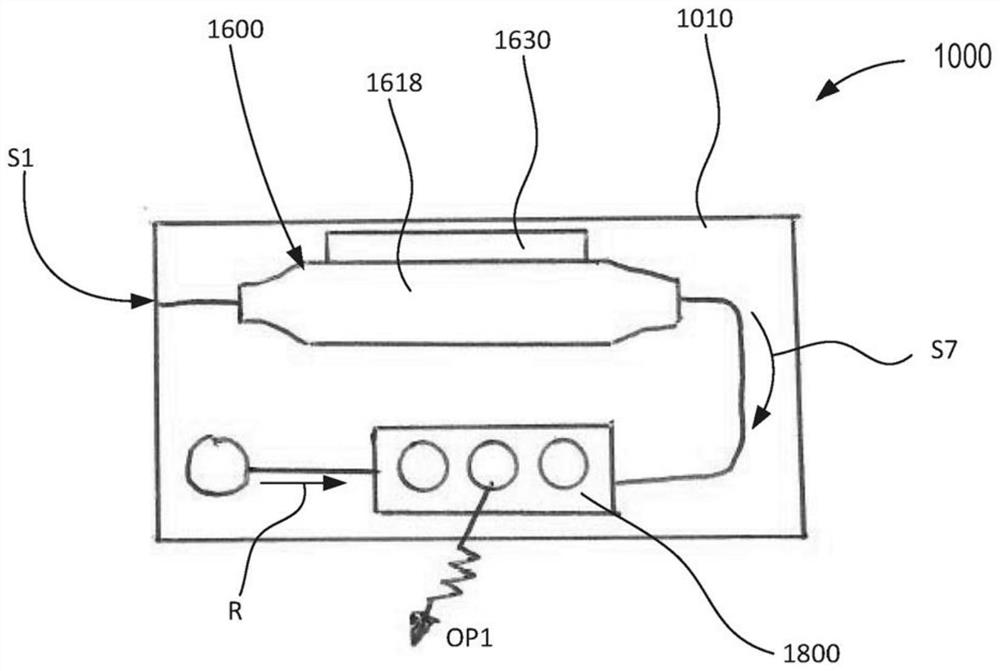
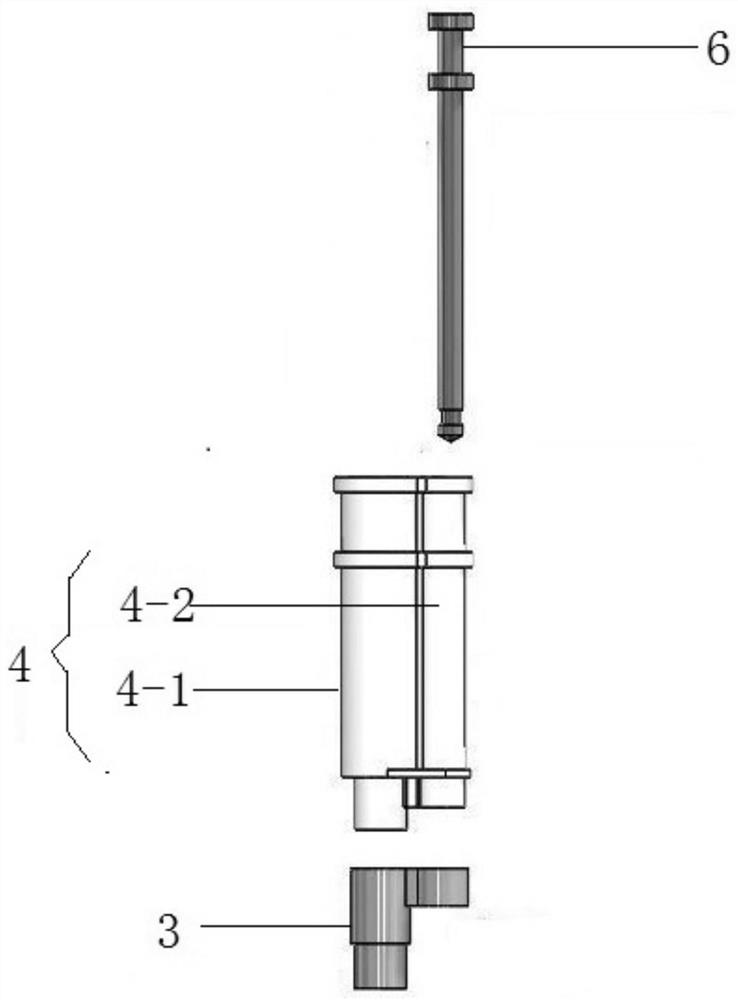
Devices and methods for molecular diagnostic testing
ActiveCN107429281AHeating or cooling apparatusLaboratory glasswaresMolecular diagnosticsMolecular Diagnostic Testing
A hand-held molecular diagnostic test device includes a housing, an amplification (or PCR) module, and a detection module. The amplification module is configured to receive an input sample, and defines a reaction volume. The amplification module includes a heater such that the amplification module can perform a polymerase chain reaction (PCR) on the input sample. The detection module is configured to receive an output from the amplification module and a reagent formulated to produce a signal that indicates a presence of a target amplicon within the input sample. The amplification module and the detection module are integrated within the housing.
Owner:VISBY MEDICAL INC
Diagnostic system
ActiveUS10820847B1Avoid Sealing ProblemsEasy to changeHeating or cooling apparatusMicrobiological testing/measurementMolecular diagnosticsBiology
Methods and systems are provided for point-of-care nucleic acid amplification and detection. One embodiment of the point-of-care molecular diagnostic system includes a cartridge and an instrument. The cartridge can accept a biological sample, such as a urine or blood sample. The cartridge, which can comprise one or more of a loading module, lysis module, purification module and amplification module, is inserted into the instrument which acts upon the cartridge to facilitate various sample processing steps that occur in order to perform a molecular diagnostic test.
Owner:TALIS BIOMEDICAL CORP
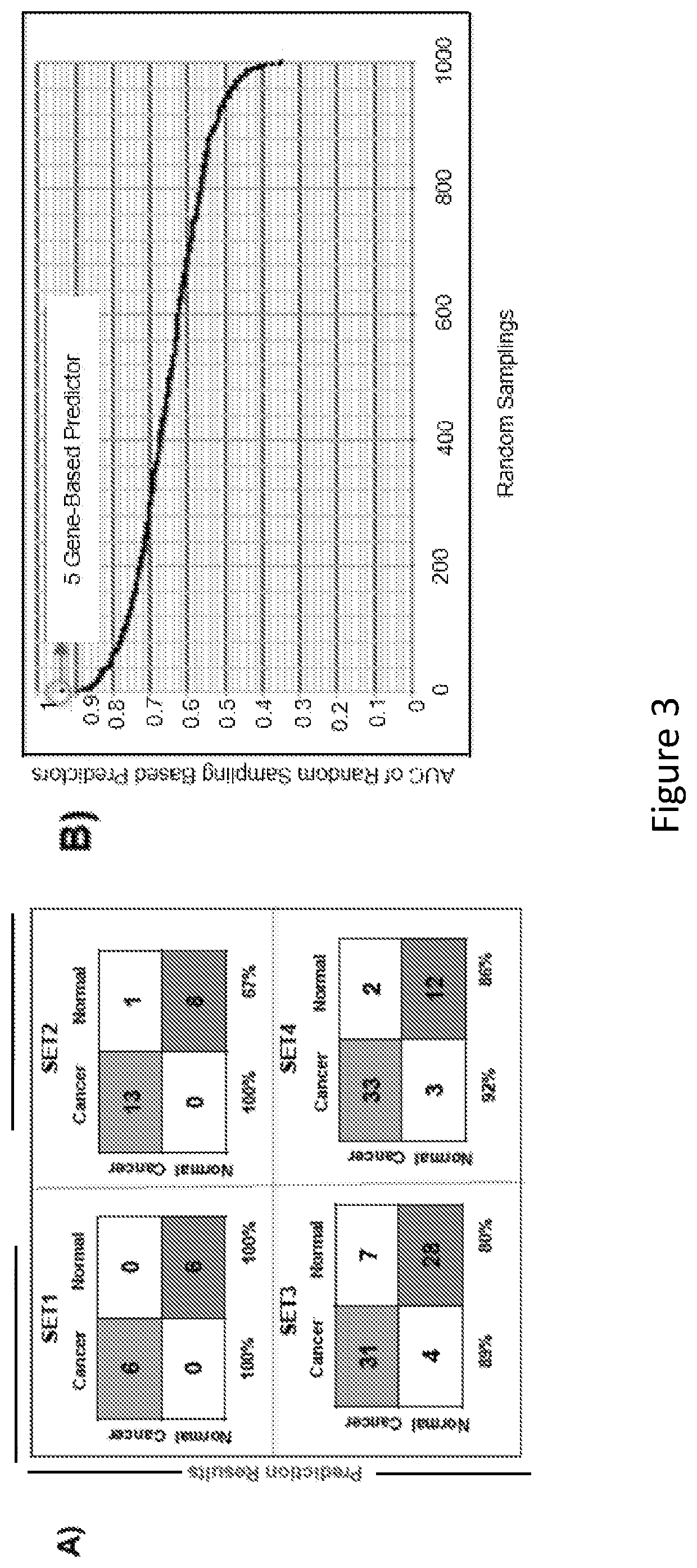
Methods and kits for the diagnosis and treatment of pancreatic cancer
ActiveUS20160348182A1Accurate diagnosisDetermine the likelihood of developing PDACMicrobiological testing/measurementMedicinePhases of clinical research
The present disclosure relates to the identification of genes and gene combinations that are correlated with patients having or being predisposed to developing pancreatic ductal adenocarcinoma (PDAC). In some instances, methods herein utilize panels of 5 or 10 genes to accurately diagnose PDAC, determine the likelihood of developing PDAC, or determine the severity / stages of PDAC. These panels may be used in a molecular diagnostic test.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
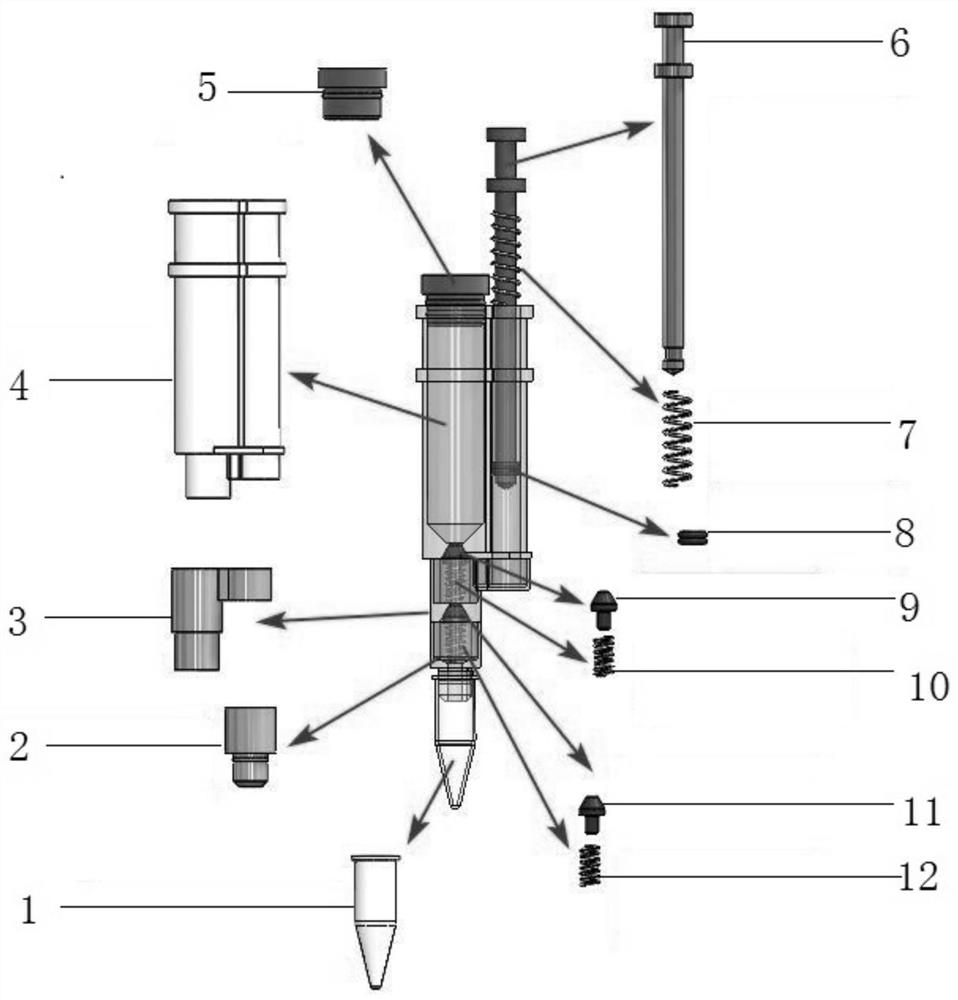
Device for use in molecular diagnostics testing
ActiveUS8992856B2Easy to operateMore flexibleAnalysis using chemical indicatorsMaterial analysis by optical meansActuatorMolecular diagnostics
A device for use in molecular diagnostics testing includes at least one sealed reagent storage container, and at least one opener for unsealing the reagent storage container. The device further includes an actuator coupled to the reagent storage container and opener such that moving the actuator brings the reagent storage container and the opener together so that the opener unseals the reagent storage container.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Molecular diagnostic test for lung cancer
InactiveUS20160222459A1Avoid overtreatmentRisk of severeMicrobiological testing/measurementRespiratory disorderChemotherapeutic drugsPharmaceutical drug
Methods and compositions are provided for the identification of a molecular diagnostic test for lung cancer. The test defines a novel DNA damage repair deficient molecular subtype and enables classification of a patient within this subtype. The present invention can be used to determine whether patients with NSCLC are clinically responsive or non-responsive to a therapeutic regimen prior to administration of any chemotherapy. This test may be used with different drugs that directly or indirectly affect DNA damage or repair, such as many of the standard cytotoxic chemotherapeutic drugs currently in use. In particular, the present invention is directed to the use of certain combinations of predictive markers, wherein the expression of the predictive markers correlates with responsiveness or non-responsiveness to a therapeutic regimen.
Owner:ALMAC DIAGNOSTICS LIMITED
Apparatus and methods for medical testing
InactiveUS8594948B2Data processing applicationsMicrobiological testing/measurementProximateSoftware system
Owner:MCGLENNEN RONALD C +5
Method of using a gene expression profile to determine cancer responsiveness to an anti-angiogenic agent
InactiveUS10260097B2Microbiological testing/measurementDisease diagnosisCancer typeAntiangiogenic therapy
Owner:ALMAC DIAGNOSTICS SERVICES LIMITED
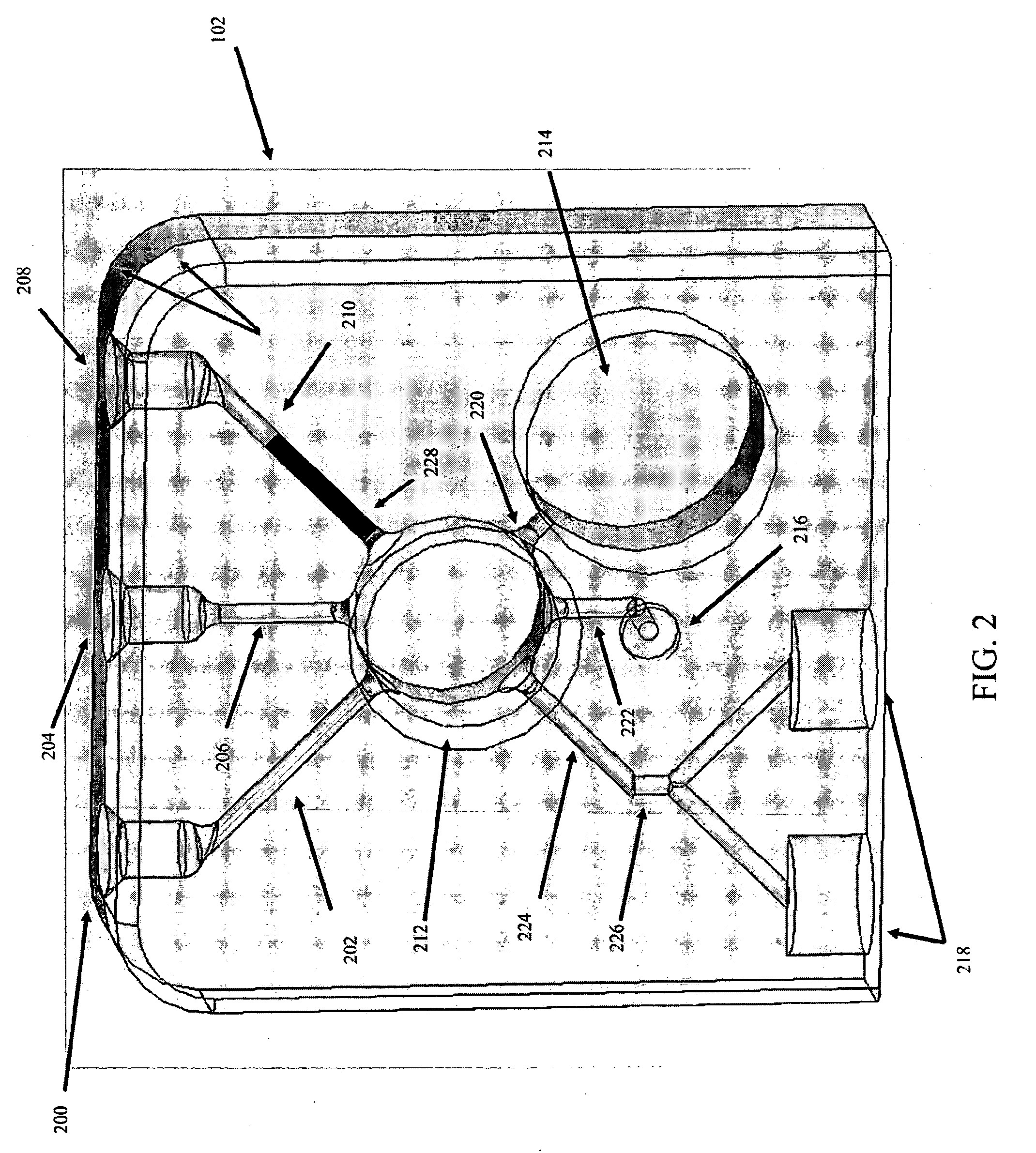
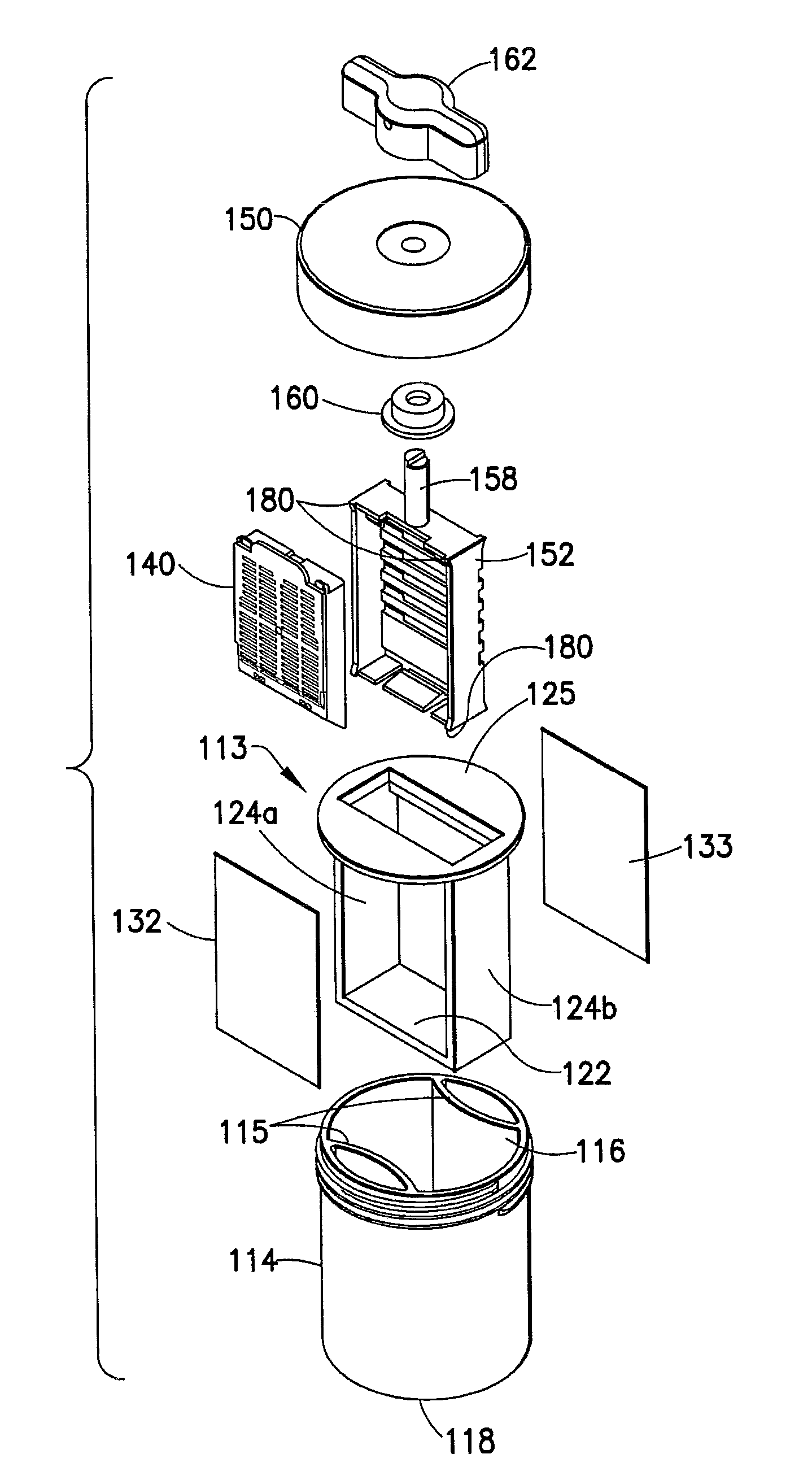
Fluid displacement tissue container for molecular and histology diagnostics
ActiveUS8813954B2Withdrawing sample devicesPreparing sample for investigationHistological diagnosisMolecular Diagnostic Testing
A container for storing a biological sample for molecular diagnostic testing and / or histological testing is provided. The container includes a first chamber for receiving a sample holder therein, a second chamber, and a closure for enclosing the container. A transitional barrier, such as a valve, is in fluid communication between the two chambers. The transitional barrier is transitional between a first position in which the first chamber is in fluid isolation from the second chamber, and a second position in which fluid can pass from at least one of the first and second chambers to the other of the first and second chambers.
Owner:BECTON DICKINSON & CO
Materials and Methods for Quality-Controlled Two-Color RT-QPCR Diagnostic Testing of Formalin Fixed Embedded and/or Fresh-Frozen Samples
InactiveUS20150225798A1Nucleotide librariesMicrobiological testing/measurementQuantitative Real Time PCRFluorescence
Described herein are materials, methods, and kits enabling accurate and reproducible two-color reverse-transcription real-time quantitative PCR (RT-qPCR) for quality-controlled molecular diagnostic testing of samples that may contain degraded RNA. In certain aspects described herein are materials, methods, and kits for use in the molecular diagnostic testing of lung cancer in FFPE samples and / or fresh-frozen samples. Also described herein are materials and methods to control for inter-experimental variation occurring during two-color RT-qPCR amplification arising from variation in fluorescence specific activity, use of different thermocyclers, and inter-laboratory differences.
Owner:UNIVERSITY OF TOLEDO
A kind of pcr instrument with multi-temperature control module asynchronous optional channel and detection method thereof
ActiveCN114181823BLow costEasy to controlBioreactor/fermenter combinationsBiological substance pretreatmentsComputer hardwareFluorescence
The invention discloses a PCR instrument with multiple temperature control modules asynchronously selectable channels and a detection method thereof; it belongs to the field of medical inspection and testing instruments and molecular diagnosis and detection instruments; its technical gist is that it includes: m temperature control modules, m A reaction pool component module and a fluorescence detection component; the fluorescence detection module is an excitation light-receiving light integrated module structure; the optical fiber fixing plate is arranged with through holes, the number of the through holes, optical fibers, and reaction pools is the same, and the optical fiber and fluorescence detection module They are respectively located on both sides of the optical fiber fixing plate; one end of the optical fiber is fixedly communicated with the through holes one by one, and the other end is inserted into the reaction pool. The present invention aims to provide a PCR instrument with multiple temperature control modules asynchronously optional channels and a detection method thereof. A set of fluorescence detection system can collect the fluorescence signals of m PCR reaction pools without interfering with each other; the channels are optional and can be To better meet the needs of users and reduce costs, the universal enhancement of equipment.
Owner:SUZHOU MOLARRAY CO LTD
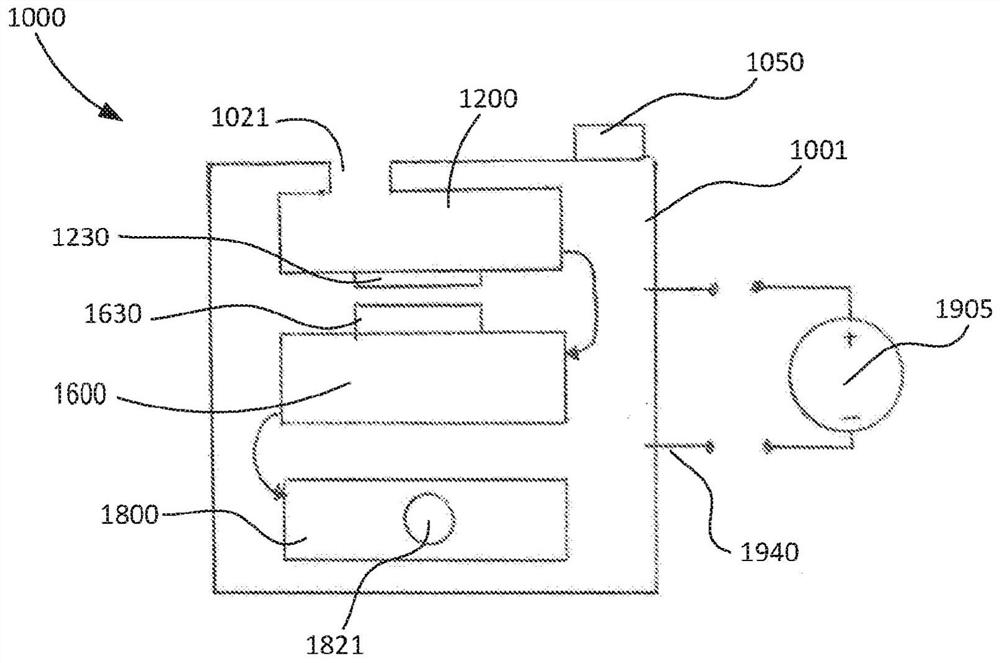
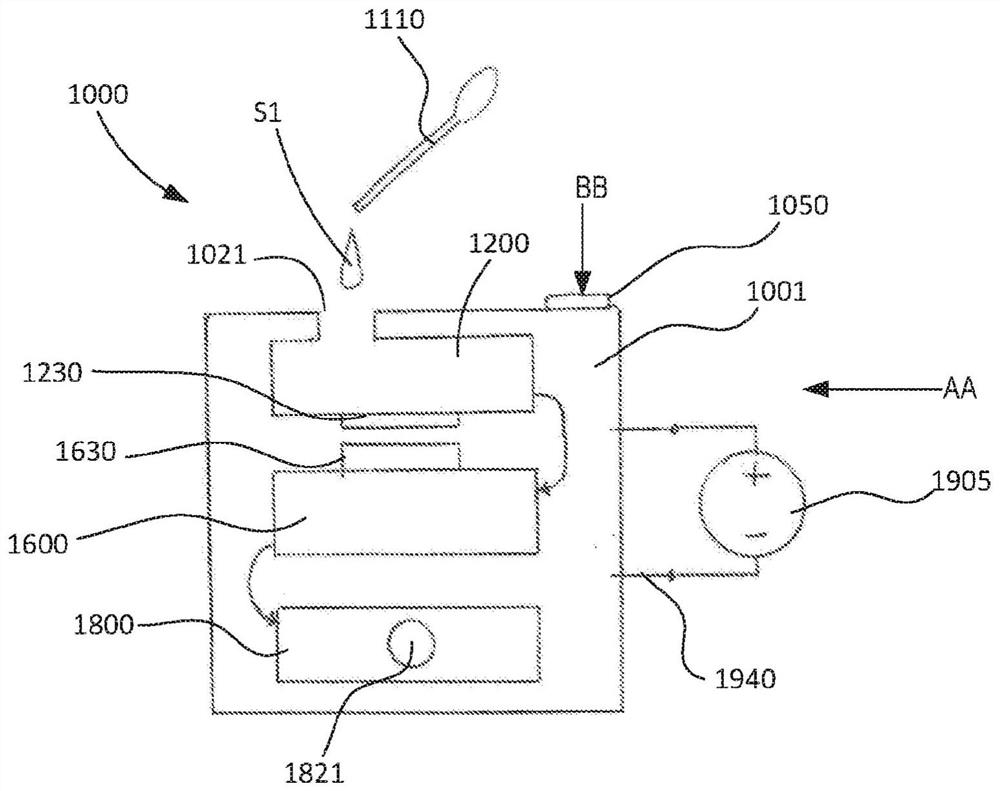
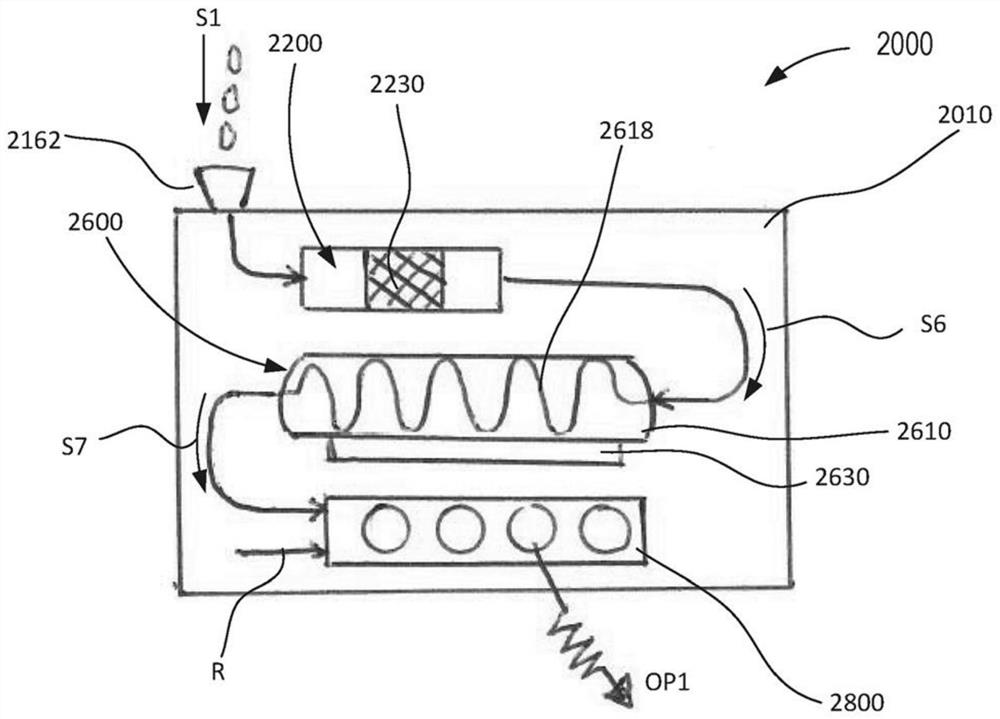
Portable molecular diagnostic device and methods for the detection of target viruses
PendingCN111655866AHeating or cooling apparatusMicrobiological testing/measurementMolecular diagnosticsMolecular Diagnostic Testing
A method includes coupling a molecular diagnostic test device to a power source. A biological sample is conveyed into a sample preparation module. The device is then actuated by only a single action to cause the device to perform the following functions without further user action. First, the device heats the sample via a heater of the sample preparation module to lyse a portion of the sample. Second, the device conveys the lysed sample to an amplification module and heats the sample within a reaction volume of the amplification module to amplify a nucleic acid thereby producing an output solution containing a target amplicon. The device then reacts, within a detection module, each of (i) the output solution and (ii) a reagent formulated to produce a signal that indicates a presence of thetarget amplicon within the output solution. A result associated with the signal is then read.
Owner:VISBY MEDICAL INC
Tissue Container for Molecular and Histology Diagnostics Incorporating a Breakable Membrane
ActiveUS20110250634A1Preparing sample for investigationSurgeryHistological diagnosisMolecular Diagnostic Testing
A container for storing a biological sample for molecular diagnostic testing and / or histological testing is provided. The container includes a first chamber for receiving a sample holder therein, a second chamber, and a closure for enclosing the container. A breakable membrane, such as a piercable foil, extends within the container and separates the two chambers. When the breakable membrane is broken, fluid can pass between the first and second chambers. The membrane may be broken through an activator on the closure, such as a depressible member or a rotatable carrier, causing the sample holder to break through the membrane.
Owner:BECTON DICKINSON & CO
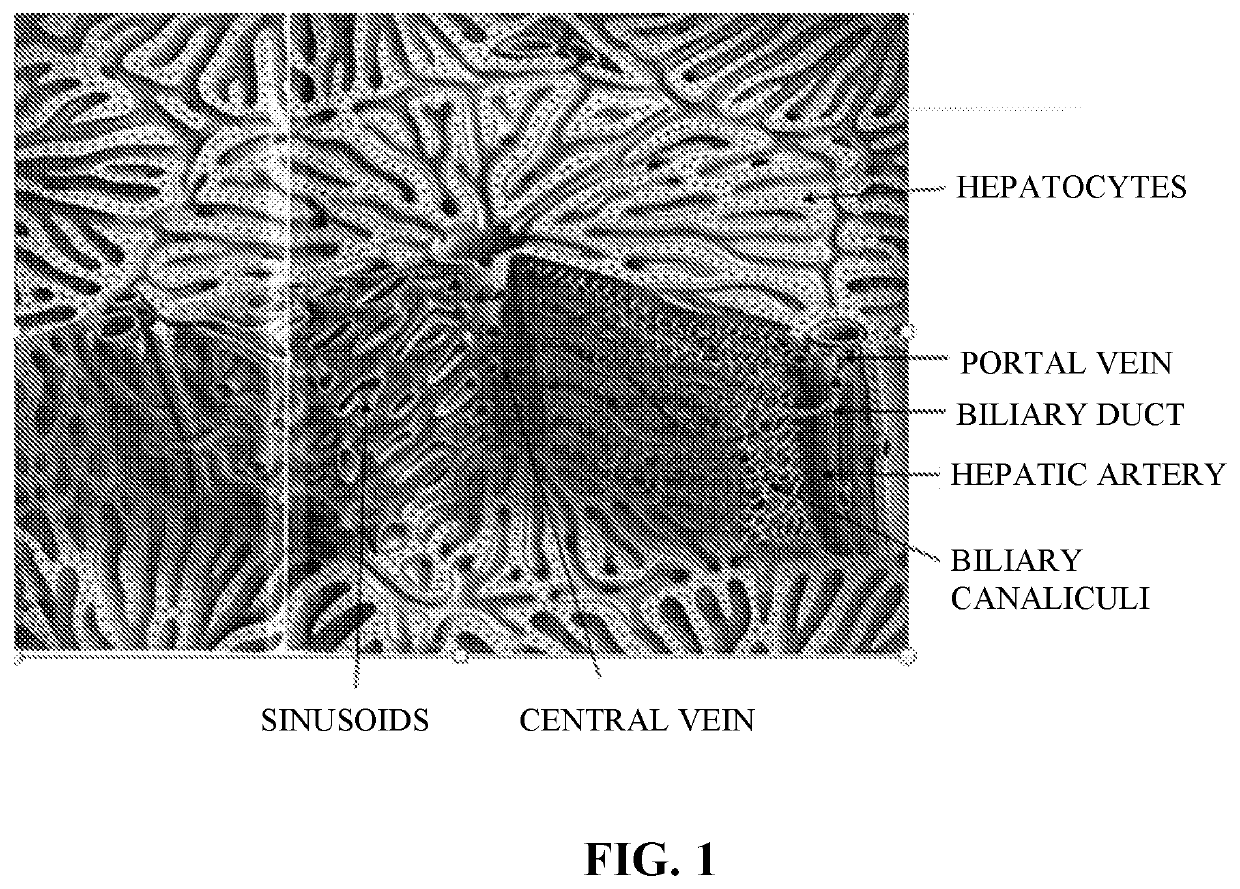
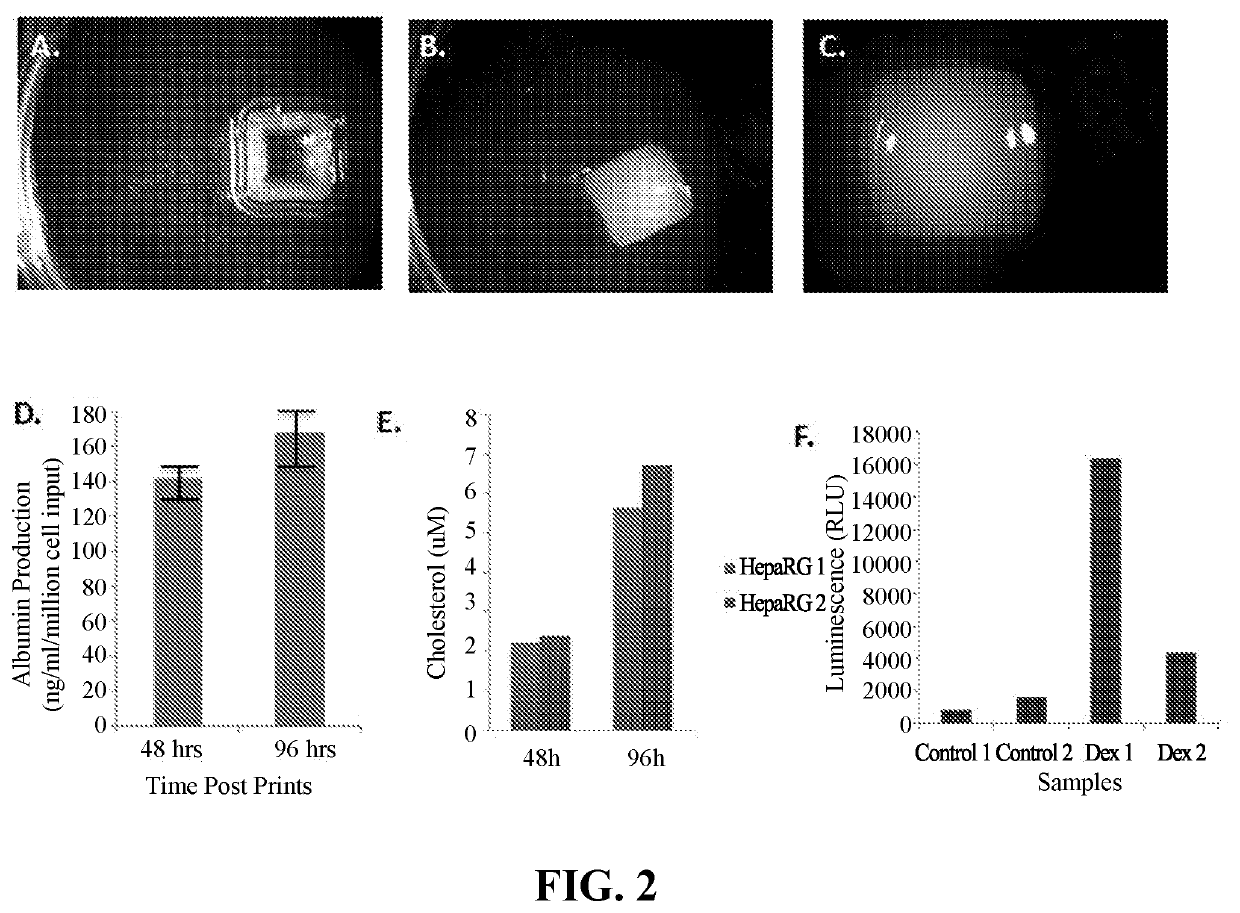
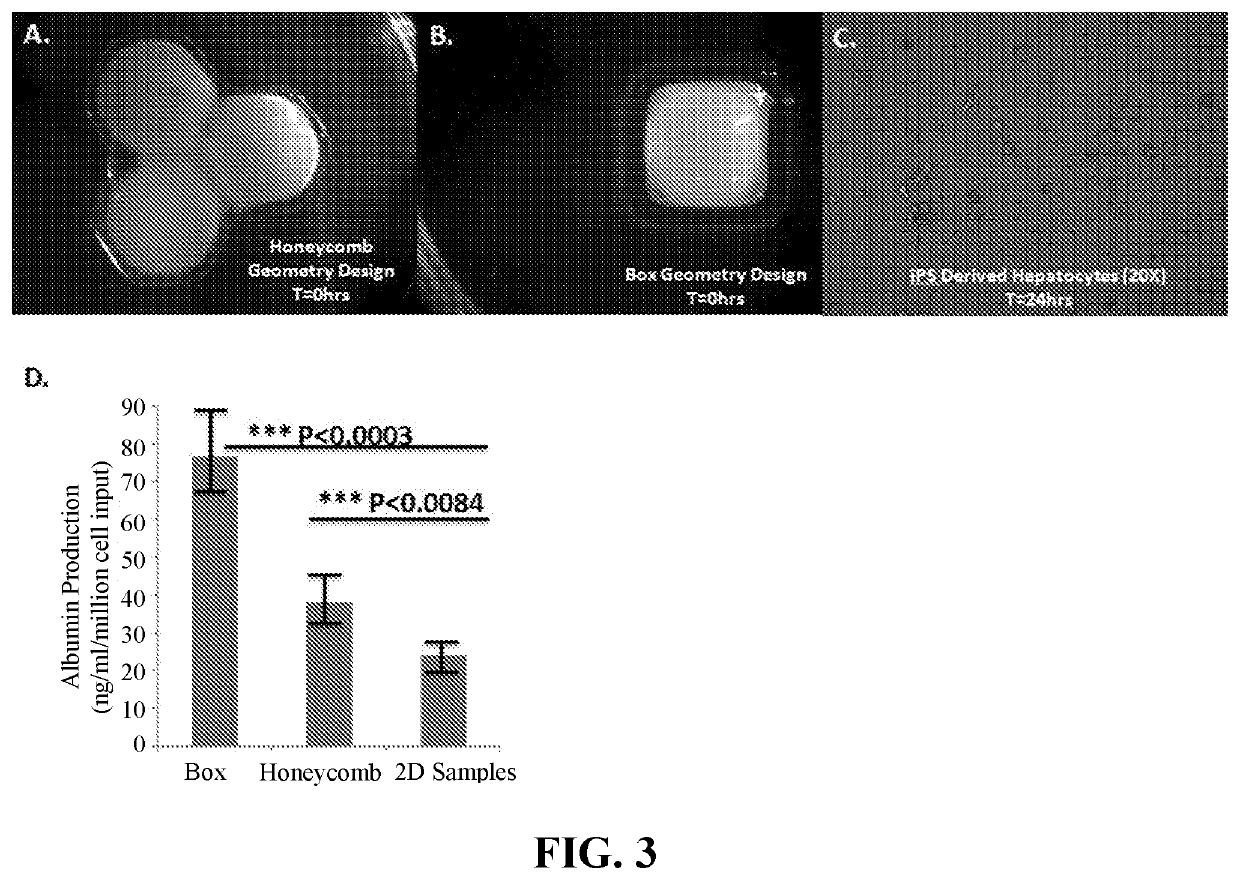
Use of Engineered Liver Tissue Constructs for Modeling Liver Disorders
The invention is directed to three-dimensional, engineered, bioprinted biological tissue constructs exhibiting a liver disorder, methods of making the constructs, and use of the constructs in assays, such as drug testing and molecular diagnostic testing, including methods of assessing the ability of a candidate therapeutic agent to reverse, reduce, or prevent a liver disorder, and methods for biomarker discovery.
Owner:ORGANOVO
Device for use in molecular diagnostics testing
ActiveUS20150196916A1Easy to operateMore flexiblePharmaceutical containersMaterial analysis by optical meansActuatorMolecular diagnostics
The invention relates to a device for use in molecular diagnostics testing comprising:at least one reagent storage container, the reagent storage container being sealed;at least one opener for unsealing the reagent storage container;an actuator coupled to at least one of the reagent storage container and the opener such that moving the actuator brings the reagent storage container and the opener together so that the opener unseals the reagent storage container.
Owner:KONINKLIJKE PHILIPS NV
Devices and methods for molecular diagnostic testing
A handheld molecular diagnostic test device includes a housing, an amplification (or PCR) module and a detection module. The amplification module is configured to receive an input sample and define a reaction volume. The amplification module includes a heater such that the amplification module can perform a polymerase chain reaction (PCR) on the input sample. The detection module is configured to receive an output from the amplification module and reagents formulated to generate a signal indicative of the presence of a target amplicon within the input sample. The amplification module and the detection module are integrated within the housing.
Owner:VISBY MEDICAL INC
Materials and methods for quality-controlled two-color RT-QPCR diagnostic testing of formalin fixed embedded and/or fresh-frozen samples
Described herein are materials, methods, and kits enabling accurate and reproducible two-color reverse-transcription real-time quantitative PCR (RT-qPCR) for quality-controlled molecular diagnostic testing of samples that may contain degraded RNA. In certain aspects described herein are materials, methods, and kits for use in the molecular diagnostic testing of lung cancer in FFPE samples and / or fresh-frozen samples. Also described herein are materials and methods to control for inter-experimental variation occurring during two-color RT-qPCR amplification arising from variation in fluorescence specific activity, use of different thermocyclers, and inter-laboratory differences.
Owner:UNIVERSITY OF TOLEDO
Methods and kits for the diagnosis and treatment of pancreatic cancer
ActiveUS10604809B2Accurate diagnosisDetermine the likelihood of developing PDACMicrobiological testing/measurementAdenocarcinomaPancreas
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
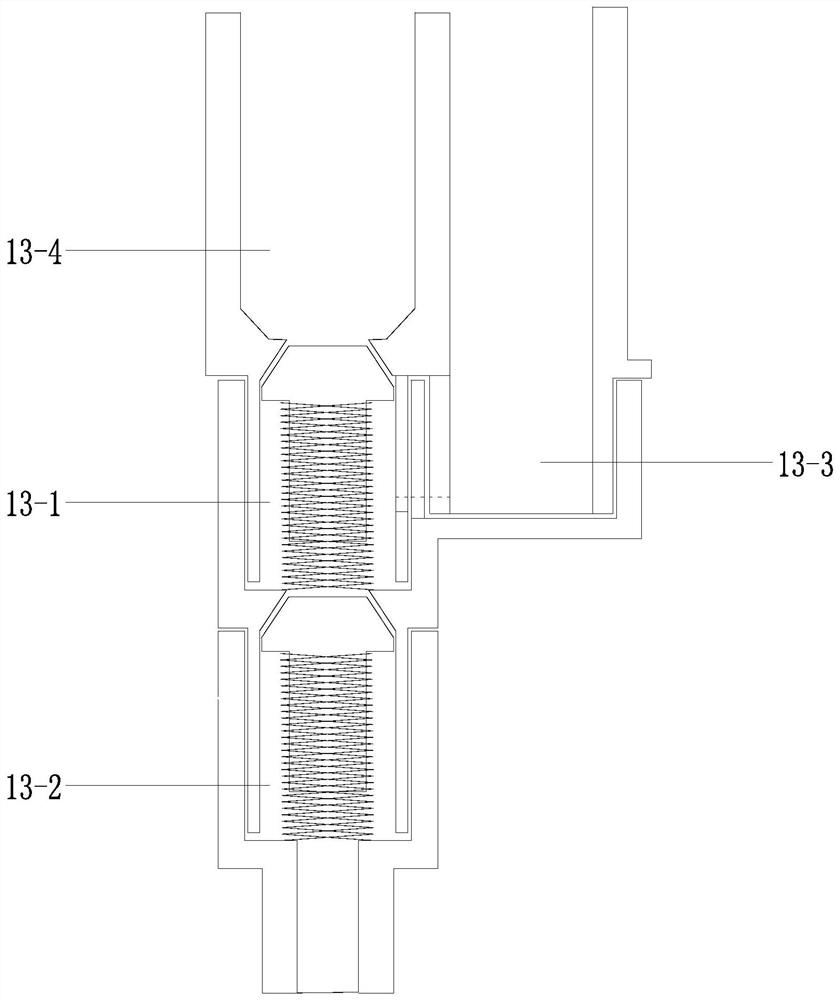
A nucleic acid extraction and amplification test tube and its working method
ActiveCN112342128BAchieve hybridImprove liquidityBioreactor/fermenter combinationsHeating or cooling apparatusEngineeringMolecular diagnostics
The invention discloses a nucleic acid extraction and amplification test tube and its working method, which belong to the field of molecular diagnosis and detection instruments, and its design points include: a PCR reaction tube, a lower tube of an extraction tube assembly, a middle tube of an extraction tube assembly, and an extraction tube assembly Upper tube assembly, top cover, piston rod, piston ring, first spool, second spring, second spool, third spring; extraction tube assembly upper tube assembly, extraction tube assembly middle tube, extraction tube assembly lower tube, The PCR reaction tubes are connected sequentially from top to bottom; the extraction tube assembly The upper tube assembly includes: two vertical parallel tube bodies: an extraction tube and a piston tube; a top cover is arranged on the top of the extraction tube; the piston rod is placed in the piston tube. The invention aims to provide a nucleic acid extraction and amplification test tube and its working method, which greatly improves the detection efficiency.
Owner:SUZHOU MOLARRAY CO LTD
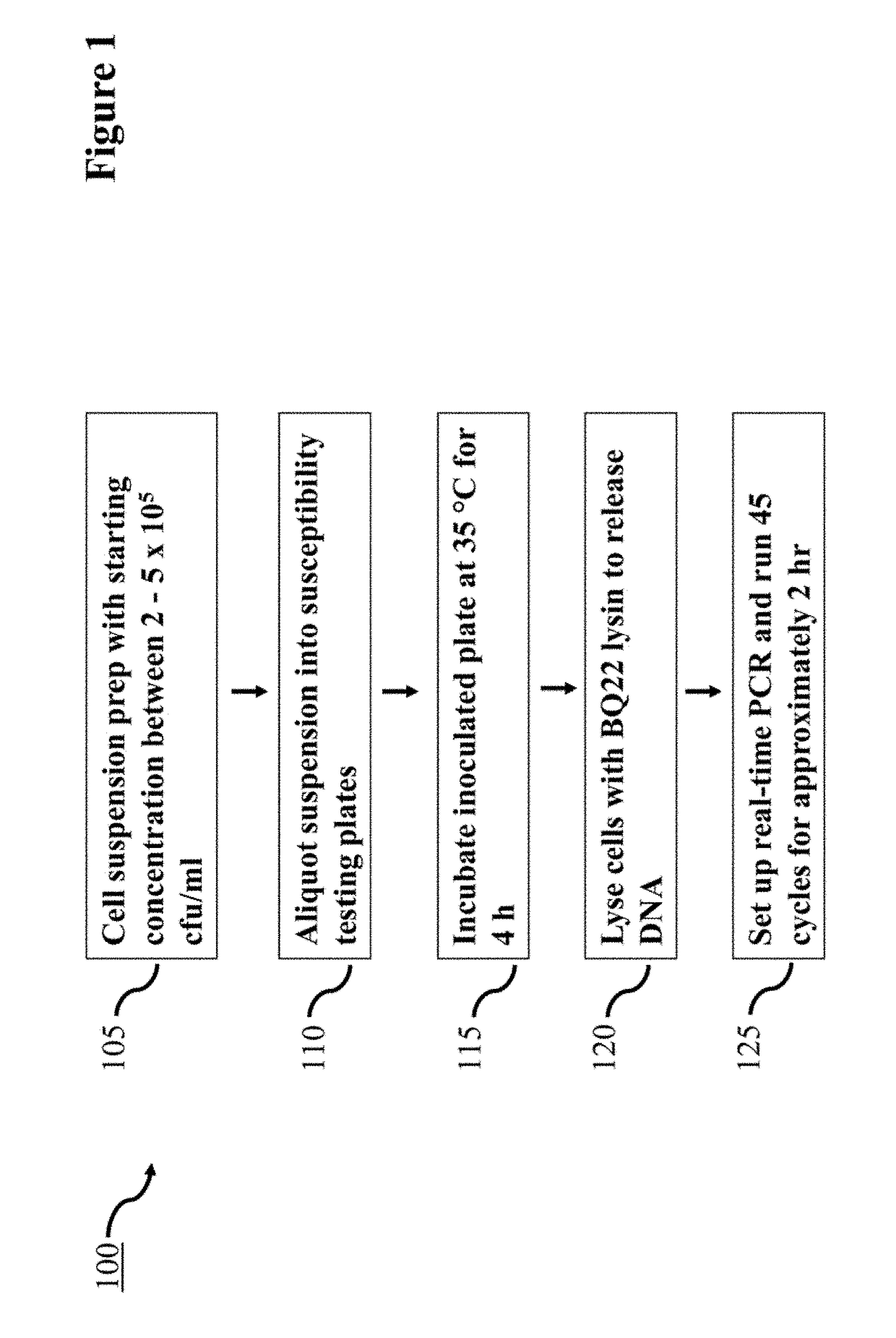
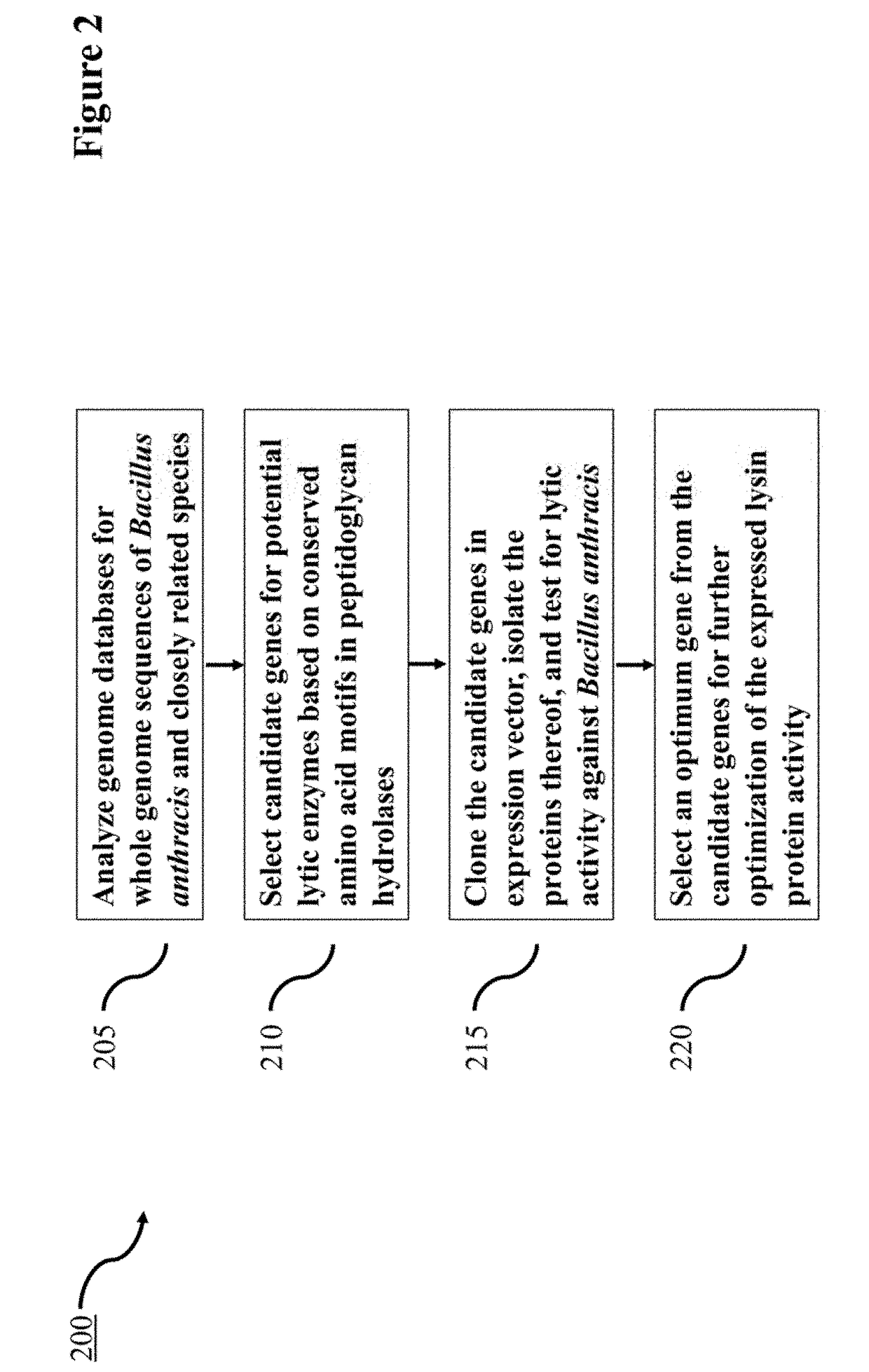
Lysin Agent and Method of Use for Diagnostic Testing
ActiveUS20170240876A1Non toxicBiocideMicrobiological testing/measurementPeptidoglycan HydrolaseNear neighbor
Disclosed herein are the identification, cloning, and optimizing the lytic activity of one or more novel Bacillus lysin proteins, a method of selecting a lysin agent for use in molecular diagnostic testing that includes analyzing genome databases for Bacillus anthracis and near neighbors, selecting candidate genes encoding potential lytic enzymes based on conserved amino acid motifs as determined in peptidoglycan hydrolases, cloning the candidate genes in expression vector, isolating proteins thereof, and testing for lytic activity against Bacillus anthracis, and selecting an optimum gene of the candidate genes for optimizing lysis conditions.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com