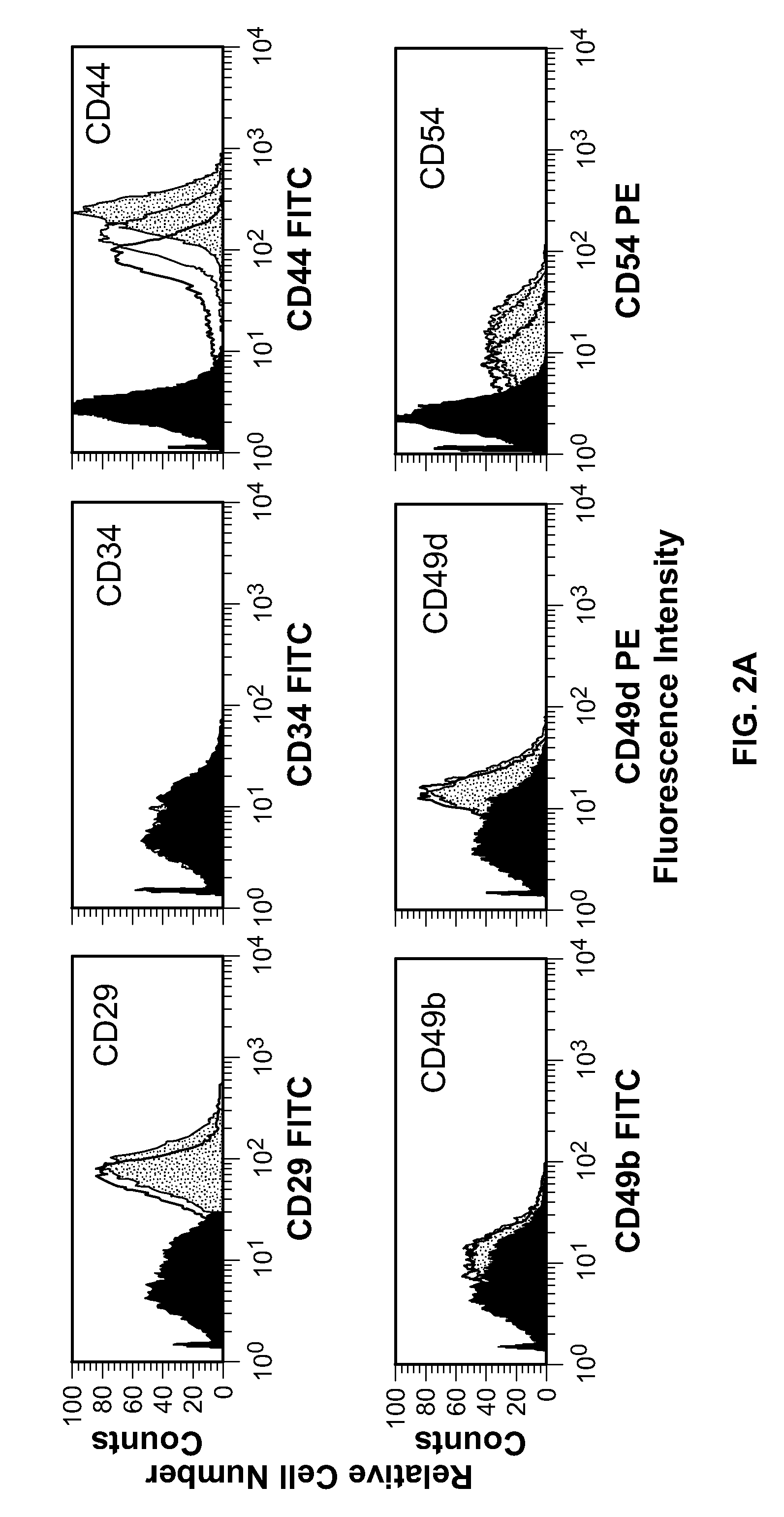
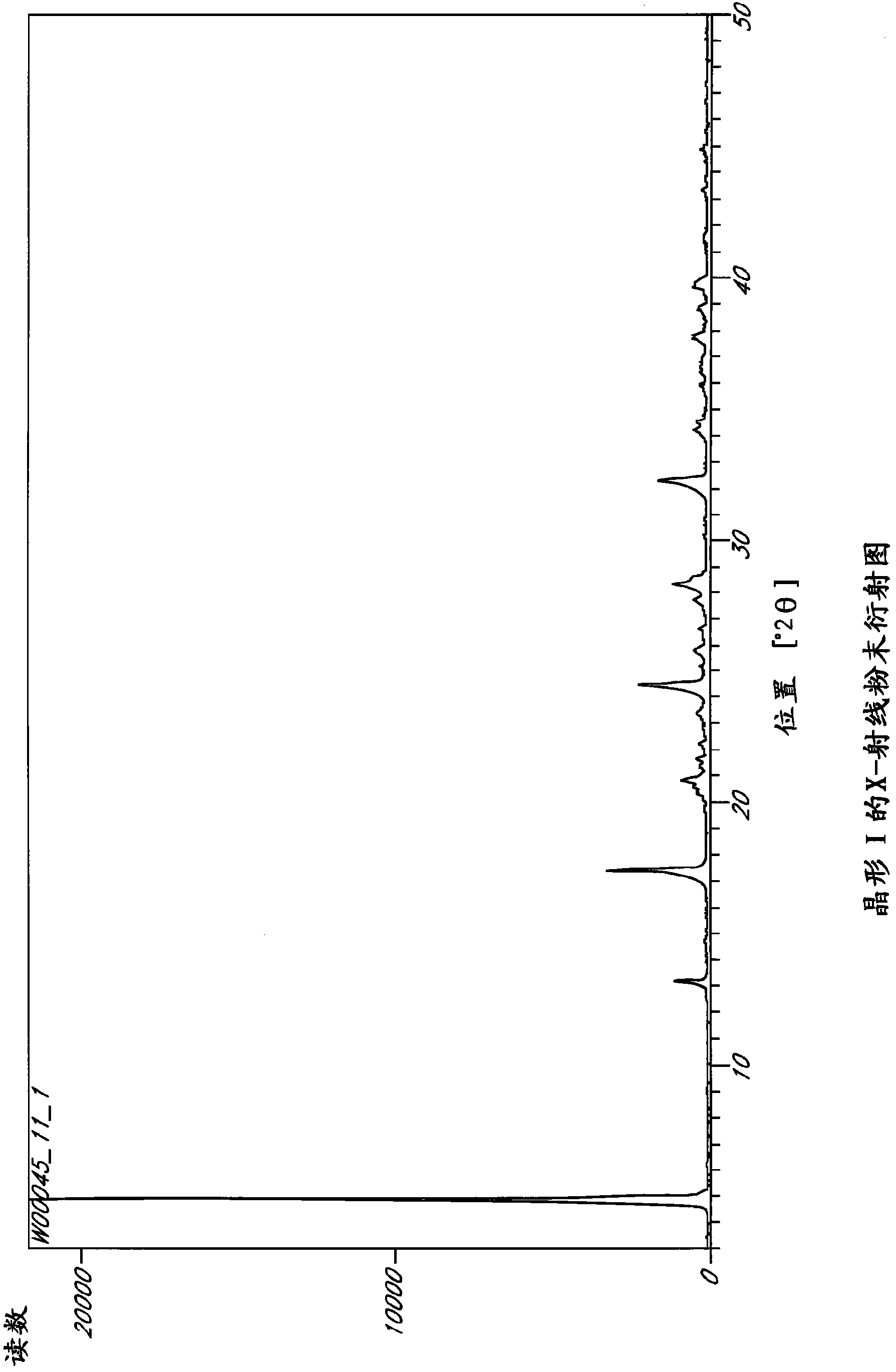
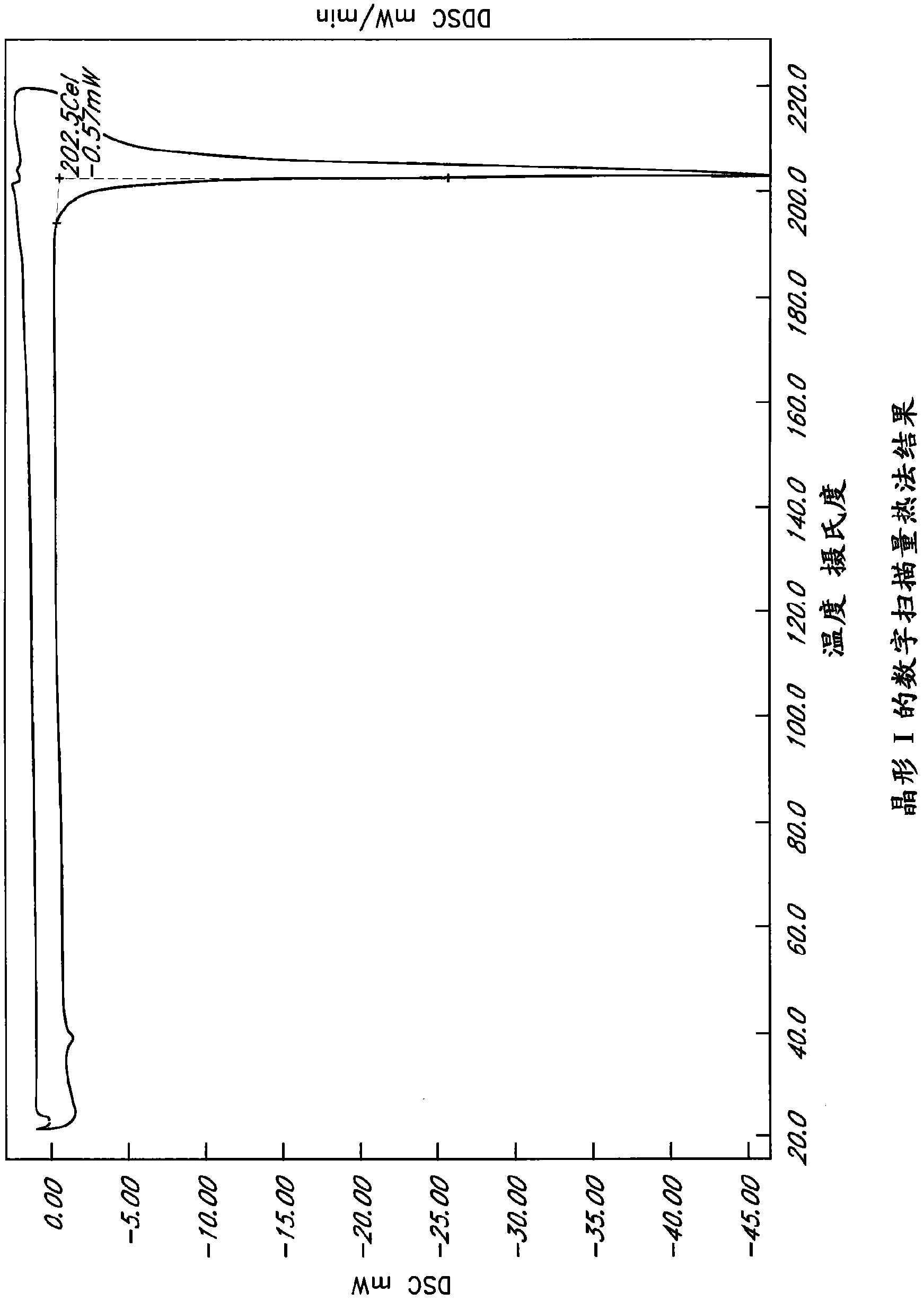
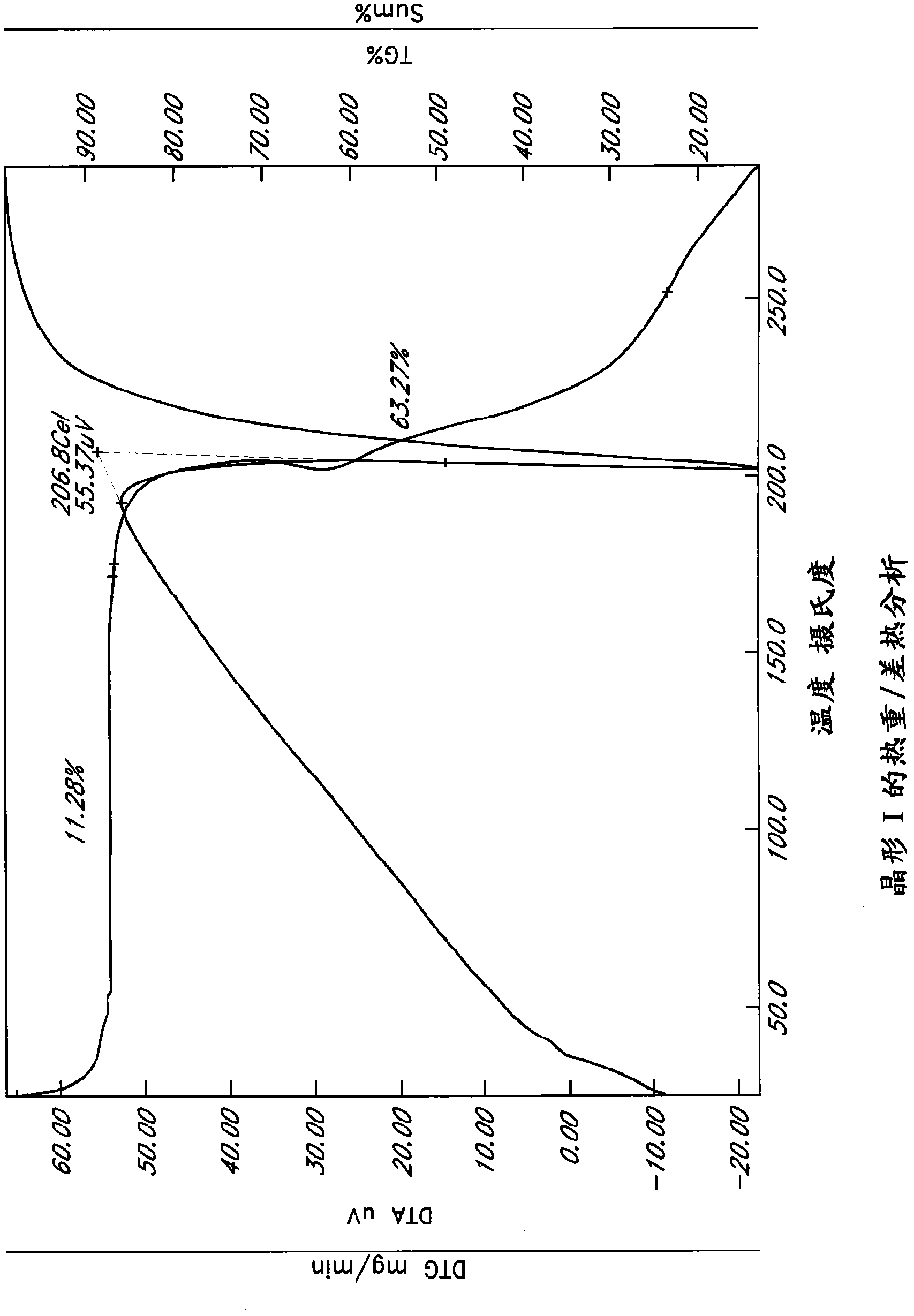
Patents
Literature
159 results about "Liver disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Impairment of health or a condition of abnormal functioning of the liver.
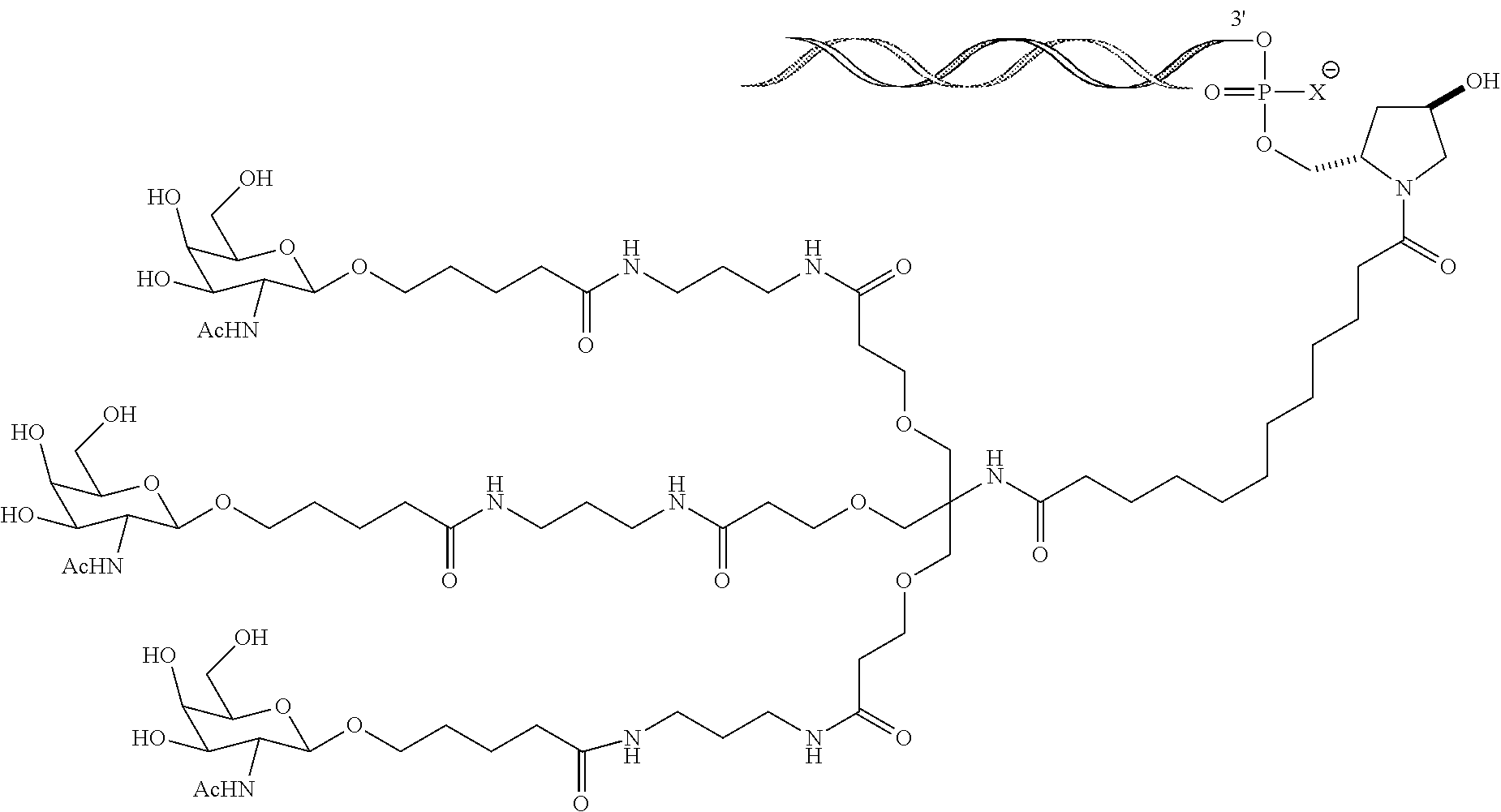
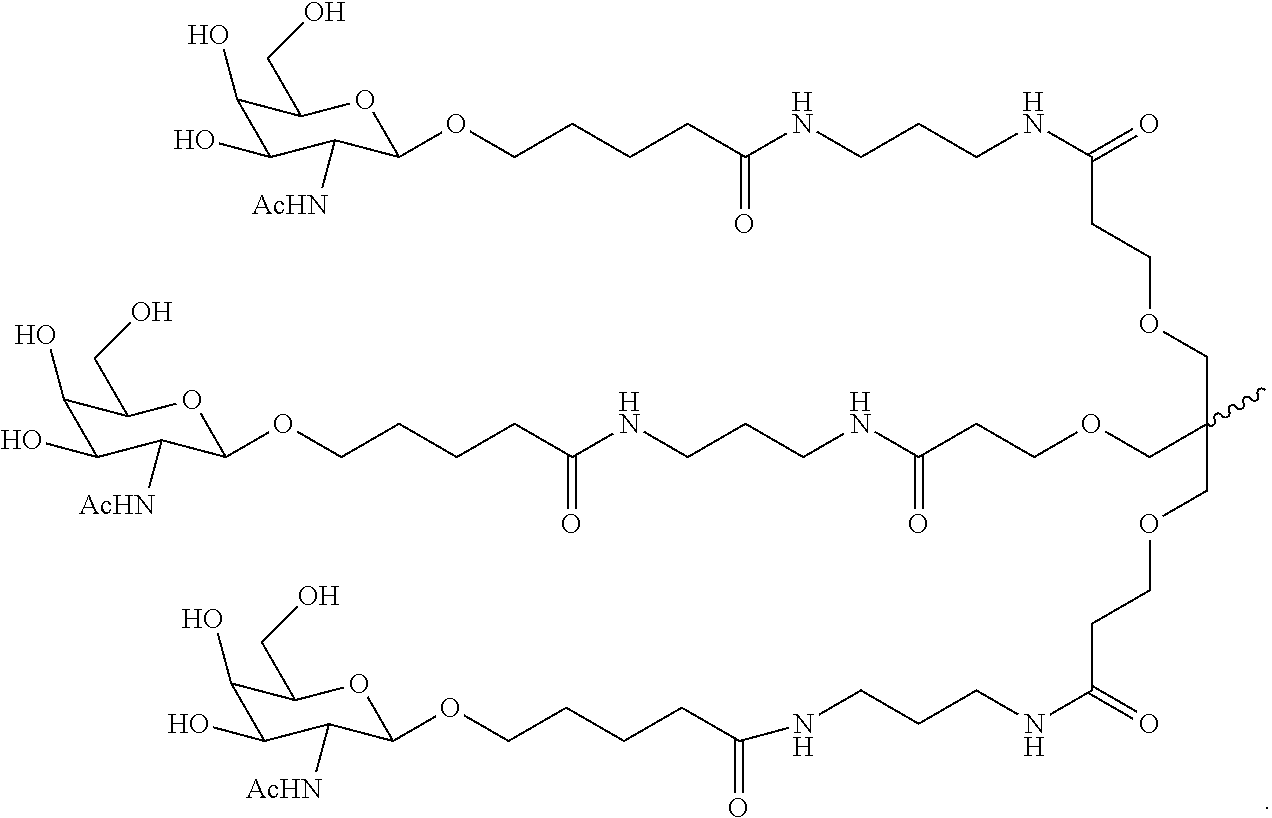
Oral delivery of therapeutically effective lna oligonucleotides
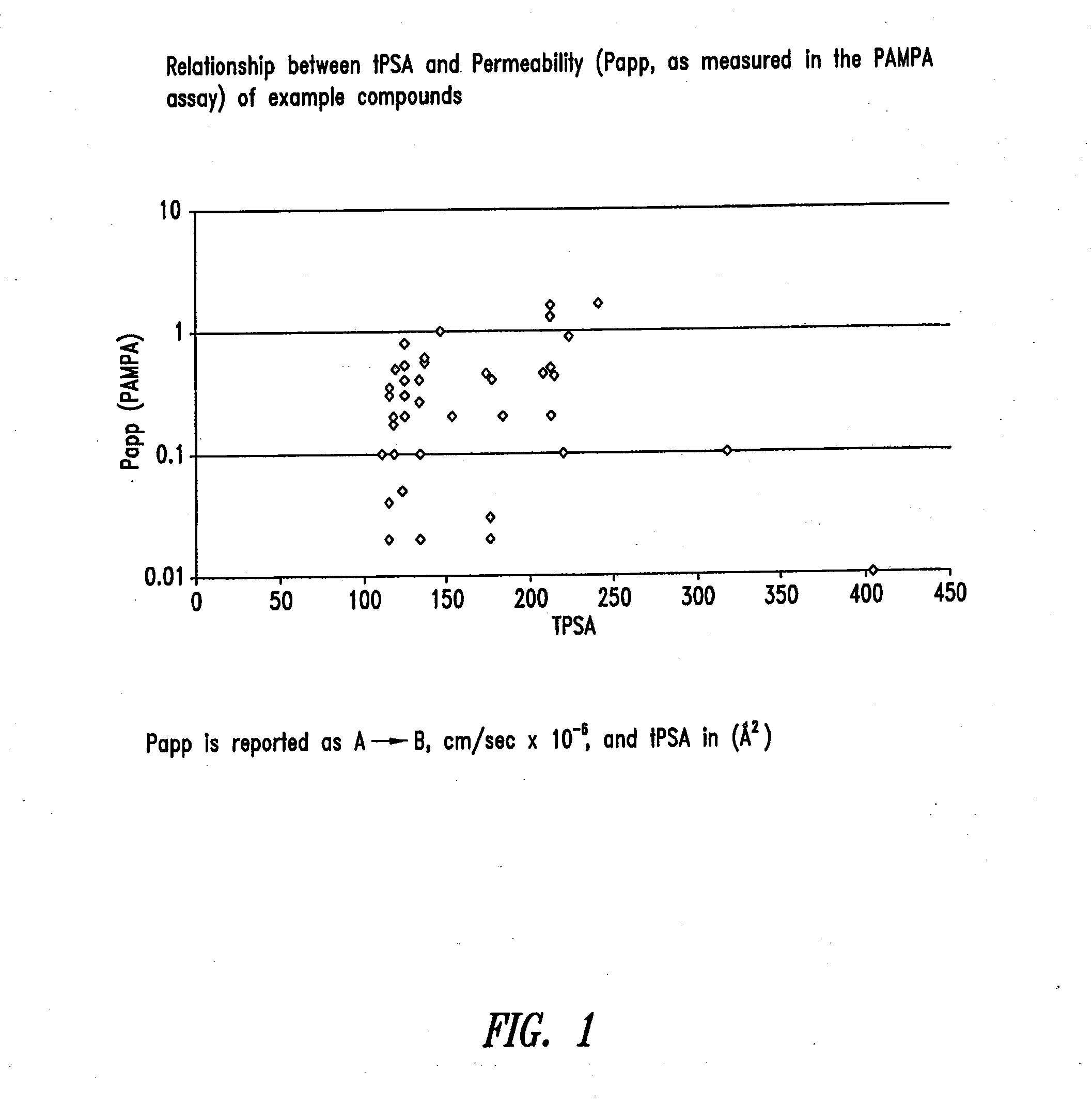
The invention provides for LNA oligomers, for the treatment of a metabolic or liver disorder, wherein the LNA oligomer is administered orally in a unit dose of less than 50 mgs / kg, wherein the LNA oligomer is administered in the presence of a penetration (permeation) enhancer.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Human TIMP-1 antibodies
Human antibodies that bind to TIMP-1 can be used as reagents to diagnose and treat disorders in which TIMP-1 is elevated, such as liver fibrosis, alcoholic liver disease, cardiac fibrosis, acute coronary syndrome, lupus nephritis, glomerulosclerotic renal disease, benign prostate hypertrophy, colon cancer, lung cancer, and idiopathic pulmonary fibrosis.
Owner:BAYER CORPORATION
Compounds and pharmaceutical compositions for the treatment of viral infections
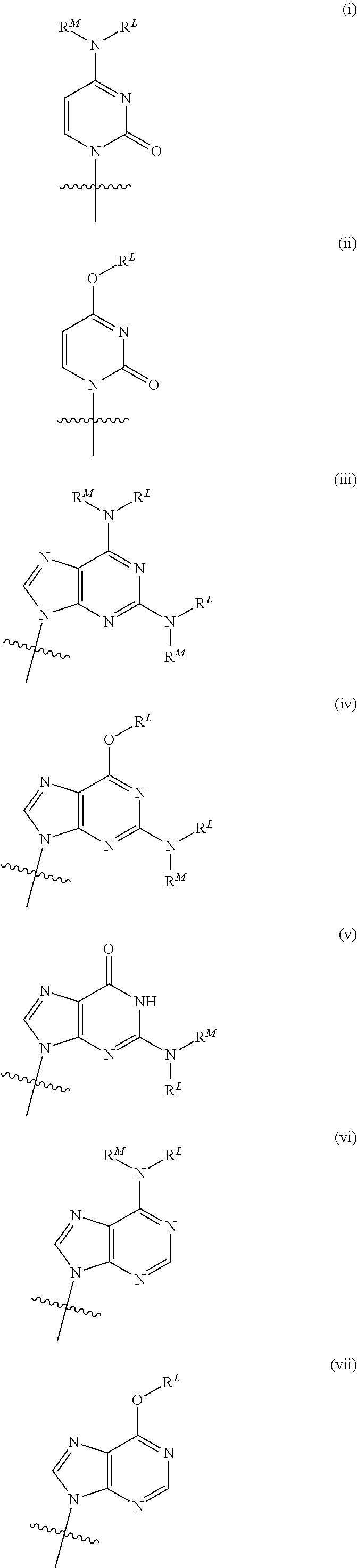
Provided herein are compounds, compositions and methods for the treatment of liver disorders, including HCV infections. In one embodiment, compounds and compositions of nucleoside derivatives are disclosed, which can be administered either alone or in combination with other anti-viral agents.
Owner:INDENIX PHARM LLC
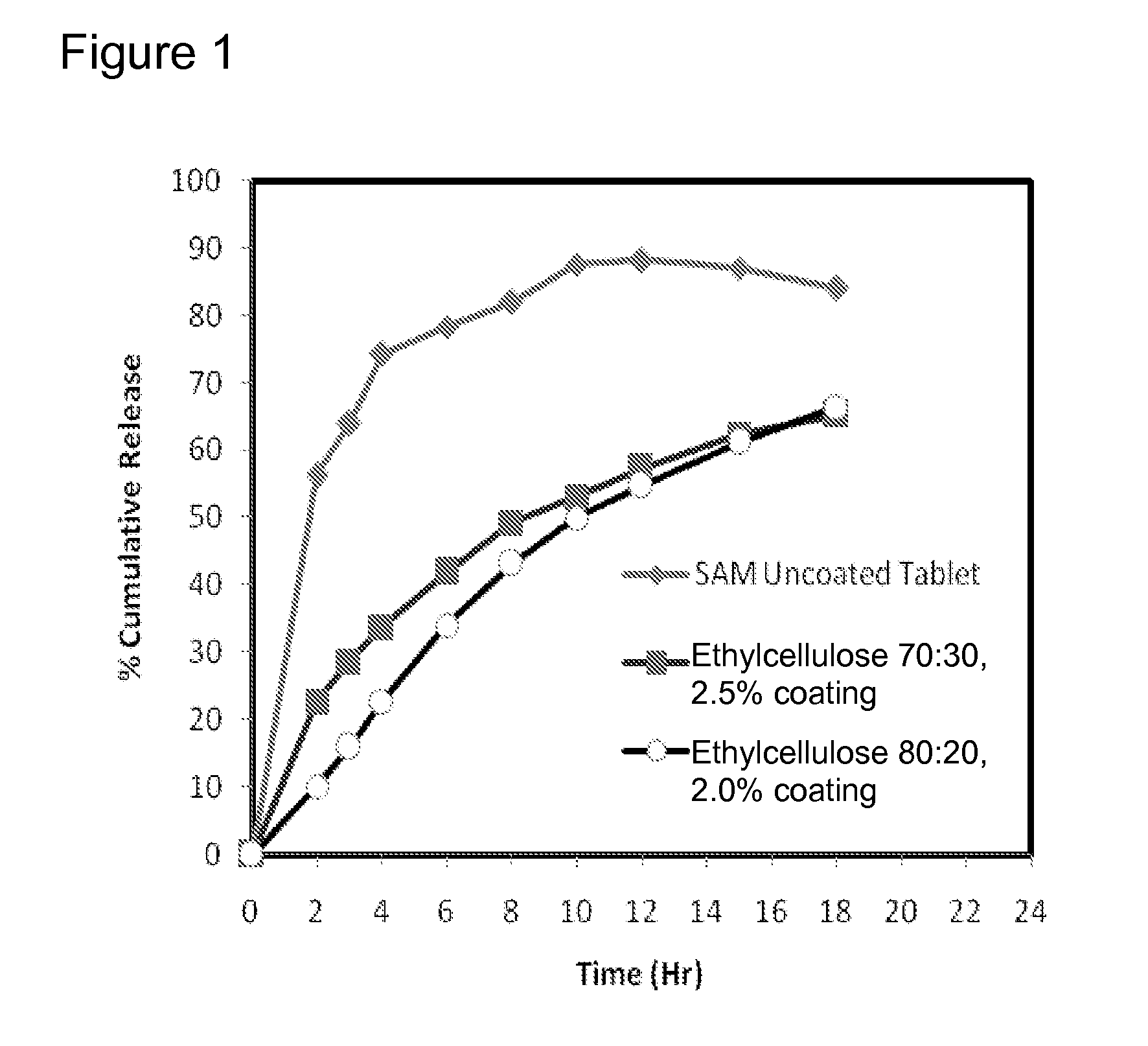
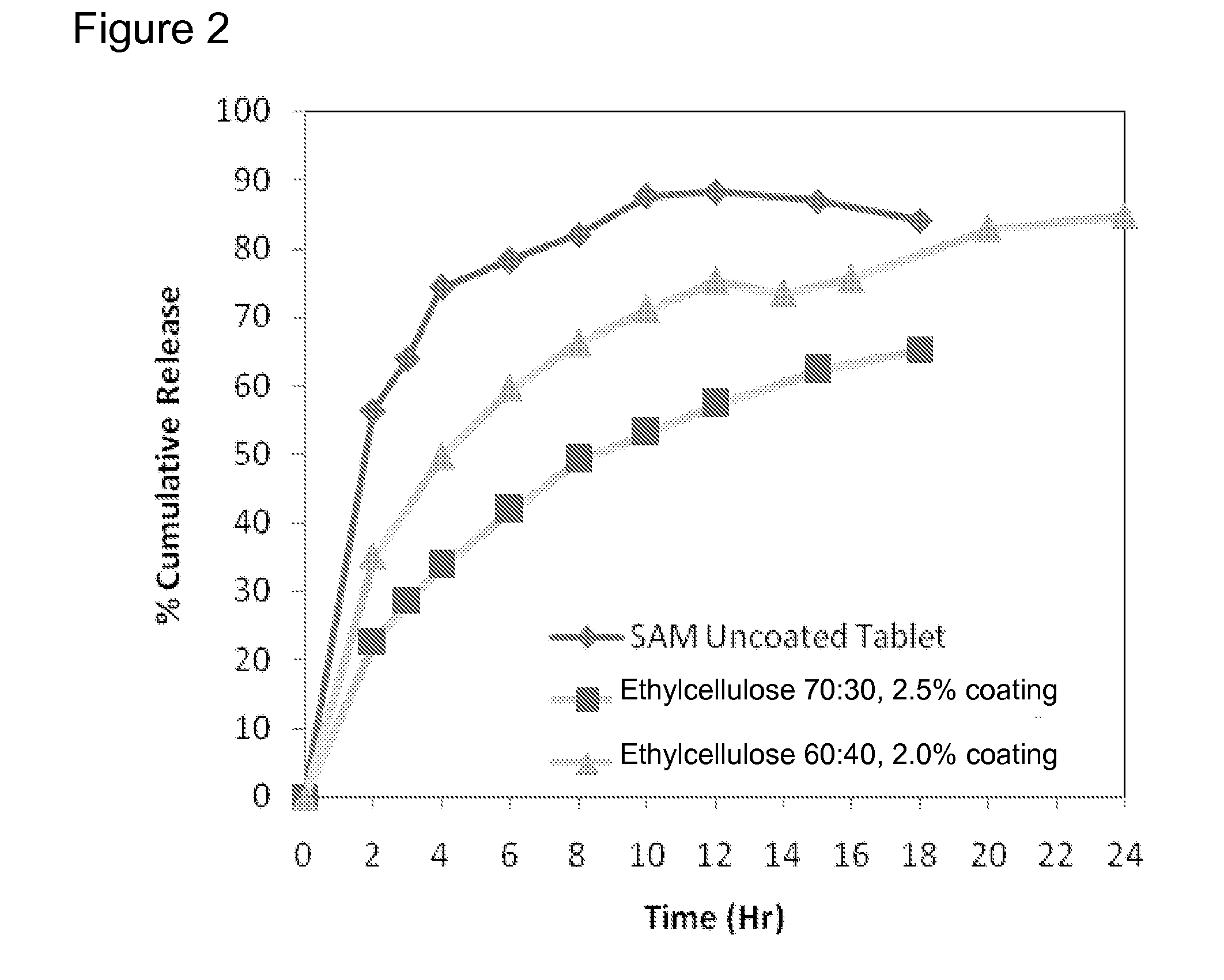
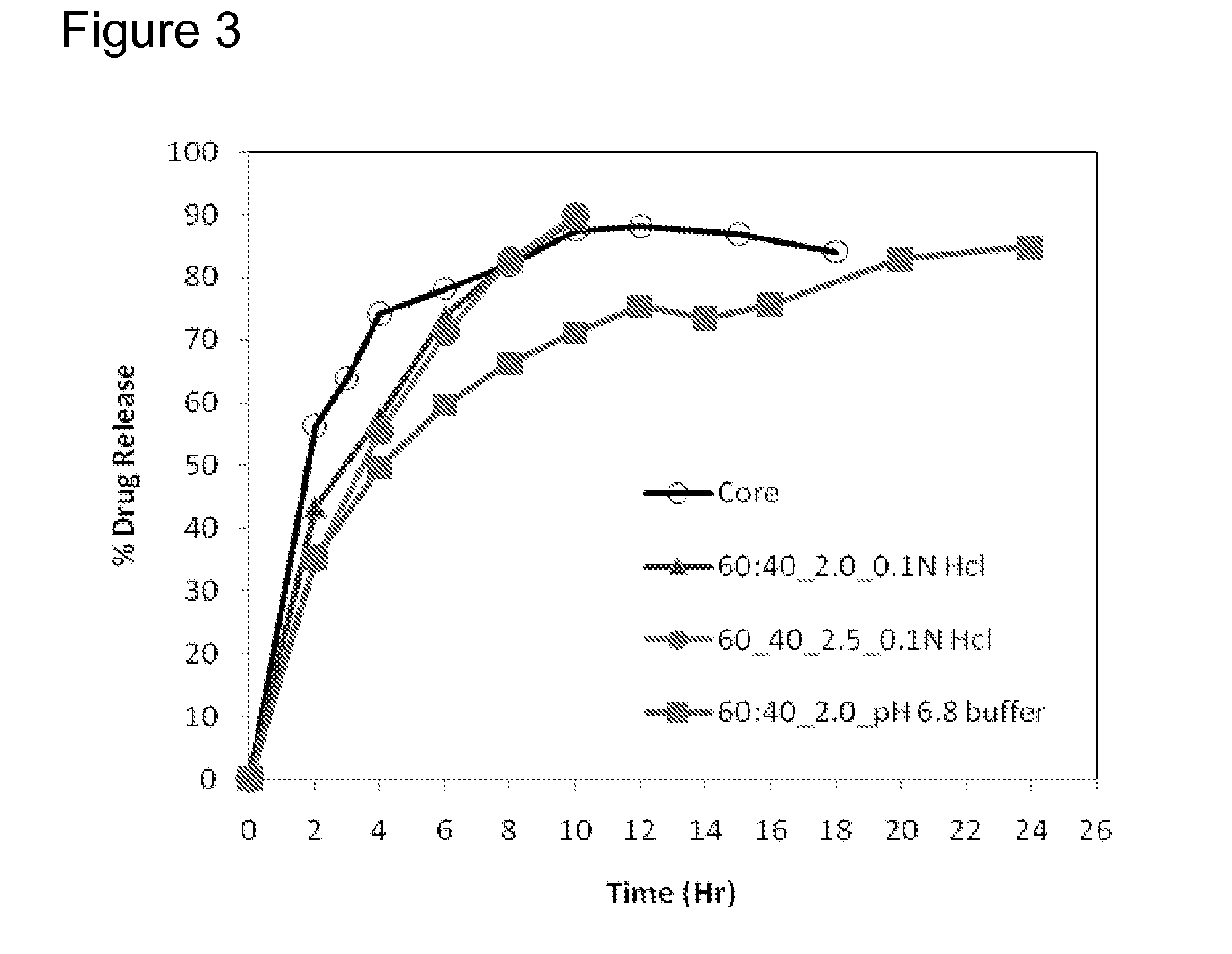
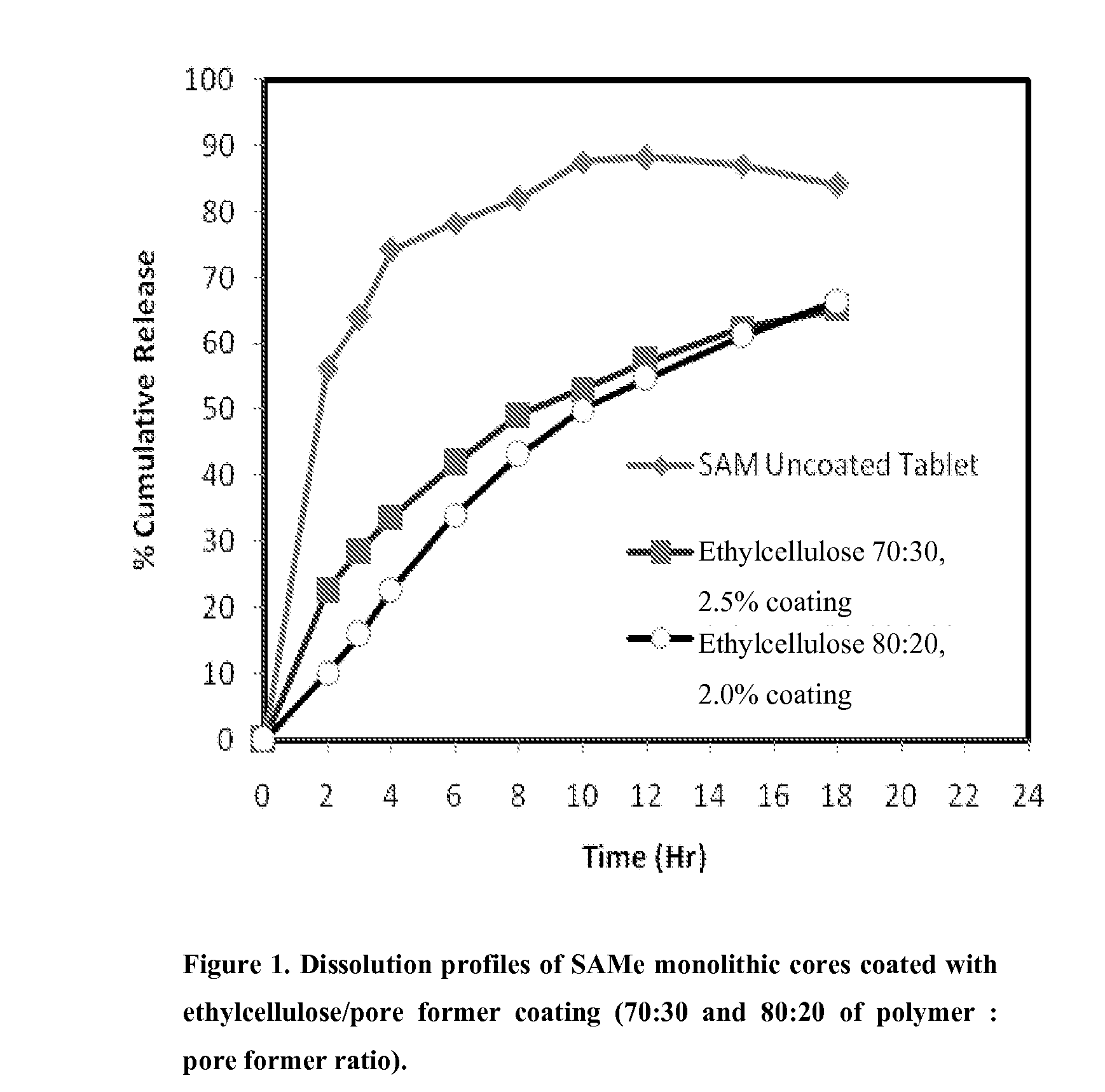
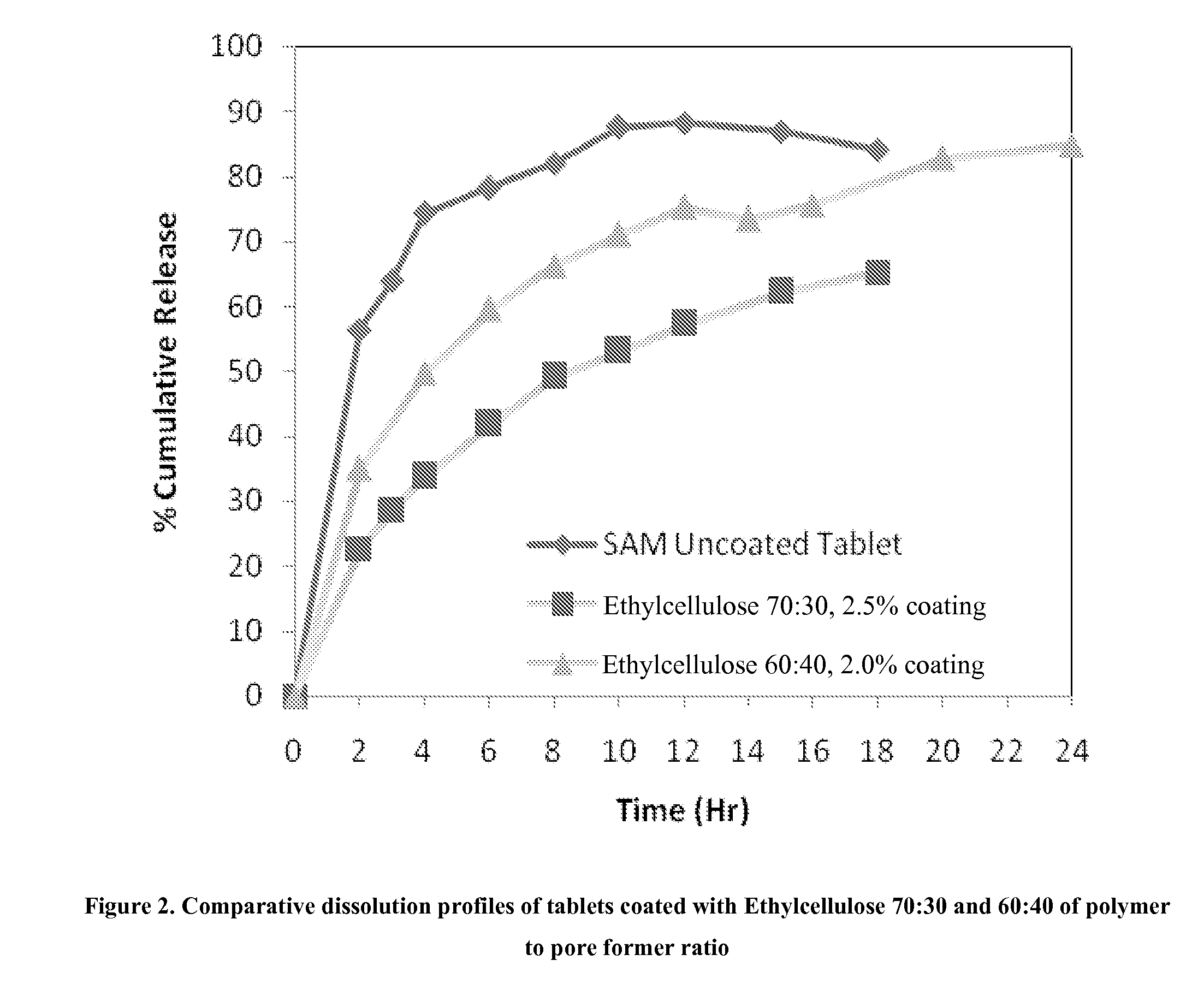
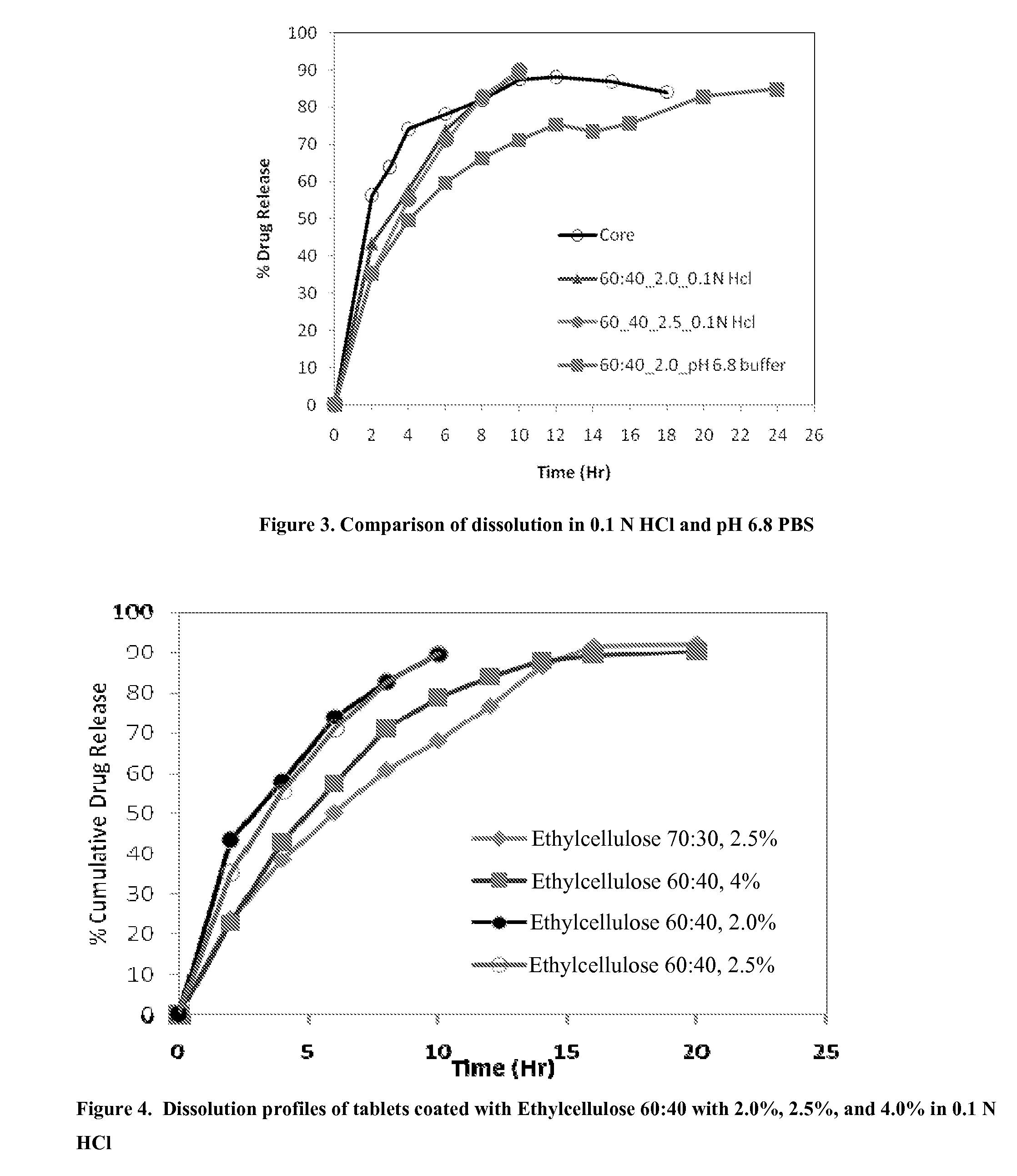
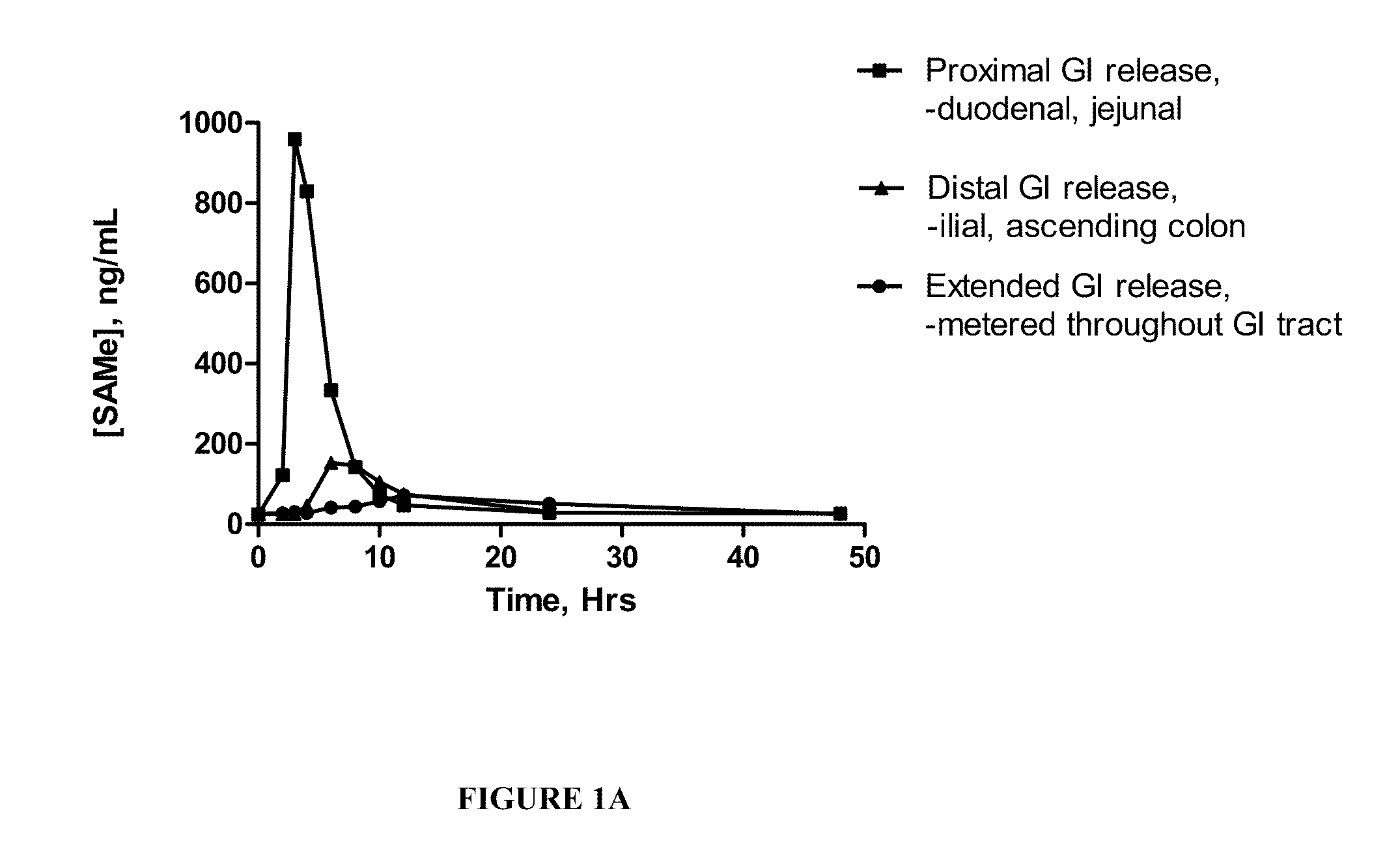
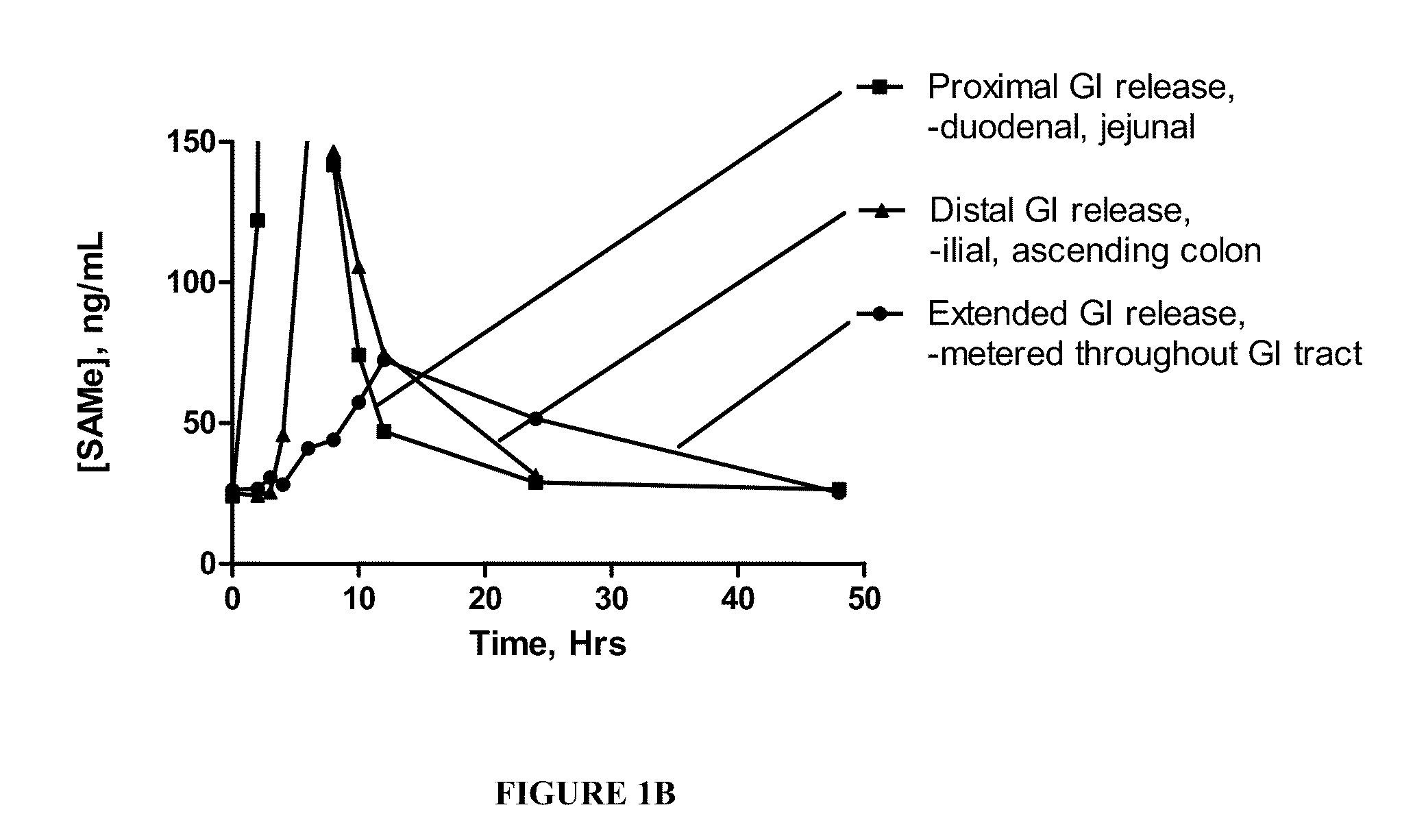
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
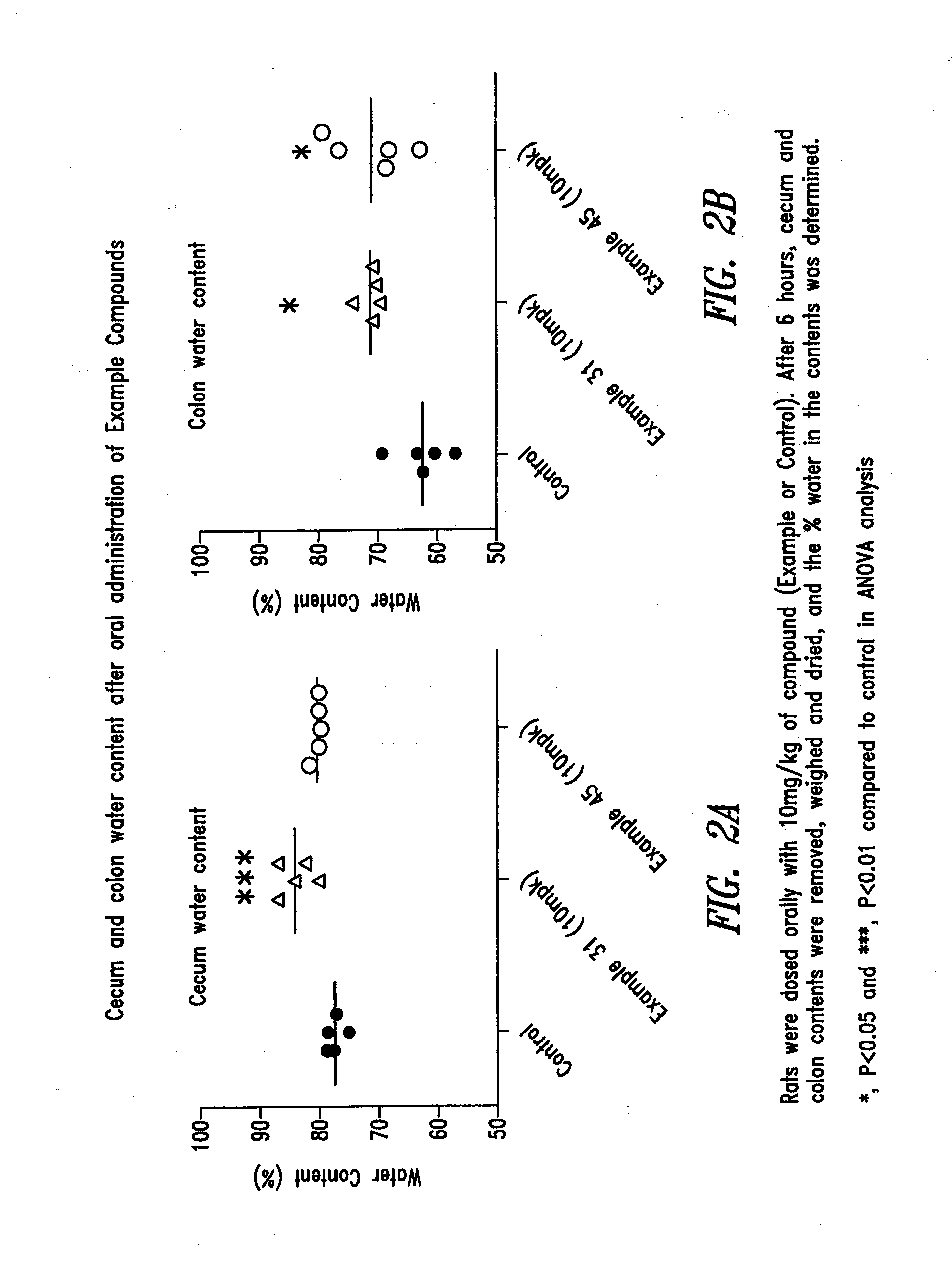
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Method of detecting portal and/or hepatic pressure and a portal hypertension monitoring system
The devices and methods generally relate to vibratable sensors for measuring ambient fluid pressure, in particular implantable sensors. The devices and methods are particularly well-suited to implantation within the body of a living animal or human to monitor physiological conditions, such as portal and / or hepatic venous blood pressure, and allow frequent, remote interrogation of venous pressure using the resonance frequency of an implanted sensor. The sensor devices are relatively small compared to conventional devices for measuring fluid pressure and can be implanted in the porto-hepatic venous system, whereas conventional devices are too large. The small size of the device is accomplished by using a thick sensor membrane, compared to conventional devices, and by limiting the size of additional elements of the device relative to the size of the sensor membrane. The thicker sensor member also obviates the need for multiple sensor arrays and maintains the accuracy and robustness of the sensor device. A data capture, processing, and display system provides a pressure measurement reading, and is particularly well-suited for detecting portal hypertension in patients with liver disorders.
Owner:MICROTECH MEDICAL TECH
SERPINA1 iRNA COMPOSITIONS AND METHODS OF USE THEREOF
ActiveUS20140350071A1Reduce accumulationAvoid developmentOrganic active ingredientsSpecial deliveryBiotechnologyDisease
The invention relates to RNAi agents, e.g., double-stranded RNAi agents, targeting the Serpina1 gene, and methods of using such RNAi agents to inhibit expression of Serpina1 and methods of treating subjects having a Serpina1 associated disease, such as a liver disorder.
Owner:ALNYLAM PHARM INC
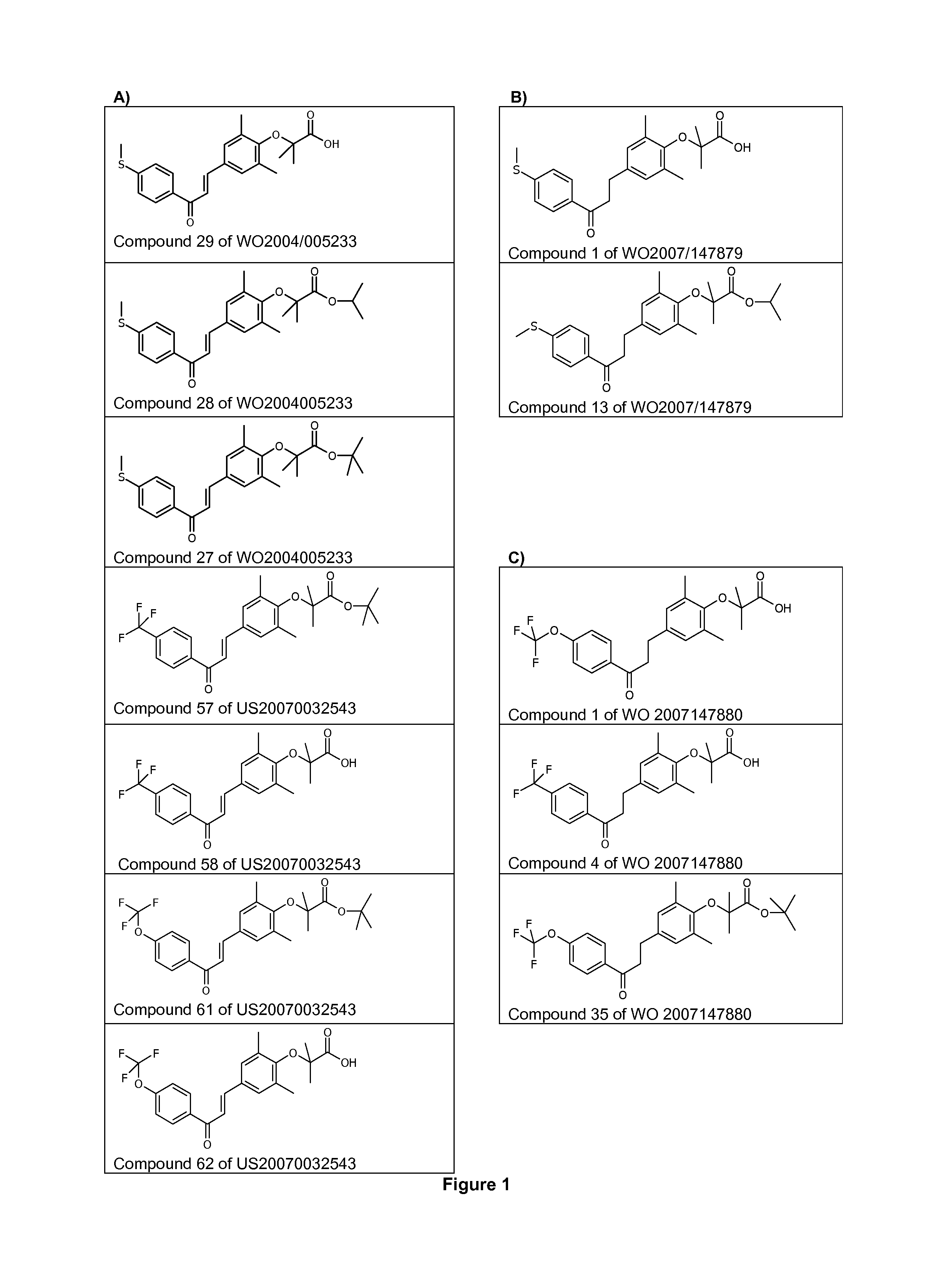
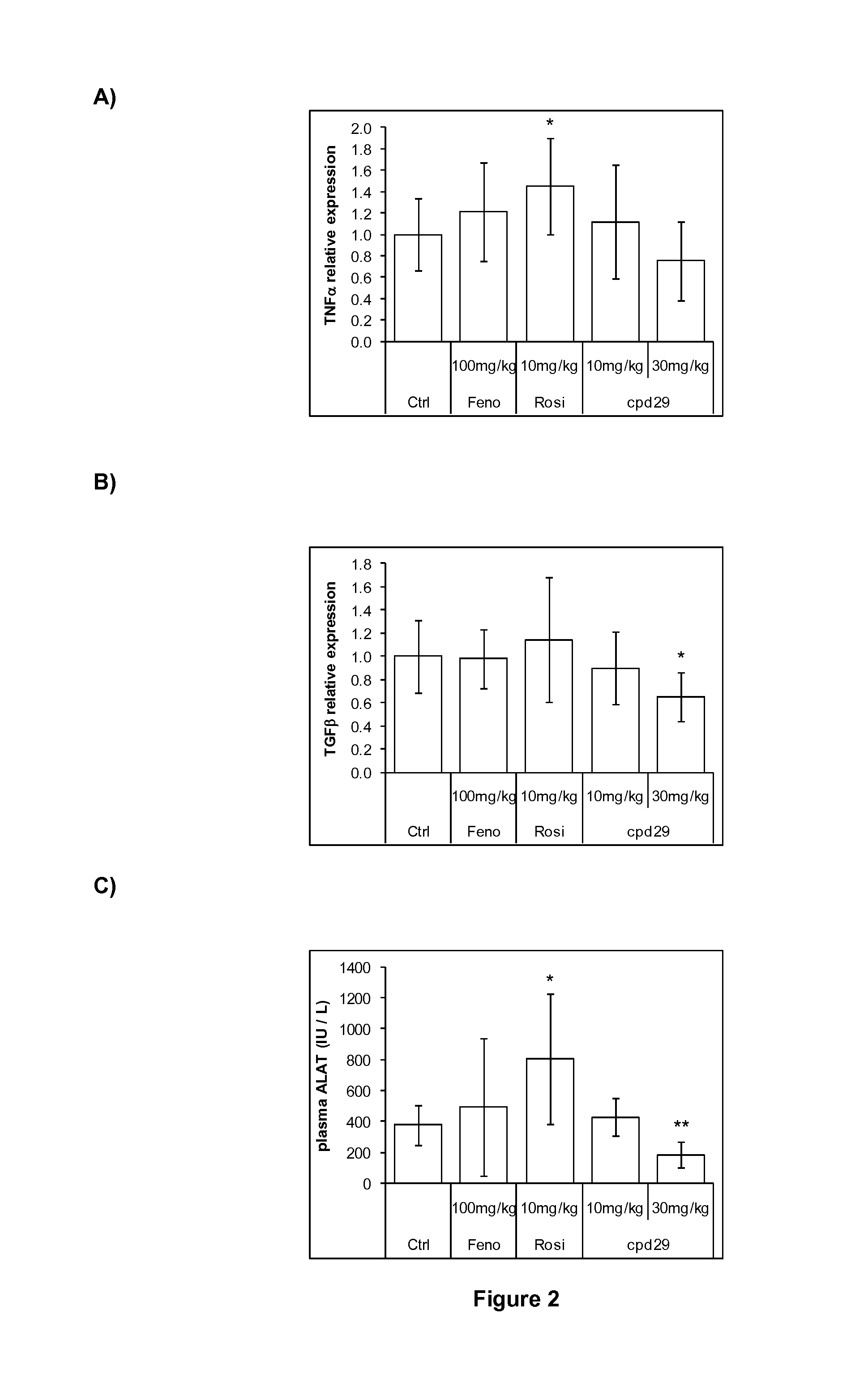
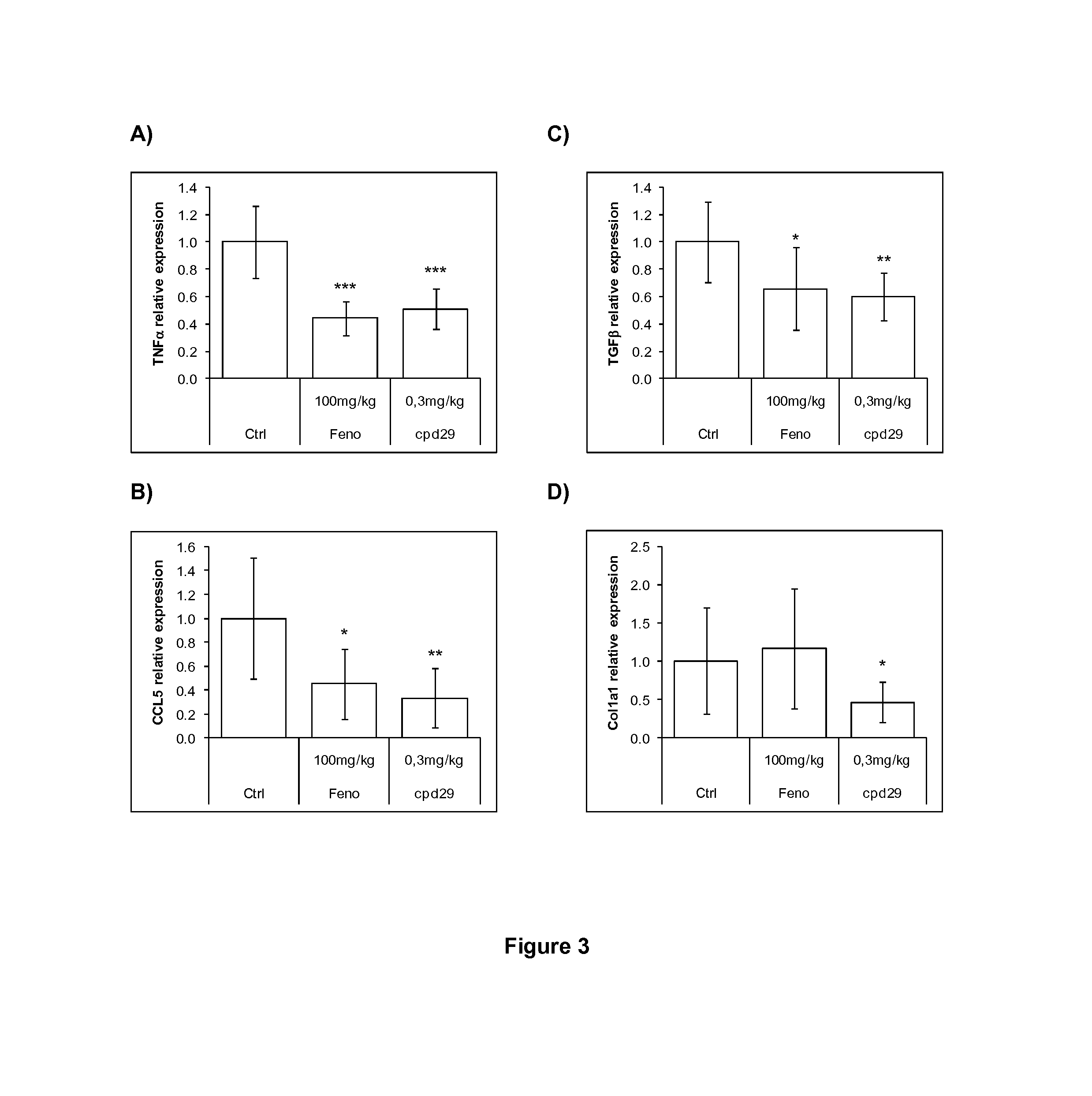
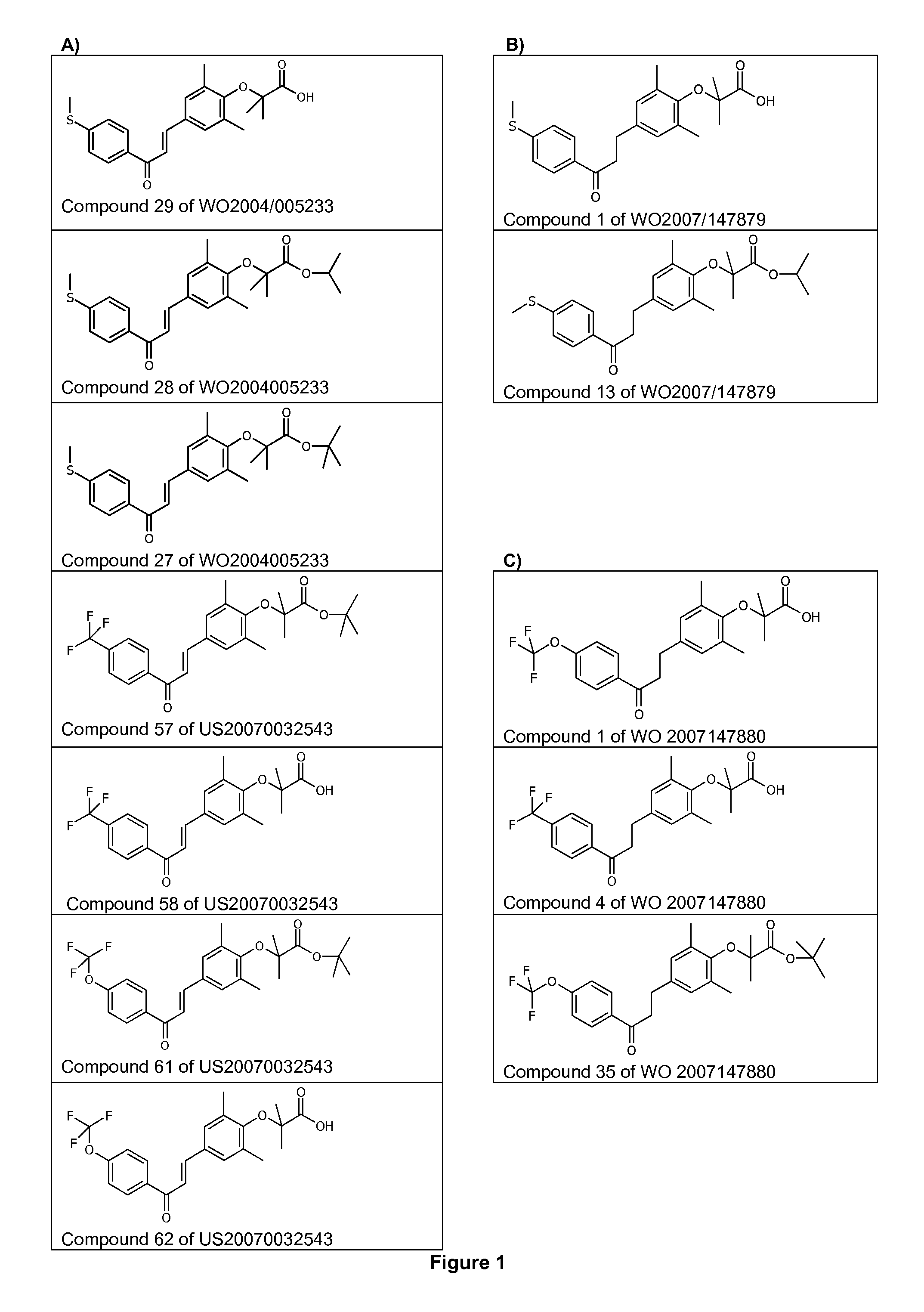
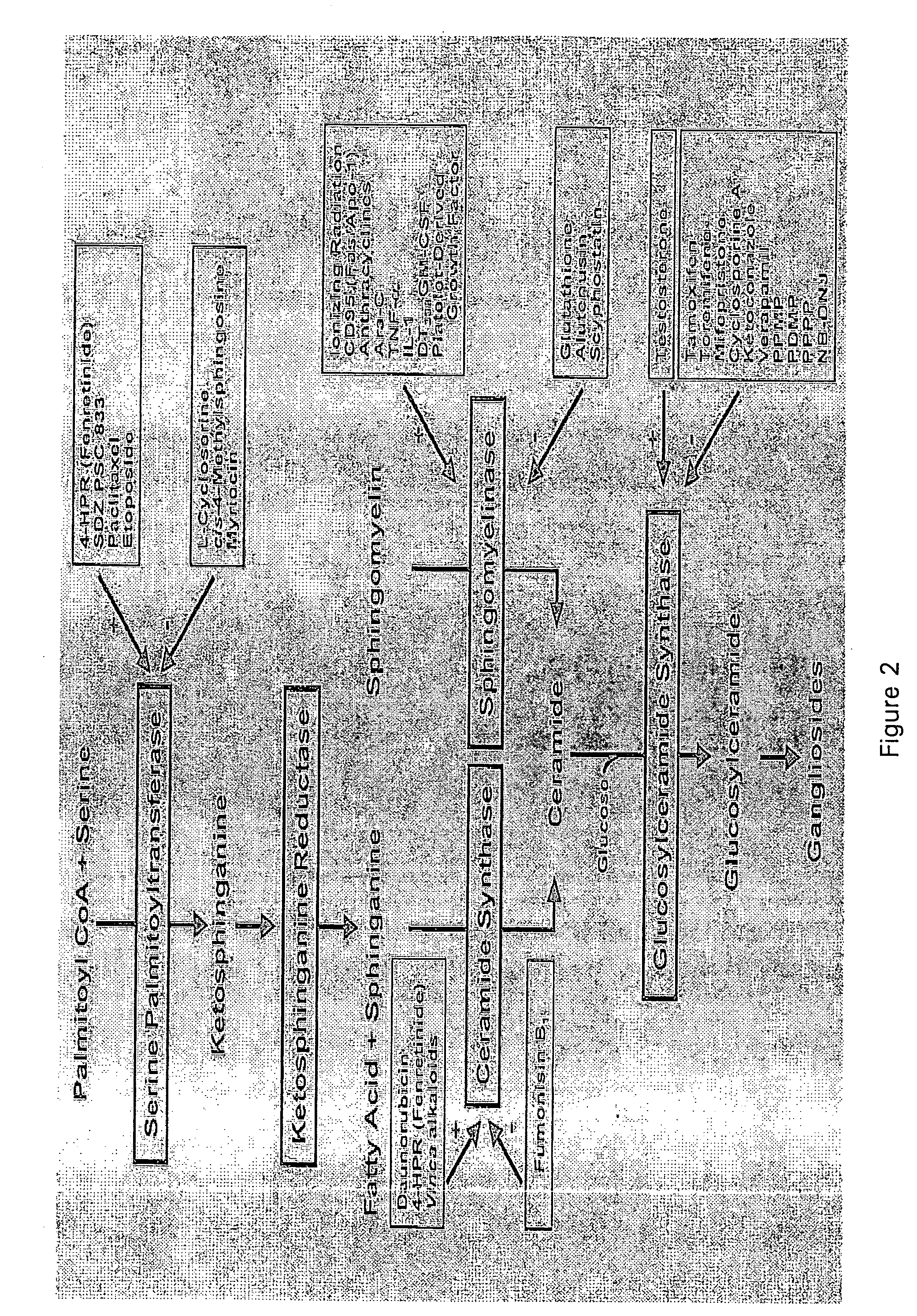
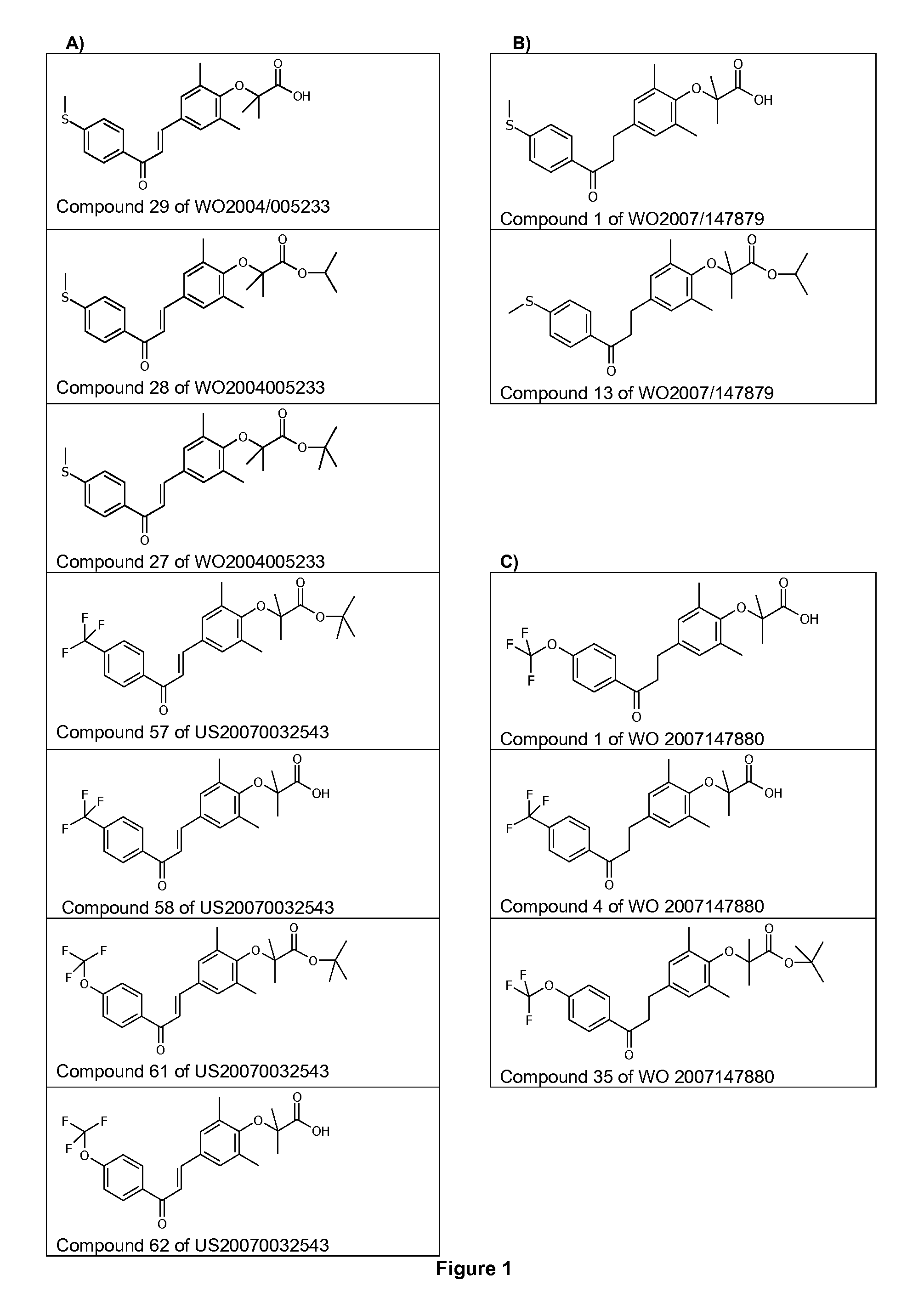
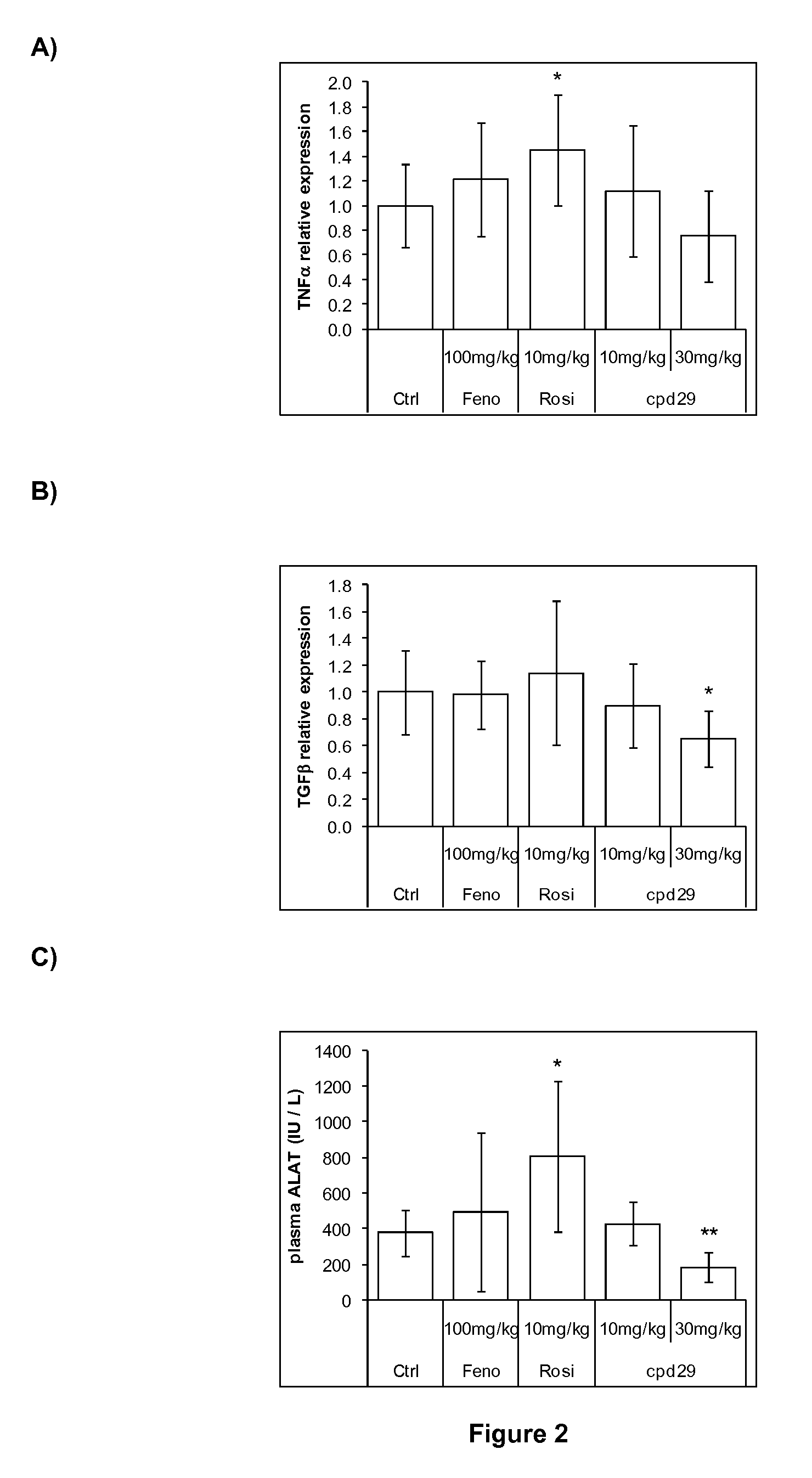
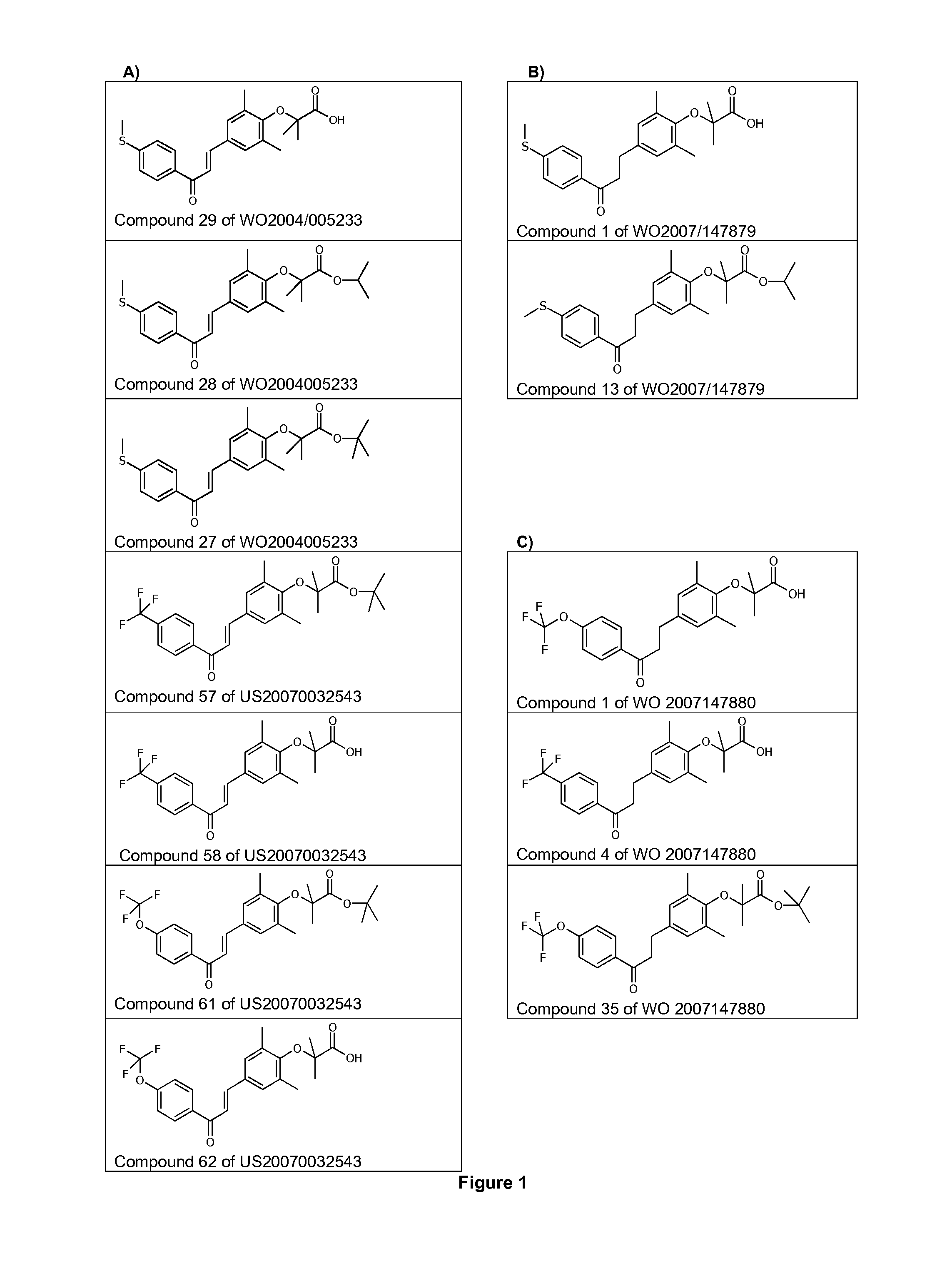
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
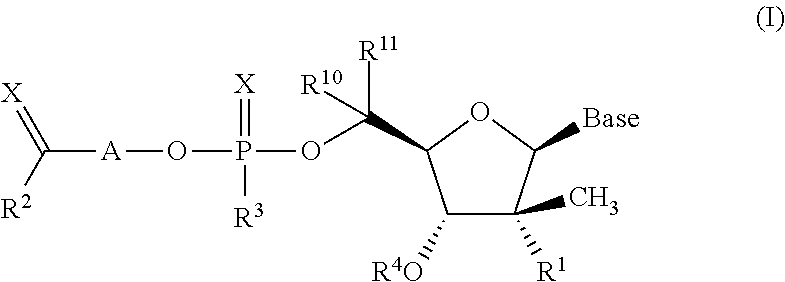
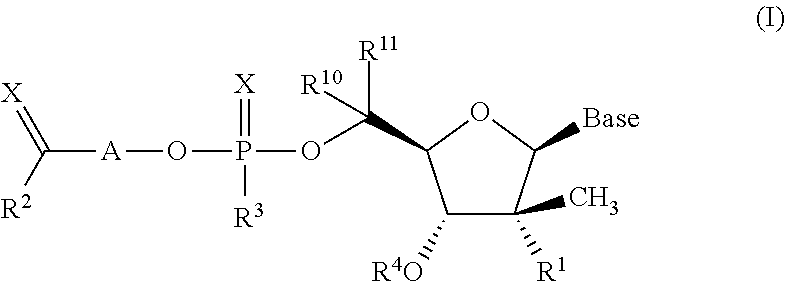
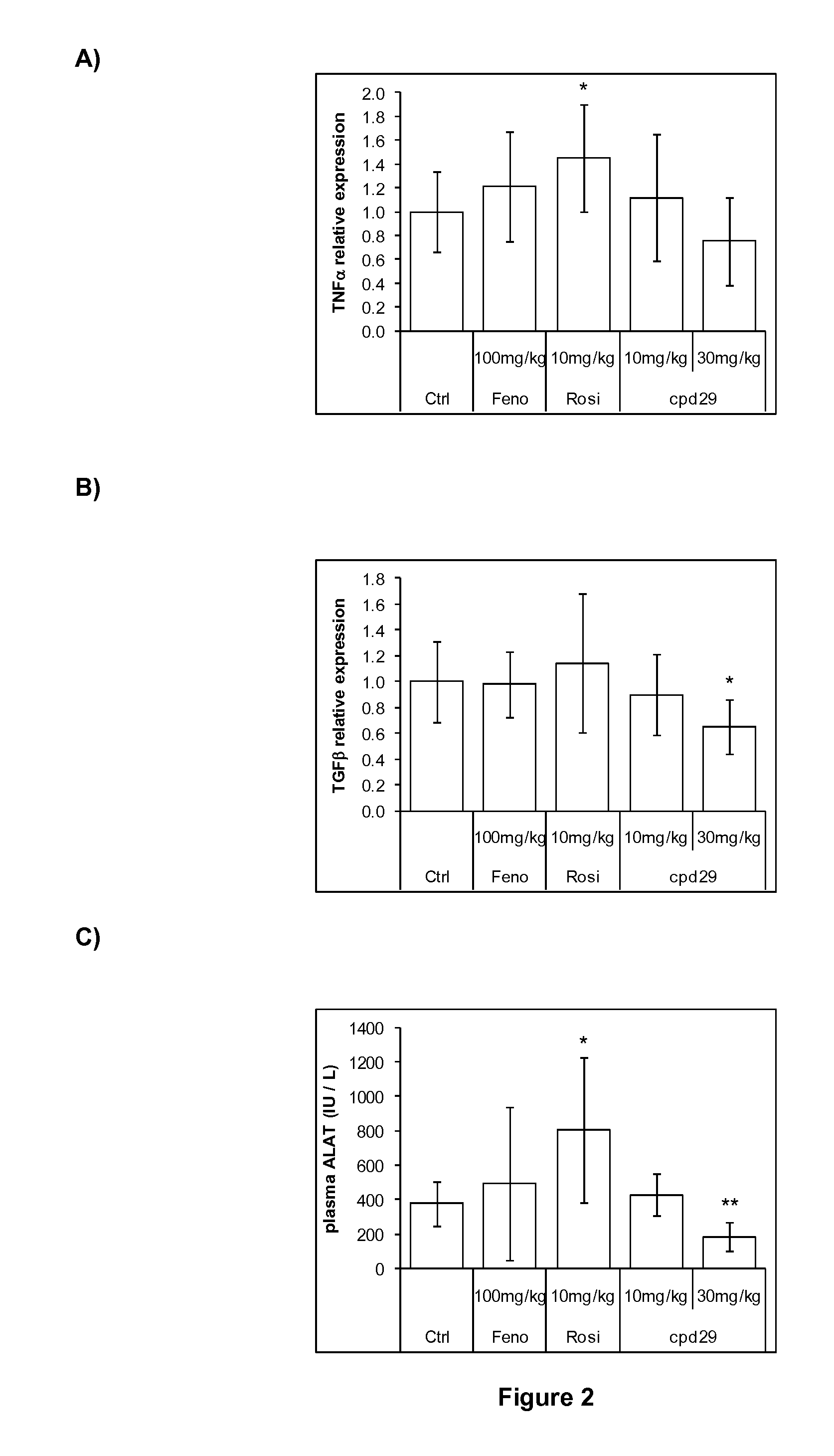
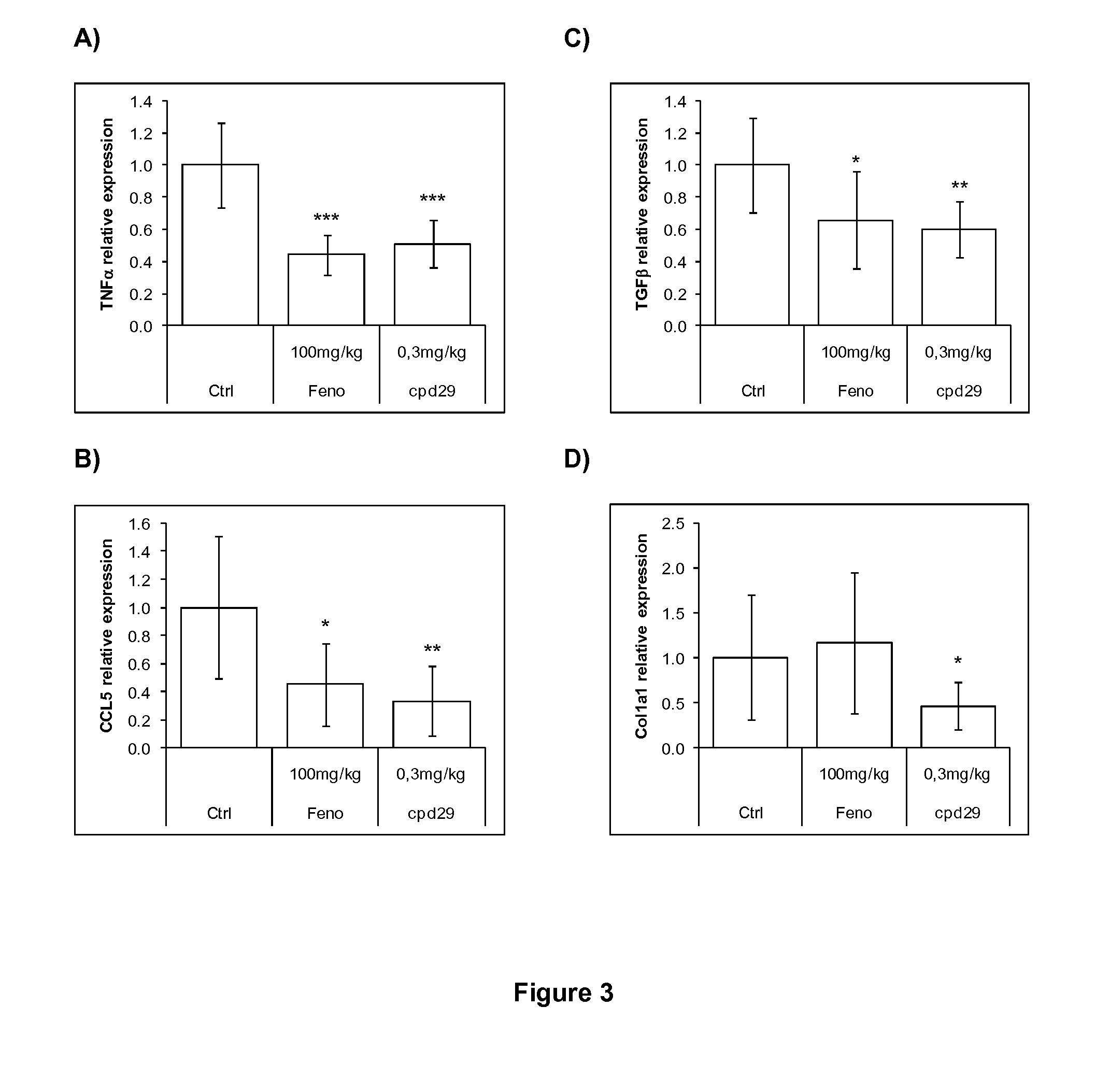
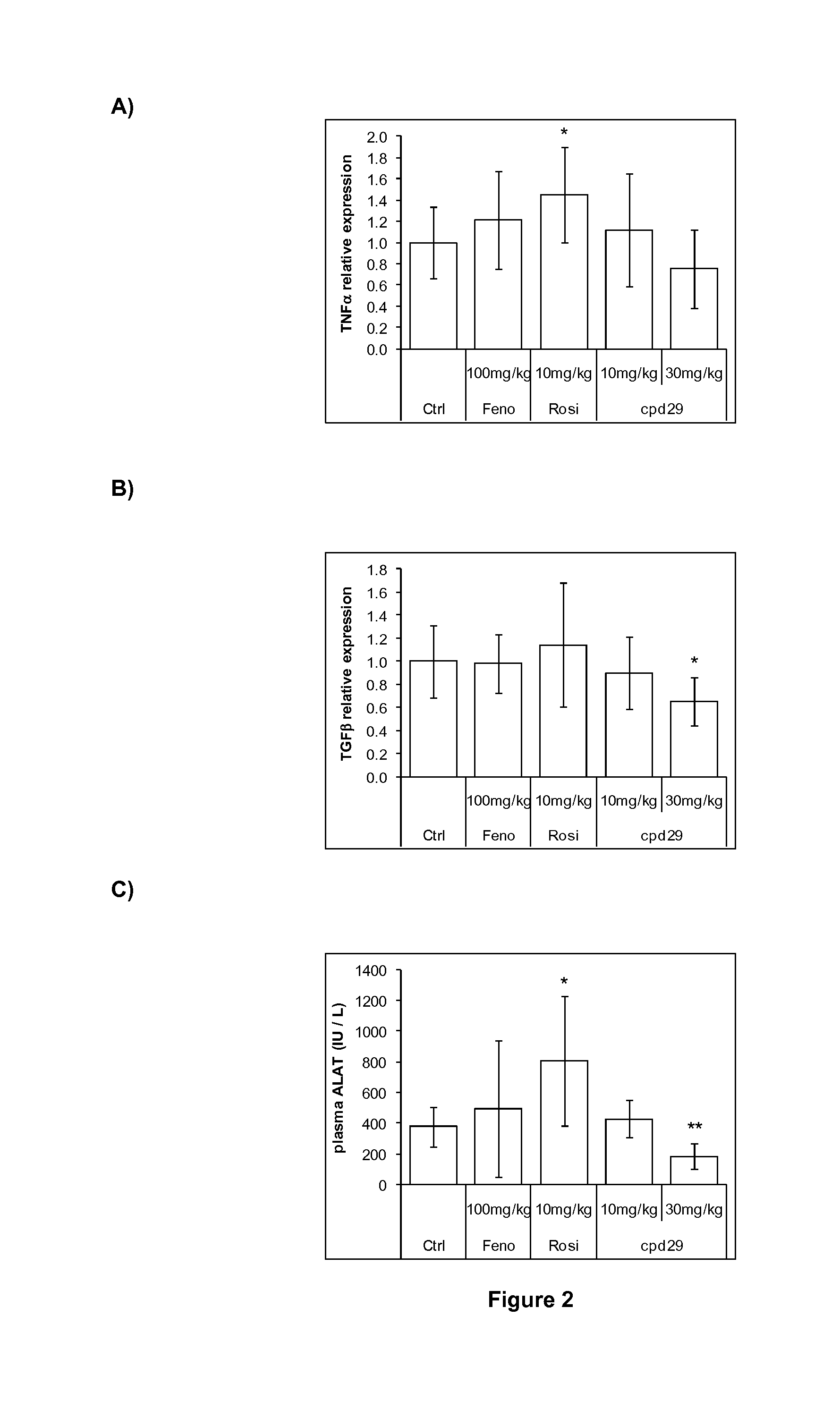
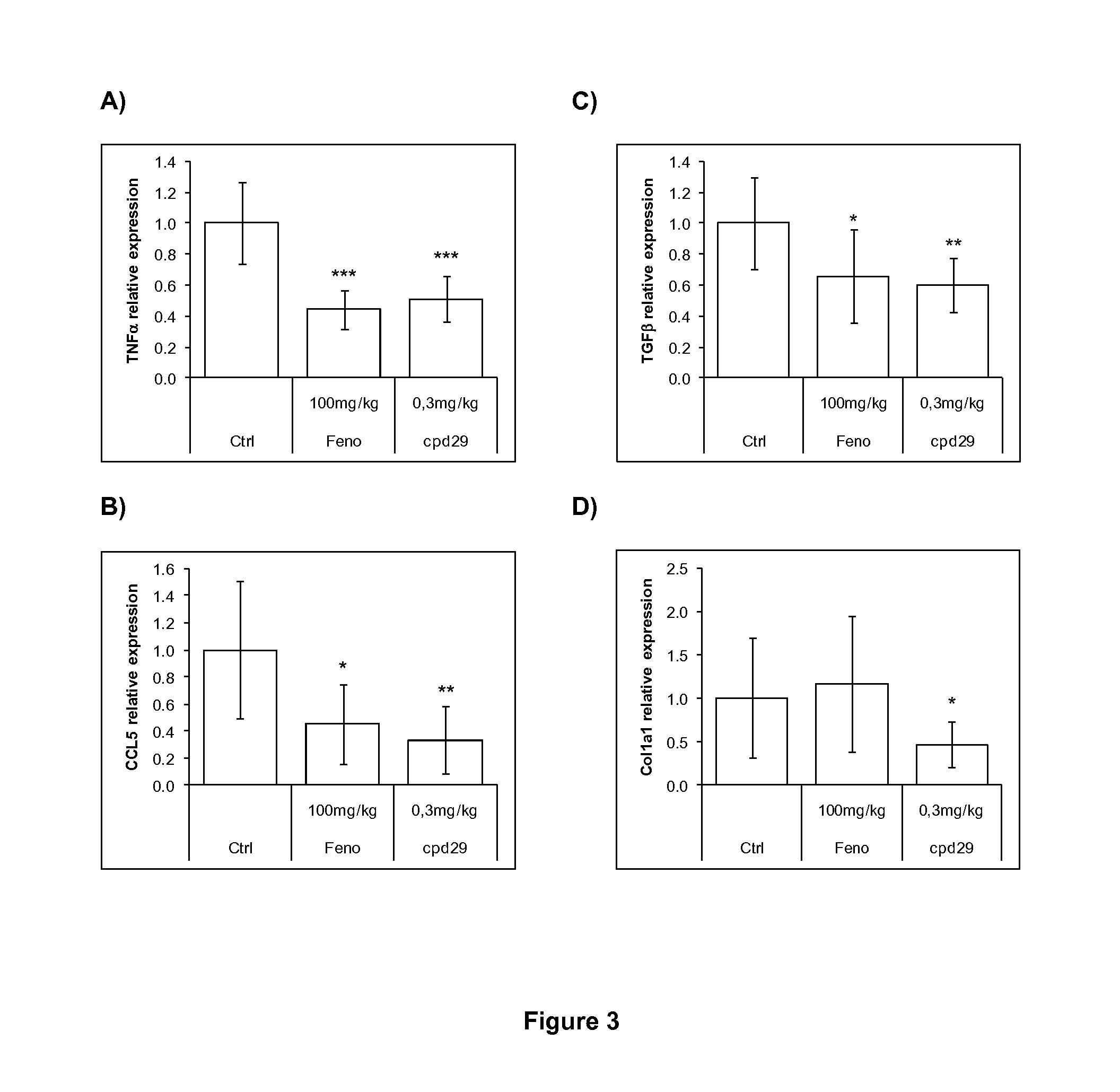
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
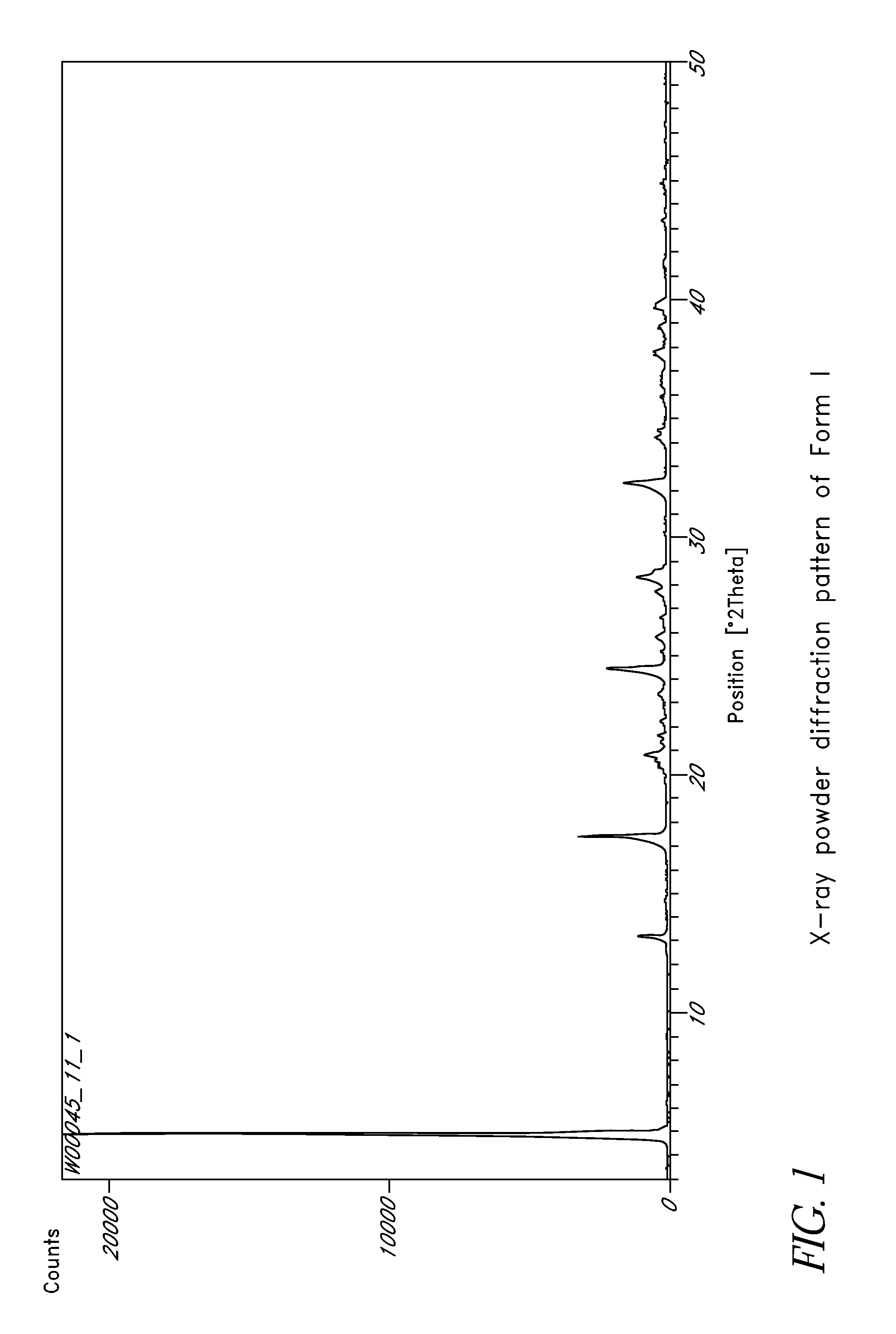
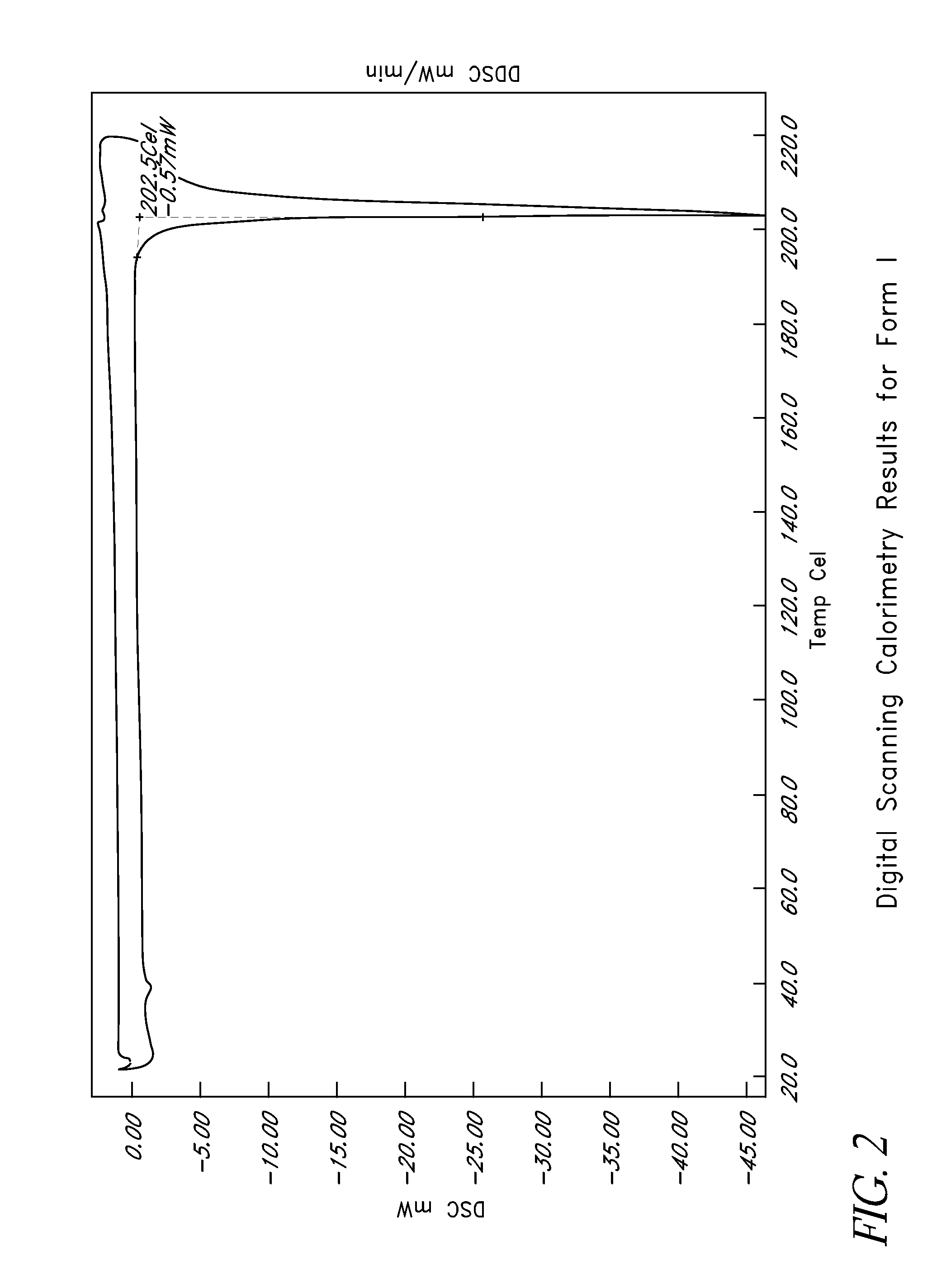
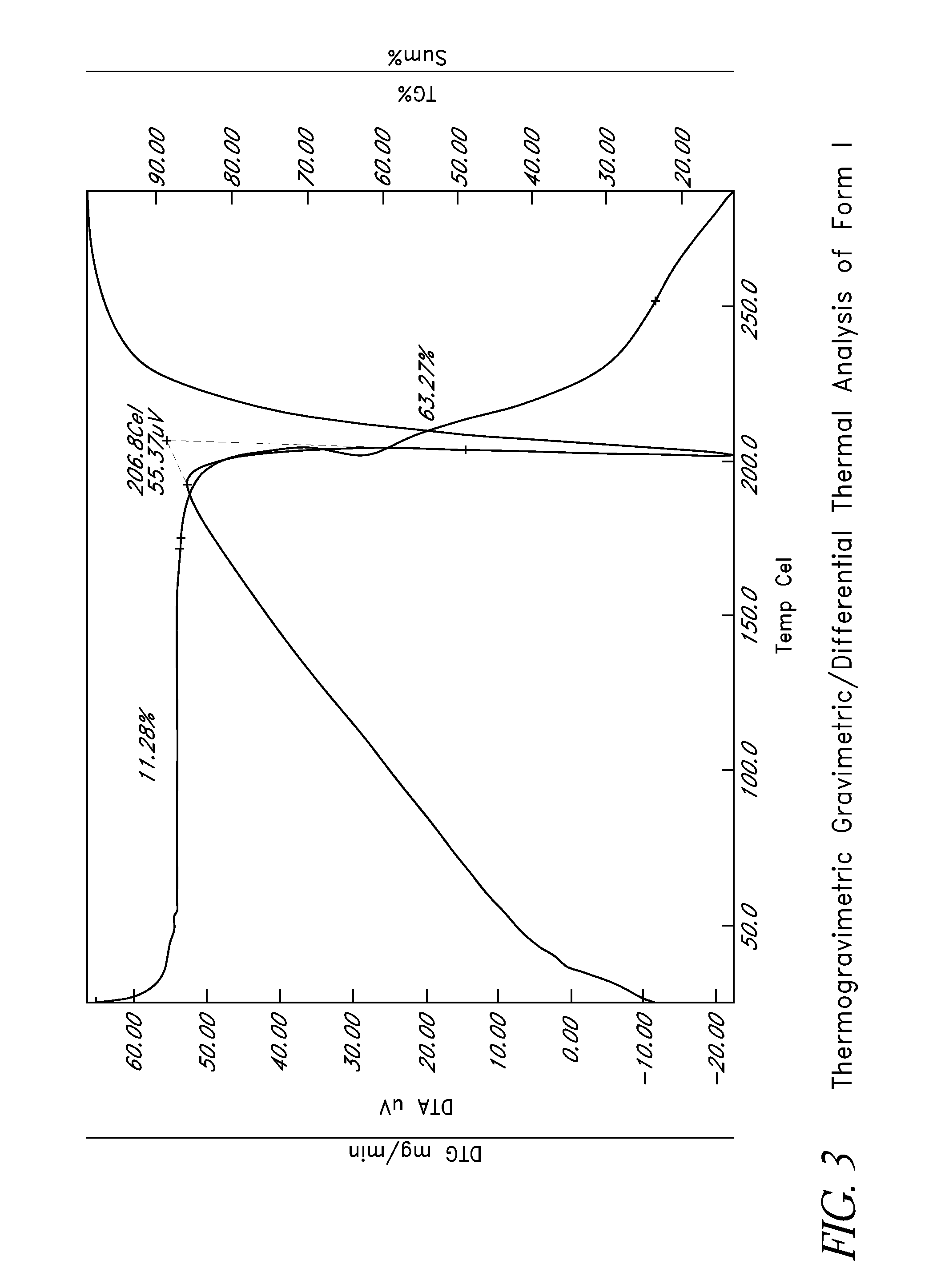
L-ornithine phenyl acetate and methods of making thereof
Disclosed herein are crystalline forms of L-ornithine phenyl acetate and methods of making the same. The crystalline form may, in some embodiments, be Forms I, II, III and V, or mixtures thereof. The crystalline forms may be formulated for treating subjects with liver disorders, such as hepatic encephalopathy. Accordingly, some embodiments include formulations and methods of administering L-ornithine phenyl acetate.
Owner:OCERA THERAPEUTICS INC
Compounds and methods for inhibiting nhe-mediated antiport in the treatment of disorders associated with fluid retention or salt overload and gastrointestinal tract disorders
ActiveUS20120263670A1Reduce interdialytic weight gainMore palatable dietBiocideSynthetic polymeric active ingredientsIntestinal tract diseasesDisease
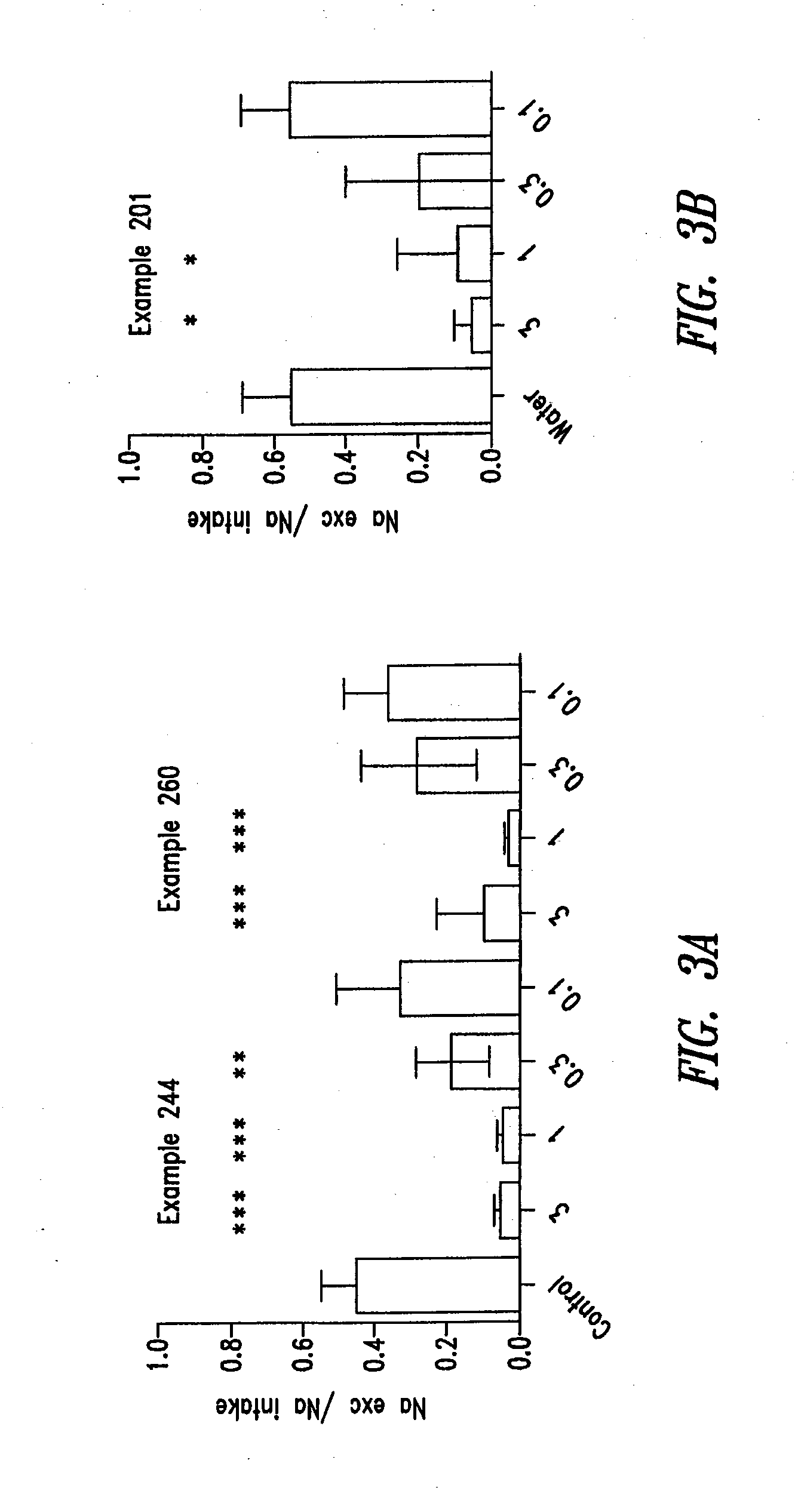
The present disclosure is directed to compounds and methods for the treatment of disorders associated with fluid retention or salt overload, such as heart failure (in particular, congestive heart failure), chronic kidney disease, end-stage renal disease, liver disease, and peroxisome proliferator-activated receptor (PPAR) gamma agonist-induced fluid retention. The present disclosure is also directed to compounds and methods for the treatment of hypertension. The present disclosure is also directed to compounds and methods for the treatment of gastrointestinal tract disorders, including the treatment or reduction of pain associated with gastrointestinal tract disorders.
Owner:ARDELYX
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090197824A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
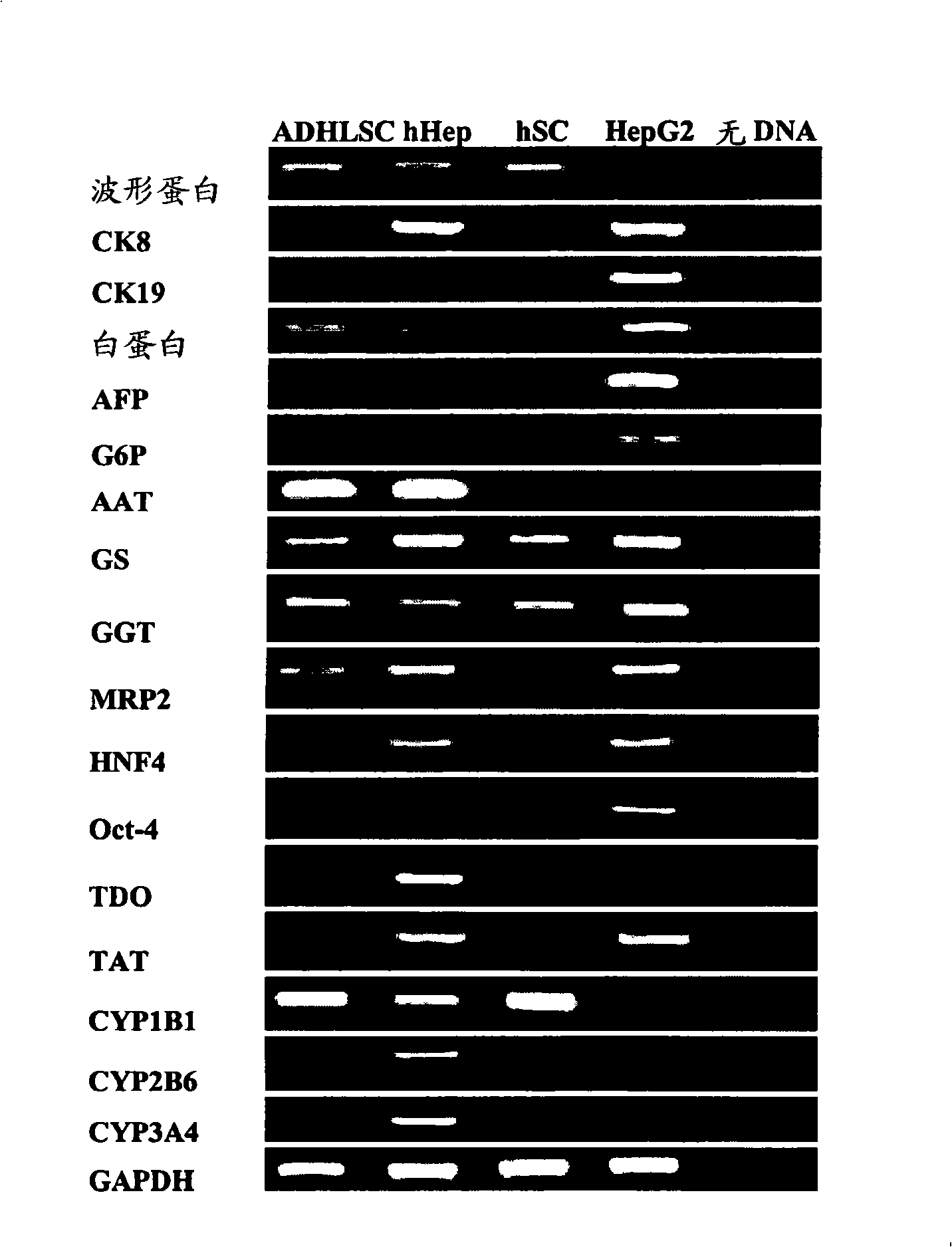
Isolated liver stem cells
The present invention relates to isolated liver progenitor stem cells, and cell population thereof, wherein said progenitor stem cells originate from adult liver, esp. of human. The present invention also relates to the use of said isolated progenitor stem cells in medicine, hepatology, inborn errors of liver metabolism, transplantation, infectious diseases, liver failure. The present invention also relates to methods of isolating these cells, their culture, characterization before and after differentiation, and their use for transplantation, animal models of human disease, toxicology and pharmacology.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN
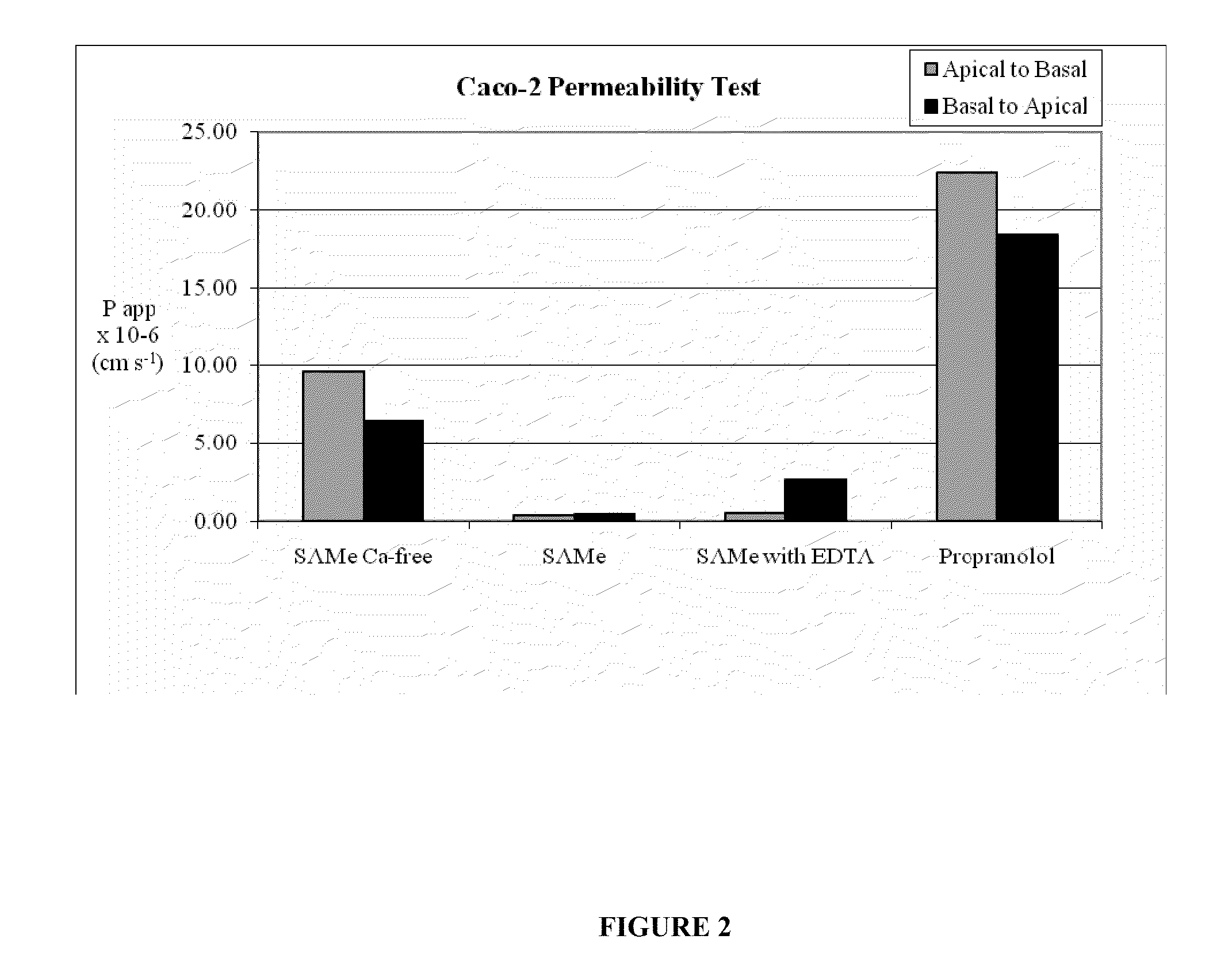
S-adenosylmethionine formulations with enhanced bioavailability
InactiveUS20110027342A1Reduce penetrationPromote absorptionBiocideNervous disorderImmunologic disordersS-Adenosyl-l-methionine
The invention relates to compositions and methods to enhance the absorption of S-adenosylmethionine (SAMe) and to methods of treating various disorders or diseases using non-parenteral SAMe formulations with enhanced-absorption and improved bioavailability. The enhanced bioavailability formulations may be used to treat a variety of diseases or disorders, such as for example, psychiatric disorders including, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, depressive disorders (e.g. major clinical depression) and dysthymia; as well as treating liver disorders, cancer, autoimmune disorders, inflammatory disorders, joint disorders, gastrointestinal disorders and cardiovascular disease.
Owner:MSI METHYLATION SCI
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as amino-transferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
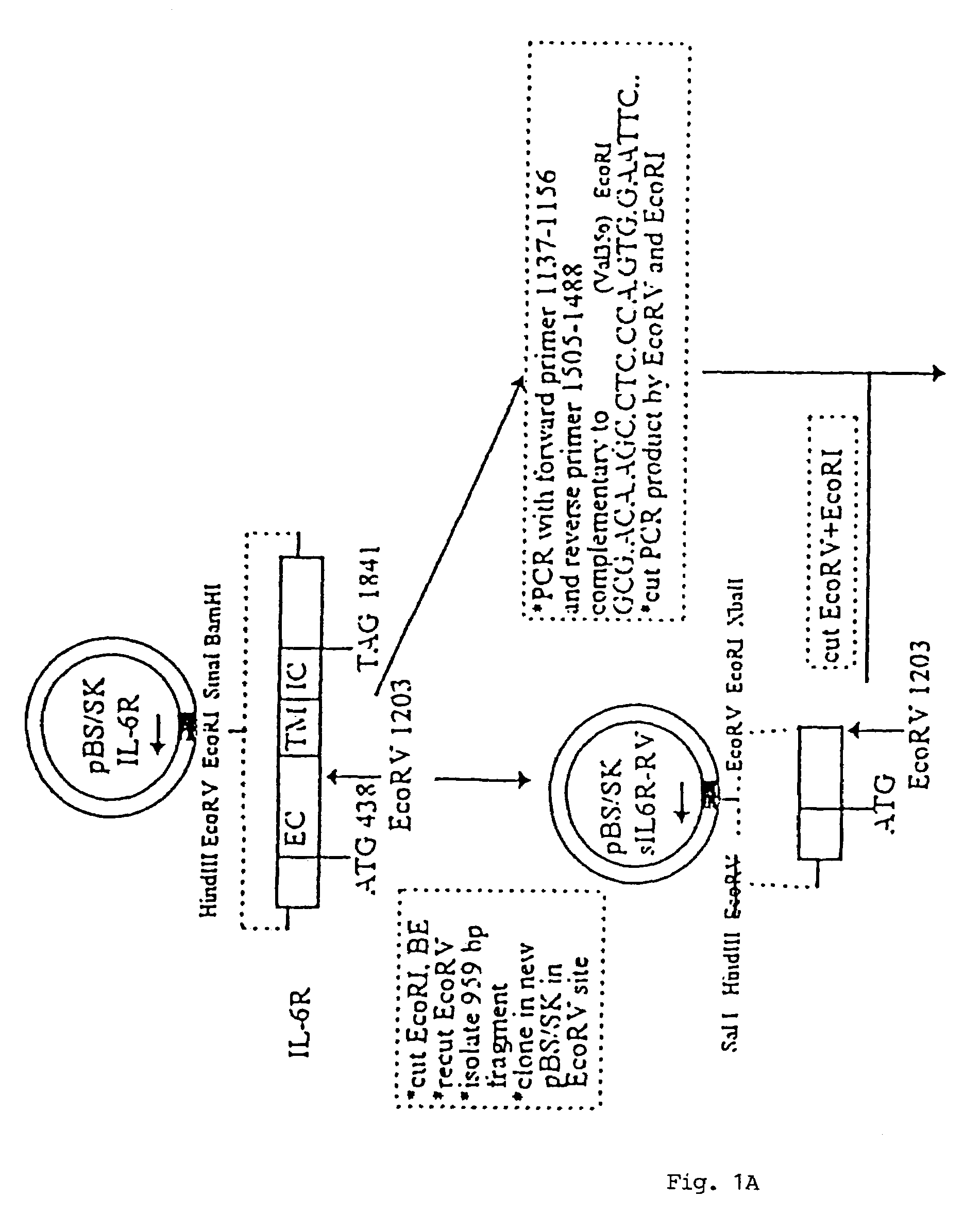
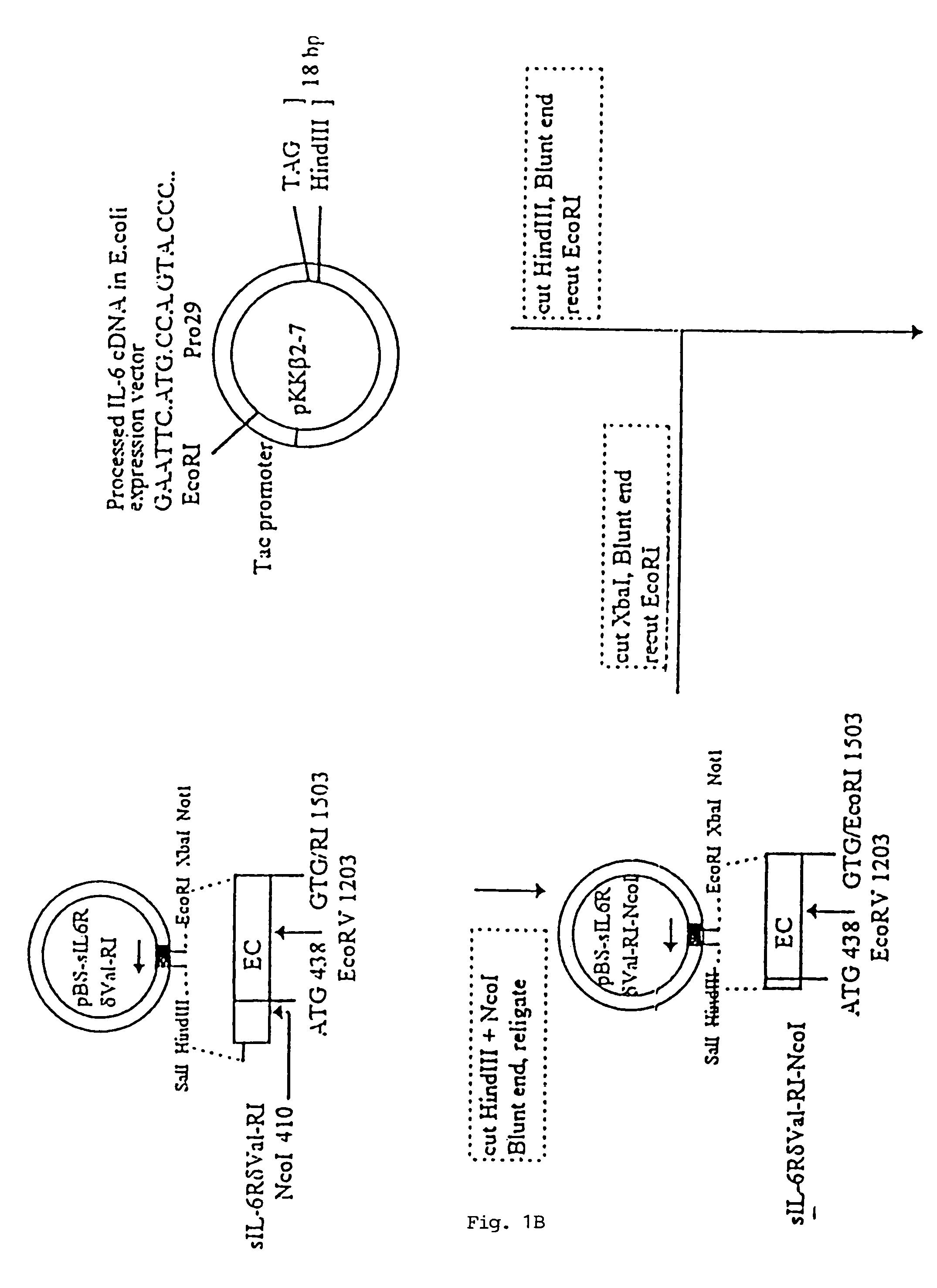
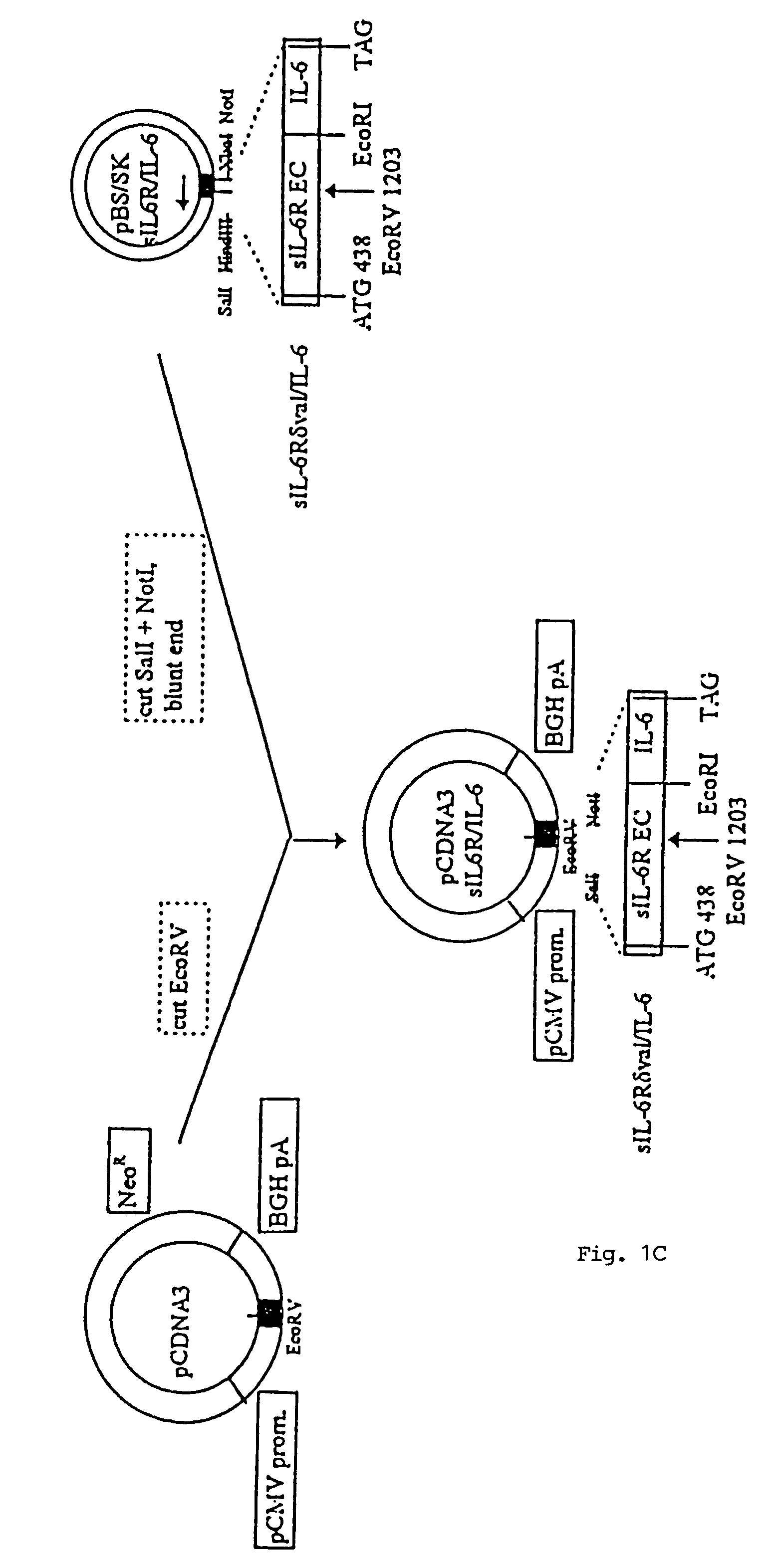
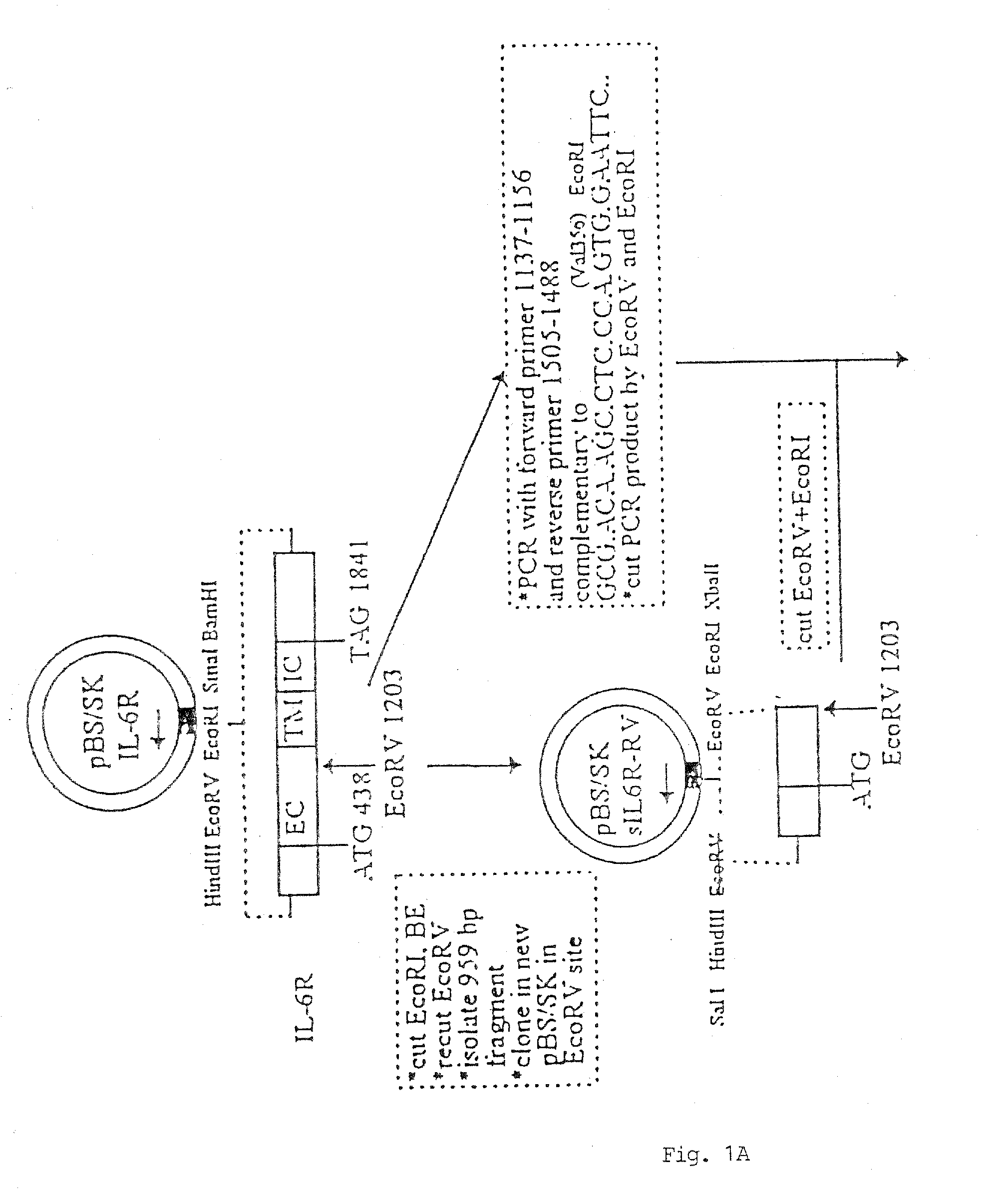
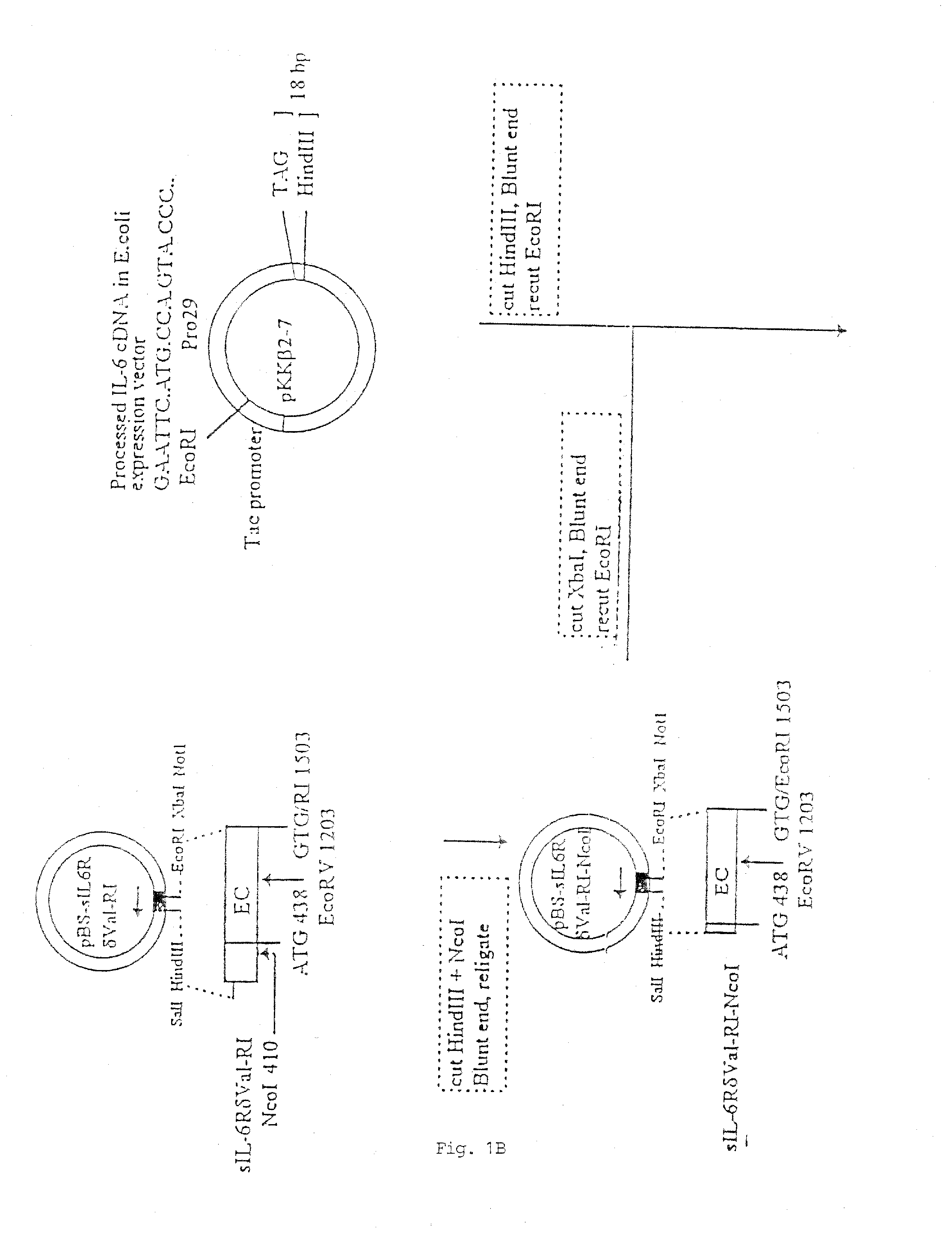
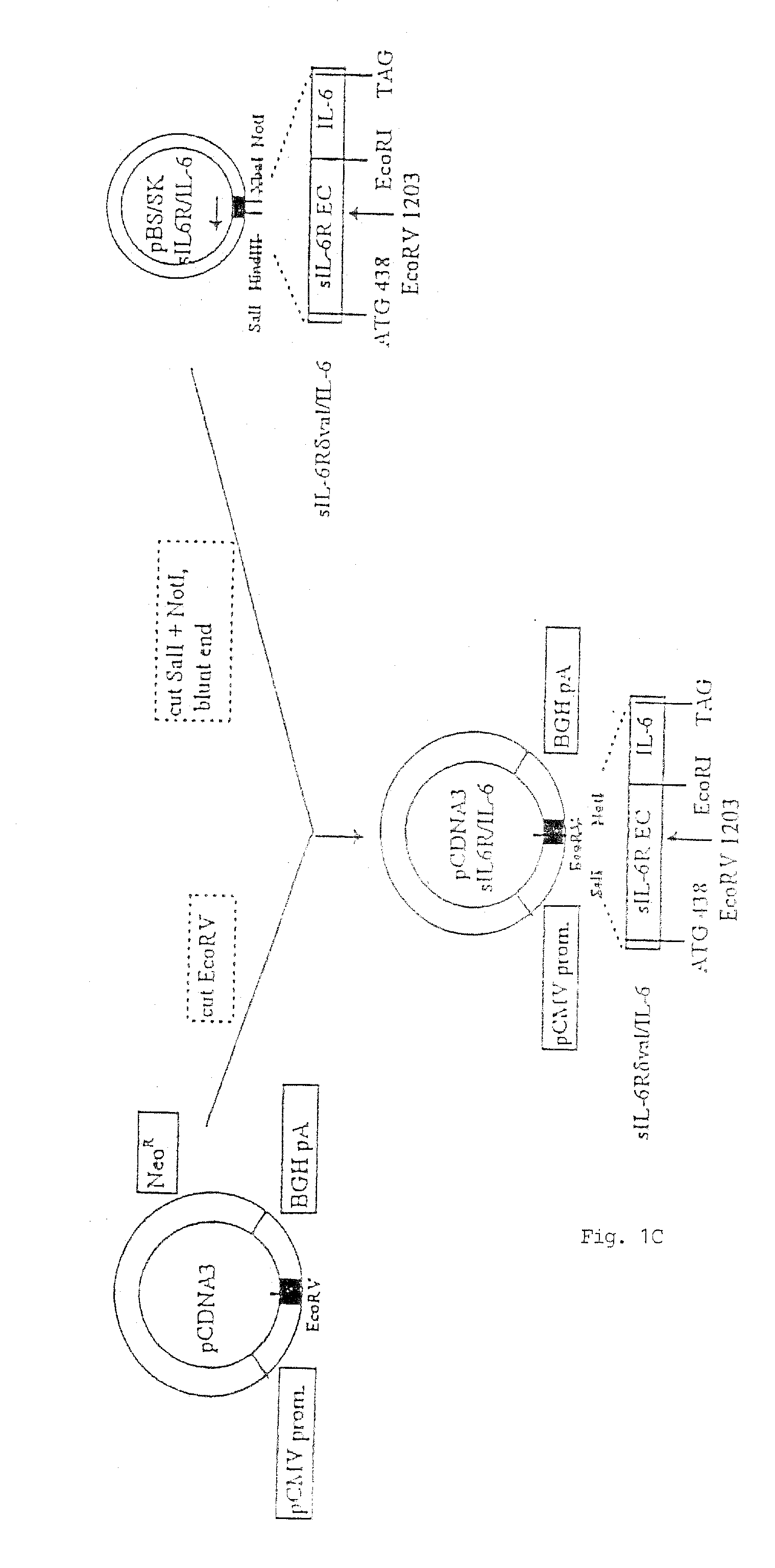
Chimeric interleukin-6 soluble receptor/ligand proteins
InactiveUS7198781B1Risk minimizationReduce potential immunogenicityNervous disorderPeptide/protein ingredientsDiseaseInterleukin 6
Chimeric proteins constructed from the fusion of the naturally occurring form of the soluble IL-6 receptor and IL-6 which are useful for treatment of cancer and liver disorders, enhancement of bone marrow transplantation, and treatment of other IL-6 related conditions are provided.
Owner:YEDA RES & DEV CO LTD
Gene up-regulated in regenerating liver
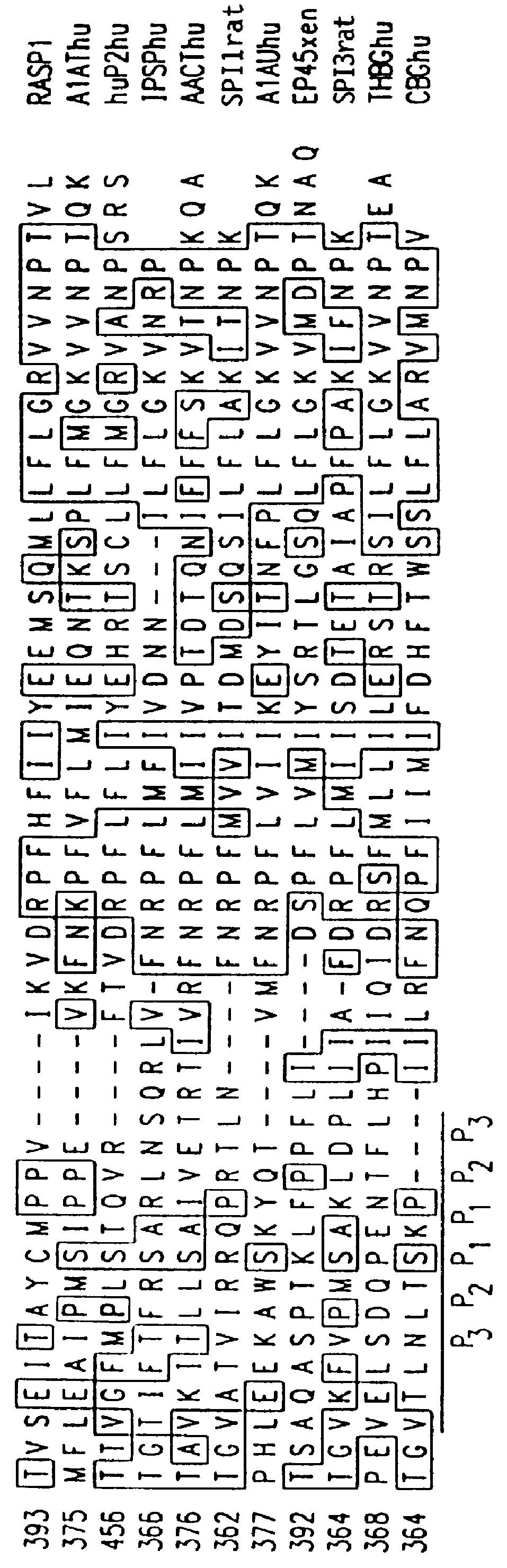
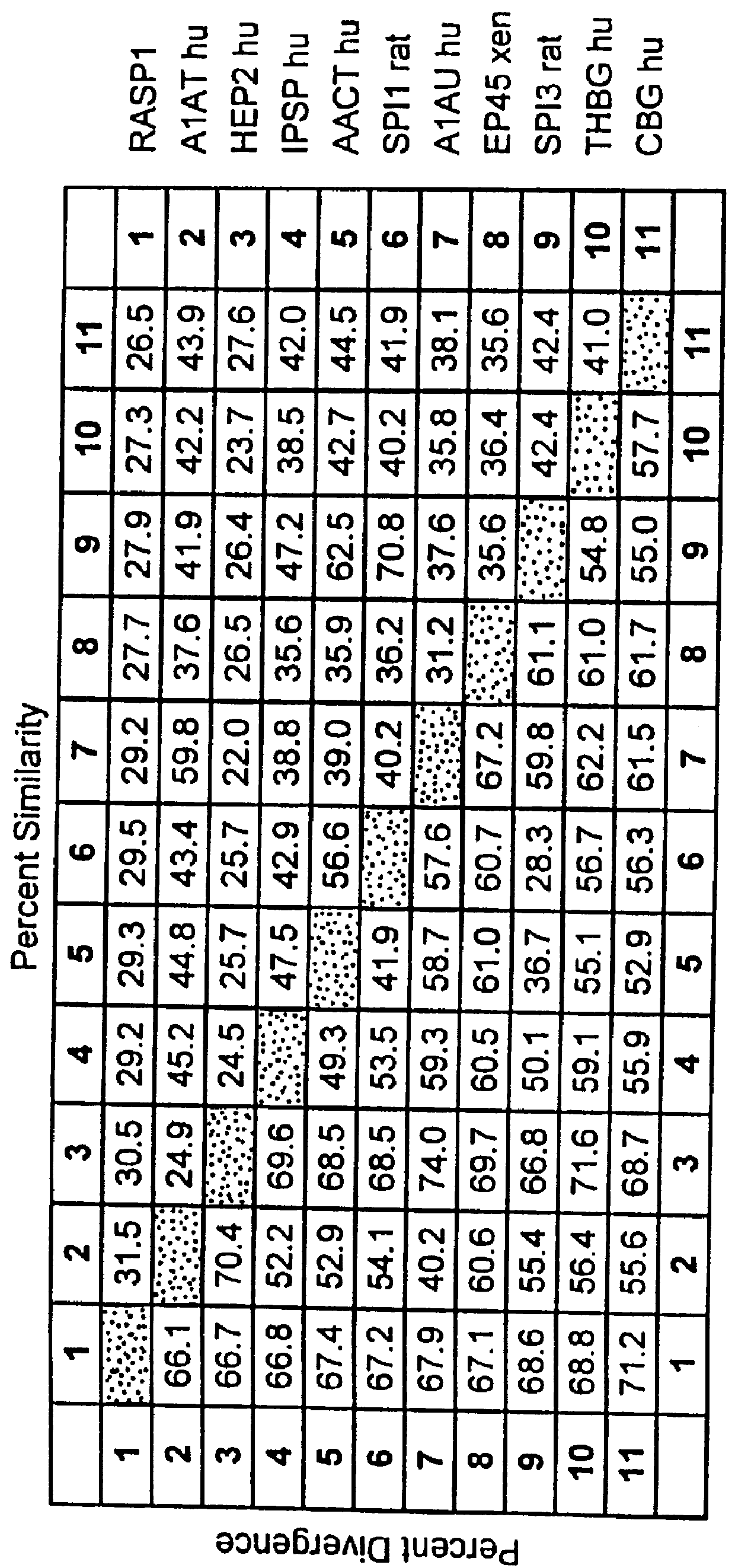
InactiveUS6027935AImprove scalabilityPromote regenerationBiocidePeptide/protein ingredientsRaspLiver disorder
The novel gene, Rasp-1, which is up-regulated in regenerating liver, is disclosed. The novel RASP-1 protein, which is encoded by the Rasp-1 gene, is also disclosed. In addition, antibodies against the Rasp-1 gene products are also disclosed. Furthermore, the use of Rasp-1 nucleic acid sequences, Rasp-1 gene products, and antibodies against Rasp-1 gene products in the treatment and diagnosis of liver disorders is also disclosed.
Owner:ADVANCED TISSUE SCIENCES INC +1
Chimeric Interleukin-6 Soluble Receptor/Ligand Protein, Analogs Thereof and Uses Thereof
InactiveUS20070172455A1Risk minimizationReduce potential immunogenicityNervous disorderPeptide/protein ingredientsDiseaseInterleukin 6
Owner:YEDA RES & DEV CO LTD
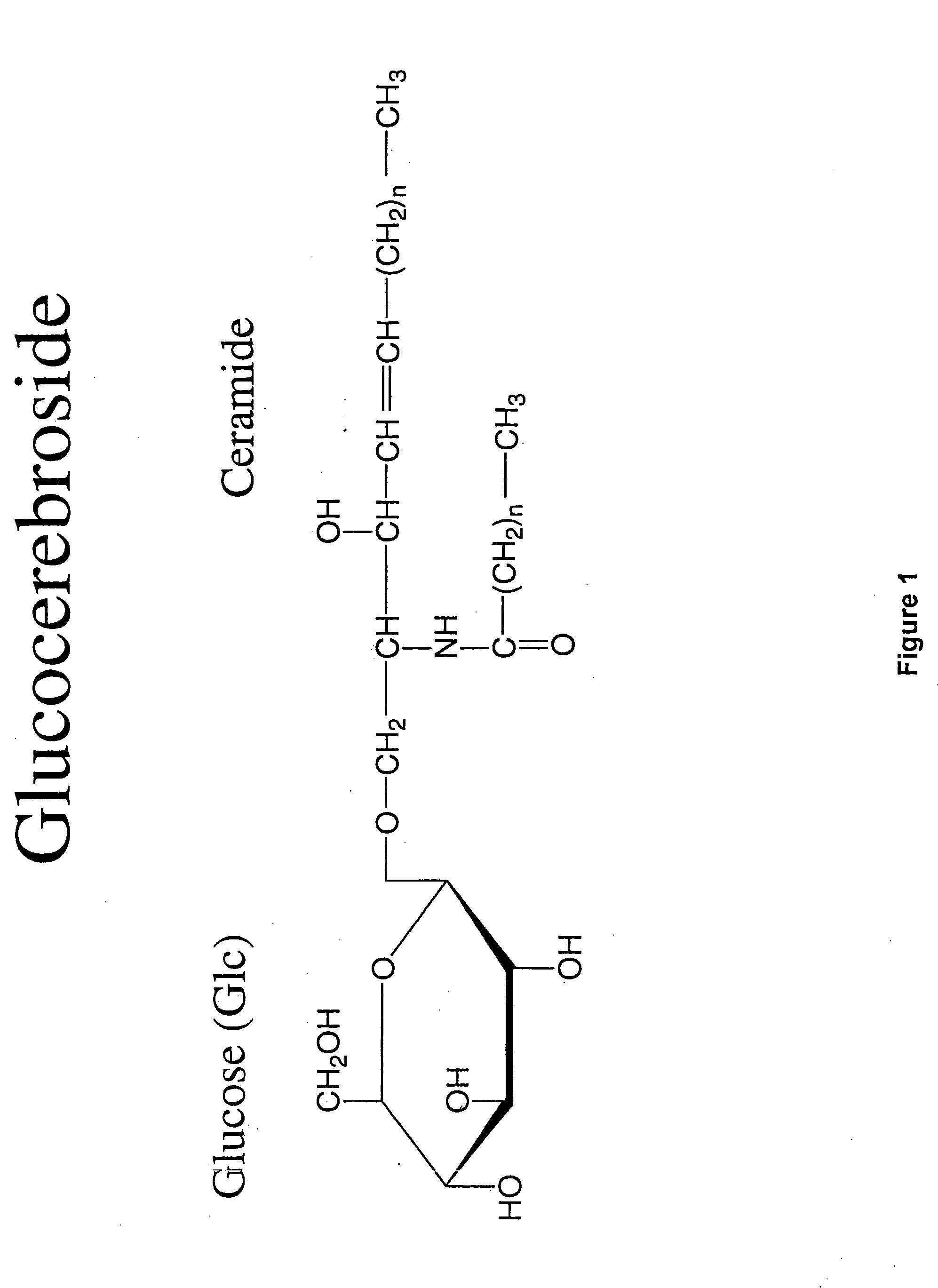
Glucocerebroside treatment of liver disorders
The present invention provides a method for the treatment of immune mediated or immune related diseases or disorders, infectious diseases, metabolic disorders and cancer in mammalian subjects. This method comprises the administration of a naturally occurring, mammalian intermediary metabolite or T cell receptor ligand, preferably a glucosylceramide, to a mammalian subject. In a preferred embodiment, such mammalian subjects are human beings.
Owner:ENZO THERAPEUTICS
Marker genes responding to treatment with toxins
InactiveUS20030165854A1Optimize treatment planEfficacy of treatmentSugar derivativesMicrobiological testing/measurementRat liverDisease
The present invention relates to a combination comprising a plurality of cDNAs which are differentially expressed in treated rat liver and in human liver and which may be used in their entirety or in part to diagnose, stage, or treat a liver disorder, to monitor diagnostic and therapeutic applications, to detect metabolic and toxicological responses, and to elucidate drug mechanism of action.
Owner:INCYTE
Primitive and proximal hepatic stem cells
InactiveUS20070099297A1Minimize formationExtensive growth potentialHepatocytesMammal material medical ingredientsProgenitorLiver disorder
Hepatic progenitors comprise two populations of human hepatic stem cells, primitive and proximal hepatic stem cells, and two populations of committed progenitors, one for biliary cells and one for hepatocytes. Human primitive hepatic stem cells are a very small fraction of the liver cell population and give rise to proximal hepatic stem cells constituting a much larger fraction of the liver. Human proximal hepatic stem cells give rise to biliary and hepatocyte committed progenitors. Primitive and proximal stem cells are the primary stem cells for the human liver. Human primitive hepatic stem cells may be isolated by immunoselection from human livers or culturing human liver cells under conditions which select for a human primitive hepatic stem cell. Proximal hepatic stem cells may be isolated by immunoselection, or by culturing human liver cells under conditions which include a developmental factor. Proximal hepatic stem cells may also be isolated by culturing colonies comprising a primitive hepatic stem cell under conditions which include a developmental factor. Resulting compositions may be used for treating liver disorders and for producing bioartificial organs.
Owner:VESTA THERAPEUTICS INC +1
Compounds and pharmaceutical compositions for the treatment of viral infections
Provided herein are compounds, compositions and methods for the treatment of liver disorder, including HCV and / or HBV infections. Specifically, compound and compositions of nucleoside derivatives are disclosed, which can be administered either alone or in combination with other anti-viral agents.
Owner:INDENIX PHARM LLC +2
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Primitive and proximal hepatic stem cells
Hepatic progenitors comprise two populations of human hepatic stem cells, primitive and proximal hepatic stem cells, and two populations of committed progenitors, one for biliary cells and one for hepatocytes. Human primitive hepatic stem cells are a very small fraction of the liver cell population and give rise to proximal hepatic stem cells constituting a much larger fraction of the liver. Human proximal hepatic stem cells give rise to biliary and hepatocyte committed progenitors. Primitive and proximal stem cells are the primary stem cells for the human liver. Human primitive hepatic stem cells may be isolated by immunoselection from human livers or culturing human liver cells under conditions which select for a human primitive hepatic stem cell. Proximal hepatic stem cells may be isolated by immunoselection, or by culturing human liver cells under conditions which include a developmental factor. Proximal hepatic stem cells may also be isolated by culturing colonies comprising a primitive hepatic stem cell under conditions which include a developmental factor. Resulting compositions may be used for treating liver disorders and for producing bioartificial organs.
Owner:VESTA THERAPEUTICS INC +1
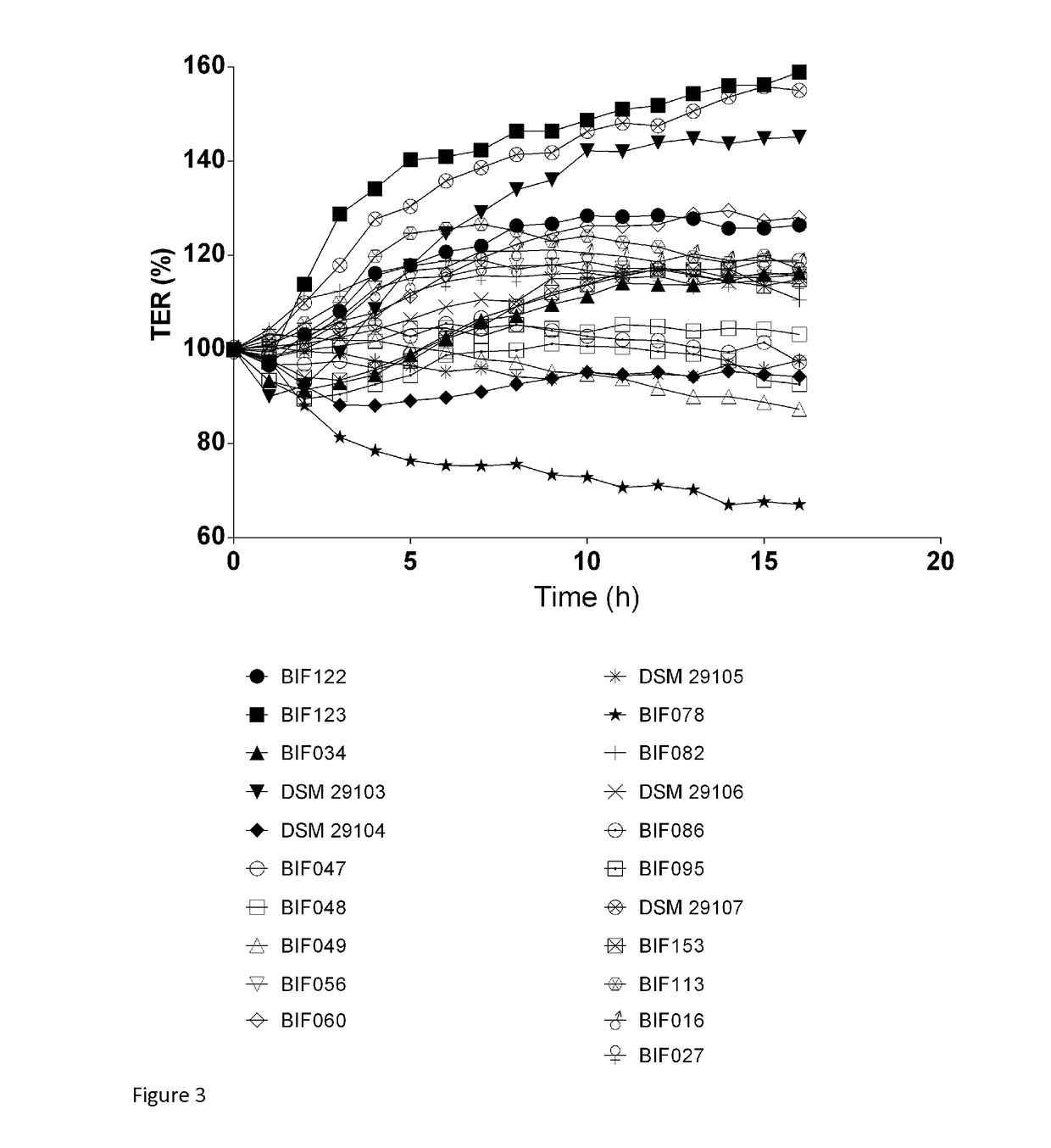
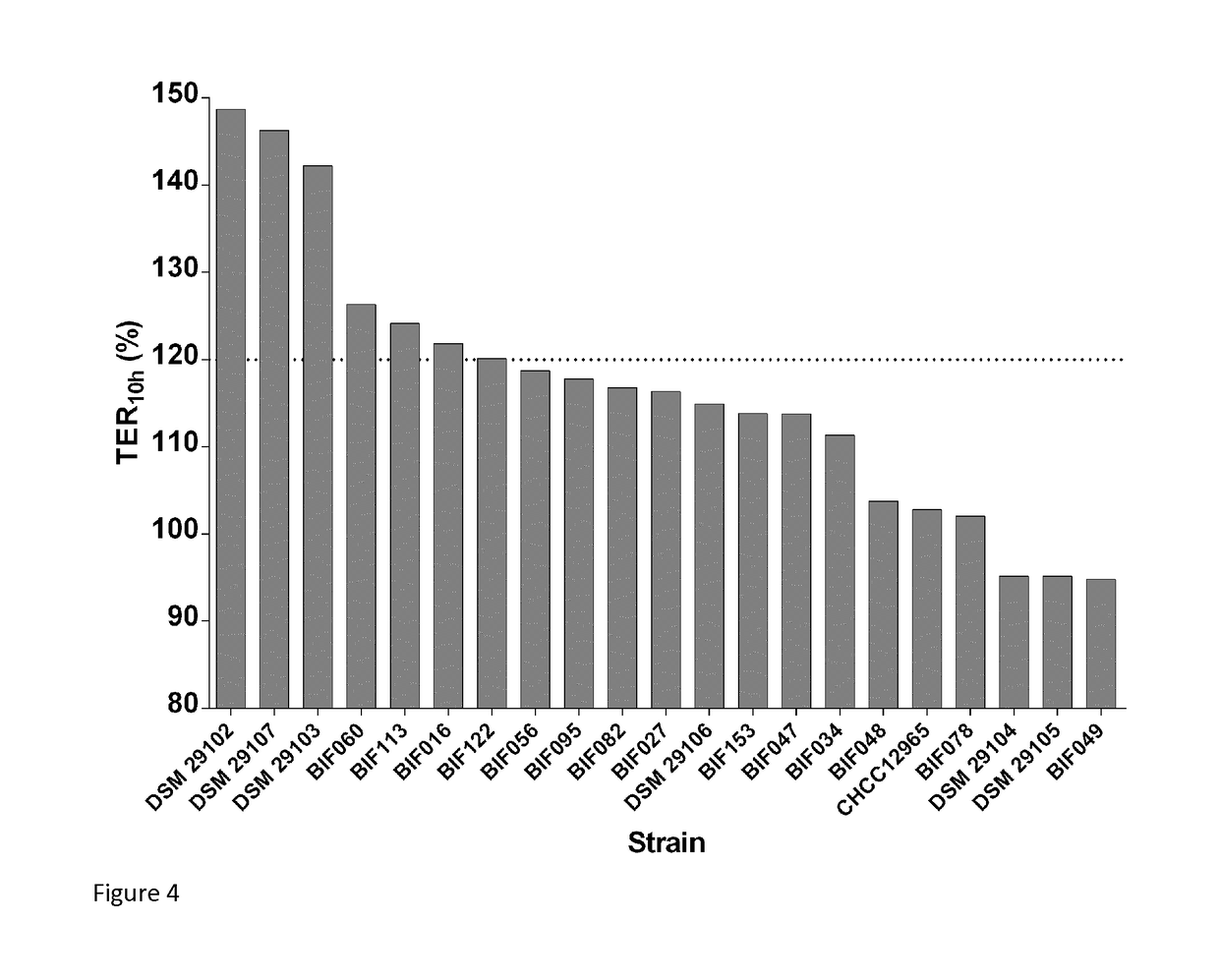
Probiotic bifidobacterium adolescentis strains
ActiveUS20170252382A1Inhibit disease severityAlleviated colitis symptomNervous disorderAntipyreticAutoimmune conditionDendritic cell
The present invention relates to novel isolated strains of Bifidobacterium adolescentis which are capable of i) increasing the trans-epithelial electrical resistance (TER) of a Caco-2 cell monolayer after 10 h treatment to more than 120% of TER at treatment start, ii) inducing secretion of >200 pg / ml of IL-10, and / or iii) inducing an IL-10:IL-12 ratio>1 when co-incubated with human PBMC derived dendritic cells. The strains may have one, two or all three of these capabilities. The present invention relates to the use of these novel strains for the in prevention, alleviation of symptoms, and treatment of diseases or conditions with an underlying impaired intestinal barrier function and pro-inflammatory activation of the mucosa. More specifically, the present invention relates to se of an isolated strain according to the invention for the prevention, alleviation of symptoms, or treatment of intestinal inflammatory conditions such as IBD and IBS, liver diseases such as NAFLD, NASH, cirrhosis, and alcohol-related liver disease, metabolic disorders such as metabolic syndrome, insulin resistance, type 2 diabetes, obesity, cardiovascular atherosclerosis, autoimmune diseases, such as celiac disease, type 1 diabetes, multiple sclerosis and rheumatoid arthritis, and mental conditions such as major depressive disorders, a mood disorder, a cognitive chronic fatigue syndrome, and anxiety.
Owner:CHR HANSEN AS
Homing in mesenchymal stem cells
InactiveUS20110165128A1Improve abilitiesImprove adhesionBiocideMammal material medical ingredientsCXCR4Liver disorder
The present invention relates to expression of CXCR4 in mesenchymal stem cells (MSCs) and homing of MSCs to sites of injury. In particular, the invention provides expanded cultures of MSCs which maintain cell surface expression of CXCR4. The MSCs are capable of homing to sites of injury and are suitable for treatment of ischemic disorders, including cardiac disorders, bone and cartilage disorders, liver disorders, inflammatory disorders, and stroke.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
L-ornithine phenyl acetate and methods of making thereof
ActiveCN102421432AOrganic active ingredientsOrganic compound preparationPhenyl acetic acidOrnithine phenylacetate
Disclosed herein are crystalline forms of L-ornithine phenyl acetate and methods of making the same. The crystalline form may, in some embodiments, be Forms I, II, III and V, or mixtures thereof. The crystalline forms may be formulated for treating subjects with liver disorders, such as hepatic encephalopathy. Accordingly, some embodiments include formulations and methods of administering L-ornithine phenyl acetate.
Owner:OCERA THERAPEUTICS INC
Diagnostic methods for liver disorders
InactiveUS20130085075A1Efficacy of treatmentImprove liver functionPeptide librariesMicrobiological testing/measurementRegimenLiver disorder
The present invention relates to methods of diagnosing a liver disorder in a patient, as well as methods of monitoring the progression of a liver disorder and / or methods of monitoring a treatment protocol of a therapeutic agent or regimen. The invention also relates to assay kits used in connection with the diagnostic methods described herein.
Owner:MESO SCALE TECH LLC
Compounds and pharmaceutical compositions for the treatment of viral infections
Provided herein are compounds, compositions and methods for the treatment of liver disorders, including HCV infections. In one embodiment, compounds and compositions of nucleoside derivatives are disclosed, which can be administered either alone or in combination with other anti-viral agents.
Owner:INDENIX PHARM LLC +1
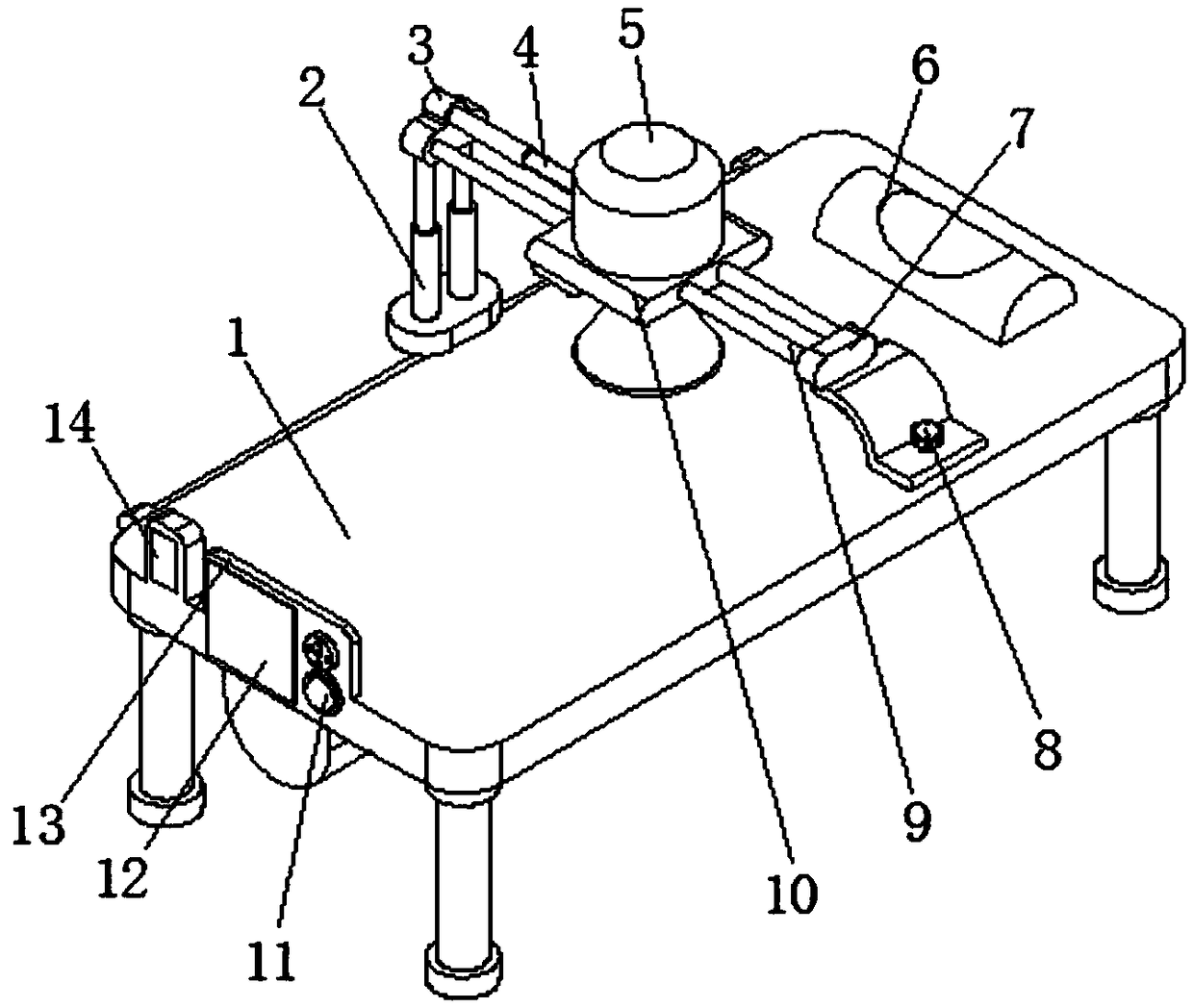
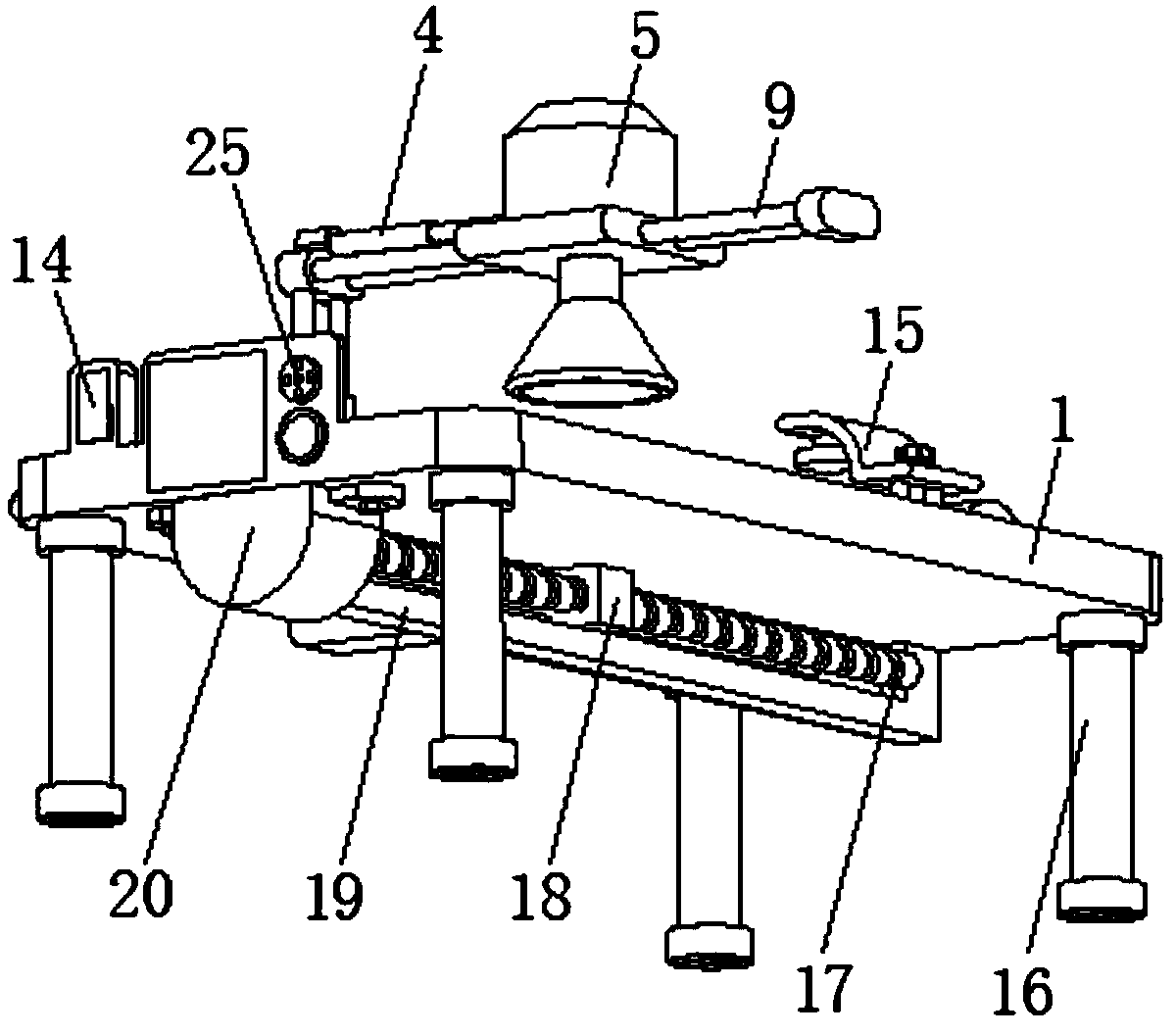
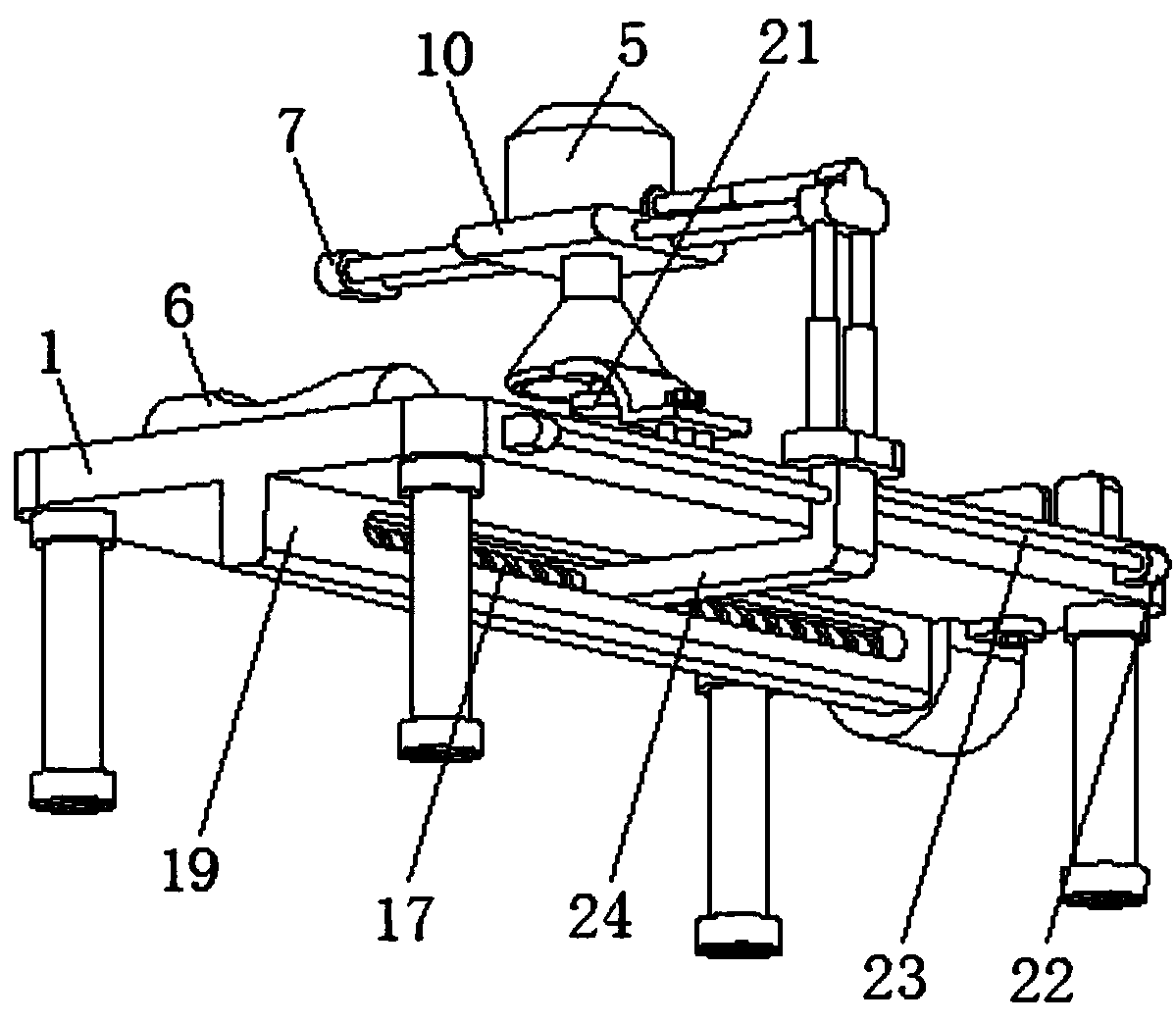
Hepatopathy infectious disease therapeutic instrument support device
The invention discloses a hepatopathy infectious disease therapeutic instrument support device comprising a bed body, wherein a middle part of a lower side of the bed body is provided with a slide rail, an internal slide groove in the slide rail is connected with a leading screw via a bearing, and one side of a slide block is connected with a connecting rod; one end, positioned far away from the slide block, of the connecting rod is connected with two groups of first electric telescoping rods; a middle part of one side, positioned close to the bed body, of the connecting rod is provided with aslide shaft; one side, positioned close to the bed body, of a connecting boss is connected with one end of each of two groups of slide rods; a middle part of each slide rod is provided with a slide plate; a middle part of each slide plate is provided with an infrared hepatopathy therapeutic instrument, an upper side of the bed body is provided with a neck pillow, vertical distance between and relative positions of the infrared hepatopathy therapeutic instrument and a patient can be adjusted rapidly and conveniently via a first electric telescoping rod and a second electric telescoping rod, the support device is simple and convenient to use, comfort of use can be realized for the patient, and medical workers can be called and alerted via an alarm lamp and a buzzer.
Owner:张波
Use of 1,3-diphenylprop-2-en-1-one derivatives for treating liver disorders
The invention provides 1,3-diphenylprop-2-en-1-one derivatives and pharmaceutical compositions comprising the same for treating liver disorders, in particular those requiring the reduction of plasma level of biochemical markers such as aminotransferases. The 1,3-diphenylprop-2-en-1-one derivatives of General Formula (I) have hepatoprotective properties and can be used in methods for treating liver disorders involving the pathological disruption, inflammation, degeneration, and / or proliferation of liver cells, such as liver fibrosis or fatty liver disease.
Owner:GENFIT SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com