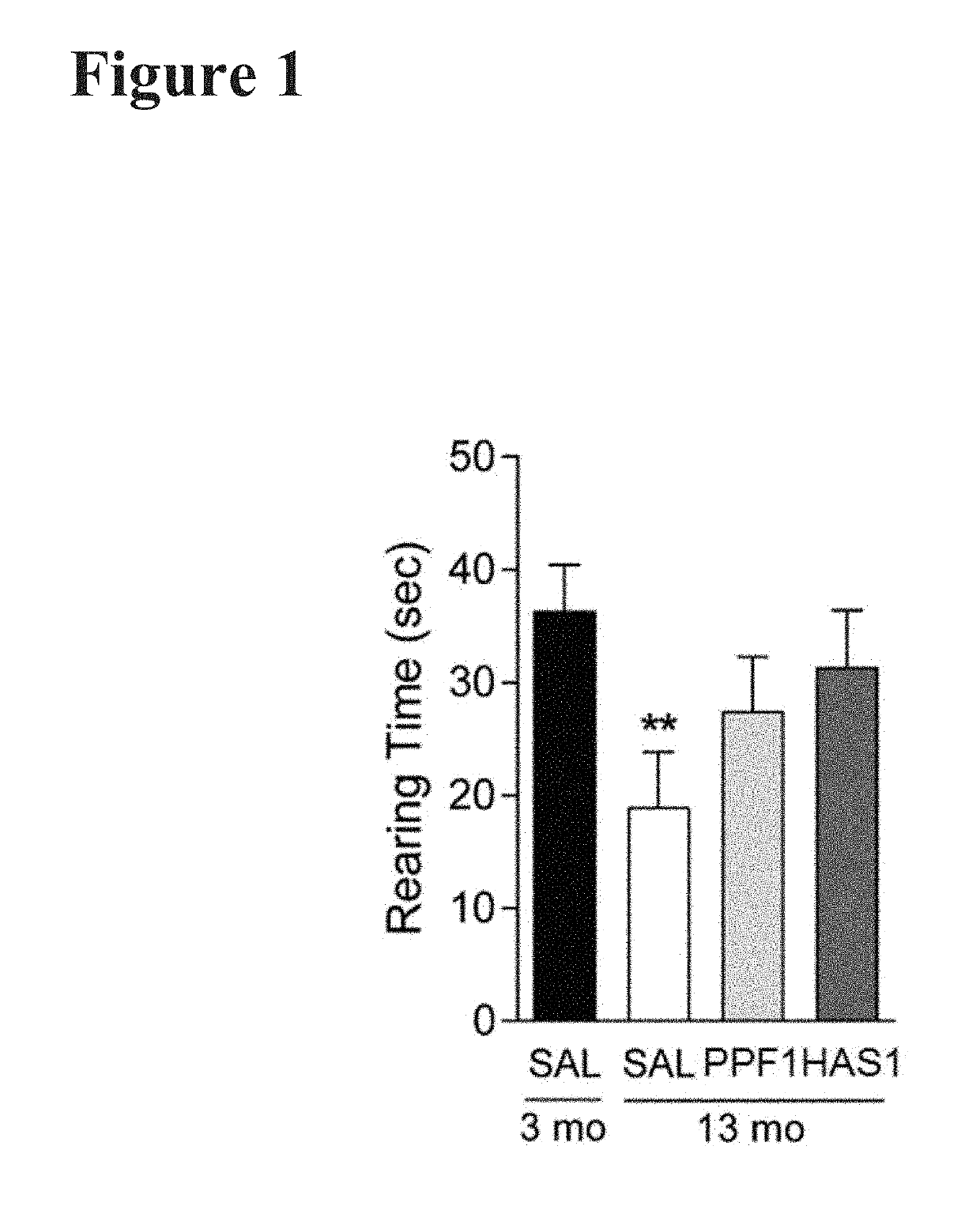
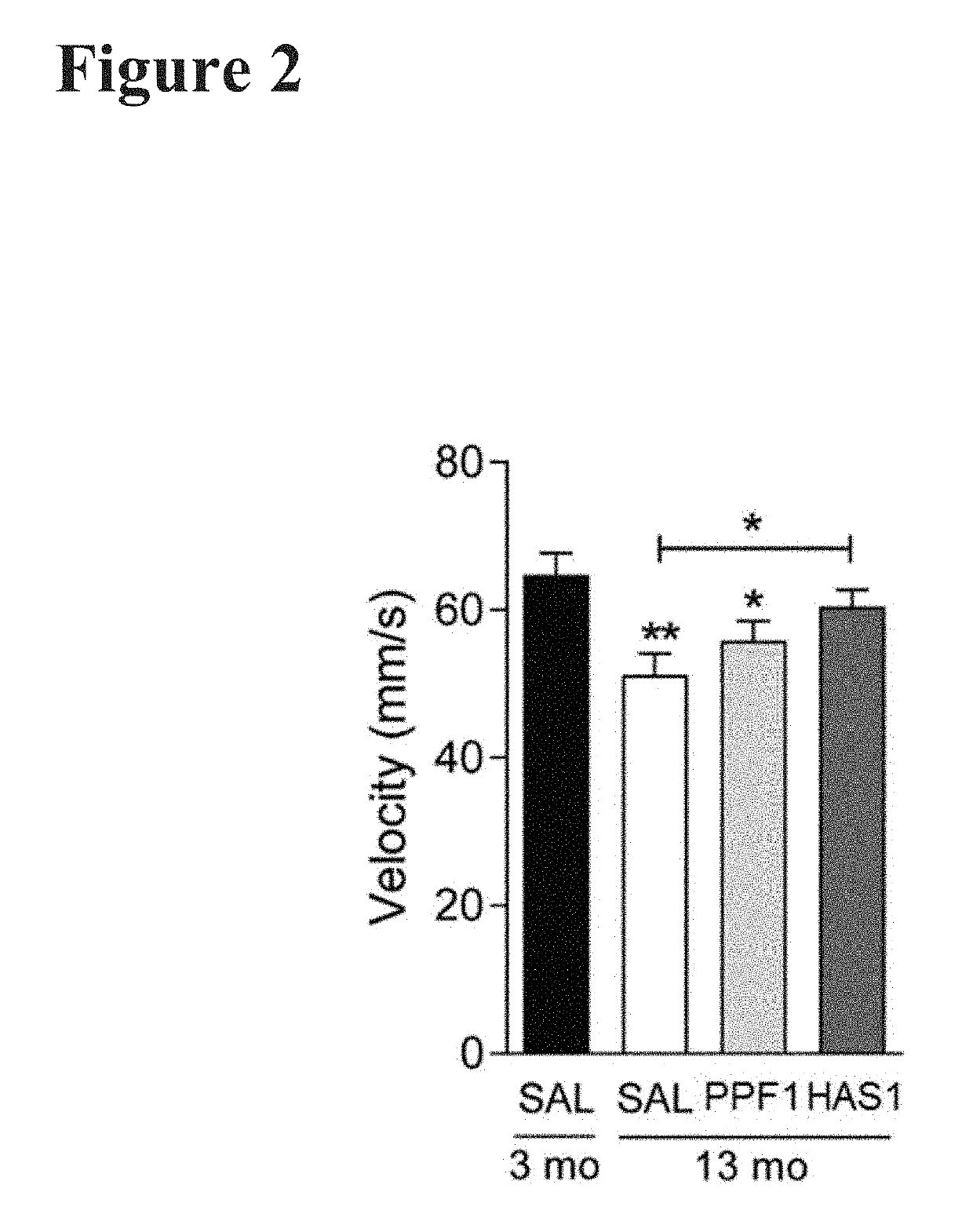
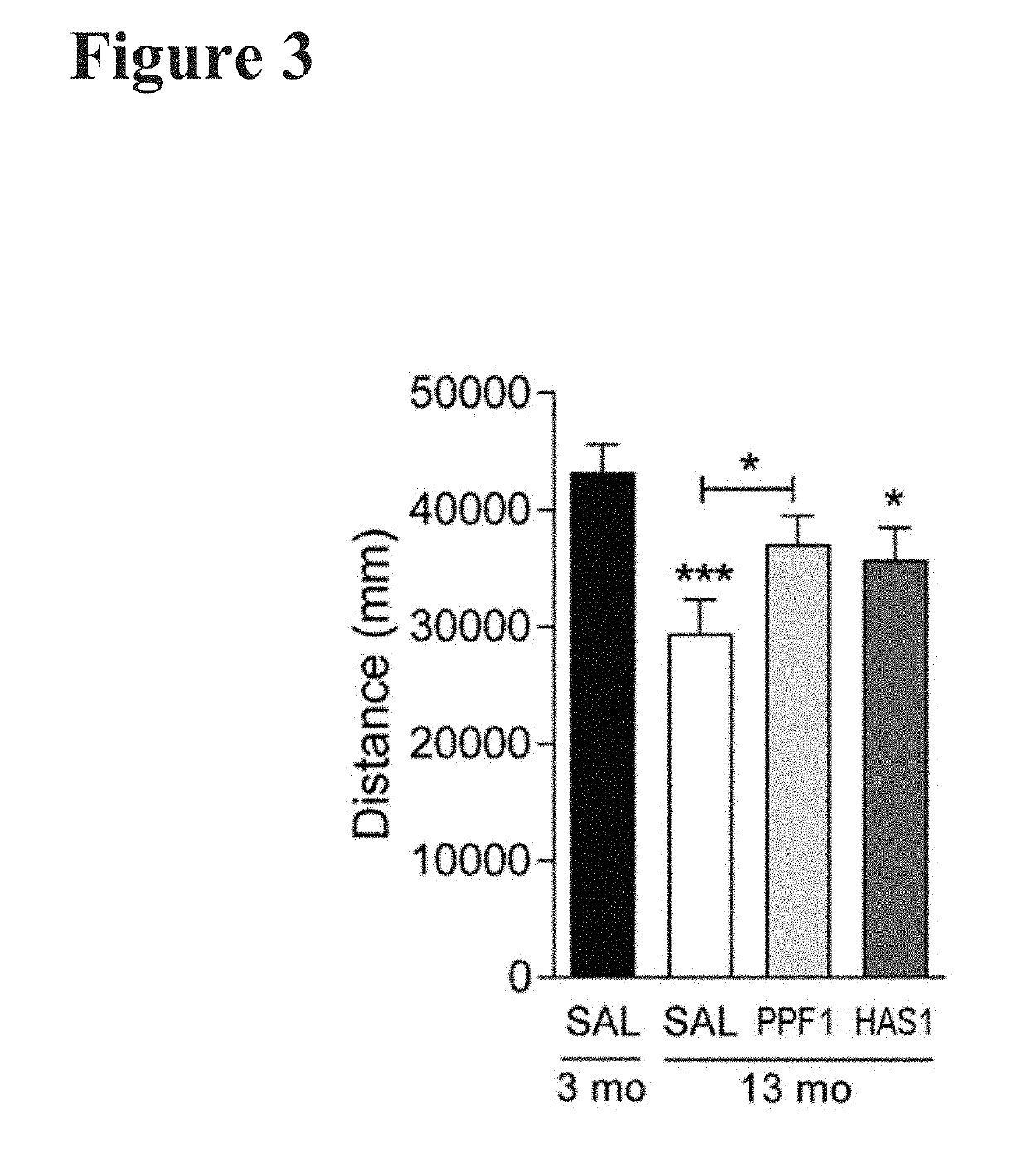
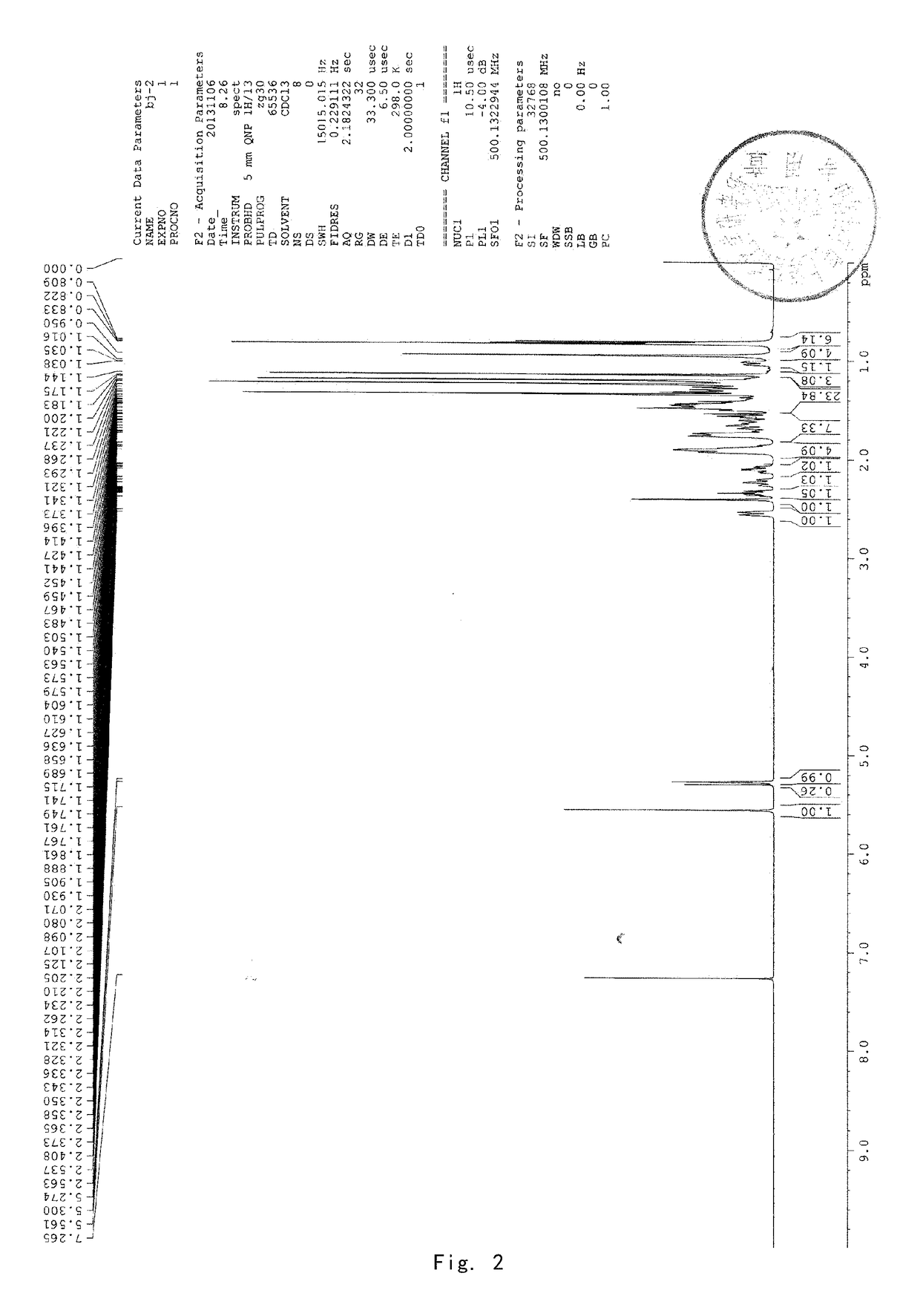
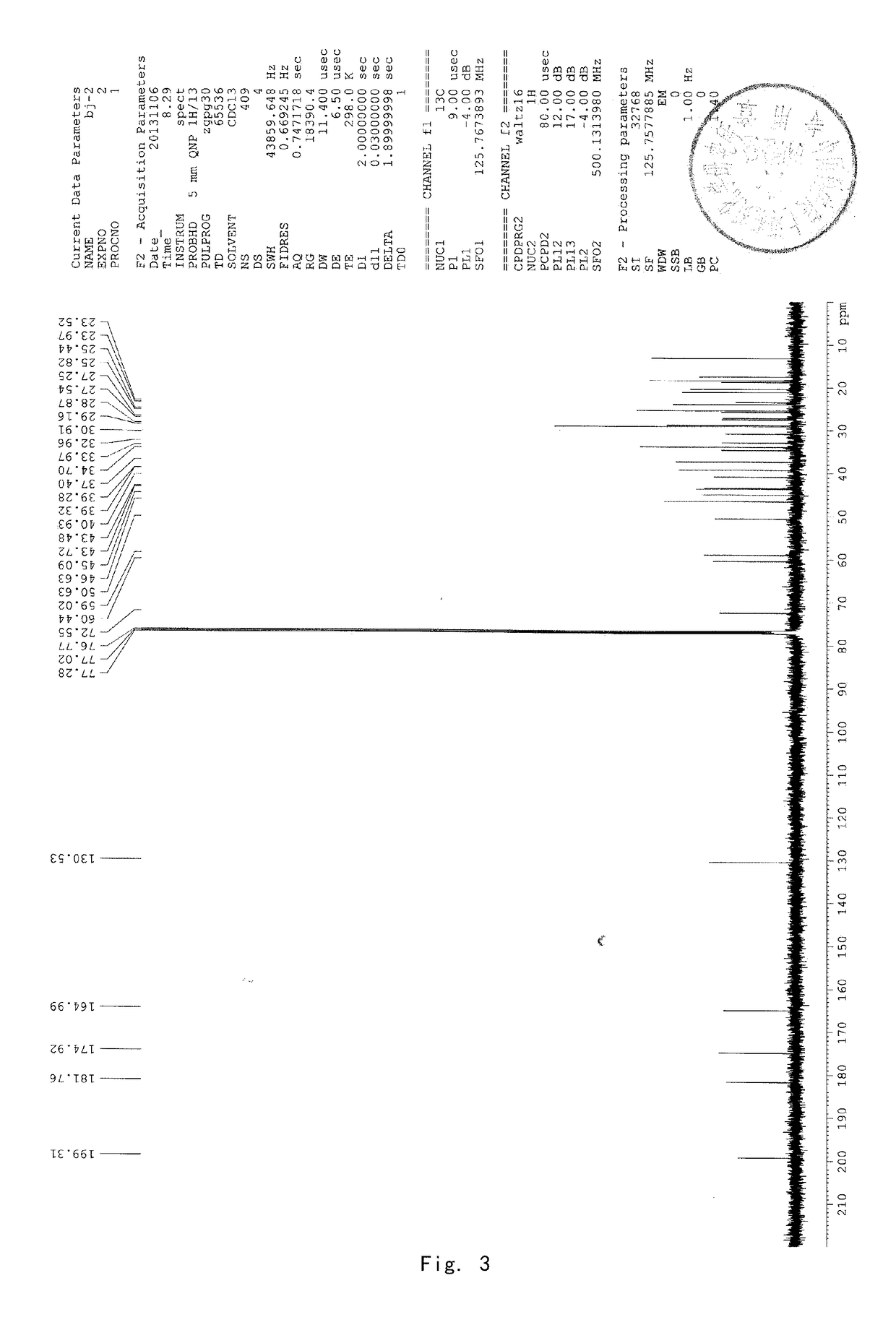
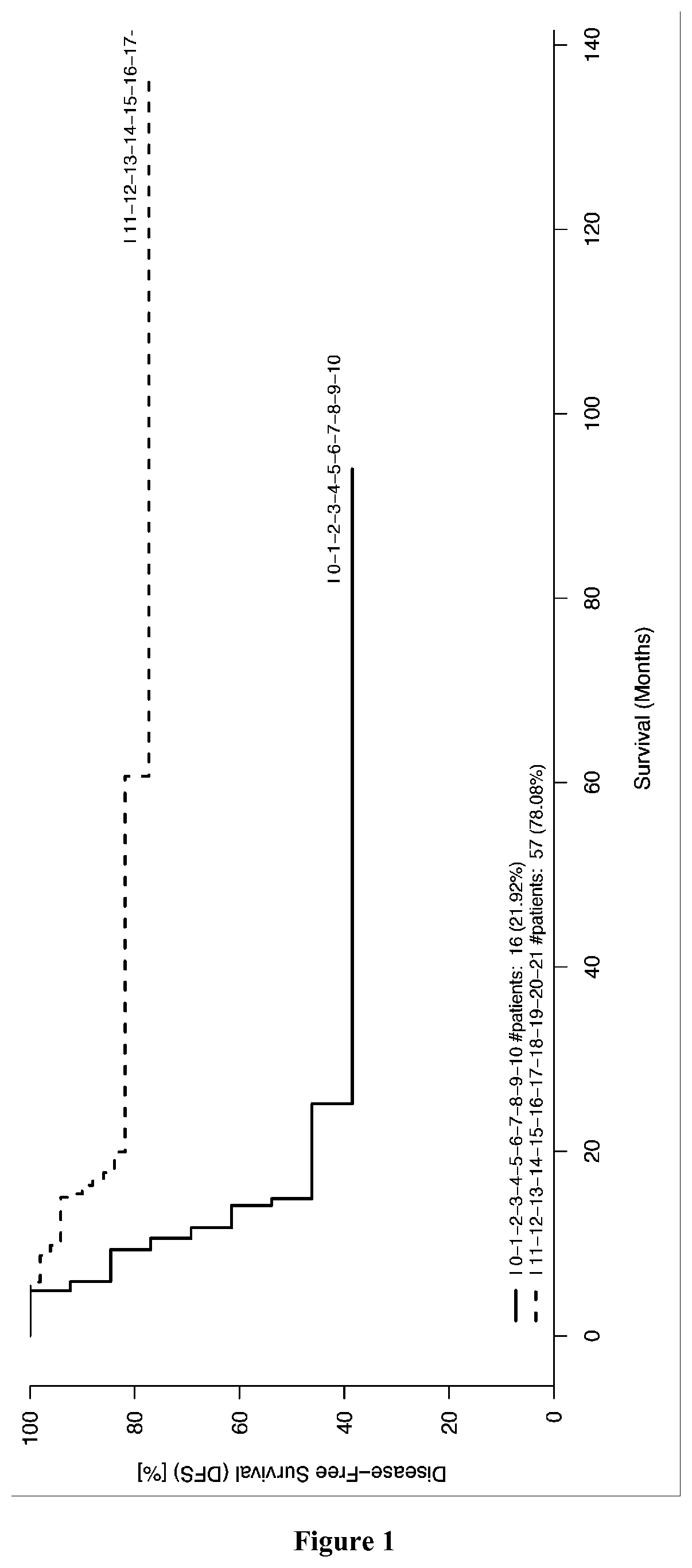
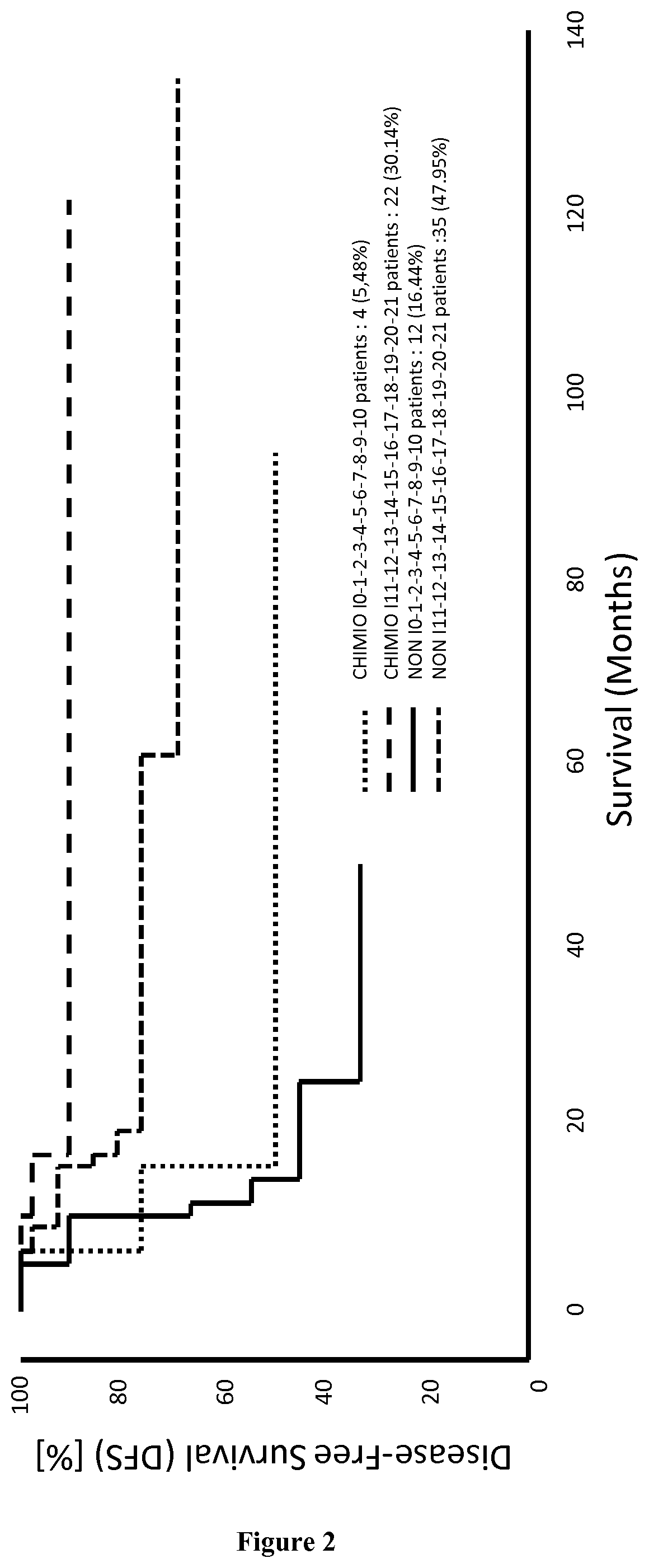
Patents
Literature
58results about How to "Efficacy of treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Two biomarkers for diagnosis and monitoring of atherosclerotic cardiovascular disease
The present invention identifies two circulating proteins that have been newly identified as being differentially expressed in atherosclerosis. Circulating levels of these two proteins, particularly as a panel of proteins, can discriminate patients with acute myocardial infarction from those with stable exertional angina and from those with no history of atherosclerotic cardiovascular disease. Such levels can also predict cardiovascular events, determine the effectiveness of therapy, stage disease, and the like. For example, these markers are useful as surrogate biomarkers of clinical events needed for development of vascular specific pharmaceutical agents.
Owner:AVIIR +1
Compositions and methods for treatment of cancer
Compositions and methods for treatment of conditions related to the overexpression of EZH2, such as late stage prostate cancer, using a DNA methylation inhibitor and / or a histone deacetylase inhibitor, optionally in combination with an EZH2 antagonist and / or an antineoplastic agent, to specifically target diseases associated with EZH2 over-expression. Further provided are reagents and kits for treatment of EZH2 overexpression.
Owner:SUPERGEN
Methods and compositions for diagnosis and monitoring of atherosclerotic cardiovascular disease
InactiveUS20070099239A1Efficacy of treatmentBiostatisticsDisease diagnosisAtherosclerotic cardiovascular diseaseExertional angina
The present invention identifies circulating proteins that are differentially expressed in atherosclerosis. Circulating levels of these proteins, particularly as a panel of proteins, can discriminate patients with acute myocardial infarction from those with stable exertional angina and from those with no history of atherosclerotic cardiovascular disease. Such levels can also predict cardiovascular events, determine the effectiveness of therapy, stage disease, and the like. For example, these markers are useful as surrogate biomarkers of clinical events needed for development of vascular specific pharmaceutical agents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
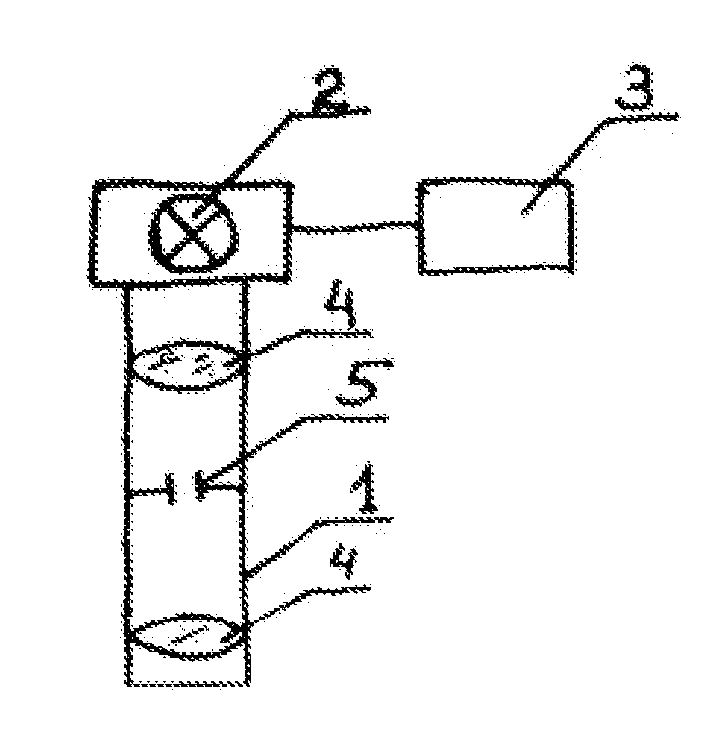
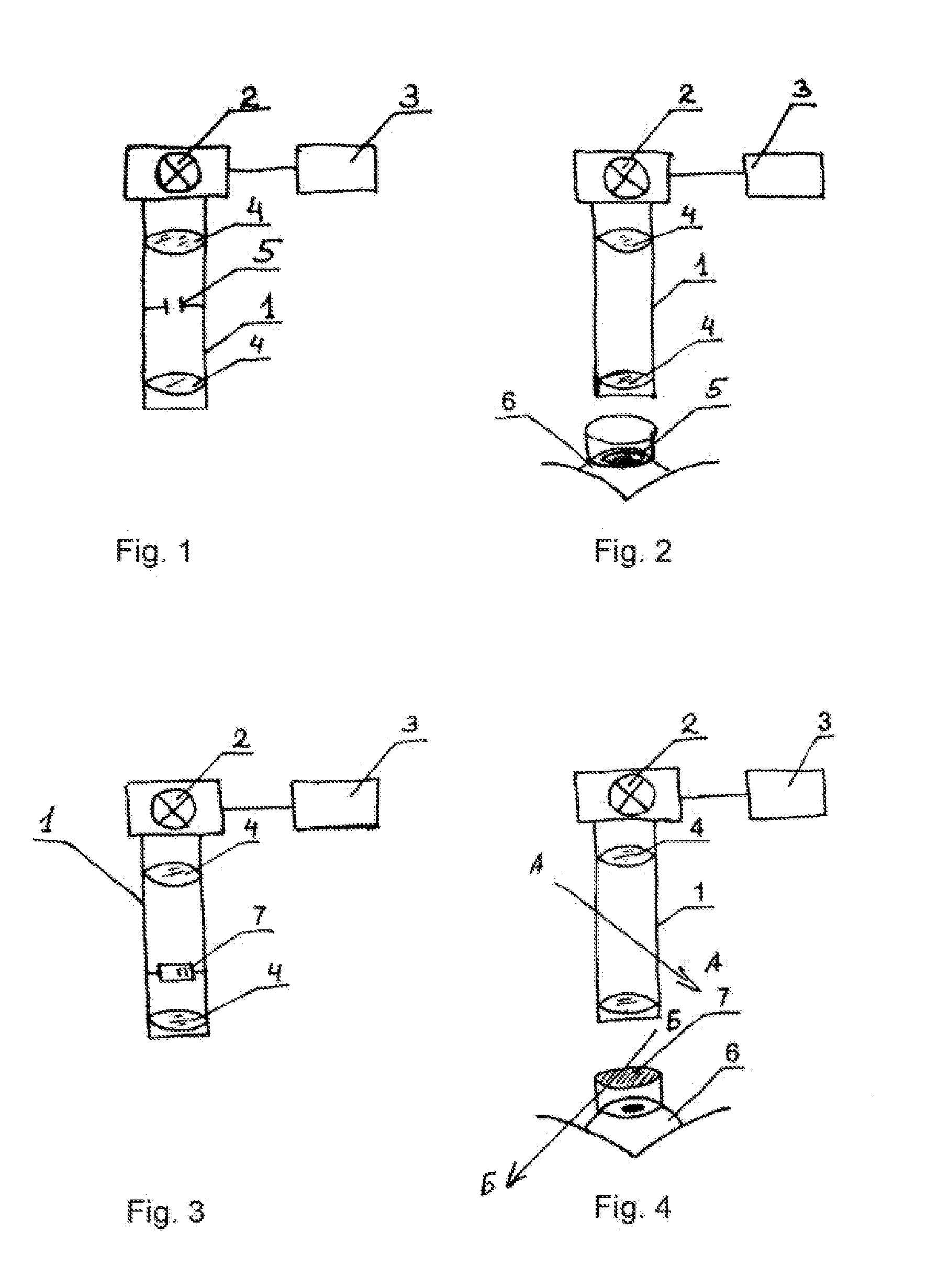
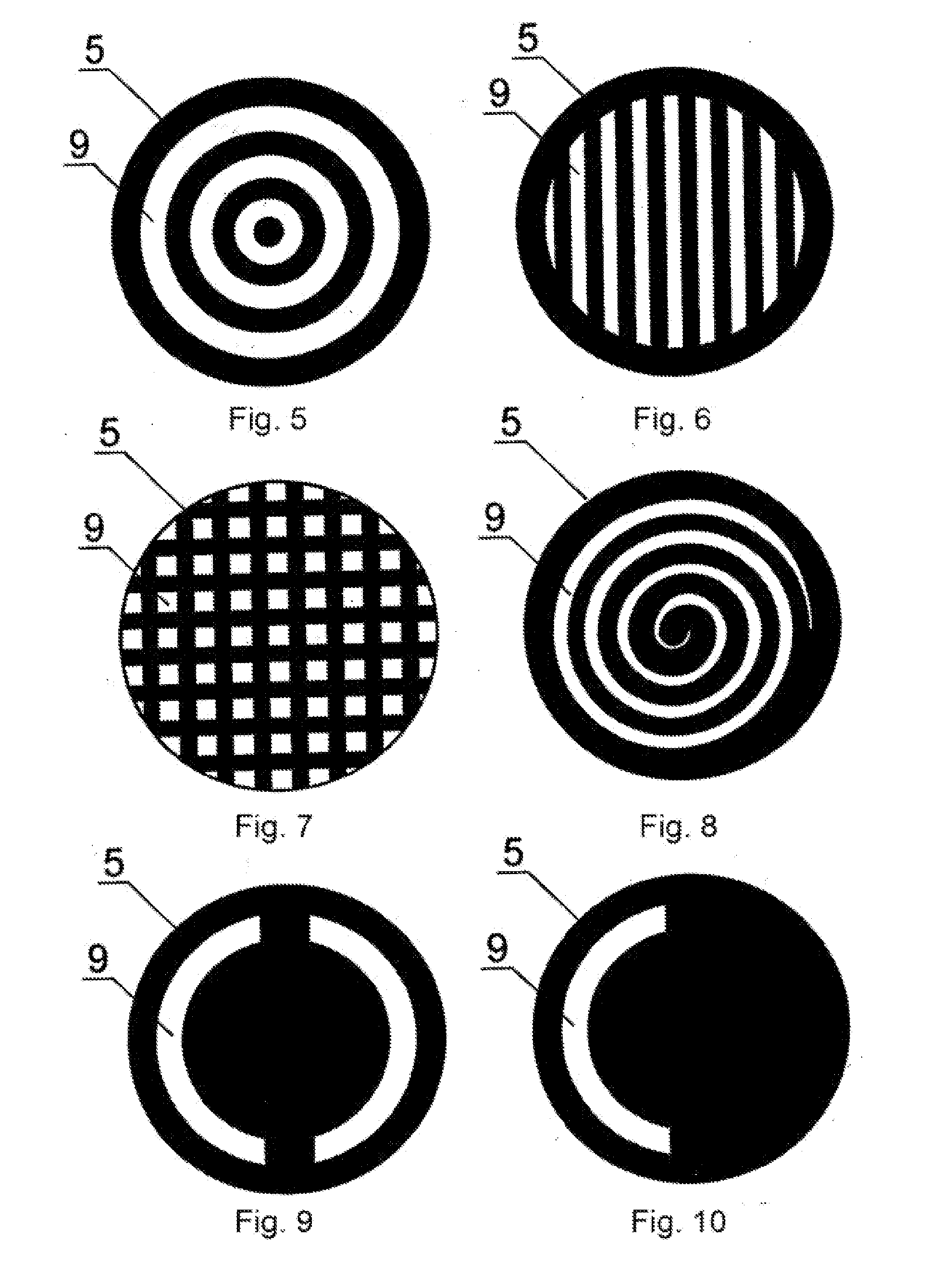
Method for treating keratoconus by UV radiation and a device for carrying out said method (variants)
InactiveUS20110190742A1Reduce adverse effectsEfficacy of treatmentLaser surgerySurgical instrument detailsOptical axisUltraviolet
The invention relates to ophthalmology and is used for treating keratoconus. The inventive method comprises forming one or more radiation areas of different shape, comprising concentric circles, arcs, parallel lines, cells, grids, spirals or other geometrical figures. In the other variant, the method comprises polarizing UV radiation and adjusting the depth of the UV radiation action by directing the UV radiation polarization plane at an angle of 1-180° to the plane of light polarization by the cornea, thereby using the effect of light polarization by the cornea. The device for treating keratoconus comprises, positioned on the optical axis common with an UV radiation source optical focusing elements and a diaphragm. The diaphragm is situated on the optical axis common with the UV radiation source and is made in the form of a mask with alternating transparent and shaded elements in the form of concentric circles, arcs, parallel lines, cells, grids, spirals or other geometrical figures. In the other variant, the device comprises, situated on the optical axis common with the UV radiation source, the optical focusing elements, the diaphragm and a polarisor.
Owner:ANISIMOV SERGEY IGOREVICH
Treatment and diagnosis of central nervous system disorders
InactiveUS20110200531A1Reduce severityImprove survivalBiocideSenses disorderDiseaseHuntingtons chorea
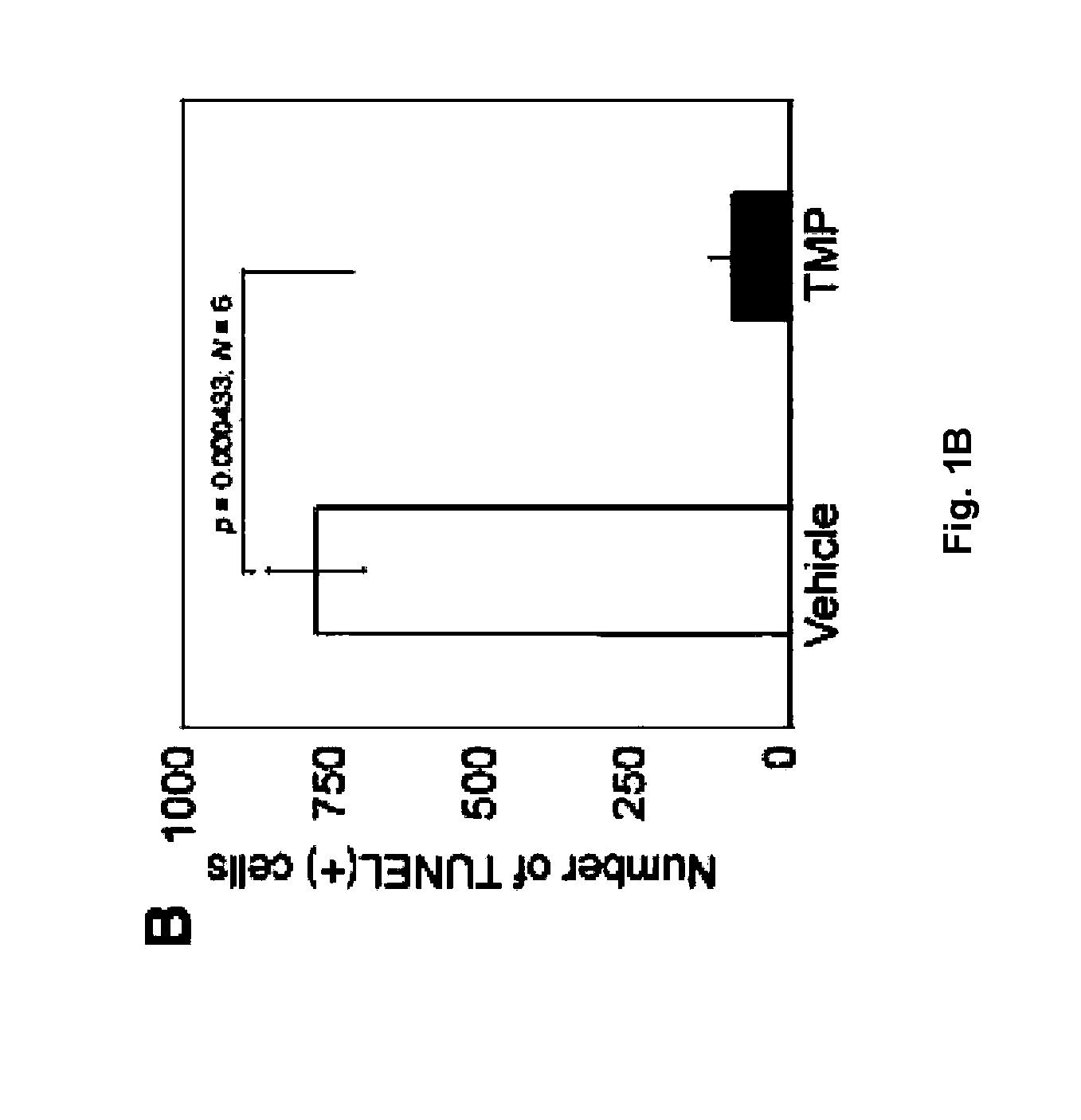
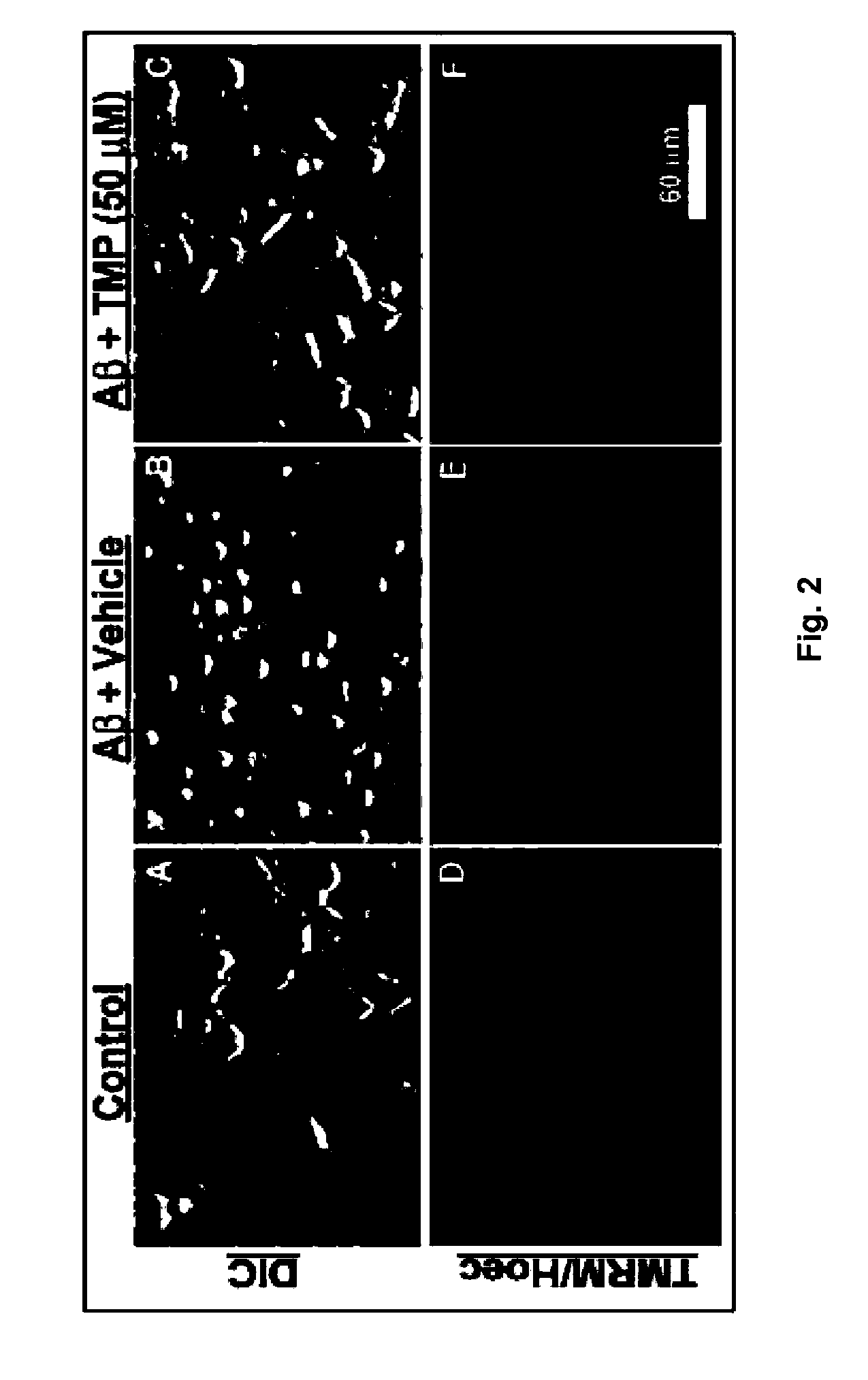
The invention relates to the use of tetramethylpyrazine (TMP) for the treatment of patients at risk for the development of, or diagnosed as having, CNS disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), glaucoma, and Huntington's Disease (HD), as well as traumatic brain injury (TBI). The present invention also relates to the use of retinal imaging to diagnose and monitor efficacy of treatments for such CNS disorders.
Owner:RGT UNIV OF CALIFORNIA
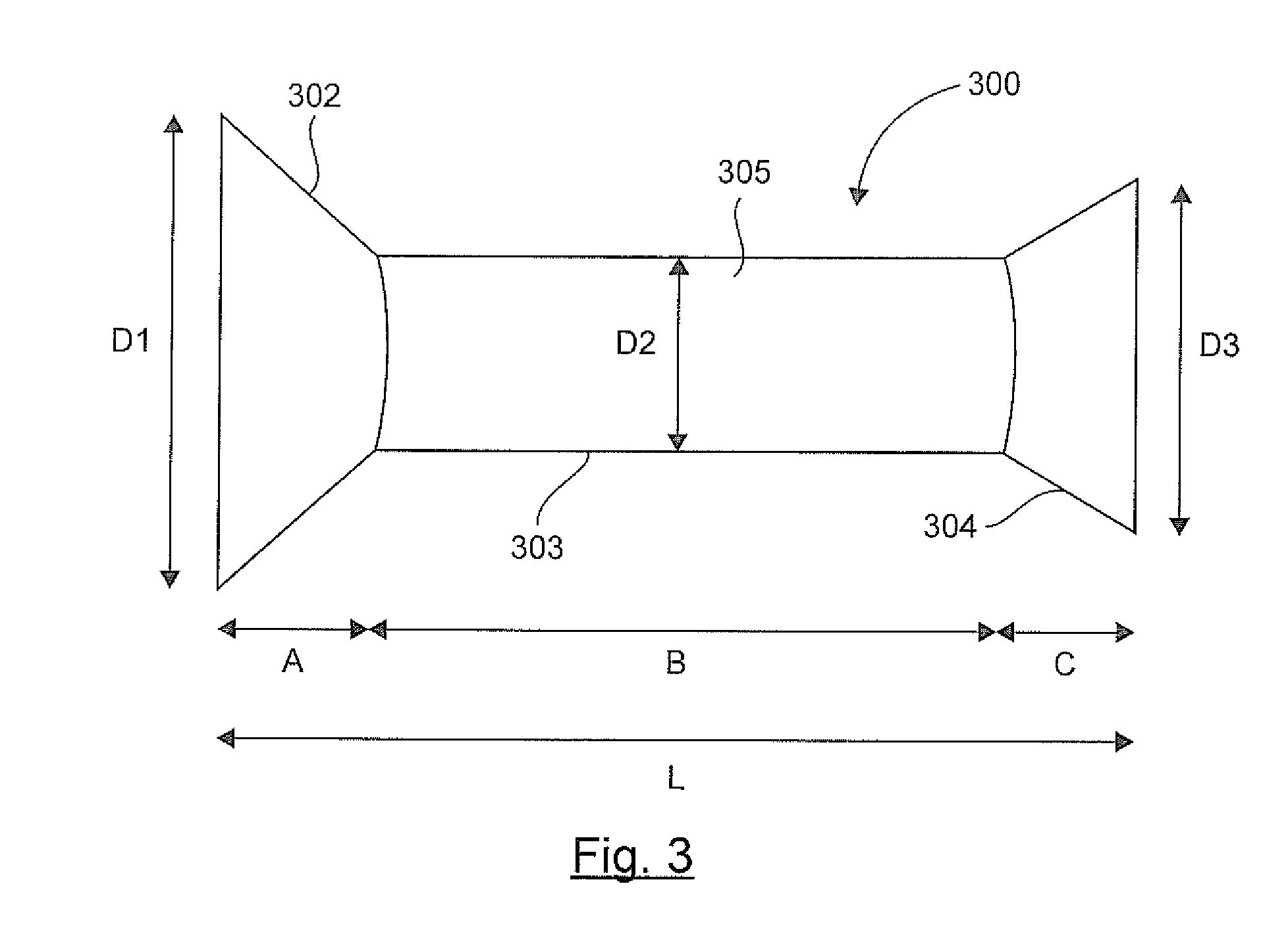
Stent prosthesis intended to be implanted in the digestive tract of a patient
InactiveUS20140364959A1Easy to disassembleNot to damageStentsDiagnosticsProsthesisBiomedical engineering
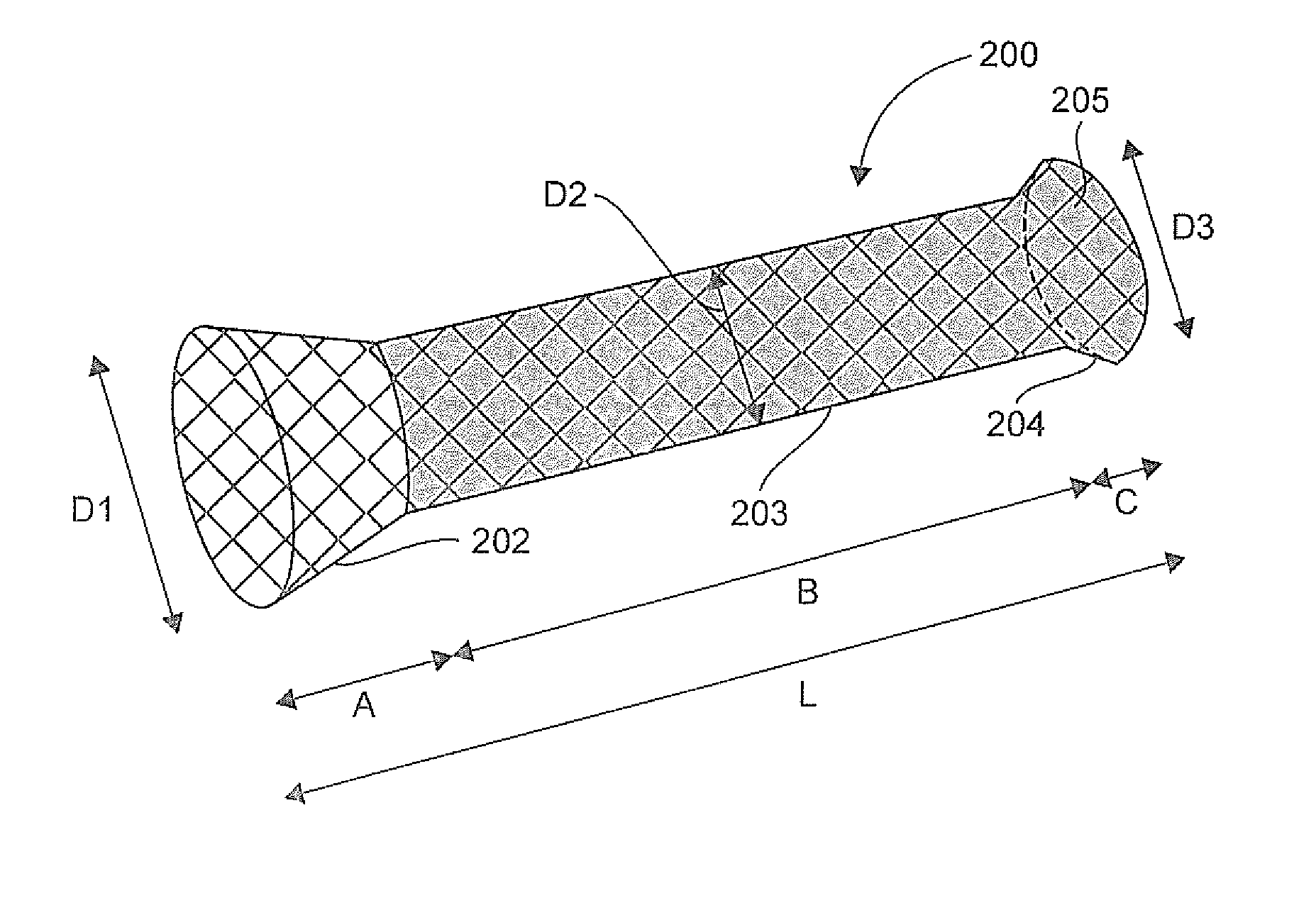
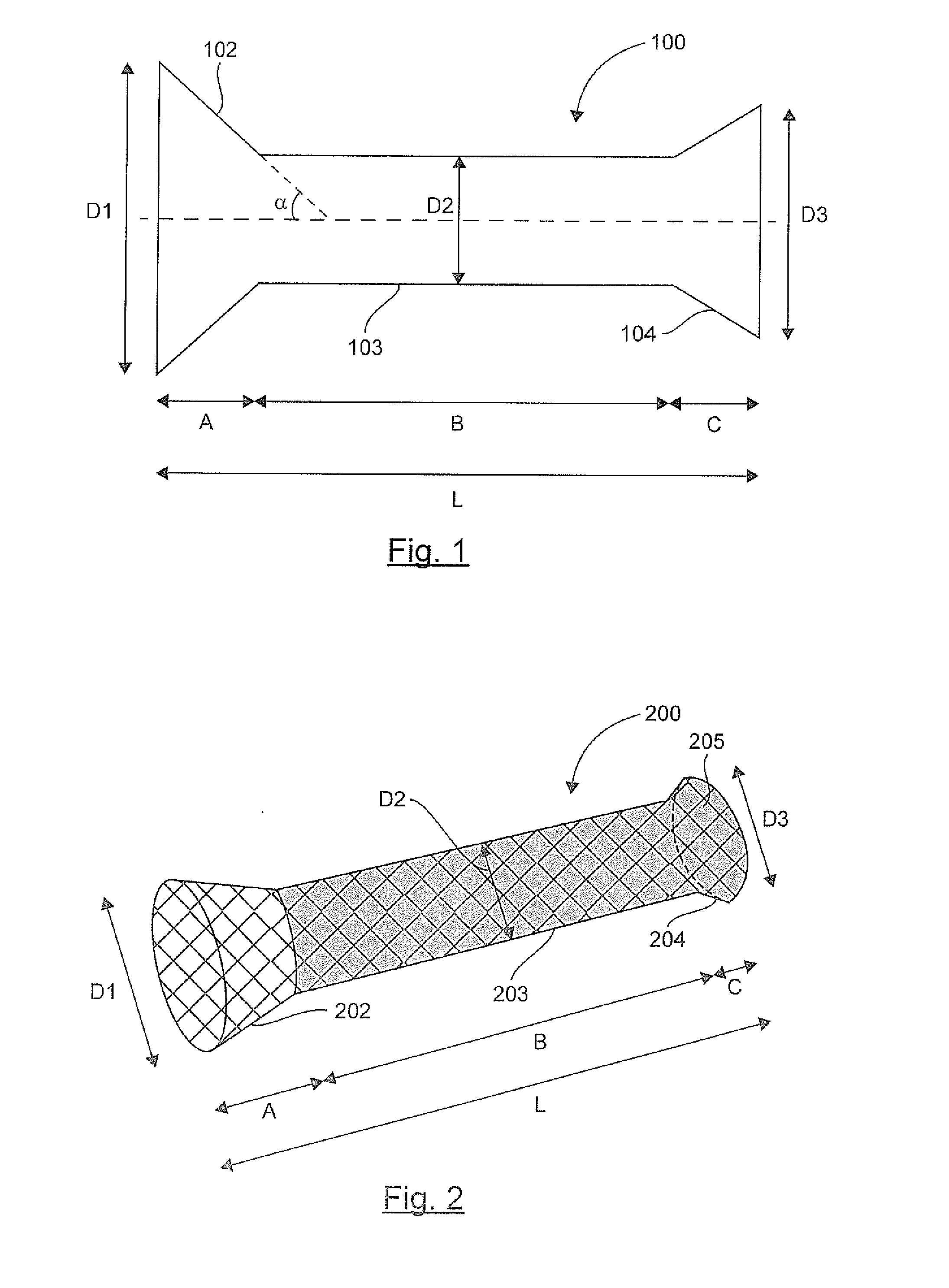
The invention relates to a prosthesis that is compressible and expandable in a radial direction, intended to be implanted in the digestive tract of a patient. According to the invention, such a prosthesis comprises: a downstream conical collar having end diameter D3, a main tabular body having diameter D2, an upstream conical collar having end diameter D1, said upstream collar not being covered by any material and having an end diameter D1 greater than the diameter D2 of said main body and greater than the end diameter D3 of said downstream collar, and said downstream collar being fully or partially covered by at least one polymer material.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS
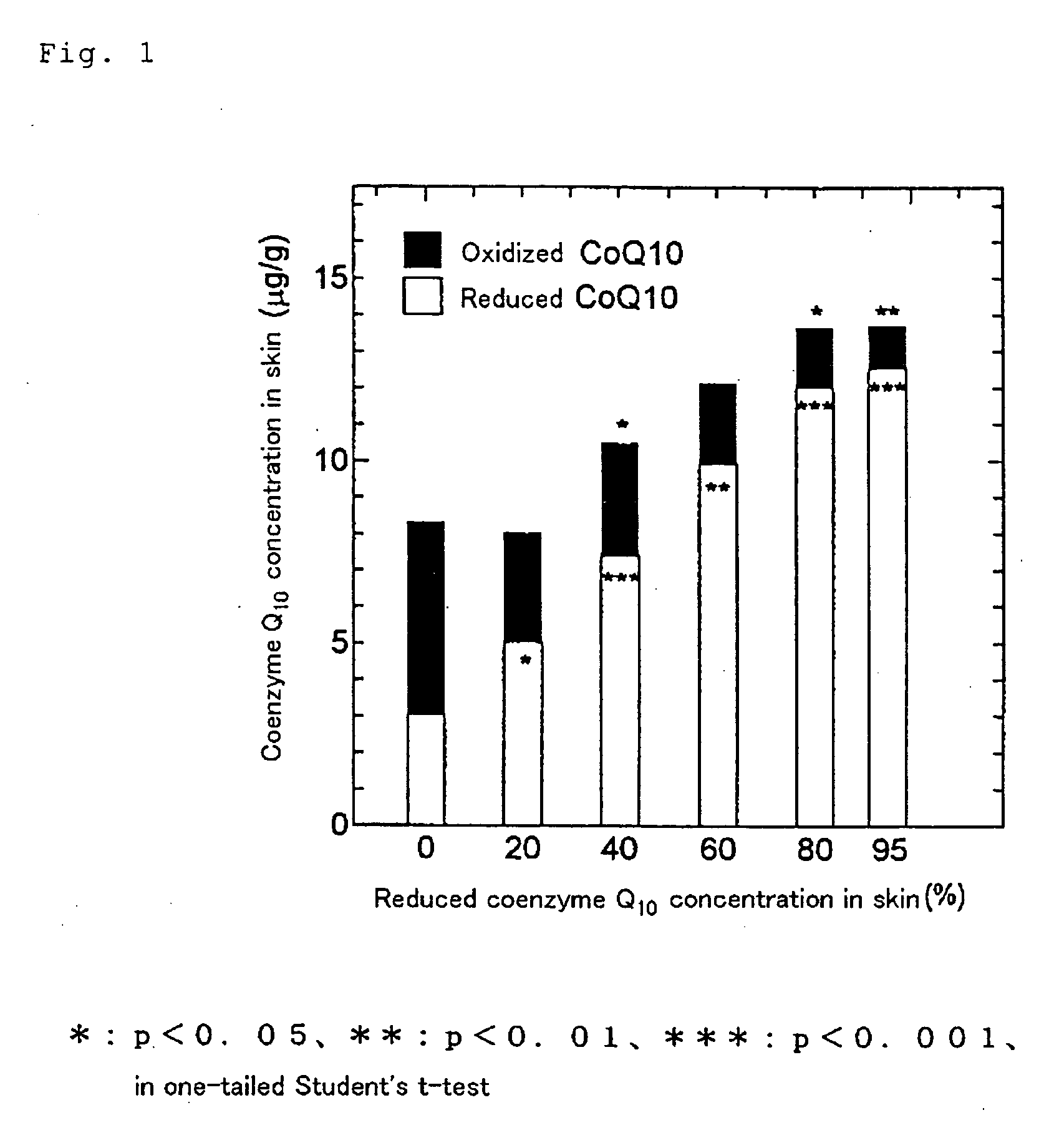
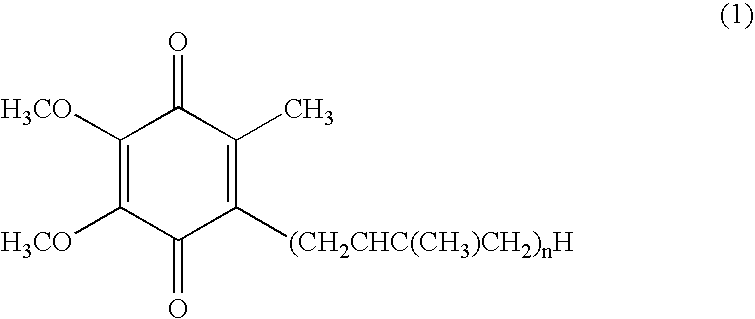
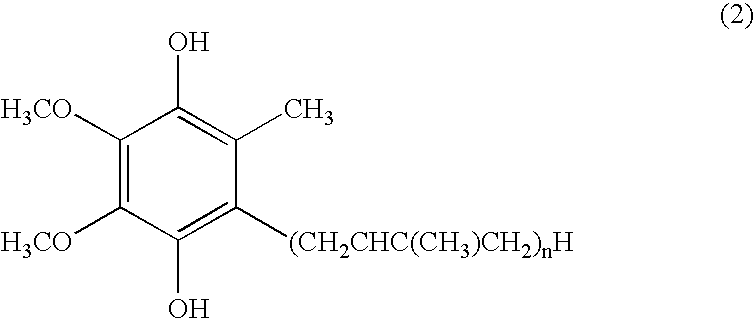
Dermal compositions containing coenzyme q as the active ingredient
InactiveUS20050070610A1Safe and highly effective therapeuticGood treatment effectCosmetic preparationsBiocideBULK ACTIVE INGREDIENTActive ingredient
The present invention provides a composition for dermal application which comprises, as an active ingredient, an oxidized coenzyme Q represented by the formula (1): in which n represents an integer of 1 to 12, and / or a reduced coenzyme Q represented by the formula (2): in which n represents an integer of 1 to 12, the total content of the oxidized coenzyme Q and reduced coenzyme Q being 0.01 to 99% by weight relative to the whole amount of the composition. The present invention also provides a therapeutic composition for skin diseases, a cosmetic composition, a skin health care composition and a bath salt composition, each comprising the above composition for dermal application. The present invention is further provides a method for the treatment of skin diseases which comprises applying, to a patient suffering from a skin disease, the above-mentioned therapeutic composition for skin diseases, or a method for the treatment of skin diseases which comprises applying, to a patient suffering from a skin disease, the above therapeutic agent for skin diseases other than the oxidized coenzyme Q represented by the formula (1) and other than the reduced coenzyme Q represented by the formula (2) in parallel with a therapeutic composition for skin diseases.
Owner:KANEKA CORP
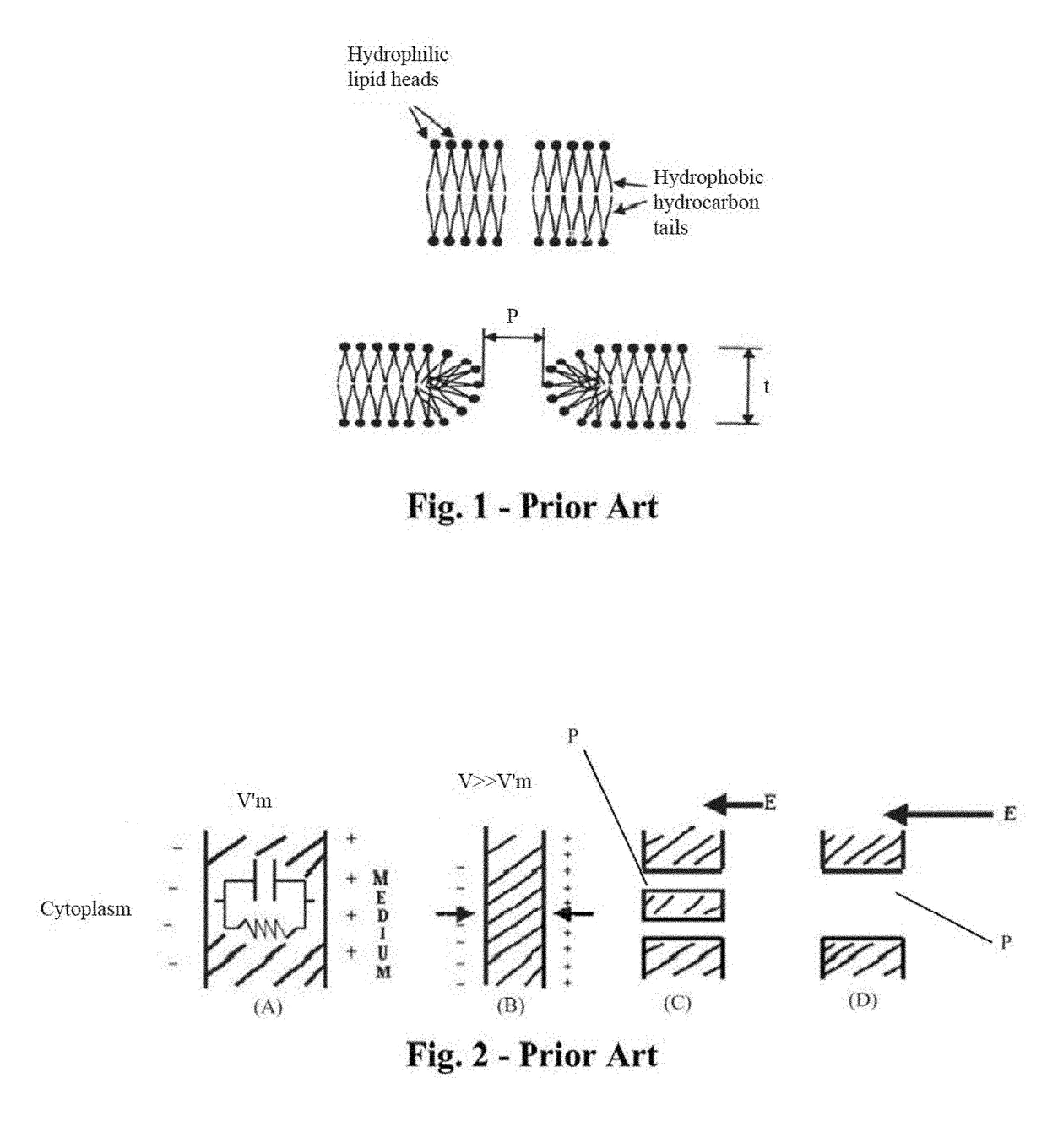
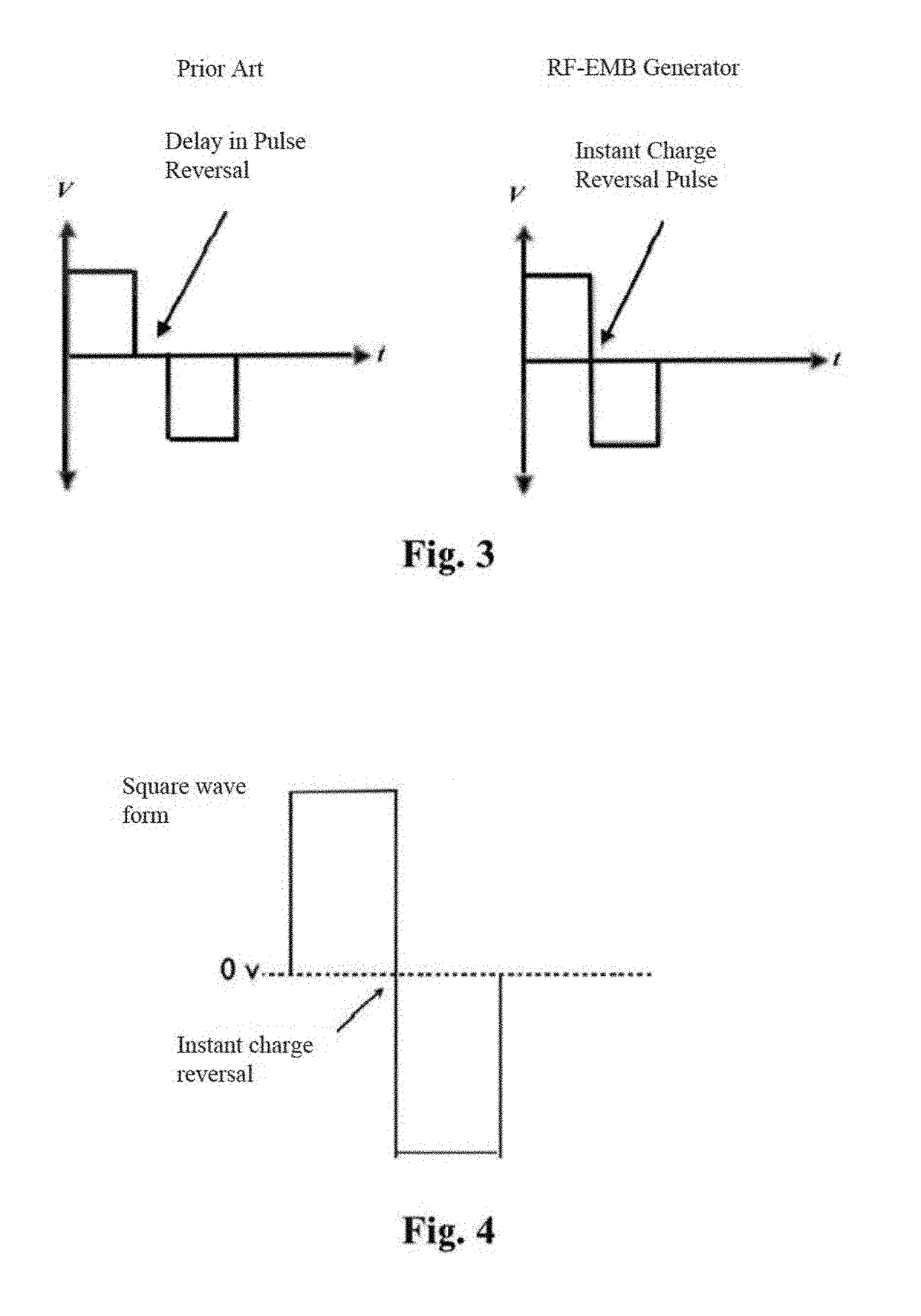
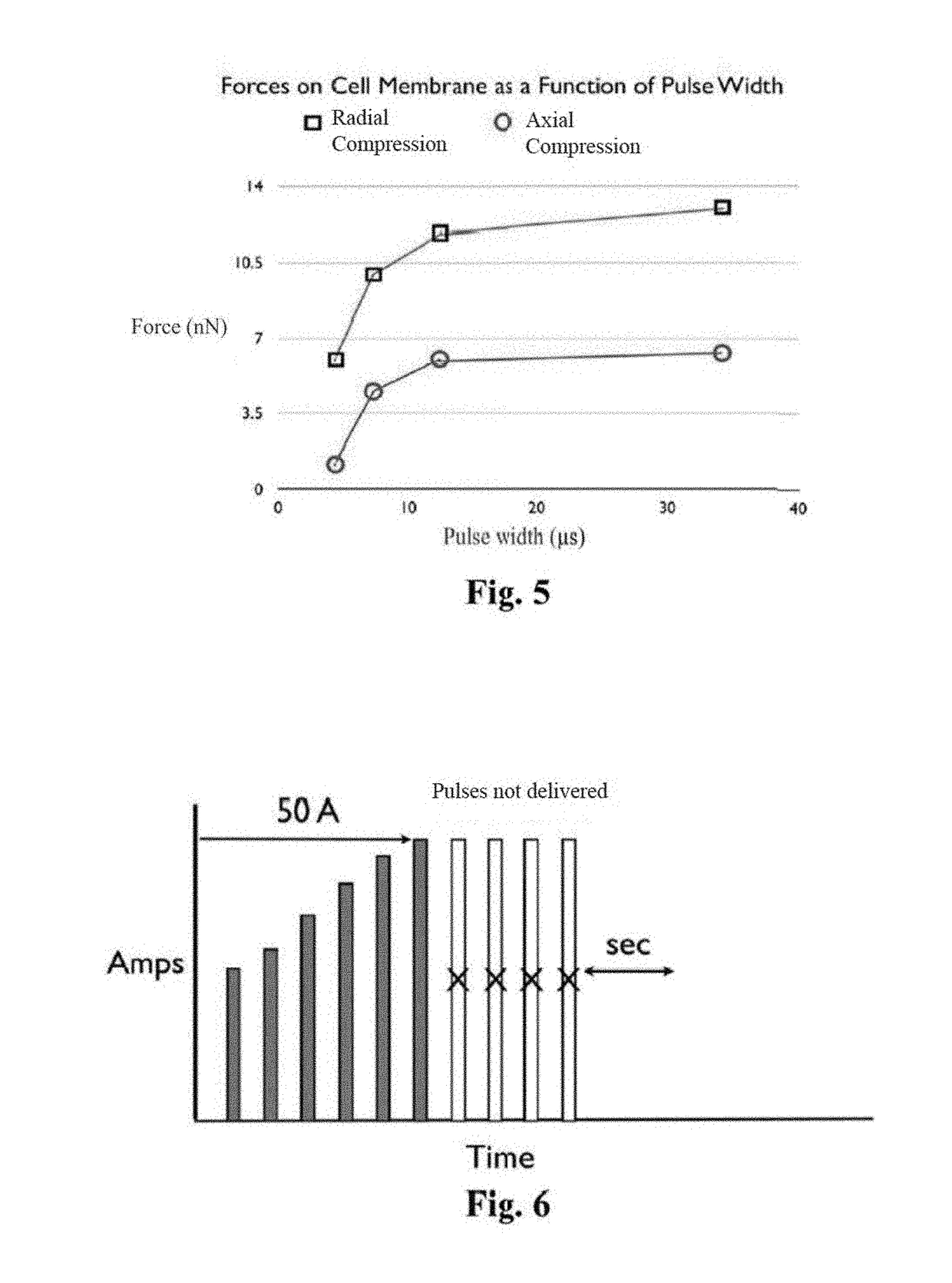
System and method for creating radio-frequency energy electrical membrane breakdown for tissue ablation
Owner:IMMUNSYS INC
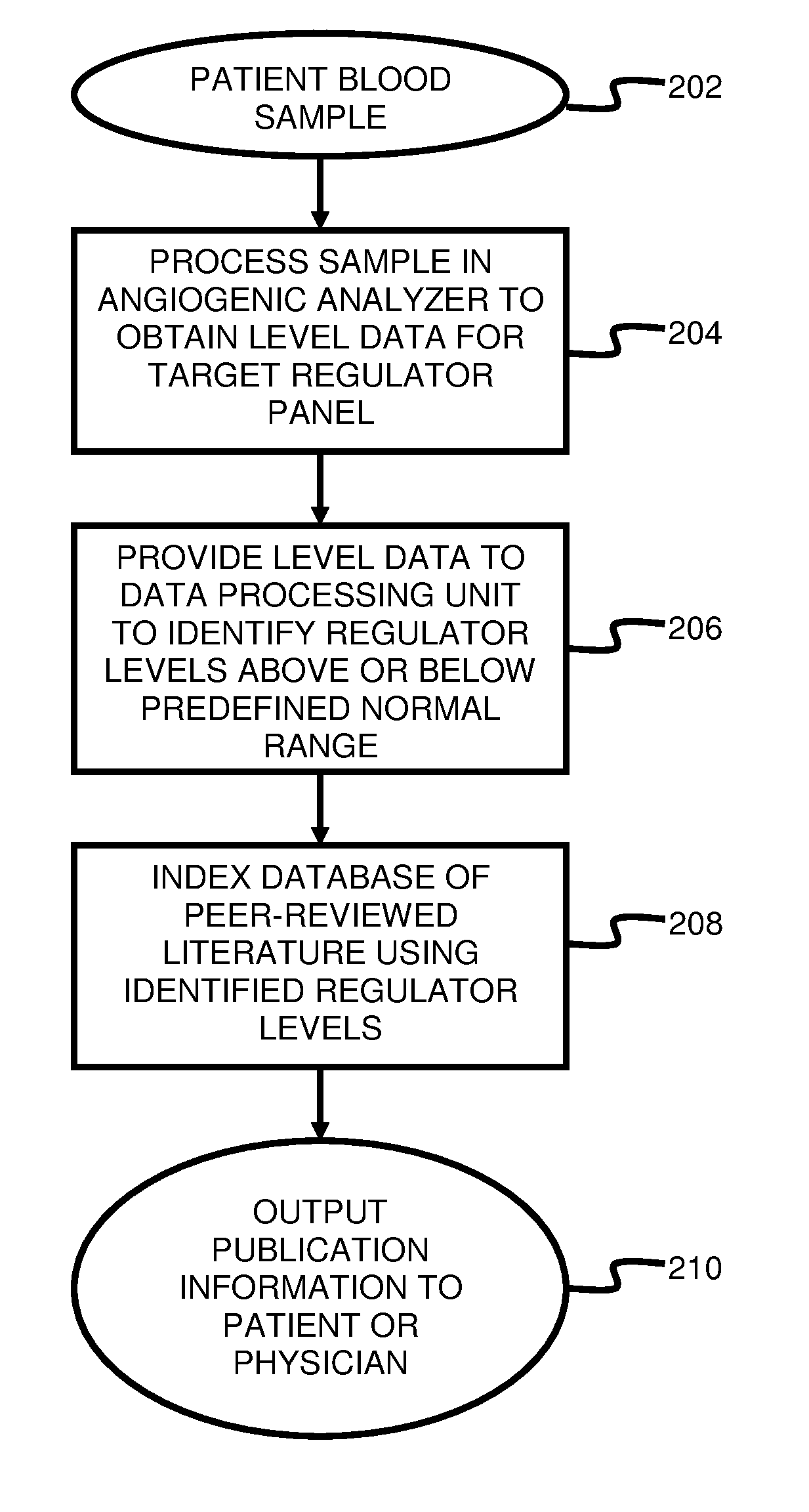
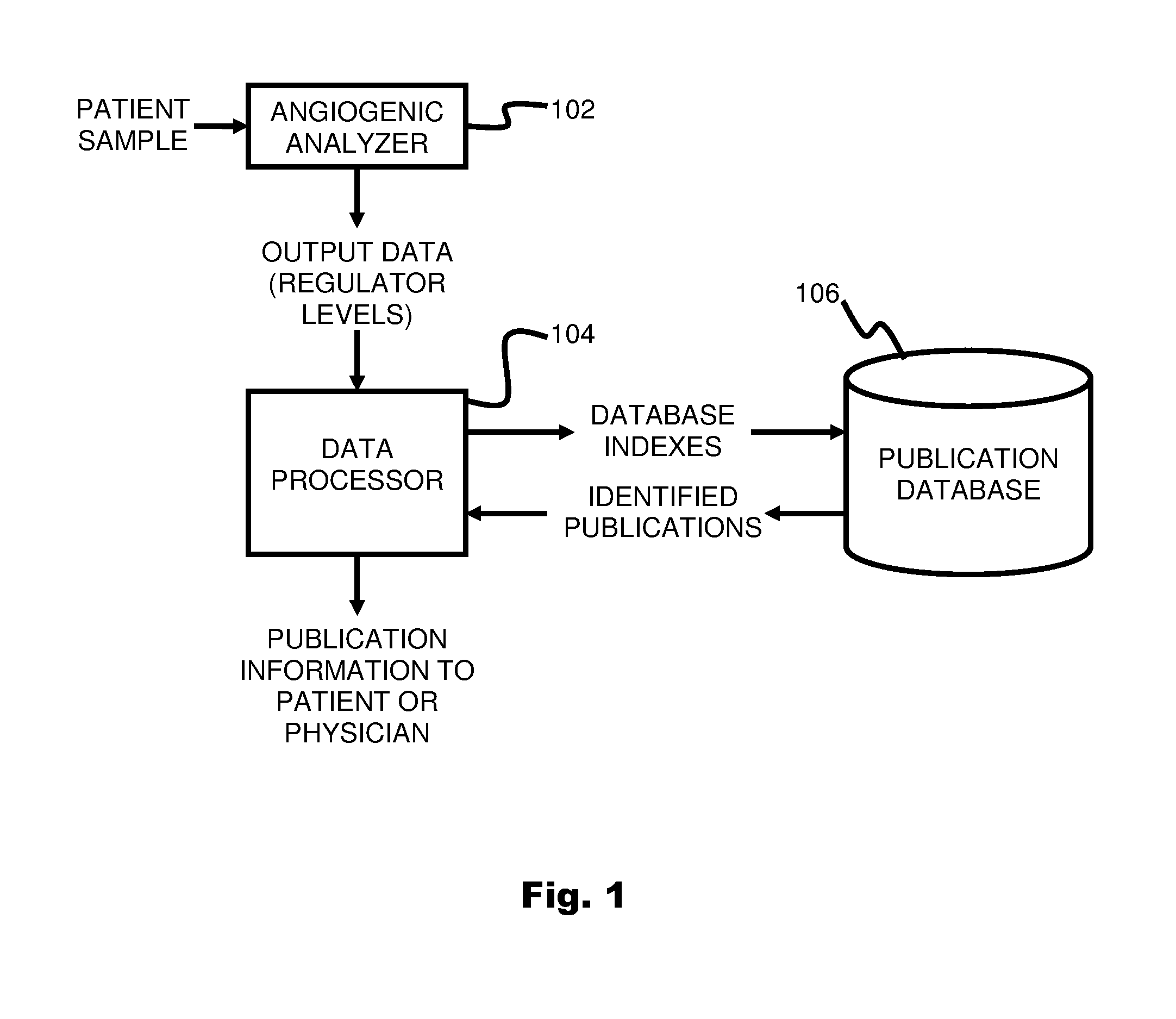
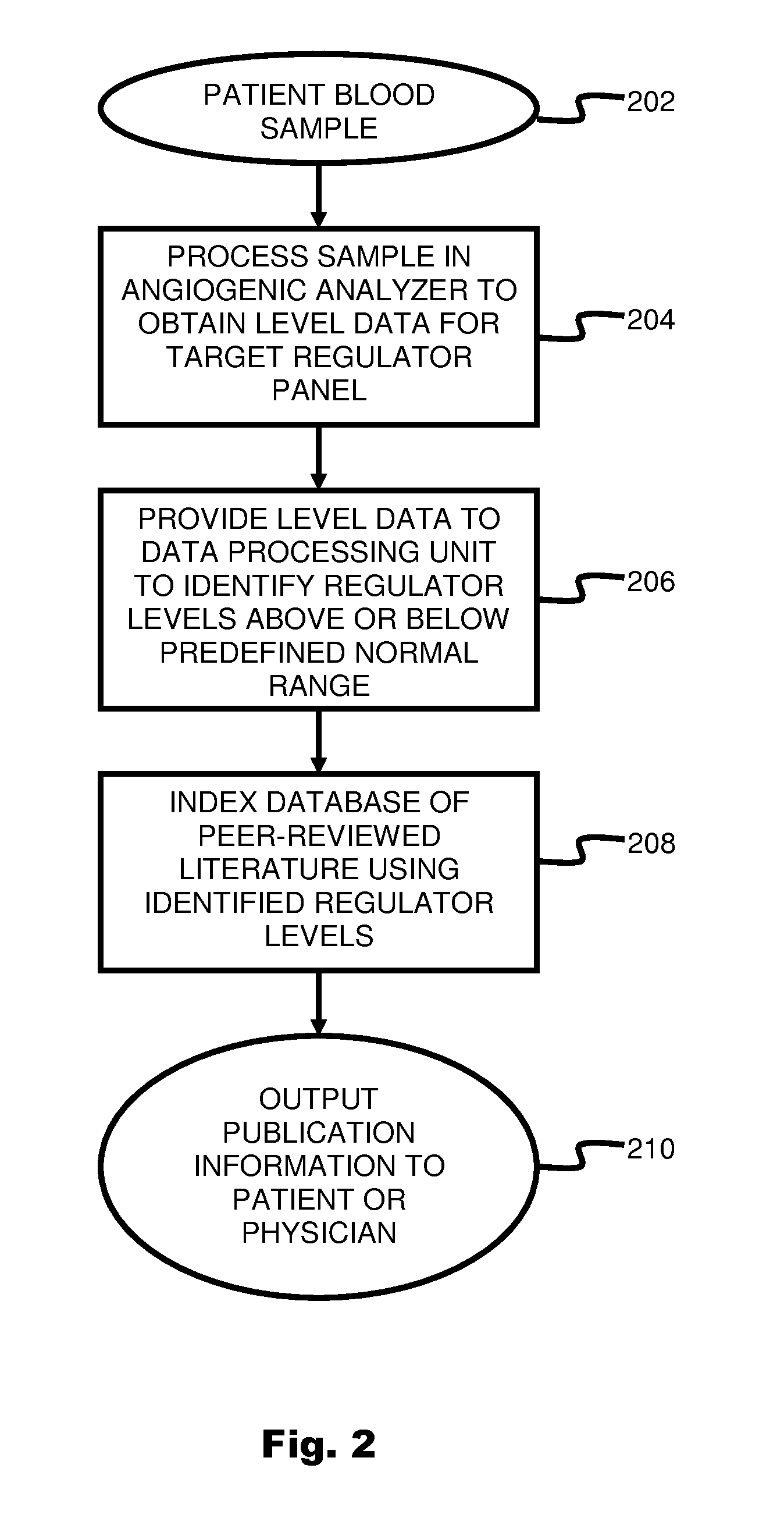
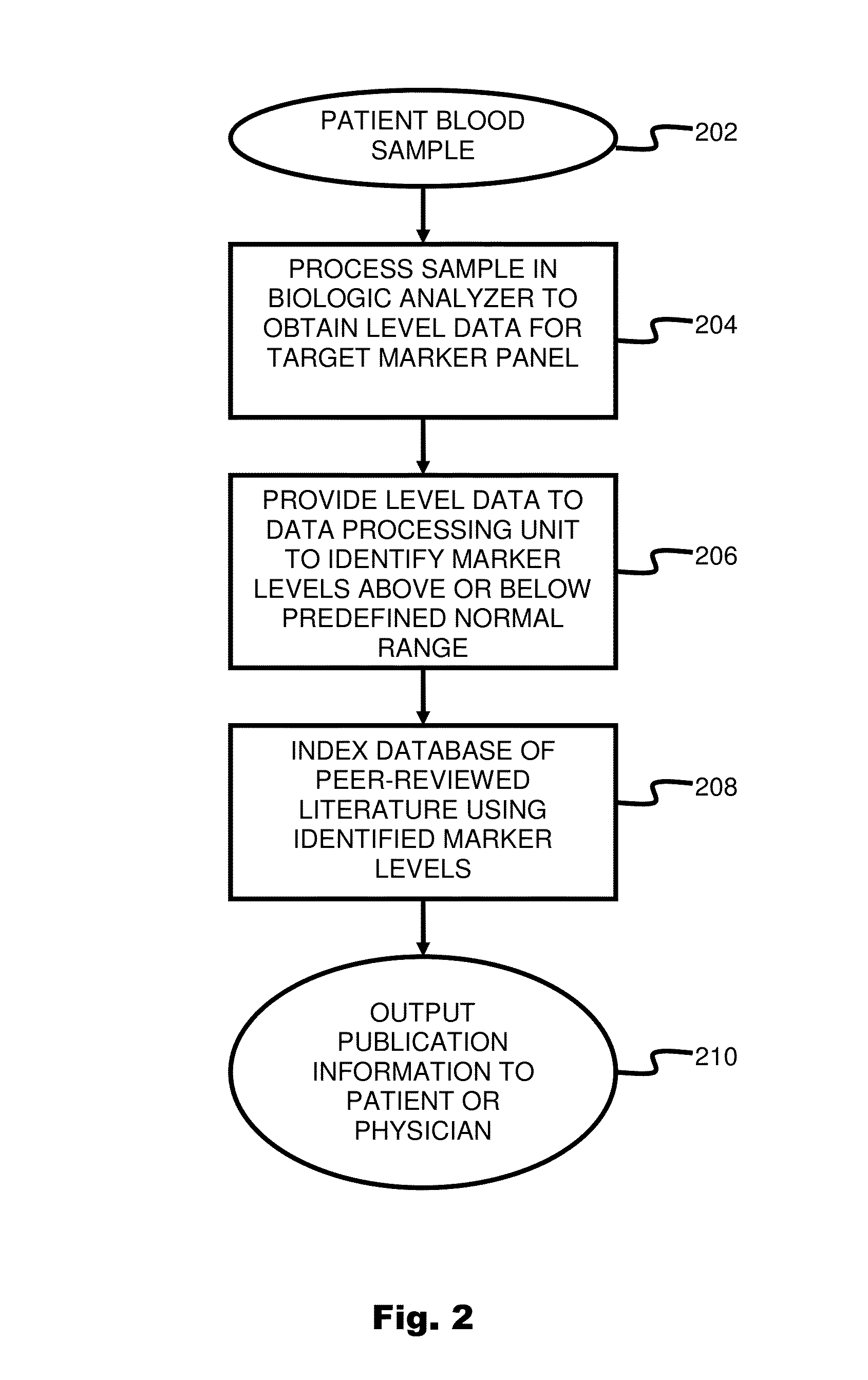
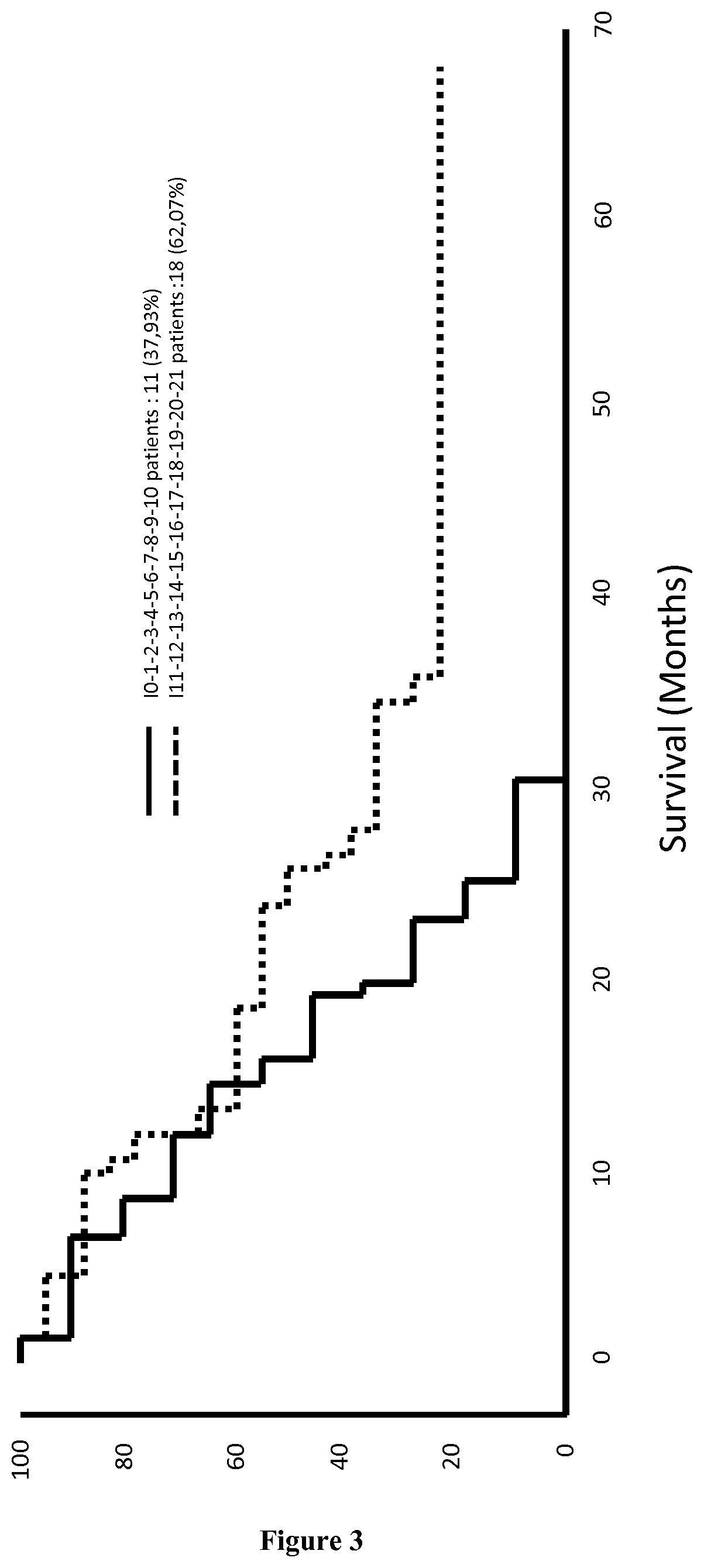
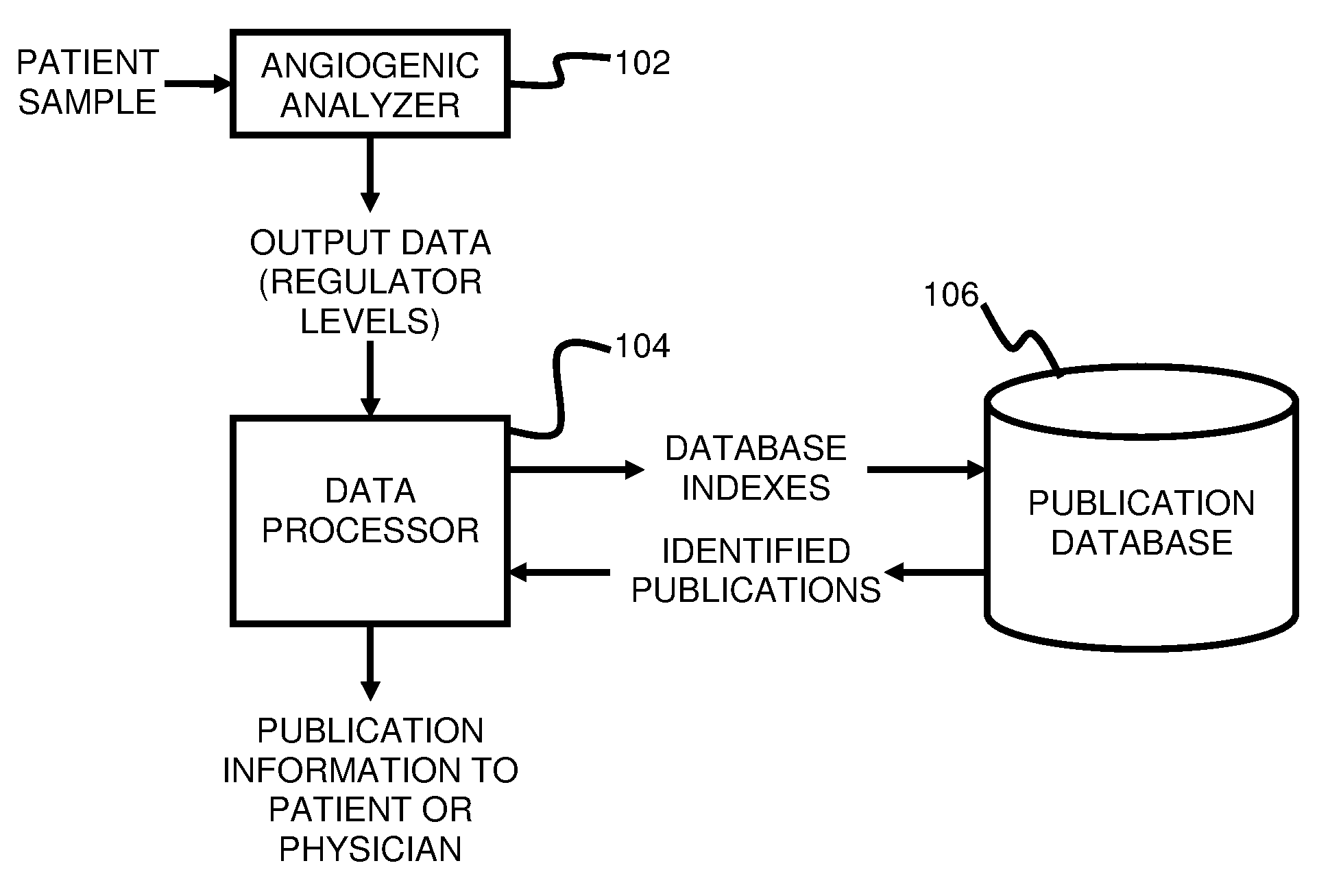
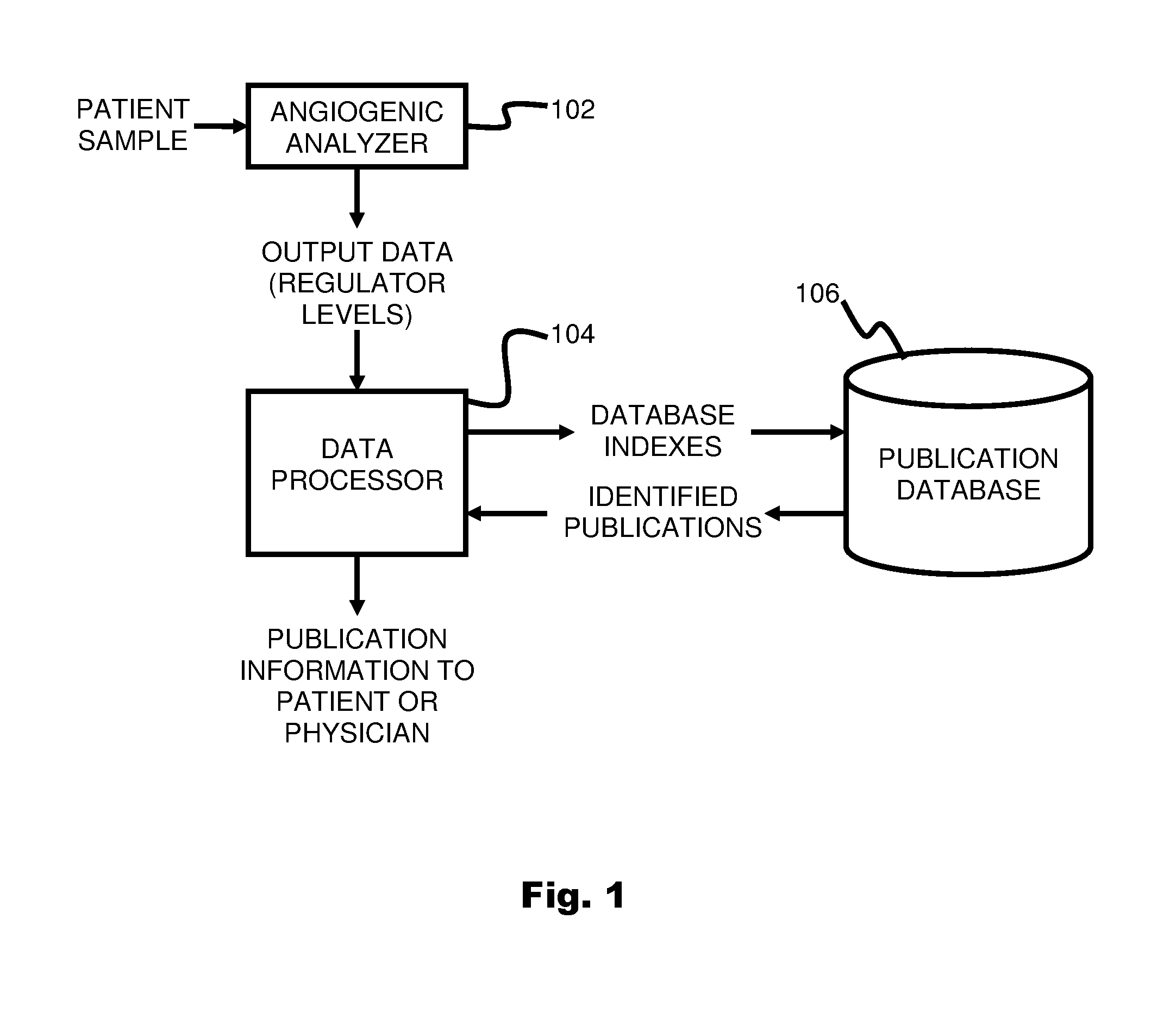
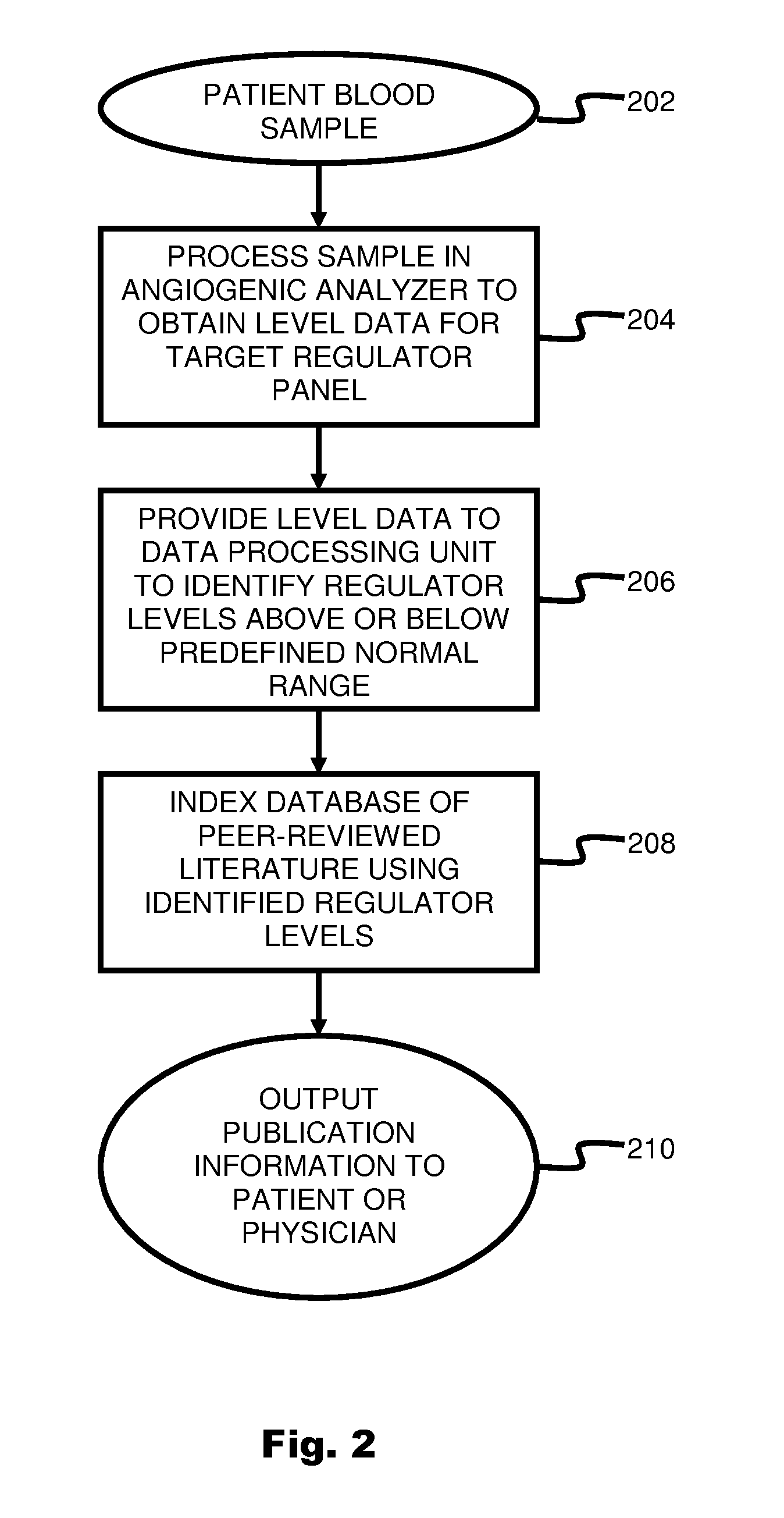
System and method for targeting relevant research activity in response to angiogenic regulator analyses
ActiveUS20110231104A1Highly targeted and unique mannerEfficacy of treatmentDisease diagnosisProteomicsAdditional diagnosesCorrelational study
A system and method for targeting relevant research activity for clinical application in response to angiogenic regulator analyses. An angiogenic analysis is performed on a patient blood sample in order to detect the level of each of at least ten angiogenic regulators. The levels of the tested regulators are used as indexes to identify relevant peer-reviewed research publications from among a large database of articles. The most relevant peer-reviewed literature reporting research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of angiogenic regulators for various disease states are then provided to the patient and / or to the patient's physician, optionally in conjunction with a summarization of the treatment recommendations gleaned from the provided literature. The customized information delivery provides the patient and physician a range of published peer-reviewed therapeutic options and published research studies for moderating the out of range regulators to within normal range or other diagnostic significant range.
Owner:LAMBERT REBECCA
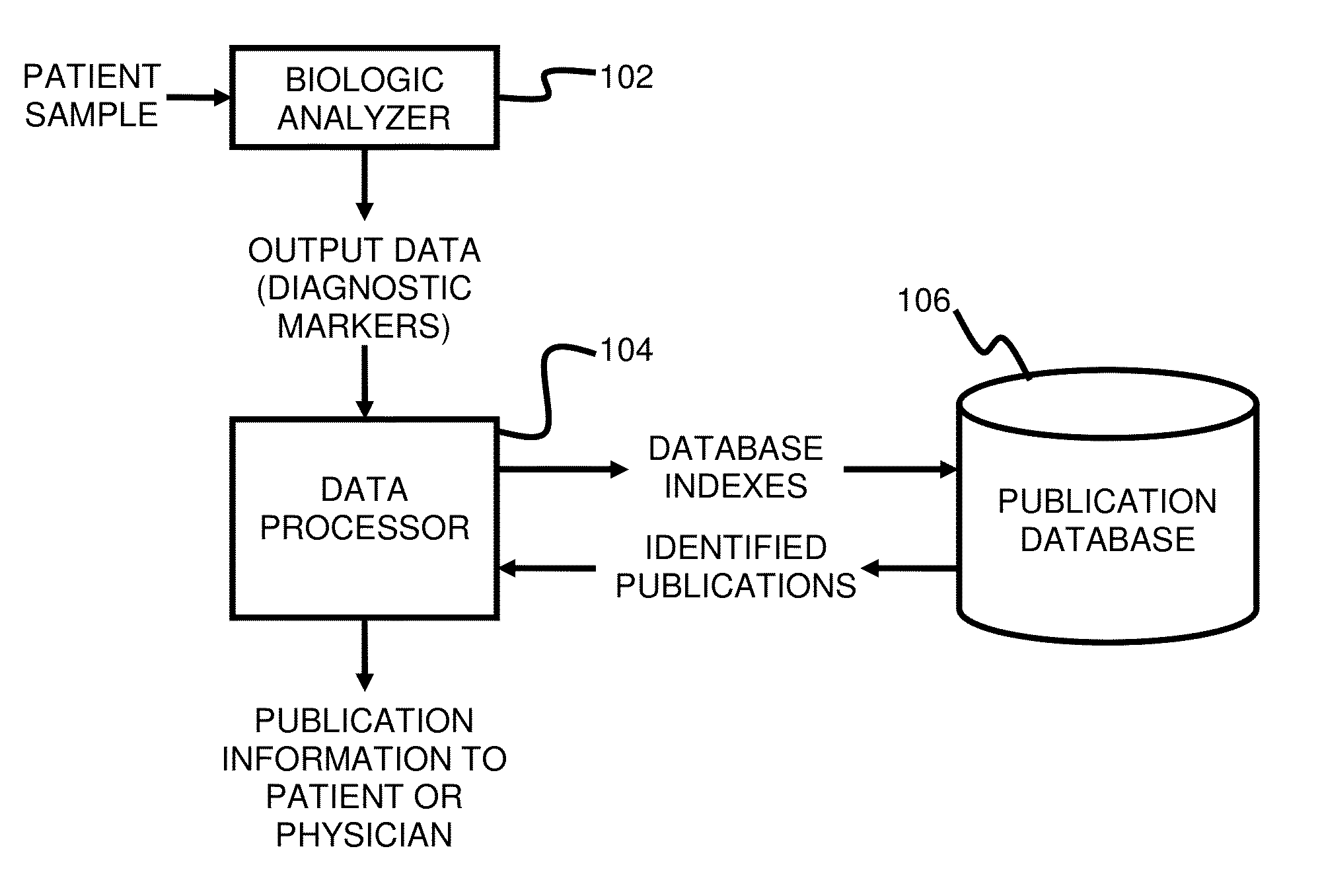
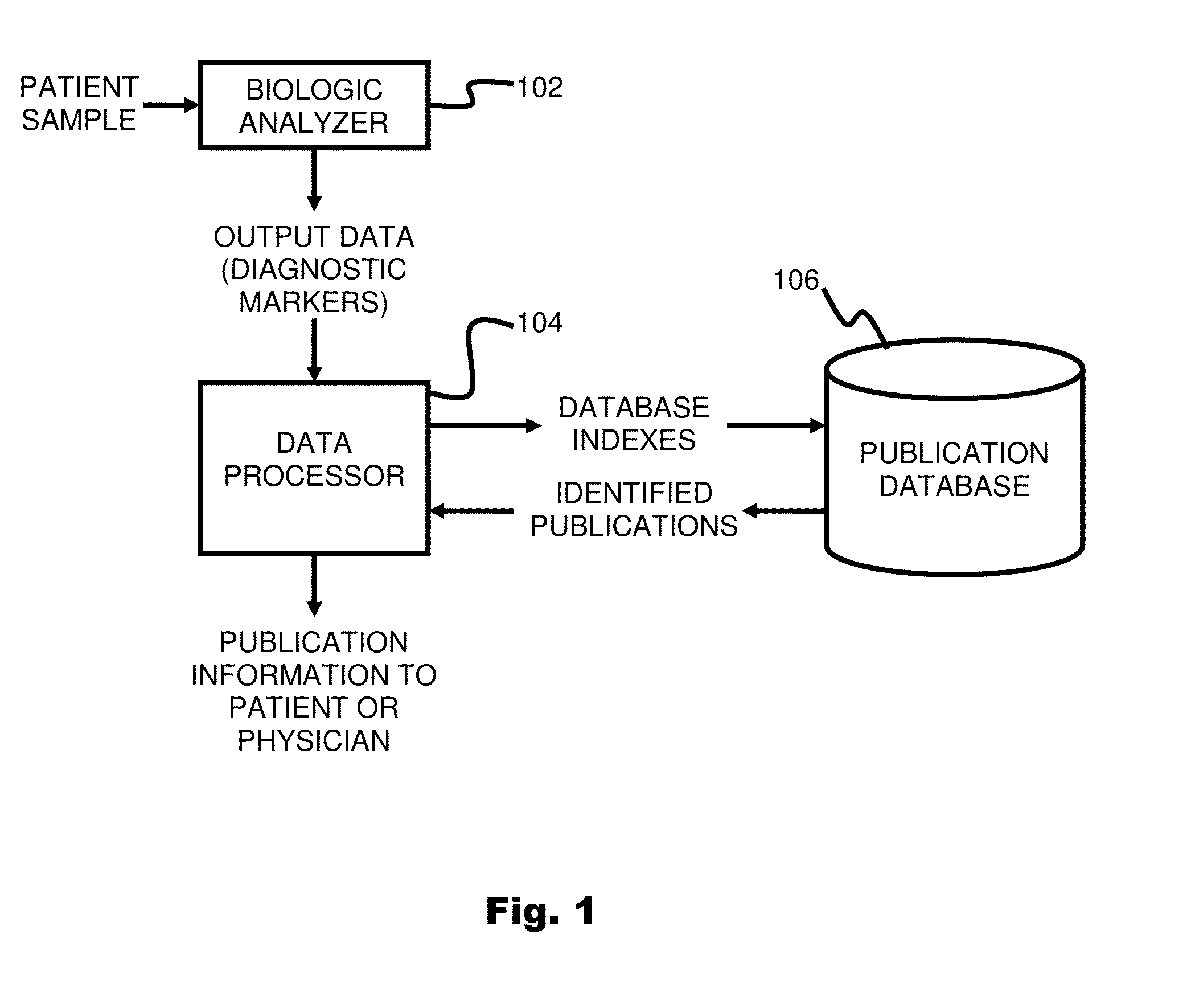
System and method for targeting relevant research activity in response to diagnostic marker analyses
InactiveUS20150046465A1Improve impactLow densityDigital data processing detailsLibrary screeningDiseaseCorrelational study
A system and method for targeting relevant research activity for clinical application in response to diagnostic markers analyses is described. Diagnostic analysis is performed to detect the level of each of at least three diagnostic markers. The levels of the tested markers are used to identify relevant publications from among a large database of articles. The most relevant literature, such as, one which reports research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of diagnostic markers for various disease states, is then provided to the patient and / or the patient's physician, optionally with a summarization of the treatment recommendations from the provided literature. The customized information delivery provides a range of published peer-reviewed therapeutic options and / or published research studies.
Owner:LAMBERT REBECCA
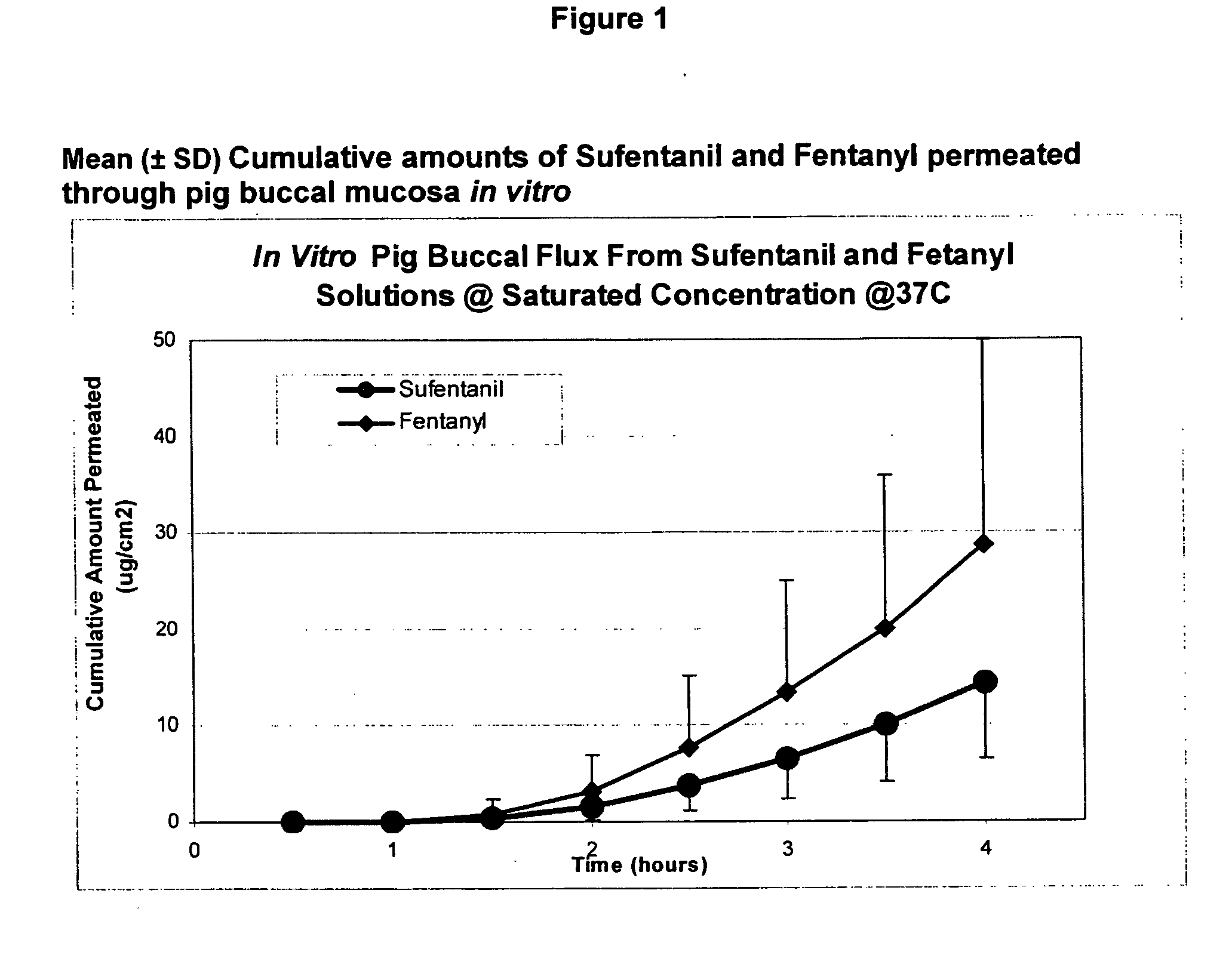
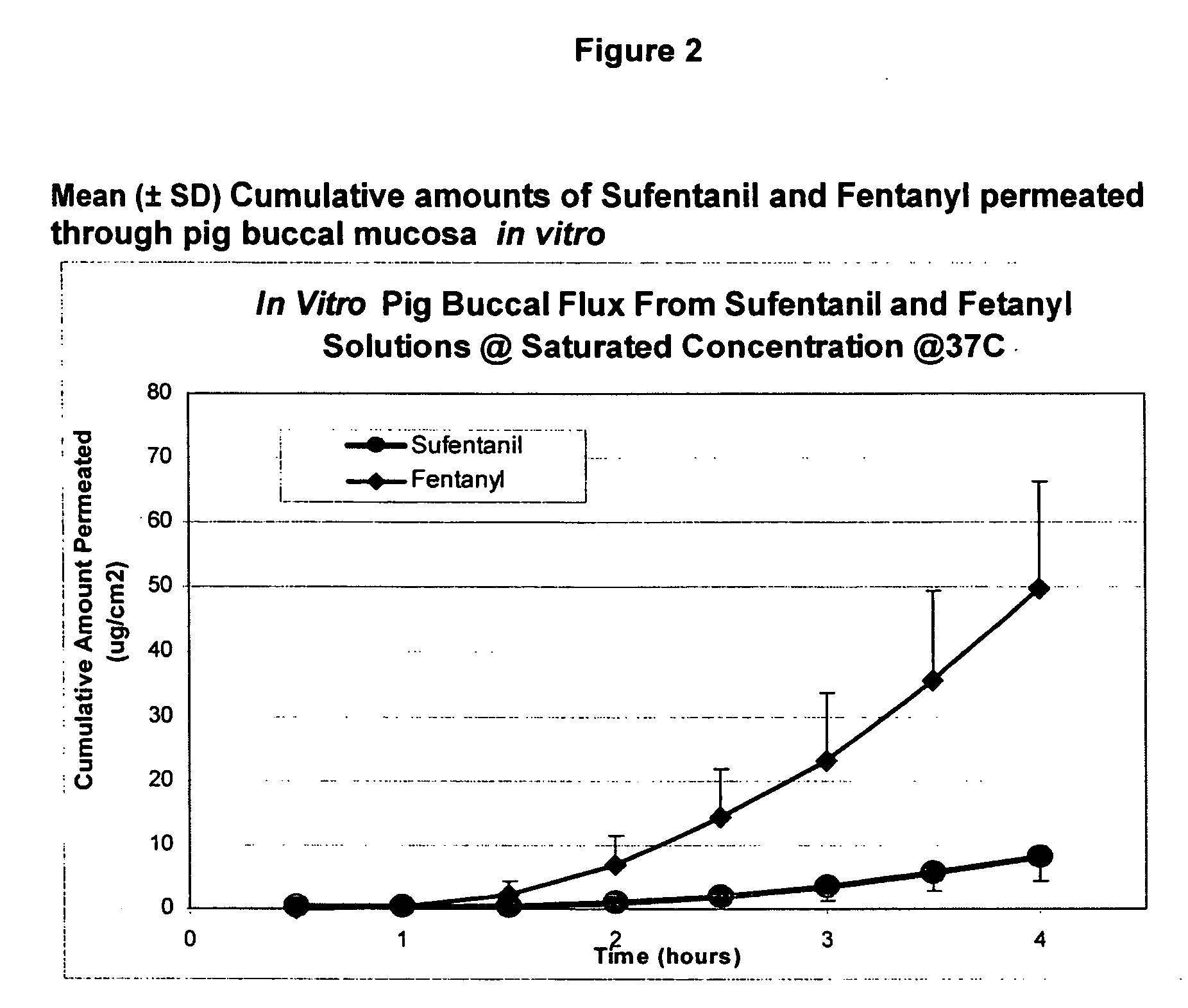
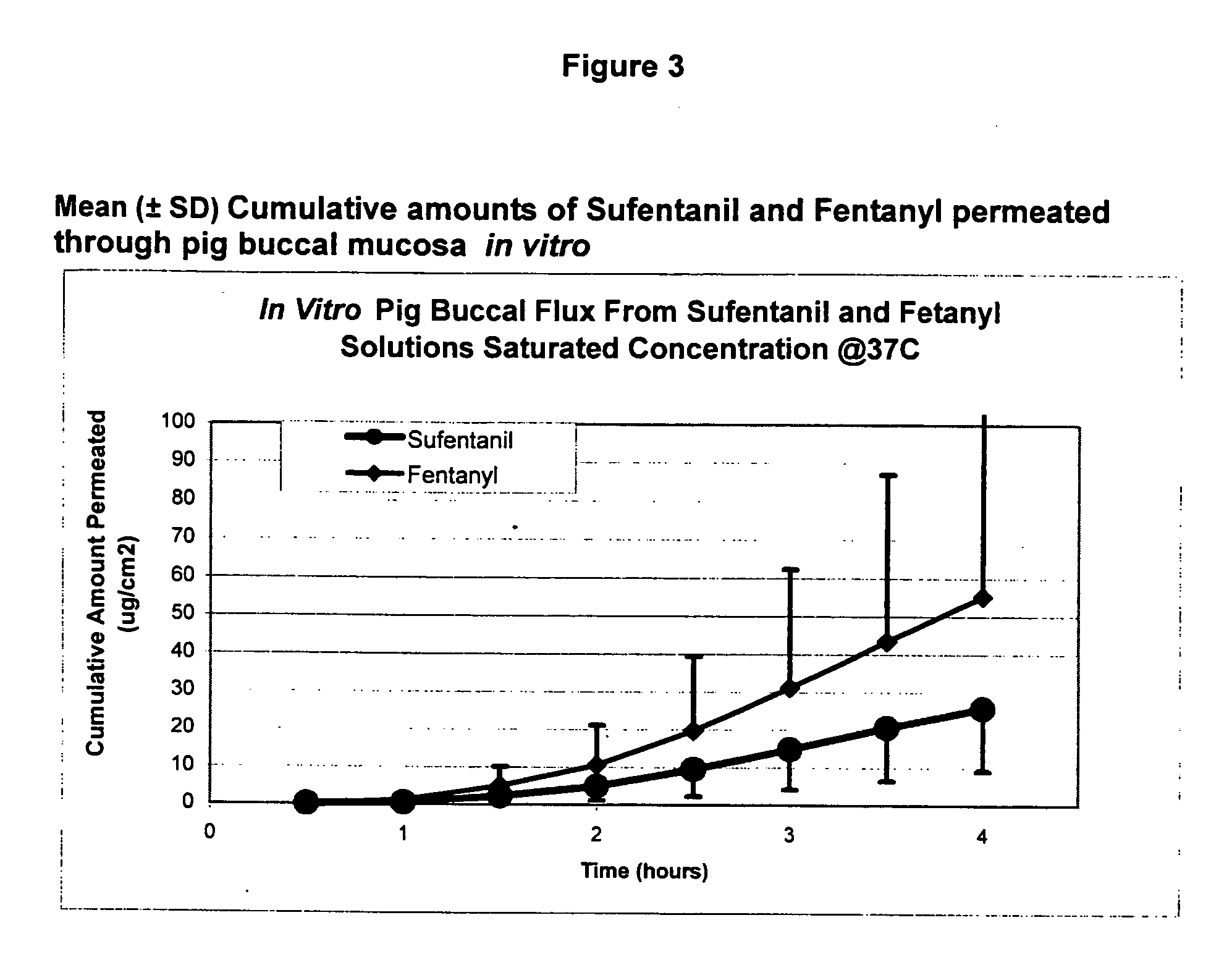
Transoral dosage forms comprising sufentanil and naloxone
InactiveUS20100010031A1Efficacy of treatmentIncreased safety marginBiocideNervous disorderMicrogramDosage form
The invention pertains to methods that include administering to a subject a transoral dosage form comprising a pharmaceutical carrier and sufentanil, and maintaining a mean pH ranging from about 3.5 to about 5.5 during a dosing period after administration of the transoral dosage form as determined using an in vitro donor media test. Related dosage forms are also disclosed. Also disclosed are transoral dosage forms and related methods, wherein a transoral dosage form may comprise: (1) about 5 to about 1000 micrograms of sufentanil; (2) about 50 micrograms to about 100 milligrams of naloxone; and (3) acidifying material in an amount sufficient to provide a mean pH ranging from about 3.5 to about 5.5 during a dosing period after administration of the transoral dosage form as determined using an in vitro donor media test; wherein the dosing period begins no earlier than about 1 minute after administration of the transoral dosage form, and ends no later than about 120 minutes after administration of the transoral dosage form.
Owner:YUM II SU +3
Ramipril Formulation
InactiveUS20070098782A1Avoids significant degradationReduce the presence of impuritiesBiocidePill deliveryPharmacologyRamiprilat
A Ramipril formulation which is suitably stabilised to control the degradation to the active metabolite ramiprilat.
Owner:SELAMINE
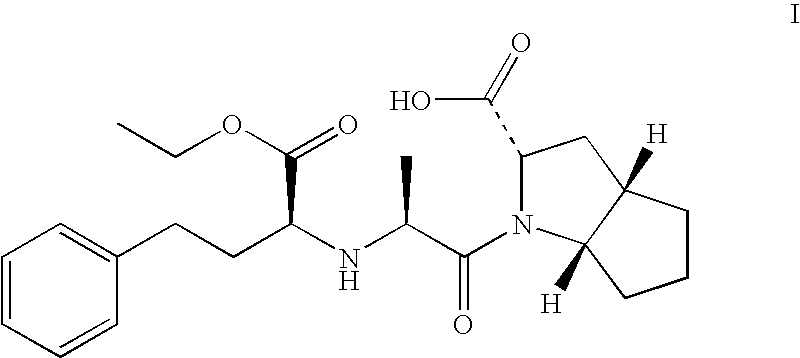
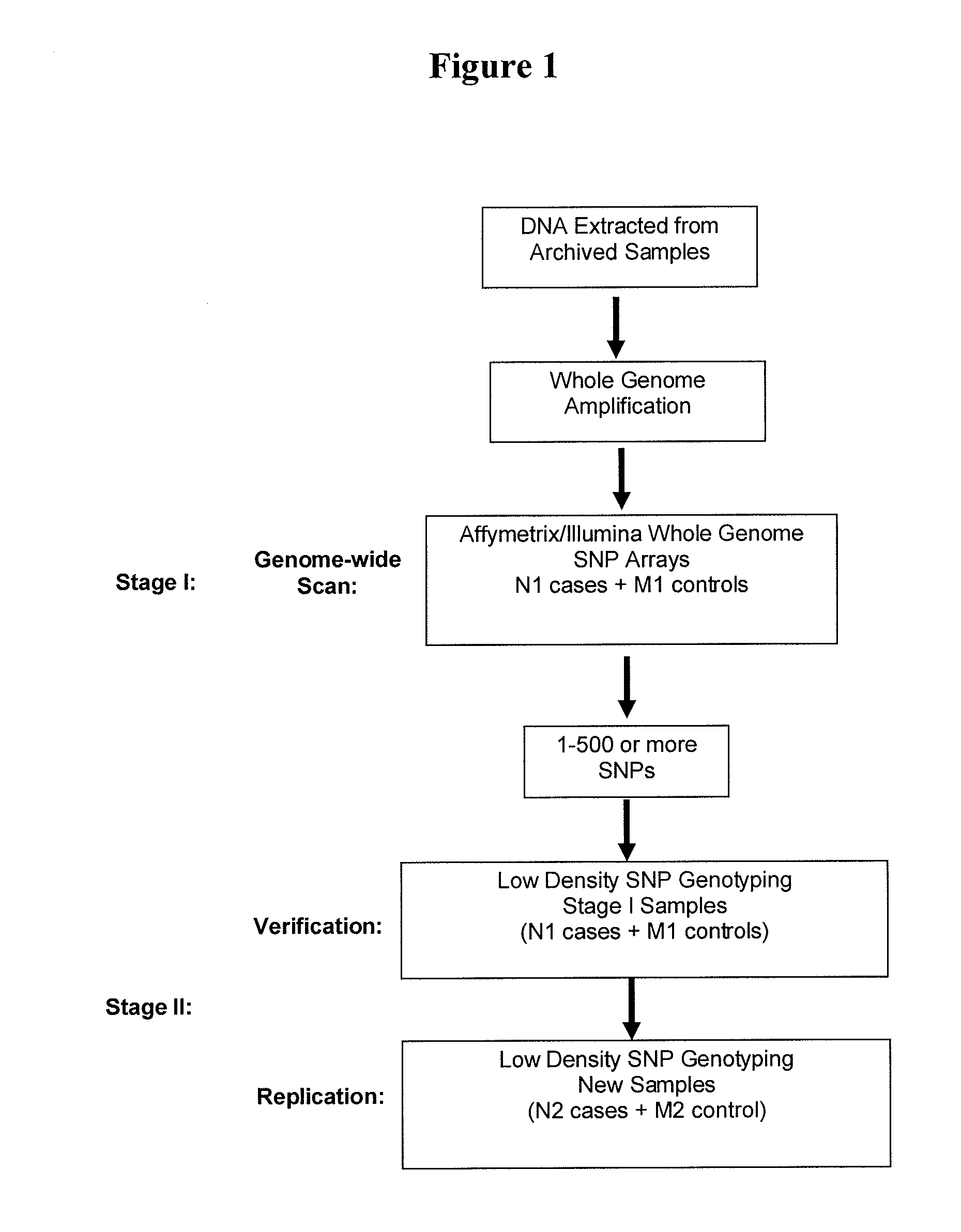
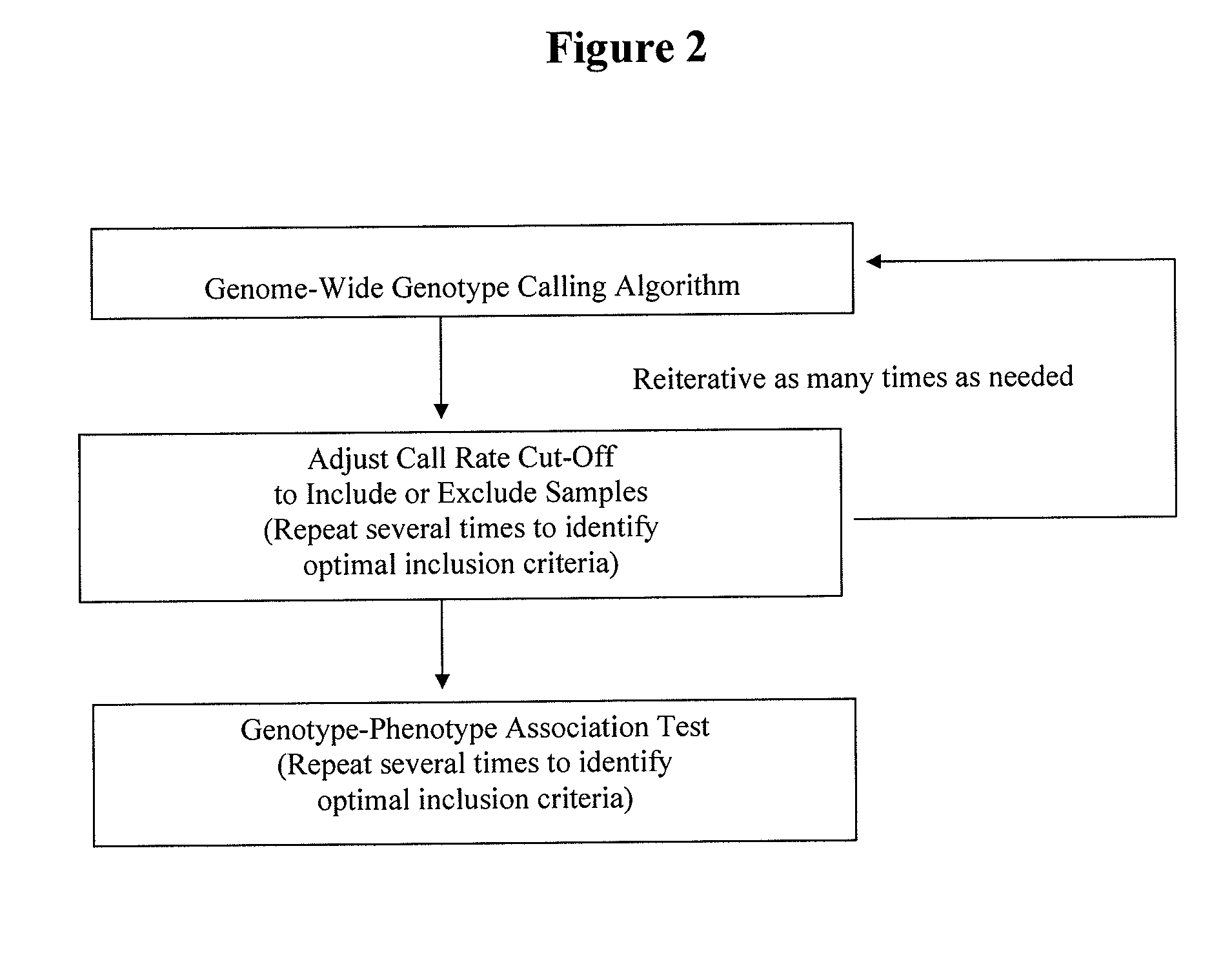
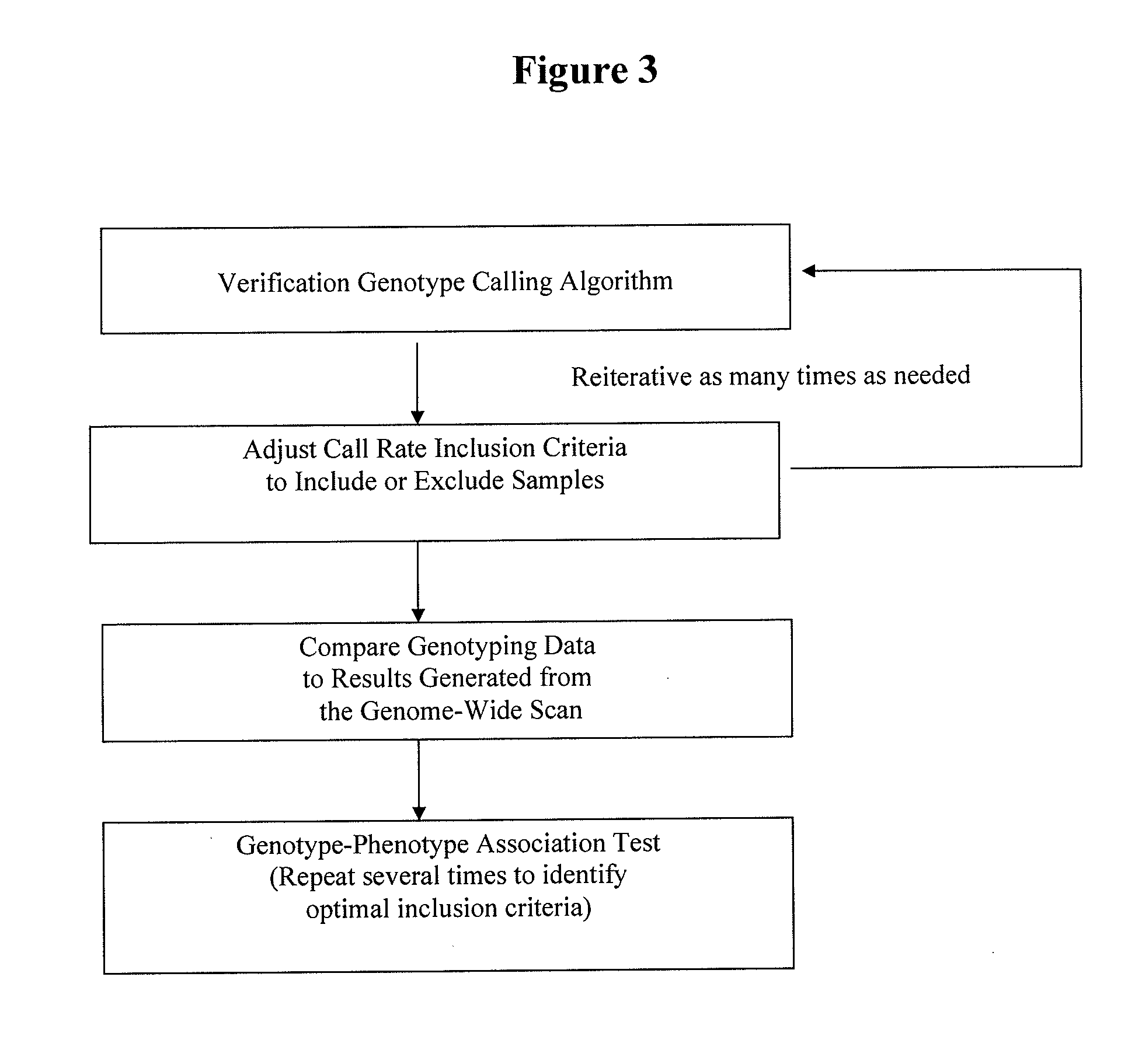
Method for discovering pharmacogenomic biomarkers
InactiveUS20140031242A1Quality improvementPredict likelihood of successSugar derivativesMicrobiological testing/measurementPharmacogenomicsDrugs response
The present invention relates to a method of discovering pharmacogenomic biomarkers that are correlated with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic agents. The present invention provides a mean to utilize archived clinical samples to perform genome-wide association study in order to identify novel pharmacogenomic biomarkers. The newly discovered biomarkers can then be developed into companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Diagnostic methods for liver disorders
InactiveUS20130085075A1Efficacy of treatmentImprove liver functionPeptide librariesMicrobiological testing/measurementRegimenLiver disorder
The present invention relates to methods of diagnosing a liver disorder in a patient, as well as methods of monitoring the progression of a liver disorder and / or methods of monitoring a treatment protocol of a therapeutic agent or regimen. The invention also relates to assay kits used in connection with the diagnostic methods described herein.
Owner:MESO SCALE TECH LLC
Ramipril formulation
InactiveUS20080108688A1Reduce the presence of impuritiesReduce degradationBiocidePill deliveryPharmacologyRamiprilat
A Ramipril formulation which is suitably stabilised to control the degradation to the active metabolite ramiprilat.
Owner:SELAMINE
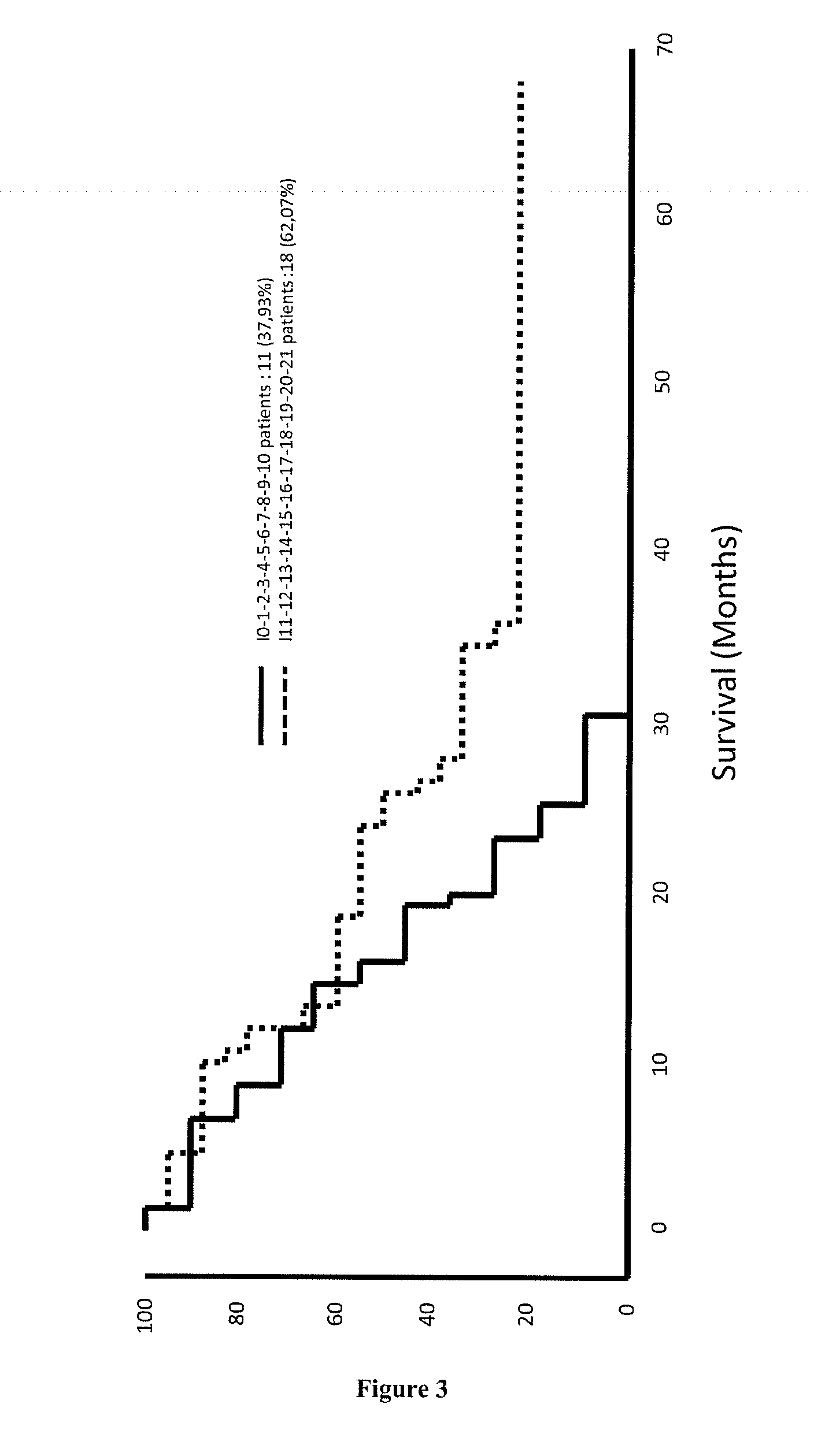
Methods for predicting the survival time and treatment responsiveness of a patient suffering from a solid cancer with a signature of at least 7 genes
ActiveUS20150203919A1Improve efficiencyEliminate side effectsBiocideMicrobiological testing/measurementCCL2Good prognosis
The present invention relates to a method for predicting the survival time of a patient suffering from a solid cancer comprising i) determining in a tumor sample obtained from the patient the gene expression level of at least 7 genes selected from the group consisting of CCR2, CD3D, CD3E, CD3G, CD8A, CXCL10, CXCL11, GZMA, GZMB, GZMK, GZMM, IL15, IRF1, PRF1, STAT1, CD69, ICOS, CXCR3, STAT4, CCL2, and TBX21, ii) comparing every expression level determined at step i) with their predetermined reference value and iii) providing a good prognosis when all expression levels determined at step i) are higher than their predetermined reference values, or providing a bad prognosis when all expression levels determined at step i) are lower than their predetermined reference values or providing an intermediate prognosis when at least one expression level determined value is higher than its predetermined value. The method is also particularly suitable for predicting the responsiveness of the patient to a treatment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
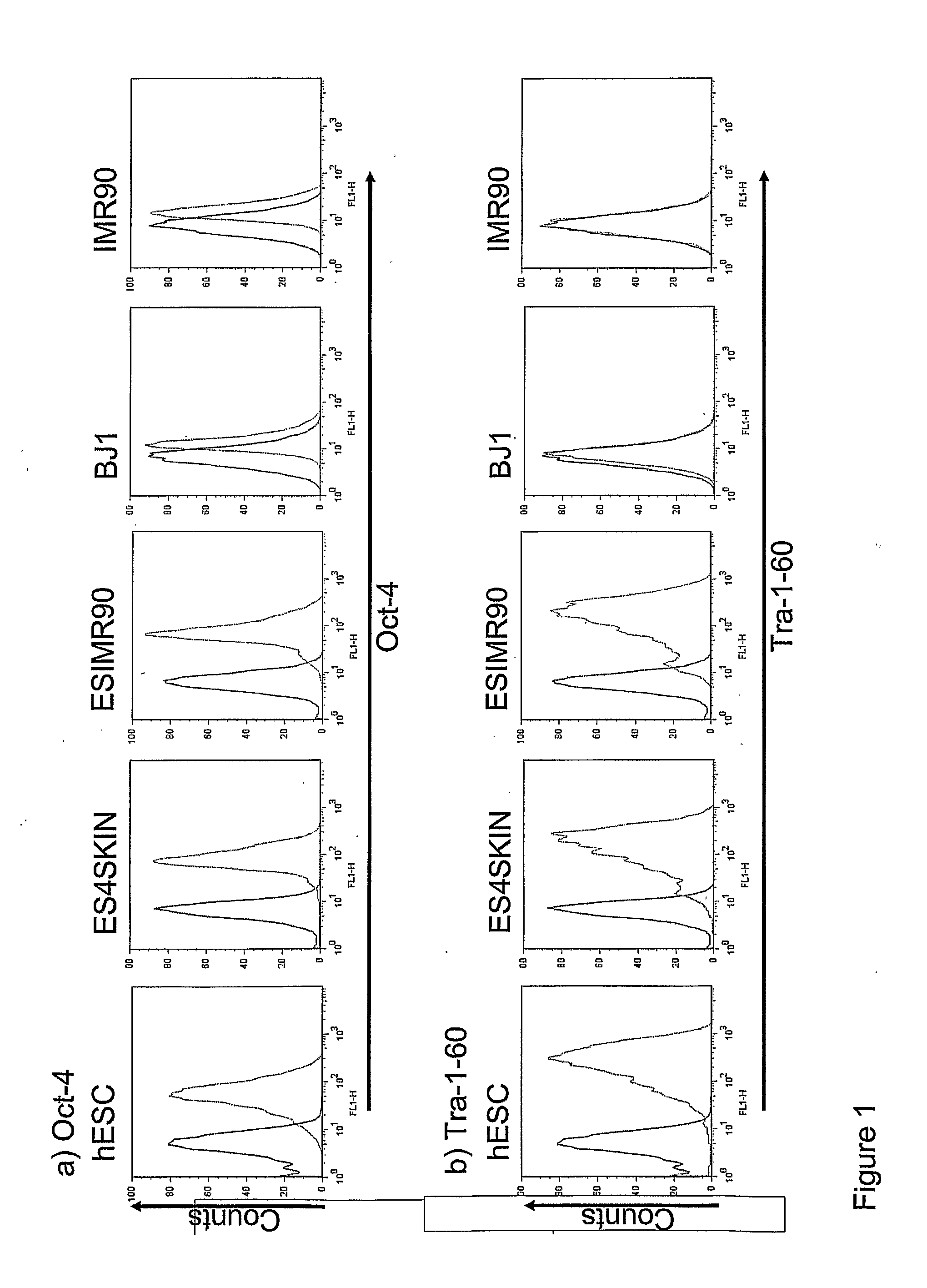
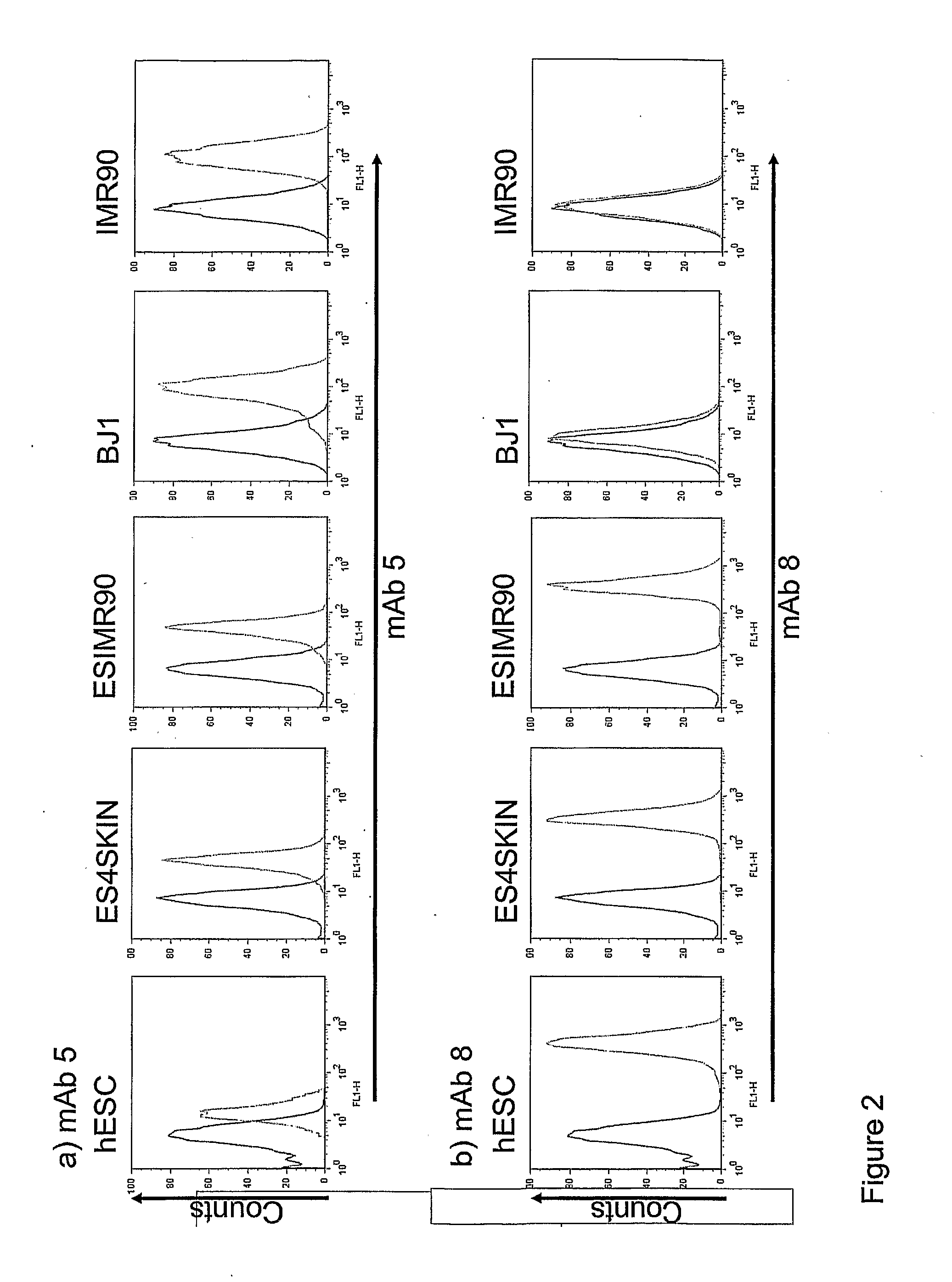
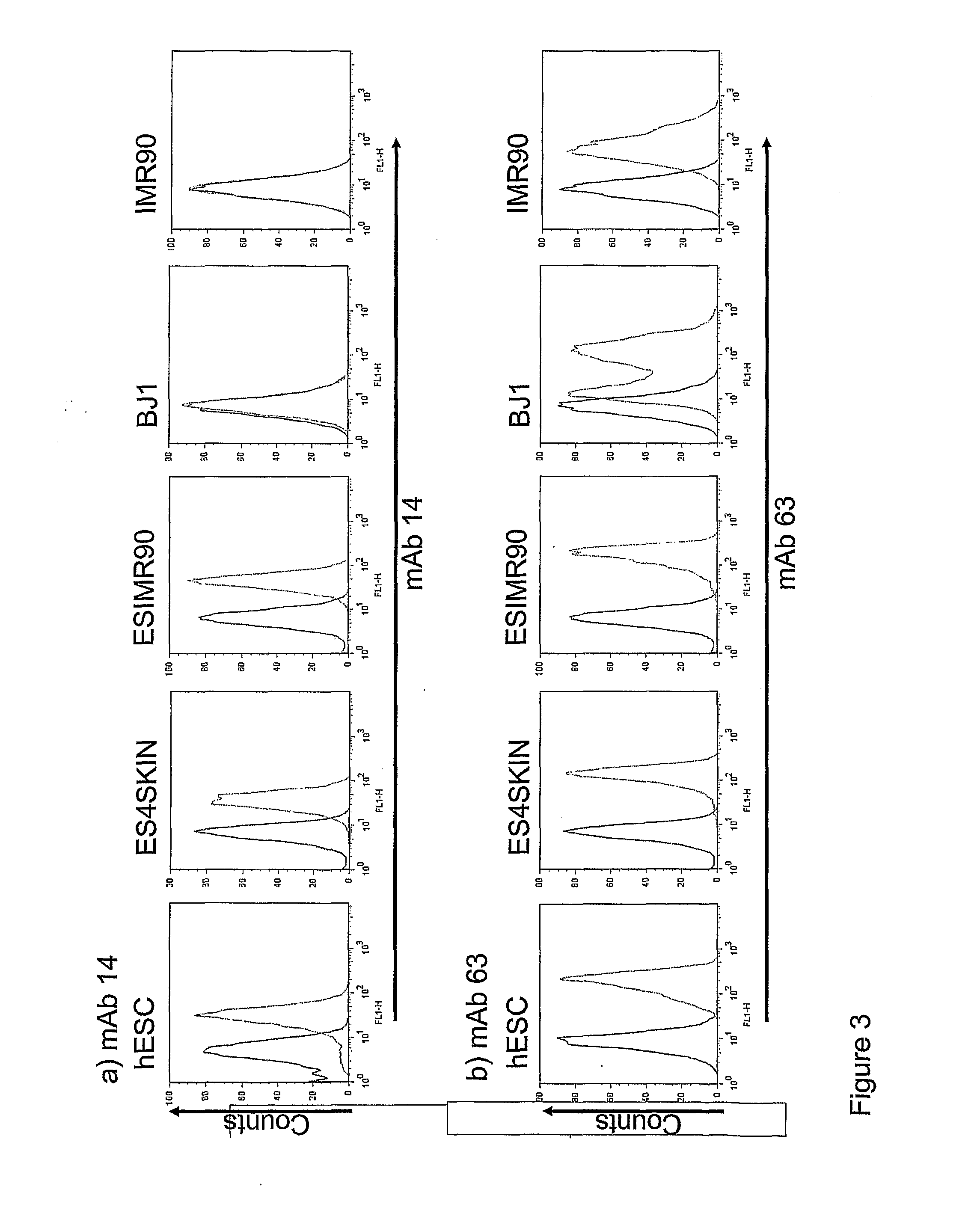
Markers of Induced Pluripotent Stem Cells
InactiveUS20110171183A1Increasing success and safetyReduce riskBiocideNervous disorderPODOCALYXIN-LIKE PROTEINHuman Induced Pluripotent Stem Cells
The disclosure relates to the expression of podocalyxin-like protein (PODXL) on the surface of induced pluripotent stem cells and particularly, although not exclusively, to the use of PODXL as a marker of induced pluripotent stem cells.
Owner:AGENCY FOR SCI TECH & RES
Blood Plasma Fractions as a Treatment for Aging-Associated Cognitive Disorders
InactiveUS20190321449A1Efficacy of treatmentNervous disorderPeptide/protein ingredientsCognitive disorderBlood plasma
Methods and compositions for treating and / or preventing aging-related conditions are described. The compositions used in the methods include fractions derived from blood plasma with efficacy in treating and / or preventing aging-related conditions such as neurocognitive disorders.
Owner:ALKAHEST INC
Methods and kits for identifying effector treg cells
ActiveUS20160169891A1Efficiently differentiate suppressiveEfficiently differentiate suppressive eTreg cellsAntipyreticAnalgesicsTreg cellPopulation
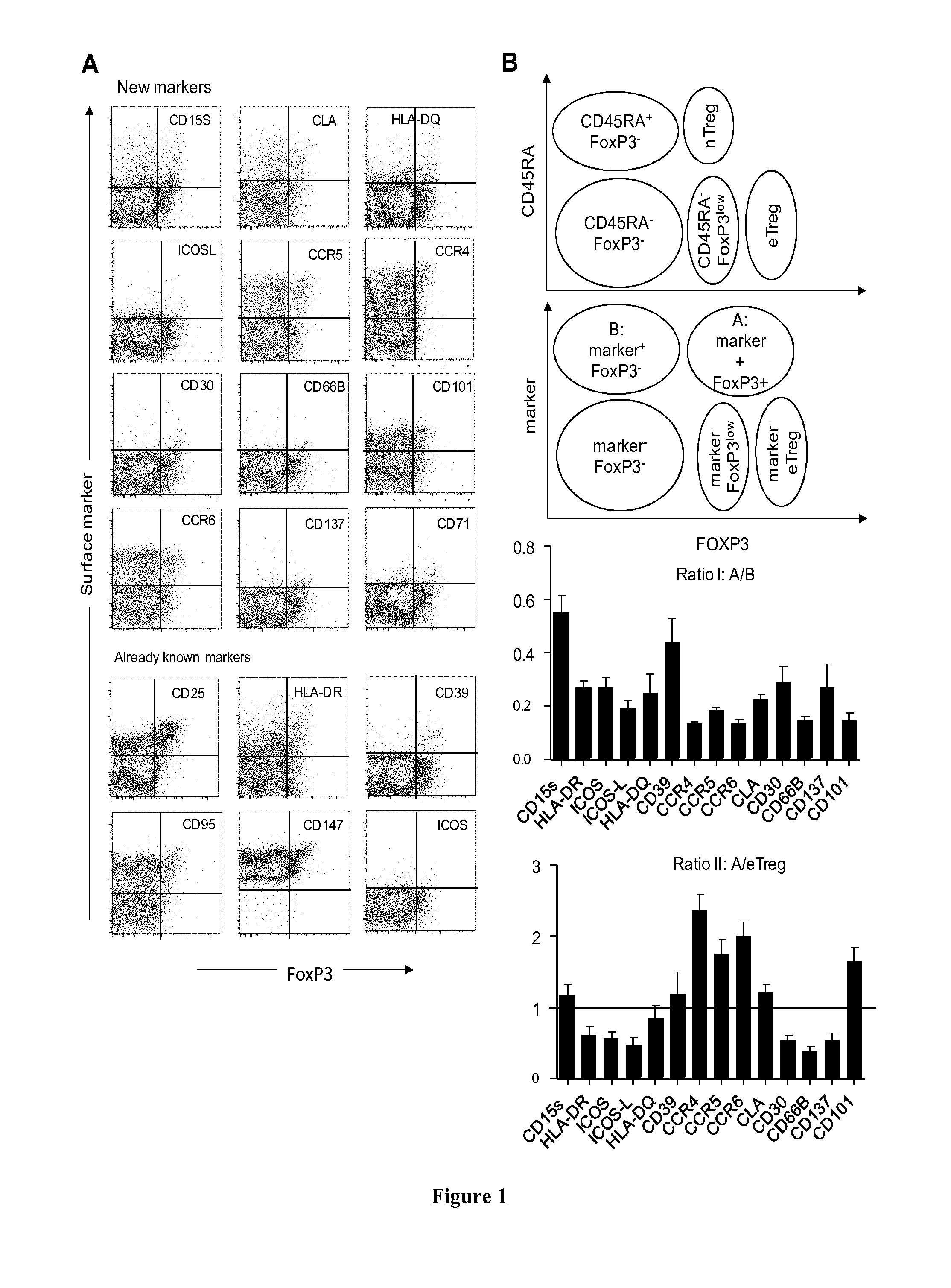
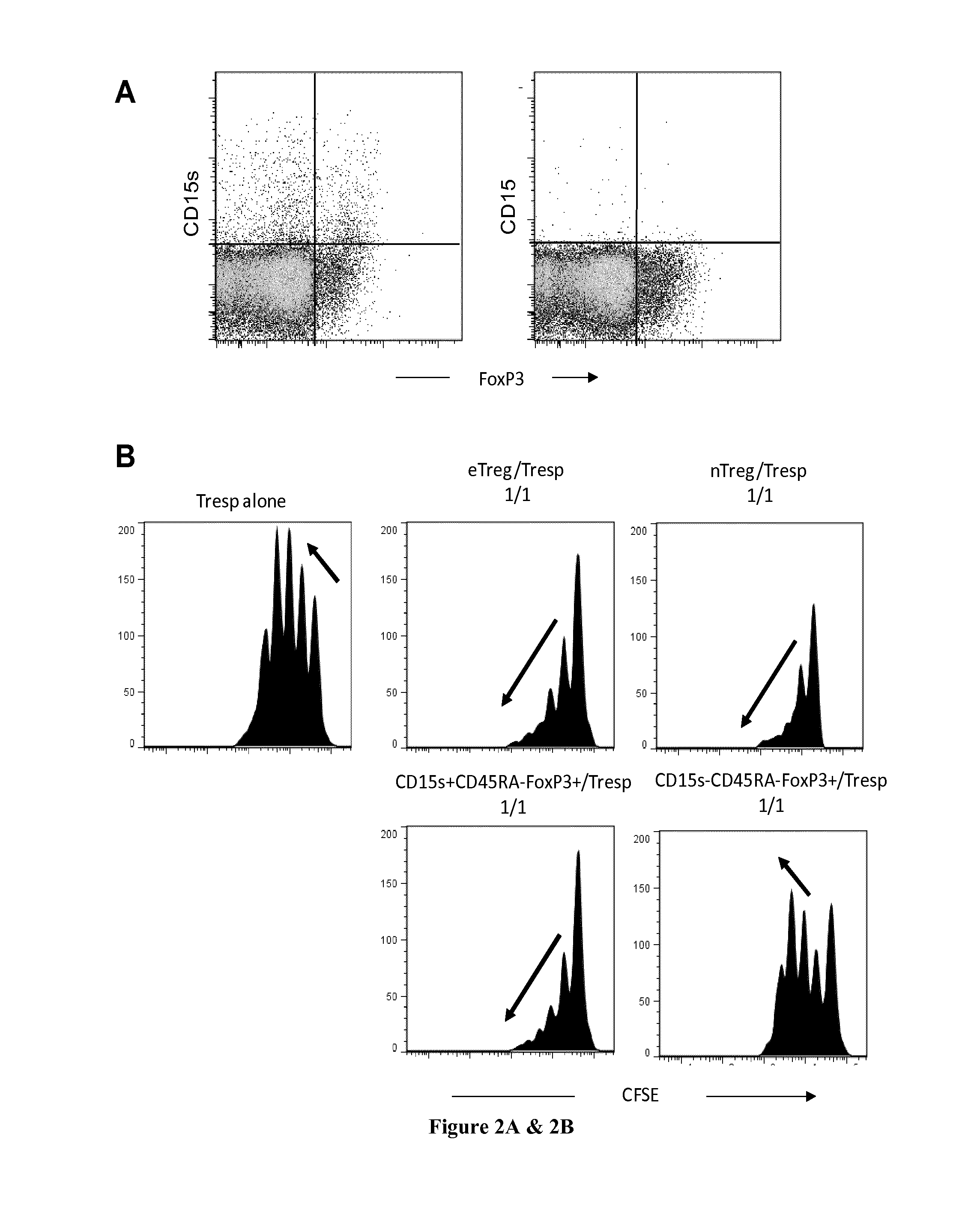
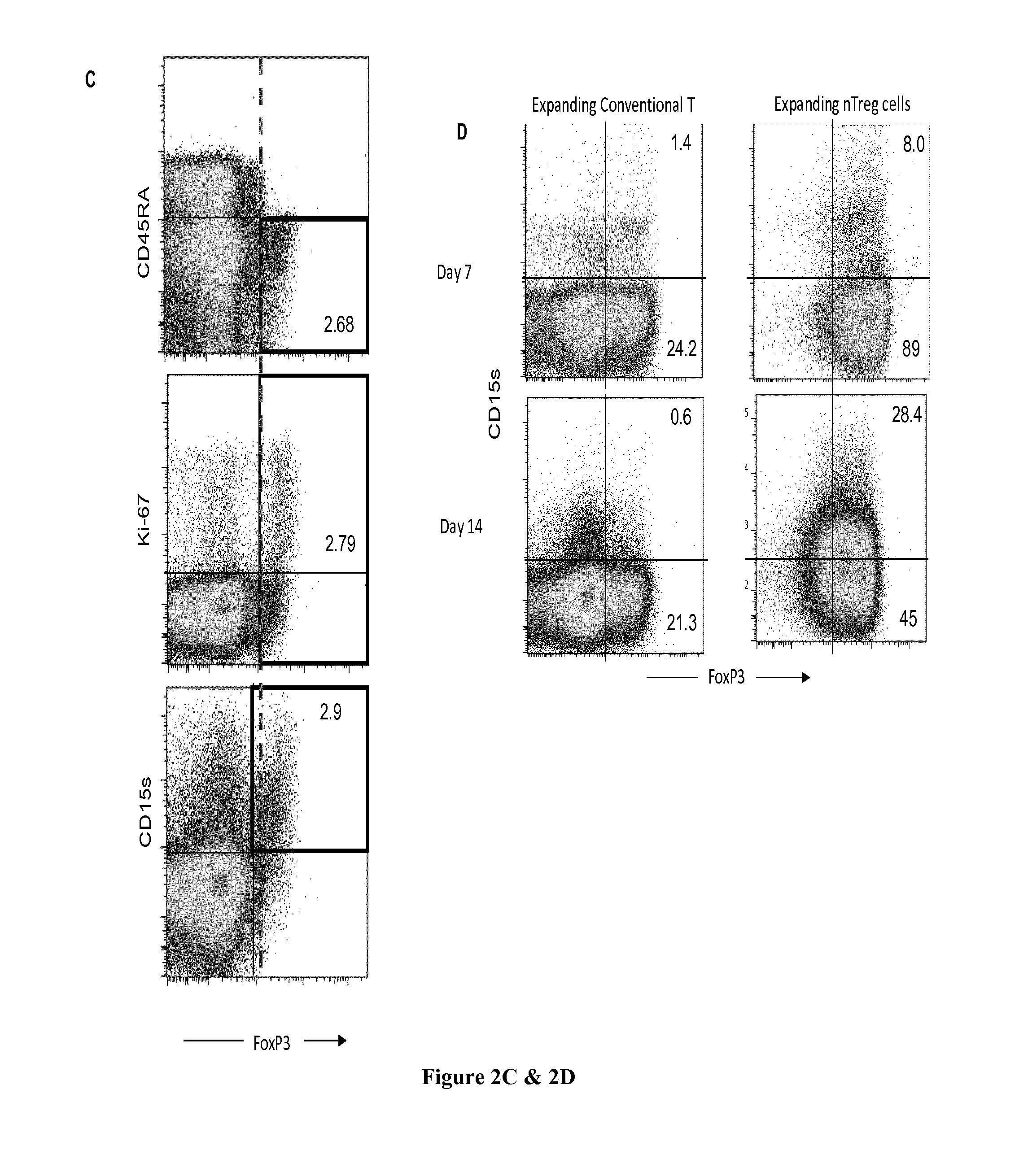
The present invention relates to methods and kits for identifying effector regulatory T cells. In particular the present invention relates to use of CD15s as a biomarker for eTreg cells. The present invention also relates to a method for identifying effector Treg cells (eTreg) in a fluid sample comprising the steps of i) detecting the cell surface expression of CD4, CD25, CD127 and CD15s markers on the cell population contained in the fluid sample and ii) concluding that the cells expressing CD4, CD25, CD127 at low levels and CD15s are the effector Treg cells. The present invention also relates to a method for identifying effector Treg cells (eTreg) in a tissue sample comprising the steps of i) detecting the cell expression of CD4, CD25, Foxp3 and CD15s markers and ii) concluding that the cells expressing CD4, CD25, Foxp3 and CD15s are the effector Treg cells.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Treatment of evolving bacterial resistance diseases including klebsiella pneumoniae with liposomally formulated glutathione
InactiveUS20150374626A1Efficacy of treatmentAntibacterial agentsBiocideAntibiotics therapyAnimal testing
The composition of the invention, liposomal glutathione, has been recently shown to have utility for having an antibiotic like effect on Klebsiella pneumonia cultures in vitro, and in vivo as demonstrated by efficacy in reducing by large multiples the presence of cultures of Klebsiella in rats in animal tests. Further, because the liposomal glutathione bolsters body defenses as well as appearing to have direct killing action, the propensity to create more and more resistant strains to antibiotic treatment is downgraded.
Owner:YOUR ENERGY SYST
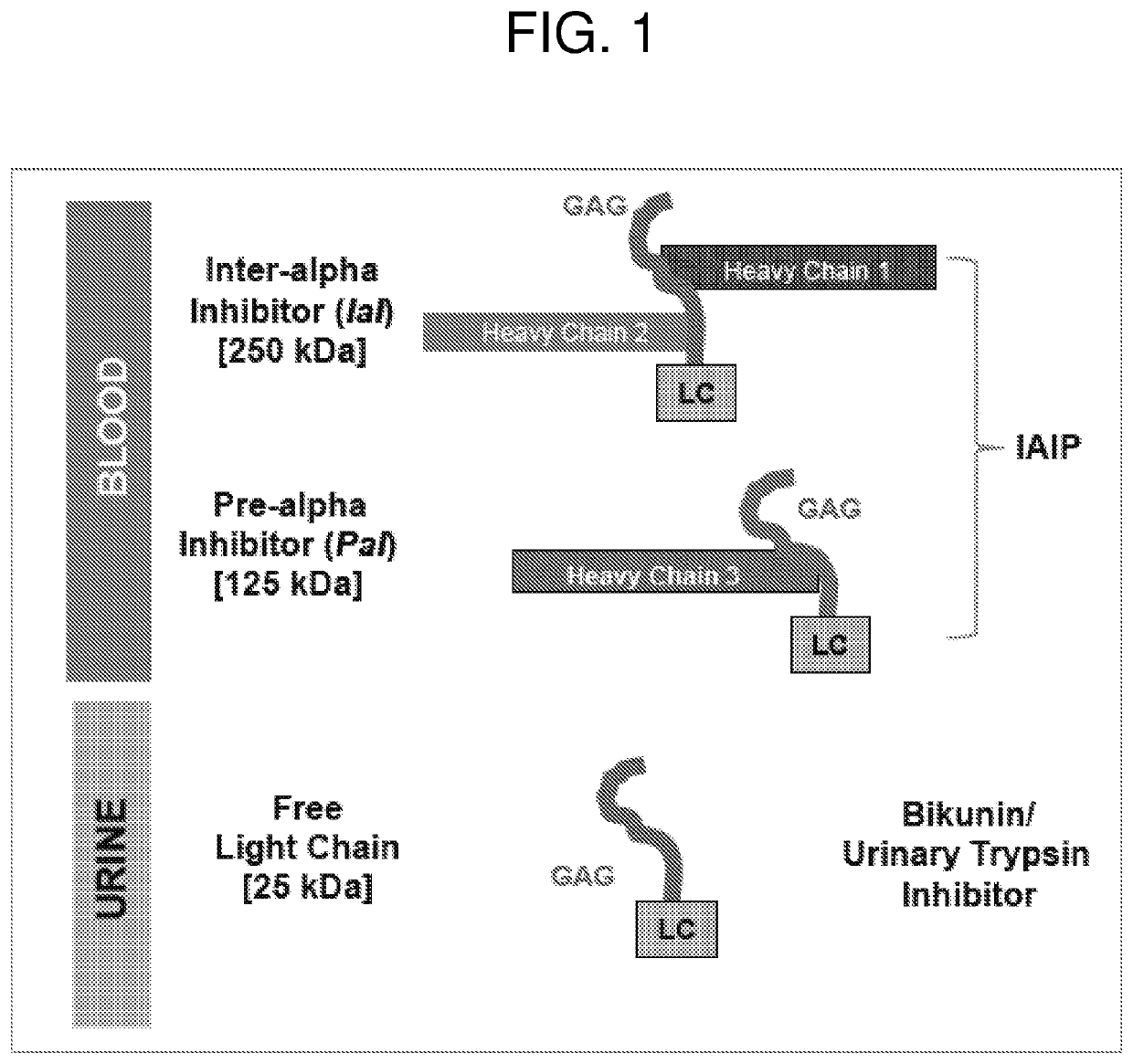
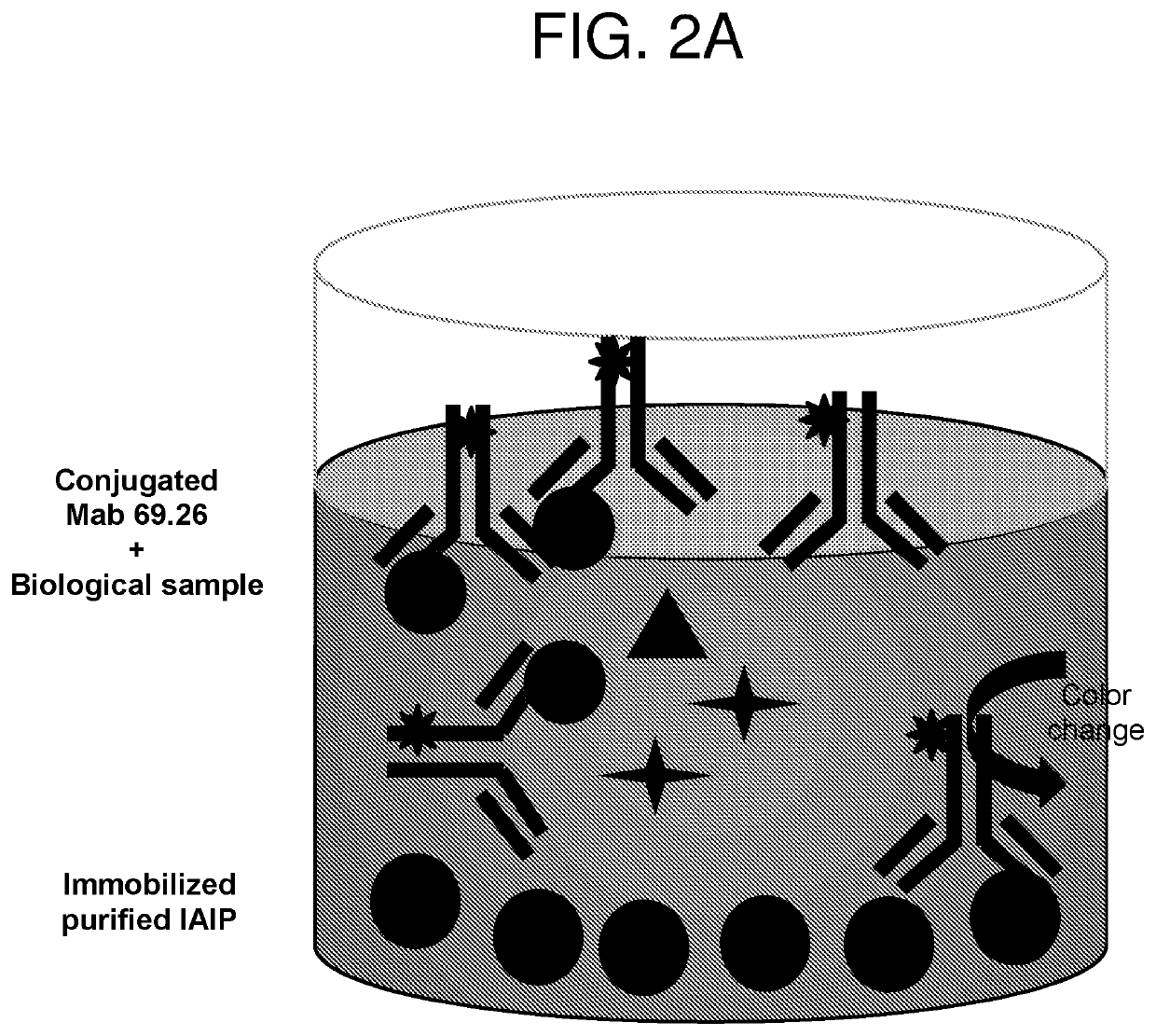
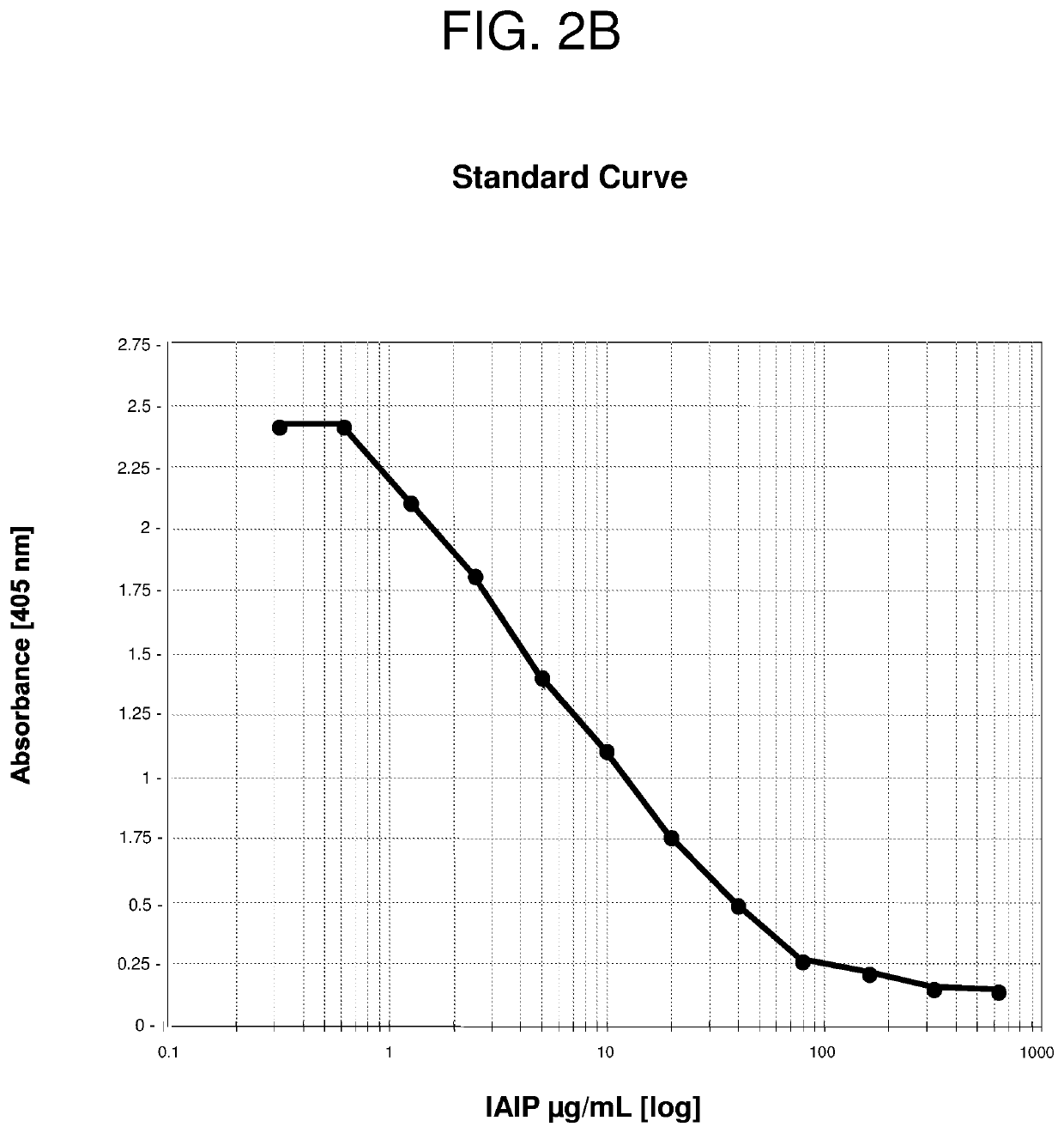
Methods for quantifying inter-alpha inhibitor proteins
InactiveUS20200057077A1Reducing and ameliorating disorderReducing and ameliorating and symptomPeptide/protein ingredientsAntipyreticAssayCell biology
Described herein are methods for quantifying lAIP levels in a sample (e.g., from a subject) using lAIP ligand-based assays. Also disclosed are methods for measuring lAIP-IAIP ligand complexes, and methods of evaluating, monitoring, and treating subjects using the aforementioned lAIP quantification methods.
Owner:POROTHERA BIOLOGY
Method for inducing selectively suppressed immune response to transplanted tissue or cells
InactiveUS7625557B2Raise the possibilitySuppress immune rejectionUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingAbnormal tissue growthDendritic cell
Transimmunization methods incorporating skin immunologic challenges are described for either selectively suppressing the immune response of recipients of transplanted tissue or cells or monitoring induced anti-cancer immunity. In one embodiment, skin from the transplant donor is allografted to the transplant recipient to induce an immunological response to the transplanted skin. A quantity of blood is taken from the recipient and treated to render the T cells in the blood apoptotic and to induce differentiation of blood monocytes into dendritic cells. The treated blood is incubated and administered to the recipient to induce formation of suppressor T cell clones which reduce the number of T cells attacking the transplanted tissue or organ. This tolerogenic approach can be complemented by also feeding the immature dendritic cells apoptotic or necrotic cells from the organ donor. In a second embodiment, dendritic cells loaded with tumor antigens are injected intradermally to monitor the anti-cancer immunity induced by Transimmunization.
Owner:YALE UNIV
Methods for identifying inflammatory bowel disease patients with dysplasia or cancer
InactiveUS20140179549A1Efficacy of treatmentMicrobiological testing/measurementLibrary screeningTherapy efficacyInflammatory bowel disease
The present invention provides methods for identifying IBD patients with dysplasia or cancer. In particular embodiments, the methods of the invention may comprise determining the presence or level of at least one or a panel of miRNAs in a sample obtained from an IBD patient to establish a miRNA expression profile, and comparing the miRNA expression profile with one or more pre-established model miRNA expression profiles. The present invention further provides methods for monitoring the efficacy of treatment of IBD patients with dysplasia or cancer.
Owner:NESTEC SA +1
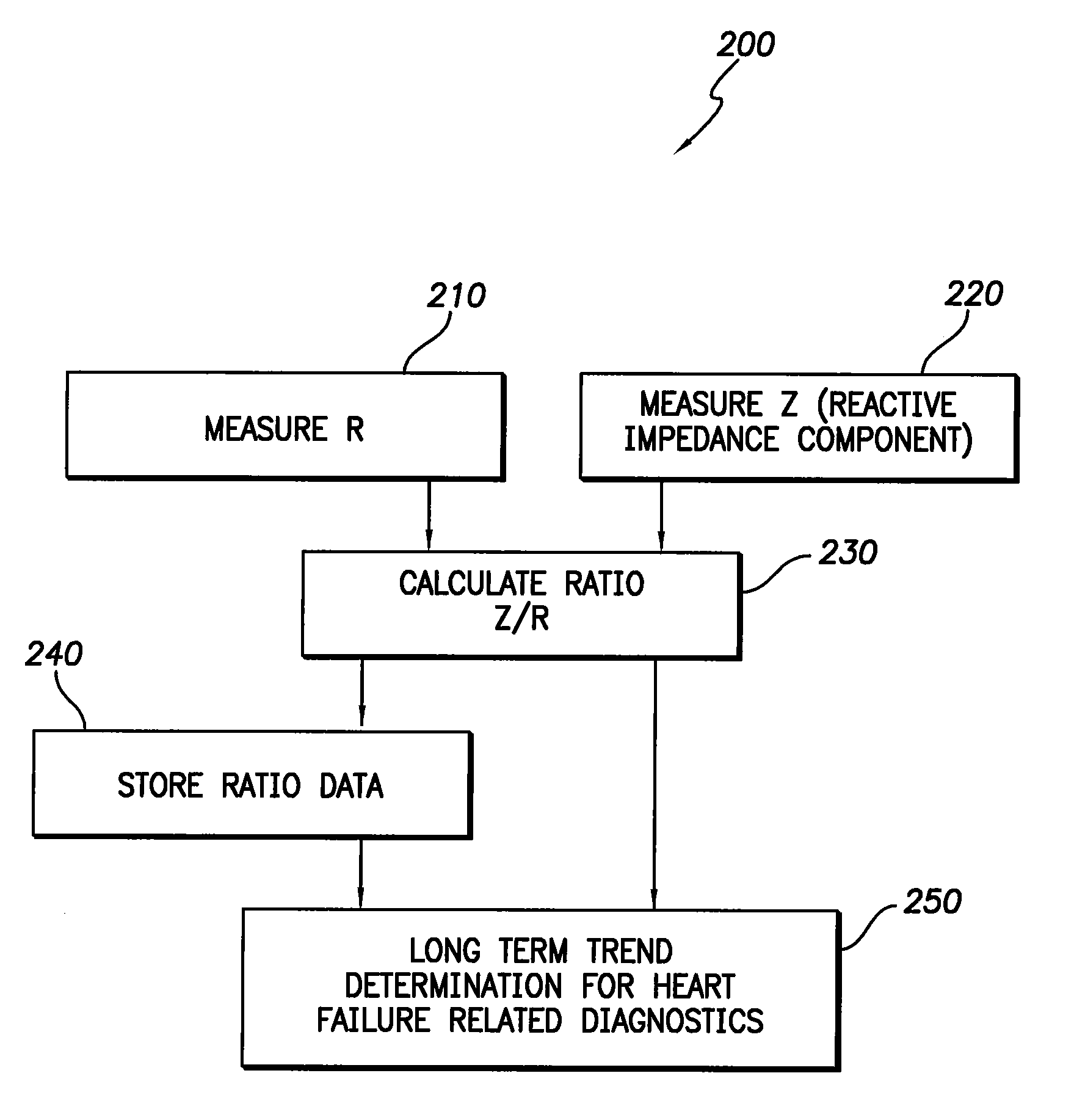
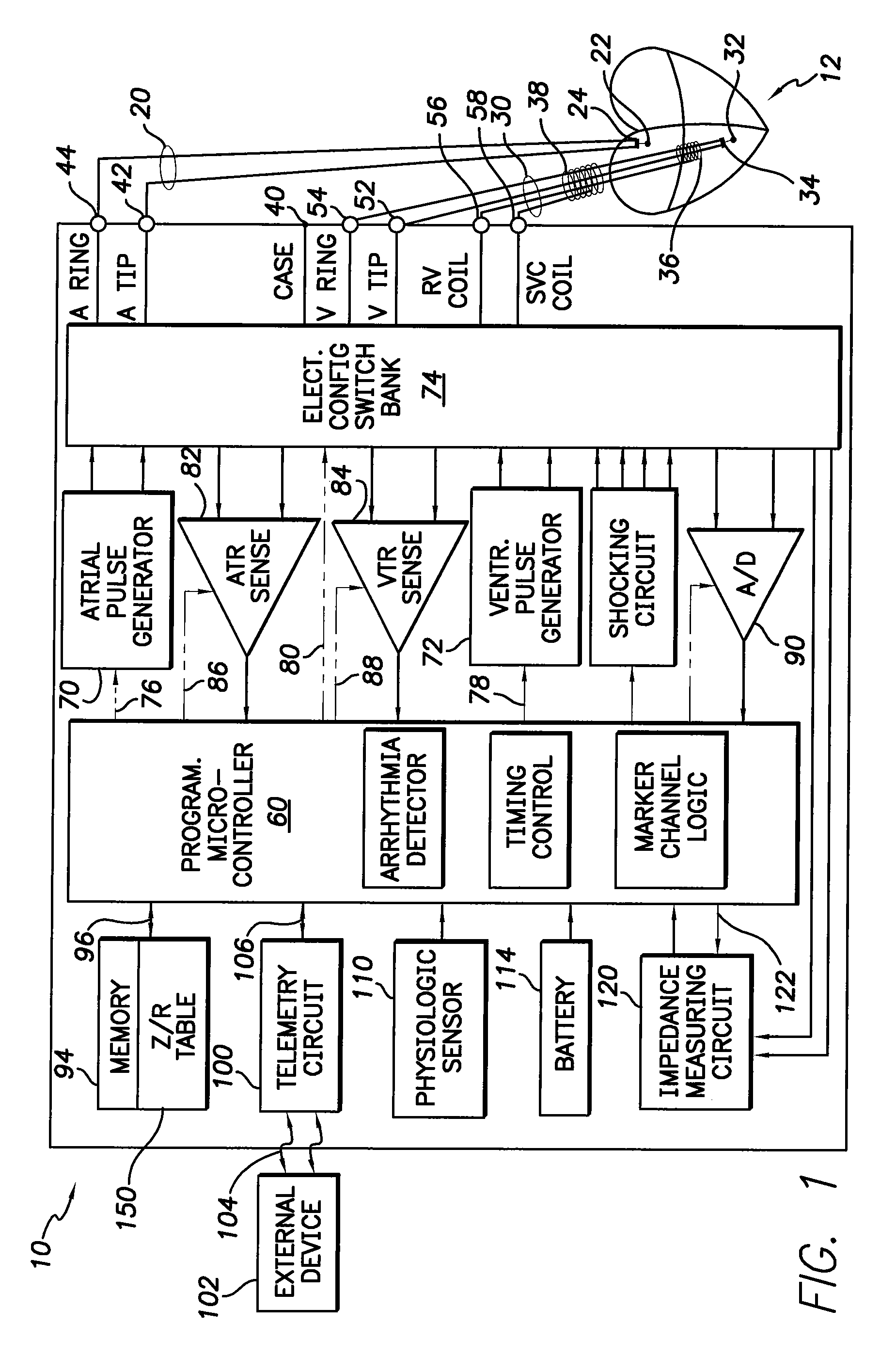
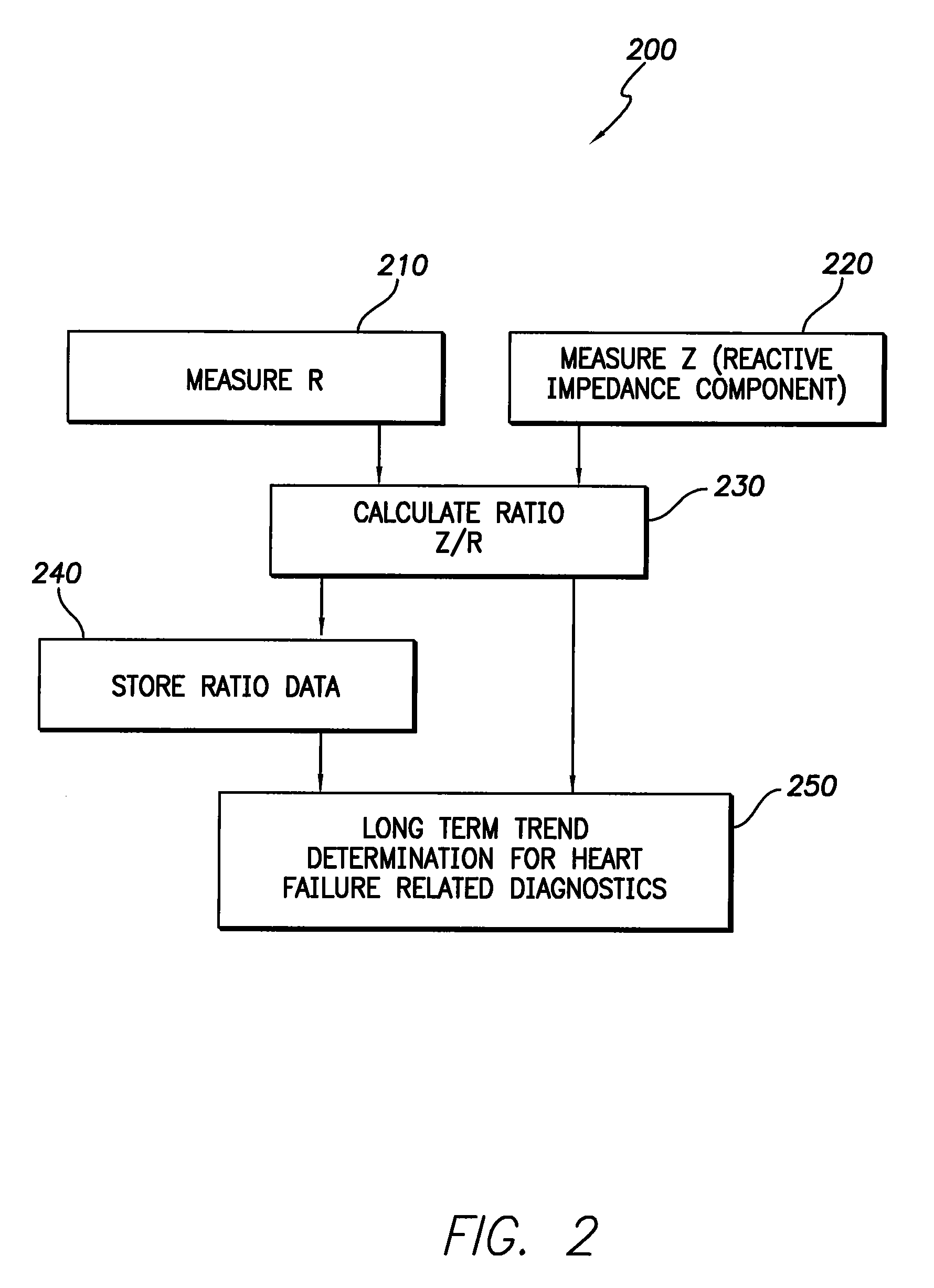
Apparatus and method for two-component bioelectrical impedance ratio measuring and monitoring
InactiveUS7596411B1Reduce and eliminate negative impactRemove Motion ArtifactsElectrotherapyDiagnostic recording/measuringElectrical resistance and conductanceMicrocontroller
An implantable cardiac stimulation and rhythm management device includes an impedance measuring circuit which determines a patient's intra-thoracic impedance and the resistance and reactance components of the impedance. The device includes a microcontroller which calculates a ratio (Z / R) which equals the reactance (Z) divided by the resistance (R). The microcontroller is configured to use the calculated ratios to establish a baseline intra-thoracic fluid level, an upper bound relative to the baseline, and a lower bound relative to the baseline, and to monitor the Z / R ratio relative to the baseline and upper and lower bounds. When the Z / R ratios are outside of the established bounds, operating parameters of the stimulation and rhythm management may be altered by the microcontroller.
Owner:PACESETTER INC
Pentacyclic triterpenoid compound with modified structure and preparation method and use thereof
ActiveUS9725482B2Efficacy of treatmentInhibition of activationSteroidsImmunological disordersAutoimmune conditionMedicine
The present invention relates to new pentacyclic triterpenes, their preparation method and use. The compounds of the present invention could effectively treat psoriasis and selectively inhibit in vitro differentiation of the TH1 and TH17 cells, thereby could be used to treat the TH1- or TH17-mediated autoimmune diseases.
Owner:SUZHOU BOTANY BIOMEDICALS
Method for inducing selectively suppressed immune response to transplanted tissue or cells
InactiveUS7988951B2Raise the possibilitySuppress immune rejectionCompounds screening/testingUltrasonic/sonic/infrasonic diagnosticsAbnormal tissue growthT-cell apoptosis
Transimmunization methods incorporating skin immunologic challenges are described for either selectively suppressing the immune response of recipients of transplanted tissue or cells or monitoring induced anti-cancer immunity. In one embodiment, skin from the transplant donor is allografted to the transplant recipient to induce an immunological response to the transplanted skin. A quantity of blood is taken from the recipient and treated to render the T cells in the blood apoptotic and to induce differentiation of blood monocytes into dendritic cells. The treated blood is incubated and administered to the recipient to induce formation of suppressor T cell clones which reduce the number of T cells attacking the transplanted tissue or organ. This tolerogenic approach can be complemented by also feeding the immature dendritic cells apoptotic or necrotic cells from the organ donor. In a second embodiment, dendritic cells loaded with tumor antigens are injected intradermally to monitor the anti-cancer immunity induced by Transimmunization.
Owner:YALE UNIV
Method and activated lymphocyte preparations for preventing recurrence of carcinoma
InactiveUS7105155B2Prevent relapseEfficacy of treatmentBiocideDrug and medicationsCancer preventionInterleukin II
Activated lymphocytes are administered to a cancer patient at least five or more times within eight months after performing surgical and chemotherapeutic treatment or radiotherapy for treating cancer particularly liver cancer, so that recurrence of the cancer can be prevented over a long period of five or more years. The activated lymphocytes to be administered while performing treatment of cancer may be autologously derived from a cancer patient or collected from the other cancer patient at need. The activated lymphocytes can be cultivated for proliferating or activating lymphocyte cells collected in the presence of solid-phase anti-CD3 antigen and interleukin 2.
Owner:LYMPHOTEC
Method and device for monitoring a therapeutic treatment regime
InactiveUS20100304361A1Easy to carryEfficacy of treatmentBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineSolid substrate
A method for monitoring a therapeutic treatment regime in an individual having a disease, comprises: providing at a first location a solid substrate capable of immobilising a biomarker characteristic of the disease and a therapeutic compound in the biological sample, contacting the biological sample with the solid substrate to immobilise the biomarker and the therapeutic compound; transferring the solid substrate with the immobilised biomarker and therapeutic compound to a second location; performing an extraction step on the solid substrate to extract the biomarker and the therapeutic compound; performing a first detection assay to detect and / or quantify the biomarker, performing a second detection assay to quantify the therapeutic compound; and correlating the detection and / or quantity of the biomarker with the disease state of the individual, and comparing the quantity of the therapeutic compound with a target level for treatment of the disease, thereby to assess the efficacy of the treatment regime.
Owner:DAS DEV PARTNERS
Methods for predicting the survival time and treatment responsiveness of a patient suffering from a solid cancer with a signature of at least 7 genes
ActiveUS11242564B2Increase productionEasier for the immune system to recognise and destroyMicrobiological testing/measurementAntineoplastic agentsCCL2Favorable prognosis
The present invention relates to a method for predicting the survival time of a patient suffering from a solid cancer comprising i) determining in a tumor sample obtained from the patient the gene expression level of at least 7 genes selected from the group consisting of CCR2, CD3D, CD3E, CD3G, CD8A, CXCL10, CXCL11, GZMA, GZMB, GZMK, GZMM, IL15, IRF1, PRF1, STAT1, CD69, ICOS, CXCR3, STAT4, CCL2, and TBX21, ii) comparing every expression level determined at step i) with their predetermined reference value and iii) providing a good prognosis when all expression levels determined at step i) are higher than their predetermined reference values, or providing a bad prognosis when all expression levels determined at step i) are lower than their predetermined reference values or providing an intermediate prognosis when at least one expression level determined value is higher than its predetermined value. The method is also particularly suitable for predicting the responsiveness of the patient to a treatment.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
System and method for targeting relevant research activity in response to angiogenic regulator analyses
ActiveUS8874378B2Highly targeted and unique mannerEfficacy of treatmentDisease diagnosisProteomicsDiseaseCorrelational study
A system and method for targeting relevant research activity for clinical application in response to angiogenic regulator analyses. An angiogenic analysis is performed on a patient blood sample in order to detect the level of each of at least ten angiogenic regulators. The levels of the tested regulators are used as indexes to identify relevant peer-reviewed research publications from among a large database of articles. The most relevant peer-reviewed literature reporting research and studies that have been conducted to identify, moderate, and define the mechanisms unique to individual and combinations of angiogenic regulators for various disease states are then provided to the patient and / or to the patient's physician, optionally in conjunction with a summarization of the treatment recommendations gleaned from the provided literature. The customized information delivery provides the patient and physician a range of published peer-reviewed therapeutic options and published research studies for moderating the out of range regulators to within normal range or other diagnostic significant range.
Owner:LAMBERT REBECCA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com