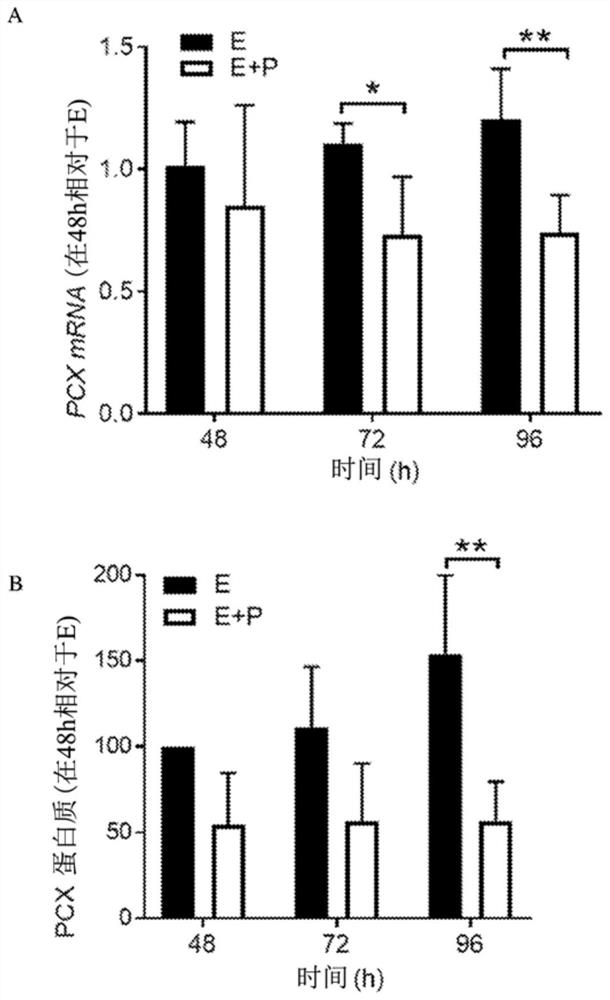
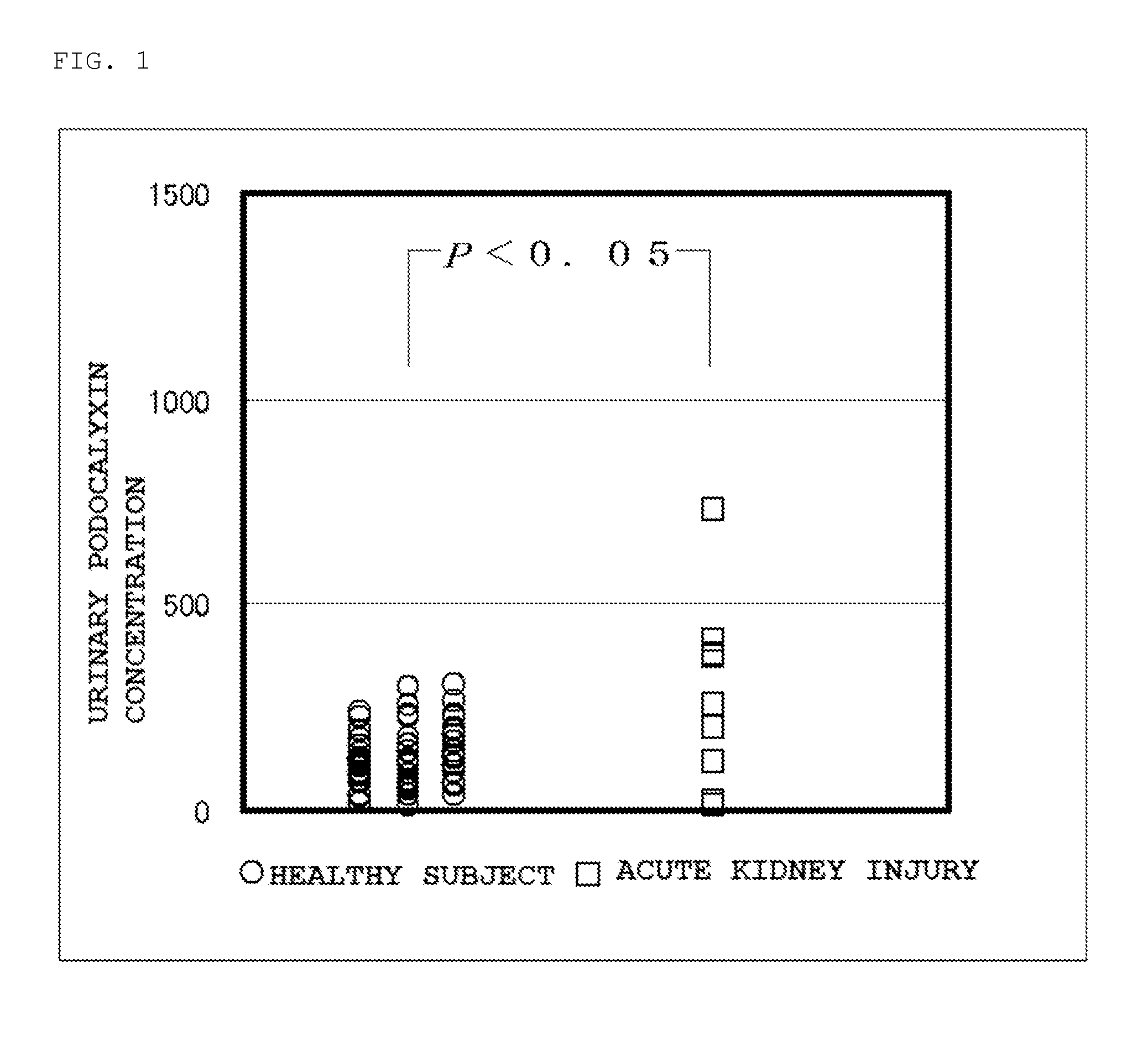
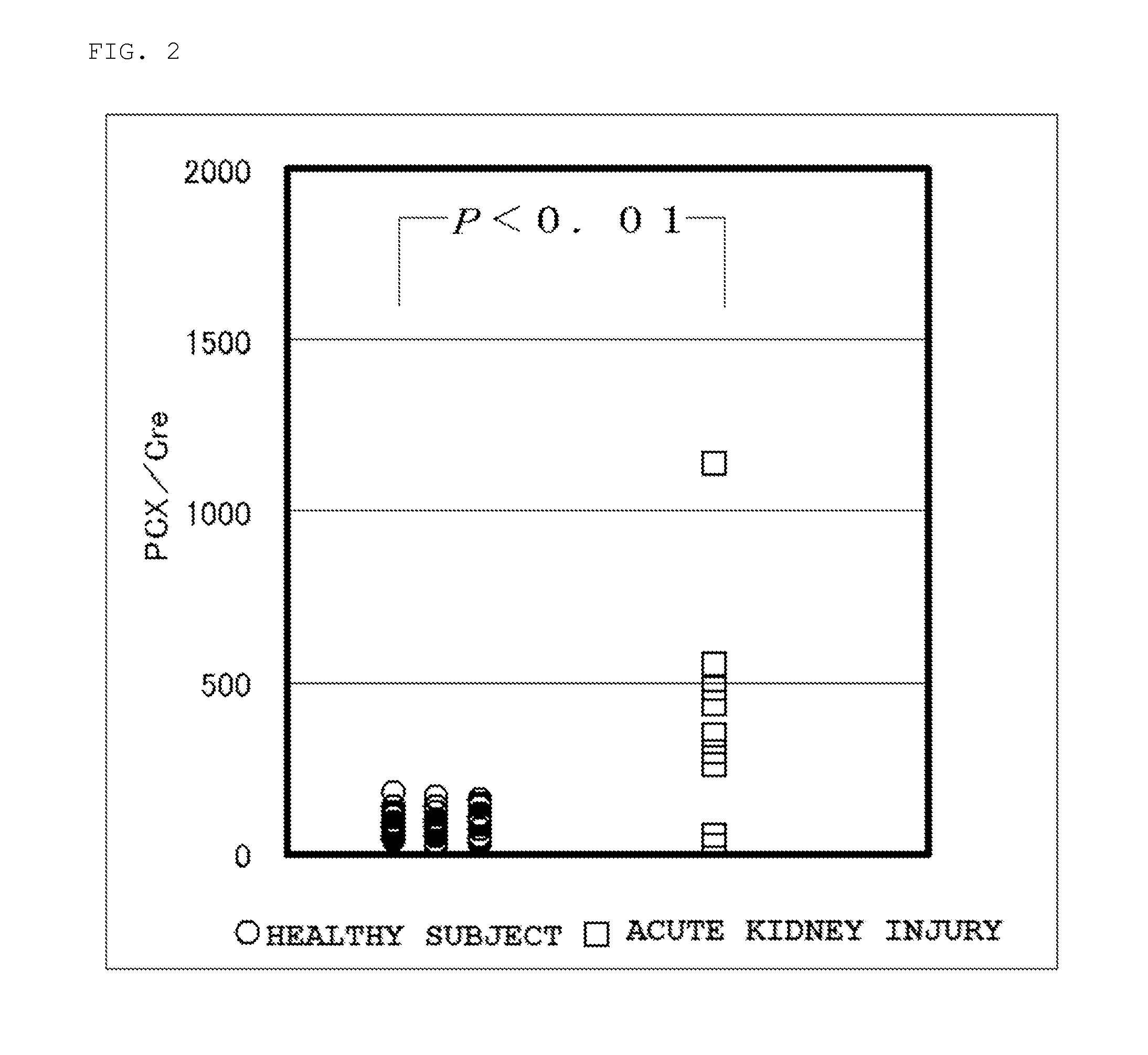
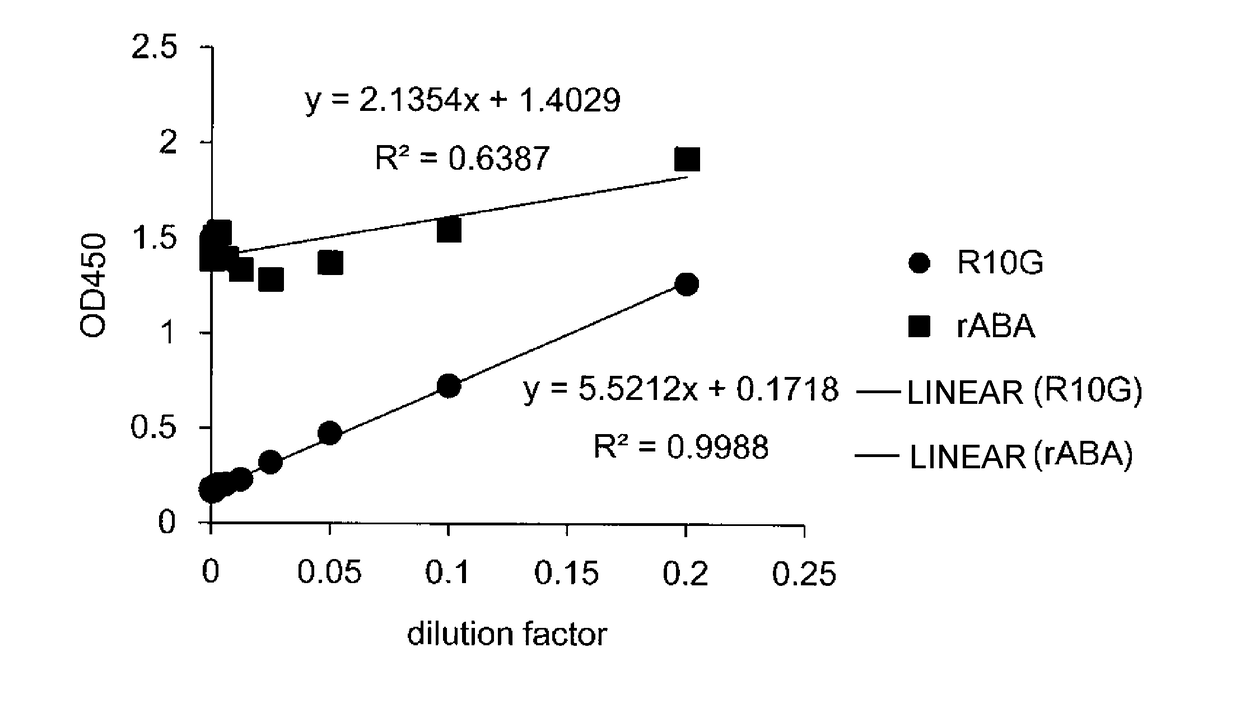
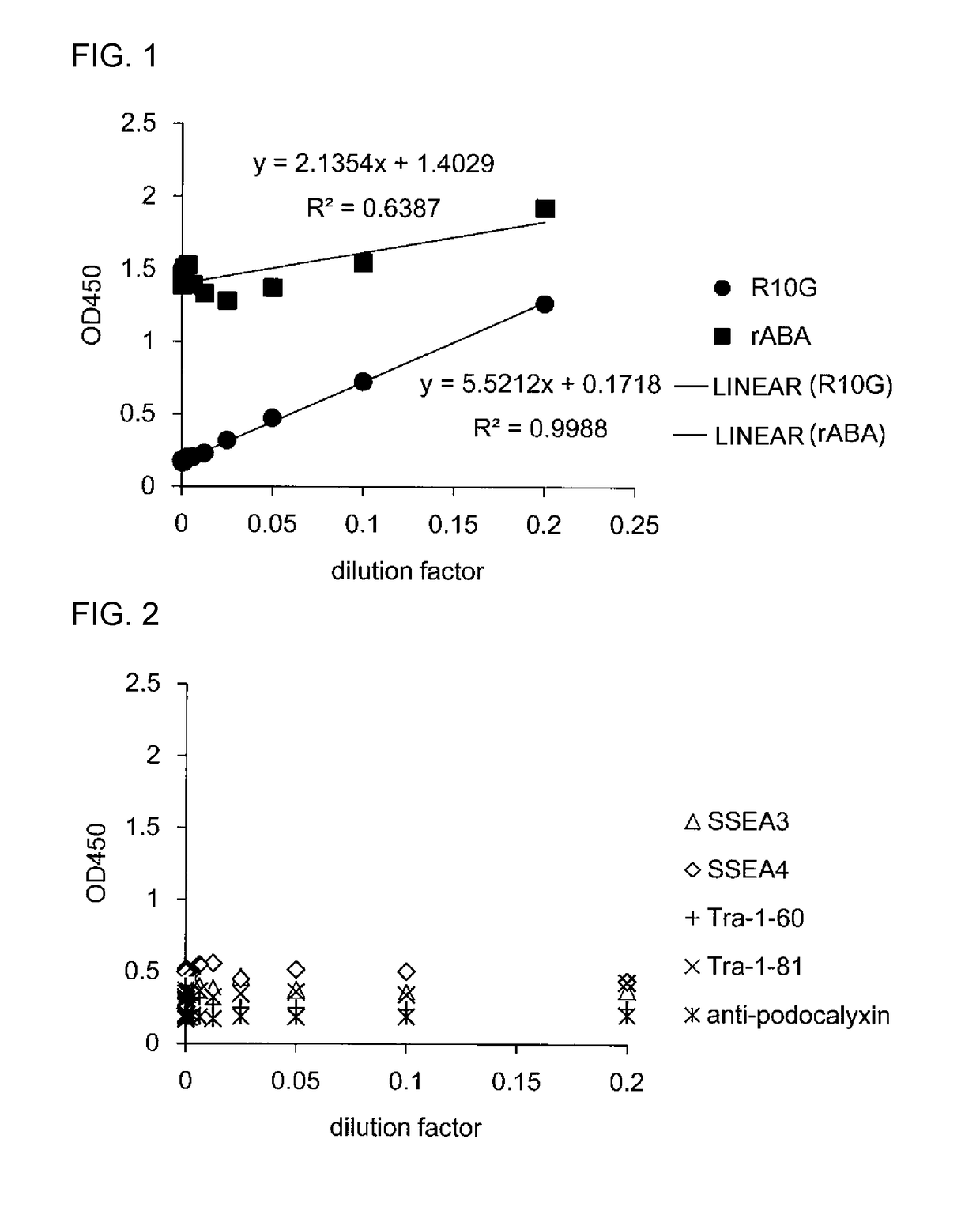
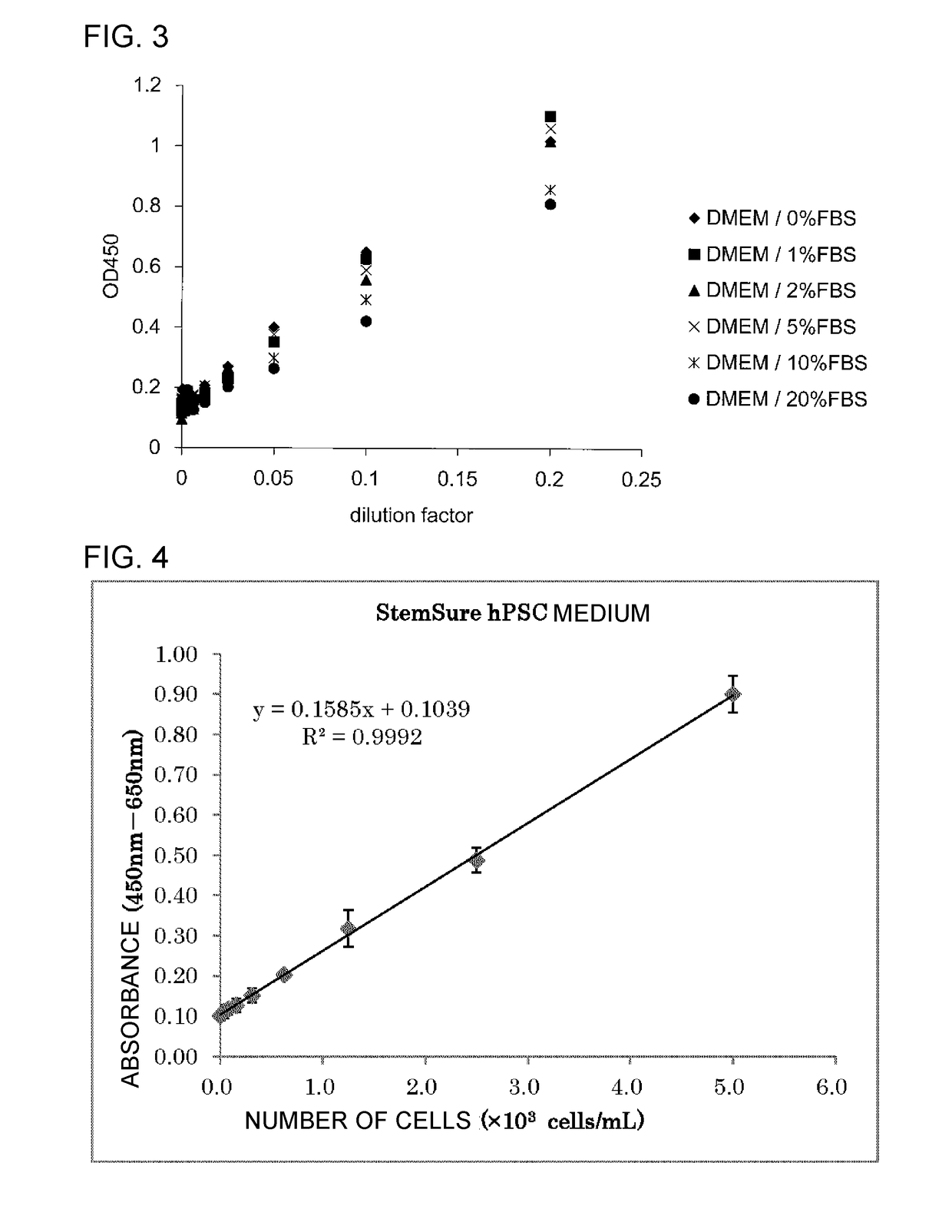
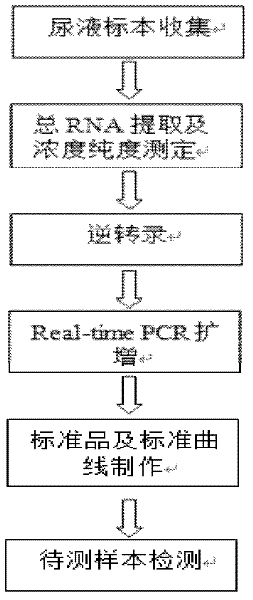
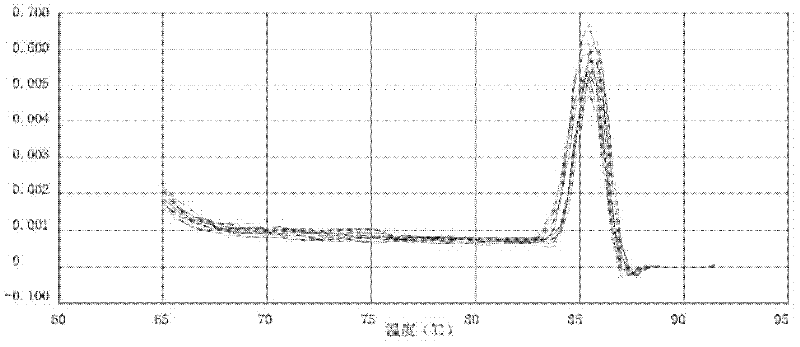
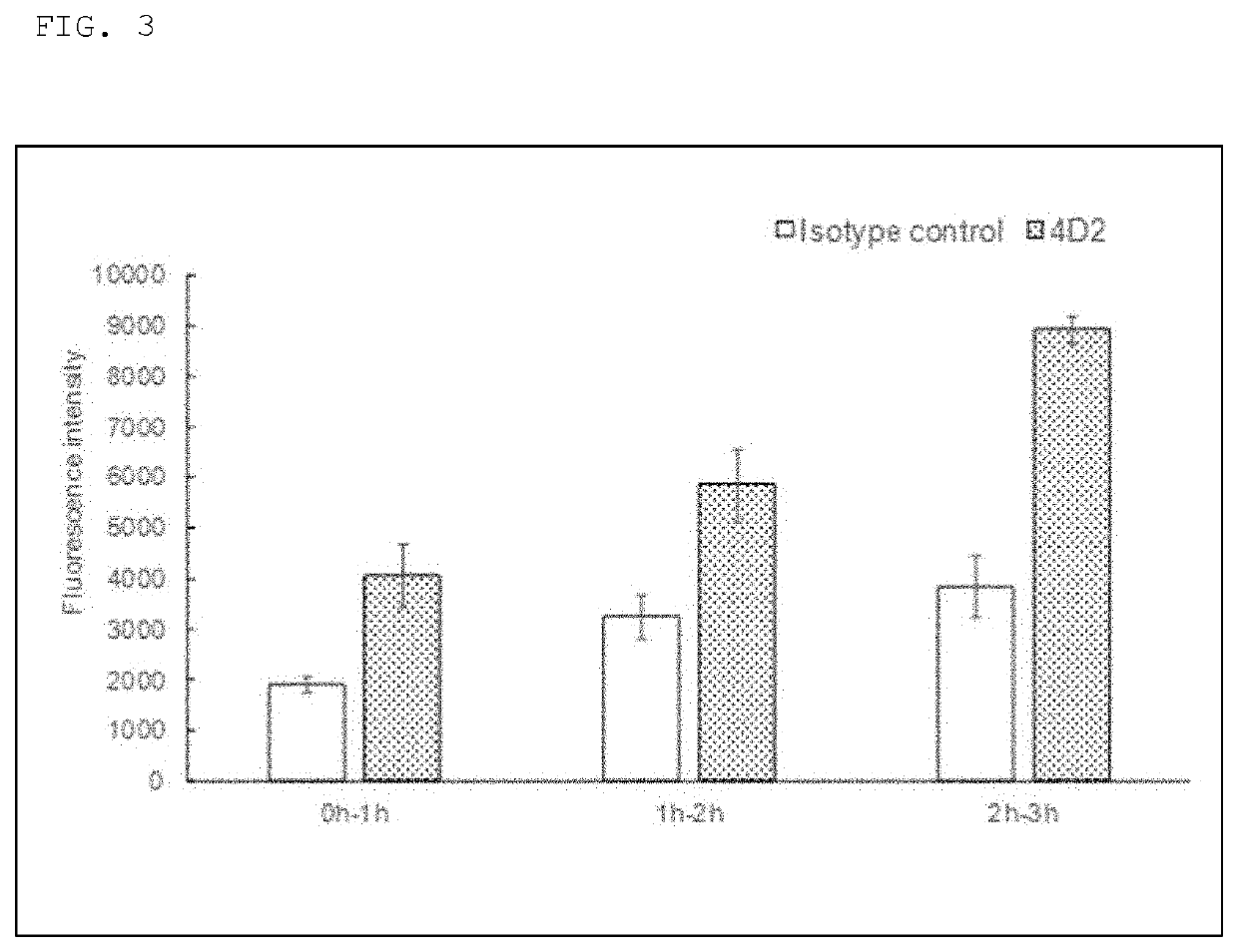
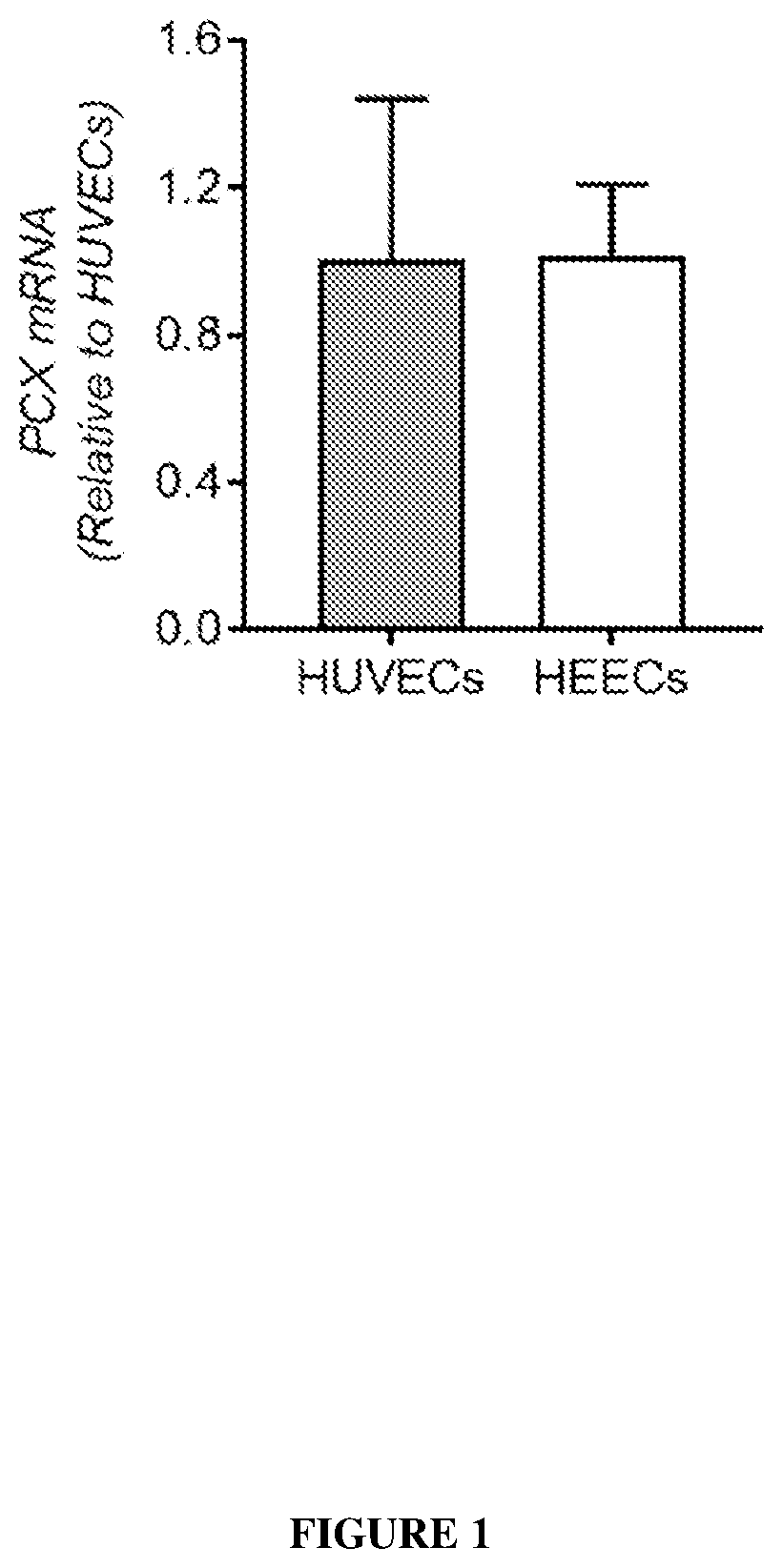
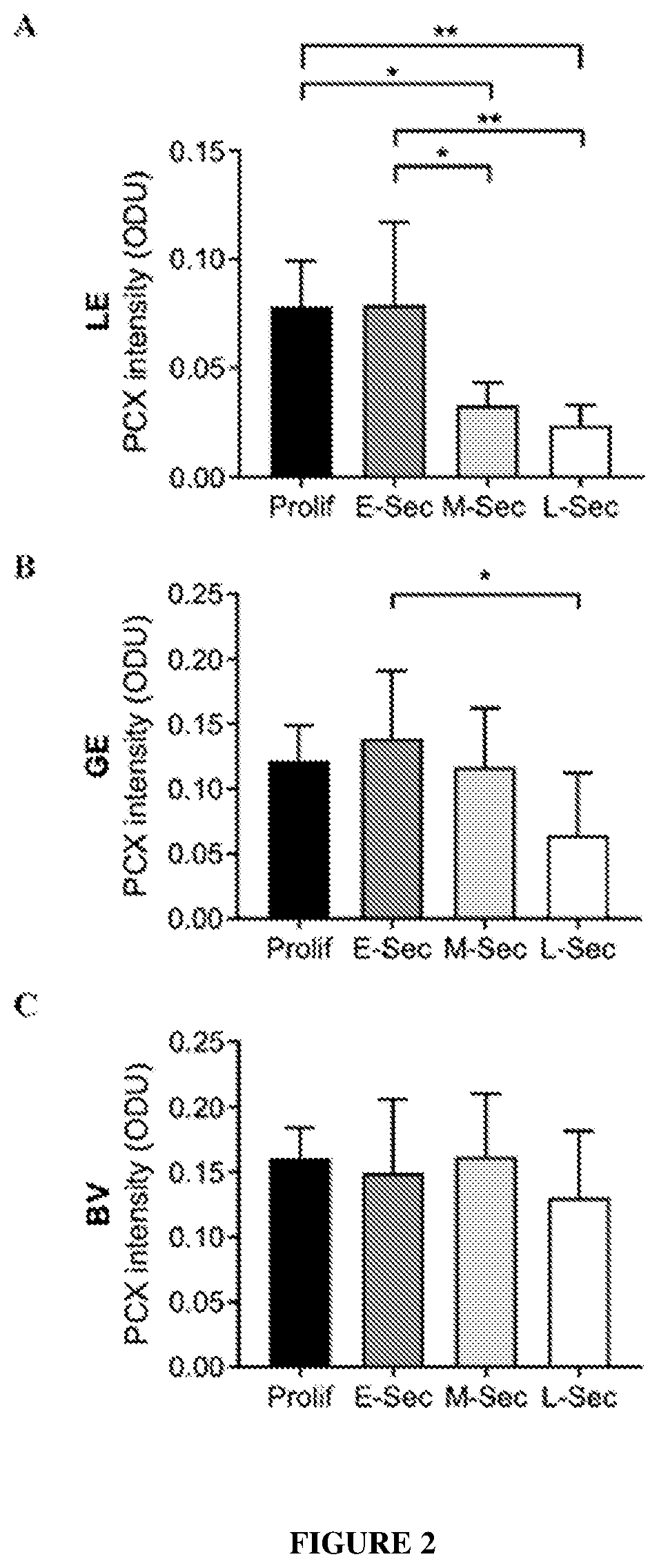
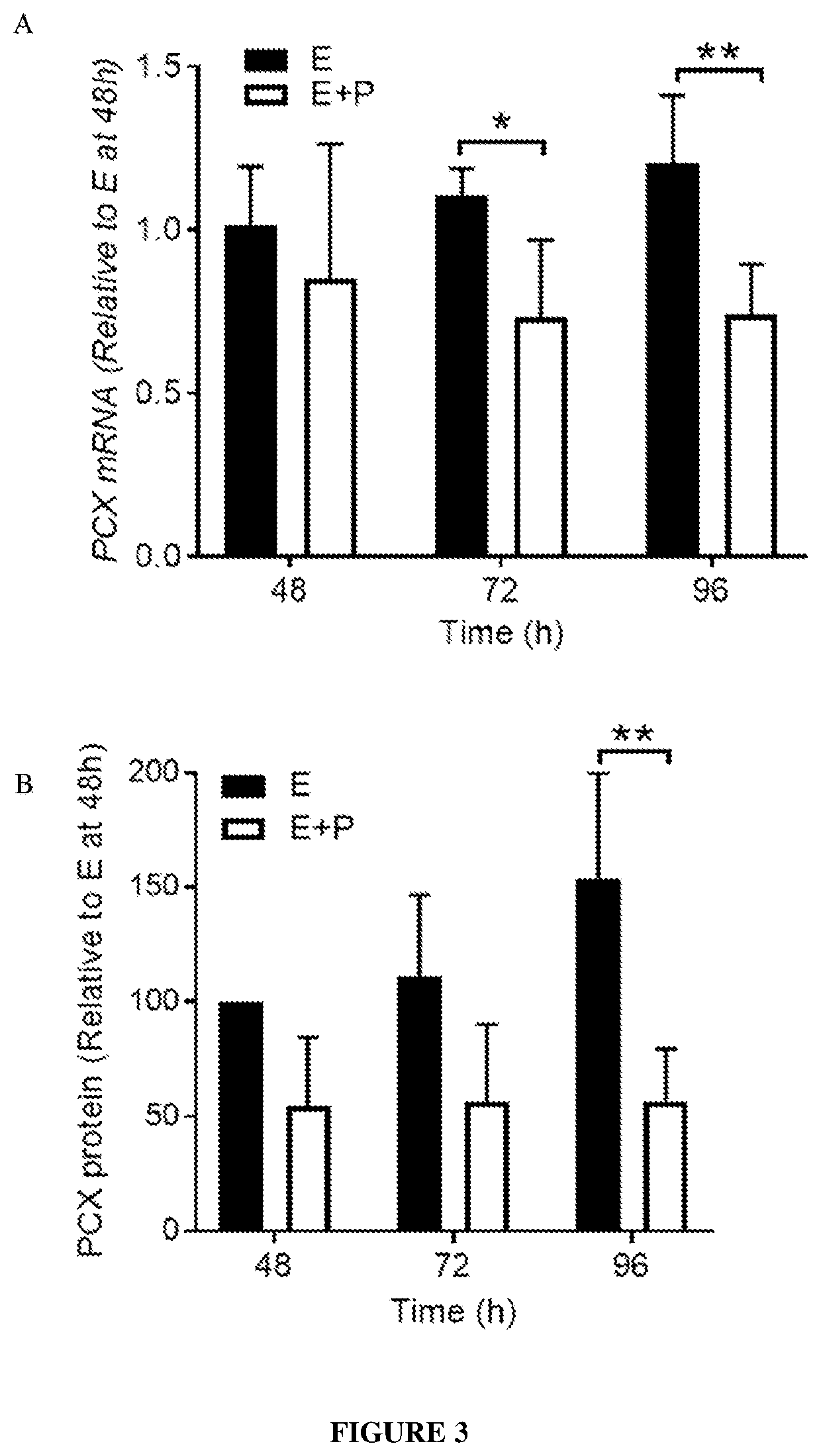
Patents
Literature
33 results about "Podocalyxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
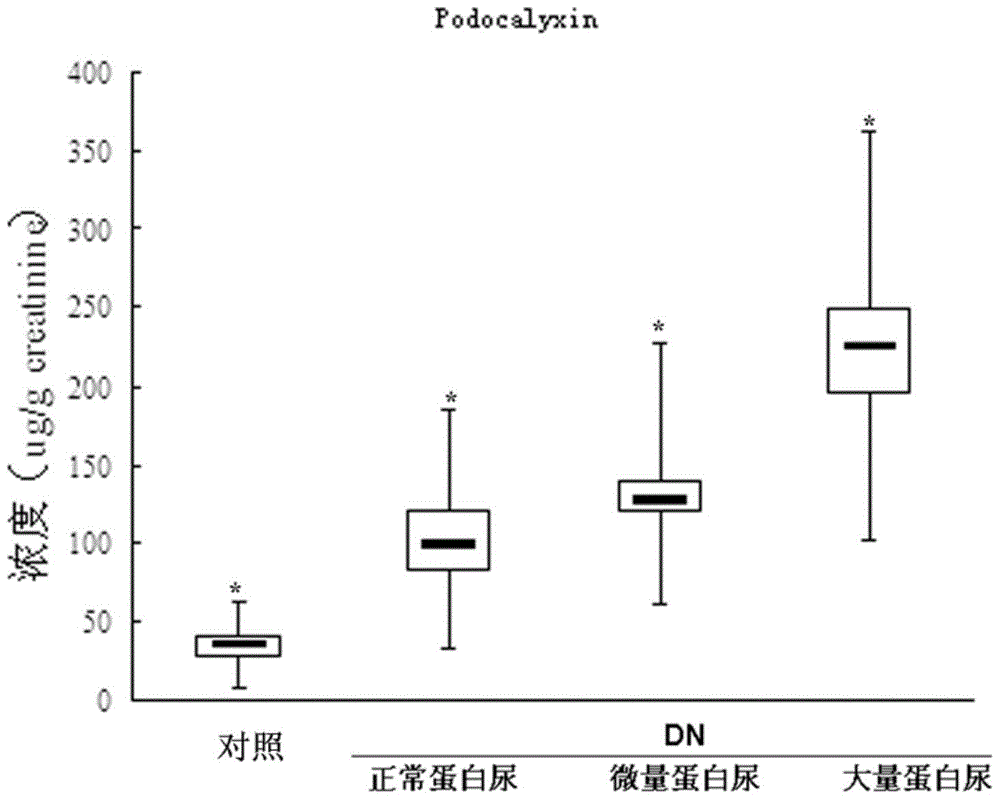
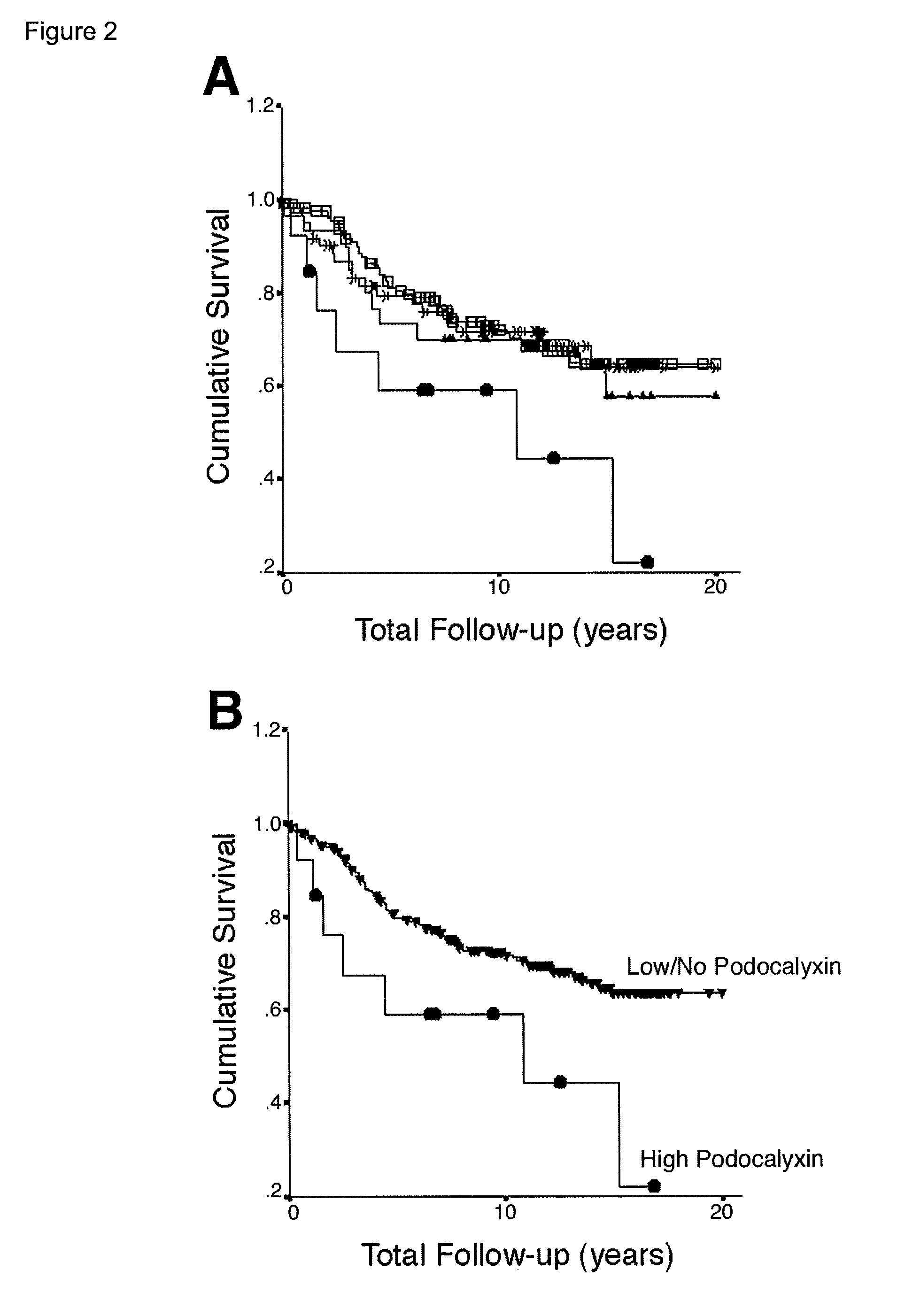
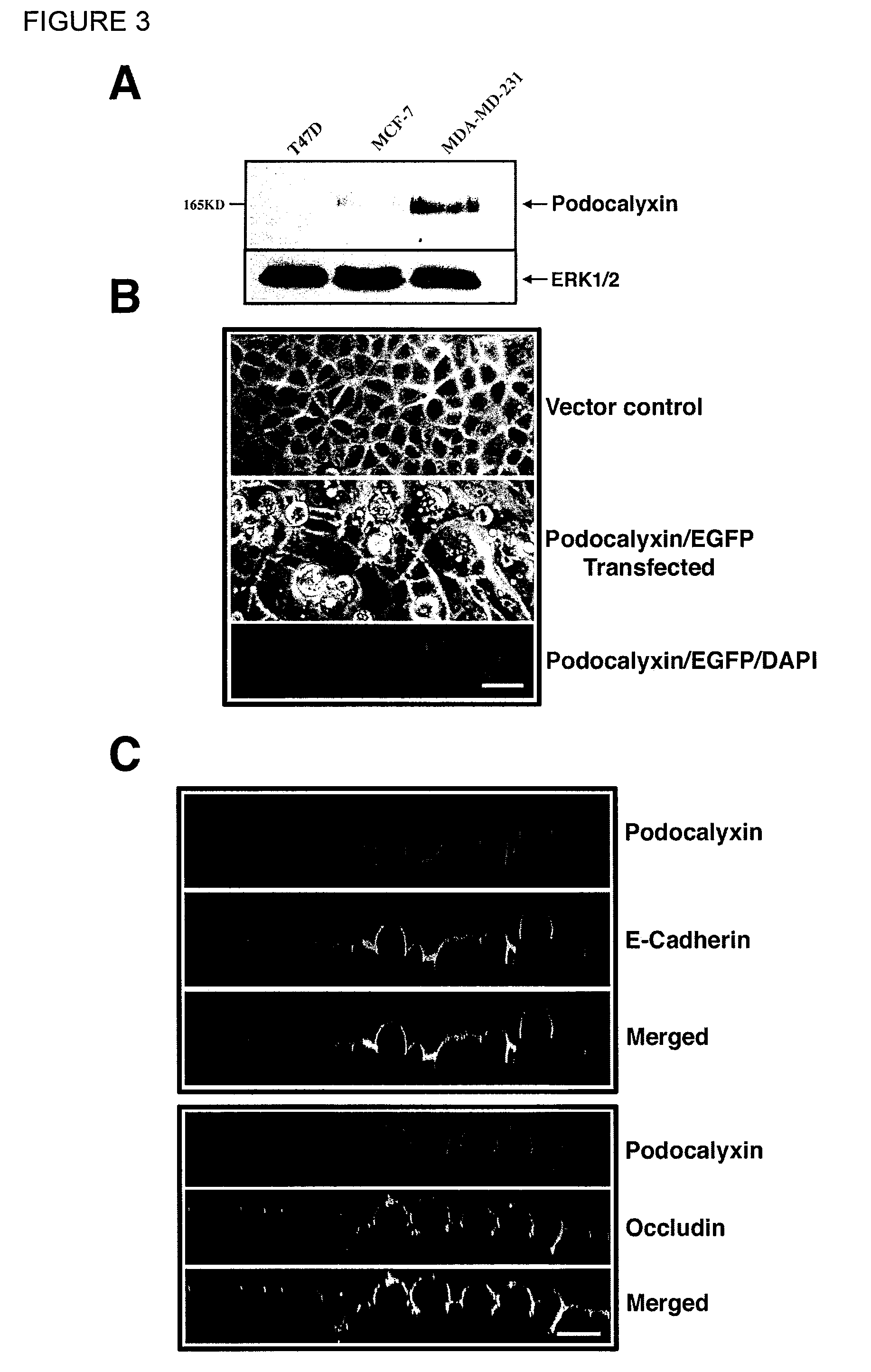
Podocalyxin, a sialoglycoprotein, is thought to be the major constituent of the glycocalyx of podocytes in the glomerulus (Bowman's capsule). It is a member of the CD34 family of transmembrane sialomucins. It coats the secondary foot processes of the podocytes. It is negatively charged and thus functions to keep adjacent foot processes separated, thereby keeping the urinary filtration barrier open. This function is further supported by knockout studies in mice which reveal an essential role in podocyte morphogenesis and a role in the opening of vascular lumens and regulation of vascular permeability. Of note, this is the only cell surface sialomucin knockout known to display a lethal phenotype. Podocalyxin is also upregulated in a number of cancers and is frequently associated with poor prognosis. Sialylated, O-glycosylated glycoforms of podocalyxin expressed by colon carcinoma cells possess L-selectin and E-selectin binding activity, and may be pivotal to the metastatic spread of colon carcinoma cells. At the cellular level podocalyxin has also been shown to regulate the size and topology of apical cell domains and act as a potent inducer of microvillus formation.
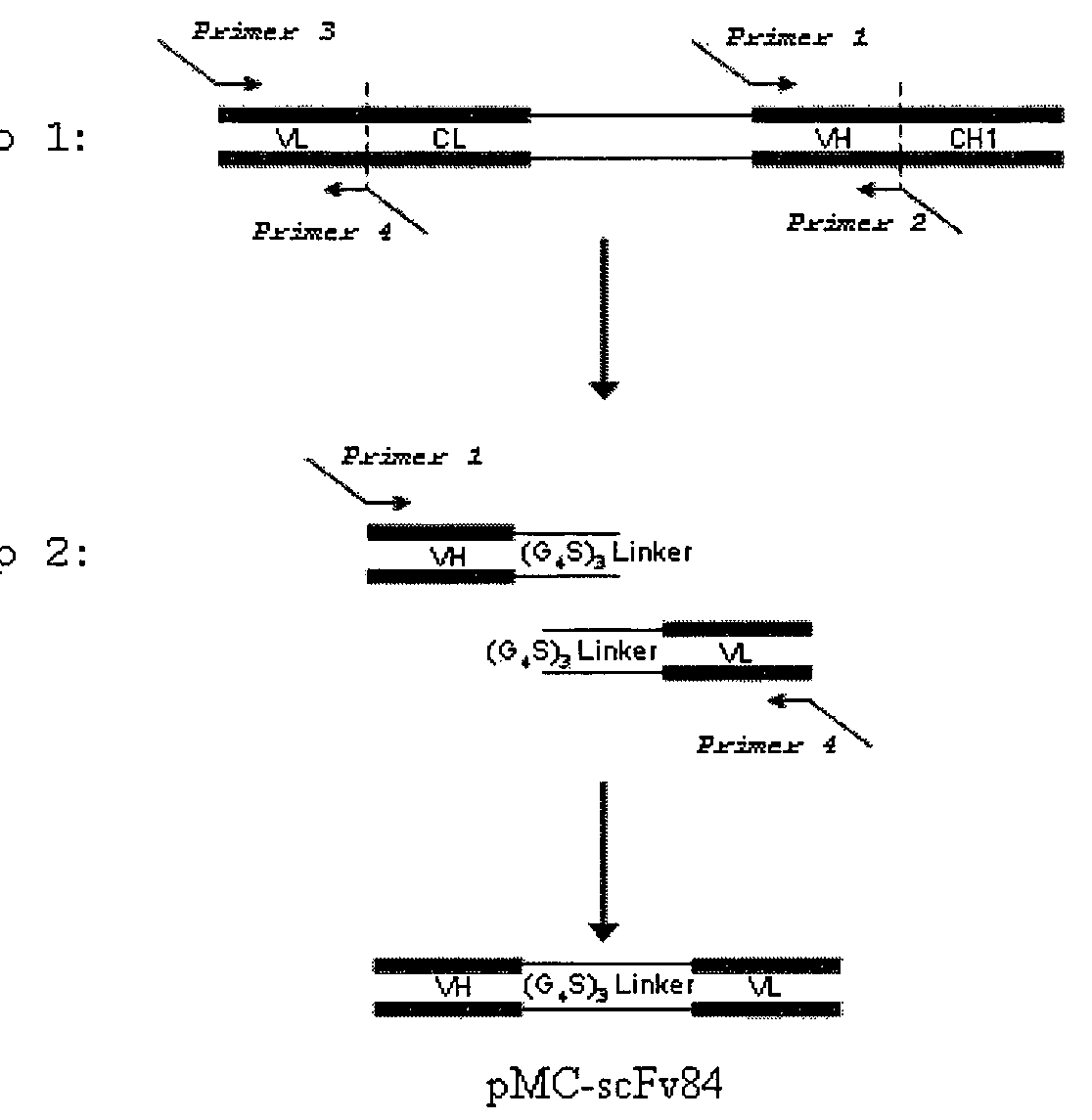
Human podocalyxin alternative-spliced forms and uses thereof
The present invention provides a method for diagnosing and detecting cancer. The present invention provides one or more proteins or fragments thereof, peptides or nucleic acid molecules differentially expressed in cancer (podocalyxin) and antibodies that bind podocalyxins. The present invention provides that podocalyxins are used as targets for screening agents that modulate podocalyxin activities. Further the present invention provides methods for treating diseases associated with podocalyxin expression.
Owner:APPL BIOSYSTEMS INC
Method for diagnosing nephropathy
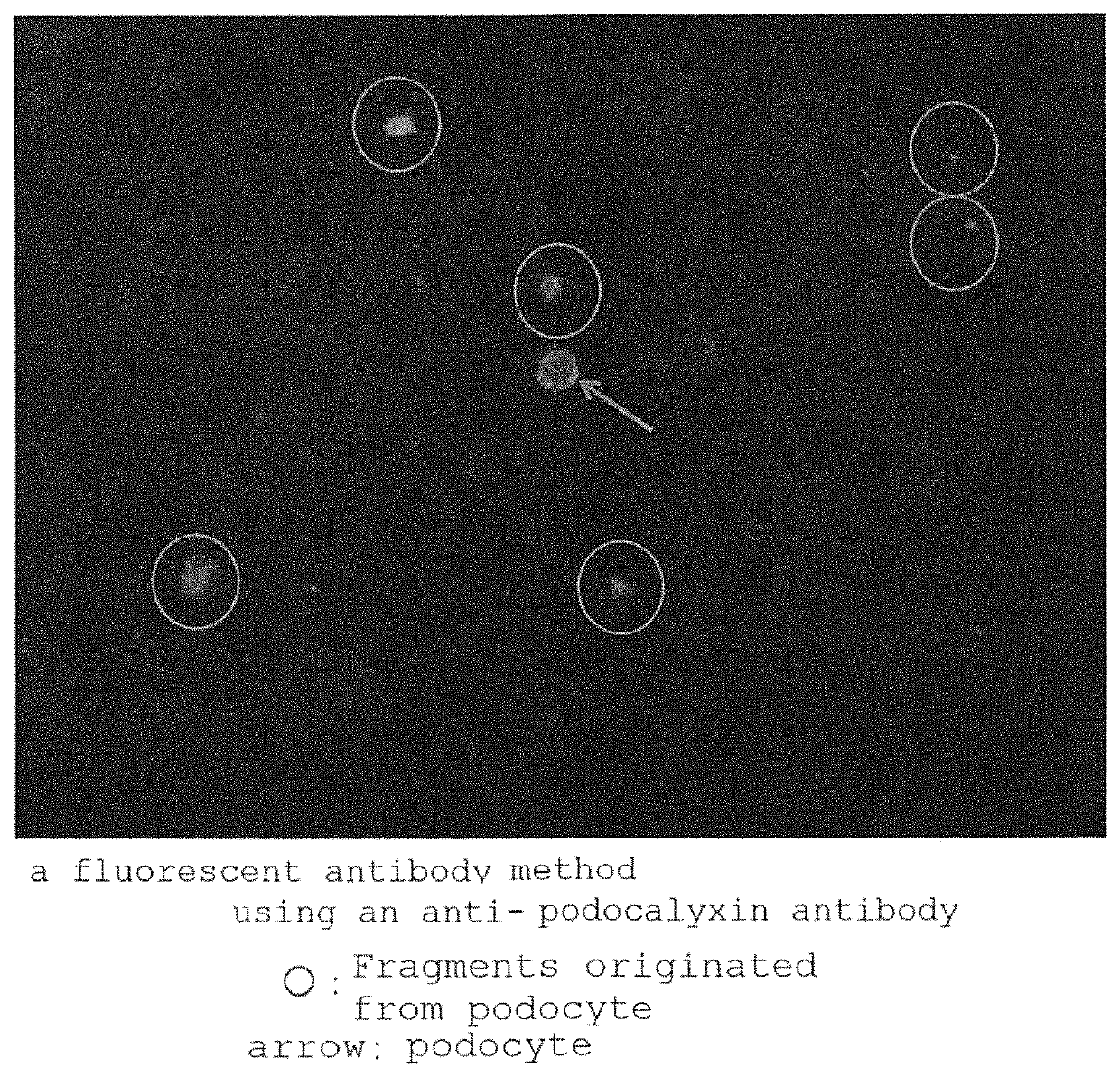
Means of conveniently examining nephropathy; and a method of assaying substance(s) in association with nephropathy (for example, podocalyxin and / or nephrin) in the urine to be used in the above means. The above assay method can be provided by releasing proteins in association with nephropathy, which exist on the surface of podocytes, from the cell surface and thus assaying the substances existing on the cell surface and / or free substances contained in the urine.
Owner:DENKA SEIKEN CO LTD +1
Methods for detecting and treating cancer
ActiveUS7833733B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsPodocalyxinCancer research
Methods and kits for detecting cancer and monitoring cancer progression are described. The method involves analyzing a sample containing nucleic acids or proteins from a patient for decreased expression of endoglycan and / or increased expression of podocalyxin.
Owner:THE UNIV OF BRITISH COLUMBIA
Method for test on diabetic nephropathy
InactiveUS20120164667A1Inhibit progressEarly detectionImmunoglobulins against animals/humansTransmissivity measurementsPodocalyxinUrinary albumin
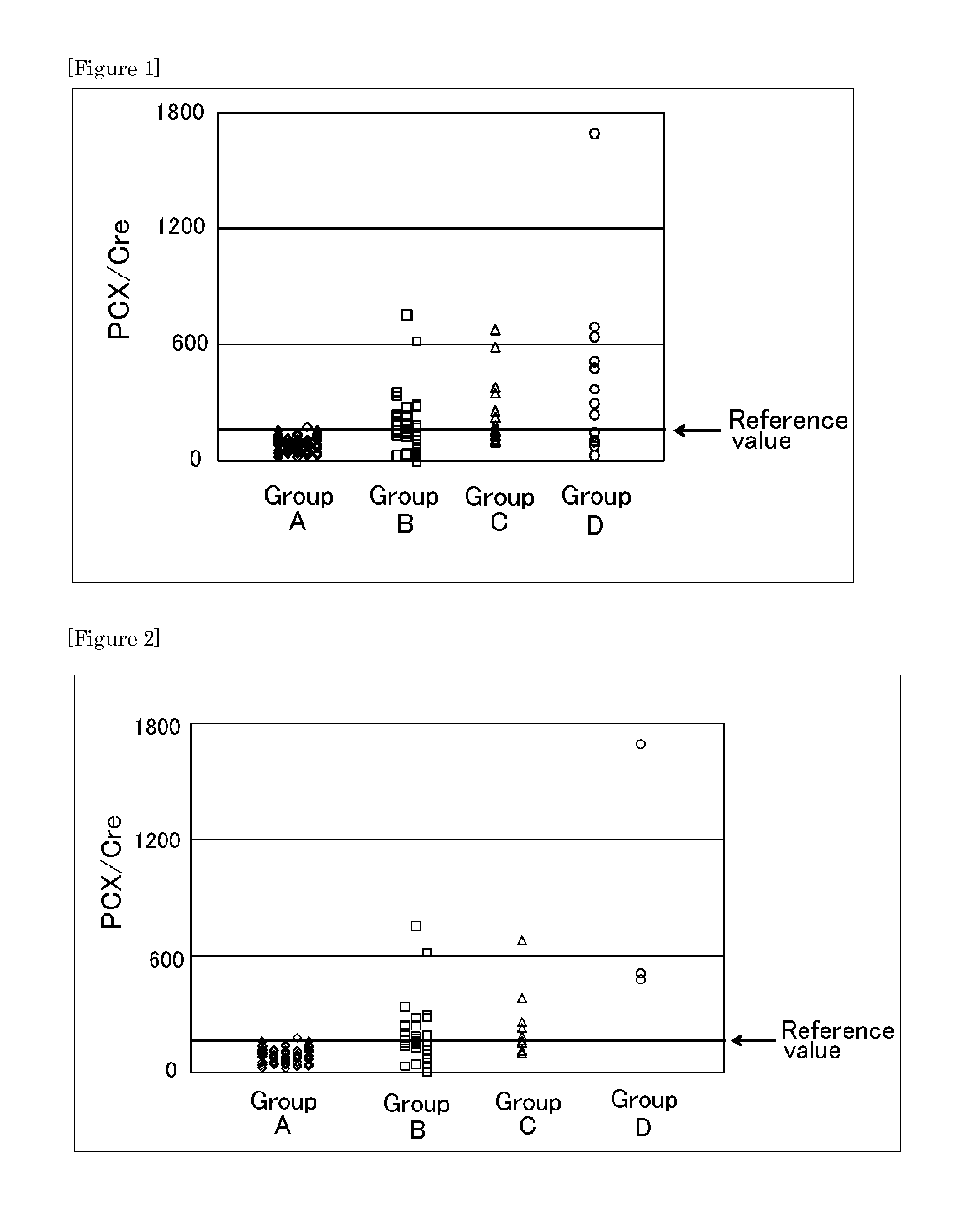
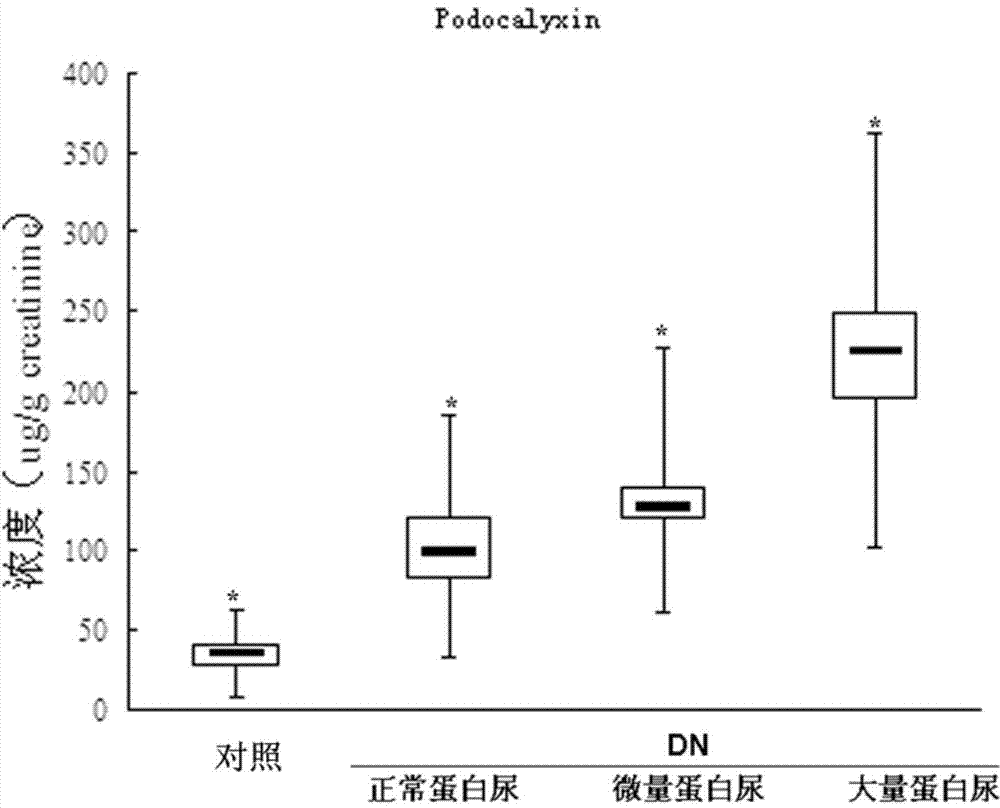
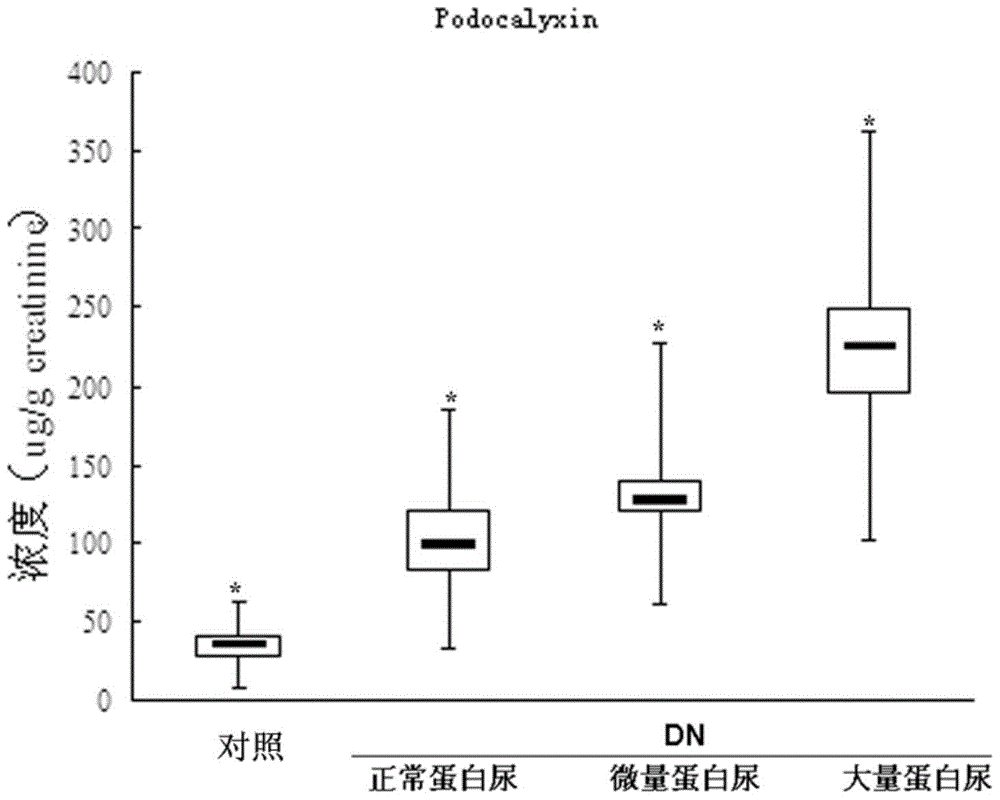
Provided is a test method for the detection of diabetic nephropathy at an early stage as compared to a conventional method. Specifically provided are: a test method for diabetic nephropathy, including detecting urinary podocalyxin; the test method, further including assessing diabetic nephropathy at least Stage I; a test reagent for use in the test method; and a test reagent kit for use in the test method. The present invention is based on a finding that urinary podocalyxin reflects the development and condition of diabetic nephropathy with high sensitivity at an early stage as compared to urinary albumin.
Owner:NIIGATA UNIVERSITY +3
Human podocalyxin alternative-spliced forms and uses thereof
The present invention provides a method for diagnosing and detecting cancer. The present invention provides one or more proteins or fragments thereof, peptides or nucleic acid molecules differentially expressed in cancer (podocalyxin) and antibodies that bind podocalyxins. The present invention provides that podocalyxins are used as targets for screening agents that modulate podocalyxin activities. Further the present invention provides methods for treating diseases associated with podocalyxin expression.
Owner:CELERA CORPORATION
Methods for detecting and treating cancer
Methods and kits for detecting cancer and monitoring cancer progression are described. The method involves analyzing a sample containing nucleic acids or proteins from a patient for decreased expression of endoglycan and / or increased expression of podocalyxin.
Owner:THE UNIV OF BRITISH COLUMBIA
Markers of Induced Pluripotent Stem Cells
InactiveUS20110171183A1Increasing success and safetyReduce riskBiocideNervous disorderPODOCALYXIN-LIKE PROTEINHuman Induced Pluripotent Stem Cells
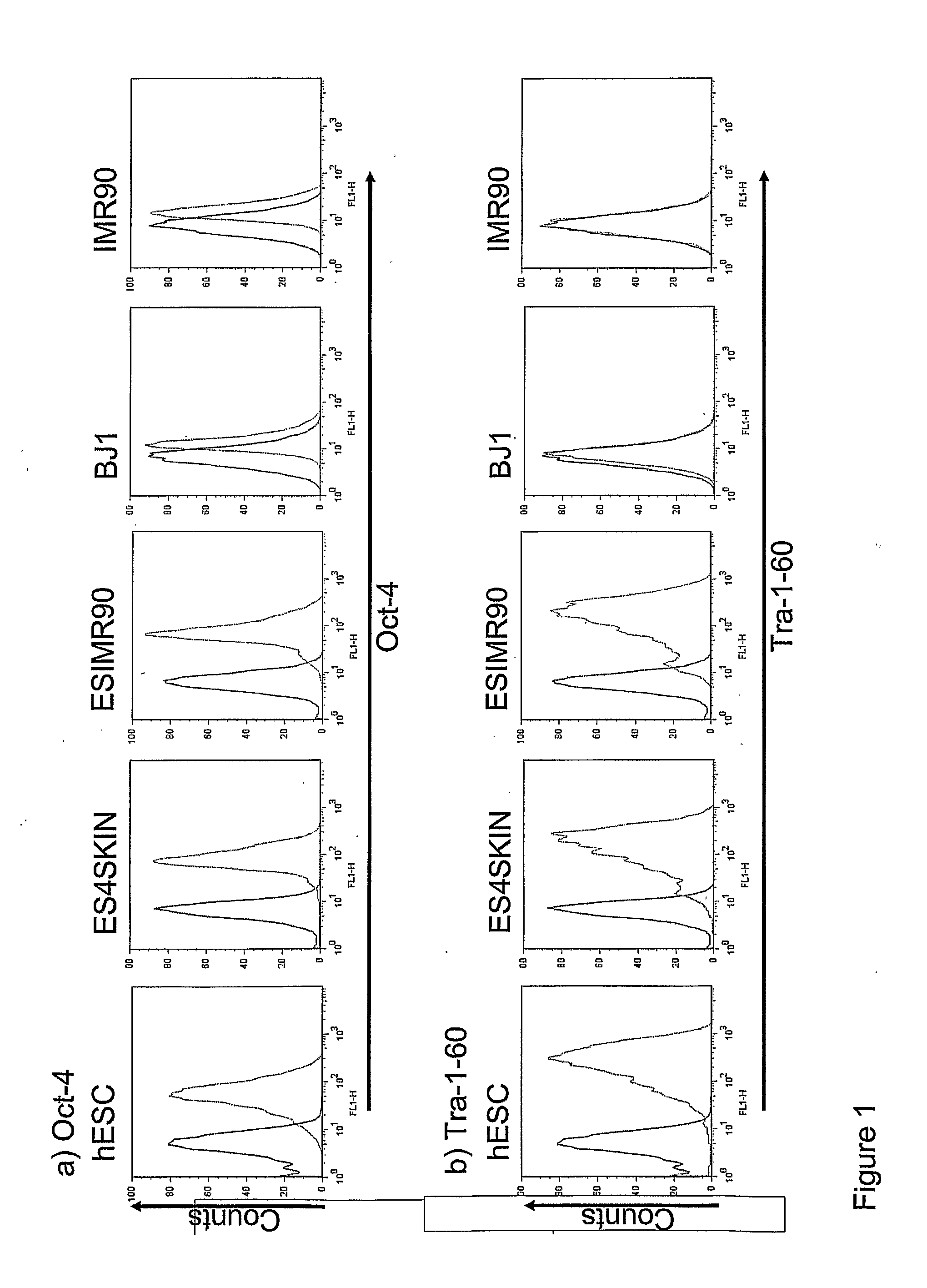
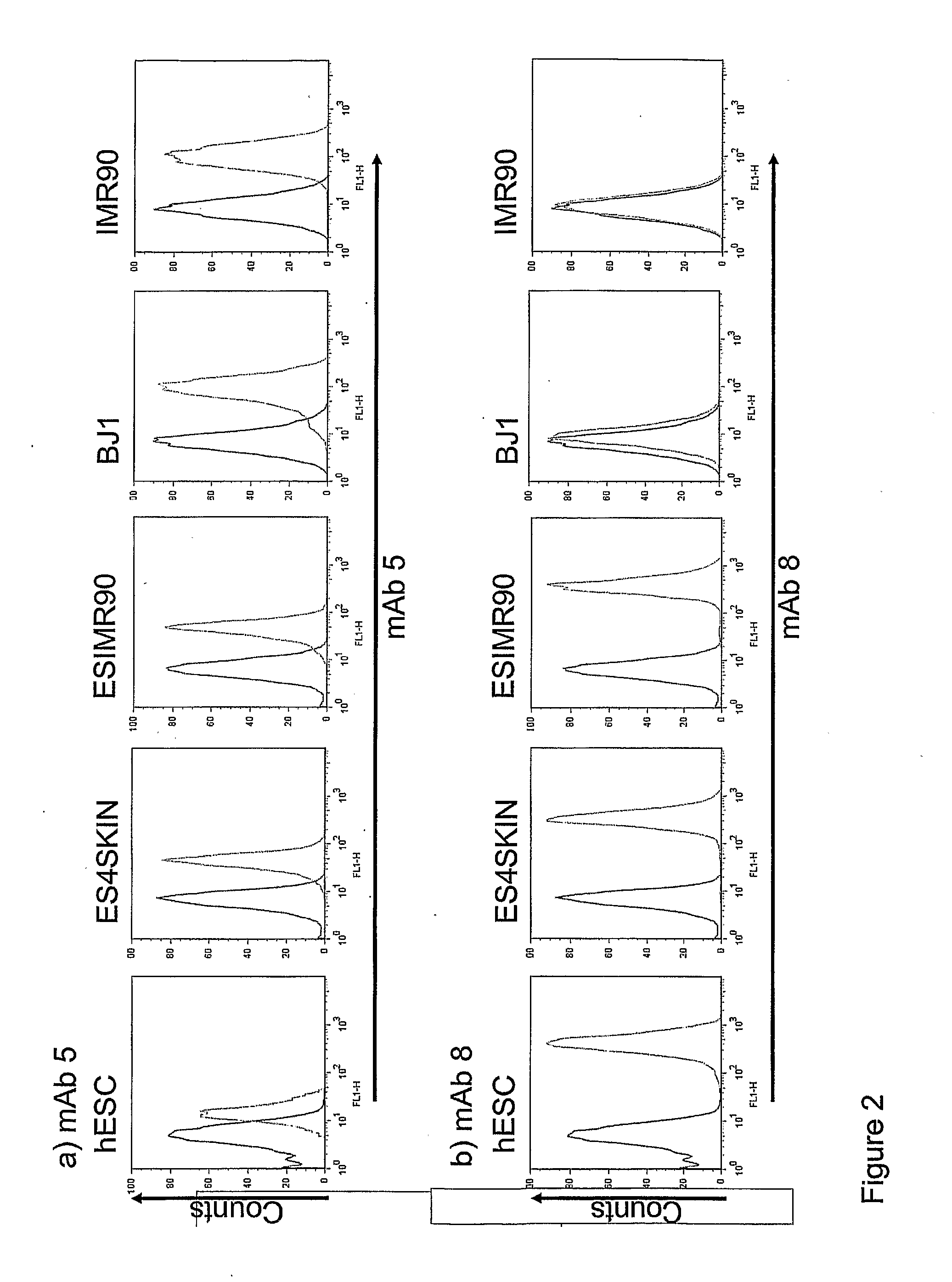
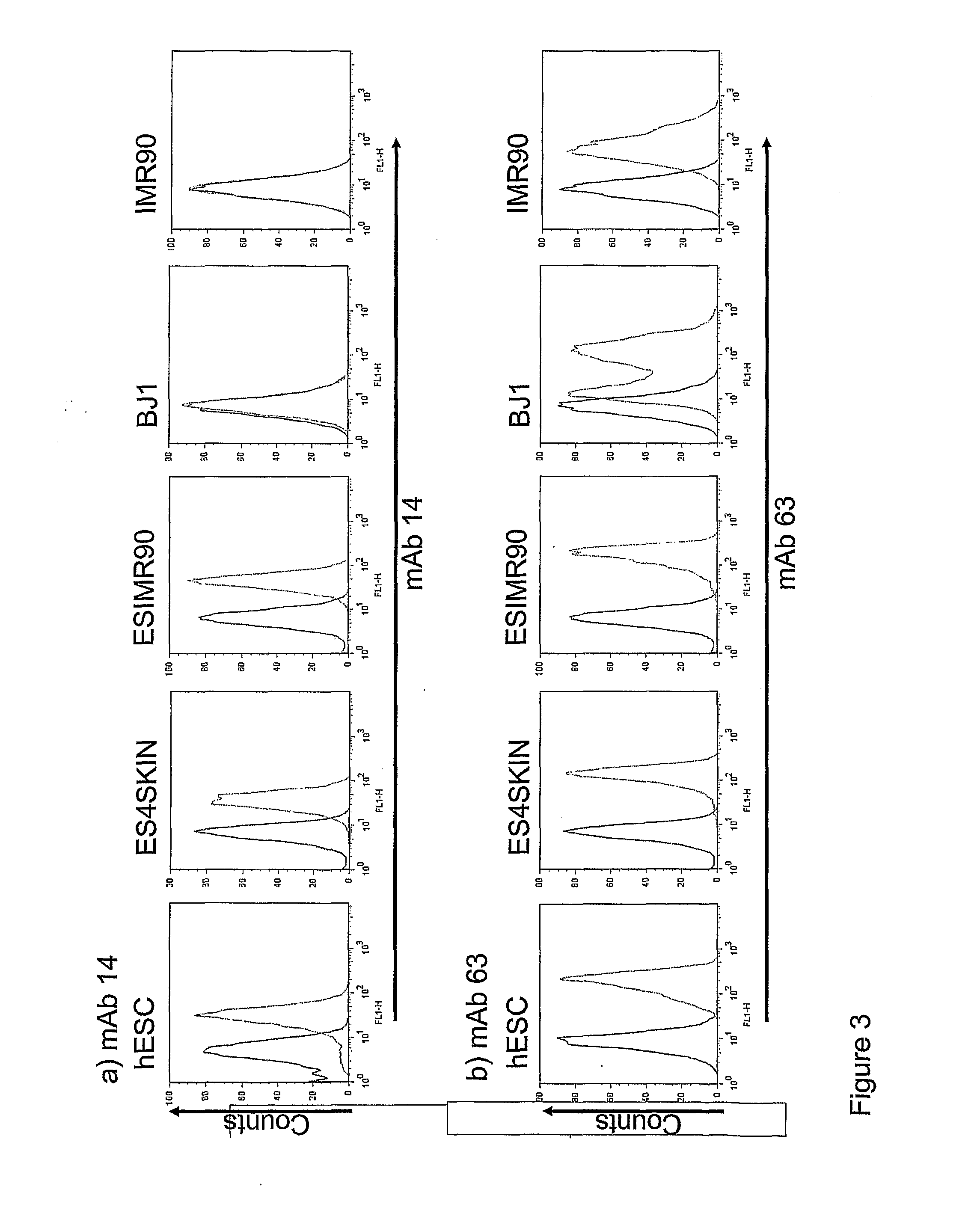
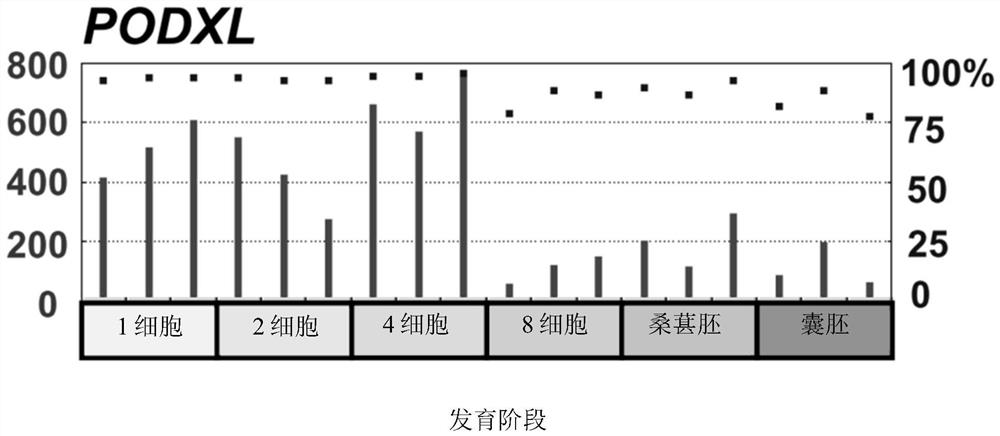
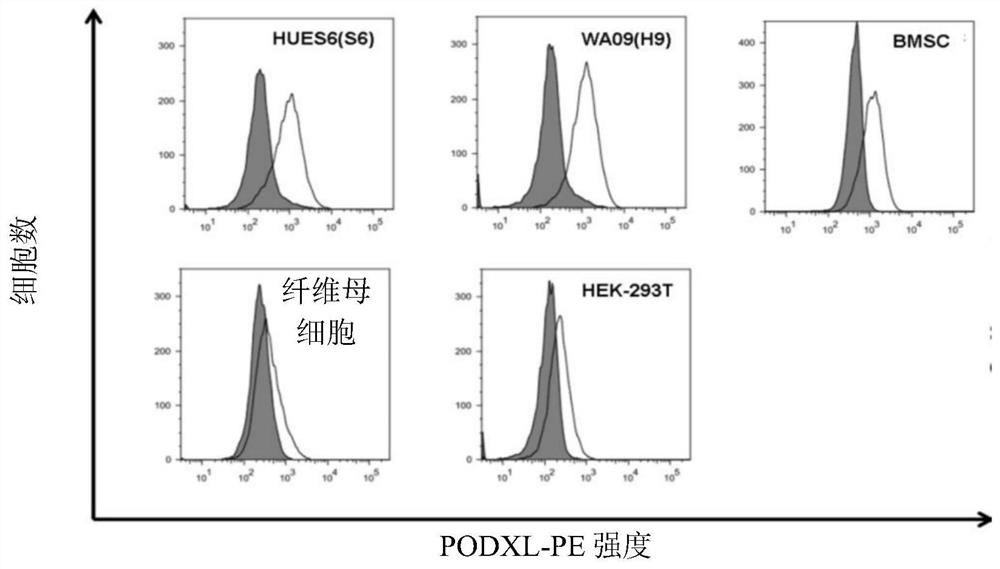
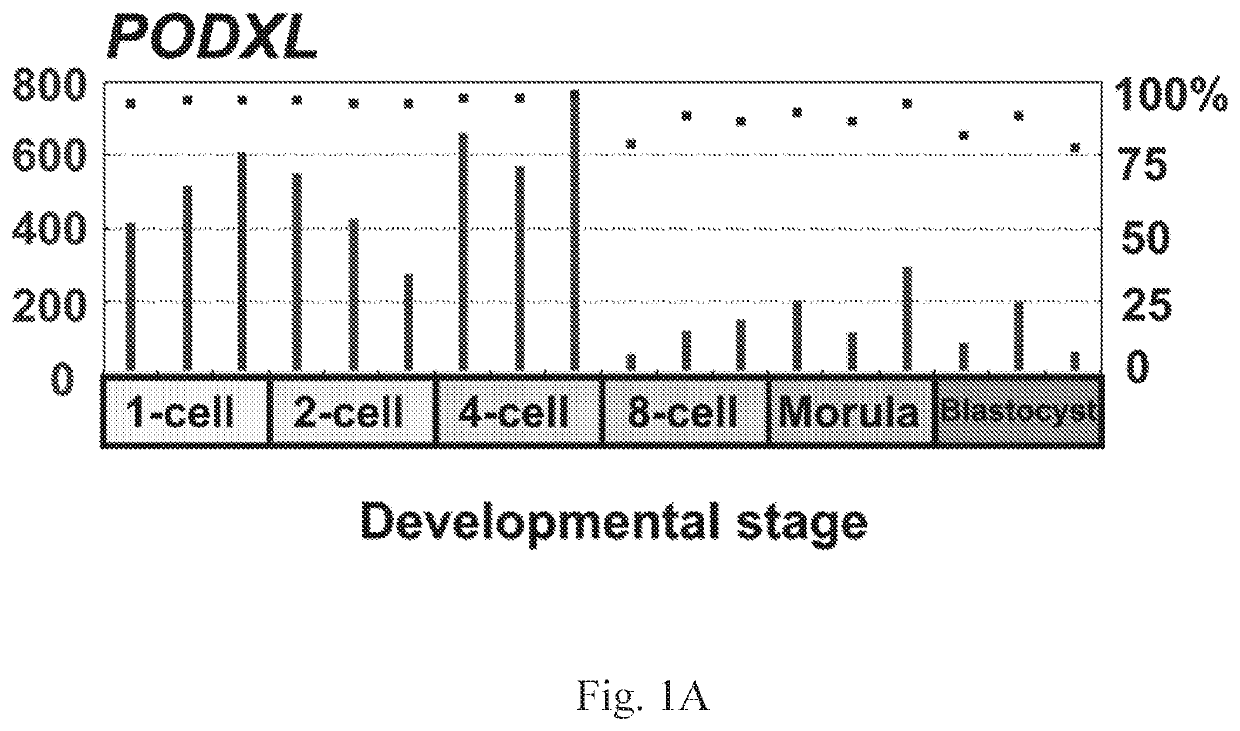
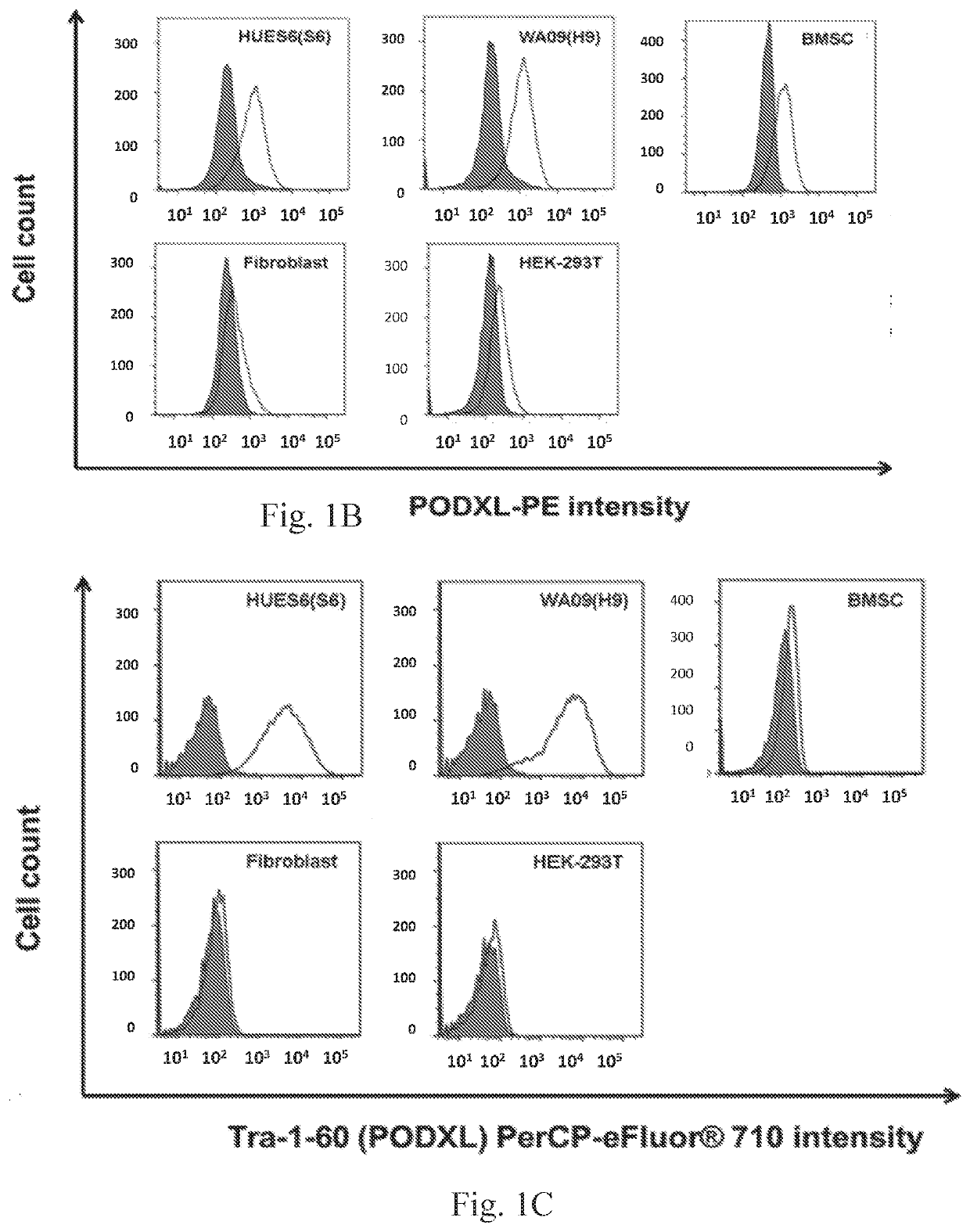
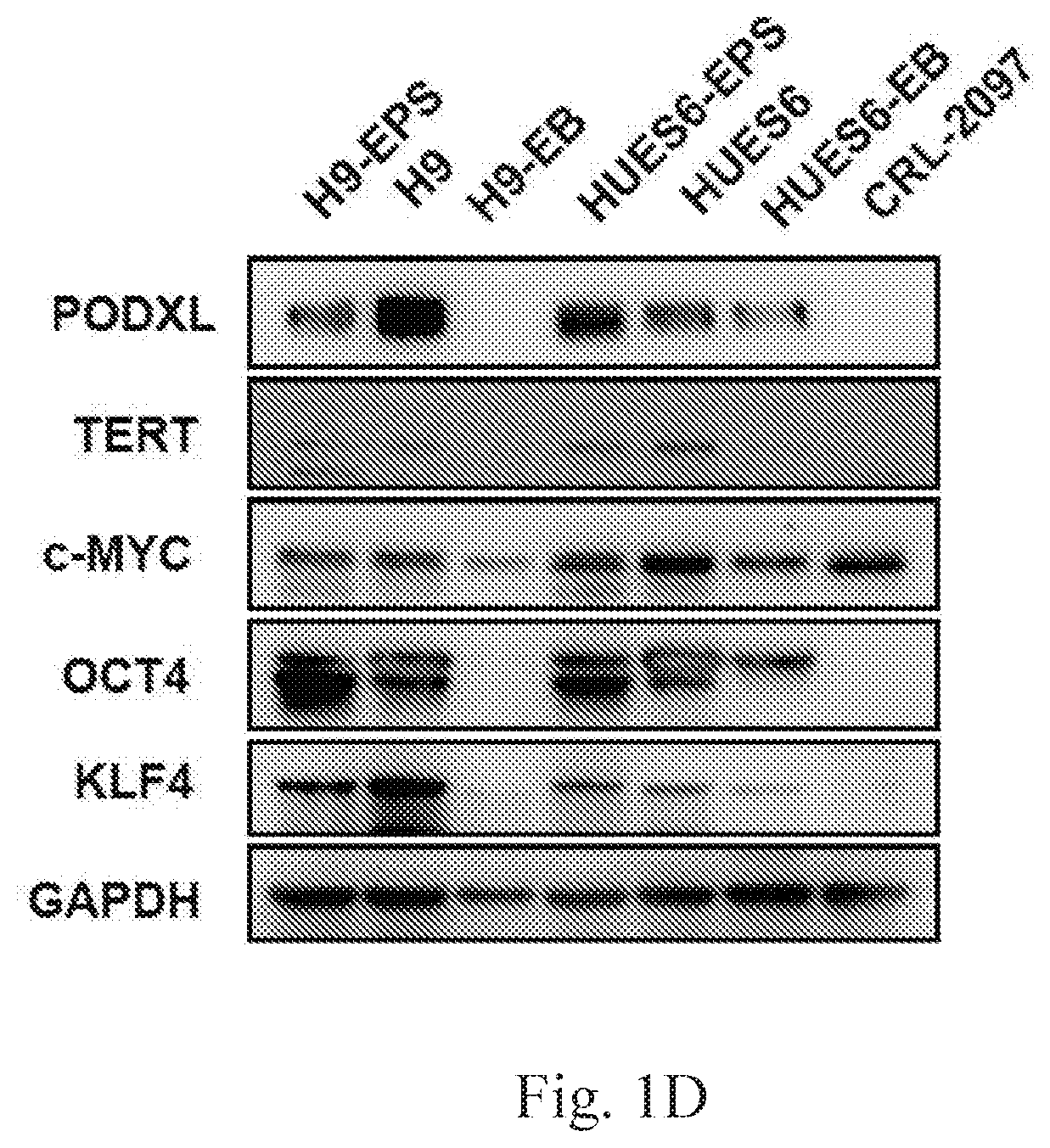
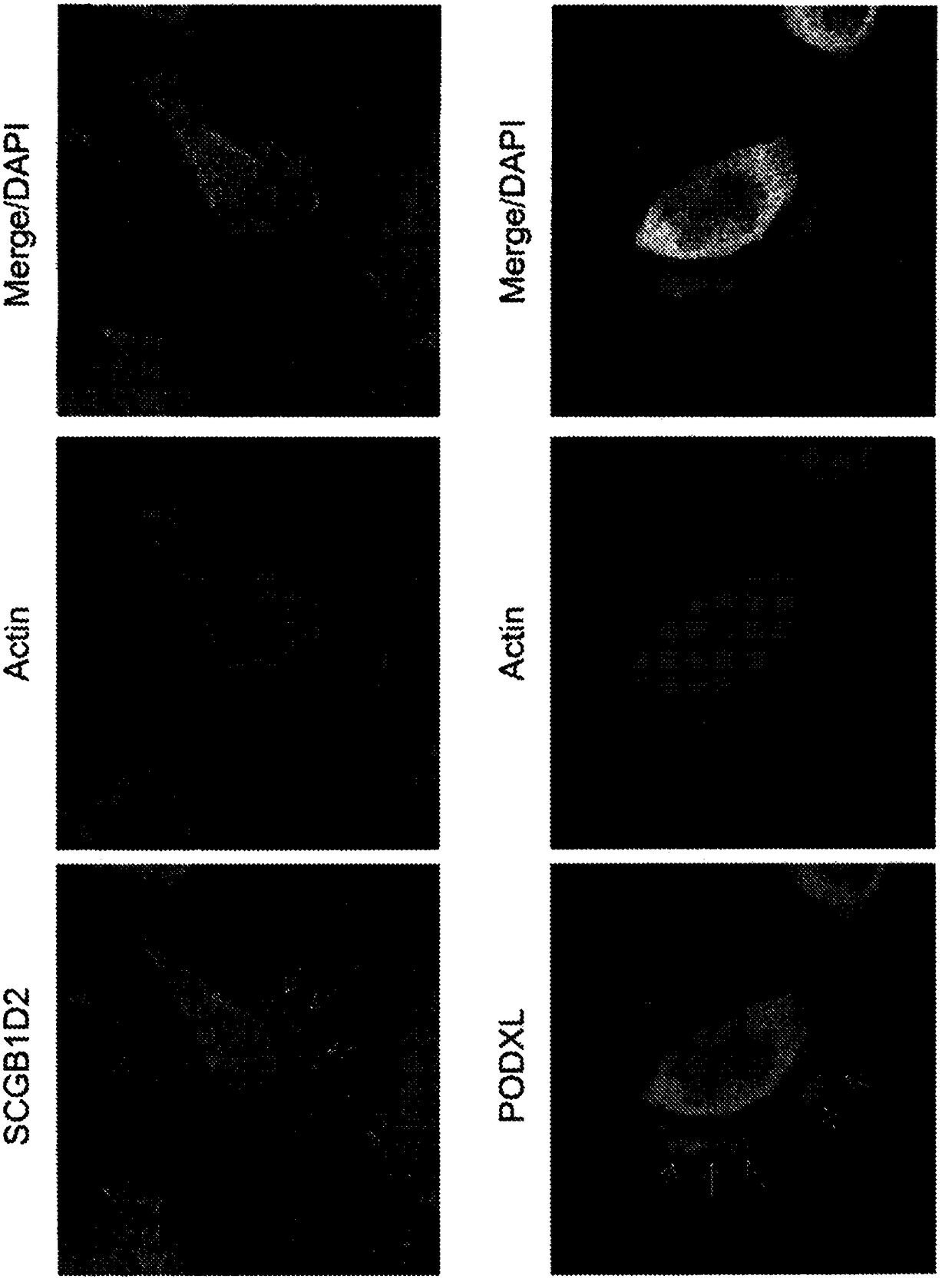
The disclosure relates to the expression of podocalyxin-like protein (PODXL) on the surface of induced pluripotent stem cells and particularly, although not exclusively, to the use of PODXL as a marker of induced pluripotent stem cells.
Owner:AGENCY FOR SCI TECH & RES
An early-stage screening kit for diabetic nephropathy, a biomarker detecting method and applications
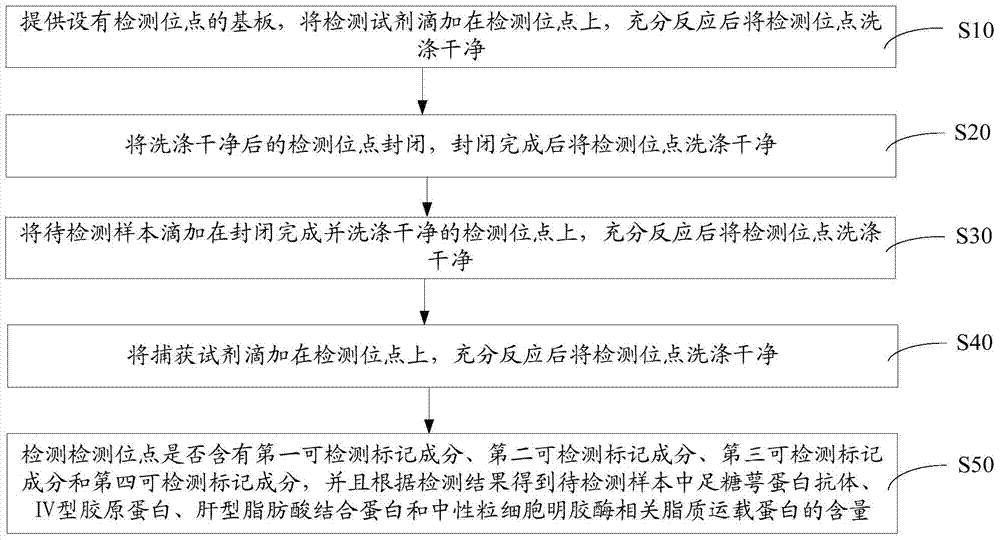
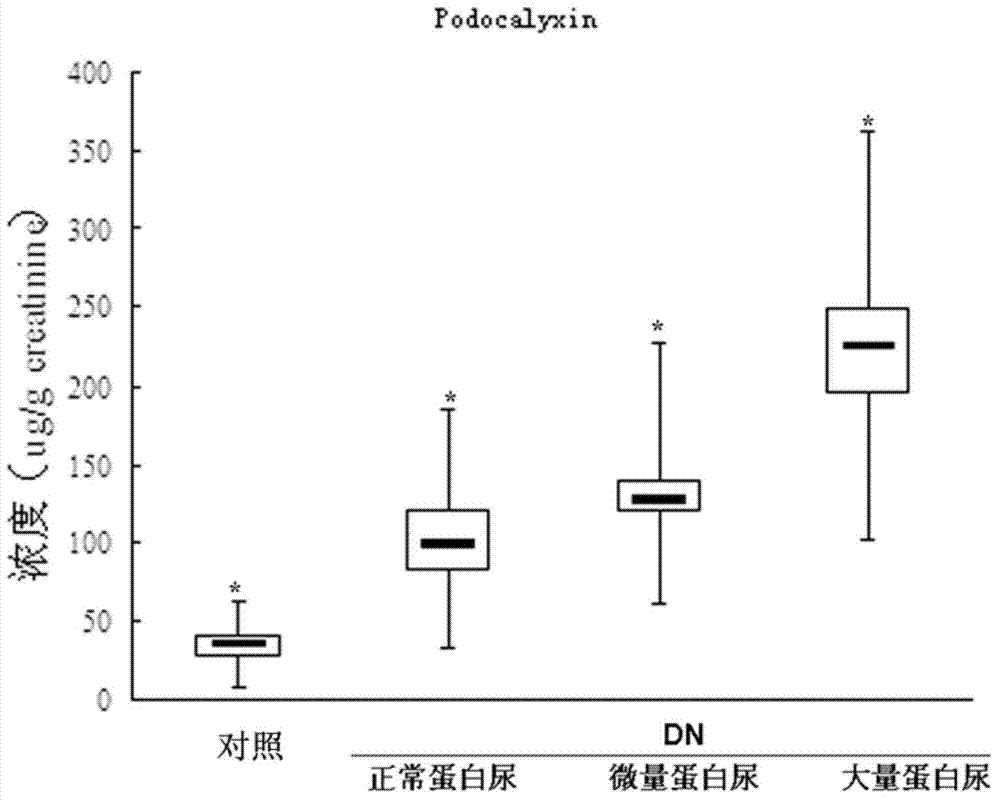
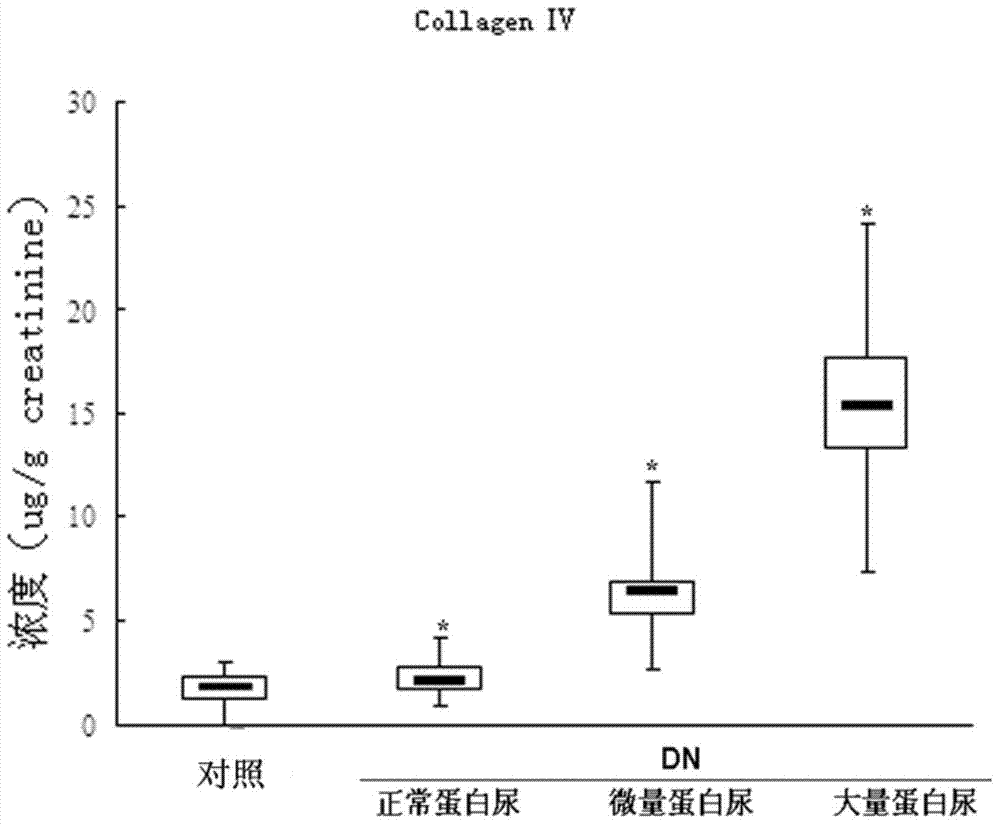
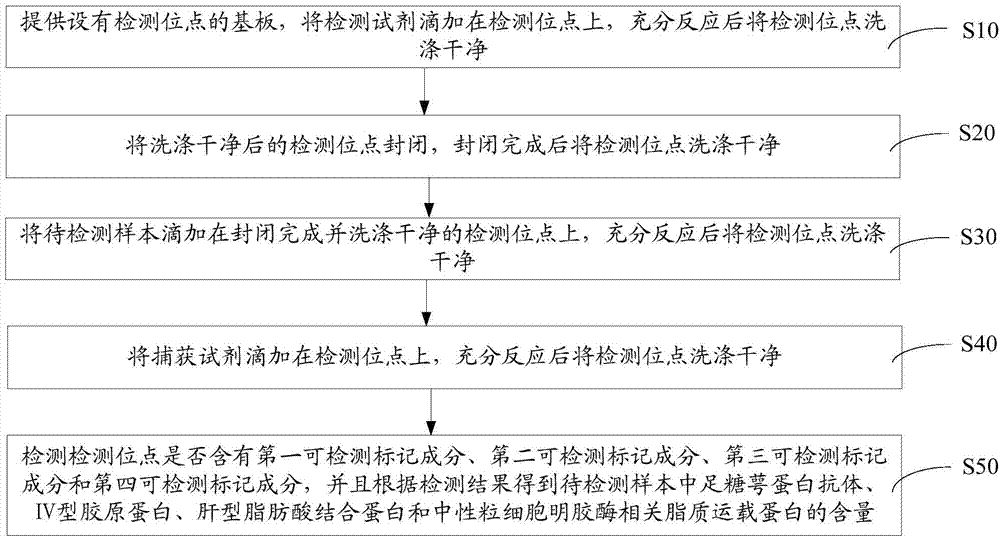
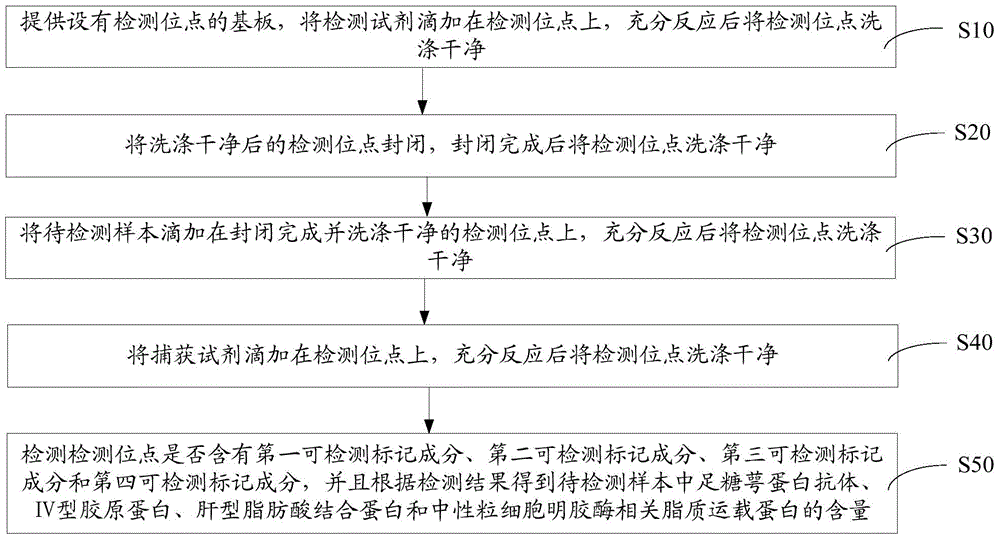
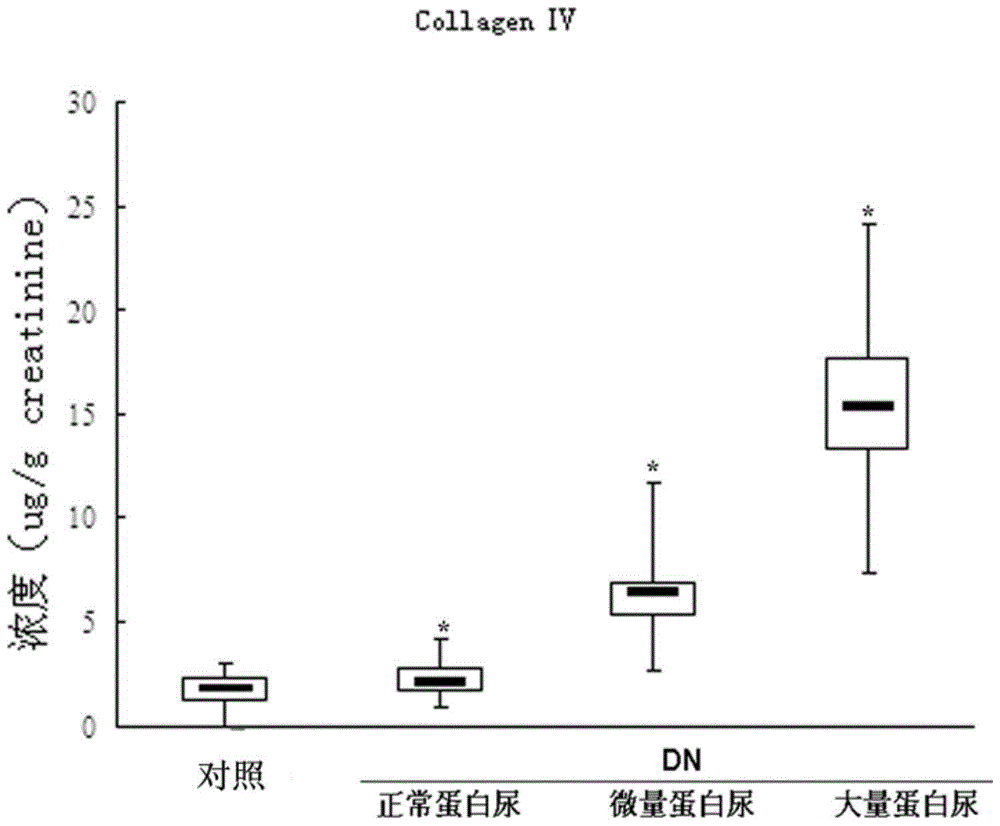
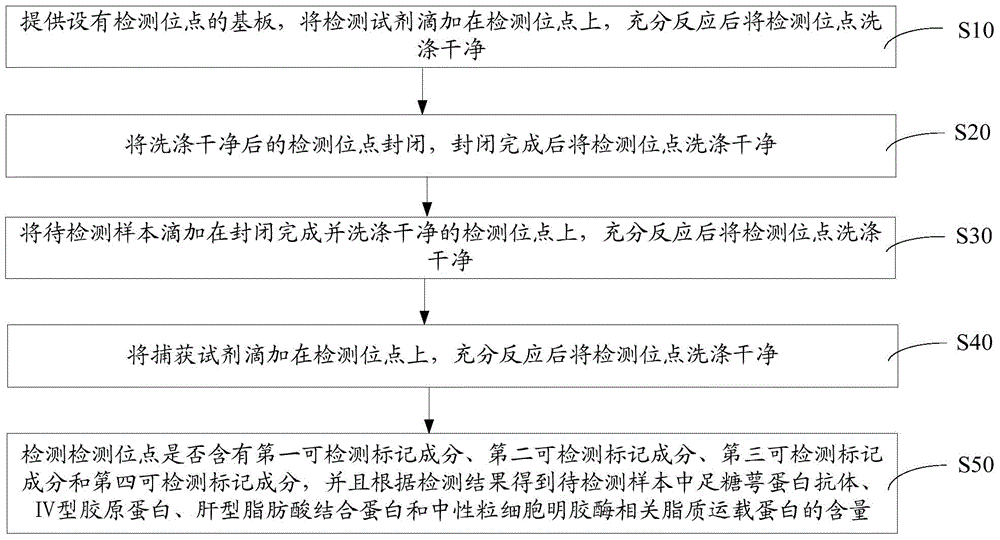
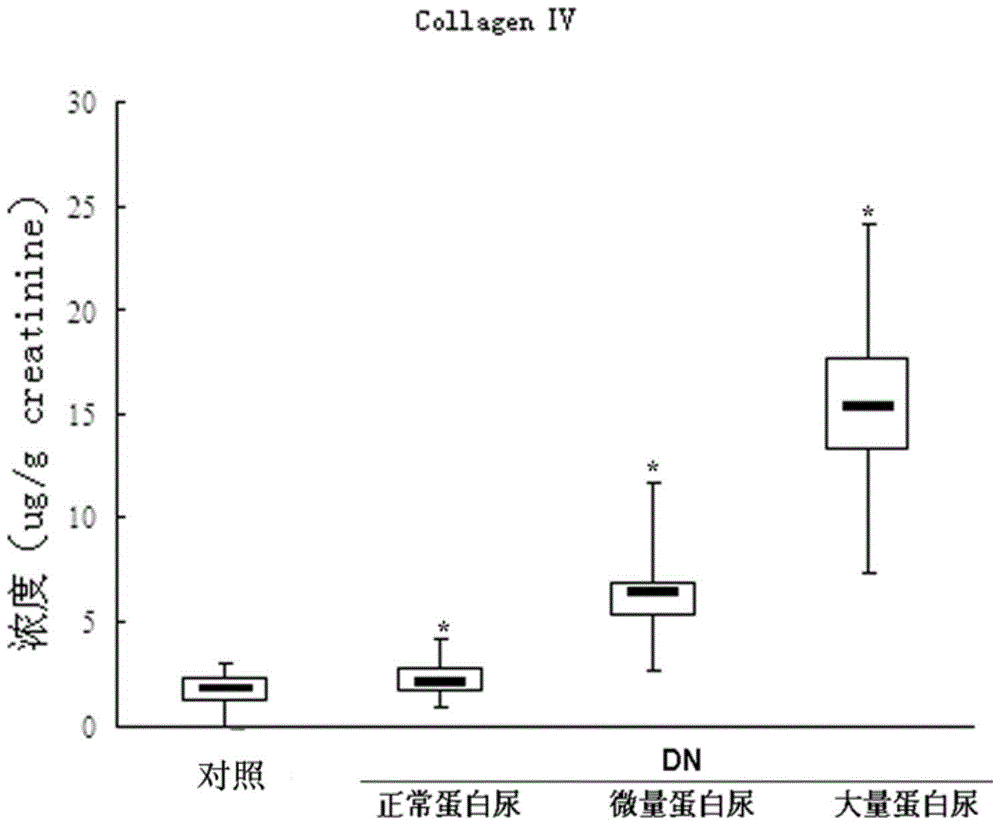
An early-stage screening kit for diabetic nephropathy is disclosed. The kit comprises detection agents. The detection agents comprise a first detection agent, a second detection agent, a third detection agent and a fourth detection agent. The solute of the first detection agent is a first podocalyxin antibody. The solute of the second detection agent is a first IV type collagen antibody. The solute of the third detection agent is a first liver-type fatty acid-binding protein antibody. The solute of the fourth detection agent is a first neutrophils gelatinase-associated lipocalin antibody. The kit adopts podocalyxin, IV type collagen, liver-type fatty acid-binding protein and neutrophils gelatinase-associated lipocalin as biomarkers, and can provide assisted determination for renal injury according to actual detection results, thus providing assistance for differentiating early-stage diabetic nephropathy.
Owner:BIO TECH ACADEMY CHINA
Podocalyxin-like-protein-1 binding antibody molecule
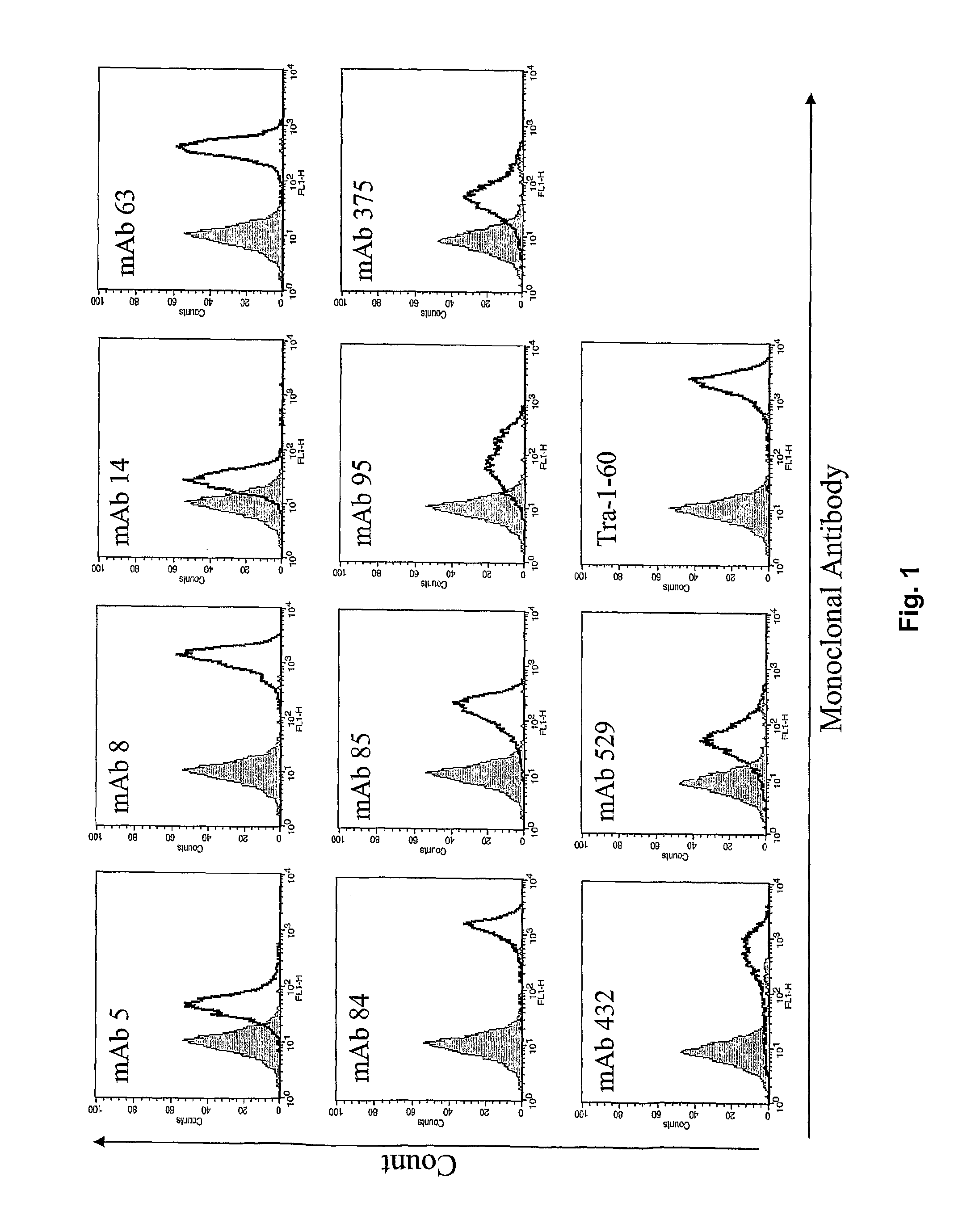
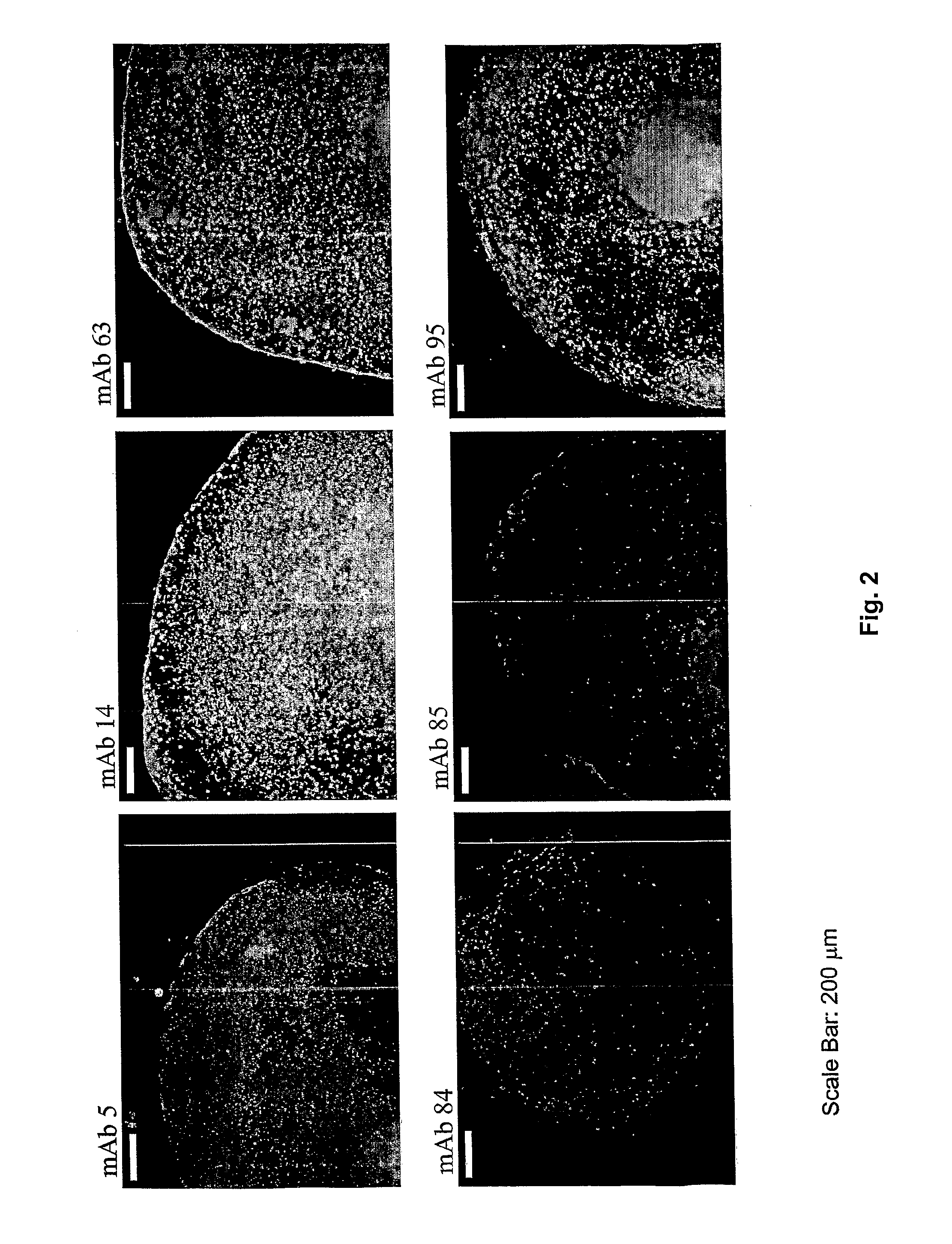
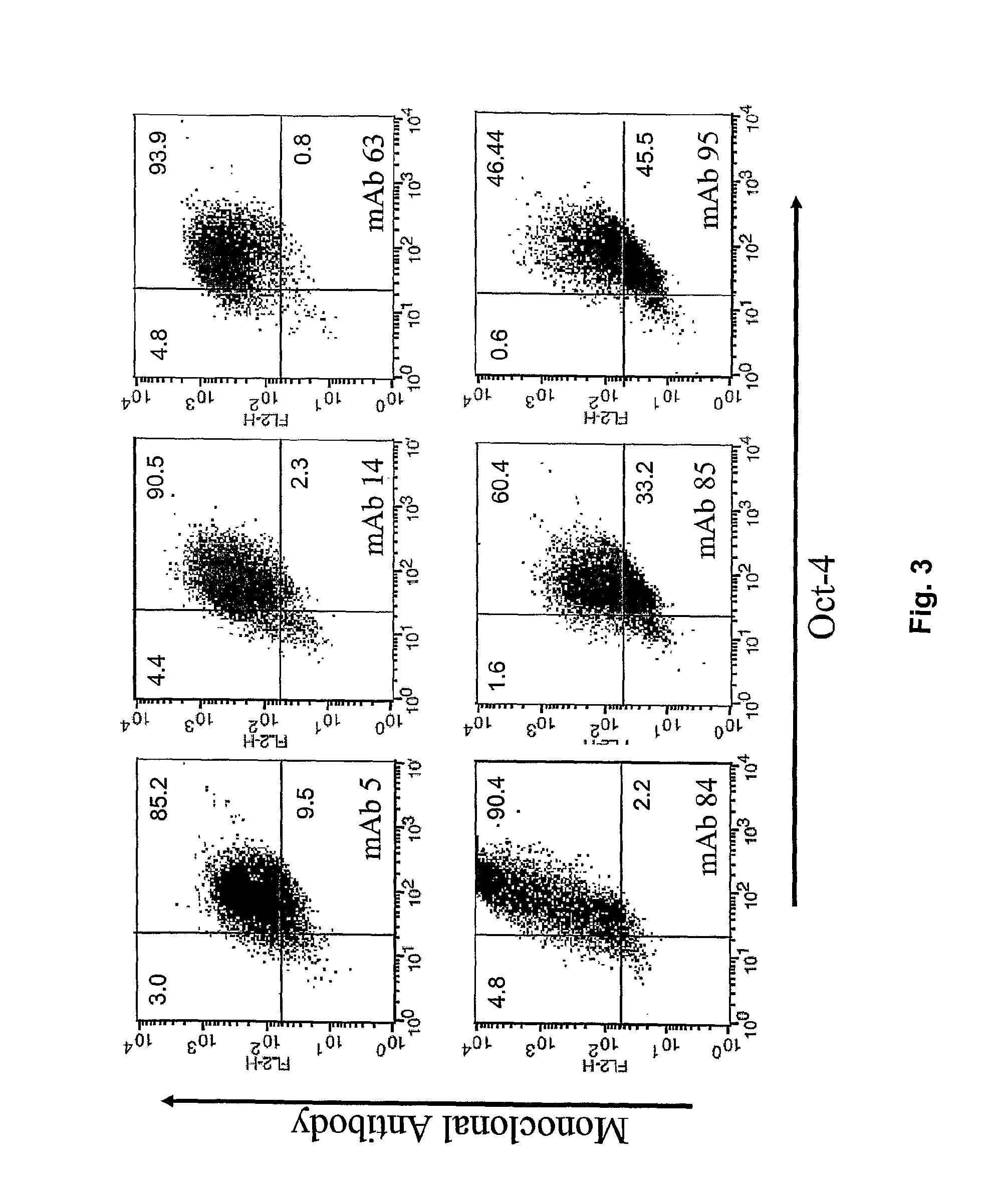
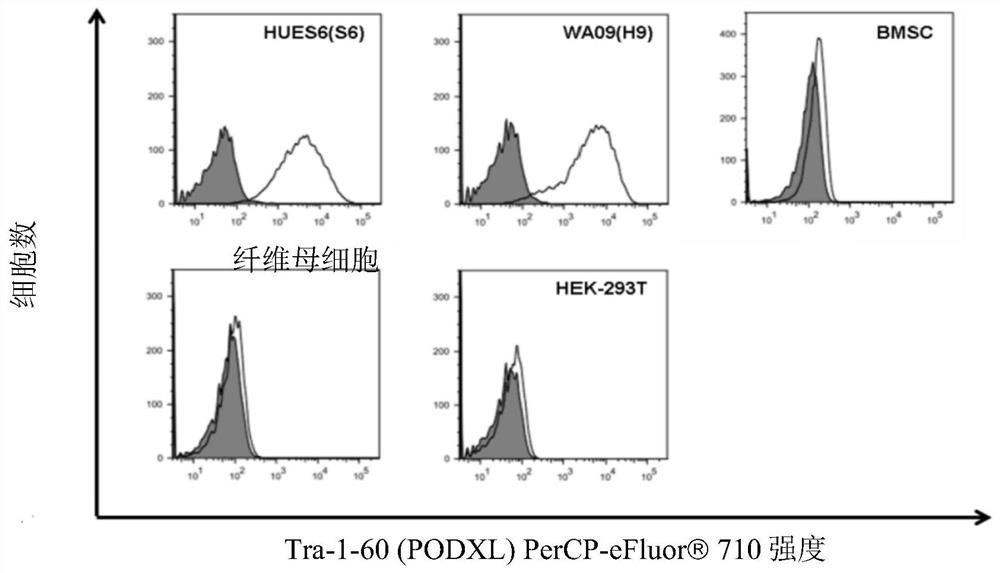
The disclosure relates to novel markers of pluripotent stem cells and uses thereof, and particularly, though not exclusively, to antibody molecules based on fragments of mAb84 which bind to undifferentiated pluripotent stem cells via podocalyxin-like protein-1 (PODXL).
Owner:AGENCY FOR SCI TECH & RES
An early-stage detection kit for diabetic nephropathy, a biomarker detecting method and applications
An early-stage detection kit for diabetic nephropathy is disclosed. The kit comprises detection agents. The detection agents comprise two or three of a first detection agent, a second detection agent, a third detection agent and a fourth detection agent. The solute of the first detection agent is a first podocalyxin antibody. The solute of the second detection agent is a first IV type collagen antibody. The solute of the third detection agent is a first liver-type fatty acid-binding protein antibody. The solute of the fourth detection agent is a first neutrophils gelatinase-associated lipocalin antibody. The kit adopts two or three of podocalyxin, IV type collagen, liver-type fatty acid-binding protein and neutrophils gelatinase-associated lipocalin as biomarkers, and can provide assisted determination for renal injury according to actual detection results, thus providing assistance for differentiating early-stage diabetic nephropathy.
Owner:BIO TECH ACADEMY CHINA
Systems and methods employing human stem cell markers for detection, diagnosis and treatment of circulating tumor cells
Owner:CUREMETA DIAGNOSTICS LLC
Systems and methods for employing podocalyxin and tra human stem cell markers as prognostic markers for aggressive and metastatic cancer
InactiveUS20140274773A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCancer cellPodocalyxin
The invention relates to systems and methods to detect podocalyxin-expressing and TRA-expressing cancer stem cells or cancer cells with stem cell-like properties in a tissue cell sample obtained from a localized tumor of a patient, and to determine a diagnosis or prognosis of aggressive and metastatic cancer by employing podocalyxin and TRA human stem cell markers as prognostic markers.
Owner:CUREMETA DIAGNOSTICS LLC
Detection method of protein marker for urine exosome
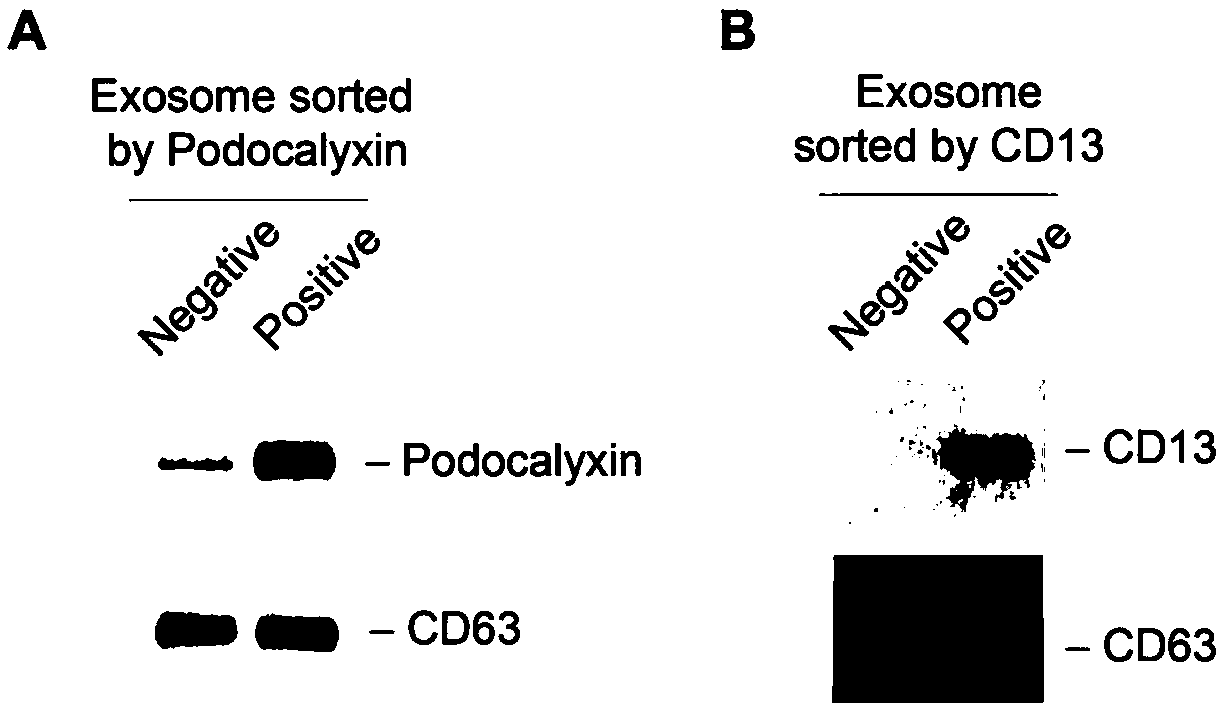
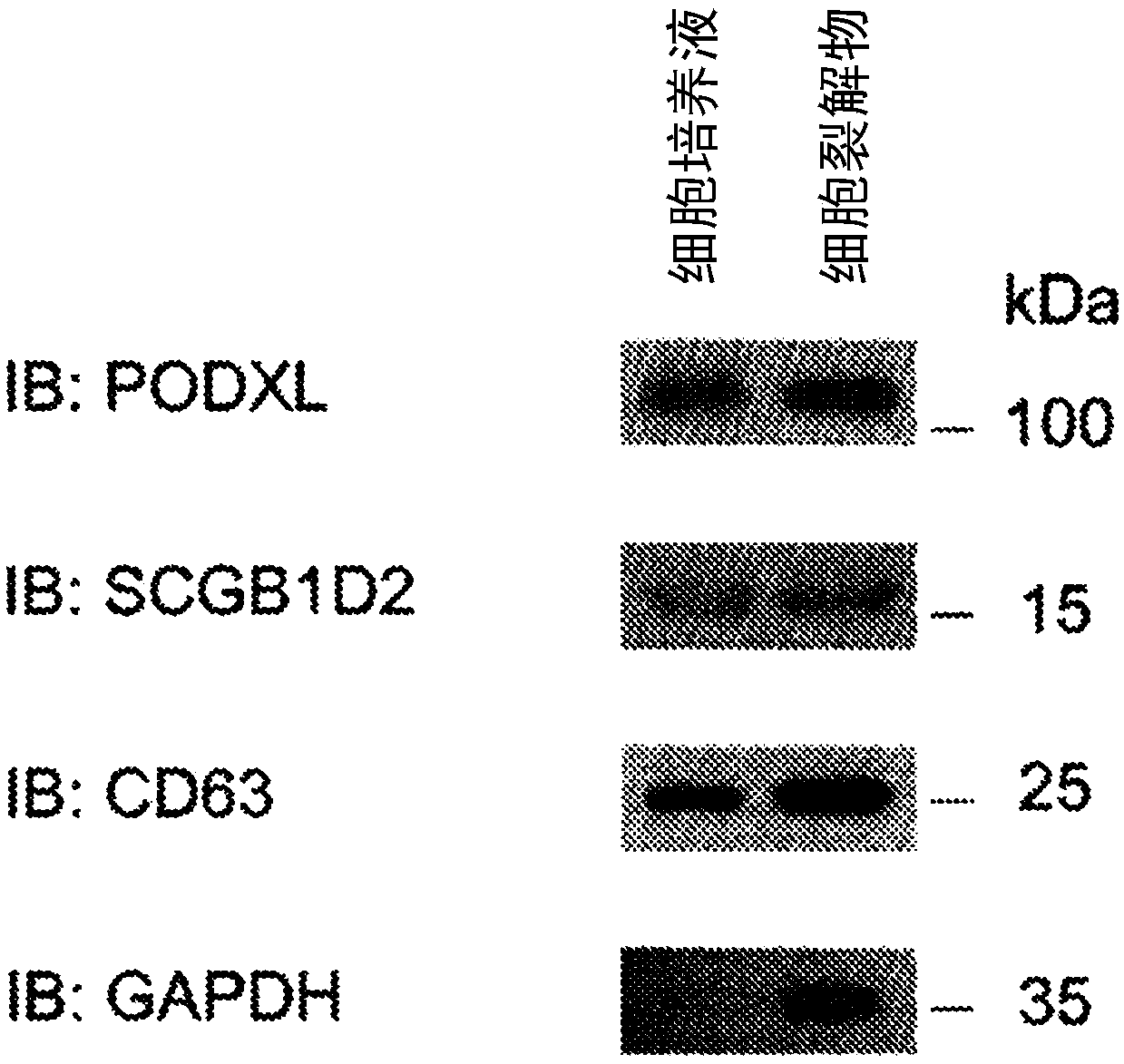
The invention discloses a detection method of a protein marker for urine exosome. The method comprises the following steps: extracting and purifying exosome from freshly collected urine, collecting precipitate, and performing resuspending, so as to obtain exosome suspension; performing separation on the exosome suspension obtained in the step (1) through magnetic beads coated by CD13 and podocalyxin respectively, so as to obtain an exosome separation fluid; measuring the protein concentration of exosome of the separation fluid through a BCA kit; preparing the separation fluid into a protein loading sample according to the protein concentration, and performing CD13, Podocalyxcin and CD63westernblot detection on the sample, so as to obtain the required protein marker after identification. The method has the advantages that positive exosome of podocalyxin and CD13 in urine can be respectively extracted, and the method is beneficial to further analysis and detection to the exosome, so thatchange of sertoli cell and proximal renal tubular epithelial cell can be accurately reflected, and a biological marker is provided for diagnosis and treatment of diseases.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Test method on renal diseases
InactiveUS20120164662A1Reduce the burden onEfficient executionImmunoglobulins against animals/humansDisease diagnosisDiseaseNephrosis
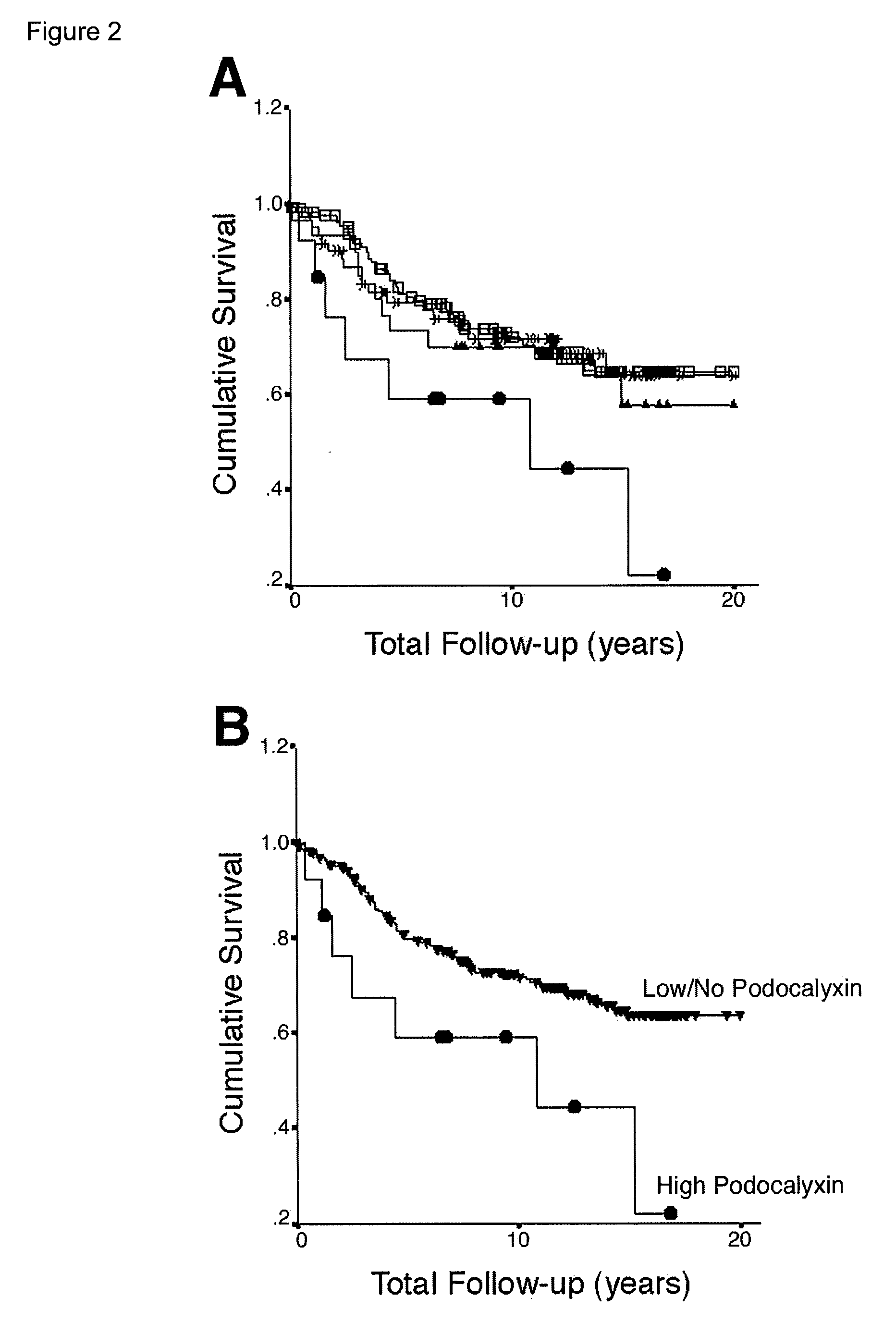
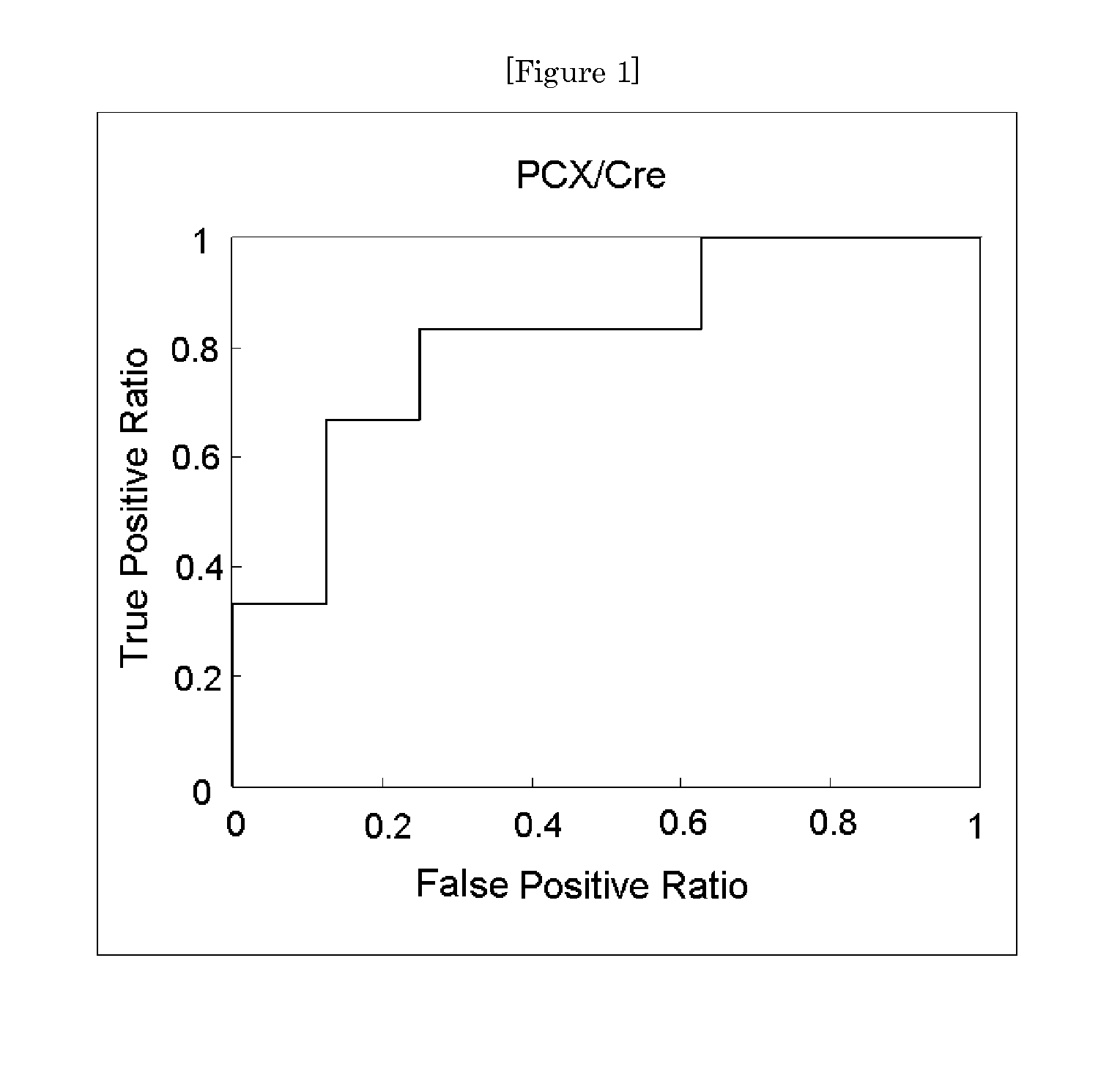
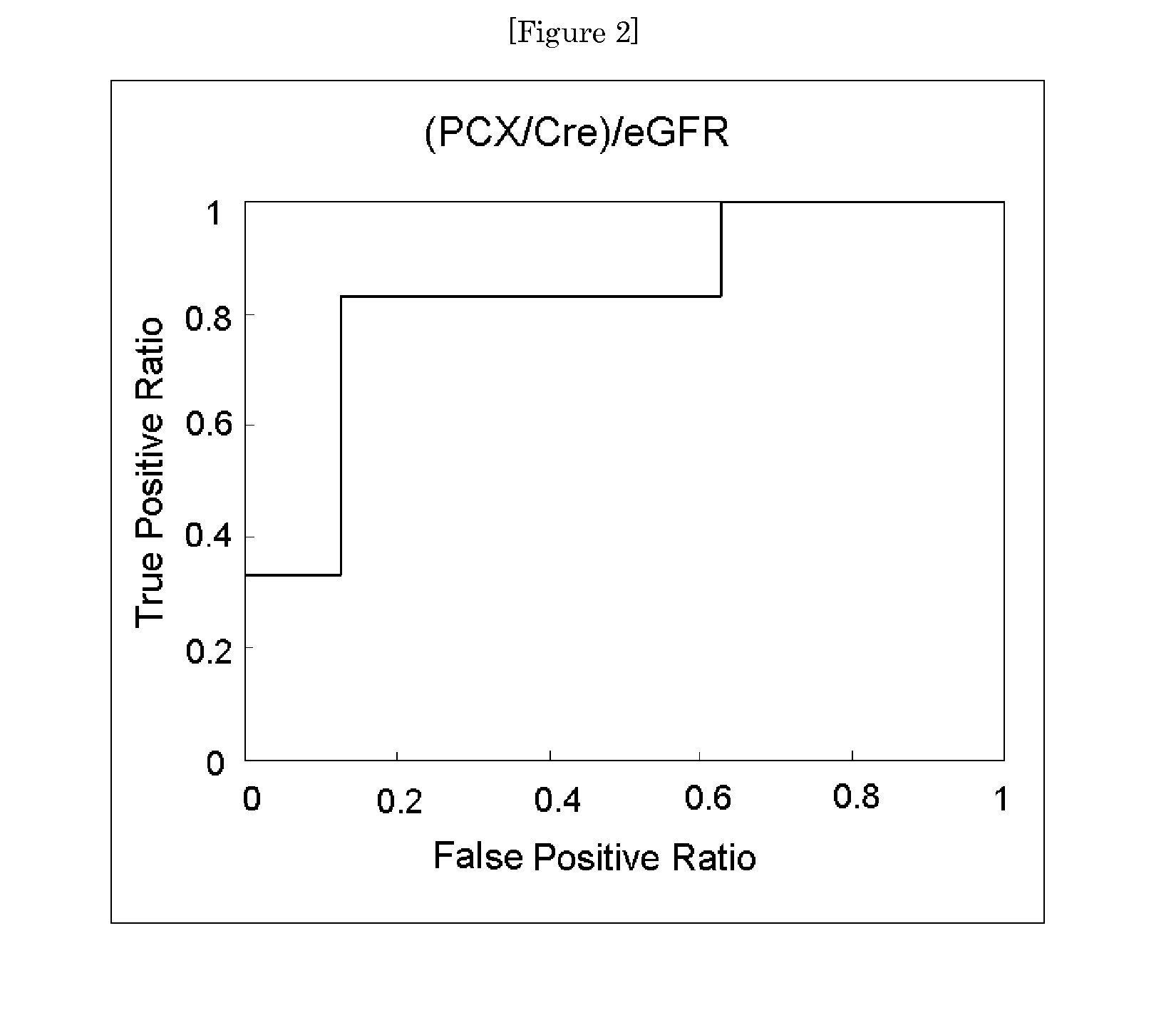
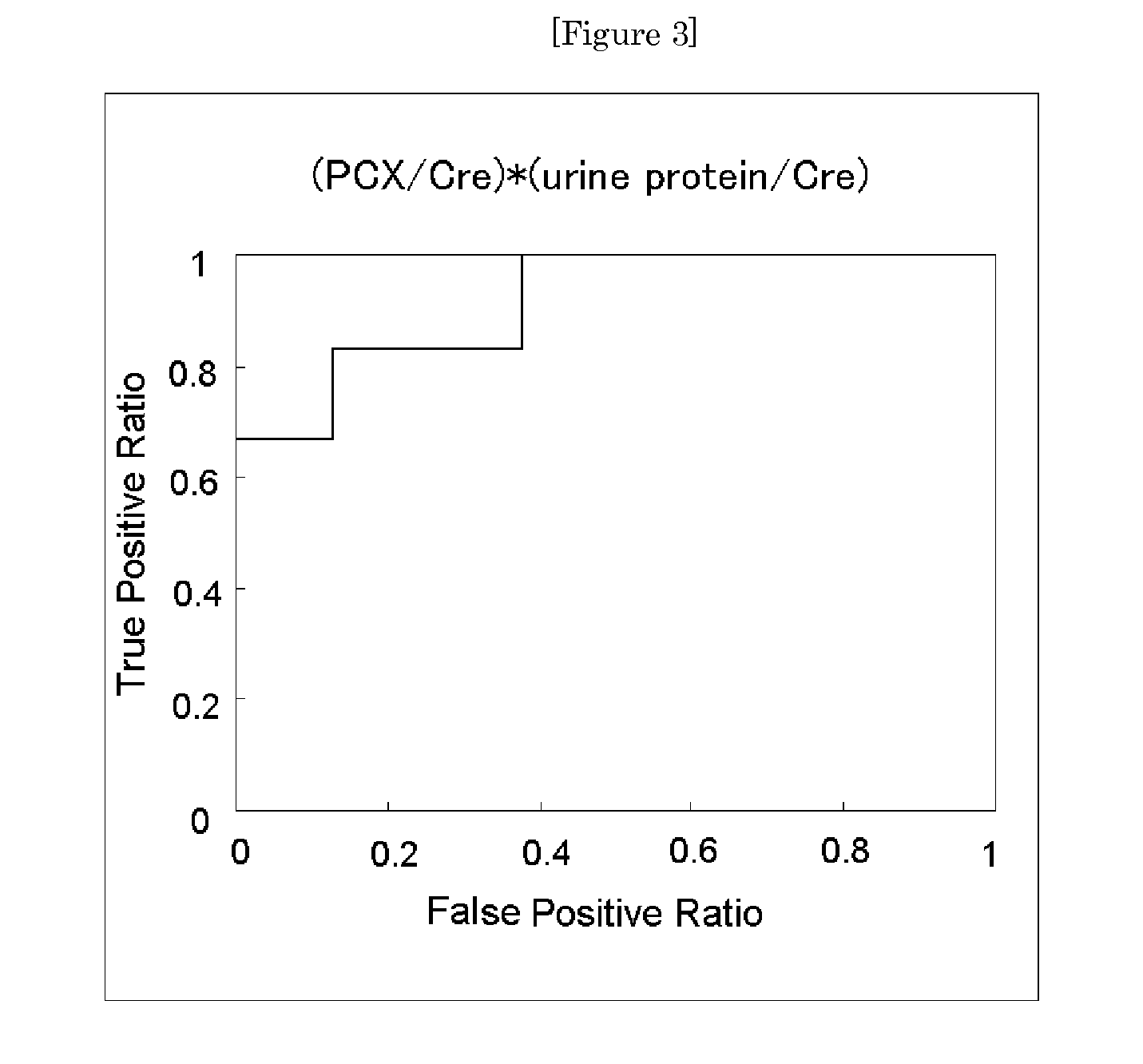
Provided is a test method for the assessment of the necessity of renal biopsy in a subject to be tested, who is suspected of having a renal disease. Specifically provided are a test method for a renal disease, including using urinary podocalyxin and one or more additional markers in combination, and a test reagent for use in the test method and a test reagent kit for use in the test method. The present invention allows the discrimination of a poor prognosis group even for poor prognosis cases with no overt findings in a conventional test method, and thus allows the assessment of a renal disease, the assessment of the necessity of renal biopsy, prognostic prediction, and the like to be performed exactly.
Owner:NIIGATA UNIVERSITY +3
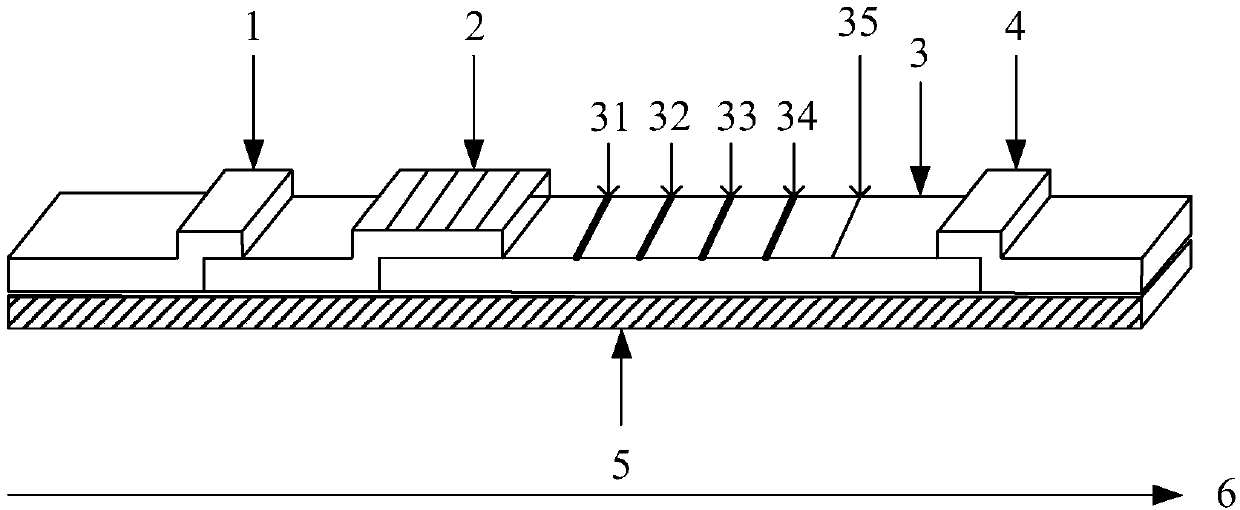
Colloidal gold kit for detecting diabetic nephropathy biomarker and method
InactiveCN110346570AReduce testing costsImprove detection accuracyDisease diagnosisBiological testingReagent stripPodocalyxin
The invention provides a colloidal gold kit for detecting a diabetic nephropathy biomarker and a method. The kit comprises a sample pad, a gold standard pad, a nitrocellulose membrane, an absorbent pad and a support pad. The nitrocellulose membrane is provided with a first detection reagent strip, a second detection reagent strip, a third detection reagent strip, a fourth detection reagent strip,and a capture reagent strip successively. The first detection reagent strip coats an anti-IV-type collagen monoclonal antibody; the second detection reagent strip coats an anti-podocalyxin monoclonalantibody; the third detection reagent strip coats an anti-neutropil gelatinase-associated lipocalin monoclonal antibody; the fourth detection reagent strip coats an anti-liver fatty acid binding protein monoclonal antibody; and the capture reagent strip coats a second antibody binding the monoclonal antibody coated on the gold standard pad. According to the invention, with the colloidal gold kit,simultaneous and quantitative detection of various biomarkers of early diabetic nephropathy can be realized; the cost for detecting early diabetic nephropathy is saved; and the detection efficiency isimproved.
Owner:BIO TECH ACADEMY CHINA
Method of predicting endometrial receptivity
The present invention relates to a method of predicting endometrial receptivity of an embryo implantation in a subject, the method comprising determining a level of podocalyxin in endometrial epithelial cells in the subject. The invention also relates to methods of monitoring epithelial cell receptivity and improving epithelial cell receptivity.
Owner:HUDSON INST OF MEDICAL RES +1
Method for examining acute renal disorder
ActiveUS20120322087A1Quick decisionEnsure correct executionImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisRenal disorderPodocalyxin
Provided is a test method for acute kidney injury, including detecting urinary podocalyxin. According to the test method, a subject to be tested who has a higher value for the urinary podocalyxin than a reference value can be assessed to have acute kidney injury. Further, as compared to a conventional method, the test method allows acute kidney injury to be assessed accurately and non-invasively, which allows a physical burden on a patient to be reduced. Thus, the test method is useful.
Owner:DENKA CO LTD +1
Method and kit for detecting stem cell
Owner:FUJIFILM WAKO PURE CHEM CORP +1
Polymerase chain reaction detection method of urinary podocyte specific messenger ribonucleic acid (mRNA)
InactiveCN102443651AStrong specificityEasy to buildMicrobiological testing/measurementHigh concentrationLinear correlation
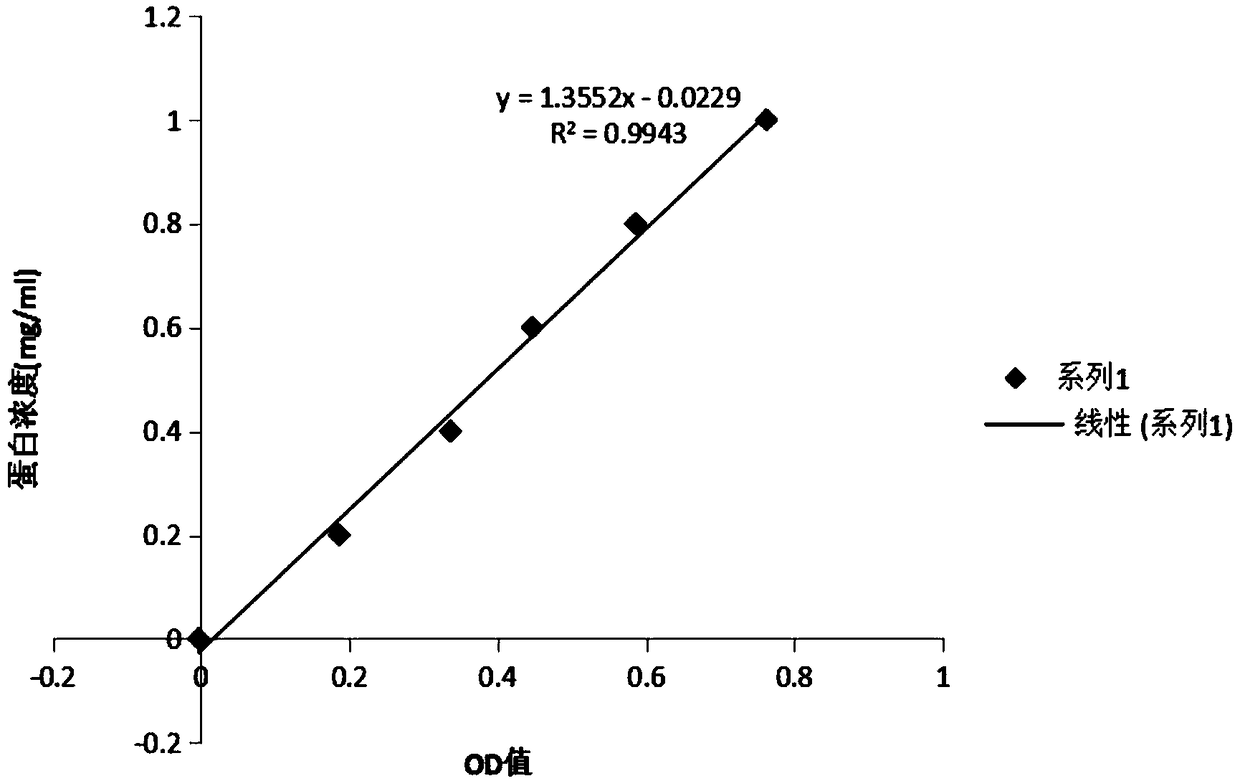
The invention provides a polymerase chain reaction detection method of urinary podocyte specific messenger ribonucleic acid (mRNA). The method is characterized by being established through the following steps of: collection of urine specimens; total RNA (ribonucleic acid) extraction as well as concentration and purity detection; reverse transcription; design and synthesis of primers; real-time PCR (polymerase chain reaction); preparation of a standard product and manufacture of a standard curve; and real-time PCR of the specimens to be detected and the standard product. The method has the characteristics of rapidness, simplicity and convenience, and can be used for acquiring an excellent quantitative linear correlation coefficient (R2>0.99); simultaneously, the established method is wide in detectable concentration range, podocalyxin and CD2-PA respectively spans 10 orders of magnitude and 8 orders of magnitude, and Ct values corresponding to minimum detectable concentrations of two types of genes respectively reach 35 and 33.2286; and furthermore, the inter-group and inter-batch differences of the high-concentration and light-concentration template Ct values, detected by the method, of the two types of genes are less than 3%, thus indicating that the method is high in stability and good in repeatability. The established method can be used for providing a stable technical platform for rapidly, simply and conveniently detecting the urinary podocyte specific mRNA.
Owner:SOUTHEAST UNIV
Diabetic nephropathy early detection test kit, biomarker detection method and application
A diabetic nephropathy early detection reagent kit comprising testing reagents. The testing reagents comprise either two or three among a first testing reagent, a second testing reagent, a third testing reagent, and a fourth testing reagent. A solute for the first testing reagent is a first podocalyxin antibody. A solute for the second testing reagent is a first collagen IV antibody. A solute for the third testing reagent is a first liver fatty acid binding protein antibody. A solute for the fourth testing reagent is a first neutrophil gelatinase-associated lipocalin antibody. By having either two or three among podocalyxin, collagen IV, liver fatty acid binding protein, and neutrophil gelatinase-associated lipocalin serving as biomarkers, the diabetic nephropathy early screening reagent kit is capable of providing assistance in determining kidney damages on the basis of an actual testing result, thus providing assistance in distinguishing early-stage diabetic nephropathy.
Owner:BIO TECH ACADEMY CHINA
Method for estimating number of podocytes in urine
ActiveUS10809265B2Estimated more easily and more accuratelyAccurate Performance EvaluationPreparing sample for investigationDisease diagnosisUrinary sedimentPodocalyxin
The present invention relates to a method of estimating the number of urinary podocytes, including detecting podocalyxin in a urinary sediment sample liquid, and more specifically, to a method of estimating the number of urinary podocytes, including the following steps (1) to (3): (1) a step of preparing the urinary sediment sample liquid by separating urinary sediment from urine collected from a test subject and solubilizing podocalyxin in the urinary sediment; (2) a step of calculating a podocalyxin excretion amount in the urinary sediment sample liquid through detection of podocalyxin in the urinary sediment sample liquid; and (3) a step of calculating the number of urinary podocytes by dividing the podocalyxin excretion amount in the urinary sediment sample liquid by a podocalyxin amount per podocyte.
Owner:HARA +1
Human embryonic stem cell methods and PODXL expression
InactiveUS8710190B2Minimise additional stepImmunoglobulins against cell receptors/antigens/surface-determinantsArtificial cell constructsPODOCALYXIN-LIKE PROTEINCell therapy
A method of identifying an undifferentiated human embryonic stem cell in a sample which may contain such cells, the method comprising identifying the cell or cells within the sample that express podocalyxin-like protein (PODXL) on their surface. A method of isolating an undifferentiated human embryonic stem cell from a sample containing such cells, the method comprising isolating the cell or cells within the sample that express PODXL on their surface. Typically, the methods use an antibody which binds to PODXL. Undifferentiated human embryonic stem cells isolated by the method may be useful in cell therapy. Also, in particular, compositions of cells differentiated from a human embryonic stem cell but which composition has been depleted of undifferentiated human embryonic stem cells are provided which are useful in cell therapy.
Owner:AGENCY FOR SCI TECH & RES
Methods for regulating potency of pluripotent stem cells and applications thereof
PendingCN113811601ASkeletal/connective tissue cellsNon-embryonic pluripotent stem cellsPodocalyxinBiochemistry
Methods for detecting and treating cancer
ActiveUS9309323B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsPodocalyxinCancer research
Methods and kits for detecting cancer and monitoring cancer progression are described. The method involves analyzing a sample containing nucleic acids or proteins from a patient for decreased expression of endoglycan and / or increased expression of podocalyxin.
Owner:THE UNIV OF BRITISH COLUMBIA
Diabetic nephropathy early screening kit, biomarker detection method and application
A diabetic nephropathy early screening reagent kit comprising testing reagents. The testing reagents comprise a first testing reagent, a second testing reagent, a third testing reagent, and a fourth testing reagent. A solute for the first testing reagent is a first podocalyxin antibody. A solute for the second testing reagent is a first collagen IV antibody. A solute for the third testing reagent is a first liver fatty acid binding protein antibody. A solute for the fourth testing reagent is a first neutrophil gelatinase-associated lipocalin antibody. By having podocalyxin, collagen IV, liver fatty acid binding protein, and neutrophil gelatinase-associated lipocalin serving as biomarkers, the diabetic nephropathy early screening reagent kit is capable of providing assistance in determining kidney damages on the basis of an actual testing result, thus providing assistance in distinguishing early-stage diabetic nephropathy.
Owner:BIO TECH ACADEMY CHINA
Transmucosal administration of agents
PendingCN114206390AImprove absorption efficiencyImprove convenienceAntibacterial agentsPowder deliveryPodocalyxinChemical compound
Provided is a drug which improves the absorption efficiency of a drug from the mucosal epithelium. This transmucosal administration agent contains, as an active ingredient, an agent compound to which a podocalyxin target molecule is bound.
Owner:DENKA CO LTD +1
Methods for regulating potency of pluripotent stem cells and applications thereof
PendingUS20220090023A1High reprogramming efficiencyDownregulating the potency of the pluripotent stem cellsSkeletal/connective tissue cellsNon-embryonic pluripotent stem cellsPodocalyxinBiochemistry
The present invention relates to a method for regulating potency of pluripotent stem cells (PSCs) by modulating expression of podocalyxin-like protein 1 (PODXL) and applications thereof.
Owner:ACAD SINIC
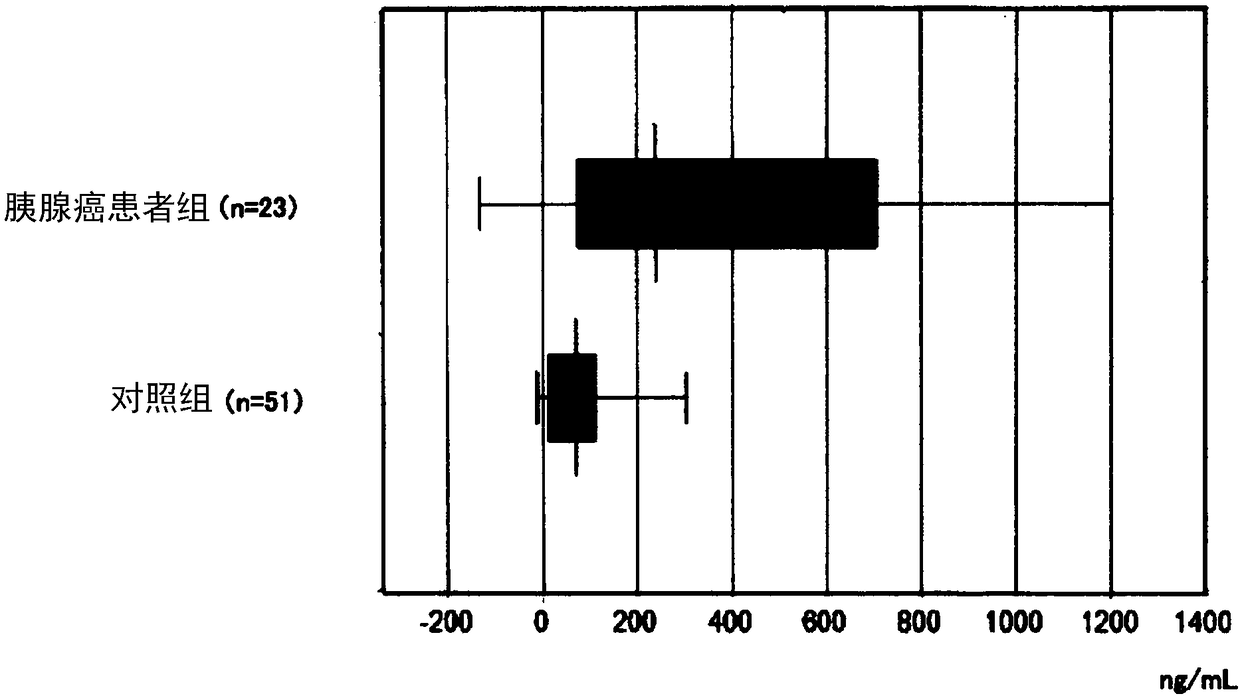
Marker for pancreatic cancer and intraductal papillary mucinous tumors
Owner:NAT UNIV CORP KOCHI UNIV
Pharmaceutical agent for transmucosal administration
PendingUS20220288224A1Effective absorptionImprove convenienceAntibacterial agentsPowder deliveryDrug compoundPodocalyxin
Provided is a pharmaceutical agent for enhancing absorption efficiency of a drug through the mucosal epithelial layer. A pharmaceutical agent for transmucosal administration comprises as an active ingredient a drug compound to which a podocalyxin targeting molecule is bound.
Owner:DENKA CO LTD +1
Methods of predicting endometrial receptivity
PendingUS20220268780A1Lower Level RequirementsImprove receptivityMicrobiological testing/measurementDisease diagnosisPhysiologyPodocalyxin
The present invention relates to methods of predicting endometrial receptivity for embryo implantation in a subject, the method comprising determining a level of podocalyxin in endometrial epithelial cells in the subject. The present invention also relates to methods of monitoring epithelial receptivity and improving epithelial receptivity.
Owner:HUDSON INST OF MEDICAL RES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com