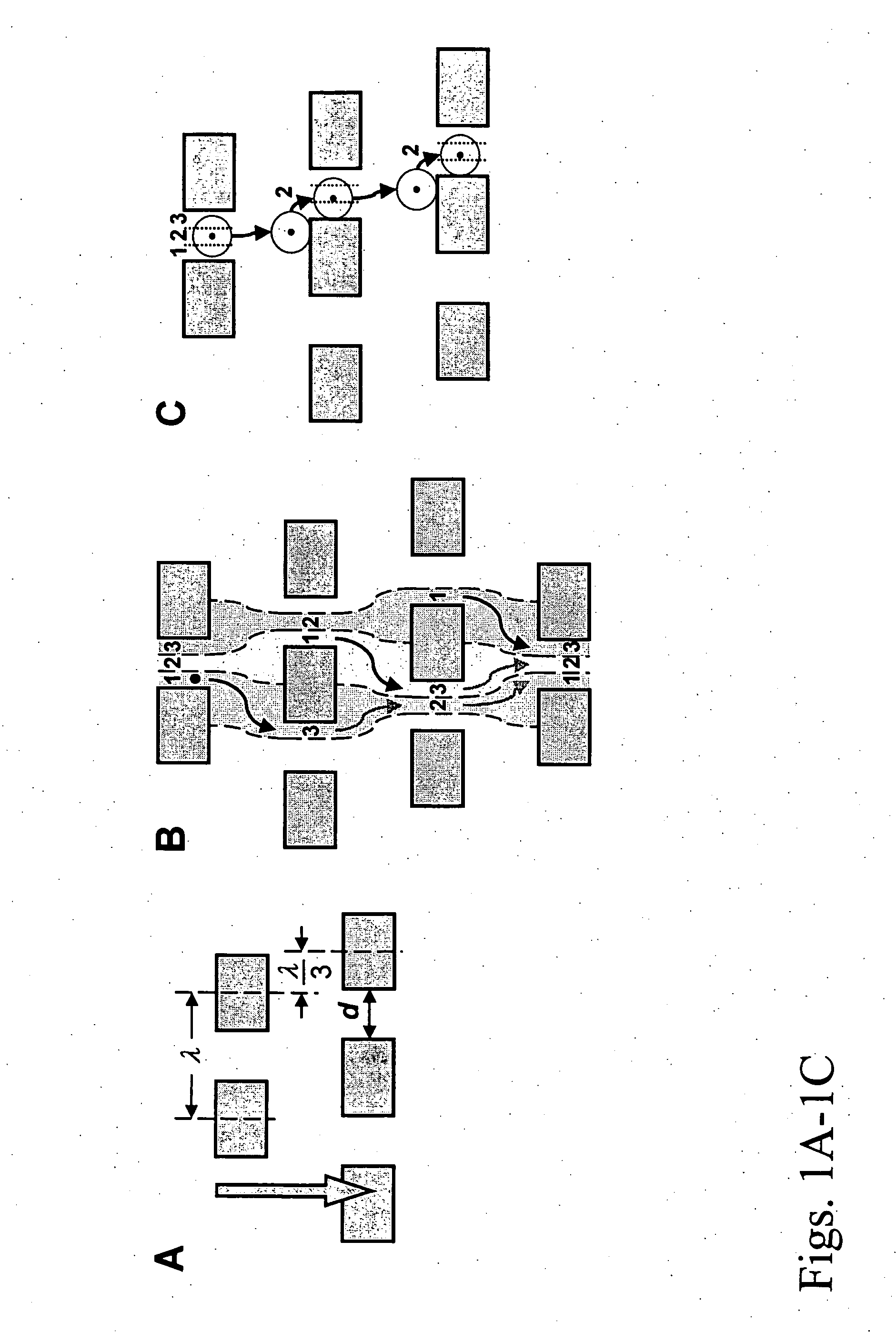
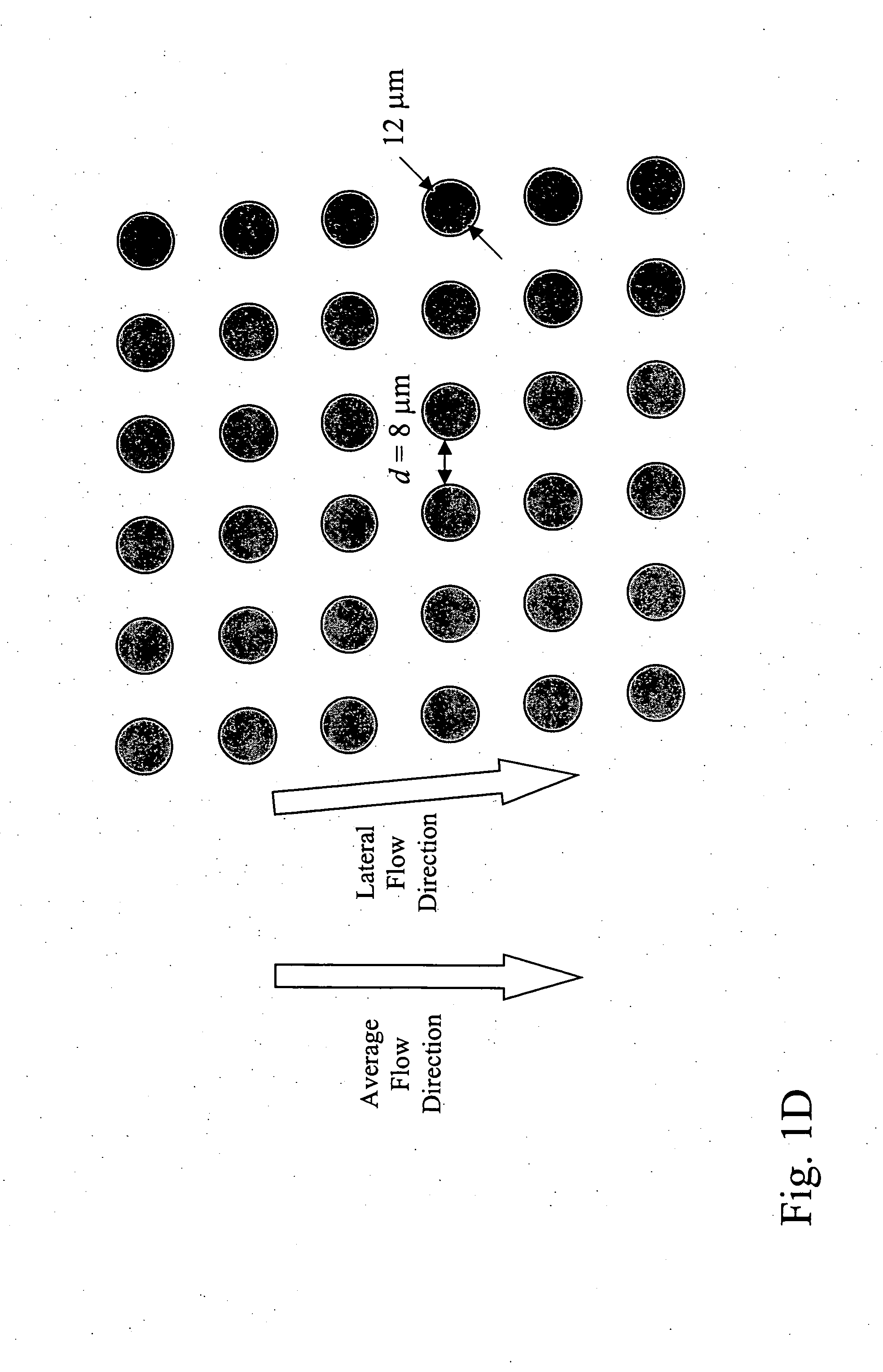
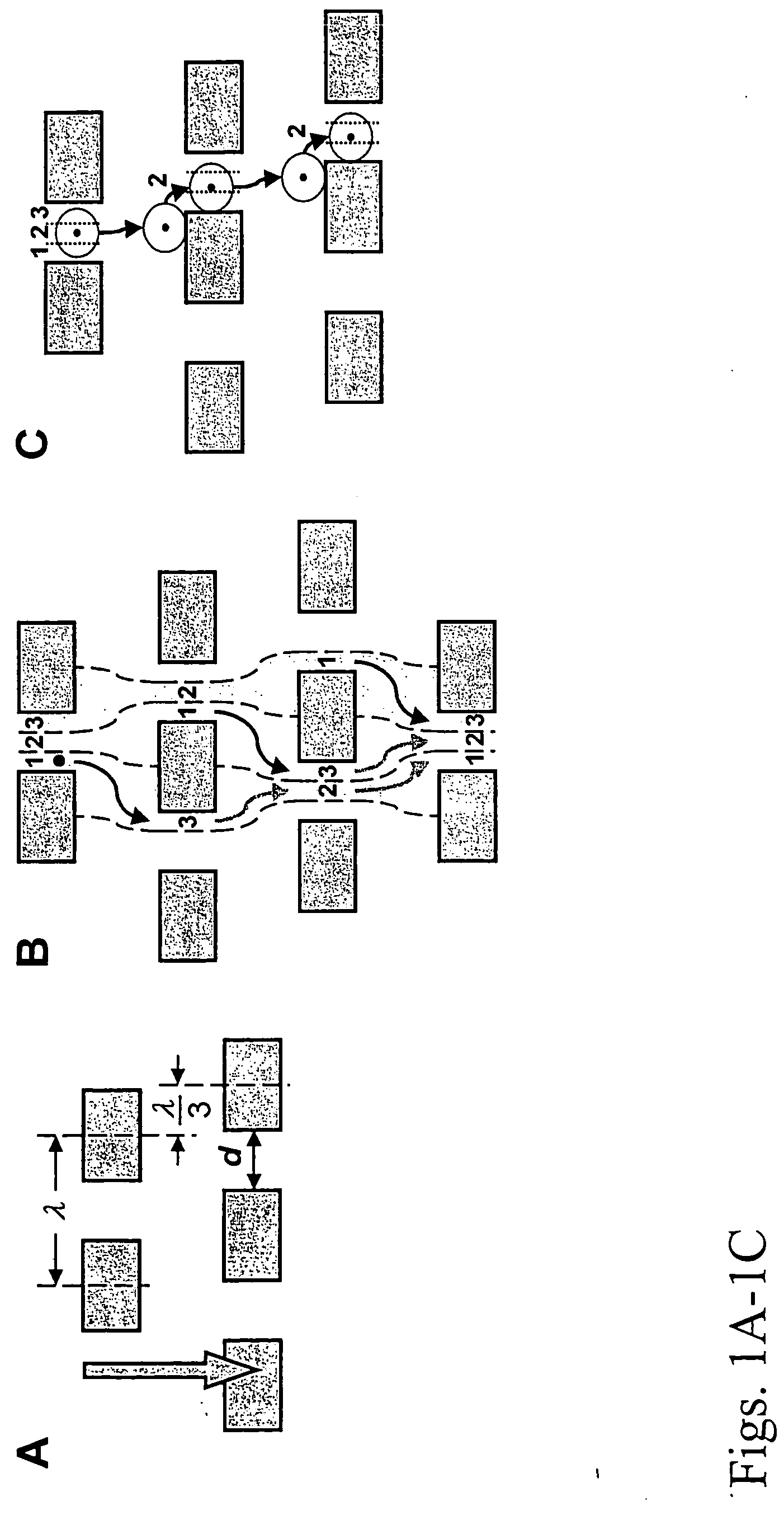
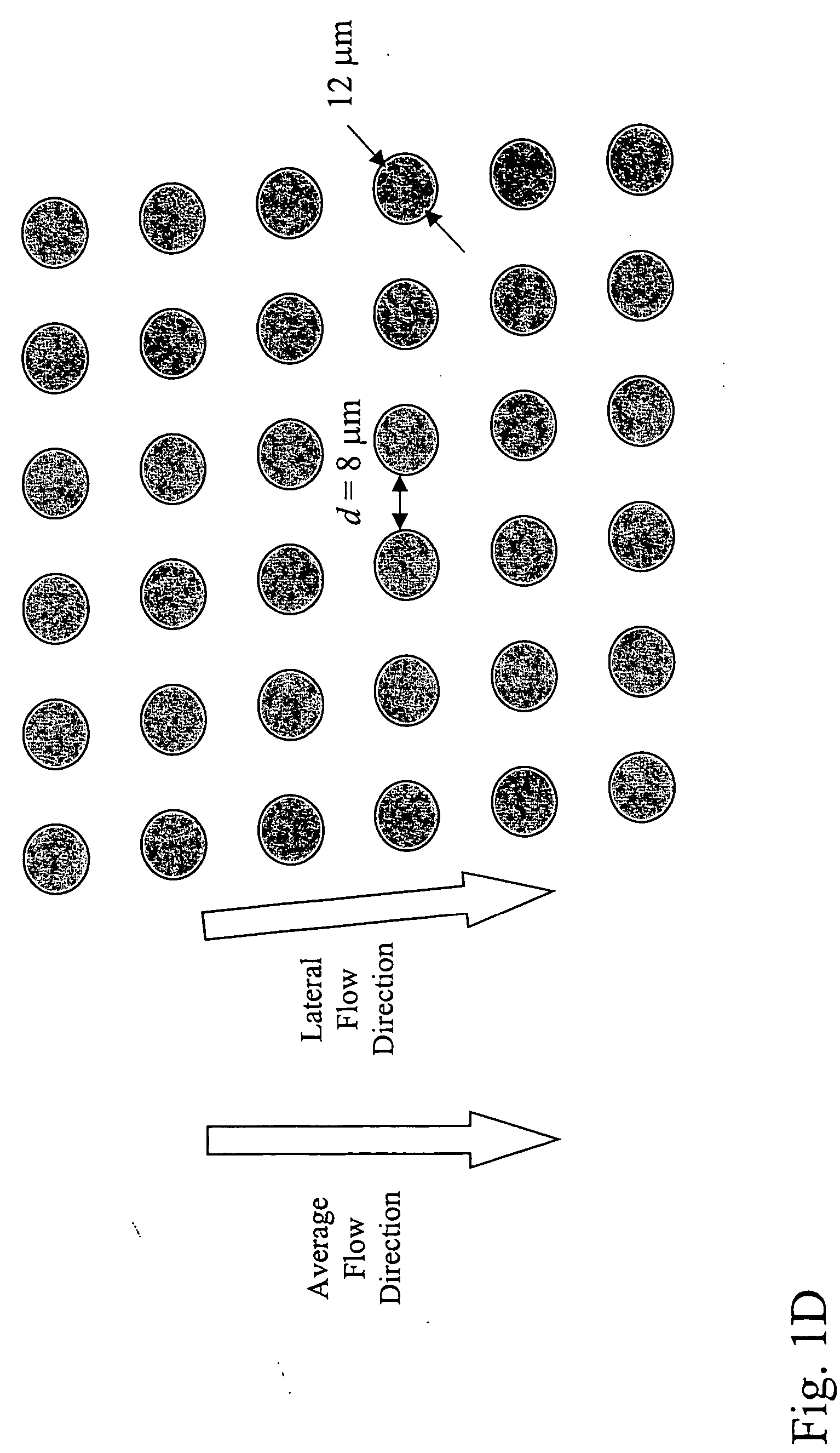
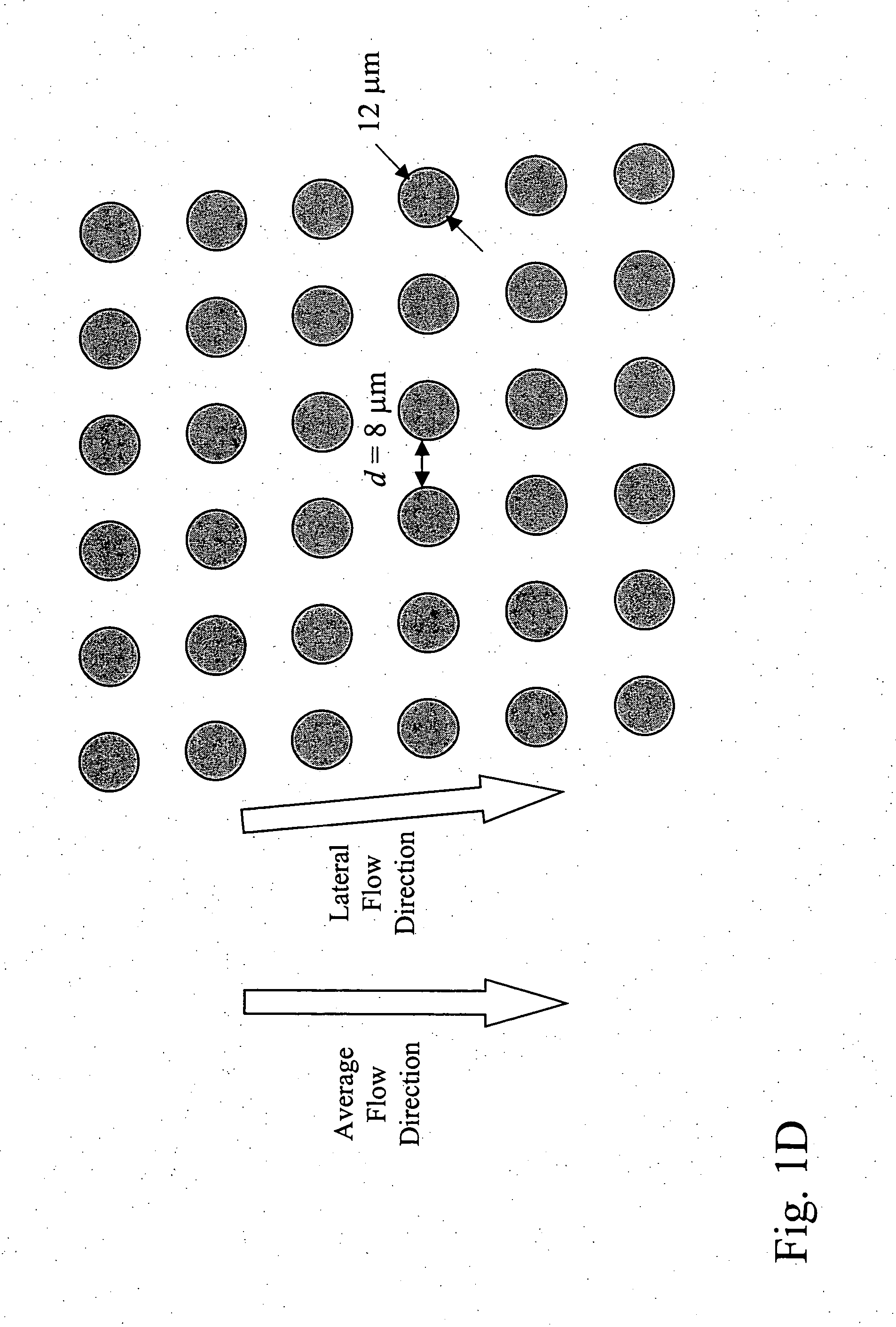
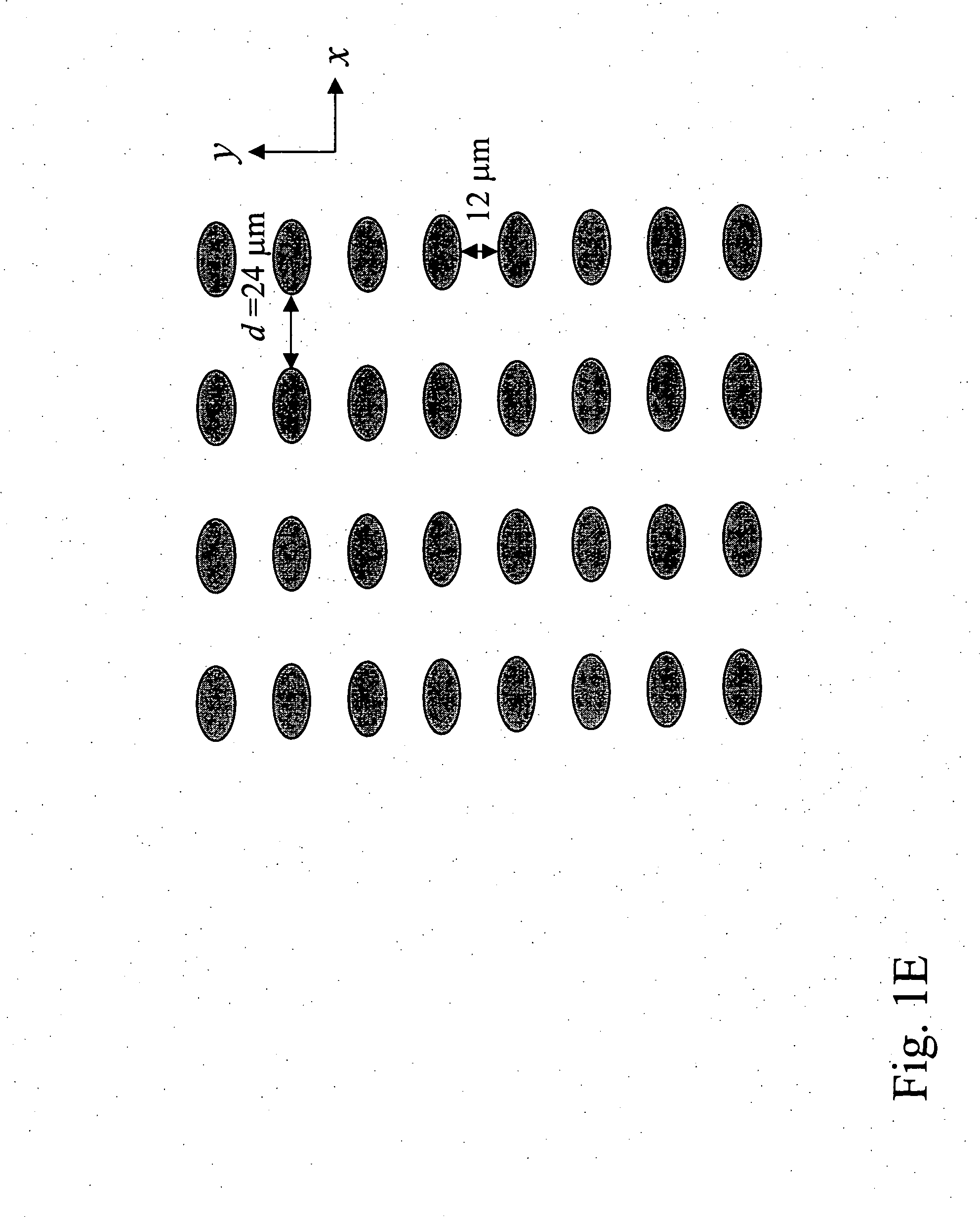
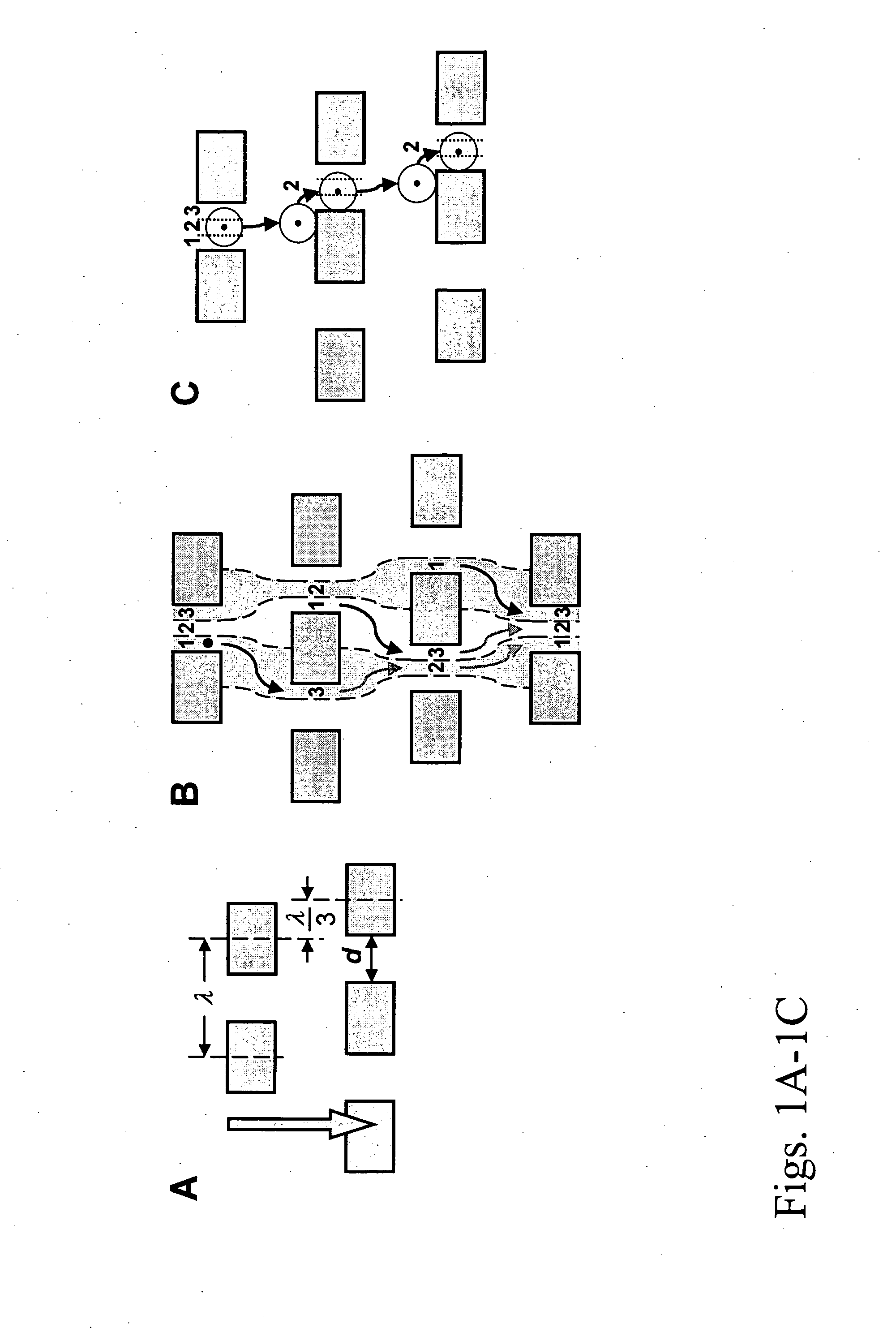
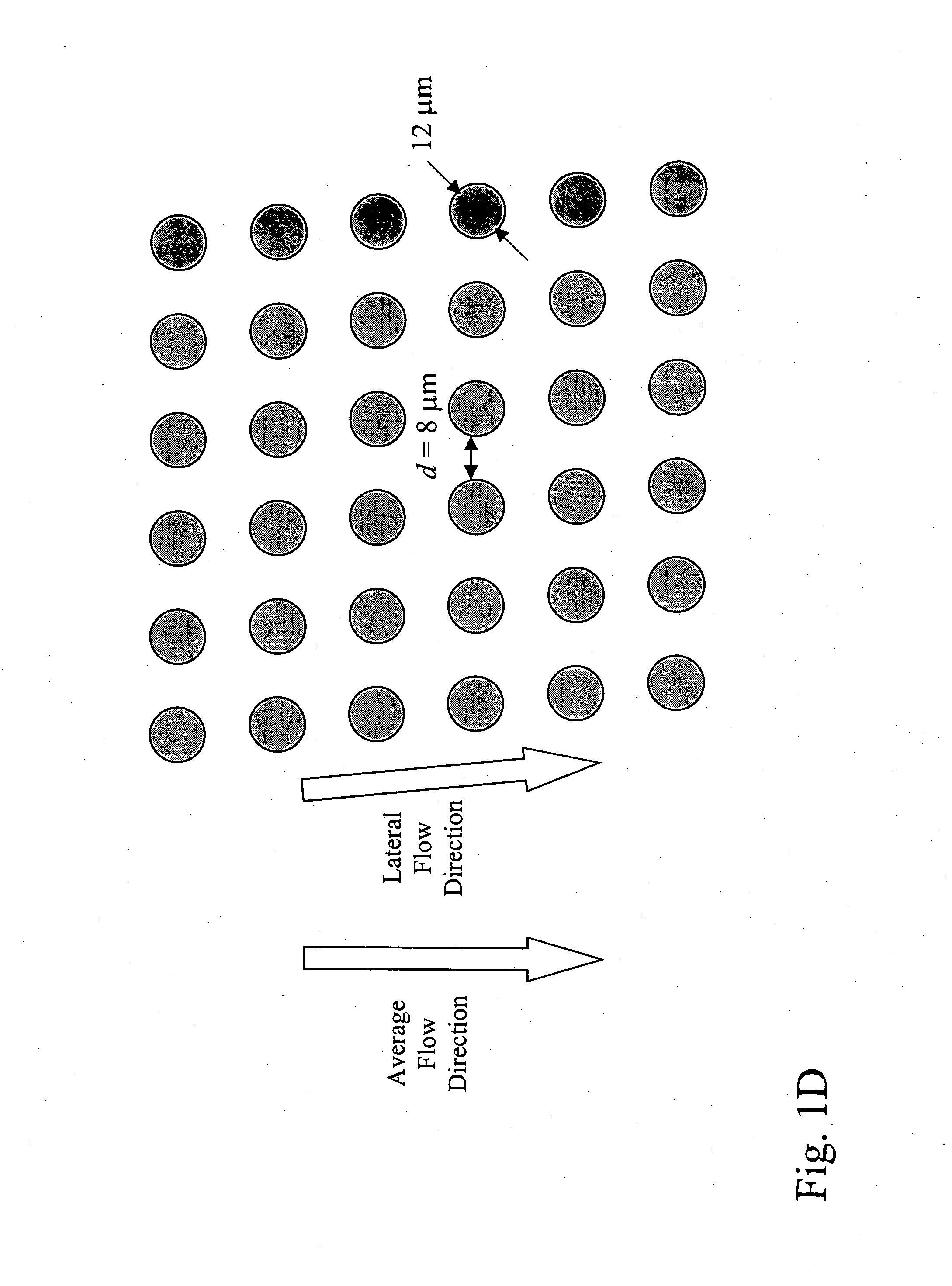
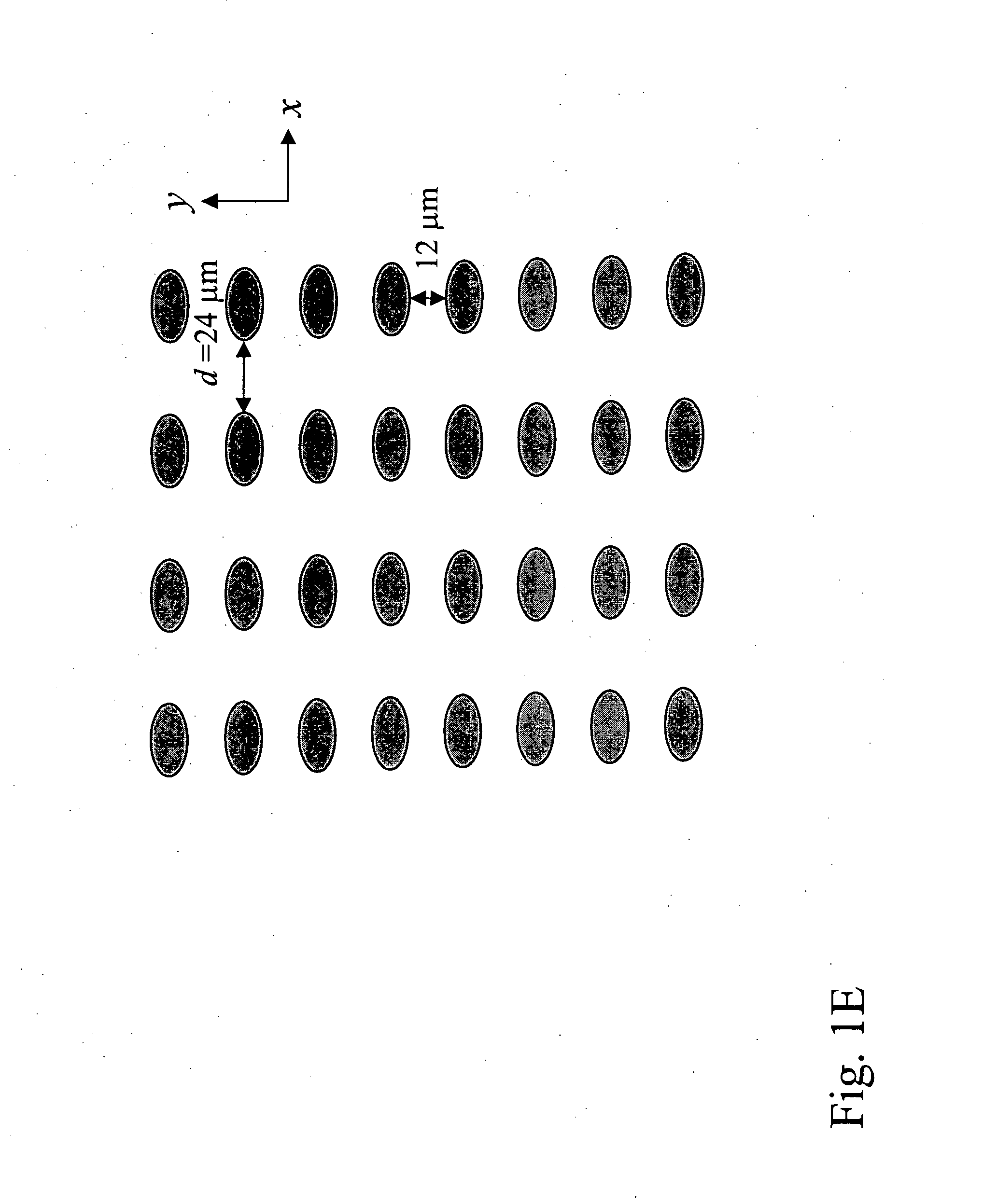
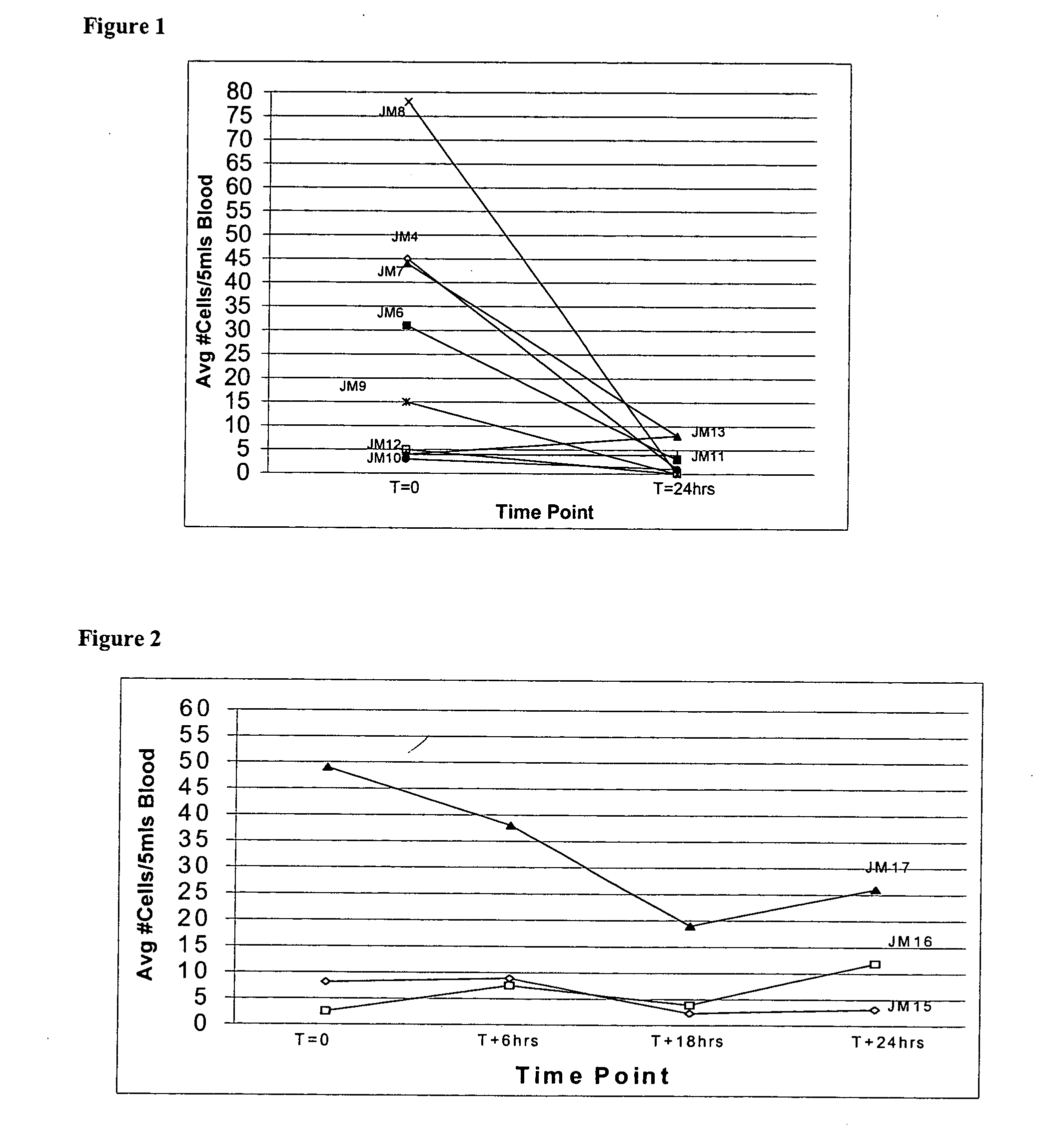
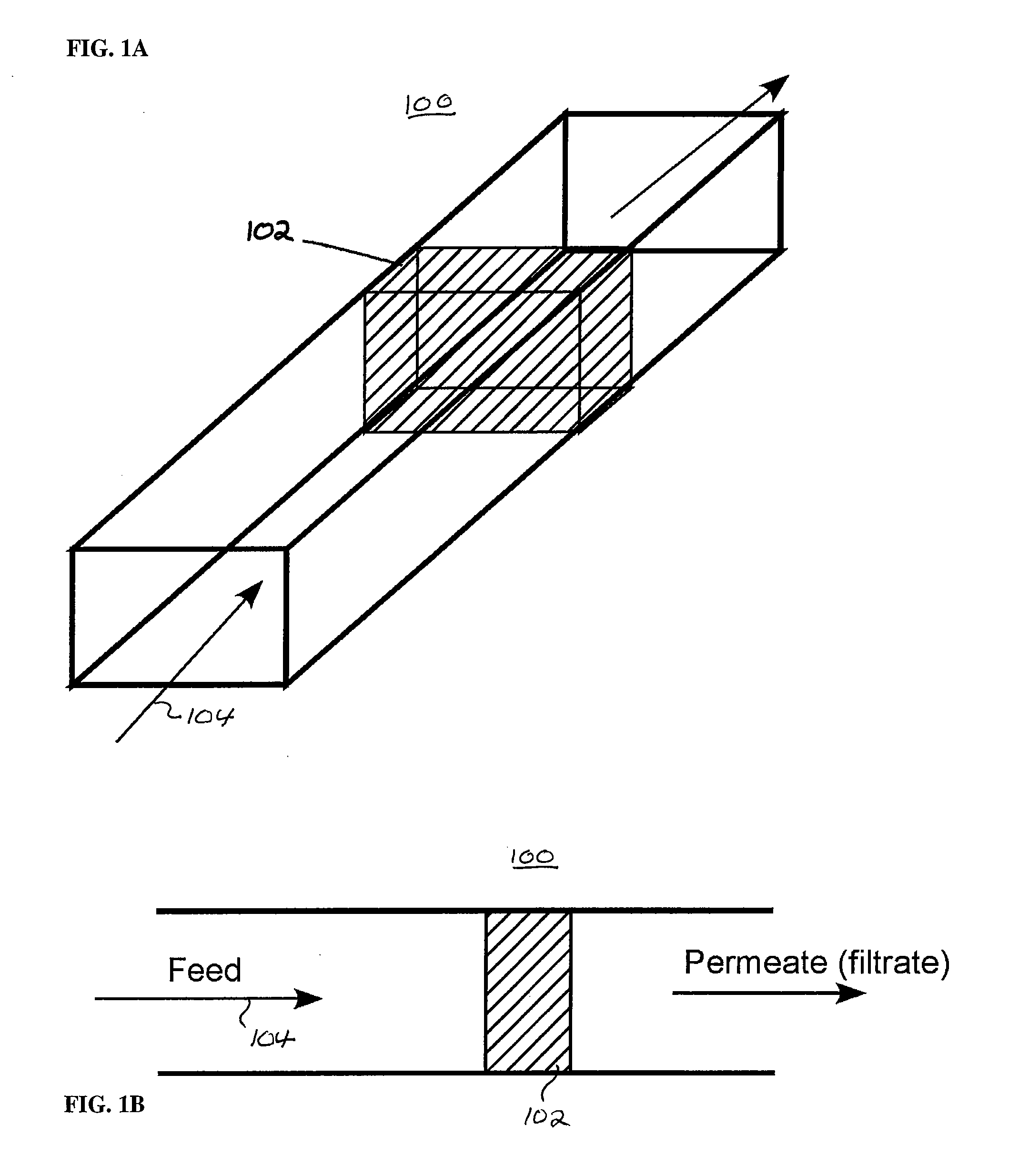
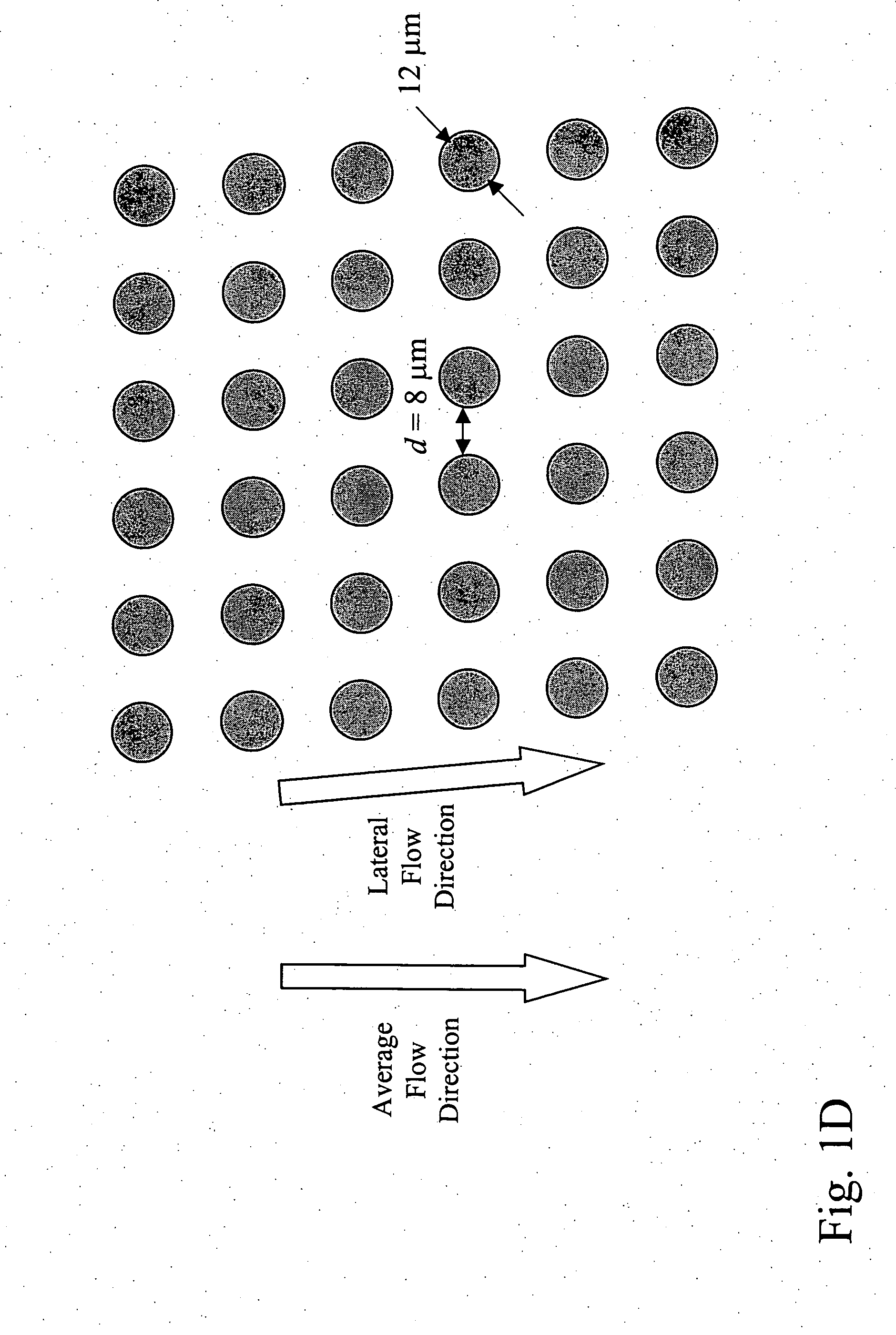
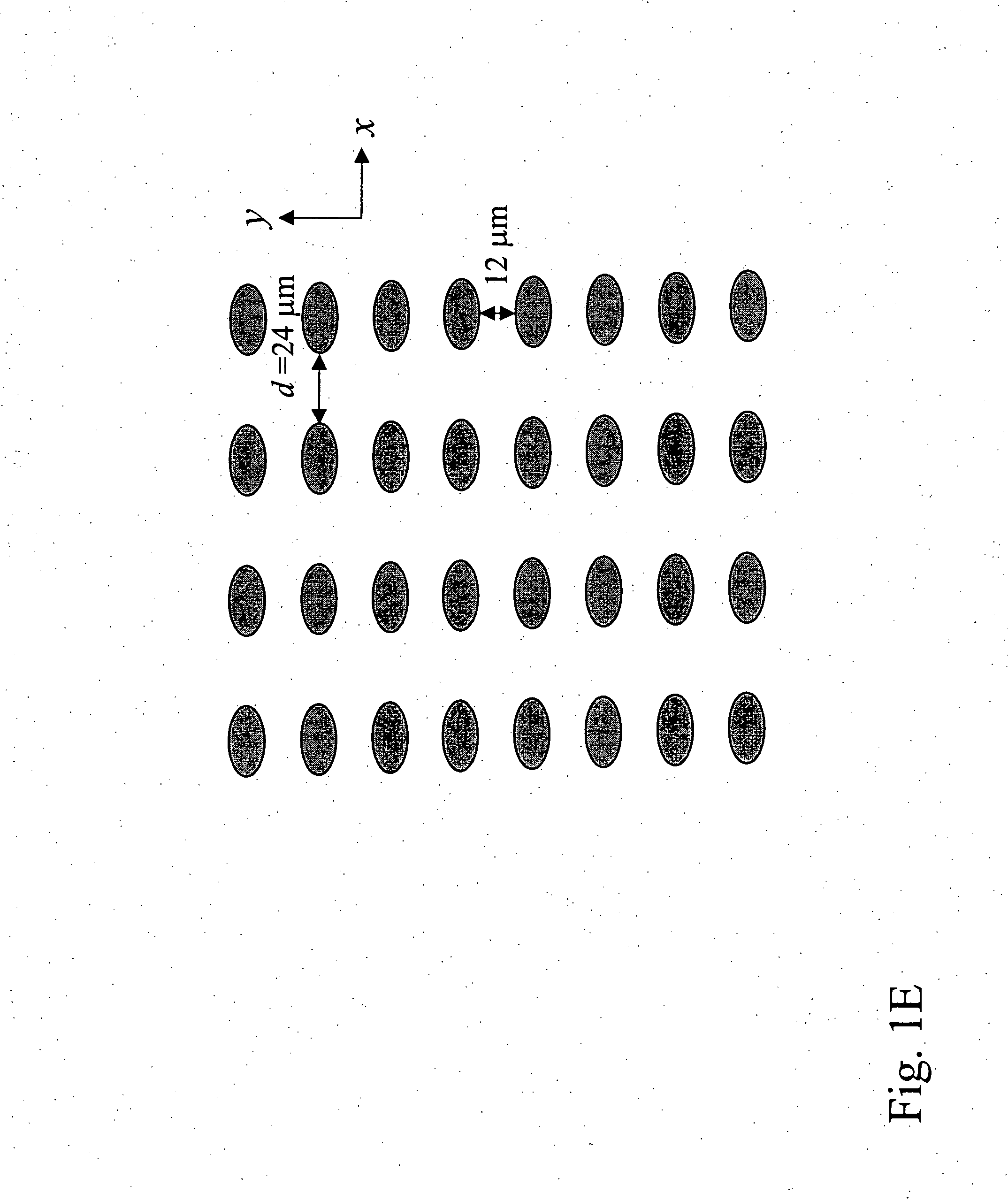
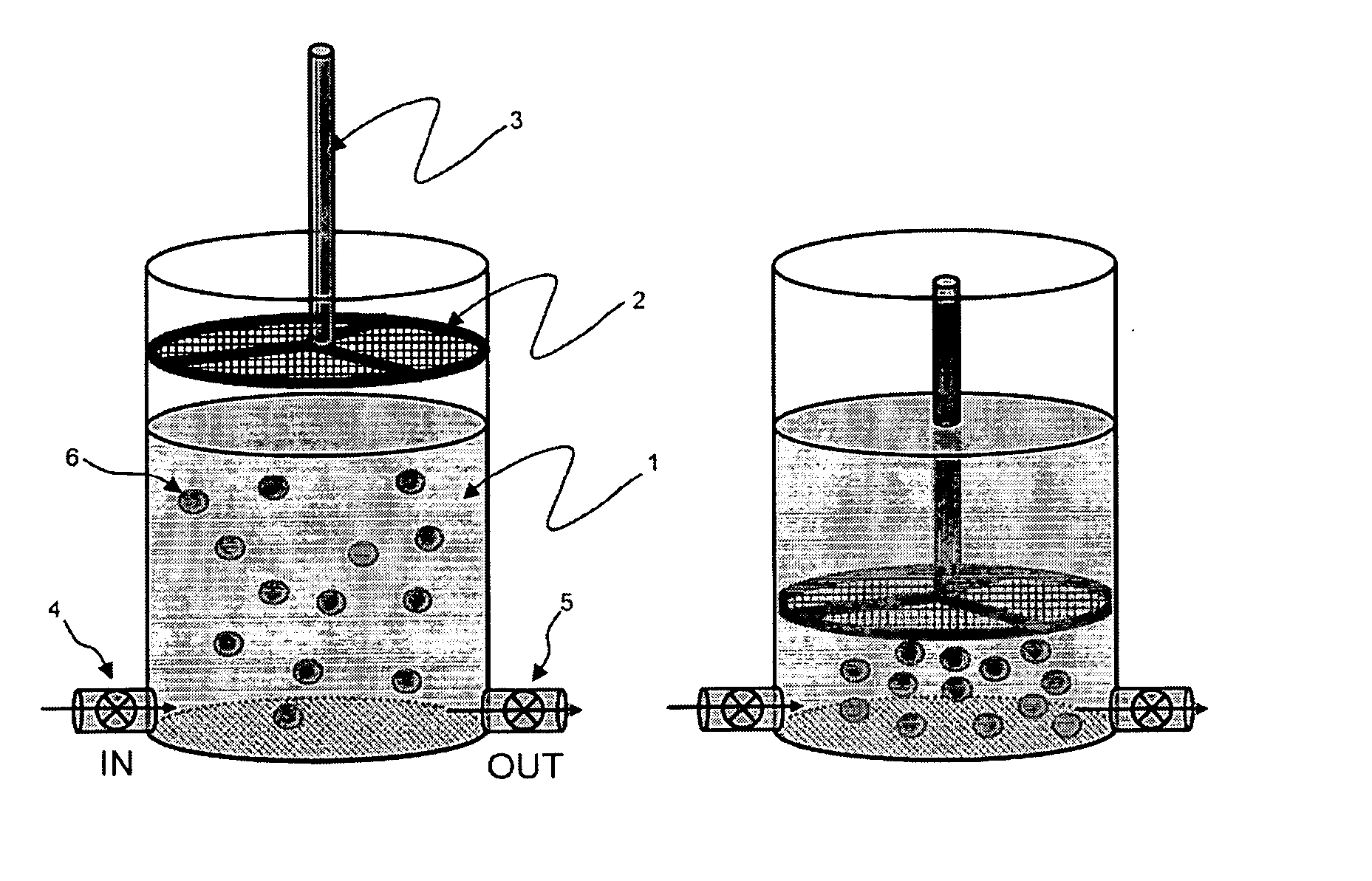
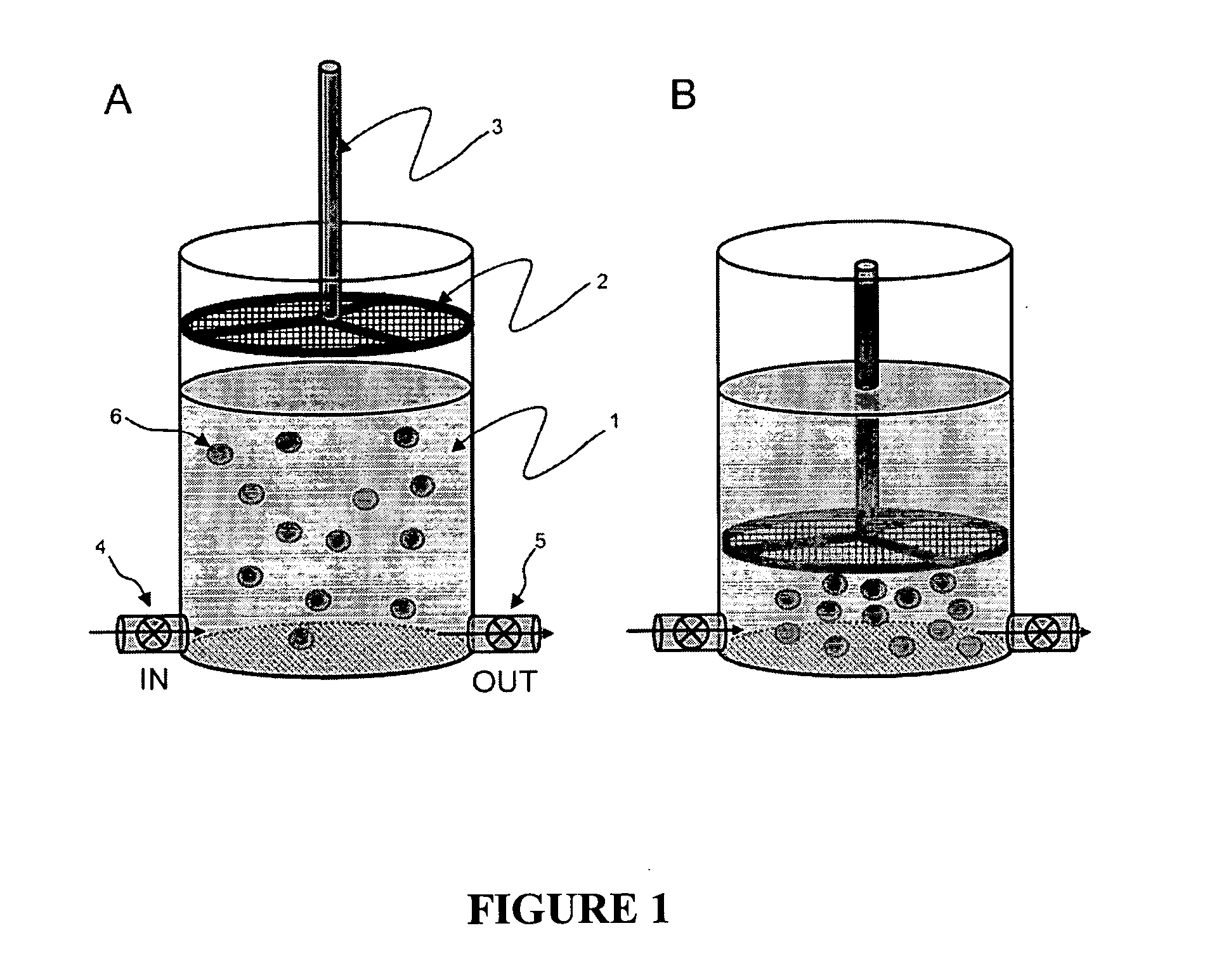
Patents
Literature
1095 results about "Circulating cancer cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
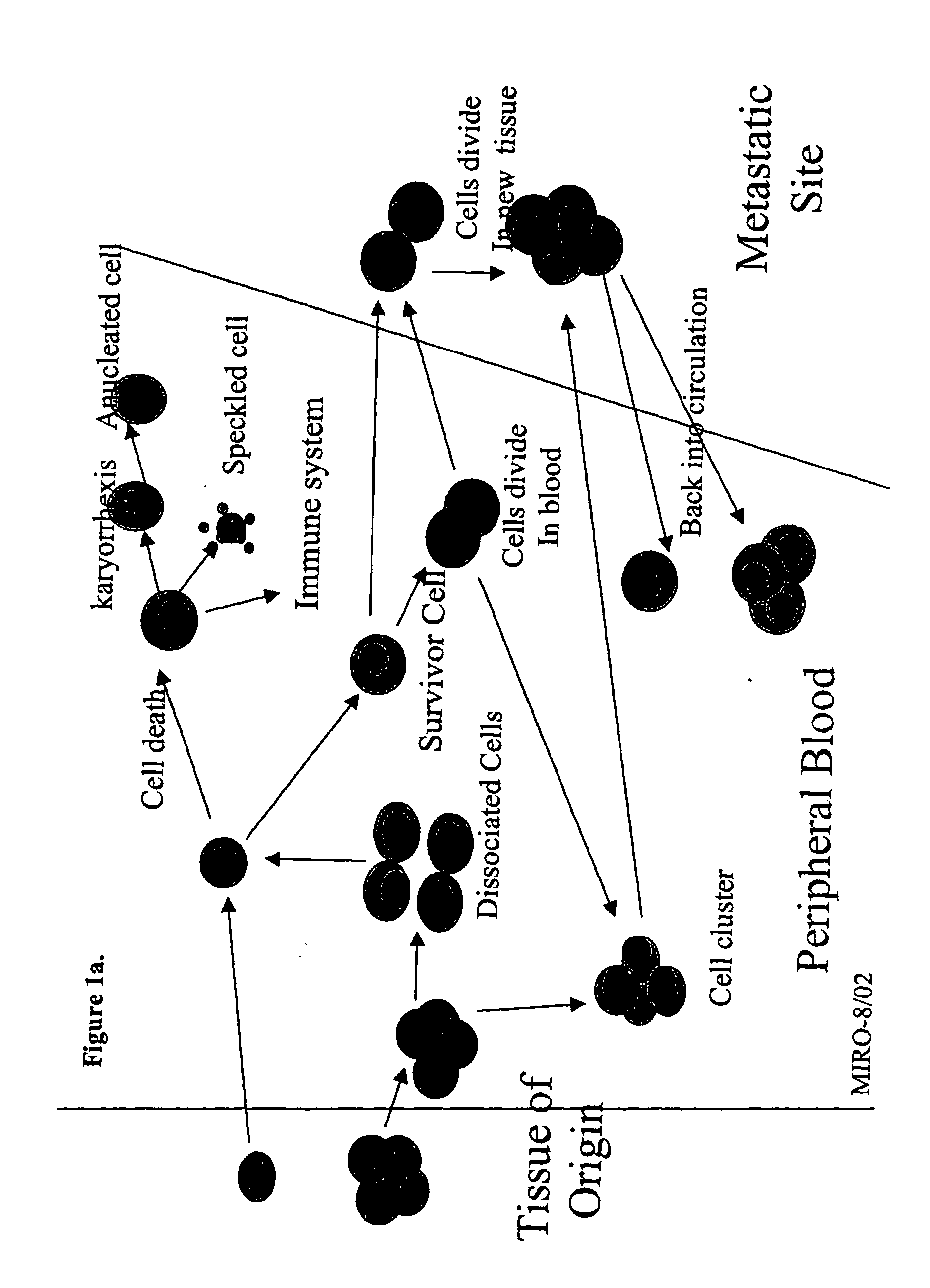
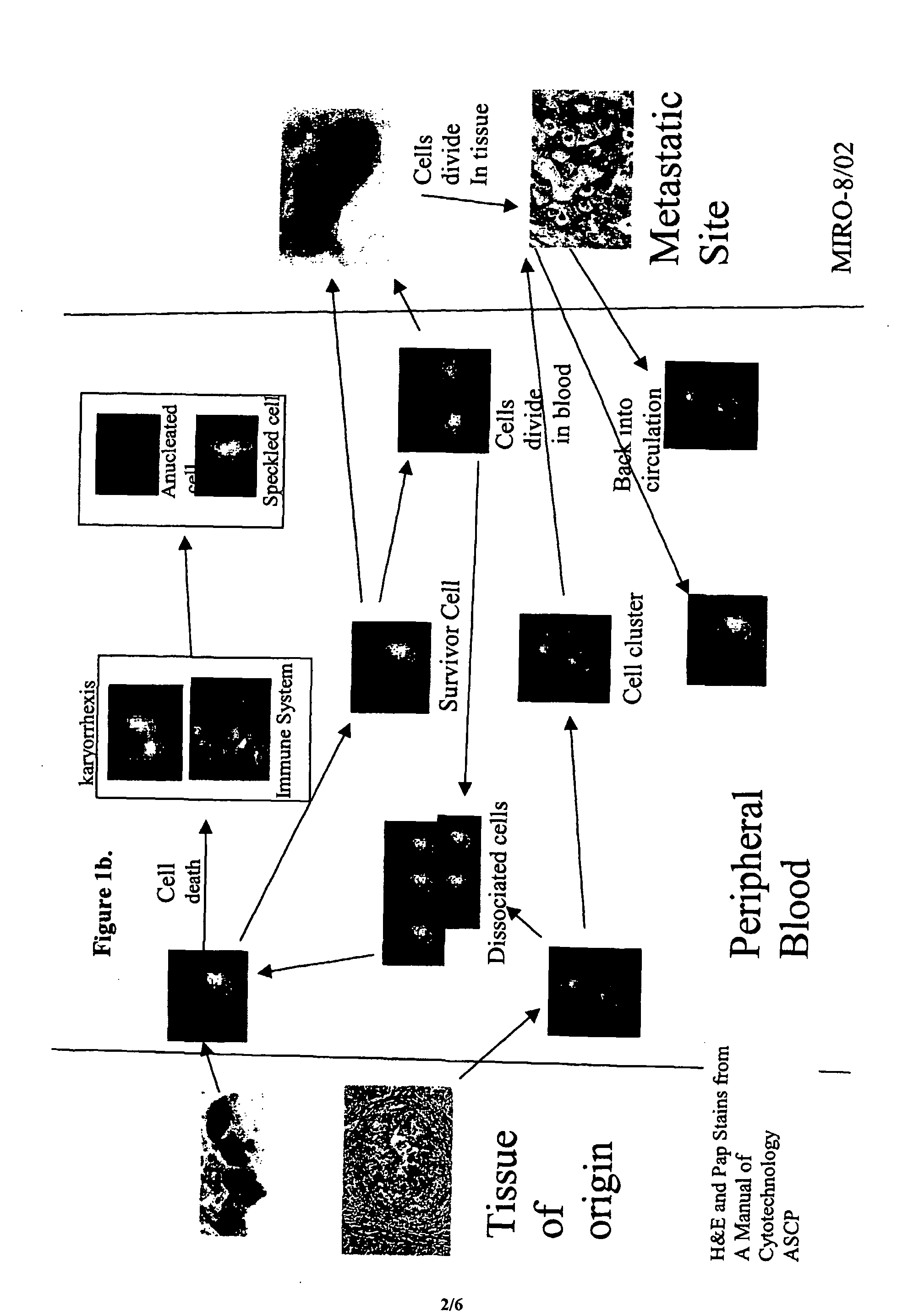
Circulating tumor cells (CTCs) are cells that have shed into the vasculature or lymphatics from a primary tumor and are carried around the body in the blood circulation.
Methods and reagents for the rapid and efficient isolation of circulating cancer cells
InactiveUS7332288B2Efficient ConcentrationEasy to detectNanomagnetismMicrobiological testing/measurementCirculating cancer cellCell sensitivity
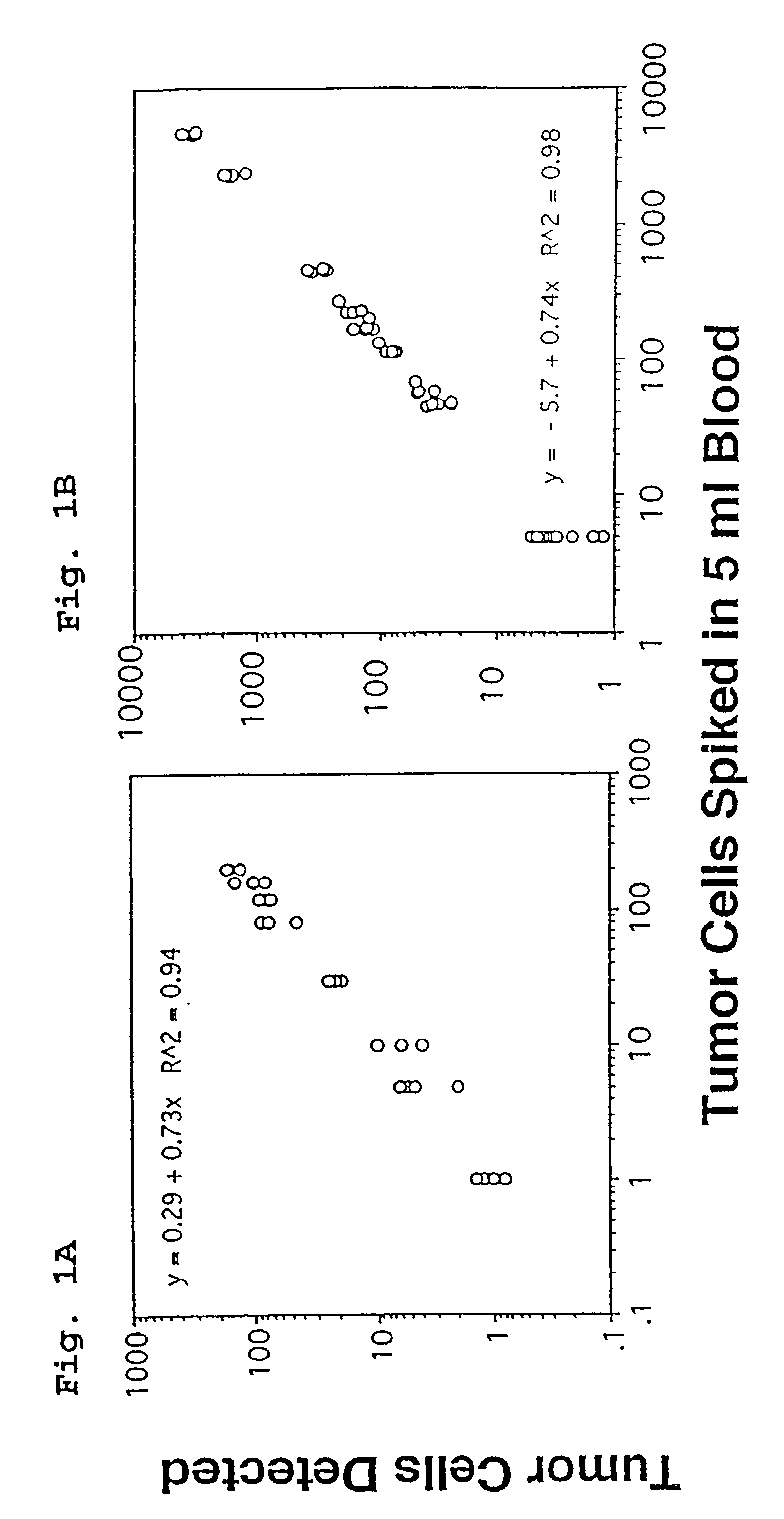
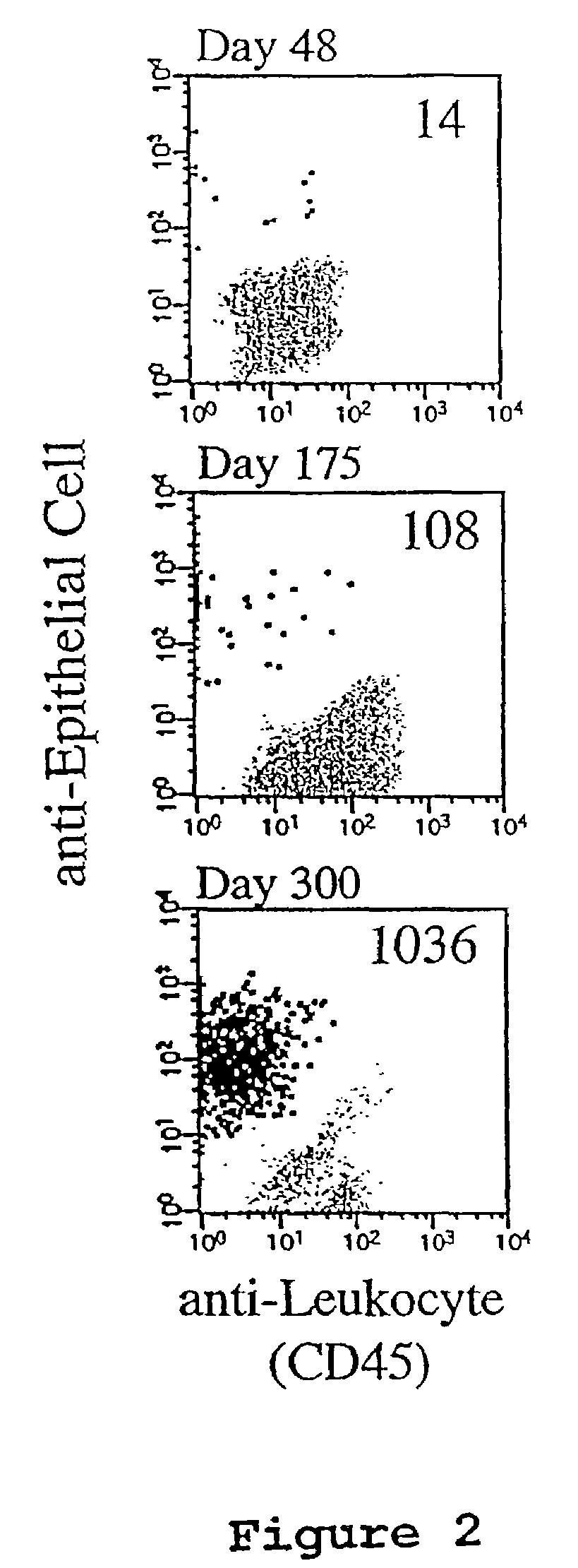
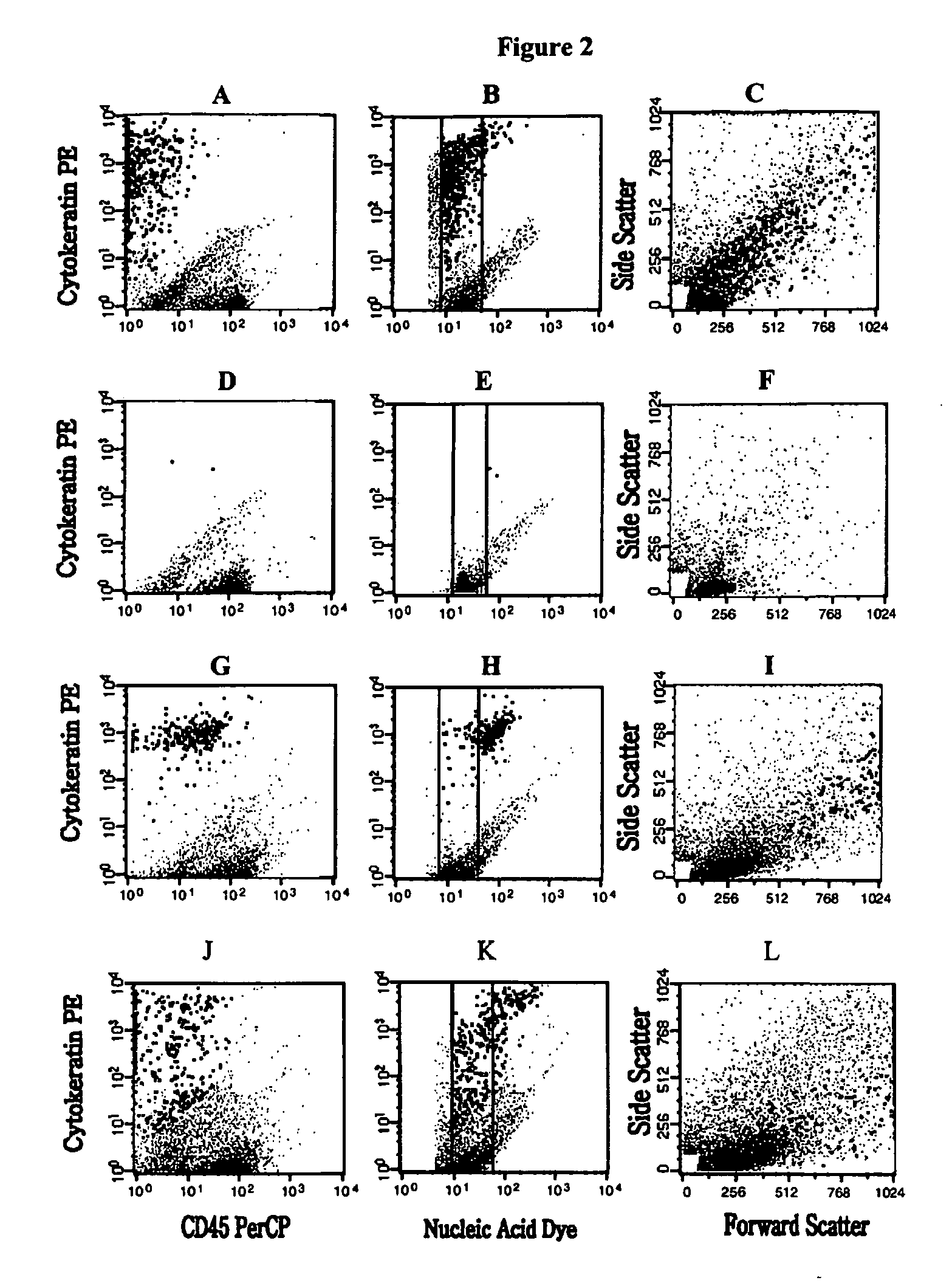
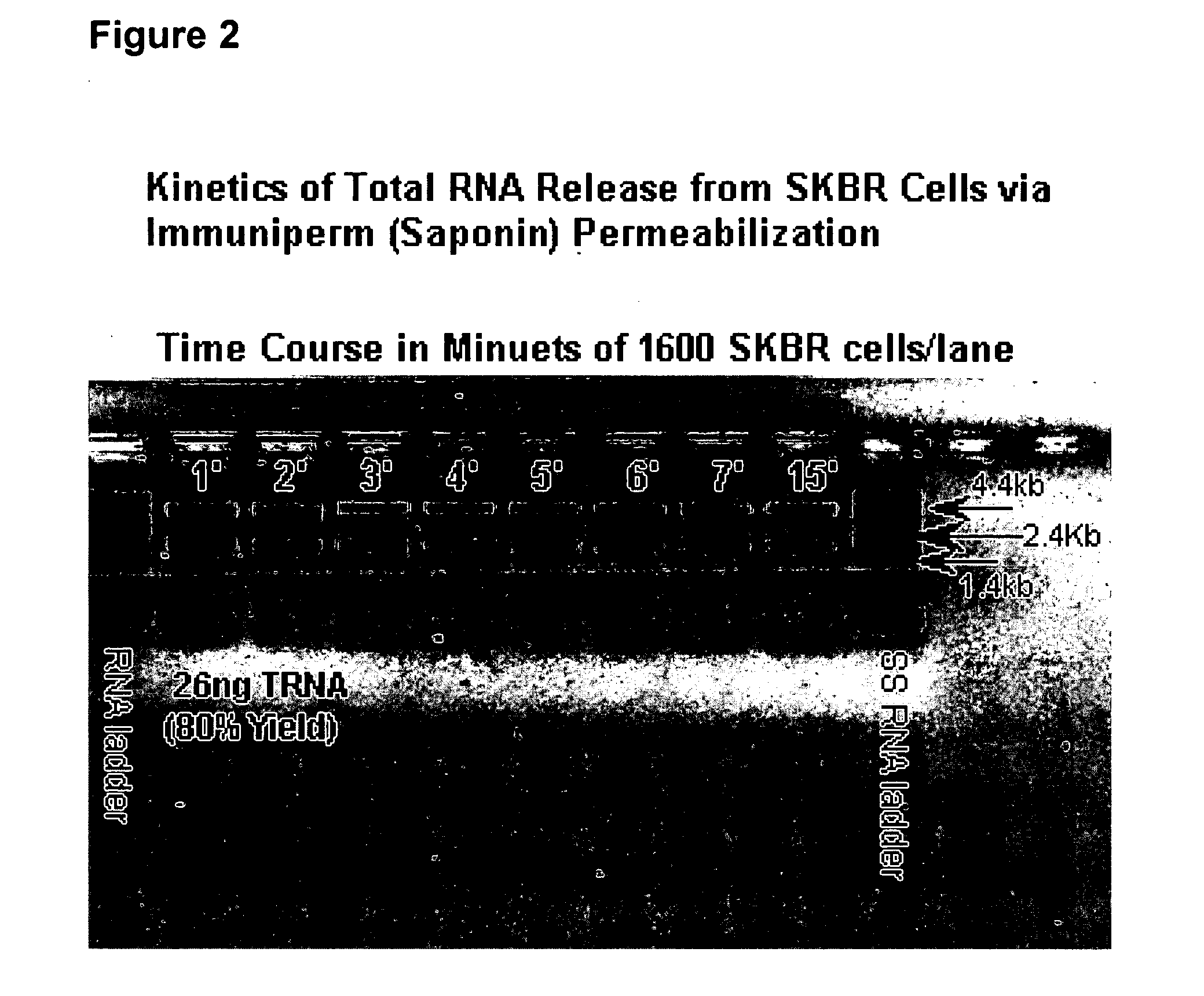
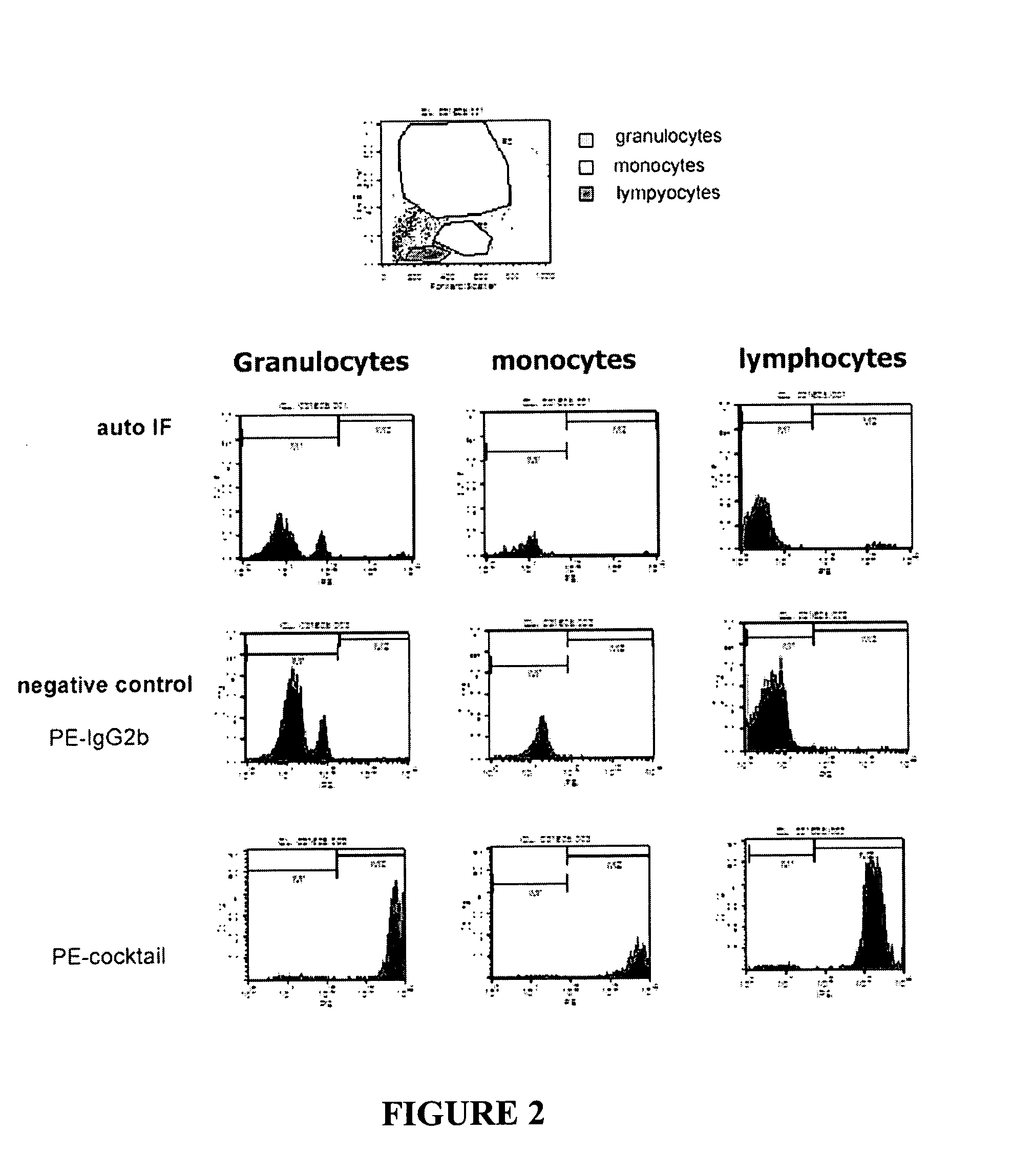
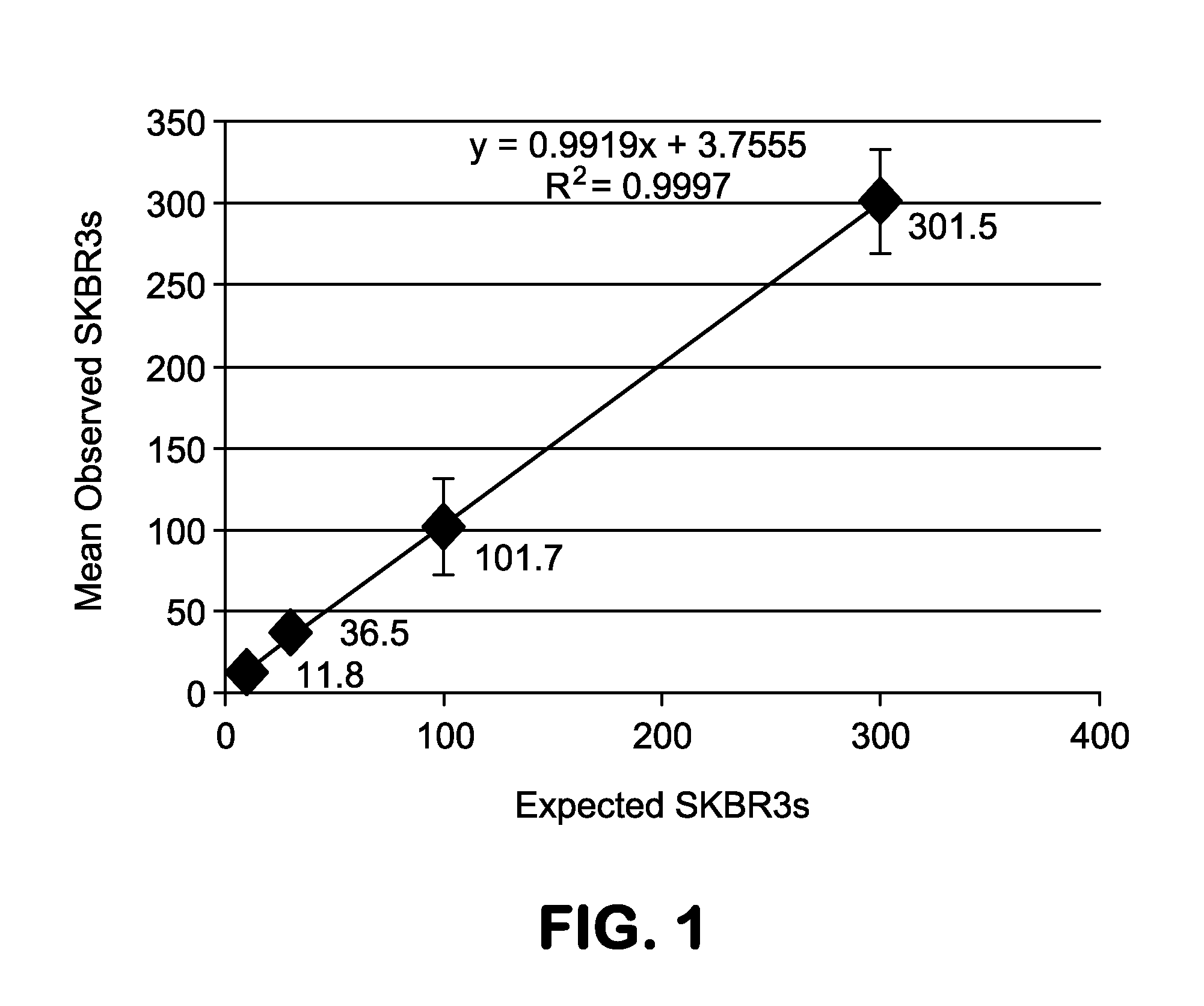
A highly sensitive assay is disclosed which combines immunomagnetic enrichment with multiparameter flow cytometric and immunocytochemical analysis to detect, enumerate and characterize carcinoma cells in the blood. The assay can detect one epithelial cell or less in 1 ml of blood and has a greater sensitivity than conventional PCR or immunohistochemistry by 1-2 orders of magnitude. In addition, the assay facilitates the biological characterization and staging of carcinoma cells.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Analysis of circulating tumor cells, fragments, and debris
InactiveUS20050181463A1Avoid further damageInhibit further damageBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceApoptosis
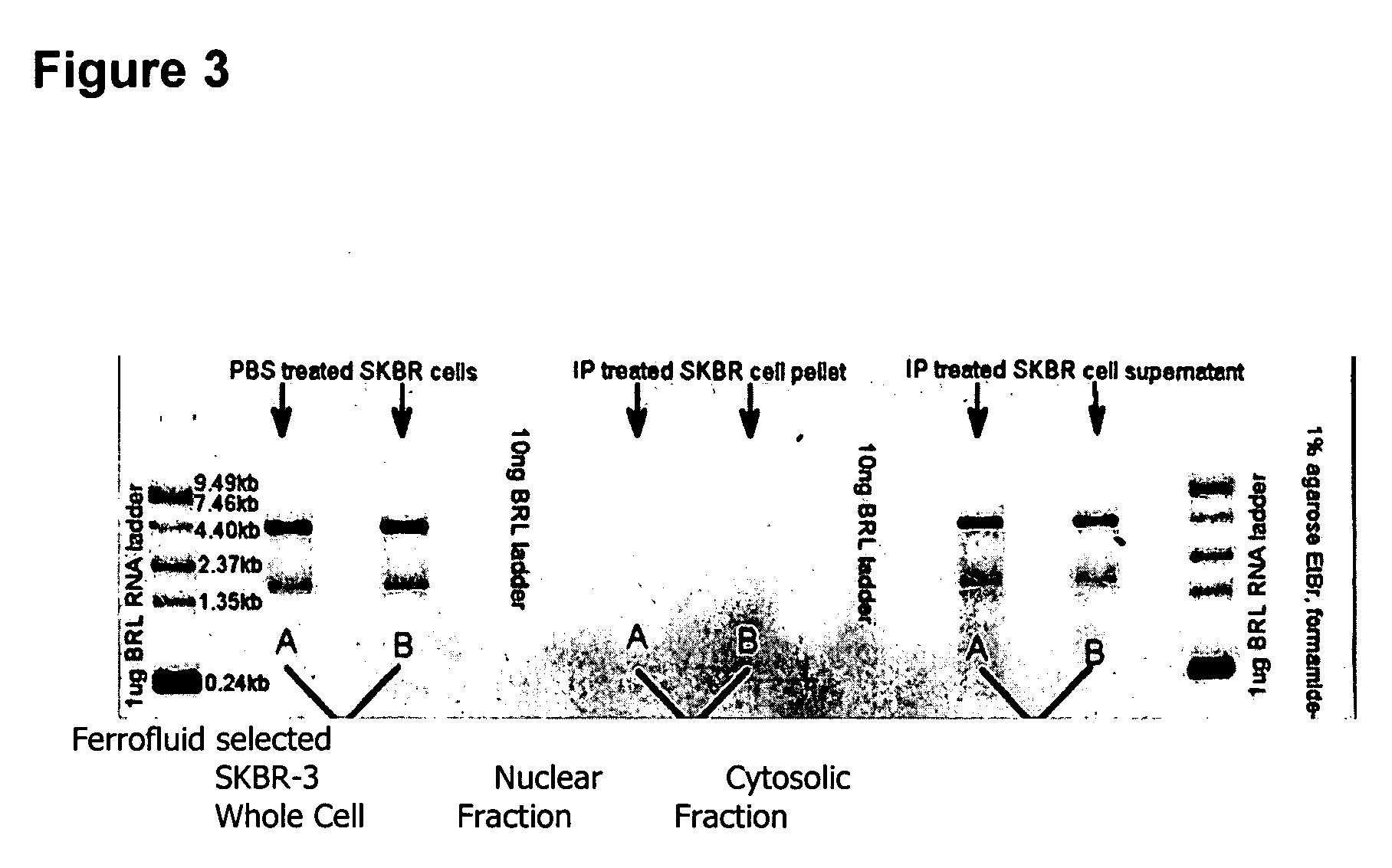
The methods and reagents described in this invention are used to analyze circulating tumor cells, clusters, fragments, and debris. Analysis is performed with a number of platforms, including flow cytometry and the CellSpotter® fluorescent microscopy imaging system. Analyzing damaged cells has shown to be important. However, there are two sources of damage: in vivo and in vitro. Damage in vivo occurs by apoptosis, necrosis, or immune response. Damage in vitro occurs during sample acquisition, handling, transport, processing, or analysis. It is therefore desirable to confine, reduce, eliminate, or at least qualify in vitro damage to prevent it from interfering in analysis. Described herein are methods to diagnose, monitor, and screen disease based on circulating rare cells, including malignancy as determined by CTC, clusters, fragments, and debris. Also provided are kits for assaying biological specimens using these methods.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Microfluidic devices and methods for cell sorting, cell culture and cells based diagnostics and therapeutics
ActiveUS20140248621A1Uniform pressure distributionReduce widthHeating or cooling apparatusMicrobiological testing/measurement3D cell cultureTumor cells
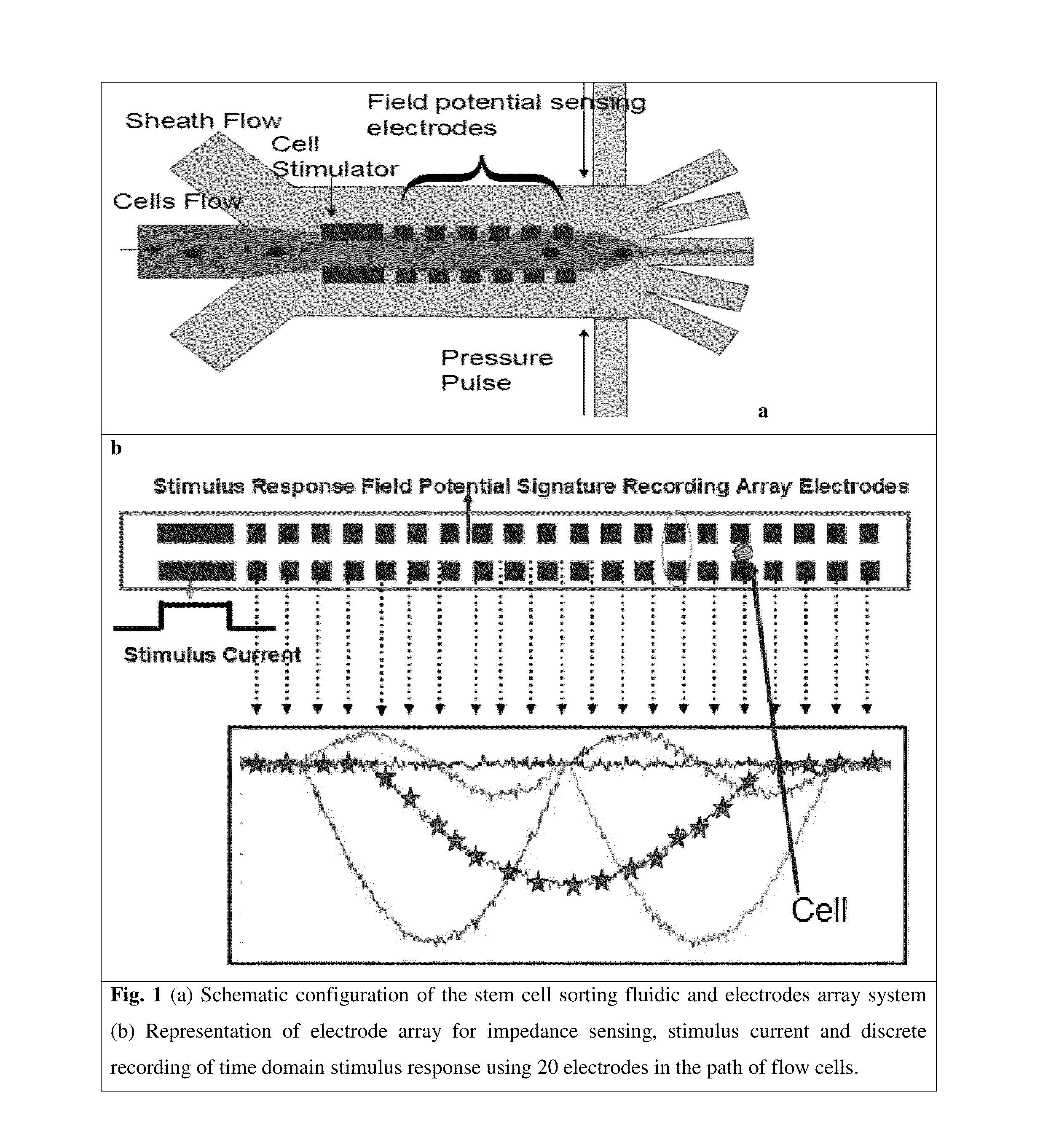
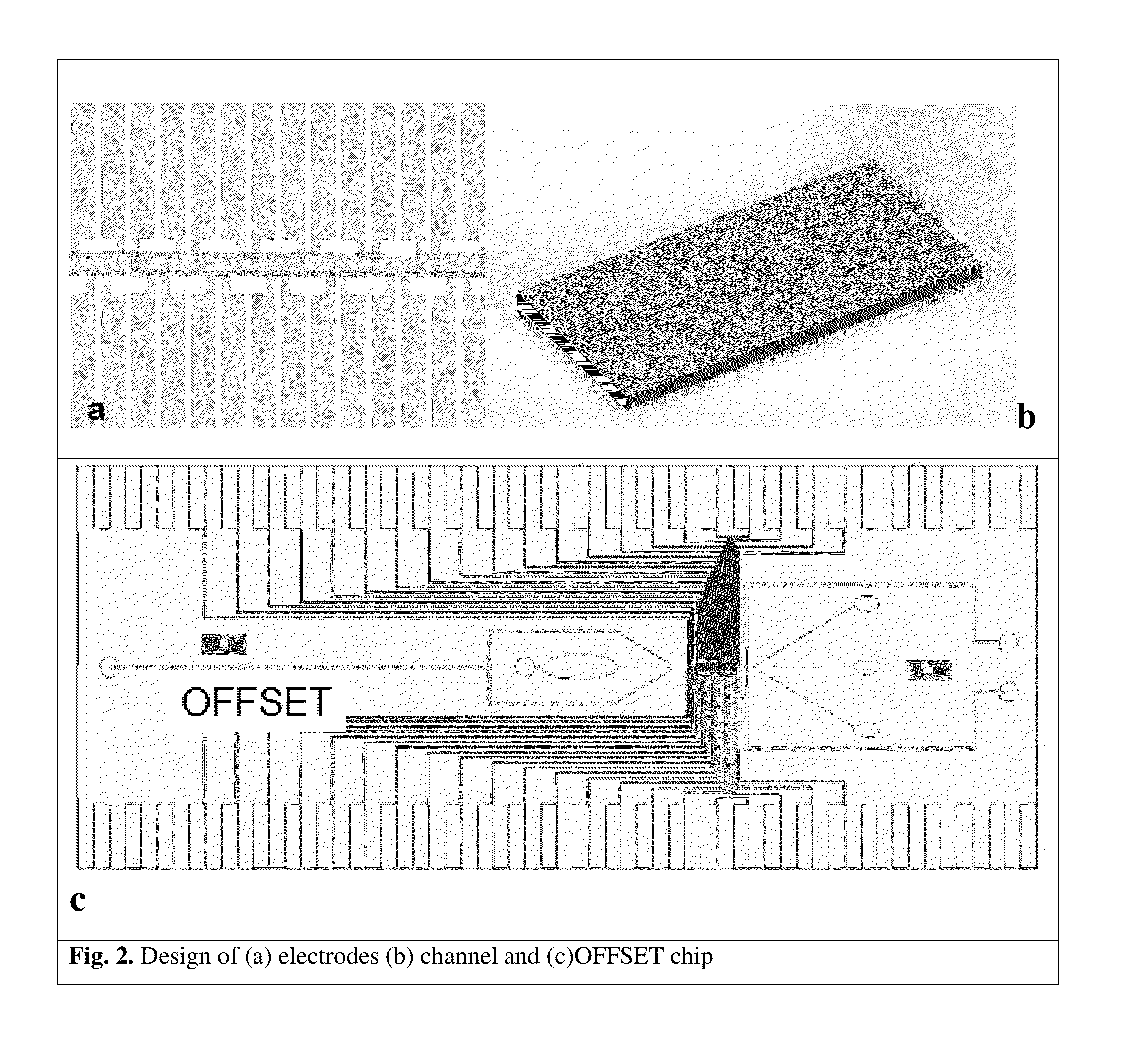
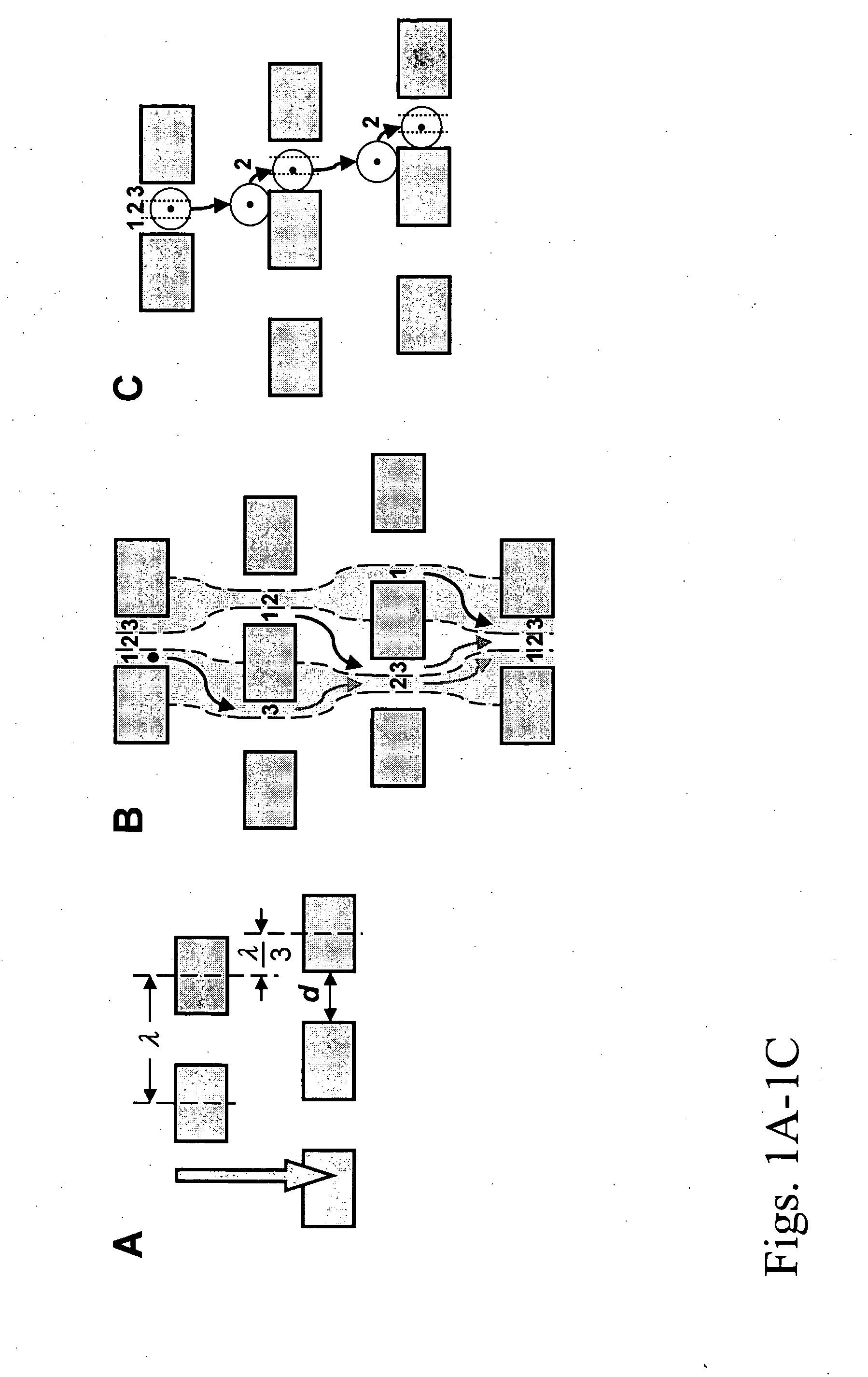
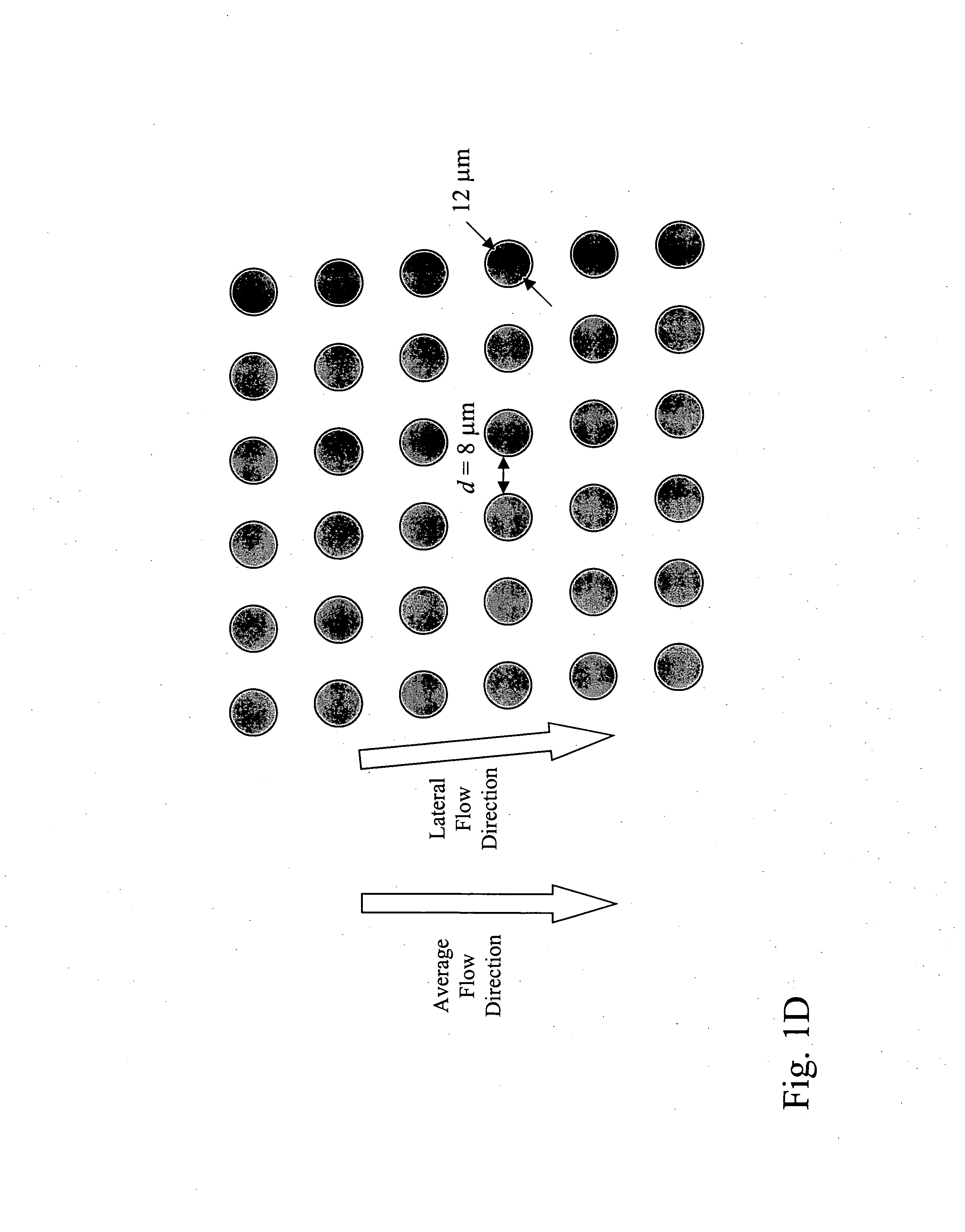
Microfluidic devices and methods that use cells such as cancer cells, stem cells, blood cells for preprocessing, sorting for various biodiagnostics or therapeutical applications are described. Microfluidics electrical sensing such as measurement of field potential or current and phenomena such as immiscible fluidics, inertial fluidics are used as the basis for cell and molecular processing (e.g., characterizing, sorting, isolation, processing, amplification) of different particles, chemical compositions or biospecies (e.g., different cells, cells containing different substances, different particles, different biochemical compositions, proteins, enzymes etc.). Specifically this invention discloses a few sorting schemes for stem cells, whole blood and circulating tumor cells and also extracting serum from whole blood. Further medical diagnostics technology utilizing high throughput single cell PCR is described using immiscible fluidics couple with single or multi cells trapping technology.
Owner:BIOPICO SYST
Class characterization of circulating cancer cells isolated from body fluids and methods of use
InactiveUS6960449B2Positive responsePreparing sample for investigationVertebrate cellsCirculating cancer cellBody fluid sample
The present invention relates to the identification and characterization of classes and subclasses of circulating cancer cells, including microtumors from body fluid samples using molecular, cytological, and morphological analyses, and methods for staging patients and measuring the efficacy of medical treatments.
Owner:GPB SCI
Methods and compositions for cell stabilization
InactiveUS20110027771A1Bioreactor/fermenter combinationsBiological substance pretreatmentsFoetal cellCirculating cancer cell
Fragile cells have value for use in diagnosing many types of conditions. There is a need for compositions that stabilize fragile cells. The stabilization compositions of the provided invention allow for the stabilization, enrichment, and analysis of fragile cells, including fetal cells, circulating tumor cells, and stem cells.
Owner:VERINATA HEALTH INC
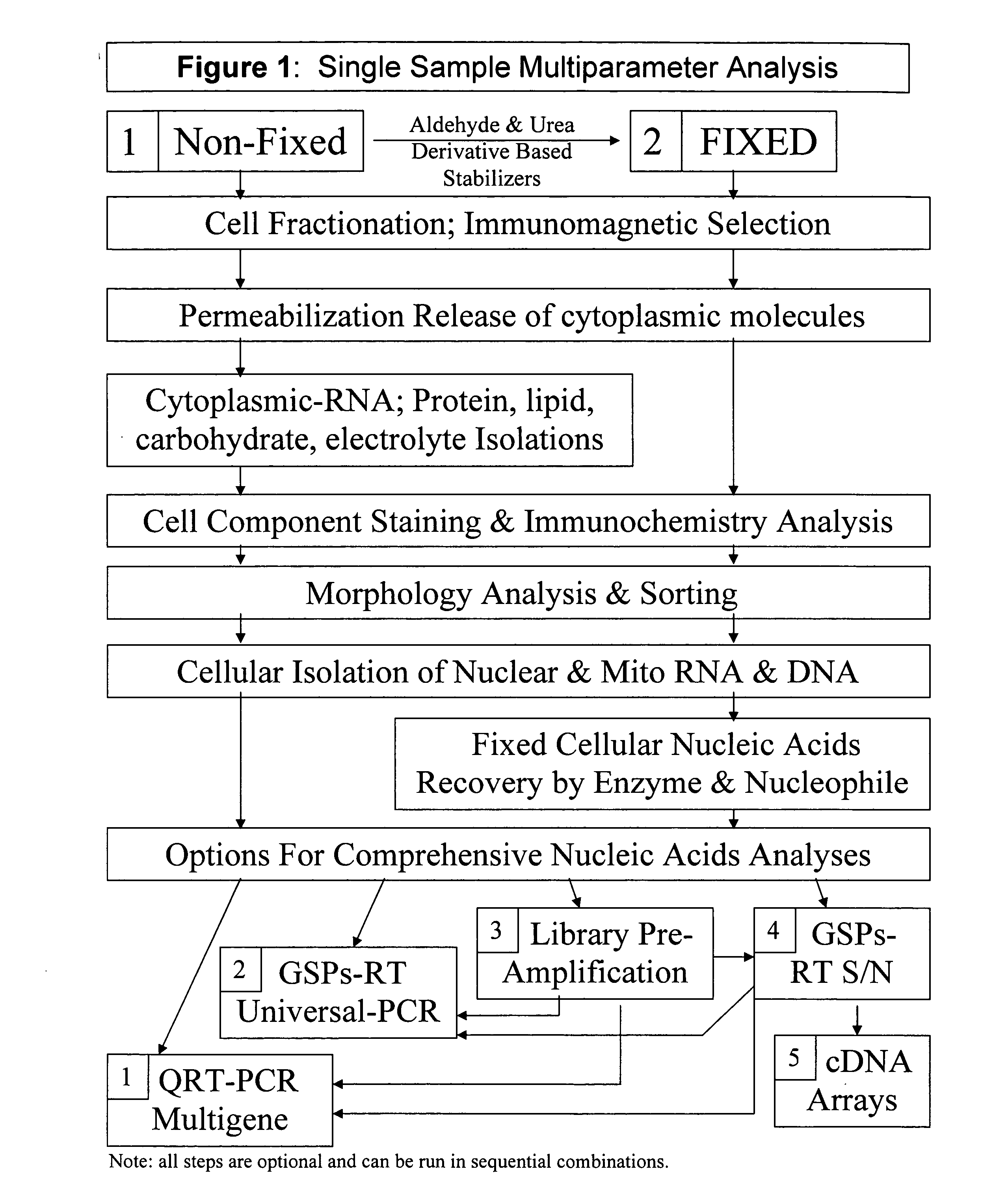
Multiparameter analysis of comprehensive nucleic acids and morphological features on the same sample
InactiveUS20060008807A1Microbiological testing/measurementMicroorganism lysisProtein profilingMass spectrometry
A highly sensitive assay is disclosed which utilizes a method for gene specific primed amplification of mRNA libraries from rare cells and rare transcripts found in blood. The assay allows detection of rare mRNA (10 copies / cell) found in 1 to 10 cells isolated through immunomagnetic enrichment. The assay is an improvement over multiplex PCR and allows efficient detection of rare coding sequences for circulating carcinoma cells in the blood. The methods are useful in profiling of cells isolated from tissues or body fluids and serves as an adjunct to clinical diagnosis of diverse carcinomas including early stage detection and classification of circulating tumor cells. Monitoring of nucleic acid and protein profiles of cells either in conventional or microarray formats, facilitates management of therapeutic intervention including staging, monitoring response to therapy, confirmation of remission and detection of regression.
Owner:VERIDEX LCC
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026415A1Increase array densitySmall particle sizeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:THE GENERAL HOSPITAL CORP +2
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026413A1Increase array densitySmall particle sizeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:THE GENERAL HOSPITAL CORP +2
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
ActiveUS20070026469A1Increase array densitySmall particle sizeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:GPB SCI
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070099207A1Increase in sizeIncrease array densityMicrobiological testing/measurementNanoinformaticsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:THE GENERAL HOSPITAL CORP +2
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026419A1Small particle sizeLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:CELLECTIVE DX CORP
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026416A1Small particle sizeLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:CELLECTIVE DX CORP
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026414A1Small particle sizeLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:THE GENERAL HOSPITAL CORP +2
Stabilization of cells and biological specimens for analysis
InactiveUS20050181353A1Improve stabilityMaintain qualityOrganic active ingredientsBiocideAbnormal tissue growthIn vivo
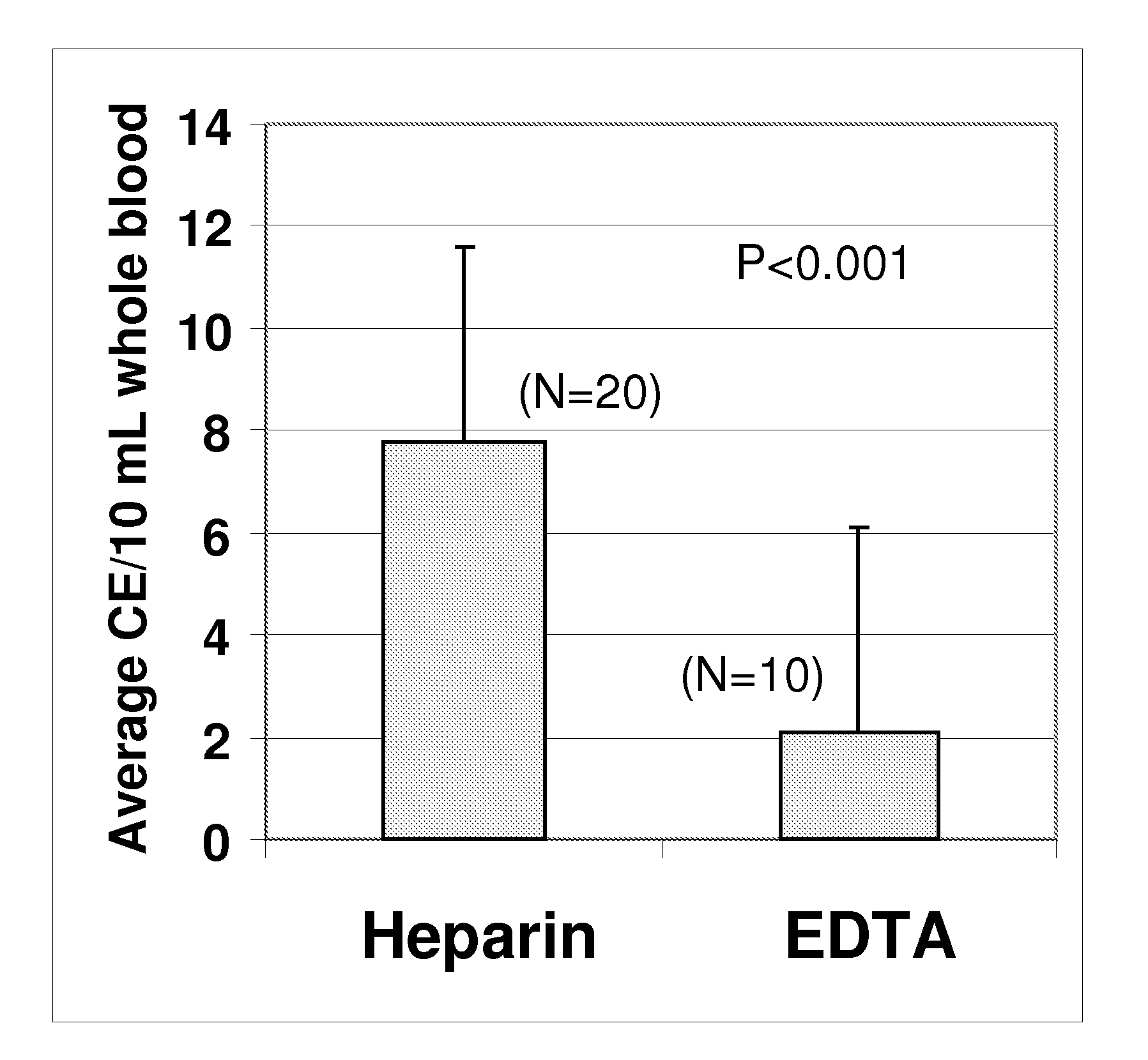
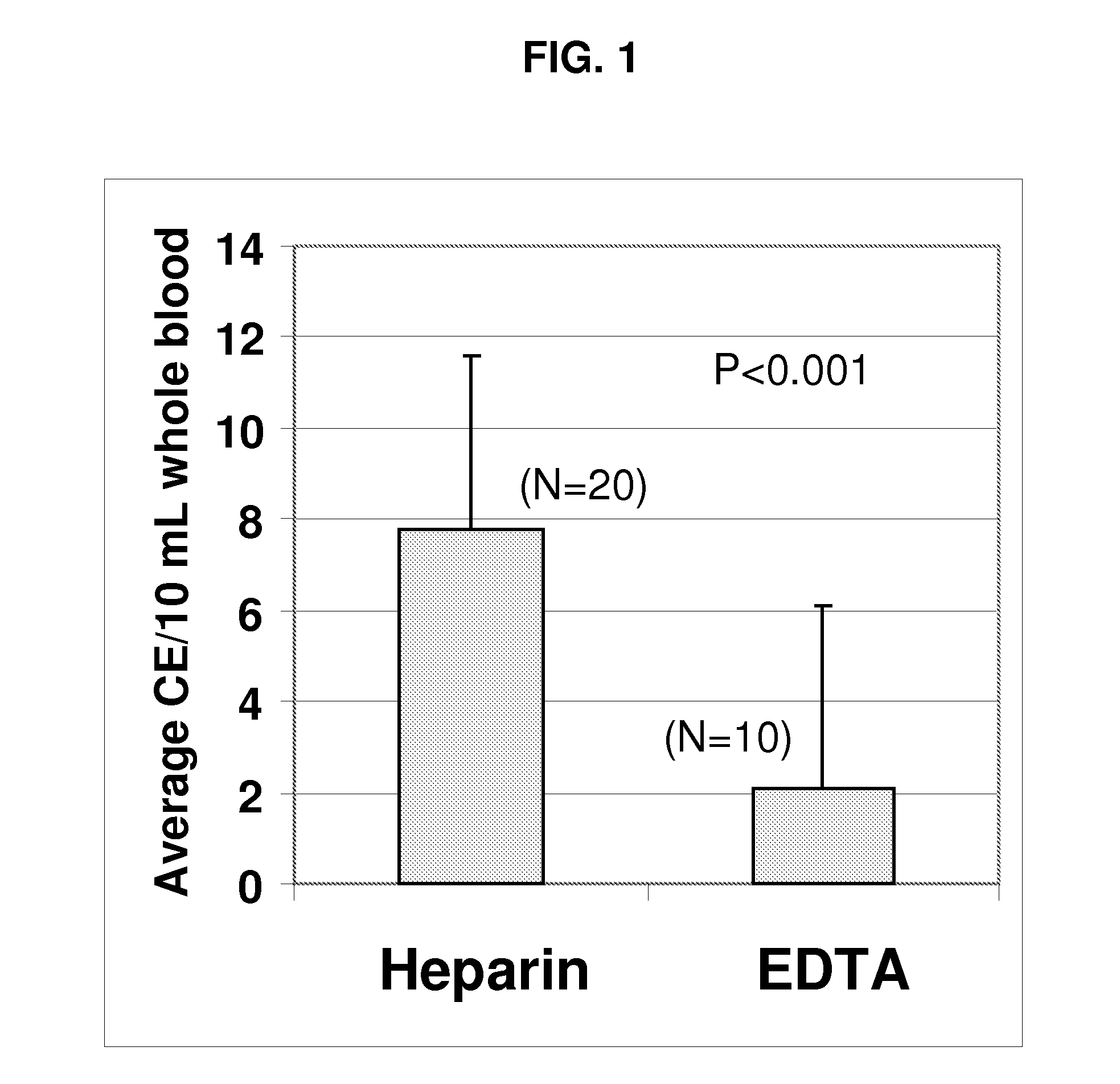
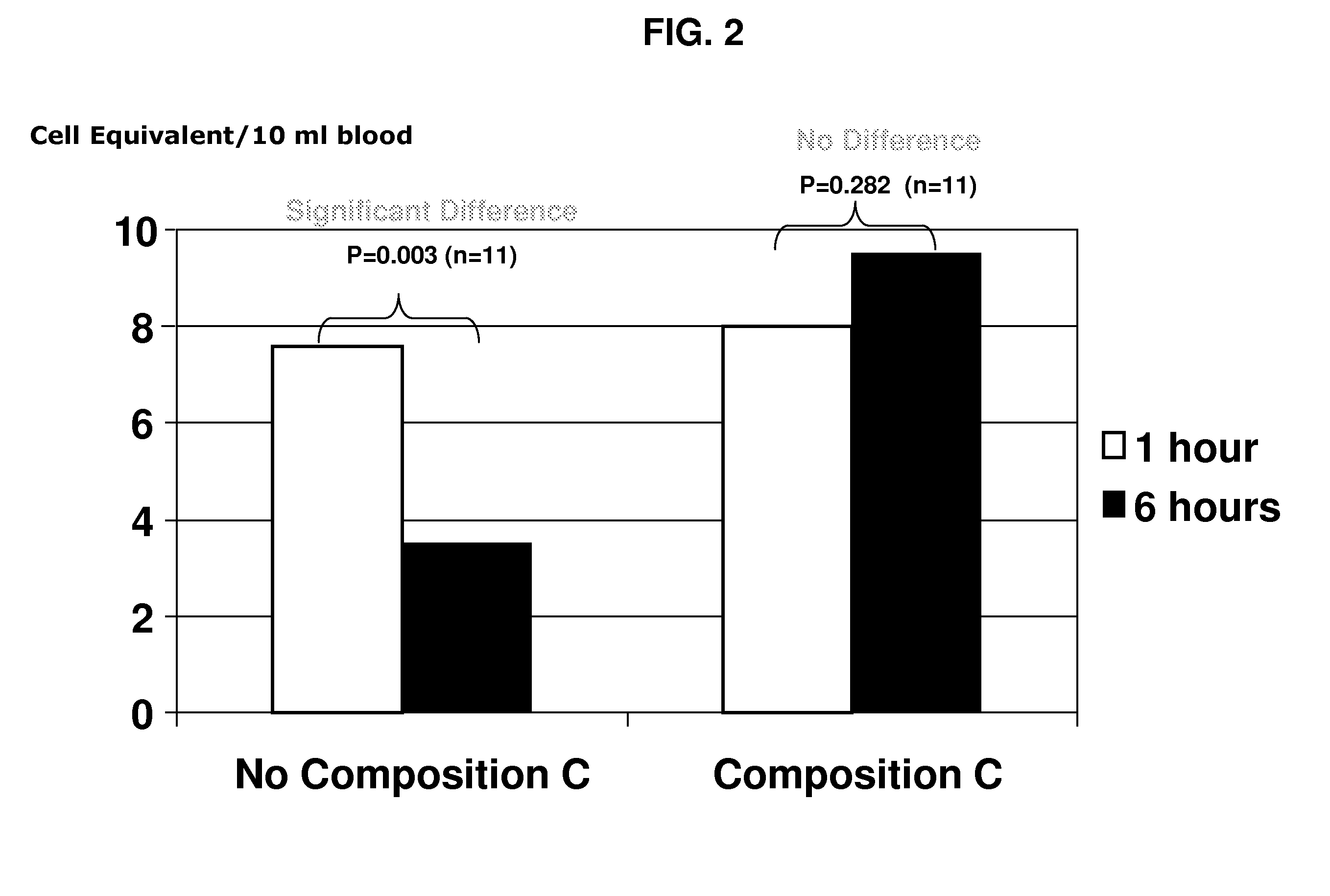
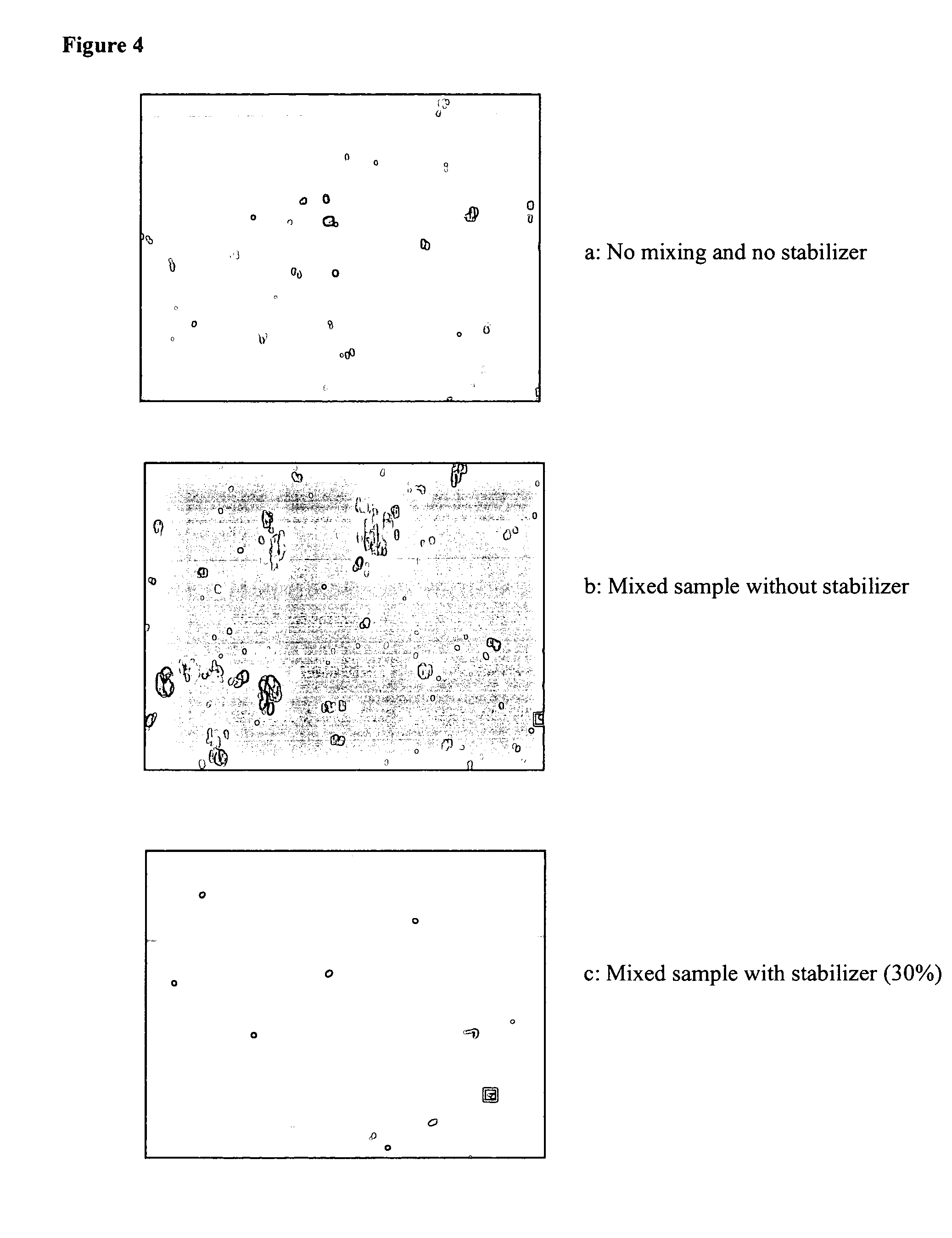
Compositions and methods for stabilizing rare cells in blood specimens, preserving the quality of blood specimens, and also serving as cell fixatives are disclosed which minimize losses of target cells (for example, circulating tumor cells) and formation of debris and aggregates from target cells, non-target cells and plasma components, thereby allowing more accurate analysis and classification of circulating tumor cells (CTC) and, ultimately, of tumor burdens in cancer patients. Stabilization of specimens is particularly desirable in protocols requiring rare cell enrichment from blood specimens drawn from cancer patients. Exposure of such specimens to potentially stressful conditions encountered, for example, in normal processing, mixing, shaking, delays due to transporting the blood, has been observed to not only diminish the number of CTC but also to generate debris and aggregates in the blood specimens that were found to interfere with accurate enumeration of target cells, if present. Stabilizers are necessary to discriminate between in vivo CTC disintegration and in vitro sample degredation.
Owner:VERIDEX LCC
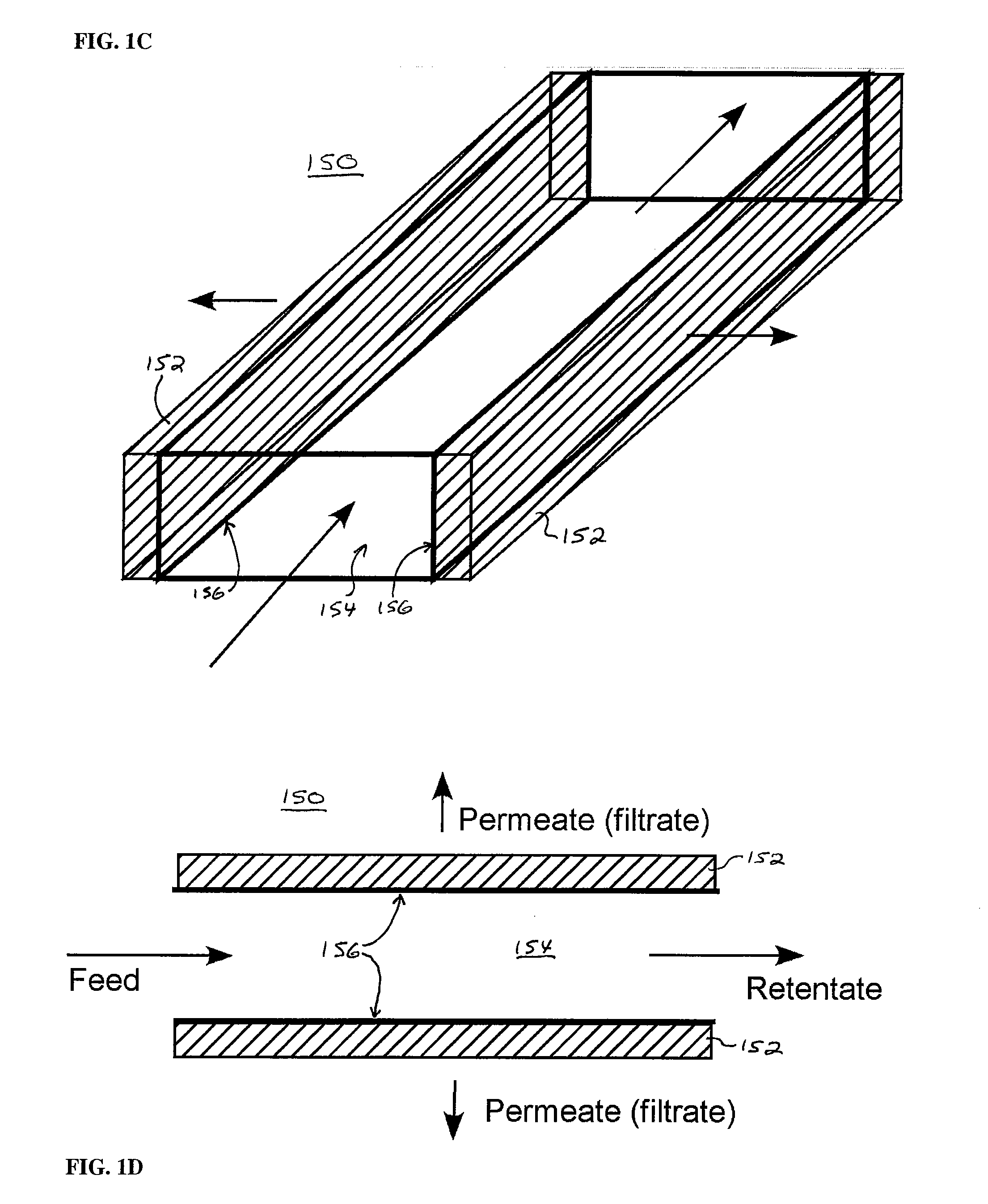
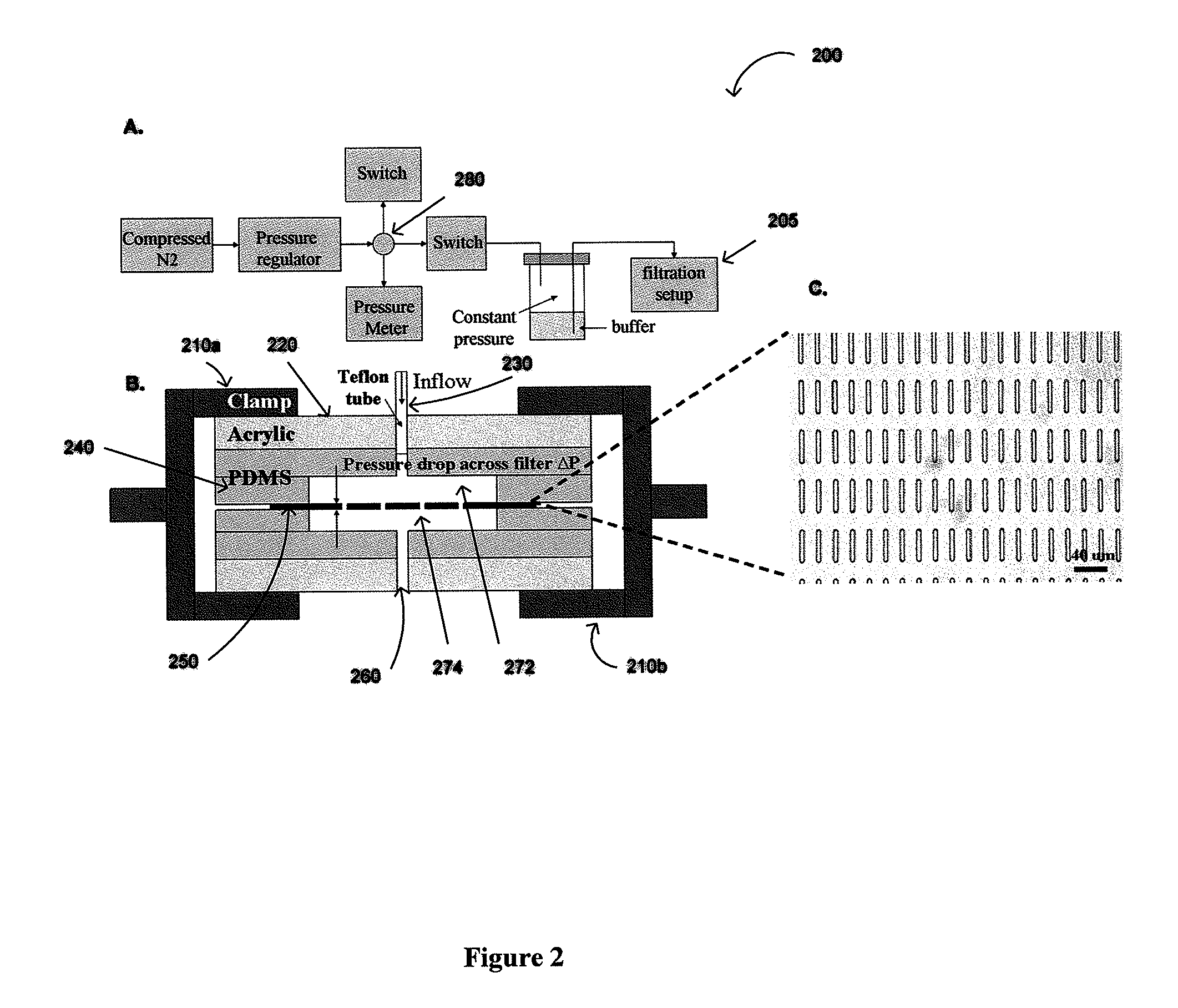
Biochip for High-Throughput Screening of Circulating Tumor Cells
ActiveUS20080318324A1Reduce direct impactShorten speedLiquid separation auxillary apparatusLaboratory glasswaresRe entryHigh-Throughput Screening Methods
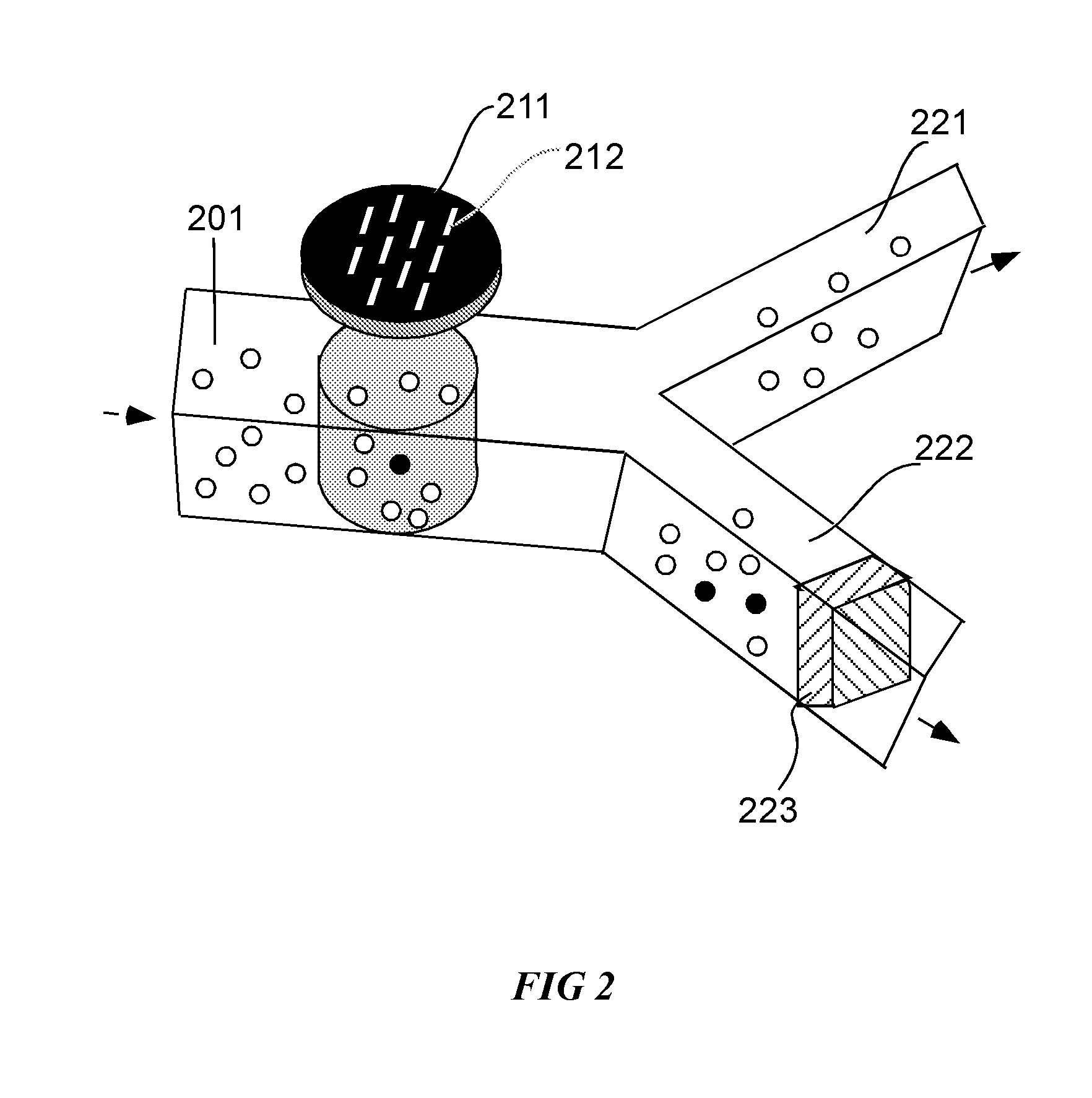
Embodiments in accordance with the present invention relate to the use of effusive filtration to segregate tumor cells from a sample of bodily fluid. In one embodiment, fluid containing a cell is flowed down a channel having a filtration medium present along at least one side wall. The tumor cell is captured when the fluid passes through the filtration medium. Accumulated pressure on the captured tumor cell is reduced by allowing the fluid that has passed through the filtration medium to re-enter the channel. In a particular embodiment, the filtration medium may comprise side wall apertures having a width smaller than that of the cell, with downstream apertures allowing re-entry of the fluid into the channel.
Owner:UNIV OF WASHINGTON
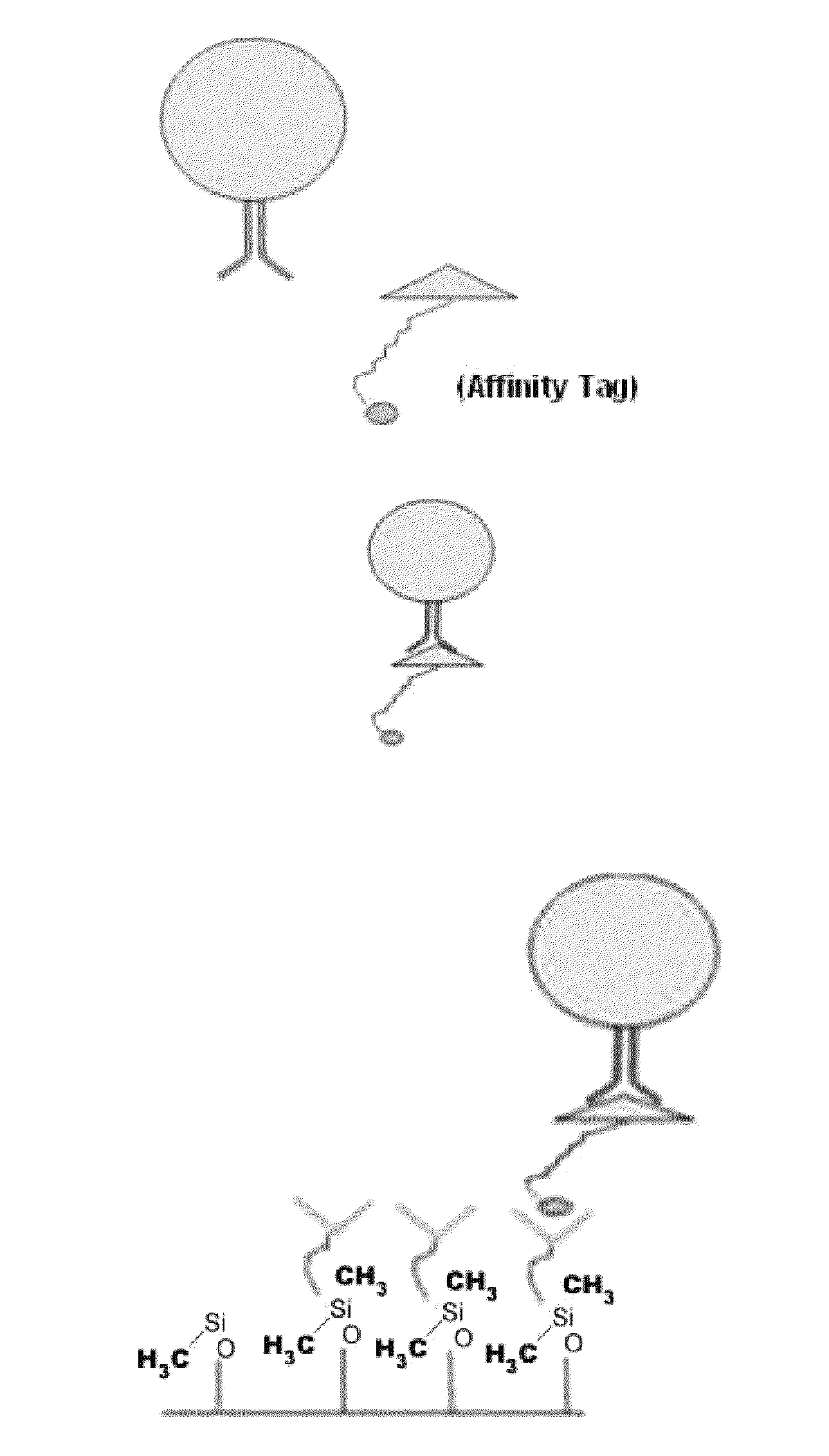
Tagged ligands for enrichment of rare analytes from a mixed sample
InactiveUS8008032B2Bioreactor/fermenter combinationsBiological substance pretreatmentsRare cellAnalyte
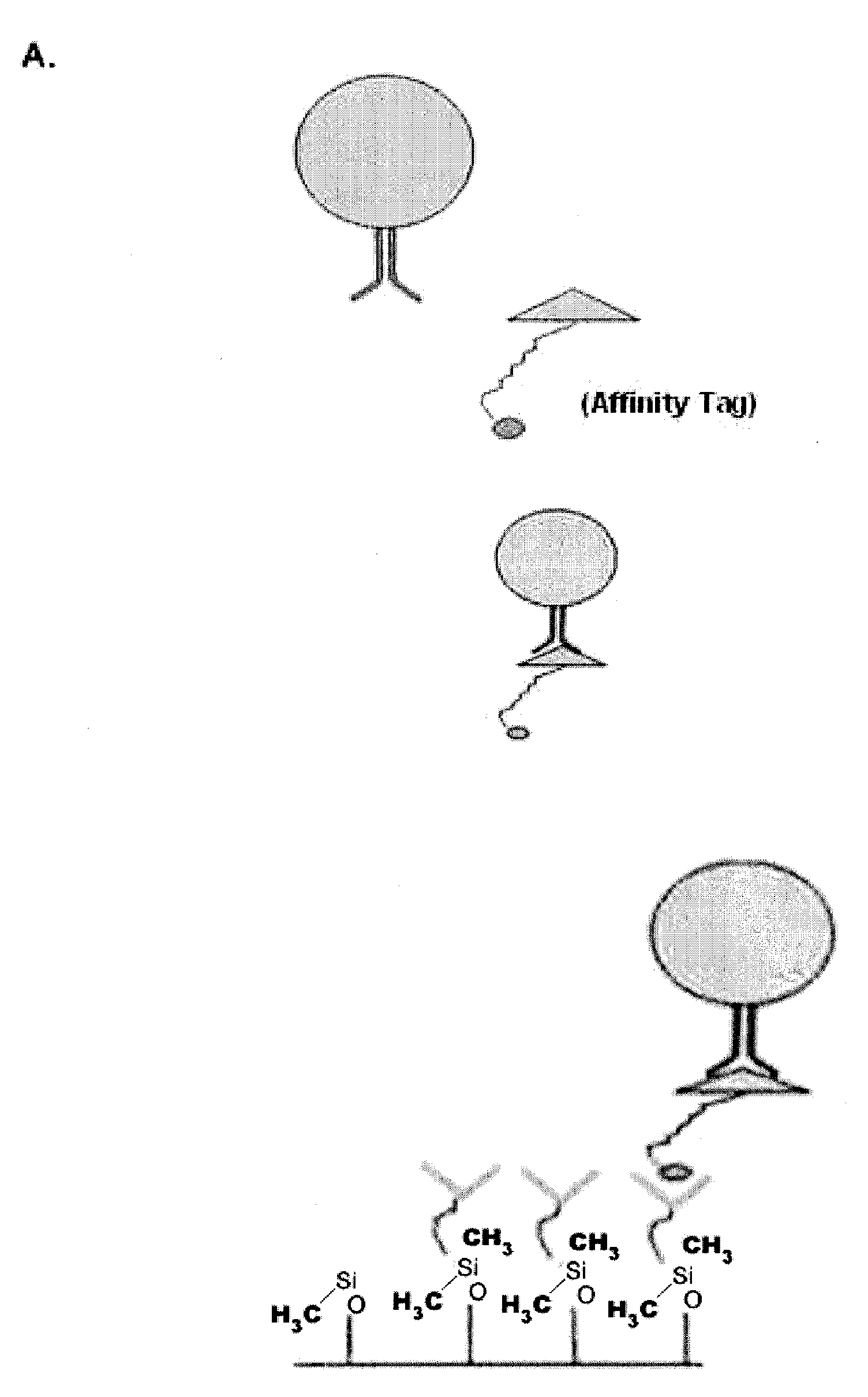
Method of enriching specific cells from cellular samples are disclosed, comprising contacting in solution a cellular sample with affinity-tagged ligands (ATLs) each comprising a first ligand linked to an affinity tag, wherein the ligand selectively binds a cellular marker of the rare cells and the affinity tag can be selectively captured by a capture moiety, wherein the affinity tags do not comprise a magnetic particle; and flowing the sample through a microfluidic device comprising the capture moiety to selectively retain ATL-bound cells. Methods for enriching circulating tumor cells, and devices for enriching specific cells from cellular samples are also disclosed.
Owner:GPB SCI
Method for cancer detection, diagnosis and prognosis
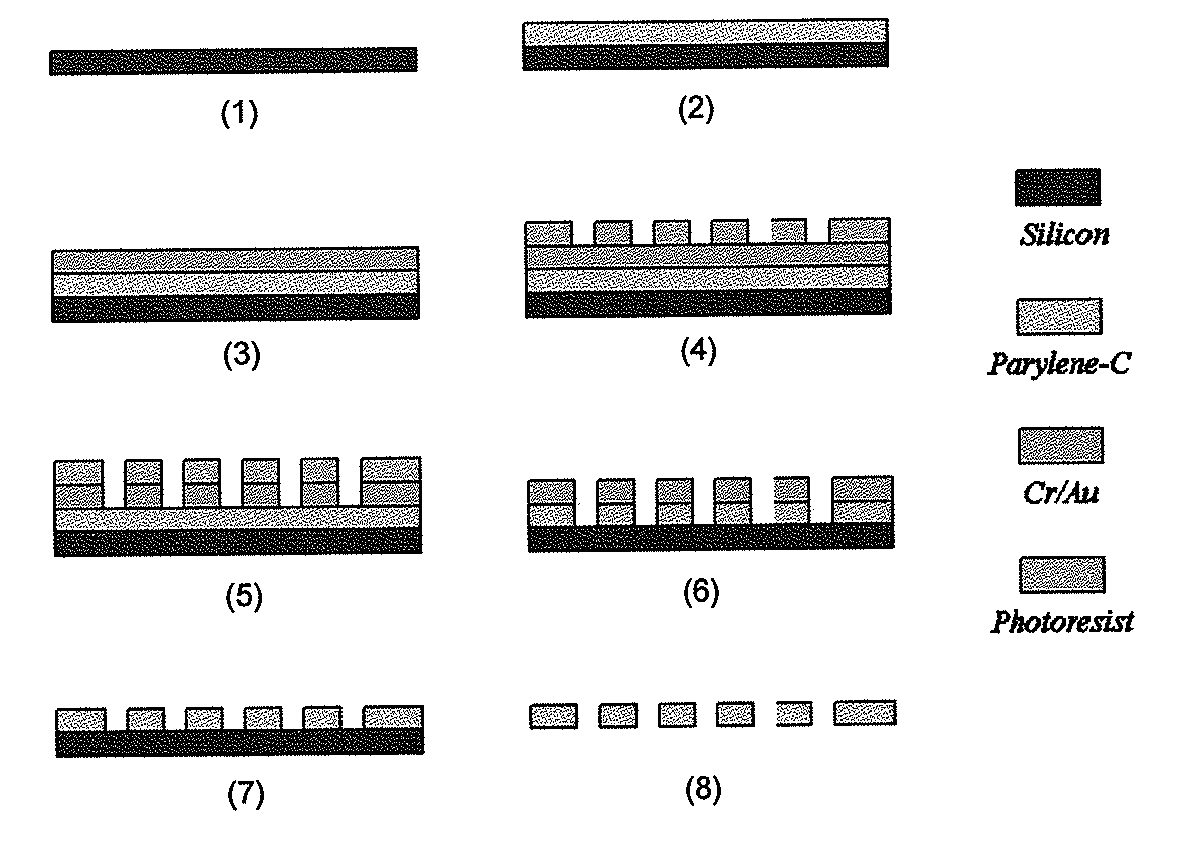
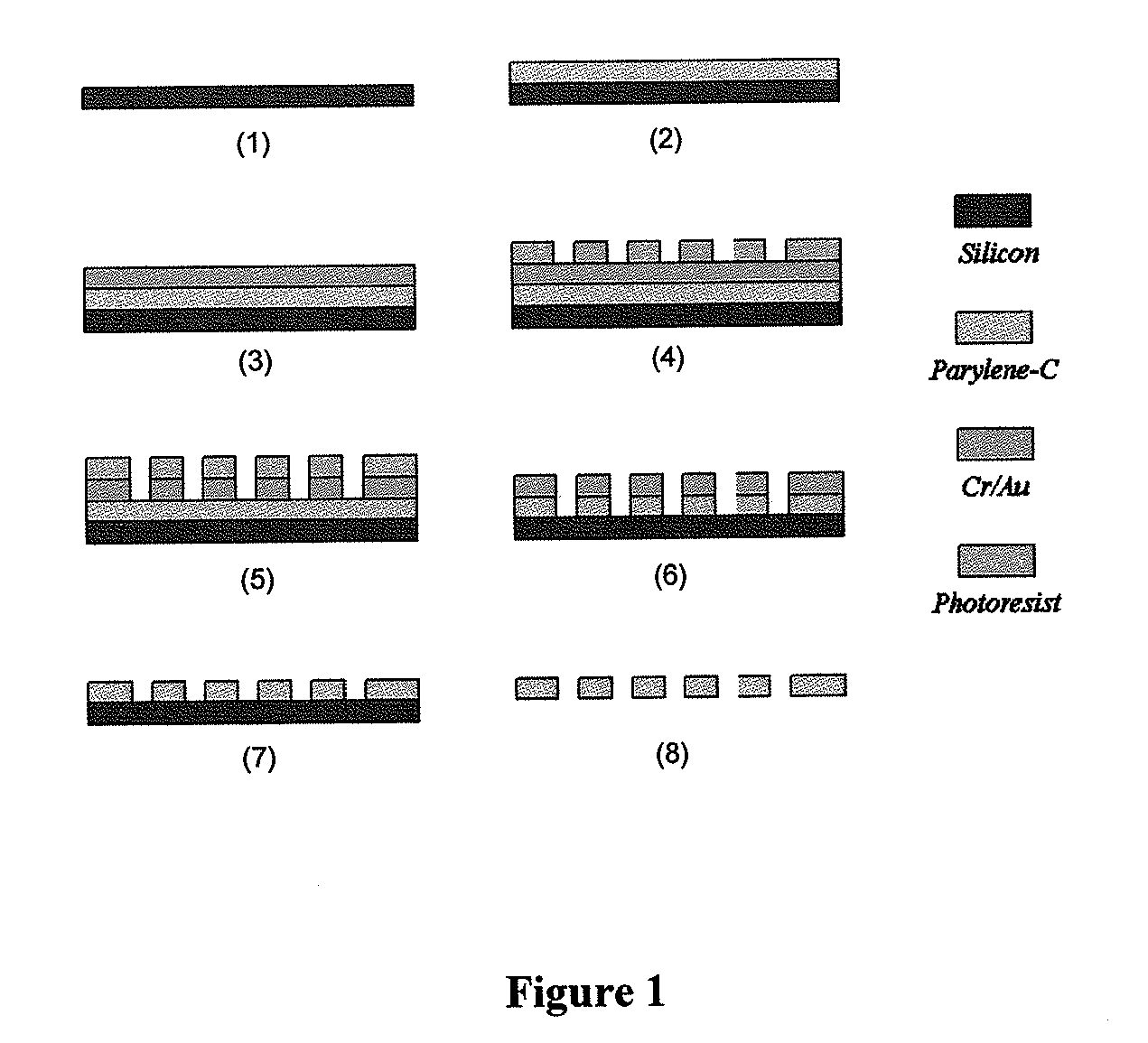
ActiveUS20110053152A1Rapidly and efficiently captureImprove capture efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsTelomeraseParylene
The present invention provides a method for diagnosing cancer, predicting a disease outcome or response to therapy in a patient sample. The method involves isolating a circulating tumor cell (CTC), for example, a viable CTC, from a sample using a parylene microfilter device comprising a membrane filter having or consisting of a parylene substrate, which has an array of holes with a predetermined shape and size; and detecting and quantifying telomerase activity in blood circulating tumor cells. The invention further provides methods of using cells live-captured in various applications.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Devices and methods for enrichment and alteration of circulating tumor cells and other particles
InactiveUS20070026418A1Small particle sizeLower the volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCirculating cancer cellTumor cells
The invention features devices and methods for detecting, enriching, and analyzing circulating tumor cells and other particles. The invention further features methods of diagnosing a condition, e.g., cancer, in a subject by analyzing a cellular sample from the subject.
Owner:CELLECTIVE DX CORP +4
Methods and compositions for detecting rare cells from a biological sample
InactiveUS20080057505A1Strong specificityEasy to identifyMicrobiological testing/measurementBiomass after-treatmentHematopoietic cellWhite blood cell
The present invention provides methods and compositions for isolating and detecting rare cells from a biological sample containing other types of cells. In particular, the present invention includes a debulking step that uses a microfabricated filters for filtering fluid samples and the enriched rare cells can be used in a downstream process such as identifies, characterizes or even grown in culture or used in other ways. The invention also include a method of determining the aggressiveness of the tumor or of the number or proportion of cancer cells in the enriched sample by detecting the presence or amount of telomerase activity or telomerase nucleic acid or telomerase expression after enrichment of rare cells. This invention further provides an efficient and rapid method to specifically remove red blood cells as well as white blood cells from a biological sample containing at least one of each of red blood cells and white blood cells, resulting in the enrichment of rare target cells including circulating tumor cells (CTC), stromal cells, mesenchymal cells, endothelial cells, fetal cells, stem cells, non-hematopoietic cells etc from a blood sample. The method is based upon combination of immuno-microparticles (antibody coated microparticles) and density-based separation. The final enriched target cells can be subjected to a variety of analysis and manipulations, such as flowcytometry, PCR, immunofluorescence, immunocytochemistry, image analysis, enzymatic assays, gene expression profiling analysis, efficacy tests of therapeutics, culturing of enriched rare cells, and therapeutic use of enriched rare cells. In addition, depleted plasma protein and white blood cells can be optionally recovered, and subjected to other analysis such as inflammation studies, gene expression profiling, etc.
Owner:AVIVA BIOSCI
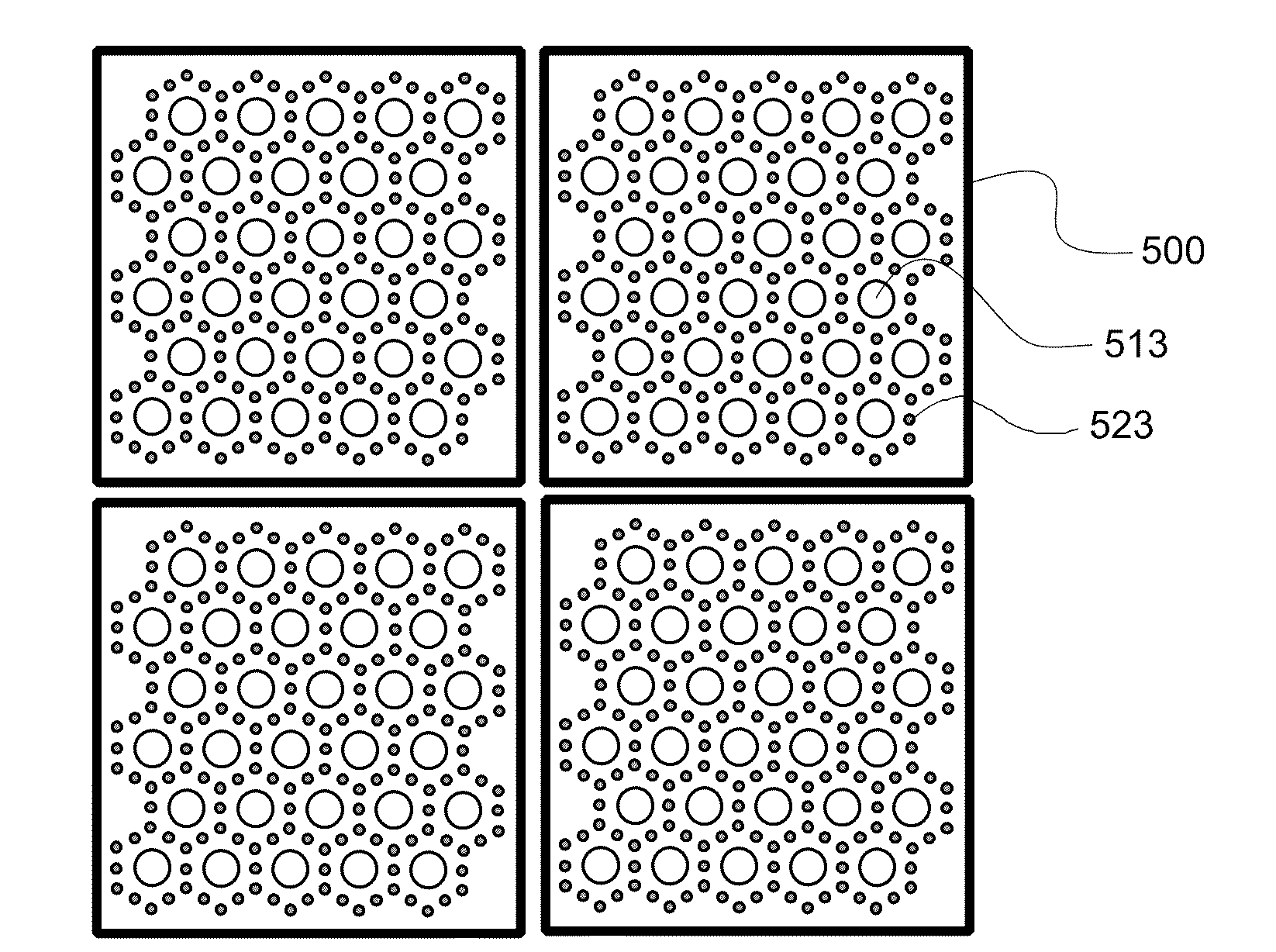
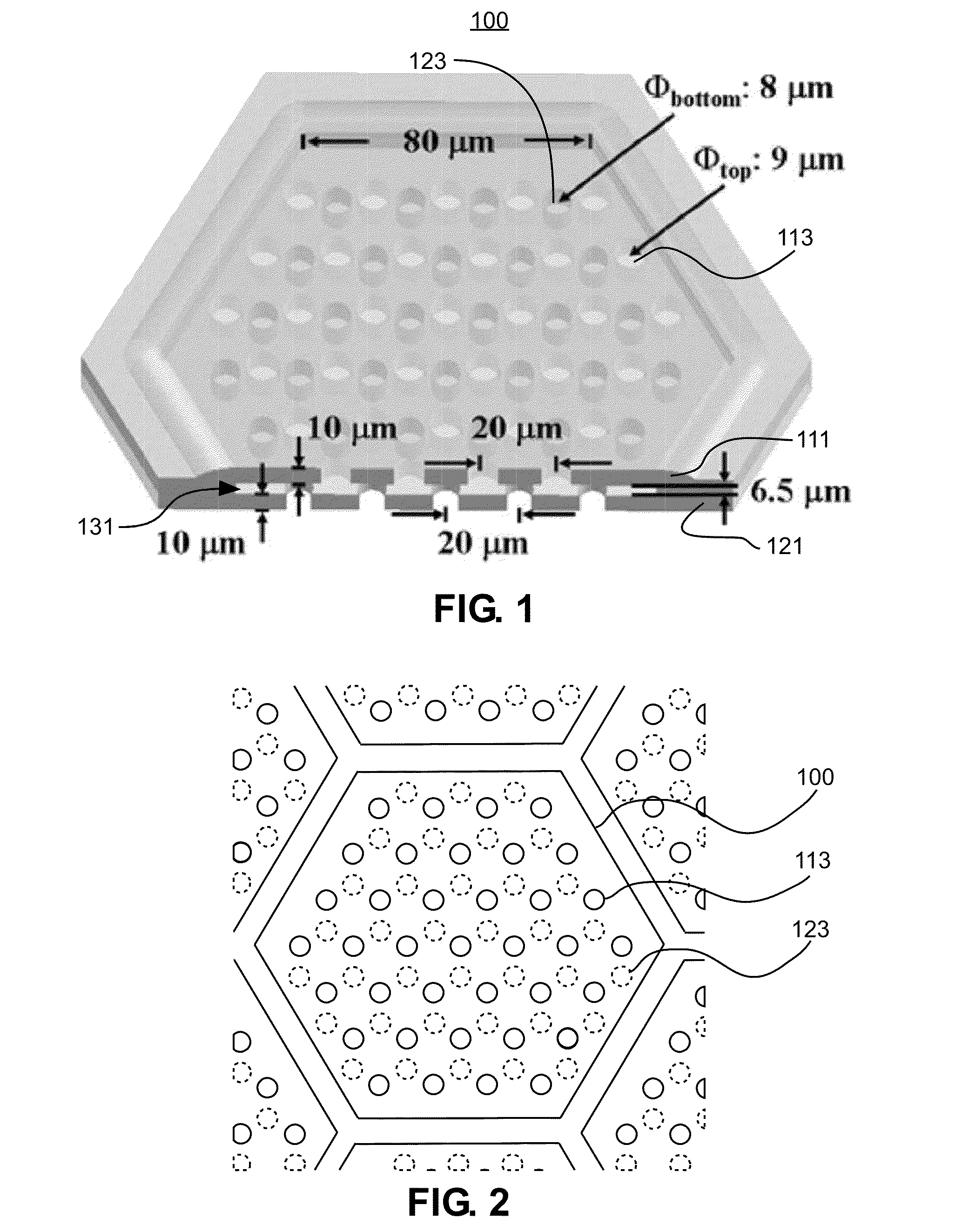
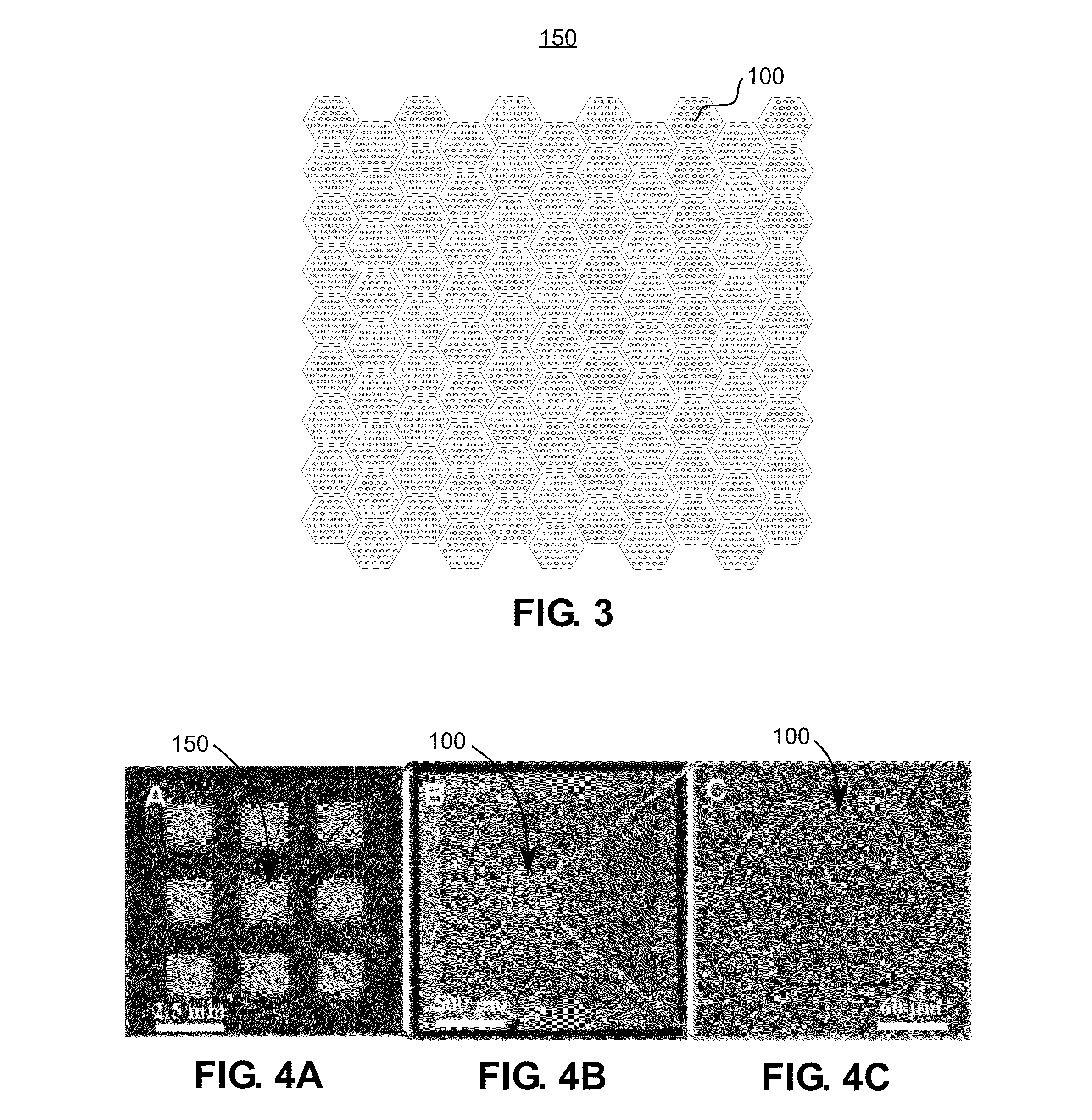
Membrane filter for capturing circulating tumor cells
ActiveUS20060254972A1Highly efficient in capturingEffective membraneBioreactor/fermenter combinationsBiological substance pretreatmentsParyleneGeometric design
The present invention provides a parylene-based membrane filter device for capturing of circulating tumor cells (CTC). The membrane filter has an array of holes having a predetermined geometric design with precisely controlled size, shape and density. In one aspect, the device has a stack of substantially parallel membrane filters with uniformly-spaced and / or monodispersed holes.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Method and apparatus for microfiltration to perform cell separation
ActiveUS20090188864A1Laborious and expensiveMembranesDecorative surface effectsParyleneMicrofabrication
A microfiltration apparatus and method for separating cells, such as circulating tumor cells, from a sample using a microfiltration device having a top porous membrane and a bottom porous membrane. The porous membranes are formed from parylene and assembled using microfabrication techniques. The porous membranes are arranged so that the pores in the top membrane are offset from the pores in the bottom membrane.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
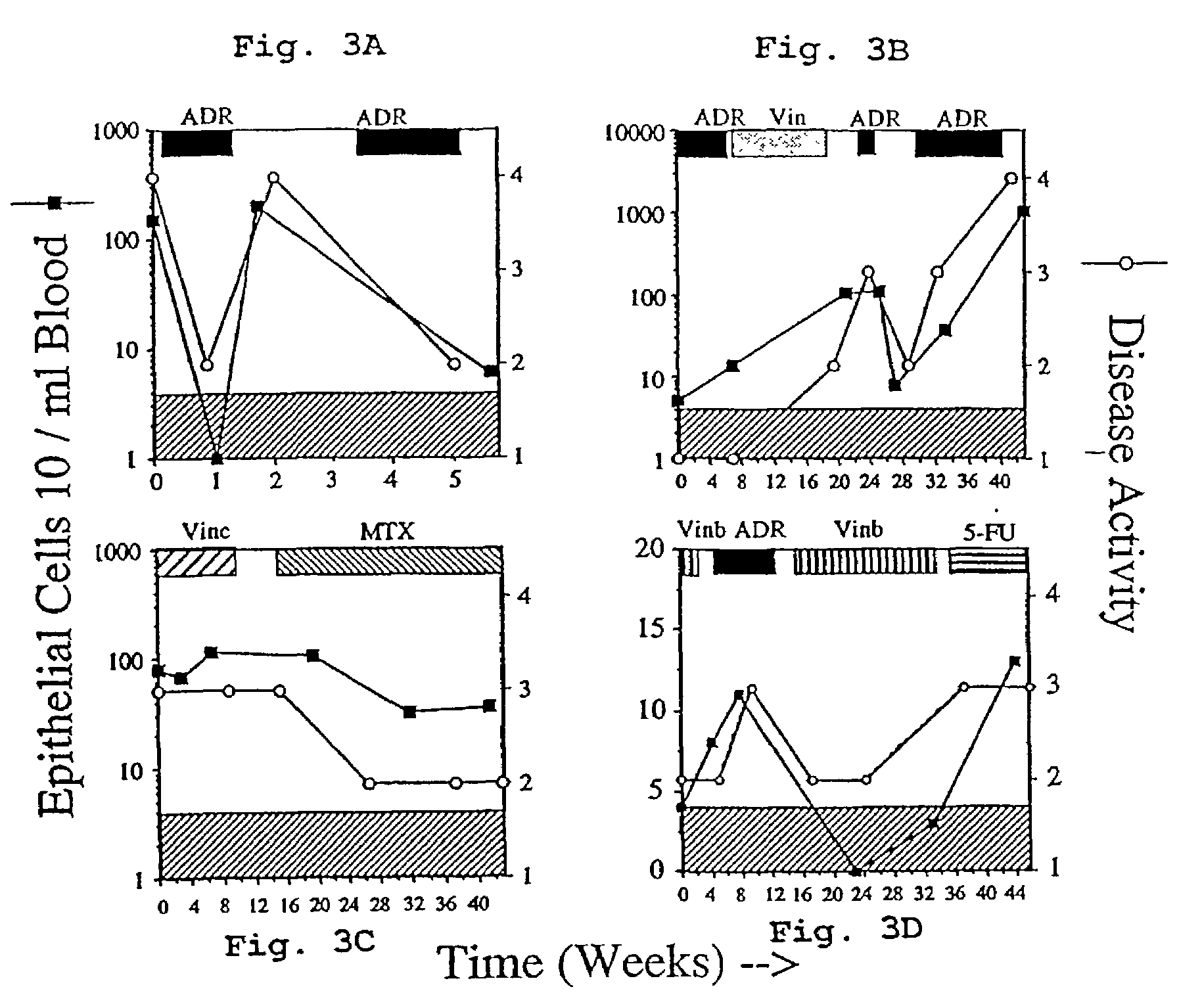
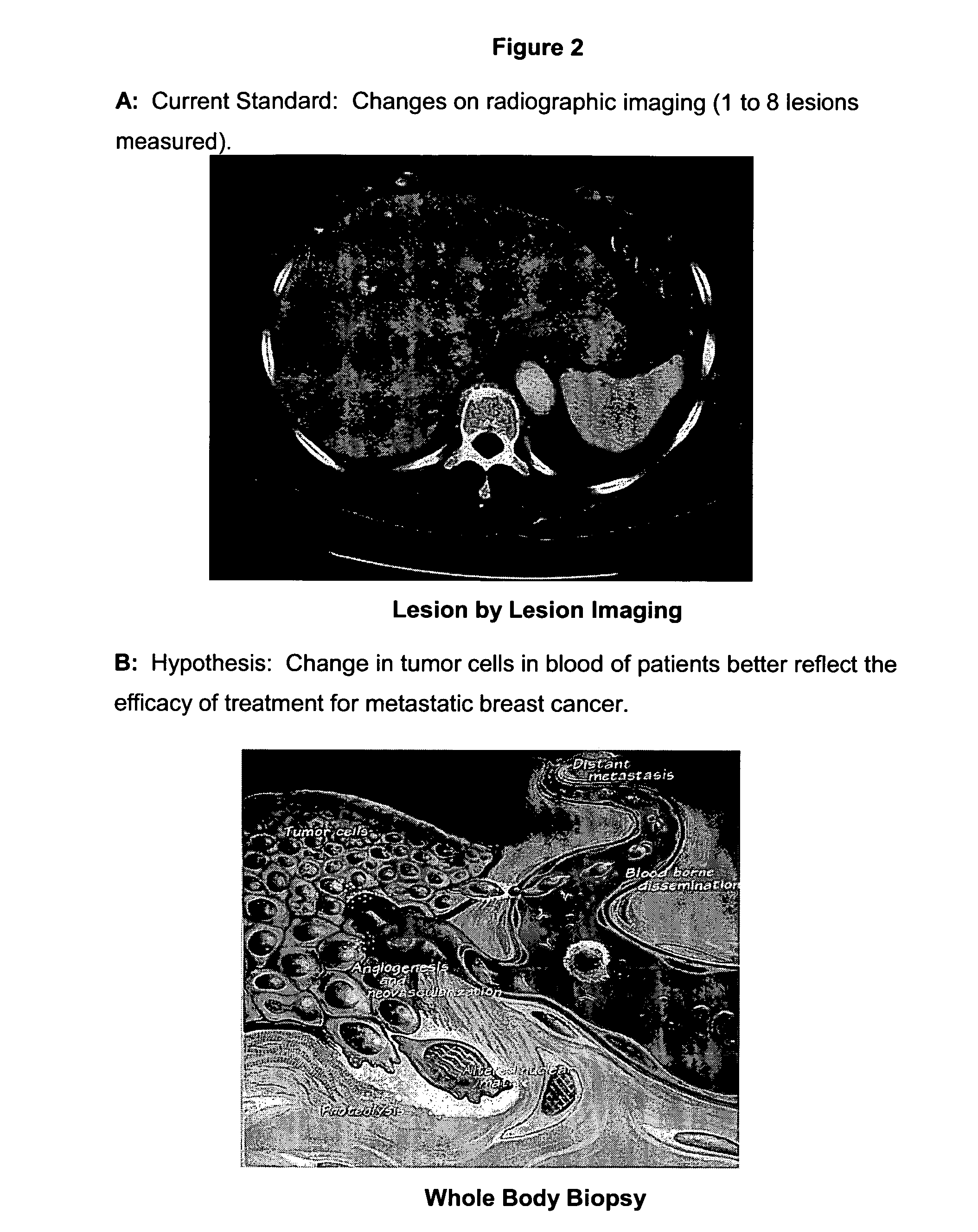
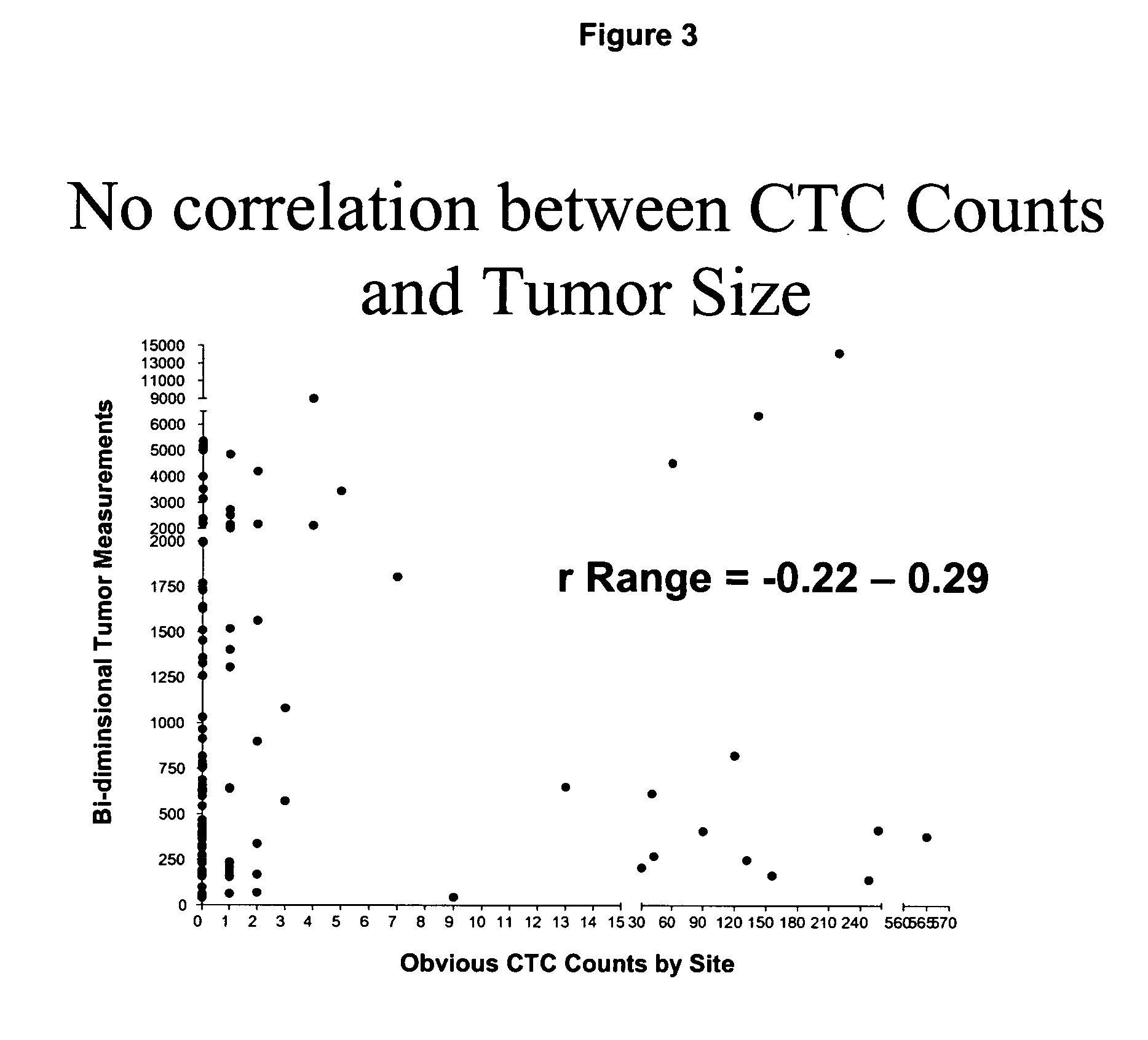
Circulating tumor cells (CTC's): early assessment of time to progression, survival and response to therapy in metastatic cancer patients
InactiveUS20070037173A1Less side effectsImprove the quality of lifeMicrobiological testing/measurementBiological testingClinical trialOncology
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:JANSSEN DIAGNOSTICS LLC
Ensemble-decision aliquot ranking
InactiveUS20120129190A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRare cellCirculating cancer cell
Provided herein, among other aspects, are methods and apparatuses for ranking aliquots from a suspension containing bioparticles. In certain embodiments, the bioparticles may be cells, organelles, proteins, DNAs, debris of biological origin, microbeads coated with biological compounds, or viral particles. As such, the methods and apparatuses provided herein may be used to quantify rare cells such as circulating cancer cells, fetal cells and other rare cells present in bodily fluids for disease diagnosis, prognosis, or treatment.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
Tagged Ligands For Enrichment of Rare Analytes From A Mixed Sample
ActiveUS20090215088A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRare cellAnalyte
Method of enriching specific cells from cellular samples are disclosed, comprising contacting in solution a cellular sample with affinity-tagged ligands (ATLs) each comprising a first ligand linked to an affinity tag, wherein the ligand selectively binds a cellular marker of the rare cells and the affinity tag can be selectively captured by a capture moiety, wherein the affinity tags do not comprise a magnetic particle; and flowing the sample through a microfluidic device comprising the capture moiety to selectively retain ATL-bound cells. Methods for enriching circulating tumor cells, and devices for enriching specific cells from cellular samples are also disclosed.
Owner:GPB SCI
Method of assessing metastatic carcinomas from circulating endothelial cells and disseminated tumor cells
InactiveUS20090191535A1Microbiological testing/measurementDead animal preservationCirculating endothelial cellPrimary breast cancer
A method for assessing cancer in test subjects is described based upon enumeration of circulating endothelial cells and / or disseminated tumor cells in a test subject. This method is used to quantify disseminated tumor cells. Correlations with circulating tumor cells provides prognostic information with high accuracy in assessing the risk of recurrence in patients with primary breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
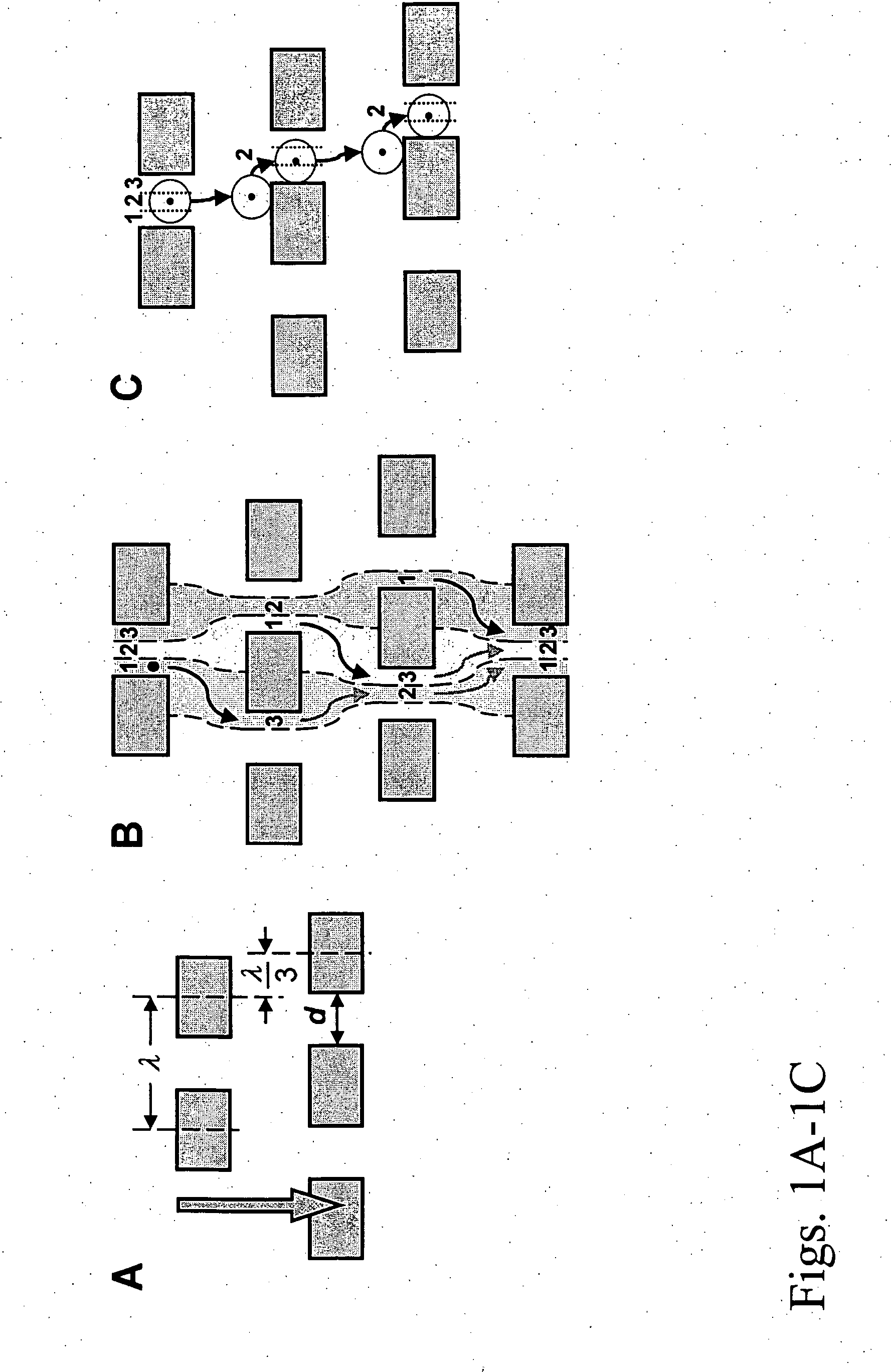
Circulating tumor cell capture on a microfluidic chip incorporating both affinity and size
InactiveUS20140154703A1Maximal flexibility/solubilityMaximal activityBioreactor/fermenter combinationsBiological substance pretreatmentsRare cellCirculating cancer cell
The invention encompasses methods and devices for diagnosing, theranosing, or prognosing a condition in a patient by enriching a sample in rare cells or other particles. The devices can be a microfluidic device comprising an array of obstacles and one or more binding moieties. The devices and methods can allow for enrichment of cells based on size and affinity, recovery of cells or other particles in locations on the microfluidic device, release of cells or other particles from the microfluidic device, flow of sample through the microfluidic device, and retention of rare cells or other particles from a sample obtained from a patient having a condition.
Owner:GPB SCI
Stabilization of cells and biological specimens for analysis
InactiveUS20060194192A1Improve stabilityMaintain qualityOrganic active ingredientsDead animal preservationAbnormal tissue growthIn vivo
Owner:JANSSEN DIAGNOSTICS LLC
Blood test to monitor the genetic changes of progressive cancer using immunomagnetic enrichment and fluorescence in situ hybridization (FISH)
InactiveUS20080113350A1Accurate measurementEasy accessMicrobiological testing/measurementLymphatic SpreadGenetic Change
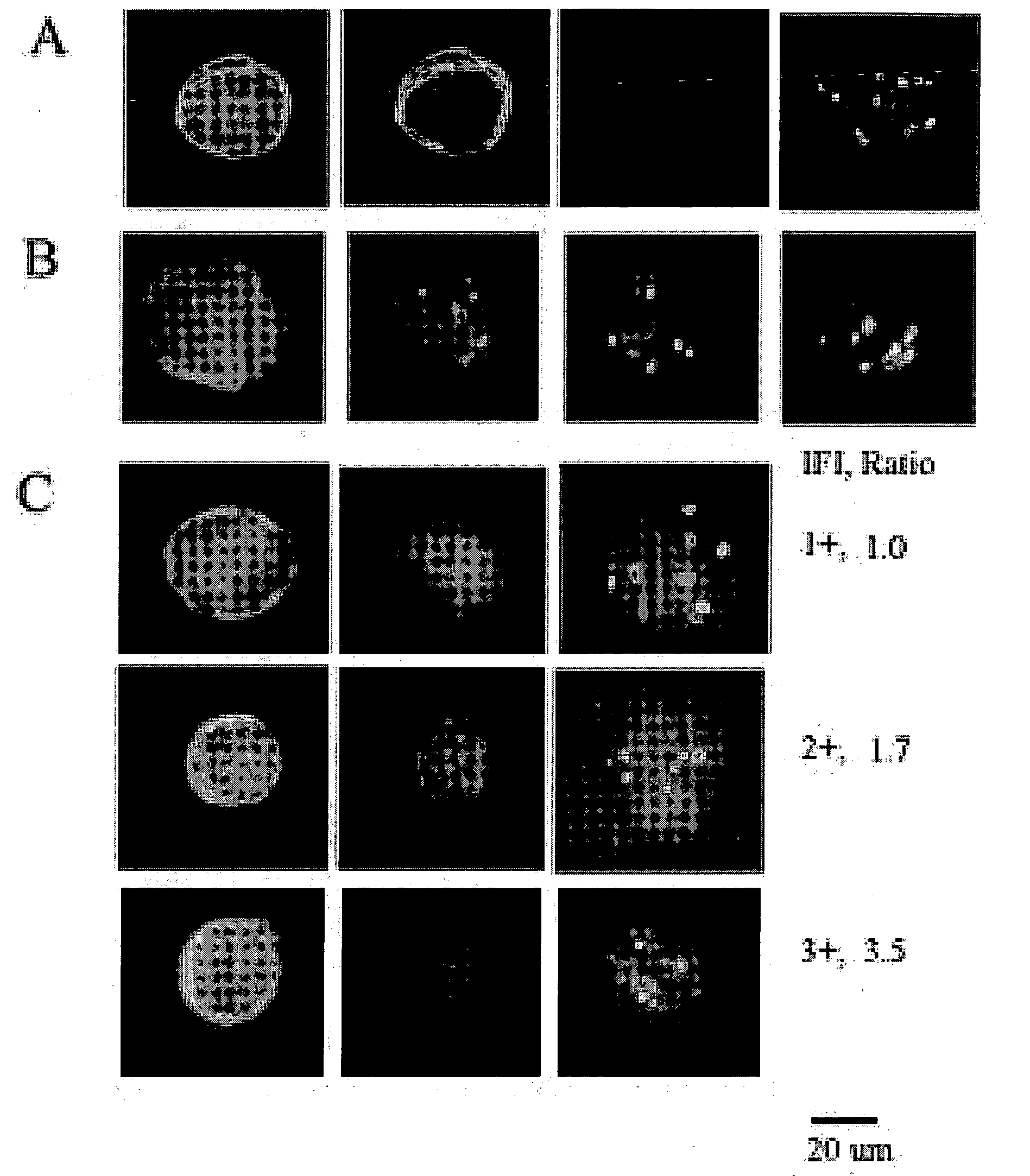
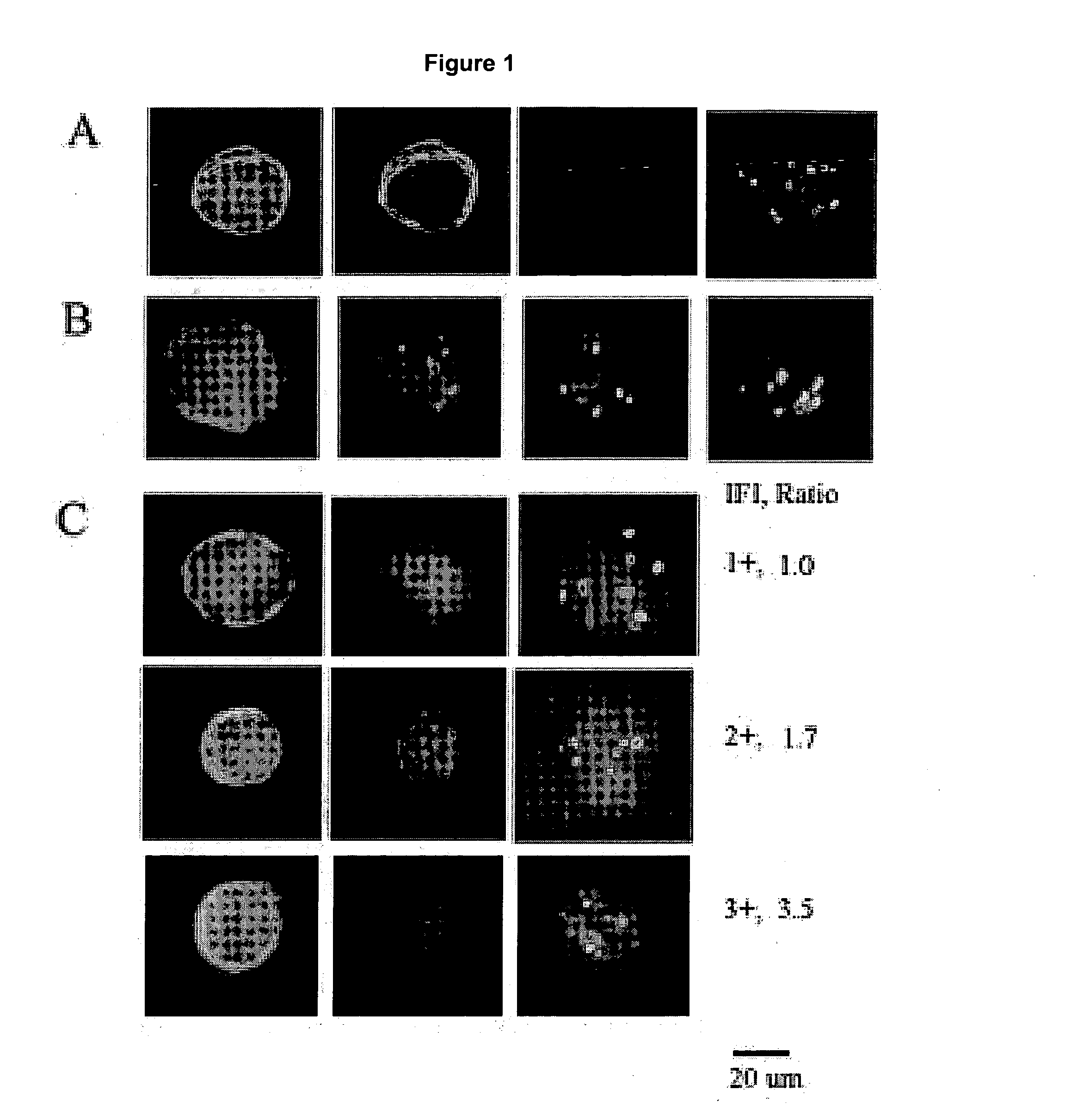
Amplification and overexpression of theHER-2 oncogene in breast cancer is felt to be stable over the course of disease and concordant between the primary tumor and metastases. Therefore, patients with HER-2 negative primary tumors will rarely receive anti-HER-2 antibody therapy. A very sensitive blood test is used to capture circulating tumor cells (CTC's) and evaluate their HER-2 gene status by FISH evaluation. The HER-2 status of the primary tumor and corresponding CTC's is used to assess the ratio of CTC's as a reliable surrogate marker. HER-2 expression of 10 CTC's is sufficient to make a definitive diagnosis of the HER-2 gene status for the whole population of CTC's in patients with recurrent breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
Method of Using Non-Rare Cells to Detect Rare Cells
InactiveUS20120276555A1Minimize biasMinimize timeDisease diagnosisFluorescence/phosphorescenceMulti dimensionalTumor cells
The invention provides seminal computational approaches utilizing data from non-rare cells to detect rare cells, such as circulating tumor cells (CTCs). The invention is applicable at two distinct stages of CTC detection; the first being to make decisions about data collection parameters and the second being to make decisions during data reduction and analysis. Additionally, the invention utilizes both one and multi-dimensional parameterized data in a decision making process.
Owner:THE SCRIPPS RES INST +1
Circulating tumor cell identification kit and circulating tumor cell identification method
ActiveCN104031993AComprehensive detectionAvoid false negativesMicrobiological testing/measurementHybridization probeFluorescence
The invention discloses a circulating tumor cell identification kit and a circulating tumor cell identification method. The circulating tumor cell identification kit comprises a detection probe for detecting mRNA of an epithelial cell marking gene and / or mRNA of a mesenchymal cell marking gene, wherein the epithelial cell marking gene is selected from one or more of CDH2, VIMENTIN, FN1 and AKT2. Multiple RNA probes are adopted in the identification method, specific genes of a plurality of circulating tumor cells (CTCs) can be marked at the same time and are divided into genes I (epithelial genes), genes II (epithelial-mesenchymal genes) and genes III (mesenchymal genes), and false positive results caused by lack of part of the specific genes of the CTCs in a process that the CTCs enter peripheral blood for circulation are reduced; the method can be finished within eight hours, and a single copied mRNA hybridization probe is combined with a corresponding fluorescent probe by virtue of a signal amplification system, so that the detection sensitivity of in-situ RNA (Ribonucleic Acid) hybridization is remarkably improved.
Owner:SUREXAM BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com