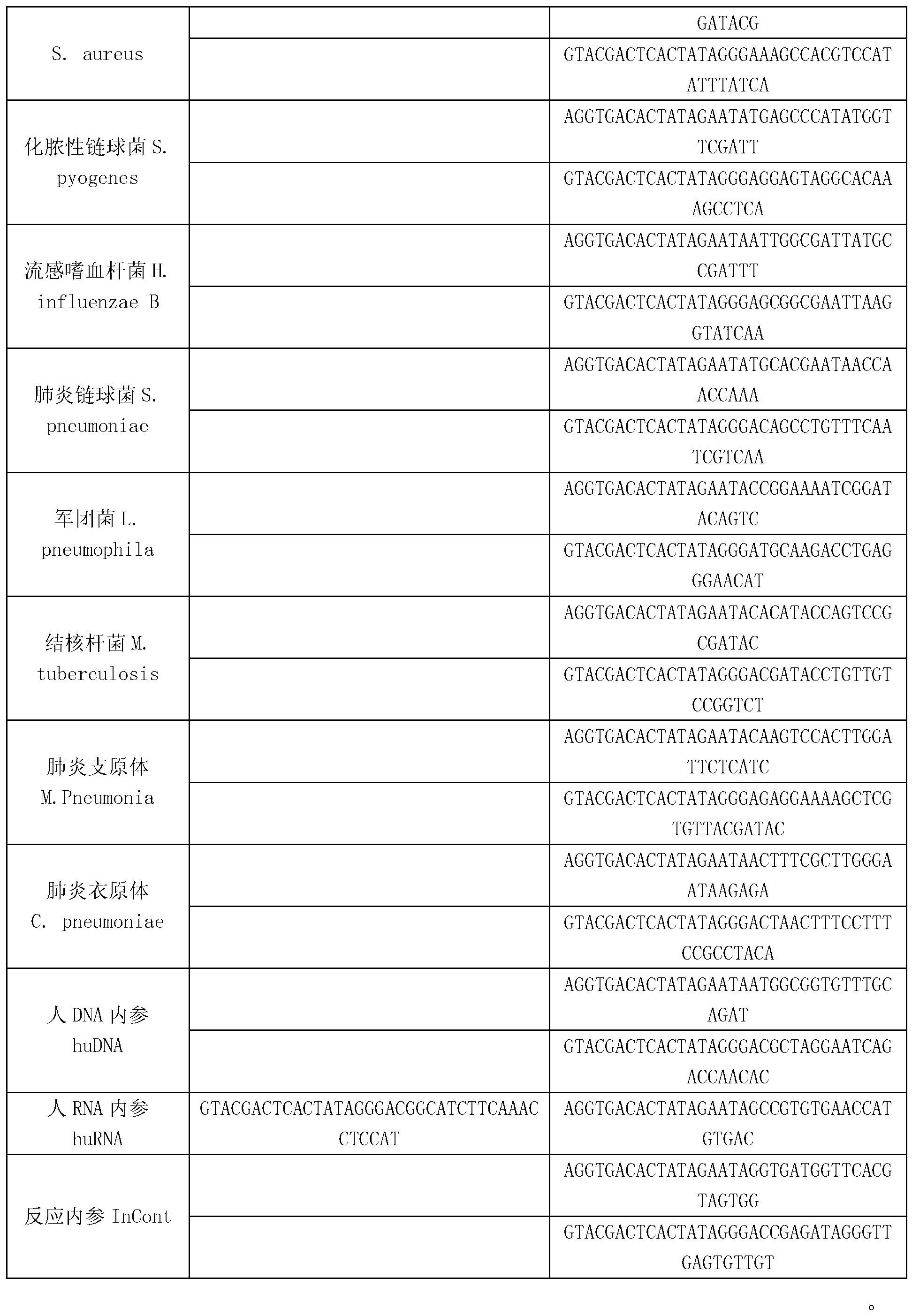
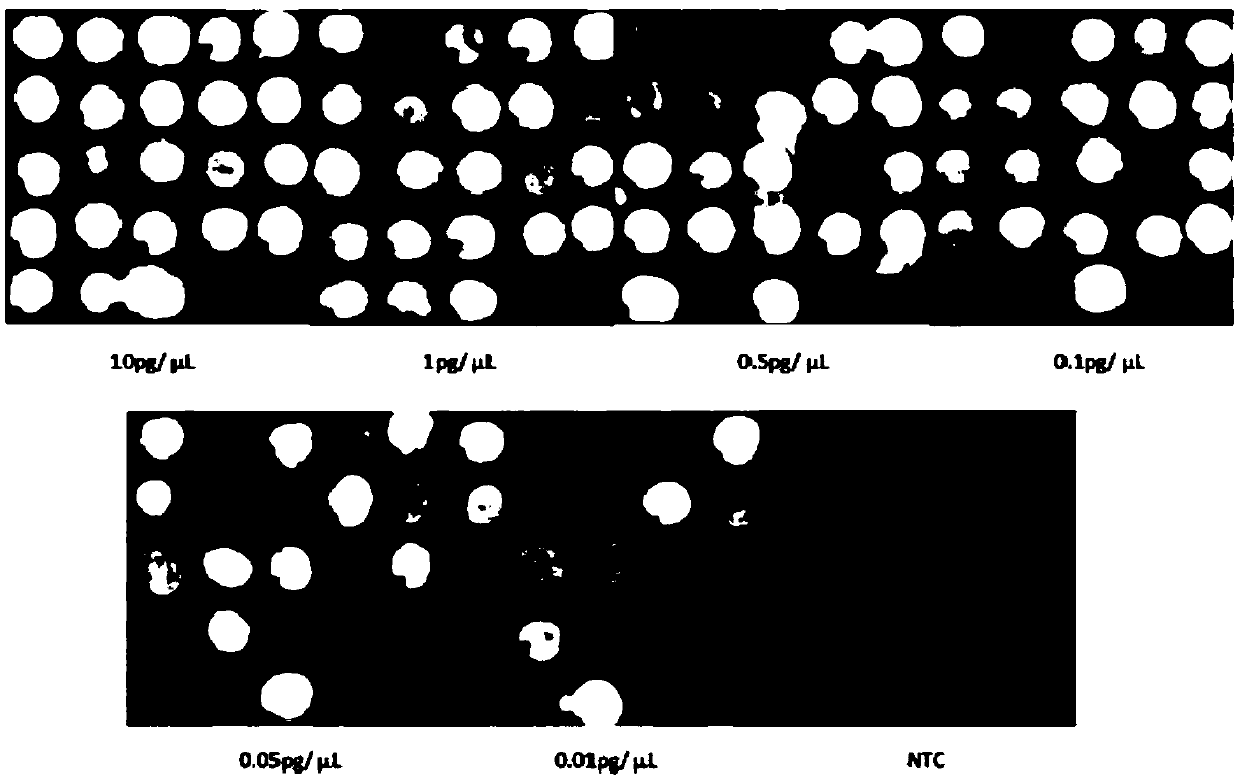
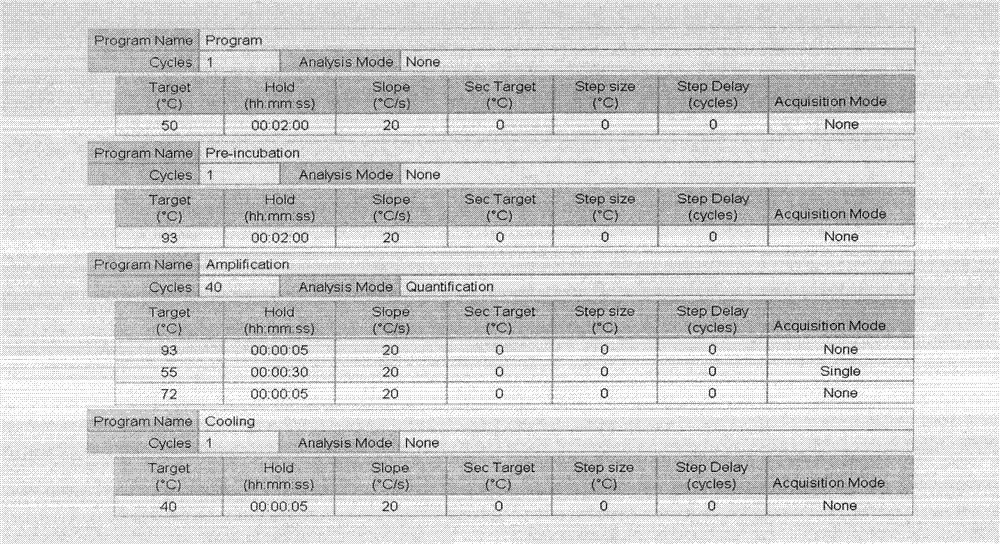
Patents
Literature
277results about How to "Avoid false negatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
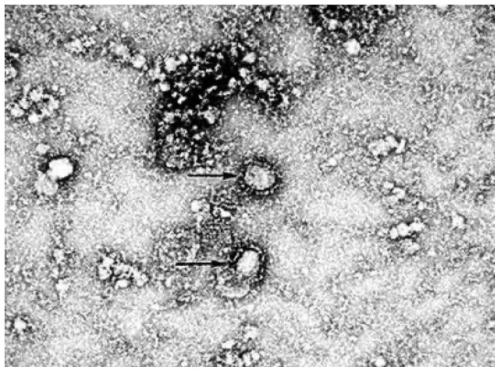
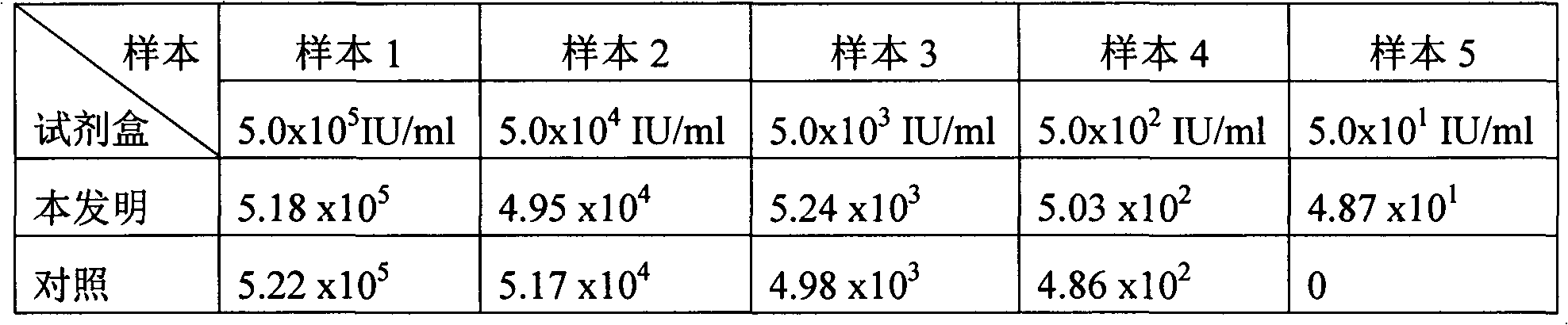
Novel coronavirus detection kit
PendingCN111187858AAccurate detectionPut an end to the possibilityMicrobiological testing/measurementMicroorganism based processesVirologyBioinformatics
The invention discloses a novel coronavirus detection kit. The kit comprises a qRT-PCR reaction liquid, a qRT-PCR enzyme liquid, an internal standard, a negative quality control substance and a positive quality control substance, wherein the internal standard is an RNA pseudovirus containing an internal reference detection gene fragment; the positive quality control substance is an RNA pseudoviruscontaining a target detection gene fragment; and the qRT-PCR reaction liquid comprises four groups of upstream and downstream primers with sequences of SEQ ID NO:1-8 and four probes with sequences ofSEQ ID NO:9-12. The kit can be used to simultaneously detect three sections of target gene fragments, so that missed detection can be effectively avoided. In addition, pseudoviruses are used in boththe internal standard and the positive quality control substance of the kit, so that safety of the kit is effectively improved, and detection accuracy of the kit is further ensured. The kit is high sensitivity and good in stability.
Owner:SICHUAN MACCURA BIOTECH CO LTD
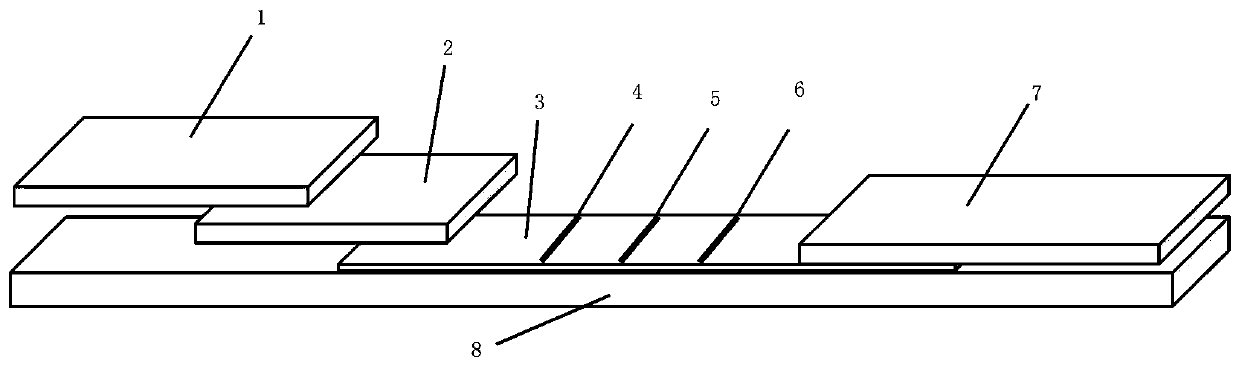
Novel coronavirus detection kit
PendingCN111337689AThe detection process is fastEasy to operateBiological testingImmunoassaysIgm antibodyColloidal au
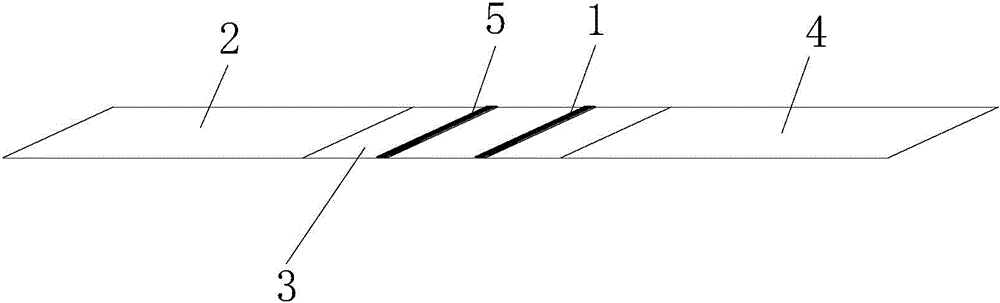
The invention discloses a novel coronavirus detection kit. The kit comprises a bottom plate, and a sample pad, a colloidal gold pad, an NC membrane and a water absorption pad which are fixed on the bottom plate, wherein a detection line and a quality control line are arranged on the NC membrane, an antigen coated on the colloidal gold pad is a mixed antigen protein composed of N protein marked bycolloidal gold and S-RBD protein marked by colloidal gold, and the detection line is a mouse anti-human IgM antibody and mouse anti-human IgG antibody detection line. The detection kit provided by theinvention takes N protein and S-RBD protein as antigens, can improve the sensitivity of COVID-19 preliminary detection, and is suitable for quick screening of large-scale crowds.
Owner:SHANXI MEDICAL UNIV
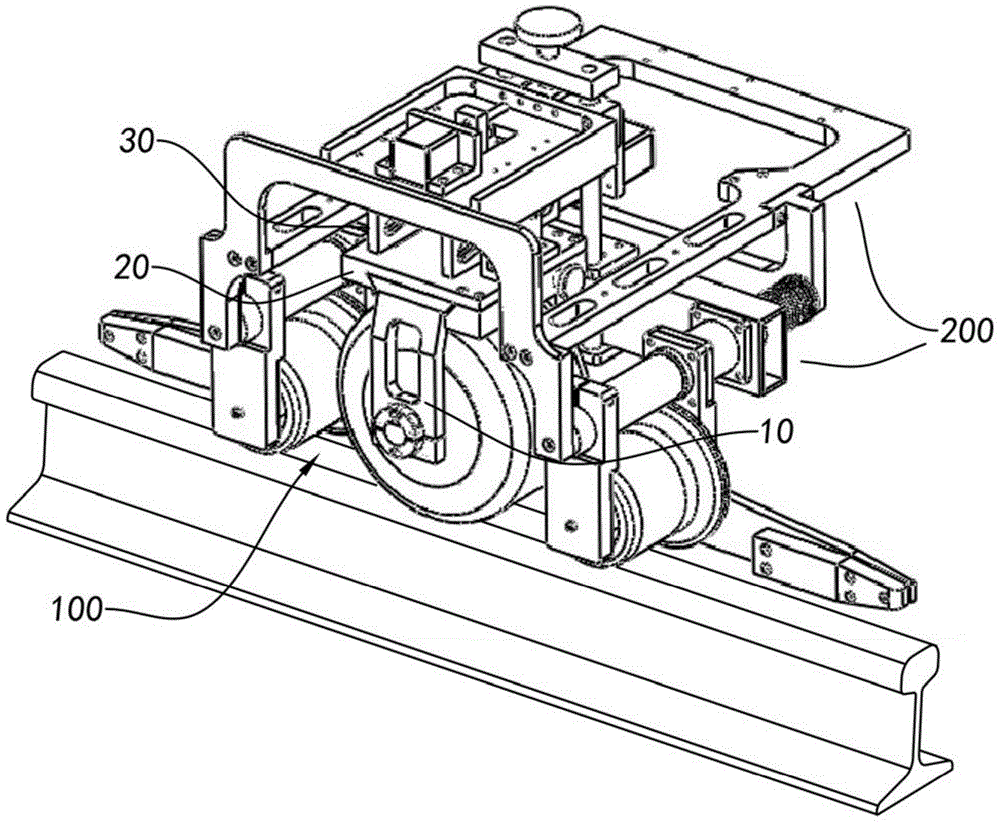
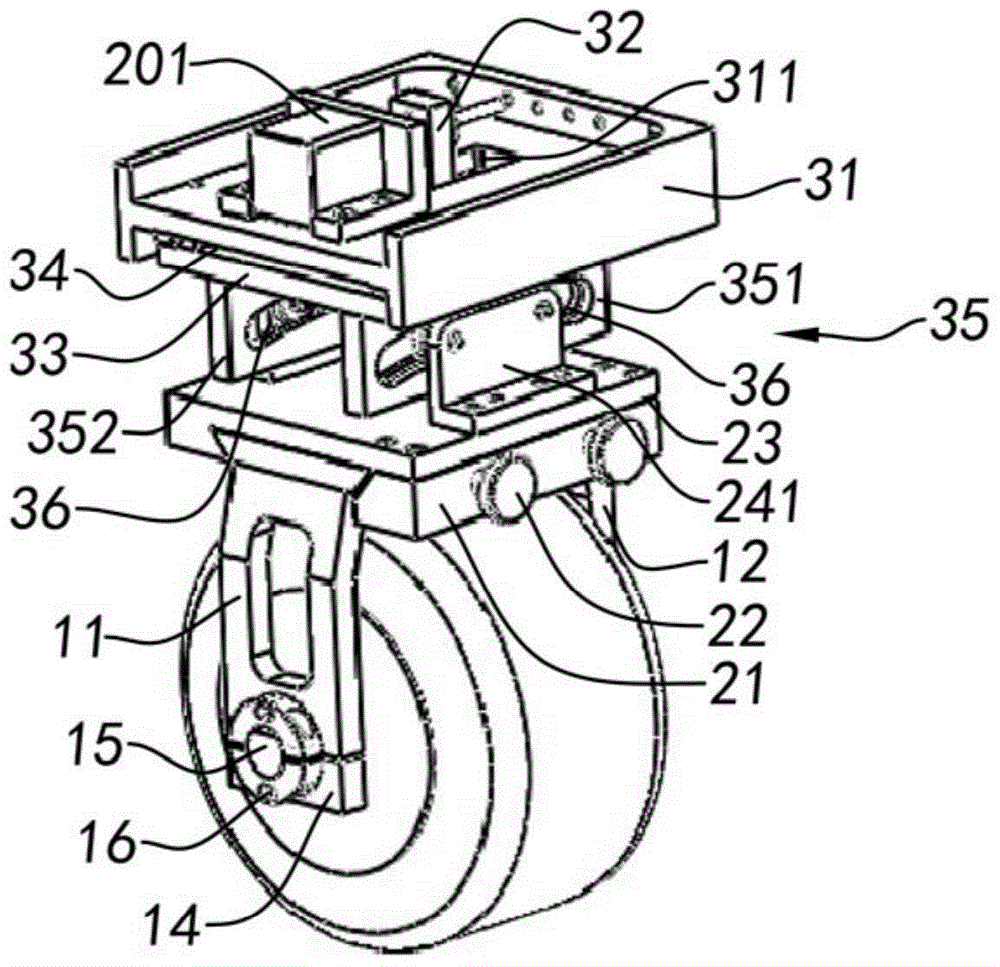
Servo centering device of rail flaw detection robot
ActiveCN104354718AAvoid false negativesRailway auxillary equipmentRailway profile gaugesEngineeringElectrical and Electronics engineering
The invention relates to a servo centering device of a rail flaw detection robot. The servo centering device of the rail flaw detection robot comprises at least one detection wheel and further comprises an integrated support, wherein the detection wheel is installed on the integrated support and is installed on a flaw detection trolley used for rail flaw detection operation, an adjusting system is formed on the integrated support, and when the flaw detection trolley conducts flaw detection operation, the adjusting system adjusts the operation state of the detection wheel in the horizontal direction, the vertical direction and the running direction of the detection wheel, so that the detection wheel and the top surface of a rail are always kept in good coupling to accurate receive, transmit flaw detection signals and avoid report mistakes and report failure.
Owner:SHANGHAI ORIENTAL MARITIME ENG TECH CO LTD
Using method for extracting virus DNA by using micro-nucleic acid releasing agent
InactiveCN104962553AGuaranteed cracking efficiencySubsequent experiment impactDNA preparationBiologyPollution
The invention discloses a method for extracting virus DNA contained in a biological sample by utilizing a micro-nucleic acid releasing agent, belongs to the technical field of molecular biology, and particularly relates to a reagent for extracting virus DNA by utilizing the micro-nucleic acid releasing reagent and a using method thereof. The micro-nucleic acid releasing agent cracks the virus DNA contained in the biological sample (a serum or a plasma) and can more effectively ensure the cracking and release efficiency through the assistance of a release promoting agent; the micro-nucleic acid releasing agent completely releases nucleic acid and also has the function of closing various factor substances, namely protein, drugs, hemolyzed blood and the like which are contained in a sample and have interference effects on PCR amplification, so that false negative in a detection process is prevented. The method disclosed by the invention is simple, convenient, flexible, fast and accurate in sample extraction, prevents the pollution and nucleic acid loss which are caused by repeated centrifugation and elution uncapping, and realizes the 'one-step' and 'one-room' operation of PCR extraction.
Owner:宝瑞源生物技术(北京)有限公司
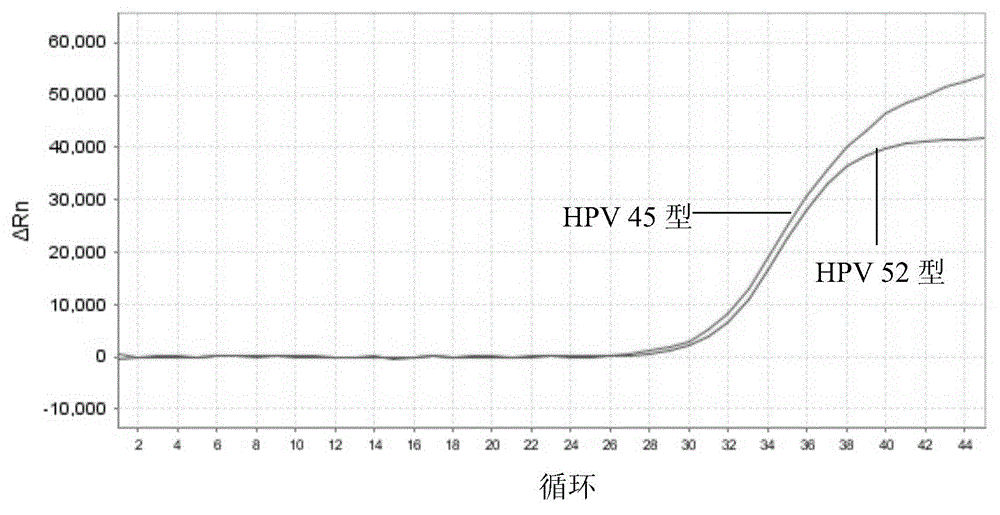
Nucleic acid detection kit for human papilloma virus, use method and application thereof
ActiveCN105506173AAvoid false negativesIncrease throughputMicrobiological testing/measurementMicroorganism based processesLower riskFluorescence
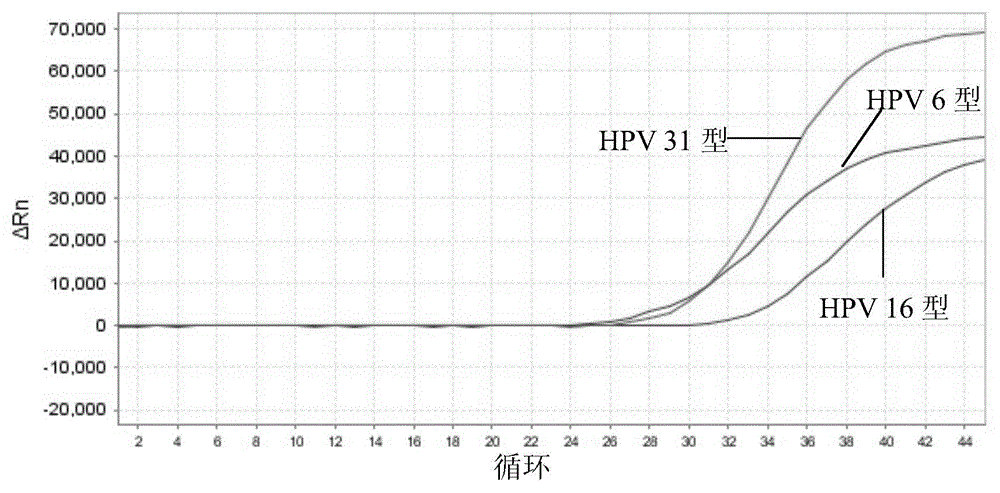
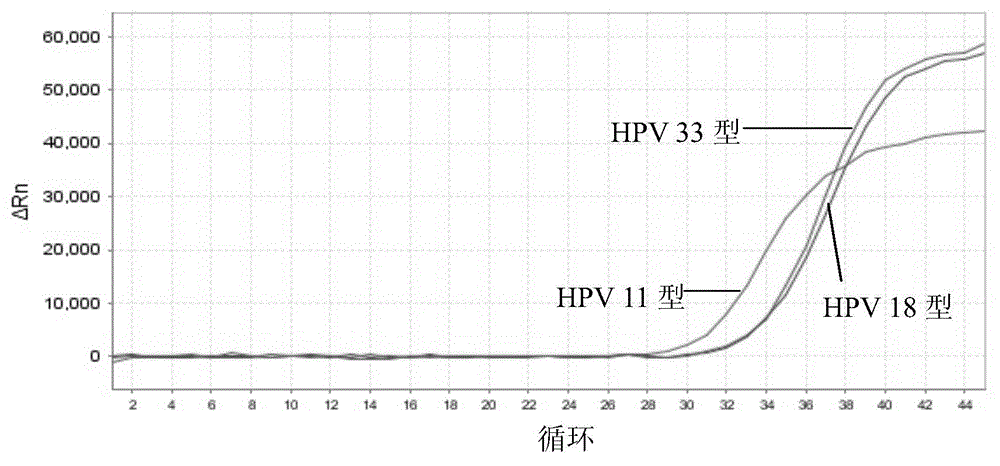
The invention discloses a nucleic acid detection kit for human papilloma virus, a use method and an application thereof. The kit includes a nucleic acid amplification reagent comprising a primer pair and a probe corresponding to the primer pair. The use method of the kit includes the following steps: 1) extracting nucleic acids from a sample; 2) preparing the reagent; 3) performing PCR amplification; and 4) performing fluorescent detection to a PCR amplification reaction product at 65-72 DEG C, and determining infection type of the HPV according to the changes on the Ct value of a target amplification curve and the Ct value of an internal standard amplification curve. The kit can be used for detecting low-risk and high-risk human papilloma virus and for typing the human papilloma virus. The kit can be used for calculating relative viral load of the HPV, can avoid pollution, can increase treatment throughput of samples, and can avoid missing detection.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
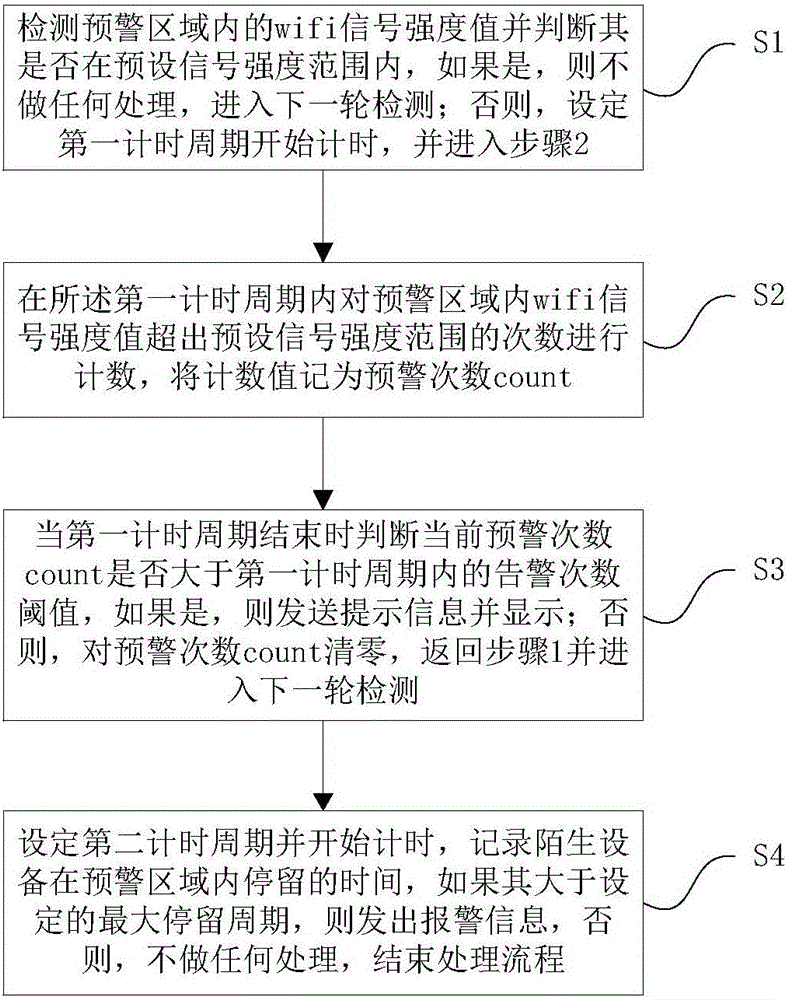
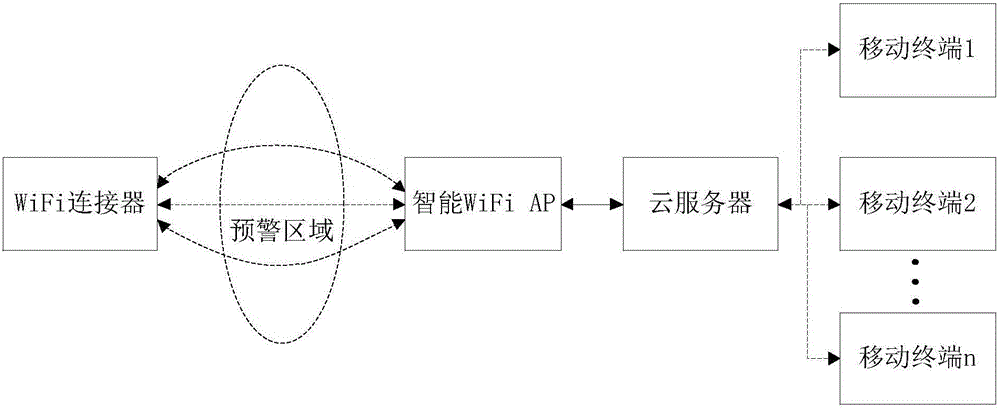
Intelligent monitoring method, device and system based on WiFi
ActiveCN105894703APrevent bandits from invadingAvoid leakage of user privacyBurglar alarm electric actuationUser privacyReal-time computing
The invention relates to an intelligent monitoring method, device and system based on WiFi. The device comprises an intelligent WiFi AP (Access Point), which is used for detecting a WiFi signal intensity value in an early warning area, judging whether the WiFi signal intensity value is within a preset signal intensity range, judging whether the current early warning count exceeds an early warning count threshold after a set timing cycle, if so, sending and displaying prompt information, otherwise, not carrying out any processing. The method, the device and the system realize intelligent monitoring using the WiFi technology, thereby effectively avoiding user privacy divulgence and missing report defect; and intelligent monitoring can be realized by adding only one WiFi connector and sufficiently utilizing the existing household routing platform, so the method, the device and the system are low in cost, high in security and very suitable for large-area popularization.
Owner:湖南领佰科技有限公司
COVID-19 nucleic acid testing primer probe composition, COVID-19 nucleic acid testing kit and COVID-19 nucleic acid testing method
PendingCN111621604AAvoid mutual interferenceStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesForward primerViral nucleic acid
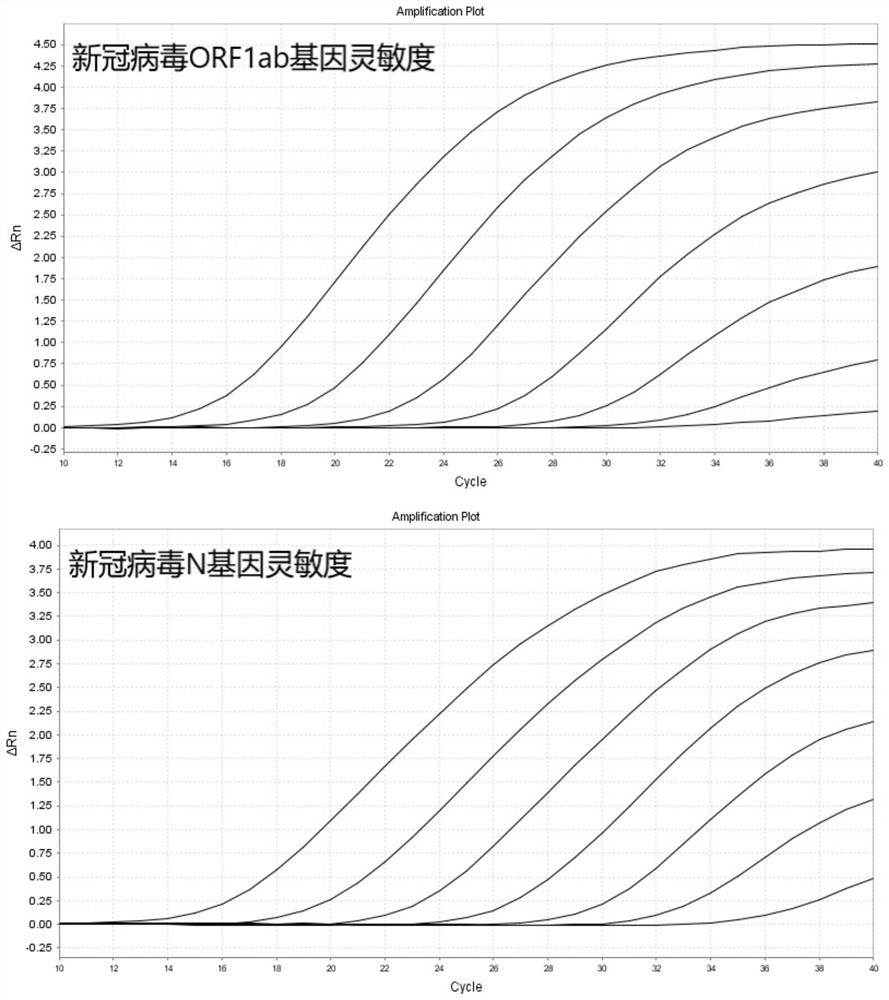
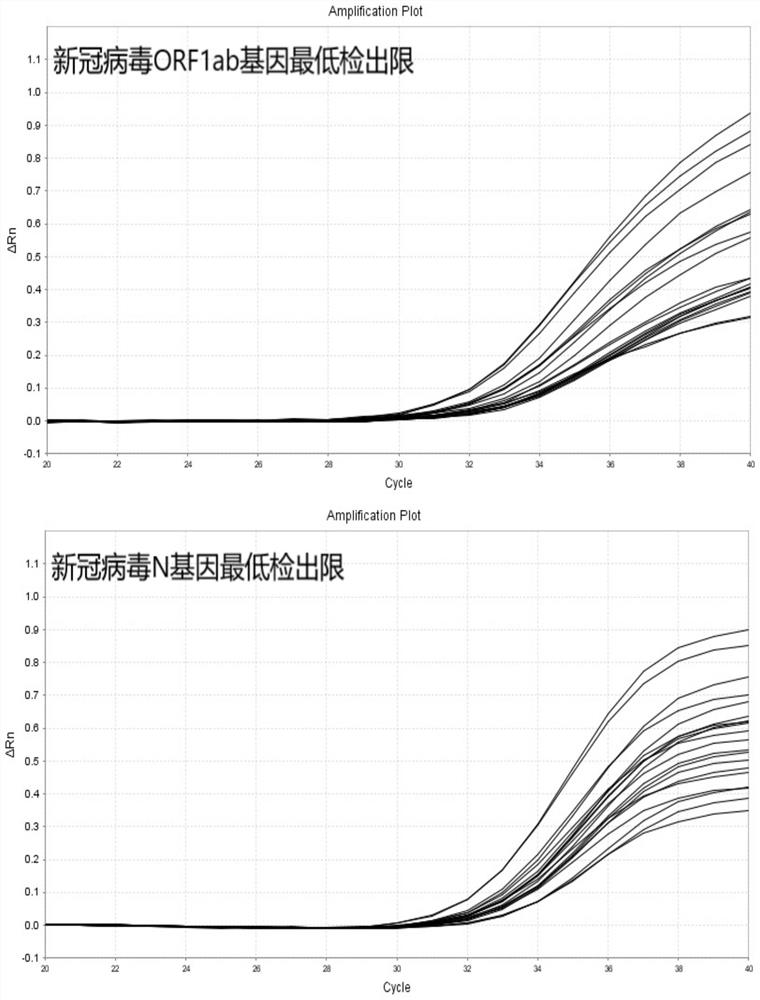
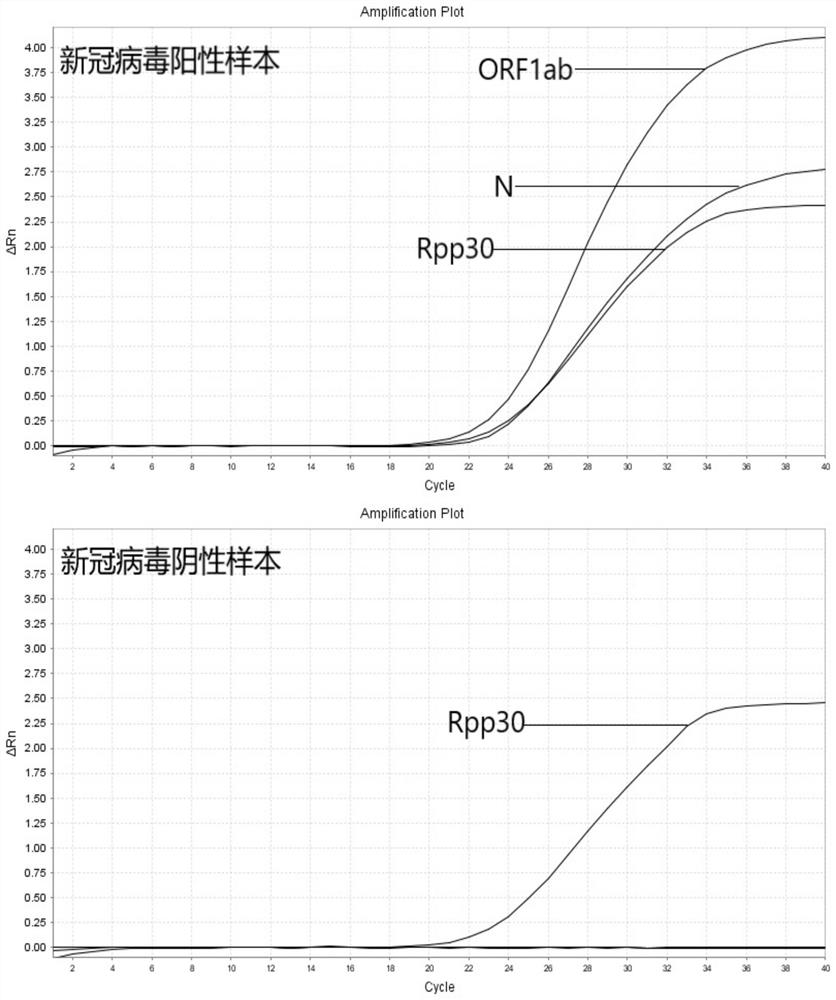
The invention relates to the field of the biological technology and the medical examination technology, and provides a COVID-19 nucleic acid testing primer probe composition, a COVID-19 nucleic acid testing kit and a COVID-19 nucleic acid testing method for solving the problems of low accuracy, poor specificity and low sensitivity of a traditional virus nucleic acid testing method. The COVID-19 nucleic acid testing primer probe composition comprises a first primer probe group, a second primer probe group and a third primer probe group, wherein the first primer probe group comprises a forward primer ORF1ab-F, a probe ORF1ab-P and a reverse primer ORF1ab-R; the second primer probe group comprises a forward primer N-F, a probe N-P and a reverse primer N-R; and the third primer probe group comprises a forward primer Rpp30-F, a probe Rpp30-P and a reverse primer Rpp30-R. According to the primer probe composition disclosed by the invention, through artful design of an amplification primer pair and a detection probe, mutual interference between a plurality of primer pairs and corresponding detection probes is avoided. The kit has a simple structure, an interior label is used for monitoring a collection, transportation and extraction process of a sample to be detected, and the false negative of a detection result is avoided.
Owner:SHANGHAI CHROMYSKY MEDICAL RES +1
DMD (Duchenne muscular dystrophy) gene capture probe and application thereof in DMD gene mutation detection
ActiveCN106834507APrecise positioningImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationDuchenne muscular dystrophyMuscular dystrophy
The invention discloses a DMD (Duchenne muscular dystrophy) gene capture probe and application thereof in DMD gene mutation detection. A preparation method of the DMD gene capture probe disclosed herein comprises: preparing N sub-probes, connecting the sub-probes to obtain the DMD gene capture probe; N is a natural number greater than or equal to 2; the N sub-probes can cover whole sequences of DMD gene; any two adjoining sub-probes on the DMD gene meet the case where there is one or more downstream nucleotide to the corresponding upstream sub-probe, which overlap with the upstream of the corresponding downstream sub-probe; the length of each of the N sub-probes is 50-150 bp. By detecting the DMD gene with the DMD gene capture probe, it is possible to detect whether the DMD gene experiences amplification mutation deletion / repetition or not, and it is also possible to precisely locate a break point region and the size of fragments and to detect DMD gene site mutations of a sample under detection.
Owner:MYGENOSTICS (CHONGQING) GENE TECH CO LTD
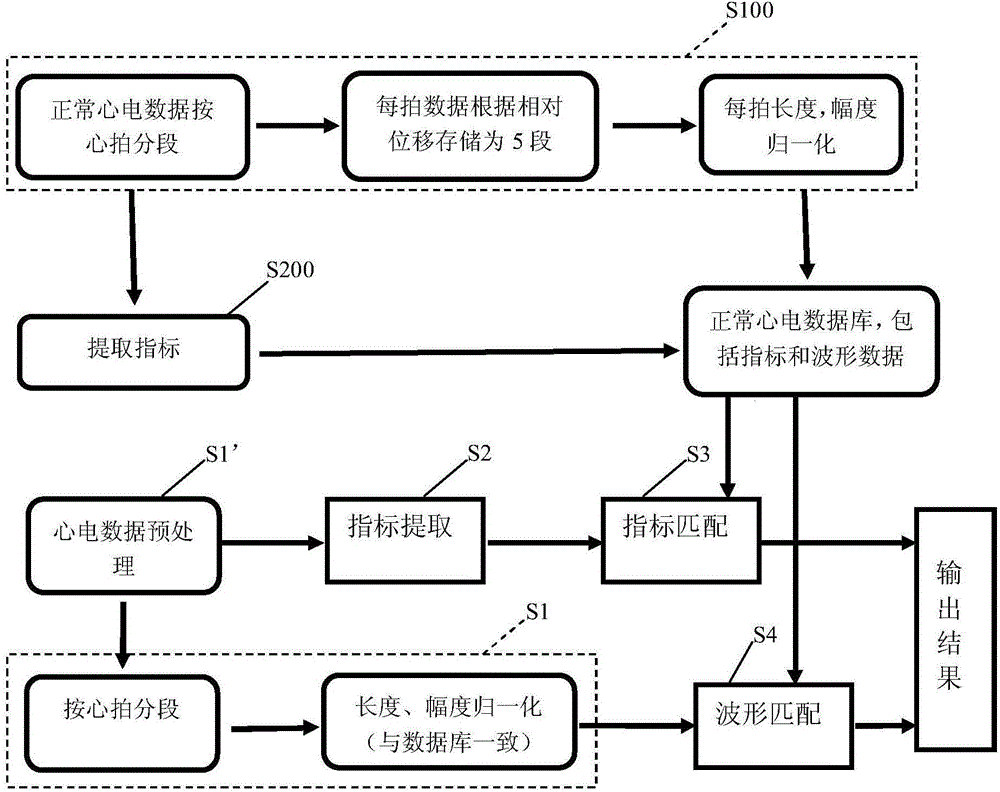
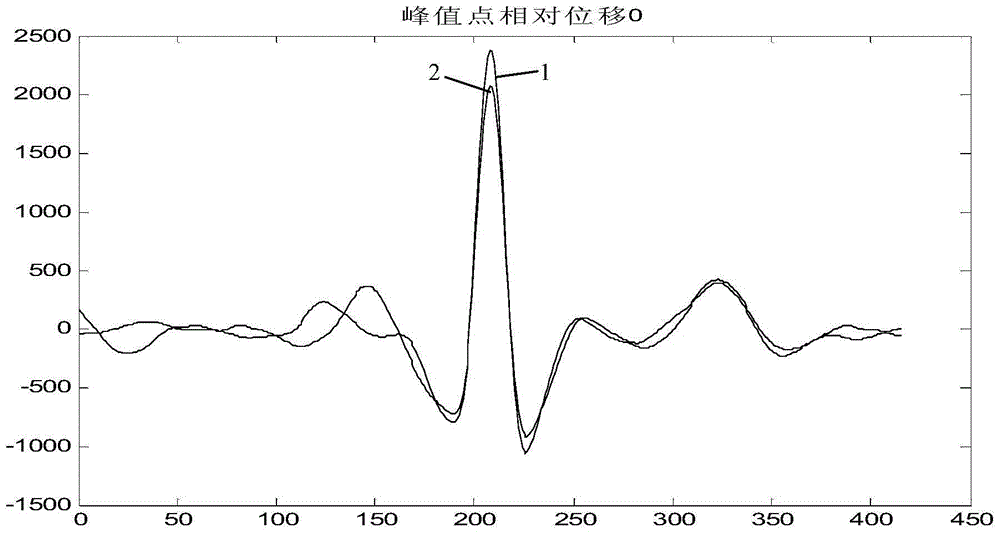
Electrocardio normality/abnormality big-data processing method and device
InactiveCN104834921AReliable identificationReduce workloadRecognition of medical/anatomical patternsWave shapeConfidence interval
The invention discloses an electrocardio normality / abnormality big-data processing method and device, and the method employs a normal electrocardio database, and comprises the following steps: S1, partitioning to-be-classified electrocardio data according to cardiac beat, and then carrying out the normalization processing of length and amplitude, thereby forming a plurality of pieces of beat-wave-shaped data; S2, extracting the index data of to-be-classified electrocardio data; S3, determining a confidence interval according to the index data stored in the database, comparing the index data of the extracted to-be-classified electrocardio data with the confidence interval, and outputting a comparison result; S4, calculating the similarity of the plurality of pieces of beat-wave-shaped data obtained through the partitioning of the to-be-classified electrocardio data with waveform data, corresponding to cardiac beat, in the electrocardio data stored in the database, comparing the similarity with a similarity threshold value, and outputting the comparison results. The device comprises the normal electrocardio database, and a plurality of modules which are used for achieving the above steps. The device can achieve the reliable classification and screening of the electrocardiogram which is not diagnosed, and avoids false negative.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
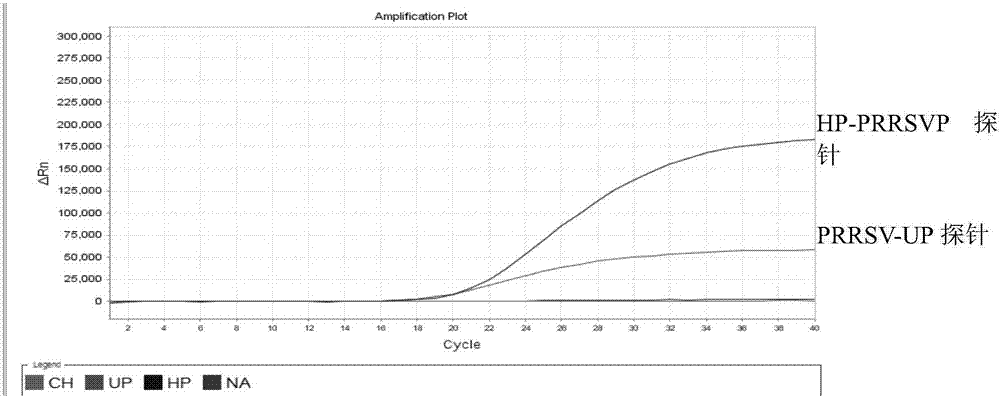
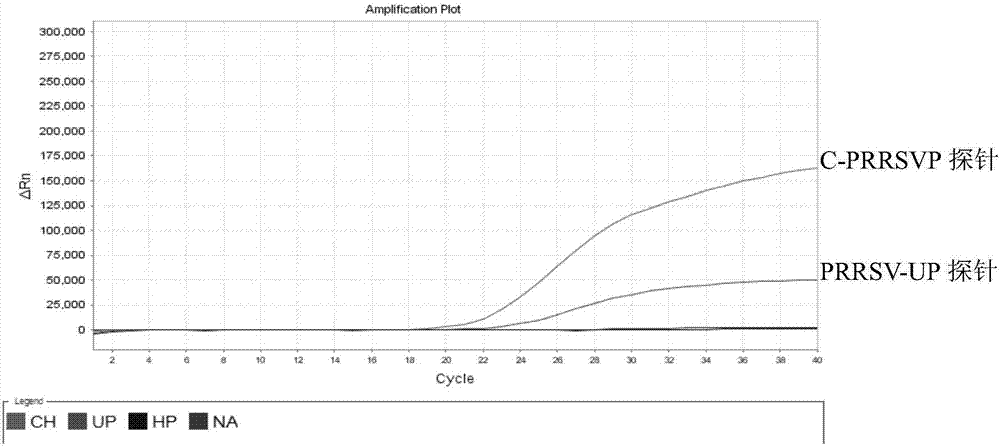
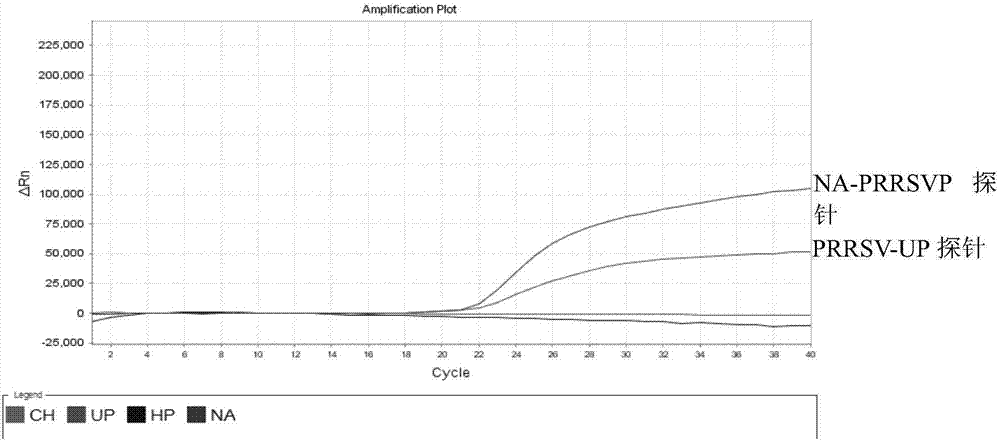
Detection method for identifying American classical PRRSV strain, HP-PRRSV strain and new-type viral NADC30 strain at the same time
ActiveCN107475459AAvoid missing detectionAvoid false negativesMicrobiological testing/measurementMicroorganism based processesHighly pathogenicFluorescence
The invention discloses a detection method for identifying the American classical PRRSV strain, the HP-PRRSV strain and a new-type viral NADC30 strain at the same time. In the method, a multiplex real-time fluorescence RT-PCR for identifying three kinds of strains at the same time is established based on an ABI7500 fluorescent quantitative PCR instrument, wherein the strains include the classical PRRSV strain, the highly-pathogenic HP-PRRSV strain with the deletion of L-amino acid at the 481st locus of the Nsp2 gene and the continuous deletion of 29 amino acids at the 533ird-561st loci of the Nsp2 gene, and NADC30like; specific primers and specific probes for the three kinds of strains are designed respectively based on the Nsp2 gene, universal primers and universal probes for PRRSV are designed according to the relatively conserved N gene, missing detection caused by Nsp2 region mutation can be effectively prevented, and false negative can be avoided.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
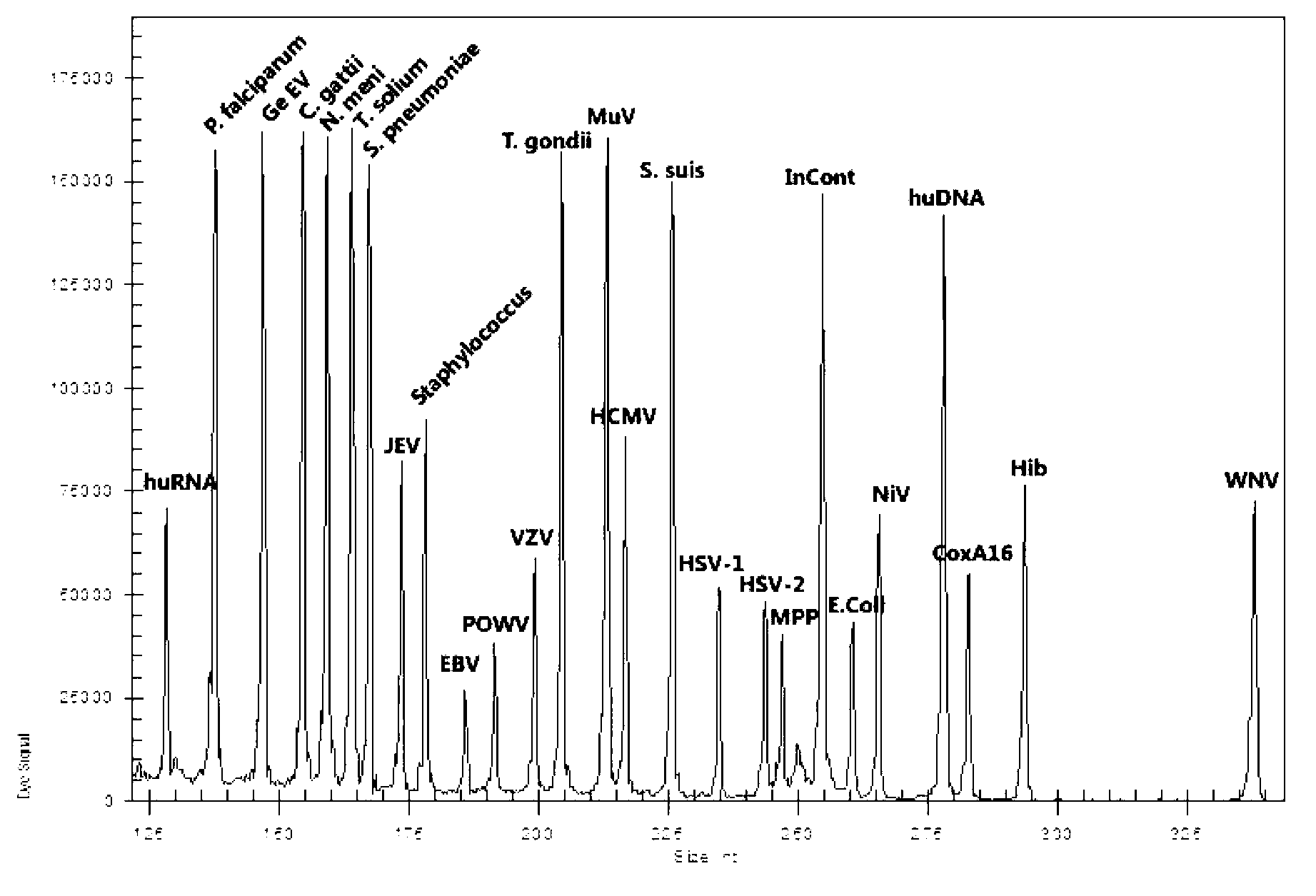
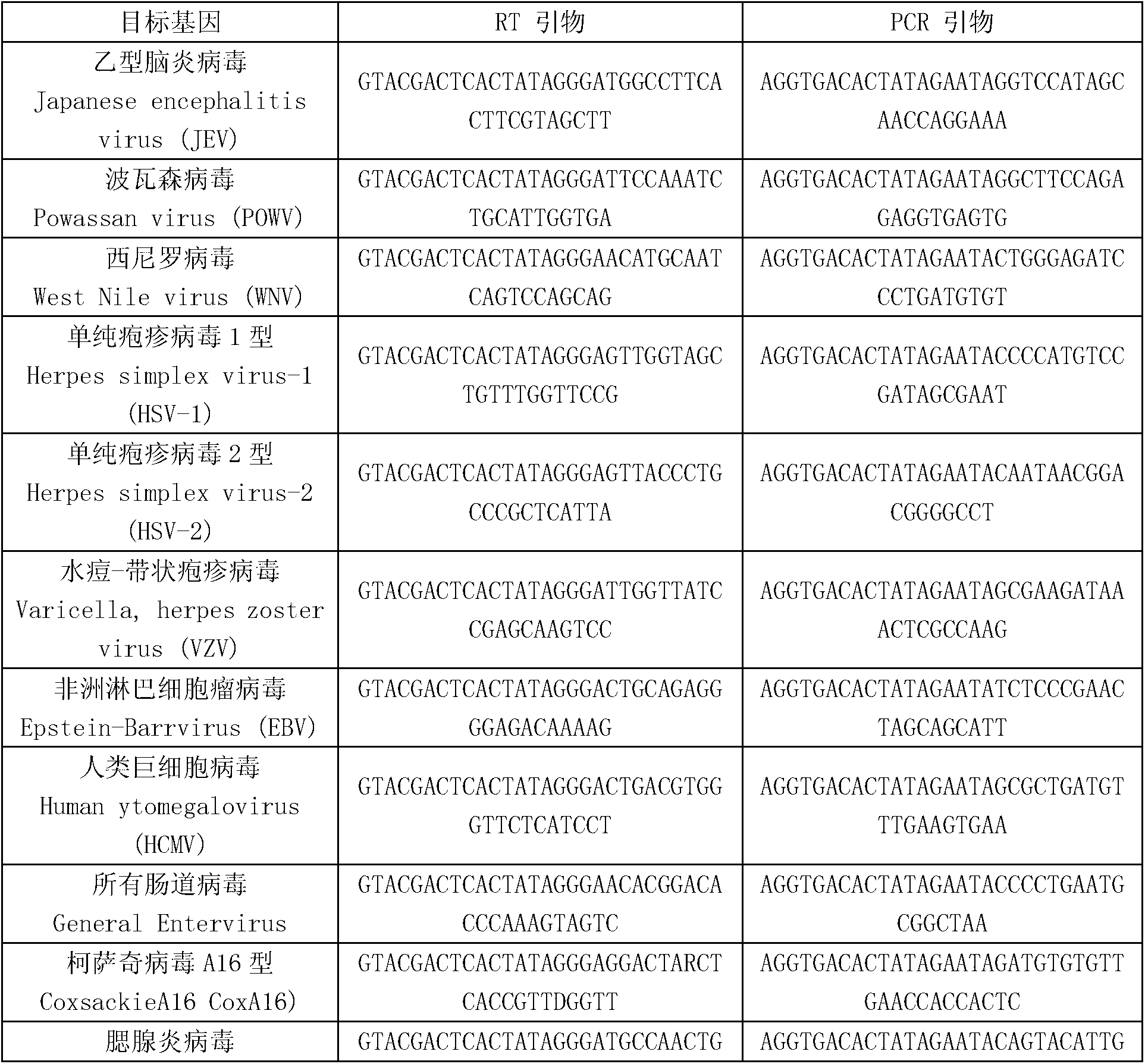
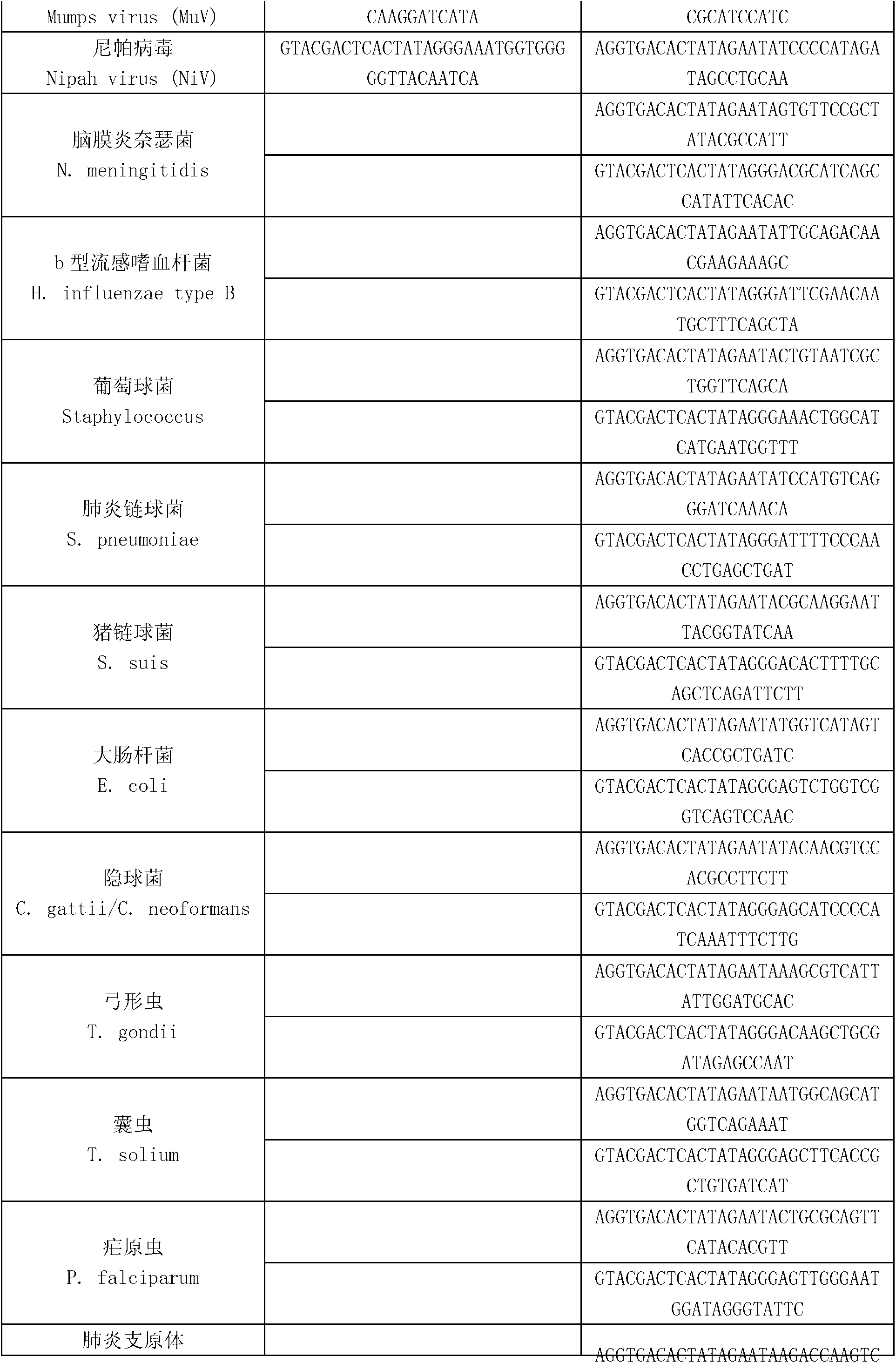
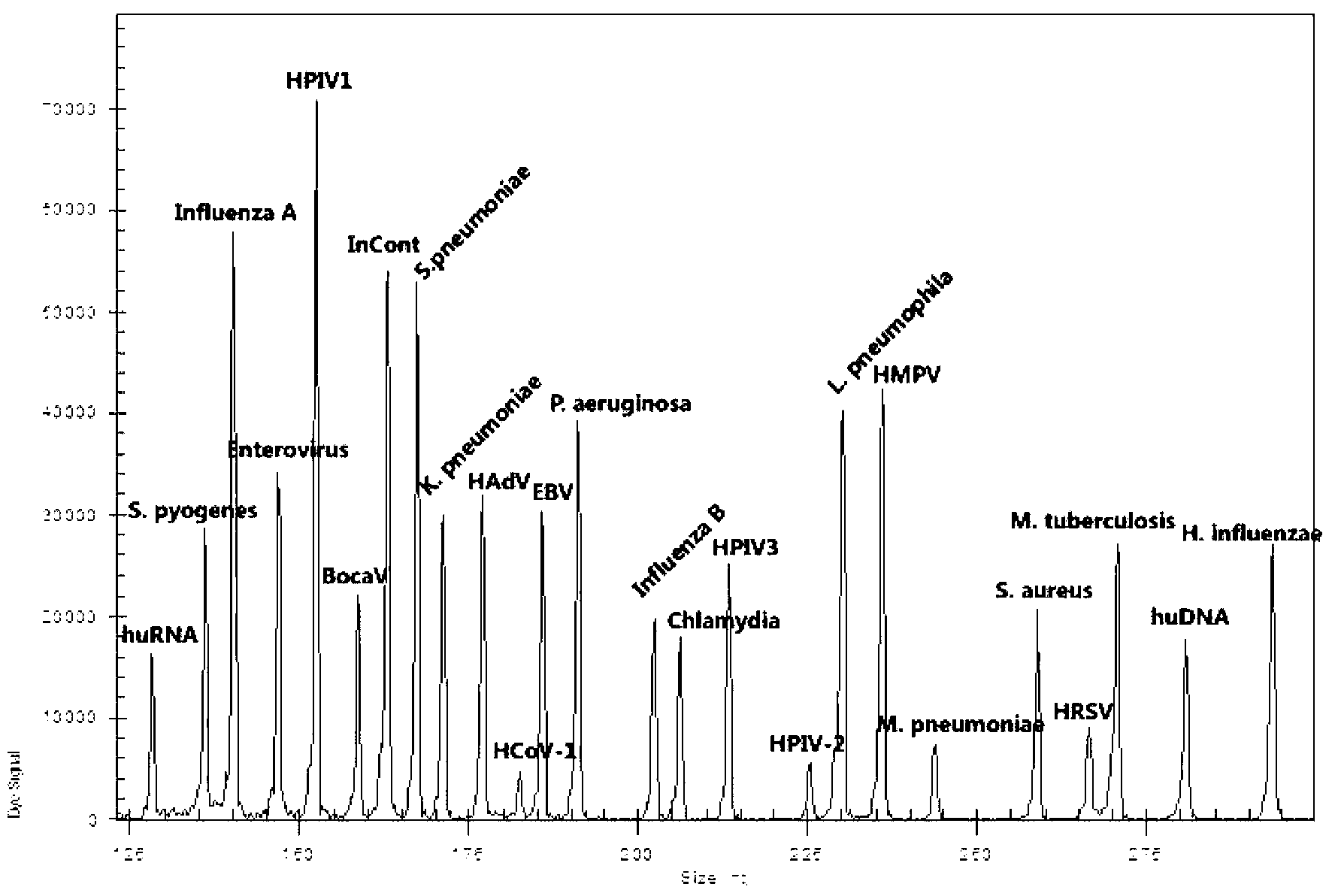
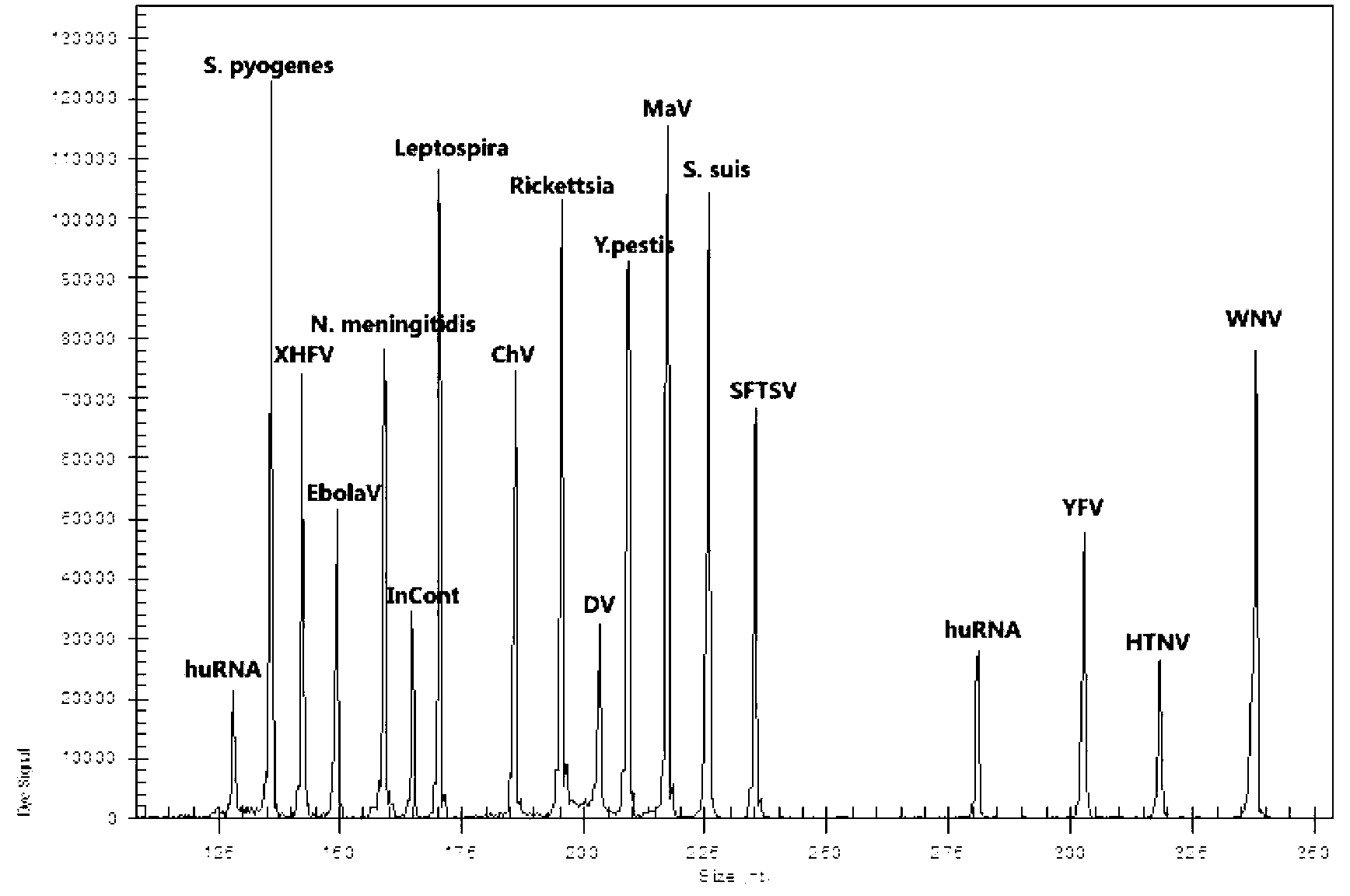
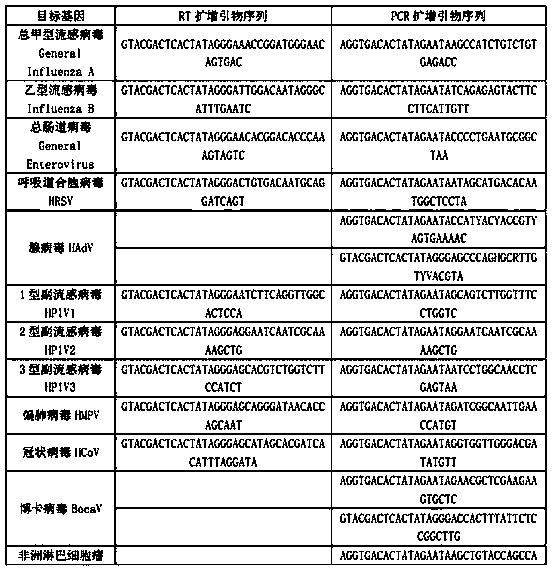
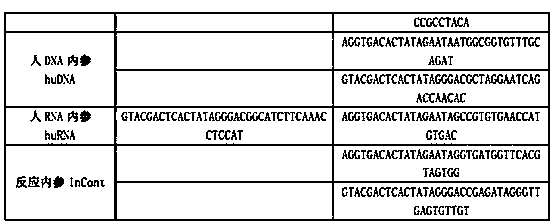
Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
ActiveCN103074448AEnsuring Quality JudgmentsStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-three meningitis pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the twelve meningitis pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-13 (sequence identifier number 1-13), and the PCR primer comprises forward and reverse PCR amplification primers of the rest eleven meningitis pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the twelve meningitis pathogens and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 14-52. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
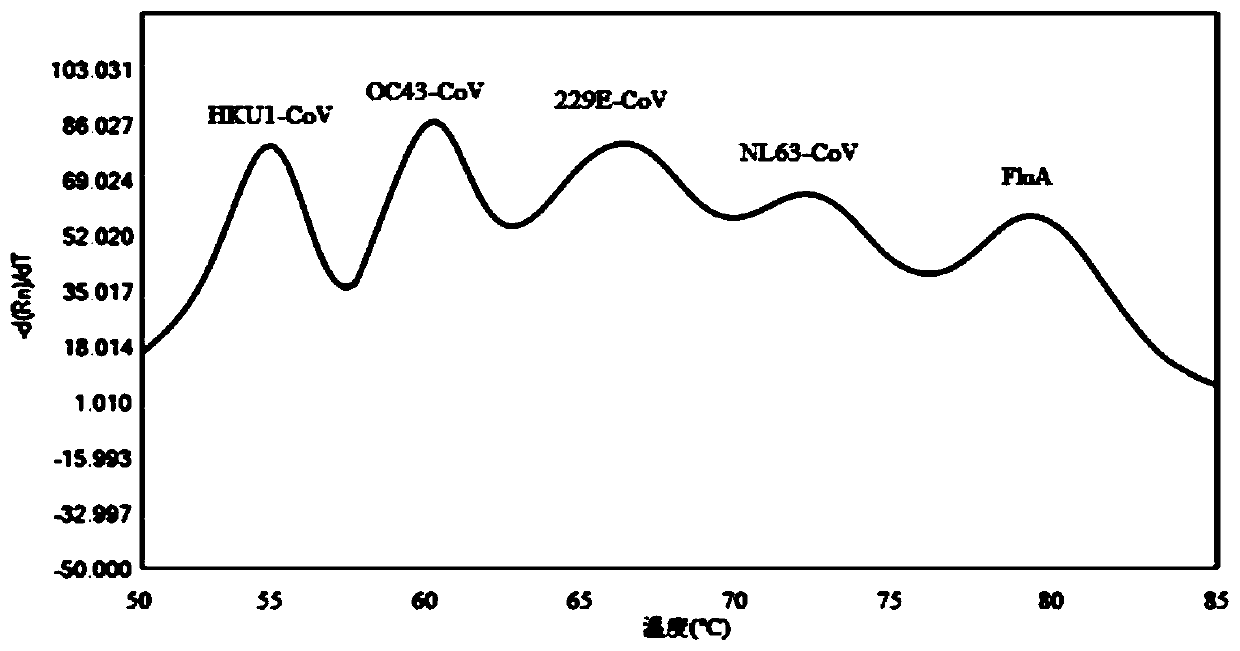
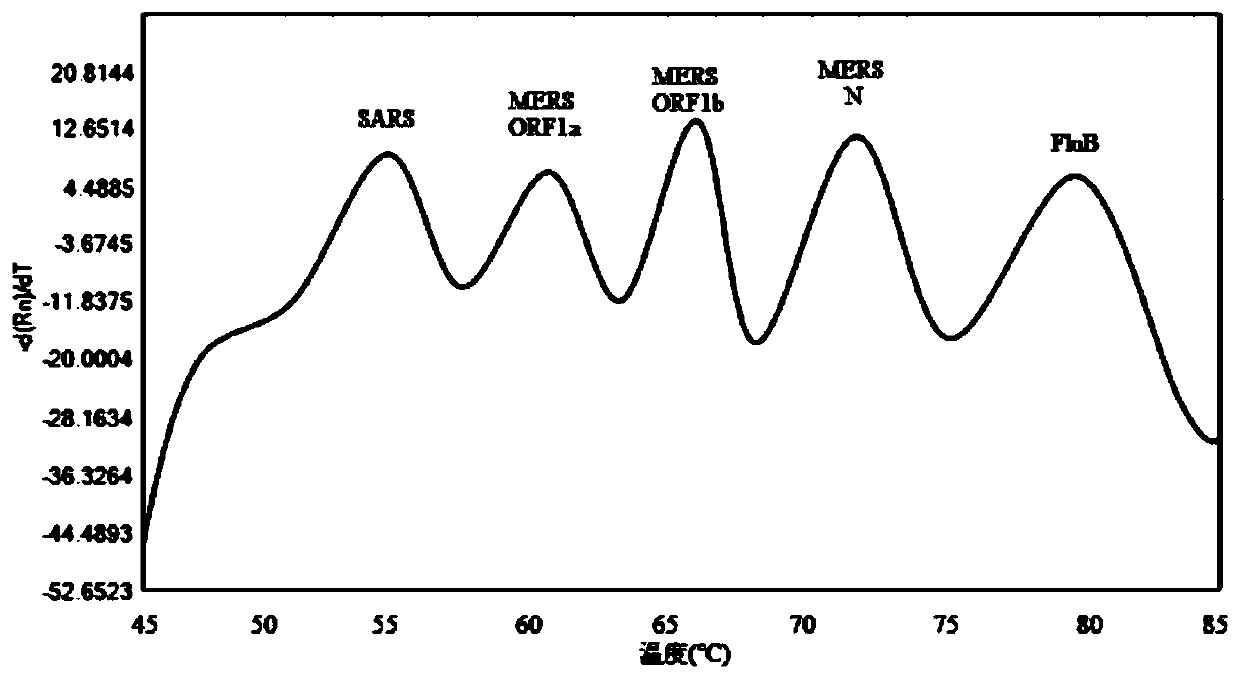
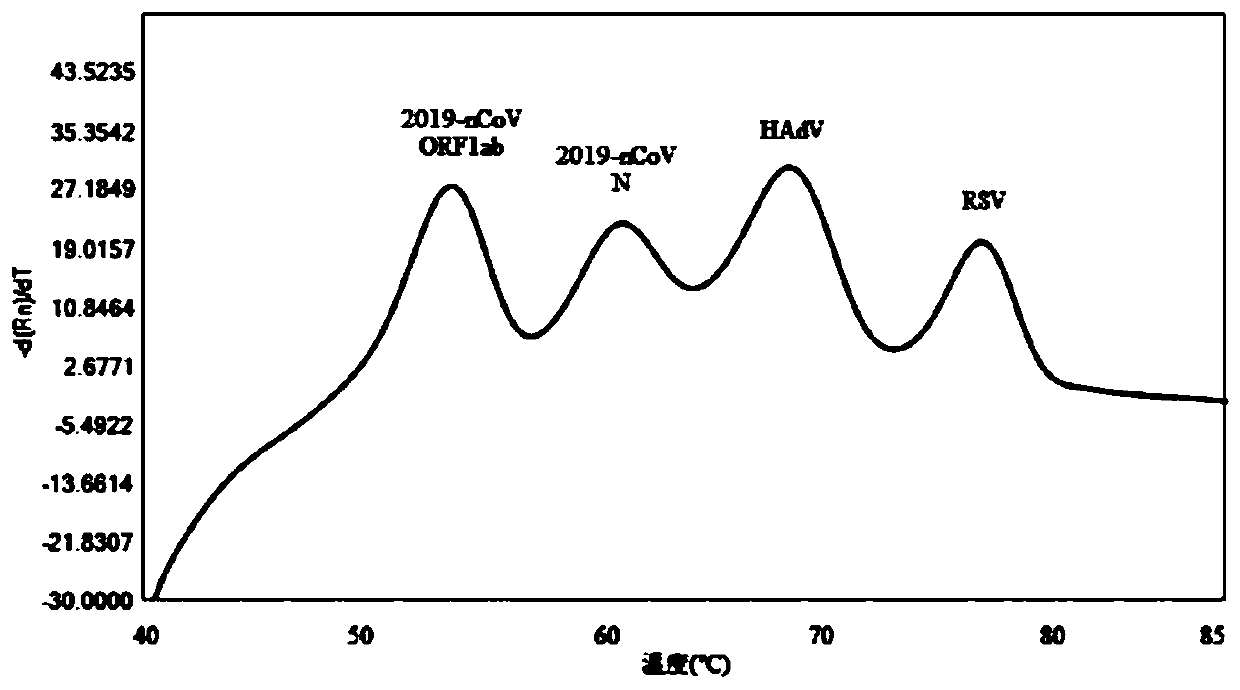
Probe and primer composition for rapidly detecting seven coronaviruses and other respiratory tract pathogens
PendingCN111440897AAvoid false negativesRealize detectionMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory pathogensMgb probe
The invention discloses a probe and primer composition for rapidly detecting seven coronaviruses and other respiratory tract pathogens. A group of specific primers and the Taqman or MGB probe are designed mainly according to the specificity of N genes of OC43-CoV, NL63-CoV, 229E-CoV, HKU1-CoV, SARS-CoV, FluB and RSV, ORF1a, ORF1b and N genes of MERS-CoV, ORF1ab and N genes of 2019-nCoV, an M geneof FluA and an HEXON gene of HadV, and by utilizing a melting curve technology, CoV-OC43, HCoV-NL63, HCoV-229E, HCoV-HKU1, SARS-CoV, MERS-CoV, 2019-nCoV, FluA, FluB, HAdV and RSV are rapidly identified according to the change of the melting temperature. By means of the design, multiple pathogens can be detected in one fluorescent channel at the same time, and a relatively high sensitivity can be met.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
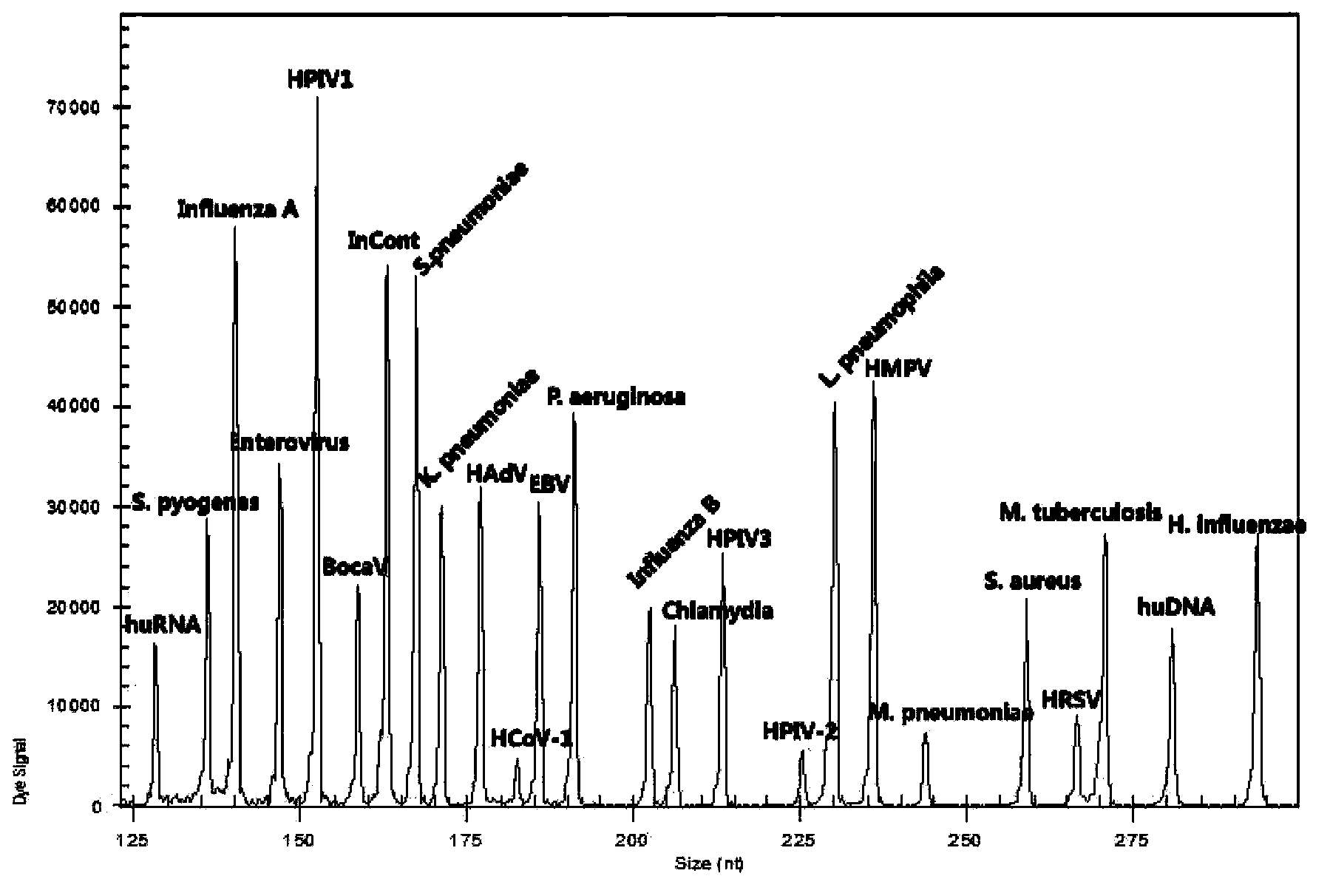
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Kit for quantum dot nucleic acid detection of urinary tract infection-causing pathogens
ActiveCN108660189AImprove throughputHigh sensitivityMicrobiological testing/measurementBiotin-streptavidin complexUpper urinary tract infection
The invention relates to the technical field of biological medicines, in particular to a kit for quantum dot nucleic acid detection of urinary tract infection-causing pathogens. The kit comprises a detection membrane strip, a fluorescent detection solution and reaction solutions, wherein the detection membrane strip comprises a nylon membrane and a capture probe fixed to the nylon membrane; the fluorescent detection solution comprises quantum dots for marking the capture probe and coupled with streptavidin on the surfaces; the reaction solutions include a reaction solution I, a reaction solution II, a reaction solution III and a reaction solution IV. The kit has the beneficial effects as follows: the high-throughput, high-sensitivity and high-specificity kit for the quantum dot nucleic acid detection of the urinary tract infection-causing pathogens is provided; the kit has fewer steps and obviously shorter detection time than the existing colorimetric gene chip, has lower equipment cost than an organic fluorescent gene chip, and is conducive to clinical popularization.
Owner:杭州千基生物科技有限公司 +1
Kit and device for detecting aneuploidy of chromosomes
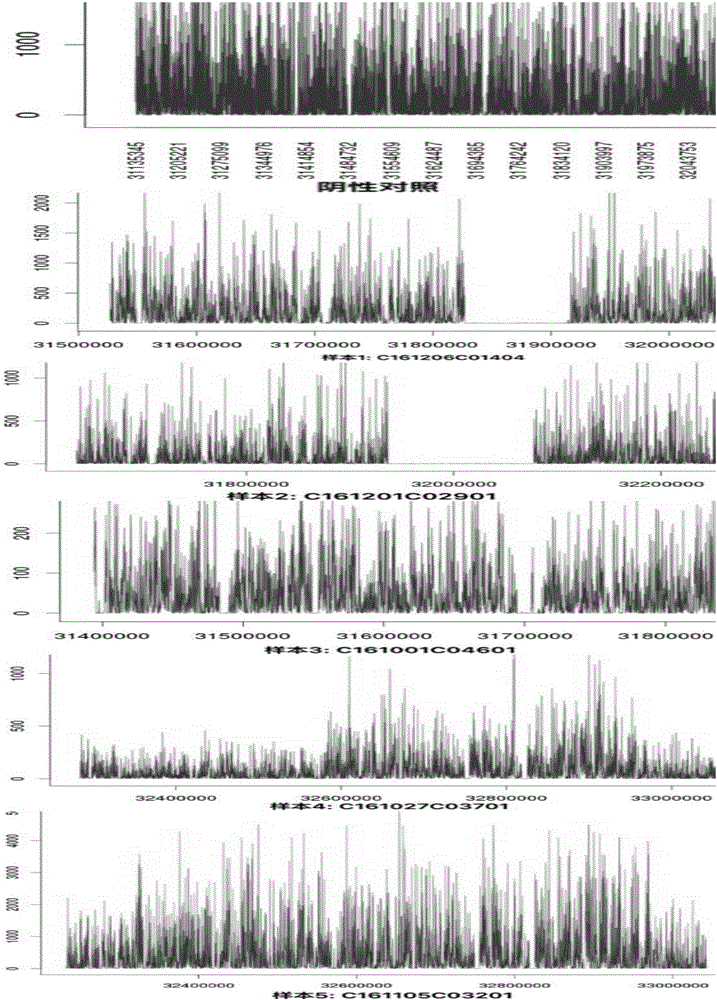
ActiveCN104789466AAvoid false negativesConfirm the result is accurateBioreactor/fermenter combinationsBiological substance pretreatmentsSingle sequenceComputer science
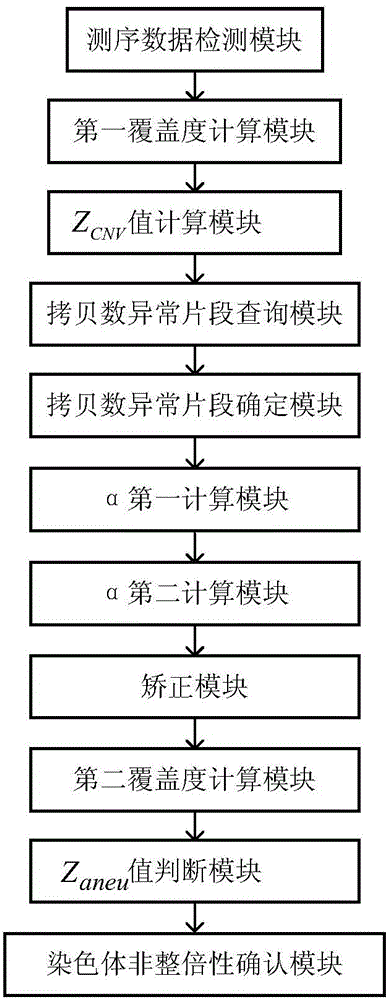
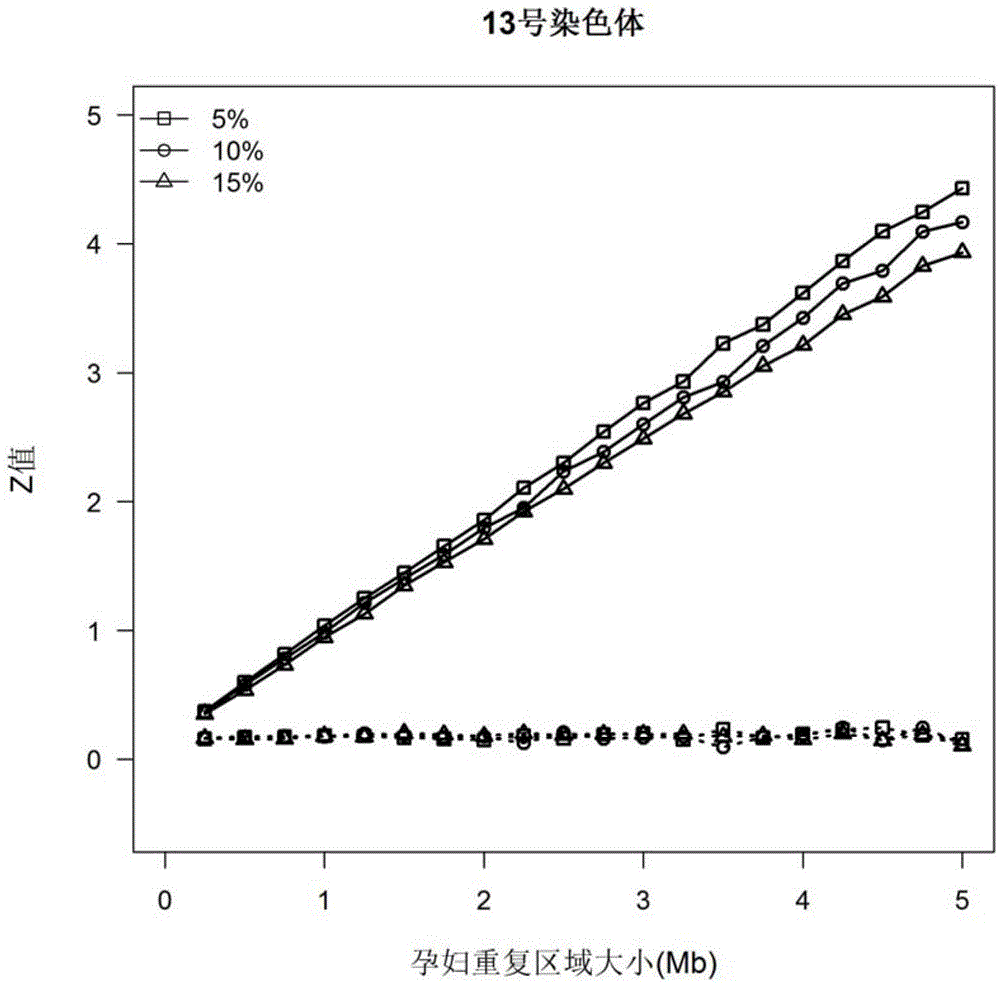
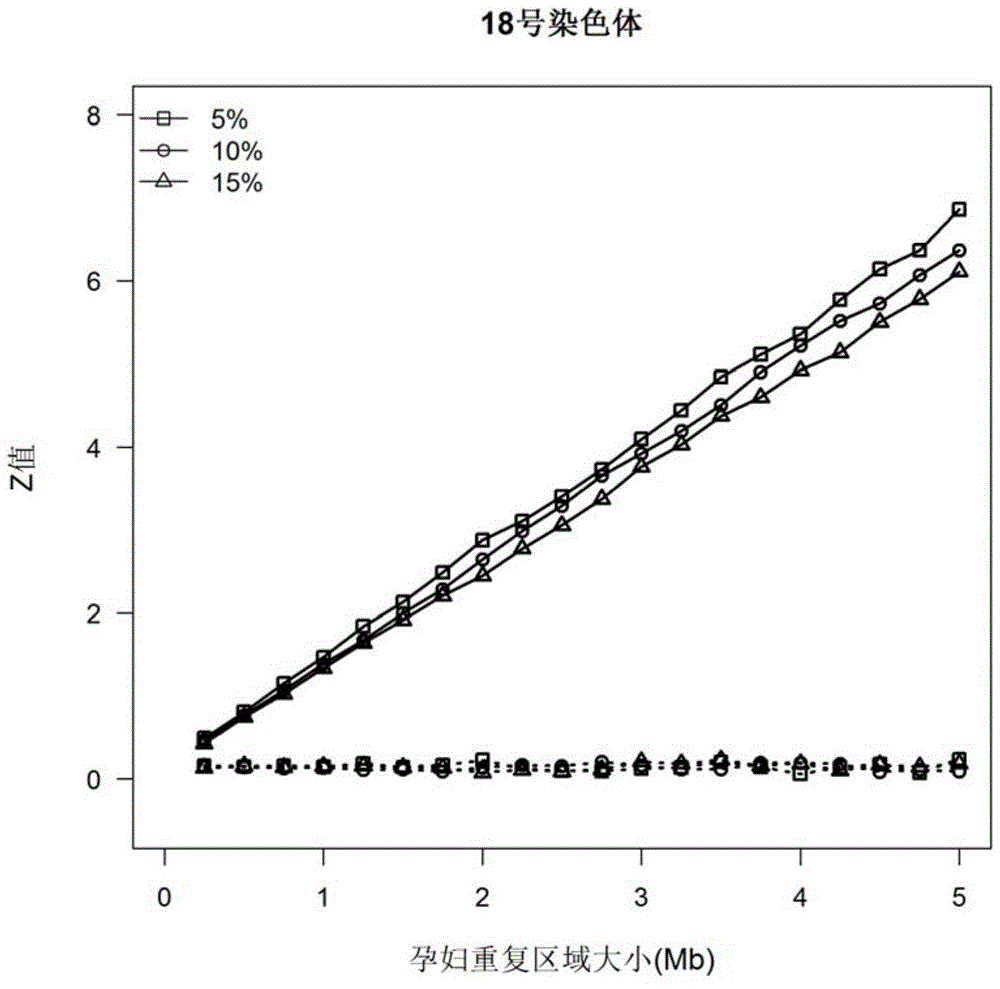
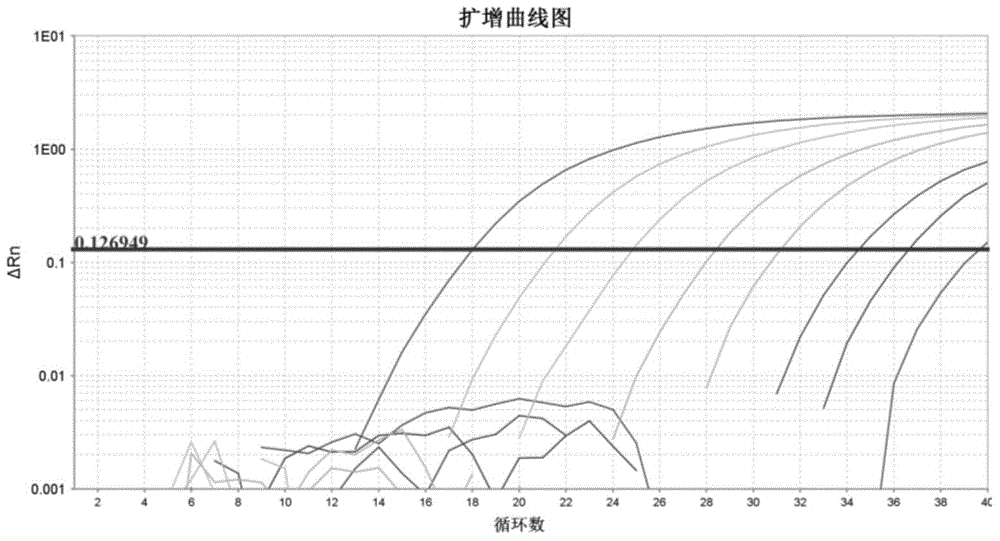
The invention discloses a kit and device for detecting aneuploidy of chromosomes. The device comprises a sequenced data detection module, a first coverage calculation module, a ZCNV value calculation module, a copy number variation fragment query module, a copy number variation fragment determination module, a first alpha calculation module, a second alpha calculation module, a correction module, a second coverage calculation module, a Zaneu value judgment module and a chromosome aneuploidy confirmation module, wherein the sequenced data detection module is used for obtaining sequenced data for all the chromosomes; the first coverage calculation module is used for obtaining uncorrected coverage of each chromosome; the ZCNV value calculation module is used for calculating a ZCNV value of a single sequence number of each window; the copy number variation fragment query module is used for querying fragments, of which the ZCNV value of over 80% of windows is greater than or equal to 4 or smaller than or equal to -4, with over 300Kb in the sequenced data; the copy number variation fragment determination module is used for determining copy number variation fragments of a pregnant woman to be detected; the first alpha calculation module is used for calculating a parameter alpha according to a formula (1); the second alpha calculation module is used for calculating the parameter alpha according to a formula (2); the correction module is used for correcting the uncorrected coverage so as to obtain corrected coverage; the second coverage calculation module is used for calculating a Zaneu value of each chromosome; the Zaneu value judgment module is used for judging whether the Zaneu value is not smaller than 3 or not; the chromosome aneuploidy confirmation module is used for determining that the chromosomes have aneuploidy. The detection is more accurate.
Owner:ANNOROAD GENE TECH BEIJING +3
Primer, kit and method for detecting CHO cell DNA residues
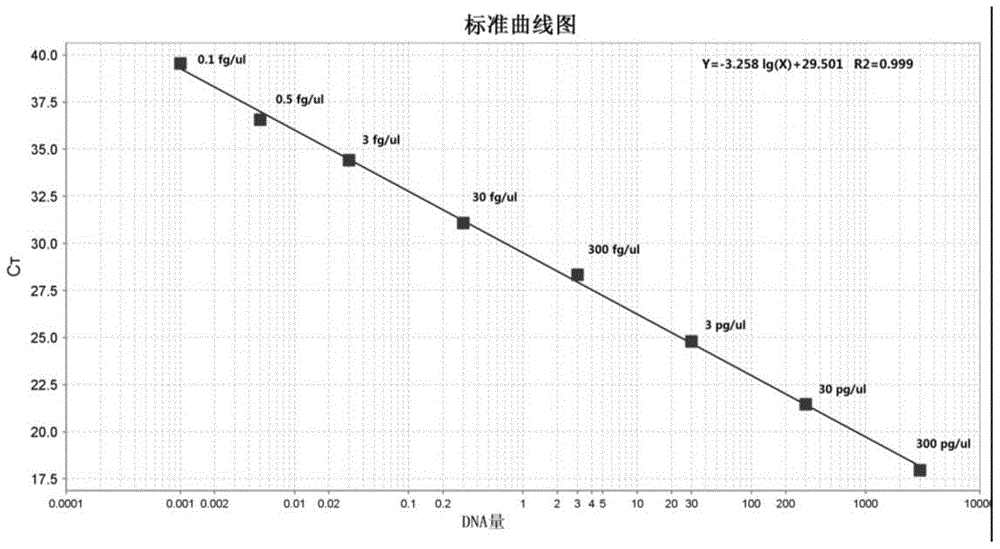
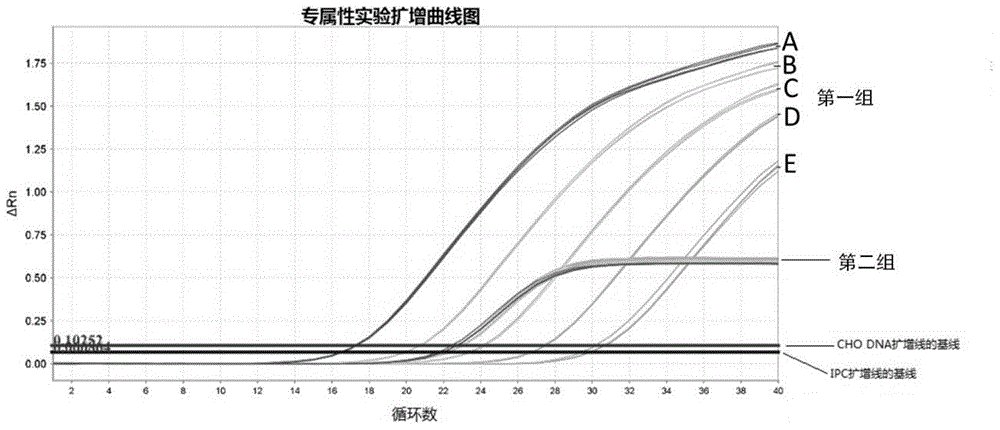
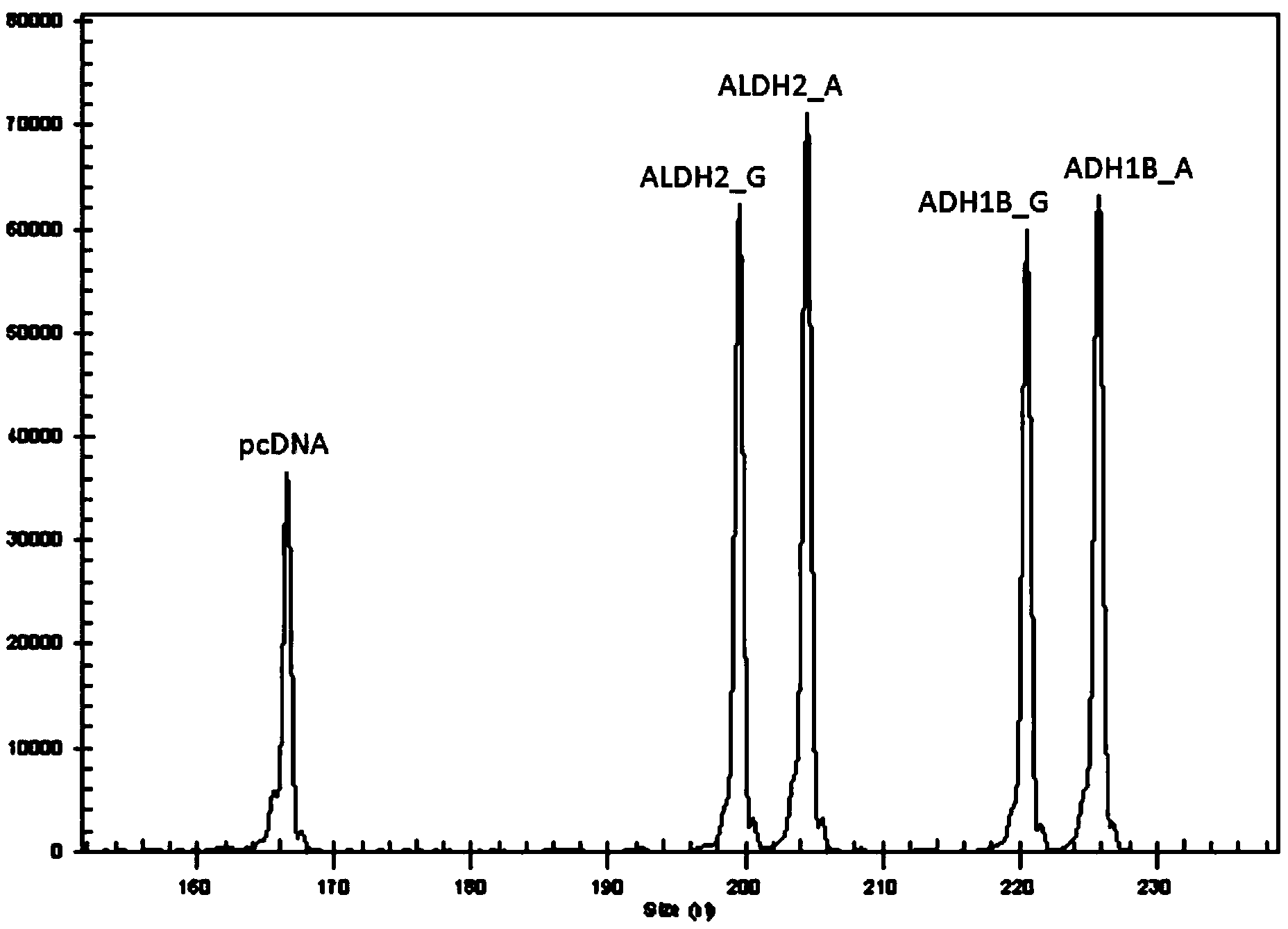
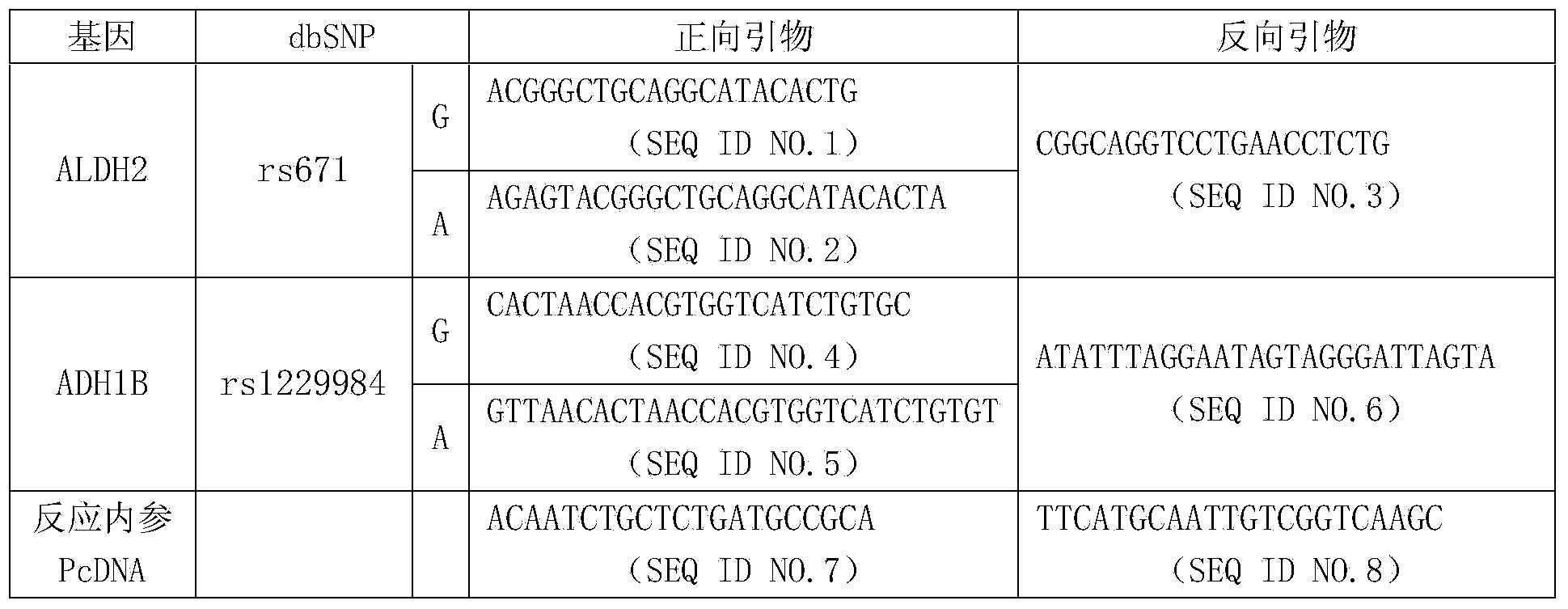
InactiveCN105861641AImprove accuracyAvoid false negativesMicrobiological testing/measurementDNA/RNA fragmentationTherapeutic proteinNucleotide
The present invention provides a primer pair for detecting CHO cell DNA residues. The invention uses a nucleotide sequence with GenBank accession number J00052.1 for design, provides a Taqman probe based on the nucleotide sequence, and a corresponding detection method. The method is a real-time PCR method; and based on an obtained standard curve, the DNA amount in CHO cells in a sample to be tested is calculated. The detection method can be used to detect bioproteins using CHO cells as an expression cell line in genetic engineering, such as antibodies, therapeutic proteins and vaccines. The real-time PCR method for detecting DNA of CHO cells and a PCR kit based on the method achieve limit of quantitation as low as 0.1fg / mul for the detection of DNA residues in CHO cells, which shows that the method has good sensitivity and high specificity.
Owner:LIVZON MABPHARM
Primer composition for guiding nitroglycerin medication and healthy drinking, multiple gene detection kit and use method of kit
ActiveCN103849681ASave production costSave testing costMicrobiological testing/measurementCapillary electrophoresisGene type
The invention discloses a primer composition for guiding nitroglycerin medication and healthy drinking, a multiple gene detection kit and a use method of the kit, and the kit comprises the primer composition, a PCR buffer solution and a positive reference substance, and the PCR buffer solution comprises ultrapure water, an X solution, a 10*PCR (polymerase chain reaction) buffer solution, a PCR primer, a 25mM magnesium chloride solution and DNA polymerase, the primer composition comprise two forward and reverse amplification primers of different gene types on the 2 SNP sites of genes related to the nitroglycerin medication and healthy drinking and forward and reverse amplification primers capable of reflecting internal reference, the gene sequences of the primers are represented as SEQ ID NO.1-NO.8; the use method comprises the step of acquiring a sample and extracting nucleic acid, the step of performing the PCR reaction by using extracted nucleic acid as a template, and the final step of separating the sample through capillary electrophoresis. The primer composition has the advantages of being strong in specificity, high in accuracy, high in flux, strong in reliability, low in cost and free from false negative result.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
HCG (human chorionic gonadotropin) colloidal gold immunoassay lateral chromatographic test strip and detection method thereof
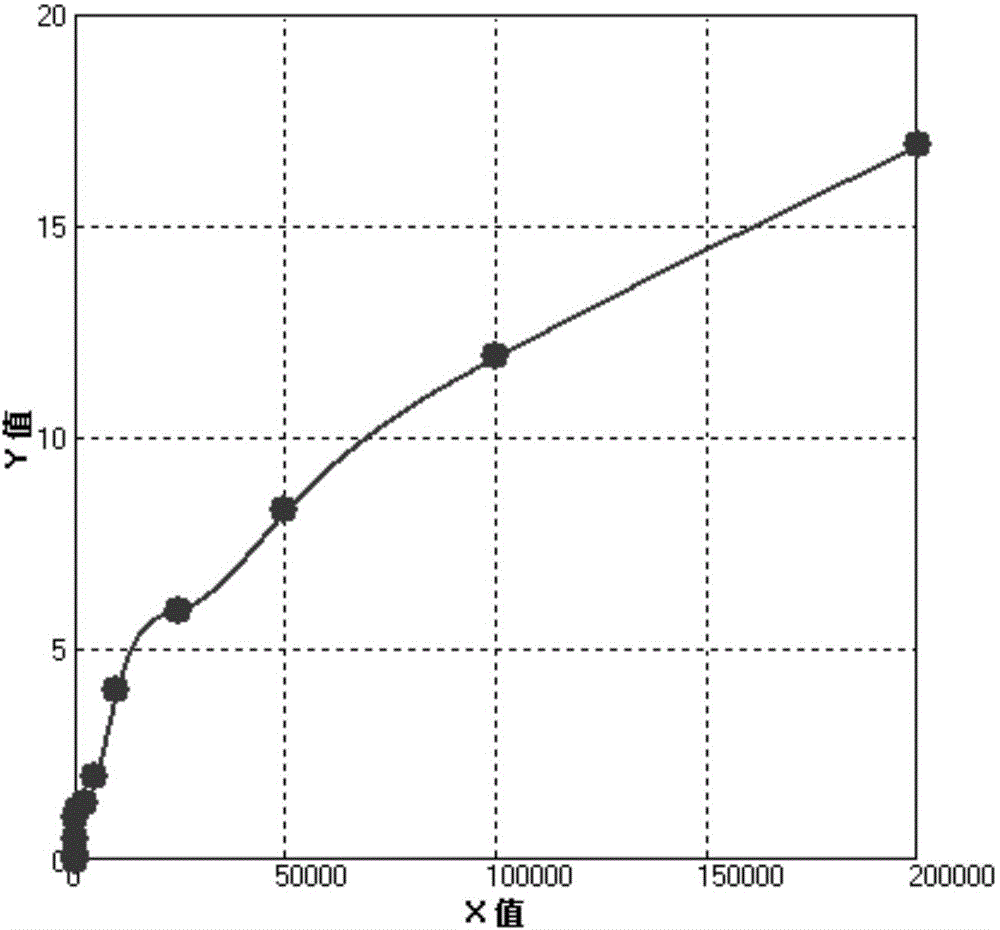
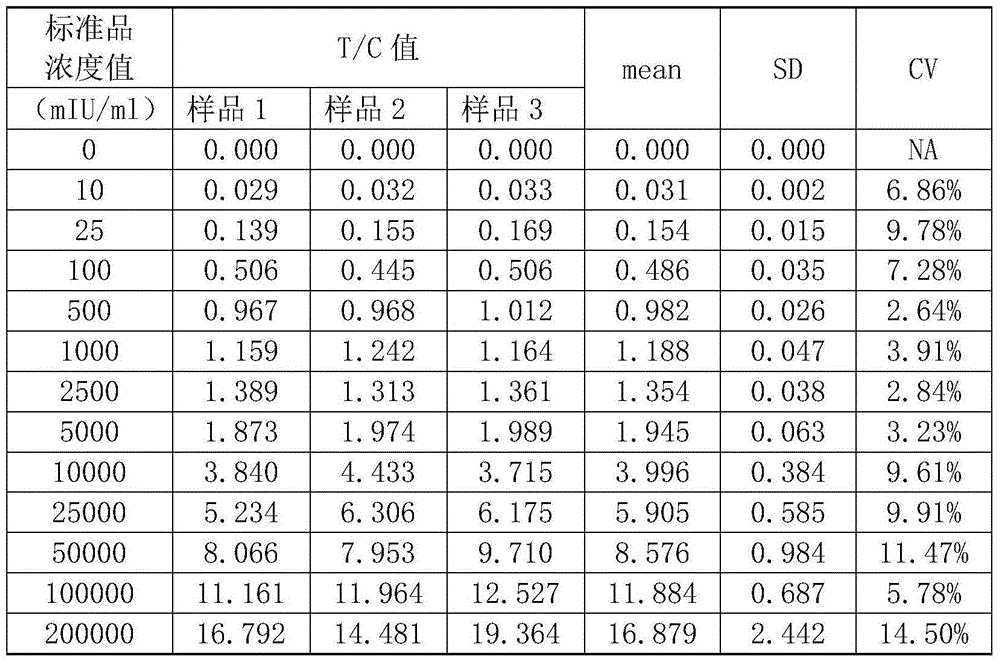
ActiveCN104991078ALower readingImprove linearityBiological material analysisBiological testingHigh concentrationHook effect
The invention belongs to the field of clinical medical diagnosis and particularly relates to an HCG (human chorionic gonadotropin) colloidal gold immunoassay lateral chromatographic test strip and a detection method thereof. The HCG colloidal gold immunoassay lateral chromatographic test strip consists of a nitrocellulose film, a Fusion 5 film and water-sucking paper, wherein a detection line T line and a quality control line C line are arranged on the nitrocellulose film; a T line solution is sprayed onto the detection line T line; a C line solution is sprayed onto the detection line C line. A standard curve line is made through different concentration values and corresponding T / C average value of a standard product, the standard curve line is subjected to interpolation fit by sample strips for three times, the curved line which is relatively good in linearity and completely raises can be obtained, HOOK effect can be overcome, and detection on high-concentration HCG is realized. The detection range is up to 10 to 200,000mIU / ml, a to-be-detected sample is not required to be diluted, the detection process is simple and convenient, and the detection cost is low.
Owner:SHANGHAI UPPER BIO TECH PHARMA
Immunochromatographic test strip, and making method and detection method thereof
ActiveCN104714008ASimple and fast operationSimple structureMaterial analysisHigh concentrationCellulose
The invention provides an immunochromatographic test strip. The test strip comprises a sample pad, a binding pad, a cellulose nitrate membrane, an absorbent pad and a backing pad, the cellulose nitrate membrane comprises a detection line and a quality control line, and the cellulose nitrate membrane also comprises a double antibody line. The double antibody line is arranged between the detection line and the quality control line. The invention also provides a making method and a detection method of the immunochromatographic test strip. The immunochromatographic test strip reserves the advantages of simple operation, simple structure and low cost of traditional immunochromatographic test strips, effectively avoids the false negative phenomenon appearing in the detection of high concentration of a substance to be tested of traditional test strips, effectively improves the detection accuracy of immunochromatography, and enlarges the linear detection range; and the test strip comprehensively utilizes the signal intensities of the above lines in a display area and the relationship among the signal intensities of the three lines not limited to the signal intensity of the detection line, so the test strip can be used to accurately determine the concentration of the substance to be tested, and the sensitivity and the specificity of immunochromatographic detection are greatly improved.
Owner:SHANGHAI JIAO TONG UNIV
Multi-gene detection kit for guiding administration of 5-fluorouracil and detection method of multi-gene detection kit
ActiveCN103074436AImprove detection efficiencyShorten the timeMicrobiological testing/measurementPositive controlPolymerase L
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
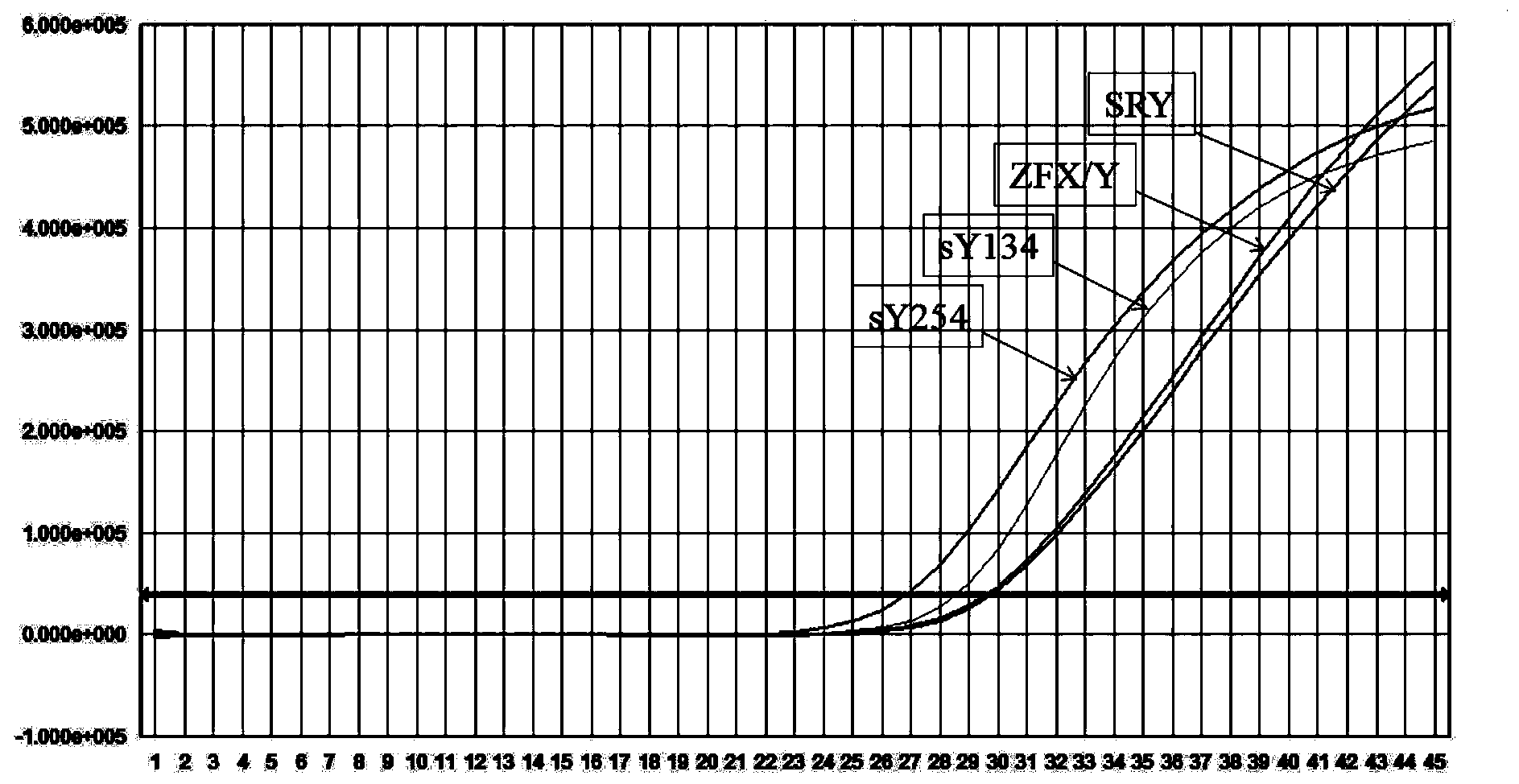
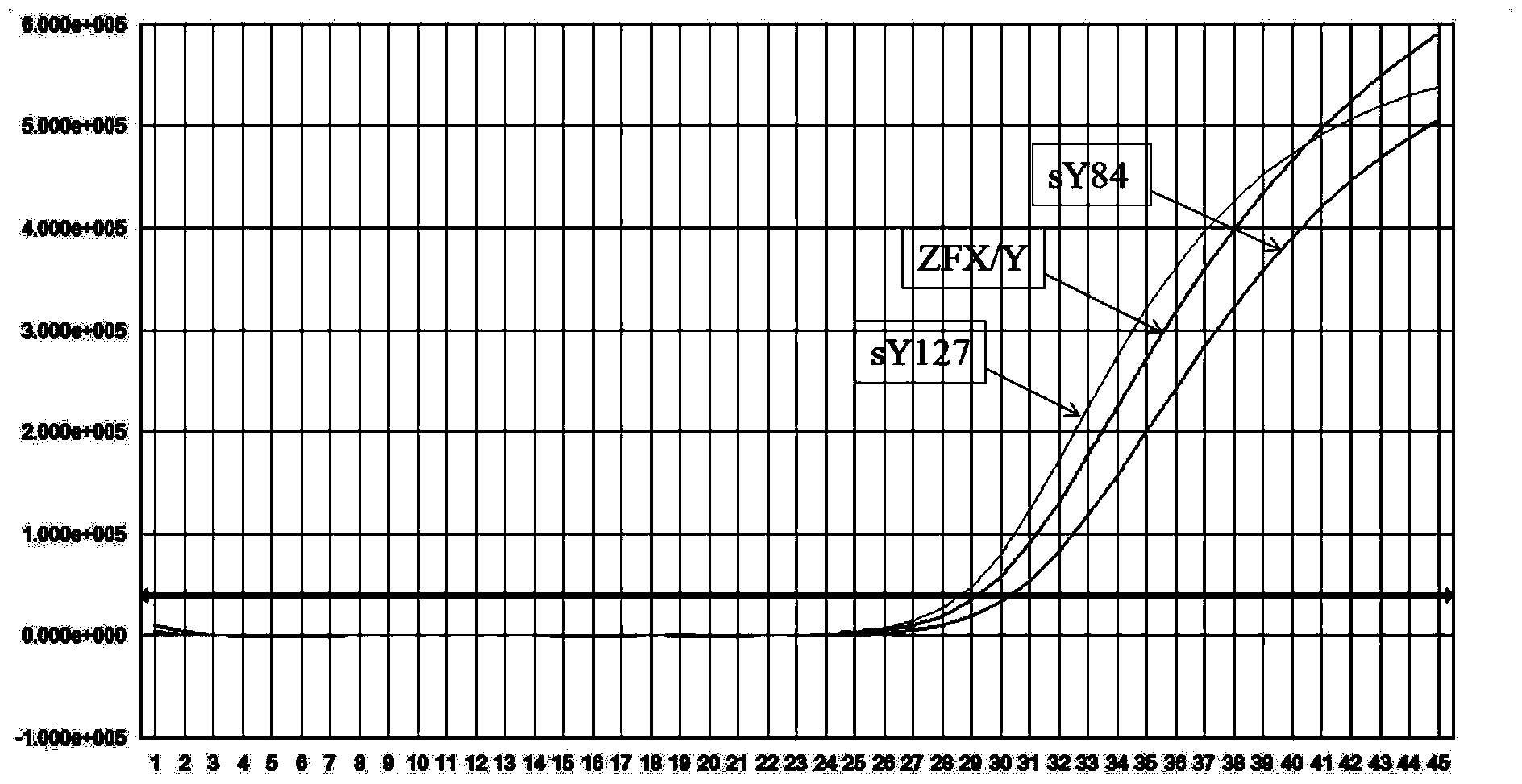
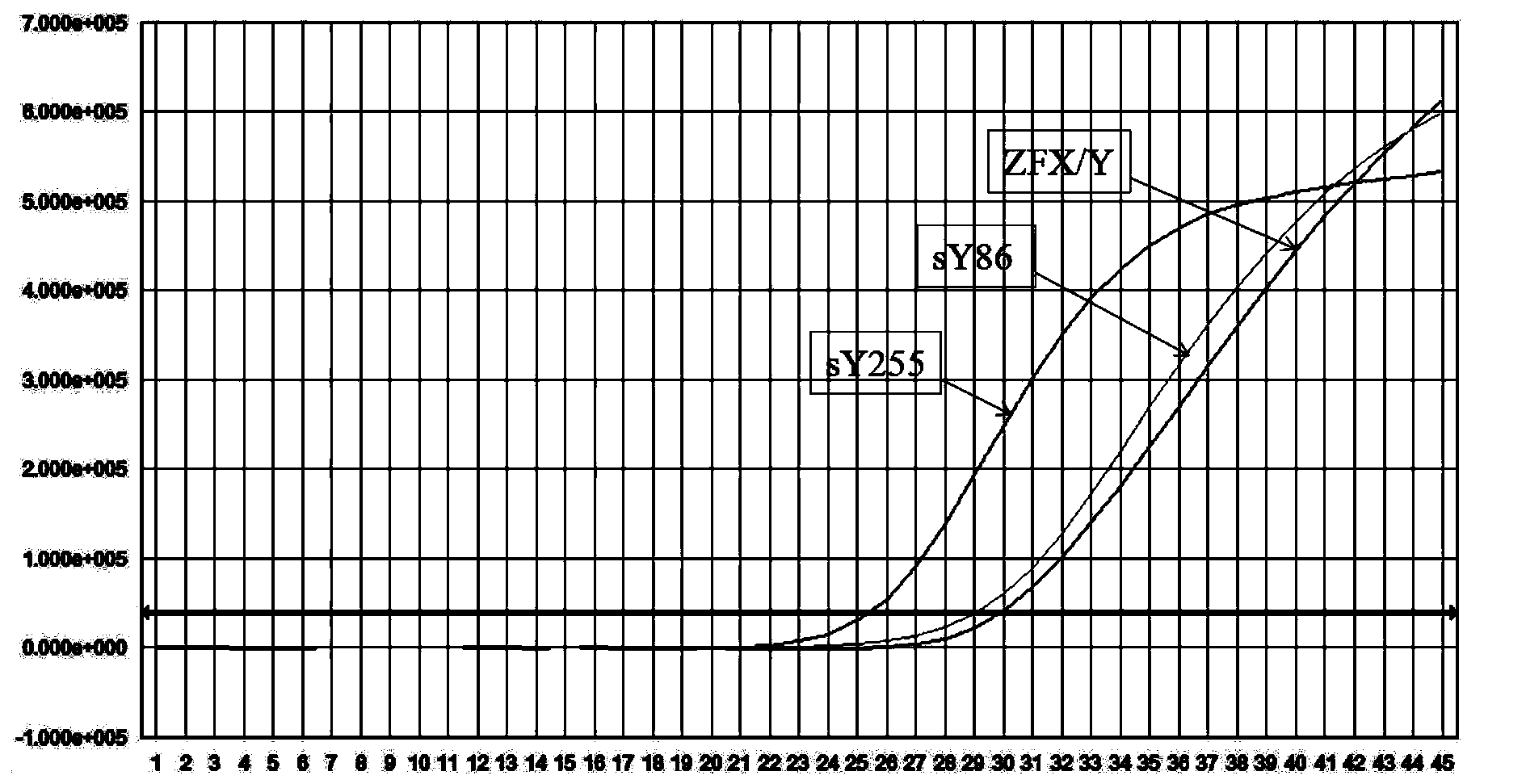
Kit for detecting microdeletion of human Y chromosome
ActiveCN104232779AMonitor stabilityReduce workloadMicrobiological testing/measurementFluorescenceReaction system
The invention relates to the field of chromosome deletion, in particular to a kit for detecting the microdeletion of a human Y chromosome. The kit comprises PCR (Polymerase Chain Reaction) reaction liquid I, PCR reaction liquid II and PCR reaction liquid III, wherein the PCR reaction liquid I comprises primers and fluorescent probes of specific amplification ZFX / Y, sY254, sY134 and SRY (Sex-determine Region of Y Chromosome) sites; the PCR reaction liquid II comprises primers and fluorescent probes of specific amplification ZFX / Y, sY84 and sY127 sites; the PCR reaction liquid III comprises primers and fluorescent probes of specific amplification ZFX / Y, sY255 and sY86 sites. According to the kit disclosed by the invention, by designing the specific fluorescent probes of different STS (Sequence Tagged Site) and three-pipe reaction systems are optimally combined, so that microdeletion types of the Y chromosome can be detected by one-time test; effective monitoring for different reaction systems is realized by the ZFX / Y.
Owner:亚能生物技术(深圳)有限公司
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
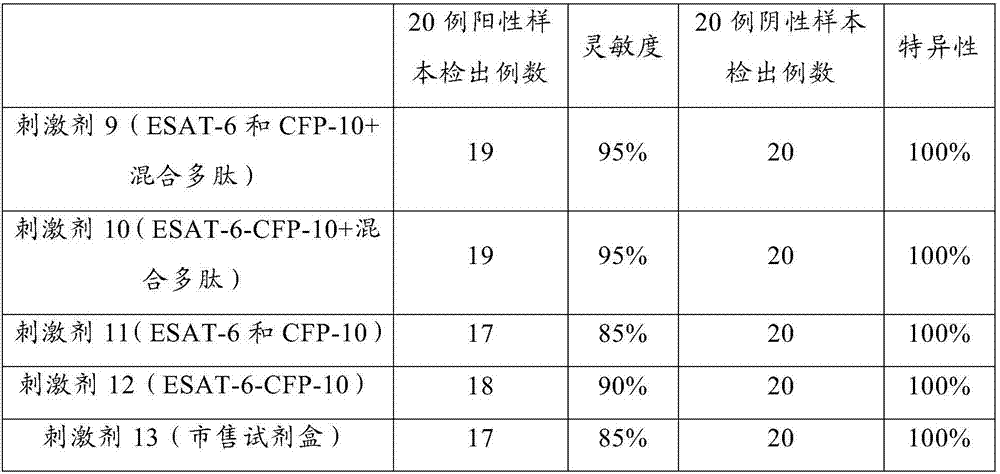
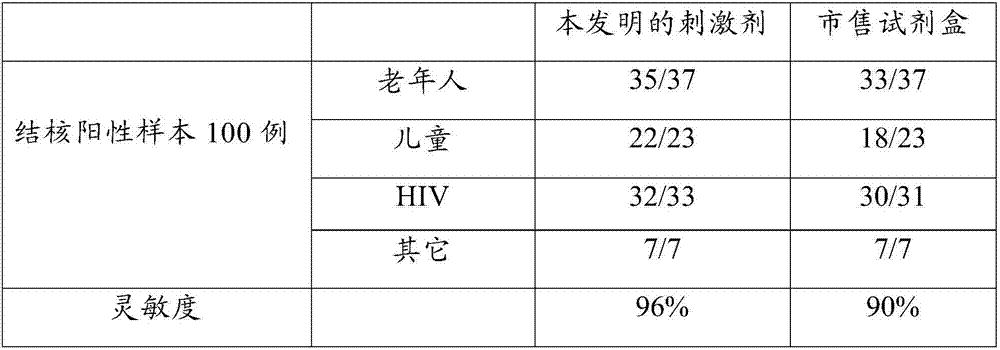
Antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection, and application thereof
ActiveCN107011418AQuick checkSpecific detectionBiological material analysisDepsipeptidesPeripheral blood mononuclear cellMycobacterium Infections
The invention discloses an antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection. The antigenic polypeptide pool specifically stimulates mycobacterium tuberculosis infected fresh whole blood to specifically secrete IFN-gama and increase the sensitivity. The invention provides a new detection reagent for detecting mycobacterium tuberculosis infection, experiments prove that peripheral blood can be directly utilized to conduct antigenic simulation without separation of peripheral blood mononuclear cell, the experimental data shows that the antigenic polypeptide pool has high sensitivity and specificity for detecting mycobacterium tuberculosis infection, and is simple and convenient to operate and low in cost, thus having high clinical application values.
Owner:武汉海吉力生物科技有限公司
Method for detecting ER gene of peripheral blood circulating tumor cells of patient suffering from advanced breast cancer
ActiveCN105510600AAvoid false negativesAvoid difficultiesBiological material analysisBiological testingFiltrationWilms' tumor
The invention discloses a method for detecting an ER gene of peripheral blood circulating tumor cells of a patient suffering from advanced breast cancer. The method comprises the steps of conducting separation with a membrane filtration device, so that CTC in peripheral blood of the patient suffering from advanced breast cancer is obtained; identifying the peripheral blood circulating tumor cells of the patient suffering from advanced breast cancer through the cellular immunofluorescence technique; preparing thin layer sections through the cell paraffin block technique; further detecting the ER expression condition of the peripheral blood circulating tumor cells of the patient suffering from advanced breast cancer through the immunohistochemical technique. According to the method, CTCs are separated and enriched by means of the ISET technique, and the CTCs are identified through the cellular immunofluorescence technique, so that the difficulty existing in CTCs identification conducted through the pure ISET technique and false negativeness existing in CTCs identification conducted through the pure immunological detection technique are overcome. The technique is not high in equipment requirement, the method is easy to grasp, and real-time monitoring can be conducted. By means of the technical method, the ER expression condition of the patient suffering from advanced breast cancer can be detected without picking breast cancer tissues.
Owner:山东发现生物技术有限公司
Antigen polypeptide pool for detecting Mycobacterium tuberculosis infection
ActiveCN107216373AImprove the detection rateHigh sensitivityBiological material analysisDepsipeptidesIfn gammaTuberculoma
The invention discloses an antigen polypeptide pool for detecting Mycobacterium tuberculosis infection. The specific antigen polypeptide pool can specifically stimulate Mycobacterium-tuberculosis-infected fresh whole blood and specifically secrete IFN-gamma, thereby increasing the detection sensitivity. The invention provides a novel detection reagent applicable to detection of Mycobacterium tuberculosis infection. The experiment proves that the peripheral blood can be directly utilized to perform antigenic stimulation without the need for separating peripheral blood mononuclear cells. The experimental data shows that when being used for detecting Mycobacterium tuberculosis infection, the antigen polypeptide pool has the advantages of higher sensitivity, higher specificity and lower cost, is simple to operate, and has higher clinical application value.
Owner:武汉海吉力生物科技有限公司
Detection kit of nucleic acid of klebsiella pneumoniae KPC (Klebsiella Pneumoniae Carbapenmase) type carbapenemases gene
InactiveCN103088144APrimer probe concentration optimizationFormamide Concentration OptimizationMicrobiological testing/measurementMicroorganism based processesFluorescencePneumonia klebsiella
The invention relates to a blaKPC (Klebsiella Pneumoniae Carbapenmase) gene detection kit, and particularly to a kit for detecting klebsiella pneumoniae KPC type carbapenemases (blaKPC) gene by utilizing a composite probe technology and a real-time fluorescence PCR (Polymerase Chain Reaction) technology. Through qualitative detection for the blaKPC gene in a sample, the kit can be widely applied to auxiliary diagnosis of bacterial infection including the blaKPC gene.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Kit for detecting FLT3-ITD (Fms-like tyrosine kinase 3-internal tandem duplication) gene mutation by using fluorescence PCR (Polymerase Chain Reaction) capillary electrophoresis
InactiveCN103103251AAvoid false negativesAvoid false positivesMicrobiological testing/measurementFms-Like Tyrosine Kinase 3Positive control
The invention relates to a kit for detecting ITD (internal tandem duplication) mutation in an FLT3 (Fms-like tyrosine kinase 3) gene juxtamembrane domain and in particular relates to a kit for detecting the FLT3-ITD in a clinical sample by using a fluorescence PCR (Polymerase Chain Reaction) capillary electrophoresis technology. The kit mainly comprises a PCR amplified reaction solution, a negative control product, a positive control product, a plurality of sealed reagent bottles or pipes which cover the kit as well as packaging boxes which are used for separating and concentratively packaging the reagent bottles or pipes, and the size and gene dosage of each amplified fragment are determined by using an electrophoresis method. The kit disclosed by the invention has high sensitivity and specificity in terms of detection and analysis of the FLT3-ITD in a whole blood or marrow sample and can be widely applied to the fields of judgment prognosis and guide treatment of leukemia and resurgence prevention of diseases.
Owner:DAAN GENE CO LTD
Hepatitis B virus nucleic acid quantitative detection method and kit
ActiveCN103184295AAvoid lossAvoid false negativesMicrobiological testing/measurementFluorescence/phosphorescenceMagnetic beadEnzyme system
The invention relates to a hepatitis B virus (HBV) nucleic acid quantitative detection method and a kit. The method includes: employing a magnetic bead technique to extract HBV and internal reference nucleic acid from a sample to serve as a template, and finishing HBV nucleic acid quantitative detection based on a fluorescent PCR technology. The introduced internal reference can be used for monitoring the loss and misoperation of the nucleic acid template or existent PCR inhibitors during operation, thus avoiding a false-negative result. An adopted dUTP-UNG enzyme system can reduce cross contamination of amplification products and prevents a false-positive result. The kit includes a nucleic acid extraction reagent, an amplification reagent, quantitative calibration substances, reference substances and the internal reference. The method and the kit solve the shortcomings of poor detection accuracy and low sensitivity in the prior art, and are suitable for application in clinical HBV nucleic acid quantitative detection.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451BEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Reagent kit of detecting human immunodeficiency virus type 1 by fluorescence quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction)
InactiveCN103045756AAvoid false negativesAvoid false positivesMicrobiological testing/measurementFluorescence/phosphorescenceReal-time polymerase chain reactionTrue positive rate
The invention relates to a reagent kit of detecting human immunodeficiency virus type 1 by fluorescence quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction), and in particular relates to a reagent kit of rapidly and quantitatively detecting infection of human immunodeficiency virus type 1 by one-step real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR). The reagent kit is high in sensitivity and specificity, is used for quantitatively detecting human immunodeficiency virus type 1 nucleic acid RNA (Ribonucleic Acid) in a serum or plasma sample and is suitable for auxiliary diagnosis of the infection of the human immunodeficiency virus type 1 and curative effect survey of drug therapy of a human immunodeficiency virus type 1 infected person.
Owner:DAAN GENE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com