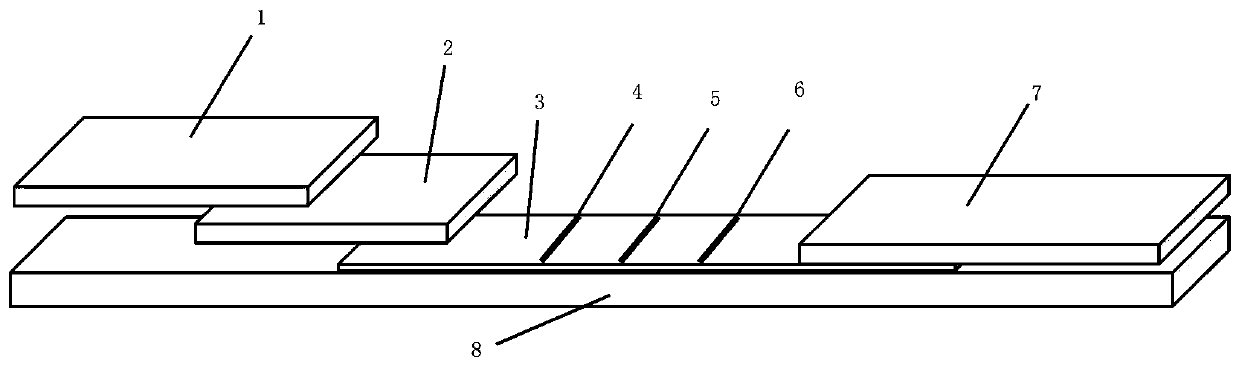
Novel coronavirus detection kit
A detection kit and coronavirus technology, which is applied in the field of colloidal gold kits and its preparation for novel coronaviruses, can solve the problems of missed detection of detection results, missed detection, N protein cannot accurately label antibodies, etc., and achieve simple sampling , avoid false negatives, and prevent the spread of the epidemic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1) Preparation of colloidal gold solution: add 500mL ultrapure water to a round bottom flask, then add 10mL of 1% gold chloride solution, heat with stirring until it just starts to boil, then quickly add 12mL of 10% trisodium citrate solution at one time, After the solution turned deep red completely, the heating was stopped, and the reaction was continued for 10 minutes using residual heat. Then cool down to room temperature with cold water, add deionized water to make up to 500mL, and store at 4°C in the dark.
[0050] 2) Antigen labeling: take 10mL colloidal gold solution, add 60μL 0.1mol / L potassium carbonate solution, quickly add 100μg N protein antigen, mix well and place at room temperature for 30 minutes. Quickly add 100 μL of 20% bovine serum albumin solution, mix well, place at room temperature for 30 minutes, and centrifuge at 12,000 rpm for 20 minutes. Discard the supernatant, then add 5 mL of 50 mmol / L, pH7.4 phosphate buffer to suspend the precipitate, ce...
Embodiment 2
[0058] Example 2: Use of the novel coronavirus detection kit.
[0059] 1) Preparation: First, put the kit and blood sample at room temperature to return to normal temperature.
[0060] 2) Tear open along the incision of the packaging bag, take out the kit and put it on the horizontal platform and press the sample number.
[0061] 3) Add 10 μL of plasma or serum to the sample well, and then immediately add 80 μL of sample diluent.
[0062] 4) After 10 minutes, read the result in the result observation area and make a judgment.
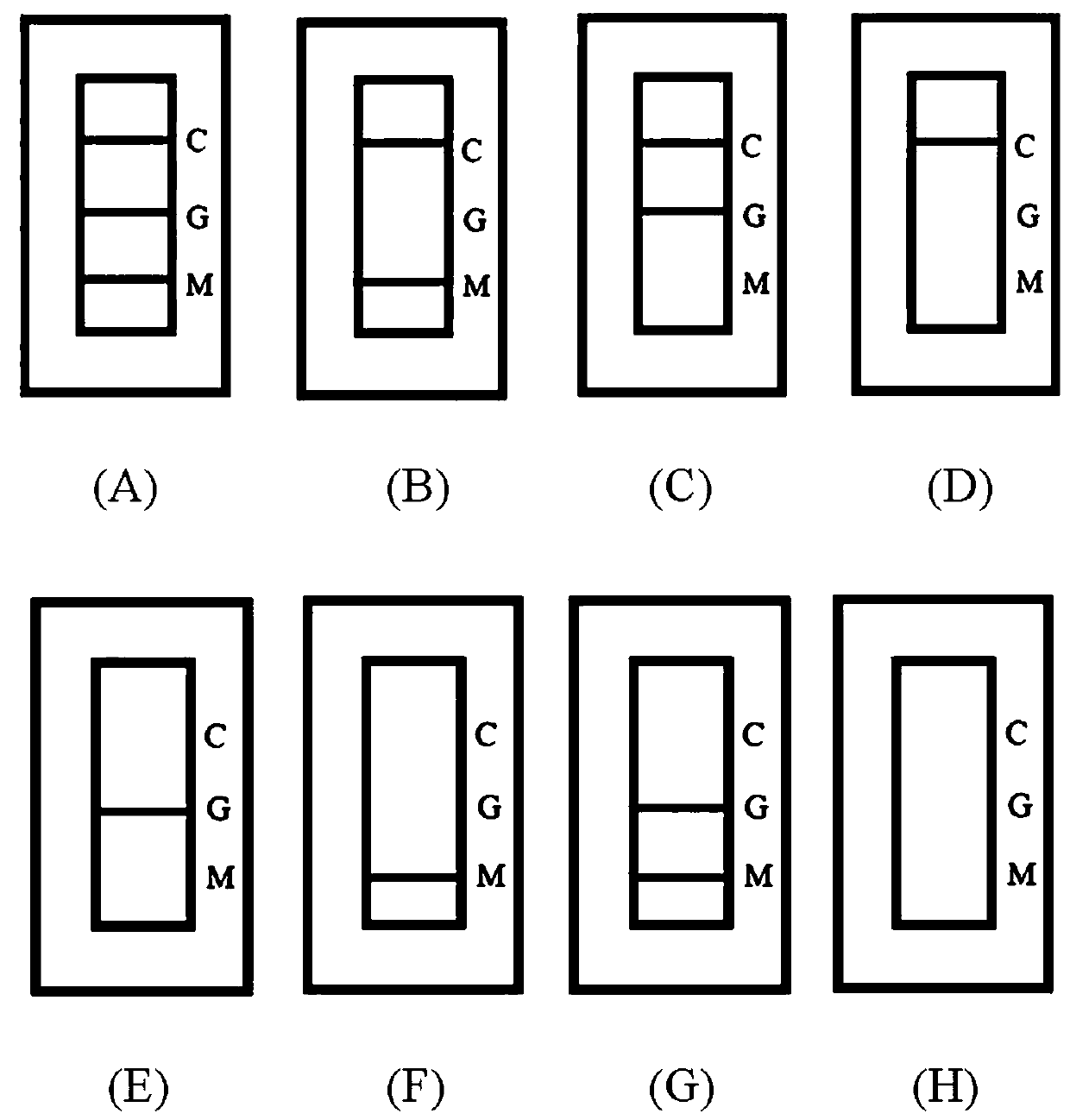
[0063] Judgment criteria such as figure 2 .
[0064] Positive: C zone (quality control line) shows a red line, T zone (test line) shows one or two red lines ( figure 2 A, B, C).
[0065] Negative: C area (quality control line) shows a red line, T area (test line) has no color ( figure 2 D).
[0066] Invalid: Area C (quality control line) has no color development ( figure 2 E, F, G, H).
Embodiment 3
[0067] Example 3: Comparison of a single antigen kit and two antigen kits.
[0068] According to the method of Example 1, a kit A in which only the N protein antigen is coated on the colloidal gold pad, and a kit B in which the antigens are N & S-RBD two proteins are prepared respectively.
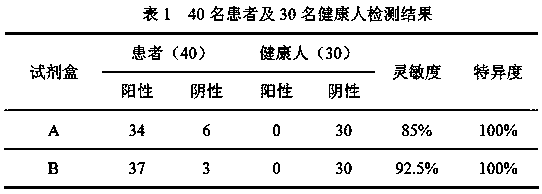
[0069] The serum / plasma of 40 patients with clinically confirmed COVID-19 from the First Hospital of Shanxi Medical University and the Affiliated Pulmonary Hospital of Shanxi Medical University and the serum / plasma of 30 healthy people were obtained, and tested with A and B kits respectively. The test results are shown in Table 1.
[0070]
[0071] It can be seen from the results in Table 1 that the detection sensitivity of kit B using the two proteins of N & S-RBD is significantly higher than that of kit A, indicating that adding S-RBD protein as an antigen can reduce the rate of false detection and missed detection, and improve the initial detection rate. screening accuracy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com