Hepatitis B virus nucleic acid quantitative detection method and kit
A hepatitis B virus and nucleic acid quantification technology, applied in the field of biochemistry, can solve problems such as low sensitivity and poor detection accuracy, and achieve the effects of simple and fast operation, improved accuracy and reduced false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of HBV nucleic acid quantitative detection kit
[0033] According to the content of the invention and the technical scheme, the preparation process of each component of the nucleic acid extraction part, amplification part, quantitative calibrator, reference substance and internal reference in the HBV nucleic acid quantitative detection kit is as follows:
[0034] (1) Lysis buffer
[0035] Contains 50mM Tris-HCl (pH8.0), 6M guanidine hydrochloride, 1% (w / v) SDS, 10% (v / v) Triton X-100.
[0036] (2) Wash buffer W1
[0037] Contains 10 mM Tris-HCl (pH 8.0), 30% ethanol.
[0038] (3) Wash buffer W2
[0039] Contains 10 mM Tris-HCl (pH 8.0), 80% ethanol.
[0040] (4) Elution buffer
[0041] It is 10mM Tris-HCl (pH8.0) solution.
[0042] (5) Magnetic beads
[0043] (6) PCR buffer
[0044] Contains 33.33mM Tris-HCl (pH8.3), 166.67mM KCl, 0.67mM dNTPs, 10mM MgCl 2 , 0.2-0.5μM HBV primer, HBV fluorescent probe and internal reference fluores...
Embodiment 2
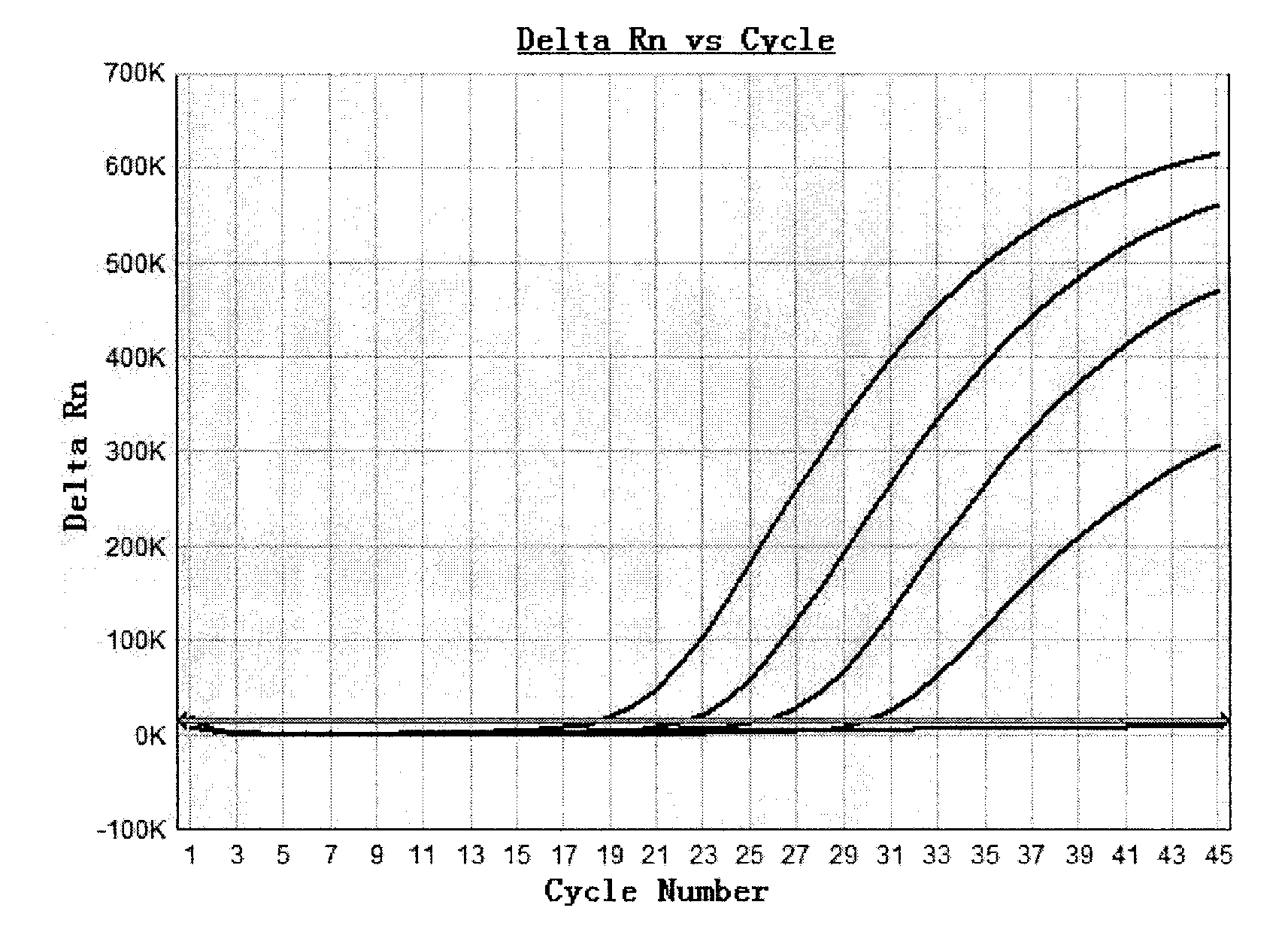
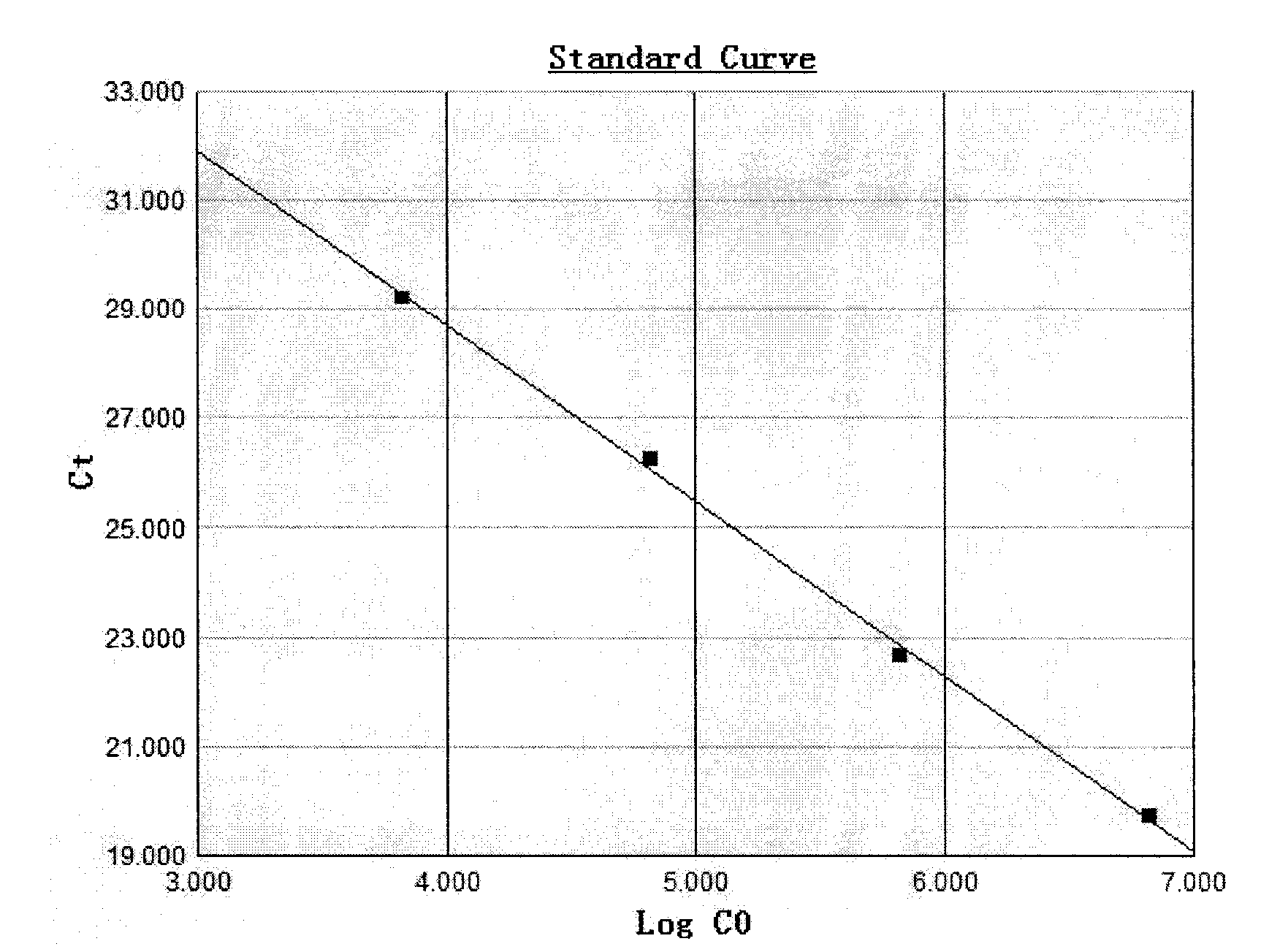
[0059] Embodiment 2: the determination of kit detection sensitivity
[0060] (1) Extraction of HBV DNA from serum samples
[0061] Three dilution gradients of HBV DNA positive serum with known concentrations calibrated by the National Institute for the Control of Pharmaceutical and Biological Products Hepatitis B virus (HBV) nucleic acid quantitative standards were the samples to be determined (each concentration was 1.0×10 1 IU / ml, 1.0×10 2 IU / ml, 1.0×10 3 IU / ml), measure the sensitivity of the test kit prepared in Example 1 to detect HBV DNA. Sample HBV DNA extraction steps are as follows:
[0062] ① Take the serum samples of the above three concentrations, add 800 μl each of the 4 quantitative calibrators of the kit, the critical positive control and the negative control, add 800 μl of lysis buffer, 20 μl of magnetic beads and 5 μl of the internal reference, and put them in a 2ml centrifuge tube to mix evenly , placed at room temperature for 10 minutes.
[0063] ②Magne...
Embodiment 3
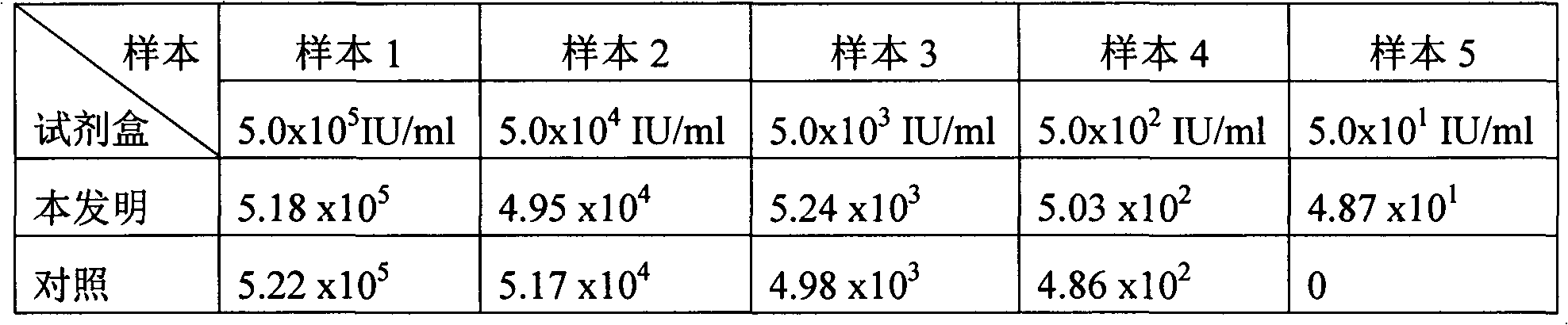
[0071] Example 3: Application of the kit in the extraction and detection of serum HBV DNA
[0072] (1) Extraction of HBV DNA from serum samples
[0073] Take 5 cases of known concentrations of HBV DNA positive serum, use the kit of the present invention prepared in Example 1 to extract and detect, and select the HBV nucleic acid fluorescent quantitative PCR detection kit of Shanghai Cloning Bio-High Technology Co., Ltd. as a control for extraction and detection.
[0074] The serum HBV DNA extraction procedure of the present invention is the same as in Example 2, and the eluted nucleic acid is taken for fluorescent PCR detection. The nucleic acid extraction method of the control kit sample of Shanghai Cloning Biotech Co., Ltd. is as follows: take 50 μl of serum, add 50 μl of nucleic acid extraction solution A, shake and mix for 10 sec, centrifuge at 13,000 rpm for 10 min, discard the supernatant; add 50 μl of nucleic acid extraction solution B to During precipitation, shake an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com