Patents
Literature
168 results about "Human dna" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV integrase inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV Integrase Inhibitors
The invention encompasses a series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
HIV Integrase Inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
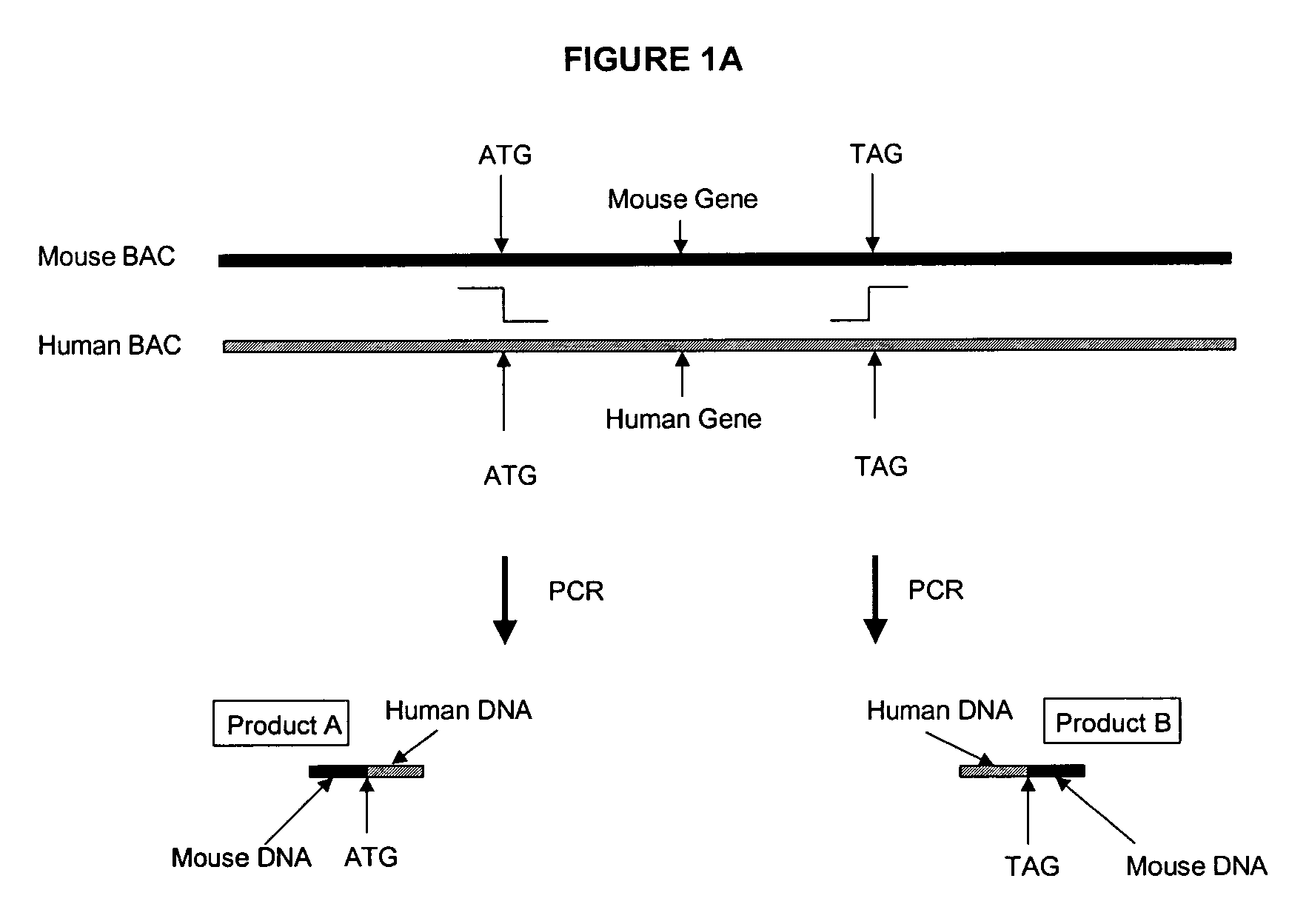
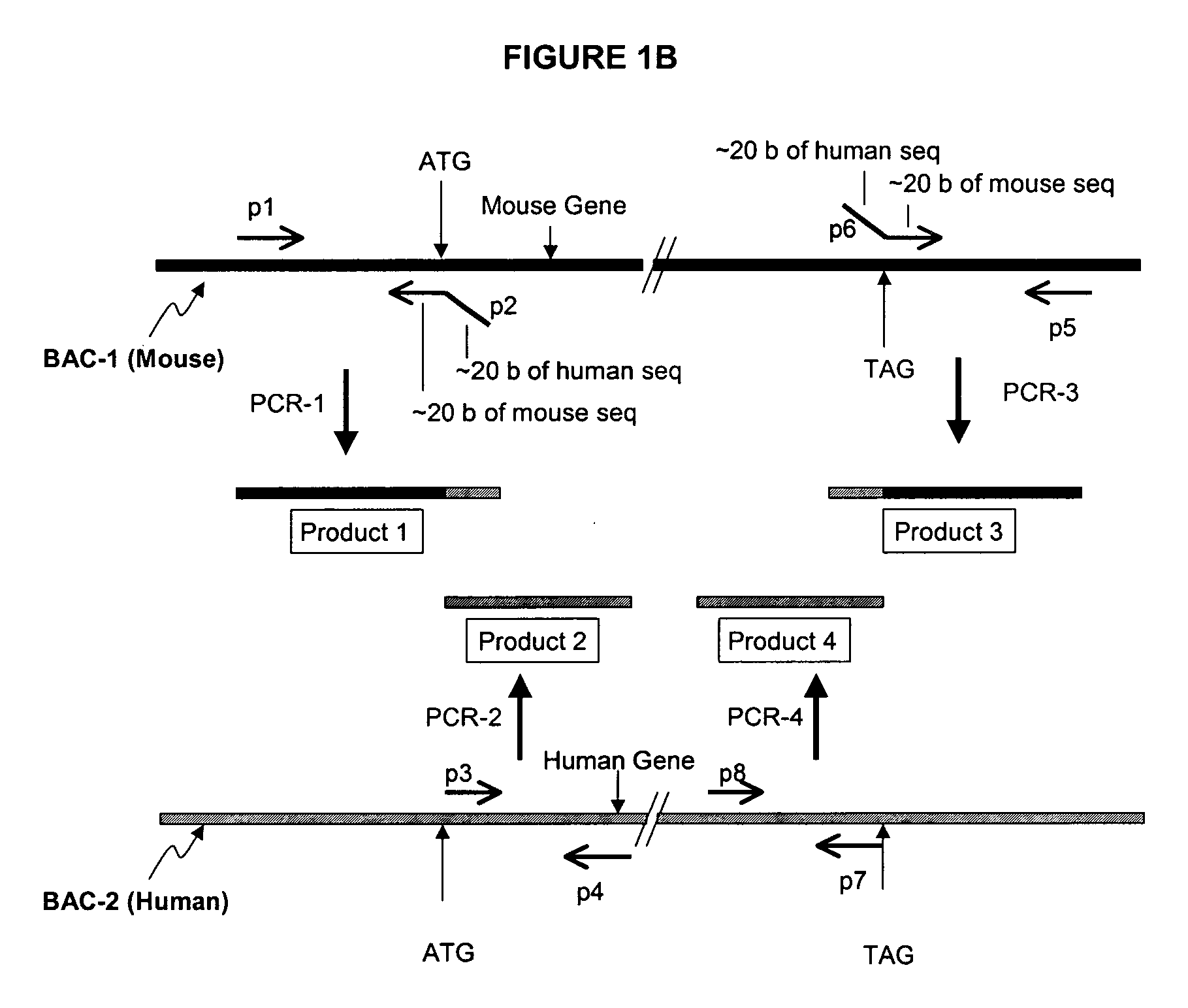
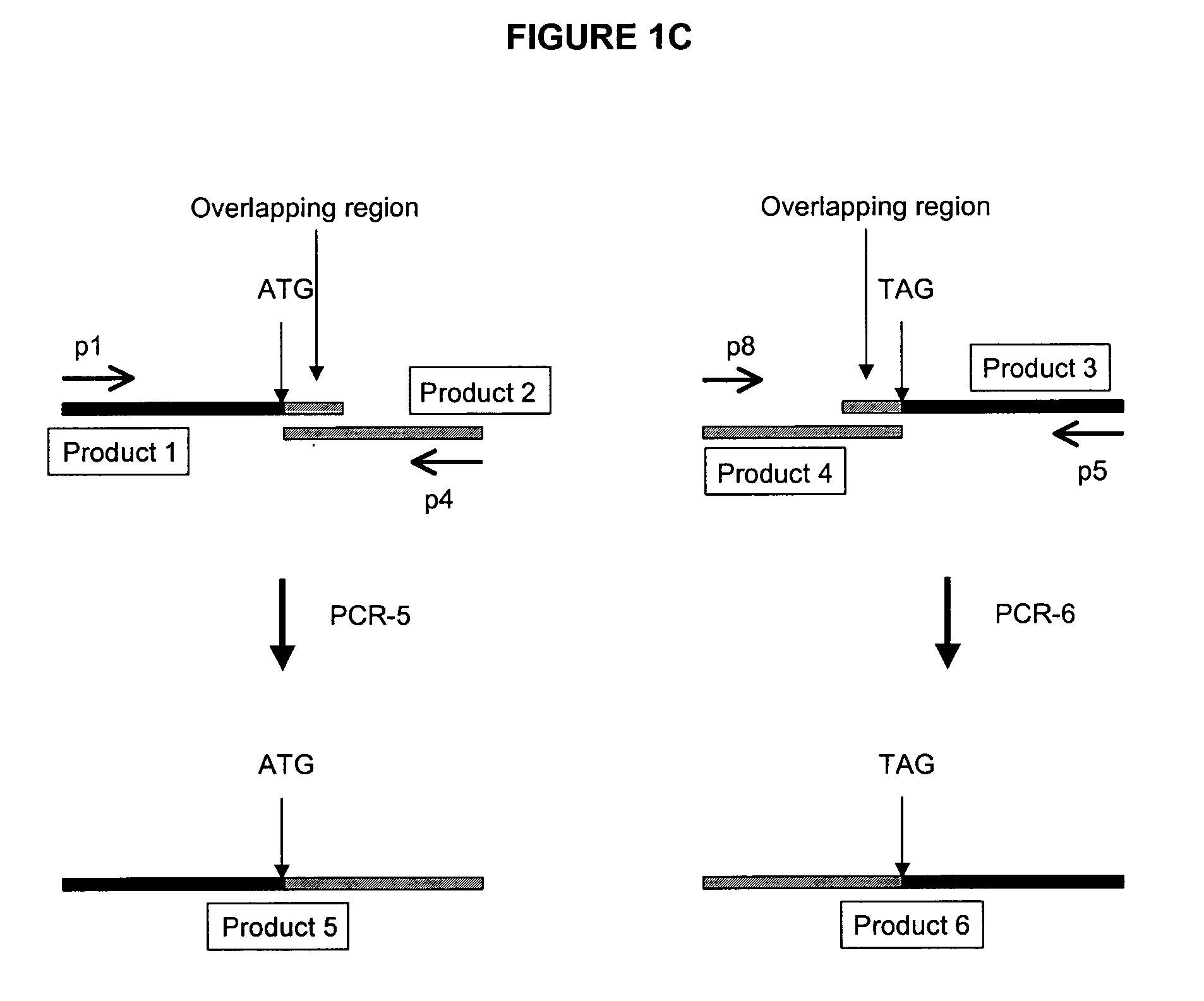
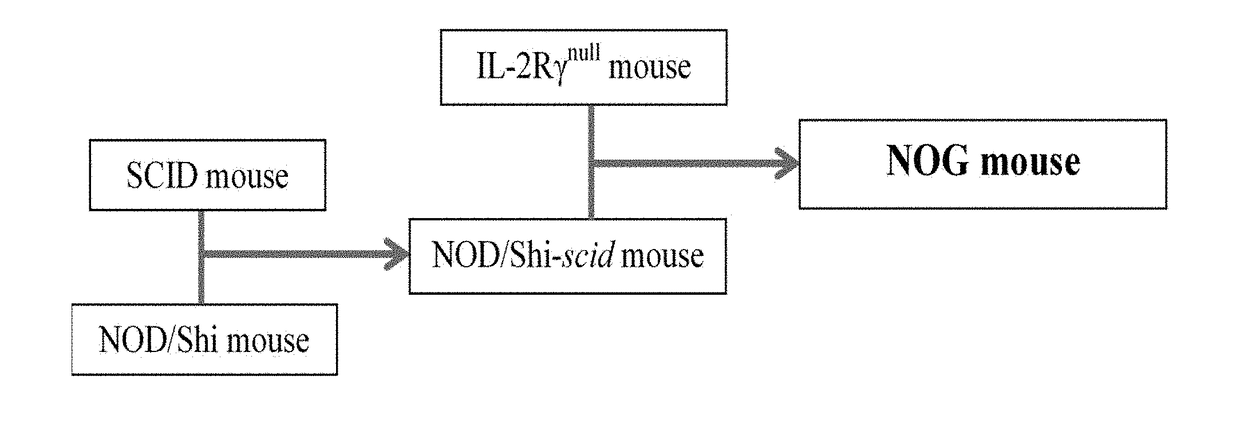
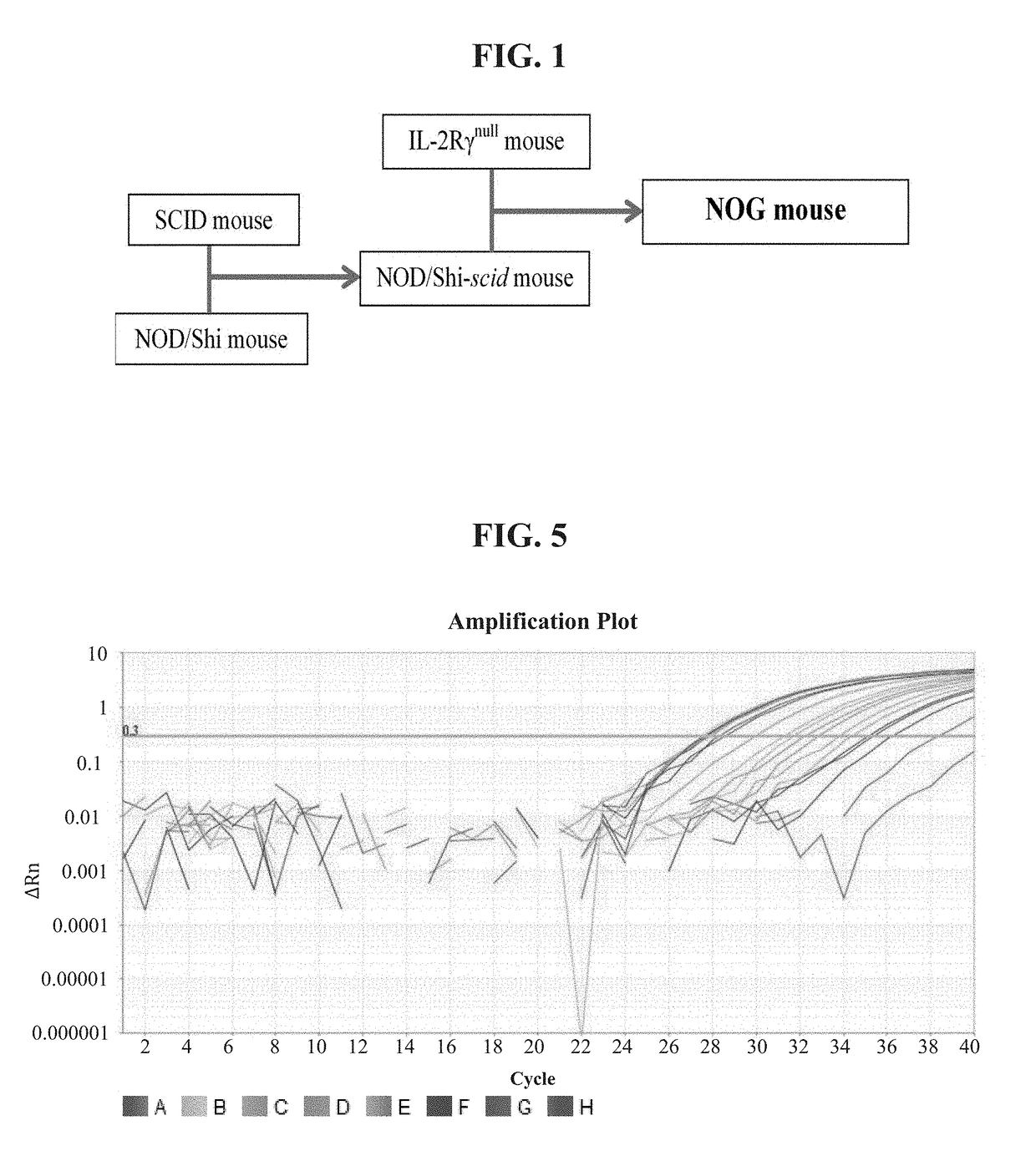
Methods and compositions for the generation of humanized mice
The invention provides methods and compositions for generating non-human transgenic animals that are humanized at one or more gene sequences. According to the methods of the invention, a DNA construct containing a human DNA sequence flanked by sequences from the non-human animal is generated by recombination in a bacterial cell, for example, in E. coli. The DNA construct that is produced can then be introduced into a non-human embryogenic stem cell where it can recombine with the genomic DNA of the non-human animal.
Owner:CALIFORNIA INST OF TECH
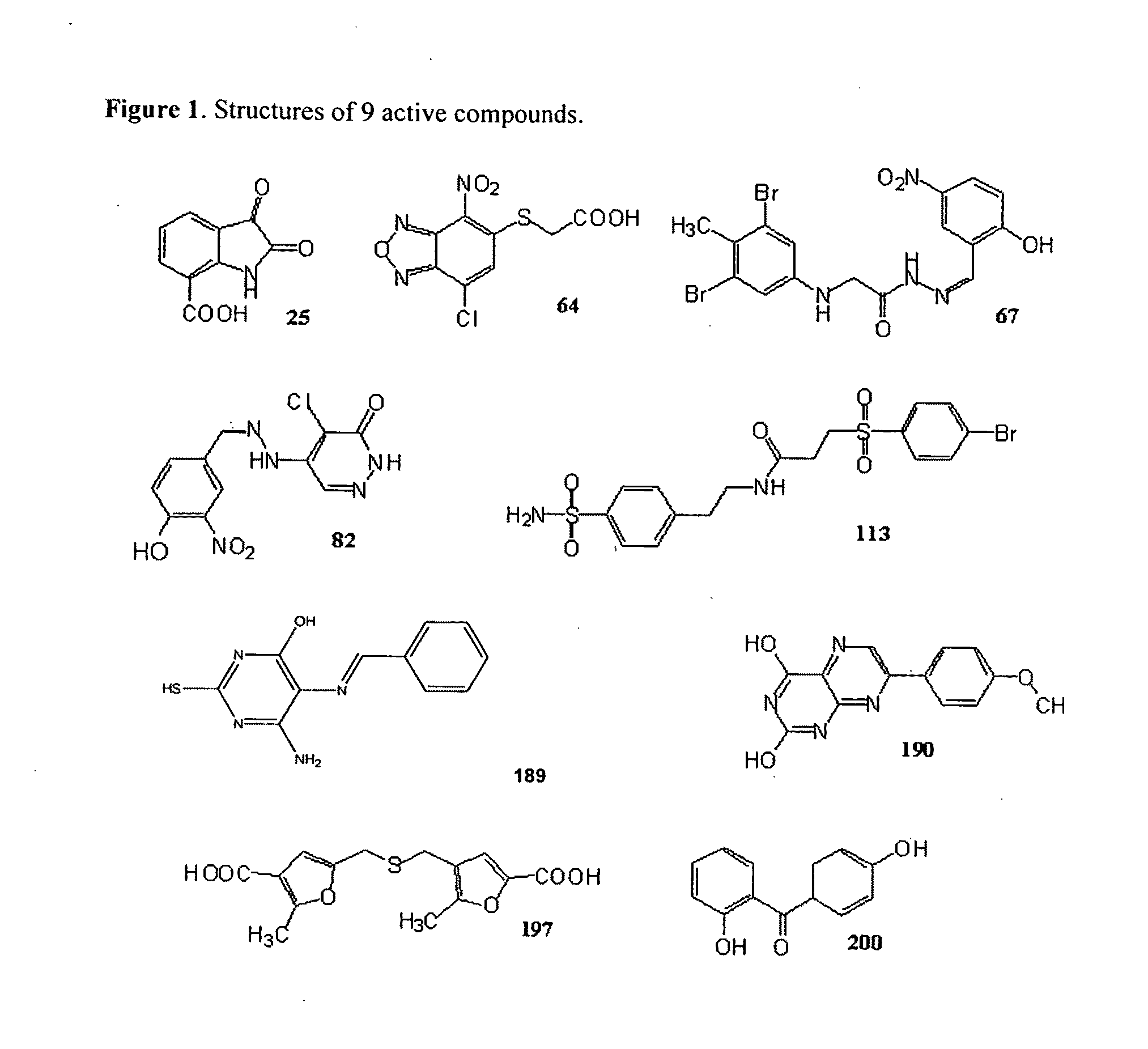
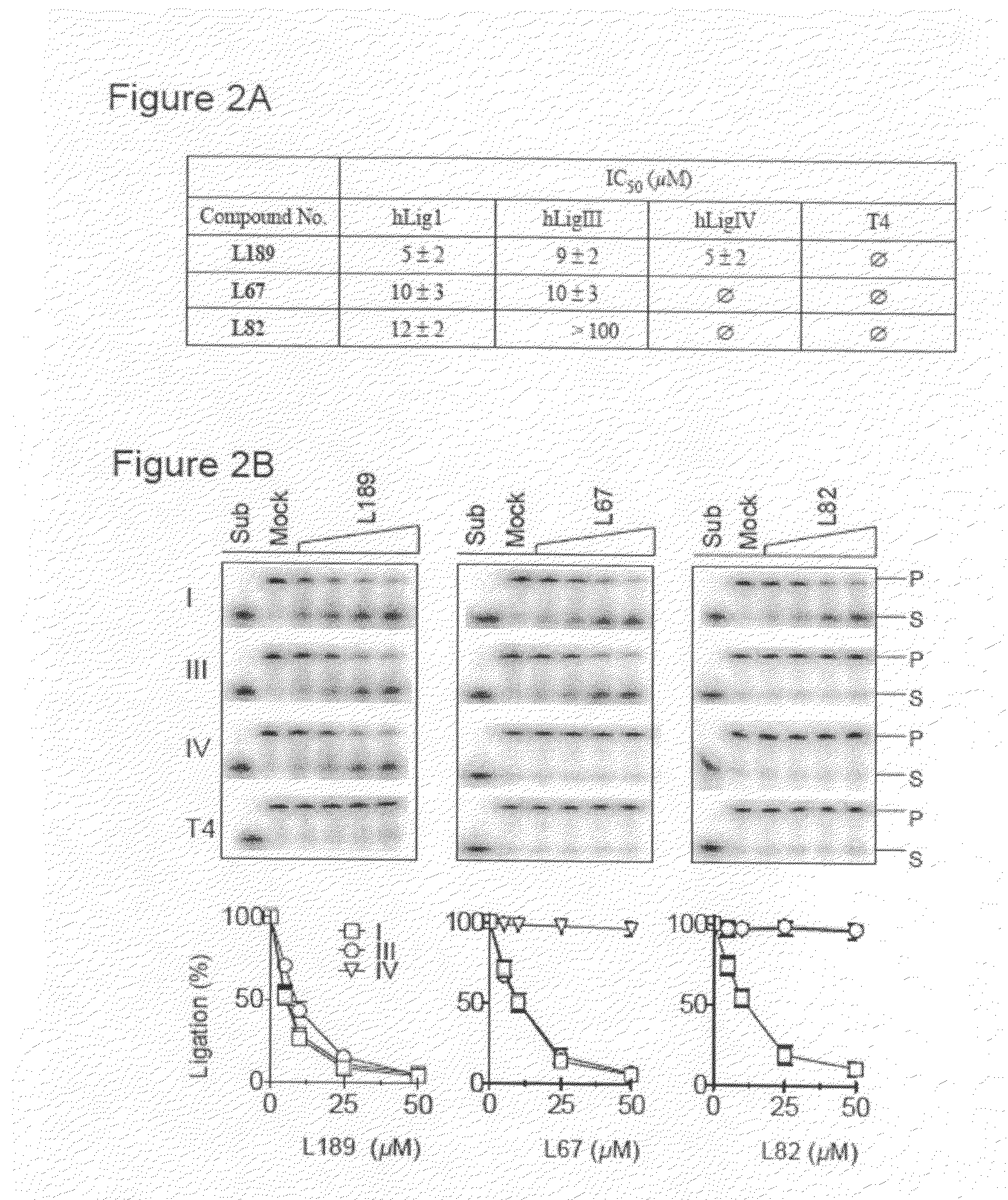
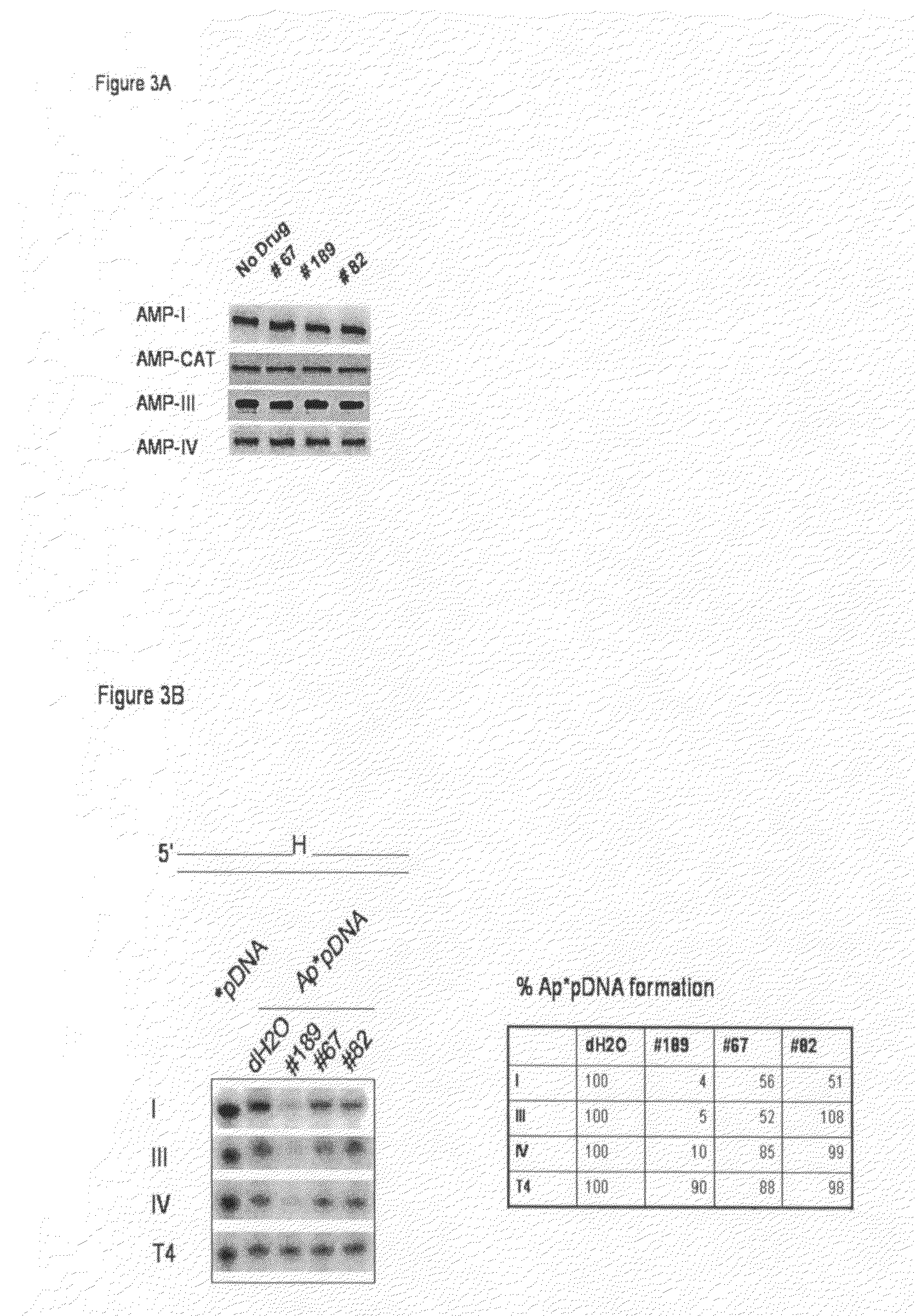
Compounds that inhibit human DNA ligases and methods of treating cancer
ActiveUS20100099683A1Strong cytotoxicityInhibit cell growthBiocideOrganic chemistryScreening methodBiochemistry
Methods for treating cancer using compounds that inhibit human DNA ligases. Methods for using compounds that inhibit human DNA ligases to provide insights into the reaction mechanisms of human DNA ligases, for example to identify the human DNA ligase involved in different DNA repair pathways. Screening methods for compounds that inhibit human DNA ligases.
Owner:UNIV OF MARYLAND
Bicyclic heterocycles as HIV-integrase inhibitors
The invention encompasses a series cyclic bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
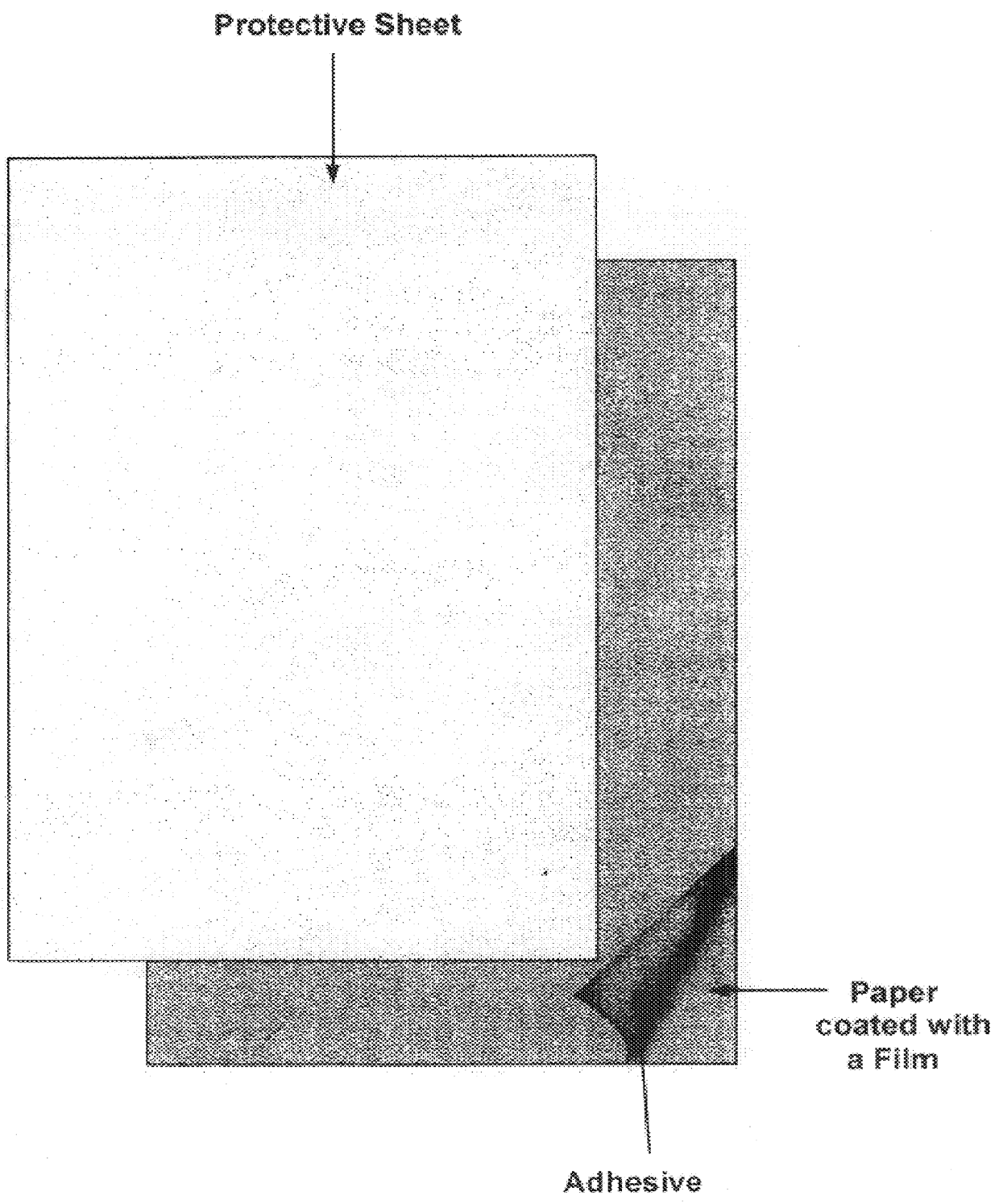
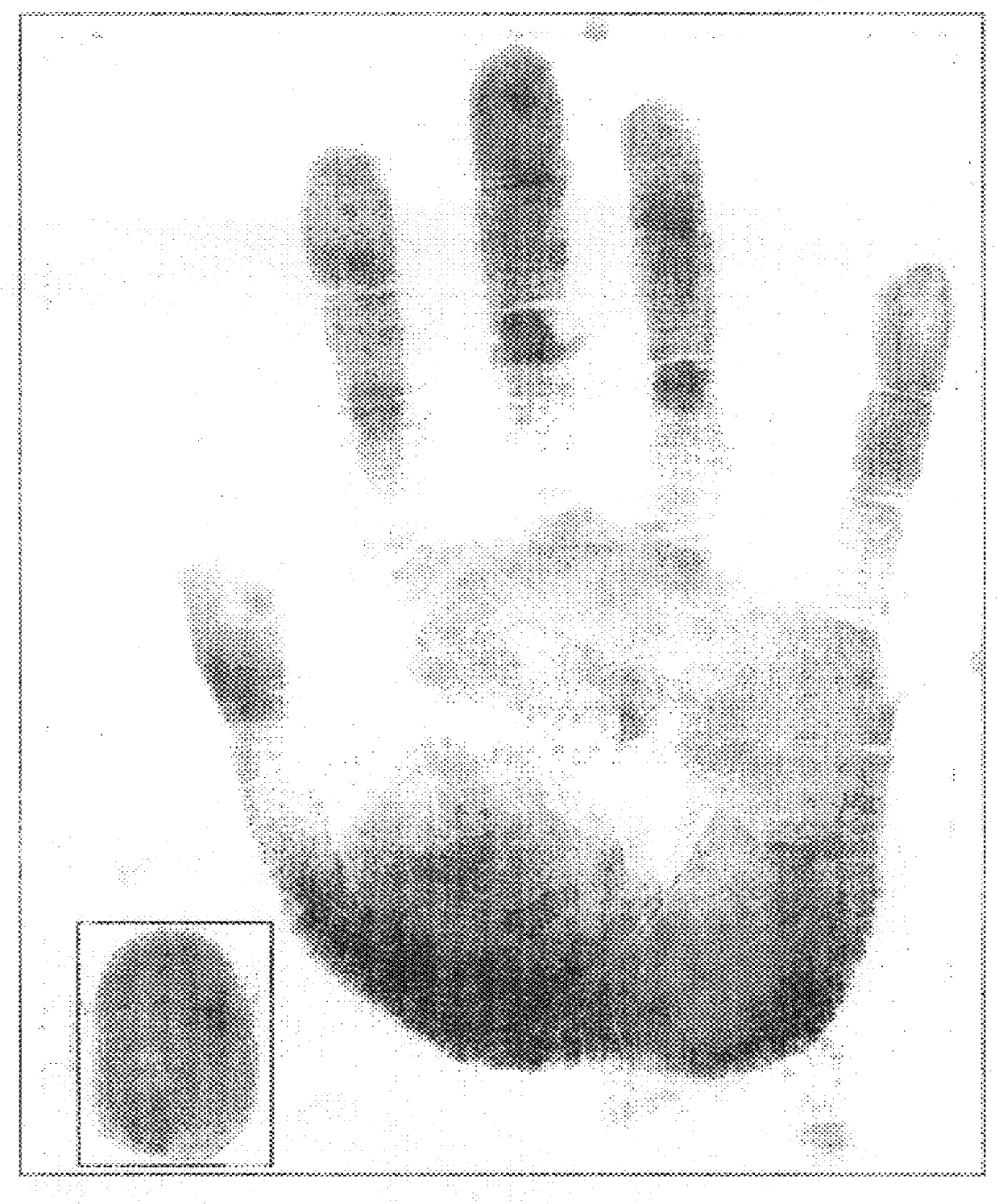
Method for obtaining human skin DNA samples with an adhesive sheet
Provided is a method for obtaining human DNA for genetic analysis, by taking the epidermis of testee by means of an adhesive sheet, and by extracting DNA from the epidermis stuck on the adhesive sheet. Provided are also combined sheets for conveniently storing DNA and a kit for taking the epidermis and analyzing DNA. Along with the kits, the method allows DNA to be easily obtained and stably stored for a long period of time. In addition, both the identification and the DNA analysis of a testee can be conducted at the same time by taking epidermal scraps from the testee, along with a figured epidermal print.
Owner:SPECIALTY LAB SOLUTION BIO CO LTD
Method for measuring tumor burden in patient derived xenograft (PDX) mice
ActiveUS20180288982A1Easy to observeMicrobiological testing/measurementScreening processCirculating tumor DNAHuman patient
Kits and methods providing measurement of tumor burden in a patient derived xenograft (PDX) mouse are described. Exemplary embodiments contemplate taking a sample, typically a blood sample, from a PDX mouse and using a real-time polymerase chain reaction (PCR) system to quantitate both human patient circulating tumor DNA (ctDNA) and mouse DNA. In preferred embodiments, both PCR amplifications are done simultaneously in a multiplex, and a highly polymorphic human DNA target sequence is amplified for high sensitivity, allowing for small volume samples, typically 50-100 μL, of mouse blood. Serial evaluations are possible because the mouse can survive withdrawal of these small volumes of blood. A related method allows for quantitation of ctDNA in the presence of human immune cells added to a “humanized” mouse. These relatively quick and easy methods of determining tumor burden in PDX mice can have predictive value for the efficacy of cancer treatments in human patients.
Owner:LIFE GENETICS LAB
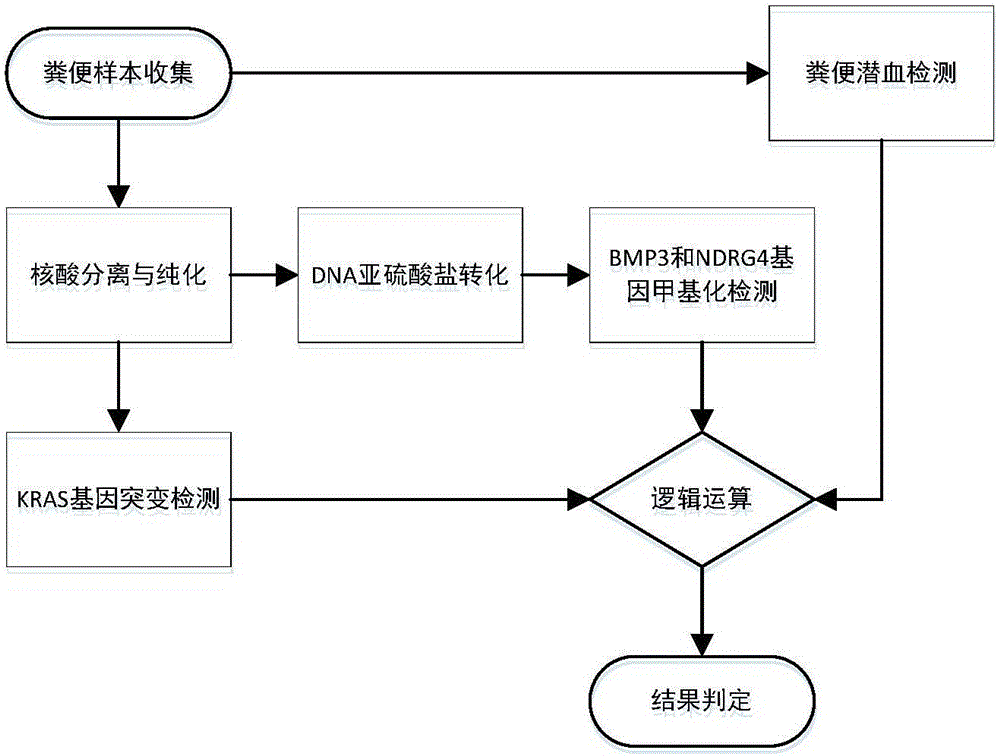
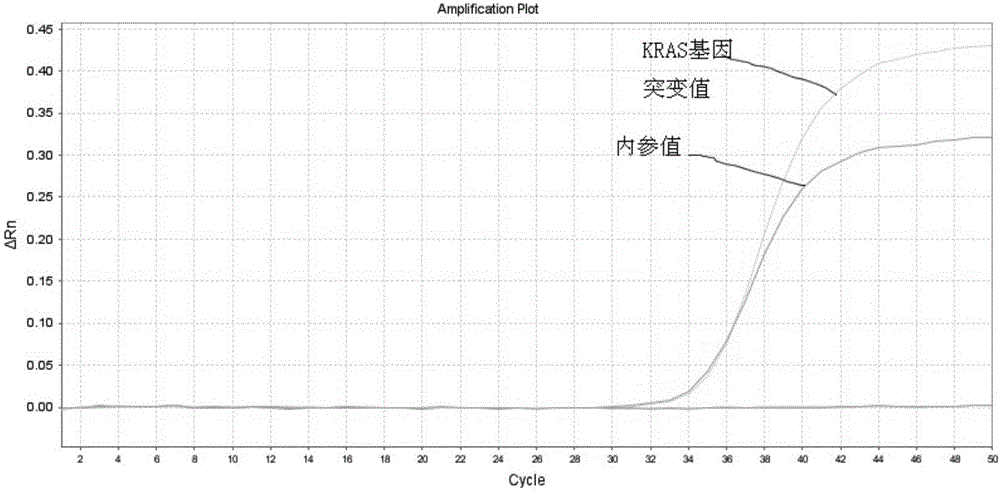
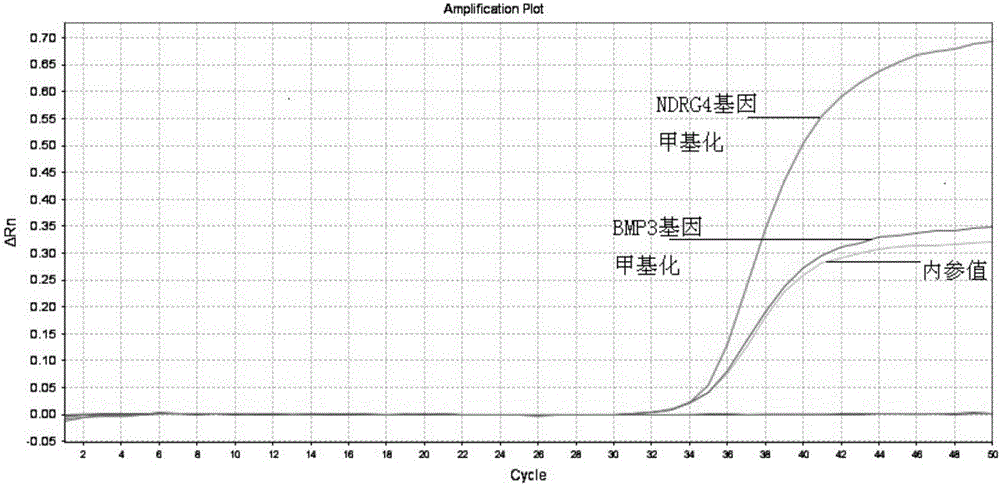
Kit for early stage colorectal cancer auxiliary diagnosis and use method and detection system thereof
InactiveCN106399570ANo detection blind zoneHigh test acceptanceMicrobiological testing/measurementBiological material analysisViral OncogeneGenes mutation
The invention provides a kit for early stage colorectal cancer auxiliary diagnosis and a use method and a detection system thereof. The kit comprises a nucleic acid isolation and purification reagent, a DNA (deoxyribonucleic acid) sulfite conversion reagent, a KRAS (kirsten rat sarcoma viral oncogene homolog) gene mutation detection reagent, a BMP3 (bone morphogenetic protein 3) and NDRG4 (N-myc downsteam regulated gene 4) gene methylation detection reagent and a fecal occult blood detection reagent, wherein the nucleic acid isolation and purification reagent is used for separating and purifying the human DNA in a faeces sample; the DNA sulfite conversion reagent is used for performing sulfite conversion on the purified partial human DNA, and is used for subsequent BMP3 and NDRG4 gene methylation detection. Through detecting two kinds of indexes of DNA and fecal occult blood in the faeces sample in a combined way, the kit provided by the invention is used for the early stage screening of the colorectal cancer. Compared with an FOBT (fecal occult blood test) detection method, the kit provided by the method can realize higher sensitivity on the detection of colorectal cancer, particularly the developing period tumor.
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
Bicyclic heterocycles as HIV integrase inhibitors
The invention encompasses a series cyclic bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
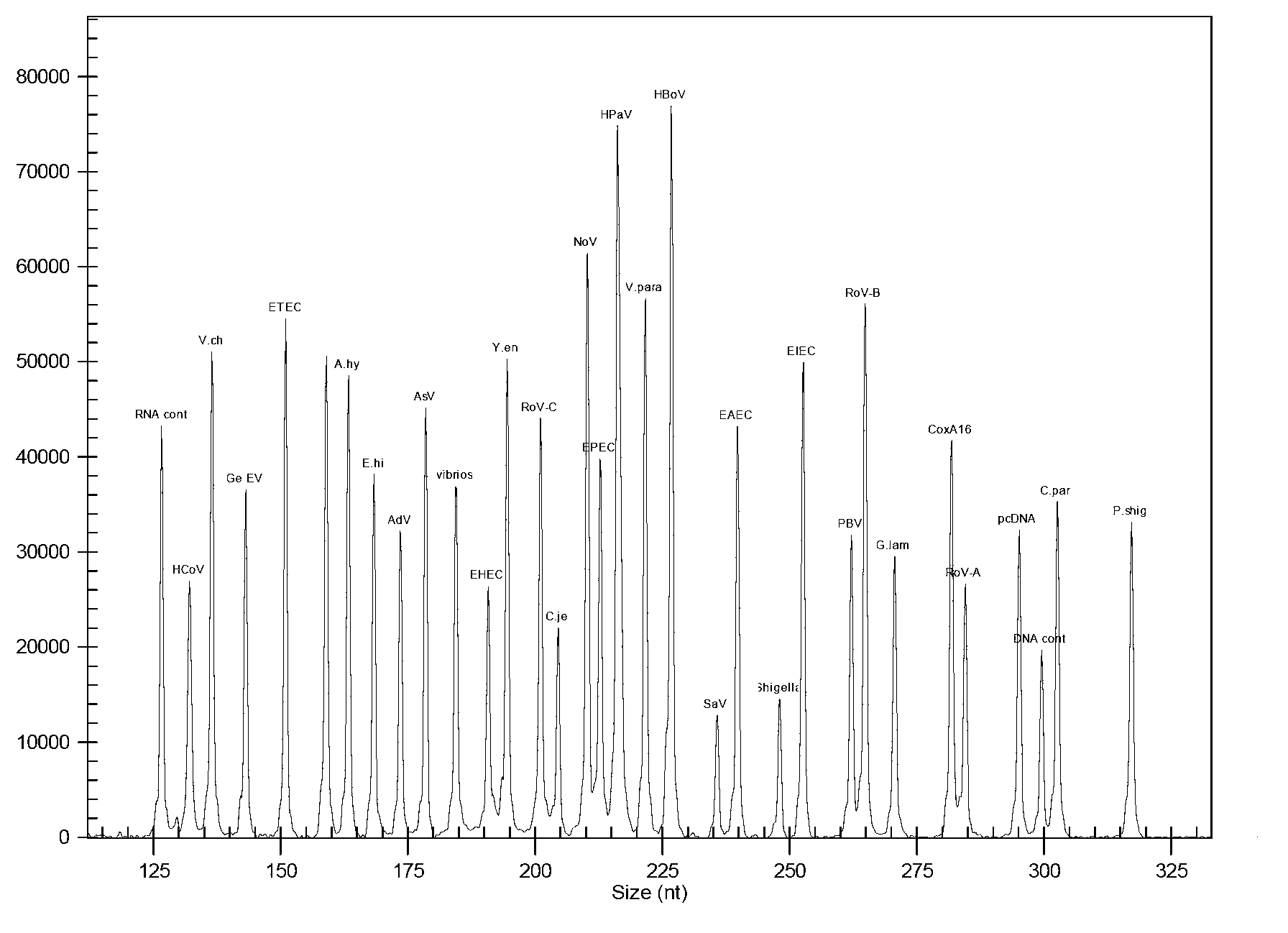
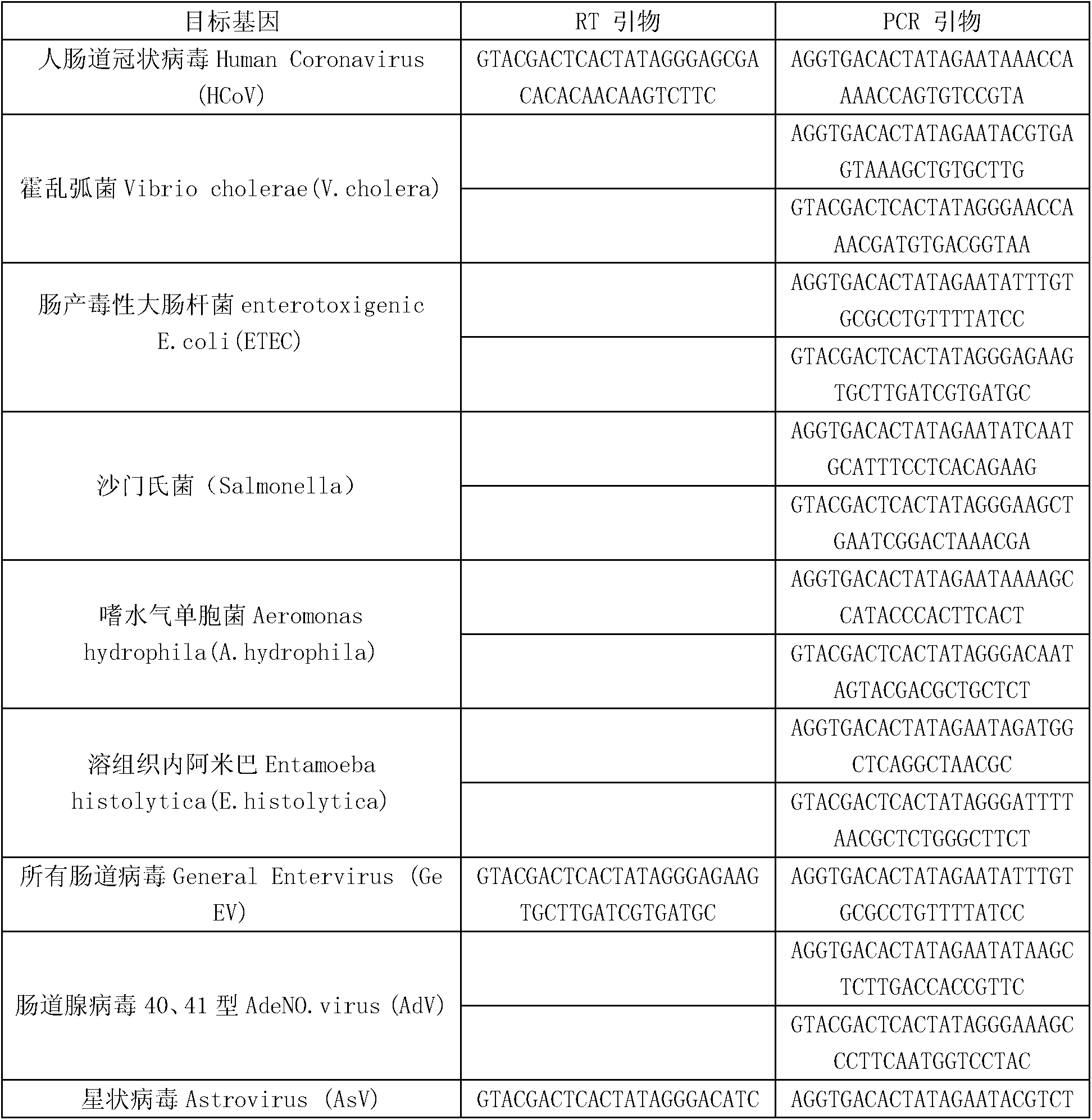
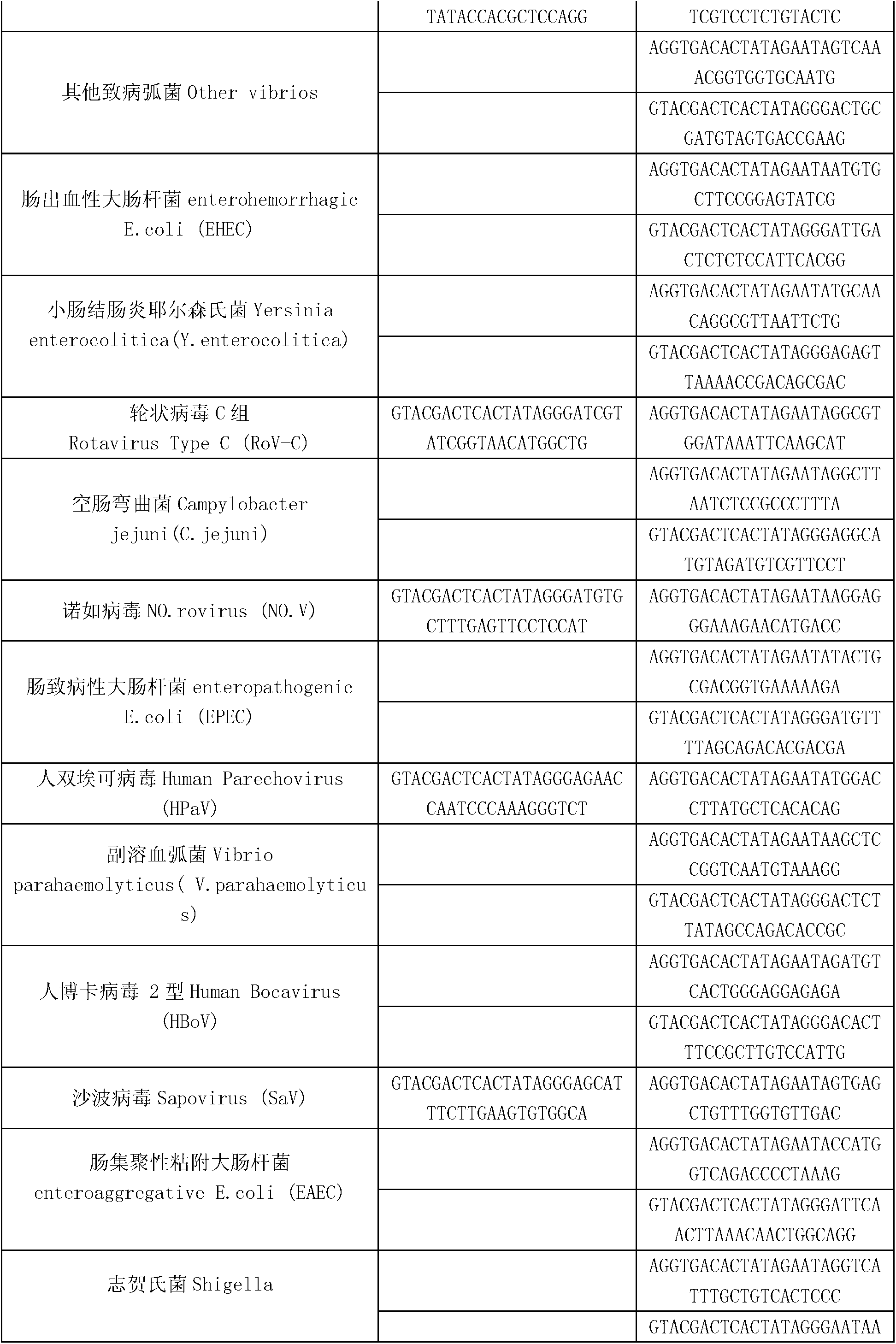
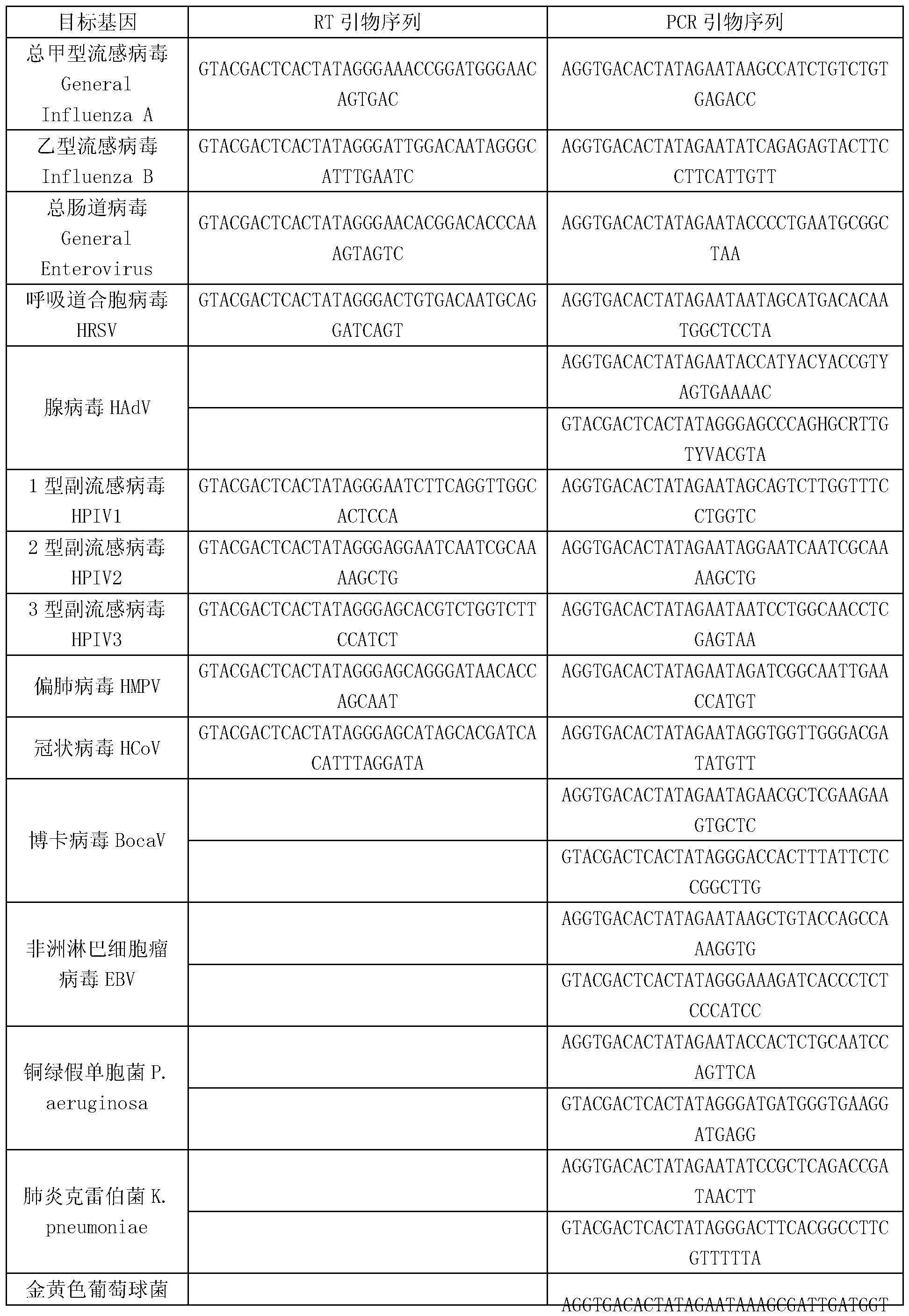
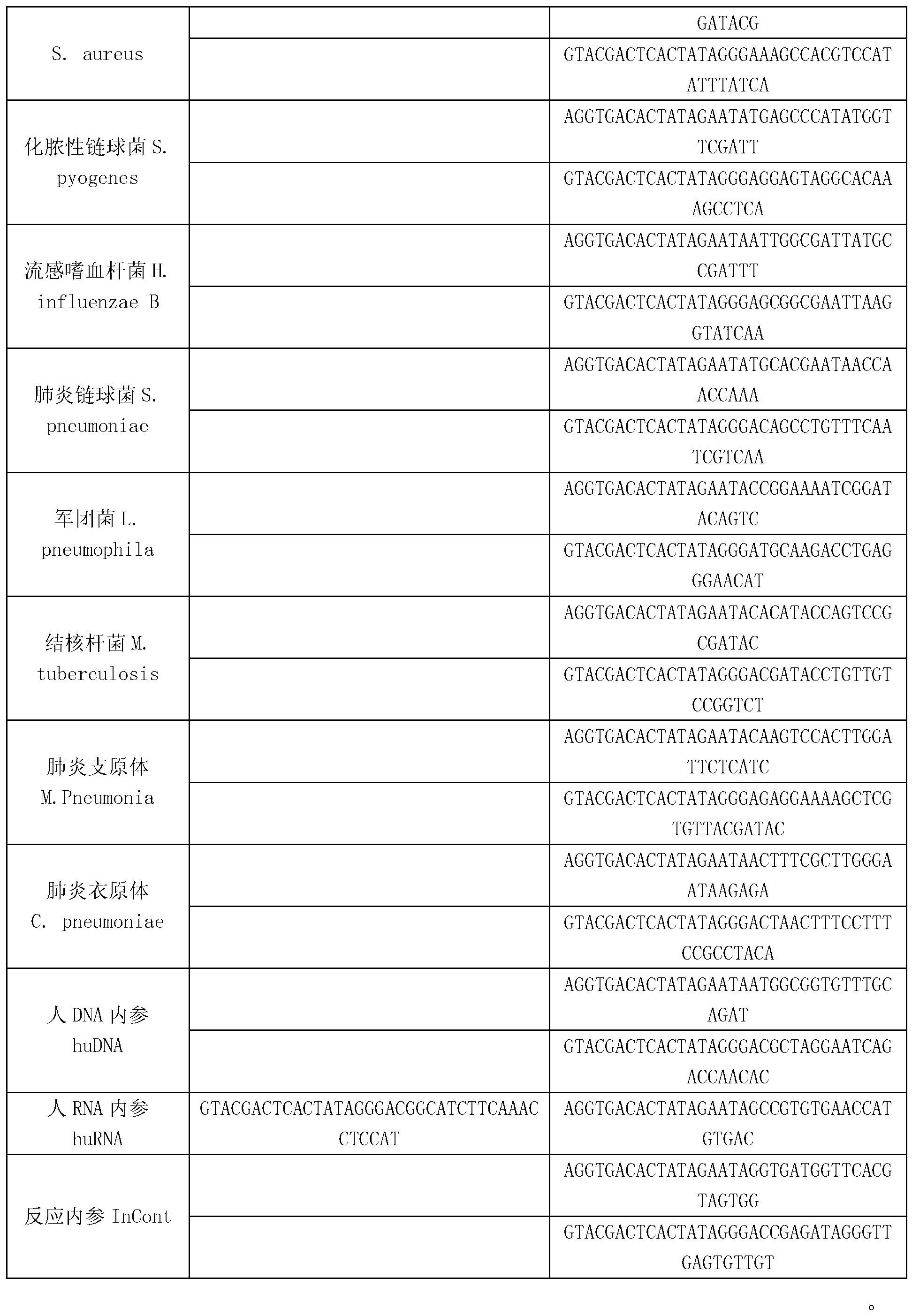
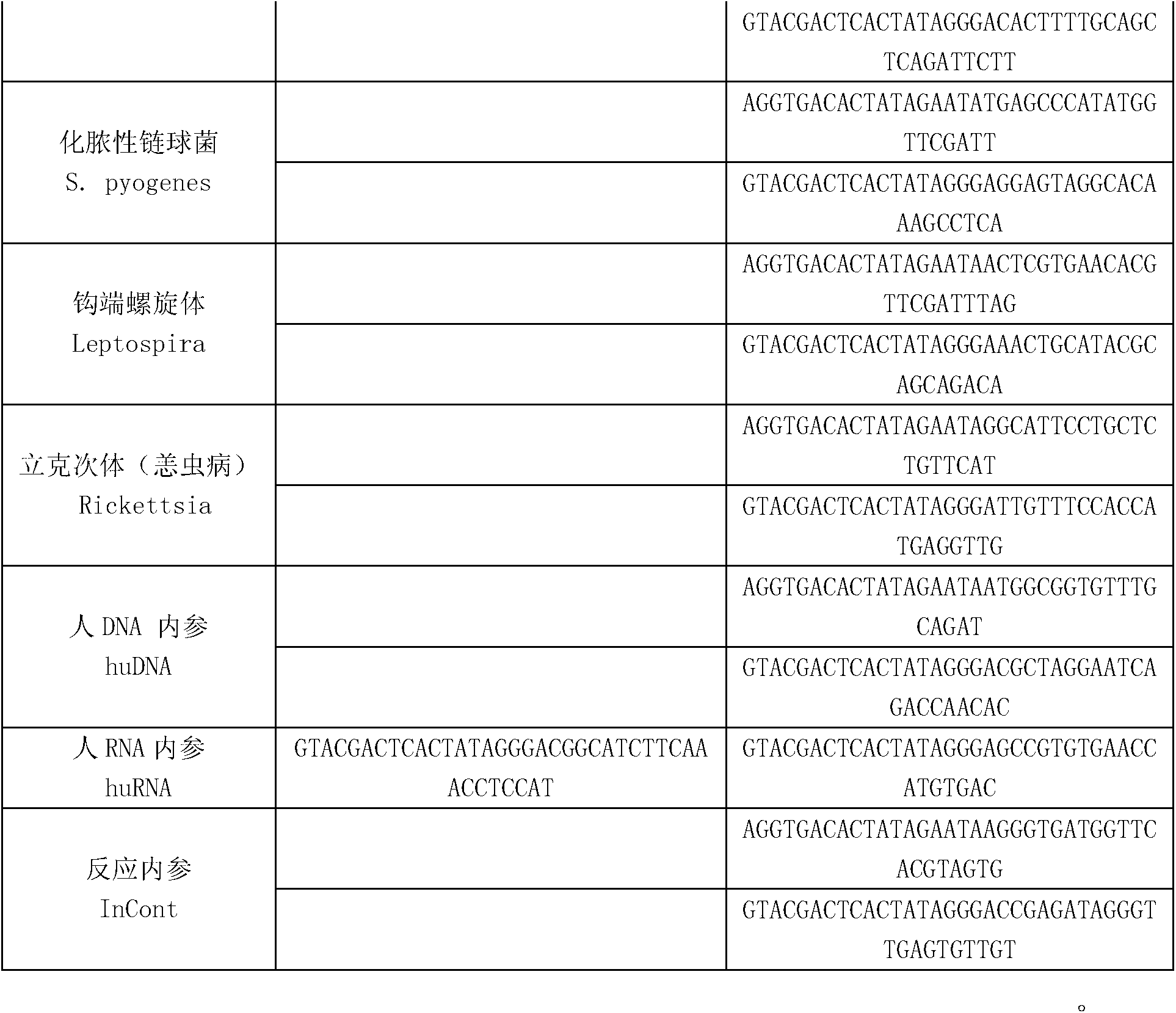
Kit for synchronously detecting thirty diarrhea pathogens and detection method of kit
ActiveCN103074450AStrong specificityImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting thirty diarrhea pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of eleven diarrhea RNA viruses and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-12 (sequence identifier number 1-12), and the PCR primer comprises forward and reverse PCR amplification primers of the rest nineteen diarrhea pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of eleven diarrhea RNA viruses and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 13-66. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed.
Owner:BRISTOL MYERS SQUIBB CO
Composite amplification kit for 47 human autosome and Y chromosome loci and application thereof
ActiveCN109750110AEffective expansionAmplification specificMicrobiological testing/measurementDNA/RNA fragmentationDNA paternity testingFluorescence
The invention relates to a composite amplification kit of 47 human autosome and Y chromosome loci and an application thereof. The invention provides a composite amplification system of the 47 human autosome and Y chromosome loci, which comprises specific primers for amplifying the 47 loci, wherein the 47 loci comprise 19 autosome STR loci, 27 Y chromosome STR loci and 1 sex recognition locus. The47 pairs of specific primers are subjected to grouping fluorescence labeling by utilizing a six-color fluorescence labeling technology, and the simultaneous high-efficiency, specific and sensitive amplification of the 47 human autosome and Y chromosome loci is achieved through the design and optimization of primer sequences and working concentrations. The detection result of the composite amplification system has high individual identification capability and good data compatibility, and can be used for paternity identification and individual identification in practice, so that the detection cost of human DNA typing is effectively reduced, and the detection working efficiency is improved.
Owner:BEIJING PEOPLESPOT TECH
Methods and materials for detecting colorectal neoplasm
InactiveUS20110236916A1Microbiological testing/measurementDisease diagnosisAbnormal tissue growthTumor-Related Protein
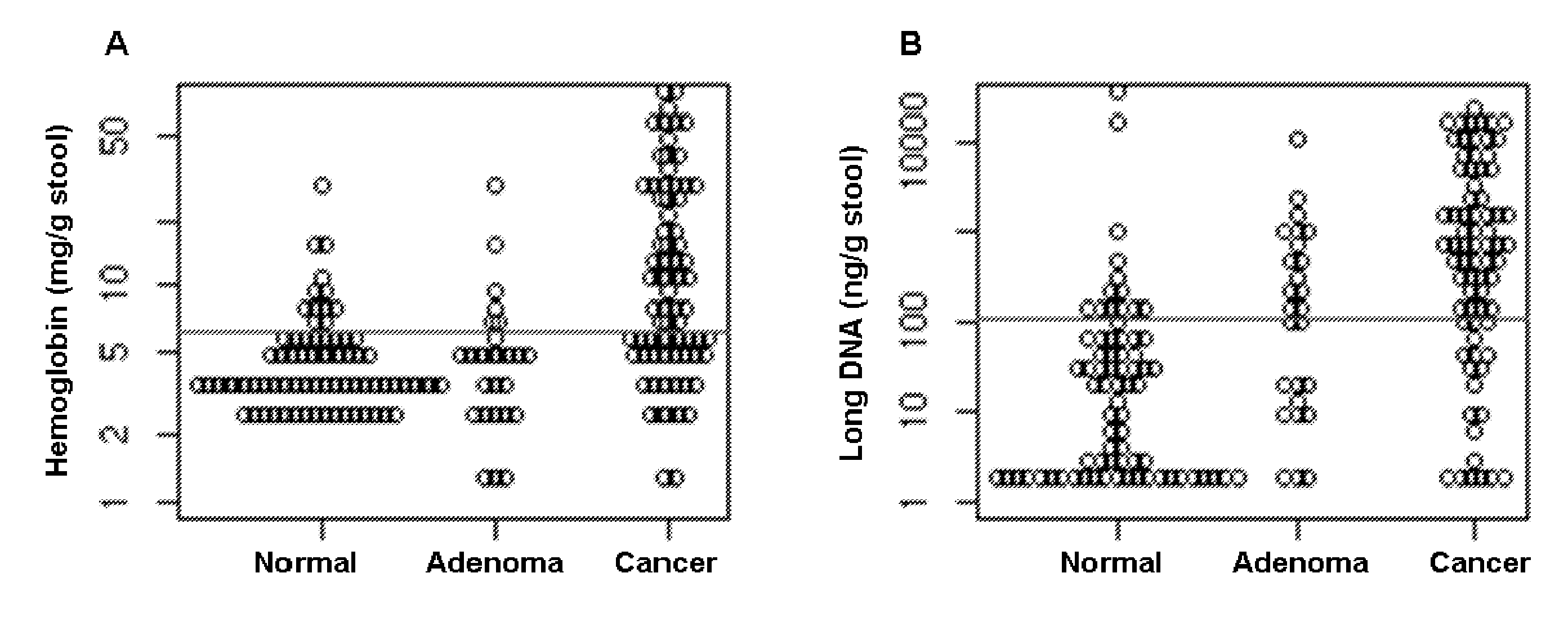
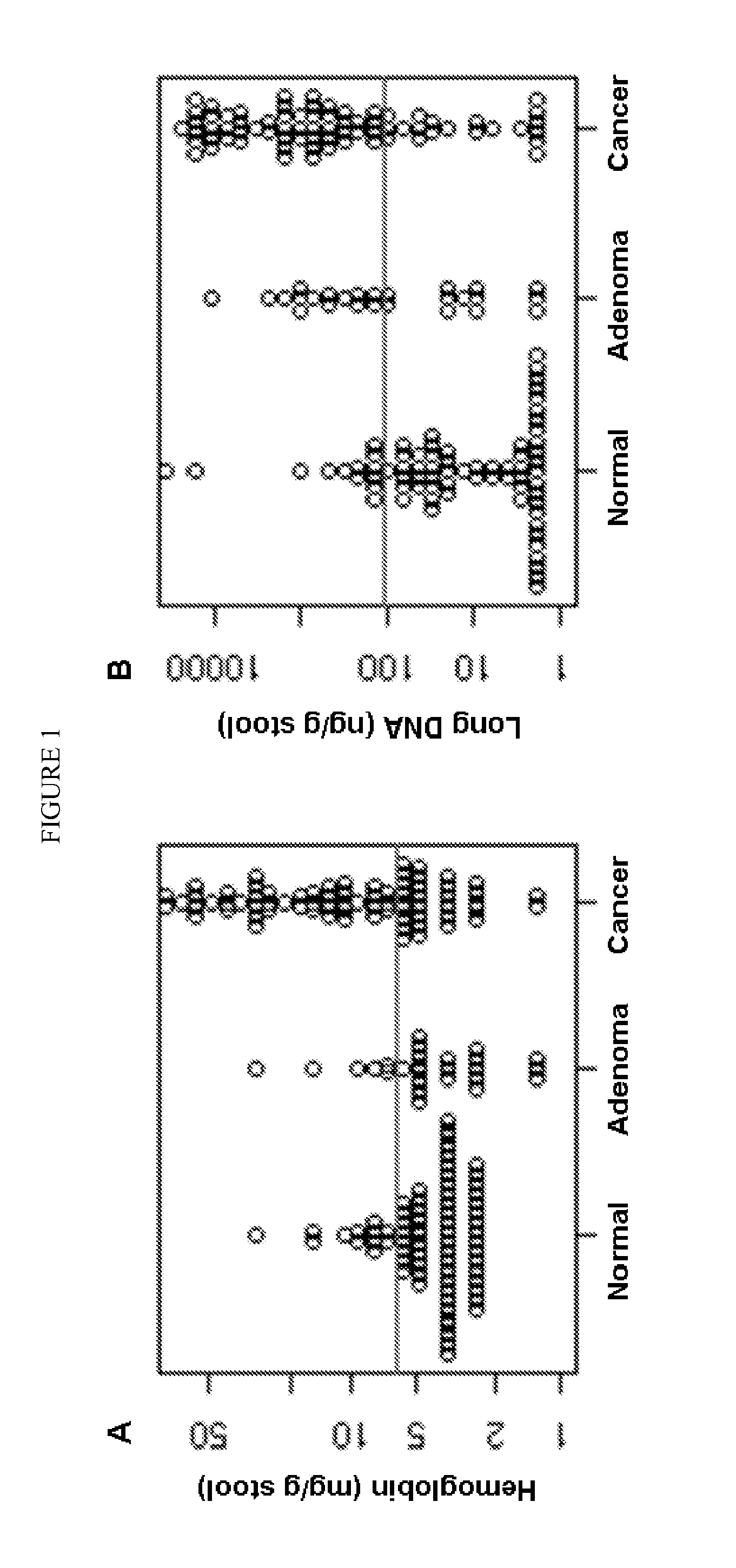
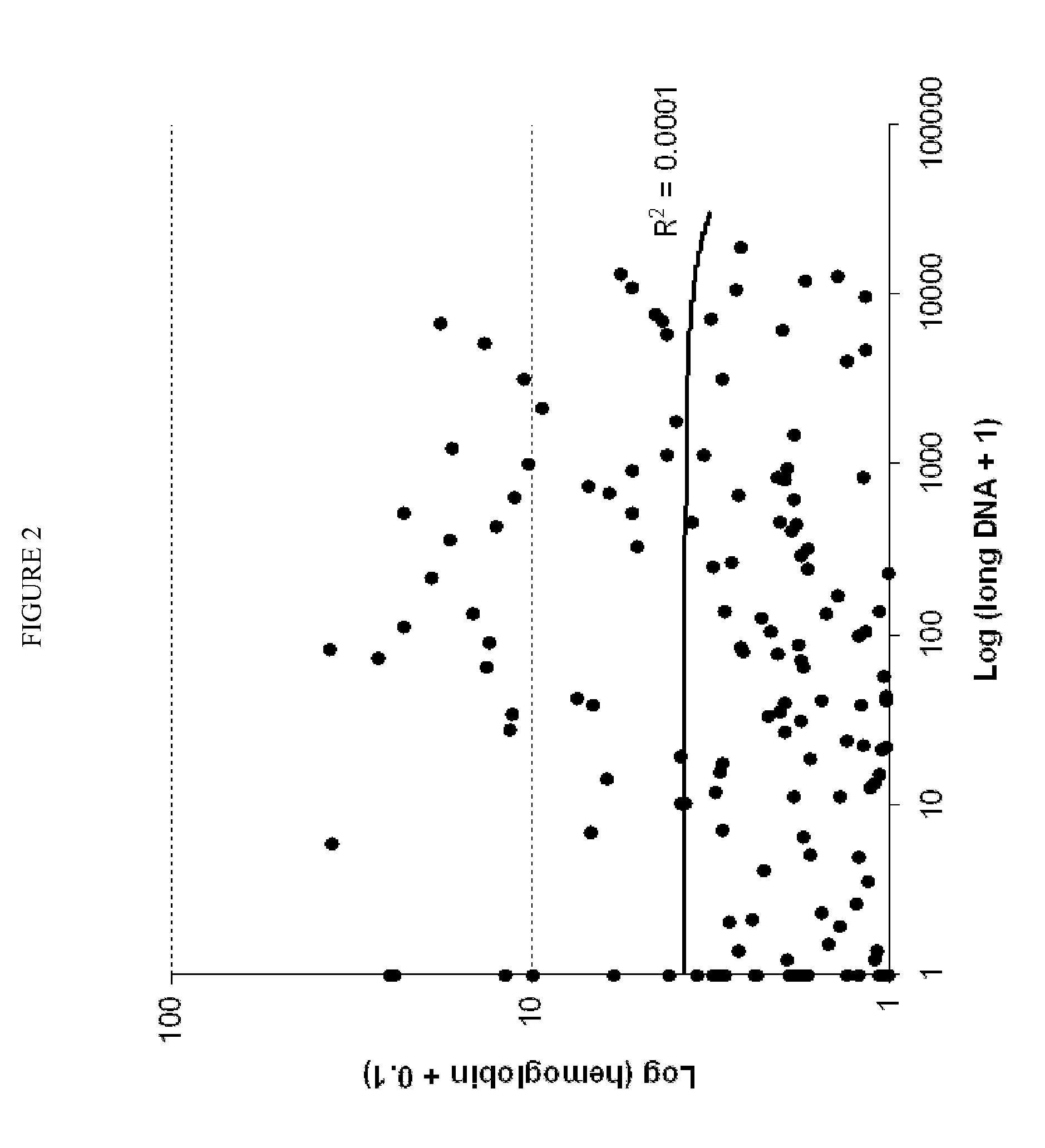
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals having a colorectal neoplasm by detecting the presence of exfoliated epithelial markers (e.g., human DNA, tumor associated gene alterations, tumor associated proteins) and blood markers (e.g., homoglobin, serum proteins) in a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Disposable, multi-use, DNA sample collection disk apparatus for field biohazard testing, DNA testing, and personal authentication
InactiveUS20050287536A1Easy to useEasily be indexedBioreactor/fermenter combinationsBiological substance pretreatmentsSmall form factorBiology
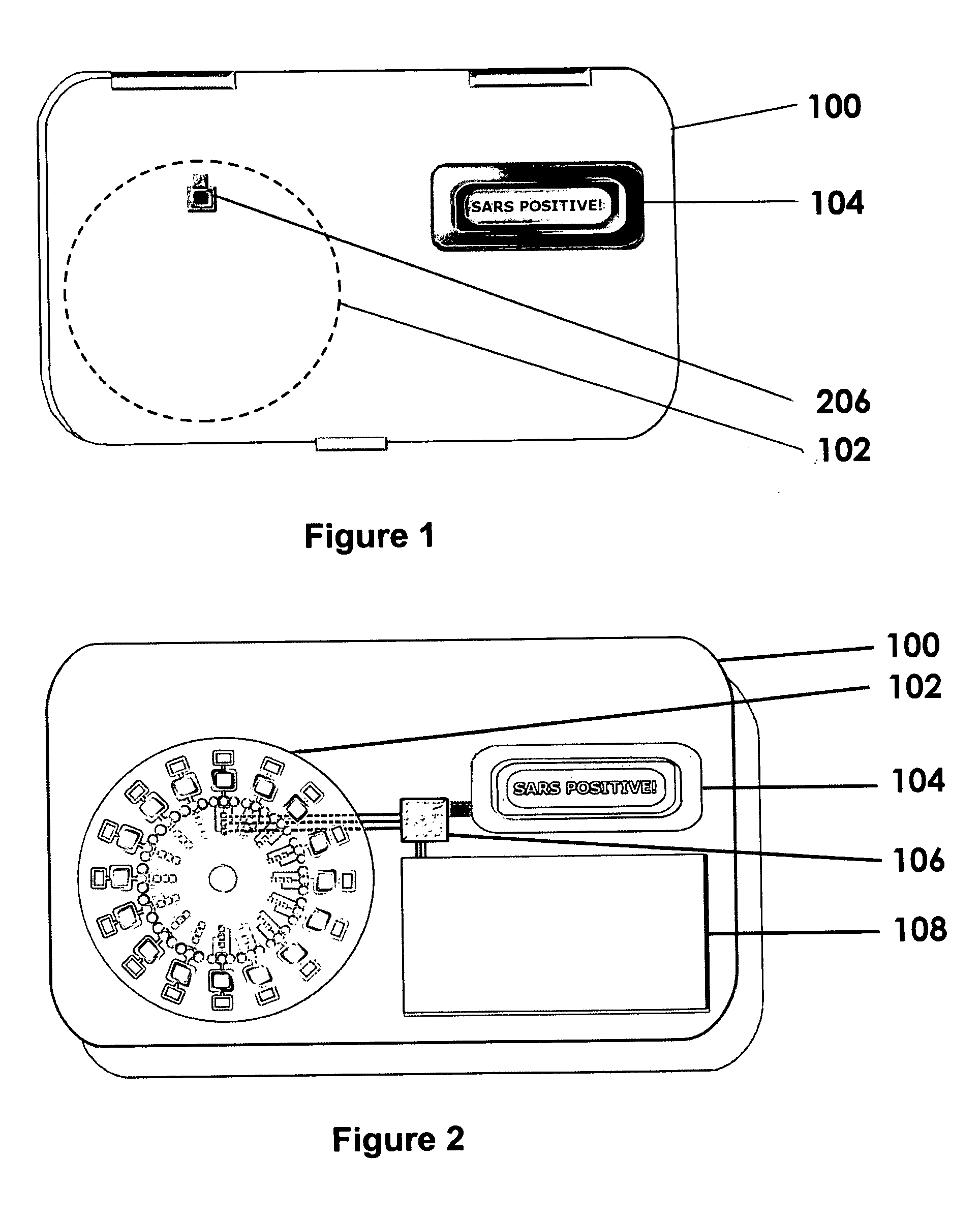
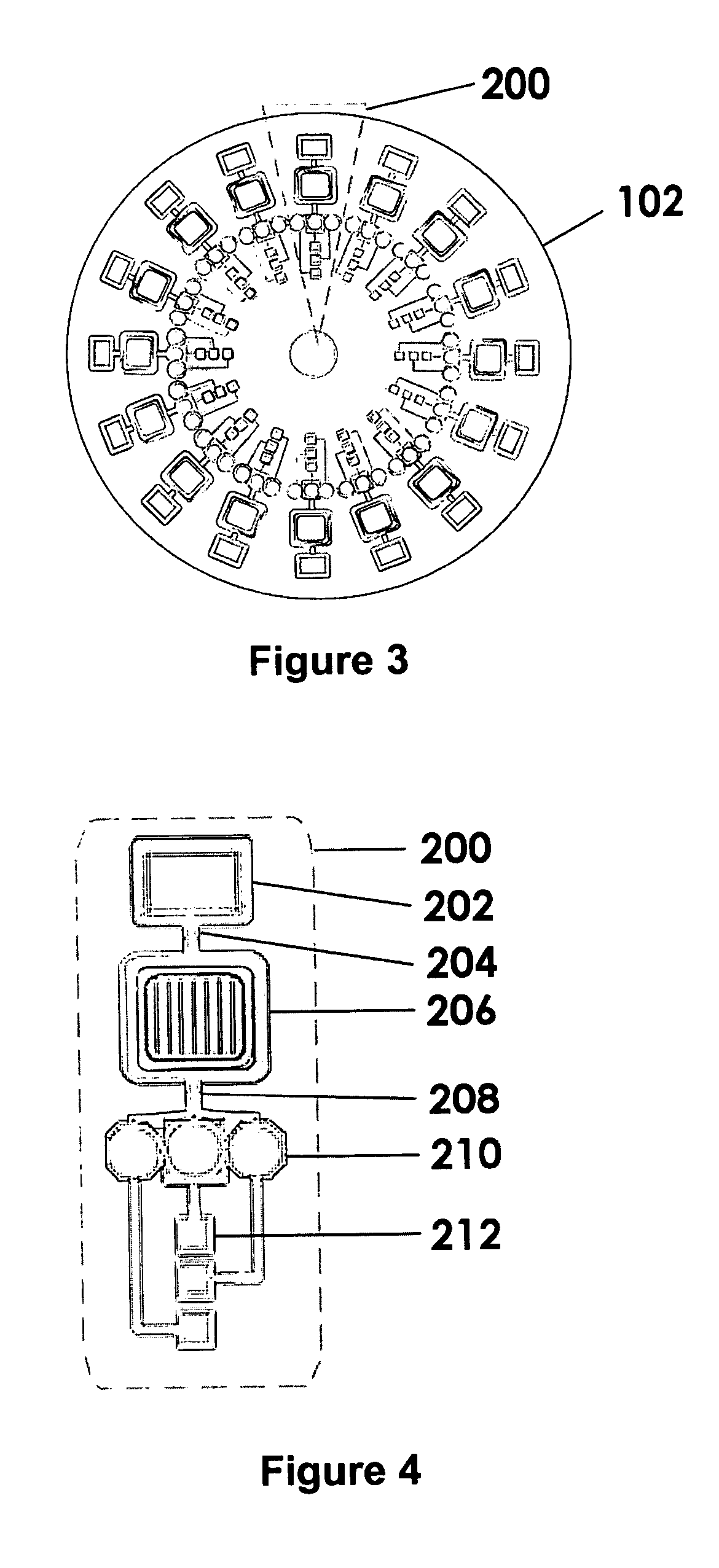
An easy-to-use, versatile, disposable, customer-replaceable, multi-use, DNA sample collection disk apparatus for use with fixed, portable, or field-based DNA sample collection, analysis, and detection systems is disclosed. General features of the invention are its' small form factor, portability, wearability, ease-of-use, and self-contained capacity to collect and analyze multiple different human DNA samples and sample types. The special utility of the invention is demonstrated in the field wherein neither trained medical personnel, nor conventional DNA testing labs, are necessary to operate the invention. Invention preferred embodiments include multipurpose cards or badges designed to (1) electronically authenticate subjects identities using DNA samples, and / or electronically detect presence or absence of biological agents (e.g., anthrax) and / or chemical agents (e.g., Sarin) using DNA samples; and (2) perform other DNA-based, protein-based, or other analytic and / or identification functions.
Owner:BIOMETRIC ASSOCS LP
HIV integrase inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
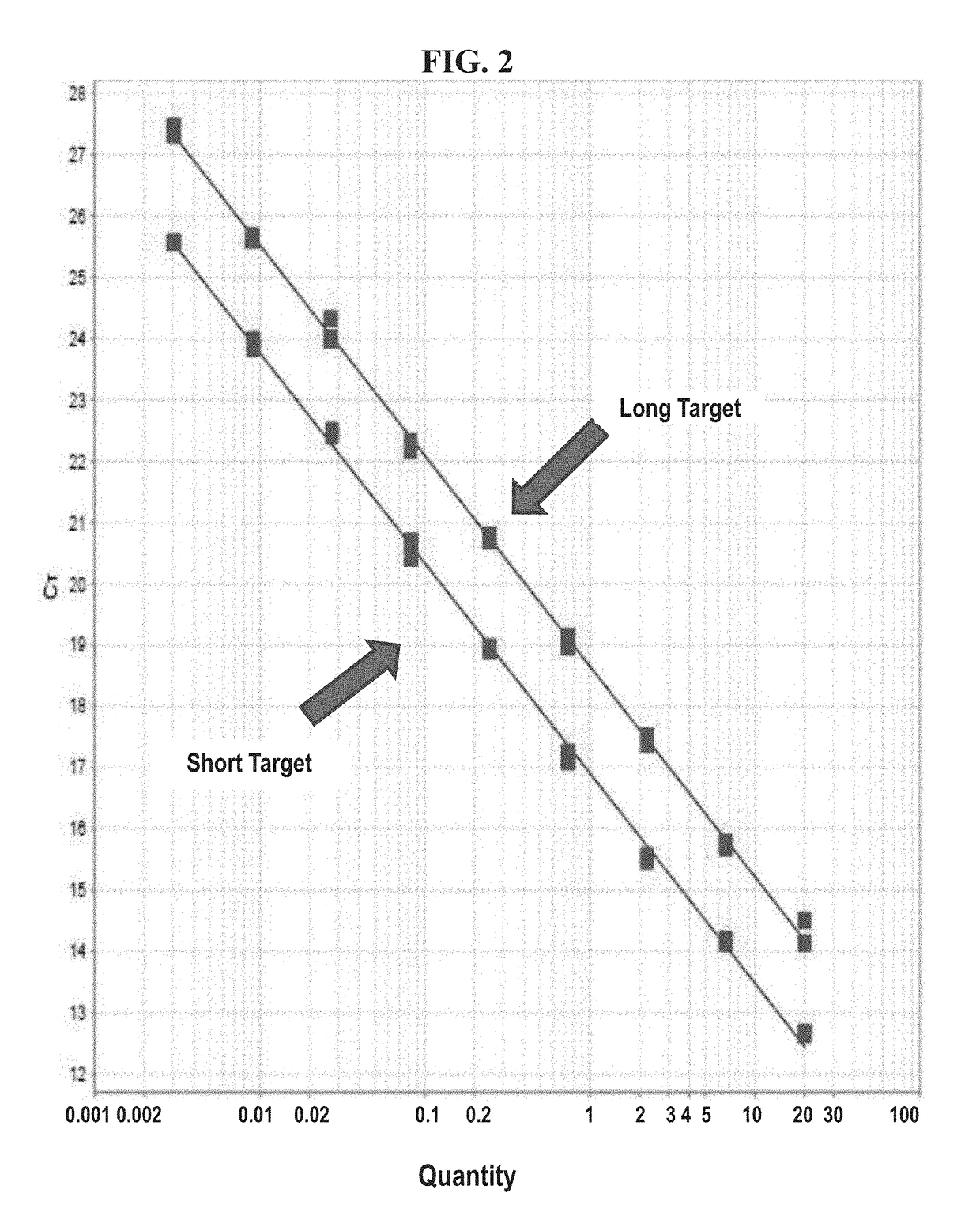
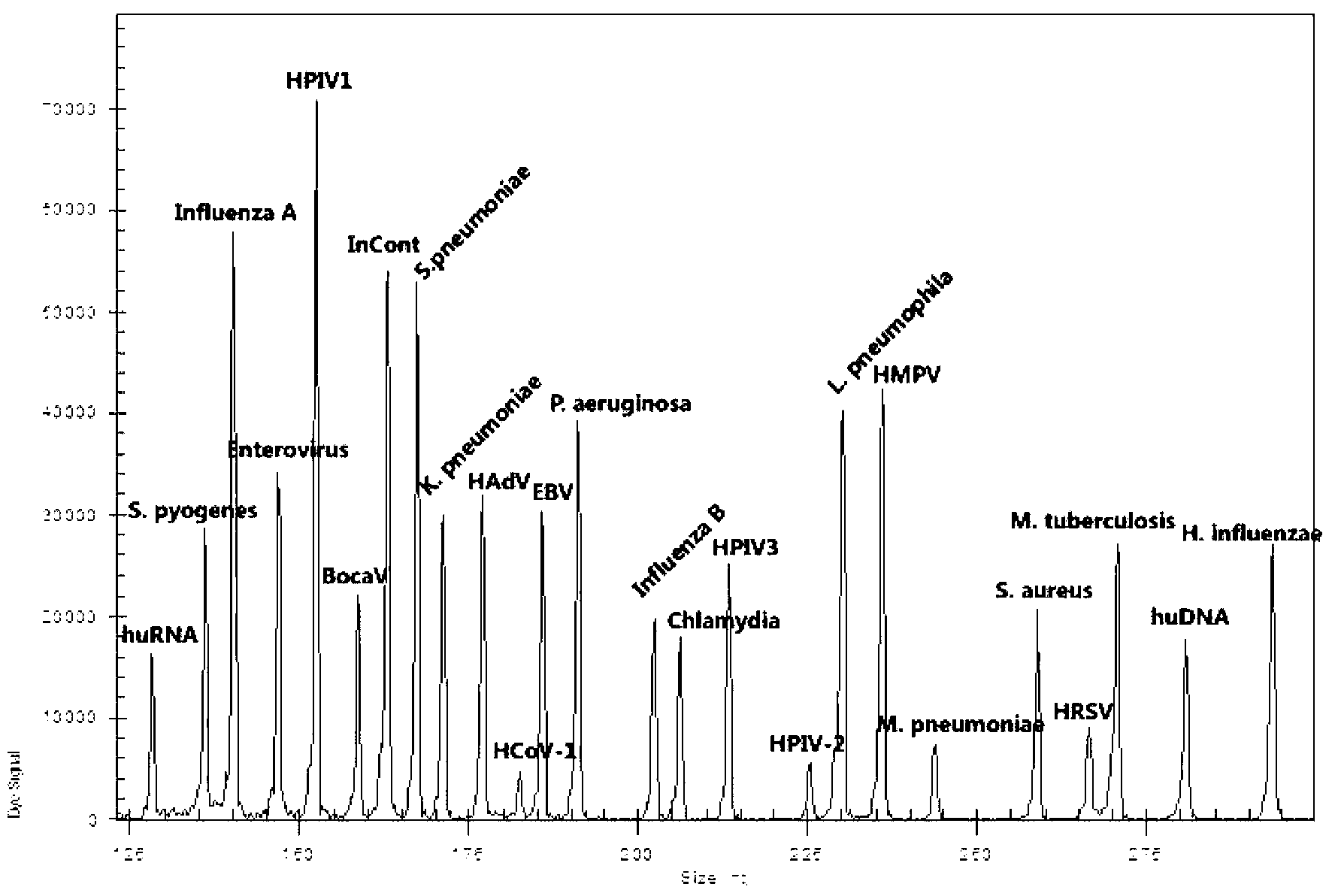
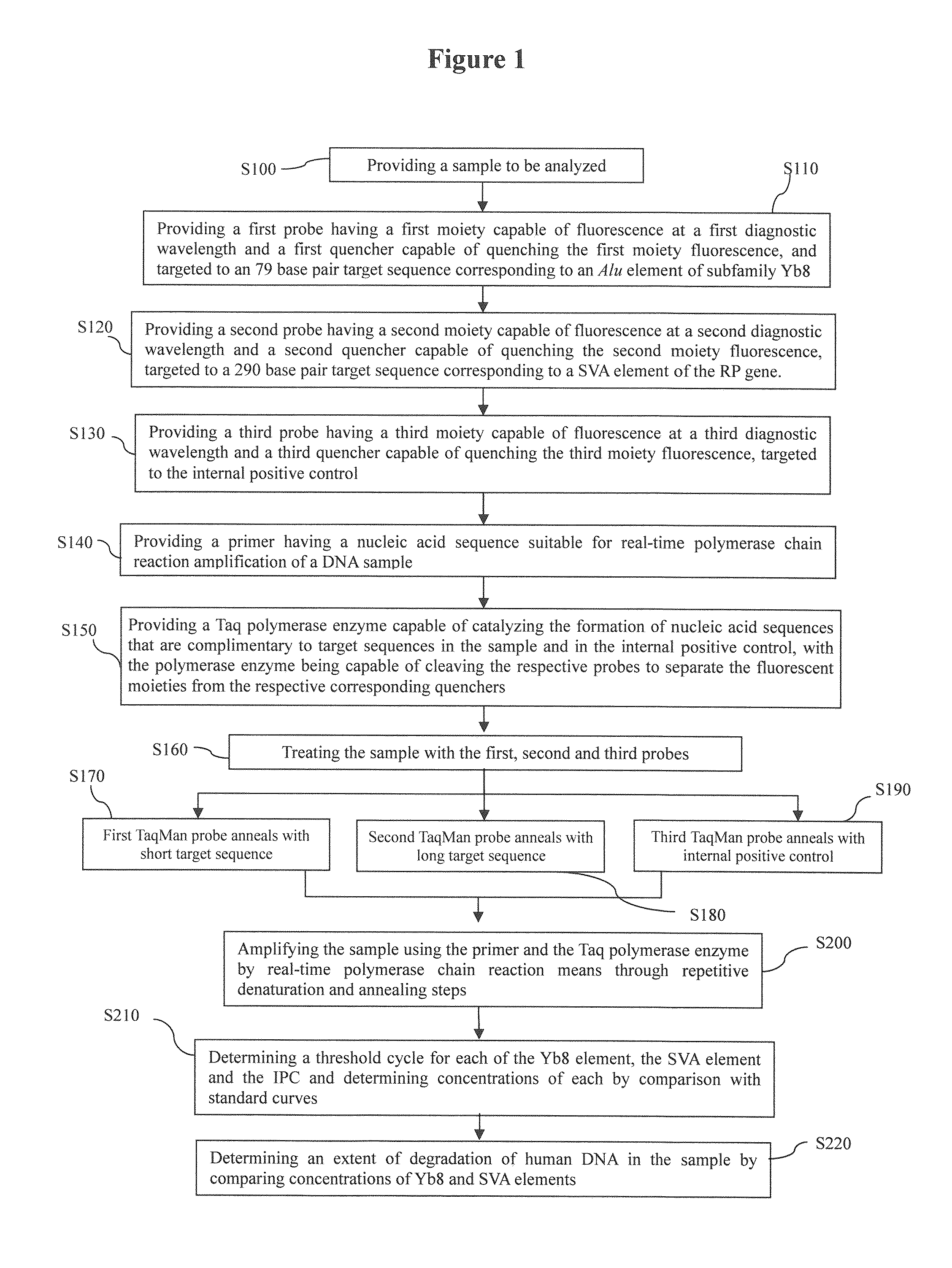
Development of a highly sensitive quantification system for assessing DNA degradation and quality in forensic samples
ActiveUS20140051075A1High sensitivitySimplified suppressionMicrobiological testing/measurementHuman DNA sequencingRetrotransposon transposition
A process of quantifying the extent of degradation present in a human DNA sample is described. The process makes use of a real time PCR system to separately quantitate within a sample a first retrotransposon interspersed element and a relatively longer second retrotransposon interspersed element, where the longer element is expected to be disrupted at a faster pace than is the shorter element as the sample degrades. In one embodiment, the process makes use of the appearance of the relatively young (on an evolutionary scale) Alu Yb-lineage subfamily sequences appearing in every human genome and their virtual absence in non-human samples. In a preferred embodiment, the process quantifies longer 290 bp sequences of “SVA” elements and shorter 80 bp sequences of Alu Yb8-lineage. Newly designed primers and TaqMan probes that are useful in the process are presented. A related process additionally quantifies male specific human DNA.
Owner:LIFE GENETICS LAB
HIV Integrase Inhibitors
The invention encompasses a series pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
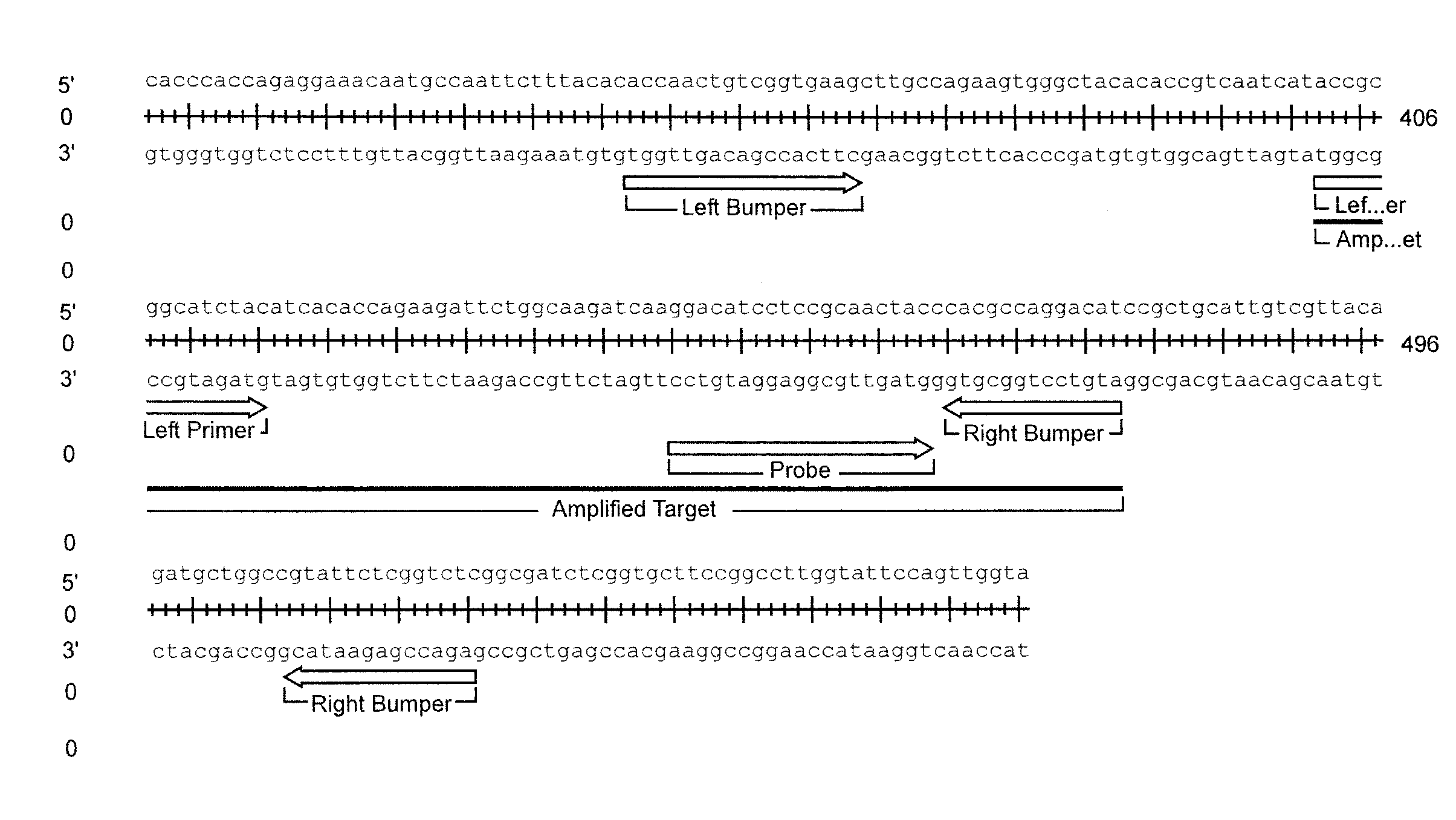
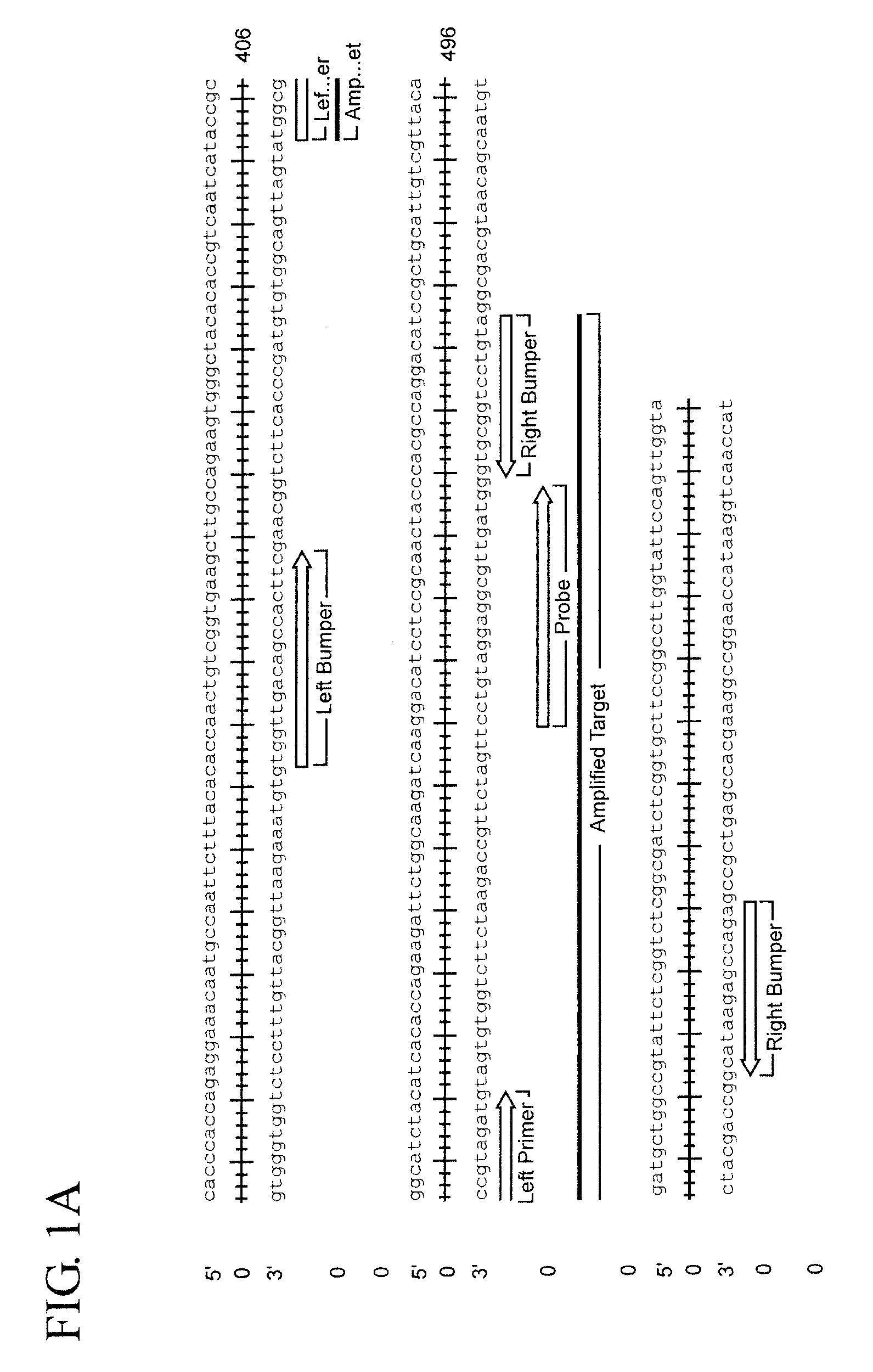
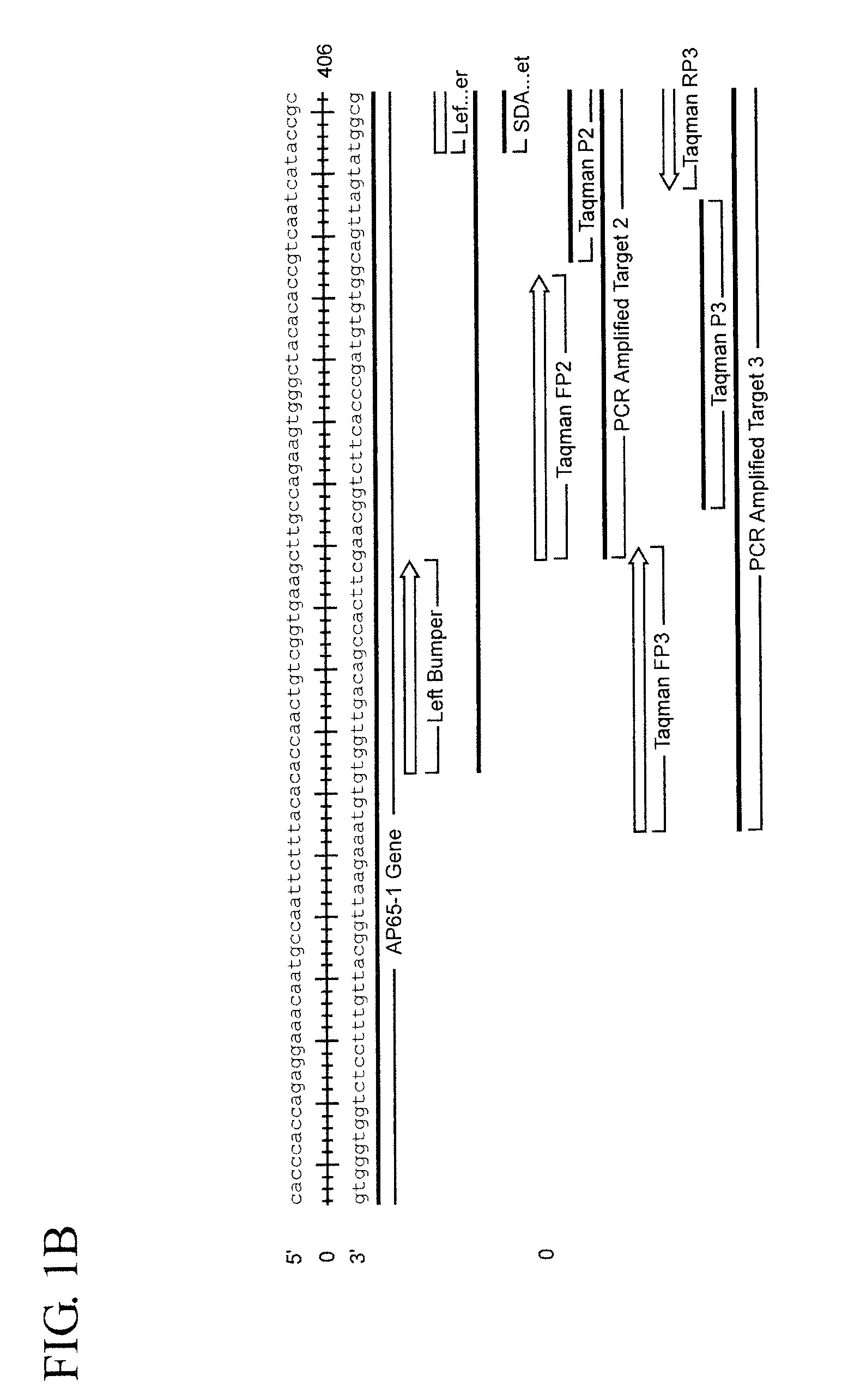
Assay for trichomonas vaginalis by amplification and detection of trichomonas vaginalis ap65-1 gene
ActiveUS20100184066A1Easy to detectIncrease temperatureMicrobiological testing/measurementMicroorganismTrichomona vaginalis
A region of the Trichomonas vaginalis AP65-1 gene has been identified which is useful for performing amplification assays to determine specifically whether T. vaginalis is present in the sample being tested. Oligonucleotides useful for performing thermal Strand Displacement Assay (tSDA) reactions on this gene are disclosed. The disclosed oligonucleotides can be used in an assay which is specific for multiple strains of T. vaginalis and which does not show cross reactivity with the genomes of other microorganisms or with human DNA.
Owner:BECTON DICKINSON & CO
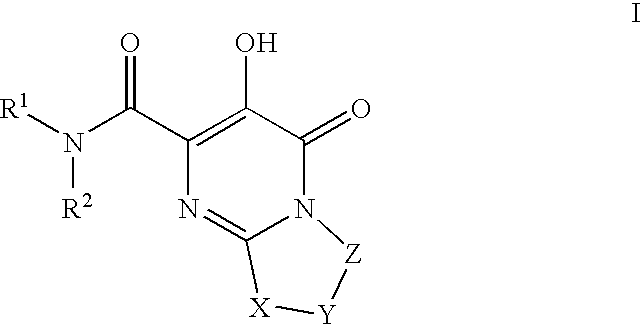
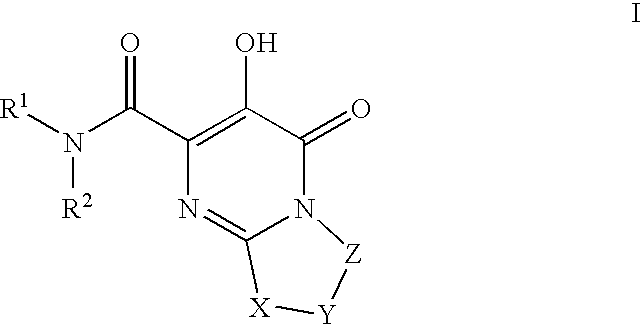
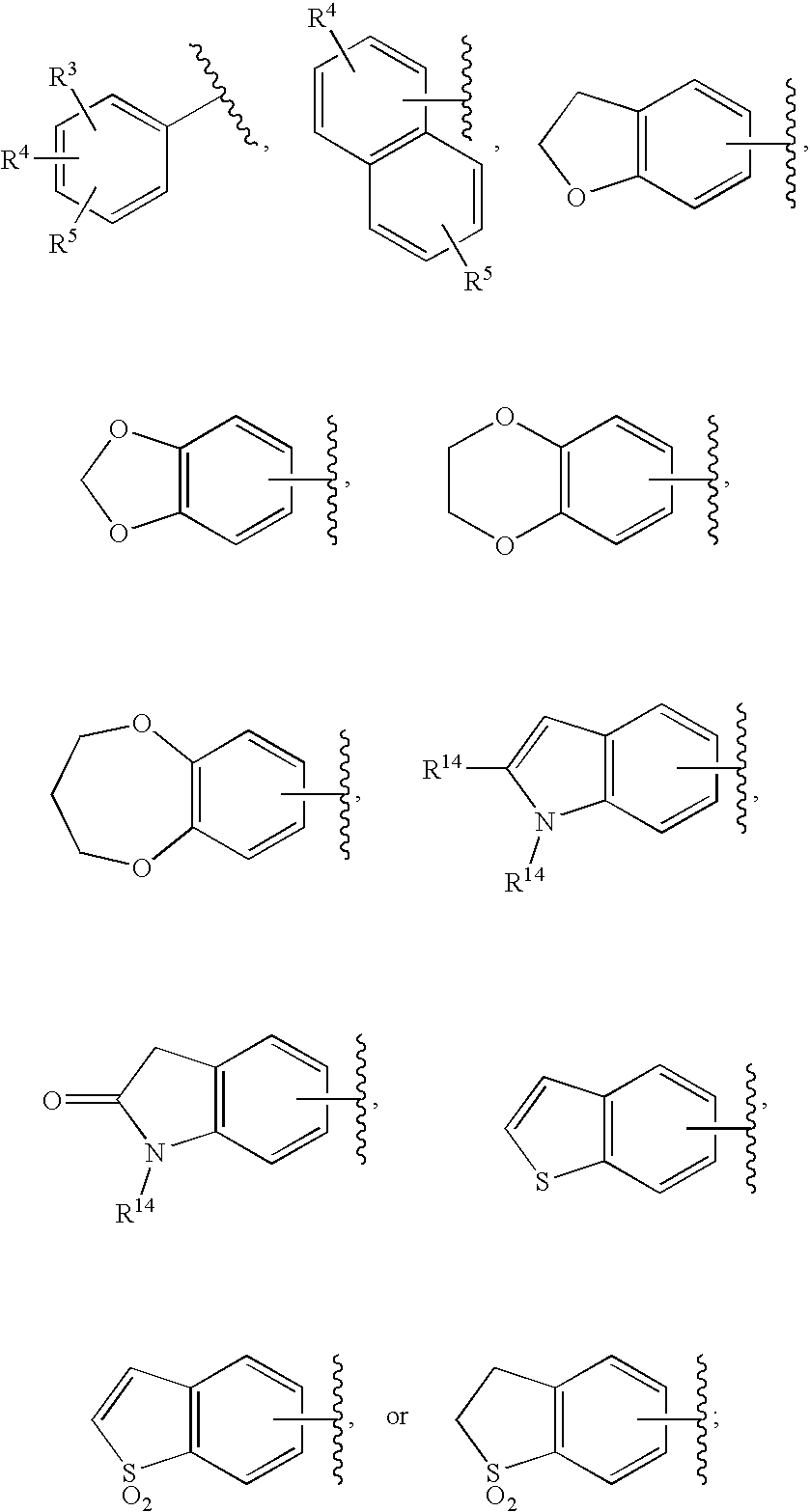
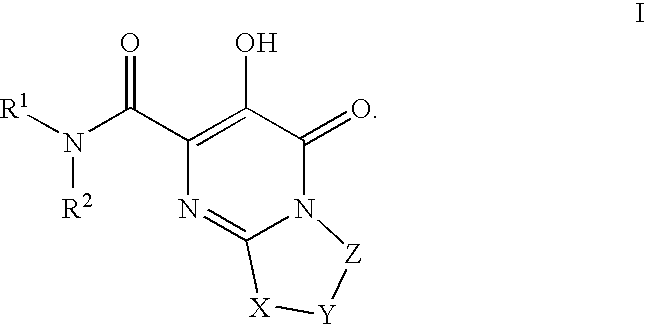
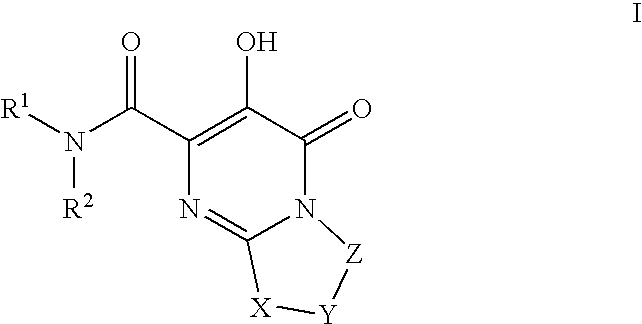
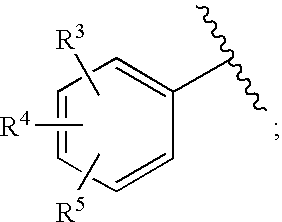
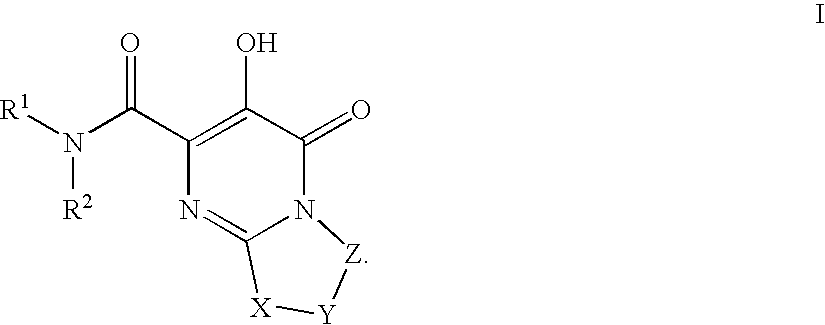
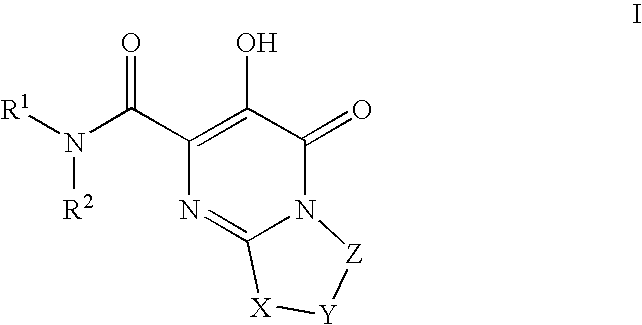
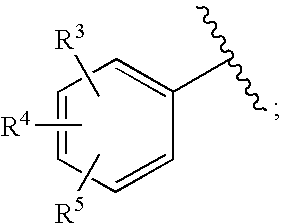
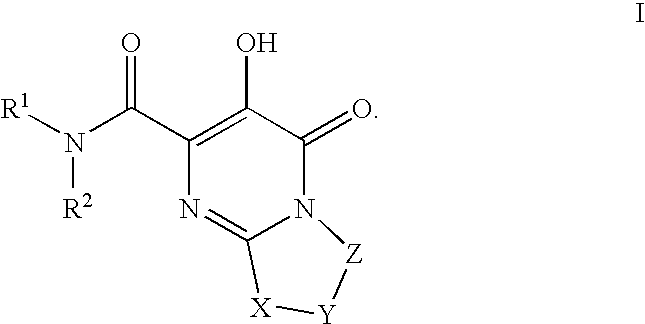
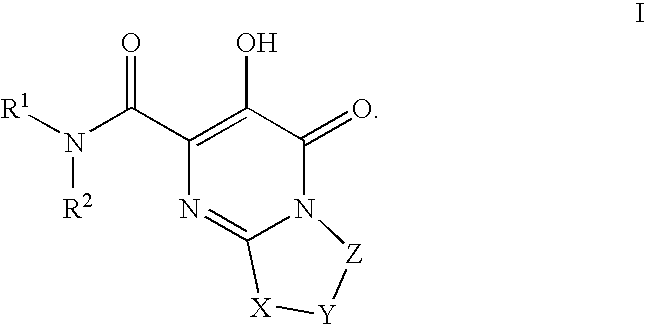
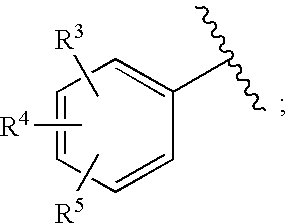
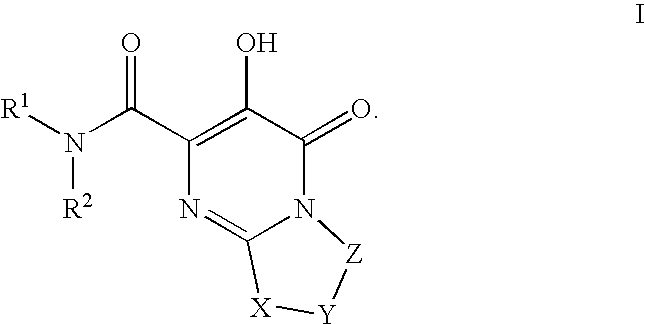
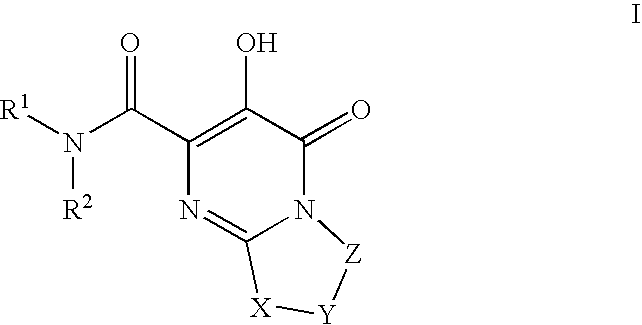
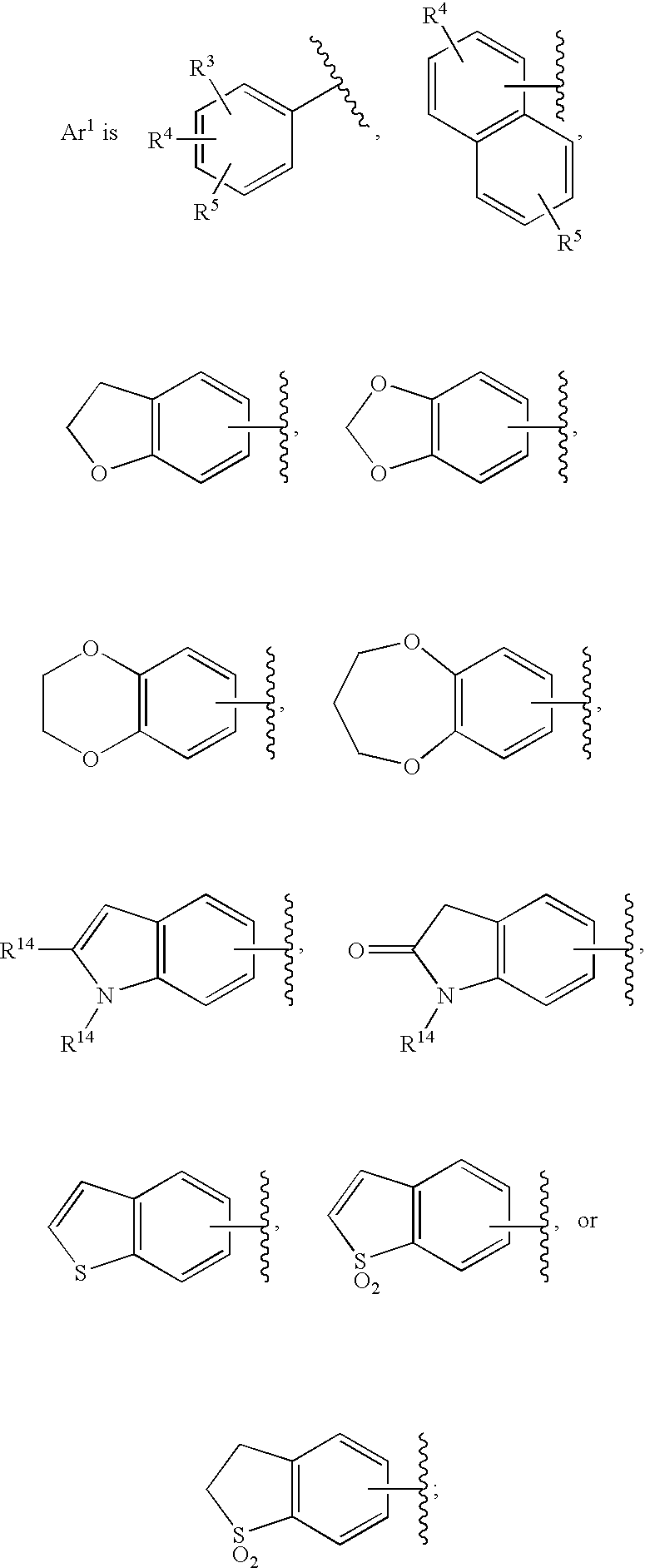
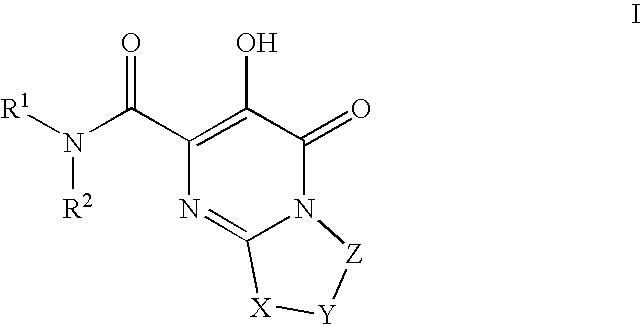
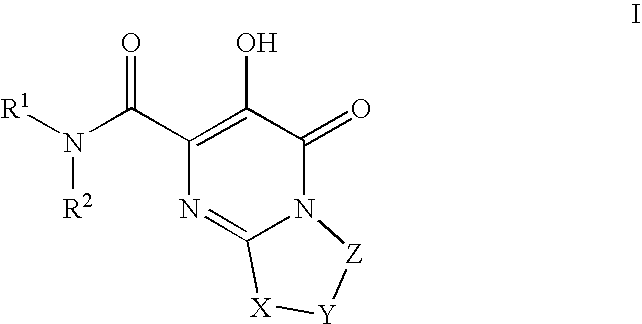
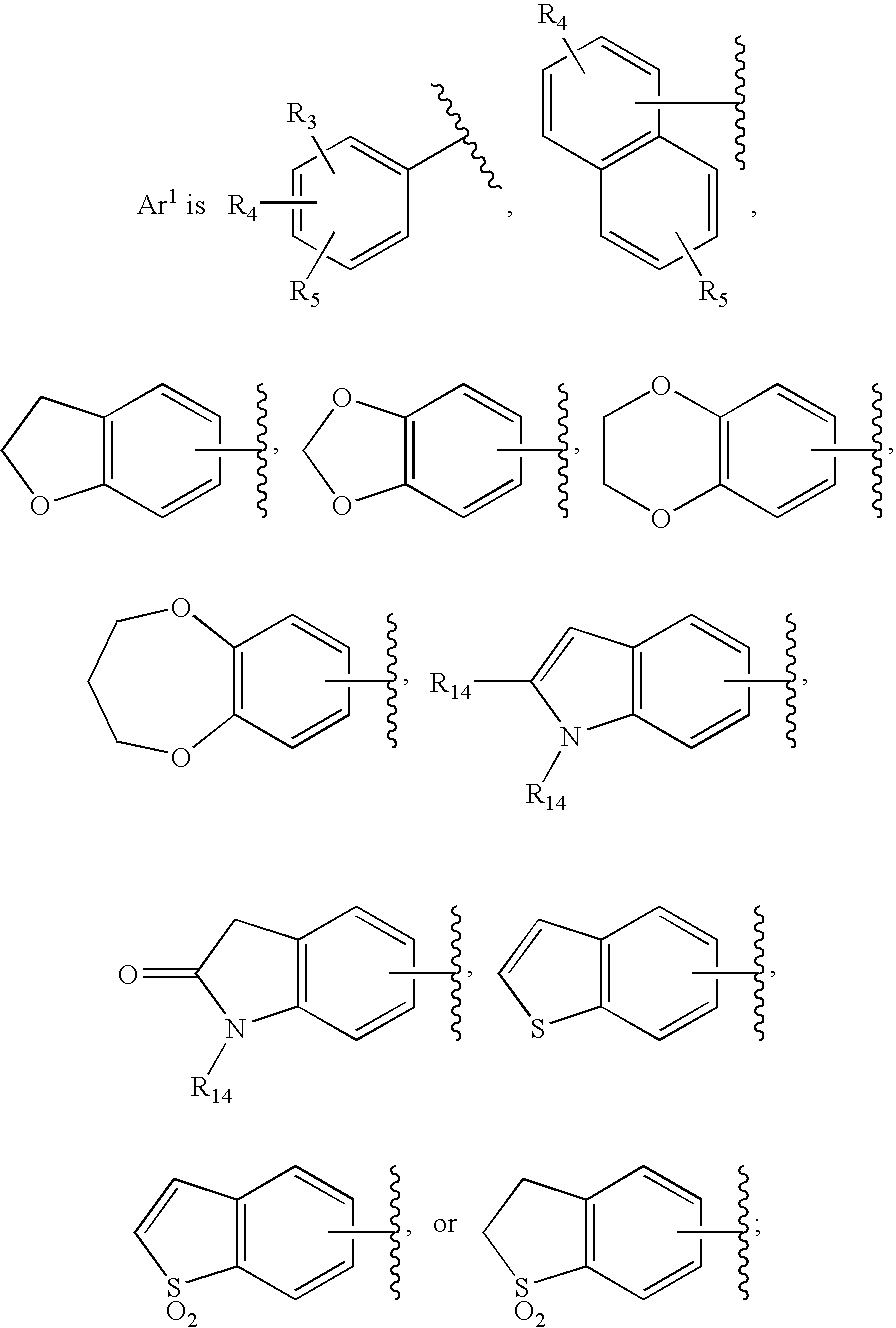
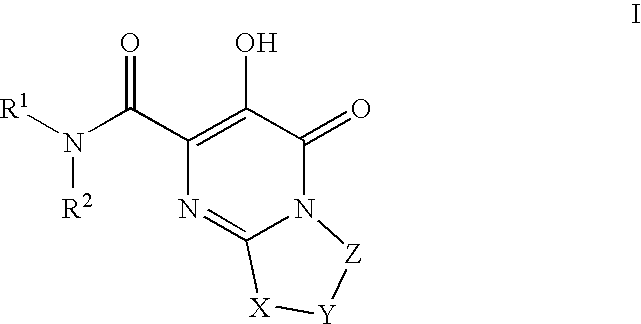
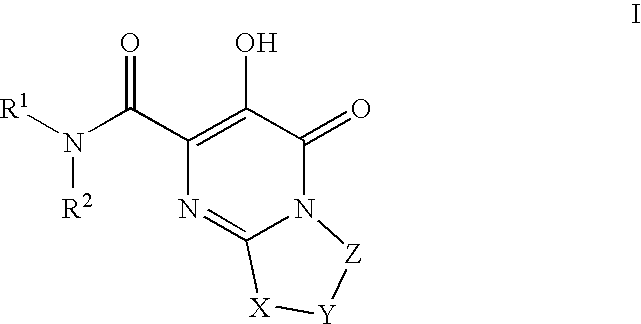
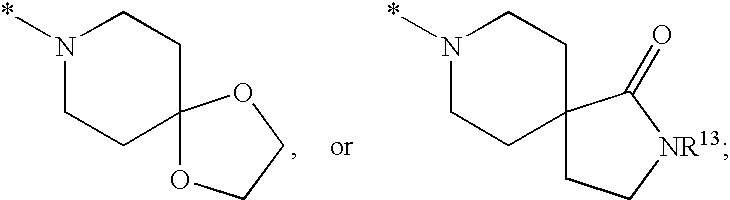
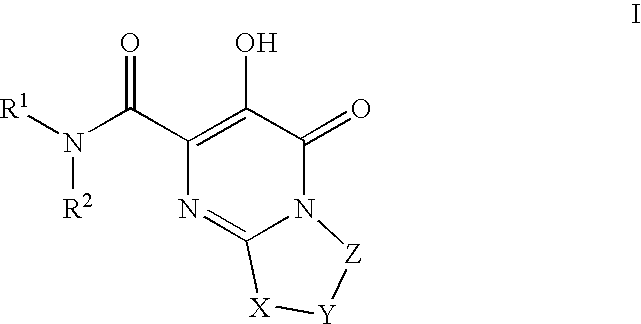
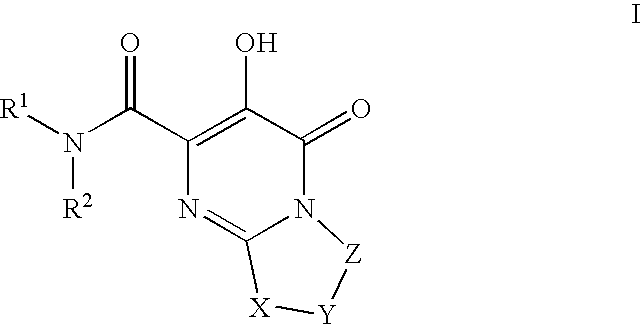
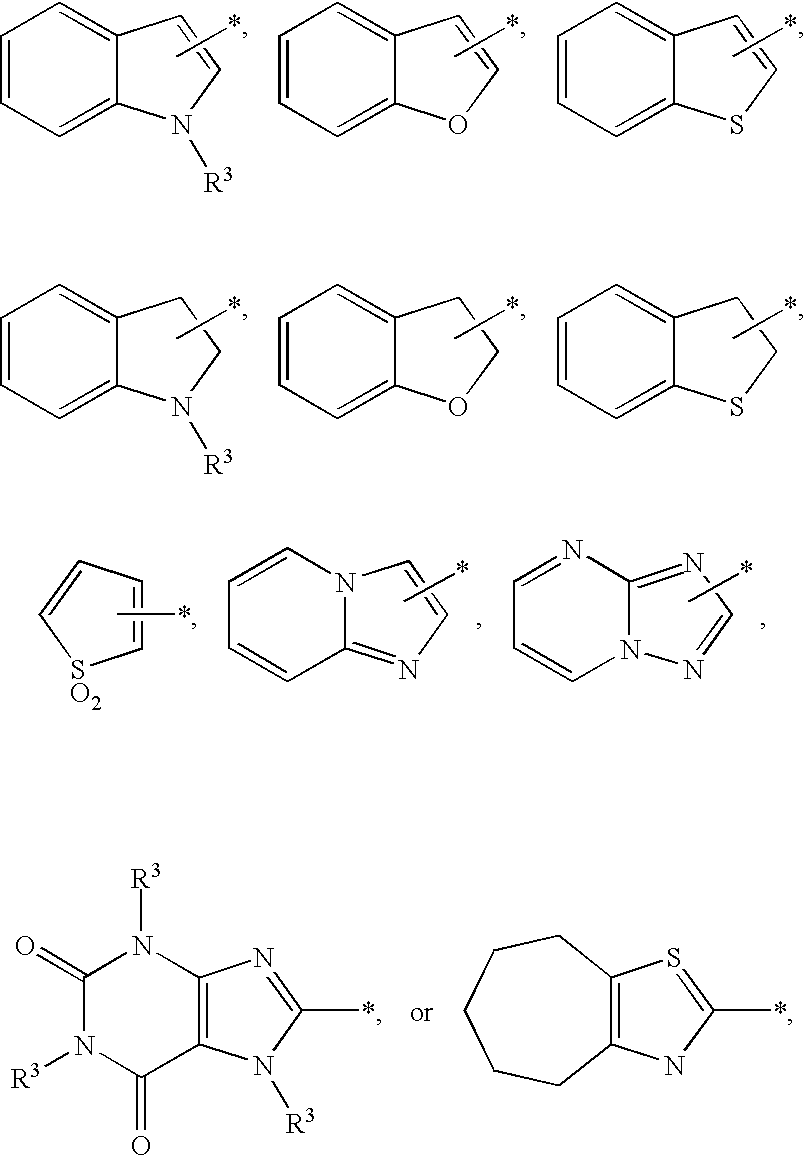
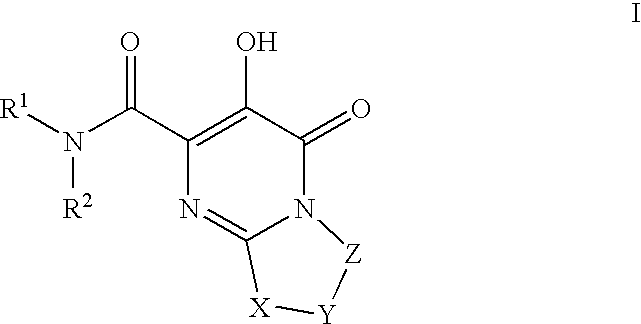
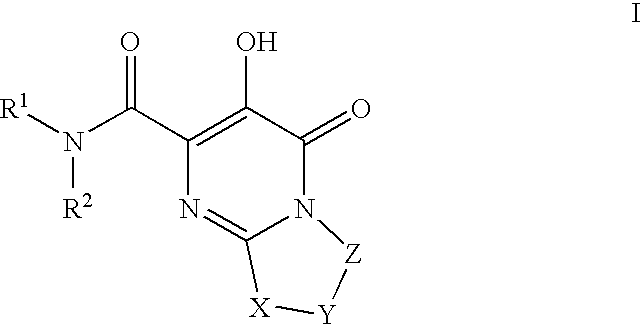
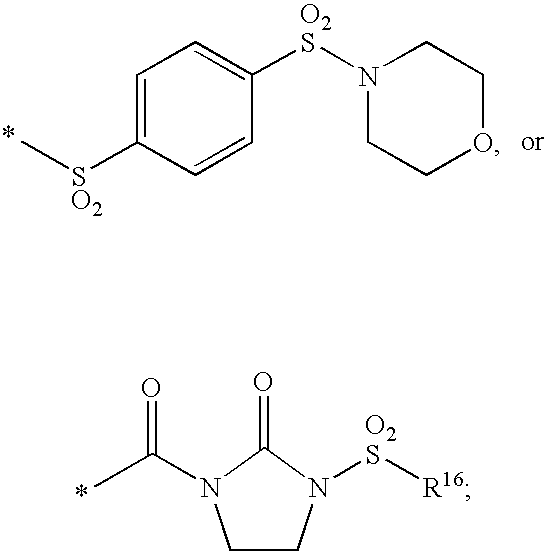
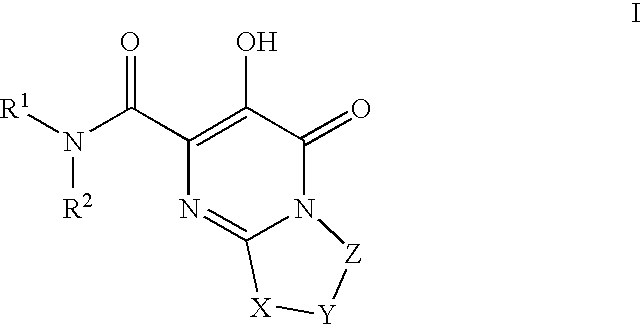
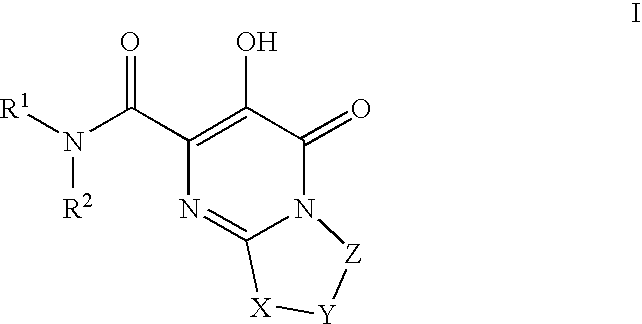
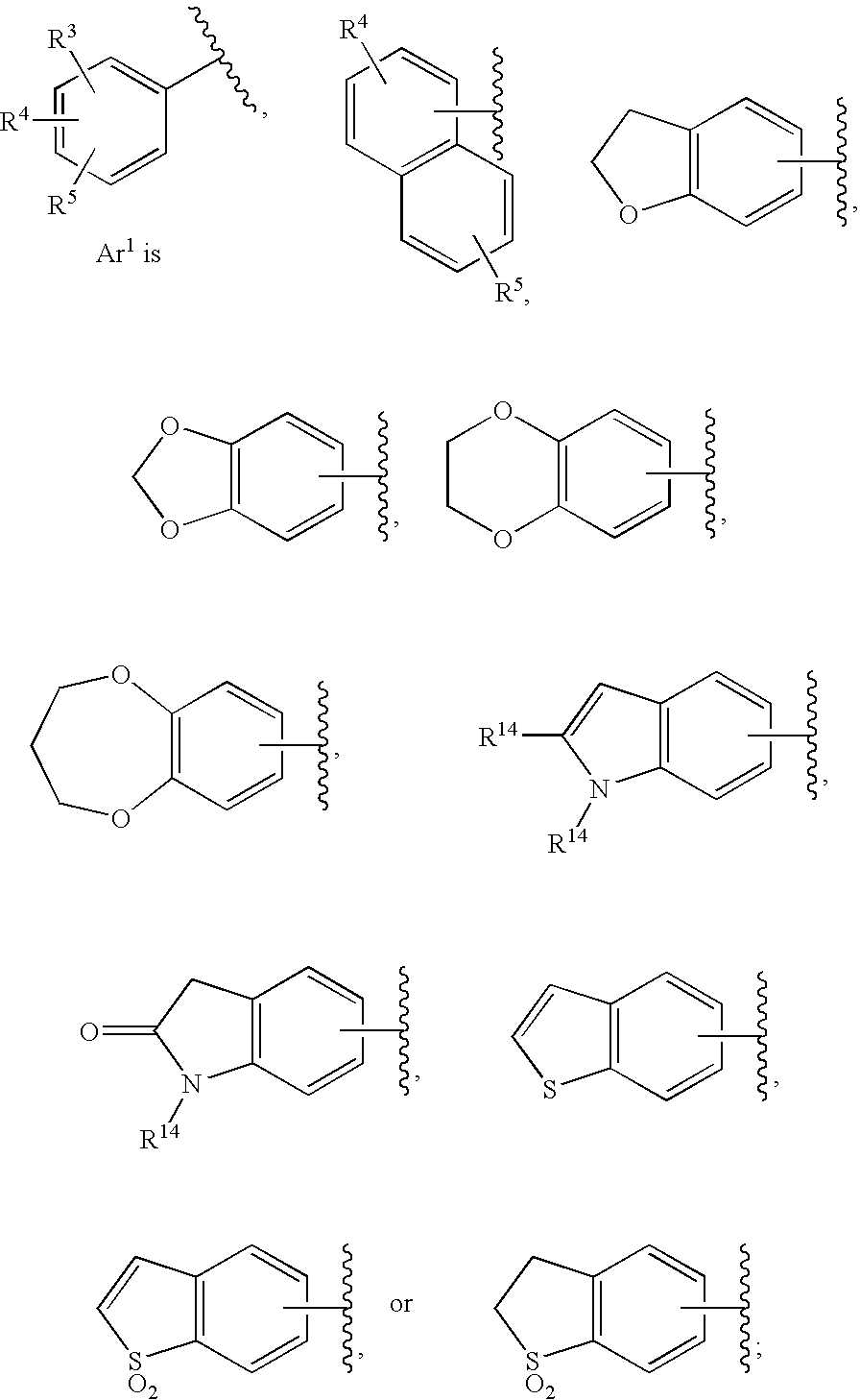
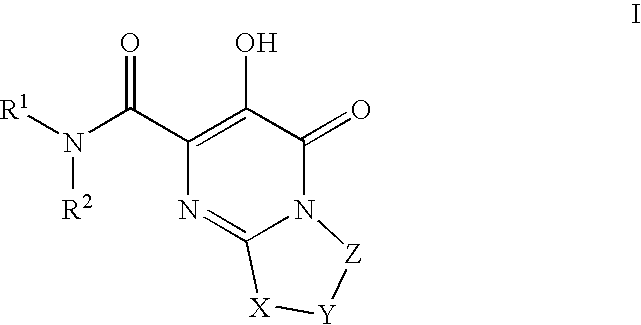
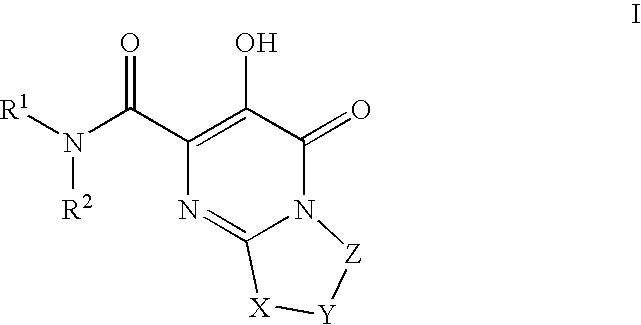
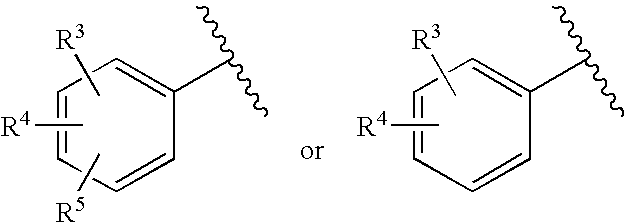
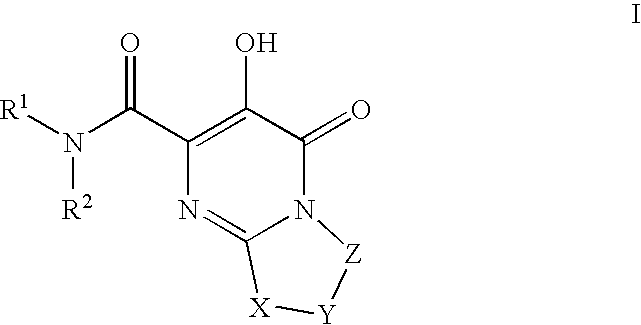
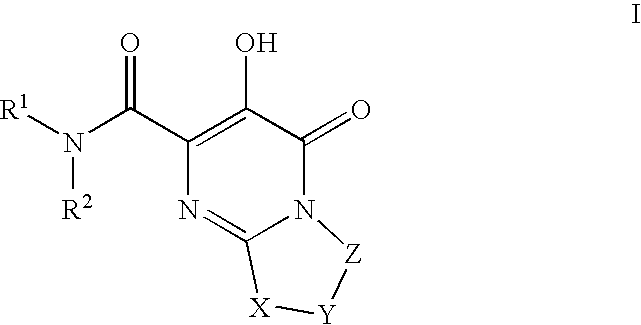
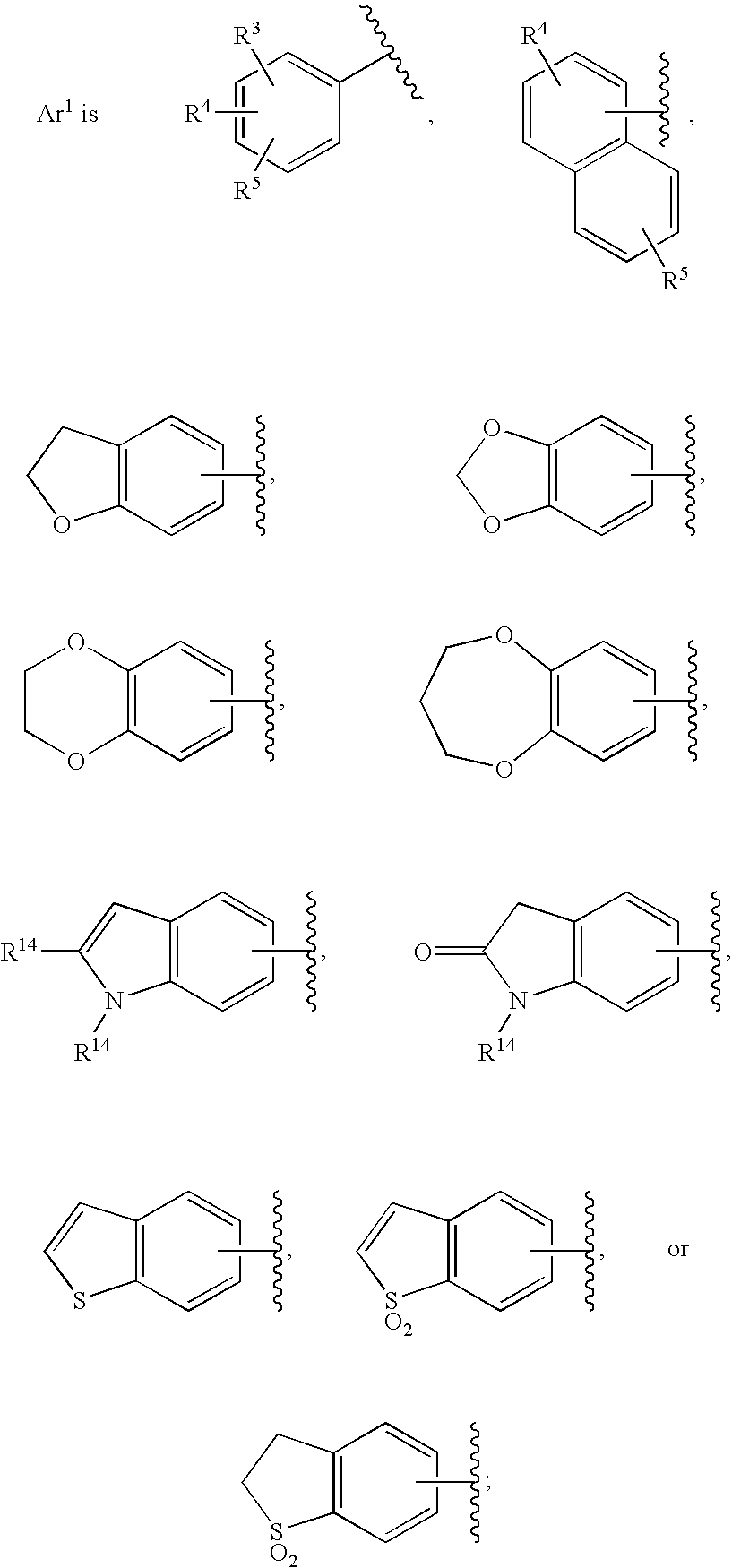
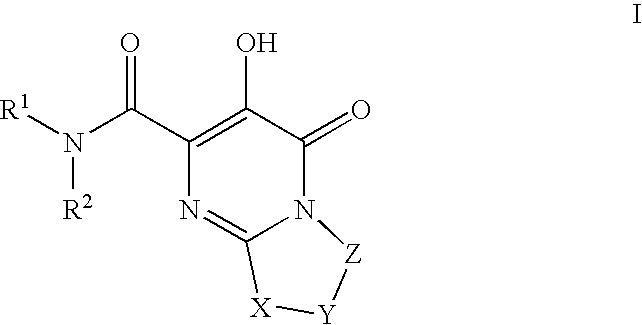
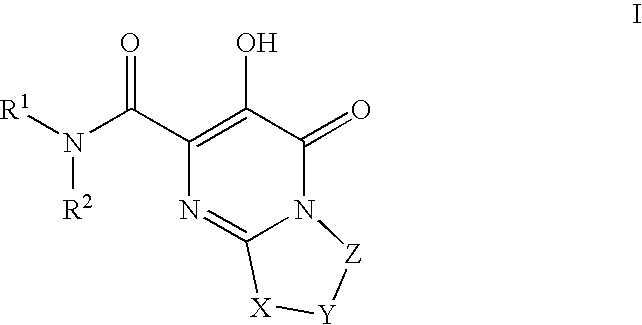
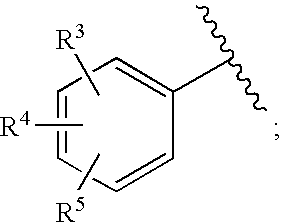
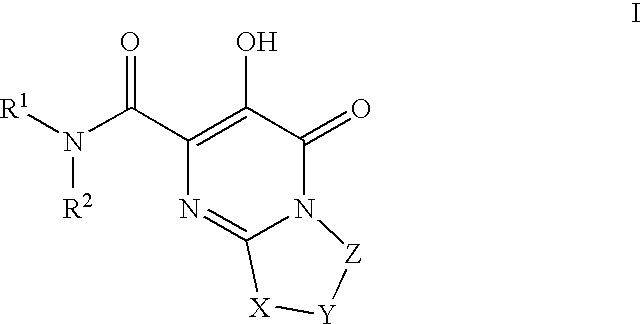
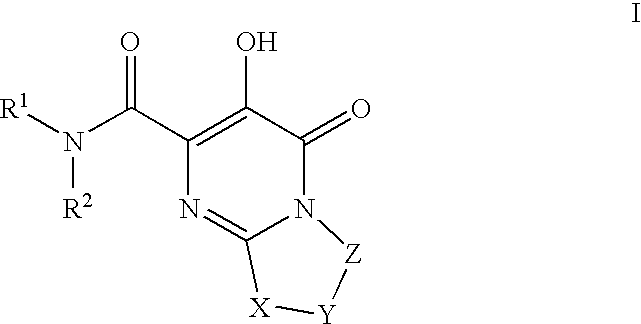
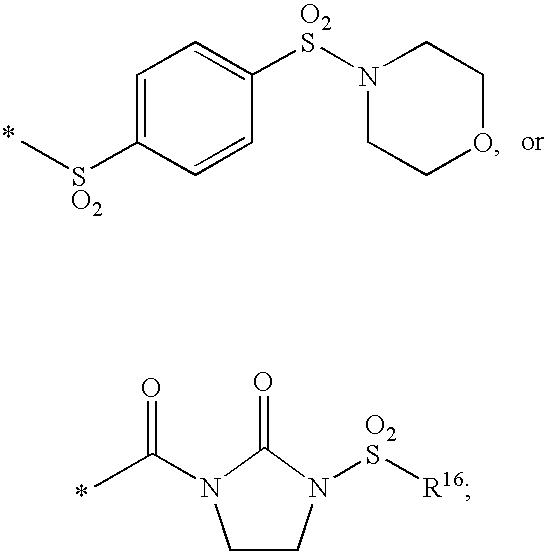
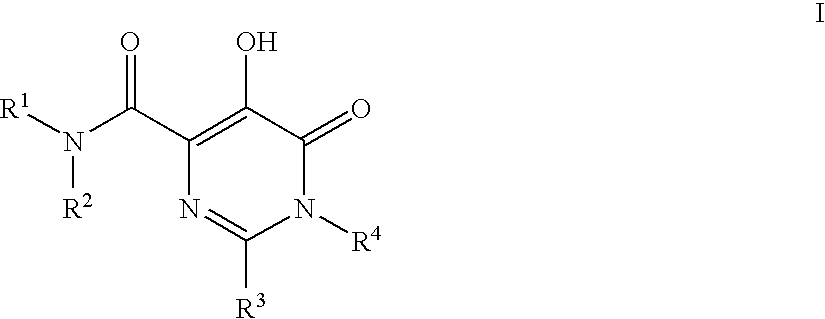
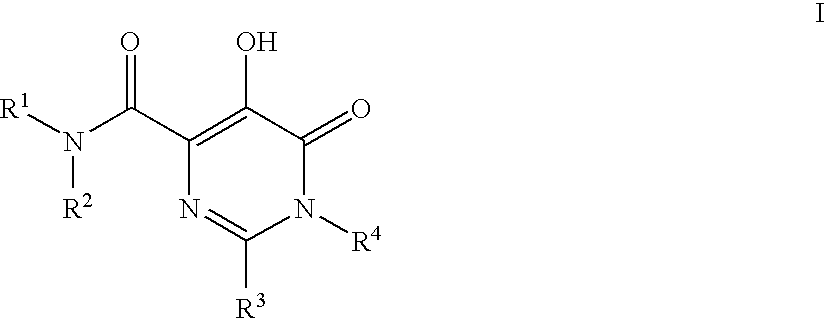
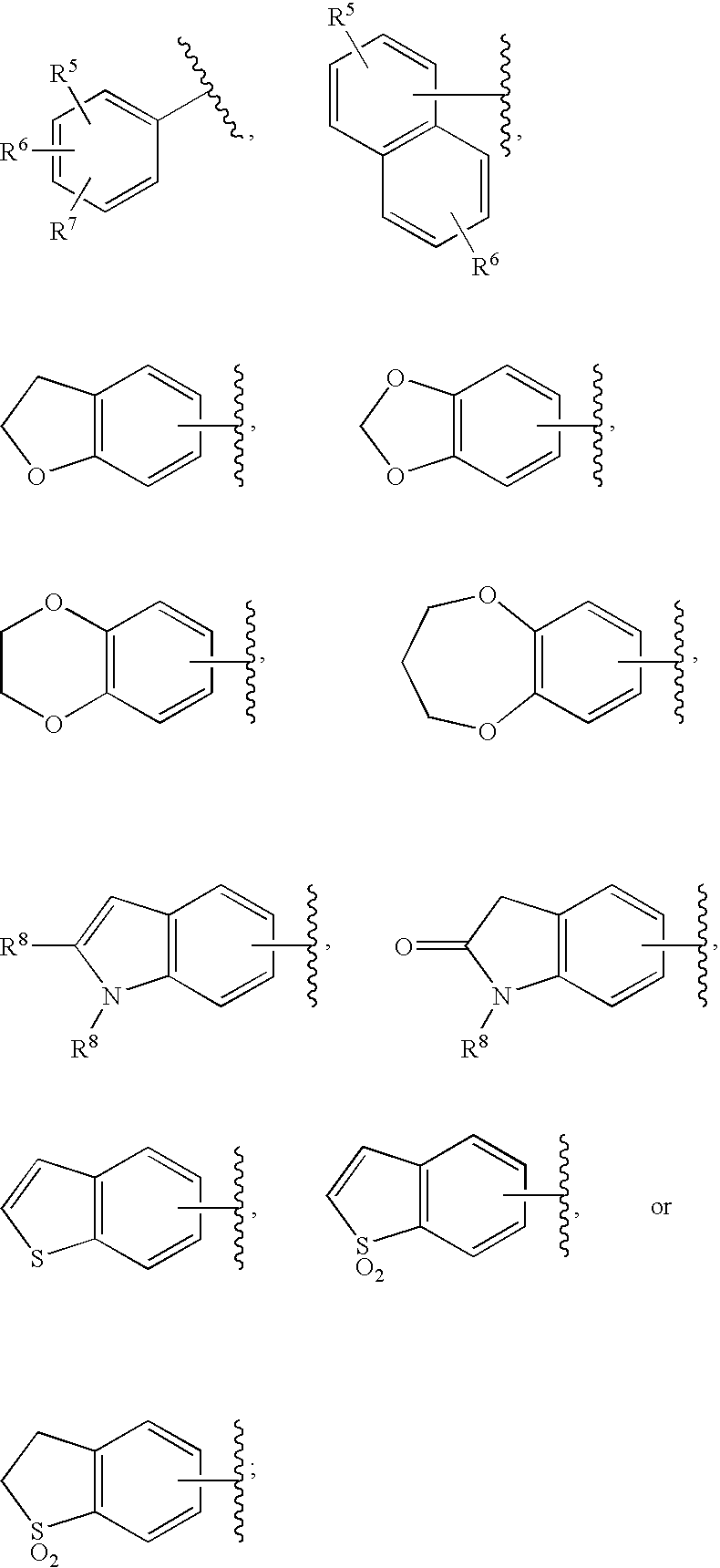
Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors
The invention encompasses a series of bicyclic heterocyclic compounds of Formula I which are inhibitors of HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Methods and compositions for improving plant growth
The present invention has surprisingly discovered that certain spore-forming microorganisms, present in soil, plants and other forms of organic matter, survive excessive heat of a fire within soil or carbonized wood. It has been determined that such microorganisms stimulate plant growth, enhance the nutritional value of plant products, and incorporate carbon dioxide. Accordingly, the present invention provides methods for isolating and identifying plant growth-stimulating microorganisms from soil and from carbonized organic materials. The present invention also provides compositions and methods useful for enhancing plant growth and nutritional properties and for producing DNA-enhanced plants which may be consumed by human individuals for enhancing human DNA. The compositions and methods of the present invention are also useful for improving soil. The invention also provides methods for using charcoal as a carrier to promote plant growth, and to transfer and relocate desirable microorganisms from one ecosystem to another.
Owner:MARRELLI JOHN CHARLES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00001.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00002.png)
![Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors Substituted imidazo[1,2-a]pyrimidines as HIV viral DNA integrase inhibitors](https://images-eureka.patsnap.com/patent_img/6adf7269-b42f-4b83-9357-b8de6749ea21/US07494984-20090224-C00003.png)