Methods and materials for detecting colorectal neoplasm
a colorectal cancer and colorectal neoplasm technology, applied in the field of colorectal neoplasm specific marker detection, can solve the problem of colorectal cancer risk profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Subjects
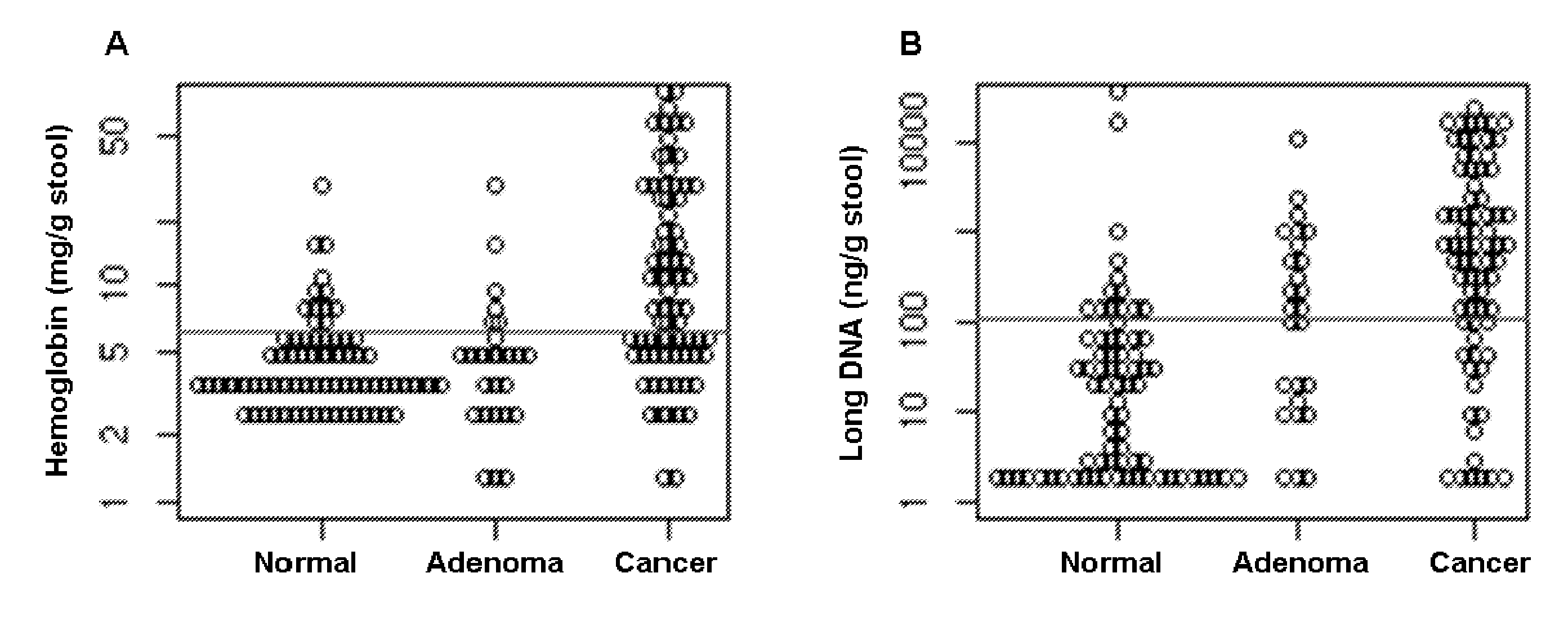
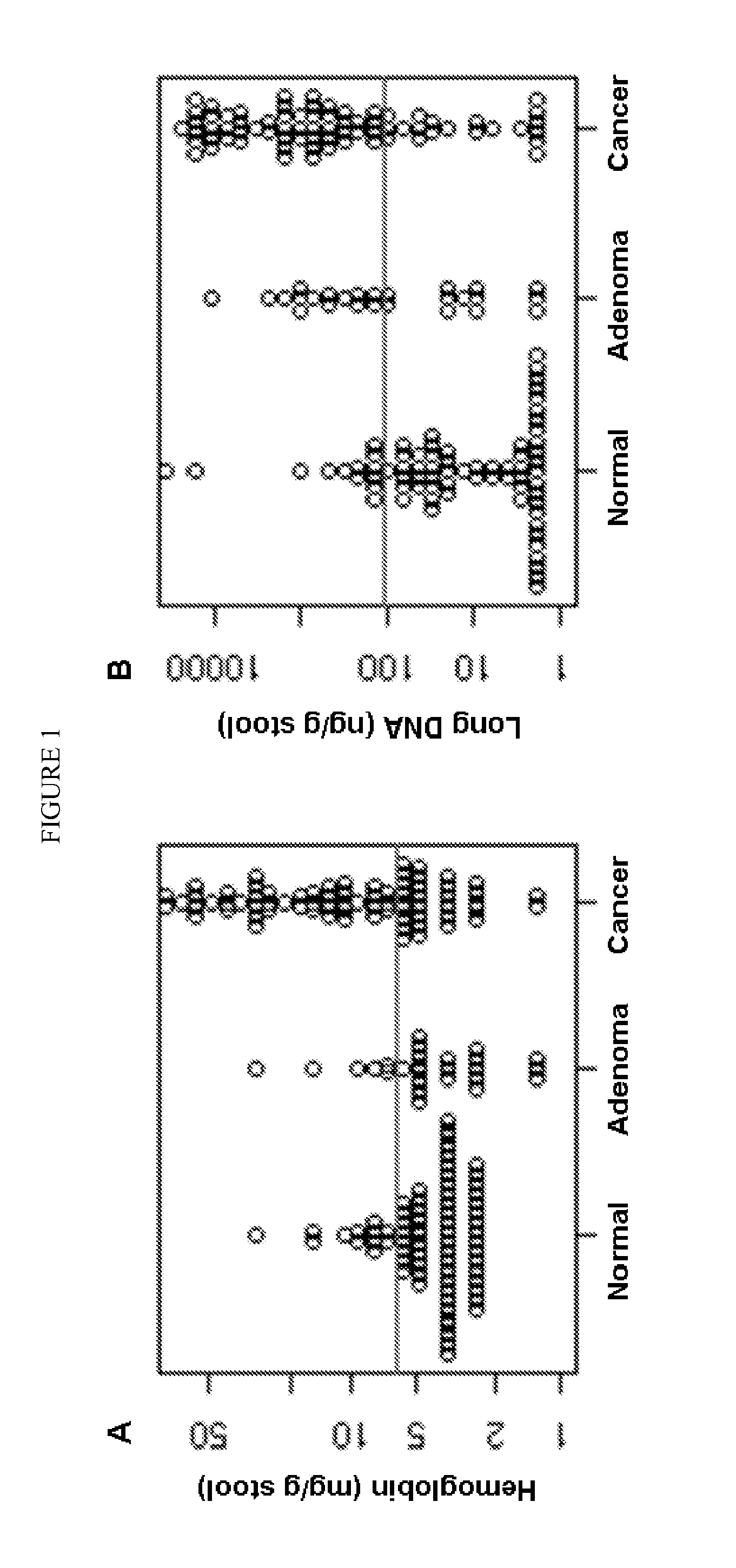
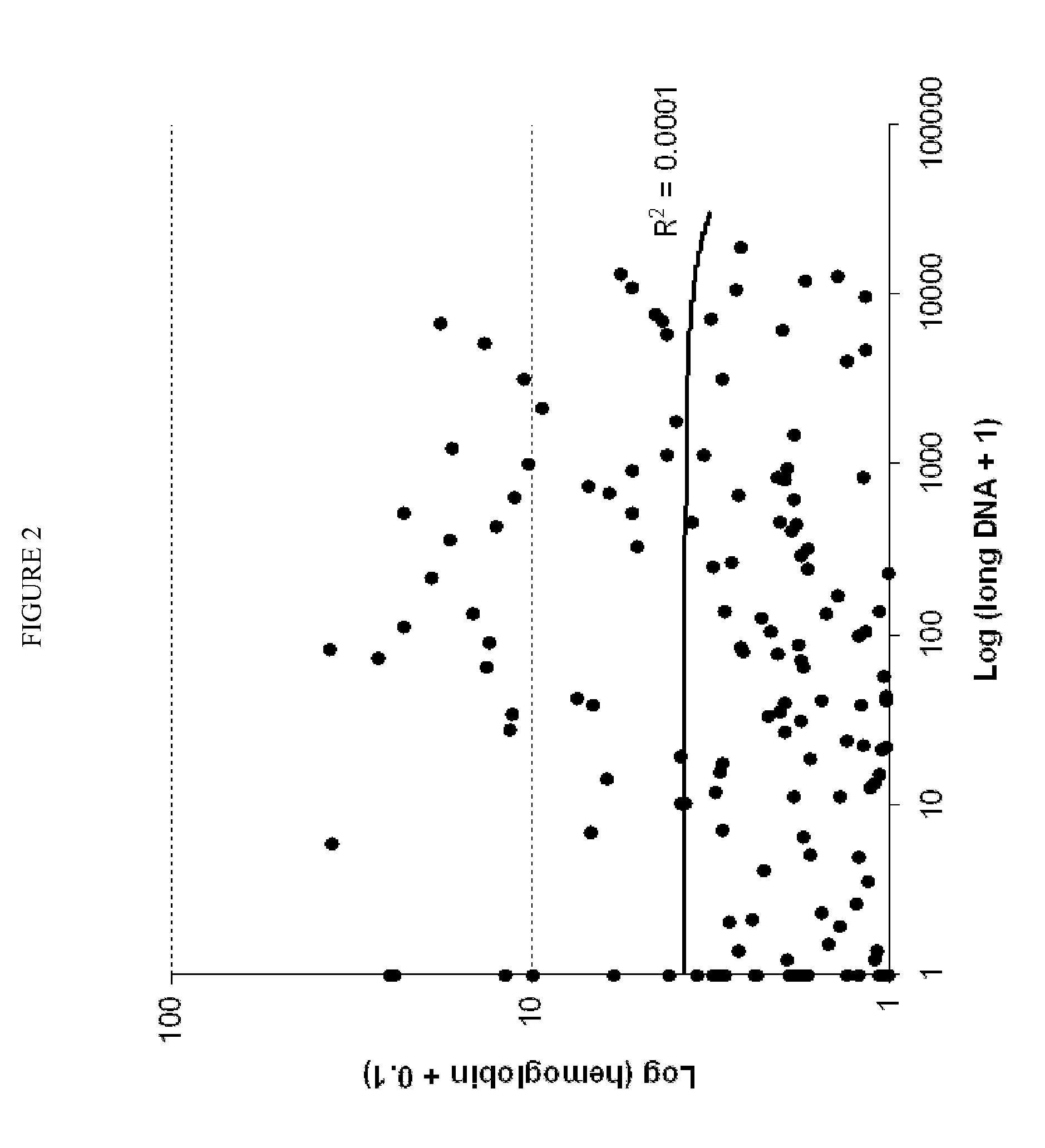
[0084]A total of two hundred and one subjects, including 74 patients with CRC, 27 with advanced adenoma (≧1 cm), and 100 colonoscopically normal individuals, were all recruited. Their demographic and clinical characteristics are described in Table 1.
TABLE 1Demographic and clinical characteristics of subjectsColorectalAdvancedcanceradenomaNormal controlNumber7427100Median Age (Range)61 (40-87) yrs67 (50-82) yrs59 (28-81) yrsSex (Male / Female)52 / 2215 / 1237 / 63Site (Right / Left)29 / 4517 / 10Stage (I / II / III / IV)13 / 16 / 27 / 18Grade (1 / 2 / 3 / 4) or0 / 4 / 55 / 520 / 5Dysplasia (Low / High)
[0085]Stool Collection
[0086]Stools were collected more than 2 weeks following any colorectal diagnostic procedure or cathartic preparation and prior to either endoscopic or surgical neoplasm resection. Patients collected whole stools in a preservative buffer (0.5 mol / L Tris, 10 mmol / L NaCl, 150 mmol / L EDTA, pH 9.0) as described (see, e.g., Olson J, et al., Diagn Mol Pathol 2005; 14:183-191; herein incorporated by refere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com