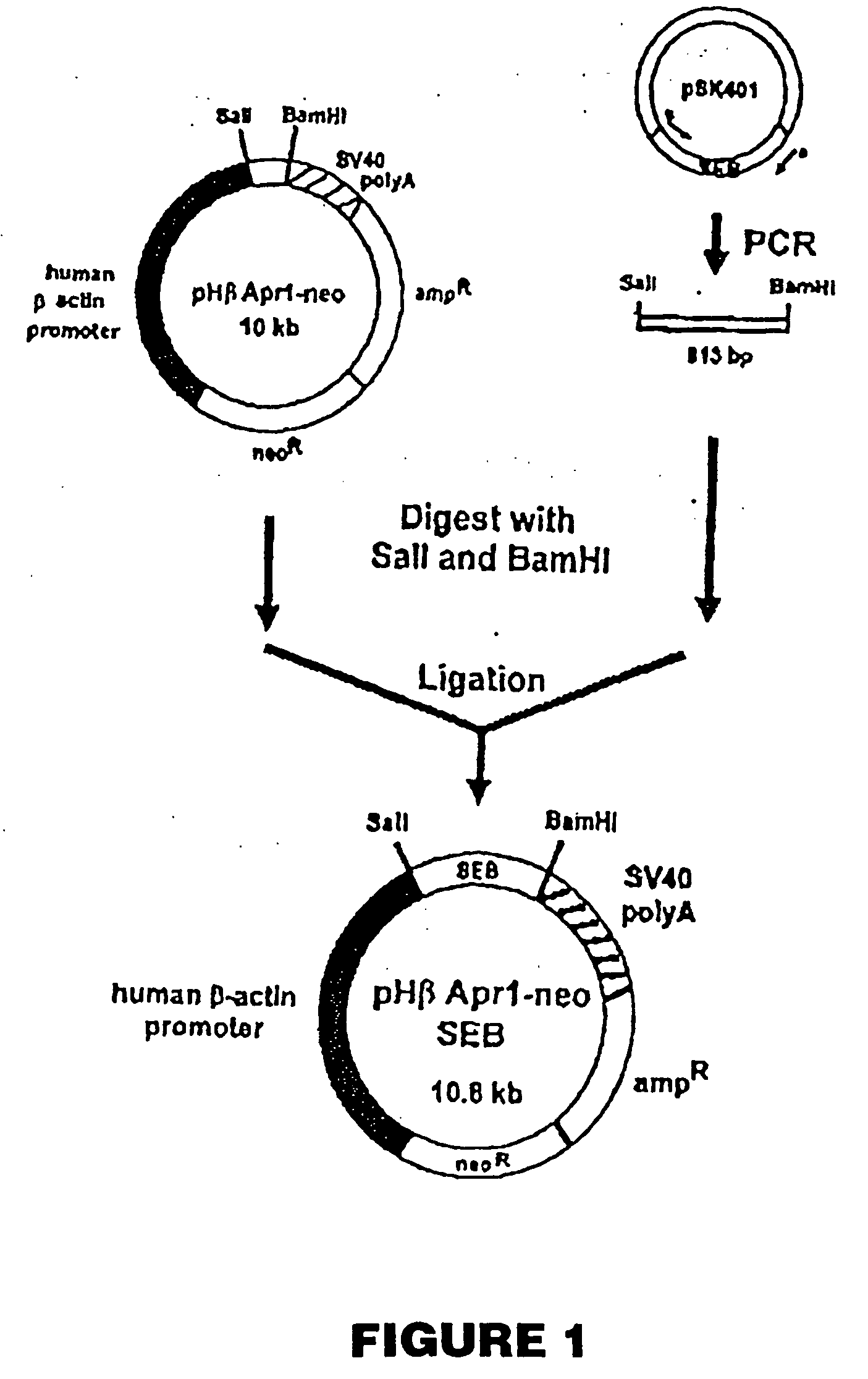
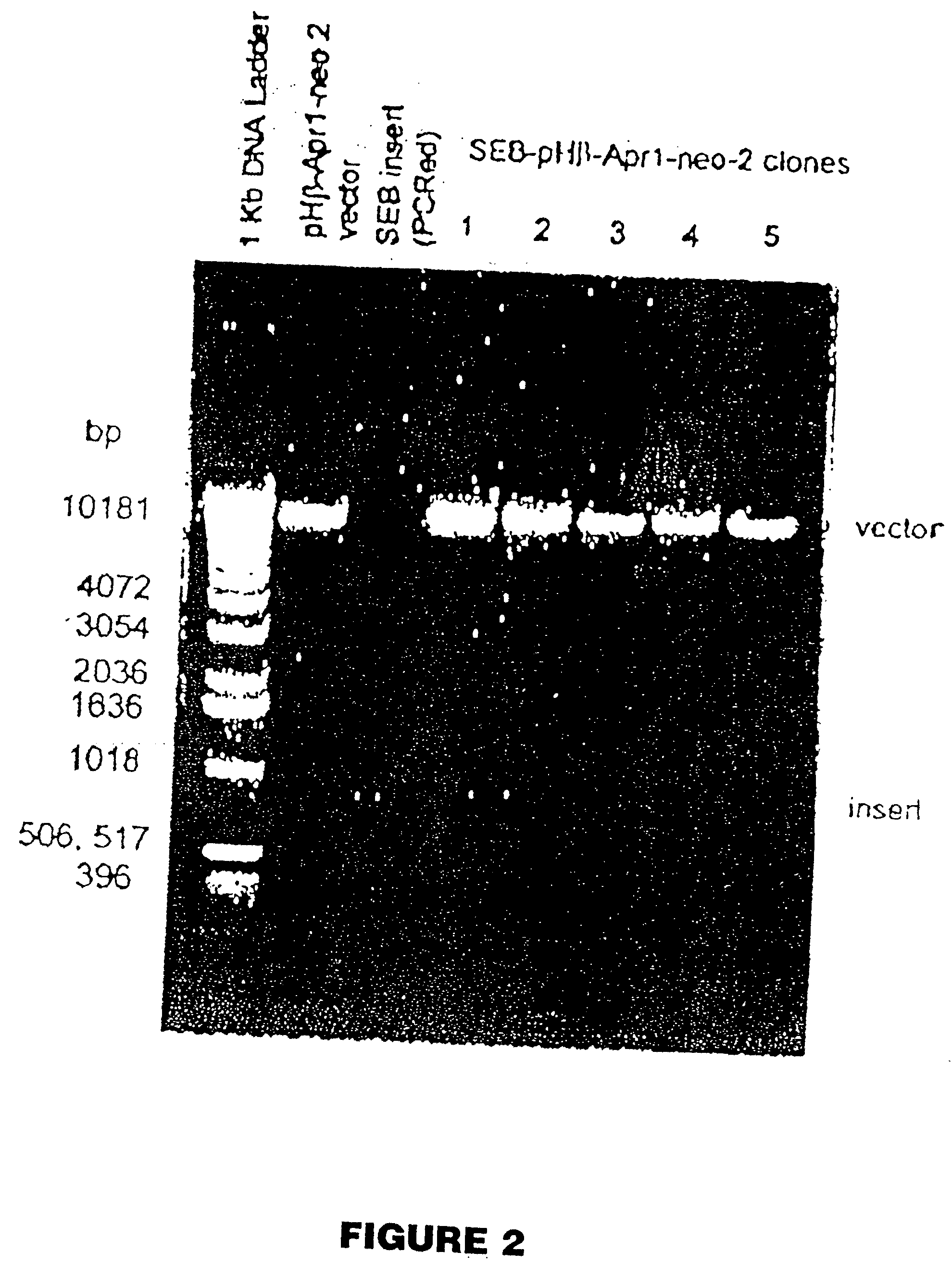
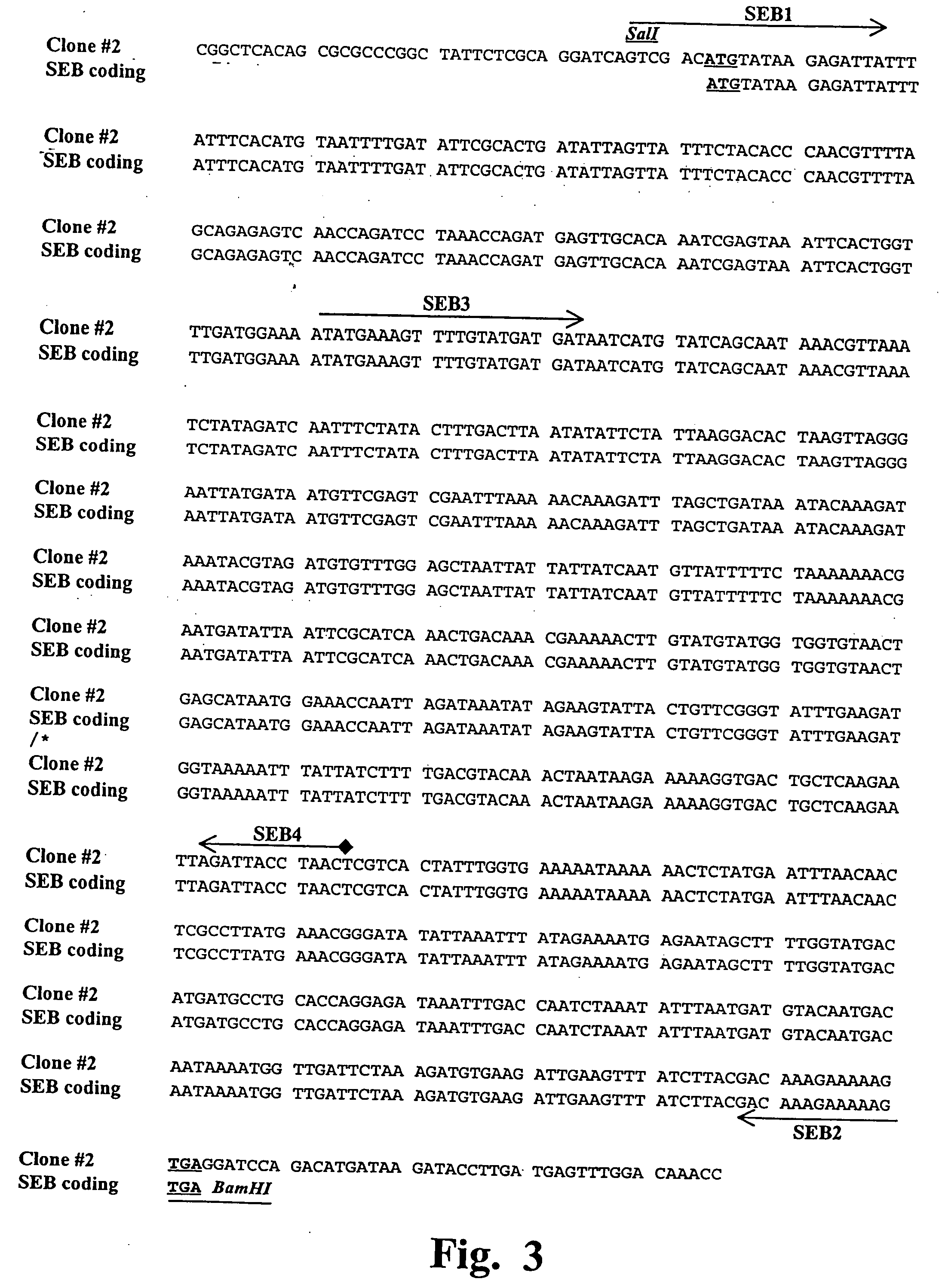
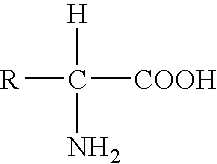
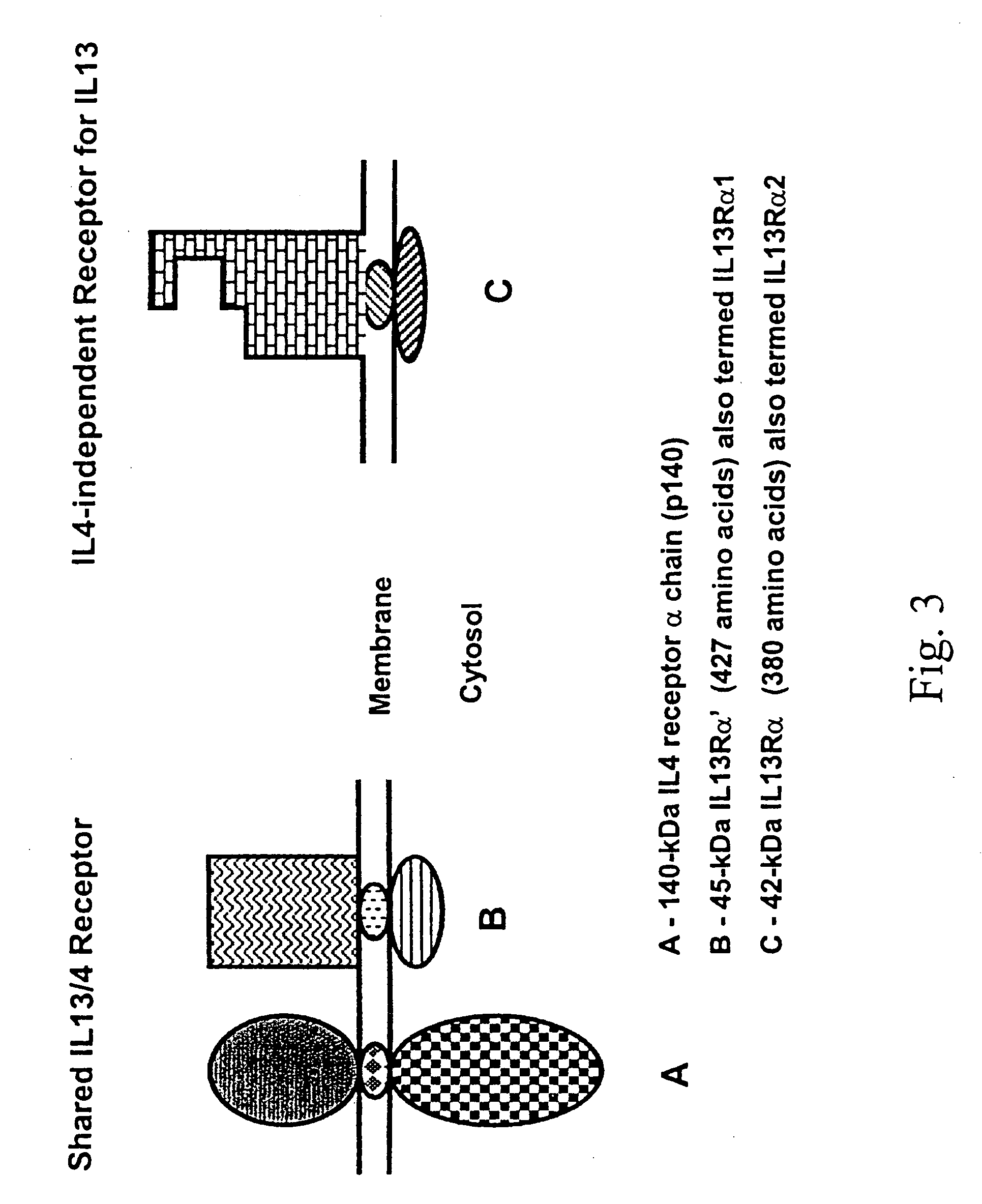
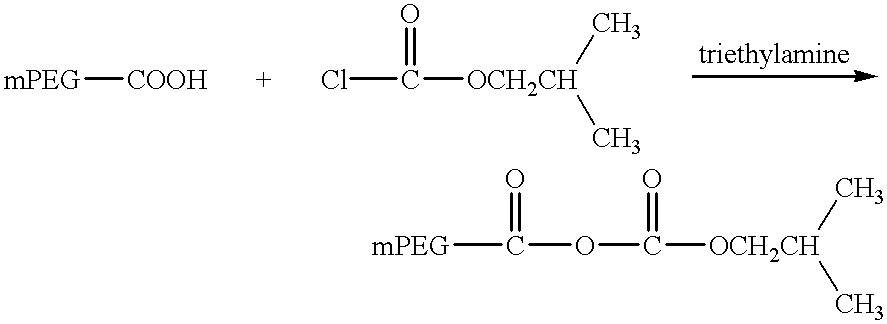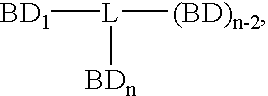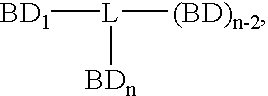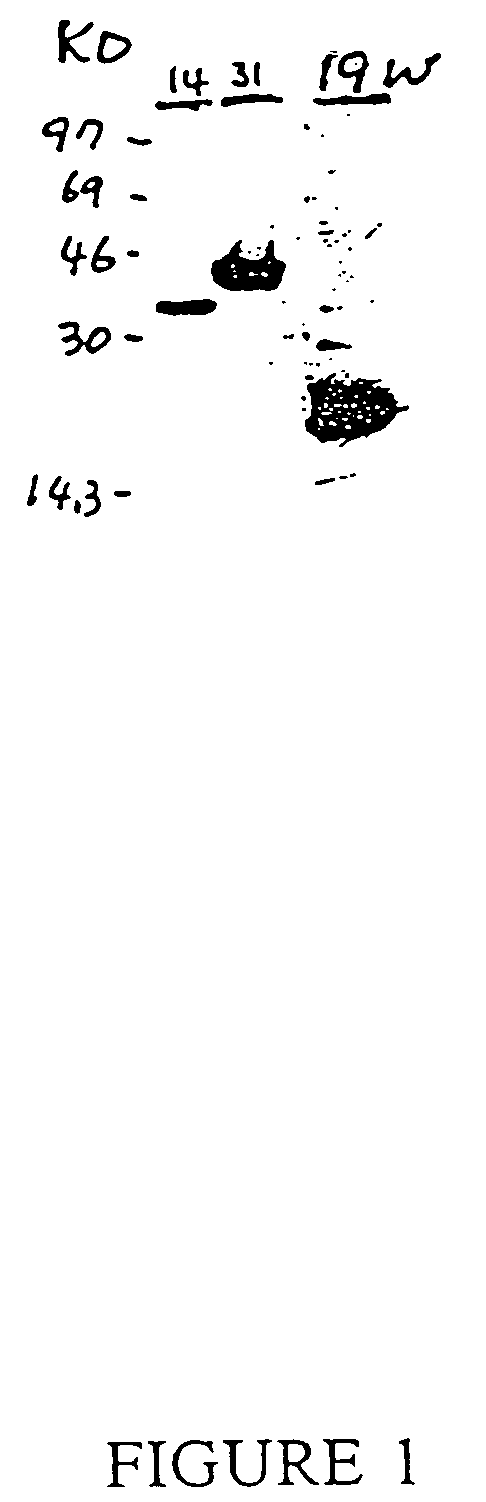Patents
Literature
861results about "Receptors for cytokines/lymphoines/interferons" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
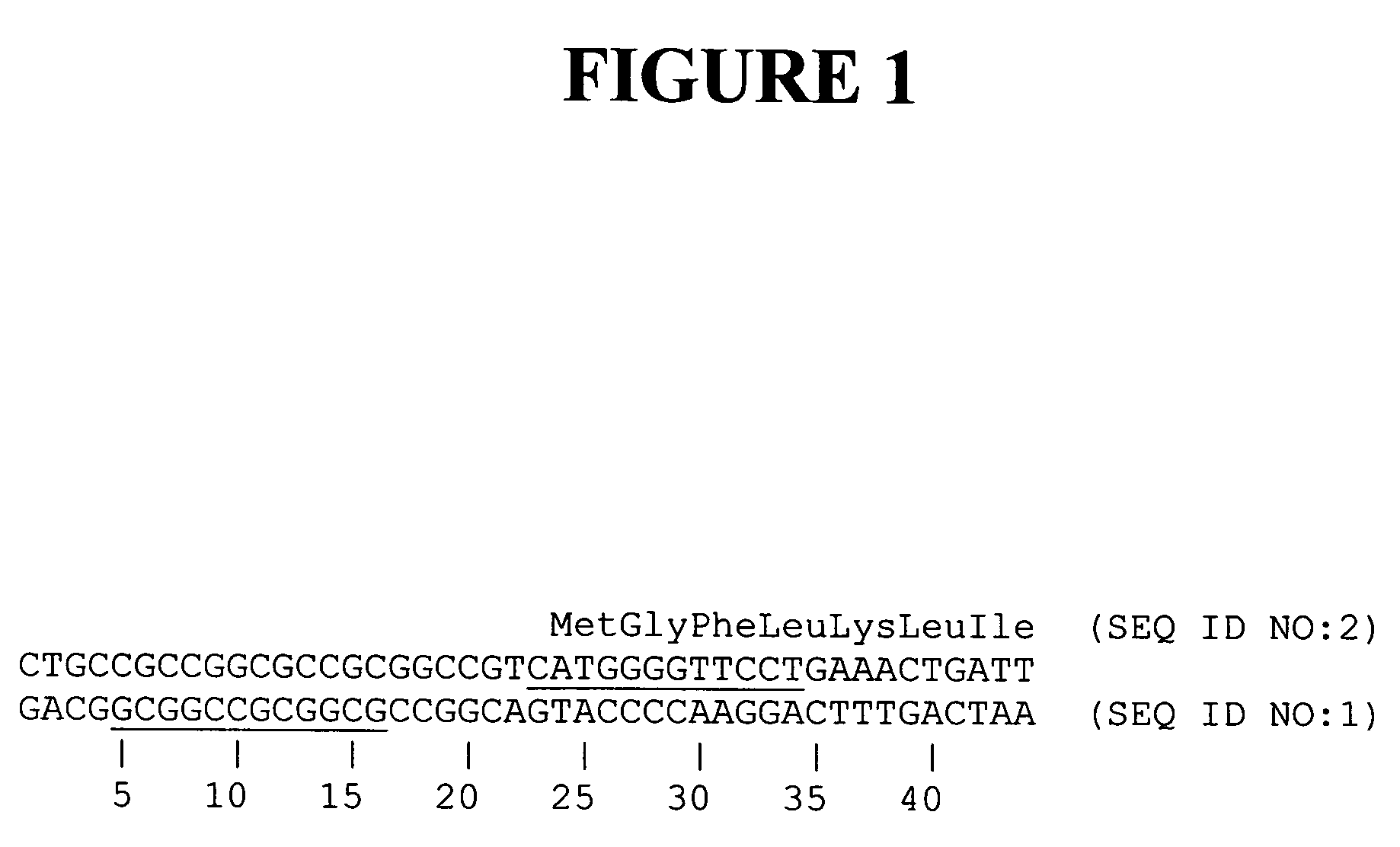
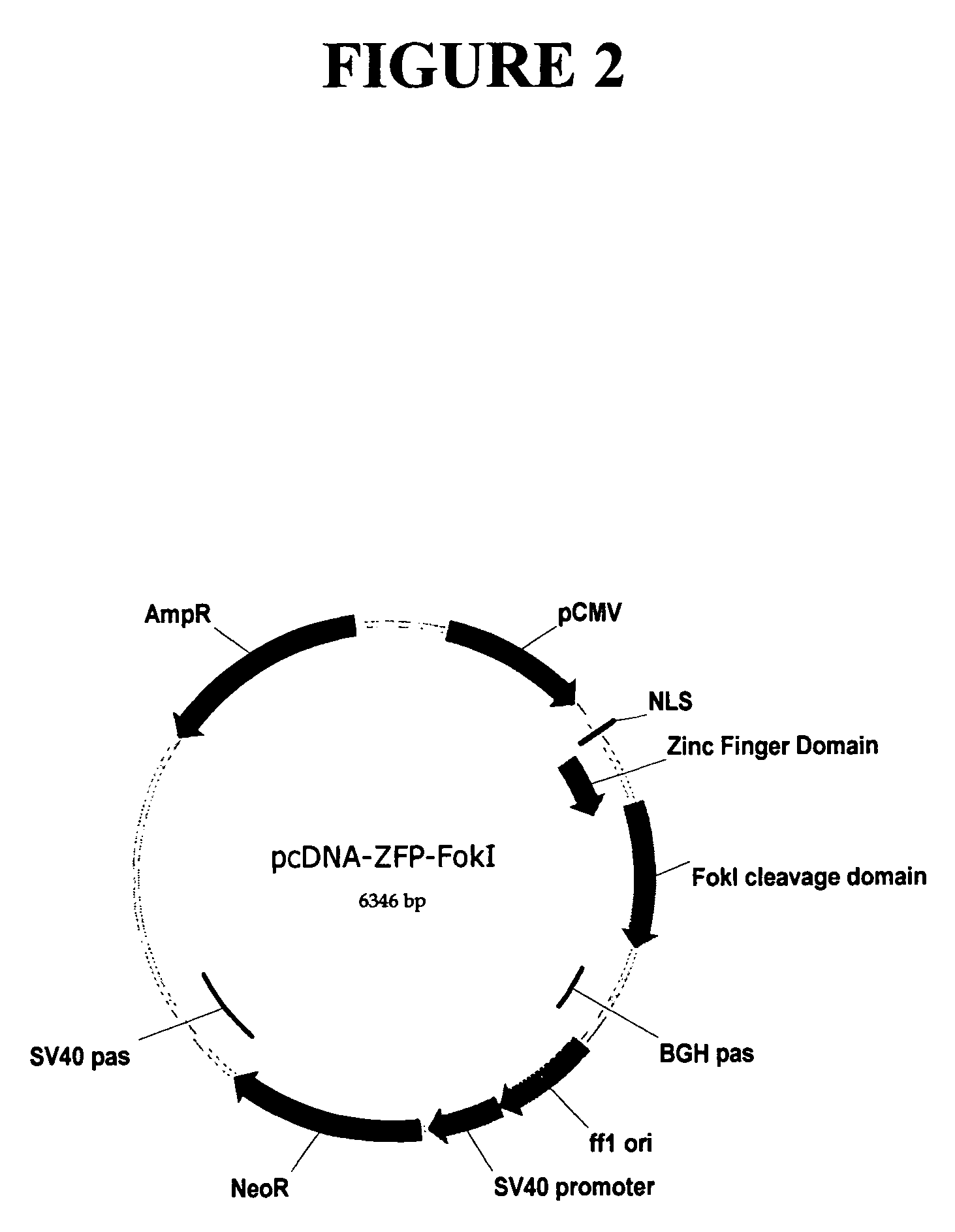
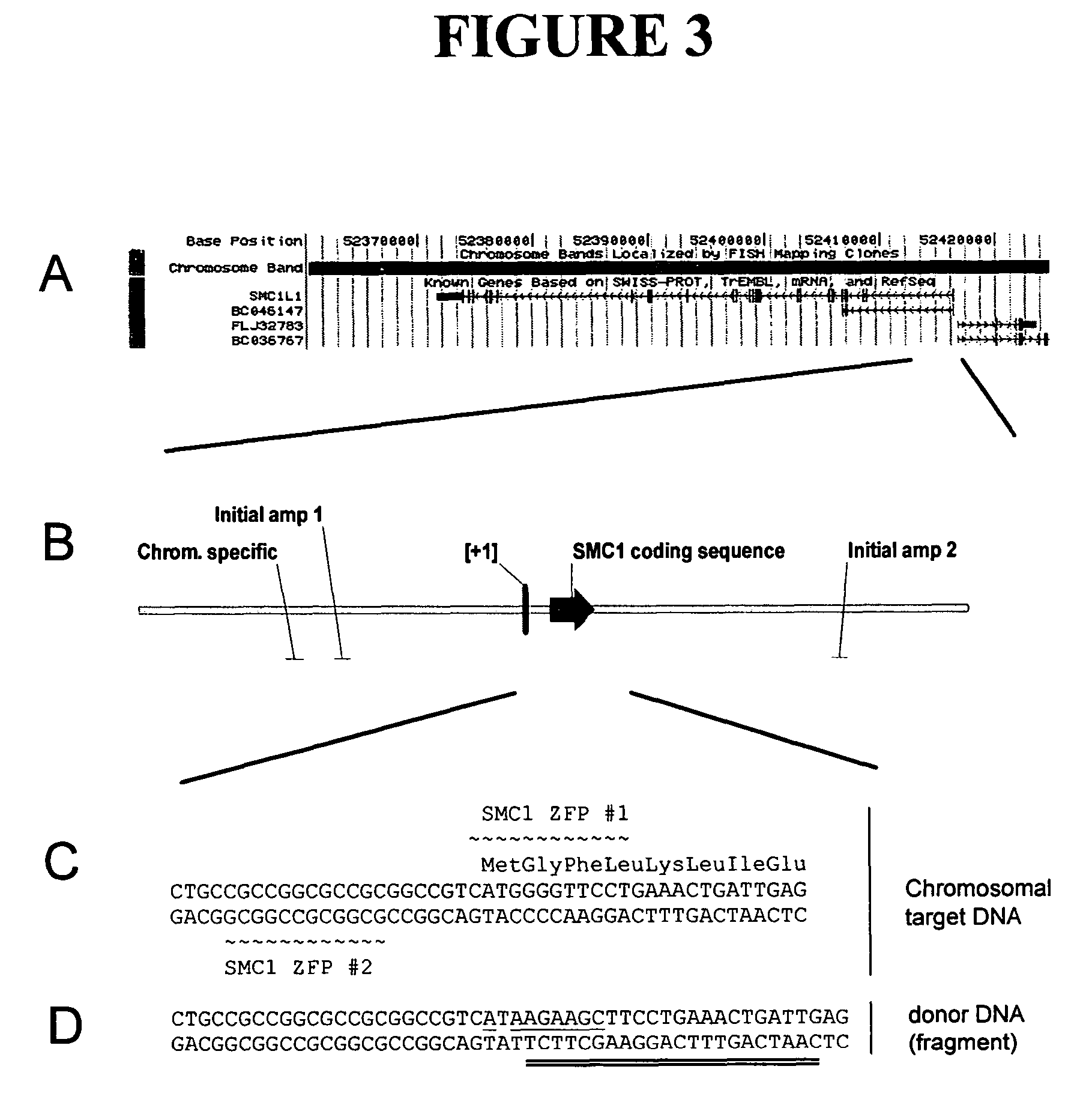
Methods and compositions for targeted cleavage and recombination
ActiveUS7888121B2High frequencyInhibitory activityFusion with DNA-binding domainHydrolasesPolynucleotideGenome
Disclosed herein are methods and compositions for targeted cleavage of a genomic sequence, targeted alteration of a genomic sequence, and targeted recombination between a genomic region and an exogenous polynucleotide homologous to the genomic region. The compositions include fusion proteins comprising a cleavage domain (or cleavage half-domain) and an engineered zinc finger domain and polynucleotides encoding same. Methods for targeted cleavage include introduction of such fusion proteins, or polynucleotides encoding same, into a cell. Methods for targeted recombination additionally include introduction of an exogenous polynucleotide homologous to a genomic region into cells comprising the disclosed fusion proteins.
Owner:SANGAMO BIOSCIENCES INC
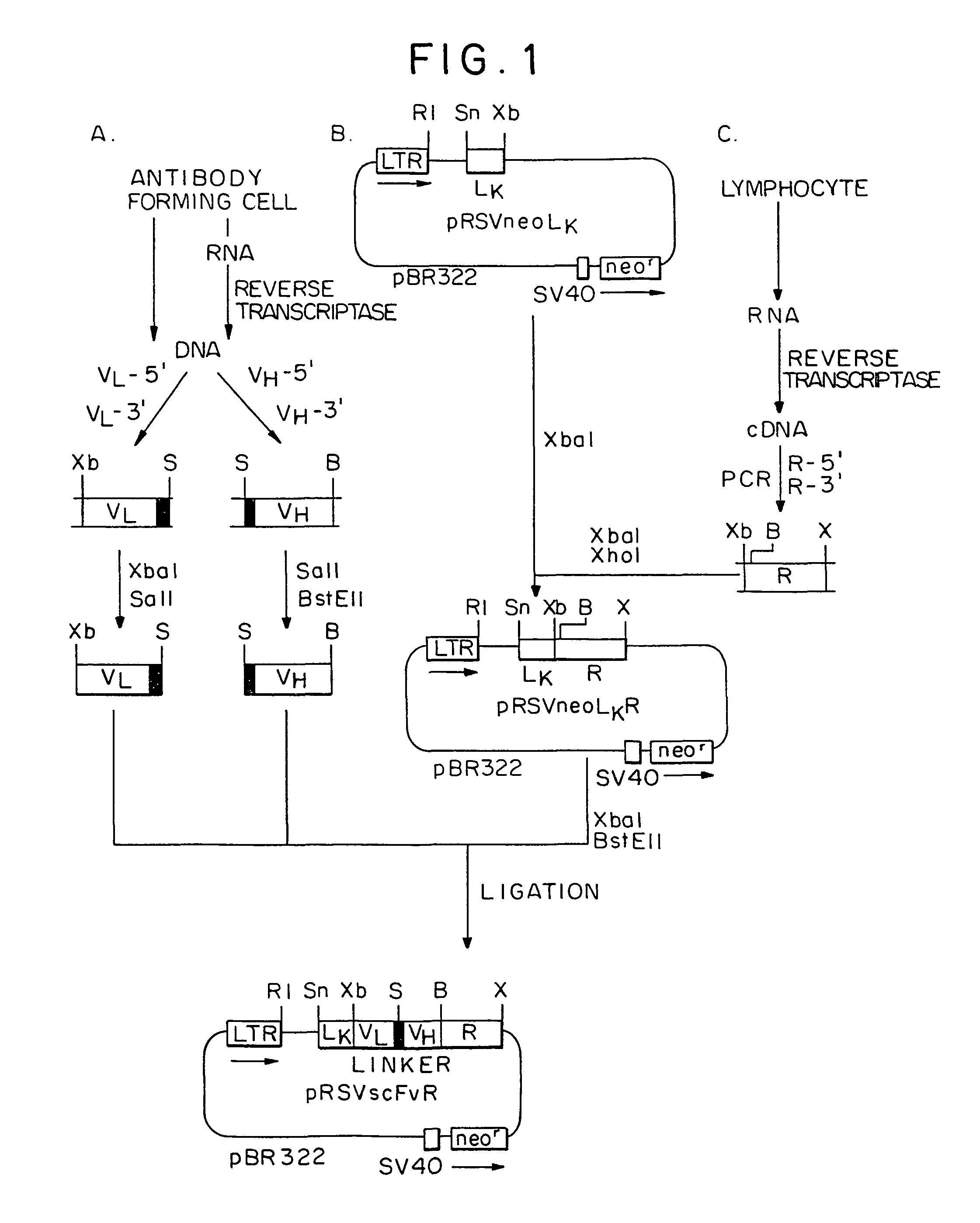
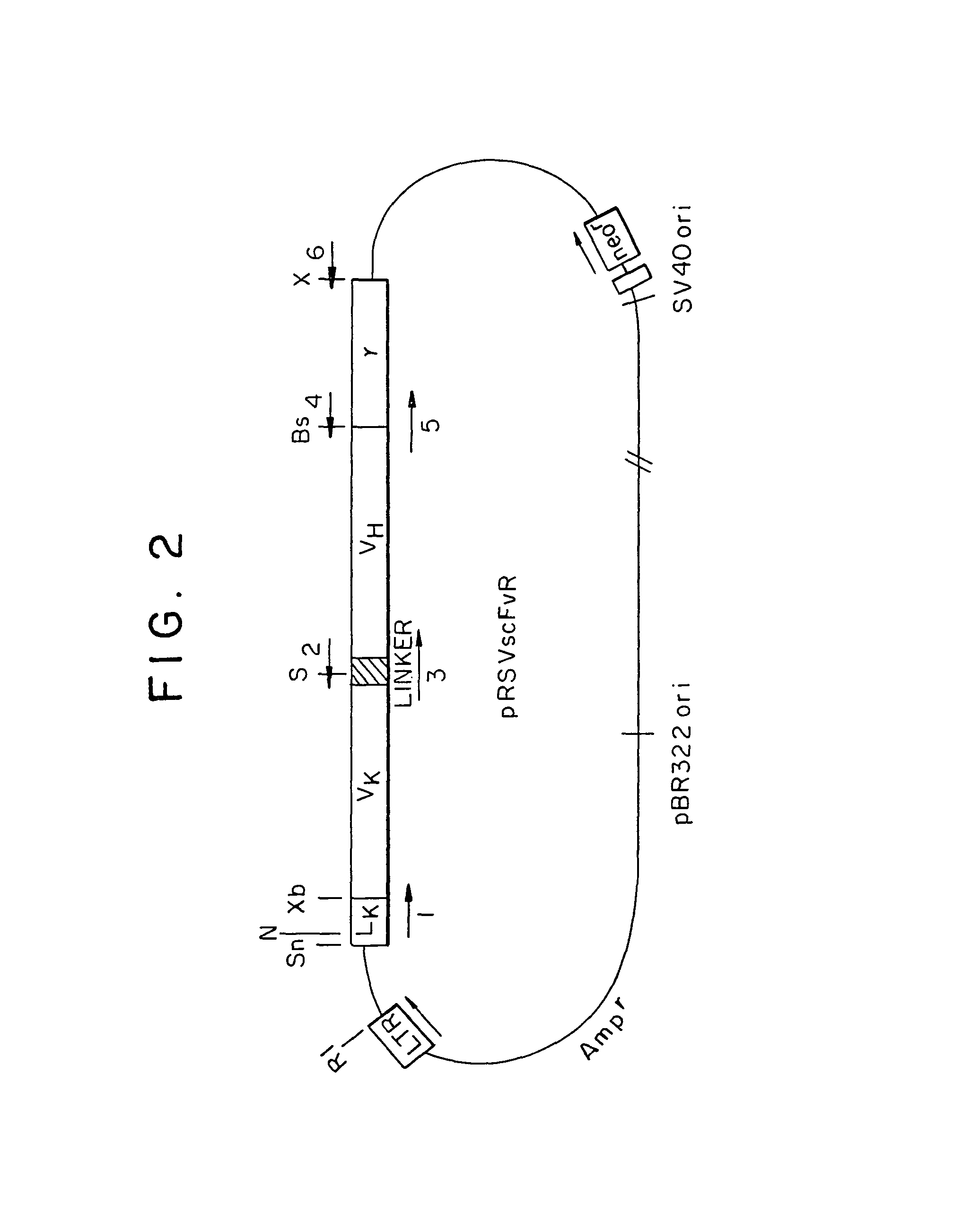
Single-chain antigen-binding proteins capable of glycosylation, production and uses thereof
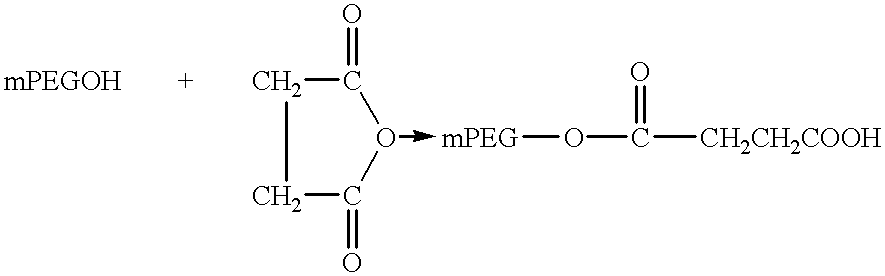
The present invention relates to single-chain antigen-binding molecules capable of glycosylation. Compositions of, genetic constructions coding for, and methods for producing monovalent and multivalent single-chain antigen-binding molecules capable of glycosylation are described and claimed. Composition of, genetic constructions coding for, and methods for producing glycosylated monovalent and multivalent single-chain antigen-binding molecules capable of polyalkylene oxide conjugation are described and claimed. The invention also relates to methods for producing a polypeptide having increased glycosylation and the polypeptide produced by the described method. Uses resulting from the multifunctionality of a glycosylated / polyalkylene oxide conjugated antigen-binding protein are also described and claimed.
Owner:ENZON PHARM INC
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
Chimeric immunoreceptor useful in treating human gliomas
InactiveUS7514537B2Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingHuman glioma
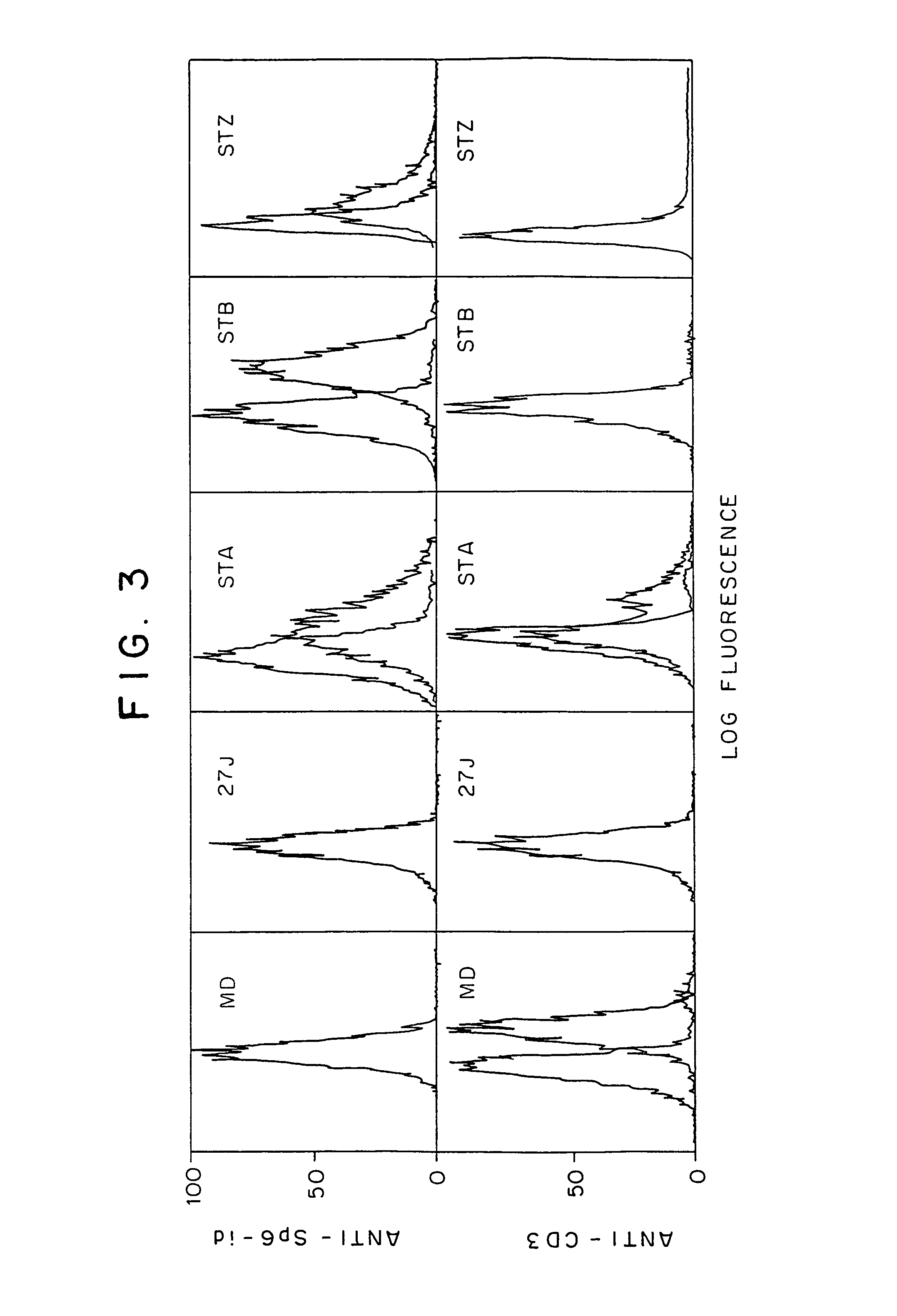
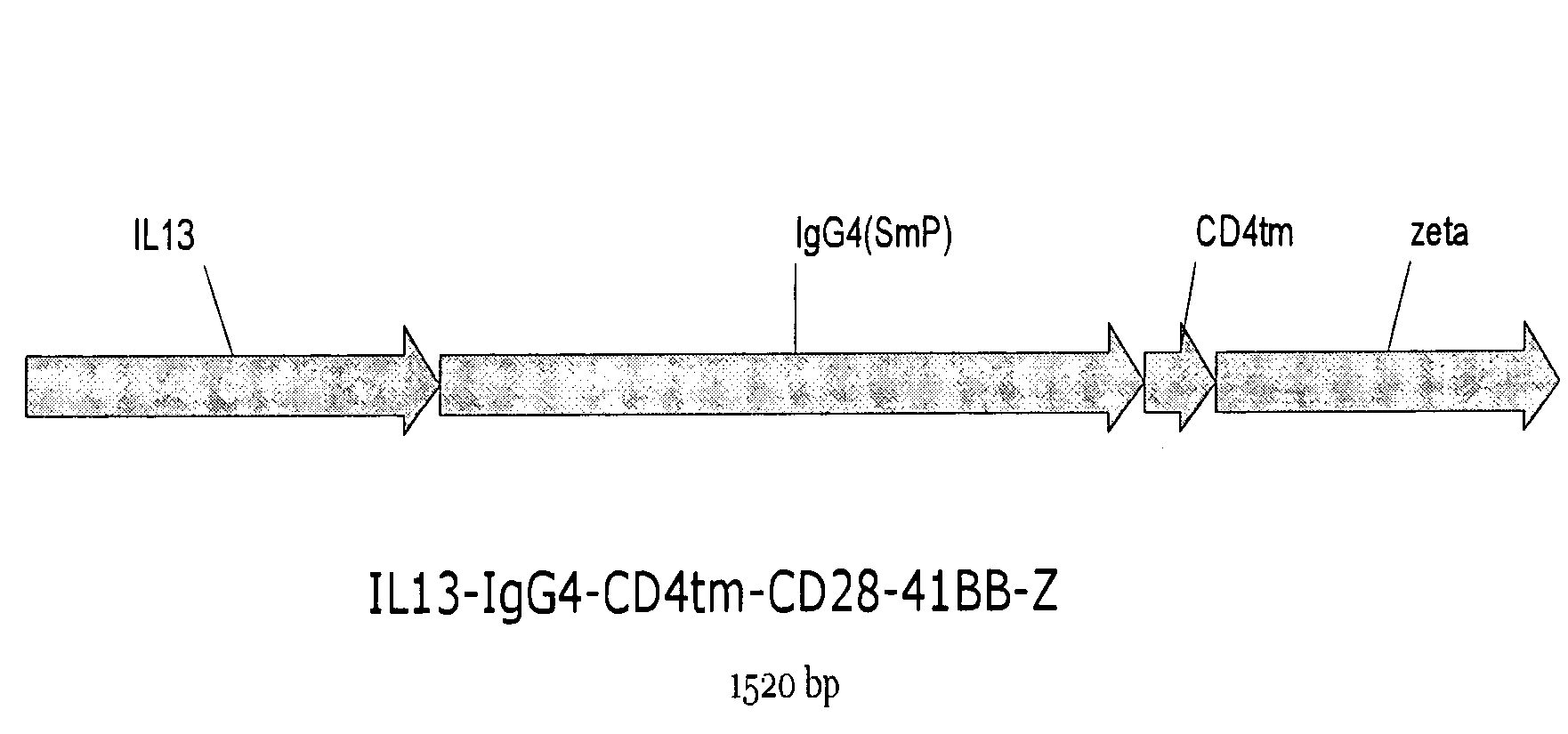
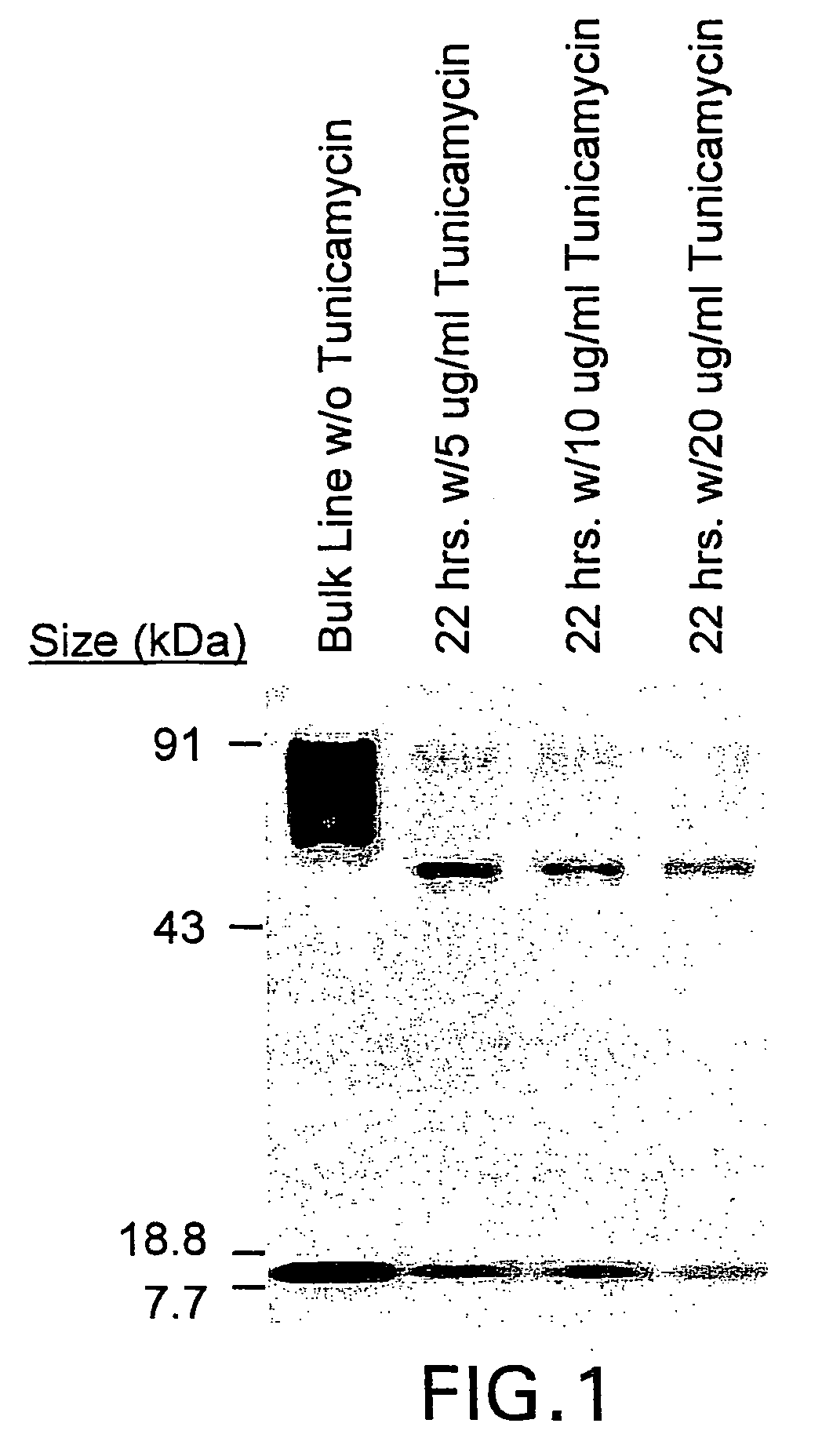
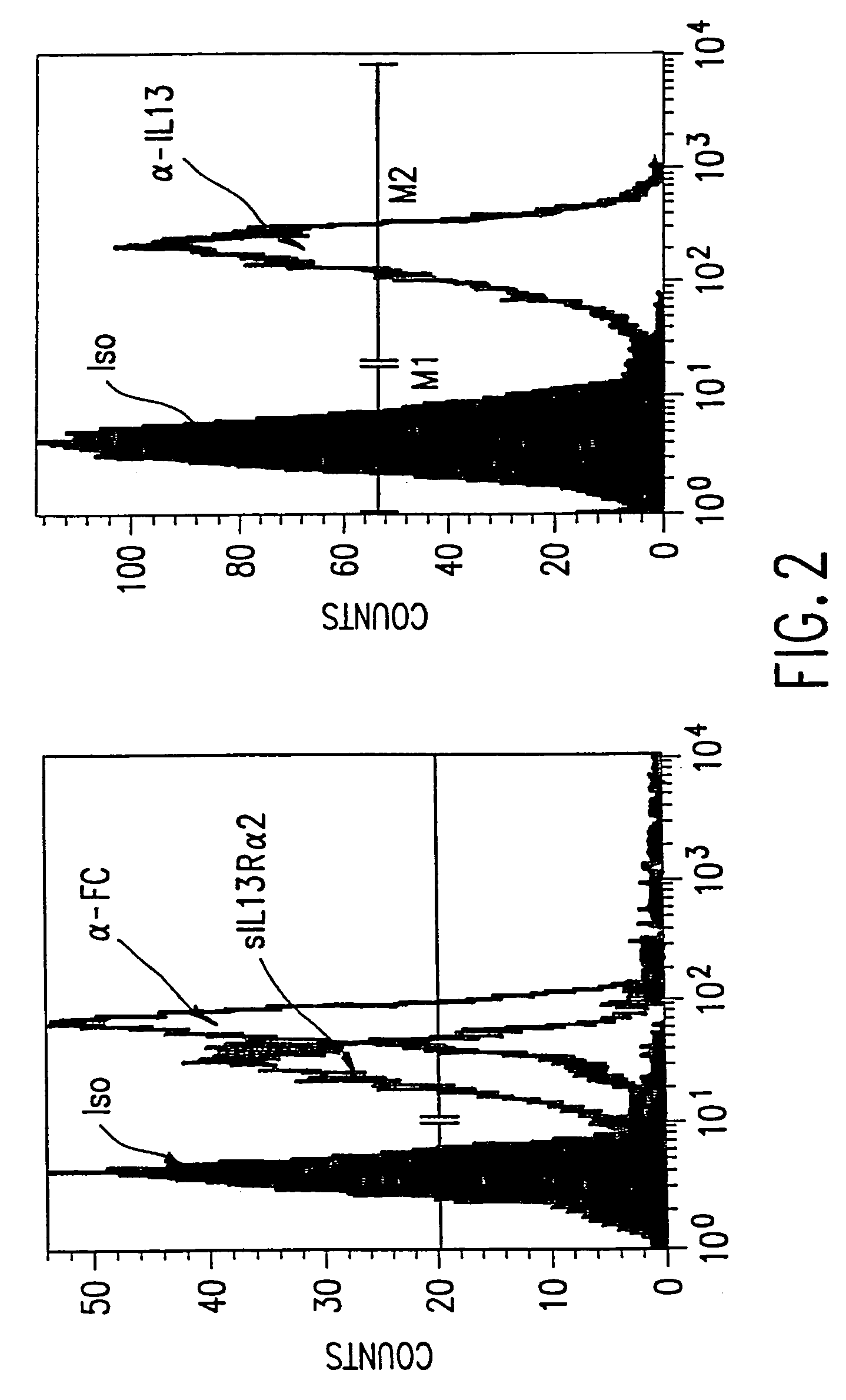
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signaling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targeting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Compositions and methods for treatment of neoplastic disease
InactiveUS20050112141A1High productFacilitates their targetingFusions for specific cell targetingReceptors for cytokines/lymphoines/interferonsAbnormal tissue growthDisease
The present invention comprises compositions and methods for treating a tumor or neoplastic disease in a host, The methods employ conjugates comprising superantigen polypeptides or nucleic acids with other structures that preferentially bind to tumor cells and are capable of inducing apoptosis. Also provided are superantigen-glycolipid conjugates and vesicles that are loaded onto antigen presenting cells to activate both T cells and NKT cells. Cell-based vaccines comprise tumor cells engineered to express a superantigen along with glycolipids products which, when expressed, render the cells capable of eliciting an effective anti-tumor immune response in a mammal into which these cells are introduced. Included among these compositions are tumor cells, hybrid cells of tumor cells and accessory cells, preferably dendritic cells. Also provided are T cells and NKT cells activated by the above compositions that can be administered for adoptive immunotherapy.
Owner:TERMAN DAVID
Novel substitution mutant receptors and their use in an nuclear receptor-based inducible gene expression system
This invention relates to the field of biotechnology or genetic engineering. Specifically, this invention relates to the field of gene expression. More specifically, this invention relates to novel substitution mutant receptors and their use in a Group H nuclear receptor-based inducible gene expression system and methods of modulating the expression of a gene in a host cell for applications such as gene therapy, large scale production of proteins and antibodies, cell-based high throughput screening assays, functional genomics and regulation of traits in transgenic organisms.
Owner:PRECIGEN INC
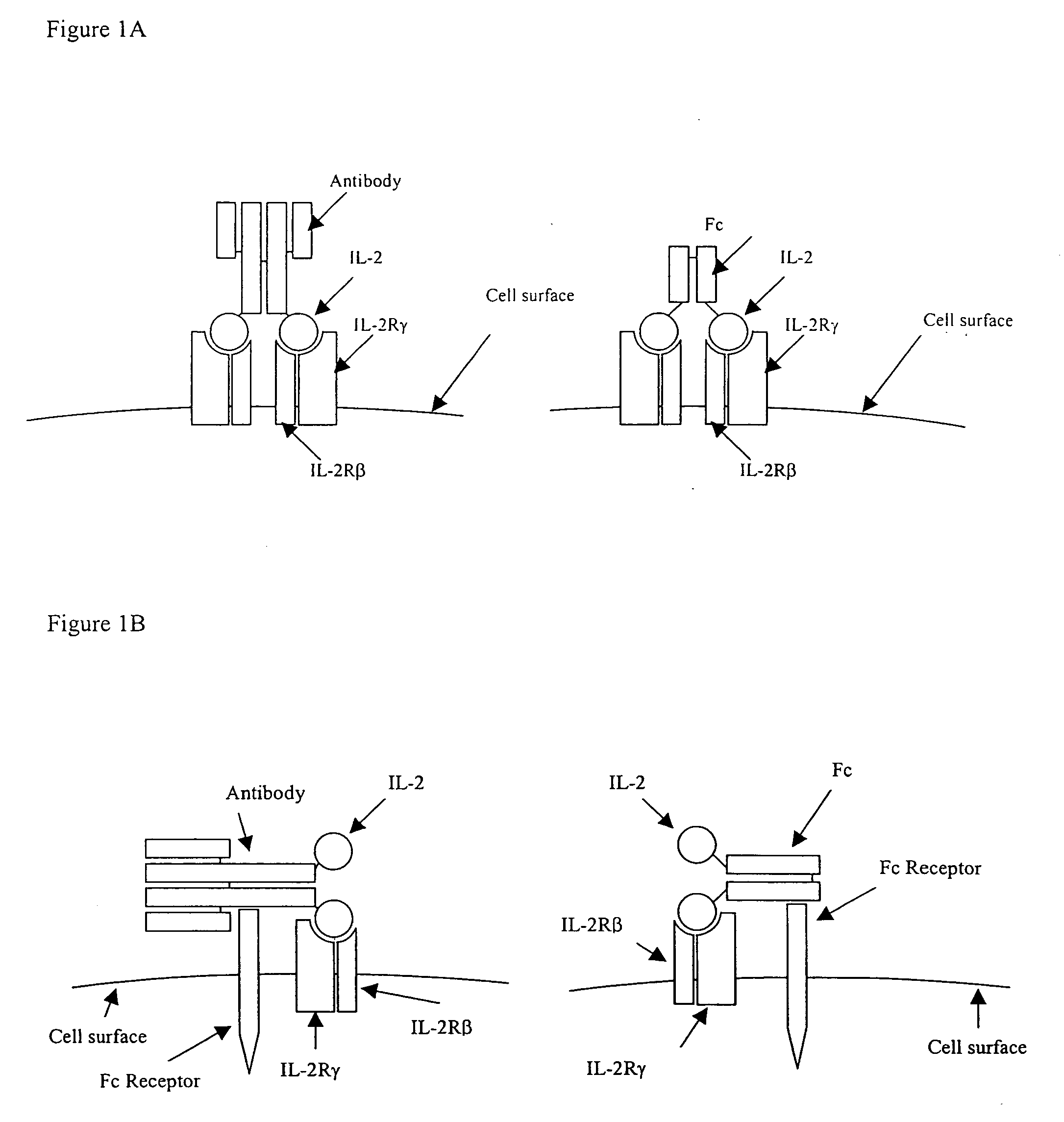
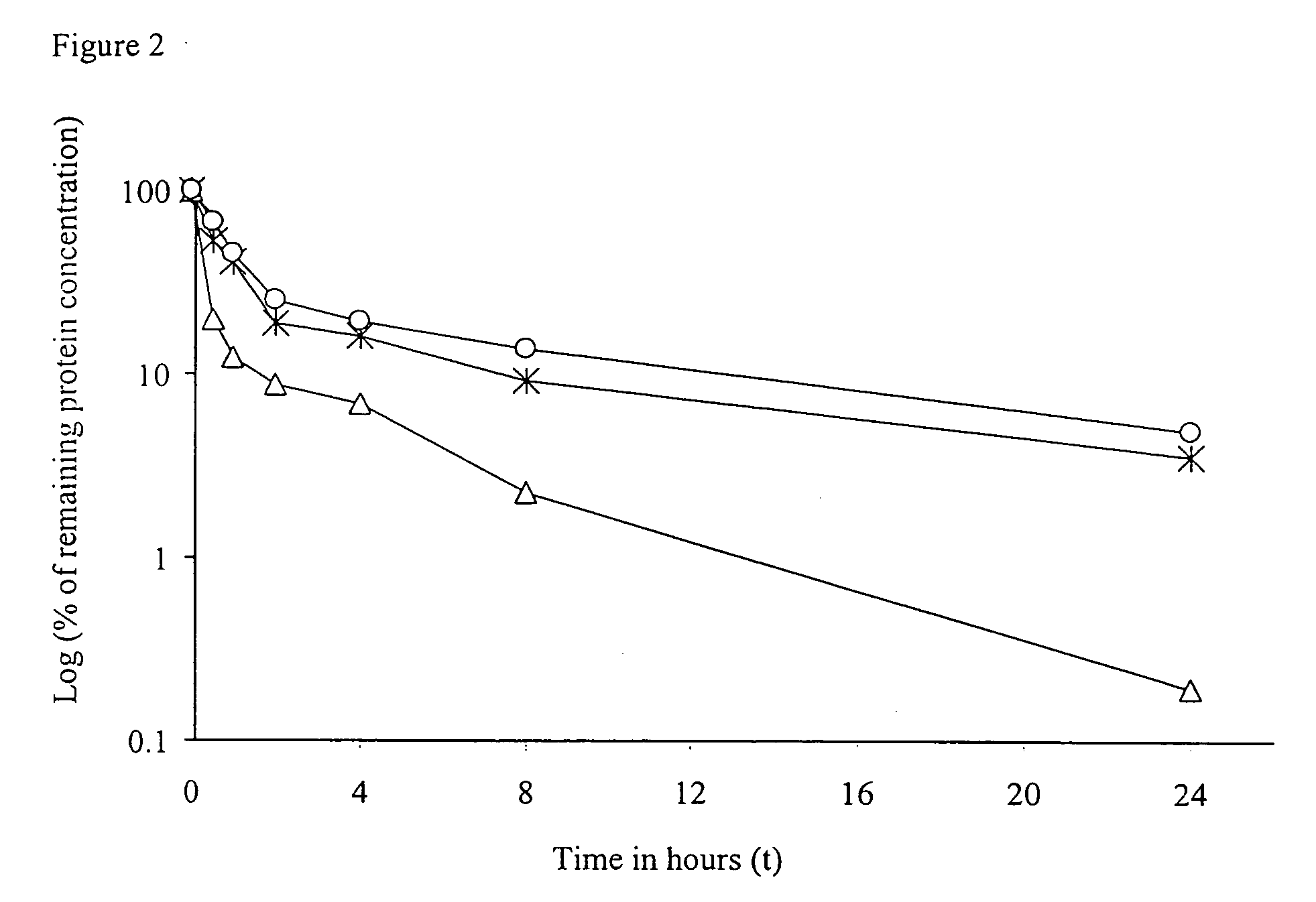
IL-2 fusion proteins with modulated selectivity
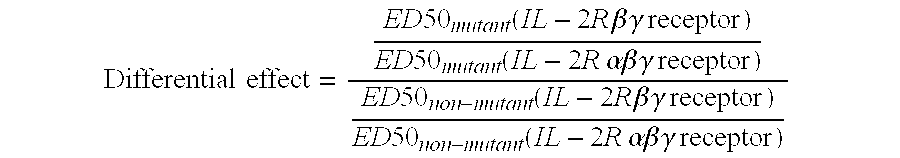
The invention provides cytokine fusion proteins with an increased therapeutic index, and methods to increase the therapeutic index of such fusion proteins. The fusion proteins of the invention are able to bind to more than one type of cytokine receptor expressed on cells and also bind to more than one cell type. In addition, the fusion proteins of the invention exhibit a longer circulating half-life in a patient's body than the corresponding naturally occurring cytokine.
Owner:MERCK PATENT GMBH
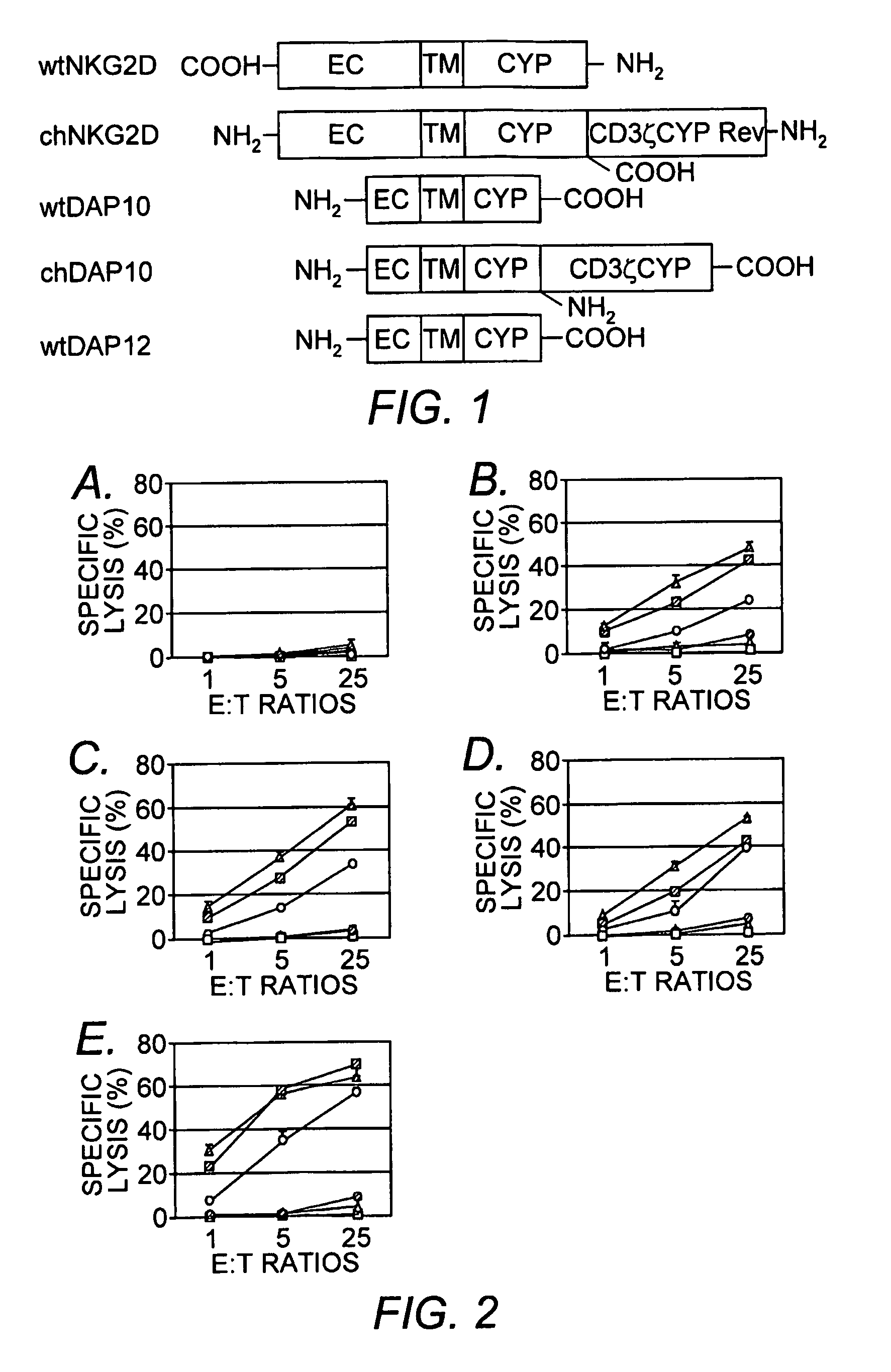
Chimeric NK receptor and methods for treating cancer
The present invention relates to chimeric immune receptor molecules for reducing or eliminating tumors. The chimeric receptors are composed a C-type lectin-like natural killer cell receptor, or a protein associated therewith, fused to an immune signaling receptor containing an immunoreceptor tyrosine-based activation motif. Methods for using the chimeric receptors are further provided.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Methods for purifying protein
ActiveUS7122641B2Antibody mimetics/scaffoldsNGF/TNF-superfamilyAntibody Affinity ChromatographyImmunoglobulin domain
A method is provided for separating a protein from one or more other proteins using hydroxyapatite chromatography in which the protein does not bind to hydroxyapatite but the other protein(s) does. In some embodiments, a second protein affixed to a solid support has been used previously to purify the protein by affinity chromatography, and small amounts of the second protein are introduced in the sample during this process. The protein being purified can comprise at least one constant antibody immunoglobulin domain. The second protein can bind to proteins comprising such a domain.
Owner:IMMUNEX CORP
Crystalline polypeptides
Owner:AMGEN INC
DNA encoding tumor necrosis factor- alpha and - beta receptors
Tumor necrosis factor receptor DNAs and expression vectors encoding TNF receptors, and processes for producing TNF receptors as products of recombinant cell culture, are disclosed.
Owner:IMMUNEX CORP
Soluble tumor necrosis factor receptor treatment of medical disorders
The invention pertains to methods and compositions for treating medical disorders characterized by elevated levels or abnormal expression of TNFα by administering a TNFα antagonist, such as recombinant TNFR:Fc.
Owner:IMMUNEX CORP
Cancer immunotherapy
InactiveUS7338929B2Increase killStimulate immune responseBiocideNervous disorderDiseaseImmuno therapy
A method for stimulating a immune response against IL-13Rα2 in a subject having or at risk for developing a disease having cells expressing IL-13Rα2 includes the steps of formulating the anti-cancer vaccine outside of the subject and administering the vaccine to the subject in an amount sufficient to stimulate an immune response against IL-13Rα2 in the subject. A composition for stimulating a immune response against IL-13Rα2 in a subject having or at risk for developing a disease having cells expressing IL-13Rα2 includes an isolated agent that can stimulate immune response against IL-13α2.
Owner:PENN STATE RES FOUND
Antibody to cytokine response gene 2(CR2) polypeptide
InactiveUS6057427AHigh incidenceEliminate the effects ofCytokine-induced proteinsPeptide/protein ingredientsAntibody fragmentsAmino acid
PCT No. PCT / US96 / 08992 Sec. 371 Date Jun. 5, 1996 Sec. 102(e) Date Jun. 5, 1996 PCT Filed Jun. 5, 1996Disclosed are an isolated antibody or antibody fragment that selectively binds a polypeptide encoded by Cytokine Response gene 2 (CR2), and in particular, selectively binds to a first polypeptide having the sequence of residues 1-60 of SEQ. ID No: 4. Also disclosed is a composition containing the antibody or antibody fragment and a diluent or carrier. Also disclosed are methods, using the present antibody or antibody fragment, of isolating or purifying a peptide comprising an amino acid sequence of residues 1-60 of SEQ. ID No: 4 or an antibody binding fragment thereof that is at least 10 to 30 amino acids long, or a fusion protein comprising any of these.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Methods of identifying compounds that modulate body weight using the OB receptor
The present invention relates to the discovery, identification and characterization of nucleotides that encode Ob receptor (ObR), a receptor protein that participates in mammalian body weight regulation. The invention encompasses obR nucleotides, host cell expression systems, ObR proteins, fusion proteins, polypeptides and peptides, antibodies to the receptor, transgenic animals that express an obR transgene, or recombinant knock-out animals that do not express the ObR, antagonists and agonists of the receptor, and other compounds that modulate obR gene expression or ObR activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or the treatment of body weight disorders, including but not limited to obesity, cachexia and anorexia.
Owner:MILLENNIUM PHARMA INC
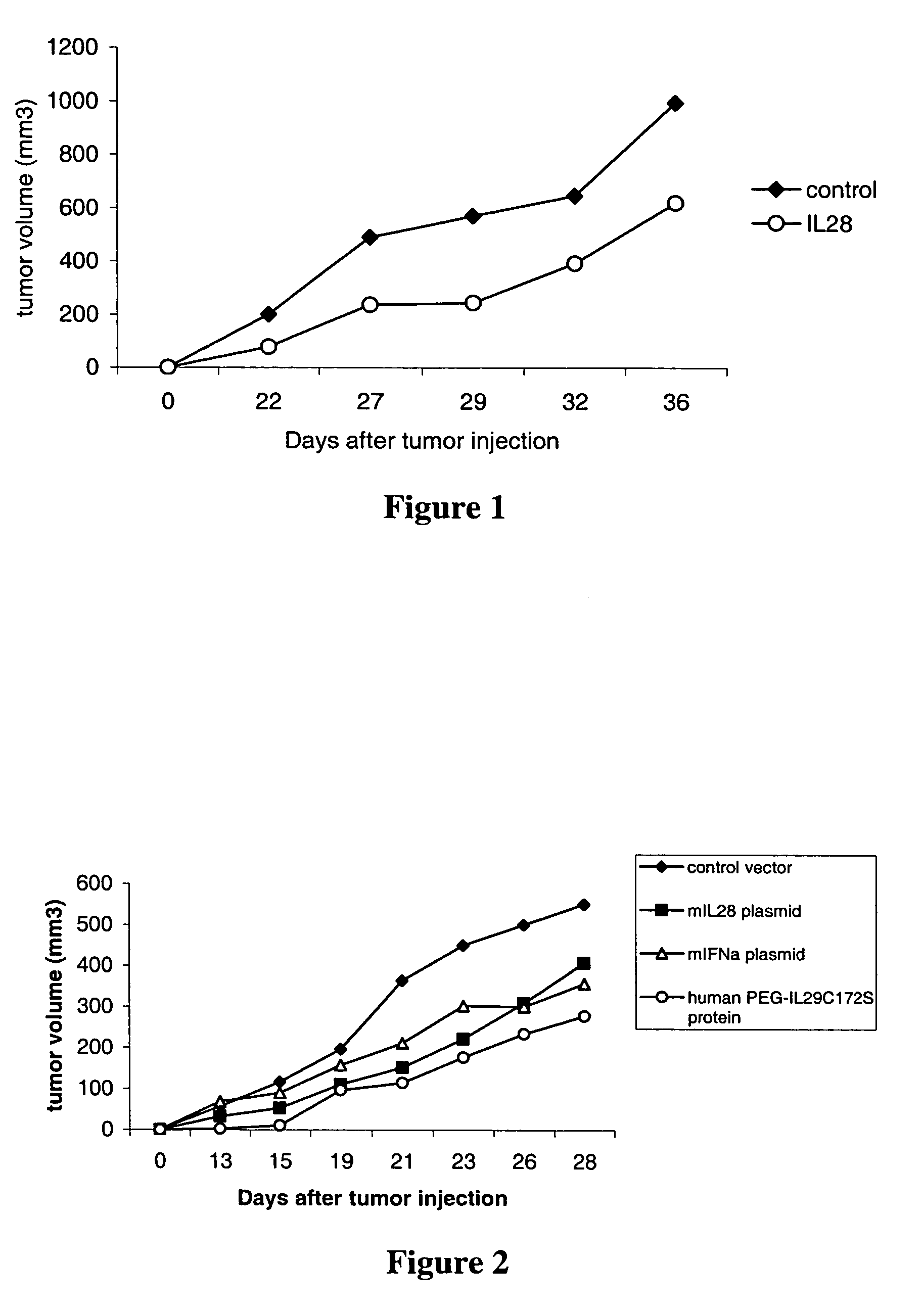
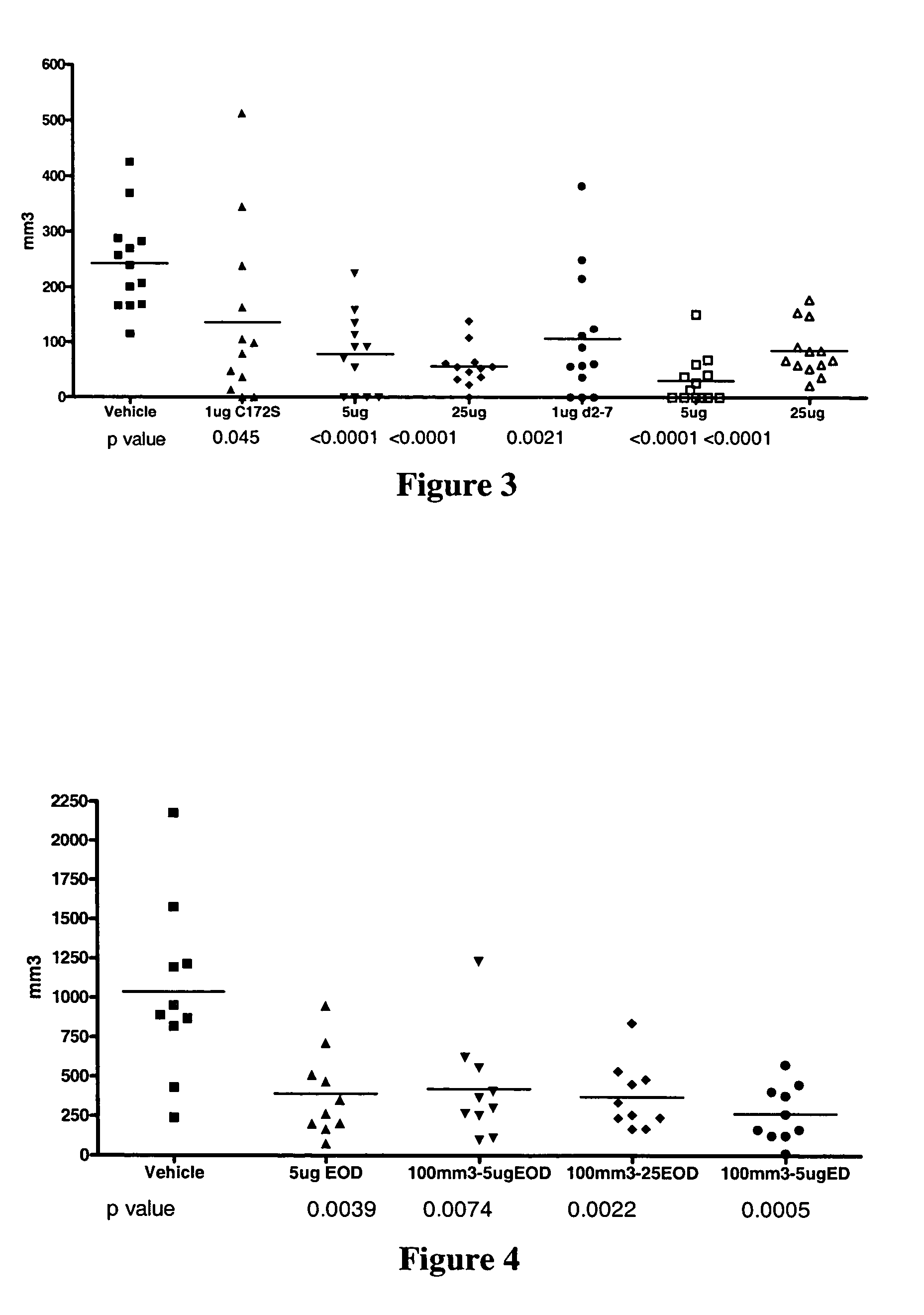
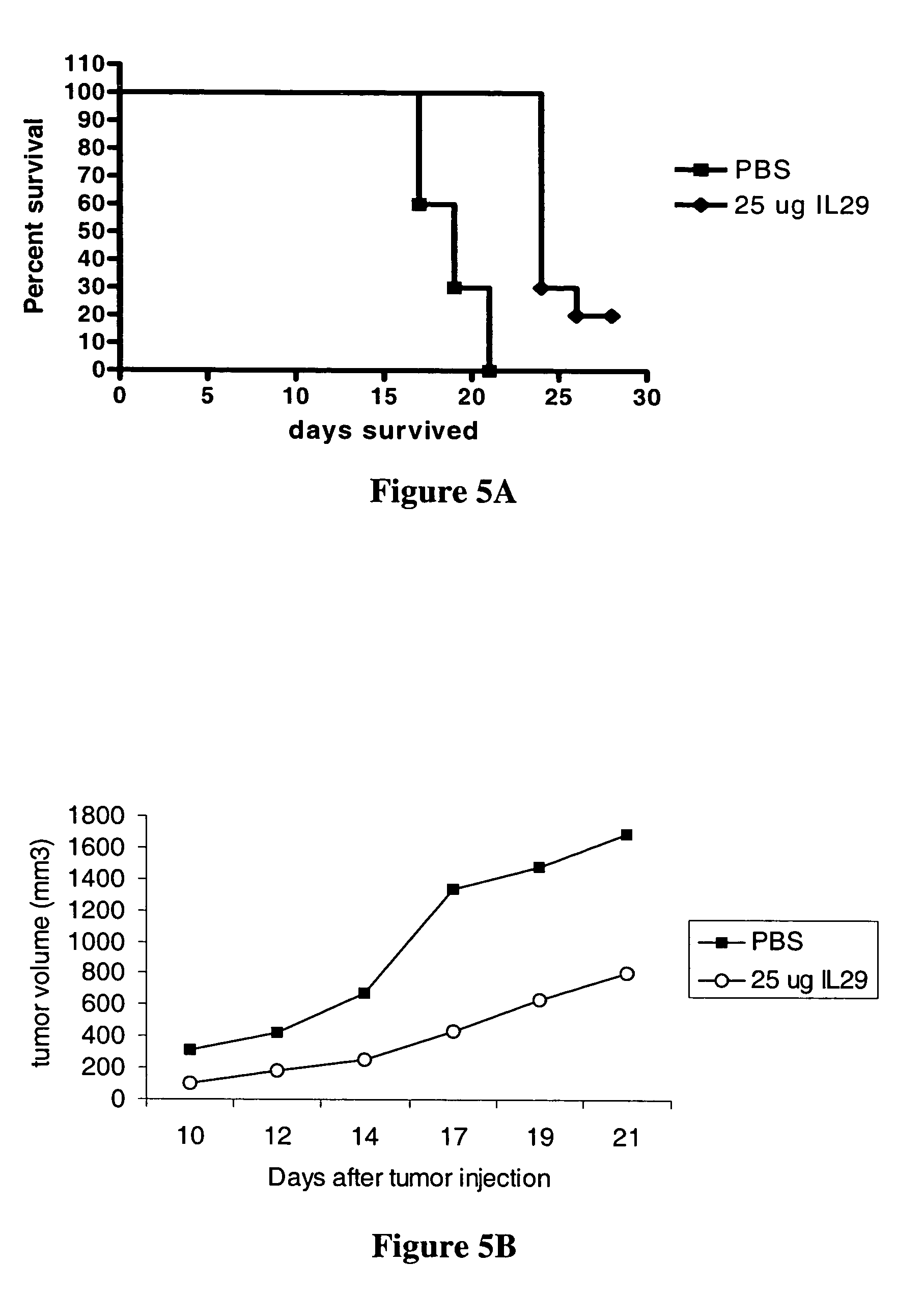
Use of IL-28 and IL-29 to treat cancer and autoimmune disorders
ActiveUS7351689B2Improve expression levelIncrease productionAntibacterial agentsBiocideDiseaseFunctional activity
Methods for treating patients with cancer and autoimmune disorders using IL-28 and IL-29 molecules. The IL-28 and IL-29 molecules include polypeptides that have homology to the human IL-28 or IL-29 polypeptide sequence and proteins fused to a polypeptide with IL-28 and IL-29 functional activity. The molecules can be used as a monotherapy or in combination with other known cancer and / or autoimmune therapeutics.
Owner:ZYMOGENETICS INC
Crystals of etanercept and methods of making thereof
The present invention relates to crystalline etanercept and to methods of making crystalline etanercept; to pharmaceutical compositions comprising crystalline etanercept; and to therapeutic uses of such compositions.
Owner:AMGEN INC
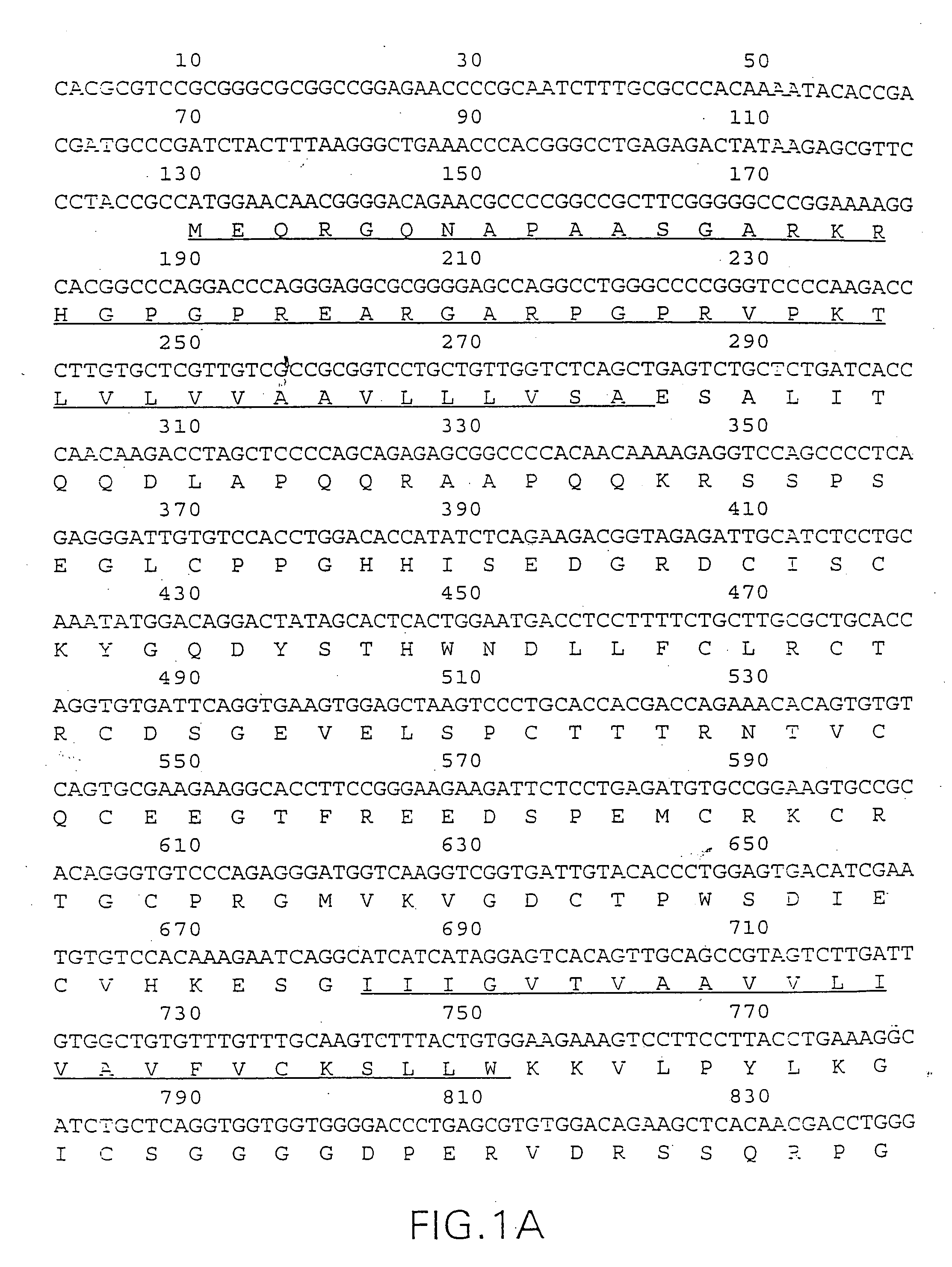
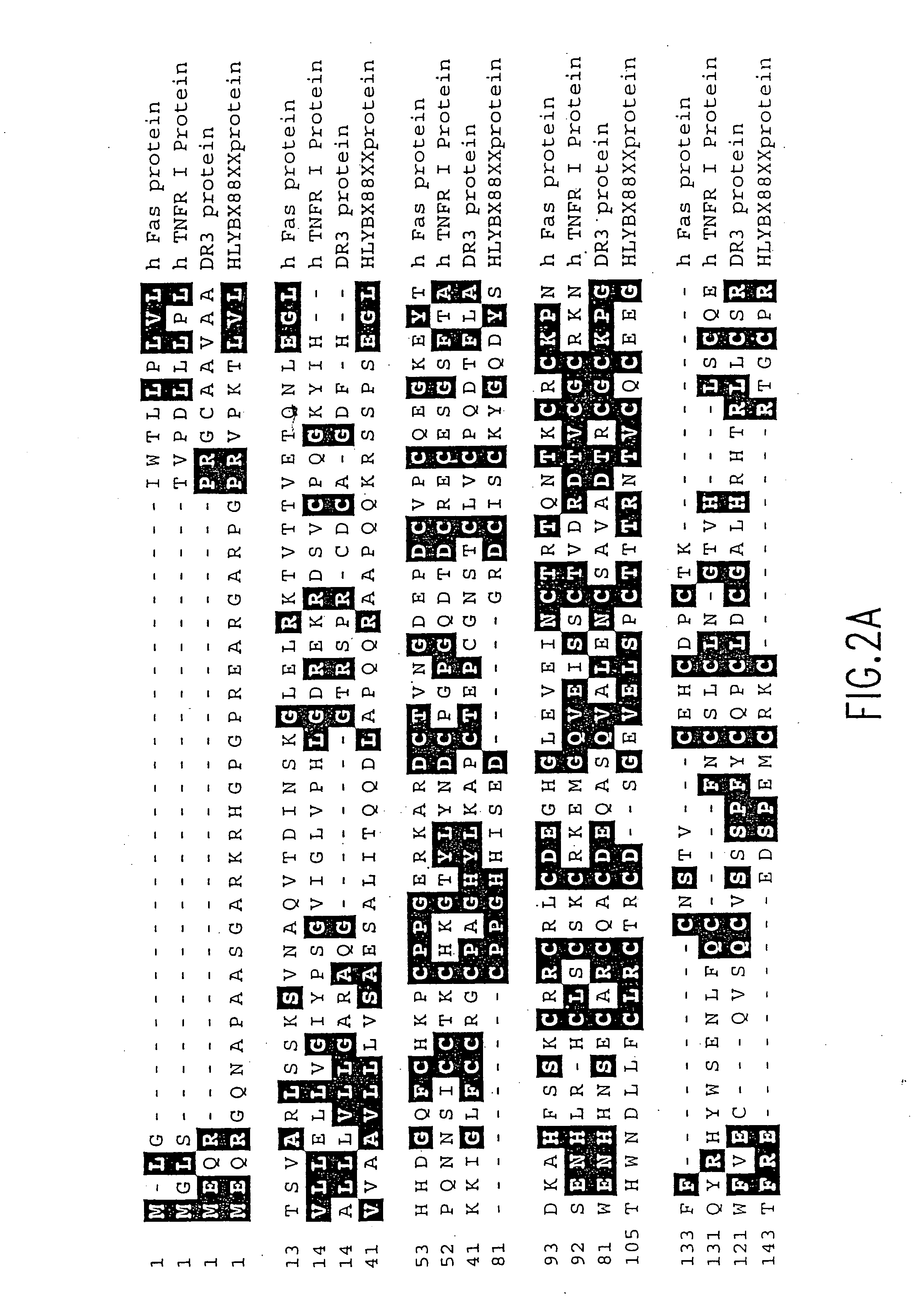
Death domain containing receptor 5
The present invention relates to novel Death Domain Containing Receptor-5 (DR5) proteins which are members of the tumor necrosis factor (TNF) receptor family, and have now been shown to bind TRAIL. In particular, isolated nucleic acid molecules are provided encoding the human DR5 proteins. DR5 polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying antagonists and antagonists of DR5 activity. The invention also relates to the treatment of diseases associated with reduced or increased levels of apoptosis using antibodies specific for DR5, which maybe agonists and / or antagonists of DR5 activity.
Owner:HUMAN GENOME SCI INC
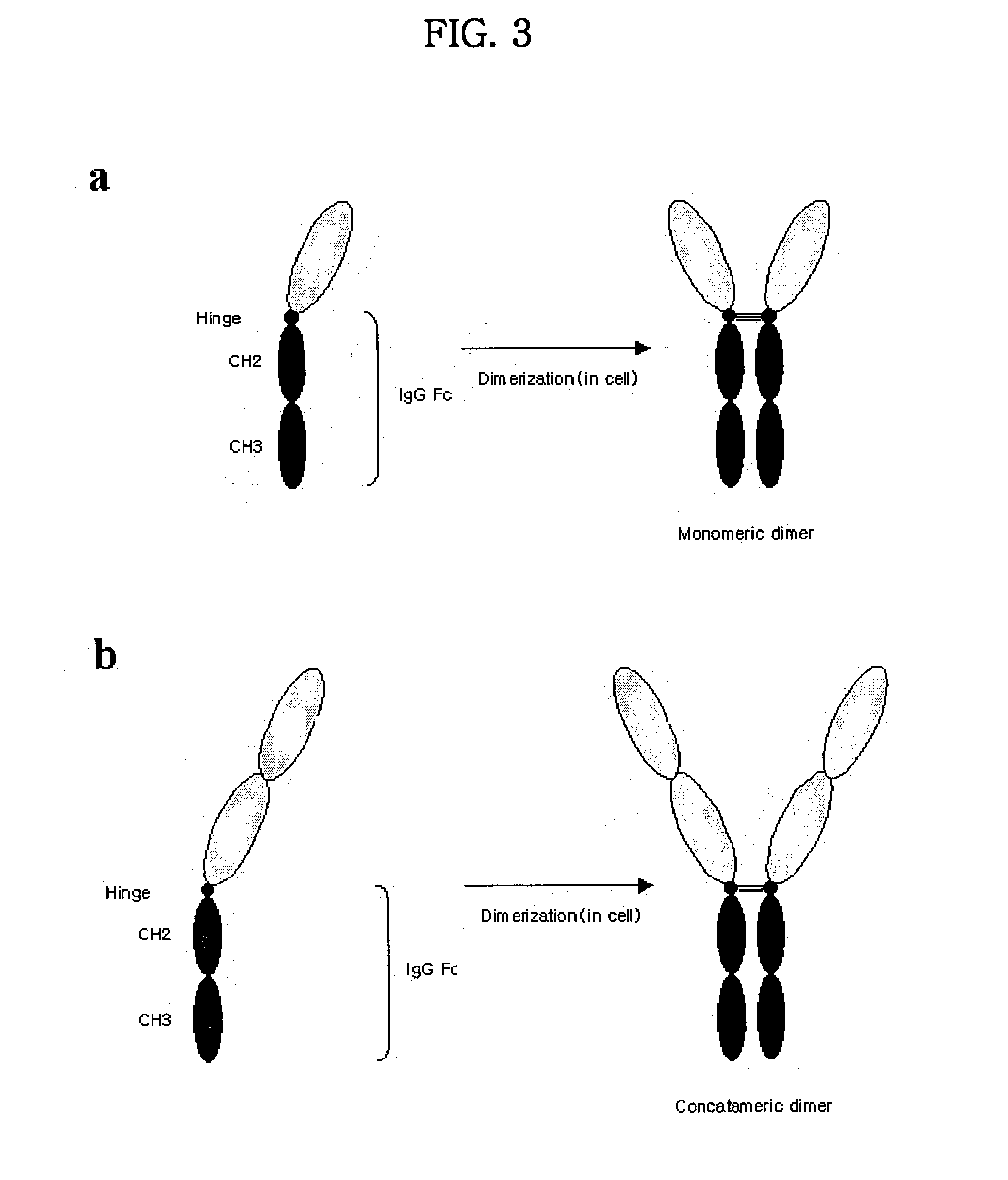
Concatameric immunoadhesion
ActiveUS20030195338A1Improve efficacyImprove stabilityAntipyreticAntibody mimetics/scaffoldsDNA constructActive protein
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
Storage stable powder compositions of interleukin-4 receptor
InactiveUS6896906B2Good chemical stabilityImprove physical stabilityPowder deliveryPeptide/protein ingredientsWhite blood cellPhysical chemistry
The present invention provides storage stable dry powder compositions of IL-4R. The powder compositions demonstrate superior chemical and physical stability over their solution counterparts, particularly upon storage under varying conditions of temperature and humidity. Moreover, the powders, as prepared, possess good aerosol properties, which are maintained upon storage.
Owner:NOVARTIS FARMA
Gene transfer for studying and treating a connective tissue of a mammalian host
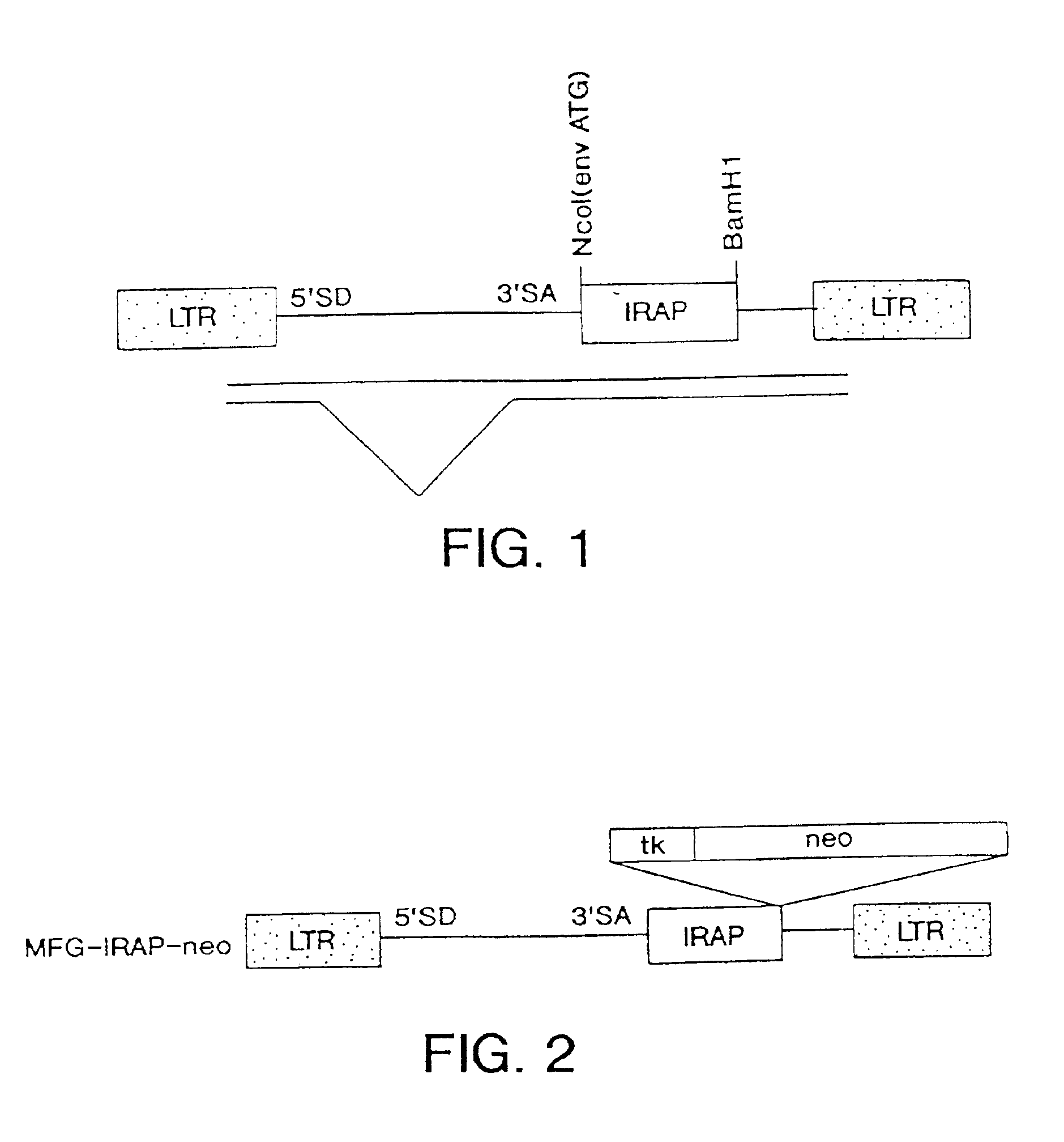
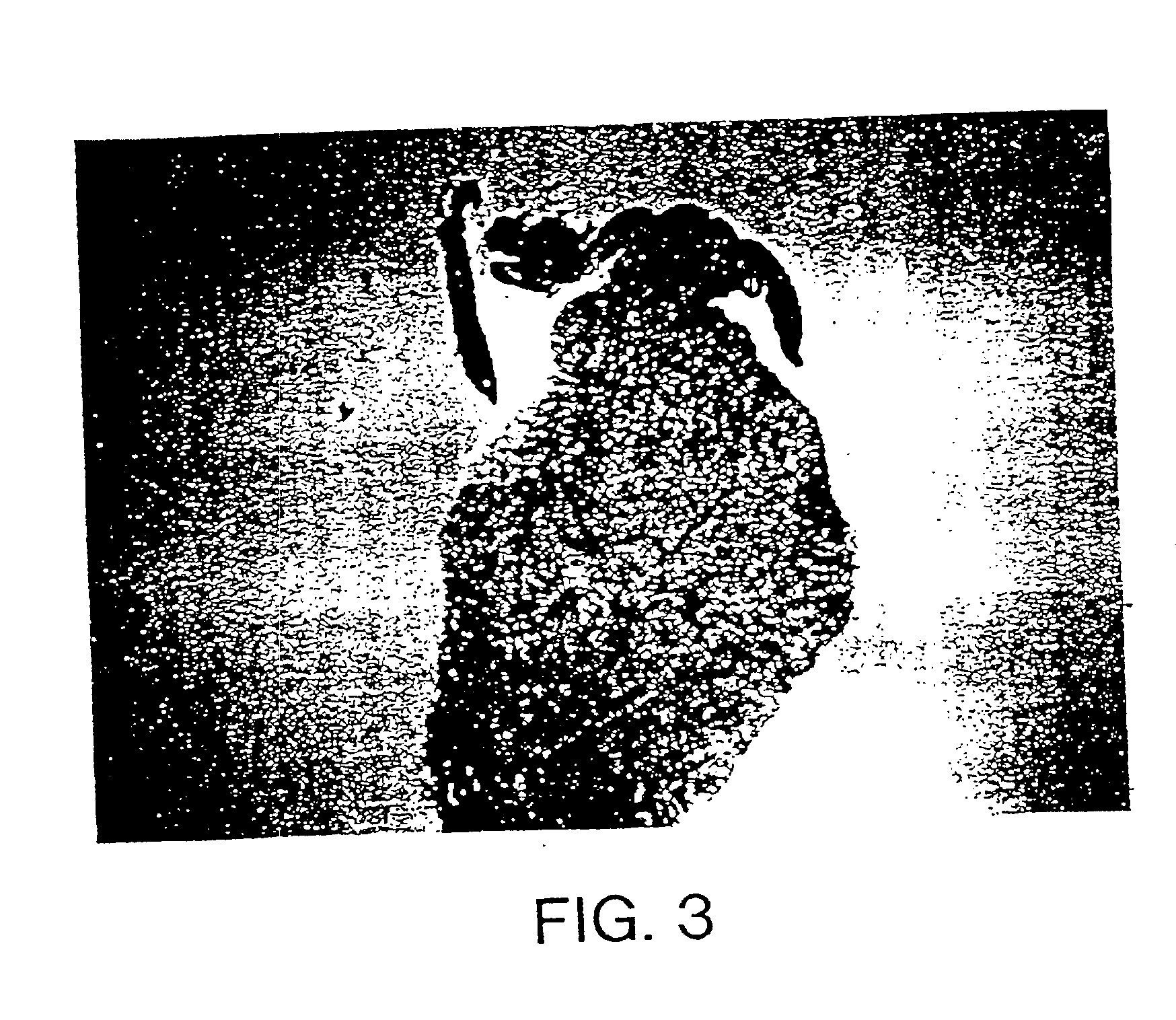
InactiveUS7037492B2Reducing at least one deleterious joint pathologyCompounds screening/testingBiocideConnective tissue fiberMammal
Methods for introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule that contains the gene encoding for the product, and infecting the target cells of the mammalian host using the DNA vector molecule. A method to produce an animal model for the study of connective tissue pathology is also disclosed.
Owner:UNIVERSITY OF PITTSBURGH
Multivalent protein conjugate with multiple ligand-binding domains of receptors
InactiveUS20030064053A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsWound healingAbnormal cell
The present invention provides compositions and methods for treating abnormal cell proliferation and for regulating angiogenesis. In particular, multivalent protein conjugates (MVPs) are constructed to include multiple ligand-binding domains of different receptors and utilized to target multiple, different ligands that are involved in regulation of cell growth and neovascularization. The MVPs of the present invention can be used to treat various conditions associated with abnormal cell proliferation and angiogenesis such as cancer and cardiovascular disorders, as well as to promote wound healing.
Owner:ABMAXIS
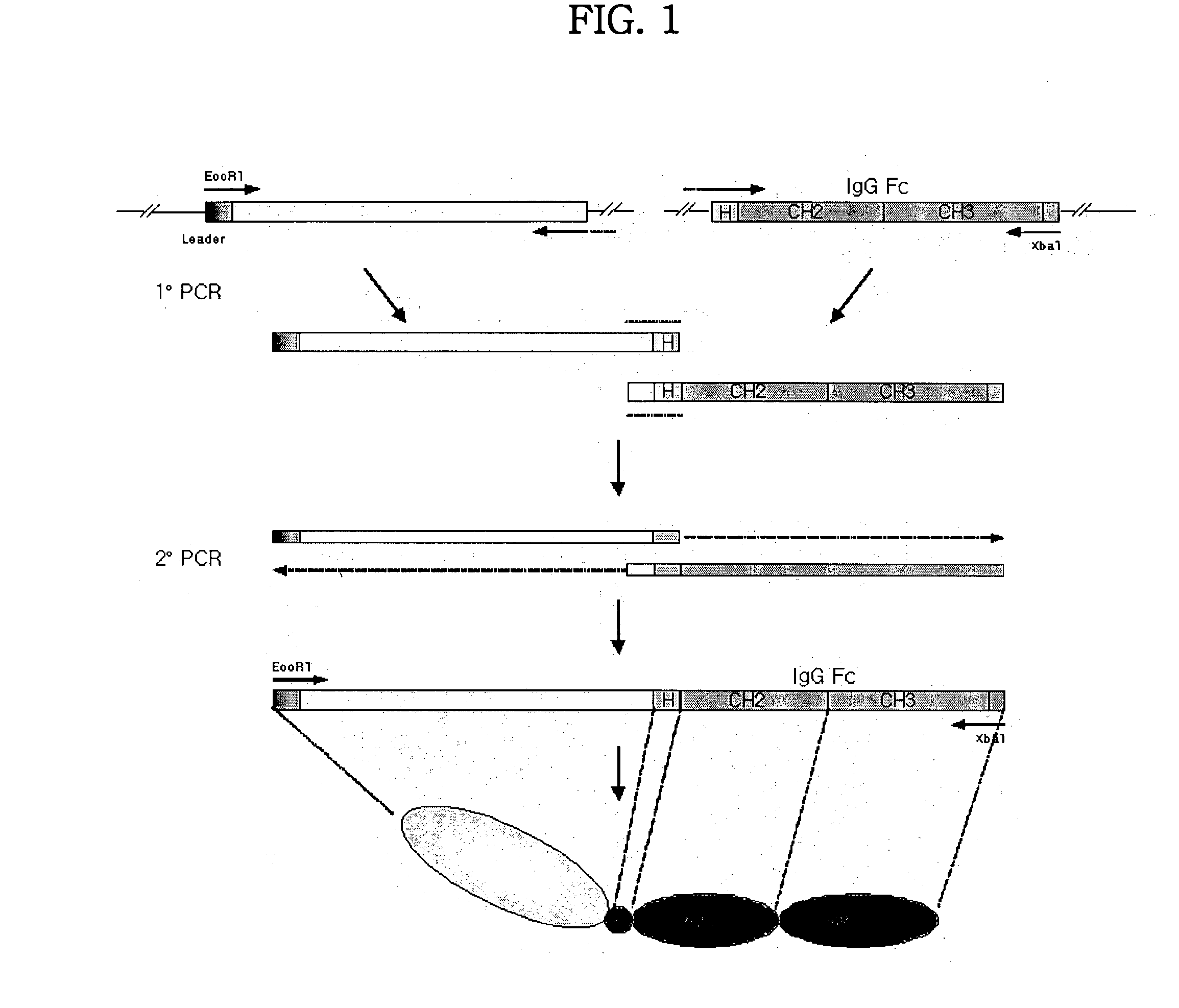
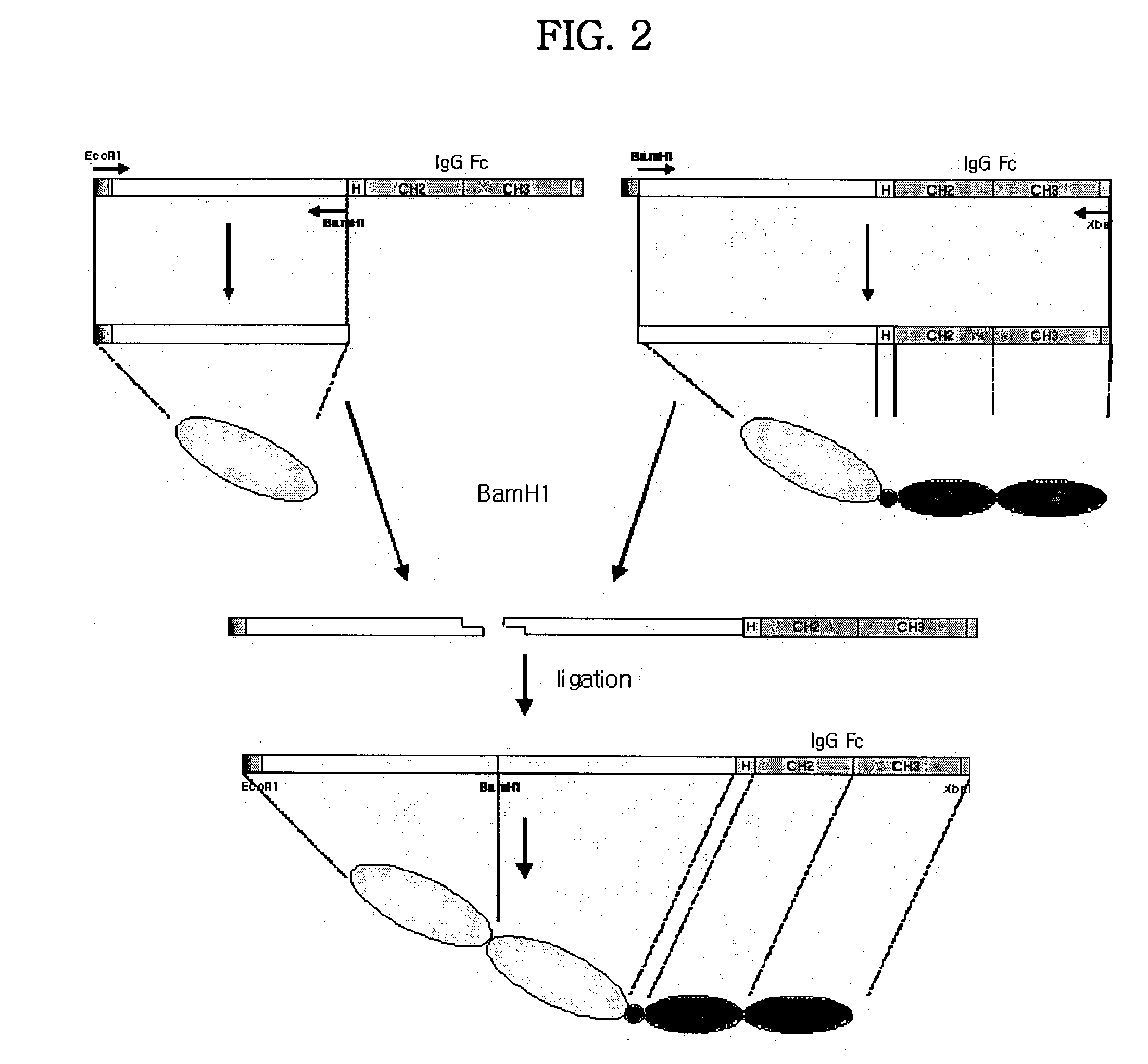
Human tumor necrosis factor-immunoglobulin(TNFR1-IgG1) chimera composition
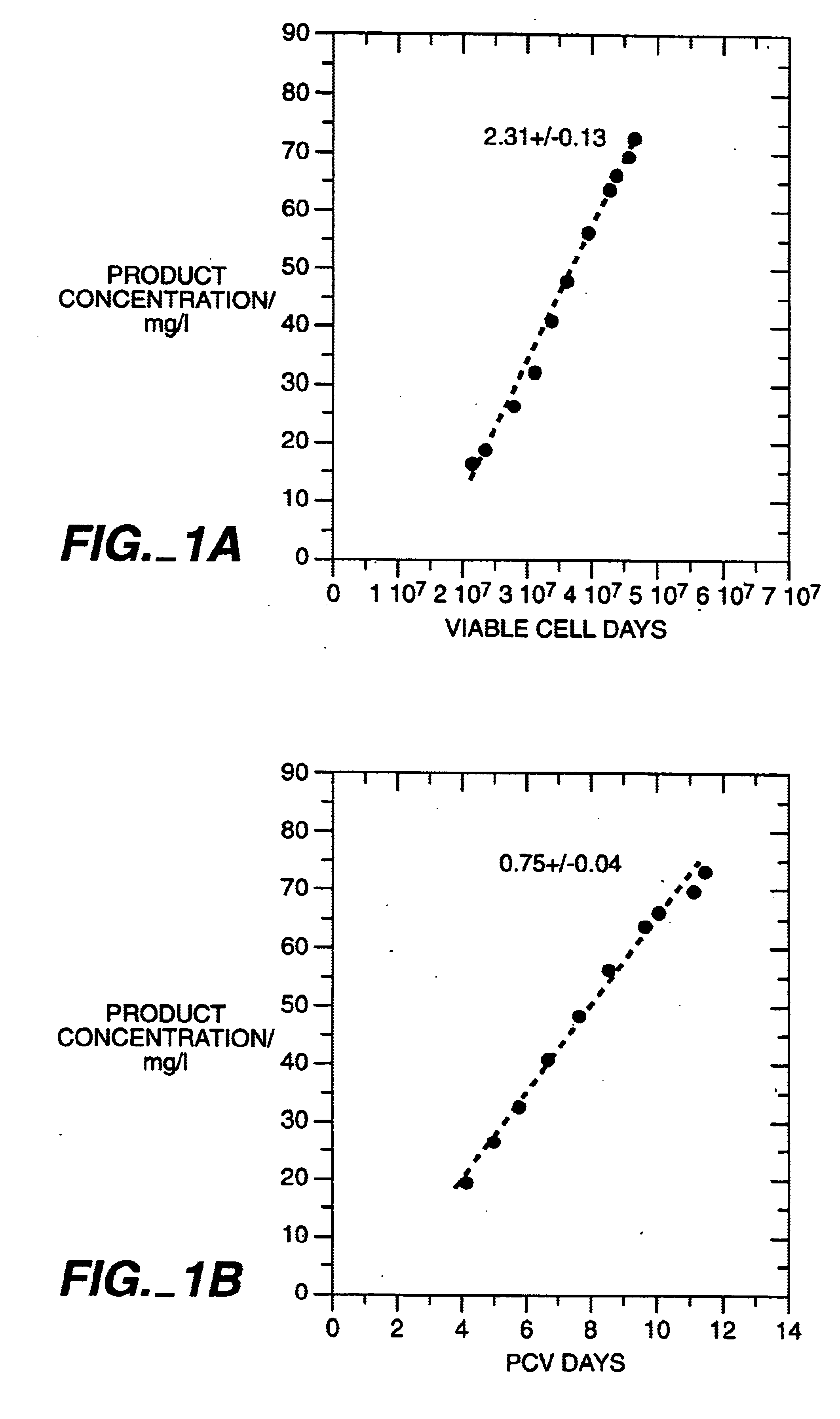
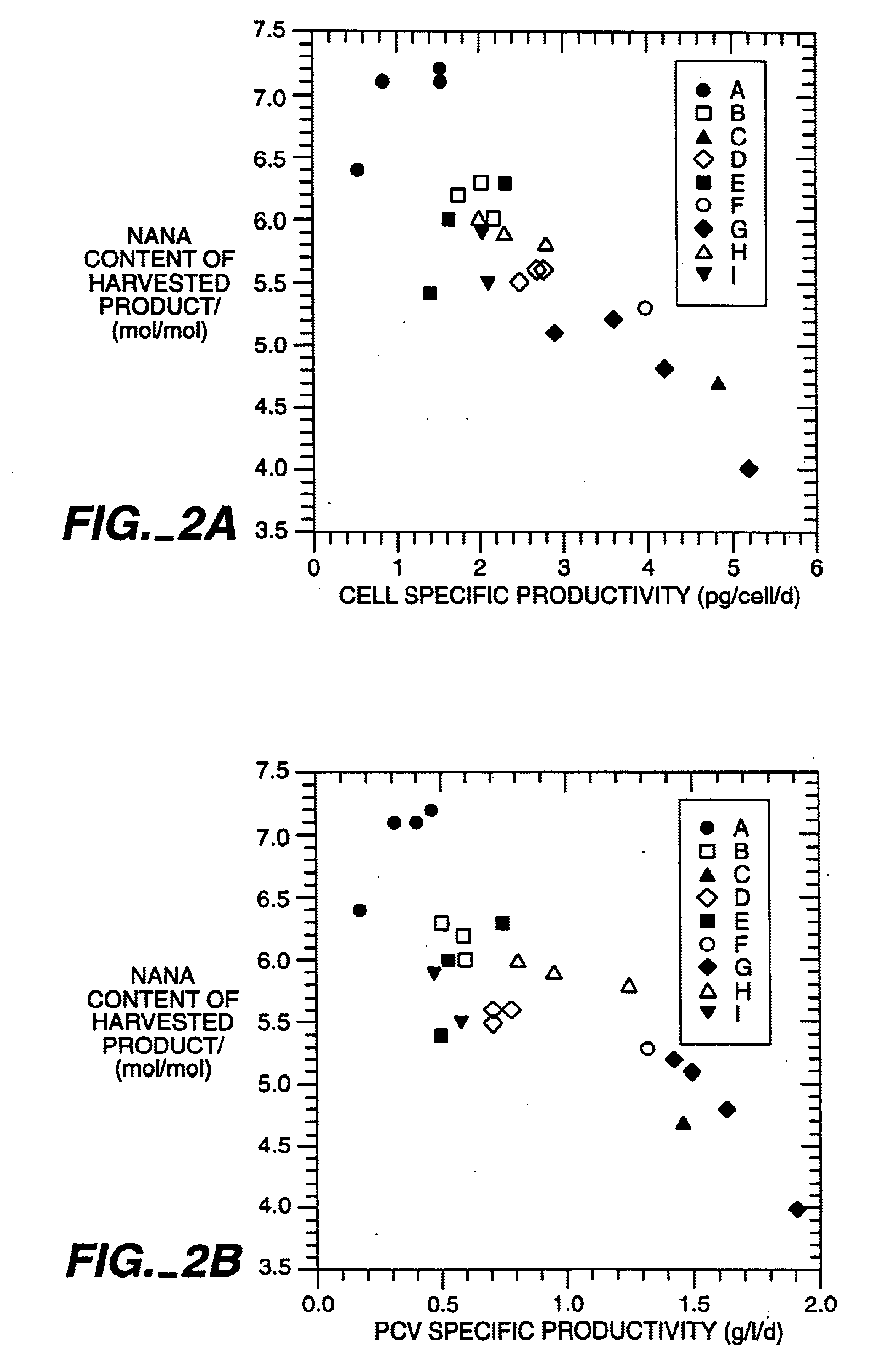
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:GENENTECH INC
Antibodies to Interleukin-4 receptors and uses thereof
InactiveUS7317090B2Peptide/protein ingredientsMicroorganism based processesAntibodyInterleukin-4 receptor
Owner:IMMUNEX CORP
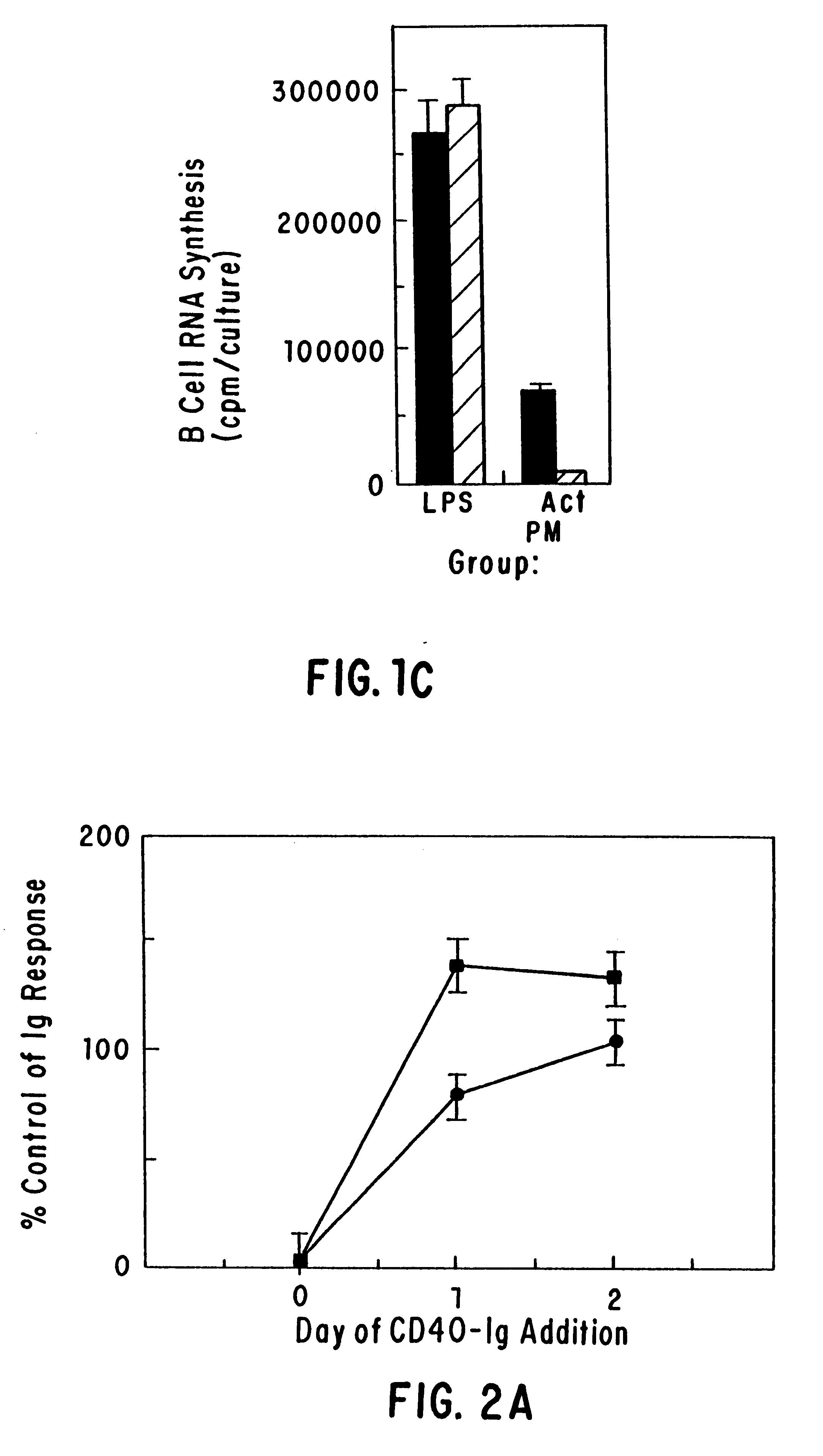
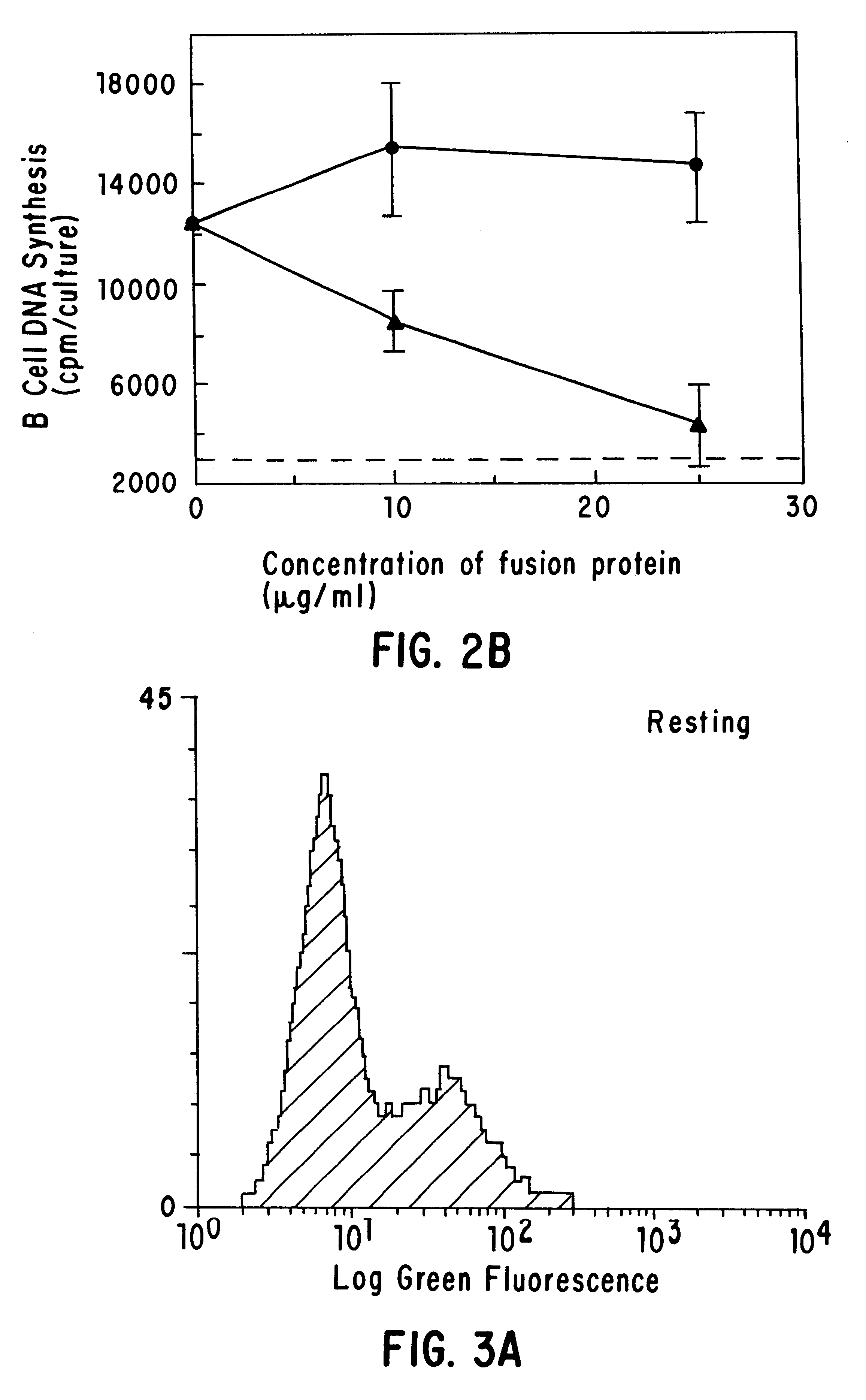
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
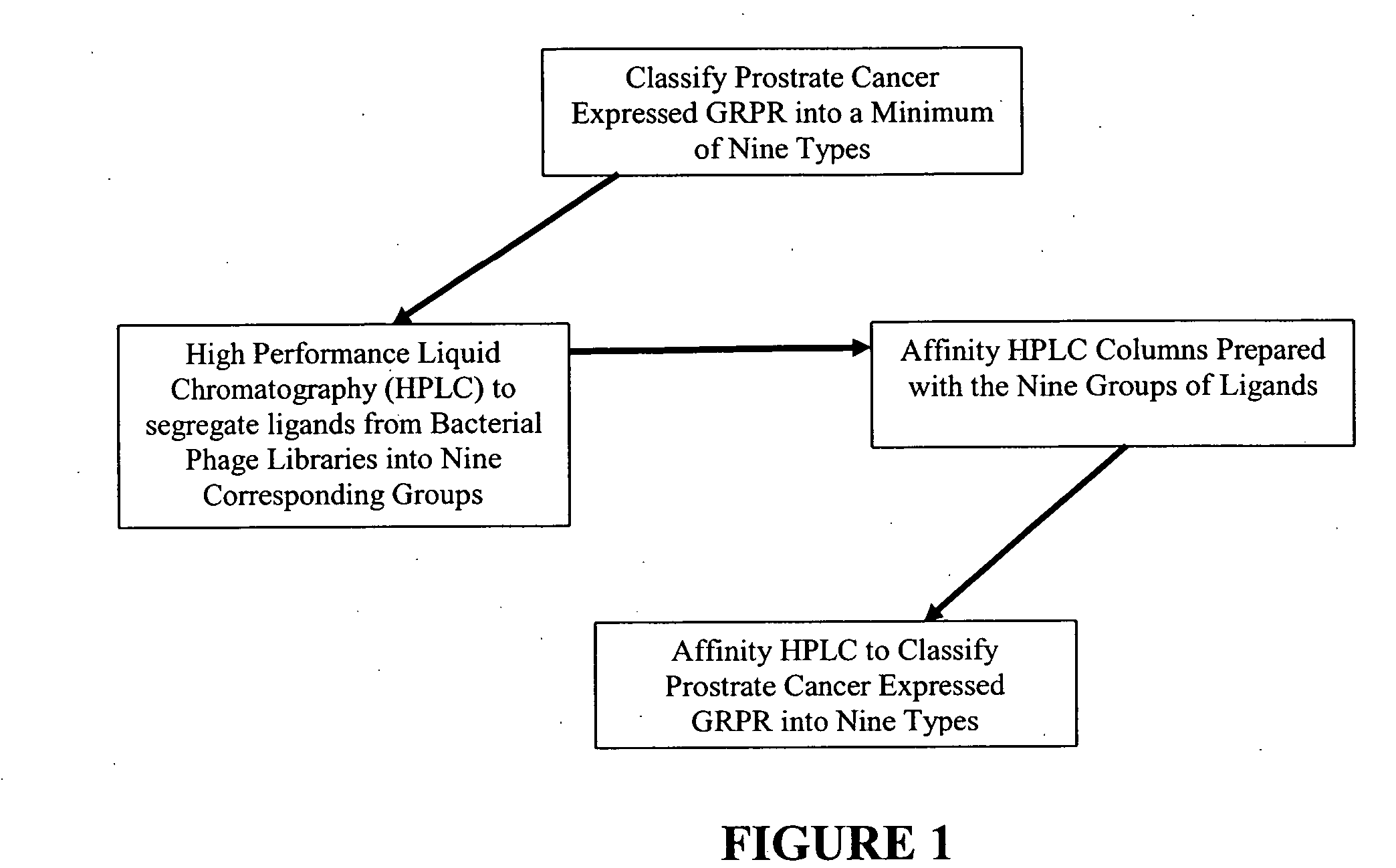
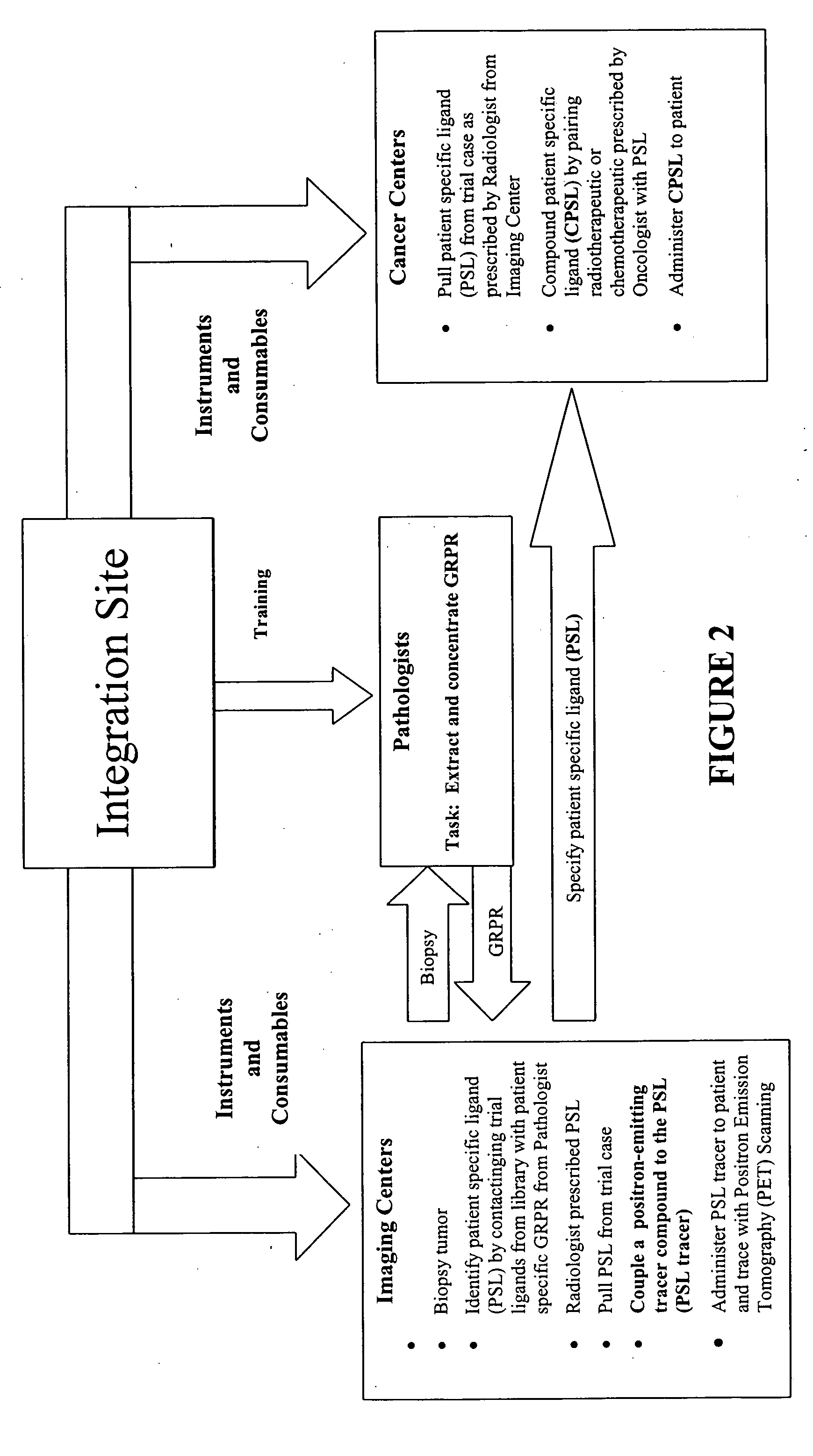
Method, compositions and classification for tumor diagnostics and treatment
InactiveUS20060160157A1Quick and easy determinationImprove survivalCompound screeningApoptosis detectionAbnormal tissue growthSide effect
The present invention is directed towards classifying tumor biomarkers, particularly membrane receptors, and more particularly the gastrin-releasing peptide (GPR) receptors, identified in patient samples, then linking therapeutic agents (chemical, radiological, or biological) to patient-specific ligands that bind to such receptors, clinicians can produce diagnostic and treatment compositions and implement treatment regimens which, by using the classified and identified biomarkers, and due to their improved accuracy, increase success and decrease undesired side effects from such treatments.
Owner:ZUCKERMAN MATHEW MARK
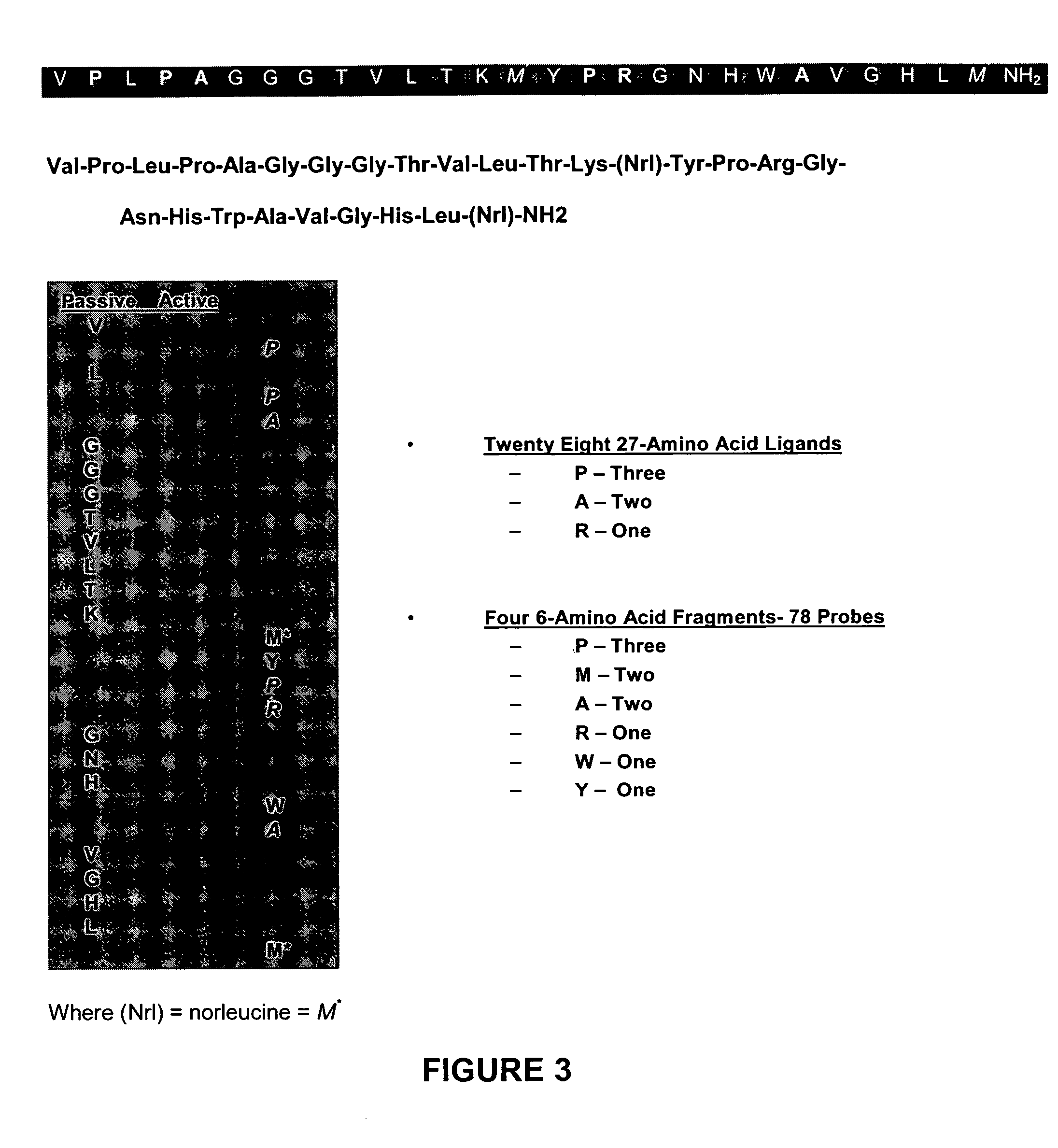
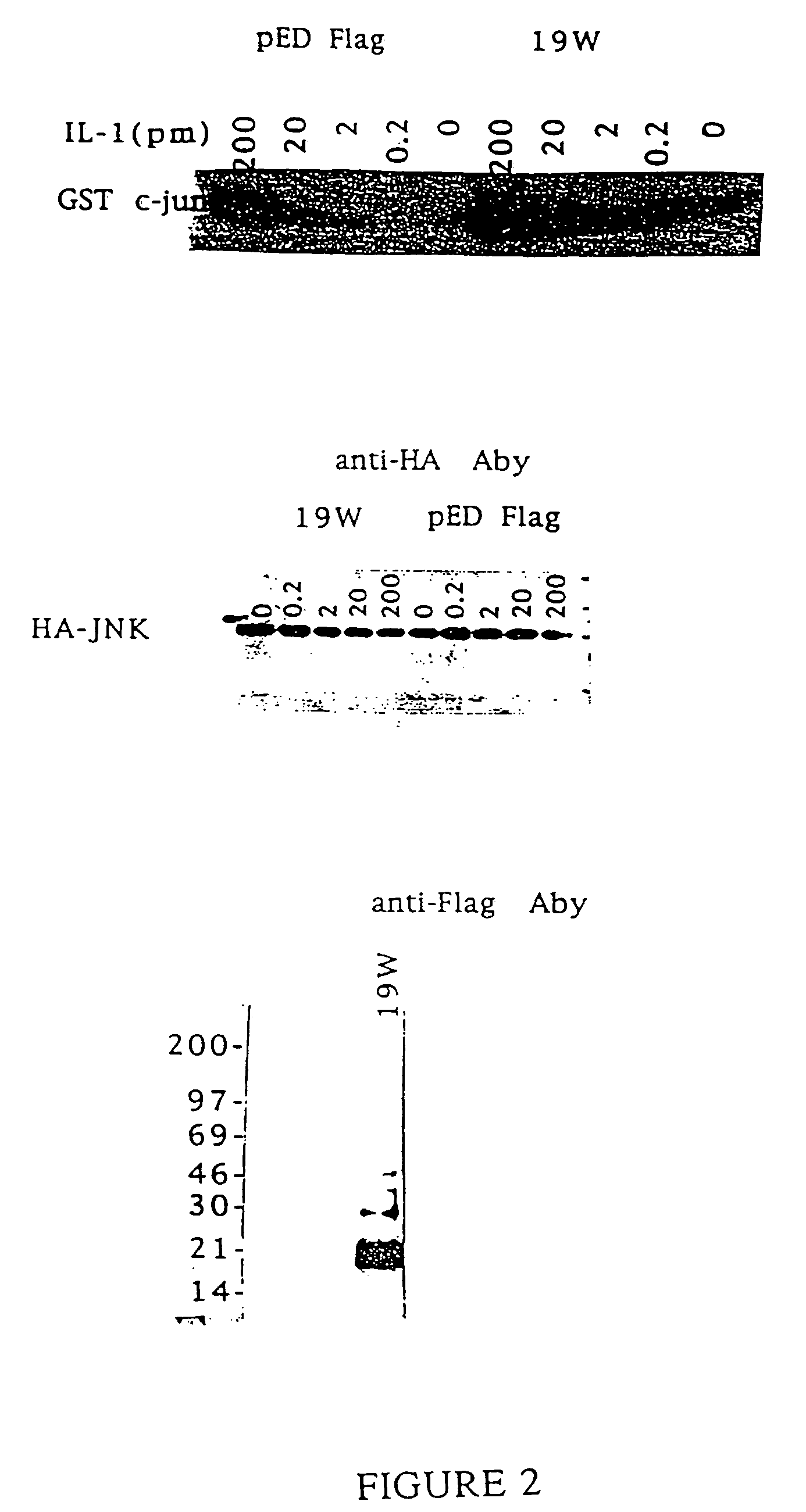
Novel interleukin-1 receptor intracellular ligand proteins and inhibitors of ligand binding
Novel IL-1-R intracellular ligand proteins are disclosed. Polynucleotides encoding the IL-1-R intracellular ligand protein are also disclosed, along with vectors, host cells, and methods of making the IL-1-R intracellular ligand protein. Pharmaceutical compositions containing the IL-1-R intracellular ligand protein, methods of treating inflammatory conditions, and methods of inhibiting IL-1-R intracellular domain binding are also disclosed. Methods of identifying inhibitors of IL-1-R intracellular domain binding and inhibitors identified by such methods are also disclosed.
Owner:GENETICS INST INC
Targeted drug delivery using EphA2 or EphA4 binding moieties
InactiveUS20050153923A1Good curative effectIncreased level of cytokineAntibody mimetics/scaffoldsGenetic material ingredientsDelivery vehicleNucleotide
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a hyperproliferative cell disease, particularly cancer. The methods of the invention comprise the administration of an effective amount of a composition that targets cells expressing an Eph family receptor tyrosine kinase, such as EphA2 or EphA4, for the treatment, management, or prevention of hyperproliferative diseases, particularly cancer. In one embodiment, the method of the invention comprises administering to a subject a composition comprising an EphA2 or EphA4 targeting moiety attached to a delivery vehicle, and one or more therapeutic or prophylactic agents that treat or prevent a hyperproliferative disease, where the therapeutic or prophylactic agents are operatively associated with the delivery vehicle. In another embodiment, the method of the invention comprises administering to a subject a composition comprising a nucleic acid comprising a nucleotide sequence encoding an EphA2 or EphA4 targeting moiety and a therapeutic or prophylactic agent that treats or prevents a hyperproliferative disease. In yet another embodiment, the method of the invention comprises administering to a subject a composition comprising an EphA2 or EphA4 targeting moiety and a nucleic acid comprising a nucleotide sequence encoding an agent that treats or prevents a hyperproliferative disease, where the nucleic acid is operatively associated with the delivery vehicle. Pharmaceutical compositions are also provided by the present invention.
Owner:MEDIMMUNE LLC
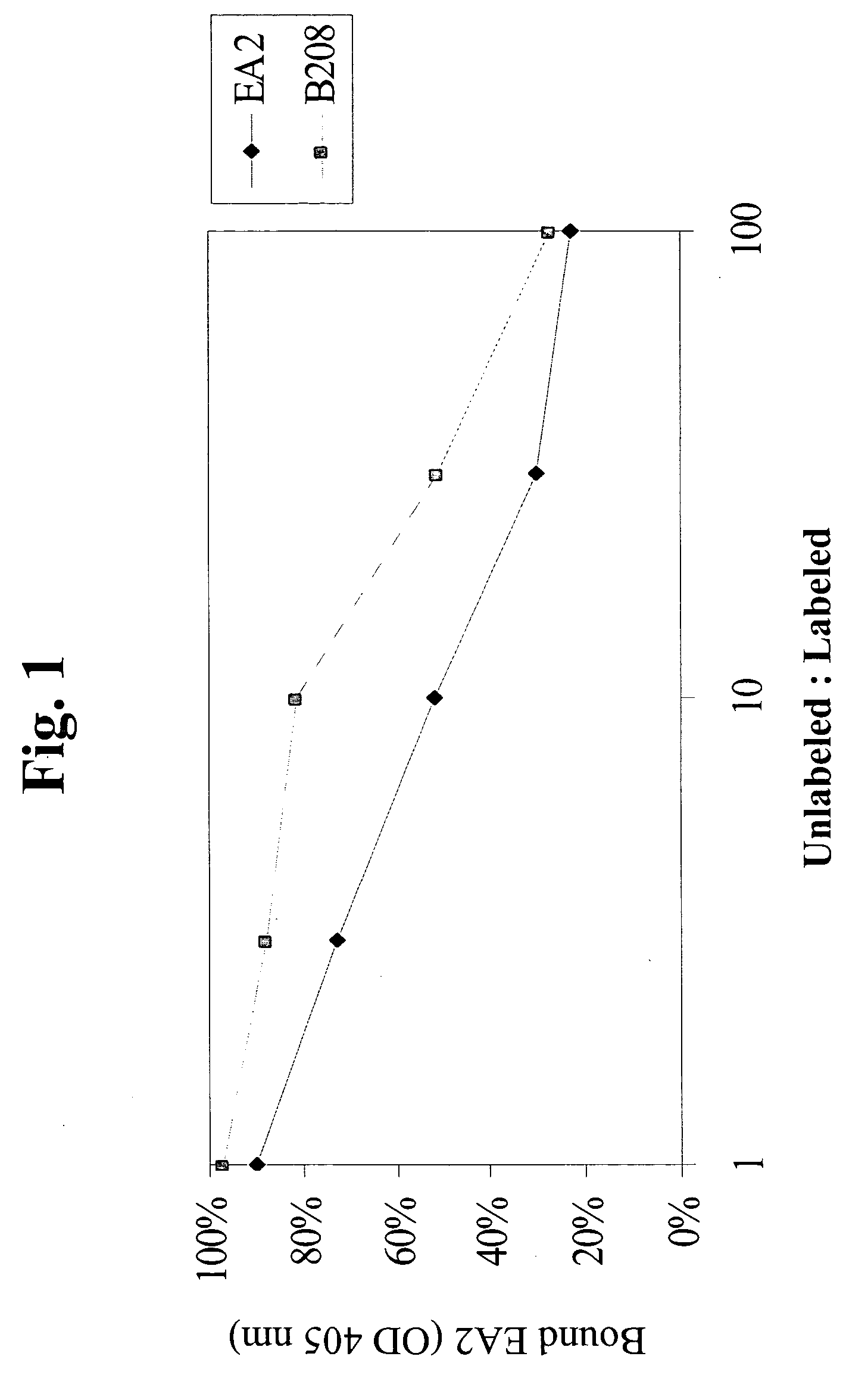
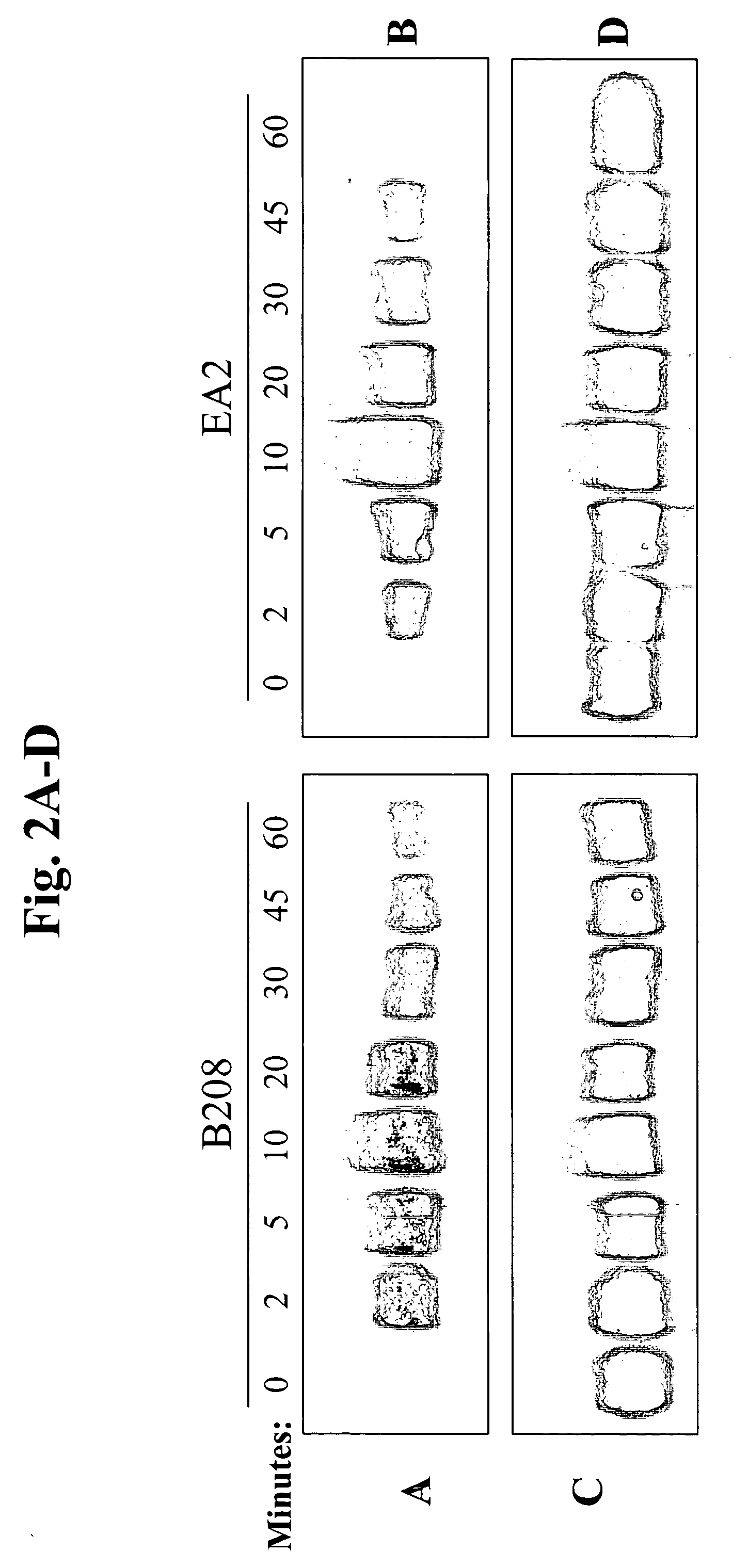
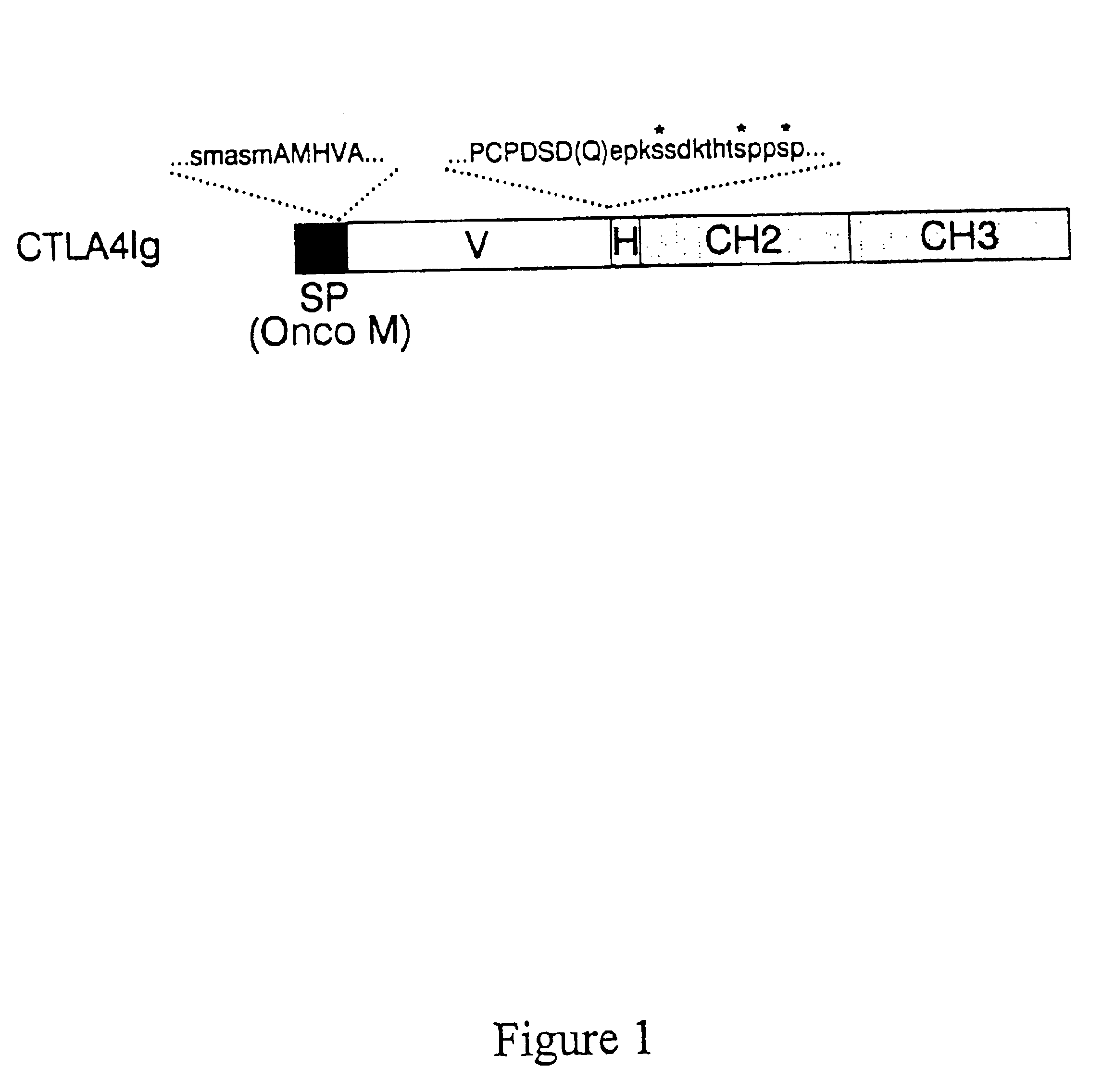
Method to inhibit T cell interactions with soluble B7
InactiveUS6887471B1Suppression problemPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellT cell mediated immunity
Owner:BRISTOL MYERS SQUIBB CO
Non-antigenic toxin-conjugate and fusion protein of internalizing receptor system
InactiveUS7033572B2Effective and less toxicIncrease valuePeptide/protein ingredientsAntibody mimetics/scaffoldsEphA ReceptorsCytokine
A conjugate of a toxin and a cytokine, and a fusion protein comprising a bispecific antibody that has a first specificity for a cell marker specific to a malignant cell and a second specificity for a region of IL-15α, each optionally further comprising a radionuclide, are useful therapeutic reagents for treating leukemias and lymphomas.
Owner:IMMUNOMEDICS INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com