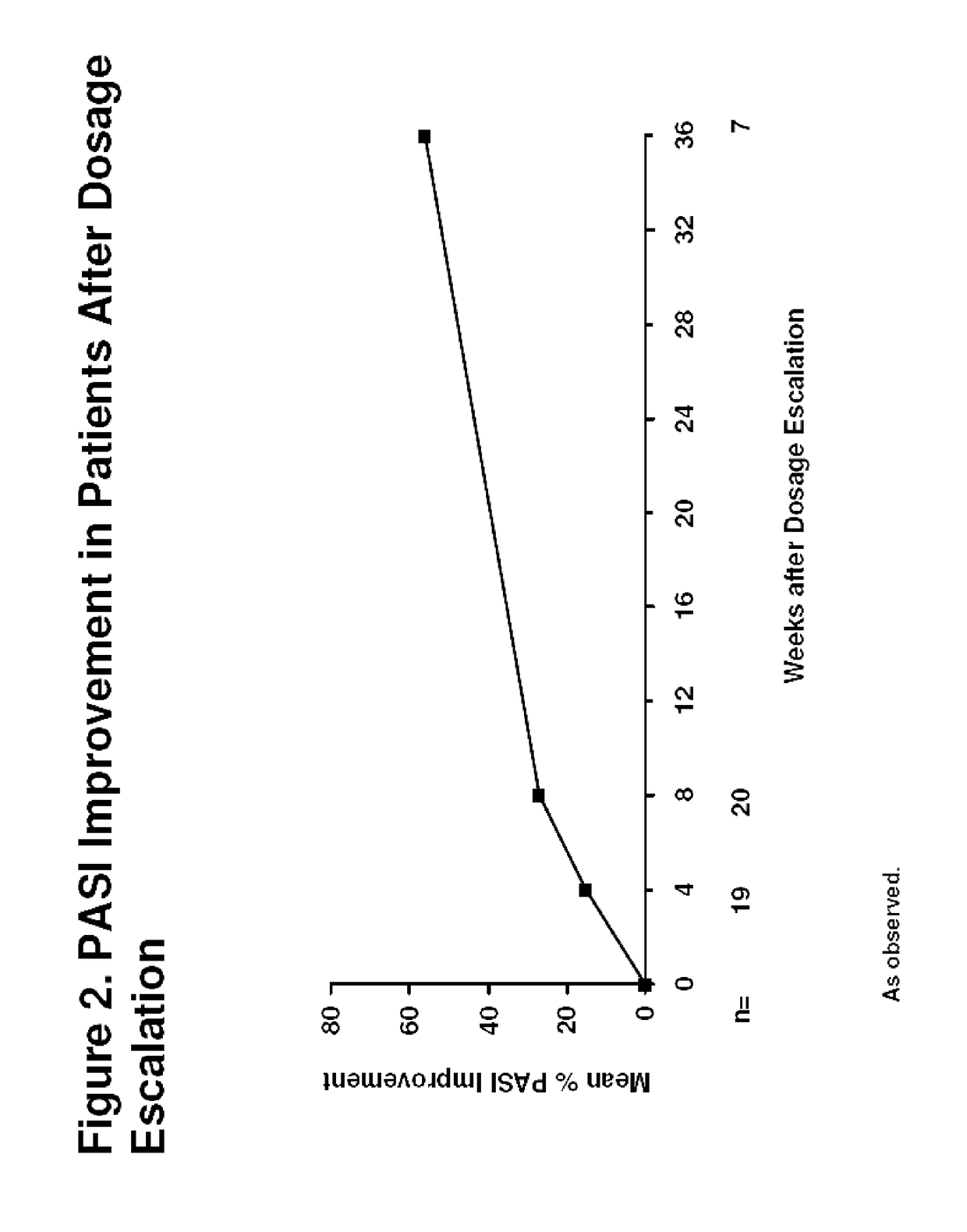
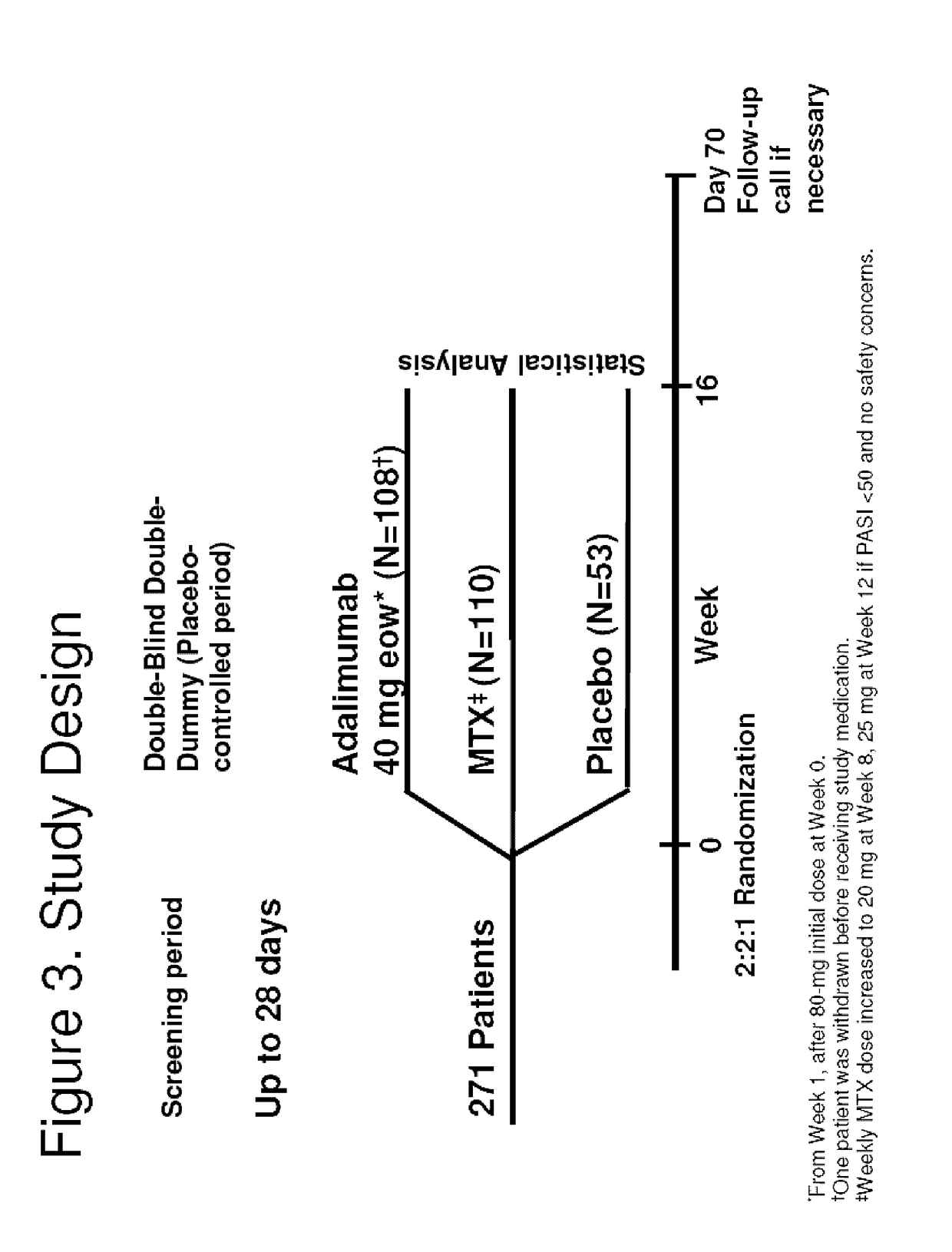
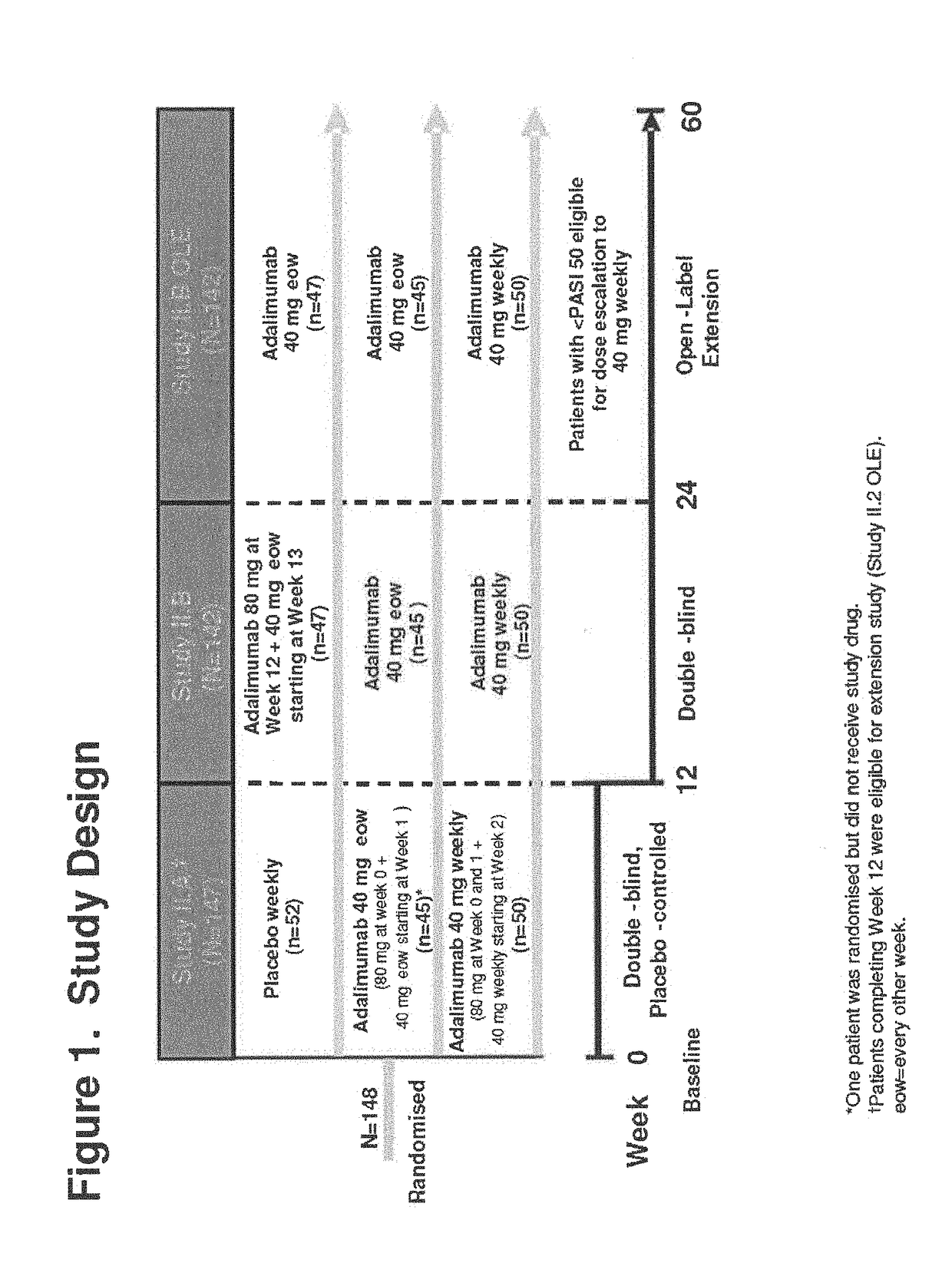
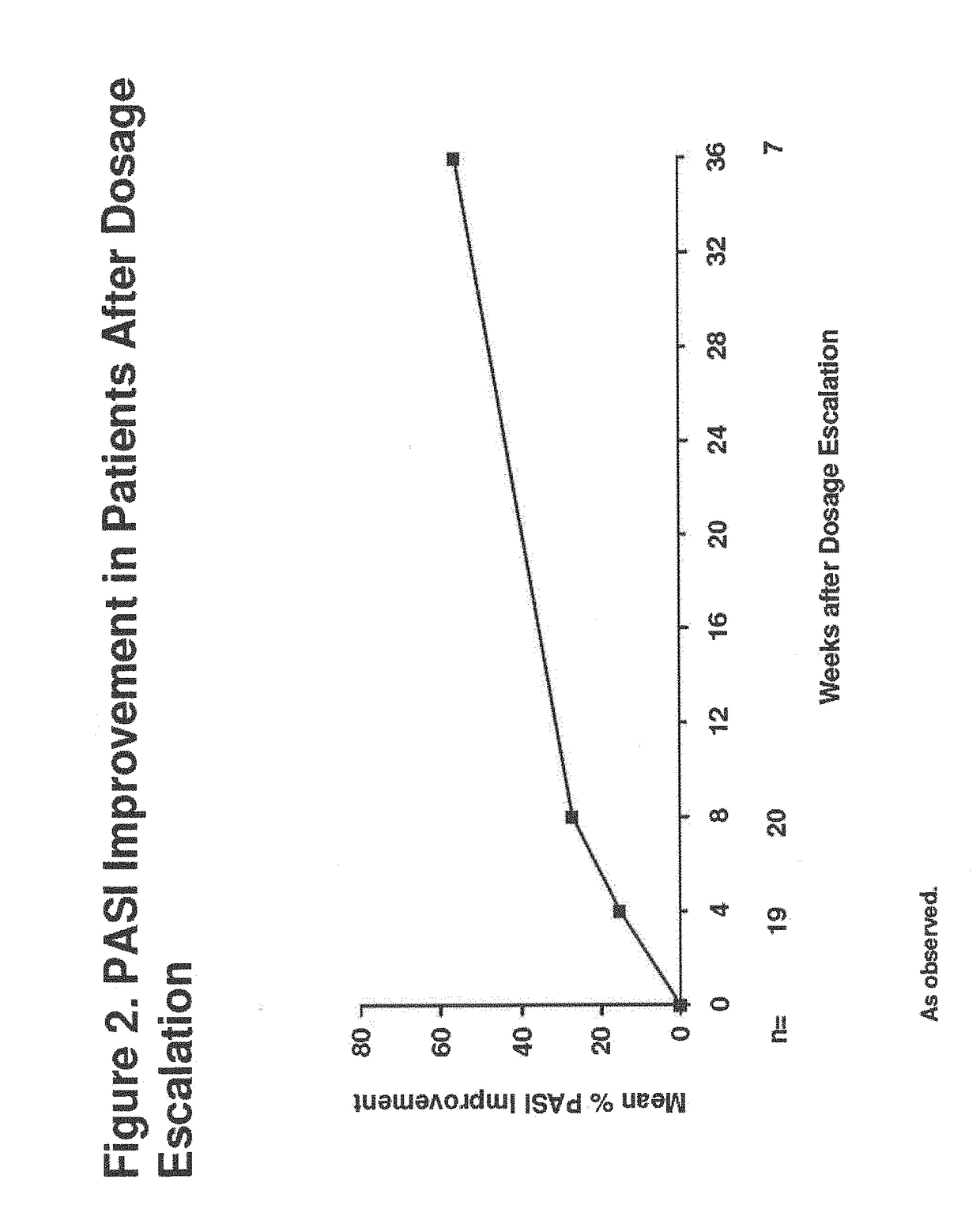
Patents
Literature
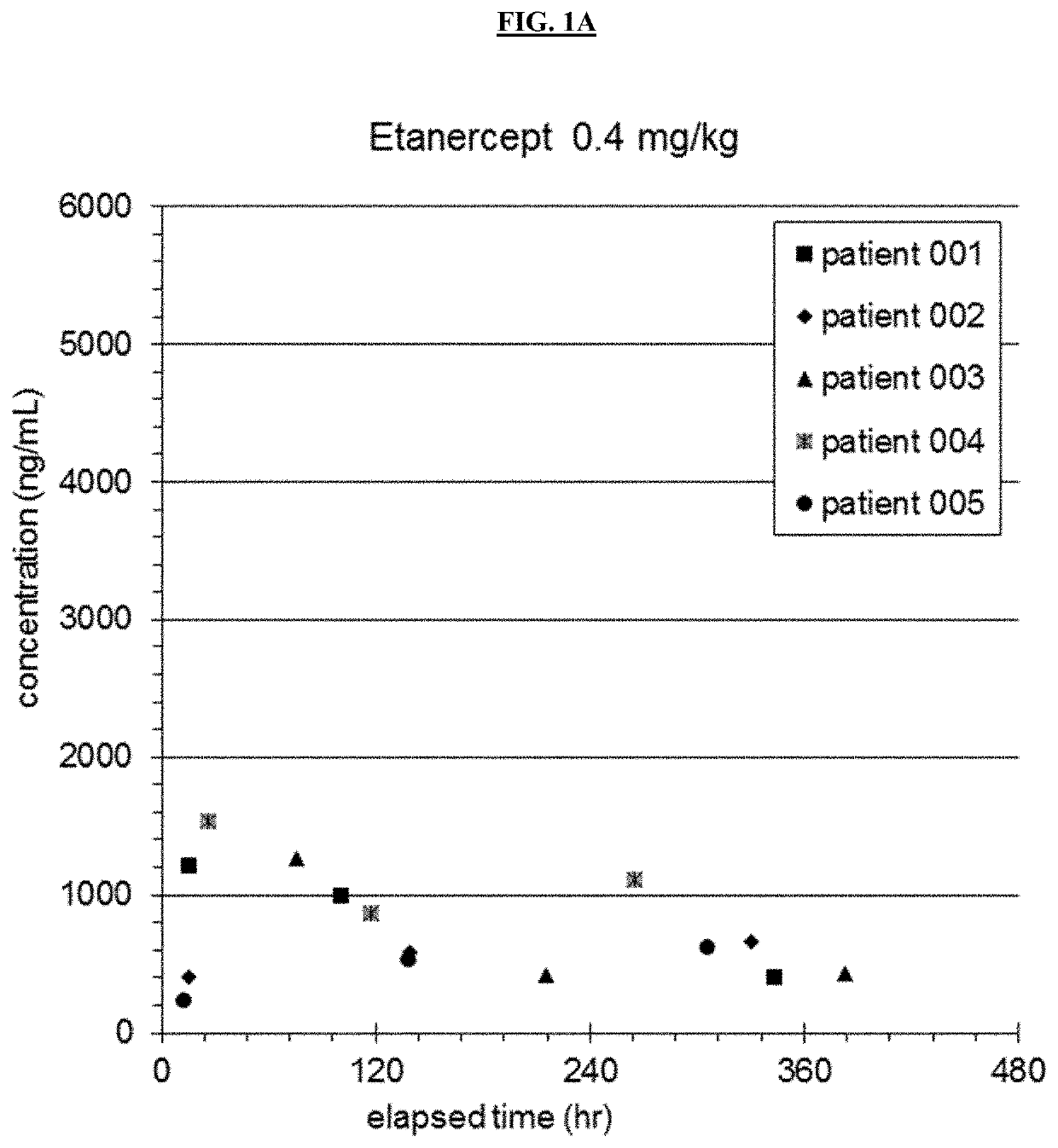
35 results about "Etanercept" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or in combination with an immunosuppressant (such as methotrexate) to treat certain types of arthritis (such as rheumatoid, psoriatic, juvenile idiopathic, and ankylosing spondylitis). Some brands of this medication are also used to treat a skin condition called psoriasis. These conditions are caused by an overactive immune system (autoimmune disease).
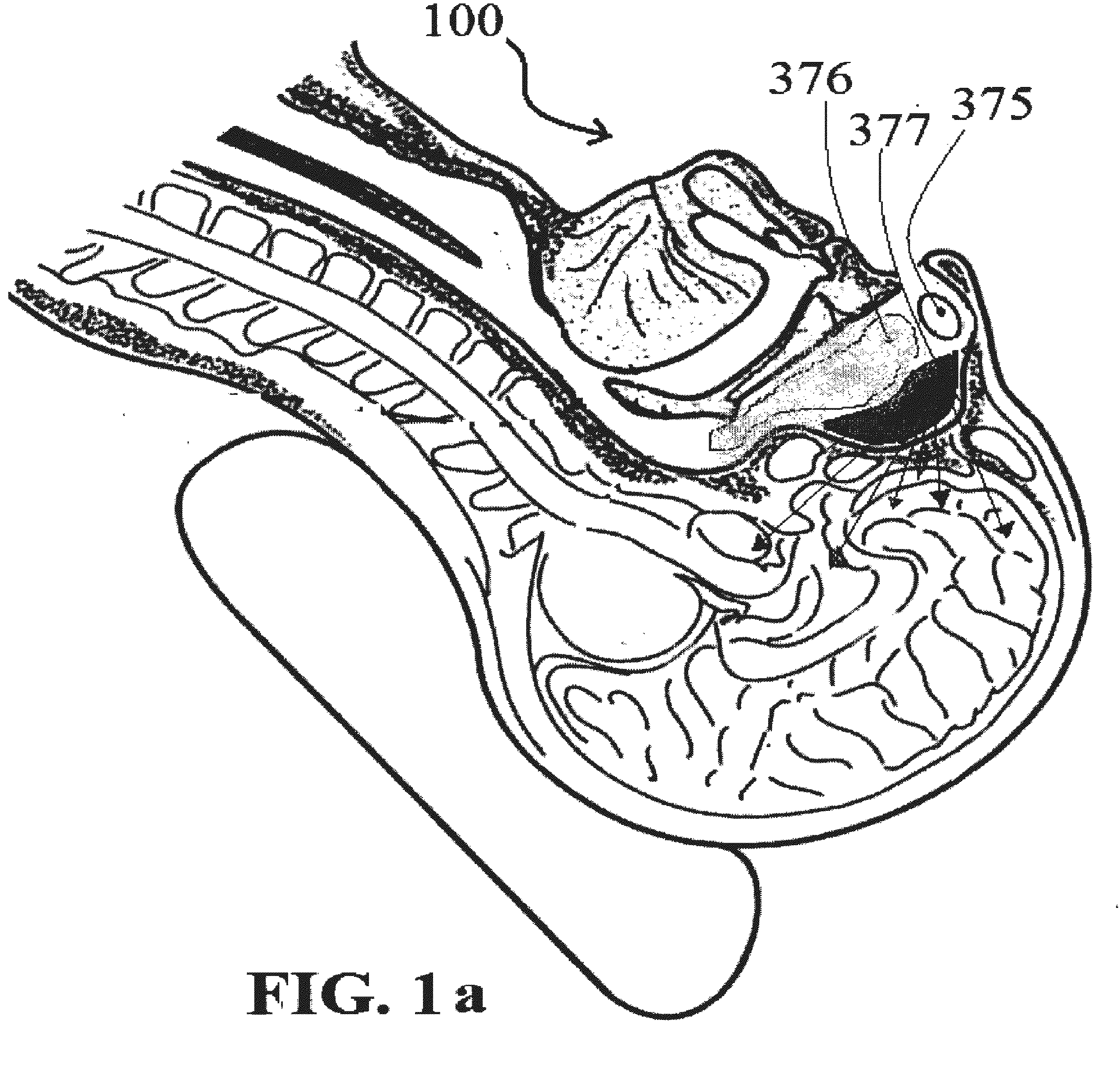
Cytokine antagonists for the treatment of sensorineural hearing loss
InactiveUS6423321B2Improve hearingReduce developmentPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsIntravenous routeSensorineural hearing loss
Specific Cytokine Antagonists, including TNF antagonists and / or Interleukin-1 antagonists, are used as novel therapeutic agents for the treatment of hearing loss, including presbycusis and other forms of sensorineural hearing loss. The present invention provides a method for inhibiting the action of TNF and / or IL-1 antagonists for treating hearing loss in a human by administering a TNF antagonist and / or an IL-1 antagonist for reducing the inflammation affecting the auditory apparatus of said human, or for modulating the immune response affecting the auditory apparatus of said human, by administering a therapeutically effective dosage level to said human of a TNF antagonist and / or an IL-1 antagonist. Administration may be systemic, through the subcutaneous, intramuscular, oral, or intravenous routes; or by delivering an anatomically localized application in the region of the head. The TNF antagonist is selected from the group consisting of etanercept, infliximab, D2E7, CDP 571, or thalidomide; and the IL-1 antagonist is either IL-1 RA or IL-1R type II receptor. Antiviral agents may be added for treating certain patients.
Owner:TACT IP
Methods and compositions for treatment of skin disorders
ActiveUS20110171227A1Undesirable effectImproving disease reductionSnake antigen ingredientsAntibody ingredientsEtanerceptPrior biologic therapy
The invention provides methods and compositions for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis. The invention includes methods for treating a skin disorder associated with detrimental TNFα activity, such as psoriasis, in a subject who has failed or lost response to prior biologic therapy, such as prior administration of etanercept. The invention further provides methods for determining the efficacy of a human TNFα antibody, or antigen-binding portion thereof, for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis.
Owner:ABBVIE BIOTECHNOLOGY LTD
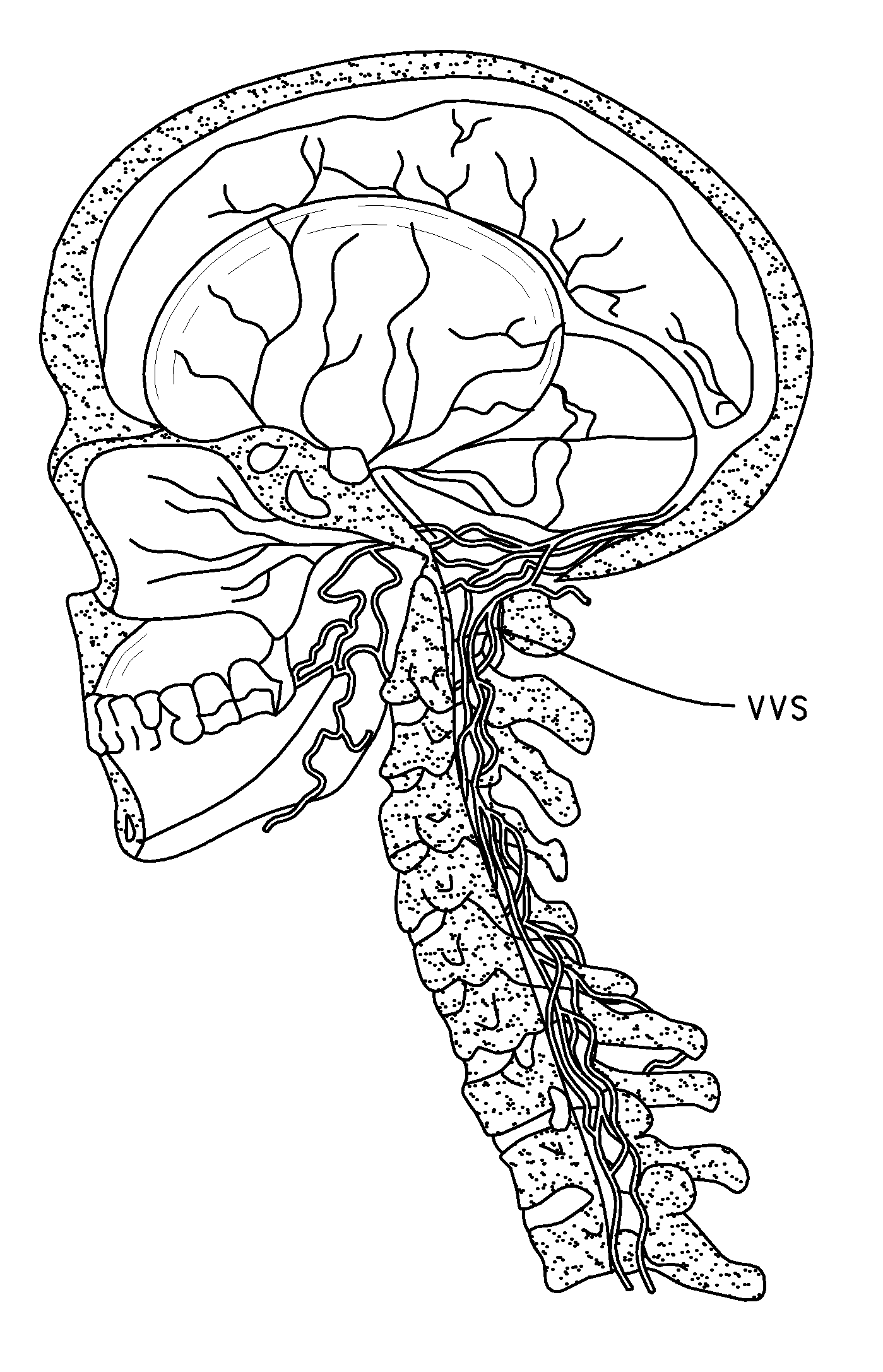
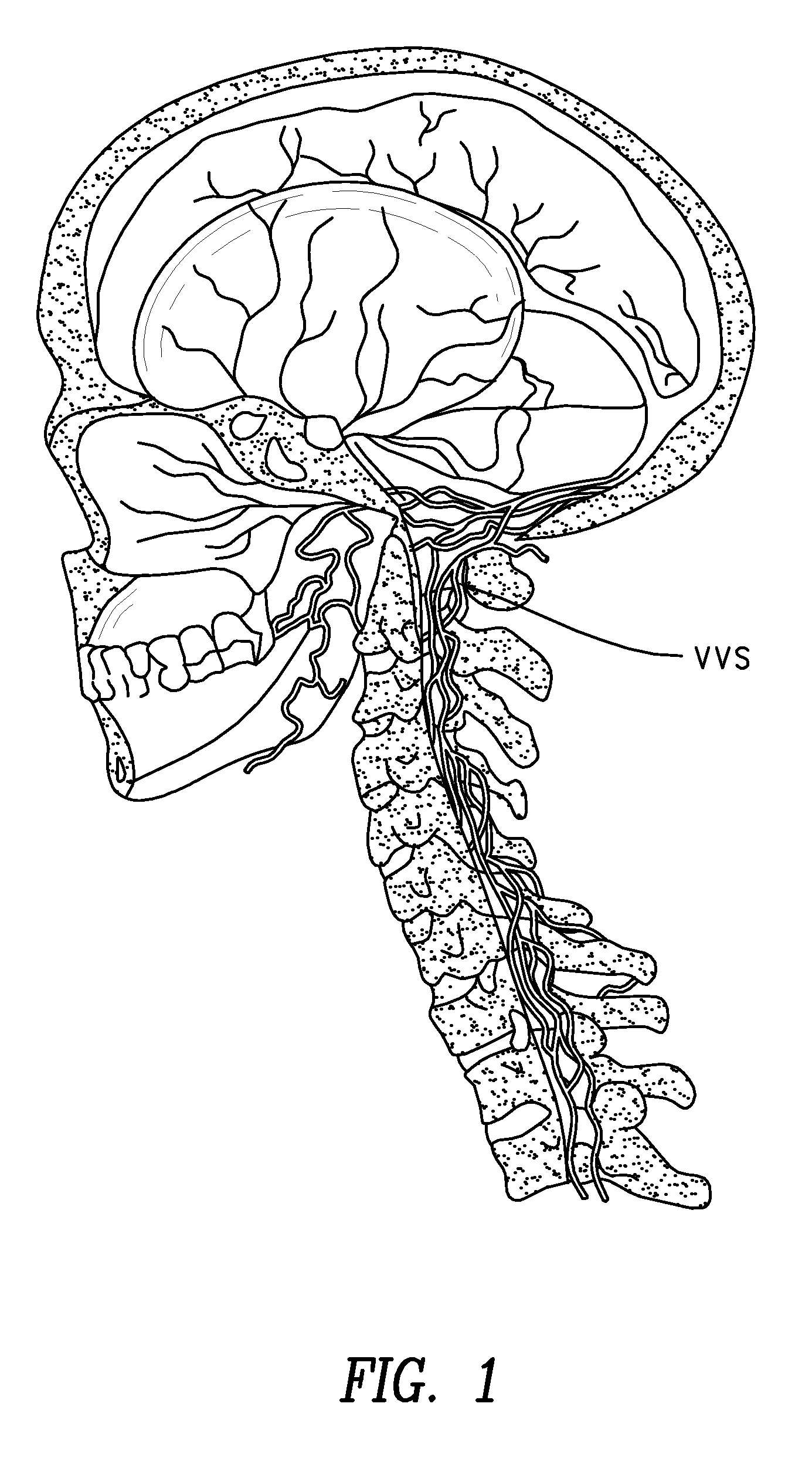
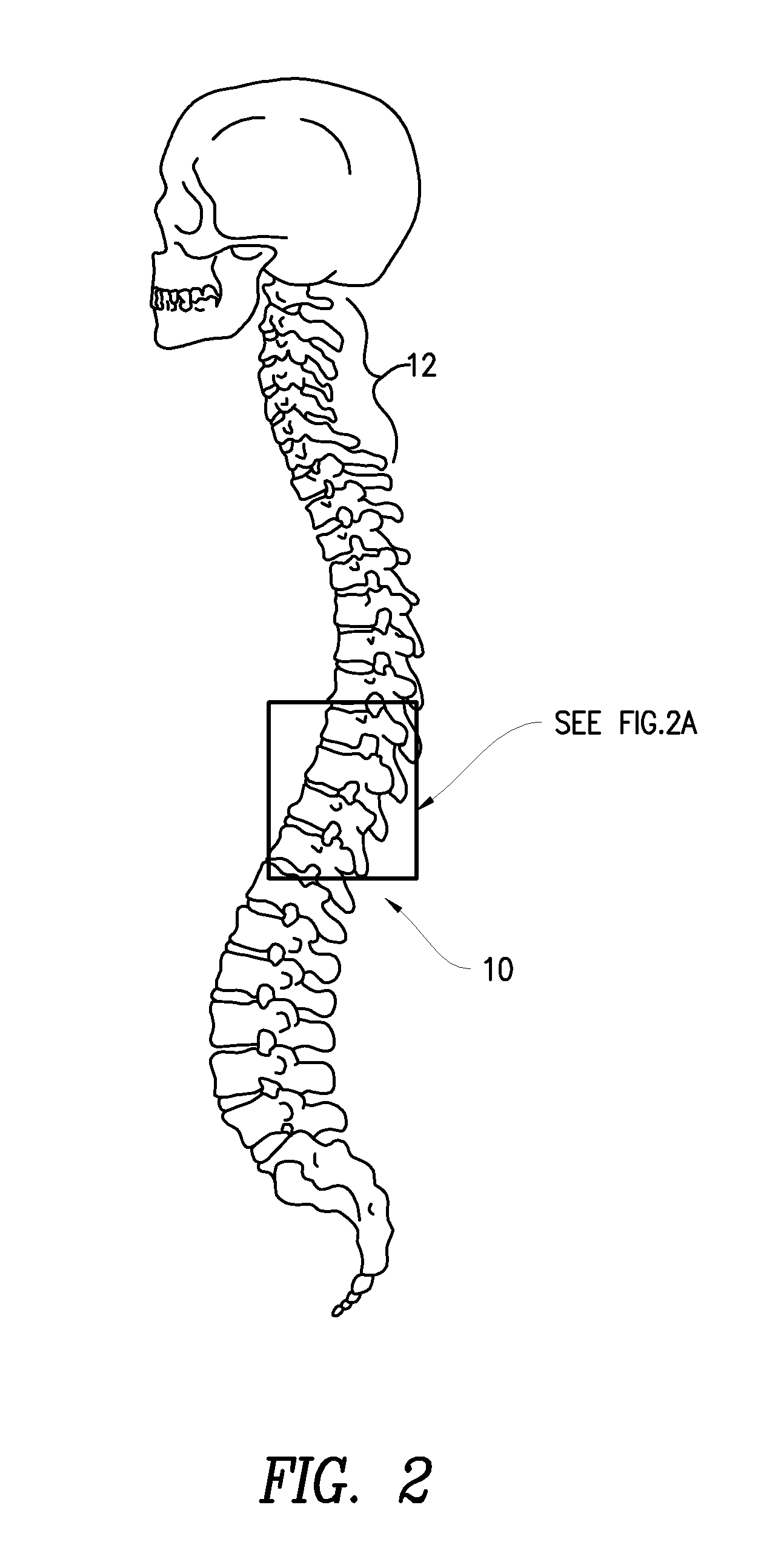
Method of delivering a TNF antagonist to the brain of a human by perispinal administration without direct intrathecal injection
InactiveUS7214658B2Improve cognitive functionReduce deliveryAnimal cellsOrganic active ingredientsEtanerceptTnf antagonists
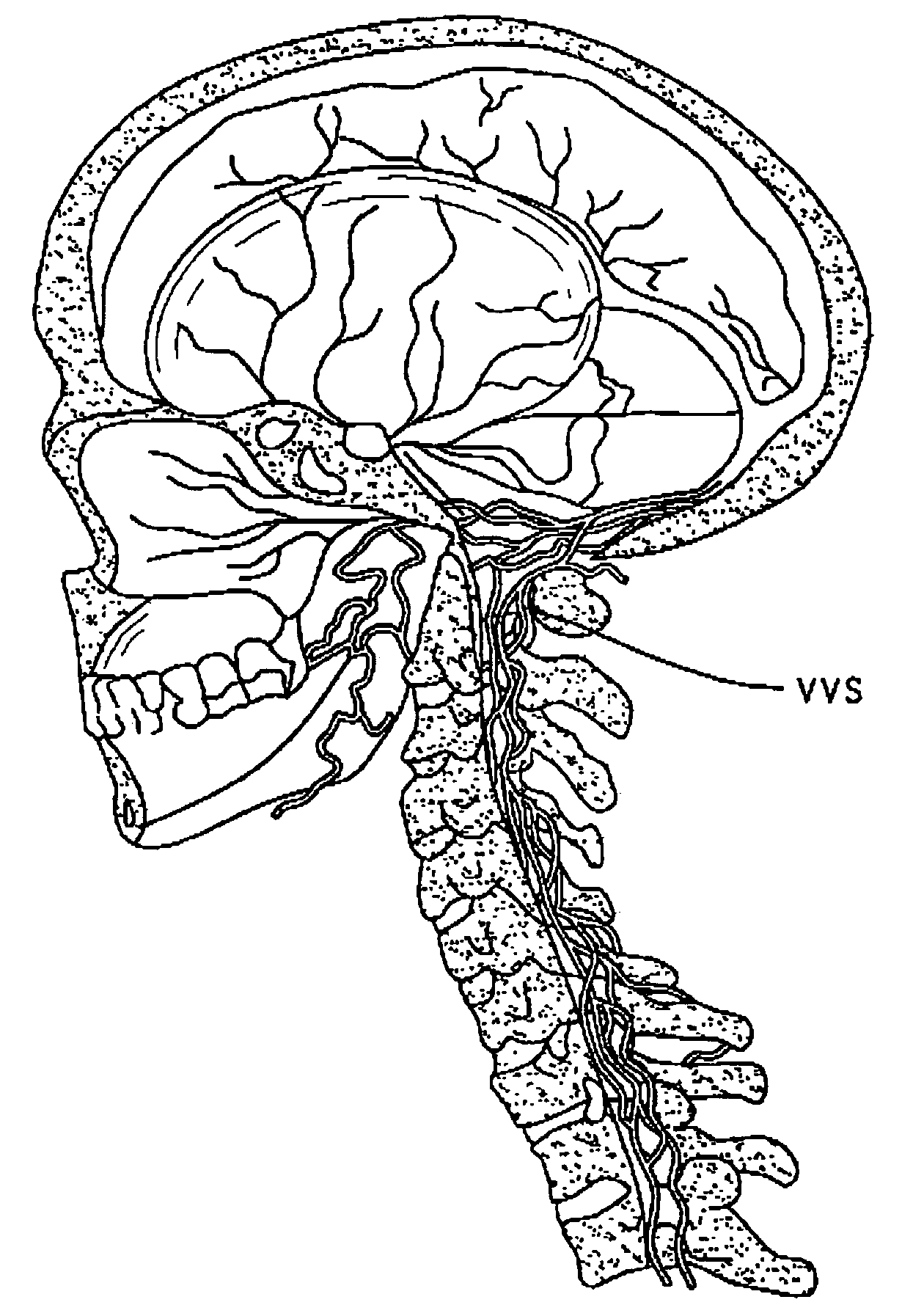
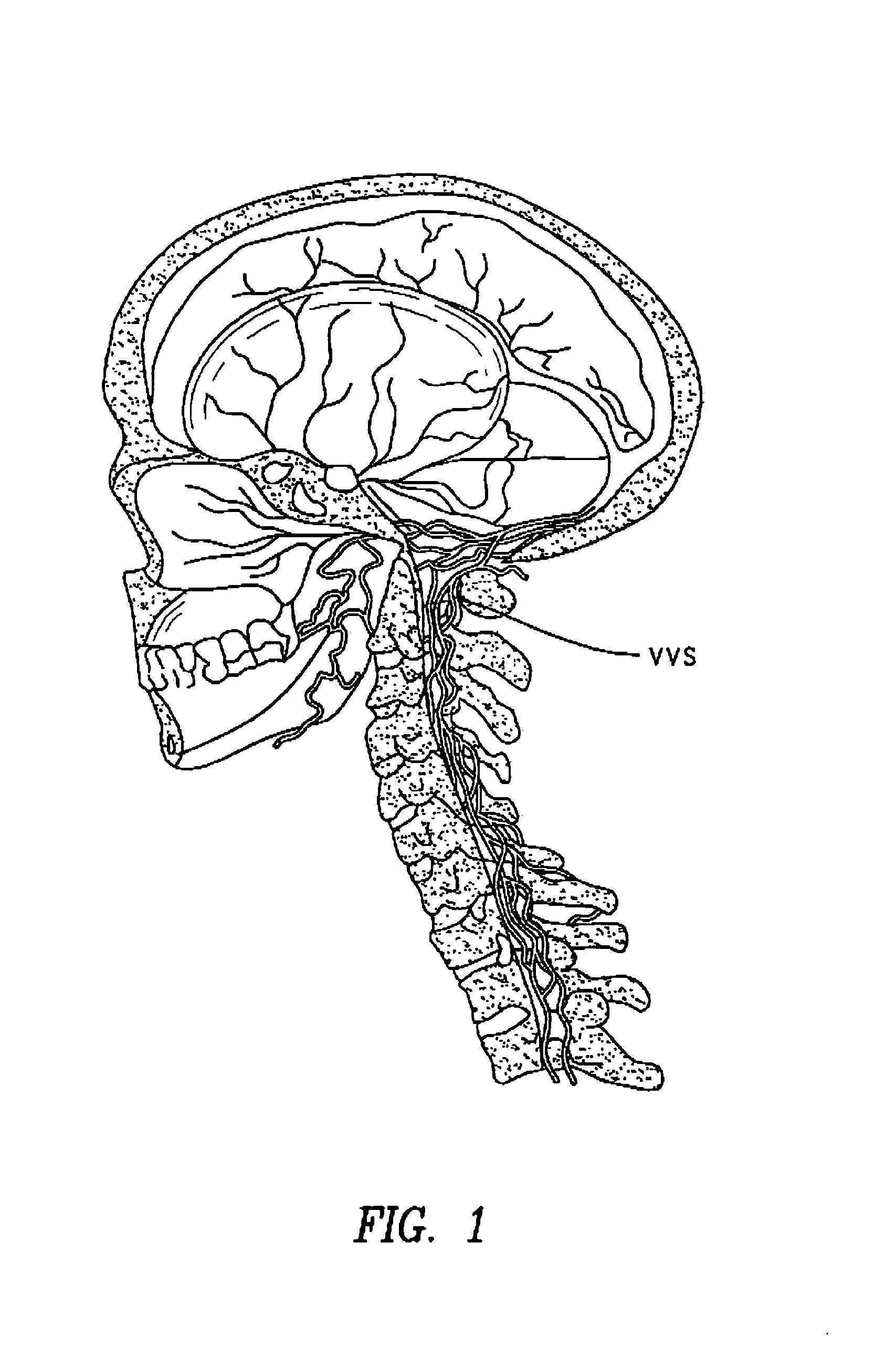
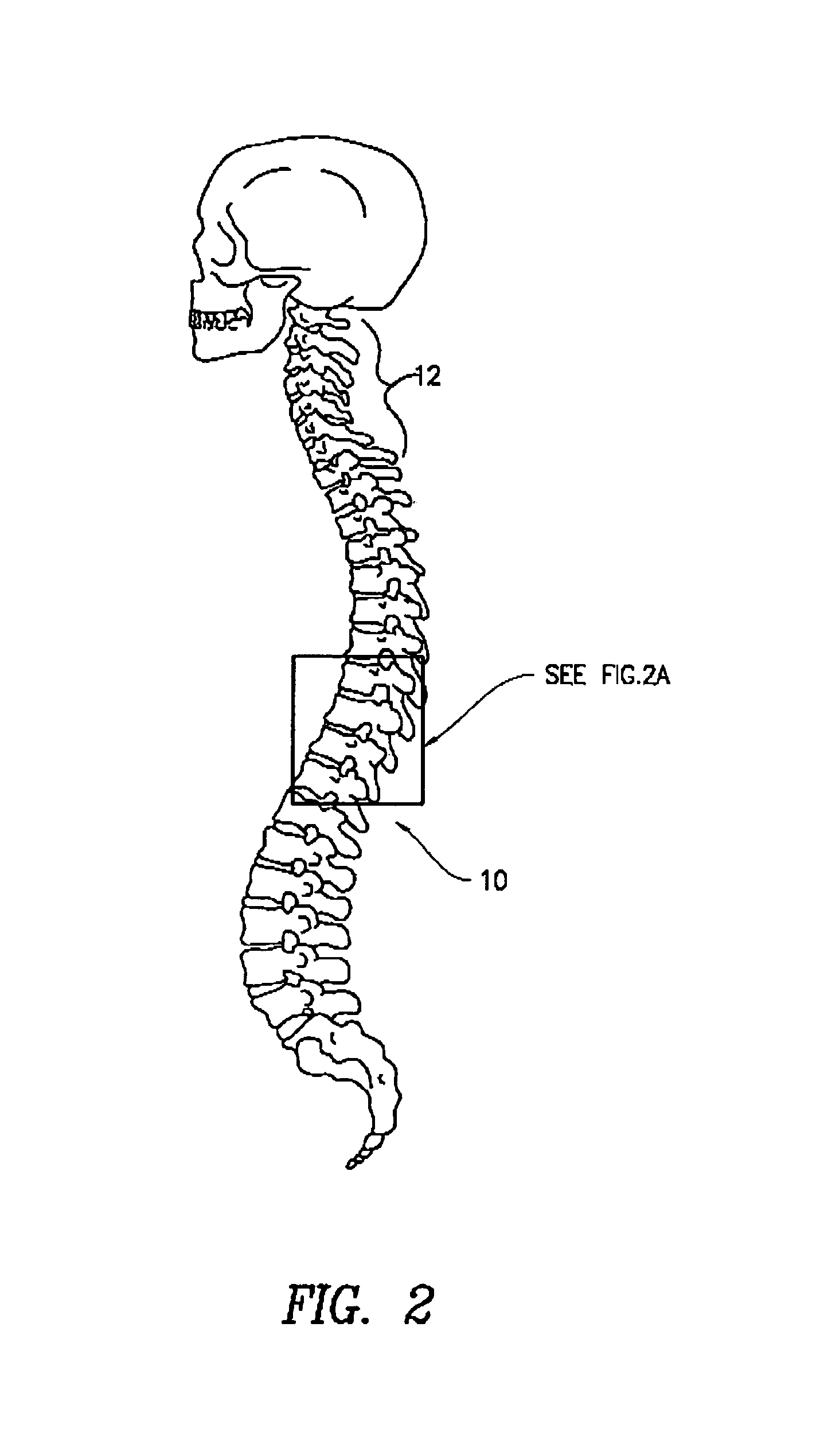
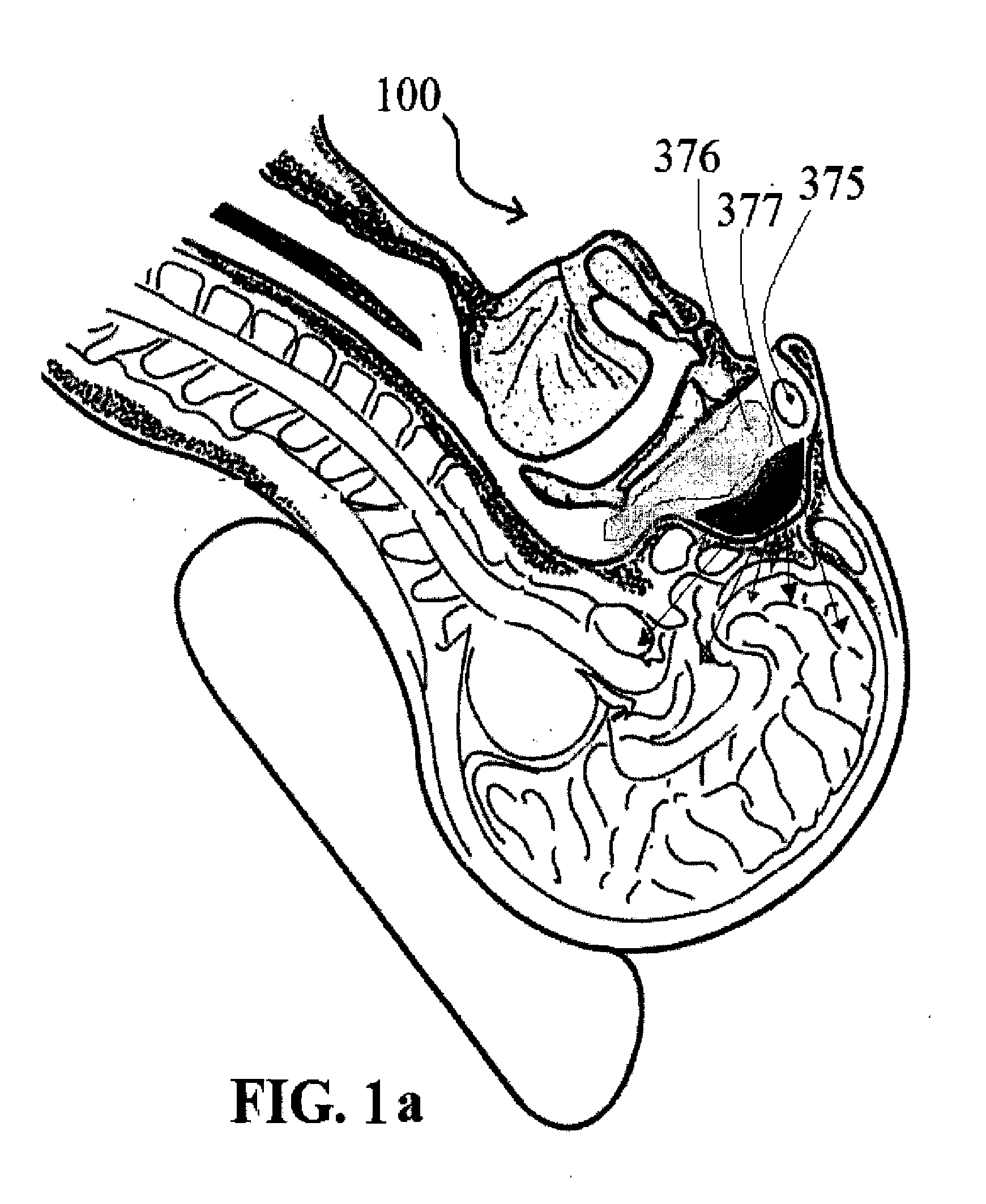
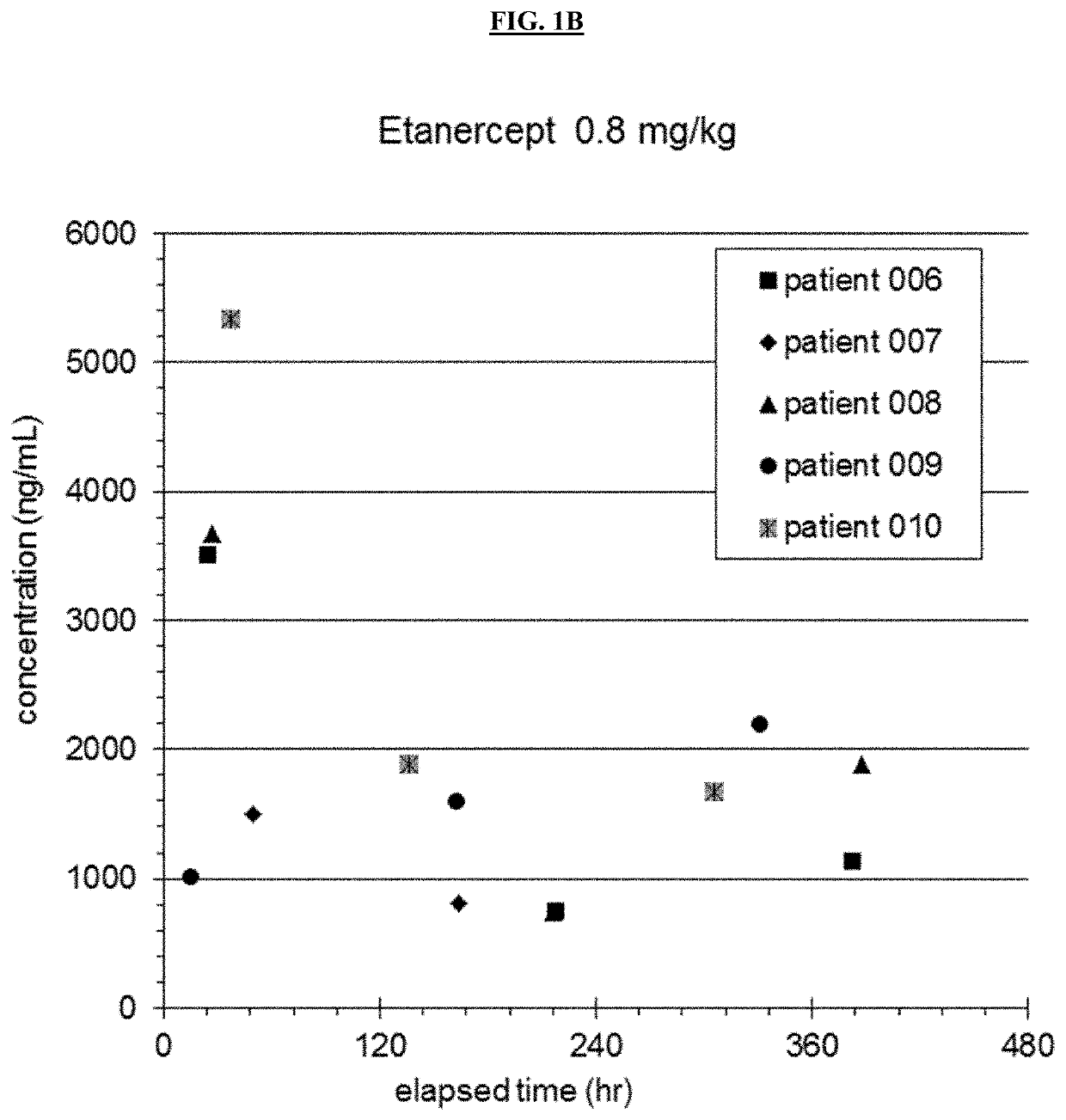
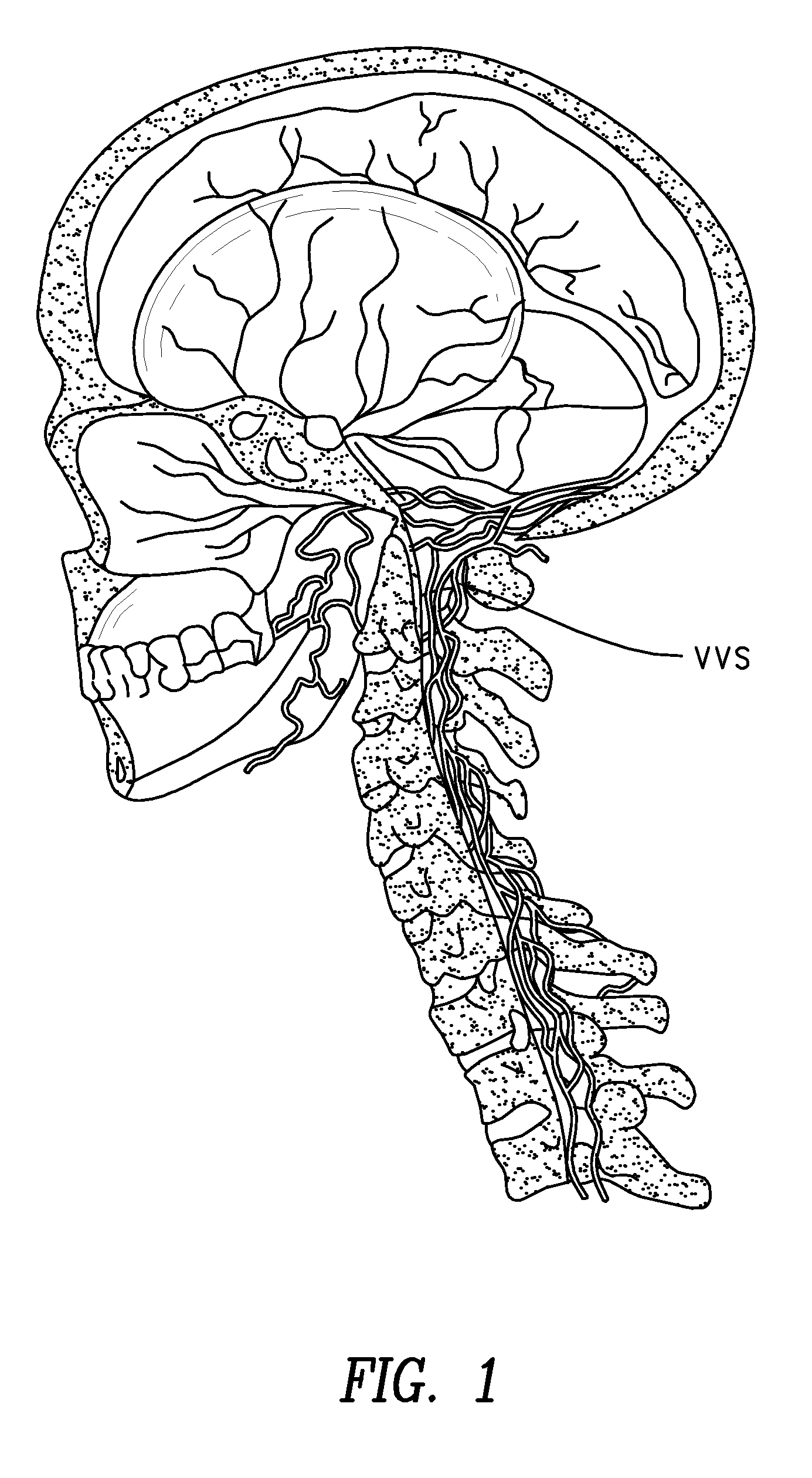
The present invention provides specific methods of using and administering etanercept to improve cognitive function in a human, for both the treatment and prevention of cognitive impairment, or, alternatively, to enhance cognitive function including Alzheimer's Disease, Idiopathic Dementia, and Traumatic Brain Injury. The methods of the present invention include the perispinal administration of etanercept. For the purposes of this patent “perispinal” is to be considered as referring to “perispinal extrathecal;” therefore direct intrathecal administration is excluded. Perispinal administration leads to enhanced delivery of etanercept to the brain in a therapeutically effective amount, via the vertebral venous system and / or the cerebrospinal fluid. Delivery of etanercept to the brain utilizing the methods of the present invention includes the use of the vertebral venous system to deliver etanercept to the brain via retrograde venous flow. Physical maneuvers are used to enhance delivery of etanercept to the brain via this route.
Owner:TACT IP
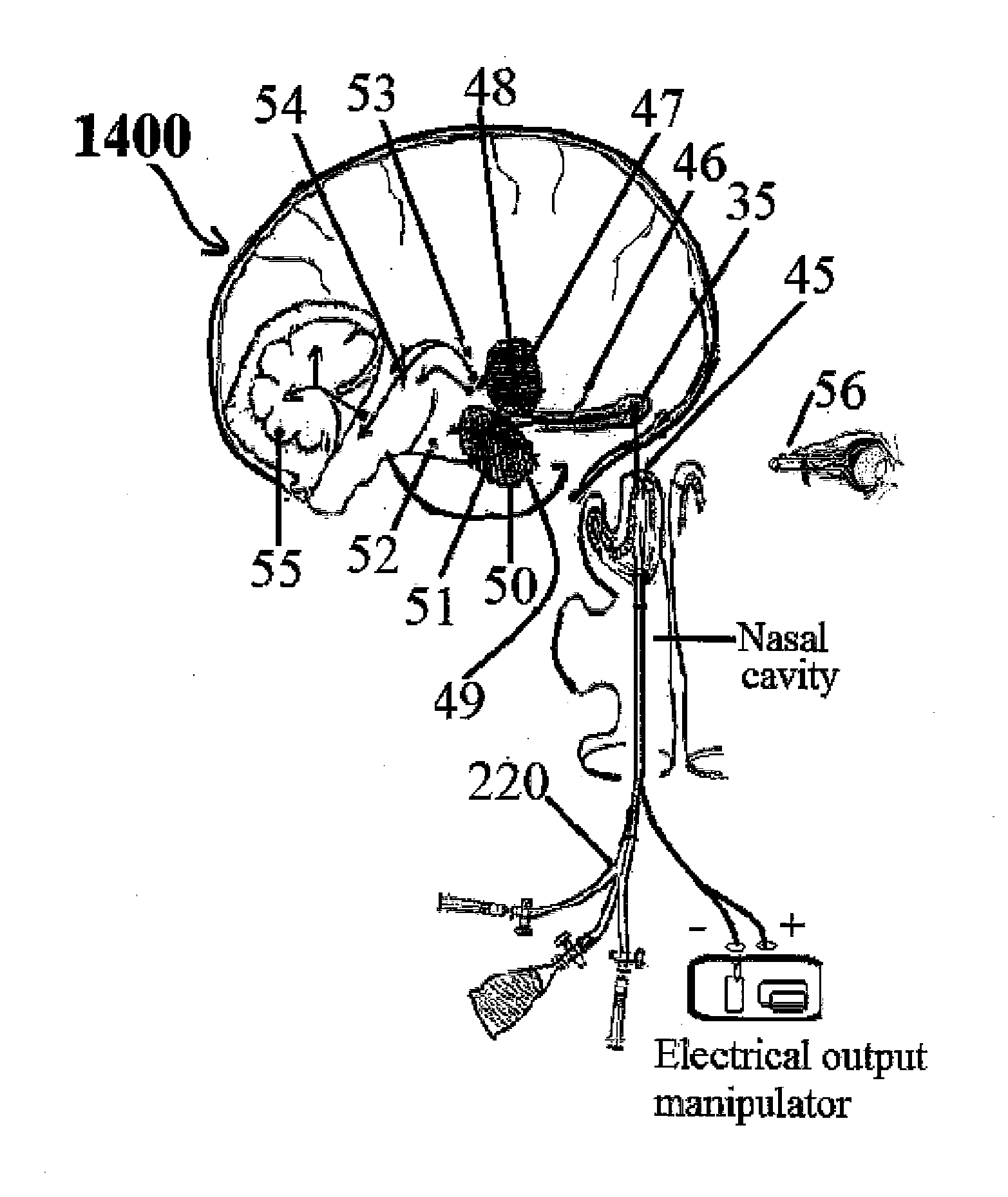
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
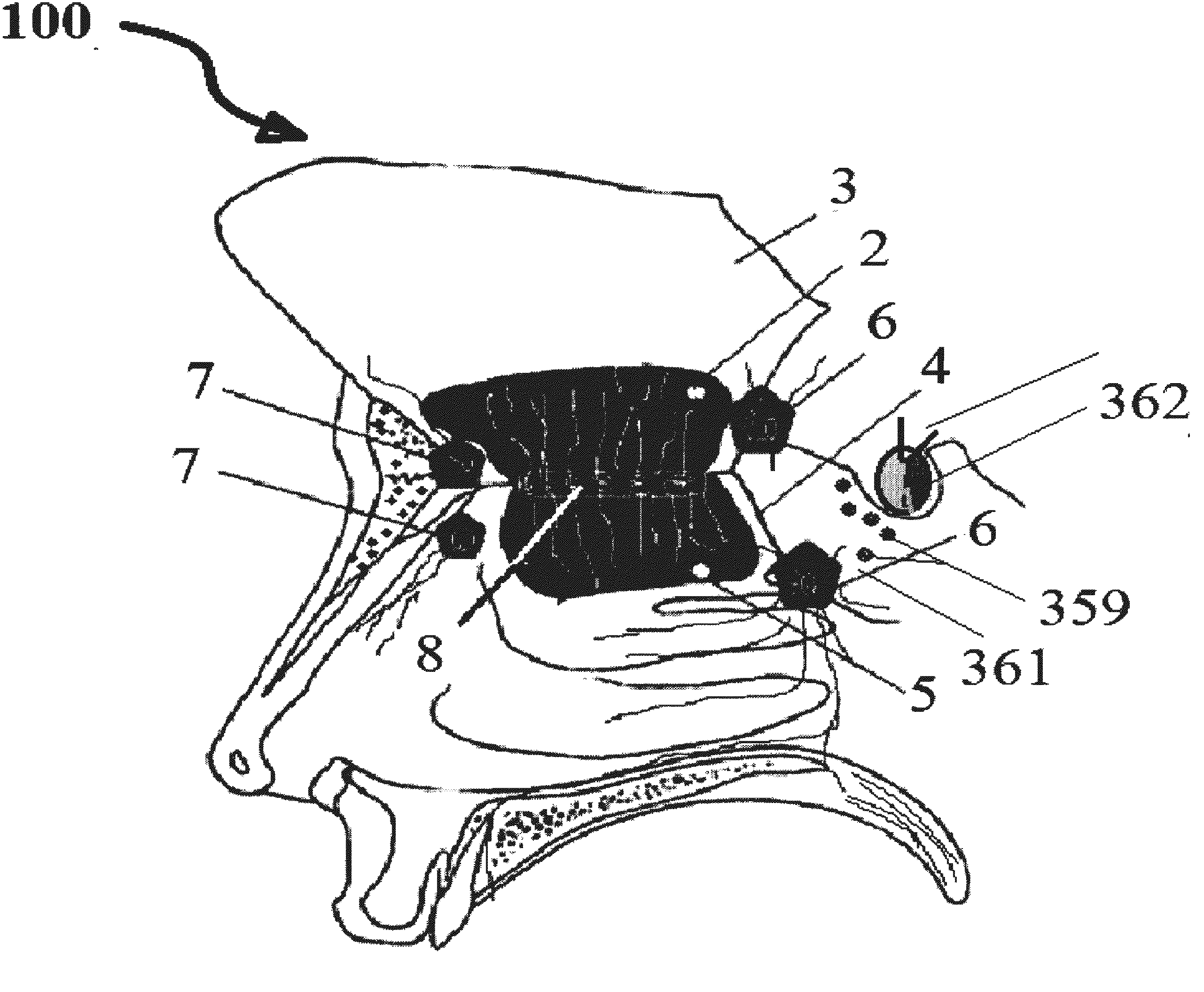
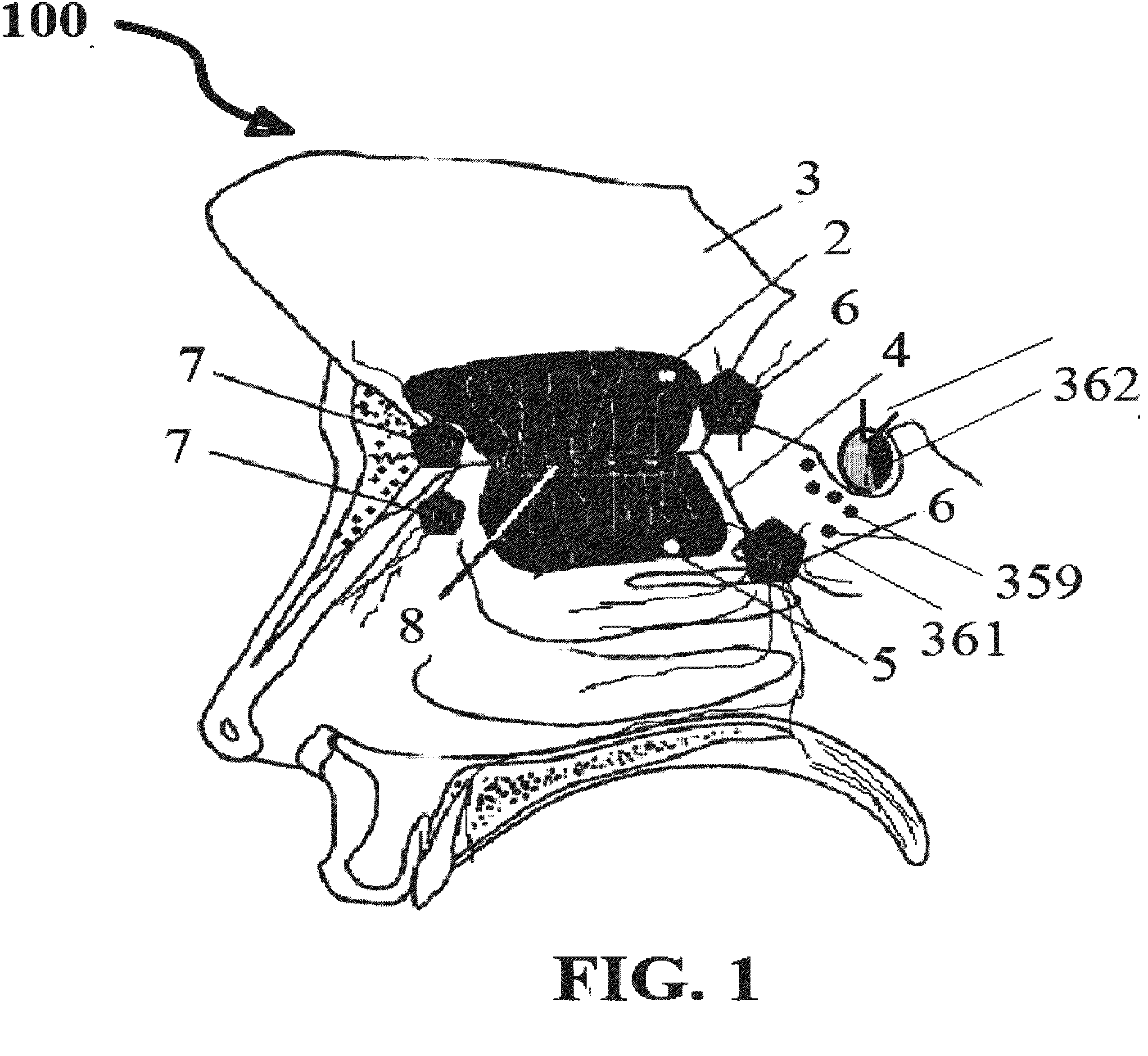
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
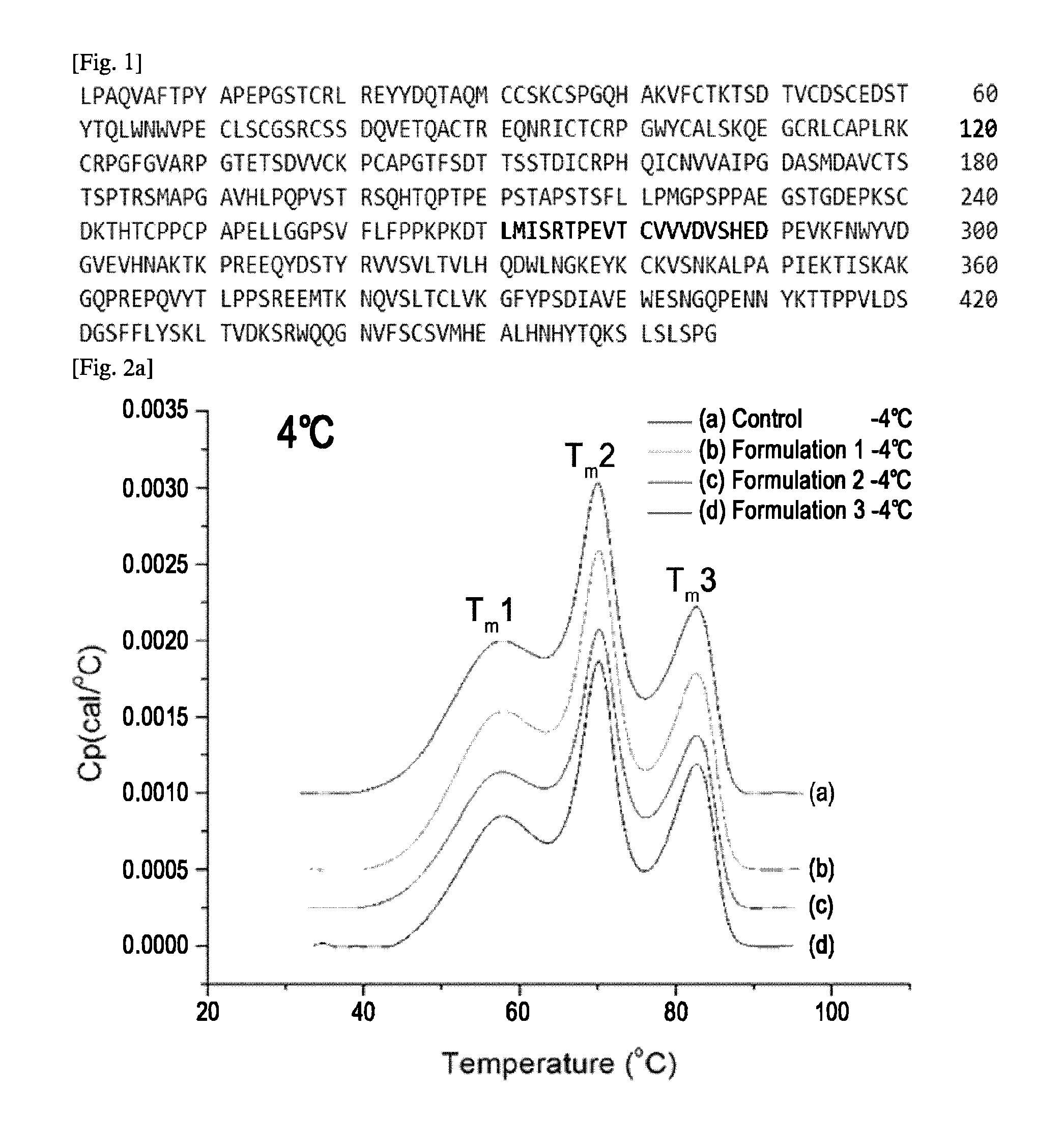
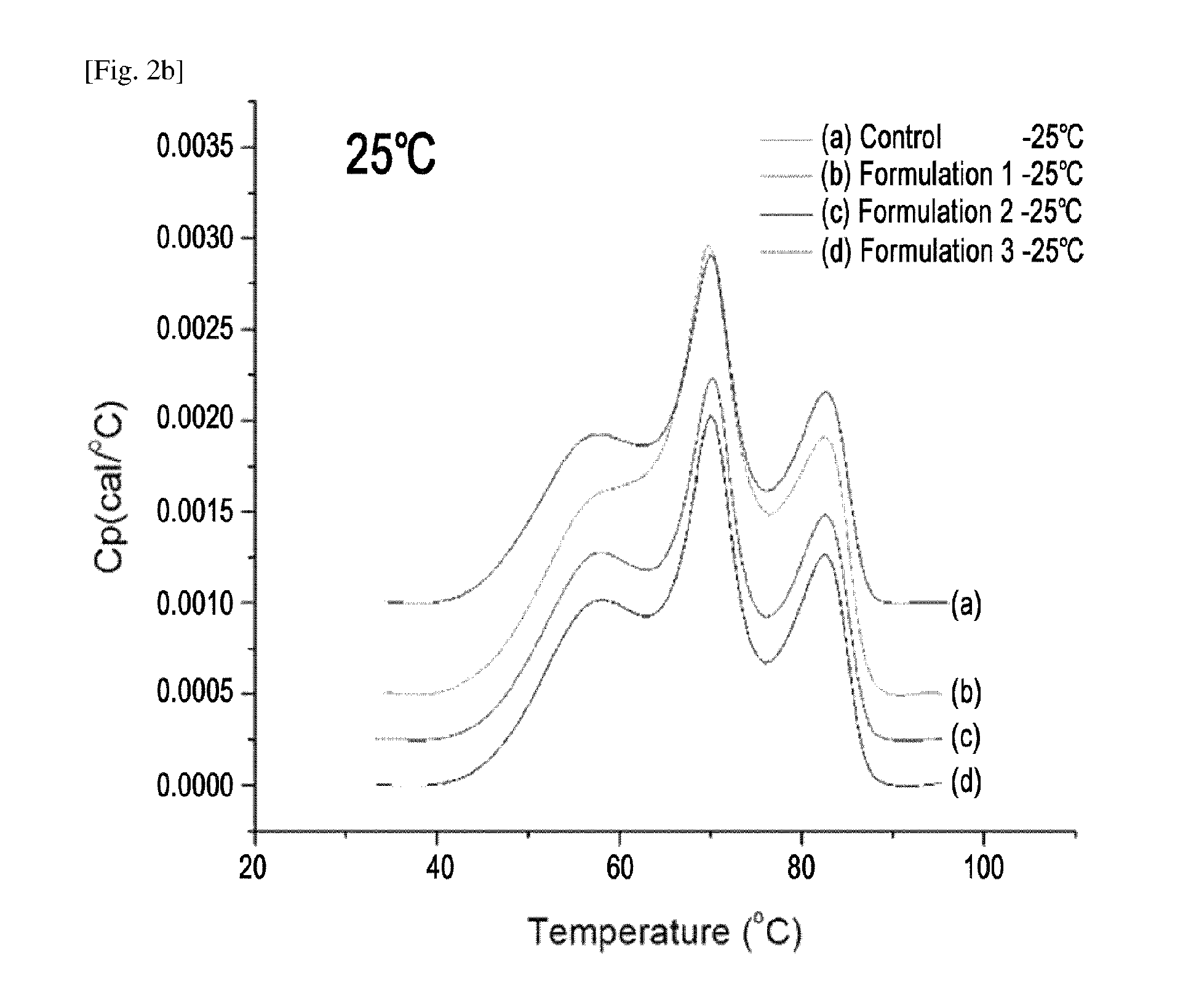
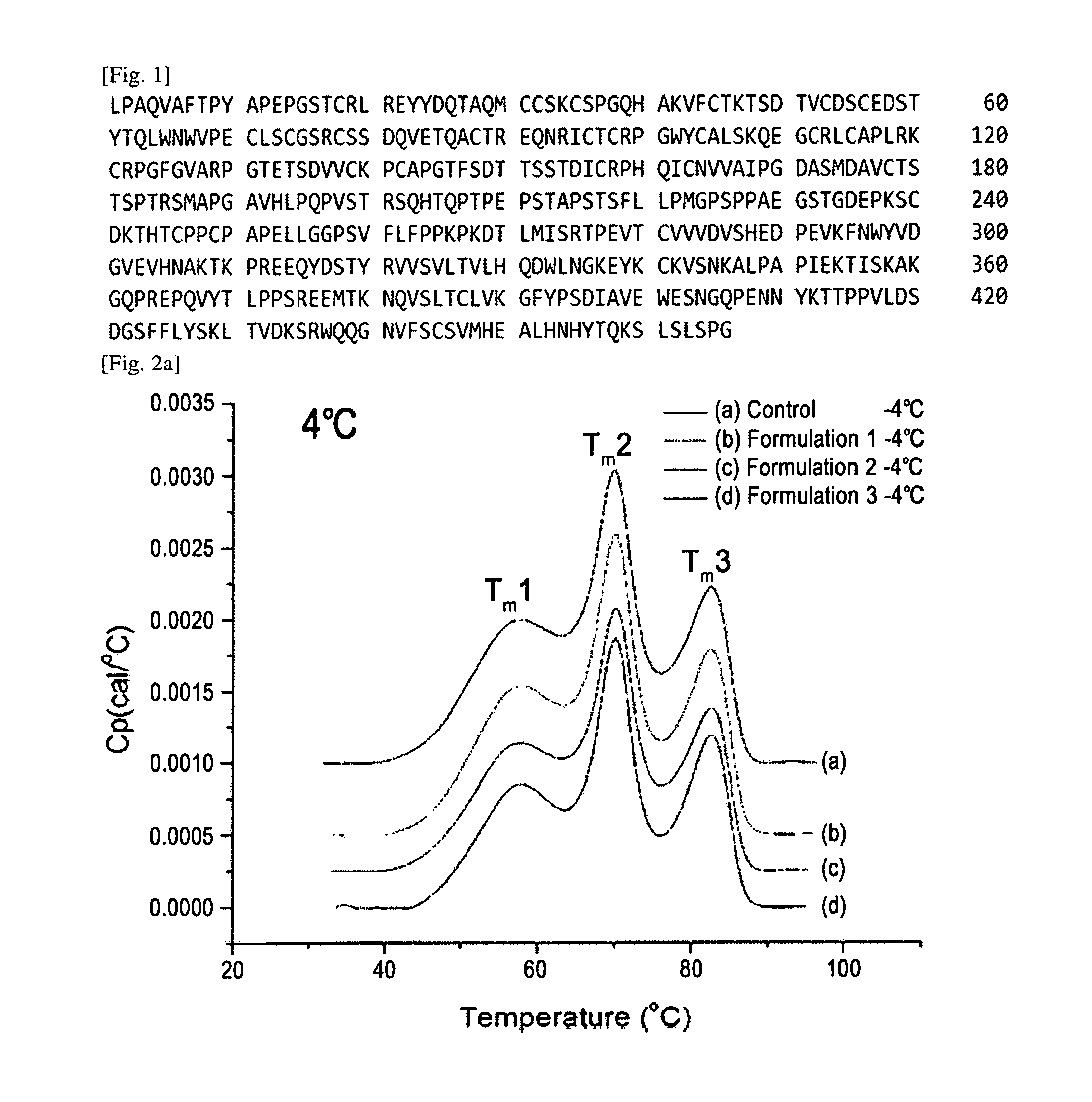
Crystals of etanercept and methods of making thereof
The present invention relates to crystalline etanercept and to methods of making crystalline etanercept; to pharmaceutical compositions comprising crystalline etanercept; and to therapeutic uses of such compositions.
Owner:AMGEN INC
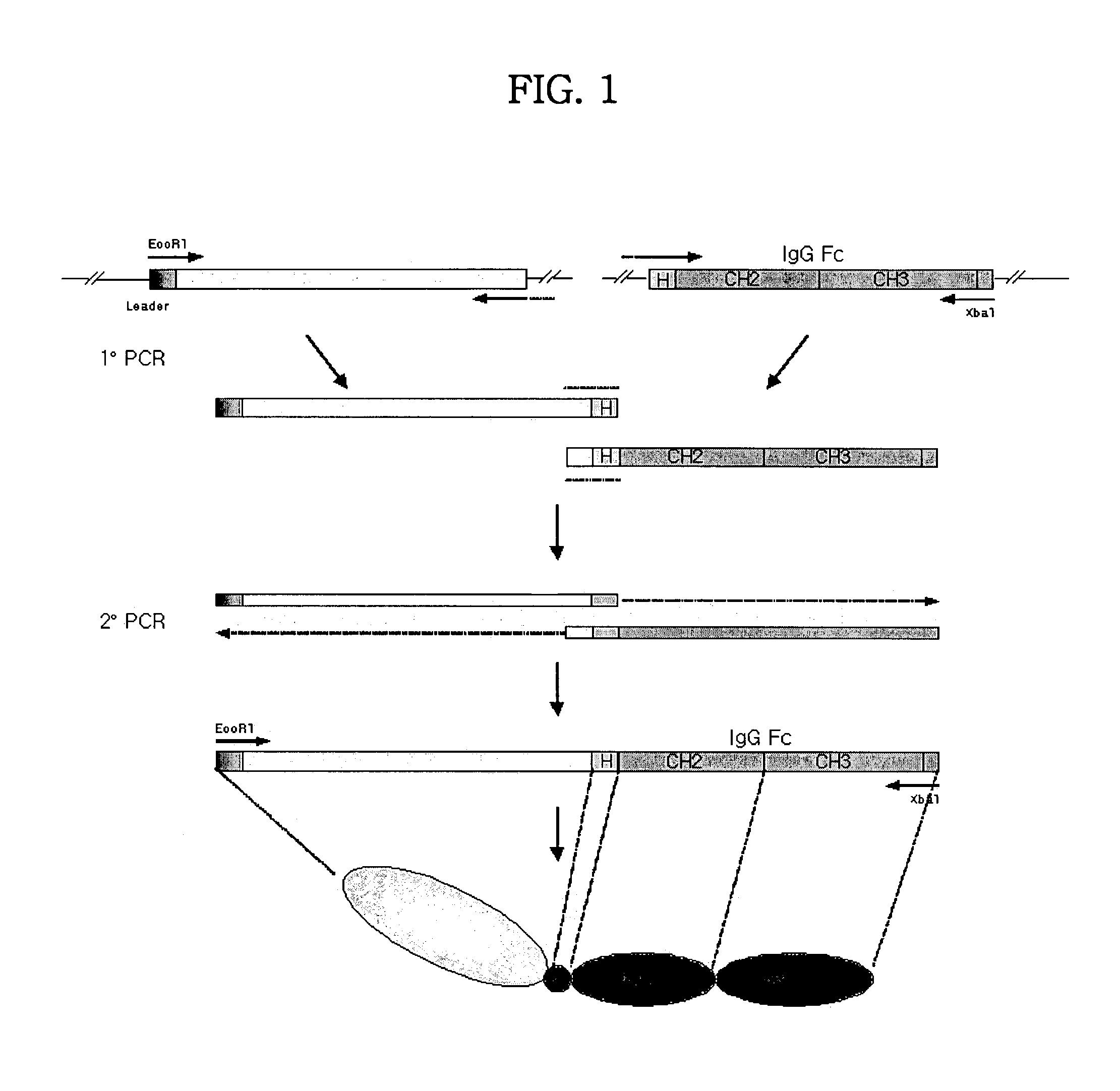
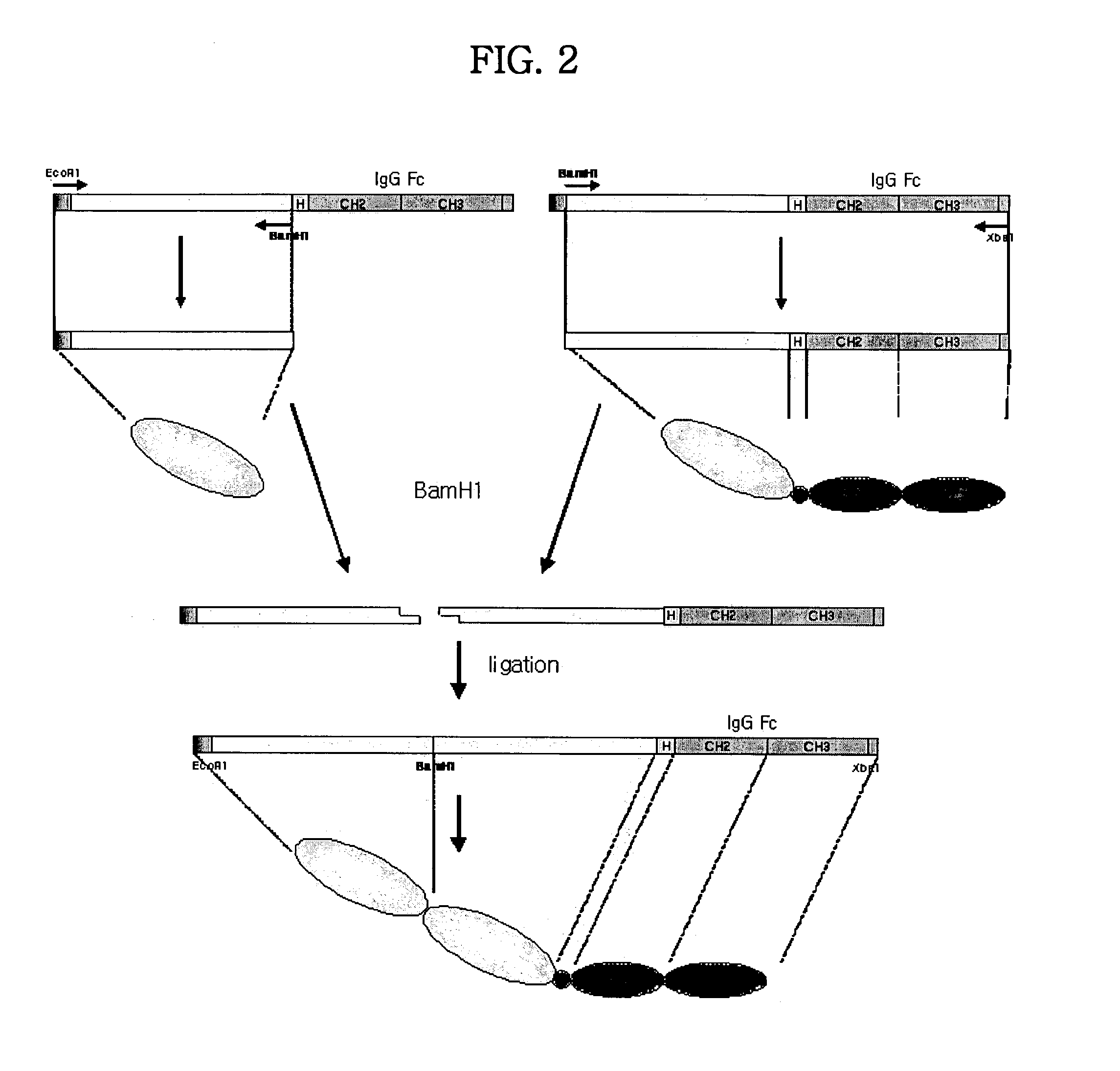
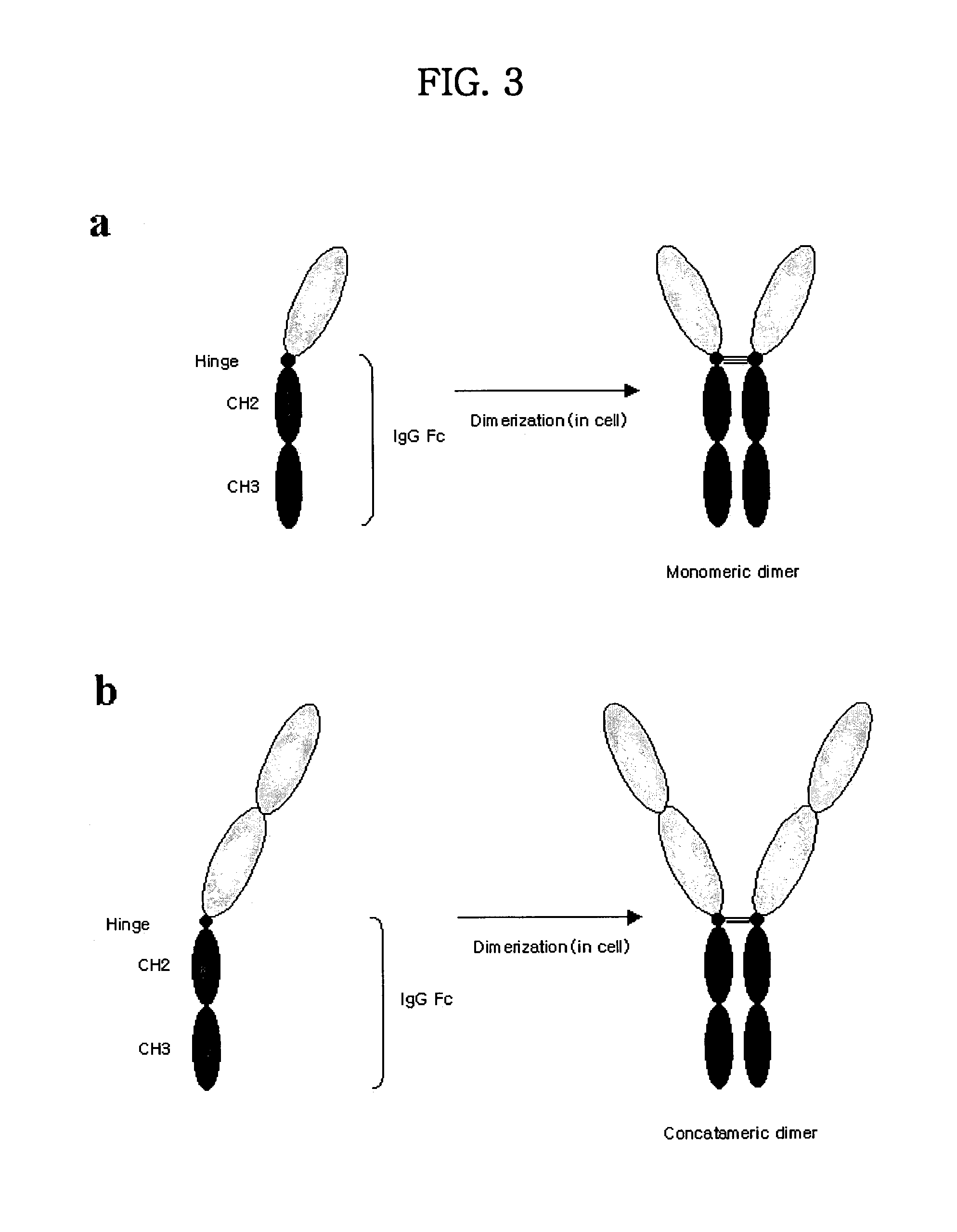
Tetravalent etanercept
ActiveUS7229962B2Improve efficacyImprove stabilityAntibody mimetics/scaffoldsAntipyreticDNA constructEtanercept
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
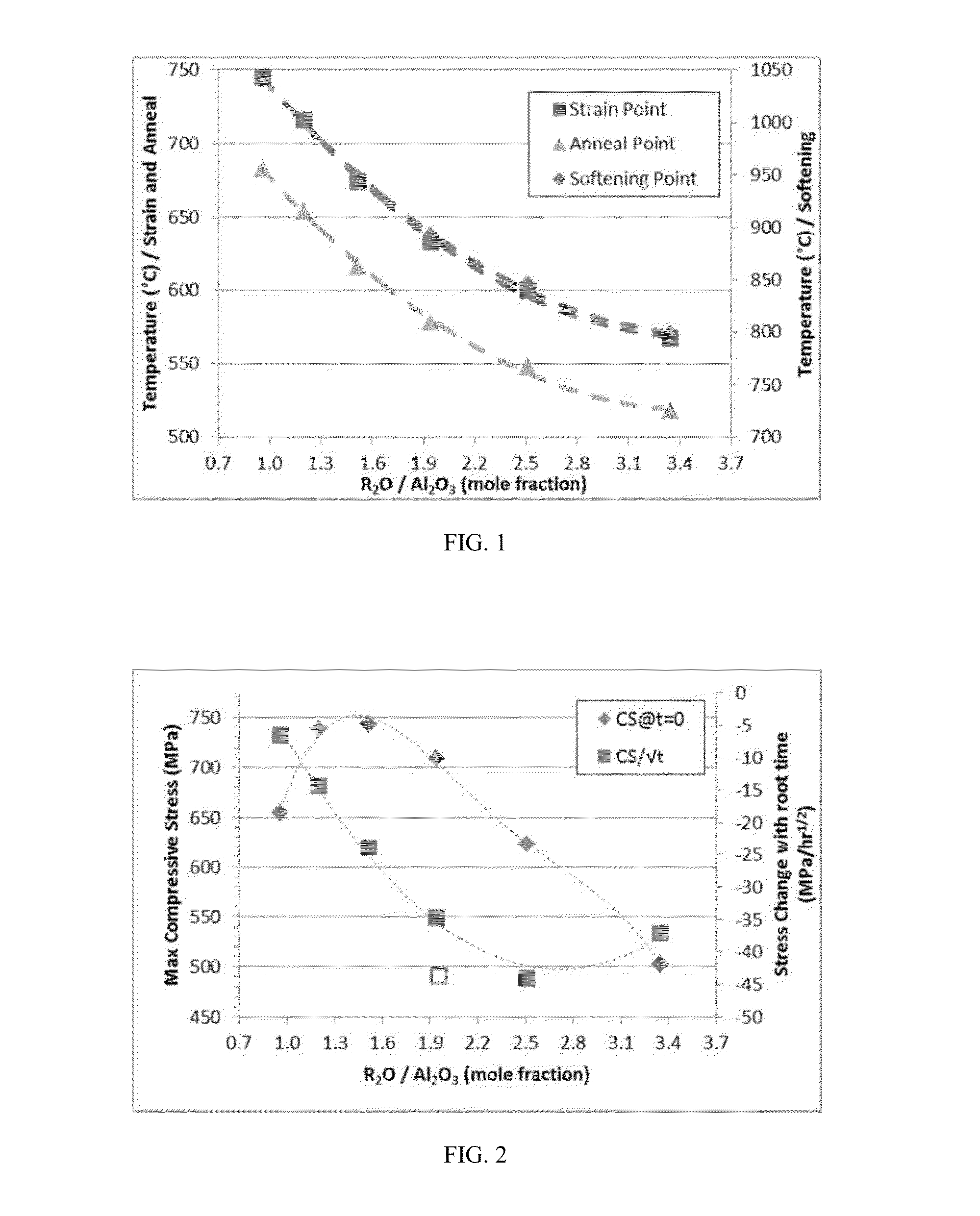
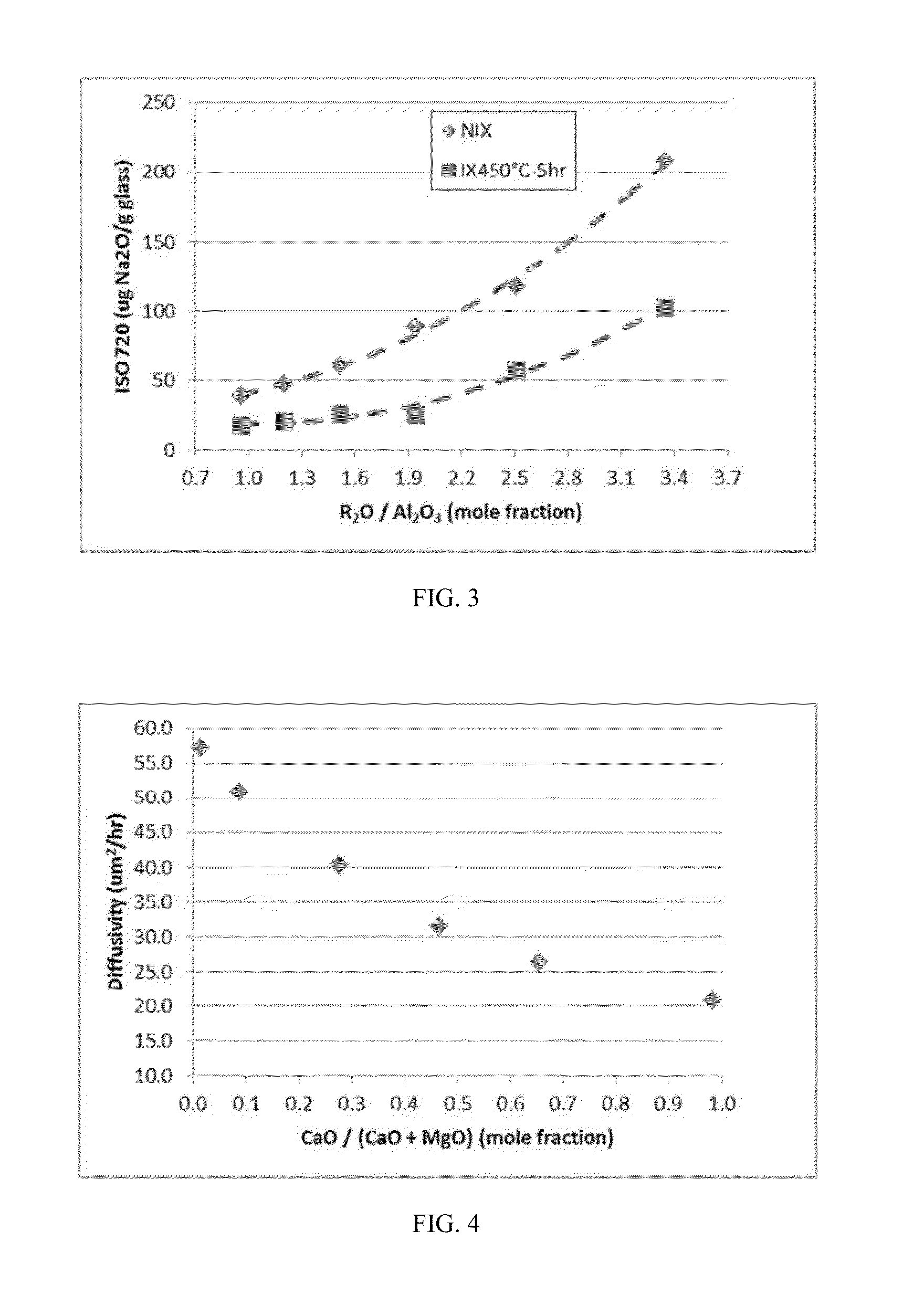
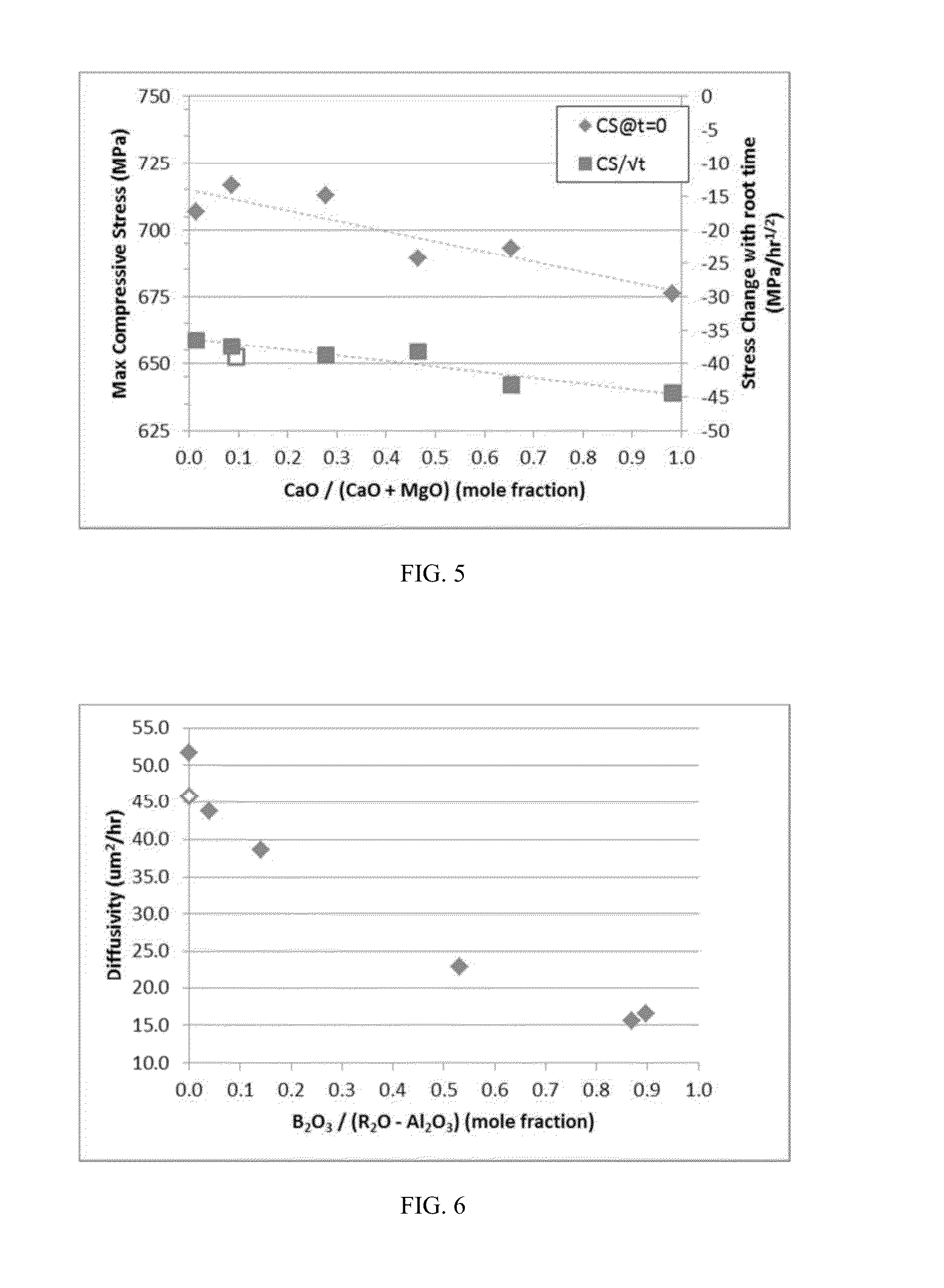
Delamination resistant pharmaceutical glass containers containing active pharmaceutical ingredients
ActiveUS20130196094A1Organic active ingredientsPeptide/protein ingredientsParticulatesBULK ACTIVE INGREDIENT
The present invention is based, at least in part, on the identification of a pharmaceutical container formed, at least in part, of a glass composition which exhibits a reduced propensity to delaminate, i.e., a reduced propensity to shed glass particulates. As a result, the presently claimed containers are particularly suited for storage of pharmaceutical compositions and, specifically, a pharmaceutical solution comprising a pharmaceutically active ingredient, for example, NEUPOGEN (filgrastim), NEULASTA (pegfilgrastim), EPOGEN (epoetin alfa) or ENBREL (etanercept).
Owner:CORNING INC
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20140012182A1Large deliveryAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
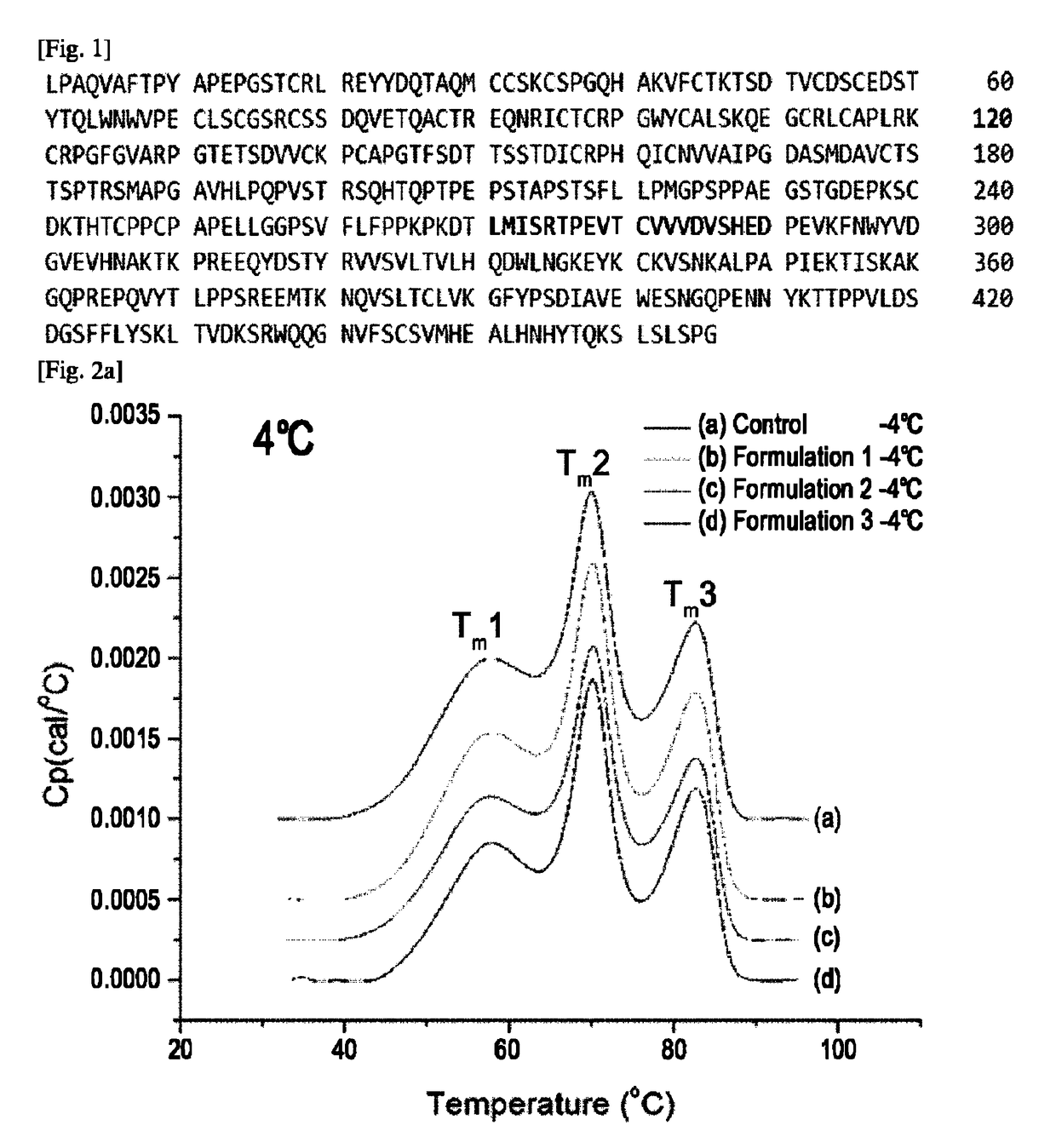
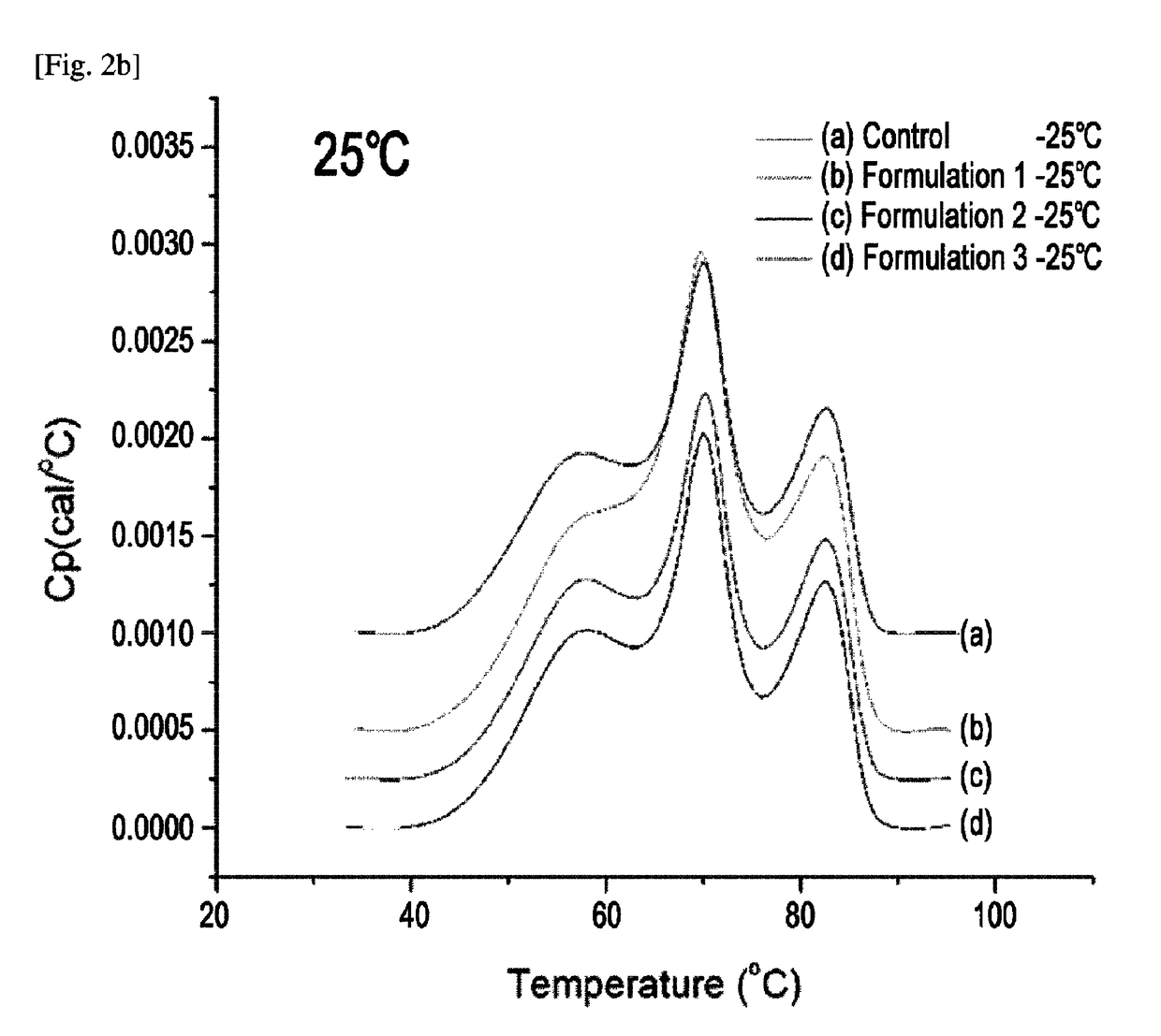
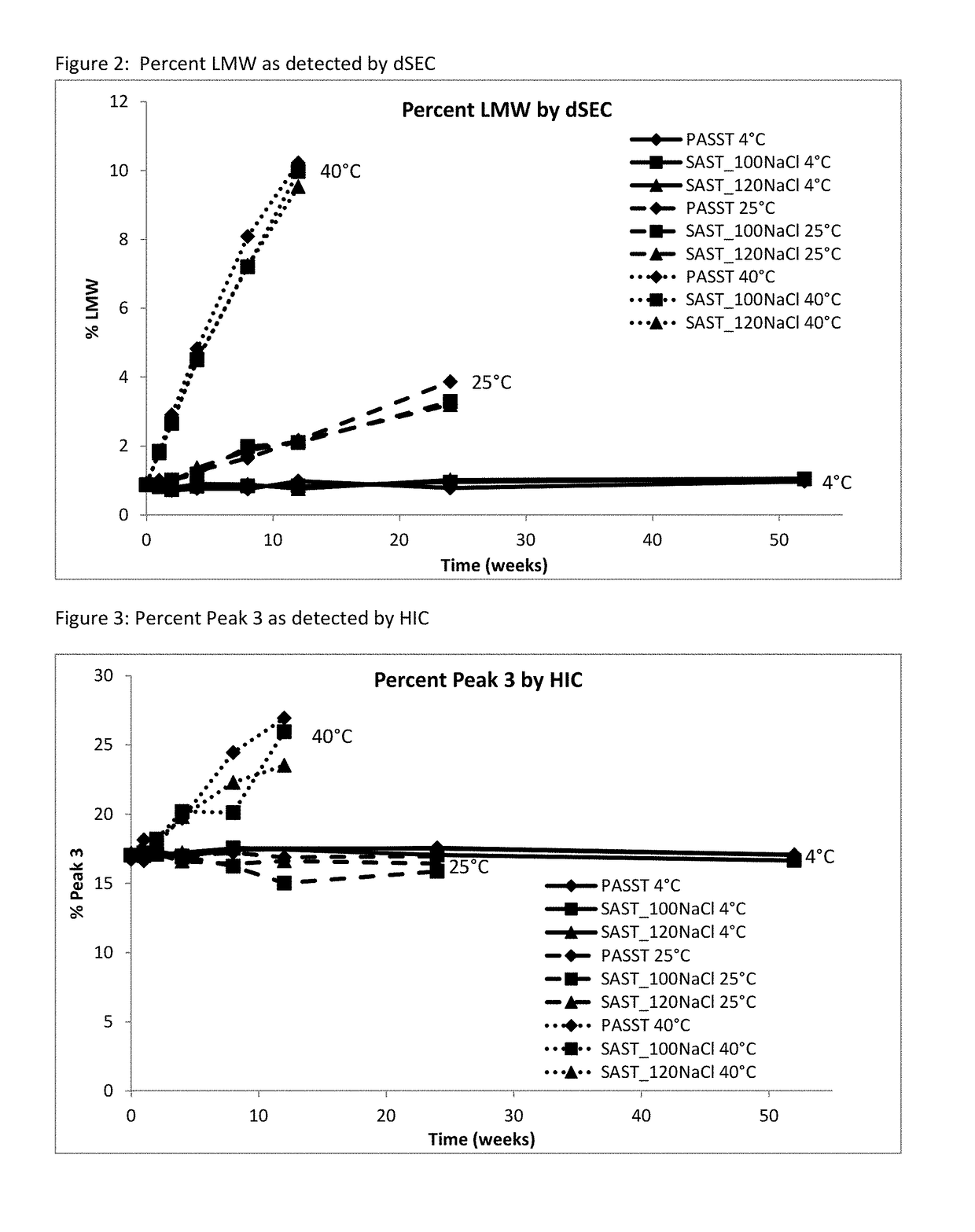
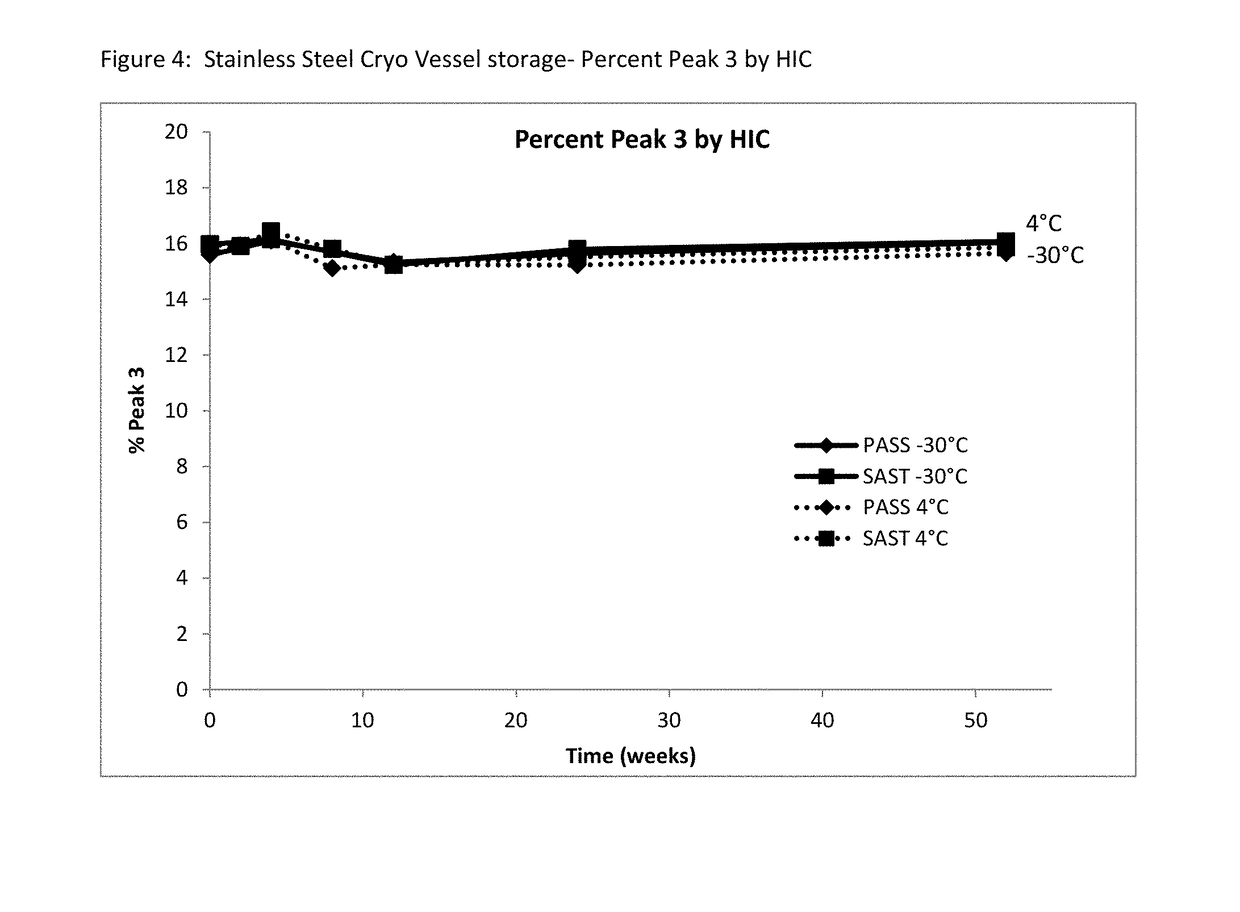
Etanercept Formulations Stabilized with Xylitol
ActiveUS20130101584A1Low costReduce of fragmentPowder deliveryNervous disorderEtanerceptPharmaceutical Substances
The invention provides stabilized aqueous pharmaceutical etanercept compositions suitable for long-term storage of etanercept, methods of manufacture of these compositions, methods of administration, and kits containing same.
Owner:COHERUS BIOSCI
Etanercept Formulations Stabilized with Meglumine
ActiveUS20130108634A1Elicit long term storage stabilityAcceptable levelPeptide/protein ingredientsAntibody mimetics/scaffoldsMedicineMeglumine
The invention provides stabilized aqueous pharmaceutical etanercept compositions suitable for long-term storage of etanercept, methods of manufacture of these compositions, methods of administration, and kits containing same.
Owner:COHERUS BIOSCI
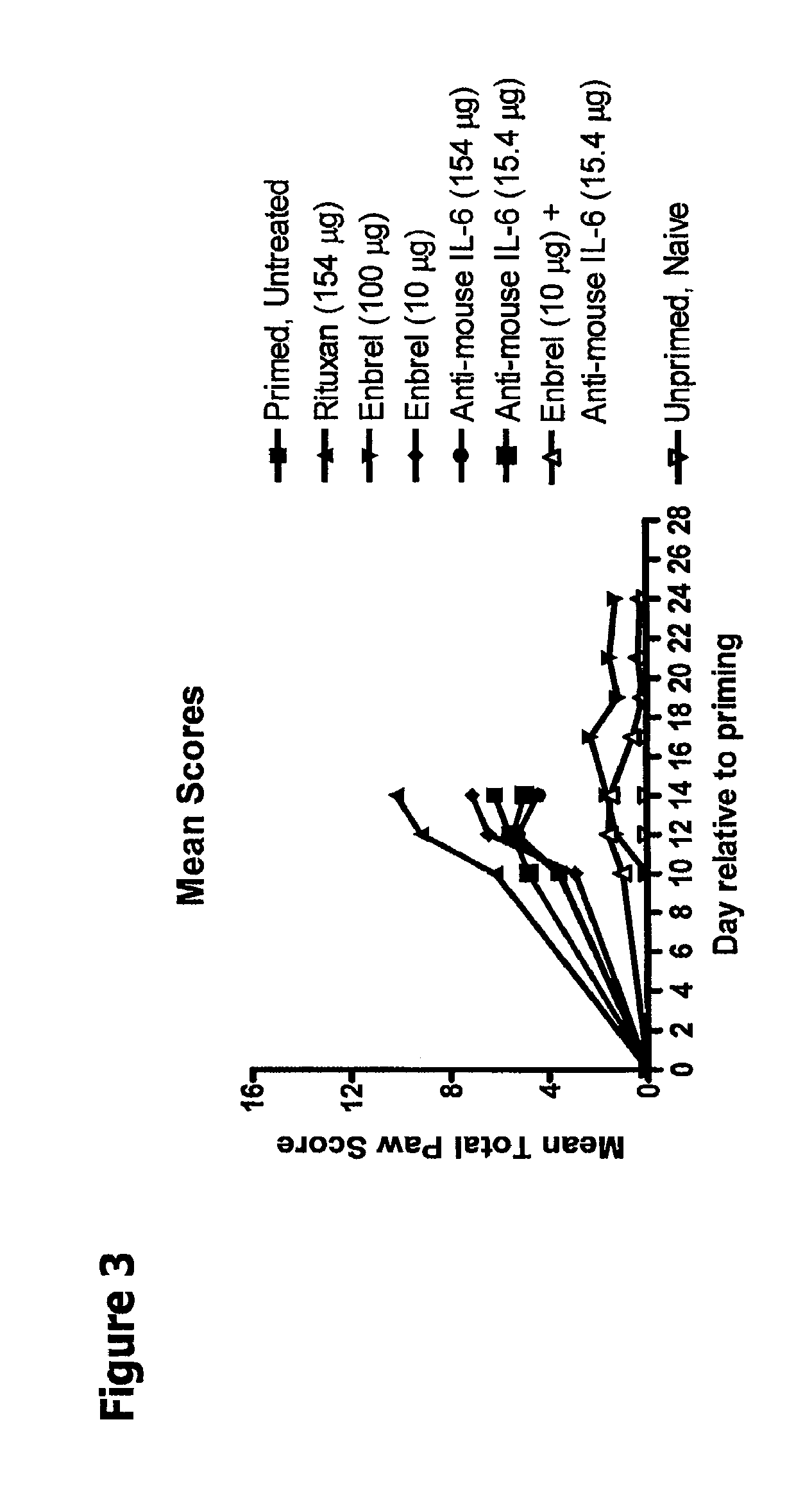
Compositions Comprising TNF-alpha and IL-6 Antagonists and Methods of Use Thereof
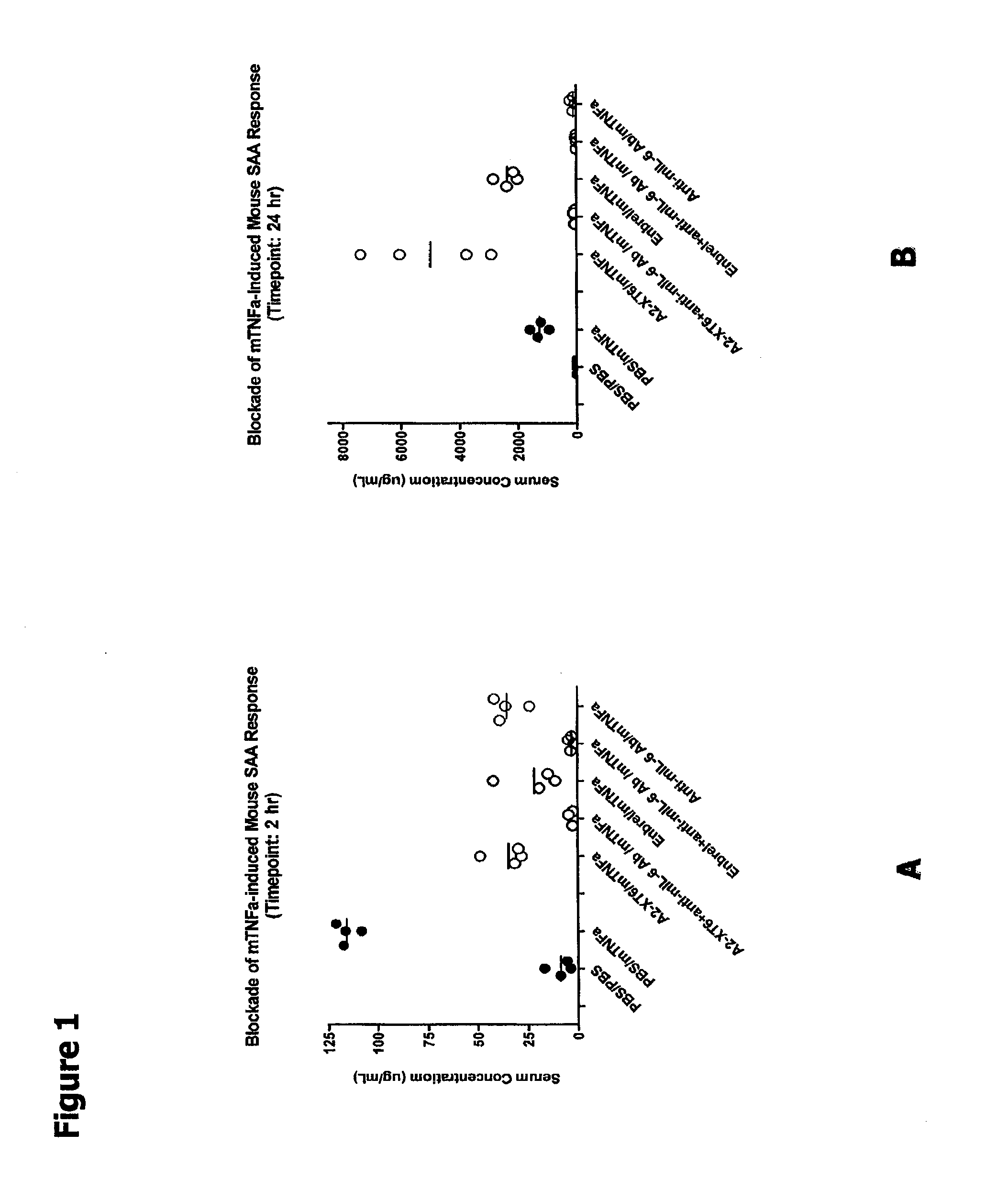
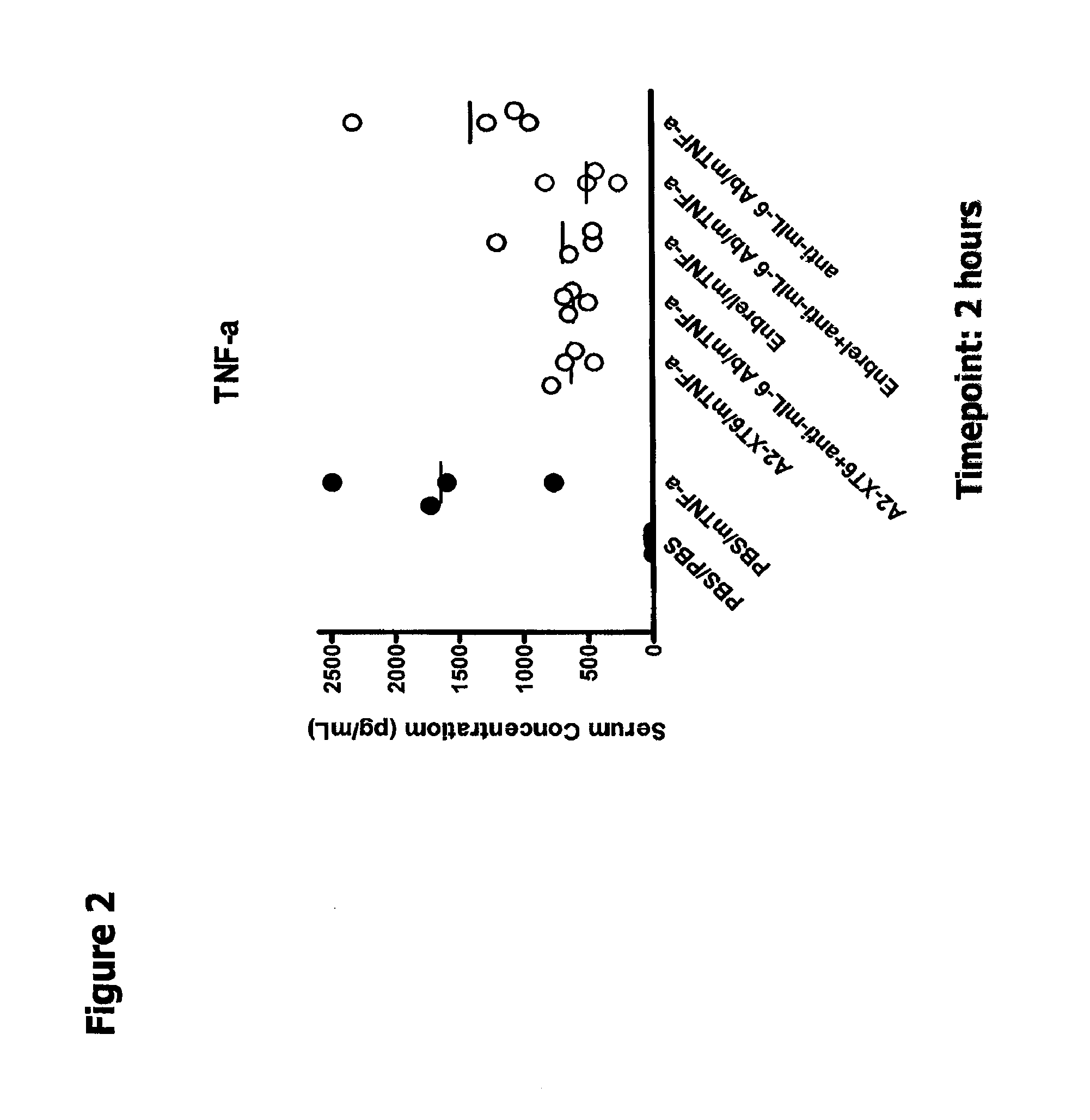
The present disclosure relates to compositions and methods for treating autoimmune diseases including rheumatoid arthritis. In particular, the present disclosure relates to compositions comprising IL-6 antagonists (e.g., anti-IL6 or anti-IL6R or anti-hyperIL6) and TNF-α antagonists (e.g., (anti-TNF or etanercept) and methods of using same in the treatment of rheumatoid arthritis.
Owner:EMERGENT PRODUCTS DEVELOPMENT SEATTLE LLC
Liquid formulation of a fusion protein comprising TNFR and Fc region
ActiveUS9700595B2Good storage stabilityEasy to usePeptide/protein ingredientsAntibody mimetics/scaffoldsCrystallographySucrose
The present invention relates to a liquid formulation comprising a TNFR-Fc fusion protein and a stabilizer, in which the fusion protein comprises TNFR (tumor necrosis factor receptor) or a fragment thereof and an immunoglobulin Fc region, and the stabilizer comprises one or more amino acids selected from the group consisting of proline and histidine, a buffer solution, and an isotonic agent containing sodium chloride (NaCl) and sucrose, and a preparation method of the liquid formulation. The liquid formulation according to the present invention provides excellent storage stability because long-term storage of TNFR-Fc fusion protein (etanercept) is possible and particular storage conditions are not needed. Since the liquid formulation of the present invention shows excellent storage stability even though the formulation is simple, it is more economical than other stabilizers or lyophilized formulations, and thus the formulation can be effectively applied for uses wherein treatment of TNFR-Fc fusion protein (etanercept) is beneficial.
Owner:ARES TRADING SA
Methods and compositions for treatment of skin disorders
ActiveUS9605064B2Improving disease reductionDecreasing a Physician's Global Assessment (PGA) scoreSnake antigen ingredientsImmunoglobulins against cytokines/lymphokines/interferonsEtanerceptPrior biologic therapy
The invention provides methods and compositions for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis. The invention includes methods for treating a skin disorder associated with detrimental TNFα activity, such as psoriasis, in a subject who has failed or lost response to prior biologic therapy, such as prior administration of etanercept. The invention further provides methods for determining the efficacy of a human TNFα antibody, or antigen-binding portion thereof, for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis.
Owner:ABBVIE BIOTECHNOLOGY LTD
Etanercept Formulations Stabilized with Metal Ions
ActiveUS20130108633A1Elicit long term storage stabilityAcceptable levelPowder deliveryNervous disorderEtanerceptCombinatorial chemistry
The invention provides stabilized aqueous pharmaceutical etanercept compositions suitable for long-term storage of etanercept, methods of manufacture of these compositions, methods of administration, and kits containing same.
Owner:COHERUS BIOSCI
Liquid pharmaceutical composition
ActiveUS20170348225A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsEtanerceptArginine
The present invention relates to novel liquid protein formulations, particularly arginine-free liquid pharmaceutical compositions of etanercept. The invention employs particular combinations and classes of buffer systems, tonicifiers, and sugar stabilisers, optionally alongside polar ionisable amino acids (e.g. aspartic acid, glutamic acid, histidine, and lysine), to afford a viable and storable drug product.
Owner:ARES TRADING SA
Methods and compositions for treatment of skin disorders
InactiveUS20180044414A1Improving disease reductionDecreasing a Physician's Global Assessment (PGA) scoreImmunoglobulins against cytokines/lymphokines/interferonsDermatological disorderEtanerceptPrior biologic therapy
The invention provides methods and compositions for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis. The invention includes methods for treating a skin disorder associated with detrimental TNFα activity, such as psoriasis, in a subject who has failed or lost response to prior biologic therapy, such as prior administration of etanercept. The invention further provides methods for determining the efficacy of a human TNFα antibody, or antigen-binding portion thereof, for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis.
Owner:ABBVIE BIOTECHNOLOGY LTD
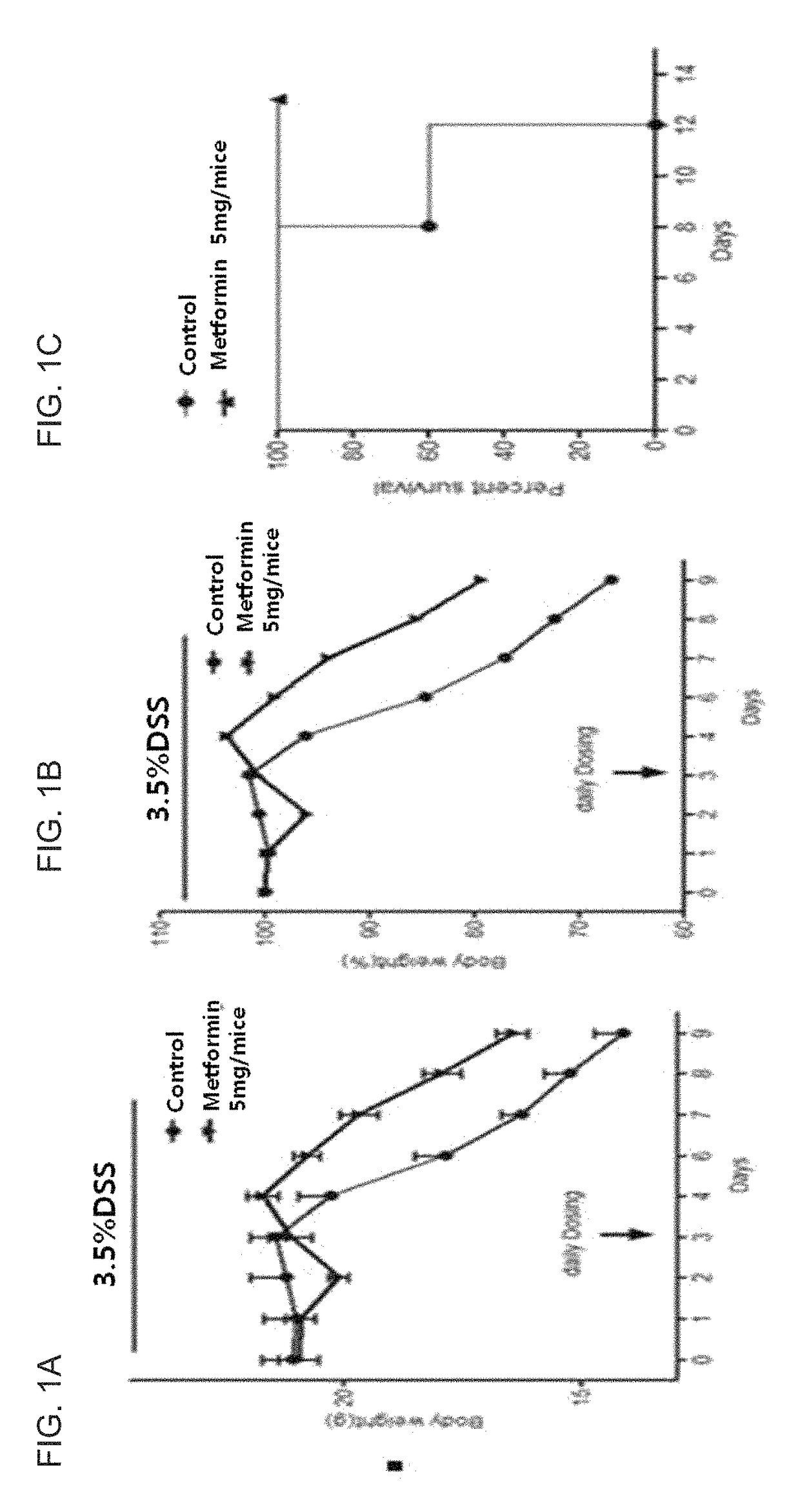
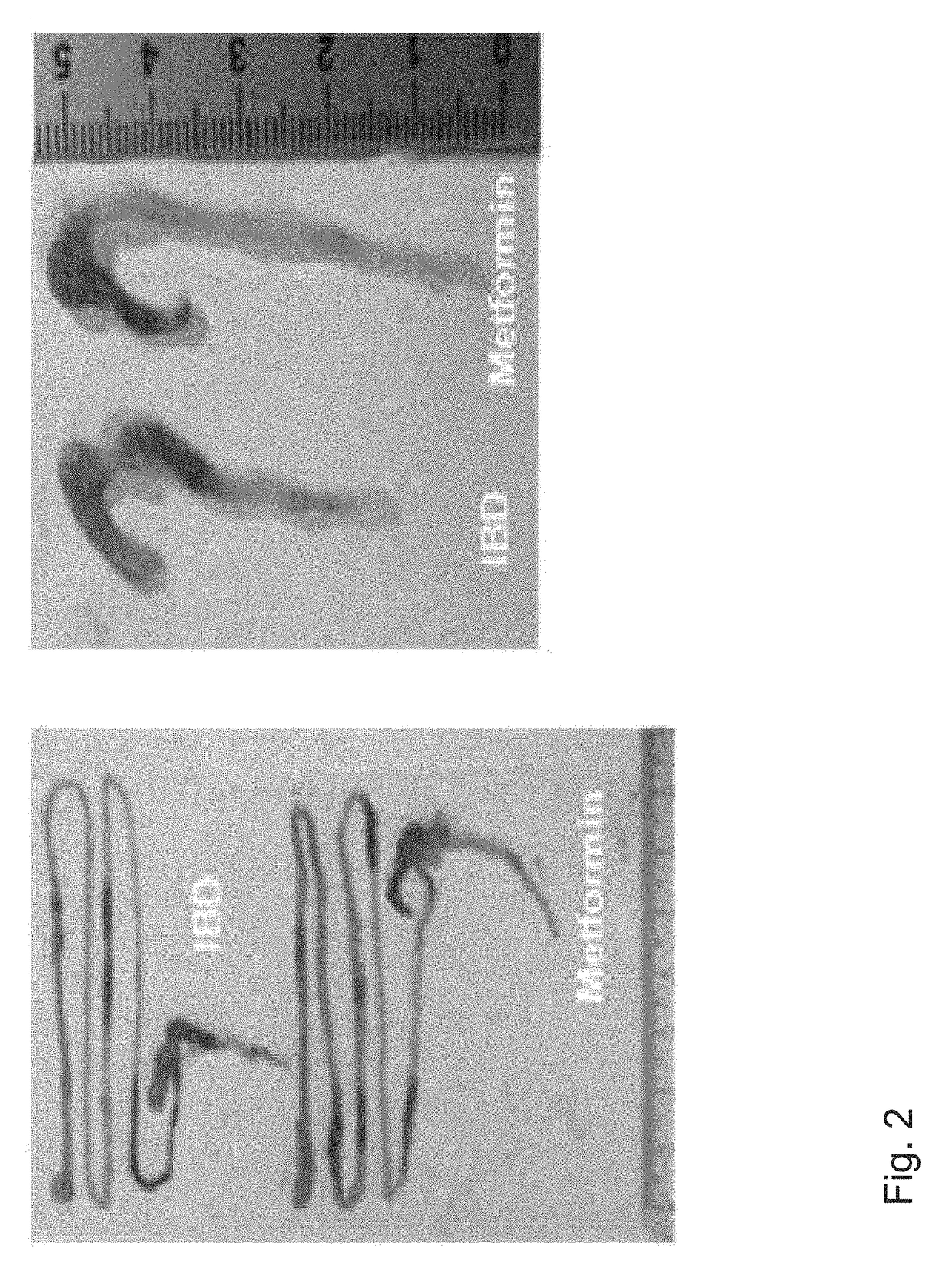
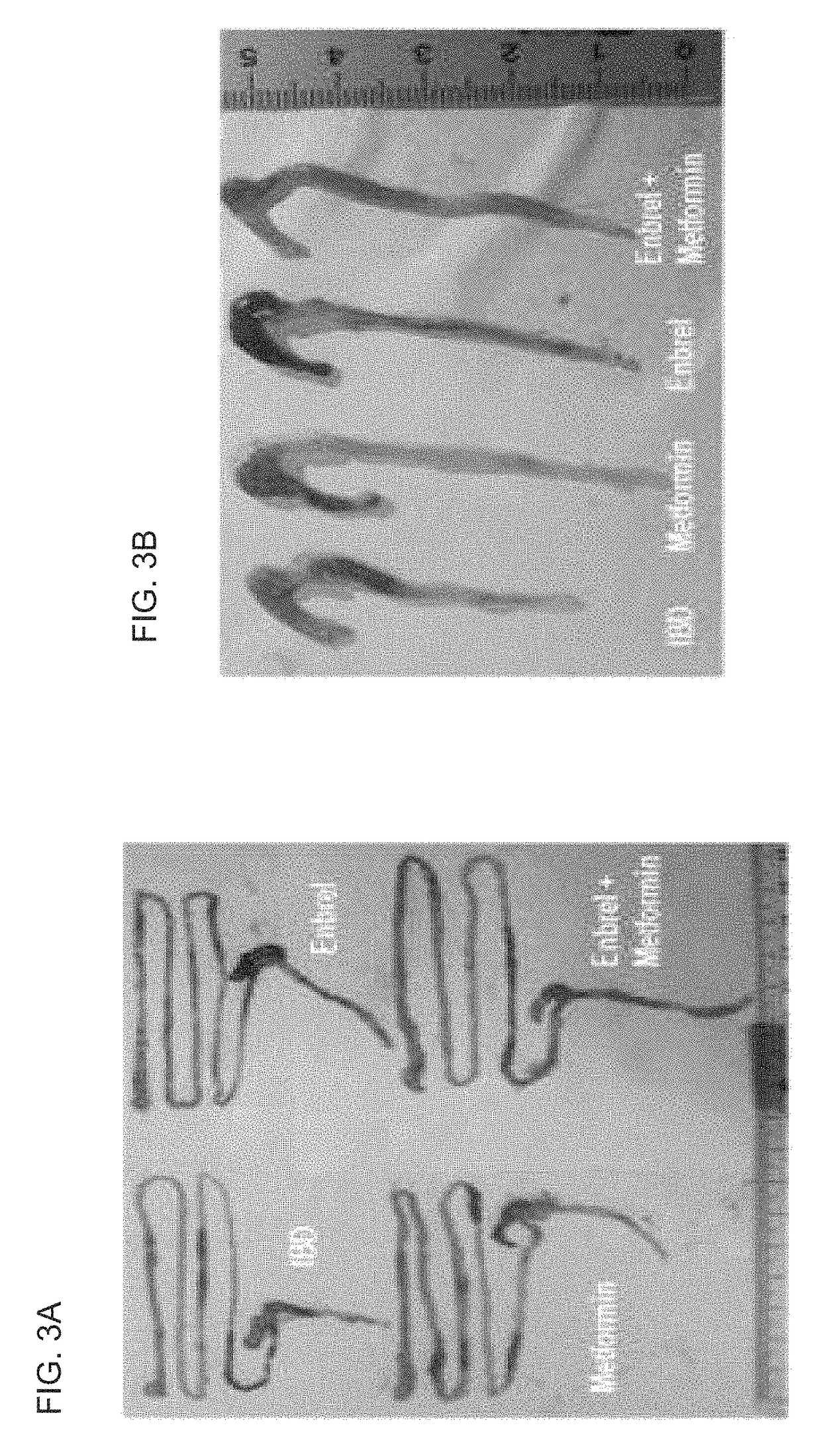
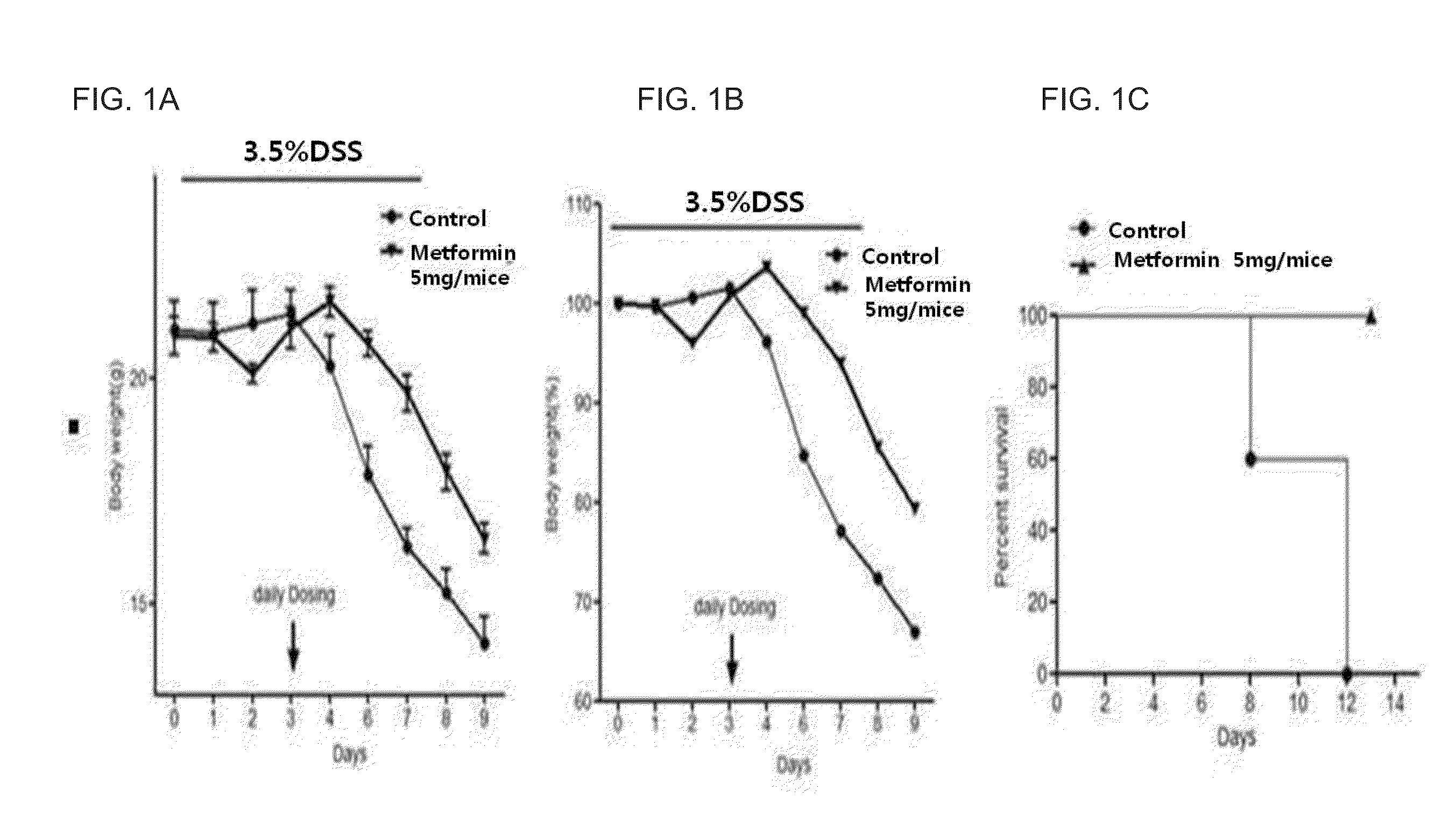
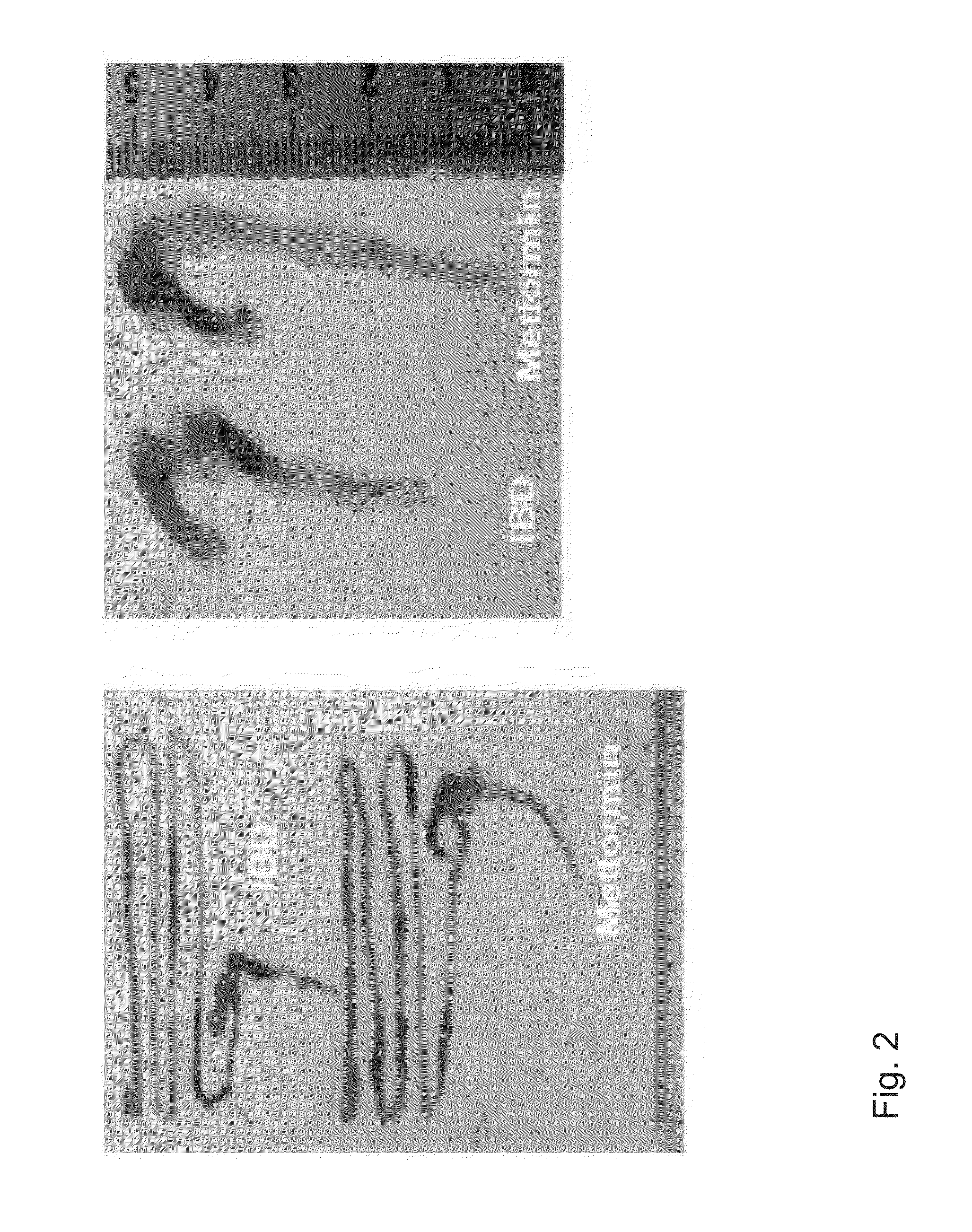
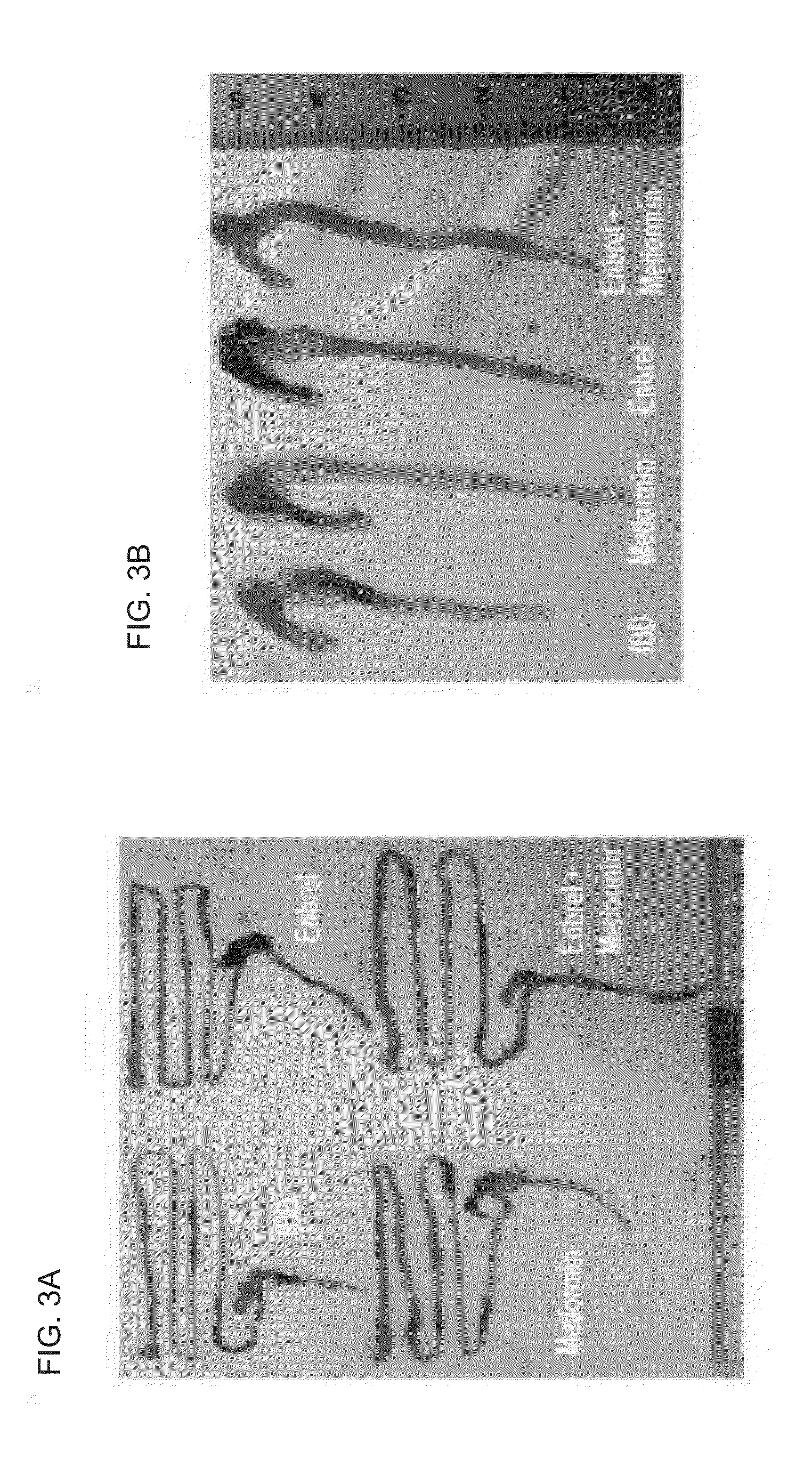
Composition comprising metformin as active ingredient for preventing or treating inflammatory bowel disease
ActiveUS9700531B2Keep the thicknessInhibiting decreasing activity of IL-1 TNF-αOrganic active ingredientsPeptide/protein ingredientsEtanerceptAutoimmune disease
The present invention relates to a composition comprising metformin as an active ingredient for preventing or treating inflammatory bowel disease. The metformin compound or the metformin-etanercept (product name: Enbrel) composite according to the present invention may have excellent effects of maintaining the thickness of the small intestine and length of the large intestine normal, inhibiting or decreasing the activity of IL-17 and TNF-a, and promoting or increasing the activity of IFNr, and therefore can be effectively used as a pharmaceutical composition for preventing or treating autoimmune diseases including inflammatory bowel disease.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Methods of treating kawasaki disease
ActiveUS20190336573A1Ameliorating and preventing progressionPromoting of dilationSalicyclic acid active ingredientsHydroxy compound active ingredientsCoronary arteriesCo administration
The present invention relates to the discovery that etanercept reduces the rate of resistance to intravenous gamma globulin (IVIG) in subjects with acute Kawasaki disease (KD). In certain embodiments, the co-administration of etanercept and IVIG more effectively treats acute KD in subjects older than 12 months than IVIG alone. In other embodiments, the co-administration of etanercept and IVIG ameliorates coronary artery dilation in high risk subjects.
Owner:SEATTLE CHILDRENS HOSPITAL (DBA SEATTLE CHILDRENS RES INST)
Pharmaceutical formulations and methods of making the same
ActiveUS20180256718A1Easy to storeLessen the pain of injectionsPeptide/protein ingredientsAntibody mimetics/scaffoldsEtanerceptPharmaceutical formulation
The invention relates to the formulation of pharmaceutical compositions of etanercept. The invention also relates to methods of removing buffer and of formulating pharmaceutical compositions of etanercept.
Owner:AMGEN INC
Liquid formulation of a fusion protein comprising tnfr and fc region
ActiveUS20170028020A1Good storage stabilityEasy to usePeptide/protein ingredientsAntibody mimetics/scaffoldsCrystallographySucrose
The present invention relates to a liquid formulation comprising a TNFR-Fc fusion protein and a stabilizer, in which the fusion protein comprises TNFR (tumor necrosis factor receptor) or a fragment thereof and an immunoglobulin Fc region, and the stabilizer comprises one or more amino acids selected from the group consisting of proline and histidine, a buffer solution, and an isotonic agent containing sodium chloride (NaCl) and sucrose, and a preparation method of the liquid formulation. The liquid formulation according to the present invention provides excellent storage stability because long-term storage of TNFR-Fc fusion protein (etanercept) is possible and particular storage conditions are not needed. Since the liquid formulation of the present invention shows excellent storage stability even though the formulation is simple, it is more economical than other stabilizers or lyophilized formulations, and thus the formulation can be effectively applied for uses wherein treatment of TNFR-Fc fusion protein (etanercept) is beneficial.
Owner:ARES TRADING SA
Stable Aqueous Formulations of Etanercept
The invention provides stabilized aqueous pharmaceutical etanercept compositions suitable for long-term storage of etanercept, methods of manufacture of these compositions, methods of administration, and kits containing same.
Owner:COHERUS BIOSCI
Methods for treatment of brain injury utilizing biologics
ActiveUS20130224197A1Improve neurological functionDelayNervous disorderPeptide/protein ingredientsInjury brainSingle-Chain Antibodies
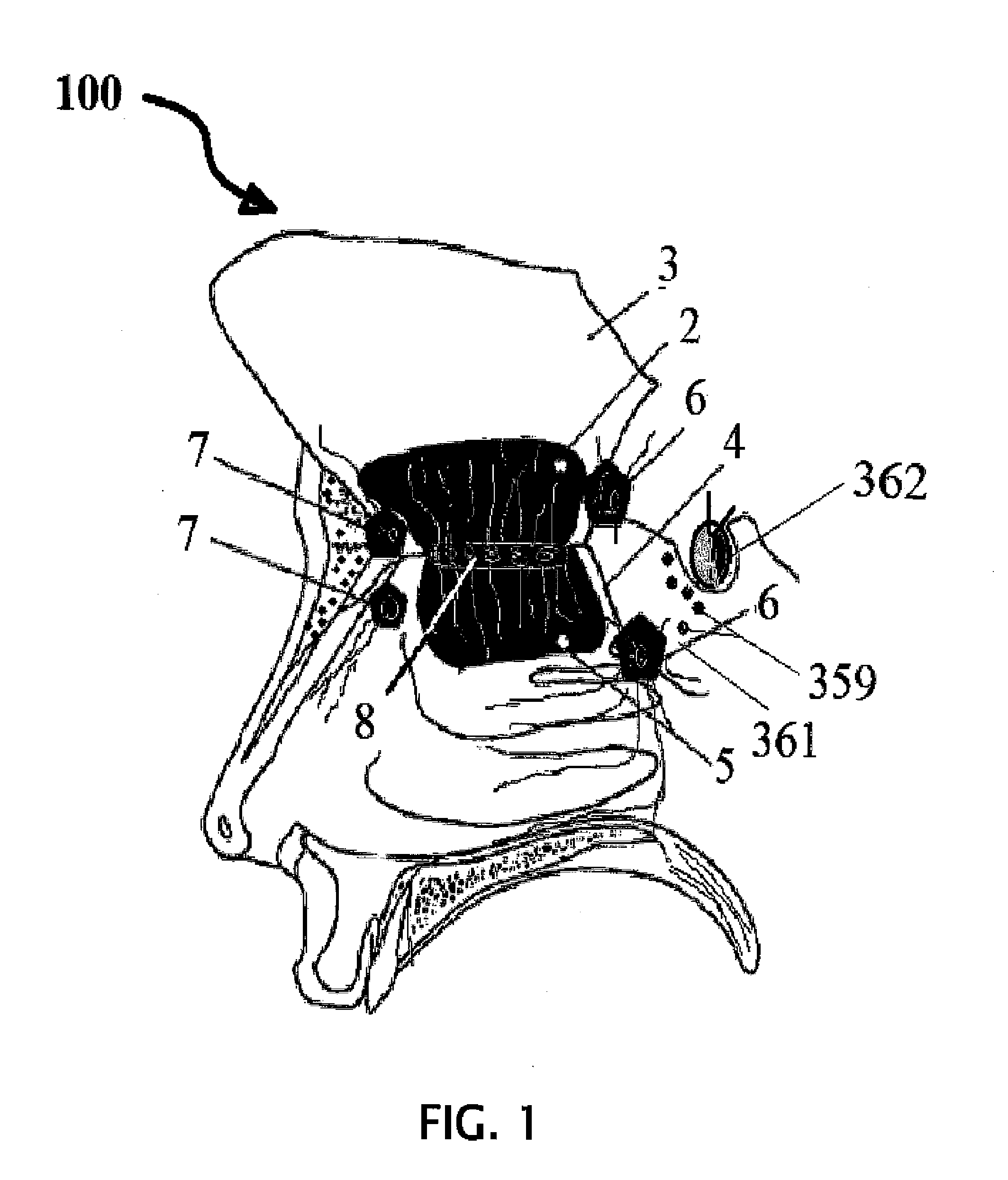
A method of using biologics to treat chronic brain injury or spasticity due to stroke, trauma and other causes. Preferred embodiments include perispinal, parenteral, transepidermal or intranasal use of TNF antagonists. The TNF antagonists include TNF receptor fusion proteins, TNF monoclonal antibodies (mAbs), humanized TNF mAbs, fully human TNF mAbs, chimeric TNF mAbs, domain TNF antibodies, mAB fragments, anti-TNF nanobodies, dominant negative TNF constructs and TNF inhibitory single chain antibody fragments. One of the preferred embodiments of this invention is the perispinal administration of etanercept for treatment of mammals following stroke. The use of Trendelenburg positioning, catheters, pumps, or depot formulations are included.
Owner:TACT IP
Composition comprising metformin as active ingredient for preventing or treating inflammatory bowel disease
ActiveUS20150196511A1Keep the thicknessInhibiting decreasing activity of IL-1 TNF-αOrganic active ingredientsBiocideEtanerceptAutoimmune disease
The present invention relates to a composition comprising metformin as an active ingredient for preventing or treating inflammatory bowel disease. The metformin compound or the metformin-etanercept (product name: Enbrel) composite according to the present invention may have excellent effects of maintaining the thickness of the small intestine and length of the large intestine normal, inhibiting or decreasing the activity of IL-17 and TNF-a, and promoting or increasing the activity of IFNr, and therefore can be effectively used as a pharmaceutical composition for preventing or treating autoimmune diseases including inflammatory bowel disease.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Methods of treating Kawasaki Disease
ActiveUS11253569B2Ameliorating and preventing progressionPromoting of dilationSalicyclic acid active ingredientsHydroxy compound active ingredientsCoronary arteriesVein
The present invention relates to the discovery that etanercept reduces the rate of resistance to intravenous gamma globulin (IVIG) in subjects with acute Kawasaki disease (KD). In certain embodiments, the co-administration of etanercept and IVIG more effectively treats acute KD in subjects older than 12 months than IVIG alone. In other embodiments, the co-administration of etanercept and IVIG ameliorates coronary artery dilation in high risk subjects.
Owner:SEATTLE CHILDRENS HOSPITAL (DBA SEATTLE CHILDRENS RES INST)
Western medicine composition for treating rheumatism
InactiveCN106729704ASmall side effectsEasy to preparePeptide/protein ingredientsHydrolasesPhosphodiesteraseWestern medicine
The invention discloses a western medicine composition for treating rheumatism. The western medicine composition comprises the following raw materials in parts by weight: 2-15 parts of rhizoma seu radix notopterygii, 0.1-1 part of methotrexate, 1-3 parts of soft soap, 0.1-1 part of phosphodiesterase, 1-5 parts of glucocorticoid drugs, 0.5-2 parts of etanercept, 10-50 parts of normal saline, 0.5-1 part of ossotide tablets, 1-3 parts of a donkey hide gelatin powder, 0.1-1 part of steroid and 1-3 parts of anisodamine. The raw materials are compounded to take a synergistic effect, and the western medicine composition has the advantages of small toxic and / or side effects, low cost and no complication, can radically cure rheumatism, ensures low probability of recurrence, is simple in preparation method, short in treatment cycle and good in curative effect, is a safe and effective medicine and protects the health of people.
Owner:ZHENGZHOU ZHANGMENG NETWORK TECH CO LTD
Arginine-free tnfr:fc-fusion polypeptide compositions and methods of use
ActiveUS20210309719A1Low costReduce morbidityPeptide/protein ingredientsAntibody mimetics/scaffoldsAutoimmune responsesArginine
Owner:BIOGEN MA INC
Methods for treatment of brain injury utilizing biologics
ActiveUS8900583B2Opportunity to healImprove neurological functionNervous disorderPeptide/protein ingredientsSingle-Chain AntibodiesInjury brain
A method of using biologics to treat chronic brain injury or spasticity due to stroke, trauma and other causes. Preferred embodiments include perispinal, parenteral, transepidermal or intranasal use of TNF antagonists. The TNF antagonists include TNF receptor fusion proteins, TNF monoclonal antibodies (mAbs), humanized TNF mAbs, fully human TNF mAbs, chimeric TNF mAbs, domain TNF antibodies, mAB fragments, anti-TNF nanobodies, dominant negative TNF constructs and TNF inhibitory single chain antibody fragments. One of the preferred embodiments of this invention is the perispinal administration of etanercept for treatment of mammals following stroke. The use of Trendelenburg positioning, catheters, pumps, or depot formulations are included.
Owner:TACT IP
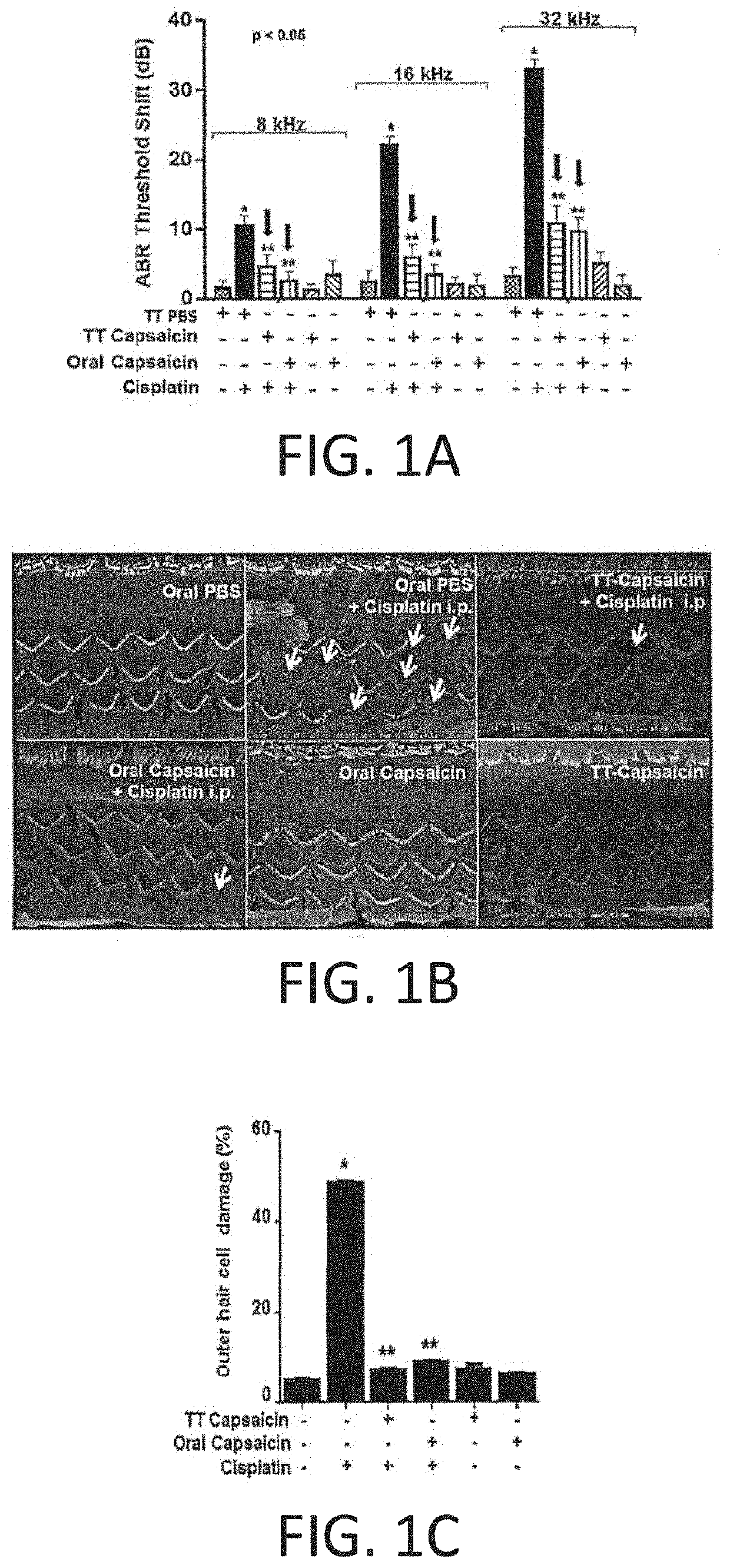
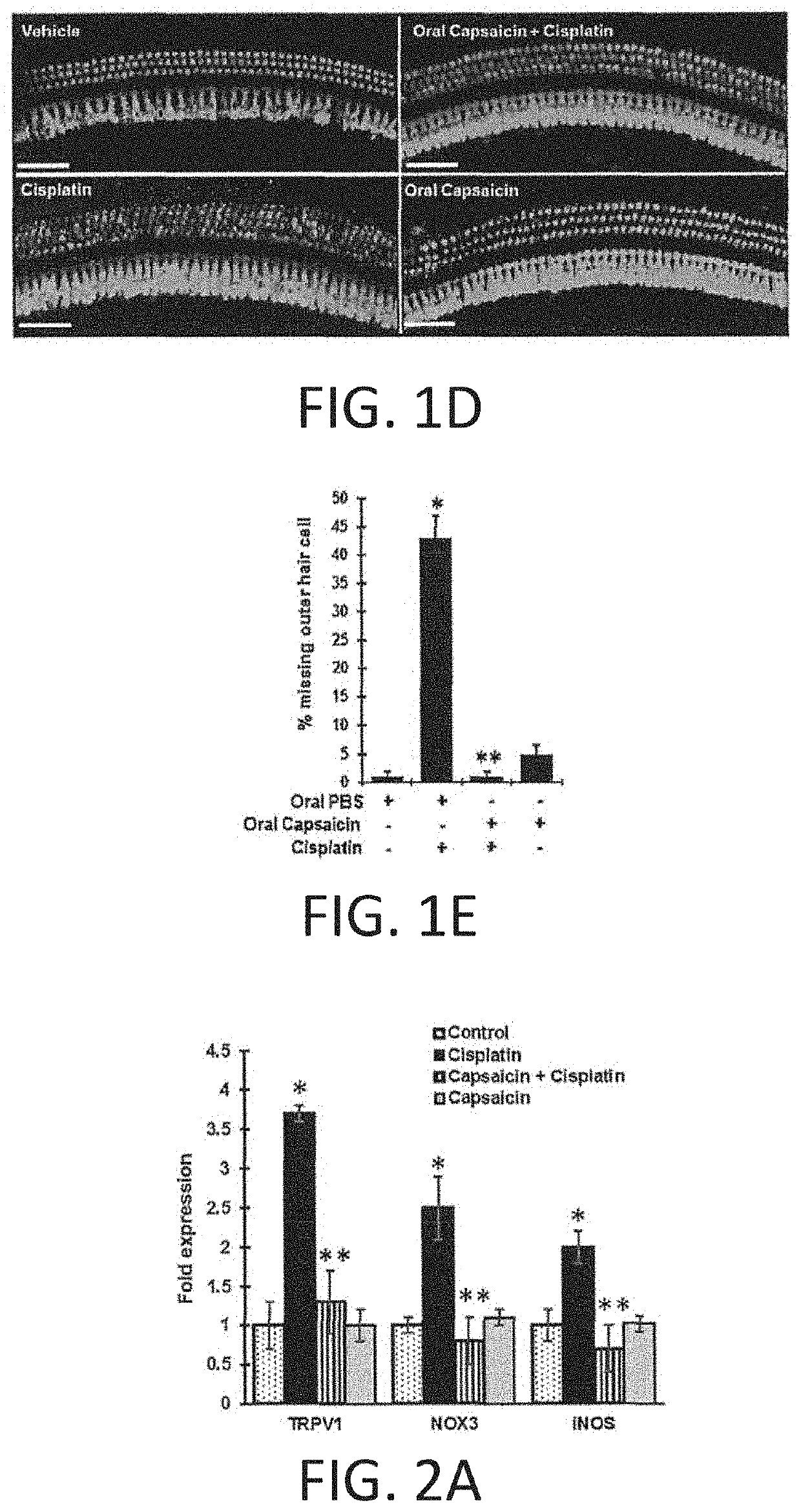
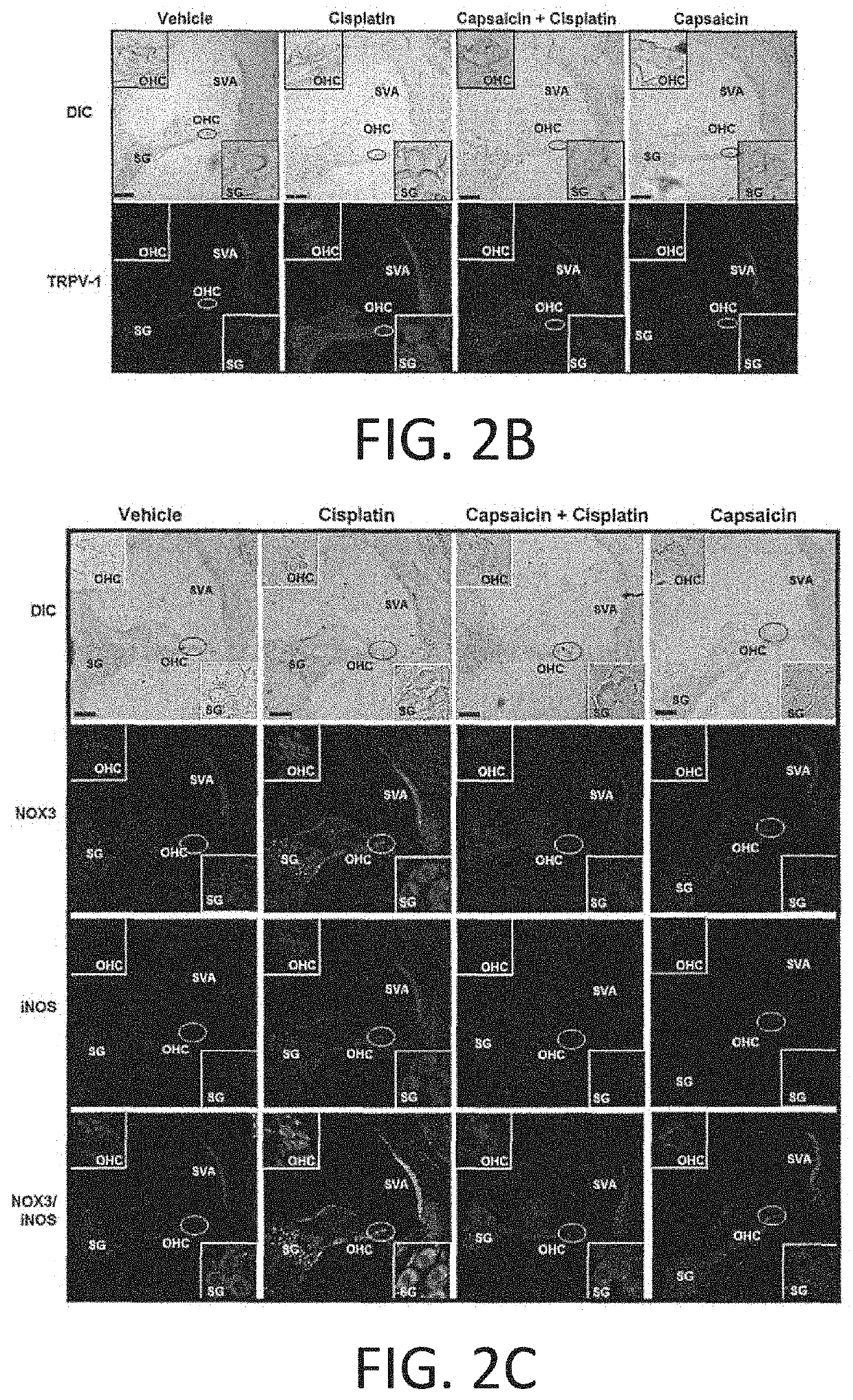
REGIMENS, COMPOSITIONS AND METHODS WITH CAPSAICIN AND TNF-alpha INHIBITOR
PendingUS20210253689A1Enhance and improve abilityEnhance and improve and levelOrganic active ingredientsSenses disorderHearing acuityEtanercept
Compositions and treatment regimen that includes the compositions, suitable for preventing, treating and restoring hearing acuity in a subject are provided. The treatment regimen includes an amount of capsaicin and an amount of a TNF-α inhibitor (such as etanercept) that are delivered relatively simultaneously to a subject. Methods of providing a pretreatment to a subject suitable for preventing hearing loss are also provided. The capsaicin and TNFα inhibitor may be provided in different delivery forms to the subject. Methods of providing a treatment for hearing loss with a TNFα inhibitor alone, such as entanercept, or with capsaicin alone, are also provided.
Owner:SOUTHERN ILLINOIS UNIVERSITY
Western medicinal composition for treating rheumatism and preparation method of western medicinal composition
InactiveCN106421783ASmall side effectsComplete relapseHydrolasesPeptide/protein ingredientsPhosphodiesteraseSide effect
The invention discloses a western medicinal composition for treating rheumatism. The western medicinal composition comprises, by weight, 2-15 parts of modafinil, 0.1-1 part of methotrexate, 1-3 parts of oxaliplatin and mannitol injection, 0.1-1 part of phosphodiesterase, 1-5 parts of glucocorticoid type drugs, 0.5-2 parts of etanercept, 10-50 parts of purified water, 0.5-1 part of bone peptide tablet, 1-3 parts of donkey-hide gelatin powder, 0.1-1 part of steroid and 1-3 parts of anisodamine. The western medicinal composition has the advantages that the synergistic effect is achieved through compounding of the raw materials, toxic and side effect is small, cost is low, complications are avoided, the rheumatism can be treated thoroughly, and reoccurrence of the rheumatism is avoided; a preparation method of the western medicinal composition is simple, treatment cycle is short, curative effect is good, and the western medicinal composition is safe and effective and protects health of people.
Owner:ZHENGZHOU RENHONG PHARMA CO LTD
Liquid pharmaceutical composition of etanercept with lysine and proline
The present invention relates to novel liquid protein formulations, particularly arginine-free liquid pharmaceutical compositions of etanercept. The invention employs particular combinations and classes of buffer systems, tonicifiers, and sugar stabilisers, optionally alongside polar ionisable amino acids (e.g. aspartic acid, glutamic acid, histidine, and lysine), to afford a viable and storable drug product.
Owner:ARES TRADING SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com