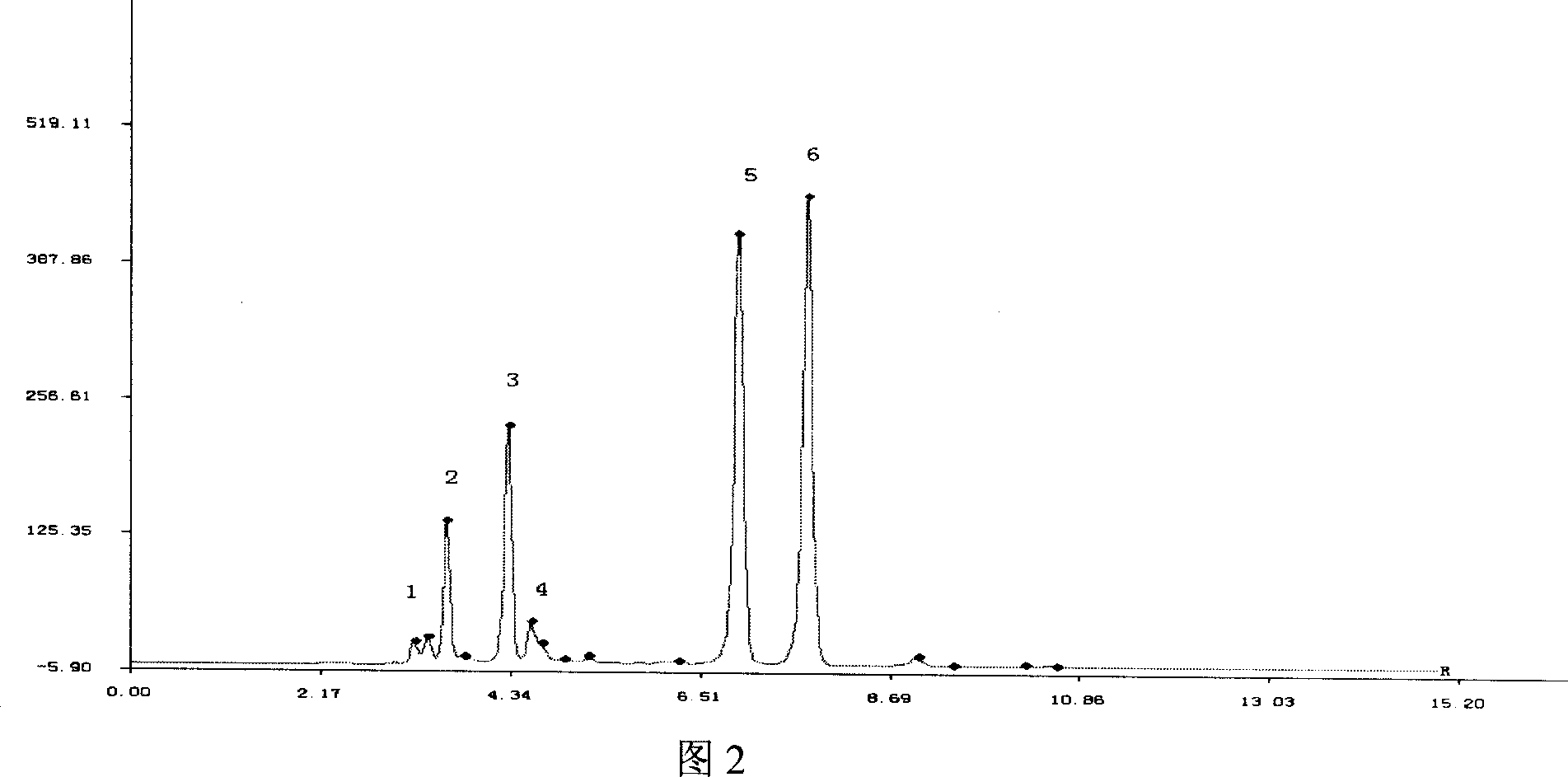
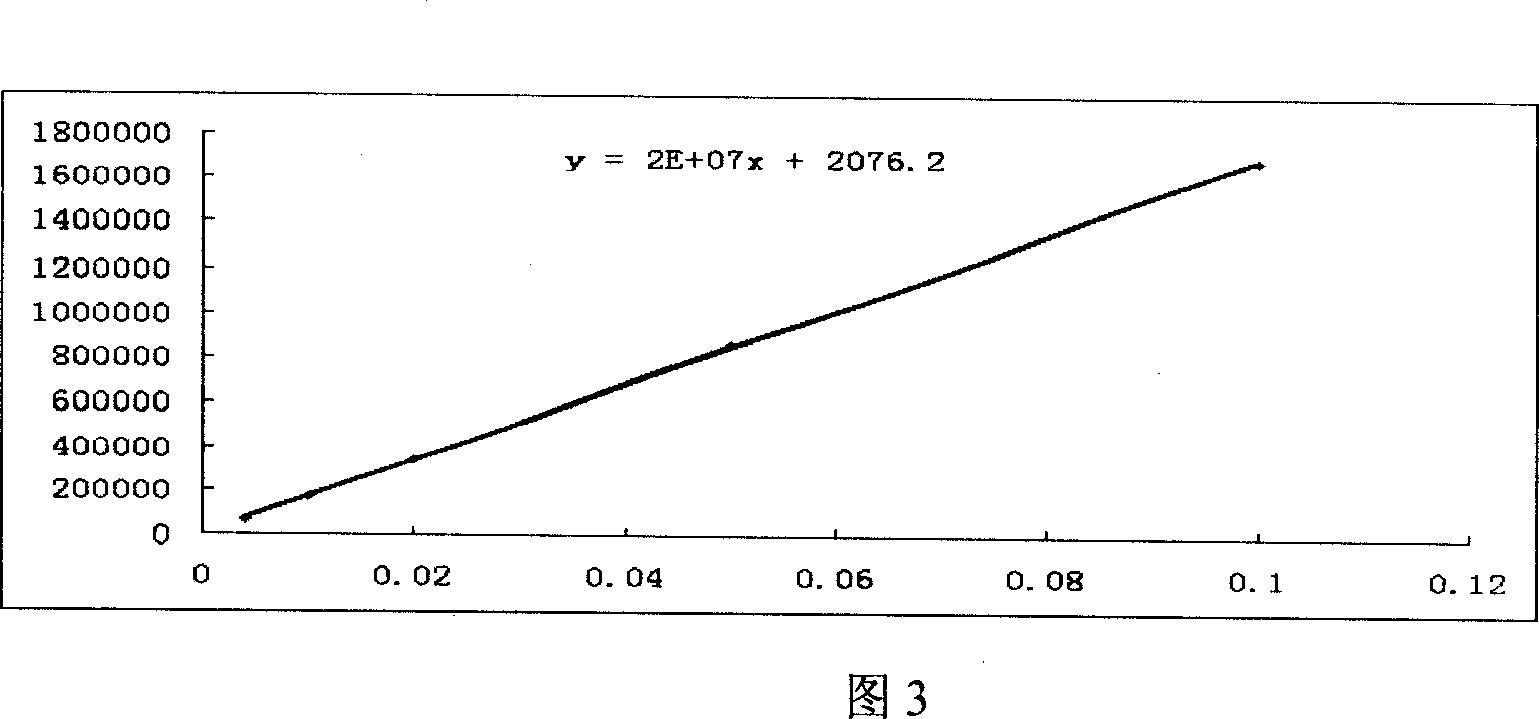
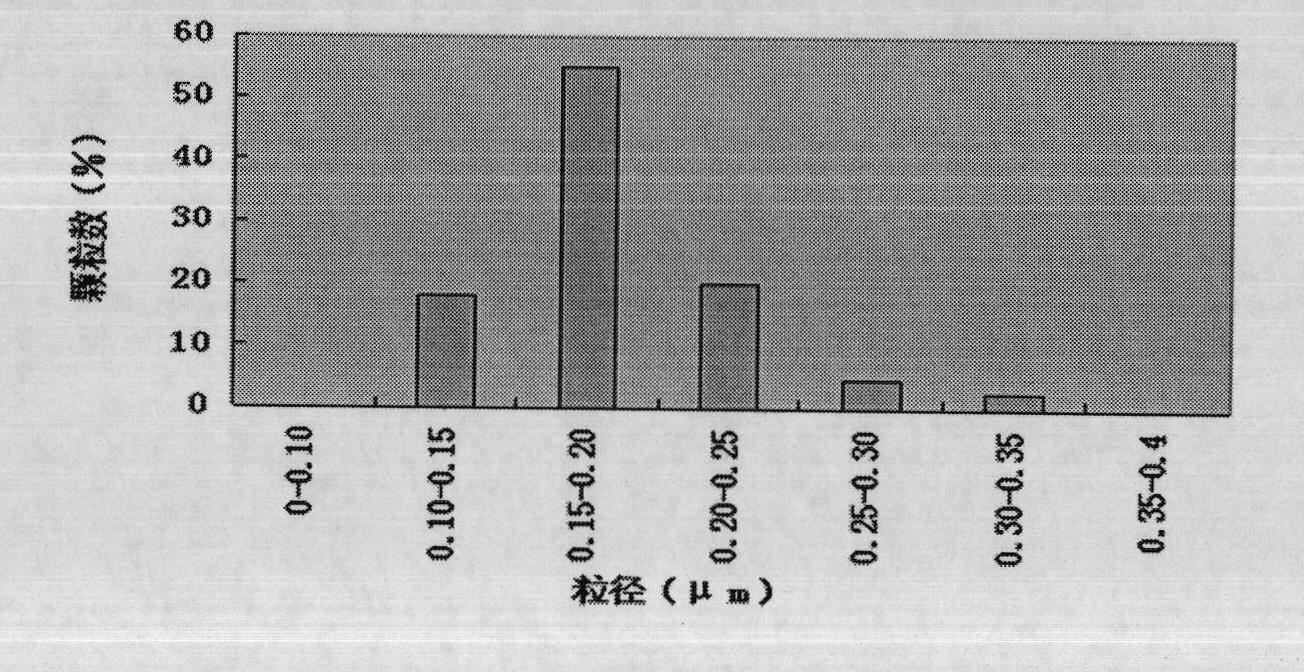
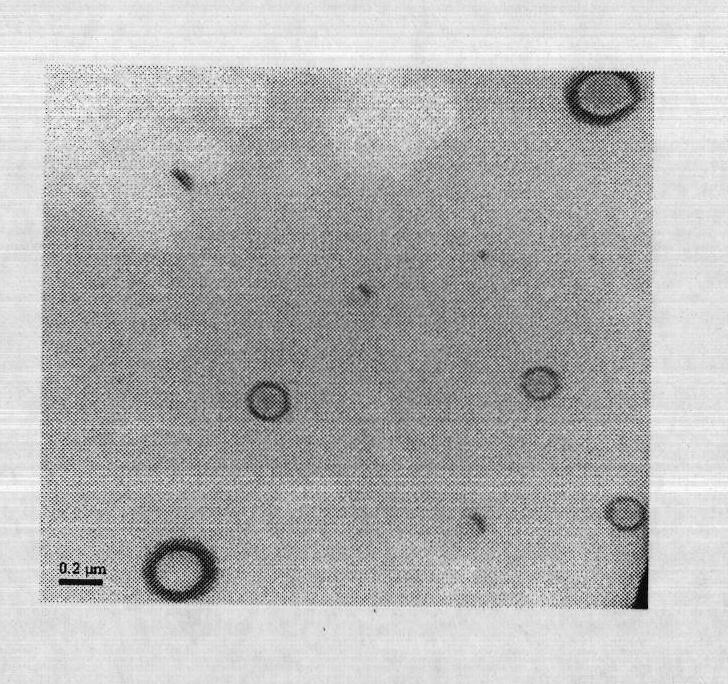
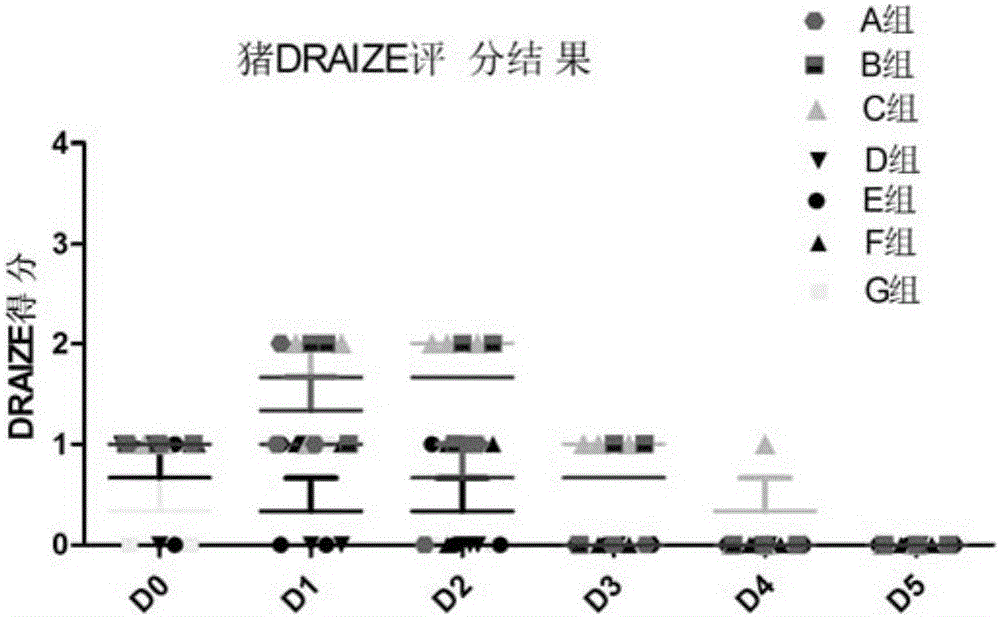
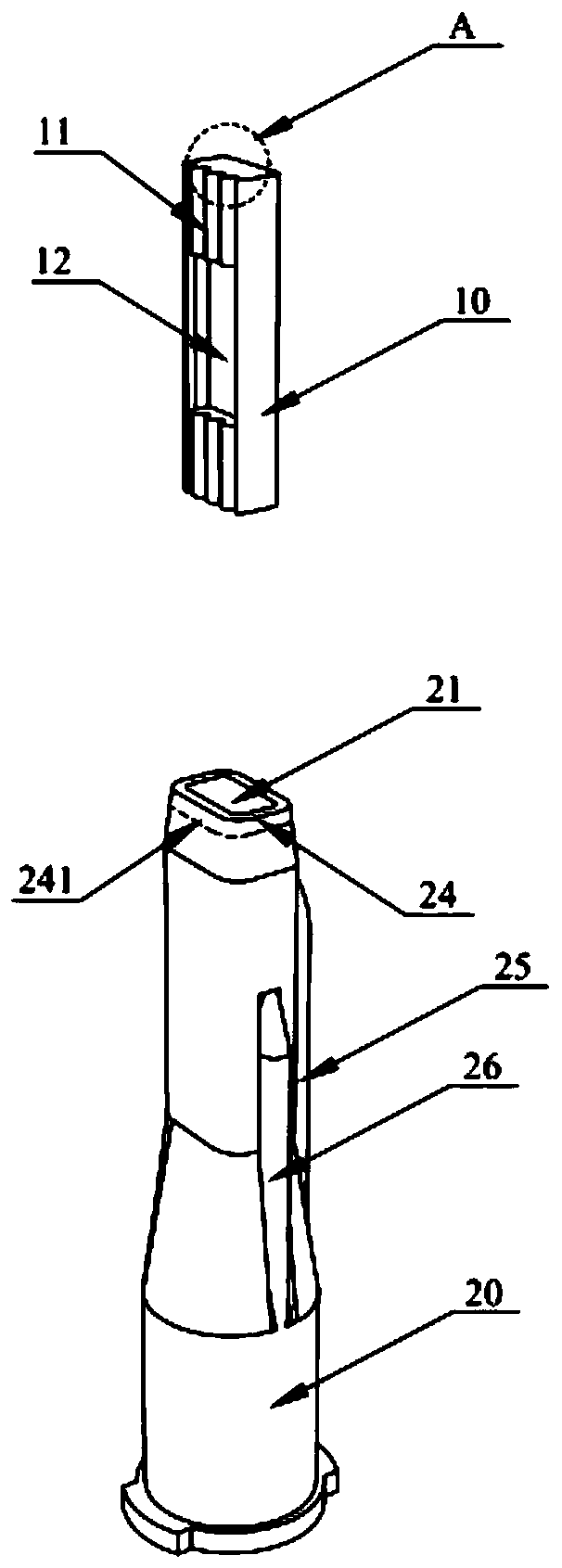
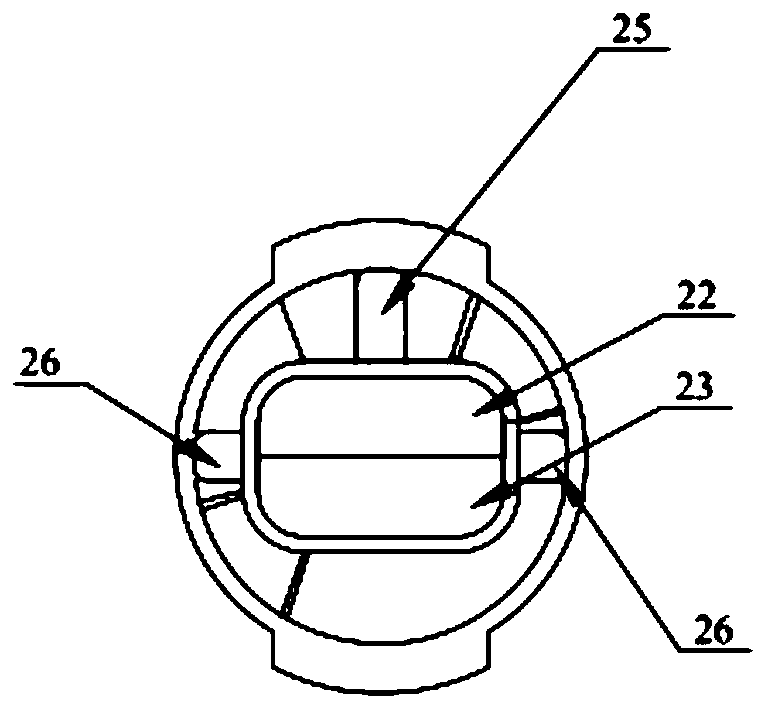
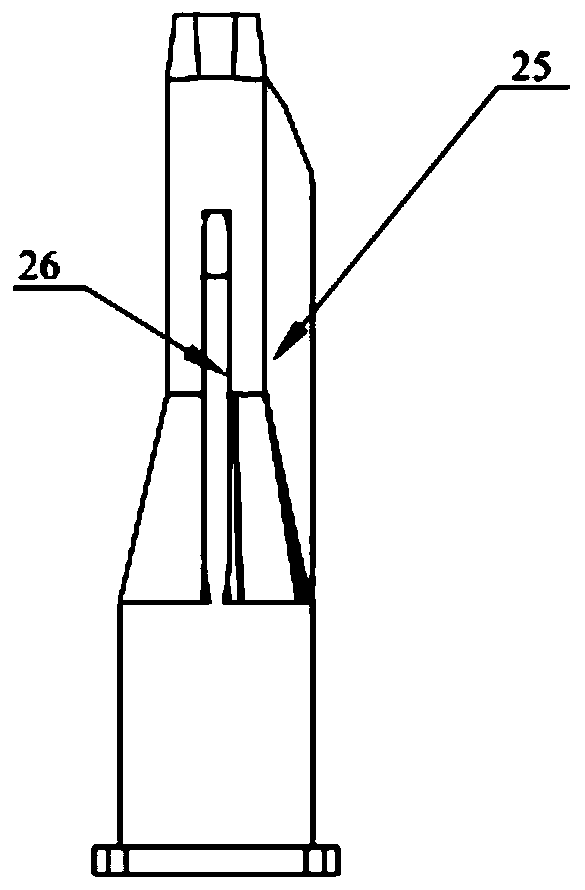
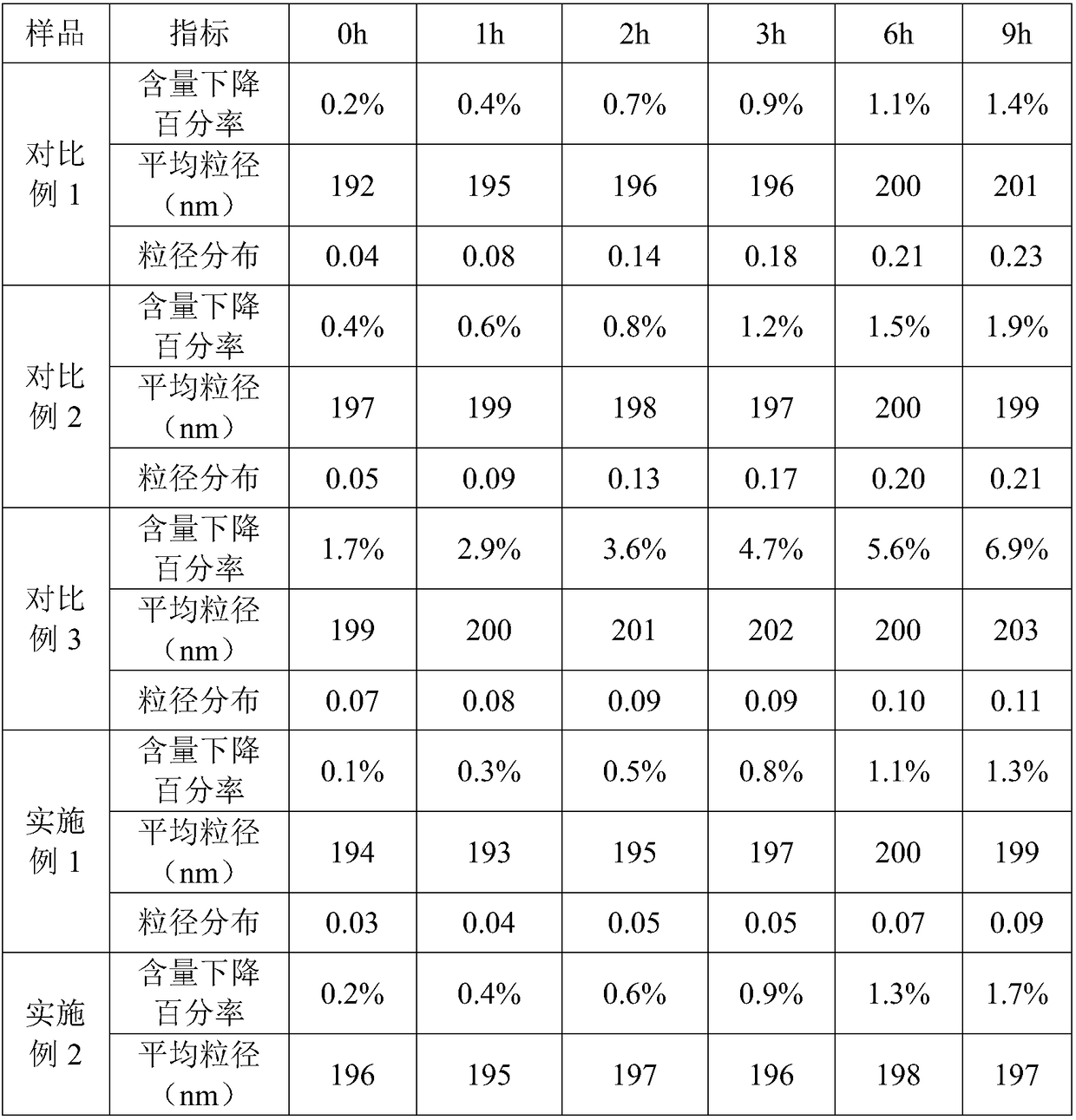
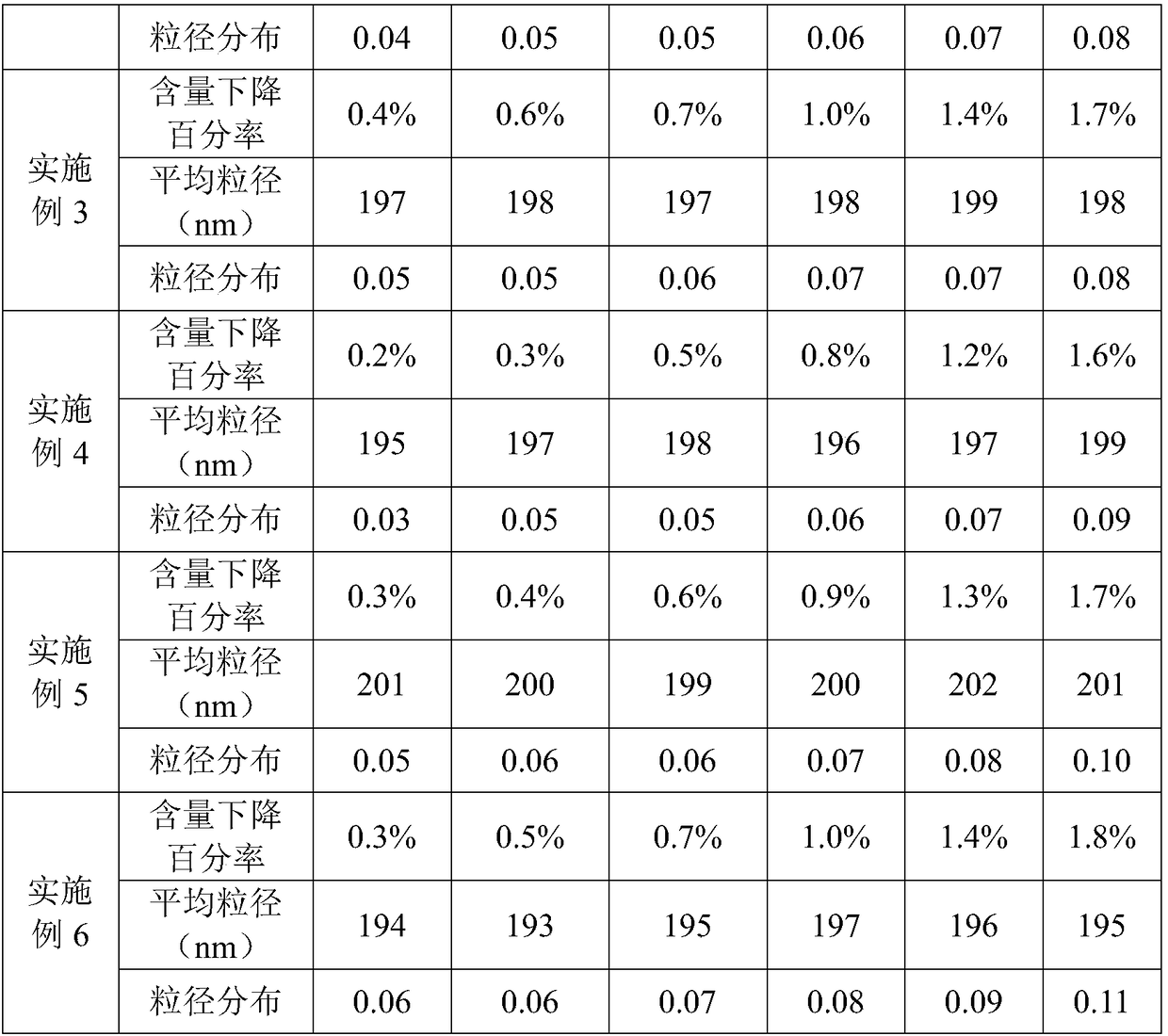
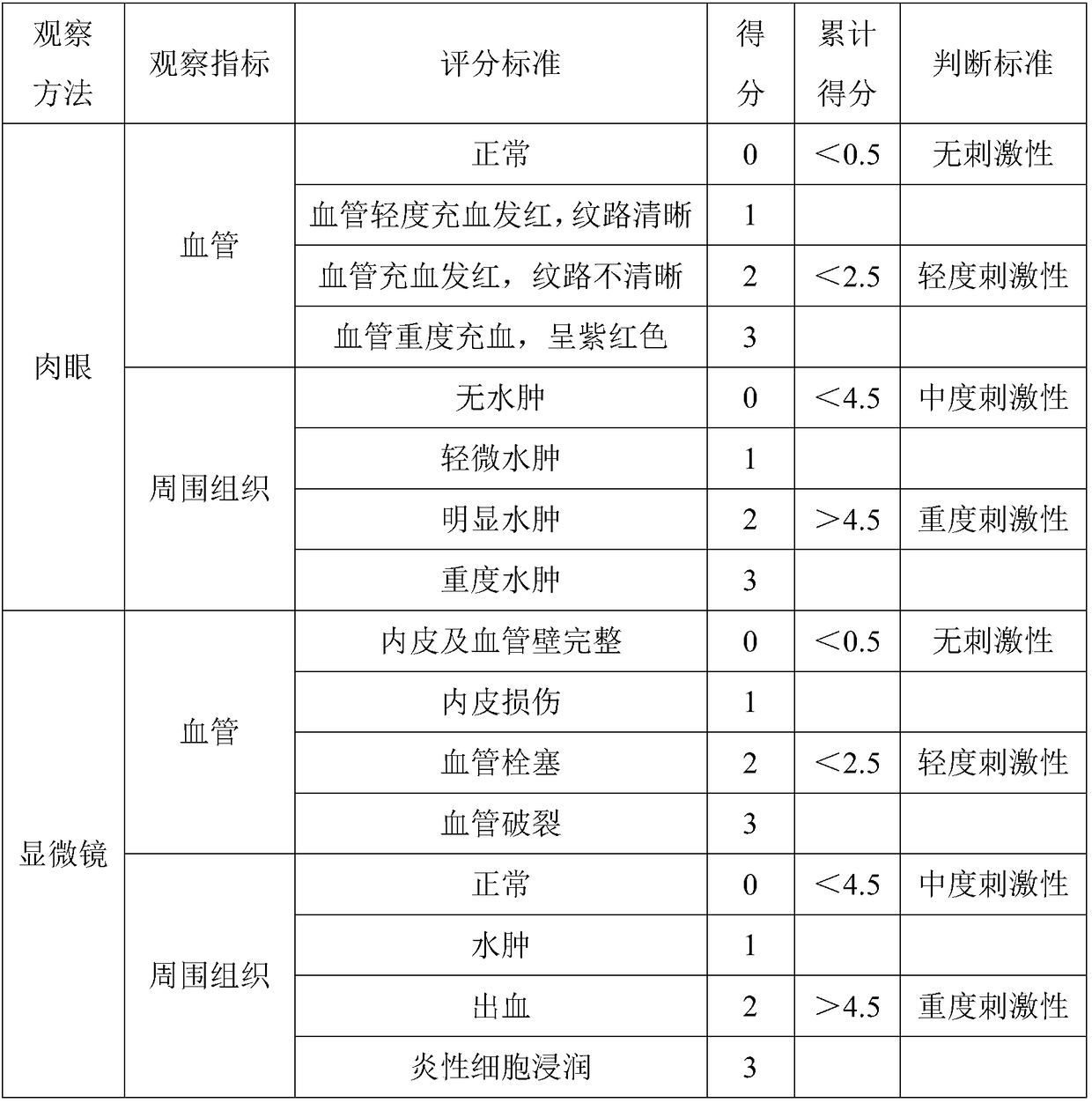
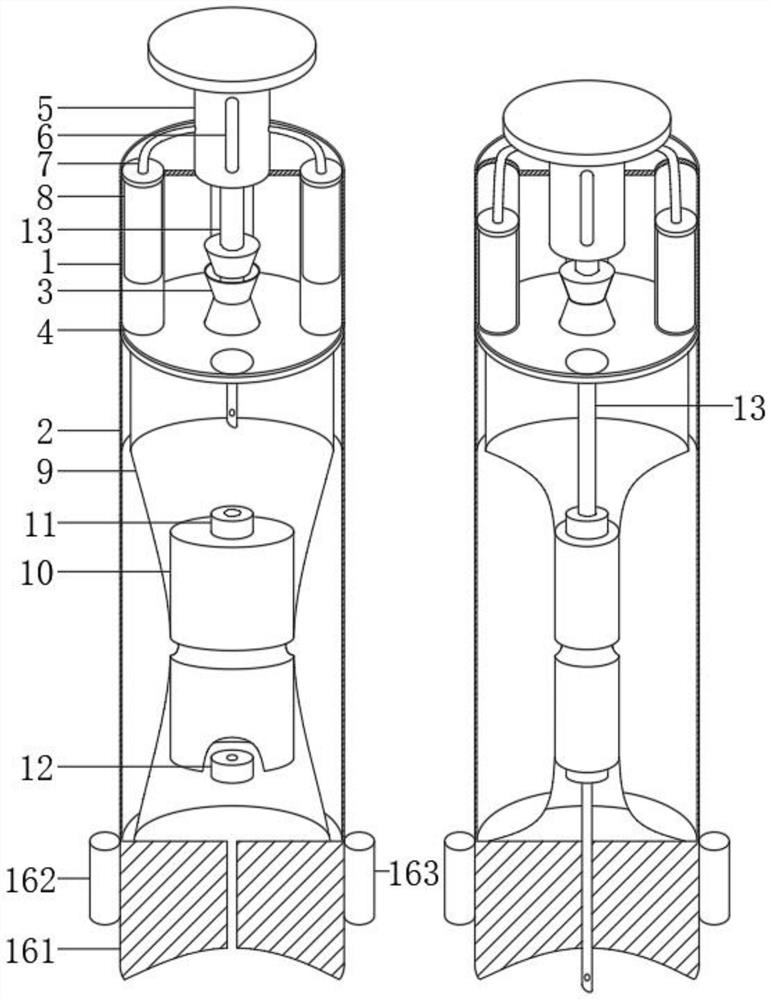
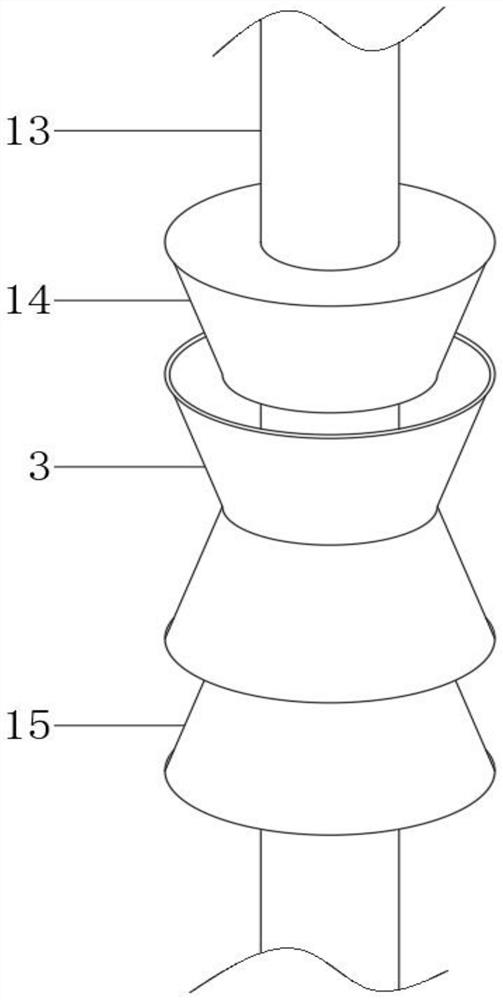
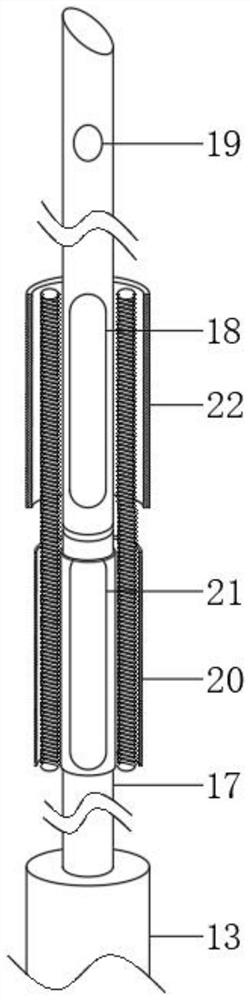
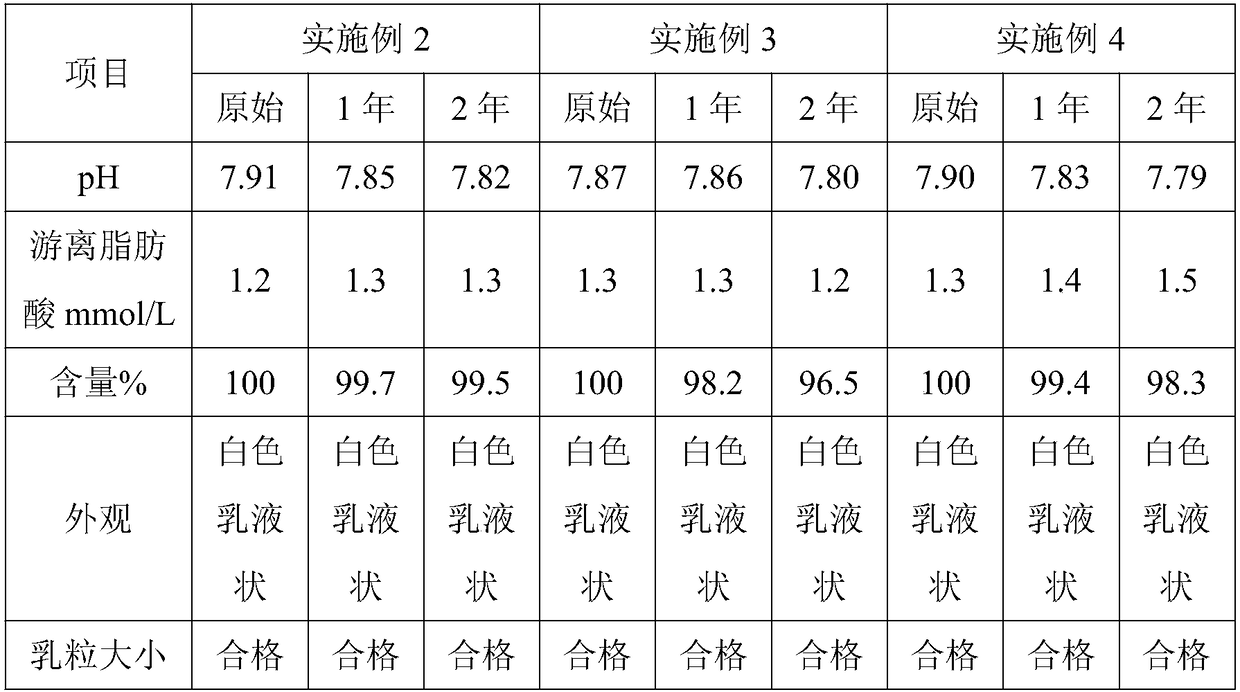
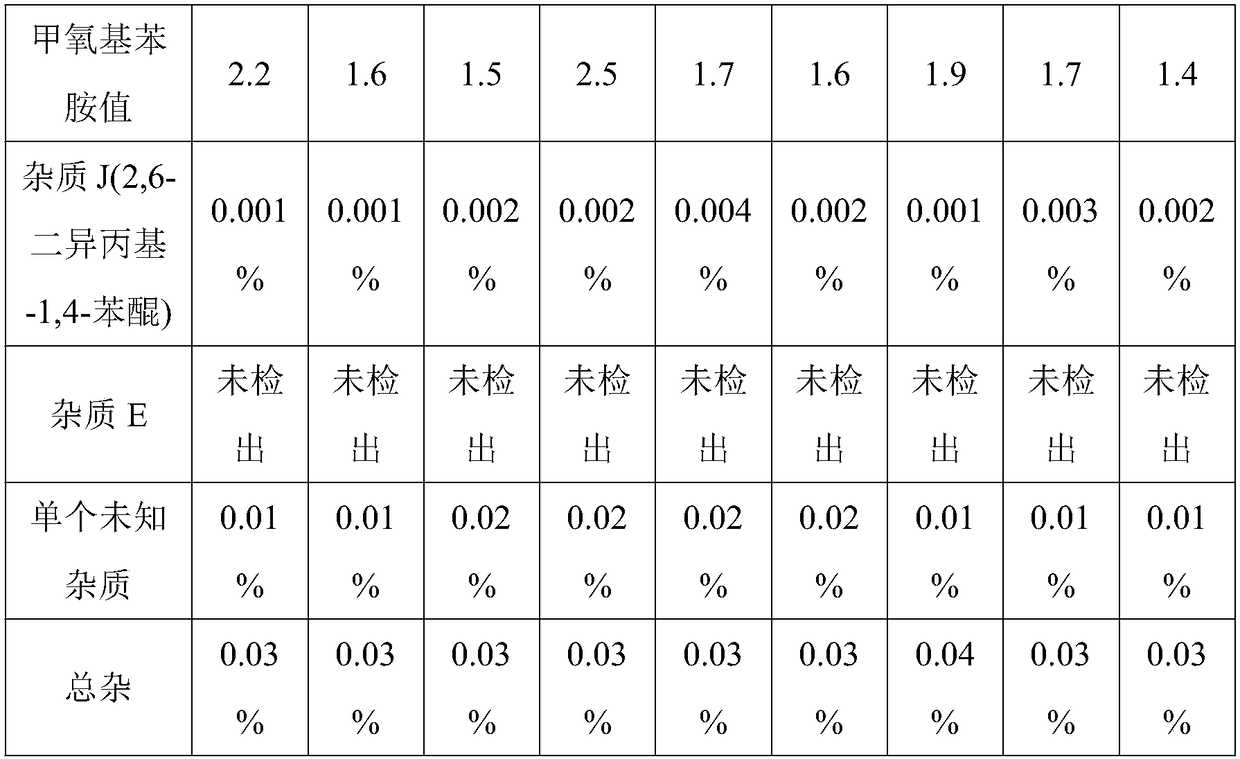
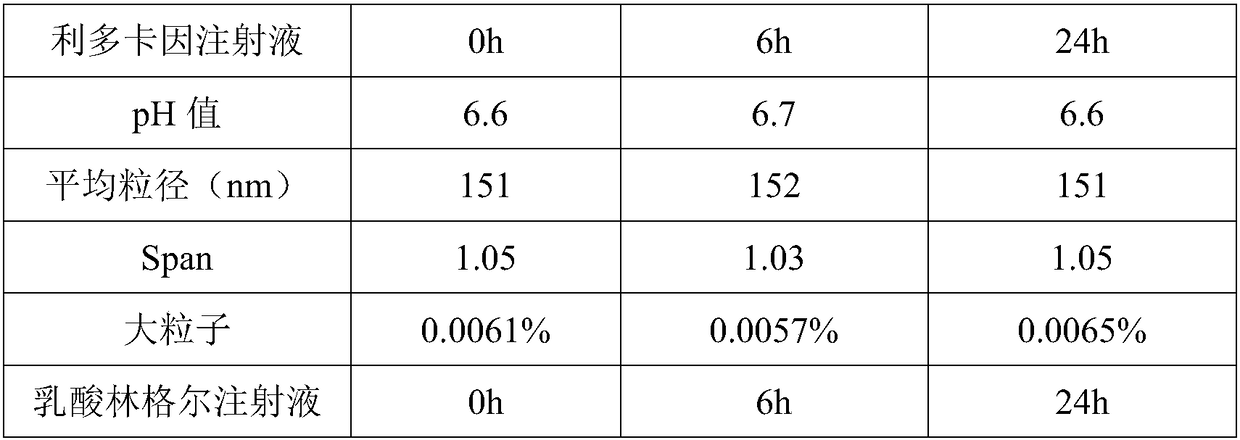
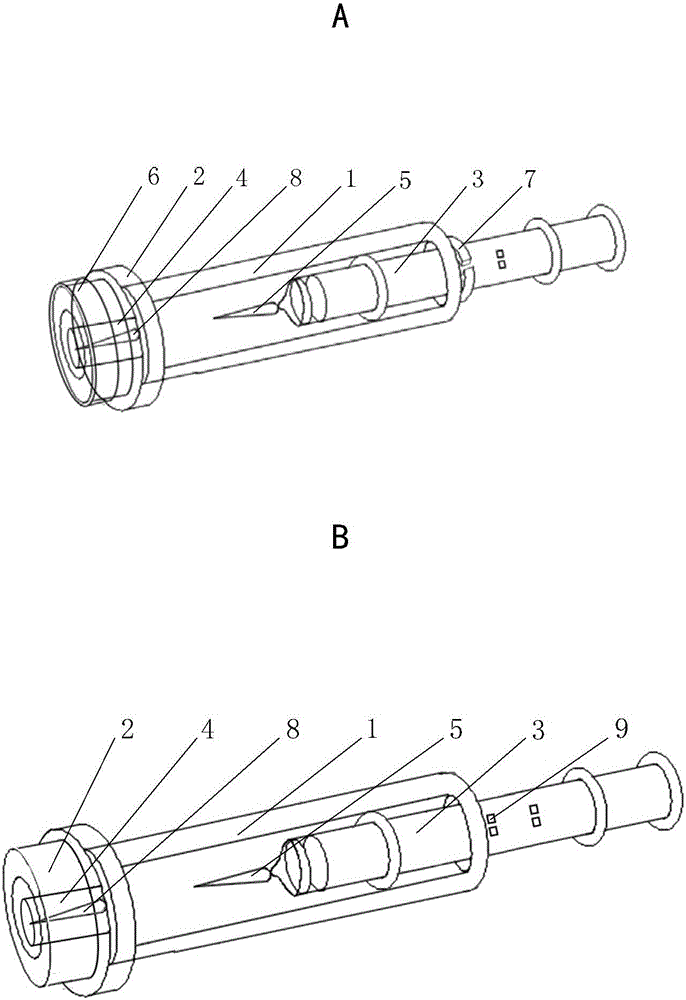
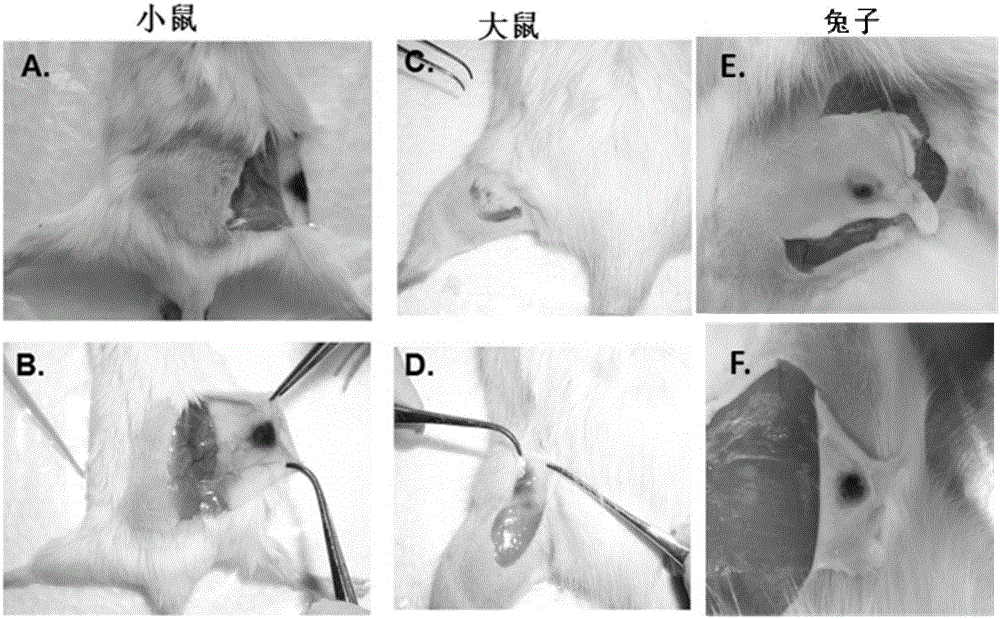
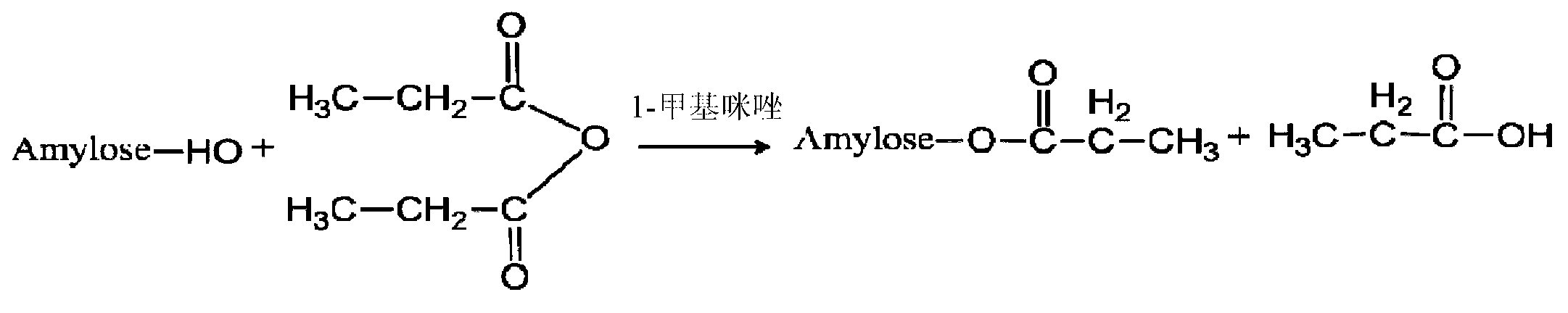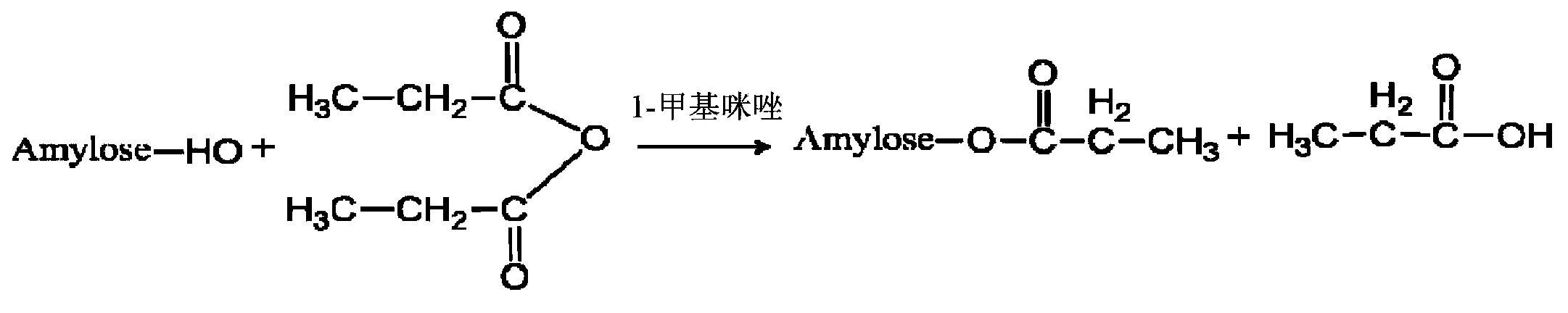Patents
Literature
32results about How to "Lessen the pain of injections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
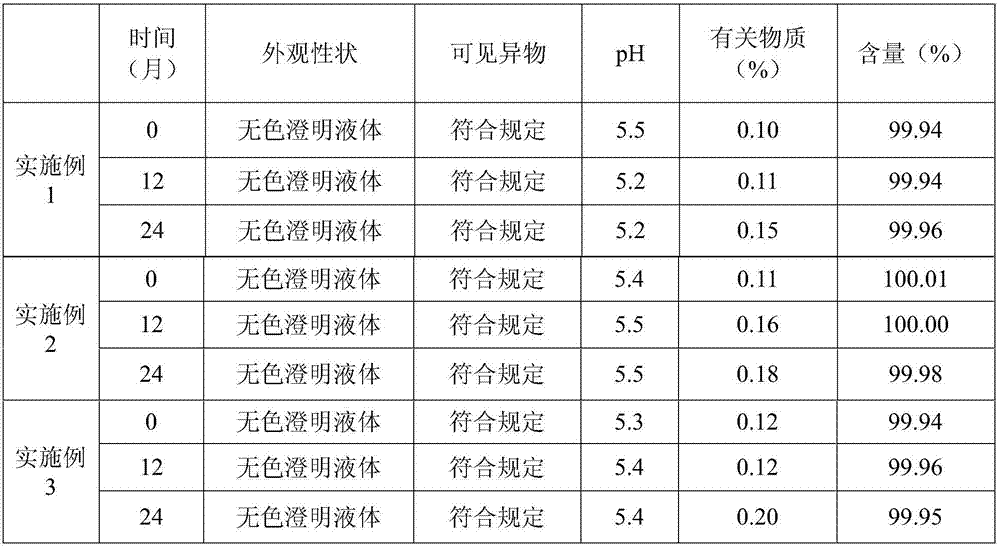
Compound recipe formula containing kurarinone prostaglandin E1 and aspirin, its preparation method and application
InactiveCN1415301ANew formulaExact therapeutic effectSalicyclic acid active ingredientsDigestive systemIsoprostaglandin E1Freeze-drying
A compound medicine containing kurarinol, prostaglandic E1 and aspirin is prepared through including the kurarinol and prostaglandin E1 by 6-0-malto-beta-cyclodextrin, mixing, adding others, and preparing the freeze dried powder injection. It can be used for treating cancers, cardiovascular and cerebrovascular diseases and hepatitis. Its advantages are sure curative effect, and no toxic by-effect.
Owner:蔡海德
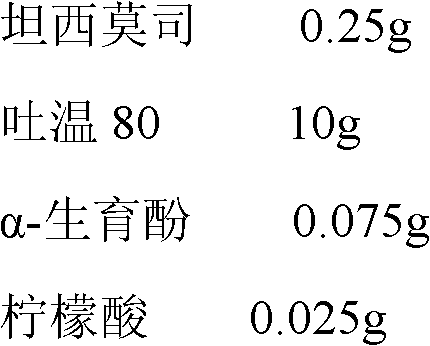
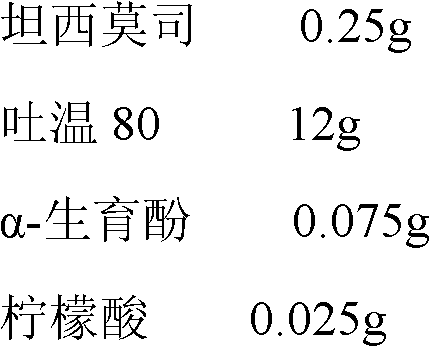
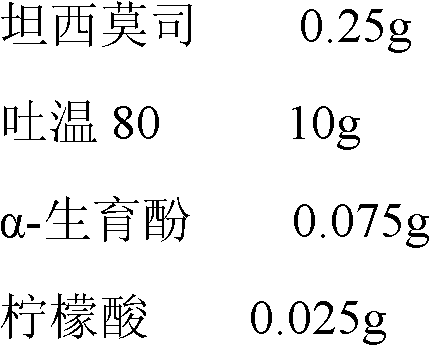
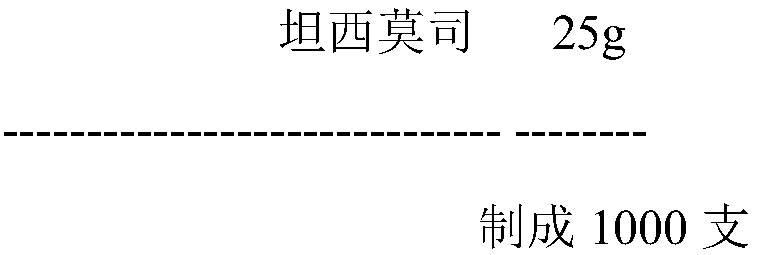
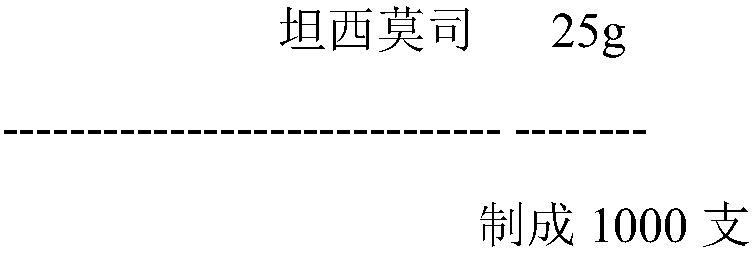
Temsirolimus for injection and preparation method thereof
ActiveCN103099806AAdvantages and Notable ImprovementsImprove stabilityOrganic active ingredientsAntineoplastic agentsAlcoholFreeze-drying
The invention belongs to the technical field of pharmaceutical preparations, and concretely relates to temsirolimus for injection and a preparation method thereof. The preparation method comprises dissolving a prescribed amount of temsirolimus and an anti-oxidant in anhydrous alcohol, mixing uniformly, adding a dispersant, re-mixing uniformly, freeze drying and removing the ethanol to obtain the temsirolimus. The preparation provided by the invention is few in prescription component; and the preparation method is convenient and easy, improves disadvantages of complex technology in present prescription, and greatly minimizes components such as alcohols and the like that may cause injection pain, thereby being relatively safe and reliable.
Owner:SHANDONG NEWTIME PHARMA
Motherwort injection
ActiveCN1973855ANot easy to removeThe content of effective components decreasedComponent separationPharmaceutical delivery mechanismHplc fingerprintMotherwort
The present invention relates to high quality motherwort injection and its preparation process. The present invention proposes the HPLC fingerprint standard, and has determined characteristic matters in motherwort injection, optimized technological process, raised injection consistency and high and stable product quality.
Owner:CHENGDU FIRST PHARMACEDTICAL CO LTD
Compound ceftiofur suspension emulsion injection and preparation method thereof
InactiveCN101953889AReduce viscosityReduce usageAntibacterial agentsOrganic active ingredientsEmulsionAntioxidant
The invention discloses a compound ceftiofur suspension emulsion injection, which comprises the following components in percentage by mass: 1.5 to 15 percent of ceftiofur hydrochloride, 1.5 to 15 percent of magnolia flower oil, 1 to 10 percent of surfactant, 3 to 30 percent of oil for injection, 0.1 to 1 percent of antioxidant, 0.01 to 0.1 percent of thickening agent, and the balance of water for injection, wherein the sum of the mass percents of the components is 100 percent. The compound ceftiofur suspension emulsion injection has simple operation for the preparation method, strong antibacterial property and lasting pharmacological effect, and avoids stresses caused by multi-time administration of conventional formulations to animals; furthermore, the preparation cost is much lower than that of an oil suspension, and the compound ceftiofur suspension emulsion injection has a very high popularization and application value.
Owner:NORTHWEST A & F UNIV
Salmon calcitonin nano liposome injection and preparation method thereof
InactiveCN102327239ASmall toxicityReduce dosagePowder deliveryNervous disorderRecombinant salmon calcitoninMedicine
The invention relates to the technical field of medicine, and discloses a cloxacillin sodium liposome preparation for injection and a preparation method thereof. The cloxacillin sodium liposome preparation is a powder injection, and is a sterile nano freeze-drying preparation which is prepared by coating salmon calcitonin with a pharmaceutically-acceptable biological carrier. The cloxacillin sodium liposome preparation comprises the following raw materials in parts by weight: 1 part of salmon calcitonin, 4-15 parts of pharmaceutically-acceptable biological carrier, 0.5-2 parts of stabilizer and 2-10 parts of freeze-drying protective agent. The product disclosed by the invention has high stability and a low side effect, can not be cracked as a result of dehydration, fusion, generation of ice crystals and the like in the freeze-drying process, and can keep a good entrapment rate after hydration and refusion, thereby facilitating the transportation and storage of the product.
Owner:傅苗青
Pharmaceutical Formulations and Methods of Making the Same
InactiveUS20180110856A1Reduce injection painConvenient storagePeptide/protein ingredientsAntibody mimetics/scaffoldsDrugPharmaceutical formulation
The invention relates to the formulation of pharmaceutical compositions of etanercept. The invention also relates to methods of removing buffer and of formulating pharmaceutical compositions of etanercept.
Owner:AMGEN INC
Compound isopropyl phenol injection contg. local anesthetic and prepn. method therefor
InactiveCN1903187ADefinite curative effectMature technologyHydroxy compound active ingredientsPharmaceutical delivery mechanismRopivacainePhenols
A compound isopropylphenol injection containing local anesthetic contains proportionally isopropylphenol, the local anesthetic chosen from procaine, lidocaine, etc, the refined plant oil for injection, emulsifier, isotonic regulator, antioxidant, pH regulator and the water for injection. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Medicinal composition and preparation method and application thereof
ActiveCN109984999ANon-irritatingHigh solubilizing abilityOrganic active ingredientsNervous disorderWater insolublePolyethylene glycol
The invention provides a medicinal composition. The composition consists of a water-insoluble antipsychotic, polyethylene glycol fatty acid ester, sorbitan ester and an aqueous intermedium; the medicinal composition forms an aqueous and injectable suspension. The water-insoluble antipsychotic drug is an aripiprazole prodrug, namely lauroyl aripiprazole, and the medicinal composition which is proper in particle size and is prepared according to a specific prescription proportion is used for preparing a clinical suspension injection which has high stability, meets the requirements for the release degree and particularly has high safety and an extremely high application value.
Owner:重庆仁泽医药科技有限公司
A kind of stable flurbiprofen axetil micro-nano emulsion and preparation method thereof
ActiveCN104188905BImprove stabilityHigh dependenceOrganic active ingredientsAntipyreticMicro nanoPolyethylene glycol
The invention relates to a stable flurbiprofen axetil micro-nano emulsion and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. A stable flurbiprofen axetil micro-nano emulsion of the present invention contains flurbiprofen axetil, vegetable oil as a solvent, lecithin as a surfactant, polyethylene glycol derivatives, etc., has good stability, Quick effect, low toxicity and side effects.
Owner:HEBEI YIPIN PHARMA
Tissue injection for improving aging
PendingCN113521258APromote Migratory GrowthWrinkles become lighter or disappearOrganic active ingredientsDipeptide ingredientsAutologous tissueNutrition
The invention discloses a tissue injection for improving aging. The tissue injection comprises the following substances, by mass concentration: 5 to 10mg / ml of collagen, 4 to 8 mg / ml of sodium hyaluronate, 5 to 10 mg / ml of tranexamic acid, 10 to 30 mg / ml of glutathione, 2 to 4 mg / ml of L-carnosine, 0.1 to 0.2 mg / ml of glycine, 0.2 to 0.4 mg / ml of proline, 5 to 10 mg / ml oflidocaine, and 9mg / ml of sodium chloride. The collagen and the sodium hyaluronate are added, so that protein and hyaluronic acid lost by an organism can be quickly supplemented, tissue lacuna is formed to stimulate autologous tissue hyperplasia, plump tissue of an injection part is promoted, the L-carnosine, the tranexamic acid and the glutathione have anti-inflammatory and anti-oxidation functions, the adaptability of skin to the environment is improved, and the chromatosis is reduced. The skin is healthier and shiny. And the glycine and the proline increase protein synthesis nutrition, so that nutrition can be provided for skin tissue cells, and the beautifying effect is more durable. In the injection process, due to the fact that lidocaine is added, injection pain and discomfort can be reduced.
Owner:北京美神煦氰美啦医疗美容门诊部有限公司
Pharmaceutical formulations and methods of making the same
ActiveUS20180256718A1Easy to storeLessen the pain of injectionsPeptide/protein ingredientsAntibody mimetics/scaffoldsEtanerceptPharmaceutical formulation
The invention relates to the formulation of pharmaceutical compositions of etanercept. The invention also relates to methods of removing buffer and of formulating pharmaceutical compositions of etanercept.
Owner:AMGEN INC
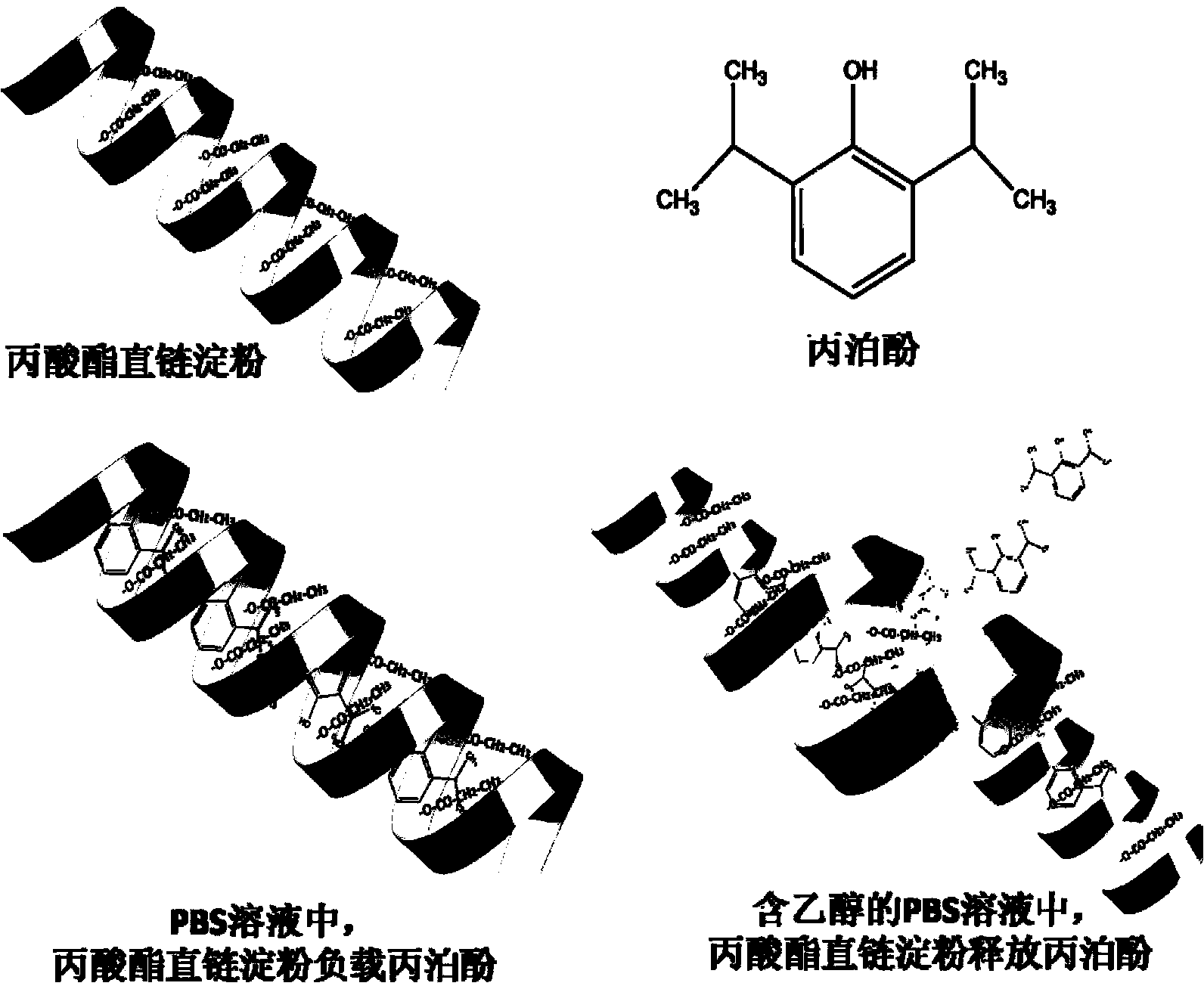
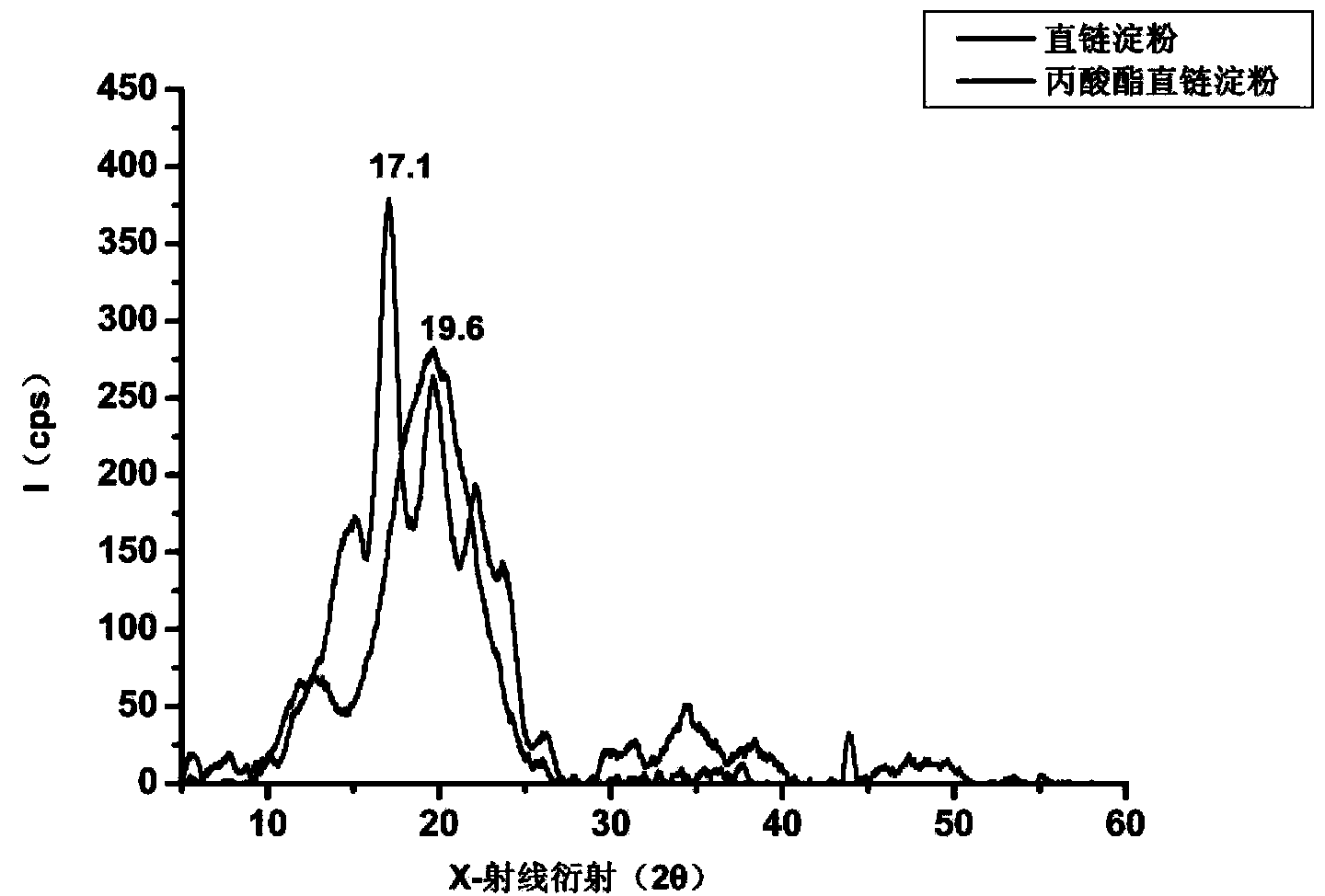
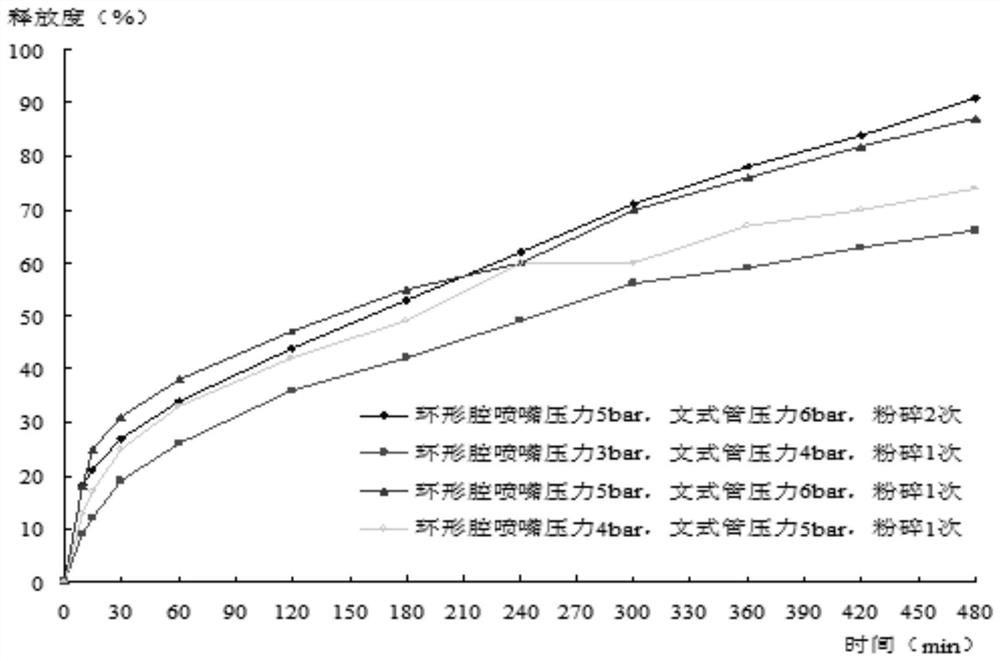
Modified amylase-based nanometer helical oil phase-triggered quick-release material and preparation method thereof
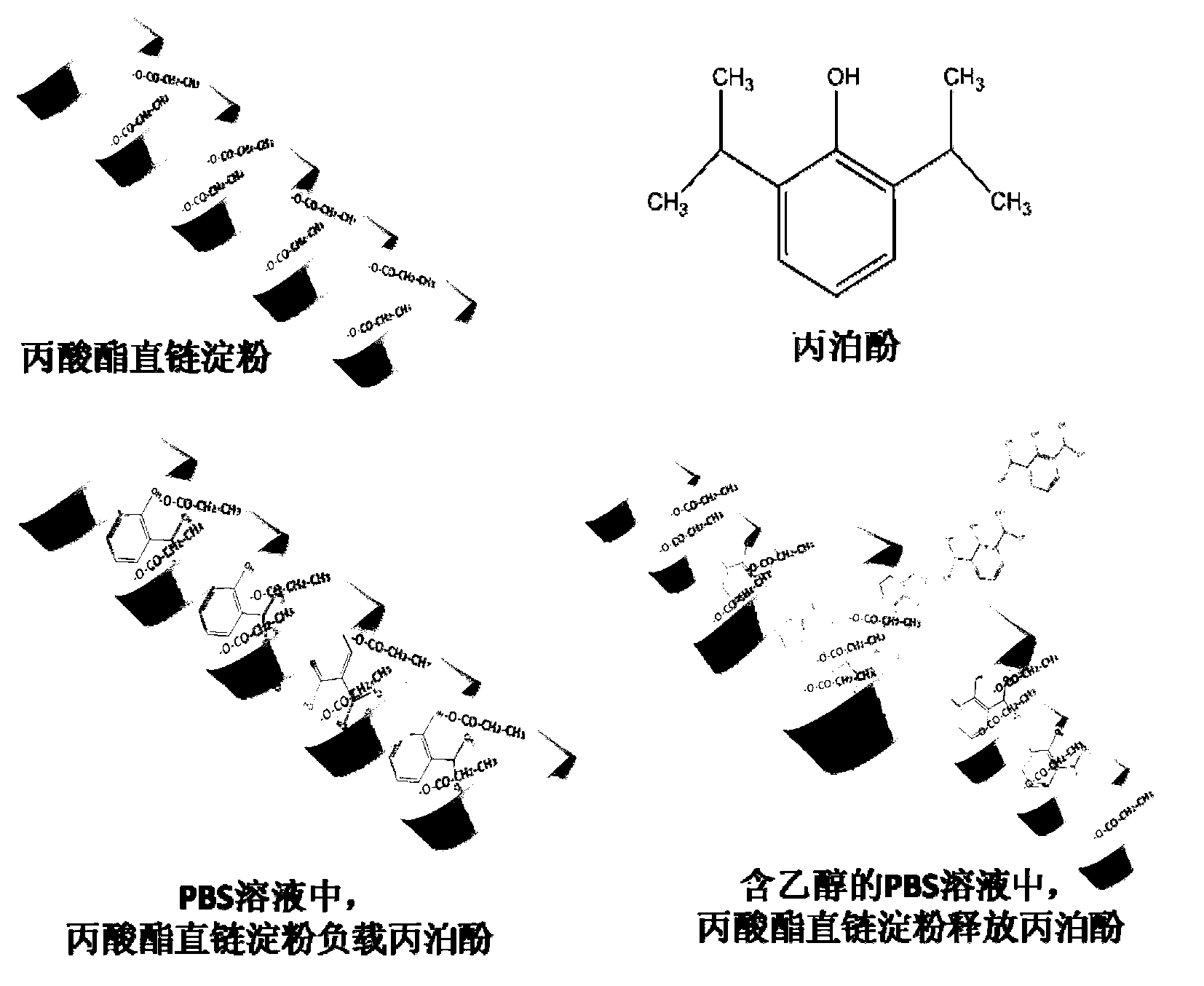
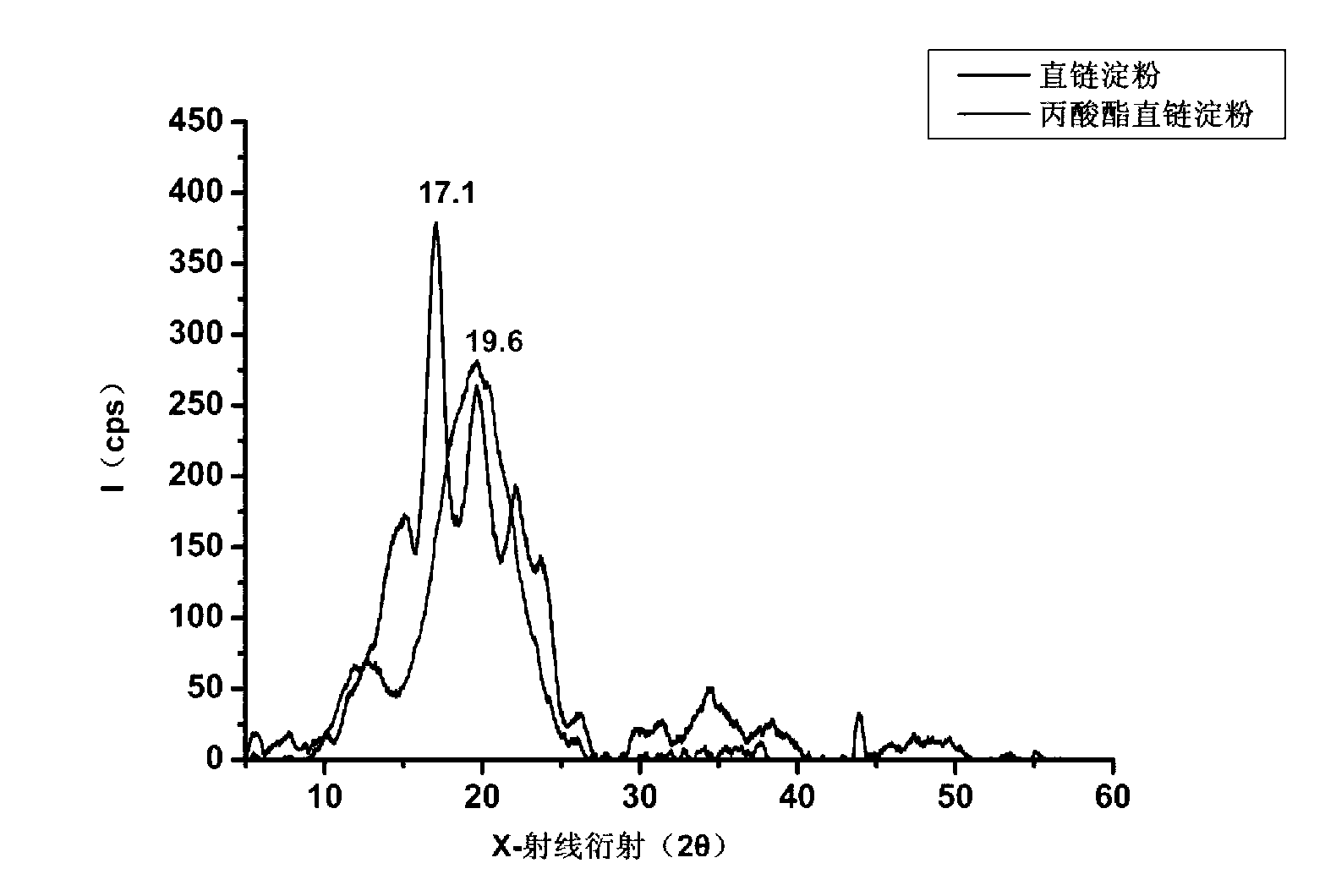
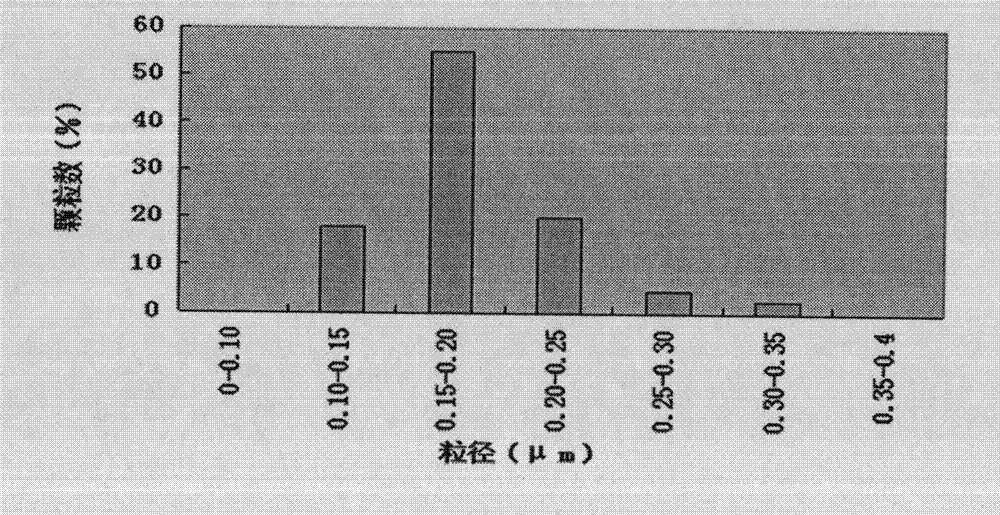
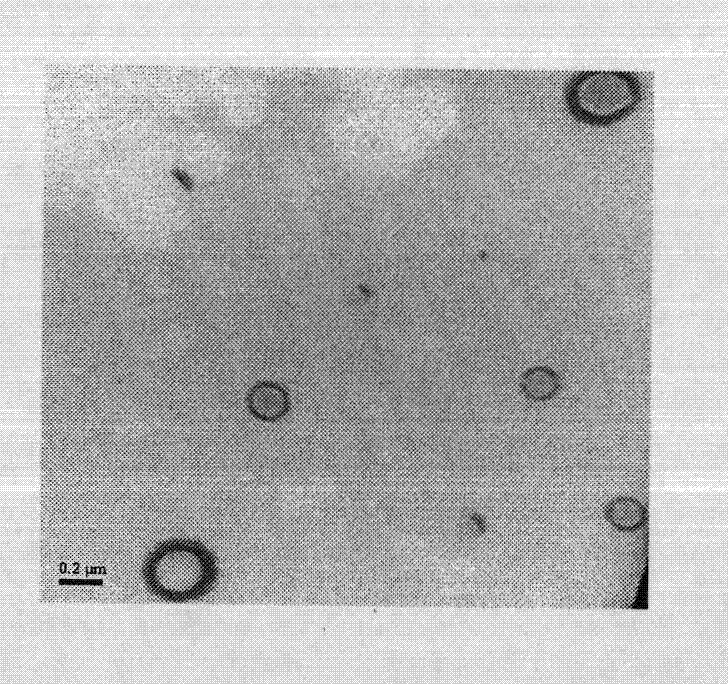
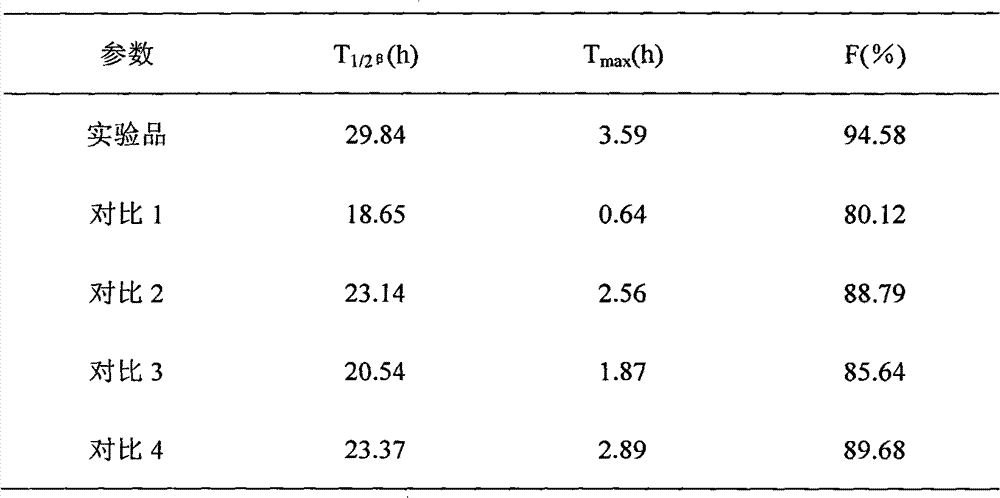
InactiveCN103222950APromote formationEasy maintenanceHydroxy compound active ingredientsPharmaceutical delivery mechanismAmylasePropofol Injection
The invention discloses a modified amylase-based nanometer helical oil phase-triggered quick-release material and a preparation method thereof. The preparation method comprises that amylase which can form a helical structure is modified, then is coated with hydrophobic vein center action drugs such as propofol and forms a helical brain localization quick-release system. The helical brain localization quick-release system has good dispersibility in a water phase, has uniform particle sizes, has a stable particle size for a long time, does not leak propofol easily, can be stored easily, and reduces propofol injection pain. After being triggered by the oil phase, the modified amylase-based nanometer helical oil phase-triggered quick-release material can fast release propofol and is conducive to fast release of propofol at the blood-brain barrier. The preparation method has simple processes, adopts the nontoxic raw materials, does not produce toxic substances in production, carries out disinfection easily and can be industrialized easily.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV +1
Brucellosis vaccine needle-free injection system and application
InactiveCN105999253APrecision injectionFast injectionAntibacterial agentsBacterial antigen ingredientsAntigenImmune effects
The invention discloses a brucellosis vaccine needle-free injection system and application. The brucellosis vaccine needle-free injection system comprises a container filled with a brucellosis vaccine and a readable carrier. Content recorded in the readable carrier includes that needle-free injection dosage of the brucellosis vaccine is not larger than the standard dosage of the brucellosis vaccine. Experiments prove that when a needle-free injection ampoule and a needle-free syringe are combined and used for immunization, the antigen dosage only accounts for part of a traditional intramuscular injection antigen dosage, and the same or better immune effect can be achieved. It is further indicated that a needle-free immune mode is an ideal strategy capable of being selected by the brucellosis immunization vaccine.
Owner:INNER MONGOLIA HUAXI BIOTECH
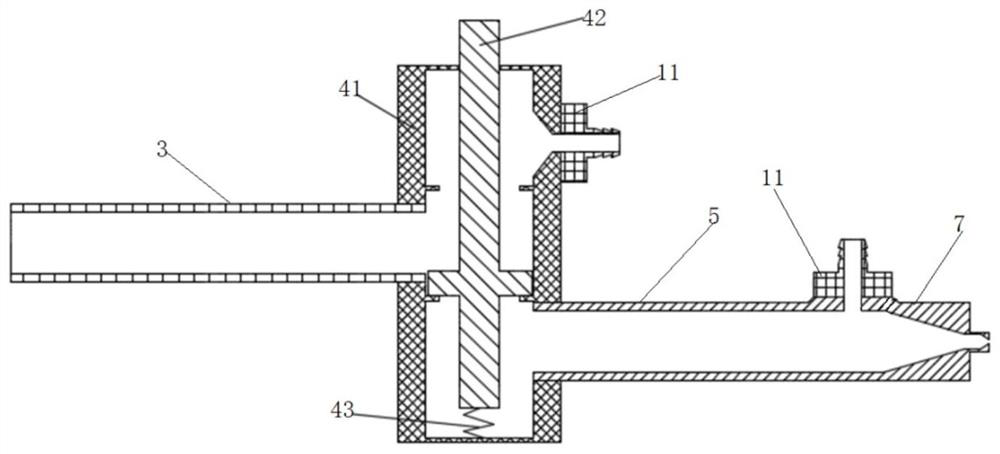
Split type multi-needle-tube injection device
PendingCN111467611AImprove assembly efficiencyGap refinementInfusion syringesInfusion needlesEngineeringBiomedical engineering
The invention relates to the technical field of medical cosmetic instruments, in particular to a split type multi-needle-tube injection device, wherein a base and a needle table are designed in a split mode, a containing groove is formed in one end of the base, the needle table is inserted into the containing groove and packaged and fixed to the containing groove through glue, a plurality of parallel needle grooves are formed in the side face of the needle table, needle tubes are fixed in the needle grooves, and the other end of the base communicates with an injector which communicates with the needle tubes. Compared with the prior art, the split type multi-needle-tube injection device has the advantages that by means of the split type design of the base and the needle table, the mode thatneedle tubes are directly and vertically inserted and assembled in a traditional process is changed, needle tube assembling efficiency can be greatly improved, and assembling precision can be improved; gaps between the needle tubes can be finer, the distance between the needle tubes is shortened, and injection pain is relieved; the number of the needle tubes can be adjusted according to the number of the needle grooves, injection devices with different requirements for the number of the needle tubes in different use scenes can be produced as required, production is flexible, flexibility is achieved easily, and product applicability is improved.
Owner:深圳中科精艺设计有限公司
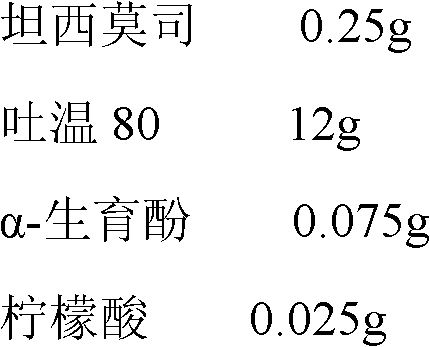
Temsirolimus for injection and preparation method thereof
ActiveCN103099806BAdvantages and Notable ImprovementsImprove stabilityOrganic active ingredientsAntineoplastic agentsAlcoholAntioxidant
Owner:SHANDONG NEWTIME PHARMA
A kind of vecuronium bromide freeze-dried preparation and preparation method thereof
ActiveCN101843593BEffective and adequateImprove solubilityPowder deliveryOrganic active ingredientsSide effectFreeze-drying
The invention discloses a novel vecuronium bromide freeze-dried preparation and a preparation method thereof. The novel preparation comprises the main components of vecuronium bromide, a freeze-dried excipient, polyethylene glycol playing a role in re-dissolving assistance, vitamin E playing a role in anti-oxidation, and glycerol serving as an isotonic regulator. According to the formula, the vecuronium bromide is developed into the novel freeze-dried preparation, and the process is simple. The novel vecuronium bromide freeze-dried preparation can improve the pH value of re-dissolved preparation solution to make the pH value reach or be close to the physiological pH range of blood, avoids or reduces stimulations caused by the preparation to a blood vessel, alleviates injection pains, and simultaneously can ensure that a medicament compound is fully dissolved to prevent insoluble substances from causing side effect injuries to a body.
Owner:XIAN LIBANG PHARMA
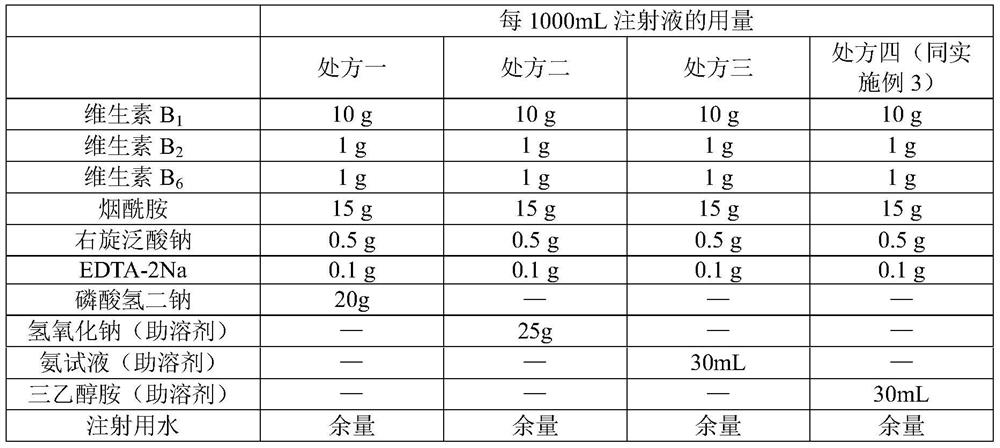
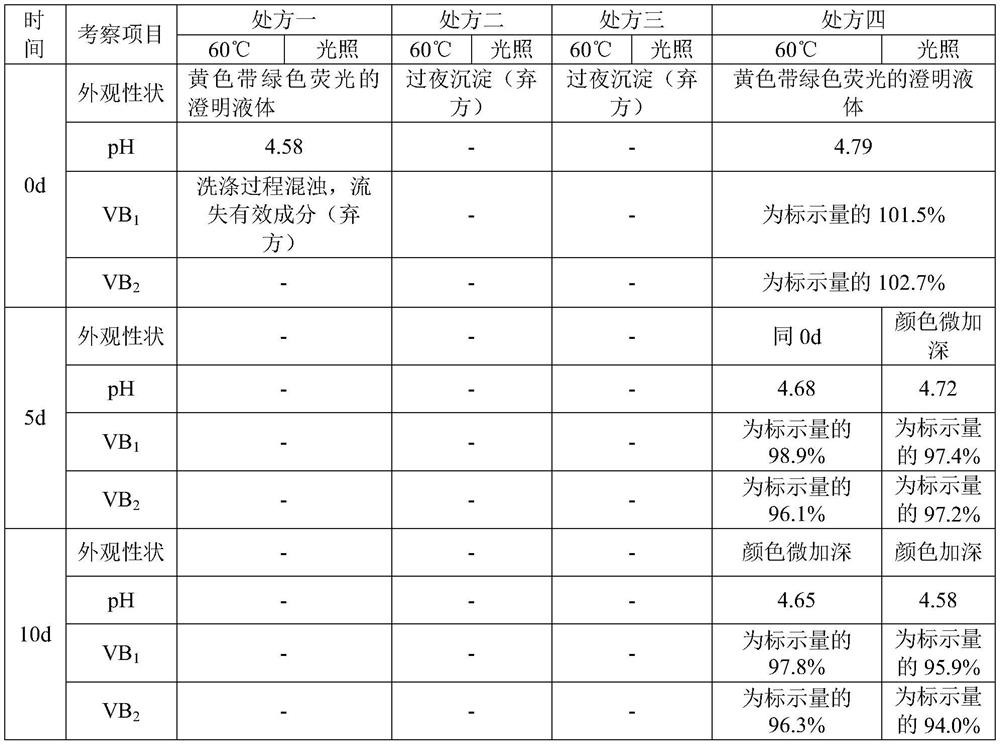
Compound vitamin B injection and preparation method thereof
ActiveCN110251461BIncrease the amount of feedLow costMetabolism disorderPharmaceutical delivery mechanismBiotechnologyVITAMIN B COMPLEX INJECTION
The compound vitamin B injection of the present invention is composed of 9.8-10.02g vitamin B per 1000mL 1 , 0.98~1.02g Vitamin B 2 , 0.98~1.02g Vitamin B 6 , 14.7~15.3g nicotinamide, 0.49~0.51g sodium d-pantothenate, 0.05~0.15g EDTA-2Na, 20~40mL triethanolamine, and the balance is water for injection. Specifically, add triethanolamine to 60% of the prescription water for injection, add vitamin B 2 , to get solution one; turn vitamin B 1 , Vitamin B 6 , Niacinamide, and D-pantothenate sodium are added to 20% of the prescribed amount of water for injection to obtain solution 2; mix solution 2 and solution 1 and adjust the pH value to 3.8-5.2, and add the remaining amount of water for injection; filter, fill, Sterilize. The compound vitamin B injection prepared by the invention has low raw material cost, high efficiency, stable quality and meets the quality standard of veterinary drugs.
Owner:河南益华动物药业有限公司
Compound ceftiofur suspension emulsion injection and preparation method thereof
InactiveCN101953889BReduce usageReduce manufacturing costAntibacterial agentsOrganic active ingredientsEmulsionAntioxidant
The invention discloses a compound ceftiofur suspension emulsion injection, which comprises the following components in percentage by mass: 1.5 to 15 percent of ceftiofur hydrochloride, 1.5 to 15 percent of magnolia flower oil, 1 to 10 percent of surfactant, 3 to 30 percent of oil for injection, 0.1 to 1 percent of antioxidant, 0.01 to 0.1 percent of thickening agent, and the balance of water for injection, wherein the sum of the mass percents of the components is 100 percent. The compound ceftiofur suspension emulsion injection has simple operation for the preparation method, strong antibacterial property and lasting pharmacological effect, and avoids stresses caused by multi-time administration of conventional formulations to animals; furthermore, the preparation cost is much lower than that of an oil suspension, and the compound ceftiofur suspension emulsion injection has a very high popularization and application value.
Owner:NORTHWEST A & F UNIV
A modified amylose-based nanohelical oil-phase triggered immediate-release body and its preparation method
InactiveCN103222950BPromote formationEasy maintenanceHydroxy compound active ingredientsPharmaceutical delivery mechanismAmylaseImmediate release
The invention discloses a modified amylase-based nanometer helical oil phase-triggered quick-release material and a preparation method thereof. The preparation method comprises that amylase which can form a helical structure is modified, then is coated with hydrophobic vein center action drugs such as propofol and forms a helical brain localization quick-release system. The helical brain localization quick-release system has good dispersibility in a water phase, has uniform particle sizes, has a stable particle size for a long time, does not leak propofol easily, can be stored easily, and reduces propofol injection pain. After being triggered by the oil phase, the modified amylase-based nanometer helical oil phase-triggered quick-release material can fast release propofol and is conducive to fast release of propofol at the blood-brain barrier. The preparation method has simple processes, adopts the nontoxic raw materials, does not produce toxic substances in production, carries out disinfection easily and can be industrialized easily.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV +1
Double-channel needle-free injection device and method with controllable jet velocity
ActiveCN112412733BPrevent splashIncrease profitJet injection syringesPositive displacement pump componentsNeedle Free InjectionEngineering
The invention discloses a double-channel type needle-free injection device and method with controllable jet velocity. The device includes a start switch, a power source, a liquid cavity, a two-position three-way electromagnetic reversing valve, an infusion tube and a hose; the liquid cavity It is used to store liquid medicine. The inlet and outlet of the liquid chamber are respectively connected to the power source and the inlet port of the two-position three-way electromagnetic reversing valve, and the two outlets of the two-position three-way electromagnetic reversing valve are connected to the inlets of the infusion tube and the hose Connection, the infusion tube is a rigid tube with a nozzle at the outlet, the hose outlet is connected to the front of the infusion tube, the power source is used to push the piston in the liquid chamber to provide power for the injection, and the power source can set the power according to the required jet velocity , the control end of the two-position three-way electromagnetic reversing valve is connected with a time-delay relay, and the start switch is connected with the power source and the time-delay relay respectively. The invention can improve the utilization rate of the medicinal liquid and reduce the risk of injection.
Owner:WUHAN UNIV
Alprostadil injection solution
InactiveCN108653208AImprove stabilityLess irritatingOrganic active ingredientsPeptide/protein ingredientsOctreotide acetateMedicine
The invention provides an alprostadil injection solution, which consists of alprostadil, phospholipid, injection oil and injection water, wherein the contents of the various components are as follows:4-8[mu]g / ml of the alprostadil, 4-20mg / ml of the phospholipid and 100-300mg / ml of the injection oil, wherein the phospholipid consts of phosphatidylcholine PC and phosphatidyl glycerol PG at the ratio of (95.5-99.7) to 0.3, and the phospholipid also consists of tocopherol in terms of the total amount of the phospholipid. The alprostadil injection solution provided by the invention can achieve good compatibility stability with an octreotide acetate injection solution; and it is accidentally discovered that the alprostadil injection solution prepared by the invention can obviously reduce vascular stimulation and injection pain.
Owner:BEIJING LANDAN PHARMA TECH
Compound isopropyl phenol injection containing local anesthetic and preparation method therefor
InactiveCN1903187BDefinite curative effectMature technologyHydroxy compound active ingredientsPharmaceutical delivery mechanismProcainePhenol
A compound isopropylphenol injection containing local anesthetic contains proportionally isopropylphenol, the local anesthetic chosen from procaine, lidocaine, etc, the refined plant oil for injection, emulsifier, isotonic regulator, antioxidant, pH regulator and the water for injection. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Foamless levo oxiracetam injection and preparation method thereof
InactiveCN107303264ANo adhesionLessen the pain of injectionsOrganic active ingredientsNervous disorderPatient complianceAdditive ingredient
The invention discloses a foamless levo oxiracetam injection and a preparation method thereof. 1mL of injection contains the following raw ingredients: 180-220mg of levo oxiracetam, 0.01-0.1mg of edetate disodium, 4-5mg of methylparaben, 10-16mg of meglumine and 1-3mg of benzyl alcohol. The preparation method for the injection comprises the steps of thickening preparation, diluting preparation, filling and sterilizing. The levo oxiracetam injection, which is prepared according to the invention by utilizing the de-foaming function of methylparaben and the fluxing stabilizing function of edetate disodium and meglumine, does not form foams, is free from liquor adhesion problem and is high in product yield. A certain amount of benzyl alcohol is added, so that the injection pain of the patient can be relieved and the patient compliance is high.
Owner:CHONGQING RUNZE PHARM CO LTD
A kind of pharmaceutical composition and its preparation method and application
ActiveCN109984999BNon-irritatingHigh solubilizing abilityOrganic active ingredientsNervous disorderAripiprazole lauroxilPsychosis drug
The invention provides a pharmaceutical composition, comprising the following components: water-insoluble antipsychotic drugs; polyethylene glycol fatty acid esters; sorbitan esters and aqueous vehicles; the pharmaceutical composition formed is aqueous, Injectable suspension. The water-insoluble antipsychotic drug is aripiprazole prodrug—lauroyl aripiprazole, and the pharmaceutical composition prepared by selecting appropriate particle size and specific prescription ratio is prepared to have good stability and meet the requirements of release, especially possesses It is a suspension injection for clinical use with good safety and high application value.
Owner:重庆仁泽医药科技有限公司
Treatment of migraine headache with diffusion of toxin in non-muscle related foraminal sites
ActiveUS20130236444A1Facilitated DiffusionNot improveBacterial antigen ingredientsNervous disorderHeadache severeMigraine
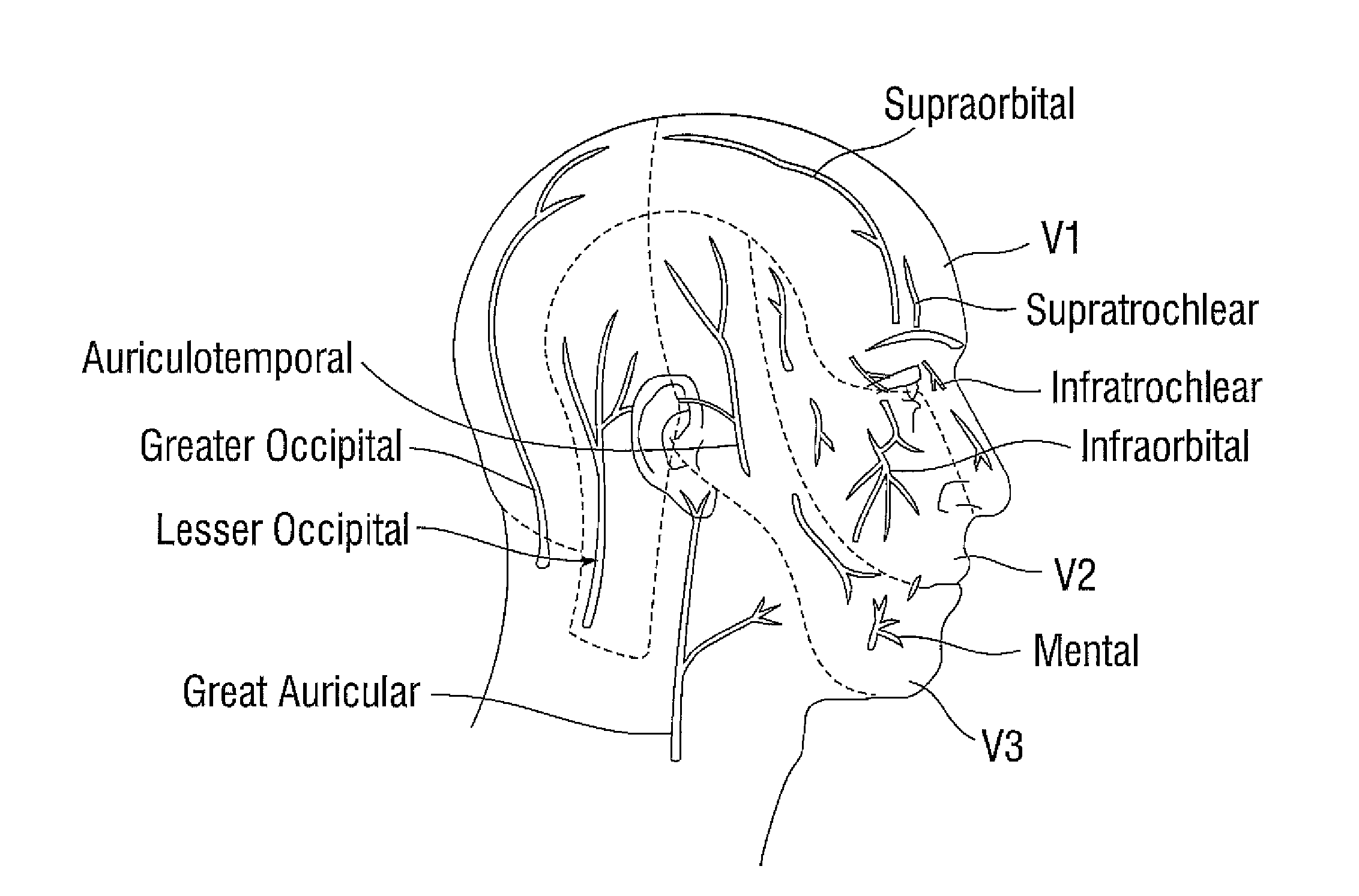
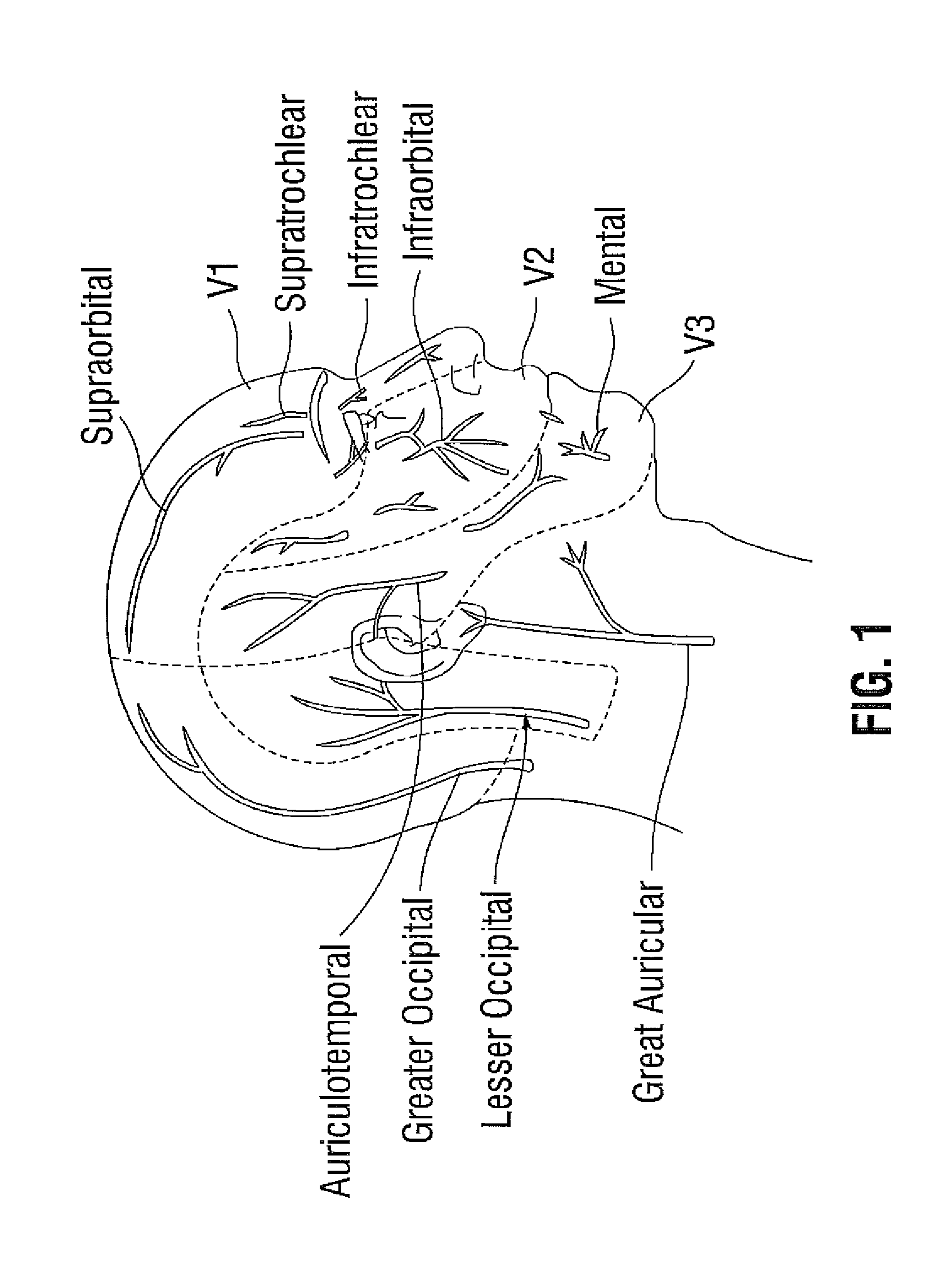
A method for treating a patient with migraine headache includes administering to the patient a therapeutically effective amount of an invertebrate presynaptic neurotoxin in a pharmaceutically safe form. The administration includes extramuscular injection of the neurotoxin to emerging nerve points including foraminal sites for enabling neurotoxin access to concentrated nerve bundles at exit points of the foramina.
Owner:MIOTOX LLC
Temsirolimus for injection and preparation method thereof
PendingCN107773539AAdvantages and Notable ImprovementsImprove stabilityPowder deliveryOrganic active ingredientsAlcoholRoom temperature
The invention belongs to the field of a medicine preparation and relates to temsirolimus for injection and a preparation method thereof. Temsirolimus raw material powder serves as an only component, no additive is added, split charging is conducted directly, and the influence on the product quality by oxygen is avoided by a vacuumizing mode or a mode of backfilling insert gas after vacuumizing. Long-term storage and acceleration experiments of powder injection indicate that the product quality is stable. The preparation has few formula components, the preparation method is convenient and practical, the defect that the existing formula process is complex is improved, components, such as alcohol, capable of bring injection pain easily, are greatly reduced, and safety and reliability are achieved. The most important thing to note is that the product prepared the technology can achieve high stability during preservation at room temperature, and product transportation and clinical use are facilitated.
Owner:LUNAN PHARMA GROUP CORPORATION
Treatment device for medical injector and use method of treatment device
ActiveCN113144347AAvoid slippingAvoid secondary useAmpoule syringesIntravenous devicesBandageBiomedical engineering
The invention discloses a treatment device for a medical injector and a use method of the treatment device, and belongs to the field of medical injections. The treatment device can integrate a piston assembly, a medicine bottle and a needle head, when the piston assembly moves downwards to conduct injection operation, liquid medicine is injected into the body of a patient through extrusion of a film on the medicine bottle, so that it is guaranteed that the liquid medicine does not make contact with the external environment in the whole process of injection operation, and pollution is avoided; meanwhile, when the injection operation is finished, through ordered conversion of space leakproofness and non-leakproofness in the piston assembly, the film is driven to expand outwards, the medicine bottle falls off from the position where the medicine bottle is clamped by the film currently, the clamping relation cannot be recovered, and the needle head is almost impossible to be aligned to the positions of an upper cover and a lower cover of the medicine bottle for the second time, so that the situation that the injector is used for the second time is effectively avoided; and in the process of injection, mutual matching between bandage sending out and bandage receiving is adopted to prevent secondary use.
Owner:山东恒智医创医疗器械科技有限公司
Propofol injection and preparation method thereof
ActiveCN108096187AImprove securitySolve the problem of blood calcium dropHydroxy compound active ingredientsInorganic non-active ingredientsDisodium EdetatePropofol Injection
The invention belongs to the technical field of medicines and particularly relates to a propofol injection and a preparation method of the injection. A formula of the propofol injection comprises an active ingredient: propofol, and auxiliary materials: structured triglyceride, egg yolk lecithin, an isoosmotic adjusting agent, water for injection and sodium hydrogen phosphate. The propofol injection has the characteristics that the propofol injection is free from a metal ion complexing agent, namely edetate disodium; soybean oil is substituted by structured triglyceride in the auxiliary materials; and sodium hydroxide is substituted by sodium hydrogen phosphate to regulate a pH (potential of hydrogen) value. The propofol injection has the advantages of reasonable compatibility, small stimulation and good stability, and has effects that an injection pain feeling of the traditional propofol injection in a clinical application is relieved and an anesthesia effect of the traditional propofol injection is improved. In addition, the encapsulation efficiency of the propofol injection is better after long-time placement, and quality parameters such as a content and relevant substances of the propofol injection comply with quality standards.
Owner:GUANGDONG JIABO PHARM CO LTD
Rabies vaccine needle-free injection system and application
InactiveCN105999259APrevent secondary useAvoid cross contaminationSsRNA viruses negative-senseJet injection syringesNeedle freeNeedle Free Injection
The invention discloses a rabies vaccine needle-free injection system and application. The rabies vaccine needle-free injection system comprises a container filled with a rabies vaccine and a readable carrier. Content recorded in the readable carrier includes that needle-free injection dosage of the rabies vaccine is not larger than the standard dosage of the rabies vaccine. Experiments prove that when a needle-free injection ampoule and a needle-free syringe are combined and used for immunization, the antigen dosage only accounts for part of a traditional intramuscular injection antigen dosage, and the same or higher ineutralizing antibody level can be generated. It is further indicated that a needle-free immune mode is an ideal means capable of being selected by the rabies immunization vaccine.
Owner:CHANGCHUN HAIJIYA BIOTECH CO LTD
Influenza vaccine needle-free injection system and application
InactiveCN105999257ARapid immunizationPrevent secondary useSsRNA viruses negative-senseJet injection syringesNeedle freeNeedle Free Injection
The invention discloses an influenza vaccine needle-free injection system and application. The influenza vaccine needle-free injection system comprises a needle-free injection ampoule filled with a vaccine which is monovalent or multivalent, and the HA content of each vaccine in monovalent or multivalent vaccines is 3-15 microgramme. Experiments prove that when the influenza vaccine needle-free injection system is used for immunization, the antigen dosage only accounts for part of a traditional intramuscular injection antigen dosage, and the same or higher HI titer can be generated. It is further indicated that a needle-free immune means is an ideal means capable of being selected by the influenza immunization vaccine.
Owner:CHANGCHUN HAIJIYA BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com