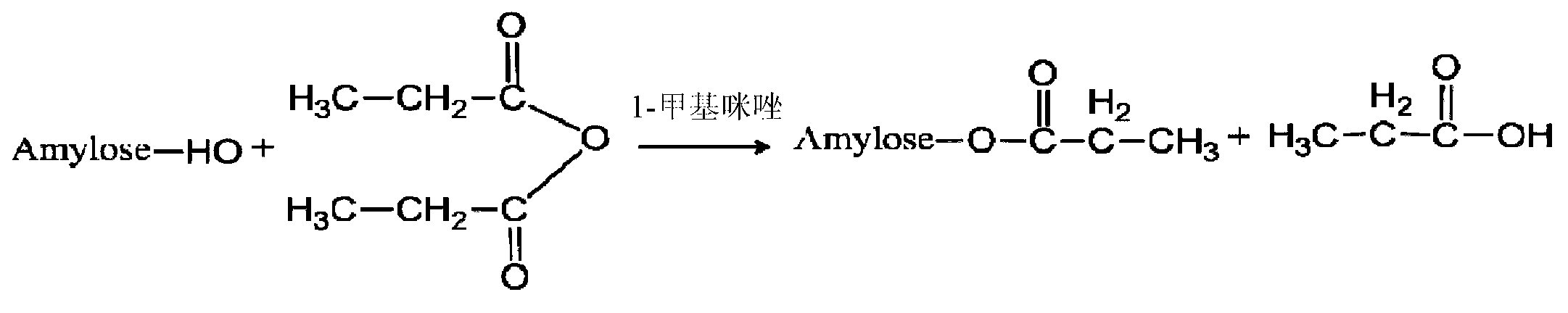Modified amylase-based nanometer helical oil phase-triggered quick-release material and preparation method thereof
A technology of amylose and oil phase, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as stimulating dilated blood vessels, unfavorable blood pressure, hyperlipidemia, etc. To achieve the effect of promoting formation and maintenance, improving hydrophilic performance and improving lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
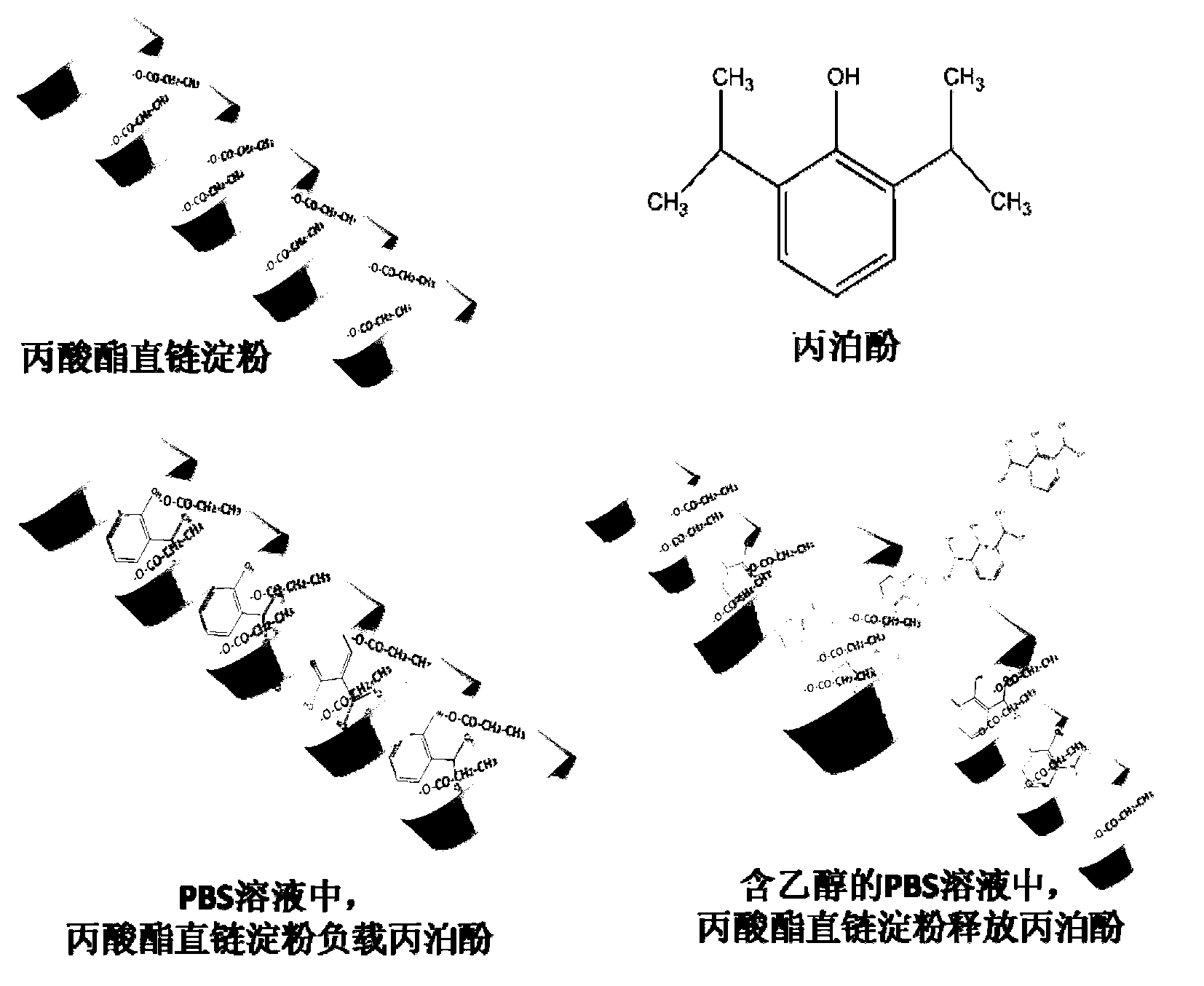
[0037] see figure 2 , the preparation method of the nano-helical oil phase triggered immediate-release body based on modified amylose, comprising the following steps:
[0038] 1) Preparation of propionate amylose
[0039] Dissolve 1 g of amylose in 50 mL of DMSO solution at 80 °C, stir for 1 h, add 0.3 mL of propionic anhydride and 0.2 mL of 1-methylimidazole, react at 80 °C for 4 h, and collect amylose propionate by suction filtration with acetone precipitation.
[0040] Identification of the propionate amylose helical structure:
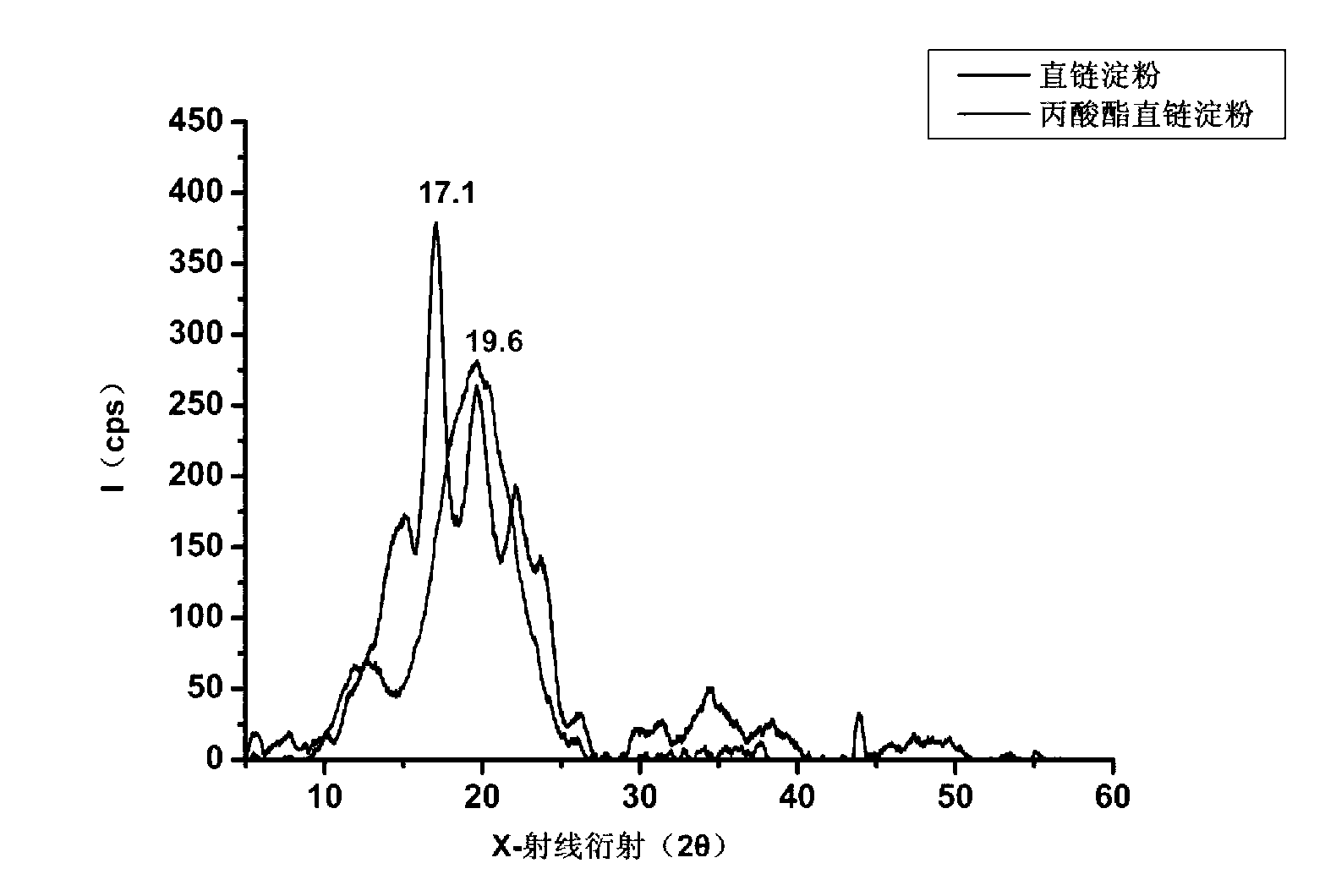
[0041] Such as image 3 X-ray diffraction patterns of amylose and propionate amylose are shown. The characteristic peaks of amylose are 14.9, 17.1, 19.6, 22.1, 23.7, etc., which are characteristic peaks of B-type starch crystallization, that is, loose crystallization; while the characteristic peaks of propionate amylose are 7.8, 12.7, 19.6, It is the characteristic peak of V-type starch crystallization, that is, helical crystallization.
[00...
Embodiment 2
[0045] The preparation method of the nano-helical oil phase triggered immediate-release body based on modified amylose comprises the following steps:
[0046] 1) Preparation of propionate amylose
[0047] Dissolve 1 g of amylose in 50 mL of DMSO solution at 60 °C, stir for 1 h, add 0.2 mL of propionic anhydride and 0.1 mL of 1-methylimidazole, react at 60 °C for 8 h, and collect amylose propionate by suction filtration with acetone precipitation.
[0048] 2) Preparation of Propofol-supported Amylose Propofol Nanohelix Immediate-release Body
[0049] Disperse 20 mg of amylose propionate in 20 mL of pH7.4 PBS solution, drop 100 mg of propofol into the solution, and stir magnetically for 15 min. The starch solution loaded with propofol was poured into a dialysis bag with a pore size of 5000D, placed in 1L of PBS solution for dialysis for 2 hours, and the dialysate was changed twice in the middle to remove free propofol. Aspirate the liquid in the dialysis bag, filter it with a 20...
Embodiment 3
[0051] The preparation method of the nano-helical oil phase triggered immediate-release body based on modified amylose comprises the following steps:
[0052] 1) Preparation of propionate amylose
[0053] Dissolve 1 g of amylose in 50 mL of DMSO solution at 90 °C, stir for 1 h, add 0.3 mL of propionic anhydride and 0.15 mL of 1-methylimidazole, react at 90 °C for 3 h, and collect amylose propionate by suction filtration with acetone precipitation.
[0054] 2) Preparation of Propofol-supported Amylose Propofol Nanohelix Immediate-release Body
[0055] Disperse 20 mg of amylose propionate in 20 mL of pH7.4 PBS solution, drop 80 mg of propofol into the solution, and stir magnetically for 15 min. The starch solution loaded with propofol was poured into a dialysis bag with a pore size of 5000D, placed in 1L of PBS solution for dialysis for 2 hours, and the dialysate was changed twice in the middle to remove free propofol. Aspirate the liquid in the dialysis bag, filter it with a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com