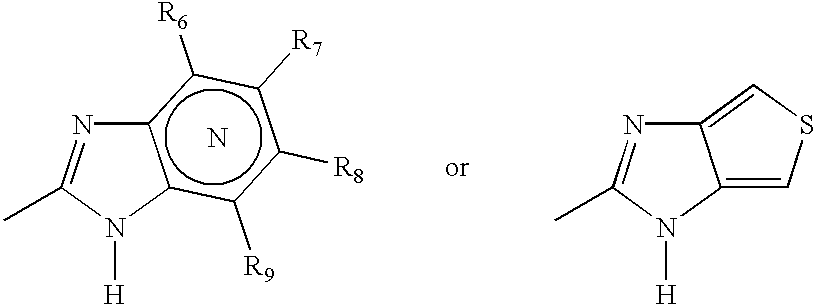
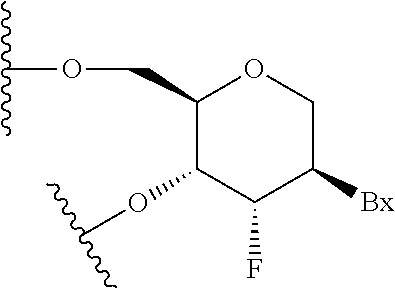
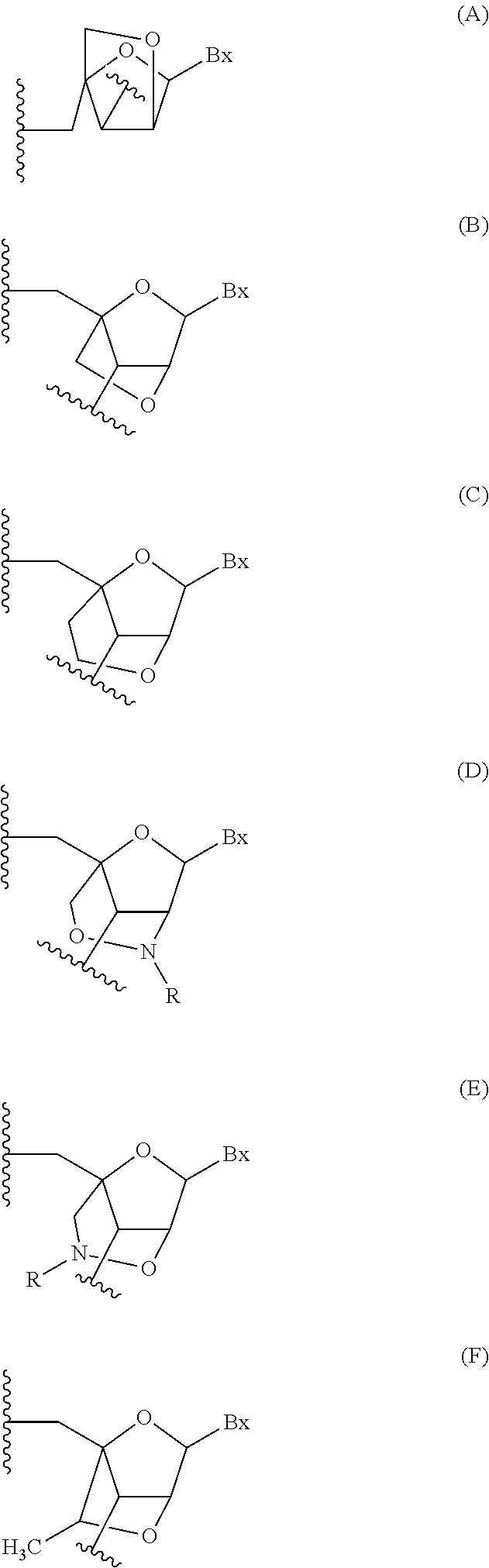
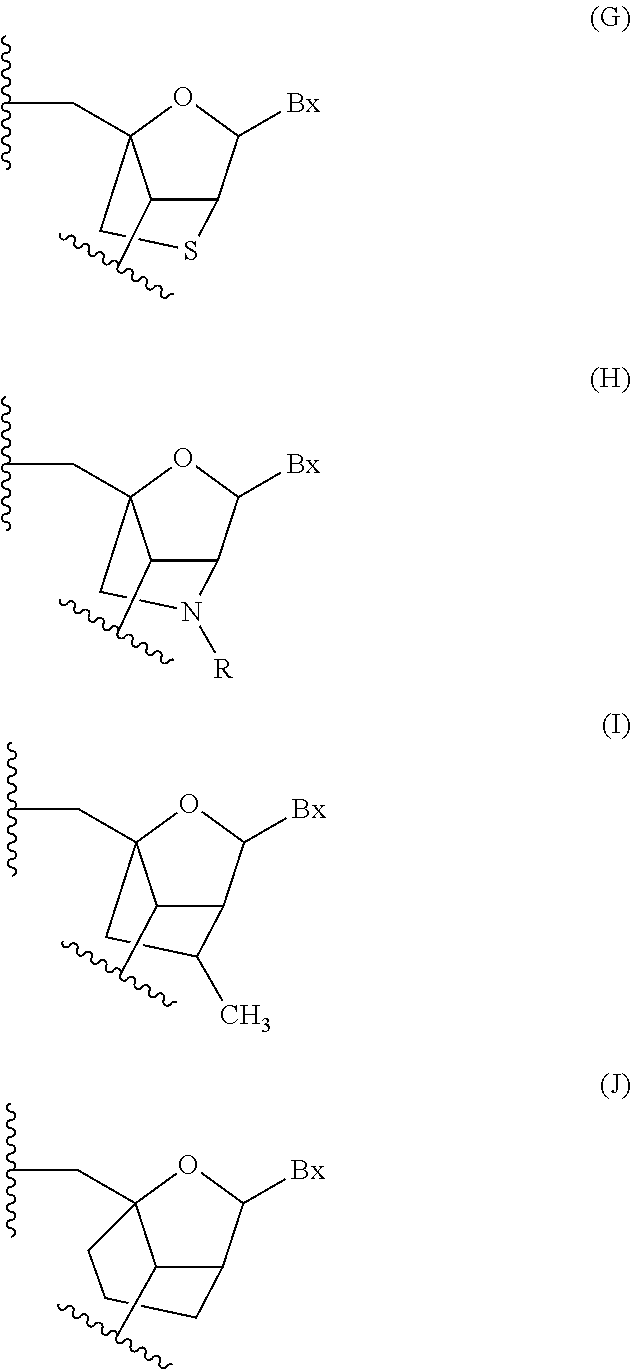
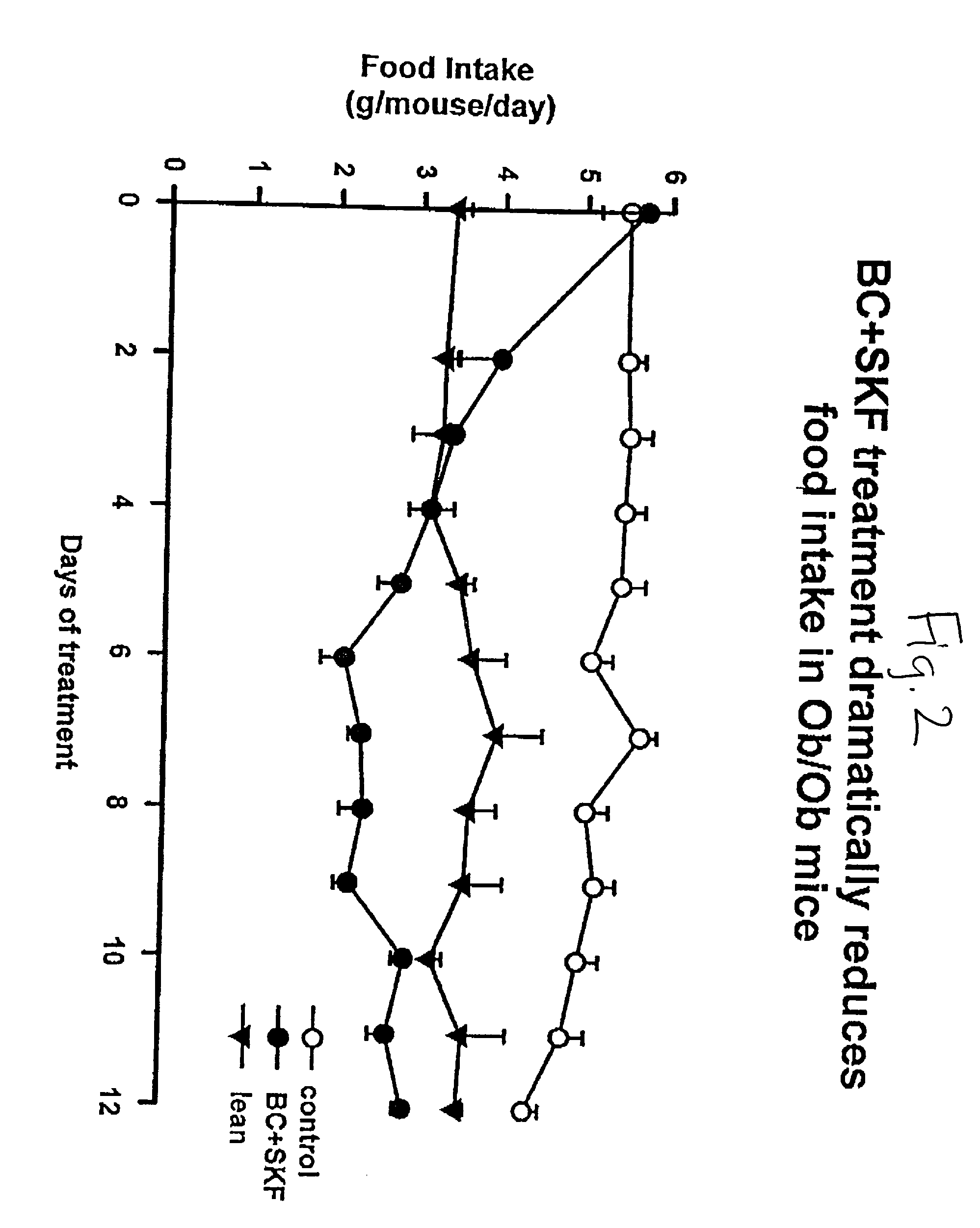
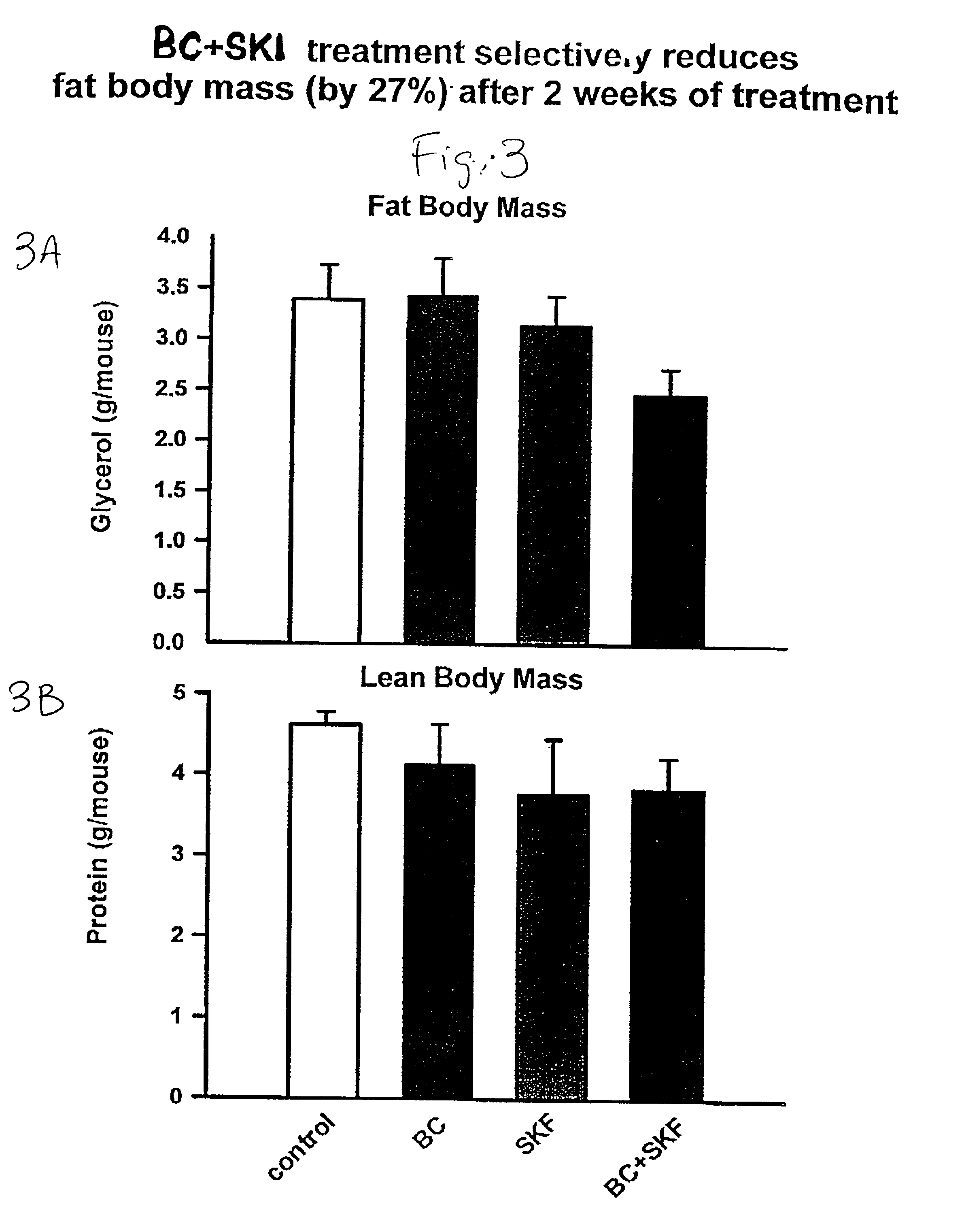
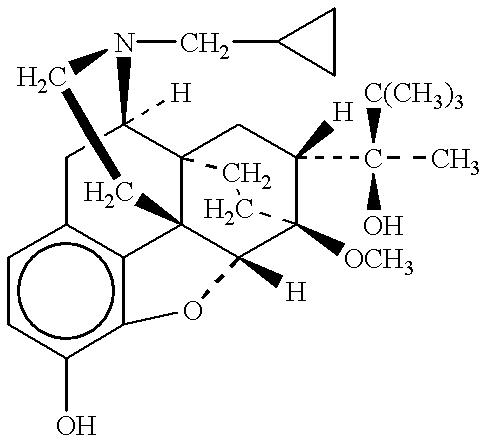
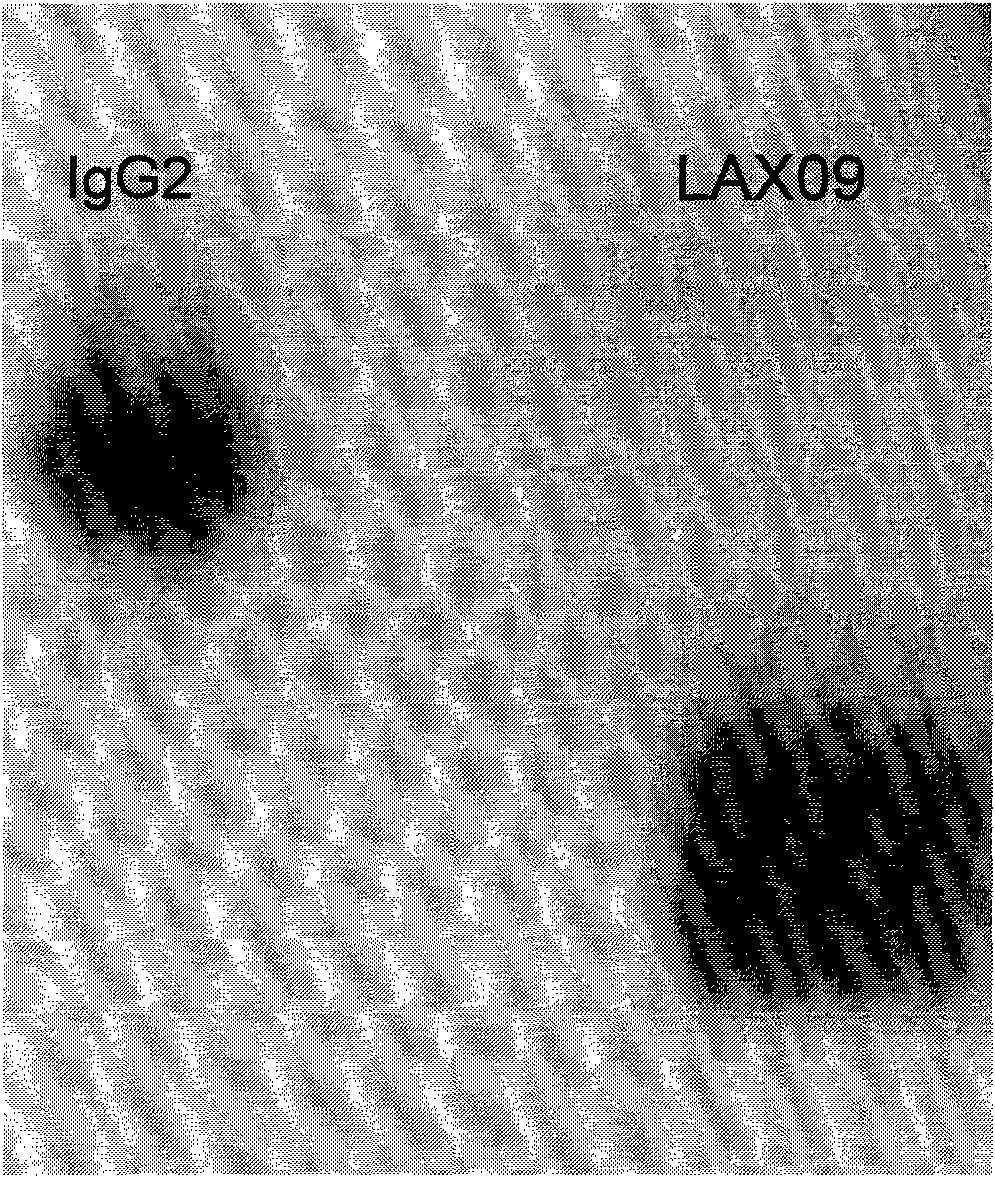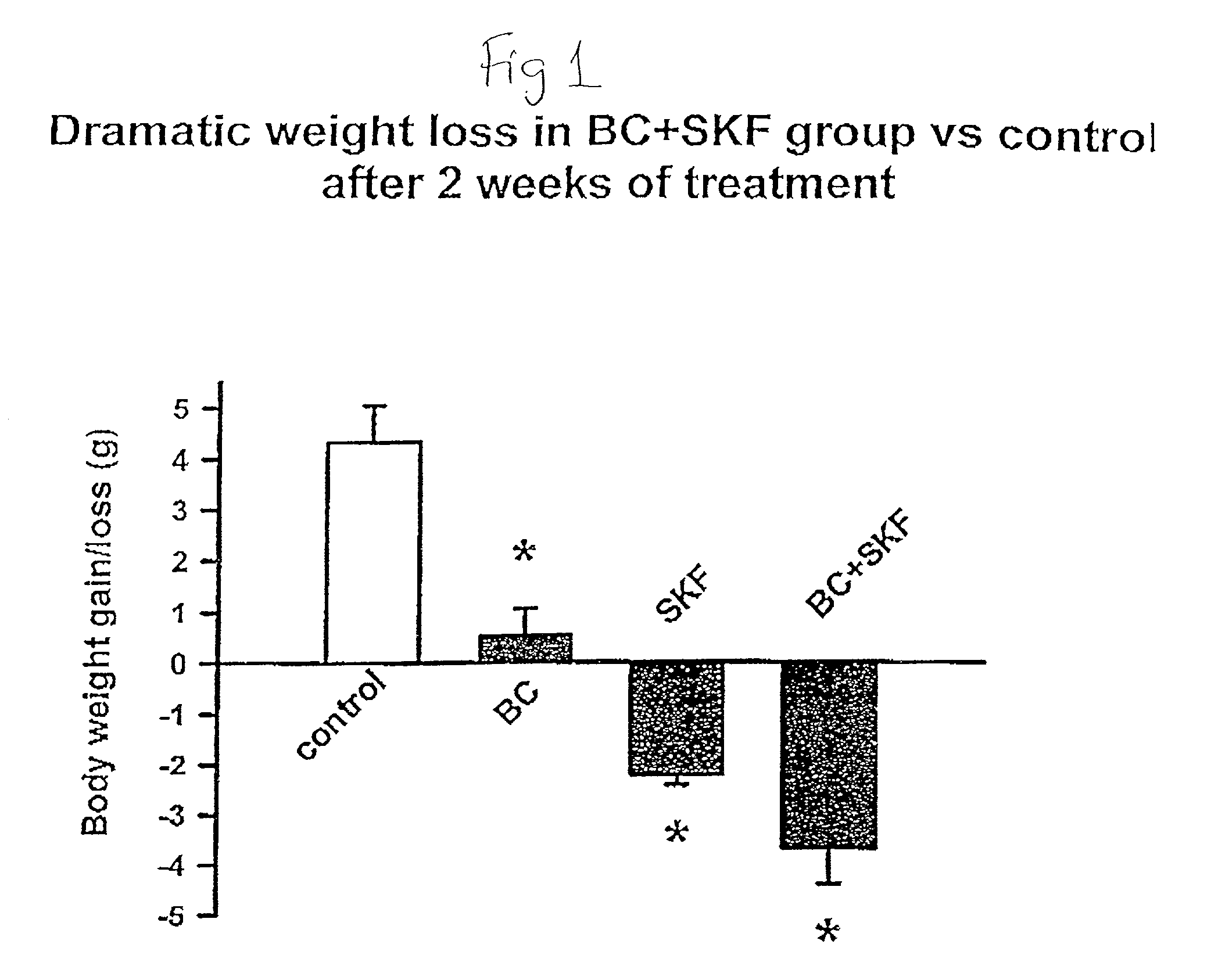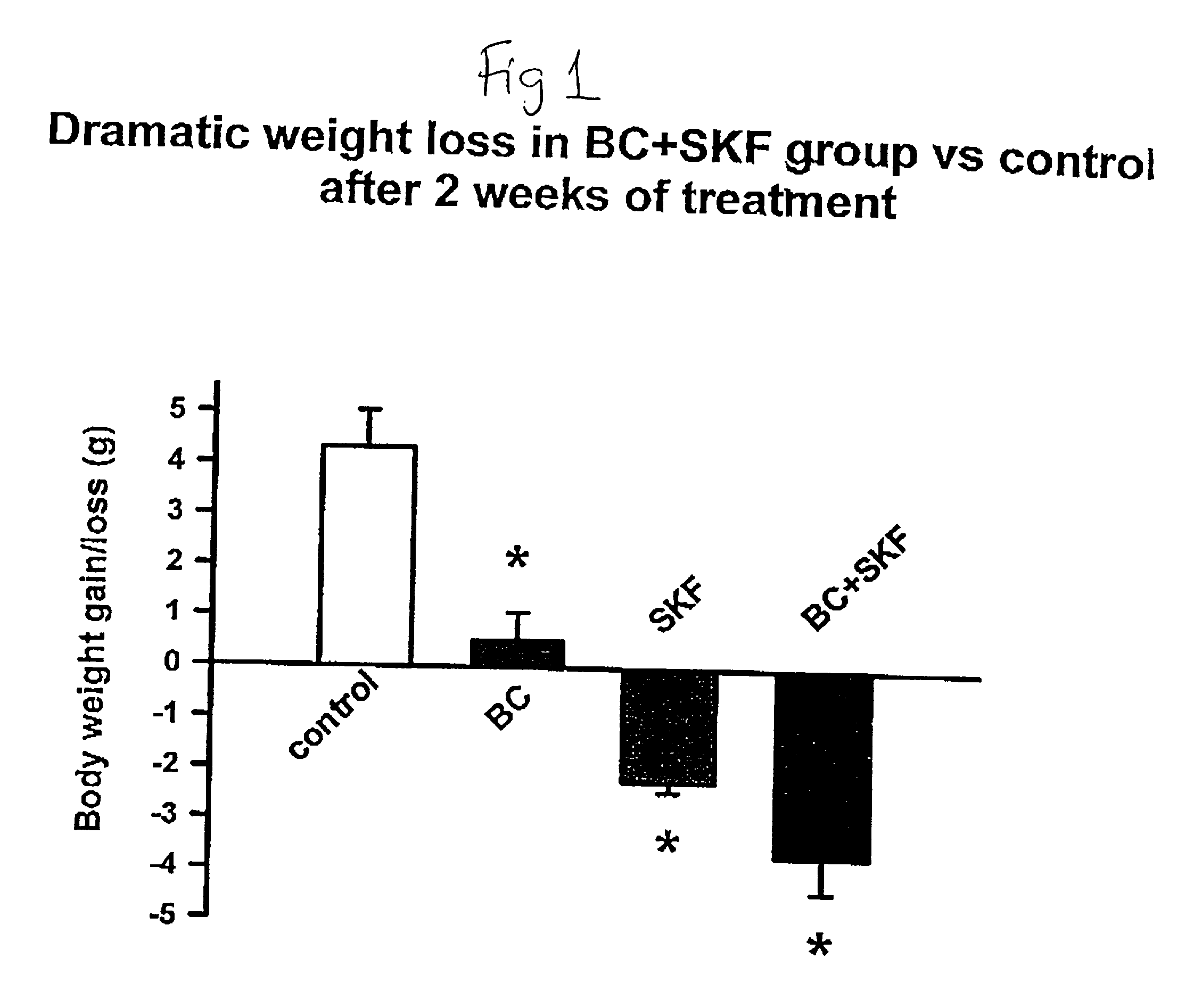Patents
Literature
451 results about "Plasma glucose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasma glucose refers to the amount of this primary sugar that is found in the liquid portion of the blood. When blood is collected using a home-testing kit or in a professional laboratory, it is whole blood. Blood sugar levels, however, are usually measured in terms of the amount of plasma glucose.
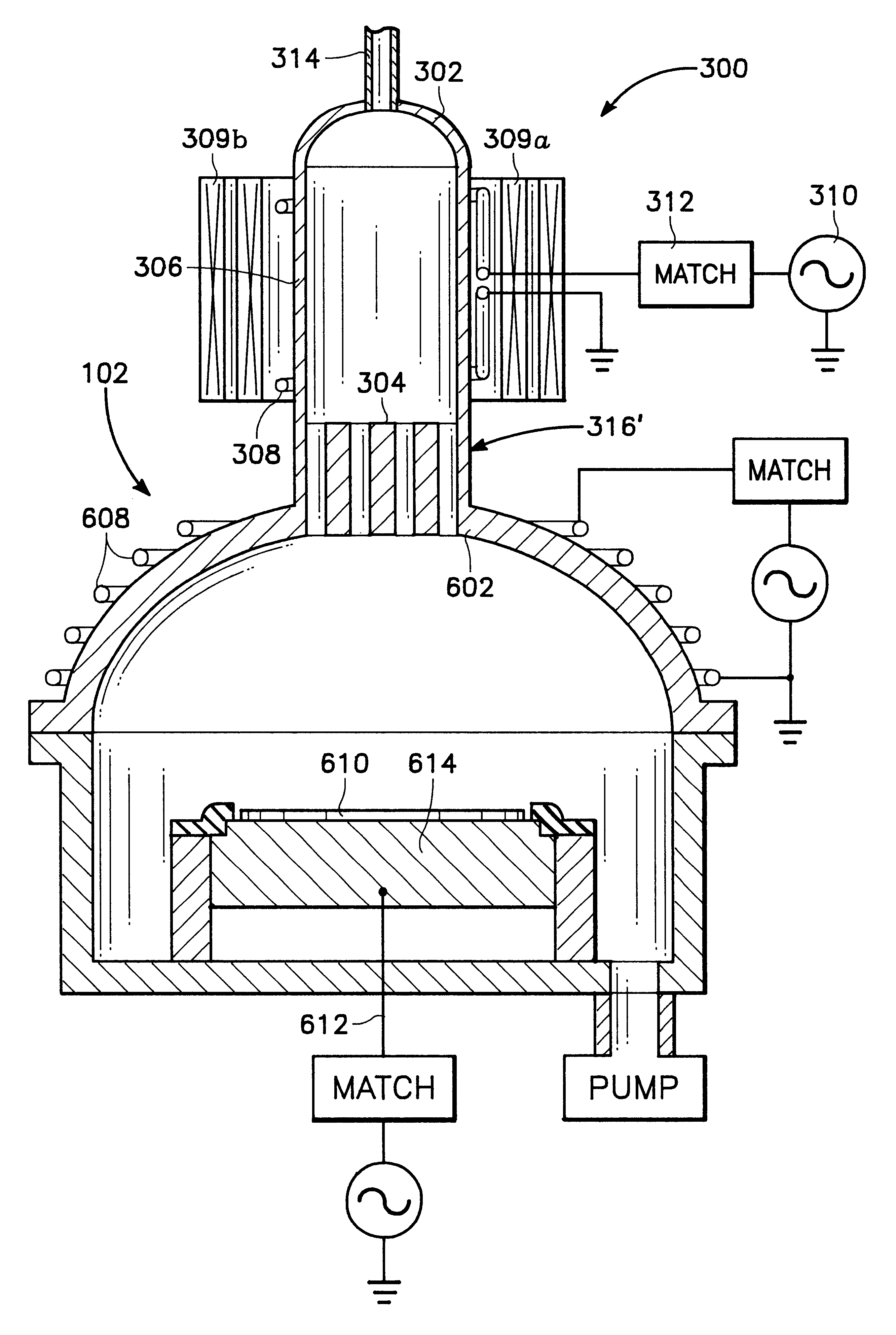
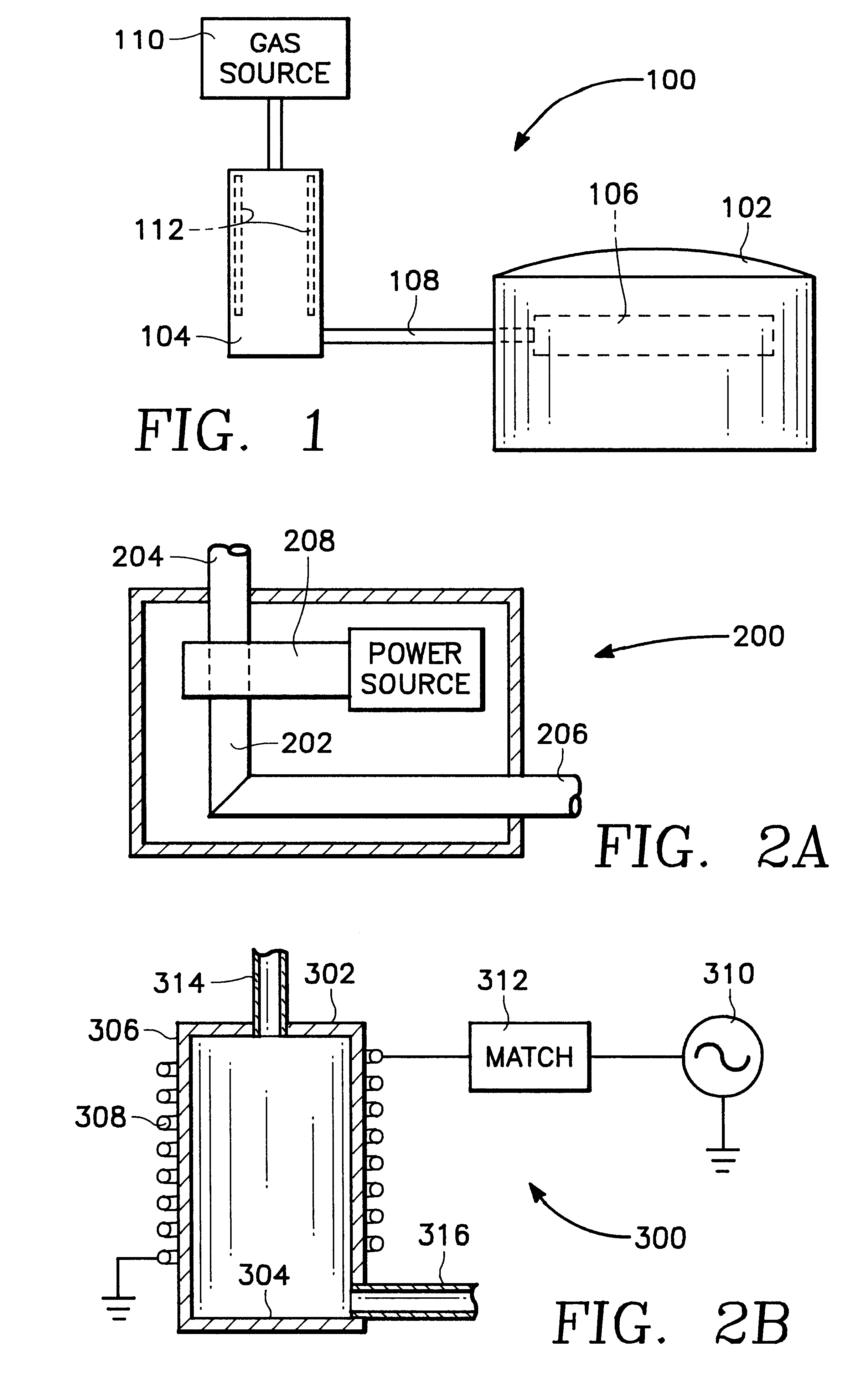
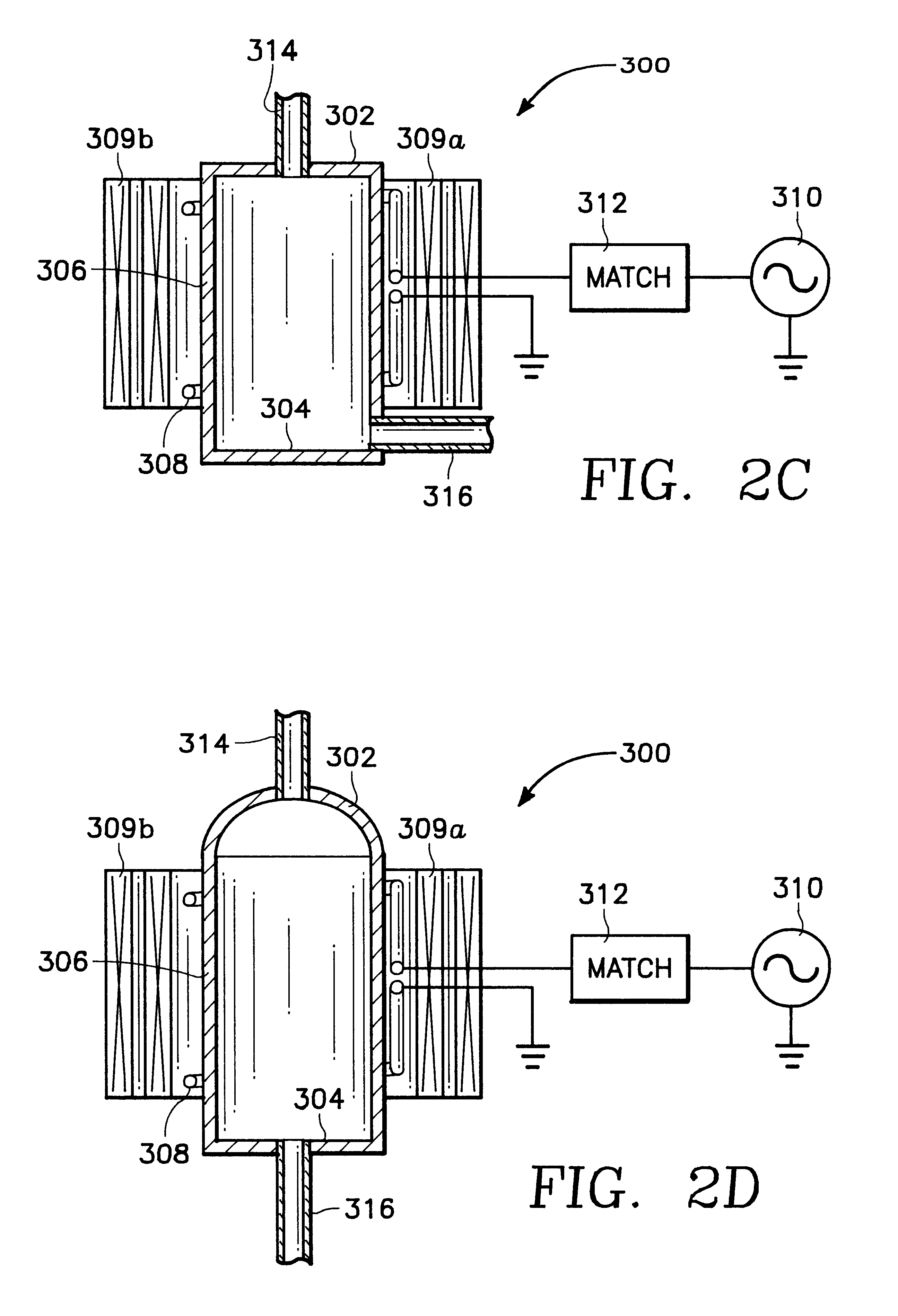
Plasma assisted processing chamber with separate control of species density
InactiveUS6352049B1Electric discharge tubesSemiconductor/solid-state device manufacturingMaterial densityPlasma glucose
The present invention provides an apparatus and method, for plasma assisted processing of a workpiece, which provides for separate control of species density within a processing plasma. The present invention has a processing chamber and at least one collateral chamber. The collateral chamber is capable of generating a collateral plasma and delivering it to the processing chamber. To control the densities of the particle species within the processing chamber the present invention may have: a filter interposed between the collateral chamber and the processing chamber, primary chamber source power, several collateral chambers providing separate inputs to the processing chamber, or combinations thereof. Collateral plasma may be: filtered, combined with primary chamber generated plasma, combined with another collateral plasma, or combinations thereof to separately control the densities of the species comprising the processing plasma.
Owner:APPLIED MATERIALS INC
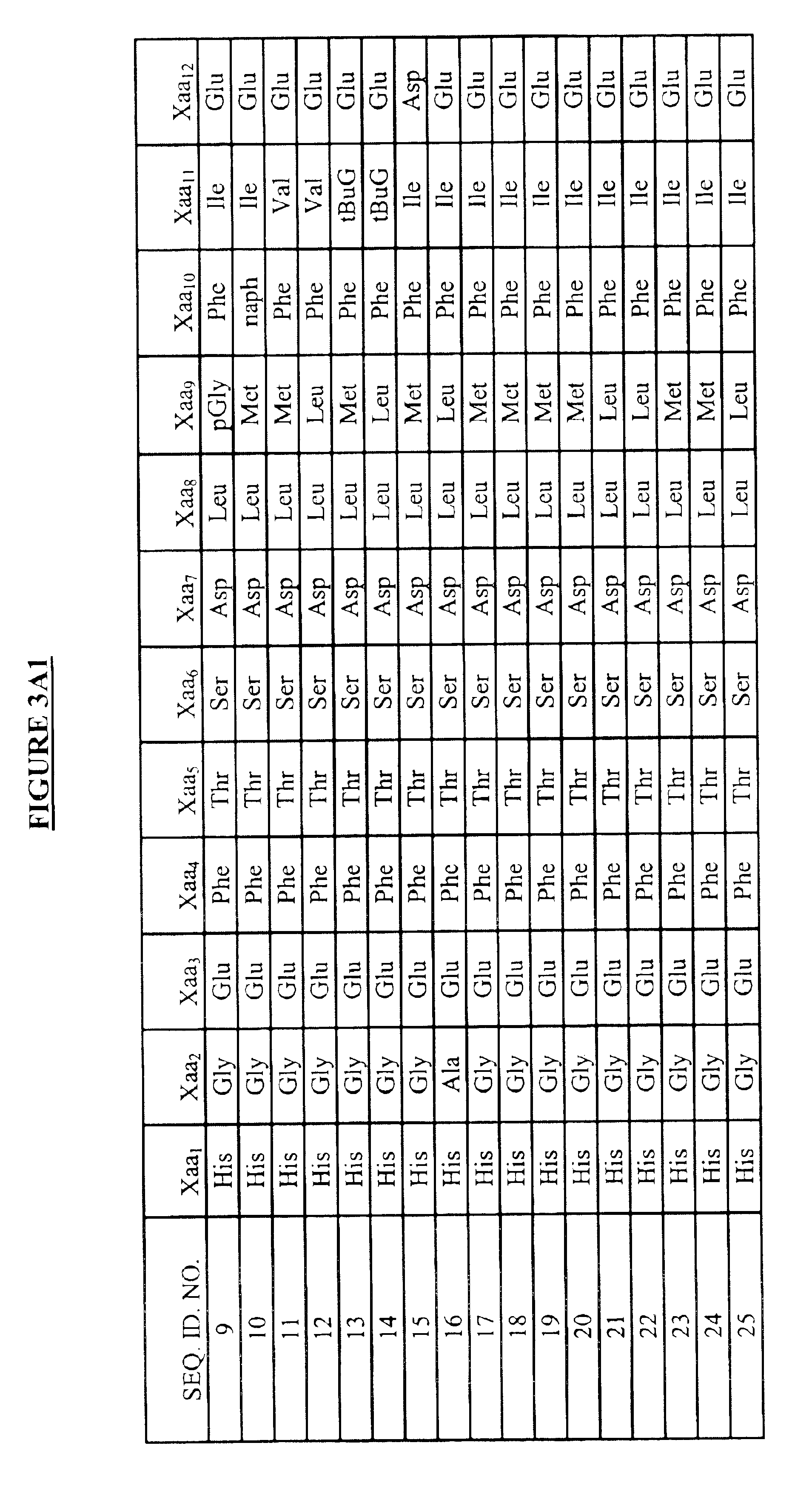
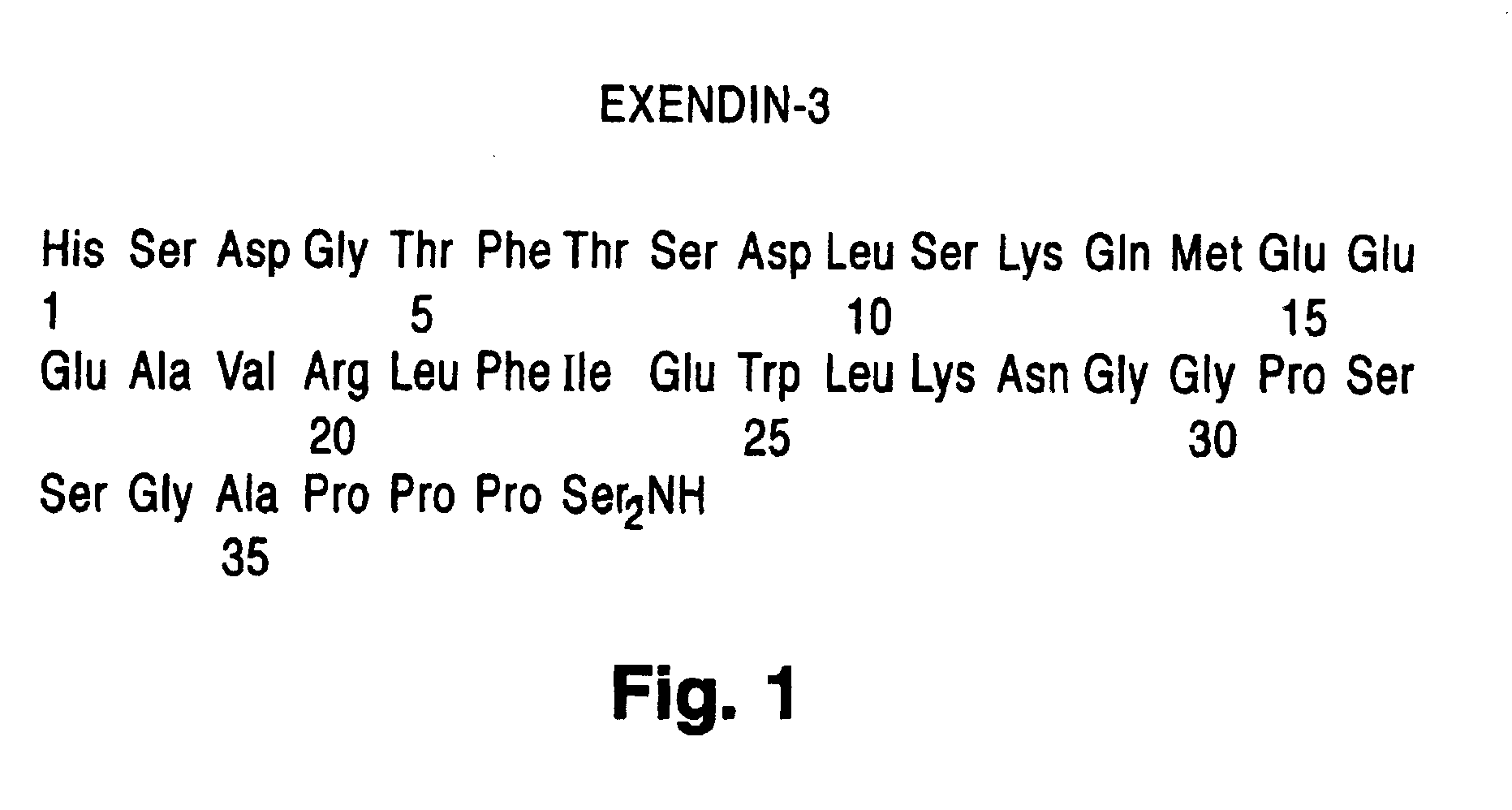
Modified exendins and exendin agonists
InactiveUS6924264B1Increase ratingsDecrease amount of potassiumMetabolism disorderSaccharide peptide ingredientsGastric emptyingPolyethylene glycol
Novel modified exendins and exendin agonist analogs having an exendin or exendin agonist analog linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonist analogs, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Exendin agonist formulations and methods of administration thereof
InactiveUS6902744B1Slow gastric emptyingLowering plasma glucose levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Modulation of apolipoprotein ciii (apociii) expression
ActiveUS20140128453A1Raise HDL levelsRaise the ratioOrganic active ingredientsNervous disorderApolipoprotein EApolipoprotein CIII
Provided herein are methods, compounds, and compositions for reducing expression of ApoCIII mRNA and protein in an animal. Also provided herein are methods, compounds, and compositions for increasing HDL levels and / or improving the ratio of TG to HDL and reducing plasma lipids and plasma glucose in an animal. Such methods, compounds, and compositions are useful to treat, prevent, delay, or ameliorate any one or more of cardiovascular disease or metabolic disorder, or a symptom thereof.
Owner:IONIS PHARMA INC
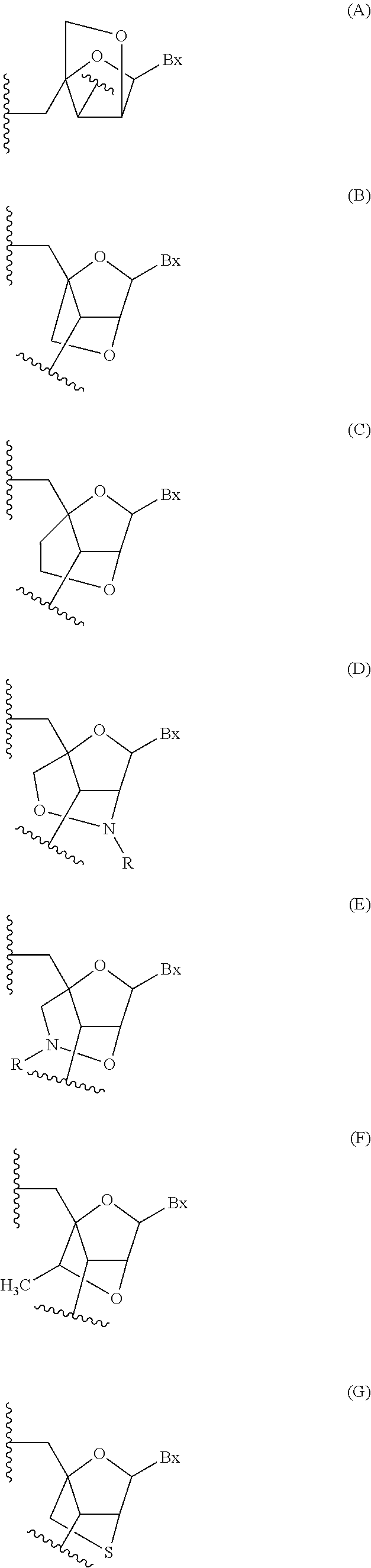
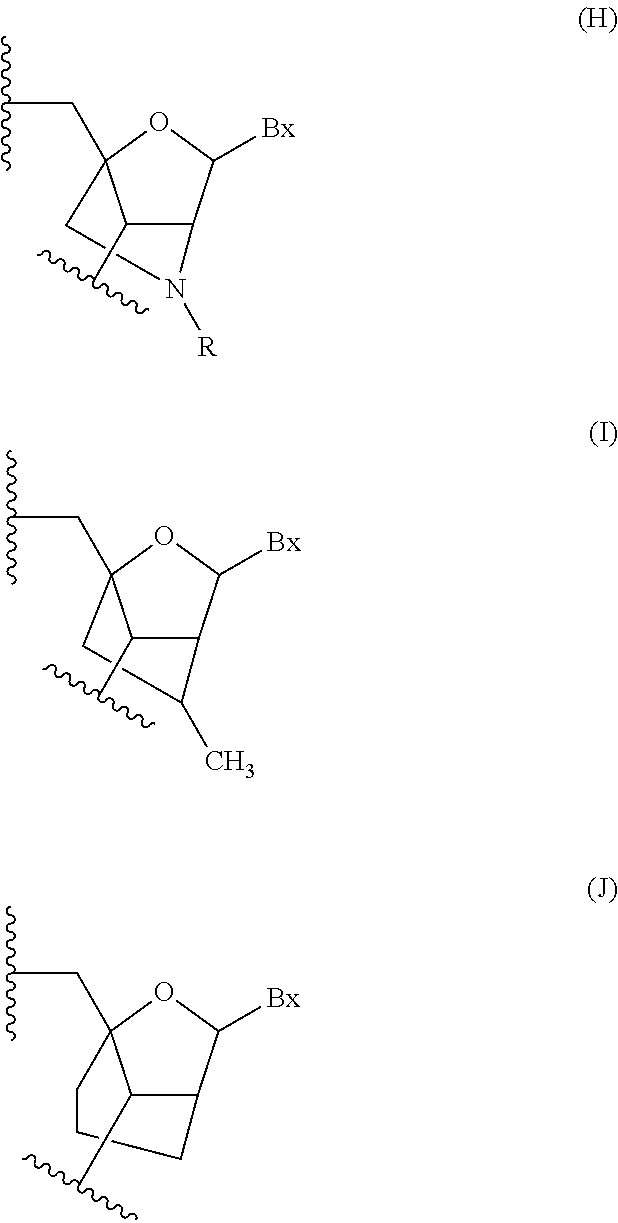
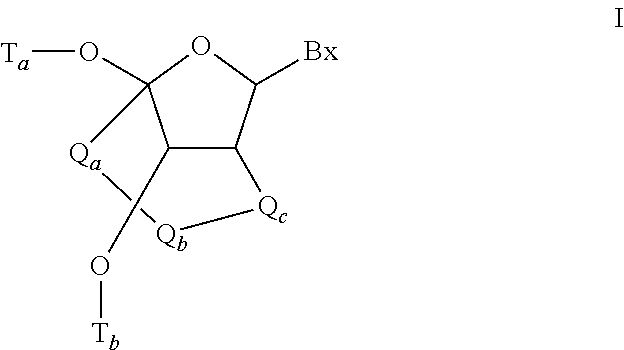
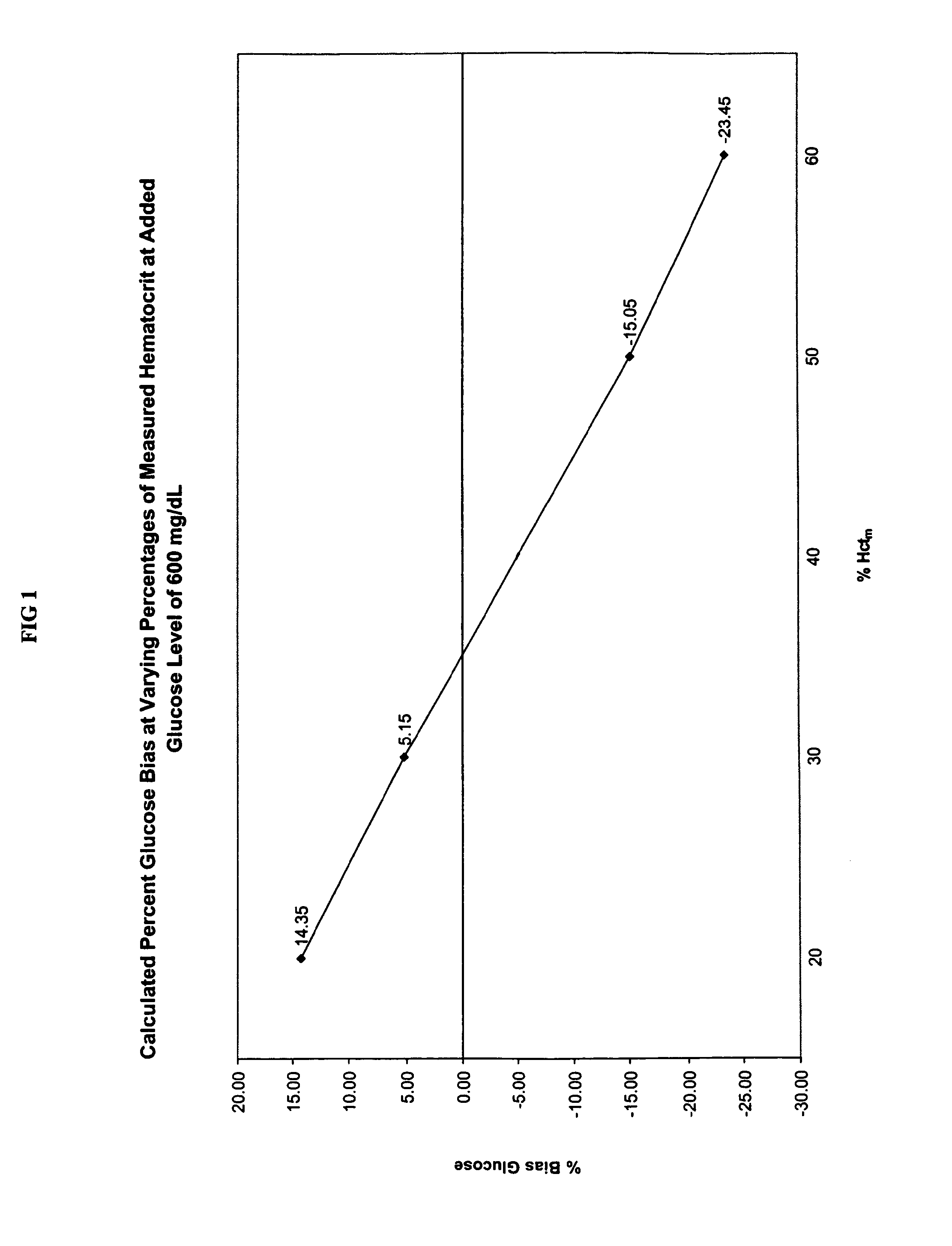
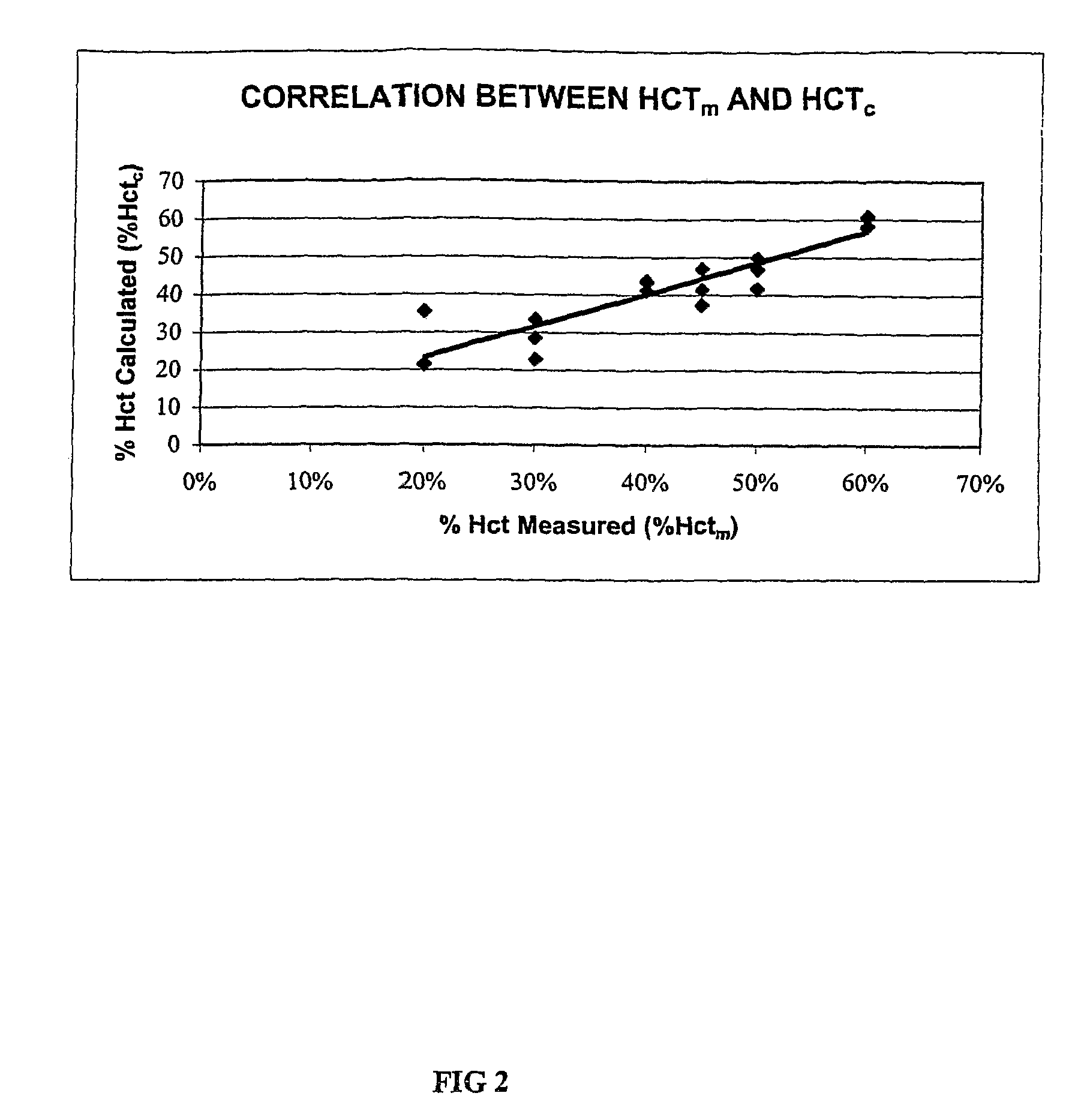
Methods for performing hematocrit adjustment in glucose assays and devices for same
ActiveUS7972861B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMedicineRed blood cell
Owner:ASCENSIA DIABETES CARE HLDG AG
Hydroxybutyrate ester and medical use thereof
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:UNITED STATES OF AMERICA +1
Synergistic use of thiazolidinediones with glucagon-like peptide-1 and agonists thereof to treat metabolic instability associated with non-insulin dependent diabetes
InactiveUS7223728B2Lower blood sugar levelsIncrease secretionPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesSide effect
Thiazolidinedione (TZD) and its pharmacologically active derivatives can be used, in combination with agonists of glucagon-like peptide-1 (GLP-1), to treat non-insulin dependent diabetes mellitus, optionally with other therapies, by improving glycemic control while minimizing side effects, such as heart hypertrophy and elevated fed-state plasma glucose, which are associate with both TZD and GLP-1 monotherapies. Thus, the co-administration of TZD and GLP-1 helps regulate glucose homeostasis in Type II diabetic patients.
Owner:ELI LILLY & CO
Method of treating insomnia
A method of treating insomnia comprising administering to a subject a formulation including zaleplon, wherein the formulation is adapted to release the zaleplon after a lag time of at least about one hour after administration of the formulation, and during which substantially no drug substance is released; provide a time of peak plasma concentration of about 3 hours to about 6 hours after administration; provide an elimination half-life after the time of peak plasma concentration of about 0.5 hours to about 0.3 hours; and provide an area under the curve of about 70 ng·h / mL to about 90 ng·h / mL.
Owner:SOMNUS THERAPEUTICS
Oral pharmaceutical extended release dosage form
An enteric coated pharmaceutical extended release dosage form of a H+, K+-ATPase inhibitor giving an extended plasma concentration profile of a H+, K+-ATPase inhibitor. The extended plasma profile is obtained by a pharmaceutical composition which comprises a core material of a hydrophilic or hydrophobic matrix, and the H+, K+-ATPase inhibitor and optionally pharmaceutically acceptable excipients. The dosage form may be administered once daily.
Owner:ASTRAZENECA AB
Modulation of angiopoietin-like 3 expression
ActiveUS20130023579A1Reducing ANGPTL expressionReduces ANGPTL expressionOrganic active ingredientsSugar derivativesPlasma lipidsPlasma glucose
Provided herein are methods, compounds, and compositions for reducing expression of an ANGPTL3 mRNA and protein in an animal. Also provided herein are methods, compounds, and compositions for reducing plasma lipids, plasma glucose and atherosclerotic plaques in an animal. Such methods, compounds, and compositions are useful to treat, prevent, delay, or ameliorate any one or more of cardiovascular disease or metabolic disease, or a symptom thereof.
Owner:IONIS PHARMA INC
Compositions comprising IMPDH inhibitors and uses thereof for treating HCV infection
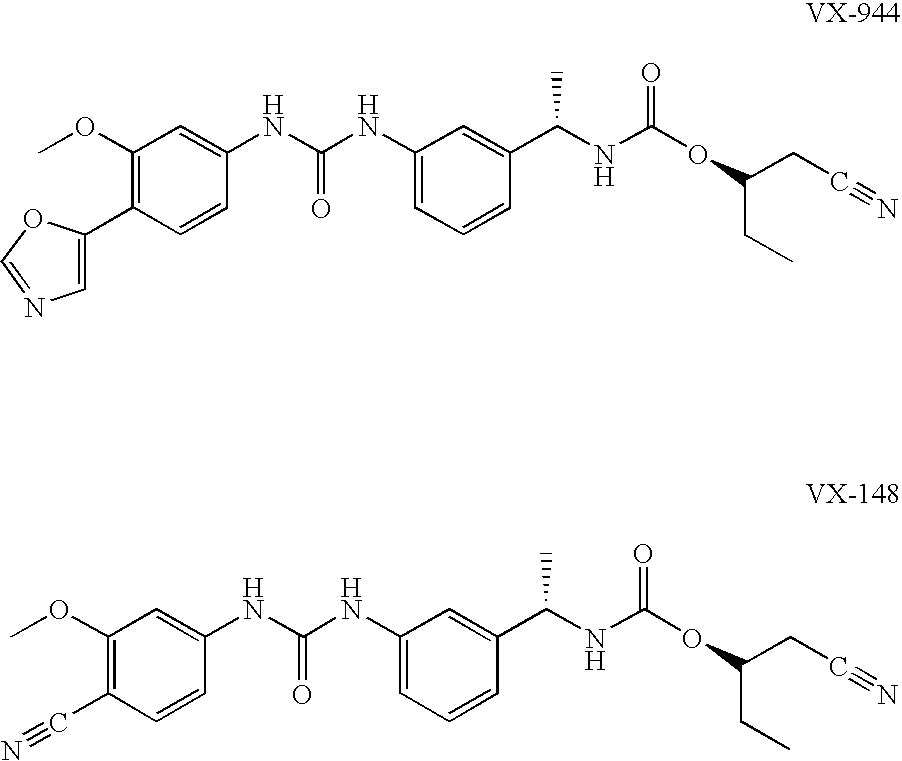
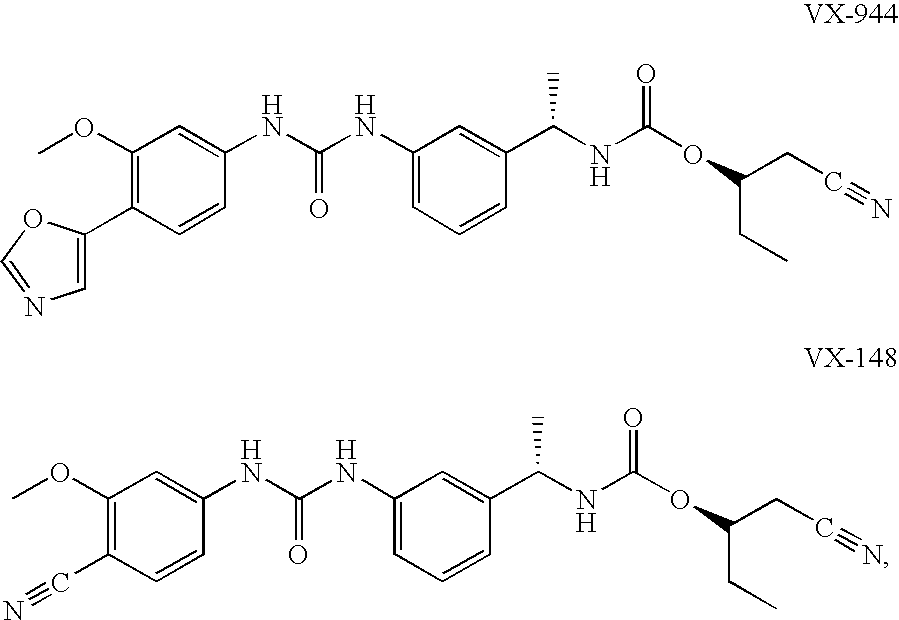
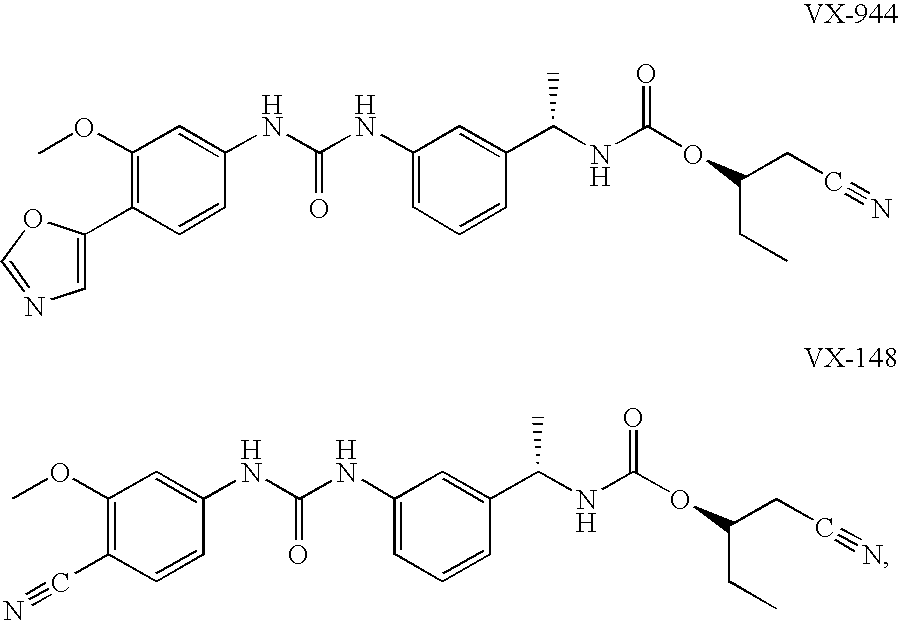
The present invention relates to optimal compositions useful in treating HCV infections in humans. These compositions comprise alpha-interferon or pegylated alpha-interferon and an IMPDH inhibitor selected from VX-148 or VX-944, wherein the IMPDH inhibitor is present in an amount such that a ratio of Cavg / Cmin is between 1 to 10, wherein:Cavg is average plasma concentration produced by said IMPDH inhibitor in said human; andCmin is estimated trough concentration produced by said IMPDH inhibitor in said human.The present invention also relates to methods of producing and using the optimal compositions to treat HCV infections in humans.
Owner:VERTEX PHARMA INC
Modified exendin and exendin agonists
Novel modified exendins and exendin agonists having an exendin or exendin agonist linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonists, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:AMYLIN PHARMA INC
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
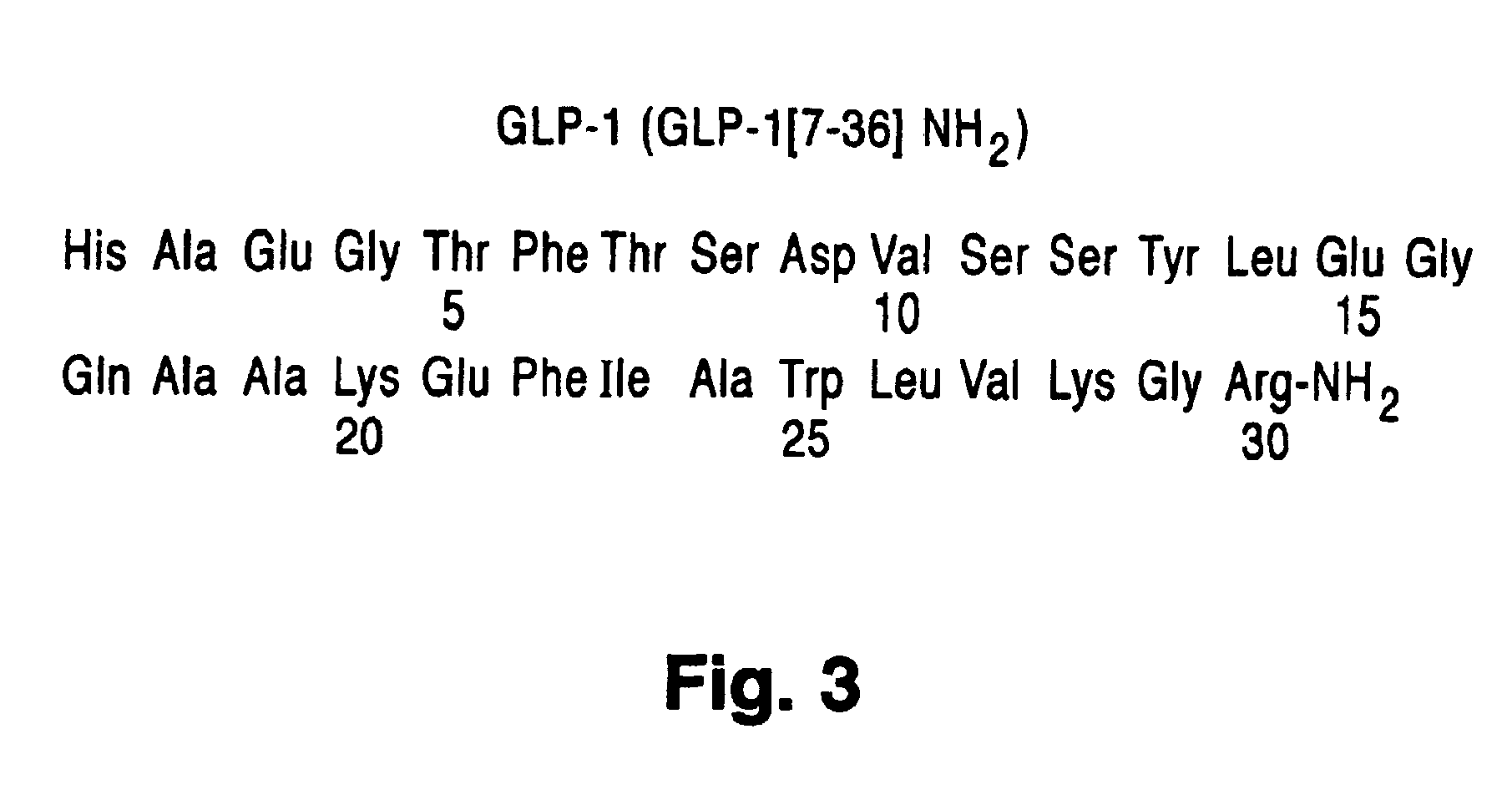
Polyethylene glycol linked GLP-1 compounds
The invention provides GLP-1 compounds coupled to at least one polyethylene glycol molecule or derivative thereof, resulting in a biologically active peptide with an extended half-life and a slower clearance when compared to that of unPEGylated peptide. These PEGylated GLP-1 compounds and compositions are useful in treating diabetes, obesity, irritable bowel syndrome and other conditions that would be benefited by lowering plasma glucose, inhibiting gastric and / or intestinal motility and inhibiting gastric and / or intestinal emptying, or inhibiting food intake.
Owner:ELI LILLY & CO
Methods of prognosis in chronic kidney disease
InactiveUS20120107420A1Reducing risk of deathAvoid deathBiocideDisease diagnosisNephrosisEnd stage renal disease
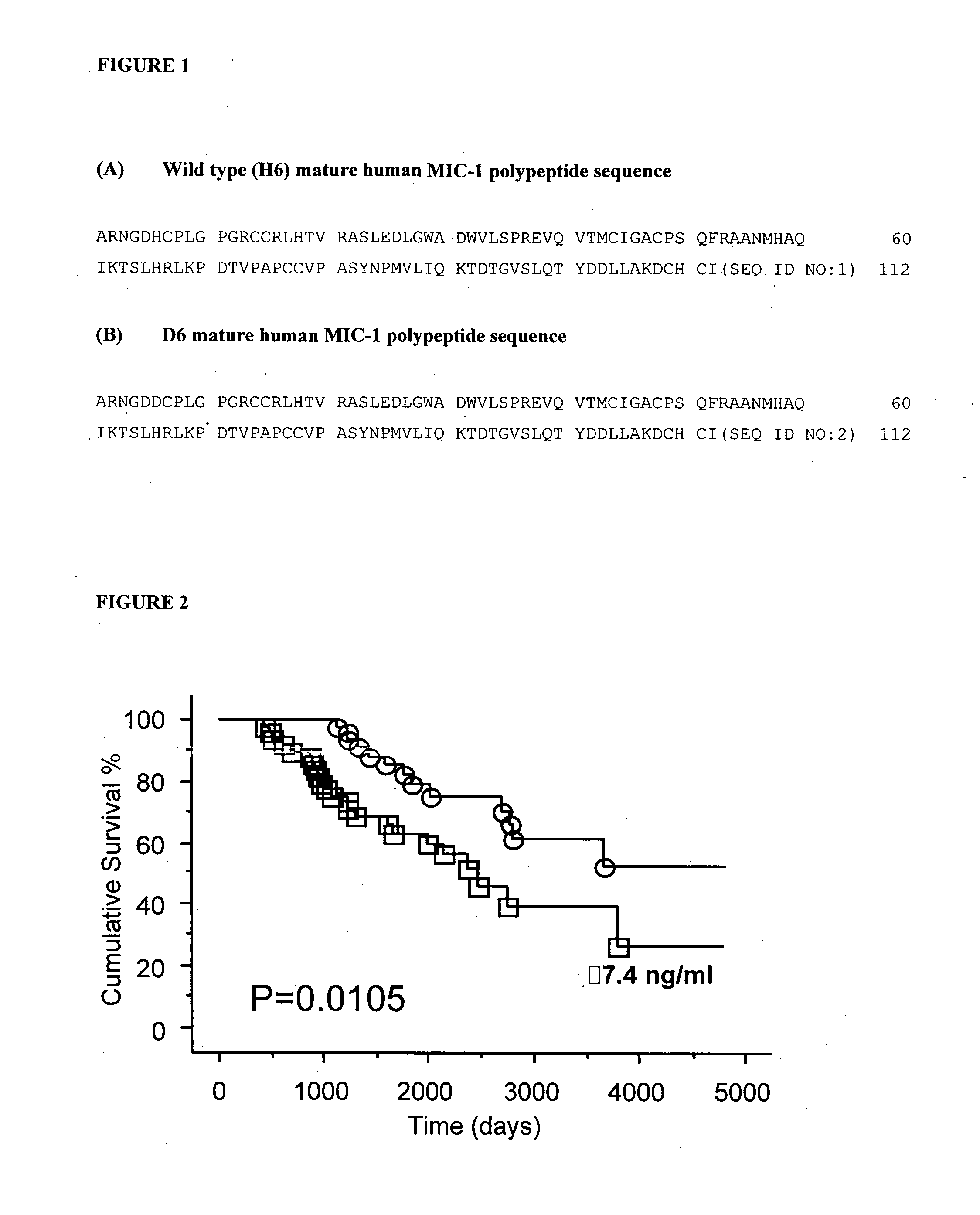
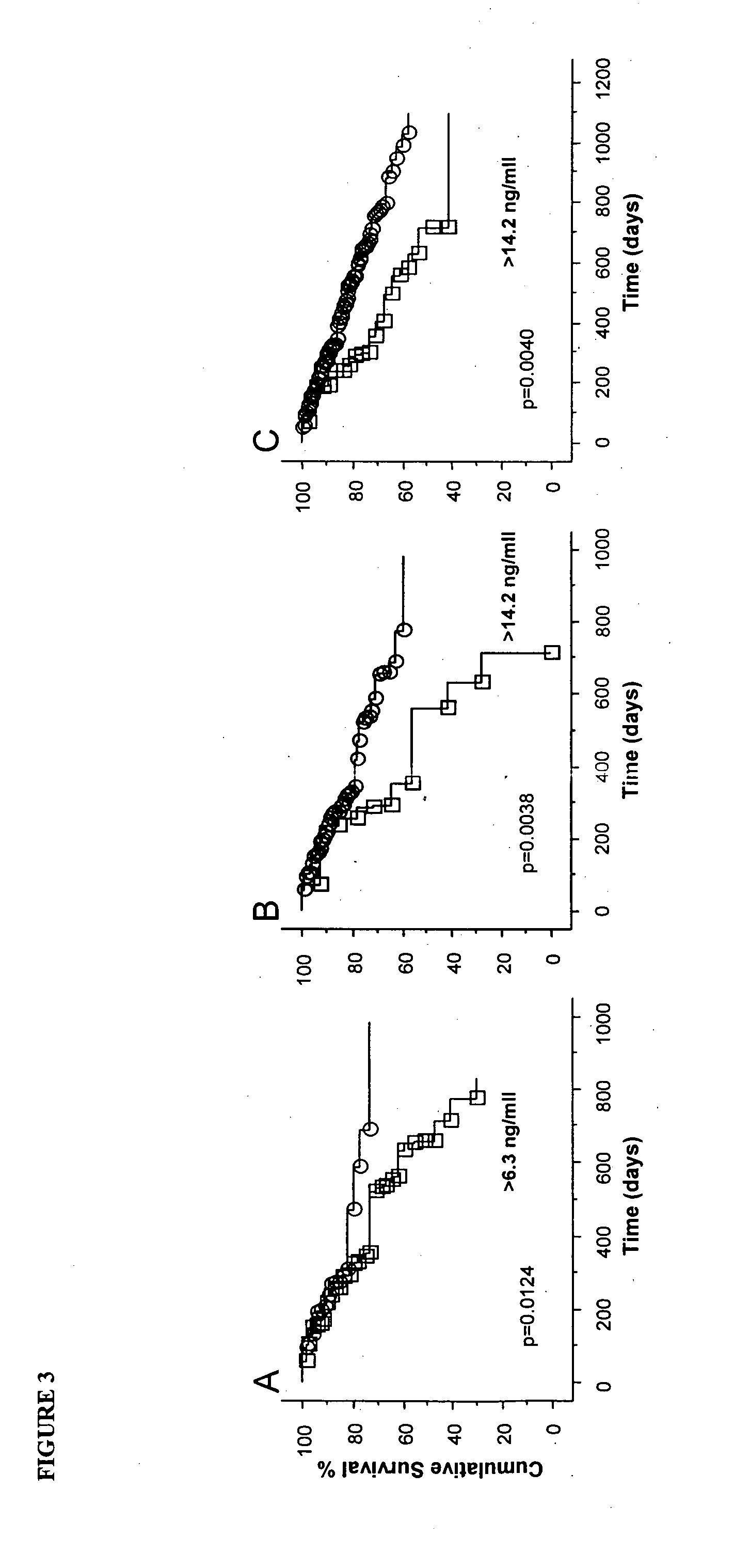
The present invention relates to methods of prognosing the survival of a diseased subject, particularly a subject with chronic kidney disease (CKD), as well as selecting an end-stage renal disease subject for a kidney transplant. The methods involve detecting an elevated amount of macrophage inhibitory cytokine-1 (MIC-1) in a test body sample from the diseased subject. A method of preventing or reducing the risk of death in a CKD subject which involves removing or inactivating MIC-1 present in the blood, plasma or serum of the subject, is also disclosed.
Owner:ST VINCENTS HOSPITAL SYDNEY
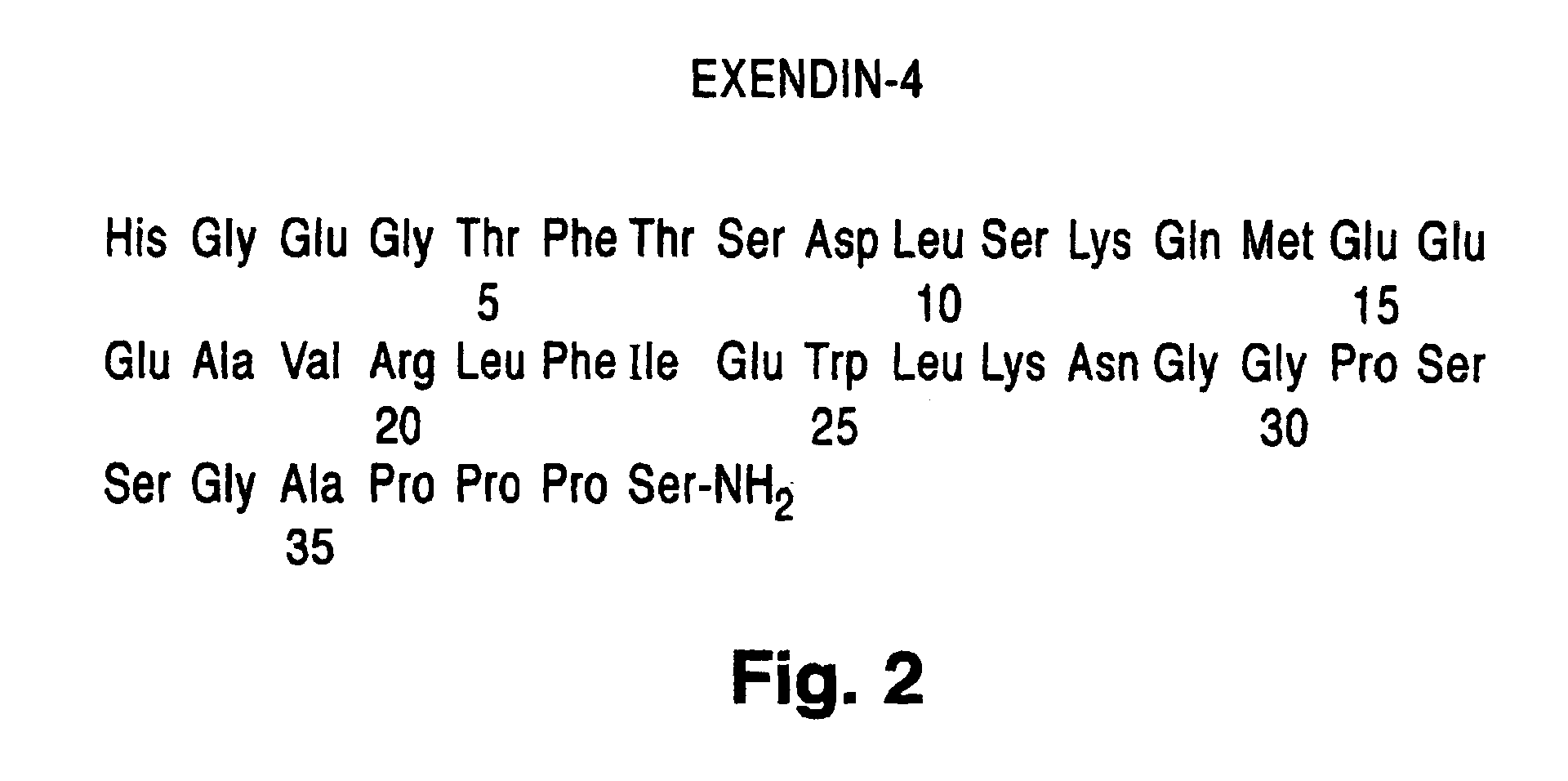
Exendin-4 and analog fusion protein thereof
ActiveCN101891823APromote regenerationPromote repairPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMotility
The invention discloses exendin-4, an analog fusion protein thereof, the corresponding polynucleotide sequence, carrier, host cell and pharmaceutical composite and a preparation method and applications of the fusion protein. The fusion protein is prepared by fusing exendin-4 and analog thereof with human immunoglobulin IgG2-Fc through special connecting peptide and has better stability and loner half-life in vivo. The fusion protein can be administered by performing local delivery, using aerosol and using injection. The fusion protein can promote the regeneration and repair of islet beta cells, increase islet beta cells, promote the secretion of insulin and improve the sensitivity of organism to insulin. The fusion protein is used to cure diabetes, adiposity and other diseases which can be benefited by reducing plasma glucose and inhibiting gastrointestinal motility and gastric emptying.
Owner:BEIJING DONGFANG BIOTECH
Plasma free nucleic acid extraction kit and application thereof
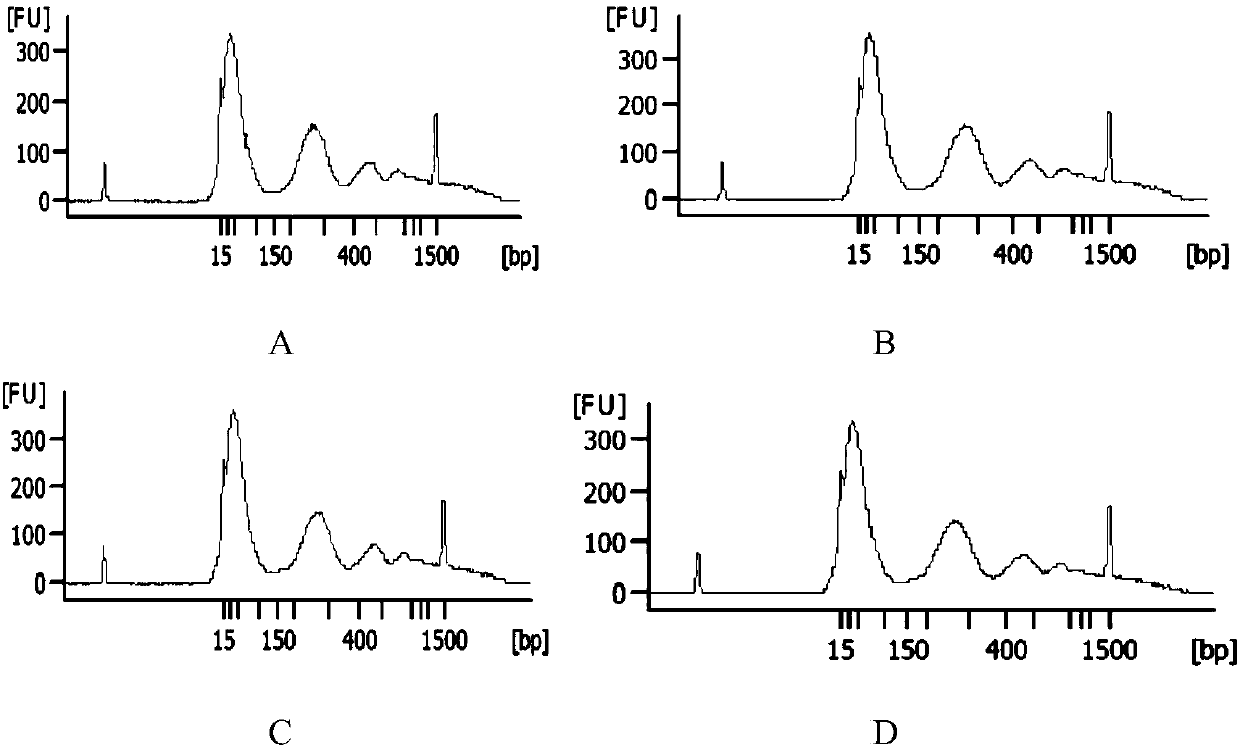
ActiveCN107663521AQuality improvementQuick extractionMicrobiological testing/measurementDNA preparationCentrifugationMagnetic bead
The invention discloses a plasma free nucleic acid extraction kit and application thereof, wherein the kit comprises (1) magnetic bead pyrolysis combination solution; (2) a first cleaning solution; (3) a second cleaning solution; (4) an elution solution. By using the plasma free nucleic acid extraction kit provided by the invention, the free desoxyribonucleic acid in the plasma can be fast extracted; in addition, the use is fast, simple and convenient; the centrifugation is not needed; once mass extraction only needs half an hour; the obtained nucleic acid has high quality; when the obtained nucleic acid is used for subsequent sequencing and detection, the result is accurate and reliable; the complete application to the existing noninvasive prenatal genetic testing technology can be realized; the important significance is realized in aspects of scientific study and clinic detection.
Owner:重庆华大医学检验所有限公司
Novel exendin agonist formulations and methods administration thereof
Owner:AMYLIN PHARMACEUTICALS LLC
Methods of providing sustained treatment with opioids
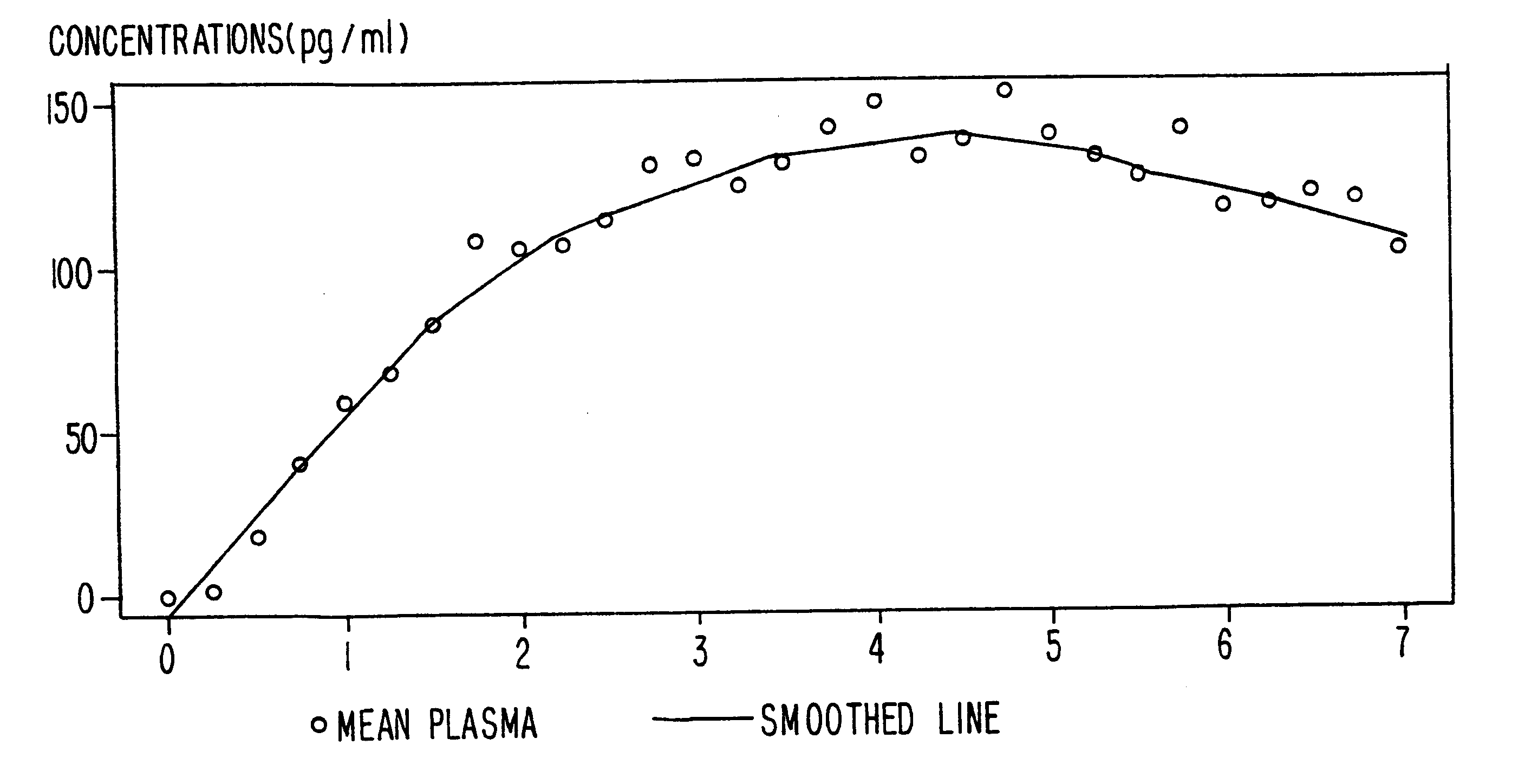
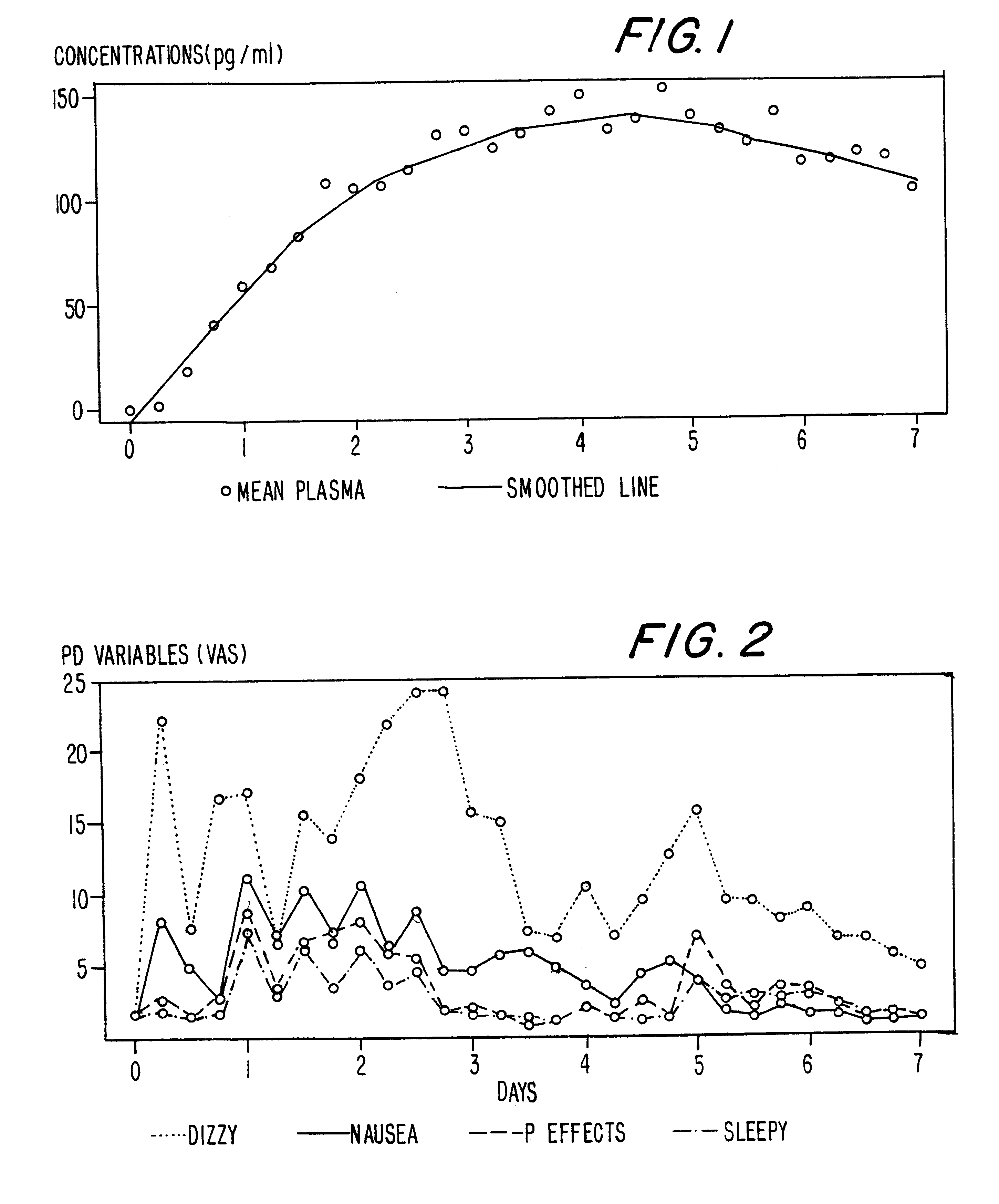
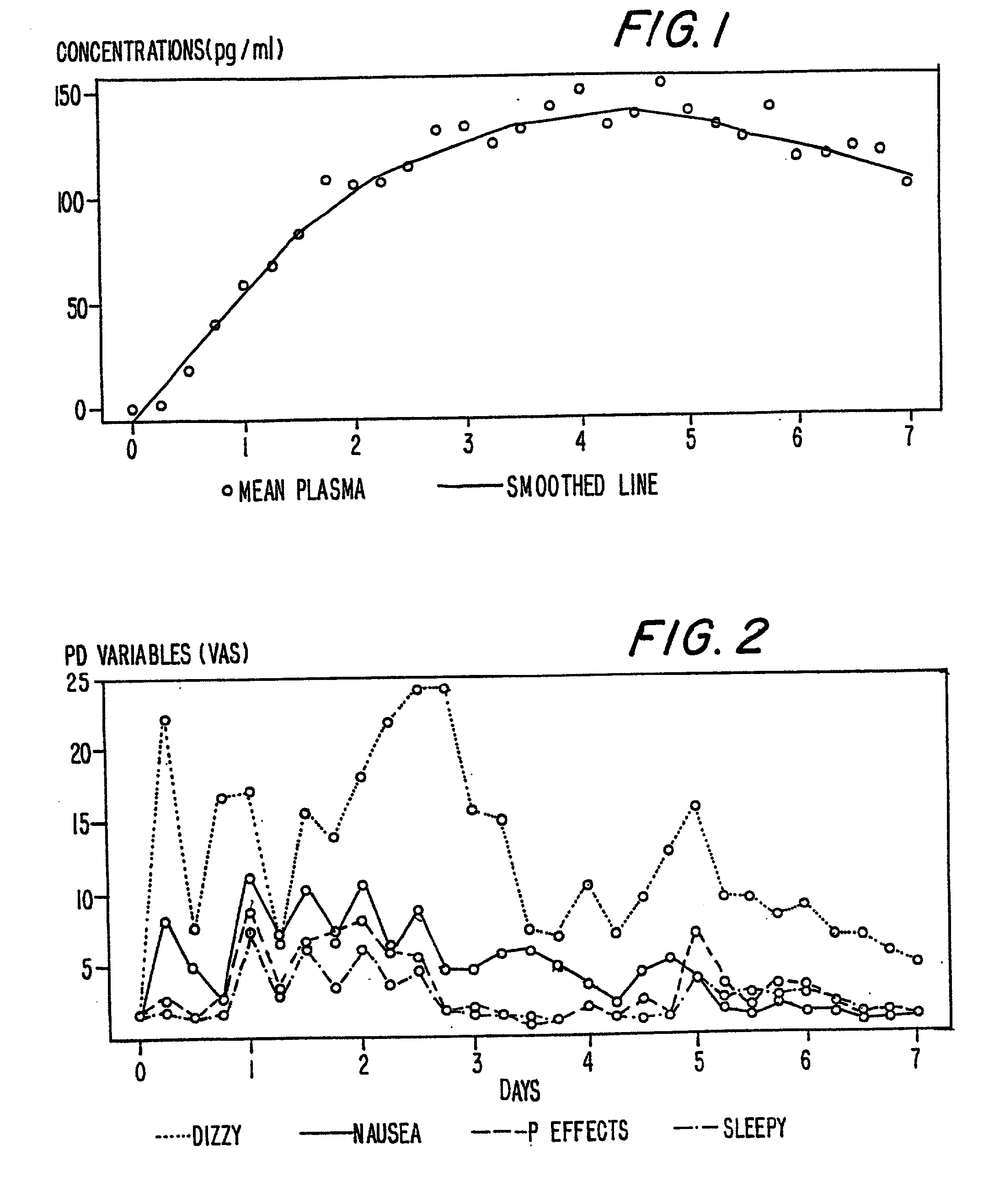
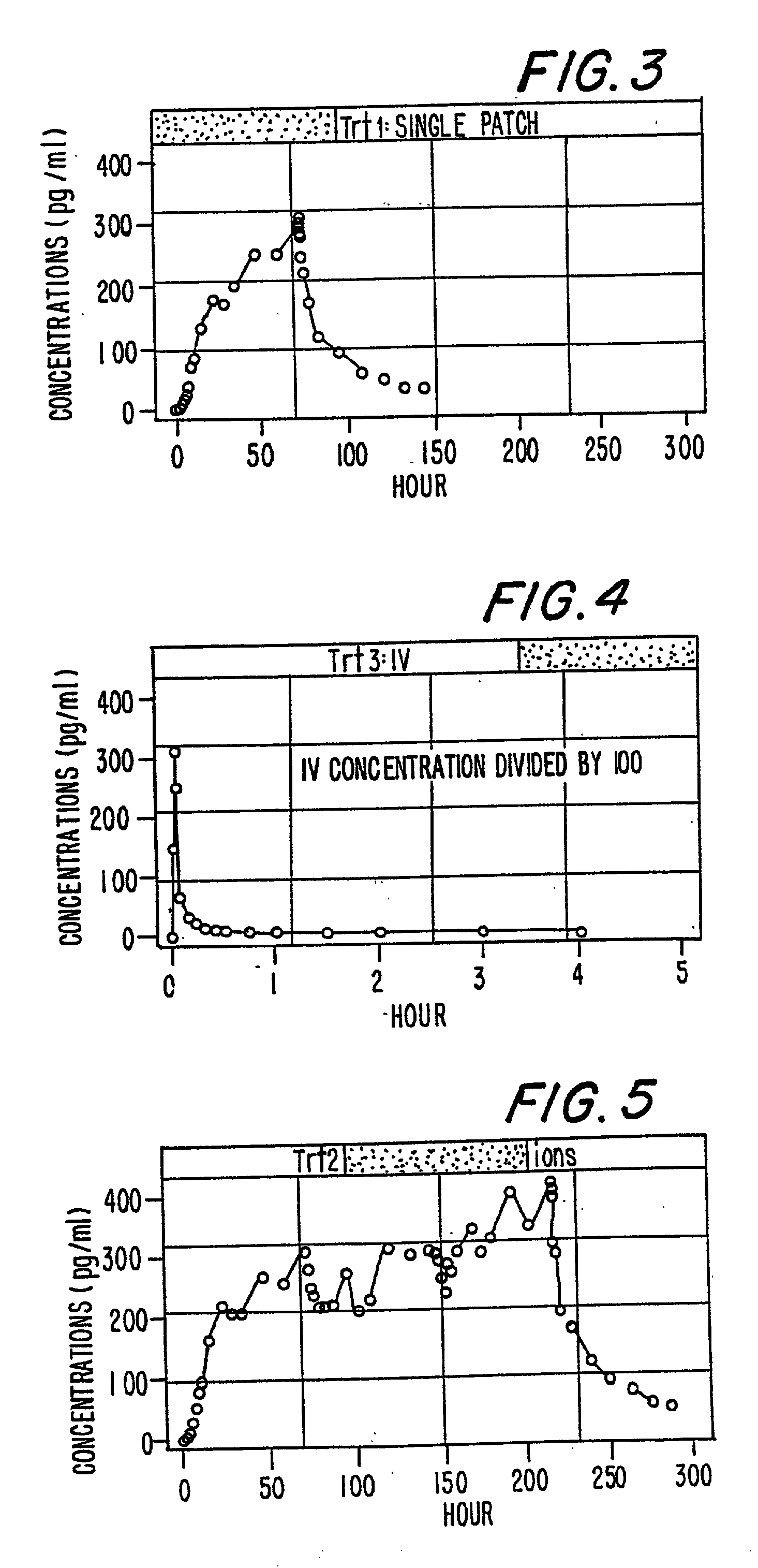
InactiveUS6231886B1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationPlasma glucose
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an addition two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Balanced sn-2 myristate-containing edible oil
A nutritional fat or oil-based composition for increasing HDL cholesterol, decreasing LDL cholesterol and decreasing the LDL / HDL cholesterol ratio in human plasma is described. The composition typically includes at least 1% by weight myristic acid esterified at the sn-2 position in triglyceride molecules, includes between 10% and 40% by weight linoleic acid, and further includes between 30% and 65% by weight oleic acid and between 15% and 40% by weight total saturated fatty acids. The ratio of sn-2 myristic acid to sn-2 palmitic acid is typically greater than 1:1 and the sum of weight percentages for saturated, monounsaturated and polyunsaturated fatty acids equals 100%. In desirable cases, the composition is substantially cholesterol-free.
Owner:BRANDEIS UNIV
Novel exendin agonist formulations and methods of administration thereof
InactiveUS20060183677A1Slow gastric emptyingLower levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:AMYLIN PHARMA INC
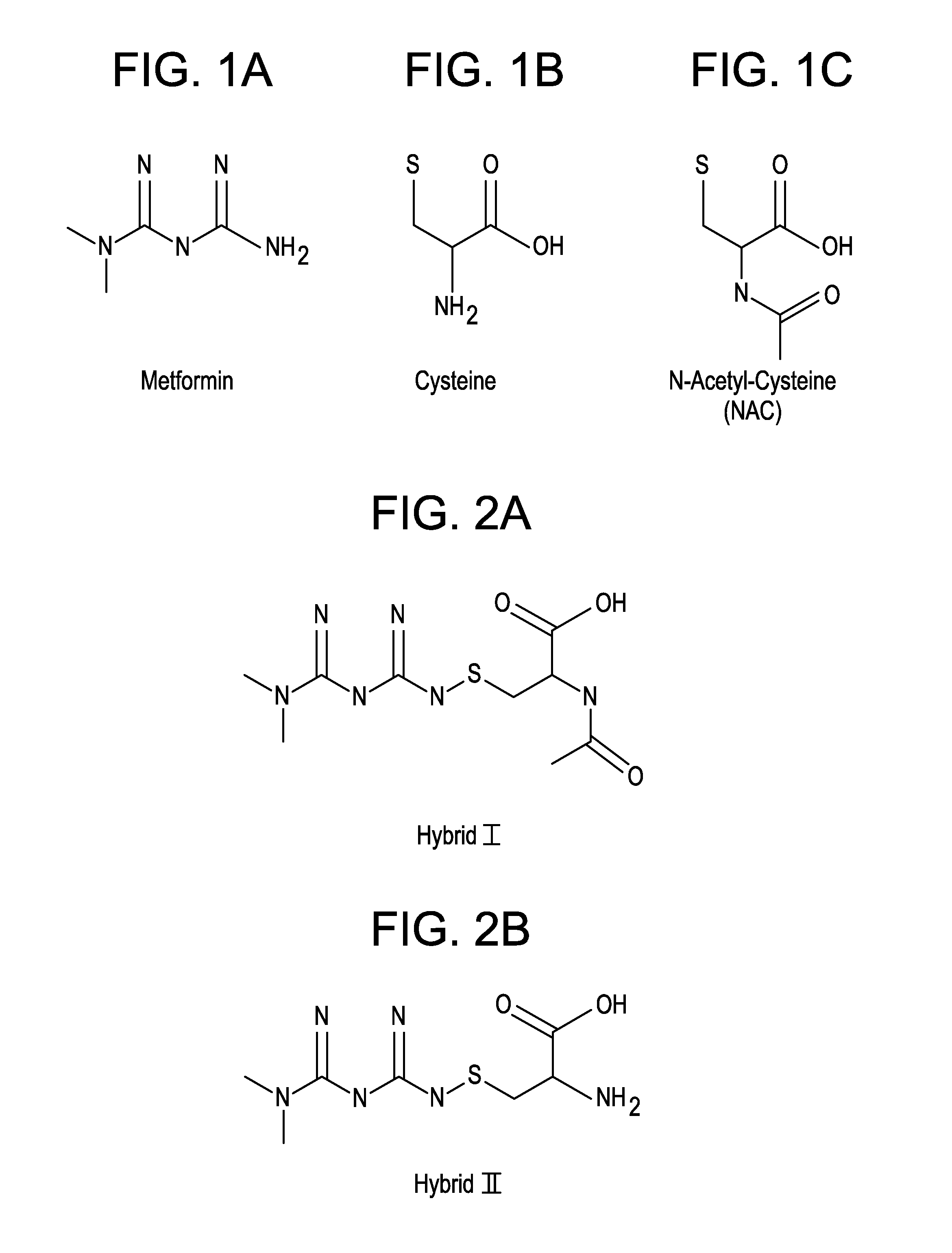
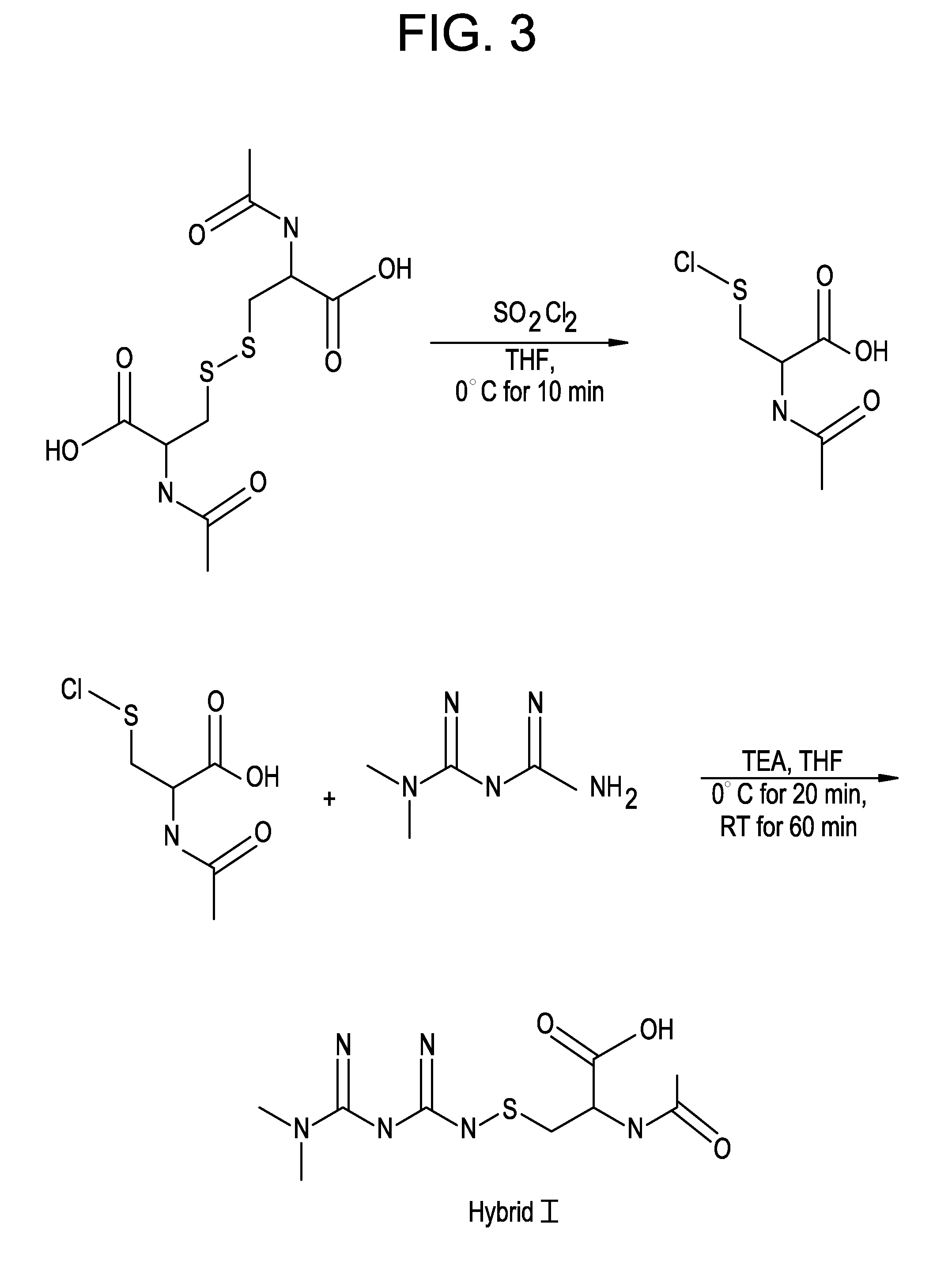
Metformin-Cysteine Prodrug
ActiveUS20110257432A1Promote intestinal absorptionEffective absorptionOrganic chemistrySmall intestinePlasma concentration
A metformin-cysteine prodrug. It is believed that the prodrug of the present invention will transport in the LAT1 and LAT2 transporter system. Because the LAT1 and LAT2 transporters are important and effective transporters of amino acids in both the small intestine and colon, it is believed that the LAT-transportable prodrugs of the present invention will be effectively absorbed both in small intestine and in the colon. The increased absorption window provided by the present invention should result in highly sustained plasma concentrations of metformin, thereby increasing the effectiveness of the medication and allowing for a single daily dose.
Owner:CODMAN & SHURTLEFF INC
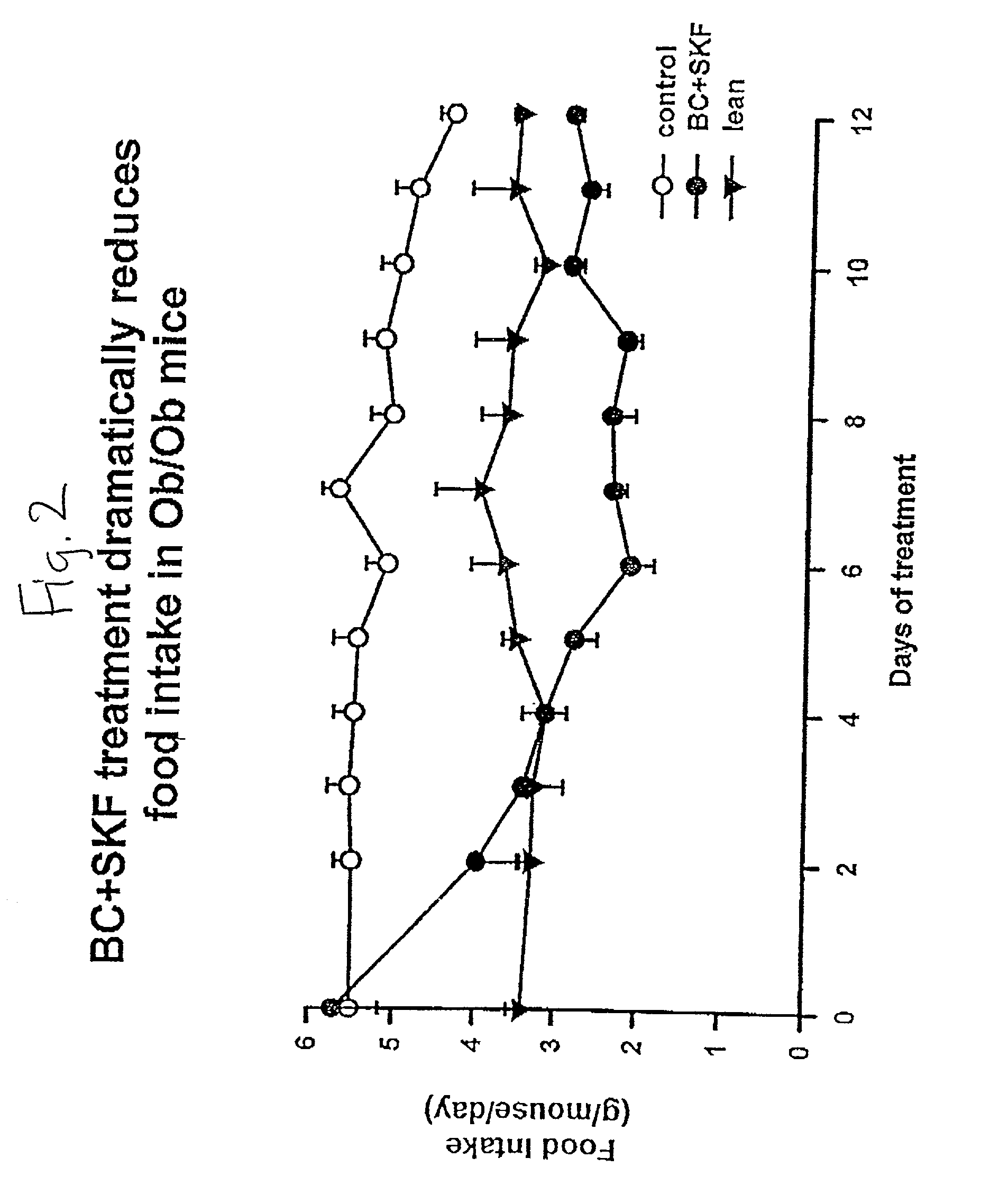
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20020187985A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideAnimal repellantsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:VEROSCI
Hydroxybutyrate ester and medical use thereof
ActiveUS20110237666A1Increase alertnessImprove cognitive functionBiocideNervous disorderFatty acidAnimal subject
A compound which is 3-hydroxybutyl 3-hydroxybutyrate enantiomerically enriched with respect to (3R)-hydroxybutyl (3R)-hydroxybutyrate of formula (I) is an effective and palatable precursor to the ketone body (3R)-hydroxybutyrate and may therefore be used to treat a condition which is caused by, exacerbated by or associated with elevated plasma levels of free fatty acids in a human or animal subject, for instance a condition where weight loss or weight gain is implicated, or to promote alertness or improve cognitive function, or to treat, prevent or reduce the effects of neurodegeneration, free radical toxicity, hypoxic conditions or hyperglycaemia.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20010016582A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideCarbohydrate active ingredientsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:CINCOTTA ANTHONY H
Method of providing sustained analgesia with buprenorphine
InactiveUS20010002259A1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationZero order kinetics
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an additional two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Methods of identifying responders to dopamine agonist therapy and treating metabolic conditions thereof
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites: dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or said patient has hypertriglyceridemai and / or hypertension . The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:VEROSCI
Methods for detecting atrial fibrillation and related conditions
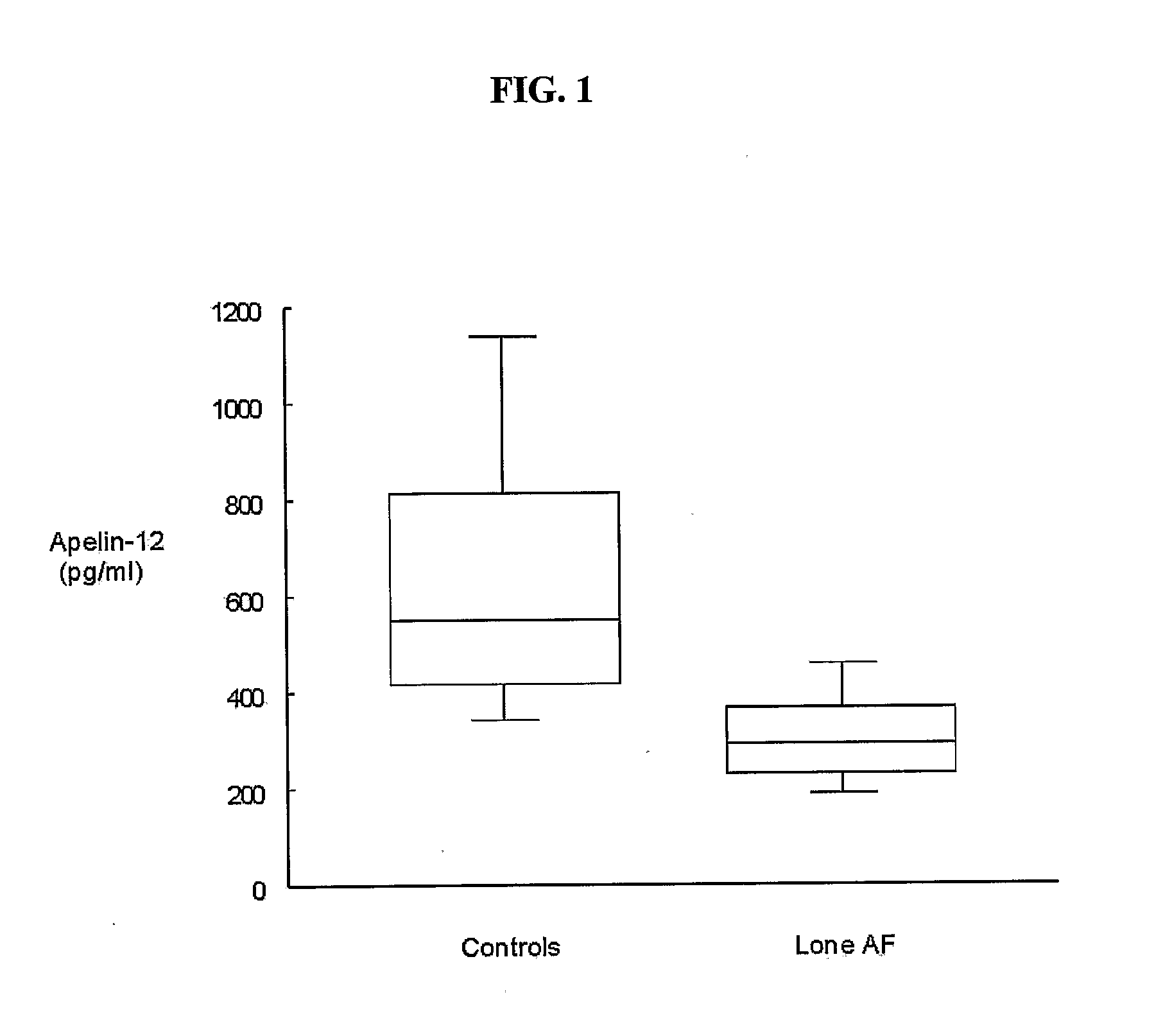
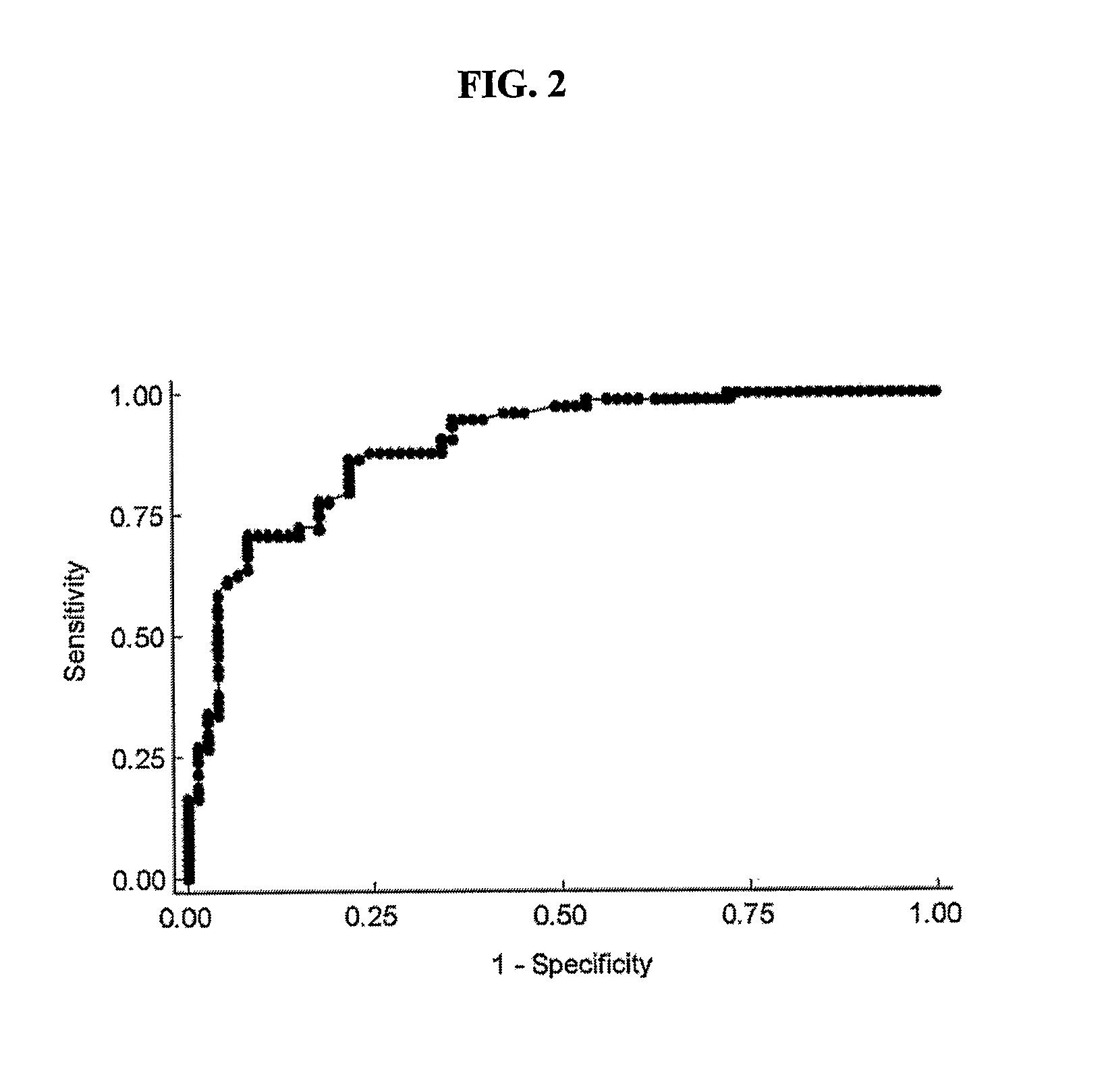
InactiveUS20110097710A1Reduce the amount requiredElectrocardiographyMicrobiological testing/measurementApelinCvd risk
Methods for detecting disorders associated with atrial fibrillation, and, thus, at risk for stroke and / or heart failure) in a subject based on a monitoring plasma levels of apelin are provided. Diagnostic compositions for the detection of such disorders are additionally provided.
Owner:THE GENERAL HOSPITAL CORP
Methods of identifying responders to dopamine agonist therapy and treating metabolic conditions thereof
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites: dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or fasting triglycerides are greater than about 150 mg / dl and / or said patient has hypertension. The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:VEROSCI
Glp-1 analog fusion protein formulations
InactiveUS20090232807A1Solution stableGood chemical stabilityPeptide/protein ingredientsMetabolism disorderIntestinal motilityObesity
The invention provides a stable solution formulation comprising a GLP-1-Fc fusion at a pH between about pH 6 and about pH 8.5. analogs fused to specific IgG4-Fc derivatives. These formulations provide unexpected and considerably greater chemical stability than when compared to GLP-1 -Fc fusions at a pH outside the described ranges. The formulations comprising a GLP-1-Fc fusion are useful in treating diabetes, obesity, initable bowel syndrome and other conditions that would be benefited by lowering plasma glucose, inhibiting gastric and / or intestinal motility and inhibiting gastric and / or intestinal emptying, or inhibiting food intake.
Owner:ELI LILLY & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com