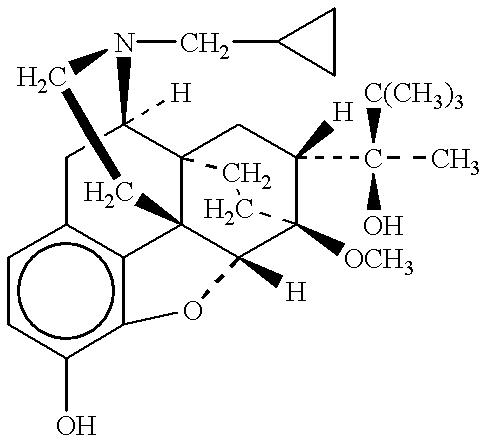
Method of providing sustained analgesia with buprenorphine
a buprenorphine and analgesic technology, applied in the direction of bandages, anti-inflammatory agents, drug compositions, etc., can solve the problem of patient starting to long for the next dosage, and achieve the effect of effective pain management, lower drug blood level, and high side effects inciden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
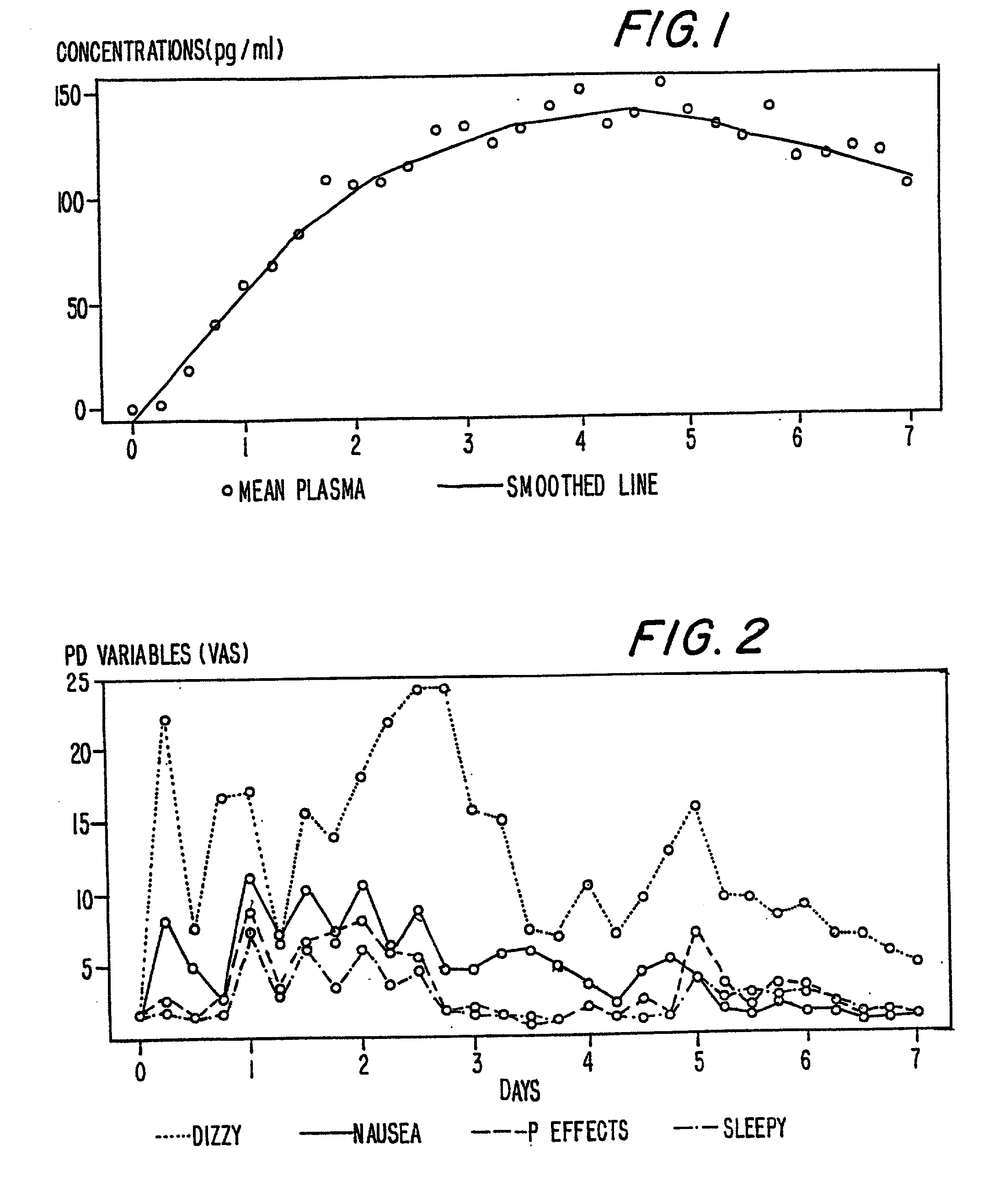
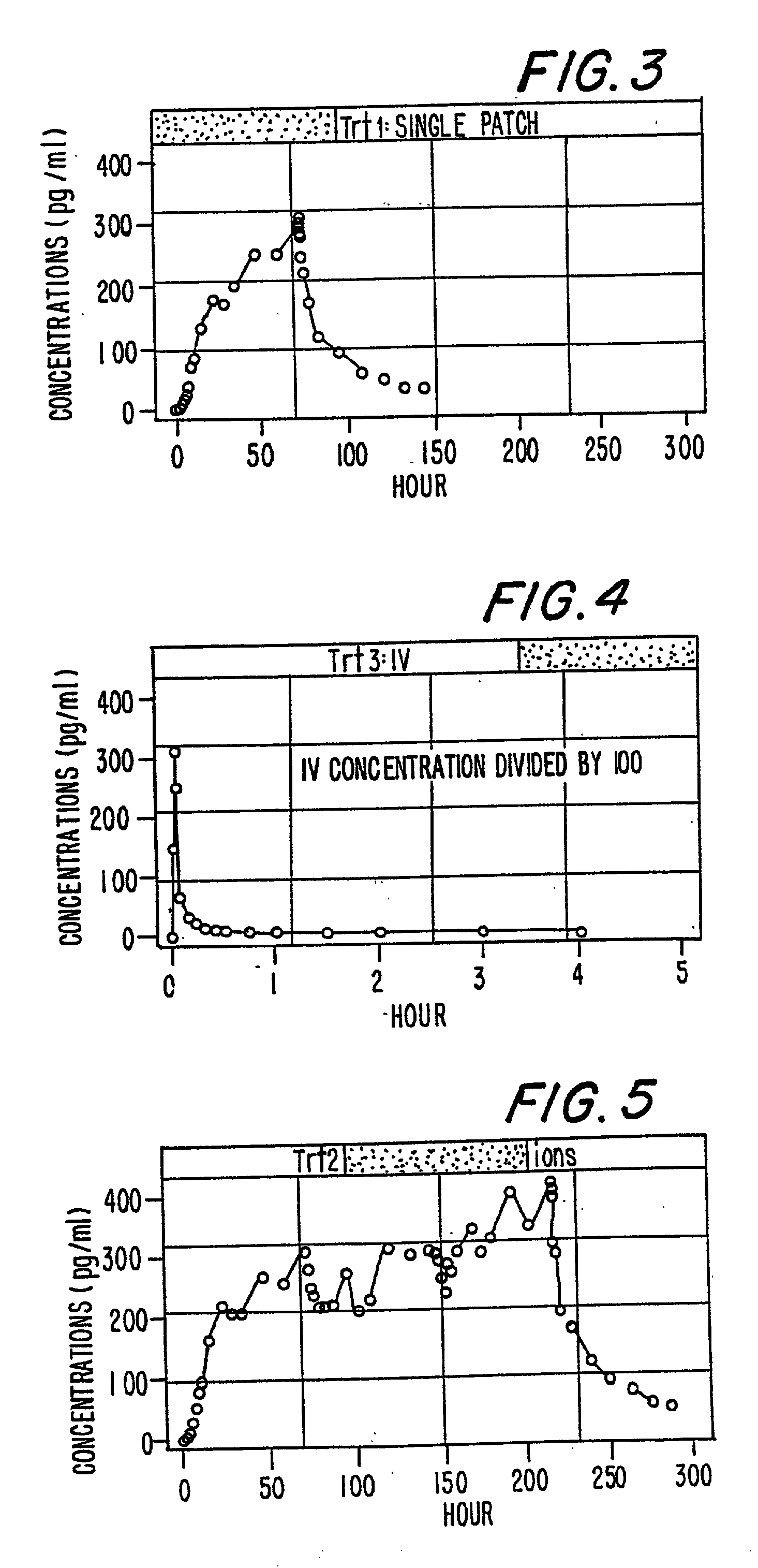
[0190] In Example 2, the method of the present invention is accomplished via a different mode of administration, i.e., intravenous infusion. The pattern of plasma concentrations seen through time in this invention can be achieved by using an intravenous infusion using the injectable, parenteral form of, e.g., buprenorphine hydrochloride suitably diluted in an intravenous infusion solution. The infusion rate would be controlled by a programable infusion pump, to provide the desired plasma concentration profile. The rate of infusion through time can be determined and adjusted based upon pharmacodynamic parameters such as pupil size (pupilometry) or pain relief (analgesia) or by the results of a suitable bioassay to determine the plasma buprenorphine concentrations at any particular point in time. In addition, it is possible to model the desired curve using pharmacokinetic modeling techniques; in this way the desired curve can be approximated without need for pharmacokinetic or pharmac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com