Patents
Literature
76 results about "Rate of infusion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacokinetics, the rate of infusion (or dosing rate) refers not just to the rate at which a drug is administered, but the desired rate at which a drug should be administered to achieve a steady state of a fixed dose which has been demonstrated to be therapeutically effective.
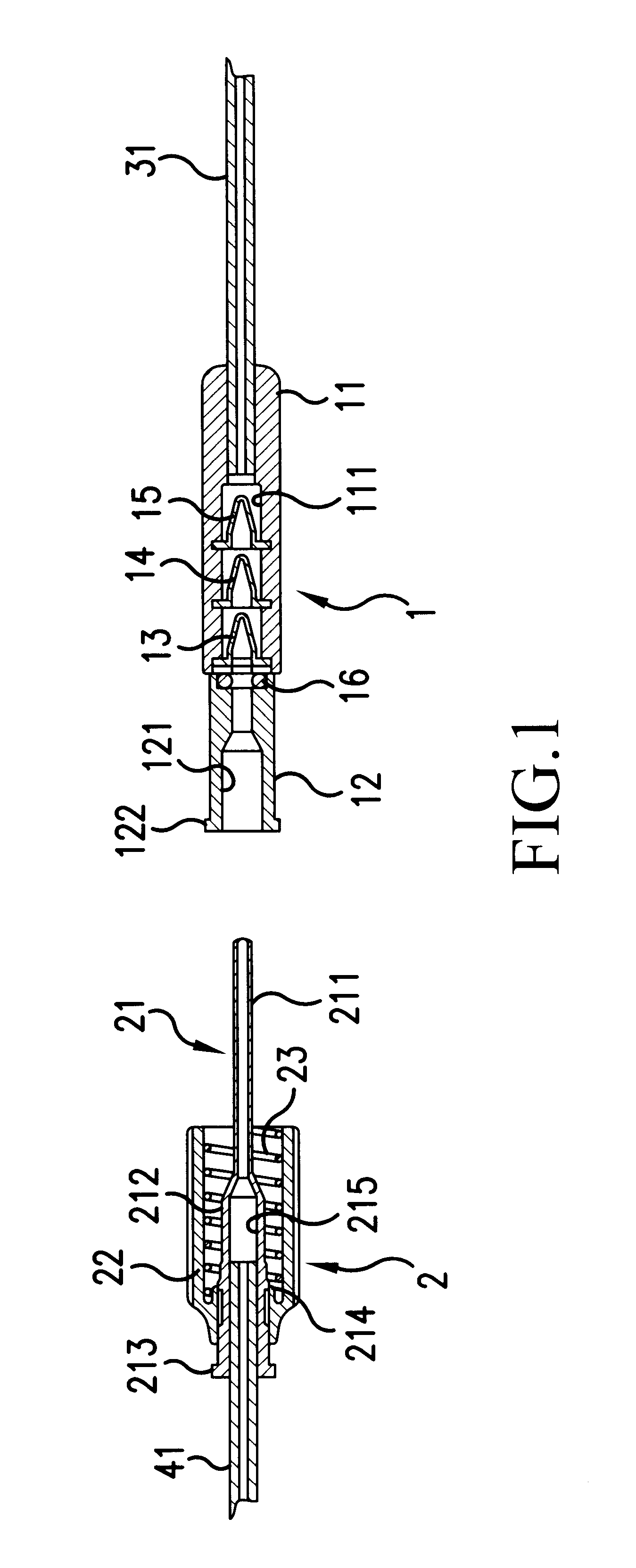
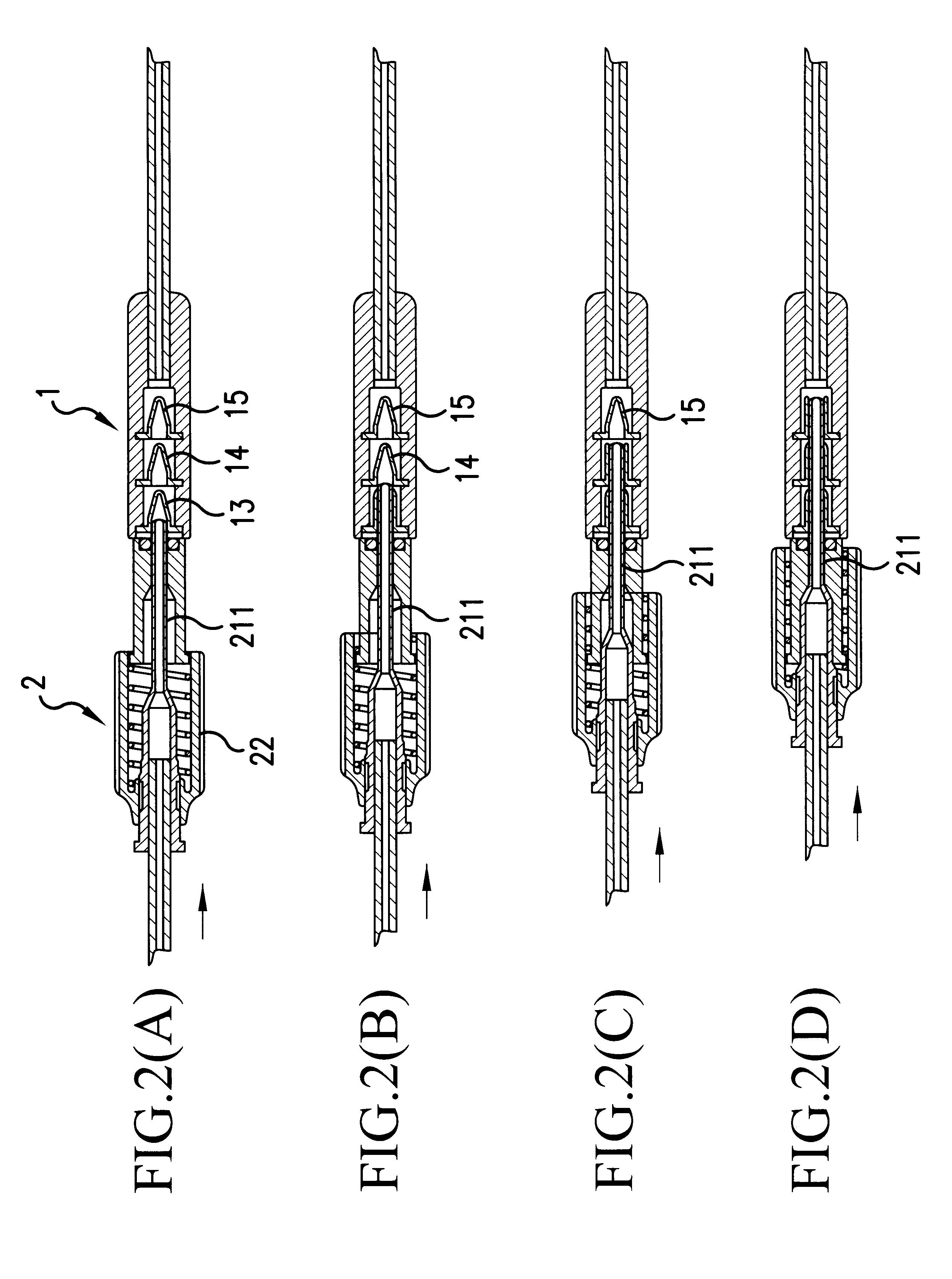
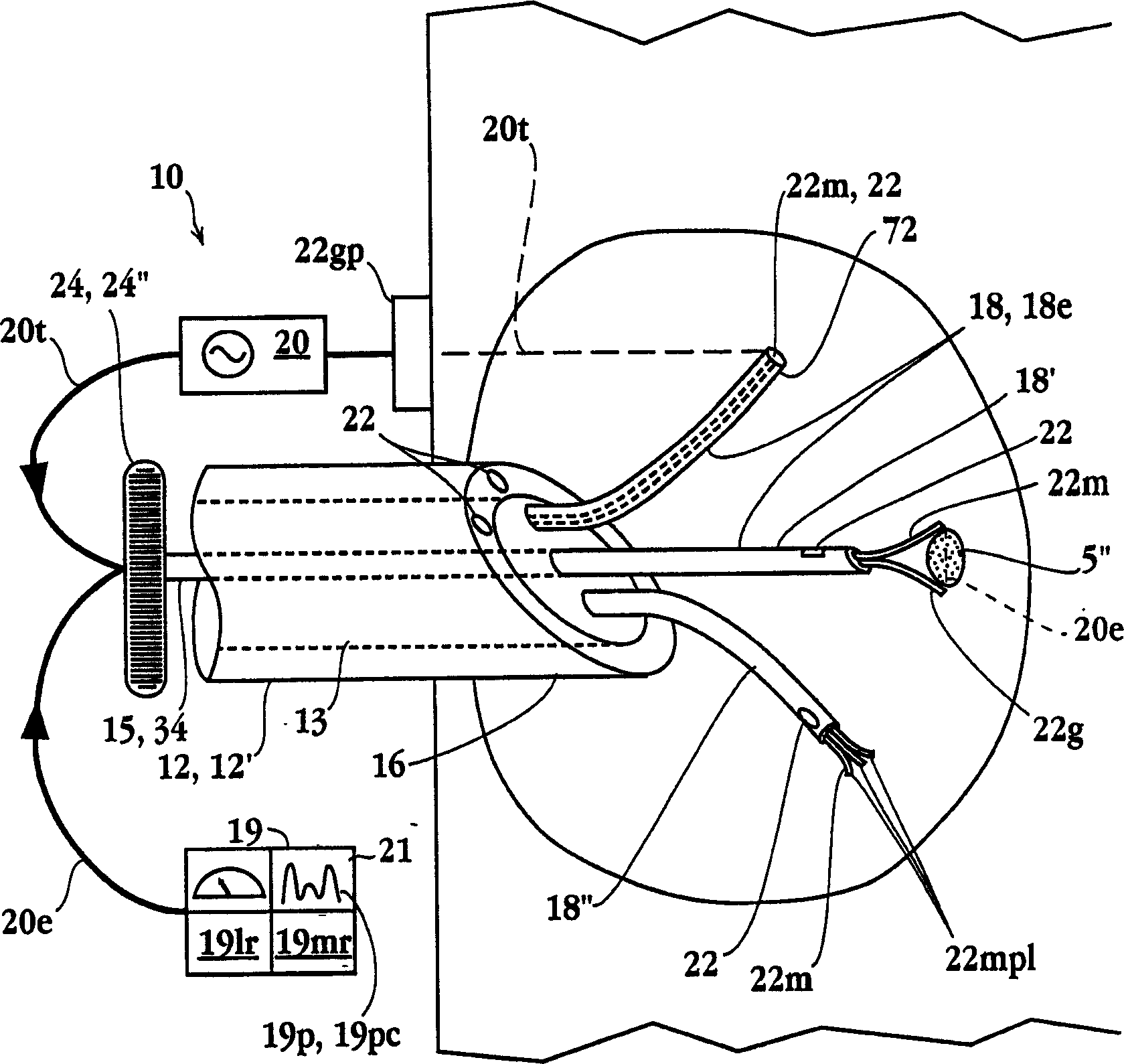
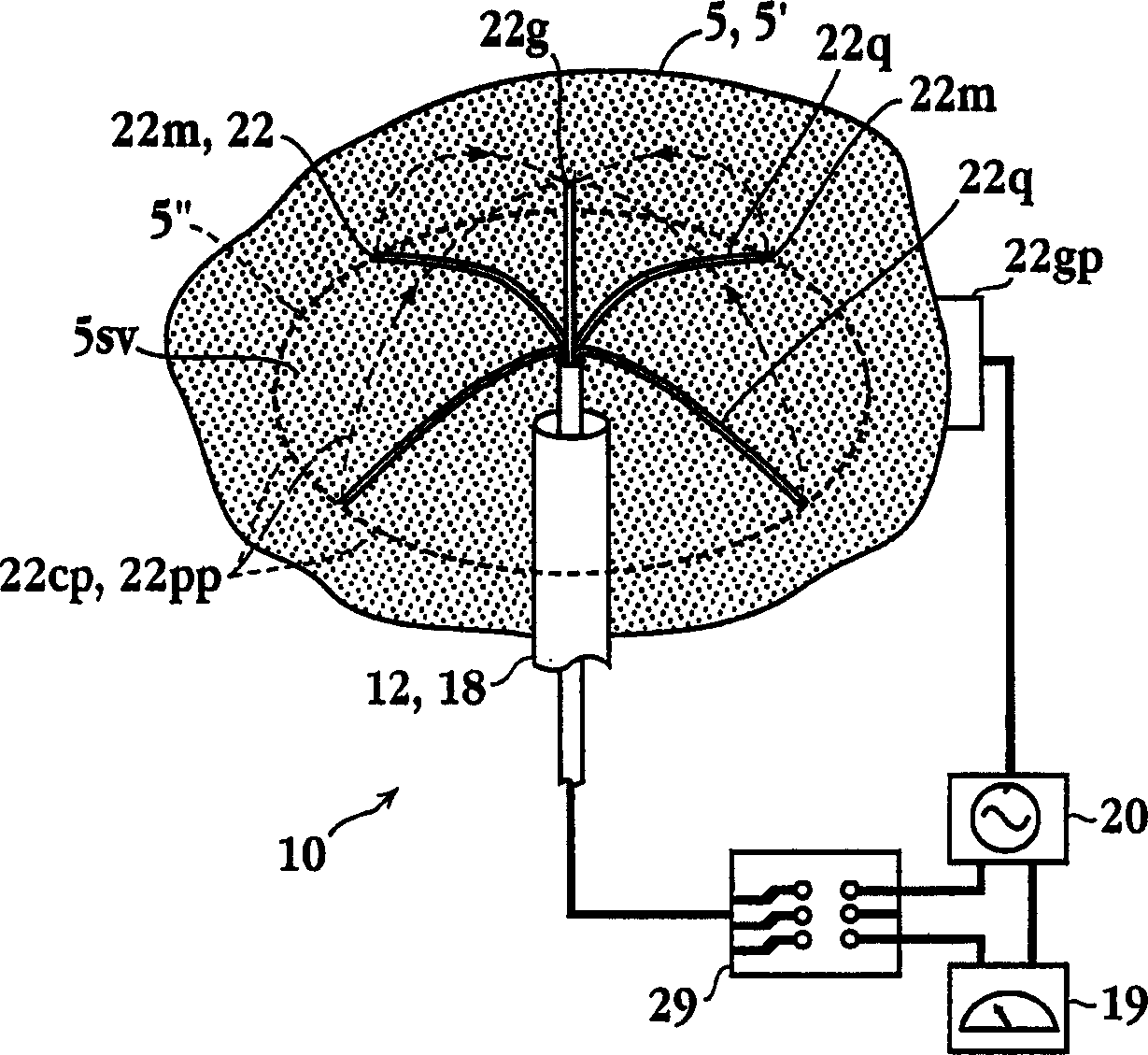
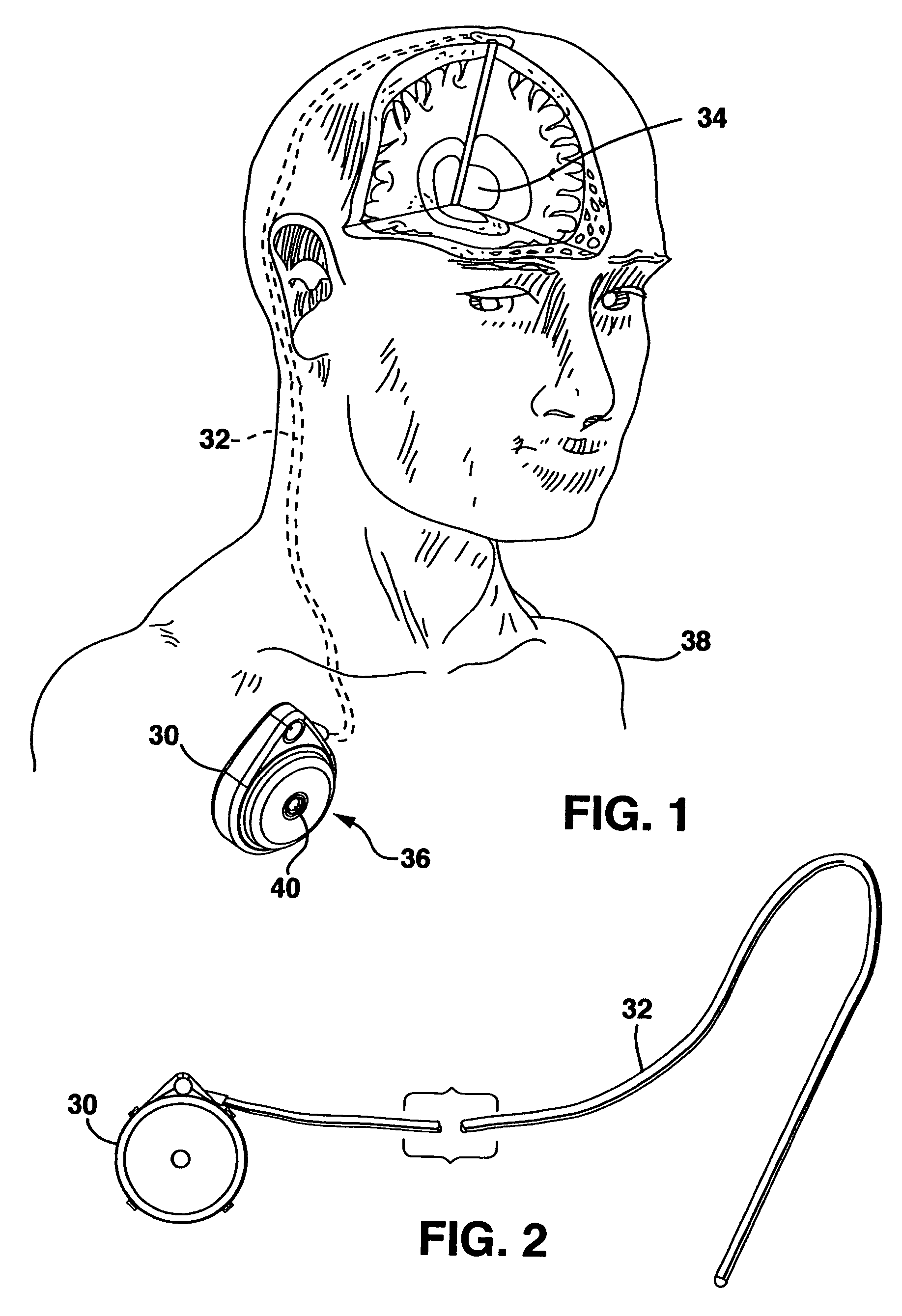
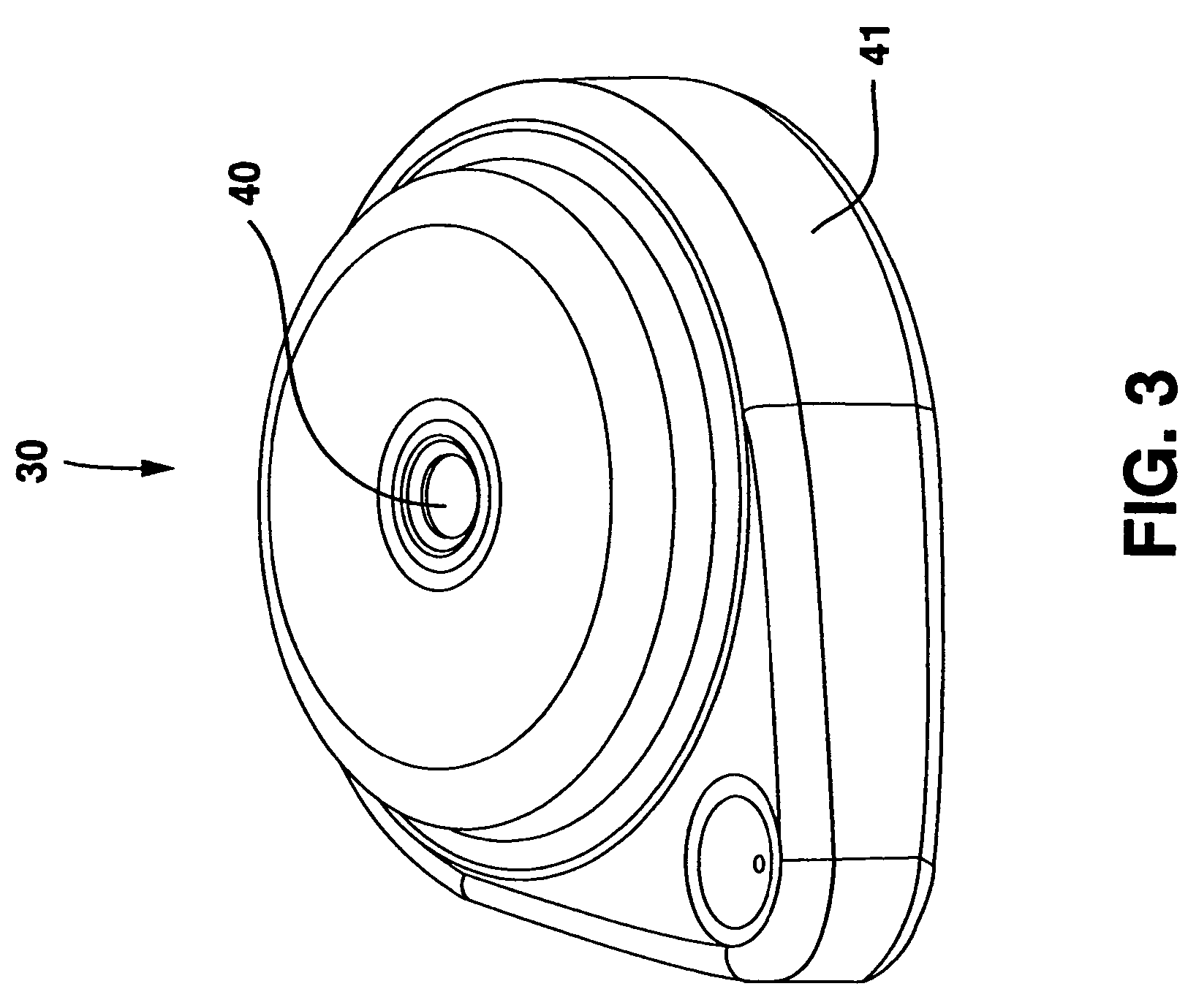
Tissue ablation apparatus and method
InactiveUS20080287944A1Improve the level ofIncrease in sizeSurgical needlesDiagnostic recording/measuringRf ablationTarget tissue
A method and apparatus for carrying our thermal ablation of target tissue is disclosed. The apparatus includes an RF ablation device having a multi-electrode electrode assembly designed to be deployed in target tissue, defining a selected-volume tissue region to be ablated, and having infusion channels for infusing a liquid into the target tissue during the ablation process. A control unit in the apparatus is operably connected to an RF energy source, for controlling the RF power level supplied to the electrodes, and to an infusion device, for controlling the rate of infusion of a liquid through the device into the tissue. During both electrode deployment and tissue ablation, impedance and or temperature measurements made within the tissue are used to control the RF source and infusion device, for optimizing the time and extent of tissue ablation.
Owner:ANGIODYNAMICS INC
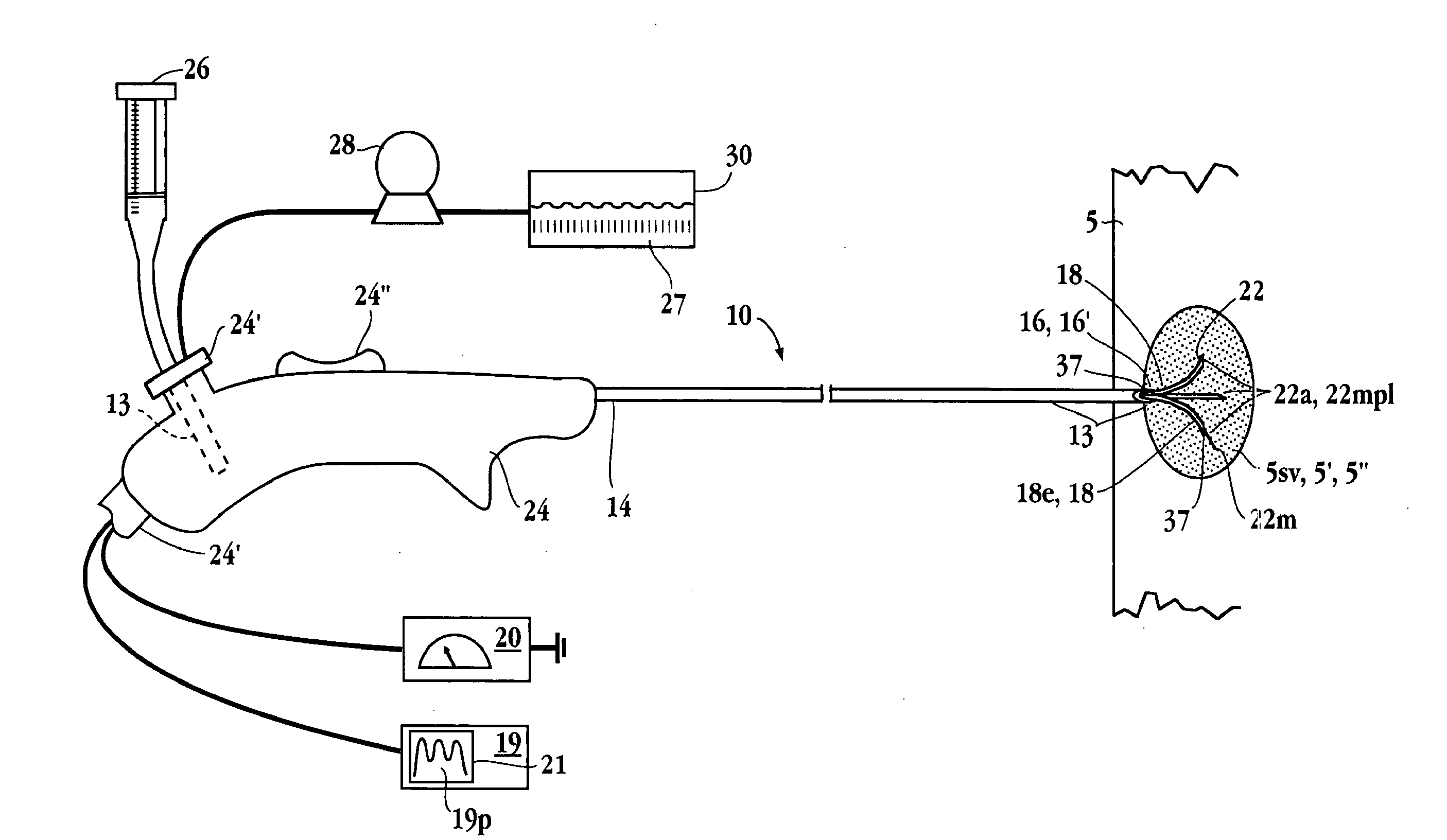
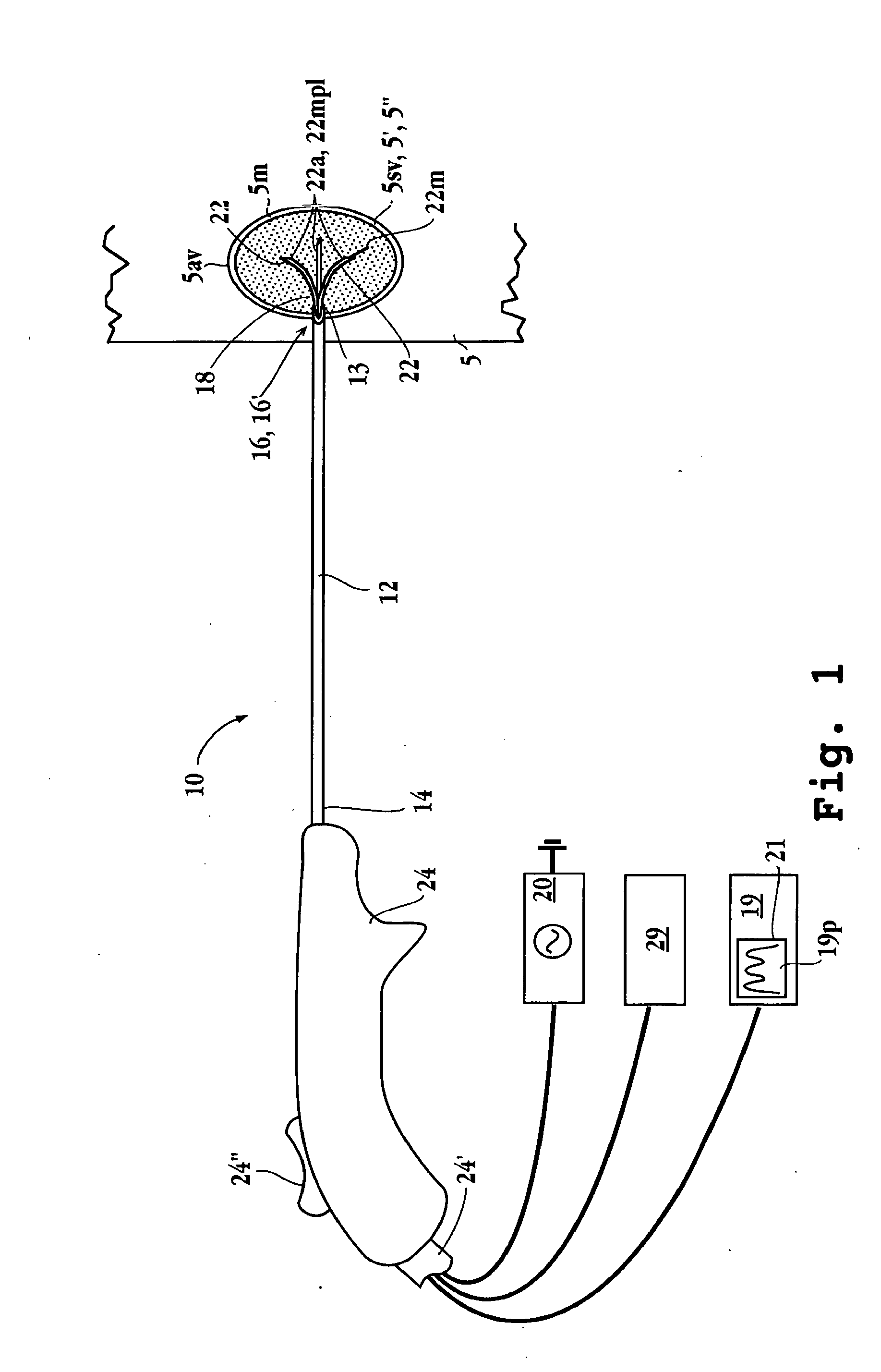
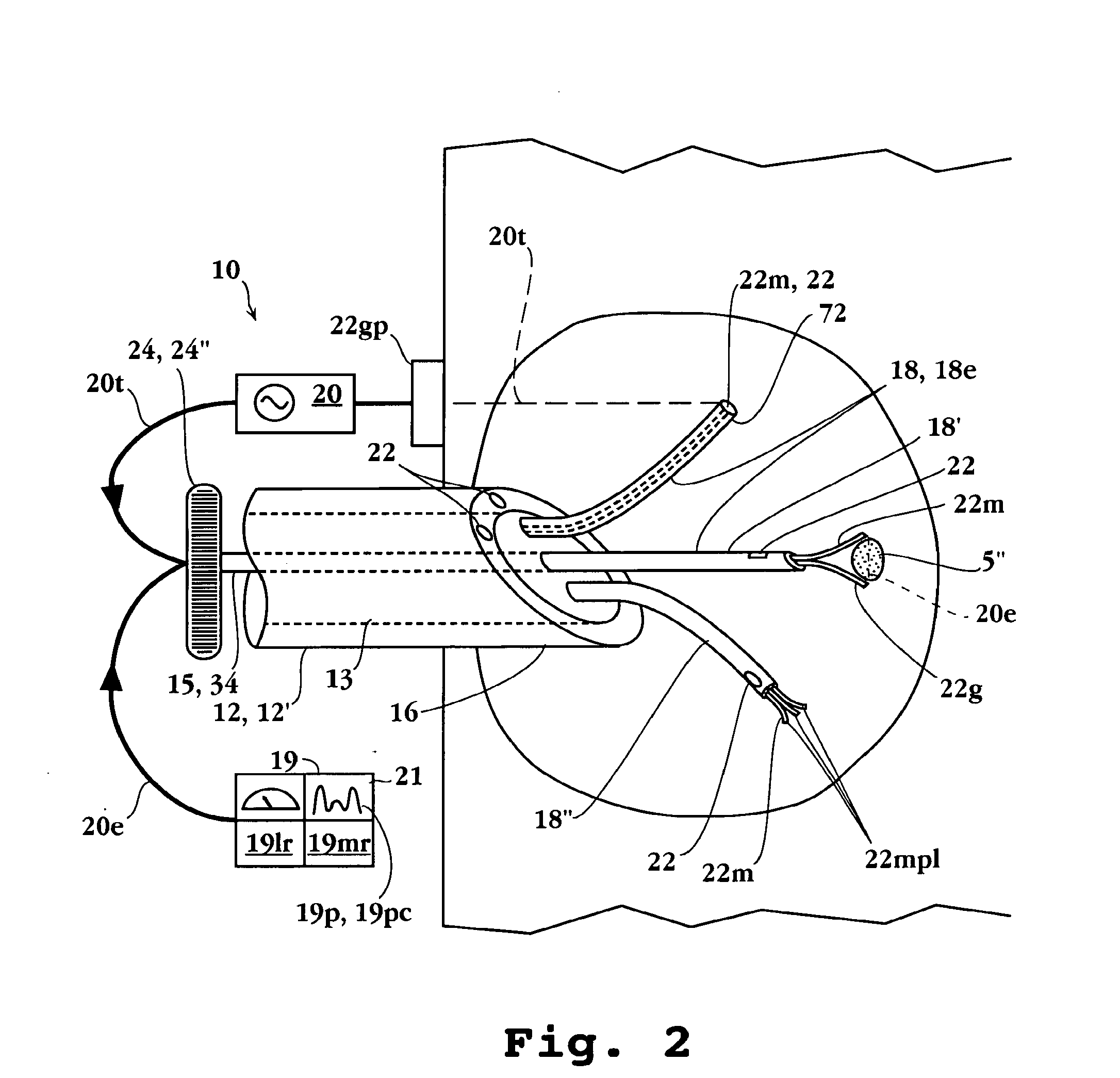
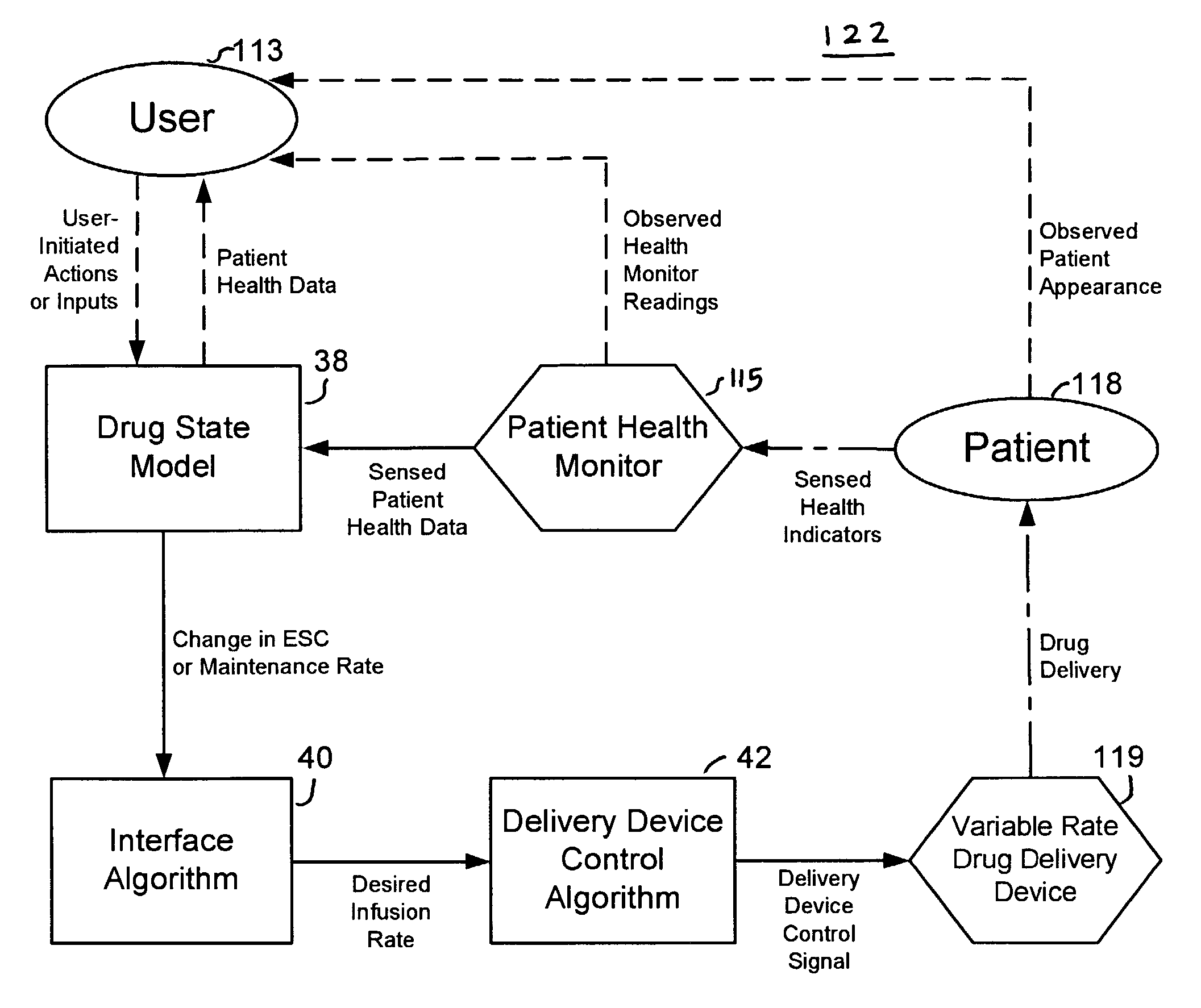
Apparatuses and methods for titrating drug delivery
InactiveUS7229430B2Reduce clinical useSafety managementMedical simulationDrug and medicationsState modelTime profile
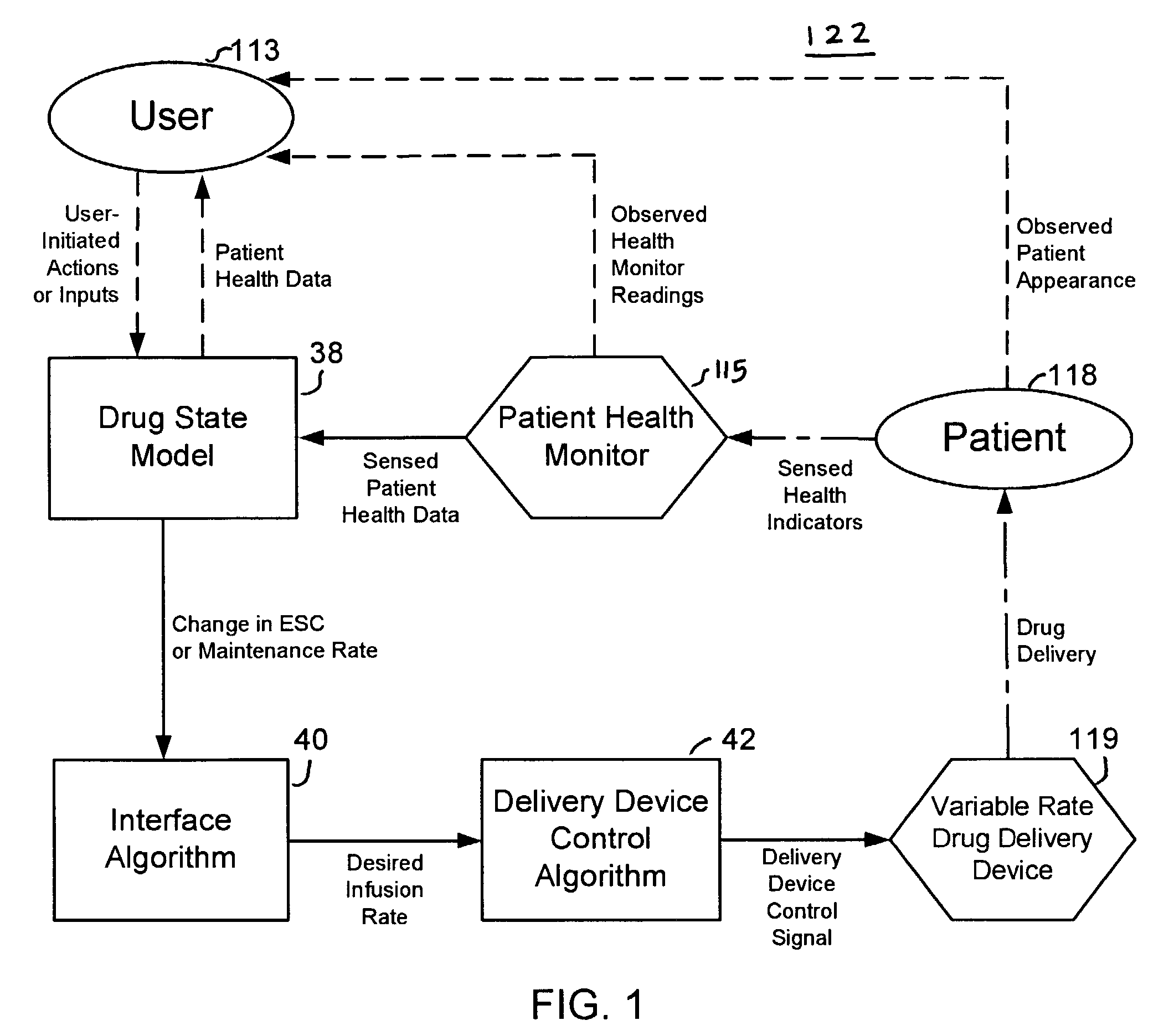
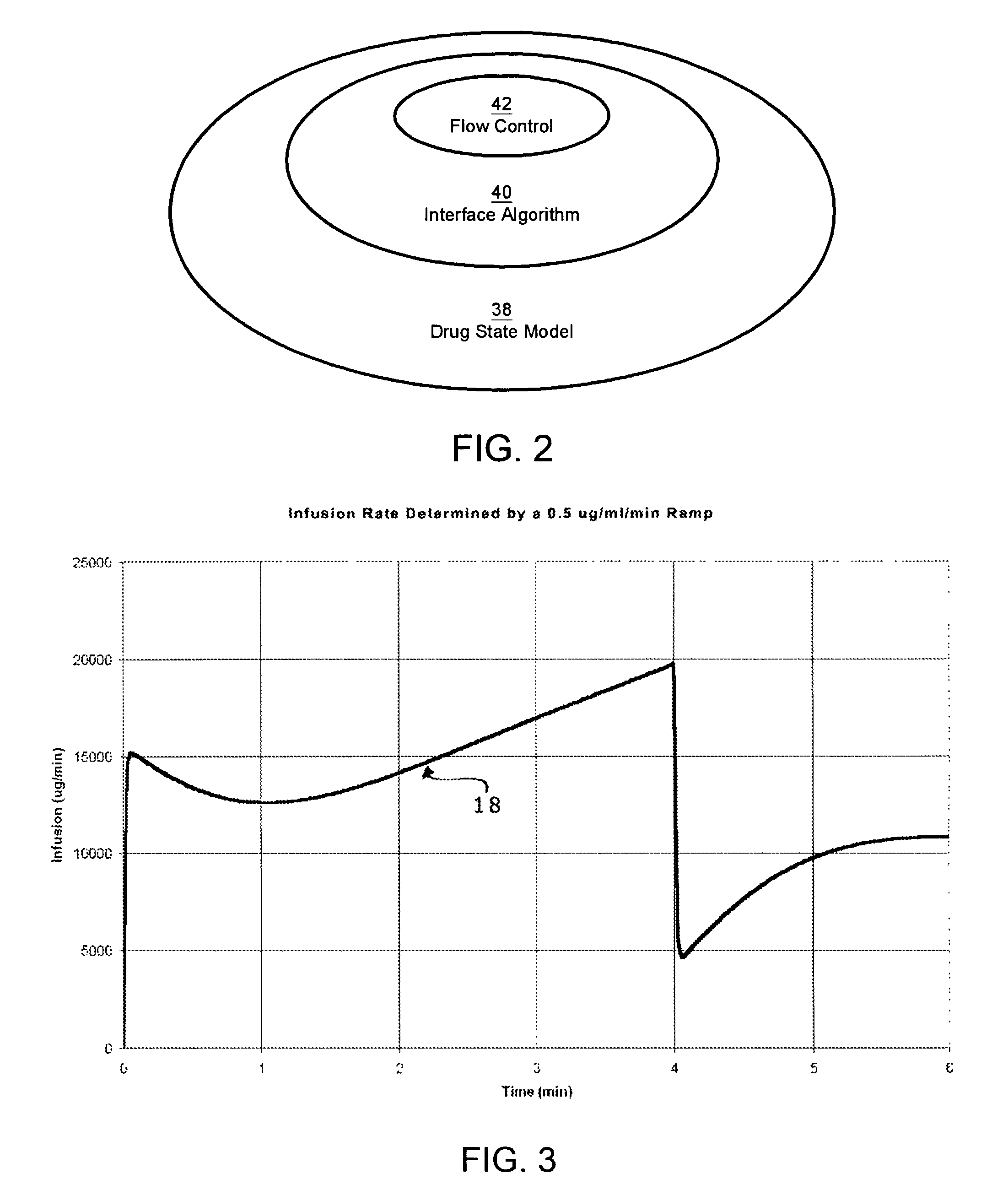
A method and apparatus for reducing the workload of titrating drug to effect while leaving clinician users in control of a related procedure is described. A drug delivery device is controlled to achieve a target drug concentration at a selected site in the patient or a predetermined infusion rate waveform. The time profile of the target drug concentration or a predetermined infusion rate waveform is controlled by a drug state model that uses clinical heuristics to implement safe, pre-defined changes in the target drug concentration or infusion rate and user-commanded changes in target drug concentration or infusion rate. The invention allows time to assess the response of the patient to changes in drug level by making small incremental and conservative changes in drug level over time.
Owner:SCOTT LAB
Osmotic pump drug delivery systems and methods
InactiveUS6471688B1Avoid mixingMedical devicesPharmaceutical delivery mechanismTreatment effectAnalgesics effects
Implantable osmotic pump devices and systems include multiple osmotic pumps and / or semipermeable membranes to extend the useful life cycle and functionality of the drug delivery system. Use of an implantable system including multiple implantable osmotic pumps allows different drugs to be administered from the same implanted system. One or more of the semipermeable membranes of the system may be initially sealed by an overlying impermeable membrane upon implantation of the system into the patient. When the patient develops a tolerance to a first drug or to a first dose of the first drug, the impermeable membrane may be breached, to expose the underlying semipermeable membrane to the osmotic pressure of the patient at the implant site. This causes the infusion rate to increase, thereby providing the patient with the needed relief and / or other desired therapeutic effect. In the case of a multiple pump system, breaching an impermeable membrane may cause the infusion of a second drug. The second drug may potentiate a therapeutic effect (such as an analgesic effect) of the first drug, as is the case with Sufentanil and Clonidine.
Owner:MICROSOLUTIONS
Permanent magnet solenoid pump for an implantable therapeutic substance delivery device
InactiveUS7288085B2Energy efficiencyPharmaceutical delivery mechanismMedical devicesGenetic MaterialsElectromagnetic pump
A medical device known as an implantable therapeutic substance delivery device is configured for implanting in humans to deliver a therapeutic substance such as pharmaceutical compositions, genetic materials, and biologics to treat a variety of medical conditions such as pain, spastisity, cancer, and many other conditions. The therapeutic substance delivery device has a permanent magnet solenoid pump that is energy efficient, accurate, small, compatible with therapeutic substances, and has many other improvements. The implantable therapeutic substance delivery device has a housing, a therapeutic substance reservoir, a power source, electronics, and a permanent magnet solenoid pump. The therapeutic substance reservoir is configured to contain a therapeutic substance and is coupled to the housing. The power source is carried in the housing to power the electronics and solenoid pump. The electronics are coupled to the solenoid pump and the solenoid pump is coupled to the therapeutic substance reservoir. The permanent magnet solenoid pump is configured for pumping therapeutic substance from the therapeutic substance reservoir through an infusion outlet at a programmed infusion rate. Many embodiments of the permanent magnet solenoid pump and its methods of operation are possible.
Owner:MEDTRONIC INC
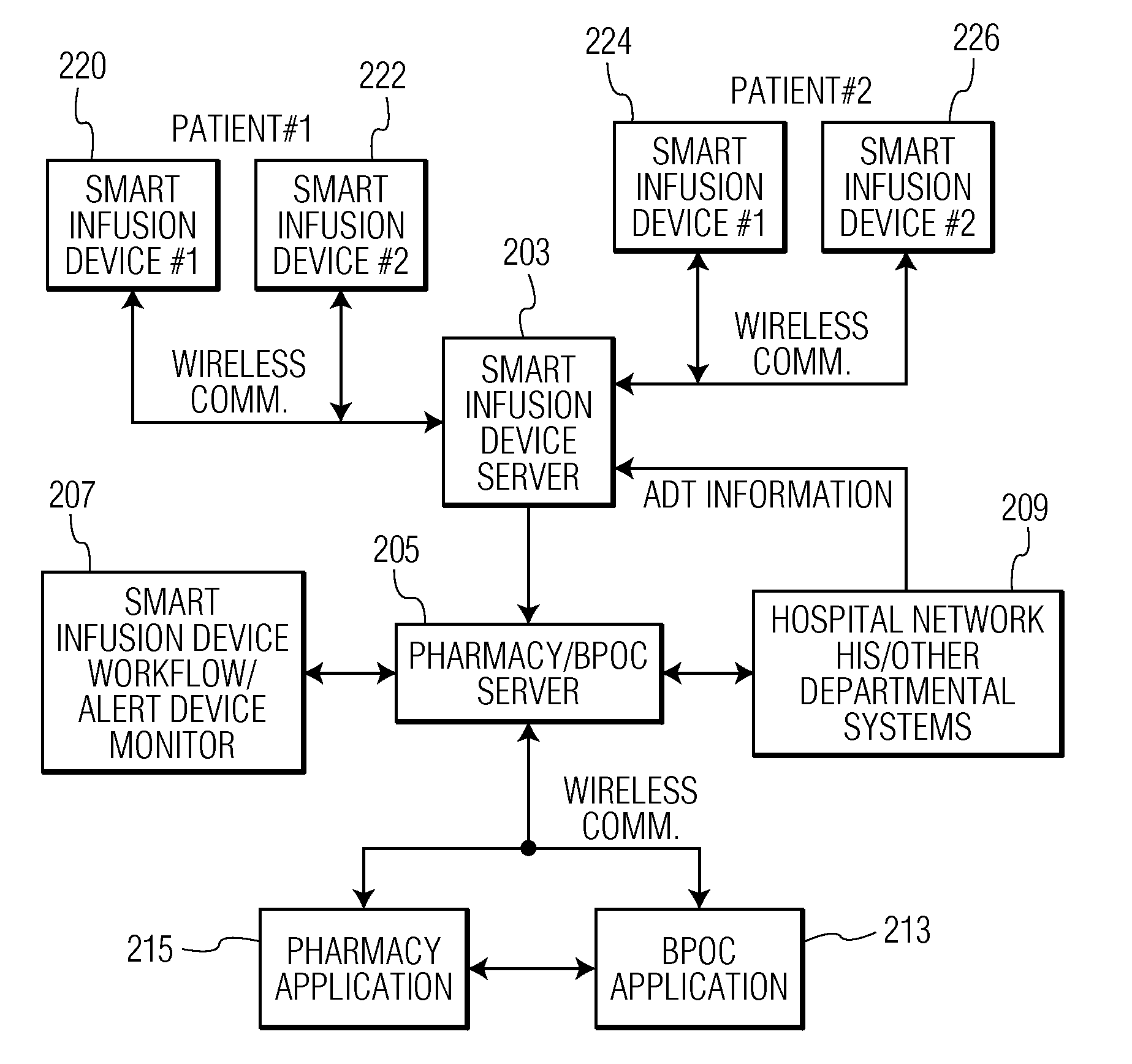
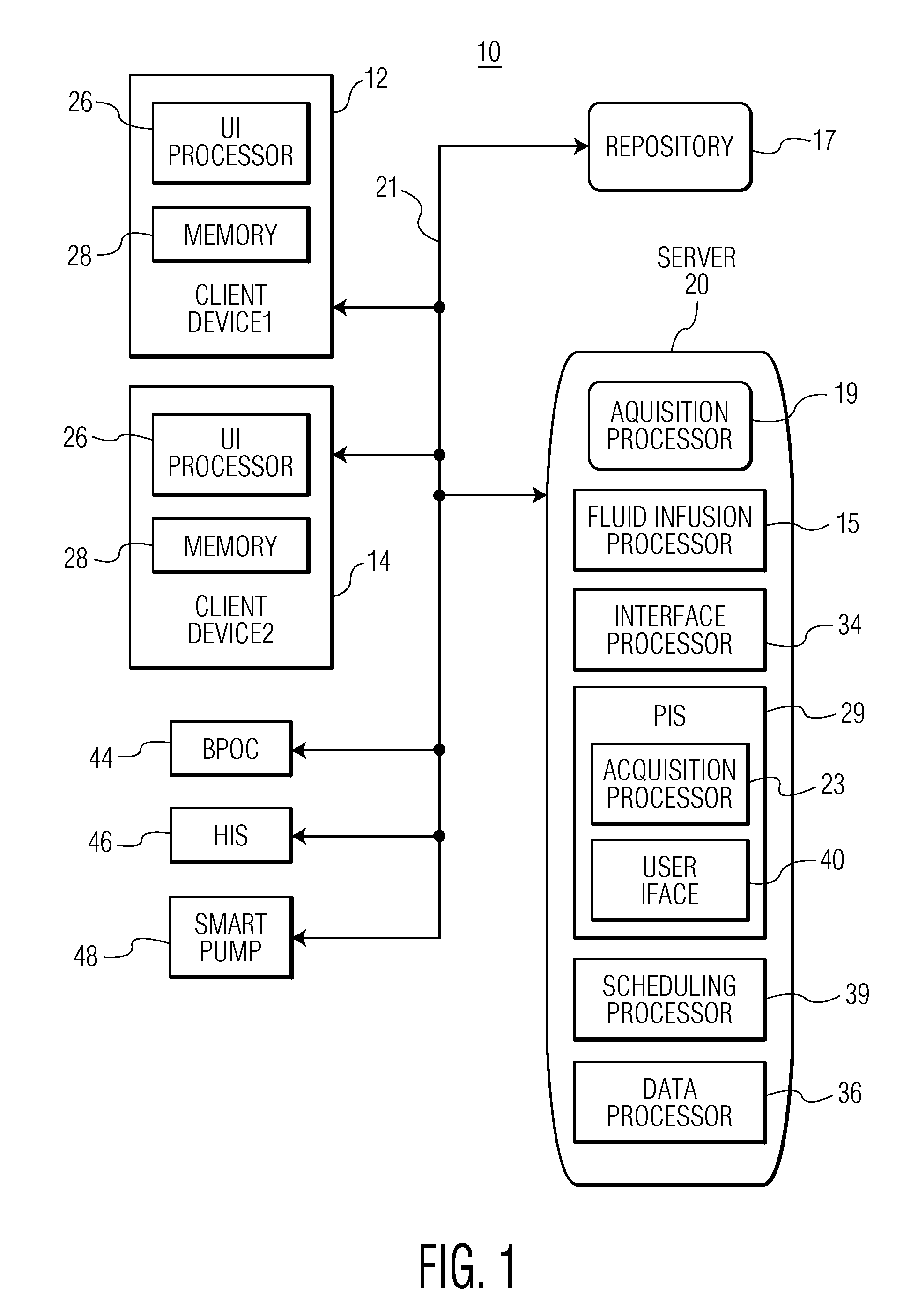
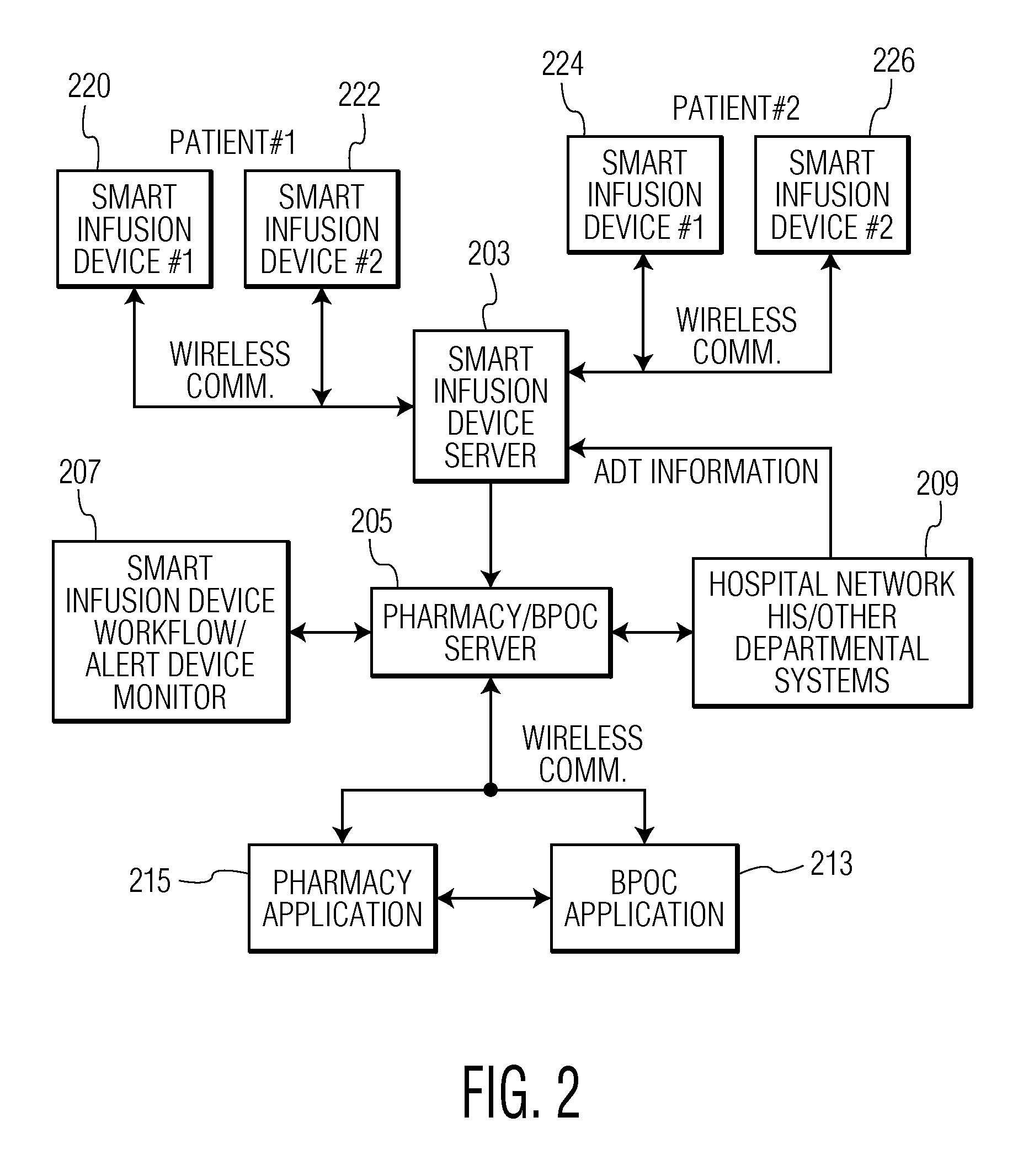
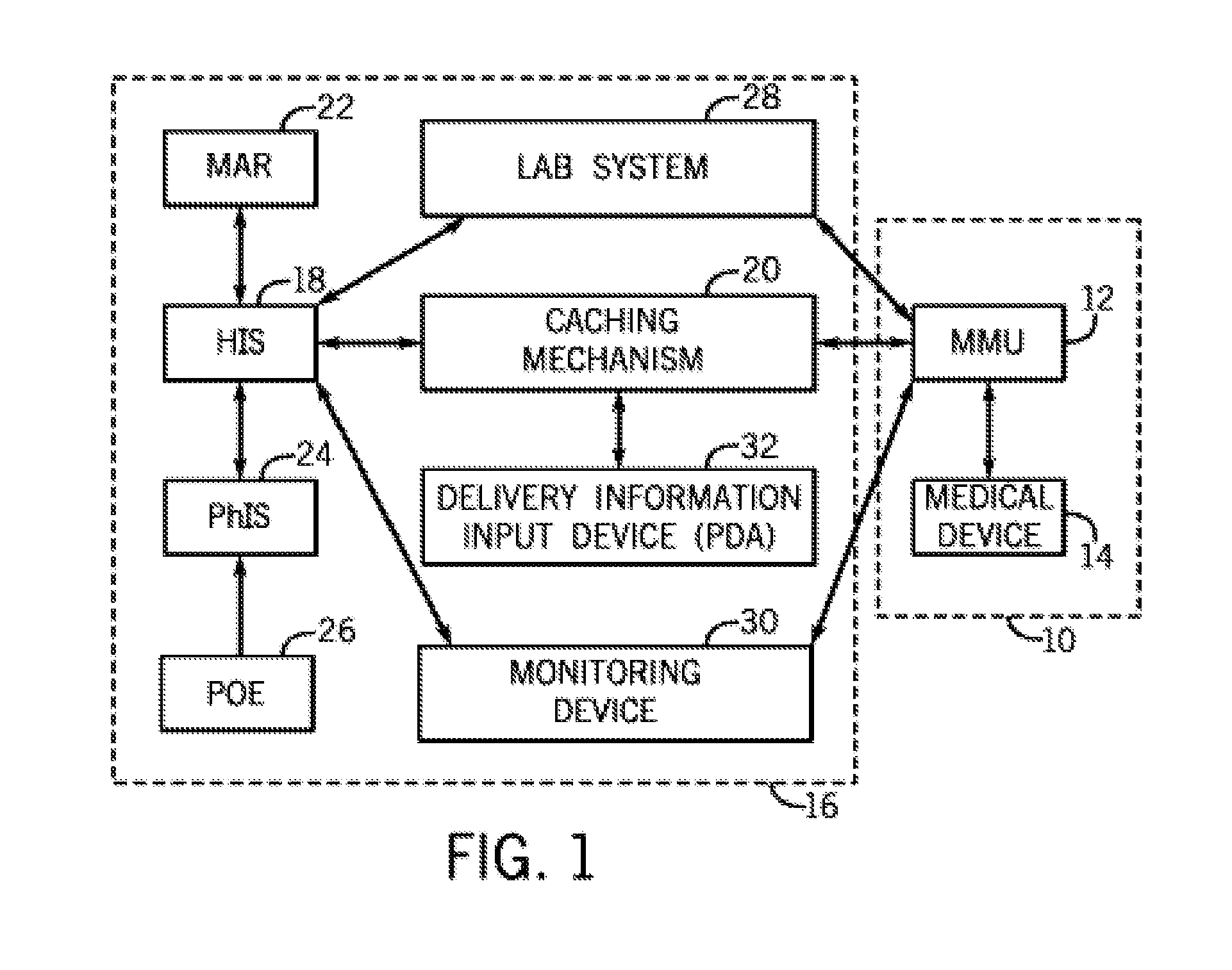
Integrated Medication and Infusion Monitoring System
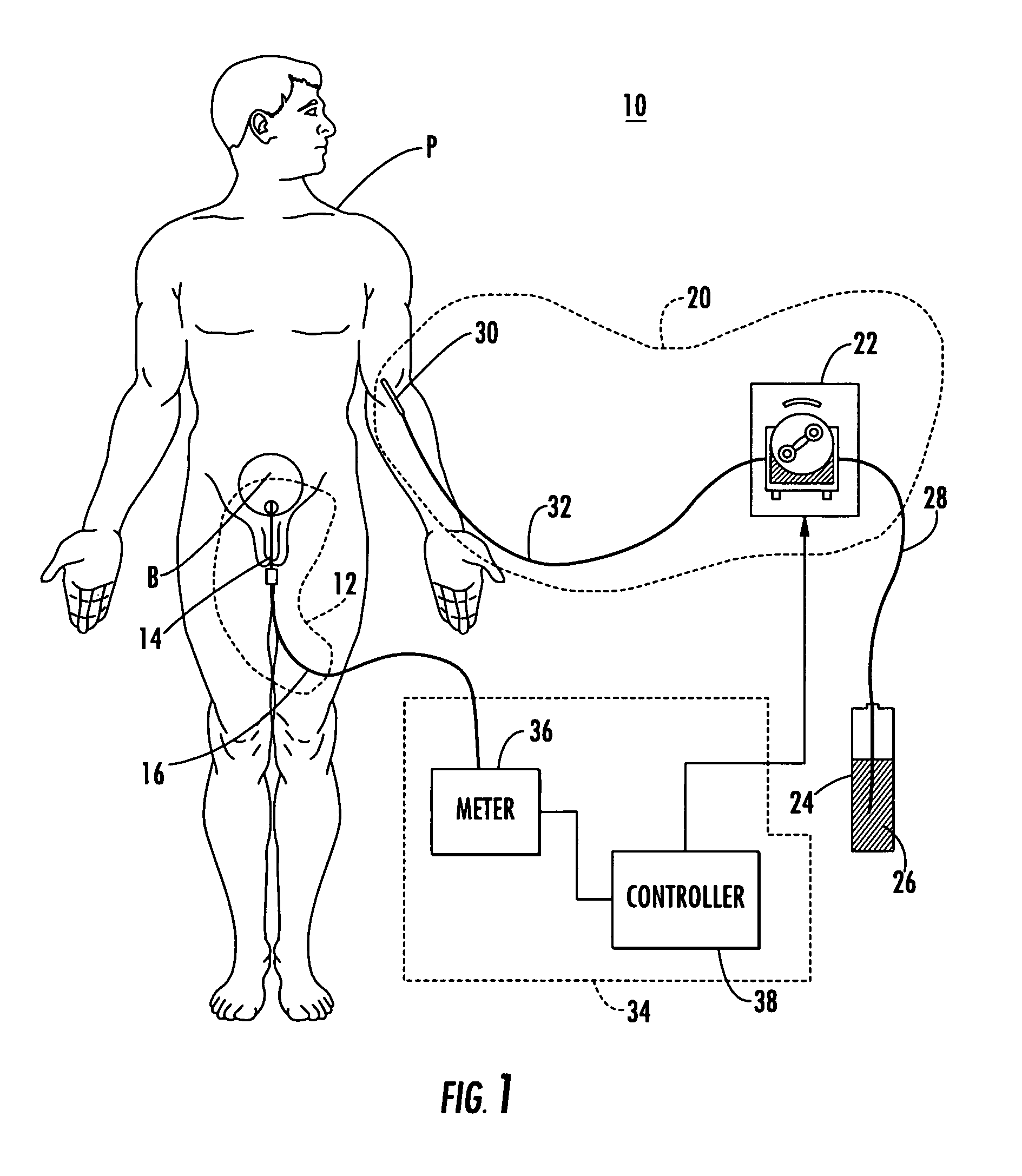
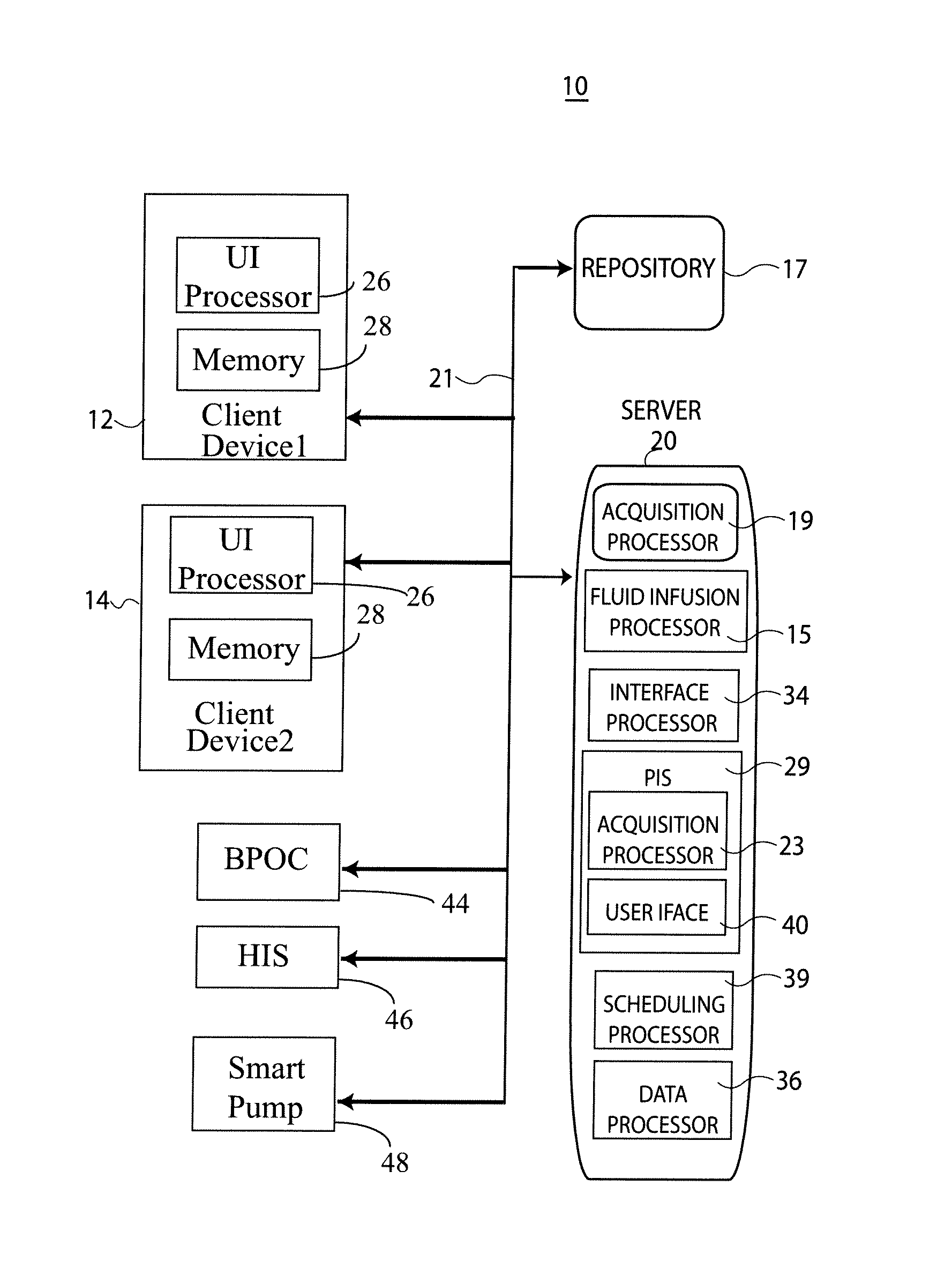
ActiveUS20100121654A1Data processing applicationsDrug and medicationsMedical recordInformation repository
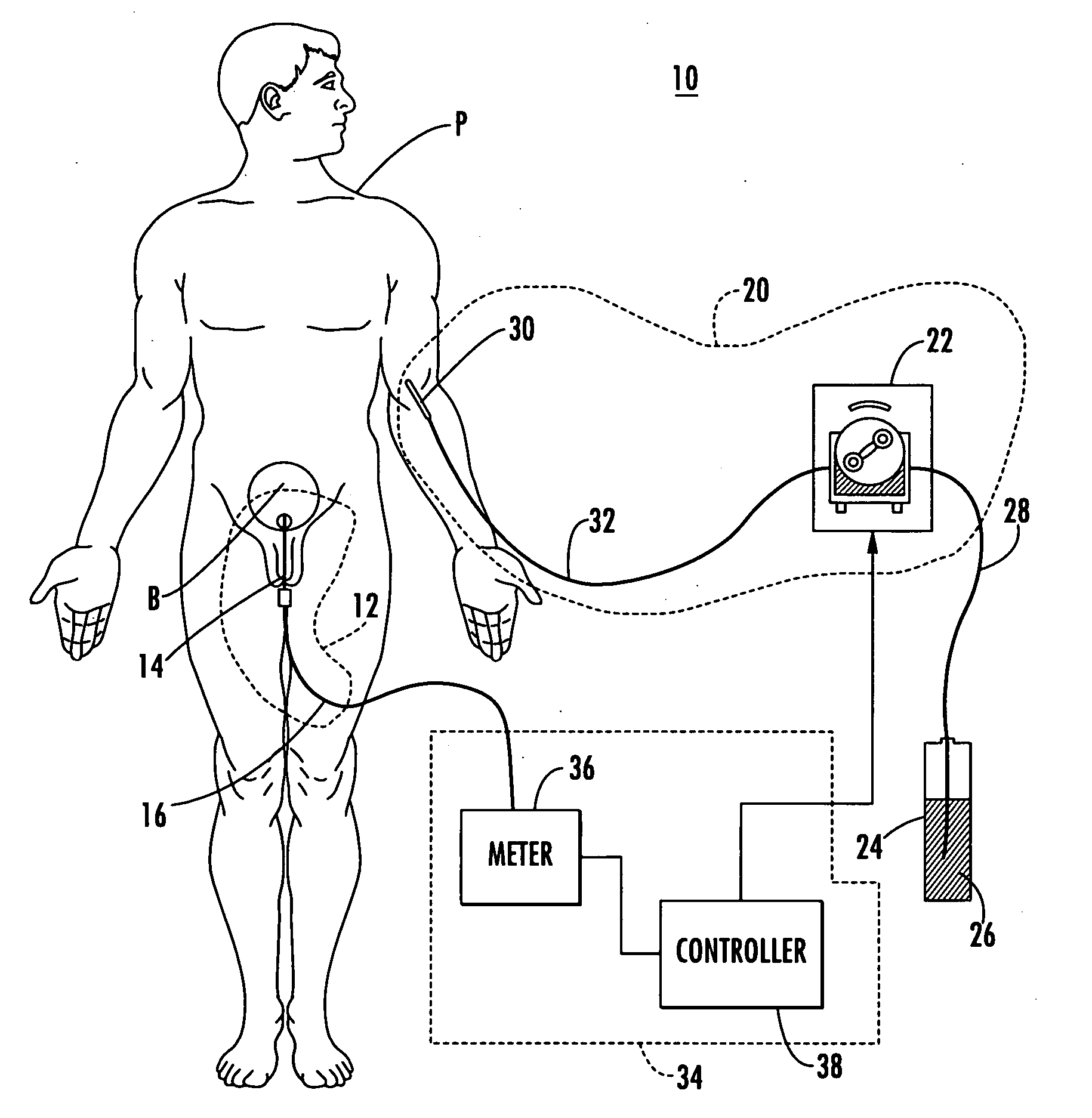
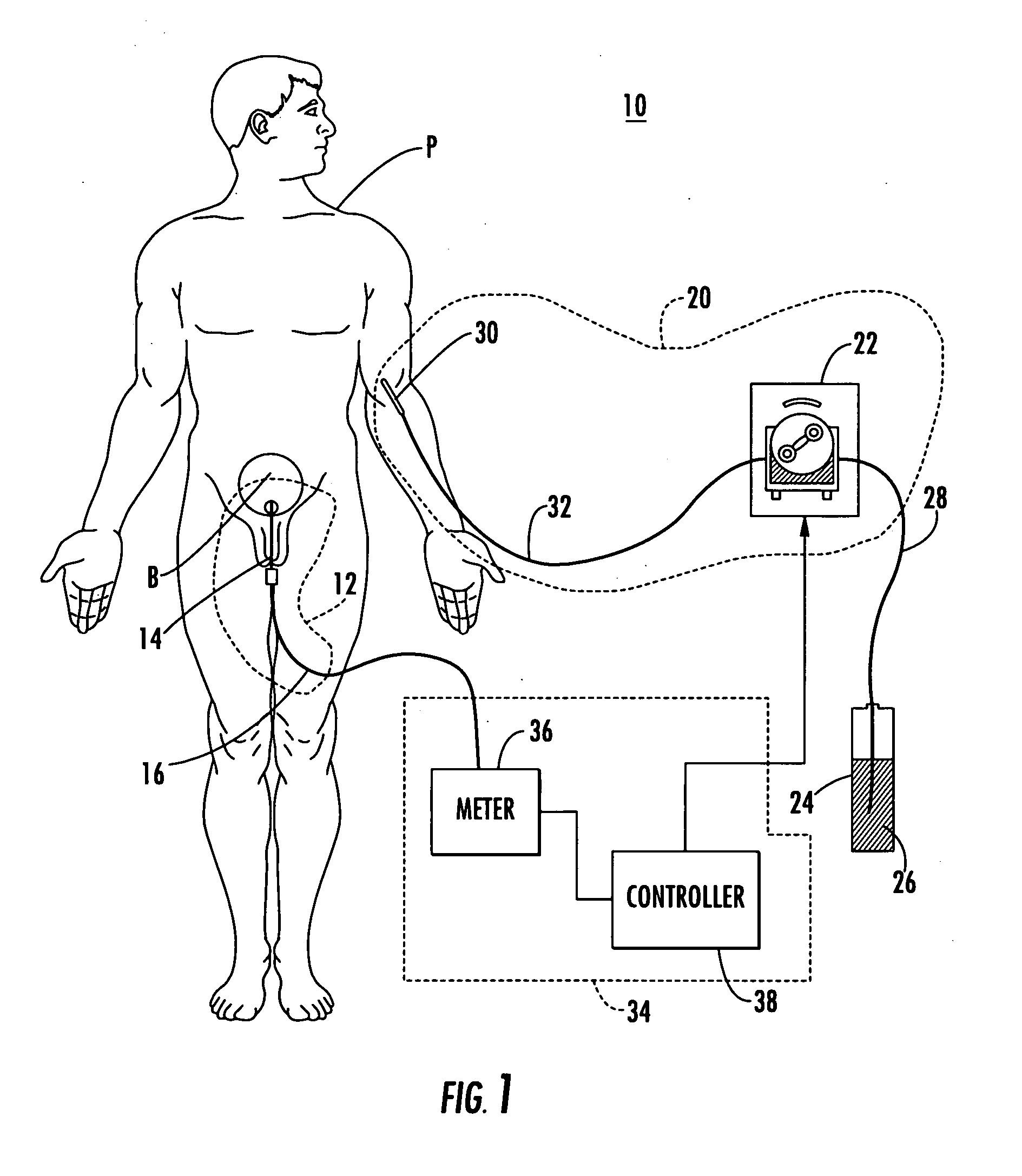
A System manages IV pumps so that clinicians automatically receive alerts, decisions, and actions required to maintain a patient IV medication therapy according to a prescribed treatment protocol. An infusion pump monitoring system, includes an acquisition processor for acquiring fluid infusion parameters comprising a patient identifier, infusion fluid identifier and a rate of fluid infusion, for administration of an infusion fluid to a patient at a point of care using an infusion pump. The system also includes a repository of patient medical record information. A fluid infusion monitor uses acquired fluid infusion parameters for automatically searching a patient medical record in the repository for information concerning rate of fluid infusion of a particular infusion fluid and determining if a rate of a previously administered dose of the particular infusion fluid was lower than a rate indicated in the fluid infusion parameters. An interface processor automatically initiates generation of a message indicating a potential adverse reaction to the particular infusion fluid in response to a determination of a lower rate being employed for previously administering the particular infusion fluid.
Owner:CERNER INNOVATION
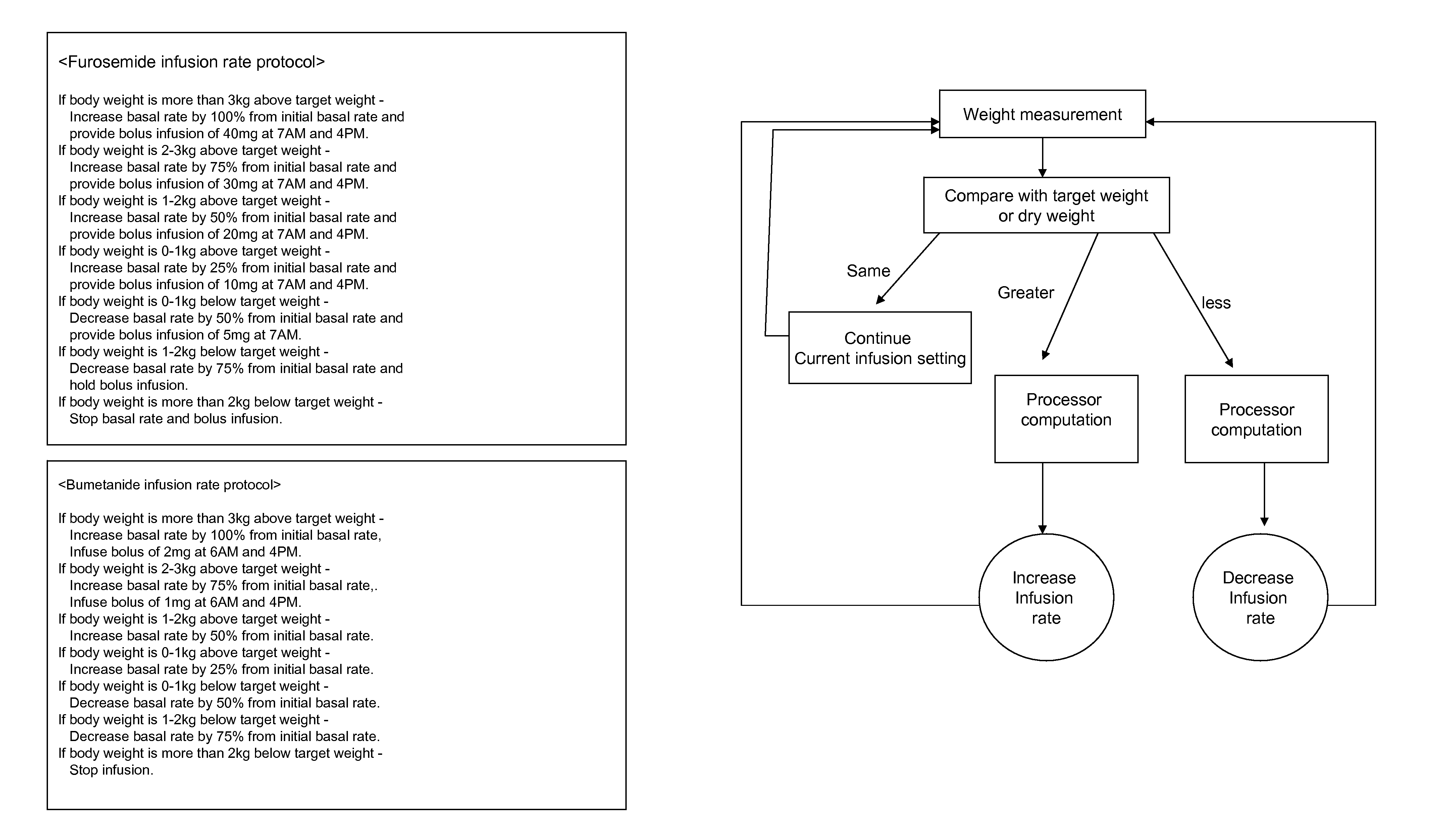
Infusion rate adjusting device for drug solution injector
Owner:NIPRO CORP
Control of body fluid condition using diuretics, based on biological parameters
InactiveUS20090062730A1Reduce deathReduce hospitalizationMedical devicesIntravenous devicesMedicineHuman patient
The system for controlling body fluids overcomes the limitations of the prior art by automatically infusing diuretic and / or other drugs into a human patient. In one approach, the rate of infusion of the diuretic is adjusted based on a measured biological parameter of the patient. For example, this biological parameter can be transmitted wirelessly to a portable diuretic infusion device attached to the patient.
Owner:WOO SAN HOON
Patient hydration system with abnormal condition sensing
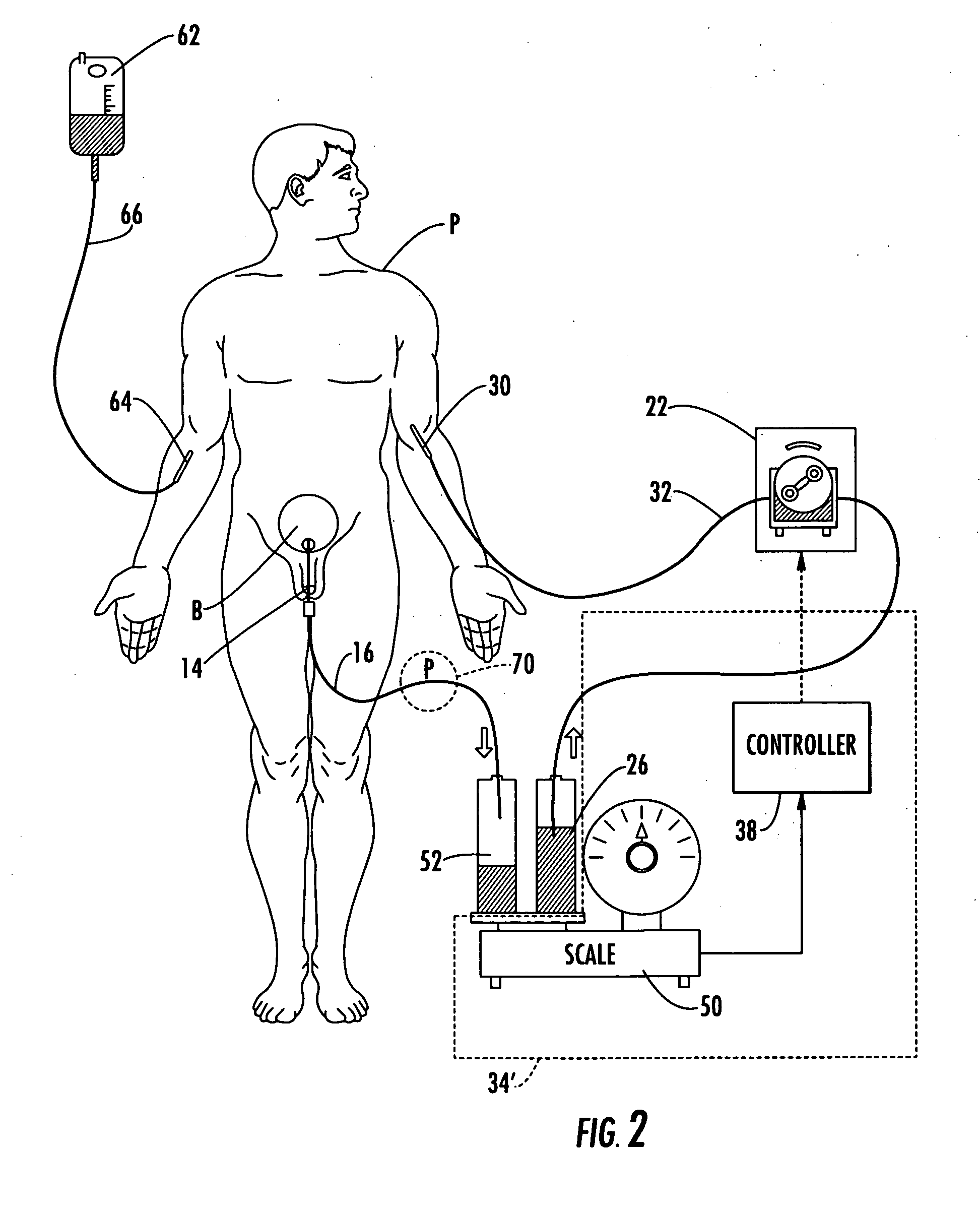
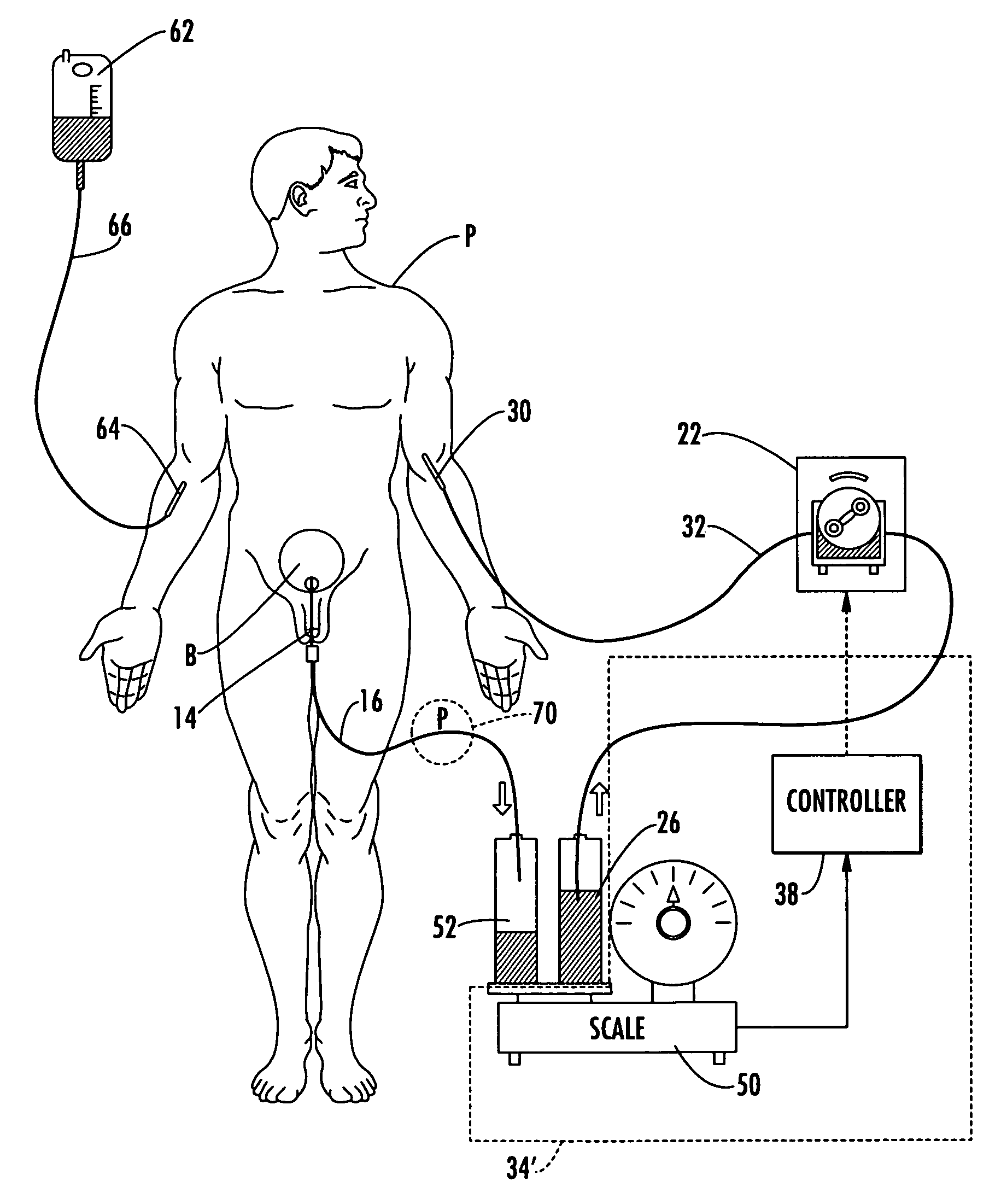
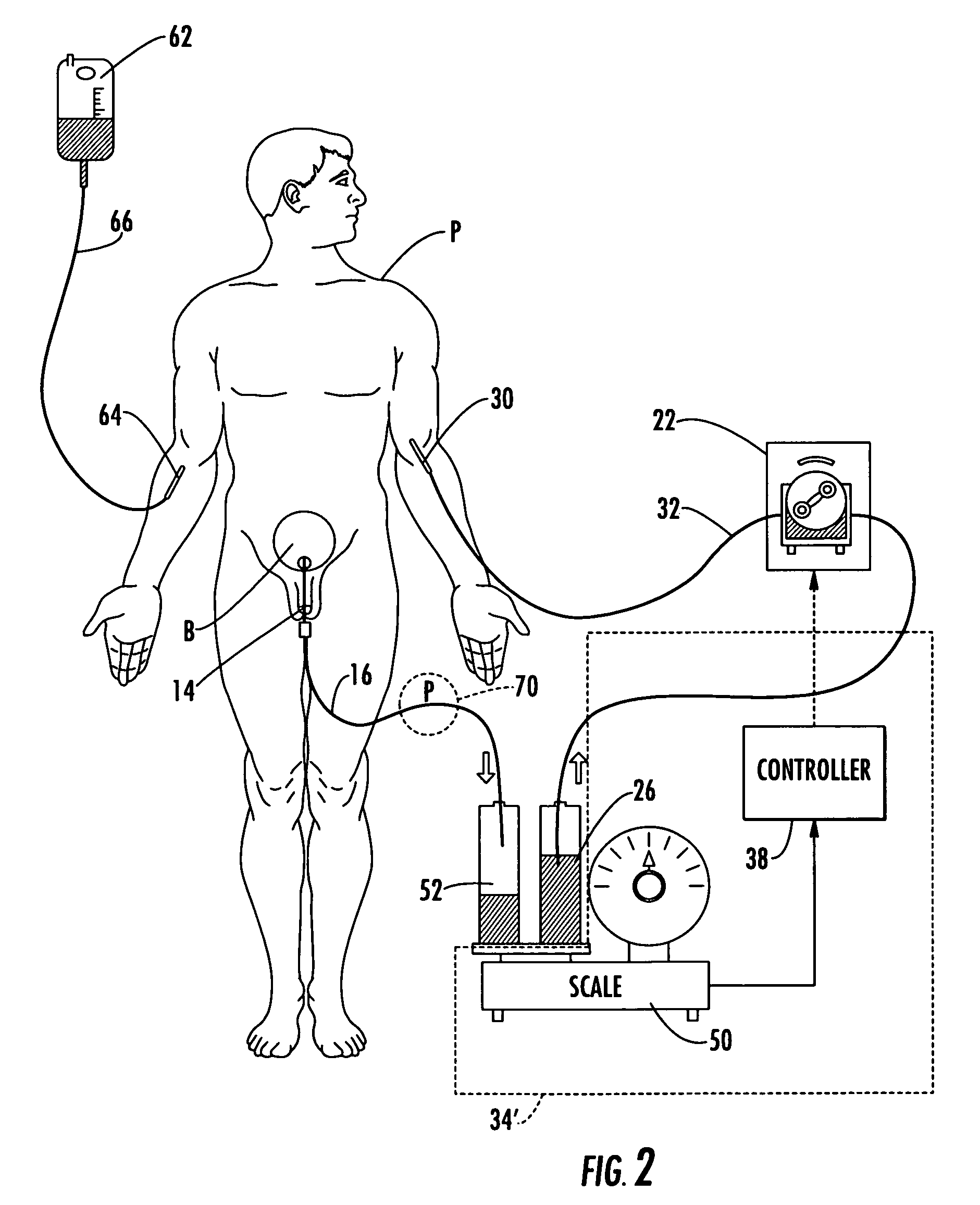
ActiveUS20060235353A1Prevent kidney damagePrevent dehydrationMedical devicesPressure infusionUrine outputIntensive care medicine
A patient hydration system and method wherein hydration fluid is administered to a patient and the patient's urine output is measured either by weight or some other suitable way. A controller is responsive to the patient's urine output measurement and configured to control the infusion rate to hydrate the patient based on the patient's urine output. The controller also detects abnormal patient urine output measurements and initiates corrective action in response.
Owner:MEDICAL SYST
Variable flow control device, system and method
InactiveUS20130138075A1Easy to controlImprove securityMedical devicesPressure infusionEngineeringInfusion pump
A device, system and method are provided for controlling the rate of infusion of fluids during infusion therapy using non-electric infusion devices. Rotation of a flow regulator dial causes an orifice connected to the inlet to modify its position relative to a particular one or more orifices or groove portions, the characteristics of which provide a certain flow rate characteristic. The regulator allows for the infusion pump to infuse at a rate that may be varied during use by the user.
Owner:BIO HEALTH FRONTIERS
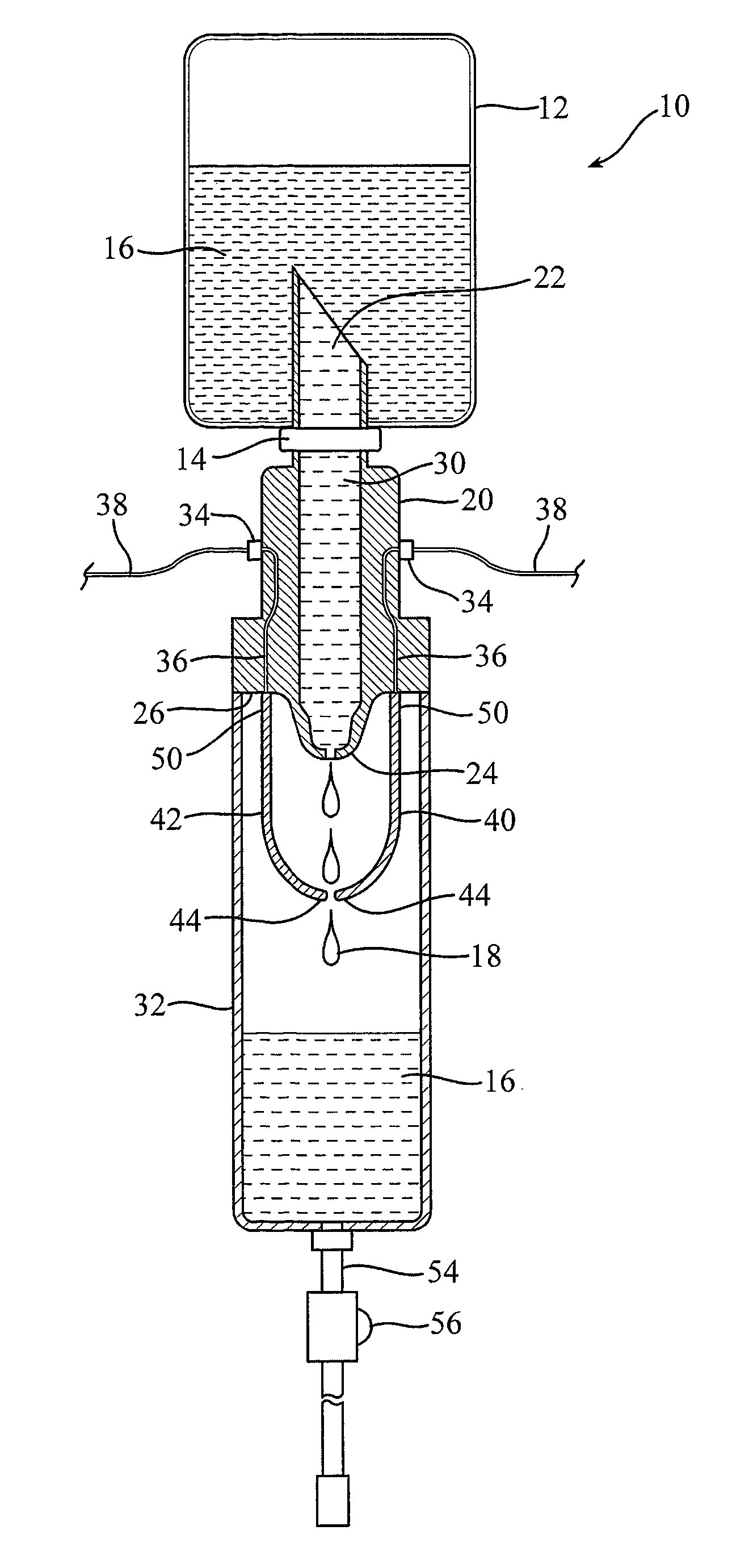
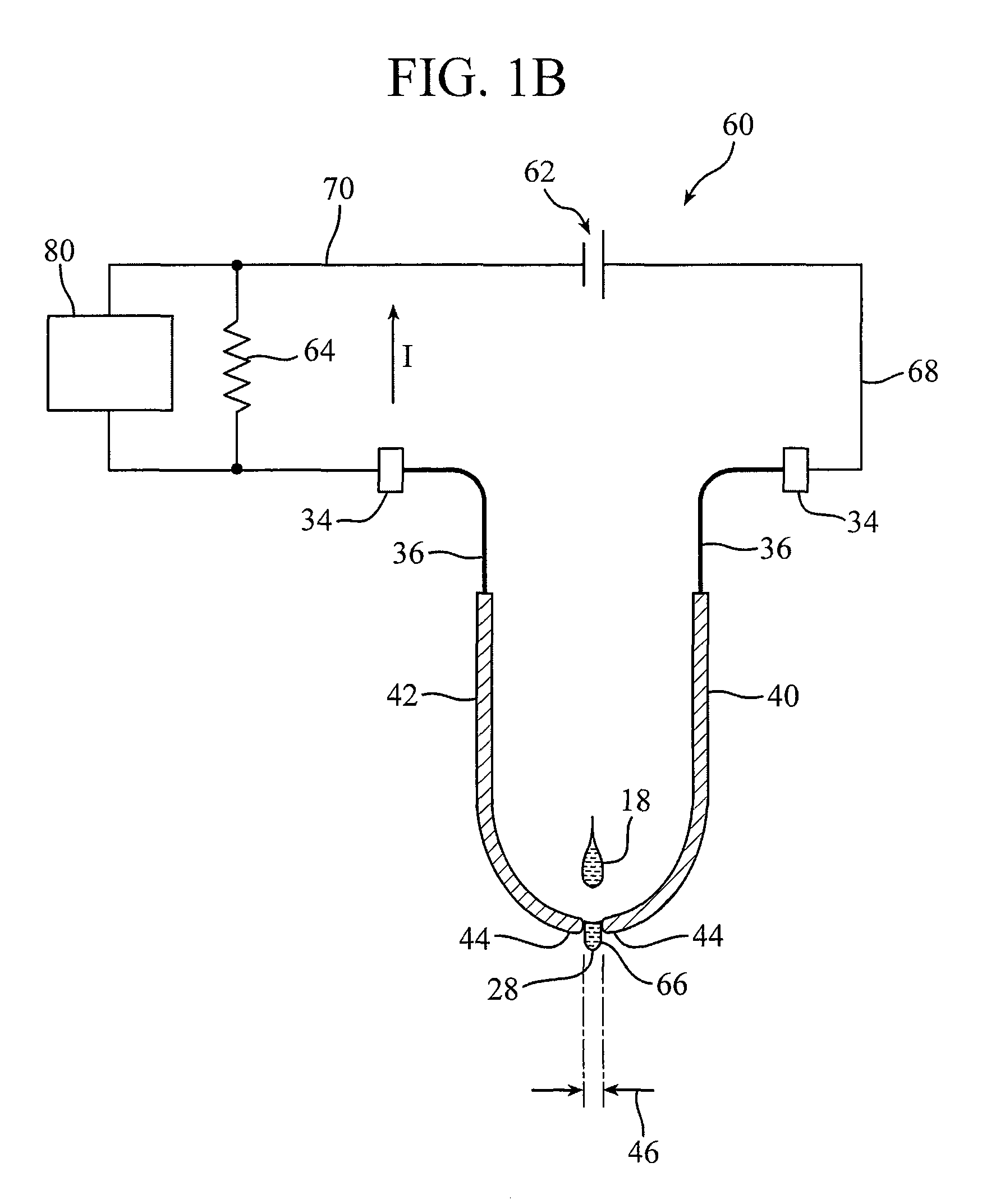
Systems and methods for providing an IV administration set incorporating drip monitoring circuitry
A circuitry for counting drips and monitoring a rate of infusion is incorporated into an IV administration set. The circuitry includes a pair of leads that are positioned in the pathway of fluid droplets, such that each droplet simultaneously contacts both leads. As such, the leads act as a virtual switch that is closed by the presence of a droplet. This event is then displayed on a drip signaling device to aid a user in adjusting the infusion rate of the IV administration set.
Owner:BECTON DICKINSON & CO
Control of Body Fluid Condition Using Diuretics, Based on Weight Measurement
InactiveUS20090062728A1Restrict infusion rateImprove approachMedical devicesIntravenous devicesHuman patientBody fluid
The system for controlling body fluids overcomes the limitations of the prior art by automatically infusing diuretic and / or other drugs into a human patient. In one approach, the rate of infusion of the diuretic is adjusted based on the measured weight of the patient. For example, this weight can be transmitted wirelessly to a portable diuretic infusion device attached to the patient.
Owner:WOO SANG HOON
Patient hydration system with abnormal condition sensing
ActiveUS7837667B2Prevent kidney damageLoss of balanceMedical devicesPressure infusionUrine outputIntensive care medicine
Owner:MEDICAL SYST
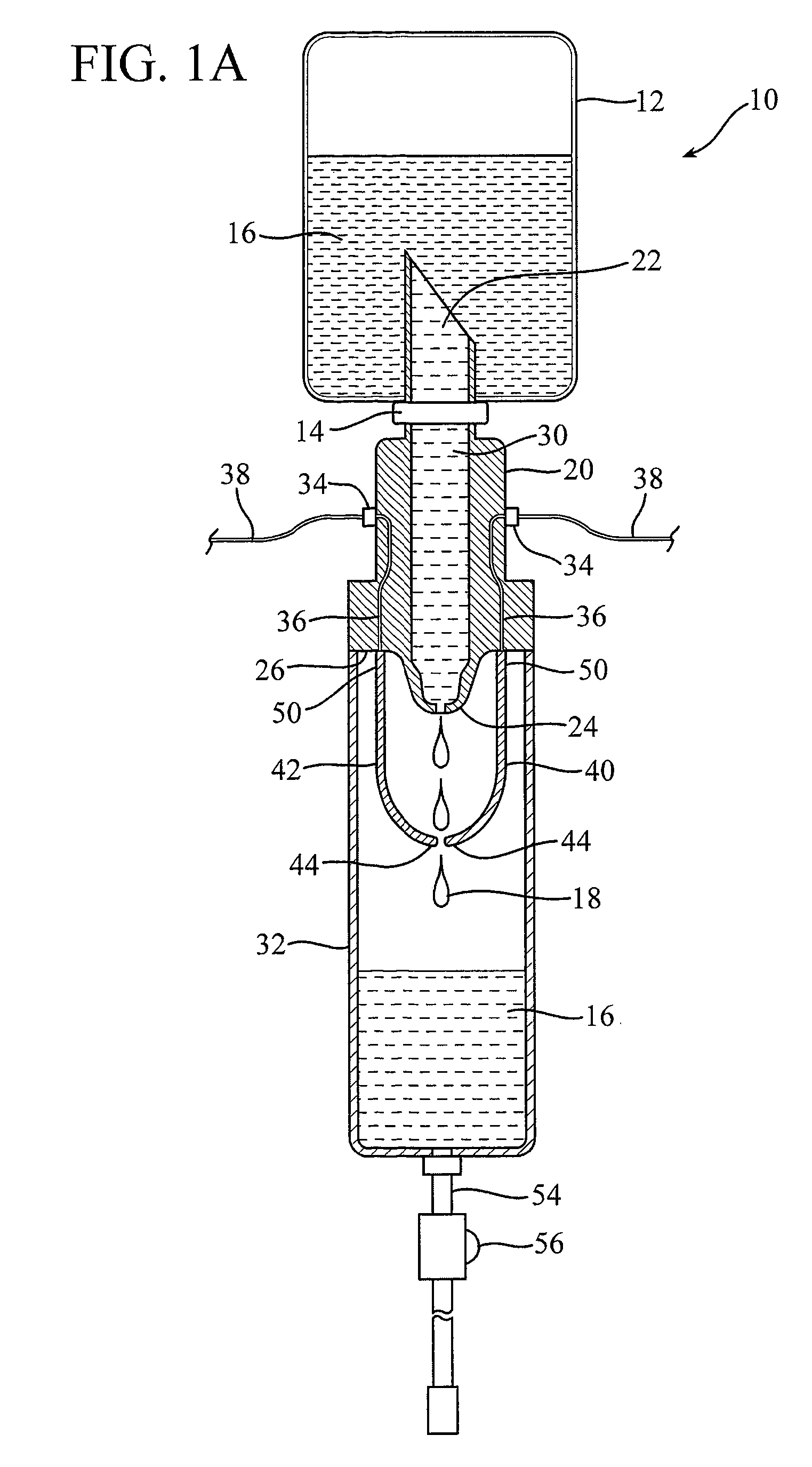
Impedance controlled tissue ablation apparatus and method
A method and apparatus for carrying our thermal ablation of target tissue is disclosed. The apparatus includes an RF ablation device having a multi-electrode electrode assembly designed to be deployed in target tissue, defining a selected-volume tissue region to be ablated, and having infusion channels for infusing a liquid into the target tissue during the ablation process. A control unit in the apparatus is operably connected to an RF energy source, for controlling the RF power level supplied to the electrodes, and to an infusion device, for controlling the rate of infusion of a liquid through the device into the tissue. During both electrode deployment and tissue ablation, impedance and or temperature measurements made within the tissue are used to control the RF source and infusion device, for optimizing the time and extent of tissue ablation.
Owner:PULSE POWER CO LTD
Fluidic capillary chip for regulating drug flow rates of infusion pumps
InactiveUS20090326517A1Avoid erosionValve arrangementsSynthetic resin layered productsDrugs solutionInfusion pump
An erosion-resistant capillary chip for use with in an infusion pump that is made from a silicon substrate having a first surface that includes a micro groove etched therein and a glass plate laminated to the first surface. The glass plate covers the micro groove so that a micro fluid conduit is created. The glass plate includes an inlet bore that connects with the micro fluid conduit and the silicon substrate includes an outlet bore that connects with the micro fluid conduit so that a drug solution entering the inlet bore from the infusion pump may pass through the micro fluid conduit at a restricted flow rate to the outlet bore and thereafter to a target site of a patient. The micro groove includes a passivation layer made from silicon nitride or silicon carbide that protects the micro groove against erosion from passing fluids having high basic or high acidic pH levels. A method for making the capillary chip is disclosed, as well as an infusion pump incorporating the improved capillary chip.
Owner:CODMAN NEURO SCI
Osmotic pump drug delivery systems and methods
InactiveUS20030032947A1Avoid mixingMedical devicesPharmaceutical delivery mechanismTherapeutic effectAnalgesics effects
Owner:MICROSOLUTIONS
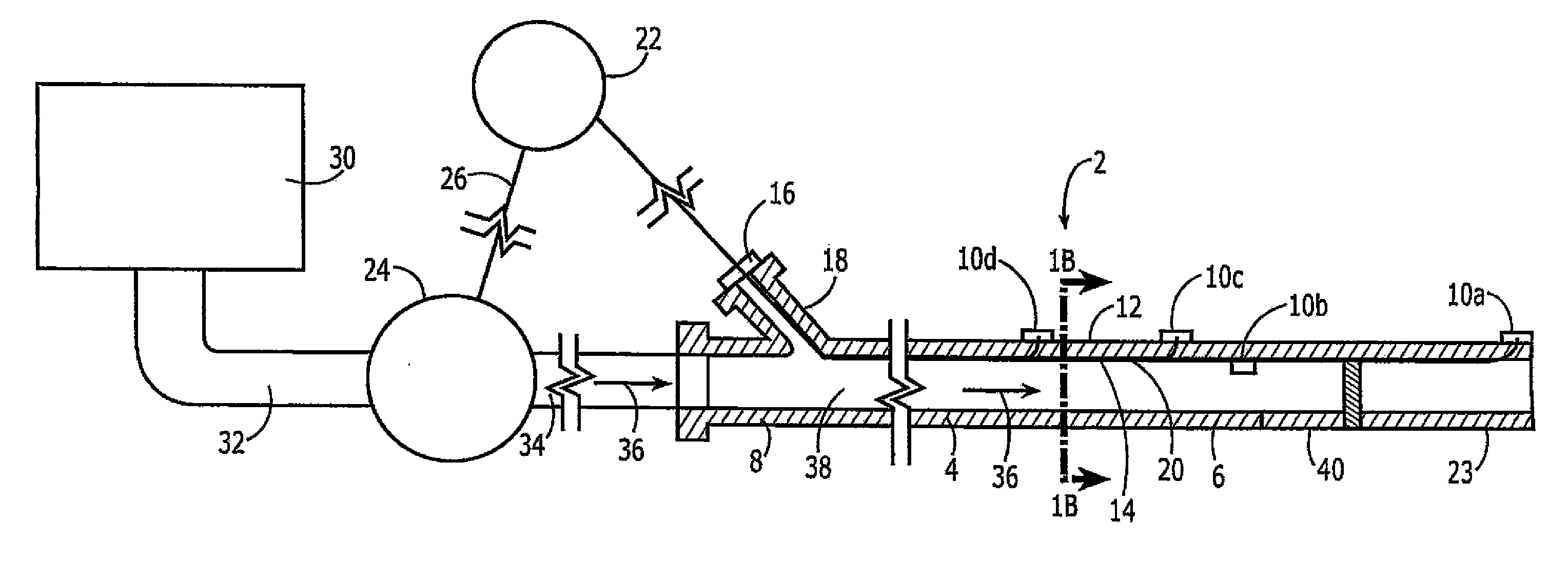
Infusion pump
InactiveUS20140243745A1Suppress reduction accuracyFlexible member pumpsPressure infusionSurgeryInfusion pump
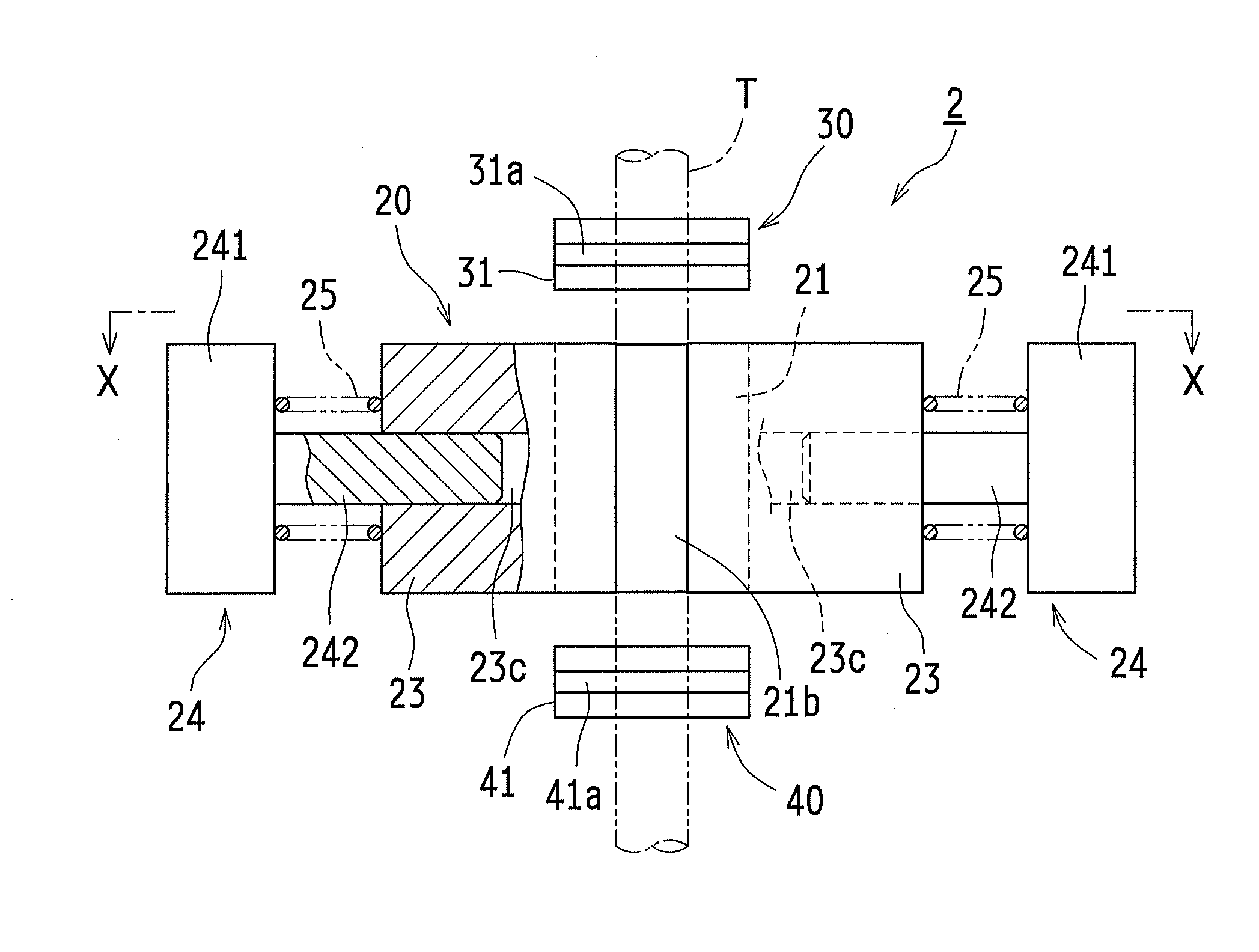
An infusion pump has a primary finger with an inclined surface, which is obliquely inclined to an advancing and retracting direction, and a secondary finger with an inclined surface, which is slidable relative to the inclined surface of the primary finger. When the primary finger is advanced, the secondary finger is retracted in proportion to the variation (increasing) in an entire width of an infusion tube, and when the primary finger is retracted, the secondary finger is advanced in proportion the variation (decreasing) in the entire width of the infusion tube. This configuration can suppress the occurrence of any gap between a tip surface of the secondary finger and an outer peripheral surface of the infusion tube in the advancing and retracting process of the primary finger. Consequently, this prevents the infusion tube from meandering, and suppresses the reduction in the accuracy of the rate of infusion.
Owner:NIPRO CORP
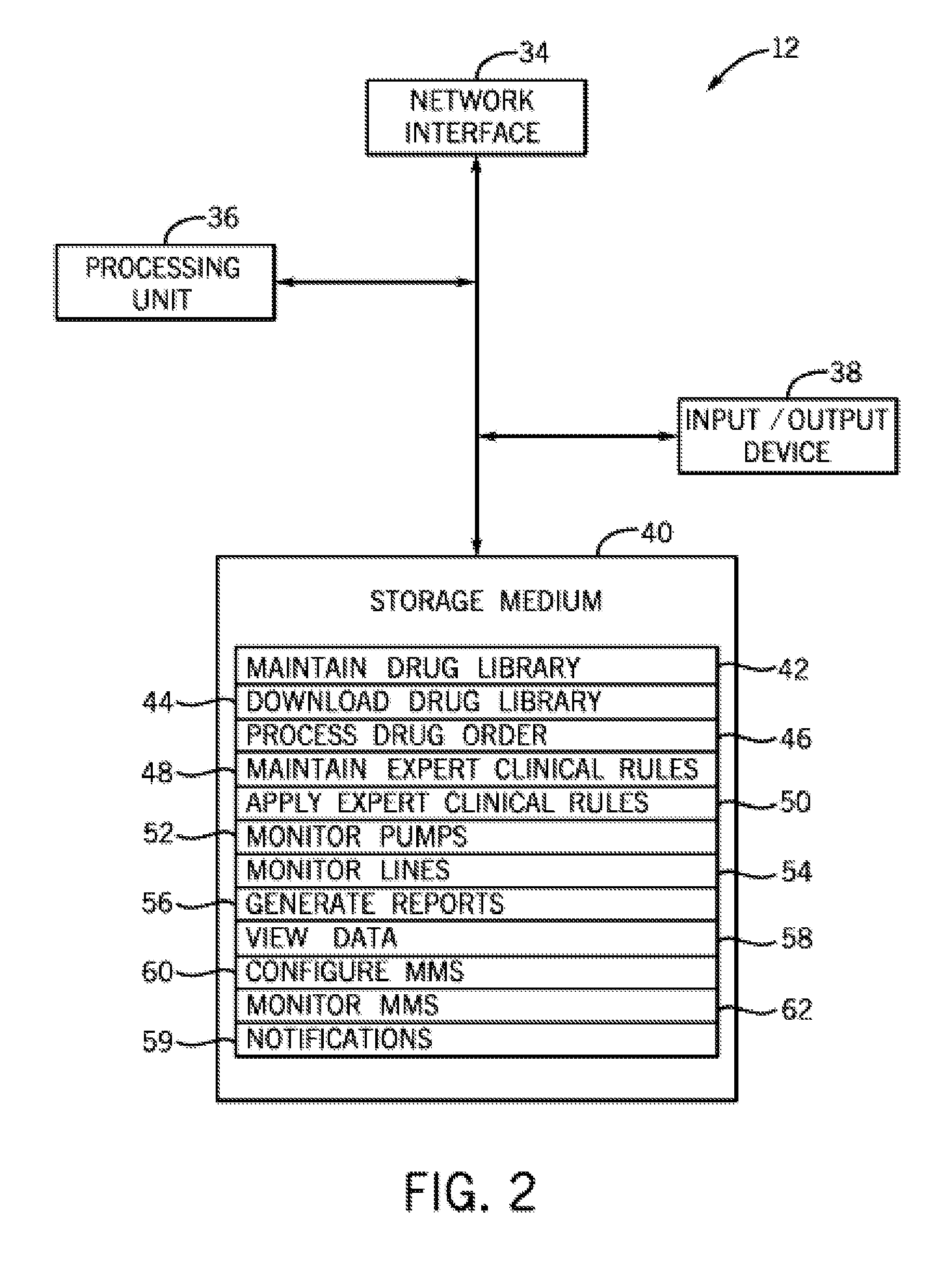
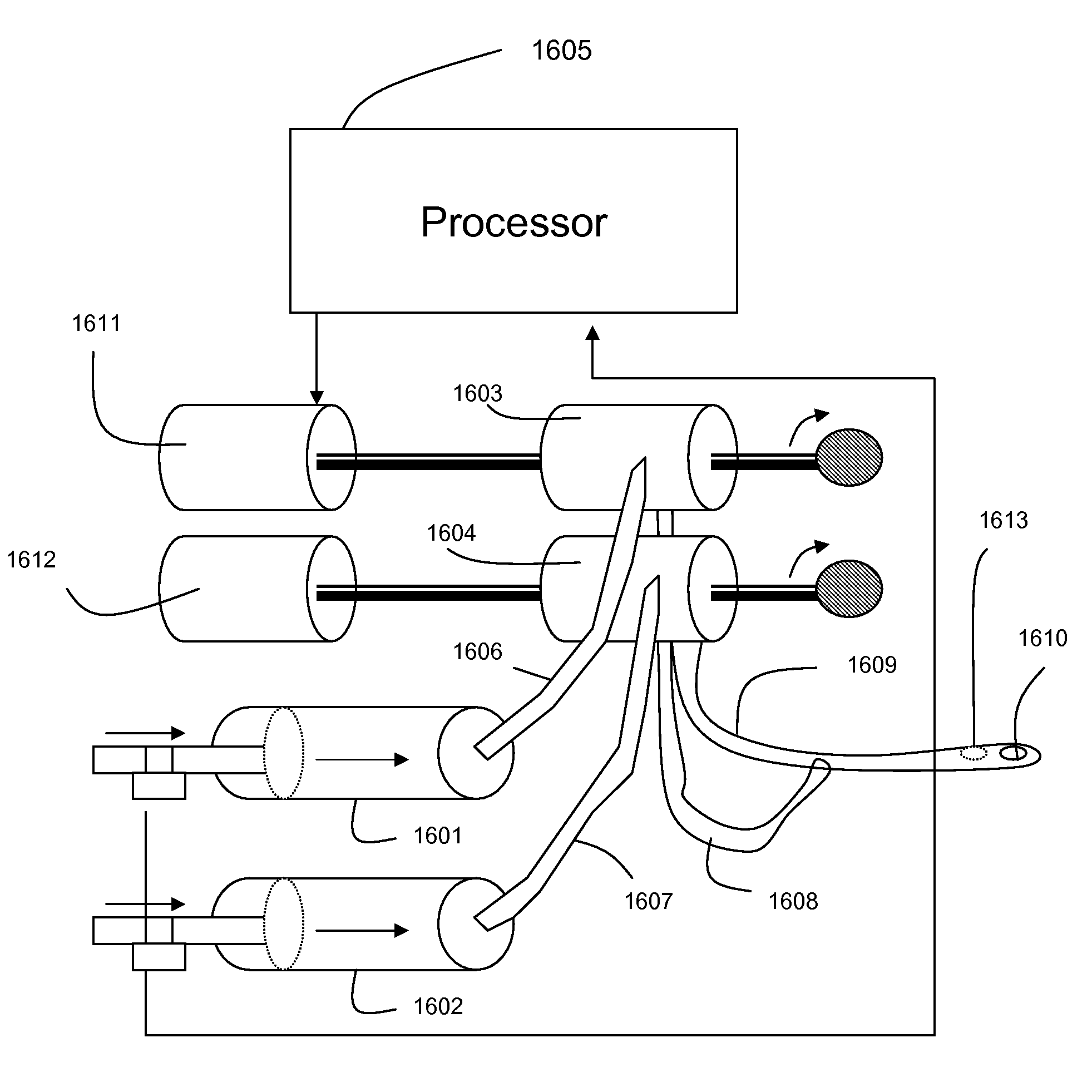
Infusion system and pump with configurable closed loop delivery rate catch-up
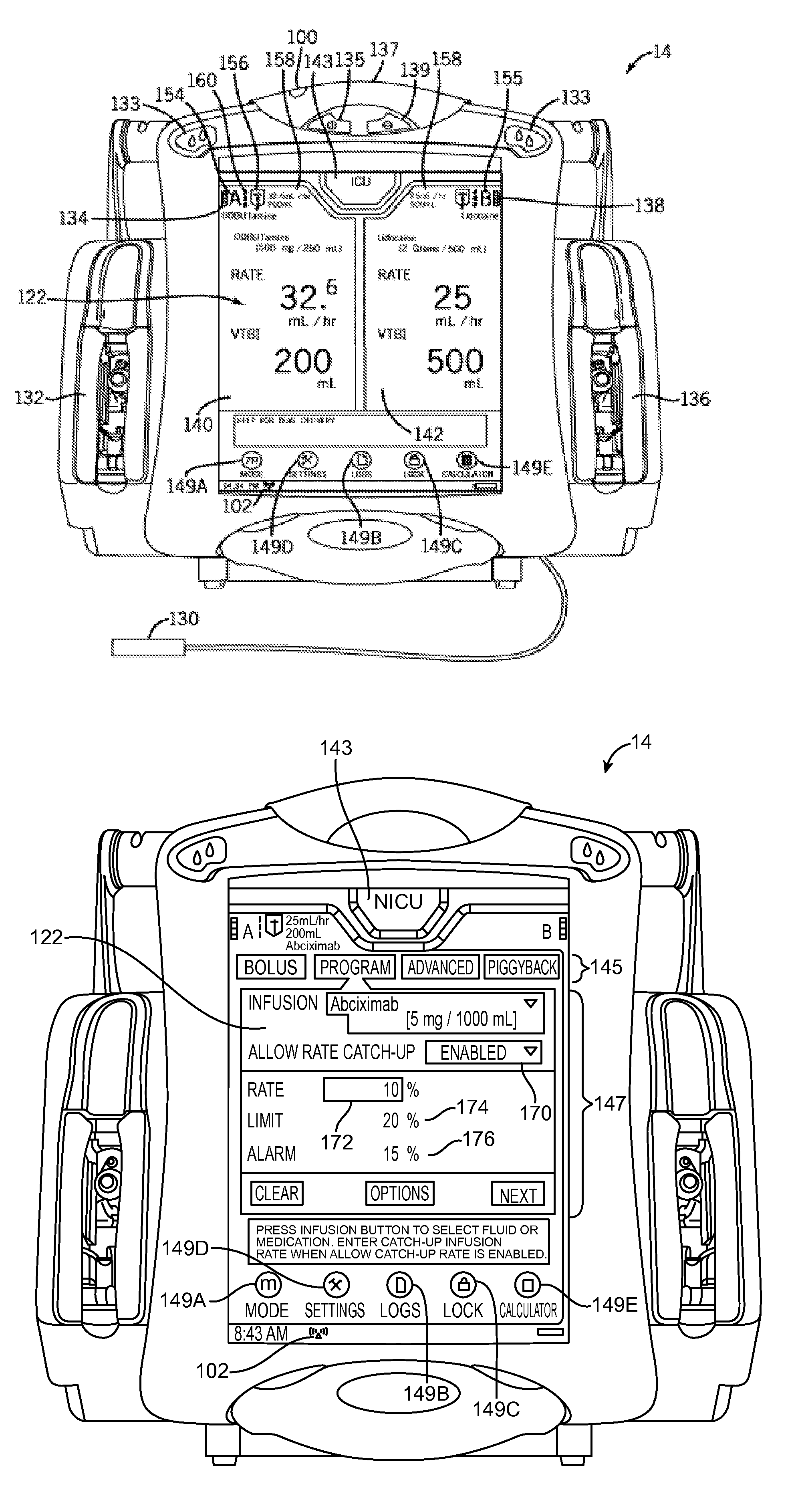
An infusion system and pump with configurable closed loop delivery rate catch-up including an infusion system having a medication management unit and a medical device. The medication management unit has programming code to: provide a graphical user interface for modifying a drug library; receive a catch-up rate factor; update the drug library with the catch-up rate factor; and transmit the updated drug library to the medical device. The medical device has programming code to: receive the updated drug library; receive a desired infusion rate; calculate expected accumulated infusion volume from the desired infusion rate; request delivery of the infusion at the desired infusion rate; determine actual accumulated infusion volume at a particular time; increase the infusion rate by the catch-up rate factor to generate a catch-up infusion rate when at the particular time the actual accumulated infusion volume is less than expected; and request infusion at the catch-up infusion rate.
Owner:ICU MEDICAL INC
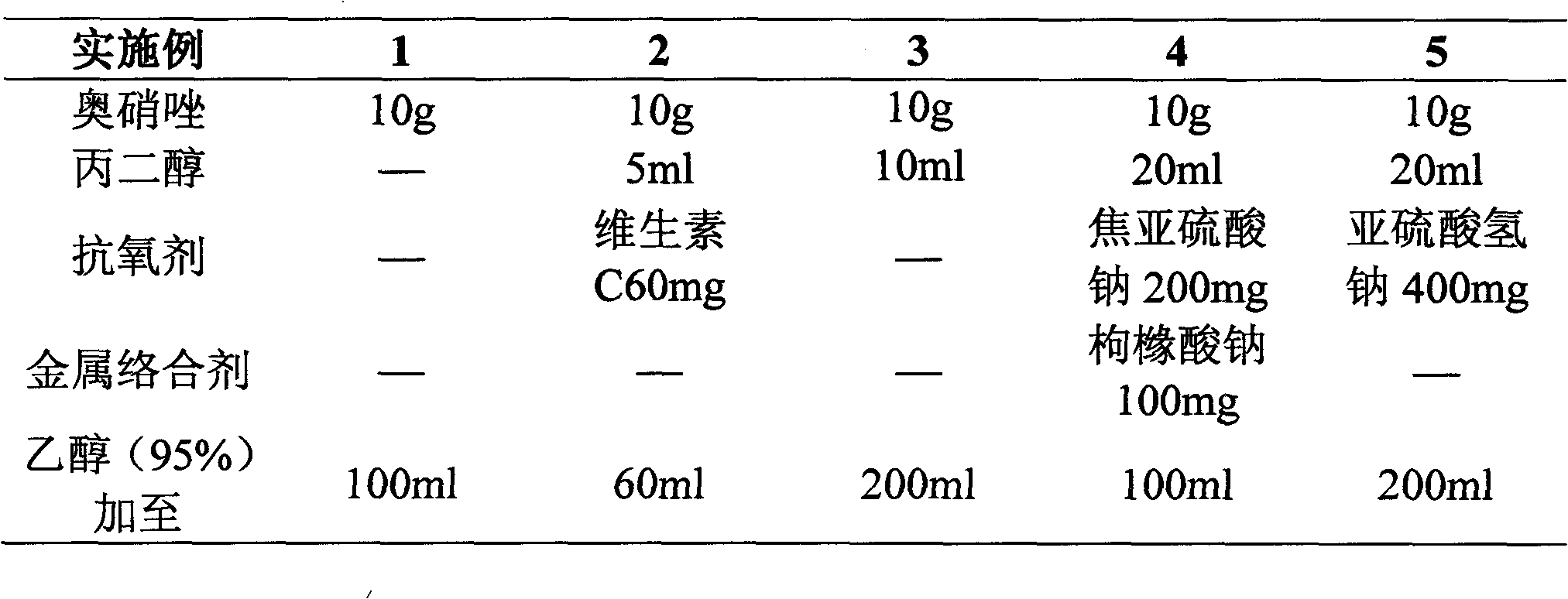
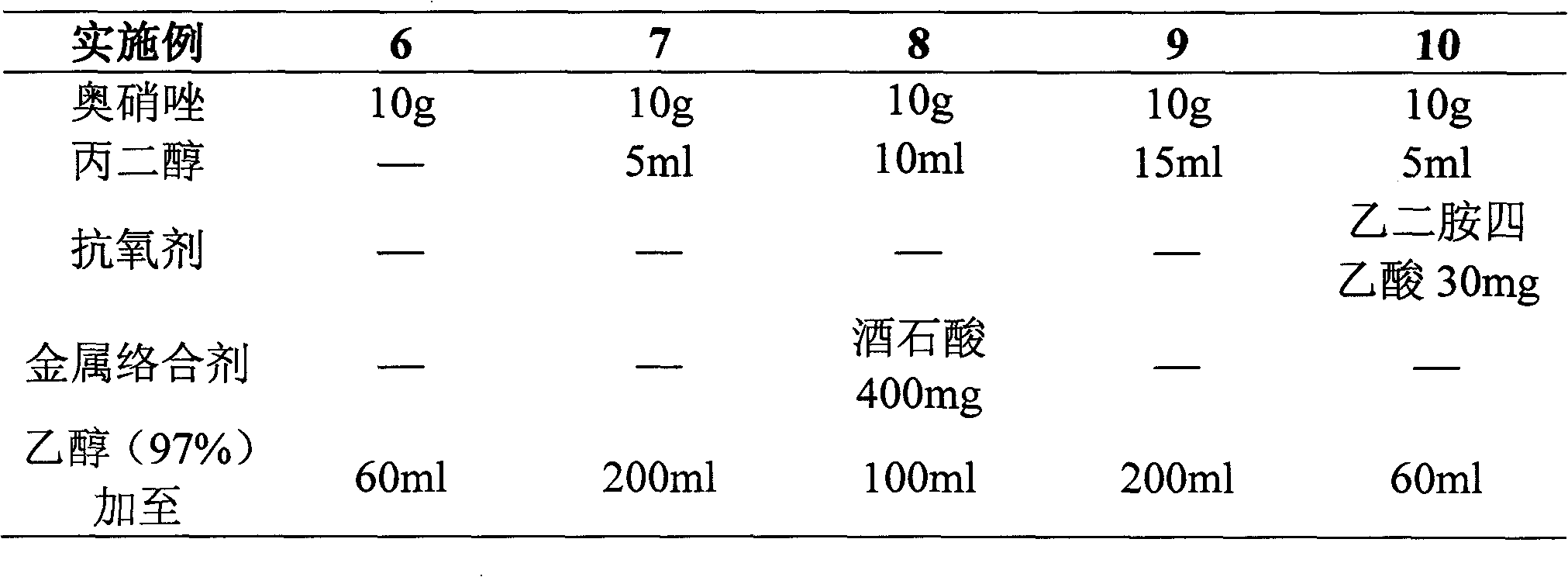
Ornidazole injection
ActiveCN102552127ALow impurity contentImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSuperficial phlebitisOrnidazole
The invention provides an ornidazole injection. The ornidazole injection has the advantages of high stability, low impurity content, pH value that is closer to the human physiological pH value, low incidence rate of infusion pain, infusion phlebitis and other adverse reactions, etc.
Owner:HEBEI RENHE YIKANG PHARMA
Beverage infusion spiral and methods of making and using the same
A liquid may be aged and infused using a wooden infusion apparatus having grain oriented along a longitudinal axis of a rod. One preferred infusion apparatus comprises machining a grain oriented wooden cylinder into the shape of a spiral having a longitudinal central axis. A preferred method is disclosed for continuously machining wooden rods into spirals, and subsequently toasting them. Another infusion apparatus comprises a plurality of wooden discs connected by a longitudinal central axis in the form of a rod, the wooden discs which are toasted. The present invention allows for more control over the time required to properly age the wine and to impart the wood flavor by giving the producer more control over the amount of wood surface area that is exposed to the wine. The rate of infusion is increased substantially when the wood grain extends along the longitudinal axis of the infusion apparatus. The shape and size of the infusion apparatus may be customized to fit any container from a huge wine barrel to a small liquor bottle. Additionally, the infusion spiral may be connected to a lid or bung of a container, and may thereby be removed when desired by merely opening the lid or bung. The infusion spiral can be replaced, by this same manner, with a different type of wood infusion spiral or a fresher infusion spiral to enhance the flavor imparted to the wine without having to fish around in the container for the infusion spiral.
Owner:RIVERSIDE ROCKETAB
Method for controlling body fluid condition using diuretics, based on weight measurement
InactiveUS8070742B2Restrict infusion rateImprove approachMedical devicesIntravenous devicesHuman patientBody fluid
The system for controlling body fluids overcomes the limitations of the prior art by automatically infusing diuretic and / or other drugs into a human patient. In one approach, the rate of infusion of the diuretic is adjusted based on the measured weight of the patient. For example, this weight can be transmitted wirelessly to a portable diuretic infusion device attached to the patient.
Owner:WOO SANG HOON
Systems and methods for intravascular cooling
ActiveUS20090018504A1Reduce the temperatureIncrease ratingsMedical devicesIntravenous devicesRefluxOxygen
Methods and systems for infusing a cooled infusate to a target location in a patient are described. A temperature of the blood and infusate admixture upstream of the catheter as well as at other locations along the catheter may be monitored and a feedback system utilized to control the volume, temperature, and / or infusion rate of the infusate so as to achieve a predetermined temperature at the target location. Control may also be based on the patient's native vessel flow rate. The system may monitor or calculate hematocrit upstream of the catheter and adjust infusion so as to provide sufficient oxygenation of the blood and infusate admixture. The system may also monitor reflux of the infusate past a distal end of the catheter and reduce infusion upon the detection of reflux.
Owner:HYBERNIA MEDICAL
Treatment regimen for administration of phenylacetylglutamine, phenylacetylisoglutamine, and/or phenylacetate
InactiveUS6943192B2High plasma concentrationFull penetrationBiocidePeptide/protein ingredientsDiseasePhenylacetylglutamine
Herein is disclosed a method of treating neoplastic disease, including cancer, comprising administering a pharmaceutical composition, the pharmaceutical composition comprising a highly concentrated aqueous solution of phenylacetylglutamine and phenylacetylisoglutamine in a 4:1 ratio, at an infusion rate of from 100 mL / hr to 400 mL / hr. In a further embodiment, herein is also disclosed a method of treating neoplastic disease, including cancer, comprising administering a pharmaceutical composition, the pharmaceutical composition comprising a highly concentrated aqueous solution of phenylacetate and (phenylacetylglutamine or phenylacetylisoglutamine) in a 4:1 ratio, at an infusion rate of from 100 mL / hr to 400 mL / hr. Herein are also disclosed the pharmaceutical compositions used in the above methods.
Owner:BURZYNSKI STANISLAW R
Systems and methods for intravascular cooling
Owner:HYBERNIA MEDICAL
Control of Body Fluid Condition Using Diuretics, Based on Weight Measurement
InactiveUS20090062727A1Restrict infusion rateImprove approachMedical devicesIntravenous devicesHuman patientBody fluid
The system for controlling body fluids overcomes the limitations of the prior art by automatically infusing diuretic and / or other drugs into a human patient. In one approach, the rate of infusion of the diuretic is adjusted based on the measured weight of the patient. For example, this weight can be transmitted wirelessly to a portable diuretic infusion device attached to the patient.
Owner:WOO SANG HOON
Integrated medication and infusion monitoring system
Owner:CERNER INNOVATION
Beverage infusion spiral and methods of making and using the same
A liquid may be aged and infused using a wooden infusion apparatus having grain oriented along a longitudinal axis of a rod. One preferred infusion apparatus comprises machining a grain oriented wooden cylinder into the shape of a spiral having a longitudinal central axis. A preferred method is disclosed for continuously machining wooden rods into spirals, and subsequently toasting them. Another infusion apparatus comprises a plurality of wooden discs connected by a longitudinal central axis in the form of a rod, the wooden discs which are toasted. The present invention allows for more control over the time required to properly age the wine and to impart the wood flavor by giving the producer more control over the amount of wood surface area that is exposed to the wine. The rate of infusion is increased substantially when the wood grain extends along the longitudinal axis of the infusion apparatus. The shape and size of the infusion apparatus may be customized to fit any container from a huge wine barrel to a small liquor bottle. Additionally, the infusion spiral may be connected to a lid or bung of a container, and may thereby be removed when desired by merely opening the lid or bung. The infusion spiral can be replaced, by this same manner, with a different type of wood infusion spiral or a fresher infusion spiral to enhance the flavor imparted to the wine without having to fish around in the container for the infusion spiral.
Owner:RIVERSIDE ROCKETAB
Controlling body fluid condition using diuretics
InactiveUS20090062729A1Restrict infusion rateImprove approachMedical devicesPressure infusionHuman patientBody fluid
The system for controlling body fluids overcomes the limitations of the prior art by automatically infusing diuretic and / or other drugs into a human patient. In one approach, the rate of infusion of the diuretic is adjusted based on the measured weight of the patient. For example, this weight can be transmitted wirelessly to a portable diuretic infusion device attached to the patient.
Owner:WOO SANG HOON
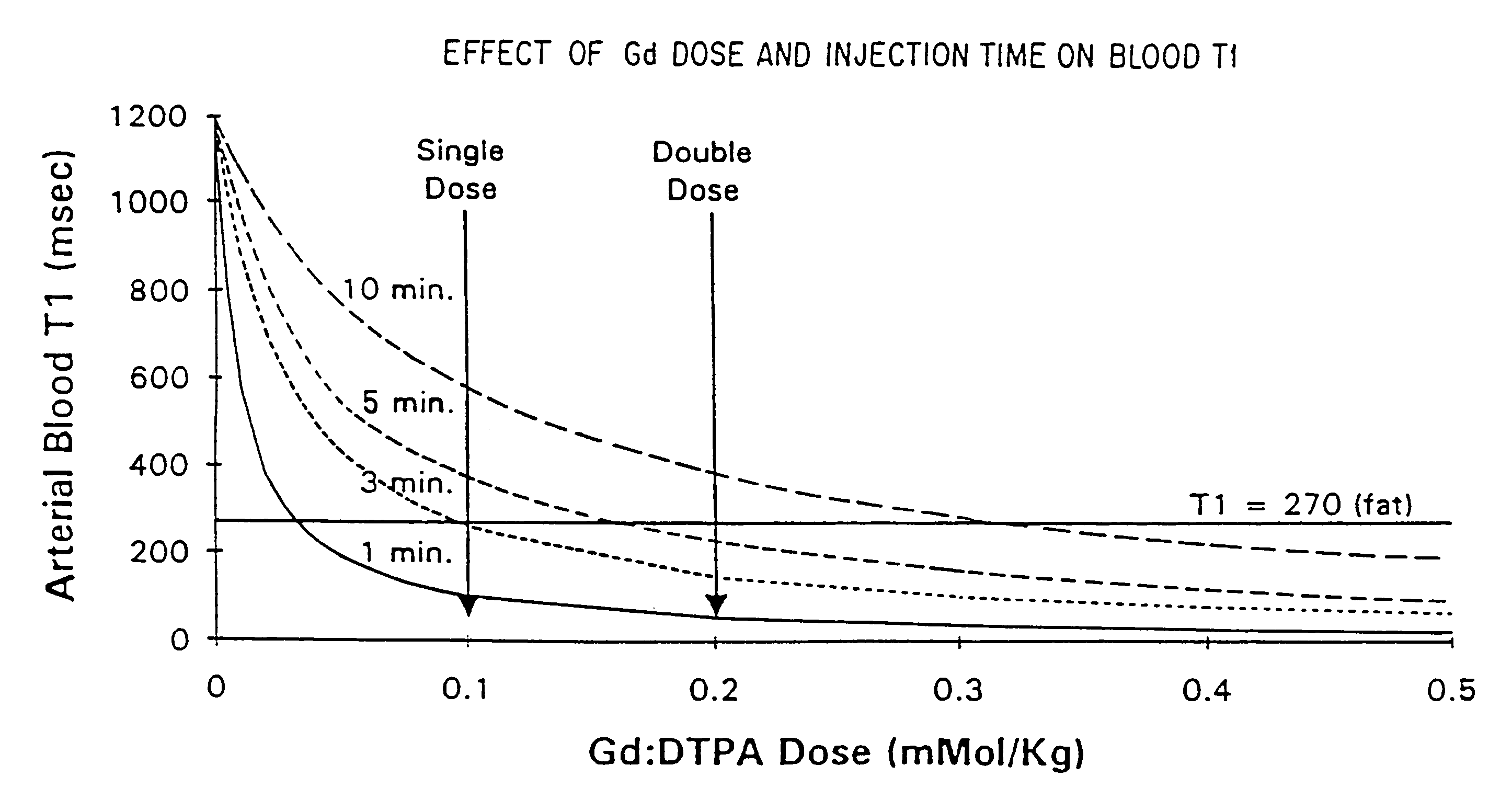
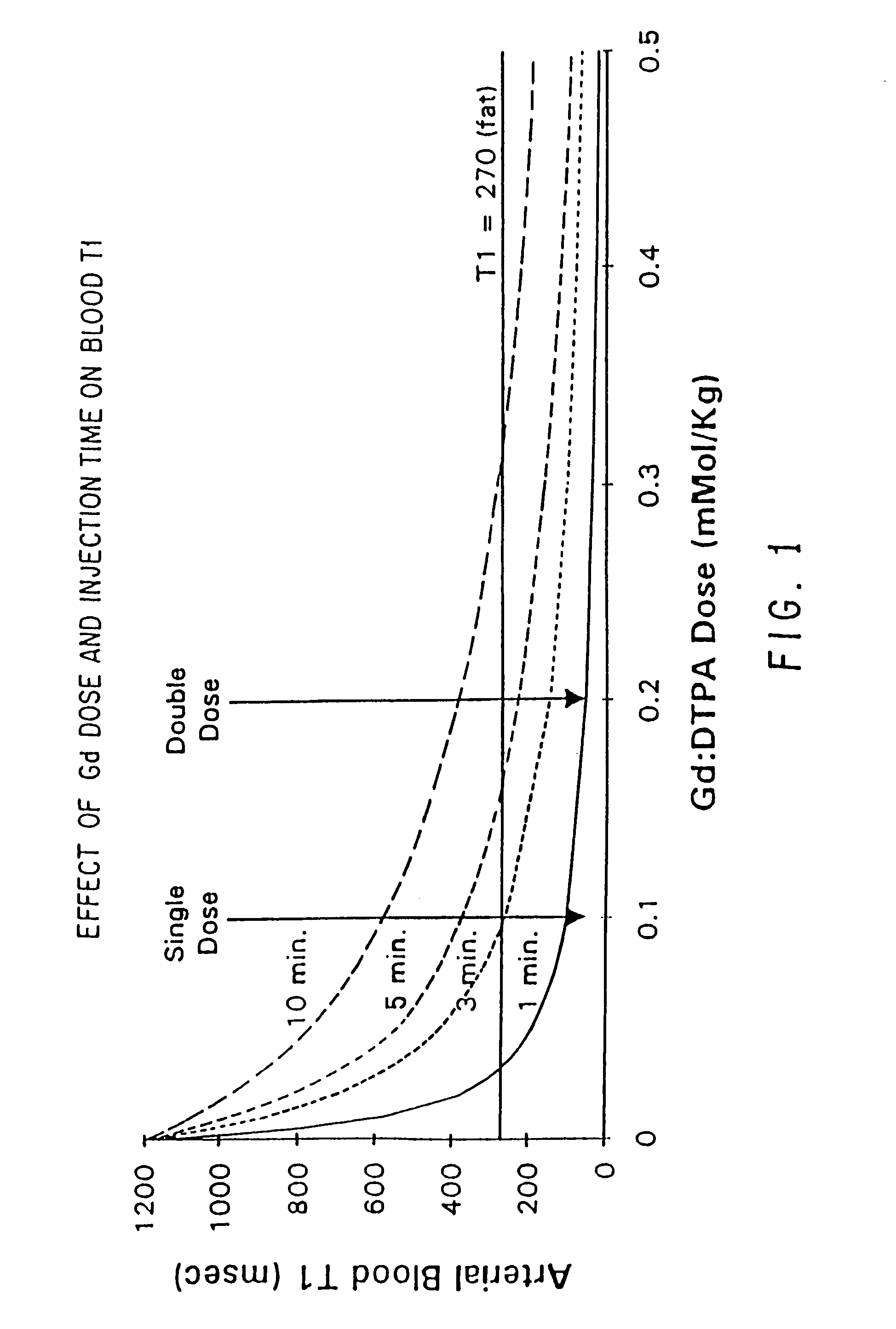
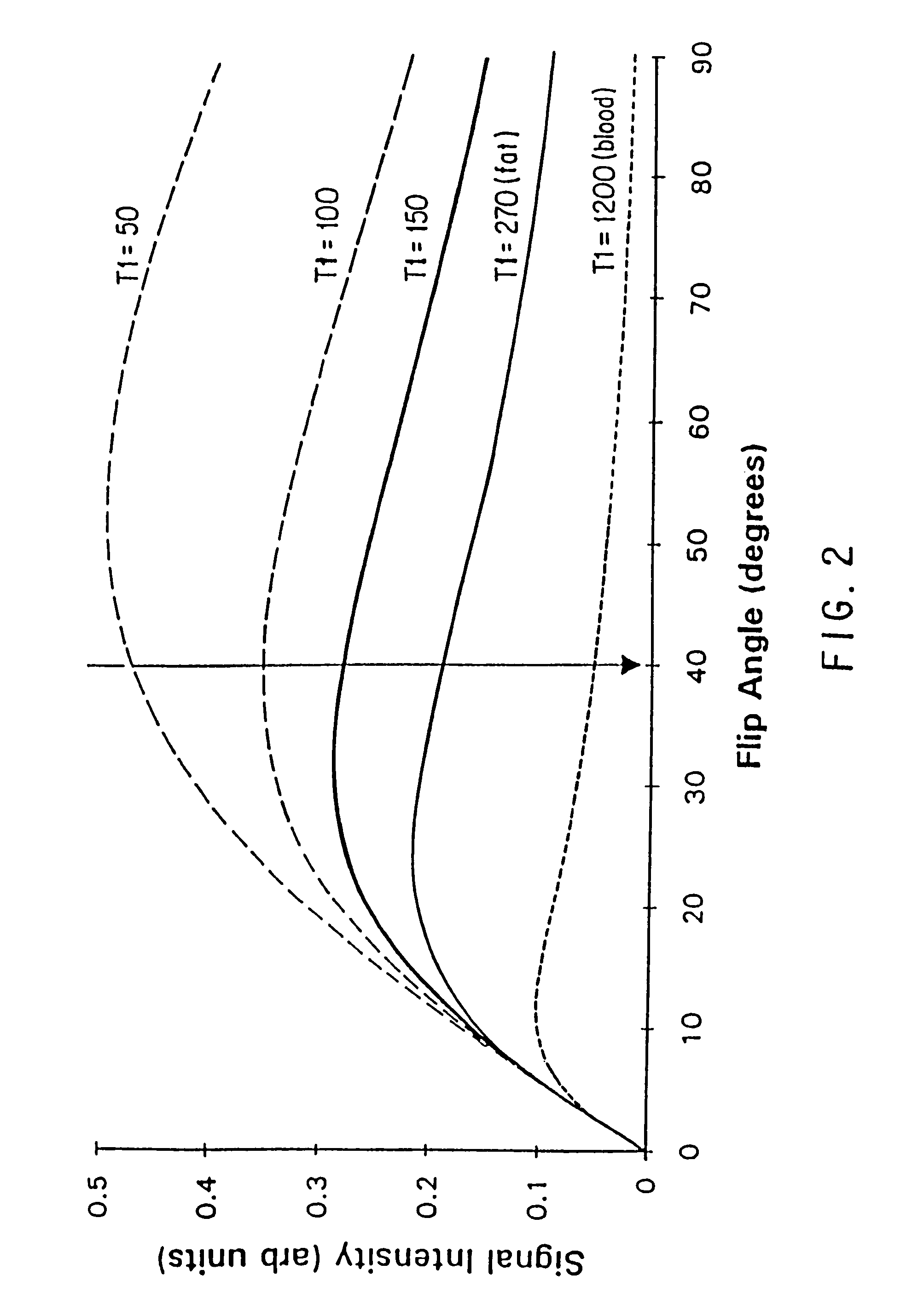
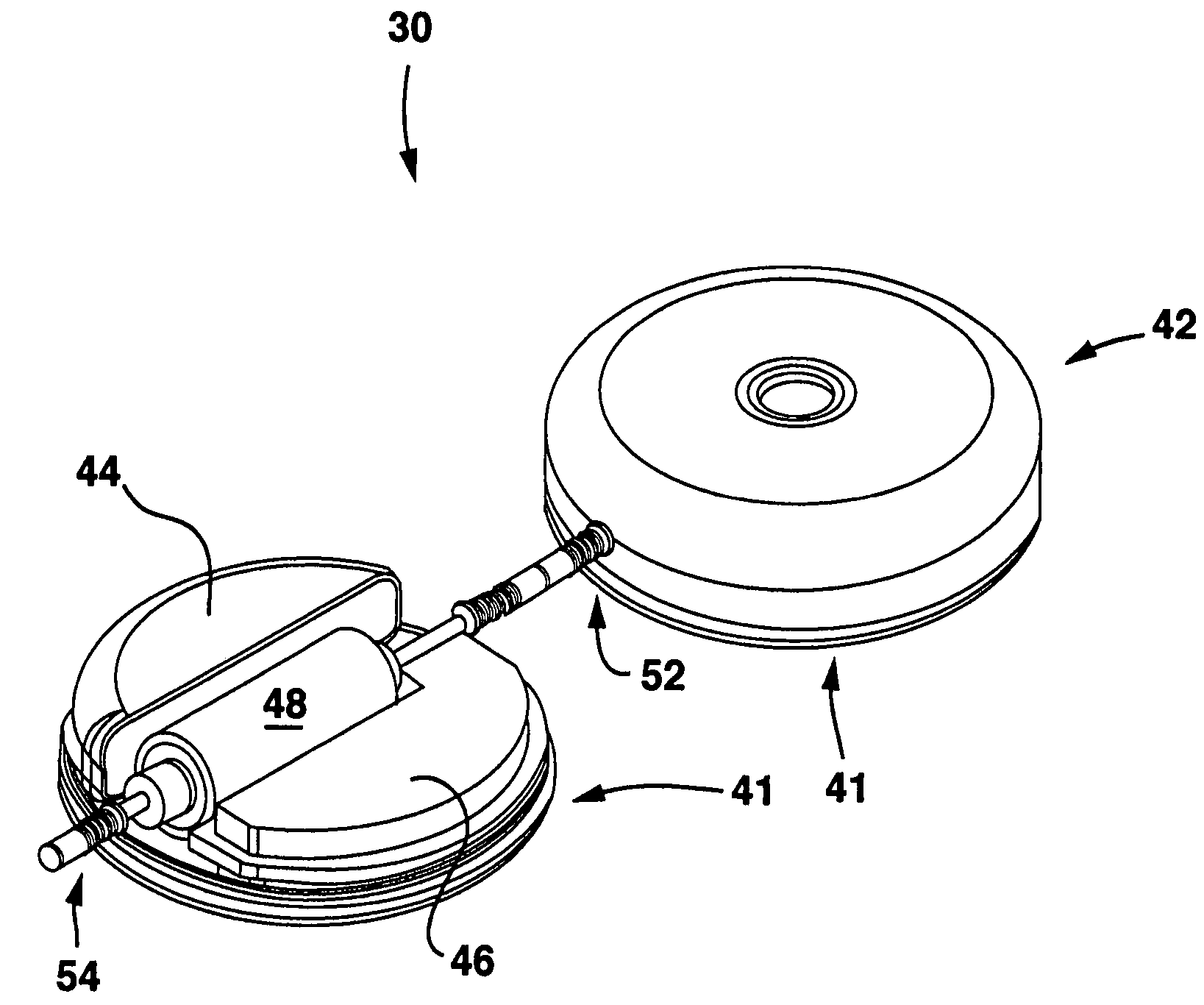
Method and apparatus for administration of contrast agents for use in magnetic resonance arteriography
InactiveUS7747309B2Eliminate riskIncrease contrastDiagnostic recording/measuringSensorsResonanceRadiology
The present invention is a method and apparatus for providing preferential enhancement of an artery of interest relative to adjacent veins and background tissue. The method and apparatus adapts the timing of a maximum or substantially elevated rate of infusion to correlate with the collection of image data corresponding to the center of k-space. The technique and apparatus temporally correlates the timing of a maximum or substantially elevated rate of infusion and the mapping of k-space according to the location of the artery of interest, the size of the artery of interest, the physical condition of the patient, the time delay due to the configuration of the contrast agent delivery system, and / or the type of pulse sequence employed by the imaging apparatus. Adapting the timing of a maximum or substantially elevated rate of infusion to correlate with the collection of image data corresponding to the center of k-space provides a period of a maximum or substantially elevated contrast concentration in the artery of interest relative to adjacent veins during collection of at least a portion of the image data corresponding to the center of k-space.
Owner:PRINCE MARTIN R
Implantable therapeutic substance delivery device
ActiveUS7758568B2Energy efficiencyMedical devicesPharmaceutical delivery mechanismDiseaseElectromagnetic pump
A medical device known as an implantable therapeutic substance delivery device is configured for implanting in humans to deliver a therapeutic substance such as pharmaceutical compositions, genetic materials, and biologics to treat a variety of medical conditions such as pain, spastisity, cancer, and many other conditions. The therapeutic substance delivery device has a permanent magnet solenoid pump that is energy efficient, accurate, small, compatible with therapeutic substances, and has many other improvements. The implantable therapeutic substance delivery device has a housing, a therapeutic substance reservoir, a power source, electronics, and a permanent magnet solenoid pump. The therapeutic substance reservoir is configured to contain a therapeutic substance and is coupled to the housing. The power source is carried in the housing to power the electronics and solenoid pump. The electronics are coupled to the solenoid pump and the solenoid pump is coupled to the therapeutic substance reservoir. The permanent magnet solenoid pump is configured for pumping therapeutic substance from the therapeutic substance reservoir through an infusion outlet at a programmed infusion rate. Many embodiments of the permanent magnet solenoid pump and its methods of operation are possible.
Owner:MEDTRONIC INC
Medicine infusion device having multiple infusion modes
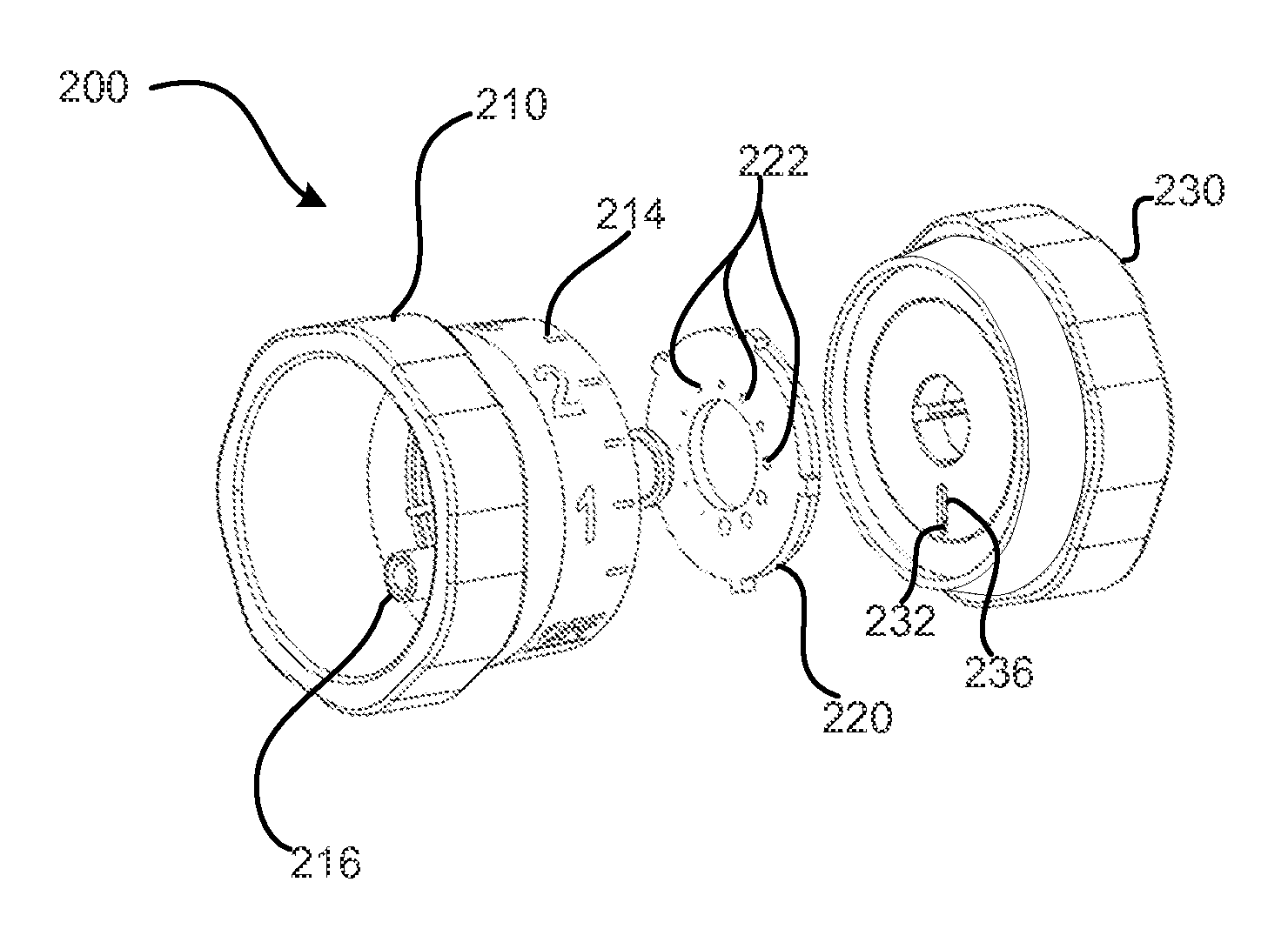
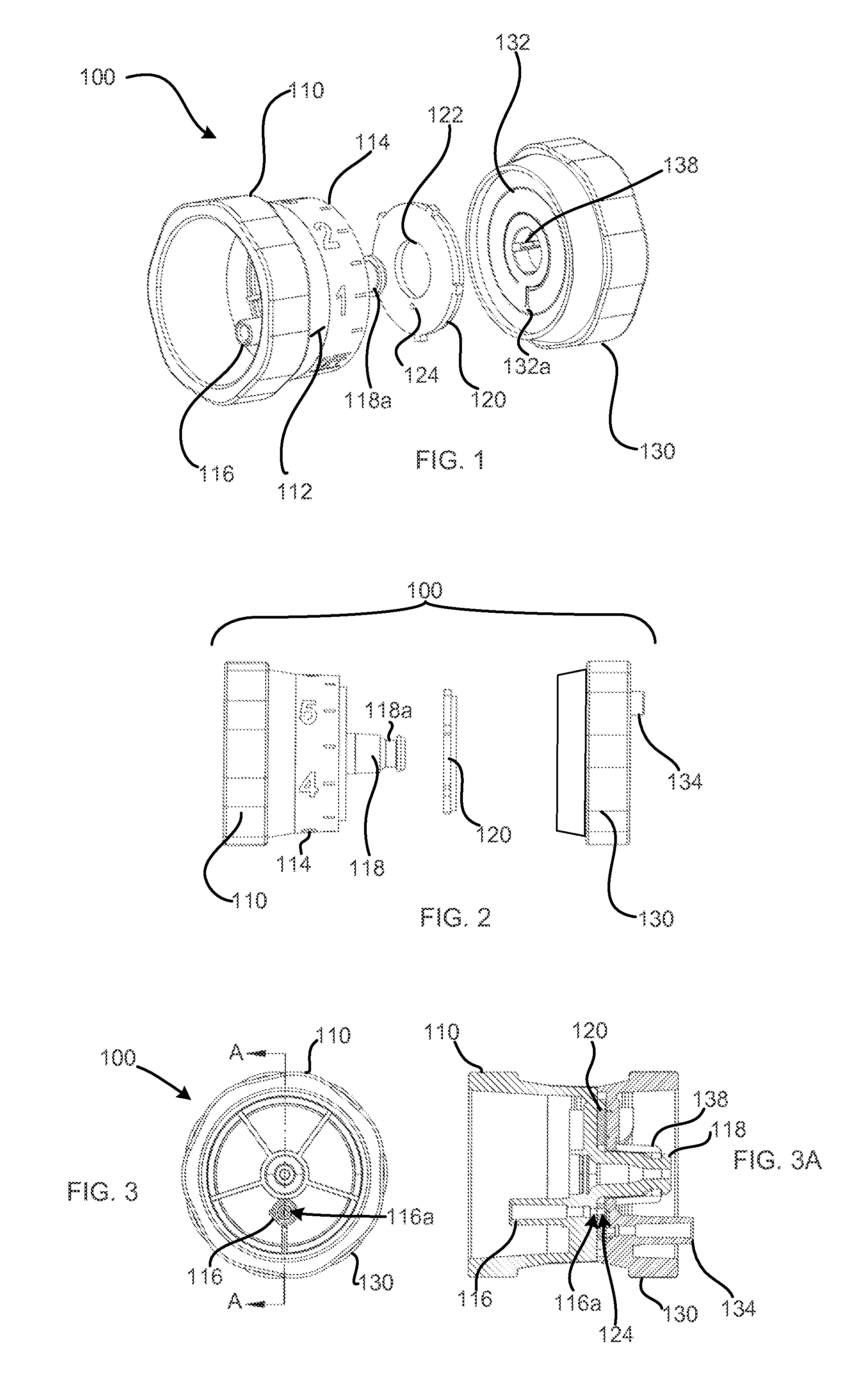
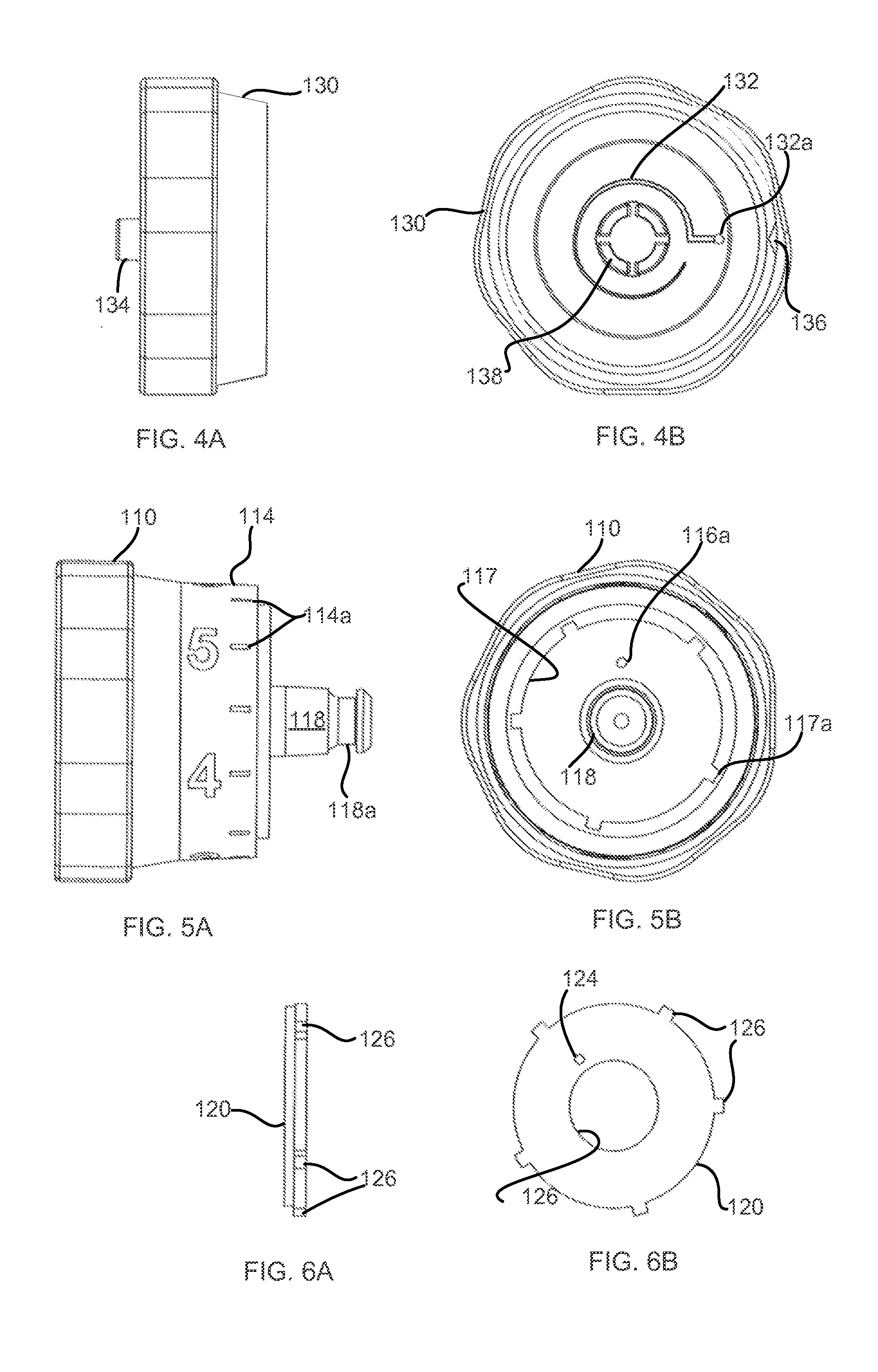
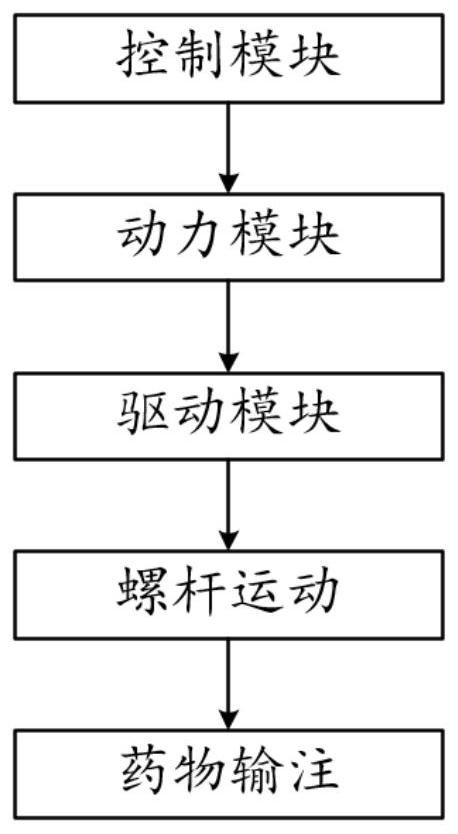
ActiveCN111939387AImprove experiencePrecise control levelAutomatic syringesMedical devicesMedication infusionPharmacy medicine
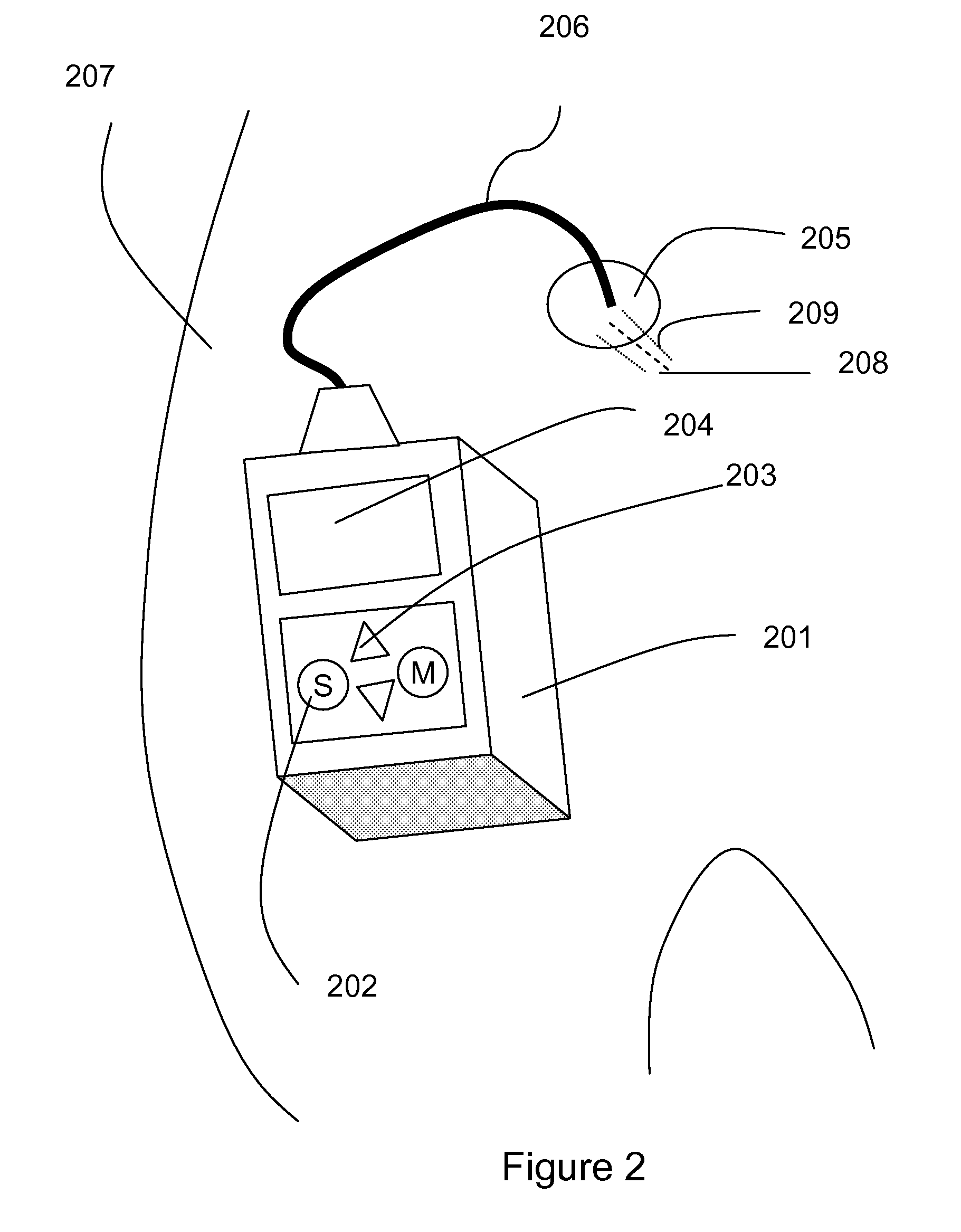
The invention discloses a medicine infusion device having multiple infusion modes. The medicine infusion device comprises a medicine storage unit, a piston, a screw rod, a driving module, a power module and a control module; the piston is arranged in the medicine storage unit; the piston is connected with the screw rod; the driving module at least comprises a first driving unit and a second driving unit, which are matched with each other to operate; the second driving unit drives the screw rod to advance; the power module is connected with the first driving unit; the control module is connected with the power module; and the control module controls the power module to apply different acting forces to the first driving unit, so that the first driving unit has multiple different operation modes, and the infusion device has multiple different unit infusion amounts or infusion rates. A user can select different infusion modes to infuse medicines, so that the user experience is enhanced.
Owner:MEDTRUM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com