Ornidazole injection
A technology of ornidazole injection and injection, which is applied in the direction of non-effective medical preparations, organic active ingredients, pharmaceutical formulas, etc., can solve problems such as easy to cause phlebitis, adverse reactions, and poor stability, and achieve The effect of low incidence of adverse reactions, high stability, and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~20
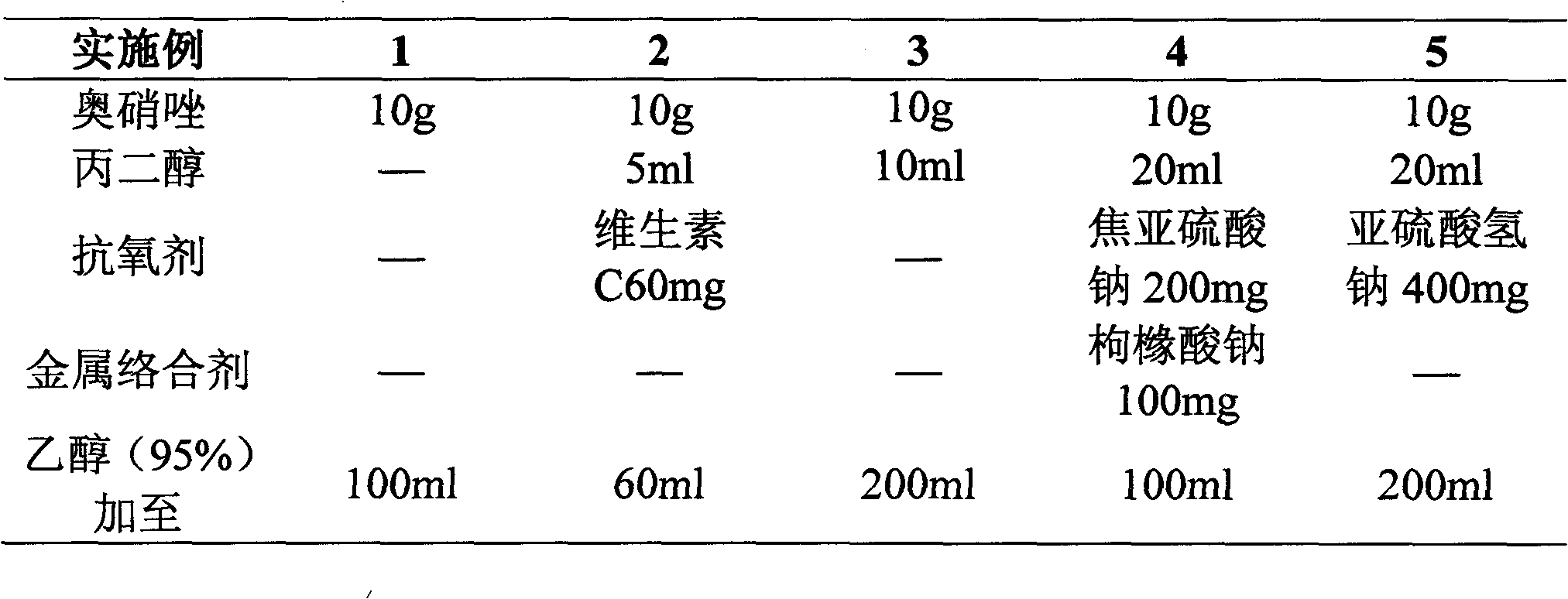
[0016] Embodiment 1~20 ornidazole injection
[0017] According to the prescription in the table below and the preparation process of the patent of the present invention, ornidazole injection is prepared, wherein Examples 16-20 are comparative examples:
[0018]
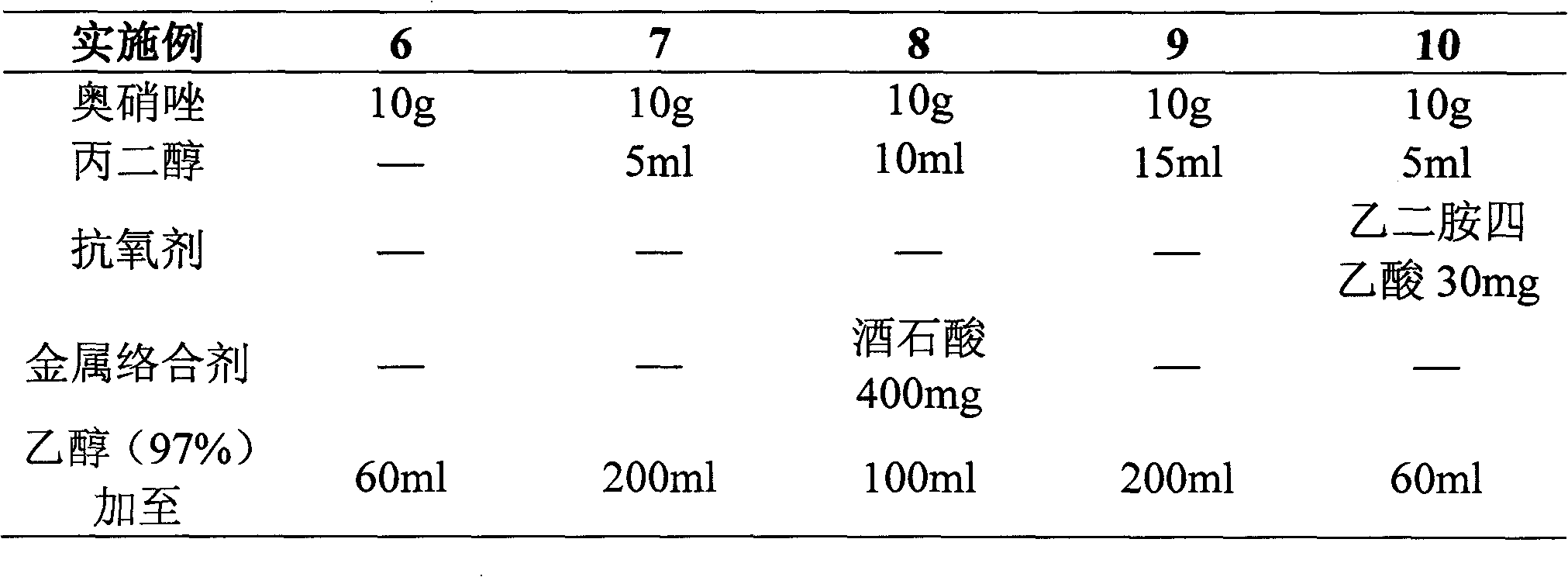
[0019]
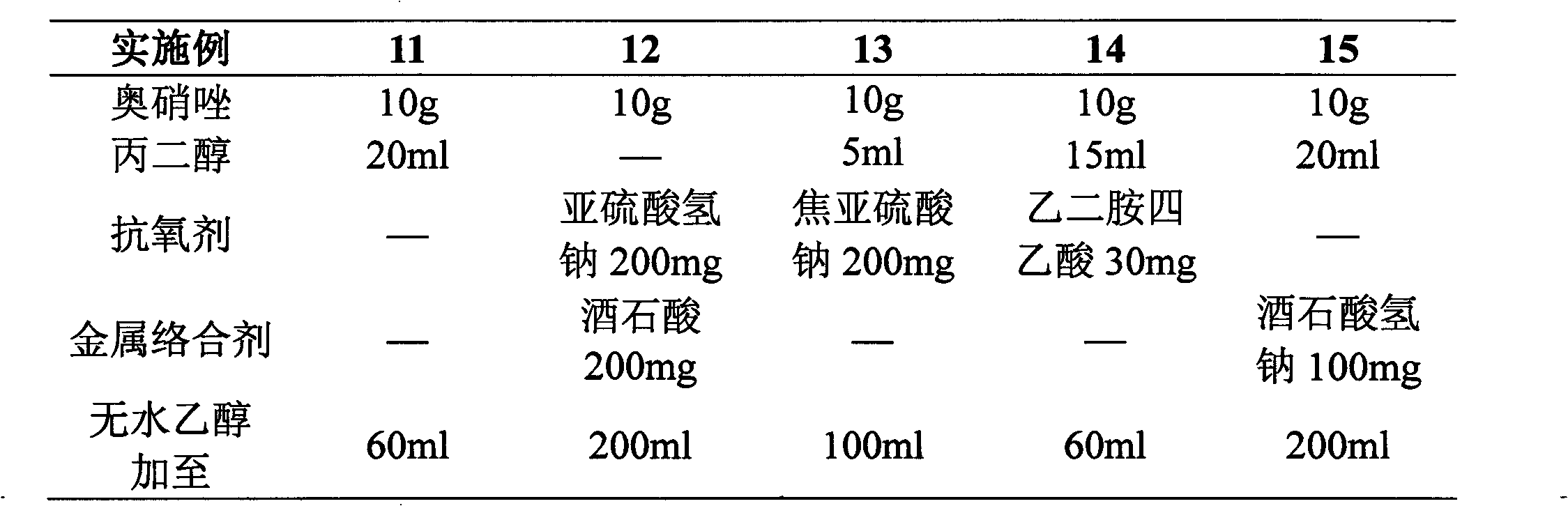
[0020]
[0021] Preparation process: Dissolve components such as ornidazole in the prescribed amount in ethanol (or a mixture of ethanol and propylene glycol), sterilize and filter, nitrogen-filled subpackage in Example 6-10, other direct subpackages, and sterilize (121° C. , 30 minutes), you can.
[0022]
[0023] Preparation process: Dissolve components such as ornidazole in the prescription amount in the mixed solution of propylene glycol and water, adjust pH, sterilize and filter, nitrogen-filled subpackage in Example 19, other direct subpackages, sterilization (121 ° C, 30 minutes), you can.
experiment example 1
[0024] Stability study of experimental example 1 ornidazole injection
[0025] According to Chinese Pharmacopoeia 2010 edition two appendices XIXC bulk drug and drug preparation stability test guiding principle, under high temperature condition (temperature is 40 ℃ ± 2 ℃, relative humidity is 75% RH ± 5% RH) carries out accelerated stability test ( 6 months), according to the method of Ornidazole Injection National Drug Standard (YBH00832004), the Ornidazole Injection that detection embodiment 1~20 makes is before sterilization, after sterilization 0 month, after sterilization 6 months. months of related substances and ornidazole content.
[0026] The result is as follows:
[0027]
[0028]
[0029]
[0030]
[0031]
[0032] To sum up, the average value of impurity content (0.14%) of the ornidazole injection (embodiment 1-15) of the present invention 0 months after sterilization is far lower than the ornidazole injection of the control example (embodiment 16-2...
experiment example 2
[0034] The pH value when experimental example 2 ornidazole injection compatibility
[0035] With the ornidazole injection that embodiment 1~20 makes, get the injection that contains principal agent ornidazole 1g, carry out compatibility with sodium chloride solution (0.9%) 200ml, glucose solution (5%) 200ml respectively, detect The pH value of the compatible solution, the results are as follows:
[0036]
[0037]
[0038]
[0039]
[0040] It can be seen that the pH value (4.5-5.7) of the ornidazole injection of the present invention after compatibility with 0.9% sodium chloride and 5.0% glucose is suitable for human infusion, and is significantly higher than that of the control example (2.7-3.7).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com