Patents
Literature
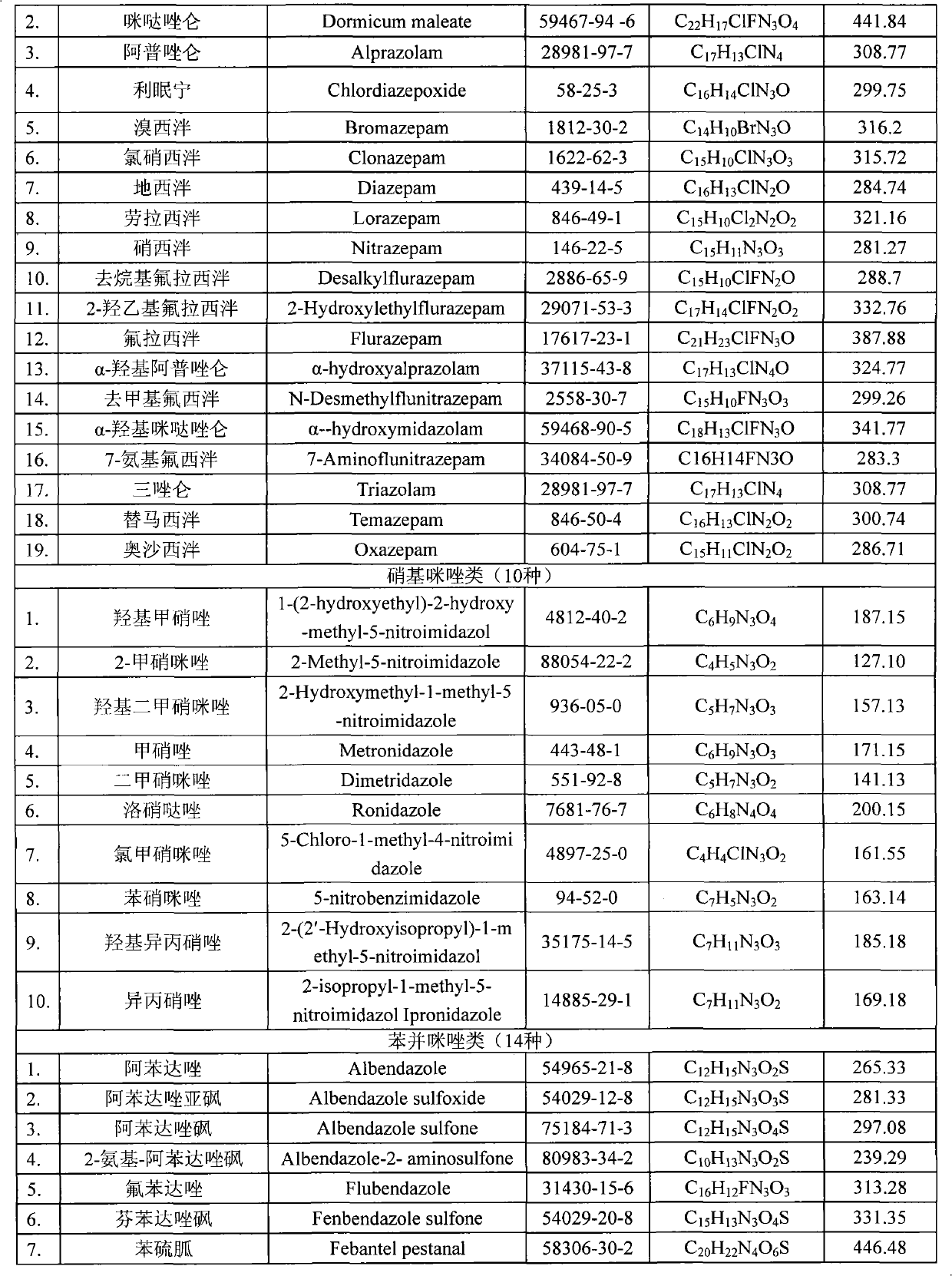
396 results about "Nitroimidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
5-Nitroimidazole is an organic compound with the formula O₂NC₃H₂N₂H. The nitro group at position 5 on the imidazole ring is the most common positional isomer. The term nitroimidazole also refers to a class of antibiotics that share similar chemical structures.
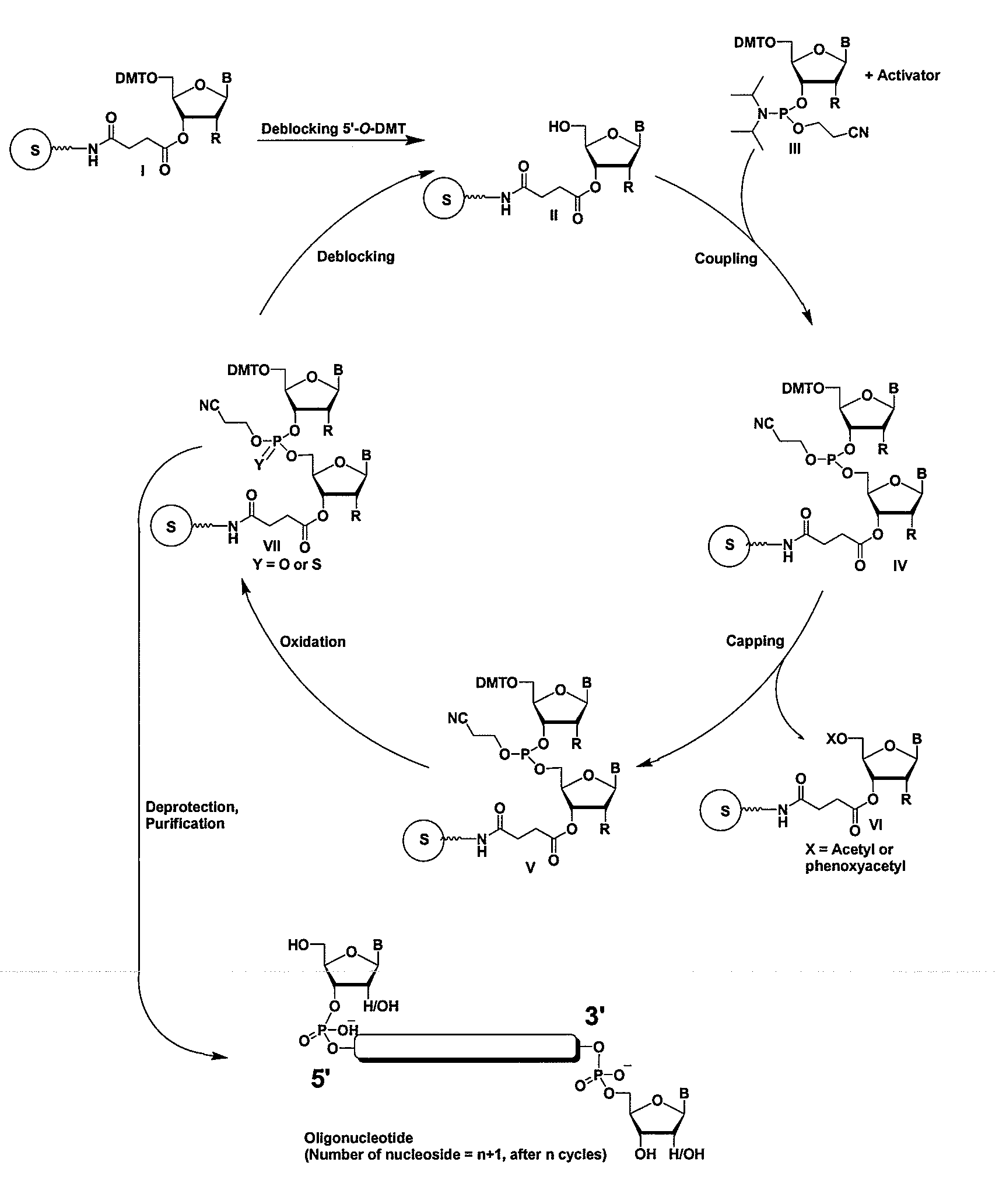
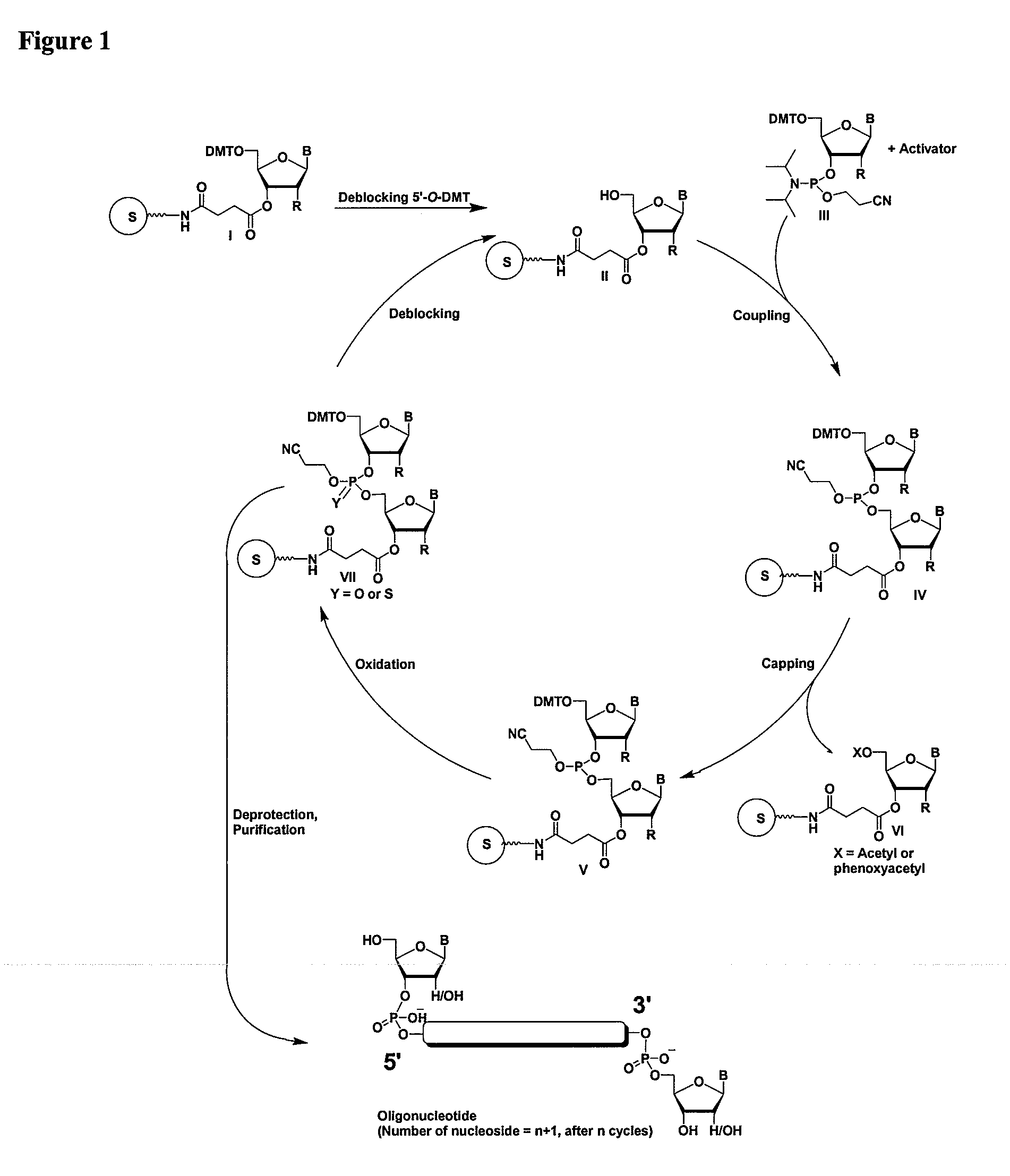
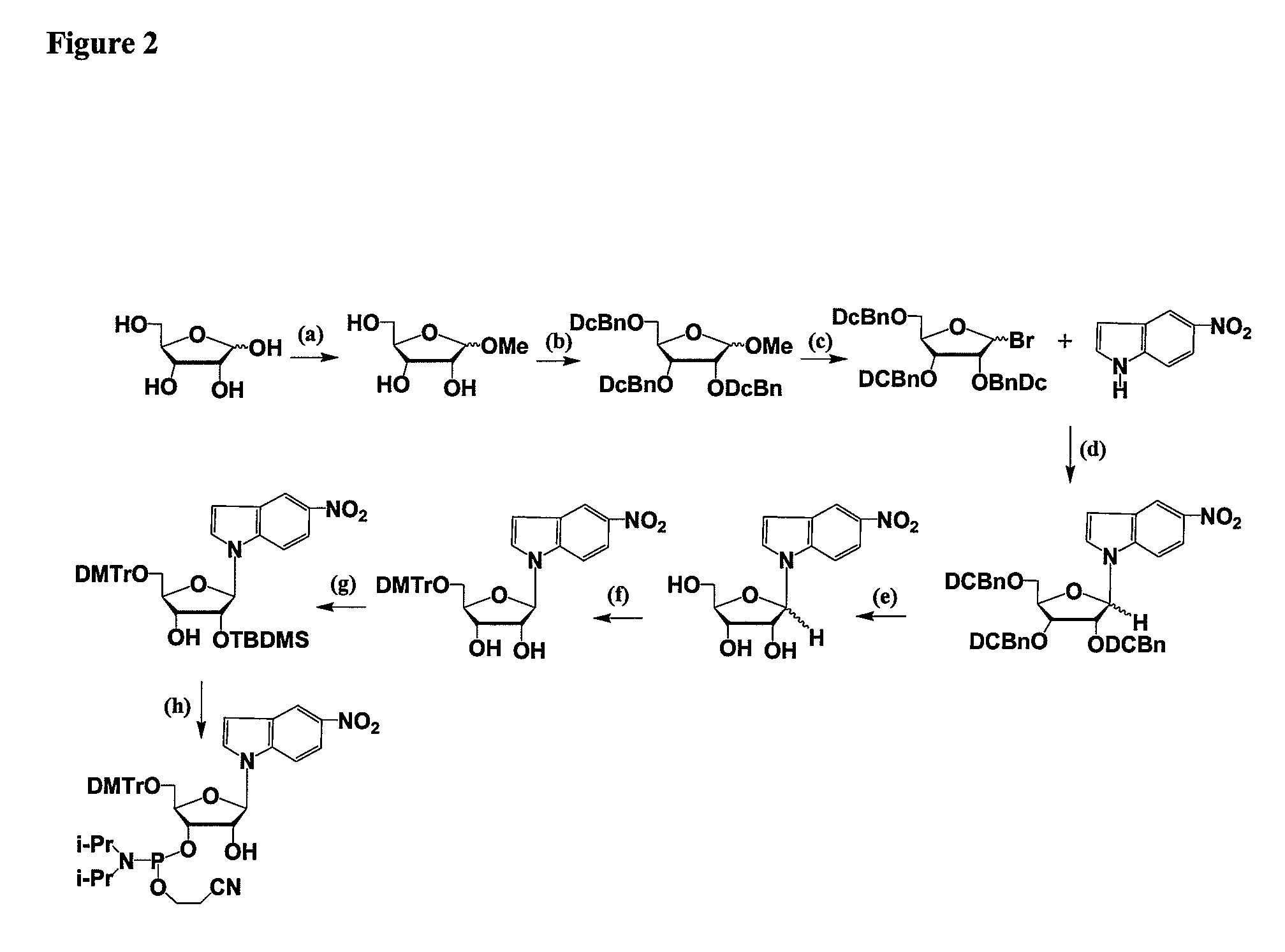
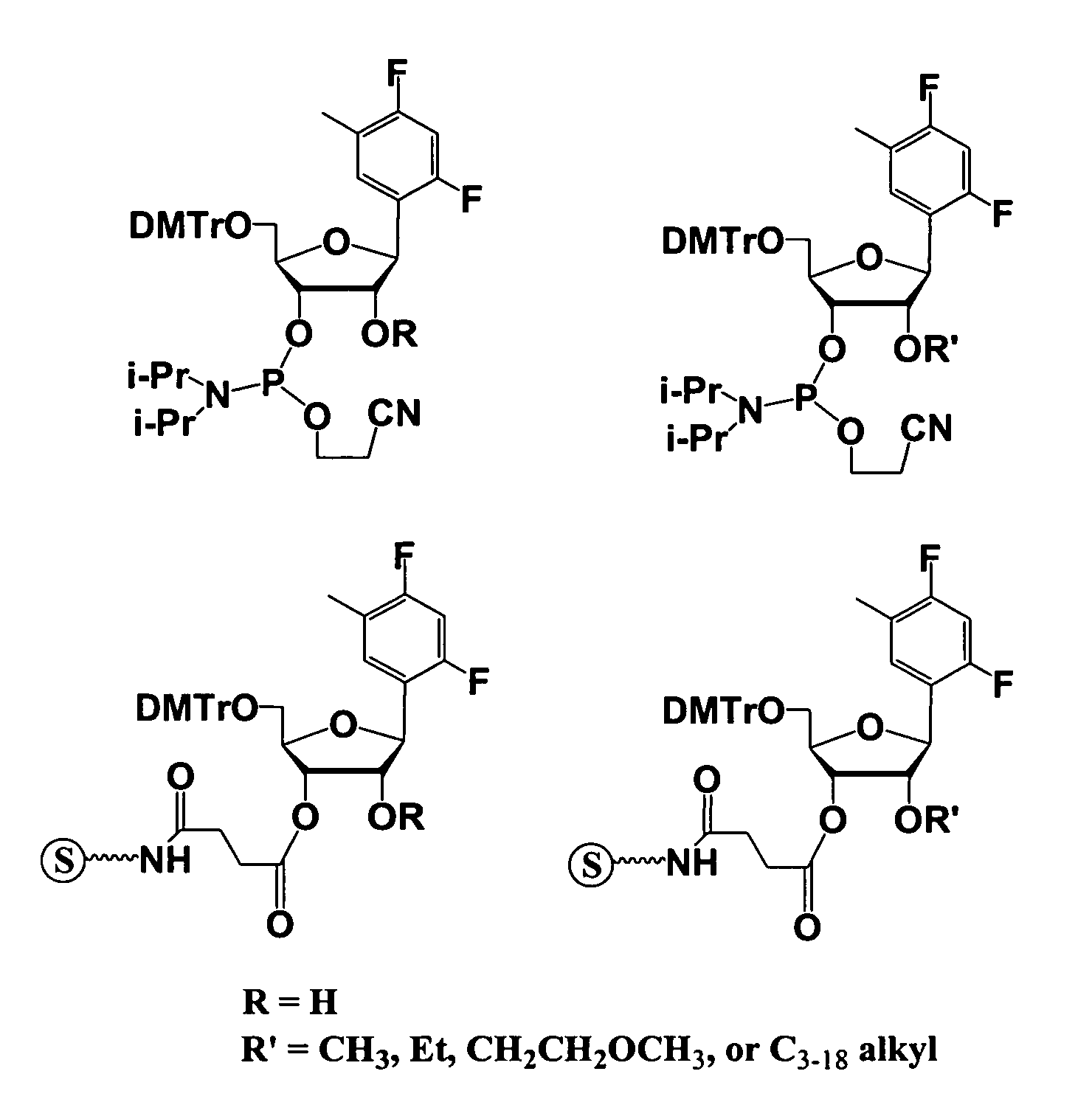
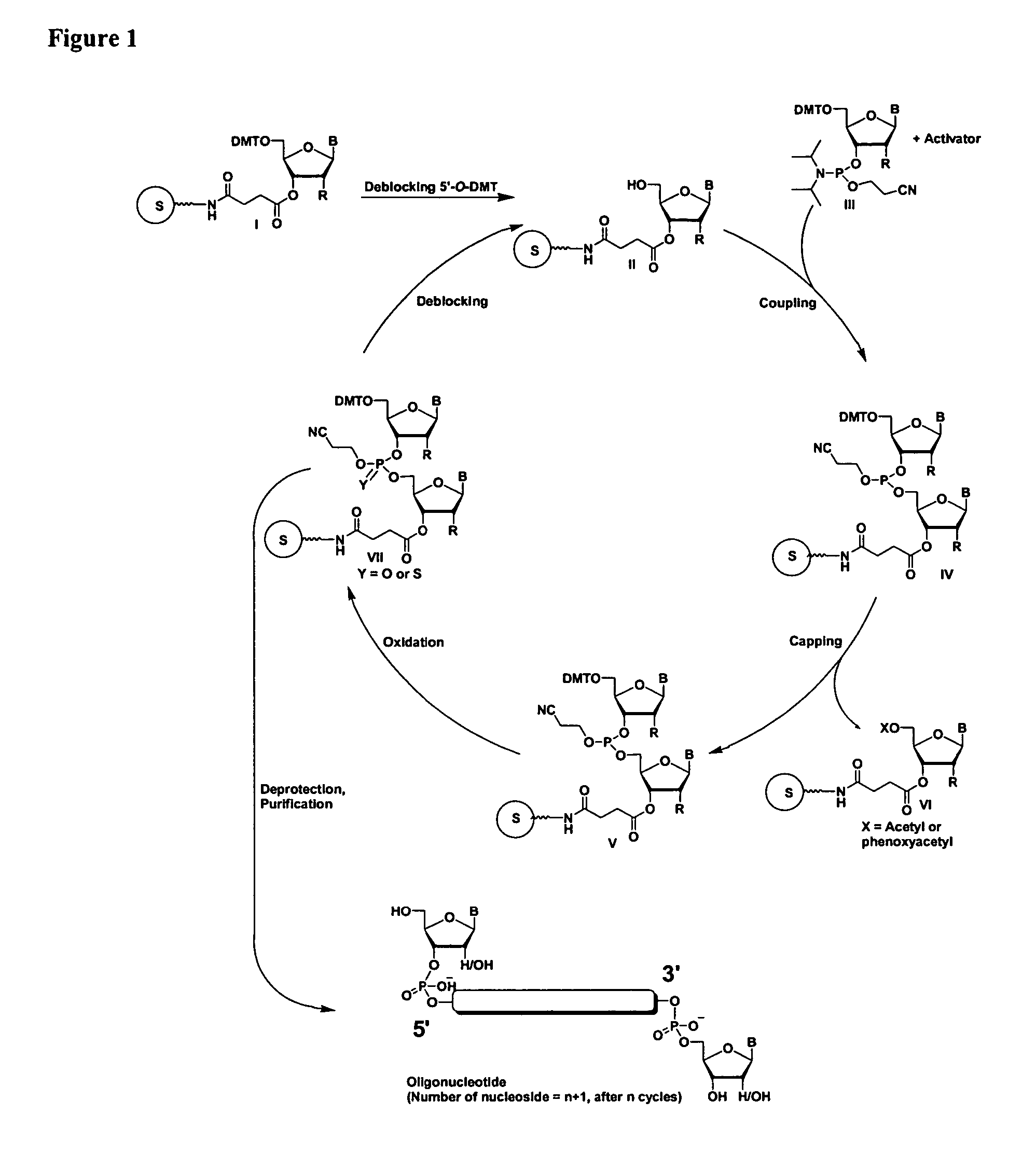
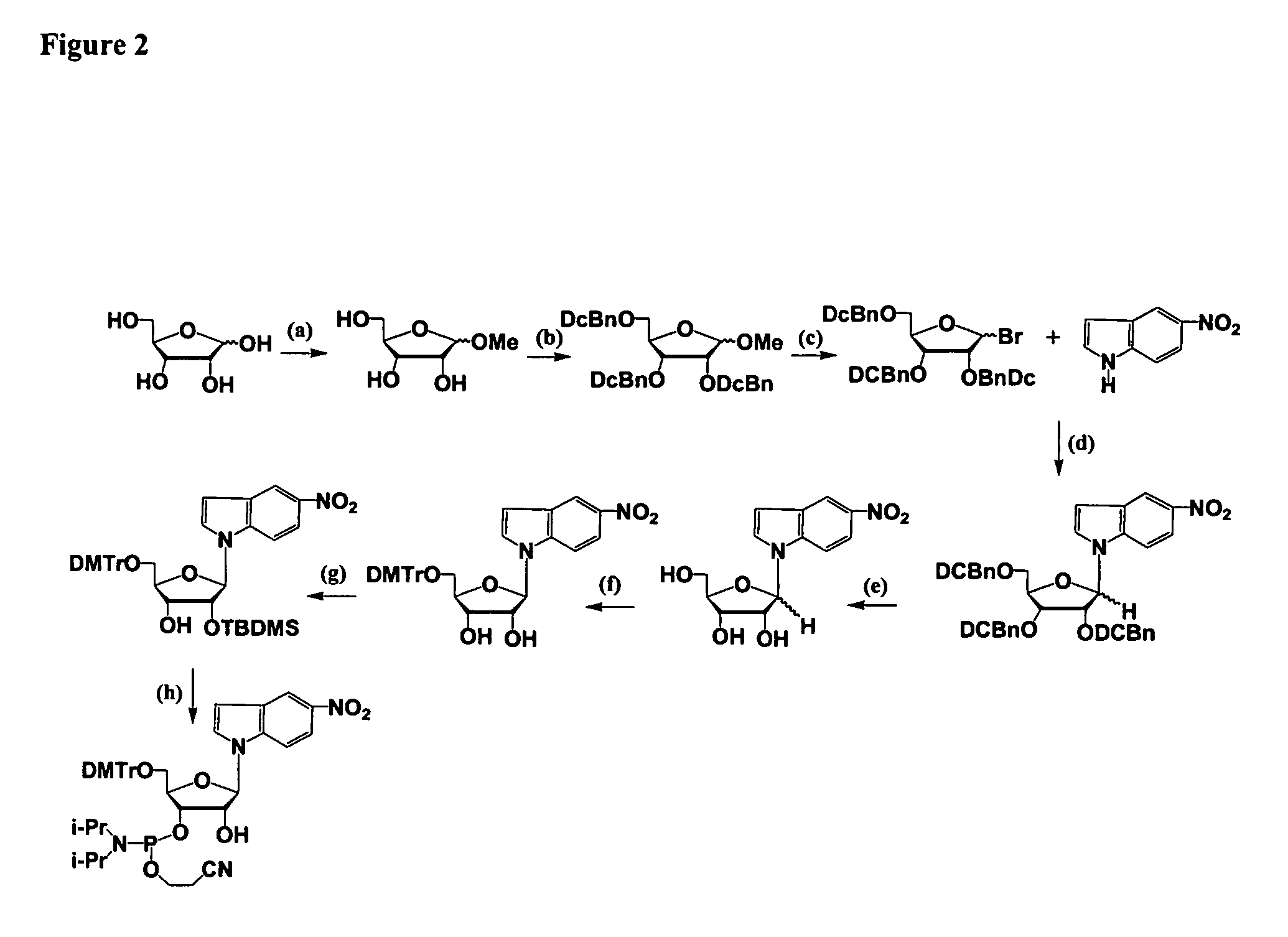
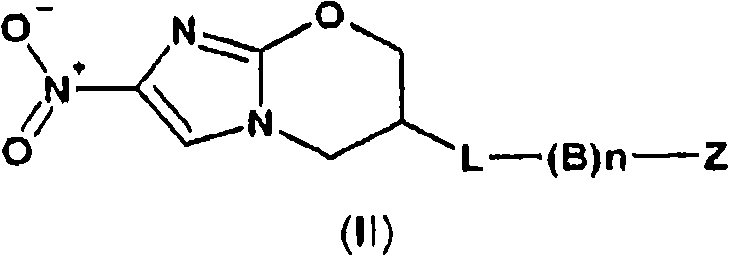
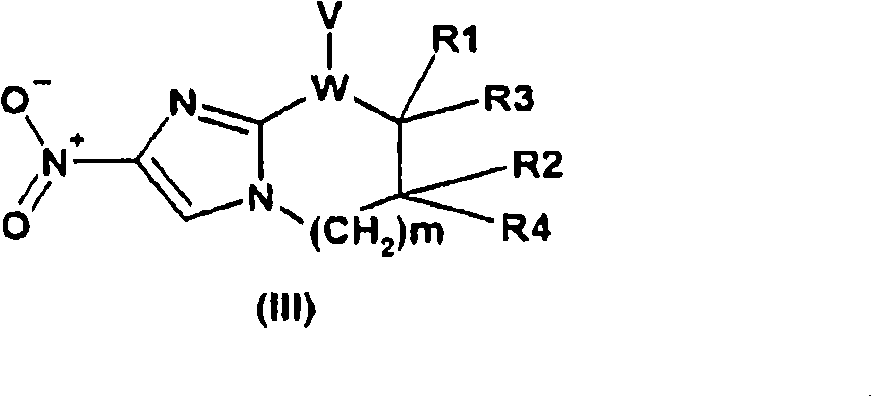
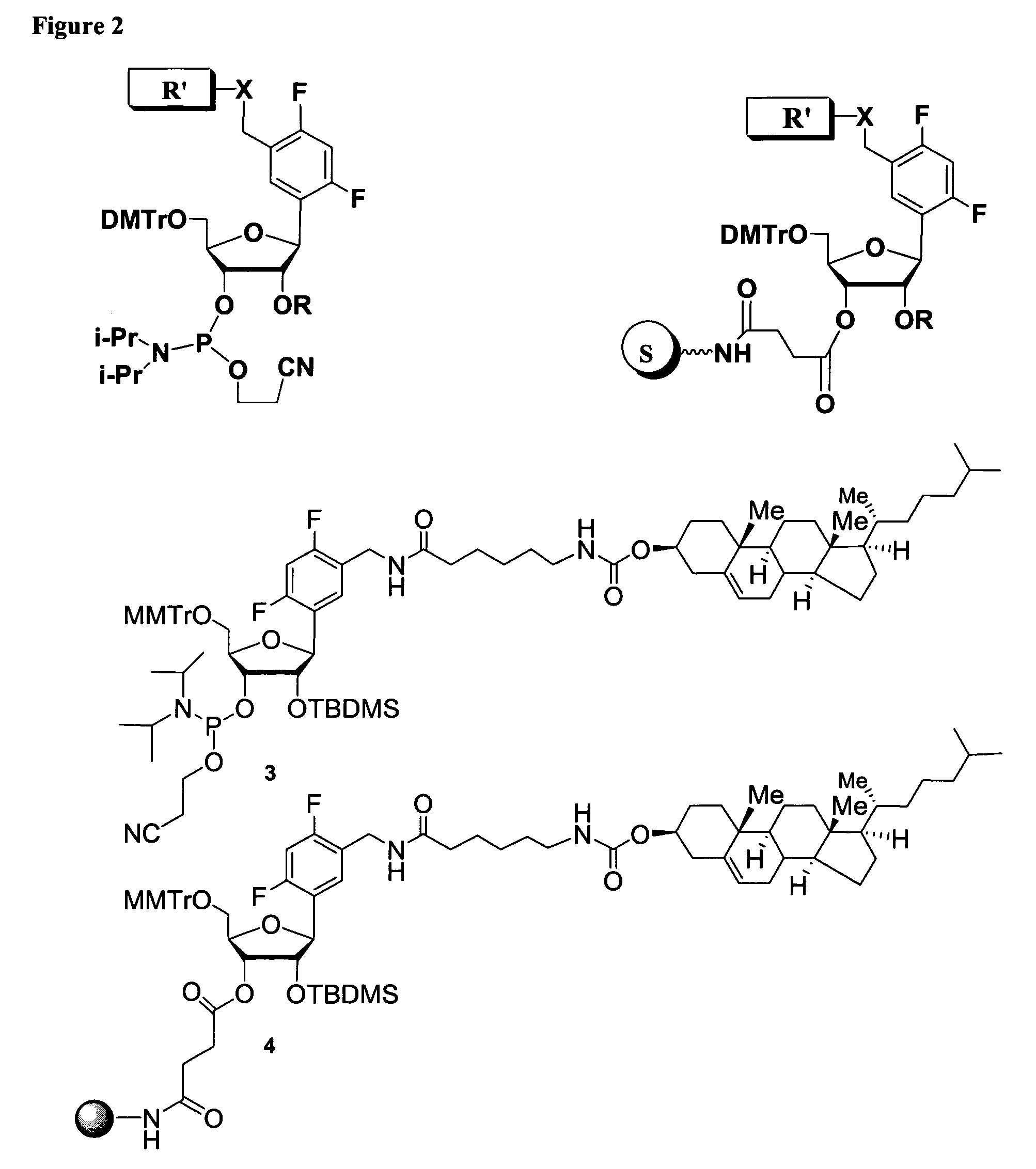
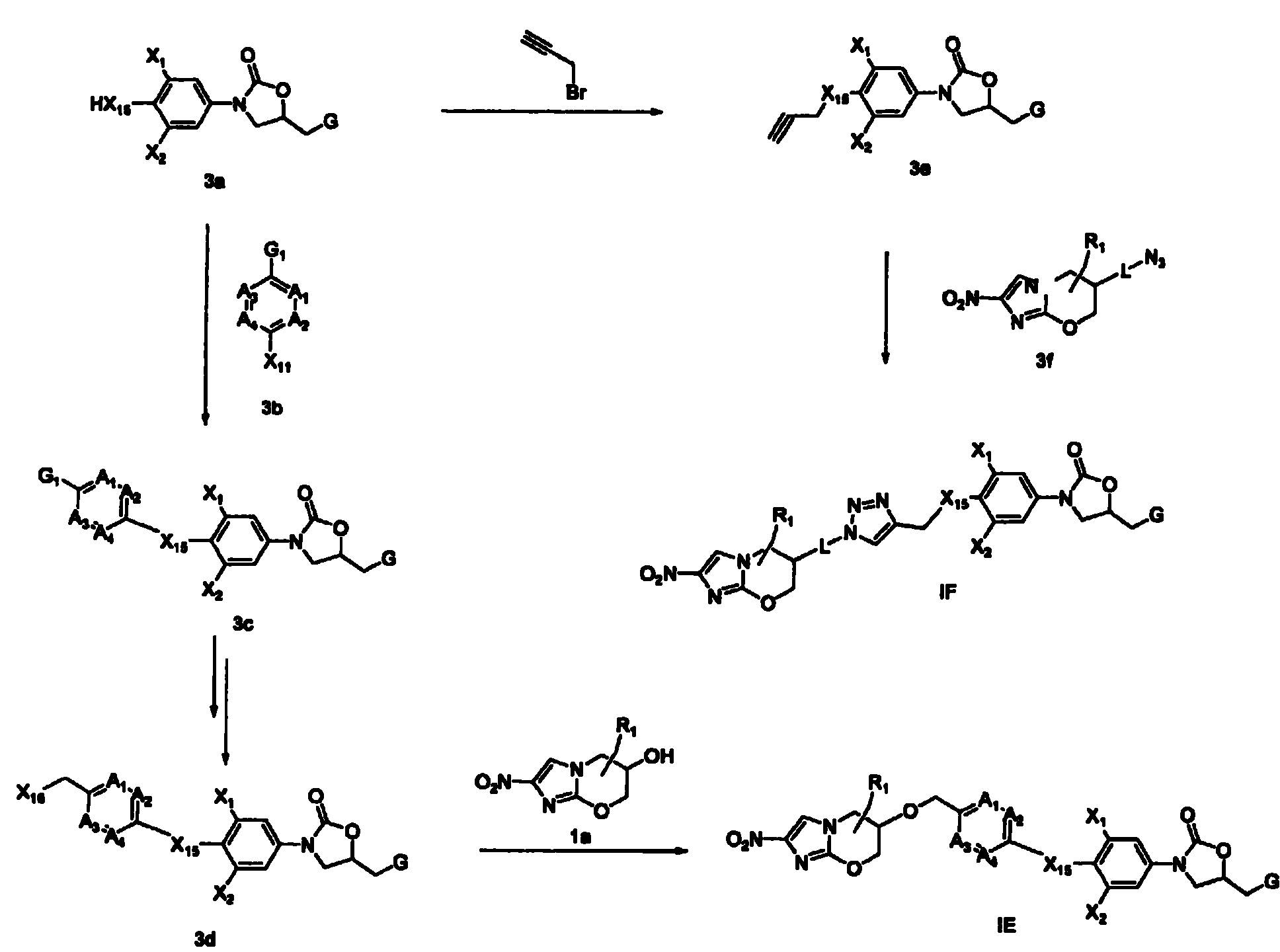
Oligonucleotides comprising a modified or non-natural nucleobase
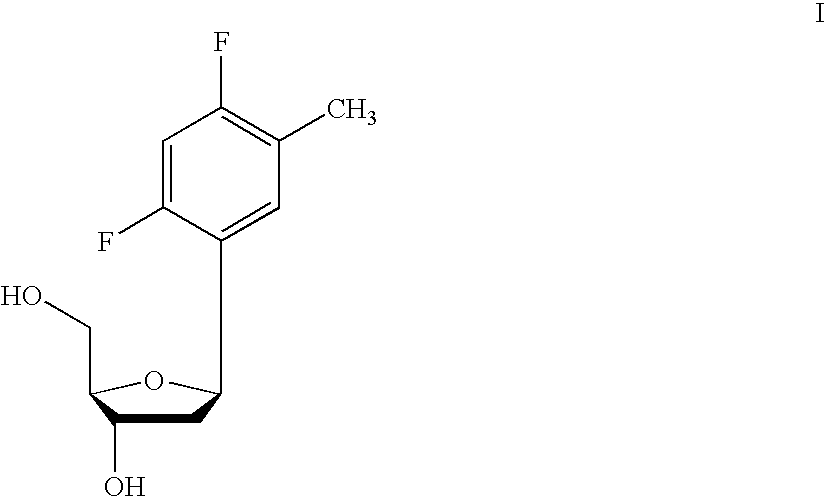
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide contains a non-natural nucleobase. In certain embodiments, both of the oligonucleotide strands comprising the double-stranded oligonucleotide independently contain a non-natural nucleobase. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one non-natural nucleobase. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, the ribose sugar moiety that occurs naturally in nucleosides is replaced with a hexose sugar, polycyclic heteroalkyl ring, or cyclohexenyl group. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage.
Owner:ALNYLAM PHARM INC
Foamable compositions containing nitro-imidazoles, processes for preparing same and methods of treatment utilizing same
A pharmaceutical or cosmeceutical foamable composition for topical application of nitroimidazoles such as Metronidazole and a process of manufacturing the same is disclosed. A method of treatment of skin and scalp disorders, especially of rosacea, acne and foul smelling lesions, by dispensing nitroimidazoles such as Metronidazole in foamable composition is also disclosed.
Owner:AGIS INDUSTRIES (1983) LTD
RNAi Agents Comprising Universal Nucleobases
InactiveUS20080213891A1Broad scopeReduce needSugar derivativesPolymorphism usesNitroimidazoleViral Genes
One aspect of the present invention relates to an oligonucleotide agent comprising at least one universal nucleobase. In certain embodiments, the universal nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the universal nucleobase is difluorotolyl. In certain embodiments, the oligonucleotide is double-stranded. In certain embodiments, the oligonucleotide is single-stranded. Another aspect of the present invention relates to a method of altering the expression level of a target in the presence of target sequence polymorphism. In a preferred embodiment, the oligonucleotide agent alters the expression of different alleles of a gene. In another preferred embodiment, the oligonucleotide agent alters the expression level of two or more genes. In another embodiment, the oligonucleotide agent alters the expression level of a viral gene from different strains of the virus. In another embodiment, the oligonucleotide agent alters the expression level of genes from different species.
Owner:ALNYLAM PHARMA INC
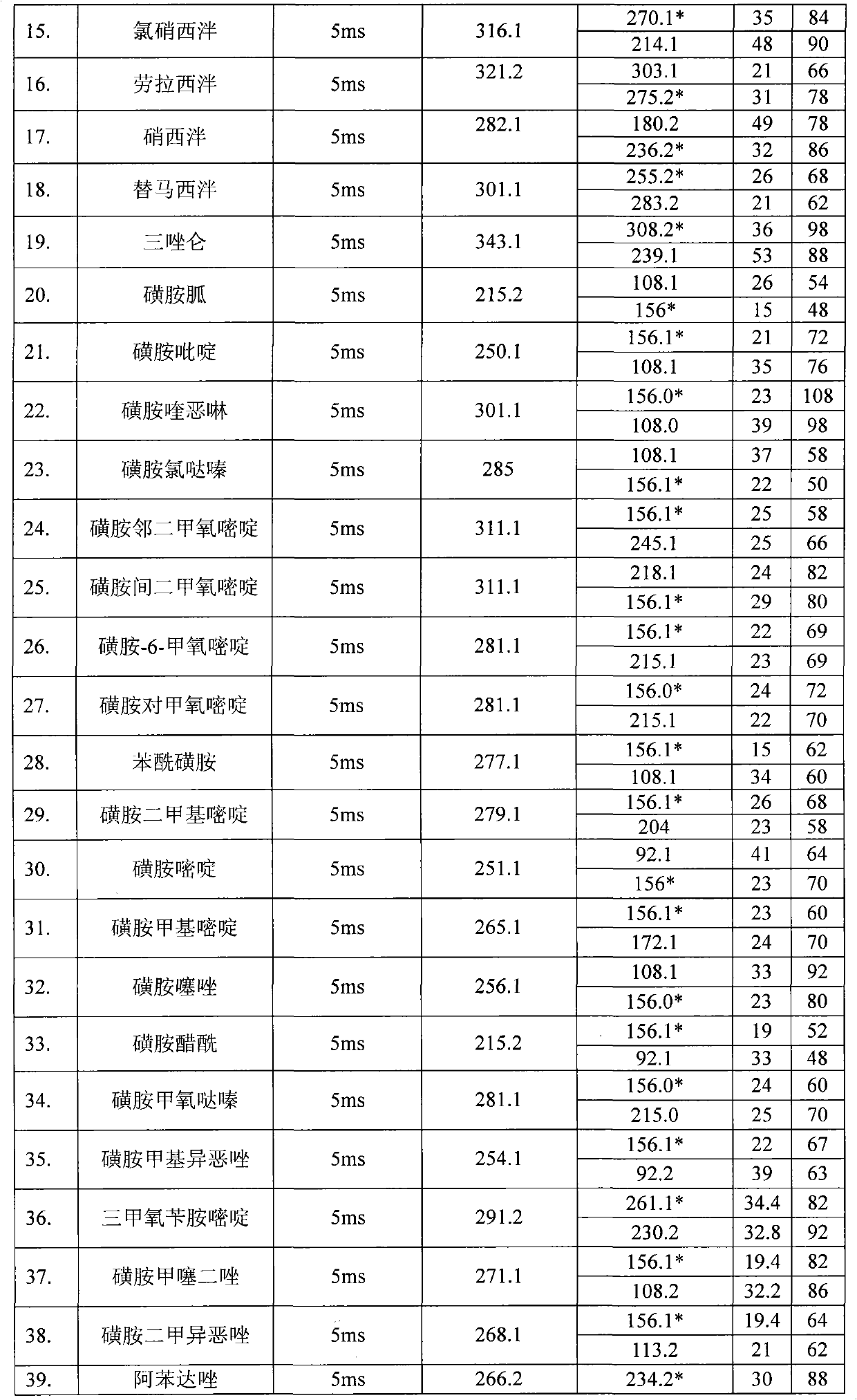
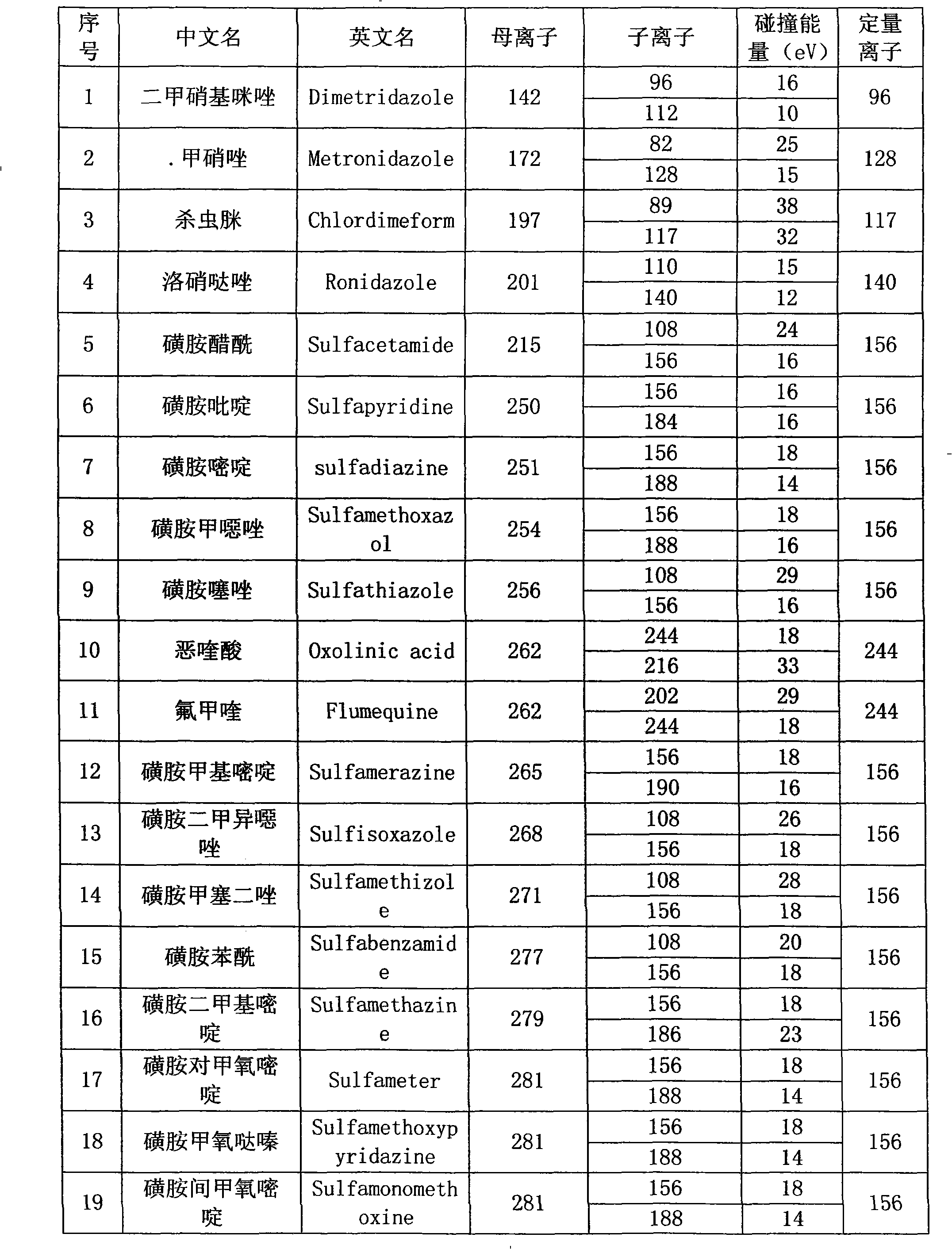
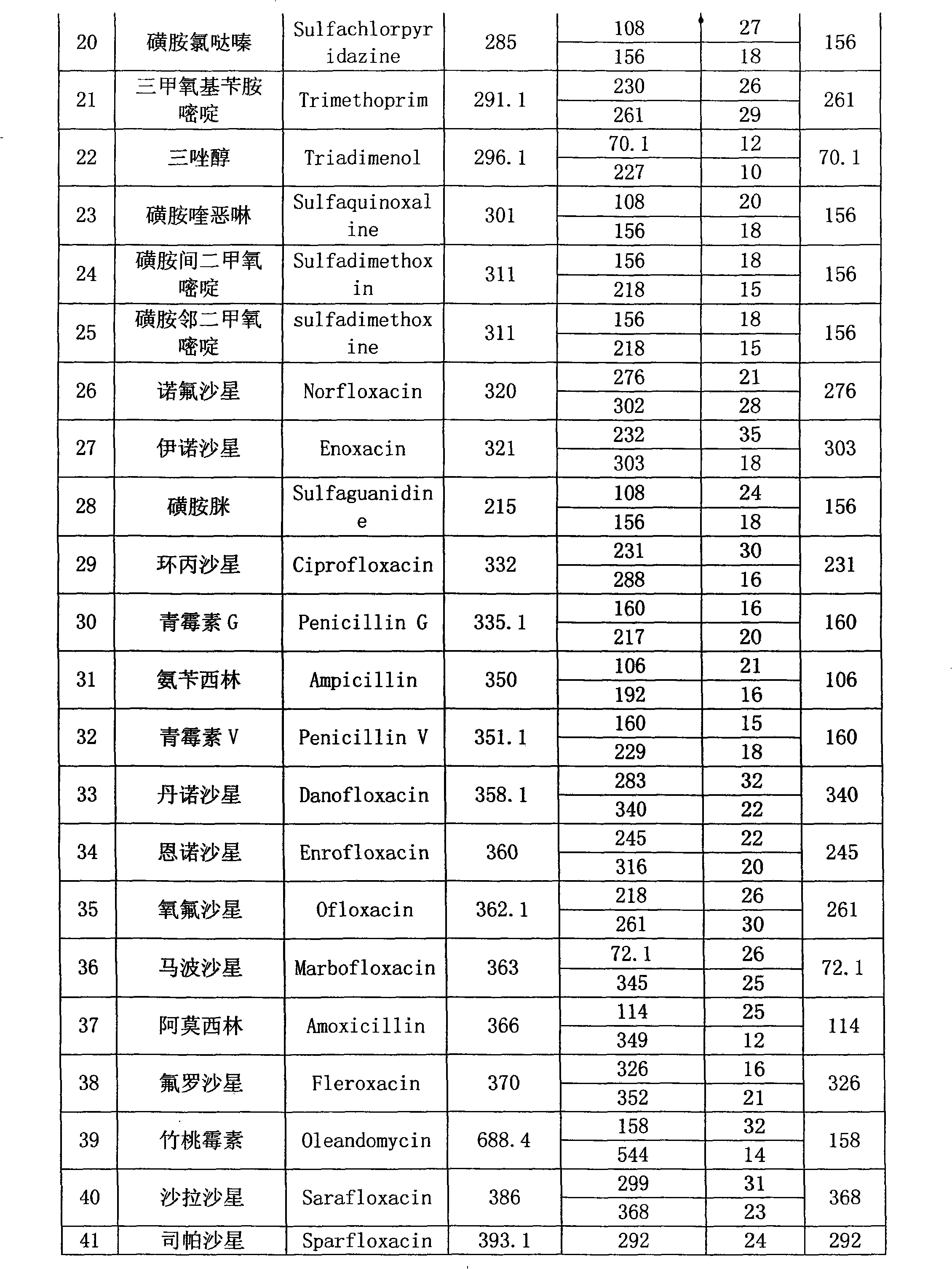
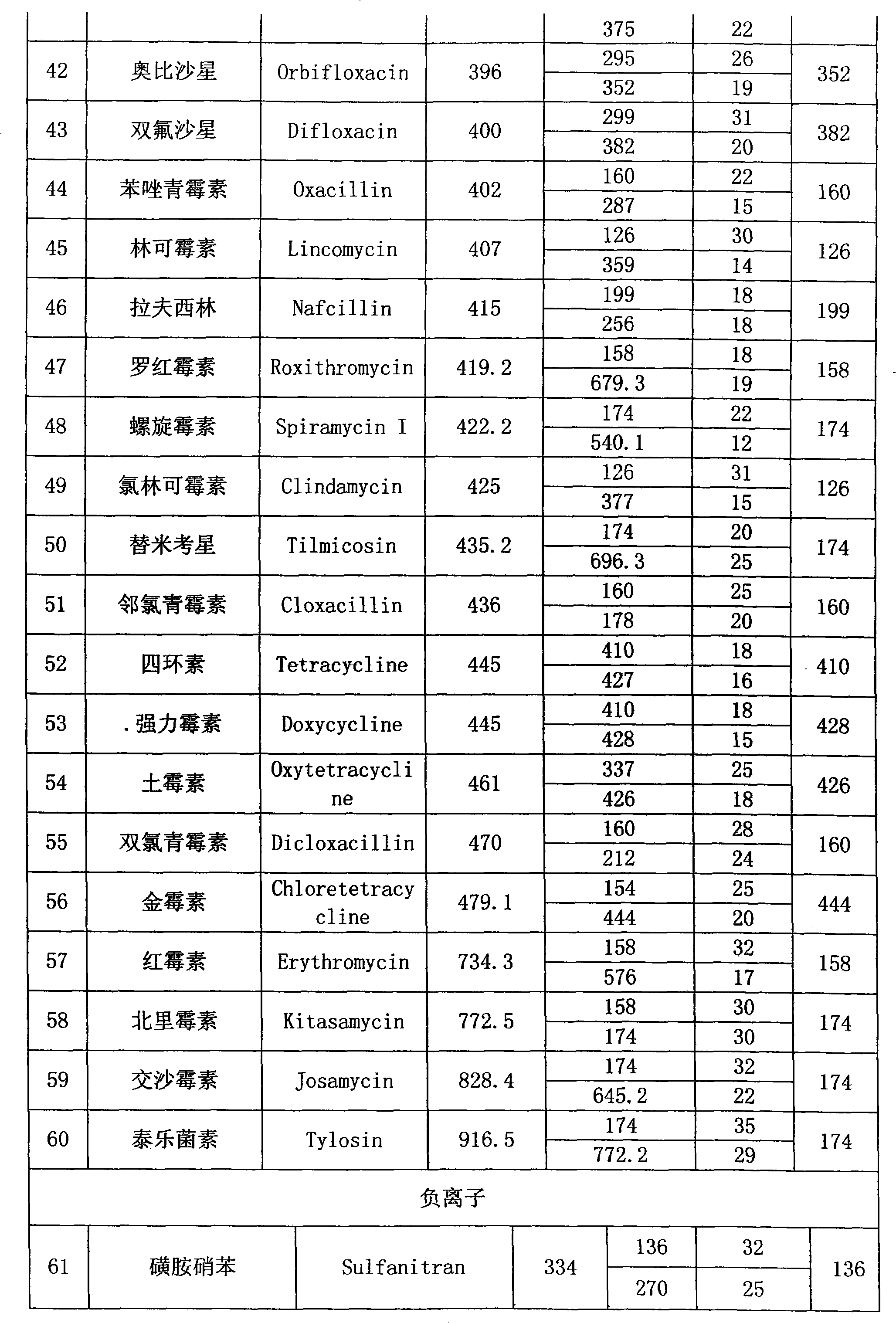
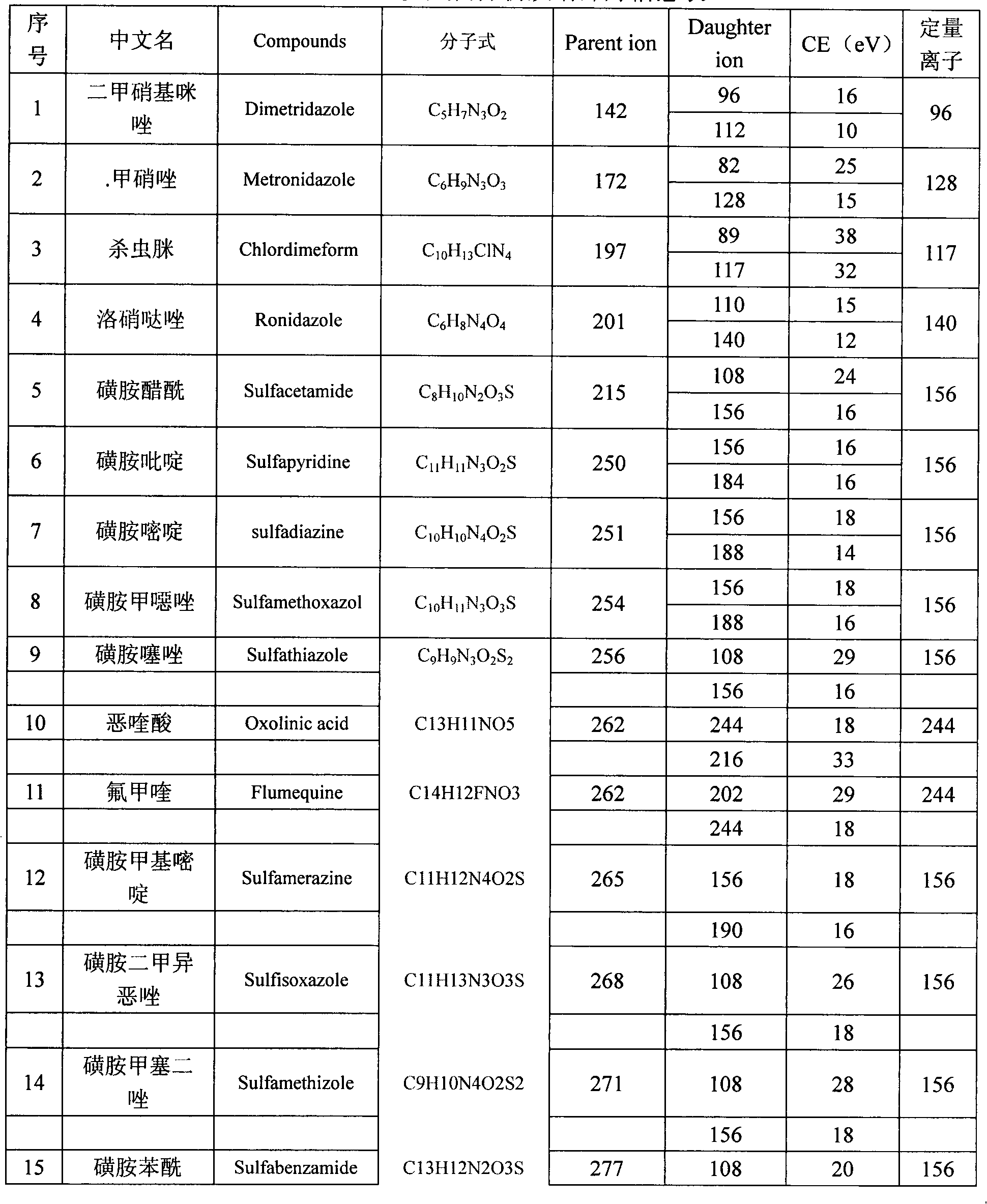
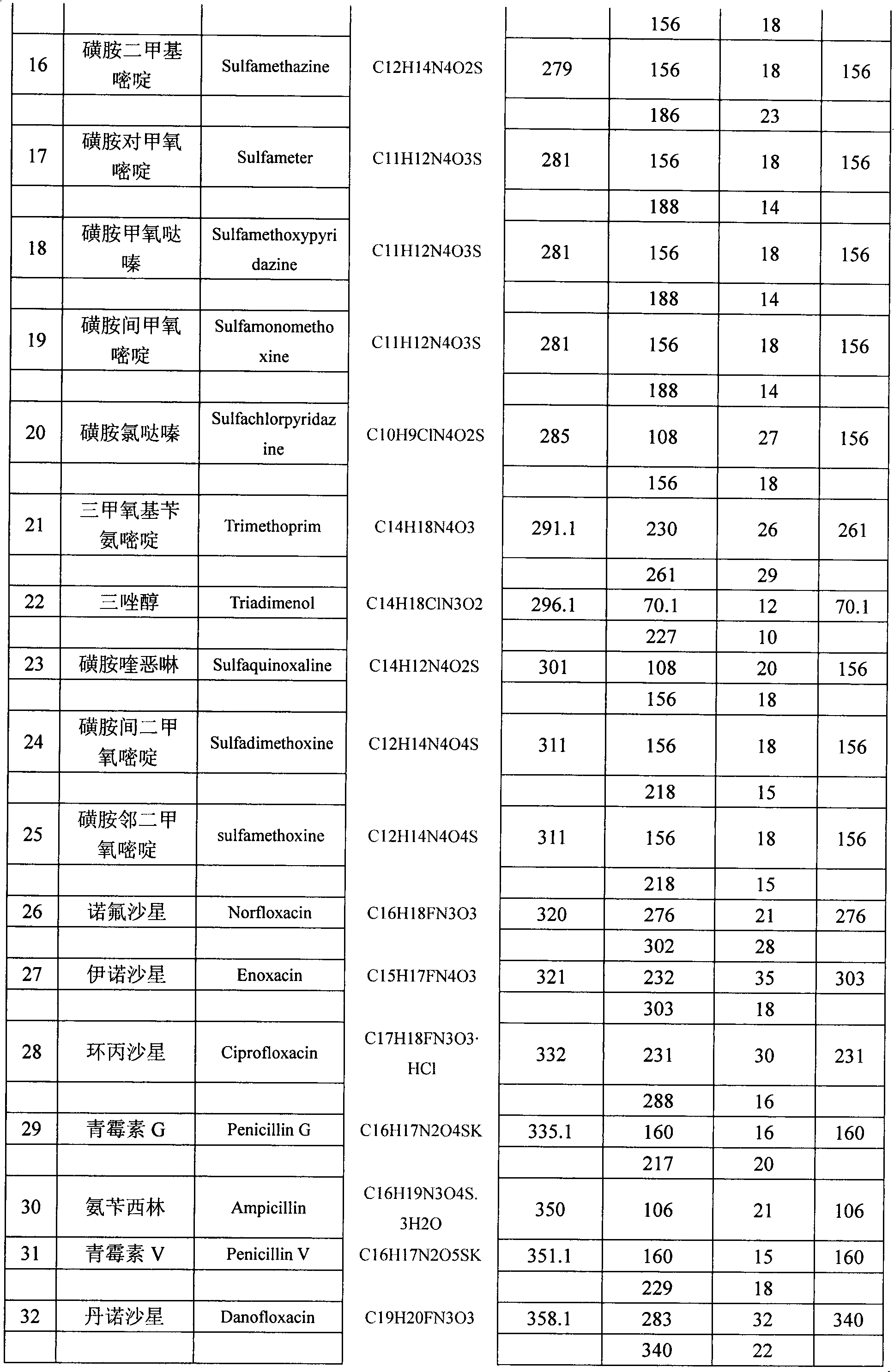
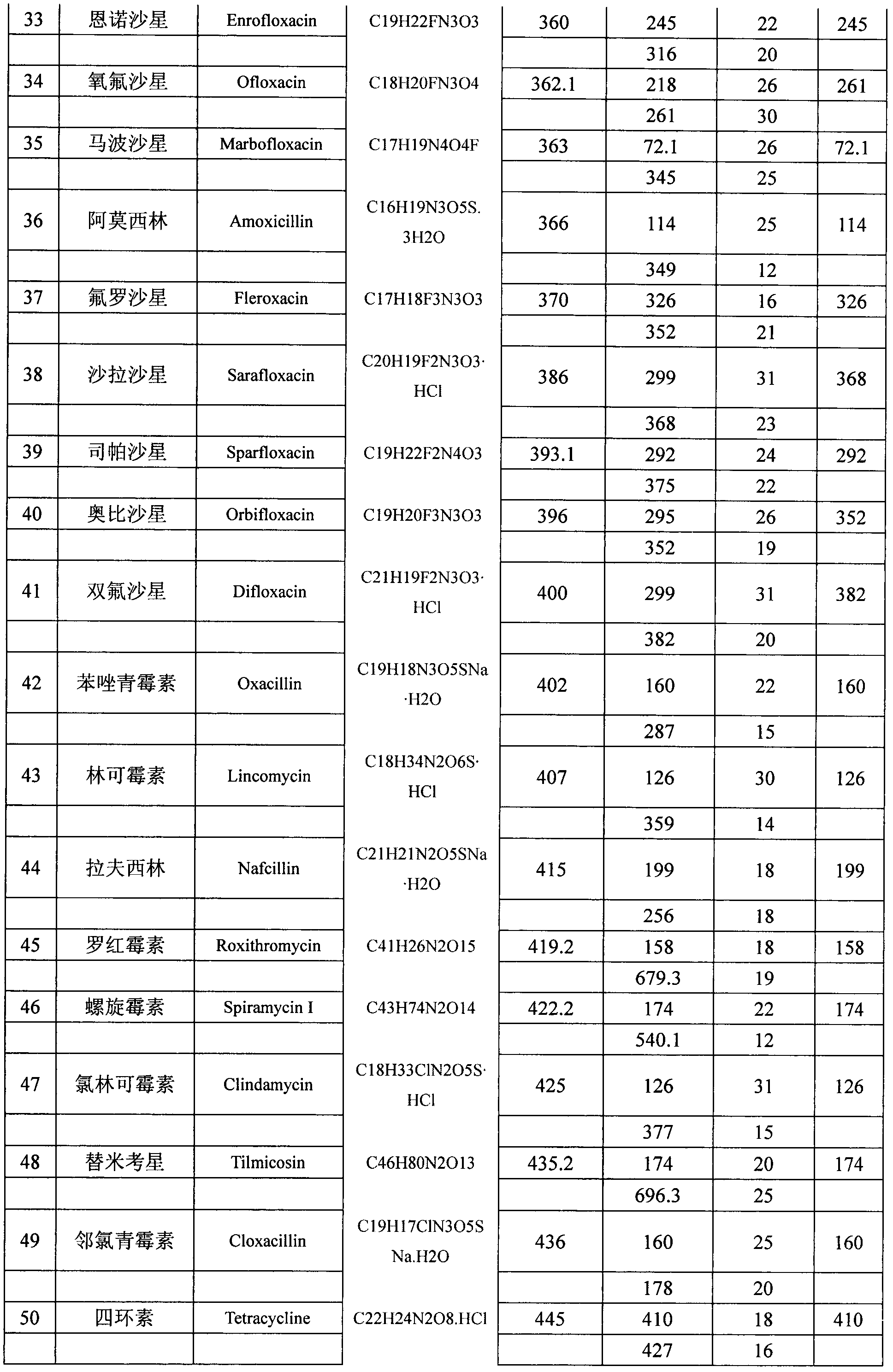
Method for detecting residual quantity of multiple alkaline drugs in animal derived food
The invention relates to the fields of analytical chemistry and food safety, in particular to a method for detecting the residual quantity of multiple alkaline drugs in animal derived food. Based on the vortex mixed extracting of acetonitrile, isopropanol and citric acid buffer solutions, the purification of a hydrophilic polystyrene-divinylbenzene solid phase extraction column and a cation exchange solid phase extraction column and the liquid phase chromatography-mass spectra determination, the method can detect the residual quantity of multiple alkaline drugs in pork, pork liver, eggs, shrimps and milk, such as beta-receptor agonists,sulfonamides, benzodiazepines, nitroimidazoles, benzimidazoles and triphenylmethanes. The method has the advantages of simple operation, fast and accurate detection and high efficiency.
Owner:SHANGHAI ANPEL SCI INSTR +1
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Method for simultaneously detecting multi-kind pesticide residues in bee products
InactiveCN101358953ASolve the problem of matrix effectFast wayComponent separationRetention timePhosphate
The present invention relates to a method of simultaneously detecting a plurality of agro-veterinary drug residues in bee products. The extracted liquid trichloroacetic acid or perchloric acid and the extracted liquid acetate, phosphate or borate solution are added into a sample; the pH value is controlled between 4.5 and 9.0; the mixed solution is centrifuged, the filtrate is added into a solid phase extraction column to be extracted, the extraction column is eluted and dried, the column is washed by oxalic acid-methanol solution, the volume of the eluent is defined by the aqueous solution of methanol, the eluent is added into liquid chromatography-tandem mass spectrometry to be analyzed and tested, the acquired chromatographic peak is contrasted with the known standard chromatographic peak of the drug, and according to the retention time and the abundance of the mass spectrum ions, the specific name of the detected drug is determined. The method only requires one pre-treatment of the sample, and thus can simultaneously extract 11 classes and more than 60 kinds of veterinary drug residues, such as sulfonamides, quinolones, macrolides, lincomycins, nitroimidazoles, beta-lactams, tetracyclines, chloromycetins, trinethoprims, chlordimeform, triadimenol and the like, the efficiency of analysis is high, and the detection cost is greatly reduced.
Owner:中华人民共和国江苏出入境检验检疫局
Oligonucleotides comprising a modified or non-natural nucleobase
Owner:ALNYLAM PHARMA INC
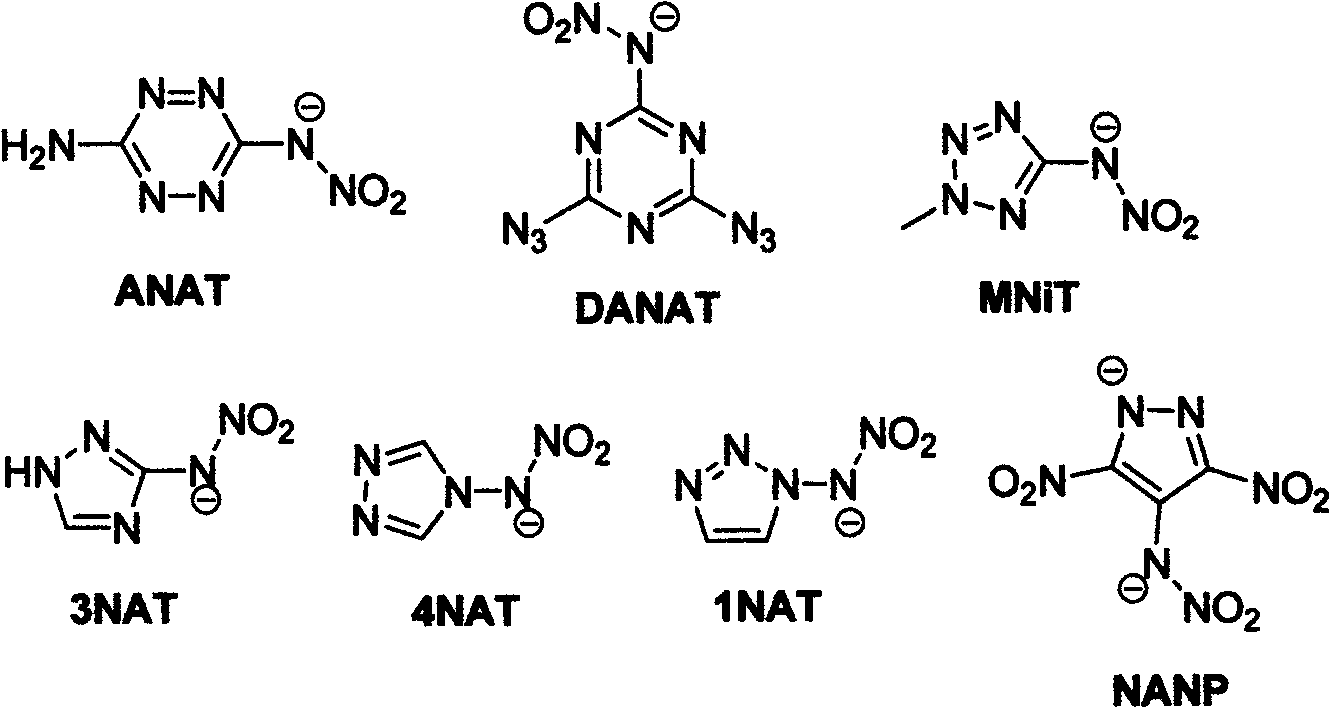
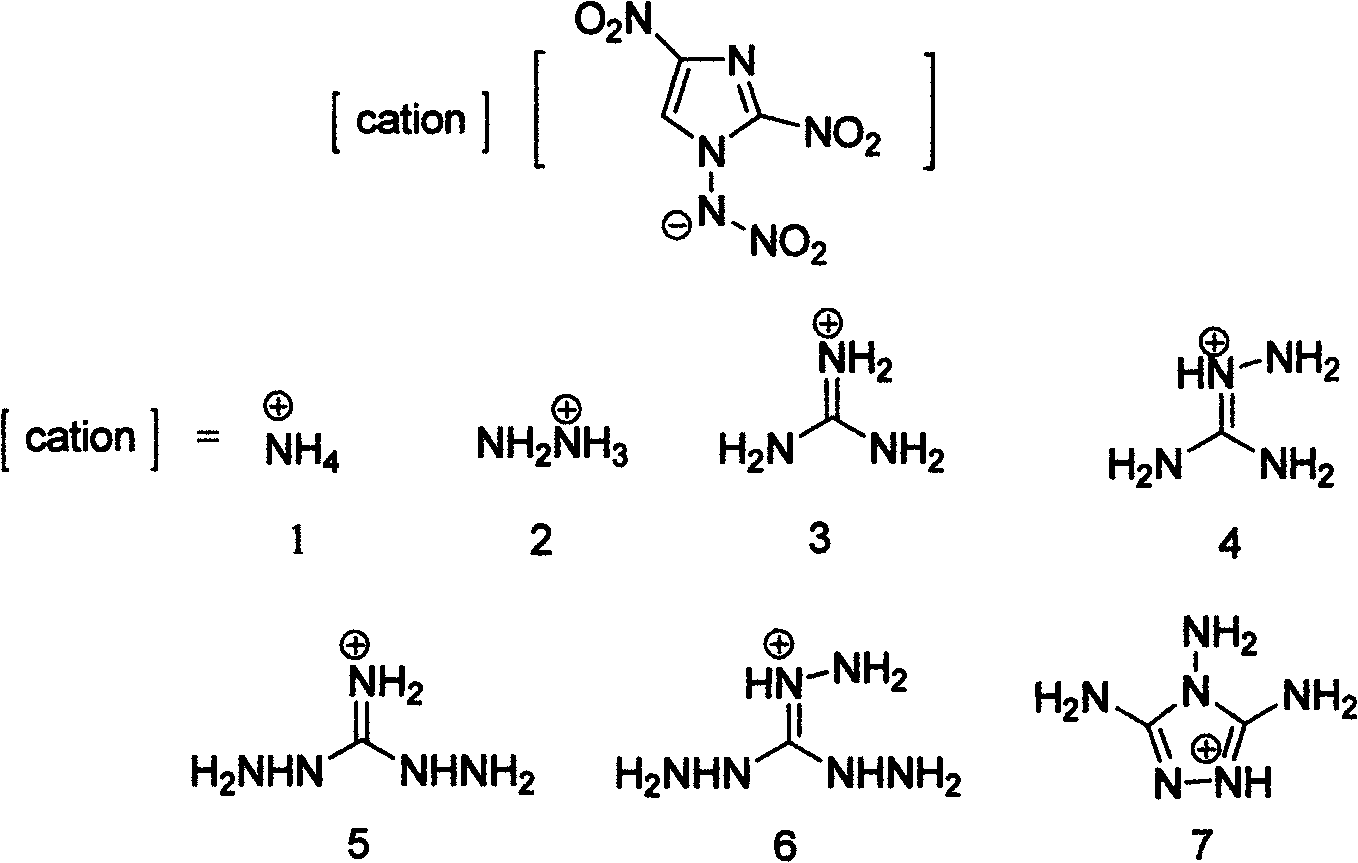
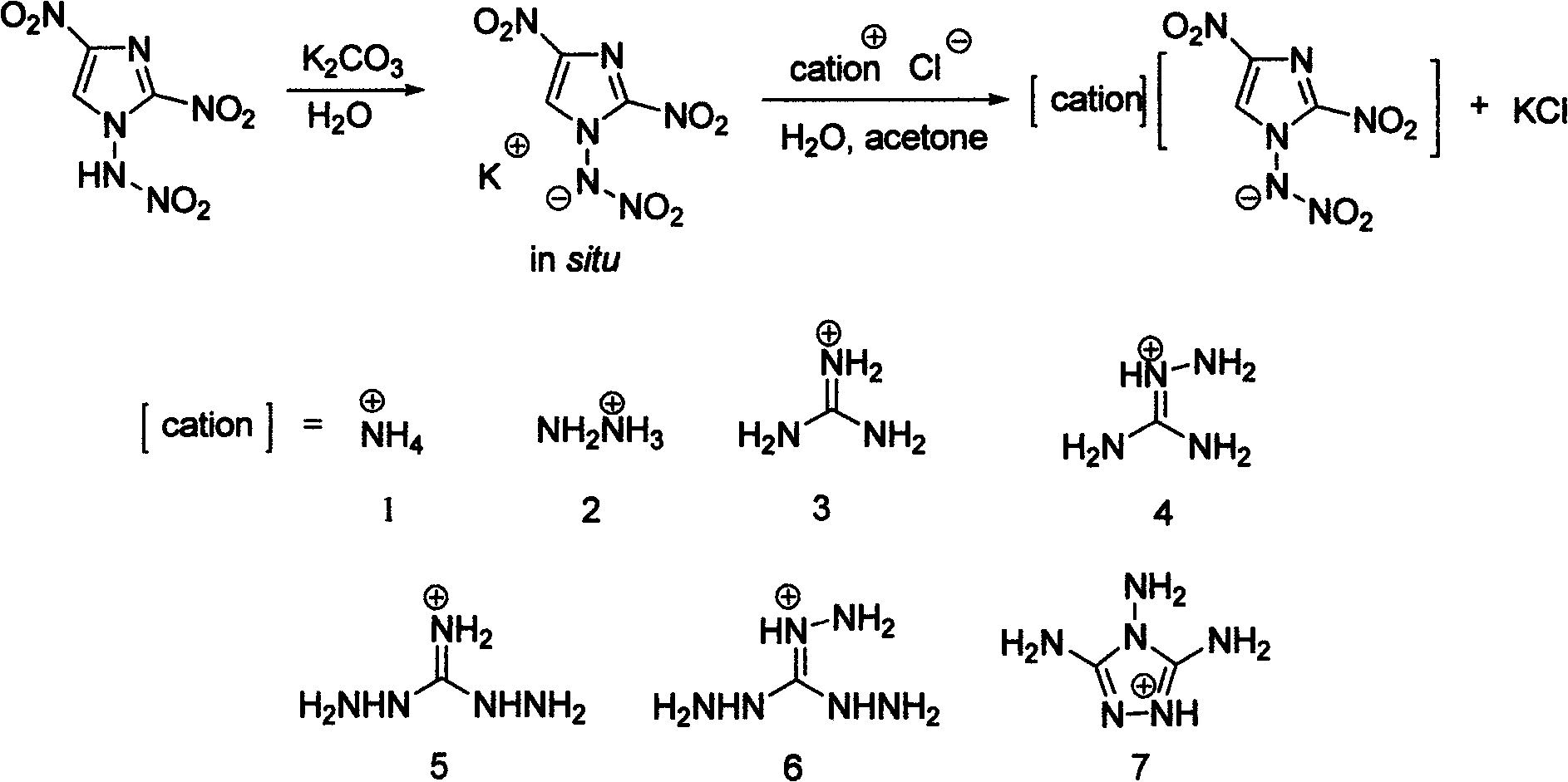
Energetic ion salts of 1-nitramine-2, 4-dimetridazloe and preparation method thereof
InactiveCN103483264AExcellent detonation speed and pressure performanceThe synthesis method is simpleOrganic chemistryOrganic compound preparationNitroimidazoleHydrazine compound
The invention discloses energetic ion salts of 1-nitramine-2, 4-dimetridazloe and a preparation method thereof, and belongs to the technical field of energetic materials. The synthetic method is as follows: dissolving the 1-nitramine-2, 4-dimetridazloe in deionized water to obtain a pale yellow clear liquid, adding with stirring 0.5 time molar equivalent of potassium carbonate at room temperature for in-situ generation of 1-nitramine-2, 4-dimetridazloe potassium salt, then adding one time molar equivalent of ammonium chloride, hydrazine hydrochloride, guanidine hydrochloride, monoaminoguanidine hydrochloride, diaminoguanidine hydrochloride, triaminoguanidine hydrochloride and 3, 4, 5-triamino-1, 2, 4-triazole hydrochloride, stirring to precipitate a pale yellow solid precipitate, after about 1 hour of reaction, filtering the pale yellow precipitate, further recrystallizing a coarse product by use of an acetone and diethyl ether mixed solvent to obtain a pure product. The synthetic method of the invention is simple, mild in condition and high in yield, and is environmental friendly due to using of the deionized water as a solvent. The density of involved seven salts is 1.70-1.93g cm<-3>, the detonation velocity calculated by EXPLO software is between 8370 and 9209 m s<-1>, the detonation pressure is between 29.3 and 40.5 GPa, the actually measured impact sensitivity is 4-40J, the detonation performance is excellent, and the energetic ion salts are potential energetic materials.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method for treating metronidazole waste water
ActiveCN102344220AAvoid pollutionAchieve recyclingPreparation from carboxylic acid saltsOrganic compound preparationNitroimidazoleSODIUM SULFATE ANHYDROUS
The invention relates to a method for treating metronidazole waste water, which comprises the following steps of: concentrating the metronidazole waste water into 1 / 3-1 / 5 of total amount, and crystallizing and filtering the concentrated metronidazole waste water at 45-100 DEG C to obtain anhydrous sodium sulfate and sodium formate; adjusting the PH to 3-6 by using sulfuric acid or formic acid, and precipitating 2-methy-5-nitro imidazole; concentrating the filter liquid to anhydrous sticky state, dissolving organic matter by using methanol or ethanol, and filtering the solution to obtain anhydrous sodium sulfate and sodium formate; merging the sodium formate and the anhydrous sodium sulfate obtained in two times, successively adding formic acid and sulfuric acid, and recovering the formic acid to obtain anhydrous sodium sulfate; and rectifying and separating the organic mixture extract of methanol or ethanol to obtain methanol or ethanol and glycol. The method for treating metronidazole waste water provided by the invention is simple and convenient to operate, the content of polluting organic matter and inorganic slats in the metronidazole waste water can be obviously reduced, environmental pollution can be preveneted, various kinds of useful organic matter and inorganic matter can be recovered, raw materials are saved, and the production cost is lowered.
Owner:HUBEI HONGYUAN PHARMA
Radiotherapy combined with hypoxic cell sensitizers
The invention discloses a method of treating cancer in a patient, comprising administering to the patient a radiation sensitizer selected from nitroimidazoles in an amount effective to sensitize a patient to radiation and subjecting the patient to radiation therapy. In certain embodiments the radiation sensitizer is etanidazole or doranidazole.
Owner:INTRAOP MEDICAL
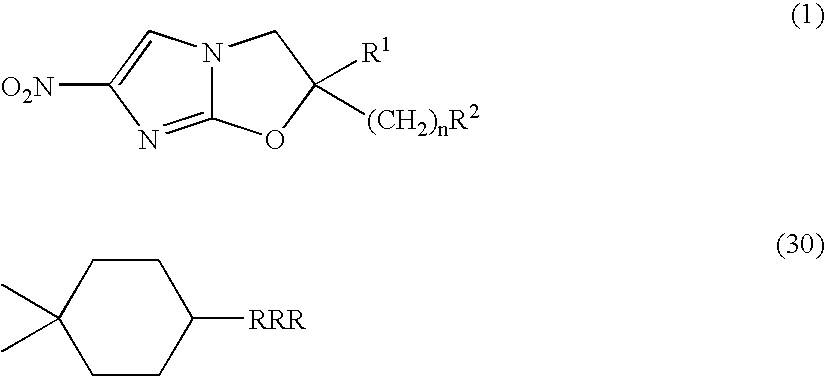
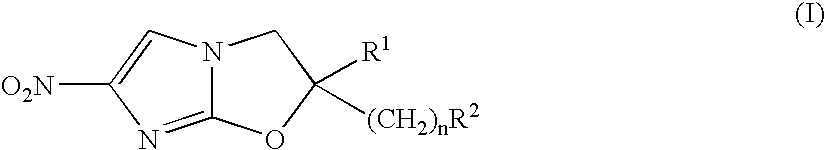
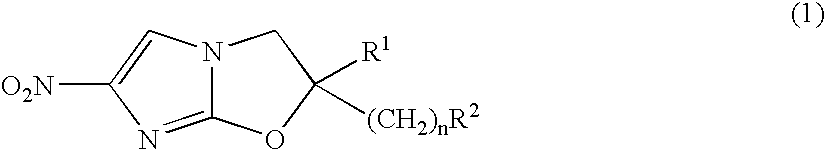
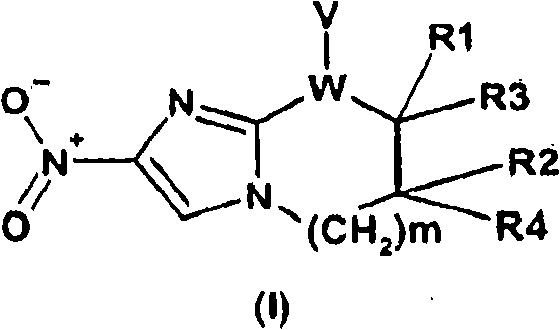
2,3-Dihydro-6-Nitroimidazo (2,1-b) Oxazole Compounds for the Treatment of Tuberculosis
InactiveUS20080119478A1Improve the bactericidal effectAntibacterial agentsBiocideNitroimidazoleHydrogen atom
The present invention provides a 2,3-dihydro-6-nitroimidazo[2,1-b]oxazole compound represented by the following general formula: (1) in the above formula (1), R1 represents a hydrogen atom or C1-C6 alkyl group, n represents an integer of 0 to 6, R1 and —(CH2)nR2 may form a spiro ring represented by the formula (30) below, together with the adjacent carbon atom (in the formula below, RRR represents a piperidyl group which may have substituents on the piperidine ring), (30) and R2 represents a benzothiazolyloxy group, quinolyloxy group, pyridyloxy group or the like. The present compound has an excellent bactericidal action against Mycobacterium tuberculosis, multi-drug-resistant Mycobacterium tuberculosis, and atypical acid-fast bacteria.
Owner:OTSUKA PHARM CO LTD
Optical antimer of a group of nitro imidazole derivatives, preparation method and uses thereof
The invention relates to a process for preparing optical enantiomers of nitroimidazole derivatives and the use of these compounds in preparing anti anaerobe medicaments.
Owner:西安新安医药科技有限公司
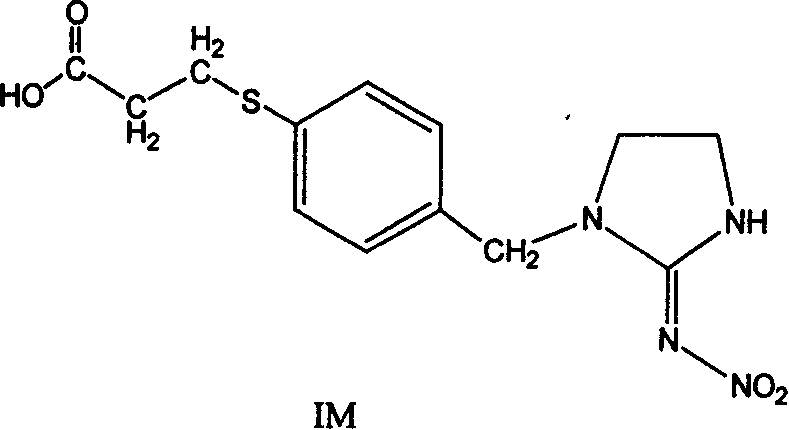
Production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody
InactiveCN1569840AEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting food2-ImidazolineImidacloprid
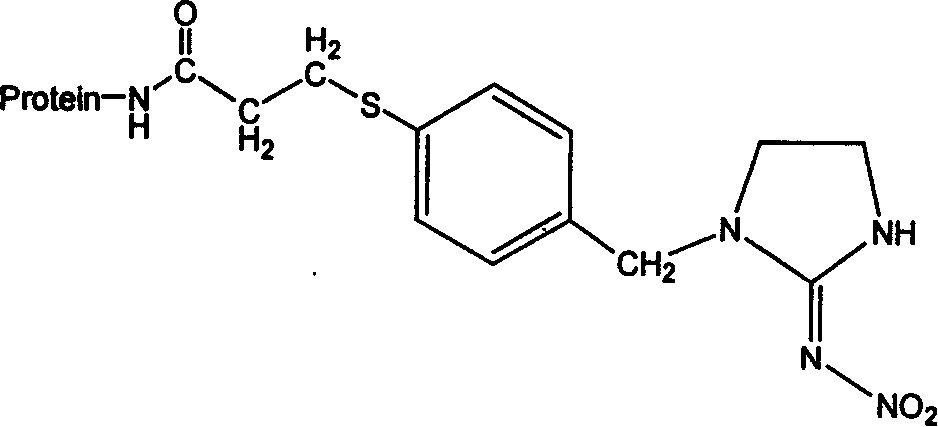
The invention discloses the production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody, wherein the production method comprises, using imidacloprid (1-(6-chlorine-3-picolyl)-N-nitro-2-imidazoline imine) as raw material for reaction with 3-mercaptopropionic acid under alkaline condition, thus synthesizing hapten 1-(6-(2-carboxyethyl) sulfo-3-picolyl)-N-nitro-2-imidazoline imines (IM), then coupling with proteins through carbodiimide method and mixed anhydride method to prepare artificial antigens (immunogens and peridium antigens).
Owner:ZHEJIANG UNIV
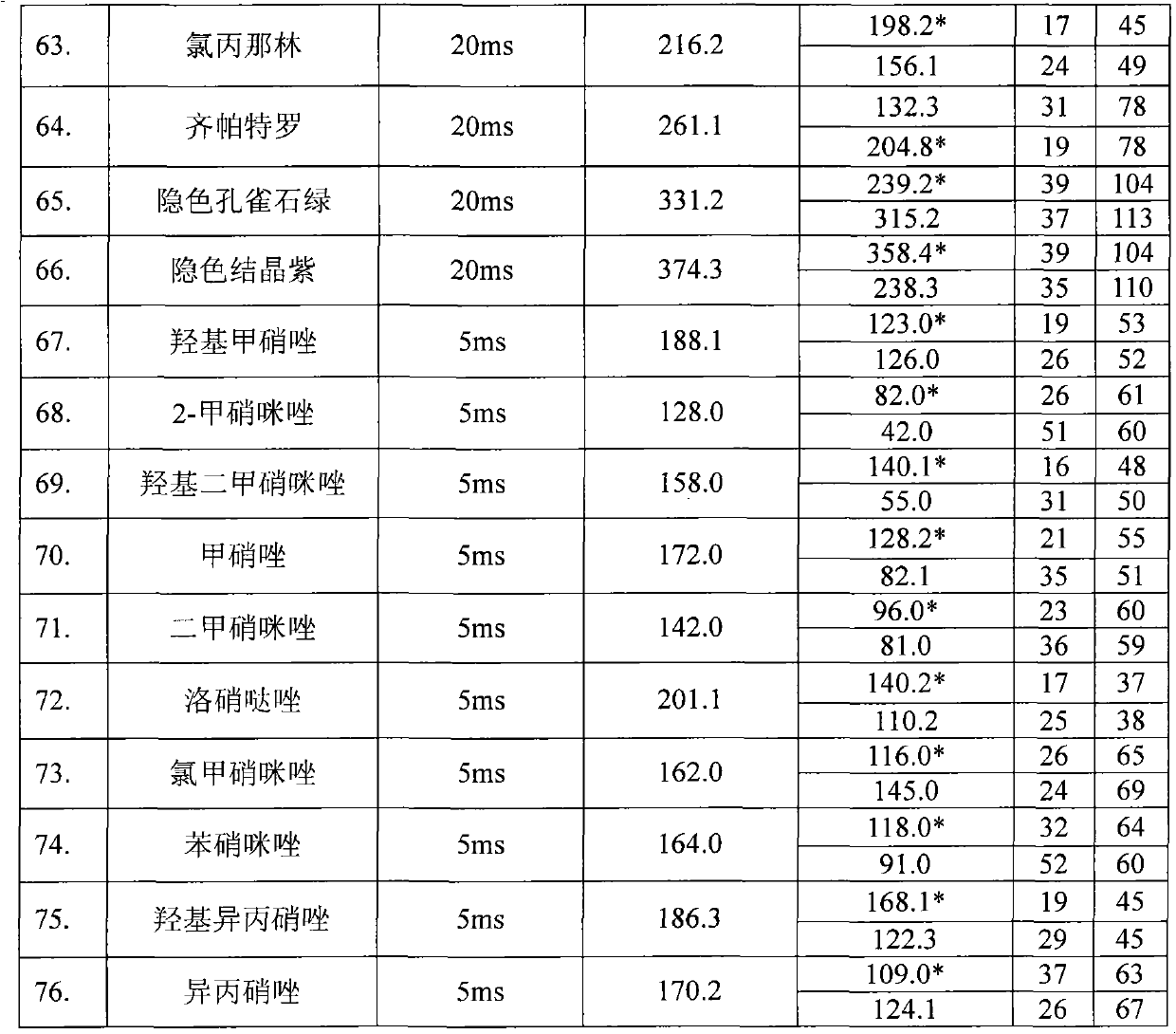
Determining method for 15 kinds of forbidden nitro imidazoles antibiotics in cosmetics
InactiveCN103048401AQuick checkThe result is accurateComponent separationNitroimidazoleRetention time
The invention relates to a determining method for 15 kinds of forbidden nitro imidazoles antibiotics in cosmetics. The determining method is characterized by comprising the step that a cosmetic sample is subjected to detection analysis by using a high-performance liquid chromatography-series quadrupole mass spectrometry after being subjected to supersonic extraction, high speed centrifugation and solid phase extraction. Compared with a parallelly operated standard sample of the 15 kinds of forbidden nitro imidazoles antibiotics such as metronidazole, the cosmetic sample disclosed in the invention is qualified by retention time and optimized monitoring ion pairs, is quantified by taking ions with a relatively high noise-signal ratio, a good peak form and low background interference as quantified ions, and then is subjected to external standard method quantification by using a standard curve. By utilizing the determining method, the detection speed is fast, and a result is accurate.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Detection method of residual quantities of various veterinary drugs in culturing or slaughtering environment
The invention provides a detection method of residual quantities of various veterinary drugs in a culturing or a slaughtering environment. With the method, rapid screening and quantitative detection can be carried out upon 61 drugs of 9 categories. The drugs include chlormycetin, beta lactams (penicillins), quinolones, sulfonamides, trimethoprims, macrolides, tetracyclines, and nitroimidazoles. According to the invention, a soil sample or an environmental water sample is added into a phosphate buffering solution; a filtrate obtained through centrifugation is extracted in a solid phase extraction column, and is eluted; the extraction column is dried by blowing, and is washed by using a methanol solution; an obtained eluent is titrated by using a methanol solution; a chromatogram peak of the sample is detected by using liquid chromatography-tandem mass spectrometry; the chromatogram peak is compared with a standard chromatogram peak, such that a specific variety of the detected drug is accurately determined. According to the invention, the sample solution is subject to liquid chromatography-tandem mass spectrometry multi-reaction monitoring selected ion analysis. Through internal standard correction, the recovering rate is 70-120%, and a relative standard deviation RSD is no larger than 18%. Compared with existing technologies, the analysis efficiency is improved by at least 5 times, and the detection cost is 30% of that of existing technologies.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
Nitroimidazole compounds
InactiveCN101341150AAntibacterial agentsOrganic active ingredientsNitroimidazoleCombinatorial chemistry
The present invention relates to certain nitroimidazole compounds, which have interesting pharmaceutical properties. In particular, the compounds are useful in the treatment and / or prevention of infections such as those caused by Mycobacterium tuberculosis, Trypanosoma cruzi or Leishmania donovani. The invention also relates to pharmaceutical compositions containing the compounds, as well as processes for their preparation.
Owner:NOVARTIS AG +1
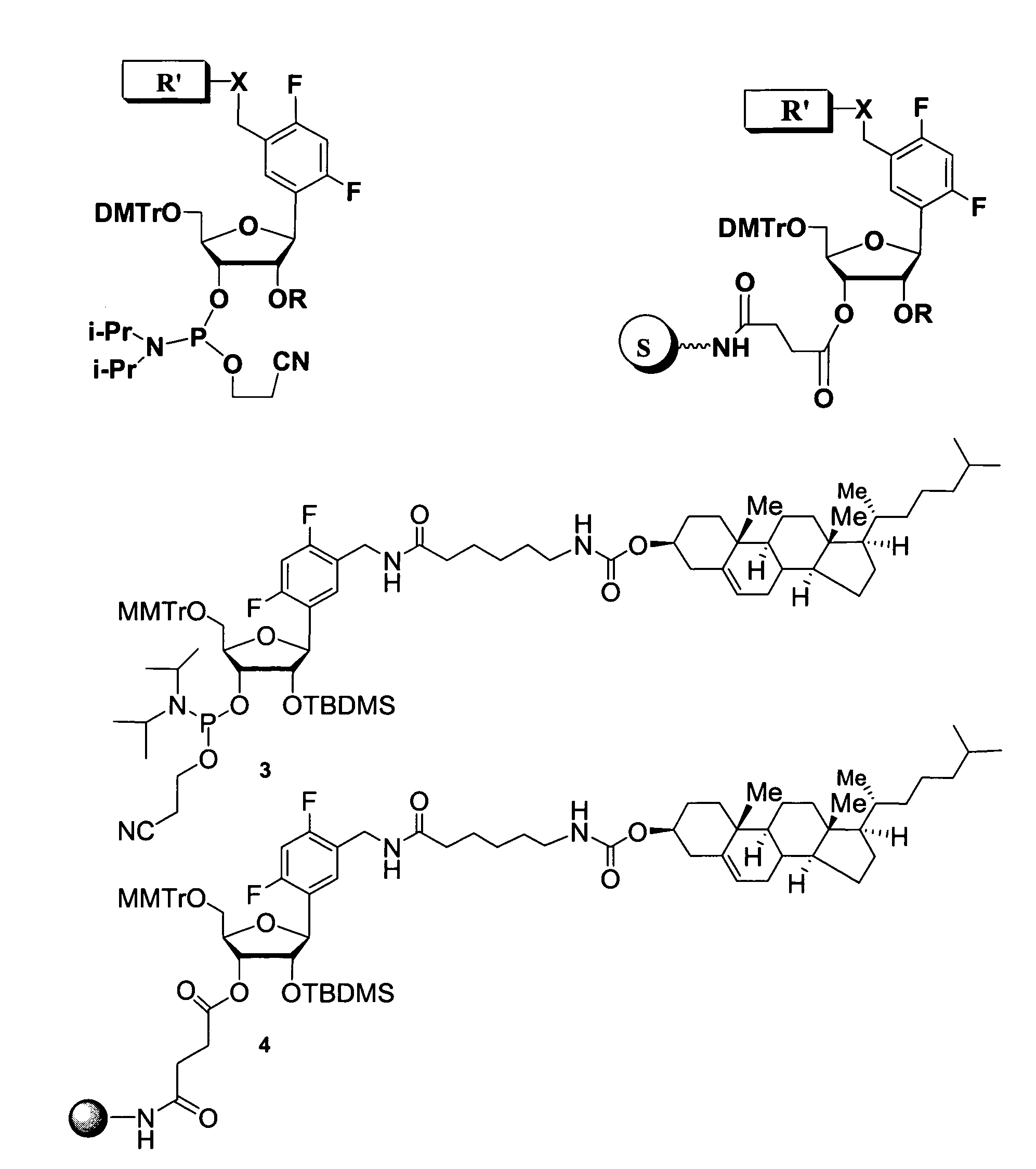
Oligonucleotides comprising a ligand tethered to a modified or non-natural nucleobase
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitropyrrolyl, or nitroimidazolyl. In certain embodiments, the ligand is a steroid or aromatic compound. In certain embodiments, only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide contains a ligand tethered to an altered or non-natural nucleobase. In certain embodiments, both of the oligonucleotide strands comprising the double-stranded oligonucleotide independently contain a ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one ligand tethered to an altered or non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitropyrrolyl, or nitroimidazolyl. In certain embodiments, the ligand is a steroid or aromatic compound. In certain embodiments, the ribose sugar moiety that occurs naturally in nucleosides is replaced with a hexose sugar, polycyclic heteroalkyl ring, or cyclohexenyl group. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage.
Owner:ALNYLAM PHARMA INC
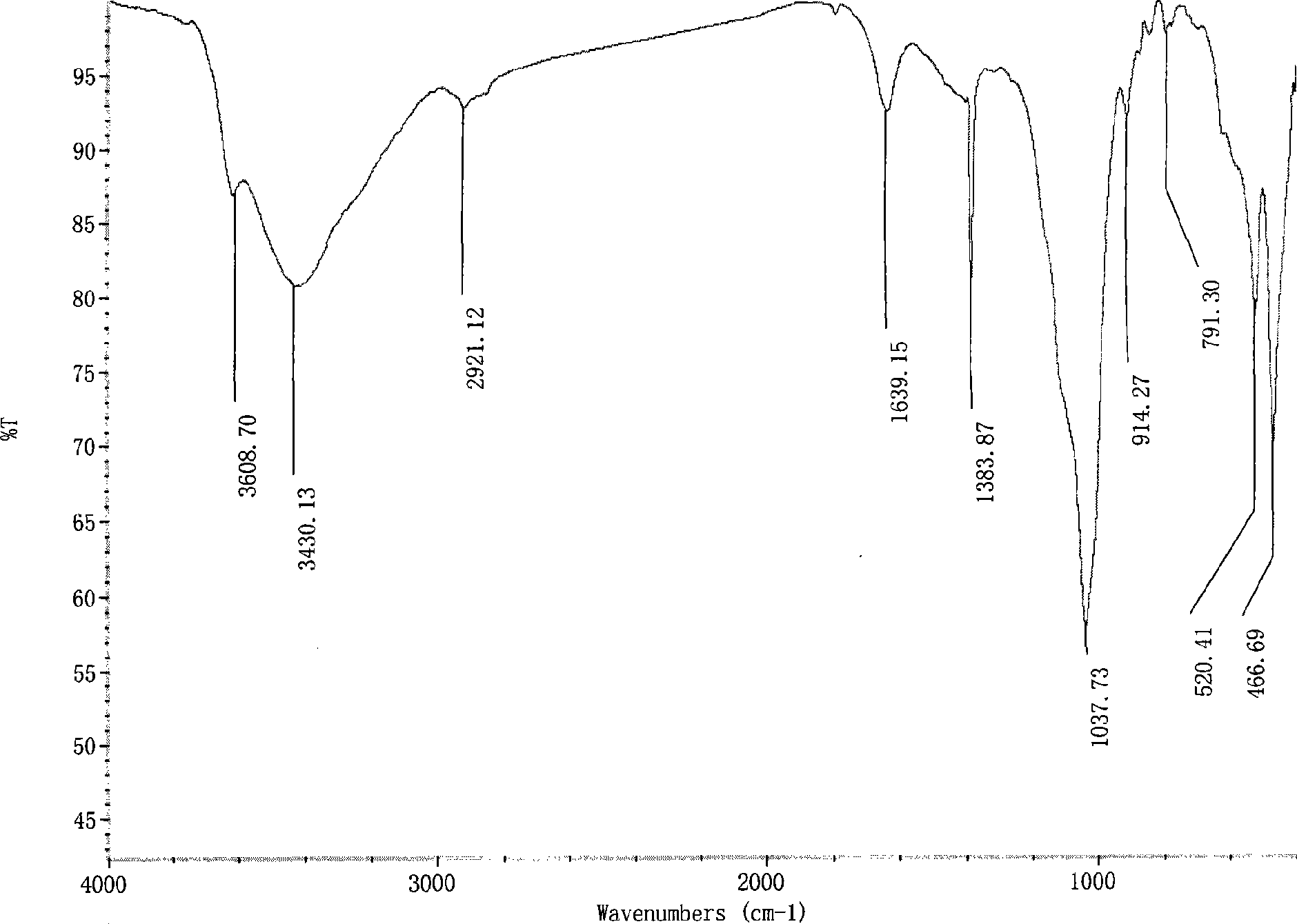
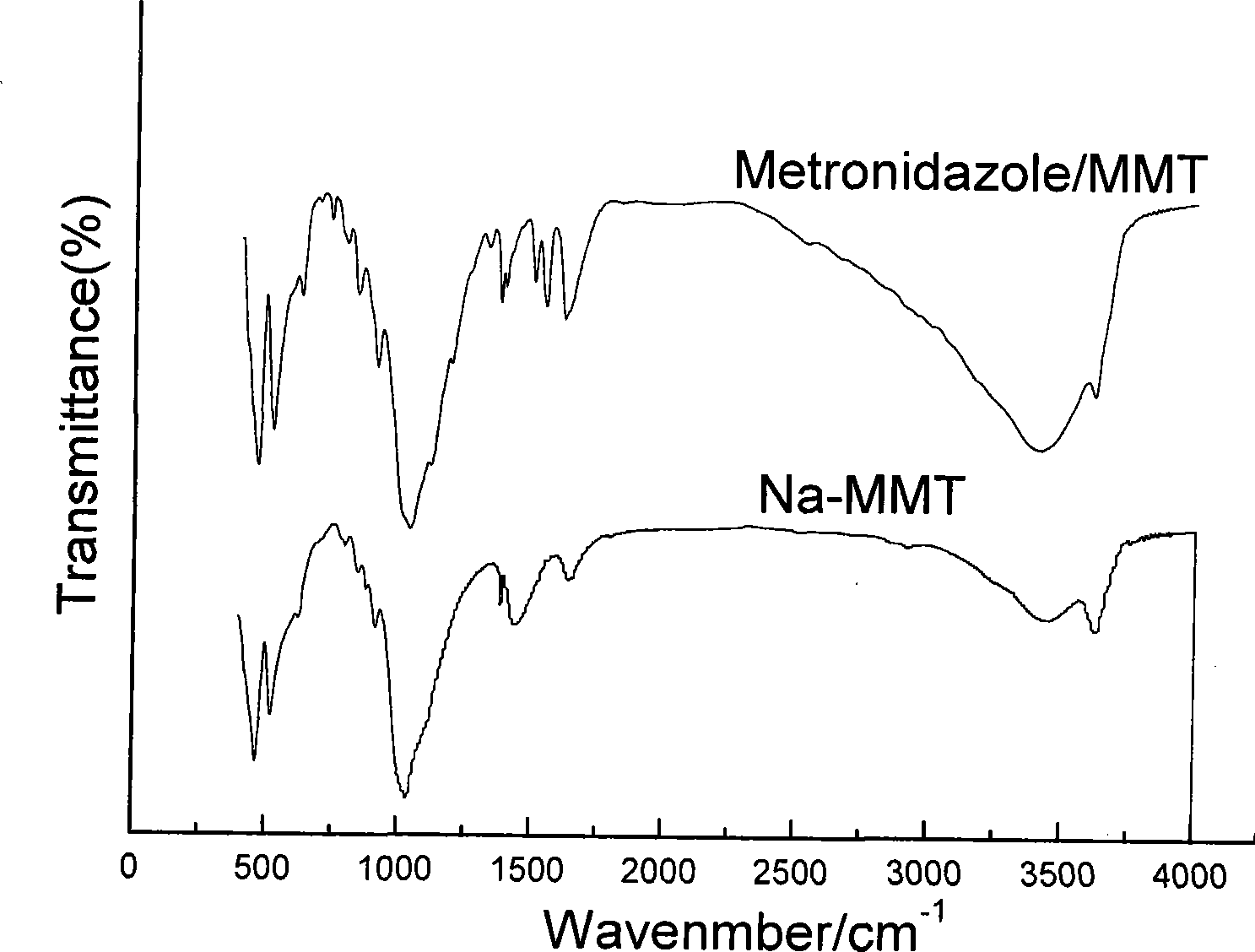
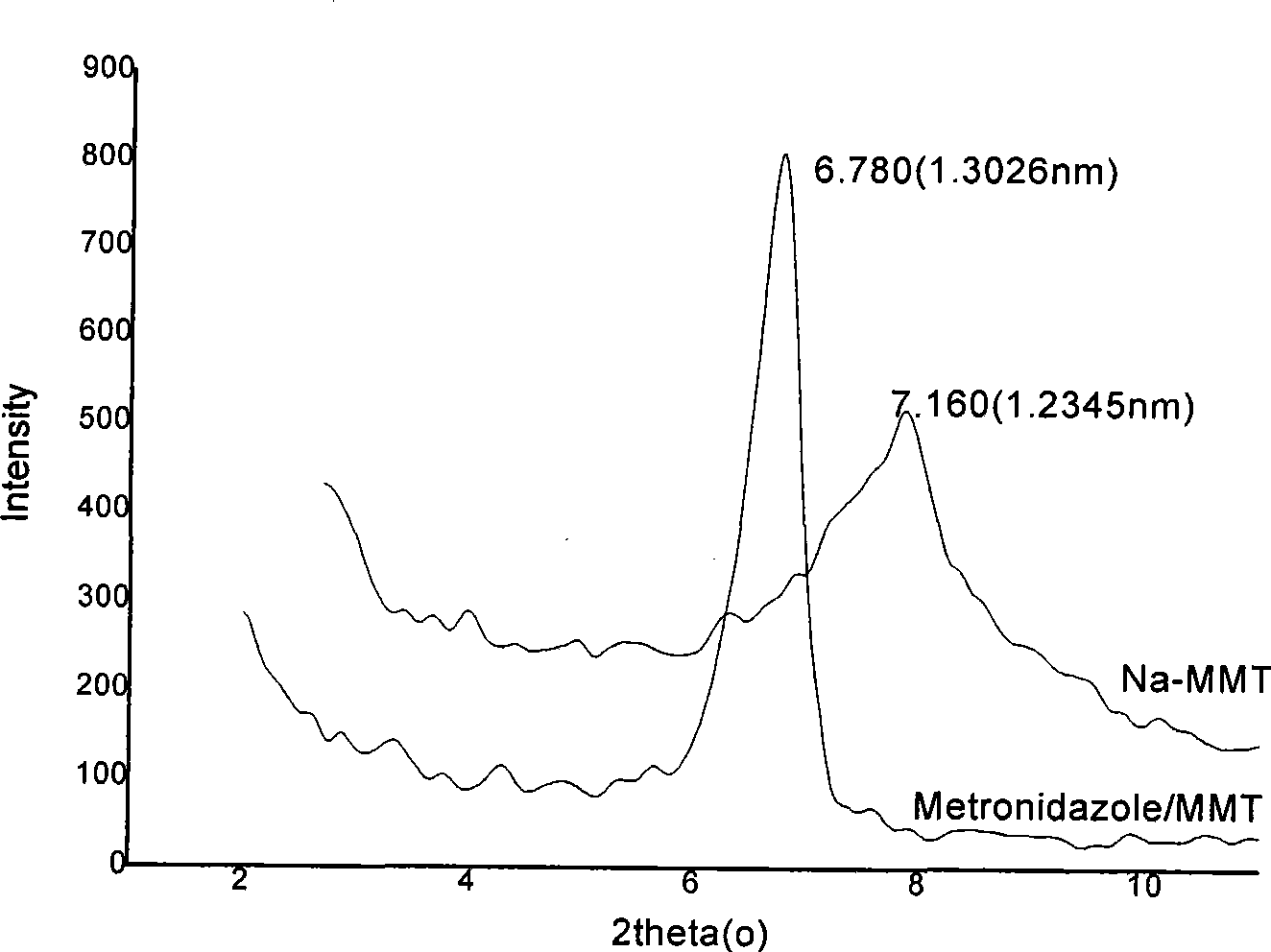
Nitroimidazoles medicine nano montmorillonite sustained-release agent and preparation method thereof
InactiveCN101422426AReduce dosageReduce typesAntibacterial agentsOrganic active ingredientsProtonationNitroimidazole
The invention provides a nanometer montmorillonite sustained release agent of an azomycin medicament The sustained release agent is prepared by inserting 10 to 45 weight portions of the azomycin medicament into the interlayer of 100 weight portions of Na-montmorillonite in a protonizing way under the condition of the pH value of 1.0 to 5.0 by adopting a liquor intercalation method; the azomycin medicament is metronidazole or tinidazole; the Na-montmorillonite is obtained by using Na<+> to replace Ca<2+> and Mg<+2> in the material of montmorillonite. The sustained release agent takes the montmorillonite as the vector of the azomycin medicament, which not only has the effects of releasing the medicament and improving the targeting, but also combines the characteristic that the montmorillonite can absorb pathogens and toxins, and can greatly improve the concentration of the partial medicament in a body, thereby reducing the medication times and species of the medicament.
Owner:SOUTHERN MEDICAL UNIVERSITY
Metronidazole preparation method
The invention relates to the field of medicine manufacturing techniques, in particular to a metronidazole API preparation method, and solves the problems that a current metronidazole preparation method is high in cost and low in yield. The method comprises the following steps: (1), acid mixing, adding 100 parts (by weight) of formic acid of concentration of 95-99 percent in a reaction kettle for stirring to a temperature of 20 DEG C, then adding 25-35 parts (by weight) of concentrated sulphuric acid, so as to prepare the mixed acid for further use; (2), synthesis reaction, adding 100-120 parts (by weight) of reaction raw materials 2-methyl-5-nitroimidazole into a reaction tank, and adding the mixed acid prepared in the step 1, stirring to a temperature of 75-80 DEG C, completely dissolving the 2-methyl-5-nitroimidazole, and maintaining 10-20 minutes. According to the invention, the design is reasonable; and the mixed acid is adopted to provide an acidic condition, an applicable reaction raw material ratio and an appropriate control reaction parameter, so as to allow the yield of the nitroimidazole to reach 65-70 percent.
Owner:SHANXI TONGJI PHARMA
F-triazole ring-polyethylene glycol-metronidazole compound and preparation method thereof
InactiveCN101709060AImprove metabolic propertiesImprove mechanical propertiesOrganic chemistryRadioactive preparation carriersNitroimidazolePolyethylene glycol
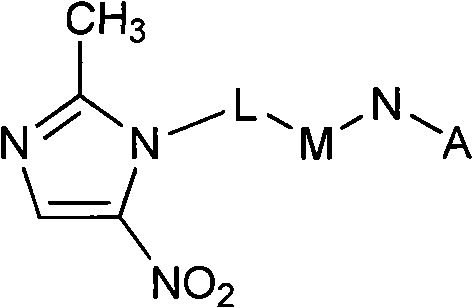
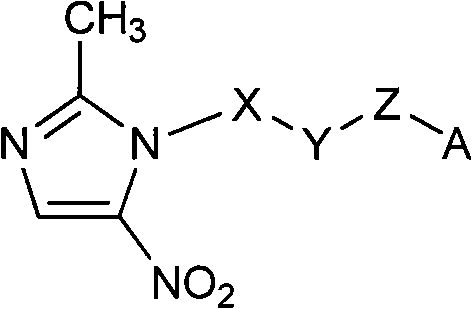
The invention relates to an F-1,2,3-triazole ring-polyethylene glycol-metronidazole compound which has the following structural general formula: L is ethyl or polyethylene glycol with the following structure: n is equal to 0, 1, 2 or 3; M is a triazole ring with the following structure: or, N is ethyl or polyethylene glycol with the following structure; n is equal to 0, 1, 2 or 3; and A is 19F or18F. Under a changeless condition that the invention keeps the metronidazole as a targeting base group, the polyethylene glycol is introduced to modify and change the molecular structure of a medicine, and the aims of improving medicine dynamics and pharmacodynamical properties and increasing the curative effect of the medicine are achieved. The triazole ring, a PEG base group, 2- metronidazole and 18 / 19F nuclide are connected by a click method, and a series of novel F-triazole ring-PEG-metronidazole derivatives are designed and synthesized. The nitro imidazole derivatives can quicken removing from normal tissues by lowering fat solubility so as to enhance a target / non-target ratio and is used for the development research of tumours, cardiac muscles or brain anoxia.
Owner:BEIJING NORMAL UNIVERSITY
Synthesis process of 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolyl-2-imine
The synthesis process of 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolyl-2-imine relates to farm pesticide imidacloprid synthesizing process. In dimethylforamide as solvent and with quaternary ammonium salt as catalyst, potassium carbonate, 2-chloro-5-chloro methyl pyridine and excessive imidazolyl alkane are condensed to produce 1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolyl -2-imine. The present invention has short reaction period, high product purity and high product yield.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
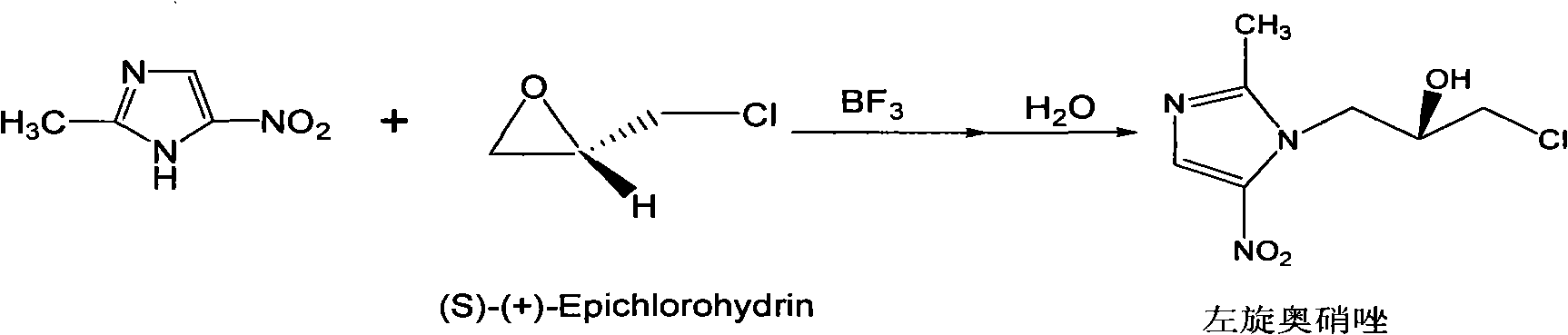
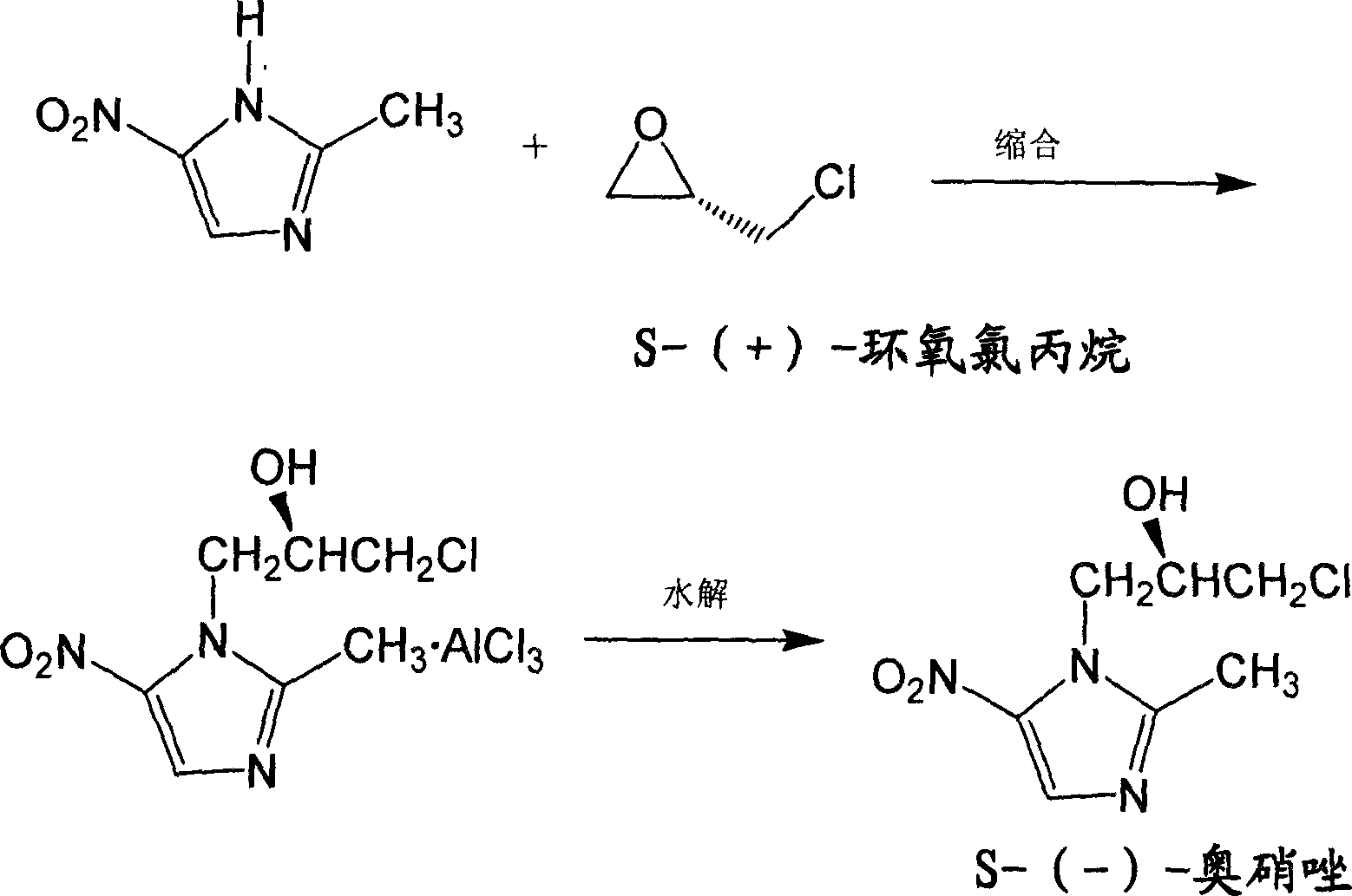
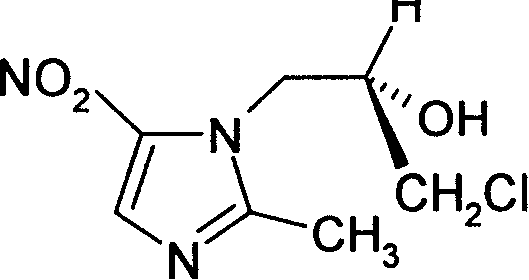
Method for preparing (S)-ornidazole
The invention provides a method for preparing (S)-ornidazole. The method comprises the following steps of: adding an organic solvent and 2-methyl-5-ornidazole, slowly adding a catalyst dropwise with stirring first, then slowly dripping S-(+)-epichlorohydrin into the mixed solution, and after the S-(+)-epichlorohydrin is dripped completely, performing a reaction for 2 to 10 hours at the temperature of between 0 and 20 DEG C; slowly adding the reaction solution into the ice-water mixture, stirring the mixed solution for a reaction for 10 minutes and 5 hours at the temperature of no less than 30 DEG C, adjusting a pH value of the mixed solution to 1.0 to 2.0 with acid, removing an organic layer, adjusting the pH value of a water layer to 6.0 to 8.0 with alkali, stirring the mixed solution for 1 to 24 hours for crystallization, filtering the reaction solution, washing the product obtained by water, and drying the product to obtain the (S)-ornidazole. The method has the advantages of high product purity, simplified procedures and the suitability for industrial production.
Owner:HC SYNTHETIC PHARMA CO LTD
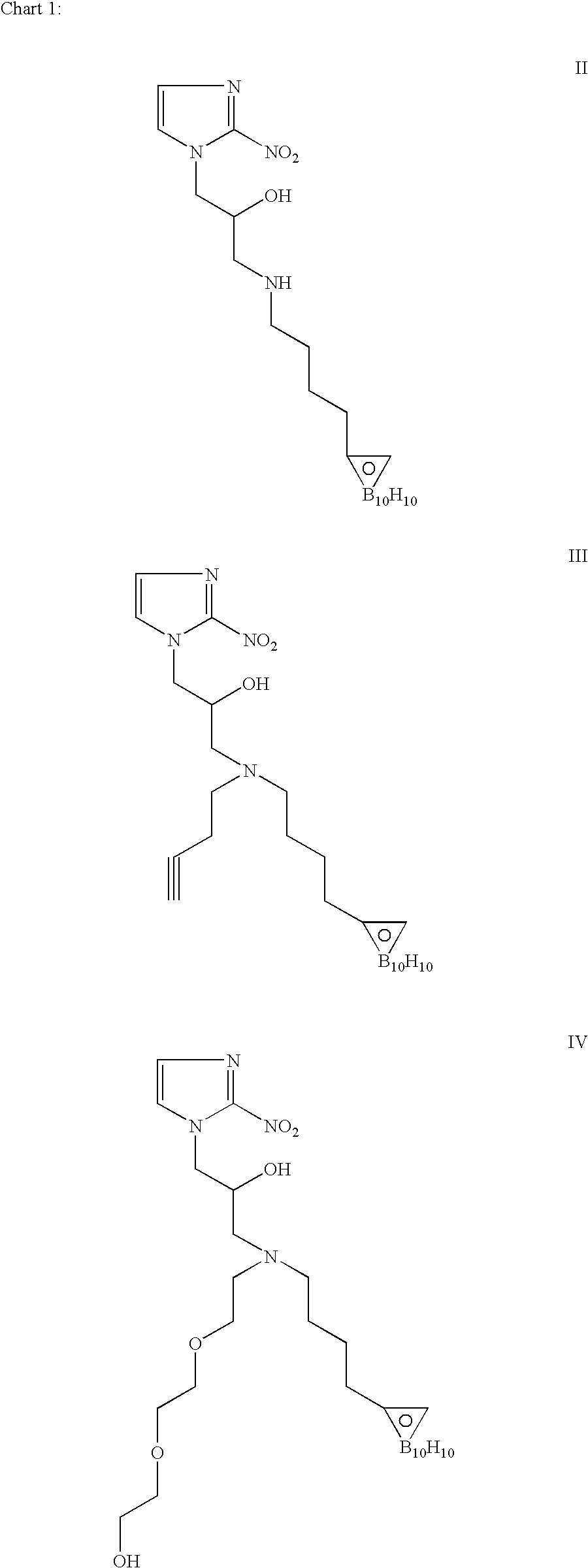
Hypoxia-selective, weakly basic 2-nitroimidazole delivery agents and methods of use thereof
InactiveUS20080102026A1Reduce deliveryImprove solubilityBiocideOrganic active ingredientsNitroimidazoleTissue concentrations
The invention features a class of 2-nitroimidazole compounds with a secondary basic nitrogen atom and a linker bearing one or more therapeutic agents, cytotoxic agents, detectable labels, or chelating groups. In particular, the invention provides 2-nitroimidazole compounds containing a cluster of boron atoms for use in boron neutron capture therapy (BNCT). The 2-nitroimidazole compounds can be used to treat hypoxic conditions, including, e.g., cancer, inflammation, and ischemia. The weakly basic 2-nitroimidazole compounds target to hypoxic tissue and provide increased tissue concentration overall.
Owner:NATURAL PHARMACIA INT
Caobonyl technetium labeled 2-azomycin composition, preparation method and application
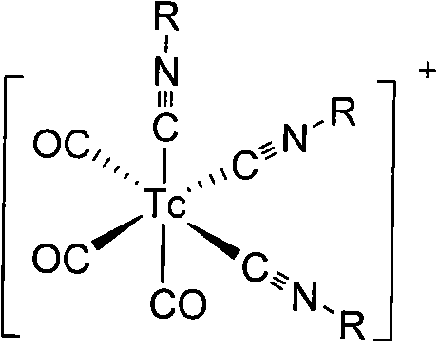
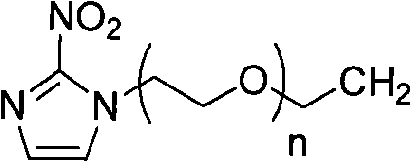
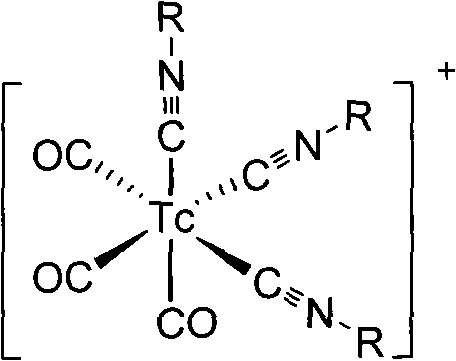
InactiveCN101654465AHigh affinityIncrease intakeRadioactive preparation carriersGroup 7/17 element organic compoundsNitroimidazoleTechnetium
The invention discloses a radioactive nuclide labeled compound which is caobonyl technetium labeled 2-azomycin composition, a preparation method of the compound and a purpose of the compound. The preparation method of the caobonyl technetium labeled 2-azomycin composition comprises the following steps: firstly synthesizing a ligand NC-PEGn-NIM and heating the ligand and a newly prepared midbody <99m>Tc(CO)3(H2O)3<+>, the pH value of which is regulated to 9 to 10, to obtain the caobonyl technetium labeled 2-azomycin composition. The caobonyl technetium labeled 2-azomycin composition is concentrated in anoxybiotic tumor cells by the targeting function of azomycin and can be used for the SPECT raster display diagnosis of anoxybiotic tumors.
Owner:LANZHOU UNIVERSITY
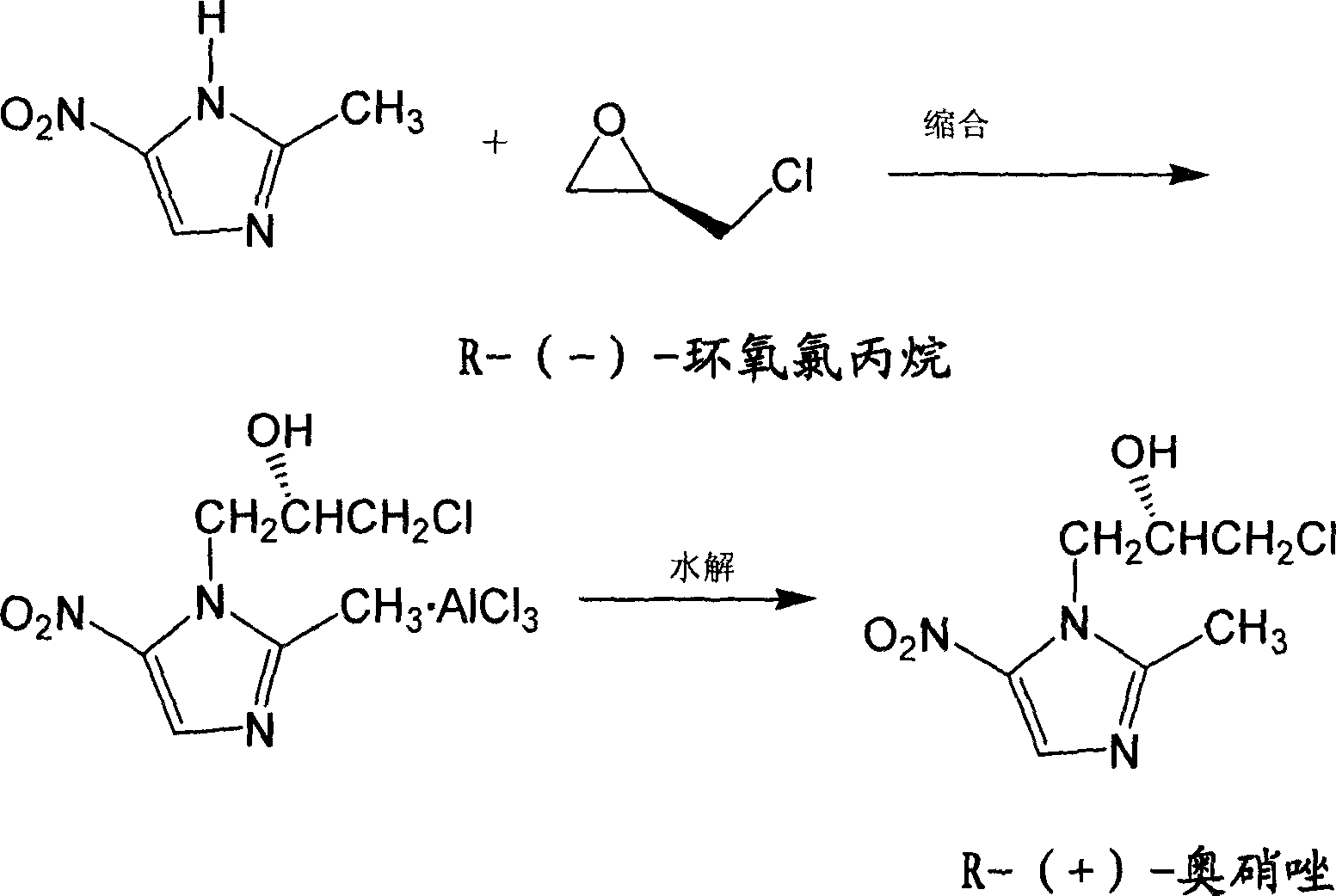
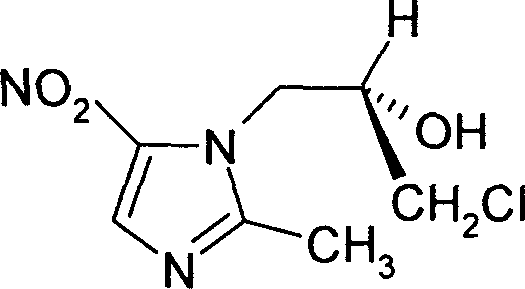
Ornidazole optical antimer preparation and purification method
The invention provides a method for preparing and purifying the optics enantiomer, which uses the 2-methyl -5-nitramisole to react with the chloropropylene oxide (S-(+)-chloropropylene oxide or R-(-) - chloropropylene oxide) to obtain the intermediate with optics activity then obtain the optics activity azoles by hydrolyzing, acidifying and inactivating then obtain the pure S-(-)-azoles or R-(+)-azoles by purifying.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
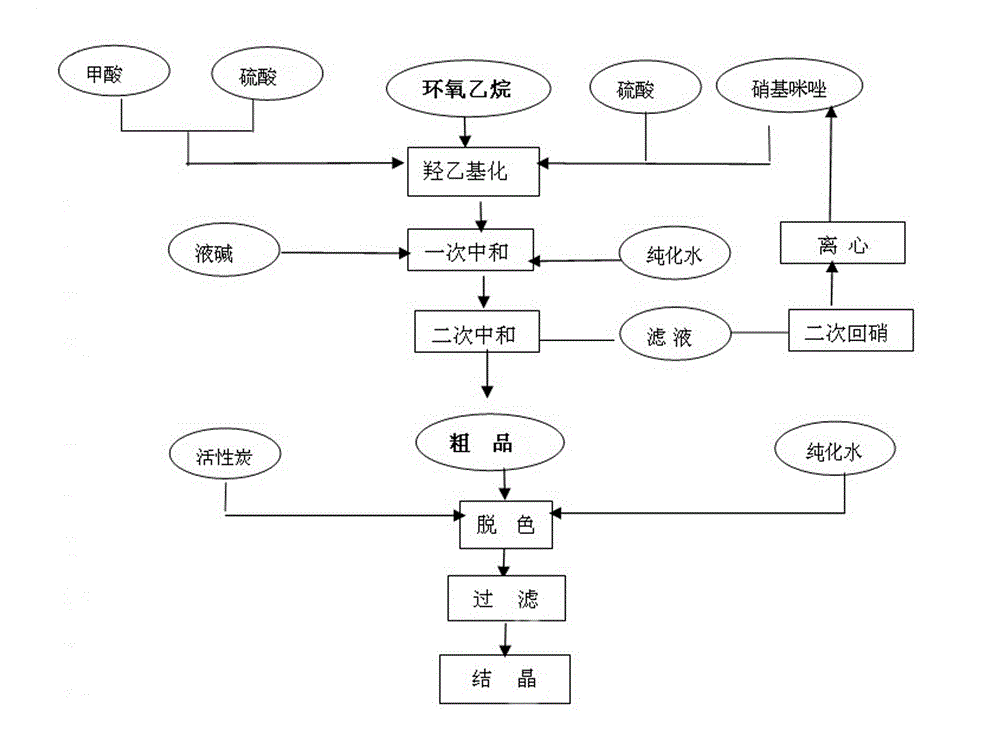
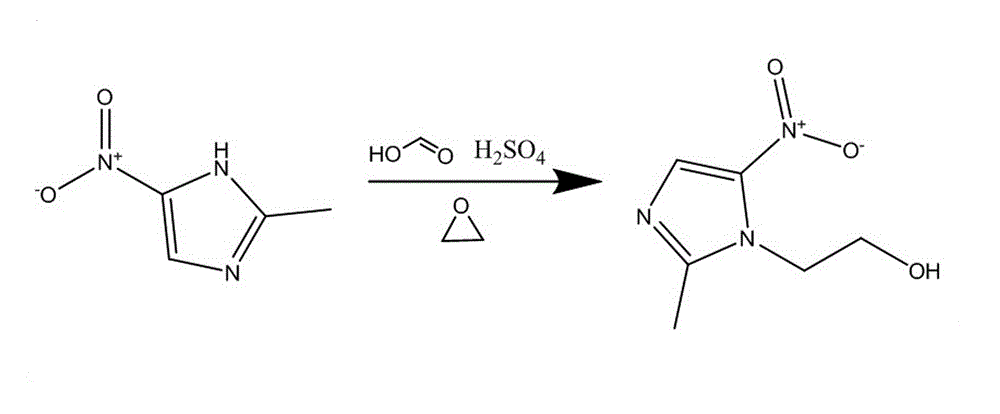
Method for synthesizing 2-methyl-5-nitroimidazole-1-ethanol
ActiveCN102321028AImprove solubilityImprove conversion rateOrganic chemistryNitroimidazoleEthylene oxide
The invention provides a method for synthesizing 2-methyl-5-nitroimidazole-1-ethanol, which comprises the following steps: the mixed solution of 2-methyl-5-nitroimidazole, formic acid and sulfuric acid is prepared according to the mole ratio of 1:0.5-0.7:1.2-1.7; ethylene oxide and concentrated sulfuric acid with the mole ratio of 1:0.08-0.3 are alternately added into the mixed solution for 3-4 times; the reaction temperature is 72-108 DEG C, the reaction time is 2.5-5 hours, and after reaction, sodium hydroxide is added to regulate the PH value of the solution to 9.5-10.5, and a finished product is precipitated through crystallization; and a 2-methyl-5-nitroimidazole-1-ethanol finished product is obtained through purification. In the method for synthesizing 2-methyl-5-nitroimidazole-1-ethanol provided by the invention, the mixed solution of formic acid and sulfuric acid is used as a solvent, the conversion rate and the yield are obviously improved, formic acid and sodium hydroxide consumption is less, and the production cost is low.
Owner:HUBEI HONGYUAN PHARMA
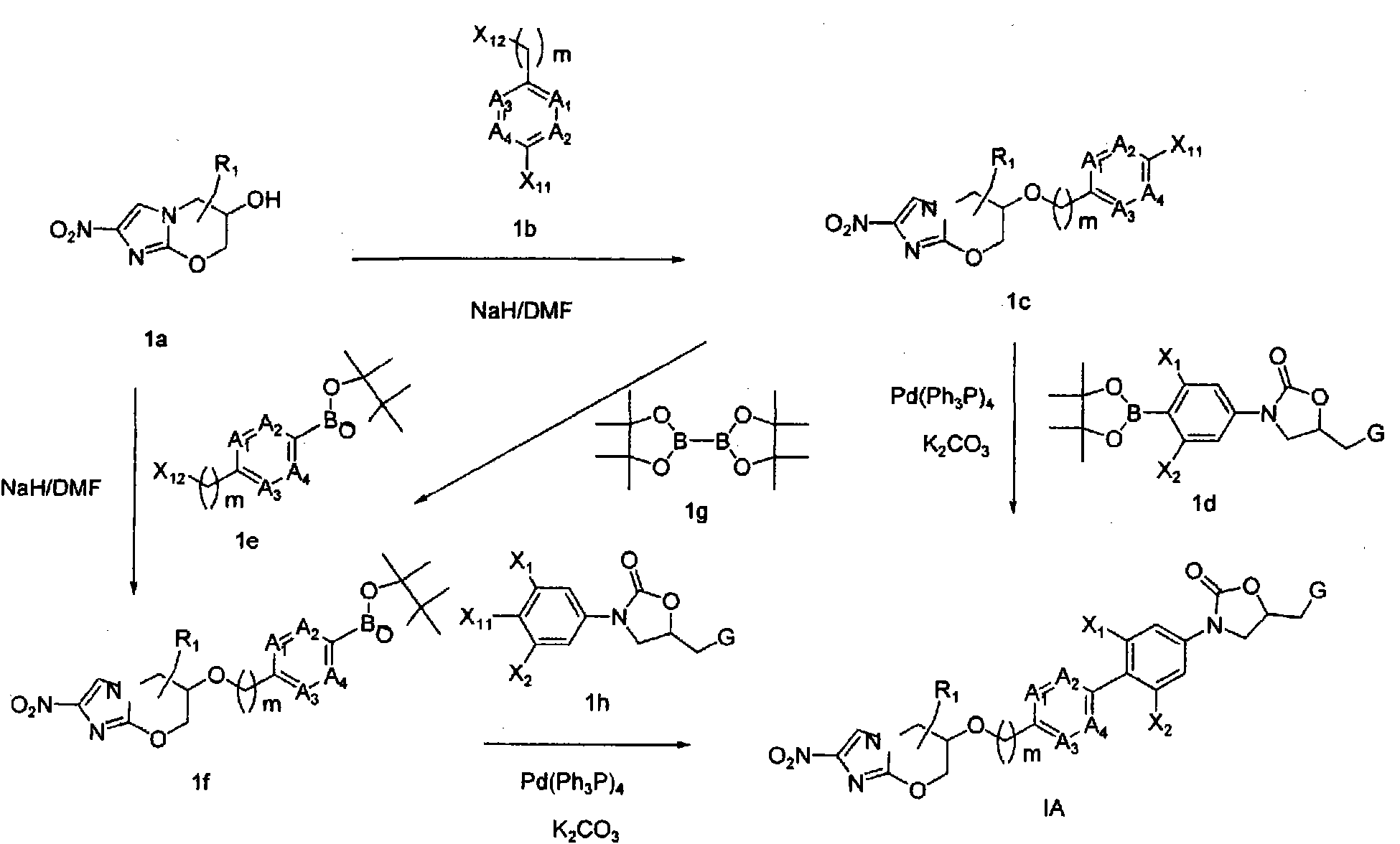
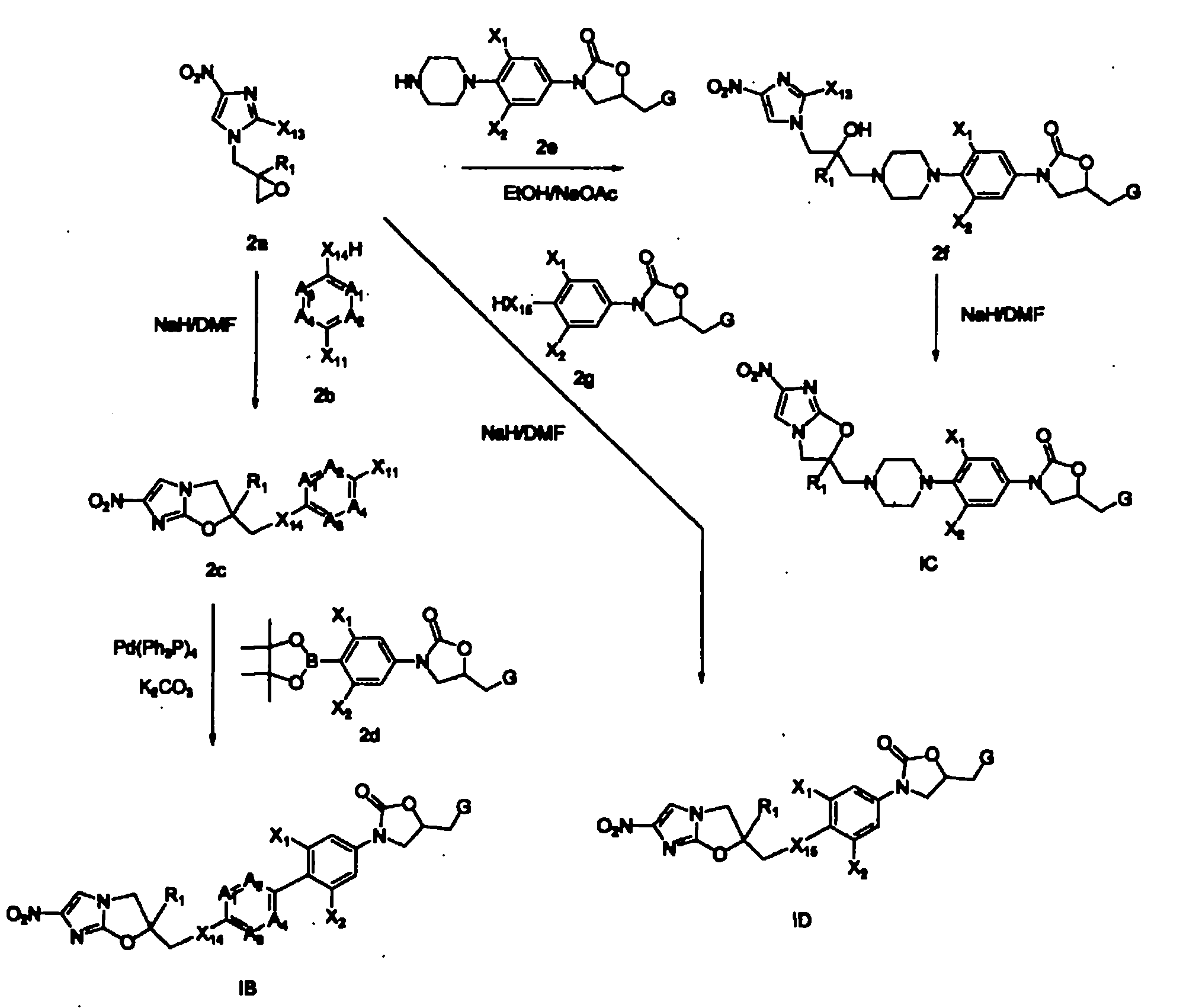
Bicyclic nitroimidazoles covalently linked to substituted phenyl oxazolidinones
The current invention provides a series of bicyclic nitroimidazole- substituted phenyl oxazolidinones in which a bicyclic nitroimidazole pharmacophore is covalently bonded to a phenyl oxazolidinone, their pharmaceutical compositions, and the method of use of the compositions for prevention and treatment of bacterial infections. The bicyclic nitroimidazole-substituted phenyl oxazolidinones possess surprising antibacterial activity against wild- type and resistant strains of pathogens, and are therefore useful for the prevention, control and treatment of a number of human and veterinary bacterial infections caused by these pathogens, such as Mycobacterium tuberculosis.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
L-ornidazole injection prepn
InactiveCN1739505AStrong pharmacodynamic activityGood curative effectAntibacterial agentsOrganic active ingredientsNitroimidazoleFreeze-drying
The present invention discloses L-ornidazole injection preparation as one kind of nitroimidazole antibiotics. The L-ornidazole injection preparation contains L-ornidazole or its salt or their hydrate. L-ornidazole is named chemically as S-(+)-1-(3-chloro-2-hydroxypropyl)-2-methyl-5- nitroimidazole. The L-ornidazole injection preparation is prepared with L-ornidazole, supplementary material and injection water, and may be prepared into injection for immediately injection, concentrated solution for injection after clinical re-compounding or powder for injection through further freeze drying. Compared with similar preparations, the L-ornidazole preparation has high pharmacodynamic activity, lower toxicity and stable quality.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Pharmaceutical compositions primarily for the treatment and prevention of genitourinary infections and their extragenital complications
InactiveUS20050208152A1Decreasing necessary dosageDecrease of possible side effectBiocideInorganic boron active ingredientsNitroimidazoleFolic acid antagonist
Compositions having synergistic effective amounts of one or more antibacterial agents, a nitroimidazole, and / or a sulfonamide or a molecule or compound having folic acid antagonist effect and nitroimidazole substitution in addition, with or without an antifungal agent effective against a Candida species. The compositions are particularly useful in the treatment of genitourinary infections and their extragenital complications. Vaccination against pathogen microbes of the vagina provides a stronger and longer immune response, then the infection.
Owner:MILANKOVITS MARTON
Method for treatment of reactive arthritis or bursitis
A method for treatment for conditions in human beings associated with either or both reactive arthritis or bursitis comprising administering a combination of a member of the family of synthetic purine nucleoside analog antiviral drugs, a member of the tetracycline family, and a member of the nitroimidazole family, or alternatively, administering a combination of a member of the family of synthetic purine nucleoside analog antiviral drugs, a member of the beta-lactam family, and a member of the nitroimidazole family, or alternatively, administering a combination of a member of the family of synthetic purine nucleoside analog antiviral drugs, a member of the macrolide antimicrobial family, and a member of the nitroimidazole family, or alternatively, administering a combination of a member of the family of synthetic purine nucleoside analog antiviral drugs, a member of the ketolide antimicrobial family, and a member of the nitroimidazole family.
Owner:BONNER ERNEST L JR +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com