Nitroimidazoles medicine nano montmorillonite sustained-release agent and preparation method thereof
A nano-montmorillonite and nitroimidazole technology, which is applied in the directions of drug delivery, pharmaceutical formulations, antibacterial drugs, etc., can solve the problems of difficulty in forming cations, improve bioavailability, and show alkalinity, so as to improve the concentration and reduce the Effects of dosage and type of drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Stir 20 g of raw material montmorillonite in 500 mL of saturated NaCl solution to form a stable suspension; stir the suspension at 35°C for 5 hours. Filter with suction, and then wash the filtrate 4 times with deionized water. Vacuum-dry at 70°C to constant weight, and ball mill until the particle size is 20-30 μm to obtain Na-montmorillonite. Store dry.
[0051] 2. Mix 10 g of Na-montmorillonite obtained above with 6 g of metronidazole, add 200 ml of deionized water, adjust the pH value to 5.0 with 3% hydrochloric acid solution, and stir the mixture at 60° C. for 2 hours. Suction filtration, then wash the filtrate several times with deionized water, detect the filtrate without metronidazole by ultraviolet spectrophotometer, dry it in vacuum at 60°C to constant weight, and ball mill until the particle size is 20-30 μm, to obtain metronidazole Nano montmorillonite. Store dry.
[0052] Collect all the filtrate in step 2, filter it with a 0.45um microporous membrane...
Embodiment 2
[0054] 1. Same as embodiment 1.
[0055] 2. Mix 10 g of Na-montmorillonite obtained above with 8 g of metronidazole, add 200 ml of deionized water, adjust the pH value to 4.0 with 3% hydrochloric acid solution, and stir the mixture at 70° C. for 3 hours. Suction filtration, and then wash the filtrate several times with deionized water, detect that there is no metronidazole in the filtrate by ultraviolet spectrophotometer, dry it in vacuum at 60°C to constant weight, and ball mill it until the particle size is 20-30 μm, then you can get metronidazole nano Montmorillonite. Store dry.
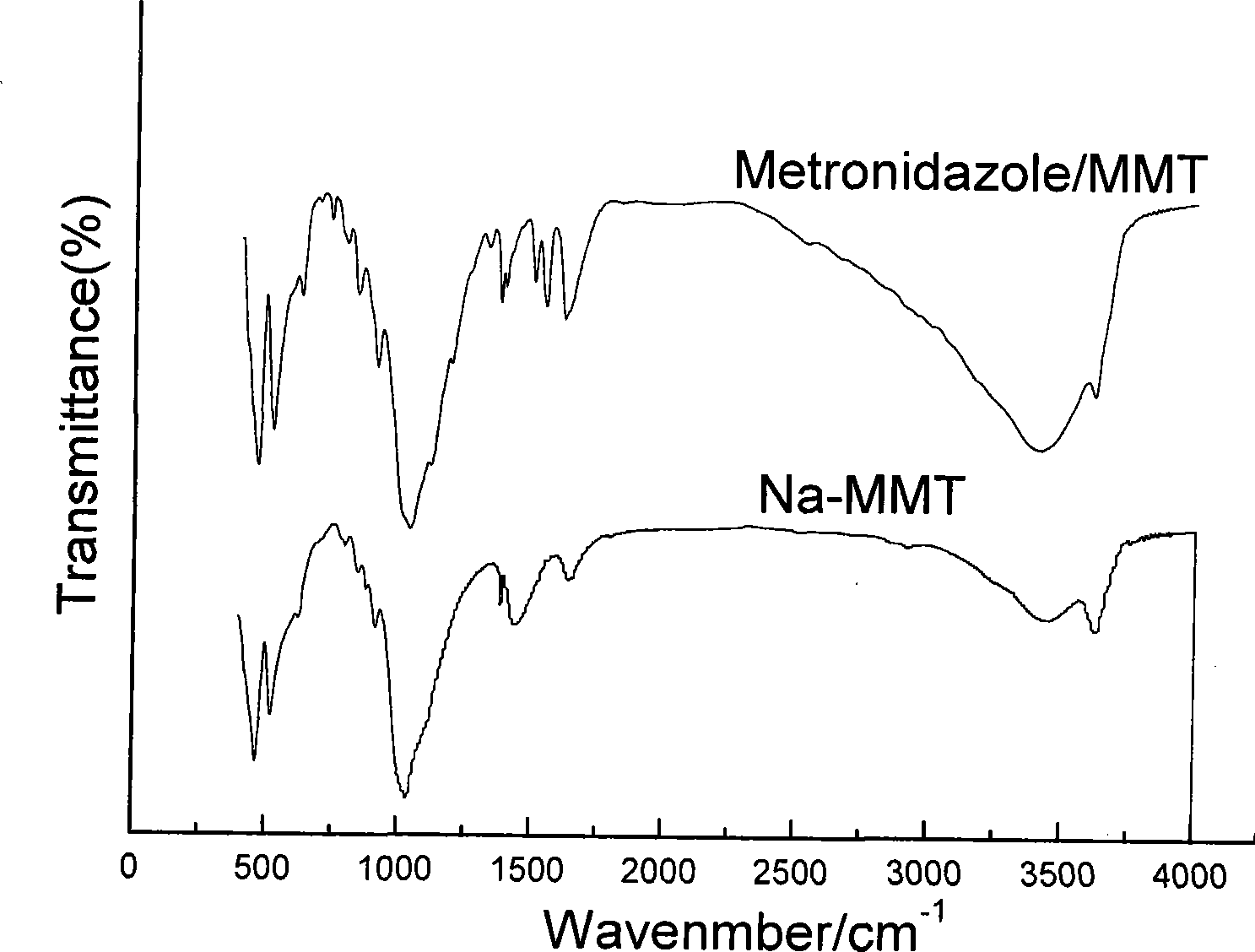
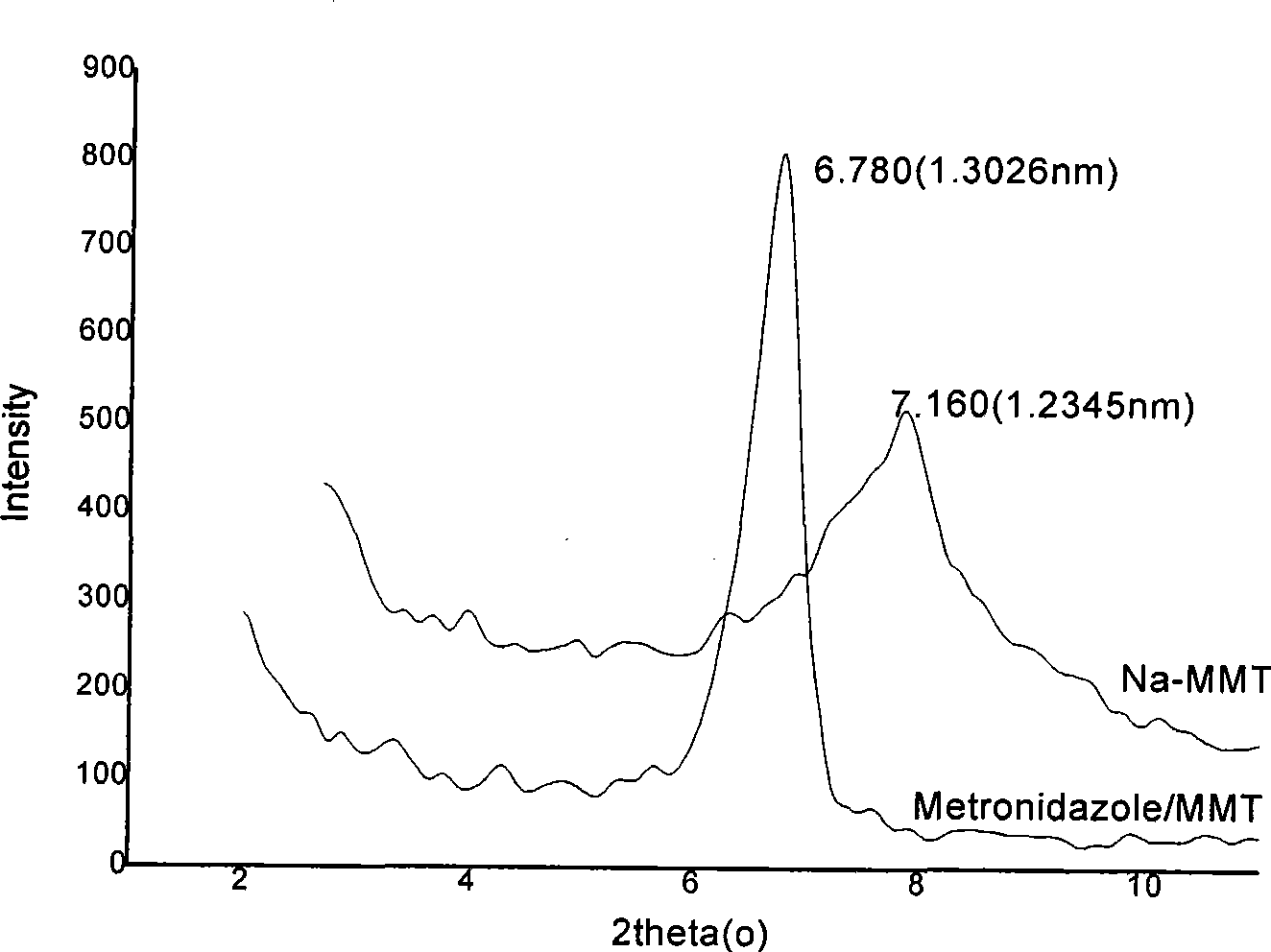
[0056] Collect all the filtrate in step 2, filter it with a 0.45um microporous membrane, take an appropriate amount of the filtrate and use a Biomat-5 ultraviolet spectrophotometer to measure the ultraviolet light absorbance value, and calculate the remaining drug 5.76g. That is, 2.24g of metronidazole was intercalated in 10g of montmorillonite; XRD test, IR test and UV spectrogravimetric analys...
Embodiment 3
[0058] 1. Same as embodiment 1.
[0059] 2. Mix 10 g of Na-montmorillonite obtained above with 8 g of metronidazole, add 200 ml of deionized water, adjust the pH to 3.0 with 3% hydrochloric acid solution, and stir the mixture at 80° C. for 4 hours. Suction filtration, and then wash the filtrate several times with deionized water, detect that there is no metronidazole in the filtrate by ultraviolet spectrophotometer, dry it in vacuum at 60°C to constant weight, and ball mill it until the particle size is 20-30 μm, then you can get metronidazole nano Montmorillonite. Store dry.
[0060] Collect all the filtrate in step 2, filter it with a 0.45um microporous membrane, take an appropriate amount of the filtrate and use a Biomat-5 ultraviolet spectrophotometer to measure the ultraviolet light absorbance value, and calculate the remaining drug 5.6g. That is, 2.4g of metronidazole is intercalated in 10g of montmorillonite; it is confirmed by XRD test, IR test and ultraviolet spectr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com