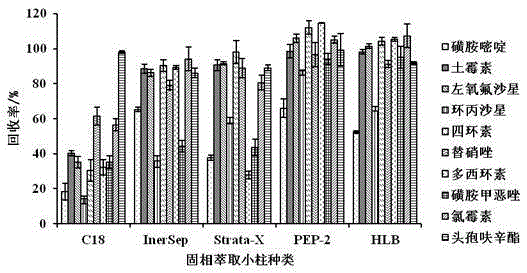
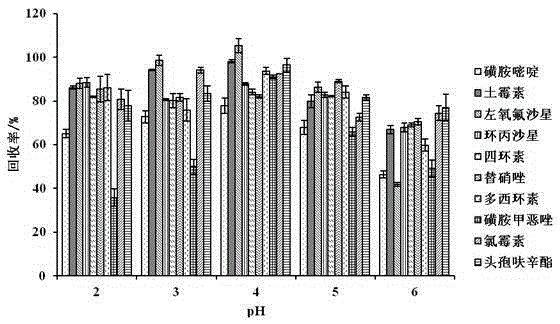
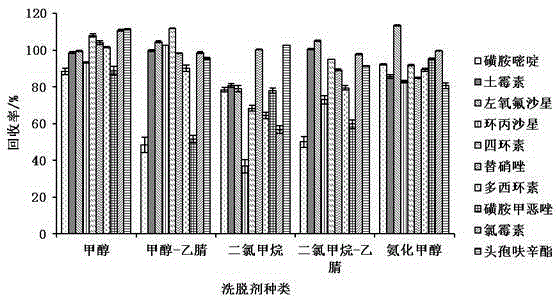
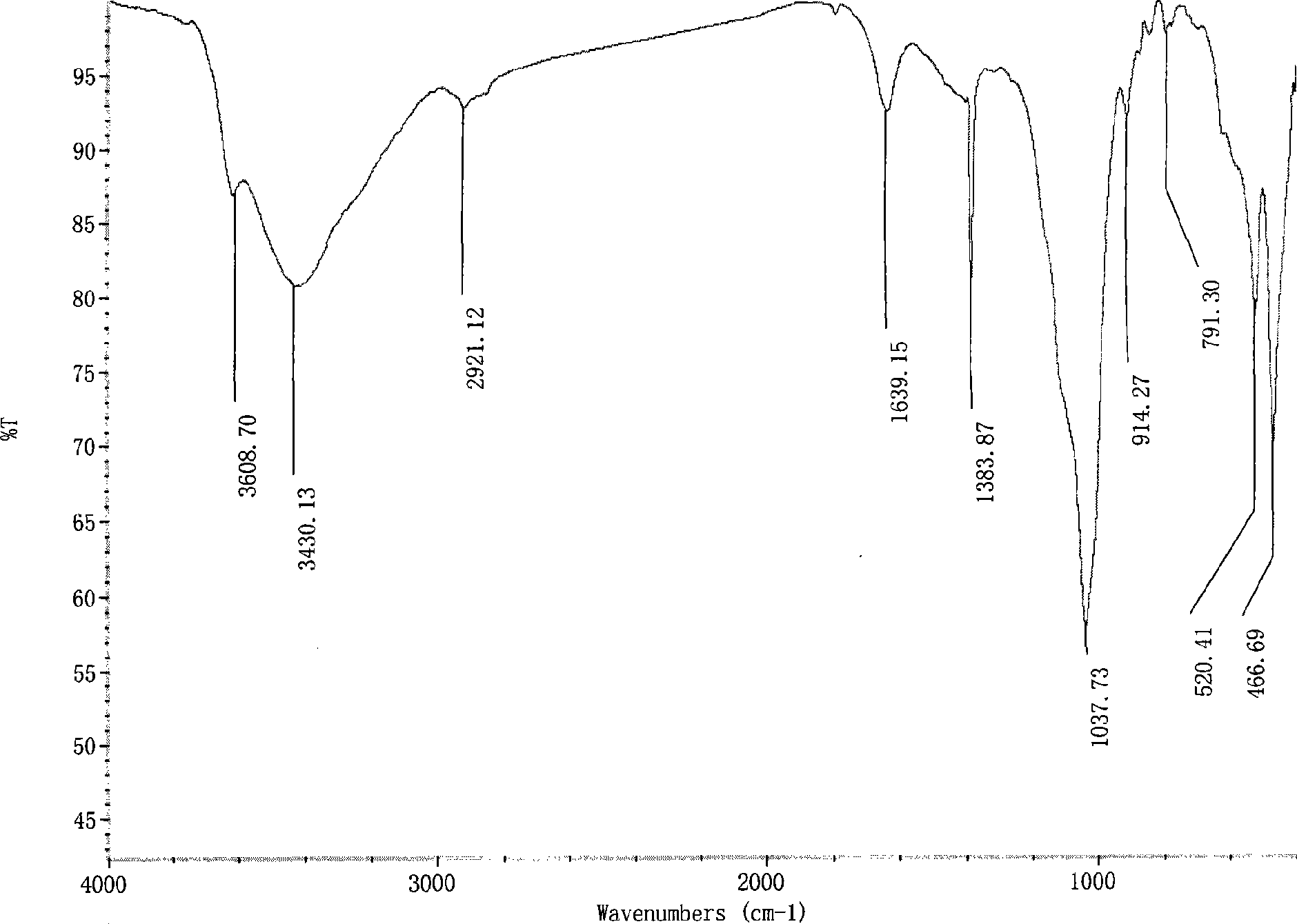
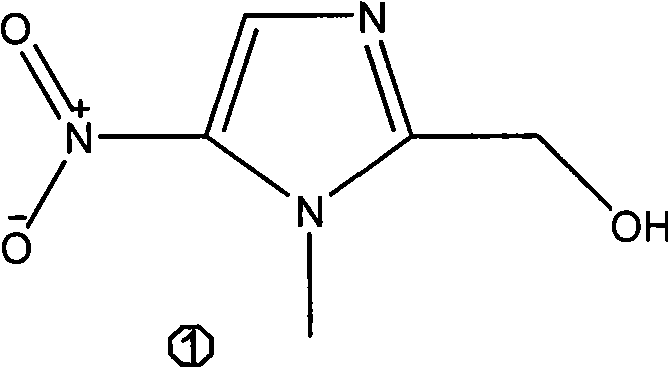
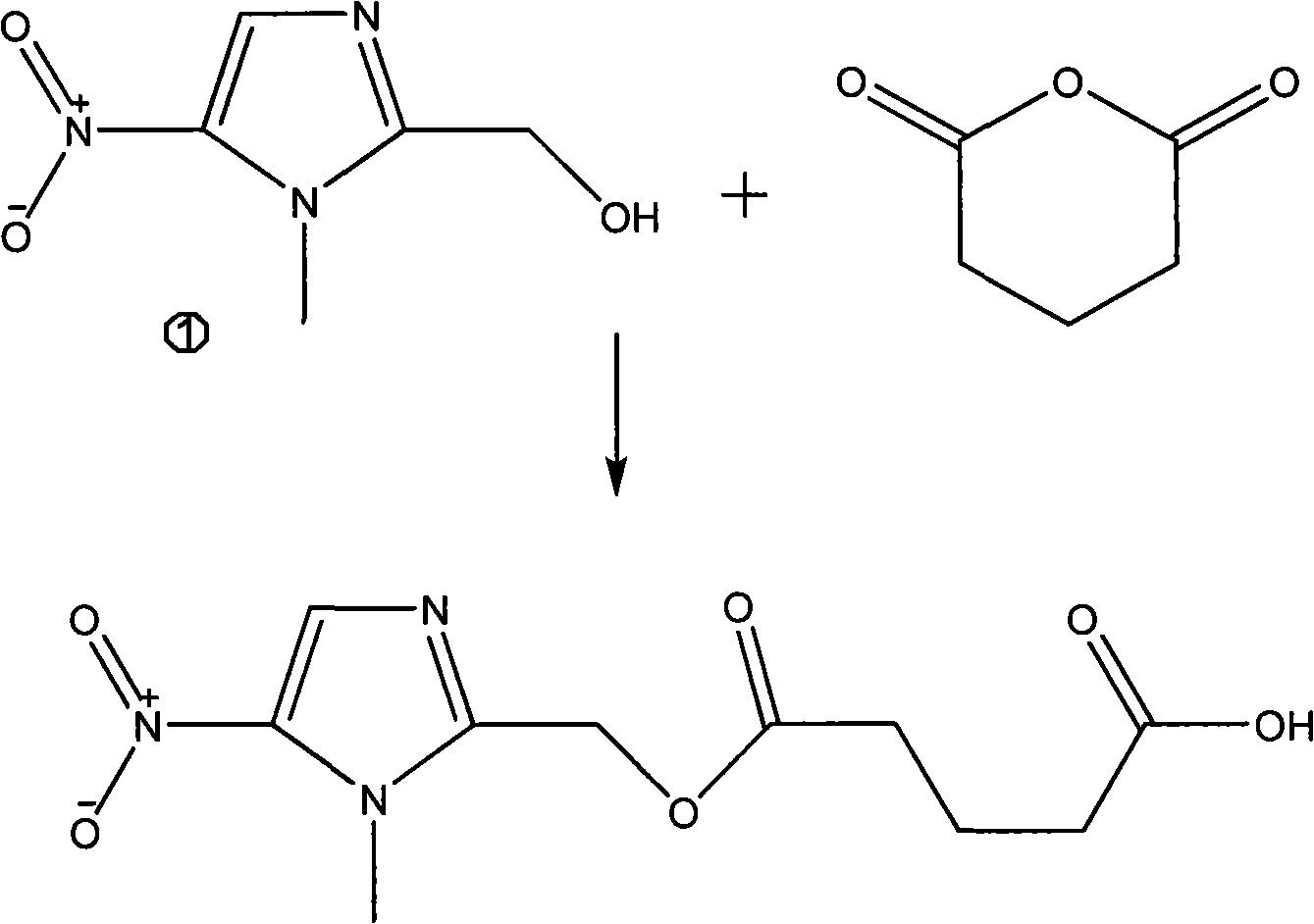
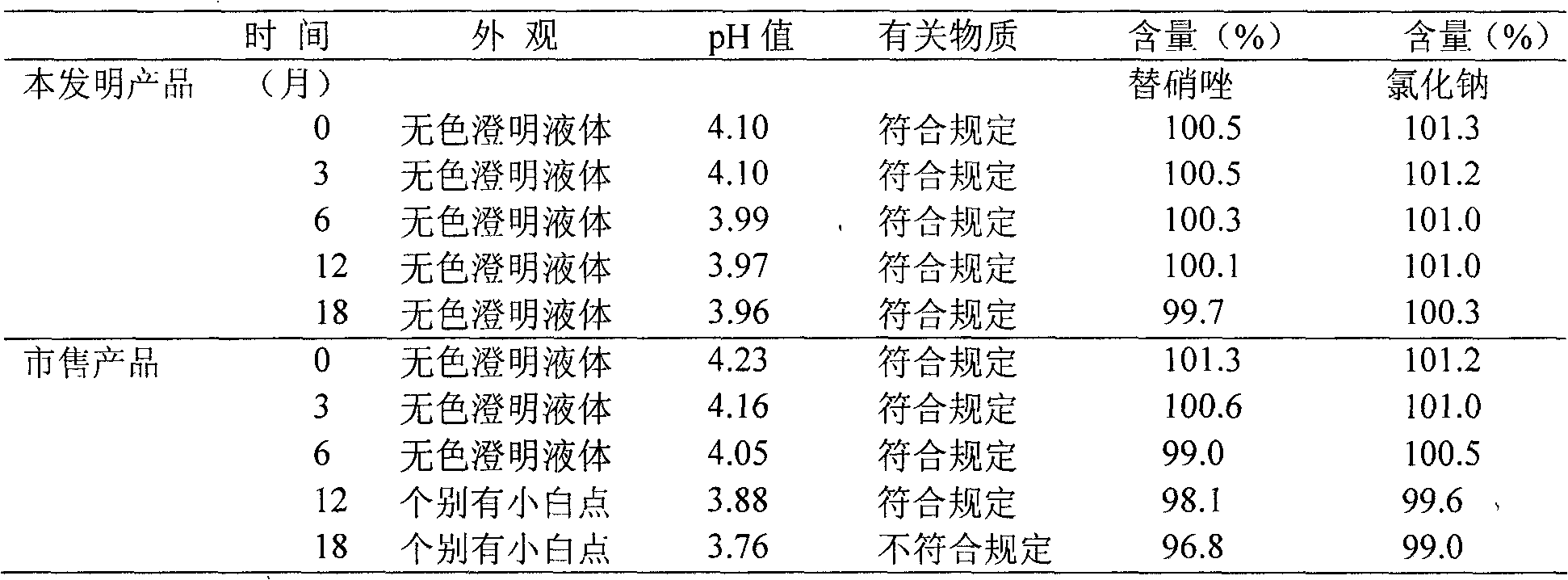
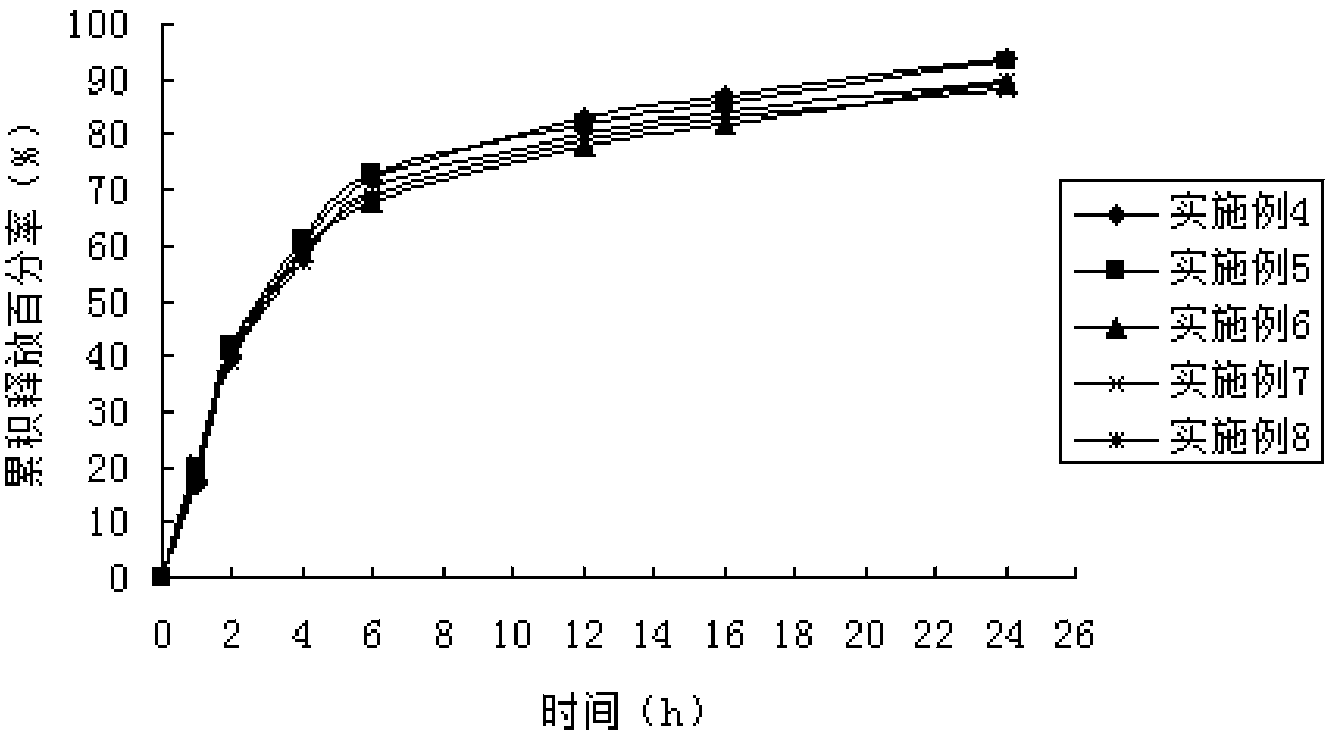
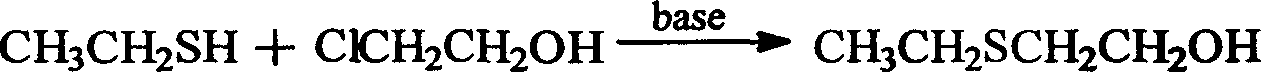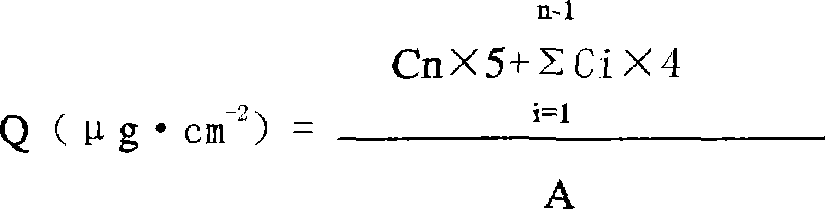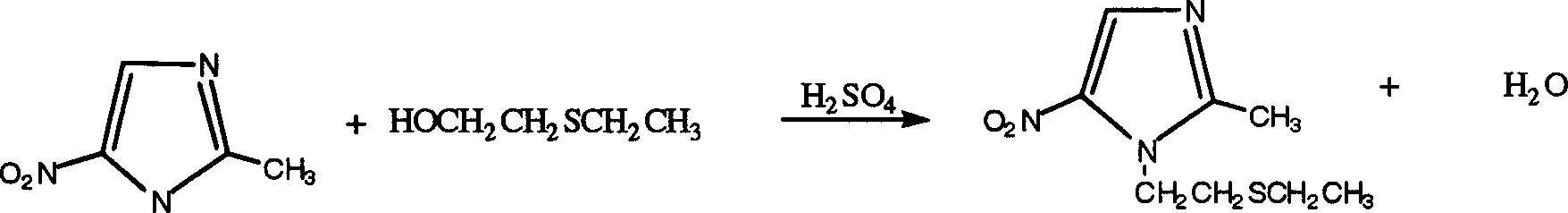Patents
Literature
133 results about "Tinidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tinidazole is an antibiotic that is used to treat certain types of vaginal infections (bacterial vaginosis, trichomoniasis). It is also used to treat certain types of parasite infections (giardiasis, amebiasis).
Association of fluconazole-tinidazole for the treatment of vaginal infections, its composition, preparation process and usage
The present invention refers to a treatment for mixed infectious diseases in the human reproductive system, wherein an association of compounds containing fluconazole and either tinidazole is used, the same being associated in doses lower to those commonly used therapeutically. The combination has proven to be highly efficacious and shown a good degree of tolerance.
Owner:ALPARIS S A DE
Method for determining 10 kinds of antibiotics in water environment through combination of sample pre-treatment technology and HPLC-MS
The present invention relates to a method for determining 10 kinds of antibiotics in a water environment through combination of a sample pre-treatment technology and HPLC-MS, and belongs to the field of detection of safety of trace organic contaminant residue in the water environment. The method is characterized in that a water sample is separated and enriched through combination of solid phase extraction and dispersive liquid-liquid microextraction (SPE-DLLME), and then an ultra-high performance liquid chromatography-mass spectrometry instrument (UPLC-MS / MS) is adopted as a detection tool to directly determine the contents of 10 kinds of common antibiotics in the water environment (drinking water, tap water, river water, sewage treatment plant influent and effluent), wherein the 10 kinds of the common antibiotics respectively are sulfadiazine, sulfamethoxazole, oxytetracycline, tetracycline, doxycycline, ciprofloxacin, levofloxacin, chloramphenicol, cefuroxime axetil and tinidazole. According to the present invention, the water sample pre-treatment method and the instrument detection conditions are investigated and optimized, and the optimal SPE-DLLME-UPLC-MS / MS method is established and is successfully applied for the real sample determination; and compared with the traditional method, the method of the present invention has advantages of high sensitivity, high extraction recovery rate, wide application objects, environmental protection, and the like.
Owner:SHENYANG PHARMA UNIVERSITY
Compsns-and methods for trapping and inactivating pathogenic microbes and spermatozoa
Antimicrobial and contraceptive compositions and methods which prevent and / or reduce the risk of transmission of sexually transmitted diseases through sexual activity as well as prevent and / or reduce the risk of pregnancy are provided. The compositions contain (1) a matrix-forming agent, (2) a bio-adhesive agent, (3) a buffering agent, (4) optionally a humectant, (5) optionally a preservative, and (6) water; wherein the composition is suitable for application within the vagina; wherein the compositions form a semisolid matrix on contact with ejaculate (thereby trapping ejaculated microbes and spermatozoa); wherein the composition causes hardening of cervical mucus (thereby decreasing the probability of sperm entry); wherein the composition forms a bio-adhesive layer over vaginal surfaces (thereby preventing or reducing the risk of contact of STD-causing microbes with the vaginal surfaces); wherein the composition maintains an acidic vaginal pH of less than about 5 in the presence of semen ejaculated from the male; and wherein the composition does not significantly impair the natural microbiological balance within the vagina. The antimicrobial and contraceptive compositions may also contain additional antimicrobial and / or contraceptive agents (e.g., nonoxynol-9, octoxynol-9, benzalkonium chloride, phosphorylated hesperidins, sulfonated hesperidins, polystyrene sulfonates, substituted benzenesulfonic acid formaldehyde co-polymers, H2SO4-modified mandelic acids, povidone iodine, itraconazole, ketoconazole, metronidazole, clotrimazole, fluconazole, teraconazole, miconazole, tinidazole, iconazole, chloramphenicol, nystatin, cyclopiroxolamine, and the like).
Owner:RUSH UNIV MEDICAL CENT
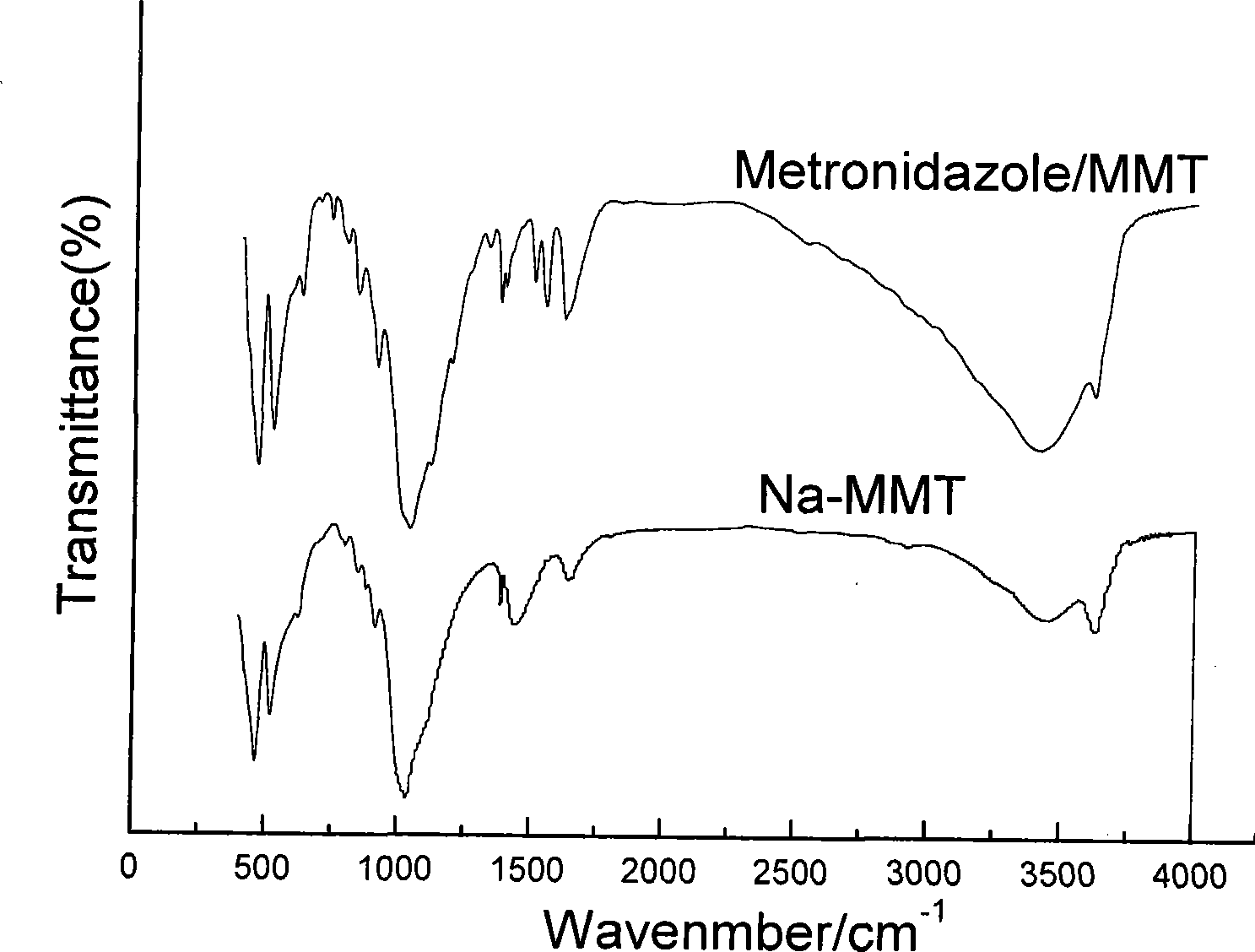
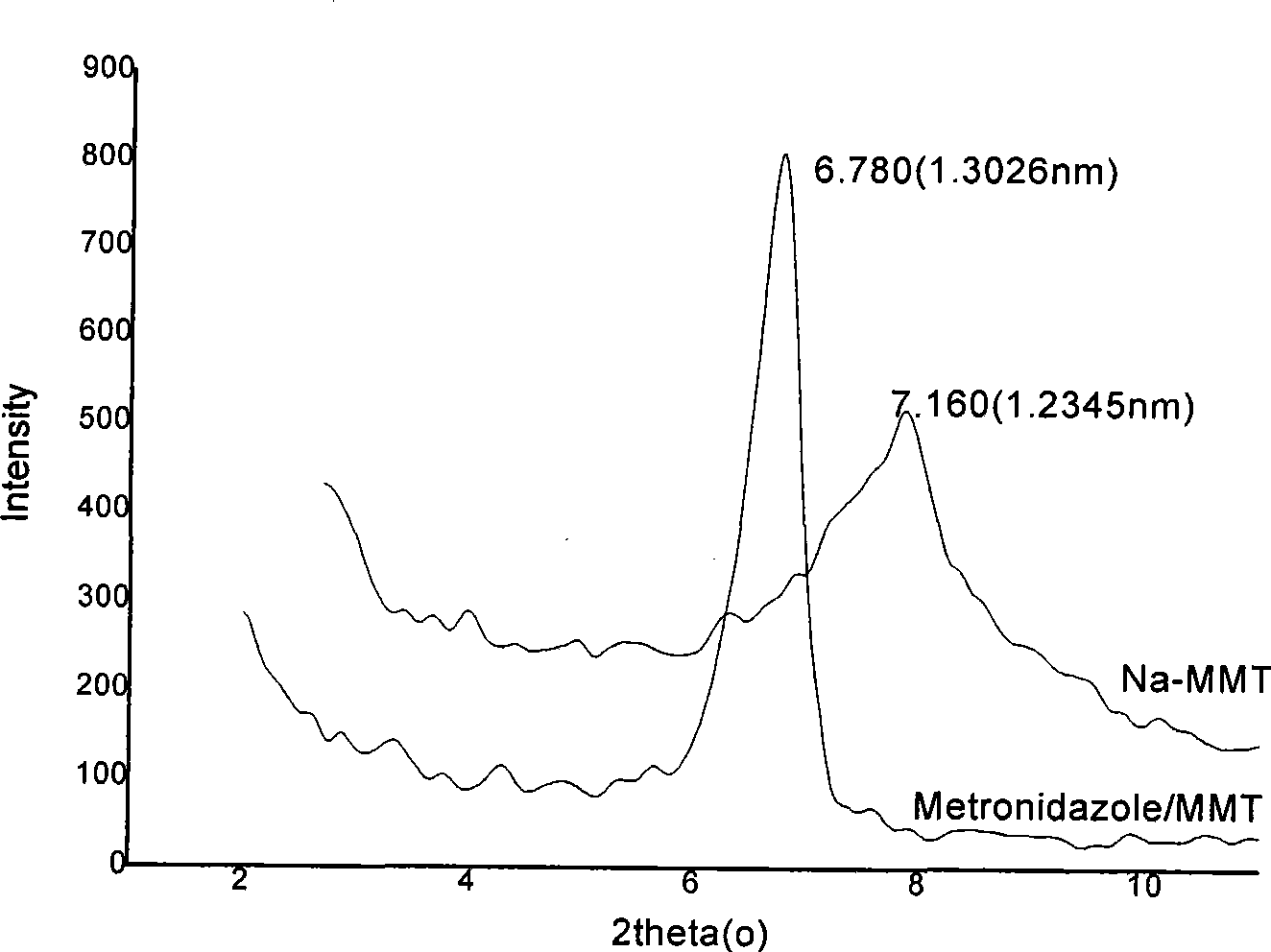
Nitroimidazoles medicine nano montmorillonite sustained-release agent and preparation method thereof
InactiveCN101422426AReduce dosageReduce typesAntibacterial agentsOrganic active ingredientsProtonationNitroimidazole
The invention provides a nanometer montmorillonite sustained release agent of an azomycin medicament The sustained release agent is prepared by inserting 10 to 45 weight portions of the azomycin medicament into the interlayer of 100 weight portions of Na-montmorillonite in a protonizing way under the condition of the pH value of 1.0 to 5.0 by adopting a liquor intercalation method; the azomycin medicament is metronidazole or tinidazole; the Na-montmorillonite is obtained by using Na<+> to replace Ca<2+> and Mg<+2> in the material of montmorillonite. The sustained release agent takes the montmorillonite as the vector of the azomycin medicament, which not only has the effects of releasing the medicament and improving the targeting, but also combines the characteristic that the montmorillonite can absorb pathogens and toxins, and can greatly improve the concentration of the partial medicament in a body, thereby reducing the medication times and species of the medicament.
Owner:SOUTHERN MEDICAL UNIVERSITY
Association of fluconazole-tinidazole for the treatment of vaginal infections, its composition, preparation process and usage
The present invention refers to a treatment for mixed infectious diseases in the human reproductive system, wherein an association of compounds containing fluconazole and either tinidazole or secnidazole is used, the same being associated in doses lower to those commonly used therapeutically. The combination has proven to be highly efficacious and shown a good degree of tolerance.
Owner:ALPARIS S A DE
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Method and special ELISA reagent kit for detecting nitryl imidazole medicament
InactiveCN101315378AStrong specificityEasy to handleFused cellsImmunoglobulinsNitroimidazoleSite monitoring
The invention discloses a method for detecting nitroimidazole drugs and a dedicated enzyme-linked immune kit. The kit comprises a special antibody, and a coating antigen and an enzyme label; nitroimidazole is at least one of the following components: metronidazole, tinidazole, imetridazole and ronidazol. The method in the invention has the advantages of simple sample pretreatment process, simple operation, low cost, good specificity, high sensitivity, high accuracy, etc.; the method can perform on-site monitoring and is suitable for screening a plurality of samples. Therefore, the method and the dedicated kit in the invention play an important part in detecting the residues of the nitroimidazole drugs in animal derived food.
Owner:BEIJING WANGER BIOTECH
Controlled release formulation of clarithromycin or tinidazol
The present invention relates to a controlled release pharmaceutical composition comprising amounts ranging from about 0.1 to about 4.5% w / w, of one or more of rate controlling cellulosic ether polymers.
Owner:RANBAXY LAB LTD
Tinidazole compound nano silver microemulsion antibacterial medicine
InactiveCN101229126AImprove solubilityLow toxicityAntibacterial agentsOrganic active ingredientsDiseaseMetabolic enzymes
The invention discloses a tinidazole nano-composite silver micro-emulsified antibiotics, 0.10 to 1.50 units of tinidazole and 0.008 to 0.020 units of nano-silver being regarded as medical components, and 96.92 to 99.67 units of micro-emulsified substrate and 0.30 to 3.00 units of auxiliary materials are evenly mixed together with a certain percentage, then the tinidazole complex nano-silver micro-emulsified antibiotics can be got which has a yellow outlook, the diameter of grains is 1nm to 100nm, the invention is stable, which can not only disturb the synthesis of DNA, but also be able to combine with oxygen metabolic enzyme (-SH) to interrupt the respiration metabolism process, so as to kill the aerobic and anaerobic pathogens and improve the recovery of wounds, and diseases such as vaginitis, endometritis can be quickly and effectively cured, and the preparation method of the invention is simple and safe.
Owner:NORTHWEST A & F UNIV
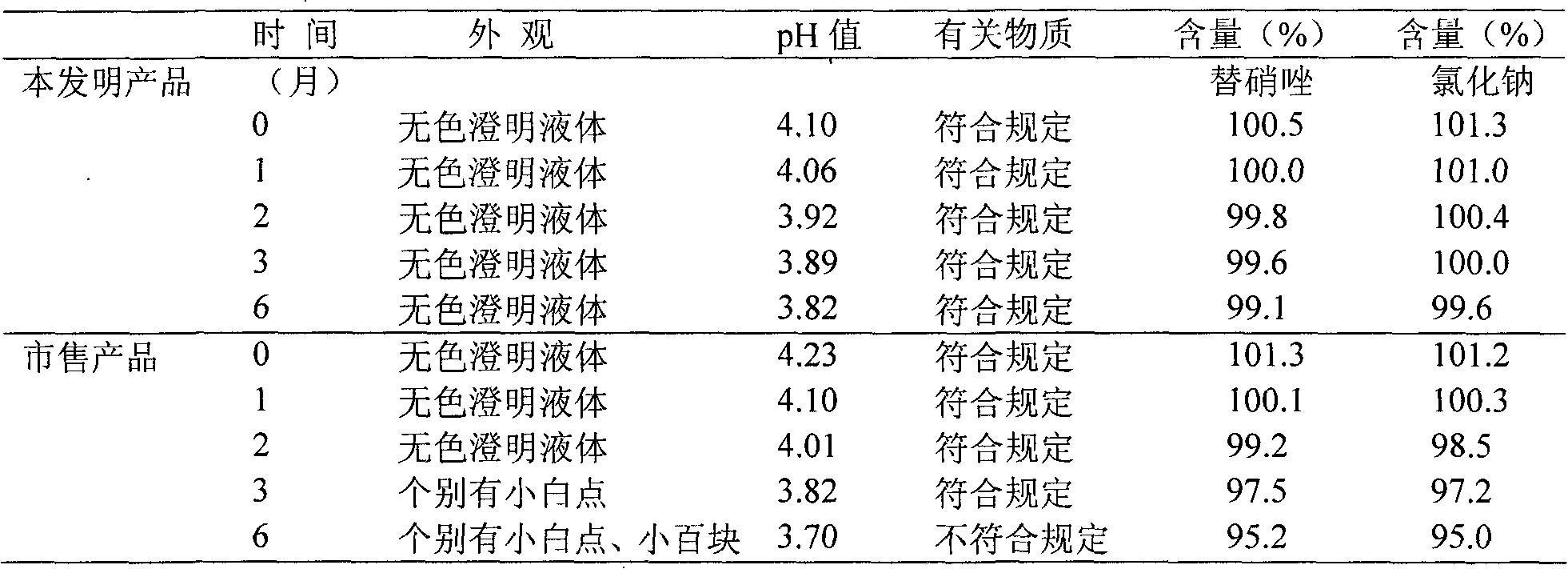
Tinidazole and sodium chloride injection and preparation method thereof
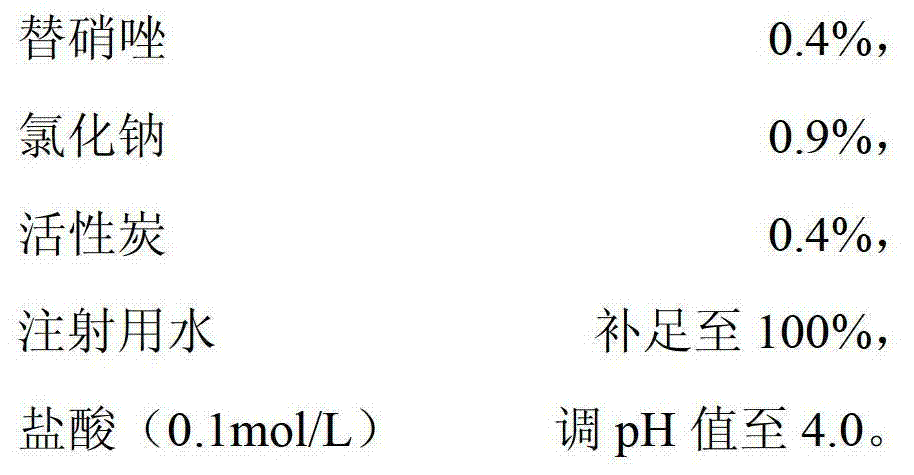
InactiveCN102525905ASolve the problem of low pass rate of clarityImprove stabilityAntibacterial agentsOrganic active ingredientsTinidazoleActivated carbon
The invention relates to a tinidazole and sodium chloride injection and a preparation method thereof. The injection is prepared from the following raw materials in percentage by weight / volume: 0.4 percent of tinidazole, 0.9 percent of sodium chloride, 0.005 to 0.5 percent of stabilizer, 0.01 to 0.05 percent of activated carbon, a proper amount of pH value regulator (the pH value is 3.5 to 5.5), and the balance of water for injection. The preparation method comprises the following steps of: putting the sodium chloride into a thick mixing tank, stirring the sodium chloride and water and dissolving to form a solution at the concentration of between 20 and 22 percent (g / ml), adding 50 percent of the activated carbon, stirring, boiling, cooling, performing pressure filtration, performing internal circulation for 10 minutes, and transferring into a thin mixing pot; putting the tinidazole into a container, adding water, stirring and dissolving, and transferring into the thin mixing pot; putting the stabilizer into the thin mixing pot, adding water, adding the residual activated carbon, adding water until the mixture reaches certain volume, stirring, refluxing for 15 minutes, and regulating the pH value to be 3.5 to 5.5 by using the pH value regulator; sampling, detecting the sodium chloride and the tinidazole, and cooling to 20 to 40 DEG C after the content of the sodium chloride and the tinidazole is qualified; and sequentially performing four-stage filtration and filling, sterilizing at the temperature of 121 DEG C, and warehousing after the product is proved to be qualified through light inspection. The problems that a tinidazole and sodium chloride injection is low in clarity qualification rate and is easy to crystallize in winter are solved.
Owner:HENAN TIANFANG HUAZHONG PHARMA
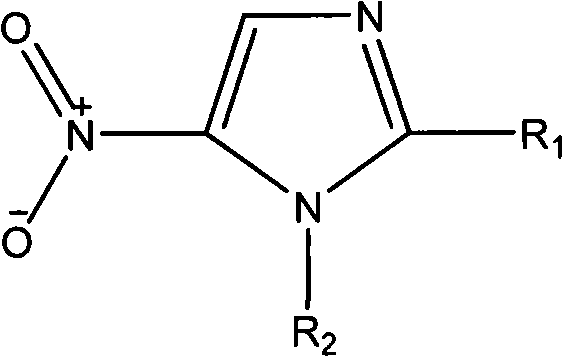
Nitroimidazole composition and method
An aqueous nitroimidazole composition comprises metronidazole, tinidazole or a combination thereof at a concentration greater than the solubility of the free base form of the nitroimidazole in water at 20° C., and a nitroimidazole crystallization-inhibiting amount of at least one organic acid. The organic acid preferably is a lower alkyl carboxylic acid (e.g., acetic acid), a polybasic acid (e.g., citric acid, tartaric acid, malic acid, polyacrylic acid, and the like), or a combination thereof. The composition can further include a thickening agent, to form a gel. The composition is free from organic co-solvents, water-soluble vitamins, and cyclodextrins; and free from nitroimidazole crystals at an ambient temperature of about 20° C. Methods of preparing the composition are also described.
Owner:CURATEK PHARMA HLDG
Tinidazole injection preparation and preparation method thereof
InactiveCN103110574ASolve the problem of high consumption and low pass rateQuality assuranceAntibacterial agentsOrganic active ingredientsTinidazoleHigh volume manufacturing
The invention discloses a tinidazole injection preparation which consists of the following components by weight percent: 0.3-0.8% of tinidazole, 0.9% of an osmotic pressure regulating agent, 0.25-0.5% of an absorbing agent, and the balance of injection water to achieve 100%, wherein pH value is regulated to be 4.0-4.2 through a pH regulating additive. Furthermore, the invention discloses a preparation method of the tinidazole injection preparation. According to the tinidazole injection preparation and the preparation method thereof, problems of high raw material consumption and low qualification rate of the tinidazole injection in production process can be effectively solved, so that an expected effect is achieved. According to the tinidazole injection preparation and the preparation method, by regulating dosages and percents of the absorbing agent and the osmotic pressure regulating agent in the formula as well as regulating main drug dissolving temperature and two absorbing time limits of the absorbing agent, the quality of a product can effectively guaranteed, and the production cost is reduced. And the tinidazole injection preparation disclosed by the invention is simple in formula, high in yield, and stable and reliable, and can implement industrial mass production conveniently.
Owner:YANGZHOU ZHONGBAO PHARMA
A kind of preparation method of tinidazole sodium chloride injection
InactiveCN102274170AImprove solubilityGuaranteed stabilityAntibacterial agentsOrganic active ingredientsTinidazoleFiltration
The invention relates to a preparation method of tinidazole sodium chloride injection, which comprises the steps of concentrated preparation, heating, filtration, dilution, adjustment of pH value, filtration, filling, sealing, sterilization, inspection and the like. The tinidazole sodium chloride injection prepared by the preparation method has good stability and can be packaged in plastic bottles.
Owner:ANHUI GLOBAL PHARM CO LTD
Mouth wash
InactiveCN104887569AWith cleanMothproofCosmetic preparationsToilet preparationsTriclosanChemical products
The invention discloses mouth wash, and belongs to the technical field of daily chemical products. The mouth wash is prepared from the following raw materials in parts by weight: 20-25 parts of radix zanthoxyli, 10-20 parts of pseudo-ginseng leaves, 10-12 parts of momordica grosvenori, 15-20 parts of lotuses, 5-8 parts of green tea, 0.5-1 part of polyphenols of grapevines, 1-5 parts of sodium chloride and 100 parts of deionized water. According to the mouth wash disclosed by the invention, active ingredients are extracted from Chinese herbal medicines of natural raw materials and are combined for use, so that the prepared mouth wash has the effects of cleaning, proofing moths, stopping bleeding and whitening; natural plant raw materials are adopted, so that the mouth wash does not contain chemical substances such as triclosan or metronidazole, tinidazole, hydrogen peroxide, an iodine solution, alcohol and the like, can be used for daily oral cavity nursing, and is easier to accept because of sweetness and faint scent.
Owner:LIUZHOU YITING TRADE
Drop for treating otitis externa mycotica and preparation method thereof
InactiveCN101766601AAntifungalHas anti-anaerobic effectSenses disorderAntimycoticsAdditive ingredientAntifungal drug
The invention relates to a compound ear drop for treating otitis externa mycotica, in particular to an ear drop for treating otitis externa mycotica. The drop is prepared by the the following ingredients: 3-30g of fluconazol, 5-50g of tinidazole, 3-30g of menthol, 100-800g of glycerine, 30-200ml of alcohol, 5-100ml of 1mol / L sodium hydroxide solution, and 1000ml of distilled water. The drop contains both antifungal drug and antianaerobic drug which enable the ear drop to have both antifungal activity and antianaerobic activity. In the process of preparation allocation, the tinidazole being slightly soluble into water need be heated by aqueous bath to be dissolved, the fluconazol and the menthol are easily dissolved into the alcohol. The alcohol is used for not only dissovling both the fluconazol and the menthol, but also being taken a transdermal accelerant of the ear drop, as well as drying the external acoustic meatus. The menthol can dispel wind to make the skin and the membrana mucosa cool and relieve the complaint and pain. A fixed amount of glycerine can protect the ear mucosa and keep the preparation tensile and adhesive.
Owner:周宣岩 +2
Tinidazole liposome injection and preparation method thereof
InactiveCN101632639AImprove stabilityPrevent crystallizationAntibacterial agentsOrganic active ingredientsTinidazoleCholesterol
The invention provides a tinidazole liposome injection and a preparation method thereof. The tinidazole liposome injection is characterized by comprising the following components by weight portion: 1 portion of tinidazole, 2-10 portions of phospholipid, 0.5-3 portions of cholesterol, 2-5 portions of tween 80 and 1-2 portions of sodium deoxycholate. The tinidazole liposome injection is prepared through reverse phase evaporation according to the proportion of specific auxiliary materials to raw auxiliary materials and achieves the unexpected effect on solving the problems of poor stability and crystallization of tinidazole injection.
Owner:HAINAN LINGKANG PHARMA CO LTD
Externally-applied skin gel as well as preparation method and application thereof
InactiveCN102641267AFunction increaseReduce in quantityAerosol deliveryOintment deliveryTinidazoleAdditive ingredient
The invention discloses externally-applied skin gel as well as a preparation method and application thereof, belongs to the technical field of medicines and relates to preparation of the externally-applied skin gel from calcium dobesilate and any one active pharmaceutical ingredient of metronidazole, tinidazole and ornidazole for the first time. In weight, per 100.0g of externally-applied skin gel contains 1.0g to 8.0g of calcium dobesilate and 0.1g to 3.0g of metronidazole, or tinidazole or ornidazole. The externally-applied skin gel can be used for treating acne rosacea and acne; the calcium dobesilate in the gel can be used for improving the blood circulation of skin and reducing the telangiectasis of the skin; and metronidazole, or tinidazole or ornidazole has an effect of exterminating worms and mites on the skin.
Owner:段亚东
Tinizadole dental plaster for treating buccal inflammation
InactiveCN1762351AIncrease concentrationLittle side effectsOrganic active ingredientsDigestive systemTinidazoleDental plaster
The invention relates to a novel dosage type of tinidazole for local and slow release administration, wherein tinidazole is used as the main pharmaceutical active composition, and biological viscous polymers are used as the pharmaceutical carriers.
Owner:BEIJING ZICHENXUAN PHARMA
Tinidazole vaginal effervescent tablet and preparation method thereof
InactiveCN102048708ASimple preparation processHigh degree of mechanizationOrganic active ingredientsAntisepticsSodium bicarbonatePolyethylene glycol
The invention relates to a tinidazole vaginal effervescent tablet and a preparation method thereof. The tinidazole vaginal effervescent tablet has the advantages of simple preparation method, good treatment effect and the like. The tinidazole vaginal effervescent tablet comprises the following components in parts by weight: 200 parts of tinidazole, 105 parts of tartaric acid, 40 parts of calcium lactate, 44 parts of lactic acid, 150 parts of starch, 6.5 parts of polyvidone K30, 20 parts of silicon dioxide, 120 parts of sodium bicarbonate, 60 parts of polyethylene glycol 4000, 4 parts of lauryl sodium sulfate and 4 parts of magnesium stearate.
Owner:赵磊
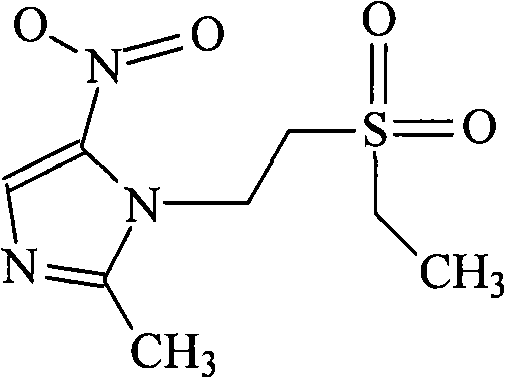
Preparation methoh of tinidazole
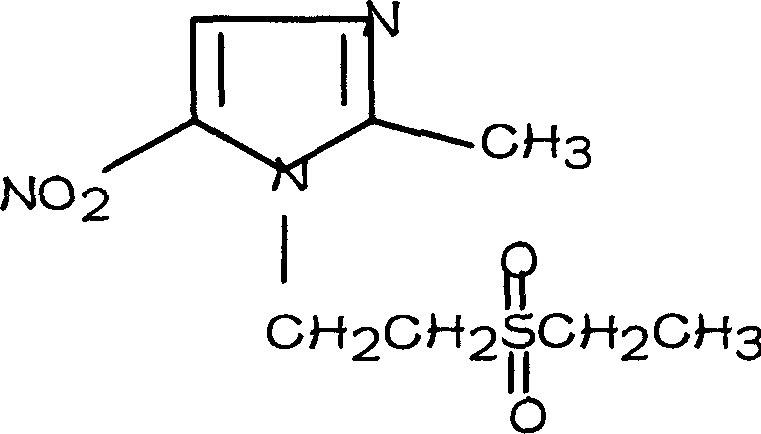
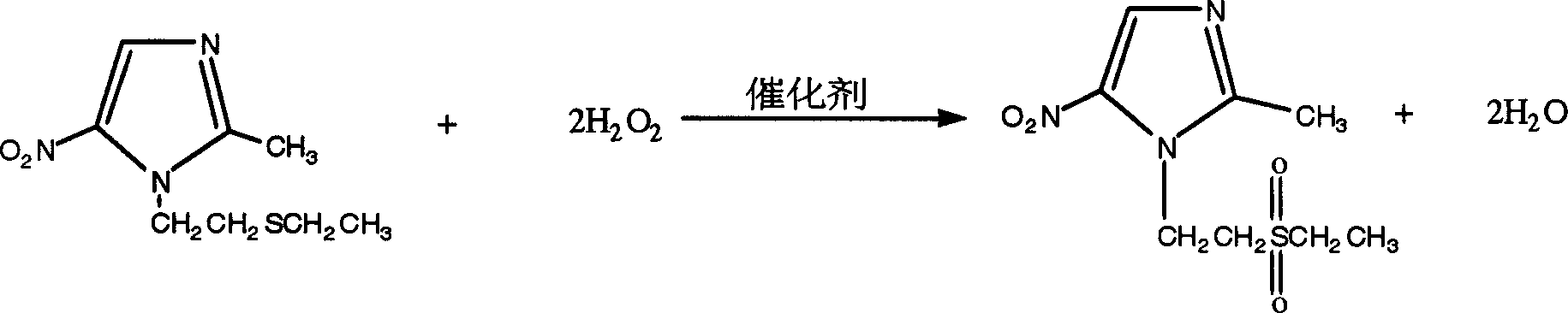
A process for preparing tinidazole from ethiol and chloroethanol includes removing sodium at ordinary temp-100 deg.C in alkaline condition, vacuum distilling to obtain beta-hydroxyethioether, condensation reaction on 2-methyl-5-nitroimidazole in methyl substituted 2-pentone under action of ZnCl2 and sodium chloride to obtain 1-(beta-ethioethyl)-2-methyl-5-nitroimidazole, and catalytic oxidizing by hydrogen peroxide and concentrated sulfuric acid. Its advantages are high output rate, low cost and less environmental pollution.
Owner:ZHEJIANG UNIV
Gargle and method for preparing same
InactiveCN106473961ALess irritatingNo side effectsAntibacterial agentsCosmetic preparationsTriclosanEugenol
The invention discloses gargle. The gargle comprises 0.5-1.2 parts of paeonol, 0.6-1.0 part of eucalyptus oil, 0.8-1.5 parts of eugenol, 0.05-0.1 part of borax, 0.1-0.5 part of epsilon-polylysine, 0.04-0.09 part of borneolum syntheticum, 0.05-0.1 part of menthol, 0.05-0.08 part of natamycin, 20-27 parts of propylene glycol, 15-21 parts of hydrogenated castor oil [PEG-40 (polyethylene glycol-40)], 0.02-0.04 part of citric acid, 1.0-1.9 parts of sweetening agents and 45-62 parts of deionized water. The gargle has the advantage that strong effects of inhibiting pyogenic bacteria in oral cavities still can be realized by the gargle without chemical components such as alcohol, cetylpyridinium chloride, chlorhexidine and triclosan or hormone such as metronidazole and tinidazole under the condition of low usage of the borax.
Owner:DANDONG KANGCHILING CLEANING PROD CO LTD
Synthesis of benzoyl ornidazole
InactiveCN102382061ASolve the problem that children with bitter taste are difficult to use drugsSolves bitter tasteAntibacterial agentsOrganic chemistryNitroimidazoleSynthesis methods
The invention relates to a new method for synthesizing an ornidazole derivative, namely benzoyl ornidazole. At present, ornidazole is a third-generation novel nitroimidazole derivative after metronidazole and tinidazole, has high anaerobe resistance and protozoan resistance, but is difficultly applied to children because of bitter taste. The invention aims to provide a new method for synthesizing the ornidazole derivative. The synthesis method comprises the following steps of: adding ornidazole, fatty acid and fatty alcohol ester, and aliphatic tertiary amine or nitrogen heterocycle organic base in turn, stirring for dissolution and clarification, heating to refluxing temperature, dripping benzoyl chloride, and reacting by keeping temperature under normal pressure; cooling, adding water, stirring, standing for demixing, adding water to wash an organic phase, cooling and freezing for crystallization, filtering, washing, and drying to obtain a crude product; and recrystallizing the crude product by using ethanol to obtain the benzoyl ornidazole. The method has the advantages that: the obtained benzoyl ornidazole is a precursor medicine of ornidazole, the original bitter taste of the ornidazole is eliminated, and a method for industrially producing the benzoyl ornidazole is realized.
Owner:SHAANXI HONGFU YIYUE PHARMA
Tinidazole expandable vaginal suppository, and preparation method and detection method thereof
ActiveCN103520090AImprove stabilityProlong the action timeWeighing by removing componentOrganic active ingredientsSecondary InfectionsTherapeutic effect
The invention relates to a tinidazole expandable vaginal suppository, and a preparation method and a detection method thereof. The expandable vaginal suppository comprises tinidazole, a matrix and an expansion carrier. After micronization treatment, tinidazole gains increased dissolution rate, so as to increase bioavailability of the suppository. A resin compound formed by the resin with micronized tinidazole can improve stability of the suppository, and a technology of medicament long-acting layered release is employed to prolong effect time of tinidazole on the vagina, extend therapeutic effect and maintain the specific acid environment and self-cleaning function of the vagina; and the pregelatinized starch can promote the mixing of each component in the suppository and improve the stability of the suppository. The tinidazole expandable vaginal suppository employs seven unique advanced technologies and has beneficial effects of prevention of outflow liquid, high stability, lasting curative effect and prevention of secondary infection.
Owner:哈尔滨田美药业股份有限公司
Medicine preparation for oral cavity
InactiveCN101028516ANon-irritatingNo obvious irritationHydroxy compound active ingredientsDigestive systemDiseaseOral medicine
An oral medicine in the form of tablet, buccal lozenge, film, or gargle for treating foul breath, periodontal abscess, pericoronitis, recurrent aphtha, etc contains the antibacterial medicine (levofloxacin and tinidazole) and menthol.
Owner:GUANGDONG PHARMA UNIV
Tinidazole preparing process
The present invention is tinidazole preparing process, and belongs to the field of chemically medicine preparing technology. The tinidazole preparing process includes the condensation between beta-hydroxyethylthio ether and2-methyl-5-nitroimidazole as main material in xylene solvent and under the action of Lewis acid; neutralization with alkali solution and water washing to obtain condensate 1-(beta-ethylthioethyl)-2-methyl-5-nitroimidazole; and direct catalytic oxidation of condensate 1-(beta-ethylthioethyl)-2-methyl-5-nitroimidazole under the action of hydrogen peroxide solution and oxidizing catalyst to obtain coarse tinidazole product. Compared with available technology, the present invention has less reaction steps, less pollution, lower production cost, short production period, higher yield and higher product quality.
Owner:ZHEJIANG SUPOR PHARM CO LTD
Controlled release preparation of clarithromycin or tinidazole
The present invention relates to a controlled release pharmaceutical composition comprising amounts ranging from about 0.1 to about 4.5% w / w, of one or more of rate controlling cellulosic ether polymers.
Owner:RANBAXY LAB LTD
Sucralfate preparation
InactiveCN109432219ATo achieve the purpose of targeted drug deliveryGood treatmentOrganic active ingredientsDigestive systemSucrose sulfateSide effect
The invention belongs to the technical field of pharmaceutic preparations, and particularly discloses a sucralfate preparation, which is prepared from the following materials in parts by weight: 30 to40 parts of sucralfate, 8 to 10 parts of tinidazole, 20 to 30 parts of an adhesive excipient, 15 to 25 parts of chondroitin sulfate, 1 to 3 parts of an acidic excipient, 30 to 40 parts of traditionalChinese medicine component, 5 to 8 parts of honey, 10 to 15 parts of a binder and 2 to 6 parts of a disintegrating agent. The sucralfate preparation is a tablet with the sucralfate, the tinidazole, the traditional Chinese medicine component, the adhesive excipient, the chondroitin sulfate, the acidic excipient and the like as main ingredients, wherein the sucralfate and the tinidazole are main drugs, which have a selective adhesive effect on gastric ulcers and enable the drug to target gastric ulcer sites; the adhesive excipient, the chondroitin sulfate and the acidic excipient can increase the viscosity of the drug, prolonging the retention time of the drug in the stomach; and the traditional Chinese medicine can relieve the side effect of the sucralfate and enhance the immunity of the body. The invention can reduce the frequency and dosage of administration, increase the therapeutic effect and reduce the toxic and side effects of the drug.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
UHPLC-MS/MS (Ultra High Performance Liquid Chromatography-Mass Spectrometry-Mass Spectrometry) analytical method for measuring rat curcumin plasma concentration
InactiveCN106841422AAccurate analysisAnalysis analysis is accurateComponent separationBlood plasmaLiquid chromatography–mass spectrometry
The invention discloses a UHPLC-MS / MS (Ultra High Performance Liquid Chromatography-Mass Spectrometry-Mass Spectrometry) analytical method for measuring rat curcumin plasma concentration. The method comprises the following steps: (1) preparing a curcumin standard solution and an internal standard tinidazole standard solution, and establishing a standard curve; and (2) adding an internal standard into to-be-measured plasma, performing pretreatment, performing LC-MS / MS analysis, and calculating the concentration of curcumin in the to-be-measured plasms by utilizing the standard curve. According to the method disclosed by the invention, the chromatographic conditions and internal standard substances are reasonably selected, the sensitivity is improved, the analysis time is shortened, and the sample pretreatment process is simplified. Meanwhile, the method disclosed by the invention has high accuracy and precision degree, and the detection requirements of non-clinical pharmacokinetic study are met.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Germicide and algicide composition and preparation method of germicide and algicide composition
ActiveCN104285955AHigh float indicatorHigh ammonia nitrogen indexBiocideScale removal and water softeningTinidazoleWater quality
The invention discloses a germicide and algicide composition and a preparation method of the germicide and algicide composition. By virtue of the germicide and algicide composition, the quality of recycled water in a recycled water system can be stabilized; the propagation of bacteria and algae is avoided or grown bacteria and algae are killed. The germicide and algicide composition is characterized by at least comprising the following two components: a first component, namely N-tinidazole-N-dodecyl dimethyl-2-hydroxypropyl ammonium chloride, and a second component, namely 2-hydroxypropyl-3-dodecyloxyl trimethy ammonium chloride.
Owner:北京拓凯化工技术有限公司
Preparation method of tinidazole
InactiveCN106632062AReduce volatilityThe reaction steps are simpleOrganic chemistryNitroimidazoleTinidazole
The invention belongs to the field of chemical pharmacy, and particularly relates to a preparation method of tinidazole. The method includes steps of applying beta- hydroxy ethyl thioether and 2-methyl-5- nitroimidazole as raw material, using xylene series as the solvent; performing condensation reaction under the function of solid superacid; water-washing and acquiring the condensate 1-(beta- ethion ethyl)-2-methyl-5-nitroimidazole; then oxidizing the condensate 1-(beta- ethion ethyl)-2-methyl-5-nitroimidazole under the combined action of hydrogen peroxide and air, and acquiring the crude product of tinidazole. The method needs not the alkali liquid to neutralize condensation reaction liquid, thus the preparation steps of tinidazole are shortened; the preparation method saves cost and reduces three wastes.
Owner:HUBEI HONGYUAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com