New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
A technology for erythromycin and preparations, which is applied in the field of preparation of pharmaceutical preparations and can solve the problems of instability, effective absorption of difficult erythromycin, and low bioavailability of enteric-coated erythromycin preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the preparation of erythromycin micropill pill
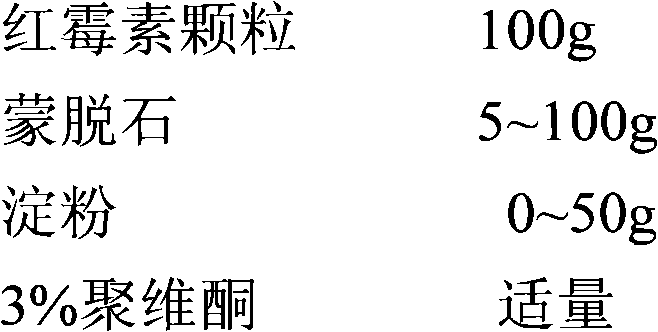
[0083] The prescription is shown in the table below
[0084] components
Take the amount
components
Take the amount
125g
1.25g~7.5g
blank core
15~30g
3% hypromellose, 1% PEG6000
Appropriate amount
0.3g~5g
Vitamin C
0.5g~5g
15~30g
[0085] Isolation layer: 3% hypromellose, talcum powder, appropriate amount.
[0086] Operating process:
[0087] (1) Preparation of wetting agent: Take 100ml of water, heat to 80°C, add 3g of hydroxypropylmethylcellulose, stir to disperse evenly while adding, then add 1g of polyethylene glycol 6000, stir to fully dissolve, cool to Fully swell at room temperature and set aside;
[0088] (2) The original and adjuvant materials of prescription quantity are mixed uniformly by the method o...
Embodiment 2
[0092] Embodiment 2: Preparation of erythromycin granules
[0093] The prescription is shown in the table below
[0094] components
Take the amount
components
Take the amount
125g
1.25g~7.5g
3% hypromellose, 1% ~ 2% Tween-80
Appropriate amount
Vitamin C
0.5g~5g
15~30g
[0095] Isolation layer liquid: 9% white gastric soluble coating premix, appropriate amount.
[0096] Operating process:
[0097] (1) Preparation of wetting agent: Take 100ml of water, heat to 80°C, add 3g of hydroxypropylmethylcellulose, stir while adding to make the dispersion even, then add the prescribed amount of Tween-80, stir to fully dissolve, cool Fully swell at room temperature and set aside;
[0098] (2) The original and auxiliary materials of the prescription amount are mixed uniformly by the method of equal addition, and set aside;
[0099] (3) Ad...
Embodiment 3
[0104] Embodiment 3: the preparation of erythromycin microchip tablet core
[0105] The prescription is shown in the table below
[0106] components
Take the amount
components
Take the amount
Erythromycin
125g
Crospovidone
1.25g~6.25g
Vitamin C
0.5g~5g
polyethylene glycol
1g~6g
20g
3% povidone, 1% ~ 2% Tween-80
Appropriate amount
[0107] Isolation layer: 9% white gastric soluble coating premix, appropriate amount.
[0108] Operating process:
[0109](1) Preparation of wetting agent: take 100ml of water, heat to 80°C, add 3g of povidone, stir while adding to disperse evenly, then add the prescribed amount of Tween-80, stir to fully dissolve, cool to room temperature and use Fully swollen, ready for use;
[0110] (2) The original and auxiliary materials of the prescription amount are mixed uniformly by the method of equal addition, and set aside;
[0111] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com