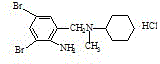
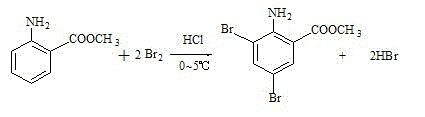
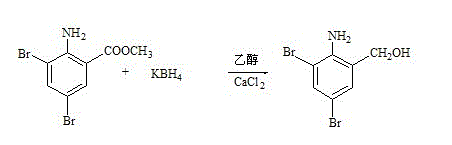
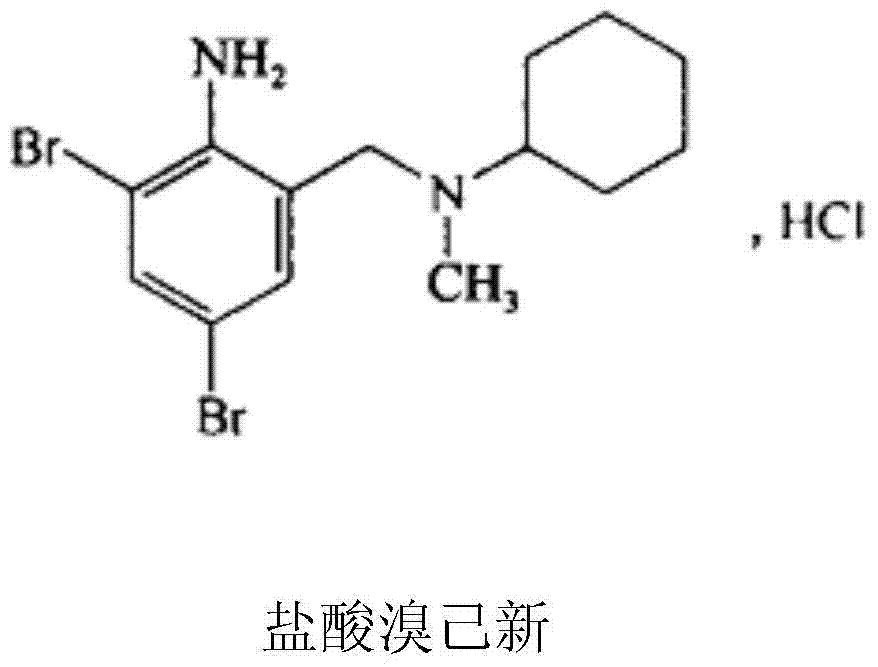
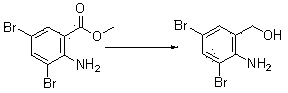Patents
Literature
42 results about "Bromhexine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bromhexine is a mucolytic drug used in the treatment of respiratory disorders associated with viscid or excessive mucus. It was patented in 1961 and came into medical use in 1966.
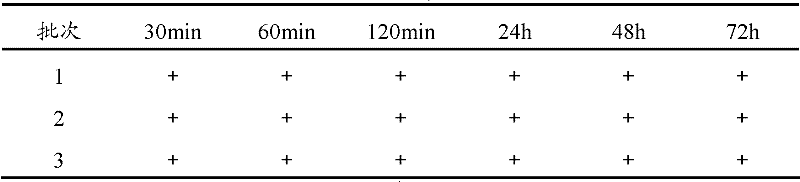
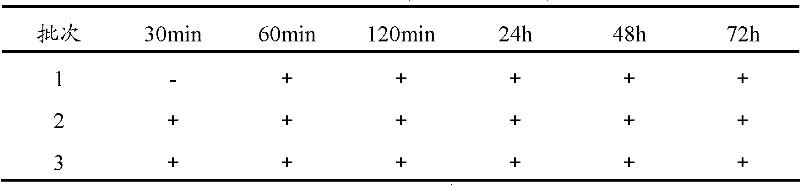
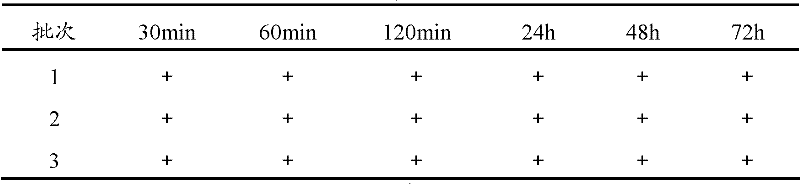
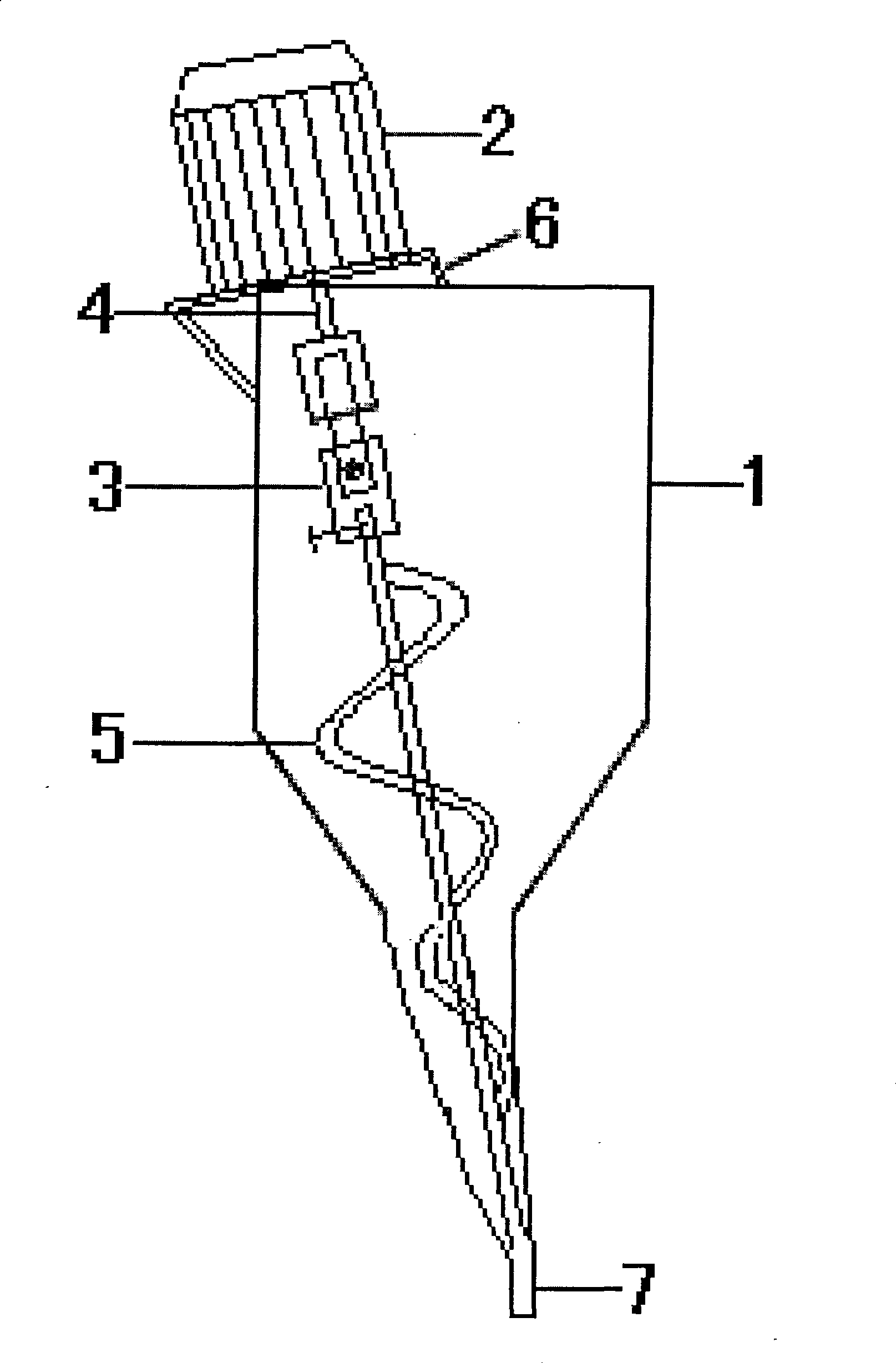
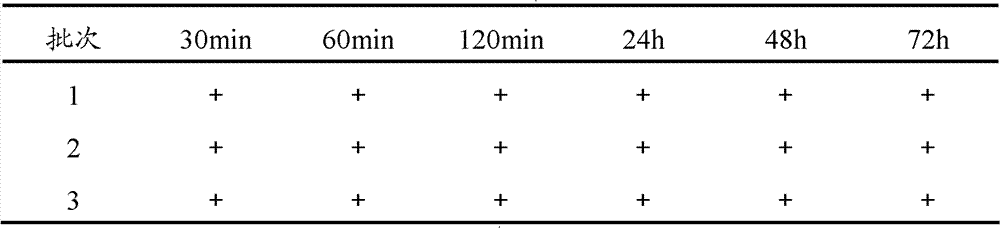
A kind of bromhexine hydrochloride injection and its preparation method and application
ActiveCN102293741AImprove stabilityReduce usageOrganic active ingredientsPharmaceutical delivery mechanismMedicineInjection solution
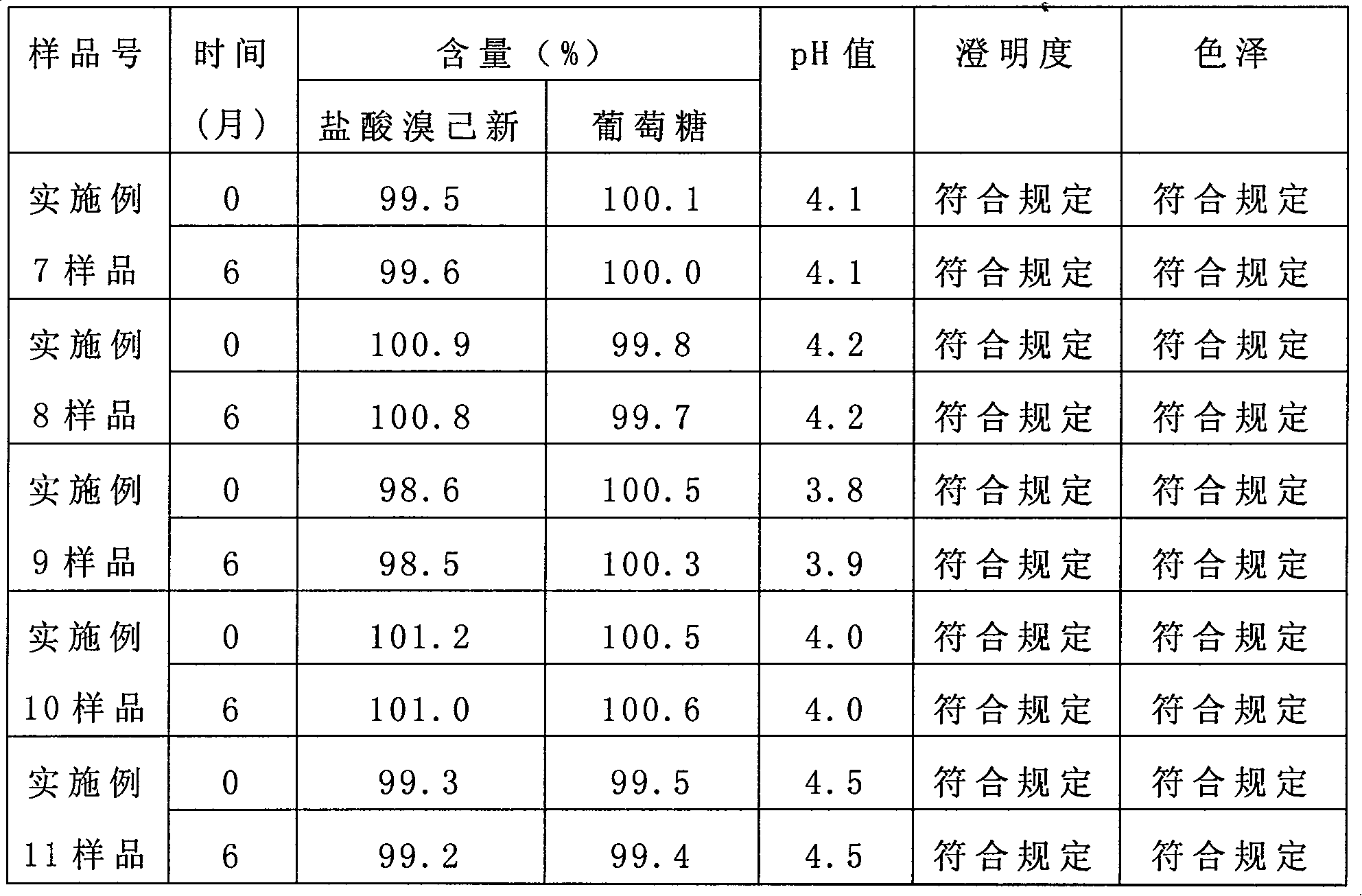
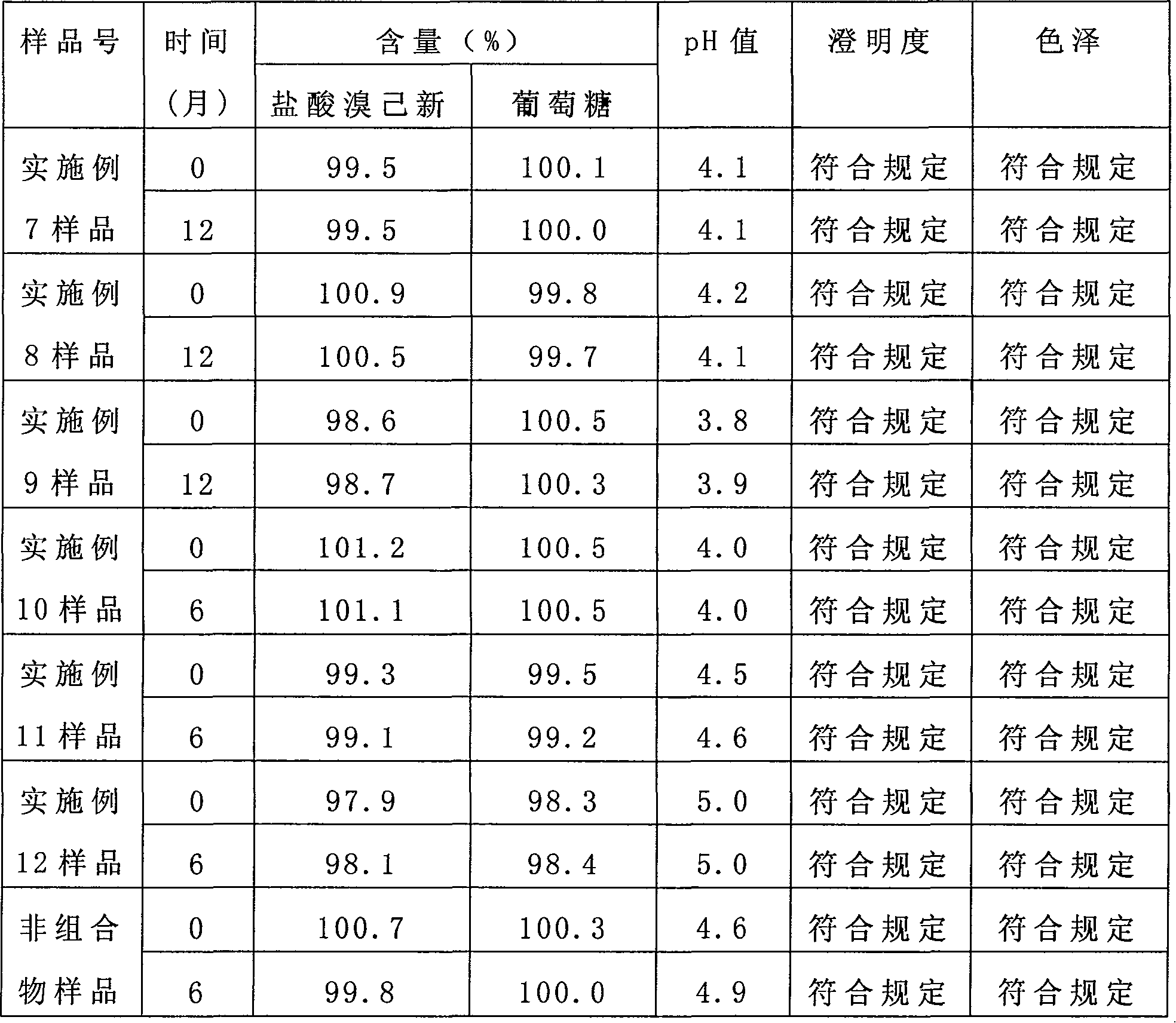
The invention provides a bromhexine hydrochlorie injection, which contains bromhexine hydrochlorie and glucose and does not contain ethanol. Without ethanol, the injection can be used together with antibiotic drugs and has good stability. In accord with national regulations for pharmaceutical preparations, the injection has stable and reliable quality, and therefore can be used safely. The invention also provides a preparation method of the bromhexine hydrochlorie injection, comprising the steps of: preparing an isotonic water solution of glucose, then predissolving the bromhexine hydrochlorie in the isotonic water solution, and conducting an ultrasonic treatment. According to the method of the invention, the use of ethanol in the process of preparing a liquid preparation of bromhexine hydrochlorie can be avoided successfully, and possible adverse reactions during clinical application are avoided. With a process simple for operation, the method provided in the invention improves the stability of the injection.
Owner:HANKANG BIOCHEMICAL & PHARMA CO LTD
Loxoprofen-containing pharmaceutical composition
InactiveCN102740854AGood storage stabilityReduce or inhibit damageOrganic active ingredientsPowder deliveryBromineLoxoprofen
Disclosed is a pharmaceutical composition containing loxoprofen or a salt thereof and codeine or the like, which has excellent storage stability. Specifically disclosed is a pharmaceutical composition which contains at least one component selected from the group consisting of codeine, carbinoxamine or a salt thereof, clemastine or a salt thereof, chlorpheniramine or a salt thereof, diphenylpyrraline or a salt thereof, bromhexine or a salt thereof, ambroxol or a salt thereof, lysozyme or a salt thereof and dextromethorphan or a salt thereof and loxoprofen or a salt thereof in such a manner that the at least one component and loxoprofen or a salt thereof are substantially not in contact with each other.
Owner:KOWA CO LTD
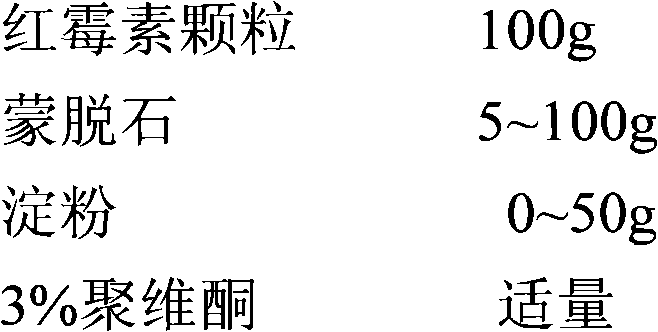
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
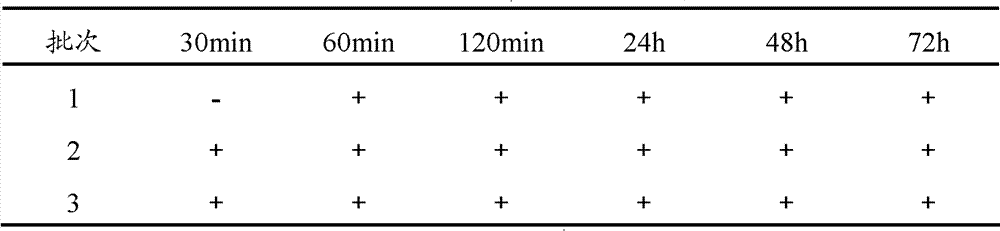
Composition and great volume injection containing bromhexine salt and the injection preparing process
ActiveCN101019826AFor long-term storageDoes not cause crystallizationOrganic active ingredientsPharmaceutical delivery mechanismHydroxyethyl celluloseBromhexine
The present invention discloses one kind of composition containing bromhexine salt, great volume injection and the injection preparing process. The composite includes bromhexine salt as the active component in 0.03-0.8 wt%, ethanol as co-solvent in 1.5-36 wt%, and stabilizer in 1.0-40 wt%, and has pH 3.0-7.0. The stabilizer is poloxamer, hydroxypropyl cellulose, hydroxypropyl methyl cellulose and / or hydroxyethyl cellulose. The composition of the present invention has high stability, and may be preserved for long period without bromhexine salt crystal separated out.
Owner:江西亿友药业有限公司
Exfoliative cell sap base preserving fluid, method thereof for flaking and kit
ActiveCN104642300ASimple and fast operationEasy to prepareMicrobiological testing/measurementDead animal preservationPotassiumMonopotassium phosphate
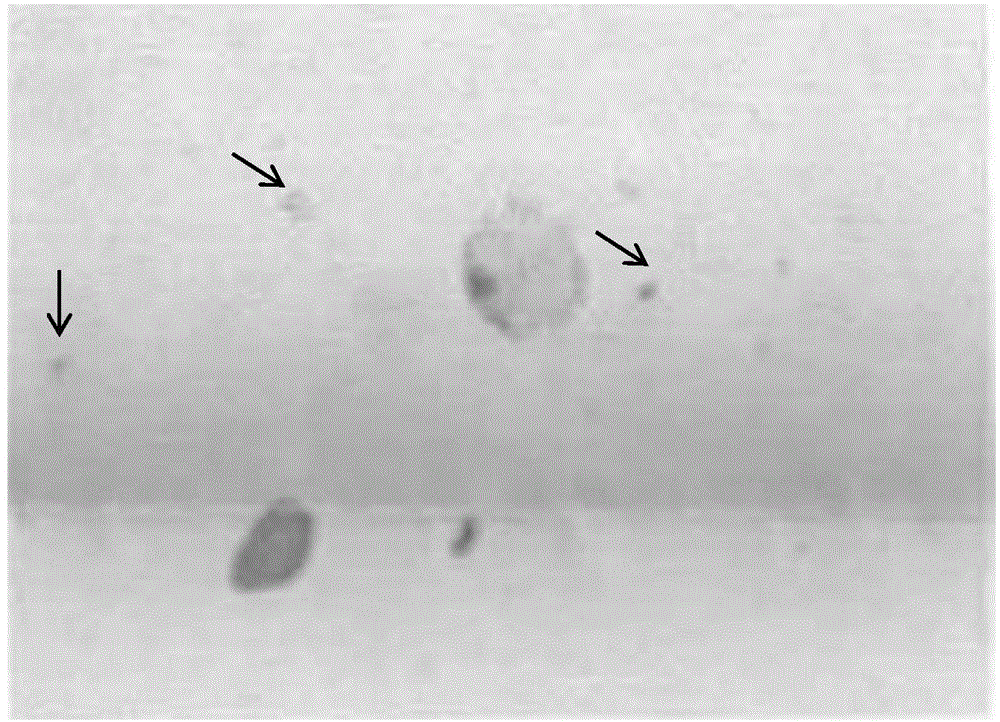
The invention discloses an exfoliative cell sap base preserving fluid, a method thereof for flaking and a kit. The exfoliative cell sap preserving fluid comprises the following components by massic volume percentage: 3.8-4.2% of paraformaldehyde, 0.8-1.5% of N-methyl-N-cyclohexyl-2-amino-3,5-bromhexine, 0.8-1.5% of glacial acetic acid, 0.1-0.2% of acetylcysteine, 0.75-0.85% of sodium chloride, 0.01-0.03% of potassium chloride, 0.12-0.15% of disodium hydrogen phosphate, 0.025-0.03% of monopotassium phosphate, and the balance of water; and pH is regulated to 7.2-7.8. The exfoliative cell sap base preserving fluid is small in shrinking to the exfoliative cell, and suitable for storage for a long time; the cell coated by the mucus in the sample is successfully separated, the positive cell stacking and loss are avoided, and the effective cell number is improved; and the preserving fluid can be used for observing whether the occult blood symptom is existent.
Owner:SHANGHAI YU KANG HOSPITAL
Bromhexine hydrochloric acid orally disintegrating tablet production method
InactiveCN101361719AOrganic active ingredientsPharmaceutical product form changeOrally disintegrating tabletCompressibility
The invention relates to a medical prescription for producing bromhexine hydrochloride orally disintegrating tablets by using powder direct compression process, a technology and a powder feeder used for a novel tablet machine. Compared with the conventional technique of the powder direct compression process, the orally disintegrating tablets produced by the invention has mouth-feel of cool-refreshing and fine-smooth, smaller tablet weight difference, good content uniformity, fast disintegration and dissolution, high dissolution speed and dissolution rate, strong hardness and good compressibility of the tablets.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
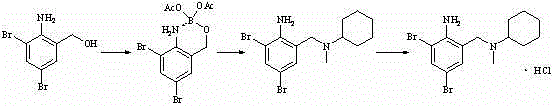
Novel preparation method for bromhexine hydrochloride
InactiveCN102531922ASimple processMild reaction conditionsAmino preparation from aminesAcetic anhydrideObstructive chronic bronchitis
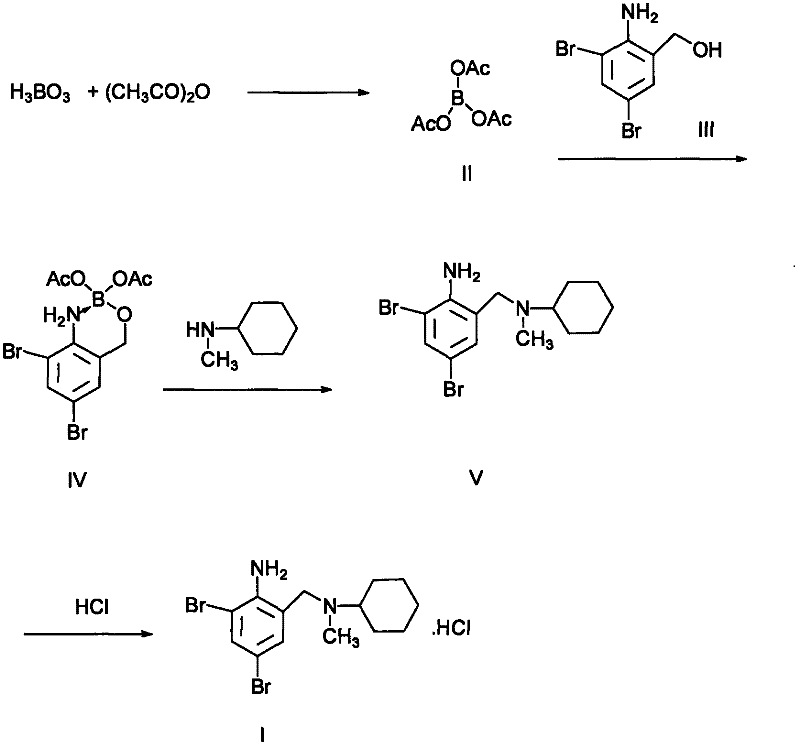
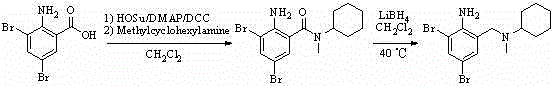
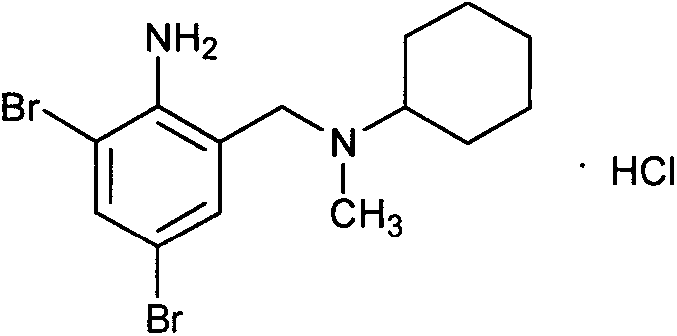
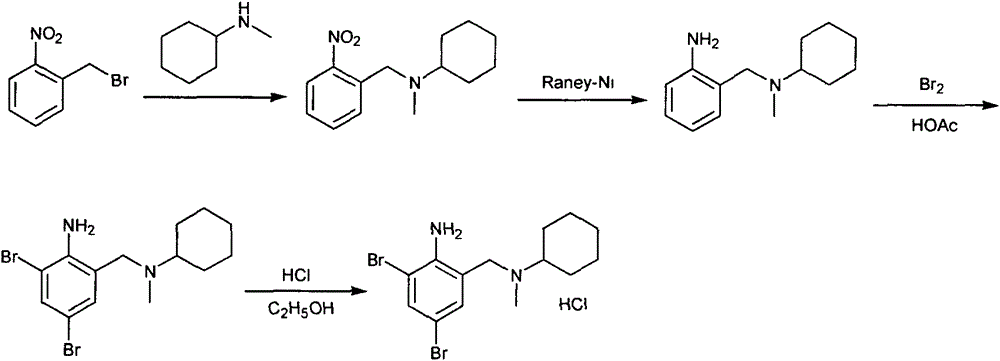
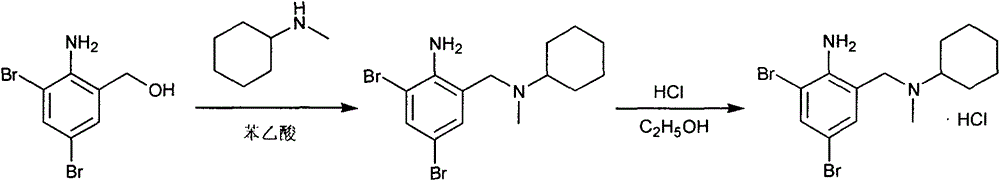
The invention discloses a preparation method for bromhexine hydrochloride. The preparation method comprises the following steps of: reacting acetic anhydride with boric acid to generate triacetic acid boric acid ester; reacting 2-amino-3,5- dibromo benzylalcohol with triacetic acid boric acid ester to generate 2-amino-3,5-dibromo benzylalcohol boron chelate; reacting 2-amino-3,5-dibromo benzylalcohol boron chelate with N- methylcyclohexylamine to generate N-(2-amino-3,5-dibromobenzyl)-N-methylcyclohexylamine (bromhexine); and reacting bromhexine with hydrogen chloride to generate crude bromhexine hydrochloride, and refining to obtain refined bromhexine hydrochloride. The bromhexine hydrochloride is suitable for treating chronic bronchitis and other respiratory diseases with phlegm which is difficult to cough out.
Owner:陈学峰
Compound cefaclor preparation and preparation method
InactiveCN102114019AGood water solubilityGreat tasteAntibacterial agentsOrganic active ingredientsSolubilitySuspending Agents
The invention relates to a preparation of a medicine and a preparation method, in particular to a compound cefaclor preparation and a preparation method, and belongs to the technical field of pharmacy. The preparation is characterized by being prepared by adding a proper amount of pharmaceutical excipients into 25 to 35 weight parts of medicine, namely cefaclor and 0.5 to 1.5 weight parts of bromhexine hydrochloride, wherein the cefaclor is calculated based on anhydrous cefaclor, and the bromhexine hydrochloride is calculated based on bromhexine; and the excipients consist of a suspending agent, a flocculating agent, a flavoring agent and a flow aid. The invention provides a formula capable of improving the dissolubility and suspension stability of medicines and the mouthfeel of finished products, and a preparation technology.
Owner:汤明昌 +1
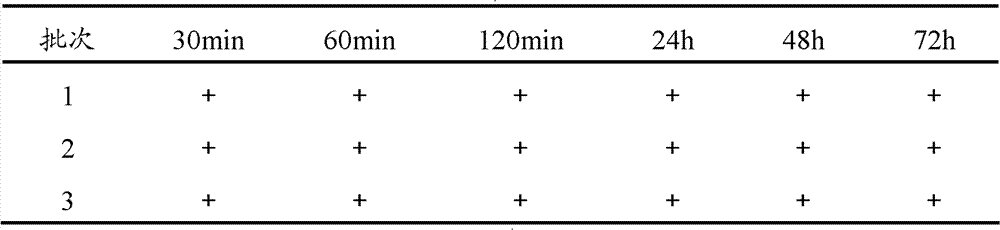
Bromhexine hydrochlorie injection, its preparation method and application
ActiveCN102293741BImprove stabilityReduce usageOrganic active ingredientsPharmaceutical delivery mechanismMedicineD-Glucose
The invention provides a bromhexine hydrochlorie injection, which contains bromhexine hydrochlorie and glucose and does not contain ethanol. Without ethanol, the injection can be used together with antibiotic drugs and has good stability. In accord with national regulations for pharmaceutical preparations, the injection has stable and reliable quality, and therefore can be used safely. The invention also provides a preparation method of the bromhexine hydrochlorie injection, comprising the steps of: preparing an isotonic water solution of glucose, then predissolving the bromhexine hydrochlorie in the isotonic water solution, and conducting an ultrasonic treatment. According to the method of the invention, the use of ethanol in the process of preparing a liquid preparation of bromhexine hydrochlorie can be avoided successfully, and possible adverse reactions during clinical application are avoided. With a process simple for operation, the method provided in the invention improves the stability of the injection.
Owner:HANKANG BIOCHEMICAL & PHARMA CO LTD
Method for synthesizing bromhexine hydrochloride
ActiveCN104628577AReduce pollutionFew reaction stepsOrganic compound preparationAmino compound preparationBenzaldehydeCyclohexylamine
The invention relates to a method for preparing bromhexine hydrochloride. The method comprises the steps of synthesizing bromhexine free alkali from 2-amino-3,5-dibromo benzaldehyde and N-methyl cyclohexylamine in the presence of a catalyst and a reducer in one step, and enabling the produced bromhexine free alkali to undergo salt forming reaction with a hydrogen chloride salt forming reagent, thereby preparing bromhexine hydrochloride, wherein the catalyst is a sulfonic acid type catalyst Amberlyst<R>15(H). According to the method, the number of reaction steps is greatly reduced, the production cycle is shortened, the reaction yield is increased, and the production cost is reduced; the method disclosed by the invention has no need of using virulent reagents, is mild in reaction conditions and little in environmental pollution, and is free from special requirements on equipment.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Production method of bromhexine hydrochloride
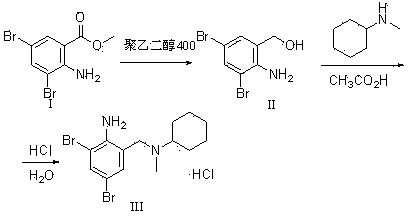
ActiveCN103396323AHigh purityReduce generationAmino compound preparation by condensation/addition reactionsBenzoic acidPotassium borohydride
The invention discloses a production method of bromhexine hydrochloride; 3,5-dibromo-2-amino benzoic acid methyl ester is used as a starting raw material, polyethylene glycol 400 is used as a solvent, 3,5-dibromo-2-amino benzalcohol is obtained by reduction using sodium borohydride or potassium borohydride, bromhexine is obtained by a condensation reaction of the 3,5-dibromo-2-amino benzalcoho and N-methyl cyclohexylamine under the effect of acetic acid, then a crude product of bromhexine hydrochloride is obtained through direct salt forming with hydrochloric acid, and finally a bromhexine hydrochloride fine product is obtained through refining with methanol. Compared with production processes in the prior art, the production method of the bromhexine hydrochloride has the advantages of few reaction steps, convenient refining, simple operation and good quality of products.
Owner:江西亿友药业有限公司
Preparation method and application of 2-amino-3,5-bibromobenzyl intermediate compound
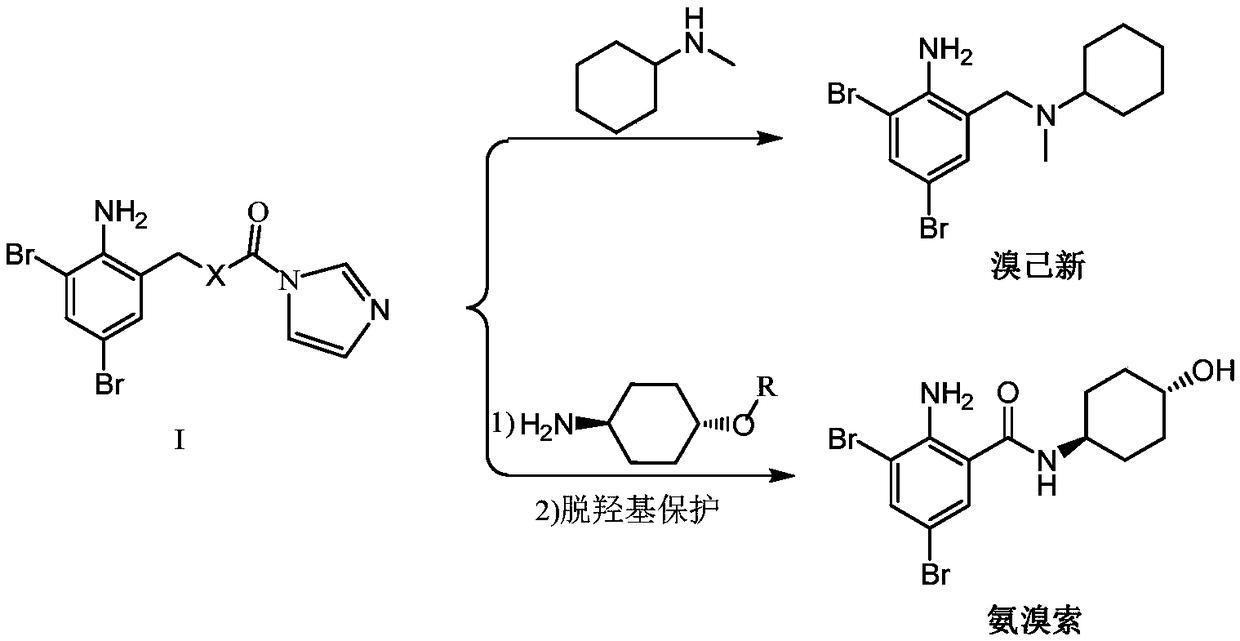
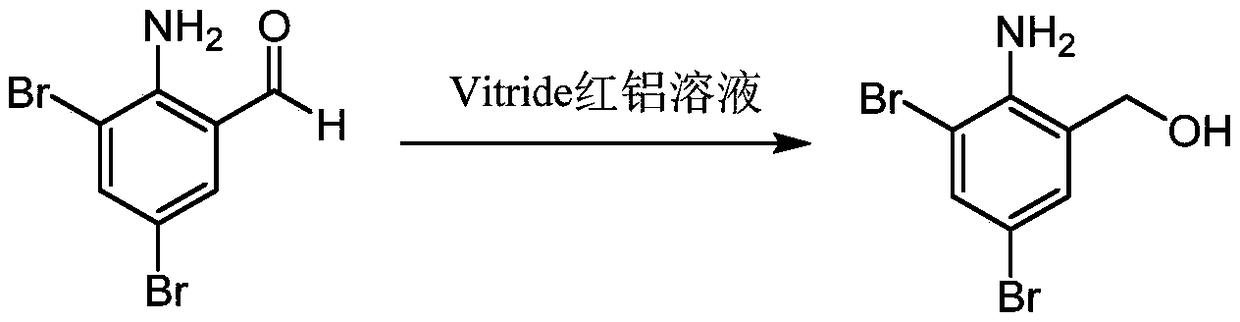
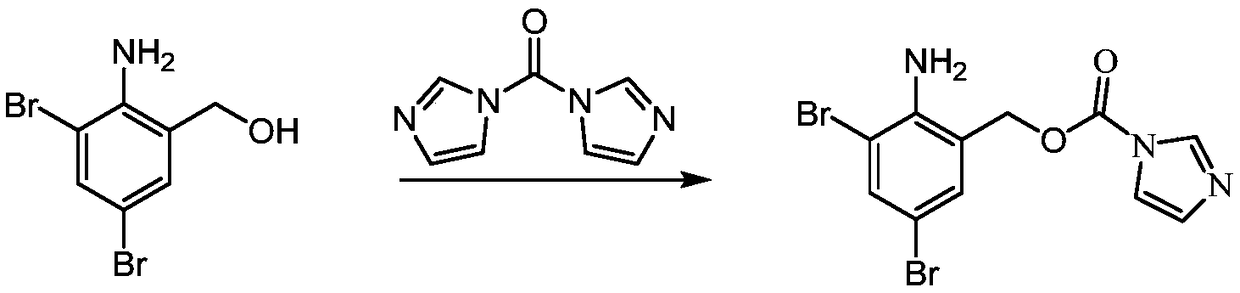
InactiveCN109096196AReduce pollutionAvoid generatingAmino preparation from aminesOrganic compound preparationCyclohexylaminesCarbonyldiimidazole
The invention provides a preparation method of a 2-amino-3,5-bibromobenzyl intermediate compound and further discloses an application of the 2-amino-3,5-bibromobenzyl intermediate compound in preparation of bromhexine and ambroxol. A cheap and available raw material 2-amino-3,5-dibromobenzaldehyde is adopted, 2-amino-3,5-dibromobenzyl alcohol is obtained through reduction, and the reaction condition is milder; N'N-carbonyldiimidazole is used for activating hydroxyl of 2-amino-3,5-dibromobenzyl alcohol to produce active ester, and the active ester is subjected to a condensation reaction with N-methylcyclohexylamine and trans-p-aminocyclohexanol to produce corresponding products respectively. The preparation method has the advantages that the reproducibility is good, the process is simple, the product purity is high and potential gene poison impurities can be avoided, and the medication safety of the 2-amino-3,5-bibromobenzyl intermediate compound can be further improved.
Owner:苏州华健瑞达医药技术有限公司
Medicine for treating radiation enteritis and preparation method and application of medicine
InactiveCN106511342AThe composition is uniqueLow priceDigestive systemAnhydride/acid/halide active ingredientsSide effectCalcium pyruvate
The invention discloses a medicine for treating radiation enteritis and a preparation method and application of the medicine. The medicine is composed of the following raw materials including, by weight, 3-7 parts of levamisole, 11-19 parts of bromhexine, 5-13 parts of taurine and 1-5 parts of calcium pyruvate. The levamisole, the bromhexine and the calcium pyruvate are mixed and added into deionized water, and the mixture is heated to 75 DEG C to 77 DEG C and stirred for 38 min to 40 min to prepare the mixture A; the mixture A and the taurine are mixed and subjected to ultrasonic treatment at 58 DEG C for 25 min, the ultrasonic power is 900W, then the mixture is stirred to dry at 63 DEG C, and the medicine is obtained. The medicine has the advantages of being unique in formula, low in price, safe and convenient to use, the effect of the medicine is superior to that of conventionally used western medicines, the medicine can effectively cure the junction and rectal injuries caused during radiotherapy and is high in cure rate, quick in effect and free of toxic and side effects and relapse after curing, simple in preparation process, short in production cycle low in cost and suitable for industrial production and large-scale popularization, and the raw materials are simple and easy to obtain.
Owner:郑州莉迪亚医药科技有限公司
Synthesis production method of bromhexini hydrochloride
InactiveCN104610073AImprove performanceReduce pollutionOrganic compound preparationAmino compound preparationBenzoic acidPotassium borohydride
The present invention discloses synthetic production method of bromhexini hydrochloride. According to the method, methyl anthranilate is adopted as a starting raw material and is subjected to a bromine and hydrochloric acid bromination reaction to synthesize methyl 2-amino-3,5-dibromobenzoate, potassium borohydride and ethanol are adopted as raw materials to carry out a reduction reaction to synthesize 3,5-dibromo anthranilic alcohol, the 3,5-dibromo anthranilic alcohol and N-methyl cyclohexylamine are condensation to form bromhexine, hydrochloric acid is added to form a salt, a bromhexine hydrochloride crude product is synthesized, and re-crystallization with ethanol is performed to obtain the bromhexini hydrochloride. The method of the present invention has characteristics of short synthesis route, simple operation, easily available starting raw material, stable intermediate performance, high product yield and low production cost, and is suitable for industrial scale production.
Owner:KAMP PHARMA
Bromhexine hydrochloride composition freeze-dried powder for injection
InactiveCN103565758AAvoid the risk of kidney failureNice appearanceAntibacterial agentsOrganic active ingredientsChitosan nanoparticlesManufacturing technology
The invention provides bromhexine hydrochloride composition freeze-dried powder for injection, which relates to the field of medicines and medicine manufacturing technology. The bromhexine hydrochloride composition freeze-dried powder for injection comprises the following raw material components in parts by weight: 1 part of bromhexine hydrochloride, 0.5-1.3 parts of chitosan nanoparticles and 5-10 parts of injection water. The bromhexine hydrochloride composition freeze-dried powder for injection provided by the invention has the advantages that: 1) the chitosan nanoparticles replace mannitol to serve as a skeleton agent of the freeze-dried powder for injection, the obtained product is good in shape, high in stability and capable of avoiding a kidney failure risk caused by clinic use of the mannitol; 2) the bromhexine hydrochloride is slightly soluble in water, the chitosan nanoparticles can also be used as a cosolvent of the hydrochloride bromhexine to improve the solubleness of the hydrochloride bromhexine and facilitate industrial production and clinic use; and 3) the chitosan nanoparticles have certain antibacterial activity, when being clinically used with the hydrochloride bromhexine, the chitosan nanoparticles can cooperatively treat respiratory tract infection accompanied with a symptom that viscous sputum is unlikely to expectorate caused by sensitive bacteria.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Novel method for preparing bromhexine hydrochloride
InactiveCN104447355AShort synthetic stepsMild reaction conditionsOrganic compound preparationAmino preparation by functional substitutionAlcoholMethylating Agent
The invention provides a novel method for preparing bromhexine hydrochloride. The method is characterized by comprising the following steps of reacting 2-amino-3,5-dibromobenzyl alcohol as a starting raw material and cyclohexylamine to obtain 2-amino-3,5-dibromo-N-cyclohexylbenzimide (referred to as Schiff base); reacting the Schiff base and a reducing agent to obtain 2-amino-3,5-dibromo-N-cyclohexyl-benzylamine (referred to as secondary amine); and reacting the secondary amine and a methylating reagent to obtain bromhexine base and then reacting the bromhexine base and hydrochloric acid to obtain bromhexine hydrochloride. The starting raw material used in the method is inexpensive and easily available, the process route is completely original, the steps are shorter, an intermediate has stable performance, and the bromhexine hydrochloride has the advantages of controllable quality, high yield and good purity.
Owner:NINGBO TEAM PHARMA
Composition and great volume injection containing bromhexine salt and the injection preparing process
ActiveCN100409835CImprove stabilityLess irritatingOrganic active ingredientsPharmaceutical delivery mechanismHydroxyethyl celluloseBromhexine
The present invention discloses one kind of composition containing bromhexine salt, great volume injection and the injection preparing process. The composite includes bromhexine salt as the active component in 0.03-0.8 wt%, ethanol as co-solvent in 1.5-36 wt%, and stabilizer in 1.0-40 wt%, and has pH 3.0-7.0. The stabilizer is poloxamer, hydroxypropyl cellulose, hydroxypropyl methyl cellulose and / or hydroxyethyl cellulose. The composition of the present invention has high stability, and may be preserved for long period without bromhexine salt crystal separated out.
Owner:江西亿友药业有限公司
Special-effect drug for treating asthma
InactiveCN103655927AGood treatment effectWide range of typesBacteria material medical ingredientsRespiratory disorderPotato starchLevamisole
The invention relates to a special-effect drug for treating asthma, which is composed of a drug I, a drug II and a drug III; the drug I comprises the following components by weight: 0.016g of dead bacillus calmette guerin vaccine, 1.071g of potato starch, 0.3g of aminophylline, 0.001g of ketotifen, 0.005g of dioxopromethazine, 0.025g of levomisole, 0.03g of bromhexine and 0.8g of green tea; the drug II comprises the following components by weight: 1.4g of fructus schisandrae, 1.4g of astragalus and 0.3g of cinnamon; and the drug IIT comprises the following components by weight: 1.2g of radix codonopsis, 1.2g of astragalus, 0.3g of epimedium and 0.4g of gypsum. The special-effect drug disclosed by the invention is prepared on the basis of combination of Chinese and western medicine sciences, has remarkable treatment effect, and treats both symptoms and root causes.
Owner:解玉启
Sputum specimen preparation staining method
The invention relates to a sputum specimen preparation staining method which comprises the following steps: collecting a to-be-detected sputum specimen, mixing the sputum specimen and sputum treatmentliquid, and shaking to completely decompose the mixture; transferring the mixture into a test tube to perform oscillatory centrifugation after the sputum solution is completely decomposed, removing the supernatant, injecting cell preservation liquid into the test tube, and performing preparation by an automatic preparation staining machine, wherein the sputum treatment liquid comprises the following components in percentage by weight: 2-8% of phosphate, 2-3% of phenol, 2-3% of bromhexine, 20-40% of ethanol and 46-74% of distilled water. The sputum specimen preparation staining method providedby the invention is simple in process and small in usage amount of the sputum treatment liquid, and when the method is matched with the automatic preparation staining machine, qualified staining sections can be prepared on a large scale at a time and are stable in quality, and the single speed can reach 2 sections per minute.
Owner:孝感市森茂激光数控设备有限公司
Chinese and western medicinal composition for treating radioactive enteritis, and preparation method and application thereof
InactiveCN106420773AThe composition is uniqueLow priceDigestive systemAnhydride/acid/halide active ingredientsSide effectRectum injury
The invention discloses a Chinese and western medicinal composition for treating radioactive enteritis, and a preparation method and application thereof. The Chinese and western medicinal composition comprises, by weight, 3-7 parts of alendronate sodium, 11-19 parts of bromhexine, 5-13 parts of taurine and 1-5 parts of calcium lactate. The preparation method includes mixing the alendronate sodium with the bromhexine and the calcium lactate, adding deionized water, increasing the temperature to 75-77 DEG C, and stirring the raw materials for 38-40 minutes to obtain a mixture A; mixing the mixture A with the taurine, performing ultrasonic treatment at the temperature of 58 DEG C for 25 minutes, and performing stirring at the temperature of 63 DEG C until drying to obtain the Chinese and western medicinal composition, wherein the ultrasonic power is 900W. The Chinese and western medicinal composition has a unique formula and low price, is safe and convenient, has better effect than regularly-used western medicine, can effectively treat colon and rectum injuries caused by radiotherapy, has high cure rate and quick action and is free from toxic and side effect; recurrence of the radioactive enteritis is avoided after curing; the preparation process is simple, raw materials are simple and easy to get, production cycle is short, cost is low, and the Chinese and western medicinal composition is suitable for industrial production and large-scale popularization.
Owner:郑州莉迪亚医药科技有限公司
Ambroxol to improve and/or extend healthspan, lifespan and/or mental acuity
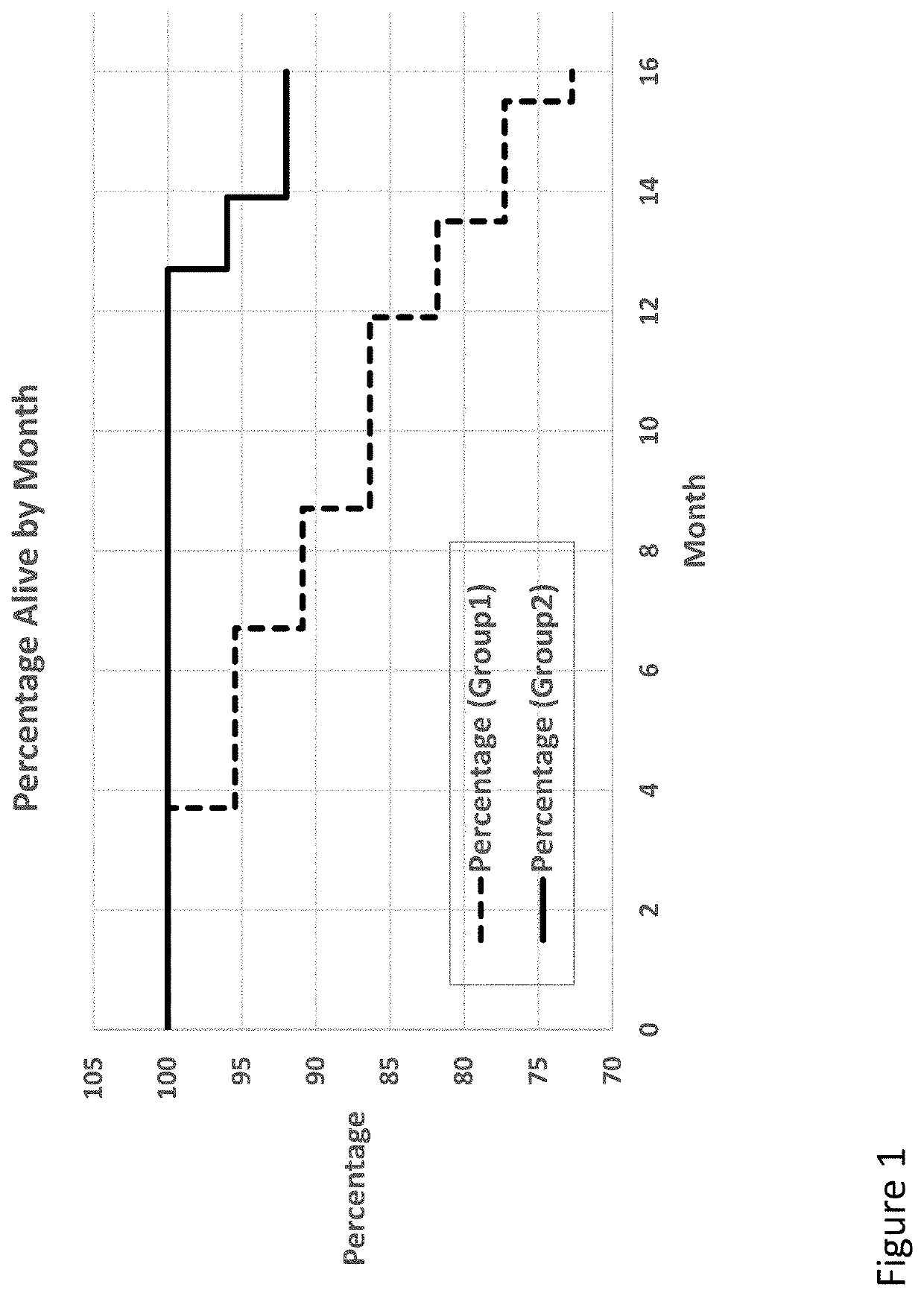
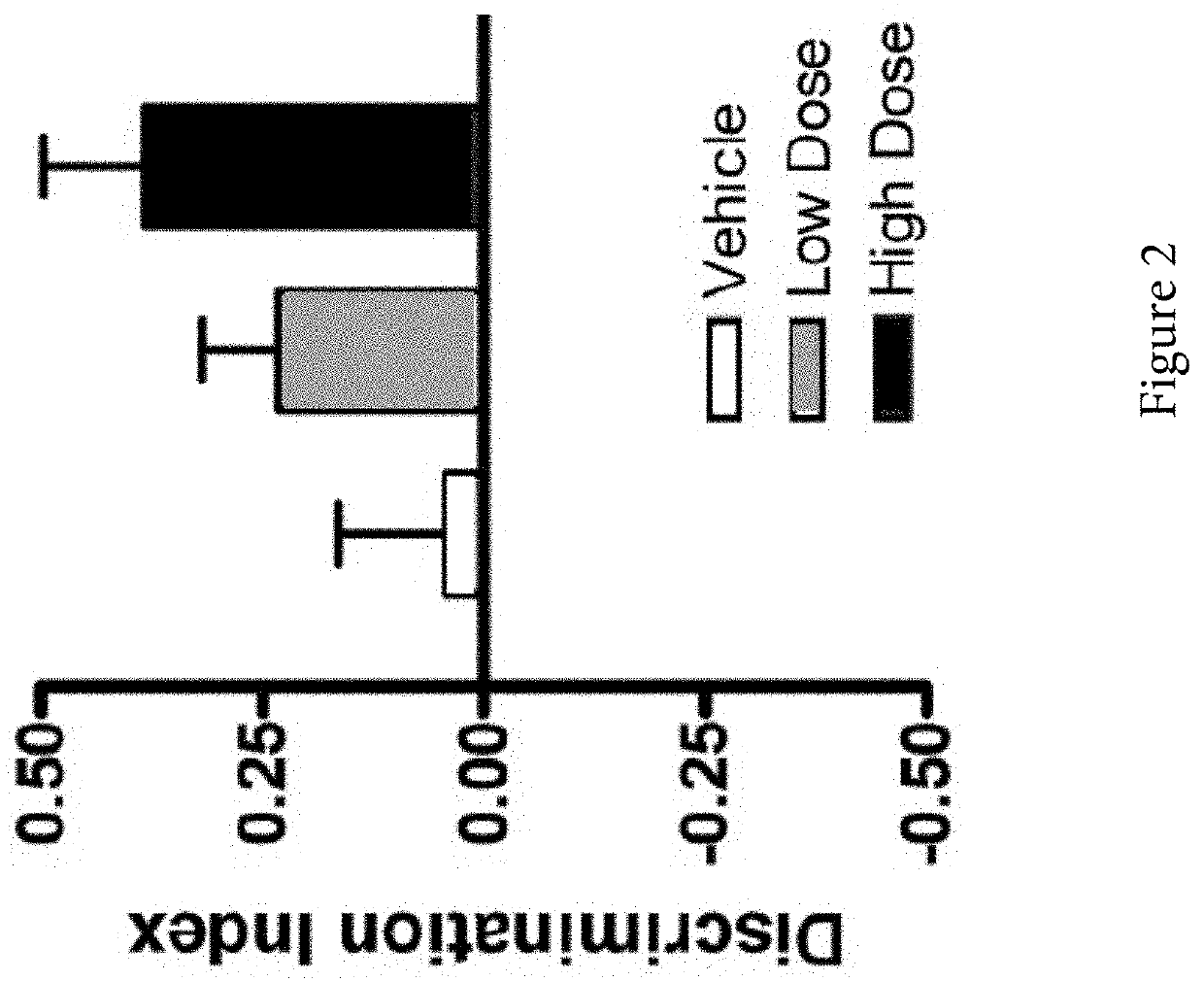
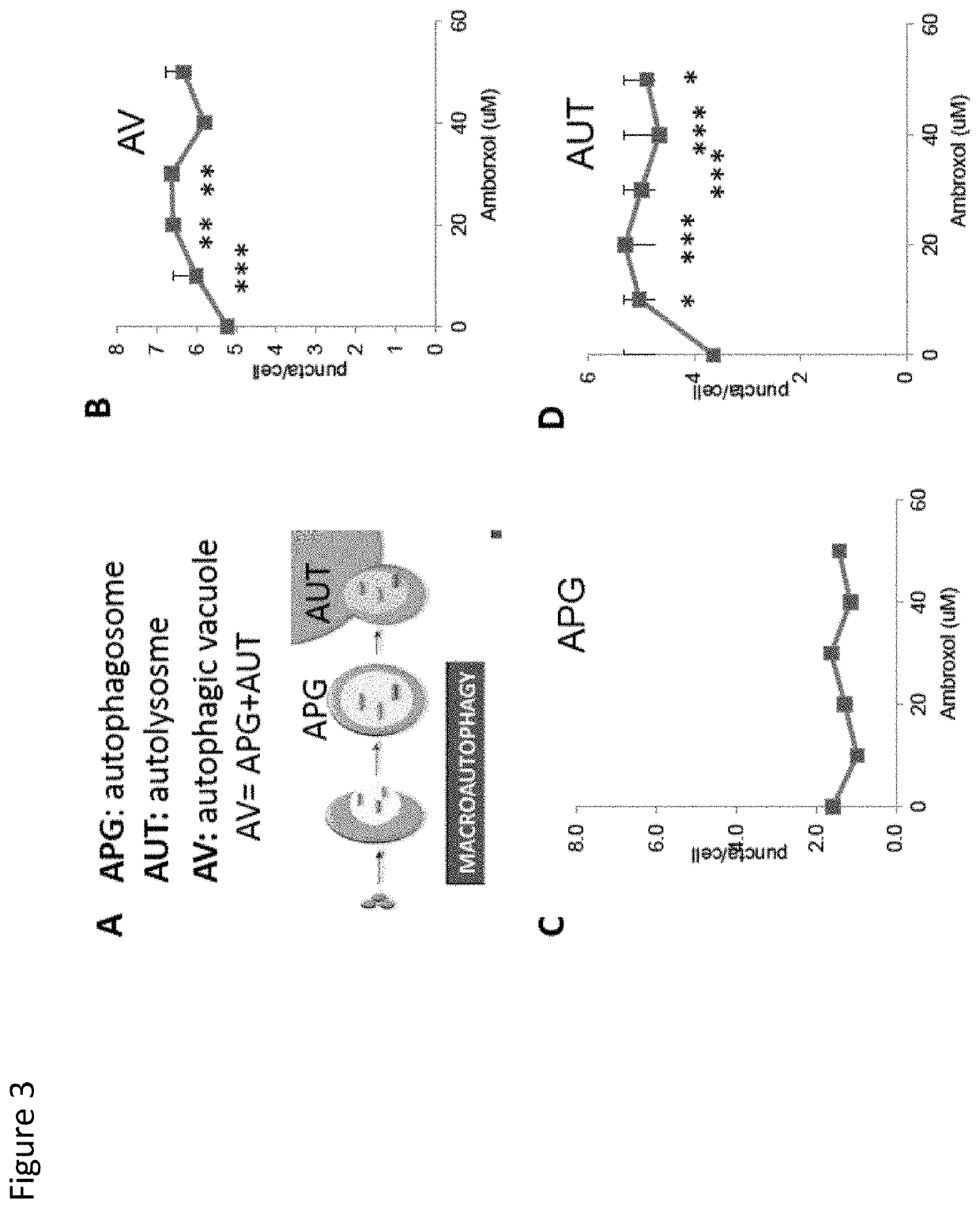
ActiveUS20200215089A1Improve acuityExtend the lifespanOrganic active ingredientsSenses disorderDiseasePhysiology
Compositions and methods for extending life expectancy are described herein. Specifically, ambroxol, ambroxol hydrochloride, and / or bromhexine can be used in a method for (a) treating, inhibiting, or reducing aging of a subject, (b) treating, inhibiting, or reducing an age-related symptom or an age-related disease in a subject, and / or (c) increasing the healthspan, lifespan, and / or mental acuity of a subject.
Owner:NEUERE LLC
Sputum specimen treatment solution and preparation method thereof
InactiveCN108240928AStable in natureEasy to handlePreparing sample for investigationPhosphateBromine
The invention provides a sputum specimen treatment solution which comprises the following components in percentage by weight: 2-8% of phosphate, 2-3% of phenol, 2-3% of bromhexine, 20-40% of ethanol and 46-74% of distilled water. The preparation method comprises the following steps: dissolving weighed phosphate into redistilled water, sequentially adding an ethanol solvent, phenol and bromhexine into the solution, and uniformly mixing, thereby obtaining the product. The sputum specimen treatment solution contains the sterilizing agent phenol, the polysaccharide cellulose decomposing agent bromhexine and the ethanol capable of achieving effects of disinfecting and dissolving, has the advantages of stable property, low cost and the like and has excellent competitive advantages compared withlike products in the market.
Owner:孝感市森茂激光数控设备有限公司
A liquid-based preservation solution for exfoliated cells, its method and kit for making sheets
ActiveCN104642300BAvoid accumulationAvoid lossMicrobiological testing/measurementDead animal preservationPotassiumMonopotassium phosphate
Owner:SHANGHAI YU KANG HOSPITAL
a medicine for asthma
InactiveCN103655927BGood treatment effectWide range of typesBacteria material medical ingredientsRespiratory disorderPotato starchLevamisole
The invention relates to a special-effect drug for treating asthma, which is composed of a drug I, a drug II and a drug III; the drug I comprises the following components by weight: 0.016g of dead bacillus calmette guerin vaccine, 1.071g of potato starch, 0.3g of aminophylline, 0.001g of ketotifen, 0.005g of dioxopromethazine, 0.025g of levomisole, 0.03g of bromhexine and 0.8g of green tea; the drug II comprises the following components by weight: 1.4g of fructus schisandrae, 1.4g of astragalus and 0.3g of cinnamon; and the drug IIT comprises the following components by weight: 1.2g of radix codonopsis, 1.2g of astragalus, 0.3g of epimedium and 0.4g of gypsum. The special-effect drug disclosed by the invention is prepared on the basis of combination of Chinese and western medicine sciences, has remarkable treatment effect, and treats both symptoms and root causes.
Owner:解玉启
Healthcare tea having efficacy of relieving asthma
InactiveCN106819215AStable and significant curative effectNo side effectsPre-extraction tea treatmentRespiratory disorderDiseaseSide effect
The invention relates to a healthcare tea having an efficacy of relieving asthma. The healthcare tea is prepared from the following raw materials by weight percent: aging tea: 10 to 35, dried mint: 5 to 35, dried orange peel: 5 to 25, and dried fructus perillae: 5 to 25. The healthcare tea capable of treating asthma has effects for relieving cough and asthma, eliminating phlegm, clearing the liver, supplementing the kidney and the like. Compared with the same drug such as theophylline sustained-release tablets, compound clorprenaline and bromhexine tablets and the like which have high toxicity and side effects, have taboos, and only can cure the symptoms not the root causes of disease, the healthcare tea can treat both the symptoms and the root causes of disease, is stable and significant in curative effect and has no toxicity and side effects; the healthcare tea is suitable for treating acute / chronic bronchitis, bronchial asthma, lung and heart diseases and the like; and from the cases treated in multiple years, the effective rate of the healthcare tea for relieving the asthma is 98 percent, and the cure rate is about 92 percent.
Owner:广州肇基生物科技有限公司
Western medicine for curing cold of bamboo rat and preparation method of western medicine
InactiveCN106581028ADoes not affect eatingEasy to solveRespiratory disorderHeterocyclic compound active ingredientsRoxithromycinEphedrine
The invention discloses a western medicine for curing a cold of a bamboo rat and a preparation method of the western medicine. The western medicine for curing the cold of the bamboo rat comprises the following components by weight: 5-8 parts of chlortrimeton, 20-25 parts of calcium gluconate, 4-8 parts of cetirizine, 10-15 parts of ephedrine, 2-8 parts of acetylspiramycin, 4-10 parts of roxithromycin, 15-20 parts of promethazine, 8-15 parts of omeprazole, 10-15 parts of etamsylate, 8-10 parts of cedilanid, 2-6 parts of bromhexine, and 2-8 parts of aminophylline. Compared with the prior art, the western medicine is mixed into a feed to feed the bamboo rat and has good effects of preventing and treating the cold of the bamboo rat, the marked effective rates of the prevention and the treatment are up to 99% and 95%, and in addition, the western medicine has no strong flavor of the western medicine, is mixed into the feed to feed the bamboo rat, does not affect the feeding of the bamboo rat, and is convenient to use.
Owner:南宁市浩特竹鼠养殖场(微型企业)
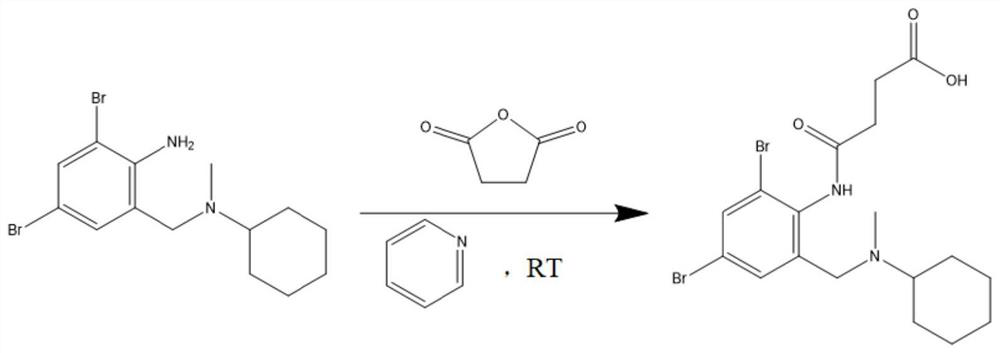
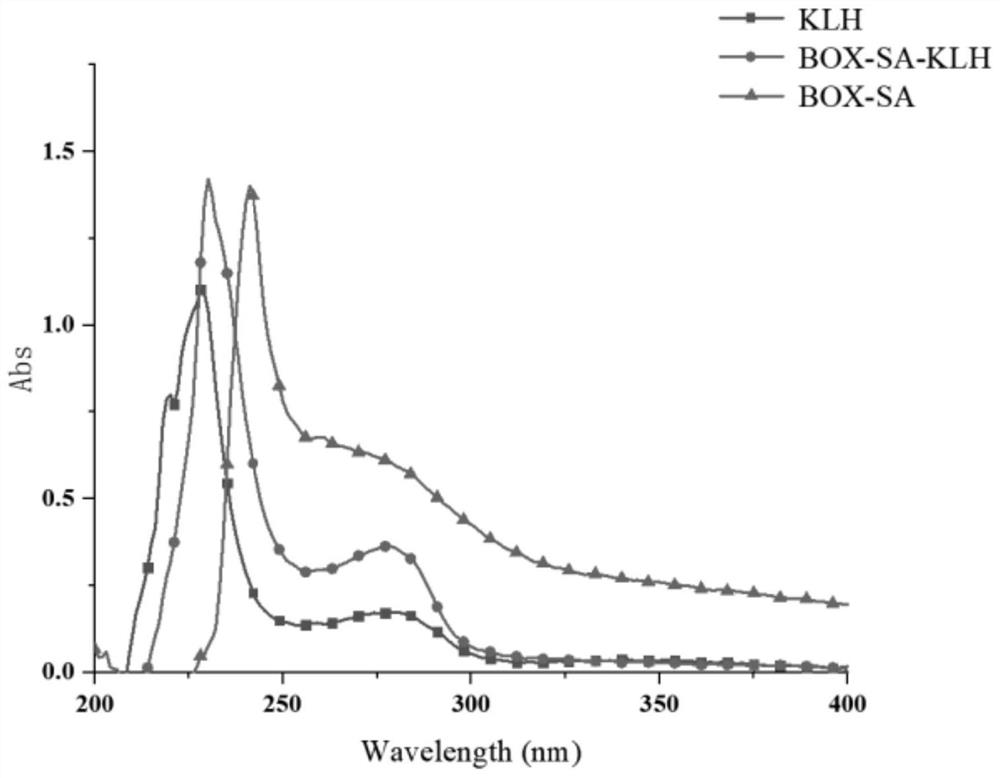
Bromhexine hapten, bromhexine artificial antigen, bromhexine antibody and preparation method and application of bromhexine artificial antigen
ActiveCN114644572AHigh sensitivityStrong specificityOrganic compound preparationTransferrinsImmune profilingBiochemistry
The invention discloses a bromhexine hapten, an artificial antigen, an antibody as well as a preparation method and application of the bromhexine hapten and the artificial antigen. The structural formula of the bromhexine hapten is shown as a formula (I), the hapten is used for preparing an artificial antigen and an antibody for detecting bromhexine, the antibody has high-sensitivity and high-specificity recognition capability on bromhexine, the semi-inhibitory concentration is 4.57 ng / mL, the lowest detection limit is 0.41 ng / mL, the quantitative detection range is 0.85-35.11 ng / mL, and the hapten can be used for detecting bromhexine. A core raw material is provided for establishing an immunoassay method for specifically detecting bromhexine. Meanwhile, the invention also establishes a bromhexine immunoassay method with higher specificity and sensitivity, in addition, a kit for detecting bromhexine residues is developed by utilizing the artificial antigen and the antibody, and the kit has the characteristics of high specificity, high sensitivity, high precision, high accuracy and the like.
Owner:SOUTH CHINA AGRI UNIV
Crystal form and preparation method of salt formed by bromhexine and fumaric acid
ActiveCN111662186BImprove stabilityImprove solubilityAmino compound purification/separationOrganic compound preparationDrugs preparationsCombinatorial chemistry
The invention discloses a crystal form of a salt formed of bromhexine and fumaric acid and a preparation method thereof. The drug co-crystal uses bromhexine as the active ingredient of the drug and fumaric acid as the reactant to form a salt in a mixed solvent; the obtained crystal form is monoclinic P2 1 / n space group, the basic structural units are composed of a protonated positively charged bromhexine molecule and a deprotonated negatively charged fumaric acid molecule, and the basic structural units are passed through N‑H…O , C‑H…O, O‑H…O hydrogen bonds are combined to form a three-dimensional space structure. The new crystal form of bromhexine fumarate of the present invention can maintain good storage stability, has improved solubility, has no hygroscopicity, and provides convenience for transportation, production, and pharmaceutical preparations.
Owner:SOUTHEAST UNIV CHENGXIAN COLLEGE
A kind of bromhexine hydrochloride injection and its preparation method and application
ActiveCN104306329BImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientBULK ACTIVE INGREDIENT
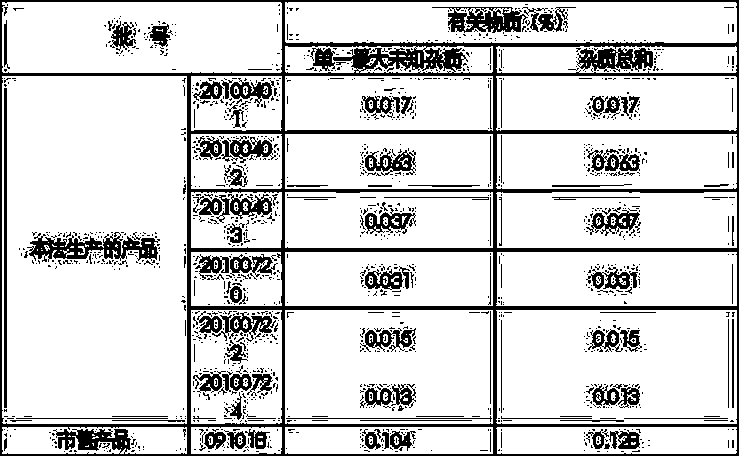
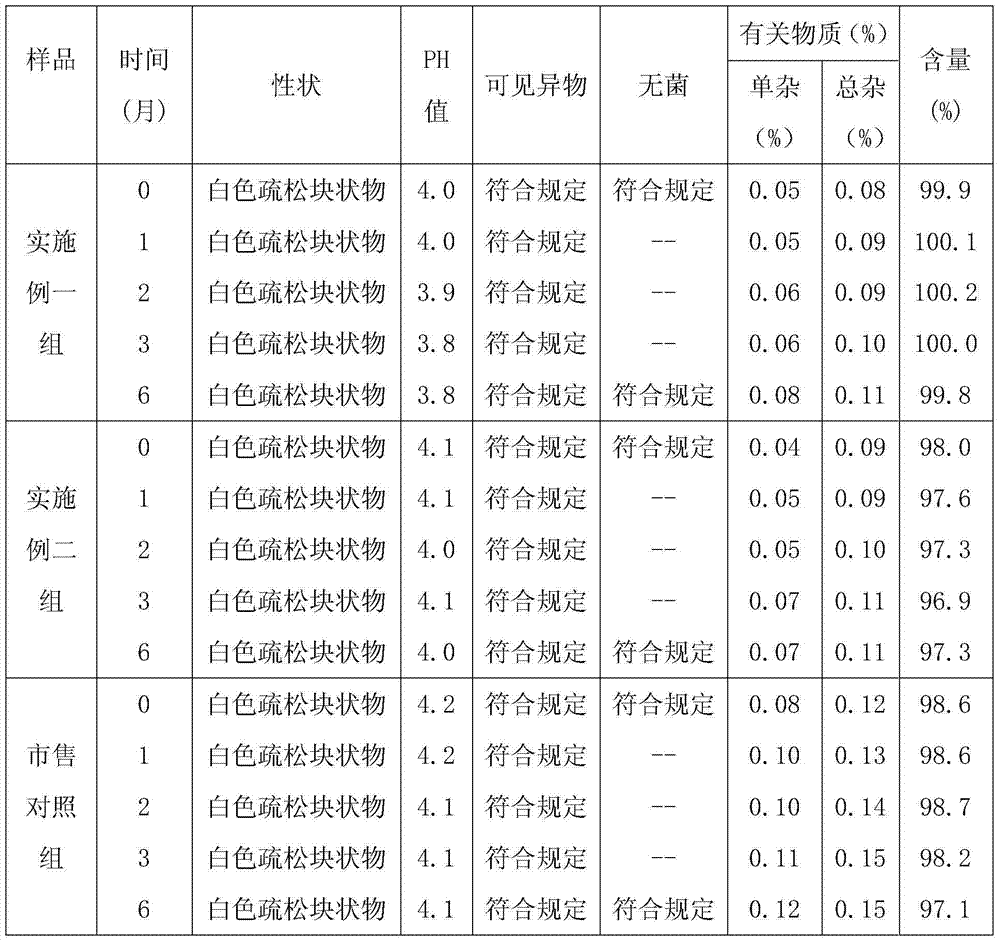
The invention provides a bromhexine hydrochloride injection and a preparation method thereof. The bromhexine hydrochloride injection of the present invention is composed of bromhexine hydrochloride active ingredient, tartaric acid, stabilizer, sodium hydroxide and water for injection. The bromhexine hydrochloride injection of the invention has the advantages of low content of related substances, high stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Bromhexine for the treatment of pain
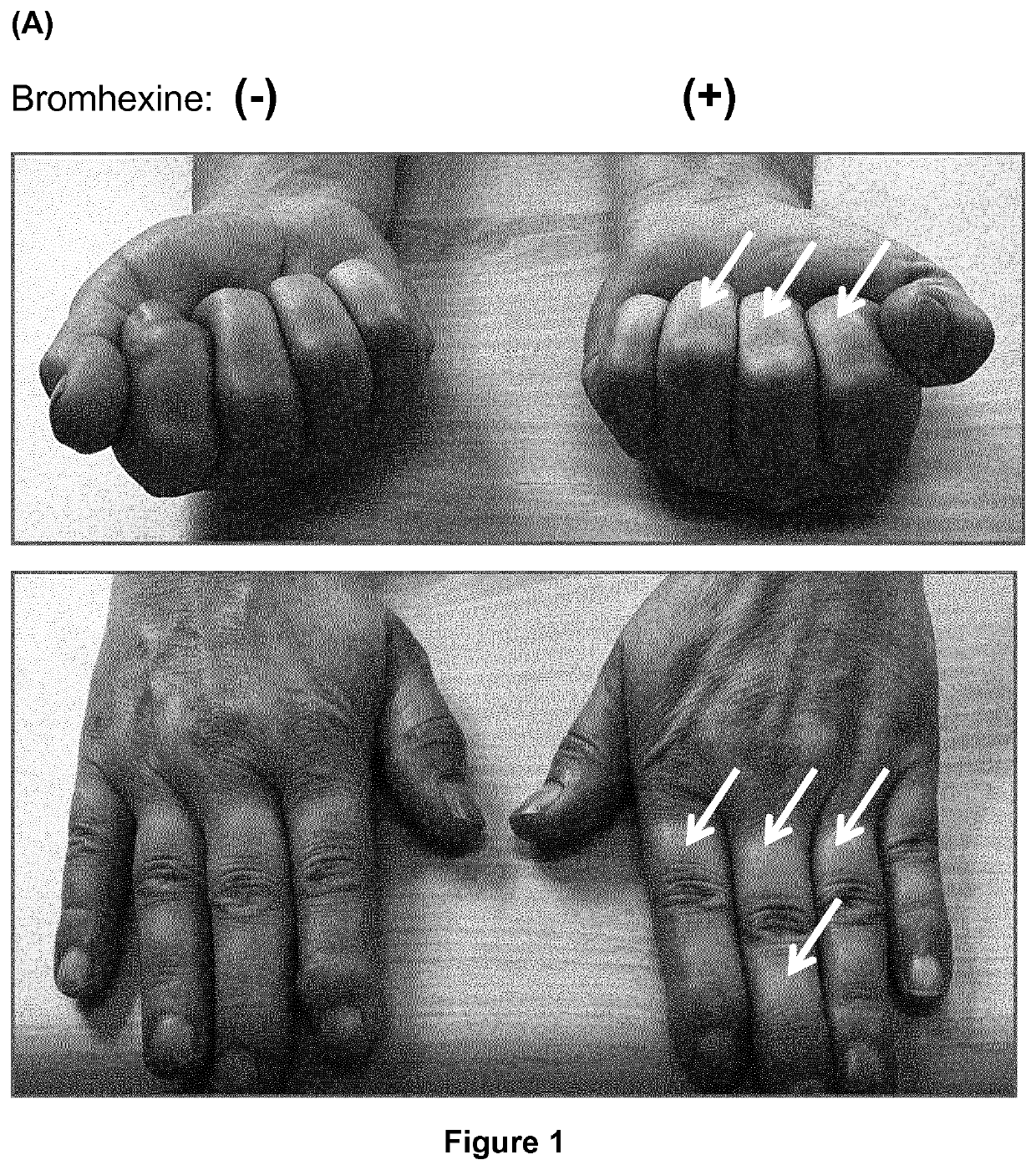
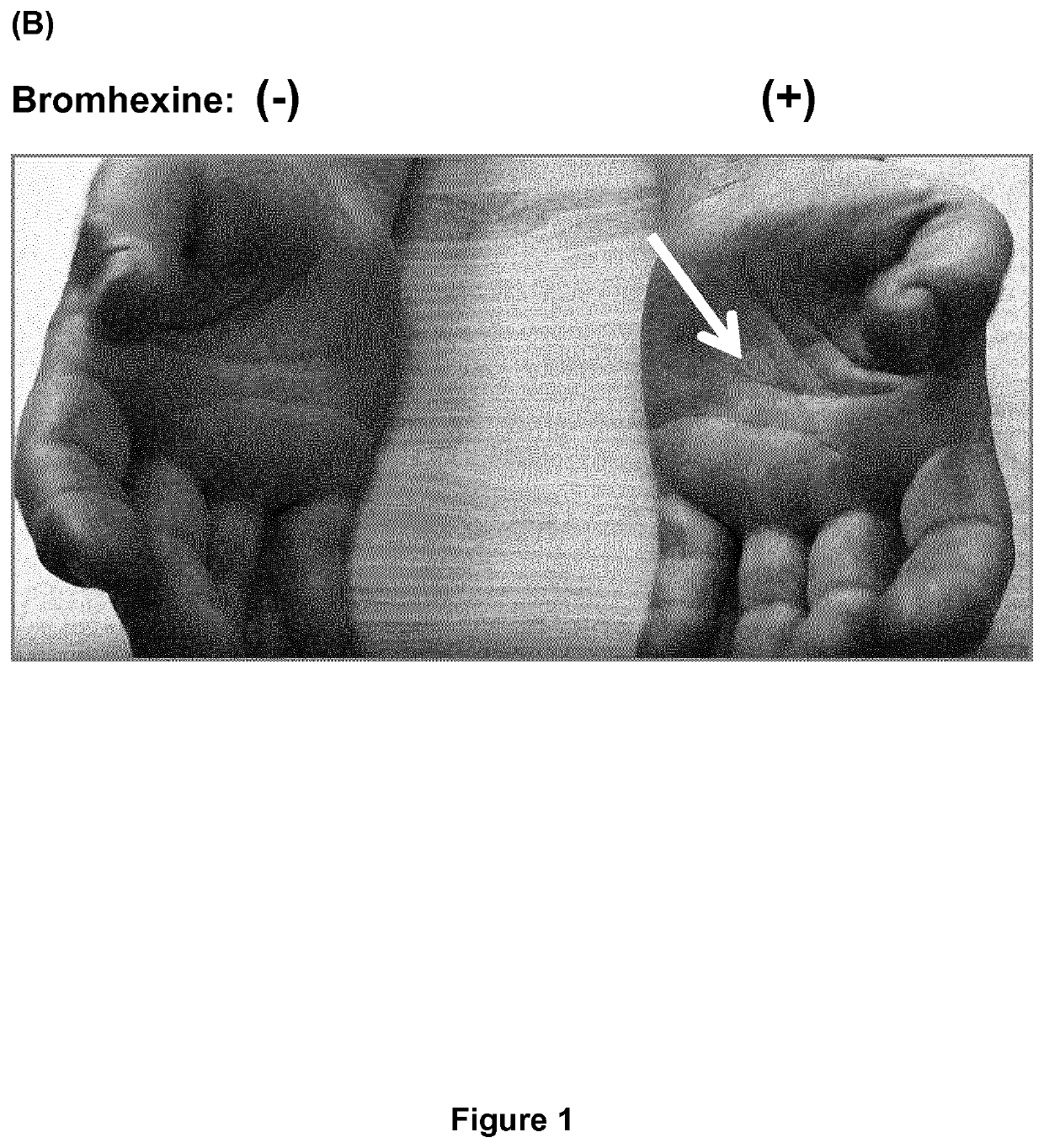
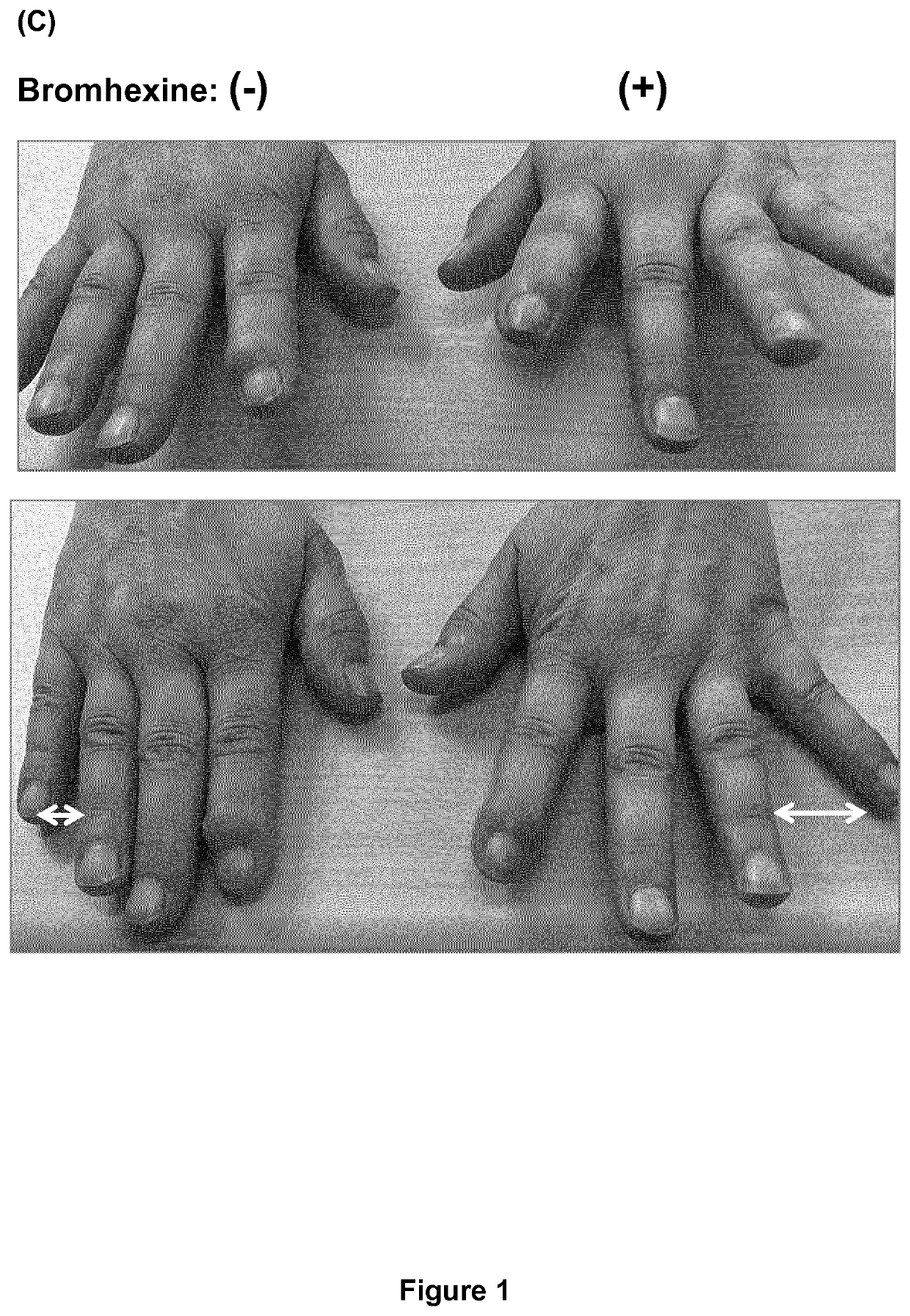
PendingUS20200197330A1Avoid excess performanceEasy to closeOrganic active ingredientsAntipyreticBromhexineTreatment pain
The invention relates to bromhexine or a salt thereof for treating acute or chronic pain in a patient. In particular, the invention relates to bromhexine or a salt thereof for use in treating nociceptive pain, neuropathic pain and or dysfunctional pain. The invention further relates to a topical pharmaceutical composition comprising bromhexine or a salt thereof and to a composition or a topical pharmaceutical composition comprising bromhexine or a salt thereof for treating acute or chronic pain.
Owner:KERN KAI UWE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com