Crystal form and preparation method of salt formed by bromhexine and fumaric acid
A technology of fumaric acid and bromhexine, which is applied in the crystal form and preparation of salt, can solve the problems of transportation, storage and use restrictions, achieve the effect of improving stability and solubility, and avoiding strong corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the crystal form of the salt formed by the bromhexine and fumaric acid of the foregoing embodiment comprises the following steps:
[0029] Put bromhexine and fumaric acid into a mixed solvent of methanol and organic solvent. The above-mentioned reactant is placed in an ultrasonic generator, and ultrasonically treated at ambient temperature (15-25° C.) for 10-30 min. After filtering and standing for volatilization, colorless and transparent massive crystals are precipitated. After filtering and washing the massive crystals, the crystals of the salt formed by bromhexine and fumaric acid are obtained after drying, which is to obtain the crystal form described in the application of the present invention.
[0030] In the above embodiments, the organic solvent is one or any combination of ethanol, acetonitrile, ethyl acetate and tetrahydrofuran.
[0031] As a preferred example, the molar ratio of bromhexine and fumaric acid is 1:1-2.
[0032] As a ...
Embodiment 1
[0045] At room temperature, weigh 200 mg (0.532 mmol) of bromhexine and 62 mg (0.534 mmol) of fumaric acid into the reactor, add 32 mL of ethyl acetate and 8 mL of methanol, place the above reactants in an ultrasonic generator, and ultrasonically 30min. Filter the solution into a clean and dry 50ml beaker with analytical filter paper, and let it stand at room temperature for volatilization. After about 2 weeks, a colorless transparent block crystal is precipitated, and the crystal form described in the application of the present invention is obtained.
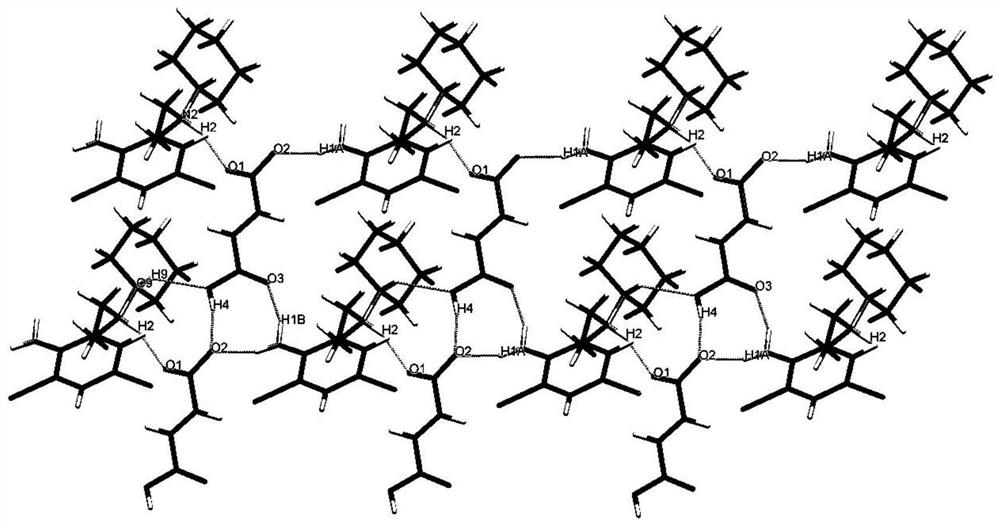
[0046] Adopt the bulk crystal obtained in embodiment 1 to carry out single crystal diffraction experiment, obtain figure 1 . The obtained crystal form is monoclinic P2 1 / n space group, the basic structural unit is composed of a protonated positively charged bromhexine molecule and a deprotonated negatively charged fumaric acid molecule, and the basic structural unit is passed through N-H...O, C-H...O, O-H...O hydrogen bonds...
Embodiment 2
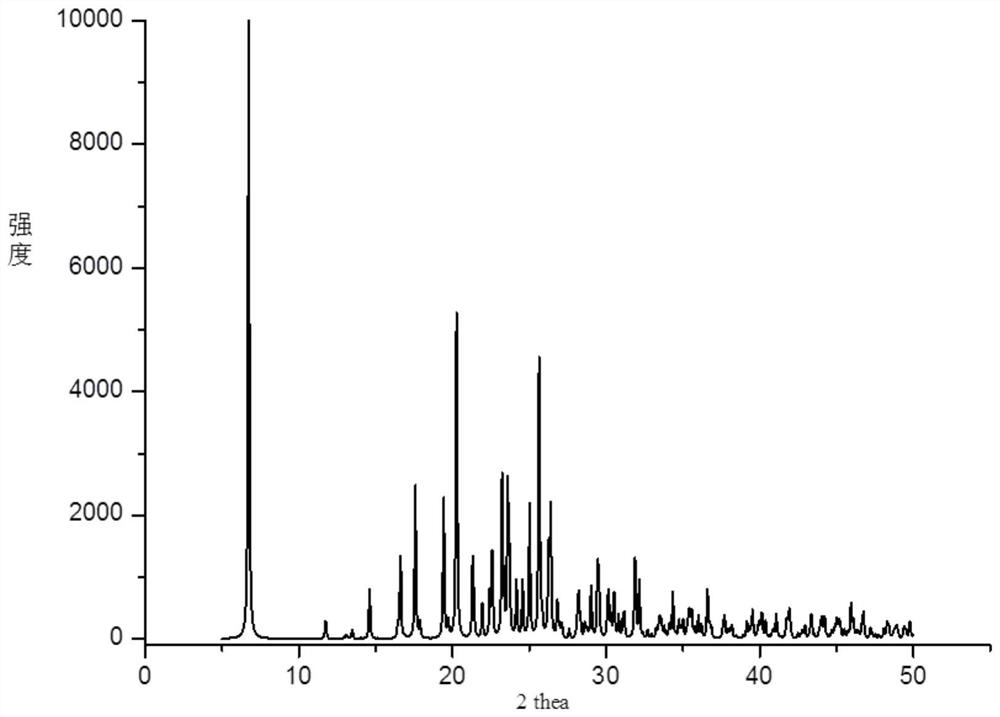
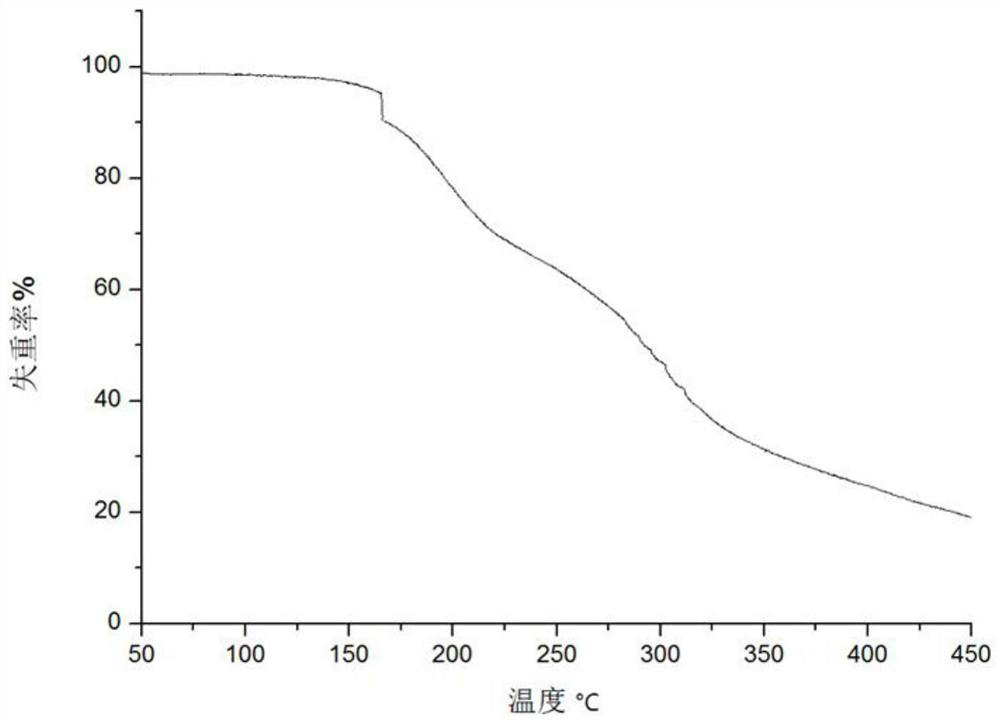
[0048] At room temperature, weigh 100 mg (0.266 mmol) of bromhexine and 34 mg (0.293 mmol) of fumaric acid into a reactor, add 20 mL of acetonitrile and 2 mL of methanol, place the above reactants in an ultrasonic generator, and ultrasonicate for 15 min at ambient temperature. Filter the solution into a clean and dry 50mL beaker with analytical filter paper, and let it stand at room temperature for volatilization. After about 2 weeks, a colorless and transparent block crystal is precipitated. The crystallographic characteristics, PXRD spectrum and thermogravimetric analysis spectrum of the bulk crystal in Example 2 are consistent with the crystal form obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com