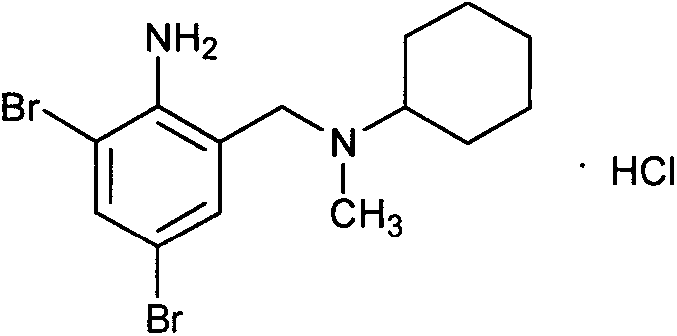
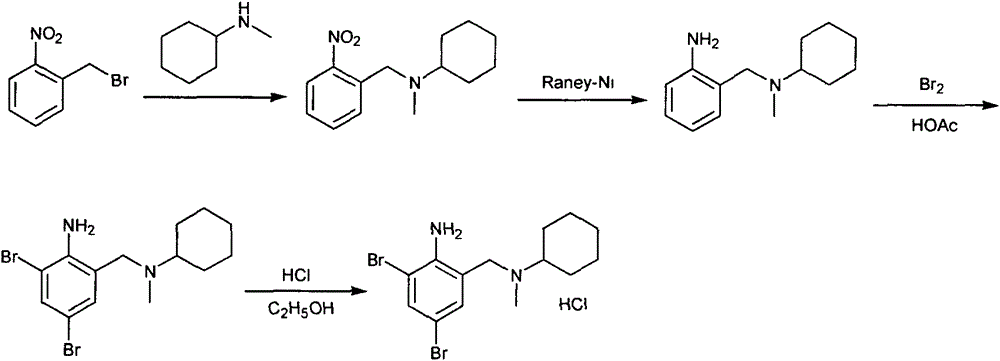
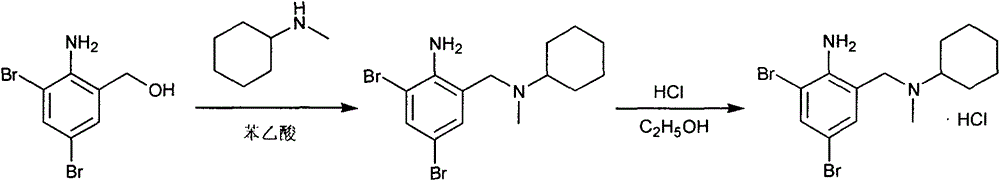
Novel method for preparing bromhexine hydrochloride
A technology of bromhexine hydrochloride and its production method, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of difficult refining and removal of impurities, corrosion of workshop equipment, and low overall yield, and achieve technological The effect of simple and feasible operation, short synthesis steps and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 83.7g of 2-amino-3,5-dibromobenzaldehyde, 59.5g of cyclohexylamine, and 600mL of dichloromethane were heated to dissolve, 1.5g of formic acid was added, refluxed for 16h, TLC showed that the reaction was complete, the solvent was evaporated under reduced pressure, and Methanol 160mL, stirred at room temperature for 30min, solid precipitated, placed in refrigerator for 30min, filtered, washed with cold methanol (30mL×2), dried at 65°C under normal pressure to obtain 100.5g, molar yield: 91.2%.
Embodiment 2
[0042] 83.7g of 2-amino-3,5-dibromobenzaldehyde, 59.5g of cyclohexylamine, and 600mL of toluene, heated to reflux and separated water for 24h, TLC showed that the reaction was complete, evaporated the solvent under reduced pressure, added 160mL of methanol, filtered, and stirred at room temperature for 30min , precipitated solid, placed in the refrigerator for 30min, filtered, washed with cold methanol (30mL×2), dried at 65°C under normal pressure to obtain 100.5g, molar yield: 91.2%.
[0043] (2) Preparation of secondary amine
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com