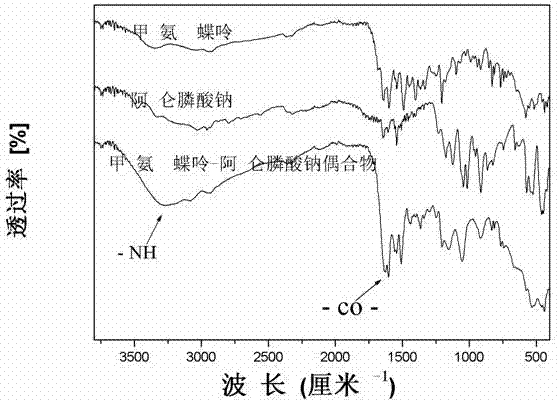
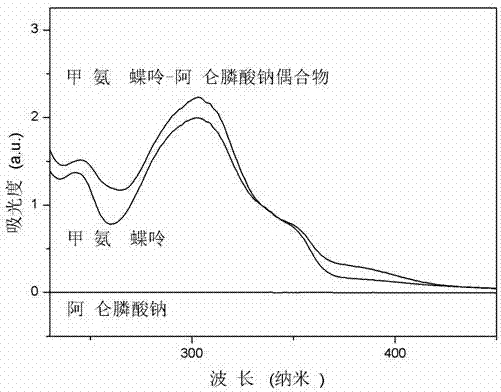
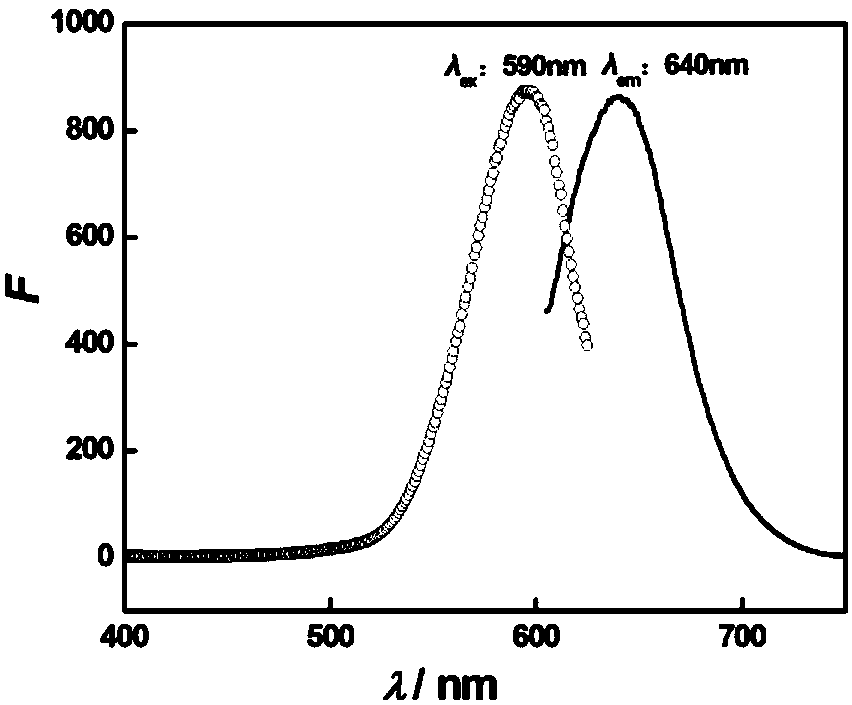
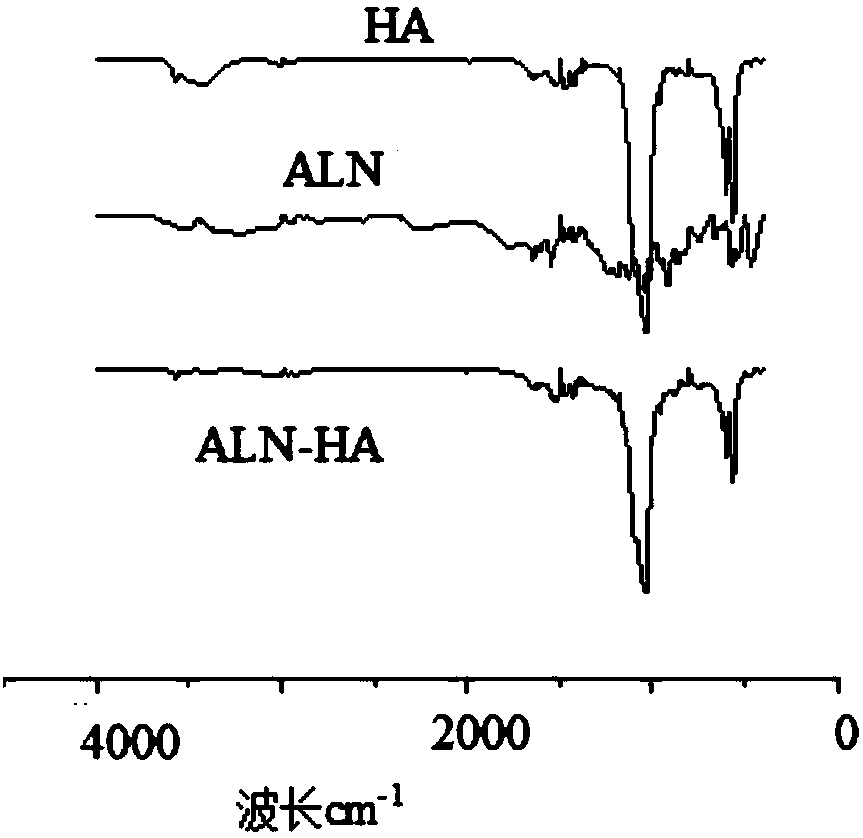
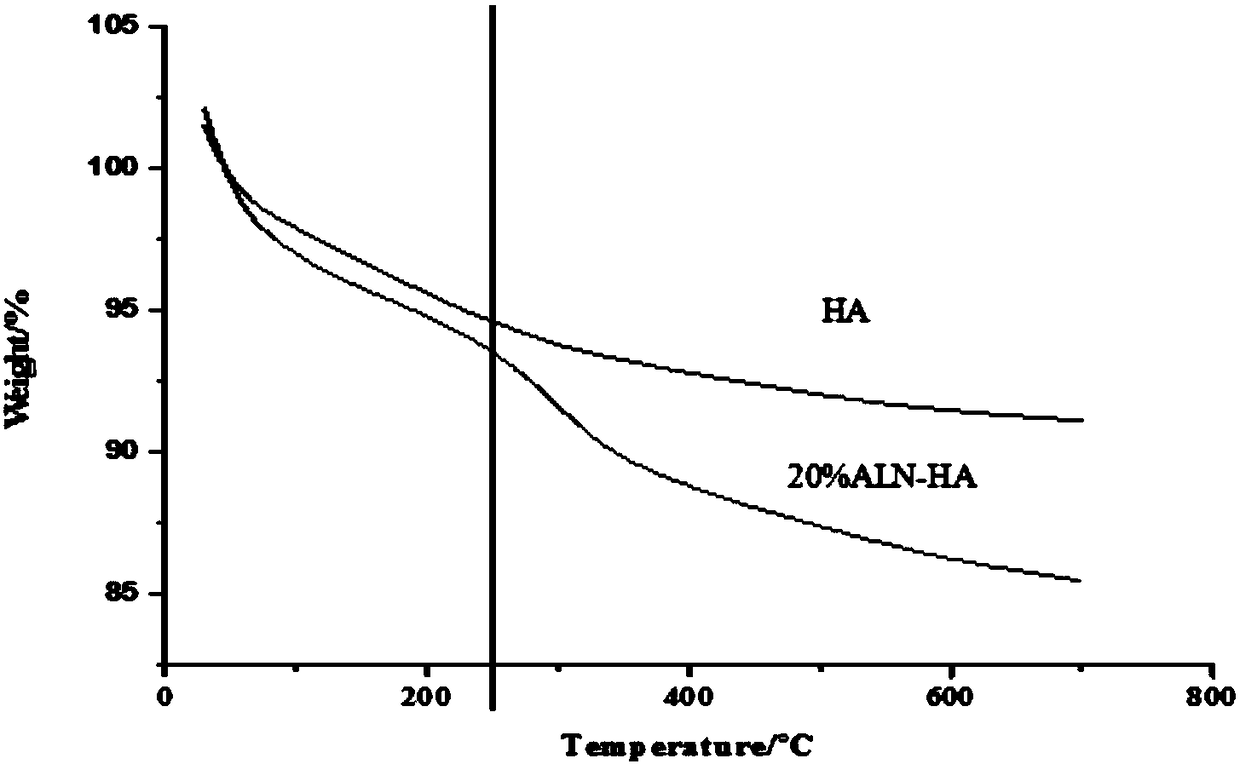
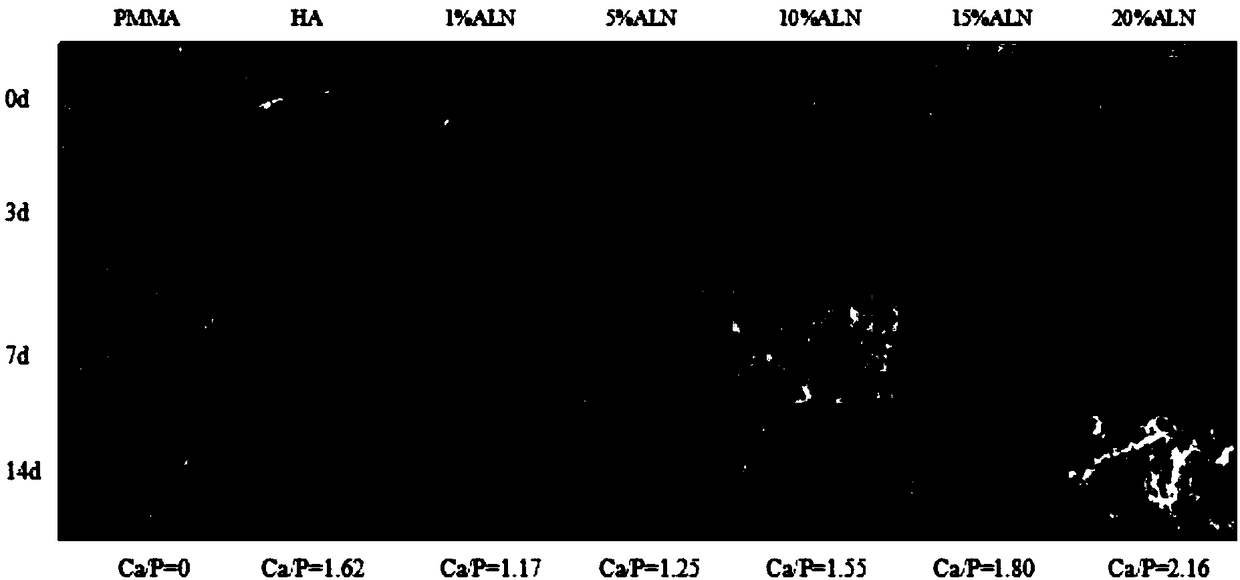
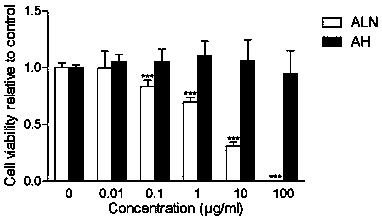
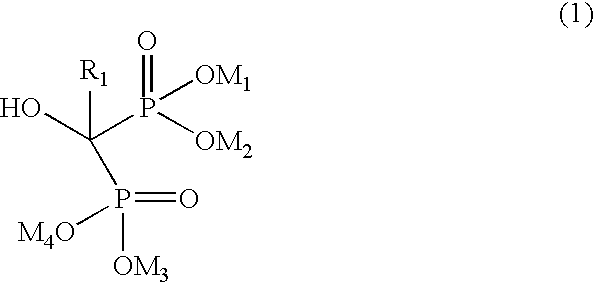Patents
Literature
62 results about "Alendronate Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
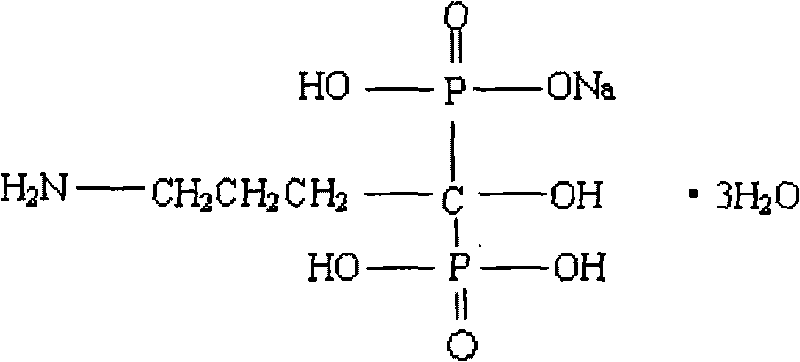
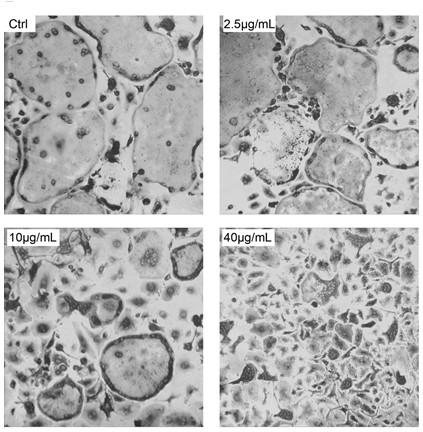
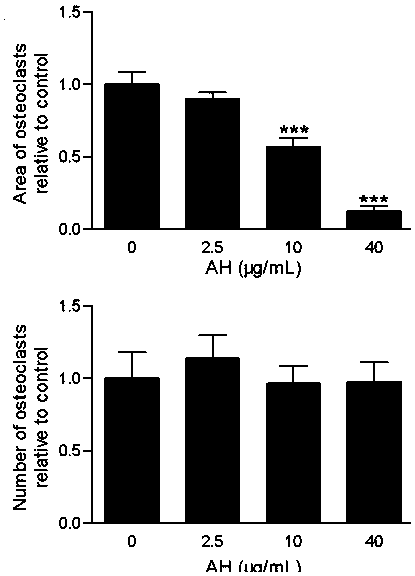
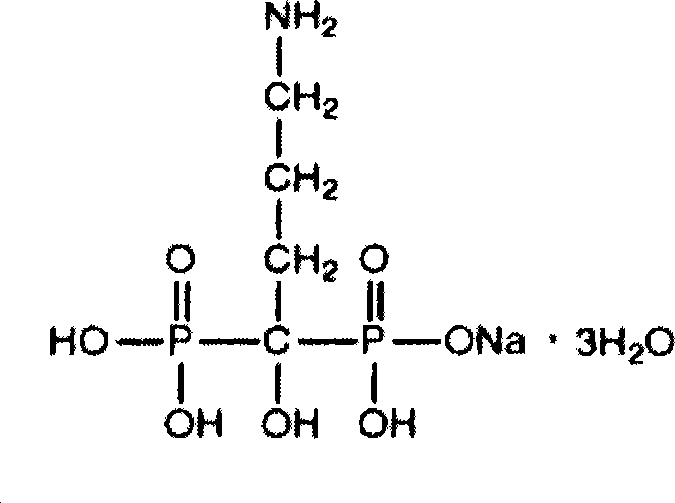
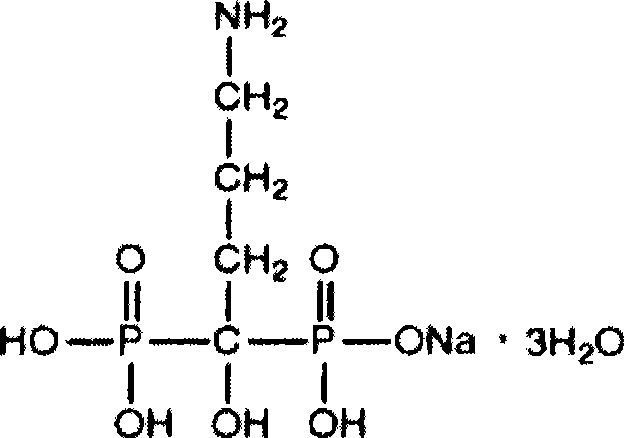
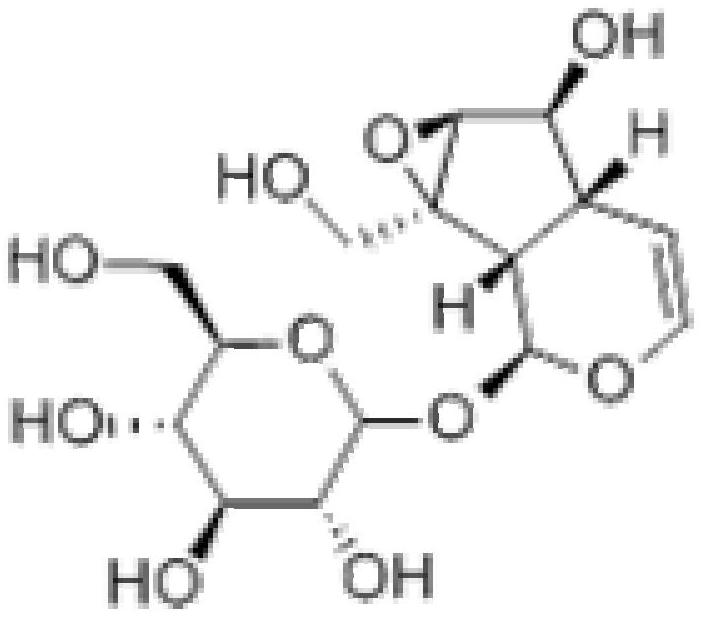
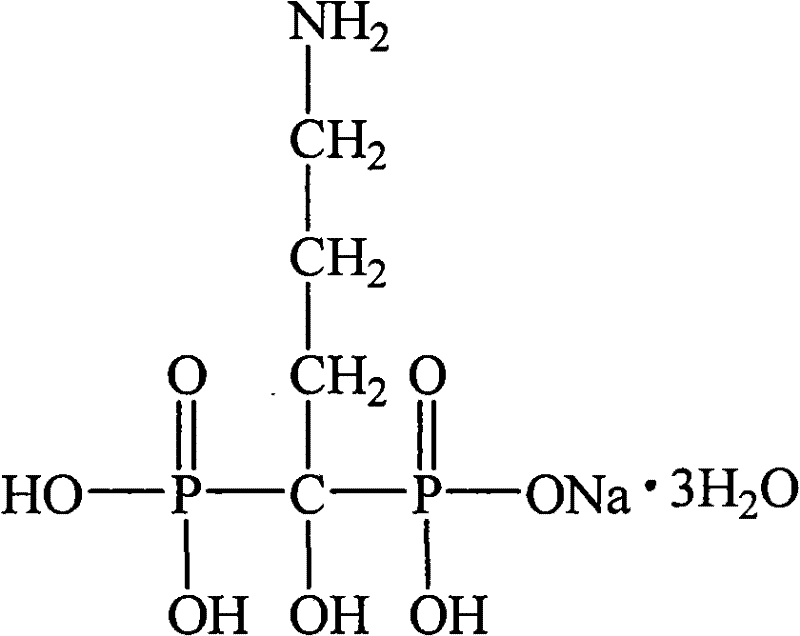
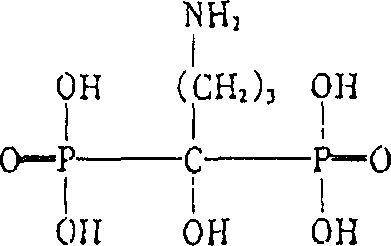
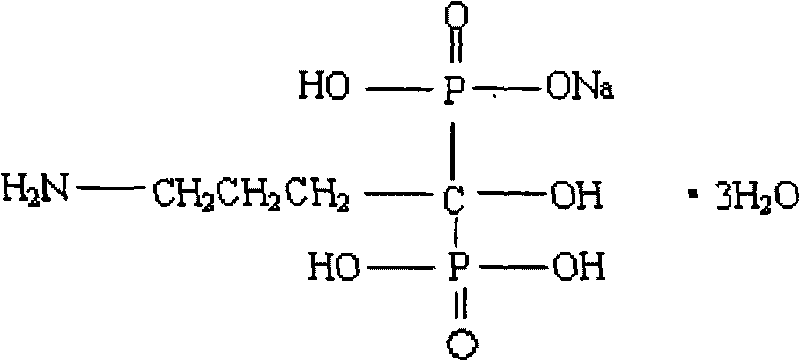
The sodium salt of alendronate, a second generation bisphosphonate and synthetic analog of pyrophosphate with bone anti-resorption activity. Alendronate sodium binds to and inhibits the activity of geranyltranstransferase (farnesyl pyrophosphate synthetase), an enzyme involved in terpenoid biosynthesis. Inhibition of this enzyme prevents the biosynthesis of isoprenoid lipids (FPP and GGPP) that are donor substrates of farnesylation and geranylgeranylation during the post-translational modification of small GTPase signalling proteins, which is important in the process of osteoclast turnover. As a result, osteoclast activity is inhibited and bone resorption and turnover are reduced.
Preparation of biphosphonic acids and salts thereof
InactiveUS7332603B2Avoid disadvantagesPromote recoveryPhosphorus organic compoundsPhosphorous acidSolvent
A process for preparing alendronate sodium includes the reaction of gammabutyric acid with phosphorous acid and phosphorus trichioride and subsequent treatment with an aqueous solution of an alkali metal hydroxide, in which the reaction is conducted in liquid ionic solvents.
Owner:CHEMI SPA
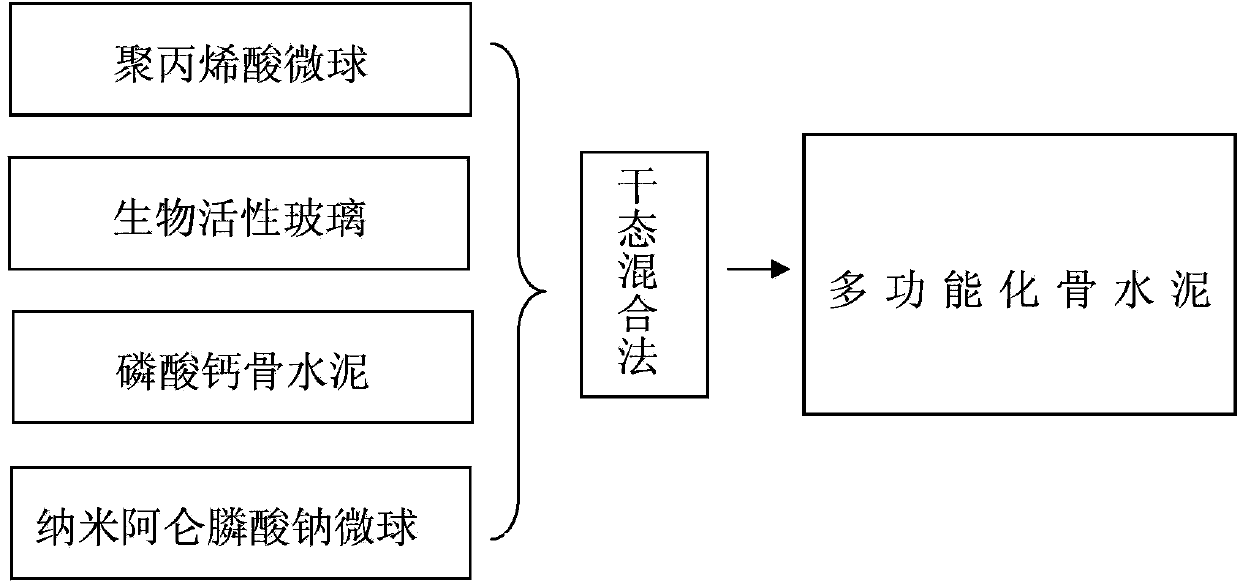
Multifunctional medical biological bone cement
The invention discloses multifunctional medical biological bone cement. The multifunctional medical biological bone cement is prepared from the following raw materials in percentage by mass: 5%-15% of polyacrylic acid microspheres, 20% of bioactive glass, 64.9%-74.9% of calcium phosphate bone cement and 0.1% of nanometer alendronate sodium microspheres, wherein the sum of the mass percentages of the raw materials is 100%. The multifunctional medical biological bone cement has the function of water absorption self-expansion; the using amount of the bone cement can be reduced, and further the leakage of the bone cement is reduced; the multifunctional medical biological bone cement has relatively good tissue compatibility, can be used for inducing the formation of new bones and enhancing the strength of vertebral bodies, is degradable in vivo without in vivo foreign matter residues; and meanwhile, the controlled-release alendronate sodium has the effects of resisting osteoporosis, reducing the absorption of bones and furthering enhancing the vertebral bodies. Therefore, the multifunctional medical biological bone cement has the advantages of little consumption, promotion of bone formation and bone absorption resistance when applied to vertebroplasty.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
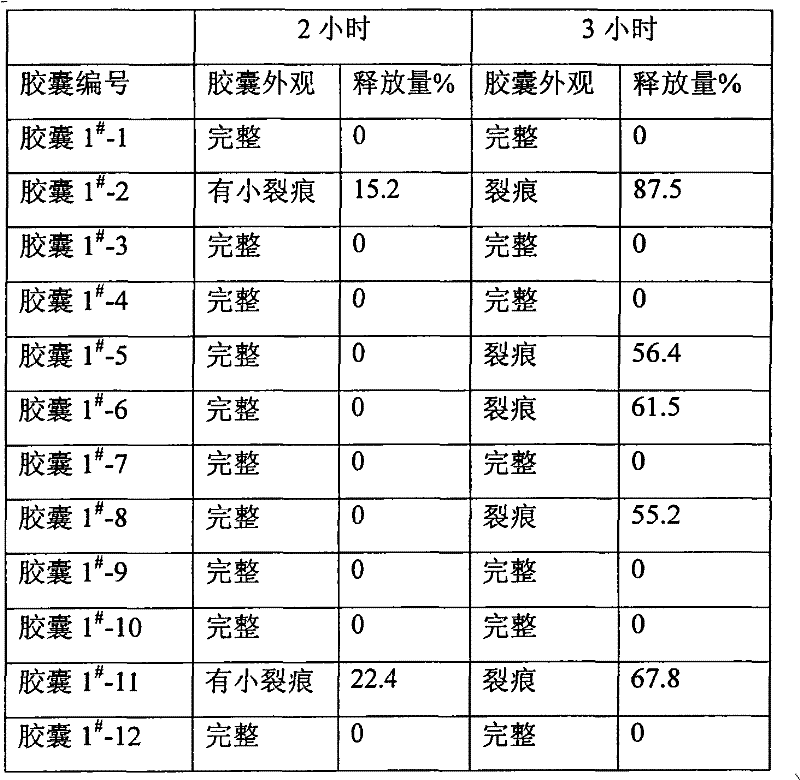
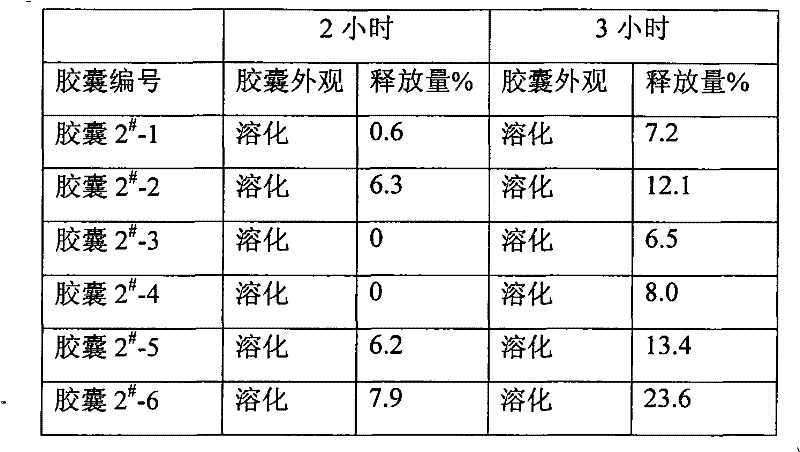
Enteric coated formulation for bisphosphonic acids and salts thereof
Pharmaceutical compositions and methods of using the composition are provided. The pharmaceutical composition comprises an inert core surrounded by an active coating containing one or more bisphosphonic acids or salts thereof, a seal coating surrounding the active coating and an enteric coating surrounding the seal coating. Alendronic acid and alendronate sodium trihydrate are the preferred active ingredients. The composition may be provided in the form of pellets in a capsule or Peltabs. The invention further provides methods for the treatment of disorders caused by the abnormal dissolution or deposition of calcium salts using the inventive compositions.
Owner:CIPLA LTD
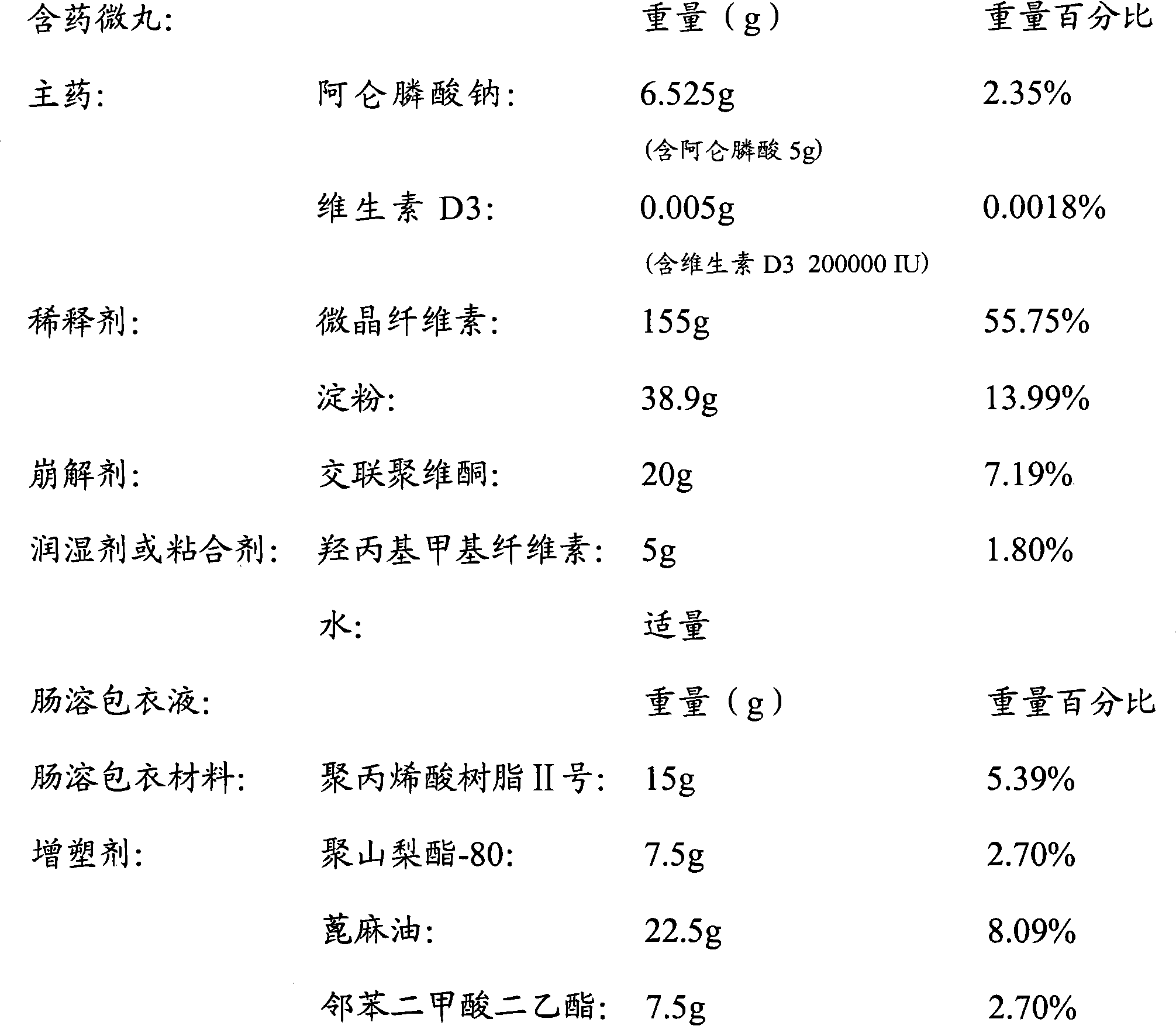
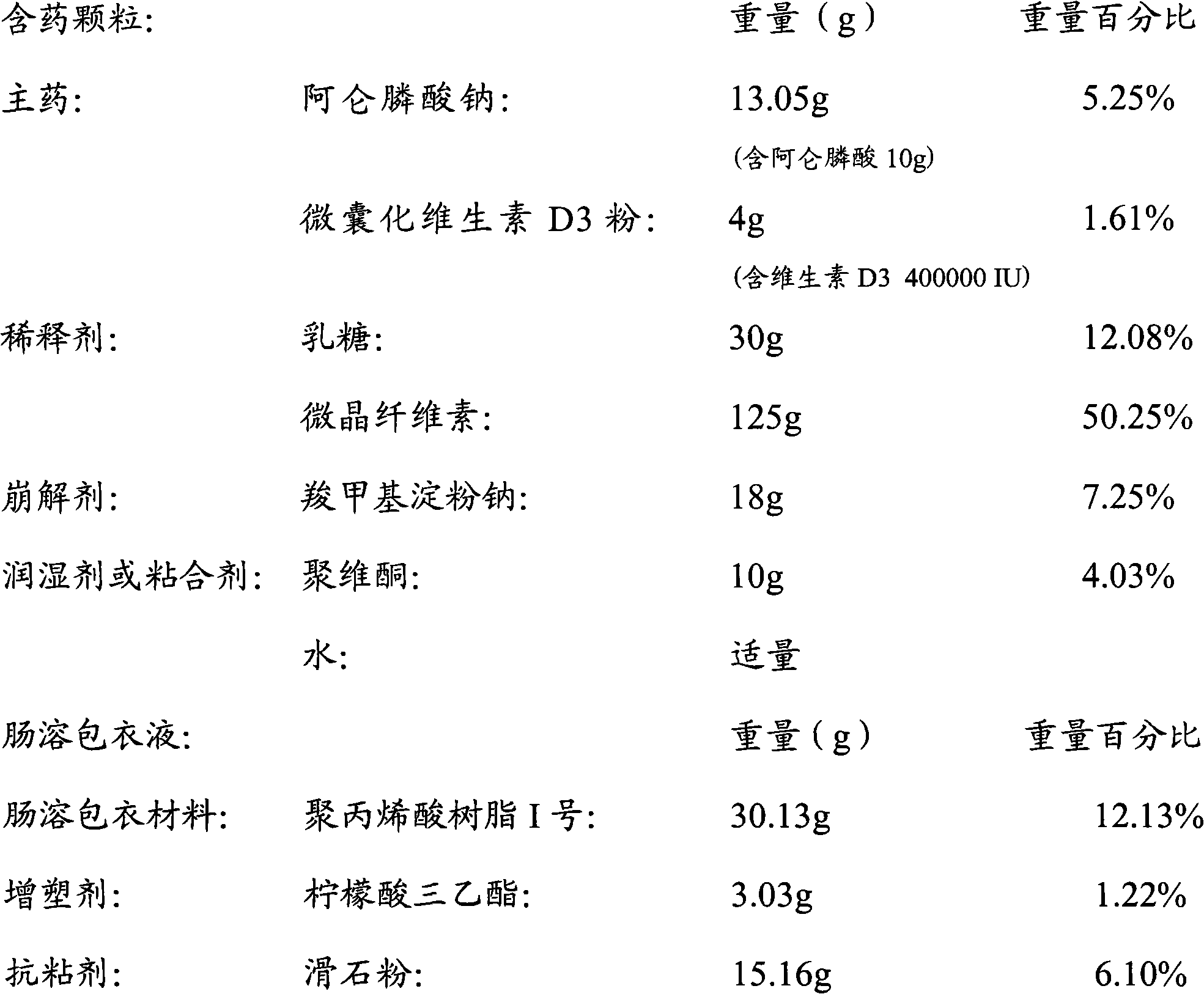
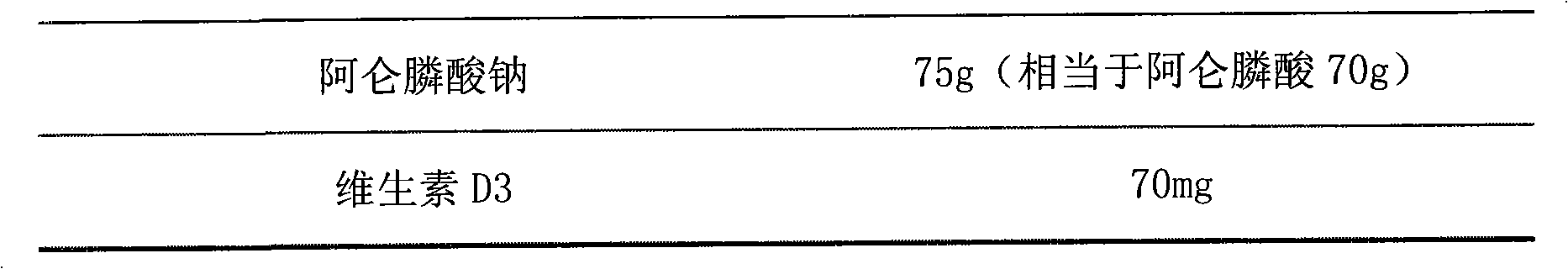
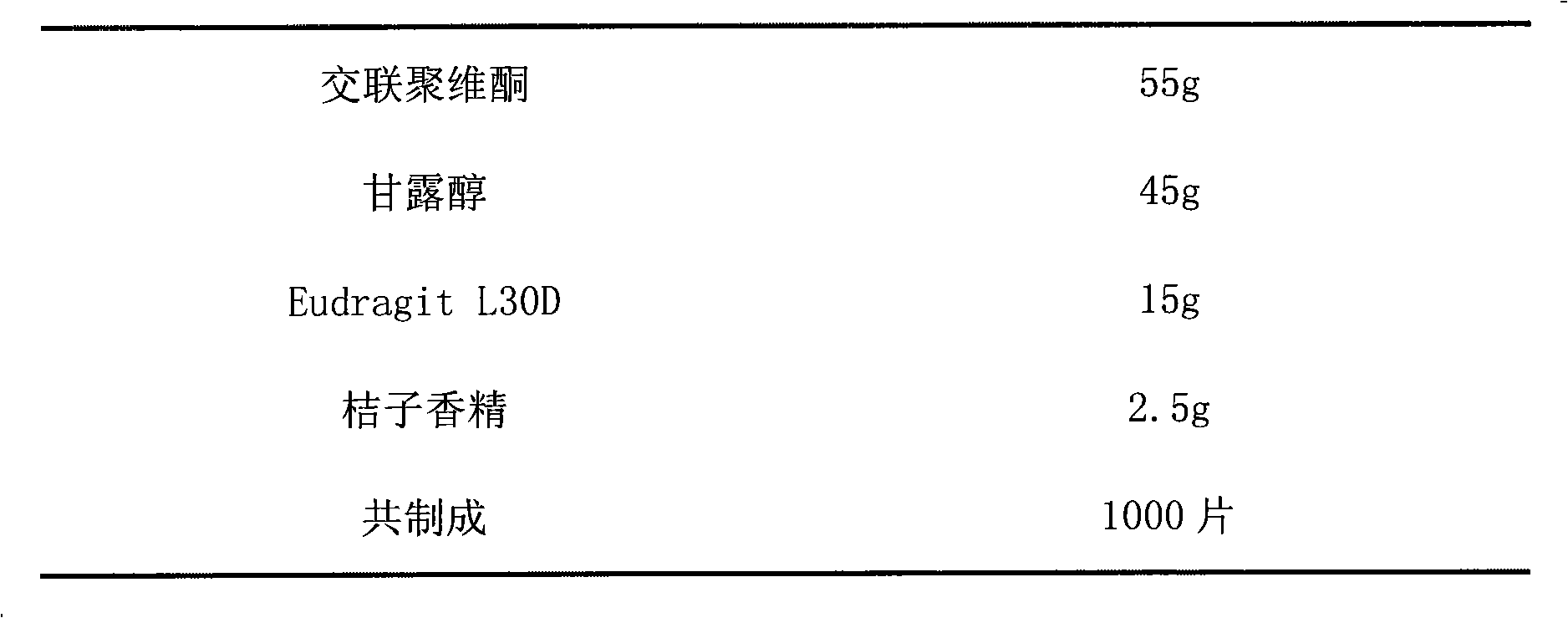
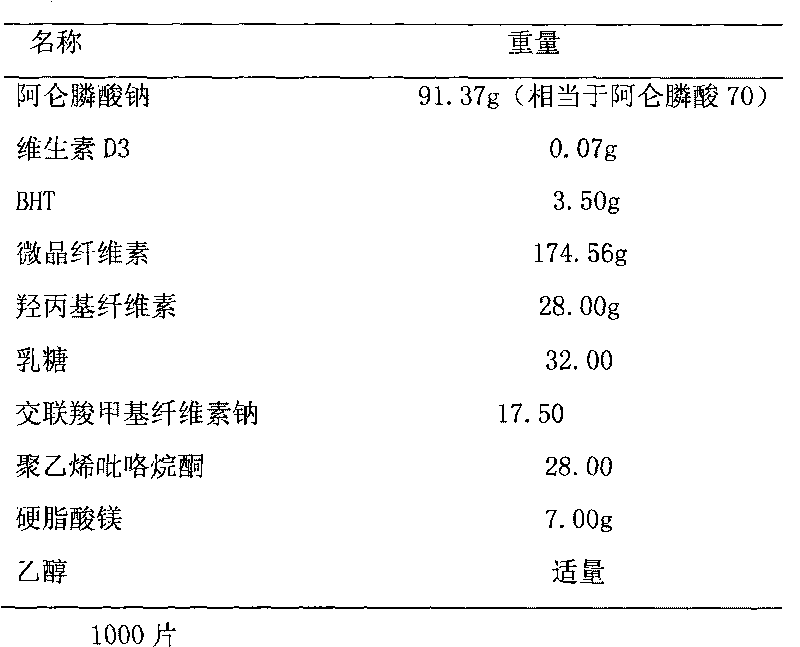
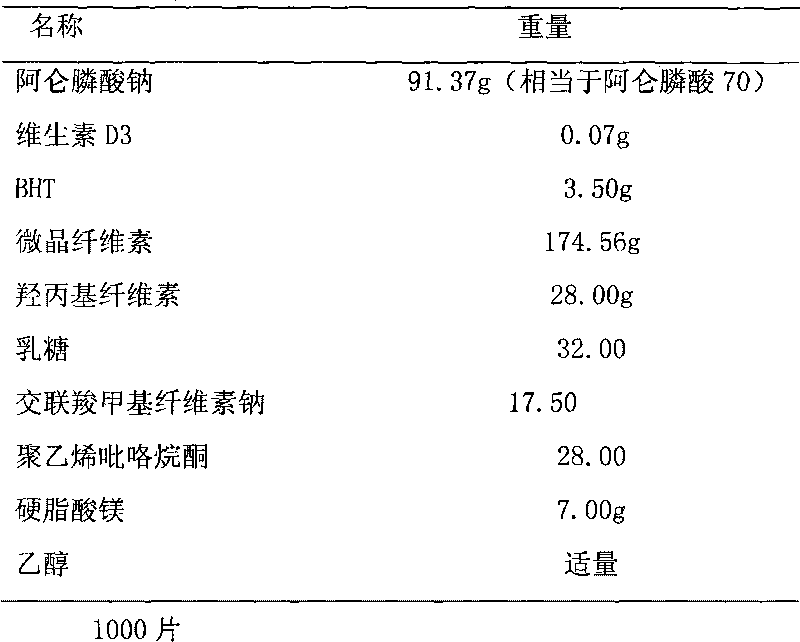
Multi-unit bowel dissolvable preparation of compound alendronate sodium and vitamin D3 and preparation method thereof
InactiveCN101623292AImprove bioavailabilityAvoid stimulationOrganic active ingredientsSkeletal disorderMulti unitMedicine
The invention discloses a multi-unit bowel dissolvable preparation of compound alendronate sodium and vitamin D3 and a preparation method thereof. The preparation is bowel dissolvable micro-pills or bowel dissolvable granules comprising the alendronate sodium, the vitamin D3, one or a plurality of bowel dissolvable coating materials and other medicinal auxiliary materials; and the preparation is undissolvable in the stomach, is evenly released in the bowel and has the characteristics of high bioavailability, light adverse reaction, convenient taking and the like.
Owner:WUXI DINGFU PHARMA
Directional orderly mineralized material for tooth enamel and preparation method thereof
ActiveCN104983583ATo remineralizeReasonable structural designImpression capsDentistry preparationsNatural toothBiocompatibility Testing
The invention provides a directional orderly mineralized material for tooth enamel and a preparation method thereof; after carboxymethyl chitosan and alendronate sodium are crosslinked, a solution containing hydrogen phosphate ions and a solution containing calcium ions are successively dropwise added, then nano amorphous calcium phosphate particles are formed, followed by, glutamic acid is added to the solution, and thus the mineralized material is obtained. The mineralized material is simple and convenient in preparation method, can be permeated to below a demineralization surface to reach deep mineralization, and is fixedly combined with a tooth body surface; newly formed hydroxyapatite is in directional orderly arrangement, and is similar to natural tooth enamel; the mineralized material has the advantages of no toxicity, no stimulation, good biocompatibility, low sensitization, and ideal use effect.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
A kind of biomineralization material and its preparation method and application
InactiveCN102294034AIncrease varietyImprove adsorption capacityOrganic active ingredientsImpression capsMedical unitEnd-group
A biomineralization material of the invention has a structural formula shown as below. In the structural formula, one part is a polyamide-amine dendritic polymer with an end group of carboxyl, and the ALN is an Alendronate sodium medical unit. The preparation method of the biomineralization material of the invention includes the following steps: (1) synthesizing polyamide-amine dendritic polymer with the end group of carboxyl; (2) partially activating the polyamide-amine dendritic polymer; (3) synthesizing the biomineralization material. According to experiments, the biomineralization material of the invention has both functions of absorbing hydroxy apatite and inducing re-mineralization of the hydroxy apatite, and can be used as a derivant for dentin in-situ re-biomineralization.
Owner:SICHUAN UNIV
Targeting gene carrier as well as preparation method and applications thereof
ActiveCN106047935AHigh affinityEfficient combinationMicroencapsulation basedGenetic material ingredientsBone tissueCholesterol
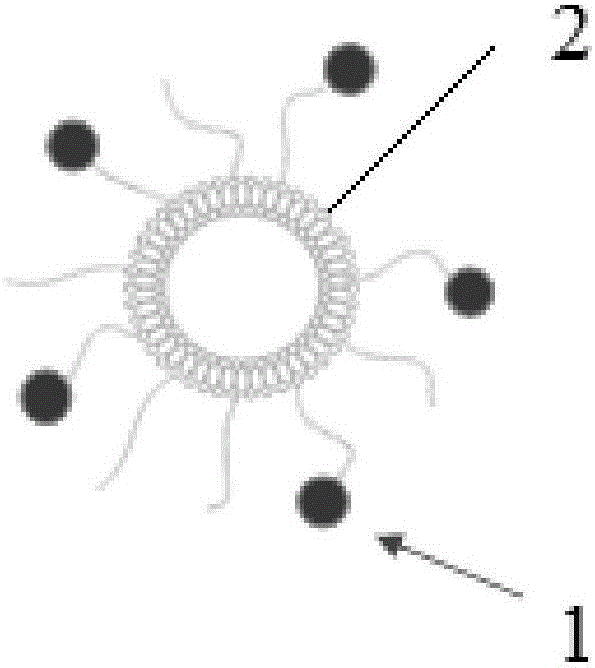
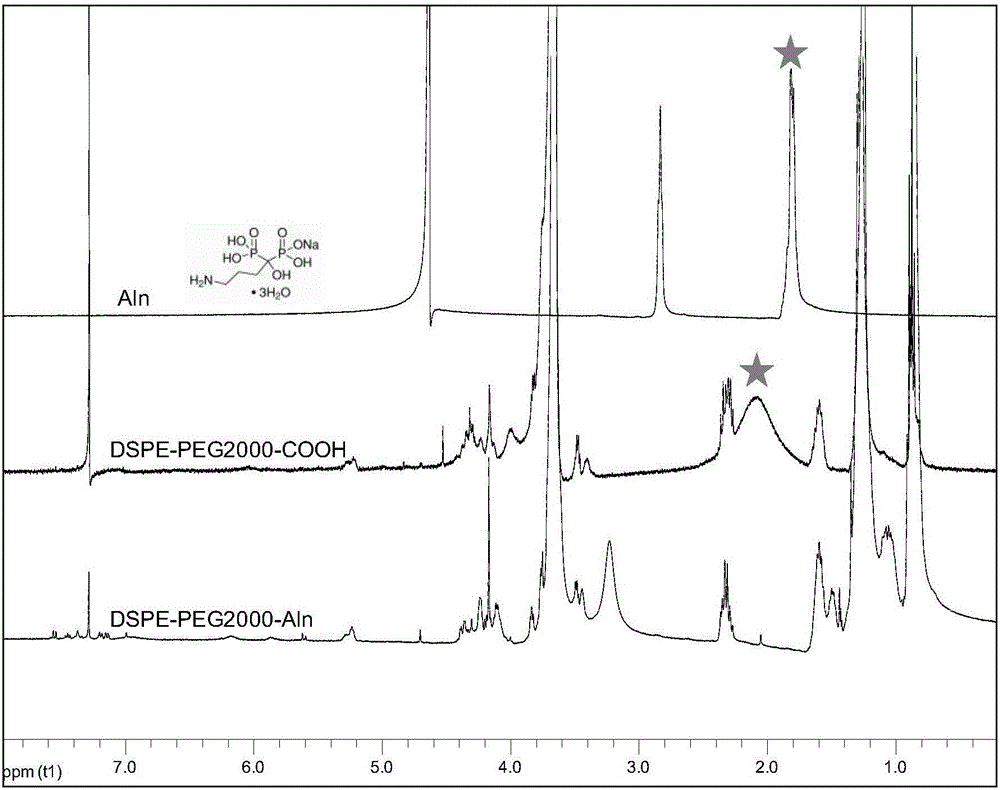
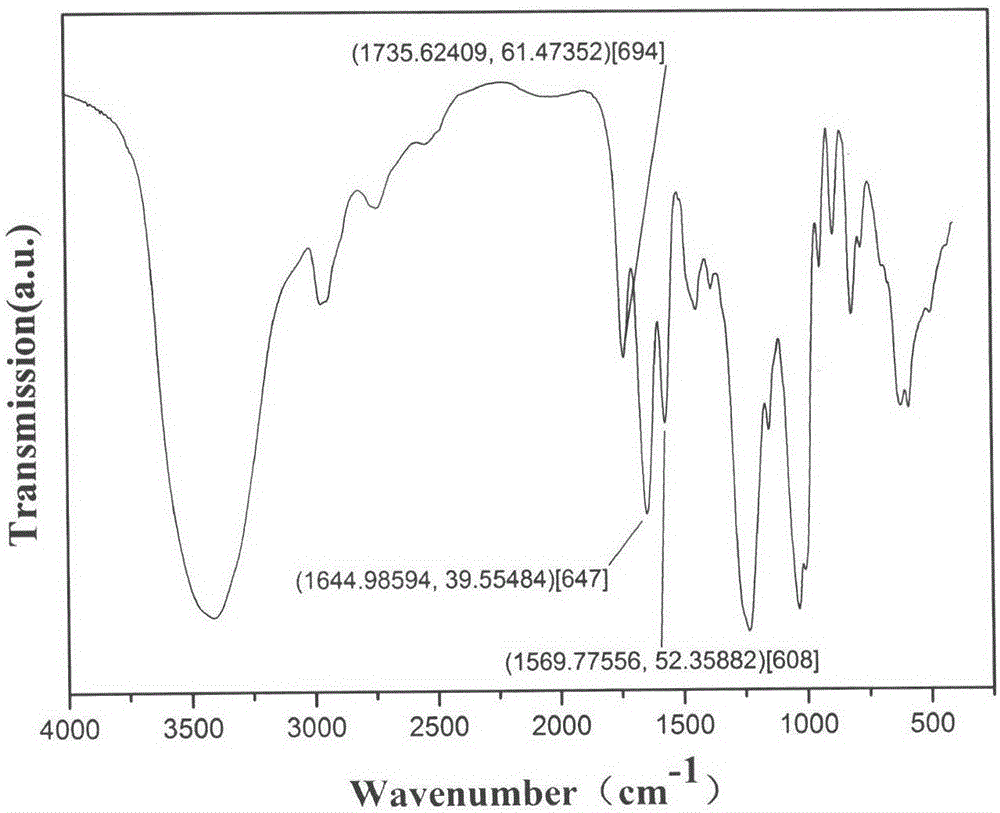
The invention provides a bone targeting gene carrier. The bone targeting gene carrier is a lipidosome modified by alendronate sodium, wherein the lipidosome modified by alendronate sodium comprises cationic lipid, neutral auxiliary lipoid, cholesterol and phospholipid modified by alendronate sodium, the cationic lipid, the neutral auxiliary lipoid and the cholesterol form a phospholipid layer, the phospholipid modified by alendronate sodium is distributed in the phospholipid layer and forms a vesica structure with the phospholipid layer, and alendronate sodium is exposed out of the phospholipid layer. The bone targeting gene carrier has relatively high targeting property and transfection efficiency for the bone tissue, and can be efficiently expressed near the bone tissue by being loaded with a gene substance. The invention further provides a preparation method and applications of the bone targeting gene carrier.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
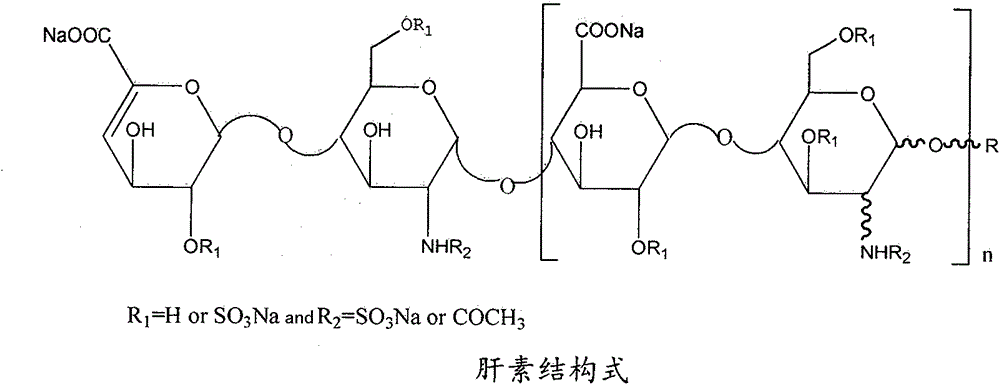
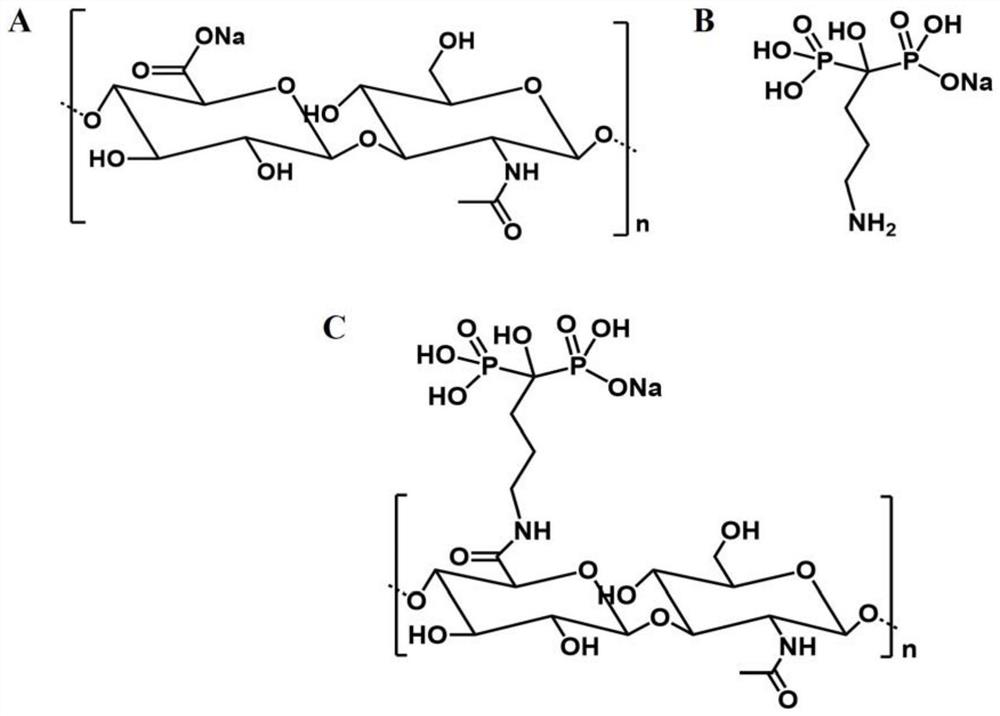
Heparin bisphosphonate derivative and synthetic method and application thereof
InactiveCN105085717ADemonstrate successful couplingEasy to prepareOrganic active ingredientsSkeletal disorderSide effectIrritation
The present invention relates to a heparin bisphosphonate derivative and a synthetic method and application thereof. The modification material for alendronate sodium is heparin; the heparin and alendronate sodium are coupled through an amide bond for drug modification of alendronate sodium. The present invention overcomes the defects of high preparation cost, complex process, complicated operation and incapability of mass production in the conventional method. The invention directly employs a chemical method for modification of alendronate sodium. The alendronate sodium improved drug is simple for preparation, low in cost, and uses readily available raw materials; besides, the improved drug can improve the permeability of alendronate sodium in the body, reduce irritation of drug on mucous membranes, increase the effective concentration of the alendronate sodium drug in targets, reduce the side effects of alendronate sodium in the body, improve the body's level of rehabilitation therapy, and improve medication safety and quality of patients.
Owner:YANGZHOU UNIV
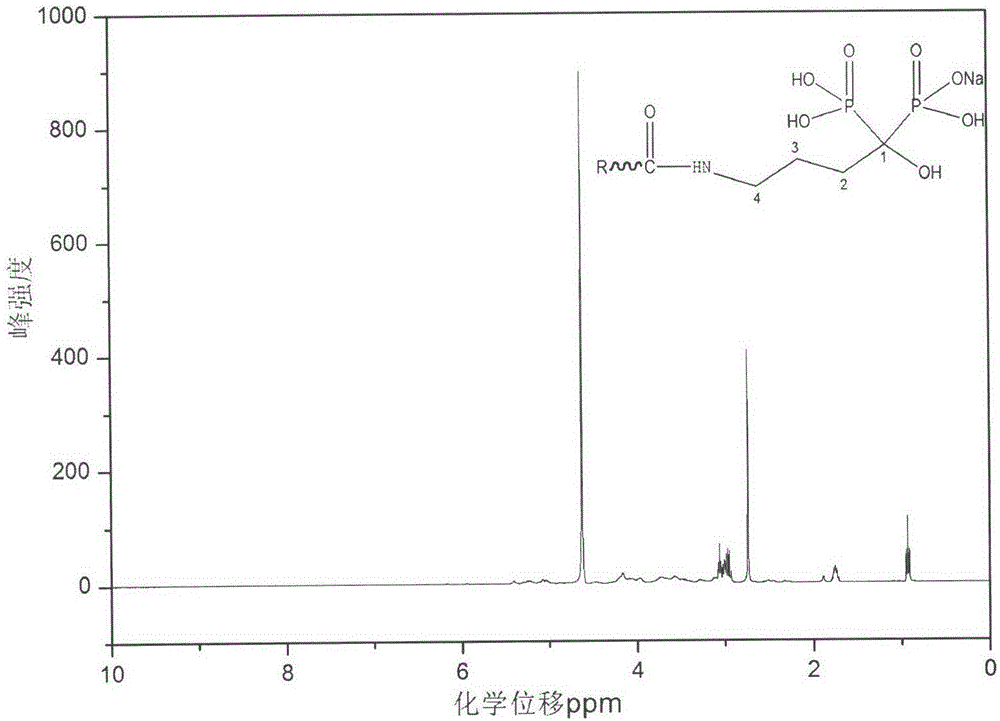
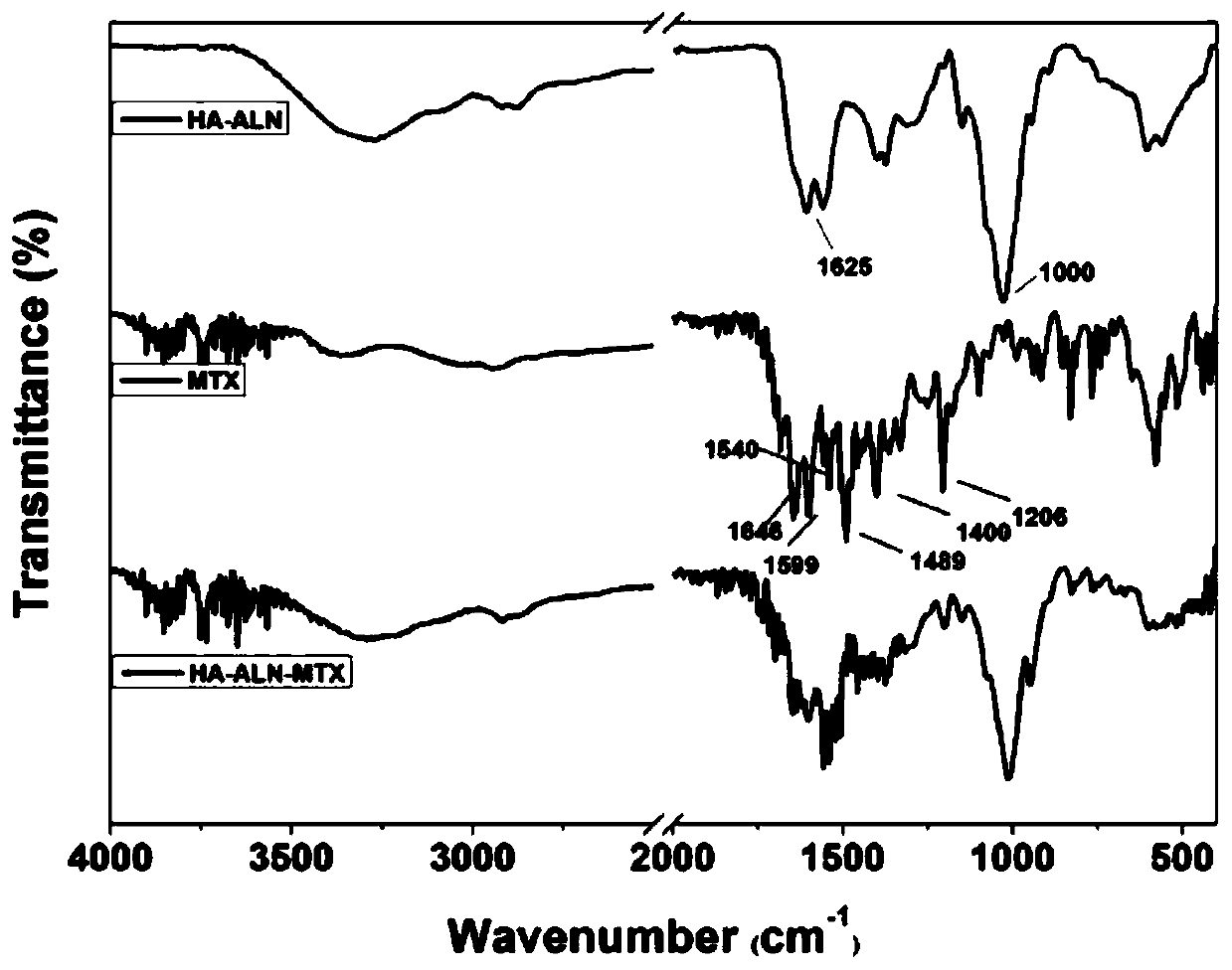
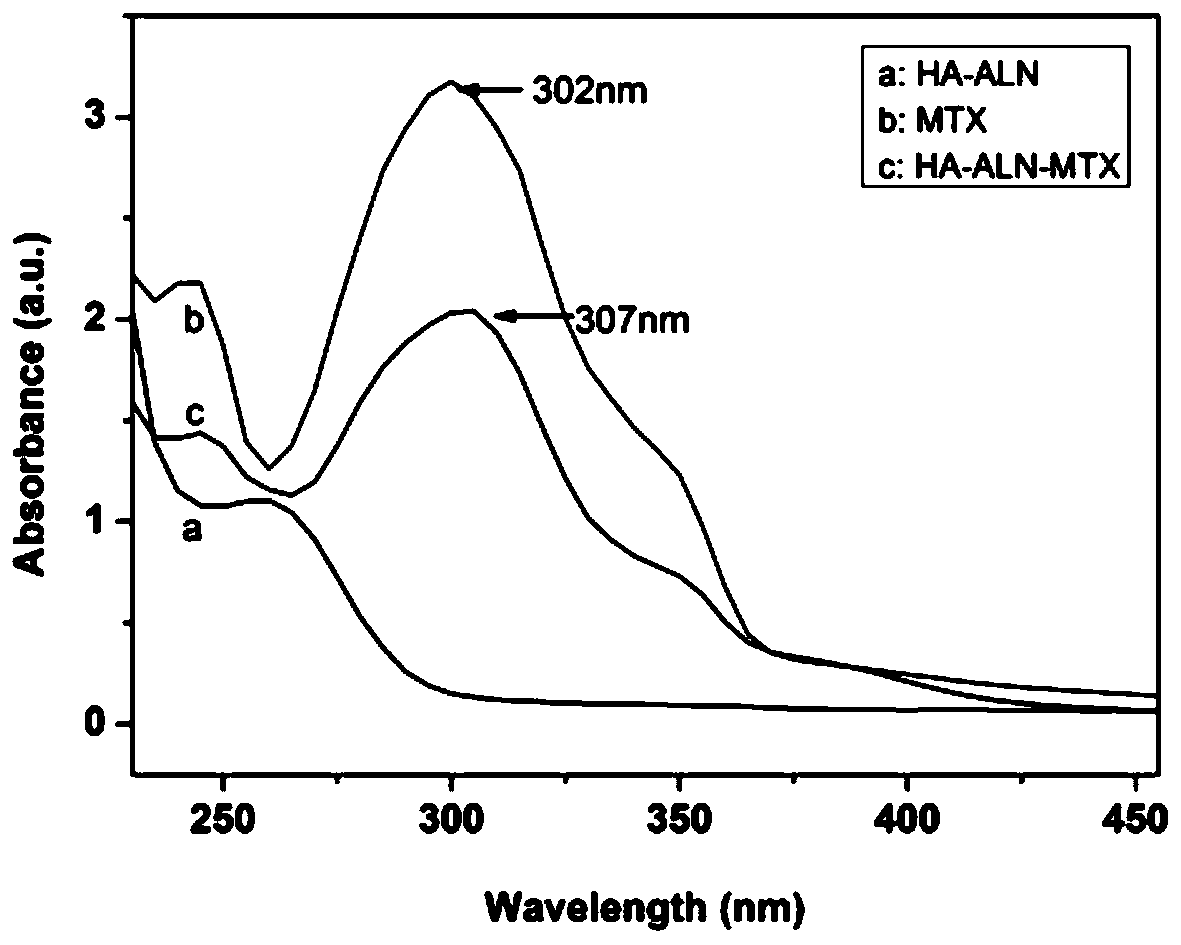
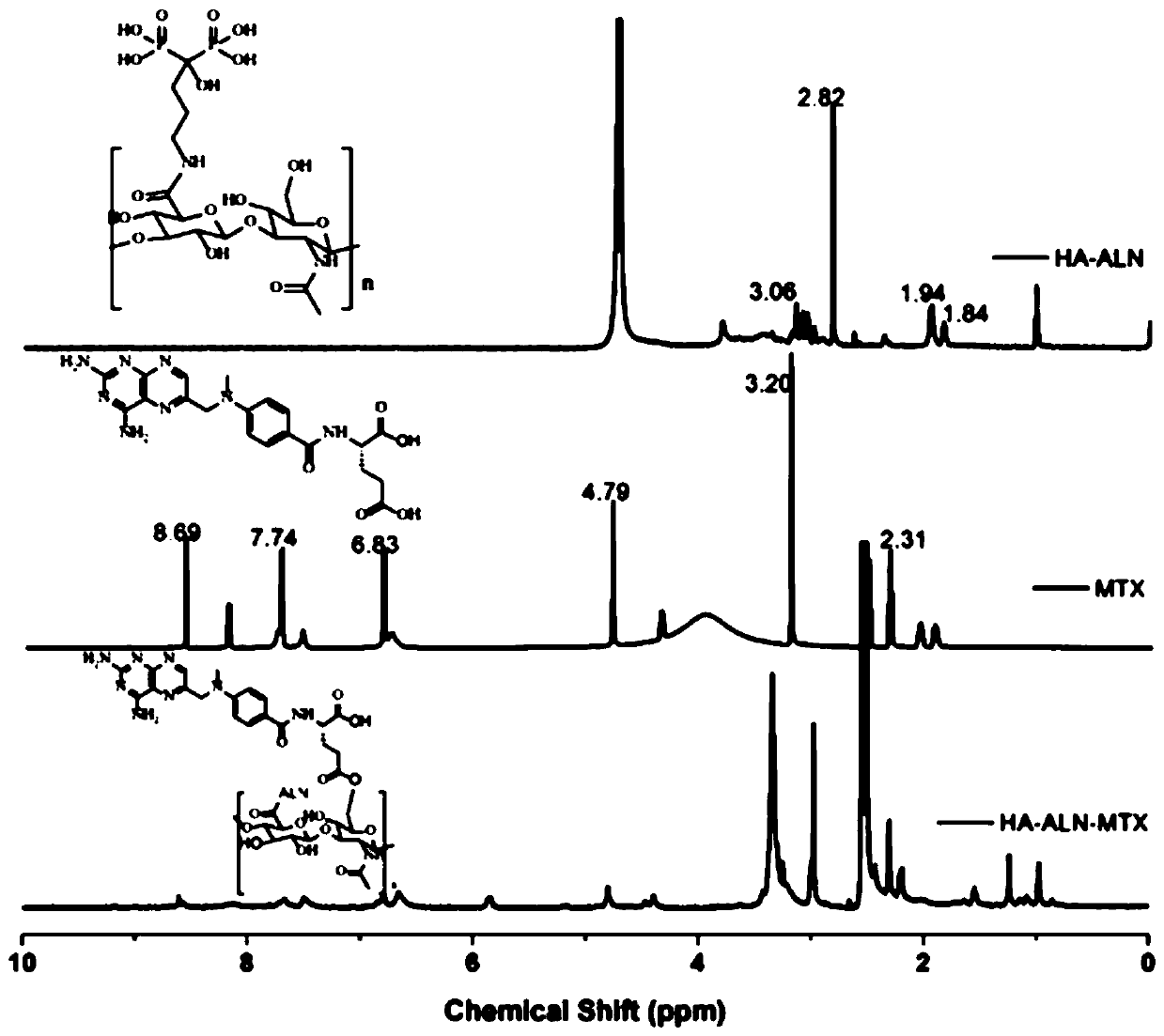
Preparation method of hyaluronic acid-alendronate sodium- methotrexate nanometer granules
PendingCN110237266AImprove stabilityGood dispersionPowder deliveryOrganic active ingredientsTumor targetSide effect
The invention discloses a preparation method of hyaluronic acid-alendronate sodium-methotrexate nanometer granules. The method comprises the steps of adding hyaluronic acid to an aqueous solution of EDC and NHS, after carboxyl activation, adding alendronate sodium, continuing a stirring reaction, and performing dialysis to obtain an HA-ALN coupling substance; then enabling the HA-ALN coupling substance to dissolve in DMF, adding DMSO, performing diluting to prepare an HA-ALN coupling substance solution, enabling methotrexate to dissolve in dimethylsulfoxide, and adding EDC and DMAP, to prepare a methotrexate solution; and finally, mixing the HA-ALN coupling substance solution with the methotrexate solution, and performing a normal-temperature reaction to prepare the hyaluronic acid-alendronate sodium-methotrexate nanometer granules. The prepared HA-ALN-MTX coupling substance is high in stability and favorable in dispersibility, can be targeted to cancer cells to effectively kill cancer cells, and besides, is low in toxic and side effect, and has application prospect in tumor targeted therapy.
Owner:YANGZHOU UNIV
Method for preparing autologous hematopoietic stem cells, kit, the stem cells and application
ActiveCN104419660AYield advantagePurity advantageAntinoxious agentsDead animal preservationInterleukin 6Culture fluid
Owner:深圳百年干细胞技术研究院有限公司
Compound alendronate sodium vitamine D3 orally disintegrating tablets and preparation method thereof
InactiveCN101305988AImprove bioavailabilityIncrease blood concentrationOrganic active ingredientsSkeletal disorderSide effectOrally disintegrating tablet
The invention relates to compound alendronate sodium vitamin D3 orally-disintegrating tablets used for preventing and treating stmenopausal osteoporosis and a preparation method thereof, aims to provide a novel preparation, i.e. the compound alendronate sodium vitamin D3 orally-disintegrating tablets to patients and medical workers; wherein, the compound alendronate sodium vitamin D3 orally-disintegrating tablets have fast absorption and high bioavailability, need no water when in administration, have little intestinal residue and insignificant side effects, and avoid the first-pass effect in liver sausage. Alendronate sodium vitamin D3 is used as a raw material, auxiliary materials are added in, and the alendronate sodium vitamin D3 orally-disintegrating tablets are prepared according to the technical means.
Owner:北京利乐生制药科技有限公司
Alendronate sodium powder inhalation used for respiratory drug delivery and preparation method and application thereof
ActiveCN105640924AAvoid side effectsSimple manufacturing processPowder deliveryOrganic active ingredientsSide effectStimulant
The invention provides alendronate sodium powder inhalation used for respiratory drug delivery and a preparation method and application thereof. Alendronate sodium powder inhalation includes single-component alendronate sodium micro powder and does not include auxiliary materials. In the production and preparation process of the alendronate sodium micro powder, other auxiliary materials or solvents are not involved, and alendronate sodium raw material medicine is directly smashed to prepare the alendronate sodium micro powder. The alendronate sodium powder inhalation can improve the breathing function of a chronic obstructive pulmonary disease (COPD) rat by about 40% and has a better effect than salbutamol sulfate with a common usage amount, and drug resistance of salbutamol sulfate and other beta2 receptor stimulants can be eliminated. The powder inhalation mainly makes sedimentation in the trachea, bronchia, bronchiole and other pulmonary respiratory ducts to directly take effect after drug-delivery inhalation, the diastolic function of the respiratory ducts becomes effective rapidly, and the drug effect lasts for a long time; the alendronate sodium powder inhalation enters pulmonary alveoli, is low in absorption and blood-entering amount, can avoid systematic side effects and is suitable for being developed to be a drug for treating COPD and asthma.
Owner:杭州东博医药科技开发有限公司
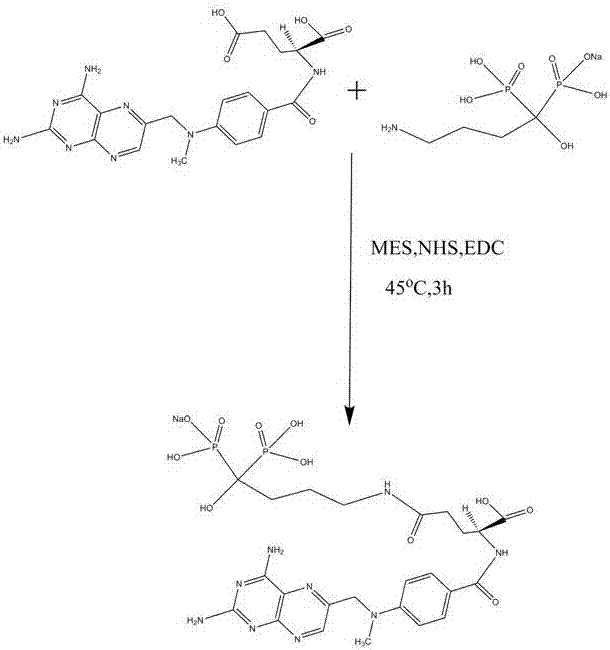
Preparation method of methotrexate and alendronate sodium conjugate
ActiveCN107200753AReduce dosageSave resourcesAntipyreticGroup 5/15 element organic compoundsTreatment effectOrganic solvent
The invention discloses a preparation method of a methotrexate and alendronate sodium conjugate, and belongs to the technical field of organic pharmaceutical production. The method comprises the following steps: mixing the methotrexate, ultra-pure water and an organic solvent to obtain a methotrexate solution; then adding morpholineethanesulfonic acid, N-hydroxysuccinimide, 1-(3-dimethylamino)-3-ethylcarbodiimide hydrochloride into the methotrexate solution to obtain a methotrexate mixed solution; mixing the methotrexate mixed solution with alendronate sodium for reaction under a magnetic force stirring effect; carrying out dialysis and lyophilization to obtain a nanoscale methotrexate and alendronate sodium conjugate. By adopting the conjugate, a novel targeting medicine for orthopedics can be obtained, so that the medicine can be enriched toward lesions of orthopedics, and a preferable treatment effect can be achieved.
Owner:YANGZHOU UNIV
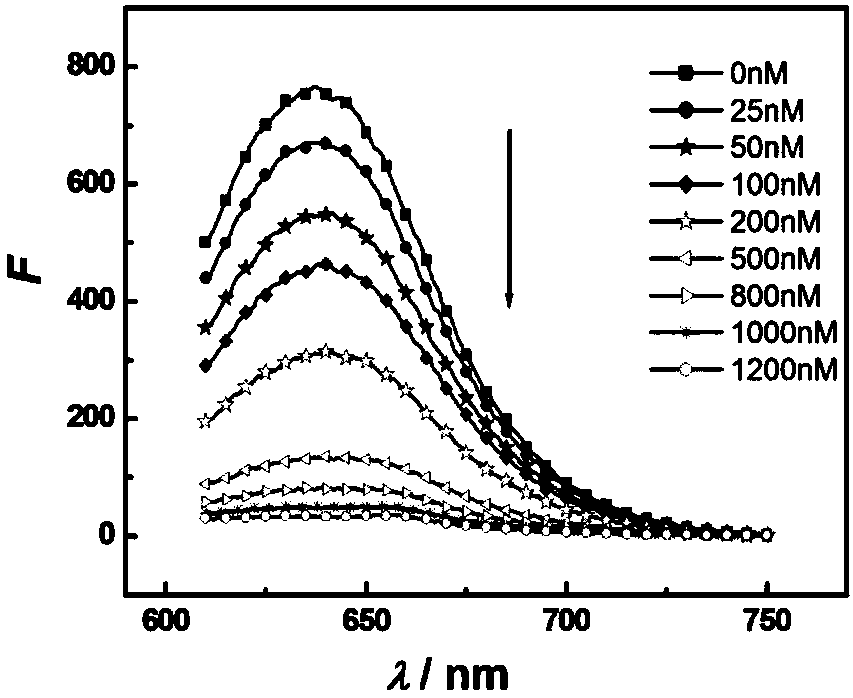
Novel method for high-sensitivity and wide detection range fluorescence detection of alendronate sodium (ALDS)
InactiveCN107655871AReduce background signalPracticalFluorescence/phosphorescenceSerum samplesCopper
The invention provides a novel method for high-sensitivity and wide detection range fluorescence detection of alendronate sodium (ALDS). A background signal is lowered by the aid of quenching effect of copper ions on silver nanoclusters in oligonucleotide-fluorescent silver nanoclusters, the ALDS in samples is combined with the copper ions to form a compound, the copper ions are enabled to be awayfrom the oligonucleotide-fluorescent silver nanoclusters to have fluorescence in the siliver nanoclusters to be restored, and content of the ALDS is calculated by monitoring the change of fluorescence intensity of the DNA-AgNCs compound probe by the concentration of the ALDS and drawing a standard curve of fluorescence recovery degree and content of the ALDS. The method is applicable to detectionof content of the ALDS in the samples (pharmaceutic preparations or biological samples such as urine samples and serum samples), and has the advantages of wide linear range, high flexibility, less interference, time saving, green and safety and the like, and the method is suitable for large-scale promotion.
Owner:CHONGQING MEDICAL UNIVERSITY
Alendronate sodium vitamine D3 enteric-coated tablet and preparation method thereof
The invention relates to an alendronate sodium vitamine D3 enteric-coated tablet and a preparation method thereof. The method comprises the following steps of: uniformly mixing alendronate sodium, vitamine D3 and an auxiliary material in a given proportion and screening; then adding adhesive to prepare a soft material; granulating, drying, straightening and pressing into tablets; then preparing enteric-coated liquid for standby; spraying and drying according to conventional coating and packaging respectively to obtain the product. The invention provides the treating selection of a medicament which is dissolved and released in the intestines to prevent gastrointestinal tract influence for a patient with osteoporosis, can meet the clinical requirement of long-term medicament use and achieve the aims of reducing taking frequency and toxic and side effect, generating synergistic effect and improving bioavailability and is suitable for industrialized production.
Owner:BEIJING HOPE HUGE PHARM SCI
Compound alendronate sodium vitamin D3 enteric-coated tablet and preparation method thereof
InactiveCN101632680ASimple processImprove stabilityOrganic active ingredientsSkeletal disorderIntestinal structurePatient compliance
The invention discloses a compound alendronate sodium vitamin D3 enteric-coated tablet and a preparation method thereof. The preparation is an enteric-coated tablet consisting of alendronate sodium, beta-cyclodextrin inclusion compound of vitamin D3, an enteric-coating material and other pharmaceutical excipients. The invention has simple process and good stability, is insoluble in stomach and can rapidly release in intestine, thereby avoiding adverse reactions stimulating upper gastrointestinal and having good patient compliance.
Owner:WUXI DINGFU PHARMA
Composite type medical biological bone cement, preparation method and application thereof
ActiveCN108478872AAvoid adverse reactionsInhibit bone resorptionProsthesisMass ratioBiocompatibility Testing
The invention discloses composite type medical biological bone cement. The composite type medical biological bone cement comprises a powder part and a liquid part in the mass ratio of (1: 1) to (1: 1.5); in parts by weight, the powder part comprises 40 to 45 parts of alendronate sodium-nano-hydroxyapatite powder, 45 to 60 parts of polymethyl methacrylate powder with the polymethyl methacrylate of0.5 to 0.7 million and 3 to 4 parts of dibenzoyl peroxide. The alendronate sodium-nano-hydroxyapatite powder is used for greatly improving the biocompatibility of PMMA bone cement, the mineralizationof the bone cement is promoted, the bone cement can be preferably combined with bone, the bone conduction effect and bone induction effect of the bone cement can be used for effectively promoting thegrowth of bone, simultaneously, the contained alendronate sodium is used for effectively resisting osteoporosis, the activity of osteoclasts is inhibited, and the bone absorption is reduced.
Owner:SUN YAT SEN UNIV
Heparin alendronate sodium conjugate synthetic method and drugs
ActiveCN110124053AHigh synthesis efficiencyThe synthesis process is simpleOrganic active ingredientsMetabolism disorderSolubilityFreeze-drying
The invention relates to a heparin alendronate sodium conjugate synthetic method and drugs in the field of medicine. The heparin alendronate sodium conjugate synthetic method comprises the steps: step1, firstly, dissolving 2-chloro-4,6-dimethoxy-1,3,5-triazine in tetrahydrofuran, adding 4-methylmorpholine, stirring for 1-2 h at room temperature, collecting by filtration and washing a precipitatefive times with THF, and thus then obtaining a DMT-MM condensation agent after 48 h of vacuum drying; and step 2, adding heparin and the condensation agent into ultra-pure water, stirring for 1 h, then adding an alendronate sodium solution into the mixed solution of heparin and the condensation agent, carrying out reaction for 24-48 h, pouring the solution into a dialysis bag, dialysing for 48-72h, changing water once every 4 h, finally, freeze-drying the product obtained from dialysis, and thus obtaining the heparin alendronate sodium conjugate drugs. In the method, alendronate sodium is modified by heparin, so the solubility of the drugs is increased, the bioavailability is improved, the cytotoxicity is reduced and the formation of osteoclasts is effectively inhibited.
Owner:YANGZHOU UNIV
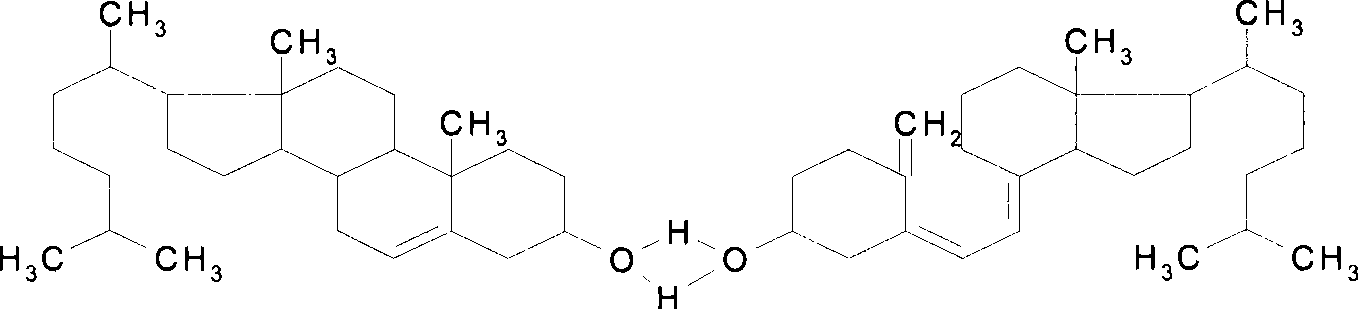
Pharmaceutical preparation containing alendronate sodium and cholecalciferol-cholesterol
ActiveCN101444521BIncrease production capacityEasy to storeOrganic active ingredientsSkeletal disorderMedicineCholesterol
The invention provides a pharmaceutical composition which contains alendronate sodium, cholecalciferol-cholesterol, antioxidizer and a pharmaceutically acceptable carrier. The invention also provides the usage of the pharmaceutical composition in treating osteoporosis.
Owner:SHANGHAI SINE PHARMA LAB
Preparation method of hydroxyapatite/modified polylactic acid composite microspheres
InactiveCN109350768AImprove hydrophilicityImprove adsorption capacityCoatingsMicrocapsulesEthylenediamineMicrosphere
The invention discloses a preparation method of hydroxyapatite / modified polylactic acid composite microspheres. The preparation method is characterized in that ethylenediamine is used as a modifier toprepare amination-modified polylactic acid microspheres by the combination of aminolysis, emulsification and thermally induced phase separation; Ca(NO3)2 and K2HPO4 solutions and the amination-modified polylactic acid microspheres are used as inorganic calcium and phosphorus sources and an organic templating agent respectively to acquire amination-modified polylactic acid microspheres by in-situbiomimetic mineralization precipitation. The preparation method has the advantages of good environmental friendliness, good process simplicity, low cost and the like; amino groups in the modified polylactic acid provide great chelating action upon Ca2+ of hydroxyapatite, so that the modified polylactic acid and hydroxyapatite have good interfacial compatibility; hydroxyapatite crystal drops rarelyand can disperse evenly. Compared with polylactic acid microspheres, the composite microspheres herein can gain evidently improved bioactivity and bone-repairing performance, can effectively carry alendronate sodium drug and is an ideal bone-repairing material.
Owner:FUJIAN NORMAL UNIV
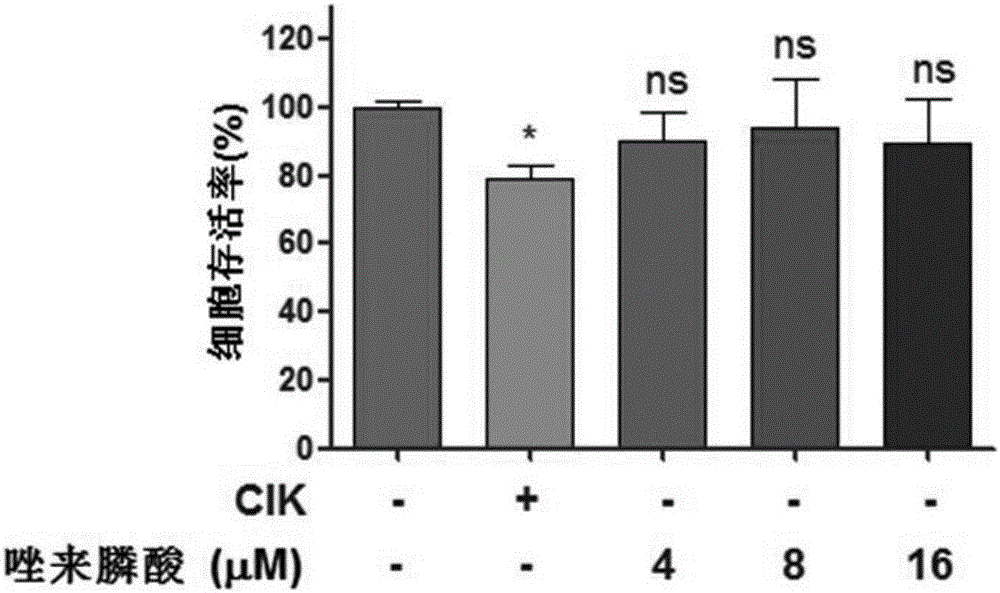
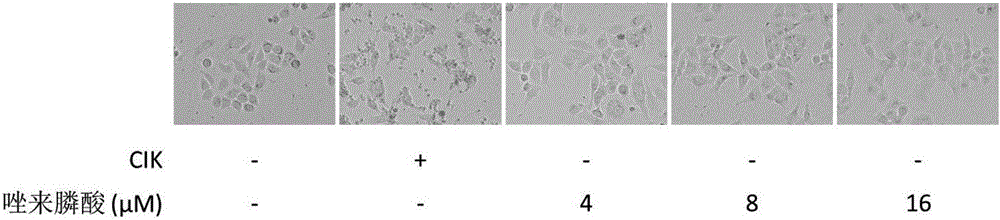
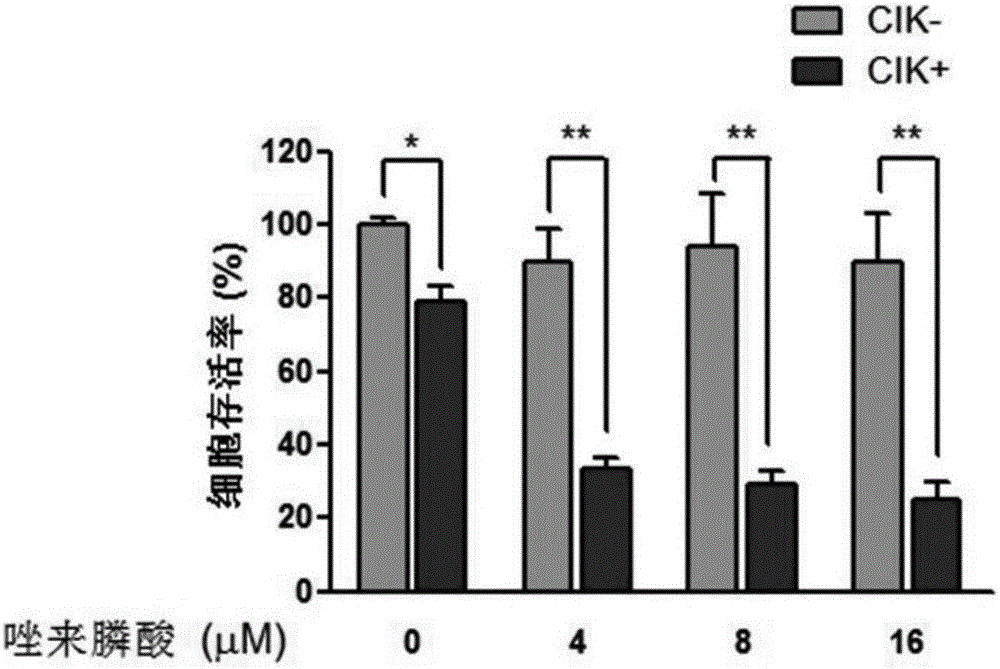
Antitumor combined medicine
ActiveCN106668067AIncrease lethalityReduce the number of treatmentsOrganic active ingredientsMammal material medical ingredientsTiludronate DisodiumEtidronate Disodium
The invention discloses an antitumor combined medicine. The combined medicine comprises a diphosphonate compound serving as an active ingredient and CIK cells, wherein the diphosphonate compound is at least one of clinical medication etidronate disodium, clodronate disodium, pamidronate disodium, tiludronate disodium, alendronate sodium, neridronate sodium, olpadronate sodium, risedronate sodium, sodium ibandronate, incadronate disodium and zoledronic acid. The method for greatly improving the tumor cell killing capability of CIK cells by utilizing combination of anti-tumor-osseous-metastasis diphosphonate medicines and the CIK cells, so that the number of CIK cells for achieving the treatment effect the same as a conventional method is greatly reduced; and compared with biological treatment of single CIK cells, the combined medicine has the advantages that the relatively high safety of CIK cells can be maintained, and the treatment effect can be remarkably improved.
Owner:JINAN UNIVERSITY
Composition and method for treating soft nails
A composition and method for treating soft nails, particularly soft fingernails, the composition including bisphosphonate, preferably alendronate sodium, in a vehicle effective for topical administration.
Owner:ROSENBERG E WILLIAM +1
Alendronate sodium solid lipid nanoparticle and preparation method thereof
InactiveCN106821984ARelease stabilityImprove bioavailabilityOrganic active ingredientsSkeletal disorderOrganic solventDistilled water
The invention discloses an alendronate sodium solid lipid nanoparticle and a preparation method thereof. The alendronate sodium solid lipid nanoparticle comprises the following components in weight percentage: 0.01-0.05% of alendronate sodium, 0.29-0.5% of solid lipid, 0.19-0.5% of a fat soluble emulsifier, 0.49-0.98% of a water soluble emulsifier and balance of distilled water. The preparation method disclosed by the invention adopts a shear-ultrasonic method, and alendronate sodium is wrapped into a solid lipid nanoparticle, so that the alendronate sodium solid lipid nanoparticle is obtained. The preparation method comprises the following steps: precisely weighing all the components in prescription dosage, firstly dissolving alendronate sodium into a small amount of organic solvent, then mixing with the solid lipid and the fat soluble emulsifier, heating and fusing, so that an oil phase is obtained; adding the water soluble emulsifier into the distilled water, and heating to the temperature the same with the oil phase, so that an aqueous phase is formed; and pouring the aqueous phase into the oil phase, and carrying out high-speed shearing and ultrasonication, so that the alendronate sodium solid lipid nanoparticle is obtained. Urine excretion dynamic effects show that the bioavailability of a rat oral solid lipid nanoparticle is 3-4 times that of an alendronate sodium solution.
Owner:NANCHANG UNIV
Pharmaceutical preparation containing alendronate sodium and cholecalciferol-cholesterol
ActiveCN101444521AIncrease production capacityEasy to storeOrganic active ingredientsPharmaceutical delivery mechanismMedicineCholesterol
The invention provides a pharmaceutical composition which contains alendronate sodium, cholecalciferol-cholesterol, antioxidizer and a pharmaceutically acceptable carrier. The invention also provides the usage of the pharmaceutical composition in treating osteoporosis.
Owner:SHANGHAI SINE PHARMA LAB
Bone targeting nano material and application thereof
PendingCN112316154APromote formationAchieve enrichmentPowder deliveryOrganic active ingredientsPolyethylene glycolTherapeutic effect
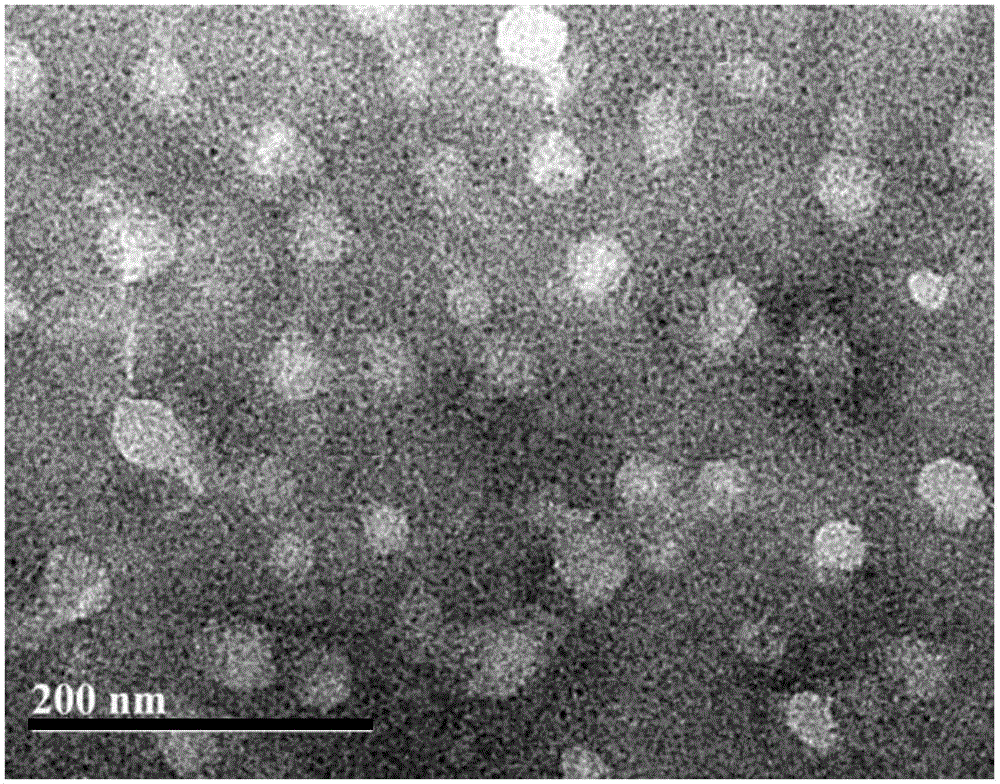
The invention relates to a nano material and application thereof, in particular to a bone targeting nano material. The bone targeting nano material comprises catalpol, alendronate sodium, polyethyleneglycol and a poly (lactic-co-glycolic acid) copolymer. TEM (transmission electron microscope) detection and diabetes mouse osteoporosis model pharmacodynamic experiments prove that the material can be better distributed in bone tissue, has a remarkable treatment effect, and has good open prospect.
Owner:XUZHOU MEDICAL UNIV
Rare earth-based nano composite hydrogel wound dressing as well as preparation method and application thereof
ActiveCN112604025AEasy to operateLow costPharmaceutical delivery mechanismBandagesWound dressingBiocompatibility
The invention provides a rare earth-based nano composite hydrogel wound dressing as well as a preparation method and an application thereof. The preparation method comprises the following steps: preparing rare earth oxide nanorods through annealing treatment by utilizing a hydrothermal reaction; dissolving sodium hyaluronate in an MES buffer solution, activating with NHS and EDC, and finally adding alendronate sodium for a reaction; reacting to obtain a hyaluronic acid-alendronate sodium polymer; dispersing the hyaluronic acid-alendronate sodium polymer into a PBS buffer solution of F127, and after the hyaluronic acid-alendronate sodium polymer is completely dispersed, adding rare earth oxide nanorods to form nano-composite hydrogel, thereby obtaining the rare earth-based nano-composite hydrogel wound dressing. The rare earth-based nano composite hydrogel wound dressing also shows good biocompatibility in vivo and in vitro, and also has good anti-inflammatory property, so that the hydrogel dressing has good application prospects in wound healing and tissue regeneration.
Owner:XI AN JIAOTONG UNIV
Alendronate sodium intestine-sol capsule and preparation method thereof
ActiveCN101601662BEnsure safetyGuaranteed releaseOrganic active ingredientsSkeletal disorderIntestinal structurePollution
The invention discloses an alendronate sodium intestine-sol capsule and a preparation method thereof; the intestine-sol capsule comprises the following compositions by weight parts: 10-20 parts of alendronate sodium, 70-100 parts of diluents, 0.5-5 parts of protective agents and 0.5-2 parts of lubricants. The alendronate sodium intestine-sol capsule is particularly added in the protective agents,so as to effectively prevent basic remedy from being released in oral cavity, esophagus and esophagus before reaching the absorption site of the intestinal canal, thereby improving the medicine quality, reducing the adverse reaction and improving the compliance of a patient; the preparation process is simple, the pollution to the environment is reduced by energy consumption and social cost is saved.
Owner:JIANGSU SHENLONG PHARMA
Compound injection contg. alendronate sodium and vitamin D3
A compound injection for treating osteoporosis is prepared from sodium allenphosphonate, VD3, cosolvent, excipient and / or pH regulator.
Owner:GUANGDONG XIANQIANG PHARMA
Alendronate sodium enteric-coated tablet and preparation method thereof
InactiveCN101756932AShort disintegration timeEasy to takeOrganic active ingredientsSkeletal disorderOral medicationAdhesive
The invention relates to an alendronate sodium enteric-coated tablet and a preparation method thereof. The method comprises the following steps of: uniformly mixing alendronate sodium with an auxiliary material in a given proportion and screening; then adding adhesive to prepare a soft material; granulating, drying, straightening and pressing into tablets; preparing enteric-coated liquid for standby; spraying and drying according to conventional coating and packaging respectively to obtain the product. By using the enteric-coated tablet formulation, the defects of the traditional preparation technology are overcome, the simulation of the alendronate sodium to the alimentary canal mucosa in the process of oral administration is reduced or eliminated, and a medicament which can be selected, is relatively safe and is convenient for taking is supplied to patients.
Owner:BEIJING HOPE HUGE PHARM SCI
Chinese and western medicinal composition for treating radioactive enteritis, and preparation method and application thereof
InactiveCN106420773AThe composition is uniqueLow priceDigestive systemAnhydride/acid/halide active ingredientsSide effectRectum injury
The invention discloses a Chinese and western medicinal composition for treating radioactive enteritis, and a preparation method and application thereof. The Chinese and western medicinal composition comprises, by weight, 3-7 parts of alendronate sodium, 11-19 parts of bromhexine, 5-13 parts of taurine and 1-5 parts of calcium lactate. The preparation method includes mixing the alendronate sodium with the bromhexine and the calcium lactate, adding deionized water, increasing the temperature to 75-77 DEG C, and stirring the raw materials for 38-40 minutes to obtain a mixture A; mixing the mixture A with the taurine, performing ultrasonic treatment at the temperature of 58 DEG C for 25 minutes, and performing stirring at the temperature of 63 DEG C until drying to obtain the Chinese and western medicinal composition, wherein the ultrasonic power is 900W. The Chinese and western medicinal composition has a unique formula and low price, is safe and convenient, has better effect than regularly-used western medicine, can effectively treat colon and rectum injuries caused by radiotherapy, has high cure rate and quick action and is free from toxic and side effect; recurrence of the radioactive enteritis is avoided after curing; the preparation process is simple, raw materials are simple and easy to get, production cycle is short, cost is low, and the Chinese and western medicinal composition is suitable for industrial production and large-scale popularization.
Owner:郑州莉迪亚医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com