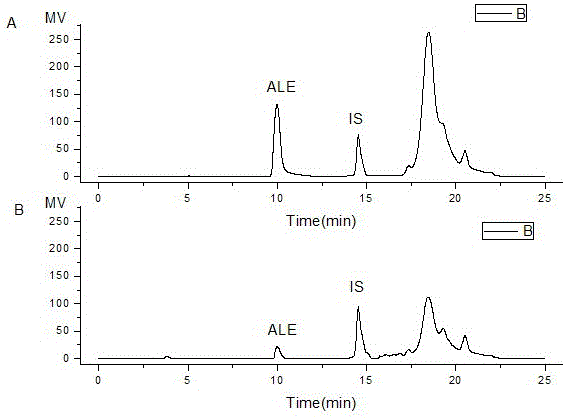
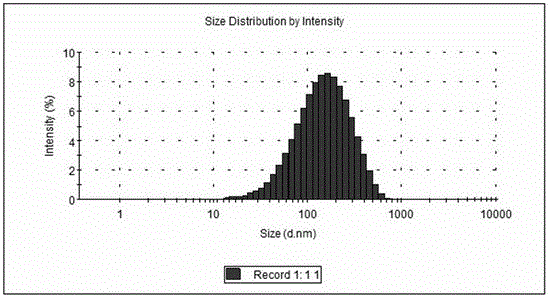
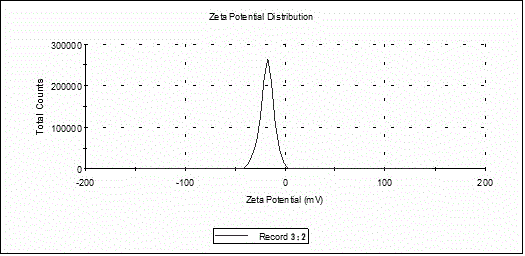
Alendronate sodium solid lipid nanoparticle and preparation method thereof
A technology of solid lipid nanometer and alendronate sodium, which is applied in the directions of liposome delivery, pharmaceutical formulations, and medical preparations of inactive ingredients, etc., can solve the problem of lack of oral bioavailability information, etc. degree, less residue, and the effect of increasing water solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of solid lipid nanoparticles of alendronate sodium; prescription: 3 mg of sodium alendronate, 30 mg of glyceryl behenate, 20 mg of soybean lecithin, 50 mg of poloxamer 188, 0.4 ml of absolute ethanol , 10ml of distilled water.
[0041] Preparation of Alendronate Sodium Solid Lipid Nanoparticles: Step 1: Accurately weigh each component of the prescription amount, dissolve Alendronate Sodium in a small amount of organic solvent (absolute ethanol), and then mix it with behenic acid Glyceride and soybean lecithin are mixed, heated to 85°C to melt the lipid to obtain an oil phase; step 2, add Poloxamer 188 to 10ml of distilled water, and heat to the same temperature as the oil phase to form a water phase; step 3. Pour the water phase into the oil phase, high-speed shear at 7000rpm for 3min, and ultrasonically break at 300w for 3min to obtain alendronate sodium solid lipid nanoparticles.
Embodiment 2
[0042] Example 2 Preparation of solid lipid nanoparticles of alendronate sodium; the prescription is as follows: 5 mg of sodium alendronate; 50 mg of glyceryl behenate; 20 mg of soybean lecithin; 50 mg of poloxamer 188; 0.4 ml of absolute ethanol ; Distilled water 10ml.
[0043] Preparation of Alendronate Sodium Solid Lipid Nanoparticles: Step 1: Accurately weigh each component of the prescription amount, dissolve Alendronate Sodium in a small amount of organic solvent (absolute ethanol), and then mix it with behenic acid Glyceride and soybean lecithin are mixed, heated to 85°C to melt the lipid to obtain an oil phase; step 2, add Poloxamer 188 to 10ml of distilled water, and heat to the same temperature as the oil phase to form a water phase; step 3. Pour the water phase into the oil phase, high-speed shear at 7000rpm for 3min, and ultrasonically break at 300w for 3min to obtain alendronate sodium solid lipid nanoparticles.
Embodiment 3
[0044] Example 3 Preparation of Alendronate Sodium Solid Lipid Nanoparticles; the prescription is as follows: Alendronate Sodium 1mg
[0045] Glyceryl behenate 50mg; soybean lecithin 25mg; poloxamer 188 30mg; absolute ethanol 0.4ml; distilled water 10ml.
[0046] Preparation of Alendronate Sodium Solid Lipid Nanoparticles: Step 1: Accurately weigh each component of the prescription amount, dissolve Alendronate Sodium in a small amount of organic solvent (absolute ethanol), and then mix it with behenic acid Glyceride and soybean lecithin are mixed, heated to 85°C to melt the lipid to obtain an oil phase; step 2, add Poloxamer 188 to 10ml of distilled water, and heat to the same temperature as the oil phase to form a water phase; step 3. Pour the water phase into the oil phase, high-speed shear at 11000rpm for 3min, and ultrasonic crush at 300w for 3min to obtain alendronate sodium solid lipid nanoparticles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com