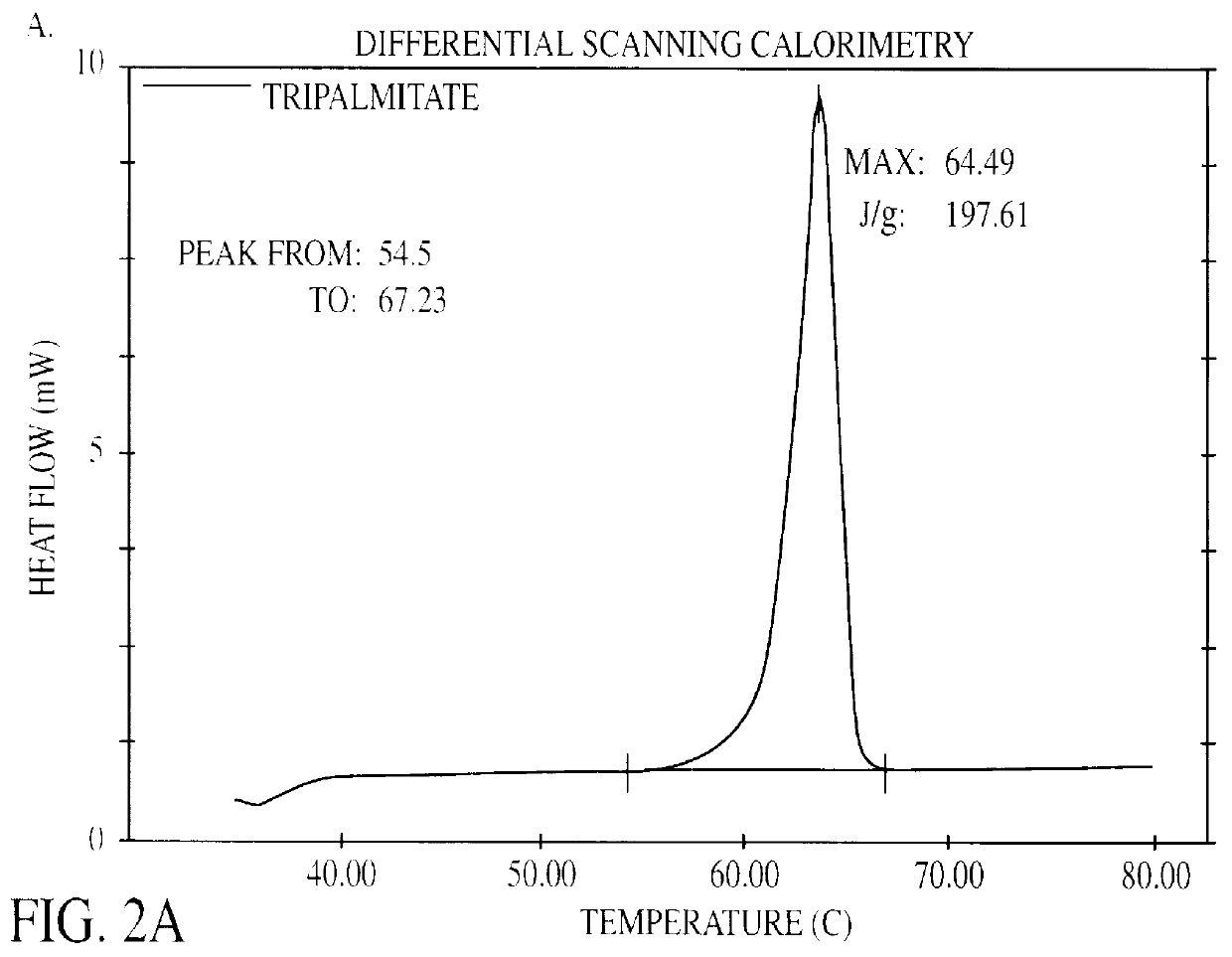
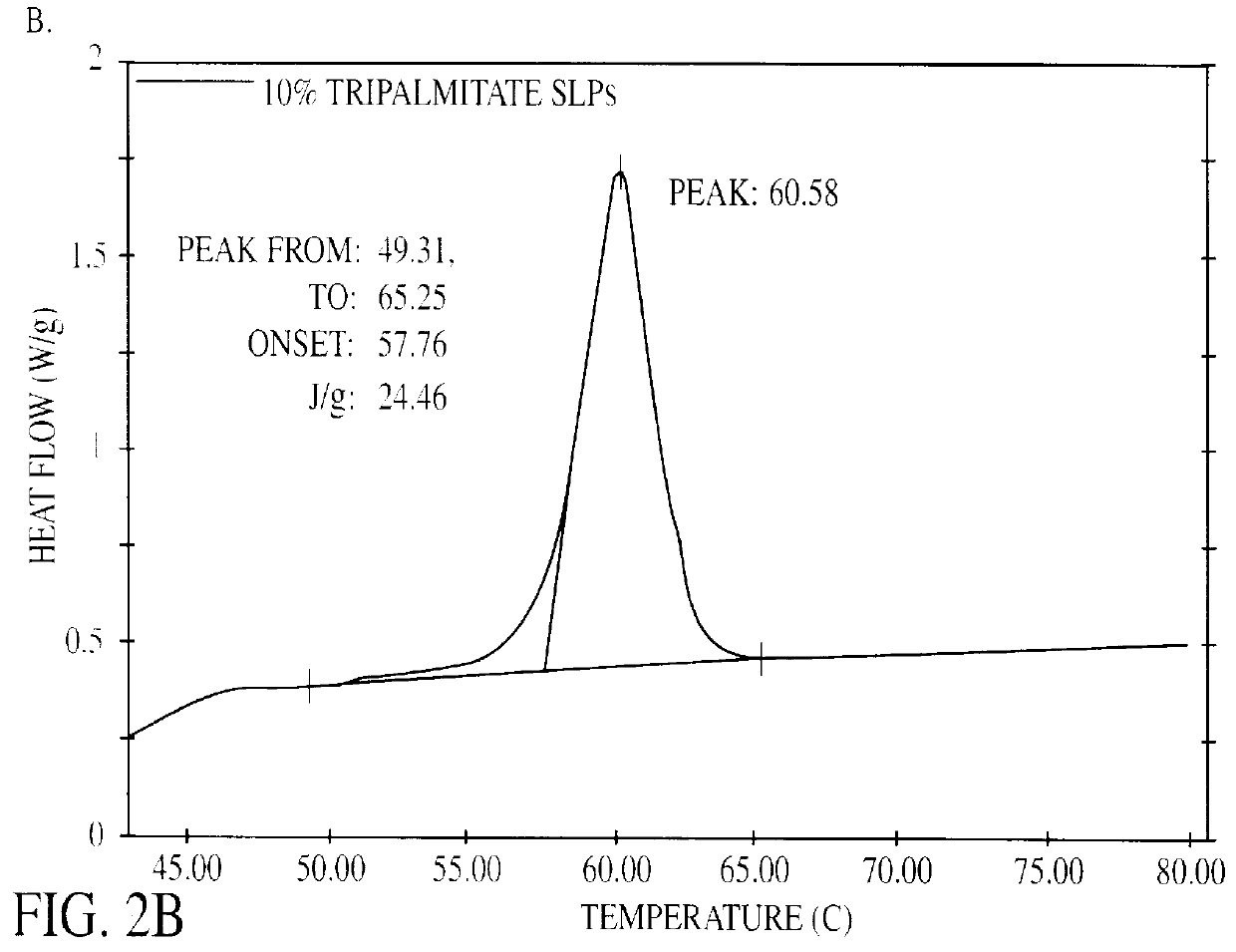
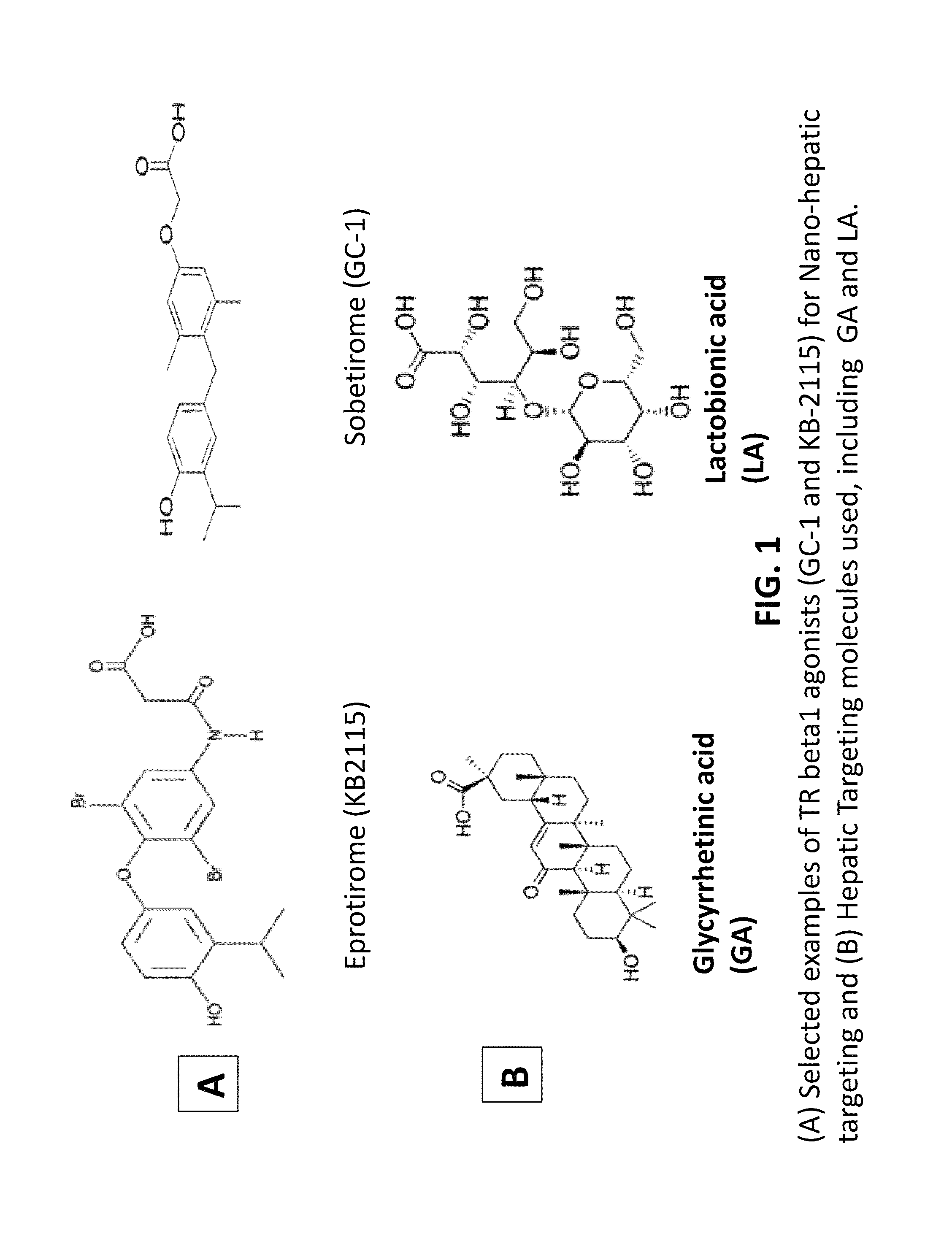
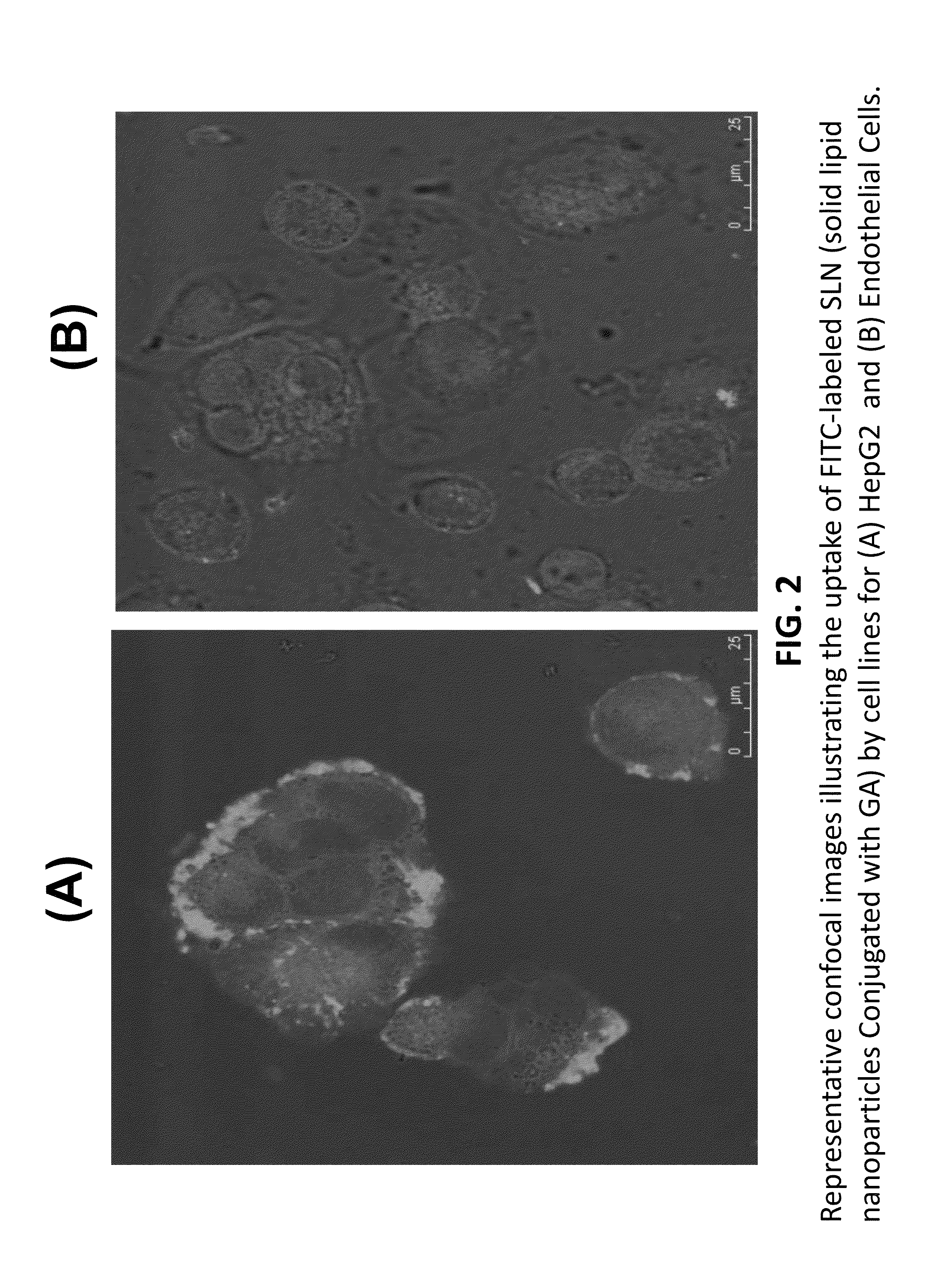
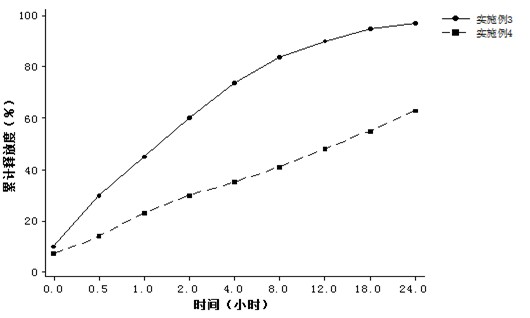
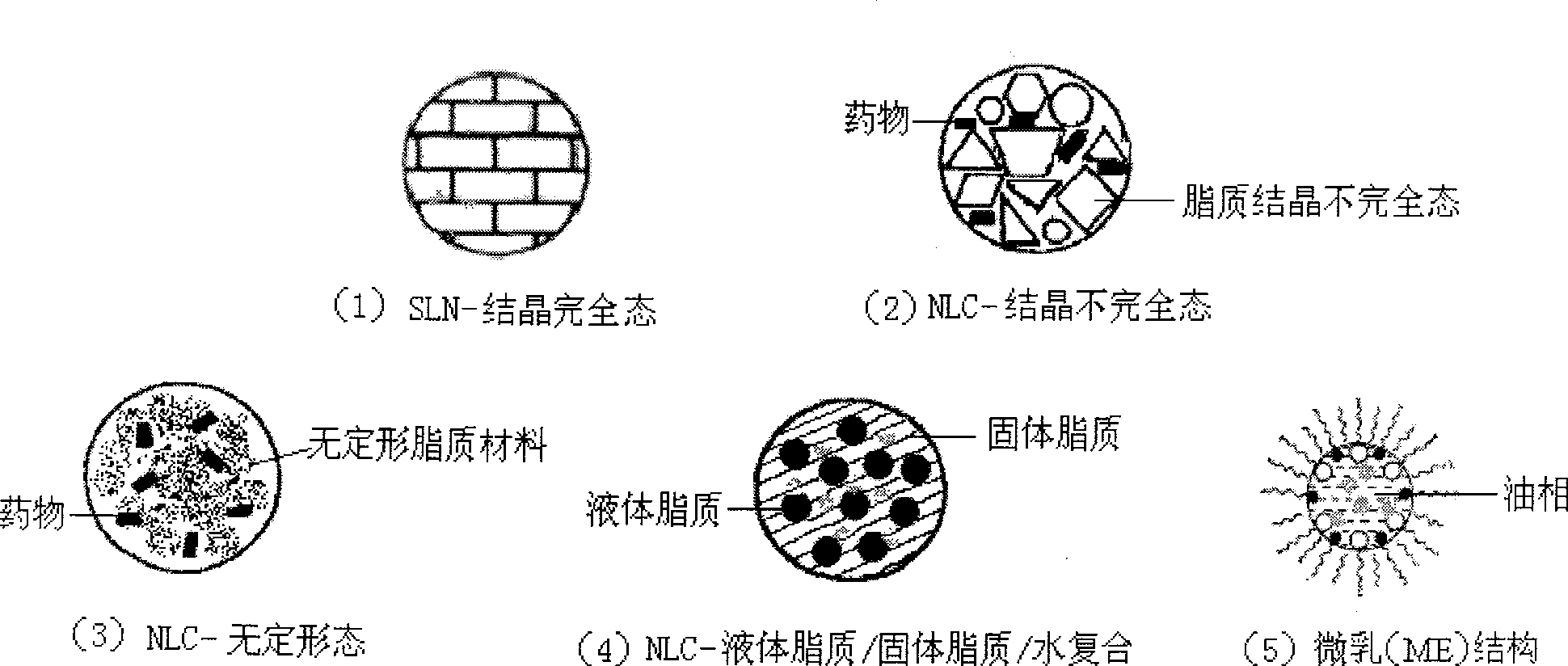
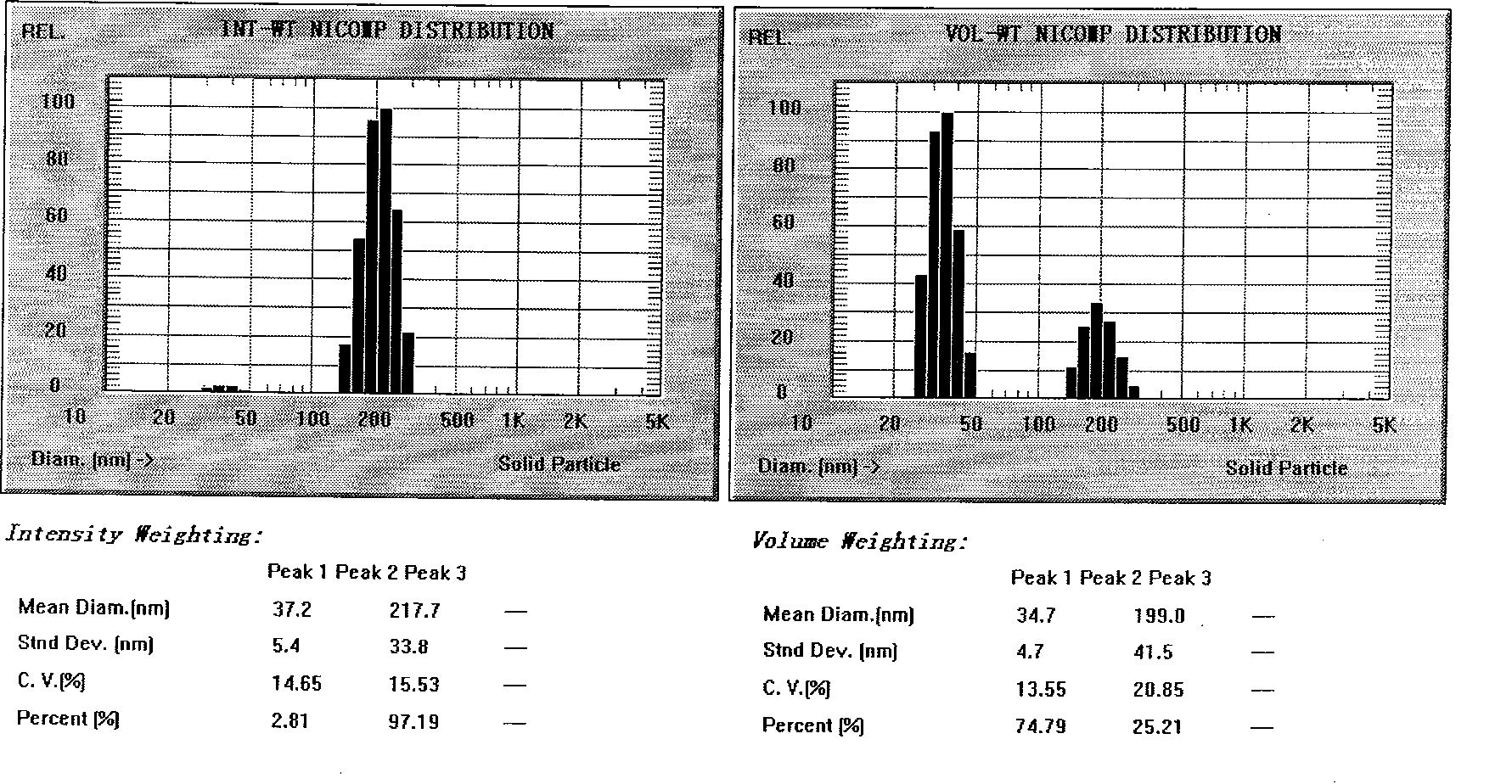
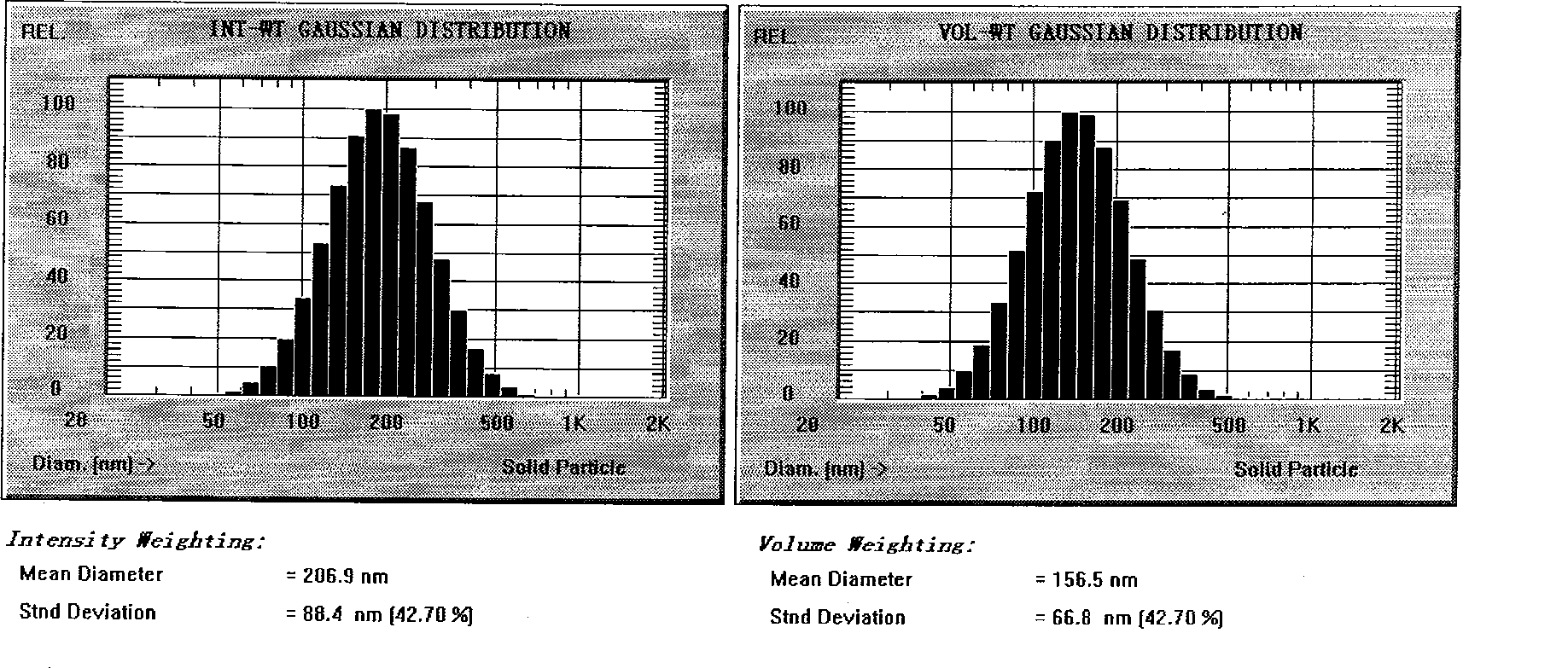
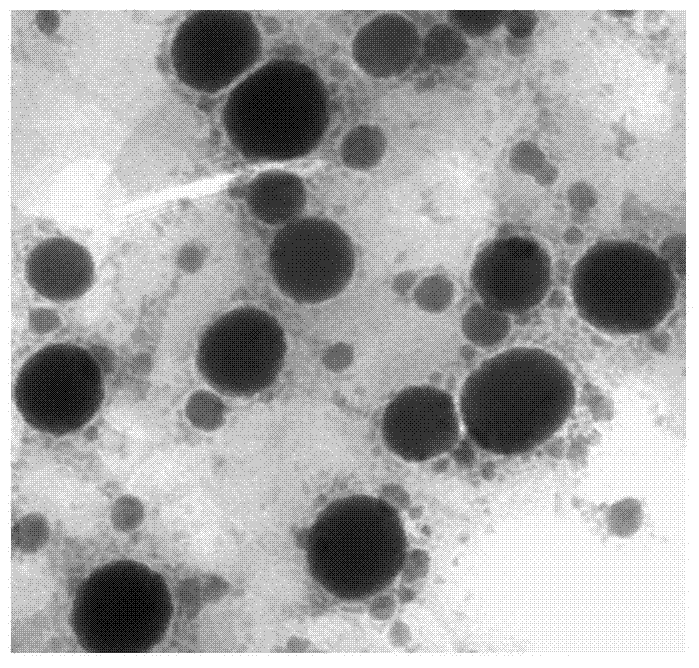
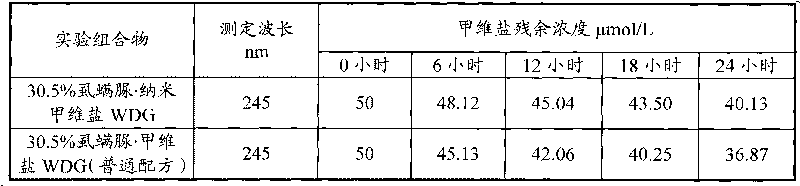
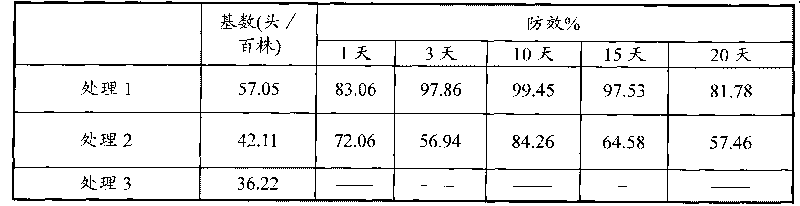
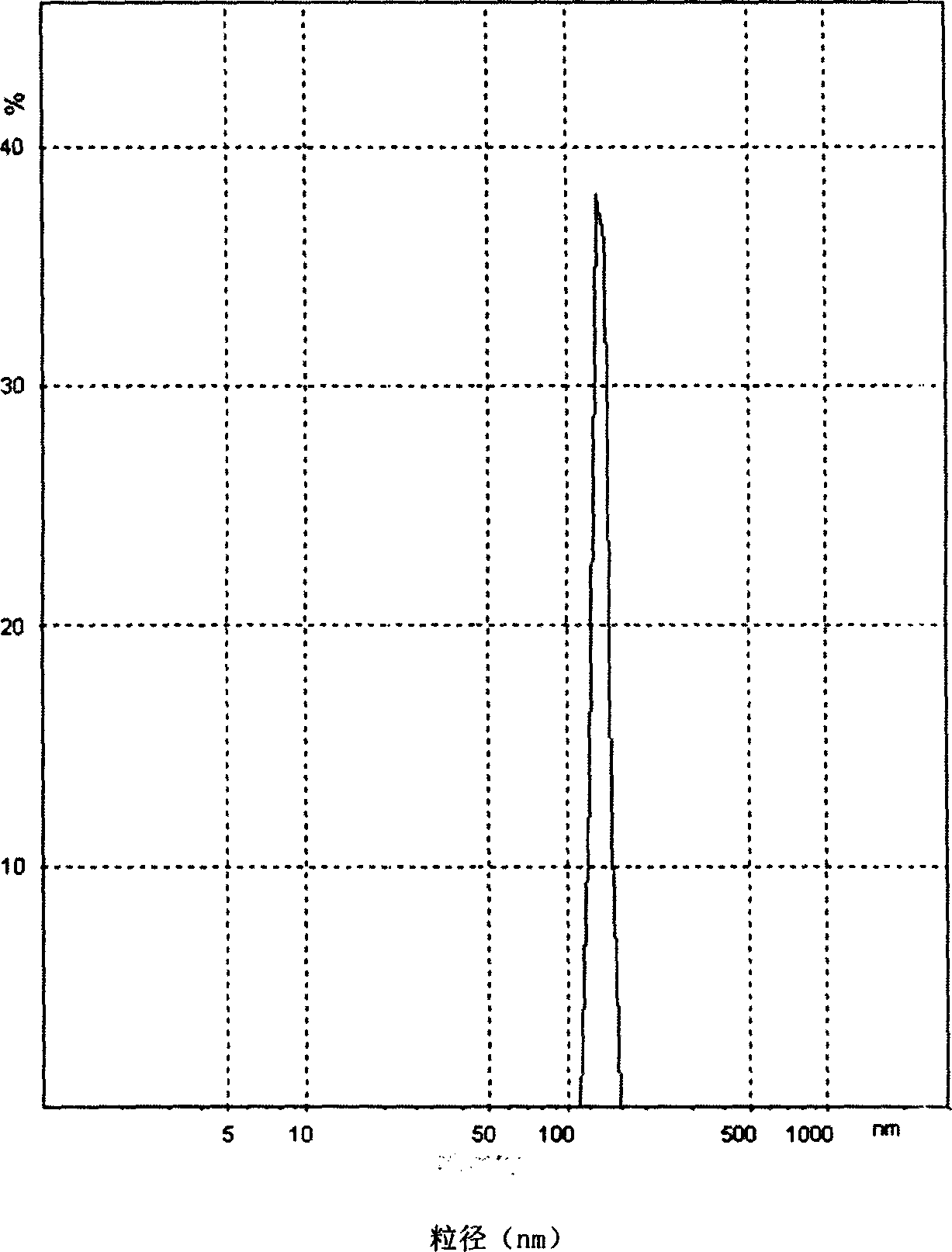
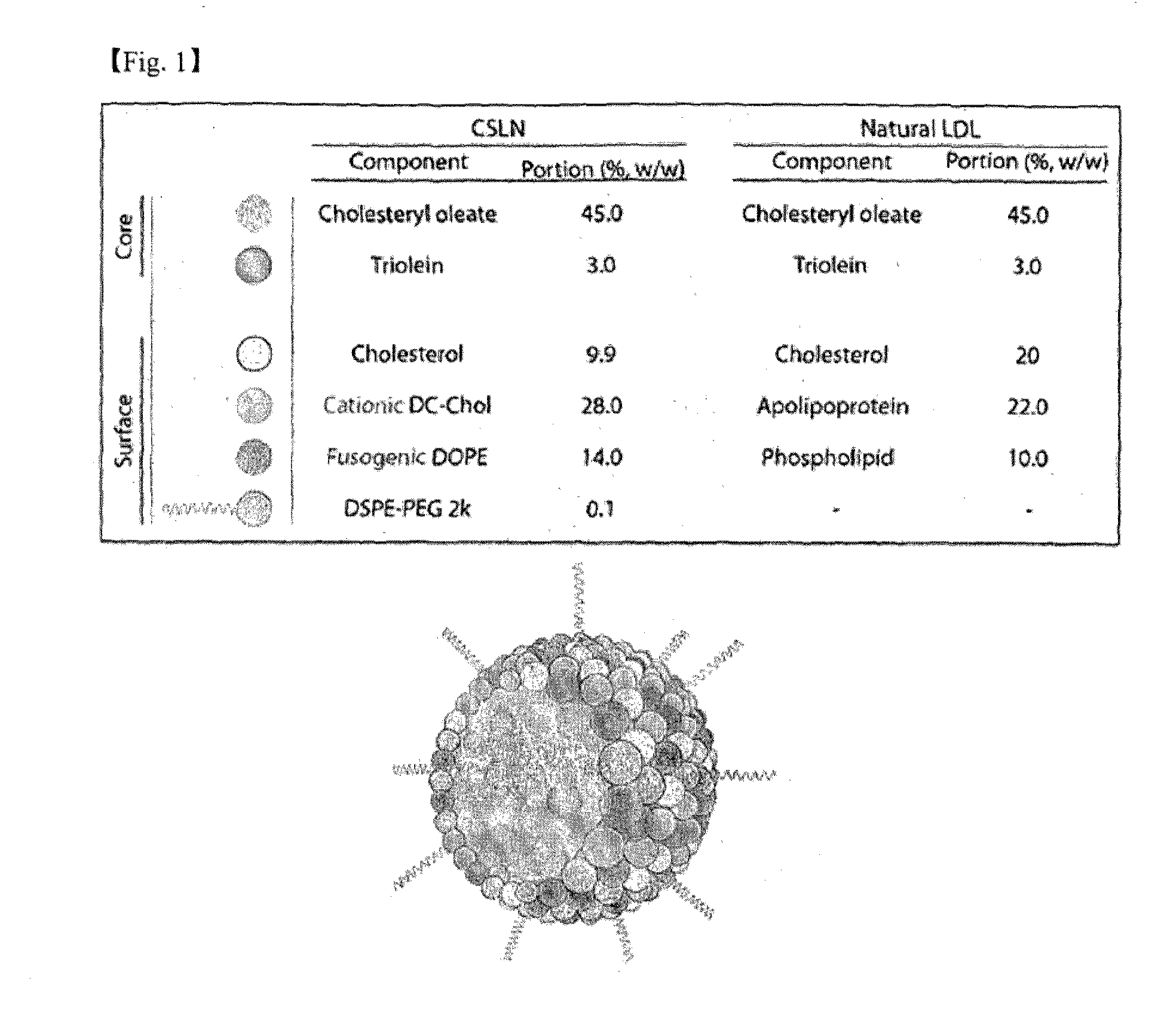
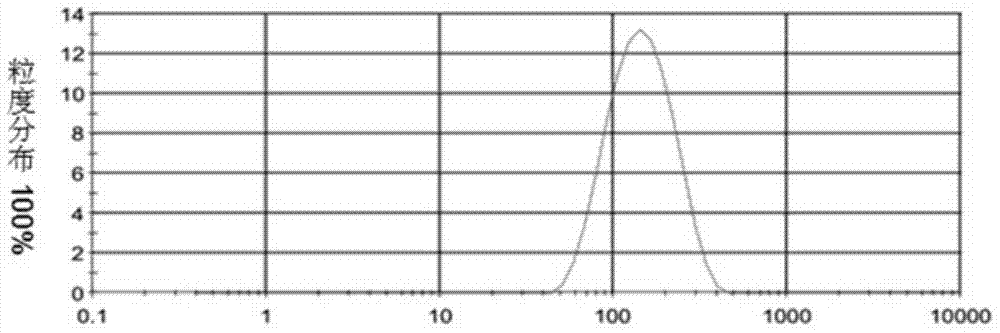
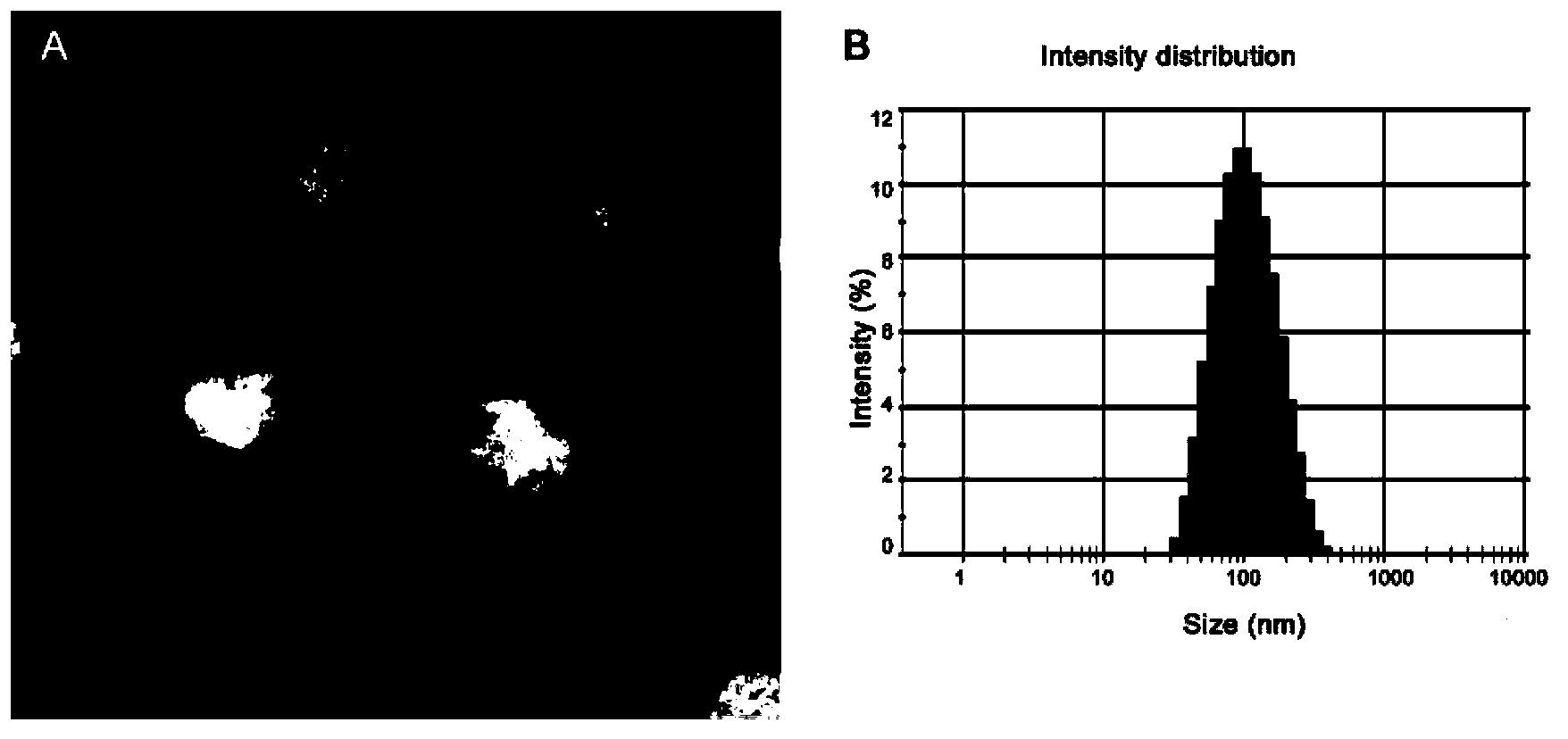
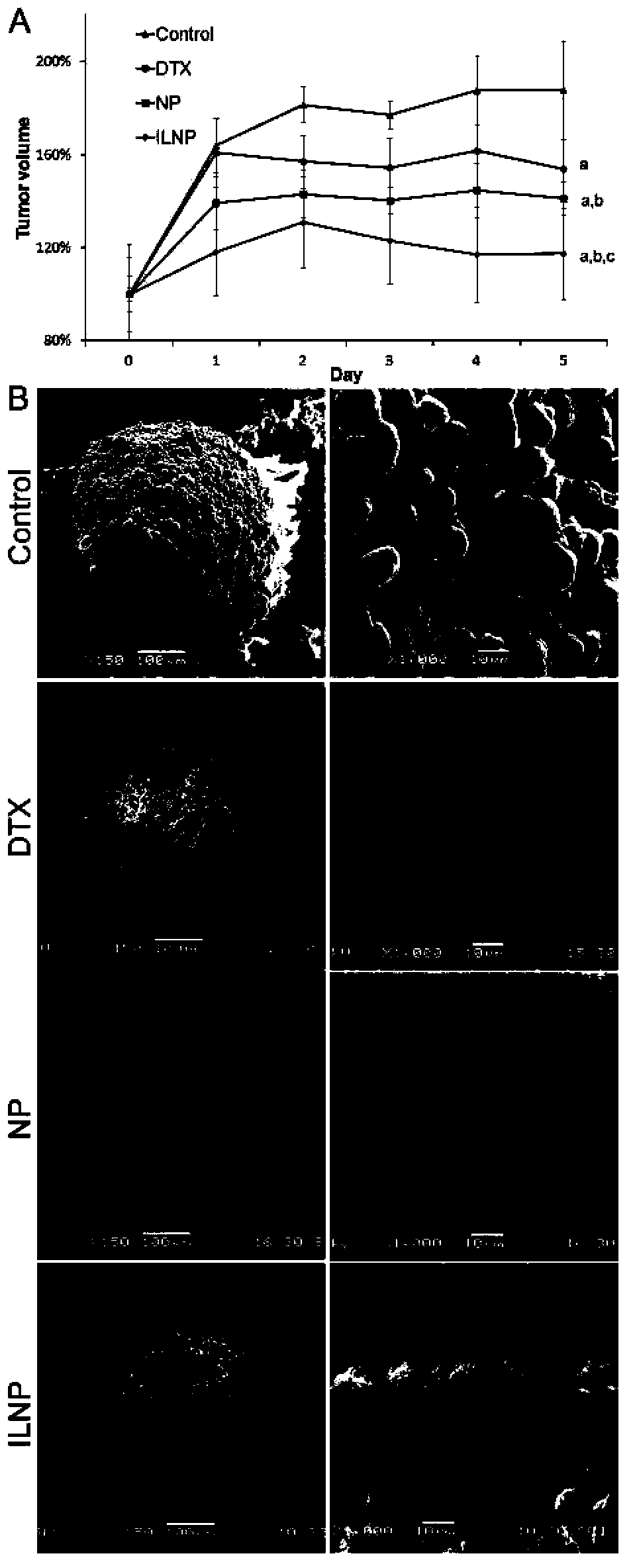
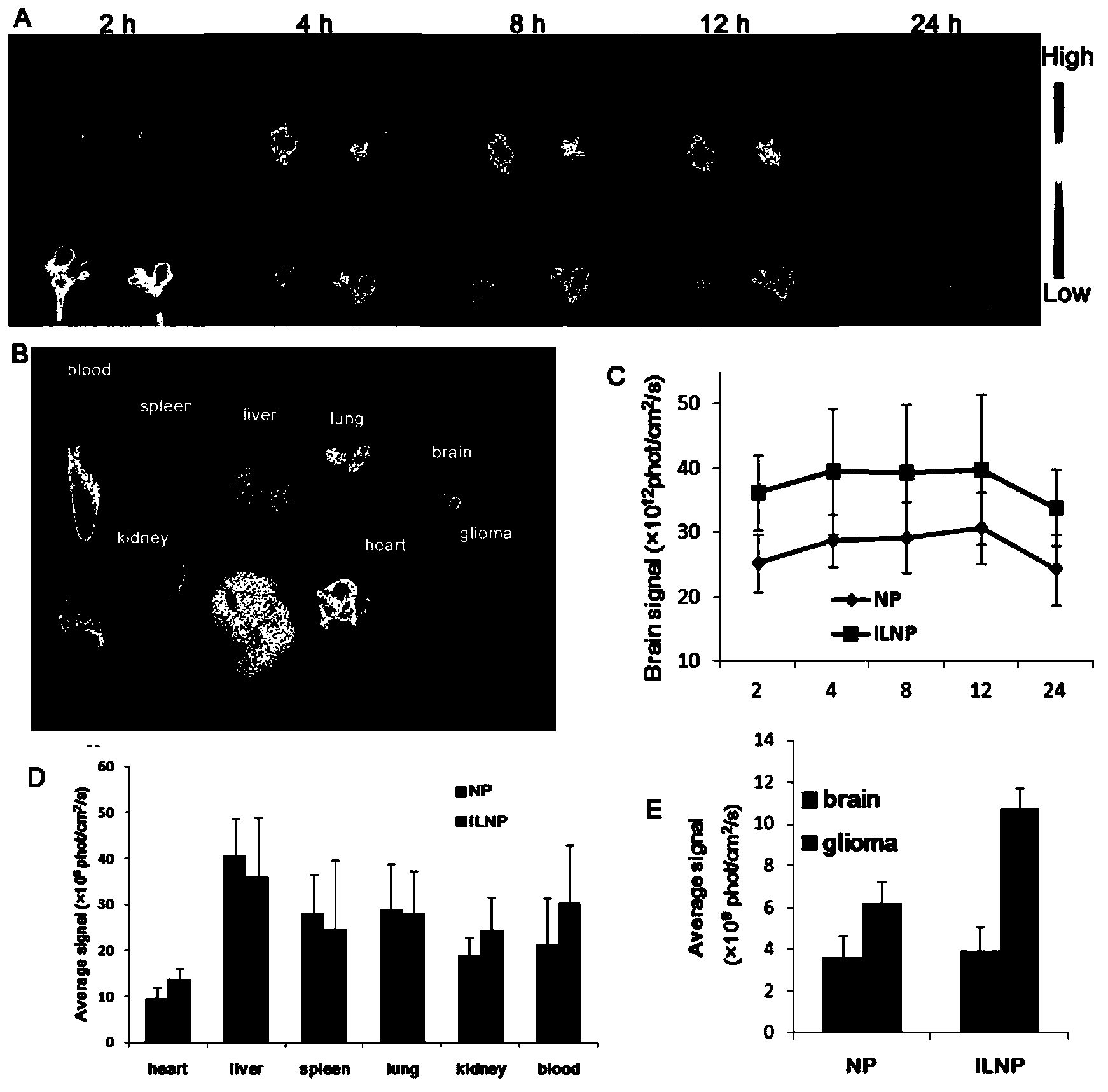
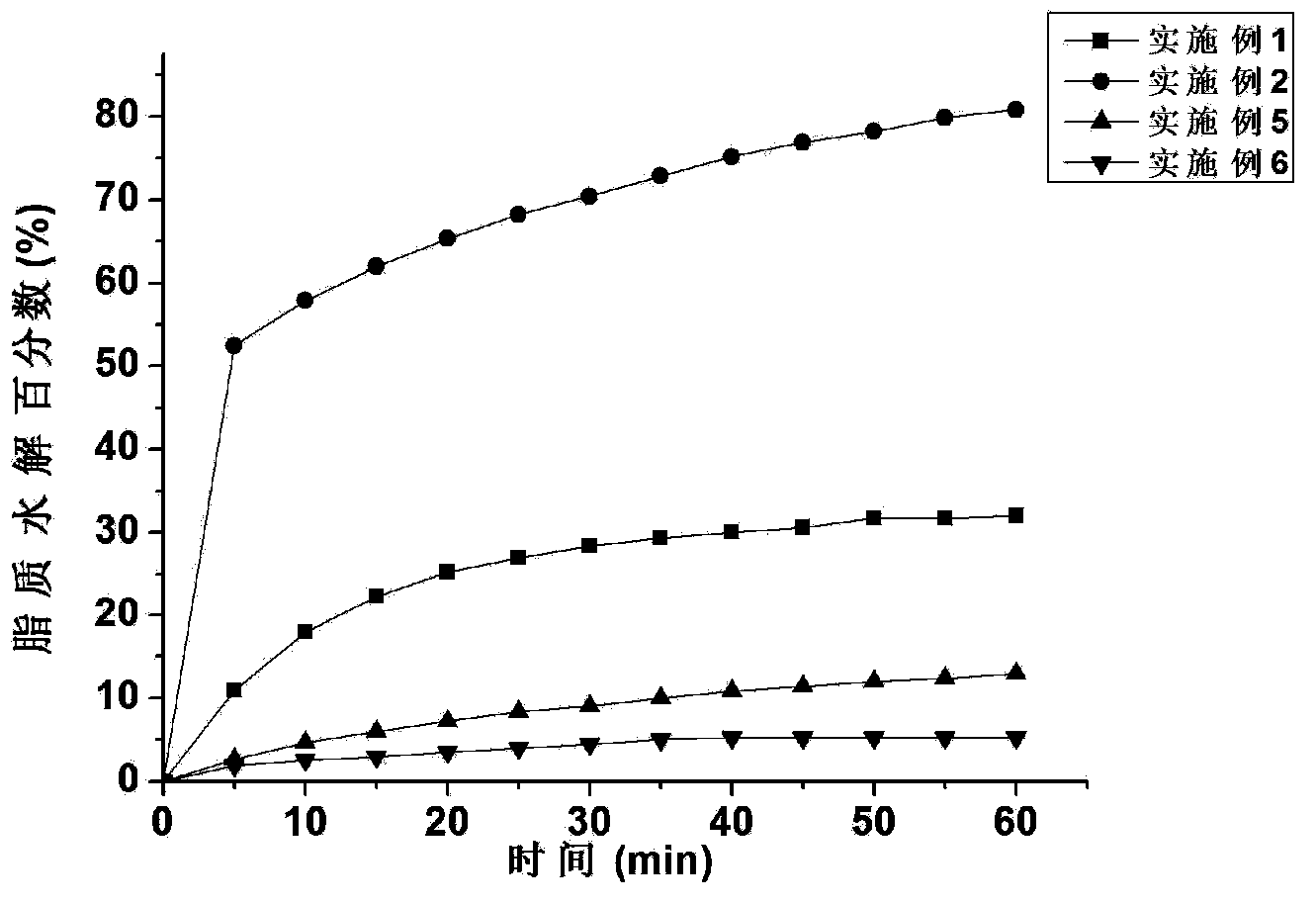
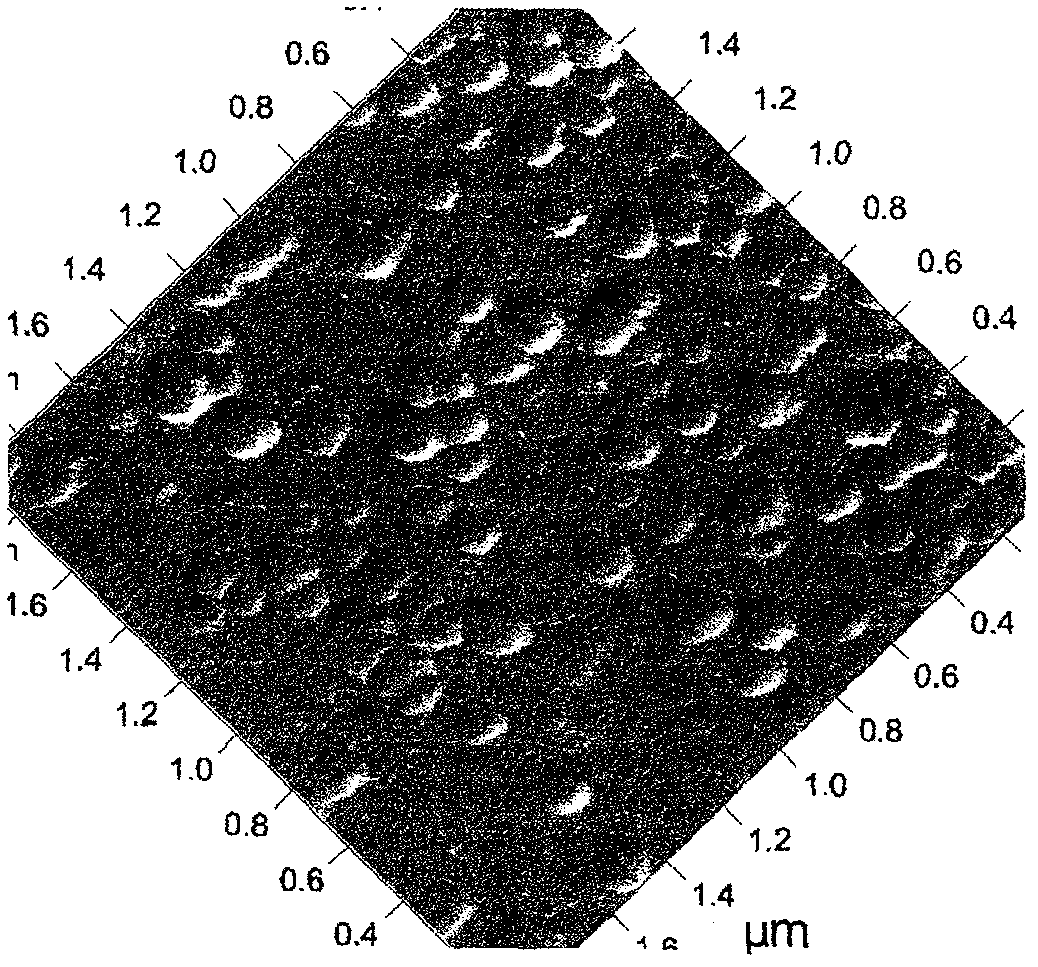
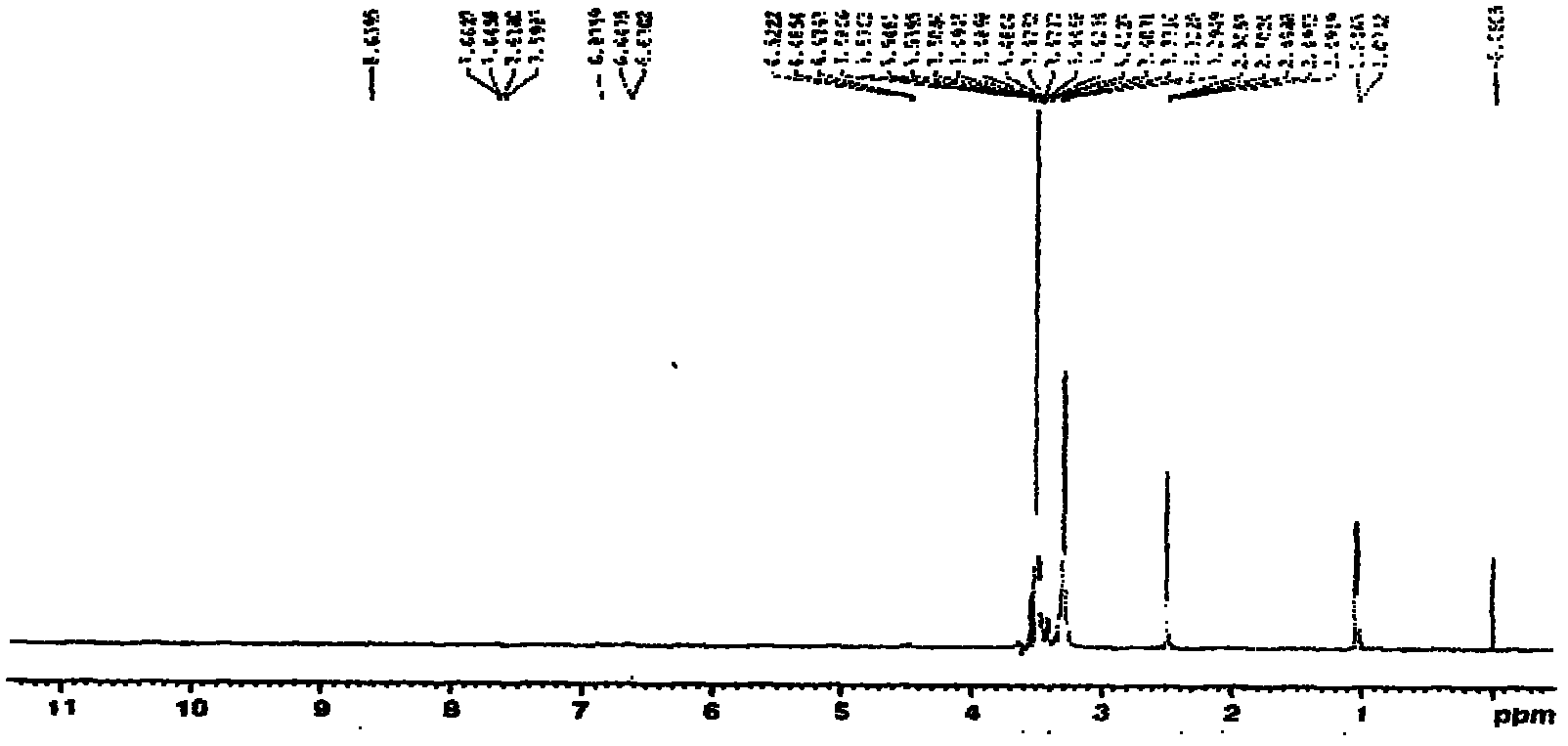
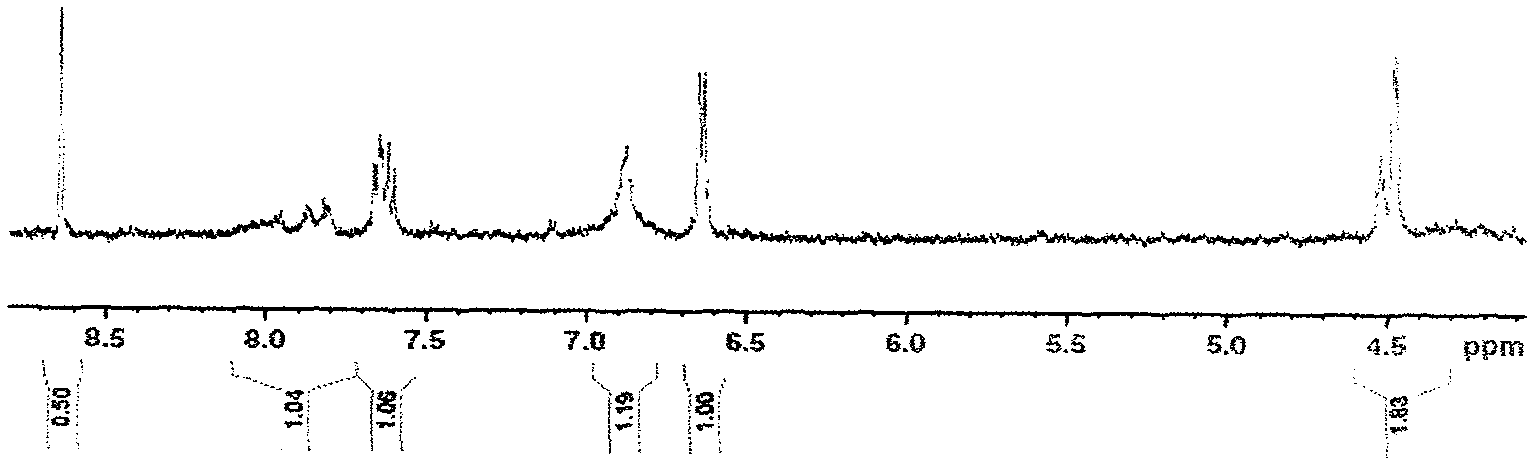
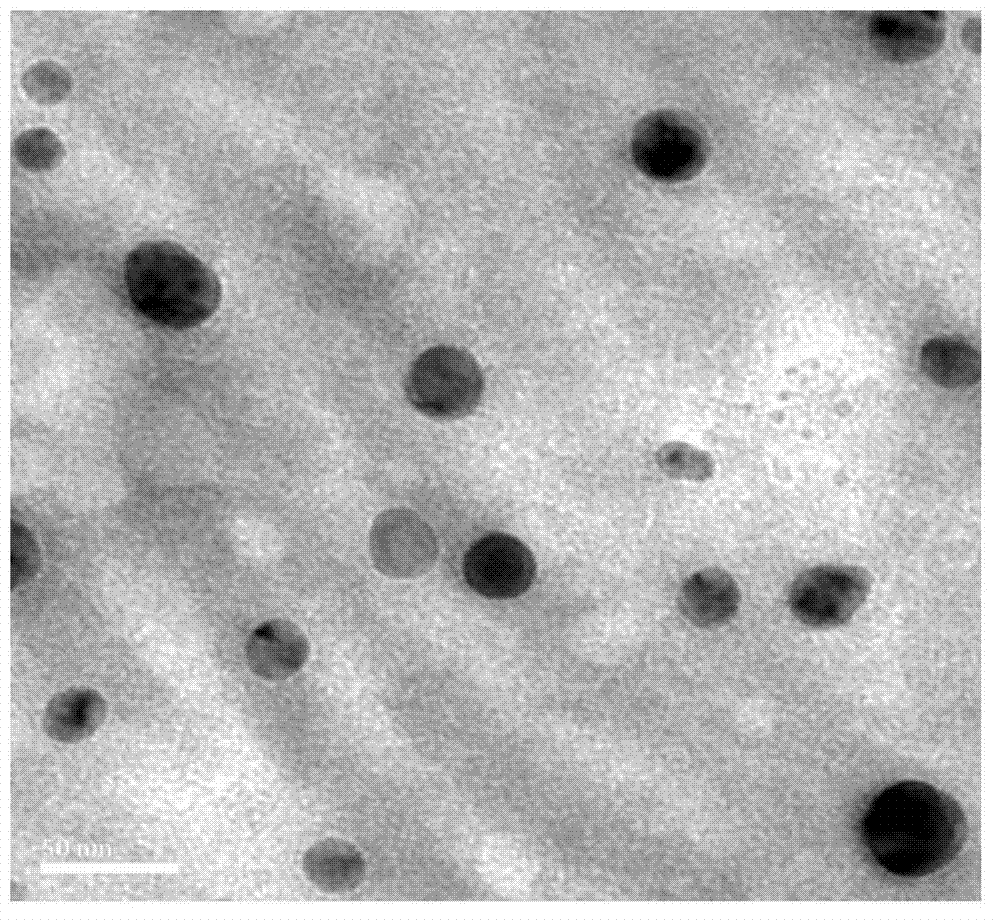
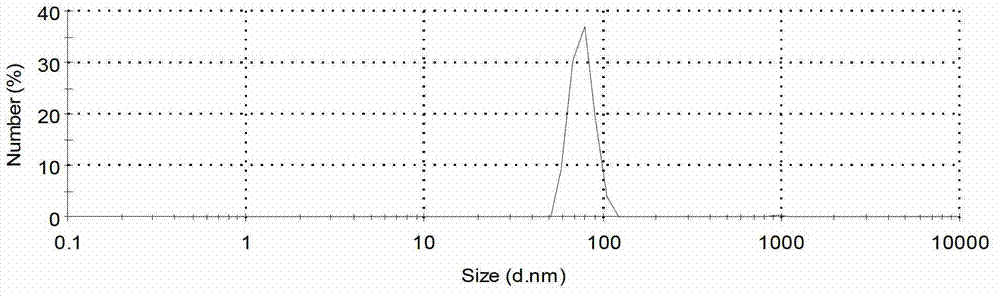
Patents
Literature
215 results about "Solid lipid nanoparticle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid lipid nanoparticles (SLNs) are a new pharmaceutical delivery system or pharmaceutical formulation. The conventional approaches such as use of permeation enhancers, surface modification, prodrug synthesis, complex formation and colloidal lipid carrier based strategies have been developed for the delivery of drugs to intestinal lymphatics. In addition, polymeric nanoparticles, self-emulsifying delivery systems, liposomes, microemulsions, micellar solutions and recently solid lipid nanoparticles (SLN) have been exploited as probable possibilities as carriers for oral intestinal lymphatic delivery.
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS7404969B2Reduce deliveryAntibacterial agentsOrganic active ingredientsLipid formationMolecular composition
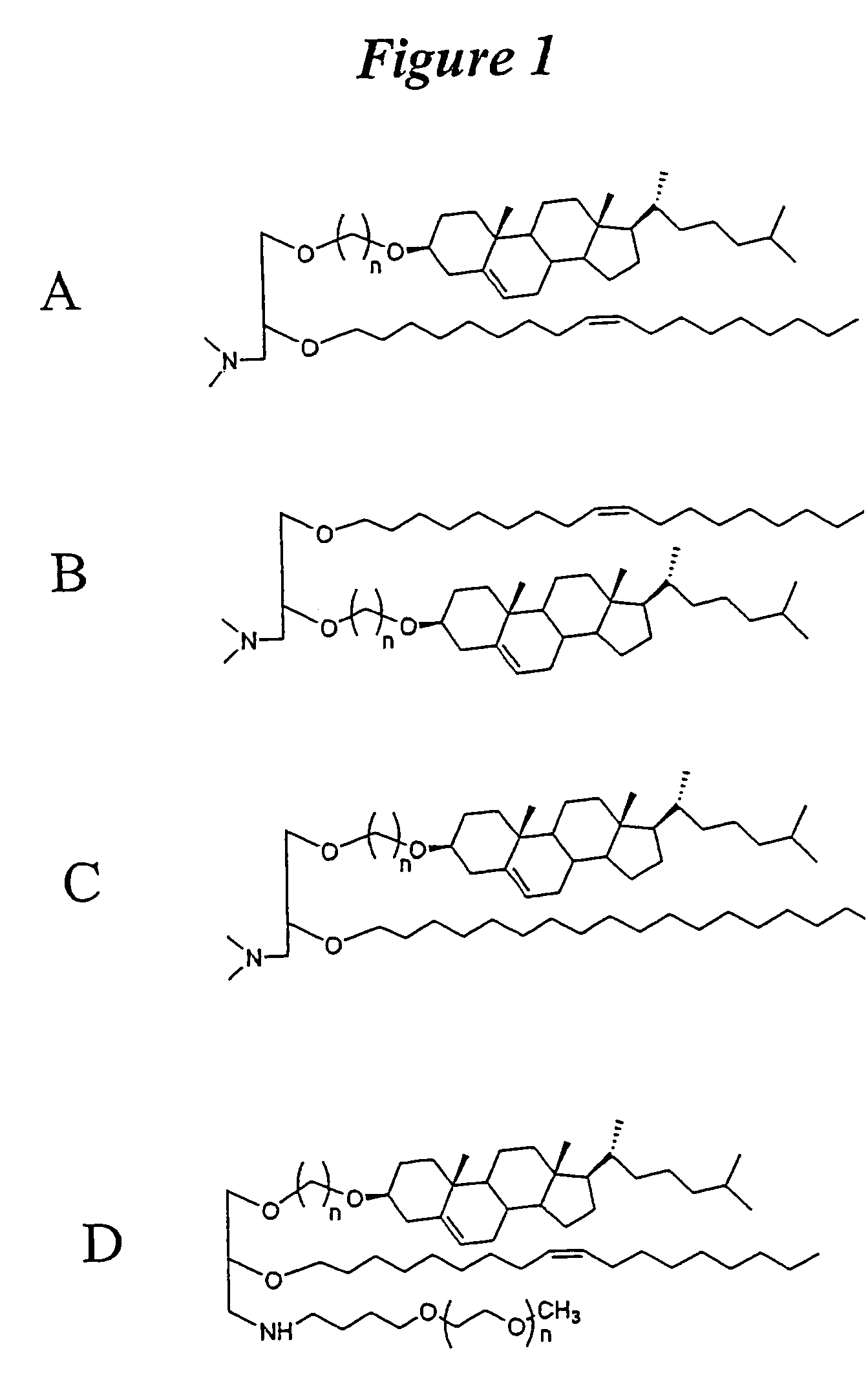
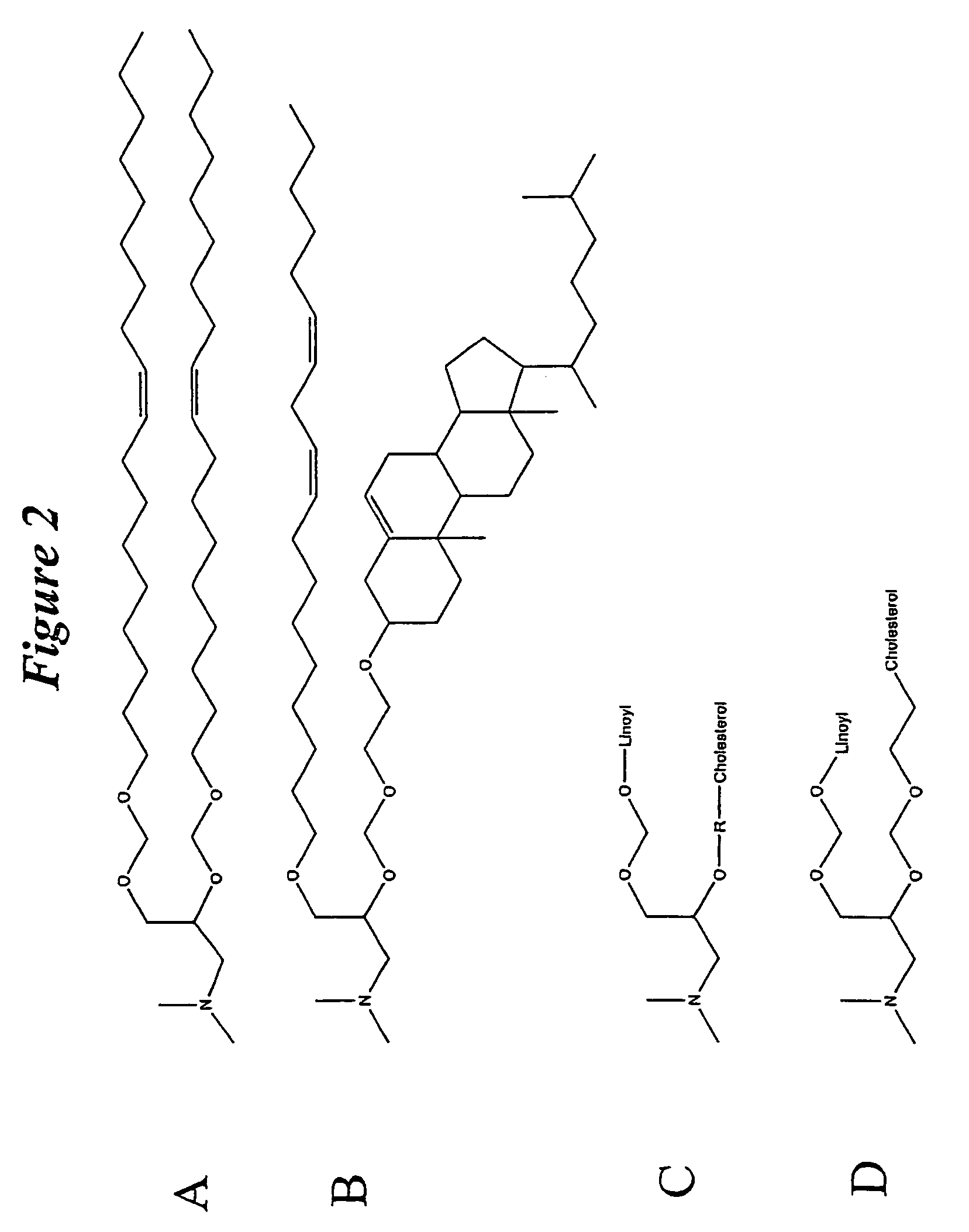
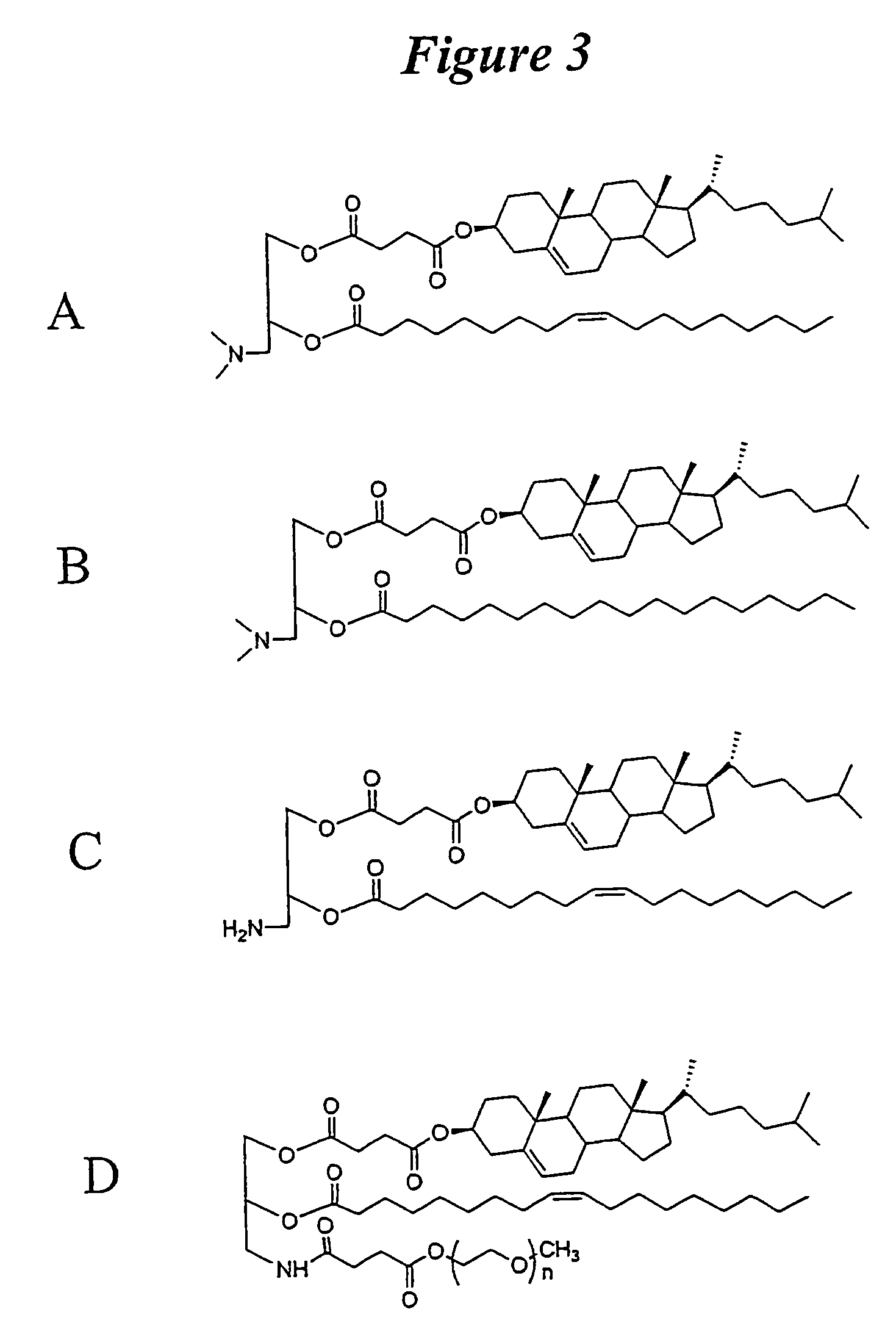
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver short interfering nucleic acid (siNA). The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Lipid nanoparticle capsules
InactiveUS20130017239A1Cosmetic preparationsToilet preparationsLipid formationBULK ACTIVE INGREDIENT
A delivery system for active ingredients which comprises lipid nanoparticles, such as solid lipid nanoparticles (SLN) or nanostructured lipid carriers (NLC), polymerically coated, and their use in the preparation of pharmaceutical, cosmetic and / or alimentary compositions.
Owner:LIPOTEC SA
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
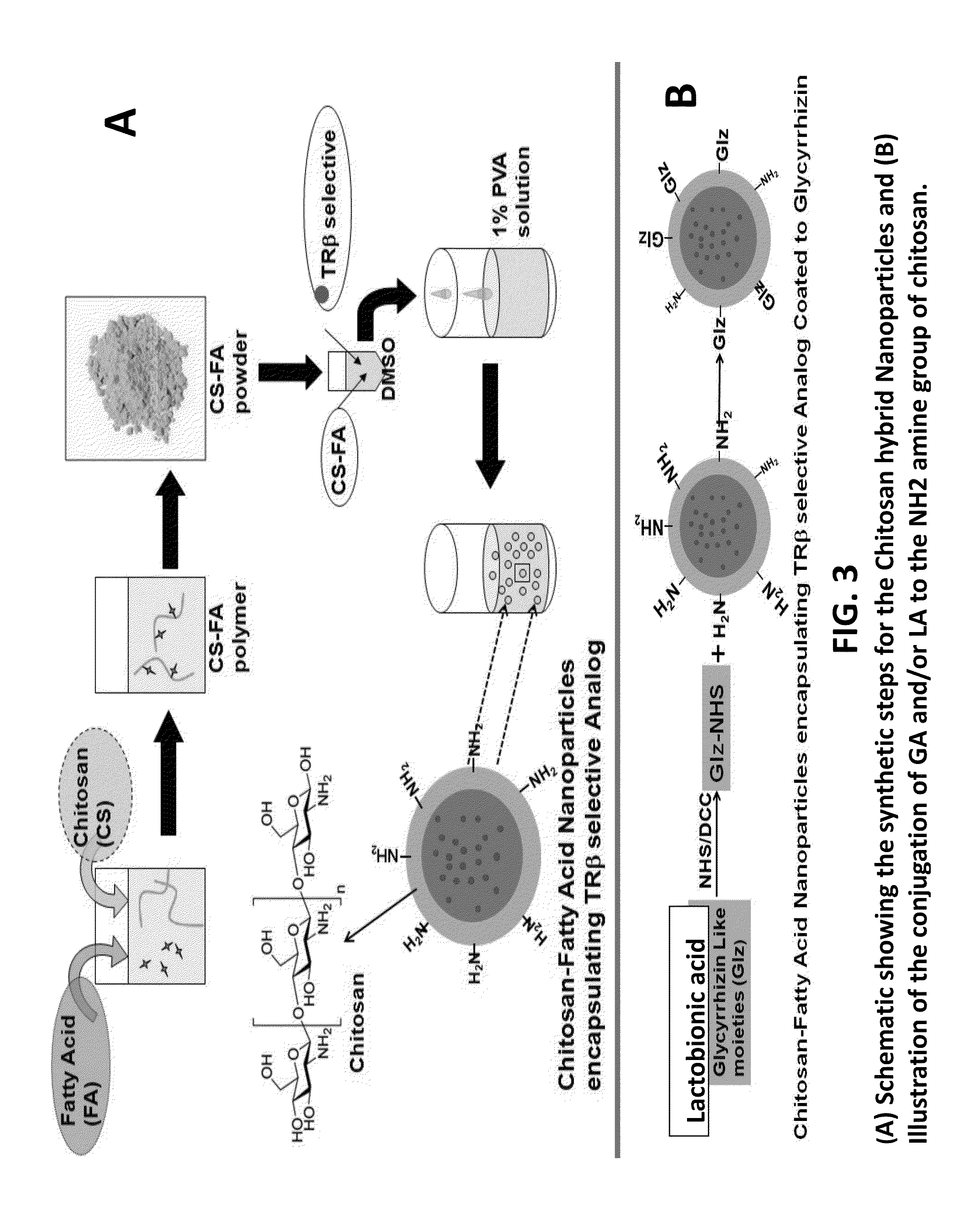
Nanoformulation and methods of use of thyroid receptor beta1 agonists for liver targeting
A composition and an associated method for hepatic targeted delivery of thyroid receptor beta1 (TRβ1) agonist to a liver of a subject. The composition includes hydrophobic nanoparticles, a liver targeting moiety exterior to each nanoparticle and covalently bonded to each nanoparticle, and at least one TRβ1 agonist encapsulated within each nanoparticle. The nanoparticles include chitosan hybrid nanoparticles, amine-modified PLGA nanoparticles, solid lipid nanoparticles, and combinations thereof. The liver targeting moiety includes Glycyrrhetinic acid (GA), Lactobionic acid (LA), or combinations thereof.
Owner:MOUSA SHAKER A
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. The invention also features compositions, and methods of use for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity in a subject or organism. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver biologically active molecules, such as antibodies (e.g., monoclonal, chimeric, humanized etc.), cholesterol, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, dsRNA, allozymes, aptamers, decoys and analogs thereof, and small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules, to relevant cells and / or tissues, such as in a subject or organism. Such novel cationic lipids, microparticles, nanoparticles and transfection agents are useful, for example, in providing compositions to prevent, inhibit, or treat diseases, conditions, or traits in a cell, subject or organism. The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Polymerized solid lipid nanoparticles for oral or mucosal delivery of therapeutic proteins and peptides
InactiveUS20080311214A1Powder deliveryPeptide/protein ingredientsAntigen deliveryTherapeutic protein
The present invention encompasses lipid nano / micro particles, which have been modified, preferably on their surface, to contain a molecule or ligand, which targets the nano / micro particles to a specific site. The invention also encompasses the use of the modified lipid nano / micro particles for the oral delivery of drugs and antigen delivery systems.
Owner:TRANSGENE BIOTEK
Colloidal solid lipid vehicle for pharmaceutical use
The invention provides a drug carrier that includes a solid lipid nanoparticle (SLN), wherein the SLN includes tocopherol or a derivative thereof. The invention also provides a pharmaceutical composition that includes a SLN and a biologically active compound, wherein the SLN comprises tocopherol or a derivative thereof. The present invention further provides a colloidal drug delivery system that includes solid lipid nanoparticles (SLNs), wherein the SLNs comprise tocopherol or a derivative thereof. Also provided are methods for preparing the drug carrier, pharmaceutical composition, and colloidal drug delivery system of the invention.
Owner:ALPHARX
Limit size lipid nanoparticles and related methods
Various lipid nanoparticles are disclosed, including nanoparticles comprising a lipid bilayer comprising a phospholipid, a sterol, a polyethylene glycol-lipid surrounding an aqueous core which comprises a therapeutic and / or diagnostic agent and nanoparticles comprising a lipid monolayer surrounding a hydrophobic core. Of particular interest are limit size lipid nanoparticles with a diameter from 10-100 nm. Such lipid nanoparticles are the smallest particles possible for a specific particle composition. Methods and apparatus for preparing such limit size lipid nanoparticles are disclosed.
Owner:THE UNIV OF BRITISH COLUMBIA
Composition of solid lipid nanoparticles for the long-term conservation of fruits, vegetables, seeds, cereals and/or fresh foodstuffs using a coating
InactiveUS20140205722A1Extended shelf lifeLess perishable productSeed preservation by coatingFatty substance preservation using additivesFresh foodLipophilicity
The invention relates to a composition of solid lipid nanoparticles taking the form of a nano-coating for natural fresh foodstuffs, such as seeds, cereals, fruits or vegetables, preferably fresh fruits and vegetables which are coated by means of fluidization, immersion or spraying. According to the invention, the composition comprises: (a) solid lipids or wax, (b) emulsifying stabilizing agents, and film-forming materials in an aqueous dispersion or solution. The inclusion of a submicronic lipophilic system in aqueous dispersion allows the application of the composition to be easily controlled since it is a fluid system with low viscosity, which is advantageous in that it can be applied easily and uniformly and provides improved coating properties, such as sheen, mechanical strength and gas permeability inter alia.
Owner:UNIV NAT AUTONOMA DE MEXICO
Intracochlear drug delivery to the central nervous system
InactiveUS20110208161A1Improved surgical easeOrganic active ingredientsEar treatmentLipid formationSubarachnoid space
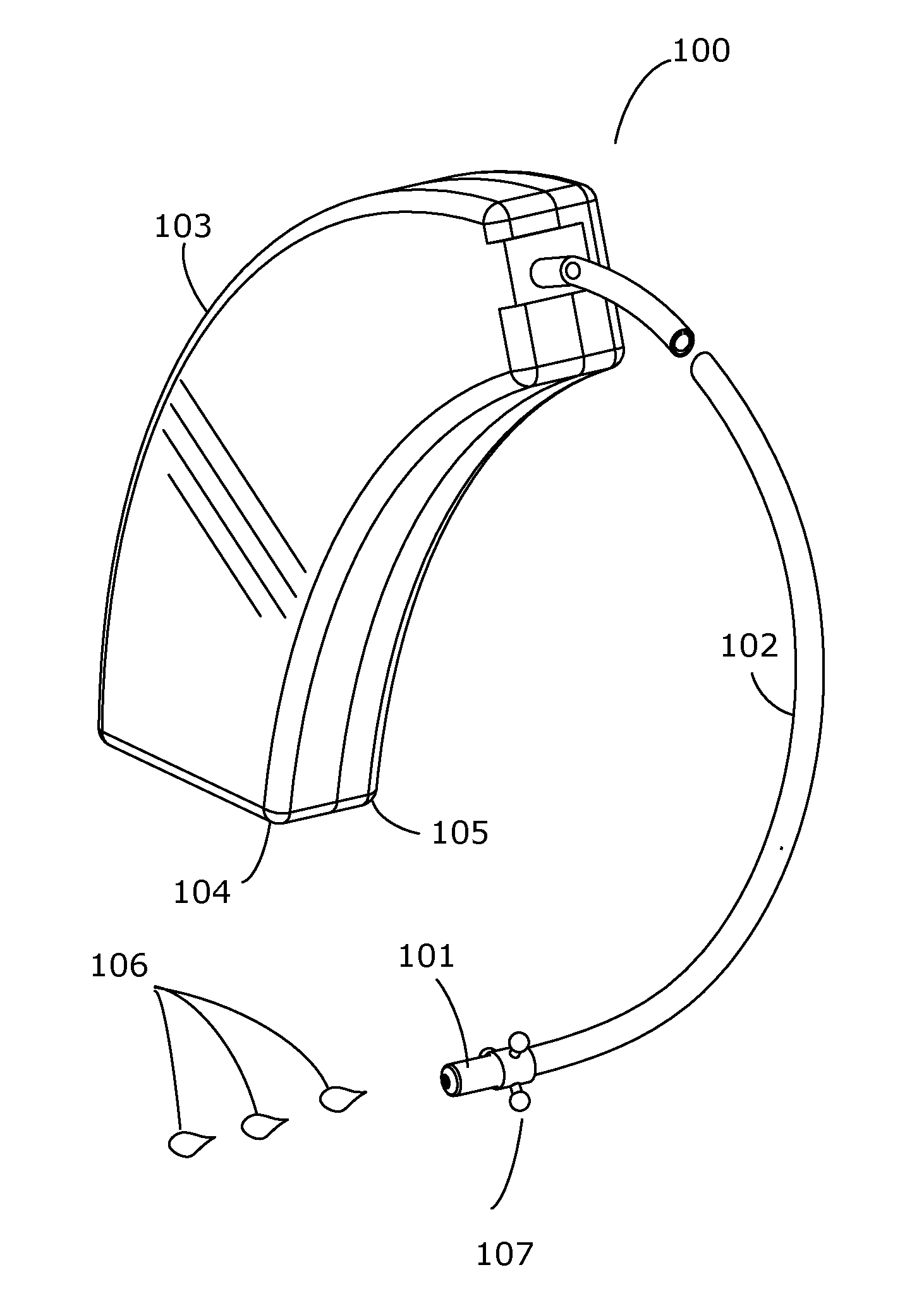
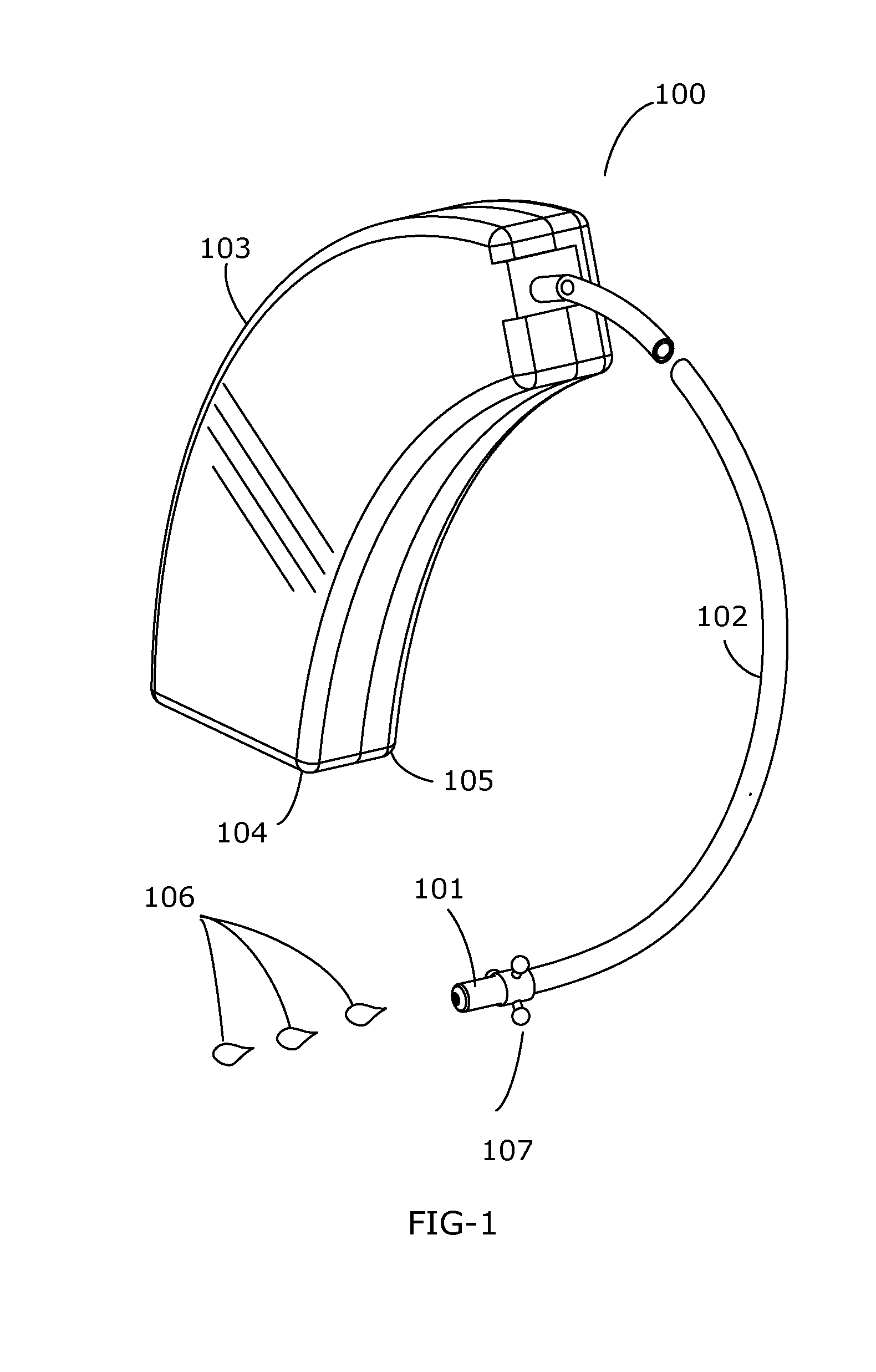
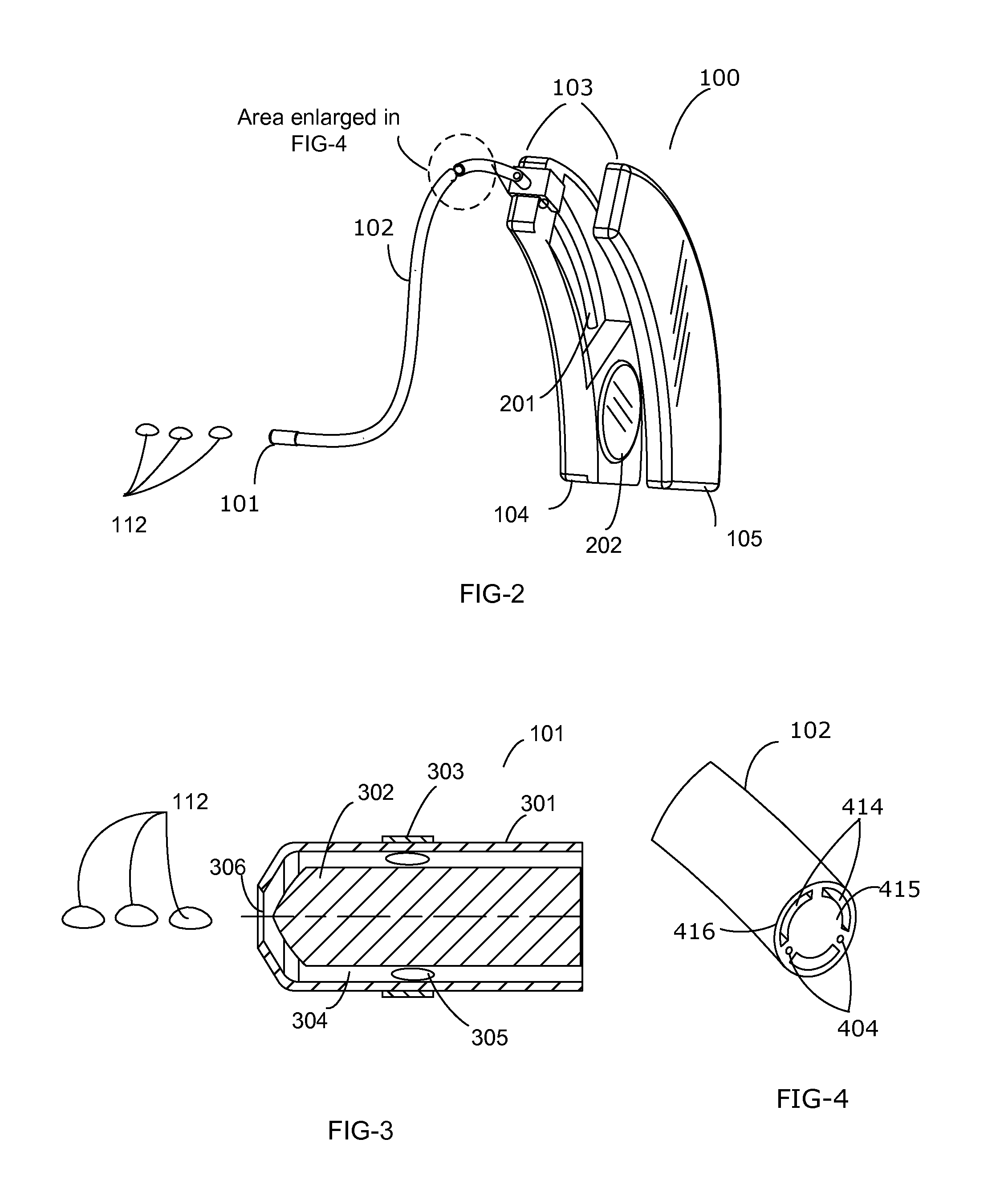
The present invention is directed to method and system for delivery of brain-targeted drugs to the cerebrospinal fluid via the perilymphatic fluid of the inner ear. The system utilizes the passage of cochlear aqueduct as a drug delivery route from the inner ear to the subarachnoid space of the brain. The delivery system includes an otological conduit which enables transfer of drugs from the auditory ear canal to the inner ear and a wearable dispenser for supplying drugs to the otological conduit. The drug composition comprises a suspension of solid lipid nanoparticles (SLN) which facilitate delivery through the cochlear aqueduct. Employing aspects of present invention, a method and system for treating chronic pain is described.
Owner:IVRI YEHUDA
Novel solid lipid nanoparticle medicament delivery system for protein-loaded medicaments
ActiveCN102106821AHigh activityGuaranteed stabilityPowder deliveryAerosol deliveryActive agentBULK ACTIVE INGREDIENT
The invention provides novel solid lipid nanoparticles for protein medicaments and a preparation method thereof. Each solid lipid nanoparticle consists of two parts, namely a core and a shell, wherein the core is that a biosurfactant forms a micelle or an aggregate to wrap active ingredients, or / and the biosurfactant and the active ingredients form a synergic aggregate; and the shell is that a solid lipid material is adopted for encapsulating the core. A novel solid lipid nanoparticle medicament delivery system has extremely high safety, can effectively protect the biological activity of polypeptides and polypeptide medicaments and remarkably improve the stability of preparations of the polypeptides and the polypeptide medicaments at the same time, can be applied in multiple ways such as injection, non-injection and the like, and achieves better bioavailability.
Owner:SICHUAN UNIV
Docetaxel solid lipid nanoparticle and preparation method thereof
InactiveCN102579341AGood chemical stabilityImprove stabilityPowder deliveryOrganic active ingredientsTumour targetingDocetaxel
The invention belongs to the field of medicine preparation and particularly relates to a docetaxel solid lipid nanoparticle and a preparation method thereof. The docetaxel solid lipid nanoparticle consists of an effective curative dose of docetaxel, solid lipid materials, liquid lipid materials, an emulsifying agent, additives, long-circulating auxiliary materials and water for injection. The docetaxel solid lipid nanoparticle provided by the invention has the characteristics of stable docetaxel structure, phagocytosis prevention of an in-vivo reticuloendothelial system and active tumor targeting, and can be stably stored.
Owner:SHENYANG PHARMA UNIVERSITY
Novel nano-lipid carrier for injection embodying paclitaxel series substances and preparation method thereof
InactiveCN101366697AImprove complianceDoes not cause toxic reactionsOrganic active ingredientsAntineoplastic agentsLipid formationFreeze-drying
The invention provides a novel nanometer lipid carrier containing a taxol substance for injection and a method for preparing the same. Mixed lipids are used as materials; liquid lipid and solid lipid with different physical states are mixed according to the proportion of between 0 and 100 percent to obtain the nanometer lipid carrier (NLC) which is prepared by a mixed solid-liquid substrate and has different crystalline states; and microemulsion (ME) prepared by a full liquid substrate and solid lipid nanoparticles (SLC) prepared by a full solid substrate are also obtained. The new nanometer lipid carrier can increase or improve the encapsulation efficiency and the release property of drug and has the characteristics of high drug-loading amount, good stability and low toxicity. The taxol substance, a carrier material, a surfactant and injection water are respectively stirred to form two phases of oil and water; the oil phase and the water phase are mixed and emulsified to obtain primary emulsion; further taxol substance nanometer lipid carrier injection or suspension type injection which meets the requirement of intravenous injection is prepared by a high-pressure homogenization process; or a protective agent is added into the injection to prepare solid powder for injection by vacuum freeze drying or spray drying; before use, normal saline, a glucose solution or a Ringer solution is used for dilution; and the solid powder can be rapidly dissolved. Experiments show that the prepared nanometer lipid carrier containing the taxol substance for injection can improve targeting property to tumor cells, improve curative effect, greatly reduce toxic and side effects, has simple preparation process and low cost and is suitable for large-scaled industrialized production.
Owner:李淑斌
Curcumin and piperine carried solid lipid nanoparticles and preparation method thereof
ActiveCN103784421AUniform particle sizeStable particle sizeKetone active ingredientsPharmaceutical non-active ingredientsLipid formationSolubility
The invention relates to curcumin and piperine carried solid lipid nanoparticles. The curcumin and piperine carried solid lipid nanoparticles are prepared from 0.1-5% of curcumin, 0.1-5% of piperine, 10%-70% of solid lipid material, 5%-30% of liquid oil phase, 10%-60% of emulsifier and the balance of water by weight percentage. A preparation method of the curcumin and piperine carried solid lipid nanoparticles can be a thin film dispersion method, a micro-emulsion method or an emulsifying evaporation-low temperature solidification method. Compared with the existing forms, the curcumin and piperine carried solid lipid nanoparticles have the advantages that the solubility of the curcumin and piperine drugs is improved, the stability and the release degree in vitro of the drugs in the nanoparticles are improved and the effects of anti-tumor and inversion of multidrug resistance of the curcumin are enhanced.
Owner:HARBIN MEDICAL UNIVERSITY
Neo-gambogic acid SLN (solid lipid nanoparticle) and preparation method thereof
InactiveCN101947204ALow toxicityImprove tolerancePowder deliveryOrganic non-active ingredientsLipid formationSolubility
The invention relates to a neo-gambogic acid SLN (solid lipid nanoparticle) and a preparation method thereof. The neo-gambogic acid SLN comprises a therapeutically effective amount of neo-gambogic acid, medicinal phosphatide, a surfactant and a lipid material. In the invention, the neo-gambogic acid is prepared into SLNs (solid lipid nanoparticles), thereby improving the solubility of the neo-gambogic acid, reducing the irritability, improving the bioavailability, and prolonging the action time of medicaments in a human body, in addition, the neo-gambogic acid SLNs can be gathered partially in the human body so as to play the targeted action of the SLNs and exert the anti-cancer therapeutic action of the SLNs better.
Owner:彭代银 +4
Emamectin benzoate solid lipid nanoparticle and preparation method and application thereof in pesticide formulation
InactiveCN101692808AHigh affinityDelayed photolysisBiocideAnimal repellantsWater dispersibleChlorfenapyr
The invention discloses an emamectin benzoate solid lipid nanoparticle and a preparation method for a water dispersible granule, a suspension concentrate and wettable powder of the composition of the emamectin benzoate solid lipid nanoparticle with lufenuron, indoxacarb, pleocidin, chlorfenapyr and chlorantraniliprole. Compared with the composition preparation which is prepared by directly adopting the emamectin benzoate raw pesticide as raw material, the emamectin benzoate solid lipid nanoparticle has the advantages of improving the chemical stability of hot and cold storage, reducing the photolysis rate, prolonging efficacy time, reducing dosage, and significantly improving insecticidal effect.
Owner:SHENZHEN NOPOSION AGROCHEM
Curcumenol solid lipid nano-particle and its preparation method
InactiveCN1765356AReduce solubilityProtectiveOrganic active ingredientsPowder deliveryLipid formationPhospholipid
The invention provides a curcumenol solid lipid nanoparticle and its preparation method, which comprises curcumenol 1 part, phospholipids 3-10 parts, grease material 5-100 parts, surface active agent 10-200 parts and excipient 10-400 parts.
Owner:NANJING UNIV OF TECH
Low-density lipoprotein analogue nanoparticles, and composition comprising same for targeted diagnosis and treatment of liver
InactiveUS20150297749A1Avoid eliminationProcess stabilityOrganic active ingredientsPowder deliveryParenchymaHepatic Diseases
This disclosure relates to a low density lipoprotein-like cationic solid lipid nanoparticle targeting liver cells including parenchyma cells and non-parenchyma cells, a composition for liver target delivery, a composition for diagnosis and / or treatment of liver disease comprising the same, and a method for liver targeting of an active ingredient.
Owner:POSTECH ACAD IND FOUND
Solid lipid nanometer particle for astaxanthin and preparation method of solid lipid nanometer particle
ActiveCN104257632AImprove solubilityHigh yieldOrganic active ingredientsAntinoxious agentsLipid formationSolubility
The invention belongs to the technical field of medicines, and particularly relates to a solid lipid nanometer particle for astaxanthin and a preparation method of the solid lipid nanometer particle. The solid lipid nanometer particle for the astaxanthin is prepared from lipid materials and an aqueous phase according to a weight ratio of 1:11, wherein the lipid materials comprise the following components in percentage by weight: 1-15% of solid fat, 85-98.9% of vegetable oil and 0.1% of astaxanthin; the aqueous phase comprises the following components in percentage by weight: 1-10% of surfactants and 90-99% of deionized water. A lipid kernel is arranged in the solid lipid nanometer particle provided by the invention, the aqueous phase is arranged outside the solid lipid nanometer particle for the astaxanthin, and the solubility of the astaxanthin in water is increased, so that the absorptivity and the bioavailability of fat-soluble components, i.e., the astaxanthin, in gastrointestinal tracts are improved; moreover, the solid lipid nanometer particle for the astaxanthin is in a subtransparent state, the indexes of emulsion, such as the average particle diameter, the Zeta electric potential and the dispersion index PDI, are good, isomerase and degradation are not easy to generate, and the solid lipid nanometer particle for the astaxanthin has better stability.
Owner:BEIJING UNIV OF CHEM TECH
Targeting nanometer drug delivery system aiming at glioma
ActiveCN103622915AAchieving Targeted TherapyIncrease intakePowder deliveryMacromolecular non-active ingredientsNanocarriersPolyethylene glycol
The invention belongs to the field of medicine preparation, and in particular to a targeting nanometer drug delivery system aiming at glioma, and a preparation method and application thereof. The drug delivery system comprises target functional molecules, a drug and nano carriers. The target functional molecules are from short chain polypeptide from interleukin 13; the drug is a micromolecular anti-glioma drug; the nano carriers are liposome with surface modified by polyethylene glycol, nanoparticles, polymeric vesicles, polymer micelles and solid lipid nanoparticles; and the drug is enveloped in the nano carriers in an enveloping or covalent connection manner, and the short chain polypeptide is connected with the polyethylene glycol on the surfaces of the nanoparticles through covalent connection. The drug delivery system can promote uptake of the glioma cells by mediated effect of an interleukin 13 receptor alpha 2 on the surfaces of the glioma cells, so as to improve the effect of anti-glioma chemotherapeutics.
Owner:FUDAN UNIV
Sunscreen spray containing solid lipid microparticles and preparation method of sunscreen spray
InactiveCN103919701AReduce stimulationReduce penetrationCosmetic preparationsToilet preparationsSunscreen agentsPreservative
Owner:SHANGHAI INST OF TECH
Curcumin solid lipid nanoparticle with P-gp inhibiting effect and preparation method thereof
InactiveCN103655519AImprove bioavailabilityImprove stabilityMetabolism disorderAntipyreticLipid formationSolubility
The invention relates to a curcumin solid lipid nanoparticle with a P-gp inhibiting effect, and a preparation method and application thereof. The curcumin solid lipid nanoparticle comprises the following components according to mass ratio: 0.05-1% of curcumin, 5-15% of lipid material, 5-15% of an emulgator and the balance of water. According to the invention, the solid lipid nanoparticle technology is adopted to encapsulate curcumin, so that the stability of medicine is increased, the solubility of medicine is improved, the excretion function of small intestines is reduced, and the bioavailability of curcumin is improved. In addition, the preparation method of the curcumin solid lipid nanoparticle, disclosed by the invention, is the emulsification evaporation and low temperature solidification method which is simple and convenient and suitable for being used in a laboratory, and has low requirements for an apparatus.
Owner:HARBIN MEDICAL UNIVERSITY
Functional edible film and preparation method thereof
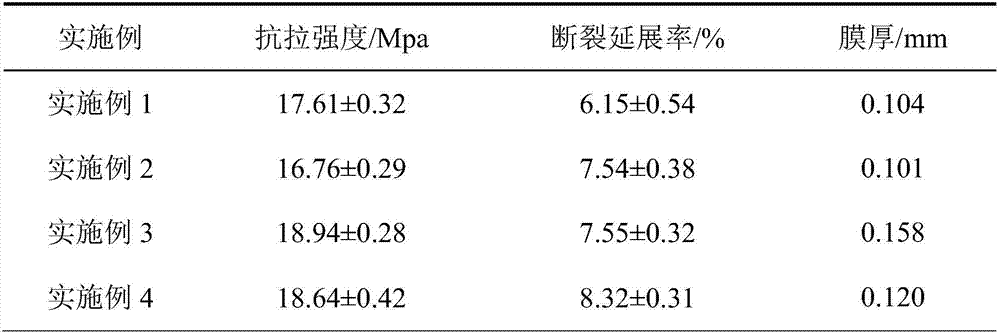
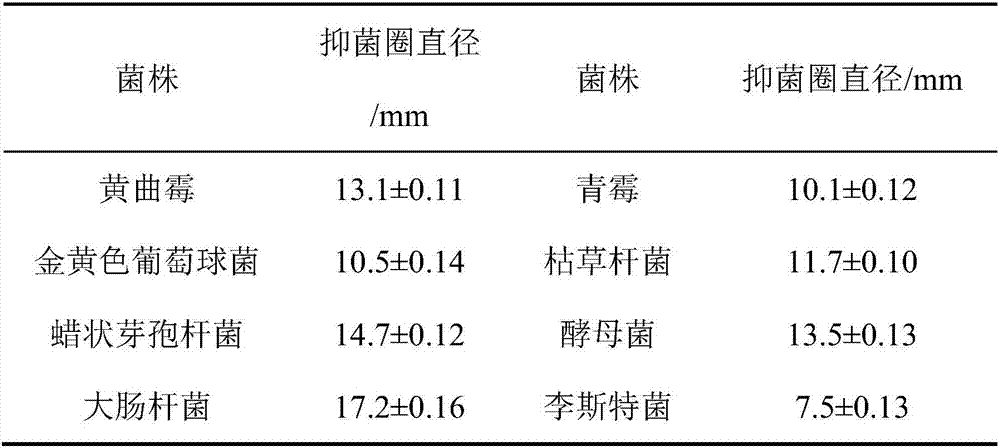
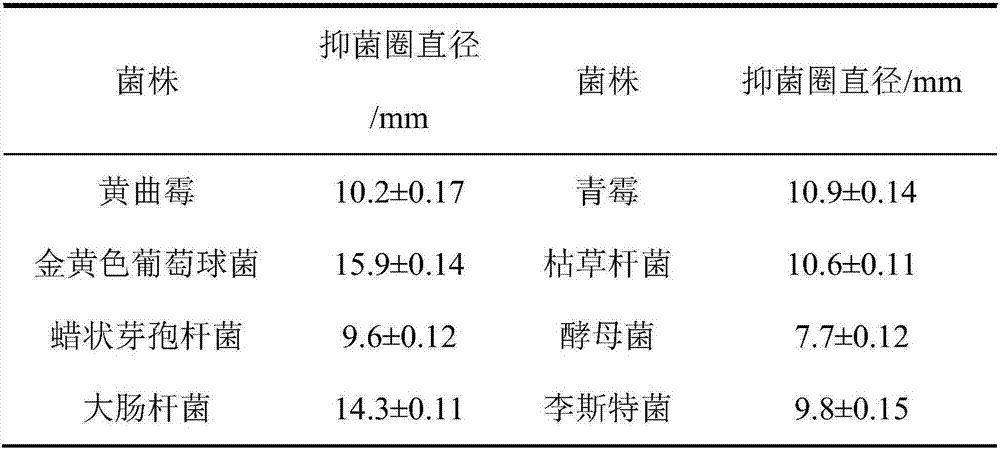
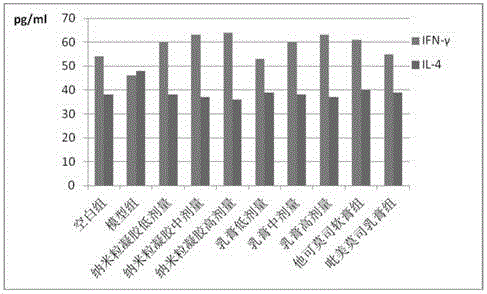
ActiveCN106905564AExtended shelf lifeNo side effectsFlexible coversWrappersFood packagingMechanical property
The invention discloses a functional edible film and a preparation method thereof. The functional edible film comprises starch, a plasticizing agent, solid lipid, active substances and an emulsifying agent. The preparation method comprises the following steps: preparing solid-lipid nanoparticles carrying the active substances and preparing the functional edible film. The functional edible film adopts the starch as a film-forming substrate, which is combined with the nanoparticles carrying the active substances; the prepared functional edible film has better bacteriostatic and anti-oxidation functions and good mechanical property, and is safe and environmental-friendly, and when the functional edible film is used for food package, the shelf life of food can be effectively prolonged.
Owner:SOUTH CHINA UNIV OF TECH
External preparation containing sirolimus as well as preparation method and application thereof
ActiveCN105663027APromote absorptionImprove stabilityOrganic active ingredientsAerosol deliveryIrritationTherapeutic effect
The invention provides a new route of administration for sirolimus, namely that sirolimus is prepared into an external preparation for treating skin diseases. The external preparation is a prescribed preparation or compound preparation containing sirolimus, and the dosage form can be any one of pharmaceutical skin external dosage forms including cream, ointment, gel, spray, coating agent and the like, and can be any one of new external dosage forms including solid lipid nanoparticles and the like. The research shows that the external preparation containing sirolimus can be used for treating multiple immune and inflammatory skin diseases including atopic dermatitis, eczema, dermatitis, lichen planus, psoriasis, vitiligo, rosacea, sarcoidosis and the like, and is good in curative effect, high in safety and free of skin irritation. The external preparation is expected to become an important pharmaceutical preparation for clinically treating skin diseases following corticosteroids.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Lipid carrier, indissolvable pharmaceutical composition and preparation method thereof
InactiveCN103877065AEvenly distributedParticle size controllableEmulsion deliveryMicrocapsulesMicroemulsionHydrolysis
The invention provides a lipid carrier, an indissolvable pharmaceutical composition and a preparation method thereof. The composition includes a self-microemulsion indissolvable pharmaceutical composition and a composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical. The self-microemulsion indissolvable pharmaceutical composition comprises, by weight, 0.05%-10% of an indissolvable pharmaceutical, 5%-60% of a liquid lipid, 20%-50% of a surfactant and 10%-35% of a cosurfactant; the composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical comprises, by weight, 0.05%-5% of the indissolvable pharmaceutical, 40%-60% of a solid lipid and 30%-59% of the surfactant. By changing the type and the proportion of the lipid in the composition composed of a solid lipid nanoparticle carrier and an indissolvable pharmaceutical, the degree of hydrolysis can be controlled between 3%-90%, wherein the hydrolysis is carried out with a lipase. Because that the releasing rate of the indissolvable pharmaceutical in the lipid carrier is related to the hydrolysis of the lipid carrier, the purpose of changing the pharmacokinetics of the indissolvable pharmaceutical is achieved by controlling the hydrolysis rate of the lipid carrier.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Targeting ginkgolide B solid lipid nanoparticle and preparation method thereof
InactiveCN102579342AAvoid devouringExtend cycle timePowder deliveryOrganic active ingredientsCell membranePhagocytosis
The invention discloses a targeting ginkgolide B solid lipid nanoparticle and a preparation method thereof. The ginkgolide B solid lipid nanoparticle targeting a blood brain barrier is prepared with an oil-in-water emulsion process by taking a ginkgolide B as a treating medicament, taking a solid lipid material as a carrier, taking a folic acid-modified surfactant as a targeting material and taking a mixture obtained by mixing a surfactant with the folic acid-modified surfactant in a certain ratio as an emulsifier. The particle diameter is 80-200 nanometers, and the polydispersion coefficient is 0.30+ / -0.10. According to the solid lipid nanoparticle, a brain targeting effect is achieved by using the folic acid-mediated phagocytosis on the surfaces of brain cells and the phagocytosis way of adsorption of a brain cell membrane by using the nanoparticle.
Owner:CHINA PHARM UNIV
Berberine hydrochloride solid lipid nano preparation and preparation method thereof
The invention discloses a berberine hydrochloride solid lipid nano preparation and a preparation method thereof, relates to berberine hydrochloride, and provides the berberine hydrochloride solid lipid nano preparation which has the advantages of simple method, low production cost, convenience in carrying and transportation and suitability for any administration route and the preparation method thereof. The berberine hydrochloride solid lipid nano preparation comprises the following raw materials in percentage by mass: 1 percent of berberine hydrochloride, 5-15 percent of solid lipid, 5-15 percent of emulsifier and 1-3 percent of surfactant. The solid lipid and the emulsifier are added into an organic solvent; after the solid lipid is fully dissolved, the berberine hydrochloride is added and is dissolved to obtain an oil phase; the obtained oil phase is added into a water phase containing the surfactant and is stirred until the organic solvent is fully removed; the obtained material is subjected to ultrasonography, is cooled and then is filtered by using a micro-porous filter membrane to obtain berberine hydrochloride solid lipid nanoparticle suspension; and the obtained berberine hydrochloride solid lipid nanoparticle suspension is added into a free-dry protecting agent to be freeze-dried to obtain the berberine hydrochloride solid lipid nano preparation which is in the form of nanoparticle freeze-dried powder.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV
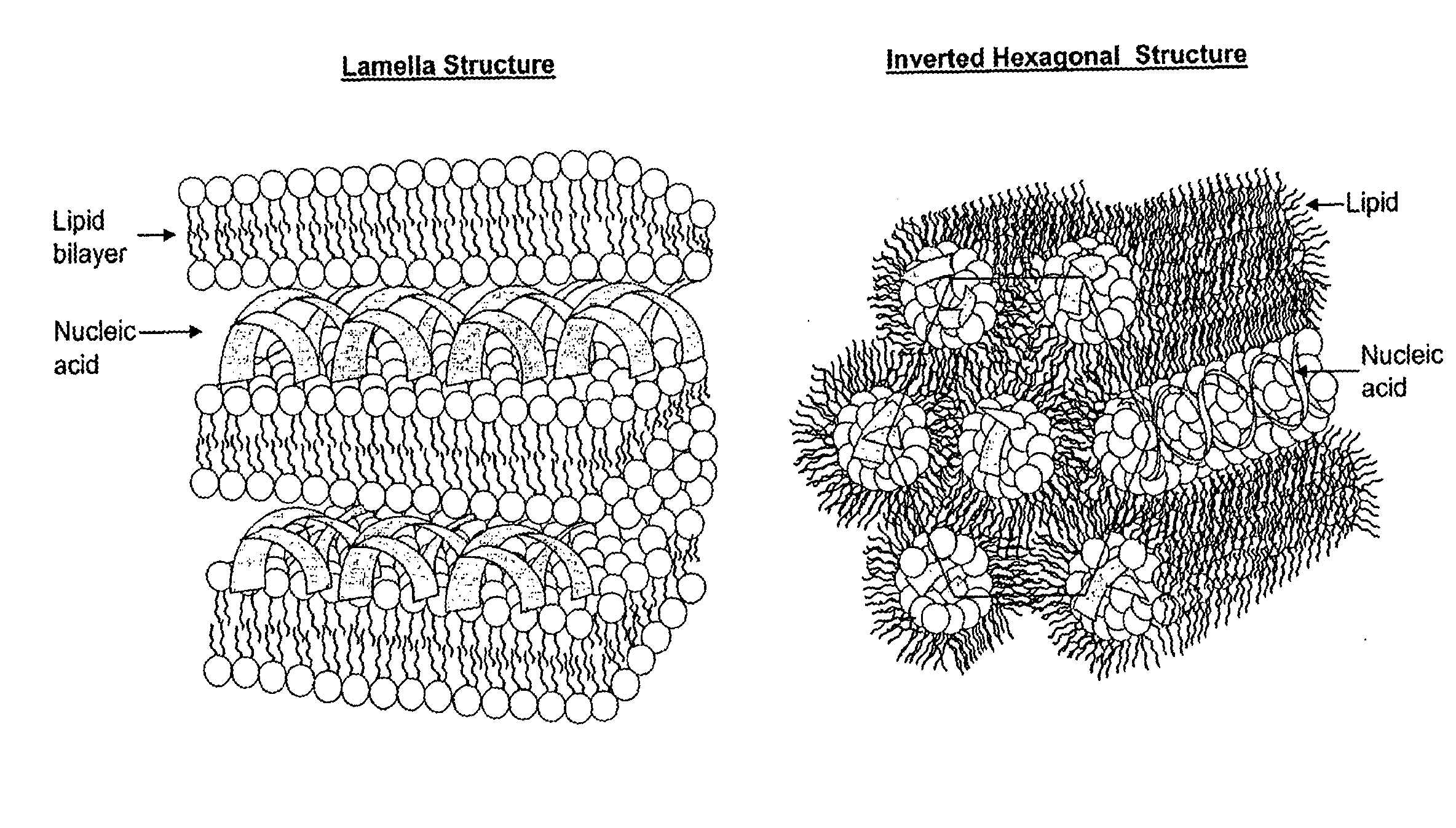
Lipid Nanoparticles as Vehicles for Nucleic Acids, Process for Their Preparation and Use
The invention relates to solid lipid nanoparticles composed of lipid material and containing, as bioactive molecule, a nucleic acid, preferably an antisense oligonucleotide, preferably modified by chemical methods to achieve a greater resistance to endo- and exo-nucleases, and to the process for preparation of the nanoparticles. In the present invention, the efficiency of the delivery system represented by nanoparticles containing synthetic or natural polynucleotides allows the use of such system for transfection. The particles are especially effective in the treatment of diseases of the posterior segment of the eye (such as diabetic retinopathy, macular degeneration, etc.) and in angiogenesis.
Owner:GASCO
Novel preparation method of solid lipid nanoparticles
InactiveCN102151250ALong term storageSimple preparation processPowder deliveryLyophilised deliveryLipid formationOrganic solvent
The invention discloses a novel preparation method of solid lipid nanoparticles (SLN) (including nanostructured lipid carriers NLC), solving the unstable problem of easiness in aggregation, agglomeration, and the like of the solid lipid nanoparticles (including NLC). The preparation method comprises the steps of: a, dissolving lipid matters and lipotrophic matters (including medicines) in an organic solvent (such as tertiary butanol) capable of being mixed and dissolved with water to form an oil phase (O), or solubilizing hydrophilic matters (including medicines) in an organic solvent (O) capable of being mixed and dissolved with water by using a surfactant, wherein the lipid matters and the lipotrophic matters are used for forming the solid lipid nanoparticles (including NLC); b, dissolving water-soluble matters in water to form a water phase (W); c, injecting the oil phase (O) into the water phase (W) under stirring condition according to a proper volume proportion to obtain a solid nanoparticle dispersing solution; d, freezing and drying the obtained dispersing solution to remove the solvent to obtain a freeze-dried product; and e, hydrating the obtained freeze-dried product to obtain the solid lipid nanoparticles (including NLC). The preparation method is simple in procedures and easy to implement.
Owner:王汀 +1
Solid lipid nanoparticles of finasteride and preparation method thereof
ActiveCN101559038AReduce usageImprove the deficiency of low bioavailabilityPowder deliveryOrganic active ingredientsSolubilityAdditive ingredient
The invention relates to the technical field of medicines, disclosing solid lipid nanoparticles of finasteride, which comprise the ingredients according to parts by weight as follows: 1 to 10 parts of finasteride, 3 to 500 parts of solid lipid materials, 10 to 200 parts of phospholipid and 10 to 100 parts of emulsifiers. The invention also discloses a preparation method of the above solid lipid nanoparticles of finasteride. The solid lipid nanoparticles of finasteride primely solve the problems of slightly solubility, low dissolution rate and low bioavailability of the finasteride, being advantageous to fabrication of medicinal preparation and quality improvement.
Owner:江苏黄河药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com