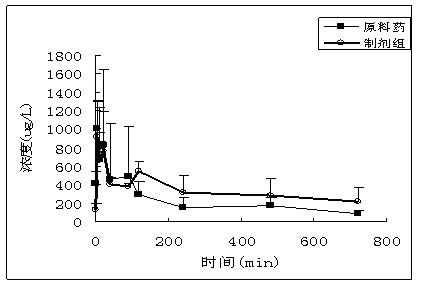
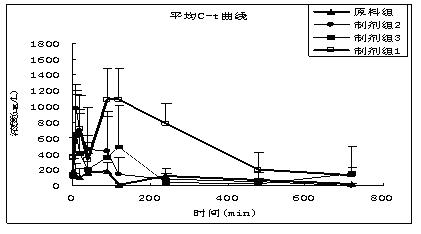
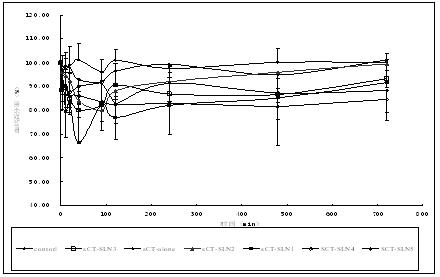
Novel solid lipid nanoparticle medicament delivery system for protein-loaded medicaments
A solid lipid nano and solid lipid technology, which is applied in drug delivery, liposome delivery, pharmaceutical formulations, etc., can solve the problems of severe preparation process conditions, poor stability of protein drugs, and low bioavailability, so as to reduce toxicity Effects of side effects, enhanced bioavailability, and enhanced therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Examine the concentration of biological surface active agents to the packet and particle size inspection of nanoparticles:
[0079] Examine the amount of sodium gall acid S The effect of CT-SLNS preparation.Precision is called salmon decreased calcium ( S CT) 3.75mg is dissolved in 0.01mol / ml HCL aqueous solution of 0.5ml, adds an appropriate amount of sodium bile acid after dissolving, 30min, and then adds to dissolved 1 mg of 1mg of glycolic acid, 10mg of Palm acid glycolin, and 20mg of soybean phospholipids.In the 1ml dichloromethane solution.Probe ultrasound 15S and 40W of power, forming colostrum.Add colostrum to a 0.1%Polosam 188 solution of 4ml, the probe ultrasonic 15S and the power of 80W, forming nanomal milk.Dilute the nano -milk with the same concentration of Polosham 188, and then steam it to dichloromethane completely.
[0080] The amount of sodium gallopate is examined in accordance with the above preparation methods to 0, 10, 20, 30, 50, 100, 150m...
Embodiment 2
[0081] Example 2 Examine the types of organic solvents on the type of nanopartal particle size and stability:
[0082] Examine different organic solvents pairs S CT-SLNS has the effects of particle size and stability. It is precisely known as salmon reduced calcium calcium or 7.5mg to dissolve in 0.5ml 0.01mol / ml HCL aqueous solution.In the 1ml organic solvent of 20mg of phospholipid, the probe ultrasonic 15S and the power of 40W, forming colostrum.Add colostrum to a 0.1%Polosam 188 solution of 4ml, the probe ultrasonic 15S and the power of 80W, forming nanomal milk.Dilute nanocal milk with the same concentration of Polosam 188 solution and steam to the organic solvents completely, that is, the SCT-SLNS dispersing solution is obtained to check the changes in particle size and stability. Among them, the stability is 'good',The evaluation of ',' difference ',' / ', of which' good 'means that after 24 hours of placement, the particle size is not changed, and the solution is a clear ...
Embodiment 3
[0087] Example 3 The effect of examining different lipid materials on the packaging rate, particle size and stability:
[0088] Precision is called salmon oligomatin 7.5mg dissolved in 0.5ml 0.01mol / ml HCL aqueous solution, adds 25 mg of sodium sodium after dissolving, 30 minutes of vortex, and then adds to dissolved (according to SLN1, 2, 4-4 in Table 2The 10th prescription requires different ratios to add mixed lipid materials) 1ml dichloromethane solution for lipid materials.Probe ultrasound 15S and 40W of power, forming colostrum.Add colostrum to the Polosam 188 solution of 4ml0.1%, the probe ultrasonic 15S and the power of 80W, forming nanomal milk.Dilute the nano -milk with the same concentration of Polosham 188, and then steam it to dichloromethane completely.The effects of each prescription on the package rate, particle size and stability (stability after 24 hours) are shown in Table 2:
[0089] Table 2: Different lipid materials Mixed -packed and particle size inspection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com