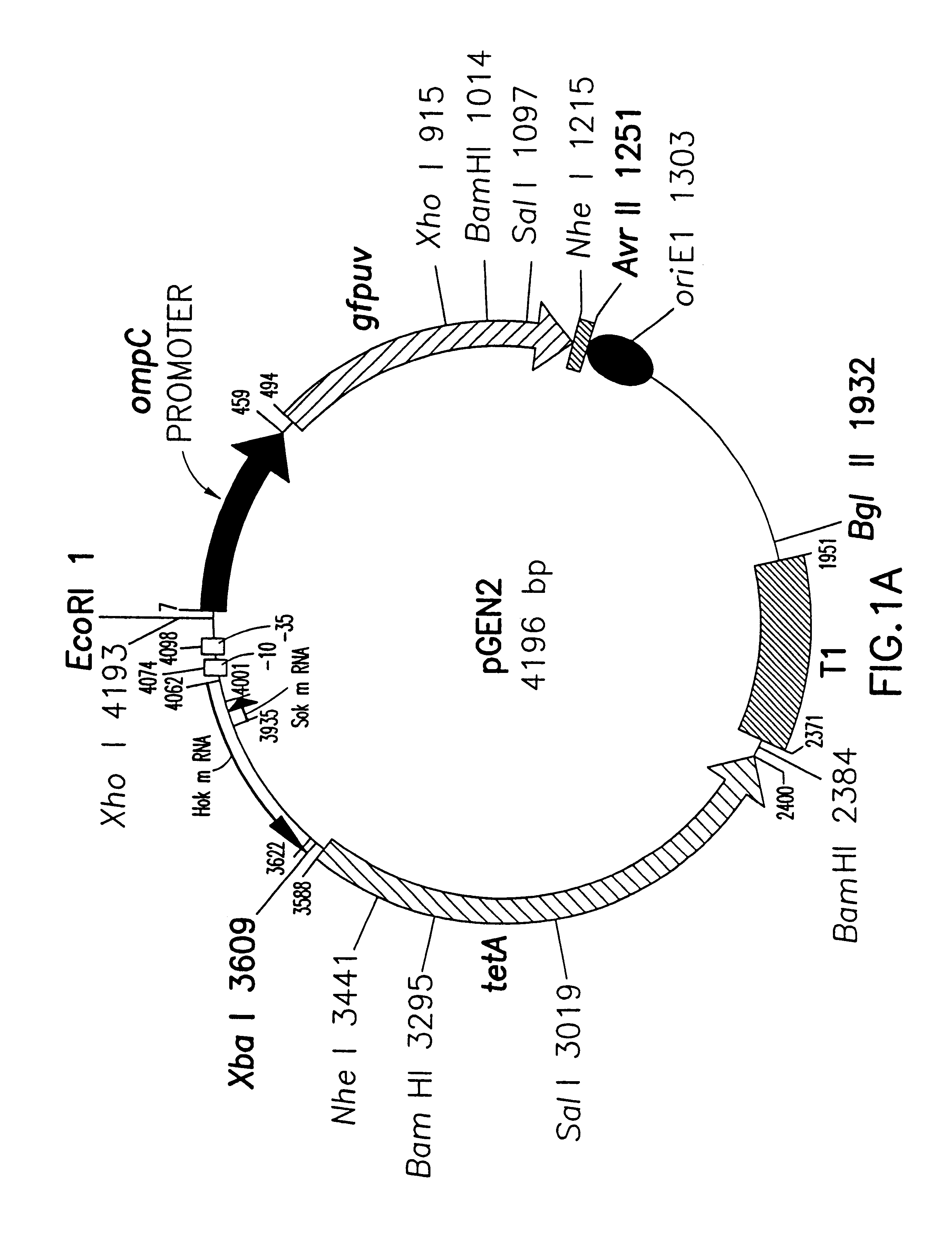
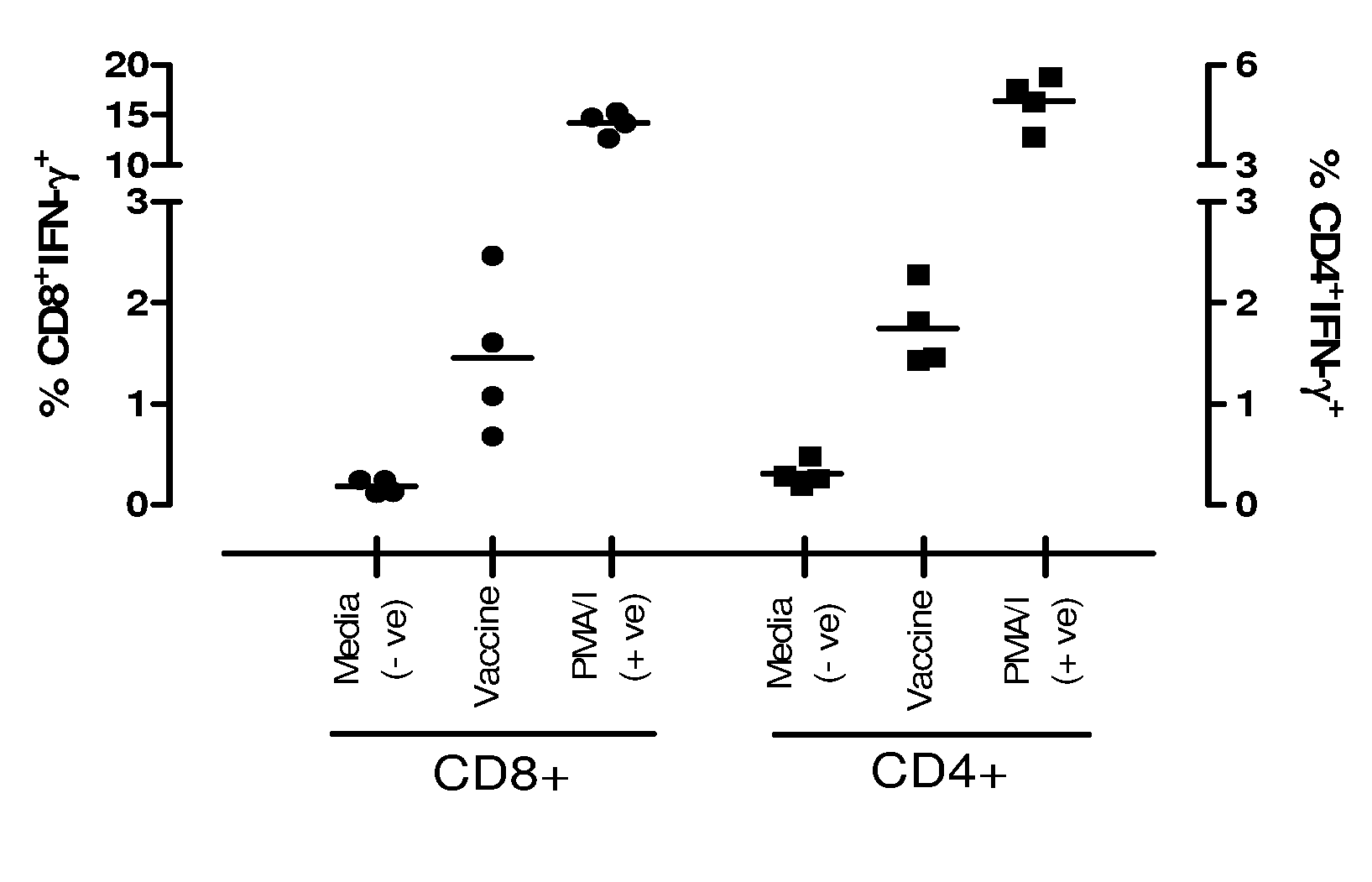
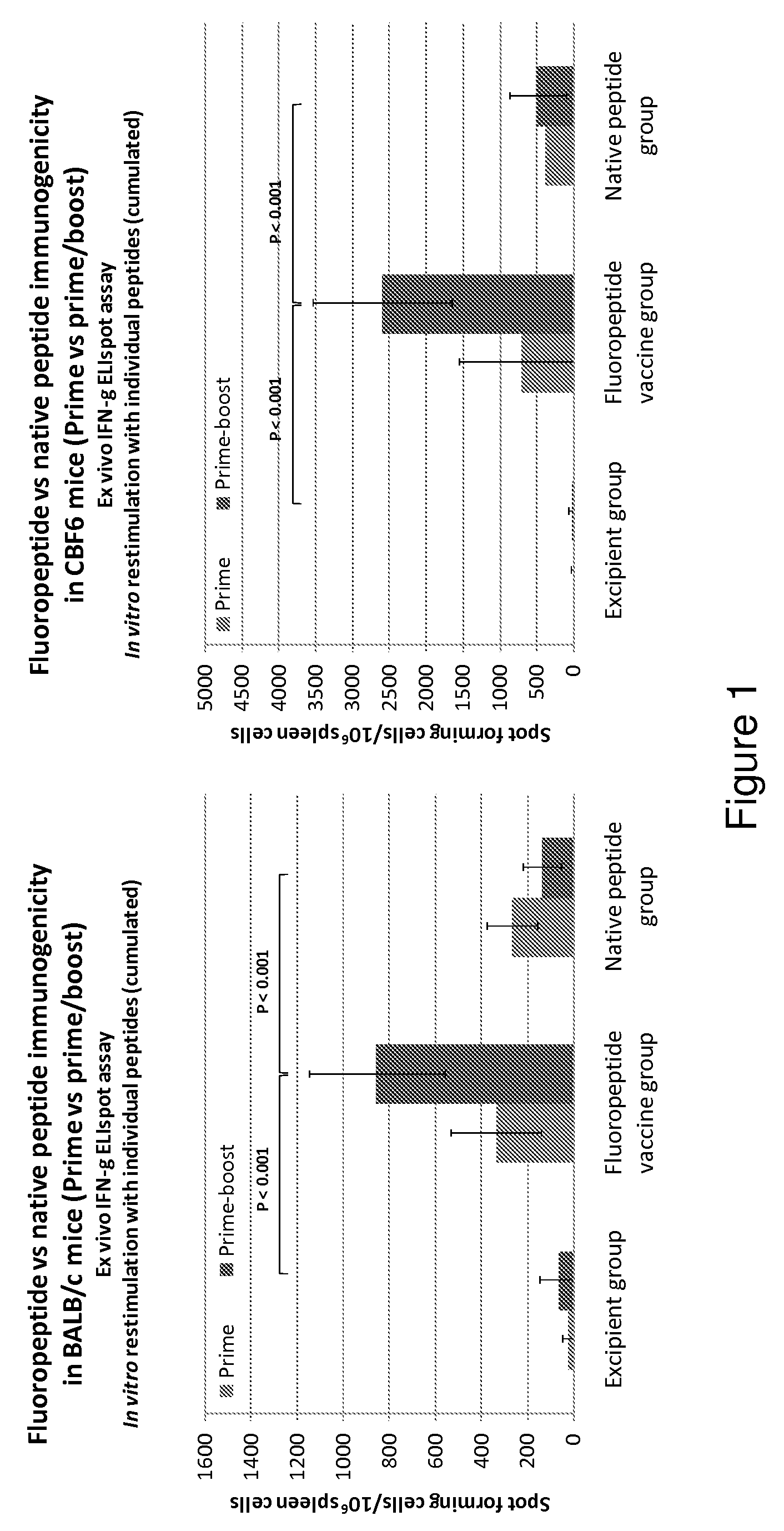
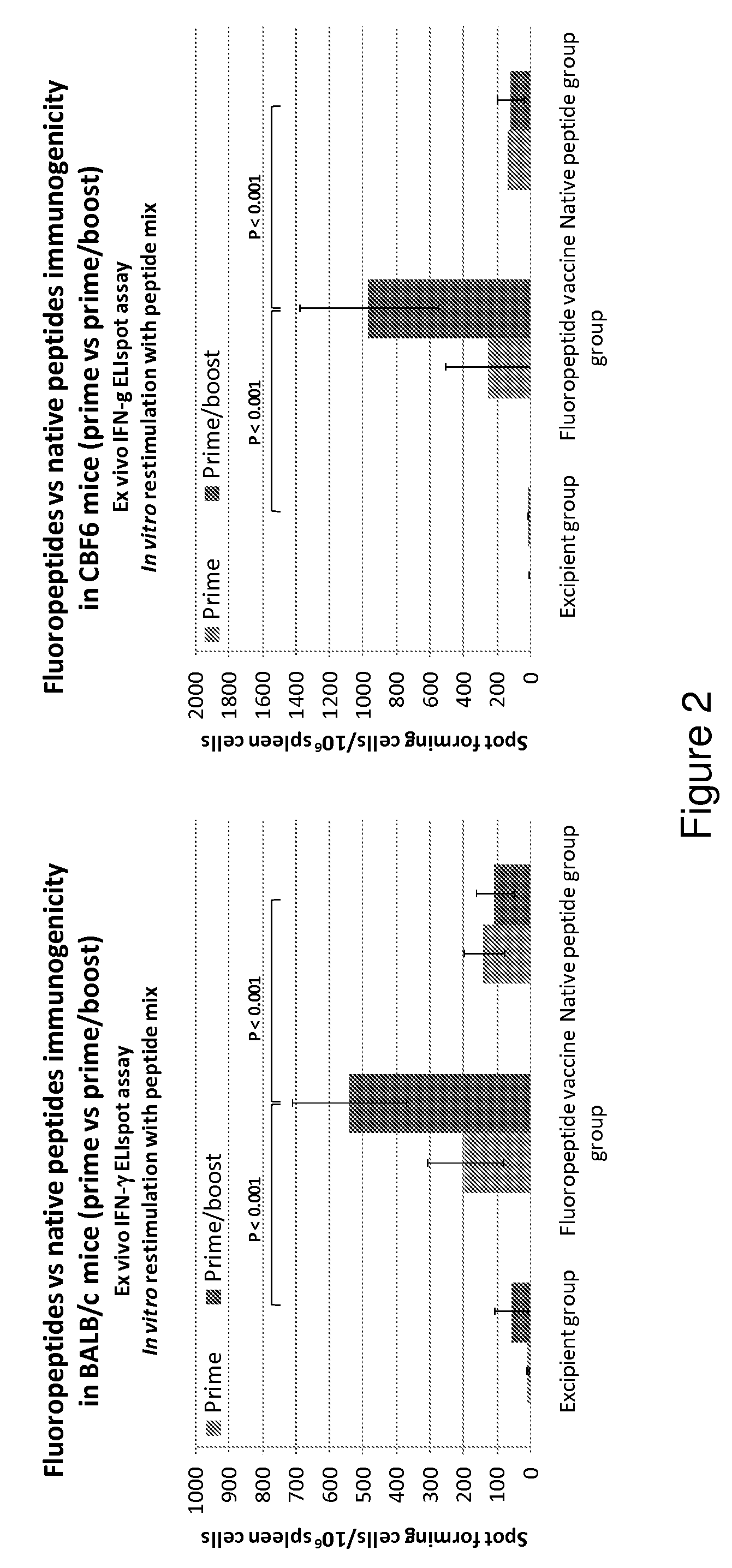
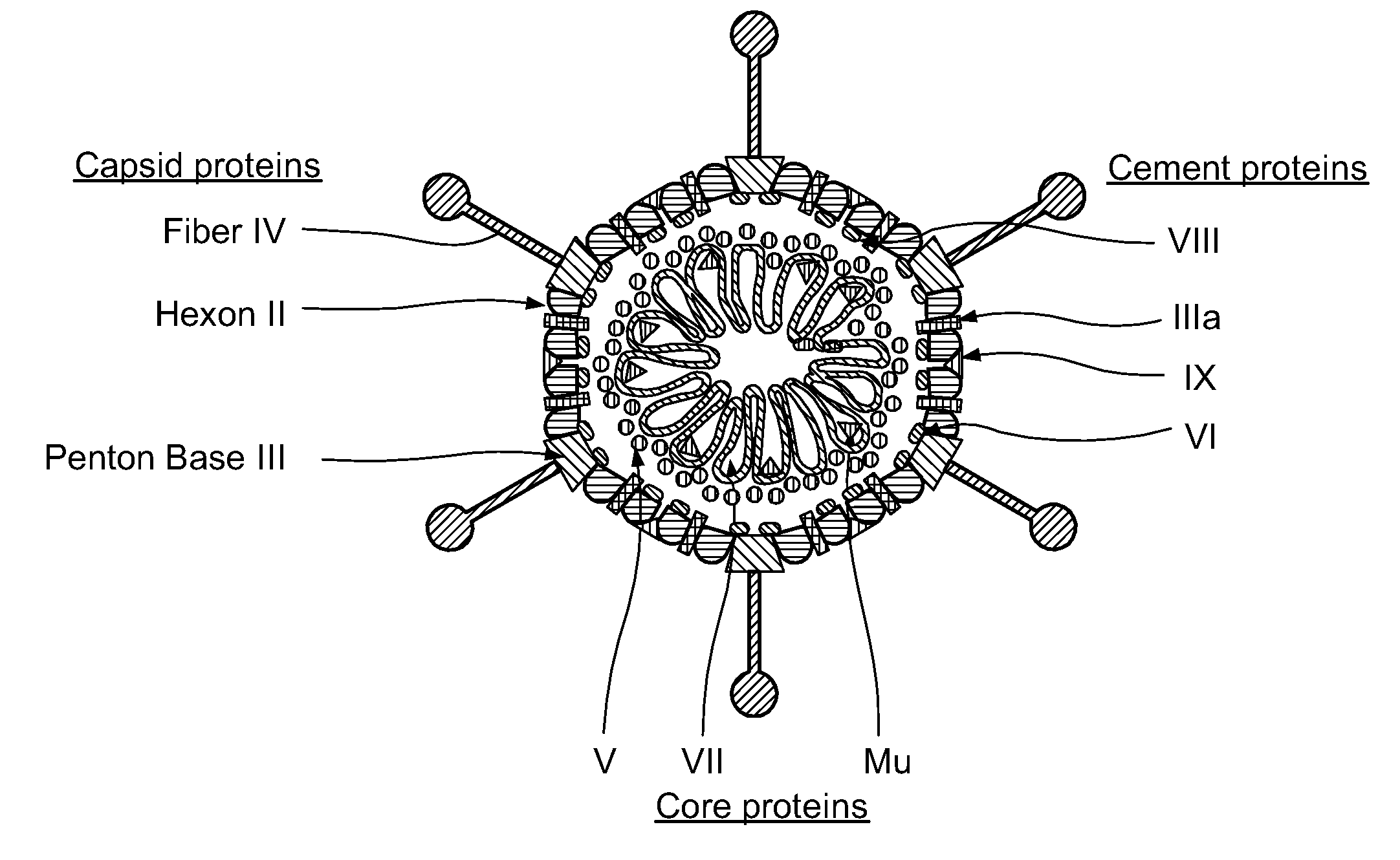
Patents
Literature
89 results about "Antigen delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
Antigen delivery compositions and methods of use
ActiveUS7303881B2Inhibition formationBacterial antigen ingredientsNanotechAntigen deliveryInfective disorder
The present invention provides antigen delivery compositions and methods of using same to prevent or to treat cancers and other infectious diseases.
Owner:PDS BIOTECH
Programmed immune responses using a vaccination node
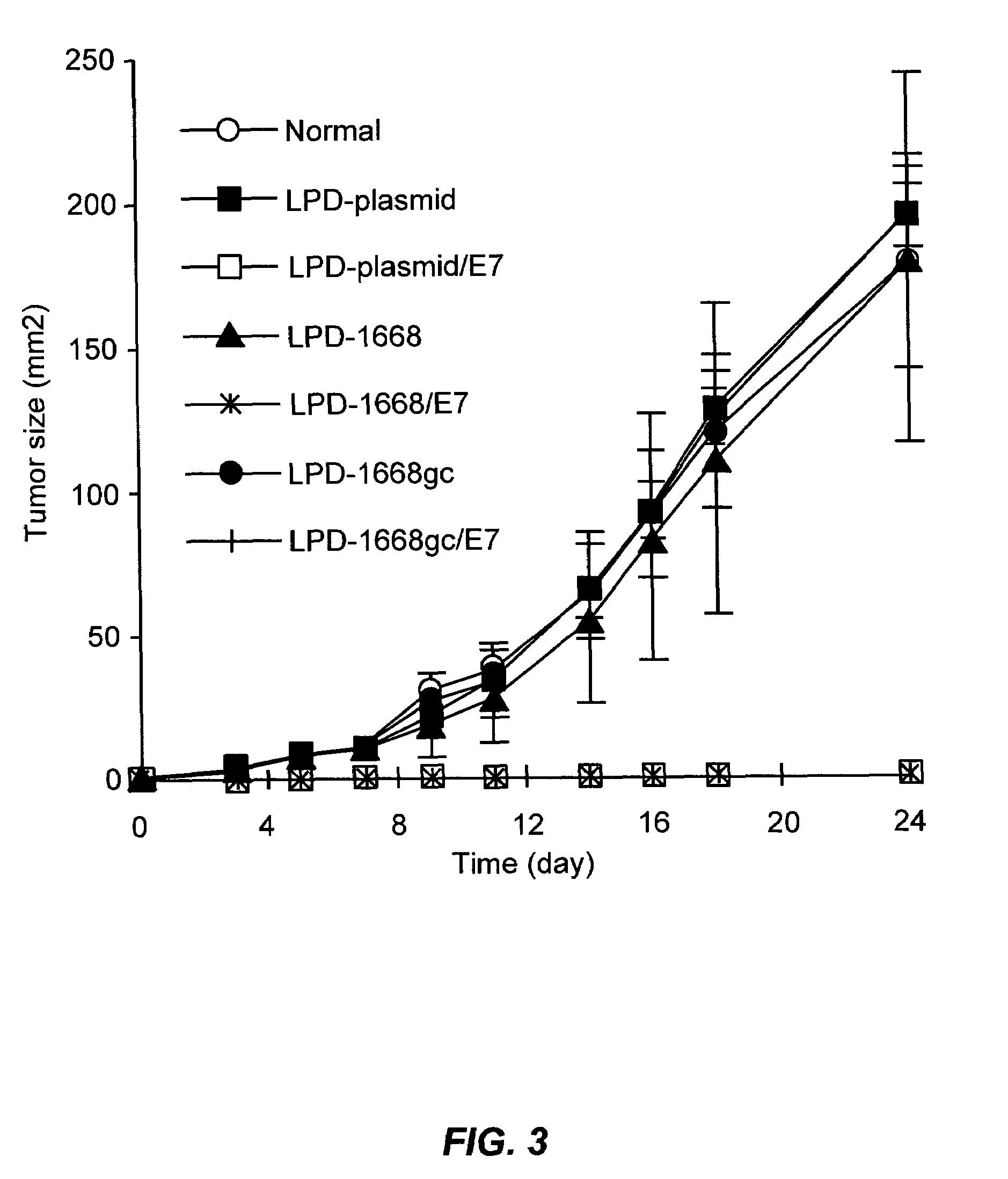
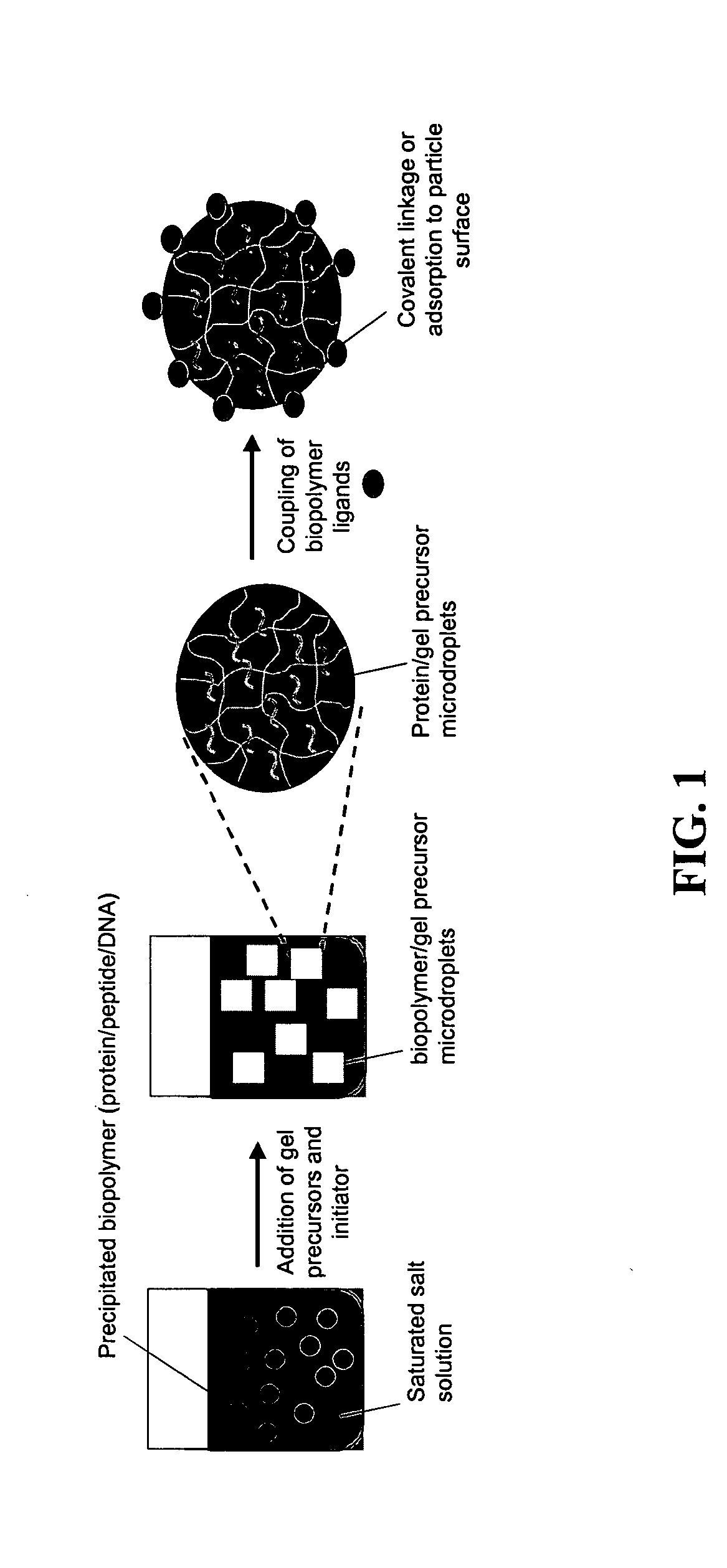
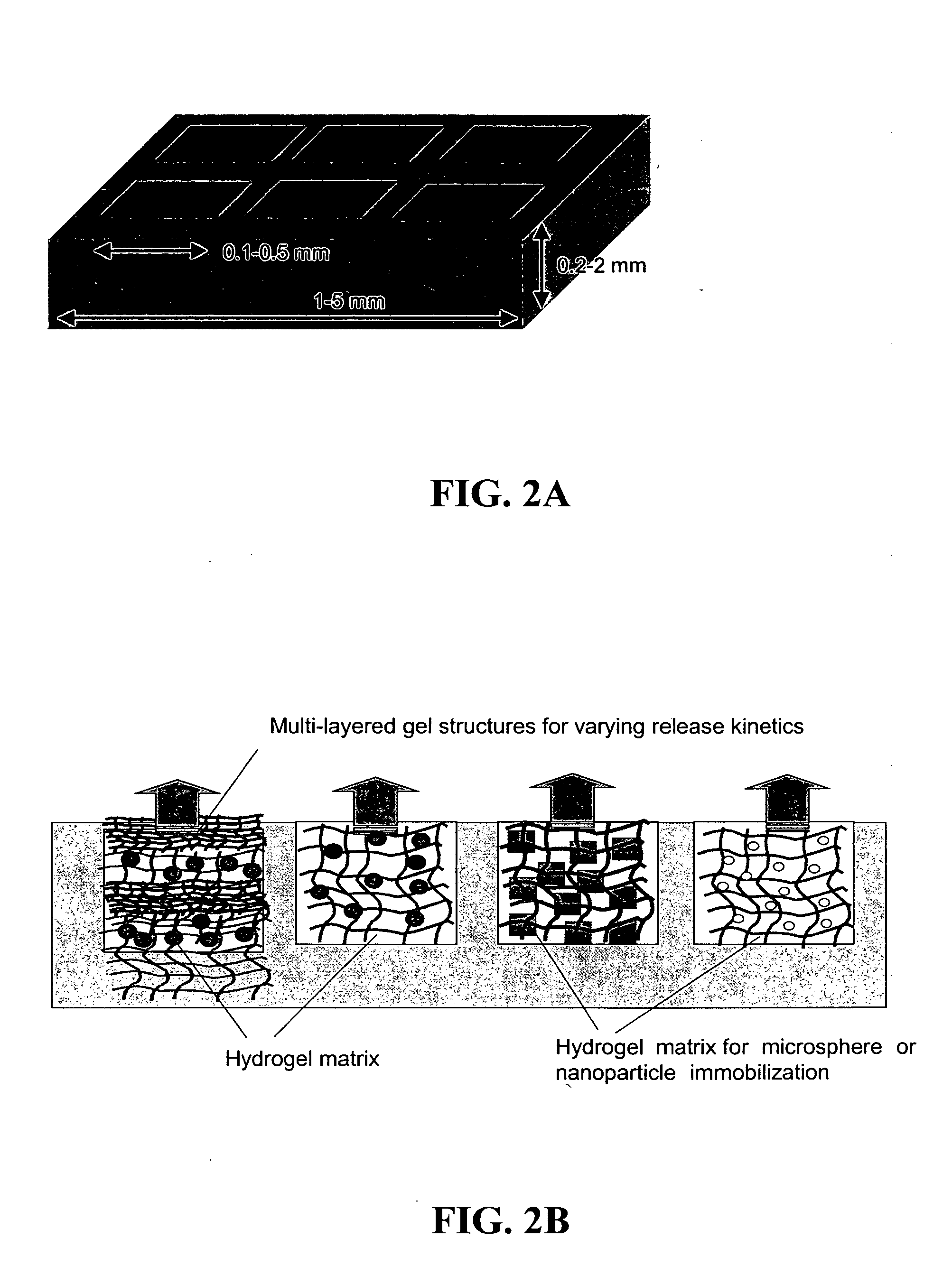
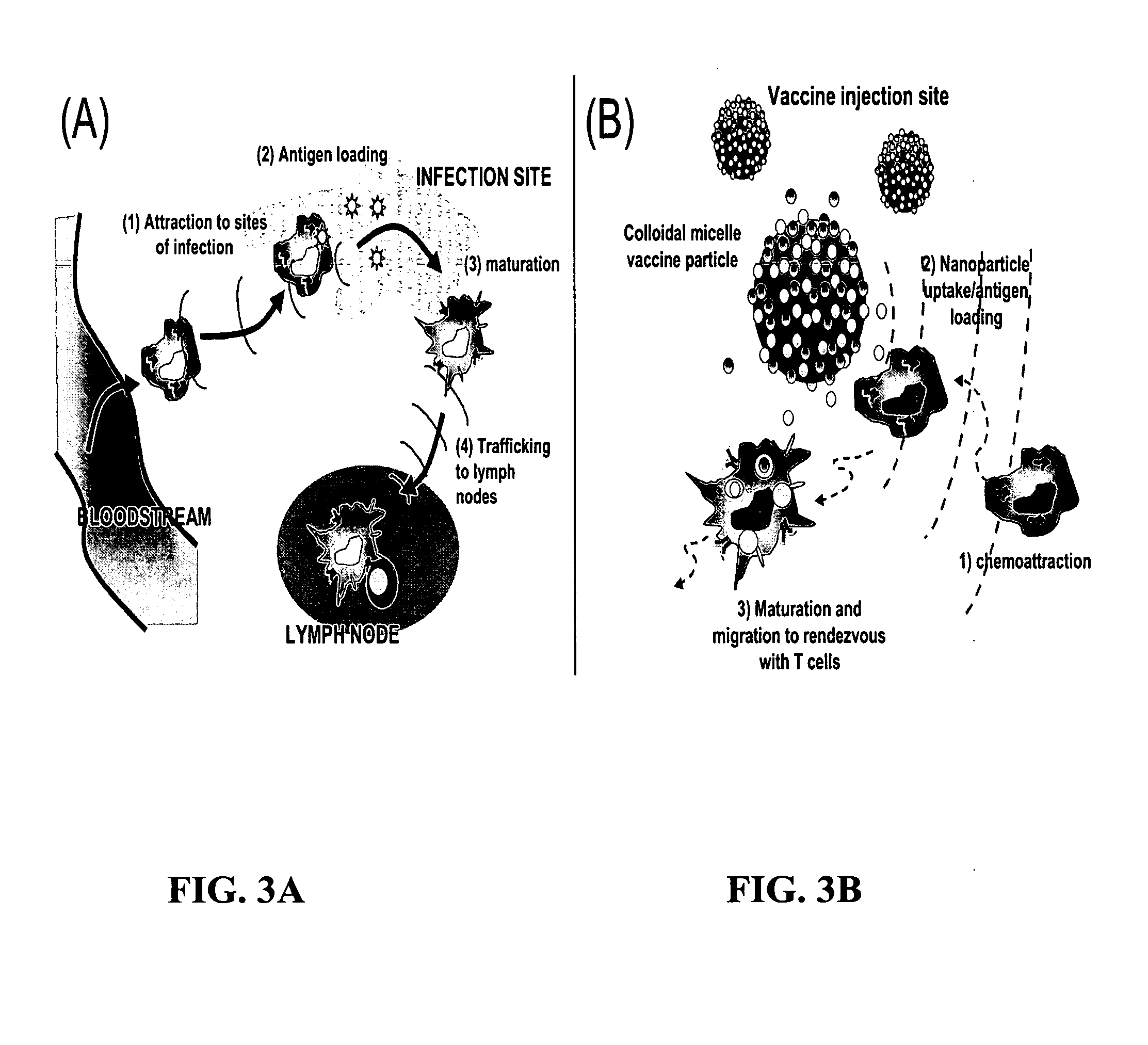
The present invention provides compositions and methods for modulating immune responses to antigens. One aspect of the present invention relates to a particle-based antigen delivery system (vaccination node) that comprises a hydrogel particle capable of both antigen presentation and DC activation. The VN may further comprise a chemoattractant-loaded microsphere capable of attracting DCs to the site of administration. Another aspect of the present invention relates to the use of the VN to modulate antigen presenting cells activation for the prevention and / treatment of various diseases, such as infectious diseases, cancers and autoimmune diseases.
Owner:VAXDESIGN
Polymerized solid lipid nanoparticles for oral or mucosal delivery of therapeutic proteins and peptides
InactiveUS20080311214A1Powder deliveryPeptide/protein ingredientsAntigen deliveryTherapeutic protein
The present invention encompasses lipid nano / micro particles, which have been modified, preferably on their surface, to contain a molecule or ligand, which targets the nano / micro particles to a specific site. The invention also encompasses the use of the modified lipid nano / micro particles for the oral delivery of drugs and antigen delivery systems.
Owner:TRANSGENE BIOTEK
Antigen delivery platforms
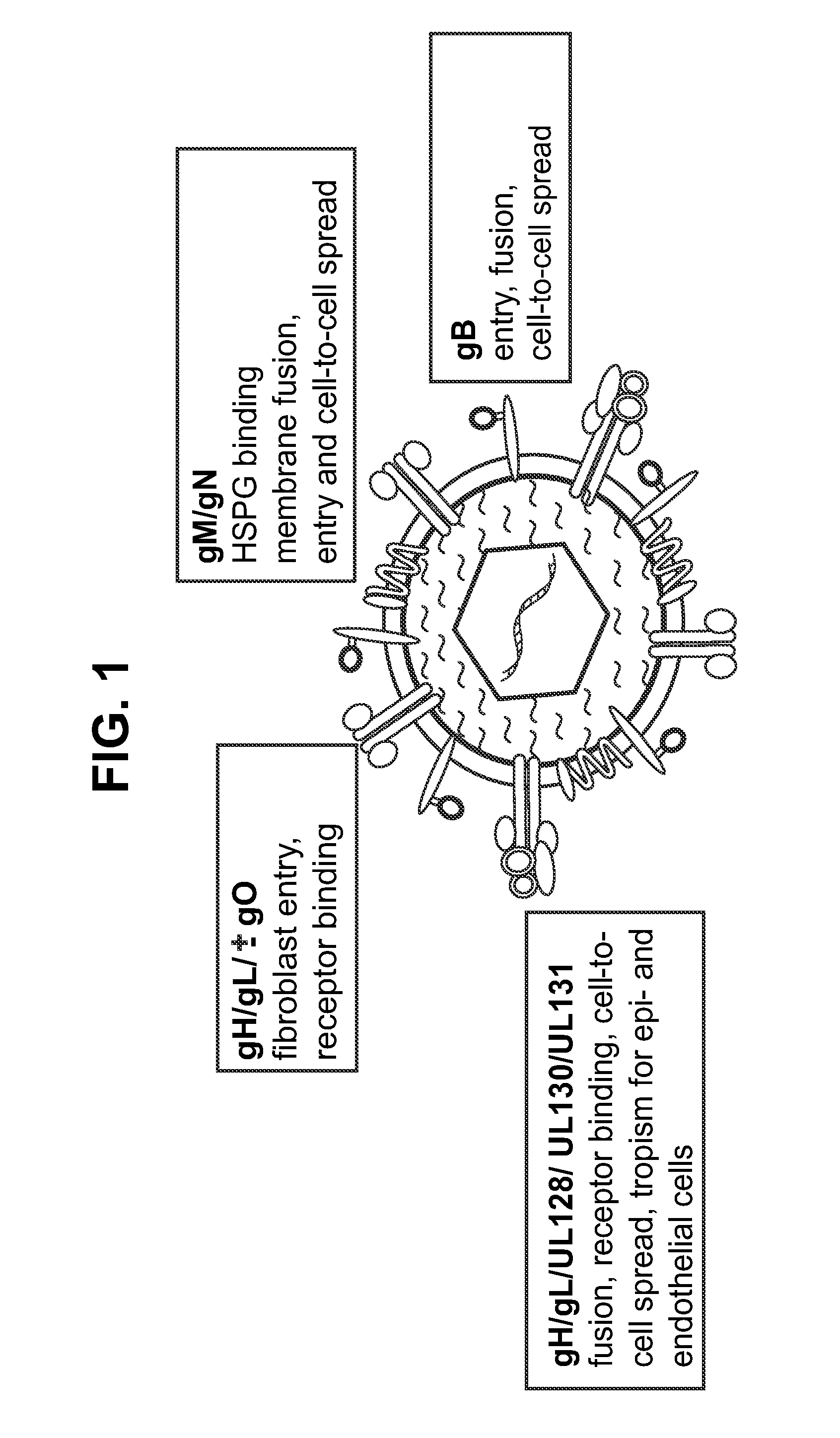
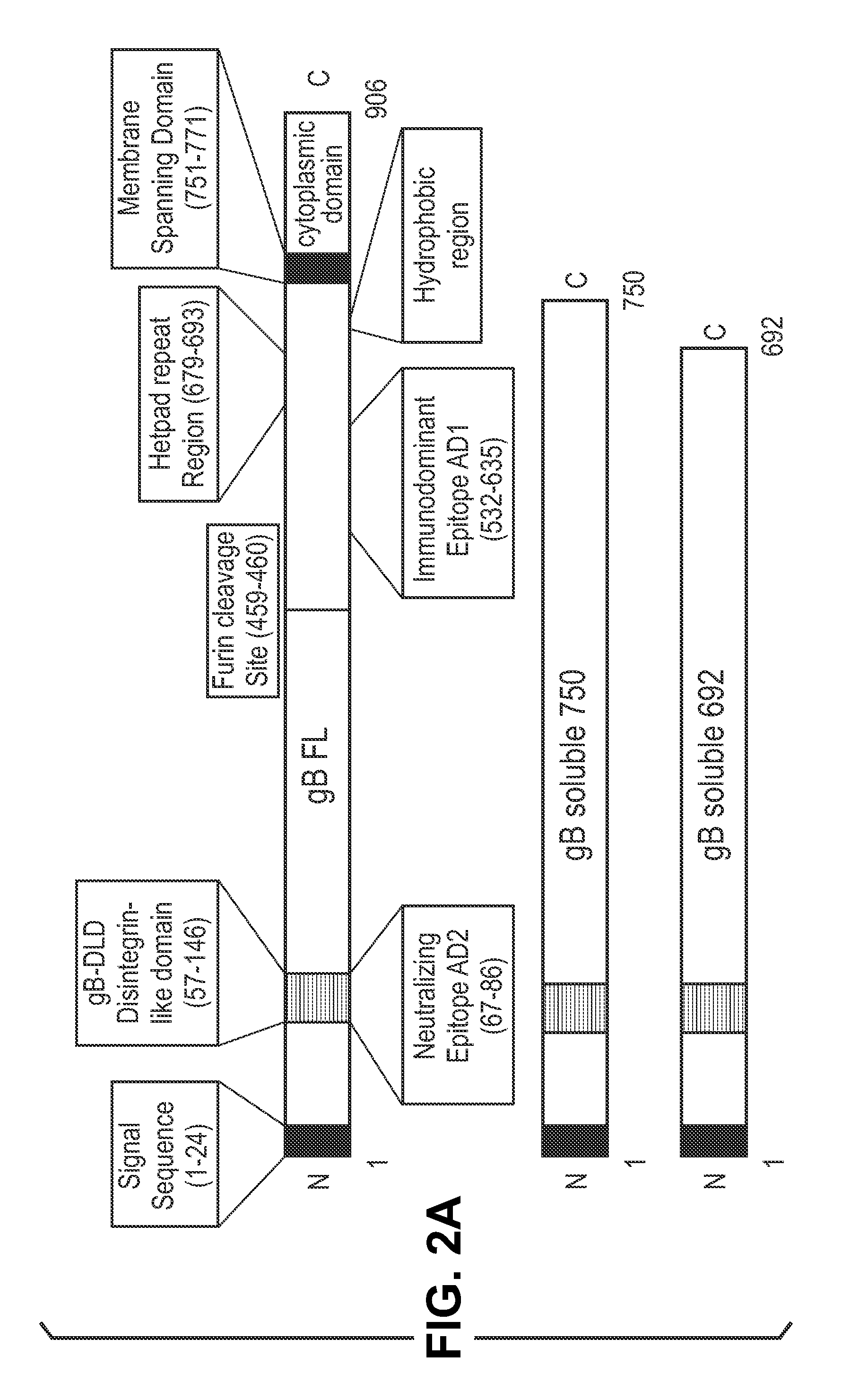
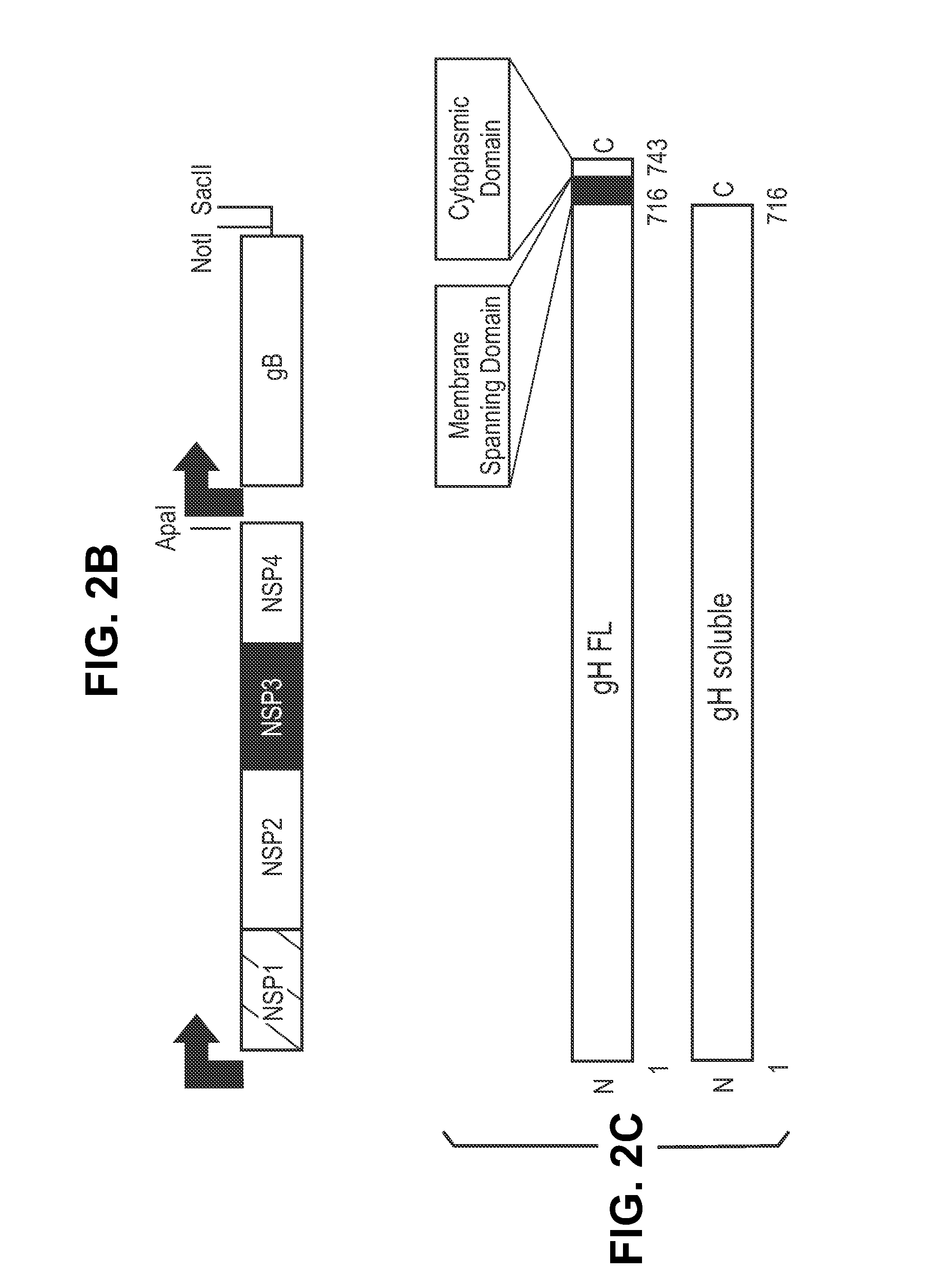
InactiveUS20140030292A1Improve stabilityAvoid reduce stimulationSsRNA viruses positive-senseFusion with post-translational modification motifAntigen deliveryHerpes simplex virus DNA
This disclosure provides platforms for delivery of herpes virus proteins to cells, particularly proteins that form complexes in vivo. In some embodiments these proteins and the complexes they form elicit potent neutralizing antibodies. Thus, presentation of herpes virus proteins using the disclosed platforms permits the generation of broad and potent immune responses useful for vaccine development.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Immunogenic compositions and vaccines comprising carrier bacteria that secrete antigens
InactiveUS20040101531A1Snake antigen ingredientsDepsipeptidesMucosal Immune ResponsesAntigen delivery
Disclosed are vaccines and immunogenic compositions which use live attenuated pathogenic bacteria, such as Salmonella, to deliver ectopic antigens to the mucosal immune system of vertebrates. The attenuated pathogenic bacteria are engineered to secrete the antigen into the periplasmic space of the bacteria or into the environment surrounding the bacteria. The vertebrate mounts a Th2-mediated immune response toward the secreted antigen.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Bacillus based delivery system and methods of use
ActiveUS20100233124A1Stimulate immune responseSlow onsetBiocideSsRNA viruses positive-senseSurface displaySpore
Herein a Bacillus exosporium antigen delivery (BEAD) system that provides a means to introduce recombinant proteins or small molecules into the exosporium of members of the B. cereus family of bacteria, i.e. B. anthracis, B. cereus, and B. thuringiensis, is disclosed. The system results in the surface display of recombinant proteins or small molecules such that they can stimulate an immune response. In addition, methods of making and using the system are described.
Owner:UNIVERSITY OF MISSOURI
Calcium phosphate particles as mucosal adjuvants
The present invention relates to mucosal immune protection, mucosal vaccine delivery, and mucosal drug delivery. Novel calcium phosphate core particles, particularly nanoparticles are used as vaccine adjuvants and compositions for inducing protective mucosal immunity. Methods of inducing an immune response to an antigen by delivering the antigen to a mucosal surface using the particles of this invention, and to methods of making such particles are also provided. The particles of this invention are also useful for delivering compositions, such as a pharmacologically active agent, to the mucosal surfaces of a patient in need thereof, to methods of delivering such compositions, and to methods of making such particles.
Owner:CAPTIVATE PHARMACEUTICALS LLC
Antigen delivery compositions and methods of use
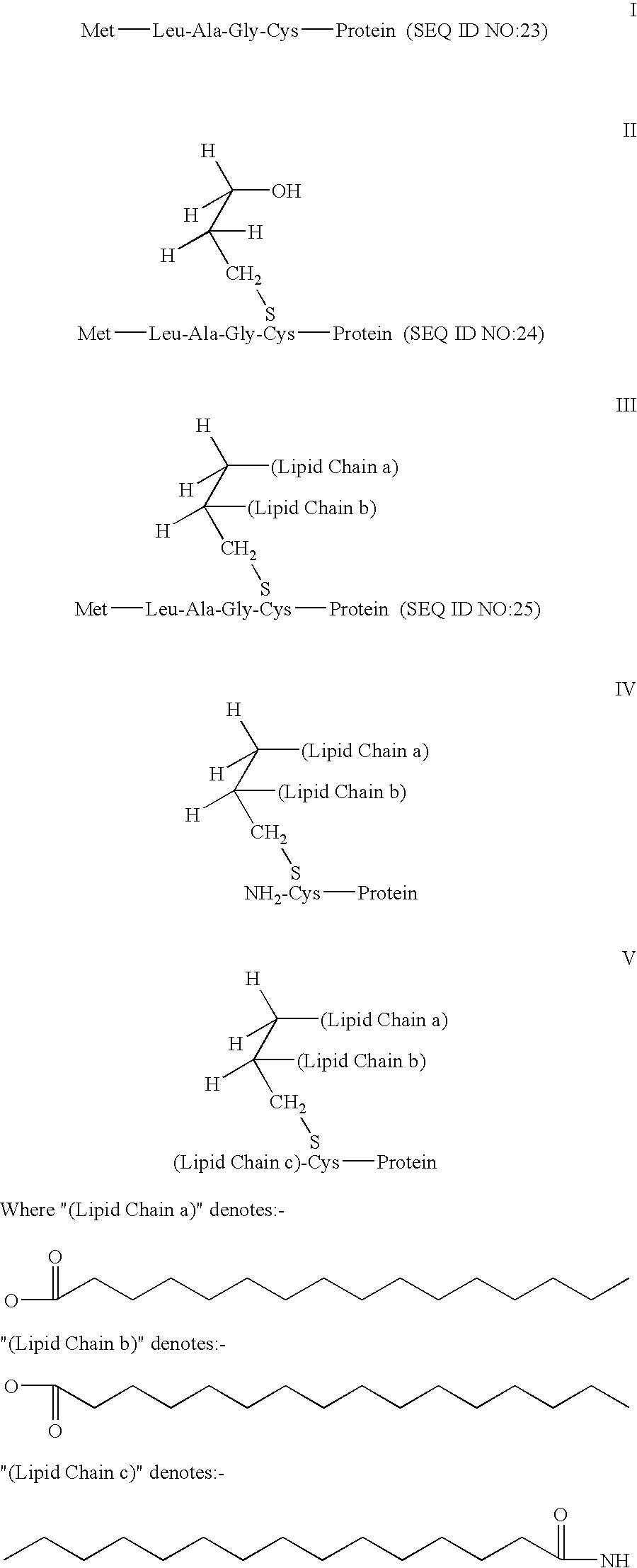
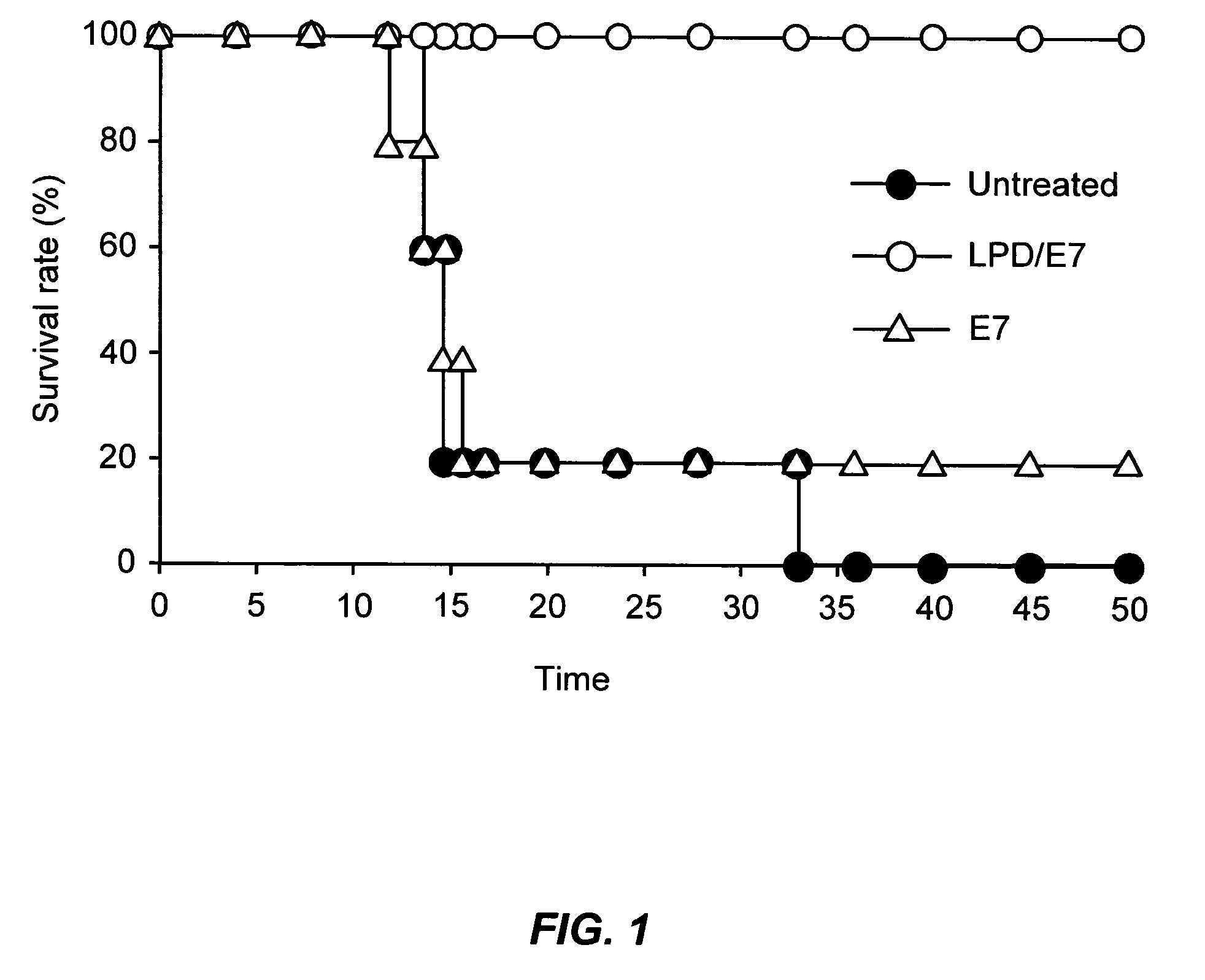
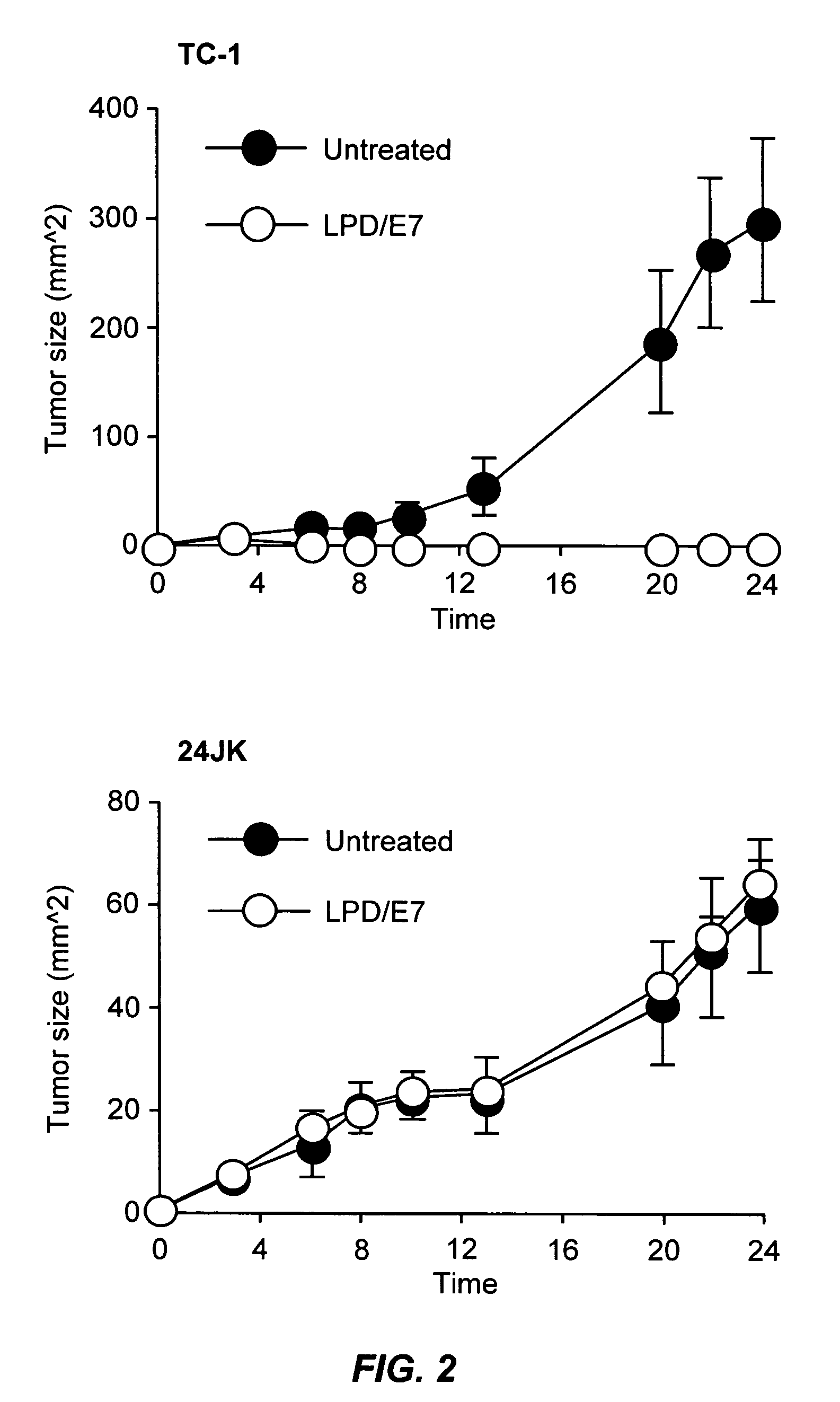
ActiveUS20060008472A1Prevent formation of newEnhance cellular immunityNanotechBacterial antigen ingredientsVaccine deliveryAntigen delivery
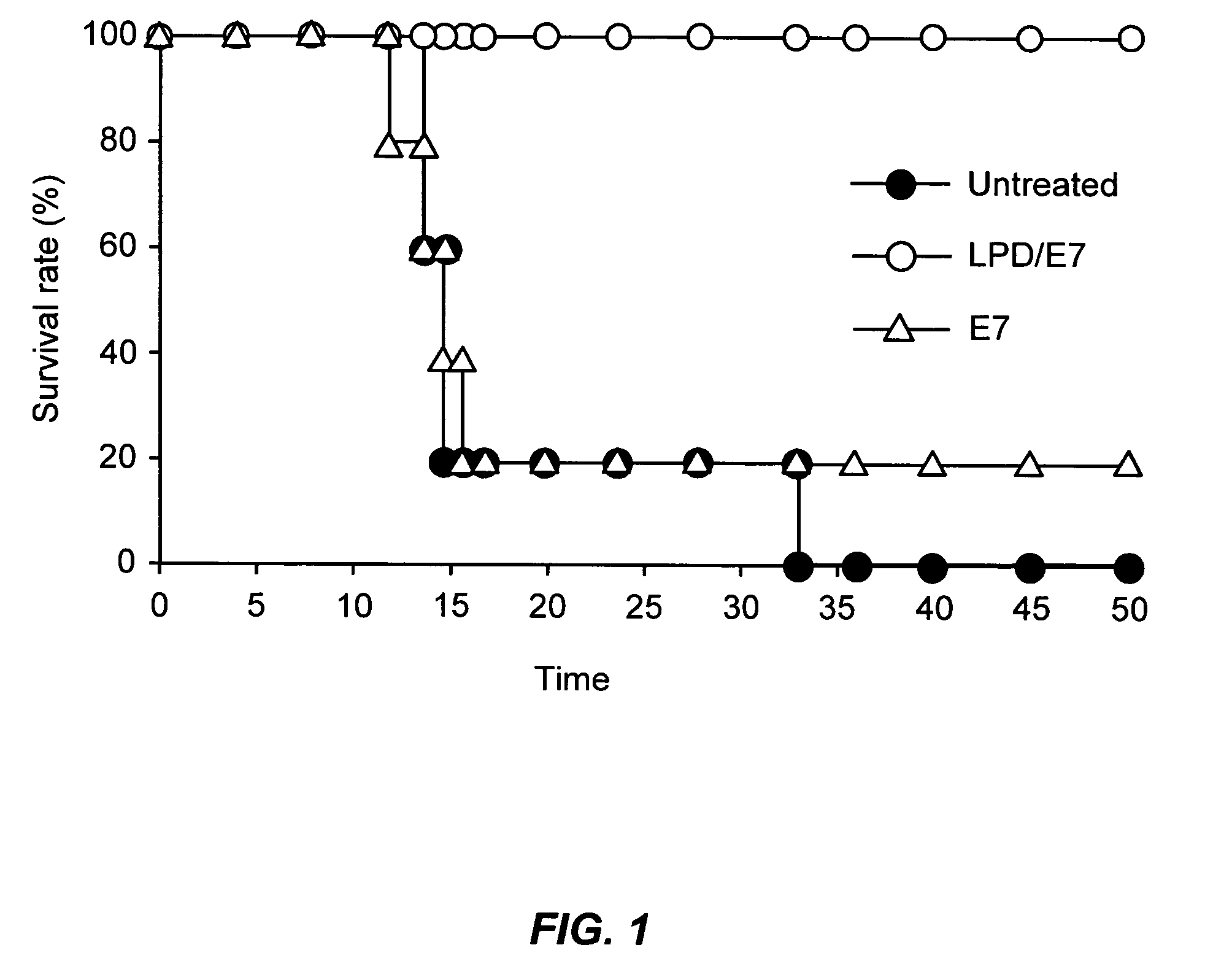
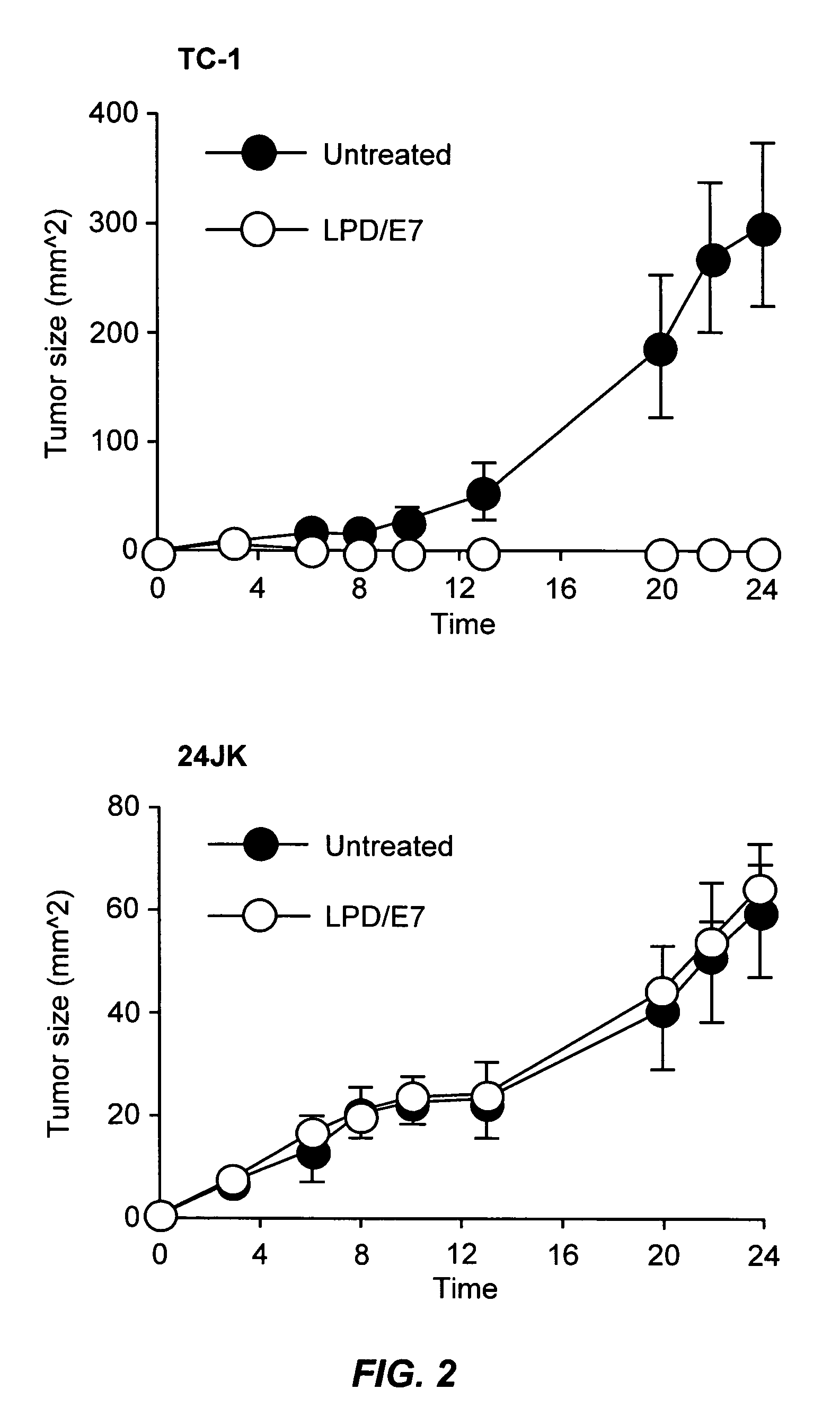
The present invention provides antigen delivery compositions and methods of using same to prevent or to treat cancers and other infectious diseases. More particularly, the present invention provides a lipid-protamine-DNA (LPD) vaccine delivery system and methods of using such LPD vaccine delivery system for the prevention and treatment of cancer and other infectious diseases.
Owner:PDS BIOTECH
Recombinant bacterial vaccine system with environmentally limited viability
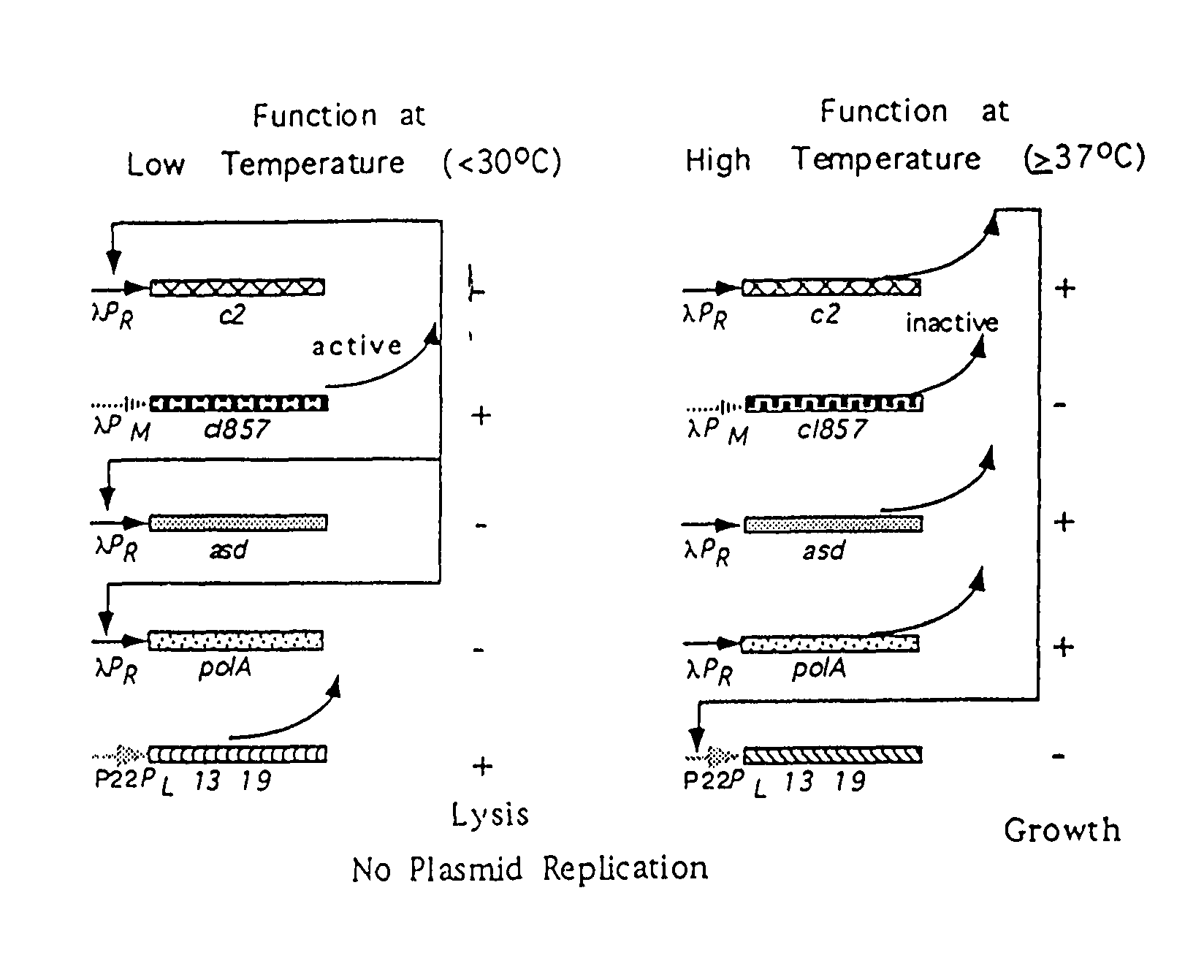
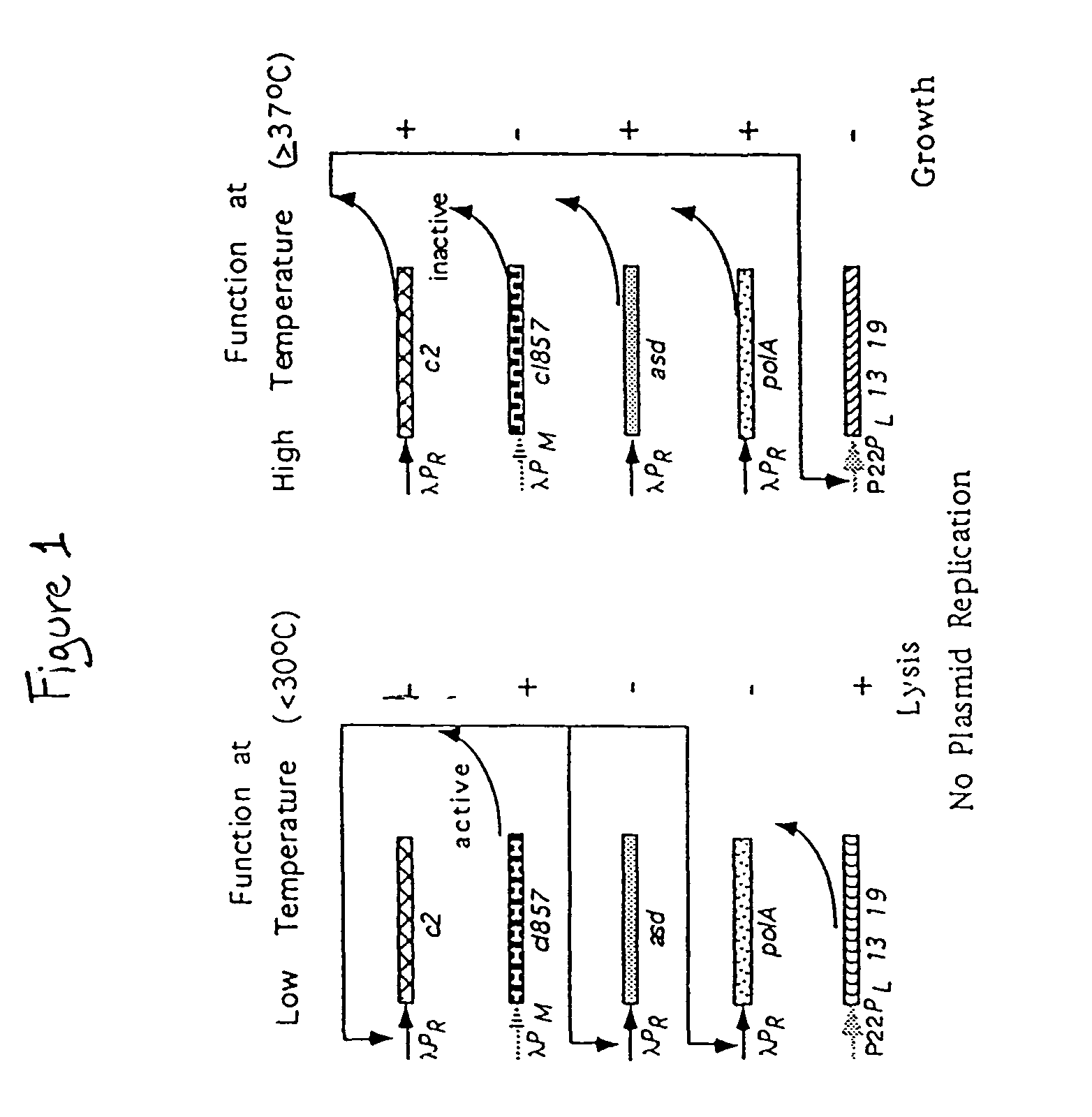
Disclosed is an Environmentally Limited Viability System (ELVS) for microorganisms based on temperature differences between permissive and non-permissive environments. Viability of the microorganisms are limited to the permissive environment by specifically expressing one or more essential genes only in the permissive environment, or expressing one or more lethal genes only in the non-permissive environment. Environmentally Limited Viability Systems are also disclosed involving coordinate expression of a combination of required genes and lethal genes. Microorganisms containing an Environmentally Limited Viability System are useful for release into a permissive environment. Temperature regulated Environmentally Limited Viability Systems are particularly suited for use with recombinant avirulent Salmonella vaccines by limiting their growth to the warmer environment inside the host. Such vaccines can be administered to protect humans or warm-blooded animals against bacterial, viral, mycotic and parasitic pathogens, especially those that colonize on or invade through mucosal surfaces. This antigen delivery system can also be used for expression of gamete-specific antigens to induce immune responses to block fertilization, or to induce immune responses to tumor antigens. In the event that an individual sheds live vaccine into the environment, the presence of the ELVS prevents survival of the vaccine. When environmentally regulated lethal genes are present on an extrachromosomal element and are regulated by chromosomal genes, transfer of the extrachromosomal element to other microorganisms will be limited by unregulated expression of the lethal genes in the recipient microorganism.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Regulated antigen delivery system (RADS)
InactiveUS7341860B2Increase heightImprove the level ofBacteriaMicroorganism based processesOrigin of replicationGene delivery
Owner:WASHINGTON UNIV IN SAINT LOUIS
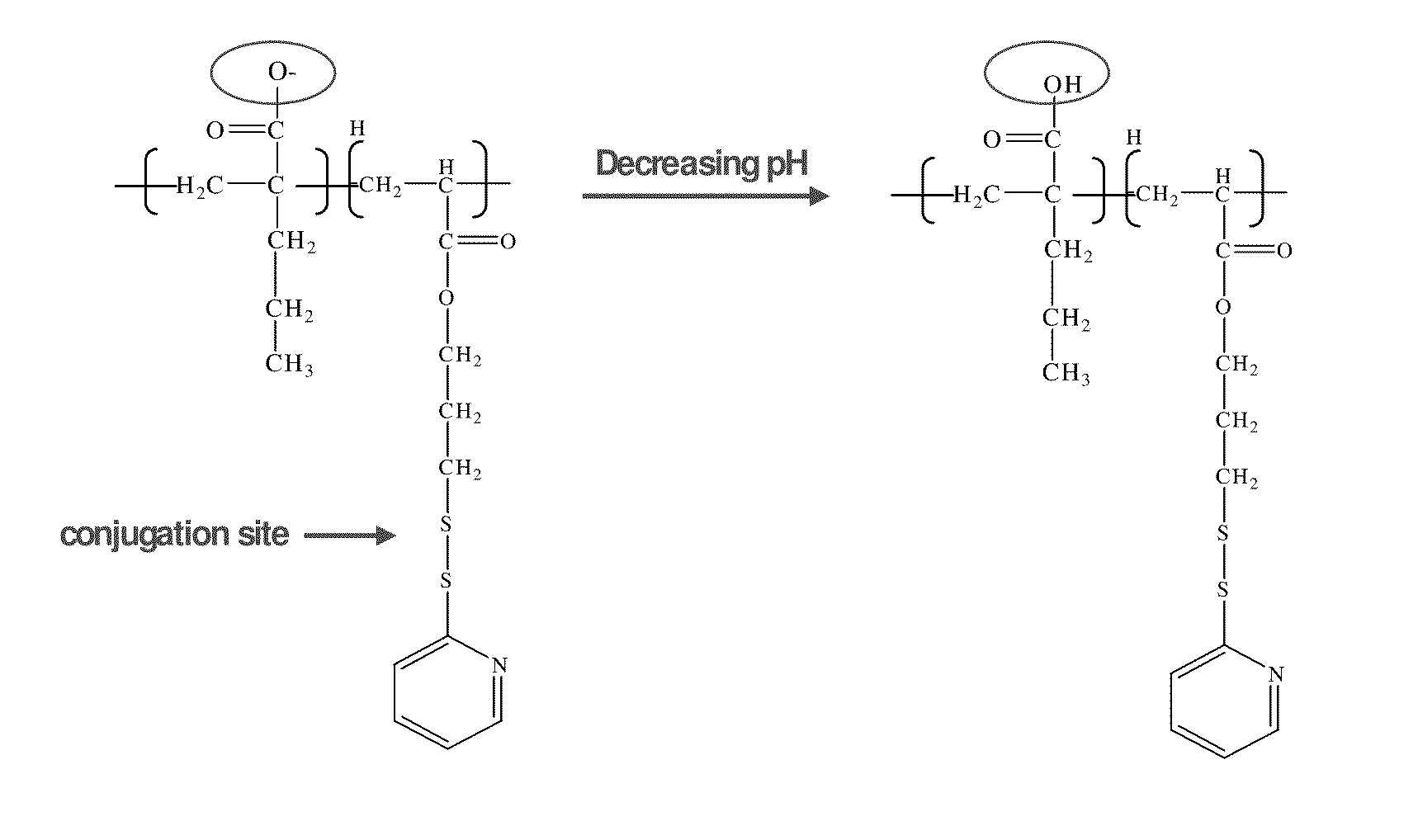
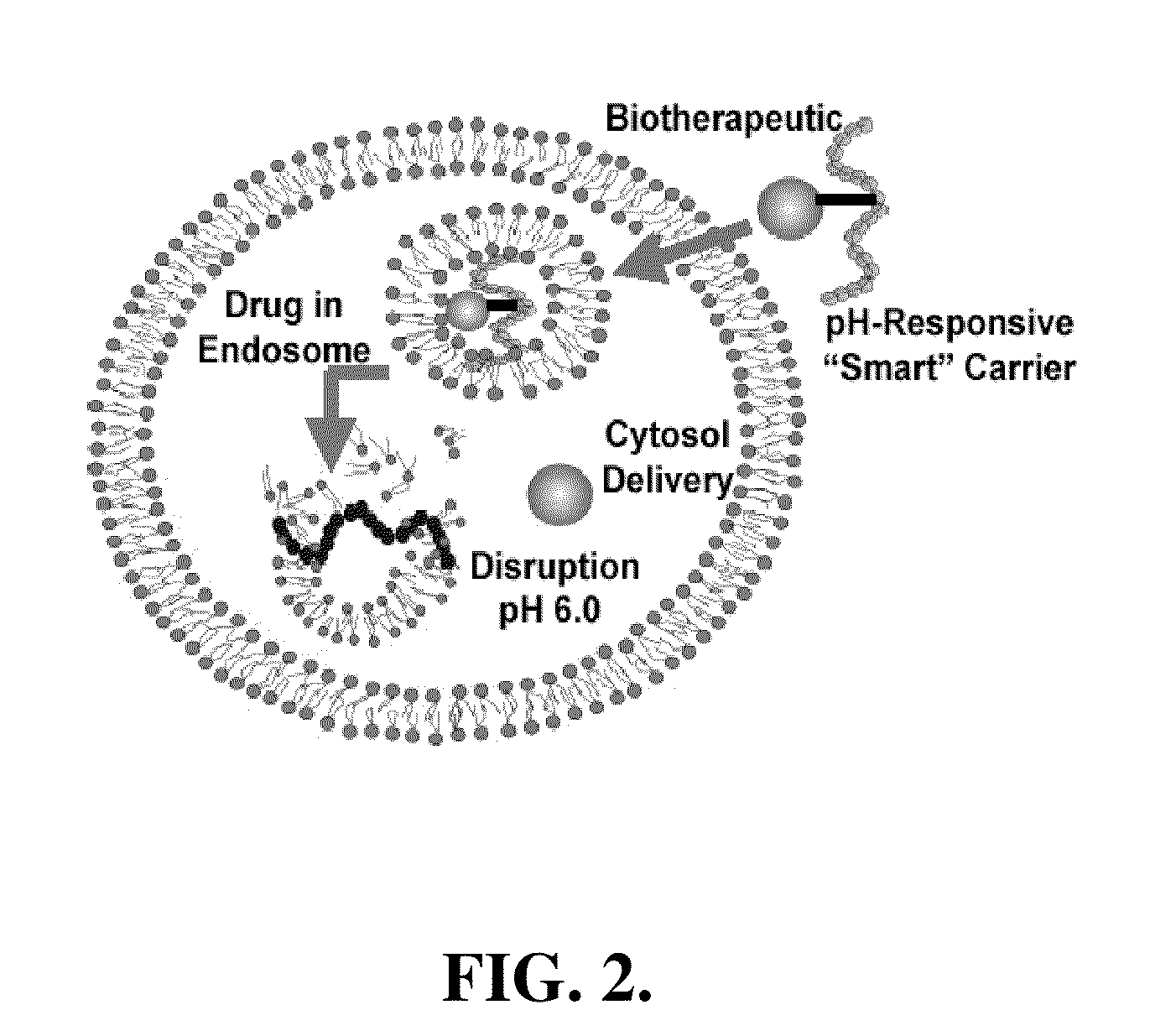
pH-RESPONSIVE POLYMER CARRIER COMPOSITIONS FOR CYTOSOLIC PROTEIN DELIVERY
InactiveUS20100150952A1Amount of remainedIncrease volumeCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsAntigen deliveryLymphocyte
pH-Responsive polymer-based protein delivery carriers and compositions, methods for making the carriers and compositions, and methods for using the carriers and compositions for intracellular protein antigen delivery, inducing a cytotoxic T-lymphocyte response, introducing a tumor-specific protein antigen to an antigen presenting cell to induce an immune response, and providing tumor protection to a subject.
Owner:UNIV OF WASHINGTON
Antigen delivery vectors and constructs
ActiveUS20060013820A1Improving immunogenicityOptimise steric presentation of antigenAntibacterial agentsOrganic active ingredientsVirologyAntigen delivery
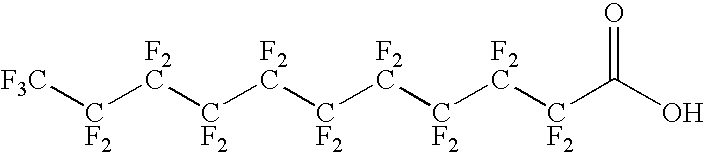
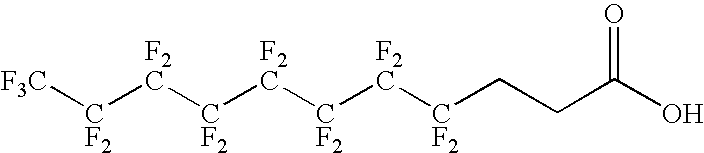
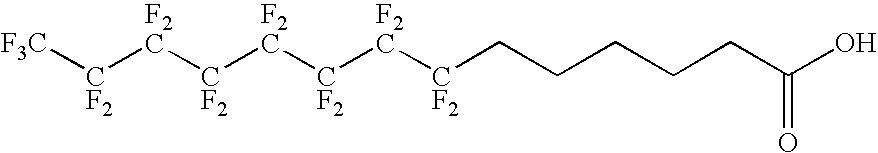
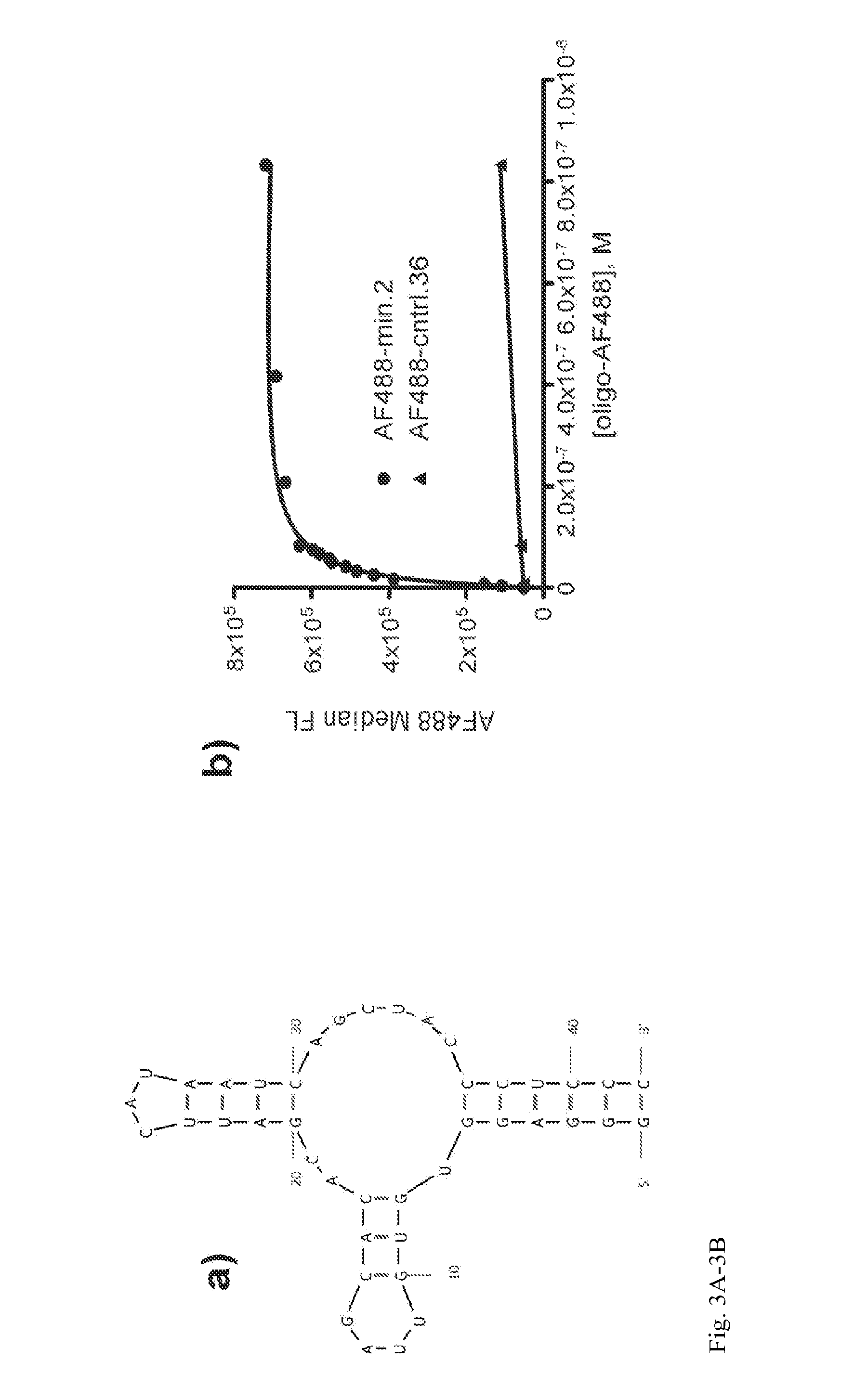
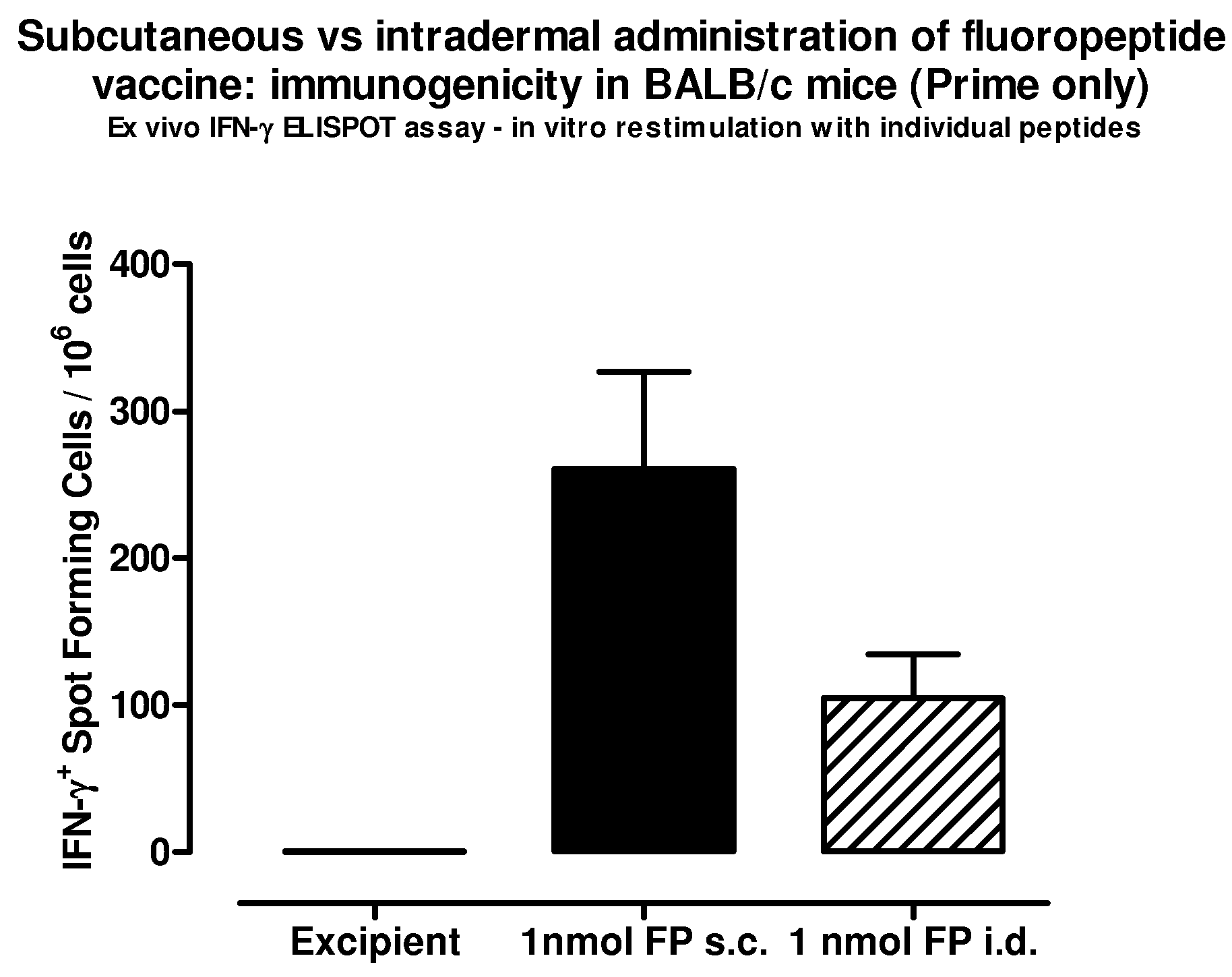
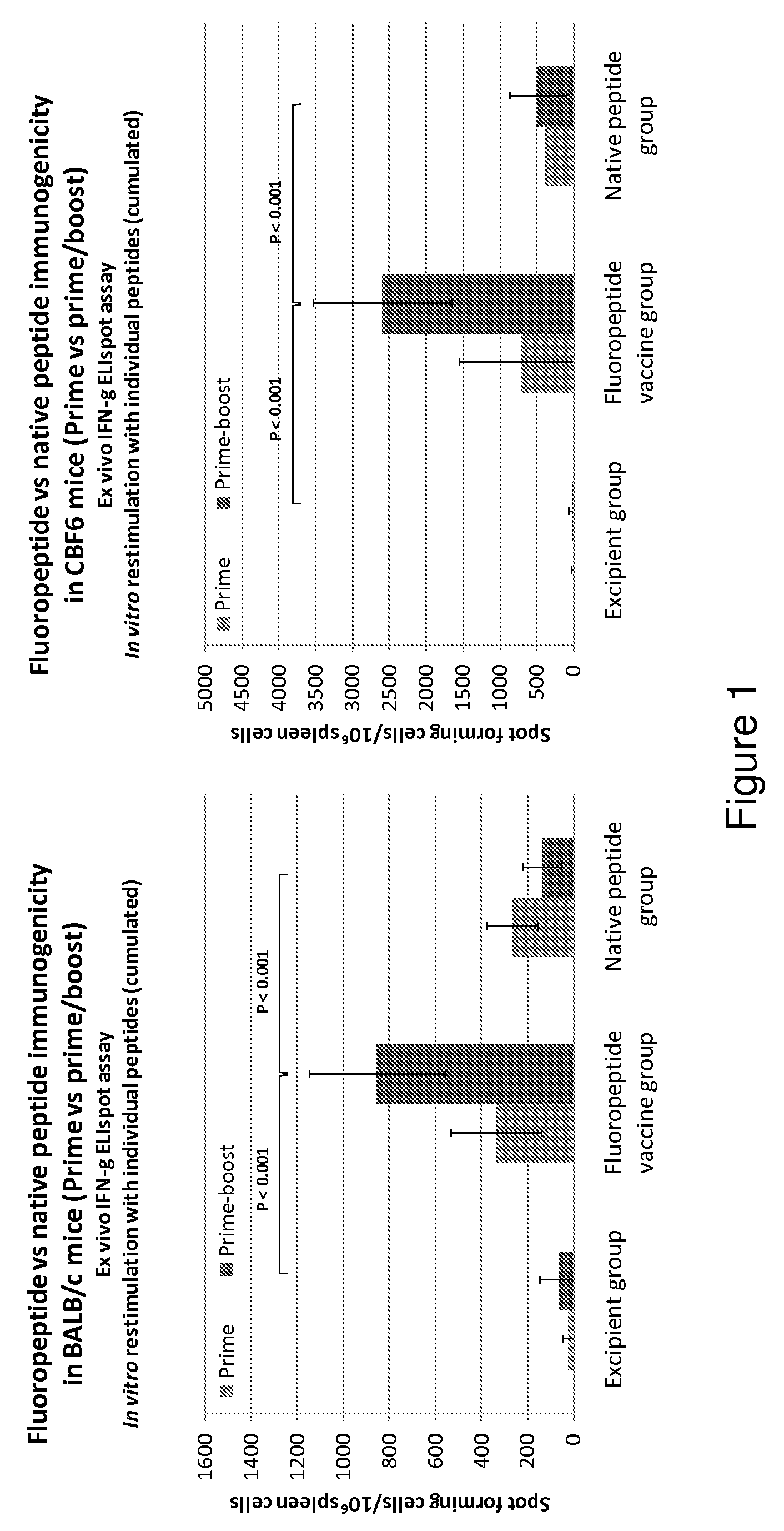
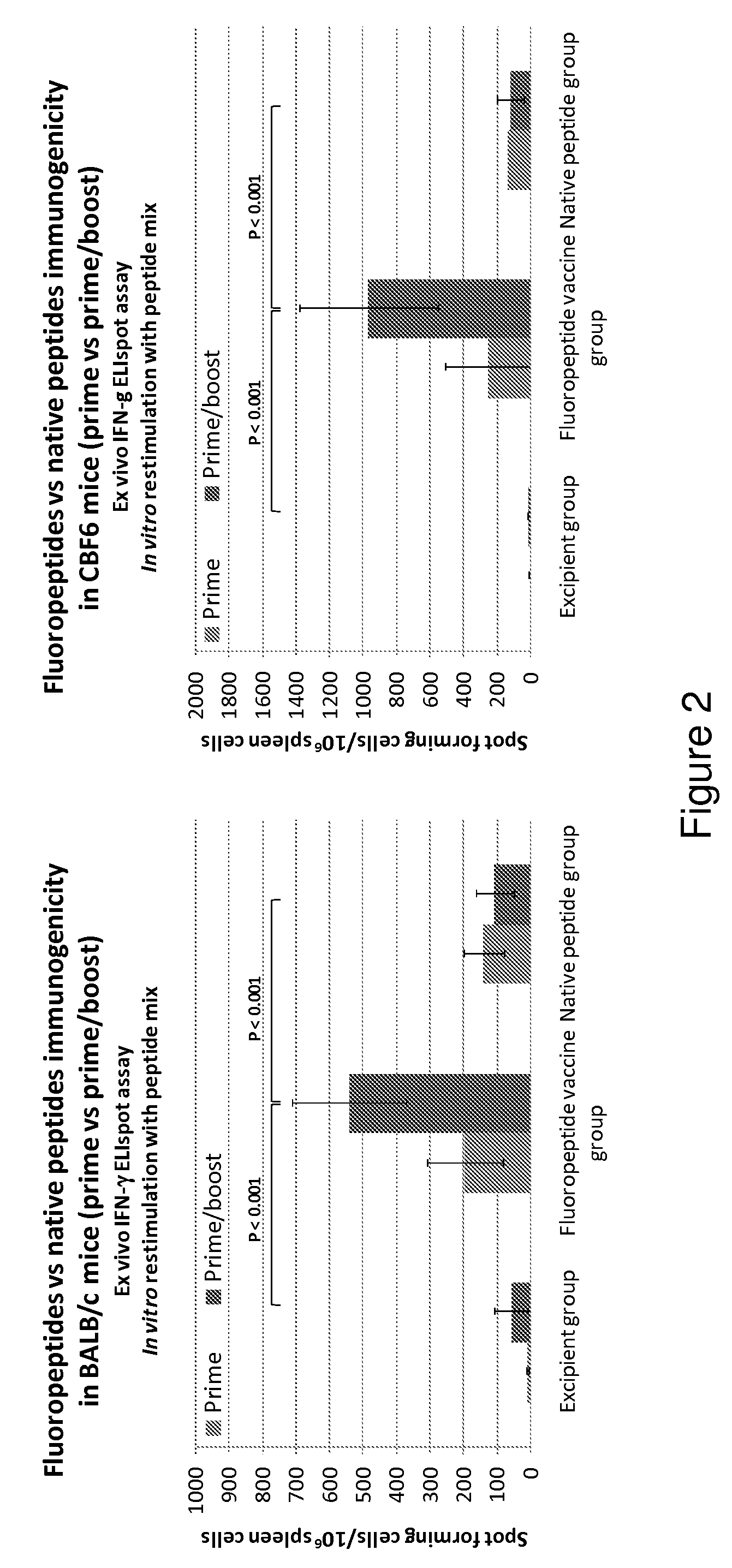
The present invention relates to fluorocarbon vectors for the delivery of antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals.
Owner:ALTIMMUNE UK LTD
Dry formulation for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells through the skin, and induces an immune response in an animal or human. For example, a skin-active adjuvant (e.g., an ADP-ribosylating exotoxin) can be used to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a dry formulation containing antigen and adjuvant to skin of the animal or human. The dry formulation may be a powder or a unit-dose patch. Use of adjuvant is not required if the antigen is sufficiently antigenic. Transcutaneous immunization may be induced with or without penetration enhancement.
Owner:IOMAI CORP
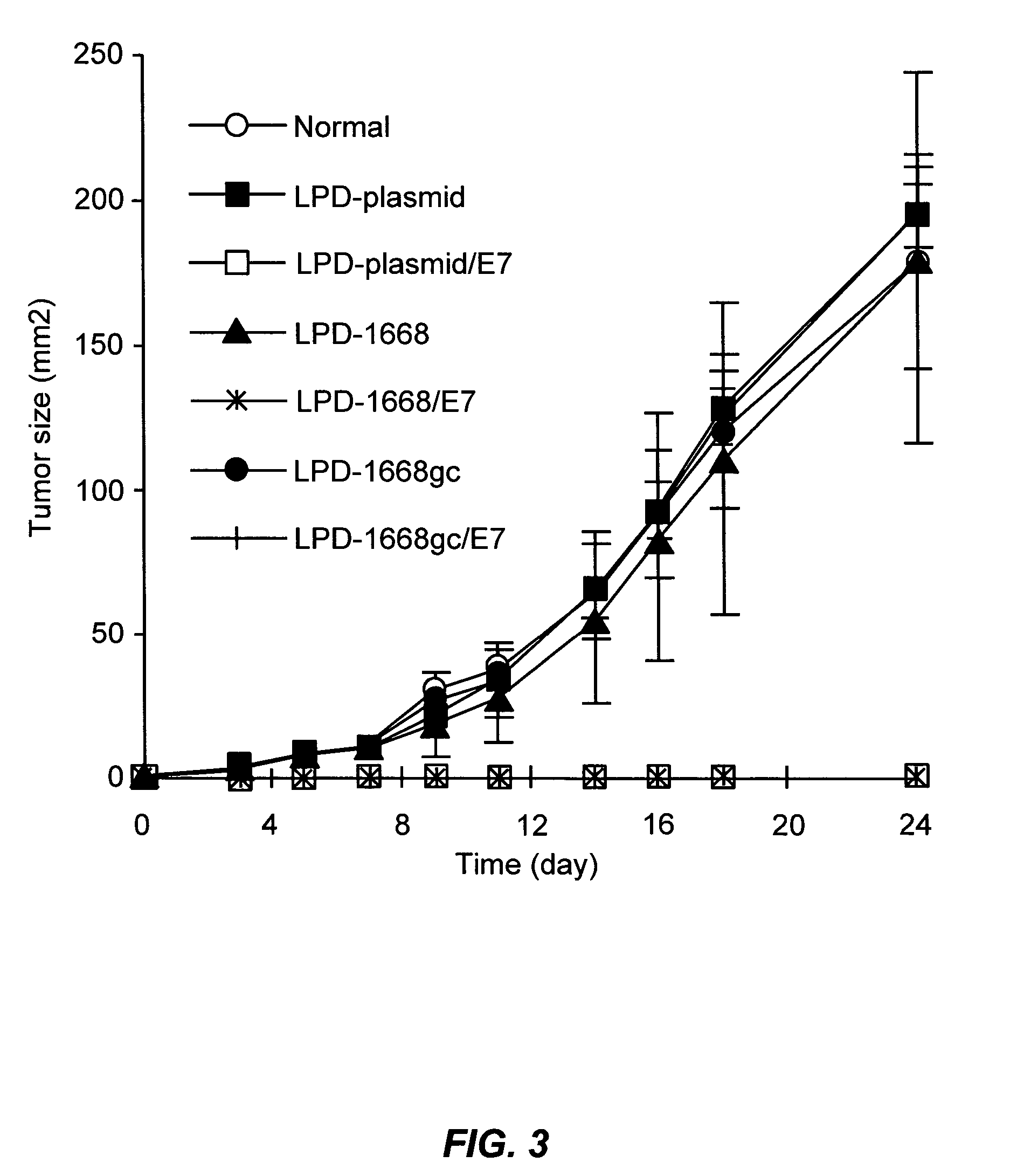
Plasmid maintenance system for antigen delivery
InactiveUS6703233B1Low toxicityGood for persistenceAntibacterial agentsBacterial antigen ingredientsGene deliveryAntigen delivery
The present invention relates generally to a Plasmid Maintenance System for the stabilization of expression plasmids encoding foreign antigens, and methods for making and using the Plasmid Maintenance System. The invention optimizes the maintenance of expression plasmids at two independent levels by: (1) removing sole dependence on balanced lethal maintenance functions; and (2) incorporating at least one plasmid partition function to prevent random segregation of expression plasmids, thereby enhancing their inheritance and stability. The Plasmid Maintenance System may be employed within a plasmid which has been recombinantly engineered to express a variety of expression products.
Owner:UNIV OF MARYLAND BALTIMORE +1
Antigen delivery vectors and constructs
ActiveUS7687455B2Improving immunogenicityOptimise steric presentation of antigenAntibacterial agentsOrganic active ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals.
Owner:ALTIMMUNE UK LTD
Regulated antigen delivery system (RADS)
InactiveUS20050106176A1Effective exposureIncrease productionBacterial antigen ingredientsBacteriaGene deliveryOrigin of replication
We describe a regulated antigen delivery system (RADS) that has (a) a vector that includes (1) a gene encoding a desired gene product operably linked to a control sequence, (2) an origin of replication conferring vector replication using DNA polymerase III, and (3) an origin of replication conferring vector replication using DNA polymerase I, where the second origin of replication is operably linked to a control sequence that is repressible by a repressor. The RADS microorganism also has a gene encoding a repressor, operably linked to an activatible control sequence. The RADS described provide high levels of the desired gene product after repression of the high copy number origin of replication is lifted. The RADS are particularly useful as live bacterial vaccines. Also described is a delayed RADS system, in which there is a delay before the high copy number origin is expressed after the repression is lifted. The delayed RADS is also particularly useful for live bacterial vaccines. Also described are several control elements useful for these systems, as well as methods for providing immunity to a pathogen in a vertebrate immunized with the RADS microorganisms.
Owner:WASHINGTON UNIV IN SAINT LOUIS
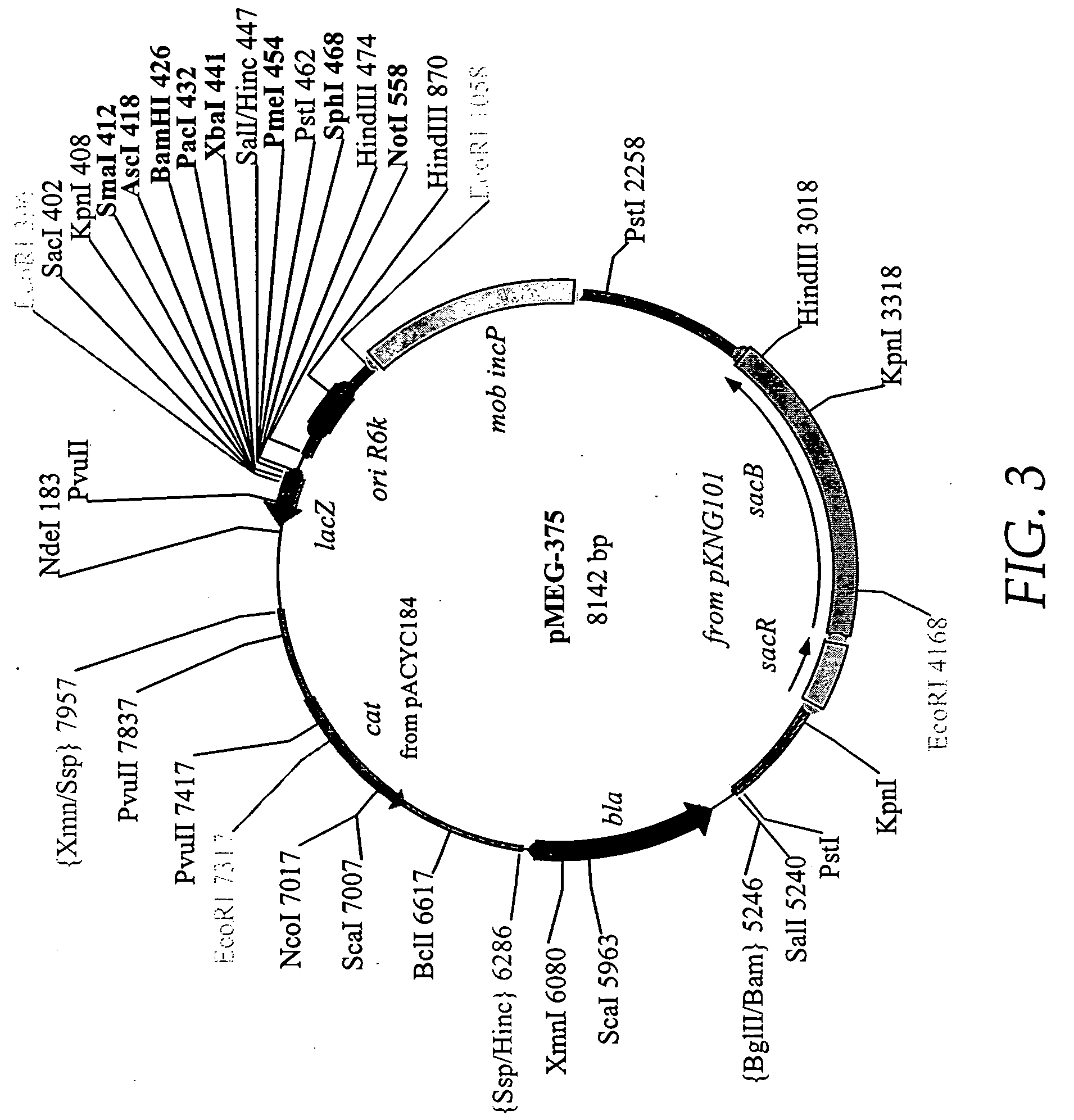
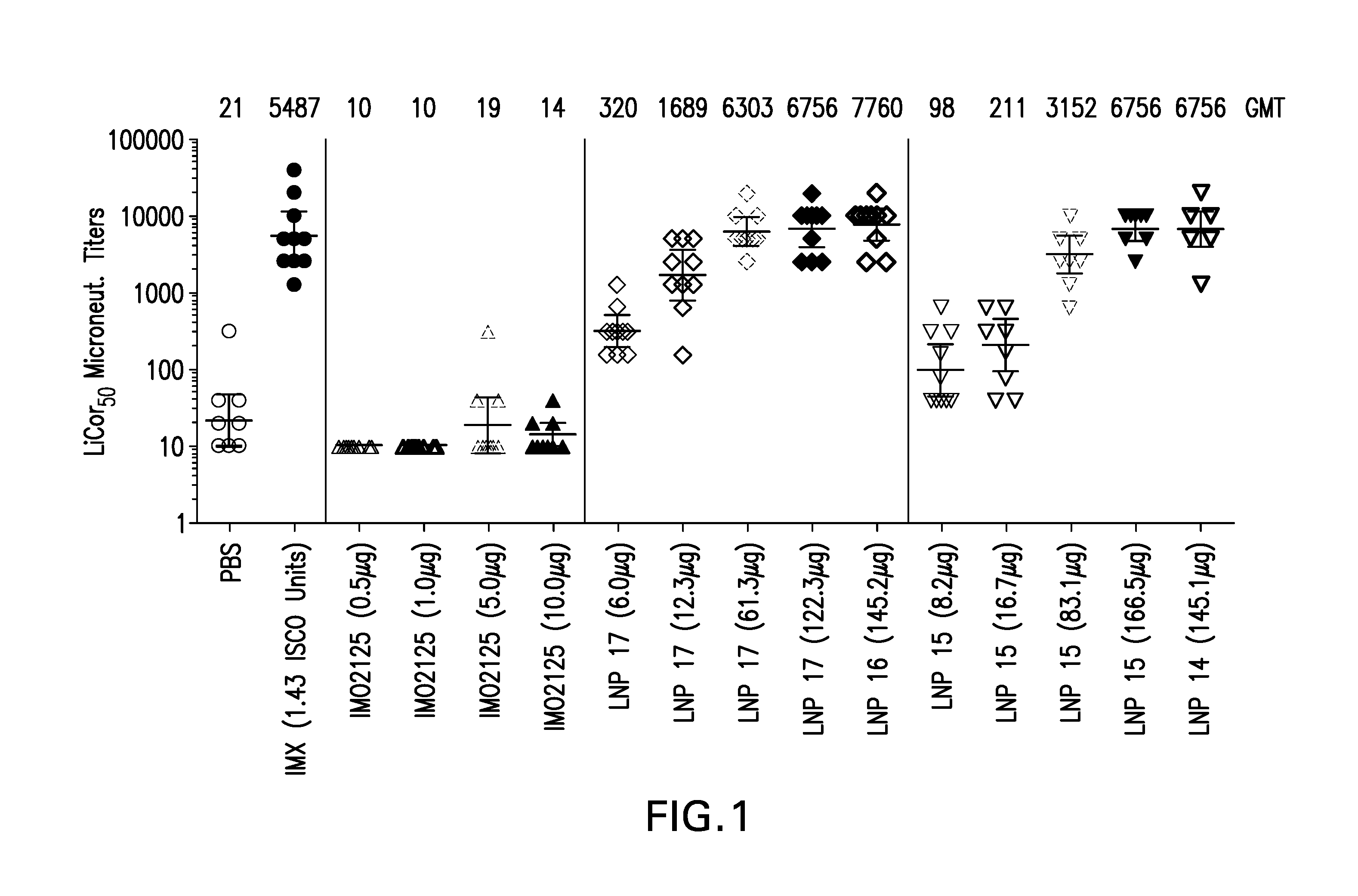
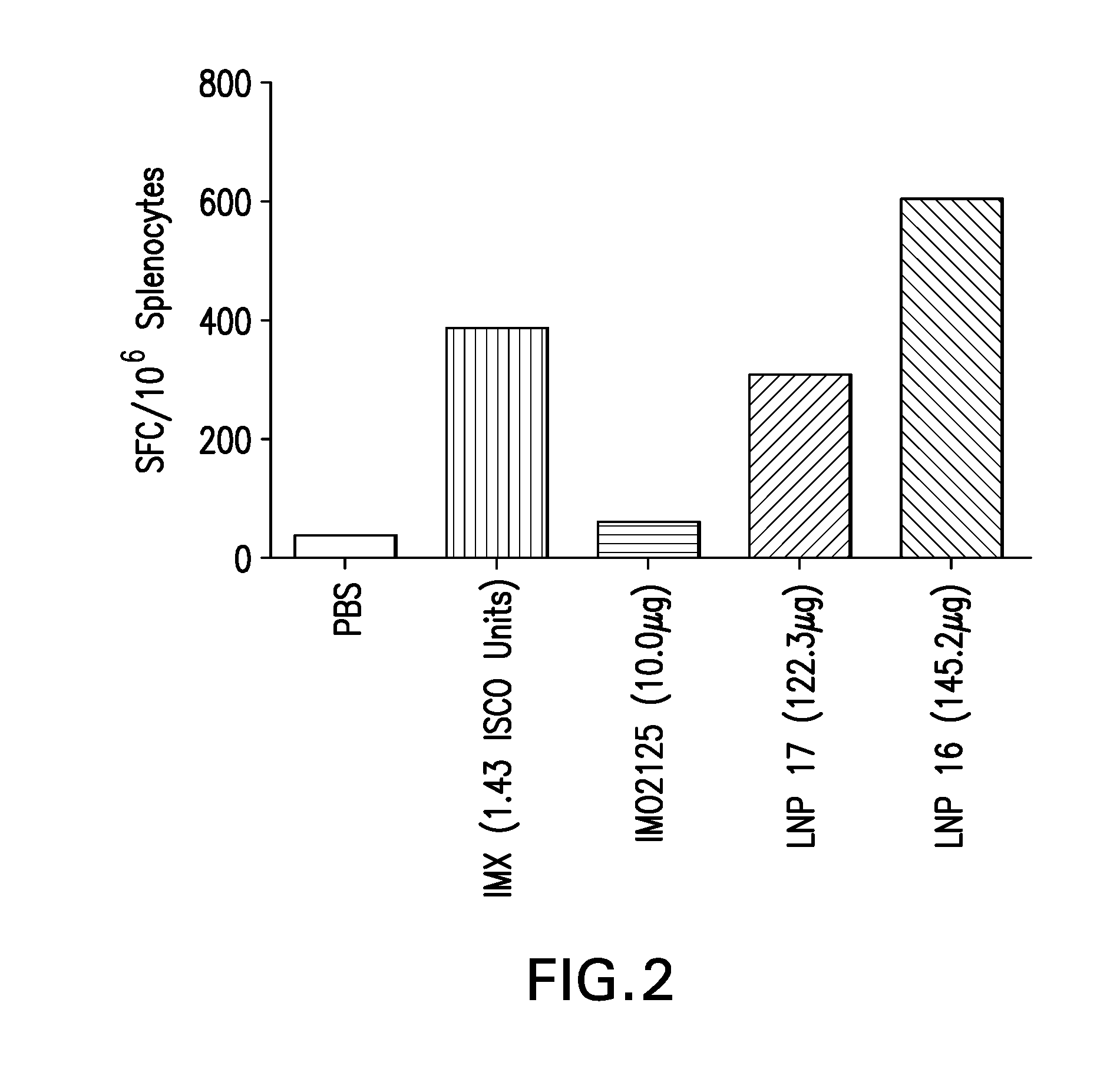
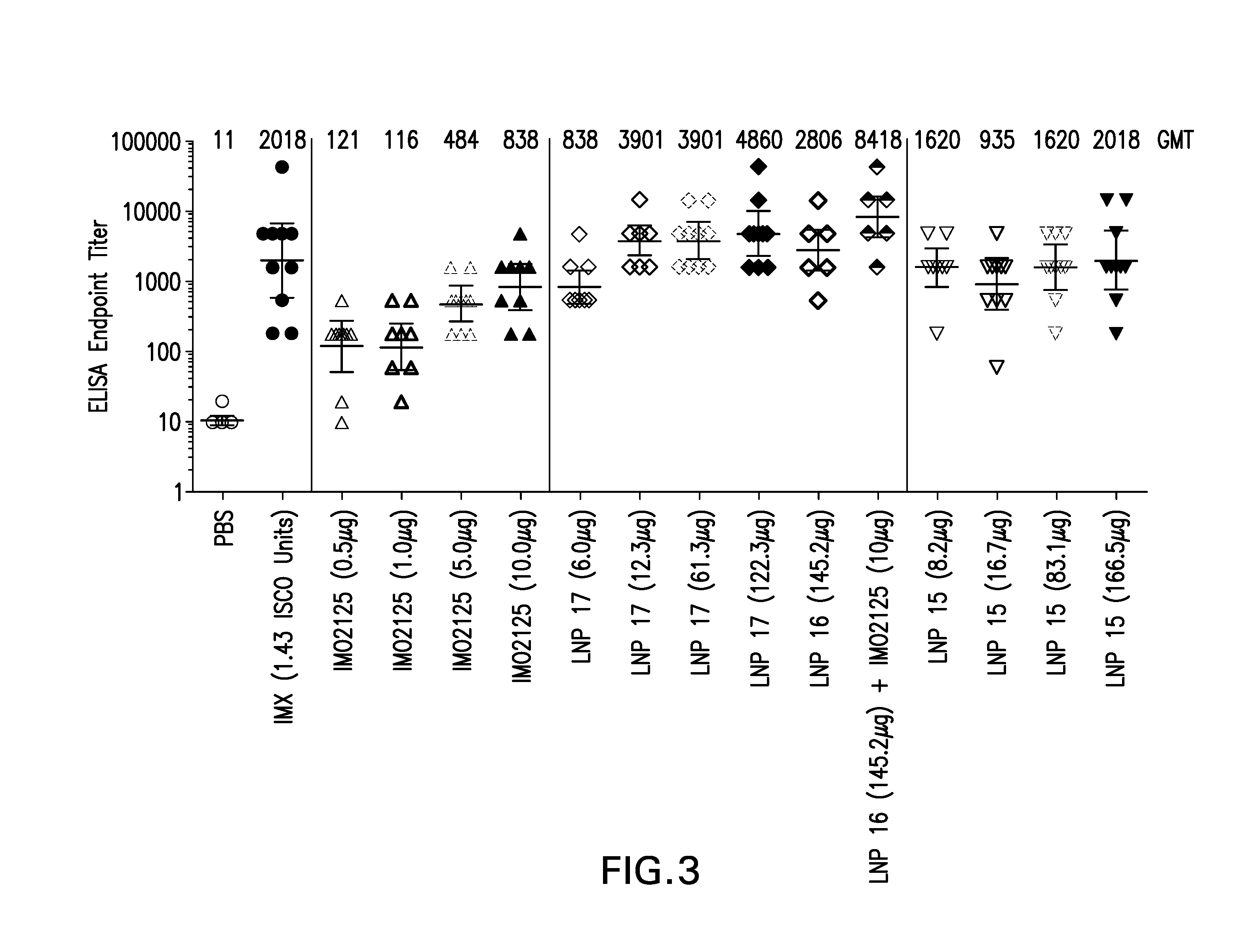
Lipid nanoparticle vaccine adjuvants and antigen delivery systems
The instant invention provides for novel lipid nanoparticle (LNP) formulations, containing cationic lipids, for use as vaccine adjuvants and / or as antigen delivery systems. It is an object of the instant invention to provide LNP formulations that demonstrate enhancements in humoral and cellular immunogenicity of vaccine antigens, particularly subunit vaccine antigens, when utilized alone or in combination with immunostimulatory agents (e.g. small molecule or oligonucleotide TLR agonists). The instant invention further identifies physical and chemical properties of the LNP formulations that can be manipulated to enhance antigen efficiency and adjuvant tolerability in vivo.
Owner:MERCK SHARP & DOHME LLC
Multiple delivery system for heterologous antigens
InactiveUS20120135033A1Bacterial antigen ingredientsSugar derivativesHeterologousHeterologous Antigens
The invention is directed to an episomal recombinant nucleic acid encoding at least two heterologous antigens each fused to a PEST-endogenous polypeptide, vaccines comprising the same, methods of preparing same, and methods of inducing an immune response, and treating, inhibiting, or suppressing cancer or tumors comprising administering the same.
Owner:ADVAXIS
Delivery of biomolecules to immune cells
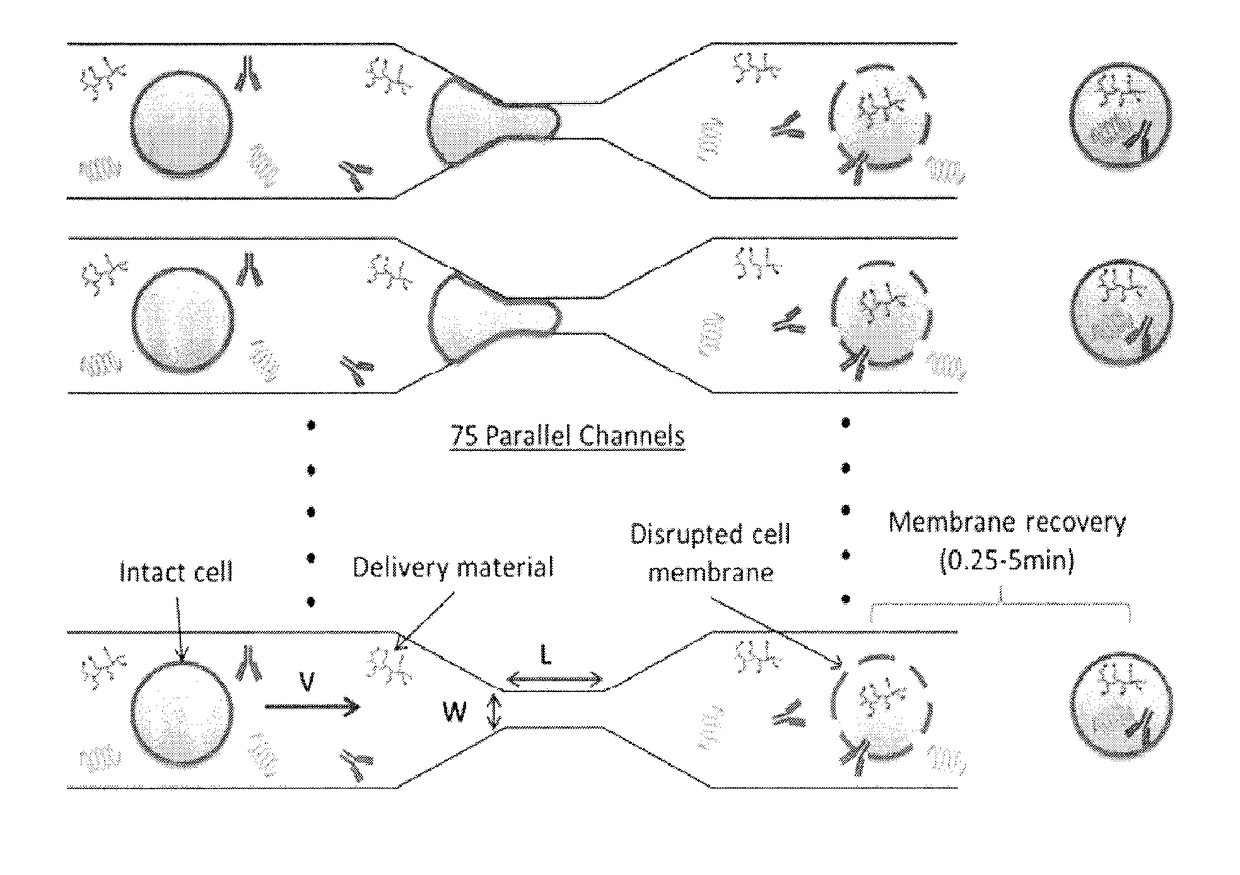
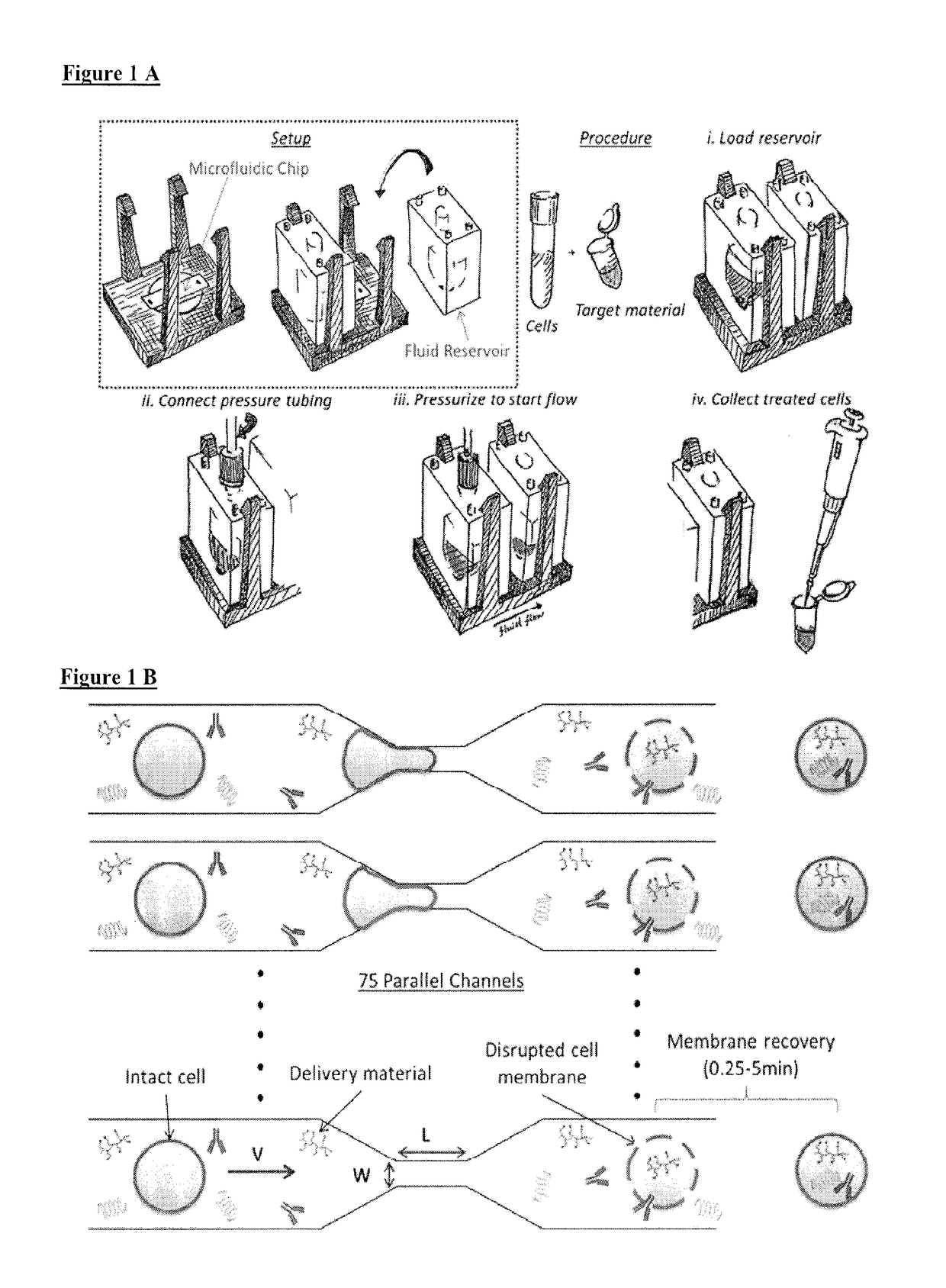
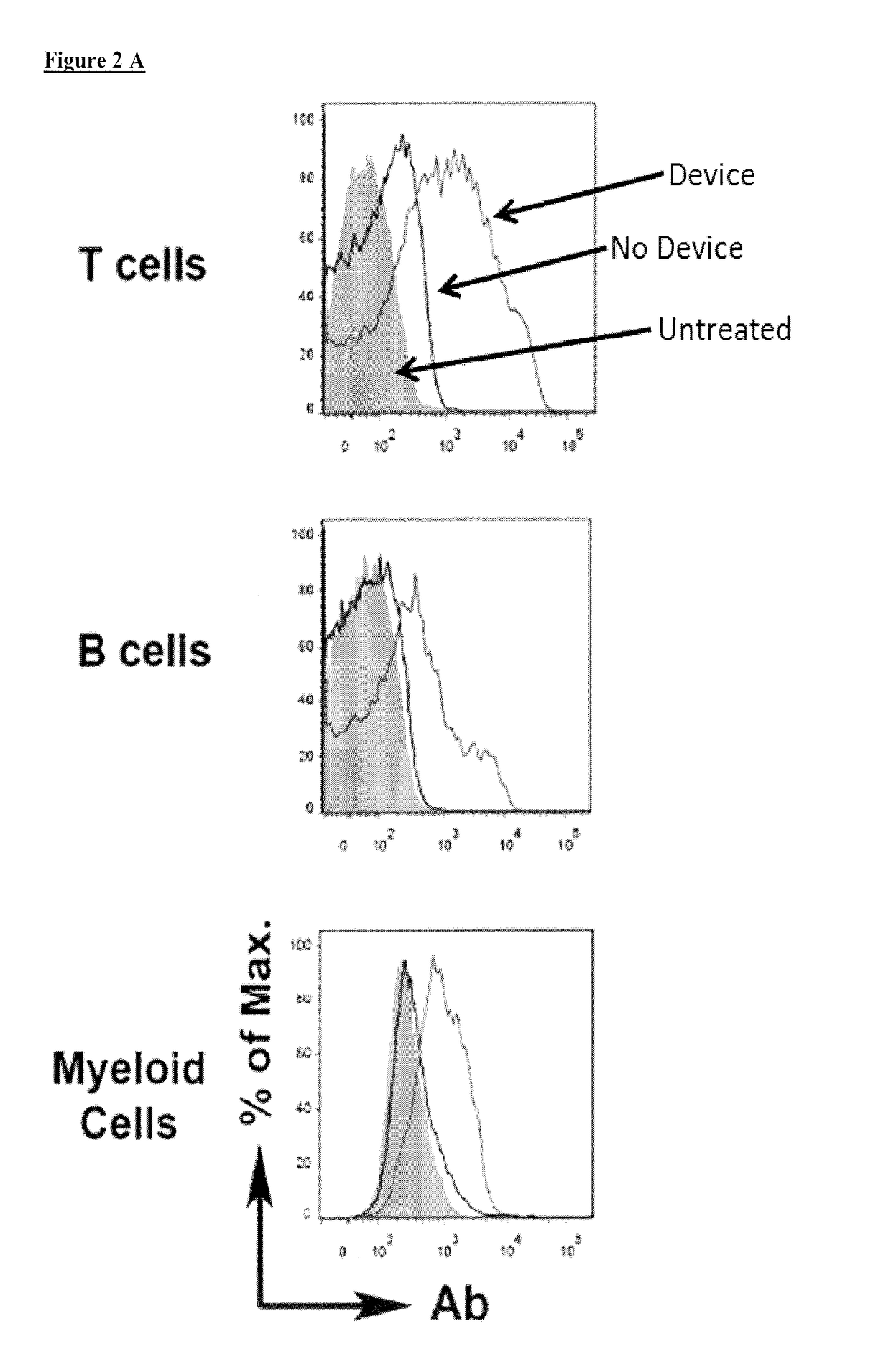
ActiveUS20180142198A1Elicit T cell responseGenetically modified cellsCulture processCytosolAntigen delivery
A method and device for preferentially delivering a compound such as an antigen to the cytosol of an immune cell. The method comprises passing a cell suspension comprising the target immune cell through a microfluidic device and contacting the suspension with the compound(s) or payload to be delivered.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
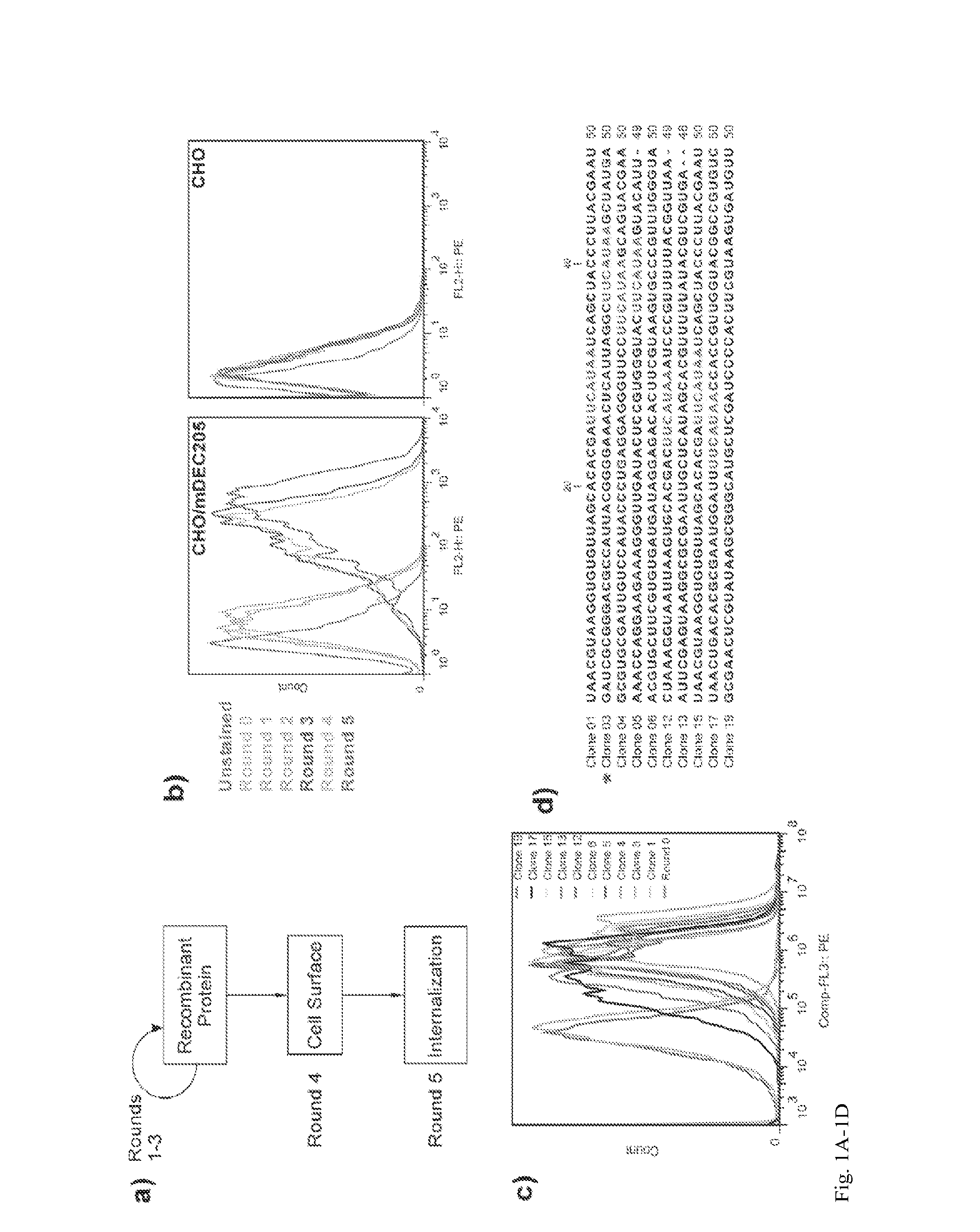
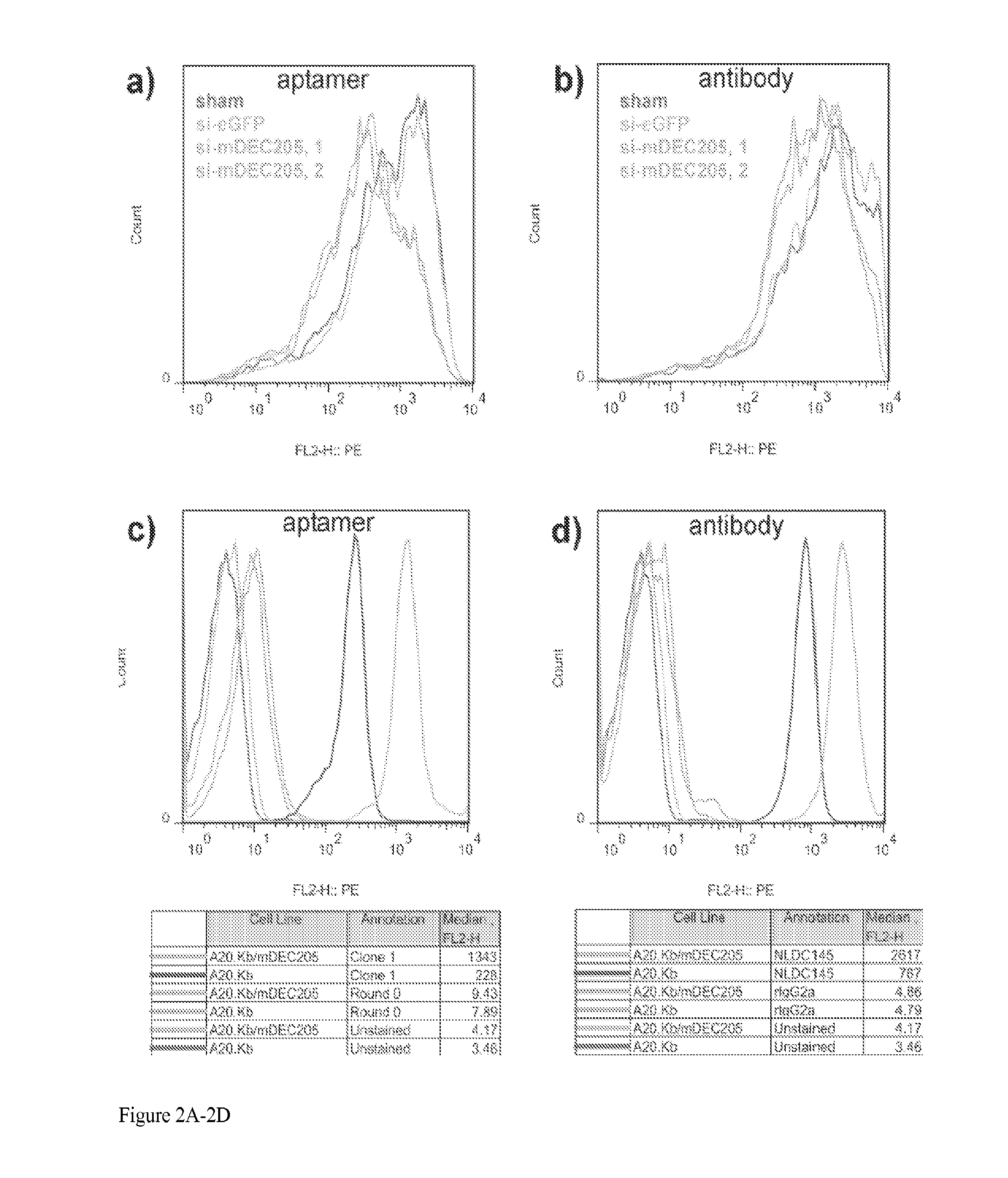
Aptamer-targetted antigen delivery
ActiveUS20150191730A1Elicit immune responseSpecial deliverySugar derivativesGene deliveryAntigen delivery
A composition is provided comprising an oligonucleotide aptamer conjugated to an antigen, wherein the aptamer is directed against a cell-surface target of an antigen-presenting cell. Also provided are methods of delivering an antigen to a dendritic cell and of eliciting an immune response in a subject.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Influenza antigen delivery vectors and constructs
ActiveUS20090191233A1Good curative effectImproving immunogenicitySsRNA viruses negative-sensePeptide/protein ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of influenza antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-influenza antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals, including humans.
Owner:ALTIMMUNE UK LTD
Bacillus based delivery system and methods of use
ActiveUS9133251B2Slow onsetImprove the immunityBiocideSsRNA viruses positive-senseSurface displayAureobasidium sp.
Herein a Bacillus exosporium antigen delivery (BEAD) system that provides a means to introduce recombinant proteins or small molecules into the exosporium of members of the B. cereus family of bacteria, i.e. B. anthracis, B. cereus, and B. thuringiensis, is disclosed. The system results in the surface display of recombinant proteins or small molecules such that they can stimulate an immune response. In addition, methods of making and using the system are described.
Owner:UNIVERSITY OF MISSOURI
Vaccine
InactiveUS8846080B2Slow linkEase with which oligonucleotideSugar derivativesViral antigen ingredientsAntigen deliveryImmunogenicity
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Adjuvant compositions
InactiveUS20100003280A1Safe and effectiveImproving immunogenicityAntibacterial agentsOrganic active ingredientsAntigen deliveryNucleic acid sequencing
Adjuvant compositions comprising type 1 interferon inducers, such as double-stranded RNA, in combination with antigen delivery systems and / or immunostimulatory molecules, such as immunostimulatory nucleic acid sequences, for enhancing the immune response of a coadministered antigen, are described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Aptamer-targetted antigen delivery
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Influenza antigen delivery vectors and constructs
ActiveUS8642531B2SsRNA viruses negative-senseHalogenated hydrocarbon active ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of influenza antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-influenza antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals, including humans.
Owner:ALTIMMUNE UK LTD
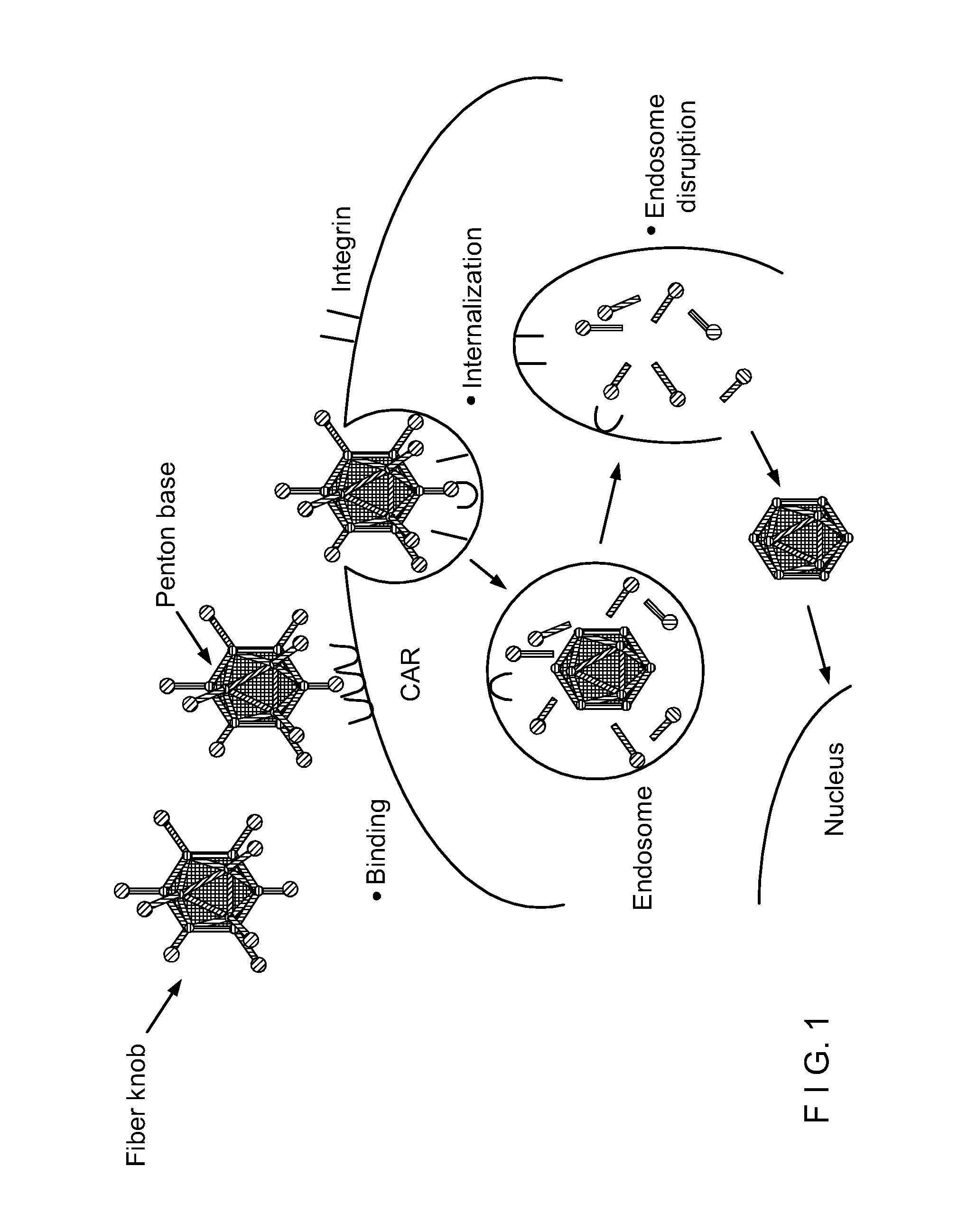
Capsid-Incorporated Antigen for Novel Adenovirus Vaccine
InactiveUS20110059135A1High affinityEnhances Ad transductionAntibody mimetics/scaffoldsAntibody medical ingredientsEpitopeAntigen delivery
This invention pertains to tropism-modified adenoviral vectors optimized for antigen delivery that induced both humoral and cellular immune responses, as well as a method of constructing and using such vectors. The vectors of the present invention may incorporate an epitope or an antigen into a capsid protein. Methods for treating of a host with an effective amount of adenovirus vector of the present invention are also provided.
Owner:KOVESDI IMRE +1
Antigen Delivery Vectors and Constructs
ActiveUS20100183650A1Improving immunogenicityOptimise steric presentation of antigenAntibacterial agentsHalogenated hydrocarbon active ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals.
Owner:ALTIMMUNE UK LTD
Antigen Delivery Vectors and Constructs
ActiveUS20100183708A1Improving immunogenicityOptimise steric presentation of antigenAntibacterial agentsHalogenated hydrocarbon active ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals.
Owner:ALTIMMUNE UK LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com