External preparation containing sirolimus as well as preparation method and application thereof
An external preparation, the technology of sirolimus, applied in the field of medicine, achieves the effect of good drug absorption, high safety and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Single Cream Prescription:
[0132] component name
Dosage
5g
40g
40g
20g
70g
Glycerin
50g
3g
3g
Laurocaprazine
2g
Appropriate amount
Full amount
1000g
[0133] Preparation Process:
[0134] (1) Oil phase: Accurately weigh stearic acid, glyceryl monostearate, vaseline, and liquid paraffin, heat and melt on a water bath, stir well, and keep warm at about 75°C;
[0135] (2) Water phase: Accurately weigh glycerin, triethanolamine, laurocapram, sodium lauryl sulfate and water, heat and stir in a water bath to dissolve, and heat to 75°C.
[0136] (3) Slowly add the above oil phase into the water phase, stir for 15 minutes, and keep stirring.
[0137] (4) Turn on the cooling water, start cooling down to about 5...
Embodiment 2
[0140] Single Cream Prescription:
[0141] components
Dosage
Sirolimus
10g
30g
60g
20g
30g
Glycerin
30g
5g
Appropriate amount
Full amount
1000g
[0142] Preparation Process:
[0143] (1) Oil phase: Accurately weigh stearic acid, glyceryl monostearate, vaseline, and liquid paraffin, heat and melt on a water bath, stir well, and keep warm at about 75°C;
[0144] (2) Water phase: Accurately weigh glycerin, triethanolamine, laurocaprazine and water, heat and stir in a water bath to dissolve, and heat to 75°C.
[0145] (3) Slowly add the above oil phase into the water phase, stir for 15 minutes, and keep stirring.
[0146] (4) Turn on the cooling water, start cooling down to about 55°C, add the prescribed amount of sirolimus, and continue stirring.
[0147] (5) When the...
Embodiment 3
[0149] Compound Cream Prescription:
[0150] components
Dosage
Sirolimus
1g
triamcinolone acetonide
2g
stearic acid
100g
liquid paraffin
120g
20g
Glycerin
40g
8ml
Appropriate amount
quantity
1000g
[0151] Preparation Process:
[0152] Weigh stearic acid, liquid paraffin, and lanolin in the same container, heat to 80°C, stir well, and use it as the oil phase for later use; take glycerin, triethanolamine and water in another container, heat to 80°C, Stir well and use as the water phase for later use; add the oil phase to the water phase and stir, and when the temperature drops to 60°C, add the prescribed amount of sirolimus and triamcinolone acetonide, and stir evenly; Pack and serve.
PUM
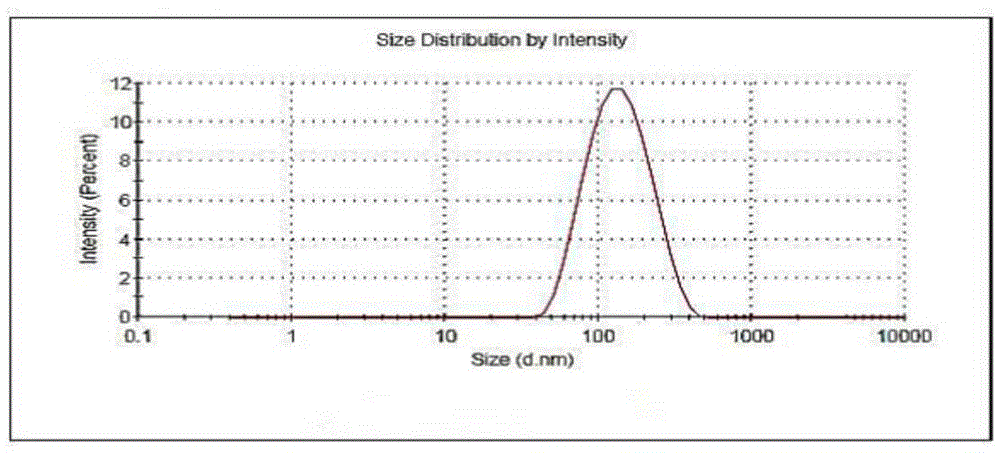
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com