Patents
Literature
72 results about "Triamcinolone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triamcinolone is a glucocorticoid used to treat certain skin diseases, allergies, and rheumatic disorders among others. It is also used to prevent worsening of asthma and COPD. It can be taken in various ways including by mouth, injection into a muscle, and inhaled.
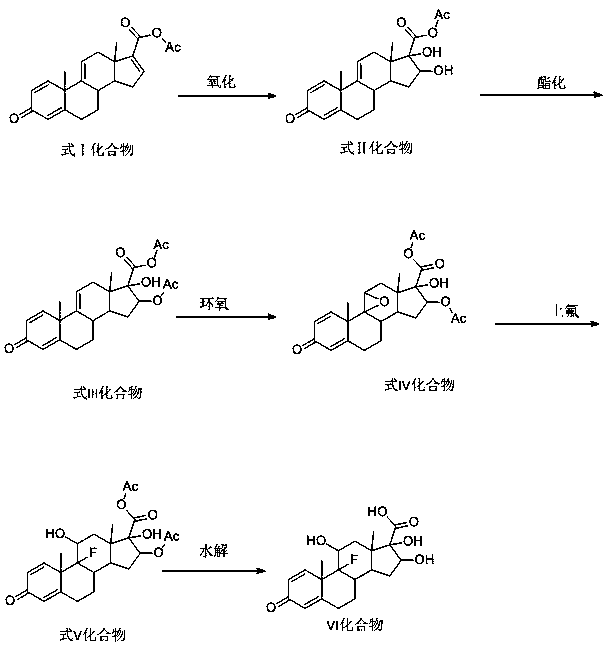
Preparation of metacortandralone and derivatives thereof
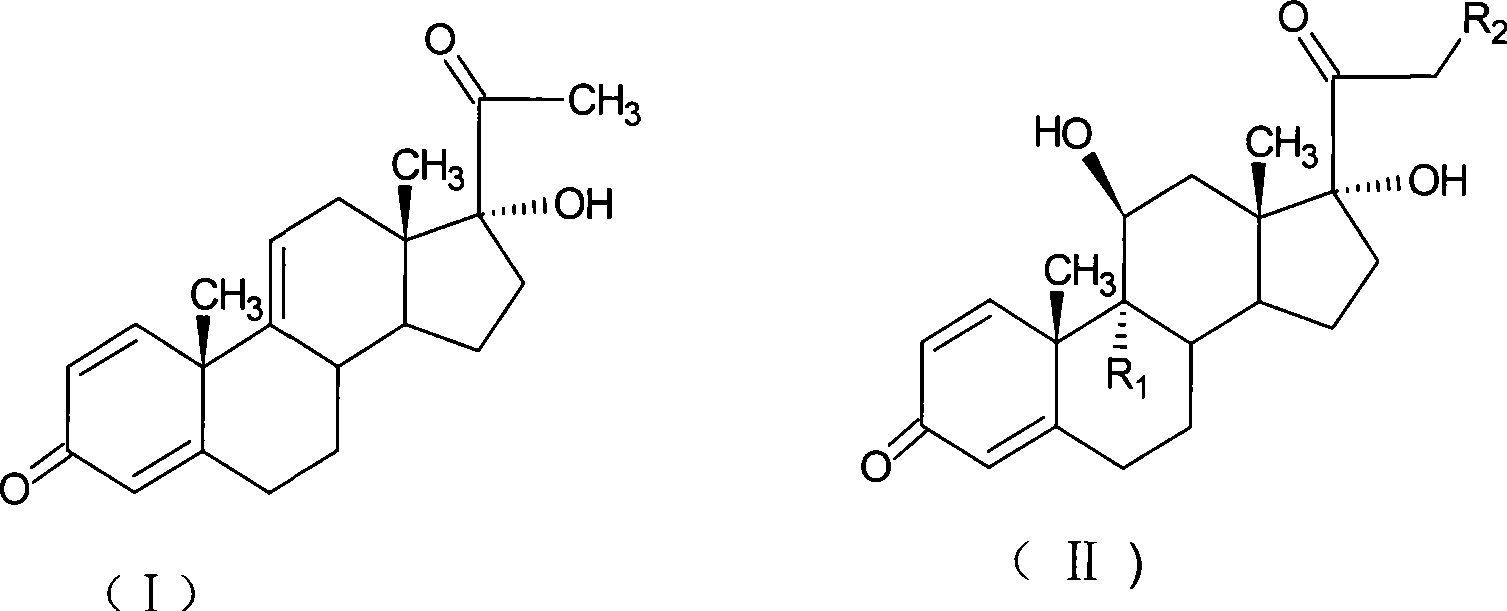
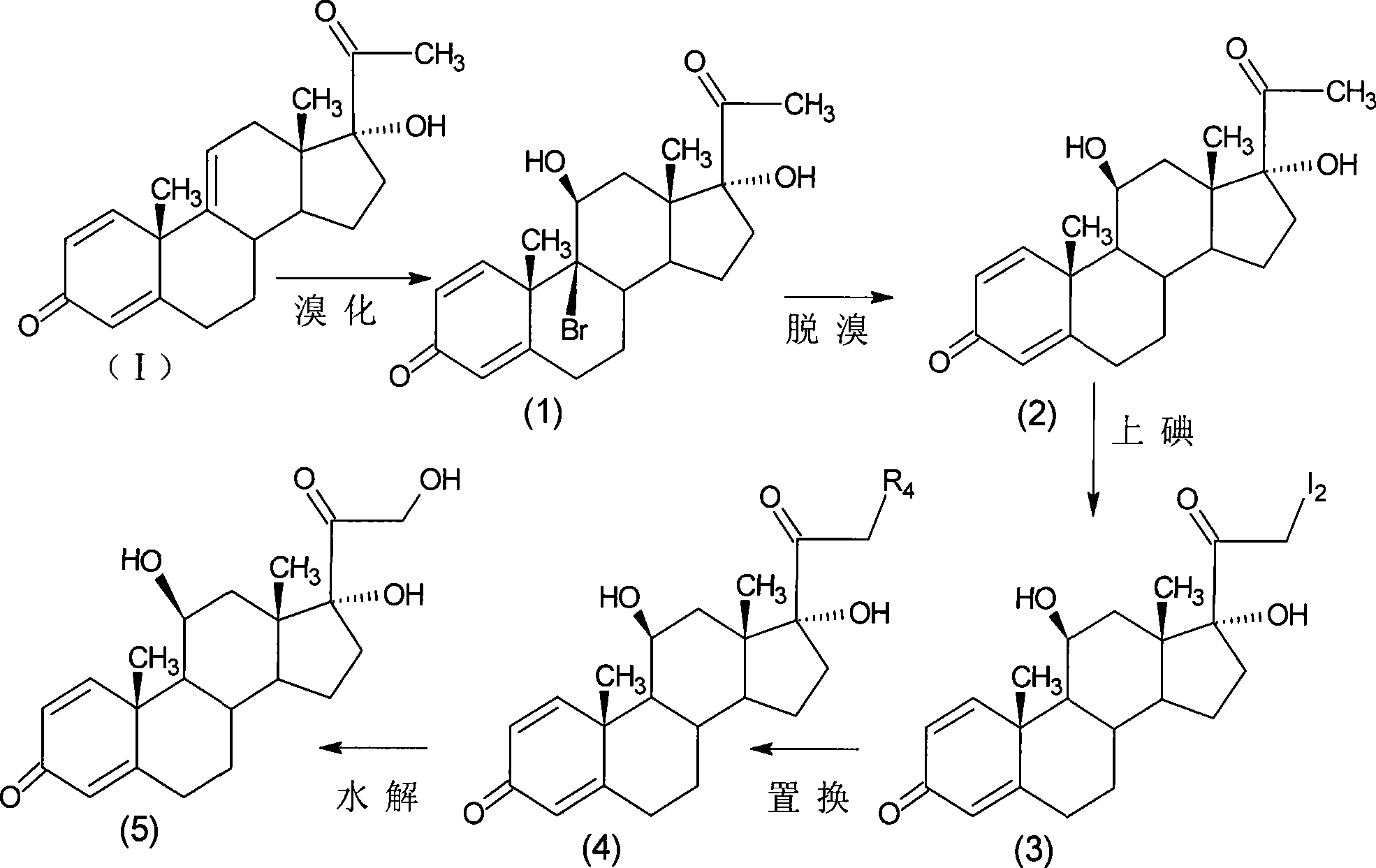
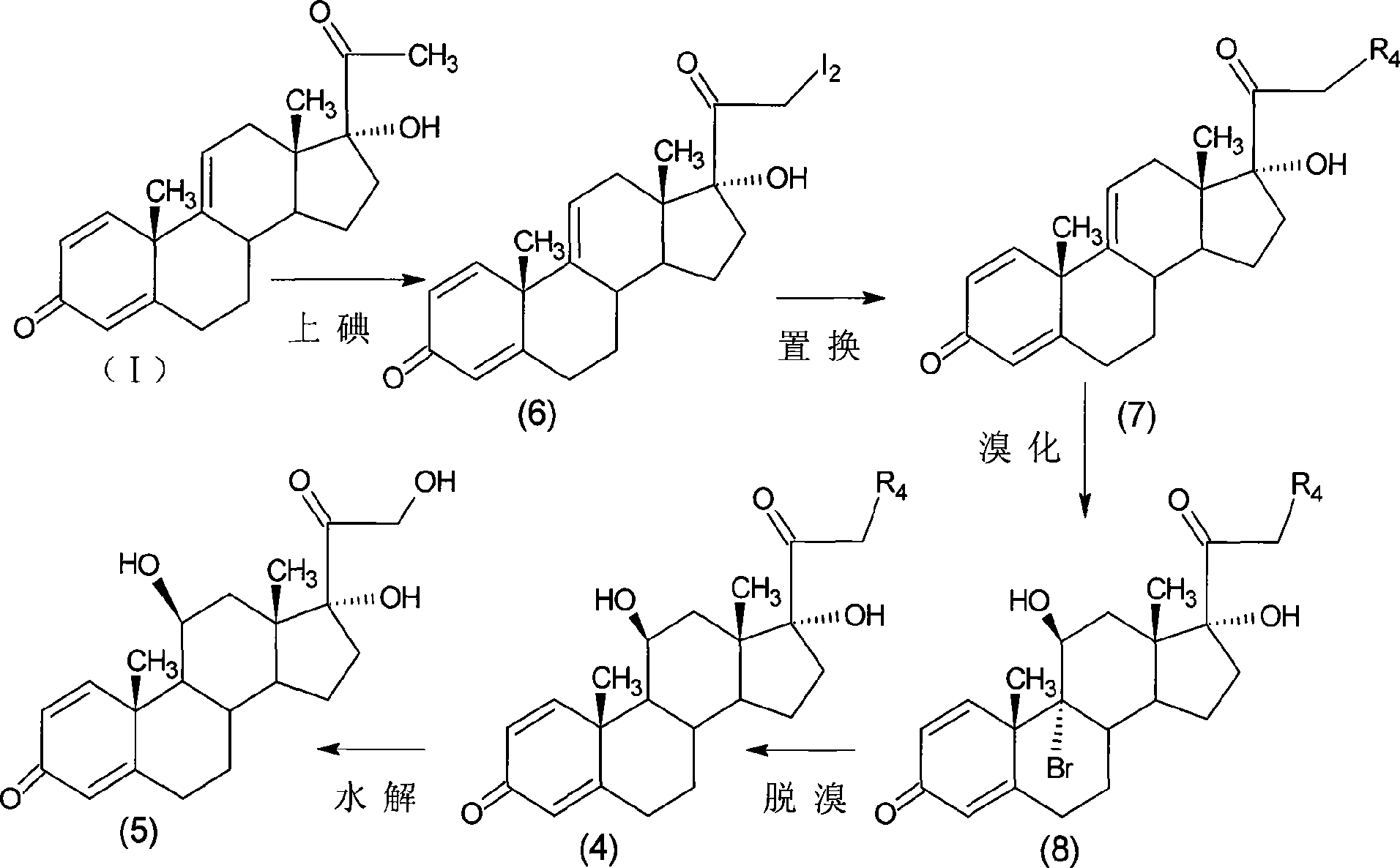
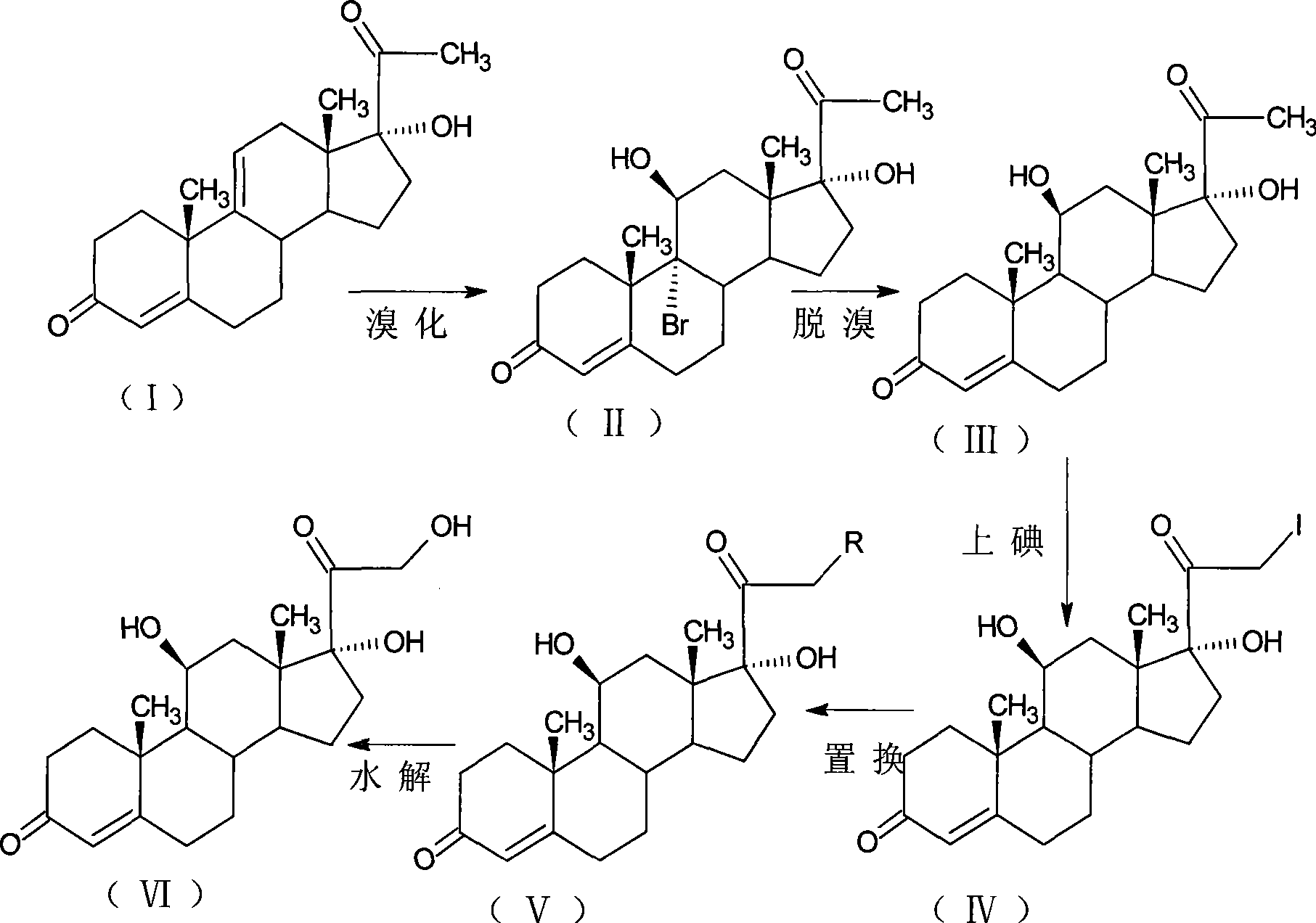
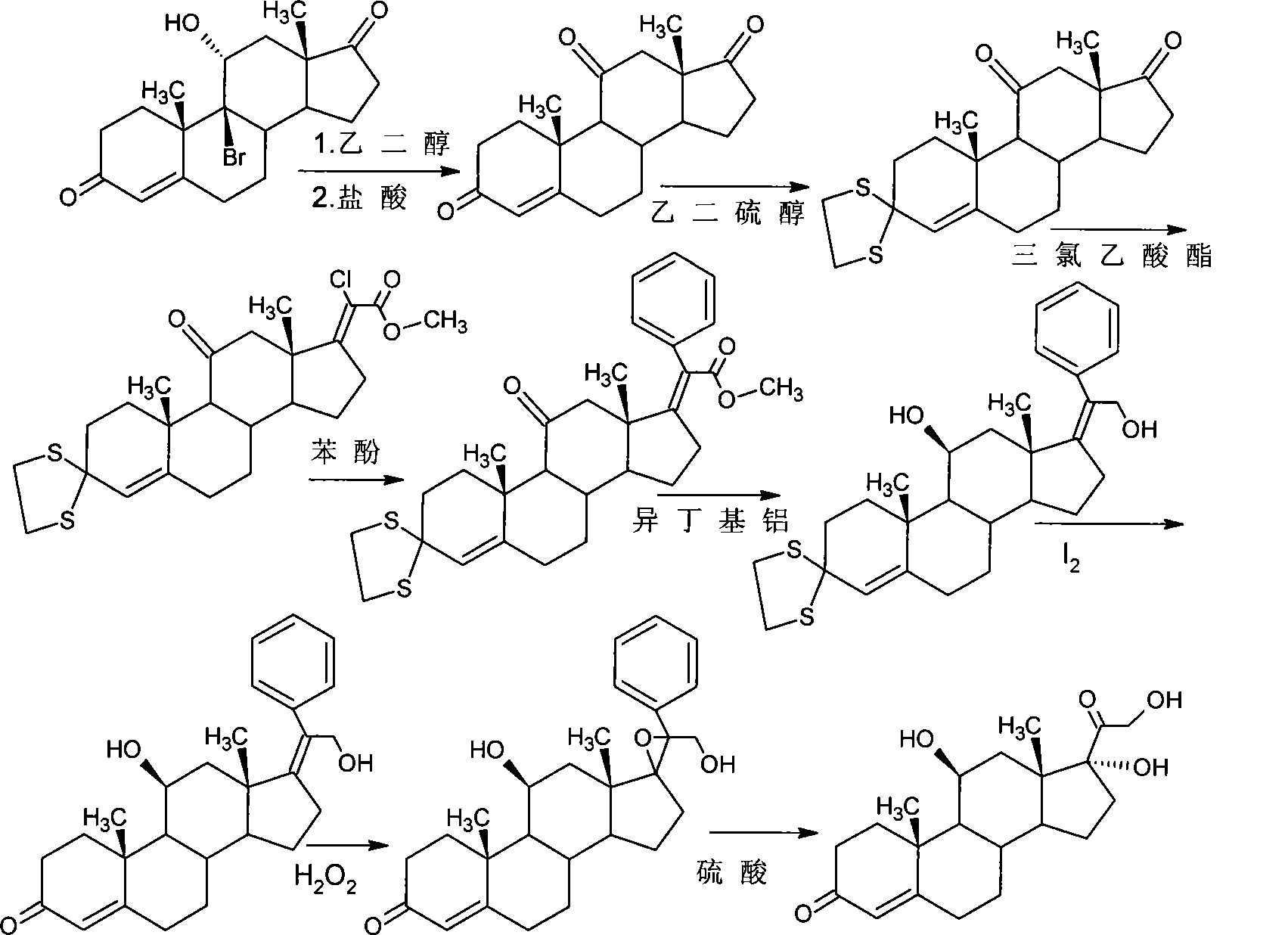
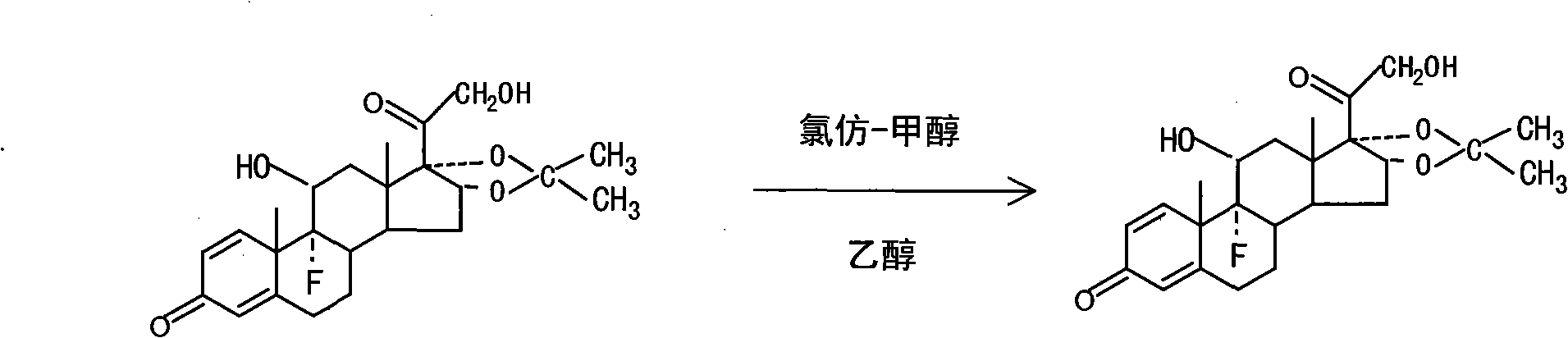
The invention relates to a preparation method of a steroid compound, in particular to the preparation for prednisolone and the derivative thereof, which takes 17-hydroxyl-1, 4, 9-triene- pregna-3, 20-diketone as the initiator and is improved by 9, 11th and 21st to obtain the prednisolone and the derivative thereof, such as prednisolone acetic ester, isoflupredone, and the like. The invention further provides the application of a compound (I) in the preparation of a compound (II). As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the prednisolone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the triamcinolone products and the prednisolone products, thus greatly reducing the production cost and industrial conditions. R1 is equal to H, F, Cl and Br; R2 is equal to H, OH and OCOR3, wherein, R3 is equal to the alkyl with less than 11carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
Preparation of hydrocortisone
InactiveCN101397323ASimple processRaw materials are easy to getSteroidsDiketoneHydrocortisone product
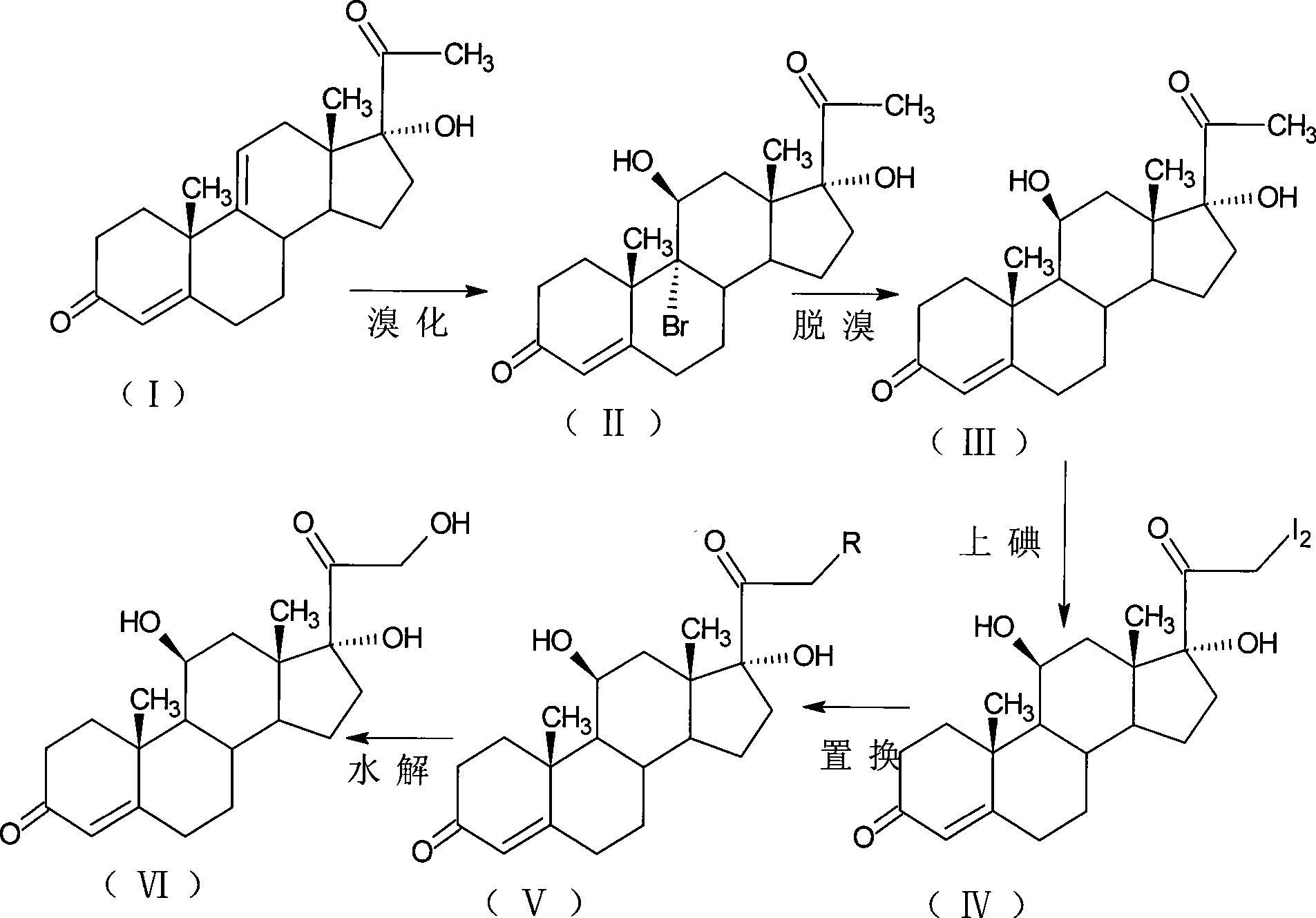
The invention relates to a preparation method of a steroid compound, in particular to the preparation of hydrocortisone, which takes 17-hydroxyl-4, 9-diene-pregna-3, 20-diketone as the initiator and is improved by 16, 17-grignard, 9, 11 th and 21st to obtain the hydrocortisone and a hydrocortisone-21st esterified ester thereof. As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, a line can be used for obtaining a plurality of products, and the yield and the cost are obviously superior to the historical synthetic method of the hydrocortisone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the triamcinolone products, the hydrocortisone products and the anecortave acetate products, thus greatly reducing the production cost and industrial conditions, wherein, R is equal to -OCOR1, R1 is equal to the alkyl with less than 11carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
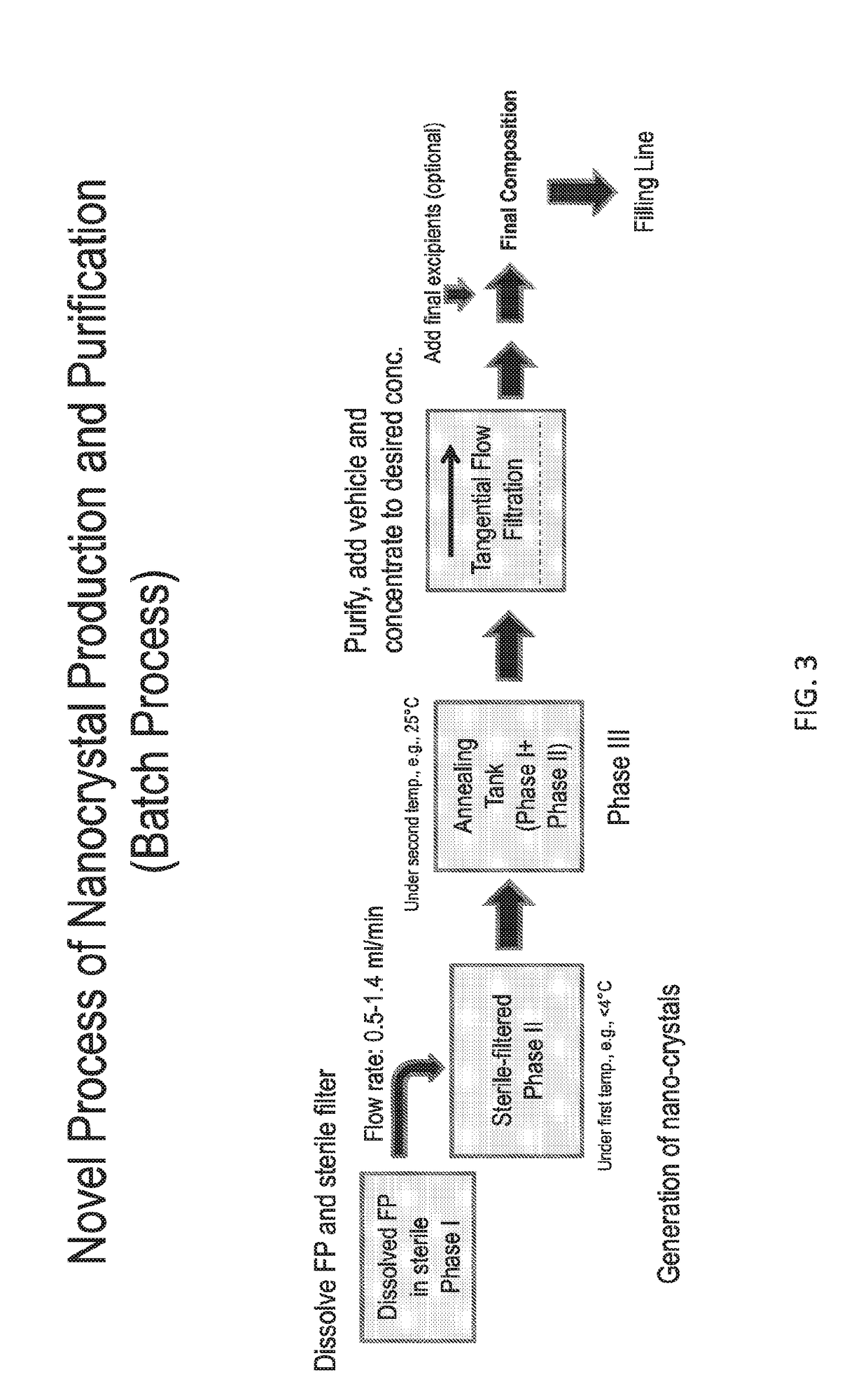
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20150337006A1Modifies release timeExtend posting timeOrganic active ingredientsSynthetic resin layered productsDiseaseRespiratory disease
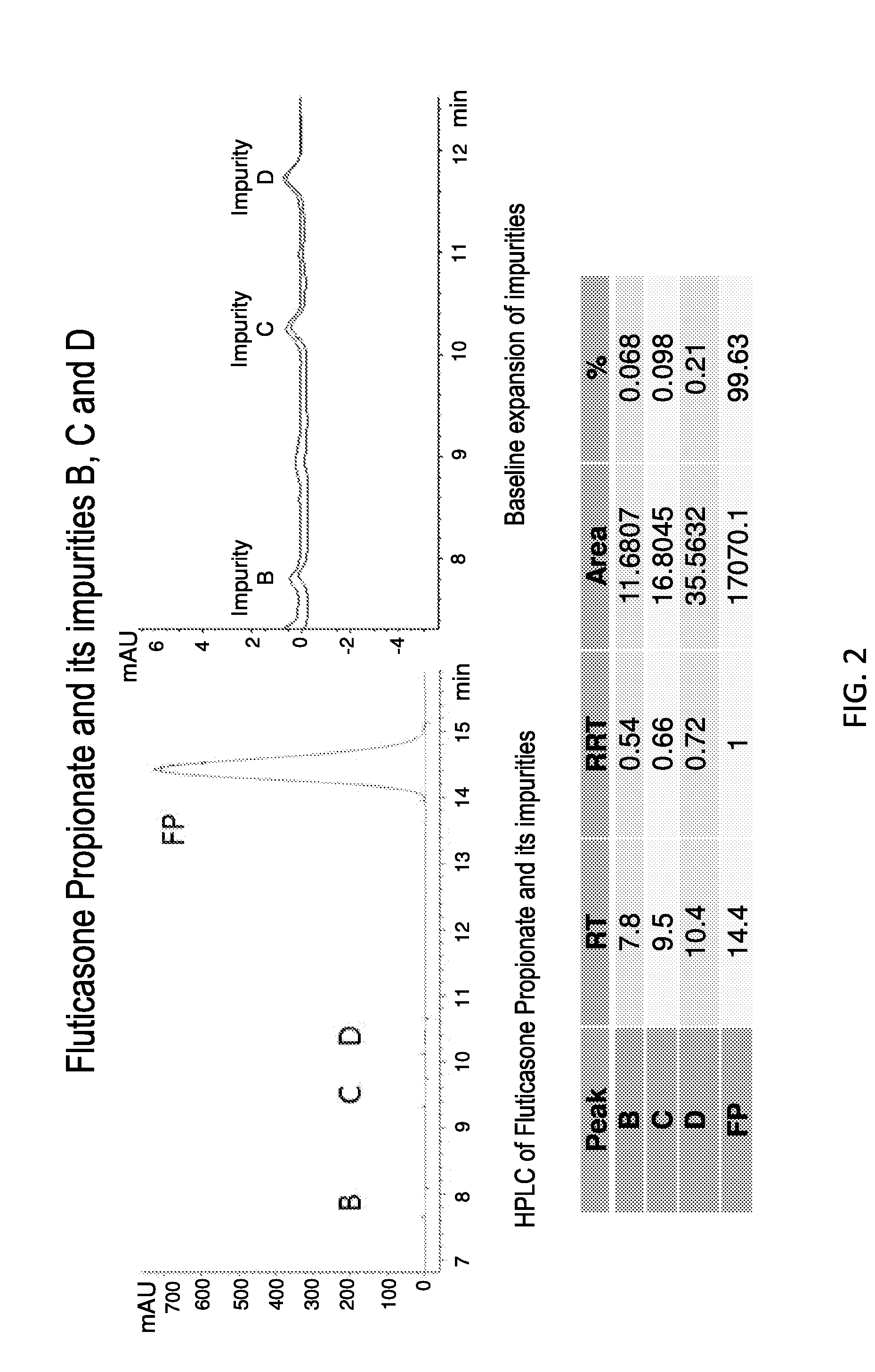
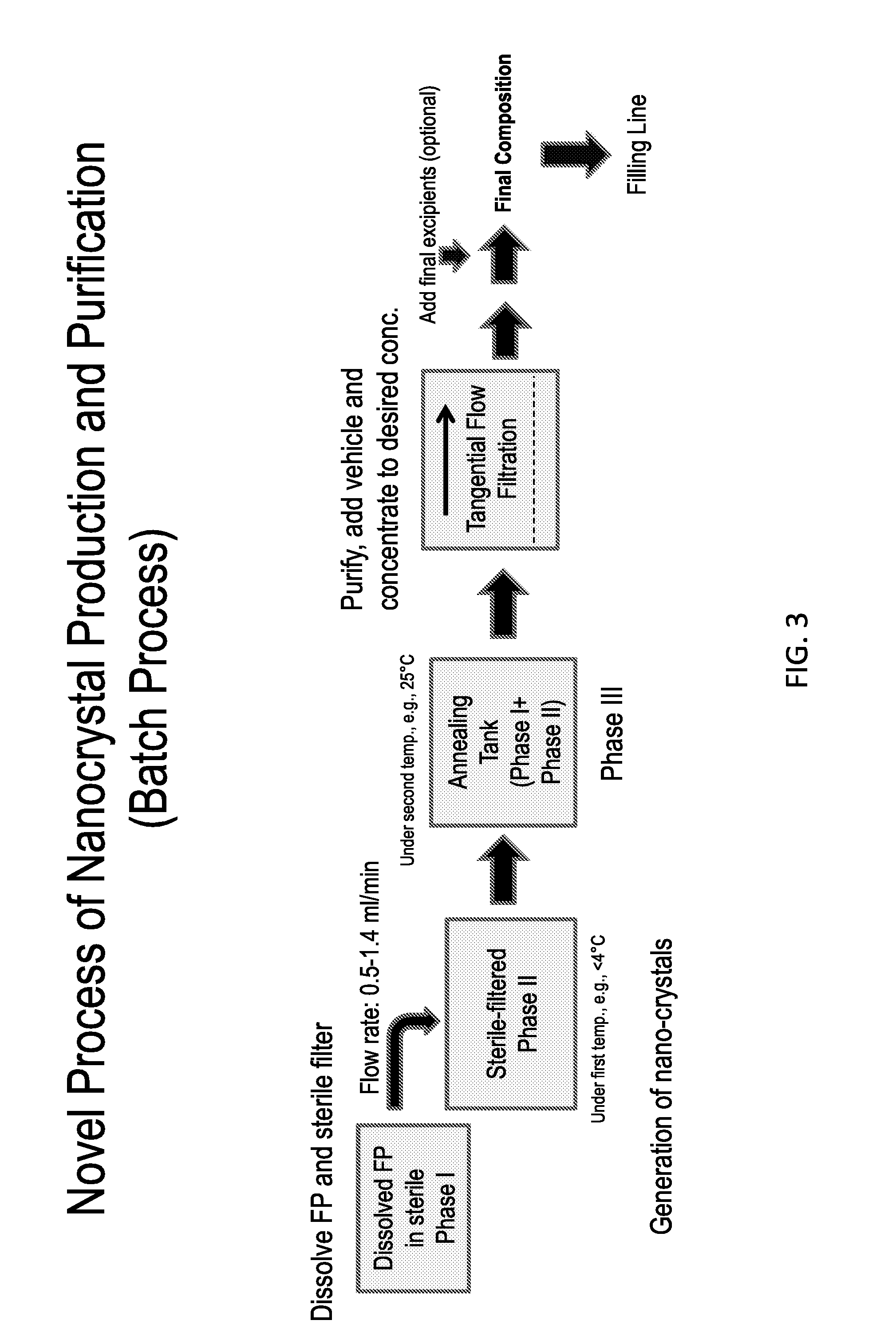
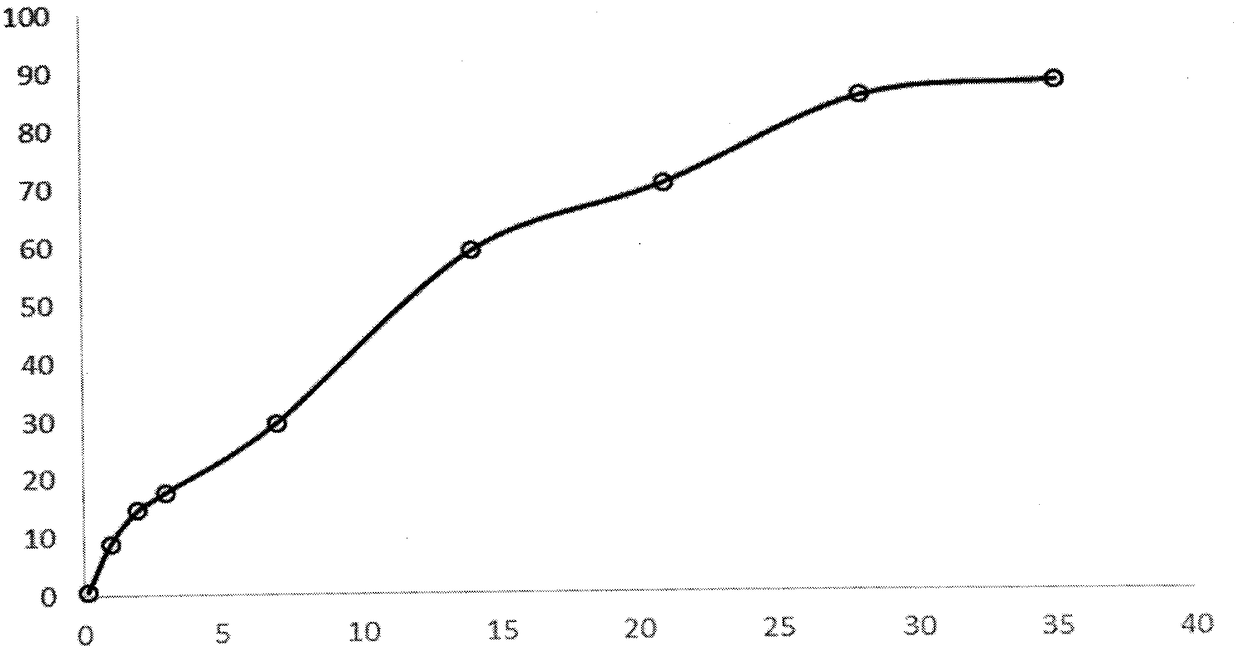
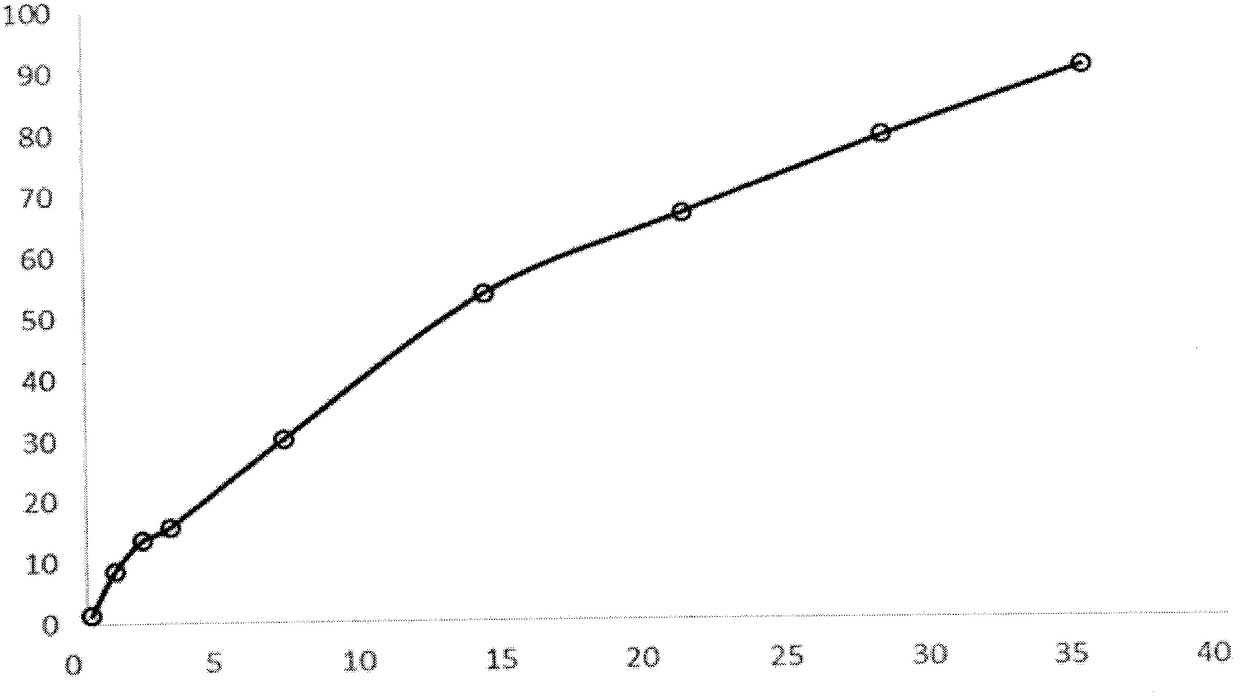
The present invention further provides method of preparing nanocrystals or microcrystals of a hydrophobic therapeutic agent such as fluticasone or triamcinolone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
Compositions and methods for treating noninfectious uveitis
InactiveUS20190269702A1Reduce inflammationReduces inflammatory scoreOrganic active ingredientsSenses disorderUveitisMacular edema
The present invention relates to methods, devices, and compositions for treating ocular disorders such as uveitis, macular edema associated with uveitis, and diabetic macular edema. For example, the methods include treatment of subjects having macular edema associated with non-infectious uveitis, or diabetic macular edema, by administering to the subjects a triamcinolone composition via non-surgical administration to the suprachoroidal space (SCS) of the eye.
Owner:CLEARSIDE BIOMEDICAL
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20150126483A1Maintaining osmolalityMaintain purityOrganic active ingredientsOrganic chemistry methodsDiseaseRespiratory disease
Owner:NICOX OPHTHALMICS
A kind of preparation method of triptorelin acetate sustained-release microspheres
ActiveCN105169366BSmall burst effectSlow release curvePowder deliveryPeptide/protein ingredientsDrugs solutionMicrosphere
The invention relates to a preparation method of triptorelin acetate sustained-release microspheres, comprising the following steps: step 1) adding water to triptorelin acetate to form a drug solution A; adding PLGA to an organic solvent to form a solution B; step 2) mixing Solution A and solution B are mixed and ultrasonicated to form colostrum, and the colostrum is added to a PVA aqueous solution saturated with an organic mixed solvent, and homogeneously emulsified to obtain double emulsion; Step 3) Stir the double emulsion at room temperature for 1 hour and then heat up to 40°C-45°C The temperature was kept at ℃ for 1 hour, and then the temperature was lowered to 10 ℃, and the particles were collected by sieving and freeze-dried. On the one hand, the technology involved in the present invention can overcome the problem of increased adverse drug reactions caused by the sudden release of triptorelin acetate microspheres; on the other hand, the blood drug concentration of the prepared microspheres is very smooth and stable, and is suitable for long-term administration treat.
Owner:LIVZON PHARM GRP INC
Refining process of triamcinolone acetonide raw medicine
The invention relates to a technology for refining a raw material drug of Triamcinolone. The method comprises the following steps that refining is carried out through recrystallization with a mixed organic solvent, wherein the raw Triamcinolone is added into the mixed solvent of chloroform and carbinol, heated to 50 to 70 DEG C, then a circumfluence under mixing is carried out, and the refined product undergoes the procedures until the content of Triamcinolone exceeds 96 percent, then the refined product undergoes heating circumfluence in ethanol for 2-3 hours, at a temperature of between 70 and 90 DEG C, is cooled down to a temperature below 0 DEG C, filtered by centrifuge, and then dried. The obtained final product is proved completely qualified according to China Pharmacopoeia. The invention can overcome the disadvantages of the prior refining art, and the final product produced by the method has the advantages of stable quality, high content, low impurities, and high yielding rate.
Owner:天津太平洋化学制药有限公司
External use medicine for treating dermatosis and its preparing method
InactiveCN1943581AEasy to useLow priceHydroxy compound active ingredientsAmide active ingredientsDiseaseMenthol
Owner:刘亚军
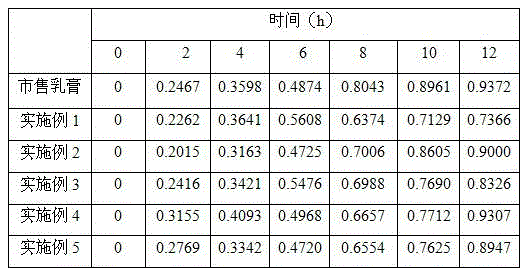
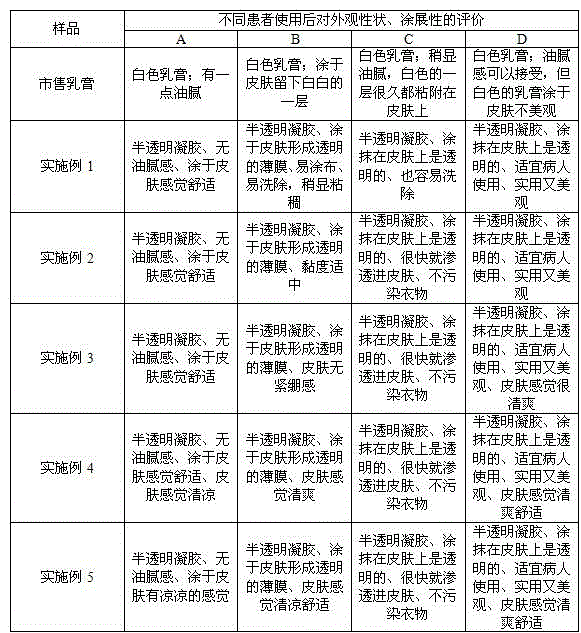
Triamcinolone acetonide and miconazole emulsion type gel
ActiveCN103142622AGood lookingNo greasy feelingAntibacterial agentsOrganic active ingredientsActive agentChemical compound
The invention discloses triamcinolone acetonide and miconazole emulsion type gel. The gel comprises econazole nitrate and triamcinolone with effective doses, as well as 1 to 9 weight percent of lipophilic phase and 90 to 98 weight percent of hydrophilic phase, wherein the lipophilic phase comprises a lipophilic compound and / or a lipophilic surfactant, and the hydrophilic phase comprises hydrophilic gel matrix, a hydrophilic compound and a hydrophilic surfactant. According to the triamcinolone acetonide and miconazole emulsion type gel prepared by the prescription disclosed by the invention, econazole nitrate and triamcinolone have good in stability and cannot be easily separated out, and the product has a good transparent appearance; due to the low content of lipophilic phase in the product, oily feeling does not exist after the gel is applied to skin; and the hydrophilic gel matrix is contained in the product, so that a transparent film can be formed after the gel is applied to skin, the texture is uniform and smooth, and the gel is easily applied, is not irritant and can be used for increasing the compliance of a patient.
Owner:TAIJI GRP SICHUAN TIANCHENG PHARMA
Triamcinolone acetonaide acetate nano controlled-release formulation, preparation method thereof and artificial lens containing same
InactiveCN102327212ATurbidity preventionImprove bioavailabilityOrganic active ingredientsAntipyreticPolyvinyl alcoholLate complication
The invention relates to a triamcinolone acetonaide acetate nano controlled-release formulation, a preparation method thereof and an artificial lens containing the same for preventing inflammation and late complication after cataract operation, wherein the triamcinolone acetonaide acetate nano controlled-release formulation is prepared from the triamcinolone acetonaide acetate-chloroform solution with the triamcinolone acetonaide acetate concentration of 1%-3%, the lactic acid-glycolic acid copolymer (PLGA)-dichloromethane solution with the lactic acid-glycolic acid copolymer (PLGA) concentration of 2%-5%, and the polyvinyl alcohol (PVA) with the concentration of 0.5%-1.5%. The invention provides the preparation method of the triamcinolone acetonaide acetate nano controlled-release formulation which can effectively prevent posterior capsular opacity, has simple preparation process, and is convenient to use and the artificial lens containing the triamcinolone acetonaide acetate nano controlled-release formulation.
Owner:严宏 +1
Sterically stabilized liposome and triamcinolone composition for treating the respiratory tract of a mammal
InactiveUS20060115523A1Extend effective lifeLiposomal deliveryEffective treatmentAerosol drug delivery
This invention relates to a composition containing a sterically stabilized liposome and triamcinolone, effective for the treatment of a mammal, with the composition being adapted for administration as an aerosol and with the composition providing effective treatment for a period of time at least 1.5 times as long as the effective time for treatment with triamcinolone alone. This invention also relates to a method for treating a mammal respiratory tract with the composition.
Owner:VGSK TECH
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
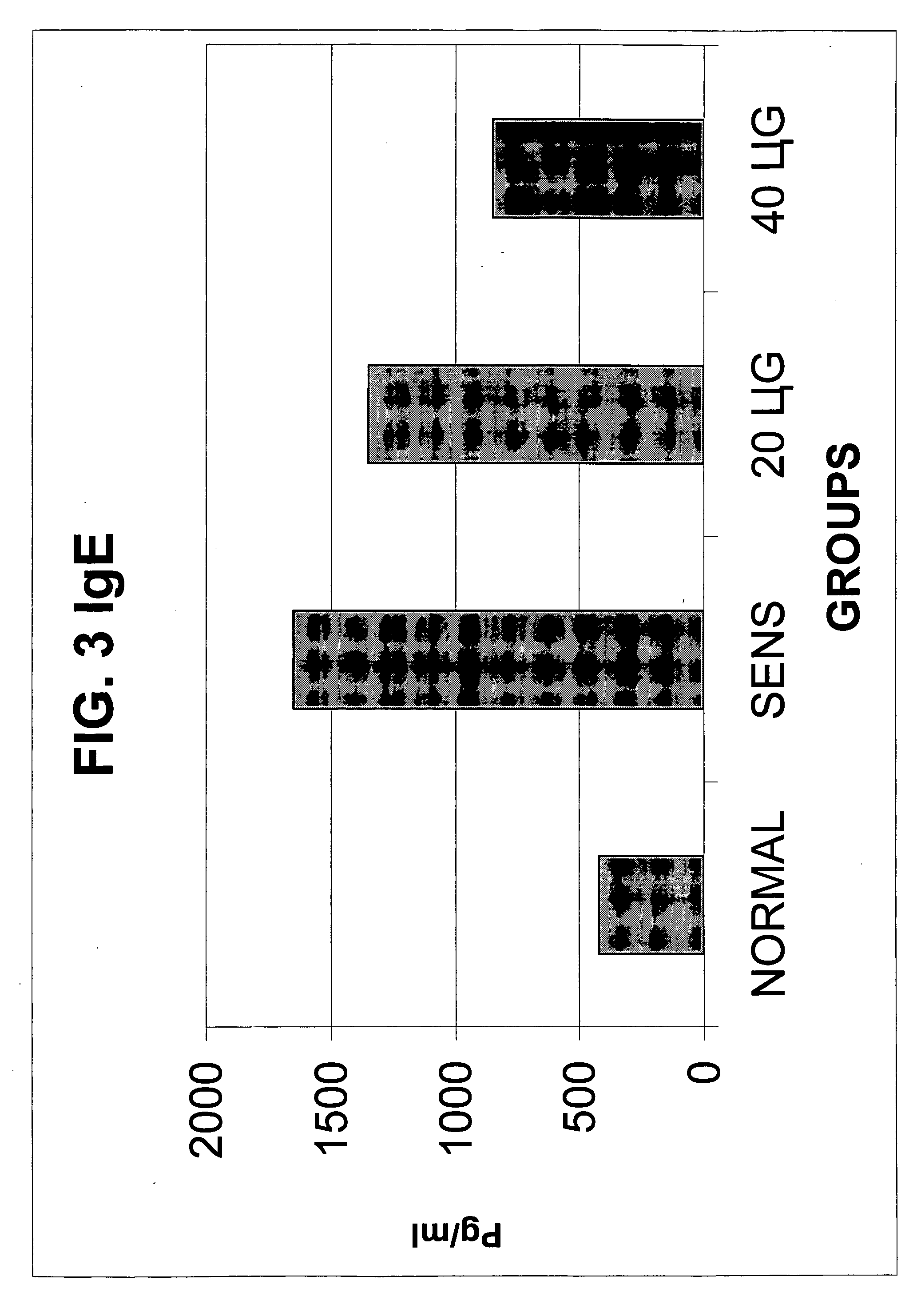
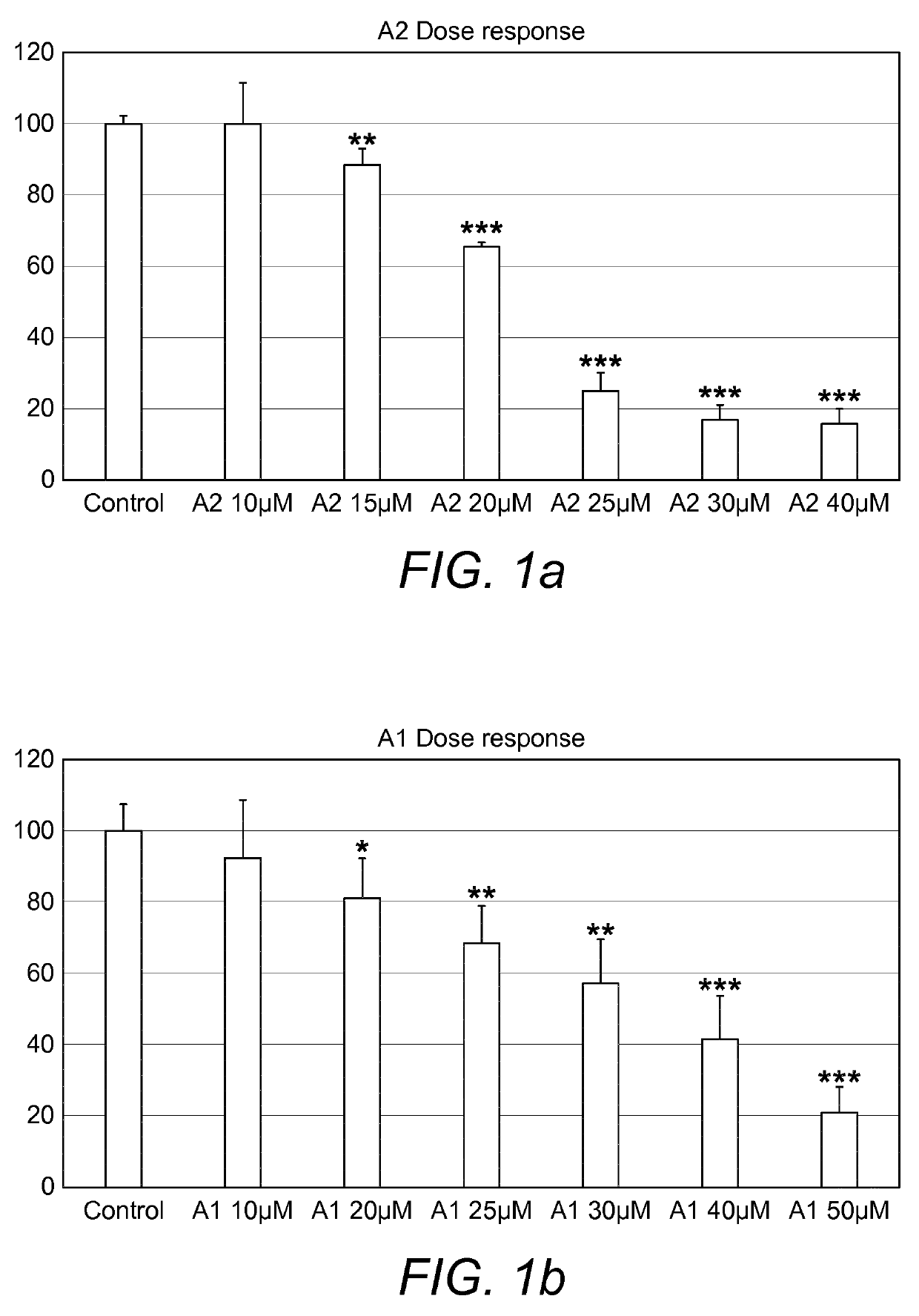
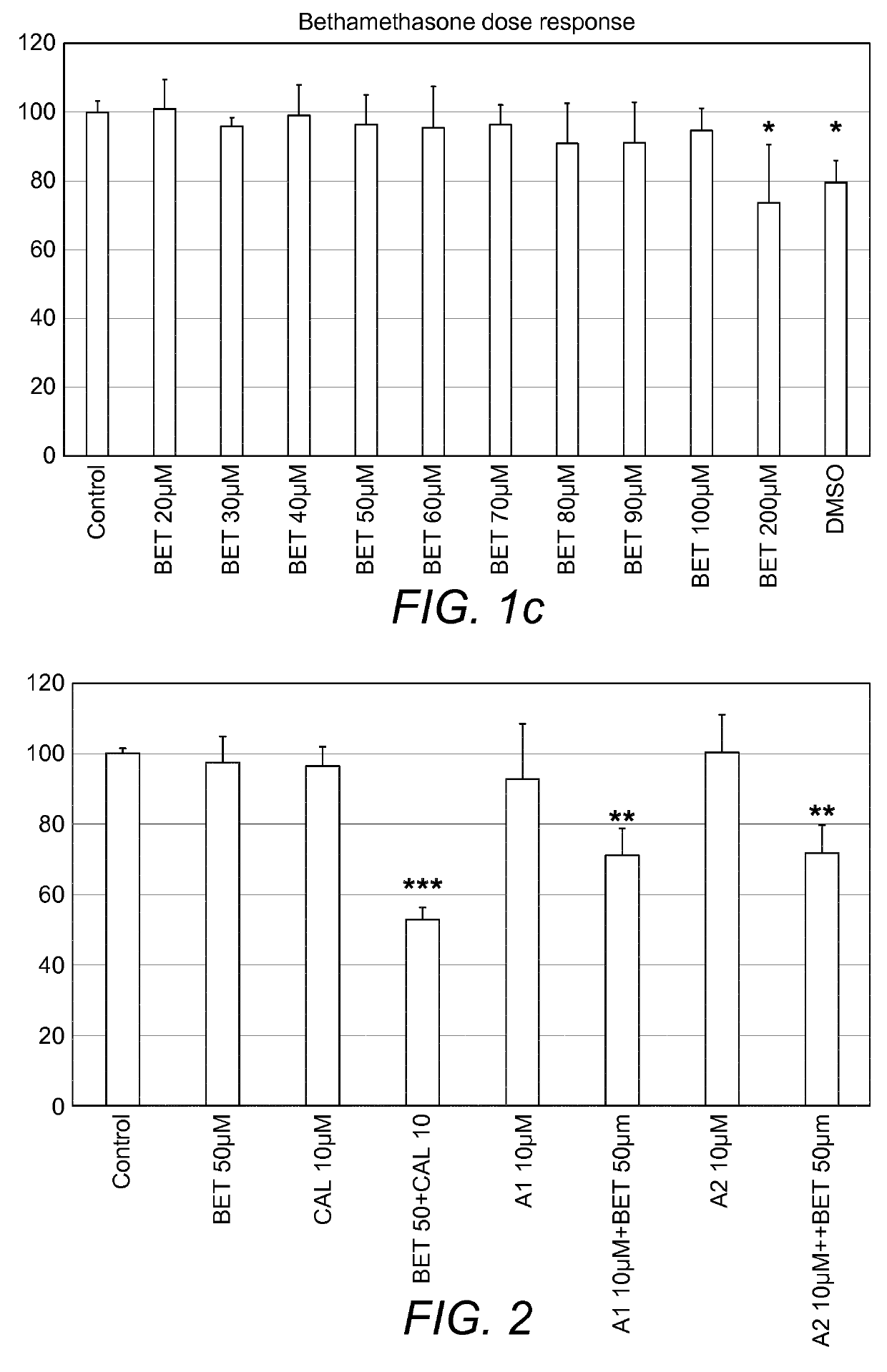
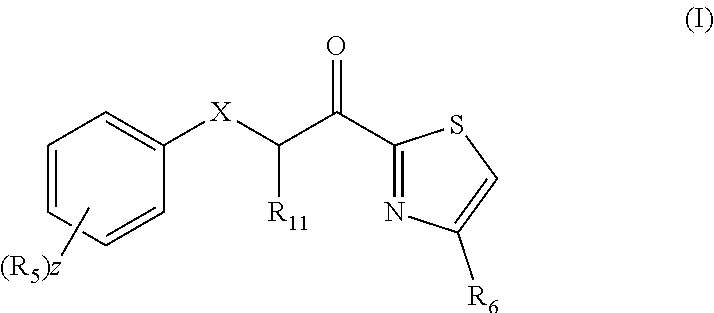
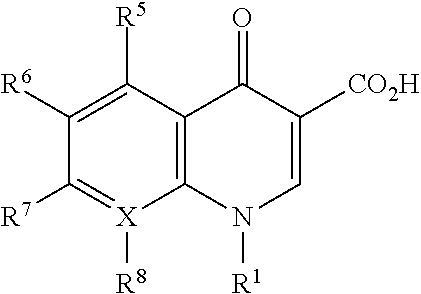
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
Triamcinolone biodegradable maltose microneedle array and preparation method thereof
The invention relates to a triamcinolone biodegradable maltose microneedle array and a preparation method thereof. Concretely, the invention provides a microneedle array and a preparation method thereof, wherein a microneedle comprises: (1) triamcinolone or its pharmaceutically acceptable salt, and (2) maltose or a hydrate thereof. The length of the microneedle is 800-1500mum. The triamcinolone biodegradable maltose microneedle array has advantages of long drug effect time, uniform absorption, easy absorption, painless feeling when dosing.
Owner:SHANGHAI SEVENTH PEOPLES HOSPITAL
Medicinal cosmetic lipoatrophy
ActiveUS20130116223A1Safe and effective regressionOrganic active ingredientsCosmetic preparationsLipoatrophyPhysiology
Methods relating to local injections of corticosteroids are provided. More specifically intralesional injections of corticosteroids and preferably Triamcinolone and its derivatives are suitable to produce medicaments to be injected in the subcutaneous fat at deep levels to provoke cosmetic lipoatrophy of small fat deposits on the face and body.
Owner:NOVA SCI LLC
Predictors for patients at risk for glaucoma from steroid therapy
InactiveUS20050192257A1Increased riskEvaluate benefitBiocideOrganic active ingredientsIntraocular pressureAnti angiogenic
A patient's risk for increased intraocular pressure after being treated with a steroid for an ocular disease, such as macular edema or age related macular degeneration, is assessed. The patient receives an intraocular challenge dose of triamcinolone, and the patient's intraocular pressure before and after the challenge dose is compared. If the intraocular pressure after the challenge is increased by at least 5 mm Hg, the patient is at risk for glaucoma if a therapeutic dose of a steroid is administered to treat the disease. This allows the physician to better manage the risk and / or provide an alternative therapy. The challenge composition may also contain an anti-angiogenic agent that will beneficially reduce the risk of new blood vessel growth in the eye.
Owner:MINU
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20180022775A1Maintaining osmolalityMaintain purityOrganic active ingredientsSenses disorderDiseaseRespiratory disease
The present invention further provides method of preparing nanocrystals of a hydrophobic therapeutic agent such as fluticasone or triamcinolone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
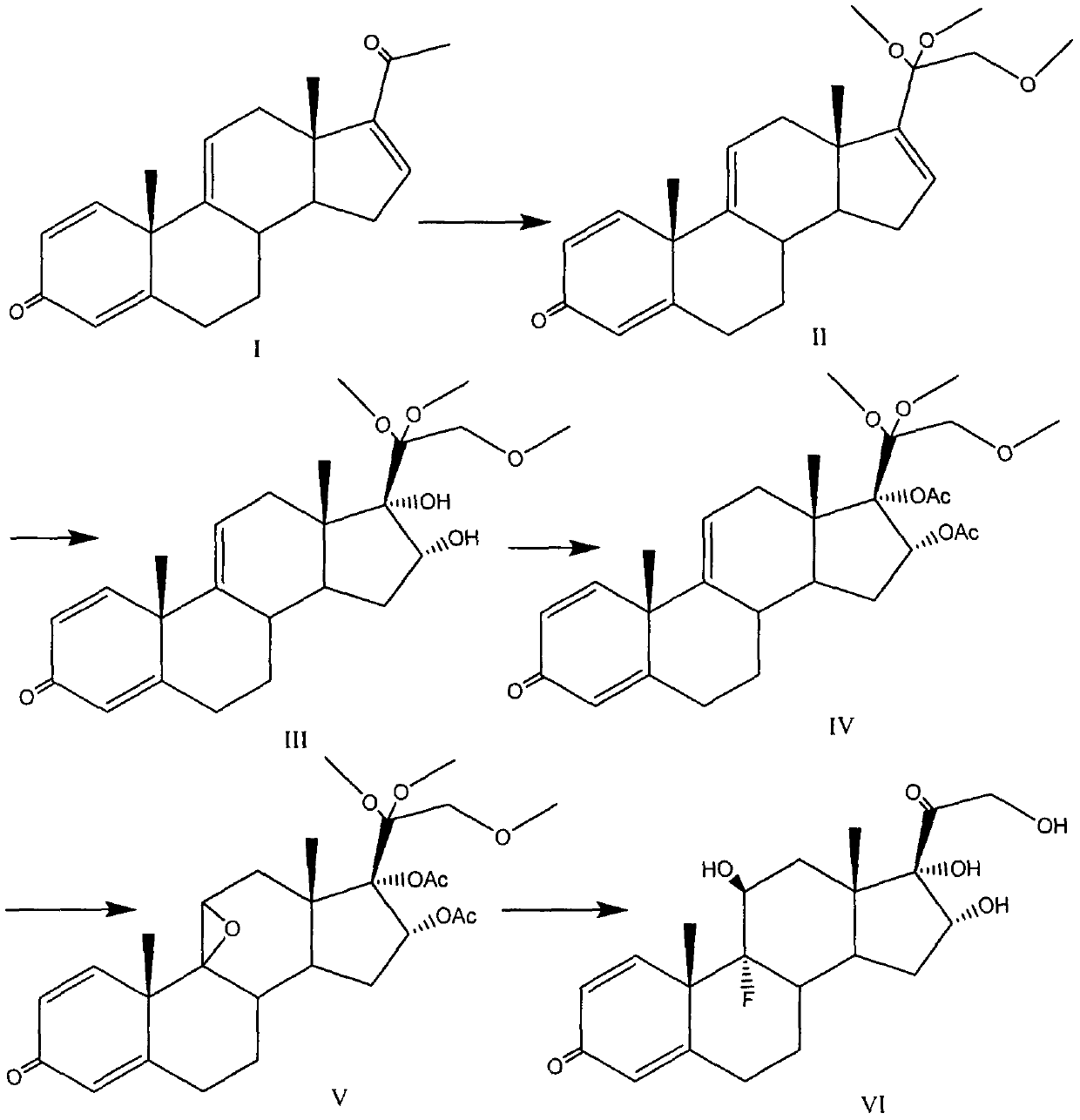
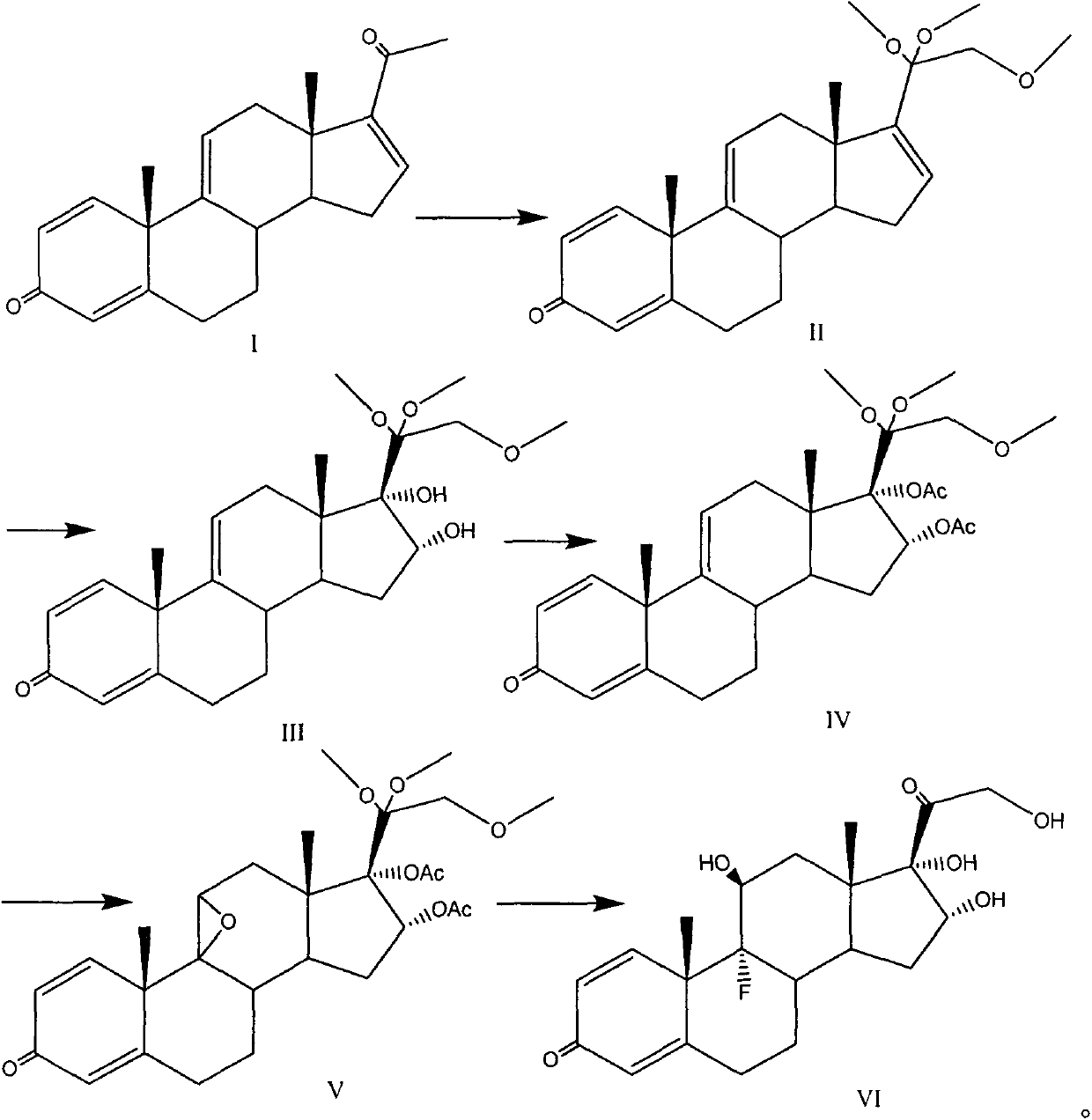
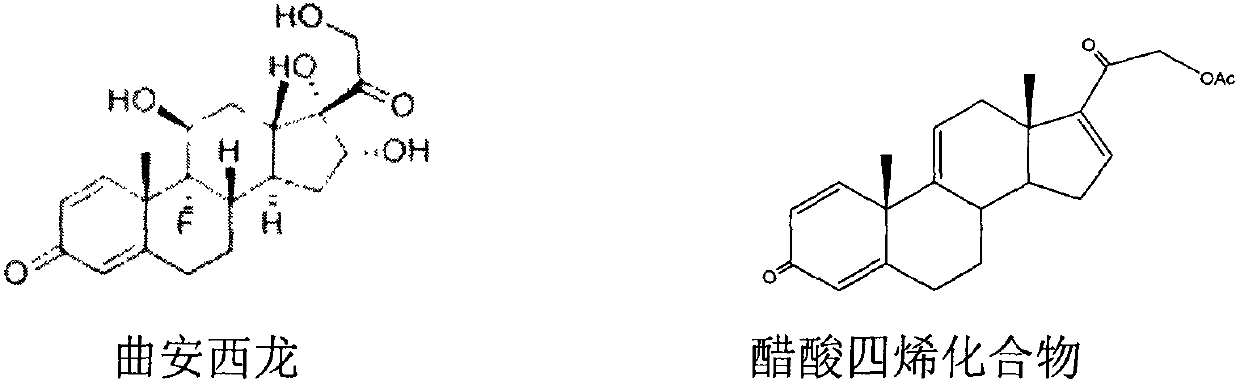
Preparation method of triamcinolone
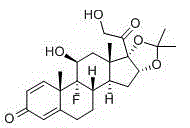
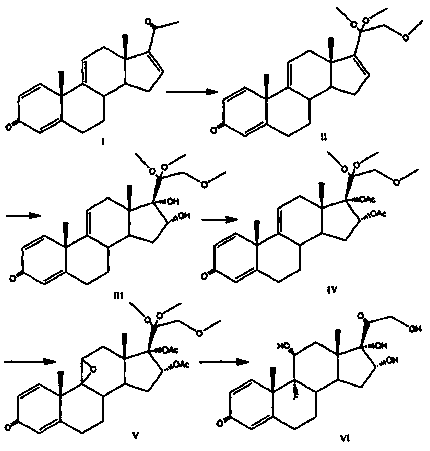
The invention provides a brand new synthesis route for preparation of triamcinolone; adopted raw materials are cheaper and easier to obtain, reactive raw materials are hydroxylated and then protectionis performed, five-membered ring double bonds are subjected to selective oxidation, generated di-hydroxyl is subjected to esterification protection, then six-membered ring double bonds are subjectedto epoxidation and are subjected to ring-opening fluorination to remove a protective group, and thus the triamcinolone product is obtained. The reaction process is easy to operate, the yield of each step is relatively high, and the purity of the obtained product is higher, the formation of by-products is effectively avoided, the production cost is reduced and industrialized production is facilitated.
Owner:TIANJIN PACIFIC PHARMA
Treatment of oral lichen planus with a combination of triamcinolone and retinoic acid
InactiveUS20140249118A1Effective treatmentClear controlBiocideHydroxy compound active ingredientsImmunologic disordersOral lichen planus
Oral lichen planus (OLP) is an immunologic disease which can be controlled and cleared by topical application of a composition of triamcinolone acetonide 0.1% and retinoic acid 0.05% in Orabase® on the affected areas of the mucosa, thrice daily for three weeks. The combined therapy is more effective than triamcinolone acetonide alone in Orabase®. By this method, clearance can be maintained by less frequent application or lower concentrations of the composition.
Owner:NAJAFI SHAMSOULMOLOUK +2
Anti-cancer sustained release agent loaded with glucocorticoid and chemical curing medicine
InactiveCN101450036AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsPolyesterAdditive ingredient
A anti-cancer slow-release agent co-loaded with glucocorticosteroid and chemotherapy medicament is a slow-release injection agent composed of slow-release microsphere and solvent, wherein, the slow-release microsphere comprises anti-cancer effective ingredient and slow-release auxiliary materials, the solvent is the special solvent containing suspending agent. The glucocorticosteroid is selected from prednisolone, methylprednisolone, dexamethasone, betamethasone, omcilon or triamcinoloneAcetonide, the chemotherapy medicament is selected from phosphoinositide 3-kinase restrainer and pyrimidine analogue or the like; the slow-release auxiliary materials are polylactic acid and copolymer thereof, polyethyleneglycol, polylactide-COOH copolymer, 2-aliphatic acid, sebacic acid polyester, poly(erucic acid dimmer-sebacic acid), poly(fumaric acid-sebacic acid), polyphenyl and polylactic acid or the like biocompatibility high molecules; the suspending agent viscosity is 100cp-3000cp (20 DEG C-30DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The anti-cancer effective compositin and the slow-release microsphere can be made into a slow-release implantation agent, by intra-tumor injection or tumor circumference injection or arrangement, the tumor growth can be effectively inhibited, the edema can be alleviated, and the curative effects of the chemotherapy and the radiation therapy can be reinforced.
Owner:JINAN KANGQUAN PHARMA TECH +1
Medicine composition for injecting triamcinolone acetonide palmitate lipid microsphere and preparation method thereof
The present invention relates to the medical technical field, in particular to a lipid microsphere injection medical combination containing triamcinolone palmitate and a preparation method thereof.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Medicinal cosmetic lipoatrophy
ActiveUS20140213562A1Safe and effective regressionBiocideOrganic active ingredientsLipoatrophyPhysiology
Methods relating to local injections of corticosteroids are provided. More specifically intralesional injections of corticosteroids and preferably Triamcinolone and its derivatives are suitable to produce medicaments to be injected in the subcutaneous fat at deep levels to provoke cosmetic lipoatrophy of small fat deposits on the face and body.
Owner:NOVA SCI LLC
Triamcinolone acetonaide acetate nano eye drops and preparation method thereof
The invention relates to triamcinolone acetonaide acetate nano eye drops and a preparation method thereof, wherein the triamcinolone acetonaide acetate nano eye drops are prepared in the way that the triamcinolone acetonaide acetate and the chitosan nano carrier are prepared into nanoparticles according to the proportion of 1 / 2 and then are mixed with the distilled water. The invention provides the triamcinolone acetonaide acetate nano eye drops which have simple preparation process, can prevent pain and complication which are caused by local injection, facilitate clinical use and are easy to be accepted by patients and the preparation method of the triamcinolone acetonaide acetate nano eye drops.
Owner:严宏 +1
Ivermectin slow-release injecta, and preparing process and use method thereof
InactiveCN1197583CSmall toxicityImprove securityOrganic active ingredientsPharmaceutical delivery mechanismAluminum monostearateEthyl acetate
Owner:陈坚
External use medicine for treating dermatosis and its preparing method
InactiveCN100435801CEasy to useLow priceHydroxy compound active ingredientsAmide active ingredientsDiseaseMenthol
The invention is to disclose a medicine of external use for treating skin disease And its preparation method, said medicine includes materials according to part by weight as follows: egocort 6-9 ; camphor 80-120;menthol 80-120;triamcinolone acetonaide acetate 8-12;carbamide 800-1200;aspirin 440-4400.
Owner:刘亚军
Triamcinolone preparation method
The invention discloses a triamcinolone preparation method, and belongs to the technical field of pharmacy. The triamcinolone preparation method comprises: selecting a tetraenyl acetate material as araw material, carrying out oxidation, esterification at 16 site, epoxidation and fluorination, and hydrolyzing after the fluorination refining to obtain triamcinolone. According to the present invention, the yield and the cost of the method of the present invention are obviously superior to the synthesis method sequentially comprising oxidation and direct epoxidation, wherein the impurity is removed after the fluorination process, such that the problem that the impurity is difficultly removed in the final refining can be avoided, and the impurity can be controlled at the intermediate stage soas to reduce the difficulty in the separation and purification.
Owner:SHANDONG TAIHUA BIO & TECH
Compound triamcinolone acetate-urea cream and preparation method thereof
The invention discloses compound triamcinolone acetate urea-cream and a preparation method thereof, relating to the technical field of medicine. The compound triamcinolone acetate-urea cream consists of triamcinolone acetate, urea and an emulsion substrate. In the compound triamcinolone acetate-urea cream, triamcinolone acetate serves as a medium-efficiency glucocorticoid which can be used for eliminating fever, flushing and swelling caused by local non-infectious inflammation; and urea has the effects of dissolving and denaturalizing keratoprotein and enhancing the cuticle layer hydration, so that skin is softened, and cracking is prevented. The compound triamcinolone acetate urea-cream has the effects of suppressing movement of inflammatory cells to inflammatory positions, preventing inflammatory media from being released, stability a lysosome membrane, suppressing immune reactions and improving the stress ability of a mechanism to noxious stimulus.
Owner:JILIN AODONG GROUP DALIAN PHARMACEUTICAL CO LTD
Trimcinolone and moxifloxacin methods
ActiveUS20220062168A1Similar and improved stabilityAntibacterial agentsOrganic active ingredientsFlocculationActive agent
Provided here are new ophthalmological methods for the treatment or prophylaxis of ocular bacterial infections and related conditions comprising delivering an effective amount of an ophthalmologically suitable composition comprising an effective amount of a moxifloxacin compound and an effective amount of a triamcinolone compound. In aspects, methods comprise delivery of the composition by injection. In aspects, the delivered compositions are stable suspensions, which maintain physical stability and chemical stability for extended periods of time (e.g., exhibiting no sustained flocculation, coagulation, or clumping after several months of storage under typical storage conditions). In aspects, compositions are suspensions that include a suspension component comprising one or more suspension agents. In aspects, the suspension component includes an ionic suspension and may also or alternatively comprises a non-ionic surfactant, non-ionic suspension agent, or both, or, an agent that provides both functions. In aspects, such compositions further comprise an effective amount of a chelating agent.
Owner:SOMERSET THERAPEUTICS LLC
A kind of triamcinolone acetonide econazole cream and preparation method thereof
ActiveCN103860566BImprove stabilityFast transdermal absorptionOrganic active ingredientsAntimycoticsPEG 400Oil phase
The invention discloses triamcinolone acetonide and econazole cream and a preparation method thereof. The cream is prepared by uniformly mixing a water phase and an oil phase, wherein the water phase is a hydroxy propyl cellulose water solution and the oil phase is prepared by virtue of the following steps: dissolving triamcinolone acetonide and econazole in isopropyl myristate, then adding dodecylicpolyethylene glycol glyceride laurate and polyethylene glycol 400 and uniformly stirring. Compared with the prior art, the medicament disclosed by the invention exists in the cream in a nanometer form, and is high in stability, rapid in transdermal absorption, simple in preparation process, free of use of complicated emulsion preparation equipment and suitable for the requirements of large-scale production.
Owner:江苏晨牌邦德药业有限公司
Medicinal cosmetic lipoatrophy
ActiveUS20160310510A1Safe and effective regressionReduce areaOrganic active ingredientsCosmetic preparationsLipoatrophyPhysiology
Methods relating to local or “intralesional” injections of corticosteroids are provided. More specifically injections of corticosteroids and preferably Triamcinolone and its derivatives are suitable to produce medicaments to be injected in the subcutaneous fat at deep levels to provoke to provoke cosmetic lipoatrophy of small fat deposits on the face and body.
Owner:NOVA SCI LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com