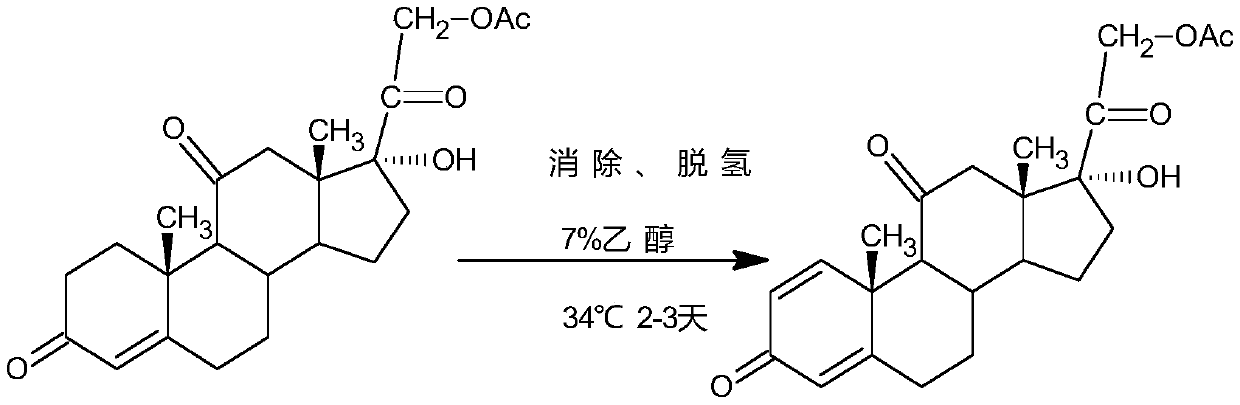
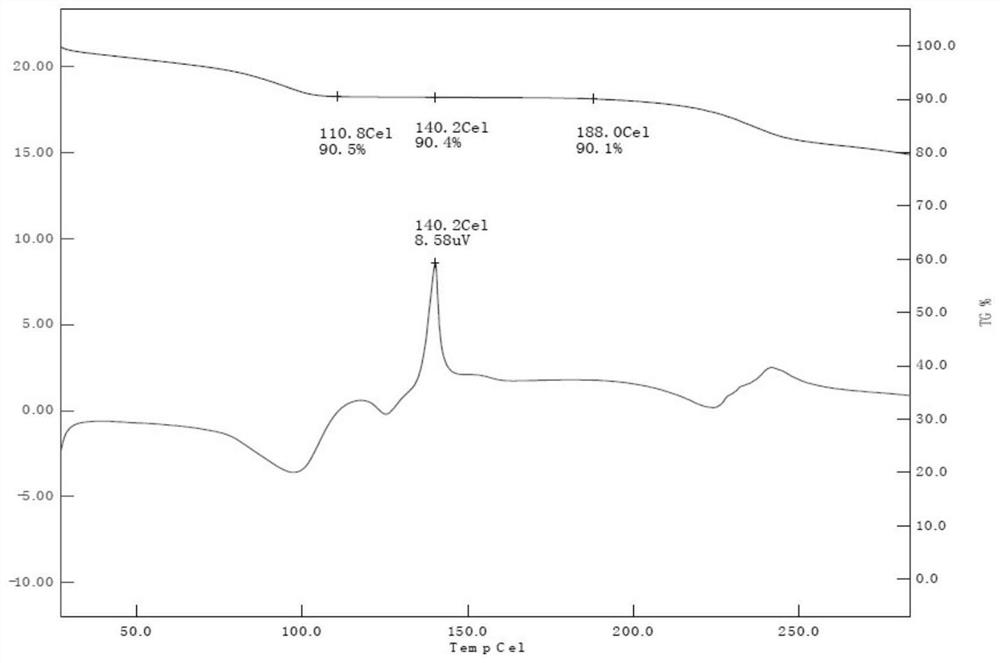
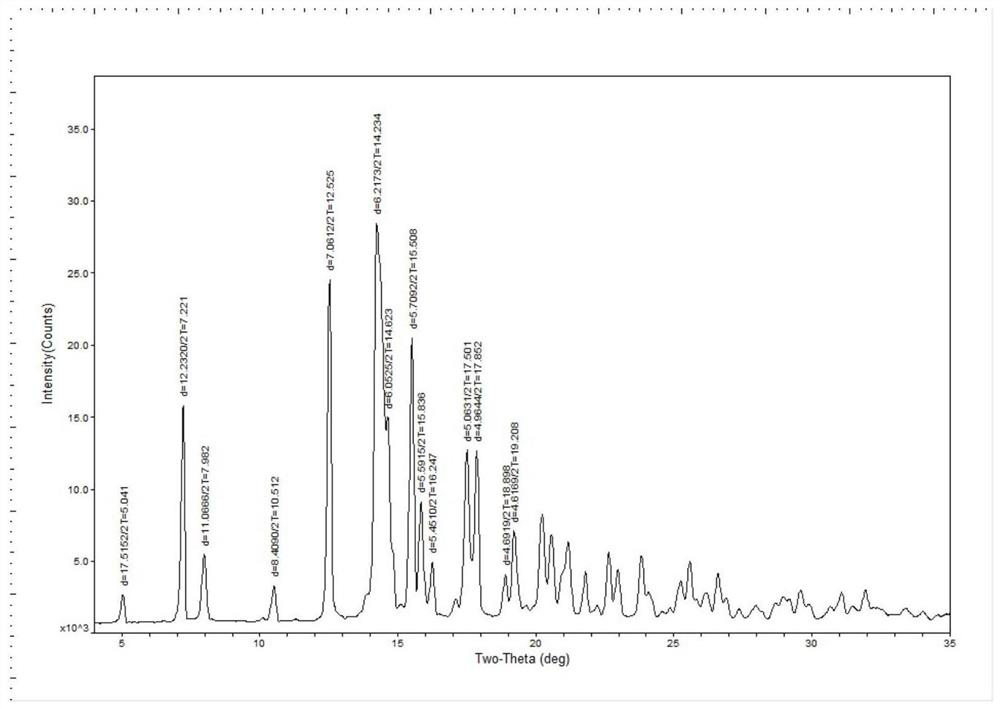
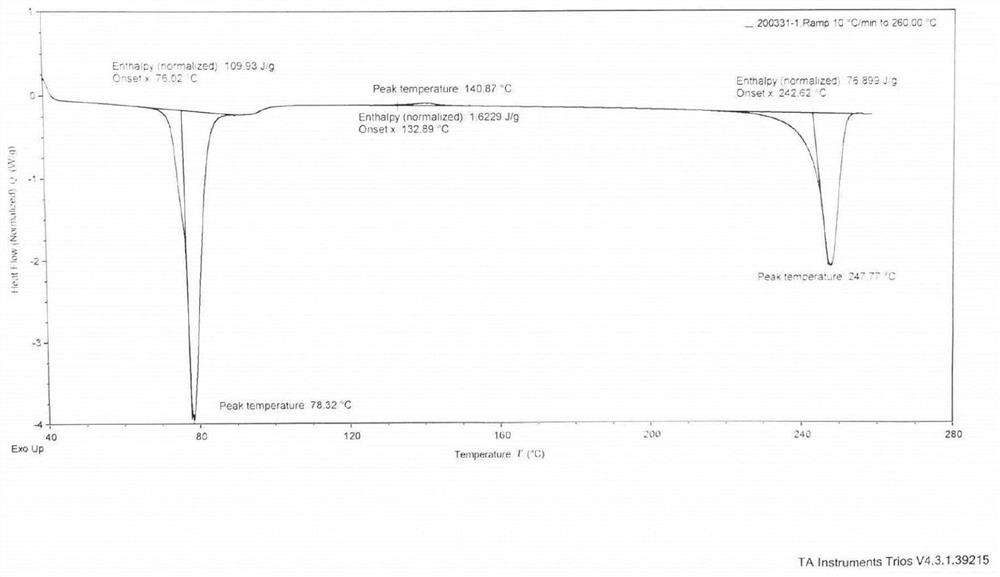
Patents
Literature
69 results about "Prednisonum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
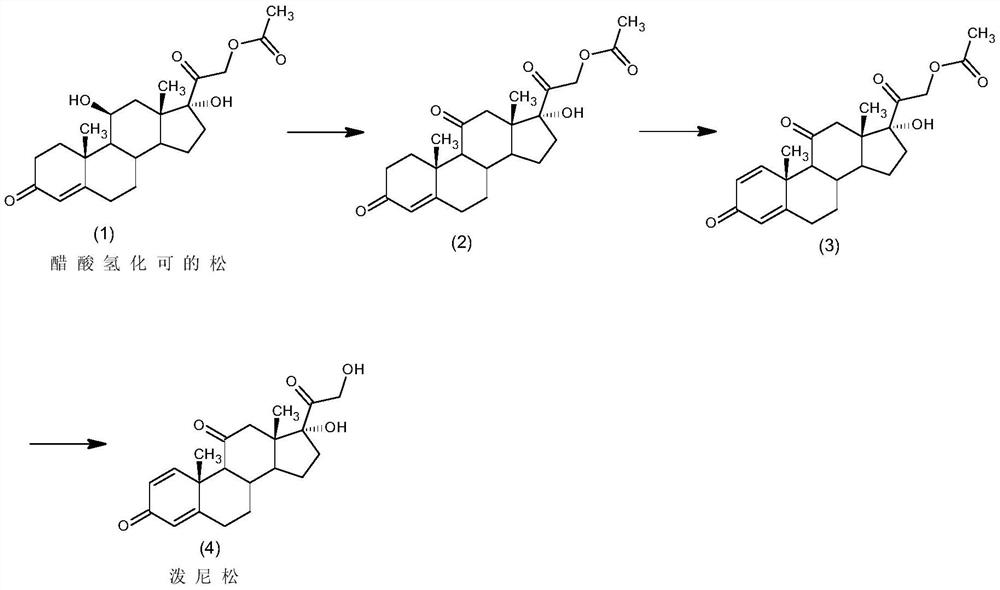
Preparation of metacortandralone and derivatives thereof
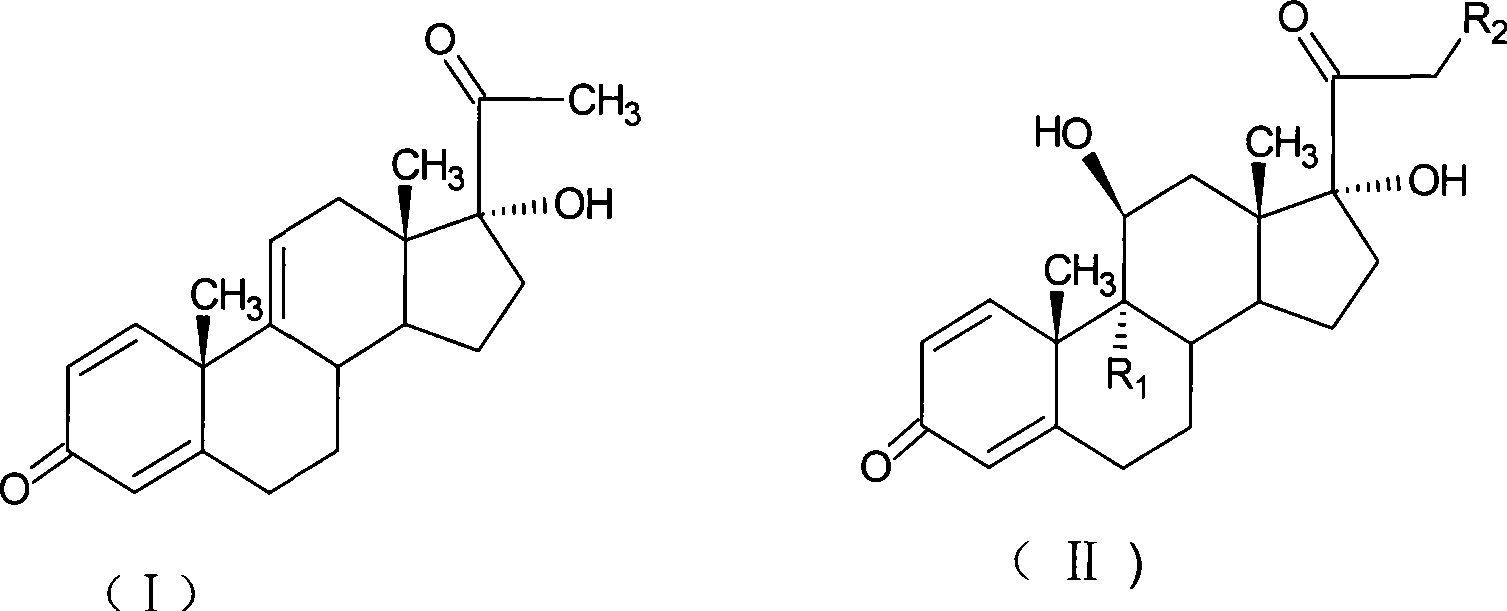
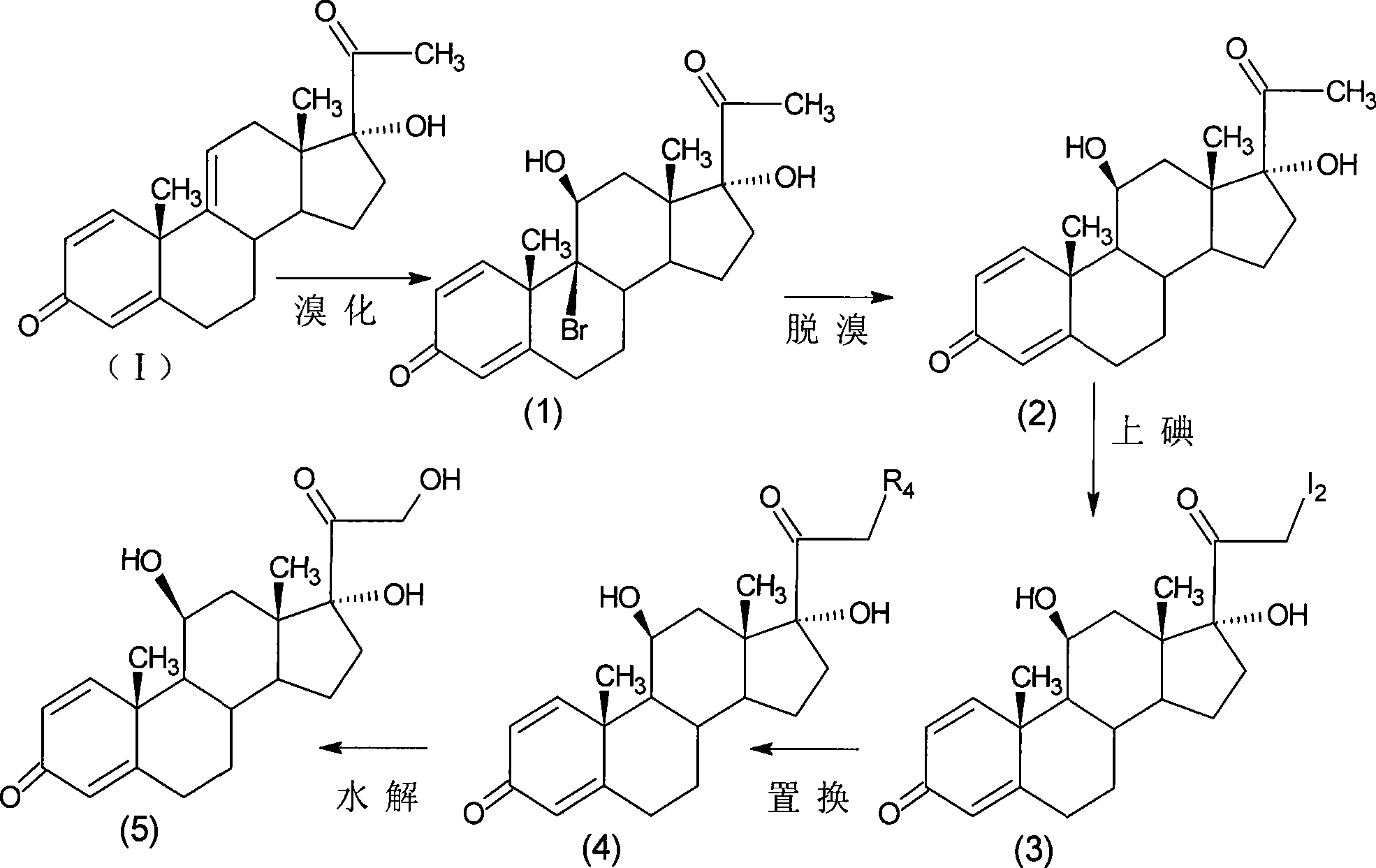
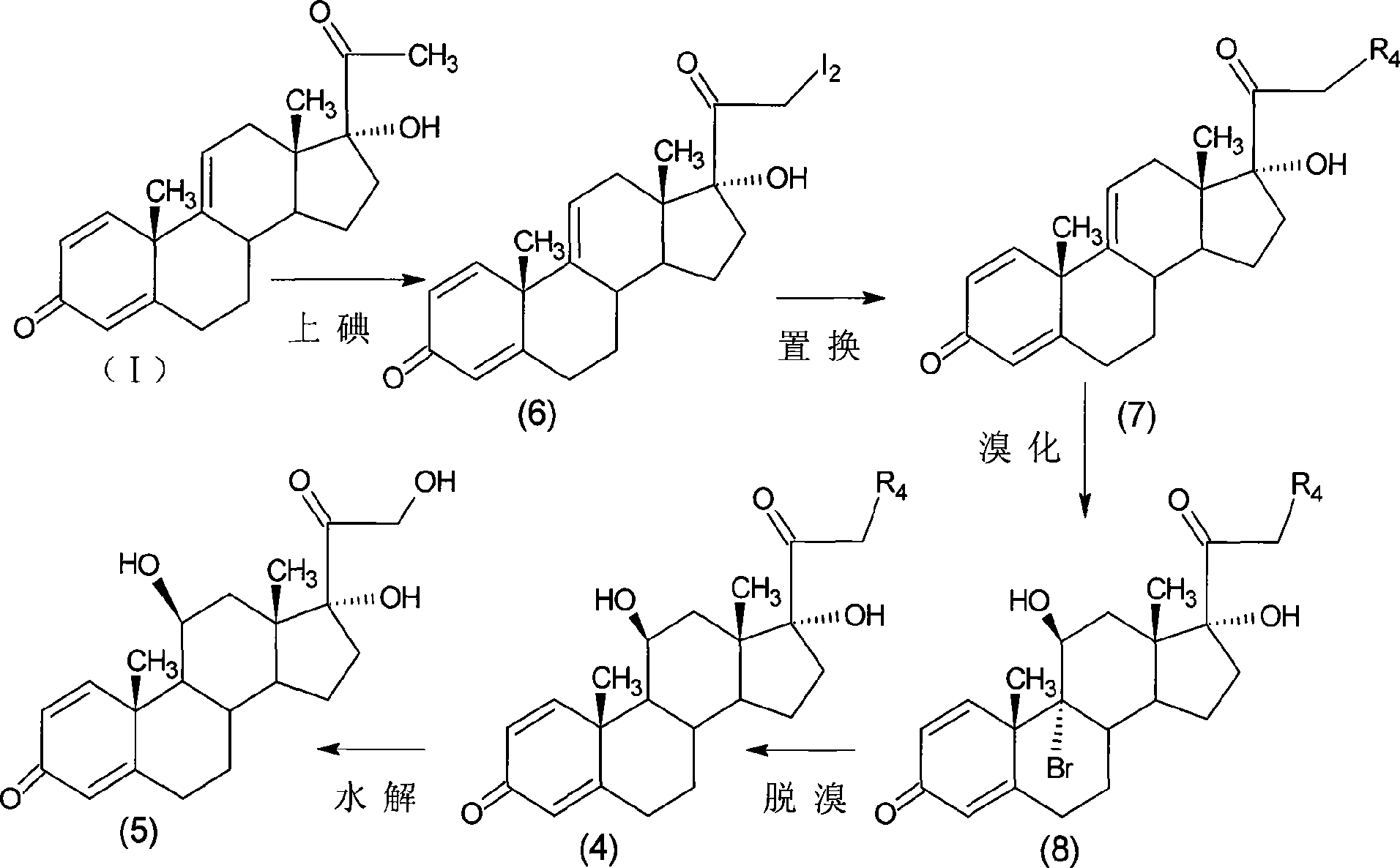
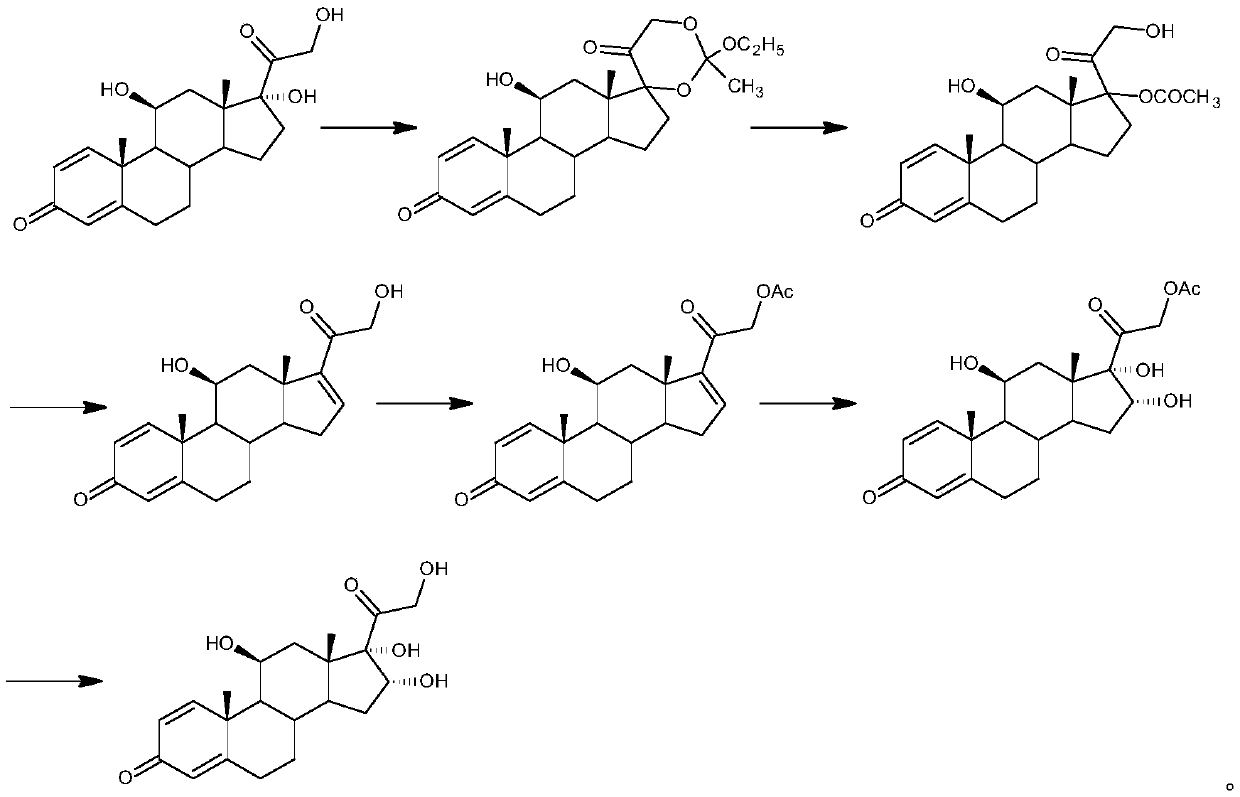
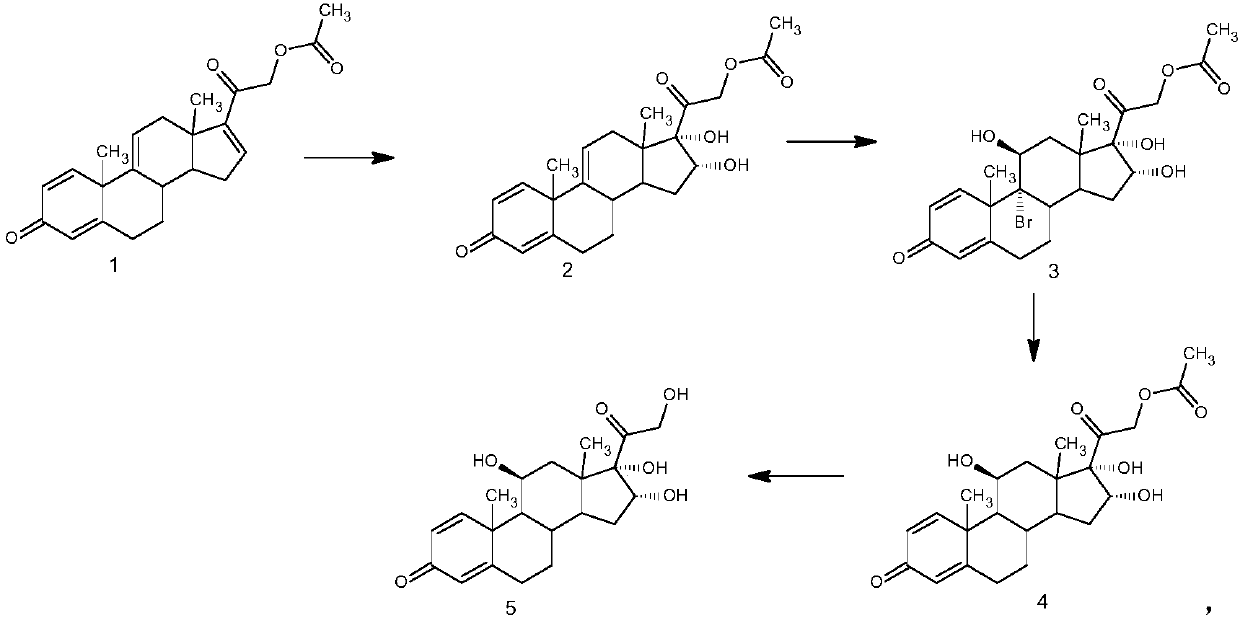
The invention relates to a preparation method of a steroid compound, in particular to the preparation for prednisolone and the derivative thereof, which takes 17-hydroxyl-1, 4, 9-triene- pregna-3, 20-diketone as the initiator and is improved by 9, 11th and 21st to obtain the prednisolone and the derivative thereof, such as prednisolone acetic ester, isoflupredone, and the like. The invention further provides the application of a compound (I) in the preparation of a compound (II). As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the prednisolone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the triamcinolone products and the prednisolone products, thus greatly reducing the production cost and industrial conditions. R1 is equal to H, F, Cl and Br; R2 is equal to H, OH and OCOR3, wherein, R3 is equal to the alkyl with less than 11carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
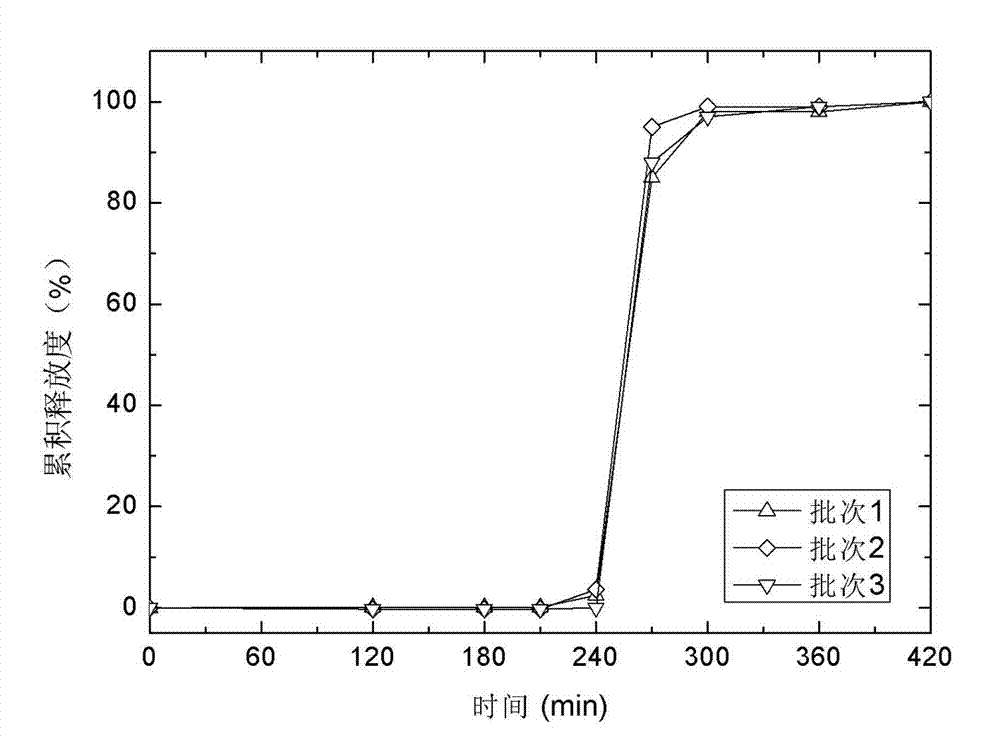
Oral prednisone time-selecting release preparation and preparation method thereof
InactiveCN103690545AGive full play to the therapeutic effectImprove balanceOrganic active ingredientsAntipyreticCelluloseFormulary
The invention discloses an oral prednisone time-selecting release preparation and a preparation method thereof. The oral prednisone time-selecting release preparation provided by the invention mainly consists of 0.3-5 parts of prednisone and derivatives thereof, 10-50 parts of glyceryl behenate and 3-30 parts of hydroxypropyl cellulose, and can further contain a disintegrating agent and other pharmaceutically acceptable excipients. The preparation method is as below: extruding tablet cores or granules containing the drug according to the formula by a tablet press or a dry granulator; and coating the tablet cores or particles containing the drug by a coating pan or a fluidized bed to attach the coating film to the tablet cores or particles containing the drug, so as to obtain the oral prednisone time-selecting release preparation. The oral prednisone time-selecting release preparation provided by the invention can achieve a good balance between the biological rhythm of the patients and the curative effects, and is safer, more convenient and effective compared with a traditional preparation. The oral prednisone time-selecting release preparation is prepared by an extrusion-coating process, which is simple for operation, and the obtained time-selecting release preparation has the advantages of drug stability and high reproducibility.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
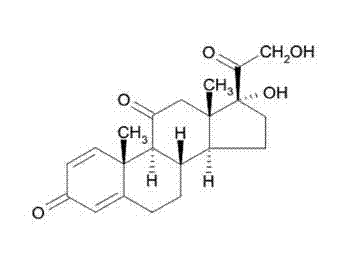
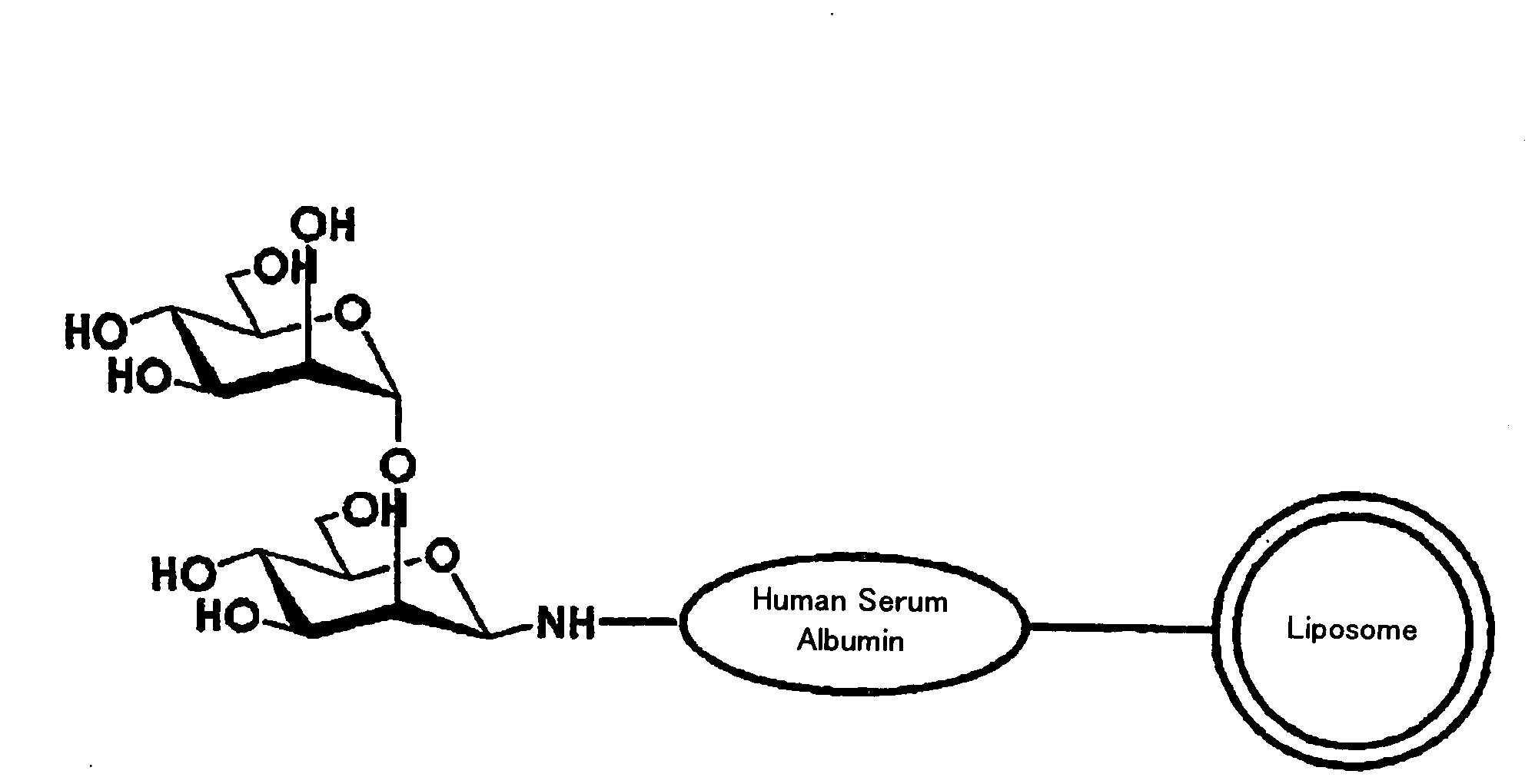
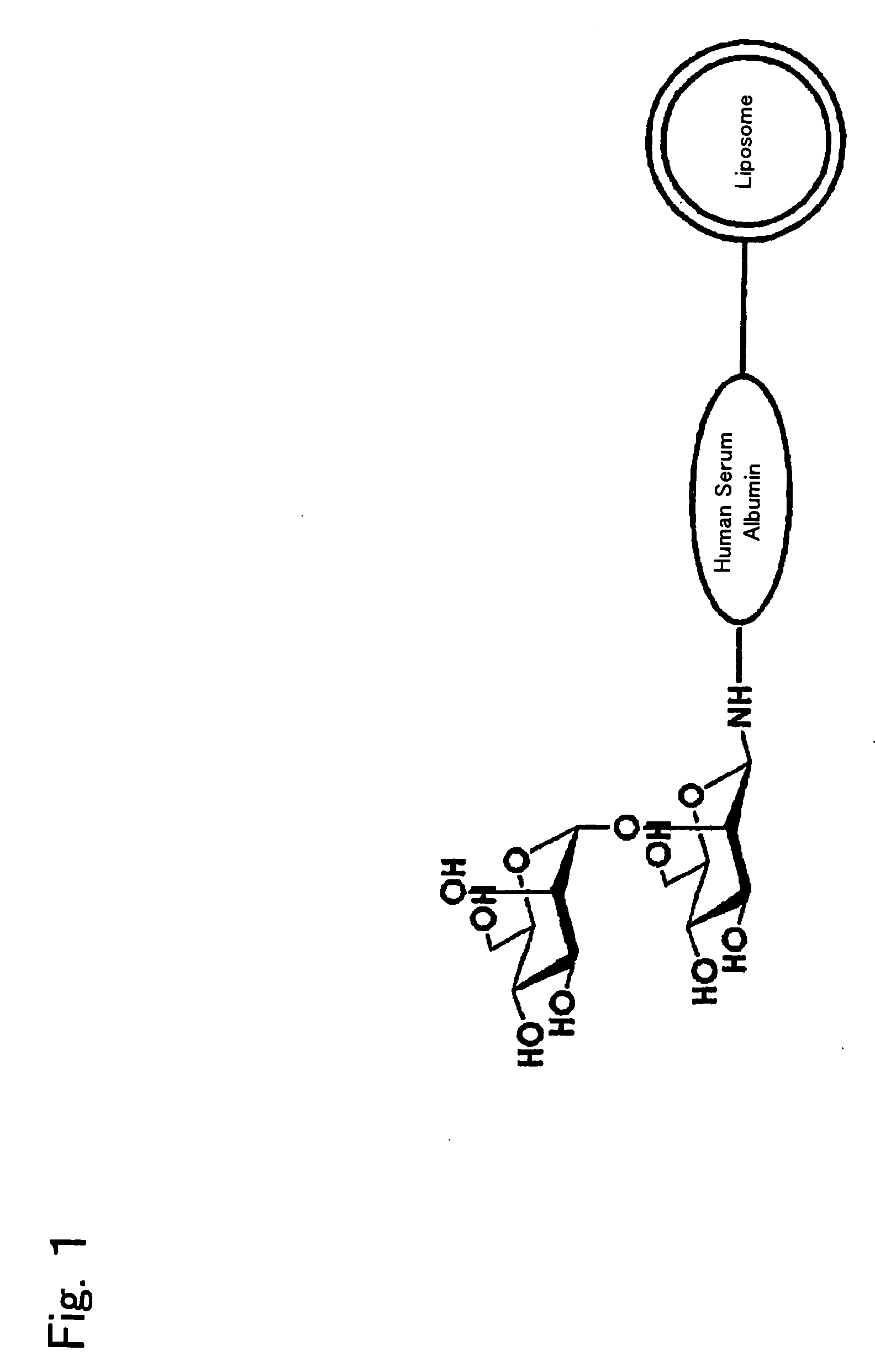
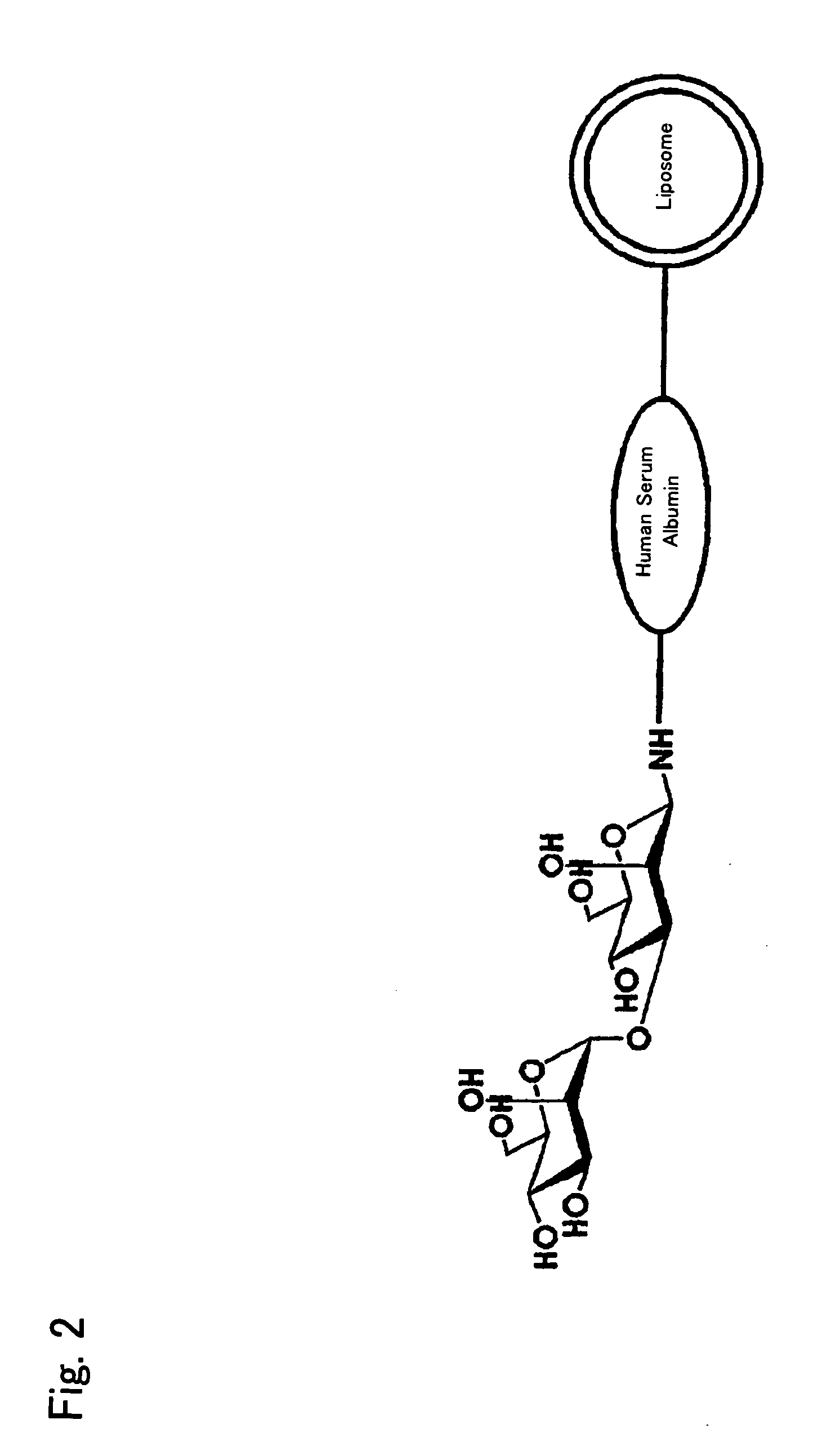
Liposome Preparation
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
Methods of Treating Multiple Myeloma
PendingUS20220062415A1Conducive to survivalReduce riskInorganic non-active ingredientsBoron compound active ingredientsAntiendomysial antibodiesPharmaceutical drug
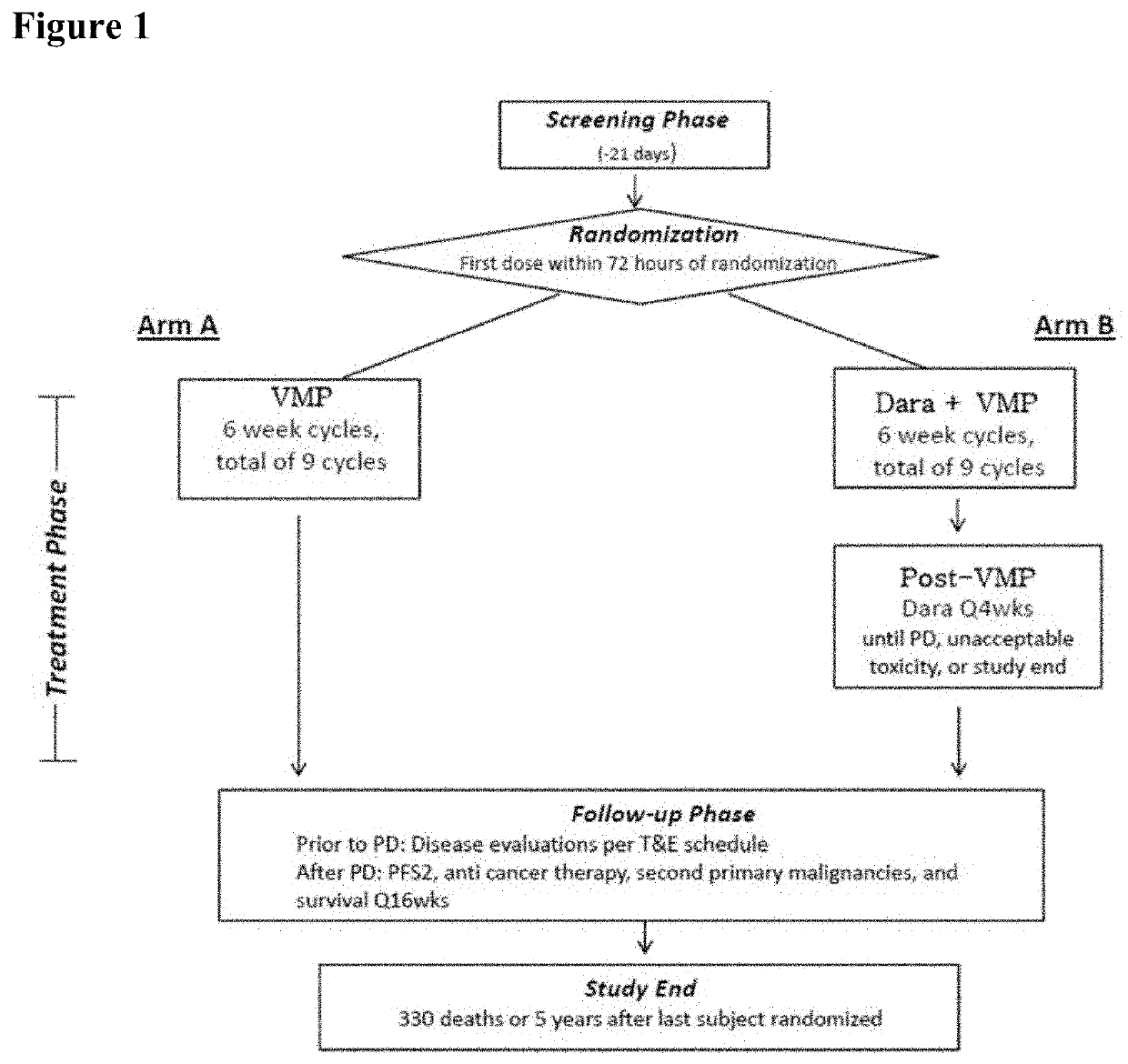
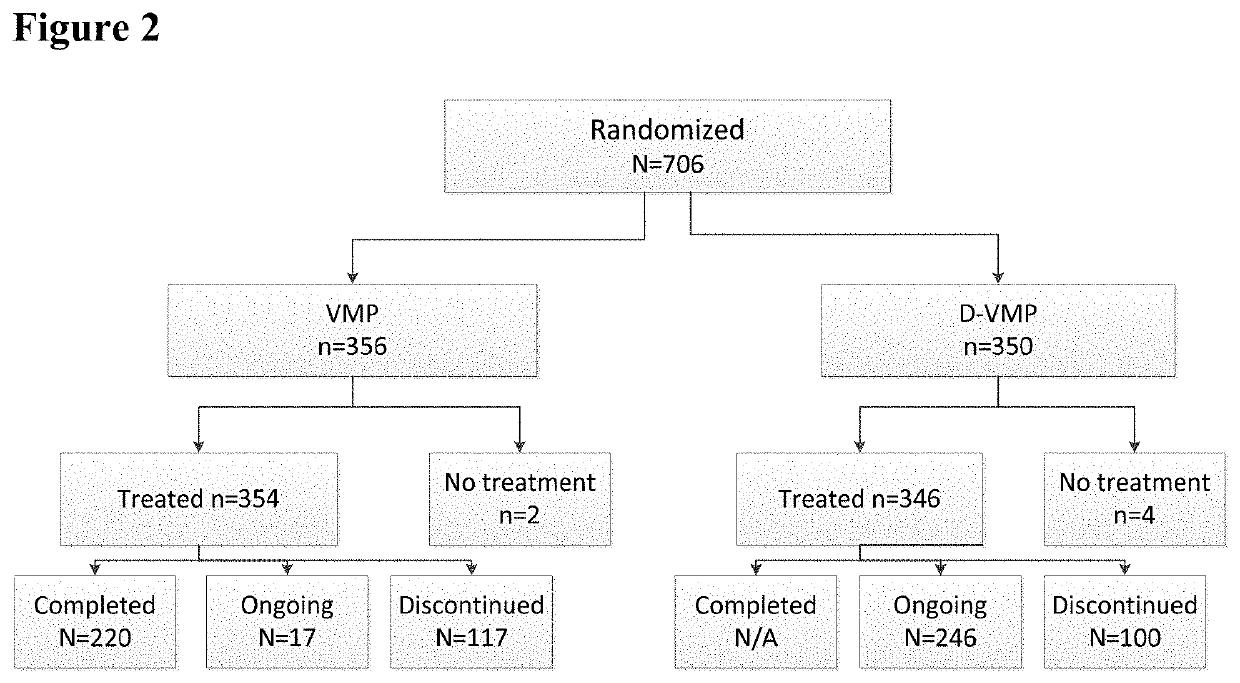
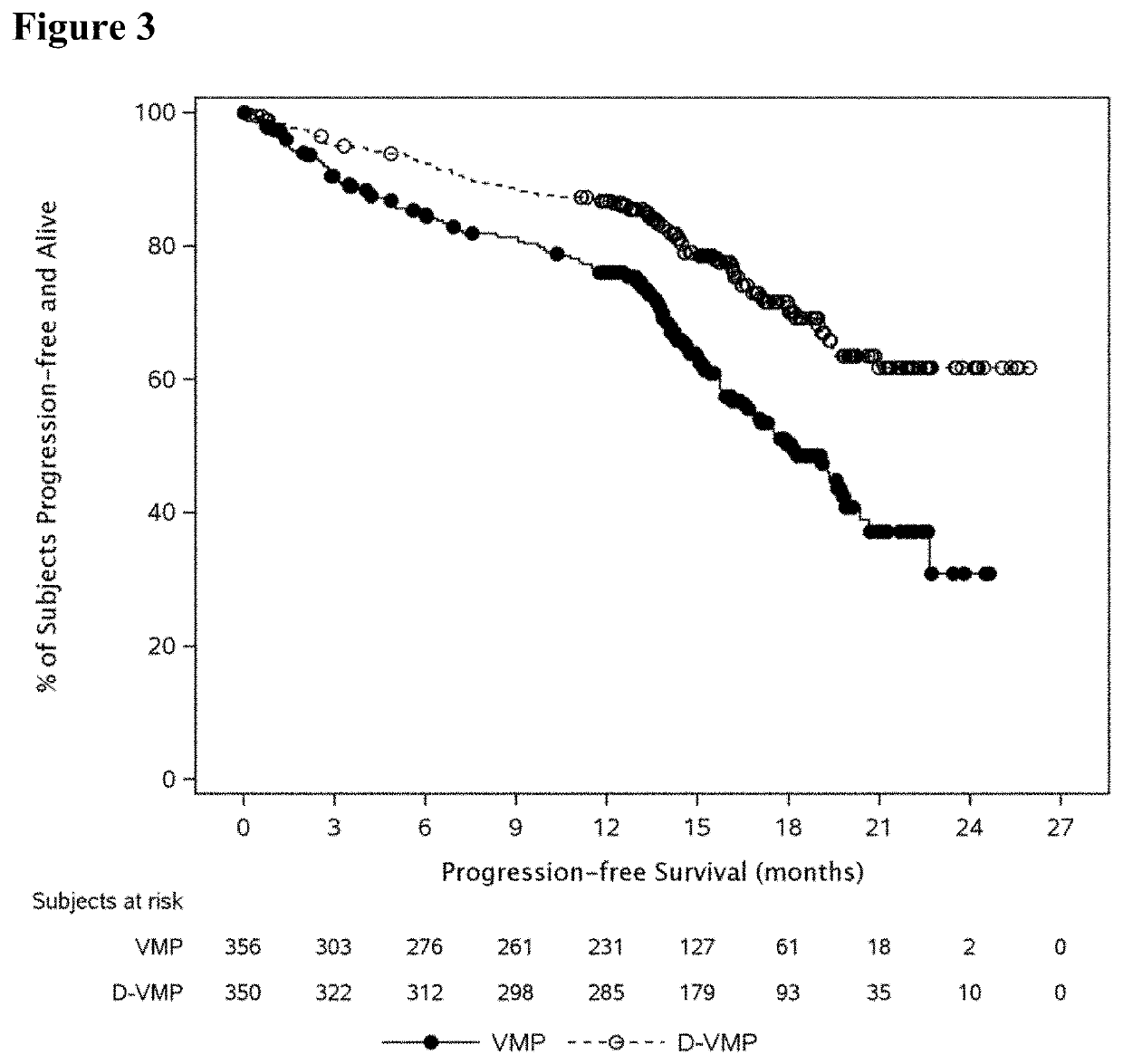
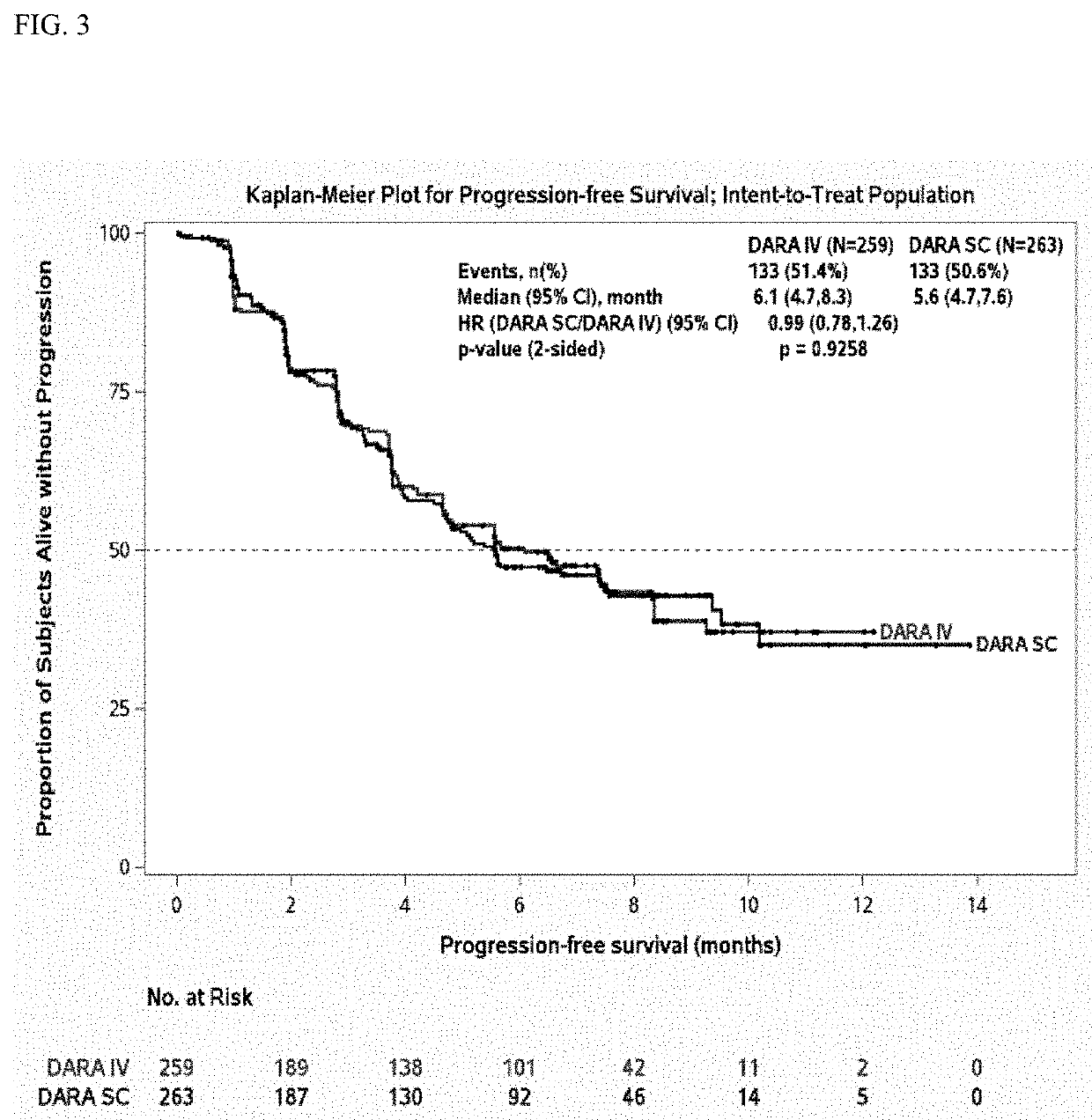
Described herein are methods of treating multiple myeloma with clinically proven safe and effective amounts of an antibody that specifically recognizes CD38 with bortezomib, melphalan, and prednisone. Also described are methods of selling or offering for sale an antibody that specifically recognizes CD38 or pharmaceutical compositions thereof with bortezomib, melphalan, and prednisone.
Owner:JANSSEN BIOTECH INC
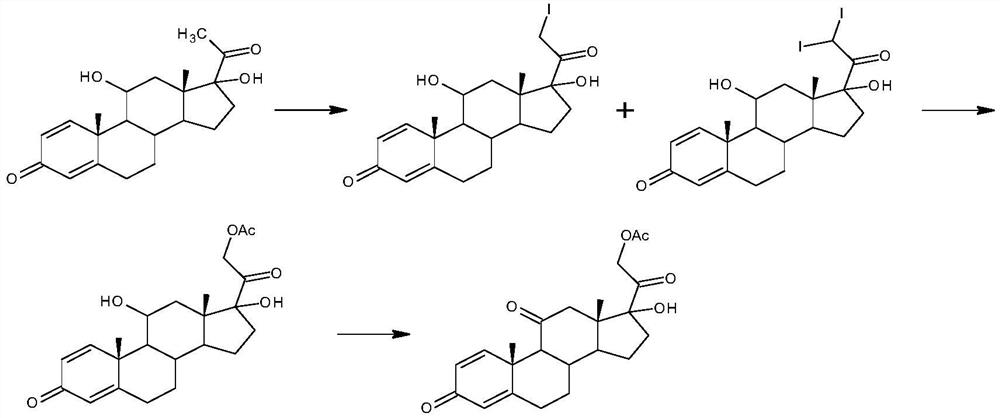
Preparation method for prednisone acetate and intermediate of same
The invention relates to the field of preparation of steroid drugs and an intermediate of the same, and in particular relates to a preparation method for prednisone acetate. The method comprises the steps of taking 11 alpha, 17 alpha-dyhydroxy Pregnene-1,4-diene-3,20-dione as an initial material, oxidizing to obtain 17 alpha-hydroxy Pregnene-1,4-diene-3,11,20-trione, and carrying out iodization reacting to obtain the prednisone acetate. The invention provides a novel oxidation technology more suitable for production of the intermediate of the prednisone acetate, the synthesis path has the characteristics of low cost and simple operation, environment pollution pressure can be greatly reduce, and the yield and quality of the prednisone acetate can reach a satisfactory level.
Owner:JIANGSU YUANDA XIANLE PHARMA
Clinically Proven Subcutaneous Pharmaceutical Compositions Comprising Anti-CD38 Antibodies and Their Uses in Combination with Bortezomib, Mephalan and Prednisone
InactiveUS20200330593A1Peptide/protein ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesPharmaceutical drug
The present invention relates to clinically proven subcutaneous pharmaceutical compositions comprising anti-CD38 antibodies and methods of their uses in combination with bortezomib, melphalan and prednisone.
Owner:JANSSEN BIOTECH INC
Method for preparing steroids and novel intermediate compound used therein
InactiveCN101117350AHigh yieldThe yield of iodine replacement is improvedSteroidsBulk chemical productionColor ScaleGlucocorticoid
The present invention relates to an improved preparation method of glucocorticoids steroid, in particular to the preparation methods of prednisolone acetate, cortisone, prednisolone or hydrocortisone. The present invention also relates to a new intermediate compound used in the process of preparing the compounds. The present invention provides a new synthetic route of iodo first and opening the ring after, improving the selectivity of final products, reducing the impurity content of the products, and satisfying the color scale of glucocorticoids drugs, thereby conquering the disadvantages of preparing 17 alpha- hydroxyl at first, and then preparing 21-hydroxyl or the derivatives during the process of preparing 17 alpha, 21-hydroxyl steroid or the derivatives of prior art.
Owner:TIANJIN PHARMA GROUP CORP
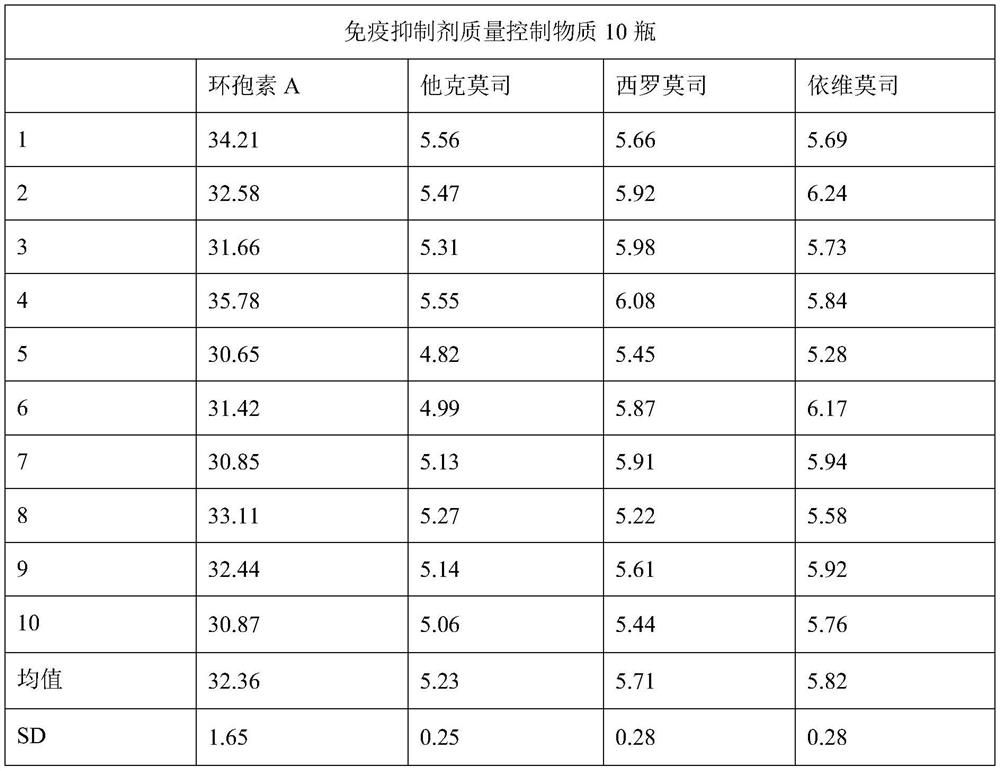
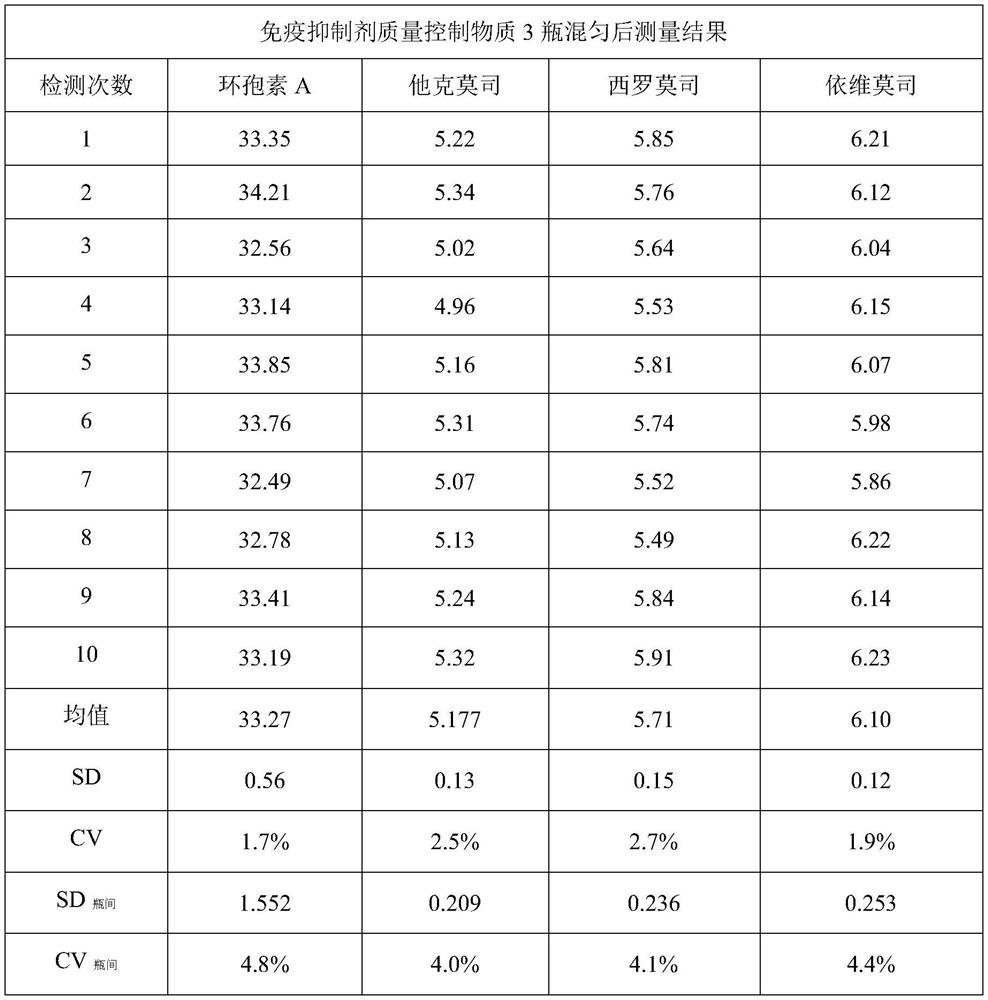
Whole blood type freeze-dried powder immunosuppressant quality control substance as well as preparation method and application thereof
The invention discloses a whole blood type freeze-dried powder immunosuppressant quality control substance as well as a preparation method and application thereof. The substance is a freeze-dried product mainly prepared from human whole blood serving as a matrix and an immunosuppressant. The immunosuppressant comprises one or more of tacrolimus, sirolimus, everolimus and cyclosporin A, or furthercomprises glucocorticoid immunosuppressants: one or more of hydrocortisone, cortisone, prednisolone, prednison and mycophenolic acid ester immunosuppressants for blood detection. The substance has theadvantages that the uniformity and the stability are good, low-temperature storage at -70 DEG C is not needed, and the substance can be used as a calibration product of immunosuppressant kits of different methodologies and can also be used as a quality control product for clinically monitoring an immunosuppressant detection system.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
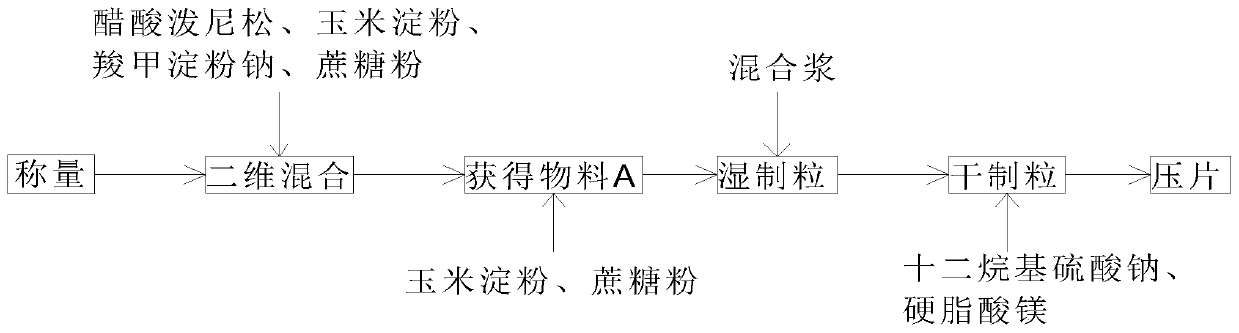
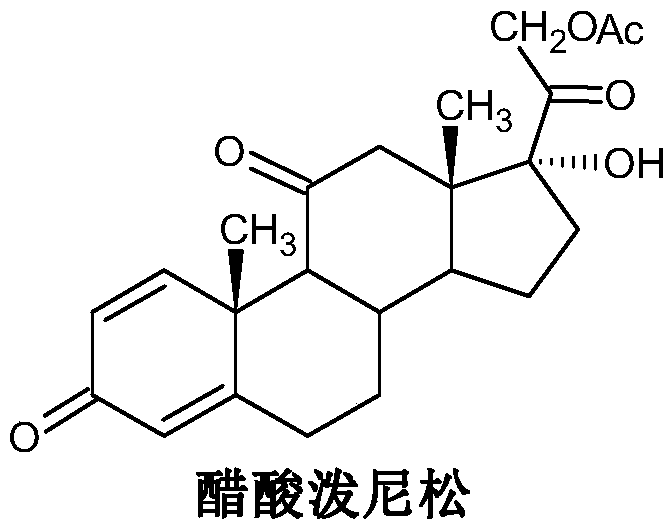
Prednisone acetate tablets and preparation method thereof
InactiveCN110840854AHigh dissolution rateQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsSucroseMedicine
The invention discloses prednisone acetate tablets and a preparation method thereof, which comprises, by weight, 4.22% of prednisone acetate, 64.50% of corn starch, 28.50% of sucrose, 1.41% of sodiumcarboxymethyl starch, 0.67% of sodium lauryl sulfate, and 0.70% of magnesium stearate; quality parameters of the prednisone acetate tablets include moisture of < / =5.0%, content of 93.0-107.0% and friability of < / =0.8%. According to the preparation method, micronization of prednisone acetate is implemented first, so that the dissolution rate of the prednisone acetate tablets is effectively improved; the prednisone acetate is fully mixed with the corn starch material; the quality is stable, the impurity content is low, the moisture is low, and the prednisone acetate tablet scan be directly absorbed and digested by a human body after being taken by the human body.
Owner:安徽金太阳生化药业有限公司
Drug-release type gel for treating fundus macular degeneration and preparation method thereof
InactiveCN109966243AAchieve sustained releaseLittle side effectsSenses disorderAerosol deliveryPeroxydisulfateNon invasive
The invention discloses drug-release type gel for treating fundus macular degeneration and a preparation method thereof. The drug-release type gel comprises an anti-angiogenesis drug, an inducing compound and gel and is characterized in that the inducing compound is used for increasing the permeation of the drug, and the gel attaches to the cornea during use and serves as the carrier of the drug;the anti-angiogenesis drug is selected from Lucentis or Compaq; the inducing compound is selected from optional one of dexamethasone, prednisone and cortisone or the combination of two of dexamethasone, prednisone and cortisone; the gel serving as the drug carrier is prepared by preparing a solution containing polydopamine and methacrylate gelatin, adding crosslinking agent N,N-methylene bisacrylamide and initiator potassium peroxydisulfate into the solution, and performing fixed-die forming to obtain the gel which does not contain the drug. The drug-release type gel has the advantages that non-invasive drug delivery is adopted, sustained drug release is achieved after the gel attaches to the cornea, and the anti-VEGF drug is allowed to act on the vitreous body by utilizing the permeability of the inducing compound.
Owner:SHANGHAI TONGREN HOSPITAL
Prednisone acetate micro-tablet and preparation method thereof
PendingCN113304115AImprove complianceImprove brittlenessOrganic active ingredientsAntipyreticEthylic acidBULK ACTIVE INGREDIENT
The invention discloses a prednisone acetate micro-tablet and a preparation method thereof. The micro-tablet comprises prednisone acetate serving as an active component and pharmaceutic adjuvants, the mass percentage of the active ingredient prednisone acetate in the micro-tablet is 2%-20%. The preparation method of the prednisone acetate micro-tablet comprises the following steps: pretreating the raw and auxiliary materials, premixing, performing wet granulation, totally mixing, and tabletting to obtain the prednisone acetate micro-tablet. The raw materials and the auxiliary materials are mixed according to a set proportion and then crushed, and the particle size of the crushed mixed powder is controlled, so that the production process is improved, the loss of the raw materials is reduced, and the problem that the dissolution speed of the tablet is relatively low is solved.
Owner:CISEN PHARMA
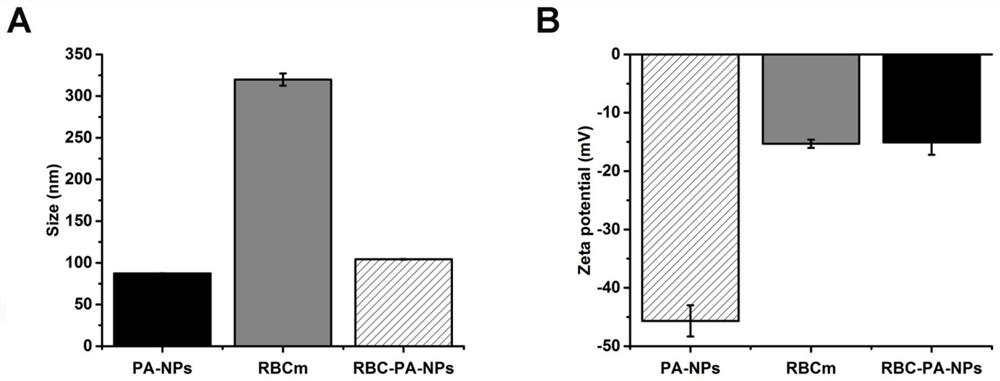
Novel kidney-targeted nano drug delivery system with biomimetic modification of erythrocyte membrane as well as preparation method and application of novel kidney-targeted nano drug delivery system
ActiveCN113133988AOrganic active ingredientsPharmaceutical non-active ingredientsEthylic acidCell membrane
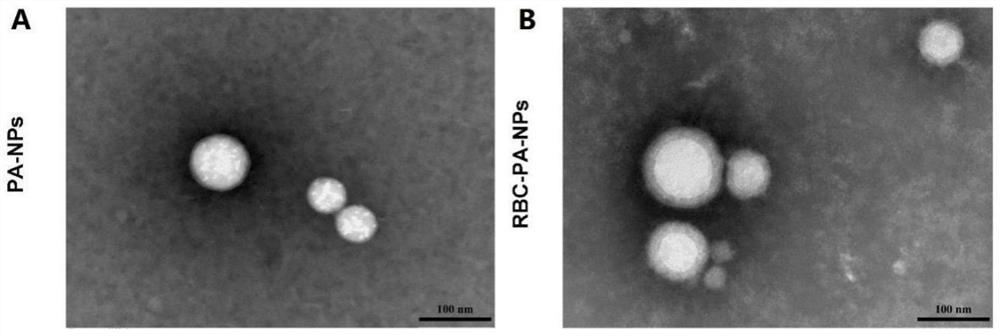
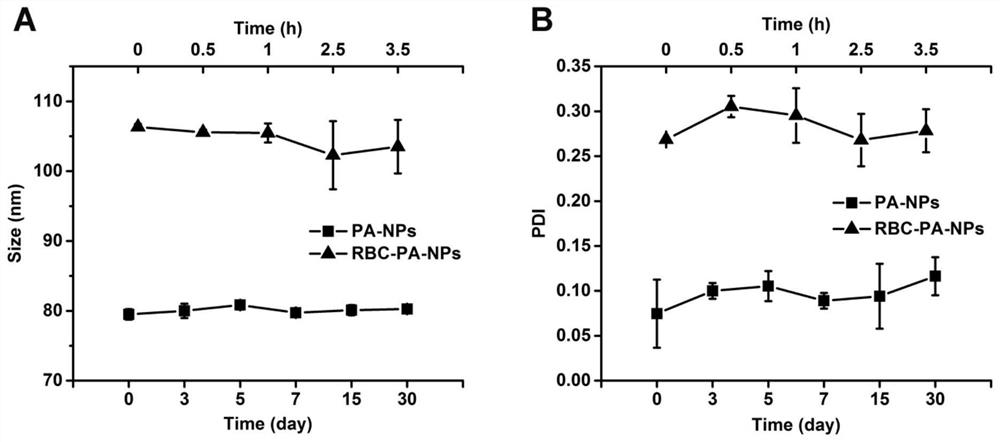
The invention belongs to the technical field of biological medicines, and particularly relates to a novel kidney-targeted nano drug delivery system with biomimetic modification of an erythrocyte membrane as well as a preparation method and application of the novel kidney-targeted nano drug delivery system. According to the application, polylactic acid-glycolic acid copolymer (PLGA) is taken as a carrier material, prednisolone acetate (PA) is taken as a model drug, and a novel bionic nano drug delivery system (RBC-PA-NPs) with kidney targeting is constructed by utilizing a strategy of coating an erythrocyte membrane on the surface. The drug delivery system combines the biocompatibility of the erythrocyte membrane and the targeting property of the nanoparticles, so that the kidney targeting property of PA is improved, and the glomerulonephritis is treated more effectively. Therefore, evaluation is carried out from the aspects of a preparation process, physicochemical properties, cytotoxicity and uptake, in-vivo targeting and the like of the drug delivery system.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
Lactococcus lactis subsp.lactis HFY14 and application thereof
PendingCN114686402AImprove immune organ indexImprove kidney functionBacteriaMicroorganism based processesBiotechnologyStaphylococcus lactis
The invention discloses a lactococcus lactis subsp. Lactis HFY14 and application thereof, and belongs to the technical field of biology, the lactococcus lactis subsp. Lactis is named as HFY14, the preservation number is CGMCC No.16647, the strain is separated from naturally fermented yoghurt, and the strain has the advantages that the strain can be used for preparing the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14; animal experiments, histopathologic observation and other experiments prove that the renal dysfunction of lupus nephritis mice induced by hypophytane through intragastric administration of viable bacterial liquid of the strain can be obviously improved, and the effect is close to that of a medicine prednisone. The invention provides a theoretical basis for the subsequent development and utilization of the lactococcus lactis subsp. Lactis HFY14 and the application of the lactococcus lactis subsp. Lactis HFY14 as a probiotic to improve the renal function of nephropathy, and the strain has the application potential of performing long-term intervention on lupus nephritis to improve the renal function.
Owner:善恩康生物科技(苏州)有限公司
Method for preparing prednisolone through bio-fermentation in one step
The invention relates to a method for directly generating prednisolone through bio-fermentation in one step by using hydrocortisone acetate as a raw material and Arthrobacter Simple ATCC 21032.
Owner:TIANJIN JINYAO GRP
Dry cow mamma perfusion agent for preventing and curing cow mastitis and preparation method thereof
InactiveCN1666744ADecreased cell countReduce incidenceAntibacterial agentsOrganic active ingredientsVegetable oilAmpicillin
The invention discloses a dried cow breast perfusion agent and its process method for treating cow mastitis. It is in the characterized in that: perfusion agents of each 10 ml prescriptions comprise ampicillin or amoxicillin of 100-150 mg; prednisolone acetate is 4-7 mg; sodium chloride is 400-550 mg; vegetable oil is 6-6.5 ml; polysorbate os 0.75-0.82 ml; left is distiller water. And the process method is: vegetable oil. Sodium chloride, and distilled water are mix uniformly to be added polysorbate for emulsion; ampicillin or amoxicillin are added to mixed after prednisolone acetate being added, then emulsified in low temperature; aseptic package to attain finish products. Said invention has significantly effect on prevention and treatment of dried cow occult mastitis without waste, and it has low process cost and low treatment cost.
Owner:NANJING AGRICULTURAL UNIVERSITY
Medicine for treating toothache
A medicine for treating toothache, dental marrow inflammation and periodontitis is prepared from VB1, VC, aminopyrine, phenacetin, coffeine, austrominal, prednisone acetate, and safflower.
Owner:王明贤
Hyaluronic acid-chitosan thermosensitive hydrogel loaded with prednisone and preparation method thereof
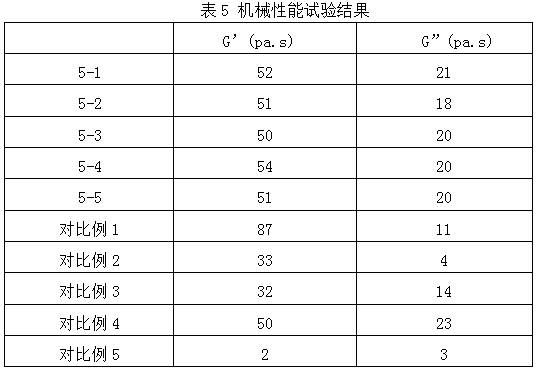
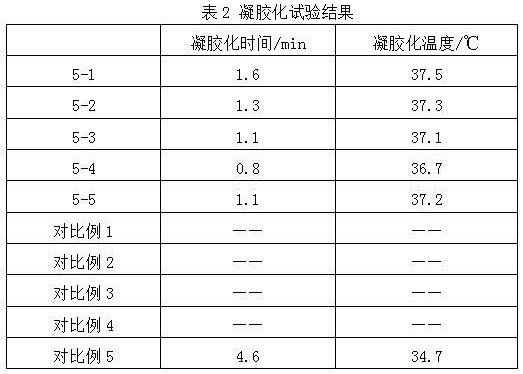
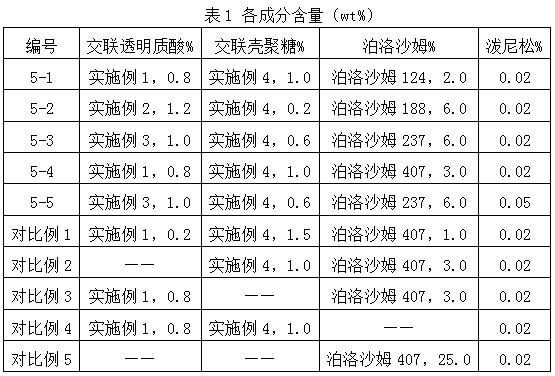
ActiveCN112516075AImprove biodegradation rateReduced release rateOrganic active ingredientsAntipyreticHuman bodyJoint cavity
The invention discloses hyaluronic acid-chitosan thermosensitive hydrogel loaded with prednisone and a preparation method thereof. The thermosensitive hydrogel is prepared from 0.8%-1.2% of cross-linked hyaluronic acid, 0.2%-1.0% of cross-linked chitosan, 0.01%-0.05% of prednisone and 2.0%-6.0% of poloxamer. The cross-linked hyaluronic acid and the cross-linked chitosan are used as main hydrogel matrixes, an articular cavity can be lubricated, the filling and supporting effects are achieved, the biodegradation rate and the release rate of the anti-inflammatory drug prednisone are decreased, and symptoms such as knee joint pain can be relieved and treated for a long time. According to the thermosensitive hydrogel, the dosage of the poloxamer is reduced, the gelation time of the obtained hydrogel is short, the gelation temperature is close to the body temperature of a human body, and articular cavity injection is easier to carry out.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Medicine for treating toothache
InactiveCN100484535CQuick cureDigestive systemHeterocyclic compound active ingredientsAminophenazonPharmaceutical drug
Owner:王明贤
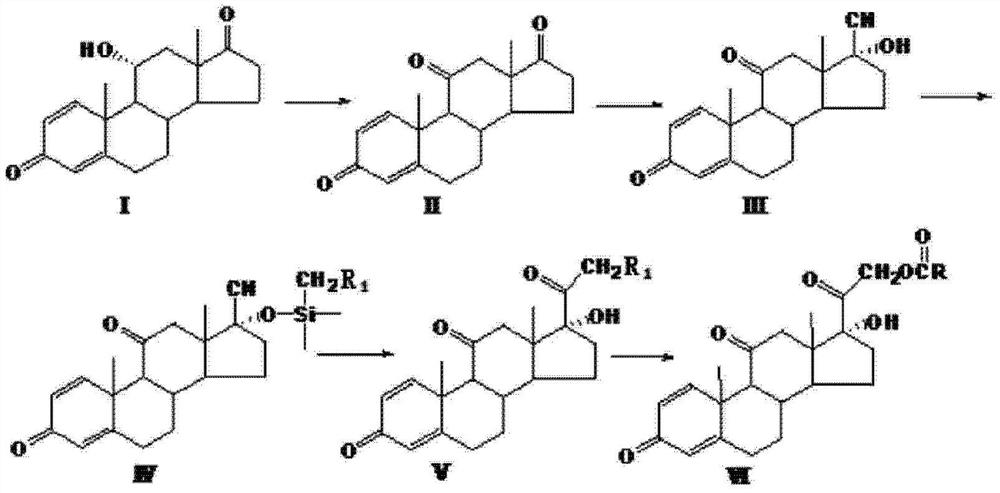
Method for preparing 16 alpha-hydroxyprednisolone
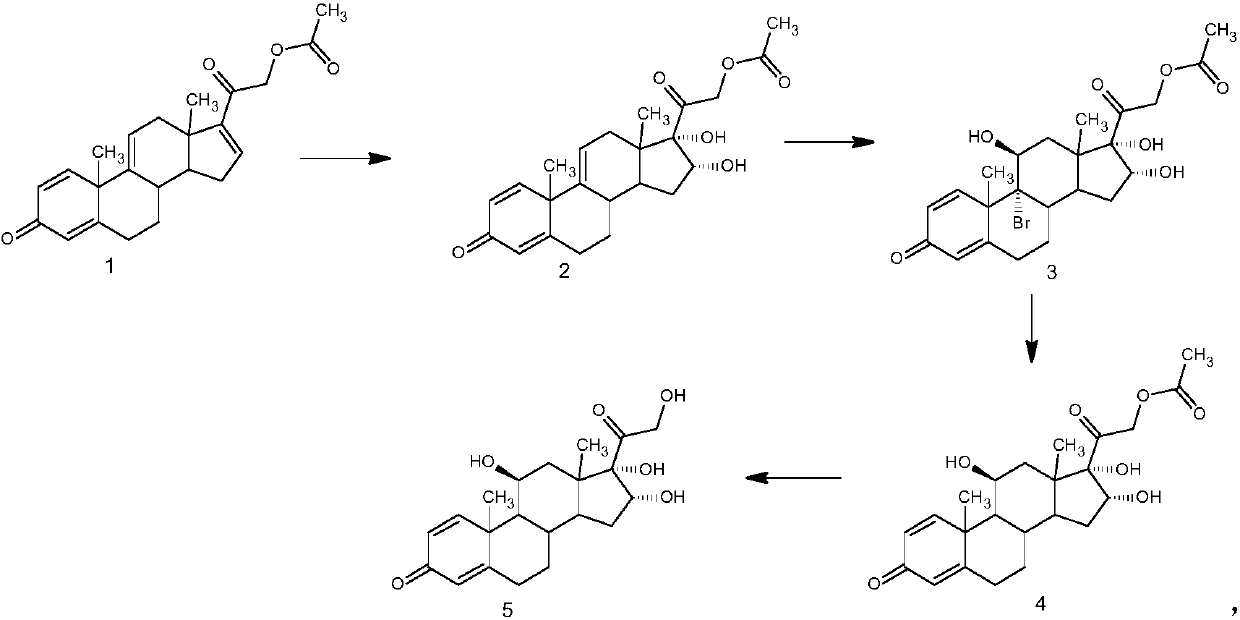
ActiveCN111253457ATo avoidImprove reaction efficiencyOrganic decompositionSteroidsPrednisoloneBiochemical engineering
The invention discloses a method for preparing 16 alpha-hydroxyprednisolone, belonging to the technical field of medicine preparation and processing. According to the method, 21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione 21-acetate is used as an initial raw material and subjected to oxidation, bromo-hydroxylation, debromination and alcoholysis to prepare 16 alpha-hydroxyprednisolone. Accordingto the method for preparing 16 alpha-hydroxyprednisolone, generation of impurities in the reaction process can be effectively controlled by improving the defects of a traditional process, reaction process is mild, and an overall conversion rate is high; and the method disclosed by the invention has the advantages of low requirements on a reaction device, low operation cost, simplicity and convenience in operation, suitability for industrial production and higher market prospects.
Owner:ZHEJIANG SHENZHOU PHARMA
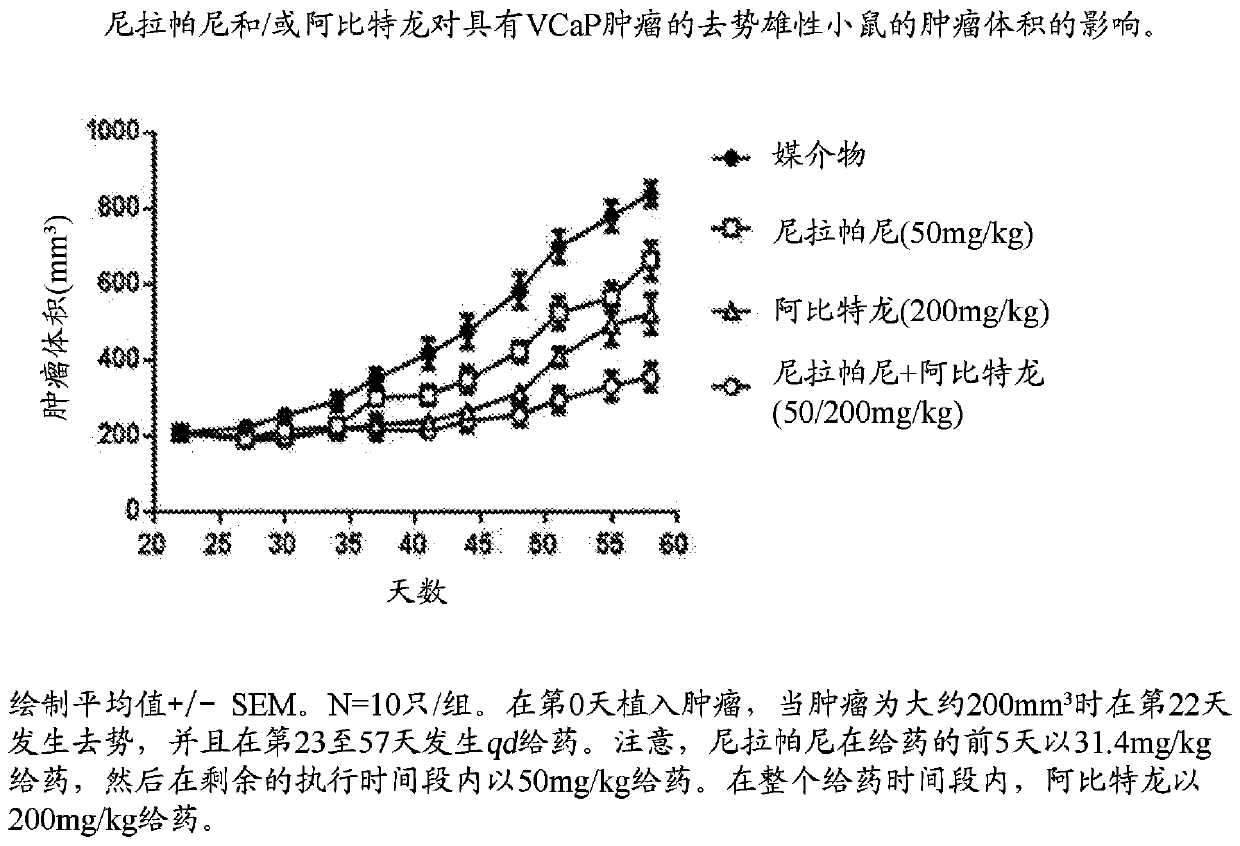
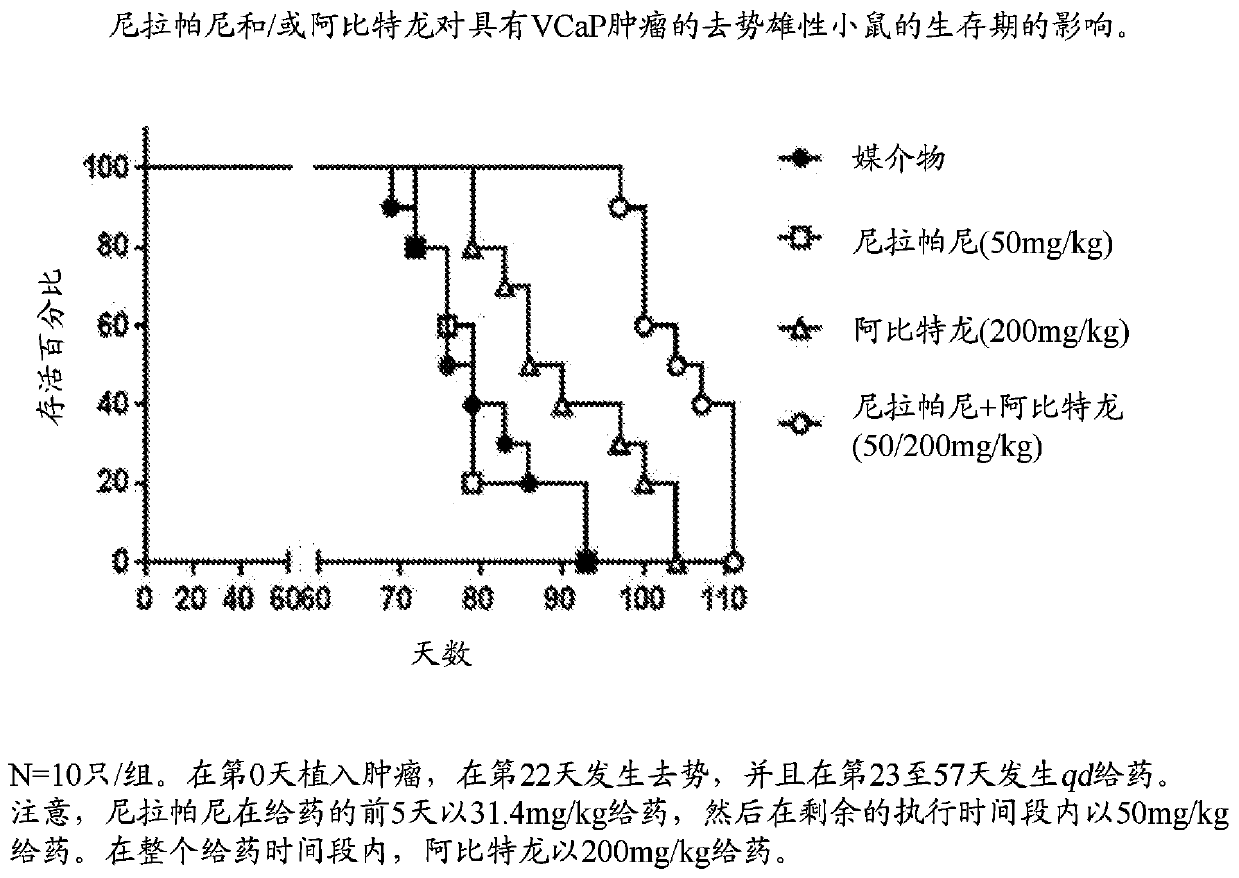
Combination therapy for prostate cancer
Provided are methods and compositions, for treating prostate cancer by administering to a patient in need thereof a therapeutically effective amount of a PARP inhibitor, e.g., niraparib; a therapeutically effective amount of a CYP17 inhibitor, e.g., abiraterone acetate, and a therapeutically effective amount of a glucocorticoid, e.g., prednisone.
Owner:JANSSEN PHARMA NV
Taste-masked prednisolone oral formulations
InactiveUS20060127472A1Effective taste-masking amountOrganic active ingredientsDispersion deliveryMedicineEther
An improved taste-masked pharmaceutical prednisolone composition contains prednisolone sodium phosphate taste-masked with an effective taste-masking amount of rum ether.
Owner:WHITEHEAD KEITH
External medicine for treating herpes zoster
InactiveCN103251683ANo damageRich sourcesOrganic active ingredientsAntiviralsSide effectChlorobenzene
The invention discloses an external medicine for treating herpes zoster, which is characterized by being prepared from the following components in parts by weight: 90-100 parts of compound sulfamethoxazole tablet, 0.25-0.45 part of chlorpheniramine maleate, 0.4-0.6 part of prednisone acetate, 12-15 parts of talcum powder, 15-20 parts of honeysuckle, 15-20 parts of folium isatidis and 15-20 parts of rhizoma corydalis. The external medicine disclosed by the invention is a medicine for external use, avoids toxic and side effects and does not hurt human bodies; the raw materials are widely available, the cost is low, the preparation method is simple, and the medicine is safe to use; the medicine realizes fast curative effects on diminishing inflammation and relieving pain; and the pain can be relieved after 1 hour of application, the shortest healing time is 4 days, and the longest healing time is 14 days.
Owner:马群
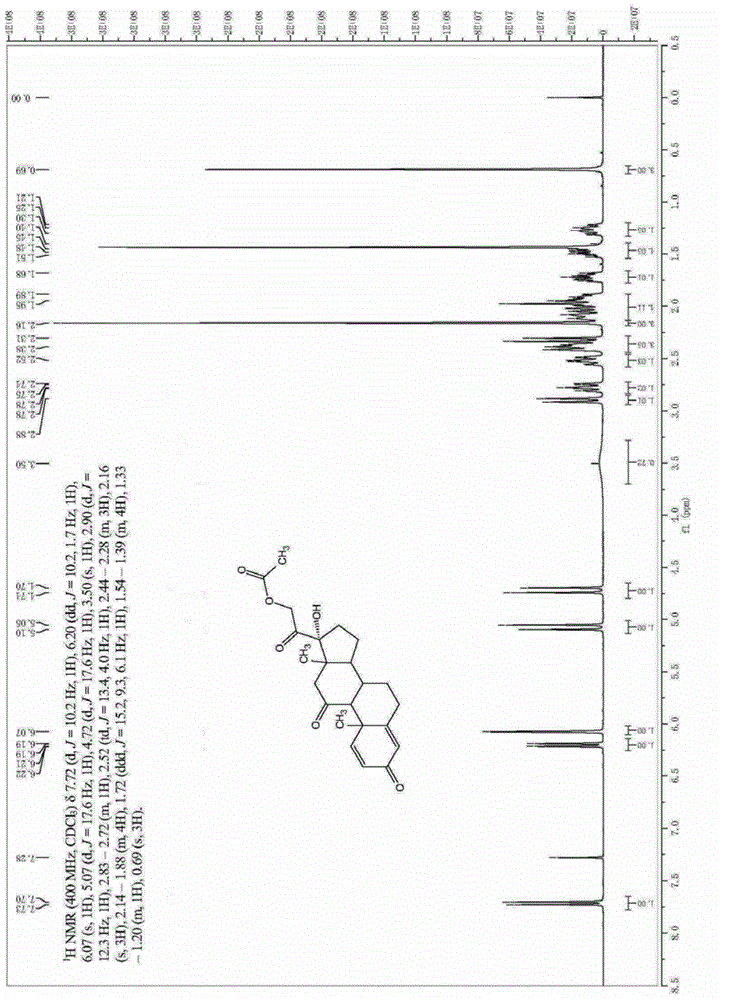
Preparation method of prednisolone acetate
The invention relates to prednisolone acetate and a preparation method of prednisolone acetate, which are characterized in that the prednisolone acetate is prepared by sequentially carrying out a biological fermentation, an esterification reaction, a bromination reaction and a debromination reaction on the raw material, the prednisolone acetate is subjected to a hydrolysis reaction to obtain prednisolone, the overall yield is up to 81.75%, and the HPLC area normalization content of prednisolone is up to 99.5%. The preparation method is short in synthetic route and low in cost, is suitable forindustrial production, and has a very high industrial value.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Preparation method of prednisone
ActiveCN111777654ALower requirementLow running costMicroorganism based processesSteroidsBiotechnologyDehydrogenation
The invention discloses a preparation method of prednisone, and belongs to the technical field of preparation and processing of medicines. According to the method, hydrocortisone acetate is used as aninitial raw material, and the prednisone is prepared through three steps of oxidation, biological fermentation dehydrogenation and hydrolysis. According to the preparation method of prednisone, the defects of a traditional process are overcome, the target product is high in purity, good in quality stability, high in yield, low in production cost and mild in reaction condition, a highly toxic cyanide reagent is prevented from being used, and the method is easy and convenient to operate, suitable for industrial production and wide in market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Natural method for eliminating HIV to be employed by type 1 and 2 HIV sufferers
A defined natural procedure that lists a series of steps that will effectively eliminate HIV from the victim's body in 2 months. The procedure starts with prayer to the God of Abraham for healing which continues throughout the process. It includes treating the yeast infection with a physician prescribed antifungal product and prednisone 7-day step down regimen (if needed). The elimination of refined sugar products and the inclusion of daily cardiovascular and weight bearing exercise will cut off the HIV's food supply. The final steps involve performing daily internal cleanse to detoxify the body, abstaining from sexual intimacy, and consuming a combination of foods in the form of a salad for breakfast with the remaining meals calorie restricted. With the HIV virus eliminated from the victim's body, the immune system will rebuild / replenish itself to the point where it can effectively combat opportunistic diseases.
Owner:LUCKETT DENESSA ROCHELLE
Compound nose drops and its prepn.
InactiveCN1336175AReduce edemaAdjust immune functionOrganic active ingredientsRespiratory disorderCurative effectTherapeutic effect
The invented naristillae is composed of naphazoline hydrochloride nose drop 3-8 ml. contianing 0.1% naphazoline hydrochloride, lincomycin hydrochloride solution 2-6 ml. containing 0.6-1.8 gm licomycin hydrochloride, prednisolone aectate solution 1-5 ml. containing prednisolone acetate 25-125 mg. Advantages include: apparent therapeutic effect quick action, easy to use it, no recurrence etc.
Owner:杨俊爱
Preparation method of high-quality prednisone acetate and intermediate thereof
InactiveCN110862431AHigh feeding concentrationGood conversion effectMicroorganism based processesSteroidsHydrocortisoneDehydrogenation
The invention discloses a preparation method of high-quality prednisone acetate and an intermediate thereof. The method includes the steps of: taking epihydrocortisone as the raw material, firstly conducting microbial dehydrogenation to obtain 11alpha, 17alpha, 21-trihydroxy-pregn-1, 4-diene-3, 20-dione intermediate, then carrying out esterification reaction to obtain 11alpha, 17alpha, 21-trihydroxy-pregn-1, 4-diene-3, 20-dione-21-acetate, and finally carrying out oxidation reaction to obtain prednisone acetate. The method provided by the invention solves the technical difficulty of non-idealintroduction of C1, 2 double bond in the traditional fermentation production process of prednisone acetate. The cortisone acetate prepared by the method provided by the invention has high quality andextremely low impurity content, greatly improves the multidirectional application of prednisone acetate, and meanwhile, the process route has the characteristics of low cost and simple operation.
Owner:HUAZHONG PHARMA
Medicament for treating bronchitis
InactiveCN104840719ACompatibility is reasonableEasy to take medicineTetracycline active ingredientsRespiratory disorderChlorobenzeneEthylic acid
The invention relates to a medicament for treating bronchitis. The medicament comprises the following raw materials in parts by weight: 8 to 12 parts of liquoric root extract powder, 0.1 to 0.3 parts of chlorpheniramine maleate, 0.068 to 0.1 parts of ketotifen fumarate, 0.18 to 0.32 parts of prednisone acetate, 2.5 to 4.1 parts of aminophylline, 6.5 to 8.6 parts of oxytetracycline, 0.2 to 0.4 parts of dioxopromethazine hydrochloride and 25 to 35 parts of unibract fritillary bulb. The medicament for treating bronchitis has the advantages of reasonable compatibility, convenience in taking, prevention of relapse, short treatment period, quick response, achievement of remarkable effect on the administration day, curative rate of up to 89.5 percent, total effective rate of up to 99 percent, simple preparation method, low cost and particularly remarkable effects on emphysema and lasting chronic bronchitis.
Owner:孟智琴
Prednisolone dihydrate as well as preparation method and application thereof
PendingCN114315944AOvercome temperatureOvercome the cycleSteroidsEndocrine system disorderPrednisolonePhysical chemistry
The invention provides prednisolone dihydrate as well as a preparation method and application thereof, and relates to the technical field of medicines. The X-ray powder diffraction of the prednisolone dihydrate has characteristic peaks when the diffraction angle 2theta is equal to 7.2 degrees + / -0.2 degrees, 12.5 degrees + / -0.2 degrees, 14.2 degrees + / -0.2 degrees, 15.5 degrees + / -0.2 degrees and 16.2 degrees + / -0.2 degrees. The invention provides a brand-new prednisolone dihydrate, the dihydrate can be dehydrated and converted into a crystal form I at a lower temperature, compared with the existing method for preparing the crystal form I by using a sesquihydrate, the required time is greatly shortened to be within 5h from the original more than 24h, and the method overcomes the defects of high baking temperature, long period and yellowing and deterioration of a product, and has the advantages that the method is simple and convenient to operate, and the production cost is reduced. The requirements of industrial production can be met.
Owner:TIANJIN TIANYAO PHARM CO LTD +1
Abiraterone acetate tablets and preparation method thereof
InactiveCN108096253ALess impuritiesReduce allergic reactionsOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkDocetaxel
The invention belongs to the technical field of biological pharmacy and discloses abiraterone acetate tablets and a preparation method thereof. The abiraterone acetate tablets are combined with prednisone to treat metastatic castration resistant prostate cancer patients who receive docetaxel-combined chemotherapy in the past. The abiraterone acetate tablets are prepared from dehydroepiandrosteroneacetate as a raw material and auxiliary materials including 3-pyridyllithium, 3-pyridineboronic acid, hydroxypropylcellulose, sodium lauryl sulfate, starch, microcrystalline cellulose, cross-linked povidone, magnesium stearate and silica. The prepared abiraterone acetate tablets contain fewer impurities and are purer, allergic reactions caused by impurity doping in the preparation process are reduced, and receivers are increased.
Owner:XUZHOU COLLEGE OF INDAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com