Combination therapy for prostate cancer
A technology for prostate cancer and composition, which can be applied to drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as inability to effectively and accurately repair DSB, cell death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 - Combination Therapy with Patient Evaluation
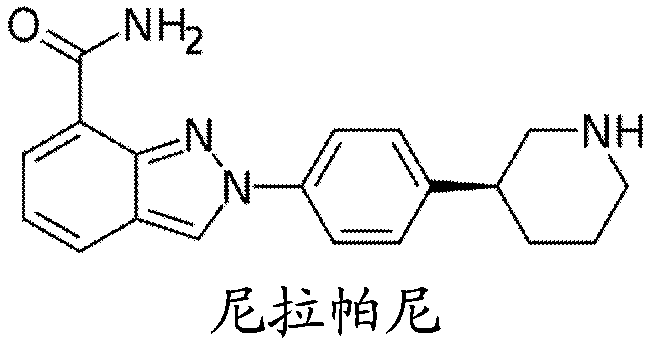
[0077] Niraparib was supplied as 200 mg capsules or tablets administered orally once daily by patients, abiraterone acetate was supplied as capsules or tablets (4 x 250 mg, total dose 1000 mg) administered orally once daily, and prednisone was administered as tablets Supplied as tablets or capsules (2 x 5 mg), administered orally twice daily (5 mg per tablet, twice daily). Dosing of niraparib was initiated at a starting dose of 200 mg once daily, which is 67% of the current clinical dose of niraparib monotherapy. The dose of abiraterone acetate 1000 mg once daily was kept constant throughout the treatment period, and the dose of prednisone 5 mg twice daily was also kept constant during the treatment period. All drugs were administered together starting on Day 1 of Cycle 1. The medicine must be swallowed whole. Patients took their doses (with or without food) in the morning, except on days when pharmacokineti...
Embodiment 2
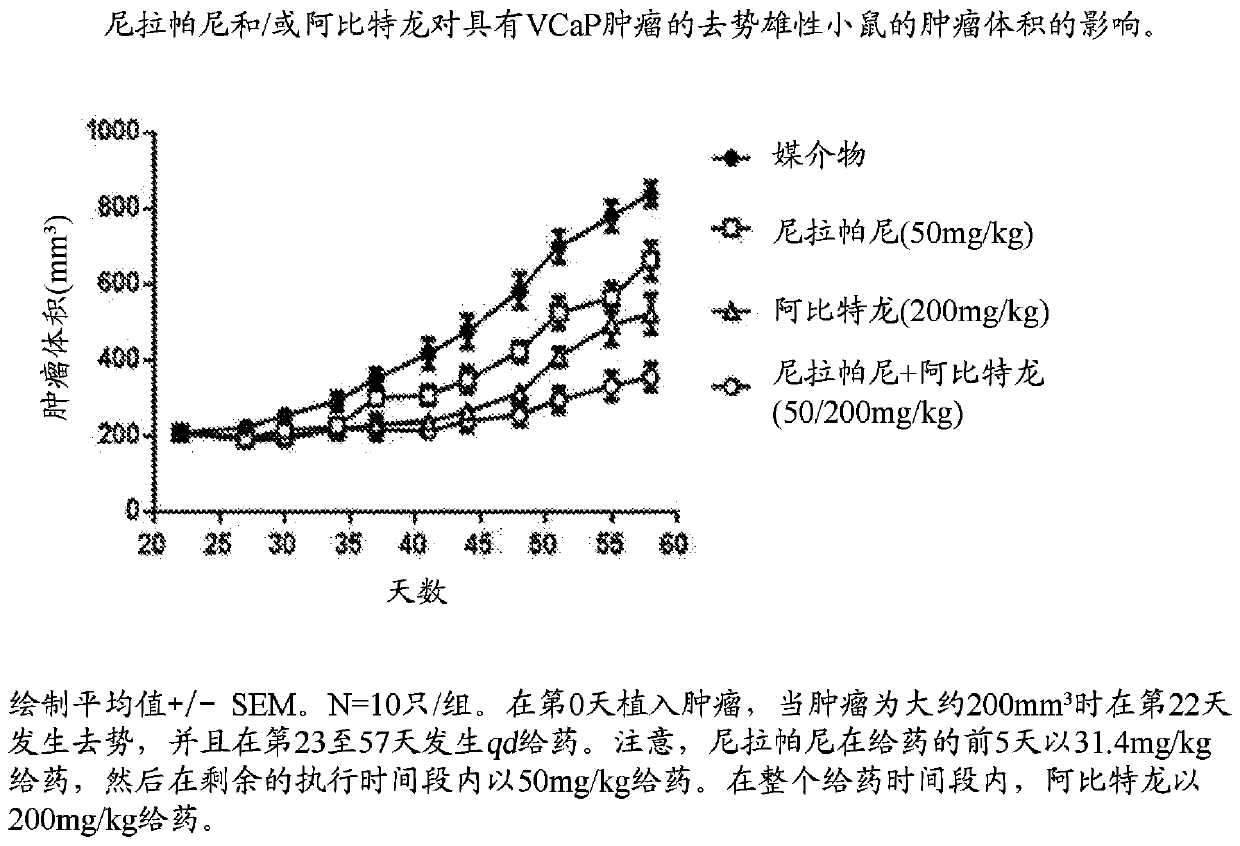
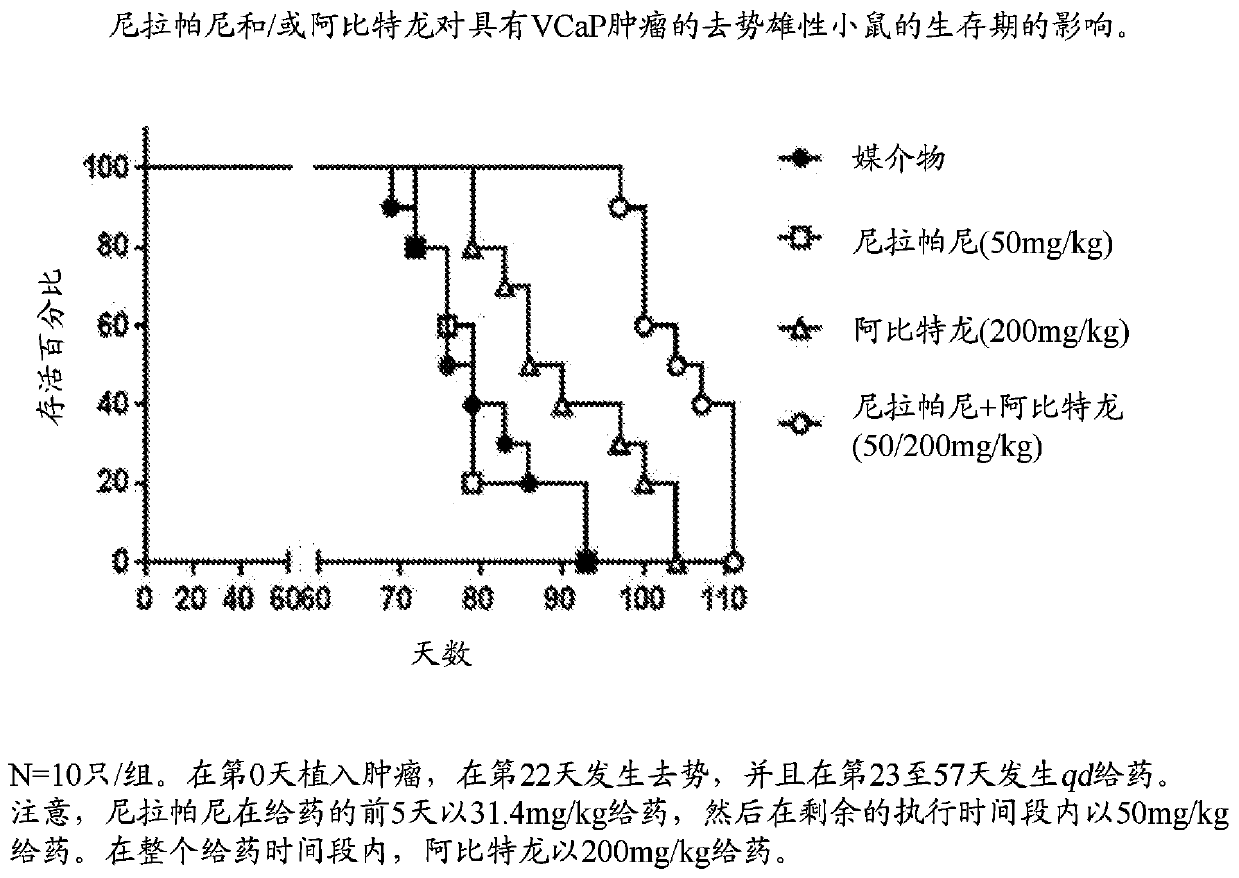
[0116] Example 2 - Niraparib Plus Abiraterone Effects on Human Prostate Tumor Model Implanted in Castrated Male Mice (VCaP) Efficacy
[0117] Objective: This study evaluated the efficacy of niraparib in combination with abiraterone in castrated male mice bearing human VCaP prostate tumors. Readouts were tumor volume and survival.
[0118] cell culture
[0119] The human VCaP prostate tumor line was derived from spinal metastases from a patient with castration-resistant prostate cancer. VCaP tumor cells carry TMPRSS2-ERG fusions and express the androgen receptor. VCaP tumor cell lines were maintained in DMEM medium supplemented with 10% fetal calf serum at 37C in an atmosphere of 5% CO2 in air. Tumor cells were subcultured twice weekly by trypsin-EDTA treatment. Cells were harvested for tumor injection when they were in the exponential growth phase.
[0120] Tumor cell injection and study design
[0121] Each mouse was subcutaneously injected with VCaP tumor cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com