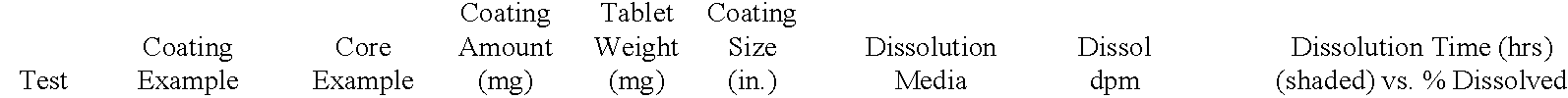Chronotherapeutic dosage forms containing glucocorticosteroid and methods of treatment
a technology of glucocorticosteroid and dosage form, which is applied in the direction of pharmaceutical product form change, inorganic non-active ingredients, drug compositions, etc., can solve the problems of osteoarthritis, affecting the treatment effect, and most likely to suffer heart attack or stroke, so as to reduce the risk of disease, less medication, and more efficient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
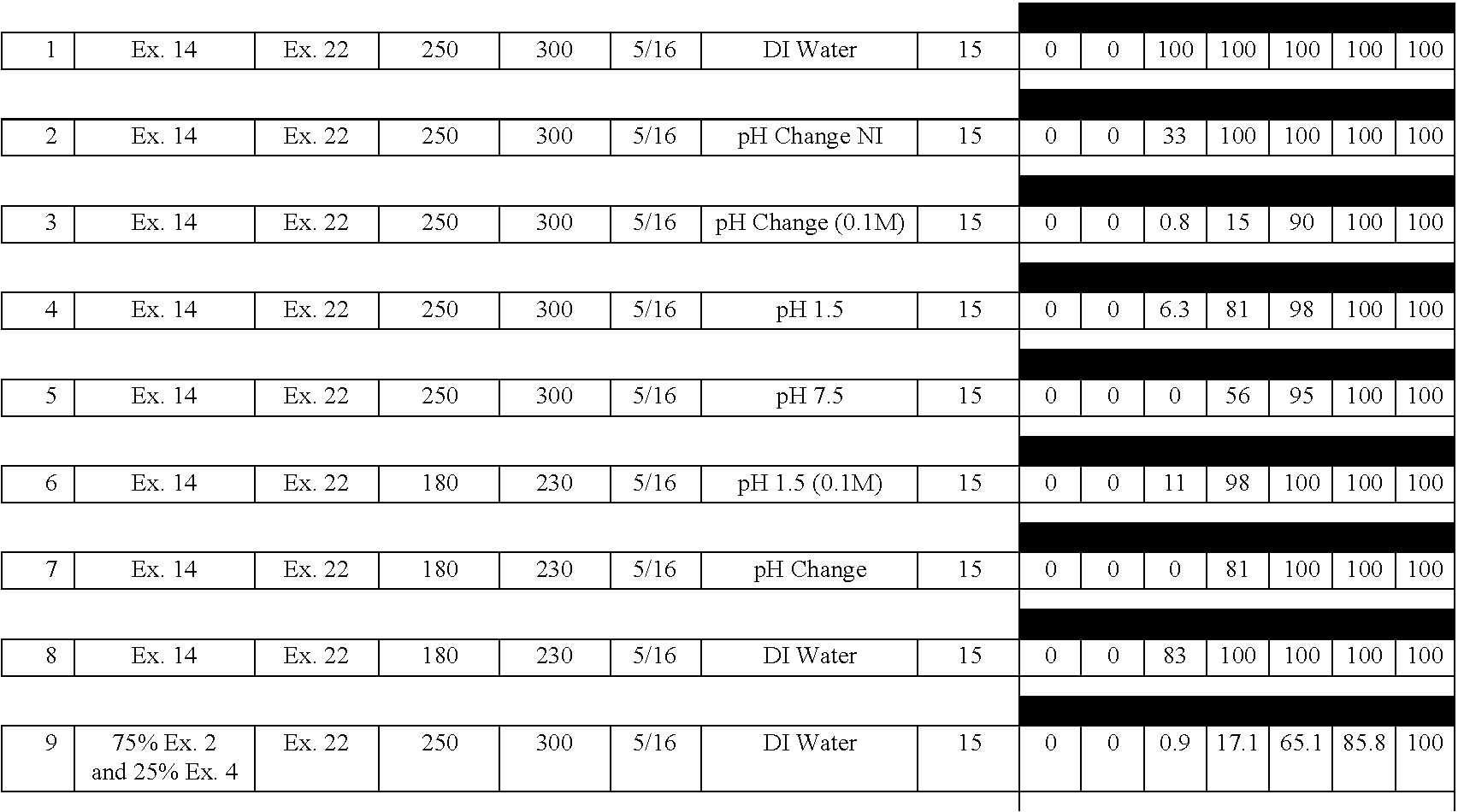
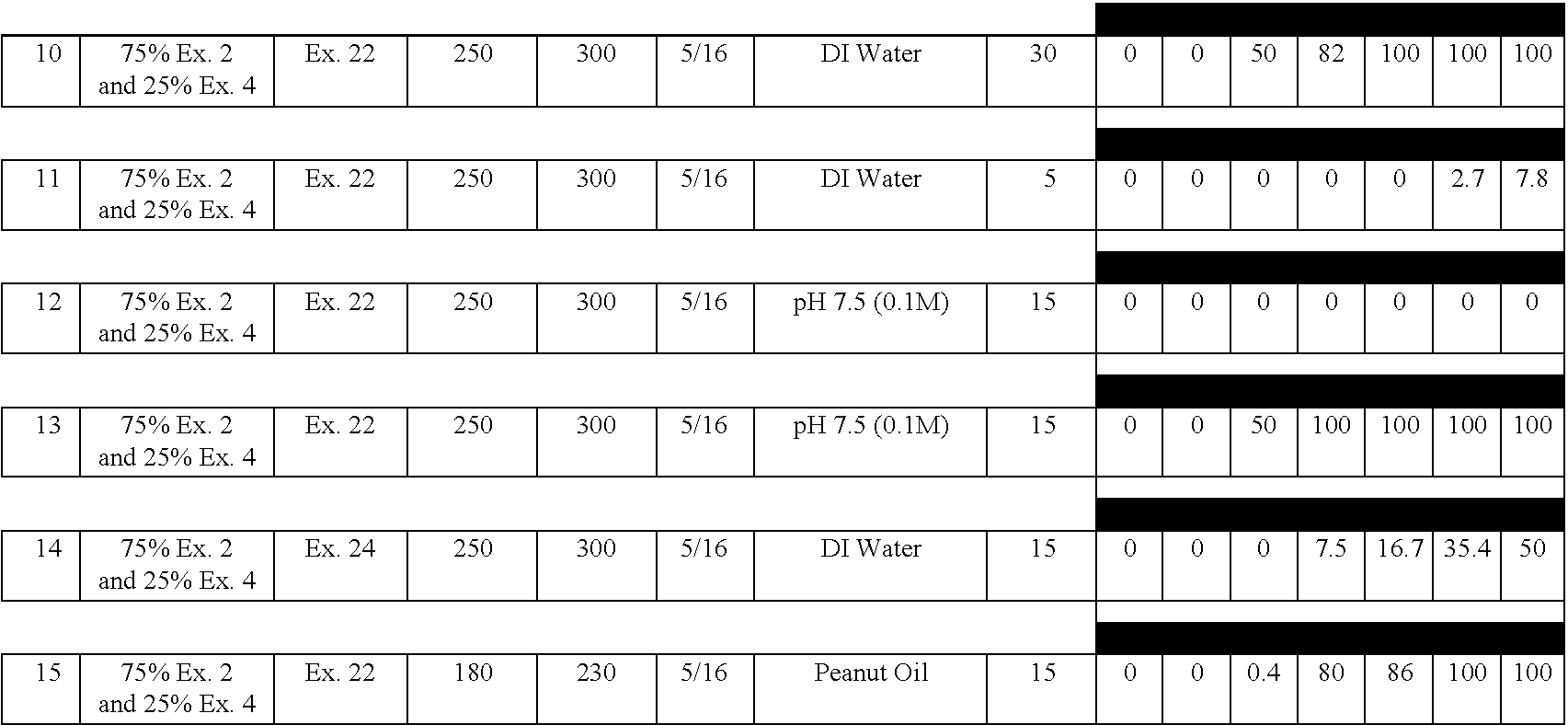
[0132] A delayed release material to be used in the compression coatings of the invention is prepared having the following formulation listed in Table 2:
3 TABLE 2 Component Percentage 1. Xanthan Gum 25 2. Locust Bean Gum 25 3. Dextrose 35 4. Calcium Sulfate Dihydrate 10 5. Ethylcellulose 5 5. Alcohol, SD3A, anhydrous* 20 6. Water* q.s. *Removed during processing
[0133] Process:
[0134] 1. The requisite amounts of xanthan gum, locust bean gum, calcium sulfate, and dextrose are dry blended in a high speed mixer / granulator for 3 minutes.
[0135] 2. A slurry of hydrophobic polymer (ethylcellulose) is prepared by dissolving ethyl cellulose in ethyl alcohol.
[0136] 3. The slurry is added to the dry blended mixture, and granulated for another 3 minutes.
[0137] 4. The granulation was then dried in a fluid bed dryer to a LOD (loss on drying) of less than about 10% by weight (e.g., 4-7% LOD).
[0138] 5.
example 3
[0139] A delayed release material to be used in the compression coatings of the invention is prepared having the following formulation listed in Table 3:
4 TABLE 3 Component Percentage 1. Xanthan Gum 15 2. Locust Bean Gum 15 3. Dextrose 60 4. Calcium Sulfate Dihydrate 10 5. Water* q.s. *Removed during processing
[0140] Process:
[0141] 1. The requisite amounts of xanthan gum, locust bean gum, calcium sulfate, and dextrose are dry blended in a high speed mixer / granulator for 3 minutes.
[0142] 2. Water (125-150 ml) is added to the dry blended mixture, and granulated for another 3 minutes.
[0143] 3. The granulation is then dried in a fluid bed dryer to a LOD (loss on drying) of less than abou-t 10% by weight (e.g., 4-7% LOD).
example 4
[0144] A delayed release material to be used in the compression coatings of the invention is prepared having the following formulation listed in Table 4:
5 TABLE 4 Component Percentage 1. Xanthan Gum 16 2. Locust Bean Gum 24 3. Dextrose 60 4. Water* q.s. *Removed during processing
[0145] Process:
[0146] The same process for Example 1 is used to prepare the delayed release coating of Example 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com