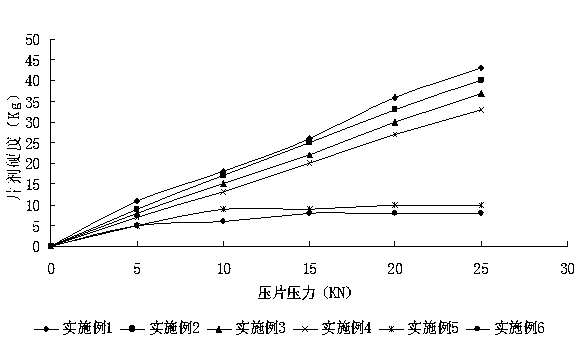
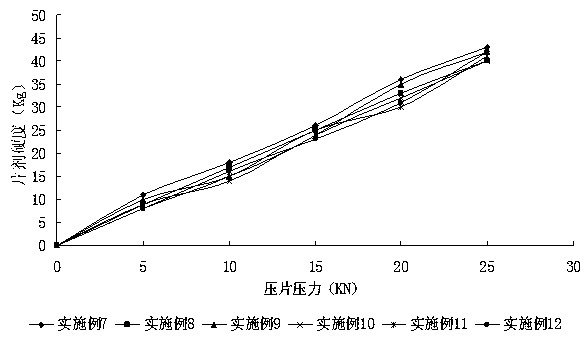
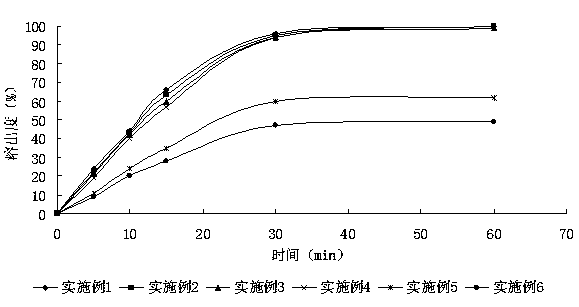
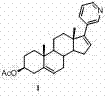
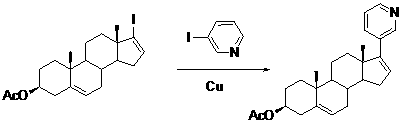
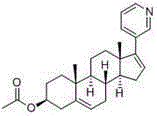
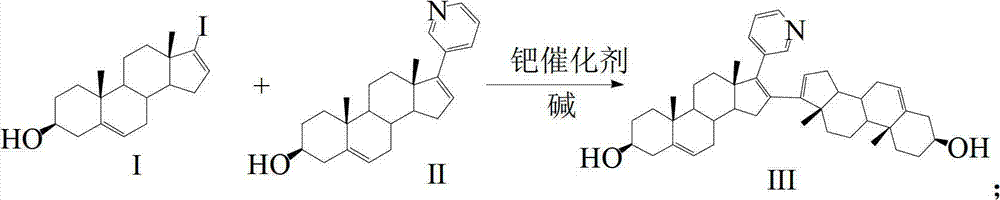
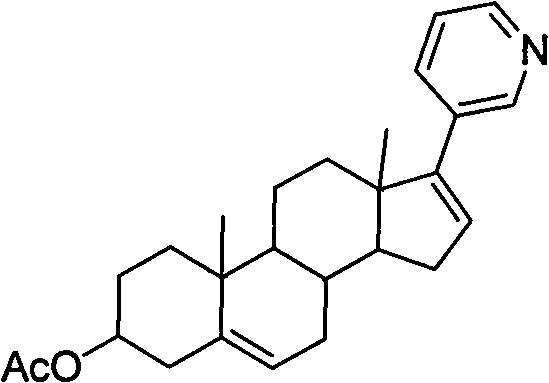
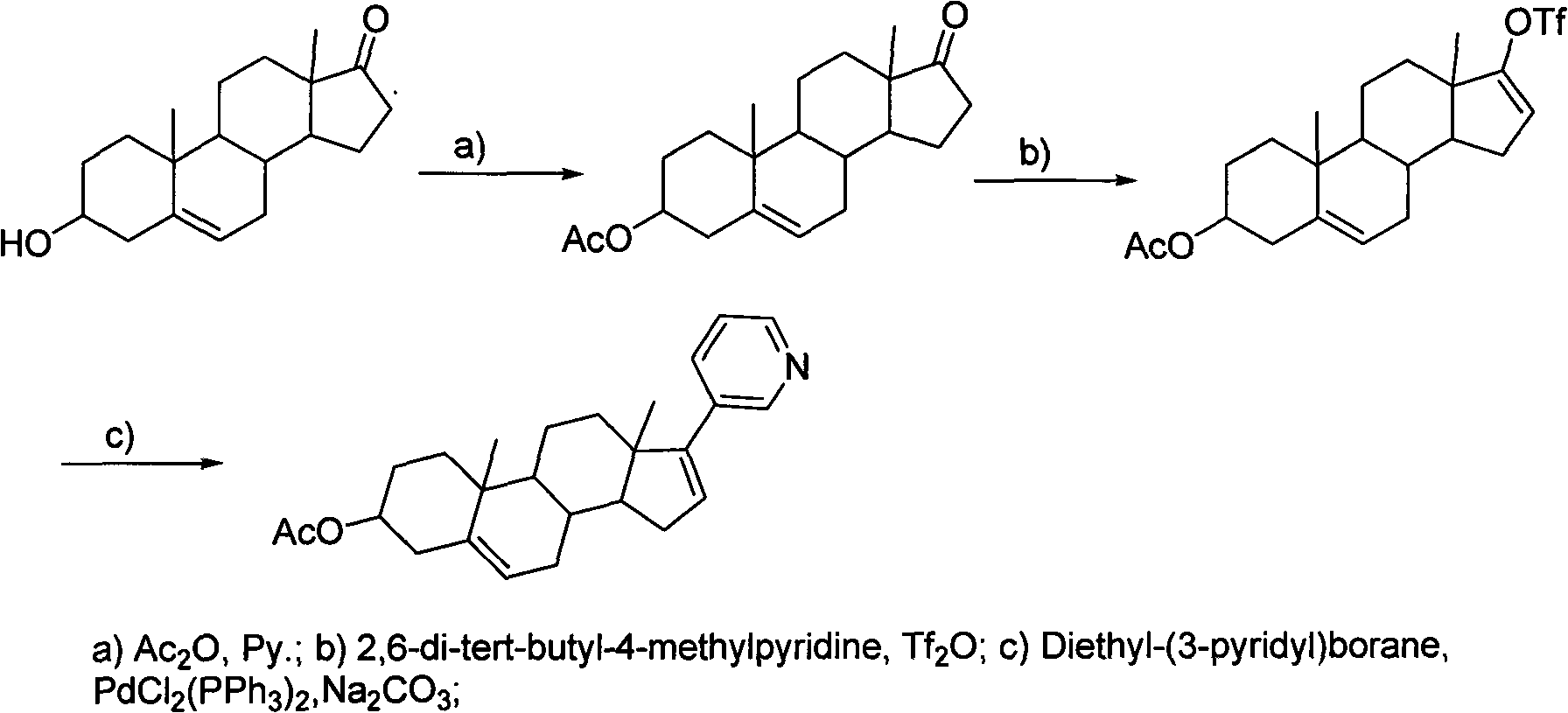
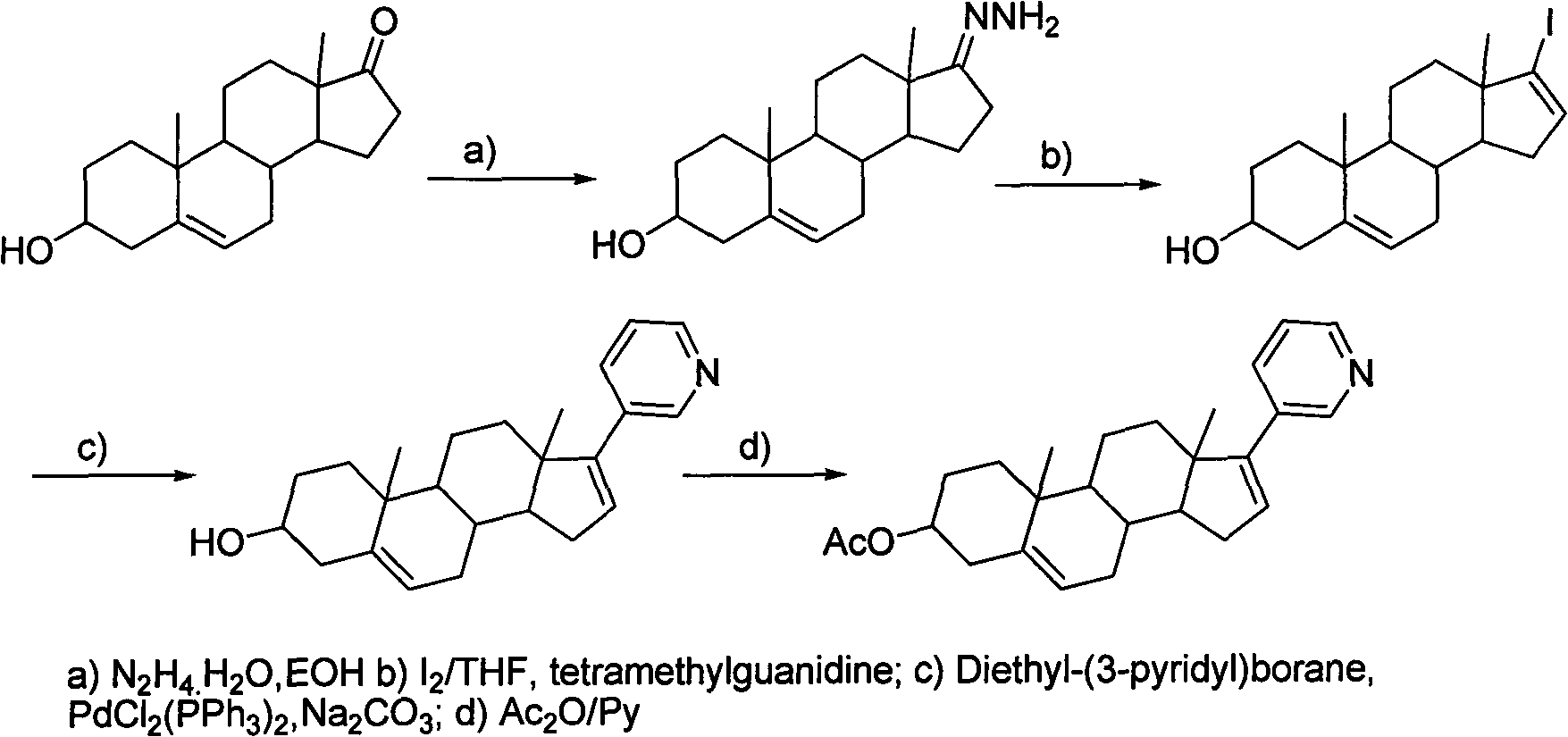
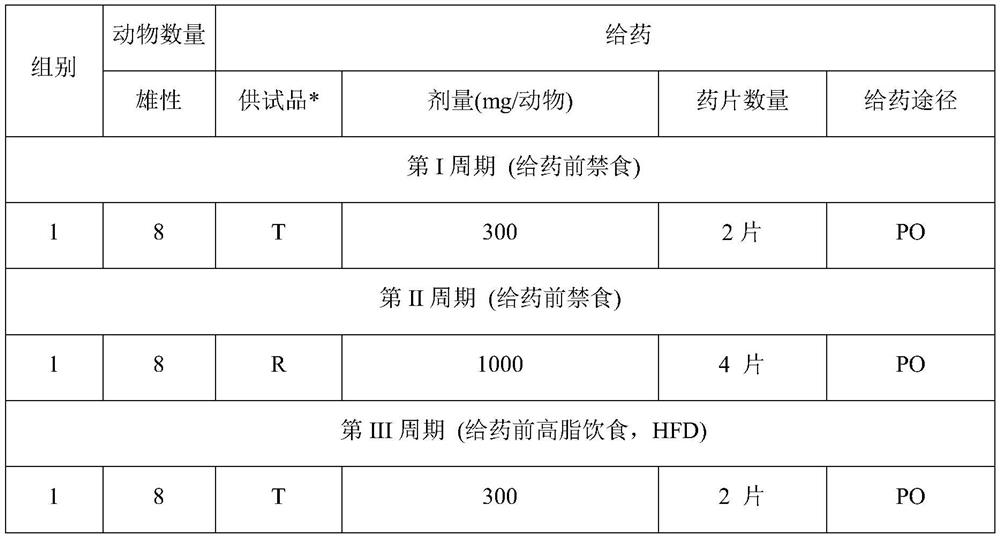
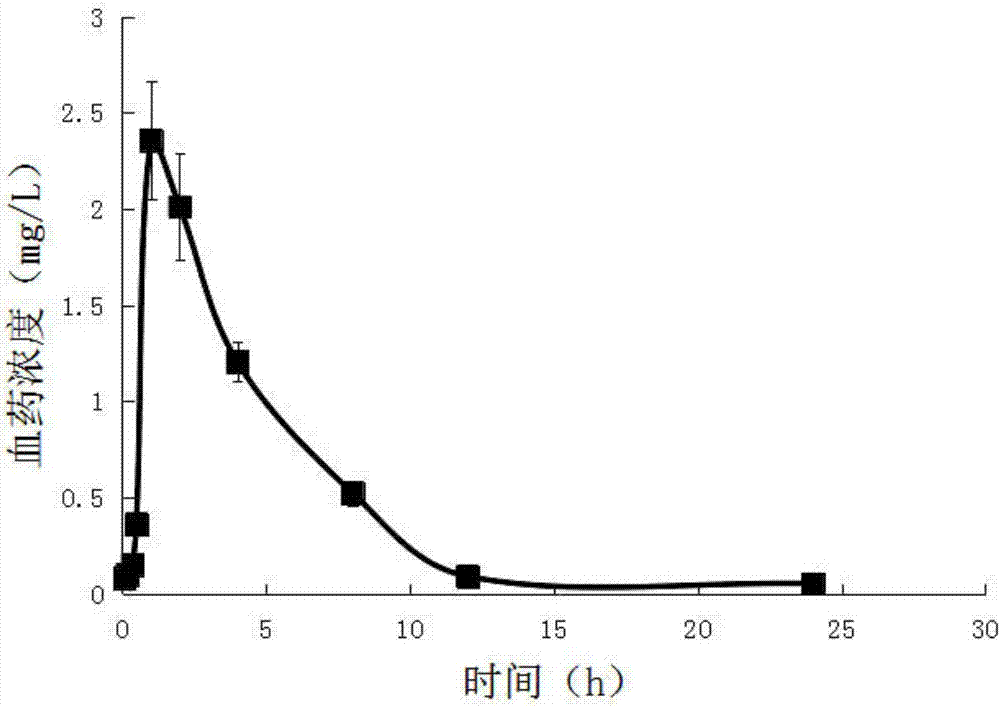
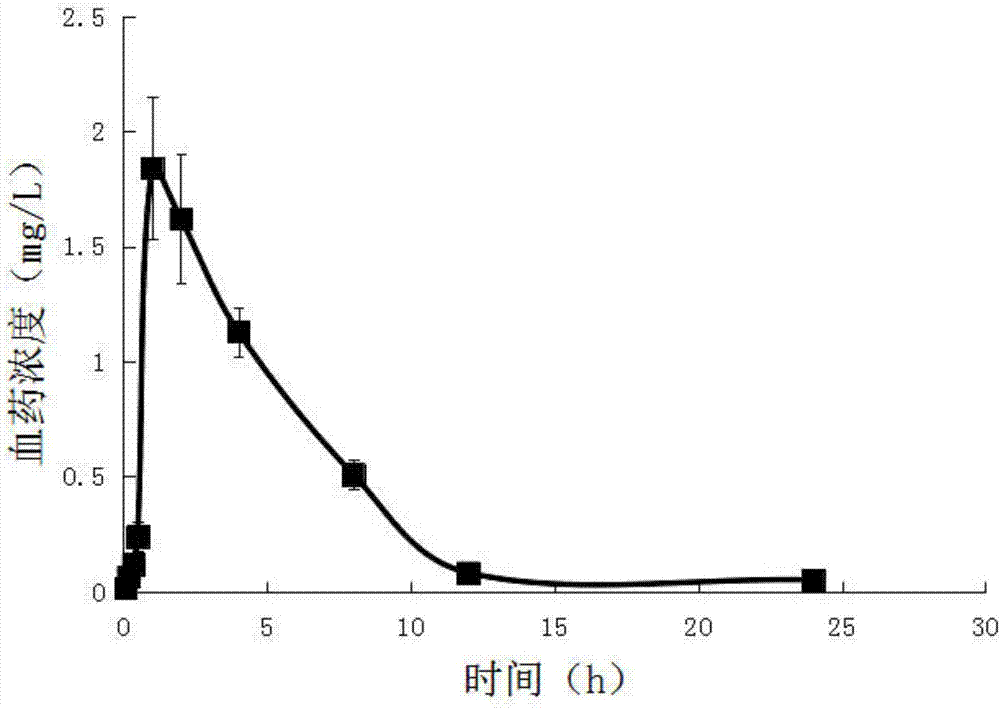
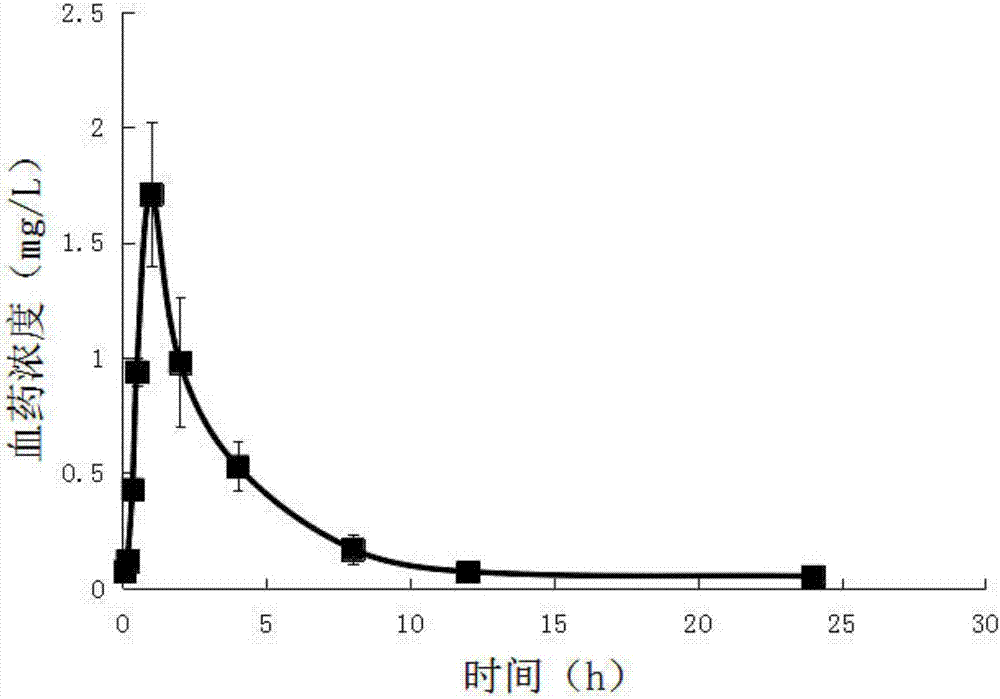
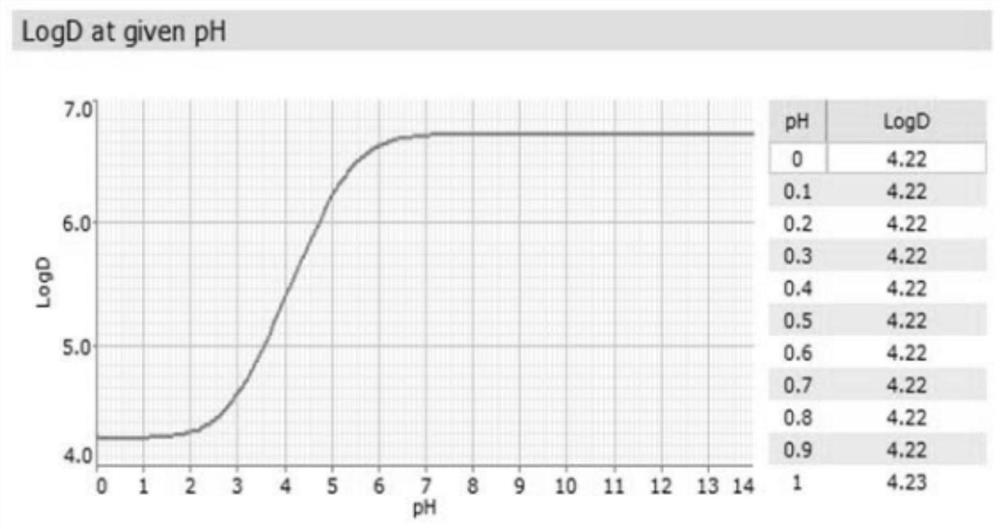
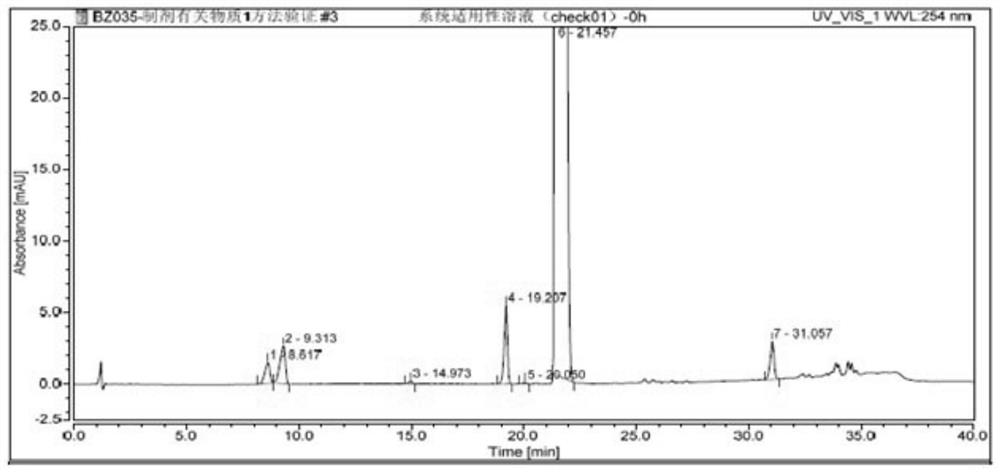
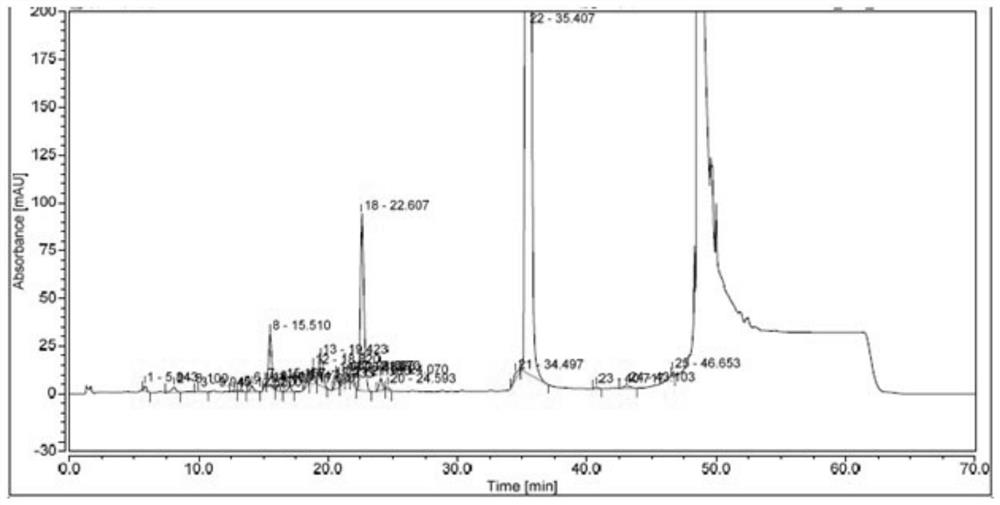
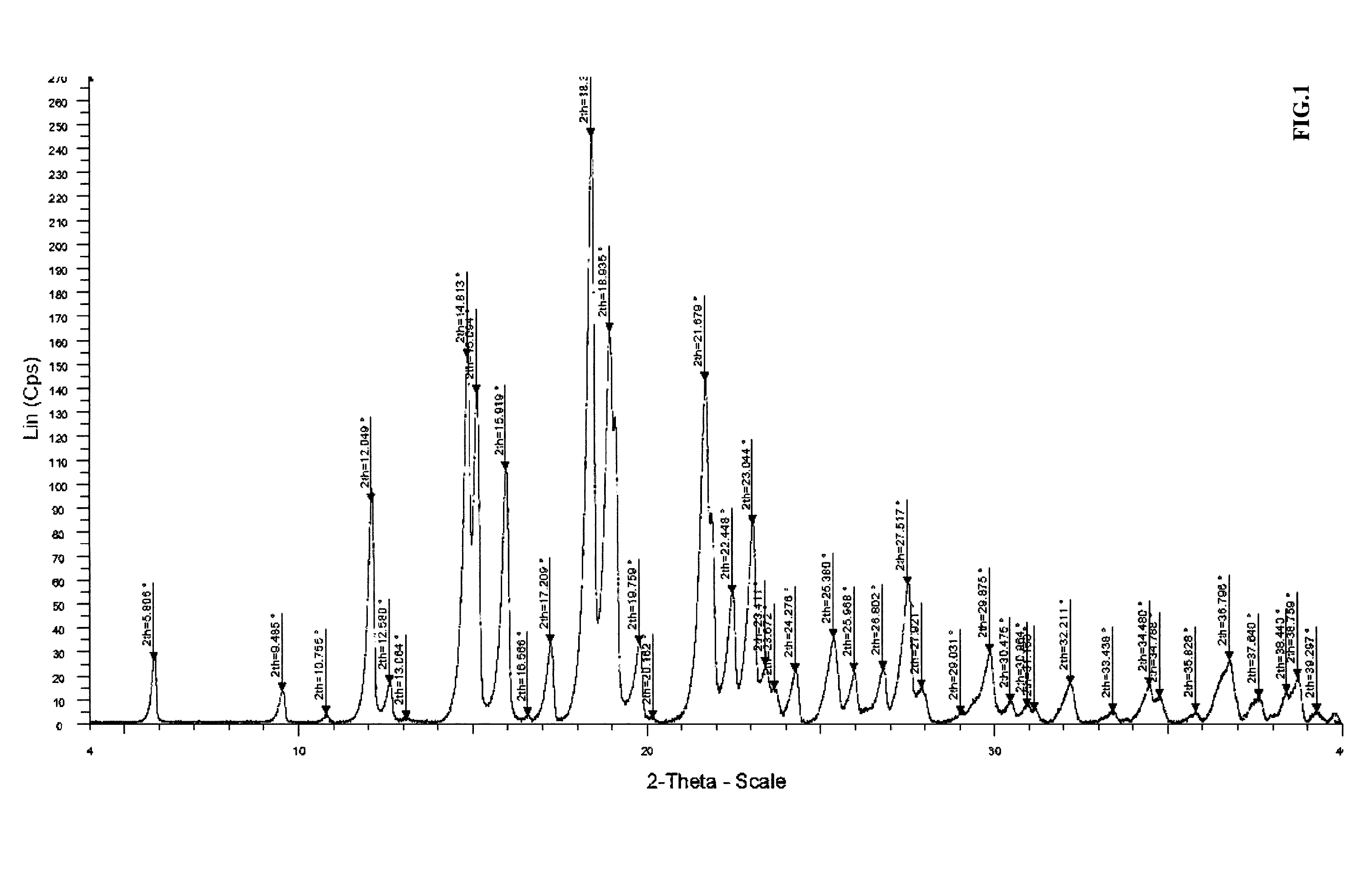
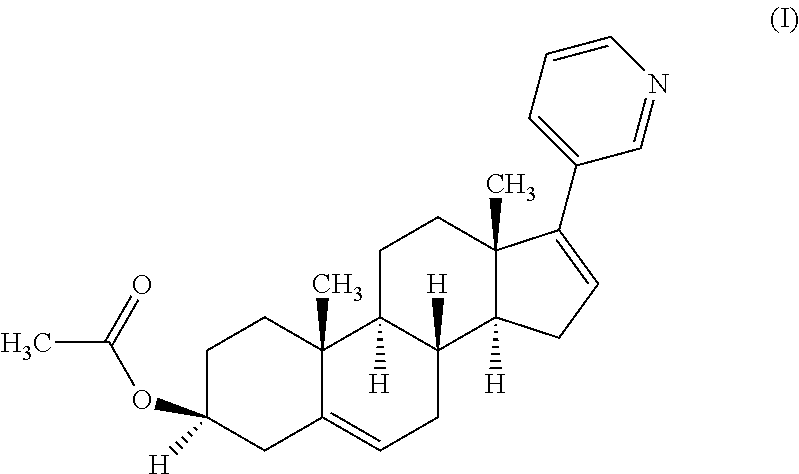
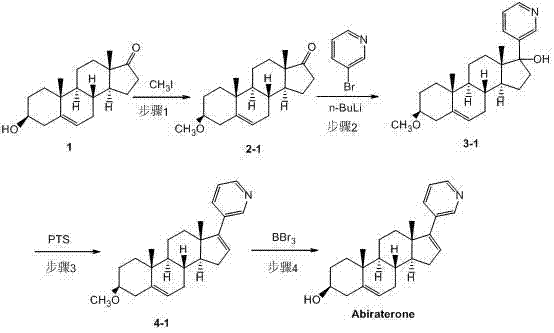
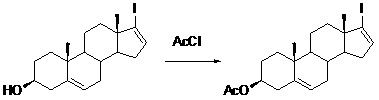Patents
Literature
110 results about "Abiraterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
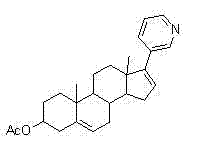
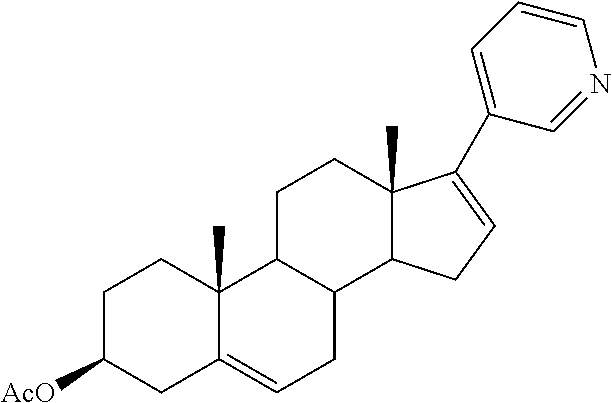
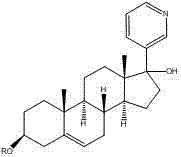
This medication is used along with prednisone to treat men with prostate cancer that has spread to other areas of the body.
Oral solid composition of abiraterone and preparation method thereof
The invention relates to an oral solid composition of abiraterone and a preparation method thereof. The composition contains abiraterone acetate with a particle size of 1-30 micrometers and pharmaceutic adjuvants.
Owner:CHONGQING PHARMA RES INST
Method for preparing abiraterone acetate
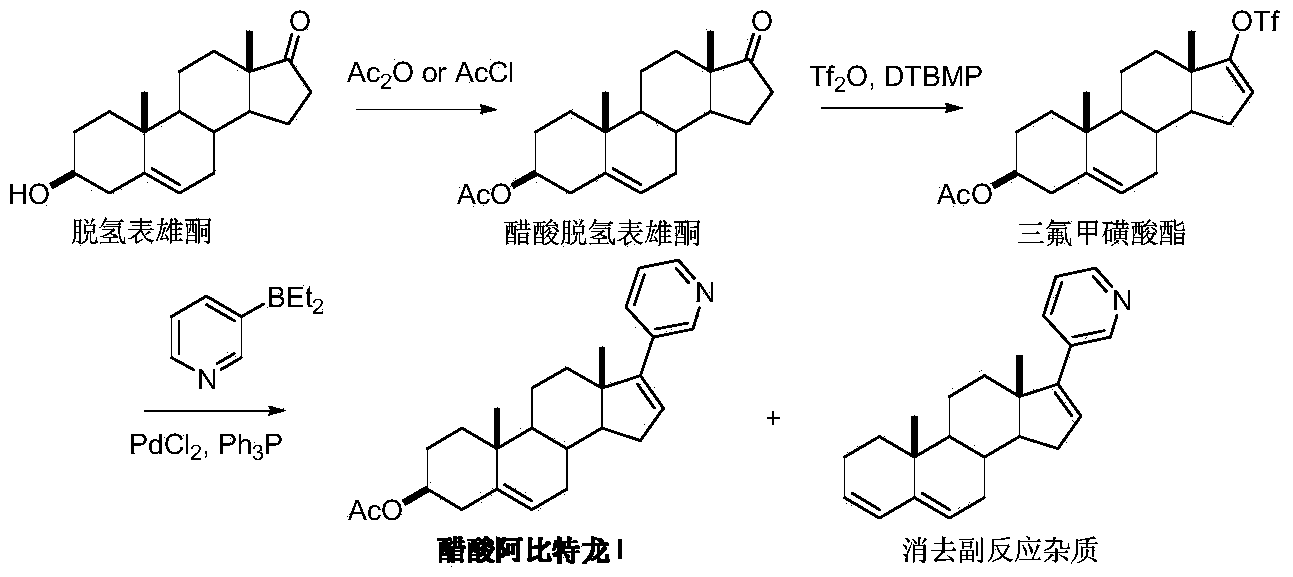
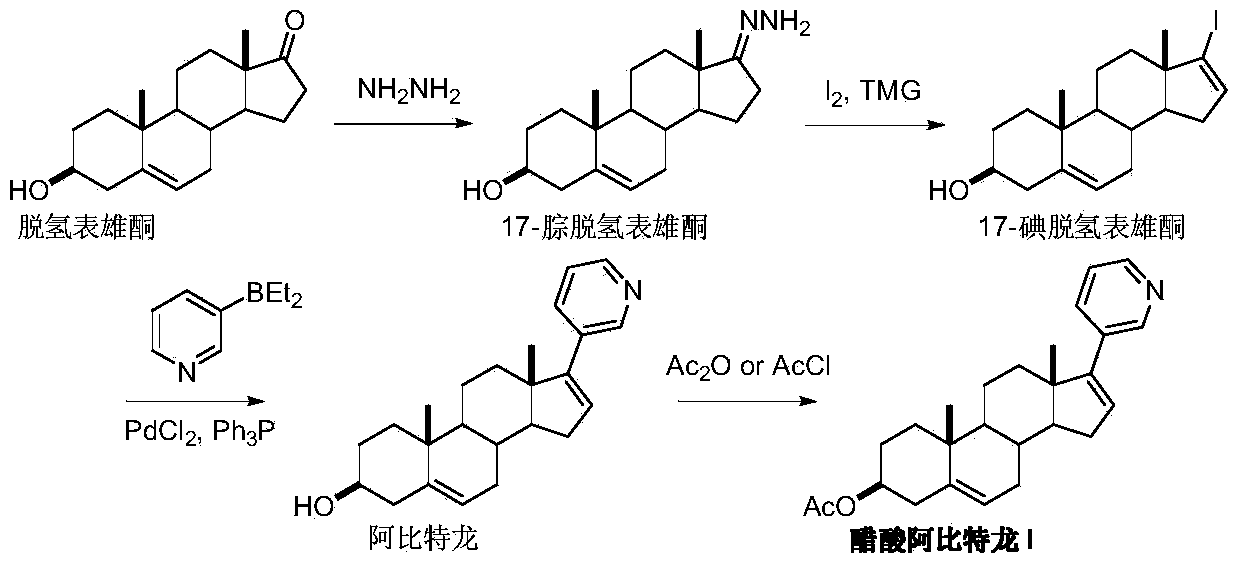
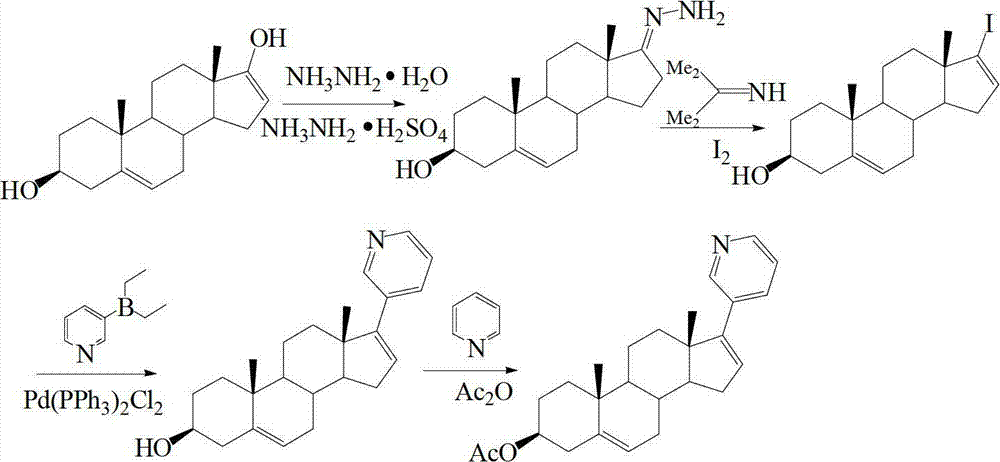
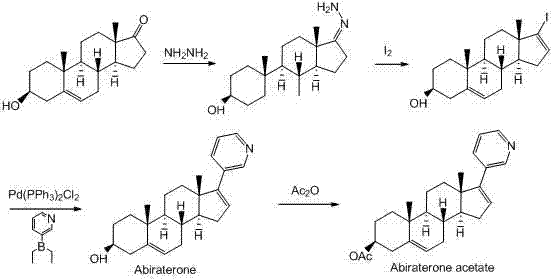
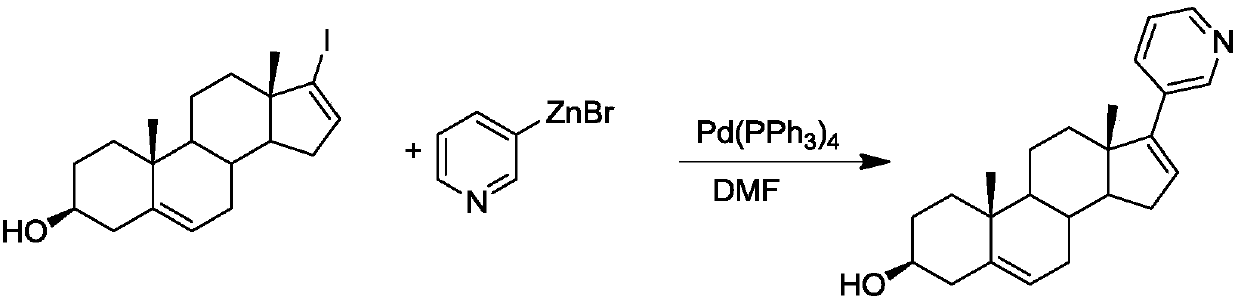
The invention discloses a convenient, rapid and economical method for massively preparing abiraterone acetate. According to the method, dehydroepiandrosterone acetate is used as a starting material, a keto carbonyl group is converted to hydrazone, and the abiraterone acetate is directly synthesized through iodination and Suzuki coupling reaction. According to the method, the operation is simple and convenient, the total yield of the three-step reaction is 34.7%, the product purity is 98.5%, the column chromatography separation purification is not required in the postprocessing, and the method is suitable for industrial production.
Owner:SUN YAT SEN UNIV
Abiraterone acetate crystal form and preparation method thereof
The invention relates to a new crystal form of a medicament, namely abiraterone acetate for treating prostatic cancer and a preparation method thereof, a pharmaceutical composition containing the new crystal form, and application of the new crystal form in preparing medicines for treating prostatic cancer.
Owner:CHONGQING PHARMA RES INST
Preparation method of abiraterone acetate
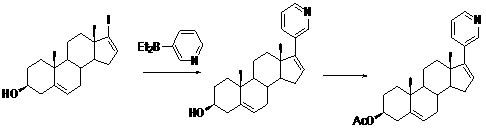
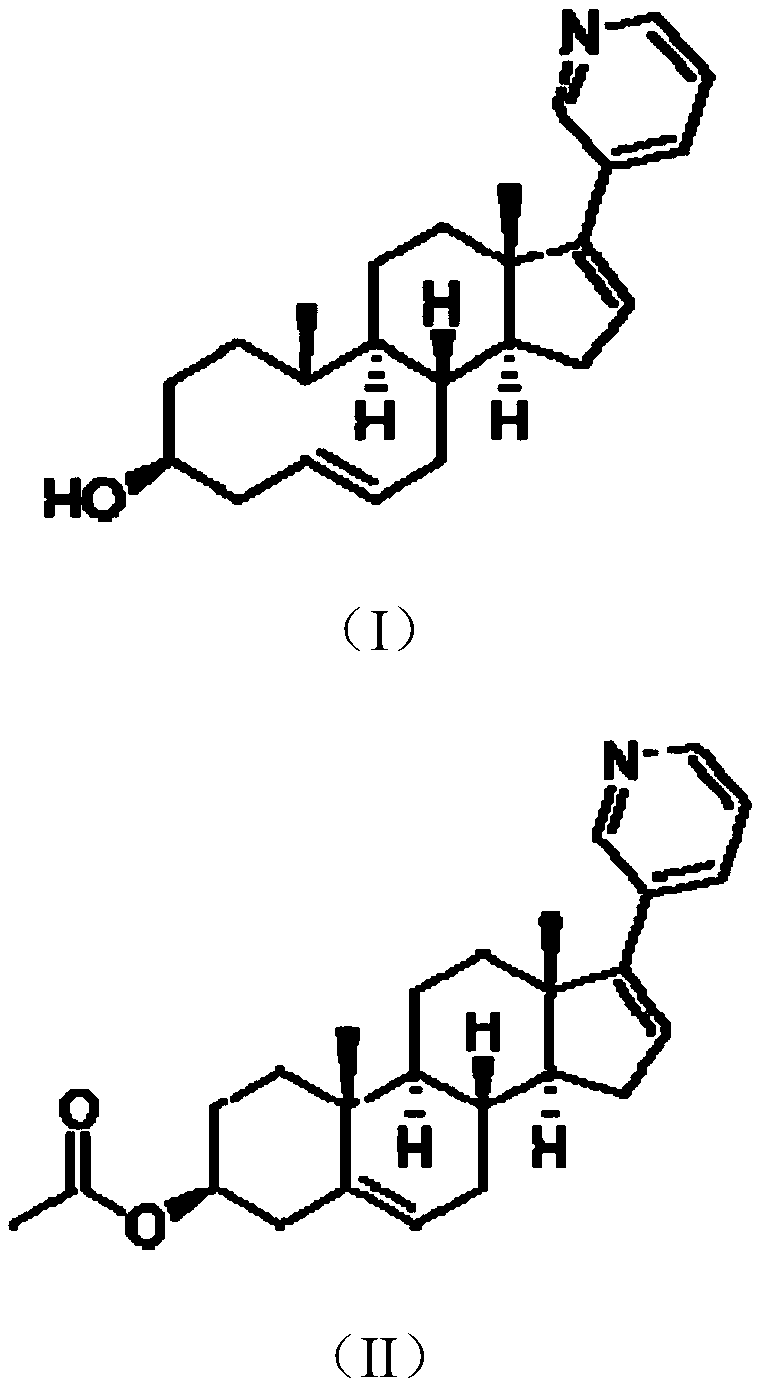
The invention belongs to the filed of medical technology, and particularly discloses a preparation method of abiraterone acetate, which comprises the following steps that compound I is acetylated to obtain compound II in an organic solvent, and compound II is coupled with halogenated pyridine to obtain the target product. The invention provides a preparation method of abiraterone acetate which can reduce the cost.
Owner:北京万全阳光医药科技有限公司
Abiraterone oral spray and use and preparation methods thereof
InactiveCN105055314ACorrectly designedTake a small doseOrganic active ingredientsAerosol deliveryPolyoxyethylene castor oilMetabolite
The invention relates to the technical field of pharmaceutical preparations and particularly relates to an abiraterone oral spray. The abiraterone oral spray comprises the following components in percent by weight: abiraterone acetate, an oil phase, a surfactant, a co-surfactant and the balance of water, wherein the oil phase is used as a carrier for prompting system micro-emulsification, and the oil phase is medium chain triglyceride, ethyl oleate or oleic acid; the surfactant is used for improving the performance of the abiraterone oral spray, expanding the range of application and realizing solubilization, and the surfactant is polyoxyethylene 40 hydrogenated castor oil or polyoxyethylene 35 castor oil; and the co-surfactant is used for reducing the surface tension of liquid and enhancing the emulsifying, moistening and blistering effects, and the co-surfactant is ethanol, n-butyl alcohol or propylene glycol. The spray has the advantages that the taking dose is reduced, so that the manufacturing cost is lowered; the formation of invalid metabolites is reduced, so that adverse reactions are effectively reduced; and the drug effect of the spray is longer than that of an oral drug.
Owner:HANGZHOU ANDE TECH CO LTD
Method for purifying abiraterone acetate
ActiveCN104447934ASolve processing problemsSuitable for industrial productionSteroidsAcetic anhydrideOrganic base
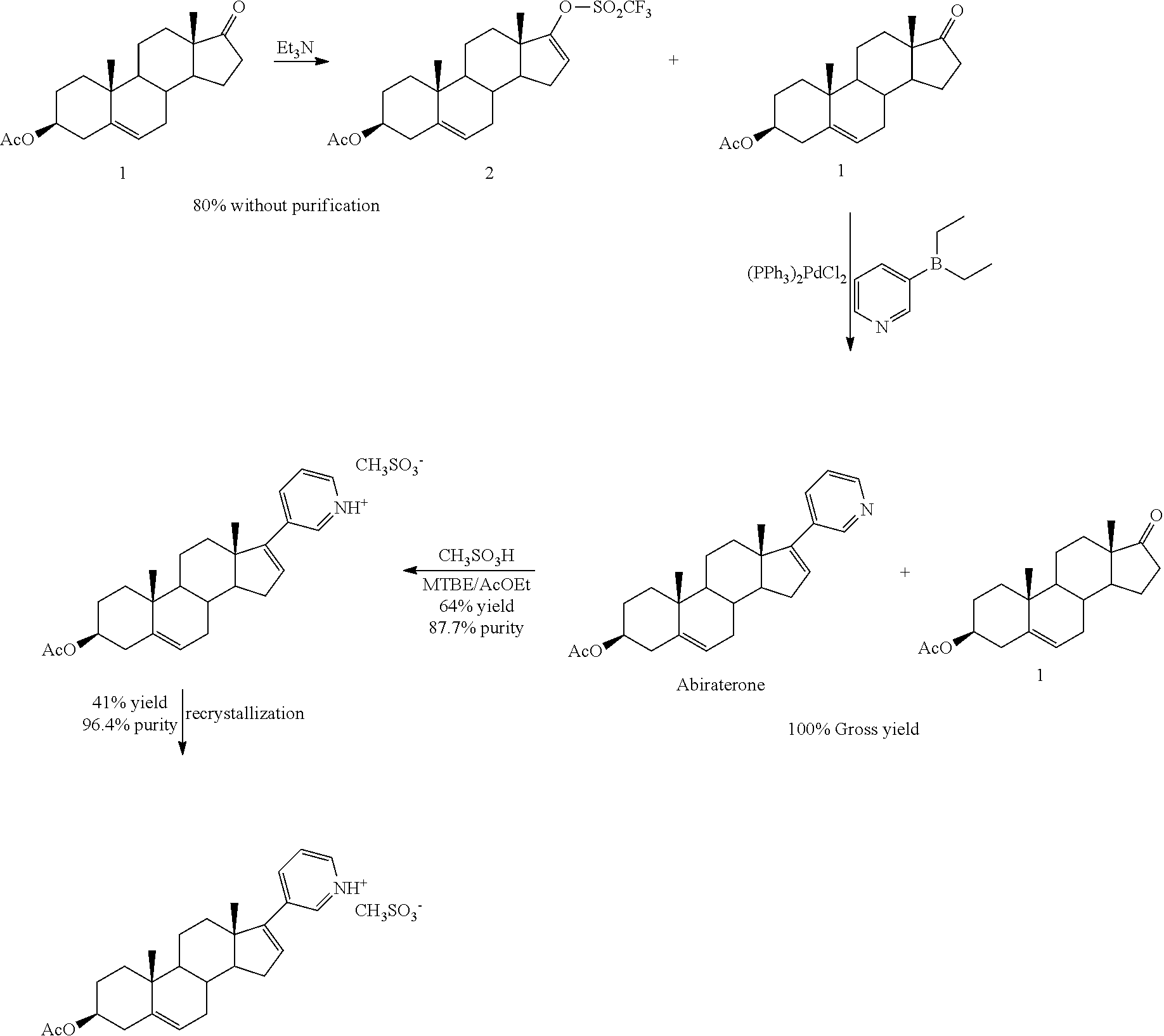
The invention belongs to the technical field of medicine synthesis, and provides a method for purifying abiraterone acetate. The method comprises the following steps: in the presence of acetic anhydride and organic bases, dissociating abiraterone acetate salt into free abiraterone acetate, wherein the high-performance liquid chromatography (HPLC) purity of dissociated abiraterone acetate is more than 99%; and then refining abiraterone acetate by virtue of acetonitrile recrystallization to obtain abiraterone acetate which has the HPLC purity of more than 99.7% and can meet the medical standards, and ensuring that the content of hydrolysis impurity abiraterone is less than 0.02%. By adopting the method provided by the invention, the problem that inevitable hydrolysis impurity abiraterone exists in a salt dissociation process of abiraterone acetate can be solved, a purification process can be simplified, and reagents can be saved; and moreover, the method is easy to operate, and is suitable for industrial production.
Owner:SHENZHEN KEXING PHARM CO LTD
Purification method of abiraterone
The invention discloses a purification method of abiraterone. The method comprises the following steps: salifying an abiraterone crude product and acid, recrystallizing in an appropriate solvent, and adding alkali for ionization to obtain the abiraterone pure product, of which the HPLC (high performance liquid chromatography) purity is higher than 99.5% and the single impurity content is less than 0.1%. The invention has the advantages of simple technique and high crystallization yield, and is easy to control.
Owner:成都伊诺达博医药科技有限公司
Methods for preparing abiraterone acetate and intermediate thereof
The invention relates to a method for preparing abiraterone which is a key intermediate of abiraterone acetate serving as a medicament for treating prostatic cancer and a method for preparing the abiraterone acetate by using the intermediate thereof. The methods are simple in processes, and the products have high purity and low content of impurities.
Owner:CHONGQING PHARMA RES INST
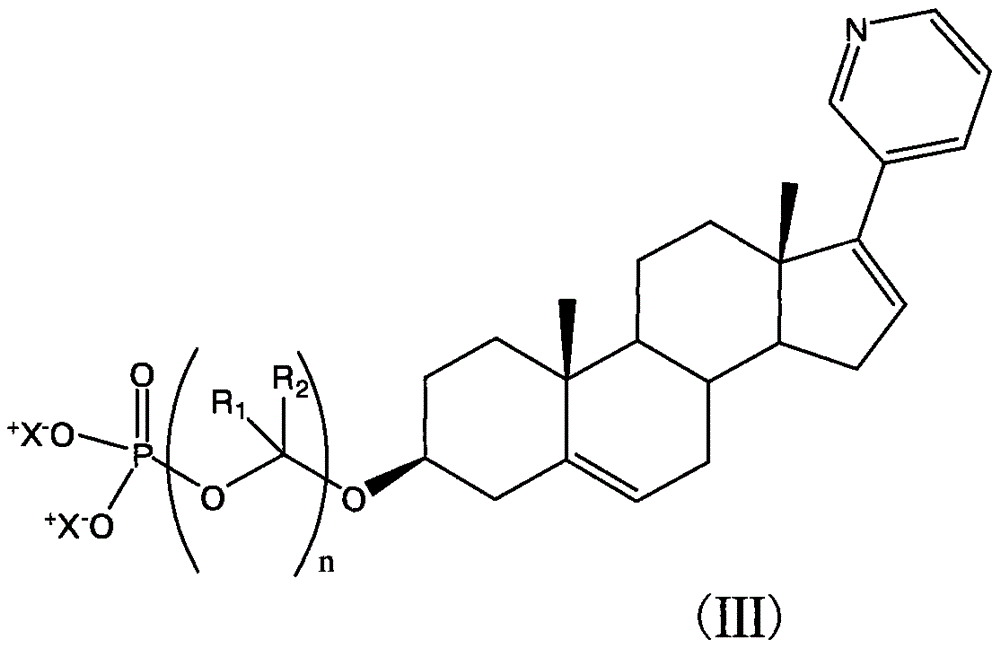
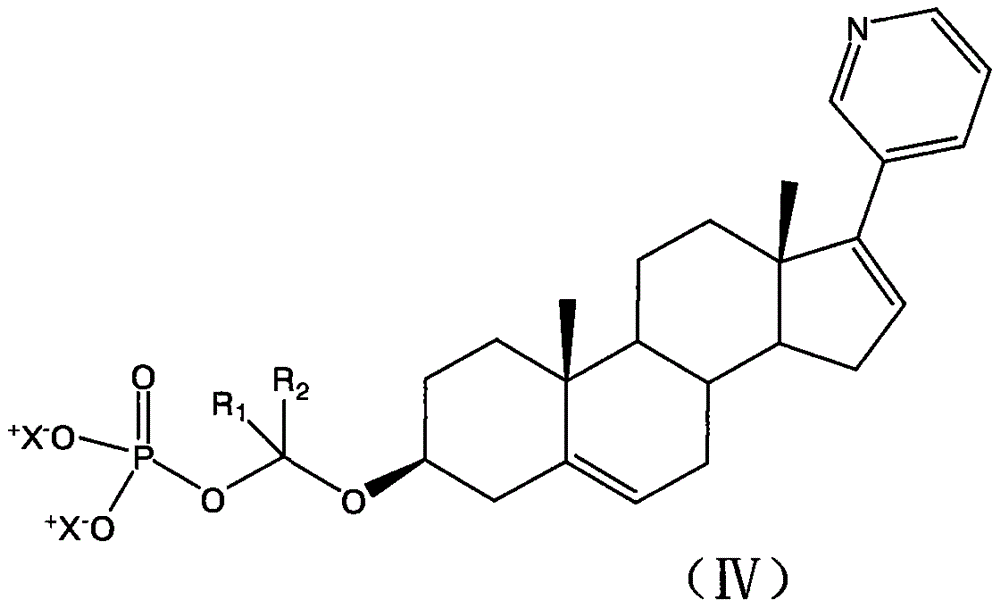
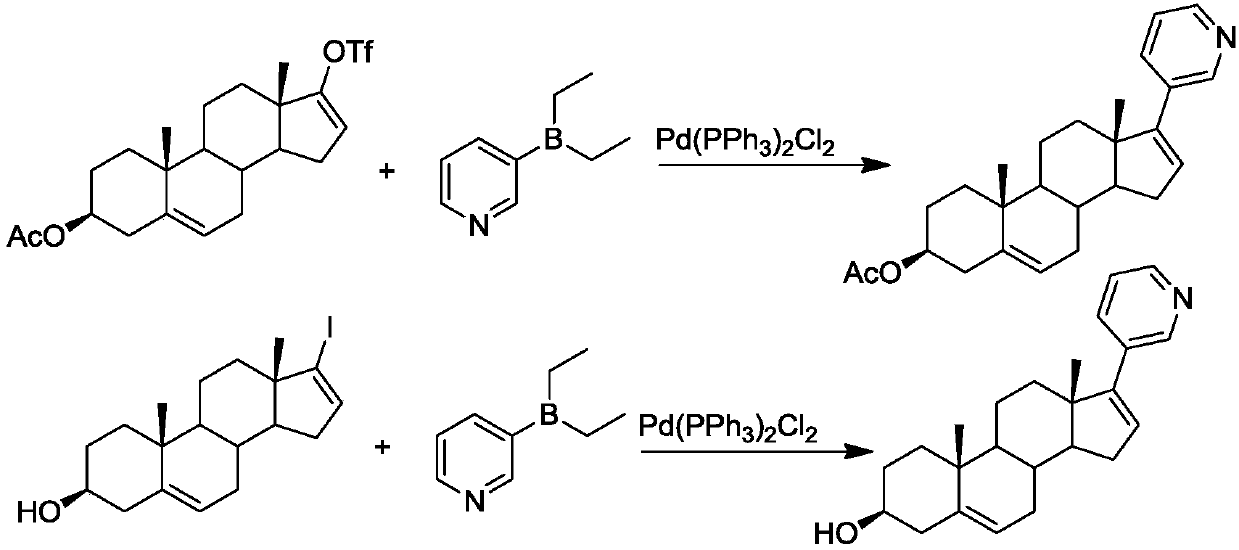
Synthesis of abiraterone and related compounds
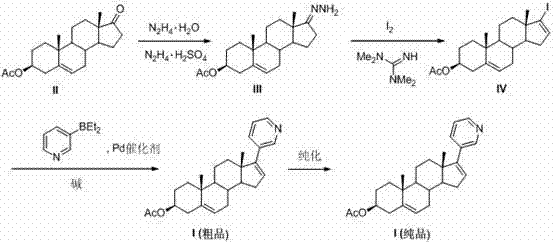
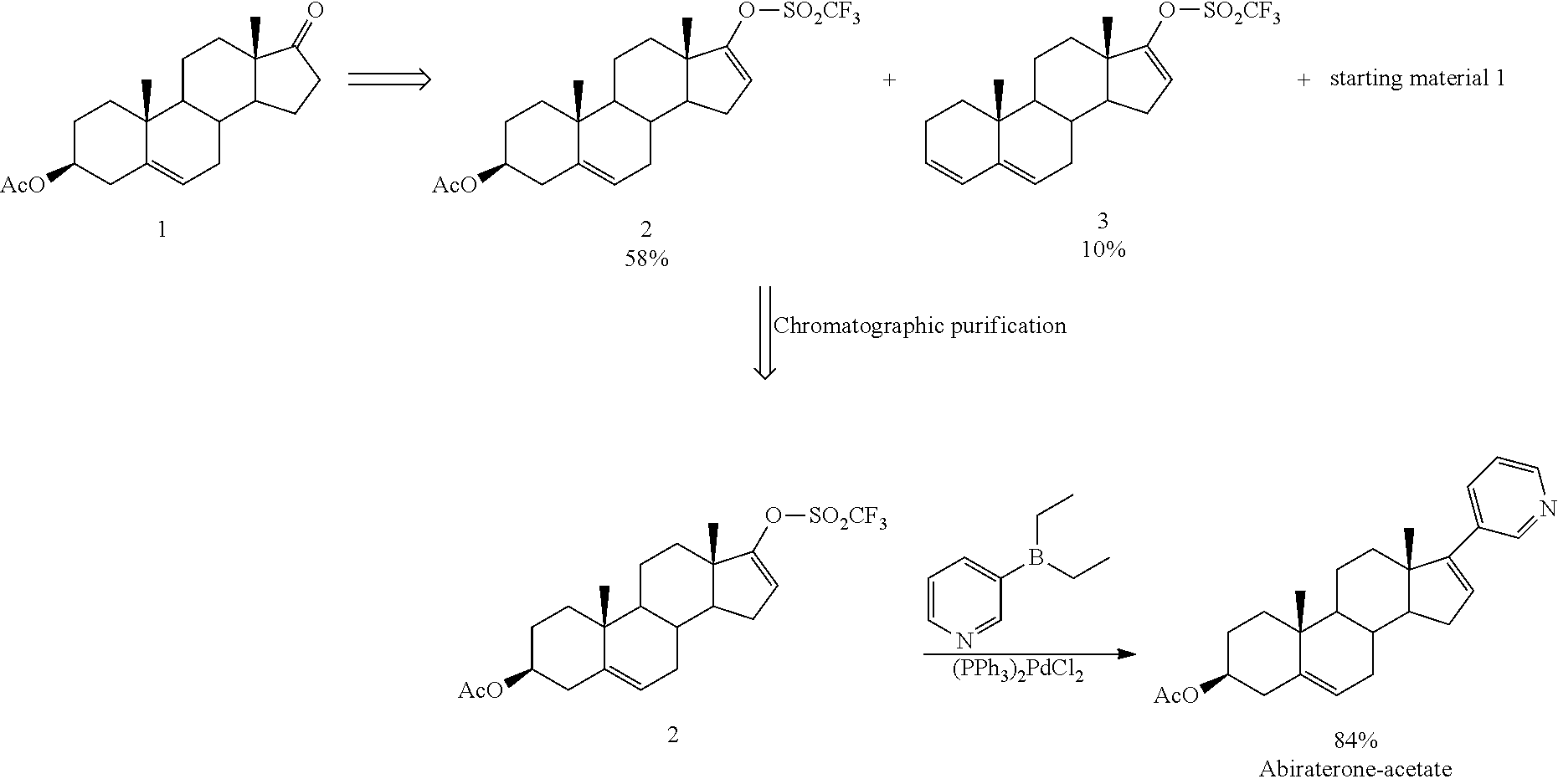
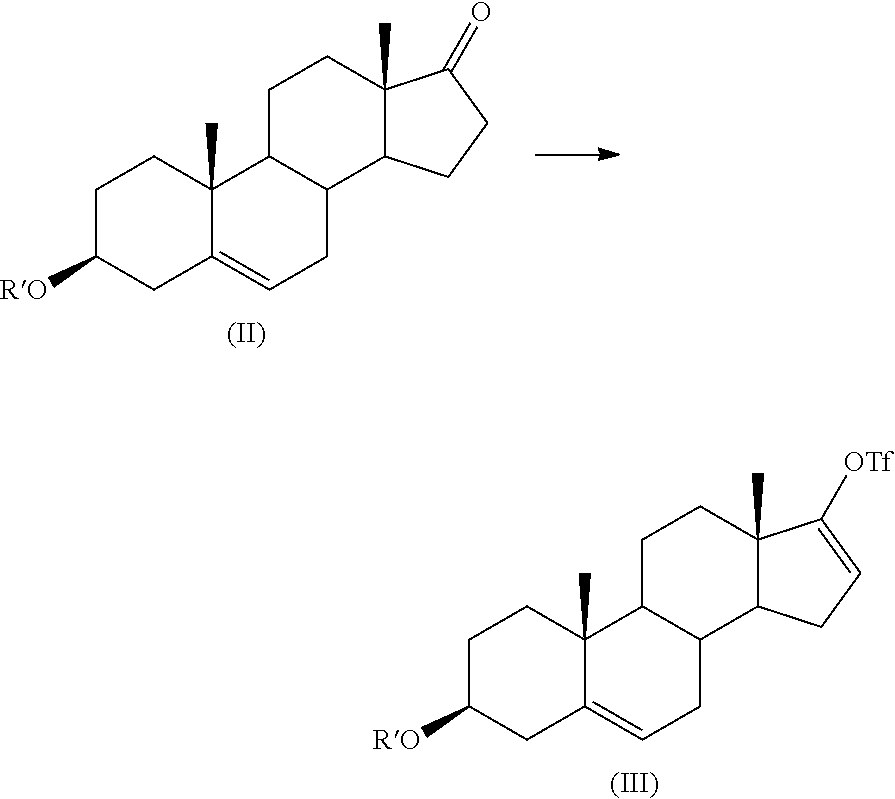
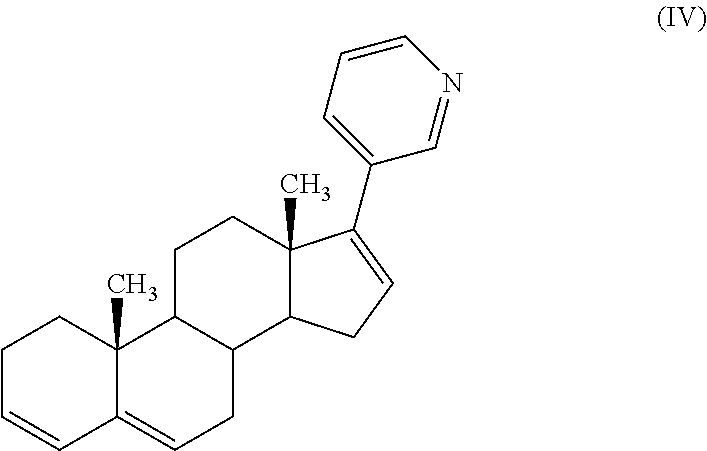
The present invention relates to processes for obtaining abiraterone and derivatives thereof, such as abiraterone acetate, by means of a Suzuki coupling through a steroid borate of general formula (IV) or a C—C coupling through a steroid hydrazone of general formula (II), as well as to intermediates useful in said processes.
Owner:CRYSTAL PHARMA SA
Preparation method of abiraterone acetate
ActiveCN103450313AHigh selectivityPromote the development of economy and technologySteroidsBurgess reagentDehydroepiandrosterone acetate
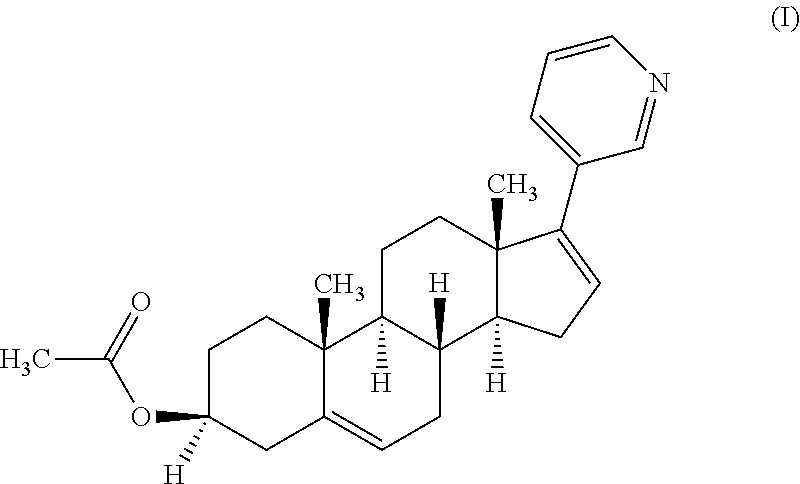
The invention discloses a preparation method of abiraterone acetate (I). The preparation method comprises the following steps of: based on dehydroepiandrosterone acetate as a raw material, performing addition reaction with 3-pyridyllithium to generate 17-(3-pyridyl)-17-hydroxy-androst-5-ene-3 beta-ol acetate (II); performing elimination reaction on the intermediate (II) under the action of a Burgess reagent to get abiraterone acetate (I). The preparation method has the advantages of simple process, easiness in obtainment of raw materials and controllable quality, and is suitable for industrial production.
Owner:南京广祺医药科技有限公司
Novel prodrug of steroid CYP 17 inhibitor as well as application and preparation method thereof
ActiveCN104017045AHigh doseGood antitumor activityOrganic active ingredientsSteroidsDiseaseAbiraterone
The invention discloses a precursor compound of a novel abiraterone drug, wherein the precursor compound provides improved oral bioavailability and drug dynamic characteristic. The drug is an irreversible inhibitor of human body CYP17 enzyme, can be used for treating urogenital system or androgen-associated cancers, diseases or sickness, for example, human prostate cancer, breast cancer and prostatic hyperplasia.
Owner:广州艾格生物科技有限公司
Stable abiraterone oral solid medicinal composition and preparation method thereof
InactiveCN103800296AImprove stabilitySuitable for long term storageOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneEthylic acid
Owner:CHONGQING PHARMA RES INST
Abiraterone oral emulsion and preparation method thereof
PendingCN111012745AHigh drug loadingImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneDigestion
The invention discloses an abiraterone oral emulsion and a preparation method thereof. The preparation comprises an active component, a solubilizer, an emulsifier and an antioxidant; the active component is abiraterone acetate or abiraterone; and the preparation comprises, by weight, 2%-10% of abiraterone acetate or abiraterone, 20%-70% of the solubilizer, 20%-40% of the emulsifier and 0.01%-1% ofthe solubilizer. According to the invention, the drug loading capacity and the stability of the abiraterone oral emulsion are high; and the abiraterone oral emulsion can be spontaneously emulsified to form an emulsion when meeting water. And the formed emulsion is stable; the digestion influence is low; the dissolution of medicines in gastrointestinal tracts is improved; the absorption of the medicines is promoted; and the bioavailability is improved.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
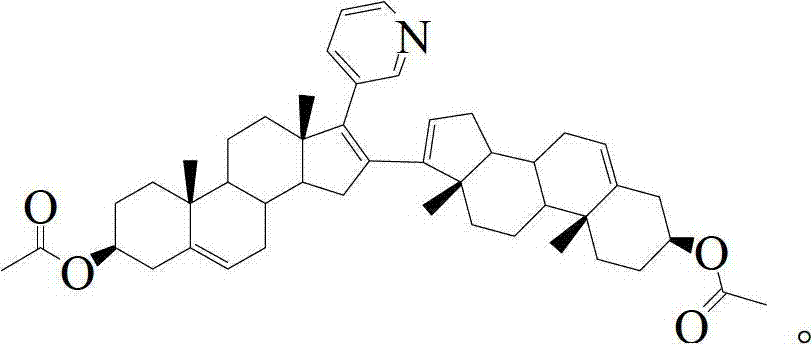
Preparation and detection method of abiraterone Acetate dimer compound
The invention discloses a preparation and detection method of an abiraterone acetate dimer compound, and relates to the of field pharmacy. The dimer compound is 3 beta-acetyl-16-(3 beta-acetyl androstane-5,16-diene-17-base)-17-(3-pyridyl) androsterone-5,16-diene. The method is as follows: adding compounds I and II into a reactor; dissolving the compounds and adding a palladium catalyst and alkali into the reactor; cooling to room temperature; filtering; concentrating; adding water; extracting; concentrating to obtain a compound III crude product; placing the crude product in the reactor; adding pyridine and an acetyl compound; stirring at room temperature till complete reaction; concentrating to remove pyridine and the acetyl compound; adding water, extracting and concentrating to obtain a dimer compound crude; and dissolving the dimer compound crude, washing, drying, filtering, concentrating and recrystallizing to obtain the abiraterone acetate dimer compound. According to high performance liquid chromatography detection and calculation by an area normalization method, the prepared dimer compound has a purity up to 98% and a yield higher than 80%.
Owner:武汉长联来福制药股份有限公司
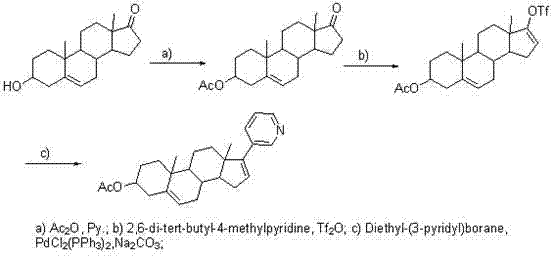
Novel synthesis method of Abiraterone acetate
The invention discloses a novel synthesis method of Abiraterone acetate. The synthesis method replaces expensive pyridine-3-diethylborane with pyridine-3-bronic acid, which is available in the market and can be easily synthesized, to carry out the Suzuki coupling reaction so as to synthesize Abiraterone ester in a high yield. The novel synthesis method of Abiraterone has the advantages of lower raw material cost, simple technology, controllable reaction conditions, and high reaction yield.
Owner:成都伊诺达博医药科技有限公司
Abiraterone derivative with anti-cancer effect
InactiveCN104974212AImprove solubilityImprove bioavailabilitySteroidsAntineoplastic agentsSolubilityAbiraterone
The invention provides an abiraterone derivative with an anti-cancer effect. The general structural formula of the abiraterone derivative is as shown in the description. The abiraterone derivative can ensure improvement of biophysics properties such as abiraterone solubility and bioavailability; the stability of the abiraterone derivative is maintained very well during pharmaceutical manufacturing and drug administration; and after drug administration, the abiraterone derivative can be degraded and release active compounds; and the abiraterone derivative has the characteristics of low toxicity and the like.
Owner:BEIJING LANG REBONE TECH CO LTD
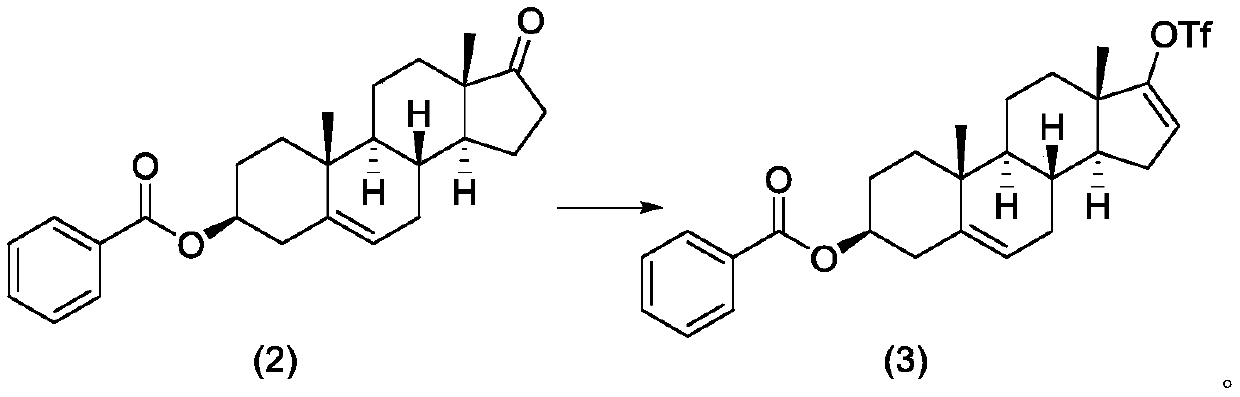
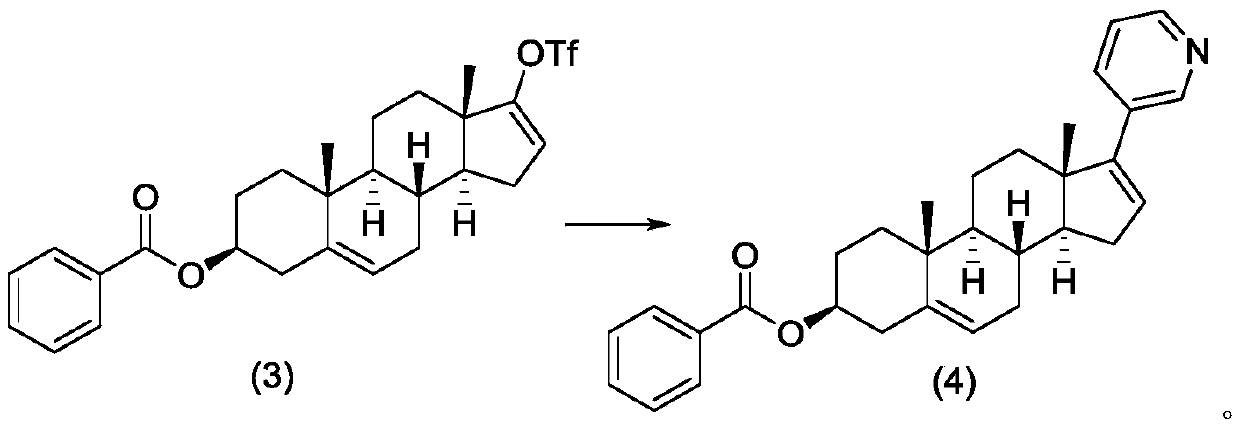
Method for preparing abiraterone acetate
The invention provides a method for preparing abiraterone acetate. Specifically, the invention relates to an improved method for synthesizing abiraterone or a derivative thereof through a key 3 beta-benzoyloxy intermediate. According to the process, intermediate DHEA 3-benzoyloxy ester is a solid, the intermediate with higher purity can be obtained through a crystallization method, and the processoperability is high. Meanwhile, benzoyl is strong in electric negative force, easy to react with hydroxyl and high in acylation rate, and a six-membered ring structure is twisted in a space structureof a benzoyl functional group, so that elimination reaction is not easy to perform, and generation of process byproducts is effectively avoided.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
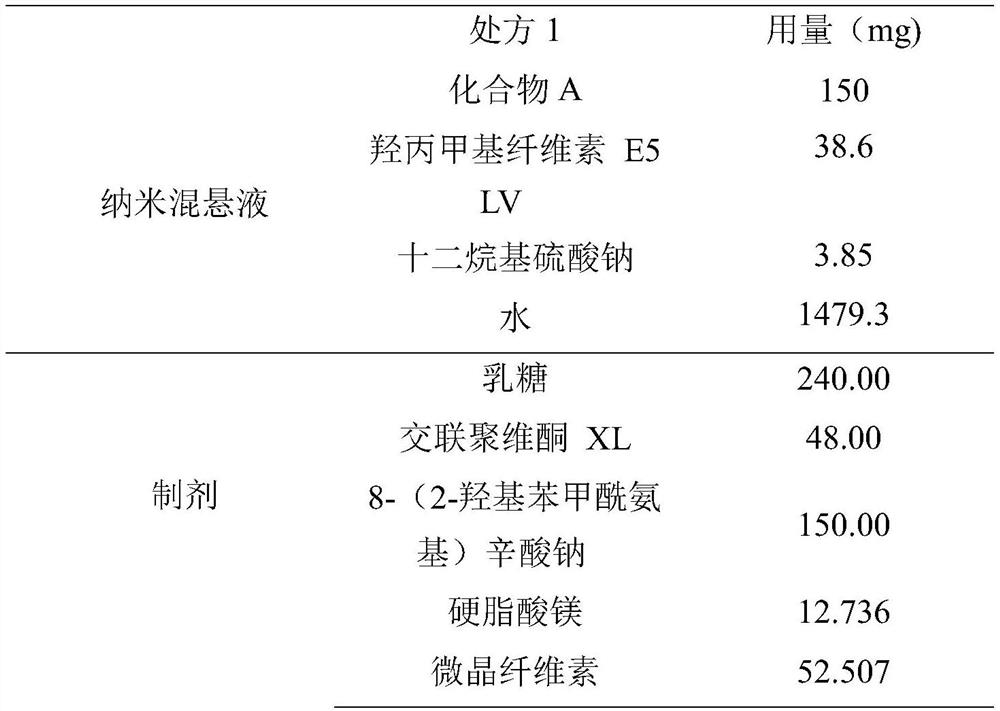
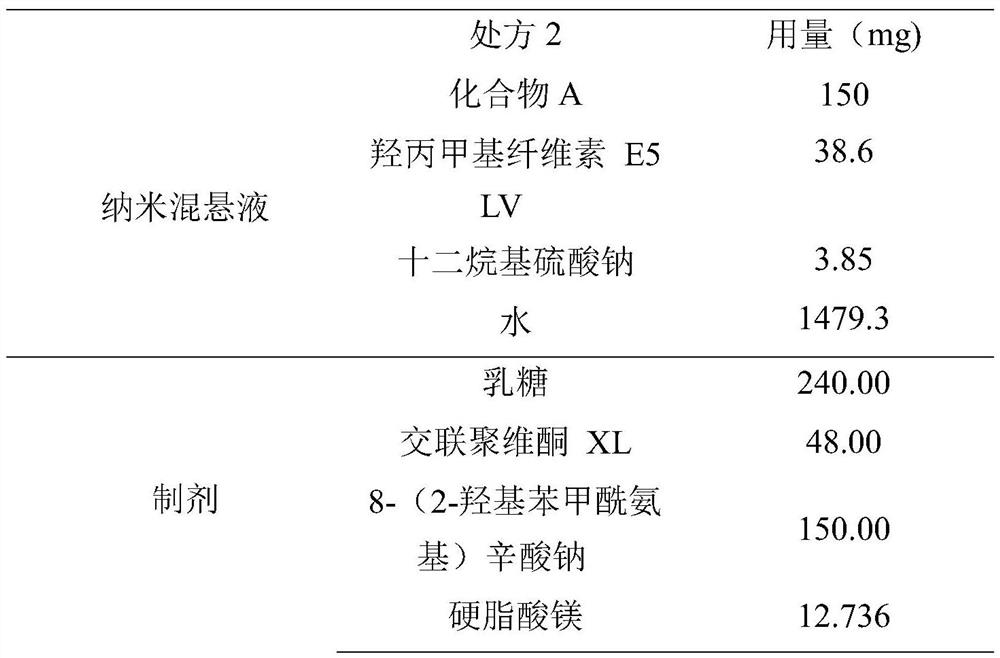
Method for preparing medical composition of abiraterone or derivative thereof, and application of medical composition
PendingCN111617258AImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneFluidized bed
The invention relates to a method for preparing a medical composition of abiraterone or a derivative thereof, and an application of the medical composition. Specifically, the method comprises the steps of mixing nanometer suspension containing the abiraterone or the derivative thereof with an absorption enhancer and one or more arbitrarily-chosen excipients, and performing granulation in a fluidized bed, wherein the particle diameter D90 value of the abiraterone or the derivative thereof is smaller than 1000nm, and is preferably 400-600nm. Compared with preparations sold in the market, the medical composition has the advantages that the biological availability is improved, and individual differences and the like of medicine administration patients are improved.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
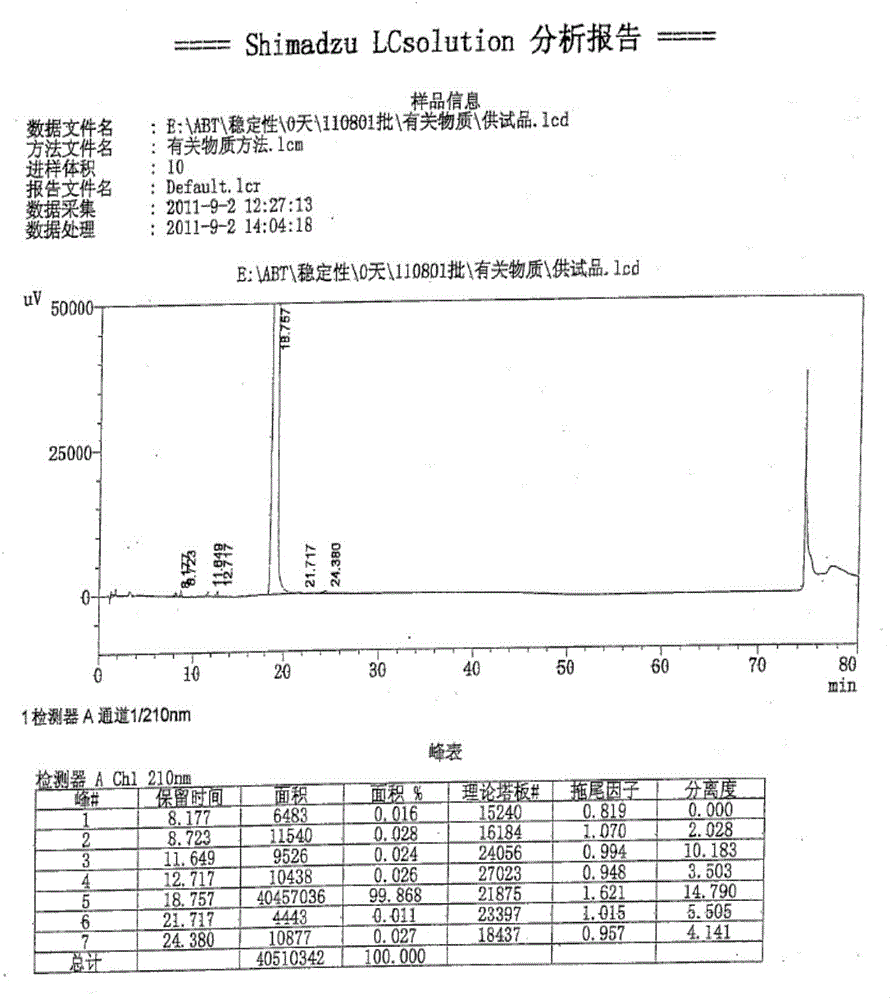
Method for quantitatively detecting abiraterone in whole blood
The invention discloses a method for quantitatively detecting abiraterone in whole blood. The method adopts whole blood as an object for detecting, and sequentially comprises the following steps: adding ethyl acetate to whole blood lysate, performing vortex shaking, and centrifuging; drying the ethyl acetate layer with nitrogen airflow by means of blowing, redissolving with 20% acetonitrile aqueous solution, centrifuging, and extracting supernatant I; weighing alkaline diatomite and putting into a glass chromatographic column, vibrating, adding the supernatant I, eluting with pure water, 20% methanol aqueous solution and chromatographic methanol sequentially, drying the eluent with nitrogen airflow by means of blowing, redissolving with 20% acetonitrile aqueous solution, centrifuging, extracting supernatant II, and analyzing with a high performance liquid chromatography system to obtain an abiraterone drug peak area Y, putting into equation Y equaling to 27524X plus 1956.6, wherein X is the abiraterone concentration of the supernatant II; and performing conversion to obtain the abiraterone concentration in the whole blood for detecting.
Owner:CHINA JILIANG UNIV
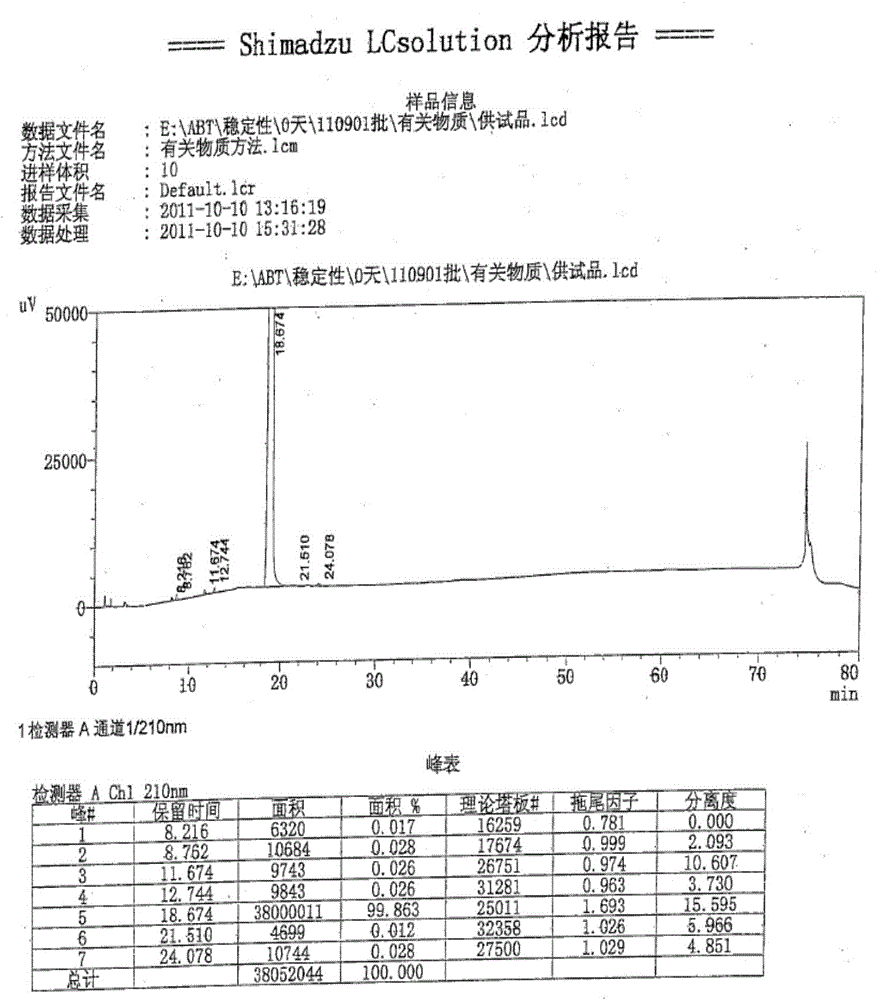
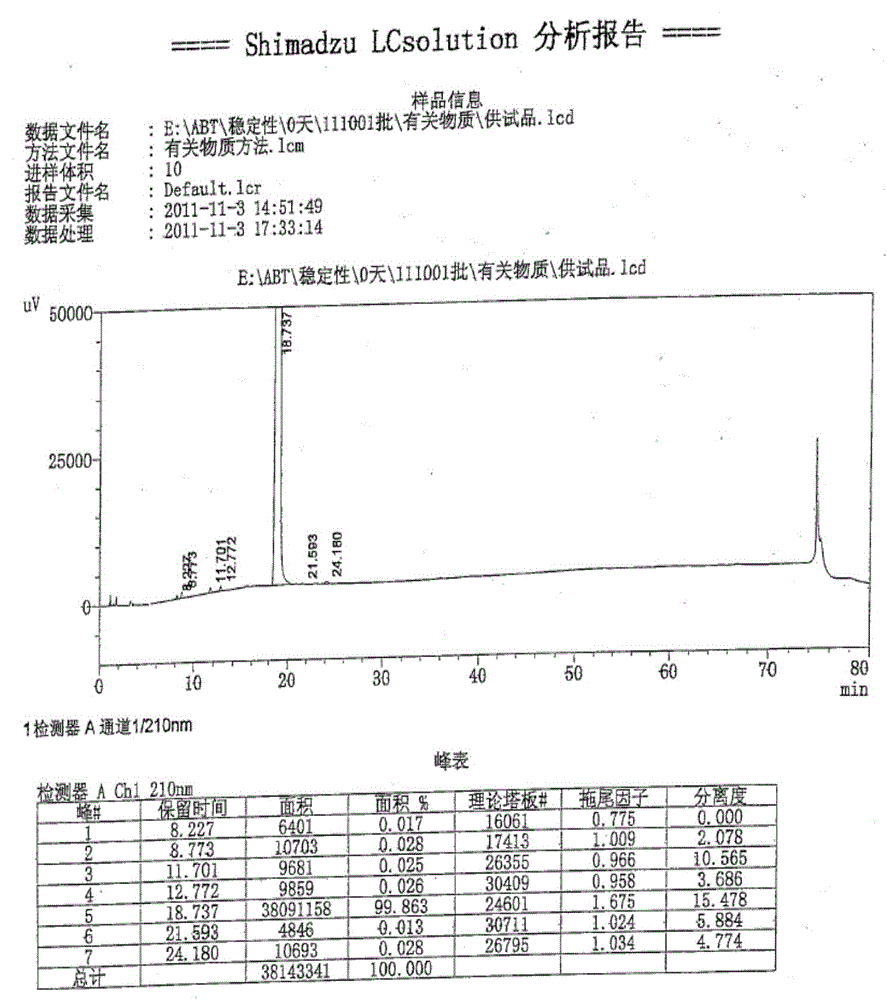
High performance liquid detection method of abiraterone acetate preparation
InactiveCN112964800AAvoid missing detectionLower reservationComponent separationO-Phosphoric AcidAbiraterone
The invention belongs to the field of medicine quality control, and particularly relates to a high performance liquid chromatography detection method of an abiraterone acetate preparation. According to the method, octadecylsilane chemically bonded silica is adopted as a filler, a 0.1% (V / V) phosphoric acid solution is adopted as a mobile phase A, acetonitrile is adopted as a mobile phase B, gradient elution is carried out, and the detection wavelength is 254 nm. The method comprises the following steps: preparing an abiraterone acetate test sample solution from an abiraterone acetate test sample and ethanol, respectively preparing a contrast solution and a sensitivity solution, respectively injecting samples, and calculating by adopting a correction factor contrast method.The detection method overcomes the defects of auxiliary material interference and too strong retention of main components and impurities, can be used for rapidly detecting abiraterone acetate impurities and degradation products, is simple to operate, has high sensitivity and good specificity, and is convenient to control the product quality.
Owner:江苏万高药业股份有限公司
Process for producing a solid form of abiraterone acetate
ActiveUS20160083416A1Synthetic resin layered productsCellulosic plastic layered productsAbirateroneImproved method
The present invention relates to a process for the preparation of 17-substituted steroids and, more particularly, to an improved method of preparing micro size abiraterone or derivatives thereof in high yield and purity by means of a spherical agglomeration process.
Owner:ZACH SYST SPA
Abiraterone acetate nanocrystal as well as preparation and preparation method thereof
ActiveCN112933053AImprove solubilityIncrease dissolution ratePowder deliveryOrganic active ingredientsAbirateroneEthylic acid
The invention belongs to the field of pharmaceutical preparations, and provides an abiraterone acetate nanocrystal as well as a preparation and a preparation method thereof. The preparation process of the abiraterone acetate nanocrystal comprises the following steps of: weighing an abiraterone acetate raw material medicine and a stabilizer in proportion; adding a proper amount of water, carrying out ultrasonic mixing uniformly; carrying out high-speed shearing on a solution; taking a lower-layer primary suspension; adding zirconium oxide grinding beads and stirrers, and carrying out medium grinding to obtain the abiraterone acetate nanocrystal. A freeze-drying protective agent can be added into the abiraterone acetate nanocrystal, and vacuum drying is carried out to obtain the abiraterone acetate nanocrystal freeze-dried powder. According to the abiraterone acetate nanocrystal as well as the preparation and preparation method thereof of the present invention, the solubility and the dissolution rate of the BCSIV drug abiraterone acetate are improved by using the nanocrystal technology; and the BCSIV drug abiraterone acetate can be prepared into the freeze-dried powder or tablets, such that the absorption of the BCSIV drug abiraterone acetate is increased, the problem of low bioavailability of the BCSIV drug abiraterone acetate is solved,and the drug cost is reduced.
Owner:CHINA PHARM UNIV
Abiraterone acetate soft capsule and preparation method thereof
PendingCN113616614AReduce solubilityEasy to storeOrganic active ingredientsCapsule deliverySoftgelAbiraterone
The invention discloses an abiraterone acetate soft capsule and a preparation method thereof. The abiraterone acetate soft capsule consists of a content and a capsule shell, wherein the content comprises abiraterone acetate, caprylic / capric acid mono / diglyceride, Tween 80, Span 80 and butylated hydroxyanisole; and the capsule shell is made of soft capsule materials and comprises gelatin, water, glycerol and a sorbitol and sorbitan solution, wherein the ratio of the gelatin to the water to the glycerol to the sorbitol and sorbitan solution is 1: 0.74: 0.35: 0.35. After the abiraterone acetate soft capsule provided by the invention is orally taken on the empty stomach, compared with an original research medicine Zytiga (the specification is 250mg), the step of dissolving out the original research medicine Zytiga in the digestive tract is not required, the oral bioavailability is improved to 12.5 times, variability among individuals is low, and the phenomenon that the exposure quantity of abiraterone is obviously increased due to postprandial administration of the original research medicine Zytiga is eliminated.
Owner:HANGZHOU HEZE PHARMA TECH +1
Synthesis method of abiraterone
ActiveCN103360458ALow priceEasy to getSteroidsBulk chemical productionOrganic solventGrignard reagent
The invention relates to a synthesis method of abiraterone. The synthesis method comprises the following steps of with dehydroepiandrosterone as a raw material, carrying out ether formation protection on hydroxyl by using a protecting group to obtain a compound; next, subjecting the compound, 3-bromopyridine and a Grignard reagent to reaction to obtain 3 beta-substituted oxo-17-(3-pyridyl)-androst-5-ene-17-ol; and heating the 3 beta-substituted oxo-17-(3-pyridyl)-androst-5-ene-17-ol, acid or a dehydrant and the like in an organic solvent, dehydrating, and then, removing the protecting group of the hydroxyl to obtain the abiraterone. The method provided by the invention has the advantages of simplicity and easiness in operation, adoption of low-toxicity raw materials and particular suitability for industrial production.
Owner:BRIGHTGENE PHARMA
Abiraterone derivative and preparation method and application thereof
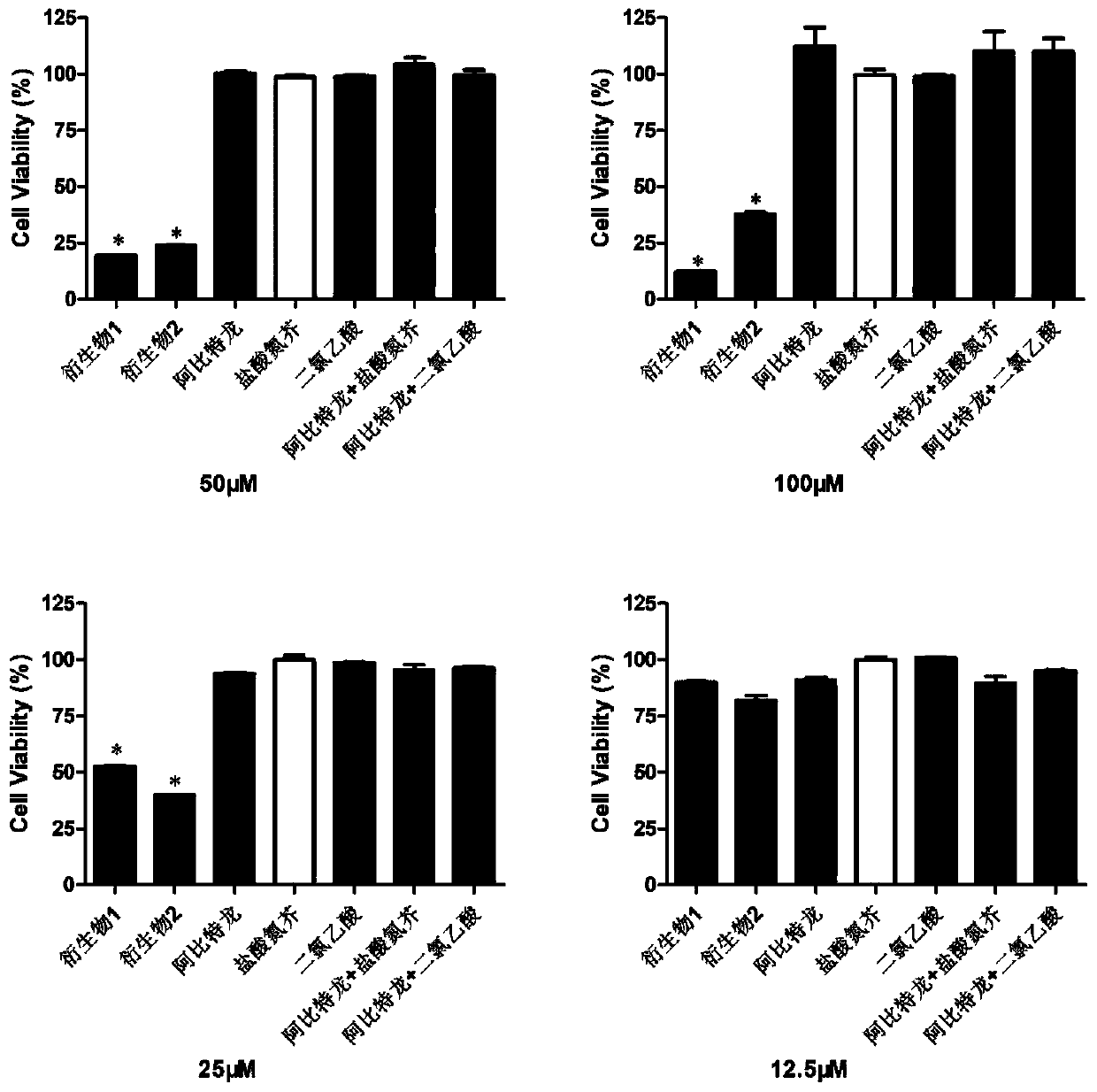
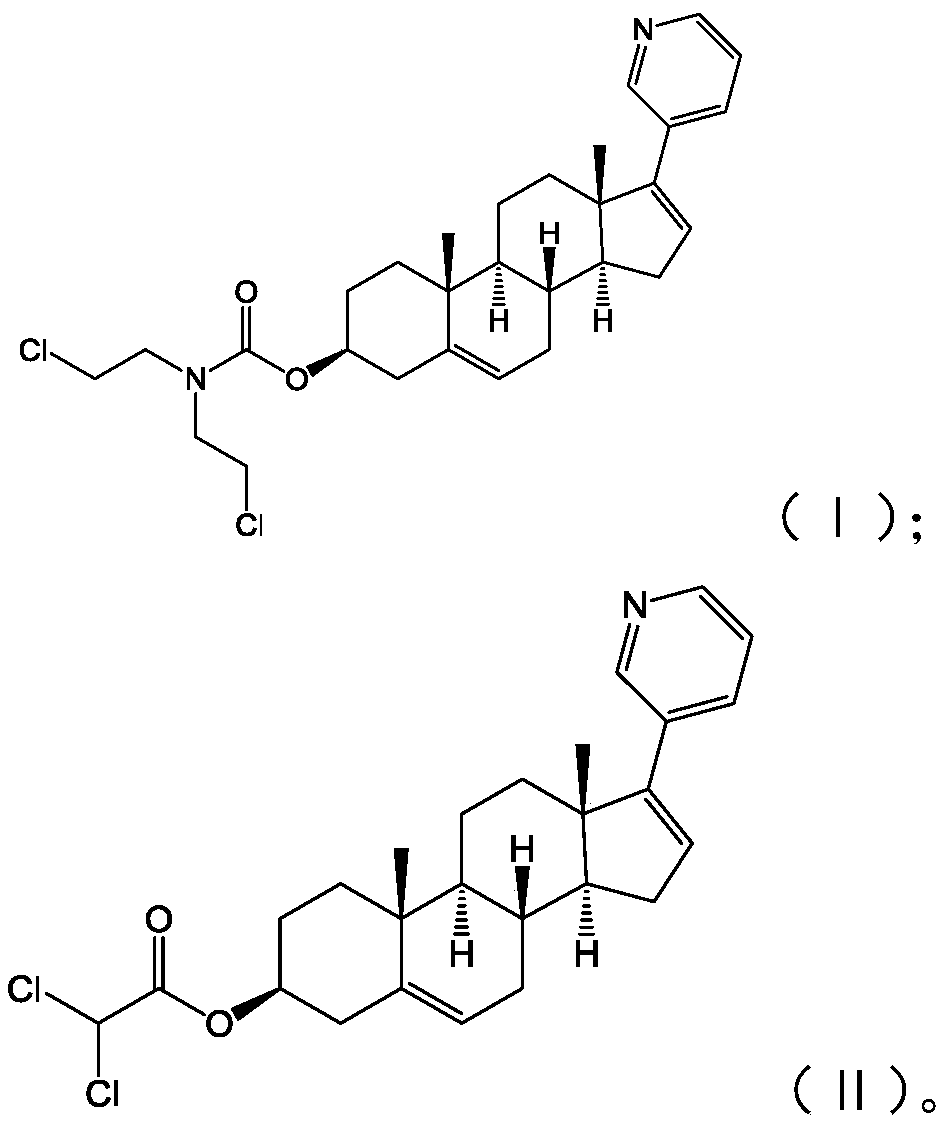
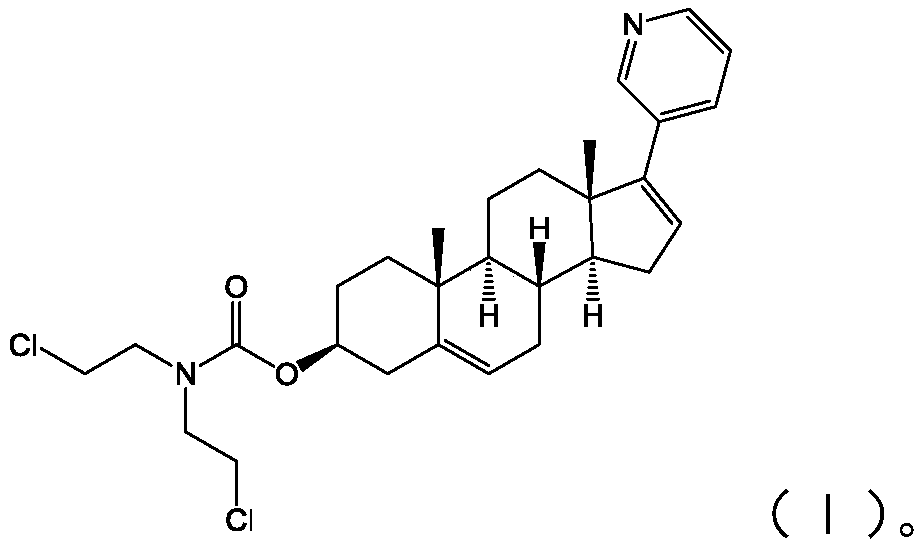
InactiveCN110272465APrevent proliferationStrong inhibitory activityOrganic active ingredientsSteroidsProstate cancer cellAbiraterone
The invention provides an abiraterone derivative shown in a formula (I) or a formula (II), provides a preparation method of the abiraterone derivative shown in the formula (I), provides a preparation method of the abiraterone derivative shown in the formula (II), and also provides application of the abiraterone derivative in preparing drugs for treating prostatic cancer. The abiraterone derivative has the advantages that a specific chlorine-containing group is introduced into the provided abiraterone derivative so that the abiraterone derivative can significantly inhibit the proliferation of prostatic cancer cells and have stronger inhibitory activity against the prostatic cancer cells.
Owner:CHENGDU BIOBEL BIOTECHNOLOGY CO LTD
Process for preparing 17-substituted steroids
The present invention relates to a process for the preparation of 17-substituted steroids and, more particularly, to an improved method of synthesizing abiraterone or derivatives thereof in high yield and purity by means of a key 3-formate intermediate.
Owner:ZACH SYST SPA
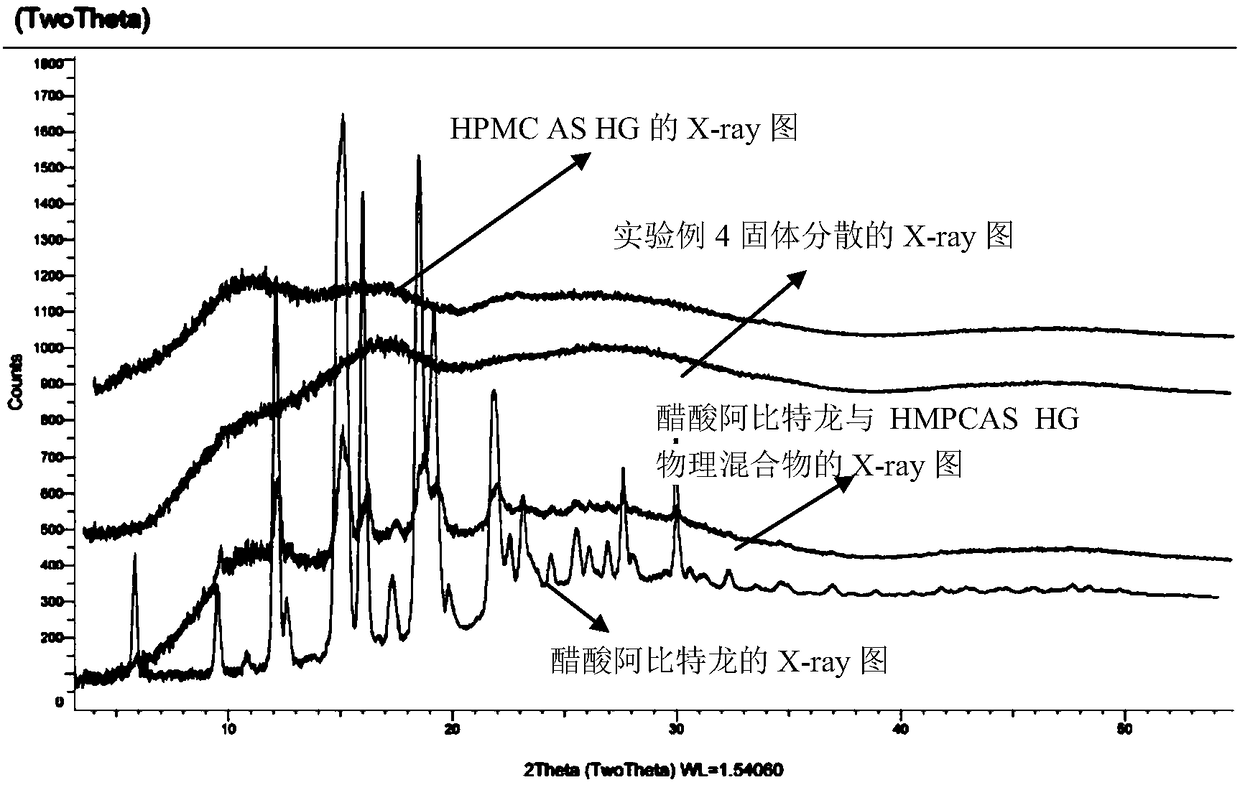
Solid dispersion and preparation method thereof
The invention relates to a solid dispersion and a preparation method of the solid dispersion, in particular to the solid dispersion. The solid dispersion contains abiraterone or a derivative of the abiraterone and a carrier material HPMCAS, wherein DS<Ac> in HPMCAS is not greater than 0.50, and DS<Ac>+DS<s> is not smaller than 0.83, The preparation prepared by the solid dispersion system has gooddissolution and stability, and can eliminate individual differences after patients take medicines and food effects and the like caused by fasting and satiety administration to some extent.
Owner:SUNCADIA PHARM CO LTD
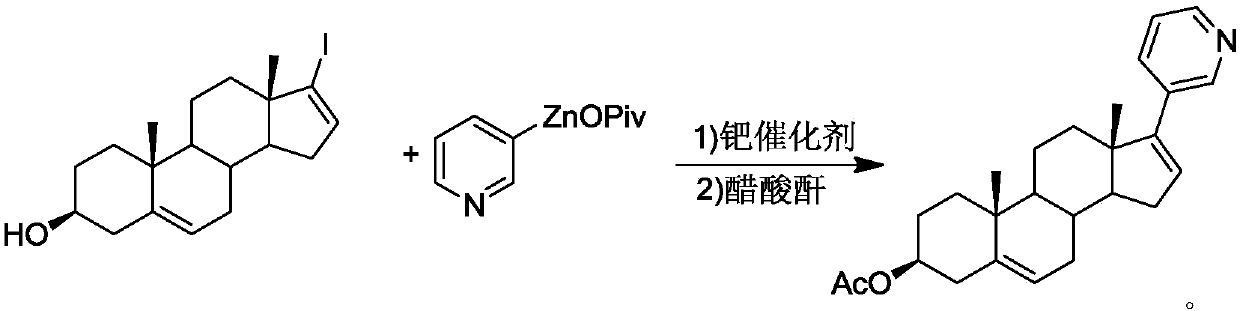
Abiraterone acetate preparation method
The invention provides an abiraterone acetate preparation method, which comprises: dissolving 17-iodoandrosta-5,16-dien-3 beta-hydroxyl and 3-pyridine zinc neopentanoate in an organic solvent, reacting at 50-80 DEG C for 3-12 h under the catalysis of a palladium catalyst, removing the solvent after the reaction is finished, and esterifying with acetic anhydride to obtain an abiraterone acetate product. According to the invention, the method overcomes the defects of expensive raw materials, high cost, harsh reaction conditions and the like in the prior art, is low in cost, mild in preparation conditions, simple and convenient in production method and suitable for industrial production, and has high application value.
Owner:AURISCO PHARMACEUTICAL CO LTD
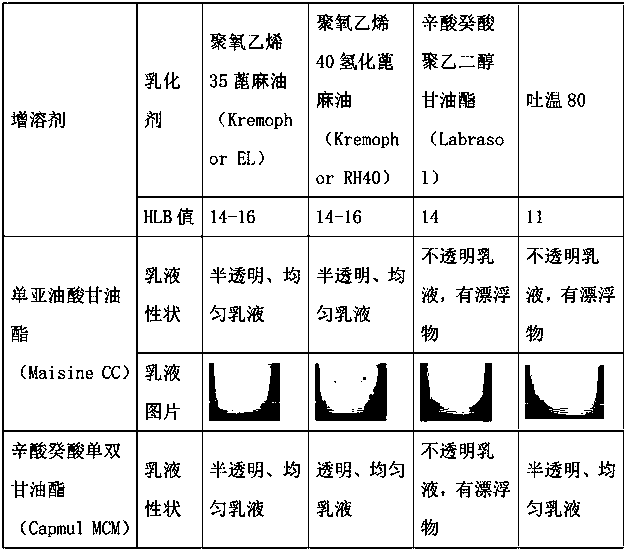
Self-microemulsion system for loading abiraterone acetate, composition and application
ActiveCN114306236AImprove solubilityImprove stabilityOrganic active ingredientsEmulsion deliveryAbirateroneEthylic acid
The invention discloses a self-microemulsion system for loading abiraterone acetate, a composition and application. The self-microemulsion system for loading abiraterone acetate has excellent solubility and stability on abiraterone acetate, and the composition formed by dissolving abiraterone acetate in the system can significantly reduce the influence of food on abiraterone acetate absorption and reduce the difference before and after meals. Therefore, the medicine can be taken under the conditions of empty stomach and satiety, and the limitation of medicine taking time is reduced.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
High performance liquid chromatography detection method of abiraterone acetate related substances
ActiveCN111505148AAchieve separationSimple methodComponent separationAgainst vector-borne diseasesAbirateroneEthylic acid
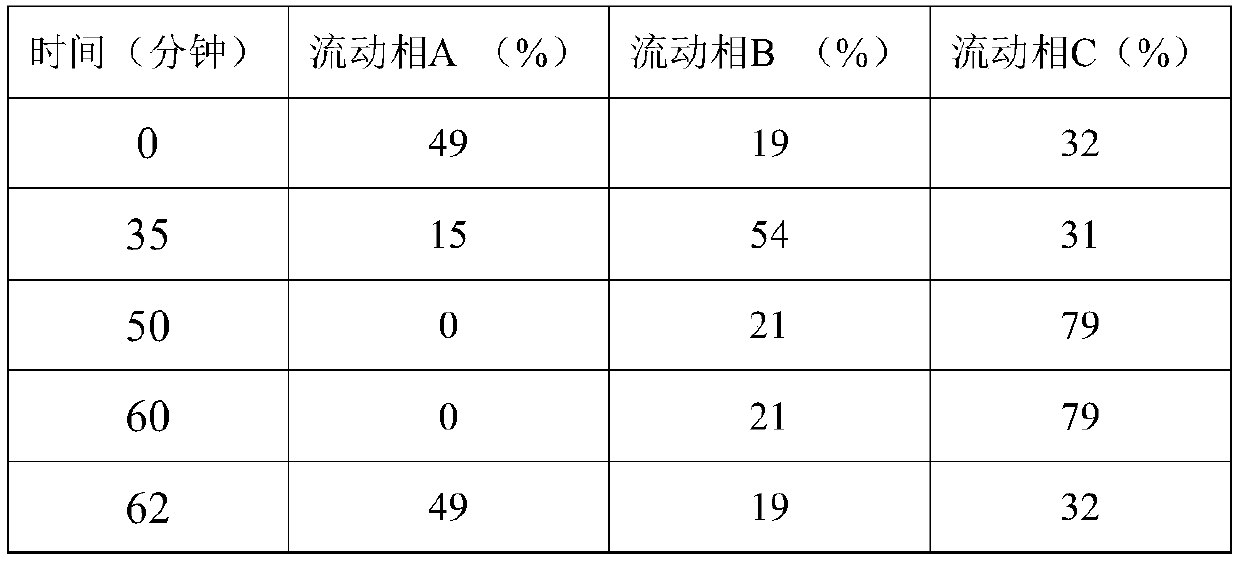
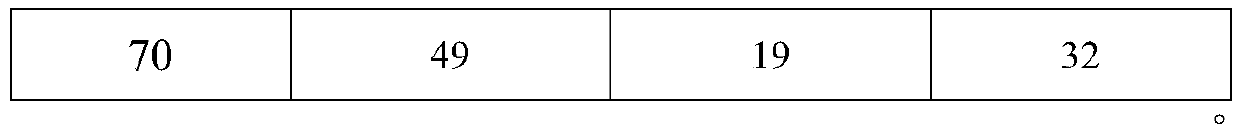
The invention discloses a high performance liquid chromatography detection method of abiraterone acetate related substances, which can detect impurities from the initial synthesis step to the final finished product process, thereby effectively ensuring the safety and effectiveness of drugs. Chromatographic conditions of the method are as follows: octadecylsilane chemically bonded silica is used asa filler, a buffer salt solution is used as a mobile phase A, acetonitrile is used as a mobile phase B, and ethanol is used as a mobile phase C for gradient elution. The method is simple, easy to operate, high in specificity and high in sensitivity, the content of multiple related substances can be measured at the same time, and therefore the product quality of abiraterone acetate is effectivelyguaranteed, and medication safety is guaranteed.
Owner:GANSU LANYAO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com