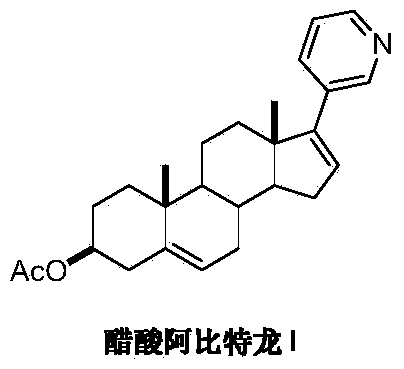
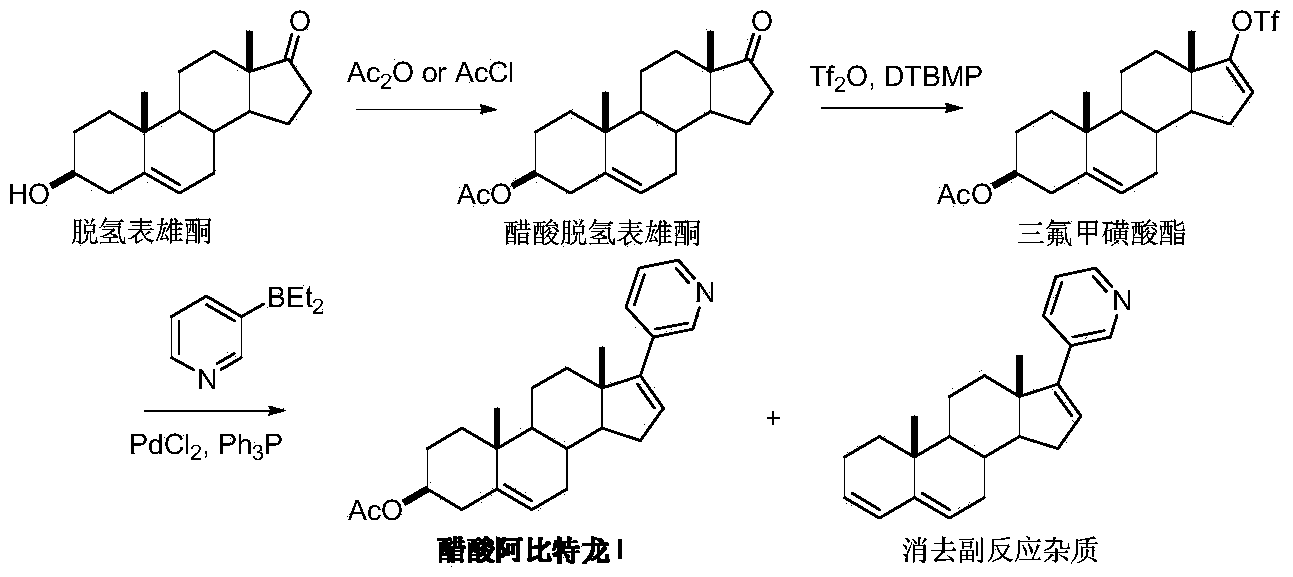
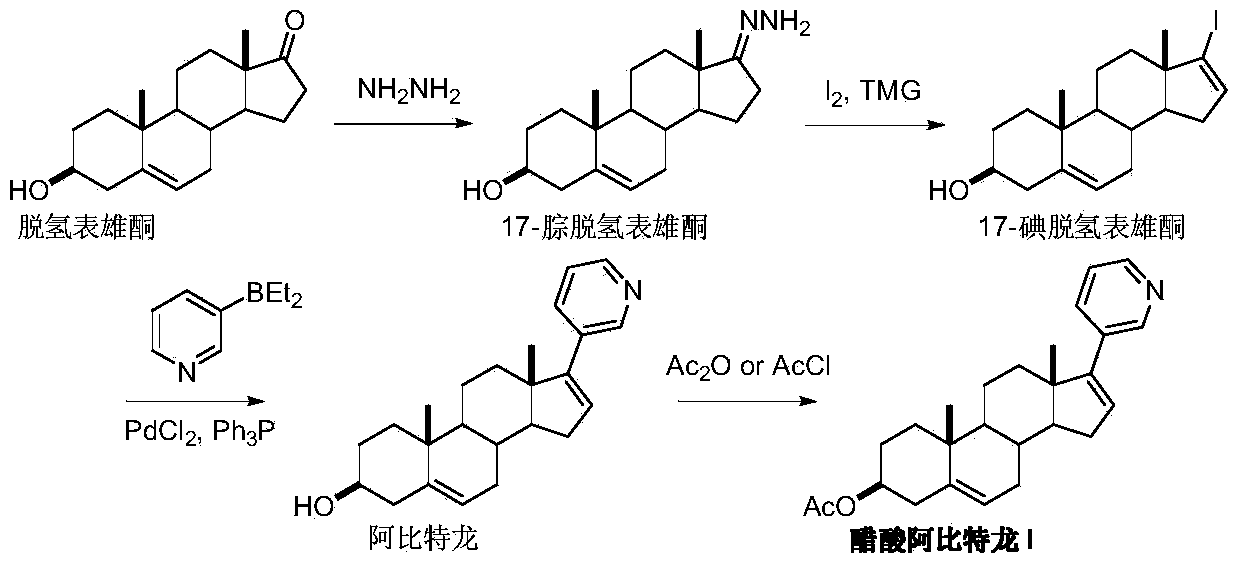
Preparation method of abiraterone acetate
A technology of abiraterone acetate and alcohol acetate, which is applied in the field of preparation of abiraterone acetate, can solve problems such as yield reduction, influence industrialization effect, and be difficult to purify, achieve reliable quality, promote economic and technological development, and improve raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under nitrogen atmosphere, 3-bromopyridine (1.88 g, 12 mmol) and 15 mL of anhydrous ether were added to a dry reaction flask, the temperature was lowered to -78 °C, n-butyllithium n-hexane solution (2.5 M, 5 mL) was added dropwise, and the mixture was stirred. The reaction was carried out for 45 minutes. Keeping at -78°C, 25 mL of tetrahydrofuran solution of dehydroepiandrosterone acetate (3.3 g, 10 mmol) was added dropwise to the system. It was naturally warmed to room temperature and the reaction was stirred for 24 hours. At 0°C, the reaction was quenched with saturated ammonium chloride solution, and after stirring, extracted with dichloromethane three times, the organic phases were combined, washed with water and saturated brine successively, and dried over anhydrous magnesium sulfate. The dichloromethane was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate / n-hexane to obtain 1.74 g of 17-(3-pyridine)-17-hydroxy-androst-5-ene-...
Embodiment 2
[0034] Under nitrogen atmosphere, 17-(3-pyridine)-17-hydroxy-androst-5-en-3β-ol acetate (II) (2.05 g, 5 mmol) and 25 mL of toluene were added to a dry reaction flask, and stirred. Methyl N-(triethylammoniumsulfonyl)carbamate (Burgess reagent) (1.28 g, 5 mmol) was added, the reaction was carried out at room temperature for 1 hour, the temperature was raised to 85°C, and the reaction was continued for 1 hour. Additional Burgess reagent (1.28 g, 5 mmol) was added, and the reaction was carried out at room temperature for 1 hour. The temperature was raised to 85° C. and the reaction was continued for 1 hour. TLC detection reaction was completed. After cooling, the reaction was quenched with ice water. The organic phase was separated and the aqueous phase was extracted twice with toluene. The organic phases were combined, washed successively with water and brine, and dried over anhydrous sodium sulfate. Toluene was recovered under reduced pressure, and the residue was recrystalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com