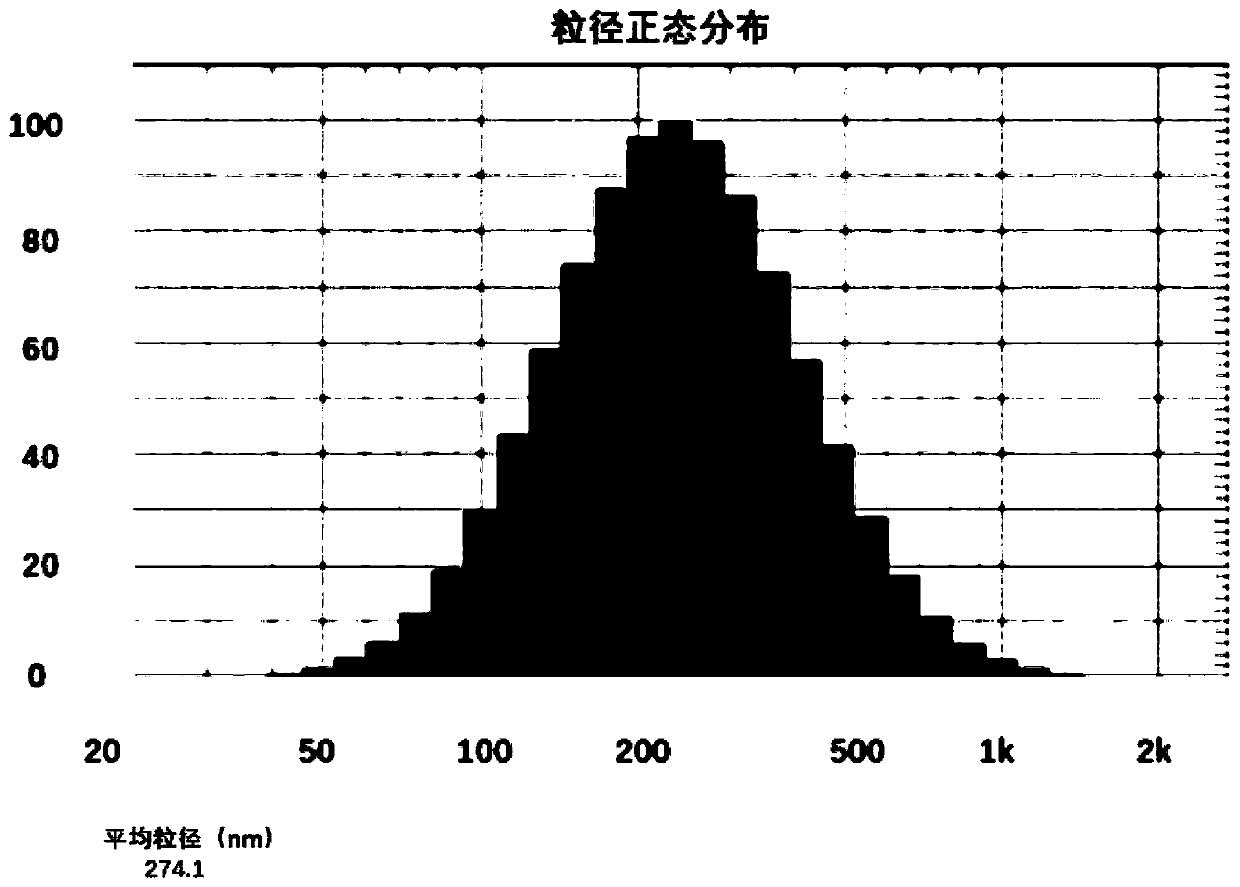
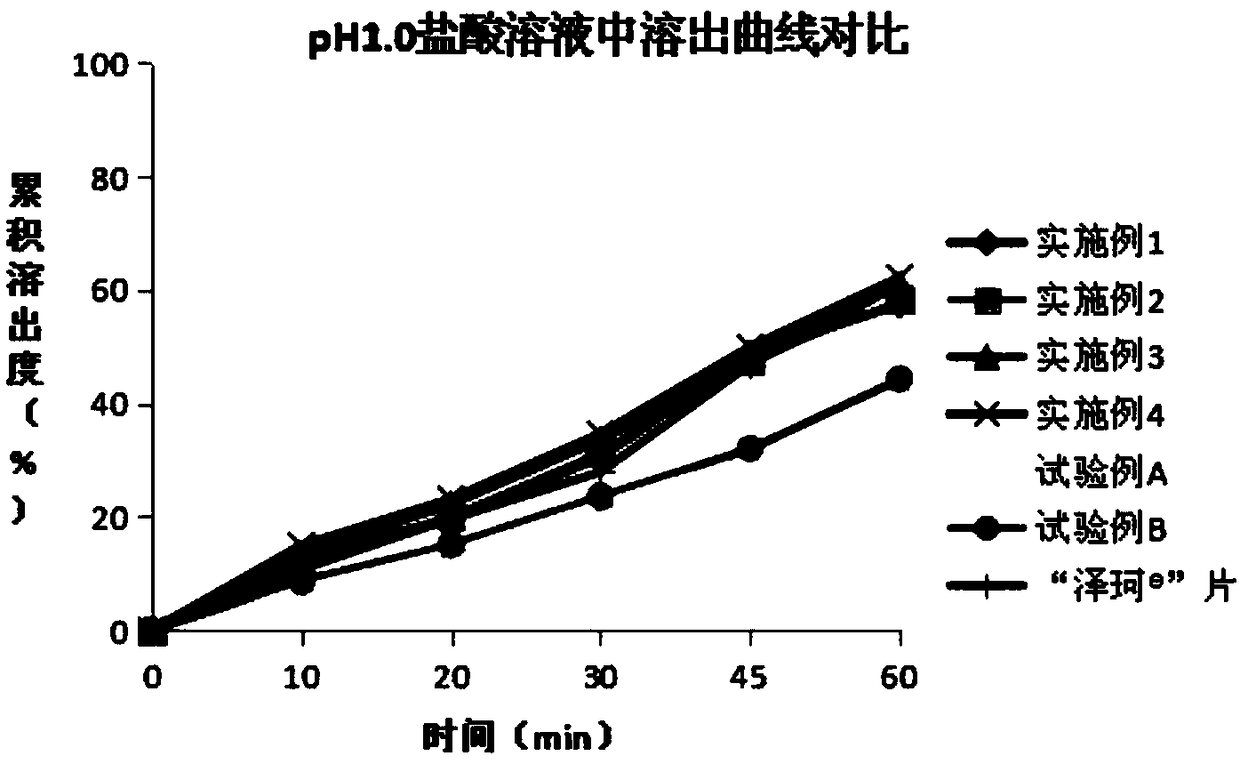
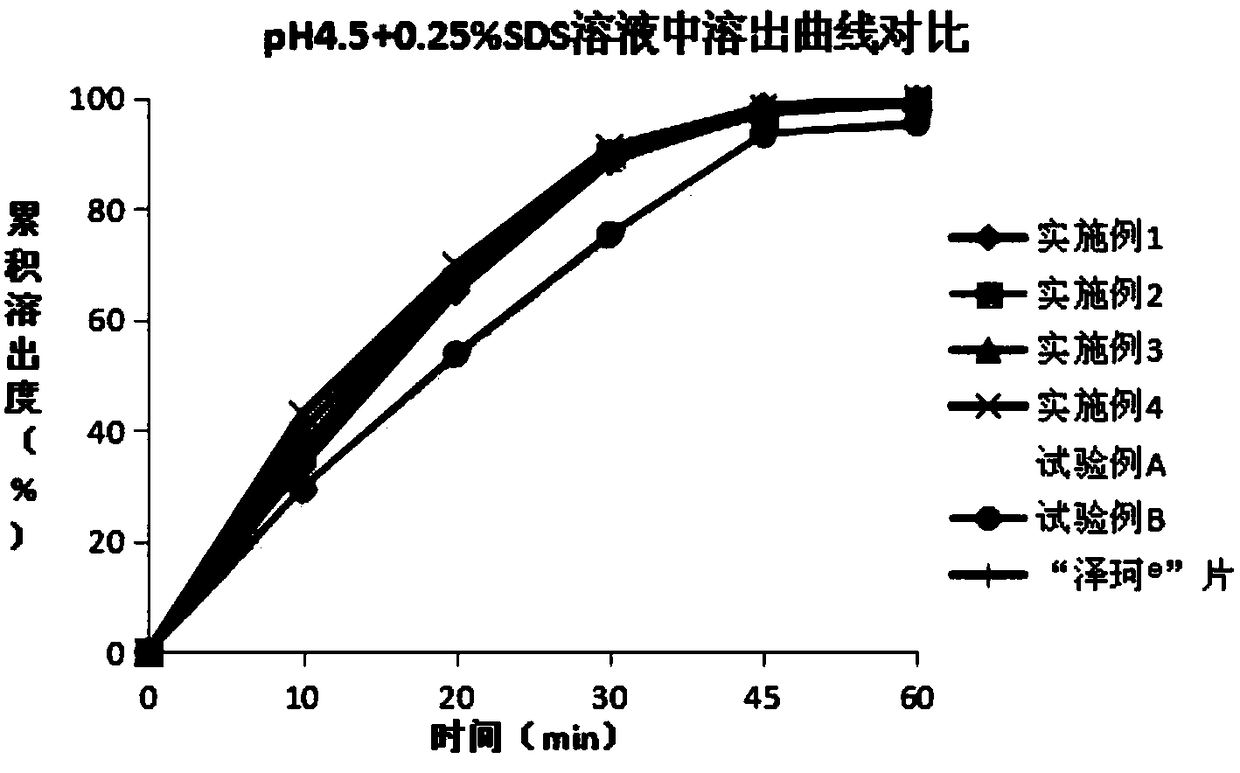
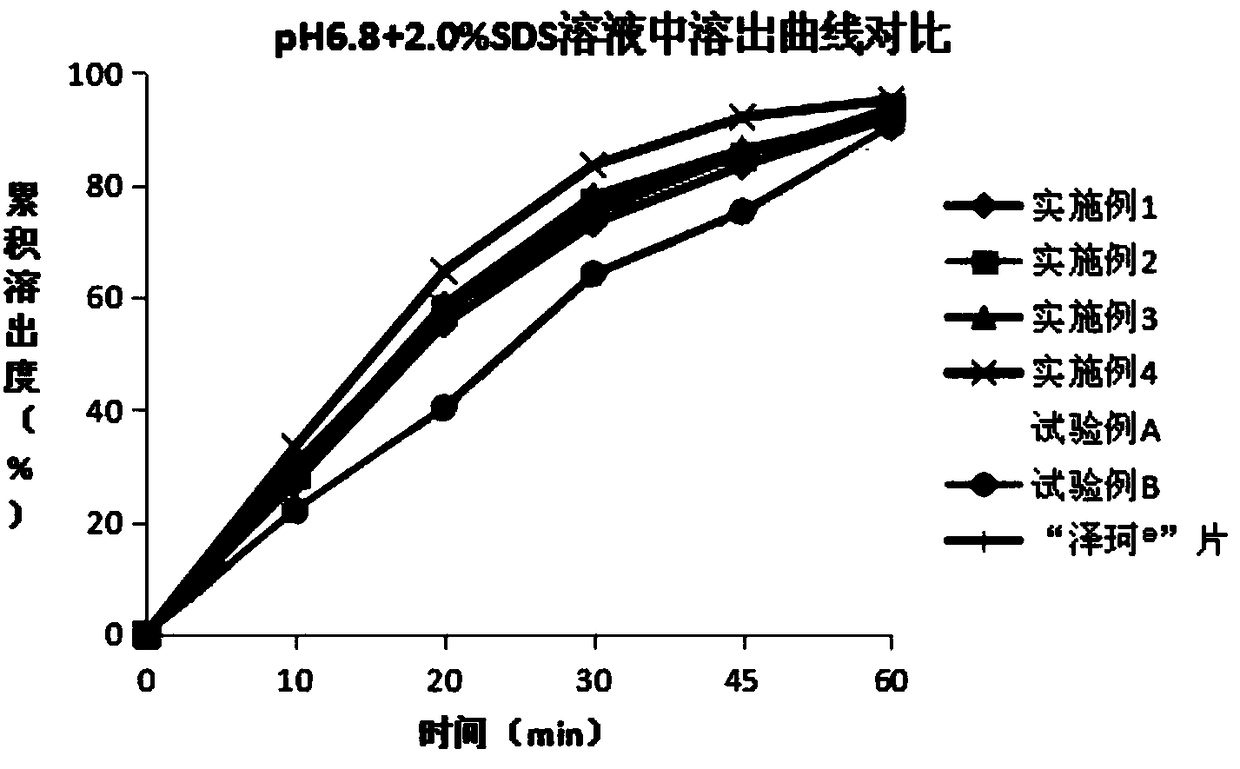
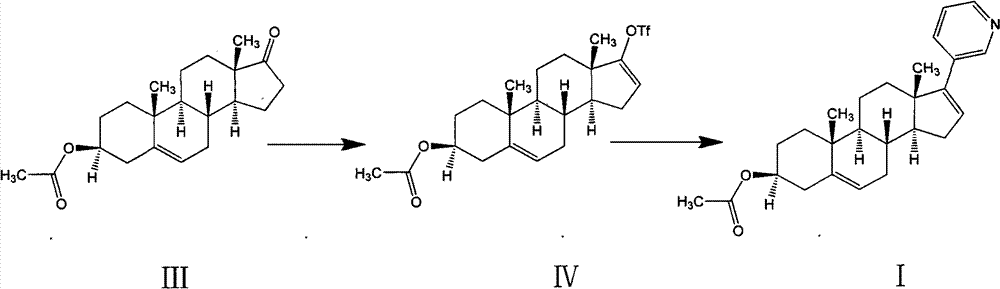
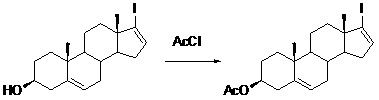Patents
Literature
174 results about "Abiraterone acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
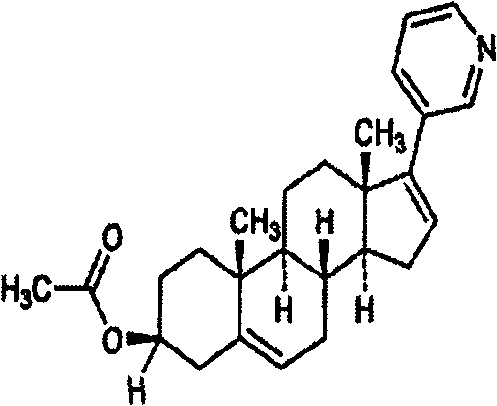
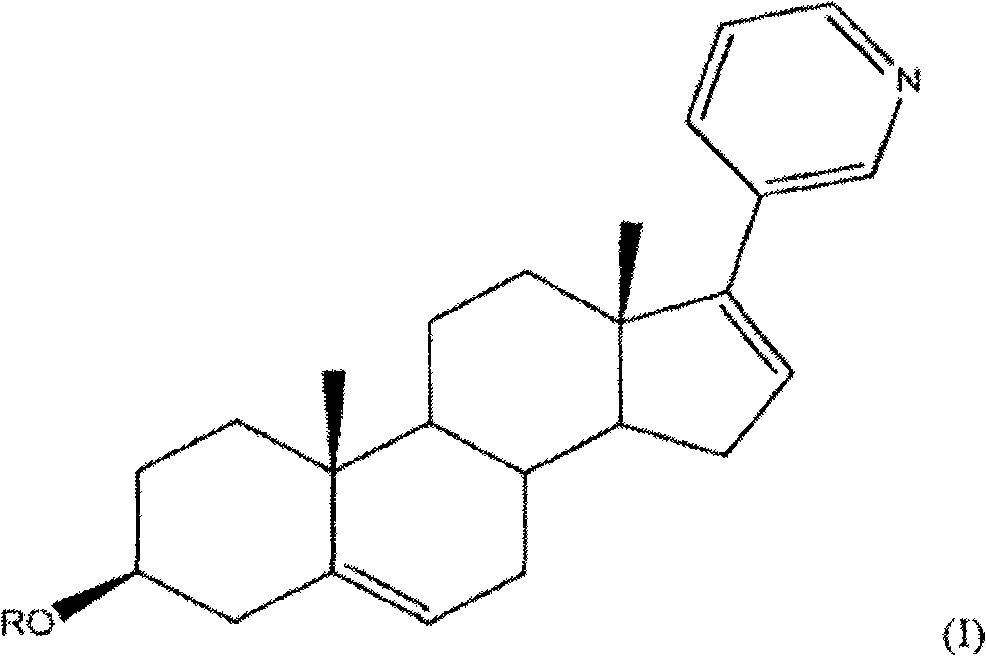
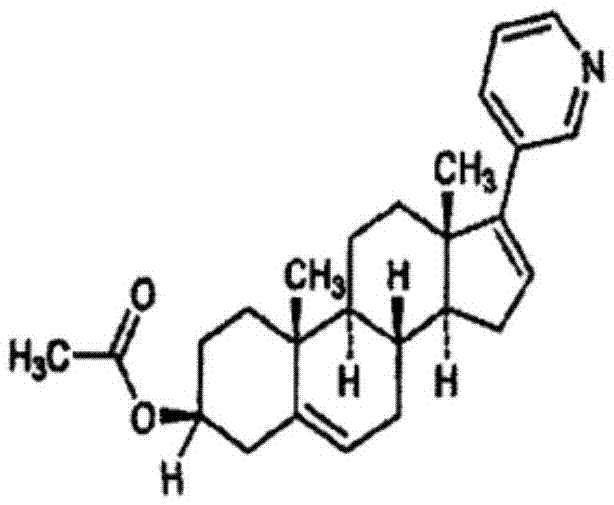
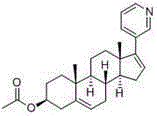
Abiraterone acetate, sold under the brand name Zytiga among others, is an antiandrogen medications used to treat prostate cancer. Specifically it is used together with castration and prednisone for metastatic castration-resistant prostate cancer (mCRPC) and in the treatment of metastatic high-risk castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
Medicinal composition containing abiraterone acetate and preparation technology thereof
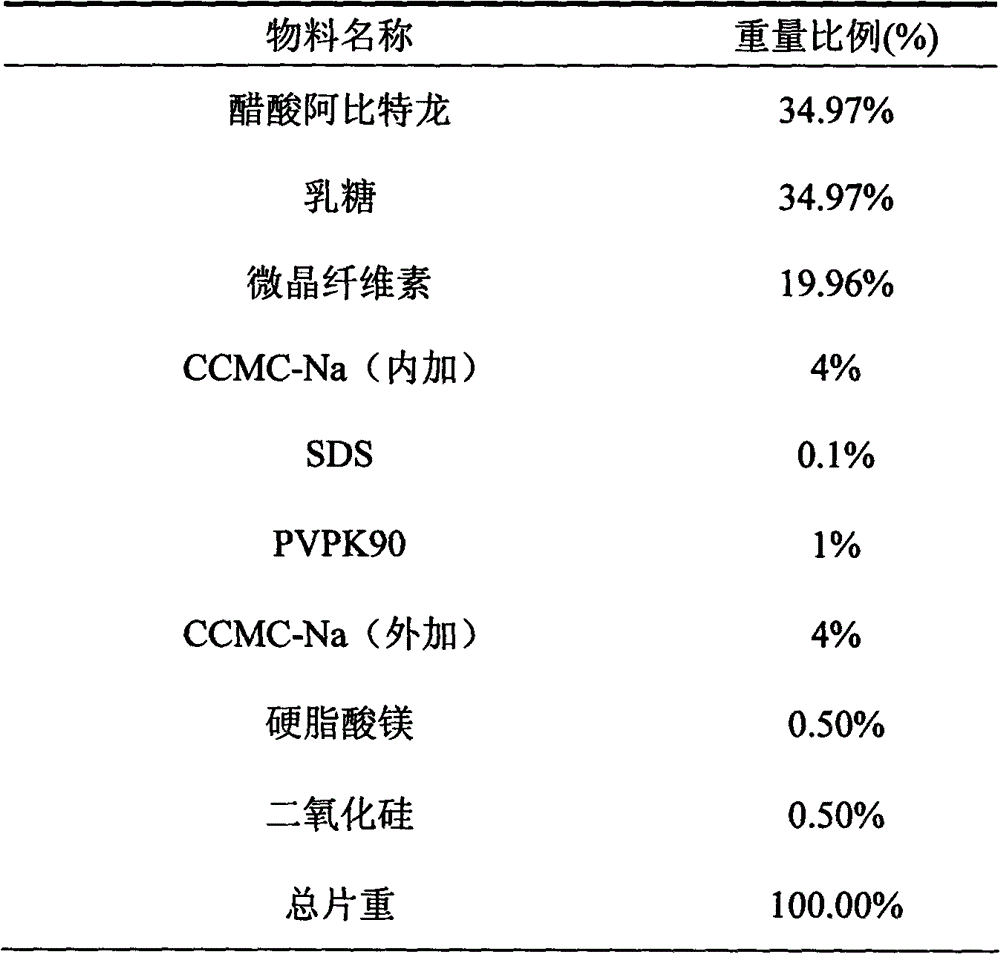
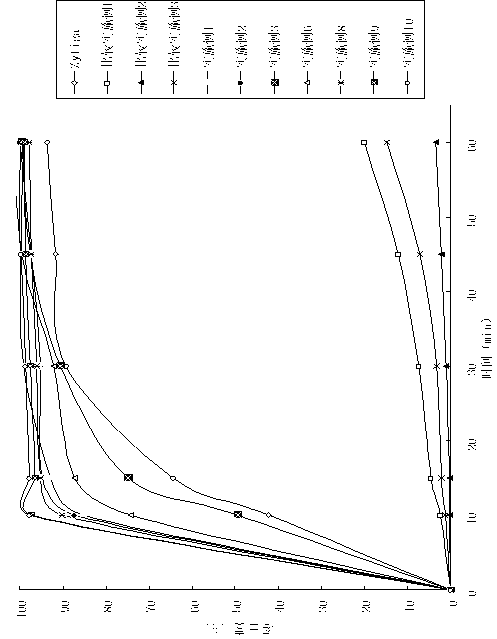
The invention belongs to the technical field of medicine, and particularly relates to a medicinal composition containing abiraterone acetate and a preparation technology of the medicinal composition. The medicinal composition comprises abiraterone acetate, a solubilizer, a binder and a disintegrating agent and can be prepared into tablets, granules or capsules. The invention is characterized in that the abiraterone acetate and hydrophilic auxiliary materials are crushed based on a proportion, and the dissolution rate and bioavailability of the medicine are effectively improved, the medicinal composition can be used for treating the prostate cancer.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Polymorphs of abiraterone acetate and preparation method thereof
The invention discloses polymorphs A, B, C and D of abiraterone acetate. A preparation method of the polymorphs comprises the step of re-crystallizing the abiraterone acetate subjected to the separation and the purification of a chromatographic column in different solvents. Through stable investigation, four polymorphs have favorable stability and flowability, can be used as raw materials for storage and transportation and are suitably applied to antitumor medicinal preparations.
Owner:深圳万乐药业有限公司
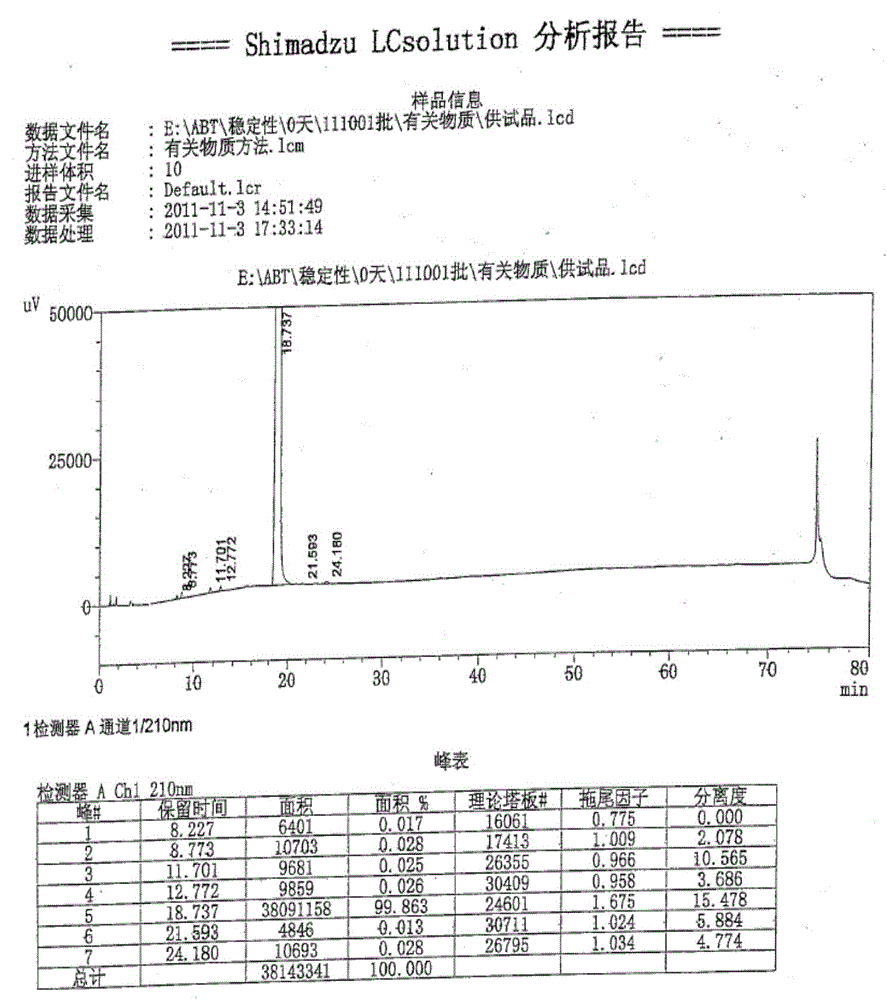
Purification method of abiraterone acetate
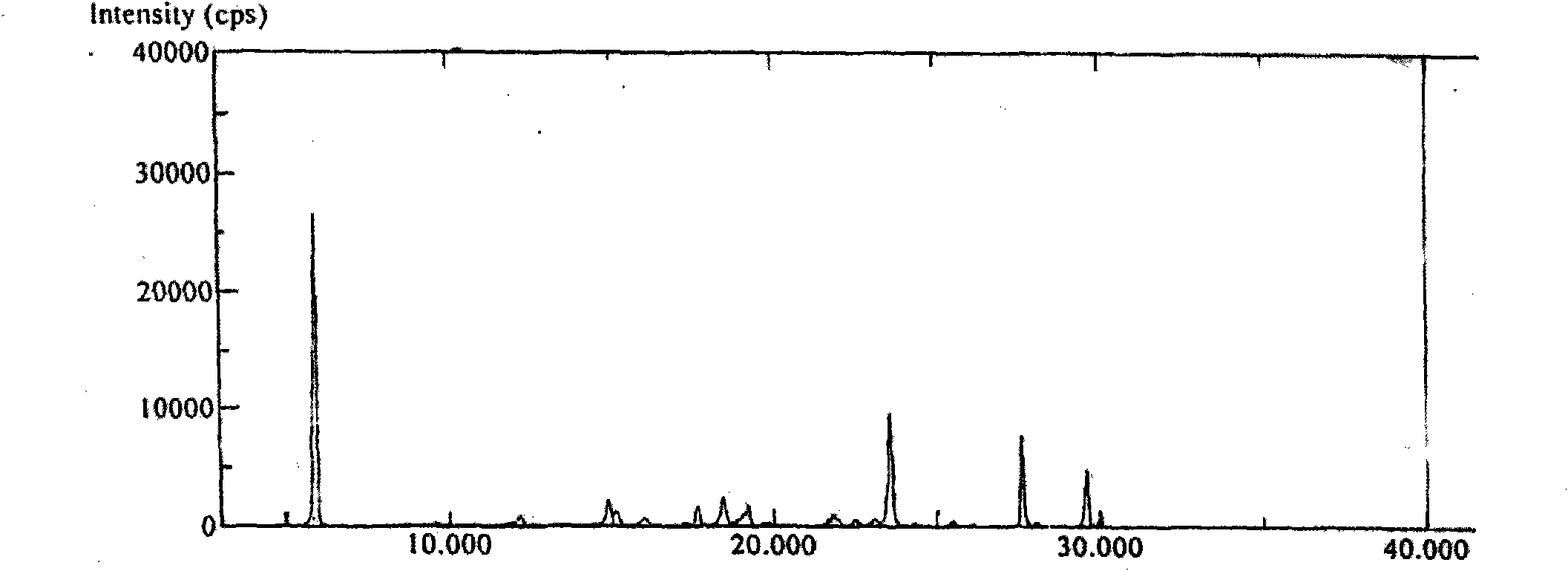
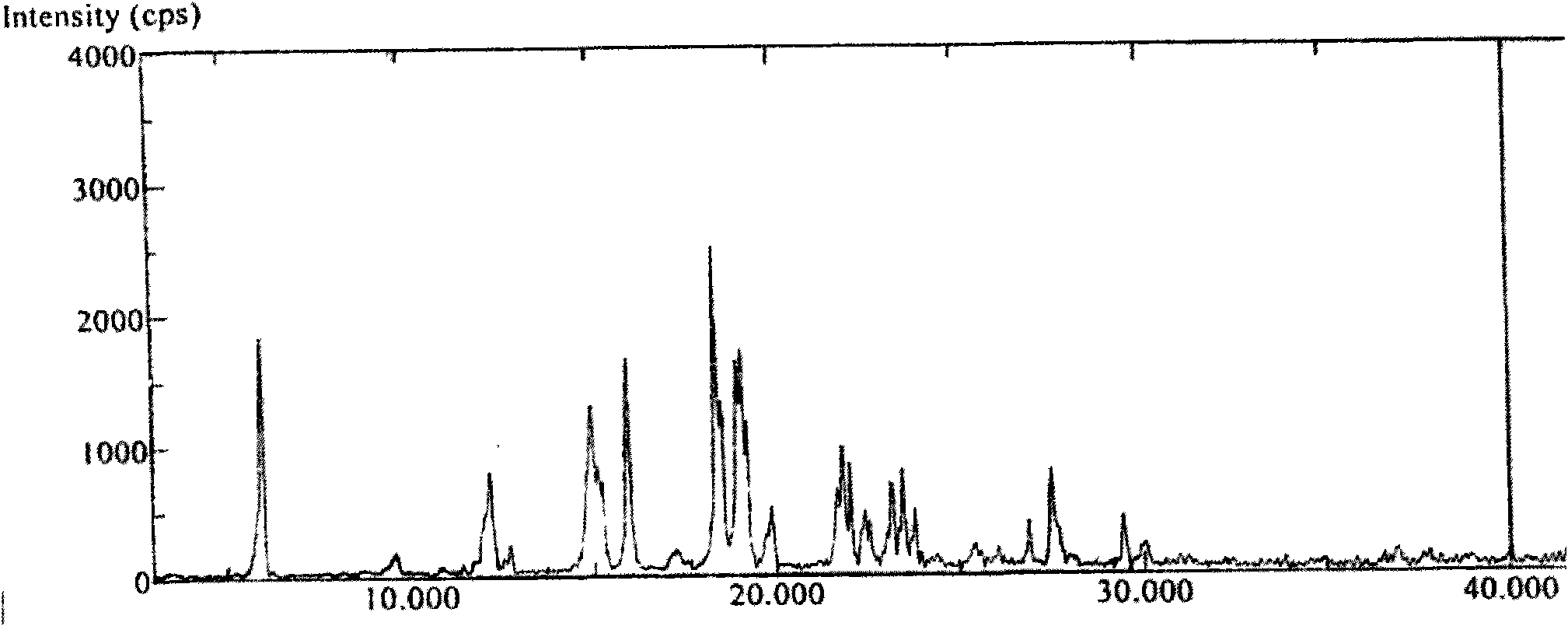
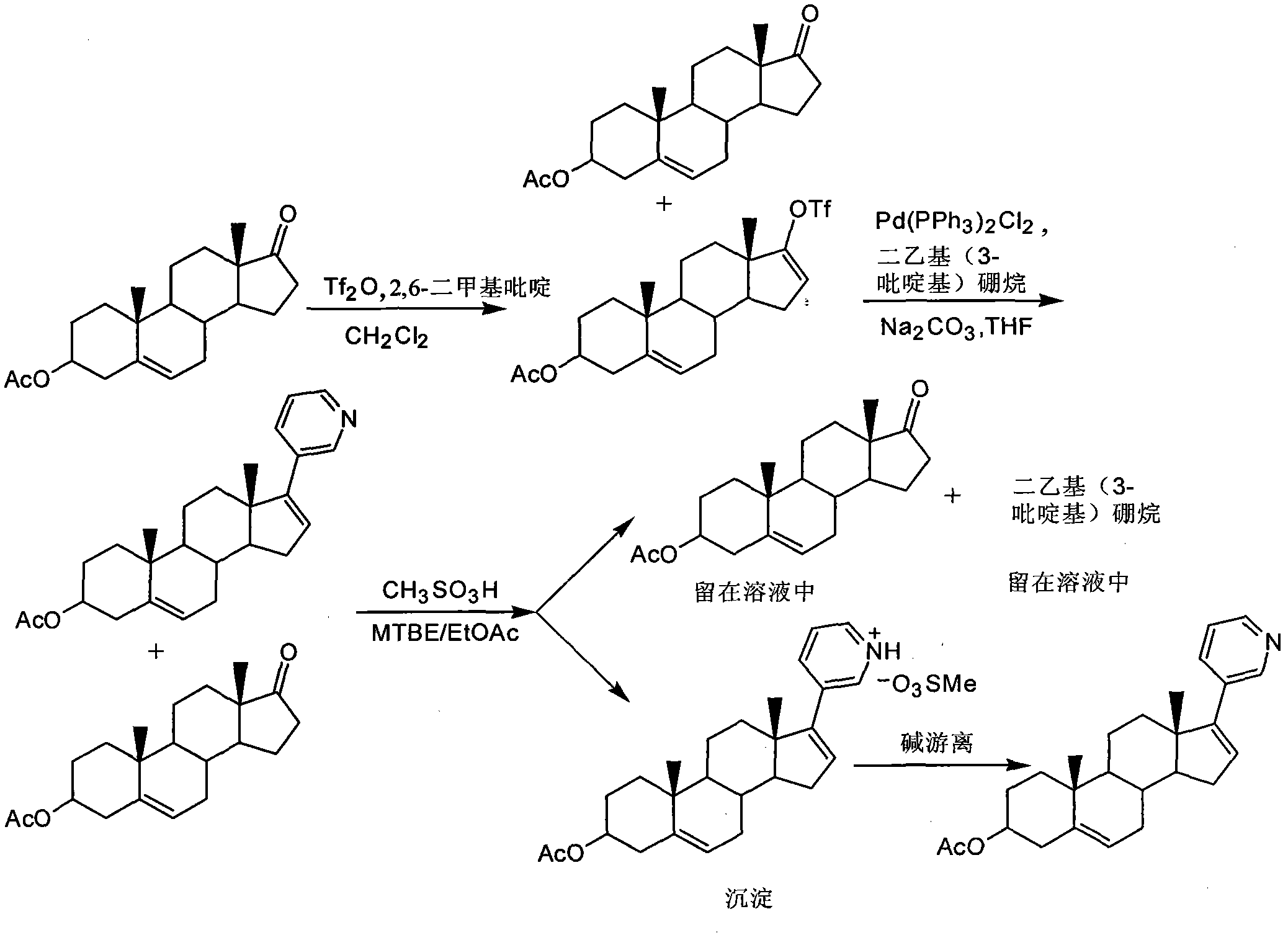
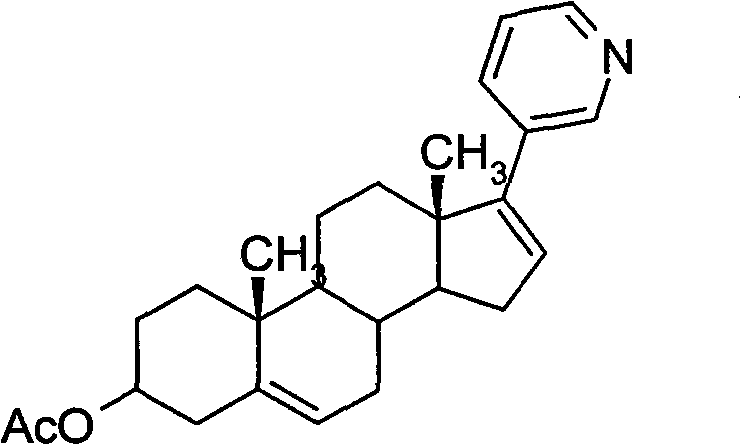
InactiveCN102030798ASimple purification processEasy to operateSteroidsPurification methodsTriflic acid
The invention provides a purification method of abiraterone acetate. The method comprises the following steps of: reacting trifloromethanesulfonic acid serving as a salt forming reagent with crude abiraterone acetate to obtain dry and easily-purified abiraterone acetate trifluoromethanesulfonic salt with the purity of over 97 percent; and performing a free reaction on the abiraterone acetate trifluoromethanesulfonic salt without a purification step so as to obtain abiraterone acetate, wherein the purity of the abiraterone acetate obtained by the free reaction is over 97 percent. The method simplifies purification process, saves reagent and is easy to operate and suitable for industrial production.
Owner:深圳万乐药业有限公司
Solid dispersion and tablets comprising abiraterone acetate, and preparation methods thereof
ActiveCN103070828AAdvantages and Notable ImprovementsGood water solubilityOrganic active ingredientsPowder deliverySolubilityMedicine
The invention relates to a solid dispersion and tablets comprising abiraterone acetate, and preparation methods thereof. The solid dispersion is prepared through the steps that: abiraterone acetate and povidone with a weight ratio of 1:0.5-4 are dissolved in chloroform; and reduced-pressure drying is carried out, such that the solid dispersion is obtained. The tablets comprises 1 part of the abiraterone acetate solid dispersion, 2-8 parts of a filling agent, 0.2-0.8 parts of a disintegrating agent, and 0.05-0.1 parts of a lubricant. According to the invention, a micronization technology and a solid dispersion technology are creatively combined, such that abiraterone acetate water solubility is greatly improved. Therefore, abiraterone acetate can be rapidly dissolved in gastrointestinal tract body fluids.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of abiraterone acetate
ActiveCN102627681AReduce usageAvoid separation and purificationSteroidsAcetic anhydrideEthyl Chloride
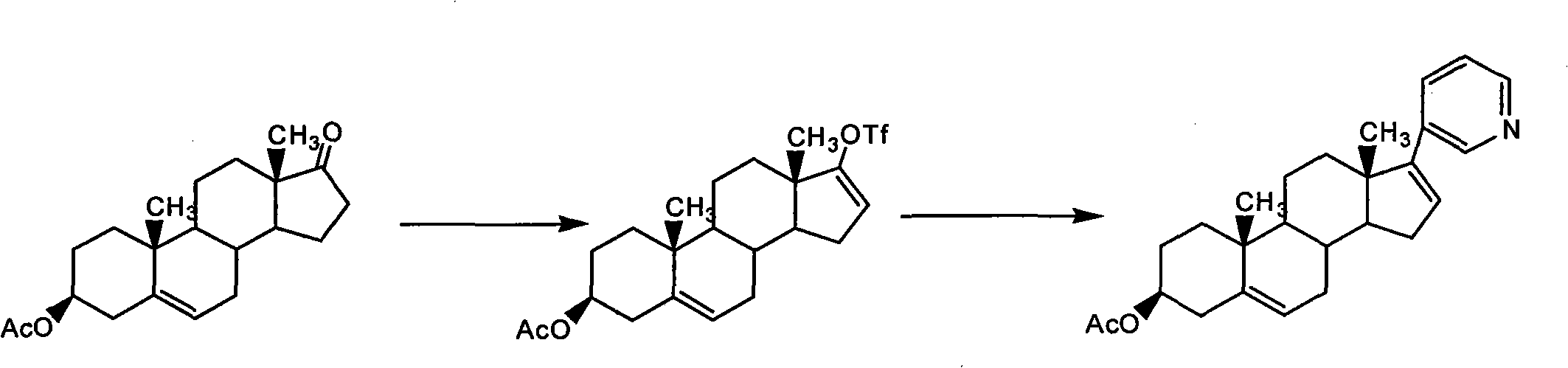
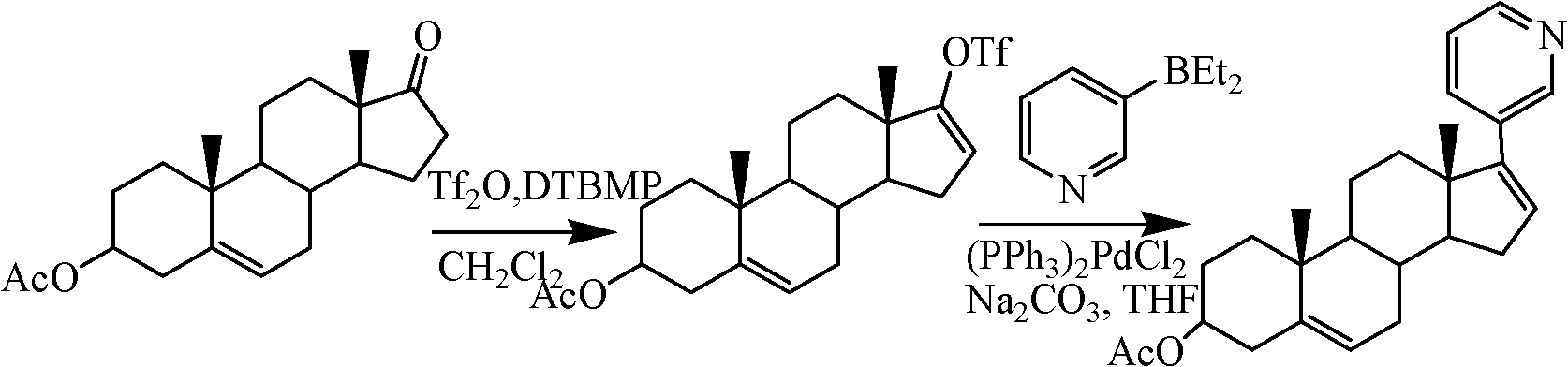
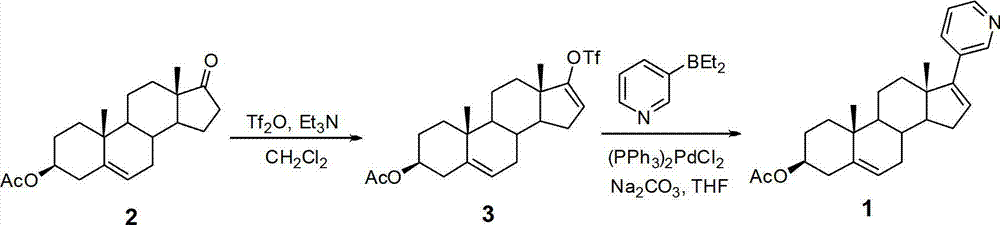
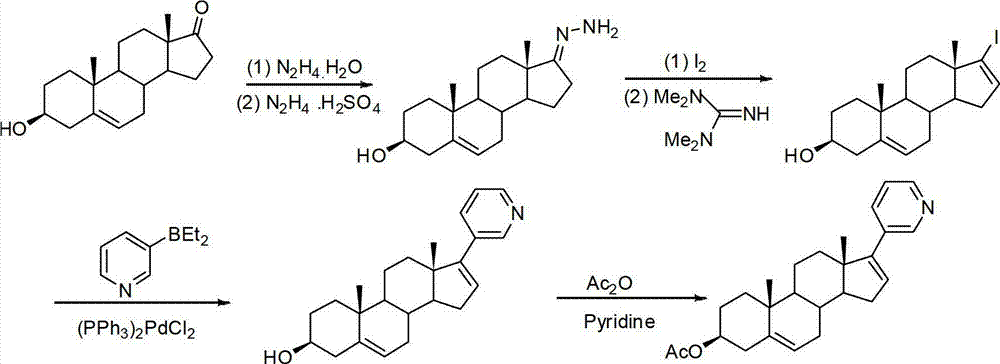
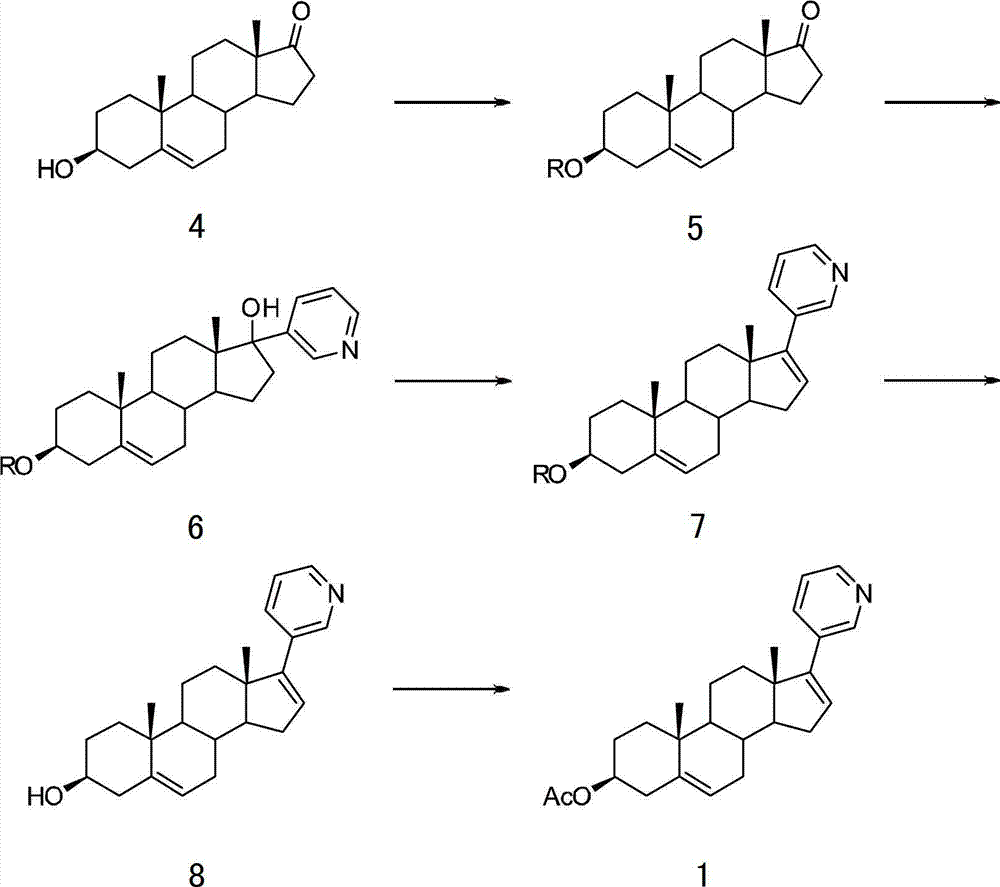
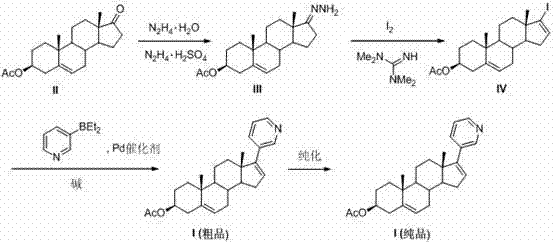
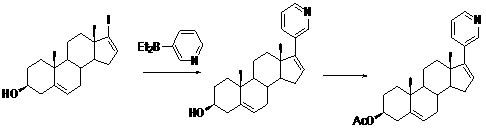
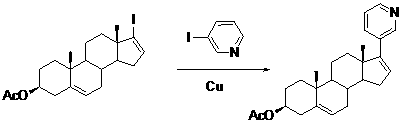
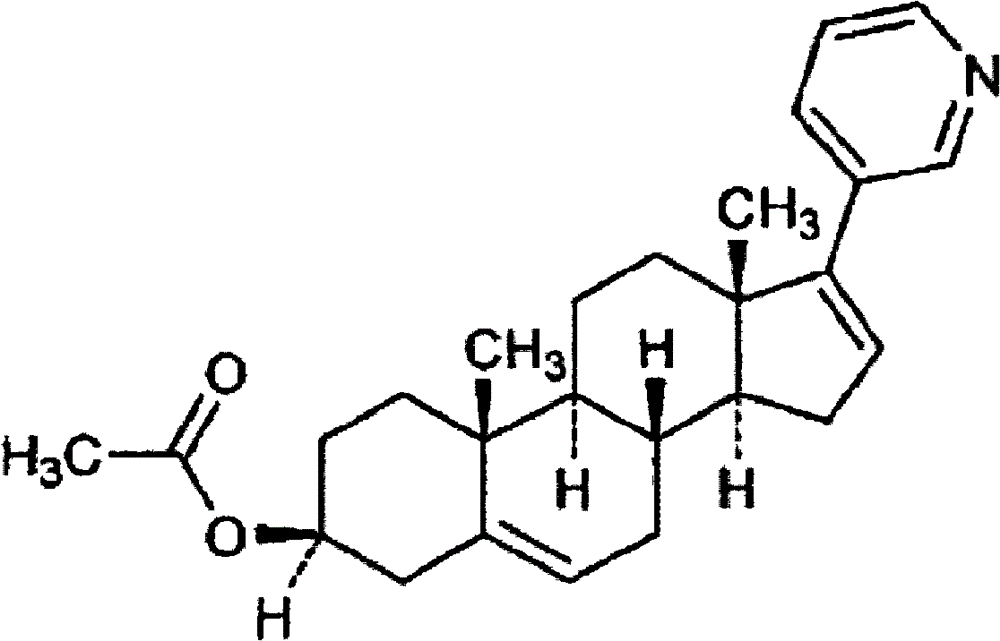
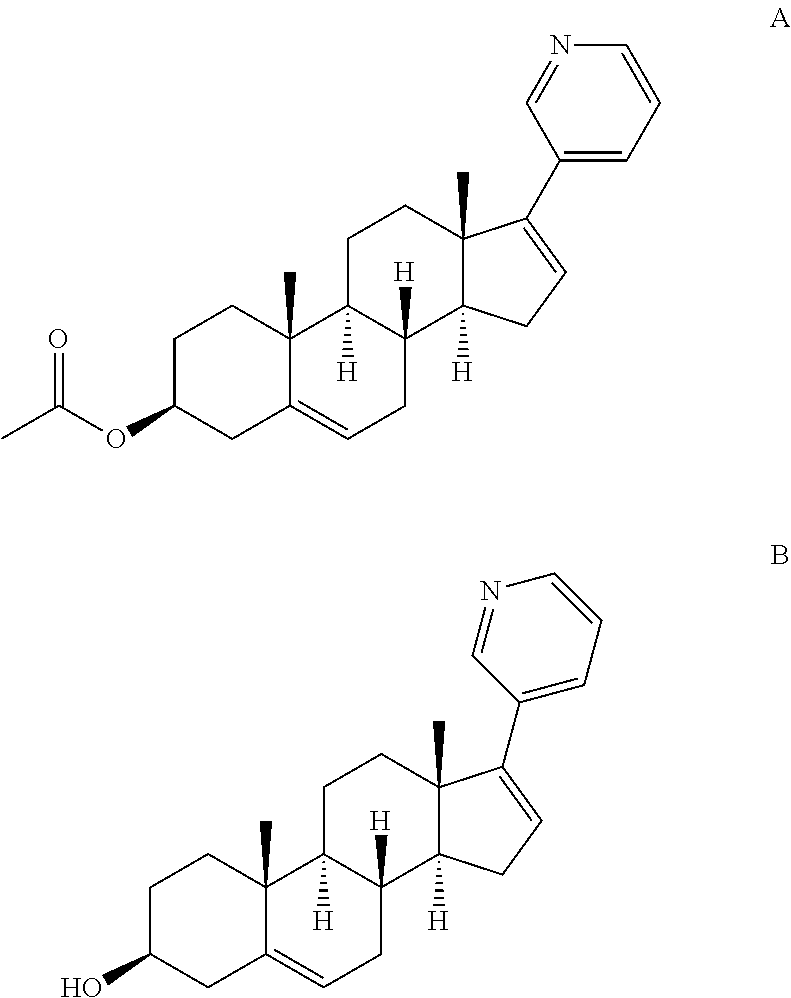
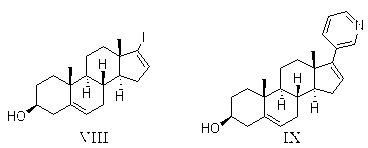
The invention relates to a preparation method of abiraterone acetate. The method comprises: taking dehydroepiandrosterone as the raw material, which successively reacts with hydrazine hydrate and idoine so as to obtain 17-iodo-adrost-5, 16-diene-3beta-ol, which then undergoes a Negishi coupling reaction with 3-pyridine zinc halide to obtain abiraterone, and finally conducting esterification with acetyl chloride or acetic anhydride, thus obtaining the target product abiraterone acetate.
Owner:SHANDONG NEWTIME PHARMA
Methods and compositions for treating cancer
ActiveUS8822438B2Retain and improve pharmacological activityBiocideOrganic active ingredientsAcetic acidAnticarcinogen
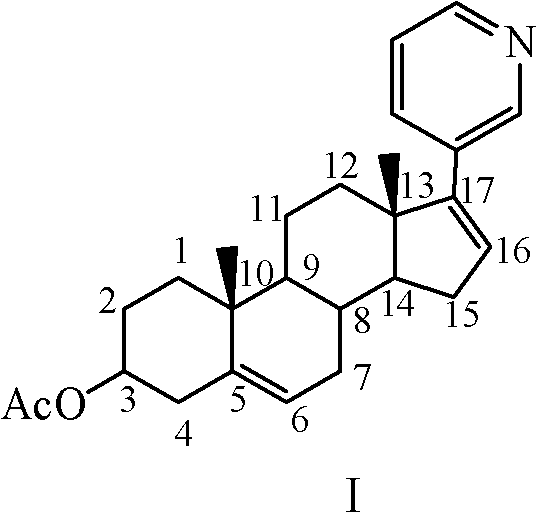
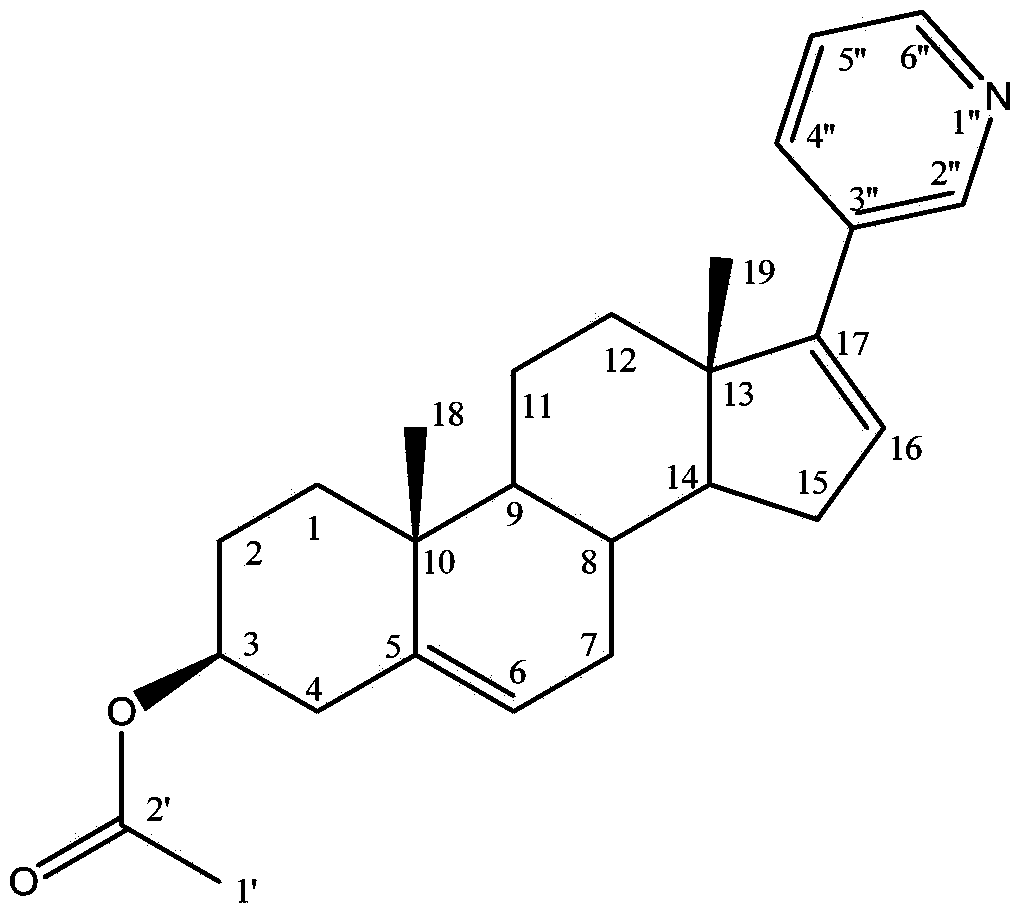
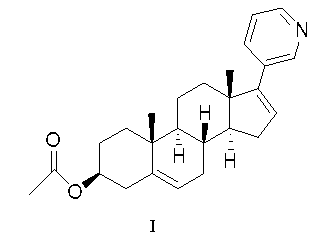
Methods and compositions for treating cancer are described herein. More particularly, the methods for treating cancer comprise administering a 17α-hydroxylase / C17,20-lyase inhibitor, such as abiraterone acetate (i.e., 3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene), in combination with at least one additional therapeutic agent such as an anti-cancer agent or a steroid. Furthermore, disclosed are compositions comprising a 17α-hydroxylase / C17,20-lyase inhibitor, and at least one additional therapeutic agent, such as an anti-cancer agent or a steroid.
Owner:BRITISH TECH GRP LTD
Oral solid composition of abiraterone and preparation method thereof
The invention relates to an oral solid composition of abiraterone and a preparation method thereof. The composition contains abiraterone acetate with a particle size of 1-30 micrometers and pharmaceutic adjuvants.
Owner:CHONGQING PHARMA RES INST
METHODS FOR TREATING CANCER USING 17alpha-HYDROXYLASE/C17,20-LYASE INHIBITORS
InactiveUS20090124587A1Reducing and avoiding progressReduced plasma concentrationOrganic active ingredientsAntineoplastic agentsMetaboliteRegimen
Owner:AUERBACH ALAN H +1
Abiraterone acetate polymorphic substance and pharmaceutical composition
InactiveCN102336801AEasy to prepareImprove stabilityOrganic active ingredientsSteroidsEthylic acidPharmaceutical Substances
The invention discloses an abiraterone acetate polymorphic substance I, and a preparation method and pharmaceutical composition thereof.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Method for preparing abiraterone acetate
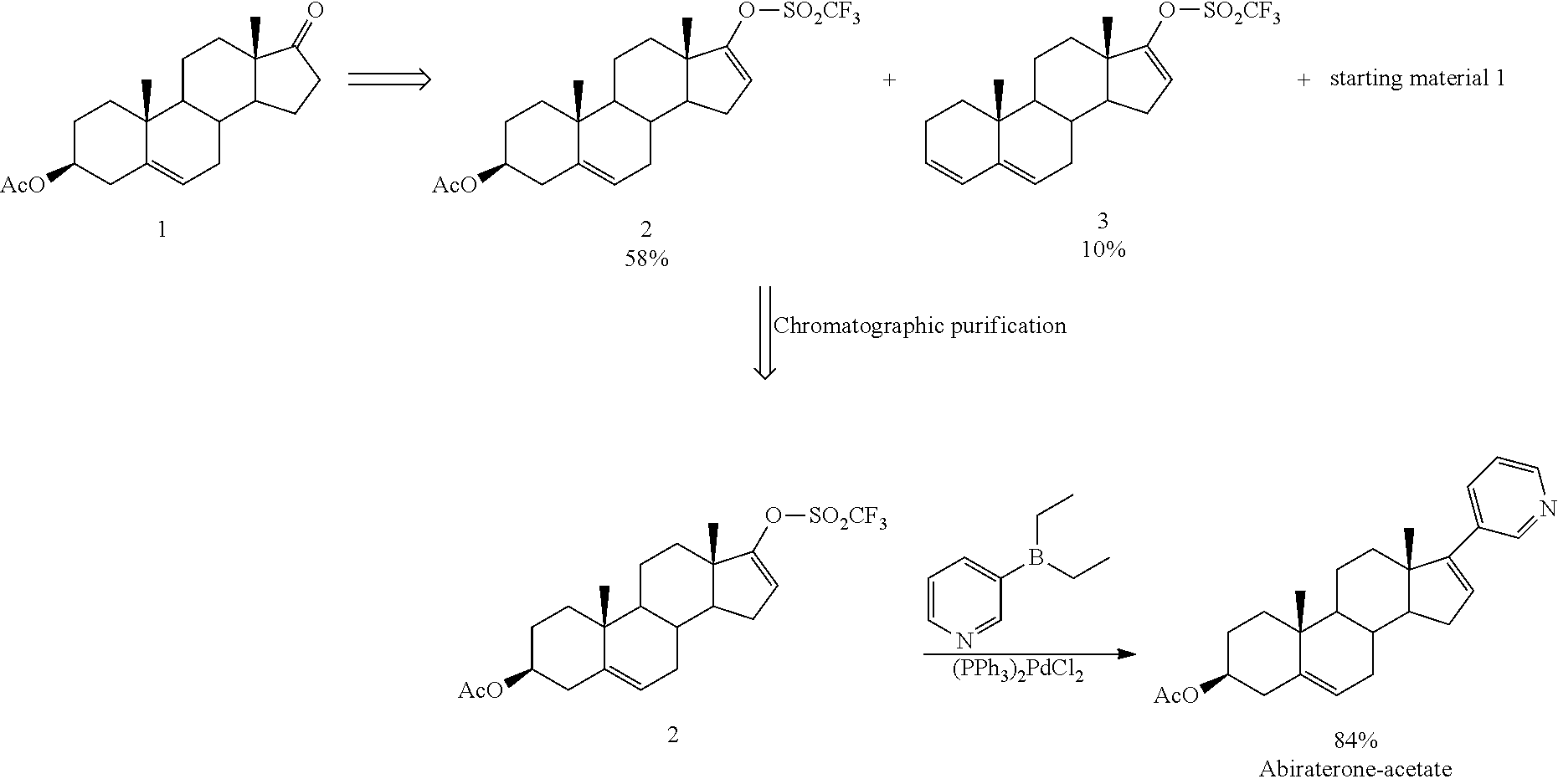
The invention provides a method for preparing (3beta)-17-(3-pyridyl)-androstane-5,16-diene-3-alcohol acetate. According to the method, the traditional Grignard addition reaction is adopted, pyridine groups are introduced in the presence of a catalyst such as cerous chloride, the method is low in raw material cost, available in raw materials, economical, practical, environment-friendly, convenient and simple in post-treatment, the reaction process is easily and continuously operated, the product quality and yield can be improved, and industrial production is promoted.
Owner:ZHEJIANG SHENZHOU PHARMA
Method for preparing abiraterone acetate
The invention discloses a convenient, rapid and economical method for massively preparing abiraterone acetate. According to the method, dehydroepiandrosterone acetate is used as a starting material, a keto carbonyl group is converted to hydrazone, and the abiraterone acetate is directly synthesized through iodination and Suzuki coupling reaction. According to the method, the operation is simple and convenient, the total yield of the three-step reaction is 34.7%, the product purity is 98.5%, the column chromatography separation purification is not required in the postprocessing, and the method is suitable for industrial production.
Owner:SUN YAT SEN UNIV
Methods and compositions for treating cancer
Methods and compositions for treating cancer are described herein. More particularly, the methods for treating cancer comprise administering a 17a-hydroxylase / C17, 20-lyase inhibitor, such as abiraterone acetate (i.e., 3beta-acetoxy-17-(3-pyridyl) androsta-5, 16-diene), in combination with at least one additional therapeutic agent such as an anti-cancer agent or a steroid. Furthermore, disclosed are compositions comprising a 17a- hydroxylase / C17, 20-lyase inhibitor, and at least one additional therapeutic agent, such as an anti-cancer agent or a steroid.
Owner:库伽尔生物科技公司
Abiraterone acetate crystal form and preparation method thereof
The invention relates to a new crystal form of a medicament, namely abiraterone acetate for treating prostatic cancer and a preparation method thereof, a pharmaceutical composition containing the new crystal form, and application of the new crystal form in preparing medicines for treating prostatic cancer.
Owner:CHONGQING PHARMA RES INST
Drug composition containing abiraterone acetate and preparation method and application of drug composition
PendingCN110538150AUniform textureDecreased pre- and post-meal differencesOrganic active ingredientsUrinary disorderEthylic acidOil phase
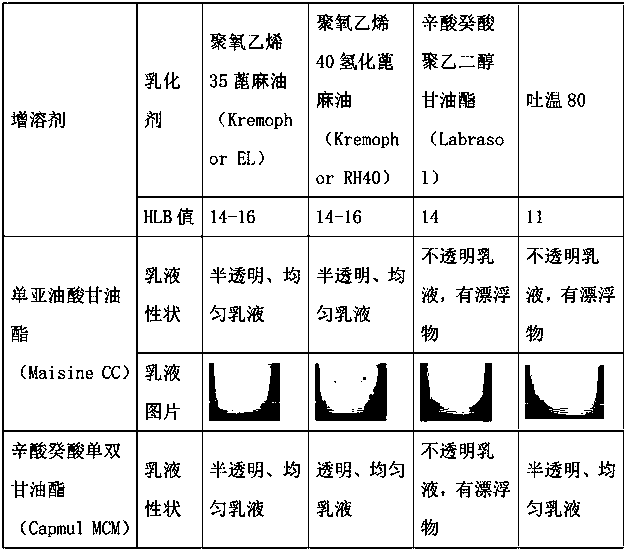
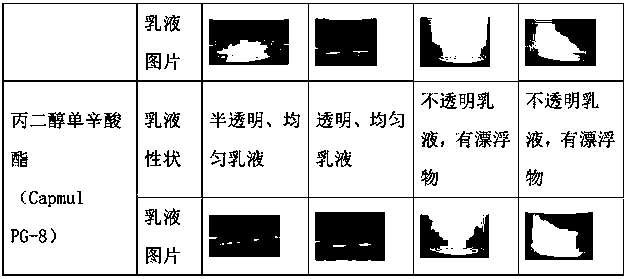
The invention relates to the technical field of drug preparations, in particular to a drug composition containing abiraterone acetate, and a preparation method and application of the drug composition.The drug composition is prepared from an active ingredient, namely the abiraterone acetate and accessories including one or more oil phases, one or more emulsifiers and one or more co-emulsifiers. After being orally taken, the drug composition meets gastrointestinal fluid to be spontaneously dispersed under gastrointestinal peristalsis to form O / W-type nanoemulsion. The formed nanoemulsion is small in particle size, the permeability of intestinal epithelial cells is improved, and the bioavailability of drugs can be significantly improved. Compared with microemulsion, a self-emulsified solution has higher stability, and the requirement of long-term preservation can be met. The drug composition is stable in content. Compared with an original drug Zytiga, the difference before and after mealis significantly reduced; and a capsule can be further prepared from the drug composition, and the capsule is stable in property.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Abiraterone acetate tablet medicine composition and preparation method thereof
InactiveCN109125276AAdvantages and Notable ImprovementsSignificant progressPowder deliveryOrganic active ingredientsDiluentHot melt
The invention relates to an abiraterone acetate tablet medicine composition and a preparation method thereof, and belongs to the technical field of medicine preparations. According to an abiraterone acetate tablet, firstly, a hot melting extruding technology is adopted for preparing a solid dispersion body of abiraterone acetate and copovidone, then the solid dispersion body, a diluent, an adhesive, a disintegrating agent and a lubricating agent are prepared into a mixture, and finally the mixture is prepared into tables. The prepared abiraterone acetate tablets improve the dissolution rate and bioavailability of a peroral solid preparation and have larger clinic application value; in addition, the prepared abiraterone acetate tablets are stable in quality, controllable and convenient to conduct industrialized production.
Owner:QILU PHARMA
Abiraterone acetate liquid capsule
ActiveCN102961358AHigh dissolution rateSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsMonoglycerideOil phase
The invention relates to an abiraterone acetate liquid capsule, comprising a capsule shell and contents, wherein the contents contain the components in percent by weight: 8-25% of abiraterone acetate and 74.8-91.8% of oil phase, wherein the oil phase comprises fatty acid monoglyceride and a solvent in a ratio of 1: 4 to 4: 1 by weight. The capsule disclosed by the invention does not contain a surfactant, as well as is good in dissolution rate, high in safety and simple in preparation process.
Owner:CHONGQING PHARMA RES INST
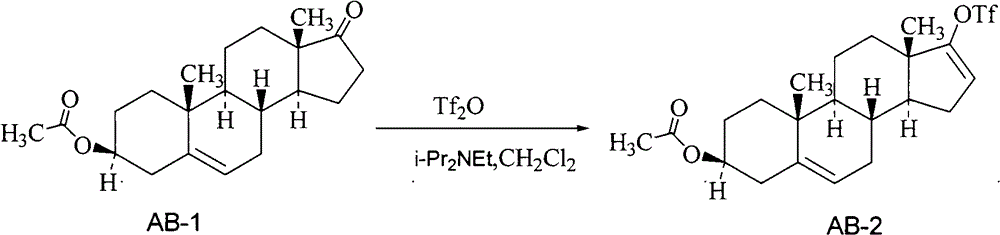
Preparation method of high-purity abiraterone acetate
ActiveCN103193849AHigh yieldHigh puritySteroidsTrifluoromethanesulfonic anhydrideDehydroepiandrosterone acetate
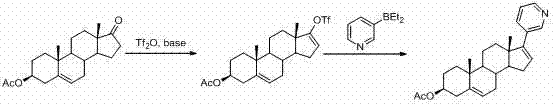
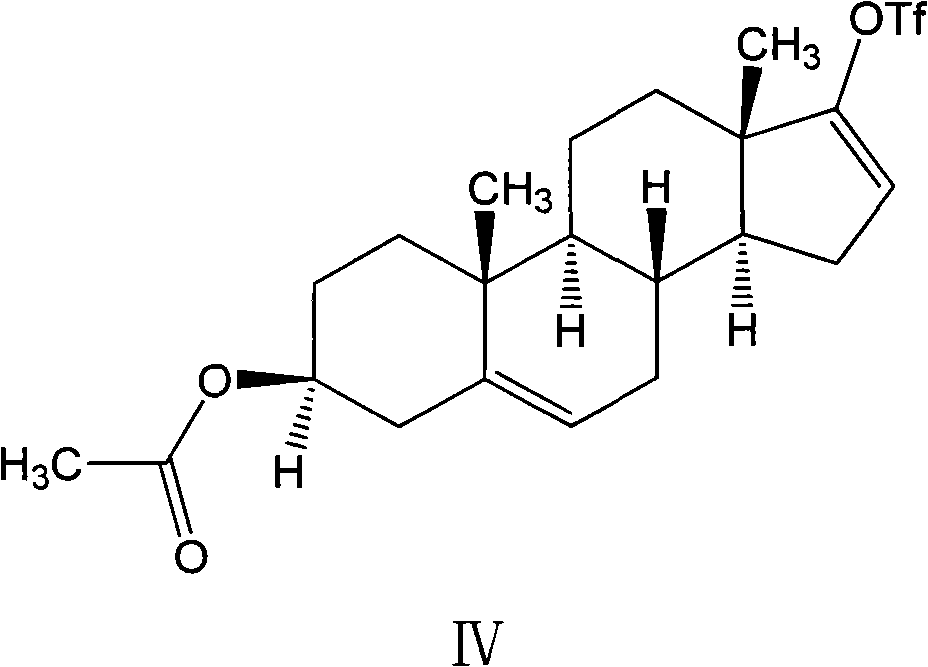
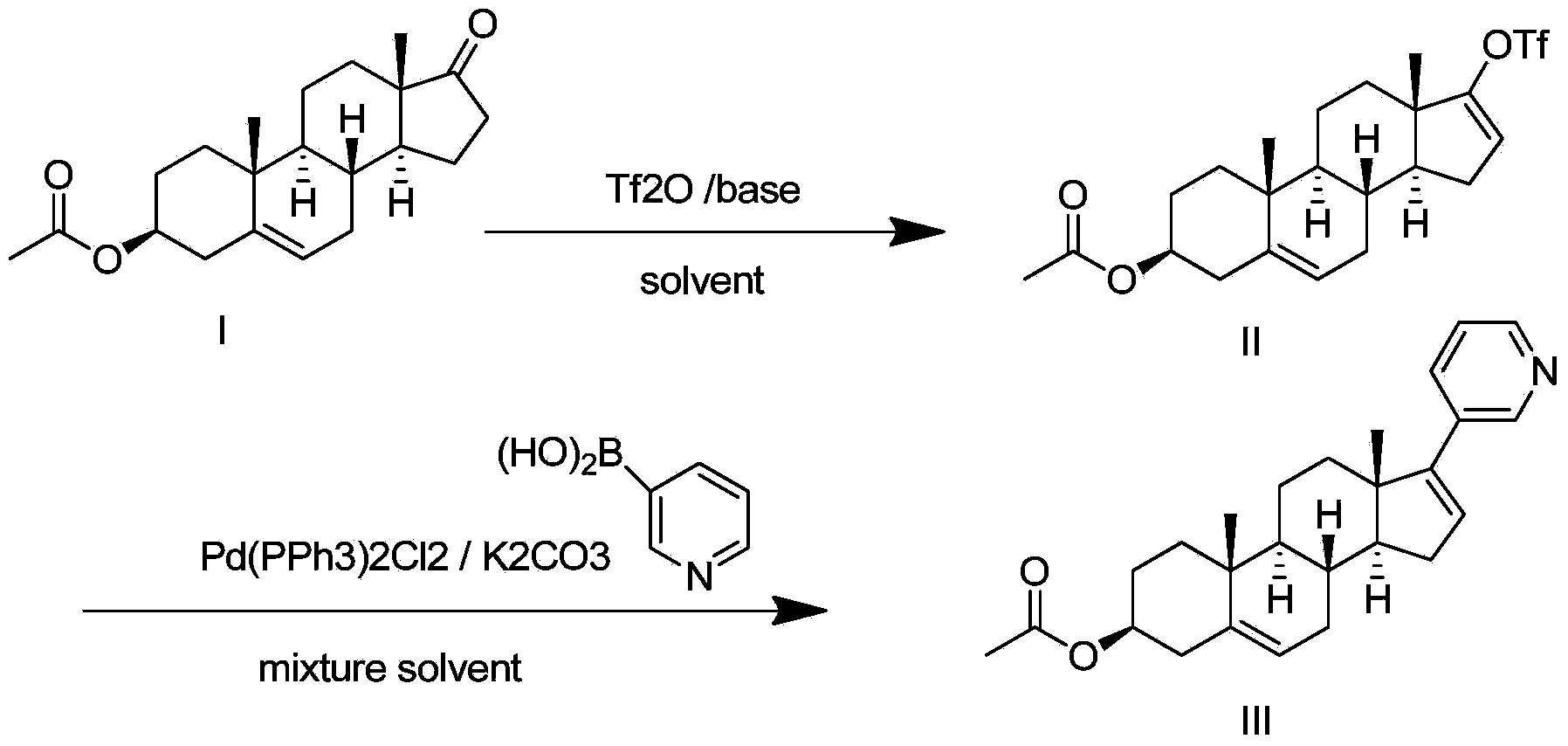
The invention relates to a preparation method of high-purity abiraterone acetate. The preparation method comprises that dehydroepiandrosterone acetate and trifluoromethanesulfonic anhydride undergo a sulfonylation reaction in the presence of an inorganic base. The preparation method obviously improves the yield of abiraterone acetate, greatly reduces by-products, has a low cost and no odor or strong smell, can be operated easily and is especially suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Abiraterone acetate sublingual tablet and preparation method thereof
ActiveCN106913539AGuaranteed curative effectAvoid stimulationOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventPolyethylene glycol
The present invention relates to a tablet, which uses water as a dissolution medium and has characteristics of rapid dissolution and good absorption. The preparation method comprises: dissolving abiraterone acetate and D-alpha-tocopheryl polyethylene glycol 1000 succinate in an organic solvent, carrying out pressure reducing drying to remove the organic solvent, mixing the obtained dried product, a filler and a disintegrant, adding a lubricant, mixing, and tableting to obtain the abiraterone acetate sublingual tablet. Compared to the abiraterone acetate sublingual tablet in the prior art, the abiraterone acetate sublingual tablet of the present invention has the following advantages that the abiraterone acetate sublingual tablet can be subjected to complete dissolution within 15 min so as to ensure the efficacy of the medicine, the complex micro-powder treatment does not required, and the bioavailability of the medicine is substantially improved.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of abiraterone acetate
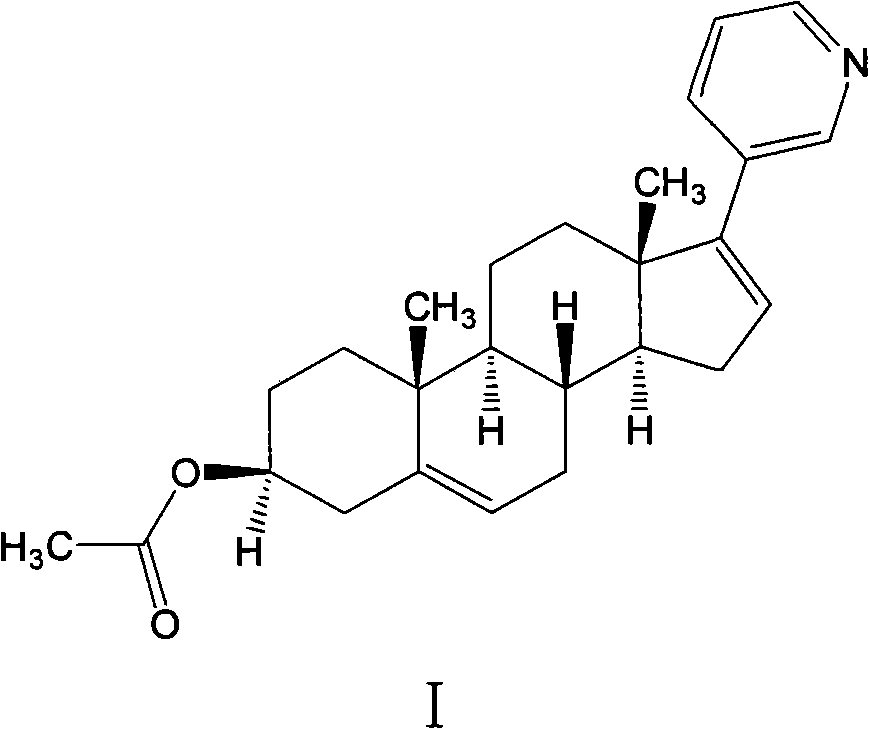
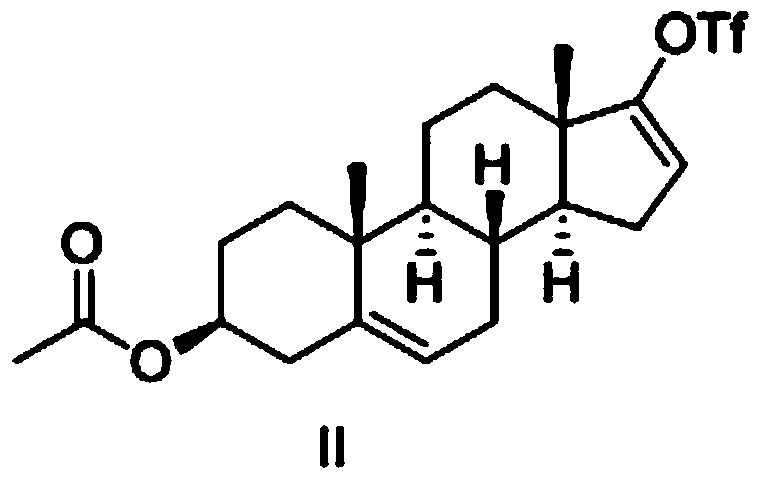
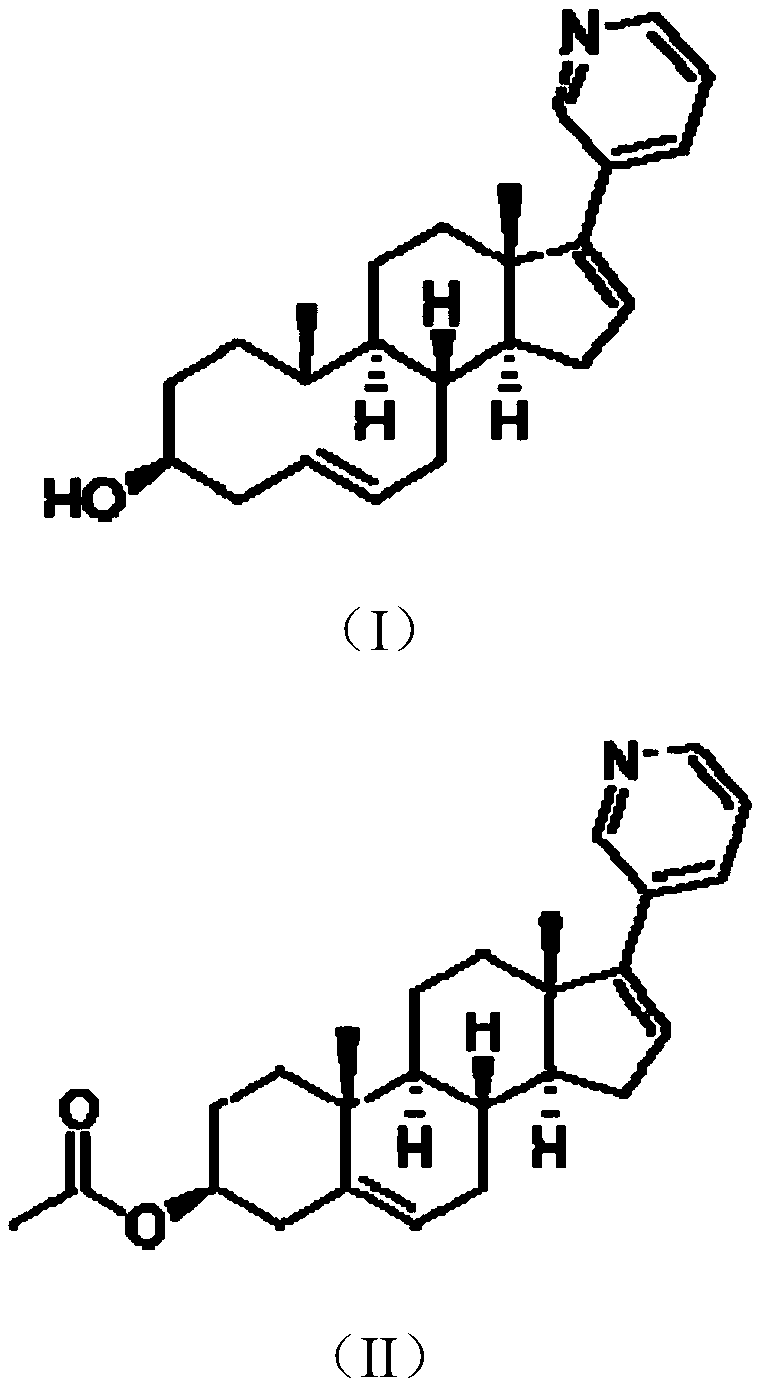
The invention belongs to the filed of medical technology, and particularly discloses a preparation method of abiraterone acetate, which comprises the following steps that compound I is acetylated to obtain compound II in an organic solvent, and compound II is coupled with halogenated pyridine to obtain the target product. The invention provides a preparation method of abiraterone acetate which can reduce the cost.
Owner:北京万全阳光医药科技有限公司
Preparation method for abiraterone acetate
ActiveCN103965282AMild reaction conditionsLow costSteroidsTrifluoromethanesulfonic anhydrideOrganic base
The invention discloses a preparation method for abiraterone acetate. The preparation method comprises the following steps: dehydroepiandrosterone acetate and trifluoromethanesulfonic anhydride are subjected to sulfonyl reaction at the presence of an organic base catalyst to obtain a compound expressed in the formula II shown in the Specification; the compound and 3-pyridine organoboron componud or 3-pyridine organosilicon compound react at the presence of triphenyl phosphine palladium dichloride catalyst to obtain crude product of abiraterone acetate; the crude product is recrystallized in a proton solvent or a non-proton solvent to obtain abiraterone acetate crystal; the crystal is put into a diffluent solvent again to heat for dissolution and then the dissolved crystal is dropwise added to a less diffluent solvent and stirred for crystallizing solids to obtain micro-powder abiraterone acetate; the diffluent solvent is a mixture of more than two of acetone, ethanol and water at will and the less diffluent solvent is water. According to the preparation method, the course is reasonable, the operation is simple and convenient, the product quality is good, the yield is high, no column flowing, salifying and refining are needed in the whole technology process, the requirement for industrial scale is met and abiraterone acetate with the approximate grain size of 10 Mu m is obtained simultaneously.
Owner:WUHAN BIOCAUSE PHARMA DEV +1
Abiraterone oral spray and use and preparation methods thereof
InactiveCN105055314ACorrectly designedTake a small doseOrganic active ingredientsAerosol deliveryPolyoxyethylene castor oilMetabolite
The invention relates to the technical field of pharmaceutical preparations and particularly relates to an abiraterone oral spray. The abiraterone oral spray comprises the following components in percent by weight: abiraterone acetate, an oil phase, a surfactant, a co-surfactant and the balance of water, wherein the oil phase is used as a carrier for prompting system micro-emulsification, and the oil phase is medium chain triglyceride, ethyl oleate or oleic acid; the surfactant is used for improving the performance of the abiraterone oral spray, expanding the range of application and realizing solubilization, and the surfactant is polyoxyethylene 40 hydrogenated castor oil or polyoxyethylene 35 castor oil; and the co-surfactant is used for reducing the surface tension of liquid and enhancing the emulsifying, moistening and blistering effects, and the co-surfactant is ethanol, n-butyl alcohol or propylene glycol. The spray has the advantages that the taking dose is reduced, so that the manufacturing cost is lowered; the formation of invalid metabolites is reduced, so that adverse reactions are effectively reduced; and the drug effect of the spray is longer than that of an oral drug.
Owner:HANGZHOU ANDE TECH CO LTD
Method for purifying abiraterone acetate
ActiveCN104447934ASolve processing problemsSuitable for industrial productionSteroidsAcetic anhydrideOrganic base
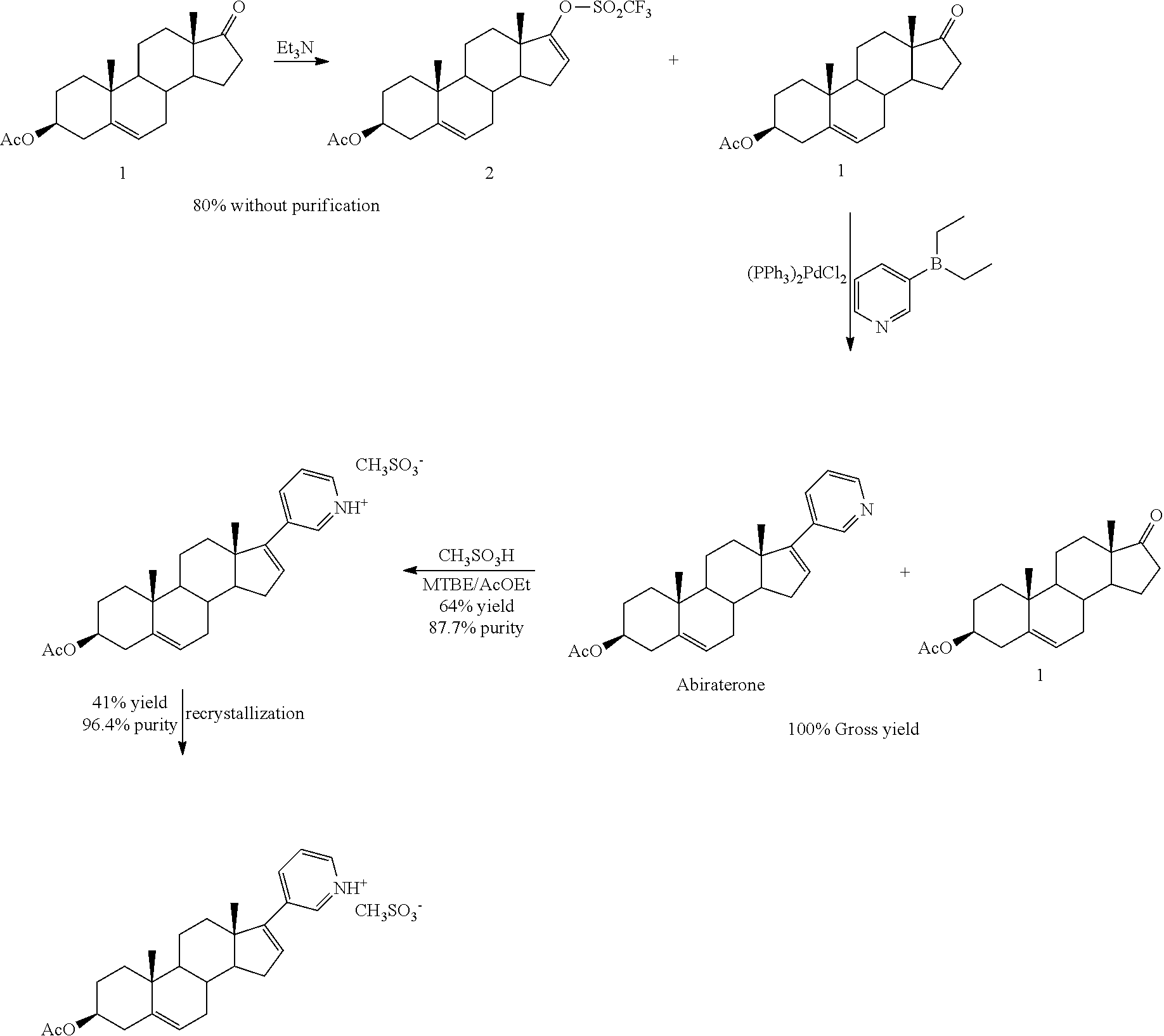
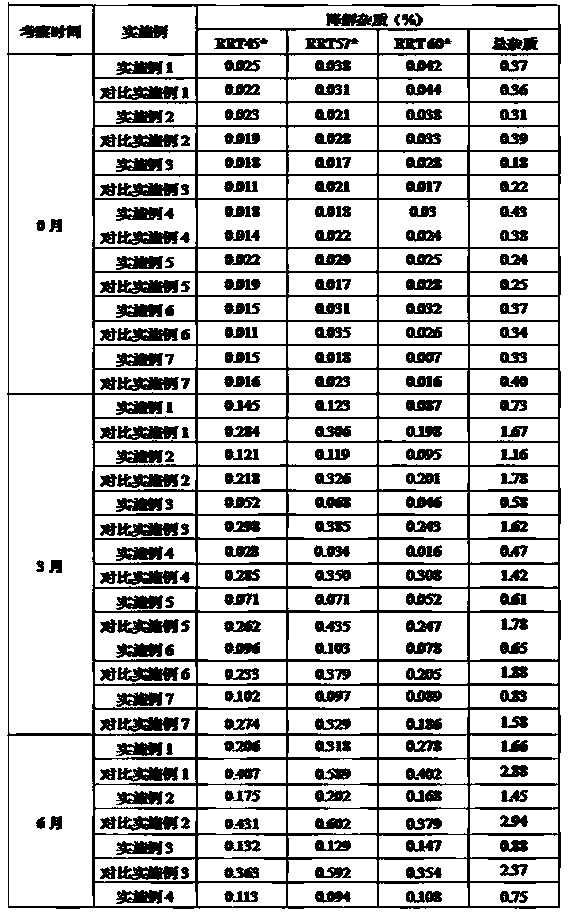
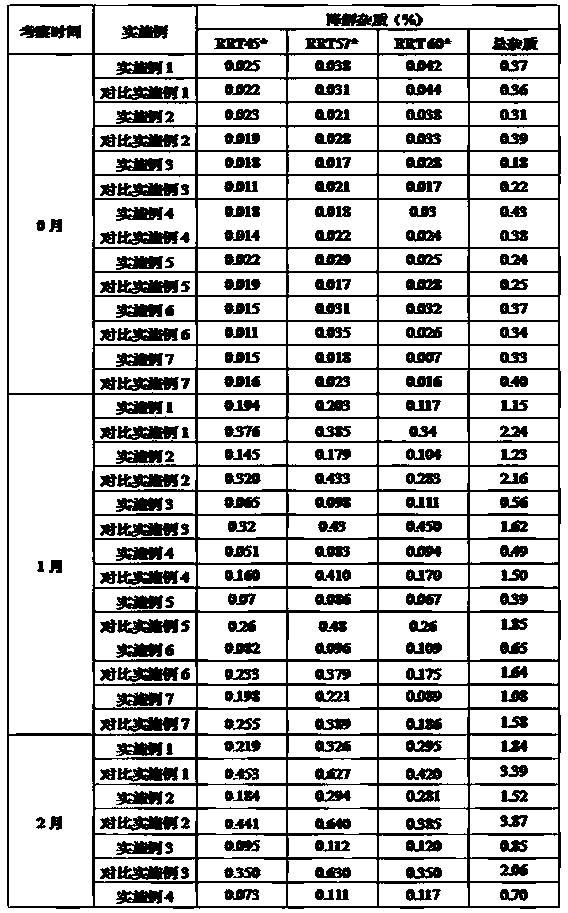
The invention belongs to the technical field of medicine synthesis, and provides a method for purifying abiraterone acetate. The method comprises the following steps: in the presence of acetic anhydride and organic bases, dissociating abiraterone acetate salt into free abiraterone acetate, wherein the high-performance liquid chromatography (HPLC) purity of dissociated abiraterone acetate is more than 99%; and then refining abiraterone acetate by virtue of acetonitrile recrystallization to obtain abiraterone acetate which has the HPLC purity of more than 99.7% and can meet the medical standards, and ensuring that the content of hydrolysis impurity abiraterone is less than 0.02%. By adopting the method provided by the invention, the problem that inevitable hydrolysis impurity abiraterone exists in a salt dissociation process of abiraterone acetate can be solved, a purification process can be simplified, and reagents can be saved; and moreover, the method is easy to operate, and is suitable for industrial production.
Owner:SHENZHEN KEXING PHARM CO LTD
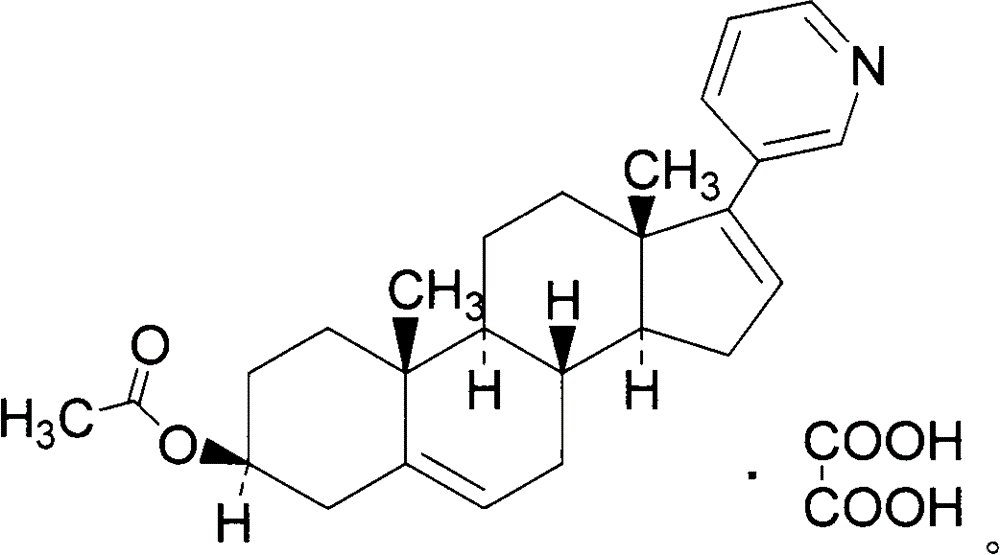
Abiraterone acetate oxalate and purification method of abiraterone acetate
ActiveCN103059090AReduce manufacturing costLow costSteroidsCarboxylic acid salt preparationOxalatePurification methods
The invention relates to an abiraterone acetate oxalate and a purification method of abiraterone acetate. Oxalate and an abiraterone acetate crude product are subjected to a reaction to obtain abiraterone acetate oxalate; and then the abiraterone acetate oxalate is subjected to a dissociation reaction to obtain abiraterone acetate.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD +1
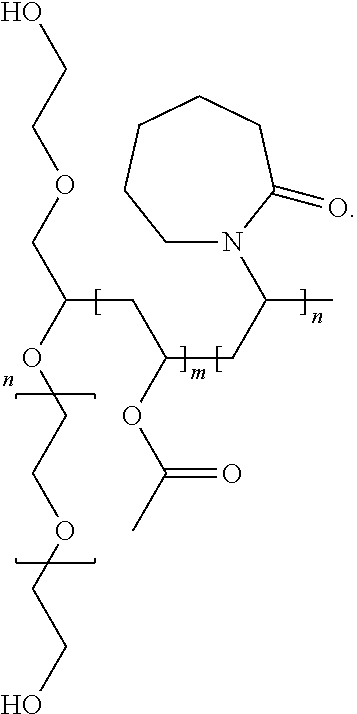
Complexes of abiraterone acetate, process for the preparation thereof and pharmaceutical compositions containing them
ActiveUS20160228455A1Improved physicochemical characteristicImprove biological performanceOrganic active ingredientsPowder deliveryFOOD EFFECTDose reduction
The present disclosure relates to pharmaceutically acceptable complex formulae comprising complexes of Abiraterone acetate and pharmaceutically acceptable excipients, process for the preparation thereof and pharmaceutical compositions containing them. The complex formulae of the present disclosure have improved physicochemical properties which results in reduced food effect which allows significant dose reduction and the abandoning of the requirement of taking the drug on an empty stomach.
Owner:TAVANTA THERAPEUTICS HUNGARY INC
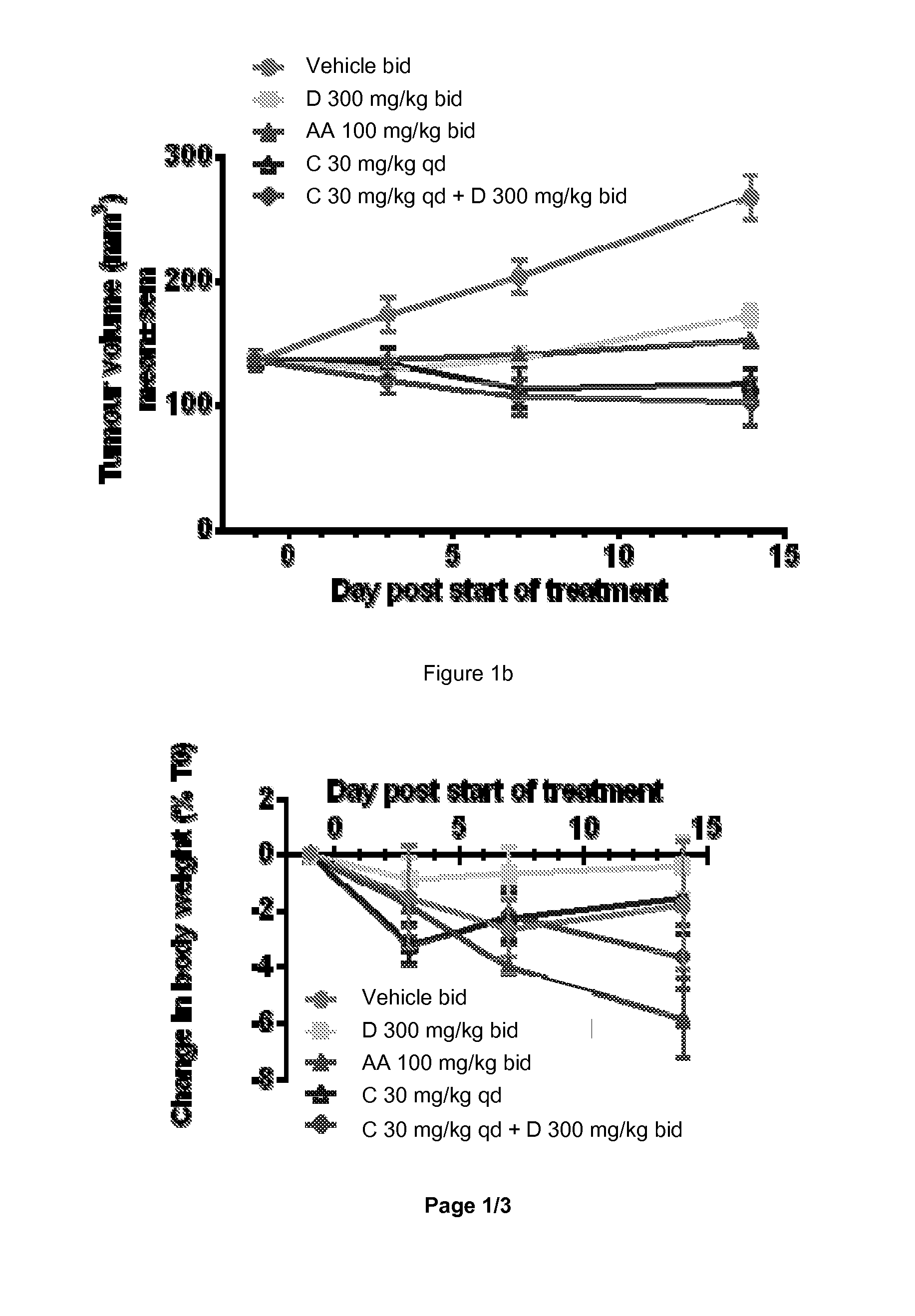
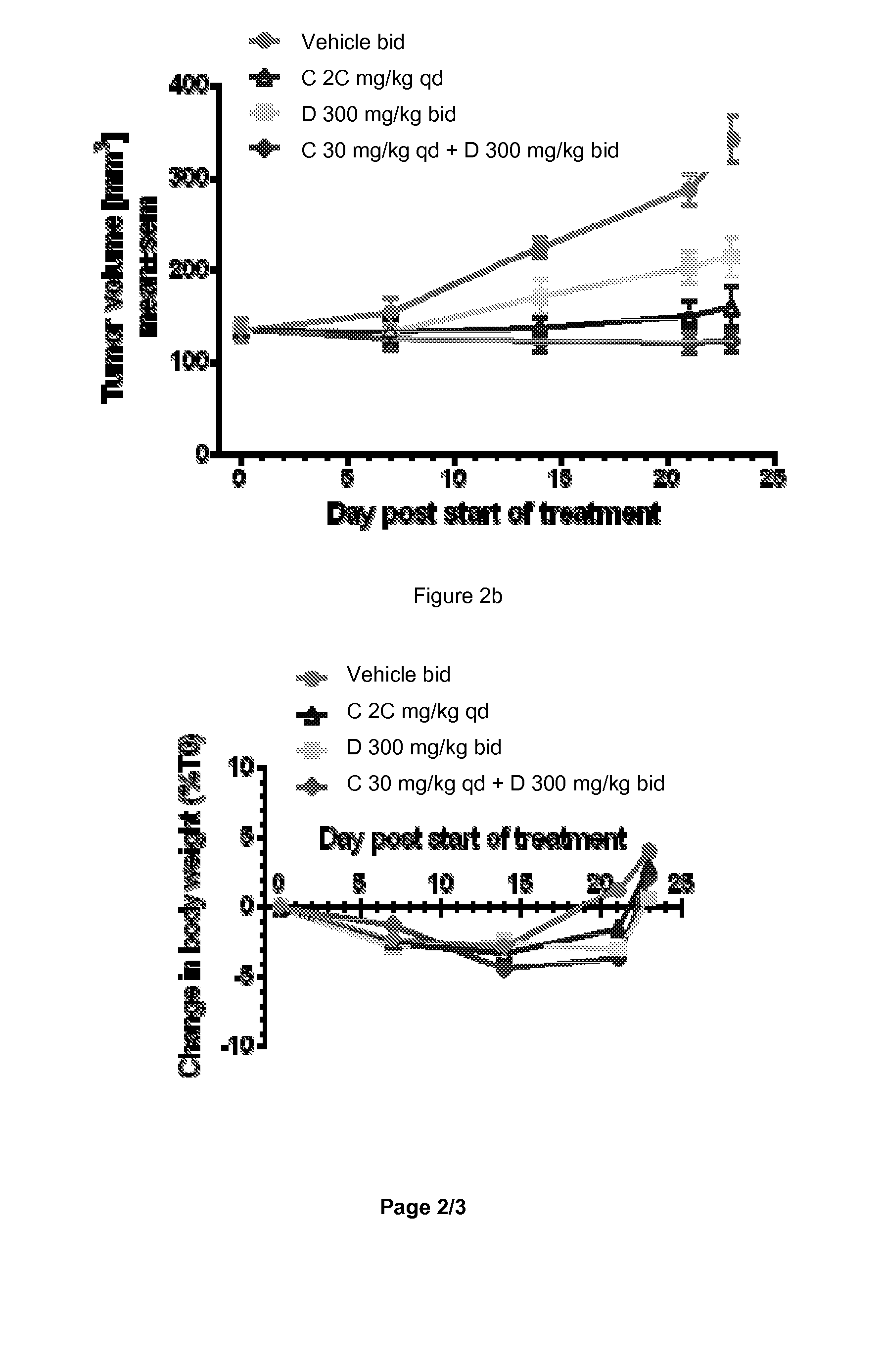
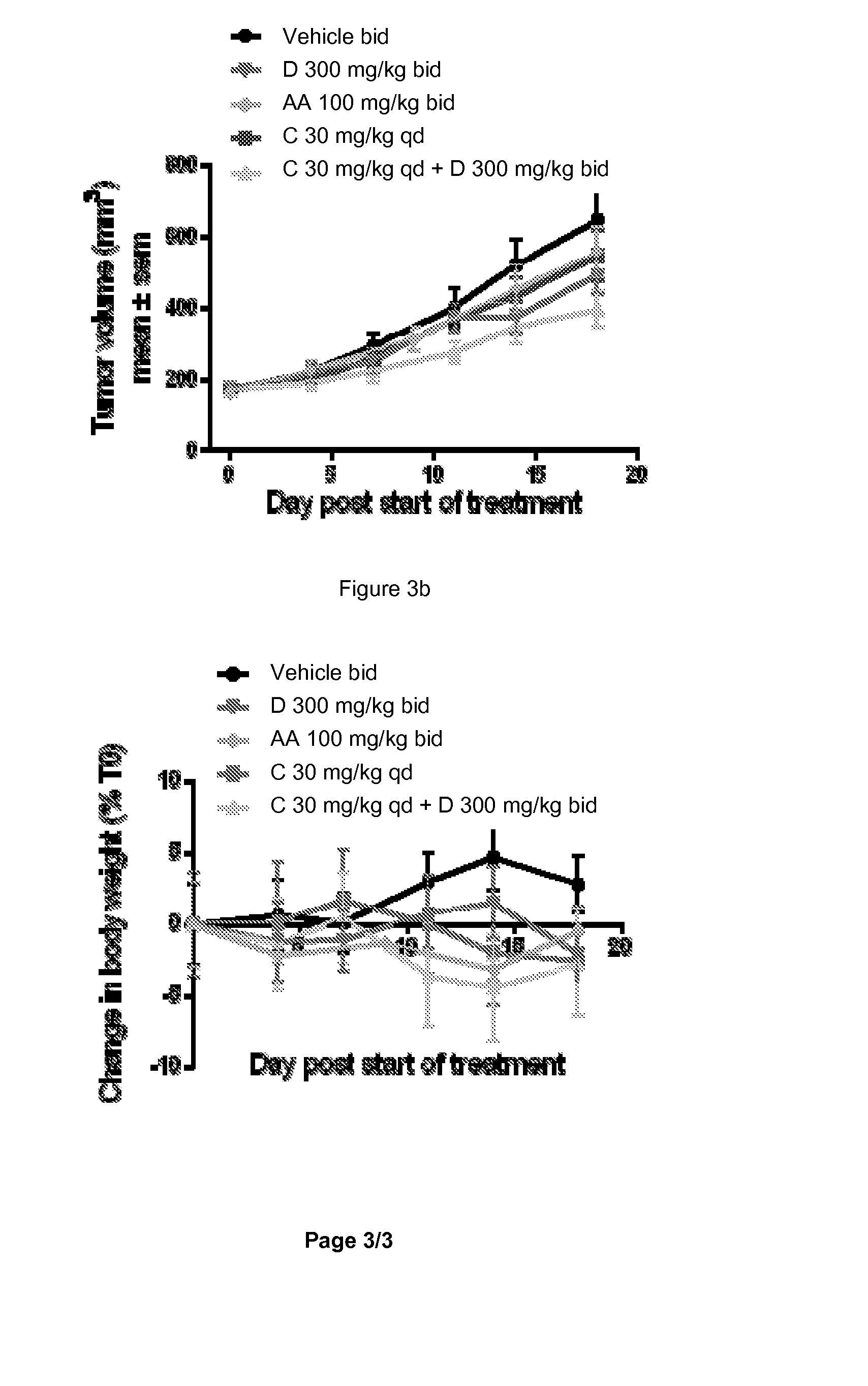
Combination of a 17-alpha-hydroxylase (c17,20-lyase) inhibitor and a specific pi-3k inhibitor for treating a tumor disease
InactiveUS20150157645A1Good effectSuitable for treatmentBiocideOrganic active ingredientsDiseaseLyase
The present invention relates to a combination which comprises (a) a phosphatidylinositol 3-kinase inhibitor selected from the group consisting of a compound of formula (I) or a compound of formula (II), or pharmaceutically acceptable salt thereof, (b) a 17α-Hydroxylase / C17,20-lyase inhibitor (CYP17 inhibitor), specifically abiraterone acetate and 1-(2-Chloro-pyridin-4-yl)-3-(4-methyl-pyridin-3-yl)-imidazolidin-2-one or pharmaceutically acceptable salt thereof, for simultaneous, separate or sequential use for the treatment of a tumor disease; a pharmaceutical composition comprising such combination; use of such combination for the treatment of a tumor disease; a commercial package or product comprising such combination; and to a method of treating a patient having a tumor disease comprising administration of said combination to a patient in need thereof.
Owner:NOVARTIS AG
Methods for preparing abiraterone acetate and intermediate thereof
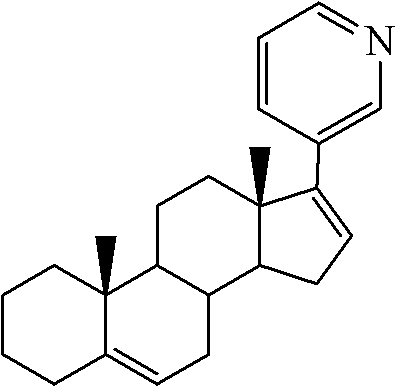
The invention relates to a method for preparing abiraterone which is a key intermediate of abiraterone acetate serving as a medicament for treating prostatic cancer and a method for preparing the abiraterone acetate by using the intermediate thereof. The methods are simple in processes, and the products have high purity and low content of impurities.
Owner:CHONGQING PHARMA RES INST
Synthesis of abiraterone and related compounds
The present invention relates to processes for obtaining abiraterone and derivatives thereof, such as abiraterone acetate, by means of a Suzuki coupling through a steroid borate of general formula (IV) or a C—C coupling through a steroid hydrazone of general formula (II), as well as to intermediates useful in said processes.
Owner:CRYSTAL PHARMA SA
Stable abiraterone oral solid medicinal composition and preparation method thereof
InactiveCN103800296AImprove stabilitySuitable for long term storageOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneEthylic acid
Owner:CHONGQING PHARMA RES INST
Preparation method of abiraterone acetate
InactiveCN105503992AHigh puritySimple and fast operationSteroidsChemical recyclingInorganic saltsMedicinal chemistry
The invention provides a preparation method of abiraterone acetate. According to the method, firstly an intermediate product, namely coarse abiraterone is purified and refined, then a purified product of abiraterone is acetylated, the abiraterone acetate is directly prepared, accordingly the coarse abiraterone product which is in an off-white color, relatively high in purity and less in impurity is obtained directly, the coarse abiraterone product is further simply crystallized, the abiraterone acetate which is high in purity and meets the medicinal requirement can be obtained, and therefore the step of chromatography or tedious purification of an inorganic salt-precipitated crystal in a traditional method can be avoided. The method has the advantages of being more convenient and faster in operation and purification treatment step, more suitable for industrialized amplification production and the like.
Owner:JIANGSU LIANHUAN PHARMA
Abiraterone oral emulsion and preparation method thereof
PendingCN111012745AHigh drug loadingImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAbirateroneDigestion
The invention discloses an abiraterone oral emulsion and a preparation method thereof. The preparation comprises an active component, a solubilizer, an emulsifier and an antioxidant; the active component is abiraterone acetate or abiraterone; and the preparation comprises, by weight, 2%-10% of abiraterone acetate or abiraterone, 20%-70% of the solubilizer, 20%-40% of the emulsifier and 0.01%-1% ofthe solubilizer. According to the invention, the drug loading capacity and the stability of the abiraterone oral emulsion are high; and the abiraterone oral emulsion can be spontaneously emulsified to form an emulsion when meeting water. And the formed emulsion is stable; the digestion influence is low; the dissolution of medicines in gastrointestinal tracts is improved; the absorption of the medicines is promoted; and the bioavailability is improved.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com