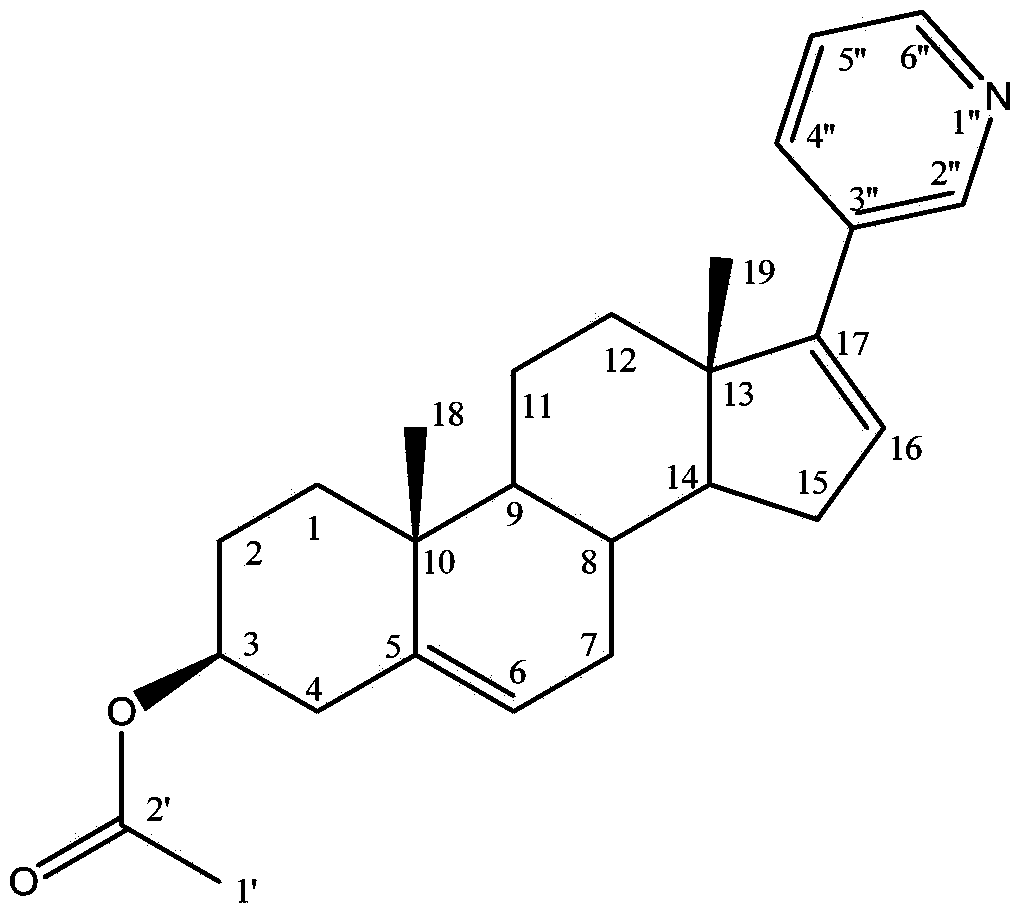
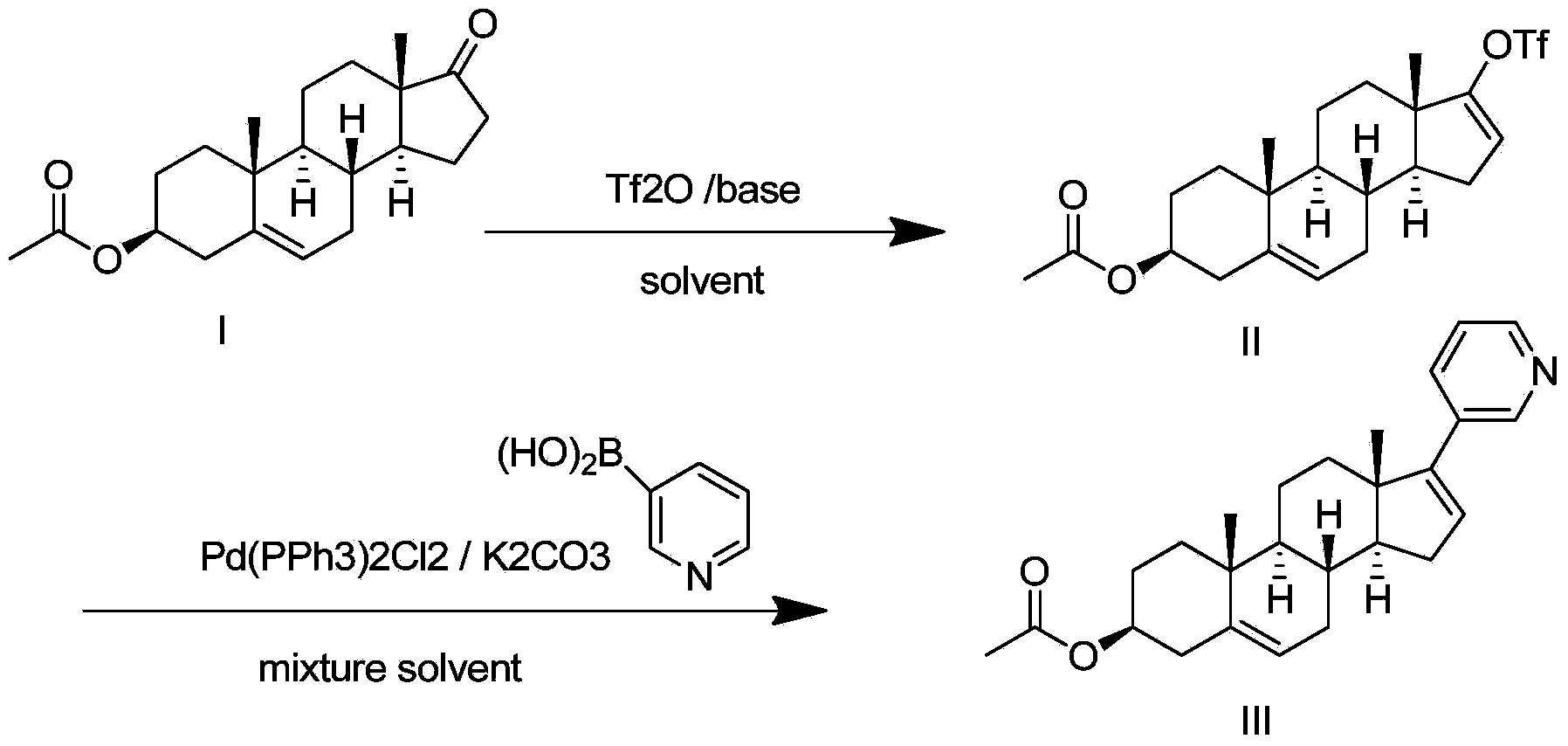
Preparation method for abiraterone acetate
A technology of abiraterone acetate and volume ratio, applied in the direction of steroids, organic chemistry, etc., can solve the problems of using too many solvents, too long reaction steps, increasing production steps and costs, etc., to achieve mild reaction conditions and low total cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 Abiraterone acetate
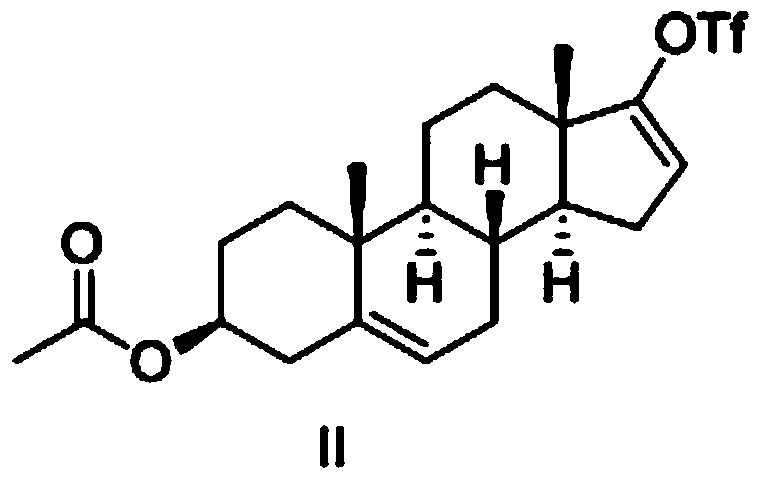
[0043] 1. In a dry 500mL reaction flask, add 10g (30.3mmol) dehydroepiandrosterone acetate and 100mL tetrahydrofuran; cool down to 0°C, then slowly add 5.6mL (33.3mmol) of trifluoromethanesulfonic anhydride dropwise, the dropwise Then continue to add 3.0mL (30.6mmol) of 2-picoline dropwise, keep the temperature not exceeding 5°C during the period, react at 0°C for 2 hours after the drop, warm up to room temperature after the reaction, stir at constant temperature for 3 hours, and go directly to the next step .
[0044] 2. Under the protection of nitrogen, cool the reaction solution in the previous step to -5°C, add 20mL of potassium carbonate aqueous solution (containing potassium carbonate 15.2g, 110mmol) to it, keep the temperature not higher than 5°C, and then add bistriphenylphosphine 97 mg (0.14 mmol) of palladium dichloride and 5.1 g (41.5 mmol) of 3-pyridineboronic acid were heated to reflux and reacted overn...
Embodiment 2
[0046] The preparation of embodiment 2 Abiraterone acetate
[0047] 1. In a dry 500mL reaction flask, add 10g (30.3mmol) dehydroepiandrosterone acetate and 100mL toluene, cool down to below -5°C, then slowly add 5.6mL (33.3mmol) of trifluoromethanesulfonic anhydride dropwise, Continue to drop the mixture of 2-picoline 1.5mL (15.3mmol) and quinoline 1.9mL (15.3mmol) after the dropwise addition, and stir at -5°C for 3 to 3.5h after the dropwise addition, the color of the reaction solution turns into Brown; after heating up to 10°C, stirring at constant temperature for 2-2.5h, the color of the reaction solution turns brown. After the reaction was over, add 150mL of water to the reaction flask to quench the reaction, stir for 10 minutes, and separate the organic layer; the aqueous phase was extracted with 50mL of toluene, and the organic phase was combined; the organic layer was washed once with 50mL of water, and then washed with saturated brine (50mL ×3) The organic layer was w...
Embodiment 3
[0051] Embodiment 3 abiraterone acetate scale-up production technology
[0052] 1. Add 20L toluene to the 100L reactor, then add 4kg (12.12mol) dehydroepiandrosterone acetate, and start stirring. When the temperature dropped below -5°C, 2240 mL (13.32 mol) of trifluoromethanesulfonic anhydride was added dropwise, and the color of the reaction solution turned light green. After the trifluoromethanesulfonic anhydride was added dropwise, the mixture was stirred at -5°C for 10-15 minutes. Start to drop 11.36L of 2-picoline and quinoline toluene solution (containing 2-picoline 600ml (6.12mol), quinoline 760ml (6.12mol), toluene 10L). After the dropwise addition, stir at -5°C for 3 to 3.5 hours, and the color of the reaction solution turns brown; then raise the temperature to 10°C, stir at a constant temperature for 2 to 2.5 hours, and the color of the reaction solution turns brown. After the reaction was over, add 60L of water to the reaction kettle to quench the reaction, stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com