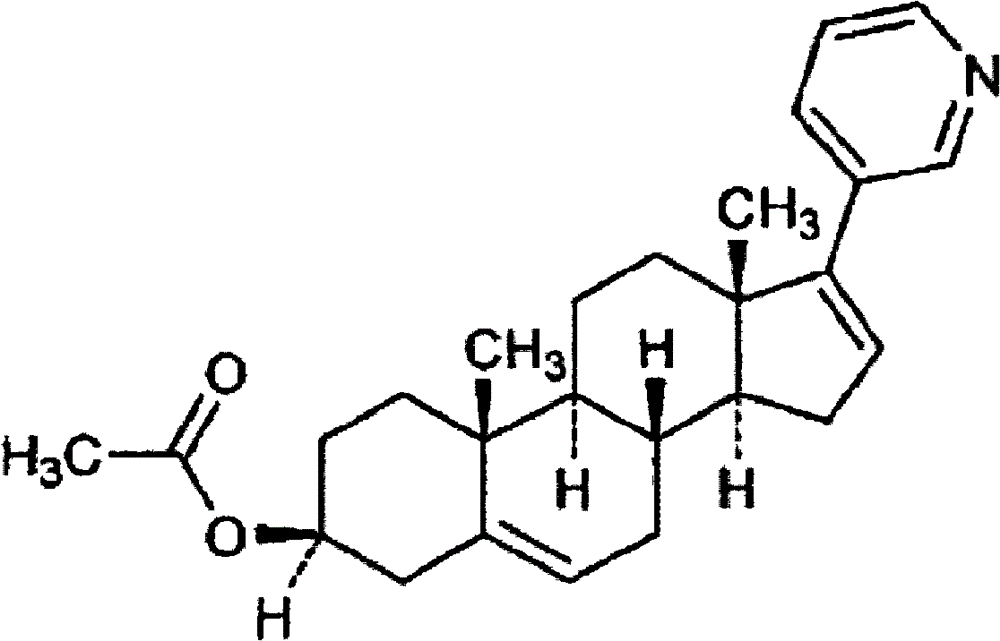
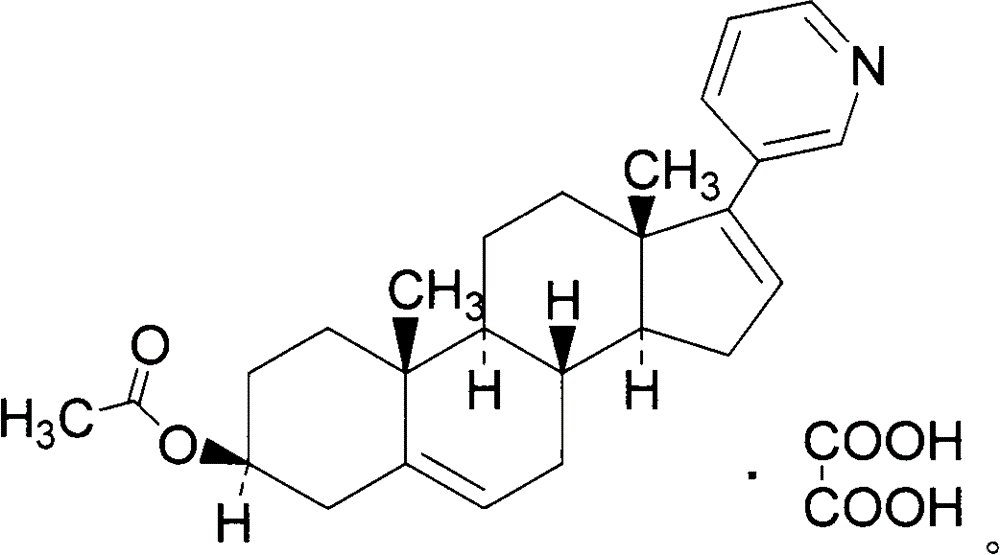
Abiraterone acetate oxalate and purification method of abiraterone acetate
A technology of abiraterone acetate and oxalate is applied in the purification field of abiraterone acetate oxalate and abiraterone acetate, can solve problems such as difficult operation, air pollution, equipment corrosion, etc. The effect of reducing production costs and equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
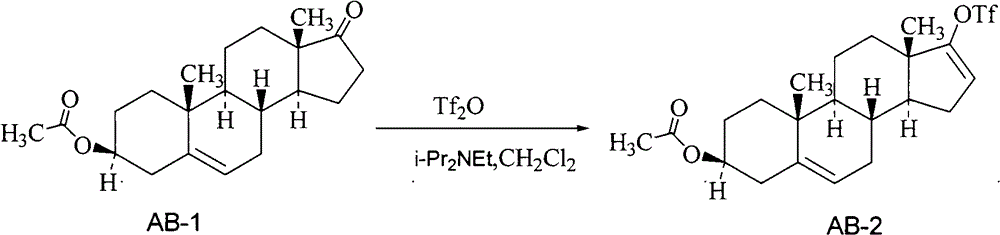
[0030] The preparation of embodiment 1 Abiraterone acetate crude product
[0031]
[0032] Dissolve 200 g of dehydroepiandrosterone acetate in 2 L of dry dichloromethane under stirring, and add Tf dropwise at 15-20°C 2 O 206g, after 0.5h dropwise, slowly add diisopropylethylamine (i-Pr 2 NEt) 96g, after 2h dripping, continue to react for 3h. Add ice water (1.5L) to quench, separate the layers, extract the aqueous phase with dichloromethane (500ml), combine the organic phases, wash with 2N hydrochloric acid and 1.5L of water successively, dry over anhydrous sodium sulfate, and concentrate to obtain a purple-black oil The product is about 280g (AB-2).
[0033]
[0034] AB-2(280g), THF(2L), Pd(PPh 3 ) 2 Cl 2 (3.7g), diethyl-(3-pyridine)borane (96g) and 2M Na 2 CO 3 (1L). Heat to an external temperature of 80°C, and react for 4 to 5 hours. Stand still to separate the liquids, extract the lower aqueous phase with ethyl acetate (1L), remove most of the THF from the up...
Embodiment 2
[0035] The preparation of embodiment 2 abiraterone acetate oxalate and the purification of abiraterone acetate
[0036] (1) Preparation of Abiraterone Acetate Oxalate
[0037]
[0038] Add 1.5 L of acetone to the crude product of abiraterone acetate (260 g), stir to dissolve, add 67 g of oxalic acid at 50 ° C, continue stirring for 1 h, then lower to room temperature and stir for 2 h. Suction filtration, the filter cake was rinsed with acetone, dried under reduced pressure to obtain off-white solid, i.e. abiraterone acetate oxalate 144.9g (AB-3), total yield 49.7% (calculated based on starting material AB-1 ), the HPLC purity detection is 96.1%.
[0039] Differential scanning thermogram analysis (DSC) of abiraterone acetate oxalate:
[0040] Instrument model: NETZSCH DSC 204S1
[0041] Experimental conditions: crucible type: standard aluminum crucible (needle piercing)
[0042] Sweep gas: N 2 20mL / min; protective gas: N 2 70mL / min.
[0043] Temperature range: 100--30...
Embodiment 3~8
[0058] The preparation of embodiment 3~8 abiraterone acetate oxalate and the purification of abiraterone acetate
[0059] Concrete operation is tested according to the method of embodiment 2, and reaction consumption and condition are operated by table 1.
[0060] Table 1
[0061]
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com