Methods for preparing abiraterone acetate and intermediate thereof
A technology of abiraterone acetate and asana, which is applied in a new preparation field of abiraterone acetate, a drug for treating prostate cancer, can solve the problems of limited purification effect, low yield of salt-forming purification operation, and low product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
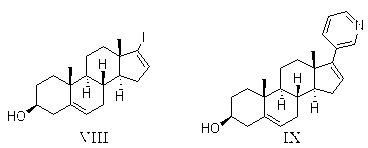
[0101] Preparation of compound VIII:
[0102] Add 100g of dehydroepiandrosterone, 400ml of ethanol, and 40ml of 80% hydrazine hydrate into the reaction kettle, heat up to reflux reaction, after the reaction, cool the reaction solution, add 1L of water, first cool to room temperature and stir for 2 hours, then cool to 0~ 10°C, stirred for 1 hour, filtered, and the filter cake was dried under reduced pressure at about 60°C to obtain 103 g of the compound of formula VII; HPLC: 98.4%.
[0103] Add 1L of tetrahydrofuran to the reaction kettle, add 168g of iodine and 160ml of tetramethylguanidine, add dropwise a solution of 100g of the compound of formula VII and 2L of tetrahydrofuran at below 10°C, after the reaction is completed, filter, concentrate the filtrate under reduced pressure, and add di Chloromethane 2L and water 2L were added, then hydrochloric acid was added to adjust the pH to acidic, the organic layer was separated, washed with water and saturated sodium chloride sol...
Embodiment 2
[0105] Preparation of Compound IX:
[0106] Take 40g of compound VIII, dissolve in 1200ml of ethanol, add 15.5g of diethyl(3-pyridyl)borane, 720mg of bis(triphenylphosphine)palladium dichloride, add 53.6g of sodium carbonate and 240ml of water solution, heated to 70~75°C for 22 hours; cooled, filtered; half of the filtrate was concentrated under reduced pressure, and 1200ml of water was added to the remaining part to stir and crystallize, and filtered to obtain the crude compound IX; HPLC purity: 86.1%, which contained impurities X 0.9%.
[0107] The above crude product was mixed with 160ml of ethanol, stirred at about 65°C for 1 hour, cooled, and filtered; the filter cake was dried under reduced pressure at about 50°C to obtain 26g of compound IX; HPLC purity: 98.6%, which contained impurities X0.5% ; Yield: 74.1%.
[0108]
Embodiment 3
[0110] Preparation of Compound IX:
[0111] Take 10g of compound VIII, add 480ml of ethylene glycol dimethyl ether to dissolve, then add 4.8g of diethyl(3-pyridyl)borane, 360mg of bis(triphenylphosphine)palladium dichloride, 60ml of water, and then add carbonic acid Potassium 21g, heated to 75~80°C for 30 hours; cooled, filtered; half of the filtrate was concentrated under reduced pressure, and 500ml of water was added to the remaining part, stirred and crystallized, filtered to obtain the crude compound IX; HPLC purity: 80.5%, containing impurities X0.6%.
[0112] The above crude product was mixed with 40ml of methanol, stirred at about 55°C for 1 hour, cooled, and filtered; the filter cake was dried under reduced pressure at about 50°C to obtain 6g of compound IX; HPLC purity: 97.7%, containing impurity X0.5% ; Yield: 68.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com