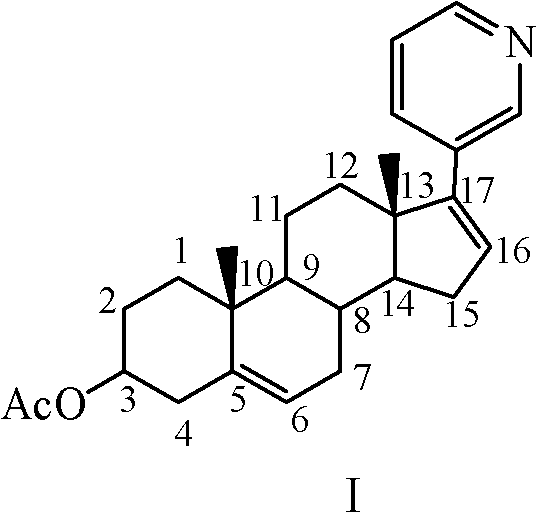
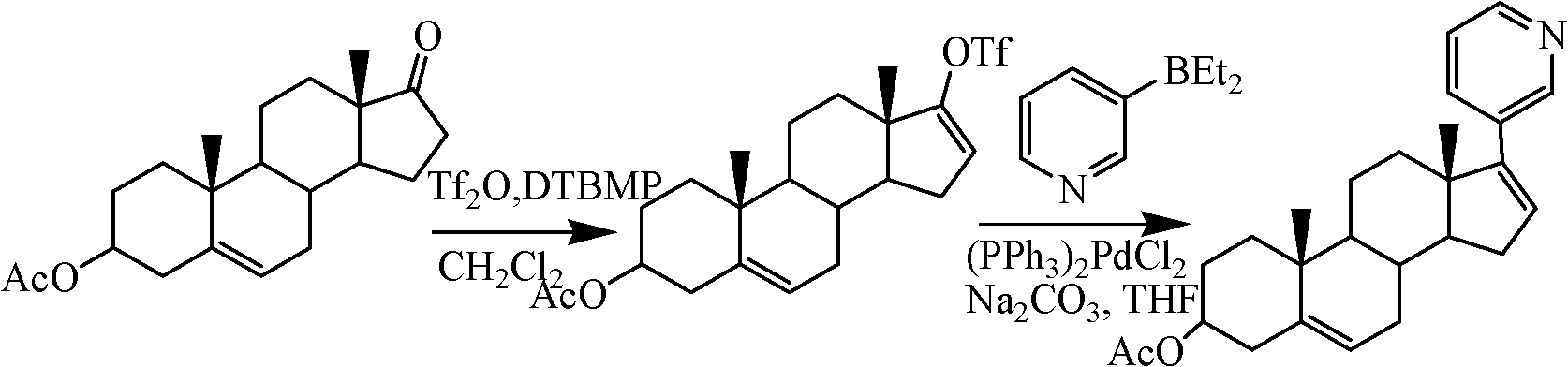
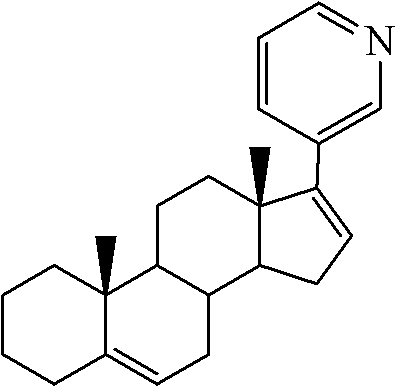
Preparation method of abiraterone acetate
A technology of abiraterone acetate and abiraterone, which is applied in the directions of steroids and organic chemistry, can solve the problems of high requirements on reaction vessels, long reaction events and high energy consumption, avoid column chromatography separation and purification, and is suitable for Industrial production, the effect of simplifying the refining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 dehydroepiandrosterone-17-hydrazone
[0027] Take a 1L three-neck flask, add mechanical stirring, add 28.8g (0.1mol) of dehydroepiandrosterone and 500mL ethanol, stir well at room temperature, then measure 20mL 80% hydrazine hydrate and 2mL 0.25mol / L dilute sulfuric acid, add, stir at room temperature Reaction for 40 hours, TLC analysis, the reaction is complete, add 450mL water and stir for 30 minutes, then pour the reaction mixture into a beaker filled with 1L water, static crystallization for 2 hours, filter, wash the solid with 2×50mL water, and vacuum dry 29.8 g of white crystals were obtained (yield: 98.7%, mp: 204-205° C.).
Embodiment 217
[0028] Preparation of Example 217-iodo-androst-5,16-diene-3β-alcohol
[0029] Take a 5L three-neck flask, add mechanical stirring, add iodine 110g (0.43mol), tetrahydrofuran 2L and diethyl ether 1.5L, nitrogen bubbles, stir well, cool down to 0°C, then add tetramethylguanidine 133mL (1.06mol), Dissolve 63.4 g (0.21 mol) of dehydroepiandrosterone-17-hydrazone in 1.6 L of tetrahydrofuran, then slowly add it dropwise to the reaction mixture, and maintain the reaction at 0 ° C for 3 hours. According to TLC analysis, the reaction is complete, and the reaction mixture is suction filtered. Concentrate the filtrate to dryness to obtain a brown oil, heat the oil to 100°C, stir and react under nitrogen atmosphere for 2.5 hours, cool to room temperature naturally, add 500 mL of ethyl acetate, stir and reflux for 30 minutes, cool, filter with suction, and adjust the pH of the filtrate with dilute hydrochloric acid To 4, the solution turns from brown to yellow, and the solution is washed w...
Embodiment 31
[0030] Preparation of Example 317-(3-pyridyl)androst-5,16-diene-3β-alcohol
[0031] (1) Take a 1L three-necked flask, install a mechanical stirrer, add 13.7mL (0.142mol) of 3-bromopyridine and 290mL of diethyl ether, respectively, blow nitrogen gas, cool down to -78°C, and slowly drop in the n-hexane solution of n-butyllithium ( 2M) 74.5mL, after the dropwise addition, continue stirring for 30 minutes, add 33.5g (0.149mol) of zinc bromide in ether solution 522mL, keep stirring and react for 1 hour, slowly rise to room temperature, concentrate under reduced pressure, blow nitrogen, add 600mLN, N -Dimethylformamide, fully stirred and dissolved, then added 17-iodo-androst-5,16-diene-3β-alcohol 39.8g (0.1mol) and tetrakistriphenylphosphinopalladium 116g (1mmol), room temperature Stir for 5 hours, adjust the pH of the reaction mixture to 1 with 1mol / L hydrochloric acid, then pour the reaction mixture into a beaker containing 500mL ethyl acetate and 500mL water, stir for 1h, separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com