Oral solid composition of abiraterone and preparation method thereof
A technology of abiraterone and abiraterone acetate, applied in the direction of drug combination, medical preparations containing active ingredients, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
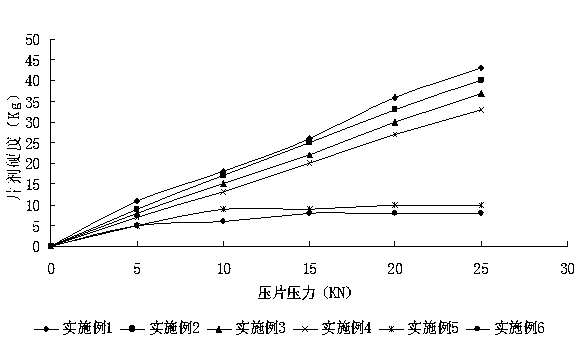
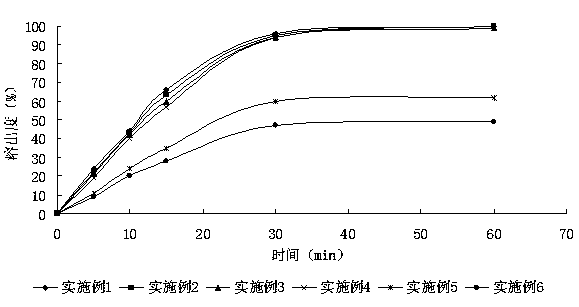
Embodiment 1~6
[0061] The prescription of embodiment 1~6 sees table 1:
[0062] The prescription of table 1 embodiment 1~6
[0063] Example Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Average particle size of abiraterone acetate particles 1.024μm 3.133μm 9.968μm 20.225μm 29.873μm 50.325μm Abiraterone acetate 250mg 250mg 250mg 250mg 250mg 250mg lactose 200mg 200mg 200mg 200mg 200mg 200mg microcrystalline cellulose 160mg 160mg 160mg 160mg 160mg 160mg Croscarmellose Sodium 40mg 40mg 40mg 40mg 40mg 40mg povidone 30mg 30mg 30mg 30mg 30mg 30mg Magnesium stearate 5mg 5mg 5mg 5mg 5mg 5mg
[0064] Preparation Process:
[0065] 1. Take the abiraterone acetate bulk drug (also known as the original drug or raw material, the same below) with the above-mentioned average particle size for later use;
[0066] 2. Pass the lactose, mi...
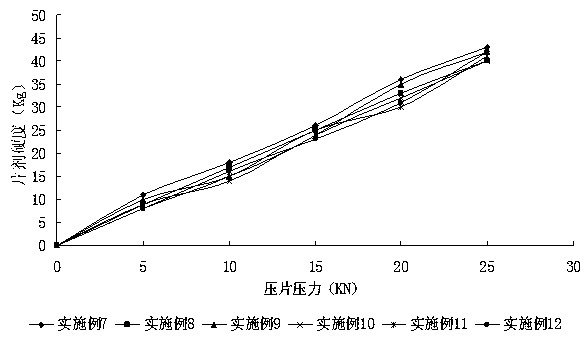
Embodiment 7~12
[0081] The prescription of embodiment 7~12 sees table 4:
[0082] The prescription form of table 4 embodiment 7~12
[0083] Example Example 7 Example 8 Example 9 Example 10 Example 11 Example 12 Average Particle Size of Abiraterone Acetate 5.492μm 5.492μm 5.492μm 12.462μm 12.462μm 12.462μm Abiraterone acetate 250mg 250mg 62.5mg 1000mg 125mg 500mg lactose 150mg —— —— 240mg 300mg 100mg microcrystalline cellulose 100mg —— 180mg —— 200mg 100mg Mannitol —— 200mg —— —— —— —— starch —— —— 150mg 100mg —— —— pregelatinized starch —— 120mg —— —— —— —— Croscarmellose Sodium 40mg —— —— 20mg 25mg —— Crospovidone —— 30mg —— —— —— 40mg Sodium carboxymethyl starch —— —— 45mg 20mg —— —— povidone 30mg —— 35mg —— —— 35mg hypromellose —— 15mg —— 15mg 10mg —— Sodium dodecyl sulfate 20mg —...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com